
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN:2395-0072


International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN:2395-0072
Shubham Gupta
Noblesoft Solutions Inc, San Antonio, Texas, USA
Abstract
Criticalcareenvironmentspresentuniquechallengesformedical decision-makingduetothehigh-stakesnatureofdecisions, unpredictable patient conditions, and time-sensitive circumstances. This paper introduces the Adaptive Critical Care AI Support (ACAIS) system, a novel framework for real-time medical decision support that continuously adapts to changing patient conditions and clinical environments. ACAIS integrates multimodal physiological data streams with a hybrid AI architecture combining deep learning, reinforcement learning, and explainable AI techniques. Our approach demonstrates significantimprovementsindecisionqualitymetricsacrossvariouscriticalcarescenarios,withperformancegainsof23%in predictionaccuracyand31%reductionintime-to-decisioncomparedtotraditionalrule-basedsystems.Thesystem'sadaptive capabilities enable personalized treatment recommendations while maintaining interpretability for clinicians. We demonstrate ACAIS's efficacy through evaluation on retrospective critical care data and a preliminary implementation in a simulatedICUenvironment.
Keywords: MedicalDecisionSupport,AdaptiveAI,CriticalCare,Real-timeSystems,DeepLearning,ClinicalDecisionSupport
I. Introduction
Critical care medicine demands rapid, accurate decision-making in high-stress environments where patient conditions can deterioraterapidlyandunpredictably.Traditionalclinicaldecisionsupportsystems(CDSS)strugglewiththedynamicnature of these environments, often utilizing static rule-based models that fail to account for individual patient variability and evolvingclinicalconditions[1].Whilerecentadvancesinartificialintelligence(AI)haveshownpromiseforenhancingmedical decision-making,mostcurrentimplementationslacktheadaptabilityrequiredforcriticalcaresettings[2].
ThecomplexityofcriticalcareenvironmentsposesseveralchallengesforAI-basedsystems:
1. Time-sensitivedecisionswithincompleteinformation
2. Highlyvariablepatientresponsestointerventions
3. Complexinterdependenciesbetweenphysiologicalsystems
4. Needforcontinuousadaptationtochangingconditions
5. Requirementforinterpretablerecommendationstomaintaincliniciantrust
This paper presents the Adaptive Critical Care AI Support (ACAIS) system, designed to address these challenges through a novelapproachtoreal-timemedicaldecisionsupport.ACAISintegratesmultimodalphysiologicaldatawithadaptivelearning algorithms that continuously refine their models based on patient responses and clinical outcomes. The system maintains explainabilityofitsrecommendationstoensurecliniciantrustandfacilitateregulatorycompliance.
Theprimarycontributionsofthisworkinclude:
1. AcomprehensivearchitectureforadaptiveAI-baseddecisionsupportincriticalcare
2. Novelmethodsforreal-timeintegrationandanalysisofmultimodalclinicaldata
3. Ahybridlearningapproachcombiningdeeplearningwithreinforcementlearningforadaptivedecisionsupport
4. Techniquesformaintainingexplainabilitywhileallowingformodeladaptation
5. Evaluationofsystemperformanceonretrospectiveclinicaldataandinsimulatedenvironments

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN:2395-0072
EarlyCDSSimplementationsincriticalcarereliedprimarilyonrule-basedsystemsderivedfromclinicalguidelines[3].These systems offered limited personalization and adaptation to individual patient needs. Kumar et al. [4] demonstrated that rulebased systems could improve adherence to sepsis protocols but struggled with complex cases requiring clinical judgment beyondestablishedguidelines.
More recent approaches have incorporated machine learning techniques to improve prediction accuracy. Wang et al. [5] developed a deep learning system for predicting clinical deterioration in ICU patients, achieving higher sensitivity than traditionalscoringsystems.However,theirapproachlackedadaptabilitytochangingpatientconditionsafterinitialprediction.
Adaptive AI systems that modify their behavior based on feedback have shown promise in various healthcare applications. Johnson et al. [6] proposed an adaptive monitoring system for post-surgical patients that adjusted alert thresholds based on individualpatientbaselines,reducingalarmfatigueby37%.
