www.idosr.org
©IDOSR PUBLICATIONS
International Digital Organization for Scientific Research
Tabakwot et al
ISSN: 2579-0781
IDOSR JOURNAL OF EXPERIMENTAL SCIENCES 8(1) 31-47, 2022. Evaluation of the Effects of Galinsoga parviflora Aqueous Leaf Extract on the Histological Patterns of Cerebellum after Mercury Chloride Exposure.
John Ayuba Tabakwot, Okesina Akeem Ayodeji and Usman Ibe M.DepartmentofHumanAnatomy,KampalaInternationalUniversity,Uganda.
ABSTRACT
The cerebellum forms a major part of the brain and its function is to control all muscular movements and coordination. Injury or damage to the cells of the cerebellum may lead to conditions like; ataxia, Joubert syndrome, and many others. These injuries may be a result of traumatic brain injuries or toxic agents such as mercury, tin, and aluminum. For example,mercury exposure has been reported to be part of our daily lives, therefore it is important to understand the pathological implication that might follow these exposures andalso with the help of a local herb; Galinsogaparviflora (GP) to finda cheap and readily available management solution to mercury toxicity in the cerebellum. This study was designed to evaluate the effects of Galinsoga parviflora aqueous leaf extract on the histological patterns of cerebellum after mercury chloride exposure. Twenty-five adult male mice of an average weight of 25g were randomly divided into 5 groups of 5 rats each (n=5). The animals in Groups 1-5 received oral administration of distilled water, 2.3mg/kg body weight of HgCl2, 2.3 mg/kg HgCl2 followed by GP extract (800mg/kg), GP extract (800mg/kg)followedby2.3mg/kgHgCl2andGPextract(800mg/kg)followedby2.3mg/kg HgCl2 concurrently respectively. These treatments were followed by behavioral tests, euthanization of the animals through perfusion fixation and tissue samples were harvested forhistologicalanalyses.There wasevidenceofpyknoticcells,degeneratingmyelin,region of lineardistributionofPurkinjecellsinthecerebellumofgroup2animals.Theanimalsin group 3 revealed reduced pyknotic cells,presence myelination in their cerebellum. In conclusion, this study revealed that mercury exposure in the cerebellum of mice caused pyknosis and reduced myelination in the axons. However, the introduction of Galinsoga parviflora in the cerebellum acted as a therapeutic agent against mercury toxicity in the cerebellumofadultmalemice.
Keywords:Galinsoga parviflora, leaf extract, histological patterns and cerebellum after mercurychlorideexposure.
INTRODUCTION
The histo-architecture of the cerebellum holds a very important, as regards the normal functionality of the cerebellum [1]. A cross-section of the cerebellum reveals the intricate pattern of folds and fissures that characterize the cerebellar cortex[2].The cerebellarcortexisdivided into three layers, the innermost layer, the granule cell layer, is made up of tightly packedgranulecells,themiddlelayer,the Purkinjecelllayerandthemolecularlayer is made of the axons of granule cells and the dendrites of Purkinje cells, as well as a few other cell types [3]. The three distinct layers of the cerebellar cortex are
all of the functional relevance [2]. The most important cell in the cerebellar cortex is the Purkinje cell; and forms the Purkinje layer [4]. The Purkinje cells are the only inhibitory cell of the cerebellum and control the rate of signal firing in the cerebellum and every motor action is coordinated [5]. Studies by Martin and colleagues linked autism in children with the loss of Purkinje cells [6]. Therefore, buttressing the effects of morphologic deviation on behavior, studies have linked mercury exposure with the loss of histo-architectural integrity of different parts of the brain including the cerebellar
www.idosr.org
cortex, manifesting as an alteration in behavior [1]. Behavioral and biochemical consequences of mercury exposure cannot be separated from its possible structural or morphological alteration [4]. Various in vivo studies have reported structural/histological consequences of mercury exposure [1]. Teixeira etal., [4] study on exposure to inorganic mercury, linked mercury exposure with oxidative stress, and functional deficits in the motor cortex. Teixeira et al., [4]also associated mercuryexposure withcellular degeneration, necrotic changes, and subsequent cell death. Furthermore, mercury exposure can cause damage or reduction in the number of both astrocytes and neurons, with consequent associated functional changes [4]. A study by Jha et al., [7] on prooxidative and neurotoxic effects of mercury chloride in rats; indicated that mercury administration showed degenerative and necrosis changes, and perivascular edema. Ibegbu et al., [1] revealed degenerative and necrotic changes in the Purkinje cells of the Purkinje cell layer of the cerebellar cortex following aluminum chloride exposure. A study by [8] study on the reduced Purkinje cell number in essential tremor, suggested that a postmortem study indicated a reduction in Purkinje cell numbers in the brains of patients with essential tremor who do not have Lewy bodies. Myelin sheath degeneration occurs when a neuron dies or if its axons have been severed, the myelin sheath surrounding the degenerating axon breaks up and is phagocytosed [9; 10; 11]. Myelin damage impairs the conduction of signals in the affected nerves. In turn, the reduction in conduction ability causes deficiency in sensation, movement, cognition, or other functions depending on which nerves are involved[12]
There is a paucity of literature on the histological consequence of GP administration during or after mercury exposure on the cerebellum. However, the phytochemical composition of GP may be useful in the composition of
Tabakwot et al
complementary regimens for the management of mercury exposure, especially in a resource-limited environment of most developing countries [13]. A study by [1] on the benefit of ascorbic acid (vitamin C) in mercury exposure on the cerebellum, reported histological improvement in the cerebellar cortex, with an explanation hinged on the antioxidant potential of ascorbicacid.Flavonoids,aromaticesters, diterpenoids, caffeic acid derivatives, steroids, phenolic acid derivatives, and vitamin C are some of the phytochemicals found in GP [14], suggesting its possible deployment in the management of mercuryexposure.Areviewby[15]onthe beneficial role of phytochemicals on oxidative stress and age-related disease, suggestedthatmostplantphytochemicals such as flavonoids and polyphenols can be deployed to protect neuronal cells during mercury exposure. A recent review by [16] on the Potential of naturally derived alkaloids as multi-targeted therapeutic agents for neurodegenerative diseases suggested that management of chronicdiseaseincludingcancer,diabetes and neurodegenerative diseases. According to [17] on the role of plantderived alkaloids and their mechanism in neurodegenerative disorders, indicated that plants which contain alkaloid have possessed potential therapeutic effects against several neurodegenerative diseases including Alzheimer's disease (AD), Huntington disease (HD), Parkinson's disease (PD), Epilepsy, Schizophrenia, and stroke. The choice of the use of GP is hinged on the fact, there are published on the effect of GP on the histology and histochemical changes the following mercury in Wistar rats, despite the enormous promises it holds in the management of mercury exposure-related health issues [14]. Hence, the finding fromthis willaddtotheavailable bodyof knowledge and the benefit of various plant materials in the management of heavy metal exposure including mercury exposure.
