
























$700,000 + In Decompression Research Funds Raised
8 New Patent Applications 2021-2023
5 New Patent Applications in Progress
Advanced by Team of 5 Engineers
3x Inc. 5000 Recipient 2022-2024
3x USF Fast 56 Recipient 2022-2024
ISO 13485-2016 Certified Medical Device Designer & Manufacturer
FDA Registered & Audited Medical Device Establishment
1,200+ Locations Using DRX9000
5 Universities Using DRX9000
Presence in 50+ Countries 18k+ YouTube Subscribers w/ 3.5M + Views 1 Million + Facebook Fans
“I was shocked to be able to reproduce the same efficacy seen in our retrospective analysis, to take patients who has had chronic back pain for greater than a 10 year duration and over the course of 2 weeks....50% reduction in pain and that continued to improve over the final 6 weeks of therapy to a level that is almost baseline, almost zero in pain.”
- John B. Leslie, MD, MBA Mayo Clinic Arizona
“I’ve been treating patients with DRX9000 for approximately 20 years, I have been very impressed with the clinical results of the DRX treatment; 90% of the patients have significantly improved! Theses improve ments are seen in pain reduction and visible improvements in all areas of the disc The DRX9000 will put the disc back in, it will help increase the disc height, it will help improve the space of the spinal cord and the spinal canal. The DRX9000 also helps decrease central spinal canal stenosis, primarily by bringing back the disc where it belongs ”
- William R. Martin, MD Stanford Alumni
“After experiencing a DRX9000 treatment at a conference, I immediately decided that this is something I have to implement into my practice. I have owned two other spinal decompression units in the past and nothing came even close the the comfort and smoothness of the DRX9000. The DRX has the most comfortable and controlled pull that I have ever experienced in my years of practice! Since replacing my other spinal decompression units my patients experienced a remarkable improvement in their outcomes. I highly recommend anyone that hasn’t physically tried the DRX to try a treatment, only then will you under stand why its the most advanced in the industry
- Sunny Kim, MD Johns Hopkins Alumni
DRX9000®
'599(E722R(S!?TT T?U U? #$% & '# V4532R(S!?T!F?@!/?!///
At Excite Medical, our mission is to enhance the quality of life for patients worldwide by providing cutting-edge, non-surgical spinal decompression solutions. Since our founding, we have been driven by a passion for innovation, quality, and excellence. Our flagship device, the DRX9000, now trusted by over 1,200 healthcare centers and leading universities around the globe, stands as a testament to our commitment to advancing healthcare.


