The Athinoula A. Martinos Center for Biomedical Imaging

1
Imaging for the Advancement of Science and Medicine 2024
Revolutionizing
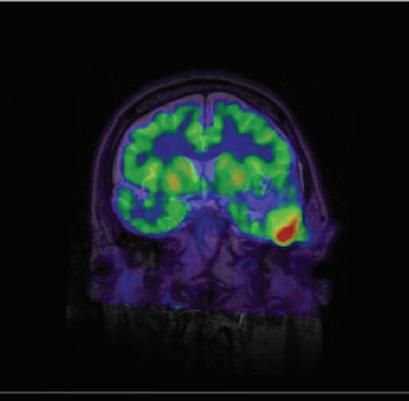
Cover image courtesy of the Martinos Center’s Alpen Ortug. PhD. The image is a 3D representation of the white matter pathways in a mouse model (top view). The representation was created using a technique known as diffusion tensor imaging and obtained with a 4.7T preclinical MRI scanner. The colors represent the directions of the nerve fibers in the white matter. The two antenna-like structures on the left side of the image are the olfactory nerves, and the red region on the right side is the cerebellum.
The stunning image earned Dr. Ortug the “People’s Choice Award” at the 2024 Image Awards hosted by the Mass General Research Institute.
2
Table of Contents
Understanding Neurological Disorders
With the Martinos Center’s help, a patient wtih epilepsy continues to live a full, happy life ... 4
Connectome 2.0: Revealing Structural Connections in the Brain with the Most Advanced Scanner for Diffusion MRI ... 6
Wielding Advanced Imaging Tools in the Fight against MS ... 8 A First-of-its-kind Study Explores the Neurobiology of Autism in Females ... 9 Uncovering the Relationship between Loneliness and Poor Health Outcomes ... 10 Working toward Early Detection—and Even Prevention—of Alzheimer’s Disease ... 12 Novel Probe Sheds Light on the Mechanisms of Alcohol Use Disorder ... 13
Advancing Clinical Care
Detecting ‘Covert Consciousness’ in the ICU ... 14
An Inexpensive, Low-Field MRI Screening Tool for Breast Cancer ... 15 ‘Fast MRI’ Yields More Efficient, More Patient-friendly Exams ... 16
Novel MR Contrast Agent Avoids Gadolinium Retention in the Body and Brain ... 17
Improving Mental Health and Supporting Vulnerable Populations
Understanding the Role of Empathy in Psychedelic-Assisted Therapy ... 18 Managing Anxiety and Depression with Mindfulness-based fMRI Neurofeedback ... 19
Elucidating the Relationship between Intimate Partner Violence and Brain Injury ... 20
Fostering Innovation
‘Martinnovate’ Seminar Series Boosts Innovation at Martinos ... 22
Wearable Device Could Assess Brain Health from the Comfort of Home ... 23 Research in Action: Introducing the Rapid Prototyping Lab ... 24 The Institute for Innovation in Imaging: A New Era in Innovation ... 25
Building Better Tools
Magnetic Particle Imaging: Exciting New Imaging Technology Will Soon Be Online ... 26 Ultrahigh-field Human MRI: The End of One Era, the Beginning of Another ... 27 Center Installs State-of-the-Art Optically Pumped Magnetometer (OPM) Device ... 28
The High Spatio-temporal Resolution ‘BrainPET’ Will Offer Unprecedented Sensitivity for Simultaneous PET/ MRI ... 29
Creating a Stronger Community
The Center Welcomes the First Athinoula A. Martinos Professor of Health Sciences and Technology ... 30
Mesoscale Brain Mapping: Bridging Scales and Modalities in Neuroimaging ... 31 Martinos Seminar Series ... 32
Other Community-Building Efforts ... 34
3


I am thrilled to report another year of successes at the Athinoula A. Martinos Center for Biomedical Imaging, one of the world’s premier research centers devoted to the development and application of advanced biomedical imaging technologies.
Our success can be measured in a variety of ways. “The Martinos,” as some of our younger colleagues have taken to calling the Center, is housed in the Department of Radiology at Massachusetts General Hospital and affiliated with both Harvard Medical School and the Massachusetts Institute of Technology (MIT). As you will see on the next page, the Mass General Department of Radiology brings in more than twice as much NIH funding as the next largest academic radiology research program, and the Center accounts for more than half of that funding. In FY2023, Center researchers were awarded over $80 million in funding, supporting projects in areas including neurodegenerative disease and aging, oncology, heart and lung disease, and much more.
“Every day in the Center, we devote ourselves to understanding better how the body and the brain work.”
Even beyond the research conducted by Martinos faculty, the Center maintains an imaging core facility with a 40+ year track record of institutional service that widely impacts scientific work at Mass General, in the greater Boston area, and across the nation, with over 1,000 users from more than 10 institutions and more than 15 departments at Mass General. The range of projects supported by the Martinos Imaging Core is extremely broad and includes studies in neurology, psychiatry, neurodegenerative disease, oncology, cardiovascular disease, pain medicine, infectious disease, and other conditions. In 2023, a record number of NIH-funded projects were supported by the Martinos Center Imaging Core, with total costs of more than $160 million.
Of course, we are most proud of some of the less quantifiable aspects of our success: the impact of our research on patient care.
Here in the Martinos Center, we work on the cutting edge of developing and applying tools to advance human health in all its forms. Imaging is, at its core, seeing. And seeing is the foundation of knowing and understanding. Every day in the Center, we devote ourselves to understanding better how the body and the brain work, often working with physicians and partnering with biotech and pharmaceutical companies to advance our clinical impact. In doing so, we contribute not only to improved diagnosis and management of disease but also to a higher quality of life for patients at all points on the care continuum.
Our success in these areas is due in part to the broad range of the types of activities ongoing in the Center. From our unique position at the intersection of world-class academic institutions and one of the nation’s most respected and highest-ranked hospitals, we engage in every aspect of the development and application of biomedical imaging tools: from technology development to basic science research and on again to translational research, in which we iteratively collaborate with clinicians at MGH and other hospitals across the Mass General Brigham system to identify clinical problems and find novel solutions to those problems.
In recent years, with an eye toward the dramatic changes underway across the medical technology landscape, we have also introduced efforts to encourage innovation among Center researchers seeking to bring new devices and new ideas to market.
In the following pages, we present highlights from the past year in the Martinos Center in each of the areas described above. If you would like to learn more about our efforts beyond this brief snapshot, please do not hesitate to reach out to us.
 Bruce Rosen, Director Athinoula A. Martinos Center for Biomedical Imaging
Bruce Rosen, Director Athinoula A. Martinos Center for Biomedical Imaging
The Martinos Center at a Glance
More than $81M in Funding in FY2023
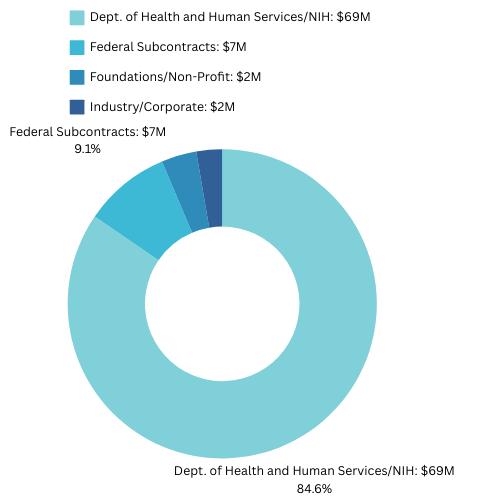





The Martinos Center for Biomedical Imaging is the flagship research center in the Department of Radiology at Massachusetts General Hospital, which receives more than twice as much in NIH funding as the next largest academic radiology research program.
The Center itself accounts for more than half of the funding awarded to the MGH Department of Radiology, making it the largest academic radiology research program in the country, even when considered apart from the rest of the department.
2
Anywhere FY2023 Snapshots 597 101 / $114M >525 136 48 Faculty, Fellows & Staff New Grants / est. total amt. Papers Published (CY2023) IP Applications Patents Issued
The Most Productive NIH-Funded Radiology Research Program

40+ Years of Innovating and Advancing Care

1984
Researchers in the MGH-NMR Center (later the Athinoula A. Martinos Center for Biomedical Imaging) produce the first magnetic resonance angiograms using the still-emergent MR technology. The technique is today used in hospitals across the globe for applications in stroke and cardiovascular disease.
1991
Center researchers introduce the technique functional magnetic resonance imaging (fMRI), which enables imaging of neural activity in the brain. fMRI has since yielded countless previously inaccessible insights into the workings of the brain in both health and disease.
2000
The Martinos Center installs the first clinical 7T MRI scanner, developed by Center researchers in collaboration with Siemens Healthcare. The ultrahigh-field scanner allows researchers and clinicians to image the structures of the brain with unprecedented resolution.
2008
Martinos researchers produce the first simultaneous human PET/MRI images in the US, with much more accurate co-registration of structural and functional/metabolic information than was previously possible. The use of simultaneous PET/MRI has contributed in myriad ways to the discovery of new targets for pharmaceuticals.
2019 and 2020
Two groups in the Martinos Center report low-field portable MRI scanners: one developed within the Center, the other developed commercially based on research and IP by a Center investigator. The technology has the potential to revolutionize MRI by bringing the scanner to the patient: at bedside, in ambulances and point-ofcare locations, and even in rural areas and developing countries.
Today and Beyond
The Martinos Center continues to advance the development of new imaging technologies and care of patients with a broad range of diseases and disorders. Read how in the following pages.
Second Chances
With the Martinos Center’s help, a patient with epilepsy continues to live a full, happy life
A retired minister who now helps people with brain disorders through a yoga therapy practice, Kristine Zakarison knows a thing or two about second chances.
“A lot of people have a point in life where they get a second chance,” she says. “Some aren’t aware of the fact that they’ve been given the chance. Others have the good fortune to be keenly aware.
“As for myself, I believe I’ve got that chance, and I’m going to make the most of it.”
Kristine has had epilepsy since she was young. For many years, it was well controlled through medication — so much so that she believes her husband, Jonathan, had never seen her have a seizure. Things began to shift, though, when she became perimenopausal. “I started having seizures again,” she says, “and they continued to happen like clockwork.”
A surgeon in Washington State, where she and Jonathan live, wanted to address the seizures by resecting a relatively large section of her brain, according to the standard of care at the time. However, because the procedure would also have impacted other, adjoining areas her brain, it likely would have left her with impaired cognitive and language functions. Hoping to avoid this, she sought a second opinion in New England, where she attended graduate school and had always maintained connections.
This is when she came to the Martinos Center.

