Detection of Drugs and Their Metabolites in Oral Fluid (Emerging Issues in Analytical Chemistry) 1st Edition White Visit to download the full and correct content document: https://ebookmeta.com/product/detection-of-drugs-and-their-metabolites-in-oral-fluid-e merging-issues-in-analytical-chemistry-1st-edition-white/
More products digital (pdf, epub, mobi) instant download maybe you interests ...
Carbon Dots in Analytical Chemistry: Detection and Imaging 1st Edition Suresh Kumar Kailasa
https://ebookmeta.com/product/carbon-dots-in-analyticalchemistry-detection-and-imaging-1st-edition-suresh-kumar-kailasa/
Detection of Drug Misuse Biomarkers Analytical Advances and Interpretation 1st Edition Rohrig
https://ebookmeta.com/product/detection-of-drug-misusebiomarkers-analytical-advances-and-interpretation-1st-editionrohrig/
FASTtrack Chemistry of Drugs 1st Edition David Barlow
https://ebookmeta.com/product/fasttrack-chemistry-of-drugs-1stedition-david-barlow/
Smartphone-Based Detection Devices: Emerging Trends in Analytical Techniques 1st Edition Chaudhery Mustansar Hussain (Editor)
https://ebookmeta.com/product/smartphone-based-detection-devicesemerging-trends-in-analytical-techniques-1st-edition-chaudherymustansar-hussain-editor/
Chemistry and pharmacology of anticancer drugs Second Edition Ilona Pysz
https://ebookmeta.com/product/chemistry-and-pharmacology-ofanticancer-drugs-second-edition-ilona-pysz/
Analytical Chemistry I 1st Edition Ulf Ritgen
https://ebookmeta.com/product/analytical-chemistry-i-1st-editionulf-ritgen/
White Magic A Story of Heartbreak Hard Drugs and Hope 1st Edition Arjun Nath
https://ebookmeta.com/product/white-magic-a-story-of-heartbreakhard-drugs-and-hope-1st-edition-arjun-nath/
Fluid Mechanics (9th Edition) Frank M. White
https://ebookmeta.com/product/fluid-mechanics-9th-edition-frankm-white/
Fluid Mechanics 9th Edition Frank M White
https://ebookmeta.com/product/fluid-mechanics-9th-edition-frankm-white-2/
DetectionofDrugsandTheir MetabolitesinOralFluid EmergingIssuesinAnalyticalChemistry SeriesEditor BrianF.Thomas Co-publishedbyElsevierandRTIPress,theEmergingIssuesinAnalyticalChemistry serieshighlightscontemporarychallengesinhealth,environmental,andforensic sciencesbeingaddressedbynovelanalyticalchemistryapproaches,methods,or instrumentation.Eachvolumeisavailableasane-book,onElsevier’sScienceDirect, andviaprint.SerieseditorDr.BrianF.Thomascontinuouslyidentifiesvolumeauthors andtopics;areasofcurrentinterestincludeidentificationoftobaccoproductcontent promptedbyregulationsoftheFamilyTobaccoControlAct,constituentsanduse characteristicsofe-cigarettesandvaporizers,analysisofthesyntheticcannabinoidsand cathinonesproliferatingontheillicitmarket,medicationcomplianceandprescription painkilleruseanddiversion,andenvironmentalexposuretochemicalssuchas phthalates,endocrinedisrupters,andflameretardants.Novelanalyticalmethods andapproachesarealsohighlighted,suchasultraperformanceconvergence chromatography,ionmobility,insilicochemoinformatics,andmetallomics.By highlightinganalyticalinnovationsandnewinformation,thisseriesfurthersour understandingofchemicals,exposures,andsocietalconsequences.
2016 TheAnalyticalChemistryofCannabis:QualityAssessment,Assurance,and RegulationofMedicinalMarijuanaandCannabinoidPreparations
BrianF.ThomasandMahmoudElSohly
2016 Exercise,Sport,andBioanalyticalChemistry:PrinciplesandPractice
AnthonyC.Hackney
2016 AnalyticalChemistryforAssessingMedicationAdherence SangeetaTannaandGrahamLawson
2017 SustainableShaleOilandGas:AnalyticalChemistry,Geochemistry,and BiochemistryMethods VikramRaoandRobKnight
2017 AnalyticalAssessmentofe-Cigarettes:FromContentstoChemicalandParticle ExposureProfiles
KonstantinosE.Farsalinos,I.GeneGillman,StephenS.Hecht,Riccardo Polosa,andJonathanThornburg
2018 Doping,Performance-EnhancingDrugs,andHormonesinSport:Mechanismsof ActionandMethodsofDetection
AnthonyC.Hackney
2018 InhaledPharmaceuticalProductDevelopmentPerspectives:Challengesand Opportunities
AnthonyJ.Hickey
2018 DetectionofDrugsandTheirMetabolitesinOralFluid RobertM.White,Sr.,andChristineM.Moore
DetectionofDrugsandTheir MetabolitesinOralFluid RobertM.White,Sr.
RetiredfromRTIInternational,ResearchTrianglePark,NC; RMWConsulting,Inc.,Naples,FL,USA
ChristineM.Moore ImmunalysisCorporation(nowapartofAbbott),Pomona,CA,USA
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright r 2018ElsevierInc.Allrightsreserved.
PublishedincooperationwithRTIPressatRTIInternational,anindependent,nonprofitresearchinstitute thatprovidesresearch,development,andtechnicalservicestogovernmentandcommercialclientsworldwide (www.rti.org).RTIPressisRTI’sopen-access,peer-reviewedpublishingchannel.RTIInternationalisatrade nameofResearchTriangleInstitute.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicor mechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem,without permissioninwritingfromthepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthe Publisher’spermissionspoliciesandourarrangementswithorganizationssuchastheCopyrightClearance CenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher (otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenour understanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecome necessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusing anyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethods theyshouldbemindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomtheyhavea professionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeany liabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceor otherwise,orfromanyuseoroperationofanymethods,products,instructions,orideascontainedinthe materialherein.
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
ISBN:978-0-12-814595-1
ForInformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: JohnFedor
AcquisitionEditor: KathrynMorissey
EditorialProjectManager: AmyClark
ProductionProjectManager: PaulPrasadChandramohan
Designer: MathewLimbert
TypesetbyMPSLimited,Chennai,India
DEDICATION ChristineM.Moore
Ishouldliketothankmysister,Anne,forherconstantsupportand mycolleaguesatImmunalysisforalltheirexemplaryresearchand scientificapproachtoallaspectsofanalyzingdrugsinoralfluid.
RobertM.White,Sr.
Toallmyfamilyfortoleratingendlessstacksofpaperthroughoutthe writingandpublicationofsixbooks.