Aim of the Study
The aim of this research was to evaluate the effects of Galinsoga parviflora aqueous leaf extract on the histological patterns of cerebellum after mercury chlorideexposure.

Study Location
Research Question
• What are the effects of Galinsoga parviflora aqueous leaf extract on the histological patterns of cerebellum after mercurychlorideexposure?
METHODOLOGY
The study was conducted in the animal house of the Kampala International University, Institute of Biomedical Research (IBR) and Histology Laboratory of Kampala International University, Western Campus (KIU-WC); which is situated in Western Uganda in Ishaka Municipality, Bushenyi District 350KM fromKampala,Uganda.
Ethical Consideration
Ethical approval (KIU-2021-14) was obtained from the Kampala International University Research Ethics Committee before conducting the research and register in Uganda National Council for Science and Technology. The experimental study was carried out at the Institute of Biomedical Research Laboratory (IBRL) Kampala International University-Western Campus (KIU-WC) and
the laboratory animal experiments were conductedfromtheKampalaInternational University Animal House. The 3Rs (Reduction,Replacement,andRefinement) were considered in determining the number of mice to be used in this study as well as the handling of animals (European Medicine Agency, 2016). Atotal of 25 mice were used in this study. The study will use an animal model system and not an in vitro system to determine the effect of Galinsogaparviflora on the cerebellum after mercury chloride is introduced then the expression of antioxidant biomarkers in cells is determined. The report by ‘resource equation’ was considered when chosen the number of animals. The animals were grouped into 5 groups with five animals pergroupandwithgroups,threeandfour receiving the same doses but in reverse
www.idosr.org
methodand also a suitable dose was used according to literature to avoid using too many animals so that to be able to achieve the reduction principle. The researcher obtains training on how to handle laboratory animals before the study and work under the supervision of his supervisors. All procedures and works on animals were conducted following a guide for the care and use of laboratory animals (IACUC, 2010). The animals were treated in a humane way to prevent them from any discomfort like excessive pain and stress. The animals were housed five per group and there was fed on a daily basic morning and evening and also their beddings werechangedweeklyso thatthe animals were comfortable to avoid the fightandsoallowtorespondtotreatment in a ventilated environment. At the end of 42 days, all animals were sacrificed humanely by subjecting them to light ether anesthesia followed by cervical dislocation. At the end of the study, all sacrificedanimalswere incinerated.
Collection and Preparation of Plant Extract
Galinsoga parviflora was obtained from the local garden in Ishaka- Bushenyi District, Uganda. A small sample of the leaves was taken to Mbarara University of Science and Technology, Faculty of Science Herbarium Unit, Mbarara, for botanical identification and deposition of voucher number. Extraction of the plant was carried out in the Biochemistry Departmental laboratory, Kampala InternationalUniversity,WesternCampus, Ishaka, Uganda. The plant’s leaves were driedatroomtemperature;groundedwith a grinding machineand followed by extraction. Sixteen grams (16g) of the powdered leaves were dissolved in 300ml of distilled water and left to stand for three days, shaken frequently until complete extraction of plant materials Then the extracts were filtered through a filter into a funnel and the extract was kept in an oven at 400 C for three days to solidified[18].
‘
Tabakwot et al
Purchase of Chemical Mercuric chloride (May and Bakers, England) was purchased from a chemical storeinIshakatown,WesternUganda.
Animals
The animals were procured from the animal facility of Kampala International University Western Campus (KIU-WC) twenty-five (25) adult male mice were used for this study weighing 20-35g. All mice were housed in a well-ventilated plastic cage; the animals were left to acclimatize for two weeks with free accesstofoodandwater.
Study Sample Size
The resource equation’ approach was used to arrive at adequate sample size. According to the ‘resource equation’ method a value “E or DF” is measured. DF = degree of freedom of analysis of variance(ANOVA).
DF can be measured by the following formula: DF=N – k n=DF/k+1
Where: N = total subjects number; k = numberofgroups;n=numberofsubjects in a group. Minimum DF range = 10 and maximumDFrange=20 [19;20].
The sample size for the study will be: N = 25malemice;K=5groups
DF = 25 – 5 = 25, n = DF/k+1 = 20/5+1 = 5(fivemicewillbeplacedpergroup)
Sampling Technique
The mice were sampled via simple random sampling procedures. A sequence of numbers was randomly used to direct how the animals will place in groups (table 1). After the randomization, the micewere placed in five groups comprising of 5 mice each labeled mercury and gallant soldier dose (MG), gallantsoldierandmercury(GM),mercury and gallant soldier dose concurrently(MGC), positive control (mercury dose) (PC) and negative control (NC)(table2).
Table 1:Randomizationassignmentsequence
Table 2:Groupplacementafterrandomization
Experimental Design
Dosage and Administration
Mercury chloride: 2.3mg/kg of Mercury Chloride was administered. The stock solution was prepared daily to prevent the risk of degradation [21] The dilution factorwas50mgin100ml.
Galinsoga parviflora: 800mg/kg of GP was administered. The stock solution was prepared daily to prevent the risk of degradation [22]. The dilution factor was 100mg in 1ml Mercury chloride and GP was administered orally for three weeks respectively
Reconstitution of Galinsoga parviflora Extract mercury Chloride for Animal Administration
Asuitable dose of GP was administeredto the animals. The right amount of the extract which was guided by the OECD’s (organization of economic corporation
and developments) guidelineswas reconstituted and the calculated doses shall be administered according to each animal's body weight daily (between 9am and 10am). The mercury chloride on the other hand was constituted for administration on the mice according to the appropriate dose which was 800mg/kgasdescribeby[21].The dosage of GP was prepared by dissolving in an appropriate volume of distilled water not exceeding 20 ml/kg [23]. The mercury chloride was guided by the OECD’S guideline and the powder be dissolved in distilled water daily. The mixture was kept in the refrigerator at 60C pending daily administration to the animals accordingtotheirbodyweight. Dosage and preparation of stock solution willbecalculatedusingtheformula: Dosage(mg)=Bodyweightanimals(g)xdose(mg) 1000g
Table 3: Animals Grouping
GROUP DOSAGE
Group1
Receivedistilledwateronly
Group2 Receivemercurychlorideonly(2.3mg/kg)
Group3 Receive mercury chloride (2.3mg/kg) followed byGalinsoga parviflora(800mg/kg)
Group4 Receive Galinsoga parviflora (800mg/kg) followed by mercury chloride(2.3mg/kg)
Group5 Receive mercury chloride (2.3mg/kg) and Galinsogaparviflora (800mg/kg)concurrently