“We can make meaning out of the bad situations by passing on the support we have received, by helping others through their own difficult times.”
Kristine had long known Center director Bruce Rosen, MD, PhD. In fact, she had introduced him to his wife, Sharon, through a blind date. While visiting Boston not long after meeting with the surgeon in Washington State, she reached out to Dr. Rosen to learn where she should get a second opinion. Of course, the answer was: at the Martinos Center and with the Center’s colleagues in MGH Neurosurgery.
Dr. Rosen introduced Kristine to Steven Stufflebeam, MD. Dr. Stufflebeam, director of the Clinical Magnetoencephalography (MEG) Service at Massachusetts General Hospital and medical director of the Martinos Center, would guide her care at the hospital.
“Steve did the complete Martinos workup,” Dr. Rosen says, “an MEG study, a PET-MR study and a Connectome study. The goal was to try to understand both the nature of the brain lesion that she had — the cause of the epilepsy — and how the adjoining parts of her brain may have been involved.”
The latter was important because resection of a brain lesion can also extend to the surrounding areas, thus impacting the functions associated
4
*****
Kristine Zakarison leads a yoga therapy session
with those areas. Understanding the wiring of Kristine’s brain would help determine which of the connections the neurosurgeon would want to preserve in treating her epilepsy.
With the additional information from the imaging studies, Dr. Stufflebeam worked with the neurosurgeon to come up with a surgical approach in which the margins of the lesion were much better delineated than they would have been otherwise. “Based on this information, they felt confident they preserve her cognitive function,” Dr. Rosen says. “They did the surgery and that’s largely what happened.”
Prior to the surgery, Dr. Stufflebeam explained to Kristine that the procedure would likely affect her functioning in two areas: numbers and facial recognition. She still has some difficulty with these, she says, but the overall impact on her wellbeing and her lifestyle is relatively minor, especially
compared to the deficits she almost certainly would have experienced otherwise.
As part of her recovery, Kristine devoted a good deal of time dancing and practicing yoga. Eventually, she enrolled in and completed an extensive training program to become a Certified Yoga Therapist, with the goal of helping others with neurological disorders or injuries.
“This is one of the ways we can find meaning after going through difficult times,” she says. “We can make meaning out of the bad situations by passing on the support we have received, by helping others through their own difficult times.”
Today, she works with people with neurological disorders — the farming community where she lives in

eastern Washington State has a high incidence of Parkinson’s disease and multiple sclerosis, both of which have been linked to pesticide use — as well as with people who have experienced the trauma of domestic violence.
She helps many in her community through her practice, which is also deeply informed by her own experiences as a patient with a neurological disorder of her own. “So many of the people I meet are frustrated,” she says. “Some don’t feel they have any hope. Sometimes I meet people who have just given up, and I think about the times in my own life when I could have gone the same way.”
Kristine never lost hope. Through persistence and hard work, and with the support of her loved ones and care providers, she continues to live a full life. And with the second chance she’s received, she continues to help many others do the same.
Kristine and Jonathan live in rural eastern Washington State, where she was born and raised. Her parents were getting older, she says, and she wanted to be able to take care of them. She is grateful that she has had the opportunity and the ability to help.
Since moving back to the region, she has also developed a new passion. The area in which she lives was originally a prairie and she now devotes much of her time and energy to reintroducing native plants to the area — repopulating parts of the family farm and other lands she has acquired, literally planting the seeds that will restore the lands to their earlier vitality.
5
*****
Connectome 2.0: Revealing Tissue Microstructure and Connectional Anatomy in the Brain with the Most Advanced Scanner for Diffusion MRI
A new state-of-the-art MRI scanner at the Martinos Center is set to provide unprecedented insights into the inner workings of the living human brain, with important implications for improving the diagnosis and treatment of neurological and mental disorders.
Dubbed “Connectome 2.0,” the scanner was developed through the support of the NIH BRAIN Initiative’s next-generation human imaging technology efforts to enable studies of neural tissue microstructure and circuits in the living human brain. It replaces the original Connectome scanner developed by Martinos researchers under the auspices of the NIH-funded Human Connectome Project.
“It’s an amazing device,” says Martinos Center director Bruce Rosen, MD, PhD. “It’s more than four times as powerful as the previous Connectome scanner, which was almost five times as powerful as anything else on the planet before we developed it.”
Imaging of the human “connectome,” as the complete set of connections within the brain has come to be known, dates to the mid-1990s, when Martinos researchers pioneered the MR imaging methods that made such imaging possible. “Diffusion” imaging enables imaging of axonal bundles— the wires that facilitate communication between different areas of the brain— by tracking water molecules as they travel along these white matter fibers.
Susie Huang, MD, PhD, a neuroradiologist at Mass General and the Martinos faculty member leading the Connectome 2.0 effort, is thrilled about the potential applications of the technology.
“Connectome 2.0 will dramatically enhance our ability to visualize the structure of the human brain by offering unprecedented spatial and diffusion resolution,” she says. “We are already seeing how the increased sensitivity of Connectome 2.0 to axonal and cellular
CONTACTS
Susie Huang, MD, PhD Susie.Huang@mgh.harvard.edu
Anastasia Yendiki, PhD ayendiki@mgh.harvard.edu
Bruce Fischl, PhD bfischl@mgh.harvard.edu
structure at the micron level is allowing us see surprising differences in how the human brain is organized at the individual level.”
Dr. Huang is now leading efforts to establish the Connectome 2.0 scanner as a unique shared resource facility and advance neuroscience collaborations around the world.
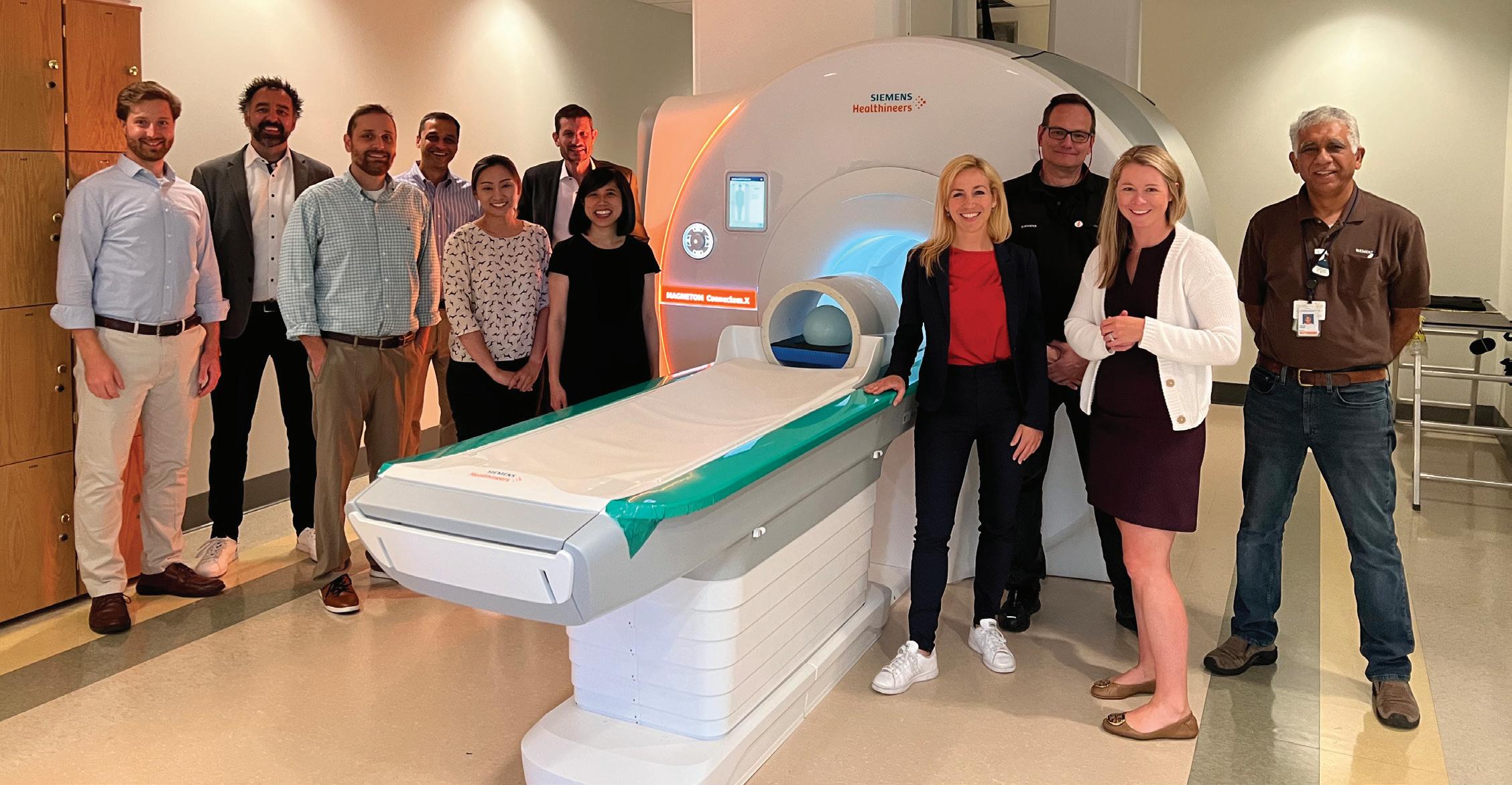
6
Susie Huang, MD, PhD (standing to the immediate left of the scannner) and Martinos Center executive director Hope Taft (second from right) with Siemens collaborators from the US and Erlangen in front of the Connectome 2.0
Research Could Benefit Treatment of Parkinson’s Disease, Other Applications
Martinos researchers are already making important strides with Connectome 2.0.
Anastasia Yendiki, PhD, is the lead principal investigator of the new Center for Large-scale Imaging of Neural Circuits (LINC), a five-year, $23.5 million project supported by the NIH by way of the BRAIN Initiative Connectivity Across Scales (CONNECTS) program.
The goal of the center is to develop innovative technologies with which to image connections in the brain down to the microscopic scale and apply the technologies to improve the accuracy of deep brain stimulation (DBS) for motor disorders, such as Parkinson’s disease, Tourette syndrome, and dystonia, as well as psychiatric disorders such as obsessive-compulsive disorder (OCD) and depression.
“We know that deep brain stimulation works, but we are not exactly clear how it works,” says Dr. Yendiki says. “We are going to map these connections in human brains at a microscopic scale for the first time to give clinicians a clear picture of how brain regions are interconnected—and hopefully improve how these diseases are targeted.”
Another BRAIN CONNECTS grant at the Center will also take advantage of Connectome 2.0.
Bruce Fischl, PhD, is director of the Laboratory for Computational Neuroimaging (LCN), which has developed and continually refined over the past 20-plus years image analysis tools including FreeSurfer, an open-source software suite for processing, analyzing and visualizing structural, functional and diffusion neuroimaging data.
With the recently awarded BRAIN CONNECTS grant “Mapping Connectivity of the Human Brainstem in a Nuclear Coordinate System,” Dr. Fischl and colleagues will leverage this experience to develop technologies to create a multiscale atlas for the human brainstem. Call it a Google Earth for the brainstem. Using the atlas, researchers will be able to visualize brainstem-wide networks, then zoom in to the level of individual cells, probing their connectivity at micrometer resolution.
The new tool will enable types of analyses of neuroimaging data that simply haven’t been possible before—and thus could lead to important advances in understanding traumatic brain injury, Alzheimer’s disease and a range of other neurological conditions.
7
Wielding Advanced Imaging Tools in the Fight against MS
The Multiple Sclerosis Imaging Laboratory in the Martinos Center employs cutting-edge imaging technologies to understand better the mechanisms of MS, while also seeking new means of diagnosing and monitoring the disease. In the Q&A below, Lab Director Caterina Mainero, MD, PhD, discusses her group’s research and the role of the advanced imaging methods available at the Center in their many significant findings.
Tell us about the work you do in the Multiple Sclerosis Imaging Laboratory.
Our lab specializes in translating novel multimodal imaging techniques for investigating the structure, function, and pathology within the brain and spinal cord of people with multiple sclerosis. Our overall mission is to integrate novel and advanced imaging methods, many of which have been developed at the Martinos Center, with clinical and biological markers of the disease to investigate the brain mechanisms underlying disease activity and progression, and to define the most sensitive neuroimaging tools for improving disease diagnosis and monitoring.
What imaging tools do you use?
For our work we often combine advanced imaging modalities to investigate the heterogenous aspects of multiple sclerosis pathology that include neuroinflammation, demyelination, neurodegeneration, and tissue repair.
For example, we use ultrahigh-field MRI at 7 Tesla (7T) for investigating the presence of very small demyelinating lesions, like those in the cortex, which are usually not easily detectable on routine MRI scans in the clinic but have been reported to be extremely
common and diffuse in post-mortem examinations of multiple sclerosis brain specimens. Our group was the first to image and characterize in vivo, using ultrahigh-field 7T MRI, the different types of cortical multiple sclerosis lesions described by neuropathology, and to show that the cortical lesion load assessed at 7T is an independent and main predictor of disease progression.
We also use, in many of our studies, positron emission tomography (PET) to assess specific molecular aspects of multiple sclerosis lesions that are related to neuroinflammation, a main driver of multiple sclerosis pathology. The aim is to understand how neuroinflammation contributes to developing cortical demyelinating lesions and their progression.
In some cases, we combine these techniques with advanced diffusion MRI methods to probe tissue microstructure and, specifically the integrity of axons that often degenerate in multiple sclerosis leading to the development of irreversible disability.
Can you describe recent findings using these tools?
A main area of focus of the lab is the study of the cortex and cortical lesions in people with multiple sclerosis. We have gathered extensive data at 7T and recently, using a machine learning approach, evaluated the role of cortical lesions in predicting disability progression along with other radiological markers of the disease (DOI:10.1093/ braincomms/fcab134). We found that cortical lesions are among the top three radiological predictors of the progression of neurological disability in patients.

