11 minute read
Biologics in TLIF—A Focus on Cost and Efficacy
Minimally invasive surgical (MIS) techniques in spine surgery are increasingly being utilized to treat a wide range of spine pathologies. With more than 400,000 spine fusion procedures performed annually in the United States, it has become one of the most common and well-established treatments for degenerative disorders, spine trauma, tumors, and structural deformities.[1] Bone graft substitutes are commonly used during spinal arthrodesis surgery, and while numerous factors influence the success of spinal arthrodesis, choosing the most appropriate biologic is essential to optimize both efficacy and safety. The current variability in the use of bone grafts and substitutes reflects uncertainty about their effectiveness, outcomes, and associated costs. An evidence-based approach to cost-effective decision-making should aim to maximize patient-centered benefits or outcomes while minimizing costs and risks. The present brief review will focus on the cost and efficacy of bone morphogenetic protein (BMP) in MIS transforaminal lumbar interbody fusion (TLIF) surgery.
BMP Utilization and Efficacy
Using the Nationwide Inpatient Sample of the Healthcare Cost and Utilization Project, Singh et al analyzed national epidemiologic trends in BMP use for spinal arthrodesis from 2002 to 2011, with a focus on the impact of the 2008 Food and Drug Administration warning. Consistent with earlier studies, they observed a steady annual increase in BMP utilization, increasing from 0.6% BMP use in 2002 to 26.9% in 2011 across various types of spinal fusions.[2] The posterior lumbar fusion cohort accounted for the majority of spinal fusions that used BMP, representing 76.8% of all spinal fusions between 2002 and 2011.[2] However, in a more recent review, Wadhwa et al retrospectively reviewed a total of 316,070 patients who underwent spinal fusion from 2006 to 2015 where BMP was used in 60,249 cases (19.1%) and found that BMP utilization rates decreased from 23.1% in 2006 to 12.0% in 2015. This was not stratified to delineate the rates for MIS TLIF, but BMP use in posterior lumbar fusions decreased from 31.5% in 2006 to 15.8% in 2015.[3]
Nevertheless, BMP is still widely used and its cost effectiveness remains in question. In a single institution retrospective cohort study of 187 patients with 2-year follow-up, Khan et al found that BMP in TLIF procedures was relatively effective for achieving bone fusion at rates similar to autograft. The fusion rate for the BMP and non-BMP groups was 92.7% and 92.3%, respectively, and the overall pseudoarthrosis rate was 7.5% (14 of 187 patients).[4] In a systematic review of clinical studies published between 2000 and 2012 comparing fusion rates after posterior lumbar interbody fusion (PLIF)/TLIF (not limited to MIS) surgery with versus without BMP, Galimberti et al found that the average fusion rate 24 months after surgery was 95.7% for PLIF/TLIF (n = 141) with BMP and 89.5% (n = 86) without BMP. This difference was not statistically significant, but the lack of significance can be explained by variability in BMP dosing, surgical methods, and the quality of the studies.[5] Although MIS TLIF was not differentiated from open TLIF, there is literature to support comparable fusion rates. In an open vs MIS TLIF retrospective cohort study with 148 MIS patients, Price et al demonstrated the fusion rate was similar for both open and MIS TLIF (91% overall). More importantly though, they found BMP use was associated with higher fusion rate (93% vs 83%) at 1 year, regardless of approach.[6]

