10 minute read
Advancing the Assessment of Degenerative Change in the Cervical Spine
Degenerative cervical spine disease (cervical spondylosis) is a form of osteoarthritis that affects the spine, characterized by the spontaneous degeneration of either the disc or facet joints, leading to axial neck pain and neurological complications that may require surgical intervention. Research on the Medicare Claims 5% Limited Data Set found that the average prevalence of spinal degeneration was 27.3% ± 1.7%.1 While the spine undergoes age-related degenerative changes that are nearly universal, they can begin as early as the first decade of life. 2 Population-based studies indicate that roughly 80% to 90% of individuals exhibit disk degeneration on magnetic resonance imaging (MRI) by age 50 years. 3,4 Due to the increasing prevalence of degenerative changes in the cervical spine among the aging US population, 5 it is essential to diagnose and treat this condition appropriately. This review will examine current methods for assessing degenerative changes in the cervical spine and highlight the necessity of enhancing the diagnosis of related conditions more effectively.
Clinical Presentation of Degenerative Changes in the Cervical Spine
Cervical spondylosis is caused by various degeneration processes, including aging, overuse, and trauma. While it can affect bone quality and joint structures, the most common changes primarily occur in the intervertebral disks and facet joints.6,7 With aging, the intervertebral disc undergoes degenerative changes characterized by the depletion of nucleus pulposus cells, which are responsible for maintaining the proteoglycan-rich extracellular matrix. Concurrently, the cartilaginous endplates become sclerotic, impairing nutrient diffusion into the disc. These processes lead to disc desiccation and progressive reduction in disc height. When disc space narrowing becomes severe, the normally aneural annulus fibrosus may undergo neo-innervation, contributing to nociceptive signaling. Additionally, marginal osteophyte formation can occur similar to that seen in other synovial joints under mechanical stress. The facet joints, which primarily function to limit axial rotation and sagittal plane motion, subsequently bear increased mechanical loading due to the loss of disc height. This increased axial stress accelerates the degeneration of facet joints.7–9 These structural changes can lead to a range of clinical manifestations. Patients with degenerative cervical spondylosis might show signs of mechanical neck pain, radiculopathy, myelopathy, or a mix of these symptoms.
Neck pain is quite common, impacting about 15% of the general population at any given time.10 Mechanical neck pain may stem from signals from intervertebral joint receptors, where abnormal nerve fibers are proliferating. These signals are communicated primarily to the cervical paraspinal muscles, leading to muscle spasms and resulting in typical interscapular and lateral neck pain.11
Cervical spondylotic radiculopathy (CSR)occurs when a nerve exiting the spinal cord is compressed. This compression can arise from a degenerated disc, such as a herniated disc, where a weak spot in the annulus allows the inner disc material to press against the nerve root. It can also result from foraminal stenosis narrowing caused by bone spur or hypertrophied ligamentum flavum on moderate to severe degenerative facet or intervertebral joint.12 Degenerative cervical myelopathy (DCM) typically involves severe disc and facet degeneration, resulting a narrowed spinal canal and subsequent deformation of the spinal cord.13 These results in DCM are also commonly secondary to disc herniation, with the C7 root being the most frequently affected, followed by C6 and C8.14,15
Current Assessment Methods
Clinical evaluation is important for assessing degenerative cervical spine disorders and depends primarily on patient history, symptom description, physical examination, and patient-reported outcome measures.
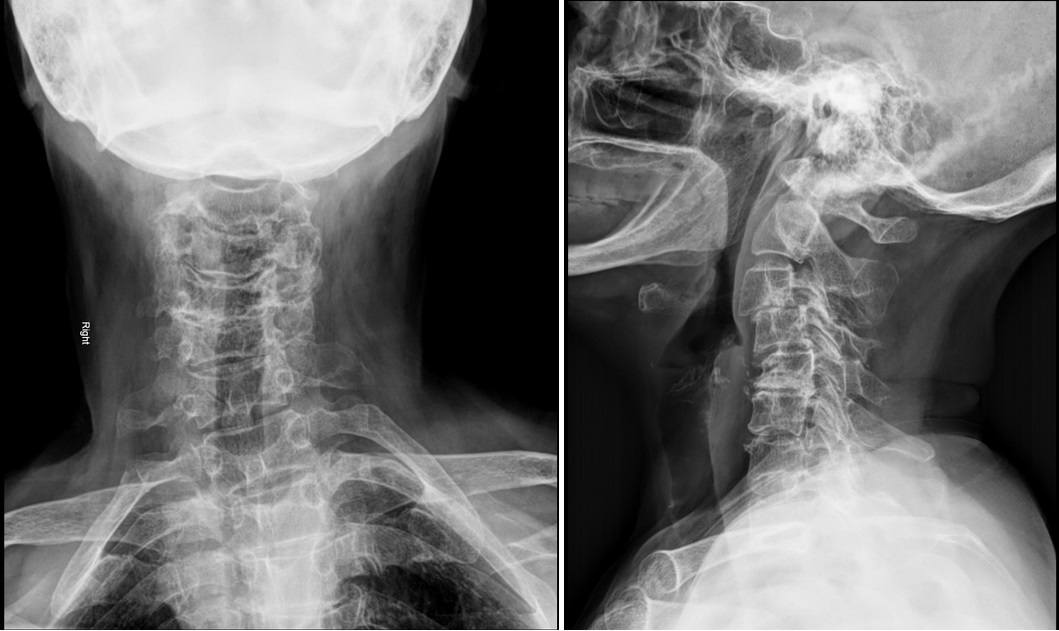
Neurological symptoms in extremities, including CSR and DCM, often necessitate detailed radiographic assessments. This typically includes anteroposterior, lateral, and oblique x-ray images, which are economical and provide information on degenerative changes and alignment,16,17 potentially showing a reduction in normal cervical lordosis, hypertrophic changes, and narrowing of the disc space. Lateral flexion or extension views may also disclose ankylosed segments, cervical instability, limited range of motion, and fused cervical spine segments.6,18 Most flexion and extension motions of the cervical spine come from C4-C6. It is most common to see the earliest and considerable degenerative changes at the C5-C6 segment.19
