Los Angeles Patient and Family Seminar

Thank you to our Sponsors!


















7:55 – 8:00 AM
Saturday Agenda
Introduction and Housekeeping
Robin Tuohy, Vice President, Support Groups, International Myeloma Foundation
8:00 – 8:30 AM
Welcome & IMF Presentation
Yelak Biru, MSc., Myeloma Patient, President & CEO of International Myeloma Foundation
8:30 – 9:00 AM
Myeloma 101: What Myeloma Patients Need to Know
Brian Durie, MD
Chairman of the Board & Chief Scientific Officer of International Myeloma Foundation
9:00 – 9:20 AM Q&A with Panel
9:20 – 9:40 AM
Frontline Therapy
Rafat Abonour, MD, Indiana University School of Medicine
10:00 – 10:30 AM Q&A with Panel
10:30 – 10:40 AM
Myeloma.org Presentation

Robin Tuohy, Vice President, Support Groups, International Myeloma Foundation
10:40 – 11:00 AM
11:00 – 11:30 AM
COFFEE BREAK
Patient Support Group Programs in Asia

Daryl Tan, MD, Hematologist at Mount Elizabeth Novena Hospital, Singapore
AM – 12:00 PM
with Panel
– 1:00 PM LUNCH BREAK
1:00 – 1:15 PM
Agenda After Lunch Break
Approaches to Relapse Therapy

Noopur Raje, MD, Director, Center for Multiple Myeloma, Massachusetts General Hospital
1:15 – 1:30 PM Q&A with Panel
1:30 – 1:45 PM
Immune Therapy Options
Ajai Chari, MD, Director of Multiple Myeloma

UC San Francisco – San Francisco, CA
–
Breakout Sessions
Managing Myeloma Symptoms and Side Effects w/ Donna Catamero - Sunset Care Partners w/ Robin Tuohy - Hollywood
Diagnosis and Treatment w/ Dr. Durie, Dr. Chari, Dr. Raje & Dr. Abonour – Beverly Ballroom
Welcome & IMF Presentation
Yelak Biru
Myeloma Patient, President & CEO of the
International Myeloma Foundation

Patient and Family Seminar

 Yelak Biru, President and CEO
Yelak Biru, President and CEO

A day in the life of a myeloma patient


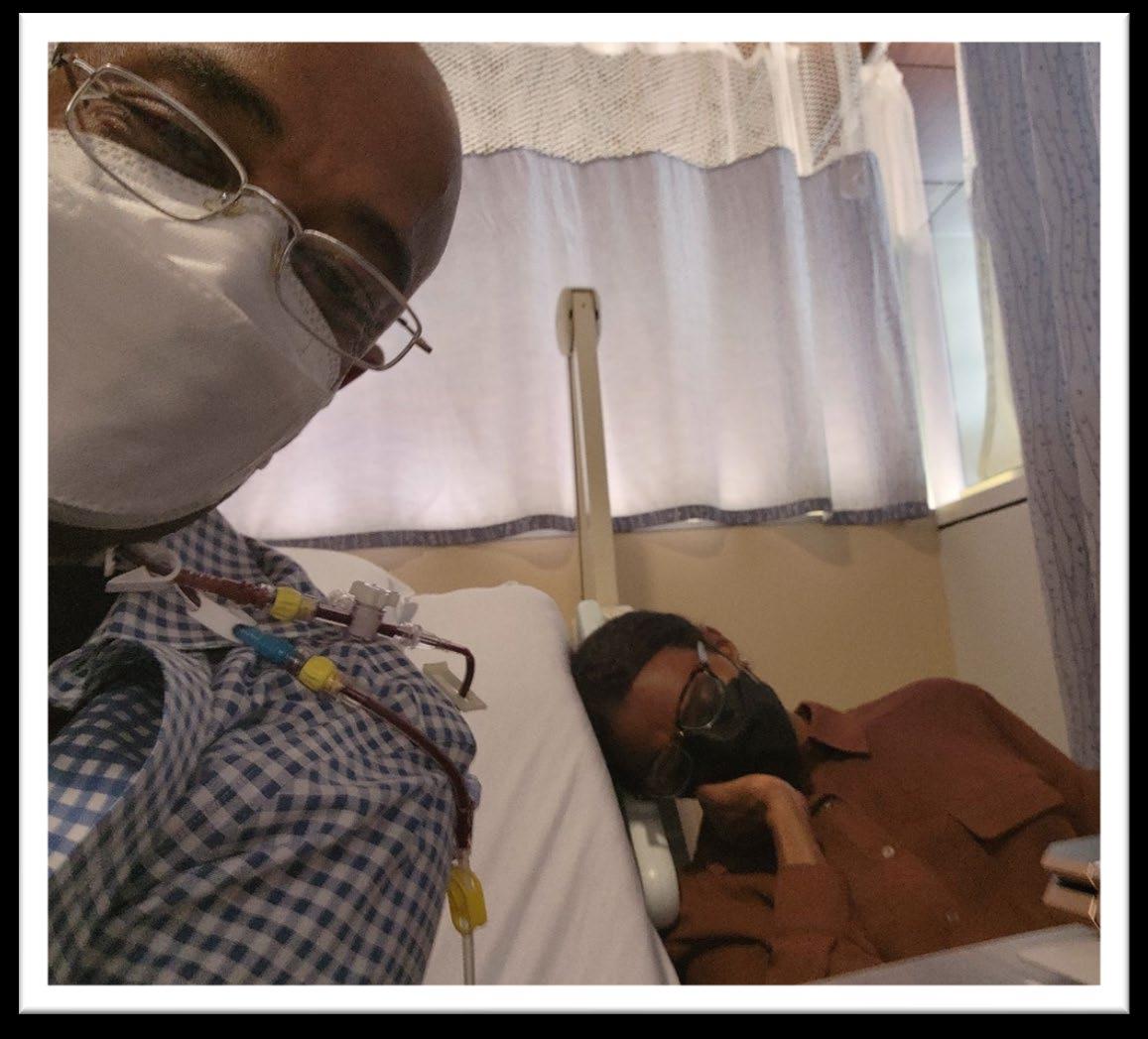
Diagnosed with myeloma at age 25 in 1995

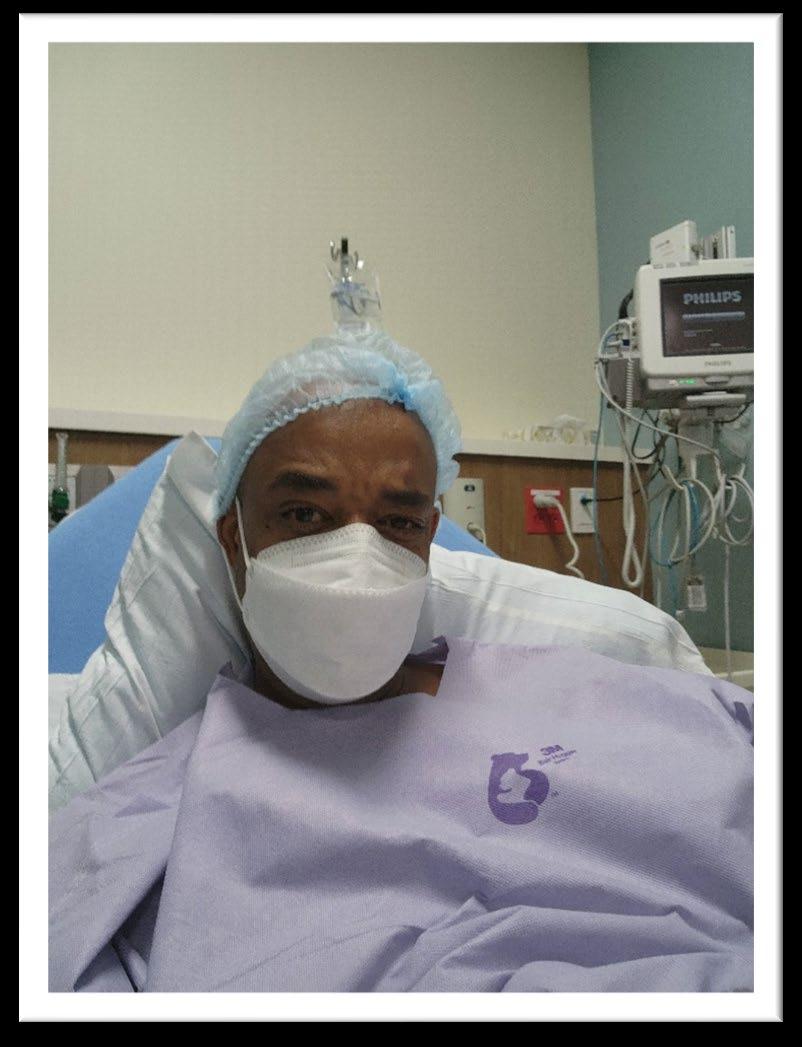





Given 2 -3 years to live
Told I will be dead before the age of 30
Huh… what is myeloma?
27 Years ago!

Know your options
Track your history
Build Partnerships

Have Plans (short and long term)
Seek Clinical Trials as part of Tx
Know your MDEs
Communicate your QOL needs
Consult a Myeloma Expert
Know you are one of a kind!

“In the absence of a mongoose, keep the snake in the basket!”
Tremendous progress over the last two decades



Early Relapse Late Relapse






IMF’s Origin Story









Patients at the center of everything we do!



IMF’s Pillar Priorities
Research
Provide ongoing, updated global diagnostics and treatment guidelines
Accelerate lab to bedside research - clinical trials

Create immunotherapy and biomarker data marketplace
Conduct research in areas of MGUS, SMM, High Risk Disease, MRD
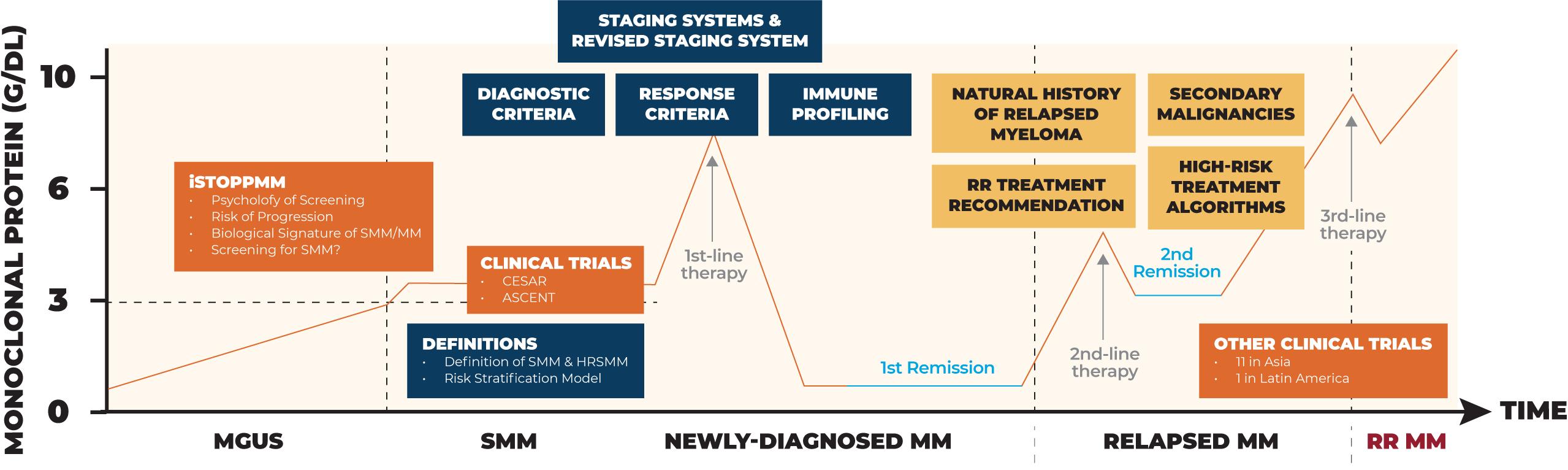
Drive signature research projects such as BSRI, iStopMM, ASCENT, …

