
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 01 | Jan 2025 www.irjet.net p-ISSN: 2395-0072


International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 01 | Jan 2025 www.irjet.net p-ISSN: 2395-0072
Santosh kumar Gupta1
1Department of Mechanical Engineering, Madan Mohan Malviya University of Technology, Gorakhpur, Uttar Pradesh -273010, India
1Mechanical Engineering, Department of Technical Education, Uttar Pradesh-India
Abstract - It is well known that the destruction of availability or exergy results from irreversible processes called combustion. For an adiabatic, constant volume system, the cycle's destruction of availability (energy) is investigated. This analysis was conducted without the use of experimental measures. For ethanol, methanol, propane, and octane-air combinations, the fraction of fuel availability that is destroyed by the irreversible processes is determined as a function of temperature, pressure, and equivalency ratio. When operating at greater temperatures, the combustion process generally results in less destruction of the fuel's available energy. Because they include some oxygen, methanol and ethanols differ greatly from gasoline. This study examines the fuel-air cycle and performs estimates for energy destruction during the compression, combustion, expansion, and exhaust process, taking into account changes in the equivalency ratio. With the adjustment in the equivalency ratio, the efficiency of the first and second laws is also determined for propane, ethanol, methanol, and iso-ocatne. Furthermore, depending on the specific operating conditions, equivalency ratio might have a large impact on the destruction of availability. In particular, the destroyed availability as a result of the combustion process varied from around 5 to 25% of the initial reactant availability under the study's conditions. There is a description of how these findings affect internal combustion engine combustion processes.
Key Words: Availability, Petrol Engine, Equivalent Ratio, Air Fuel Ratio, Methanol, Ethanol
1.INTRODUCTION
Theavailabilityofagivensystemisdefinedasthemaximumusefulworkthatcanbeobtainedinaprocessinwhichthesystem comestoequilibriumwiththesurroundingsorattainsadeadstate Theavailabilityofenergyconversionsystemsisonedirect result. The second law of thermodynamics is a potent exposition of pertinent physical facts with many applications in engineeringandenergyconversionsystemoperation.Forexample,thesecondlawcomputesthemaximumperformanceof thermalsystems,establishesequilibriumconditions,determinesthedirectionofprocesses,andidentifiesthecomponentsof processeswhichareusedtodetermineoverallperformance.Theframeworkprovidedbythermodynamicssecondtypelaw allowsforadeepercomprehensionofcombustionprocesses.thoroughunderstandingoftheenergyconversionprocess.[1-3] Numerousinvestigationsontheoperationandexhaustemissionsofspark-ignitionengine(SIengine)basedonblendsoffuel likegasolineandalcoholhavebeenconducted;resultshowthattheemissionsarereduced [8-13].Forexample,Cevizand Yüksel[14]lookedintohowblendsofgasolinewithoutethanolaffectedemissionsandcyclicvariabilityinaspark-ignited engine.Accordingtothestudy'sfindings,utilizingfuelmixesofethanol-freegasolinedecreasedthecoefficientofvariationin thedisplayedmeaneffectivepressure,aswellastheconcentrationsofCOandHCemissions,whileraisingtheCO2contentin thefuelblendupto10%ethanolbyvolume[6].Inagasolineengine,Çelik[18]employedethanolasfuelatahighcompression ratio.Incomparisontorunningwithpuregasolinefuel,hediscoveredthatusingE50fuelatahighcompressionratioenhanced enginepowerwhileloweringspecificfuelconsumption(sfc)andexhaustpollutants.Inadifferentinvestigation,Shenghuaetal. [35]ranthree-cylinderSparkIgnitionengineatfullloadusingdifferentmethanolpercentagesingasoline(10%,15%,20%, 25%, and 30%). They discovered that when the fuel blend's methanol content increased, the engine's power and torque droppedandthebrakes'thermalefficiencyincreased.Gasoline-methanolmixeswerealsousedasfuelsinaportfuelinjection (PFI)gasolineenginebyFanetal.[36]withoutanymodifications.Accordingtothestudy'sfindings,methanol-gasolineblended fuelshadnoeffectonengineperformance,andtherewasnodiscerniblechangeinthecylinderpressureorheatreleaserateas theblendedfuel'smethanolconcentrationincreased[37,38].
Ethanol,methanol,andpropanearethealternativefuelsunderconsideration.Anengineerisinterestedindeterminingthe mostusefulworkthatcanbeobtainedfromasysteminagivenstate.[20,27,28]Obviously,thegreatestamountofworkwillbe producedonlywhenthesystem'stheultimateconditionisinharmonywithitsenvironment.Inotherwords,untilthesystem's interaction with the environment reaches a point where it can no longer function in any way. A system's availability is determinedbythemaximumamountofusefulwork whichmaybefindthroughoutaprocessthatendswhenthesystem reacheswithstateofinactivityorequilibriumwithitssurroundings.[4]Itisobviousthatasystem'savailabilityisdependent

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 01 | Jan 2025 www.irjet.net p-ISSN: 2395-0072
uponbothitsinternalandexternalconditions.Theentropylawofthermodynamics,acomprehensiveandpotentstatement thatrelatedphysicalinvestigationswithnumerousimplicationsfortheengineeringdesignandoperationofenergyconversion systems,directlyleadstoavailability.[5].
Theaimofthepresentinvestigationwastoevaluationandanalysisindetailthedestructionofavailabilityforprocesseslike combustionandcompression.Thecompressionprocessinadiabatic,constantvolumecombustionsystemwasstudied.The noveltyoftheresearchischangingtheequivalencyratio.Astheratioofequivalencychanged,forcomputationsandanalysis. Ethanol,Methanolareverydifferentfromgasolineasitcontainssomeamountofoxygeninit.Inthispaper,afuel–airactual cycleisbeingconsideredandcalculationsarebeingdoneforenergydestructionduringcompression,combustion,expansion andexhaustprocesswiththechangeinequivalenceratio.
