Understanding TECVAYLI®


The IMF InfoLine team is here to support you and your loved ones with the most up-to-date information about myeloma
Call the IMF InfoLine at 1.800.452.CURE (toll-free in the U.S. & Canada) or 1.818.487.7455 (worldwide), or email InfoLine@myeloma.org with your questions, or if you wish to discuss the contents of this booklet.
Want answers and don’t want to wait?
Get the answers you need anytime from Myelo® , your 24/7 generative AI assistant that is designed to support you on your journey with myeloma. Ask Myelo your questions at myeloma.org.
Join the Myeloma Knowledge Platform
Visit myprofile.myeloma.org and create your personal online IMF account to receive the most helpful content recommendations tailored to support your unique myeloma journey.
You are not alone
The International Myeloma Foundation (IMF) is here to help you. The IMF is committed to providing information and support for patients with multiple myeloma (which we refer to simply as “myeloma”) and their care partners, friends, and family members.
The IMF supports the myeloma community with a broad range of resources available on our website myeloma.org, and through numerous programs and services such as seminars, webinars, workshops, and the IMF InfoLine.
Contact the IMF InfoLine with your myeloma-related questions and concerns, and receive the most up-to-date information in a compassionate and caring manner. Call 1.818.487.7455, email InfoLine@myeloma.org, or visit mmsm.link/infoline to schedule a convenient time to talk with an IMF InfoLine Coordinator.
What you will learn from this booklet
Myeloma is a cancer that is not known to most patients at the time of diagnosis. To play an active role in your own medical care and to make good decisions about your care in partnership with your doctor, it is important and helpful to learn about myeloma, as well as its treatment options and supportive care measures.
If you are a patient with myeloma, we suggest that you read the IMF’s publication, Patient Handbook for Multiple Myeloma, which presents an overview of the disease and its treatment options. In addition, this booklet will direct you to resources that may be relevant in your particular case.
The IMF’s Understanding-series publications address specific drugs, drug classes, combination therapies, and supportive care measures that may help you manage the symptoms and side effects of myeloma and its treatments.
Words in bold+blue are explained in the IMF’s companion publication, Understanding Myeloma Vocabulary, which you can access directly at glossary.myeloma.org. If you prefer to read any of the IMF’s publications in electronic format, the light blue links will take you to the corresponding resources. All IMF publications are free-of-charge and can be read, downloaded, or requested in printed format at publications.myeloma.org.
This booklet discusses Tecvayli® (also known as teclistamab-cqyv, its generic drug name), the first in a drug class of bispecific antibodies to be used for the treatment of myeloma. Tecvayli was granted accelerated approval by the U.S. Food and Drug Administration (FDA) in October 2022 for the treatment of adult patients with myeloma.
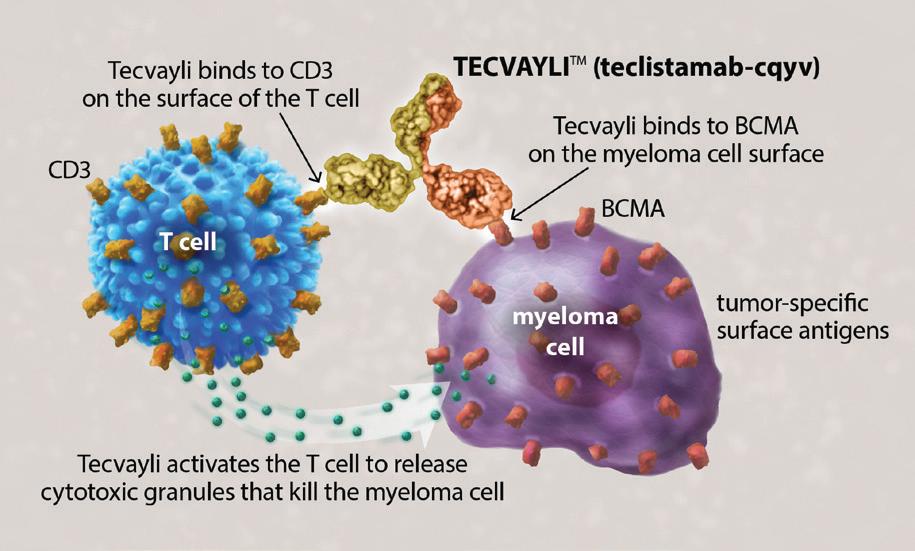
How Tecvayli works
Tecvayli is an immunotherapy that enhances a patient’s own immune system to attack their myeloma cells. Tecvayli is a bispecific antibody, a laboratory-made monoclonal antibody that binds to two (“bi”) targeted cells.