Support
Enable increased connection to others catering to the mind, body and spirt
Create a real sense of belonging for diverse myoma patients
Empower those coping with myeloma to achieve optimal outcome by eliminating obstacles to care
Education
Provide the most up-to-date, accurate, and trusted myeloma related information in a patient friendly format
Increase capacity of myeloma treatment by providing education to community physicians and centers

Advocacy


Advocate for everyone to benefit from myeloma diagnostics and treatment advances globally
Regulatory Evolution (Evolve the conversation from cost to value)

Brian N. Jr/Sr Research Grant

International Myeloma Working Group
Nurse Leadership Board
Asian Myeloma Network
Latin America Myeloma Network
Global Myeloma Action Network


RESEARCH
Black Swan Research Initiative
I2 Team for Endpoint Approval of Myeloma MRD
Beyond Medicine Barriers
M-Power
PATIENT
CME + NON-CME
Conference Series
InfoLine
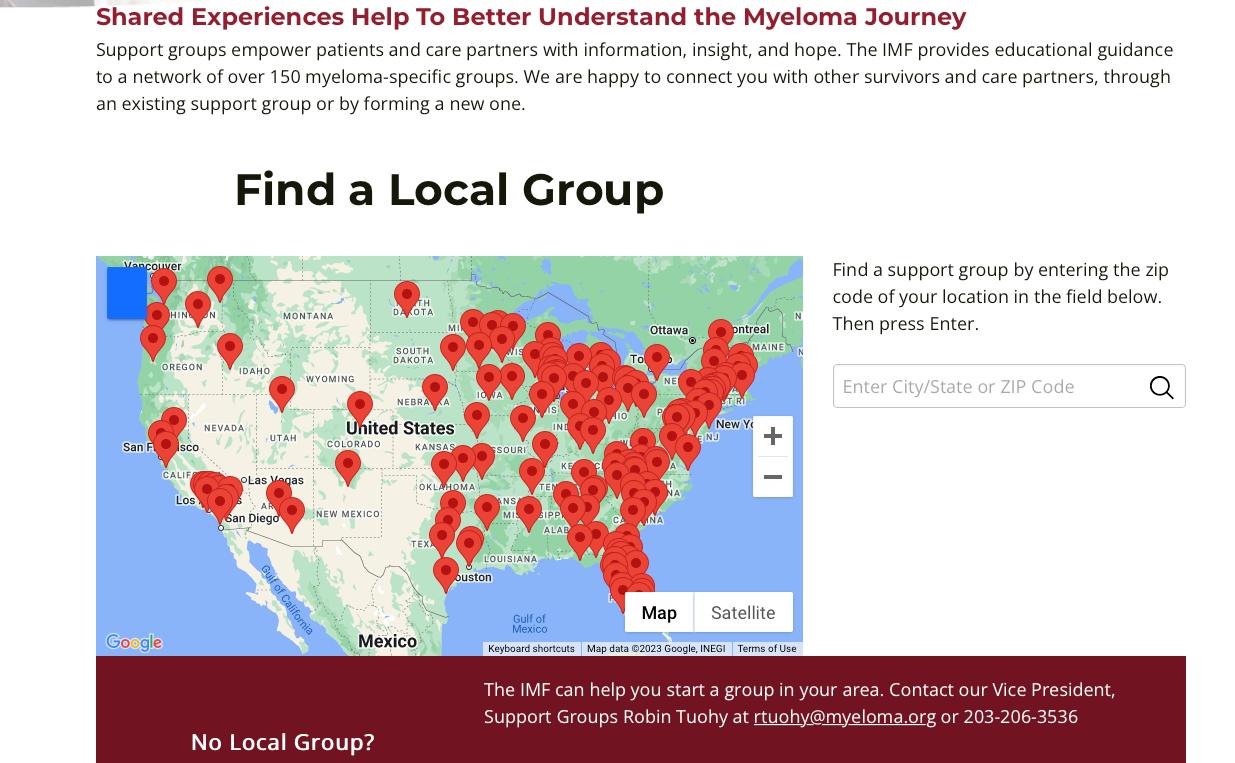
>150 Support Groups
Wellness Programs
SG Toolkit
SUPPORT
Coalition to Improve Access to Cancer Care
Myeloma Action Team
Education
Peer Reviewed Publications
Guidelines
Chart Studies
Clinical Trials
PFS | RCW
Master Classes
Myeloma University


Ask Dr. Durie
Podcasts | Blogs
Whitepapers
Videos | Publications
Veterans Against Myeloma

All Access Congress

IMF’S CONTRIBUTION & INFLUENCE ON MYELOMA RESEARCH






GLOBAL MYELOMA ACTION NETWORK


GOALS
01 BUILDING CAPACITY
0 2 0 3
INCREASING AWARENESS
IMPROVING ACCESS
M-Power = Myeloma Power

The core vision of this initiative is to improve the short- and longterm outcomes of African American patients with myeloma.
ENGAGE
Engage the community to increase awareness and provide support
We want to empower patients and communities to change the course of myeloma…

empower
ENHANCE EDUCATE
Enhance access to optimal care by educating myeloma providers about the disparity and how to reduce it
Shorten the time to diagnosis by educating primary care providers to recognize the disease and order the right tests
Volunteer! Myeloma ACTION Team




INDUSTRY TRENDS

BARRIERS TO HEALTH
What We at the IMF Have Seen
Disconnection Self Navigation or Avoidance

• Care
• RX Approval – FDA, EMA,
• Payment – HTA, Single Payor, Country level capacity, Regional Health Systems
• Care Coordination – single hospital, across specialty

Inequitable Distribution Health Disparities
• Innovation – R&D and Supply Chain
• Resources – Rx, Tx, Clinical Trials, Information

Exponential Treatment Options Care Friction
• Complexity of Care
• Need to better understand patient journey
• Generation of Patient Reported Outcomes

OUR APPROACH

OUR STRATEGY FLYWHEEL
Serve Patients Broadly | Deepen Our Relationships | Provide Awesome Experiences
1. Primary Destination
• Research, Support, Education, Advocacy
• Omnichannel way – Online, In Person, Telephone
2. Patients & Care Partners
• Shorten time from diagnosis to Hope –Time To Hope (TTH)
• Increase Time To next Treatment (TTT)
• Education
4. Partner Ecosystem



• Credible partners in development, access, and policy

• Clinical trials – remove entry barrier + increase patient participation
• Research – Novel testing & therapy approaches
• Black Swan Research Initiative – BSRI
• Immunotherapy Data Marketplace
Gen-X (42-57)

Baby Boomers (58-75 Traditionalists (75 -95)
• Connection
• Overcome obstacles to access
• Address health equity
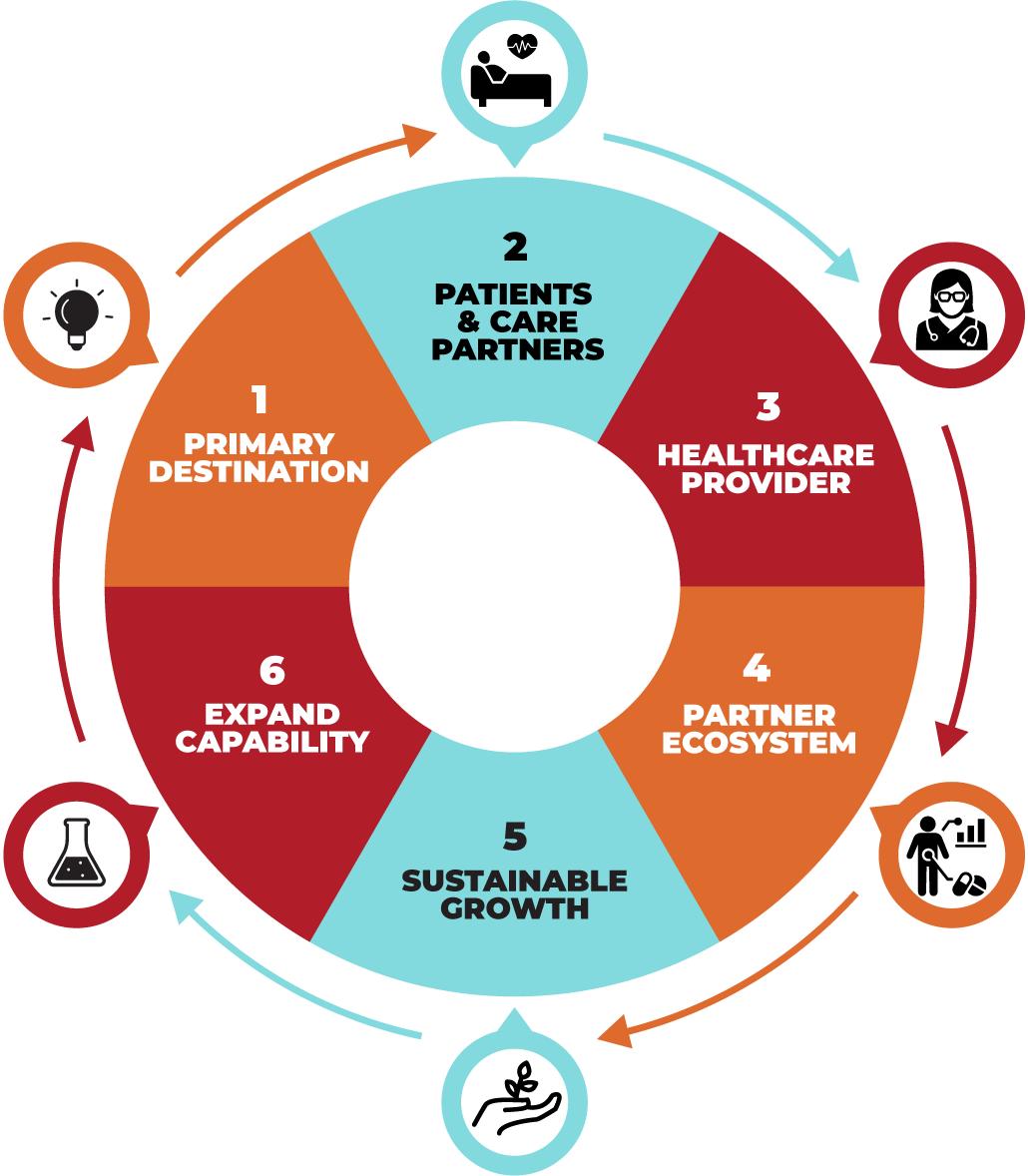