2.1
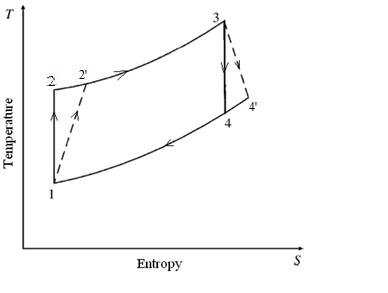
Fortheanalysis,arealfour-strokefuel-aircycleforsparkignitionhasbeentakenintoaccount.Theinvestigationonlylooksat thedestructionofenergyduringcombustion. Figure 1 depictstheentireSIenginecycle.Theprocess1-2sisanexampleof reversibleadiabaticcompression,whereastheprocess1-2isactualcompressionprocess.Processes2-3and3-4areexamples ofreversibleandirreversibleadiabaticprocesses,respectively,andcombustionandexpansion,respectively.Process4-1's exhaustisrunningatasteadyvolume.Itisassumedthattheprocessofconstantvolumecombustionisadiabatic.Thereisno energytransmittedasaresultofheattransferbecausetheprocessisadiabatic.Sincethesystemvolumeisclosed,massflows donotresultinthetransmissionofenergy.Consequently,duringa worktransfer,noenergyisexchanged.Therefore,the combustionprocessaloneisresponsibleforanychangeinexergyduringachangeofstate.Thisstudy'sanalyticalmethodology isbasedonrelatedworkthatemployedenginecyclecalculationswithcompressionchanges.
Whenperformingconstantvolumeadiabaticcombustion,thefollowingpresumptionsaremade.
1.Theclosedchambercontentisthethermodynamicsystem.
2.Itisbelievedthatthecylinder'scontentsarehomogeneousinspaceandonlyoccupyonezone.
3.Ithasbeenincludedtovarythespecificheatwithtemperatureatvariousprocesspoints.
4.Thereisquickcombustion.
5.Itisconsideredthatallofthefuelhasevaporatedandcombinedwiththereactantair.
6.Thereisspatialuniformityinthethermodynamicproperties,suchastemperatureandpressure.
Table 1 showing the engine specification which are used in analysis of exergy analysis during various operations like compressionandexpansionsetc.
Table 1 Enginespecification
DiaofBore 120mm
Inletvalvetimeopening 35o BeforeTDC
Inletvalvetimecloses 45o BeforeTDC
Equivalenceratio Various
Fuel Different
CompressionRatios
SparkignitionTime
Varying
30oBeforeTDC
Strokelength 80mm
EngineSpeed3000rpm 3000rpm
Lengthofconnectingrod
mm

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 01 | Jan 2025 www.irjet.net p-ISSN: 2395-0072
3.1 Availability
Athermodynamicpropertyofasystem,availabilityisalsoknownasexergyorexergy(essenceofenergy)byvariousauthors.It measuresthemaximumamountusefulworkthatanysystemachievewhenitisallowedtoreversiblychangeintoaconditionof thermodynamicequilibriumwithitsenvironment.[23][24]
3.2 Restricted Dead State
Thelocalenvironment'scircumstancesarecharacterizedbytheconfineddeadstate.Thiswasselectedbecause,whenany systemreachesperfectbalancewithitsimmediatesurroundings,itcannolongergenerateanymorevaluableworkandis deemedtobe"dead."Thisdeadstateisincompleteandcalled"restricted"becausethecontentsofthesystemcannotmixor reactwiththesurroundings.[25][26]
For the dead state conditions, 298.15 K and 101.325 kPa which is 1 atm are the recommended temp and pressure. The elementsthatmakeupthedeadstateneedstobespecifiedinadditiontothetemperatureandpressureofdeadstate.
3.3 Irreversibility
Accordingtothesecondlaw,theentropyofasystemplusitssurroundings,oranisolatedsystem,canneverdecrease.The secondlawstates:[26]
(ΔS)System +(ΔS)surr.=0,whereΔ=final–initial
>0irreversibleforrealworld
=0reversiblefrictionless,ideal
IfQistheheatfromasourceatT,theninanidealsituation,itsavailabilityorthemaximumamountofwork whichitcan produceisQ(1-T0/T), whereT0 istheambienttemperature.Itwillalwaysbelessthanthisamount.Wecallthedifferenceasirreversibility. Availability(Exergy)=Maximumpossiblework-Irreversibility W useful =W rev –I
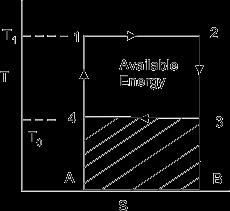
Thedifferentialamountofworkdonebytheengineisgivenby
If the Carnot engine works tillthetemperatureofthebodyattainsavalueT2(Figure 2)thetotalworkdonebythereversibleengineisgivenby W= = Q(1- )= -To =Q-To (1.3)
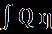
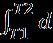
3.4
Afterdeterminingthethermodynamiccharacteristicsofaspecificsetofcircumstances,determiningavailability(Exergy)is comparativelystraightforward.Thepotentialandkineticenergiesareignoredinthisdevelopment(andcanbeshowntobe negligible).Allthewaythroughthesystem,everytime.[29-31][39] a=(u-u0)+p0(v-v0)-T0(s-s0) (1.4) dW=dQ =dQ(1-To/T) (1.2)
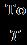
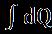

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 01 | Jan 2025 www.irjet.net p-ISSN: 2395-0072
theavailabilitybalancereducesto adest=a1-a2 (1.5) changeinentropyduetoirreversibility’sasfollows: adest=T0Sgen/m=I/m (1.6)
whereSgen isthedifferenceinentropyduetoirreversibility
3.5 Exergy Concept
A system's exergy is the maximum shaft work it can accomplish within a specific reference environment. The reference environmentisthoughttobeinfinite,harmonious,andencompassesallothersystems.[17]
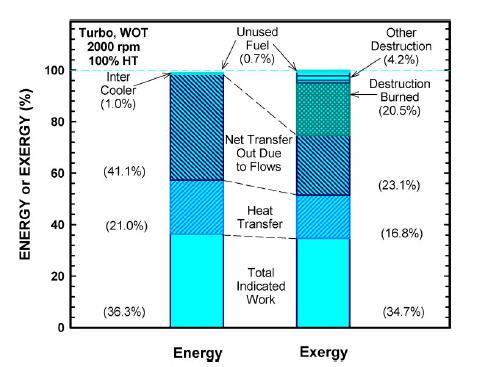
Itindicatesenergydestructionofvariouscomponentswasexaminedatfullload.Itisveryessentialtoanalyzingheattransfer levelandworktransfer(Figure 3).Afterexaminesengineperformancethermodynamicallychemicalbalancearenecessaryfor differentfueluse
4 Analysis
4.1 Chemical balance equations
Table2showsthepropertiesofFuelsUsedinAnalysis.Thistable2isusedtoanalysisofchemicalbalanceequation.