PREFACE AccordingtoIrwinMandel,saliva “lacksthedramaofblood,the sincerityofsweatandtheemotionalappealoftears.” However,oral fluid,ofwhichsalivaisthemaincomponent,hasfoundnumeroususes inclinicalandforensictoxicologyandmedicine.Likeitscounterpart plasma,itcontainsproteinsandpolypeptidesofdiagnosticsignificance.Itisusedinthediagnosisofnon-microbialdiseasestatessuch asSjögren’ssyndrome,Cushing’sdisease,andceliacdisease.Itis usefulfordiseasesthathaveaspecificmicroorganismastheirroot cause,suchasherpesvirusassociatedwithKaposisarcoma; Helicobacterpylori associatedwithgastritis,pepticulcer,andpossible stomachcancer;andhumanimmunodeficiencyvirus(HIV).High qualityDNA(deoxyribonucleicacid)maybeextractedfromoralfluid inusablequantity.However,atthetimeofthiswriting,themainvalue oforalfluidliesintheanalysisofsmallorganicmoleculesincluding, butnotlimitedto,hormonessuchascortisolanddrugs,boththerapeuticandillicit.Themainpurposeofthisshortbookistoidentify whereoralfluiddruganddrugmetabolitetestingisuseful,whatthe limitationsofthetestsare,andhowtoaccomplishtestingforavariety ofspecialties.
MandelID.Thediagnosticusesofsaliva. JOralPatholMed. 1990;19(3):119 25.PubMedID 2187975.
ACKNOWLEDGMENTS ChristineM.Mooreacknowledgesthesupportofallthestaffat Immunalysisduringthisproject.SheisanemployeeofImmunalysis (nowapartofAbbott),whichmanufacturestheQuantisaloralfluid collectiondeviceaswellasnumerousimmunoassaysintendedforthe analysisofdrugsinoralfluid.Inaddition,Abbottmanufacturesseveralpoint-of-collectionrapidtestsfororalfluid,includingtheDDS2 device,alsodiscussedinthisbook.
RobertM.White,Sr.,retiredfromRTIInternationalAugust2017, acknowledgesthesupportofRTIInternationalandtheSubstance AbuseandMentalHealthServicesAdministrationfororalfluid projectsandprogramsreferencedinthisbook.
CHAPTER 1 1 Introduction GENERAL Beforeconsideringoralfluidasamatrixfordruganddrugmetabolite testing,itisworthwhiledescribingthesourcesandformationofhuman oralfluidinordertounderstanditsstrongpointsandlimitations.
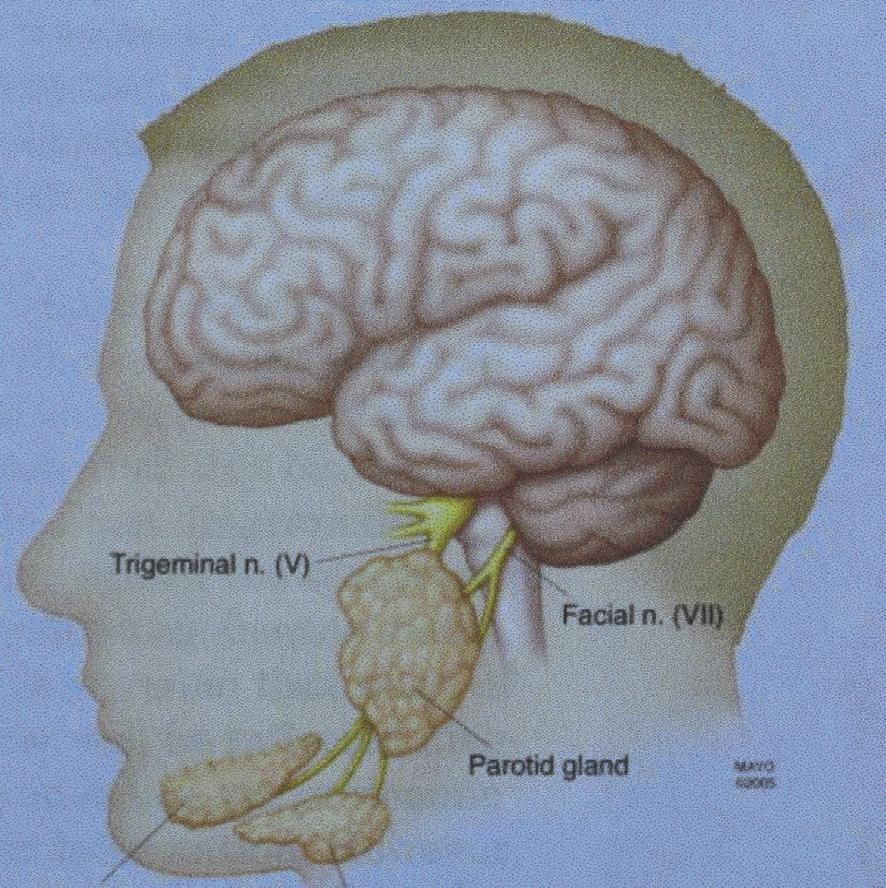
Saliva,themajorcomponentoforalfluid,isproducedprimarilyby threebilateralpairsofsalivaryglands:parotid,submandibular,and sublingual,assummarizedin Table1.1 1
Fig.1.1 showstheapproximatelocationofeachpairofglands.2
Minoraccessorysalivaryglands,whichareabout450 750innumber,arelocatedonthetongue,thebuccalmucosaofthelips,andthe palate.Theydonothaveacommonduct,andtheyproduceaviscous secrete.1 VonEbner’sglands(notshownin Fig.1.1),whichareoral exocrineglandsthatinpartsecretelipase,areserousglandsthatreside atthebaseofthecryptsthatsurroundthecircumvallateandfoliate papillaeonthetongue,justanteriortotheposteriorthirdofthe tongue.3
Ahealthyindividualproducesabout500 1500mLofsalivaper day,whichisabout0 6mLperminute,dependinghighlyonwhether theindividualisawakeorasleepandwhetherthesalivaryglandsare stimulatedornot,asillustratedin Table1.2 1
Asalivaryglandconsistsofaciniandaductthatleadseither directlytotheoralcavityortoacommonductthatleadstotheoral cavity. Fig.1.2 showsthatcirculatingbloodexchangescomponentsat theaciniandtheductus.2 Inbothareas,acapillarybedexiststofacilitatetheexchange.
In Fig.1.2,itismostnotablethatseveralpathways,notjustsimple filtration,existforthetransferofsmallmoleculessuchasdrugsand
Table1.1MajorSalivaryGlandsWithTheirAssociatedDuctsandSecretions
GlandDuctSecreteMajorNon-WaterProduct
ParotidStensonSerousAmylase,proline-richprotein;nomucins
SubmandibularvanWhartonSeromucousMucins
SublingualBartholinMucousonlyMucins
Figure1.1Approximatelocationsofmajorhumansalivaryglands.
Table1.2PercentContributionofEachSalivaryGlandbyStimulationType
GlandSleepNoStimulationMechanicalStimulationa CitricAcidStimulationb
Parotid0215845
Submandibular72703345
Sublingual14222
Minorglands14778 aThistypeofstimulationmaybeaccomplishedbychewingonaninertsubstancesuchasparaffin. bAlsocalledgustatorystimulation.
drugmetabolitesfromcapillaryplasmaintosalivathatisbeingformed inthesalivarygland.
Salivabecomesoralfluidwhenformedsalivaenterstheoralcavity andmixeswithcrevicularfluid,whichcontainssmallamountsofnormalhumanplasmaconstituentssuchasimmunoglobulinG(IgG),oral
Figure1.2Mechanismsoftransportofproteinsandionsfromplasmaintothesalivaryducts.a,ultrafiltration; b,activetransportorpassivediffusionacrossthecellmembrane;c,simplefiltrationthroughcellmembranepores; d,transepithelialmovementofwateralongNaClgradientviachannelproteins;e,creationofhypotonicsalivarysolutionviaductalNa1 reabsorption;f,acinarcellmembrane;g,cellmembranepore;h,intercellularspace;i,acinarcell.
cavitymicrobes,nasopharyngealsecretions,andanyfoodparticles thatmaybepresent.