Tissue Collection and Preparation
Trans-cardiac Perfusion Fixation
Trans-cardiac perfusion fixation was done using 4% paraformaldehyde as described below. A perfusion tube with one side open was placed in a beaker having 4% paraformaldehyde (in icebox) filled in it. 20ml/minofthe%paraformaldehydewas adjusted to drip slowly and steadily. An appropriate amount of anesthetic agent (Ketamine 20 mg/kg) was given intramuscularly to the animals. The animal wasplaced back down on the operation table under the influence of anesthesia. On the operation board, the animal appendageswere pinned down. A pinch response method was done to determine the depth of anesthesia. The incision was made by a scalpel through the abdomen to the length of the diaphragm. The diaphragm was cut with aid of sharp scissors through the connective tissues to have access to the rib cage, then the rib cage was cut close to the midline with the aid of large blunt scissors was used. The thoracic cavity was open, through the rib cage a two-end center horizontal cutwas done. A Clamp was used to expose the heart and provide drainageforbloodandfluids.Thebeating heart held steady with forceps. A needle wasinsertedinto the protrusion of the left ventricle which extends to about 5mm. I was careful not to go deeper so as not to damage any interior wall and compromise circulation. A 0.9% saline solution was allowed slowly at 20ml/mm through the valve. For about 7-8 minutes the atrium was cut with sharp scissors to allow the solution to pass freely. When the blood
has been cleared from the body, the solution was changed to a 4% paraformaldehyde solution.Perfusion was confirmed by spontaneous movement (formalin dance) and the lightened color of the liver was observed. Perfusion was stopped and the brain was removed and fixed in the same fixative for another 2 hours at 4oc overnight before starting dehydration and embedding (this fixation method is important when the target organisthebrain)[24]
Histological/ Histochemical Analyses of Cerebellar Tissue Tissue processing
The act of removing water from a celland using a medium to replace the water and a medium then solidifies the cells for cutting with microtome is term tissue processing. Clearing, Dehydration and infiltration are steps used for the tissue process that is normally done after the tissue is properly fixed and the tissue processing machine carries out this process. Paraffin is used to carry out dehydration in tissues because it is immiscible for you to carry out infiltrationthewaterneedstoberemoved completely, 70% to 100% concentration of alcohol is used to remove water; Clearing is done to remove alcohol Since alcohols and paraffin are not miscible, xylene is used to clear alcohol in the tissue than theparaffininfiltrationcaneasilybedone so that section of the tissue can be done withmicrotone[24]
Staining Procedure
Hematoxylin and Eosin (H and E): Staining was used to demonstrate the general histoarchitecture of the cells of
www.idosr.org
the cerebellar cortex. Sections were immersed in already filtered Harris Hematoxylin for a minute after that they were rinsed with running tap water. The sections were now immersed in the Eosin stainfor2minutes.Afterwhichtheywere rinsed with tap water. Then dehydration was done with ascending grades of alcohol (50 %, 70 %, 80 %, 95 % x2, and 100%). Dehydration was repeated as before (100%, 95%.80%, 70%, and 50%), The sections were cleared with xylene which will be also done twice. After which, the coverslip was mounted onto a labeled glass slide with a permanent mounting medium to cover the sections [24]
Myelin Staining (Kluver and Barrera, 1953)
The luxol fast blue–modified Kluver's myelin sheath method was created by Heinrich Klüver and Elizabeth Barrerain 1953 [25] and modified by [26] and [27] wasused.Thetissuesweredeparaffinized andhydratedto95%alcohol.Then keptin Luxol fast blue solution, overnight at 60°C. Sections were rinsed in 95% alcohol and distilled water. Then sections were placedinLithiumcarbonatesolutionfor5 seconds, before being passed through 70% alcohol, two changes, 10 seconds each. Then sections were washed in distilled water. The sections were repeatedly passed through lithium carbonate solution, 70% alcohol and washedindistilledwateruntiltherewasa sharp contrast between the blue of the white matter and the colorless gray matter. Sections were later rinsed in 70% alcohol,stainedinEosinfor1minute,and rinsed in distilled water. Sections were stained in Cresyl violet for 1 minute, rinsed in distilled water and dehydrated through 95% and 100% alcohol, before
Tabakwot et al
being cleared in xylene, and coverslip (when is dark blue to green shows abnormal while light blue to dark indicatesnormal)[8].
Motor Coordination
Beam walking test: This test was conducted to assess motor coordination and balance. 1m long narrow wooden beam (1-2cm wide) suspended from its ends at an elevation of 30cm to the darkroom. The animal was gently be placed in the center of the Beam, facing one of the ends. The rat was allowed to walk to the end of the darkroom; the procedure was repeated three times, the animals were trained for a week once a day before administration and the test wasconductedthreetimesaweekandthe timetoreachtheendwasrecorded[29].
Forearm grip test:
The neuromuscular/skeletal muscle functions were determined by the maximal peak force developed by the mice. The mice were placed on the apparatus a 120cm by 50cm wire forearm grip apparatus. The wire was placed horizontally and the mice were placed in the center to balance the time it takes to reach the other end or falls will be recorded. The training was done for a week once a day before administration and the test was conducted three times a week[29].
Statistical Analysis
All the data of behavior and the biochemical study was statistically evaluated with SPSS 25 using ANOVA to compare the mean. Where necessary, Tukey post hoc test was used to find where the significant difference lies across the groups. All the results were expressed as mean ± SD or SEM value was significantat p≤0.05. Histology study was done with the aid of H and E and KluverBarrerastain.
RESULTS
Histology and Histochemical Result
Hematoxylin and Eosin (H & E)
ThePhotomicrographofthecerebellumof adult male mice treated with distilled water group 1 (normal control), revealed relatively normal cyto-architecture; showing the Purkinje neurons and showing the molecular layer (ML),
Pyramidal cell layer (PL), Granular cell layer (GL) (Fig 2). Histological observations based on H & E revealed evidenceofpyknosisinthepyramidalcell layer of the cerebellar cortex of group 2 (Fig. 3). In group 3 the Section revealed relatively normal pyramidal cells with
www.idosr.org
reduced normal of cells undergoing pyknosis (Fig. 4). In the Group 4 animals, the section of the cerebellum revealed evidence of both pyknosis and normal cells in the pyramidal cell layer of the
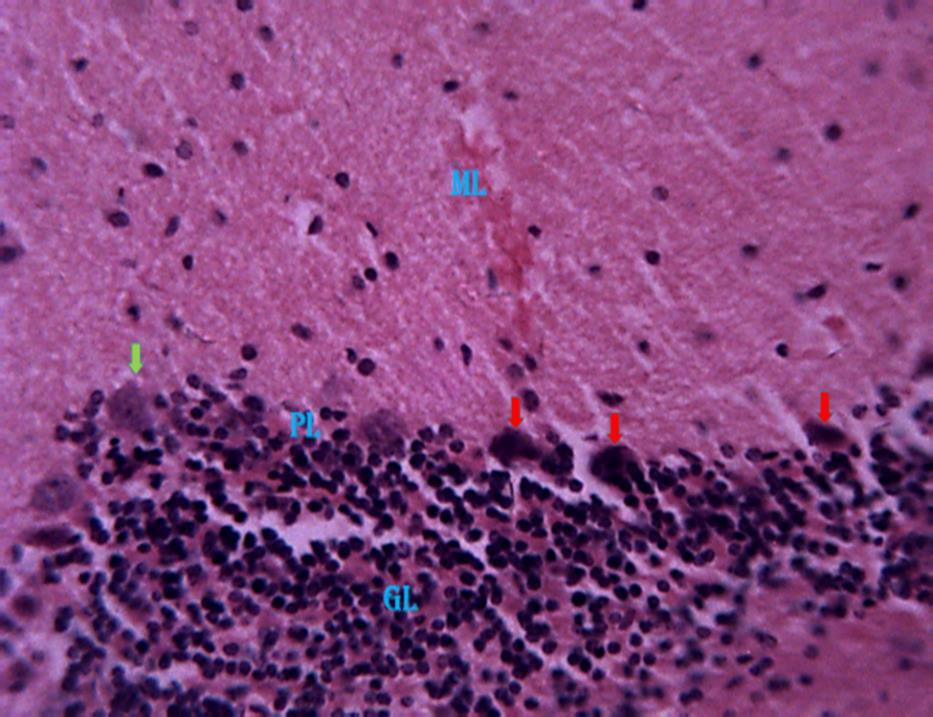

Tabakwot et al
cerebellar cortex (Fig 5). Also, group 5 revealed a relatively high normal pyramidal cell(with intactnucleus) as against the reduced normal of cell undergoingpyknosis(Fig.6).