Table –2 PropertyofDifferentFuels[15,16]

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
ChemicalEquationsforPropane[11][40-44]
0.8C3H8 +5O2 +18.8N2 =2.4CO2 +O2 +3.2H2O+18.8N2
0.9C3H8 +5O2 +18.8N2 =2.7CO2 +0.5O2 +3.6H2O+18.8N2
1.1C3H8 +5O2 +18.8N2 =2.3CO2 +CO+4.4H2O+18.8N2
1.2C3H8 +5O2 +18.8N2 =1.6CO2 +2CO+4.8H2O+18.8N2
ChemicalEquationsforIso-Octane
0.8C8H18 +12.5O2 +47N2 =6.4CO2 +2.5O2 +7.2H2O+47N2
0.9C8H18 +12.5O2 +47N2 =7.2CO2 +1.25O2 +8.1H2O+47N2
1.1C8H18 +12.5O2 +47N2 =6.3CO2 +2.5CO+9.9H2O+47N2
1.2C8H18 +12.5O2 +47N2 =4.6CO2 +5CO+10.8H2O+47N2
ChemicalEquationsforMethanol
0.8CH3OH+1.5O2+5.64N2=0.8CO2+0.3O2+1.6H2O+5.64N2
0.9CH3OH+1.5O2+5.64N2=0.9CO2+0.15O2+1.8H2O+5.64N2
1.1CH3OH+1.5O2+5.64N2=0.8CO2+0.3CO+2.2H2O+5.64N2
1.2CH3OH+1.5O2+5.64N2=0.8CO2+0.3CO+2.2H2O+5.64N2
ChemicalEquationsforEthanol
0.8C2H5OH+3O2+11.28N2=1.6CO2+0.6O2+2.4H2O+11.28N2
0.9C2H5OH+3O2+11.28N2=1.8CO2+0.3O2+2.7H2O+11.28N2
1.1C2H5OH+3O2+11.28N2=1.6CO2+0.6CO+3.3H2O+11.28N2
1.2C2H5OH+3O2+11.28N2=1.2CO2+1.2CO+3.6H2O+11.28N2
Alloftheaboveequationswerederivedfromtheanalysissection'sequatingandbalancing[32-34]
4.2 Thermodynamic Relations
Inordertodefinethecompositionandfuelairequivalencyratio,itisusefultoknowaboutfuelairequivalencyratio,whichis defines Φ=(F/A) actual/(F/A) stoichiometric (4.1)
Themolecularweightsofthereactantsandproductswerecalculatedusingthefollowingrelations. MR=∑niMi/N (4.2)
Thequantityofreactantorproductmolesperworkingfluidmassunitisdenotedbyni.ThespecieshasamolecularmassofMi andatotalmolecularmassofN.Therelationshipisusedtodeterminethespecificheatofthereactantandproduct.
R=Cp -Cv (4.3)
Cv = ∑ni Cvi/N (4.4)
Cp = ∑ni Cpi/N (4.5)
ThevalueofCpandCvwereevaluatedattwopointsatthestartofthecompressionprocesses
Attheaveragetemperature(temperaturefollowingcompressionplusadiabaticflametemperature)2duringthecombustion processequationslikethefollowingareusedtocomputethevaluesofCpandCvofvariousfuels.Therepresentationofspecific heatsasafunctionoftemperatureis Cp=a+bT+cT2 +dT3 (4.6) (TinK,Cpinkj/kmol K)
Thevaluesoftheconstantsa,b,canddaretakenfromthetable3.
Thecompressionefficiencycanbedefinedas(Forthereversibleadiabaticprocess1-2s)
ηc =T2s –T1/T2-T1 (4.7)
T2s/T1 =(V1/V2)γ-1 (4.8)
WhereγistheadiabaticindexanditsvalueisCp/Cv
Nowpolytropicindexiscalculatedbyusingthefollowingequation
T2/T1 =(V1/V2) n-1 (4.9) whereVrepresentsthegasinthecylinder'svolume.
Abalanceequationforavailabilitywasusedtodeterminethemixture'savailabilityduringcompression.(1–2).[39] a2 –a1 =(u2 –u1)+P0 (v2 –v1)–T0(s2 –s1) (4.10)
Theenergywithworktransferisrepresentedbythesecondterminthisequation,andtheenergydestructionis representedbythethirdterm.
Duringprocessofcompression,entropygenerationisgivenby
Volume: 12 Issue: 01 | Jan 2025 www.irjet.net p-ISSN: 2395-0072 © 2025, IRJET | Impact Factor value: 8.315 | ISO 9001:2008 Certified

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 01 | Jan 2025 www.irjet.net p-ISSN: 2395-0072
Sgen =(Cp lnT2/T1 –RlnP2/P1 +Q/T0) (4.11)
Inthiscycle,thechoiceofconstantvolumeadiabaticcombustionallowsforthecombustionprocesstobeheldresponsiblefor anyvariationsinavailability.Sincecombustionisadiabatic,therewon'tbeanyavailabilitytransferasaresultofheatloss throughthecylinderwall.Undesirablyhightemperaturesarereachedatthemaximumcycletemperaturewhenallofthefuel's energyisdeployed.
Qin ×ηc ×LHV=mf Cv (T3 –T2) (4.12)
Whereasmf ismassflowrateoftheairfuel(AF)mixture.
