

Webinar will start at 3pm BST 2nd October



Webinar will start at 3pm BST 2nd October

Dr Bev Isherwood
Opening Remarks - Dr Bev Isherwood
Introduction to PACE – Dr Clive Mason
Funding Call : Overview and Key Features – Dr Peter Coombs
Process, selection criteria and resources – Dr Tanja Taylor


Dr Clive Mason


Bring together the sector to help innovators with early-stage antimicrobial and diagnostics projects move forward with greater speed and confidence, accelerating the delivery of new innovations to tackle AMR

A collaboration between three leaders in the UK’s health innovation and research community


The UK’s innovation agency, inspiring, involving, and investing in innovation Self-funded medical research charity with the ambition to transform the way diseases are identified and treated

Independent not-for-profit, national innovation centre reshaping drug discovery for patient benefit

Diagnostic testing is a pivotal tool to help address AMR
Diagnostics have the potential to reduce inappropriate use of antibiotics that can allow drug resistance to develop
Early diagnosis and appropriate management of infections can improve patient outcomes
Enablement of precision medicines
Diagnostics are pivotal for effective deployment of novel antimicrobial therapeutics
Diagnostics can rapidly detect and contain resistant infections







Only 7% of AMR R&D investment goes towards diagnostics
Total AMR R&D Investment*
3x Less investment than therapeutics*
4-5% RTI, UTI & BSI each receive 4% of the total Dx funding Sepsis receives 5%*

Grow a pipeline of highquality antimicrobial drugs and innovative diagnostics aligned with unmet needs
Provide innovative researchers in Academia and SMEs the funding, advice and support they need to build derisked and investmentready assets
Convene collaborative network(s) to identify barriers to innovation, advance programmes, and drive new collaborative R&D
The right funding, at the right time with the right support
Funding and wrap-around incubator-like support

• Translating academic and SME innovation
• Guidance and mentorship to support assets and their developers
• Access to Scientific Infectious Disease and Diagnostic expertise
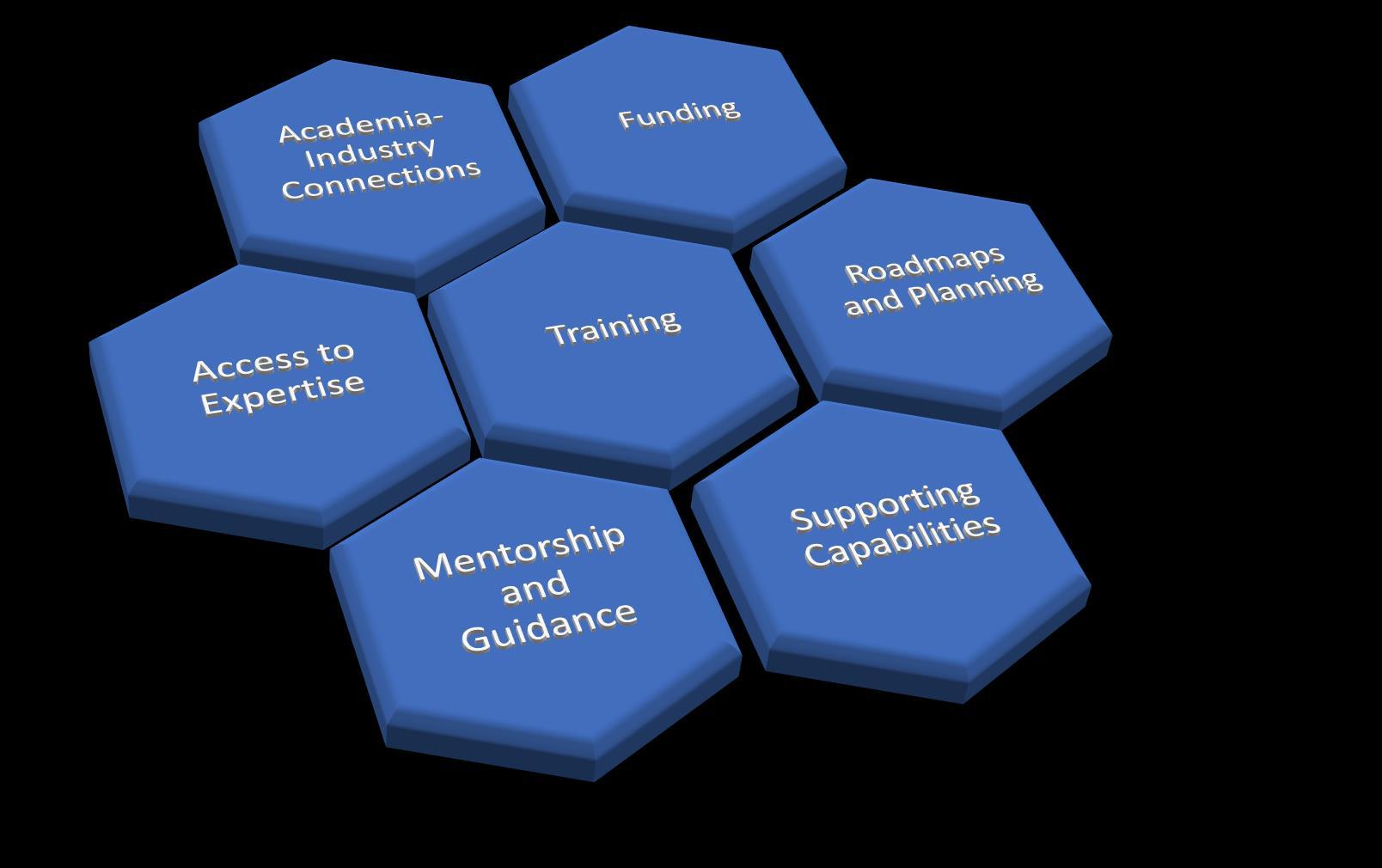