Theactionsoftheaciniproduceanultrafiltrateofbloodwitha compositionthatisvastlydifferentfromplasma,whichisthenoncellularcomponentofwholeblood(asillustratedin Table1.3)fromwhich theoralfluidwasderived.2,3
IncreasedsecretionflowrateresultsinincreasedpHprimarilydue toincreasedbicarbonate.Also,assalivaryflowrateincreases,saliva sodiumion(Na 1 )concentrationincreasesduetoreducedopportunity foruptakeimmediatelypriortotheductus.Acompensatoryriseinthe
Table1.3ComponentsofSaliva(NormallyMeasuredasOralFluid)Comparedto BloodPlasma2
ParameterPlasma4
pH(arterial, wholeblood exceptfororal fluid)
Na1 (mmol/L; mEq/L)
7.35 7.45(children,adults)6.8(6.2 7.4)5 Increased.Pleasesee text.
7.31 7.42(60 90years)6.7(5.6 7.9)6
7.26 7.93(.90years)
137 143(16 49years;male)520 80
137 142(16 49years;female)
136 143(50 79years)
K1 (mmol/L; mEq/L) 3.8 4.9(6 79years)2220
TotalCa11 (mmol/L) 9.1 10.4(20 39years;male)1 41 4 9.0 10.1(20 39years;female)
9.0 10.2(40 79years)
Mg11 (mmol/L); serum 0.68 1.07( .12years)0.20.2
Cl (mmol/L)101 106(12 29years;male)1530 100
100 107(12 29years;female)
102 108(30 79years)
TotalCO2; primarilyHCO3 (mmol/L)
19 24(arterial,6 79years)515 80
22 26(venous,6 79years)
PO 3 4 (mmol/L)2.9 4.7(16 47years)64
2.8 4.7(48 79years;male)
3.1 4.8(48 79years;female)
SCN (mmol/L); serum ,0.4(nonsmokers)2.52 ,1.2(smokers)
NH3 (mmol/L)15 4563 (NH2)2CO (mmol/L)
2.9 7.0(8 19years;male)3.32 4
3.3 7.9(20 39years;male)
3.5 8.6(40 59years;male)
76.7(8 59years;female)
3.6 9.2(60 79years)
Totalprotein (g/L)
68 82(6 19years)33 65 83(20 29years)
65 78(30 79years)
Mucin(g/dL)Notdetected0.27(0/08 0.6) aFromReference1exceptasnoted.
majorcounterion,Cl ,occursduetotheincreasedsodiumions. Increasedbicarbonatealsomeansanincreasedbufferingcapacity.It hasbeentheauthors’ experiencethateventhoughoralfluidisapproximately99%water,ithasanunexpectedlyhighbufferingcapacity.Itis wellworthalaboratory’stimetovalidatetheabilityoftheircollection devicebyascertainingthatitmaintainsitsinitialpHinoralfluidfrom atleast10separatedonors.Forsuchastudy,approvalfromaninstitutionalreviewboardshouldbeobtained.Ithasbeentheexperienceof theauthorsthatvitaminC(ascorbicacid)canbeusedsuccessfullyasa salivarystimulantwhenlargebatchesoforalfluid(e.g., $ 50mL)are required.ThepHofthemixedoralfluidusuallyisaround6.6 6.8, notelevatedasmightbeanticipated.Ifascorbicacidisused,itsinfluenceorlackthereofonanalyticalmethodologyneedstobevalidated.
Anoralfluidspecimenisonecollectedfromadonor’soralcavity andisacombinationofphysiologicalfluidsproducedprimarilybythe salivaryglands.7 Wholeoralfluid,manytimesmisreferencedaswhole salivaormixedsaliva,isamixtureoforalfluidsandincludessecretionsfromtheminorandmajorsalivaryglandsandconstituentsof nonsalivaryorigin,includinggingivalcrevicularfluid,expectoratednasal andbronchialsecretions,bloodandbloodderivativesfromoralwounds, bacteriaandbacterialproducts,viruses,fungi,desquamatedepithelial cellsandothercellularmaterial,andfoodparticles.AlbuminandIgG ariseinoralfluidprimarilyfromcrevicularfluid.Itisnotablethatcrevicularfluidisreducedorabsentinedentulouspeople.8
Smallmoleculessuchasdrugs,drugmetabolites,otherxenobiotics, andnaturallyproducedhormonescommonlyarethoughttobepresent inoralfluidduetosimplefiltrationinthesulcusoftheoralfluidgland withiontrappingofbasic(usuallynitrogen-containingdrugsandtheir metabolites)molecules,sincethepHoforalfluidusuallyislowerthan thatofthearterialbloodfromwhichthesmallmoleculesaretransferred.However,theprocessbywhichsmallmoleculesinthearterial capillarylumenendupinthelumenofthesalivaryglandismorecomplexthanoftenconsidered.Usually,atleastfivebarriersmustbe passedforasubstancetotravelfromthevascularlumentotheductal systemwhereinitiallyformedsalivaistransformedintothefinalproduct(pleasesee Fig.1.2 and Table1.3)thatexitstheduct.2
1.Thecapillarywall
2.Theinterstitialspace
3.Thebasalcellmembraneoftheacinarcell
4.Thefluidinsidetheacinarcell
5.Theluminalcellmembrane
Atleastfiveknownfactorsaffectthemovementofsubstancesfrom capillarybloodintosalivathatwilleventuallyexitthesalivaryduct intotheoralcavity.2
1.Molecularmassandsize:Massplaysaminorroleinregulatingdiffusion.Thediffusioncoefficientisinverselyproportionaltothe molecularradius.Rod-likelargermoleculespassmoreeasilythan globularmolecules.
2.Lipophilicity:Lipophilicsubstancesusuallydiffusemoreeasilythan lipophobicsubstances.
3.Ionization:Nonionizedorweaklybasicsubstancesusuallydiffuse moreeasilythanacidicsubstances.
4.pH:WhenthepHofthesalivainthesulcusislowerthanthatof thearterialbloodinthecapillarybedthatisincontactwiththe acinarcells,basicsubstancesareconcentratedinthesalivarelative towholearterialblood.
5.Membranetransit:Thefreeorunboundsubstanceofinterestusuallyis requiredtopassacrossthemembranesintotheinitiallyformedsaliva.
Asshownin Fig.1.2,sero-salivarysubstancesalsoemployfiltration throughwater-filledpores,activetransport,orfacilitateddiffusionasa meanstoentertheinitiallyformedsaliva.
DISEASESTATESTHATMAYINFLUENCESALIVARYFUNCTION Severaldisorderscanhaveanadverseeffectonsalivation.Followingis apartiallist.