Tecvayli has two “arms.” One arm binds to the myeloma cell surface by adhering to the B-cell maturation antigen (BCMA). The other arm binds to a local T cell by its CD3 antigen. Tecvayli brings the myeloma cell and the T cell together and activates the T cell to release cytotoxic granules to destroy the myeloma cell.
A similar strategy is already used in myeloma. Chimeric antigen receptor (CAR) T-cell therapy involves collecting the patient’s T cells, engineering them in a laboratory to express a receptor that can attach to BCMA on the surface of a myeloma cell, multiplying the engineered T cells in a laboratory, then re-infusing them into the patient to attack their myeloma.
Tecvayli differs from CAR T-cell therapy in that there is no need to collect the patient’s T cells. Instead, Tecvayli engages the patient’s T cells directly after injection. Not having to collect, engineer, and manufacture T cells over the course of several weeks shortens the time-to-treatment for the myeloma patient. The “off-the-shelf” availability of Tecvayli makes it easier to access treatment.
Indications for treatment with Tecvayli
Tecvayli is FDA-approved for adult patients with relapsed or refractory multiple myeloma (RRMM) who have received at least 4 prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent , and an anti-CD38 monoclonal antibody.
Tecvayli administration, dosing, and schedule
When Tecvayli is injected, there is a risk that the patient’s immune system may react in a way that causes a cytokine release syndrome (CRS), which is explained in the next section of this booklet. Therefore, Tecvayli is given at lower doses to begin with so it can be tolerated better by the patient’s immune system.
At the discretion of the treating doctor, the patient may be admitted to the hospital for 48 hours after the administration of each “step-up” dose of Tecvayli. Alternatively, the individual may be monitored on an outpatient basis for CRS and the risk of neurologic toxicity.
The recommended dosing schedule for Tecvayli is provided in Table 1. The two step-up doses of Tecvayli are 0.06 mg/kg then 0.3 mg/kg. The regular once-weekly treatment dose is 1.5 mg/kg given until disease progression or unacceptable side effects. Pretreatment medications are given prior to each dose of the Tecvayli step-up dosing schedule, which includes step-up dose 1, step-up dose 2, and the first treatment dose.
a. See Table 2 for recommendations on restarting Tecvayli after dose delays.
b. Step-up dose 2 may be given between 2 to 4 days after step-up dose 1 and may be given up to 7 days after step-up dose 1 to allow for resolution of side effects.
c. First treatment dose may be given between 2 to 4 days after step-up dose 2 and may be given up to 7 days after step-up dose 2 to allow for resolution of side effects.
Warnings and precautions
The most common side effect occurring in 20% or more patients treated with Tecvayli in the MajesTEC-1 clinical trial are fever, CRS, musculoskeletal pain, injection site reaction, fatigue, upper respiratory tract infection, nausea, headache, pneumonia, and diarrhea.
The most common severe laboratory abnormalities occurring in 20% or more of patients treated with Tecvayli are decreased lymphocytes, decreased neutrophils, decreased white blood cells, decreased hemoglobin, and decreased platelets.
Tecvayli is available only through a Risk Evaluation and Mitigation Strategy (REMS) restricted program. The FDA requires a REMS program if a specific drug or treatment has serious safety concerns. REMS programs support the use of such drugs or treatments and help ensure that the potential benefits outweigh the risks.
Cytokine release syndrome (CRS)
Cytokines are proteins that are produced all over the body, including in the bone marrow. Cytokines circulate in the bloodstream, stimulating or inhibiting the growth and/or activity of other cells. CRS is a potentially fatal, uncontrolled immune reaction in which levels of cytokines become highly elevated and trigger an overwhelming immune system response. A “cytokine storm” can seriously damage body tissues and organs.