A connected community
Innovators
Teams
• Overcoming barriers together
• Provision/ access to capabilities and materials
• Navigating Regulatory complexity
• Applying best practice and lessons learned
Academia
Acceleration & Innovation AMR
Funders &
investors
Regulators, Payers & HTA bodies
Patients, Charities, Advocacy Groups and Professional associations

With this support, our science community will be better supported and collectively we will have the best chance of making bigger strides towards tackling AMR.
A more connected, better supported community with a new focus
The AMR community will feel more connected and more effective, ultimately leading to a better pipeline of tests and treatments with the potential to help save lives.

Increased R&D in AMR with more investment ready assets

A strengthened pipeline of medicines and diagnostics aligned with patient need

Dr Peter Coombs

PACE’s second funding call: £5m available to support up
Aim to provide participants with funding and support that secures delivery of key data for onward development and investment
Focused on technologies to tackle antimicrobial resistance and improve patient outcomes through innovation in diagnostics
The portfolio will be actively managed. Projects will receive support and advice from the PACE team





















• New in-vitro diagnostics in development at TRL stage 3-5 (late TRL 2 considered)
• Between technical feasibility to early prototype incl. field studies for generating data in relevant environment
• We are looking for projects addressing unmet diagnostic needs in bacterial infections with the highest burden of antimicrobial resistance, with the aim to:
✓ Reduce inappropriate antibiotic prescriptions
✓ Provide faster results that indicate which antibiotic should be used
✓ Catalyse the move to personalised, narrow-spectrum treatments

Rapid tests suitable for use in level 1 health care settings that can 1) identify whether a bacterial infection is present and/ or 2) identify the most appropriate treatment and, ideally, the underlying pathogen(s).
Innovative solutions for use at-home or in the community setting are also encouraged.
We are prioritising solutions for the following scenarios:
Rapid tests to identify presence of a bacterial infection applicable to level 1 healthcare settings, community care and hospital triage settings.
Rapid, near-patient tests that identify the most appropriate treatment and, ideally, the underlying pathogen for pneumonia applicable to primary care or hospital settings; minimally invasive/ burdensome sample types will be prioritised.
Hospital-based tests that can provide faster identification of pathogens and antibiotic susceptibility profile for suspected BSI/ sepsis cases direct from sample1 .
Includes diagnostic innovation for detection and management of the bacterial component of neonatal sepsis.