In contrast, a challenge when assessing patients with only neck pain is that nearlyall individuals older than 50 years show degenerativecervical changes on radiographicimaging, often without specific findings.3,4 Degenerative changes such as the narrowing of the intervertebral disc space, facet and uncovertebral joint osteoarthrosis, the presence of osteophytes, and end-plate sclerosis are common among adult patients and are not diagnostic.17,20 Thus, diagnostic imaging is recommended for selected patients experiencing ongoing neck, shoulder, or arm pain that either does not respond to standard conservative treatment or occurs with suspected neurological symptoms.6,11,21
In contrast, diagnostic images is often not recommended for patients presenting with nontraumatic neck pain without neurologic symptoms, signs, or red flags for malignancy. Cervical spine MRI without contrast is the preferred imaging method for patients with progressive neurologic impairments or signs of myelopathy, as it offers insights into osseous structures, soft tissues, and spinal cord structures.22 It is also indicated for neck pain in patients with a history of malignancy, prior cervical surgery, or suspected infection.21 It often shows abnormalities, making it essential to correlate these imaging results with clinical findings.23 If an MRI cannot be performed or is not accessible, a CT scan or CT myelography of the cervical spine should be considered. Compressive osteophytes, foraminal stenosis, and ossification of the posterior longitudinal ligament are most effectively detected using CT scans. Additionally, CT myelography, which is an invasive procedure, is said to outperform MRI in differentiating between osseous and soft-tissue compressions of neural structures, as well as in identifying foraminal stenosis.24,25
Current cervical disc degeneration assessment employs both qualitative and quantitative methods, each with distinct strengths. The Pf irrmann classif ication (grades I-V), initially developed for the lumbar spine, remains widely used in cervical evaluations due to its familiarity. This classification evaluates disc structure, signal intensity, and height loss on T2-weighted MRI, demonstrating almost perfect intraobserver reliability (κ = 0.858) but only fair interobserver agreement (κ = 0.265).26,27 In contrast, the Suzuki classification (grades 0-III), designed specifically for cervical discs, provides a more accurate representation of cervical pathology and a more even grade distribution, although its interobserver reliability is similarly modest (κ = 0.29).27,28 Both systems cluster most cases into midgrade categories (Pfirrmann III-IV, Suzuki II), which limits their sensitivity to early changes.29
Meanwhile, Kettler and Wilke’s review26 highlights reliability challenges across 42 spine degeneration systems, recommending Pfirrmann and Suzuki for discs (κ >0.6) while advocating for standardized scales starting at grade 0 for normal anatomy. Kellgren and Lawrence et al30 focused on osteophytes and disc narrowing, but their study lacked sensitivity for early degeneration, limiting its utility compared to MRI’s soft-tissue detail. Using CT, Huang et al31 adapted the Kellgren and Lawrence system to assess uncovertebral joint degeneration, grading changes from normal (Grade 0) to joint fusion or severe osteophyte articulation (Grade 4). Facet joint degeneration is best evaluated by CT, as exemplified by Park et al’s grading system (grades I–IV), which identifies the upper cervical levels (C2/3–C4/5) as degeneration-prone with high reliability (ICC = 0.87–0.89). 32
Emerging quantitative tools, such as the Disc Signal Intensity Index (DSI2), address subjectivity limitations by normalizing the disc T2 signal to cerebrospinal fluid, achieving superior interobserver reliability (ICC = 0.90). 33 DSI2 correlates linearly with Pfirrmann grades but detects early degeneration that is missed by qualitative systems and identifies novel associations (eg, diabetes is linked to higher DSI2, possibly due to metformin’s protective effects). 33 Large-scale MRI studies 28 validate that degeneration begins at C5/6, progresses contiguously, and rarely skips levels—critical for understanding adjacent-segment disease. While qualitative systems remain clinically practical, DSI2 and similar metrics show promise for research requiring precision, particularly in tracking subtle degenerative changes and evaluating therapeutic interventions.