Alcoholiclivercirrhosis:Inapproximately50%ofpatients,theparotid glandsareenlarged,givingrisetoa50%reductioninsalivaryflowrate andareductionofsalivarysodium,bicarbonate,andchloridelevels.1
Burningmouthsyndrome:Thetermappliestoseveralconditionsof unknownetiologythatarecharacterizedbyaburningsensationand pain.Itismostoftenencounteredinmiddle-agedwomenorpeoplewith candidiasiswhohaveusedantibioticsforanextendedperiodoftime.1,9
Cysticfibrosis(CF):CFisoneofthemostcommonautosomal recessivediseasesinpeopleofnorthernEuropeanancestry.10 Ithas
beenmappedtoband31.2ofthelongarmofchromosome7(7q31.2). Becauseitisautosomalrecessive,twoaberrantgenesmustbepresent forittobemanifestedphenotypically.Severityrangesfromfatalin childhoodtoundetectablewithoutunusualcircumstancesortargeted genotyping.WhenCFispresentphenotypically,theoralfluidshows increasedviscoelasticitywithseveralelectrolyteandproteinconcentrationdifferences,comparedtonon-CFindividuals.1
Graft-versus-hostdisease:Thisconditionisprecipitatedbythe immuneresponseofhistoincompatible,immunocompetentdonorcells againsttissuesofanimmunocompetenthost.Itmayoccurasaresultofa complicationofbonemarrowtransplantationorofmaternal fetalblood transfusionoratherapeuticbloodtransfusioninwhichtherecipienthasa cellularimmunodeficiencydisease.9 Amongthemanymanifestations, destructionofthesalivaryglandsresultsinreducedsalivaryflowrate.1
Sjögrensyndrome:Thisconditionoccursusuallyinmiddle-agedor olderwomen.Itiscomplexandofunknownetiology.Itincludeskeratoconjunctivitissiccawithorwithoutlacrimalglandenlargement,xerostomiawithorwithoutsalivaryglandenlargement,andconnective tissuediseasewhichusuallyisrheumatoidarthritisbutmaybesystemiclupuserythematosus,scleroderma,orpolymyositis.Anabnormal immuneresponsemaybeimplicated.9
Nervedamage:Sincesalivationisunderthecontrolofboththe sympatheticandparasympatheticnervoussystems,headandneck nervedamagemayresultinxerostomia.
Salivaryglandremoval:Anobviousbutrarecauseofxerostomiais removalofthesalivaryglandseithersurgicallyorbycessationoffunctionthroughexposuretoradiation.
THEINFLUENCEOFDRUGSONSALIVARYFUNCTION Whenoralfluidiscollectedfordrugtesting,themainpurposeisthe detectionand,whereappropriate,thequantificationofdrugsandtheir metabolites.However,notonlymaydrugshaveasignificanteffecton anindividual’sperformance,theyalsomayaffectsalivaryfunction, possiblycompromisingoralfluidcollection.
Anydrugthatinterfereswiththecentralnervoussystemorthe peripheralnervoussystemwillinfluencetheproductionofsaliva.1
Analgesics,antiarrhythmics,anticonvulsants,antidepressants,antiemetics,antihistamines,antihypertensives,anti-nauseaagents, antiparkinsondrugs,antipsychotics,antispasmodics,cytoxins,decongestants,diuretics,expectorants,monoamineoxidaseinhibitors,and minortranquilizers,whicharealltherapeuticdrugs,willreducesalivation. Table1.4 showssometherapeuticdrugsthatareanticholinergic (parasympathetic)andreducesalivaryfloweitheraspartofadeliberate anticholinergicaction(leftcolumn)orasasideeffect(rightcolumn).
Table1.5 showsparasympatheticdrugsthatincreasesalivaryflowrate.
Table1.4AnticholinergicTherapeuticDrugsThatReduceSalivaryFlow1
AnticholinergicEffectAnticholinergicSideEffect
Muscarineblockers atropine scopolamine clidinium ipratropium oxybutynin pirenzepine propantheline
Anti-Parkinsondrugs benztropine biperiden orphenadrine trihexyphenidyl procyclidine
Antidepressants amitriptyline desipramine imipramine lithium lofepramine maprotiline nortriptyline oxypropylene
Antihistamines cyclizine diphenhydramine promethazine tripelennamine
Antiarrhythmics disopyramide
Antipsychotics Chlorpromazine Butyrophenones
Table1.5ParasympatheticDrugsThatIncreaseSalivaryFlow DirectlyActingIndirectlyActing arecolinecisapride bethanecholneostigmine carbamylcholinenizatidine cevimelinephysostigmine methacholine muscarine oxotremorine pilocarpine
Whereappropriatetotestingandinterpretingresults,referencesto drugsanddiseasestatesnotedabovewillbecited.
REFERENCES 1. ApsJKM,MartensLC.Review:Thephysiologyofsalivaandtransferofdrugsintosaliva. ForensicSciInt.2005;150:119 131.
2. FordeMD,KokaS,EckertSE,CarrAB.Systemicassessmentsutilizingsaliva.Part1. Generalconsiderationsandcurrentassessments. IntJProsthodontics.2006;19:43 52.
3. CarpenterGH.Thesecretioncomponents,andpropertiesofsaliva. AnnuRevFoodSci Technol.2013;4:267 276.
4. AdeliK,CeriottiF,NieuwesteegM.ReferenceinformationfortheclinicalLaboratory. In:RifaiN,HorvathAR,WittwerCT,eds. TietzTextbookofClinicalChemistryand MolecularDiagnostics.6thed.Elsevier;2018.
5. HöldKM,deBoerD,ZuidemaJ,MaesRAA.Salivaasananalyticaltoolintoxicology. Int JDrugTest.2011;1 34.
6. RitschelWA,TompsonGA.Monitoringofdrugconcentrationsinsaliva:anon-invasive pharmacokineticprocedure. MethFindExptlClinPharmacol.1983;5(8):511 525.
7.SAMHSA,May15,2015FederalRegister,80FR28053,OralFluidMandatoryGuidelines; Washington,DC,GovernmentPrintingOffice.
8. TerraponB,MojonP,MensiN,CimasoniG.Salivaryalbuminofedentulouspatients. Arch OralBiol.1996;41(12):1183 1185.
9.Dorland’sIllustratedMedicalDictionary.31sted.Philadelphia,PA:SaundersElsevier;2007.
10. Vnencak-JonesCL,BestDH.Genetics.In:RifaiN,HorvathAR,WittwerCT,eds. Tietz TextbookofClinicalChemistryandMolecularDiagnostics.6thed.Elsevier;2018.