3. Healthcare Providers
• Timely Information + Education
• Treatment guidelines for newly diagnosed & initial relapse
• Expert 2nd opinion
5. Sustainable Growth
• Strategic Plan
• Digital Innovation
• Investment in our People
• Bold Collaboration
6. Expand Capability
• Research
• Technology and Data
AWESOME EXPERIENCES
Repeatable and Scalable Model Enabling
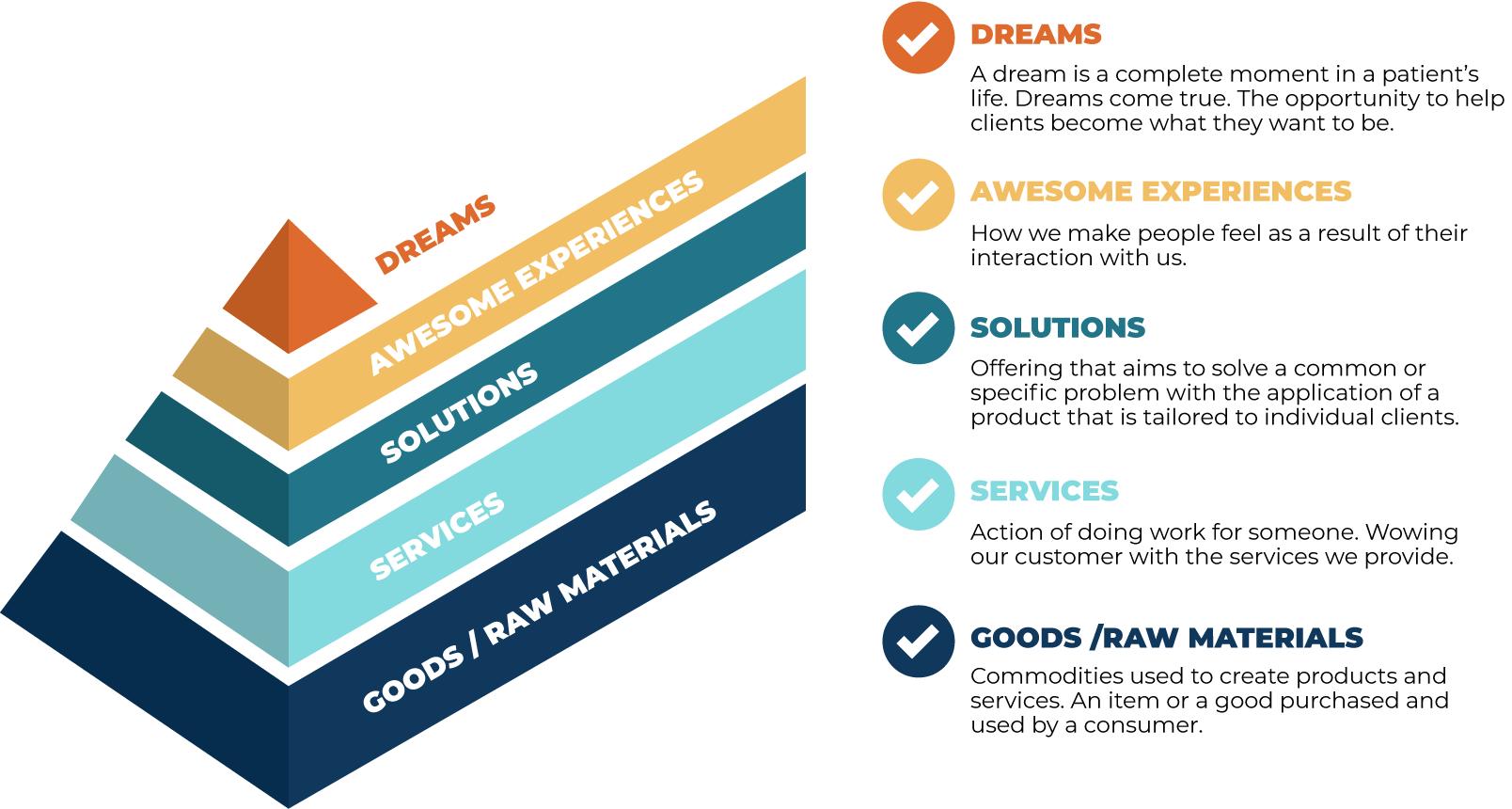

We want to accelerate the time from diagnosis to hope using digital assets
We want to be the third place for Myeloma patients and their families. The place between the hospital and home, where a combination of information, community and connection will give patients the information they need, the support they desire and the research needed to extend their life meaningfully.

STRATEGIC PLANNING UPDATE




DIGITAL STRATEGY

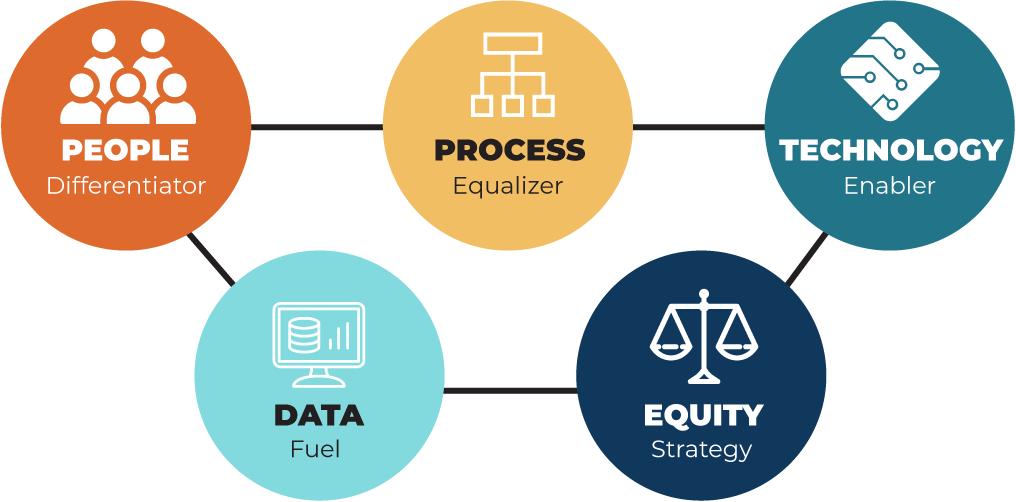
Data saves lives; we use this term often when discussing the opportunities data driven technologies are providing today to deliver patient centered care. In healthcare, data is the very fabric from which smart and evidence-based decisions can be made in both clinical and nonclinical settings. With the continued expansion of bigdata, it will become even more important to leverage cognitive and machine learning capabilities.

Think Big Vision
CRM - Salesforce
- Engaging Networks
Clinical/Research
- Partner sites
- Myeloma Manager
Finance
- Quickbooks
-
Form 990
- Audit/Annual Report
- Infoline
Events
- Cvent
Zoom Webinar
Member Fundraiser
Patient 360
Events Attended
Patient Profile




Social Media
Brand Mentions
- Agoura Pulse
- Give Panel
Website Visited
Patient Cohort Journey
Infoline Visit
Social Media Engagement
HR/Operations
- Sharepoint
- Employee Handbook
- Inventory
- Distribution
- GoToConnect
- Videos
- Publications
- Podcasts
- Myeloma.org
- Myeloma.org

- Satellite Sites
Web Analytics
GA4/Google UA
SEO/SEM Rush
Google Adwords
Support Group Participation
Surveys Participated
Secure
Products, Services, and Patient data are secure with process in place to mitigate enterprise risk, including encryption, health industry level certification, HIPPA compliance.
AWESOME EXPERIENCES
Customer Personas

Patient

Newly diagnosed or newly relapsed
A multiple myeloma patient at a point in their experience where critical decisions need to be made regarding treatment and their care but comes at a time where they are most vulnerable from an emotional state.

IMF Team Member who is interacting directly with patients/caregivers. Understanding their needs and guiding them to information, support, and services they need and make informed decisions. This includes InfoLine Team Members, Support Group Leaders, Research opportunities and design of education Curriculum

Community-based HCP treating people with many different types of cancers. Someone who is empathetic and committed to ensuring their patients understand their diagnosis and treatment path, however, have little time and resources to spend to educate themselves on the latest advancements in multiple myeloma

Impact on Patient Life and Outcomes



RESOURCES


Multiple Myeloma affects patients and families

. The IMF provides FREE resources to help both patients and families.
Established in 1990, the IMF’s InfoLine assists over 4600 callers annually and answers questions across a wide variety of topics including:
Frequent topics:

Newly Diagnosed MM/Myeloma 101
Treatment Options
Transplant
Maintenance
Side Effects
Relapsed/Refractory MM

Clinical Trials
Resources for Drug Access/Financial Support/Local Support
Referrals to Myeloma Specialists Within/Outside the US
Health Issues Related to Myeloma


Caregiver Support
Paul Hewitt, Missy Klepetar, and Deb Verla
Educational Publications

























A core mission of the IMF is to provide thorough and cutting- edge education to the myeloma community.











Regional Community Workshops




ROBUST SUPPORT AND EDUCATION PROGRAMS
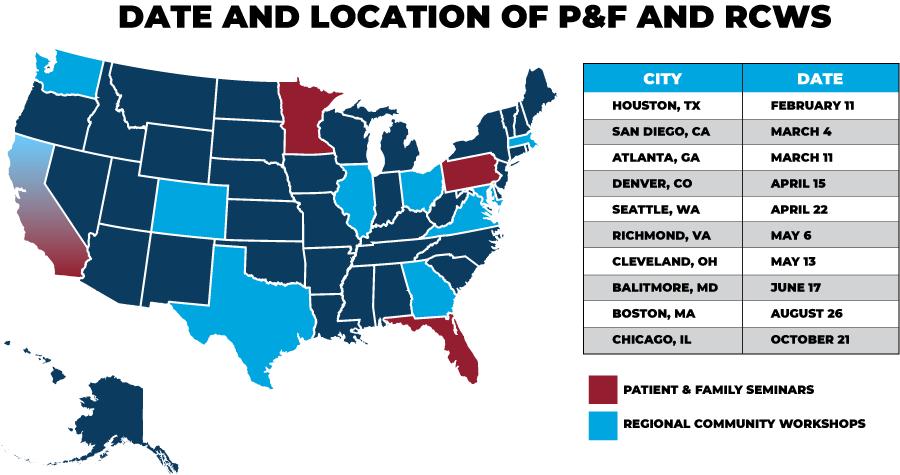

150 support groups, including three special groups (Spanish Speaking, Young Parents with Children, Solo and Strong, Smoldering MM )
Less Isolated
More Connected

Better Outcome
Reuse Playbooks
Better Informed through Shared Experiences
CONCLUSION


Conclusion














THANK YOU! WITHOUT YOU, WE COULD NOT DO WHAT WE DO!

