TheApplication ofGreenSolvents inSeparation Processes
Editedby
FranciscoPena-Pereira
CampusAsLagoas-Marcosendes/n,Vigo,Spain
MarekTobiszewski
Gda ´ nskUniversityofTechnology,Gda ´ nsk,Poland
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright r 2017ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicor mechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem,without permissioninwritingfromthepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthe Publisher’spermissionspoliciesandourarrangementswithorganizationssuchastheCopyrightClearance CenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher (otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroaden ourunderstanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecome necessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingand usinganyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationor methodstheyshouldbemindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomthey haveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeany liabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceor otherwise,orfromanyuseoroperationofanymethods,products,instructions,orideascontainedinthe materialherein.
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
ISBN:978-0-12-805297-6
ForInformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher:JohnFedor
AcquisitionEditor:KathrynMorrissey
EditorialProjectManager:AnnekaHess
ProductionProjectManager:MohanapriyanRajendran
Designer:MatthewLimbert
TypesetbyMPSLimited,Chennai,India
ListofContributors
DeeneshK.Babi NovoNordiskA/S,Kalundborg,Denmark;TechnicalUniversity ofDenmark,KongensLyngby,Denmark
TakeshiBamba KyushuUniversity,Fukuoka,Japan
PetrBo ˇ cek InstituteofAnalyticalChemistryoftheCzechAcademyofSciences, Brno,CzechRepublic
CarmenCaballo UniversityofCo ´ rdoba,Co ´ rdoba,Spain
MartaCostas-Rodrı´guez GhentUniversity,Ghent,Belgium
LarissaP.Cunico LundUniversity,Lund,Sweden
InmaculadadelaCalle UniversityofVigo,Vigo,Spain
MarceloM.R.deMelo UniversityofAveiro,Aveiro,Portugal
PabloDomı´nguezdeMarı´a SustainableMomentum,LasPalmas,Spain
RafiqulGani TechnicalUniversityofDenmark,KongensLyngby,Denmark
TadeuszGo ´ recki UniversityofWaterloo,Waterloo,ON,Canada
KariHartonen UniversityofHelsinki,Helsinki,Finland
UdiJumhawan KyushuUniversity,Fukuoka,Japan
AlisaKammafoo SCGChemicals,Bangkok,Thailand
PiotrKonieczka Gda ´ nskUniversityofTechnology,Gda ´ nsk,Poland
PavelKuba´ˇn InstituteofAnalyticalChemistryoftheCzechAcademyofSciences, Brno,CzechRepublic
KusumaKulajanpeng SCGChemicals,Bangkok,Thailand
HianKeeLee NationalUniversityofSingapore,Singapore,Singapore
KhavinetLourvanij SCGChemicals,Bangkok,Thailand
SeyedMohammadMajedi NationalUniversityofSingapore,Singapore,Singapore
MariuszMar ´ c GdanskUniversityofTechnology,Gdansk,Poland
AhmedMostafa UniversityofDammam,Dammam,KingdomofSaudiArabia
JacekNamie ´ snik Gda ´ nskUniversityofTechnology,Gda ´ nsk,Poland
EvangelosK.Paleologos GeneralChemicalStateLaboratory,ChemicalServiceof EpirusandWesternMacedonia,Ioannina,Greece
FranciscoPena-Pereira UniversityofVigo,Vigo,Spain
xiii
Ine ˆ sPortugal UniversityofAveiro,Aveiro,Portugal
JoselitoP.Quirino UniversityofTasmania,Hobart,TAS,Australia
Marja-LiisaRiekkola UniversityofHelsinki,Helsinki,Finland
SoledadRubio UniversityofCo ´ rdoba,Co ´ rdoba,Spain
MałgorzataRutkowska Gda ´ nskUniversityofTechnology,Gda ´ nsk,Poland
HebaShaaban UniversityofDammam,Dammam,KingdomofSaudiArabia
Marı´aD.Sicilia UniversityofCo ´ rdoba,Co ´ rdoba,Spain
CarlosM.Silva UniversityofAveiro,Aveiro,Portugal
ArmandoJ.D.Silvestre UniversityofAveiro,Aveiro,Portugal
Monika ´ Smiełowska GdanskUniversityofTechnology,Gdansk,Poland
K.H.Smith TheUniversityofMelbourne,Parkville,VIC,Australia
G.W.Stevens TheUniversityofMelbourne,Parkville,VIC,Australia
MarekTobiszewski Gda ´ nskUniversityofTechnology(GUT),Gda ´ nsk,Poland
AnanyaTongrod SCGChemicals,Bangkok,Thailand
CharlottaTurner LundUniversity,Lund,Sweden
J.Vovers TheUniversityofMelbourne,Parkville,VIC,Australia
AlainWuethrich UniversityofTasmania,Hobart,TAS,Australia
Bo ˙ zenaZabiegała GdanskUniversityofTechnology,Gdansk,Poland
xiv ListofContributors
InitialConsiderations
FranciscoPena-Pereira1 andMarekTobiszewski2
1UniversityofVigo,Vigo,Spain, 2Gda ´ nskUniversityofTechnology(GUT),Gda ´ nsk,Poland
1.1THENEEDTOUSESOLVENTS
Solventsareubiquitousauxiliarysubs tancesmainlyusedinpaints,coatings,adhesives,cleaners,andcosmetics,butalsoinchemicalandpharmaceuticalprocesses.Aworldwidesolventconsumptionofabout30million metrictonsperyearhasbeenrecentlyestimated [1] .Fromthem,alcohols suchasmethanol,ethanol, n -butanol,andiso-propanolarethemoretypicallyusedsolvents,withanannualconsumptionof6.5milliontonsin accordancewitharecentmarketstudy [2] .Aromatics,ketones,esters,and ethersarealsowidelyused.Theglobalsolventconsumptionhascontinuouslyincreasedinthelastyears,andthistrendseemslikelytopersistand evenintensifyinthefuture.Theexpectedincreaseintheshorttermhas beenmainlyattributedto thegrowingdemandforsolventsbytheemerging marketeconomies,especiallytomeettherequirementsinconstructionand automobileindustries.
Solventsarealsoincorporatedinmanyfieldsofchemistry.Theyseldom playtheleadingroleinchemicalactivities.Chemistsaremoreinterestedin theminoramountsofcompoundsthataredissolvedorobtainedinthem.Itis verycommonthatsolventsbuildthegreatmajorityofthemassofchemicals usedinchemicalprocessesandtheyareoftenoutofdirectchemists’attention.Thisisespeciallyvalidincaseoffinechemicalsandpharmaceutical industries,wheregreatmassofsolventisutilizedpermassofproduct [3]. Chemicalsynthesisandseparationprocessesaretwomainareasinvolving organicsolvents.Thus,solventsallowcarryingoutcountlesschemicalreactions,isolationandpurificationoftargetcompounds,formationofazeotropes forseparation,ortemperaturecontrol,amongotherapplications,withexcellentperformance.Solventlessprocessesarenowadaysfavored,andalternativeapproachesareintroducedtoreducetheenvironmentalimpactand safetyissuesofvolatileorganicsolvents.
Chapter1
3 TheApplicationofGreenSolventsinSeparationProcesses.DOI: http://dx.doi.org/10.1016/B978-0-12-805297-6.00001-2 © 2017ElsevierInc.Allrightsreserved.
Organicsolventsshowaparamountroleinawiderangeofscientificand technologicalapplications.However,severalproblemscanbeascribedtothe useofmostoftraditionalsolvents.Apartfromthegreatamountsofsolvents used,thereishighriskconnectedtotheapplicationofsolventsofveryhigh concern.Theyoriginatefromvariousclassesofchemicals,sotheyarecharacterizedbyverydiverseproblemstheymaycause.Historically,halogenated solventswerewillinglyappliedbecauseoftheirexcellentpropertiestodissolvemanyorganiccompounds.However,severalofthemarecurrently underregulation,andtheirapplicationisonlyallowedforthosespecific activitieswhereappropriatealternativesarenotavailable.Otherfrequently usedsolventsarealiphaticoraromatichydrocarbons.Allofthesecompoundsarecharacterizedbythebenefitofpoorwatersolubility,whatgives thepossibilityforeasyseparationofthesesolventsfromaqueousfractions. Anotherissuerelatedtotheutilizationoftraditionalorganicsolventsistheir toxicity,especiallyconcerninghydrocarbonsandchlorinatedsolvents.These arecharacterizedbyhighoralandinhalationtoxicities.Theyareoftencharacterizedbychronicnegativeeffects,suchasmutagenicity,teratogenicity, andsomeofthesolventsarelistedascarcinogens [4].
Inaddition,awiderangeoforganicsolventsshowenvironmentalpersistence.Whilepolarsolventsarereadilydegradableintheaquaticenvironment viahydrolysisreactionandbiodegradation,othersolventssuchaschlorinated volatileorganiccompounds(VOCs)arehardlydegradable,withtheiraquatic environmenthalf-livesreachingdecades.Presentintheatmosphere,solvents cancontributetoglobalwarming,ozonedepletion,ortheoccurrenceof troposphericozone.
Becauseoftheirhighvolatility,severalorganicsolventscanalsocause risktotheirusers.Theiroccupationalexposurecannotbeneglectedandthey provedtocauseacuteandchronicthreats.Whatismore,someofthemare difficulttohandleastheyareflammableandexplosive.
Importanteffortshavebeenmadetocontroltheproductionandconsumptionofhazardousorganicsolventsinthelastdecades.Forinstance,the InternationalLaborOrganization(ILO)adopted,in1971,theBenzene Convention,regardingtheprotectionagainsthazardsarisingfrombenzene [5].Accordingly,theILOestablishedthatharmlessorlessharmfulsubstitute productsshouldbeusedinsteadofbenzeneorproductscontainingbenzene wheneverpossible.Inaddition,theMontrealProtocolonSubstancesthat DepletetheOzoneLayerset,in1989,alistofozone-depletingsubstances withtheaimofcontrollingtheirproductionandconsumption [6]. Fig.1.1 showsasummaryofproposedphaseoutschedulesfordevelopedanddevelopingcountriesundertheMontrealProtocolthatincludeseveralhalogenated solventstypicallyusedinchemicalprocesses.Furthermore,TheRegistration, Evaluation,AuthorizationandRestrictionofChemicals(REACH)aimedto
1.2TRADITIONALSOLVENTS
4 SECTION|I Introduction
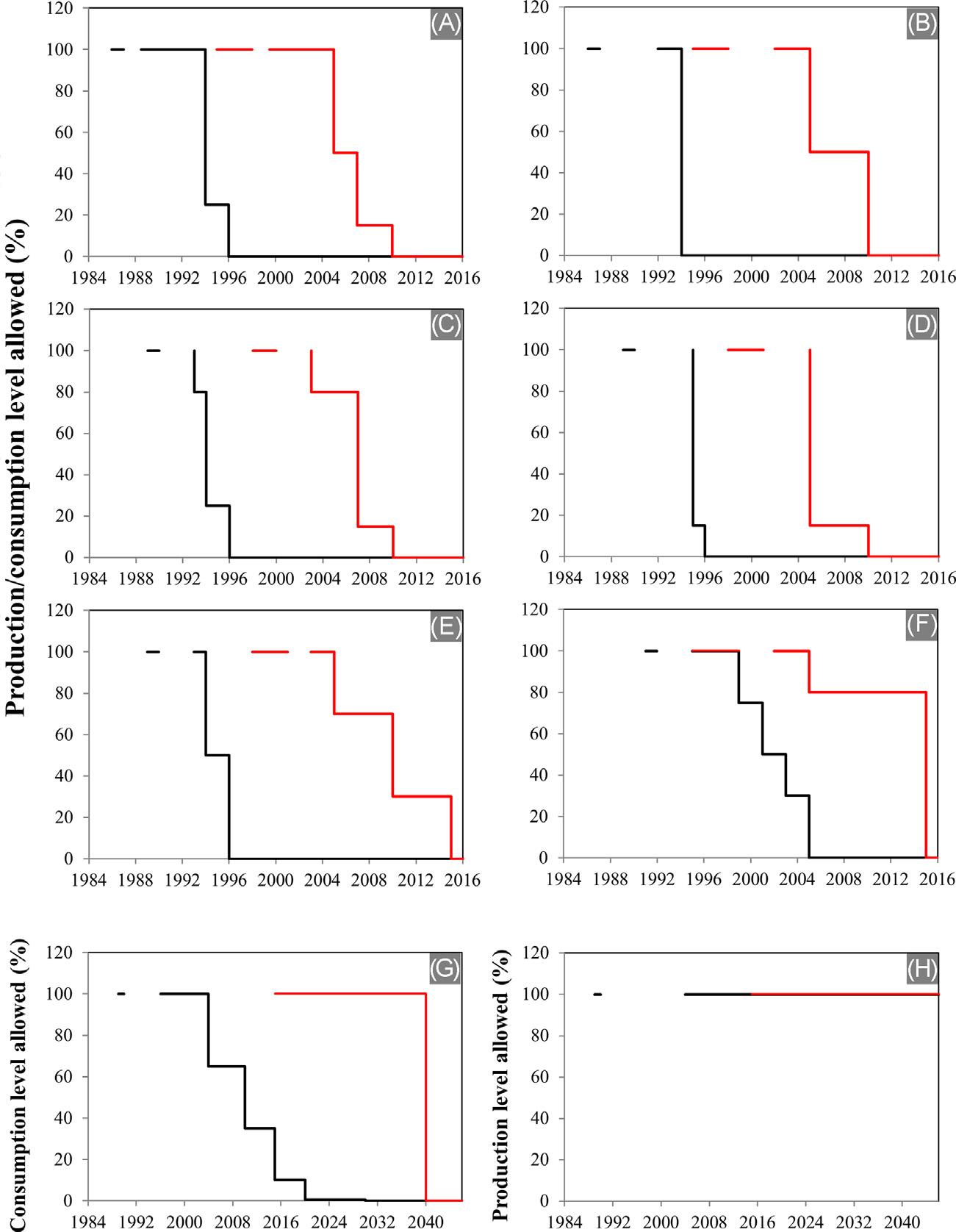
FIGURE1.1 SummaryofcontrolmeasuresundertheMontrealProtocol:(A) Chlorofluorocarbons(CFC-11,CFC-12,CFC-113,CFC-114,CFC-115);(B)halons(halon1211, halon1301,halon2402);(C)otherfullyhalogenatedCFCs(CFC-13,CFC-111,CFC-112, CFC-211,CFC-212,CFC-214,CFC-215,CFC-216,CFC-217);(D)carbontetrachloride;(E)methyl chloroform;(F)methylbromide;(G)hydrochlorofluorocarbons(HCFCs,consumption);(H)HCFCs (production) [6].Controlmeasuresestablishedfordevelopedcountriesarerepresentedbyblack lines,whilethoseestablishedfordevelopingcountriesareshowninred.