CONTACT
Caterina Mainero, MD, PhD cmainero@mgh.harvard.edu
8
A First-of-its-kind Study Explores the Neurobiology of Autism in Females

CONTACT
Nicole Zürcher Wimmer, PhD nzurcherwimmer@mgh.harvard.edu
An estimated one in 36 children in the US, and one in 45 adults, has autism spectrum disorder (ASD). Yet, there is still a great deal about the disorder that researchers don’t know.
Nicole Zürcher Wimmer, PhD, is working to change this. Dr. Zürcher Wimmer, an assistant professor in the Martinos Center, uses magnetic resonance imaging (MRI) and simultaneous positron emission tomography (PET)-MRI to study brain function and dysfunction in health and disease, with a primary focus on ASD and related conditions. She has made significant contributions to the body of literature on the disorder: including, for example, studies of the role of epigenetics—the complex relationship between genes and behavioral and environmental factors—in the etiology of ASD.
Now, she and colleagues have uncovered important sex differences in the disorder. They reported their findings in a recently published paper (DOI:10.1038/s41386-024-01859-6).
Sex differences in the prevalence and clinical presentation of ASD are well documented. Researchers have established, for instance, that males are diagnosed with ASD four times as often as females, and that females may have fewer and/or less pronounced core symptoms. Little is known, though, about the neurobiology of ASD in females. Over the past two decades, more than 90 percent of ASD neuroimaging studies either have
enrolled male subjects only or, if females were included, have not looked at sex effects.
Dr. Zürcher Wimmer and team have begun to address this relative lack of understanding. Previous studies had found altered levels of a mitochondrial protein, TSPO, in males with ASD. In the present work, the first of its kind, the researchers used the advanced PET-MRI technologies in the Martinos Center with the [11C]PBR28 radioligand to measure TSPO levels in adult females with the disorder.
Their findings were striking. PET-MRI scans in 12 adult females with ASD and 10 healthy controls showed higher TSPO levels in specific brain areas in the former than in the latter. No brain areas in the females with ASD had lower TSPO levels. This contrasted sharply with previous findings in males with ASD, which showed generally lower TSPO levels than in healthy controls.
This has important implications for understandings of ASD. “Given the connection between TSPO and neuroimmune function, the findings suggest that neuroimmune mechanisms may be involved in autism,” Dr. Zürcher Wimmer says.
The researchers are now planning an ultra-high-field (7-Tesla) MRI study to learn more about how brain regions with atypical TSPO might be altered in autism.
9
Uncovering the Relationship between Loneliness and Poor Health Outcomes
Ongoing work by the Martinos Center’s Daphne Holt, MD, PhD, could help tackle the worsening epidemic of loneliness and social isolation in the general population.
Public health experts have called attention to this epidemic since even before the COVID-19 pandemic. At the same time, researchers have shown that loneliness and isolation are particularly common in those suffering from serious mental illnesses, including psychotic disorders, affecting approximately 80% of people with these conditions.
This societal problem has broad public health implications. A growing body of literature has linked loneliness and isolation to poor cardiometabolic health and premature death. Little is known, though, about the brain mechanisms underlying this relationship and how they might vary across different groups of people.
Clues have emerged to help connect the dots. For example, studies have shown that certain cognitive and behavioral biases are associated with

CONTACT
Daphne Holt, MD, PhD
dholt@mgh.harvard.edu
loneliness, including a bias towards social mistrust and a related tendency to maintain a greater physical distance (“personal space”) from others.
Now, Dr. Holt and colleagues, including Faye McKenna, PhD, Louis Vinke, PhD, and Mona Avanaki, MD,

have leveraged these biases to help identify changes in brain functioning associated with loneliness and social isolation — in other words, neural markers of loneliness — in people with and without psychotic disorders.
They did this by scanning subjects with functional MRI while showing them videos with either faces approaching them and appearing to violate personal space boundaries — thus triggering the sensitivity to social threat that has been associated with loneliness — or faces moving away from them, signaling social withdrawal.
In fact, the study revealed an association between loneliness and a greater response to the withdrawing faces in the hippocampus and a related network of subcortical brain regions involved in emotion and homeostatic regulation. This pattern of responses was similar across the psychotic disorder and healthy control groups, suggesting that it may represent a marker of loneliness.
“Understanding how the brain responds when a person’s social needs are not being met gives us an objective way to measure and monitor this type of deficit,” says Dr. Holt, “which will help us understand the effects of loneliness on the body and how to reverse those effects.”
In ongoing work, the researchers are testing whether the potential marker of loneliness is linked to some of the known biological effects of loneliness (including cardiometabolic and inflammatory dysregulation), and whether these effects play a role in the poor health outcomes and earlier mortality associated with psychotic disorders and loneliness across populations.
10

Working toward Early Detection and Even Prevention of Alzheimer’s Disease
Heidi Jacobs, PhD, a principal investigator in the Martinos Center, is making significant strides in the study of Alzheimer’s disease: specifically, in examining the earliest affected regions of the brain in Alzheimer’s and applying the insights she gains to improving early detection of the disease and advancing noninvasive interventions to delay cognitive decline.
In a 2023 study reported in the journal Neurology (DOI:10.1212/ WNL.0000000000207646), Dr. Jacobs and colleagues, including first author Prokopis Prokopiou, PhD, a faculty member in Dr. Jacobs’ lab, looked at specific neuronal changes associated with memory decline in preclinical Alzheimer’s and how improved understandings of these changes might lead to earlier and thus more effective interventions.
We recently spoke with Dr. Jacobs about the study. Here is what we learned.
What question were you seeking to address in this work?
Alzheimer’s disease is characterized by the accumulation and aggregation of two proteins, beta-amyloid and tau. In most individuals, these pathologies follow a stereotypical pattern of progression throughout the brain, with the first accumulations of tau starting in the locus coeruleus. (The locus coeruleus is a tiny nucleus in the brainstem providing the brain with norepinephrine, an important neurotransmitter involved in emotion, memory and arousal.)
As pathology progresses in this predictable topography in the brain, it has been suggested that interactions between neurons may be part of the
 Heidi Jacobs, PhD hjacobs@mgh.harvard.edu
Heidi Jacobs, PhD hjacobs@mgh.harvard.edu
mechanism underlying spreading of tau. Recent work in animal models indicated that not only the level of neuronal activity, but also the mode of activity of these neurons plays a critical role in disease progression, with burst-like (phasic) activity of locus coeruleus neurons having a more protective effect on brain health and cognition.
Given that high arousing or novel events elicit phasic firing in the locus coeruleus, we aimed to examine whether lower novelty-related activity in the locus coeruleus would be associated with higher levels of tau in the entorhinal cortex—the first cortical region affected by tau following the locus coeruleus—and in the progression of cognitive symptoms.
What were the most significant findings?
Consistent with findings in animal models, we found that lower noveltyrelated locus coeruleus activity was related to higher entorhinal tau.
There were two important additional findings: first, that elevated entorhinal tau may constitute part of the mechanism explaining how lower noveltyrelated LC activity leads to faster memory decline; and second, that beta-amyloid plays a facilitating role: in particular, in the downstream effects of tau, as the relationship between elevated entorhinal tau and memory decline is much more pronounced at higher levels of beta-amyloid.
In what ways could your findings inform clinical care with respect to Alzheimer’s?
These findings highlight that if we want to prevent Alzheimer’s disease, we need to detect individuals at-risk earlier and start interventions earlier, at a time when pathology is not yet too widespread in the brain and when individuals are still cognitively healthy.
The field has now entered an exciting time as anti-amyloid antibodies are approved for the treatment of Alzheimer’s disease. These findings elicit the question whether treatment with these antibodies in earlier stages, the asymptomatic stages, could delay the cascade of initial tau-related cognitive decline.
Another exciting possibility that will require further research is whether activating individuals to engage in novel and stimulating activities can strengthen the health of the locus coeruleus and delay tau spreading.
12
CONTACT
Novel Probe Sheds Light on the Mechanisms of Alcohol Use Disorder
Alcohol use disorder (AUD) is defined “an impaired ability to stop or control alcohol use despite adverse social, occupational or health consequences.” It is considered a brain disorder, albeit one involving a complex interplay of genetic and environmental factors: a phenomenon known as “epigenetics.”
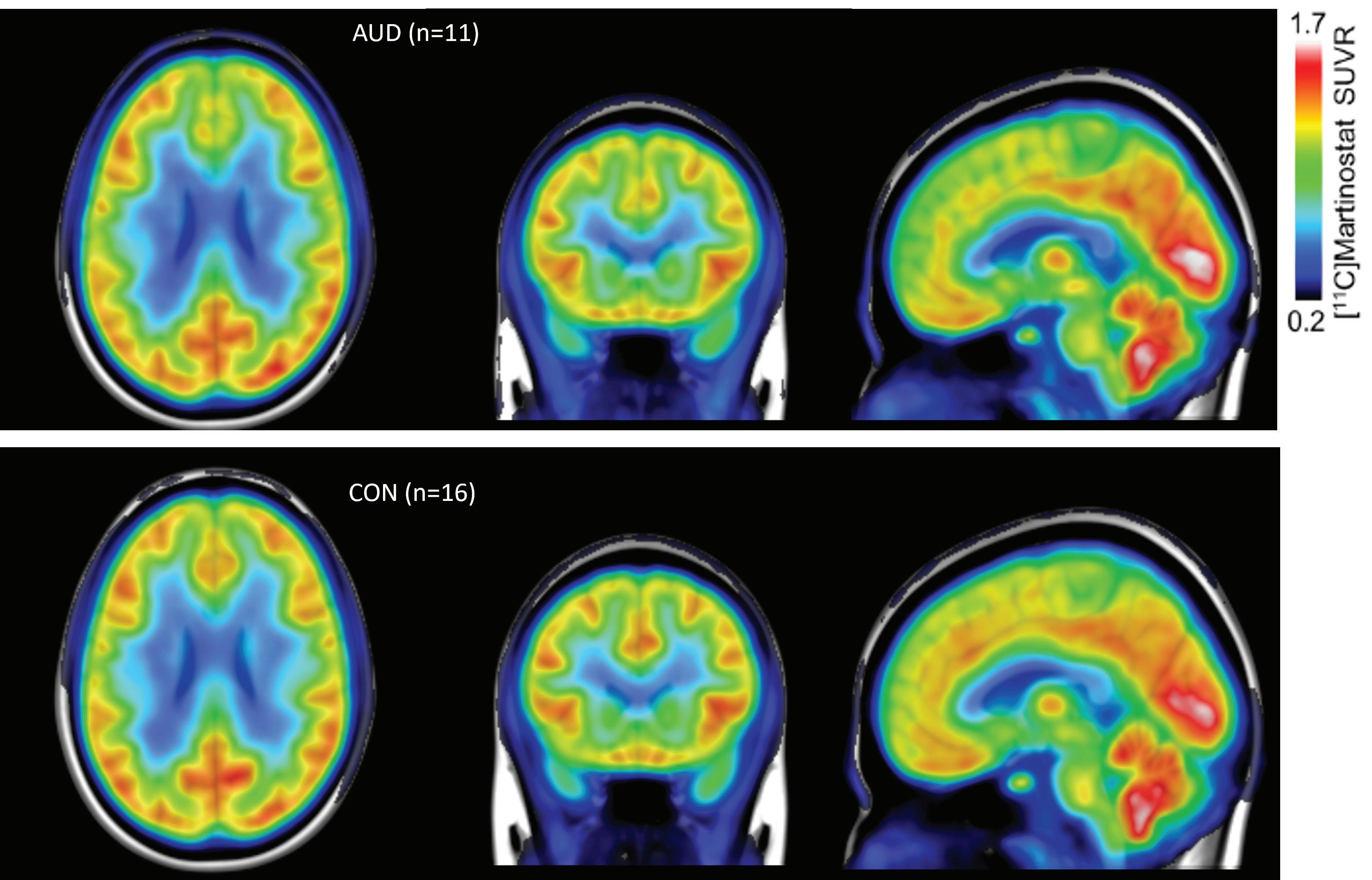
At the Martinos Center, Changning Wang, PhD, and colleagues study epigenetics using simultaneous positron emission tomography (PET) and magnetic resonance imaging (MRI). Specifically, they have developed and applied a PET probe called Martinostat that allow them to visualize the mechanisms underlying gene-environment interactions by tracking enzymes such as histone deacetalyses (HDACs).