Table 2. Restarting therapy with Tecvayli after dose delay
Last
Step-up dose 1
More than 7 days
8 days to 28 days
Step-up dose 2
Any treatment dose
More than 28 daysb
8 days to 28 days
More than 28 daysb
Recommendations
Restart Tecvayli step-up dosing schedule at step-up dose 1 (0.06 mg/kg).a
Repeat step-up dose 2 (0.3 mg/kg)a and continue Tecvayli step-up dosing schedule.
Restart Tecvayli step-up dosing schedule at step-up dose 1 (0.06 mg/kg).a
Continue Tecvayli weekly dosing schedule at treatment (1.5 mg/kg).a
Restart Tecvayli step-up dosing schedule at step-up dose 1 (0.06 mg/kg).a
a. Pretreatment medications are given prior to Tecvayli dose and patients are monitored accordingly.
b. Discuss with your doctor the benefit-risk of restarting Tecvayli if you require a dose delay of more than 28 days due to a side effect.
In the MajesTEC-1 clinical trial of Tecvayli in RRMM, CRS occurred in 72% of patients who received Tecvayli at the recommended dose, with Grade 1 CRS occurring in 50% of patients, Grade 2 in 21%, and Grade 3 in 0.6%. Most patients experienced CRS following step-up dose 1, step-up dose 2, or the first treatment dose. After subsequent doses of Tecvayli, less than 3% of study participants developed CRS. The median time to onset of CRS was 2 days (range of 1 to 6 days) after the most recent dose, with a median duration of 2 days (range of 1 to 9 days). Clinical signs and symptoms of CRS included, but were not limited to, fever, hypoxia, chills, hypotension, sinus tachycardia, headache, and elevated liver enzymes.
At the 2024 American Society of Clinical Oncology (ASCO) annual meeting, conclusions were presented from the phase I/II MajesTEC-1 clinical trial cohort of patients receiving prophylactic tocilizumab (toci) for the reduction of CRS (van de Donk). In patients receiving Tecvayli, the use of prophylactic toci resulted in 65% relative reduction of the overall incidence of CRS. Longer-term follow-up did not reveal new safety concerns or compromise the efficacy of Tecvayli.
Prevention and treatment of CRS
To reduce risk of CRS, therapy with Tecvayli should be started with the step-up dosing schedule. Pretreatment medications are given to reduce risk of CRS. Patients are monitored following administration of Tecvayli. At the first sign of CRS, immediate evaluation should be made to consider the patient for hospitalization. Supportive care is administered based on severity of CRS. Further management should follow current practice guidelines. Tecvayli administration may be interrupted or discontinued, depending on the severity of the CRS.
Neurologic toxicities
Immune effector cell-associated neurotoxicity syndrome (ICANS) has occurred in patients following treatment with Tecvayli. ICANS often correlates with CRS but it can also occur in the absence of CRS. After treatment with Tecvayli, ICANS can occur before, during, or after CRS onset, or after CRS resolution. ICANS may be severe, life-threatening, or fatal. Symptoms of neurologic side effects include but are not limited to confusion, disorientation, loss of consciousness, seizures, tremor, slower movements, changes in personality, depression, tingling and numbness of hands and feet, leg and arm weakness, facial numbness, and difficulty speaking, reading, or writing.
In the MajesTEC-1 clinical trial, neurologic toxicity occurred in 57% of patients who received Tecvayli at the recommended dose, with Grade 3 or 4 neurologic toxicity occurring in 2.4% of patients. The most frequent
neurologic toxicities were headache (25%), motor dysfunction (16%), sensory neuropathy (15%), and encephalopathy (13%). With longer follow-up of patients who received Tecvayli, Grade 4 seizure occurred in 1 patient and fatal Guillain-Barré syndrome (GBS) occurred in 1 patient. ICANS was reported in 6% of patients who received Tecvayli at the recommended dose. Recurrent ICANS occurred in 1.8% of patients. Most patients experienced ICANS following step-up dose 1 (1.2%), step-up dose 2 (0.6%), or the initial treatment dose (1.8%). Less than 3% of patients developed first occurrence of ICANS following subsequent doses of Tecvayli. The median time to onset of ICANS was 4 days (range of 2 to 8 days) after the most recent dose with a median duration of 3 days (range of 1 to 20 days).