We are proud to work with renowned institutions such as the University of South Florida, where our research partnerships push the boundaries of medical technology and science. This relentless pursuit of knowledge ensures that our products are not only e ective but are supported by evidence-based research, contributing to improved patient outcomes.
As we continue to grow, our focus remains steadfast on delivering the highest quality medical devices and o ering exceptional customer support. With the dedication of our talented team, partners, and healthcare providers, we strive to make a lasting, positive impact in the medical field.
Thank you for your continued trust in Excite Medical.
Sincerely,
Saleem Musallam Founder & President, Excite Medical
Saleem N. Musallam
Mr. Saleem Musallam, a Johns Hopkins-educated Healthcare System Engineer, is the CEO of Excite Medical and US Orthotics, both FDA-registered medical device manufacturers. In addition to leading these companies, he serves on the University of South Florida’s Board of Medical Engineering and the Board of Directors for the Florida Medical Manufacturers Consortium, representing over 900 FDA-registered medical device manufacturers. Mr. Musallam is also the Chairman of the Board of Advisors for Excite Medical, where he continues to guide the company’s vision and innovation in the medical device industry. Board Members:
Michael Foreit, DO
Dr. Michael Foreit, a board-certified Family Physician with extensive experience in Occupational Medicine, brings valuable expertise to Excite Medical’s Board of Advisors.
Born in South Chicago and raised in Northwest Indiana, Dr. Foreit earned his Bachelor of Science degree from Loyola University before obtaining his Doctor of Osteopathic Medicine degree from the New York Institute of Technology College of Osteopathic Medicine in 1994. After completing his postdoctoral training at The Chicago College of Osteopathic Medicine/Midwestern University, he served as Director of Occupational Medicine at St. Margaret Mercy’s Lake Ridge Clinic. In 2003, Dr. Foreit co-founded Comprehensive Care with Dr. Frank Messana, demonstrating his commitment to advancing healthcare. His deep experience in family and occupational medicine provides Excite Medical with invaluable insight and leadership.
Samson Jarso, PhD
Dr. Samson Jarso has an impressive academic and professional background. He completed his Ph.D. in Biophysics at the University of California, Berkeley, followed by a postdoctoral fellowship at Johns Hopkins University in the FM Kirby Center. Dr. Jarso then joined the junior faculty in the Department of Radiology at Johns Hopkins University. His work later expanded to focus on biomedical engineering and global health, where he has contributed to healthcare strengthening and capacity building in various settings around the world.
Bruce Langone
Mr. Bruce Langone brings a wealth of experience and leadership. He holds a Master’s degree from NYU in Interactive Telecommunications and is a U.S. Marine Corps veteran, having served as an Infantry Platoon Commander with the 1st Battalion, 7th Marines.
With over 30 years of experience on Wall Street, Bruce currently serves as President of Invemed Securities, where he manages multi-manager investment portfolios, oversees private placement activities, and provides strategic advisory in Power Systems. His diverse background adds significant value to Excite Medical's advisory board.
Matthew Montoya, PhD
Dr. Matthew Montoya serves as a mentor-advisor, instructor, researcher, and professor at The Johns Hopkins University in the Systems Engineering, Healthcare Systems Engineering, and Lifelong Learning programs, having received both the JHU Distinguished and Outstanding Instructor Awards. For 35 years, Dr. Montoya was also a Principal Chief Engineer and Executive Portfolio Director at The Johns Hopkins University Applied Physics Laboratory, where he directed multi-million and billion-dollar systems projects related to national security and healthcare. His extensive educational background includes degrees in physics, mathematics, systems engineering, public health, and business from prestigious institutions such as Colorado State University, The Johns Hopkins University, Loyola University of Maryland, Dartmouth College, and The George Washington University.
Mark Sanna, DC
In addition to serving on Excite Medical's Advisory Board, Dr. Mark Sanna is a board member of the Foundation for Chiropractic Progress. He earned his undergraduate degree from Auburn University and his Doctor of Chiropractic degree from the Northeast College of Health Sciences. As the President and CEO of Breakthrough Coaching, Dr. Sanna has been instrumental in advancing practice management for healthcare providers. His leadership and extensive experience provide invaluable insights to Excite Medical, reinforcing its commitment to innovation in the medical field.
Nathen Schilaty, DC PhD
Dr. Nathan Schilaty, is a valued member of Excite Medical's Board of Advisors. He serves as the Lincoln Endowed Chair of Chiropractic & Biomechanics Research and Assistant Professor of Neurosurgery & Brain Repair and Medical Engineering at the University of South Florida. After earning his Doctor of Chiropractic degree from Parker University in 2007 and practicing for five years, Dr. Schilaty pursued a PhD in Neuroscience, followed by postdoctoral training at Ohio State University and Mayo Clinic. His research, funded by the NIH and other sponsors, focuses on injury prevention and rehabilitation, with a passion for expanding chiropractic research globally.
Establishment: Excite Medical of Tampa Bay, LLC
4710 Eisenhower Blvd Suite A-12
Tampa, FL 33634 – USA
Registration Number: 3008816697
FEI Number: 3008816697
Owner/Operator Number: 10057708