Previous studies have linked changes in HDAC expression to several brainrelated disorders. Now, Dr. Wang and colleagues have shown that such changes can also be found in patients with AUD. At the same time, they have demonstrated the potential of using HDAC PET imaging in conjunction with artificial intelligence for personalized diagnosis of AUD, complementing current approaches to diagnosis and treatment while advancing the field of precision medicine.
For this study, Dr. Wang collaborated with Roger Weiss, MD, a physicianresearcher at McLean Hospital. Tewodros Mulugeta Dagnew, PhD, a postdoctoral fellow working with Dr. Wang at the Martinos Center, also played an important role.
13
CONTACT Changning Wang, PhD CWANG15@mgh.harvard.edu Group-averaged images for AUD (top) and control (bottom) populations. Red indicates higher HDAC enzyme presence and blue indicates lower.
Detecting ‘Covert Consciousness’ in the ICU
Every year, more than one million people across the globe are impacted by severe brain injury (TBI). Bedside behavioral examination has long been the gold standard test for assessing the level of consciousness in patients who experience such injury. Still, not all patients can respond to the clinician’s commands during the test because they cannot express themselves by speaking or writing, they have arm and leg weakness that prevents them from moving in response to a command, or another reason.
Studies have shown that 15%-20% of patients in the intensive care unit (ICU) may have higher levels of consciousness than their bedside behavioral examination suggests. Consciousness that cannot be detected with the bedside exam is labeled “covert consciousness.”
At Massachusetts General Hospital, Brian Edlow, MD, associate director of the Center for Neurotechnology
and Neurorecovery in the Department of Neurology and a Martinos Center faculty member, is leading the charge in developing a fuller understanding of covert consciousness.
This work is especially important because the bedside behavioral exam results often inform families’ decisions on whether to continue life support. Clinicians’ assessments of the patient’s level of consciousness have been cited as the most significant factor in families’ decisions. Without a fuller understanding of what is happening in the brain, they may choose to withdraw the support prematurely. Discontinuation of life-sustaining therapy is the cause of death for up to 80% of patients with acute severe brain injuries.
In March 2023, Dr. Edlow was announced as an MGH Research Scholar. Funded entirely by philanthropic gifts, the MGH Research Scholars Program provides forward-thinking
researchers with the unrestricted support they need to take their work into new and uncharted territories.
The five-year grant accompanying the honor will help advance his project, “Detecting Covert Consciousness in the Intensive Care Unit.” Here, he will lead a multidisciplinary team that will translate TMS-EEG to the ICU, optimize the technique for use in patients with acute severe brain injuries, and assess its ability to detect and predict recovery of consciousness in these patients.
“TMS-EEG has shown unparalleled accuracy at detecting consciousness in patients with chronic brain injuries, providing a compelling clinical and ethical rationale for translating TMS-EEG to the ICU,” he says. “This project has the potential to save lives by detecting signs of covert consciousness and preventing premature withdrawal of life-sustaining therapy.”
CONTACT
Brian Edlow, MD bedlow@mgh.harvard.edu
14
An Inexpensive, Low-Field MRI Screening Tool for Breast Cancer

CONTACT
Matt Rosen, PhD msrosen@mgh.harvard.edu
An ongoing MGH Research Scholar project could lead to more women screening for breast cancer earlier, potentially improving outcomes for a large swath of the population.
Approximately one in eight women will develop breast cancer in their lifetime. X-ray mammography is the most used imaging-based screening tool for breast cancer, but it misses about 13% of actual tumors in normal tissue and possibly as much as 30% in higher density tissue. In 2015, only 65% of women in the US had their recommended mammogram.
Women opt out of mammography for several reasons: if they are susceptible to radiation effects; if the required compression is uncomfortable; and if there is a high uncertainty about the results of mammography in dense tissue, which can increase patient anxiety.
MRI-based methods can visualize differences in soft tissues without obfuscations from dense tissue, but are very expensive, with MRI scanners costing upwards of $3M.
With support from a 2022 Kiyomi and Ed Baird MGH Research Scholar award, Matt Rosen, PhD, and col-
Christina Pfeifer-Mattig (October 18, 1970 - October 9, 2022) was a friend whose breast cancer journey inspired Dr. Rosen to pursue the work that is now supported by the MGH Research Scholar Award.
“She was one of my very closest friends,” he says. “She found a lump during self-exam at age 38 and, when she told her doctor, she was told she was too young for a mammogram. By the time she got her first imaging, at age 39, she was diagnosed with stage 4 breast cancer.
“She did beat the odds and lived nearly 14 years with Stage 4 breast cancer, but early diagnosis could have changed the outcome significantly.”
leagues are developing a new MRI technique called T1rho dispersion for use in combination with a special inexpensive low-magnetic field MRI scanner. This new technique is expected to have increased accuracy in dense tissues and enable inexpensive portable low-field MRI to be used as a screening tool for breast cancer.
Low-field MRI systems for portable neuroimaging are now available for roughly $50,000, and similar safe portable MRI systems could be used in settings similar to mammography centers.
If shown to be effective as breast screening tools, the techniques developed by Dr. Rosen and his lab will significantly improve women’s access to health care, and enable frequent, safe, comfortable and diagnostically accurate breast cancer screening in high-risk populations.
Preliminary work supported by the Research Scholar award has already led to a new, NIH-funded project: “Novel Technique for Breast Cancer Screening and Evaluation Using Ultra Low field MRI,” with Dr. Rosen and Kathryn Keenan, PhD, of the National Institute of Standards and Technology as principal investigators.

15
‘Fast
MRI’ Yields More Efficient, More Patient-friendly Exams
Launched in 2022, the Mass General Brigham Outpatient Imaging Center at Assembly Row in Somerville not only provides a convenient location for an array of outpatient exams but also offers access to cutting-edge imaging techniques and the latest innovations in workflow optimization, enabling exam times of 30 minutes or less.
Taken together, the many features of the center add up to an unparalleled patient experience, and what Susie Huang, MD, PhD, a neuroradiologist in the Martinos Center for Biomedical Imaging who played an integral role in developing the center, describes as a “model practice of the future.”
The convenient location and optimized workflow aren’t the only ways the imaging center is enabling shorter visits for patients. Dr. Huang and colleagues are also reducing the length of exams by incorporating specialized MRI protocols. Here, they looked to a longstanding collaboration between researchers at the Martinos Center for Biomedical Imaging and engineers at Siemens Healthineers, a collaboration that has yielded new parallel imaging technologies enabling what the researchers have dubbed “fast MRI.”

Fast MRI offers tremendous benefits. The typical MRI brain exam can take up to 40 minutes, producing a considerable burden: a burden in terms of cost and efficiency—because of the relatively low throughput associated with the exams—but more importantly a burden to the patient, who needs to lie still in the scanner for long periods of time, a requirement that can prove uncomfortable and even distress-
ing. By incorporating fast MRI into its exams, the outpatient imaging center at Assembly Row helps relieve this burden, reducing the exam times to 30 minutes or even less.
In fact, scans using the technology aren’t just faster, enabling higher throughput and increased efficiency for the center and less anxiety and discomfort for the patient, they are better. This counterintuitive proposition— spending less time on a scan can give you a higher-quality image—can be explained by considering the problem of motion artifacts: anomalies in an image that result from the patient squirming or otherwise moving around during a scan. Developers of the technology have found that patients are better able to hold still for the relatively short lengths of time required for fast MRI. This leads to fewer motion artifacts and thus results in better images.
In a recently completed study, Dr. Huang and colleagues, including first author Min Lang, MD, a neuroradiology fellow and incoming faculty member in the department, evaluated the time savings achieved with the fast MRI techniques and optimized workflow in the outpatient imaging center. They found that the time savings from both the use of fast MRI and the unique design of the Assembly Row facility accounted for the dramatic decrease in imaging slots from 45 minutes in 2019 at the reference outpatient imaging center to 30 minutes at Assembly Row.
Just as important, the fast exams contribute to a more positive and less stressful experience for patients visiting the new outpatient imaging center. In the recently completed study, Drs. Huang and Lang reported a patient satisfaction score of 93.6 averaged over a year at Assembly Row, as compared to an average score of 85.8 at other Mass General outpatient imaging facilities in 2019, prior to adopting the fast exams.
16
CONTACT Susie Huang, MD, PhD Susie.Huang@mgh.harvard.edu
Novel MR Contrast Agent Avoids Gadolinium Retention in the Body and Brain
Gadolinium-based contrast agents (GBCAs) have long been used to improve the quality of images for some MR exams, but they are not entirely without risk. In 2017, the U.S. Food and Drug Administration (FDA) issued a class warning that gadolinium—the rare earth metal used in GBCAs—remains in patients’ bodies and brain for months or even years after injection of the contrast agents.
The FDA noted that gadolinium retention was not directly linked to adverse health effects in patients with normal kidney function, and that the benefit of approved GBCAs continued to outweigh any potential risks. Still, in the wake of the new warning, researchers focused renewed attention on gadolinium retention and on possible alternatives to GBCAs.
In October 2023, for example, the Martinos Center’s Peter Caravan, PhD, Mariane Le Fur, PhD, and colleagues reported in Radiology (DOI:10.1148/ radiol.230984) a study in which they compared the retention of four clinically used GBCAs in animal models.
“Our motivation for this study was to help better understand the clinical significance of the issue of gadolinium retention in vivo and help determine whether, in some cases, specific gadolinium-based contrast agents (GBCAs) should be preferred on a safety basis,” Dr. Le Fur said.

