6 minute read
Breaking the chain of infections
By Barbara Otieno and Lucy Nganga
Inadequate sterilization can be costly to hospitals. The Antibiotic-Resistant Bacterial Infection outbreak in 2015, was a consequence of improper sterilization of contaminated duodenoscopes that resulted in 400 people being infected with superbugs and further led to 35 deaths.
Advertisement
Emphasis on the importance of proper decontamination methods and increased awareness of Healthcare-Acquired Infections (e.g. Hepatitis B or HIV) after surgical procedures is key.
According to the Centers for Disease Control and Prevention, decontamination of tools and objects ensures that all pathogenic microorganisms are removed so that they are safe to handle and use on another patient.
Centralizing the reprocessing of single-use patient items helps ensure that high standards of sterilization practices are upheld. Decontamination of medium and high-risk instruments that are in contact with a break in skin or body fluids is performed at the Central Sterilization Service Department (CSSD).
Single-use items such as hypodermic needles and syringes are not reprocessed in the hospital - they pose a high risk of infection transmission since they cannot be thoroughly cleaned,” explained Mr. Nazarino Kithaka, Senior Nursing Officer, Theatre Sterile Surgical Unit.
The success of surgical departments at Kenyatta National Hospital (KNH) is dependent on the CSSD’S complimentary unit; Theatre Sterile Surgical Unit (TSSU), which is located right next to the main theatres. Ms. Lilian Kabua, Assistant Chief Nurse, Head of Central Sterilization Service Department points out; “The department is the hospital’s backbone, and without it, the dayto-day hospital operations will be disabled.”
TSSU’s sole focus is intently to decontaminate surgical instruments prepared for surgical procedures.
The CSSD is mapped out to streamline the movement of items through 4 main central process areas; decontamination, packaging or assembly, sterilization, and storage. Access to the processing area is strictly controlled and staff has to comply with the department’s dress code policy.
They are provided with appropriate Personal Protective Equipment (PPEs) that are laundered and donned at the facility.

Roselida Sunguri(right), Theatre Sterile Assistant,TSSU demonstrating to Lucy Nganga, Volunteer - M&C (left) how Sterile supplies are transported in transparent bag to minimize crosscontamimation from the Sterile storage area to the place of usage.
PHOTO | BARBARA OTIENO
The purpose is to minimize microbial dispersal from undergarments and to protect them from exposure to microorganisms. CSSD has a total of 100* trained staff; thirty-five at the TSSU to support the operating theatres seven days a week.
From the staff entrance, a long corridor leads the Newsline team to the receiving area. After each use, patient items from the wards, operating rooms, and outpatient clinics are handed over in covered carts at the dirty area counter.
The hospital generates 13 tonnes of contaminated patient care supplies daily. The functionality and intactness of instruments sets must be checked and disassembled to eliminate 80% of microbial contamination. Low, medium, and high-risk items are cleaned before disinfection and sterilization.