The ratio of the net chemical energy released to the total energy released during combustion is known as combustion efficiency.[22]
ηc =HR (TA)–HP(TA)/mf ×LHV (4.13)
Where HR (TA)–HP(TA)=m(∑ni∆hfi) R -(∑ni∆hfi) P (4.14)
∆hfi isthespeciesiatstandardenthalpyofproductionatroomtemperatureTA.
Foreverysystemgoingthroughanyprocess,includingreactingsystems,theentropybalanceisexpressedas[25][26]
Sin –Sout +Sgen=ΔSsys (4.15)
Sin –Sout isthemassandheat-inducednetentropytransfer.Sgen isgenerationofentropyandΔSsys istheentropychange
∑Qk/Tk +Sgen =Sprod -Sreact (4.16)
WhereTkistheboundarytemperaturewhereQkcrossesit.
Nevertheless,entropygenerationforanadiabaticprocess(Q=0)isprovidedby
Sgen =Sprod -Sreact (4.17)
TheidealgasentropychangerelationprovidesabsoluteentropyvaluesforanytemperatureTatpressuresotherthan1 atm.[19]
(T,P)= 0(T,Po)– lnP/Po (4.18)
(T,Pi)= 0(T,Po)– lnyi Pm/Po (4.19)
WherePiispartialpressure,andyiismolefractionofthecomponent,Pm ismixture’stotalpressure.
Exergydestructionduringcombustionprocessisprovidedby[19-20][48].
Edest =T0Sgen (4.20)
PercentageofenergydestructionisdefinedasEnergydestructionintheprocessofcombustion/Chemicalenergyofthefuel (4.21)
TABLE–3ConstantValuesintheEqn.(4.6)[21]
Sincetheequivalencyratiosofdifferentfuelsvary,theequivalencyratiosusedintheanalysisare0.8,0.9,1.1,and1.2.Thisis becausetheentireexergyanalysisisbasedonthesedifferences.Thisissobecausethesediscrepanciesformthefoundationof
8.315

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 01 | Jan 2025 www.irjet.net p-ISSN: 2395-0072
theentireexergyanalysis.Consequently,allchemicalequationsarebalancedwhentheequivalencyratiovaluesaboveare taken. This research contains a tabular representation of the calculated results for every parameter that was used in the analysis(suchasthepercentageofenergydestructionduringcompressionandcombustion,thechangeinavailabilityduring compressionandcombustion,andtheenergydestructionduringcompressionandcombustion).Additionally,thisresearch providesathoroughexplanationalongwiththenecessarygraphsandcurves.
Theall-calculatedresultsofallparameterswhichhavebeentakeninthisanalysis(i.e.energydestructionduringcompression and combustion, change in availability during compression and combustion, percentage of energy destruction during compressionandcombustionetc.)withrespecttoequivalenceratio
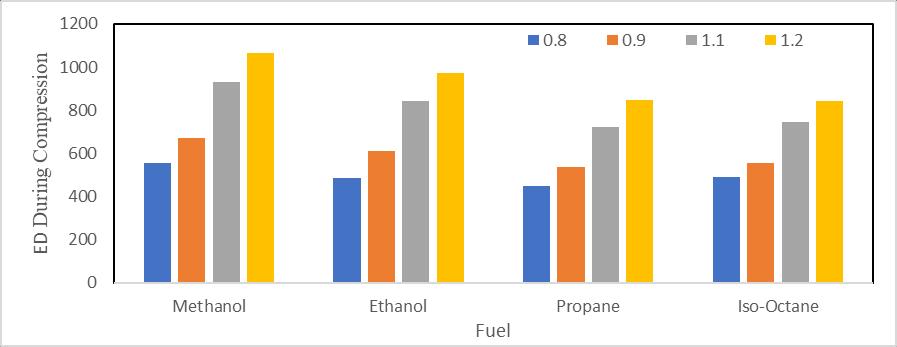
Figure 4 illustrateshowtheenergydissipationduringcompressionchangesthemethanolandethanolequivalencyratios. Energydestructionduringcompressionisdemonstratedtodecreaseuptothestoichiometriccondition(Φ=1),andthisis because,forallselectedfuels,temperatureaftercompressiondecreasesuptoanequivalencyratioof0.8to1.Thisreducesthe amountofheattransferthroughthewall.Figure4illustrateshowenergydestructionintheprocessofcompressionrisesinrich conditionsforanequivalencyratioof1.1andthenfallsfurtherforeveryselectedfuels.Thetemperatureatanequivalencyratio of1.1ismorethantemperatureforanequivalencyratio1.2,whichexplainswhy.Becausetemperatureisatitslowestduring stoichiometricconditions,energydestructionduringcompressionisatitslowest.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 01 | Jan 2025 www.irjet.net p-ISSN: 2395-0072
The energy loss during combustionmodifies the methanol and ethanol equivalency ratios, as shown in Figure 5. Becausetemperaturefallsuptoanequivalencyratioof0.8to1forallselectedfuels,itis depictsthatenergydestructionin combustionprocessreducestillstoichiometriccondition,whichis(Φ=1).
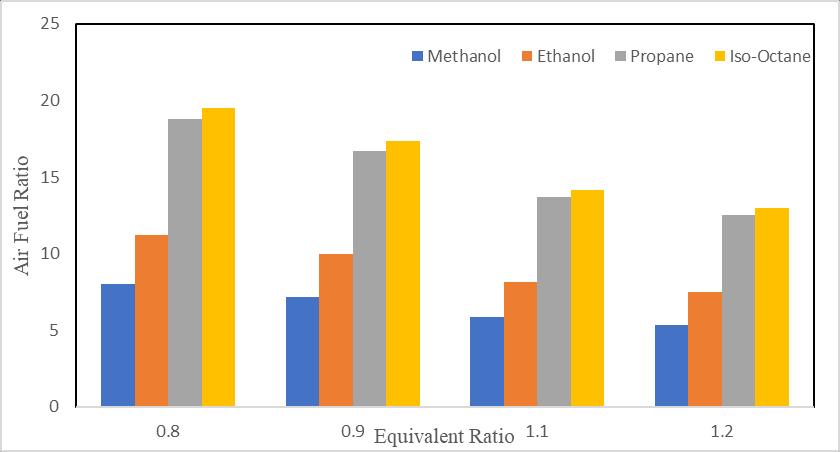
Figure6explainstheAirfuelRatiovsEquivalenceratiofortheall-selectedfuels.Itisshownthatchangesinairfuelratioforthe all-selectedfuels.