“Using MRI and ex vivo elemental imaging techniques, we found that [the GBCA] gadoteridol was excreted faster and had lower retention in rat kidneys compared to the other GBCAs included in the study. We also found that GBCAs are retained in human kidney samples for several weeks after GBCA administration, but additional studies are needed to know whether gadoteridol is also less retained in human kidneys.”
Exploring the Alternatives
In Dr. Caravan’s lab at the Martinos Center, the retention of gadolinium also motivated development of a gadolinium-free contrast agent RVP001 (also known as Mn-PyC3A). The company launched to spearhead this development has now completed a Phase 1 clinical trial to assess its safety, tolerability, and pharmacokinetics in healthy volunteers and is about to
start a Phase 2 clinical trial in patients. The multi-center trial is based at MGH with John Chen, MD, PhD, a neuroradiologist and co-director of the Institute for Innovation in Imaging, as principal investigator and imaging performed at the Martinos Center.
The researchers are also exploring possible applications of the contrast agent. In April 2023, in collaboration with Christopher Nguyen, PhD, a Martinos alum and current director of the Cardiovascular Innovation Research Center at the Cleveland Clinic, they reported in Journal of the American Heart Association a study testing the potential of RVP-001 for cardiac MRI. There is a clinical need for gadoliniumfree contrast agents, the authors noted in the paper, both because of gadolinium retention generally and because gadolinium-based agents are contraindicated in patients with acute and chronic renal insufficiency.
The study showed that RVP-001 appears to be an effective contrast agent for assessment of myocardial infarction location, size and transmurality, and thus could provide a useful alternative to gadolinium-based agents
CONTACTS
Mariane Le Fur, PhD mlefur@mgh.harvard.edu
Peter Caravan, PhD pcaravan@mgh.harvard.edu
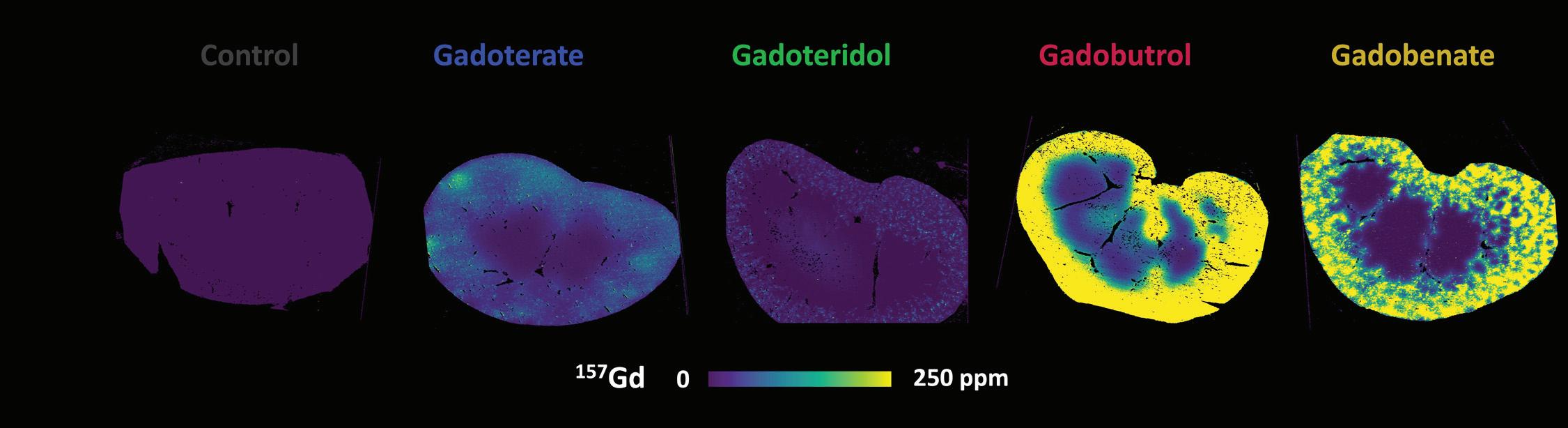
Comparison of GBCA retention in the kidney in animal models
17
Understanding the Role of Empathy in Psychedelic-assisted Therapy
Recent years have seen a resurgence of interest in the potential of psychedelics—including mushrooms (psilocybin), ecstacy (MDMA) and ketamine—for a host of clinical applications: not least, for treatment of neuropsychiatric conditions such as depression and post-traumatic stress disorder.
Now, a novel approach to MRI at the Martinos Center could help advance the use of psychedelics.
Vitaly Napadow, PhD, and his team in the Center for Integrative Pain NeuroImaging (CIPNI) at the Martinos Center have been exploring the use of “hyperscan neuroimaging,” in which two or more MRI scanners are connected to enable simultaneous tracking of the neural responses in individuals interacting with one another.
Since the mid- to late 2010s, they have used this technique to probe the brain mechanisms by which the patient-clinician relationship and therapeutic alliance can contribute to improvements in clinical outcomes:

CONTACT
Vitaly Napadow, PhD vitaly@mgh.harvard.edu
essentially, to understand better the benefits of good bedside manner.
Now, they are also applying it to understand better the mechanisms involved in psychedelic-assisted therapy.
We recently spoke with Dr. Napadow about this work and its broader implications. Here is what we learned.
What led you to study the patient-clinician relationship in psychedelic-assisted therapy?
Psychedelic-assisted therapy such as with MDMA (or Ecstasy) is gaining interest for several neuropsychiatric disorders, but the mechanisms are not well understood. Since psychotherapy is widely acknowledged as an important component of this therapy, many experts now think of the psychedelic drug itself as simply an important tool that is necessary but not sufficient for the beneficial clinical effects.
Coincidentally, we have been applying hyperscan neuroimaging to better understand the brain mechanisms by which the patient-clinician relationship and therapeutic alliance can contribute to improvements in clinical outcomes such as pain, with recent research publications in Science Advances, Translational Psychiatry, and PNAS. We are now exploring whether this extends to how MDMAassisted therapy might help patients, particularly chronic pain patients.
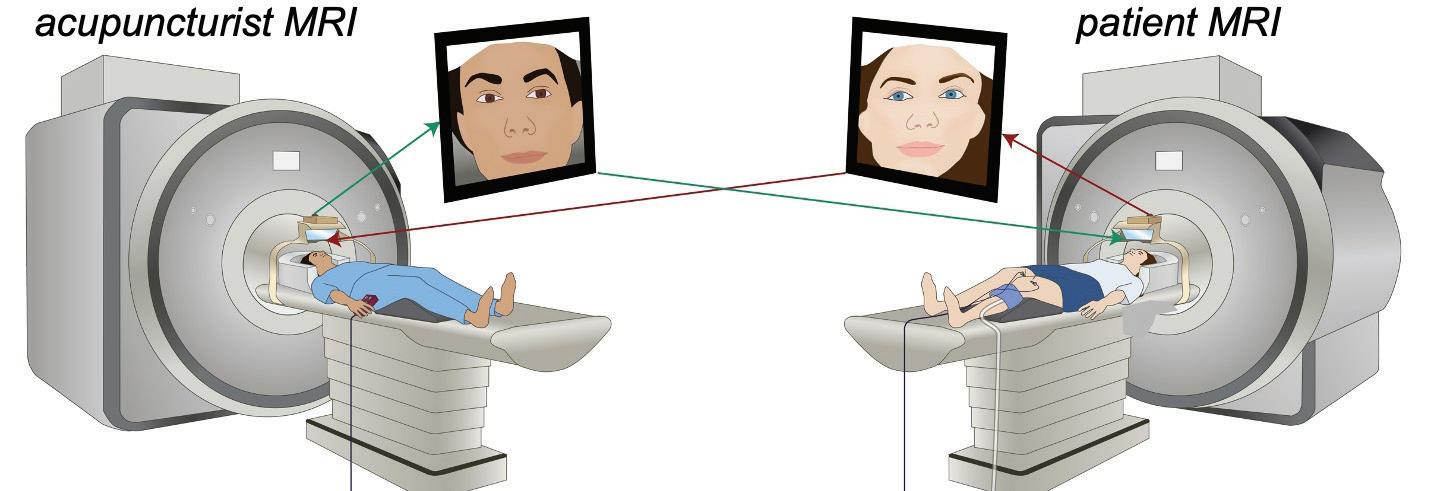
18
How can hyperscan neuroimaging held shed light on the impact of the patient-clinician relationship generally?
Our prior research showed that brain regions important for empathy and social communication — the socalled social mirroring network which includes ventrolateral prefrontal cortex, temporoparietal junction, and anterior insula — are activated during patientclinician interaction and exhibit brainto-brain concordance in dynamics during this interaction. Greater behavioral mirroring and brain-tobrain concordance was linked with therapeutic alliance and pain relief, suggesting that this concordance may be important for better clinical outcomes.
How might your findings inform clinical practice in psychedelicassisted therapy?
Linking specific brain mechanisms to the clinical response for psychedelicassisted therapy is important for understanding how such therapies are beneficial and which patients might benefit most with this therapy. There’s a lot of inter-individual variability in clinical response and hopefully underrating brain mechanisms can lead to a biomarker that will help clinicians make important decisions in the future. It’s our goal to be part of that development, while at the same time exploring new and exciting clinical applications for psychedelic-assisted therapy.
For instance, ours is the first Multidisciplinary Association for Psychedelic Studies (MAPS) approved study of MDMA-assisted therapy for fibromyalgia pain and
Managing
will be a collaboration between the Martinos Center, MGH Department of Psychiatry, and the Schoen Adams Discovery Center for Chronic Pain Recovery at Spaulding Rehabilitation Hospital. Additionally, we will also support neuroimaging for a new study of psilocybin therapy for Irritable Bowel Syndrome (IBS), a collaboration between the Martinos Center and MGH Gastroenterology. Such crossdepartmental collaborations are essential to move our understanding of psychedelic-assisted therapy forward.
Will this work have any broader implications for the study of the patient-clinician relationship?
Hyperscan research can also help us better understand therapeutic alliance more broadly. In other words, MDMA is a pharmacological intervention upregulating the psychological constructs we are so interested in studying with our hyperscan neuroimaging methods.
Anxiety and Depression with Mindfulness-based fMRI Neurofeedback