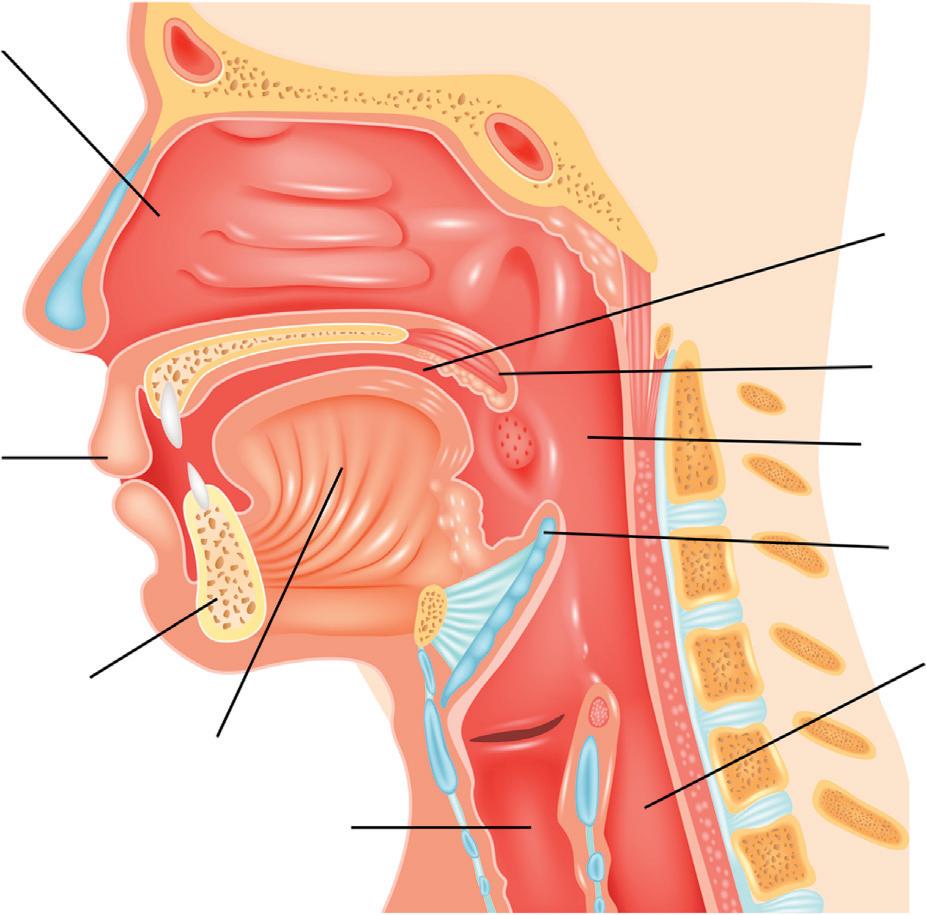
CHAPTER 2 2 OralFluidPharmacokinetics INTRODUCTION Fluidgeneratedintheoralcavityarisesprimarilyfromthemajorsalivaryglands,theminorsalivaryglands,thegingiva(crevicularfluid, minorcontribution),andnasopharyngealdischarge,thecontribution ofwhichcanvarydependingonthestateofhealthoftheindividual’ s nasalcavity(Fig.2.1).Fluidfromthelungsalsomayentertheoral cavityviathepharynx.Exceptunderunusualcircumstances,theoral cavityisfedprimarilybythebloodsystem,withminorcontributions fromthenasalcavity,pulmonaryefflux,andtheenvironment(e.g., smokeordust).Exceptforoccasionalexpectoration,essentiallyallthe 500 1500mLoforalfluidgeneratedperdaydrainsintothestomach viatheesophagus.Fromthestomach,drainedoralfluidisprocessed likeanyotherliquidpresentedtothelowergastrointestinaltract.The oralcavitycanbeconsideredasabodycompartmentpharmacokinetically.Thissectionwilldescribethebasicpharmacokineticsofdrugsof interestandtheirmetabolites.
Whenadrugisadministeredintravenously,thedruganditsmetabolitesmaytransferfrombloodintothecontinuouslyformingoral fluid.Foradrugormetabolitetodothis,thebarriersenumeratedin Chapter1needtobeovercome.Topassthroughthosebarriers,adrug ormetaboliteusuallymustbeinthefreeornon-protein-boundform, nonionized,andofappropriatesize.Rareexceptionsmayexistwhena drugisboundtoaproteinthatisexcretedintosalivaactively.
ThepHofwholehumanbloodisverytightlycontrolledbyan extensivebloodbufferingsystem.AnydeviationsfromthepHrange statedinTable1.3generallyresultinclinicallyobservablesymptomatology.ThetableshowsthatthepHofhumanoralfluidisusually 6.2 7.4or5.6 7.9,dependingonthereference.Understresssuchas thatinducedbyprovidinganoralfluiddonorwithsourcandyor anothersalivarystimulant,thepHmayriseashighas8.0.Theterm “iontrapping” ofabasicdrugismanytimesquotedwiththe DetectionofDrugsandTheirMetabolitesinOralFluid.DOI: https://doi.org/10.1016/B978-0-12-814595-1.00002-7 © 2018ElsevierInc.Allrightsreserved.
HUMAN DIGESTIVE SYSTEM appearanceofadrugfromthebloodinoralfluid.Iontrappingis whereabasicdrugdemonstratestheusualproteinunbound:bound equilibriuminwholeblood(pH 5 approximately7.35 7.45)buttransfersreadilyintoformingorformedsaliva,whichhasalowerpH.The pHoforalfluidisusually,butnotalways,belowthatoftheblood thatfeedsthesalivaryglands.BasedsolelyonpH,onemightintuitivelyconcludethefollowing:
BasicDrugorMetabolite:OralFluid/Plasma . 1
AcidicDrugorMetabolite:OralFluid/Plasma , 11
However,otherfactorssuchaslipophilicityandproteinbinding mayenterintotheequation.Additionally,notalldrugsandtheir metabolitesfitneatlyintotheacidic/basic/neutralcategorization,as willbediscussedundereachdrugorclassbelow.
Pleaseseethediscussionbelowoneffervescentfentanyltabletfora commentaryonthebloodtosalivatransfersystem.
Nasal cavity
Lips
Jaw
Tongue
Larynx
Esophagus
Epiglottis
Pharynx
Uvula
Oral cavity
Figure2.1Humanoralcavityandassociatedphysiologicalfeatures. FromShutterstock;TeguhMujiono.
Table2.1AverageOralFluid-to-BloodConcentrationRatiosforSelectedDrugs
Averageoralfluid-to-bloodratiosofseveraldrugsarepresentedin Table2.1 2 Thesenumbersneedtobeusedwiththeunderstandingthat manyfactors,especiallyoralfluidpH,caninfluencetheratio.
Theoralcavityisnotaclosedcompartment.Itisopennotonlyat themouthbutalsothroughthenostrilsandnasalcavityandtheesophagus.Althoughrarelytakenintoaccount,adrugthathasbeenswallowedmayrefluxintotheoralcavityandcauseanunexpectedly elevatedoralfluiddruglevel.Likewise,smoke(e.g.,fromamarijuana jointoracigarettesoakedincocaineormethamphetamine)maycontaminatetheoralcavityandgiverisetoamarkedlyelevateddruglevel.
Insufflation(“snorting”)isacommonwaytoingestcocaineinits saltform(usuallyhydrochloride).Insufflateddrugthatisnotabsorbed inthenasalpassagesmaymakeitswayintotheoralcavityvianasopharyngealfluids,resultinginanextremelyelevatedfluidconcentrationrelativetoacommondose.
Manydrugscanbeusedbysimpleoralingestion.Thisusuallycontaminatestheoralcavity,producinganunexpectedlyhighoraldrug level.Theexactoppositecanoccurifacoatedtabletisingestedwithoutchewingordegradationofthecoating.Thedruginthecoatedtabletisnotreleaseduntilitarrivesinthelowergastrointestinaltract, whereitisabsorbed;sotheoralfluidlevelreflectsonlywhatisinthe blood,assumingthedrugwilltransferfrombloodtooralfluid.
Followingisadiscussionofthefundamentalclassesofabused drugsofinteresttotoxicologists,withunusualcircumstanceskeptin mind.Experiencewiththeactualhandlingoforalfluidmatricesand commondrugsisreviewedinChapter9.