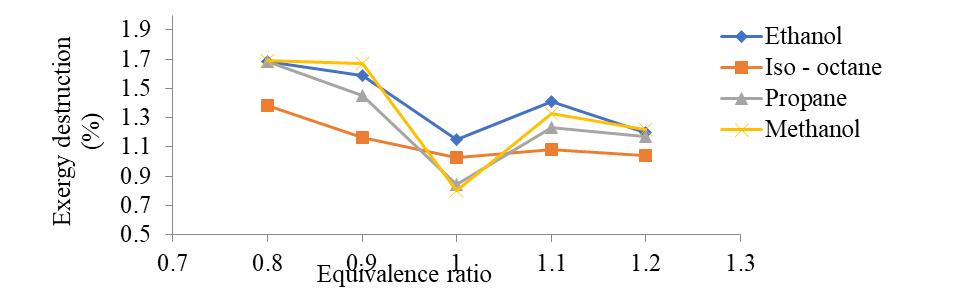
ExergyDestructioninpercentageisshownin Figure 7. OnchangingtheEquivalentrationavailabilityofeachofthechosen fuel’schangesfromtimetotime,Itdecreasesuptoequivalentratio1afterthatitincreases.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 01 | Jan 2025 www.irjet.net p-ISSN: 2395-0072
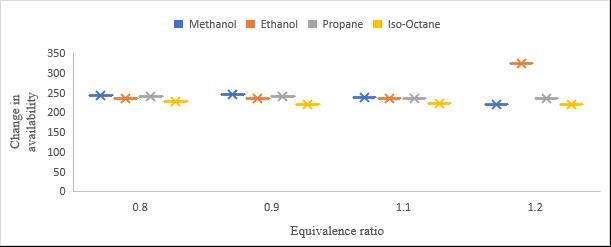
Figure 8 Changeinavailabilitywiththechangeinequivalenceratio
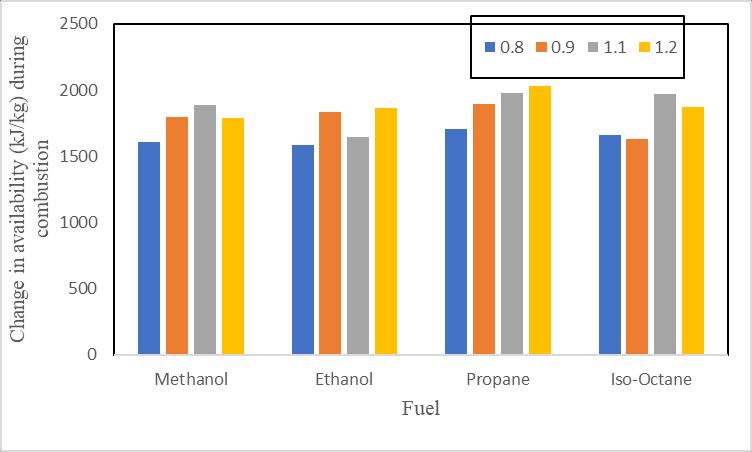
Changeinavailabilitywiththechangeinequivalenceratiointhe Figure 8. Itisdemonstratedthatavailabilityincreasesforall selectedfuelsfollowingcompression,andthisisbecauseworkissuppliedduringthisprocess.Additionally,itisdemonstrated thatthetrendsformethanolandethanoldiffer.
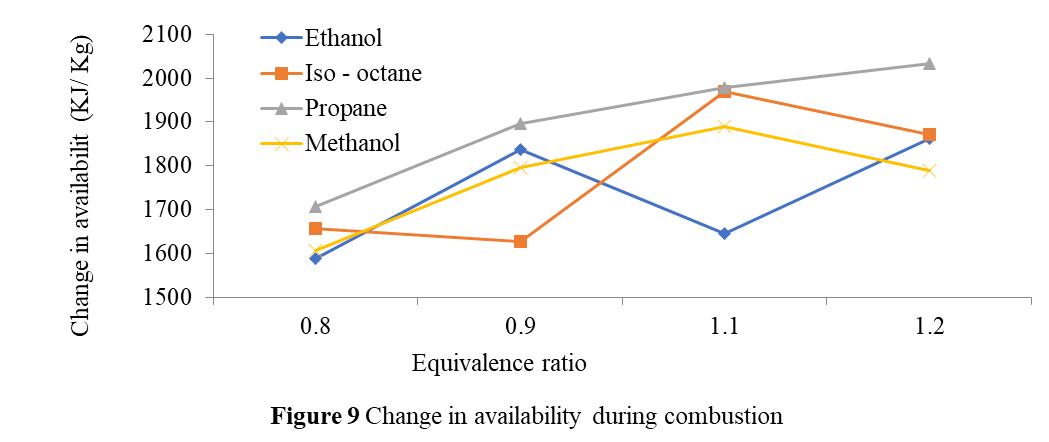
Figure9illustrateshowtheequivalencyratiochangesinrelationtoavailabilityduringcombustion.Ithasbeendemonstrated thatforpropanefuels,availabilitychangesastheequivalencyratiorises.However,theavailabilityofiso-octaneandethanol changesinawavypatternduringthisprocess,whichisbecausetheairfuelratioandcombustionefficiencyplayaroleinthe availabilitychanges.Theairfuelratioandcombustionefficiencybothdropwhentheequivalencyratiorises.Itisadditionally demonstratedthatunderleanconditions(Φ=0.8and0.9),availabilitychangeswhentheequivalencyratiosforpropane, methanol,andethanolgrow.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 01 | Jan 2025 www.irjet.net p-ISSN: 2395-0072
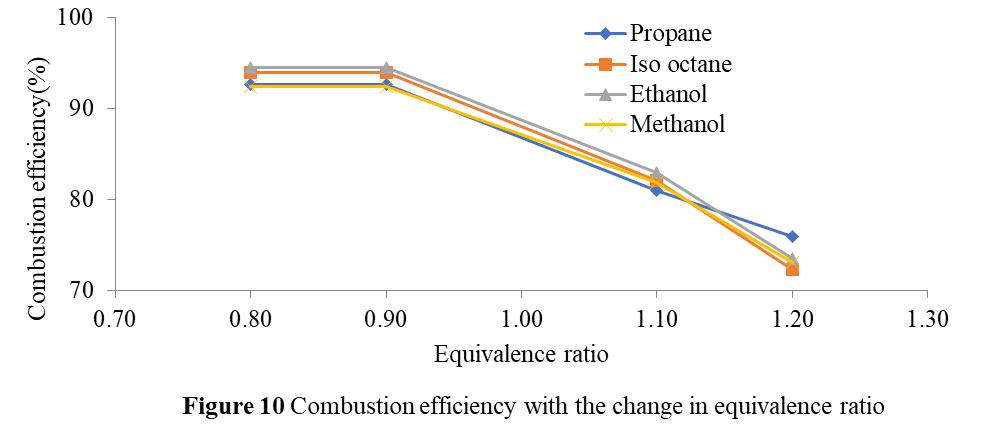
Forethanol,methanol,propane,andiso-octane, Figure 10 illustrateshowtheequivalencyratiochangesinrelationtothe varianceincombustionefficiency.Itisdemonstratedthat,forallofthefuelsthatwerechosen,combustionefficiencyfallsasthe equivalencyratiorises.Thisisbecause,athigherequivalencyratios,fueldoesnotreceiveenoughoxygentoburn,leavingsome fuel unburned. It is also demonstrated that methanol has a minimum combustion efficiency and ethanol a maximum. Furthermore,itisnotedthatthedifferenceincombustionefficiencyislowestforpropane(17.99%)andlargestforiso-octane (23.04%).