CONTACT
Susan Whitfield-Gabrieli, PhD s.whitfield-gabrieli@northeastern.edu
Martinos researcher Susan WhitfieldGabrieli, PhD, and colleagues have developed a mindfulness-based fMRI neurofeedback approach that could play an important role in treating adolescents with a history of anxiety and depression.
The work was done in collaboration with the Center’s Anastasia Yendiki and the Martinos-based Boston Adolescent Neuroimaging of Depression and Anxiety (BANDA) study.
In the Q&A below, Dr. Whitfield-Gabrieli discusses the approach and the impact it could have on the management of anxiety and depression.
What motivated the study?
In adolescents, rates of depression and anxiety (which are highly comorbid with ADHD) have been rising over the past decade, with the COVID-19 pandemic exacerbating these alarming trends. As a result, the American Academy of Pediatrics, American Academy of Child and Adolescent Psychiatry, Children’s Hospital Association and the U.S. Surgeon General have declared a national state of emergency for youth mental health.
Strategies for supporting these populations are inadequate and unequitable: large-cohort studies have shown that a majority of patients are initially prescribed ineffective pharmacologi-
19
cal treatments, and it frequently takes years of trial and error to get to an effective therapy. As currently implemented, both pharmacological and behavioral interventions are effective for only about 50% of patients.
In what ways are the strategies inequitable?
Both pharmacological treatments and behavioral treatments can be expensive — and they are especially difficult to access now because there is a dramatic shortage of available therapists. Not everyone has the financial resources to support such interventions. For example, a primary barrier is the lack of health insurance, which prevents families from being able to afford standard treatments. However, mindfulness meditation is free and can be done by anyone, anywhere and anytime.
How does the approach you and colleagues have described work? How does it help address the need for more effective and more equitable strategies?
Depression, at the neural level, is consistently characterized by hyperactiva-
tion and hyperconnectivity among a set of regions belonging to the ‘default mode network’ (DMN), including key nodes in the medial prefrontal cortex (MPFC) and the posterior cingulate (PCC). The DMN, an intrinsic brain network associated with our inner mental lives, becomes engaged during mental time travel — such as reminiscing about the past or planning for future. It is over-engaged in patients suffering from depression/anxiety — reminiscing about the past may become ruminating or rehashing the past and planning for the future may become obsessively worrying about the future.
In depression, the DMN “ropes in” the subgenual anterior cingulate (sgACC) which is known to be an effective direct, or associated target, in stimulation-based treatment options such as deep brain stimulation and transcranial magnetic stimulation.
Instead of relying on externally delivered neurostimulation that may require invasive surgery and/or can only be performed at a clinic, we aim to teach patients how to modulate their DMN, via mindfulness meditation augmented with real-time fMRI neurofeedback,
Elucidating
with the goal of teaching them how to volitionally modulate their brain networks and mitigate symptoms in a context specific manner.
In what ways would you like to implement the approach?
Ultimately, we are hoping to leverage the power of real-time fMRI triggered experience sampling and neurofeedback to inform highly individualized behavioral therapies and rebalance patterns of aberrant brain network activity that underlie a broad range of mental disorders (e.g., psychosis, depression, anxiety, ADHD).
Our vision is to create a technologybased training platform that supports individuals suffering from mental illness, ultimately enabling them to (1) recognize the onset of negative mental states (e.g., repetitive negative thinking [RNT] such as rumination, worry) in real-time in their daily lives; (2) initiate context-/symptom-specific interventions (e.g., mindfulness meditation, exercise) that are targeted to their own precise neural architecture and activity patterns, and (3) create brain network dynamics that are conducive to positive mental health.
the Relationship between Intimate Partner Violence and Brain Injury
Intimate partner violence (IPV) is a common problem in the United States, affecting millions yearly. According to the Centers for Disease Control and Prevention (CDC), approximately 41% of women and 26% of men experience contact sexual violence, physical violence, and/or stalking by an intimate partner, whether a current or former spouse or a dating partner. Nearly one in three women has experienced physical or sexual violence, mostly by an intimate partner. The impacts of IPV range from missing work to symptoms of posttraumatic stress disorder (PTSD) and a variety of physical
injuries—the latter including partnerinflicted injuries to the brain.
Eve Valera, PhD, a researcher in the Department of Psychiatry at Massachusetts General Hospital and an affiliated faculty member in the Martinos Center, has studied intimate partner violence for the past quarter century, focusing especially on using imaging and other tools to understand the neural, neuropsychological, and psychological consequences of traumatic brain injuries (TBIs) resulting from IPV. In a recent conversation, she elaborated on her work.
What led you to pursue research into intimate-partner violence?
The primary objective of my work in IPV is to understand the effects of partner-inflicted brain injuries from blunt force trauma and strangulation. In graduate school, over 25 years ago, while working with women who had experienced IPV, I realized the abuse these women were sustaining could contribute to brain injuries. I was very curious about what was known about this issue and discovered that no papers were examining this topic. I decided that needed to
20
change and began my dissertation with the goal of changing it.
How do you use imaging?
I was very curious about what was known about this issue and discovered that no papers were examining this topic. I decided that needed to change and began my dissertation with the goal of changing it.
How do you use imaging?
Currently, we are using resting-state fMRI, diffusion MRI, and magnetic resonance spectroscopy to understand better how neural networks and certain neurometabolites are affected by repetitive brain injuries. I emphasize ‘repetitive’ because most women who sustain partner-inflicted brain injuries sustain more than one. We published the first and—at the time— only imaging papers in this area showing associations between a brain injury severity score (based on the number and recency of brain injuries) and specific functional and structural connectivity metrics in the brain. Although there are a couple of other studies out there now and a few groups working towards this goal, there are still very few people using imaging to address this issue.

CONTACT
Eve Valera, PhD evalera@mgh.harvard.edu
Can you describe recent findings with respect to your IPV work?
We have more recently found that even in women living in the community—that is, women not seeking partner violence support or intervention or medical assistance—the rates of IPV-inflicted brain injuries are high (64%) and that the associations with neurobehavioral symptoms are found long after the most recent brain injury. Furthermore, we found an association between balance and the number of

brain injuries. We had not previously looked at balance. This is important as it tells us that these associations are not limited to women who are still in crisis or are merely “acute” issues. It is also important as balance problems can predispose women to falls that could result in even more brain injuries. We need to follow up on these findings.
How might your research contribute to broader awareness or prevention of IPV?
There is a tremendous misunderstanding of IPV and how women are affected. Yet, on the flip side, there is a growing understanding of how dangerous brain injuries are in athletics and the military. I hope that if people can understand the high prevalence and consequences of brain injuries from partner violence, there will be a greater awareness of the need for resources and intervention for these women, as there has been an awareness of such a need for athletes and military personnel.
What’s next in your work with IPV?
A couple of exciting newer projects include our qualitative study as well as the transgender arm of our main study. The qualitative study allows us to hear more directly from women about what they know about brain injuries and how they addressed their injuries when they were in their relationships. This will include answers to questions we may not have thought to ask using a more quantitative approach.
Regarding the transgender arm of the study, although all women have always been welcomed into our studies, there were no trans-specific measures. We have now included such measures and hired experts to help us in these efforts. As we know that interpersonal violence, as well as partner violence, is higher for transgender women than for cisgender women, we must increase our efforts to hear their voices and find out how brain injuries are affecting them as well.
21
‘Martinnovate’ Seminar Series Boosts Innovation at Martinos
The Martinos Center has always been a hotbed of entrepreneurship. Over the years, innumerable investigators and staff have launched companies seeking to commercialize products they have developed as part of their research.
A broad effort in the Center aims to support this entrepreneurial spirit while building a network of scientists and entrepreneurs interested in scientific innovation.
Introduced in early 2023, the “Martinnovate” series offers monthly presentations by invited speakers who share their experiences from across the innovation spectrum. The brief, informal talks are followed by lively discussions, with the goal of empowering attendees hoping to commercialize their own science.
Martinnovate emerged from a series of conversations between Martinos faculty member Bastien Guerin, postdoctoral research fellow Nikou Louise Damestani, and Hope Taft, executive director of the Center.
“We noticed an increasing number of investigators engaging with the commercialization pipeline,” Damestani says, “and wanted to provide an avenue for them to disseminate their knowledge of the innovation process to see how much interest already existed in the community.
“Our first speaker almost packed the room, so we realized we were onto something!”

In May, the Martinos Center hosted its first annual Innovation Challenge. This year’s challenge focused on Mass General Brigham’s Home Hospital Program. A major initiative across the system, Home Hospital seeks to deliver hospital-level care where
patients live, thus maximizing patients’ time in their homes and with their loved ones and freeing up capacity for patients who require treatment in the hospital. Home Hospital also extends beyond home health, providing care for patients facing a wide range of conditions, including heart failure, COPD, pneumonia and urinary tract infections, among others.
22
Innovation Challenge Showcases Home Health Technologies of Tomorrow
From L to R: Center director Bruce Rosen, MD, PhD; Jerry Ackerman, PhD; Hope Taft; and Glenn Miller, PhD, MGB Innovation, a panelist for the event
CONTACTS
Bastien Guerin, PhD bguerin@mgh.harvard.edu
Hope Taft htaft@mgh.harvard.edu
Jerry Ackerman, PhD jlackerman@mgh.harvard.edu
The 2023 Innovation Challenge sought to harness the innovation power and expertise of the Martinos Center to develop game-changing technologies to improve care delivery at home. The goal of the challenge was to identify high-potential innovations and showcase them to leaders across MGB and beyond—leveraging resources and funding mechanisms to support future development and commercialization, and ultimately enhance the home hospital care delivery model.
The evening’s two winners were Anthony Samir, PhD, director of the Center for Ultrasound Research & Translation in the Department of Radiology at Massachusetts General Hospital (panel selection), and Jerry Ackerman, PhD, director of the Biomaterials Laboratory within the Martinos Center (audience selection).
Samir presented a point-of-care surgical robotic platform that combines machine learning algorithms, ultraportable ultrasound imaging, and low-cost surgical robotics to automate vascular access. Applications of the system include blood draws and the remote administration of injectable medications.
Ackerman proposed a fleet of trucks offering curbside, mobile MRI. The project takes advantage of novel “dry” (liquid-helium free) superconducting magnet technology, which enables MRI scanners that are highly compact, portable, and less expensive than conventional magnets. The team behind this project also included Clarissa Cooley, PhD, Jason Stockmann, PhD, Andre van der Kouwe, PhD, and Susie Huang, MD, PhD.
Wearable Device Could Assess Brain Health from the Comfort of Home
The first cerebral oximeter for adults can be added to the rapidly expanding list of wearable health monitors, thanks to the efforts of PhD candidate Kuan-Cheng (Tony) Wu, postdoctoral fellow Marco Renna, PhD, Maria Angela Franceschini, PhD, and others at the Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital.
The team has developed a wireless, low-cost, open-source cerebral oximeter and a corresponding mobile application for Android smartphones, built on the noninvasive technology nearinfrared spectroscopy (NIRS), which was pioneered by Martinos researchers. Named FlexNIRS, the device will be especially useful for monitoring

CONTACT
Maria Angela Franceschini, PhD mfranceschini@mgh.harvard.edu
older adults, who are generally at risk of a range of brain diseases.
So that FlexNIRS can reach underresourced communities, Mass General has made the hardware (circuit schematic and 3D drawing) and software files available online for non-commercial use.
Ultimately, FlexNIRS will provide personalized monitoring and cerebral health assessment that can be performed daily at home. In addition, cerebral SO2 monitoring at a community level and in telehealth applications may aid in the prevention or early detection of sleep apnea, stroke, and other cerebrovascular diseases.
23
Research in Action: Introducing the Martinos Center Maker Space
As of this writing, the Athinoula A. Martinos Center for Biomedical Imaging is preparing for the opening of its new Maker Space, scheduled for summer 2024.
The mission of the Maker Space is to create a collaborative and safe environment for innovation in biomedical imaging through cutting-edge rapid prototyping techniques that facilitate the translation of ideas into tangible solutions.
Forging a New Path for MRI Hardware
The Martinos Center has a long legacy of leading the way in MRI research through the development of custom MRI instrumentation. Making hardware capable of functioning in the harsh electromagnetic environment of an MRI scanner is no easy task. Advancements in computer simulation

Jeff Short jshort5@mgh.harvard.edu
FEM tools and AI-driven optimization offer new opportunities for MRI signal acquisition and image reconstruction while adding manufacturing complexity. The new Make Sspace will bridge the gap between these simulations and the real world.
For example, the Maker Space will focus on facilitating the advancement of MRI coils used by Martinos Center researchers to acquire the MR signal. Applications for these MRI coils range from cancer detection with MR spectroscopy to better understanding how the human brain functions with diffusion imaging. The Maker Space will serve as a space where Center researchers and engineers can collaborate and innovate on the way MRI coils are designed and created.
In time, the Maker Space may provide a hardware engineering service capable of meeting the novel MRI