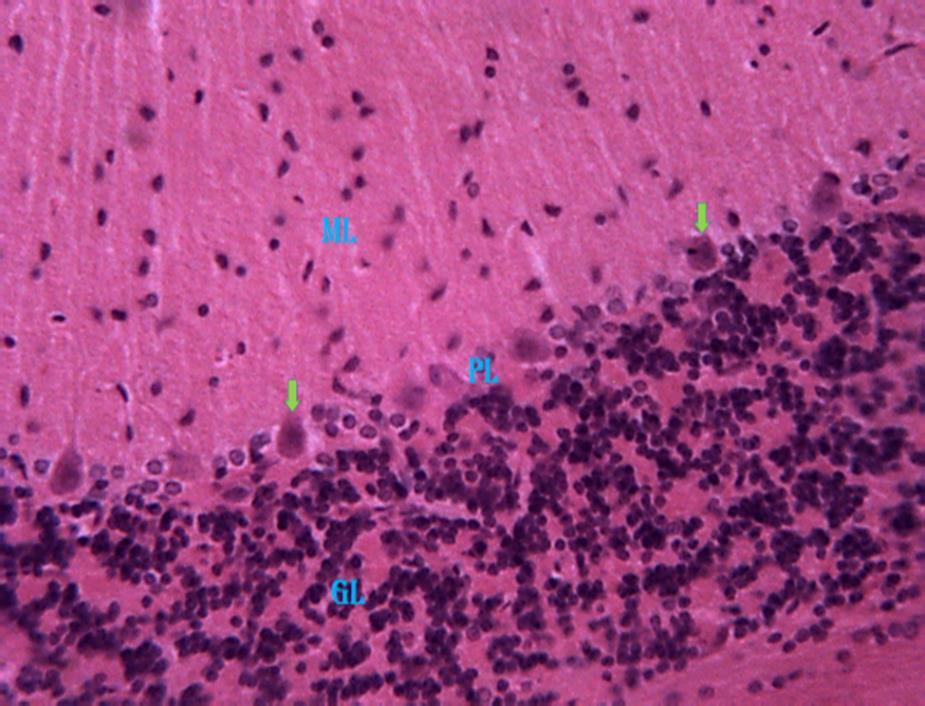

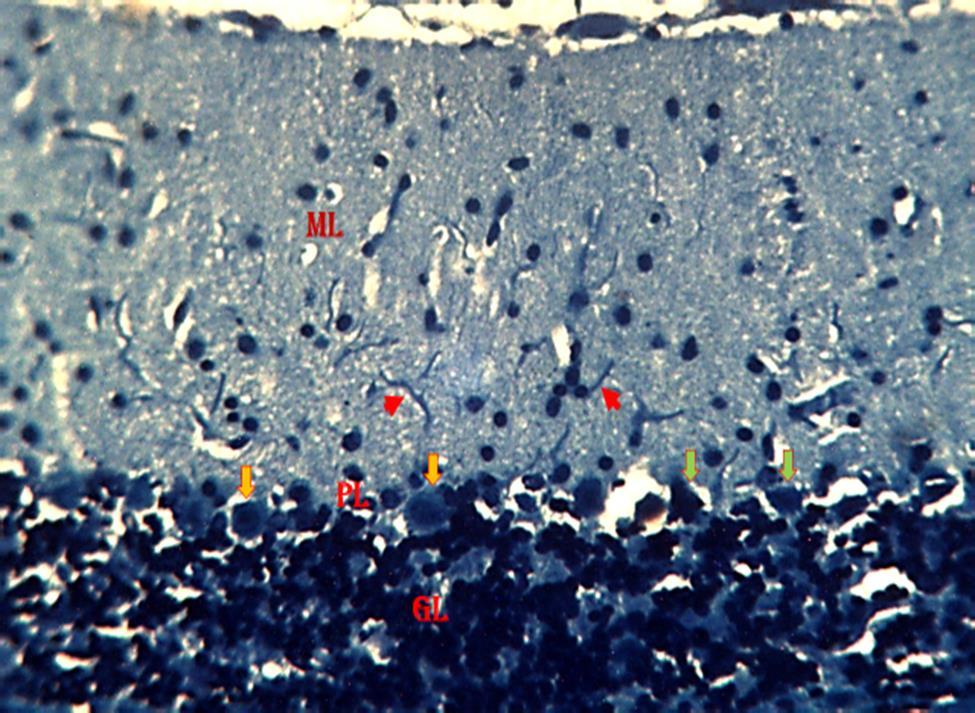
Kluver-Barrera
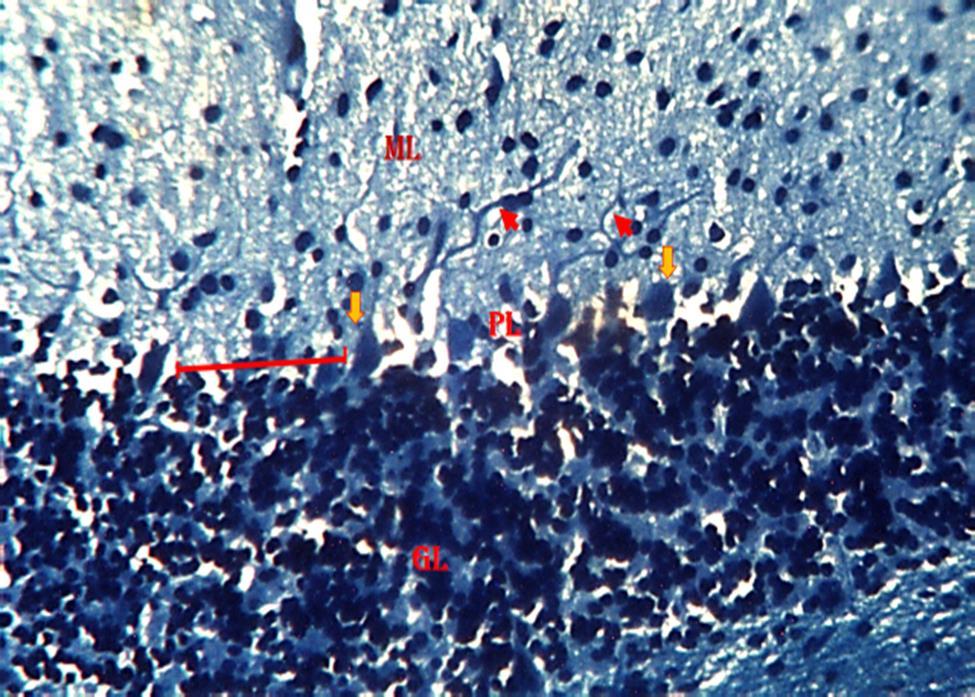
ThePhotomicrographofthecerebellumof adult male mice treated with distilled water group 1 (normal control), revealed relatively normal cyto-architecture; having normal myelination (Fig. 7). Group 2 a section of the cerebellum revealed degenerating myelin and a region of reduced linear distribution of Purkinje cells (Fig 8). Group 3 animals revealed a
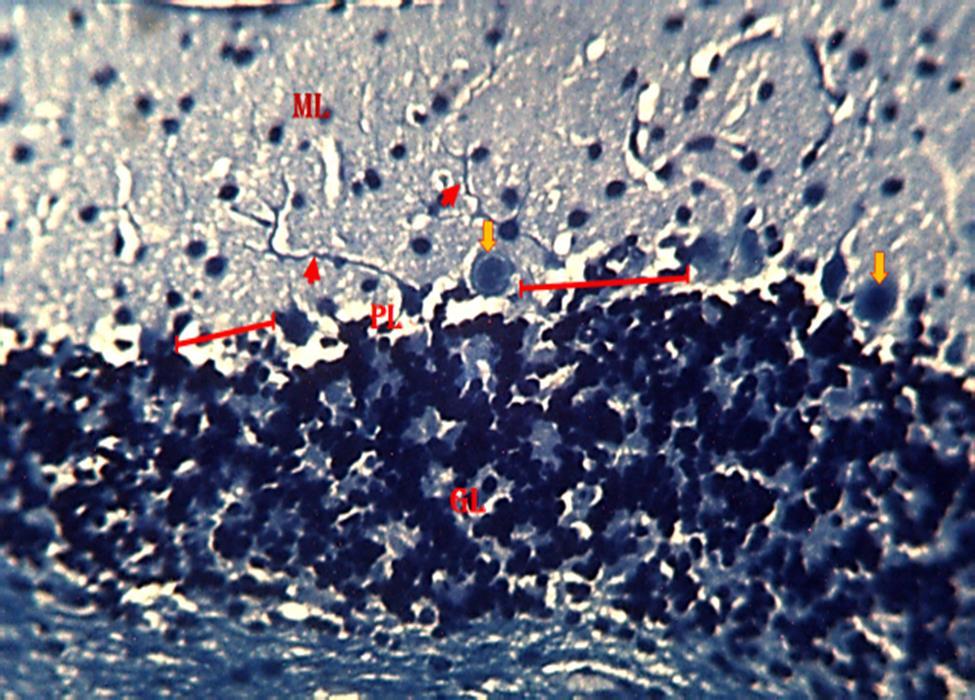
section of the cerebellum with degenerating myelin (Fig. 9). In group 4,the section of the cerebellum revealed a region of reduced linear distribution of Purkinje cells and evidence of myelination. (Fig. 10). In group 5,the section of the cerebellum shows a region of reduced linear distribution of Purkinje cells and presence of axonal myelination (Fig11)
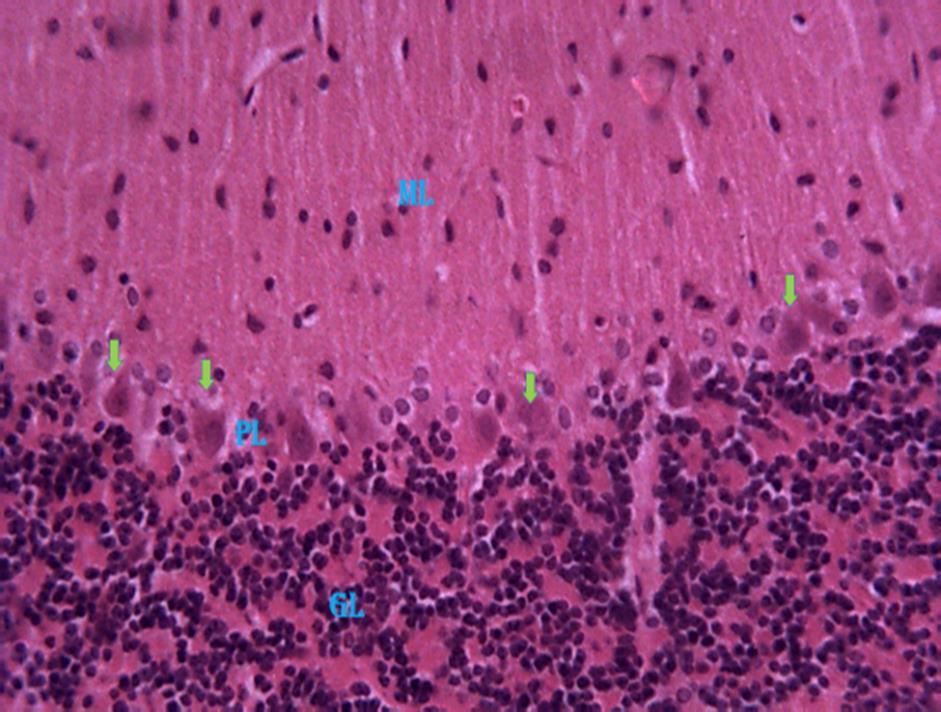
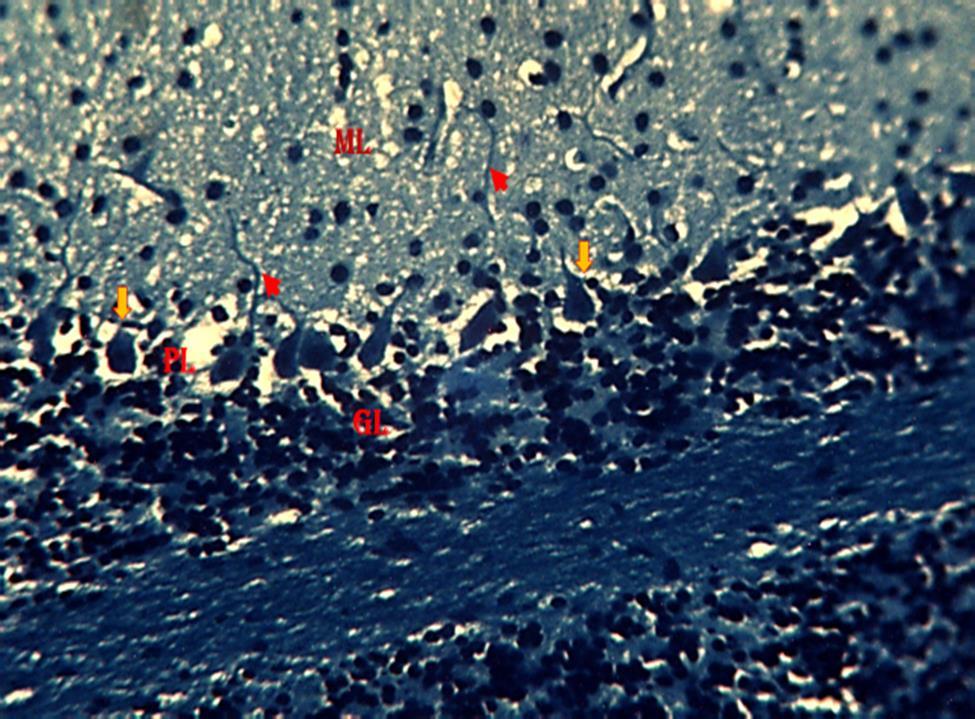
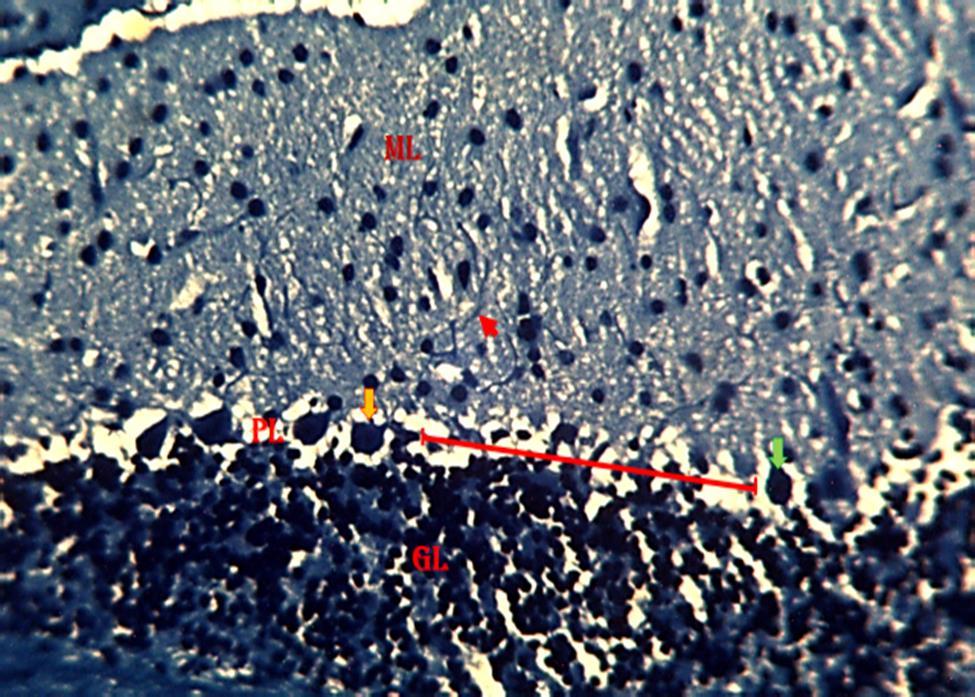
Figure 9: Photomicrograph from the cerebellum of mice treated with 2.3 mg/kg HgCl2 followed by 800 mg/kg G. pav. Showing the molecular layer (ML), Pyramidal cell layer (PL), Granular cell layer (GL), relatively normal pyramidal cells (Orange arrow), degenerating myelin(Greenarrow),nervefibers(Redarrowhead).(KluverBarrera;×250).
DISCUSSION
This study was centered on assessment effects of Galinsoga parviflora (cav) aqueous leaf extract on the cerebellum during mercury exposure in adult male mice, using histomorphological to observe the specific effect of this chemical on the brain using the mice as a model. Histomorphological study of sections of cerebellum of mice in