Use of microfluidics technology to model α-synuclein aggregation and seeding in Parkinson’s disease in vitro
Eve Corrie1, Rebecca Kelly1, Matthieu Trigano1, and Emma V. Jones1
1Medicines Discovery Catapult, Block 35, Alderley Park, Cheshire, SK10 4ZF, UK

• Many neurodegenerative diseases are associated with the presence of misfolded, aggregating proteins within the brain, leading to cytotoxicity and cell death
• The prion hypothesis states that these toxic species spread through the brain via anatomically connected regions, leading to widespread neurodegeneration
• α-synuclein is a protein that is implicated in the pathogenesis of Parkinson’s disease, as well as other synucleinopathies such as dementia with Lewy bodies and multiple system atrophy
• Mutations in the SNCA gene that encodes α-synuclein are known causes of familial, early-onset Parkinson’s disease and are thought to change the structure of the protein to increase beta-sheet formation2 – promoting the misfolding, aggregation and the prion-like spread of α-synuclein pathology through the brain

An early-onset Parkinson’s disease-associated mutation increases the rate of α-synuclein phosphorylation and aggregation Introduction


Exposure to α-synuclein preformed fibrils triggers phosphorylation and aggregation of native α-synuclein in iPSC-derived neurons
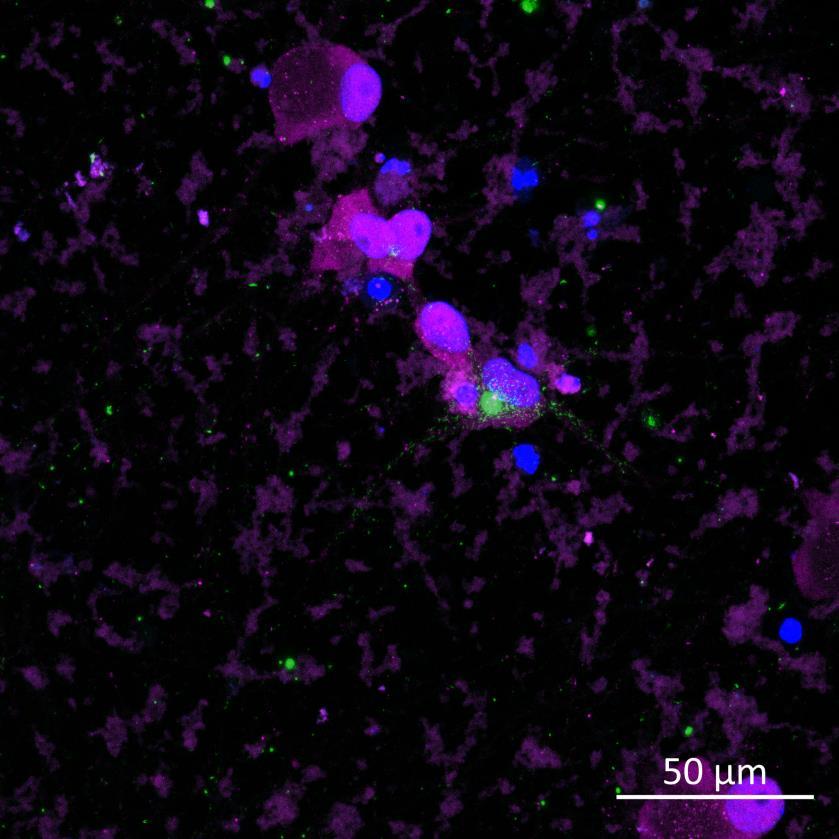
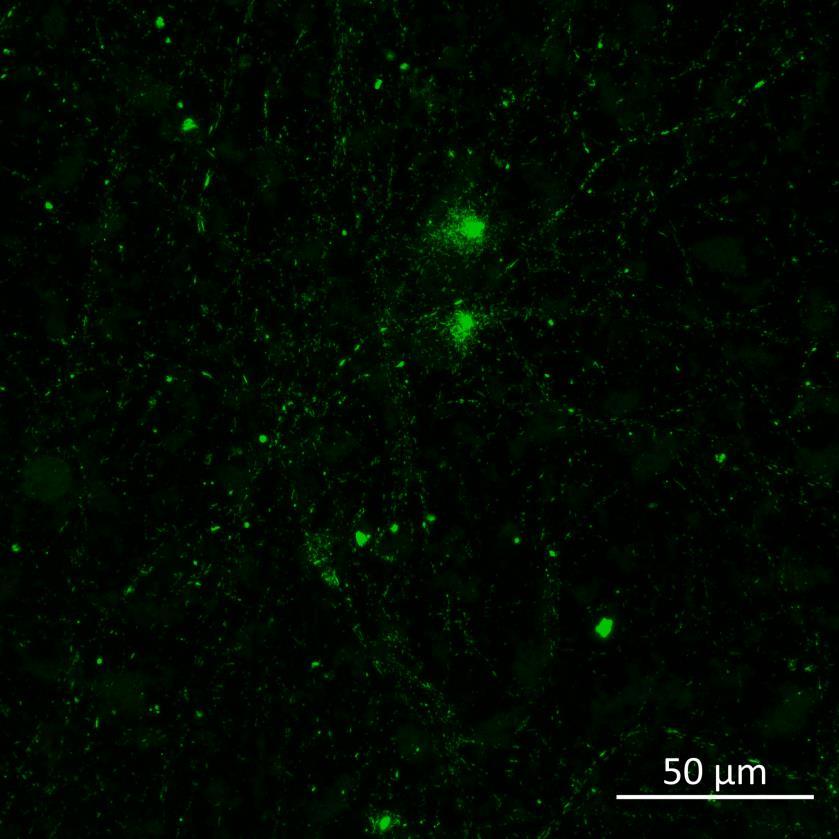
• Human iPSC-derived cortical neurons (ioGlutamatergic neurons, bit.bio) were exposed to recombinant, aggregated α-synuclein preformed fibrils (PFFs) for 24 hours
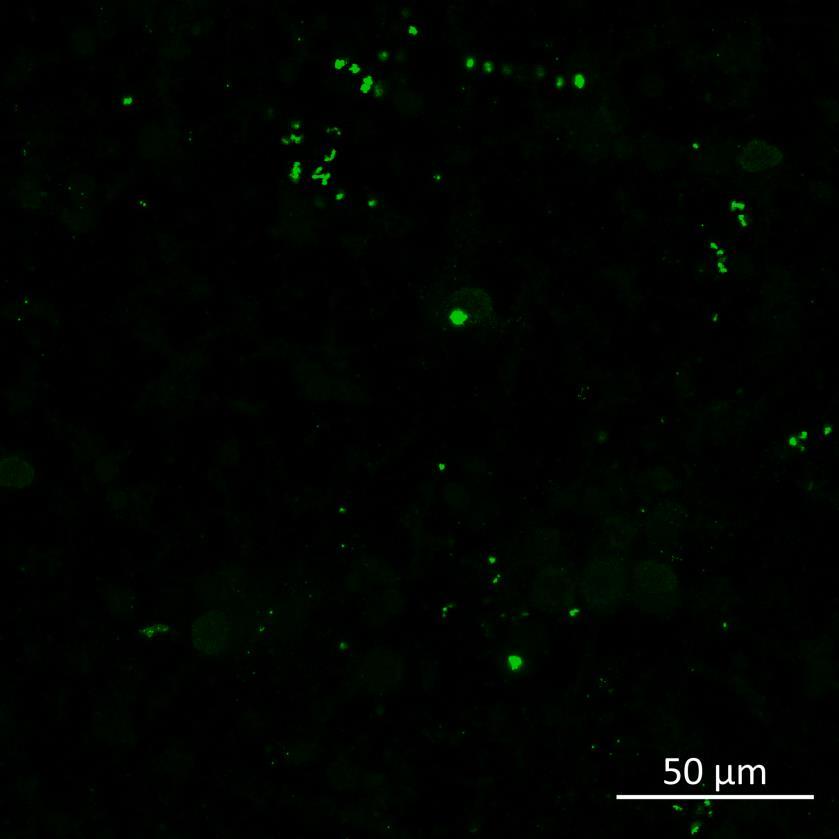
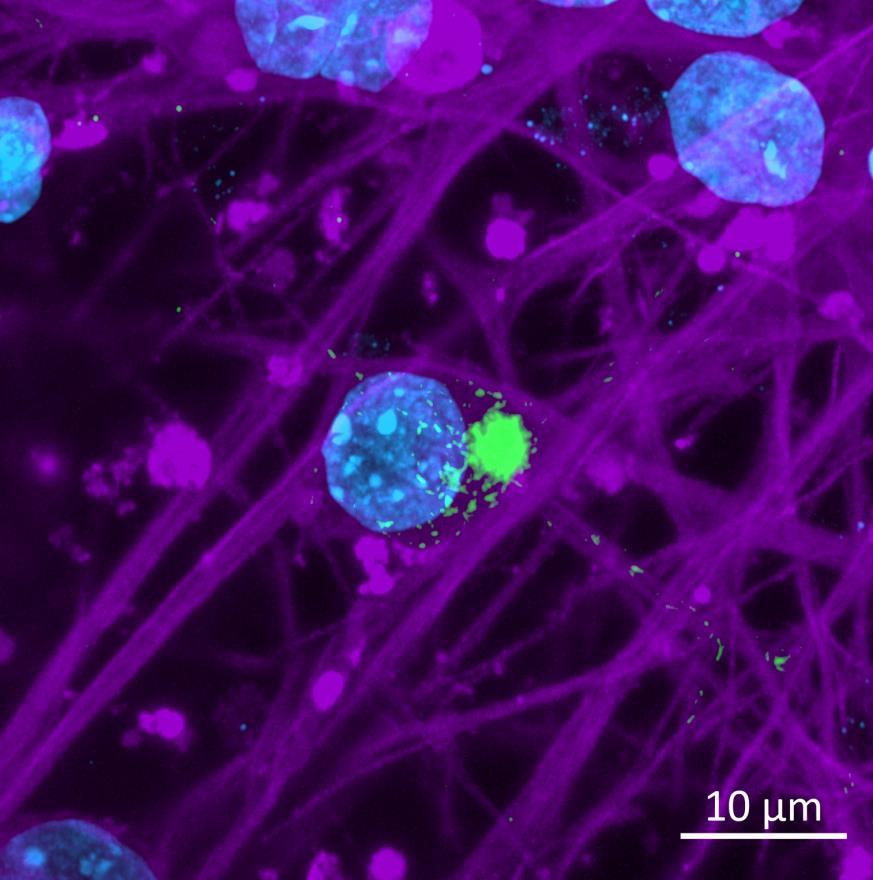
• 2-3 weeks following PFF addition, the neurons were fixed and immunocytochemistry for phosphorylated serine 129 α-synuclein was carried out
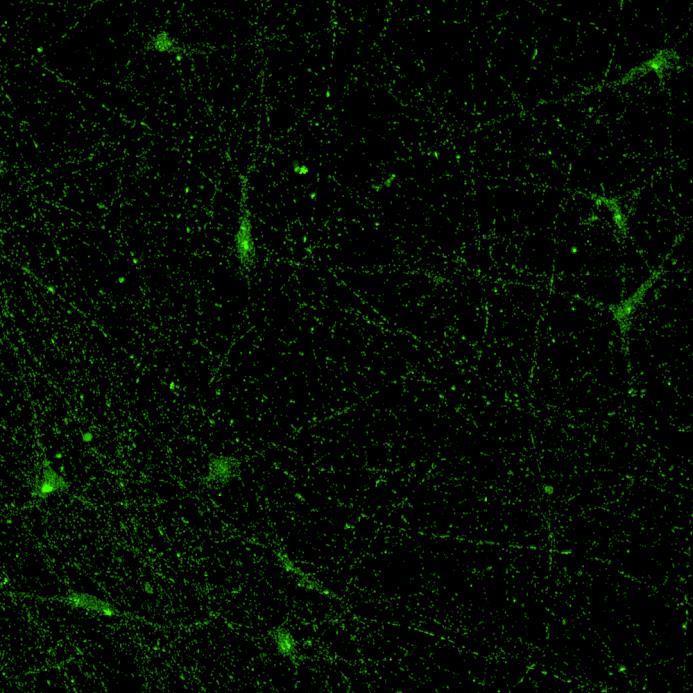
• Lewy body-like perinuclear accumulation of phosphorylated α-synuclein was often observed in response to PFF application