InitialConsiderations Chapter|1 5
achieveaminimizationofsignificantadverseeffectsonhumanhealthand theenvironmentinbothproductionanduseofchemicalsby2020 [7].
REACHregulationspursuedtoruleoutdangeroussubstancesorprogressivelyreplacingsubstancesofconcernbylessdangeroussubstanceswhere suitablealternativesareavailable.Severalcommonlyusedsolventsare includedamongalargenumberofsubstancesclassifiedascarcinogenic, mutagenic,ortoxictoreproductionthatarecontrolledbyREACHwith specificconditionsofrestriction.
Regulationshavebeenimplementedtodecreasetheconsumptionof solventsshowingimportantissuesor,inthecaseofsubstancesofveryhigh concern,ceaseoftheirusewhentheycancauseseriousandirreversible harmtohumanhealthandtheenvironment.Regulationsshowanimportant roleinordertominimizethedeleteriouseffectsofsolventconsumption.For instance,nonmethaneVOCemissionshaveexperiencedadecreaseof3.4% peryearintheEuropeanUnionintheperiod2000 13 [8].Eventhoughthe correspondingdata(7milliontonsofnonmethaneVOCemissionsin2013) isstillimprovable,itcanbeconsideredthattheimplementationofstricter regulationsandthecontrolofsolventuseandemissionshavecontributedto thereductionofVOCconsumptioninEurope.
Regrettably,hazardousorganicsolventsareyetusedinawiderangeof scientificandtechnologicalactivities,thuscontributingtothegenerationof significantamountsofwastes.Thus,activitiessuchaspurificationofwastes andvaporstreams,removalofcontaminants,ormonitoringofenvironmental pollutantssignificantlycontributetofurtherpollutionduetotheorganicsolventsusedinthecorrespondingprocesses.Bearinginmindtheenvironmental, health,andsafetyissuesassociatedwiththeuseofmanyconventionalorganic solvents,thedevelopmentofgreeneralternativesisbecomingincreasingly important.Anotherimportantaspectregardingorganicsolventsisthattheyare mainlyderivedfromfossil-basedfeedstocks.Thus,thepossibilityofobtaining valuablesolventsfrombiomasswastewithoutmakinguseofadditional solventsderivedfromcrudeoilisachallengingresearchareathathasbeen exploredinthelastyearswithhighlypromisingresults [9,10].Productionof greensolventsfromrenewablerawmaterialsisthereforeofutmostinterestto developmoresustainablechemicalprocessesandmethodologies.
Alltheabovementionedissuesrelatedtotheapplicationoftraditional solventsaregoodreasonsforsearchinggreenersolutions.Inthissense,the inceptionofgreenchemistrypavedthewayforamorecarefulandconscientioususeofsolvents.Regardingextractionandseparationprocesses,several aspectsshouldbeconsideredtoselectagivensolvent,includingnotonlythe environmental,health,andsafetyissuesofconsideredsolventsbut,also importantly,metrologicalandeconomicaspects.Thus,theextractionand/or separationperformance,energydemand,possibilityofsolventrecovery,and recycling,aswellassolventcompatibilitywithanalyticalinstrumentation (inthecaseofanalyticalmethoddevelopment),shouldbecarefullyassessed.
6 SECTION|I Introduction
1.3GREENSOLVENTS
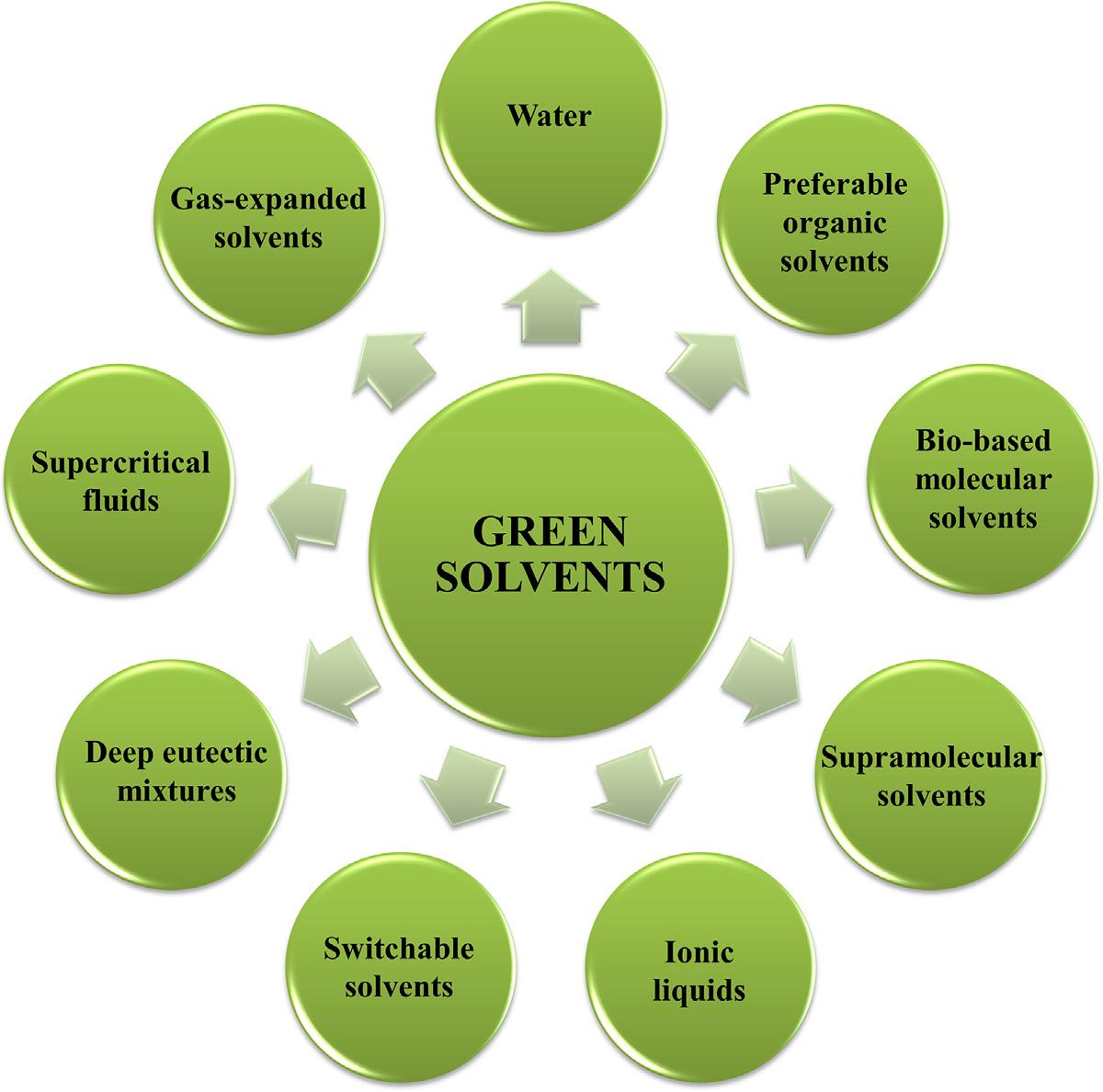
Inthelastdecades,scientistshavepaidincreasingattentiontotheadverse effectsofreagentsandsolventsusedinchemicalprocesses.Theintroduction ofthe12principlesofgreenchemistryrepresentedaturningpointtowarda reductionofenvironmental,safety,andhealthhazardsassociatedtoconventionalchemicalprocesses [11,12].Thedevelopmentandapplicationofgreener solventsiscurrentlyahottopicinavarietyofscientificandtechnological areas.Greensolventscanbedefinedasthosesolventsthatdisplayreduced health,safety,andenvironmentalissuesandareducedlifecycleimpact [13 15]. Fig.1.2 showsanumberofalternativesolventsthatfulfill,toa greaterorlesserextent,thisdefinitionandaredescribedindepthinthisbook.
Twomainmeasureshavebeenconsideredtomakechemicalprocesses greenerwhensolventlessapproachesarenotfeasible.Themoreconservative one,yetchallenging,involvesasignificantreductionoforganicsolventconsumptioninagivenchemicalprocess.Moredesirably,thesecondmeasure involvesareplacementofharmfulsolventsbygreeneralternatives.Needless
InitialConsiderations Chapter|1 7
FIGURE1.2 Greensolventsusedinextractionandseparationprocesses.
tosaythatthecombinationofbothmeasuresrepresentthemostadvantageousapproach.Significanteffortshavebeenmadeinbothdirections. Substitutionofaharmfulsolventbyamorebenignalternativeis,ofcourse, nottrivialinmanycases,asnovelchallengesandobstaclescanarisedueto thedifferentphysicochemicalpropertiesofthesolventsconsidered.
Watercanbeconsideredasthegreenestsolventfromthewidelistof substancestraditionallyusedinthechemicalindustry.Itisenvironmentally benign,nonflammable,andeasilyobtainableonalargescalewithhigh purity.Remarkably,itsphysicochemicalpropertiescanbetunedbytemperatureandpressure.Thus,watercanevenbeusedforextractionofnonpolar compoundswithnoneedforadditionalorganicmodifiers [16,17].However, waterhaslimitedapplicabilityinchemicalindustryandtheneedforsearchingothergreensolventsisconsideredveryurgent [14].Insomecases,conventionalsolventswithreducedhealth,environmental,andsafetyissues havebeenrecommended.Forinstance,someanalyticalmethodsinvolving extractionswithharmfulsolventshavebeenrevisedsoasalternative,less harmful,solventsarenowadaysrecommended.Forinstance,USEPAmethods413.1and413.2fortestingoilandgreaseinwaterwerereplacedbythe EPAmethods1664Aand1664Badecadeago.Inpractice,Fluorocarbon113,anozone-depletingsubstancecontrolledbytheMontrealProtocol [18], wasreplacedby n-hexaneinthesemethods [19].Inthisvein,therecent effortsmadetowardthedevelopmentofsolventselectionguidesallows choosingappropriatesetsofsolventsforagivenapplicationtakinginto accounttheirenvironmental,health,andsafetyissues,potentialtobe recycled,possibilitytobeobtainedfromrenewablefeedstocks,etc. [20 22]
Thus,organicsolventscanberelativelyeasilyfoundforproblemsolving, wherepolarsolventscanbeapplied.Insuchcasesalcohols,esters,orcarboxylicacidsarefrequentlyincorporated.Thisisespeciallytrueinthecase ofconventionaloxygenatedsolventsthatcanbepreparedfromrenewable feedstocks(e.g.,acetone,methylethylketone,methanol,ethylacetate,and 2-methyltetrahydrofuran) [23].
Recently,thereisatrendtosearchforandapplybio-basedsolvents [24] .Asustainableconversionofbioma sstohighvalue-addedchemicalsis achallengingresearcharea.Biorefine riesaresystemsthatproducechemicals,includingsolventsfromagivenplantfeedstock,usuallyseaweedor wastesfromcertainfoodindustrialprocessesandagriculture [25]
Bio-basedsolventsareincreasinglyofinterestforscientificandtechnologicalapplications.Anumberofplatformmolecules,suchaslacticacid,levulinicacid,furfural,5-h ydroxymethylfurfural,or γ-valerolactone [26],have beenusedforthesynthesisofbio-basedsolvents.Productionofbenign solventsfromrenewablefeedstocki sinagreementwithgreenchemistry principles.Adetailedassessmentoftheirenvironmentalimpactis,however,requiredsincetheirgenerationmayrequiremoreenergeticinputthan obtainingsolventsfromfossils.