• New in-vitro diagnostics or adaptation of existing diagnostics that could be implemented in new healthcare settings/ target regions/ countries, or deployed for new indications/ use-cases
• Proposals that include potential for community care (e.g., pharmacy, care home, school, community diagnostic centre) or ‘at-home’ use
• Analytes can be a direct measurement of bacterial markers or host-based biomarkers, or combination thereof
• Applications providing solutions tailored to an intended setting. This includes those tailored towards resource-limited settings, including in low- and middle-income countries (LMICs)













• Later-stage diagnostic development (TRL6+), incl.








• Longer term studies in support of regulatory filings such as long-term stability studies
• Manufacturing of the final instrument/ scale-up activities
• Marketing support including submission of marketing approvals
• Tests that require central lab infrastructure
• Standalone: pathogen ID, technology/ method/ device development, algorithm/ ML model development, biomarker identification/ validation, pilot lot production of reagents and instruments
• Surveillance-only approaches and solutions to improve empiric treatment based on surveillance data
• Diagnostics focused solely on fungal/ viral/ parasitic infections, sexually-transmitted infections or TB
• Projects over 2 years in duration


Feasibility:
Technology concept formulated

▪ Intended Use: outline clinical unmet need, market/ competitor landscape, patent landscape
▪ Design: demonstration of approach/ concept (breadboard, basic device)
▪ Performance: demonstrate clinical validity of biomarker(s), proof-of-concept data on contrived samples is required


Feasibility:
Proof of concept demonstrated
▪ As TRL 2 plus:
▪ Intended Use: Intended Use Statement (intended user/ setting/ purpose)
▪ Design: design components integrated, develop reagents and buffers
▪ Performance: Finalise diagnostic targets and methods for detection, quantifying targets, lab-based demonstration of end-to-end process, proof-of-concept data on clinical† specimens is required
▪ Implementation: Business Plan developed, risk analysis, regulatory strategy (preferred)
Design optimisation & characterisation /
Design Specifications completed


▪ As TRL 3 plus:
▪ Design: product requirements documented (Design Inputs), all design variables set (Design Outputs) for Minimum Viable Product, regulatory strategy (finalised)
▪ Performance: Data on real samples is required, early clinical evidence gathered to indicate safety and accuracy, stress testing/ characterisation††, relevant performance characteristics demonstrated†††

• Academics or researchers from SMEs worldwide, including consortia (large diagnostic industry partners permitted, but must cover own costs)
• Applications should have a single lead applicant, however co-applicants are welcome
• Should last up to two years
• Will be milestone driven with clear go/ no go progression criteria defined upfront
• With up to £1 million GBP of direct costs – for product development projects (TRL 4-5)
• With up to £300K GBP of direct costs – for technical feasibility projects (TRL 3*)
*late stage TRL 2 with reasonable evidence considered

Our intention is not just to fund but to add additional support to enable the best chances of success for projects to attract further onward investment
Support throughout the project funding journey tailored to individual project needs, working in partnership with the PACE team:
✓ Support through the application process (e.g., 1:1s, advice on full application, Catalyser workshop)
✓ Diagnostic development & disease area insight, advice & mentorship
✓ Access to a collaborative network of potential project delivery partners
✓ Facilitate and support project delivery where applicable (e.g., development of statements of work and acting as point of contact with delivery partners)
✓ Facilitate scientific advisory boards, and public and patient engagement panels
✓ Business support (e.g. orientation to potential downstream funders, commercial know-how such as pitch-deck preparation)


For innovators to maximise impact & reach their potential, it is essential that funded recipients and the PACE team work closely together. Therefore, we have the following expectations of applicants & funded recipients:
✓ Be engaged in the process and work collaboratively with the PACE team, especially R&D advisors, in all aspects of project development and lifecycle if successful (note: our PACE R&D advisers will be separated from funding governance and will provide support not monitoring)
✓ Each applicant will, if successful, attend (or send a delegate to) the Catalyser workshop (virtual)
✓ Successfully funded projects to start within 3 months of the receipt of an award letter and be committed to the project length specified
✓ Legal and ethical requirements are followed (e.g., award agreement terms, good laboratory practice in conducting work)
✓ Applicant will highlight and raise any issues or concerns in a timely manner throughout the process