ETHANOL EthylAlcohol, “Alcohol”
Wherelegaloratleastreadilyavailable,ethanol(Scheme2.1)isthe mostusedandabuseddrugworldwide.Formostclinicalandforensic toxicologylaboratories,determinationofethanolinbloodandblood products,urine,andoralfluidisoneofthemostcommonlyperformed analyticaltests.Theuseofethanolbyhumanshasbothacuteand chroniceffects,anditisaknownteratogen.Acutely,ethanolisalmost uniqueinthatwholebloodandbloodproductlevelscorrelate extremelywellwithclinicalsymptoms.3 Pharmacokinetically,ethanol’ s rateofdisappearanceisunusualinthatitiszeroorder;i.e.,aplotof apostabsorptivebloodorbloodproductlevel(y-axis)againsttime (x-axis)yieldsastraightline.3 Anotheralmostuniquepropertyisthat ethanolisavolatileliquidthatiscompletelymisciblewithwater.4 In bloodandbloodproducts,itexistsinthefreeformandisnotbound toprotein.ItdoesnotionizeatanypHcompatiblewithlife.5 Its uniquephysiologicalandphysicalpropertiesmakeithighlysuitableas anoralfluidanalytebothqualitativelyandquantitatively.
Ethanolistakenintothehumanbodyalmostexclusivelybythe oralroute.
Perhapsduetothesignificantlygreateramountofproteininplasma thaninoralfluid(essentiallysaliva),thesaliva-to-plasmaratioforethanolhasbeenstatedtobe1.1.1 Asistruewithotherstatedratiosfor ethanol(e.g.,serum-to-wholeblood),thesaliva-to-plasmaratioisa rangeratherthananabsolutevalue.Jonesstatedittobe1.077witha rangeof0.84 1.36andlateradjustedthatto1.094witharangeof
0.88 1.36.Sinceaconstantrangeexistsforsaliva-to-plasma,oralfluid ethanolcanbeusedtodemonstratethe correlationbetweenoralfluid levelandclinicalsymptoms.Itisnotablethatbeforemeasuringaquantitativeoralfluidethanollevel,a20-minobservedabstinenceperiod shouldbeusedpriortosamplecollection,asisrequiredforbreathalcohol testing.Asistrueforbloodandbloodproductethanoleliminationstudies,theoralfluiddonormustbepostabsorptivetobeinthelinearphase.
CANNABINOIDS Δ9-Tetrahydrocannabinol(THC)(Scheme2.2)isusedasanantiemetic,analgesic,andappetitestimulant4 aswellasarecreational drug.Itismostcommonlysmoked,butoraladministrationviasocalled “edibles” appearstobegainingpopularity,especiallyinareas wheremarijuanahasbeenlegalized.Italsomayenteroralfluidby passiveexposuretosmoke.ExposuretoTHCandothercannabinoids canbeclassifiedasactiveorpassive,oralorsmoked,andfrequentor occasional,andthetypeofexposurecanaffectpharmacokinetics.The followingparagraphssummarizecurrentknowledgeoforalfluidTHC pharmacokineticsineachtypeofexposurebrieflyandwithinwhatis currentlyknownatthetimeofthiswriting.
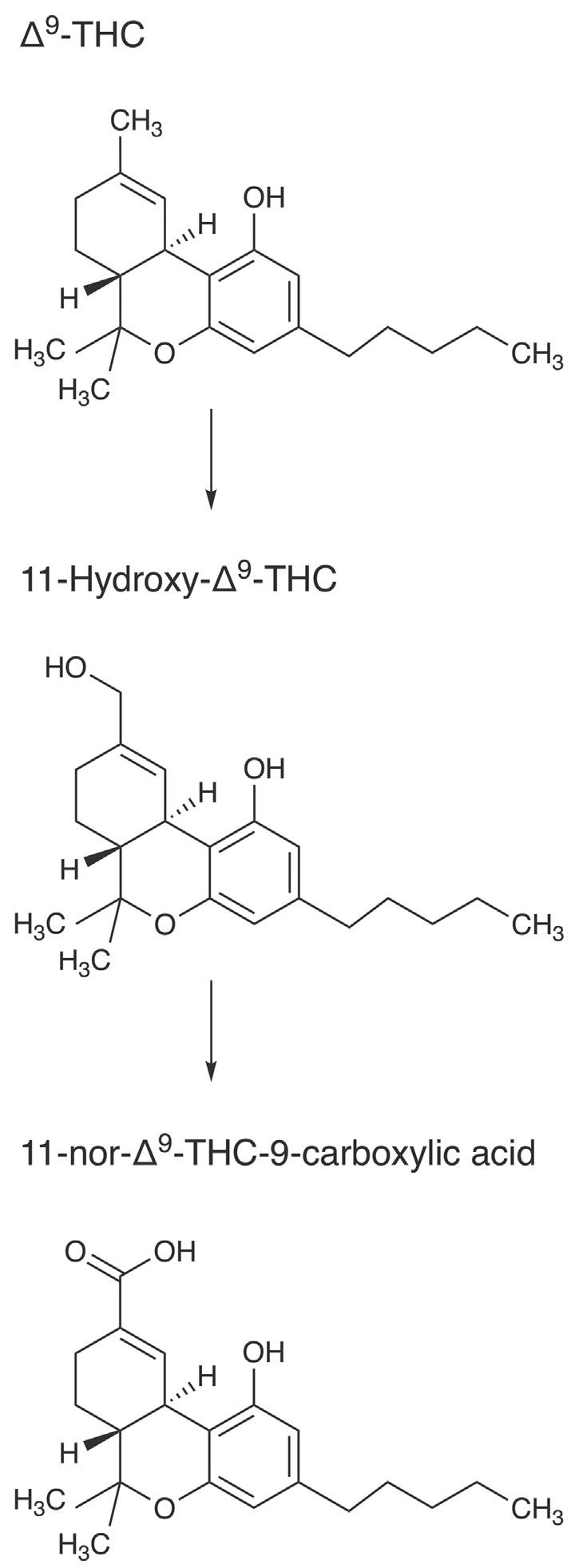
Althoughthetargetsubstanceinurinedrugtestingusuallyis11nor-Δ9-tetrahydrocannabinol-9-carboxylicacid(THC-COOH,also calledTHCA),whichistheprimarymetaboliteofTHC,itistheparentsubstanceitselfthatisofprimaryconcerninoralfluiddrugtesting. Thesinglenon-hydrocarbonmoietyonTHCisaphenolicgroupwhich isonlyweaklyacidic(pKa 5 10.6).ParentTHCistightlyboundto bloodprotein(Fb 5 0.97).6 Thus,THCisapoorcandidatefortransfer frombloodtooralfluid.ConsistentwithparentTHC’sphysicalproperties,contaminationoftheoralcavitywithsmokemostlydetermines smokedTHCoralfluidlevels,ratherthantransferofabsorbedTHC frombloodtooralfluid.7 Thesaliva-to-plasmaratioforparentTHC hasbeencalculatedas0.099 0.1291 andisstatedas1.2inalaterreference. 2 At0.060 0.099,theratiooftheactivemetaboliteofTHC, 11-hydroxy-Δ9-THCisevenlower.1
Smokedmarijuanainastudyinvolvingsixhealthymalesubjects demonstratedtheanticipatedinitialmaximumfororalfluidTHCat about12min.Incorrespondingplasmaspecimens,THCalsopeaked
at12min.Ahalf-life(t1/2)forTHCinoralfluidwascalculatedtobe 0.80h,whichwassimilartotheplasmahalf-lifeof0.75h.Theauthors suggestedthattransmucosalabsorptionofTHCoccursduringsmokingandelevatesexistingbloodlevels.8 Inaseparatestudy,themedian Cmax forTHCinoralfluidaftersmokingacigarettecontaining20mg ofTHC(500mgofcannabiswith4%THC)addedtotobaccowas 55.4 123,120.0 μg/L.Thecorresponding Cmax inplasmawas 1.6 160.0 μg/L.Itwasstatedthatthevariationsinoralfluidranges aretoobroadtopredictplasmaTHCfromtheoralfluidvalues.9 Ina studythatinvolvedtheuseoftheStatSuredeviceforfluidcollection, nostatisticallysignificantdifferencesintheoveralltimecourseofthe absorption,distribution,andeliminationofcannabinoidswasobserved exceptforTHC-COOH.ThepresenceofTHC-COOHidentifiedfewer occasionalsmokersthanTHC.However,thepresenceofTHC-COOH ruledoutacutepassiveenvironmentalexposure.10
Inlookingatcannabisedibles,Newmeyeretal.11 generatedthe pharmacokineticdatashownin Table2.2 followingingestionof approximately50.6mgofTHC,1.5mgofcannabidiol,and3.3mgof cannabinolbakedintoabrownie.Thebrowniewasconsumedwithin 10min.Theparticipantswere10frequentand7occasionalmarijuana smokers.