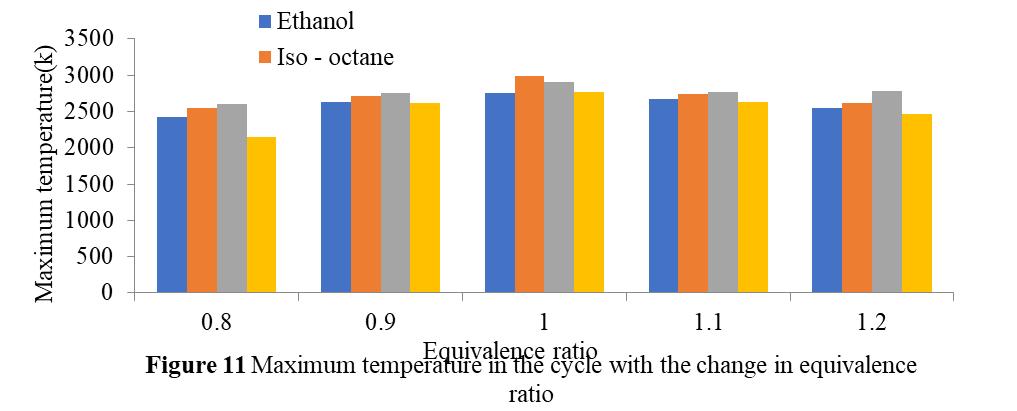
Figure 11 illustrateshowthetemperaturechangesinrelationtotheiso-octane,propane,methanol,andethanolequivalency ratios. The maximum temperature at which the product is found to occur after combustion is found to correspond to an equivalencyratioofone.Thisisbecauseallfuelsburnatstoichiometricconditions,andthemaximumtemperatureforricher fuelsdecreasesbecausetheydonotreceiveenoughoxygentoburn.Leanerfuelshaveenoughoxygentoburn,butbecausethey contain less fuel, less heat will be released during combustion, lowering the maximum product temperature above stoichiometricconditions.Additionally,itisnotedthattheiso-octaneinstoichiometricconditionshasamaximumtemperature.
6. Conclusion
By all analysis and calculations, it is concluded that the energy destruction during combustion process is maximum in comparisonofcompressionprocess.Foraconstantvolumeprocessinvolvingethanol,methanol,iso-octane,andpropane,the impactofthereactantmixtureequivalencyratiosonthepercentageofenergylostthroughcombustionandcompressionis beingassessedwithrespecttothechangeinequivalencyratio.Theeffectofthereactantmixtureequivalencyratiosonthe percentageofenergydestroyedbycombustionandcompressionisbeingevaluatedwithrespecttothechangeinequivalency

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 01 | Jan 2025 www.irjet.net p-ISSN: 2395-0072
ratioforaconstantvolumeprocessinvolvingpropane,ethanol,methanol,andiso-octane.Theavailabilityvariationsduring compressionandcombustionarealsoexamined,inadditiontothechangeinequivalencyratio.Sincethecombustionefficiency andmaximumtemperaturehaveaneffectonthe engine'sperformance,theimpactoftheequivalencyratioadjustmentis assessed.Itisdeterminedthatforallofthechosenfuels,theamountofenergydestroyedduringcombustionisminimalatthe stoichiometriccondition.Intheprocessofburning,ethanolhadanenergydestructionrangingfrom20.8to30.1%,iso-octane from21.2to25.05%,propanefrom19.54to25.1%,andmethanolfrom14.07to29.6%.
Thecalculationsandanalysispresentedaboveleadtotheconclusionthat,incomparisontothecompressionprocess,the combustionprocessdestroysthemostenergy.Theeffectofthereactantmixtureequivalencyratiosonthepercentageofenergy devastated for a constant volume process due to combustion and compression is being evaluated because methanol and ethanolvarywiththein-equivalencyratioindifferentways.Thevariationinavailabilityduringcompressionisalsoanalyzed using the shift in the equivalency ratio. The effect of the equivalency ratio change is evaluated because the engine's performanceisinfluencedbyboththemaximumtemperatureandcombustionefficiency.Itisfoundthatatstoichiometric conditions,energydestructionduringcombustionisnegligibleforbothselectedfuels.