Michael Foreit, DO
Dr. Michael Foreit, DO, serves on Excite Medical's Medical Advisory Board, bringing decades of clinical expertise to the team. As a board-certified Family Physician with extensive experience in Occupational Medicine, Dr. Foreit's career includes directing occupational health programs and co-founding Comprehensive Care. His background in patient care, leadership, and operational oversight provides Excite Medical with strategic insights into improving patient outcomes. His commitment to advancing healthcare aligns with Excite Medical's mission of delivering non-surgical spinal decompression solutions to healthcare providers globally.
Sunny R. Kim, MD
Dr. Sunny Kim is the founder and president of Progressive Rehabilitation Medicine, a nationally recognized clinic in Cedar Rapids, IA, specializing in non-surgical and natural chronic pain relief. He earned his medical degree from Rutgers New Jersey Medical School, completed an internal medicine fellowship at Johns Hopkins University/Sinai Hospital, and a residency in Physical Medicine and Rehabilitation (PM&R) at the Rutgers/Kessler Institute, where he served as Academic Chief Resident. Board certified and a fellow of the American Academy of Physical Medicine and Rehabilitation, Dr. Kim is also a leader in regenerative medicine and trains physicians nationwide.
William R. Martin, MD
Dr. William Martin is a distinguished interventional radiologist and the founder and CEO of Upper Valley Interventional Radiology in McAllen, TX. He graduated from the University of Utah School of Medicine in 1976 and completed his residency in diagnostic radiology at Stanford University in 1980. Dr. Martin co-authored the first major study on non-surgical spinal decompression, which was published in the Journal of Neurosurgery in 1994. His work in advancing spinal care and his dedication to minimally invasive procedures continue to benefit patients across the Rio Grande Valley.
Alaa Montaser, MD, PhD
Dr. Alaa Montaser is a highly accomplished neurosurgeon at the Mayo Clinic with an impressive record of over 50 published research articles. His contributions to the field of neurosurgery reflect a deep commitment to advancing medical science and improving patient outcomes. Dr. Montaser's expertise spans a wide range of neurosurgical procedures and research, positioning him as a leader in the field and a vital asset to the medical community.
Devang Padalia, MD
Dr. Devang Padalia is a fellowship-trained interventional pain medicine physician with over 16 years of experience, holding dual board certifications in anesthesiology and pain medicine. He specializes in complex pain management, with expertise in spinal and vertebral pathologies, automotive injuries, and cancer pain management. Dr. Padalia serves as an Associate Professor at the University of South Florida, where he has been honored as “Educator of the Year” three times. Widely published in pain medicine, he has trained over 50 physicians and has presented at prestigious medical conferences across the country.
Dr. Nathan Schilaty, DC, PhD, serves on Excite Medical's Medical Advisory Board, where his vast research background and clinical expertise play a key role in advancing non-surgical spinal care. As the Lincoln Endowed Chair of Chiropractic & Biomechanics Research and Assistant Professor at the University of South Florida, his research spans neuroscience, biomechanics, and neuromechanics, with a focus on injury prevention and rehabilitation. With over 100 publications and more than 1,200 citations, Dr. Schilaty's contributions help guide Excite Medical in developing evidence-based, innovative treatments.
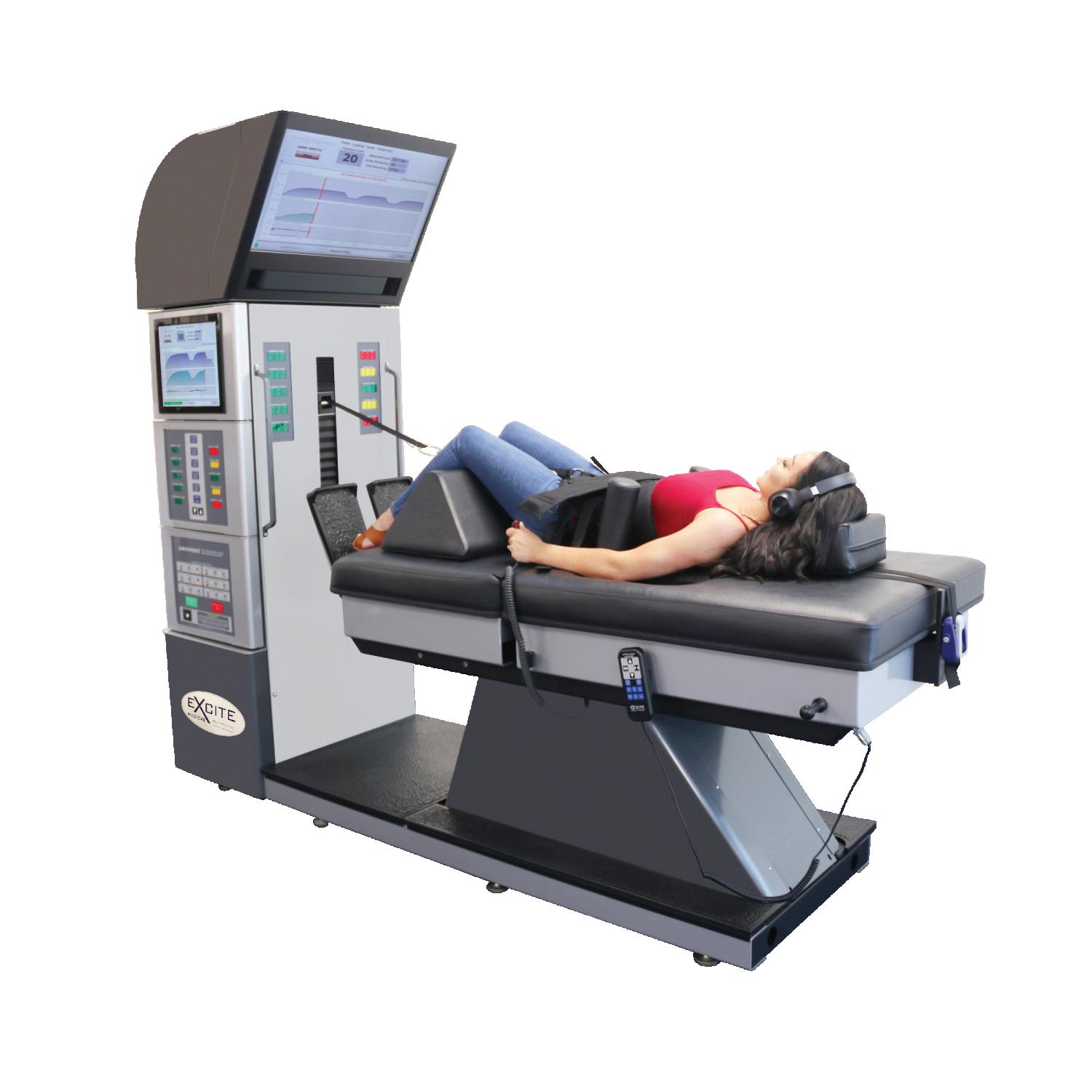
True Non-Surgical Spinal Decompression System ®
The DRX9000 ® True Non-Surgical Spinal Decompression System ® created to assist healthcare providers in their effort to treat lower (lumbar) back pain.
The DRX9000 ® True Non-Surgical Spinal Decompression® provides a primary treatment modality for the management of pain and disability for patients suffering with incapacitating low back pain and sciatica.
The DRX9000 ® True Non-Surgical Spinal Decompression System ® is designed to provide pain relief for compressive and degenerative injuries of the spine. Through the application of spinal decompressive forces to these injuries, the DRX9000 ® has given patients relief from back pain and has allowed them to resume the activities they love.