24
CONTACT
hardware needs of researchers in the broader Mass General Hospital community and, eventually, the MRI research community as a whole.
The Maker Space will offer two separate research spaces:
A Machine Shop will allow researchers to fabricate precise components using subtractive manufacturing methods. Equipment in this area will include a knee mill, CNC mill, CNC lathe, bandsaw, drill press, and other essential machinery.
A second area will feature cuttingedge 3D printers, complemented by electrical engineering workbenches, and an extensive selection of hand tools and hardware, creating a versatile and innovative space for prototype development.
The Institute for Innovation in Imaging: A New Era in Innovation
The Institute for Innovation in Imaging (i3) had a landmark year in 2023. With the official launch of a new facility located in close proximity to the Martinos Center, it is now a fully operational Core at Massachusetts General Hospital, offering a wide range of services to help researchers bridge the gap between pre-clinical innovation and first-in-human proof of concept.
Led by co-directors Peter Caravan, PhD, (Martinos Center for Biomedical Imaging) and John Chen, MD, PhD, (Center for Systems Biology, also at Mass General) and associate director Matt Dubach (Martinos Center for Biomedical Imaging), the i3 draws upon faculty across the Department of Radiology and seeks to accelerate the translation of new tools for early disease detection and monitoring of emergent treatments. To this end, it collaborates with researchers and companies innovating in these areas, working with them to assess their needs and create customized technology translation strategies drawing on the i3 faculty’s capabilities and the broad array of resources at the institute’s disposal.
An example of a recent success story can be found in the benchto-bedside translational work the i3 performed for the company TransCode Therapeutics. Transcode is developing a nanomedicine co-discovered by Dr. Zdravka Medarova of the Martinos
Center that is designed to treat cancer patients with metastatic disease. In collaboration with TransCode, the i3 scientists developed a method to tag the nanodrug with an isotope that would allow the drug to be detected by PET imaging. The i3 developed a manufacturing method, performed imaging studies in models, and provided TransCode with the documentation to file an investigational new drug (IND) application, which was approved by FDA. In collaboration with the company, the MGH Cancer Center and the Martinos Center, the team is now performing an early phase clinical study to detect whether the nanodrug targets metastatic tumors in patients. All of this work was performed in the space of about a year.
A Robust Research Environment
The i3 initially functioned in a virtual setting with the co-directors and faculty using lab space in their parent centers. All of this changed, though, with the recently completed expansion.
The institute now encompasses 2,322 square feet of office space, accommodating 25 faculty, staff and trainees, 3,500 square feet housing wet labs, with an additional 2,310 square feet of shared space. The wet lab space comprises facilities for radiopharmaceutical production for human use; a PET-CT facility for imaging nonhuman primates and rodents; a deep content mesoscale

CONTACT
Peter Caravan, PhD pcaravan@mgh.harvard.edu
imaging facility for mass specbased tissue imaging; an electronics lab; a computation lab; advanced microscopy labs; molecular biology lab; as well as synthetic, analytical and radiochemistry labs.
The expansion also created an additional 3,300 square feet for a shared Clinical Translational Research Unit (CTRU), also located in close proximity to the Martinos Center. Serving i3-supported as well as other human imaging studies performed at the Martinos Center, the CTRU contains exam and evaluation rooms, a clinical laboratory, and patient reception and waiting rooms.
25
Magnetic Particle Imaging: Exciting New Imaging Technology Will Soon Be Online
An emerging technology could give functional magnetic resonance imaging (fMRI) a run for its money. With dramatically higher sensitivity than is currently available with fMRI, it could boost a range of clinical applications.
In the Martinos Center for Biomedical Imaging at Massachusetts General Hospital, Larry Wald, PhD, a principal investigator in the Magnetic Resonance Physics & Instrumentation Group, and colleagues are nearing completion of a magnetic particle imaging (MPI) scanner for imaging of the human brain. Once validated, the scanner will provide an exciting new means to study activity in the brain.
Introduced in the late 2000s, MPI shares many of the same technologies as MRI, but it differs in one crucial way: It directly detects the magnetization of nanoparticles injected into the body, rather than relying on secondary effects of magnetic resonance relaxation times. Directly imaging the source of contrast like this is what allows for the vastly improved sensitivity.
Dr. Wald started working with MPI in 2014. With the support of one of the first BRAIN Initiative grants awarded by the National Institutes of Health, he and his group set to work assessing the potential of the technology, examining the barriers to its use for neuroimaging in humans and, through simulations, testing the performance of MPI scanner designs.
The results of the work were encouraging. The researchers concluded that a first-generation human-scale MPI scanner could offer tenfold higher sensitivity than conventional functional MRI with similar spatial resolution, with the possibility of improvement with further development.
The opportunity to construct the scanner came in 2017, when Dr. Wald ap-

plied for and was awarded his second BRAIN Initiative grant. This grant would support the construction and validation of an MPI device for use in humans, as outlined during the earlier research. Wald knew there would be challenges to face. “There is currently no human MPI scanner,” he said at the time. “And there is a reason for this: It’s hard.” But he had no doubt that the team he had assembled—which also included Clarissa Cooley, PhD, Ken Kwong, PhD, Emiri Mandeville, PhD, Joe Mandeville, PhD, Erica Mason, PhD, and Wim Vanduffel, PhD—would be able to address them. (The team quickly expanded to include Eli Mattingly, who focused on the project for his PhD thesis, Monika Sliwiak, and Jorge Chacon-Caldera, PhD.)
The greatest difficulties lay in translating the technology for human-size imaging—scaling up the field generation and detection, for example. Wald also anticipated a variety of “industrial type” challenges in designing and building the scanner. Not least: figuring out how to keep a two-ton, water-cooled electromagnet spinning around the subject’s head.
The researchers have now tackled all of these challenges and are putting the finishing touches on the first-ever
human-scale functional MPI scanner. The scanner is capable of continuously imaging objects the size of the human head with a 5-second temporal resolution and a spatial resolution comparable to conventional fMRI (about 5 mm). Its sensitivity to changes in cerebral blood volume accompanying brain activation is expected to significantly exceed that of fMRI.
The new scanner could advance a range of applications. “We are betting that it will be a valuable tool for clinical neuroscience, where the sensitivity must be sufficient to see functional differences in individuals rather than averaging over a cohort of individuals with a disorder,” Wald says today. “But also, as a diagnostic imaging modality, it could monitor for hemorrhage, assess stroke volume or, with targeted agents, provide a sensitive new molecular imaging modality to detect cancer or other disease processes.”
26
CONTACT
lwald@mgh.harvard.edu
Larry Wald, PhD
In 2015, the Center’s Larry Wald, PhD, received an MGH Research Scholar Award for his project, “Magnetic Particle Imaging for Breast Cancer Screening and Monitoring.” The goal of the project was to demonstrate the first use of magnetic particle imaging (MPI) in humans. Dr. Wald and colleagues chose breast cancer as a target because of the societal importance of the disease and because the breast, as a small, localized, externally accessible body part, was well suited for a proof-of-principle study with MPI.
The MPI team completed the five-year project in 2020. In 2021, they reported a study (DOI:10.1038/s41598-021-92644-8) in which they described a prototype device they had developed and demonstrated its potential for breast conserving surgery, a widely used treatment for early-stage breast cancers but with relatively high re-excision rates. They showed that MPI could provide a fast, specific, sensitive, easy-to-use tool for assessing tumor margins during the procedure itself and thus could reduce the need for additional surgeries.

Ultrahigh-field Human MRI: The End of One Era, the Beginning of Another
Members of the Martinos community gathered inn December to say goodbye to the Center’s original 7T scanner. Installed more than 20 years ago, the scanner was the first-ever ultrahigh-field MR system built on a clinical platform. Researchers used it
to produce amazing science over the two-plus decades. But when one of its compressors stopped working, the Center decided it was time to let it go.
Every ending is a new beginning, though. The Center has already bro-
ken in a Terra 7T FDA-approved scanner, which is continuing the tradition of providing unprecedented insights into the inner workings of the human body. And the retirement of the original 7T will clear the way for yet more advanced MR technology.

group of
27
A
7T users with Martinos Center director Bruce Rosen, MD, PhD (seated on the scanner bed) celebrating 20 years of science on the original 7T scanner
Center Installs State-of-the-Art Optically Pumped Magnetometer (OPM) Device


Optically pumped magnetometers (OPMs) are a promising sensor technology for non-invasive on-scalp measurement of human electrophysiological signals. OPM-MEG offers the potential for higher spatial resolution, naturalistic MEG studies involving movements, and higher signal-to-noise ratio (SNR) for superficial cortical sources.
Members of the Center’s MEG team developed background field cancellation coils on a two-layer Printed Circuit Boards (PCB). Built by Hopetimepcb, the PCB coils were mounted on a sliding aluminum frame for easy access to the subject during experiments. Preliminary results suggest that PCB cancellation coils are more accurate than hand-wound coils and can be easily replicated and mass-manufactured at a lower cost. This innovation paves the way for cheaper and more robust commercial OPM-MEG systems.
Shown unpacking and installing the PCB coils in the photos above: Chunling Dong, Yoshio Okada, Jack Kamataris, Abbas Sohrabpour, Teppei Matsubara, Mainak Jas, Padma Sundaram.
28
The
Positron emission tomography (PET) provides unique information about metabolic or biochemical activity in the body and the brain. Because this information is complementary to that recorded by magnetic resonance imaging (MRI), researchers in recent years have been developing PET devices that can be inserted into the bore of an MRI scanner to faciltate simultaneous PET and MR imaging.
The Martinos Center’s Ciprian Catana, MD, PhD, works on the cutting edge of this development. Currently, he is leading the team building a PET camera compatible with 7-Tesla MRI offering greater than 10x improved sensitivity over any existing PET insert. When completed, the device will enable dynamic PET imaging of brain neurotransmission, neuromodulation and other dynamic molecular events with unprecedented temporal resolution and beyond state-ofthe-art spatial resolution.