Itispossibletoconductfurtherresearchonthetwootherprocesses theexpansionandexhaustprocesses,inparticular.After that, the total amount of energy destroyed can be calculated. The energy losses over the course of the full cycle are then estimated.Consequently,wecanincreasetheefficiencyoftheSIengineor,ontheotherhand,theefficiencyoftheentirecycle withthehelpoftheaforementionedcomputations.
Nomenclature:
η efficiency
φ equivalenceratio
γ ratioofspecificheats
m mass[kg] a air
T absolutetemperature[K] c combustion
s entropy[kjkg-1K-1] f fuel
S absoluteentropy[kjK-1] i ith species
a availability[kjkg-1] mix mixture
p pressure[bar] P product
V volume[m3] R reactant
h specificenthalpy
adest AvailabilityDestruction
AFR AirFuelRatio
Cp AtConstantPressureSpecificHeat
Cv AtVolumePressureSpecificHeat
ED EnergyDestruction
HHV HigherHeatingValueofFuel
I Irreversibility
LHV LowHeatingvalueoffuel
0,1,2,3,4 statepoints

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 01 | Jan 2025 www.irjet.net p-ISSN: 2395-0072
MR MolecularWeight
mf TheMassFlowRateofTheAirFuelMixture
ni MolesofWorkingFluid
N TotalMolecularMass
η Efficiency
ηc CombustionEfficiency
p0 InitialPressure
Q TotalHeat
R NetChangeinSpecificHeat ChangeinEntropy
Sgen EntropyGeneration
ΔSsys ChangeinEntropyofsystem
T0 AmbientTemperature
u Internalenergy
u0 InitialInternalenergy
v Volume
v0 InitialVolume
W WorkDonebyReversibleEngine
REFERENCES
[1]J .A. Caton , “On the destruction of availability (energy) due to combustion process –with specific application to internal-combustionengines,”Energy,vol.25,pp.1097-1117,2000.
[2]J.A.Caton,“Operatingcharacteristicofasparkignitionengineusingthesecondlawofthermodynamicseffectsof speedandloads”In:SAEpaperno.2000-01-0952.SocietyofAutomotiveEngineers,2000.
[3]JACaton,“Thethermodynamiccharacteristicofhighefficiency,internalcombustionengines”,EnergyConversionand Management,58(2012)84-93.
[4]K.Y.Teh,S.L.Miller,andC.F.Edwards,“Thethermodynamicrequirementsformaximuminternalcombustionengine cycleefficiency”,Int.J.EngineRes.Vol.9,2008.
[5]J.A.Caton,“Areviewofinvestigationsusingthesecondlawofthermodynamicstostudyinternalcombustionengine,” In:SAEpaperno.2000-01-1081.SocietyofAutomotiveEngineers,2000.
[6]W.RDunbar,N.Lior“Sourcesofcombustionirreversibility,”Combst.Sci.Technol,Vol.103,pp.41-61,1994.
[7]ShiparoandMoranetal.,AFundamentalofThermodynamics,seventhedition,JohnWiley&Sons,Ltd(2014).
[8]M. Koç, Y. Sekmen, T. Topgül, H.S. Yücesu, (2009) “The effects of ethanol–unleaded gasoline blends on engine performanceandexhaustemissionsinaspark-ignitionengine”RenewableEnergy34,2101-2106.
[9]H.S.Yücesu,T.Topgül,C.Çınar,M.Okur,(2006)“Effectofethanol-gasolineblendsonengineperformanceandexhaust emissionsindifferentcompressionratios”AppliedThermalEngineering26,2272–2278.
[10] İ.Sezer,İ.Altın,A.Bilgin,(2009)“ExergeticAnalysisofUsingOxygenatedFuelsinSpark-Ignition(SI)Engines” EnergyFuels23,1801-1807.
[11] Z.Fan,Z.Xia,S.Shijin,X.Jianhua,W.Jianxin,(2010)“UnregulatedEmissionsandCombustionCharacteristics ofLow-ContentMethanol-GasolineBlendedFuels”EnergyFuels24,1283–1292.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 01 | Jan 2025 www.irjet.net p-ISSN: 2395-0072
[12] M.Eyidoğan,A.N.Ozsezen,M.Çanakçı,A.Türkcan,(2010)“Impactofalcohol–gasolinefuelblendsonthe performanceandcombustioncharacteristicsofanSIengine”Fuel89,2713-2720.
[13] M.Abu-Zaid,O.Badran,J.Yamin,(2004)“EffectofMethanolAdditiononthePerformanceofSparkIgnition Engines”EnergyFuels18,312-315.
[14] M.A.Ceviz,F.Yüksel,(2005)“Effectsofethanol–unleadedgasolineblendsoncyclicvariabilityandemissions inanSIengine”AppliedThermalEngineering25,917-925.
[15] BayraktarH.2005. “Experimentalandtheoreticalinvestigationofusingsparkignitionandethanolblendsin sparkignitionengines”,RenewableEnergy30:1733-1747.
[16] L.Shenghua,E.R.C.Clemente,H.Tiegang,WYanjv,“Studyofsparkignitionenginefueledwithmethanol/ sparkignitionfuelblends”,Appl.Therm.Eng. 27(11-12)(2007)1904-1910.
[17] SomS.K.,DattA.,”Thermodynamicirreversibilitiesandexergybalanceincombustionprocesses”,Progressin EnergyandCombustionScience34(2008)351–376
[18] L. Shenghua, E.R.C. Clemente, H. Tiegang, W. Yanjv, (2007) Study of spark ignition engine fueled with methanol/gasolinefuelblends”AppliedThermalEngineering27,1904-1910.
[19] CatonJeraldA.,”Implicationsoffuel selectionforan SIengine:Resultsfrom thefirstandsecondlawsof thermodynamics”Fuel89(2010)3157–3166,insciencedirect.com
[20] İsmetSezerandAtillaBilgin, “ExergyanalysisofSIengines”Int.J.Exergy,Vol.5,No.2,2008.
[21] C.D.Rakopoulos,E.G.Giakoumis,“Secondlawanalysesappliedtointernalcombustionenginesoperation”, Prog.Energ.Combust.Sci.32(2006)2-47.