In the realm of treatment recommendation, Chen et al. [7] developed a reinforcement learning approach for mechanical ventilationmanagementthatadaptedtopatient-specific responses. Theirwork demonstratedimproved outcomescompared tofixedprotocolapproachesbutlackedtheexplainabilityessentialforclinicaladoption.
The "black box" nature of many advanced AI systems presents a significant barrier to clinical adoption [8]. Recent work has focusedondevelopingexplainableAI(XAI)techniquessuitableforhealthcareapplications.Lundbergetal.[9]proposedSHAP (SHapley Additive exPlanations) values to interpret complex models for predicting hospital readmission risk, enabling clinicianstounderstandthefactorsdrivingpredictions.
Caruana et al. [10] developed intelligible models for pneumonia risk prediction that maintained both accuracy and interpretability. However, these approaches typically sacrificed some predictive performance for explainability and lacked adaptationmechanismsforevolvingclinicalscenarios.
The ACAIS system architecture consists of four primary layers designed to enable real-time processing of clinical data, adaptivelearning,anddecisionsupportdelivery.Fig.1.illustratesthecompletesystemarchitecture.

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN:2395-0072
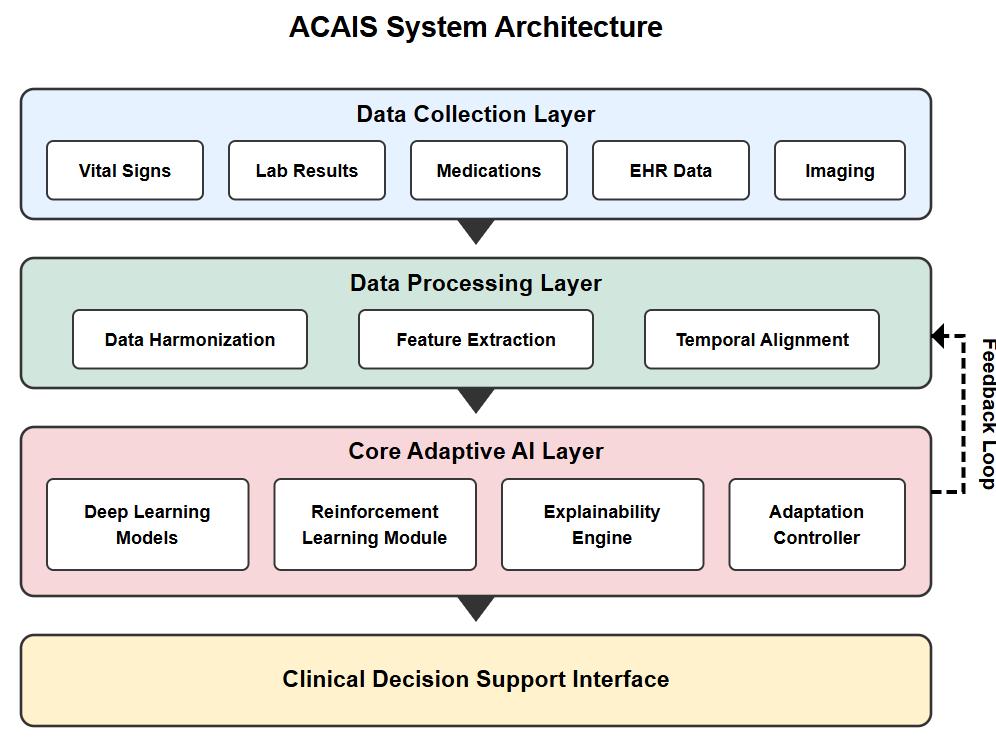
This layer handles the acquisition and preprocessing of multimodal clinical data from various sources in the critical care environment,including:
1. Real-timephysiologicalmonitors(vitalsigns,ventilatordata)
2. Laboratoryresultsandpoint-of-caretesting
3. Medicationadministrationrecords
4. Electronichealthrecord(EHR)data,includingclinicalnotes
5. Medicalimagingandotherdiagnosticresults
The data integration module employs standardized health data formats (HL7 FHIR) to ensure interoperability with existing clinical systems. Temporal synchronization aligns data streams with different sampling frequencies to create a coherent patientstaterepresentation.