Advanced System Diagnostics
Constantly monitors the device’s critical subsystems.
Bluetooth Audio Headphones
Allows patient to listen to music, educational video, music, or movie.
Automatic Shoulder
Support System
Improves patient positioning and comfort.
DRX® Cervical Pillow
Provides patient comfort and allows patient to view DVD.
Floating Lower Mattress
Allows natural elongation of patient’s spine.
DRX® Knee Rest
Improves patient comfort.
Lumbar Selector
Adjusts system to proper angle for
No-Slip Harness Tensioner
patient harness to bed.
Operator Remote Hand Control
Allows healthcare provider to easily position patient.
Patient Media System
Provides healthcare provider a tool for advanced patient education through multimedia player.
Patient Safety Switch
Gives patient the ability to immediately stop treatment if necessary
Platform Scale
Weight scale data transfers directly into treatment computer.
Power On Switch & Emergency Stop
15-amp circuit breaker rocker switch
initializes system. Stop button immediately releases tension and pressure.
Table Positioner
Automated vertical & horizontal bed tilt positioning.
PCAP Touch Screen Computer w/ Windows 10 Based DR X® Software
Easily select treatment parameters without the need of a separate keyboard and mouse.
Treatment Dynamics
Highly visible displays - monitor treatment from across a room
Treatment Positioner & Tensioning Cable
Therapeutic forces are delivered to the patient through the tensioning cable, which can be raised and lowered by the treatment positioner.
PCAP Touch Screen
Computer
Lumbar Disc
Level Selector
Advanced System Diagnostics
Power On Switch & Emergenc y Stop
Platform Sc ale
Floating Lower Mattress
Patient Media System
BOSE® Sound Bar
Treatment D ynamics
Knee Rest
Cervical Decompression Device
T he DR X9 000 C® can be a dded to any e xistin g
DR X9 000 ® True Non-Surgical Spinal Decompression System® or purchased as a combination system. The DR X9 000 C® relieves patients’ neck (cervical) pain through a detachable headrest.
Excite Medical’s DRX9000C® is an effective option for treating:
• Herniated discs
• Degenerative disc disease
• Bulging discs
• Posterior Facet Syndrome


The DRX9000C provides patients a program of treatments for relief from neck pain. Application of cyclical forces, decompress intervertebral discs, achieving pain relief associated with herniated discs, protruding discs, degenerative disc disease, posterior facet syndrome, and bulging discs.
Cervical Disc Selector
Adjust system to proper angle for speci c cervical disc treatment.
Detachable Headrest
Features interchangeable conforming head supports, real-time
ultra-precise tension feedback system
Additional headrest features include:
• Automatic Cervical Isolator
• Automatic Locking System
• Open design doesn’t grab jaw
Patient Safety Switch
Gives patient the ability to immediately stop treatment if necessary
Treatment Dynamics
Highly visible displays - monitor treatment from across a room
Power On Switch & Emergency Stop
15-amp circuit breaker rocker switch initializes system Stop button immediately releases tension and pressure.
Touch Screen Computer
Easily select treatment parameters without the need of a separate keyboard and mouse.
Treatment Positioner & Tensioning Cable
Therapeutic forces are delivered to the patient through the tensioning cable, which can be raised and lowered by the treatment positioner.