Advancements in Diagnostic Tools
New imaging modalities are improving diagnostic precision by providing more detailed, functionally relevant information. Dynamic MRI enhances the detection of motion-dependent foraminal and canal narrowing, which may be missed on static imaging. 34 In preoperative patients, dynamic MRI identified occult stenosis in nearly half of the cases, 34 leading to improved surgical planning and postoperative outcomes. 35,36 Although it is useful, issues with motion artifacts and acquisition duration have hindered broader adoption.
Building on these imaging advancements, artificial intelligence (AI), particularly deep learning (DL) models, are becoming increasingly used to predict outcomes after cervical spine surgery. Expanding upon these diagnostic capabilities, AI systems are progressively evolving from primarily focusing on diagnosis to actively supporting clinical decision-making. New DL models have been developed for MRI-based clinical decision support, specifically for degenerative cervical spine disorders, aiming to evaluate alignment with treatment recommendations from experienced spine specialists.37 Three-dimensional (3D) Deep Learning–Enhanced MRI (3D-DLRecon) enhances the visualization of foraminal stenosis and bony detail with high-resolution, multiplanar reconstructions. 38 It provides better diagnostic reliability than standard two-dimensional (2D) sequences and could be helpful for preoperative assessment of disc space narrowing and facet degeneration.39
Beyond structural imaging, novel techniques are also emerging to evaluate early biochemical and mechanical changes in the cervical spine. T1ρ MRI, which is sensitive to proteoglycan depletion, has demonstrated utility in detecting early disc degeneration before morphological changes appear. 40 DualMRI, which quantifies voxel-level strain under physiological loading, may offer a noninvasive mechano-biomarker of disc health and treatment response.41 18F-NaF PET/CT, a metabolic imaging modality, has demonstrated uptake in vertebral and facet joints that correlates with age-related degeneration, suggesting its potential use as a biomarker for disease activity and progression.42 Finally, genomic and epigenetic profiling represent a promising frontier for risk stratification in DCM. While no genome-wide significant SNPs were identified, variants in genes such as COL6A1, APOE, and RUNX2, along with nongenetic risk factors, including age, sex, and socioeconomic status, have been implicated in DCM susceptibility.43
Clinical Implications and Future Directions
With the growing incidence of degenerative cervical spine disease due to an aging population, the importance of timely and precise diagnosis, as well as tailored management strategies, is becoming more critical. Traditional assessment methods remain foundational but are often limited by nonspecific findings and a lack of correlation with symptom severity. The combination of advanced imaging techniques, including dynamic MRI, 3D-DLRecon, and PET/CT, along with cutting-edge technologies such as AI applications and genomic profiling, signifies a pivotal advancement toward precision medicine in cervical spine care. These innovations enable earlier detection of pathology, provide enhanced clinical care, and facilitate superior risk stratification for patients with complex presentations. Moving forward, collaboration across disciplines and ongoing research into AI-driven diagnostics, molecular biomarkers, and imaging-based mechano-biomarkers will be valuable assets for enhancing patient outcomes and creating a more proactive, personalized strategy for managing degenerative cervical spine disorders.
References
1. Parenteau CS, Lau EC, Campbell IC, Courtney A. Prevalence of spine degeneration diagnosis by type, age, gender, and obesity using Medicare data. Sci Rep. 2021;11(1):5389.
2. Benoist M. Natural history of the aging spine. Eur Spine J. 2003;12(0):S86-S89.
3. Teraguchi M, Yoshimura N, Hashizume H, et al. Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population-based cohort: the Wakayama Spine Study. Osteoarthritis Cartilage. 2014;22(1):104-110.
4. Brinjikji W, Luetmer PH, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. Am J Neuroradiol. 2015;36(4):811-816.
5. Oglesby M, Fineberg SJ, Patel AA, Pelton MA, Singh K. Epidemiological trends in cervical spine surgery for degenerative diseases between 2002 and 2009. Spine. 2013;38(14):1226-1232.
6. Rao RD, Currier BL, Albert TJ, et al. Degenerative cervical spondylosis: clinical syndromes, pathogenesis, and management. Instr Course Lect. 2008;57:447-469.
7. Theodore N. Degenerative cervical spondylosis [review]. N Engl J Med. 2020;383(2):159-168.
8. Van Der Werf M, Lezuo P, Maissen O, Van Donkelaar CC, Ito K. Inhibition of vertebral endplate perfusion results in decreased intervertebral disc intranuclear diffusive transport. J Anat. 2007;211(6):769-774.
9. Feng C, Liu H, Yang M, Zhang Y, Huang B, Zhou Y. Disc cell senescence in intervertebral disc degeneration: causes and molecular pathways. Cell Cycle. 2016;15(13):1674-1684.
Contributors:
Atahan Durbas, MD1
Courtney S. Harris, BA2
Sophie C. Kush, BS2
Tomoyuki Asada, MD, PhD1
Sheeraz Qureshi, MD1
From the 1Department of Spine Surgery at the Hospital for Special Surgery in New York, New York, and 2Weill Cornell Medical College in New York, New York.