8 SECTION|I Introduction
Supramolecularsolventsrepresentappealingsolventsthatconsistin nanostructuredsolventsformedfromminuteamountsofamphiphilemoleculesbyself-assemblyandcoacervation [27,28].Theselectionofamphiphilessignificantlyaffectsthephysicochemicalpropertiesoftheobtained supramolecularsolventsand,inturn,theirapplicability.Thus,supramolecularsolventscanbeconsideredastunablesolventswithpotentialapplicability forextractionofcompoundsoverawidepolarityrange.
Asmanyoftheenvironmentalproblemswithtraditionalsolventsare relatedtotheirvolatility,ionicliquidshavegainedmuchattentionofchemicalsociety.Theirnegligiblevaporpressures,abilitytodissolvemanycompoundsandpossibilitytorecoverthem,goodthermalstability,low flammability,andotherdesirablefeaturesmakethemattractivegreen solvents [29,30].Thegreatamountofcation anioncombinations,offersthe chancetoadjusttheirphysicochemicalpropertiestochemists’requirements. However,itturnedoutthatcertainionicliquidsareevenmoretoxicto aquaticorganismsthantraditionalsolvents,andtheirenvironmentalinertness hasbeenquestioned [31].Theirsynthesisandapplicationthusrequiresmore detailedassessmentprocedures.
Deepeutecticsolvents(DESs)areanotherclassofsolventsthatare concernedgreen [32 34].Theyareobtainedbyusing,atleast,twocomponentsthatbehaveashydrogenbonddonorandacceptor,respectively.DESs havesimilarphysicochemicalparameterstoionicliquidsbutarealsocharacterizedbycertainadvantagesoverthem.Themostimportantarereduced toxicity,tenability,andeaseofsynthesiswithhighefficiency.Remarkably, theycanbeobtainedfromrenewablefeedstockssuchascholinechlorideand carbohydratesoraminoacids.
Switchablesolvents,alsoknownassmartsolventsystems,areappealing solventsthatreversiblychangetheirphysicochemicalpropertiesunderan externalstimulus [35,36].Theadditionorremovalofagas(e.g.,CO2)ora temperaturechangegivesrisetoareversiblereactionthatenablestheformationofsolventswithswitchableproperties.Theswitchingbehaviorofthese solventscanbeexploitedforsolutepurificationinseparationprocessesand facilitatestheirrecycling,thusminimizingwastegeneration.
Finally,supercriticalfluidsandgas-expandedsolventshavealsosignificantlycontributedtogreeningawiderangeofseparationprocesses [37 39]. Anumberofsolventshavebeenusedundersupercriticalconditions.From them,CO2 isthemostcommonlyusedsupercriticalfluidsinceitissafeand environmentallybenign,showsalowcriticaltemperature,highdiffusivity, andaffordability.Furthermore,solvent-freecompoundsofinterestcanbe easilyobtainedafterseparationbymeansofsupercriticalCO2.Amongthe drawbacksofCO2 assupercriticalfluid,itcanbehighlighteditsreduced polarity,whichmakesnecessarytheuseofadditionalorganicmodifiersfor separationofhighlypolarcompounds.Gas-expandedsolventsaremixed solventsconsistinginorganicsolventsdissolvedincompressedgases.
InitialConsiderations Chapter|1 9
Amongthedifferentpossiblecompressiblegases,CO2 isthemostcommonly used.Gas-expandedsolventsarehighlytunablebysimplepressurevariations,showingintermediatepropertiesbetweenliquidsolventsandsupercriticalfluids.Arangeofapplications,includingchemicalreactions,separations, oradvancedmaterials,havebeenreportedintheliteraturebasedontheuse ofthesetunablesolventsystems.
Asbrieflyintroducedinthissection,anincreasingnumberofgreen solventshavebeenreportedintheliterature.Whileseveralofthemarewell establishedindifferentseparationprocesses,evenatindustrialscale,more recentalternativesolventsarestillintheirinitialstages.Design,preparation, characterization,andapplicationofnovelsolventsinscientificandtechnologicalactivitiesthusrepresentachallengingareaofresearch.Greener solventsbrieflydescribedearlierhavebeendiscussedindepthinSectionII, GreenSolvents,ofthebook.
1.4GREENEREXTRACTIONTECHNIQUES
Extractiontechniquesallowtheisolation,purification,and/orenrichmentof targetcompounds,samplecleanup,andtransferofrelevantmoleculesintoa moreconvenientphaseforfurtherprocessing.Variousextractiontechniques havebeenreportedintheliteratureforpretreatmentofliquid,gaseous,and solidmatrices.Conventionalextractiontechniquessuchasliquid liquid extractionandSoxhletextractionhavebeenextensivelyused,forinstance,to theisolationofhighvalue-addedproducts,removalofpollutants,andanalyticalmethoddevelopment [40,41].However,theseextractiontechniquesare nowadaysconsideredinefficient,takingintoaccounttheextendedtimes neededtoextracttargetcompoundswiththerequiredextractionefficiency, andthereducedenrichmentyieldstypicallyachieved.Moreimportantly fromthepointofviewofgreenchemistry,theymakeuseoflargesolvent volumespersampleand,asaresult,largeamountsofwastesaregenerated [42].Severalexamplesofextractionproceduresbasedontheseconventional techniquescanbefoundintheliterature,forinstanceinreferencemethods ofanalysis.Greenerextractiontechniqueshavebeenincorporatedinthelast yearswiththeaimofimprovingextractioncapabilitywhileminimizingand evenavoidingsolventconsumption.Inaddition,theapplicationofmicrowavesandultrasoundsallowsareductionofsolventvolumesconsumedand energysavingsduetoshortenedextractiontimesandmoreefficientenergy delivery [43 46].Thesesystems,commonlyconsideredas“cleanenergies,” arewidelyusedinorganicsynthesis,foodindustryandanalyticalchemistry.
Applicationofmembraneshasalsobeenhelpfulinthereductionof organicsolventsvolumes [47,48].Suchsystemsaredevelopedforindustrial andanalyticalpurposes.Theselectivityachievedbymeansofthevarious typesofmembranesisoneoftheirmainadvantagesinseparationprocesses.
10 SECTION|I Introduction
Supercriticalfluidextractionallowsforselectiveextractionofcompounds fromvariousmedia.ThemostfrequentlyusedCO2 initssupercriticalstate isnontoxic,nonflammable,anditssupercriticalpointisrelativelyeasytobe reached [38,49].Addingmodifierstothesupercriticalfluidallowstheadjustmentofitspolarity,whatextendsitsapplicabilityeventotheextractionof polarcompounds.
Extractiontechniqueshaveenabledthedevelopmentofgreenermethodologiesandprocesses.SectionIII,GreenExtractionTechniques,ofthebook isdevotedtogreenextractiontechniques.
1.5GREENSAMPLINGANDSAMPLEPREPARATION
ThetechniquesdescribedinSectionIII,GreenExtractionTechniques,ofthis bookareappliedinchemicalindustryandinthesmallerscaleforanalytical determinations.However,analyticalchemistryhasitsown,specificwaysfor greeningseparationprocesses,mainlybyeliminationorsignificantreduction ofsolventvolume.
Therearestrategiesappliedatsamplecollectionstagethatdeterminethe environmentalimpactofananalyticalprocedureatitsfurtherstages.The classicalexampleissamplecollectionwithpassivesamplingfollowedby thermaldesorptionofanalytes [50].Similarstrategies,implementedat samplecollectionstage,leadingtogreeningofthewholeprocedureare basedonmicroextractiontechniques.Thesetechniquesallowforseparation ofanalytesevenfromverycomplexbiologicalmatrices [51].Sample pretreatmentisakeystepoftheanalyticalprocessrequiredtoimproveboth thesensitivityandselectivityofagivenanalyticalmethodologythatcommonlyinvolvesrelativelylargeamountsoforganicsolvents.Thus,significantimprovementshavebeenperformedtomakegreenerthiscriticalstep. Theimportanteffortsperformedinthelasttwodecadestowardminiaturizationofconventionalsamplepreparationtechniquessuchasliquid liquid extractionandsolid-phaseextractionresultedintheintroductionofsolventlessorsolvent-minimizedsamplepreparationapproaches [52 54].Solidphasemicroextractionandliquid-phasemicroextractionarenowadayswellestablishedtechniquesthatenabletheenrichmentoftargetcompoundsprior totheirdeterminationbythemoreappropriateanalyticalinstrumentation.In spiteoftheevidentslownessintheimplementationofmicroextractiontechniquesinstandardmethodselection,thenumberofreferencemethods involvingmicronizedanalyticalsystemsisexpectedtoincreaseinthenear futurebearinginmindtheiradvantageousconditionsintermsofmetrology, economy,andenvironmentalimpact.
SectionIV,GreenSamplingandSamplePreparationTechniques,ofthe bookisdevotedtosamplingandsamplepreparationtechniquesthathave significantlycontributedtogreeningtheseimportantstepsoftheanalytical process.
InitialConsiderations Chapter|1 11
Chromatographicandelectrophoreticseparationtechniquesarewidelyused forseparationanddeterminationoftargetcompoundsinmatricesofdifferent complexity.Alargenumberofreferencemethodsofanalysisinvolveseparationtechniquescoupledwithappropriatedetectors.Nonchromatographic methodscansometimesbeusedfordeterminationofrelevantanalyteswitha negligibleconsumptionofsolvents [55].Nevertheless,analyticalseparations canbeunavoidableorevenmoreconvenientthannonchromatographicmethodsincertaincases.Anissuethatcannotbeomitted,however,istheapplicationofsolventsinseparationtechniques,especiallyinthecaseofliquid chromatography(LC).Separationtechniquesimportantlycontributetothe totalsolventconsumptioninanalyticallaboratoriesandlargeamountsof liquidwastesareconsequentlyproduced.Themagnitudeofthisactivityis farfrombeingnegligible.Infact,ithasbeenestimatedthatanamountof wastesofaround150,000tonscouldbegeneratedbyLCsystemsonayearly basis [15,56].Whileeffortshavebeenmainlydevotedtosolventreduction, reuse,andrecycling,recently,muchattentionisgiventoapplygreener solventsasmobilephasesinsteadofconventionalsolventssuchasacetonitrileordichloromethane [57 59].Itshouldbehighlightedthatapartfrom theirabilitytoenablesuccessfulseparationsofagreatvarietyofrelevant compounds,thecompatibilityofalternativesolventswithtypicaldetection systemsusedincombinationwithanalyticalseparationtechniquesisalsoof paramountimportanceandshouldthereforebedemonstrated.Miniaturization ofchromatographicsystems,aswellasreductionofpackedcolumnsdimensionsorapplicationofcapillarycolumnsinLCareeffortstothedecreasing ofmobilephasesconsumption.
AlternativetechniquestoLCallowforseparationofcompoundswithout applicationoforganicsolvents.Supercriticalfluidchromatography [60],gas chromatography [56],andcapillaryelectrophoresis [61] areconsideredtobe greenseparationtechniques,withtheirownadvantagesanddisadvantages,but theyallapplymobilephasesthataremorebenignintheirnature.Thecombinationofthesegreeneranalyticalseparationtechniqueswithsolventlesssamplepreparationapproachesprovidesexcellentpossibilitiesfordeterminationof targetcompoundsattraceandultra-tracelevelswhilefulfillingtheprinciples ofgreenchemistry.Greenaspectsofanalyticalseparationtechniquesare discussedinSectionV,GreenAnalyticalSeparations,ofthebook.
1.7CONCLUDINGREMARKS
Asdescribedintheearliersections,thedesign,synthesisandapplicationof solventswithreducedenvironmental,health,andsafetyhazardsrepresent afundamentalareaofresearchtowardsustainablechemicalprocesses. Thisbookisaimedtoprovideanup-to-dateviewontheapplicationofgreen
1.6SOLVENTSFORANALYTICALSEPARATIONS
12 SECTION|I Introduction
solventsinextractionandseparationtechniques.Importantaspectsregarding morebenignsolventsareaddressed.Inaddition,challengingaspectsderived fromtheuseofgreenersolventsassubstitutesofharmfulsolventsinextractionandseparationprocessesarediscussed.Furthermore,relevantscientific andtechnologicalapplicationsarealsoprovidedinthebook.Fromthe tableofcontentsofthebookitcanbeeasilyseenthatitissubstantially focusedonanalyticalchemistry.Theanalyticalperspectivehasnotbeen presentedyetintheapplicationofgreensolventsinseparationprocesses. Atthesametimewewantedtokeepthebookinterestingfornonanalytical audience.
ACKNOWLEDGMENTS
Atthispointwewouldliketoexpressourgratitudeforthehardworkanddedicationof allcontributorsthatconcurredtothedevelopmentofthisbook.
F.Pena-PereiraacknowledgesXuntadeGaliciaforfinancialsupportasapostdoctoral researcheroftheI2Cprogram.
REFERENCES
[1]E.Linak,S.N.Bizzari,GlobalSolvents:OpportunitiesforGreenerSolvents,2013.
[2]Ceresana,MarketStudy:Solvents,thirded. ,http://www.ceresana.com/en/marketstudies/ chemicals/solvents/.,2014(accessed23.08.16).
[3] R.A.Sheldon,TheEfactor:fifteenyearson,GreenChem.9(2007)1273 1283.