Cervical Decompression Device
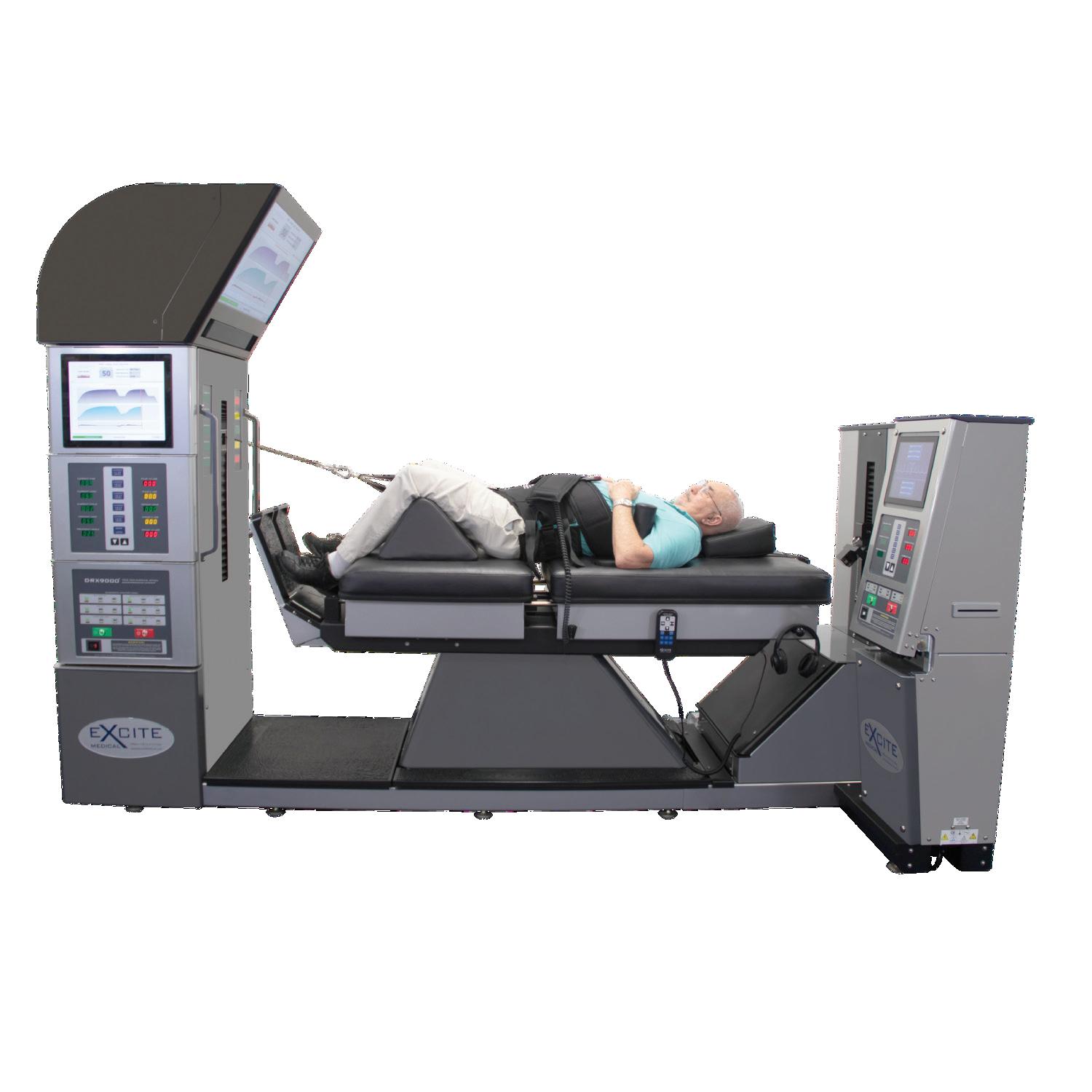
T he DRX9500 ® is a stand-alone system with an integrated bed that provides a comprehensive approach to relieving neck (cervical) pain.
With the DRX9500 ®, healthcare providers can treat patients’ neck pain caused by:
• Herniated discs
• Degenerative disc disease
• Bulging discs
• Posterior Facet Syndrome


The DRX9500® provides patients a program of treatments for relief from neck pain. Application of cyclical forces decompresses intervertebral discs, achieving pain relief associated with herniated discs, protruding discs, degenerative disc disease, posterior facet syndrome, and bulging discs.
Cervical Disc Level Selector Adjusts system to proper angle for speci c cervical disc treatment.
Headrest features:
• Conforming head support
• Automatic Cervical Isolator
• Automatic Locking System
• Open design doesn’t grab jaw
Treatment Dynamics Highly visible displaysmonitor treatment from across a room.
Patient Safety Rails Assists patient entry and exit.
Patient Safety Switch Gives patient the ability to immediately stop treatment if necessary.
Platform Scale
Weight scale data transfers directly into treatment computer.
Power On Switch & Emergency Stop
15-amp circuit breaker rocker switch initializes system. Stop button immediately releases tension and pressure.
Operator Remote Hand Control
Allows healthcare provider to easily position patient.
Table Positioner
Automated vertical & horizontal bed tilt positioning.
PCAP Touch Screen Computer w/ Windows 10 DRX® Software Easily select treatment protocol without the need of a separate keyboard and mouse.
Treatment Positioner & Tensioning Cable
Therapeutic forces are delivered to the patient through the tensioning cable, which can be raised and lowered by the treatment positioner.

