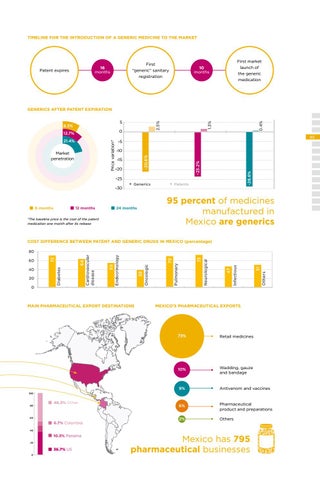
TIMELINE FOR THE INTRODUCTION OF A GENERIC MEDICINE TO THE MARKET
First “generic” sanitary registration
16 months
Patent expires
First market launch of the generic medication
10 months
-10
-20 -25 -30
12 months
• Generics
-28.6%
-15
-23.2%
Market penetration
85
-5
-20.6%
Price variation*
21.4%
1.3%
0
12.7%
6 months
2.5%
5
8.3%
0.4%
GENERICS AFTER PATENT EXPIRATION
• Patents
95 percent of medicines manufactured in Mexico are generics
24 months
*The baseline price is the cost of the patent medication one month after its release
COST DIFFERENCE BETWEEN PATENT AND GENERIC DRUGS IN MEXICO (percentage)
MAIN PHARMACEUTICAL EXPORT DESTINATIONS
51 Others
47
Infectious
Neurological
73
70 Pulmonary
39 Oncologic
0
55 Endocrinology
20
64 Cardiovascular disease
40
Diabetes
60
72
80
MEXICO’S PHARMACEUTICAL EXPORTS
73%
Retail medicines
10%
Wadding, gauze and bandage
9%
Antivenom and vaccines
6%
Pharmaceutical product and preparations
2%
Others
100
80
60
40
46.3% Other 6.7% Colombia 10.3% Panama
20
36.7% US 0
Mexico has 795 pharmaceutical businesses