Intellectual Property and Ownership of results
• Funding is non-dilutive
• Recipient background and arising IP will remain property of the recipient
• No requirement to revenue share upon successful commercialisation
Funding
Awards shall cover 100% of the direct costs of the project, provided such costs are Permitted Expenditure.
Permitted Expenditure is expenditure which is reasonably incurred and properly evidenced and is directly related to and necessary for the delivery of the Project. Typically, this will cover:
• An appropriate %FTE of staff, plus consumables, directly related to funded work
• Access to specialist equipment/ reagents
• Contract Research Organisation work
• Reasonable travel and subsistence
Receipt of funding will be linked to achieving milestones and standard payment terms will be quarterly in arrears

Dr Tanja Taylor

• EOI Opens 26th Sept 2024
• Applicants apply online
• Queries can be submitted via web form
• EOI closes at 23:59 GMT on 30th Oct 2024
Sept – Oct 2024
• Internal review by PACE team to select proposals to progress to full application
• Successful applicants invited to Catalyser workshop
• Sign Confidentiality Agreements
• 14th Jan: Catalyser workshop for Applicants
• Full application developed with support from PACE team
• Application deadline: Apr 2025
• Due diligence conducted on applications by PACE team
• Review of applications by an external expert panel
• Applicants notified of outcome
Project initiation
• Finalise project plans based on panel feedback
• Finalise budget and resource requirements
• Award letters issued
• Project launch
Nov – Jan 2024/25
Jan - Apr 2025 Apr - Jun 2025
Projects to start within 3 months from receipt of award letters
• Name, affiliations of lead applicant and any co-applicant(s)
• Application summary: Title, funding requested, duration, category e.g. diagnostic modality/approach/sample type/target biomarker, primary indication, intended setting and development stage
• Project summary (max 200 words)
• Alignment to clinical need (max 200 words)
• Differentiation of approach (max 200 words)
• The scientific rationale (max 400 words) - includes option for 1 file upload
• Research plan – key research objectives, funding request, work packages, milestones and deliverables (max 400 words)
• How each investigator/ collaborator holds the necessary expertise and experience to deliver the project (max 200 words)
• Outline any gaps in current resources or capabilities that PACE could advise on or support to improve the impact of the proposed project (max 200 words)
• Exploitation/Translational plan (max 200 words)

To be considered as a participant on the programme your expression of interest must be submitted between 26th September and 23:59 GMT on 30th October via the online portal


Selection criteria
We will prioritise projects that can provide evidence or a strong rationale for diagnostics that are:
▪ Rapid: Point of care bacterial infection diagnostics with a focus on rapid time-toresult, low cost, and ease-of-use (i.e., requirement for minimal training)
▪ Innovative: Novel technologies, or innovations on existing technologies
▪ Accurate: Sensitivity and Specificity appropriate for the situation in which they will be used
▪ Clinical value and patient benefit: Must provide evidence or an implementation roadmap to demonstrate improved outcomes such as: reduced length of hospital stays, reduced mortality, faster time to cure, reduction of unnecessary prescription of antibiotics, improved tailoring of antibiotic prescriptions
▪ Suitable: Must have potential to fit into the target healthcare setting. Proposal as to how the innovation would integrate into the target healthcare setting and care pathway (e.g., integration with IT systems, reporting, sample preparation)
▪ Affordable: Good potential for cost-effectiveness in the chosen setting. Feasible proposal for delivering test at an affordable price. Also considers environmental sustainability
• Every application is required to have a clearly articulated target profile outlining the target indication(s), use and diagnostic profile.
• These should demonstrate consideration for patient and clinical needs, as well as key diagnostic characteristics such as:
• Target analyte and sample type
• Patient population
• Setting of use
• Diagnostic performance
• User and infrastructure requirements
• Diagnostic workflow integration
• Cost and value proposition


• Priority will be given to solutions for indications associated with the highest burden of AMR and suitable for use at the point of care.
• Examples of TPPs include those developed by WHO, CARB-X and CF AMR Syndicate.