The DRX9000® True Non-Surgical Spinal Decompression System is designed to provide pain relief for compressive and degenerative injuries of the spine. Through the application of spinal decompressive forces to these injuries, the DRX9000® patients relief from back pain and has allowed them to resume the activities they love.
The DRX9000® True Non-Surgical Spinal Decompression System provides relief of pain and symptoms associated with herniated discs, bulging or protruding intervertebral discs, degenerative disc disease, posterior facet syndrome, and sciatica The therapy is non-invasive and non-surgical.
The theory behind spinal decompression is a process whereby forces are applied to the spine in a manner that maximizes spinal elongation Spinal elongation is maximized when paraspinal muscles, the muscles that guard the spine from injury, are relaxed. When paraspinal muscles relax, applied spinal decompressive forces spread apart the bony vertebra of the spine. This relieves pressure on nerves and intervertebral discs Where this spinal elongation occurs, pressure drops within the disc which facilitates movement of fluid, carrying nutrients and oxygen inside the disc. Additionally, the reduction in pressure can help draw in herniated disc fluids, reducing the size of the herniation


Spinal decompression on the DRX9000® involves the application of forces along a treatment curve to elongate the spine without causing the muscles that guard the spine to contract. The technology required to apply spinal decompressive forces is very advanced
Spinal decompression involves application of forces, logarithmically to elongate the spine without causing the muscles that guard the spine to contract The technology required to apply spinal decompressive forces precisely is very advanced. Engineering research and development efforts involved in the evolution of the True Non-Surgical Spinal Decom pression System® have been ongoing since 2001!


Located below the Pelvic Harness is a pnumatic pump and air bladder system. It is this air bladder that maintains the lordotic curve and acts as a fulcrum to the angle of the pull. The pull is key to the DRX9000® ’ s technology because the pull is generated on a logarithmic curve. Research indicates that by using a logarithmic curve, propriocepter response is avoided. Therefore, there are no muscle spasms, allowing for decompression in the targeted lumbar disc to occur

The DRX9000® utilizes high speed treatment computers to calculate the logarithmic spinal decompression treatment curve for each patient A servo-motor/servo- amplifier consistently checks (several thousand times per second) and corrects theservomotor’s movement Measurement devices inside the DRX9000 monitor changes in decopressive force experienced by each patient All of This data is constantly fed back into the treatment computers. The treatment computers constantly calculate corrections and ensure the therapy is true to each patient’s logarithmic curve. This constant monitoring, measuring, and correcting process is called a Nested Closed-Loop Feedback System This methodology is one of the hallmarks of the DRX® technology suite
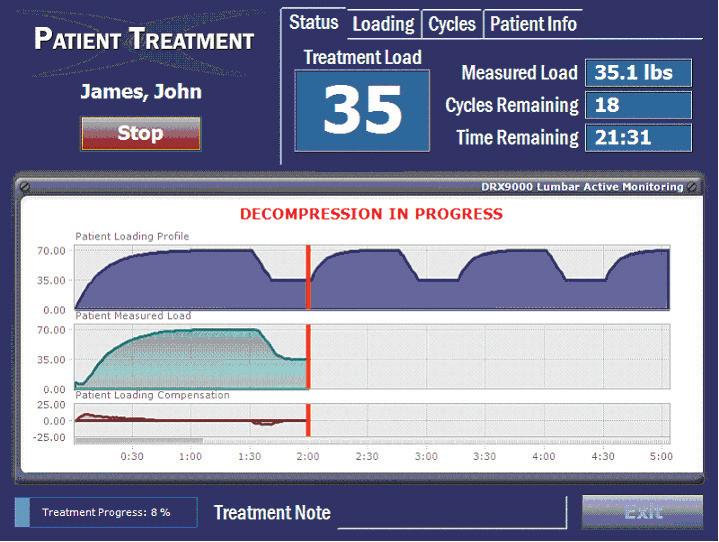
All of Excite Medical’s DRX ® ® devices contain Nested Closed- Loop Feedback Systems that ensure therapy stays true to each patient’s logarithmic spinal decompression treatment curve. Closed-loop feedback can be defined as an automatic control system that can adjust and self-correct its operation according to differences between the actual and desired output Closed-loop feedback systems improve overall system performance. Closed-loop feedback can be explained by relating it to a common example: The temperature control system that heats and cools your home. Using this example, your preferred temperature is set on the thermostat control, which continuously compares the actual temperature reading of your thermometer with your set-point Whenever the thermometer reading is different than the set point, the generator of warm or cool air is activated
As a result of this adjustment action, the temperature in your home is adjusted in the direction of your setpoint This continuous closed-loop adjustment cycle ensures that your home stays at your determined temperature, keeping you comfortable and allowing you to conserve energy The effectiveness of closed-loop feedback systems vary based on the variables measured, how they’re measured, and how often they’re measured. With respect to spinal decompression or traction systems, the measured variable is most often force, which is the amount of tension used to distract the intervertebral segments of the spine. In systems where the lower back is forced to move with the table or motor, fixed measurements are made between the motor and the patient. In systems that incorporate a floating lower mattress, force is usually measured via the tension strap. How often feedback is monitored varies significantly between currently marketed devices These rates range from several times per minute, to several times per second, to thousands of times per second