• Neurons were also lysed at 24 hours or 3 weeks after PFF addition and lysate was analysed using Luminex multiplexed immunoassay technology for phosphorylated serine 129 α-synuclein and total α-synuclein protein
A higher percentage of total α-synuclein was phosphorylated at 3 weeks compared to 24h following PFF exposure
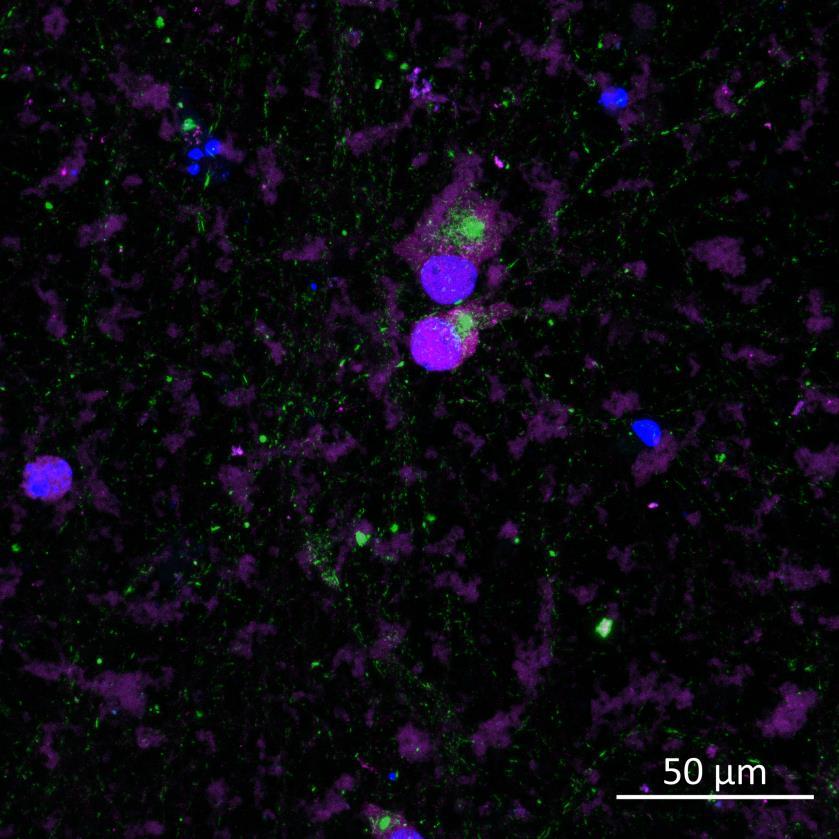
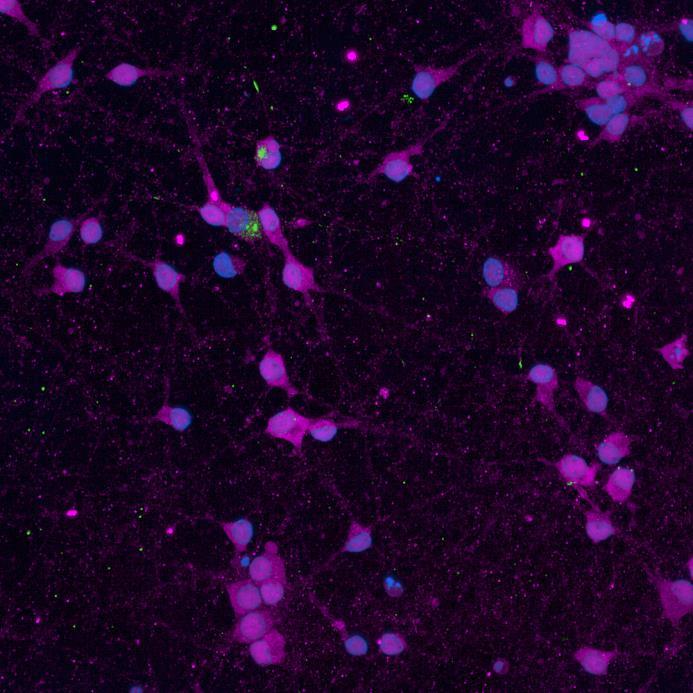
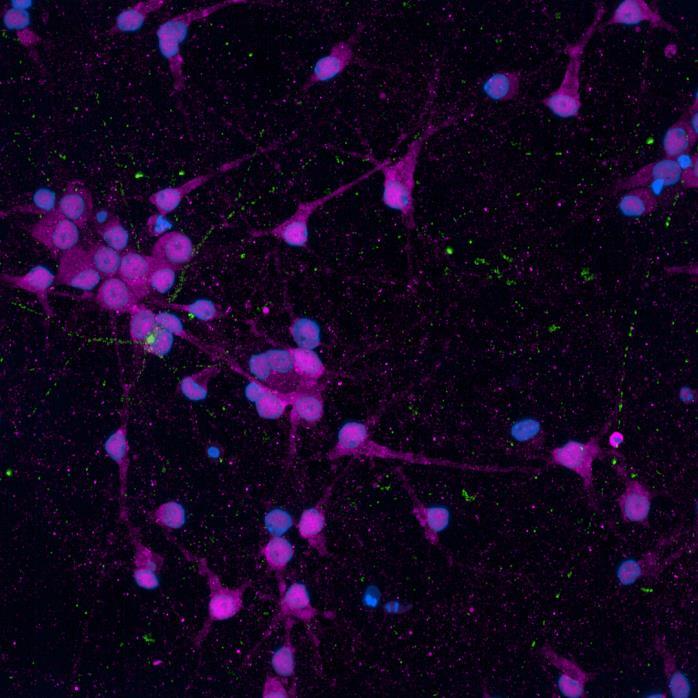
• iPSC-derived neurons expressing familial, early-onset Parkinson’s disease-related mutations (ioGlutamatergic neurons, bit.bio) were immunolabelled for α-synuclein phosphorylation following PFF addition