Myeloma 101: What Myeloma Patients Need to Know
 Brian G.M. Durie, MD Chairman of the Board & Chief Scientific Officer of International Myeloma Foundation
Brian G.M. Durie, MD Chairman of the Board & Chief Scientific Officer of International Myeloma Foundation
IMF Patient and Family Seminar




Myeloma 101: What Patients Need to Know



August 19, 2023

Outlook for 2023 & Beyond

➢ Excellent Initial Response Typical
➢Long Remissions Common

➢Many Effective Relapse Options
➢CURE on the Horizon

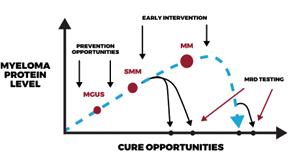
Myeloma Disease Evolution



BEST TESTING
SCREENING
BEST INTERVENTIONS/TREATMENTS

Importance of Early Diagnosis

➢ Screening can be a new tool

➢ Start discussions with HR SMM

Potential
Potential New Myeloma or Smoldering Myeloma

Any Myeloma Defining Events?
• CRAB,
• >60% PC,
• FLC >100,
• MRI >1 focal
No Myeloma Defining Events (SMM)


High Risk SMM (Median TTP ~2 years)
Intermediate or Low Risk SMM
Treat as Myeloma
Early Therapy with Len or Rd x 2 years
Clinical Trials
Observation
Evolution of Treatment Options 15 Years Ago










Treatment Options in 2023



First Therapy Relapse
VRd +/- Dara or Isa
+/- ASCT
Dara/Isa Rd
CAR T & Bispecifics
CELMoDS
Many new agents/trials




Immunotherapy




Immunotherapy Timeline




Immunotherapy Trials











Alkylators
Steroids
Anthracyclines









Active Drugs in MM
Anti-SLAMF7 monoclonal antibody (moAB)
Elotuzumab
Selinexor (XPO1 inhibitor)
Venetoclax (BCLinhibitor, unique MOA)

Anti-BCMA CAR-T
Cilta-cel
Ide-cel
JCARH125
IMiDs
Thalidomide
Lenalidomide
Pomalidomide
Anti-CD38 moABs
Daratumumab
Isatuximab
Felzartamab (MOR202)
TAK 079
SAR 442085
TAK 573 (Modakafusp Alfa)
Anti-BCMA Bispecifics
Teclistamab
Linvoseltamab (REGN5458)
Alnuctamab
Elranatamab
TNB 383B
Novel Bispecifics
Talquetamab (GPRC5D/CD3)
Proteasome Inhibitors
Bortezomib
Carfilzomib

Ixazomib
Anti-BCMA antibody drug conjugate

Belantamab*
CELMoDs
Iberdomide
Mezigdomide
*Belantamab was recently withdrawn from the US market
Cevostamab
(FcRH5/CD3)




Key Points for Ongoing Care
Keep Track of Follow-up Tests
- CBC; SPEP/UPEP; Freelite; SCANS; BM if needed
- FiSH for t[11;14] or high-risk [17P-; 1q+…]
Alert Doctor to Changes (myeloma/side effects)



Be Aware of Potential Future Options
Be Proactive in Seeking Expert Advice
Set Expectations with Your Primary Doctor



Q&A

Frontline Therapy Rafat Abonour, MD
University of Indiana School of Medicine

Initial therapy for Multiple Myeloma Rafat Abonour, M.D.
Harry and Edith Gladstein Professor of Cancer Research
Professor of Medicine, Pathology and Laboratory Medicine
Director, Multiple Myeloma, Waldenstrom's Disease and Amyloidosis Program
Indiana University School of Medicine

Treatment Goals
• Symptom Control
• Ameliorate pain and other disease-related symptoms
• Prevent further organ damage
• Preserve and improve performance status and quality of life
• Disease Response and Survival
• Rapid cytoreduction to relieve symptoms
• Minimize treatment-related toxicity
• Prolong survival – Overall Survival

Depth of Response Influence Time to Relapse
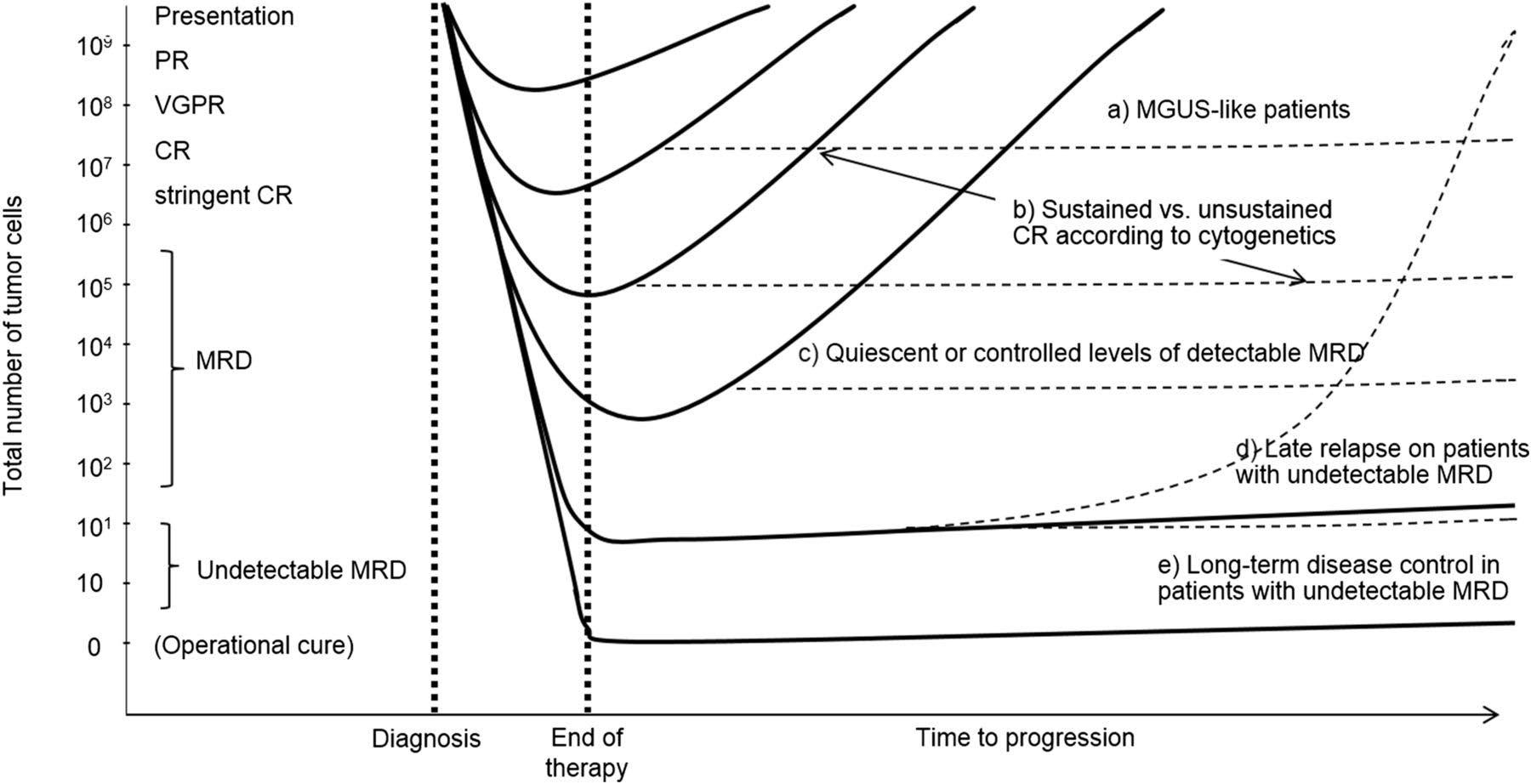
Getting rid of myeloma is a good thing
PR=partial response, VGPR=very good partial response, CR=complete remission, MRD= minimal residual disease
Minimal Residual Disease (MRD) Testing
• MRD is emerging as an important marker of lasting clinical benefit
• MRD can be tested: Using flowcytometry. Sensitive at 10-5 and can be done anytime on bone marrow samples and does not require knowing what the patient myeloma sequences were at diagnosis
− Using next generation sequences Sensitive at 10-6 but require knowing what the patient myeloma sequences were at diagnosis.
MRD Strongly Predicts outcomes
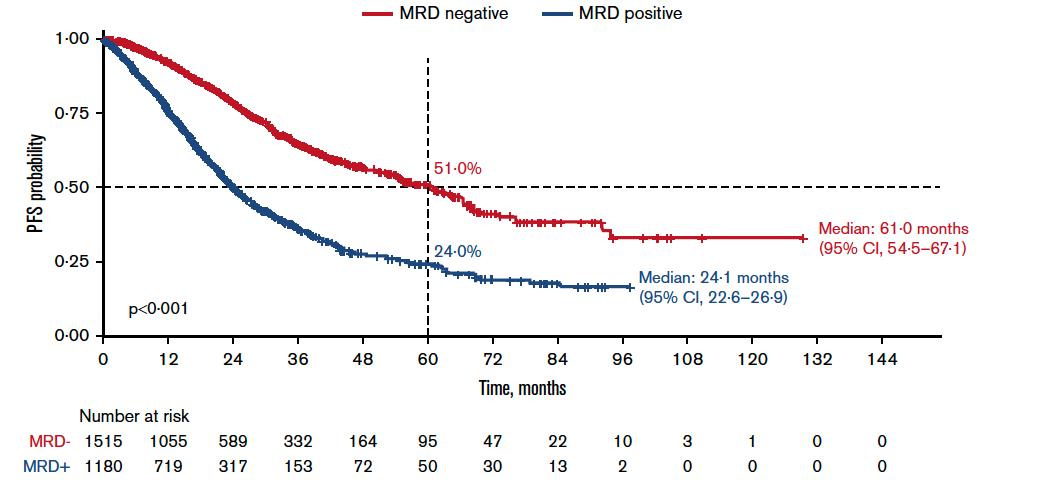

If patient achieved Negative MRD their myeloma stay under control longer and they live longer


Munshi et al. Blood Advances 2020; 4:23

MRD May Abrogate Other Risk Factors
risk patients achieving MRD do better Goicochea
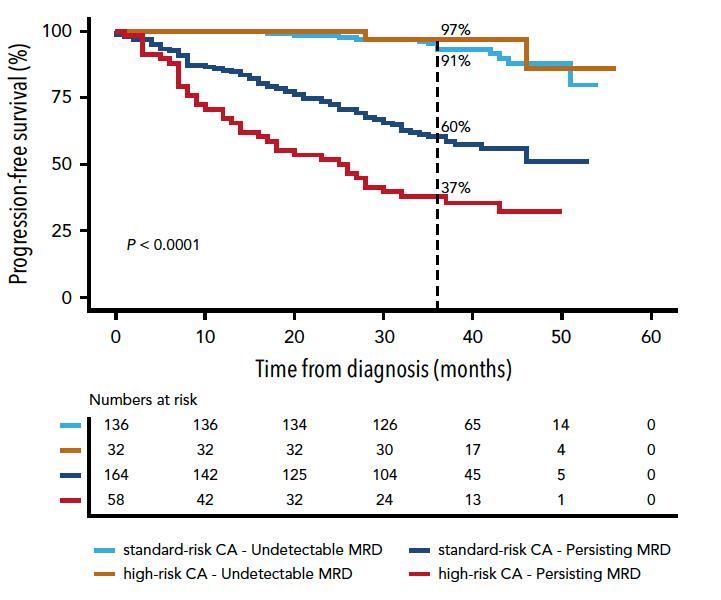
High
Opportunity for de-escalation

Need to employ new approaches
PETHEMA/GEM2012
(MRD < 2 x 10-6)






The Evolution of Myeloma Therapy
Many New Drugs and Many New Combinations
Panobinostat
Lenalidomide
Carfilzomib
Pomalidomide
Selinexor
Daratumumab
Ixazomib
Elotuzumab
Isatuximab
Belantamab mafodotin
Melphalan flufenamide
Idecabtagene autoleucel
Daratumumab?
Carfilzomib?
CAR
Therapy
Cell Modifying Agents
Venetoclax?
Inhibition?
Multiple
ASCT,
autologous stem cell transplant; CAR, chimeric antigen receptor; Cy, cyclophosphamide; d- daratumumab; D/dex, dexamethasone; isa, isatuximab; K, carfilzomib; M, melphalan; PD-L1, programmed death ligand-1; PI, proteasome inhibitor; Rev, lenalidomide; V, bortezomib
How does your doctor decide?
Upfront therapy
• Combination therapy continues to prove superiority in providing long remission and long survival.
• The patient is at good place as options are becoming more prevalent.
• Lenalidomide, bortezomib and dexamethasone continues to be an effective regimen.
• Daratumumab in combination with lenalidomide is an excellent option for “frail patient”
• But wait there are more to come
How did we start?
Is three drugs better than two?