The DRX ® device series uses an accurate (±0.01lb) force measurement device to record force on the tension strap during treatment This measurement is sent to the treatment computer and is used to calculate corrective spinal decompressive forces The treatment computer sends corrective force commands 13x per second to the servo amplifier and servo-motor, which then apply the spinal decompressive forces to the patient The closed-loop feedback system runs from the force measurement device, to the treatment computer, to the servo-amplifier / servo- motor and back again, 13x per second. Therapy on the DRX ® series of spinal decompression devices takes this concept one step further.
The DRX ® device series incorporates a closed-loop feedback system within a closed-loop feedback system, referred to as nested closed-loop feedback. As described above, one of the three stops on the closed-loop feedback path in the DRX ® devices is the servo-amplifier and servo-motor. The servo amplifier receives corrective force commands from the treatment computer 13x per second. The servomotor itself contains accurate measurement devices that measure position, speed, and power consumption The servo-amplifier monitors these variables, correcting the actions of the servo-motor 4,000x per second. This explains the nested closed loop feedback difference that separates the DRX® series from all other decompression devices
To summarize, the DRX ® device series contains a closed-loop feedback system that updates the logarithmic force curve 13x per second. Within that closed-loop feedback path exists an additional closed loop feedback path that updates the logarithmic force curve 4,000x per second.
Together, these closed-loop feedback paths form a nested closed-loop feedback system The nested closed-loop feedback system is one of the technological hallmarks of the DRX ® device series
By utilizing a servo-amplifier and servo-motor system, Excite Medical’s DRX ® series of spinal decompression equipment is capable of staying true to the logarithmic force curve. Development of the servo-motion system is an ongoing effort involving both Excite Medical’s technical staff and engineers from Danaher-Motion Danaher-Motion’s Kollmorgen Servo Amplifier and Motor and Thompson Planetary Gearhead couple the spinal decompressive forces to the patient
Excite Medical and Danaher-Motion have formed an exclusive relationship in the spinal decompression / traction market, ensuring consistency and quality and a level of expertise that can only be found in the DRX ® device series


DRX9000® Dimensions:
Length ................ (Overall) 113.00 in (288cm)
Width 32.50 inches (83cm)
Height .................. 85.50 inches (218cm)
Weight ................. 1,280 lbs. (580 kg)
Crated Weight ...... 1,730 lbs. (784 kg)
3.1m x 3.7m

in (321cm)
DRX9000® with DRX9000C Dimensions:
Length ................ (Overall) 126.00 in (312cm)
Width ................... 32.50 in (83cm)
Height .................. 85.50 in (218cm)
Weight ................. 1,780 lbs. (807 kg)
Crated Weight ...... 2,530 lbs. (1147 kg)


DRX9500® Dimensio
Length ................ (Overall) 112.00 in
Width ................... 52.00 inches (133c
Height .................. 57.00 inches (145c
Weight 850 lbs. (385 kg)

Crated Weight 1,150 lbs. (521 kg)


in (145cm) s: (Overall) 35.50 in (91cm) m) m)
500 lbs. (226kg)
Crated Weight ...... 800 lbs. (362kg)
115V Unit Voltage
(VAC)
NOTE:
Unit
(VAC)
An agency-approved cord appropriate for the target country will be provided
Only plug the supply cord into a hospital-grade, voltage matched receptacle that is properly grounded and polarized.
Leakage Current
UPS
DRX9000® True Non-Surgical Spinal Decompression System weight (mass)
Less than 500 micro amp, with power on, forward or reverse polarity
Ground resistance less than 0.1 ohm
1000VA Minimum 10 minute operation Recharge to 80% of capacity in 4.5 hours
1280 lbs. (581 kg.)
The UPS may discharge during prolonged shipment or storage times, or if the machine was disconnected from AC power for more than two months. Where possible, connect the machine to AC power when not in use for extended periods of time.
Type of protection against electric shock
Class I / Internal electric power source
Degree of protection against electric shock Type BF
Mode of operation Continuous
Degree of protection against ingress of liquids Ordinary
Recommended methods of sterilization or disinfection
Refer to Maintenance Section 8 of the DRX9000® True Non-Surgical Spinal Decompression System Operator’s Manual
Degree of safety of application in the anesthetic mixture with air, oxygen or nitrous oxide.