CONTACT
Ciprian Catana, MD, PhD ccatana@mgh.harvard.edu
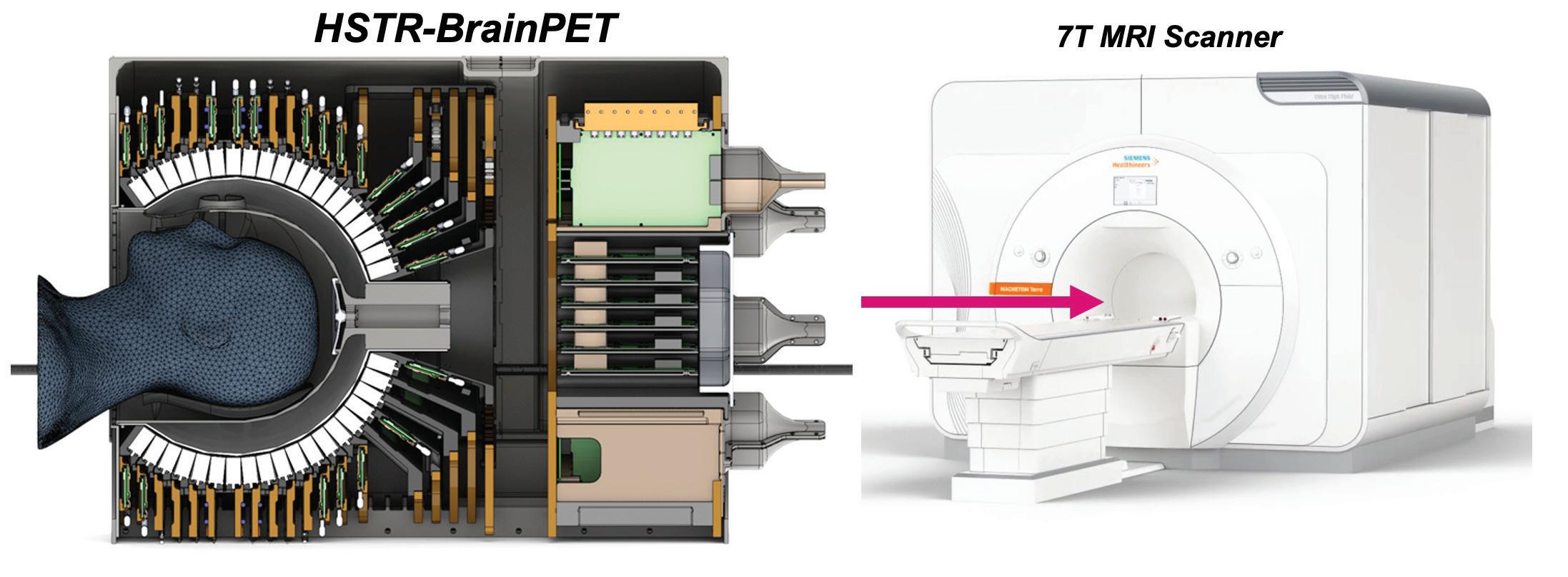
29
for
High Spatio-temporal Resolution ‘BrainPET’ Will Offer Unprecedented Sensitivity
Simultaneous PET/MRI
The Center Welcomes the First Athinoula A. Martinos Professor of Health Sciences and Technology
In February 2023, Laura Lewis, PhD, returned to the Martinos fold as the inaugural Athinoula A. Martinos Professor of Health Sciences and Technology (HST) within IMES and the Department of Electrical Engineering and Computer Sciences (EECS) at MIT, and as an investigator within the Martinos Center at MGH.
Dr. Lewis received her PhD in Neuroscience from MIT and conducted postdoctoral work at the Martinos Center before joining the faculty of Boston University in 2019. In her new role at MIT and MGH, she will continue to pursue her research interests in the basic neurophysiology of sleep and arousal, teach at MIT and, importantly, help further connections between the Martinos Center and her colleagues and students at MIT more broadly.
“One of the reasons I’m so excited about the position is that it has an unusual structure across institutions,” she says. “It’s a faculty position at MIT, in the Department of Electrical Engineering and Computer Science (EECS) as well as in the Institute for Medical Engineering & Science (IMES). It’s also a faculty position at the MGH Martinos Center for Biomedical imaging. The goal is to do biomedical imaging research at the intersection of MIT and MGH, to draw from the strengths of each and to help the two communities connect.”
Dr. Lewis’ research has garnered considerable attention in recent years. In late 2022, for example, using fast fMRI at ultrahigh field (7T), she and colleagues identified a specific cascade of neural events associated with the transition from sleep to wakefulness. The findings of the study shed intriguing new light on the neural dynamics of this transition.

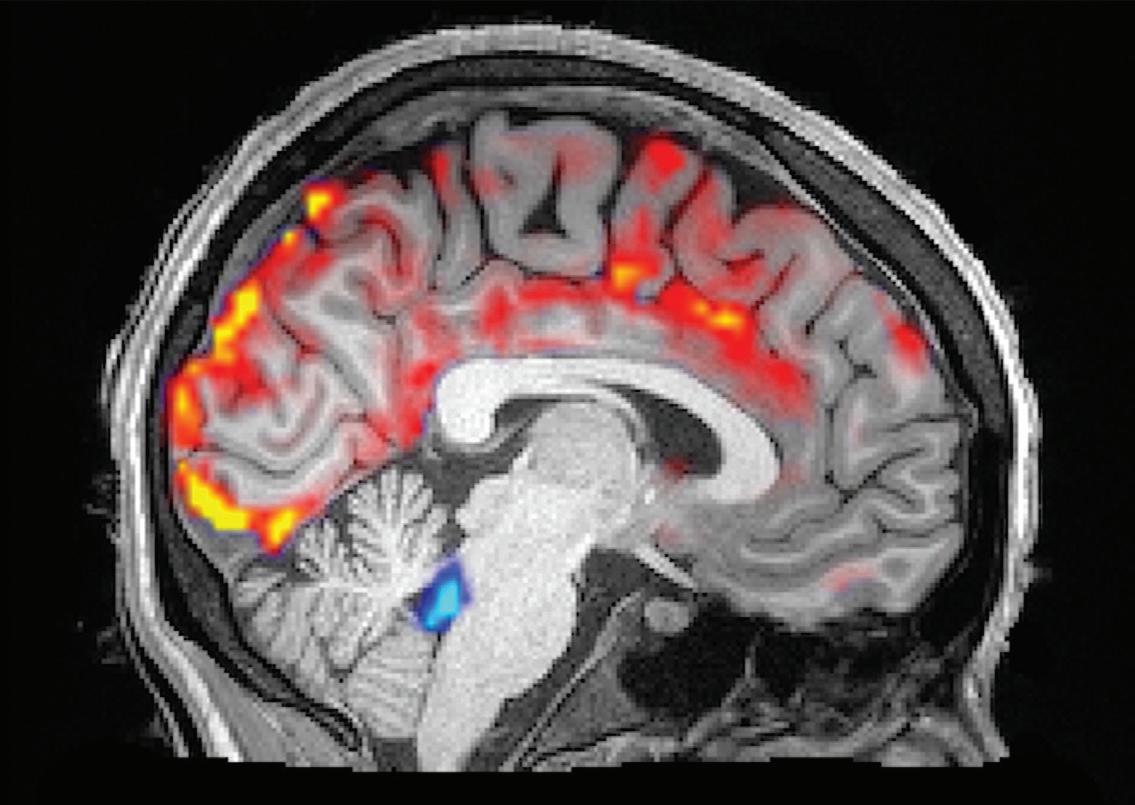
In 2019, Dr. Lewis and colleagues reported a study in which they found, using imaging in human volunteers, waves of cerebrospinal fluid washing over the brain while the volunteers slept, flushing toxins from the brain. Ongoing work in the wake of the study is providing insights into disorders such as Alzheimer’s disease that are associated with disruptions in sleep, as well as impairments linked to normal aging.
CONTACT
Laura Lewis, PhD ldlewis@mit.edu
30
Mesoscale Brain Mapping: Bridging Scales and Modalities in Neuroimaging
Recent advances both in the MGH Martinos Center for Biomedical Imaging and elsewhere are driving the convergence of microscopic- and macroscopic-scale study of the brain for human translational neuroscience, by developing and applying tools to study the spatial distribution and temporal orchestration of mesoscopic events in the brain. On October 10, 2023, the Center for Mesoscale Mapping (CMM) at the Martinos Center and the HSTbased Neuroimaging Training Program (NTP) hosted a symposium exploring the state-of-the-art in this burgeoning area of inquiry.
“Mesoscale Brain Mapping: Bridging Scales and Modalities in Neuroimaging” brought together researchers using a broad range of imaging techniques to study brain function at the intersection of the micro- and macro- scales. The daylong event was
divided into two sessions, each covering topics centered on areas the CMM is currently exploring. The morning session—which delved into the “Why” of mesoscale mapping—addressed the clinical translational and basic neuroscience rationale for developing mesoscale capabilities. What are the current gaps in knowledge that mesoscale mapping can address, for example? In what areas is it likely to have the greatest impact?
The morning session featured presentations by Laura Lewis, PhD, and Jonathan Polimeni, PhD, and a panel discussion moderated by Randy Gollub, MD, PhD, and including Shahin Nasr, PhD, Roger Tootell, PhD, and Brian Edlow, MD, as well as Lewis and Polimeni.
The afternoon talks—the “How” portion of the day—focused on the technologies either currently available or under development for mesoscale mapping and the ways in which researchers are applying them. This session featured presentations by Elizabeth Hillman, PhD, (Columbia University) and Anastasia Yendiki, PhD, and a panel discussion moderated by Susie Huang, MD, PhD, and including Hui Wang, PhD, Larry Wald, PhD, Aapo Nummenmaa, PhD, and Berkin Bilgic, PhD, as well as Hillman and Yendiki.
The session—and the symposium— ended with a fireside chat with Martinos Center director Bruce Rosen, MD, PhD and Harvard University professor Jeff Lichtman, MD, PhD. Both neuroimaging pioneers, Rosen and Lichtman discussed the next frontiers in mesoscale mapping, the opportunities the many new technologies afford.



32
Science on Tap Seminar Series
Science on Tap Trivia Contest
Martinos Seminar Series
The Martinos Center hosts several popular seminar/webinar series organized by members of the community. Clockwise from below:
The BrainMap seminar series (below) is offered by and for researchers using imaging to study the inner workings of the body. Presented by invited speakers from around the world, as well as by investigators who call the Martinos Center home, the talks cover the gamut of topics in the biomedical imaging arena.
Science on Tap (opposite, bottom) is a weekly Friday afternoon social where members of the Martinos community gather to have a snack, socialize, and get to know each other’s work a little better. Every meeting features a 10 to 15 minute talk by a Martinos investigator about their latest research—including work in progress, or not yet published efforts—followed by informal discussion over chips and guacamole or some other treat.
Science on Tap organizers also put together a variety of special events, helping bolster the sense of community within the Center. In 2023, for example, they held a Halloween trivia contest. The winning team (opposite, top) impressed everyone in attendance with their in-depth knowledge of biomedical imaging, the history of the Martinos Center, and more!

BrainMap Seminar Series
33
Other Community-Building Efforts
The Martinos Center is home to an active Women in Science group (opposite, top) devoted to generating thoughtful conversation about disparities in STEM related to gender & gender identity, culture, race, sexual orientation and background while providing tools and a safe environment to achieve equity. Since the late 2010s, the group has organized a number of presentations, panel discussions and workshops as well as symposia and a variety of other events.
In January 2023, the Martinos Center staged its first-ever talent show, aptly titled: “The Martinos Center’s Got Talent!” (opposite, bottom).The event showcase the many, varied talents of folks from across the center, from accordion playing to digital drumming (with sounds sampled from MRI!), from stand-up comedy to Rubik’s cube solving. It was, without questiion, a night to remember.
Last, but by no means least, is a Center favorite: the annual Apple/Pumpkin Day, held every fall (below). Here, members of the Martinos community gather to share good times, good company and their favorite apple, pumpkin or other seasonal dish, dessert or beverage.

34
Apple/Pumpkin Day

35
The Martinos Center’s Got Talent!
The Women in Science Group
Revolutionizing imaging for the advancement of science and medicine
As one of the world’s premier research centers devoted to biomedical imaging, the Athinoula A. Martinos Center for Biomedical Imaging has a long history of innovation and demonstrated impact in both research clinical care. Our investigators continue to develop first-of-a-kind tools and apply them to solve challenges in neuroscience, oncology, cardiology and a variety of other clinical domains.
Please consider giving to help us continue to advance health for patients across the care continuum.

36

37



 Bruce Rosen, Director Athinoula A. Martinos Center for Biomedical Imaging
Bruce Rosen, Director Athinoula A. Martinos Center for Biomedical Imaging























 Heidi Jacobs, PhD hjacobs@mgh.harvard.edu
Heidi Jacobs, PhD hjacobs@mgh.harvard.edu






