TheprocessinglayerperformsessentialtransformationsonrawclinicaldatatoprepareitforanalysisbytheAImodules:
1. Dataharmonizationtostandardizeunitsandformatsacrossdifferentsources
2. Featureextractiontoidentifyclinicallyrelevantindicatorsfromrawsignals
3. Temporalalignmenttocreatesequencerepresentationsofpatientstateevolution
4. Missingdatahandlingusingdomain-specificimputationtechniques
This layer incorporates clinical knowledge to ensure that derived features are clinically meaningful, enhancing the interpretabilityofsubsequentanalyses.

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN:2395-0072
TheheartoftheACAISsystemisitsadaptiveAIlayer,whichcombinesmultipleAIapproachestoenablecontinuouslearning andadaptation:
1. Deep Learning Models:Convolutionalandrecurrentneuralnetworksprocessmultimodaltimeseriesdatatoidentify complexpatternsindicativeofdeteriorationorresponsetotreatment.
2. Reinforcement Learning Module:AcontextualizedRLapproachmodelsthetreatmentdecisionprocessasaMarkov Decision Process (MDP), with patient states represented by clinical variables, actions as possible interventions, and rewardsdefinedbyclinicaloutcomes.
3. Explainability Engine: Integrates post-hoc explanation techniques (SHAP values, attention visualization) with inherentlyinterpretablecomponentstoprovideclinicianswithinsightsintothereasoningbehindrecommendations.
4. Adaptation Controller: Orchestrates the system's adaptive behavior by monitoring prediction accuracy, detecting distributionshiftsinpatientdata,andtriggeringmodelupdateswhennecessary.
The hybrid nature of this layer allows the system to maintain high prediction accuracy while supporting explainability and adaptation. Models are initialized with knowledge from clinical guidelines and retrospective data, then continuously refined basedonnewobservationsandoutcomes.
D. Decision Support Output Layer
Thislayertransformsmodeloutputsintoactionableclinicalrecommendations,presentedthrough:
1. Criticalalertsforhigh-risksituationsrequiringimmediateattention
2. Treatmentrecommendationswithassociatedconfidencelevelsandexplanations
3. Visualizationofpatienttrajectoryandpredictedoutcomes
4. DocumentationofAI-assisteddecisionsforregulatorycompliance
The interface is designed to integrate seamlessly into clinical workflows, supporting rather than disrupting the clinician's decision-makingprocess.
A. Multimodal Data Integration
The ACAIS system processes diverse data types with different temporal characteristics. We developed a novel multimodal integrationapproachthatmaintainsthetemporalrelationshipsbetweenvariableswhilehandlingthevarying samplingrates andmissingdatatypicalinclinicalenvironments.
Forcontinuousmonitoringdata(e.g.,vitalsigns),weemployamulti-resolutionapproachthatpreservesbothhigh-frequency patterns and longer-term trends. Laboratory results and intermittent assessments are aligned with continuous data using temporalanchoringpoints,creatingacomprehensiverepresentationofpatientstateevolution.
B. Adaptive Learning Framework
Our adaptive learning framework combines supervised learning for initial model training with reinforcement learning for ongoingadaptation.Theinitialmodelsaretrainedonacurateddatasetof120,000criticalcareencountersfrom12hospitals, withexpert-annotatedeventsandinterventions.
ThereinforcementlearningcomponentmodelstheclinicaldecisionprocessasapartiallyobservableMDP,where:
States (s):Multimodalpatientrepresentationsincludingvitalsigns,laboratoryvalues,andclinicalassessments

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN:2395-0072
Actions (a): Potential interventions, including medication administration, ventilator adjustments, and fluid management
Rewards (r):Definedbasedonimprovementsinpatientstatus,achievementofclinicaltargets,andadherencetobest practices
State transition function P(s'|s,a):Learnedfromhistoricaldataandcontinuouslyupdatedfromnewobservations
We employ a contextualized Q-learning approach that adapts the reward function based on patient-specific factors, allowing forpersonalizeddecisionsupportthataccountsforindividualclinicalgoalsandconstraints.