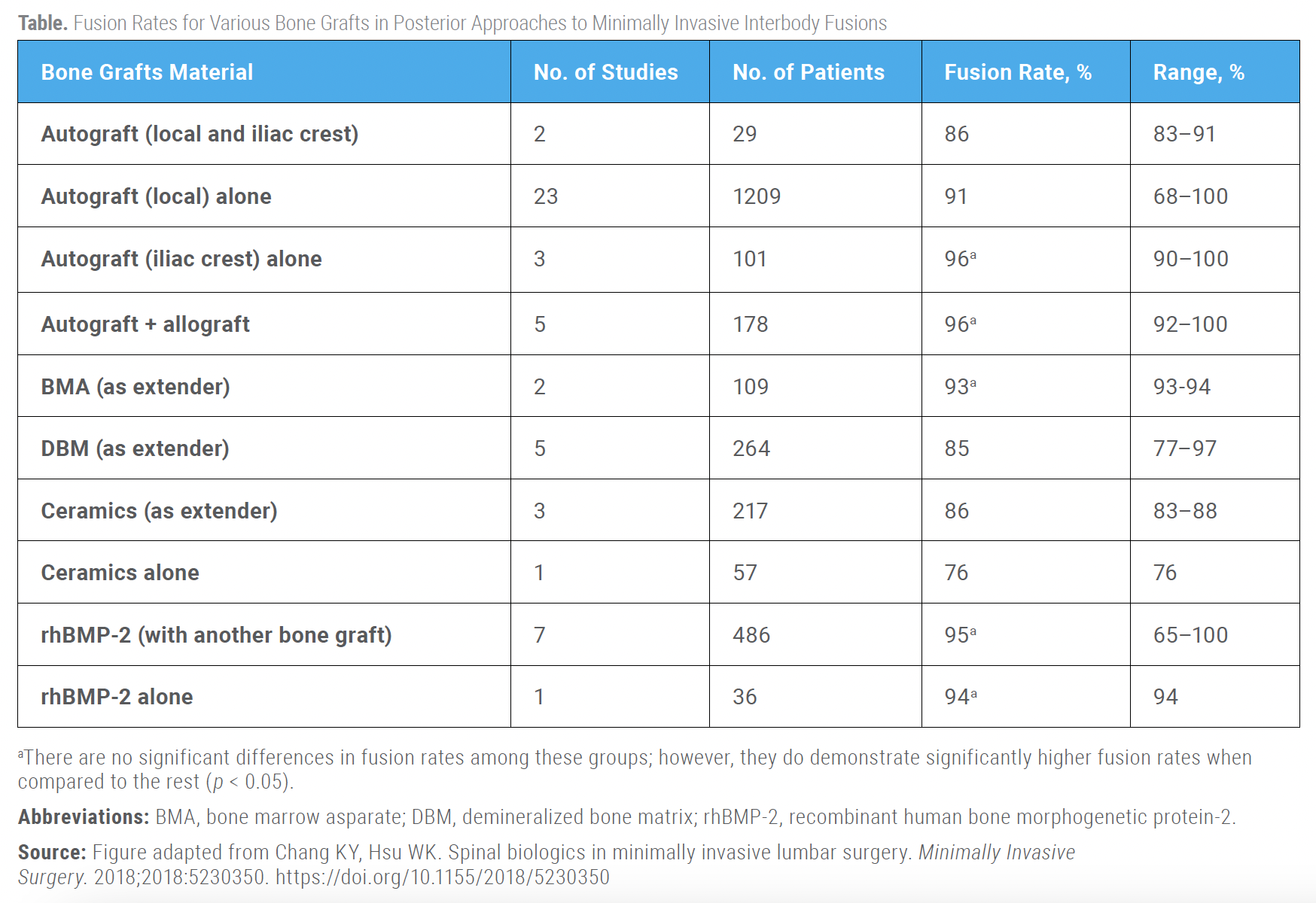
Furthermore, there has been a dose-dependent relationship demonstrated for BMP in MIS TLIF. Lytle et al retrospectively reviewed 690 patients who underwent MIS TLIF from 2009 to 2014 and found that odds of fusion increased by 2.02 when BMP dose range was increased from 0.16–1 mg/level to 1.0–2 mg/level, but fusion odds did not increase when BMP dose increased to more than 2 mg/level. Overall fusion was 95.2% with a mean follow-up of 19 months.[7] BMP also performs well in combination with autograft. In a meta-analysis that compared fusion rates of different graft materials used in MIS-TLIF, Parajón et al reported a 95.3% fusion rate after local autograft + BMP compared to 91.8% with local autograft alone (no statistics reported). The highest fusion rate was observed with a combination of local autograft with bone extender and BMP (99.1%).[8]
BMP Complications and Costs
The primary limitations of the frequency of BMP use have been related to reported complications and questionable cost effectiveness. Some complications associated with BMP include osteolysis, graft subsidence, retrograde ejaculation, and urological issues.[9-13] In the previously mentioned retrospective cohort study, Khan et al also found similar complication rates, with the BMP group exhibiting slightly higher rates of radiculitis and seroma (OR = 4.53, 95% CI, 1.42–14.5).[4] However, these complications are rare. Crandall et al reviewed 509 patients undergoing open TLIFs and discovered similar complications at an average 5-year follow-up, with pseudoarthrosis in 0.92%, seroma in 0.4%, and ectopic bone growth in 0.6%.[14]
More specifically in MIS TLIFs, in a large case series of 573 patients, Singh et al reported that 10 patients (1.7%) required additional procedures due to persistent radiculopathy, neuroforaminal bone growth, vertebral body osteolysis, or cage migration. Thirty-nine patients (6.8%) underwent reoperations for symptomatic pseudarthrosis. In their cost analysis for this single-surgeon, single-institution study, the average cost per procedure was $19,224, while the costs for reoperation were $14,785 per case for neuroforaminal bone growth and $20,267 for pseudarthrosis.[15] These are some of the potential costs that should be considered when weighing the risks and benefits of BMP use. The Medicare database study by Wadhwa et al also reported that BMP reimbursement does not increase proportionally with BMP cost: BMP use was associated with a total cost increase of about $4,597 in posterior lumbar fusions but a reimbursement increase of $964.[3]
Alternative Biologics
Traditionally, autologous bone grafting, particularly iliac crest bone graft (ICBG), was regarded as the gold standard for spinal arthrodesis. However, due to concerns about complications at the harvest site, limited availability, and associated morbidity, surgeons have increasingly turned to alternative bone graft options that offer osteogenic, osteoinductive, and/or osteoconductive properties. These alternatives include allografts, demineralized bone matrix, ceramics, mesenchymal stem cells, and recombinant human bone morphogenetic proteins.[16] ICBG provides both cortical and cancellous grafts without the added cost of commercial alternatives. It offers a substantial amount of bone and eliminates the risk of disease transmission or histoincompatibility. However, ICBG harvesting can lead to complications, including infection, pain, scarring, and graft site fractures.[17,18]