[4] B.Huang,C.Lei,C.Wei,G.Zeng,Chlorinatedvolatileorganiccompounds(Cl-VOCs) inenvironment—sources,potentialhumanhealthimpacts,andcurrentremediation technologies,Environ.Int.71(2014)118 138.
[5]InternationalLabourOrganization.Benzeneconvention:conventionconcerningprotection againsthazardsofpoisoningarisingfrombenzene. ,http://www.ilo.org/dyn/normlex/en/f?p5
NORMLEXPUB:12100:0::NO::P12100_ILO_CODE:C136.,1971(accessed24.07.16).
[6]UnitedNationsEnvironmentalProgramme(UNEP),HandbookfortheMontrealProtocol onSubstancesThatDepletetheOzoneLayer,ninthed.,Nairobi,2012.
[7]Regulation(EC)No1907/2006oftheEuropeanParliamentandoftheCouncilof18 December2006,Off.J.Eur.Union,2006,L396/1 L396/849.
[8]EurostatSustainabledevelopmentintheEuropeanUnion—2015monitoringreportofthe EUSustainableDevelopmentStrategy,2015.
[9] C.O.Tuck,E.Pe ´ rez,I.T.Horva ´ th,R.A.Sheldon,M.Poliakoff,Valorizationofbiomass: derivingmorevaluefromwaste,Science337(2012)695 699.
[10] Y.Gu,F.Je ´ ro ˆ me,Bio-basedsolvents:anemerginggenerationoffluidsforthedesignof eco-efficientprocessesincatalysisandorganicchemistry,Chem.Soc.Rev.42(2013).
[11] P.T.Anastas,J.C.Warner,GreenChemistry:TheoryandPractice,OxfordUniversity Press,NewYork,NY,1998.
[12] P.Anastas,N.Eghbali,Greenchemistry:principlesandpractice,Chem.Soc.Rev.39 (2010)301 312.
[13] W.M.Nelson,Greensolventsforchemistry:perspectivesandpractice,OxfordUniversity Press,Oxford,2003.
InitialConsiderations Chapter|1 13
[14] C.Capello,U.Fischer,K.Hungerbuhler,Whatisagreensolvent?Acomprehensive frameworkfortheenvironmentalassessmentofsolvents,GreenChem.9(2007) 927 934.
[15] F.Pena-Pereira,A.Kloskowski,J.Namie ´ snik,Perspectivesonthereplacementofharmful organicsolventsinanalyticalmethodologies:agenerationofeco-friendlyalternatives, GreenChem.17(2015)3687 3705.
[16] J.Kronholm,K.Hartonen,M.L.Riekkola,Analyticalextractionswithwateratelevated temperaturesandpressures,TrendsAnal.Chem.26(2007)396 412.
[17] R.M.Smith,Superheatedwaterchromatography—agreentechnologyforthefuture, J.Chromatogr.A.1184(2008)441 455.
[18]TheMontrealProtocolonsubstancesthatdepletetheozonelayer.2000(accessed1.2.17).
[19]E.P.A.,Method1664,RevisionA: n-hexaneextractablematerial(HEM;oilandgrease) andsilicageltreated n-hexaneextractablematerial(SGT-HEM;non-polarmaterial)by extractionandgravimetry,Washington,DC,1999.
[20] R.K.Henderson,C.Jime ´ nez-Gonza ´ lez,D.J.C.Constable,S.R.Alston,G.G.A.Inglis,G. Fisher,etal.,ExpandingGSK’ssolventselectionguide—embeddingsustainabilityinto solventselectionstartingatmedicinalchemistry,GreenChem.13(2011)854 862.
[21] M.Tobiszewski,S.Tsakovski,V.Simeonov,J.Namie ´ snik,F.Pena-Pereira,Solventselectionguidebasedonchemometricsandmulticriteriadecisionanalysis,GreenChem.17 (2015)4773 4785.
[22] D.Prat,J.Hayler,A.Wells,Asurveyofsolventselectionguides,GreenChem.16(2014) 4546 4551.
[23] P.J.Dunn,Theimportanceofgreenchemistryinprocessresearchanddevelopment, Chem.Soc.Rev.41(2012)1452 1461.
[24] Z.Li,K.H.Smith,G.W.Stevens,Theuseofenvironmentallysustainablebio-derived solventsinsolventextractionapplications—areview,Chin.J.Chem.Eng.24(2016) 215 220.
[25] D.Esposito,M.Antonietti,Redefiningbiorefinery:thesearchforunconventionalbuilding blocksformaterials,Chem.Soc.Rev.44(2015)5821 5835.
[26] P.Gallezot,Conversionofbiomasstoselectedchemicalproducts,Chem.Soc.Rev.41 (2012)1538 1558.
[27] A.Ballesteros-Go ´ mez,M.D.Sicilia,S.Rubio,Supramolecularsolventsintheextraction oforganiccompounds.Areview,Anal.Chim.Acta.677(2010)108 130.
[28]F.J.Lo ´ pez-Jime ´ nez,M.L.Lunar,M.Sicilia,S.Rubio,Supramolecularsolventsinthe analyticalprocess,Encycl.Anal.Chem.(2014)1 16.Availablefrom: http://dx.doi.org/ 10.1002/9780470027318.a9396
[29] R.D.Rogers,K.R.Seddon,Ionicliquids—solventsofthefuture?Science302(2003) 792 793.
[30] G.Cevasco,C.Chiappe,Areionicliquidsapropersolutiontocurrentenvironmental challenges?GreenChem.16(2014)2375 2385.
[31] M.CvjetkoBubalo,K.Rado ˇ sevi ´ c,I.Radoj ˇ ci ´ cRedovnikovi ´ c,J.Halambek,V.Gaurina Sr ˇ cek,Abriefoverviewofthepotentialenvironmentalhazardsofionicliquids, Ecotoxicol.Environ.Saf.99(2014)1 12.
[32] S.Khandelwal,Y.K.Tailor,M.Kumar,Deepeutecticsolvents(DESs)aseco-friendly andsustainablesolvent/catalystsystemsinorganictransformations,J.Mol.Liq.215 (2016)345 386.
[33] Q.Zhang,K.DeOliveiraVigier,S.Royer,F.Je ´ ro ˆ me,Deepeutecticsolvents:syntheses, propertiesandapplications,Chem.Soc.Rev.41(2012)7108 7146.
14 SECTION|I Introduction
[34]
M.Francisco,A.vandenBruinhorst,M.C.Kroon,Low-transition-temperaturemixtures (LTTMs):anewgenerationofdesignersolvents,Angew.Chem.Int.Ed.Engl.52(2013) 3074 3085.
[35] P.G.Jessop,D.J.Heldebrant,X.Li,C.A.Eckertt,C.L.Liotta,Greenchemistry:reversible nonpolar-to-polarsolvent,Nature436(2005)1102.
[36] P.Pollet,C.A.Eckertabc,C.L.Liotta,Switchablesolvents,Chem.Sci.2(2011) 609 614.
[37] R.M.Smith,Supercriticalfluidsinseparationscience—thedreams,therealityandthe future,J.Chromatogr.A856(1999)83 115.
[38] R.P.F.F.daSilva,T.A.P.Rocha-Santos,A.C.Duarte,Supercriticalfluidextractionof bioactivecompounds,TrendsAnal.Chem.76(2016)40 51.
[39] P.G.Jessop,B.Subramaniam,Gas-expandedliquids,Chem.Rev.107(2007)2666 2694.
[40] D.E.Raynie,Modernextractiontechniques,Anal.Chem.78(2006)3997 4003.
[41] M.D.LuquedeCastro,F.Priego-Capote,Soxhletextraction:pastandpresentpanacea, J.Chromatogr.A1217(2010)2383 2389.
[42] S.Garrigues,S.Armenta,M.D.LaGuardia,Greenstrategiesfordecontaminationofanalyticalwastes,TrendsAnal.Chem.29(2010)592 601.
[43] V.Camel,Microwave-assistedsolventextractionofenvironmentalsamples,TrendsAnal. Chem.19(2000)229 248.
[44] M.Jacotet-Navarro,N.Rombaut,S.Deslis,A.-S.Fabiano-Tixier,F.-X.Pierre,A.Bily, etal.,Towardsa“dry”bio-refinerywithoutsolventsoraddedwaterusingmicrowaves andultrasoundfortotalvalorizationoffruitandvegetableby-products,GreenChem.18 (2016)3106 3115.
[45] F.Chemat,N.Rombaut,A.-G.Sicaire,A.Meullemiestre,A.-S.Fabiano-Tixier,M.AbertVian,Ultrasoundassistedextractionoffoodandnaturalproducts.Mechanisms,techniques,combinations,protocolsandapplications.Areview,Ultrason.Sonochem.34(2017) 540 560.
[46] M.Koubaa,H.Mhemdi,F.J.Barba,S.Roohinejad,R.Greiner,E.Vorobiev,Oilseed treatmentbyultrasoundsandmicrowavestoimproveoilyieldandquality:anoverview, FoodRes.Int.85(2016)59 66.
[47] P.Kuba´ˇn,P.Bo ˇ cek,Micro-electromembraneextractionacrossfreeliquidmembranes. instrumentationandbasicprinciples,,J.Chromatogr.A1346(2014)25 33.
[48] A.Figoli,T.Marino,S.Simone,E.DiNicolo ` ,X.Li,T.He,etal.,Towardsnon-toxic solventsformembranepreparation:areview,GreenChem.16(2014)4034 4059.
[49] M.D.LuquedeCastro,M.M.Jime ´ nez-Carmona,Whereissupercriticalfluidextraction going?TrendsAnal.Chem.19(2000)223 228.
[50] A.Charriau,S.Lissalde,G.Poulier,N.Mazzella,R.Buzier,G.Guibaud,Overviewofthe Chemcatchers forthepassivesamplingofvariouspollutantsinaquaticenvironmentsPart A:Principles,calibration,preparationandanalysisofthesampler,Talanta.148(2016) 556 571.
[51] E.A.SouzaSilva,S.Risticevic,J.Pawliszyn,RecenttrendsinSPMEconcerningsorbent materials,configurationsandinvivoapplications,TrendsAnal.Chem.43(2013)24 36.
[52] J.Pawliszyn,HandbookofSolidPhaseMicroextraction,ChemicalIndustryPress,Beijing, 2009.
[53] J.M.Kokosa,A.Przyjazny,M.A.Jeannot,SolventMicroextraction,TheoryandPractice, Wiley,Horboken,NJ,2009.
[54] F.Pena-Pereira(Ed.),MiniaturizationinSamplePreparation,DeGruyterOpen,Berlin, 2014.
InitialConsiderations Chapter|1 15
16 SECTION|I Introduction
[55] A.Gonzalvez,M.L.Cervera,S.Armenta,M.delaGuardia,Areviewofnon-chromatographicmethodsforspeciationanalysis,Anal.Chim.Acta.636(2009)129 157.
[56] J.Płotka,M.Tobiszewski,A.M.Sulej,M.Kupska,T.Go ´ recki,J.Namie ´ snik,Greenchromatography,J.Chromatogr.A1307(2013)1 20.
[57] F.M.Chardon,N.Blaquiere,G.M.Castanedo,S.G.Koenig,Developmentofatripartite solventblendforsustainablechromatography,GreenChem.16(2014)4102 4105.
[58]
[59]
E.A.Peterson,B.Dillon,I.Raheem,P.Richardson,D.Richter,R.Schmidt,etal., Sustainablechromatography(anoxymoron?),GreenChem.16(2014)4060 4075.
C.J.Welch,N.Wu,M.Biba,R.Hartman,T.Brkovic,X.Gong,etal.,Greeninganalytical chromatography,TrendsAnal.Chem.29(2010)667 680.
[60] A.Dispas,P.Lebrun,P.Sassiat,E.Ziemons,D.Thiebaut,J.Vial,etal.,Innovativegreen supercriticalfluidchromatographydevelopmentforthedeterminationofpolar compounds,J.Chromatogr.A1256(2012)253 260.
[61] M.Koel,Doweneedgreenanalyticalchemistry?GreenChem.18(2016)923 931.
WaterastheFirstChoiceGreen Solvent
KariHartonenandMarja-LiisaRiekkola
UniversityofHelsinki,Helsinki,Finland
2.1INTRODUCTION
Duringthelastdecade,environmentallyfriendly(green)solventshave attractedcontinuingandincreasinginterestincleanchemicalprocessesof biorefineries [1,2],chemicalreactions/synthesis [3],energytechnology [4,5], andchemicalseparations [6].In-depthresearchondifferentgreensolvents, suchasgasexpandedliquids,ionicliquids,supercriticalfluids,andwater, hasgreatlycomplementedthesestudies [7].Althoughcoldorroomtemperaturewaterhasalsomanyinterestingapplications,forexample,intheextractionofbioactivecompounds [8 10],thischapterwillmainlyfocusonthe useofwateraloneasagreensolventindifferentchemicalseparationsat elevatedtemperatures.Namelywater,mixedwithorganicsolventsisalso widelyemployedinmanyseparationtechniquesandprocessestoenhance solvationoflesspolaranalytes.Specialattentionhereisgiventomostrecent developmentsandapplications.
2.1.1WhytoUseWaterasaSolvent?