• The A53T mutation in the α-synuclein gene and the R275W mutation in the Parkin gene were inserted via CRISPR allowing an isogenically controlled wildtype background
• High content confocal imaging and automated analysis were used to count NeuN-masked neurons positive for phosphorylated serine 129 α-synuclein, with a threshold for positivity set at the mean + 5xStDev of the no fibril control intensities
• The α-synuclein mutation increased the proportion of neurons positive for phosphorylated α-synuclein at both 2 weeks and 3 weeks after PFF addition, but mutation of the Parkin gene had no effect
WT/WT
Phospho-S129 α-synuclein NeuN
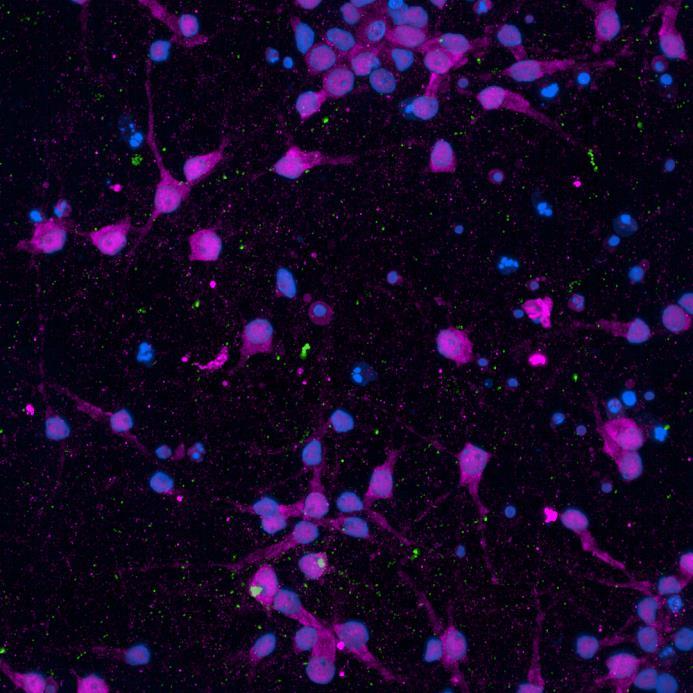

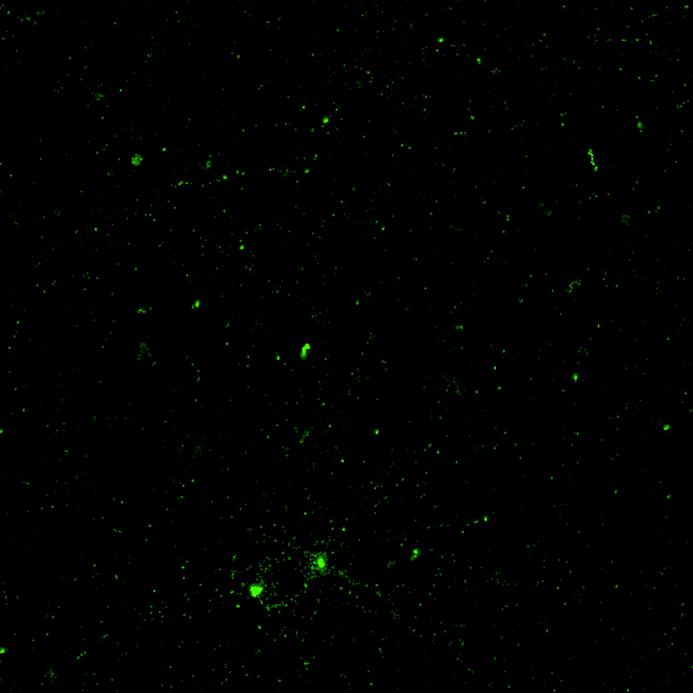