CSSD ensures satisfactory quality control by observing Physical parameters, chemical and biological indicators in line with the International Standards Organization (ISO) and Association for the Advancement of Medical Instrumentation to ensure each step in the reprocessing cycle is carried out effectively. Disinfected, inspected and assembled items are double wrapped in blue polypropylene plastic in the parcel fold technique for heavier sets allowing steam or sterilizing agent (stem or hydrogen peroxide gas plasma) to penetrate and aeration post-sterilization. Paper-plastic peel pouches are designed for packaging small single instruments used in theatres.
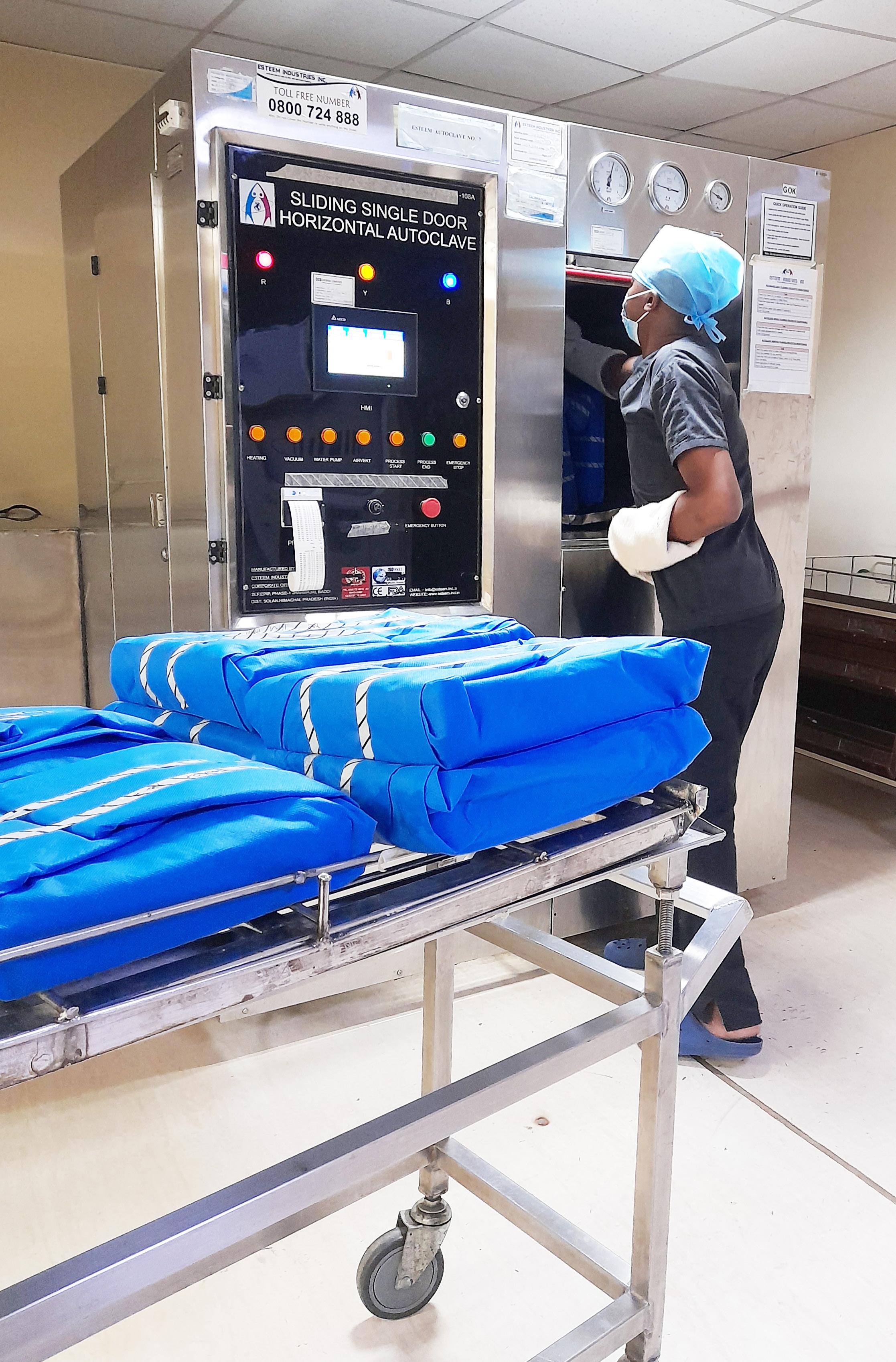
Before loading packaged sets in the sterilizer, identification labels are placed outside the wrap and must include the name of the product, wrapper, the sterilization, and expiration date (28 days). Chemical indicators e.g. Sterilization indicator sealing tape must be affixed outside of each load placed in the autoclave.
Steam sterilization (Autoclaving) is the final step in the decontamination of heat-stable high-risk items. According to specific instructions provided by the device manufacturer, saturated steam flows around heat-stable items to achieve a chamber temperature of at least 134°C for 10 minutes to complete one successful cycle.
Diagonal Black stripes on autoclave tape indicate that steam has penetrated the packs however this alone is not a satisfactory standard of efficacy.
Use of appropriate biological indicators ((B. stearothermophilus spore strips) to monitor autoclave sterility is conducted every month.
Evaluation of the performance of pre-vacuum sterilizers is performed daily before the first process load using the Bowie Dick. Gases often shield bacteria from the steam and the items after numerous autoclaving cycles preventing proper sterilization. Results of chemical and biological monitoring and log sheet records (name of the operator, time of run, and amount being autoclaved) are filed in the CSSD logbook.
Sterilized items are transferred and each instrument set is stacked on its shelf in the sterile storage area. Surgical instrument trays must be delivered shortly before they are needed in the theatres.
All sterile items from patient care areas are returned to the applicable departments in transparent paper plastic sterilization bags to prevent microbial contamination during handling.
Since 2021, five support staff have undergone the Theatre Sterile Assistant training program at the affiliated KNH School of Nursing.
KNH is dedicated to assisting the department and its staff in improving the quality of sterilized items used on patients. “I’m grateful to KNH for the theoretical and practical knowledge acquired on decontamination” expressed Rosalida Sunguri, Theatre Sterile Assistant. She adds “I have a fresh approach towards infection control.”
Decontamination of singleuse patient items is crucial to controlling healthcareassociated infections in hospitals and its processes are not as straightforward as many presume. Despite little or no interaction with patients, CSSD’s final output is used directly by the patients. Minimizing the environmental impact that the facility and its operations have is a concern of the department alongside patient and staff safety. The replacement of Ethylene oxide (ETO) to hydrogen peroxide plasma as a low-temperature sterilant is a prime example. Toxic by-products of ETO are carcinogenic and may harm personnel in the long run.
Before exiting the processing area, we had to adhere to the department’s guidelines on the safe disposal of recyclable PPE’s. We discarded our cover apparel, face masks, and shoe covers into separate designated bins.