Modern surgical techniques have reduced these risks by preserving the inner cortex and carefully closing wounds, helping to maintain the iliac crest’s shape and reduce chronic pain. Despite the potential complications, ICBG remains an effective bone graft option, supported by high fusion rates of up to 93% in MIS TLIF.[8] Haws et al reviewed 149 patients who underwent MIS TLIF with ICBG or BMP, and the authors found the ICBG cohort demonstrated increases in intraoperative blood loss and longer operative times but no significant differences in complication or reoperation rates.[19] In a prospective series of patients undergoing primary, single-level MIS TLIF with ICBG was compared to a historical cohort of patients that received BMP-2. The authors found that ICBG was associated with decreased total direct costs ($19,315 vs $21,645, P < 0.001) as compared to BMP-2.[20]
Among the allograft options, i-Factor, also known as anorganic bone matrix combined with Peptide 15 (P-15), has been utilized to augment lumbar spine fusion.[21] In a 2-year prospective clinical trial, Lauweryns et al reported the efficacy of i-Factor in PLIF surgeries. By 24 months, i-Factor had a fusion rate of 95.56% and autograft had a rate of 93.33%. In this study, i-Factor not only promoted fusion but also achieved it more rapidly than traditional autografts in PLIF procedures.[21] The unit cost of i-Factor can range from $757 to $2090, depending the size.[22] In anterior cervical discectomy and fusion, Thaci et al found the incremental cost-effectiveness ratio of i-Factor use was $13,333 per quality-adjusted life year at 90 days and demonstrated a greater benefit and a lower cost at 1 year when compared to the autograft cohort.[23] However, further studies are needed to assess the cost utility of i-Factor in MIS TLIFs.
Conclusion
The present review briefly explores the usage, efficacy, and complications associated with BMP in MIS TLIF surgeries alongside a couple of alternative biologics. Over the years, the use of BMP in spinal arthrodesis has fluctuated due to Food and Drug Administration warnings and changing trends, as analyzed by Singh et al and Wadhwa et al. Despite reductions in usage, BMP remains commonly used, showing fusion rates comparable to autografts but raising concerns about cost-effectiveness and complication risks. The discussion extends to alternative graft materials like autografts, allografts, and innovative products like i-Factor, which have their benefits and limitations. Cost considerations remain critical, and randomized controlled trials are warranted to directly compare the efficacy and costs in MIS TLIF. The narrative underscores the need for balancing efficacy, safety, and costs in choosing the appropriate graft material for MIS TLIF procedures, advocating for an evidence-based approach in clinical decision-making.
References
1. Rajaee SS, Bae HW, Kanim LE, Delamarter RB. (2012). Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine. 2012;37(1):67-76.
2. Singh K, Nandyala SV, Marquez-Lara A, Fineberg SJ. Epidemiological trends in the utilization of bone morphogenetic protein in spinal fusions from 2002 to 2011. Spine. 2014;39(6):491-496.
3. Wadhwa H, Wu JY, Malacon K, Ames CP, Ratliff JK, Zygourakis CC. Trends, payments, and costs associated with BMP use in Medicare beneficiaries undergoing spinal fusion. Spine J. 2023;23(6):816-823.