Molecularstructureofwateranditscapabilitytoformhydrogenbonds (andhydrogen-bondednetwork)resultinuniquephysicochemicalproperties makingwateraninterestingsolventandvitalsubstanceforlife.Ionicand polarcompoundsarereadilydissolvedinwateratambientconditionandless polarcompoundsatelevatedtemperatures.Waterisalsonontoxic,nonflammable,cheap,andwidelyavailableinpureform.Inaddition,itexistsin liquidstateatrelativelyhightemperatures(0 100 C)atatmosphericpressure.Thankstoitsgoodsolvationproperties,waterplaysanimportantrole inthetransportofmanynutrientsandothersubstancesintheenvironment, enablingvariousbiologicalprocesses.Duetoitshighheatcapacity,italso stabilizestemperaturesoforganismsandinvariousregionsoftheearth.
Chapter2
19 TheApplicationofGreenSolventsinSeparationProcesses.DOI: http://dx.doi.org/10.1016/B978-0-12-805297-6.00002-4 © 2017ElsevierInc.Allrightsreserved.
Basedonallthesefacts,itisnowonderthatwateristhemostnaturalchoice forthesolvent.However,aqueouswastegeneratedneedtobecleanedto guaranteethequantityandqualityofourwaterresourcesalsointhefuture.
2.1.2Water—TheMostGreenChoice
Waterisdefinitelythesafestandthemostenvironmentallyfriendlysolvent thatcanbeconsideredforuseassolventindifferent,widelystudied,and developedgreenseparationprocesses.Itisfavorablyassessedinrecentsolvent selectionguides [11,12].Inadditiontoaqueousseparations,waterisalsopopularmediumandreagentinsynthesis/reactions [3,13],oxidationofwaste materials [14,15],andconversionofbiomassintofuelsandchemicals [4,5,16].
2.2SOLVENTPROPERTIESOFWATER
Watercanmostlikelysolubilizemoredifferentkindsofcompoundsthan anyothersolvent.Itssolventpropertiescanbecharacterizedbyitsdipole moment,relativepermittivity εr,whichisameasureofsolutesolventinteractions,andbyitspolarizability π .Forwateratambientconditions(25 C), allthesethreeparametershavehighvalues1.85,78.5,and1.12,respectively [17].Inaddition,forbettersolventcharacterization,oneshouldinclude hydrogenbonddonating(α 5 1.2at25 C)andaccepting(β 5 0.37at25 C) abilitiesincludedinsolvatochromicKamlet-Taftparameters [18].Thevalues listedaboveexplainwhywaterissuchagoodsolventforionicandpolar compounds.However,itshouldbenotedthatatelevatedtemperatures,it becomeslesspolarbeinganalternativetoorganicsolvents.
Ascanbeseenfrom Table2.1 and Fig.2.1,relativepermittivity(dielectricconstant),polarizability,anddegreeofhydrogenbondinginwaterare decreasedwhentemperatureofwaterisincreased.Atthesametime,its viscosityandsurfacetensionaredecreased.Theselargechangesresultin remarkablechangesinsolventpropertiesofwaterthatareveryuniquecomparedtoanyothersolvent.Basically,waterpolarityisdecreasedwith temperature,allowingthefine-tuningofsolventpropertiesofwaterforeach casebyjustadjustingtemperature.
Separationsusingwateratelevatedtemperaturescanbedividedinto differentcategories:(1)hotorhightemperature(HT)waterfromambientto 100 C,(2)pressurizedhot(alsosubcriticalorsuperheated)water(PHW) from100 Cto374 C,and(3)supercriticalwater(SCW).Thisclassification isnotverystrictsincethetermHTisalsooftenusedattemperaturesabove 100 C.Temperatureplaysadominantroleinthedeterminationofthesolvent propertiesofwater,andpressurehasonlyaminoreffect.However,because pressureaffectsgreatlytowaterpropertieswhenthechangeofstatefrom gastoliquidorviceversaoccurs,theusersshoulddistinguishwhetherthe waterisinthestateofliquidorsteam.
20 SECTION|II GreenSolvents
CalculatedWithNIST/ASMESteamPropertiesDatabaseVersion2.01. aAtsaturationpressure. bAt0.1MPaoratsaturationpressure.
Property25 C100 C150 C200 C250 C300 C Density, ρ (g/dm3)1001962.9922.3870.9805.7715.3 Dynamicviscosity, η (mPas)0.890.280.180.140.110.09 Self-diffusioncoefficient, D (m2/s)a [19,20] 2.30 3 10 9 8.36 3 10 9 14.2 3 10 9 21.5 3 10 9 30.4 3 10 9 40.4 3 10 9 Surfacetension, γ (N/m)a 0.0720.0590.0490.0380.0260.014 Relativepermittivity, εr 78.855.944.435.127.320.3 Dissociationconstant,pKw b [21] 13.9912.2511.6411.3111.2011.34 Specificheatcapacity, Cp (kJ/kgK)4.154.194.284.454.795.68
TABLE2.1 ChemicalandPhysicalPropertiesofLiquidWateratDifferentTemperaturesandat10MPaPressure
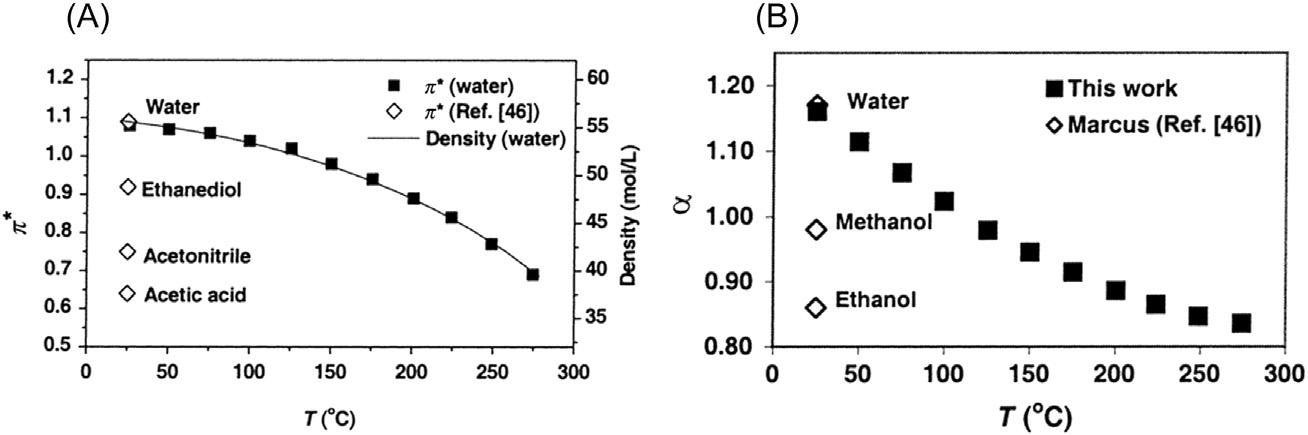
2841 [22].Copyright r 2002American ChemicalSociety.
Forseparationscarriedoutatdifferenttemperaturesinwater,itisvitalto haveinformationondifferentphysicochemicalpropertiesofanalytes.With increasingtemperature,changesinselectivityandsolutetransferthermodynamicswereobservedinreversed-phaseliquidchromatography(RPLC) usingPHWduetodisruptionofhydrogen-bondingnetwork [23].Binary diffusioncoefficientsinPHWweredeterminedforphenoliccompounds bySrinivasetal. [24].Moreover,itisessentialtoknowanalytesolubilities inPHWthatcanbeeasilydeterminedexperimentally,forexample,via correlationwithretentionfactor k byliquidchromatographyutilizingPHW aseluent [25].Comparedtoconventionalseparationcarriedoutin aqueous organicsolventeluent,extrapolationtopurewateristhusavoided inthisapproach.RecentsolubilitydatainPHWareavailablealsoforparabens [26] andforseveralbioactivecompounds [27].Staticpermittivities [28],viscosities [29],andvaporpressures [30] ofpuresolventsandbinary mixturesatHTshavebeendeterminedtoestimatetheirsuitabilityforhightemperatureliquidchromatography(HT-LC).
WhenperformingseparationsusingHTwater,itiscrucialtohaveinformationonthermalstabilityorpossibledegradationoftheanalytes.At elevatedtemperatures,thereactivity(hydrolysis,oxidation)inwateralso increases;thus,causingproblemsinmaterialdurabilityoftheseparation system(stationaryphase,column,tubings,extractionvessel,etc.).
Extractionofthermolabilecompounds(polyphenols)hasbeensuccessfully carriedoutbydynamicextractionat110 Candhighflow-rateof4mL/min leadingtoabout90%recoveries(residencetimeanddegradationwere minimized) [31].Mixtureofwater,ethanol,andformicacid(94:5:1,v/v/v) wasusedasextractionsolvent,andrecoveriesweremuchbetterthanin batch(static)modeorwithconventionalmethanolextraction.Severalstability/ degradationstudieshavealsobeenperformedwithwateratHTs.Coetal. studieddegradationofantioxidantsduringtheirpressurizedhot-water
22 SECTION|II GreenSolvents
FIGURE2.1 Polarizability π ofwater(A)andhydrogenbonddonatingacidity α (B)asa functionoftemperature. ReprintedwithpermissionfromJ.Lu,J.S.Brown,E.C.Boughner, C.L.Liotta,C.A.Eckert,Solvatochromiccharacterizationofnear-criticalwaterasabenign reactionmedium,Ind.Eng.Chem.Res.41(2002)2835
extraction(PHWE)frombirchbark [32].Stabilitiesofpreservativesusedin cosmeticswerestudiedinPHWat100 200 CbyKapalavavietal. [33]. TheynoticedthatPHWupto150 Ccanbeappliedforthechromatographic separationofthesepreservatives.HT(upto150 C)andpHstabilityofstationaryphasesusedinHT-LCwereevaluatedbyHaunetal. [34].Theyconcluded thatethylene-bridgedhybrid(BEH)technologyprovidesstillthemosttemperatureandpHstablesilica-basedcolumns.Thermalstabilitiesofthiazideand otherdiureticsinPHWwerealsostudiedduringchromatographicseparation [35].Insomecases,thedegradationdoesnotcauseanyproblemsormightbe evendesiredespeciallyifPHWisusedforsoilremediationtoextract,e.g., explosives [36] andpolyaromatichydrocarbons(PAHs) [37],inpurification processwheremycotoxinsareremovedinbeverageproduction [38] orwhen newvaluable(e.g.,bioactive)compoundsareformedduringextractionorprocessingwithPHW [39,40]
2.3SEPARATIONTECHNIQUESUTILIZINGWATER
Aswellknown,waterisconventionalsolventinmanyseparationtechniques. Typicallyitisusedincapillaryelectrophoretic(CE)separations.As describedinmoredetailinChapter17,CapillaryElectrophoresisasaGreen AlternativeSeparationTechnique,onlytwoexamplesaregivenherewhere CEhavebeenappliedtooff-lineanalysisofaminesandaminoacidsfrom PHWextracts [41] andalkaloidsfromPHWextractsof Sophoraflavescens Ait. [42].Aqueousphaseseparationsarealsomostcommononesinfieldflow-fractionationforparticles,polymersandmacromolecules [43,44],and manydistillation(hydrodistillationandsteamdistillation)processesaredone inwater,forexample,forthepurificationorisolationofcompoundsoroils fromnaturalproducts(Fig.2.2),andinsomestudiesdistillationiscompared toextractionwithPHW [45,46].PHWassteamstatehasalsobeensuccessfullyusedforcrudeoildistillationwheretheparametersofthedistillation systemwereoptimizedandmodeled [47].Techniques,suchasextraction andliquidchromatography,thatfullyutilizethechangesinsolventpropertiesofwaterwhentemperatureisincreasedaredescribedinmoredetailin thefollowingsubchapters.
2.3.1ExtractionatHTs
ExtractionofhydrophobicorganiccompoundswithorganicsolventcaneasilybereplacedbyPHWE(alsoknownassubcriticalwaterextraction,SWE orsuperheatedwaterextraction).Inadditiontochangesinsolventproperties (discussedin Section2.2)ofwaterwithtemperature,initialthermaldesorptionanddiffusivityoftheanalytesfromsamplematrixareenhanced,thus speedinguptheextractionprocess.PHWEisofgreatinteresttodayasa greentreatmenttechnologyforthebiomasstoisolate/producedifferent
WaterastheFirstChoiceGreenSolvent Chapter|2 23
FIGURE2.2 Greenextractionanddistillationprocessesforisolationofessentialoilsfrom plantmaterials. ReprintedwithpermissionfromA.Filly,A.S.Fabiano-Tixier,C.Louis, X.Fernandez,Waterasagreensolventcombinedwithdifferenttechniquesforextractionof essentialoilfromlavenderflowers,C.R.Chim.19(2016)707 717.Copyright r 2016 ElsevierMassonSAS.Allrightsreserved.
productsandchemicals(biorefineryconcept).Usuallywithbiomass,the extractiontemperaturesforvaluable,bioactiveandthermolabilecompounds havebeen100 200 C [31,48,49],whereastemperatureshigherthan200 C arelessfrequentlyusedandwhenemployed,theextractedanalyteshave beenthermallystable [50 52].SCW(supercriticalfluidextractionisdiscussedinChapter7:SupercriticalFluidsandGasExpandedLiquids)iseven morerarelyusedinextractionduetohighcriticaltemperatureofwater.