Three drugs better in some Patients
Myeloma under control longer ad you live longer
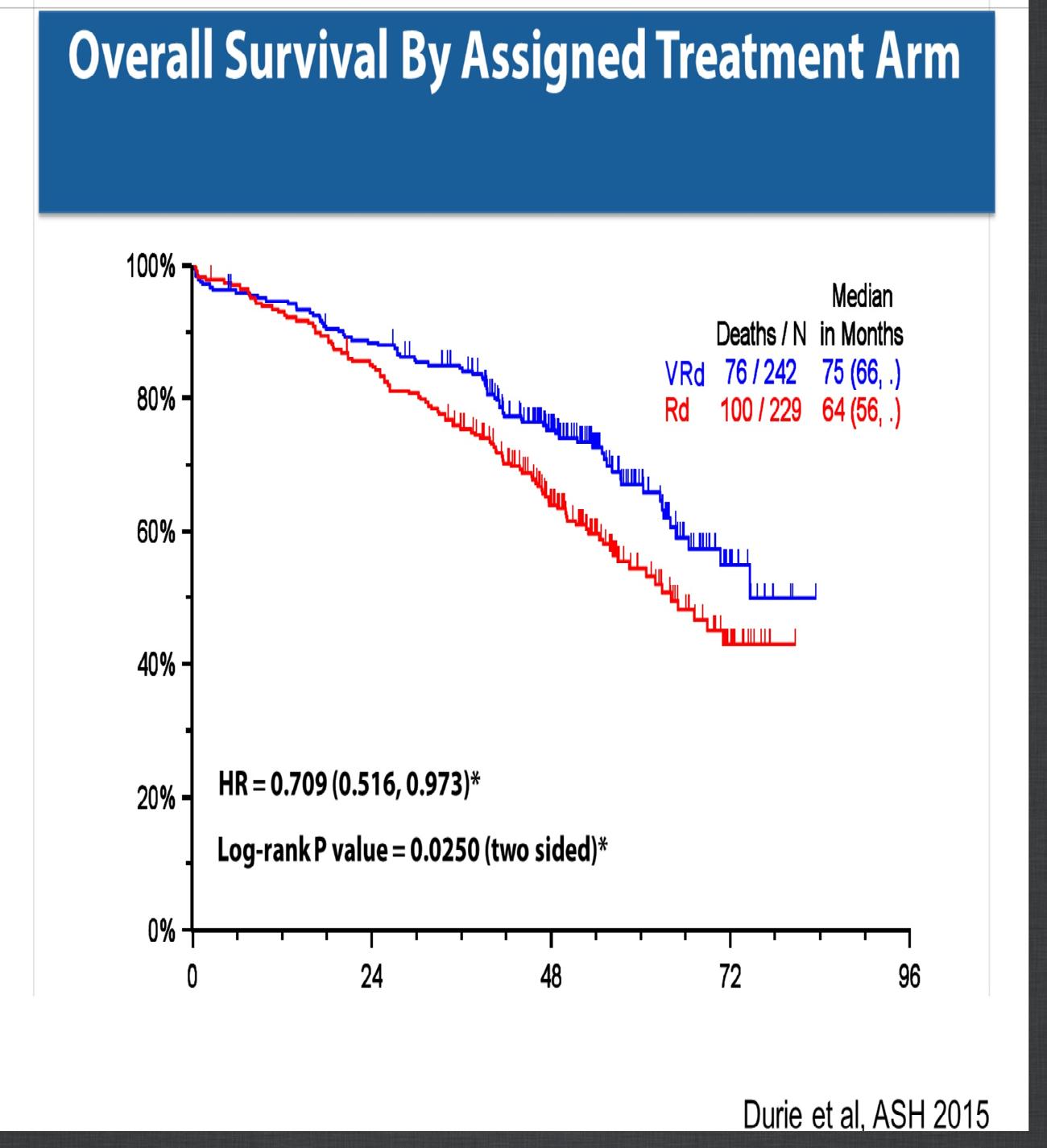
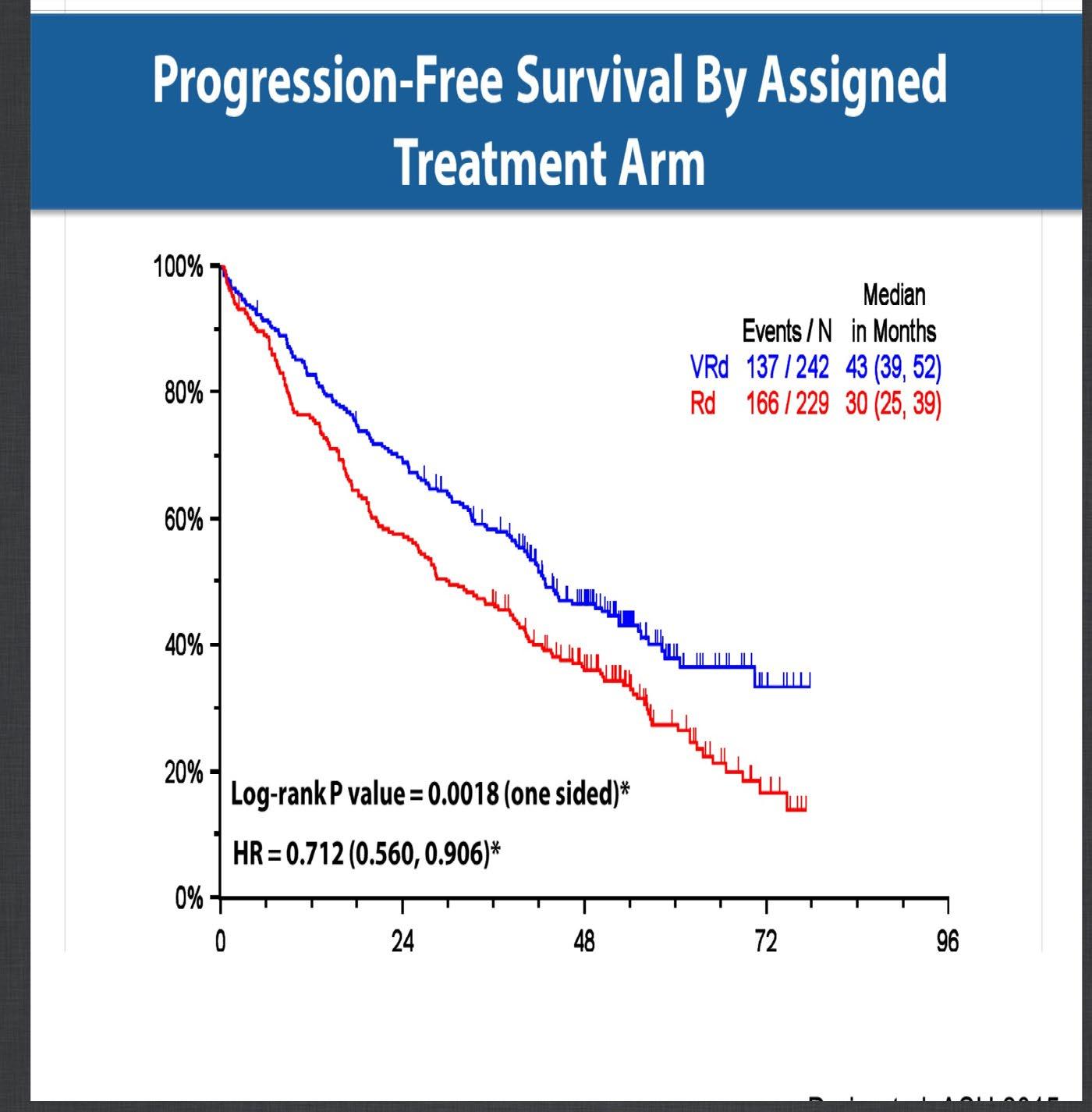
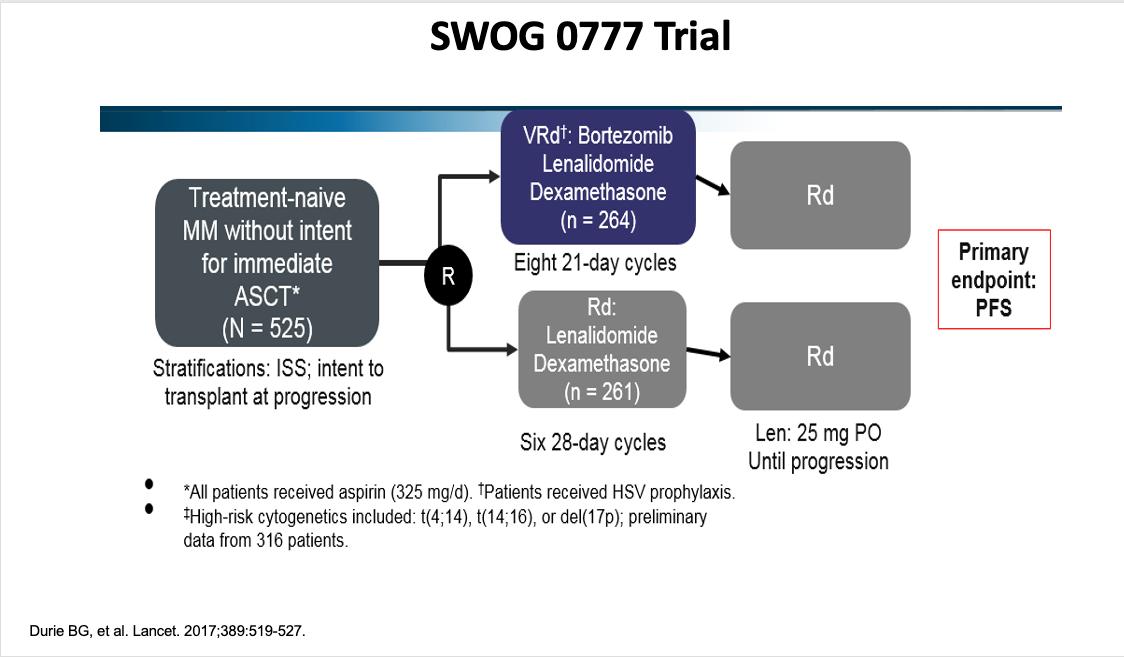
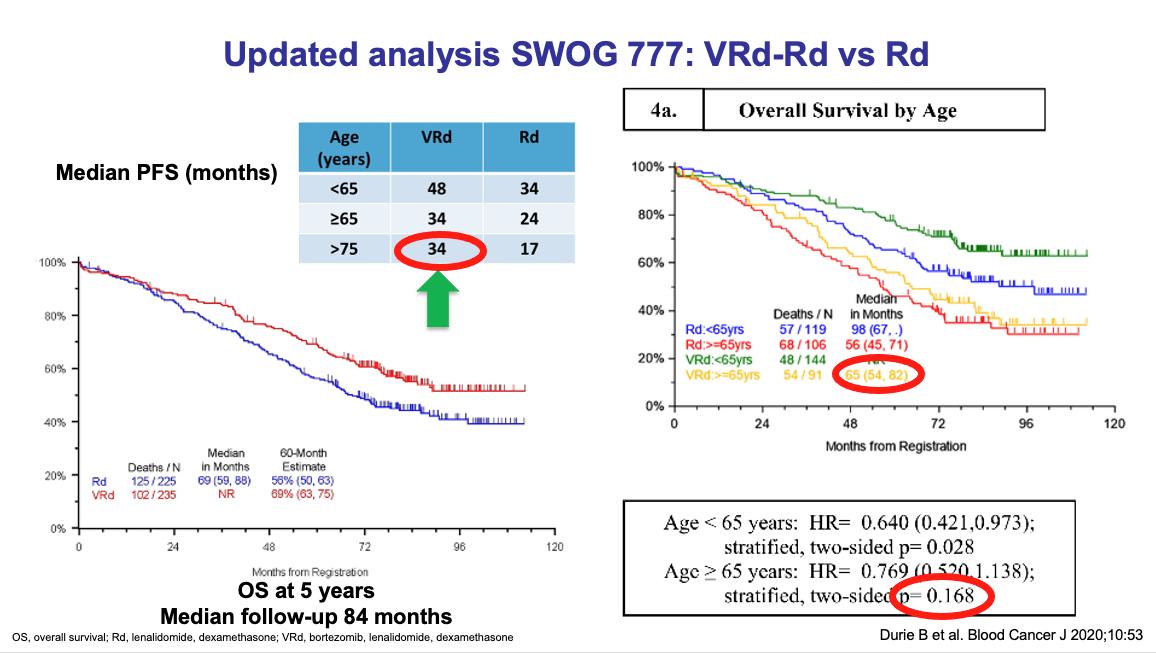
SWOG 0777
Phase III MAIA Study Design: Adding daratumumab to lenalidomide and steroid regimen
Daratumumab 16 mg/kg IVa +
Lenalidomide 25 mg/d PO, d 1-21 +
Dexamethasone 40 mg/w PO or IV (n = 368)
ECOG PS 0-2
CrCl ≥30 mL/min (N = 737)