figures 3 and 5 revealed evidence of pyknosis in the pyramidal cells of the cerebellum when compared to figure 1 (control). The presence of pyknotic cells in the Purkinje layer of the cerebellum denotes chromatin condensation in the nucleus of thePurkinjecellswhichcausedcelldeath. According to [30,32,33,34,35,36,37,38] reported that degenerative, pyknotic and necrotic changes in the Purkinje cells of the Purkinje cell layer of the cerebellar cortex following aluminum chloride exposure. This means that the pyknosis seen in pyramidal cells of the cerebellar cortex from this study are features of
neuronal death as reported by [30] Therefore, cerebellar damage produces disorders in fine movement, equilibrium, posture, and motor learning [31,39,40,41,42]. Damage to the Purkinje cells of the cerebellum can impair fine movementandmotorlearning.
Sections of the cerebellum of mice in Figures 8,10 and 11 revealed a reduced linear distribution of Purkinje cells of the cerebellum when compared to figure 7 (control). Which indicates scanty cells in thePurkinjelayerasaresultofcelldeath.
According to [8,43,44,45,46] study on the reduced Purkinje cell number in essential tremor, suggested that a postmortem study indicated a reduction in Purkinje cell numbers in the brains of patients with essential tremor who do not have Lewy bodies. This indicates that the reduction in linear distribution of the Purkinje cells cerebellar cortex might be a result of cell death or Lewy bodies. The presence of phytochemicals such as
www.idosr.org