TheresultsofNewmeyeretal.11 reinforcedthefindingofMarsot etal.9 thatareliableconversioncannotbeobtainedbetweenoralfluid andbloodcannabinoidvalues.Newmeyeretal.alsonotedthatthere wasnooromucosalcontaminationbythedrugdronabinol,becauseit wasencapsulated.InaseparatestudybyVandreyetal.,12 18participantsingestedbrowniescontaining9.4,23.6,or48.5mg(target values 5 10,25,and50mg)THC,andpharmacokineticvalueswere determined(Tables2.3and2.4).
Althoughpharmacodynamicsarebeyondthescopeofthissection, thestudybyVandreyetal.didincludeinterestingcannabispharmacodynamicoutcomes.Apreviouspaperthatincludedtheauthorsofthe 2017paperfocusedonthepharmacodynamicsofcannabinoidsfrom thesecond-handsmokeandeffectsofventilationorlackthereof.13
Severalstudiesonoralfluidcannabinoidsresultingfrompassive inhalationhavebeendone.Mooreetal.foundamaximumof17ng/mL ofTHCfromexposurefor3hinDutchcoffeeshops.Atacutoff
Table2.2OralFluidPharmacokineticValuesforCannabinoidsandMetabolites
AfterBrownieConsumption11 ParameterFrequentSmokersOccasionalSmokers
Mean,Median(Range)Mean,Median(Range) THC
Cmax, μg/L573,464(39.3 2111)362,392(115 696)0.8670.401
tmax,h0.330.33
tlast,h39,44(20 .48)23,26(20 26)3.876 0.003
Cmax, μg/L0.6,0.7(0.2 1.2)0.4,0.4(0.3 0.6)1.5210.156
tmax,h0.40,0.33(0.33 1.0)0.60,0.33(0.33 1,5) 0.9830.347
tlast,h1.1,1.0(0.33 3.5)1.4,1.3(0.33 3.5) 0.3330.745
Cmax,ng/L329,262(123 1009)318,191(27.7 1281)0.0620.951
Adjusted Cmax,ng/L285,186(123 849)315,191(27.9 1263) 0.1790.861
tmax,h12,5(3.5 48)10,10(0.33 20)0.3570.726
tlast,h .4841,44(26 .48)1.9430.100
THCVd
Cmax, μg/L7.4,6.8(1.3 19.4)5.4,4.7(1.6 10.6)0.8000.436
tmax,h0.330.33
tlast,h2.4,1.5(1.5 3.5)1.9,1.5(1.0 3.5)0.8520.409
CBDe
Cmax, μg/L14.0,12.4(2.0 29.7)10.8,9.0(4.0 18.4)0.80700.433
tmax,h0.330.33
tlast,h3.0,3.5(1.5 5.0)2.6,3.5(1.0 3.5)0.7080.491
CBGf
Cmax, μg/L31.2,27.2(3.5 90.1)21.2,22.6(7.5 33.9)0.9360.365
tmax,h0.330.33
tlast,h3.6,3.5(1.5 5.0)4.6,3.5(1.0 14) 0.6430.531
aStudent’ s t-test.
bBold P-valueindicatessignificance. c11-hydroxytetrahydrocannabinol. dTetrahydrocannabivarin. eCannabidiol. fCannabigerol.
of $ 20pg/mL,noTHC-COOHwasfound,indicatingthatanabsence ofTHC-COOHmightbeusefulinsomecasestoshowthatTHCinoral fluidwasduetopassiveinhalationandnotuse.14 Inastudyby Niedbalaetal.,itwasnotedthattheoralfluidcollectiondevice employedinpassiveinhalationstudiesmustbestoredandusedoutside theareawhereitmightbeexposedtomarijuanasmoke.15
Table2.3OralFluidConcentrationsofTHCandTHC-COOHAfterBrownie Consumption(Vandreyetal.)
TargetDoseofTHC(mg)THCTHCTHC-COOHTHC-COOH
10191.5(47 412)0.2(0.2 0.5)0.051(0 0.231)1.0(0 3)
25477.5(70 1128)0.2(0.2 0.5)0.140(0.023 0.251)9.8(3 30)
50597.5(350 1010)0.2(0.2 0.5)0.314(0 0.822)17.4(0 54)
11-Hydroxy-THC Cmax and tmax werenotdeterminedinoralfluid.
Table2.4DetectionTimetoFirstandLastPositivesintheBrownieStudyby Vandreyetal.
DetectionTimetoFirstPositive(h)
TargetDoseofTHC (mg) THCTHC-COOHTHCTHC-COOH ELISAELISALC-MS/MSLC-MS/MS (Cutoff 5 4ng/ mL) (Cutoff 5 4ng/ mL) (h,LOQ 5 1ng/ mL) (Cutoff 5 0.050ng/ mL)
100.17(0.2 0.2)0.17(0.2 0.2)0.17(0.2 0.2)3.0(3.0 30)
250.23(0.2 0.5)0.23(0.2 0.5)0.23(0.2 0.5)3.6(0.2 8.0)
500.17(0.2 0.2)0.17(0.2 0.2)0.17(0.2 0.2)1.6(0.2 4.0)
DetectionTimetoLastPositive(h)
101.5(1,2)0.7(0 2)1.9(2,3)1.0(0 3)
252.3(1 4)16.4(1 50)3.0(2 6)26.3(0 70)
5010.0(2 22)25.2(3 70)9.5(3 22)37.3(0 78)
THC’smajormetaboliteTHC-COOH,whichisinactive,usually canbefoundinoralfluidbutatonlyabout1-1000thoftheconcentrationoftheparent.16 ItisformedbytheactionofcytochromeP4502C9 (CYP2C9),17 whichproducestheactive11-hydroxy-THC,whichisfurtheroxidizedtotheinactiveTHC-COOHbyaseriesofhydroxylases. Itisfoundinoralfluidasabouttwo-thirdfreeandone-thirdglucuronideconjugate.18 Theglucuronideisformedbyuridinediphosphate glucuronosyltransferase1A3and1A1.19 THC-COOHinoralfluidis presumedtobeformedfromTHCpresentedtothehumanliver.It reentersthebloodstreamandistransferredtosaliva.However,atthe timeofthiswriting,theformationofTHC-COOHinoralcavitycells thatarebathedwithoralfluidcontainingTHChadnotbeenruled out.20 22 Inonecontrolledsmokingstudyof10subjects,themaximum THC-COOHconcentrationoccurred1 2hafterthestartofsmoking exceptfortwoparticipantsinwhomitoccurredat0.25h.22
COCAINE Parentcocaine(Scheme2.3),whichistheactiveprincipal,canbe insufflated,injectedintravenously,smoked,andtakenorally.Theoral routeiswastefulduetotheamounthydrolyzedinstomachacid. Parentcocaineisbasic(pKa 5 8.6),withabloodproteinbindingof onlyslightlyless(Fb 5 0.92)23 thanTHC.Thehalf-lifeinbloodis approximately0.7 1.5h.Thesalivaterminalhalf-lifehasbeen reportedas7.9h.1
Notunexpectedlyforabasicdrug,thesaliva-to-plasmaratioshifts from5orgreaterinunstimulatedsalivadownto0.5 3instimulated saliva.1
Scheme2.3Metabolismofcocaine.