Lenalidomide 25 mg/d PO, d 1-21 + Dexamethasone 40 mg/w PO or IV (n = 369)
28-day cycles until PD
Primary endpoint: PFS
Secondary endpoints: CR, VGPR, MRD negativity, ORR, OS, safety
Stracation:
• ISS (I, II, III)
• Region (N America vs other)
• Age (<75 y vs ≥75 y)
Median age: 73 years (45-90)
99% of patients age ≥ 65 years
aQW cycles 1-2, Q2W cycles 3-6, Q4W cycle ≥7.
Phase III MAIA: better response rates, and Sustained MRD-Negativity Rates
Median follow -up: 64.5 months
Daratumumab favored in most subgroups, including for both PFS and OS endpoints
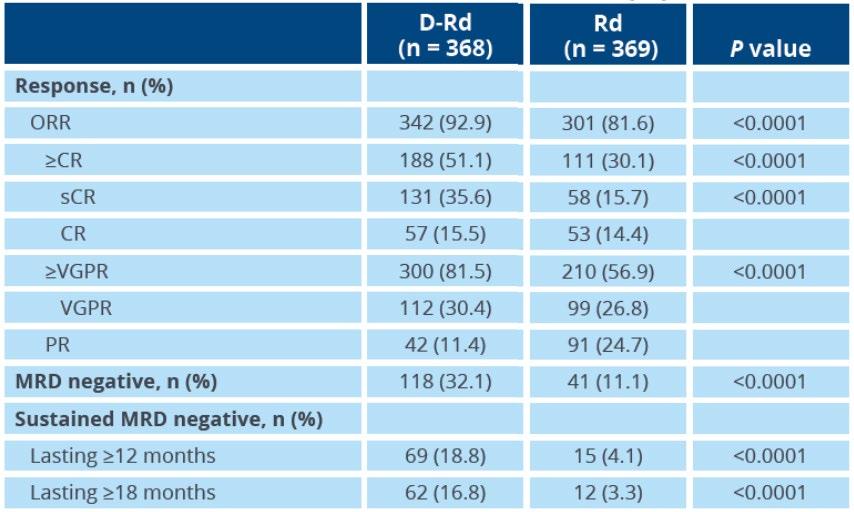
Reduced risk of progression or death with MRD negativity in both arms
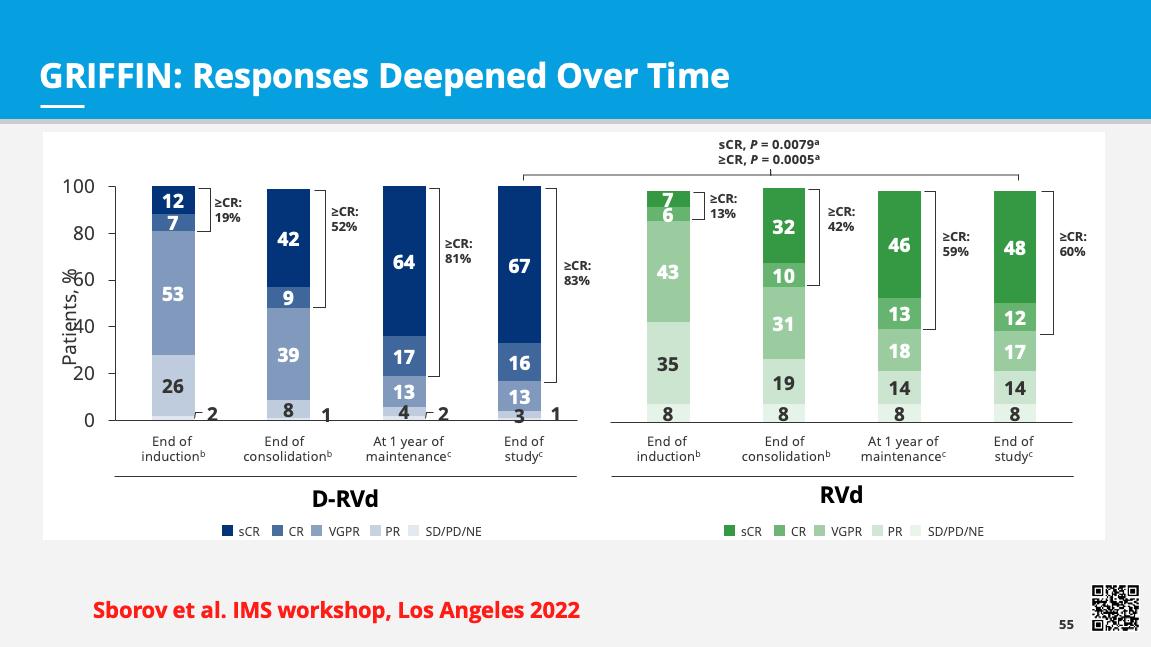
Responses deepened over time
Phase III MAIA: Safety
No new safety concerns were identified with longer follow up
IFM 2009: RVD ± ASCT for Transplant-Preferred NDMM
Transplant-preferred NDMM


RVD x 3 cycles, ASCT collection, Cy 3 g/m2
• RVD cycles 4-8
RVD x 3 cycles, ASCT collection, Cy 3 g/m2
• ASCT with MEL 200
• RVD cycles 4, 5
Lenalidomide 10-15 mg daily x 12 months
44-mo follow up
PFS=Progression free survival), OS= overall survival
IFM 2009 Study of RVD± ASCT: PFS by MRD Status
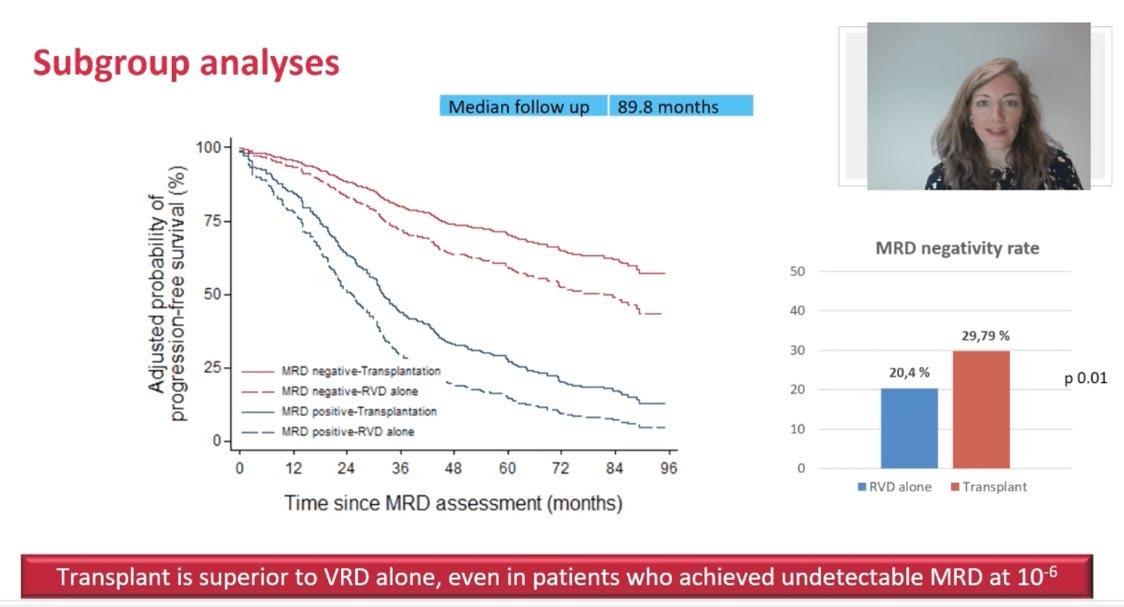
Why is this French Study Important!
Downsides of Continuous Maintenance Therapy
IFM 2009


No therapy
Deferring Lenalidomide for 7 years:
-”Saving” of $1.5 million!
-Avoid ~3.5% absolute increase in risk of SPM
-Avoid dozens of extra lab checks/MD visits

