5 minute read
Mara Riminucci
Seed International Research Project (Last year report)
CELL MECHANICAL PROPERTIES OF INFECTED ERYTHROCYTES IN THE MATURATION OF PLASMODIUM FALCIPARUM TRANSMISSION STAGES IN THE BONE MARROW NICHE INVESTIGATED WITH A HUMANIZED MOUSE MODEL
Advertisement
MARA RIMINUCCI Istituto Pasteur Italia Department of Molecular Medicine mara.riminucci@uniroma1.it
CATHERINE LAVAZEC Institut Pasteur Paris
RESEARCH AREA: INFECTIOUS AGENTS AND ASSOCIATED DISEASES
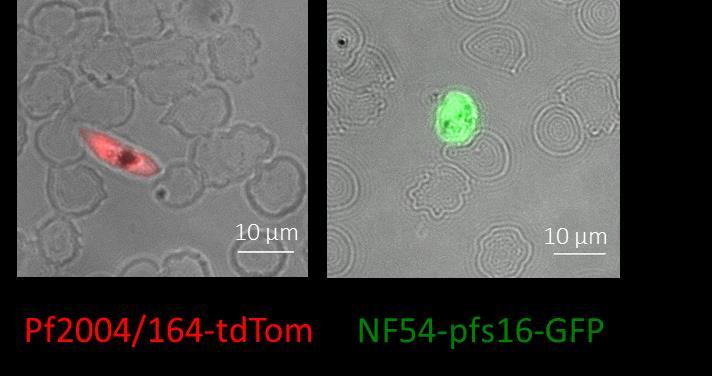
We established an in vivo experimental model to investigate the behavior of the human malaria parasite Plasmodium falciparum within the human bone marrow microenvironment. The model is based on the infusion of transgenic parasites producing fluorescent gametocytes in mice bearing heterotopic human bone marrow organoids/ossicles (Bone Marrow Humanized Mouse, BMHM). By using this model, we analysed the homing and distribution of immature P. falciparum gametocytes in the human bone marrow microenvironment and started to investigate their interaction with the local cell components. Two P. falciparum transgenic lines, the Pf2004/164-tdTom line and the NF54-pfs47pfs16-GFP line, were preliminarily analysed for the effect of histology processing on the fluorescent signal. Comparable levels of fluorescence, Tomato Red and GFP respectively, were detected in immature gametocytes after treatment for 24 hours with 4% formaldehyde (Figure 1) and the Pf2004/164-tdTom line was selected for further work.
Figure 1. Representative images of immature gametocytes of P. falciparum acquired by fluorescence microscopy after treatment with 4% paraformaldehyde for 24 hours. Both Tomato Red and GFP fluorescence signals remain detectable after exposure to the fixative.
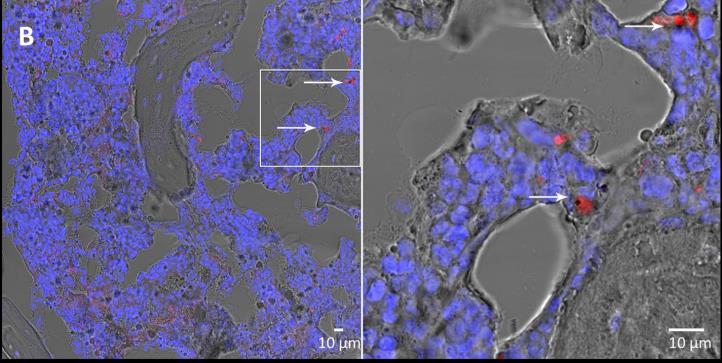
Pf2004/164-tdTom immature gametocytes (7,5x106) were injected into the tail vein of ossiclebearing mice. Multiple experiments were performed in which the mice were sacrificed at three different time points (10 minutes, 1 hour and 24 hours) after the injection of the fluorescent gametocytes. The ossicles were harvested, fixed with 4% paraformaldehyde for 24 hours, decalcified with 4% EDTA and embedded in optimal cutting temperature (OCT) compound. Histological sections were analysed by conventional light microscopy and by confocal microscopy. Through transmitted light microscopy, P. falciparum immature gametocytes were identifiable for the crescent- or sausage-shape and for the presence of the black pigment. Through confocal microscopy, they were easily recognizable by the red fluorescence and by the presence of the black pigment. Specifically, P. falciparum immature gametocytes were readily detectable within and outside marrow blood vessels at all the different time points (Figure 2) thus demonstrating that they home to the chimeric ossicle and migrate in the humanized extravascular marrow space.
Figure 2. Confocal microscopy images of 10 µm histological sections of formalin-fixed, OCTembedded ossicles harvested from the BMHM model at 10 minutes (A), 1 hour (B) and 24 hours (C) after the injection of immature gametocytes. P. falciparum gametocytes are indicated by arrows (blue stain = nuclei).