SNCA A53T/A53T


Compartmentalised microfluidics devices
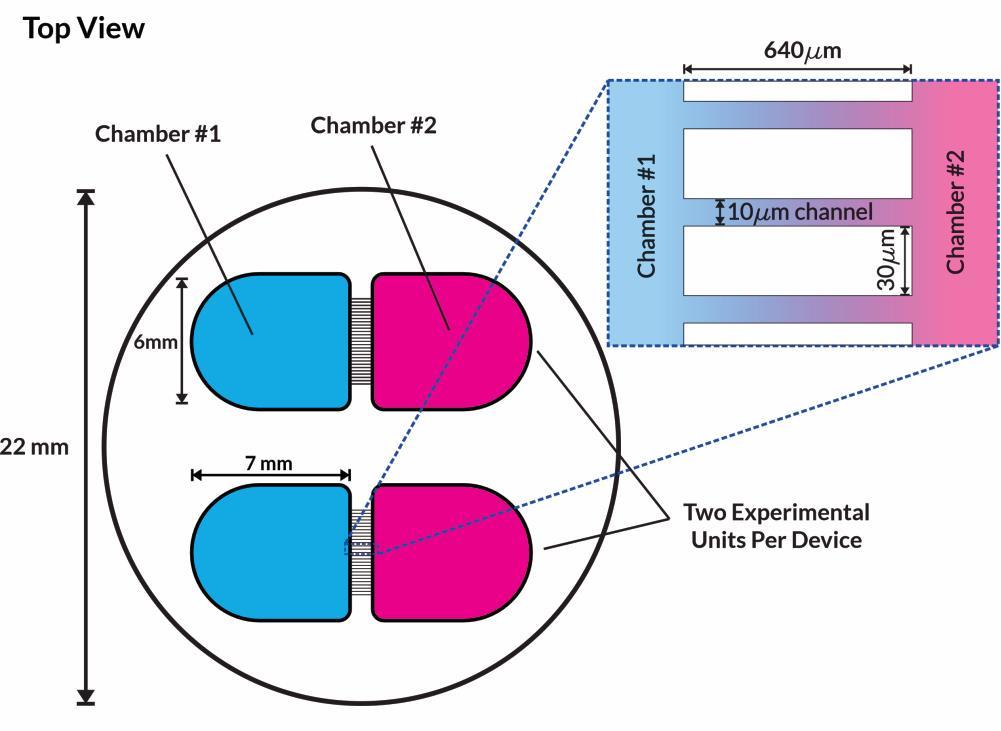
eNuvio Omega4 device
• Microfluidics devices consist of two open chambers connected by 10µm microchannels – narrow enough to maintain separation of cell bodies but allow projection of neurites between chambers
• Fluidic isolation is maintained by asymmetric volume loading to ensure unidirectional flow of fluid
• PFFs can therefore be added to a single chamber and spread of the fibrils is via inter-neuronal connections only
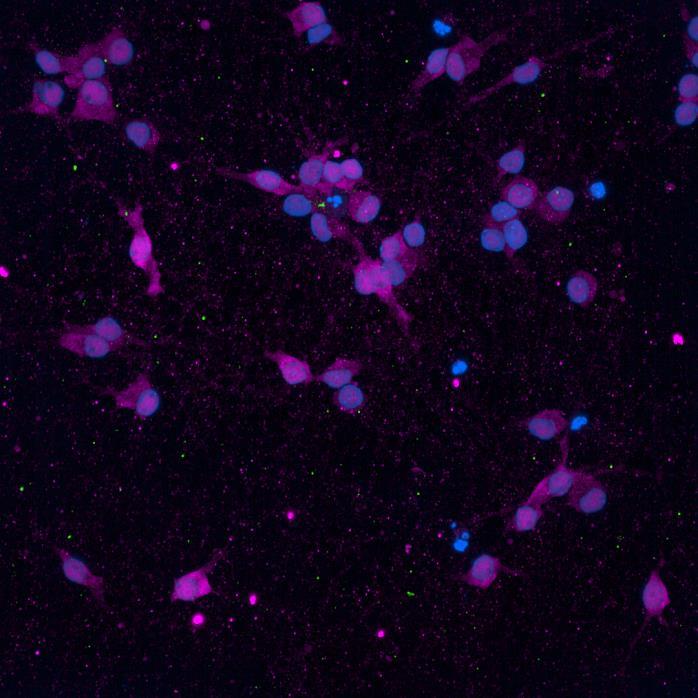
PRKN R275W/R275W


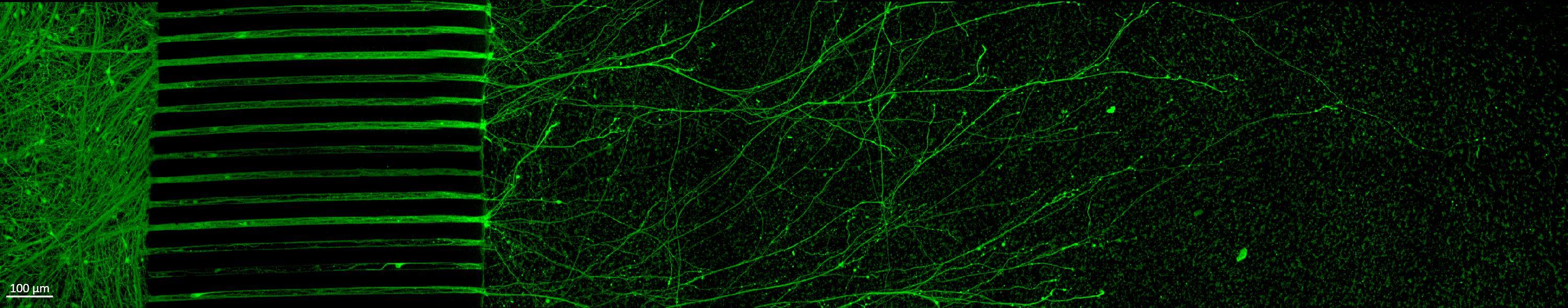