Highreactivity(corrosion)ofSCWcanalsolimititsapplications,although corrosioncanbeminimizedbytuningthedensityinSCW.Thecorrosionis worseatPHWjustbelowcriticaltemperaturedespiteofdensity [53] However,interestingseparationsystemfordesalinationwasintroducedby Leanetal. [54],whereSCWwasutilizedtoprecipitatesaltsandothertype ofparticlesandwastematerialintreatedwaterfollowingtheirseparationin spiralseparator.BothPHWathighertemperaturesandSCWaresuitablefor variousreactions,oxidation,andseparationprocesses,wheredegradation ofthecompoundsorbiomoleculesisnotaproblemorevendesired
24 SECTION|II GreenSolvents
(i.e.,reactiveextraction)asmentionedalreadyearlierin Section2.2.InreactivePHWE,oftensomecatalystsorotheradditivesareincluded.SCWcan alsobeusedasapretreatmentofthebiomassorwastematerialtobeprocessed,likefortherecoveryofmetalsfromprintedcircuitboardwaste [15].
AfterthePHWE,thetemperatureoftheextractisoftenreducedresulting indecreasedsolubilitiesoforganicmolecules.Thiscanbebenefitwhen combiningPHWE,e.g.,withsomemicroextractiontechniquestorecover andconcentrateanalytesfortheanalysis.Solid-phasemicroextraction (SPME)asasolventlesstechniqueiswellsuitedforthispurpose [55] and canbeusedfordirectorheadspacesampling.Automatedon-fiberderivatizationhasalsobeensuccessfullyusedinPHWE-SPME-GC-MS/MSofsewagesludge [56].Similarly,stirbarsorptiveextraction(SBSE)hasbeen usefulafterPHWEforaqueousandsolidenvironmentalmatrices [57,58]. Liquid-phasemicroextractionhasalsobeenutilizedforthepreconcentration ofPHWEextractpriortoanalysiseitherbyhollowfibers [59],viaVortexassistedliquid liquidmicroextraction [60] orusingdispersiveliquid liquid microextraction [61].Additionally,monolithiccapillary [62] andmolecularly imprintedpolymer(MIP) [63] havebeenusedfortrappingoftheextracted analytesinPHWE.PHWhasalsobeenexploitedfordesorptioninonline SPE-HPLC [64],desorptionof1,1-bis-(4-chlorophenyl)-2,2-dichloroethene fromnanofibersorbents [65],andforefficientremovalofMIPtemplate moleculesduringpreparationofMIPmaterialwithminimaltemplatebleeding,asshownin Fig.2.3[66].Membranescanalsobeusedinconjunction
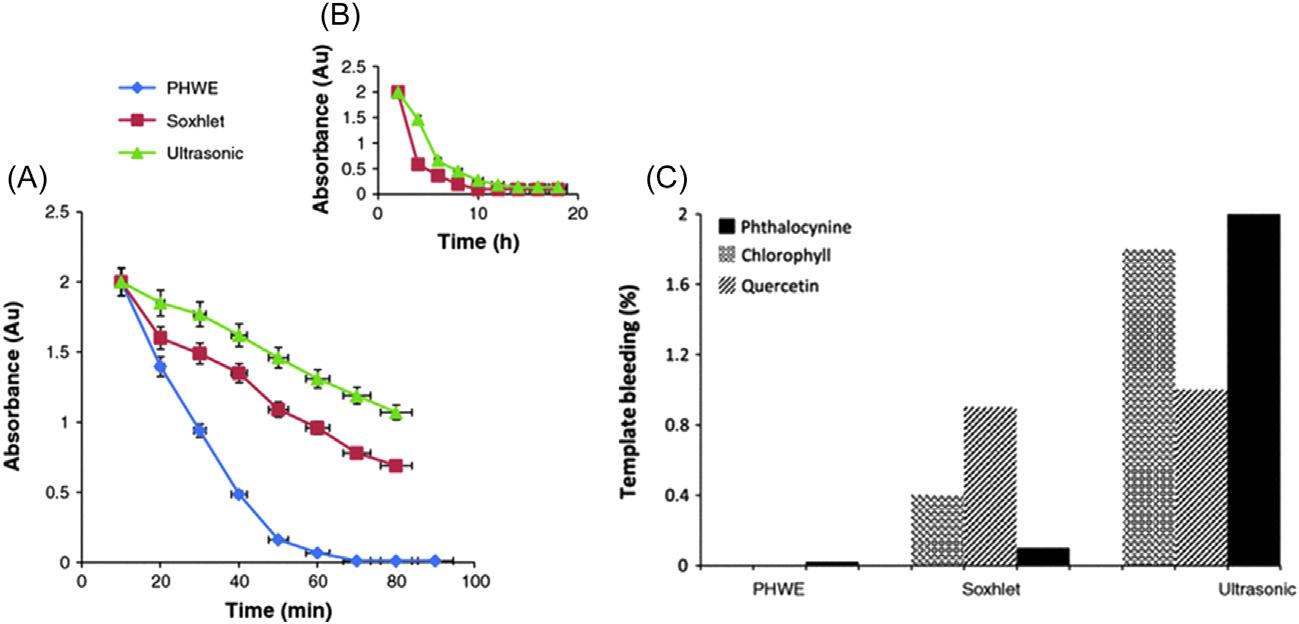
FIGURE2.3 Absorbanceofquercetinineachwashingsolutionat10minintervalsforthe differentwashingtechniques(A),at2hintervalsfortheSoxhletandultrasound(B),andtemplatebleedingafterwashingoftheMIPmaterial(C). ReprintedwithpermissionfromB.S. Batlokwa,J.Mokgadi,T.Nyokong,N.Torto,Optimaltemplateremovalfrommolecularly imprintedpolymersbypressurizedhotwaterextraction,Chromatographia73(2011)589 593. Copyright r 2011Springer-Verlag.
WaterastheFirstChoiceGreenSolvent Chapter|2 25
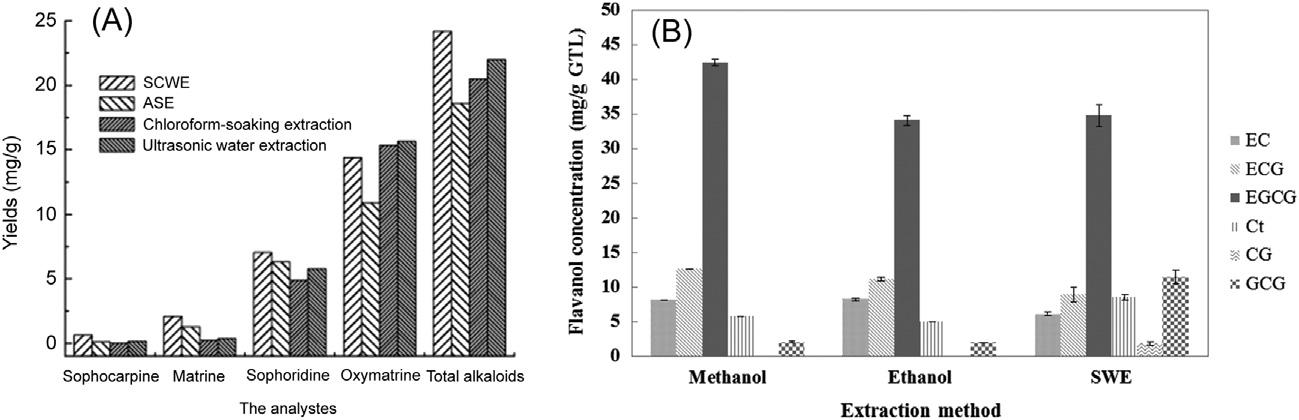
FIGURE2.4 Yieldofalkaloidsin S.flavescens Ait.(A),andflavanolsingreentealeaves (B)withdifferentextractiontechniques.PHWE(SCWE)inpanel(A)wasdoneat100 Candin panel(B)at150 C(SWE),ASEwithethanolat100 C. EC,epicatechin; ECG,epicatechingallate; EGCG,epigallocatechingallate; Ct,catechin; CG,catechingallate;and GCG,gallocatechingallate. ReprintedwithpermissionfromH.Wang,Y.Lu,J.Chen,J.Li,S.Liu,SubcriticalwaterextractionofalkaloidsinSophoraflavescensAit.anddeterminationbycapillaryelectrophoresiswith field-amplifiedsamplestacking,J.Pharm.Biomed.Anal.58(2012)146 151(copyright r 2012Elsevier)andfromM.-J.Ko,C.-I.Cheigh,M.-S.Chung,Optimizationofsubcriticalwater extractionofflavanolsfromgreentealeaves,J.Agric.FoodChem.62(2014)6828 6833. (copyright r 2014AmericanChemicalSociety).
withPHWEbeforetheanalysis,asinPHWE-miniaturizedmembraneassistedsolventextractionofsediments [67] (membraneextractioniscoveredinChapter8:GreenMembraneExtraction).
PHWEcanbeacceleratedinseveralways,suchasbyassistingextraction withmicrowaves [68,69],ionicliquids [70],enzymes [71],additionofethanol [72] orphosphate,andsonication [73].PHWEhasprovidedbetteror equivalentextractionyieldswhencomparedtomanyothertechniques (Fig.2.4),likehydrodistillation [45],sonication,refluxingandleaching [74], Soxhlet [46],solventextractionwithmethanolorethanol [49],andacceleratedsolventextraction(ASE) [61]
2.3.2LiquidChromatographyatHTs
RPLCseparationcanbecarriedoutfasterwithlowerbackpressureby increasingcolumntemperature.Generally,mildtemperaturesupto100 C havebeenusedinseparations.Whatismoreinteresting,moresustainable separationscanbeachievedbyreplacingconcentrationgradient(organicsolvent)withHTortemperaturegradient.Insteadofaddingmethanoloracetonitrileintowater,thesameeffecttoelutionstrengthcanbeobtainedbyraising thetemperaturewhileatthesametimeusingpurewateraseluent.Toachieve polaritychangesneeded,temperaturesfrom100 Cto200 Careoftenrequired. TheupperlimitoftheHTseparationisdeterminedbythethermalstabilities oftheanalytes(discussedearlier)andthestabilityofthecolumnstationary phasematerial.Usually,temperaturesaround200 Ccanbetoleratedby
26 SECTION|II GreenSolvents
zirconiumoxide,polymericorcarbon-basedphasesinadditiontosomeother specificstablephases [75 78].HT-LC,subcriticalwaterchromatographyand superheatedwaterchromatographyaretermsgenerallyusedforHT-LC separations.Inthischapter,thetermHT-LCispreferredandusedfromnow on.InadditiontoRPLC-typeseparations,alsoion-exchangechromatography (IEC)separationshavebeenconductedatHTs [79].
HT-LChasbeensuccessfullyusedwithincreasingnumberofdifferent detectors.Inductivelycoupledplasma massspectrometry(ICP-MS)and inductivelycoupledplasma-atomicemissionspectrometry(ICP-AES; Fig.2.5)havebeenutilizedafterHT-LCtodetectmetals,organometals,and organiccompoundsfromvarioussamplematrices [80 82].Evaporativelight scatteringdetector(ELSD)hasalsobeenusedafterHT-LCforcarbohydrates infoodanalysiswherefast3minseparationswereachievedat150 C [83]. Inthesamestudy,temperaturegradientfrom25 Cto175 Cwasalso employed.ELSDprovidedfivefoldincreaseinresponsewhentemperature wasincreasedfrom30 Cto180 CinHT-LC [84].Atthesametime,much smallertemperaturedependencewasnoticedforcorona-chargedaerosol
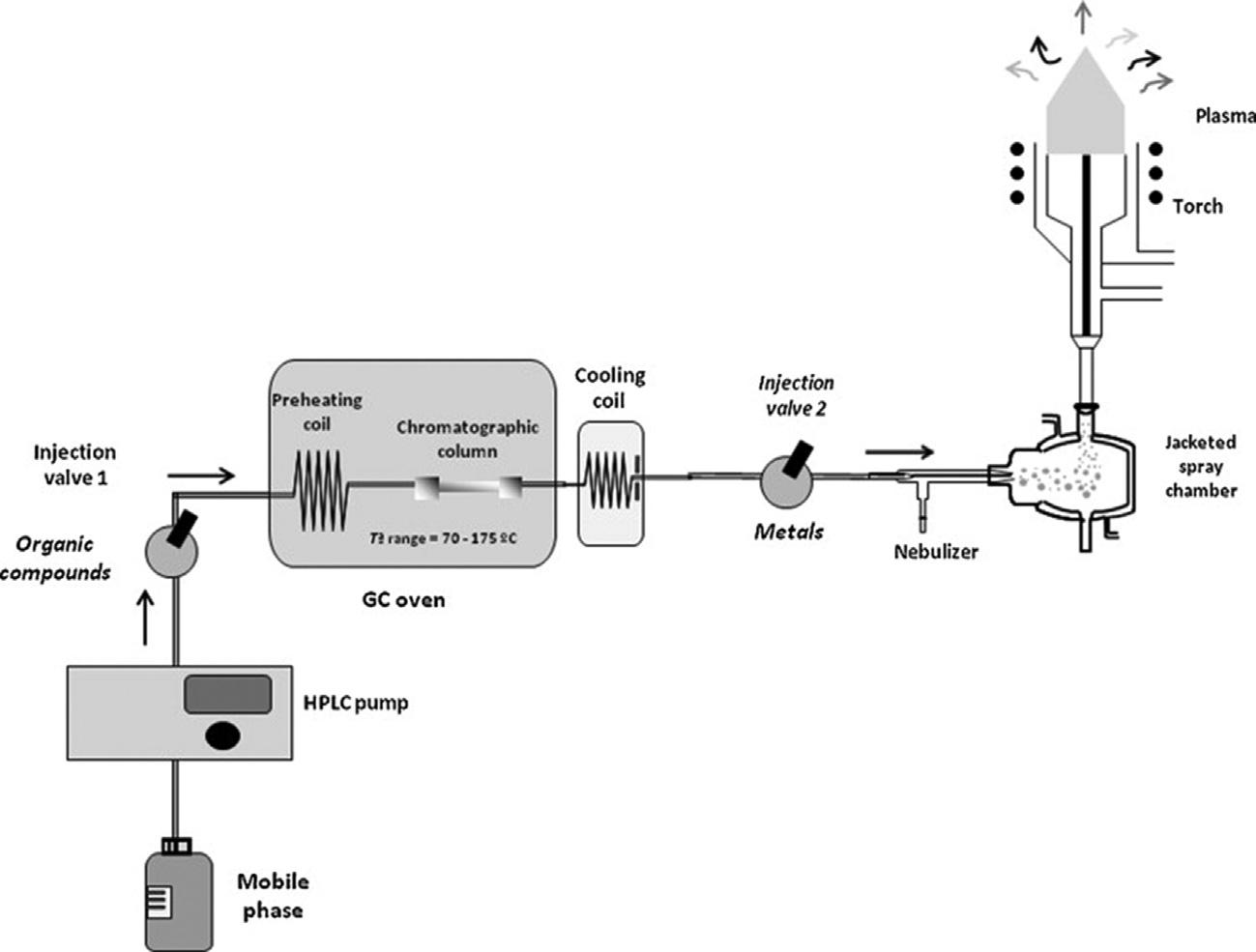
FIGURE2.5 HT-LC-ICP-AESsystemwithpneumaticnebulization. Reprintedwithpermission fromA.Terol,E.Paredes,S.E.Maestre,S.Prats,J.L.Todolı´,High-temperatureliquidchromatographyinductivelycoupledplasmaatomicemissionspectrometryhyphenationforthecombinedorganicandinorganicanalysisoffoodstuffs,J.Chromatogr.A1217(2010)6195 6202. Copyright r 2010Elsevier.