[22] Moran,M.J. “EngineeringThermodynamics“Mechanical EngineeringHandbook,CRCPressLLC,1999.
[23] GaneshanV,ACourseinInternalCombustionEngine,secondedition,Tata McGrawhill(2005).
[24] Vanwylen,Sonttag,Brognakke,Fundamentalof Thermodynamics,sixthedition.
[25] Cengeletal,thermodynamicsAnengineeringapproach,fifthedition.
[26] Cengeletal,introductiontothermodynamicsandheattransfer,secondediton.
[27] IbrahimDincerandMarcA.Rosen“Exergy,Energy,EnvironmentAnd SustainableDevelopment”2nd edition(2013).
[28] Sezer,IsmetandBilgin,Atilla”MathematicalanalysisofSparkignitionengineoperationviathecombinationof thefirstandsecondlawofthermodynamics”.fromhttp://rspa.royalsocietypublishing.org/onJune25,2015.
[29] S.Peucheret,M.L.Wyszy´nski,R.S.Lehrle,S.Golunski,H.Xu,”UseofCatalyticreformingtoaddnaturalgas HCCIcombustioninengines:experimentalandmodelingresultofopenloopfuelreforming”InternationalJournalof HydrogenEnergy30(2005)1583–1594
[30] NagP.K.,BasicandApplied Thermodynamics,second edition(2002),TataMcgrawhillpublications.
[31] AdnanA.Dahadha,NesrinTalatandSalemBarakat,“Studyoftheresearchoctanenumberdepressionof domestic kerosene-doped automotive spark ignition”, Pelagia Research Library Advances in Applied Science Research,2013,4(4):129-134.
[32] C.D.Rakopoulos,E.G.Giakoumis,”SecondLawanalysesappliedtointernalcombustionenginesoperations”, ProgressinEnergyandCombustionScience32(2006)2–47.
[33] A. Sakhrieh, E. Abu-Nada, I. Al-Hinti, Al-Ghandoor, B. Akash, “Computational thermodynamic analysis of compressionignitionengine”,InternationalCommunicationsinHeatandMassTransfer37(2010)299–303
[34] H. Serdar Yucesu , Adnan Sozen , Tolga Topgu¨ , Erol Arcakliog (2007) did “comparative study of mathematicalandexperimentalanalysisofsparkignitionengineperformanceusedethanol–sparkignitionblend fuel”,AppliedThermalEngineering, 27(2007)358–368.
[35] M.B.Çelik,(2008)“Experimentaldeterminationofsuitableethanol–gasolineblendrateathighcompression ratioforgasolineengine”AppliedThermalEngineering28,396–404.
[36] Z.Fan,Z.Xia,S.Shijin,X.Jianhua,W.Jianxin,(2010)“UnregulatedEmissionsandCombustionCharacteristics ofLow-ContentMethanol-GasolineBlendedFuels”EnergyFuels24,1283–1292
[37] Caton,J.A.‘Onthedestructionofavailability(exergy)duetothecombustionprocess-withspecificapplication totheinternalcombustionengine’,Energy,Vol.25,pp.1097–1117.
[38] Rakopoulos,C.D.andGiakoumis,E.G.‘Secondlawanalysesappliedtointernalcombustionenginesoperation’, ProgressinEnergyandCombustionScience,Vol.32,pp.2–47.
[39] Y.A.Cengel.,M.A.Boles,“Thermodynamics–anengineeringapproach,4thedition”,McGraw-Hill,NewYork, (2002).
[40] Stuart Daw, K. Chakravarthy, J Conklin and Ron L. Graves, “Minimizing destruction of thermodynamic availabilityinhydrogencombustion”,Internationaljournalofhydrogenenergy,vol.31,pp.728-736,2006
[41] C. Sayin, M. Hosoz,y, M. Canakci and I. Kilicaslan, Energy and exergy analyses of a spark ignition engine, InternationalJournalOfEnergyResearch,2006.

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 12 Issue: 01 | Jan 2025 www.irjet.net p-ISSN: 2395-0072
[42] J.S.Jadhao,D.G.Thombare,“AReviewofexhaustgasheatrecoveryofI.C.Engine” InternationalJournalof EngineeringandInnovativeTechnology(IJEIT)Volume2,Issue12,June2013
[43] Kumar Umesh and M.N.Karimi “ low grade waste heat recovery for optimized energy efficiencies and enhancedsustainabilityinprocessindustries:acomprehensivereview” Internationaljournalofmultidisciplinary sciencesandengineering,vol.5,no.4,april2014.
[44] DhayaprasadS.N.SrinivasaandParameshwarivN.“afeasibilitystudyonwasteheatrecoveryinanicengine usingelectroturbogeneration”YMCAuniversity(2012)
[45] PawarHarshalR.andLawankarShailendraM.”WasteplasticPyrolysisoilAlternativeFuelforCIEngine–A Review”ResearchJournalofEngineeringSciencesVol.2(2),26-30,February(2013)
[46] FanhuaMa,YuWang,HaiquanLiu,YongLi,JunjunWang,ShuliZhao,”Experimentalstudyonthermalefficiency andemissioncharacteristicsofaleanburnhydrogenenrichednaturalgasengine”,InternationalJournalofHydrogen Energy32(2007)5067–5075.
[47] VJLawandCaton,“Theeffectsofheattransferonperformanceandenergydestructionforaturbocharged, spark-ignitionengine”,TexasA&MUniversity,CollegeStation,TX,USA,17May2010.
[48] HeywoodJB.1998.“Internalcombustionenginefundamentals”,McGraw-Hill,NewYork.
BIOGRAPHIES

SantoshKumarGuptaisaMechanicalEngineeringgraduatefromVishveshwaryaInstitute ofEngineering&Technology,PostgraduatefromKamlaNehruInstituteofTechnologyand pursuingPhDfromMadanMohanMalviyauniversityofTechnology.HeisworkingasHead ofDepartmentunderTechnicalEducationDepartmentwithexpertiseinmechanicalengineering speciallyinSolarEnergyandThermalengg.Adynamiclearnerandproblem-solver,hehas experiencein15yearsofteachingand2yearsofindustrialexperience