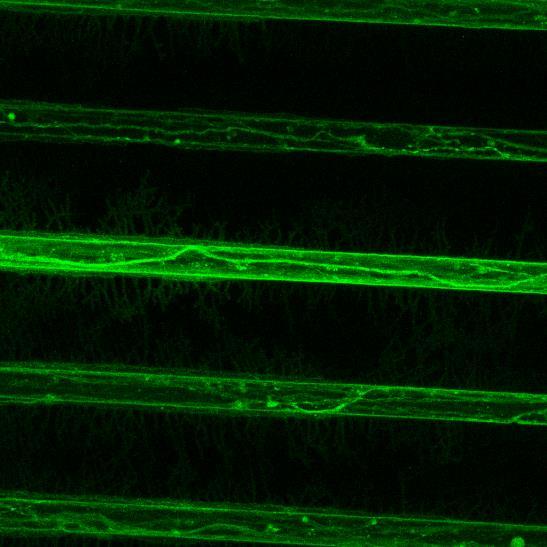
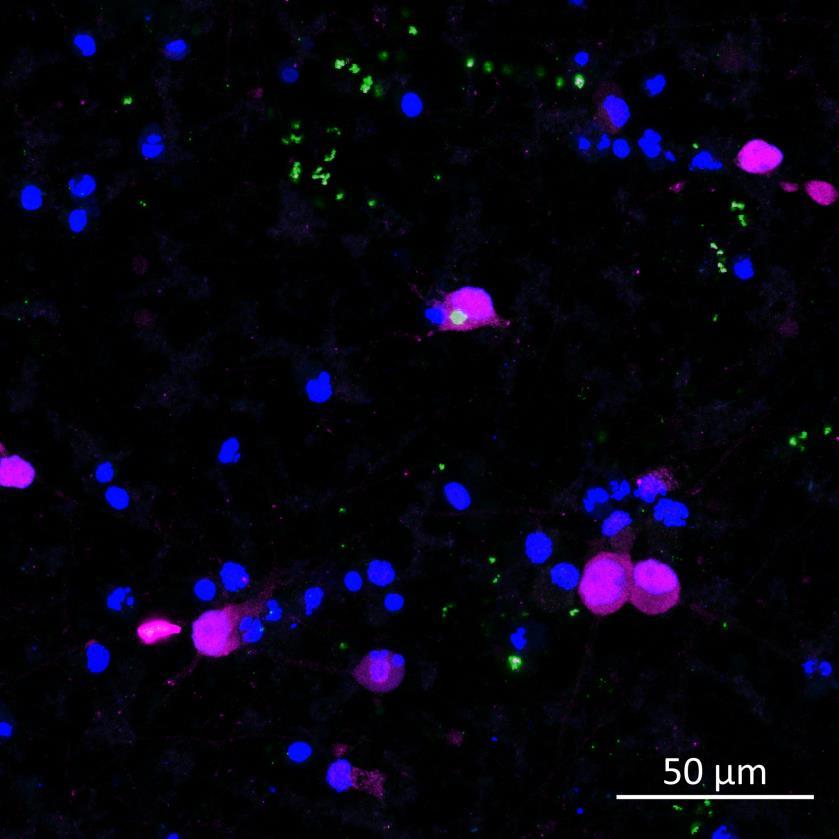
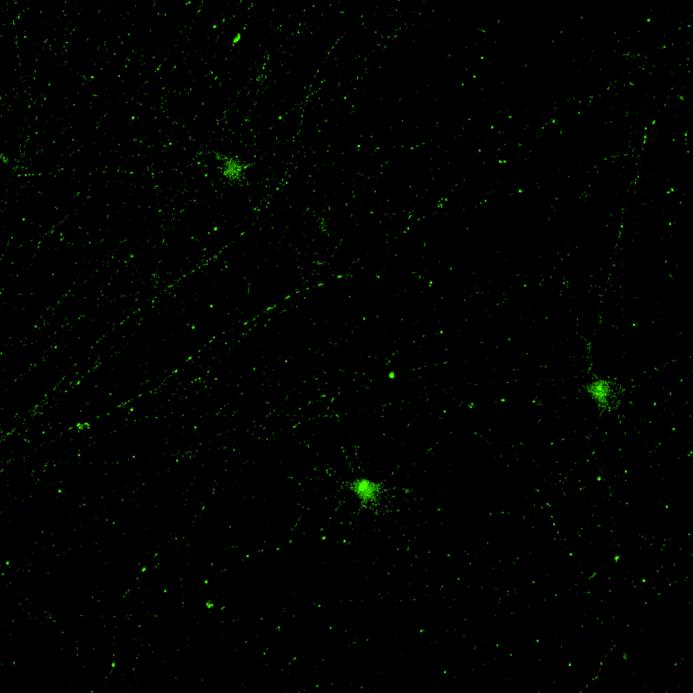
Projection of iPSC-derived neurons through microchannels, imaged at 2 weeks in culture
• iPSC-derived neurons (ioGlutamatergic neurons, bit.bio) were labelled with a live neuron-specific dye
• Axons can be visualised within the channels and projecting well into the adjacent chamber
Phosphorylation and aggregation of α-synuclein is induced by inter-neuronal seeding in microfluidics devices and is exacerbated by disease mutation
• A53T or isogenically controlled wildtype neurons were plated in both chambers of a microfluidics device then cultured to allow the neurons to connect via the microchannels
• α-synuclein PFFs were applied for 24h to the donor chamber
• 3 weeks after PFF addition, the donor and acceptor chambers were fixed and labelled for phosphorylated serine 129 α-synuclein
• In the donor chamber, a higher proportion of A53T neurons contained phosphorylated α-synuclein compared to wildtype neurons In the acceptor chamber we did observe a few wildtype neurons containing phosphorylated α-synuclein, although none were detected by the unbiased, automated imaging and analysis pipeline
• The rate of phosphorylation in the acceptor chamber in response to seeded fibrils was also increased by A53T mutation