Prevention and treatment of neurologic toxicities
Patients must be monitored for signs and symptoms of neurologic toxicity during treatment with Tecvayli. At the first sign of neurologic toxicity, including ICANS, patients are provided supportive therapy based on severity, and Tecvayli treatment may also be withheld.
Patients are advised to refrain from driving or operating heavy or potentially dangerous machinery during and for 48 hours after completion of Tecvayli step-up dosing schedule and in the event of new onset of any neurologic toxicity symptoms until neurologic toxicity resolves.
Hepatotoxicity
Tecvayli can cause hepatotoxicity. Drug-induced hepatotoxicity is an injury to the liver or an impairment of the liver function caused by exposure to a specific compound.
In the MajesTEC-1 clinical trial, there was 1 fatal case of hepatic failure. Elevation of the liver enzyme aspartate aminotransferase (AST) occurred in 34% of patients, with Grade 3 or 4 elevations in 1.2%. Elevated alanine aminotransferase (ALT) occurred in 28% of patients, with Grade 3 or 4 elevations in 1.8%. Elevated total bilirubin occurred in 6% of patients with Grade 3 or 4 elevations in 0.6%. Liver enzyme elevation can occur with or without concurrent CRS.
Prevention and treatment of hepatotoxicity
Liver enzymes and bilirubin should be measured at baseline and during treatment as clinically indicated. Tecvayli may be withheld or discontinued based on severity.
Infections
Tecvayli can cause severe, life-threatening, or fatal infections. In patients who received Tecvayli at the recommended dose in the MajesTEC-1 clinical
trial, serious infections occurred in 30% of patients, with Grade 3 or 4 infections in 35%, and fatal infections in 4.2%.
Prevention and treatment of infections
It is essential for your doctor to monitor you for signs and symptoms of infection prior to and during treatment with Tecvayli. It is also essential for you to promptly alert your doctor if you experience any infection. Based on the severity of your infection, your doctor may prescribe antibiotics and/or withhold or discontinue Tecvayli. Your immunoglobulin levels should be monitored to assess if you need intravenous immunoglobulin (IVIG) therapy.
Neutropenia
Neutropenia is a reduced level of neutrophils, a type of white blood cell necessary to combat bacterial infections. Having too few neutrophils can lead to infection. Fever is the most common sign of neutropenia. If you have a fever, you must get immediate medical attention.
Tecvayli can cause neutropenia and febrile neutropenia. In patients who received Tecvayli at the recommended dose in the MajesTEC-1 clinical trial, decreased neutrophils occurred in 84% of patients, with Grade 3 or 4 decreased neutrophils in 56%. Febrile neutropenia occurred in 3% of patients.
Prevention and treatment of neutropenia
Complete blood count (CBC) should be measured at baseline and periodically during treatment. Patients may need growth factors. Signs or symptoms of infections should be discussed with the healthcare team.
Hypersensitivity and other ARR
Tecvayli can cause both systemic and local administration-related reactions (ARR). In the MajesTEC-1 clinical trial, 1.2% of patients who received Tecvayli at the recommended dose experienced systemic ARR (including Grade 1 recurrent fever and Grade 1 swollen tongue), and 35% of patients experienced local ARR (with 30% Grade 1 and 4.8% Grade 2).
Prevention and treatment of hypersensitivity and other ARRs
Based on the severity of ARR, Tecvayli may be withheld or discontinued.
Embryo-fetal toxicity
Embryo-fetal toxicity is an exposure of an embryo or a fetus to a toxic substance. Females of reproductive potential and males with female partners of reproductive potential should ask the treating doctor if the use of effective contraception is necessary before treatment begins, during treatment, and/or after the last dose of treatment is administered.