alkaloid, carbohydrate, flavonoid, polyphenol and glycoside which are found in GP might be the reason why treated groups 3, 4 and 5 have some cells which are yet to revealed evidence of pyknosis. Although, they have reduced the linear distribution of the Purkinje cells when compared with group 2 which have pyknosis and a long linear distribution of Purkinje cells. Hussain et al.,[17] studyon the role of plant-derived alkaloids and their mechanism in neurodegenerative disorders, indicated that plants that contain alkaloids have possessed potential therapeutic effects against several neurodegenerative diseases. This means that the groups which have some cells yet to revealed the evidence of pyknosis or reduced linear distribution of the Purkinje cells might be due to the presence of those abovementionedphytochemicals[47,48,49]
Results from myelin-stained sections of the cerebellum of the adult male mice in
Tabakwot et al
animal groups treated with 2.3 mg/kg HgCl2 and 2.3 mg/kg HgCl2 followed by 800 mg/kg G. pav (figures 8 and 9 respectively) revealed evidence of degenerating myelin when compared to the control group(figure 7). This also suggests that cerebellar functions may have been impaired. Myelin sheath degeneration occurs when a neuron dies or if its axons have been severed, the myelin sheath surrounding the degenerating axon breaks up and is phagocytosed [9; 32; 11]. Myelin damage impairs the conduction of signals in the affected nerves. In turn, the reduction in conduction ability causes deficiency in sensation, movement, cognition, or other functions depending on which nerves are involved [12]. Degradation of myelin in neuronsofthecerebellarcortexasseenin the study may have affected the conduction of electrical signals in neurons, this could, in turn, impair normal functions of the cerebellar cortex.