4. Khan TR, Pearce KR, McAnany SJ, Peters CM, Gupta MC, Zebala LP. Comparison of transforaminal lumbar interbody fusion outcomes in patients receiving rhBMP-2 versus autograft. Spine J. 2018;18(3):439-446.
5. Galimberti F, Lubelski D, Healy AT, et al. A systematic review of lumbar fusion rates with and without the use of rhBMP-2. Spine. 2015;40(14):1132-1139.
6. Price JP, Dawson JM, Schwender JD, Schellhas KP. Clinical and radiologic comparison of minimally invasive surgery with traditional open transforaminal lumbar interbody fusion: a review of 452 patients from a single center. Clin Spine Surg. 2018;31(2):E121-E126.
7. Lytle EJ, Slavnic D, Tong D, et al. Minimally effective dose of bone morphogenetic protein in minimally invasive lumbar interbody fusions: six hundred ninety patients in a dose-finding longitudinal cohort study. Spine. 2019;44(14):989-995.
8. Parajón A, Alimi M, Navarro-Ramirez R, et al. Minimally invasive transforaminal lumbar interbody fusion: meta-analysis of the fusion rates. What is the optimal graft material? Neurosurgery. 2017;81(6):958-971.
9. Vaidya R, Weir R, Sethi A, Meisterling S, Hakeos W, Wybo CD. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007;89(3):342-345.
10. Smoljanovic T, Siric F, Bojanic I. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am. 2010;92(15):2614-2615.
11. Jarrett CD, Heller JG, Tsai L. Anterior exposure of the lumbar spine with and without an “access surgeon”: morbidity analysis of 265 consecutive cases. Clin Spine Surg. 2009;22(8):559-564.
12. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471-491.
13. Carragee EJ, Mitsunaga KA, Hurwitz EL, Scuderi GJ. Retrograde ejaculation after anterior lumbar interbody fusion using rhBMP-2: a cohort controlled study. Spine J. 2011;11(6):511-516.
14. Crandall DG, Revella J, Patterson J, Huish E, Chang M, McLemore R. Transforaminal lumbar interbody fusion with rhBMP-2 in spinal deformity, spondylolisthesis, and degenerative disease–part 1: large series diagnosis related outcomes and complications with 2-to 9-year follow-up. Spine. 2013;38(13):1128-1136.
15. Singh K, Nandyala SV, Marquez-Lara A, et al. Clinical sequelae after rhBMP-2 use in a minimally invasive transforaminal lumbar interbody fusion. Spine J. 2013;13(9):1118-1125.
16. Kannan A, Dodwad SNM, Hsu WK. Biologics in spine arthrodesis. Clin Spine Surg. 2015;28(5):163-170.
17. Robertson PA, Wray AC. Natural history of posterior iliac crest bone graft donation for spinal surgery: a prospective analysis of morbidity. Spine. 2001;26(13):1473-1476.
18. Sengupta DK, Truumees E, Patel CK, et al. Outcome of local bone versus autogenous iliac crest bone graft in the instrumented posterolateral fusion of the lumbar spine. Spine. 2006;31(9):985-991.
19. Haws BE, Khechen B, Yoo JS, et al. Impact of iliac crest bone grafting on postoperative outcomes and complication rates following minimally invasive transforaminal lumbar interbody fusion. Neurospine. 2019;16(4):772-779.
20. Haws BE, Khechen B, Narain AS, et al. Iliac crest bone graft for minimally invasive transforaminal lumbar interbody fusion: a prospective analysis of inpatient pain, narcotics consumption, and costs. Spine. 2018;43(18):1307-1312.
21. Lauweryns P, Raskin Y. Prospective analysis of a new bone graft in lumbar interbody fusion: results of a 2-year prospective clinical and radiological study. Int J Spine Surg. 2015;9:2.
22. Bernatz JT, Fisher MW, Pinter ZW, Sebastian AS. Controversies in spine surgery: is i-Factor superior to bone morphogenic protein for achieving spine fusion? Clin Spine Surg. 2023;36(6):224-226.
23. Thaci B, Yee R, Kim K, Vokshoor A, Johnson JP, Ament J. Cost-effectiveness of peptide enhanced bone graft i-Factor versus use of local autologous bone in anterior cervical discectomy and fusion surgery. Clinicoecon Outcomes Res. 2021;13:681-691.
Contributors:
Daniel Shinn, MD
Vincent P. Federico, MD
Arash Sayari, MD
From the Department of Orthopaedic Surgery at Rush University Medical Center in Chicago, Illinois.