Overview of what we expect from a research plan at the EOI stage (including key definitions):
• Objectives – the research endpoints that will be successfully delivered by the end of the project
• Deliverables/ outputs – the quantifiable results that arise from a project
• Work packages – defined strands of work within a project plan, often resulting in the delivery of independent research deliverables
• Milestones - a specific point in time within a project plan that aligns to a critical go/ no go point in which the results generated up until that point, possibly over multiple work packages, must be critically evaluated to assess whether the project should continue. Milestones will have associated success criteria which will be agreed by PACE prior to award

Further questions –contact via website form
Terms and Conditions
Key Date: Online EOI closes 23:59 GMT on 30th October
Please note – all applicants must complete the online EOI before 23.59 GMT on 30th October 2024 to be considered for this funding call

Webinar recording



Rapid tests suitable for use in primary care that can 1) identify whether a bacterial infection is present and/ or 2) that identify the most appropriate treatment and, ideally, the underlying pathogen(s). Innovative solutions for use at-home or in the community setting are also encouraged.
▪ Setting: Health Care Level 1 (e.g., Primary Care Physician, Health Centre, Pharmacy) and/ or Urgent Care and Emergency in Health Care Level 2
▪ Diagnostic Type: Rapid, point-of-care triage tests that determine whether an antibiotic is needed
▪ Indication(s): suspected UTI
▪ Impact Measures: Reduced antibiotic prescribing, reduced return visits, faster infection resolution
Bacterial vs Non-bacterial Triage Tests
• Detection of bacterial infection should fit within normal clinical consultation timeframe (e.g., in Level 1/primary care setting < 10 mins)
• Low cost
• Easy to use (minimal training and infrastructure requirements)
• Fully integrated, or semi-integrated with simple sample preparation steps
▪ Setting: Health Care Level 1; Return Visits (unresolved)/ Level 2/3; Referral or In-Patient
▪ Diagnostic Type: Rapid AST technologies (ideally incl. pathogen ID) that indicate which antimicrobials should be used
▪ Indications: suspected UTI
▪ Impact Measures: Reduced hospital admissions/ re-admissions, reduced hospital lengths of stay, reduced recurrence/ severe disease, more appropriate antibiotic prescribing, faster infection resolution
▪ AST + pathogen ID available for same-day follow-up to fit within primary care or hospital workflows (< 6hrs)
▪ Easy to use (minimal training and infrastructure requirements – should be usable outside of a (reference) lab setting)
▪ AST/ ID could be linked with a triage test

Test Features
Example diagnostic technologies/ platforms
• Diagnostics for use in low resource settings should align with REASSURED
• Improved accuracy over UTI dipstick/ host-marker (e.g. CRP) or equivalent
• Ideally suitable for elderly/ pregnant/ young patients
• Triage test could be linked with AST/ ID test
• Host- or pathogen-based marker detection LFIA
• LOC (lab-on-a-chip, e.g., microfluidic test chips with integrated functions)
• Closed-cartridge molecular diagnostic systems (compact)
• Novel technologies
Example diagnostic technologies/ platforms
▪ Automated combined ID + phenotypic AST systems (compact)
▪ Culture-independent molecular methods, e.g., non-phenotypic systems for detecting pathogens and AMR
▪ Novel AST systems including use of metagenomics and AI
NOTE: These are ideal test characteristics, proposals for products that do not meet all the criteria will still be considered
Rapid tests to identify presence of a bacterial infection applicable to primary care, community care and hospital triage settings. Rapid, near-patient tests that identify the most appropriate treatment and, ideally, the underlying pathogen for pneumonia applicable to primary care or hospital settings; minimally invasive/ burdensome sample types will be prioritised.
▪ Setting: Health Care Level 1 (e.g. Primary Care Physician, Health Centre, Pharmacy) and/ or Urgent Care and Emergency in Health Care Level 2
▪ Diagnostic Type: Rapid, point-of-care triage tests that determine whether an antibiotic is needed
▪ Indication(s): suspected CA-LRTI
▪ Impact Measures: Reduced antibiotic prescribing, reduced return visits, faster infection resolution
Bacterial vs Non-bacterial Triage Tests
• Detection of bacterial infection should fit within normal clinical consultation timeframe (e.g., in Level 1/primary care setting < 10 mins)
• Low cost
• Easy to use (minimal training and infrastructure requirements)
• Fully integrated, or semi-integrated with simple sample preparation steps
▪ Setting: Health Care Level 1; Return Visits (unresolved)/ Health Care Level 2/3; Referral or In-Patient
▪ Diagnostic Type: Rapid AST technologies (ideally incl. pathogen ID) that indicate which antimicrobials should be used
▪ Indications: suspected LRTI (incl. CA and HAP/VAP)
▪ Impact Measures: Reduced hospital admissions/ re-admissions, reduced hospital lengths of stay, reduced recurrence/ severe disease, more appropriate antibiotic prescribing, faster infection resolution
▪ AST + pathogen ID available for same-day follow-up to fit within primary care or hospital workflows (< 6hrs)
▪ Easy to use (minimal training and infrastructure requirements – should be usable outside of a (reference) lab setting)