CONCLUSION
In conclusion, the study revealed that mercury exposure in the cerebellum of mice caused pyknosis. However, the introduction of Galinsoga parviflora in
the cerebellum acted as a therapeutic agent against mercury toxicity in the cerebellumofadultmalemice.
REFERENCES
1. Ibegbu, A., AnimokuAbdulrazaq, A., Micheal, A., Daniel, B., Adamu Sadeeq, A., Peter, A., and Musa, S. A. (2017). Histomorphological effect of ascorbic acid on mercury chlorideinduced changes on the cerebellum of adult Wistar rats. Journal of Morphological Sciences, 31(4),219-224.
2. Styliadis, C., Ioannides, A. A., Bamidis, P.D.,andPapadelis,C.(2014).Distinct cerebellar lobules encode arousal and valence in specific time windows: an MEGstudy. OHBM.
3. Weth, O. and Renkawitz, R. (2011). CTCF function is modulated by neighboring DNA binding factors. In Biochemistry and Cell Biology https://doi.org/10.1139/o11-033
4. Teixeira,F.B.,Fernandes,R.M.,FariasJunior, P., Costa, N. M., Fernandes, L. M., Santana, L. N. and Lima, R. R. (2014). Evaluation of the effects of chronic intoxication with inorganic mercury on memory and motor
controlinrats. InternationalJournalof Environmental Research and Public Health,11(9),9171-9185.
5. Mariën,P.,Ackermann,H.,Adamaszek, M., Barwood, C. H. S., Beaton, A., Desmond, J., De Witte, E., Fawcett, A. J., Hertrich, I., Küper, M., Leggio, M., Marvel, C., Molinari, M., Murdoch, B. E., Nicolson, R. I., Schmahmann, J. D., Stoodley,C.J.,Thürling,M.,Timmann, D. and Ziegler, W. (2014). Consensus paper: Language and the cerebellum: An ongoing enigma. Cerebellum. https://doi.org/10.1007/s12311-0130540-5
6. Martin, L. A., Goldowitz, D. and Mittleman, G. (2010). Repetitive behavior and increased activity in mice with Purkinje cell loss: a model for understanding the role of cerebellar pathology in autism. European Journal of Neuroscience, 31(3),544-555.
www.idosr.org
7. Jha, A., Saidullah, B. and Bubber, P. (2019). A Study on Prooxidative and Neurotoxic Effects of Mercury ChlorideinRats. ECPharmacologyand Toxicology
8. Axelrad, J. E., Louis, E. D., Honig, L. S., Flores, I., Ross, G. W., Pahwa, R. and Vonsattel, J. P. G. (2008). Reduced Purkinje cell number in essential tremor: a postmortem study. Archives ofneurology,65(1),101-107.
9. Perry VH, Brown MC 1992: Role of macrophages in peripheral nerve degeneration and repair. Bioessays 14:401–406,
10. Valent, F., Mariuz, M., Bin, M., Little, D., Mazej, D., Tognin, V., Tratnik, J., McAfee, A. J., Mulhern, M. S., Parpinel, M., Carrozzi, M., Horvat, M., Tamburlini, G. and Barbone, F. (2013). Associations of prenatal mercury exposure from maternal fish consumption and polyunsaturated fatty acids with child neurodevelopment: A prospective cohort study in Italy. Journal of Epidemiology. https://doi.org/10.2188/jea.JE201201 68
11. Lassmann, H., Ammerer, H. P. And Kulnog, W. (1978). Ultrastructural sequence of myelin degradation. I. Wallerian degeneration in the rat optic nerve. Acta Neuropathology (Berl) 44:91–102,1978
12. Swanson, J. W. (2017). Demyelinating Disease; What Can You Do About It. Mayo Foundation for Medical Education and Research (MFMER). https://www.mayoclinic.org/diseasesconditions/multiple-sclerosis/expertanswers/demyelinating-disease/faq20058521accessedon19/09/2018
13. Lazarus, S. S., Adebisi, S. S., Tanko, Y., Agbon, A. N.and Budaye,M. N. (2018). Histological and histochemical assessements on the effect of ethanol fruit extract of Phoenix dactylifera L.(Date Palm) on cerebral cortex of lead acetate treated wistar rats. African Journal of Cellular Pathology, 10(1),1-9.
14. Ali, S., Zameer, S. and Yaqoob, M. (2017). Ethnobotanical, phytochemical
Tabakwot et al
and pharmacological properties of Galinsoga parviflora (Asteraceae): A review. In Tropical Journal of Pharmaceutical Research. https://doi.org/10.4314/tjpr.v16i12.2 9
15. Forni, C., Facchiano, F., Bartoli, M., Pieretti, S., Facchiano, A., D’Arcangelo, D. and Jadeja, R. N. (2019). Beneficial role of phytochemicals on oxidative stress and age-related diseases. BioMedresearchinternational, 2019.
16. Kong, Y. R., Tay, K. C., Su, Y. X., Wong, C. K., Tan, W. N. and Khaw, K. Y. (2021). Potential of naturally derived alkaloids as multi-targeted therapeutic agents for neurodegenerative diseases.Molecules,26(3),728.
17. Hussain,G.,Rasul, A., Anwar, H., Aziz, N., Razzaq, A., Wei, W. and Li, X. (2018). Role of plant derived alkaloids and their mechanism in neurodegenerative disorders. International journal of biological sciences,14(3),341.
18. Bazylko, A., Boruc, K., Borzym, J. and Kiss, A. K. (2015). Aqueous and ethanolic extracts of Galinsoga parviflora and Galinsogaciliata. Investigations of caffeic acid derivatives and flavonoids by HPTLC and HPLC-DAD-MS methods. Phytochemistry Letters. https://doi.org/10.1016/j.phytol.2014 .11.005.
19. Festing,M.F.andAltman,D.G.(2002). Guidelines for the design and statistical analysis of experiments Using laboratory animals. ILAR Journal.43(4):244–258.
20. Arifin, W. N. and Zahiruddin, W. M. (2017). Sample size calculation in animal studies using resource equation approach. MalaysJournalof Medical Sciences 24(5):101–105. https://doi.org/10.21315/mjms2017.2 4.5.11
21. Emanuelli, B., Peraldi, P., Filloux, C., Sawka-Verhelle, D., Hilton, D. and Van Obberghen, E. (2000). SOCS-3 is an insulininduced negative regulator of insulin signaling J.Biol.Chem. 275, 15985–15991
www.idosr.org
22. Chakrabarti, R., Megginson, W. and Yadav, P. K. (2008) Corporate Governance in India. Journal of Applied Corporate Finance, 20, 59-72. http://dx.doi.org/10.1111/j.17456622.2008.00169.
23. OECD. (2000). Guidance Document on Acute Oral Toxicity. Environmental Health and Safety Monograph Series on Testing and Assessment. 2000. No.24.