WaterastheFirstChoiceGreenSolvent Chapter|2 27
detector.HT-LCwithelectrosprayionization-MS(ESI-MS) [85] andisotope ratio-MS(IRMS) [86,87] havebeenutilizedfortheanalysisofpharmaceuticals,drugmetabolites,andsteroids,forexample.Asflameionizationdetector(FID)hasearlierbeenfrequentlyemployedasauniversalHT-LC detector,onlysomeattemptshaverecentlybeenmadetoimproveLC-FID interfacedesign [88].
TheperformanceofstationaryphasesatHTiscriticalforthesuccessful HT-LC.Withmonolithiccolumn,excellentcolumnperformancewasmaintainedaftercontinuoususeatover200 Cduring1000columnvolumes [89]
Specialbondedhybridcolumnwasusedfortheseparationofsteroids [90] andzirconia-basedcolumns(oftenusedinHT-LC)wereutilizedforparabensfrom100 Cto180 C [75].Applicabilityofnewstationaryphases, includingpoly(2-hydroxyethylmethacrylate-N-methacryloyl-(L)-histidinemethyl-ester)(NA-PHEMAH)polymericphase [77] andmonodisperse porouspoly(glycidylmethacrylate-co-ethylenedimethacrylate)microspheres [91],hasbeenstudied.NA-PHEMAHwasshowntobestableover500hat 150 Candcouldbeusedevenat200 Cwithpurewaterorfewpercentmethanolinwater.Polymericmicrosphereswerestableat150 Cduring160 injectionsandchromatographicpeakswerefoundsymmetricalwithcolumn efficiencyupto47,000plates/m.
ExperimentaldataobtainedforvanDeemtercurvesatHTswereutilized tofurthercalculatekineticplotsandtooptimizecolumngeometryforHTLC-IRMSanalysisbyErmischetal. [92].Sulfadiazine,sulfamerazine,and sulfamethazinewereusedasmodelcompoundsinthisstudy.
2.4RECENTAPPLICATIONSINEXTRACTION
Today,hotorPHWEiswidelyexploredoptionsinbiorefineriesfortheisolationofdifferentchemicalsorasbiomasspretreatmenttechniques.An exampleofthiskindofprocessisgivenin Fig.2.6.
2.4.1Biorefinery(ChemicalsFromBiomass)
Reactiveextractionorothersimilartreatmentofbiomasstoobtainvarious chemicalsisusuallybasedonthedegradationofitsbiopolymers(lignin,cellulose,hemicellulose).Biomassinconcerncanbewood,plants,grains,etc. Inmostcases,thewastestreamsofthebiomass,suchaswoodbark,knot wood,spentgrains,nutshells,andsoyahulls,areutilized.
Chenetal.found160 CtobetheminimumtemperaturetoremovecarbohydratesfromtheAspenwoodwithPHWE,andat170 Cabout20%ofthe lignincouldbeextractedduring60min [93].Norwaysprucehasbeen extractedwithdynamic(flow-through)PHWEsystemattemperaturesranging from120 Cto240 C [94].Inthisstudy,theextractionofhemicellulosesneeds temperatureshigherthan160 Candashighas220 Ctocompletetheir
28 SECTION|II GreenSolvents
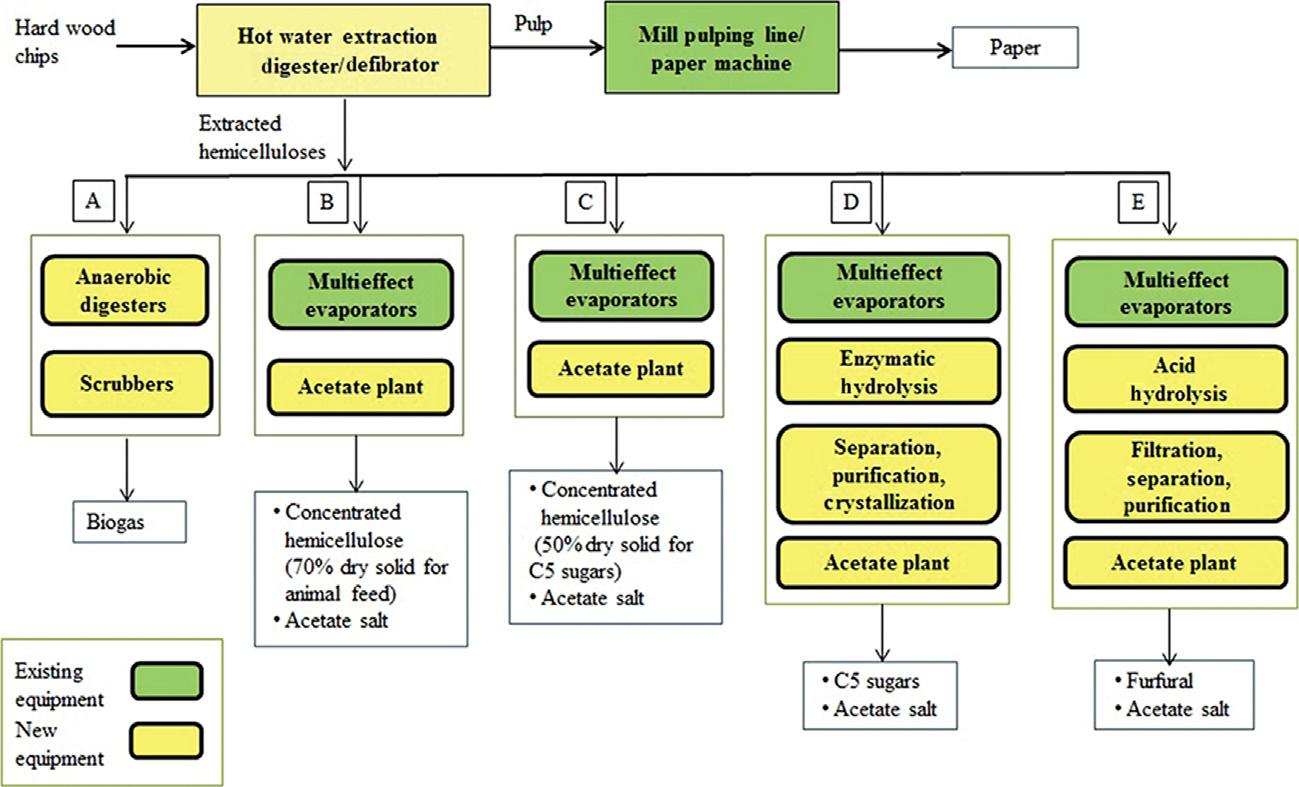
FIGURE2.6 Hotwaterextraction-basedbiorefineryprocessoptions. Reprintedwithpermission fromB.Gilani,P.R.Stuart,Lifecycleassessmentofanintegratedforestbiorefinery:hotwater extractionprocesscasestudy,BiofuelsBioprod.Bioref.9(2015)677 695.Copyright r 2015 SocietyofChemicalIndustryandJohnWiley&Sons,Ltd.
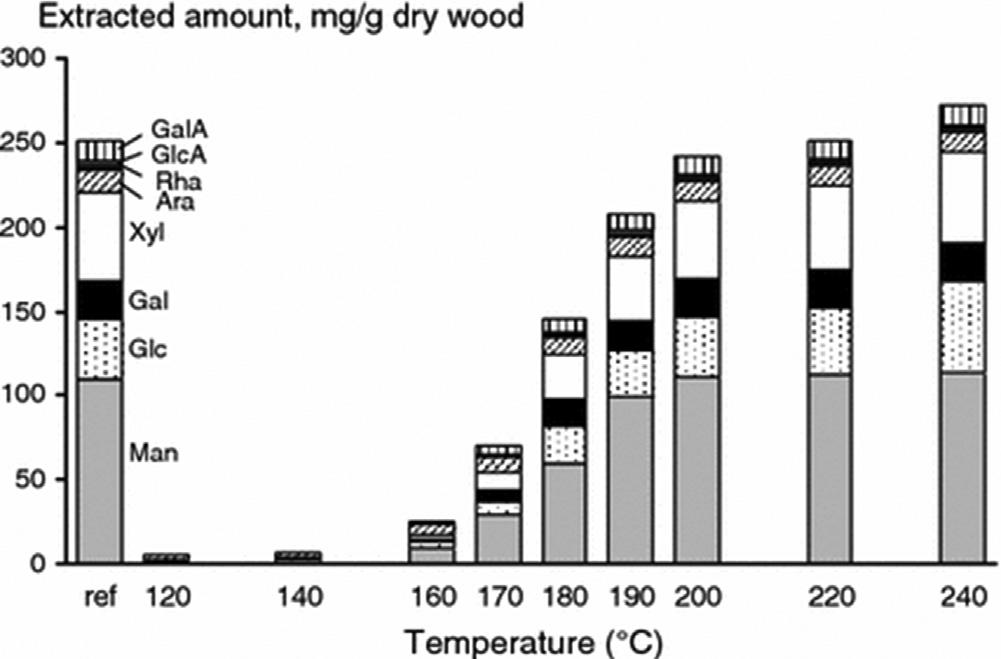
FIGURE2.7 AmountsandcompositionofcarbohydratesfromtheNorwayspruceobtained withPHWEatdifferenttemperatures. ReprintedwithpermissionfromK.Leppanen,P.Spetz, A.Pranovich,K.Hartonen,V.Kitunen,H.Ilvesniemi,PressurizedhotwaterextractionofNorway sprucehemicellulosesusingaflow-throughsystem,WoodSci.Technol.45(2011)223 236.
Copyright r 2011Springer-Verlag.
extraction(seetherecoveryofcarbohydratesin Fig.2.7).Fifteenpercentof ligninwasalsoremovedat220 C.Itwasconcludedthathemicellulosesshould beisolatedat170 180 Candhighertemperature,suchas200 C,shouldbe usedforethanolproductionsincesomehydrolysistomonosaccharideswill
WaterastheFirstChoiceGreenSolvent Chapter|2 29
alreadyoccur.Two-stagePHWEmethodwaslaterusedbyPranovichetal. [95] torecovergalactoglucomannansfromgroundsprucesapwoodat170 C. With1hextractiontime,about25%ofthewoodcouldbedissolved.They alsofractionatedthePHWextractfurtherwithorganicsolventsandobtained hemicelluloseswithsmallamountofpectins(83 90%oftheprecipitated material).Contentofthegalactoglucomannanswas80%.Larchwoodwaste hasalsobeenutilizedasbiomasstostudyisolationofphenoliccompounds withPHWE [96].Thehighestextractionyieldwasobtainedat150 C,butthe phenoliccontentwashighestat300 C.Neitheradditionofethanolnor increaseofextractionflowrateaffectedthephenolicamount.Economicsof thepilotandindustrialscalePHWEprocesseswerealsoevaluatedinthis study.Extractorwith350Lcapacitywasfoundtobethemosteconomical withdailyproductionof1200kgat300 C(221USD/kg).Extract(21.6% yield)contained71.5%phenoliccompoundsattheseconditions.