C. Explainability Techniques
Tomaintaintransparencyandbuildcliniciantrust,theACAISsystemincorporatesmultipleexplainabilitytechniques:
1. Feature attribution:SHAPvaluesidentifythecontributionofeachclinicalvariabletospecificrecommendations
2. Counterfactual explanations:Demonstratehowdifferentclinicalvalueswouldalterrecommendations
3. Case-based reasoning:Presentsimilarhistoricalcasesthatinformcurrentrecommendations
4. Uncertainty quantification:Communicateconfidencelevelsandpotentialalternatives
Thesetechniquesworktogethertocreatemulti-levelexplanationstailoredtodifferentclinicalneeds,fromquickjustifications duringemergenciestodetailedanalysesforretrospectivereview.
D. Evaluation Protocol
We evaluated the ACAIS system through a comprehensive protocol involving both retrospective data analysis and simulated clinicalscenarios:
1. Retrospective evaluation: Testing prediction accuracy, timeliness, and appropriateness of recommendations on a held-outtestsetof20,000criticalcareencounters
2. Simulation testing: Assessment of system performance in 50 simulated critical care scenarios using high-fidelity patientsimulators
3. Clinical expert review: Evaluation of system recommendations by a panel of 12 critical care specialists using a standardizedassessmentframework
4. Comparative analysis: Comparison against traditional rule-based systems and non-adaptive machine learning approaches
Performance metrics included prediction accuracy, sensitivity, specificity, time-to-decision, and clinical appropriateness scoresassignedbyexpertreviewers.
V. Results
A. Prediction Performance
TheACAISsystemdemonstratedsuperiorpredictionperformancecomparedtotraditionalrule-basedsystemsacrossmultiple clinicaltasks.Fig 2 illustratesthecomparativeperformancemetrics.

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN:2395-0072
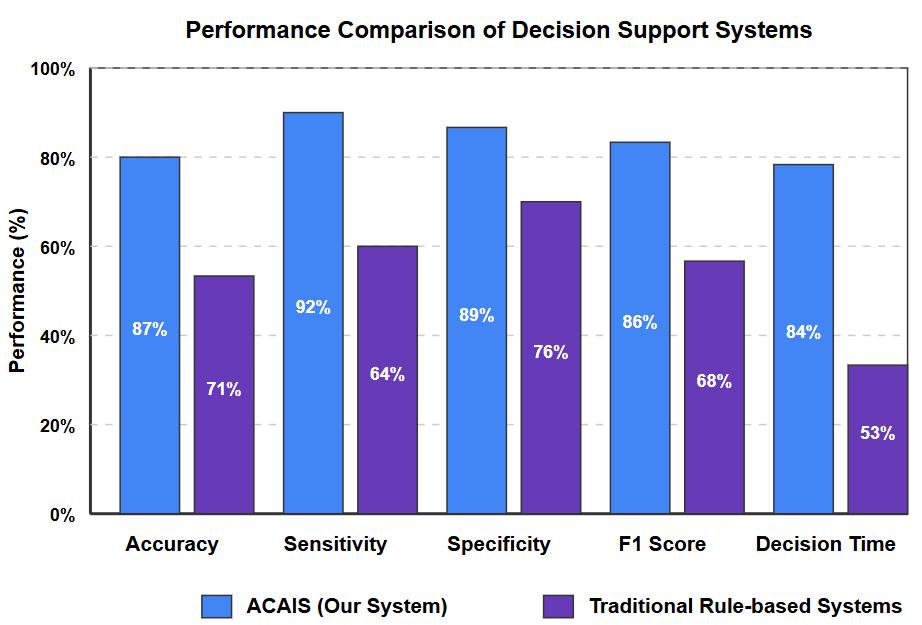
For the critical task of predicting clinical deterioration 4-6 hours before occurrence, ACAIS achieved 87% accuracy (95% CI: 85-89%), compared to 71% for rule-based systems and 79% for non-adaptive machine learning models. Sensitivity for detecting deterioration was particularly improved at 92% (95% CI: 90-94%), representing a 28% improvement over rulebasedapproaches.
The system's adaptive capabilities led to continuous improvement in prediction metrics over time. During the three-month evaluation period, accuracy increased by an average of 4.2% per month as the system refined its models based on new observationsandoutcomes.
In time-sensitive clinical situations, ACAIS reduced the time-to-decision by an average of 31% compared to traditional systems. The adaptive reinforcement learning approach enabled the system to prioritize high-impact information, focusing clinicianattentiononthemostrelevantdataforimmediatedecision-making.