24. Okesina, A B., Oparinde, D. P. and Akindoyin, K A. (2019). Prevalence of some risk factors of coronary heart disease in a rural Nigerian population. East Afr Med J 199976212–216.
25. Kluver,H., and Barrera, E. (1995). A method for the combined staining of cellsandfibersintheNervoussystem. J. Nueropath.Exp. Neurol. 12:400-403, 1953.
26. Sheehan,D C andHrapchak,B B. (1980).Theoryandpracticeof histotechnology.BattellePress,Ohio, USA
27. Crookham, J. and Dapson, R. (1991) Hazardous chemi- cals in the histopathology laboratory, 2nd ED, Anatech.
28. PCU Okechukwu, E Nzubechukwu, ME Ogbansh, N Ezeani, MO Nworie, (2015).The Effect of Ethanol Leaf Extract of Jatropha curcas on Chloroform Induced Hepatotoxicity in Albino Rats. Global J Biotech & Biochem10,11-15.
29. Jouihan, H. (2012). Measurement of liver triglyceride content.Bio Protoc., 2 (13) (2012)
30. AC Nwaka, MC Ikechi-Agba, PCU Okechukwu, IO Igwenyi, KN Agbafor, (2015).Theeffectsof ethanolextractsof Jatrophacurcasonsomehematological parameters of chloroform intoxicated rats.American-Eurasian Journal of Scientific Research10(1),45-49
31. Luong, T. N., Carlisle, H. J., Southwell, A. and Patterson, P. H. (2011). Assessment of motor balance and coordinationinmiceusingthebalance beam. Journal of visualized experiments:JoVE,(49),2376.
Tabakwot et al
32. ACCOMB Ugwu Okechukwu Paul Chima (2022) The Effect of Methanol Leaf Extract of Rauwolfia vomitoria on Hepatic Markers of Chloroform Intoxicated Albino Wistar Rats IAA JournalofAppliedSciences8(1),76-86
33. Ibegbu, A. O., Animoku, A. A., Ayuba, M.,Brosu,D.,Adamu, S. A.,Akpulu, P., and Musa, S. A. (2014). The effect of ascorbic acid on mercury-induced changes on the histomorphology of the cerebellum of adult wistar rats. AfricanJournalofCellularPathology, 3(9),9-15
34. OROOMB Ugwu Okechukwu Paul Chima (2022) TheEffectofEthanolLeafExtract of Rauwolfia vomitoria on Hepatic Markers of Chloroform Intoxicated AlbinoWistarRats IAA Journal of Applied Sciences8(1),87-97
35. Gray’s Anatomy (2008) (40th ed.). Chapter 20. p. 297. ISBN 978-0-80892371-8.
36. Valat, J., Fulcrand, J. and Marty, R. (1978). Reactivitecellulairepostnatale du nerf optiqueapres enucleation chez le rat. Acta Anaomyt (Basel) 100:5–16, 1978
37. Ugwu Okechukwu P.C. and Amasiorah V.I.(2020).The In Vivo Antioxidant Potentials of the Crude Ethanol Root Extract and Fractions of Sphenocentrum jollyanum on Oxidative Stress Indices in Streptozotocin-Induced Diabetic IDOSR Journal of Biology, Chemistry AndPharmacy 5(1):26-35.
38. PCU Okechukwu, CE Offor, UA Ibiam, AL Ezugwu, AJ Uraku, CN Igwe (2015).The Effect of Ethanol Extract of Jatropha curcas on Renal Markers of Chloroform Intoxicated Albino Wister Rats. European Journal of Biological Sciences 7(1),21-25.
39. Ssempijja Fred, Marta VicenteCrespo, Dare Samuel Sunday and Edmund Eriya Bukenya (2022). The effect of Imperatacylindrica(L.)P. Beauv. on Brain tissue Morphology of Drosophila Melanogaster transgenic flies overexpressing the paralytic Gene. IDOSR Journal of Biochemistry, Biotechnology and AlliedFields 7(1):1-11.
www.idosr.org
40. Ssempijja Fred, Marta VicenteCrespo, Dare Samuel Sunday1 and Edmund Eriya Bukenya (2022).The Effect of Imperata Cylindrica (L.) P. Beauv on Learning and memory performance in a single and prolonged exposure treatments of Drosophila Melanogaster transgenic flies overexpressing the paralytic gene. IDOSR Journal of Biochemistry, Biotechnology and AlliedFields 7(1):12-22
41. Ssempijja Fred, Marta VicenteCrespo, Dare Samuel Sunday and Edmund Eriya Bukenya(2022). The Effect of Imperatacylindrica (L.) P. Beauv. on Myelin and Nissl Substance of Drosophila melanogastertransgenic Flies Overexpressing the Paralytic Gene. IDOSR Journal of Biology, ChemistryAndPharmacy 7(1)1-13.
42. Hussein Osman Ahmed, Joy Muhumuza and Musiime James Nabaasa (2022). Evaluation of the factors associated with immediate adverse maternal outcomes among referred women in labor at Kampala International University Teaching Hospital. IAA Journal of BiologicalSciences8(1):228-238
43. Hussein Osman Ahmed, Joy Muhumuza and Musiime James Nabaasa (2022). Factors associated with Immediate Adverse Maternal Outcomes among Referred Women in Labor attending Kampala International University Teaching Hospital. IAA Journal of Applied Sciences8(1):117-125.
44. Hussein Osman Ahmed, Joy Muhumuza and Musiime James Nabaasa (2022). The composite immediate adverse maternal outcomes among women in labor
Tabakwot et al
referred to Kampala International University Teaching Hospital IAA Journal of Scientific Research 8(1):149-156.
45. Daniel Asiimwe, Herman Lule and Izimba Daniel (2022). Epidemiology of Assault Injuries among Trauma Patients Presenting at Kampala International University Teaching Hospital and Jinja Regional Referral Hospital. INOSR Applied Sciences 8(1):111119.
46. E.O.Ikuomola,O.S Akinsonmisoye, R.O. Owolabi and M. B. Okon (2022). Assessment of Toxicity Potential of Secnidazole on Reproductive System of Male WistarRats. INOSRAppliedSciences 8(1):120-133
47. Ugwu Okechukwu, P. C., Onwe, S. C. and Okon, M. B. (2022). The effect of Methanol Extract of Rauwolfia vomitoria on Lipid Profile of Chloroform intoxicated Wistar Albino Rats. IAA Journal of ScientificResearch,8(1),73-82
48. E.O.Ikuomola,O.S.Akinsonmisoye, R.O. Owolabi and M. B. Okon (2022).Evaluation of the Effect of Secnidazole on Sperm Motility, Morphology, Viability and Total Sperm Count of Wistar Rats. INOSR Experimental Sciences 8(1): 74-83, 2022.
49. E.O. Ikuomola , O.S Akinsonmisoye, R.O. Owolabi and M.B.Okon(2022).Evaluationof the effect of secnidazole on the histology of the testes and epididymis of male Wistar rats. INOSR Experimental Sciences 8(1): 84-94,2022.