Microwave-assistedPHWEhasbeenappliedtoextractmannansfrom usedcoffeeground [69] andtorecoverarabinoxylansandarabinoxylooligosaccharidesfrombrewers’spentgrains [97].Five3-minextractionsteps at200 Cwereneededformaximumof69%recoveryofmannans.The remaininginsolublematerialfromcoffeegroundcontainedmainlycellulose (84%),andthebestyield(43%)forthearabinoxylansandarabinoxylooligosaccharideswasobtainedat210 C.
PHWEat160 Cfor2hhasbeenusedtostudychemicalcompositionof apricotpitshells [98].Highyield(77%)wasachievedforxylanandabout 24%ofligninwasalsorecovered.Smallincrease(1.6%)intheenergy ofcombustionoftheapricotpitshellwasalsoobtainedduringthe PHWEtreatment.Agro-industrialresidue,soyahulls,wasrecentlysubjectedto phosphate-assistedPHWEat121 Cwithultrasoundtreatment [73].Surface activepectinwasefficientlyrecoveredbythistechniquewiththebetteryieldand puritycomparedtothetraditionalacidextraction.PHWextractcontainedmore proteinmoieties,thusprovidingbetteremulsifyingandfoamingproperties.
PHWEtreatmentat160 Candelectronbeamirradiation(1000kGy) werealsoevaluatedintheprocessingofsugarmaple [99] priorpyrolysisGC/MSanalysis.PHWEtreatmentincreasedtheglucancontentby24%and decreasedxylancontentby54%.Levoglucosan,hydroxyacetaldehyde, 5-hydroxymethylfurfural,andfurfuralyieldswereincreasedbyPHWtreatmentandtherecoveriesofaceticacid,hydroxyacetone,andotherketones weredecreased.Additionally,PHWEincreasedtheyieldsofbenzene,toluene,andxyleneby46%,35%,and26%,respectively.
PHWEofcornstoverhasbeenusedtofractionatehemicellulose [100], andabout70%ofthetotalxylosewasrecoveredat160 Cafter210min. Duringtheextraction,0.9g/Lfurfuraland0.1g/L5-hydroxymethyl 2-furaldehydeweregeneratedfrompentoseandhexoseundertheabove-mentioned conditions.Bleachabilityofthepulp,extractedfromcornstoverbyPHW, wasimproved.
30 SECTION|II GreenSolvents
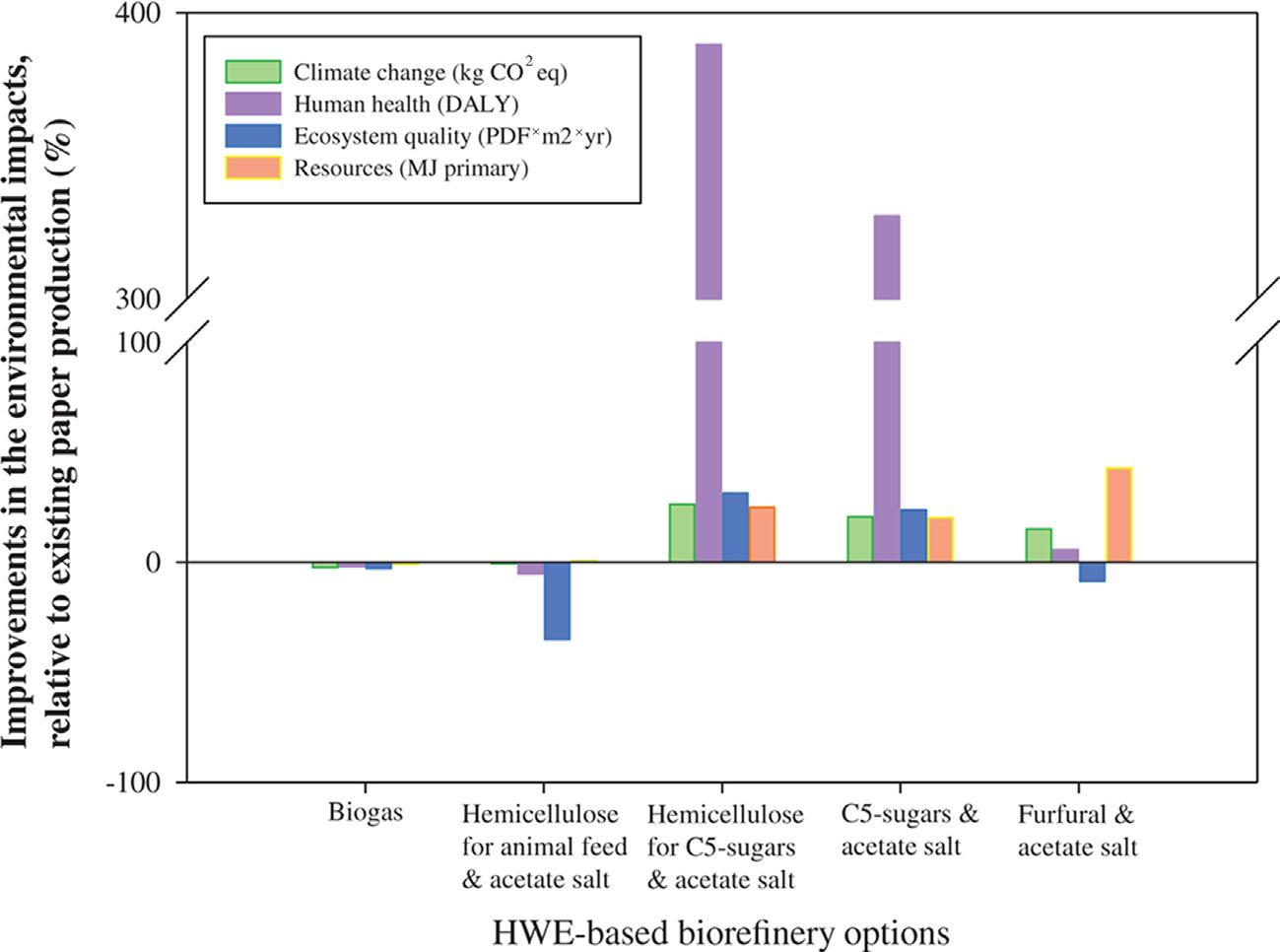
FIGURE2.8 EnvironmentalimprovementsoftheHWE-basedbiorefineryoptions. Reprinted withpermissionfromB.Gilani,P.R.Stuart,Lifecycleassessmentofanintegratedforestbiorefinery:hotwaterextractionprocesscasestudy,BiofuelsBioprod.Bioref.9(2015)677 695. Copyright r 2015SocietyofChemicalIndustryandJohnWiley&Sons,Ltd.
LifecycleassessmentforthePHWEprocessinforestbiorefineryhas alsobeenperformed [101].Theresultsindicatedthatbarkconsumption, chemicals,andbioproductstransportationhadsignificantenvironmental impacts.PHWEprocesstoproduceC5sugarsorhemicelluloseforC5sugars outperformedotheralternativeswithagreenhousegasreductionof68%and 80%,respectively.Thesetwoalternativesimprovedalsosignificantlytheir environmentalimpacts(Fig.2.8).
2.4.2BioactiveCompounds
Hot-waterextraction(HWE)andPHWEhaveextensivelybeenstudiedwith thepurposetogetvariousbioactivecompoundsfromdifferentbiomasses, suchasplants,fruits,andbarkoftree.Oftenwastematerialstreamsofthese biomasseshavebeenstudiedaspotentialsourcesforthesevaluablechemicals.PHWEprocessfordifferentphytochemicals(carbohydrates,phenolics, andantioxidants)inpotatopeelwasoptimizedbyAlvarezetal. [102]. HigheramountsofphytochemicalswereobtainedwithPHWE(190 C, 40bar)thanwithaqueousethanolextraction.
WaterastheFirstChoiceGreenSolvent Chapter|2 31
Betulinicacidisavaluablecompoundthathasawiderangeofmedicinal activities.PHWEwasdemonstratedtobeexcellenttechniquetoextractbetulinicacidfromouterlayerofbirchbark [74].Betulinicacidyieldwas 2.8mg/gbirchbarkextractedat184.5 Cfor27.4minwithsolvent/solidratio of59.6mL/g.Barkof Shorearoxburghii G.Don.hasalsobeenextracted withPHWinordertoobtaintrans-resveratrolwhichisknowntohaveanticancer,antifungal,anti-inflammatory,andantiagingactivities [103] Resveratrolisofteninthenatureintheformoftrans-piceid,andat190 C, 10MPaandwithflow-rateof3mL/min,recoverieswere23.2and 350.3 μg/gfortrans-resveratrolandtrans-piceid,respectively.
HighcytotoxicactivityinthePHWextractsofginsengleaf/stemwas noticedinthestudyofLeeetal. [104].Sampleswereextractedat190 Cand theactivityagainsthumancancercelllineswashigherthanthatofethanol extracts.Highcorrelationwasalsoobservedbetweencytotoxicactivityand flavonoidcompositionofthesample.
PHWextractobtainedat110 C(10min)of Brassicajuncea hadahigh antiviralactivityagainstinfluenzavirusA(H1N1)asshownin Fig.2.9[105]. Thisextracthasagreatpotentialasantiviralfoodsupplementinnonfatmilk.
β-Glucansareassociatedwiththereductionofpostprandialbloodglucose andcholesterollevels.ThesecompoundswereprocessedwithPHWEfrom waxybarleyat155 Cand50barfor18min [106].Extractionyieldwas53.7% andgreatreductionwasobtainedinextractiontimeandaboutfourtimes increaseinmolecularweightof β-glucancomparedtoconventionalprocess.
Differentantioxidantshavebeenstudiedfromnumeroussamplematrices (biomasses)usingPHWE.Plazaetal. [107] extractedflavonolsatvarious
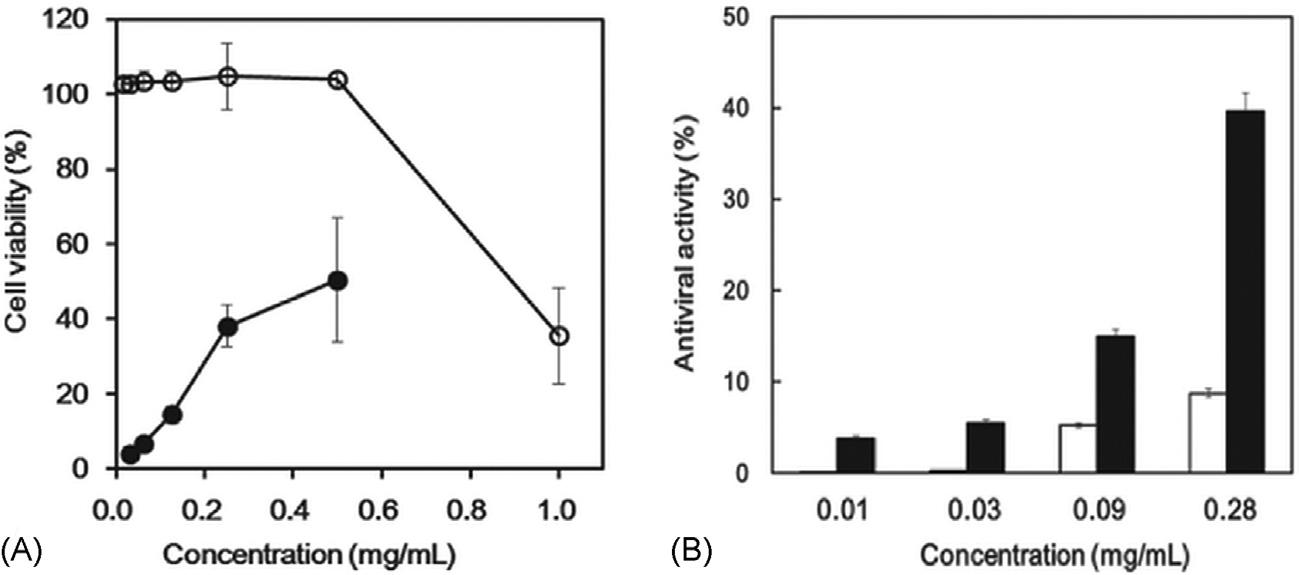
FIGURE2.9 (A)Antiviralactivity(K)andcellviability(x)ofPHWextractof Brassicajuncea againstinfluenzavirusA(H1N1)oncaninekidneycells.(B)Antiviralactivityofnonfat milk(&)andnonfatmilksupplementedwithdifferentamountsofPHWextractof Brassicajuncea (’)oncaninekidneycells. ReprintedwithpermissionfromN.-K.Lee,J.-H.Lee,S.-M.Lim, K.A.Lee,Y.B.Kim,P.-S.Chang,H.-D.Paik,Shortcommunication:antiviralactivityofsubcriticalwaterextractofBrassicajunceaagainstinfluenzavirusA/H1N1innonfatmilk,J.DairySci. 97(2014)5383 5386.Copyright r 2014AmericanDairyScienceAssociation.
32 SECTION|II GreenSolvents