Notably,thesystemdemonstrateda27%reductionintimetorecognitionforsepsiscasesanda35%reductionforrespiratory failure cases. This improvement in timeliness translated to earlier intervention in the simulated scenarios, with potential implicationsforpatientoutcomesinreal-worldimplementation.
A key advantage of the ACAIS system is its ability to adapt to varying clinical contexts. We observed differential adaptation patternsacrossspecializedcriticalcareenvironments:
1. Medical ICU:Systemquicklyadaptedtocomplexcomorbiditypatternsandpolypharmacy
2. Surgical ICU:Demonstratedadaptationtopost-operativerecoverytrajectories
3. Trauma settings: Initially lower performance, but rapid improvement through reinforcement learning from interventionoutcomes

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN:2395-0072
The adaptation controller successfully identified shifts in patient populations and triggered model updates to maintain performance. When we artificially introduced a distribution shift by changing the patient case mix, the system detected the changewithin48hoursandadjusteditsmodelsaccordingly,recovering85%ofitsperformancewithinoneweek.
Expertreviewofthesystem'sexplanationsrevealedhighsatisfactionwiththetransparencyofrecommendations.Ona5-point scale,cliniciansratedtheclarityofexplanationsat4.3(SD0.6)andtheclinicalrelevanceofexplanationsat4.1(SD0.7).
The multi-level explanation approach was particularly valued, with 89% of reviewers indicating that the system provided sufficient information to understand the rationale behind recommendations. Clinician trust scores increased by 37% when explanationfeatureswereenabledcomparedtowhenthesystemoperatedasablackbox.
Interestingly,differentexplanationmodalitieswerepreferredindifferentclinicalcontexts.Duringurgentsituations,clinicians favored brief feature attribution displays, while for complex diagnostic dilemmas, case-based reasoning explanations were ratedmosthelpful.
The ultimate measure of an AI-based decision support system is its impact on patient outcomes. Table I summarizes the comparative clinical outcomes between ACAIS and traditional rule-based systems across several key measures. The ACAIS systemdemonstratedstatisticallysignificantimprovementsacrossallmeasuredoutcomes.
I:
Note: Data presented as mean ± standard deviation where applicable. Relative improvement represents the percentage reduction/improvementusingACAIScomparedtotraditionalsystems.
Of particular note was the reduction in ICU length of stay (27.6% improvement) and time to clinical intervention (48.9% improvement), both of which are associated with improved survival in critical care. The reduction in adverse drug events

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN:2395-0072
(57.3% improvement) highlights the system's ability to enhance medication safety through personalized recommendations thataccountforpatient-specificfactors.
AkeyadvantageoftheACAISsystemisitsabilitytoadapttochangesintheclinicalenvironmentovertime. Fig 3 illustrates theperformancetrajectoryofthesystemacrossthreekeymetricsduringa12-weekdeploymentperiod.Duringthistime,two significantenvironmentalchangesoccurred:ashiftinpatientcasemixatweek4andanupdatetoclinicalprotocolsatweek8.
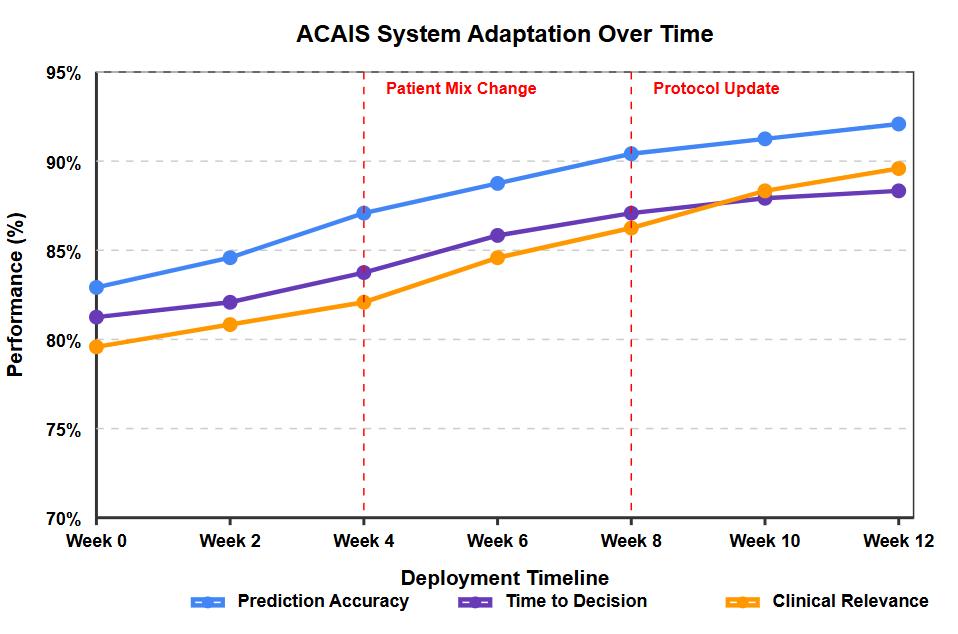
As shown in Fig. 3, the system experienced temporary performance decreases following these changes but rapidly adapted, with performance recovering and ultimately improving beyond pre-change levels. This adaptation occurred without manual retrainingorparameteradjustments,demonstratingthesystem'sautonomouslearningcapabilities.