Test Features
Example diagnostic technologies/ platforms
• Diagnostics for use in low resource settings should align with REASSURED
• Ideally suitable for elderly/ pregnant/ young patients
• Triage test could be linked with AST/ ID test
• Host- or pathogen-based marker detection LFIA
• LOC (lab-on-a-chip, e.g., microfluidic test chips with integrated functions)
• Closed-cartridge molecular diagnostic systems (compact)
• Novel technologies
Example diagnostic technologies/ platforms
▪ LRTI: Alternative sample types to sputum and bronchiolar lavage encouraged for LRTI (e.g., whole blood, saliva)
▪ AST/ ID could be linked with a triage test
▪ Automated combined ID + phenotypic AST systems (compact)
▪ Culture-independent molecular methods, e.g., non-phenotypic systems for detecting pathogens and AMR
▪ Novel AST systems including use of metagenomics and AI
NOTE: These are ideal test characteristics, proposals for products that do not meet all the criteria will still be considered

Hospital-based tests that can provide faster identification of pathogens and antibiotic susceptibility profile for suspected BSI/ sepsis cases direct from sample. Includes diagnostic innovation for detection and management of the bacterial component of neonatal sepsis.
▪ Setting: Health Care Level 2/3; referral, emergency department or in-patient
▪ Diagnostic Type: Tests that rapidly detect blood stream infections, and, ideally, the underlying pathogen and susceptibility profile to indicate which antibiotics should be used
▪ Indications: suspected BSI, incl. (neonatal) sepsis
▪ Impact Measures: reduced mortality, faster time to resolution, reduced hospital lengths of stay, faster time to antibiotic de-escalation/ increased ratio of narrow- vs broad-spectrum antibiotics
▪ Provide results that are generated faster than standard blood culture methods (within one hospital shift, < 8hrs)
▪ Straight from sample and without separate pre-culture or extensive sample prep

Example diagnostic technologies/ platforms
▪ Improved, standardised sample collection
▪ Reduced risk for contamination
▪ Detection element can be de-linked from AST/ ID
▪ Needs to be cost-effective given high volume of negative blood cultures
▪ Automated combined ID + phenotypic AST systems (ideally compact)
▪ Culture-independent molecular methods, e.g., non-phenotypic systems for detecting pathogens and AMR
▪ Novel technologies
NOTE: These are ideal test characteristics, proposals for products that do not meet all the criteria will still be considered

National Reference Laboratory,
Academic Medical Centres
Tertiary Care: Hospital Clinics and Public Health Laboratories
Specialist treatment
Secondary Care: Emergency and urgent
care clinics and walk-in centres
Urgent and emergency care and planned or elective care in hospital
Primary care practitioner’s clinics, pharmacies and wider community health programmes and initiatives including virtual ward services
National Reference Laboratories
Specialised labs and networks, provide assistance with clinical trials, evaluate new technology, conduct surveillance, national reference labs and conduct AST for 1st and 2nd line drugs. Senior health specialists.
Regional/provincial laboratories
Lab capability as for Levels 1 and 2 but also usually cultures samples and conducts pathogen ID and AST for first line drugs. Specialists/senior technicians to operate.
District Hospitals
Lab capability is as for level 1 but also ability for e.g. gram staining and have some automated kit. Technicians and assistants to operate.
Health Centres
Health centres, health posts and outreach
Centres can perform simple lab POCT inc. simple microscopy. Clinicians & other healthcare workers on site.
Health Posts often don’t have lab capability but healthcare workers can perform some POCT
(WHO,