Tecvayli in clinical trials
A clinical trial is a medical research study with people who volunteer to test scientific approaches to a new treatment or a new combination therapy. Each clinical trial is designed to find better ways to prevent, detect, diagnose, or treat a specific condition, and to answer scientific questions. If you have an interest in participating in a clinical trial, be sure to discuss with your myeloma doctor all the potential risks and benefits that may apply to your particular case.
At this time, there are numerous ongoing clinical trials for patients with myeloma, including studies with Tecvayli, and some of these clinical trials may be enrolling patients. To help myeloma patients with personalized support for identifying and exploring clinical trial options, the IMF has partnered with SparkCures. Visit myeloma.org/sparkcures or contact the IMF InfoLine for more information.
The U.S. government maintains clinicaltrials.gov, an online database of research studies. However, the government does not review the safety and science of the studies submitted to this website.
For more information about what’s involved in study participation, read the IMF’s publication Understanding Clinical Trials in Myeloma.
Tecvayli assistance program
If you are prescribed Tecvayli, you can sign up for the “TECVAYLI withMe” program at tecvayli.com/savings-support/ or by calling 1.833.565.9631 Monday – Friday, 8 a.m. – 8 p.m. (ET). You will be connected with an English-speaking or Spanish-speaking Care Navigator for personalized help with health and wellness resources, appointment reminders, transportation-related services, access to nurse educators, and cost support options regardless of your insurance type.
In closing
This booklet is not meant to replace the advice of your doctors and nurses who are best able to answer questions about your specific healthcare management plan. The IMF intends only to provide you with information that will guide you in discussions with your healthcare team.
To help ensure a good quality of life through effective treatment, you must play an active role in your own medical care. We encourage you to visit myeloma.org for more information and to join the Myeloma Knowledge Platform at myprofile.myeloma.org.
To receive the most up-to-date information about myeloma in a caring and compassionate manner, call the IMF InfoLine at 1.818.487.7455,
email InfoLine@myeloma.org, or visit mmsm.link/infoline to schedule a convenient time to talk with an IMF InfoLine Coordinator.
To get answers to your questions without having to wait, ask Myelo® anytime 24/7 at myeloma.org. This generative AI assistant is designed to help you find the right resources.
Use the hyperlinks and web addresses included in this publication for quick access to resources from the IMF. Sign up at subscribe.myeloma.org for our quarterly journal Myeloma Today and weekly e-newsletter Myeloma Minute, as well as alerts about IMF news, events, and actions.
Notes
The International Myeloma Foundation (IMF) is the global leader in myeloma. Our mission is to improve the quality of life of myeloma patients while working toward prevention and a cure. Since 1990, the IMF has been serving the myeloma community through the following four pillars:
RESEARCH At the IMF, finding a cure for myeloma is our top priority. The IMF Scientific Advisory Board (SAB) of leading myeloma experts identifies key opportunities to drive research forward. The IMF Black Swan Research Initiative® (BSRI®) is pushing the boundaries with early screening for a precursor condition of myeloma as well as cure-focused myeloma clinical trials. The IMF International Myeloma Working Group (IMWG) provides trusted guidelines for diagnosing, treating, and managing myeloma. We also fund innovative research through the IMF Brian D. Novis Research Grants.
EDUCATION Myeloma is a complex and unique journey for each patient. The IMF offers hundreds of videos and free publications in multiple languages to inform and empower patients and care partners to navigate their myeloma journey. All IMF seminars, webinars, and workshops are free-of-charge and designed to directly connect the patient community with expert myeloma clinicians. The IMF Nurse Leadership Board (NLB) provides recommendations for the management of myeloma. The IMF M-Power Project works to break down barriers and ensure health equity in underserved populations.
SUPPORT Studies show that social support can greatly improve the quality of life of people with cancer. The IMF offers more than 160 myeloma support groups across North America, including specialized groups for Spanish-speakers, people with smoldering myeloma, care partners of patients with myeloma, and patients who do not have care partners. The IMF InfoLine answers myeloma-related questions. Myelo®, the IMF’s generative AI assistant, is available 24/7 to help you find the right resources.
ADVOCACY In the U.S., the IMF Advocacy team represents your interests at the federal and state levels. Internationally, the IMF Global Myeloma Action Network (GMAN) works to improve patient access to treatments.