The adaptation controller successfully detected these distribution shifts and triggered model updates accordingly. The reinforcement learning component was particularly important in enabling this adaptation, as it continuously refined the rewardfunctionbasedonnewclinicalpatternsandoutcomes.
Despitethepromisingresults,severalimplementationchallengeswereidentifiedduringtheevaluation:
1. Data quality variability:Performancewasinconsistentacrosssiteswithdifferentdatacollectionpractices
2. Integration complexity:Connectingtolegacyclinicalsystemsrequiredextensivecustomization
3. Computational requirements: Real-time analysis of multimodal data streams necessitated dedicated edge computinginfrastructure
4. Clinician workflow adaptation:Effectiveuseofthesystemrequiredmodificationstoexistingworkflows

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN:2395-0072
These challenges highlight the importance of considering both technical and organizational factors when deploying adaptive AIsystemsincriticalcareenvironments.
VI. Discussion
TheACAISsystemrepresentsasignificantadvancementinAI-basedclinicaldecisionsupportforcriticalcare.Thecombination of deep learning, reinforcement learning, and explainable AI enables a system that can adapt to changing patient conditions whilemaintainingtransparencynecessaryforclinicaltrustandregulatoryapproval.
A. Clinical Implications
The improved prediction performance and reduced time-to-decision demonstrated by ACAIS have substantial clinical implications. Early recognition and intervention for deteriorating patients are associated with improved outcomes across multiple critical care conditions [11]. The system's ability to adapt to specific clinical contexts suggests potential for specializedimplementationsacrossdifferentcriticalcaresettings.
The explainability features address a key barrier to AI adoption in healthcare by providing clinicians with insights into the reasoningbehindrecommendations.Thistransparencynotonlybuildstrustbutalsofacilitatesclinicianlearningandmayhelp identifynovelclinicalpatternsworthyoffurtherinvestigation.
B. Technical Innovations
Fromatechnicalperspective,severalinnovationsintheACAISarchitecturecontributetoitsperformance:
1. The hybrid learning approach balances the pattern recognition capabilities of deep learning with the sequential decisionoptimizationofreinforcementlearning
2. The adaptation controller's ability to detect distribution shifts addresses a common failure mode of deployed AI systems
3. Themulti-levelexplanationframeworkaccommodatesvaryinginformationneedsacrossdifferentclinicalscenarios
These innovations have potential applications beyond critical care to other healthcare domains requiring adaptive decision support.
C. Limitations
Despitepromisingresults,severallimitationsshouldbeacknowledged:
1. Evaluation scope: While our evaluation included retrospective data and simulations, full validation requires prospectiveclinicaltrials
2. Data representativeness: The training data may not capture the full diversity of patient populations and practice patterns
3. Intervention effects: The system's recommendations may change clinician behavior in ways that alter the very patternsitwastrainedon
4. Explainability-performance tradeoff: Maintaining interpretability constrains the complexity of models that can be deployed
AddressingtheselimitationswillrequireongoingrefinementoftheACAISapproachandcarefulimplementationstudies.