-Avoid diarrhea, fatigue, rash
Most patients have not progressed in 7years despite no therapy
-Avoid “daily reminder I have cancer”
Perrot A et al. ASH meeting 2020
DISCLOSURE NOT ALL MYELOMA EXPERTS AGREE ON THIS
Randomized Phase 2 GRIFFIN Trial of D-RVd vs RVd with transplant
• 35 sites in the United States with enrollment between December 2016 and April 2018
Induction: Cycles 1-4
Consolidation: Cycles 5-6c
•
•
•
•
•
•
Maintenance: Cycles 732
Endpoints and statistical assumptions
Primary endpoint:
• sCR rate (by end of consolidation); 1-sided alpha of 0.10
• 80% power to detect 15% improvement (50% vs 35%), N = 200
Other endpoints:
• Rates of MRD negativitye (NGS), ORR, ≥VGPR, CR, PFS, OS
Stem cell mobilization with G-CSF ± plerixaforb; High-dose therapy (melphalan 200 mg/m2)
• Median age ~60; ISS3 14%, High risk 15%
• Lower ASCT rate in RVd arm due to early discontinuations
ECOG PS, Eastern Cooperative Oncology Group performance status; CrCl creatinine clearance; IV, intravenous; PO, oral; SC, subcutaneous; G-CSF, granulocyte colony-stimulating factor; D-R, daratumumab plus lenalidomide; Q4W, every 4 weeks; Q8W, every 8 weeks; NGS, next-generation sequencing; ORR, overall response rate; VGPR, very good partial response; CR, complete response; PFS, progression-free survival; PFS2, PFS on next subsequent line of therapy; OS, overall survival. aLen dose adjustments were made for pts w/ CrCl ≤50 mL/min. bCyclophosphamide-based mobilization was permitted if unsuccessful. cConsolidation was initiated 60 to 100 days post-transplant. dProtocol amendment 2 allowed for the option to dose DARA Q4W based on pharmacokinetic results from study SMM2001 (NCT02316106). eTo measure MRD negativity at a minimum threshold of 10–5 bone marrow aspirates were collected at first evidence of suspected CR or sCR (including patients with ≥VGPR and suspected DARA interference), after induction but before stem cell collection, at the post-transplant consolidation disease evaluation, and at 12 mo and 24 mo (±3 weeks) of maintenance therapy.
Sborov D, et al. IMS Annual Meeting 2022.
GRIFFIN Final Analysis: PFS in the ITT Population (Median Follow-up: 49.6 Mo)
• Median PFS was not reached in either group
• PFS was longer for D-RVd/D-R versus RVd/R, with a clinically meaningful 55% reduction in the risk of disease progression or death
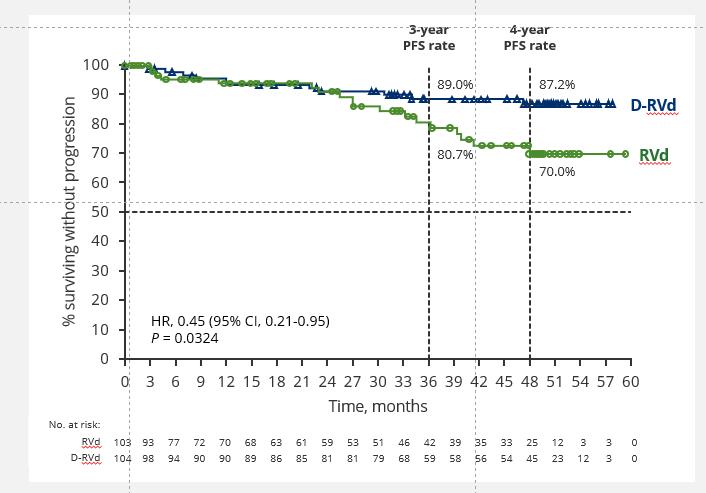
• Regardless of R therapy continuation after end of study treatment, a PFS benefit was observed in the D-RVd group versus the RVd group
• OS not reached/not mature; longer follow up would be needed to evaluate; 4-year OS: 92.7% D-RVd vs 92.2% RVd
GRIFFIN: Rates of Durable
Lasting ≥ 6 Months or ≥ 12 Months—D-RVd vs RVd


Summary: Upfront therapy with SCT
Daratumumab, Carfilzomib, Lenalidomide and Dexamethasone (Dara-KRd), Autologous Transplantation and MRD Response-Adapted Consolidation and Treatment Cessation-Final Primary Endpoint Analysis of the MASTER Trial
Luciano J. Costa1, Saurabh Chhabra2, Natalie S. Callander, MD3 , Eva Medvedova4, Bhagirathbhai Dholaria5, Rebecca Silbermann4, Kelly Godby1, Binod Dhakal2, Susan Bal1, Smith Giri1, Anita D’Souza2, Timothy Schmidt3, Aric
COMMIT- Academic Consortium to Overcome Multiple Myeloma through Innovative Trials
 Hall3, Pamela Hardwick1, Robert F. Cornell5, Parameswaran Hari2
1- University of Alabama at Birmingham; 2- Medical College of Wisconsin; 3- University of Wisconsin at Madison; 4- Oregon Health and Science University; 5- Vanderbilt University
Hall3, Pamela Hardwick1, Robert F. Cornell5, Parameswaran Hari2
1- University of Alabama at Birmingham; 2- Medical College of Wisconsin; 3- University of Wisconsin at Madison; 4- Oregon Health and Science University; 5- Vanderbilt University
Treatment
Dara-KRd
• Daratumumab 16 mg/m2 days 1,8,15,22 (days 1,15 C 3-6; day 1 C >6)




• Carfilzomib (20) 56 mg/m2 Days 1,8,15
• Lenalidomide 25 mg Days 1-21
• Dexamethasone 40mg PO Days 1,8,15,22
MRD assessment by NGS
*24 and 72 weeks after completion of therapy
MASTER trial
”MRD-SURE” -Treatment-free observation and MRD surveillance*
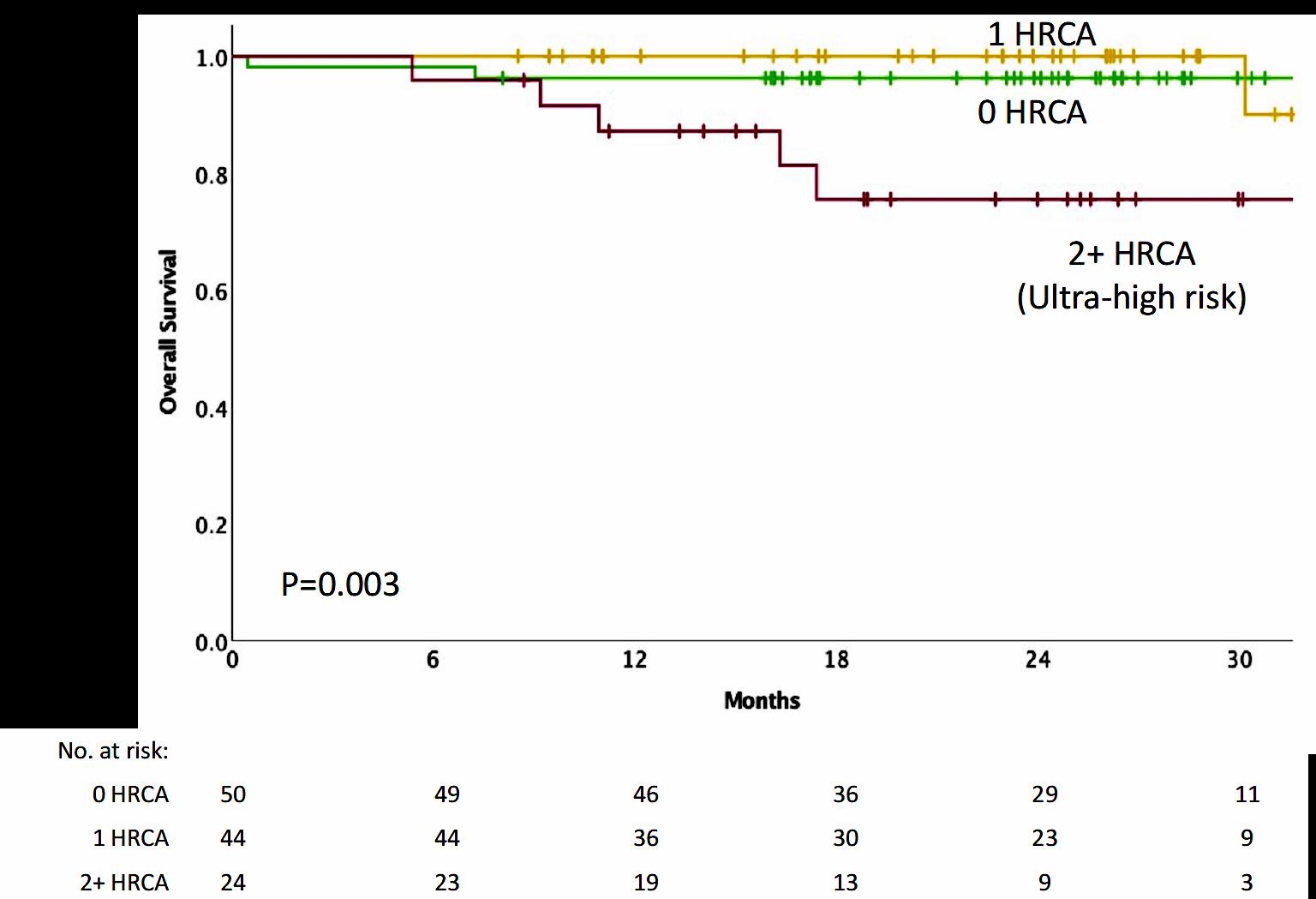
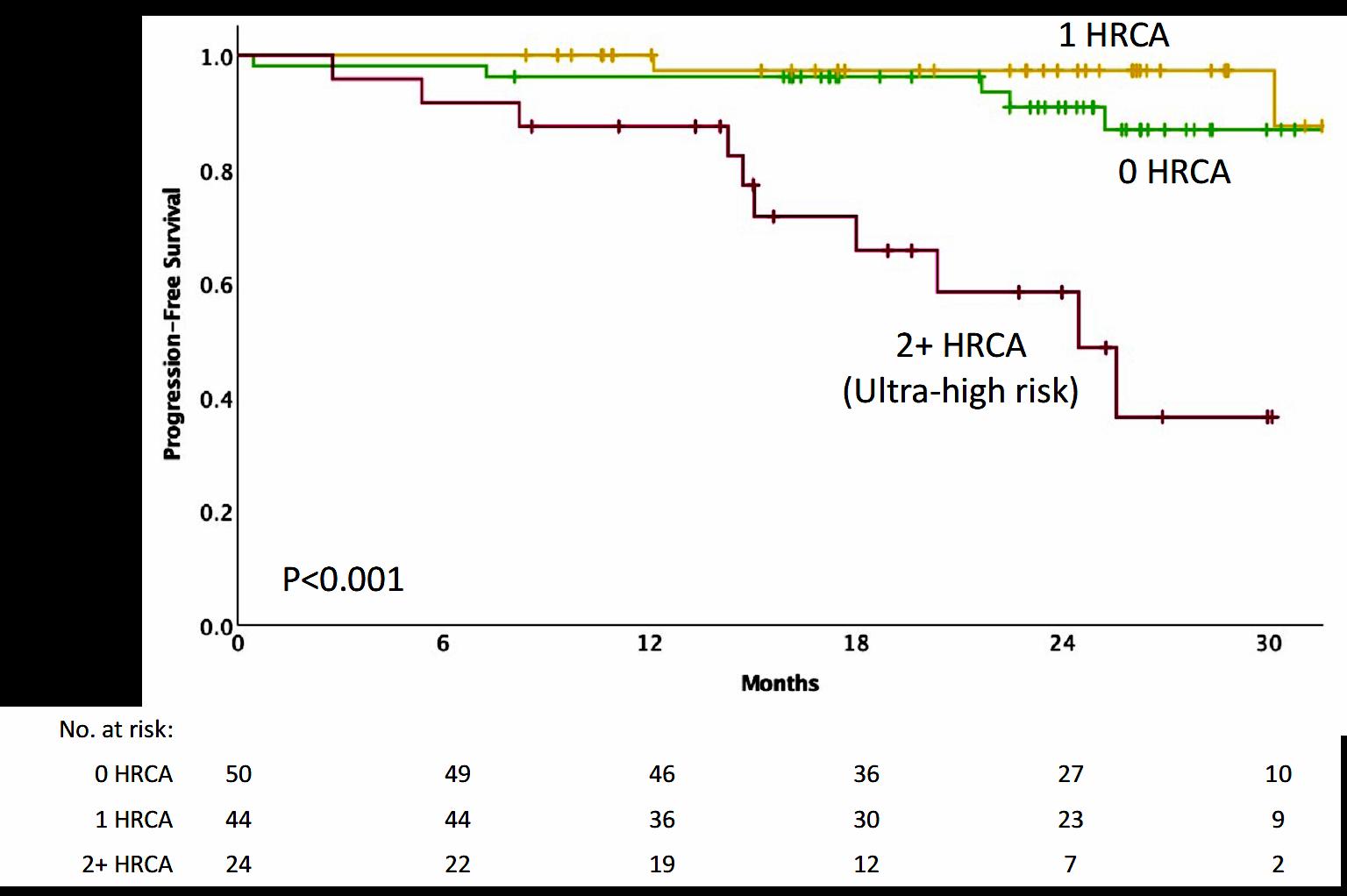
Progression-Free and Overall Survival Based on Presence of High-Risk Features
Conclusions
• NGS-MRD response-adapted therapy is feasible in ~96% of patients in multi center setting – 72% reaching MRD-SURE.
• Patients with standard and high-risk NDMM have similar depth of response and low risk of MRD resurgence or progression when treated with Dara-KRd/AHCT and MRDadapted treatment cessation.
• Quadruplet therapy and achievement of confirmed MRD (-) responses enables the exploration of treatment cessation and “MRD-SURE” as alternative to continuous therapy.
Effective novel consolidative strategies should be explored to clear MRD and improve outcomes in patients with ultra-high-risk MM
Addition of Isatuximab to Lenalidomide, Bortezomib and Dexamethasone as Induction Therapy for
Newly-Diagnosed, Transplant-Eligible Multiple Myeloma:
The Phase III GMMG-HD7 Trial