Cocaine
Norcocaine
UnlikeTHC,whencocaineisadministeredintravenously,itappears rapidlyinoralfluid.Inastudyof10cocaineusersadministered25mg aloneandincombinationwithoralacetazolamideandoralquinine, thepharmacokineticparametersforcocaineanditsmajormetabolite benzoylecgonine(BZE)weremeasured. Tables2.5and2.624 showthe resultswhencocainewasadministeredalone.
Regardlessofthetypeoforalfluidcollector,thehalf-lifefor cocaineinoralfluidwasfoundtobesimilartothatforbloodplasma.
PleaseseeChapter7foradiscussionoftheeffectoforalfluidcollectiondeviceondrugdetectiontimes.
AMPHETAMINES Amphetamine,methamphetamine,andmethylenedioxyamphetamine (MDA)aremorebasicthancocaine(Table2.7). Methylenedioxymethamphetamine(MDMA,ecstasy)isaboutasbasic ascocaine,butitsproteinbindingisintermediatebetweencocaineon thehighsideandamphetamineand d-methamphetamineonthelow
Table2.5OralFluidPharmacokineticsforCocaineandItsMajorMetaboliteUsing
Table2.6OralFluidPharmacokineticsofCocaineandItsMajorMetaboliteUsing theStatSureCollector(RangeinParentheses)
Table2.7PharmacokineticPropertiesofCocaineandAmphetamines23,25
cocaine8.60.921.6 2.70.7 1.5 amphetamine9.90.163.2 5.67 34(urinepH-dependent)
d-methamphetamine9.90.10 0.203.0 7.06 15(urinepH-dependent)
l-methamphetamine9.9?3 511 16 MDA9.7???
MDMA8.70.653 75 9
MDMA,methylenedioxymethamphetamine(“ecstasy”).
Deamination, p-Hydroxylation, and conjugation Scheme2.4Metabolismofmethamphetamine.
side.Thusamphetamine,bothmethamphetamineisomers,MDA,and MDMAarealltransferredeasilyfrombloodtosaliva.
Methamphetamine Methamphetaminecanbesmoked,butmostamphetaminesare ingested(Scheme2.4).
Theoralfluid-to-bloodratiosofamphetamine,methamphetamine, andMDMAhavebeenestimatedas7.4,4.5,and5.1,respectively, usingthefirst-generationInterceptcollectiondevice.30
InastudybySchepersetal.,fouroral10-mgsustained-release d-methamphetaminedoseswereadministeredtoeightparticipantswithin 7days.Threeweekslater,fiveparticipantsreceivedfouroral20-mg doses.Oralfluidwascollectedforupto72haftereachadministration. Bloodwascollectedforupto24h.Acitricacidsourballcandywas usedtostimulateoralfluidproductioninthefirsttwosessions.Inthe thirdsession,oralfluidwascollectedbyputtingacottonswabtreated
Table2.8OralFluidPharmacokineticsfor d-MethamphetamineintheStudyby Schepersetal.31
Parameter10-mgMethamphetamineDose20-mgMethamphetamineDose
Table2.9 tmax and Cmax fortheMetaboliteAmphetamineAfter d-Methamphetamine intheStudybySchepersetal.31
Parameter10-mgMethamphetamineDose20-mgMethamphetamineDose
with20mgofcitricacidintothedonor’smouth.Inthefourthsession, cottonswabswithoutcitricacidwereused.31 Tables2.8and2.9 show thepharmacokineticresults.
Table2.9 showstheresultsforthemetaboliteamphetamine.
Itisnotablethatthehalf-lifeformethamphetamineinoralfluidis similartothatinplasma,althoughittendstowardthelowsideofthe plasmarange.Thevolumeofdistribution(Vd)isdecidedlylowerthan inplasmabutstillonthesameorderofmagnitude.ThepeakconcentrationofamphetamineinthestudybySchepersetal.islessthan10% ofthatformethamphetamine.Notunexpectedly,the tmax foramphetamineisslightlylongerthanformethamphetamine.
InthestudybySchepersetal.,themeanpHoforalfluidspecimens harvestedwiththecitricacid treatedswabswasonaverage1.5pH unitslowerthanthosecollectedaftercitricacidcandy(swab2.8 6 0.3, candy4.3 6 0.8).Thecandystimulationyieldedvaluesapproximately 1.7pHunitslowerthanneutralcottonswabs(6.0 6 0.6).Thedatasuggestedthatthedispositionofmethamphetamineinoralfluidmaybe dose-related.However,highintrasubjectandintersubjectvariability restricttheuseofasingleoralfluidmeasurementtopredictasimultaneouslyobtainedplasmaconcentration.
Table2.10 Cmax, tmax,andTimetoFirstPositiveatLOQforMethamphetamineand itsMetaboliteAmphetamine
Inanotherstudyofmethamphetaminedisposition,Huestisand Conelookedat C max , t max ,andtimetofirstpositiveatastated LOQ(limitofquantificationorquantitation)formethamphetamine anditsmetaboliteamphetamine 32 ( Table2.10 ).Withincreasing methamphetaminedose,the C max forbothmethamphetamineand amphetamineincreasedbutwashigherthananticipatedforthe higherdose.Likewise,the t max andtimetofirstpositiveshiftedto lowervaluesforboththedruganditsmetabolite.Itwasnotedthat urinetestingusuallygiveshighdetectionratesformethamphetamine anditsmetaboliteforupto3daysfollowingdrugcessation.In contrast,oralfluidtestingyieldsonlyamoderatedetectionrate for24h.
PleaseseeChapter7forfurtherpresentationonchiralityofamphetamineandmethamphetamine.
Methylenedioxymethamphetamine Barnesetal.studiedMDMAanditsmetaboliteMDA ( Scheme2.5 ),andincludedmonitoringof4-hydroxy-3methoxymethamphetamine,and 4-hydroxy-3-amphetamine ( Table2.11 ). 33 TheauthorsconcludedthatoralfluidisagoodalternatematrixformonitoringMDMAandcandetectasinglerecreationaldoseof70 150mgforupto1or2daysafteruse.Itis notablethatthehalf-lifeofMDMAi noralfluidisdose-relatedand verysimilartoitshalf-lifeinplasma( Table2.11 ).