4 minute read
Giulia D’Amati
MITOCHONDRIAL tRNA RELATED DISEASES: IMPLEMENTATION OF
Advertisement
AND RESCUING MECHANISMS OF THERAPEUTIC MOLECULES
GIULIA D’AMATI
RESEARCH AREA: NOVEL THERAPEUTIC INTERVENTIONS
Department of Radiological, Oncological and Pathological Sciences giulia.damati@uniroma1.it
Mutations in genes coding for mitochondrial (mt)-tRNAs (MTTs) are responsible for a wide range of currently untreatable pathologies. Previous studies on the mechanisms underlining mt-tRNA associated diseases have been performed on cell types that are freely available from patients, such as fibroblasts, and the trans-mitochondrial hybrid model (cybrid). Cybrid cells have the advantage to be easily obtained in large amounts and with low costs. However, beingundifferentiated neoplastic cells, they do not entirely reproduce the biochemical phenotype observed in patients’ most seriously affected tissues, for instance heart, muscle and brain. Thus, the development of new cellular models able to recapitulate the tissue-related effects of mt-DNA mutations is envisaged. Recently, production of induced pluripotent stem cells (iPSCs) obtained from patients with either the m.3243A>G mt-tRNALeu(UUR) or the m.8344A>G mt-tRNALys point mutations (1, 2), has opened up the possibility to develop cellular models able to recapitulate the tissue-specific effects of mt-tRNA point-mutations. Accordingly, the aims of our project are: - to develop neurons, cardiomyocytes and myotubes from iPSCs obtained from patients carrying either the homoplasmic mutation m.4277T>C in mttRNAIle gene (causing mitochondrial cardiomyopathy) or the heteroplasmic mutations m.3243A>G in mt- tRNALeu(UUR) gene and m.8344A>G in mttRNALys gene (causing severe mitochondrial syndromes); - to analyze the pathologic phenotype of iPSCs and iPSC-derived cells; - to evaluate the possible rescuing effect of therapeutic molecules on these cellularmodels. We have already available in our laboratory iPSCs bearing all the mt-tRNA point mutations listed above.
According to the aims of the project, during the first year: - we generated and characterized NSCs (neural stem cells) and cardiomyocytes bearing the m.4277T>C mutation, the m.3243A>G
mutation and the m.8344A>G mutation(AIM 1); - we demonstrated that the β32_33 peptide (our therapeutic molecule), is able to penetrate both the plasma and mitochondrial membranes and exert the rescuing activity when exogenously administered to cybrids bearing both m.3243A>G and m.8344A>G mutation (see publications section). This result is preliminary to the analysis of the rescuing effect of our molecule upon exogenous administration to iPSC- derived cells (AIM 2).
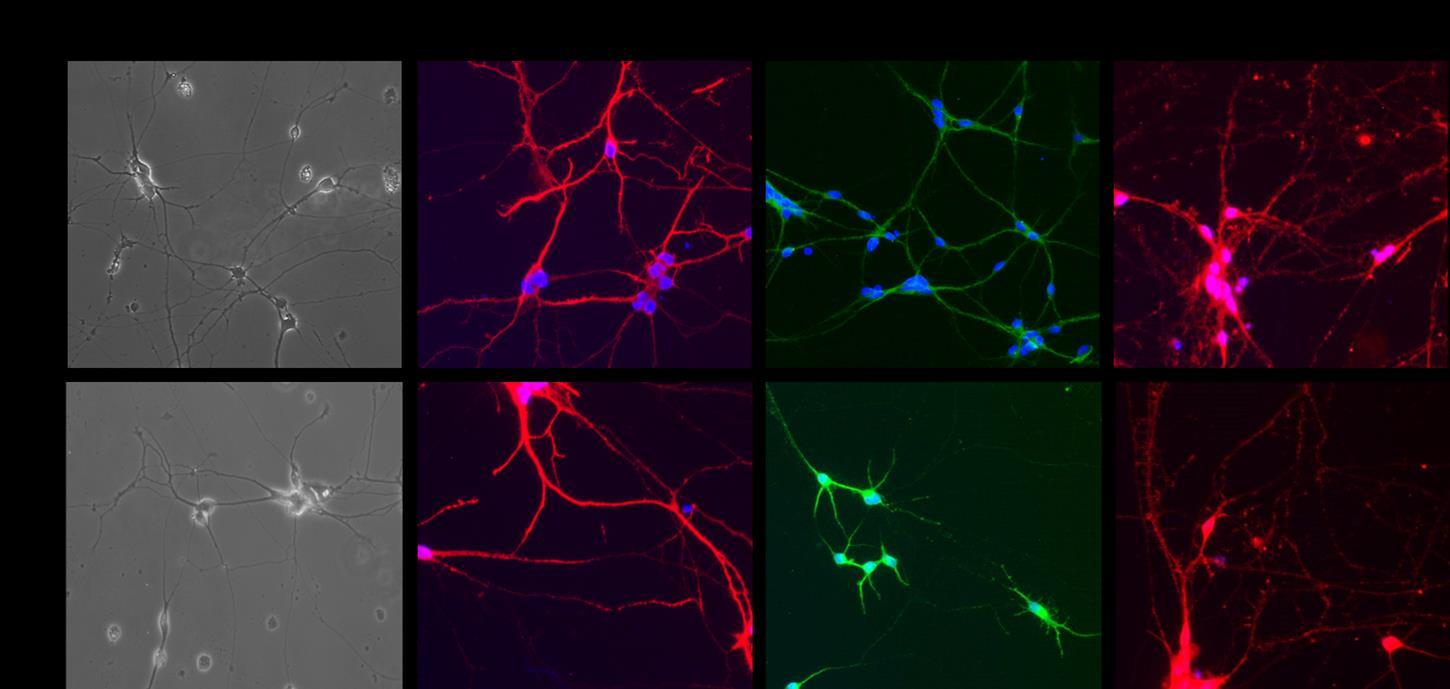