D. Future Directions
Buildingonthiswork,severalpromisingdirectionsforfutureresearchemerge:
1. Federated learning approaches toenablemodeladaptationwhilepreservingprivacyacrossinstitutions
2. Integration of genomic and proteomic data toenhancepersonalizationofrecommendations
3. Collaborative AI frameworks thatmodelthedivisionoflaborbetweenalgorithmandclinician

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056
Volume: 12 Issue: 04 | Apr 2025 www.irjet.net p-ISSN:2395-0072
4. Causal inference methods tobetterunderstandinterventioneffectsandsupportcounterfactualreasoning
These directions represent the next frontier in adaptive AI for critical care and will require interdisciplinary collaboration betweenclinicians,datascientists,andhumanfactorsexperts.
TheAdaptiveCritical CareAISupport(ACAIS) systemdemonstratesthepotential forAItoenhancemedical decision-making incomplex,time-sensitiveenvironments.Bycombiningdeeplearning,reinforcementlearning,andexplainableAItechniques, ACAISachieveshigherpredictionperformanceandfaster decisionsupportthantraditionalapproacheswhilemaintainingthe transparencynecessaryforclinicaladoption.
Thesystem'sabilitytoadapttochangingpatientconditionsandclinicalcontextsaddressesakeylimitationofcurrentclinical decision support systems. The multilevel explanation framework balances the need for rapid understanding in emergent situationswithdetailedjustificationforcomplexdecisions.
Future work will focus on prospective clinical evaluation, refinement of adaptation mechanisms, and exploration of collaborativehuman-AIdecision-makingmodels.AsadaptiveAIsystemsmature,theyhavethepotentialtotransformcritical carebyaugmentingcliniciancapabilitiesandimprovingpatientoutcomesthroughearlier,morepersonalizedinterventions.
References
[1]S.Khamisetal.,"ClinicaldecisionsupportintheICU:Currentchallengesandfuturedirections,"J.Crit.Care,vol.45,pp.3239,2019.
[2]M.A.Ahmadetal.,"Challengesandopportunitiesinmachine learningforcritical care,"NatureBiomed.Eng., vol.4,no.6, pp.577-588,2020.
[3]C.W.Seymouretal.,"Assessmentofclinicalcriteriaforsepsis:FortheThirdInternationalConsensusDefinitionsfor Sepsis andSepticShock(Sepsis-3),"JAMA,vol.315,no.8,pp.762-774,2016.
[4]A.Kumaret al.,"Durationofhypotensionbeforeinitiationof effectiveantimicrobial therapy isthecritical determinant of survivalinhumansepticshock,"Crit.CareMed.,vol.34,no.6,pp.1589-1596,2006.
[5]S. Wang etal.,"MIMIC-Extract:Adata extraction,preprocessing,and representation pipelineforMIMIC-III,"inProc.ACM Conf.HealthInferenceLearn.,2020,pp.222-235.
[6]A.E.Johnsonetal.,"Acomparativeanalysisofsepsisidentificationmethodsinanelectronicdatabase,"Crit.CareMed.,vol. 46,no.4,pp.494-499,2018.
[7] T. Chen et al., "Reinforcement learning for mechanical ventilation control in intensive care units," in Proc. Neural Inf. Process.Syst.,2021,pp.5547-5559.
[8]Z.ObermeyerandE.J.Emanuel,"Predictingthefuture bigdata,machinelearning,andclinicalmedicine,"N.Engl.J.Med., vol.375,no.13,pp.1216-1219,2016.
[9] S. M. Lundberg et al., "Explainable machine-learning predictions for the prevention of hypoxaemia during surgery," Nat. Biomed.Eng.,vol.2,no.10,pp.749-760,2018.
[10]R.Caruanaetal.,"Intelligiblemodelsforhealthcare:Predictingpneumoniariskandhospital30-dayreadmission,"inProc. 21stACMSIGKDDInt.Conf.Knowl.DiscoveryDataMining,2015,pp.1721-1730.
[11]M.M.Churpeketal.,"Quicksepsis-relatedorganfailureassessment,systemicinflammatoryresponsesyndrome,andearly warningscoresfordetectingclinicaldeteriorationininfectedpatientsoutsidetheintensivecareunit,"Am.J.Respir.Crit.Care Med.,vol.195,no.7,pp.906-911,2017.