The device complies with the requirements of IEC 601-1-2. The following basic EMC standards were applied to verify conformance.
Environment
Emissions
Immunity
IEC 601-1-2.
CISPR 11 Group 1, class B
IEC 1000-4-2, 8kV, 3kV contact
IEC 1000-4-3, 3V/m
IEC 1000-4-4, 2kV power, 500V sensor cable
IEC 1000-4-5, 2 kV line to earth, 1kV line to line
Symptoms of possible electromagnetic interference include sudden variations in the measured load graph or weight scale display that do not correspond to patient movement or normal, expected load measurements. If interference from external sources is suspected, discontinue treatment and move suspected electrical sources farther away from the machine until the interference ceases.
Excit e M edica l has gon e to great len gt hs to esta blis h relatio ns hi ps wit h inno vati ve tec hno lo gy s uppli ers t hat s ha re o ur vision . Fo r t hat reason , on ly com po ne nts t hat me et o ur st rin gent q ua lity sta nda rds a re inco rpo rat ed into any Excit e pro duct Excit e is pro ud to ha ve di rect relations hi ps wit h t he fo llo win g feat ure pa rtn ers :
Alt ra In dust ria l Motion is a premi er glo ba l desi gn er an d pro duc er o f a wi de ran ge o f motion cont ro ls so lutions . Wit h c los e to 1 0,000 em ploy ees an d o ver $1 .7 bi llion in reven ue, Alt ra is on e o f t he la rgest in its i ndust ry in t he wo rld. Excit e purc has es its Ko llmo rgen S ervo - moto r an d Boston Gea rbox di rect ly from Alt ra Ko llmo rgen ’ s s ervo moto r pro ducts is uti li zed by t he DRX pro duct s eri es to precis ely replicat e fo rc e a pplication inst ructio ns from t he t reatm ent com put er. T he Boston -bran d matc hin g plan eta ry gea rhea d devi ces pro vi de smoot h an d effici ent gea rin g di rect ly lin kin g t he s ervo-moto r to t he pati ent In 2001 , Ko llmo rgen en gin eers helped i nco rpo rat e t he s ervo-motion syst em into t he DRX9 000® T he Ko llmo rgen S ervo Moto rs a re man ufact ured in Vi rginia , US A an d Boston Gea rbox man ufact ures in No rt h Ca ro lina , U SA.


Ni ppon Bea rin g (NB), is hi ghly reco gni zed an d wi dely s uppo rt ed glo ba lly a s a pion eer o f lin ea r motion pro ducts man ufact urer. C ritica l mo vin g com pon ents gli de a lon g on NB precision bea rin g t ec hno lo gy in t he DRX ® ® pro duct s erie s. NB’s res ea rc h an d develo pm ent , as well as pro duction is bas ed in Nii gata , Ja pan
Lina k di rect ly pro vi des Excit e M edica l wit h 6 di fferent ac tuato rs t hat can be fo un d in t he DRX9 000 an d DRX9 000C 5 o f t hes e act uato rs a re ma de in St Lo uis , M O US A an d 1 is im po rt ed from Denma rk. Lina k has s ubsi dia ri es in mo re t han 35 co unt ri es wo rldwi de an d is wi dely consi de re d an int ernatio na l inno vato r in elect ric act uato r t ec hno lo gy .
ADV ANC ED Motion Cont ro ls ® ® ® ® (AMC ) desi gns , man ufac tures an d suppli es hi gh perfo rmanc e s ervo dri ves t hat Excit e M edica l uti li zes to cont rol t he s ervo -moto r fo un d in t he DRX syst ems AMC is hea dq ua rt ered in Cama ri llo , C A, U SA, where it man ufact ures t he s ervo dri ves t hat Excit e M edica l purc has es
We also offer our customers the tools to help market our products to increase patient volume and practice revenues
B y com b inin g c re ati ve t h in k in g, insi gh t ful st r at eg y an d le a d in g-edg e t e c h nolo g y , we a re a ble to pr o v i de effe cti ve int egr at ed bu sin e s s solutions.
Here are just a few of the many marketing tools we offer:
•
• Print Ads
• Brochures
• Postcards
• Billboards
• Editorials
• Press Releases
• Posters/Displays
• Yellow Page Ads
• Direct Mail Campaigns
• Educational Report
• PowerPoint Presentation
• Tradeshow Booth Design and Development
• Consultation Video
• Customized Promotional Tools
• Complete Media Buying Services
• TV Commercials: 30 & 60 Second
• 30 Minute Infomercials
• Spanish Commercials
• Custom Voice Overs
• DRX® Website Development
• Landing Page Campaigns
• Ongoing Consulting







3D Patient Educational Videos DRX9000®
Research Book




Subscribe to our YouTube channel to be Video Uploads
Like our DRX9000® Facebook page where we have over 1 million fans!