In the last year of the project, we progressed with: i) the generation and characterization of neurons from NSCs with different mutations and ii) the generation of myotubes from iPSCs bearing the m.3243A>G mutation. i) We were able to produce neurons from NSCs bearing the m.4277T>C mutation, the m.3243A>G mutation and the m.8344A>G mutation. The differentiation efficiency was assessed by immunofluorescence analysis with specific neuronal markers (Figure 1). By sequence analysis of whole mtDNA we excluded the presence of further mtDNA mutations, depletions or major rearrangements, which could be possibly induced by the differentiation process.
Figure 1. Immunofluorescence confirms the phenotype of iPSC-derived neurons. Differentiated cells were stained with doublecortin (red), MAP2 (green), synapsin (red) and nuclear DNA (DAPI, blue). Representative images of neurons bearing the m.3243A>G mutation. NMS-8: neurons with high 3243A>G mutation load; NMS-3: neurons with low 3243A>G mutation load, used as isogenic control. (Original magnification 20X).
To evaluate the tissue-specific effects of the mutations, we are now analysing the pathologic phenotype of neurons, starting from the investigation of calcium transients, since in the nervous system calcium influx is involved in exocytosis of neurotransmitter-containing synaptic vesicles and in the induction of activity-dependent synaptic plasticity (3). We are evaluating intracellular calcium transients using the membrane permeable fluorescent calcium indicator Fura 2-AM (Sigma-Aldrich, 4) (data in progress).
2) In parallel, we are starting to produce myotubes from iPSCs bearing the m.3243A>G mutation. We already generated myogenic progenitor cells, and we are assessing the differentiation efficiency by immunofluorescence analysis with specific myogenic cell markers such as CD56 and CD82 (data in progress)
Cited literature
1) Hämäläinen RH et al. (2013) Tissue- and cell-type-specific manifestations of heteroplasmic mtDNA 3243A>G mutation in human induced pluripotent stem cell-derived disease model. Proc Natl Acad Sci USA 110(38):E3622-30. 2) Chou SJ, Tseng WL, Chen CT, et al. (2016) Impaired ROS Scavenging System in Human Induced Pluripotent Stem Cells Generated from Patients with MERRF Syndrome. Sci Rep. 30; 6:23661. 3) Grienberger C and Konnerth A. (2012) Imaging calcium in neurons. Neuron. 73(5):862-85. 4) Capetian P et al. (2018) Altered glutamate response and calcium dynamics in iPSC-derived striatal neurons from XDP patients. Exp Neurol. 308:47-58.
Publications
Perli E, Pisano A, Pignataro MG, Camperse AF, Pelullo M, Genovese I, de Turris V, Ghelli AM, Cerbelli B, Giordano C, Colotti G, Morea V and d’Amati G. Exogenous peptides are able to penetrate human cell and mitochondrial membranes, stabilize mitochondrial tRNA structures, and rescue severe mitochondrial defects. FASEB J. 2020 Jun;34(6):7675-7686. doi: 10.1096/fj.201903270R. Epub 2020 Apr 18. IF: 4,966
Research Group
Bruna Cerbelli, Carla Giordano, Elena Perli, Annalinda Pisano, Maria Gemma Pignataro
Collaborations
Monika Madej (Dept. of Molecular Neuroscience, UCL, London, UK); Elisabetta Cerbai, Raffaele Coppini (University of Florence).