In this set of experiments, we also assessed the distribution of the gametocytes in different mouse organs such as liver, lung, spleen, kidney, femur and brain. Tissue samples were processed for OCT embedding and multiple sections were scanned by confocal microscopy. Gametocyte specific-red fluorescence was identified in the extravascular space in the liver, spleen and femur, while the analysis of brain, lung and kidney did not reveal the presence of gametocytes (data not shown). To investigate in detail the behaviour of the P. falciparum immature gametocytes in the bone marrow microenvironment, we refined the protocol for histological analysis by changing the embedding medium. Ossicles harvested at the three different time points were fixed with 4% formaldehyde for 24 hours, decalcified with 4% EDTA and embedded in a gelatin embedding solution (EBM, Kusumbe et al, Nat Protoc. 2015) to better preserve tissue architecture and to achieve a higher morphological resolution. In these samples, we started to analyse in detail the migration of the gametocytes within the ossicle and their interaction with human non-hematopoietic bone marrow cells, in particular bone marrow adipocytes. To this end, EBM-embedded samples were immunostained with an antiserum against fatty acid binding protein 4 (FABP4), which is expressed by both endothelial cells and adipocytes. We showed that at 10 minutes after the infusion, the majority of P. falciparum immature gametocytes are found in the perivascular region (within 10 m from the blood vessel wall) (Figures 3 and 4) and that their distance from blood vessels increases over time (Figure 4).

Figure 3. Confocal microscopy of formalin-fixed, EBM-embedded ossicles harvested at 10 minutes and immunostained with anti FABP4 antibody (green). Ten minutes after injection, tomato gametocytes within the blood vessels are rare, (A), whereas they are numerous in the perivascular space (B) (bv = blood vessels).
100
F r e q u e n c y
50
10min 1h 24h
0
10
40
Distance from vessels (mm) Figure 4. Histogram showing the frequency of P. falciparum immature gametocytes within 10 and 40 m from marrow blood vessels at different time points after intravenous inoculation.
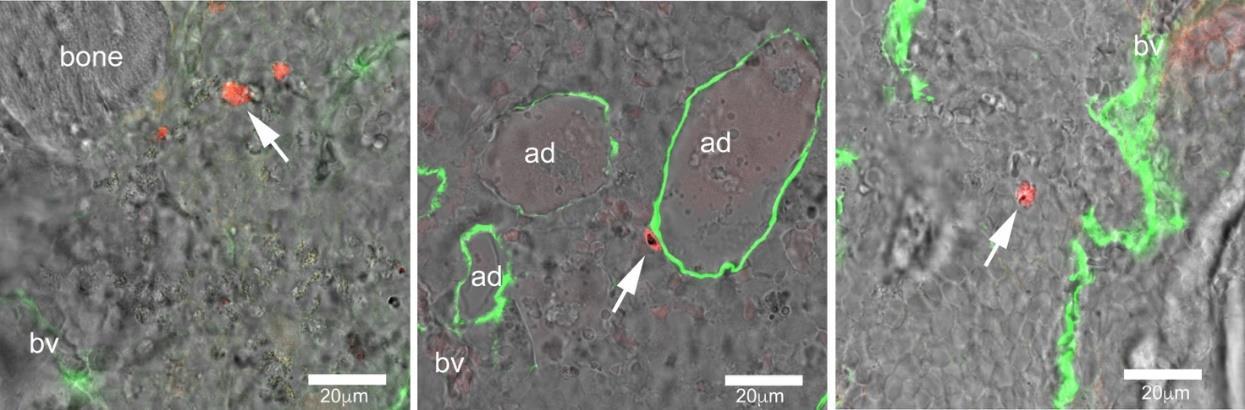
In addition, we showed that extravascular gametocytes were often in close contact with A human bone marrow adipocytes (Figure 5). This observation is in agreement with previously published work showing the interaction of the P. Falciparum with peripheral B fat depots (e.g. Mejia et al, Sci Adv. 2021), and strongly point to a role of this marrow cell type in the human malaria infection Altogether, these results clearly demonstrate that our BMHM model is a powerful experimental tool to dissect the behaviour of the P. falciparum immature gametocytes within the bone marrow, to identify their interactions with local cells and to test novel therapeutic strategies.
A B C
Figure 5 Confocal microscopy images of formalin-fixed, EBM-embedded ossicles immunostained for FABP4 (green) showing P. falciparum immature gametocytes (red) in ossicles harvested at 10 minutes (A), 1hour (B) and 24 hours (C). Note in B the close contact of gametocytes with the plasma membrane of a human marrow adipocyte (bv= blood vessels; ad= adipocytes).
Publications
Rostovskaya M, Donsante S, Sacchetti B, Alexopoulou D, Klemroth S, Dahl A, Riminucci
M, Bianco P, Anastassiadis K. Clonal Analysis Delineates Transcriptional Programs of Osteogenic and Adipogenic Lineages of Adult Mouse Skeletal Progenitors. Stem Cell Reports. 2018 11 :212-227. doi: 10.1016/j.stemcr.2018.05.014. IF 5,499
Bravenboer N, Bredella MA, Chauveau C, Corsi A, Douni E, Ferris WF, Riminucci M, Robey PG, Rojas-Sutterlin S, Rosen C, Schulz TJ, Cawthorn WP. Standardised Nomenclature, Abbreviations, and Units for the Study of Bone Marrow Adiposity: Report of the Nomenclature Working Group of the International Bone Marrow Adiposity Society. Frontiers in Endocrinology 2020 Jan 24;10:923. doi: 10.3389/fendo.2019.00923. IF. 2,776
A manuscript reporting the model and the methods established in this project and the preliminary results is in preparation.
Research Group
Alessandro Corsi, researcher; Samantha Donsante, post-doc fellow;
Collaborations
Pietro Alano and collaborators, Istituto Superiore di Sanità, Roma. Valeria de Turris, Centre for Life Nano Science, Istituto Italiano di Tecnologia, Roma.