Hartmut Goldschmidt1,2, Elias K. Mai1, Eva Nievergall1, Roland Fenk3, Uta Bertsch1,2, Diana Tichy4, Britta Besemer5, Jan Dürig6, Roland Schroers7, Ivana von Metzler8, Mathias Hänel9, Christoph Mann10, Anne Marie Asemissen11, Bernhard Heilmeier12, Stefanie Huhn1, Katharina Kriegsmann1, Niels Weinhold1, Steffen Luntz13, Tobias A. W. Holderried14, Karolin Trautmann-Grill15, Deniz Gezer16, Maika Klaiber-Hakimi17, Martin Müller18, Cyrus Khandanpour19, Wolfgang Knauf20, Markus Munder21, Thomas Geer22, Hendrik Riesenberg23, Jörg Thomalla24, Martin Hoffmann25, Marc-Steffen Raab1, Hans J. Salwender26, Katja C. Weisel11 for the German-speaking Myeloma Multicenter Group (GMMG)
1Department of Internal Medicine V, University Hospital Heidelberg, Heidelberg, Germany; 2National Center for Tumor Diseases Heidelberg, Heidelberg, Germany;
3Department of Hematology, Oncology and Clinical Immunology, University Hospital Düsseldorf, Düsseldorf, Germany; 4Division of Biostatistics, German Cancer Research Center (DKFZ) Heidelberg, Heidelberg, Germany;


5Department of Internal Medicine II, University Hospital Tübingen, Tübingen, Germany; 6Department for Hematology and Stem Cell Transplantation, University Hospital Essen, Essen, Germany;
7Medical Clinic, University Hospital Bochum, Bochum, Germany; 8Department of Medicine, Hematology/Oncology, University Hospital Frankfurt, Goethe University, Frankfurt am Main, Germany;
9Department of Internal Medicine III, Clinic Chemnitz, Chemnitz, Germany; 10Department for Hematology, Oncology and Immunology, University Hospital Gießen and Marburg, Marburg, Germany;
11Department of Oncology, Hematology and BMT, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; 12Clinic for Oncology and Hematology, Hospital Barmherzige Brueder Regensburg, Regensburg, Germany; 13Coordination Centre for Clinical Trails (KKS) Heidelberg, Heidelberg, Germany; 14Department of Oncology, Hematology, Immuno-Oncology and Rheumatology, University Hospital Bonn, Bonn, Germany;
15Department of Internal Medicine I, University Hospital Dresden, Dresden, Germany; 16Department of Hematology, Oncology, Hemostaseology, and Stem Cell Transplantation, Faculty of Medicine, RWTH Aachen University, Aachen, Germany; 17Clinic for Hematology, Oncology and Palliative Care, Marien Hospital Düsseldorf, Düsseldorf, Germany; 18Clinic for Hematology, Oncology and Immunology, Klinikum Siloah Hannover, Hannover, Germany; 19Medical Clinic A, University Hospital Münster, Münster, Germany; 20Center for Hematology and Oncology Bethanien, Frankfurt am Main, Germany; 21Department of Internal Medicine III, University Hospital Mainz, Mainz, Germany; 22Department of Internal Medicine III, Diakoneo Clinic Schwäbisch-Hall, Schwäbisch-Hall, Germany; 23Hematology/Oncology Center, Bielefeld, Germany; 24Hematology / Oncology Center, Koblenz, Germany; 25Medical Clinic A, Clinic Ludwigshafen, Ludwigshafen, Germany; 26Asklepios Tumorzentrum Hamburg, AK Altona and AK St. Georg, Hamburg, Germany
Primary endpoint: MRD negativity at the end of induction phase
Induction phase (3 x 6-week cycles)
Maintenance phase (4-week cycles)
MRD (bone marrow aspirate)
Primary endpoint:
• MRD negativity at the end of induction treatment (NGF, sensitivity 10-5) stratified according to RISS
Secondary endpoints:
• CR after induction
• Safety Data cut-off:
• April 2021
ASCT, autologous stem cell transplant; CR, complete response; d, dexamethasone; HDT, high-dose therapy; Isa, isatuximab; MRD, minimal residual disease; NDMM, newly diagnosed multiple myeloma; NGF, next-generation flow; PD, progressive disease; R, lenalidomide; R-ISS, Revised International Staging System; Te, transplant eligible; V, bortezomib 1. ClinicalTrials.gov: NCT03617731

Patients with MRD negativity at the end of induction therapy
1.34–2.51)
Low number of not assessable/missing† MRD status: Isa-RVd (10.6%) and RVd (15.2%)
Isa-RVd is the first regimen to demonstrate a rapid and statistically significant benefit from treatment by reaching a MRD negativity of 50.1% at the end of induction and to show superiority vs. RVd in a Phase 3 trial
*P value derived from stratified conditional logistic regression analysis †Missing NGF-MRD values were due to either patients’ loss to follow-up during induction therapy or to missing bone marrow samples or technical failures in measurement counted as non-responders, i.e. NGF-MRD positive CI, confidence interval; d, dexamethasone; Isa, isatuximab; ITT, intent-to-treat; MRD, minimal residual disease; NGF, next-generation flow; OR, odds ratio; R, lenalidomide; V, bortezomib

First primary endpoint, end of induction MRD negativity by NGF (10-5), was met in ITT analysis
Response rates after induction therapy
Although the rates of CR after induction therapy did not differ between the Isa-RVd and RVd arms, there was a significant increase in ≥VGPR rates and ORR with Isa-RVd
*P values derived from Fisher’s exact test
†Data adjusted per M-protein interference CR, complete response; d, dexamethasone; Isa, isatuximab; nCR; near-complete response; ORR, overall response rate; PR, partial response; R, lenalidomide; V, bortezomib; VGPR, very good partial response

Addition of Isa to RVd had limited impact on safety profile

A comparable number of patients discontinued induction therapy due to AEs in the
vs. RVd arm
*SOC considered as “Investigations” as defined by the CTCAE †Includes five episodes of febrile neutropenia during induction: Isa-VRd (n=3) vs. VRd (n=2) ‡Infusion-related reactions of CTCAE grade 2 or higher in the Isa-RVd arm were n=42 (12.7%) AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Events; d, dexamethasone; Isa, isatuximab; NA, not applicable; PT, preferred term; R, lenalidomide; SOC, system organ class; V, bortezomib
Upfront Therapy Status…Evolving

• Adding a CD38 antibody to VRD or KRD is feasible with limited incremental toxicity
• Depth of response is improved, including MRD negativity
• MRD will be an even more important measure going forward
• There is a trend to improved PFS
• This may overcome the high-risk feature of some patients
• Ultra high-risk disease remains challenging to treat with current strategies
• What about transplant?? It is still part of most upfront studies.
The future: MASTER 2 The Dream Trial
Sequential Therapy in Multiple Myeloma Guided by MRD Assessments
*MRD-SURE – Treatment-free observation and MRD surveillance




MRD (-) randomization MRD (+) randomization



Quadruplet X 3 cycles CD38MoAb-R X3 cycles
AHCT












maintenance)

We are after Myeloma

Q&A

Myeloma.org

Robin Tuohy Vice President, Support Groups
International Myeloma Foundation
COFFEE BREAK

Thank you to our Sponsors!














Patient Support Groups in Asia

Daryl Tan, MBBS, M Med, MRCP, FAMS
Mount Elizabeth Novena Hospital
Patient Support Groups
in Asia (IMF Patients and Family Symposium 2023)
Singapore
 Daryl Tan Mount Elizabeth Novena Hospital
Daryl Tan Mount Elizabeth Novena Hospital
Disclosures
Research Support/P.I. Novartis, Janssen
Employee . NA
Consultant . Janssen, BMS-Celgene, Aztra Zeneca, Abbvie
Major Stockholder . NA
Speakers Bureau NA NA
Honoraria Celgene, Janssen, Takeda, Amgen, Aztra Zeneca
Scientific Advisory Board Celgene, Janssen, Takeda, Novartis, Abbvie, Roche, Amgen, MSD
Age-Standardized
Incidence Rate of Multiple Myeloma Incidence
4.2 billion people (61% of world’s population) live in the Asia-Pacific

Asian-Pac Countries
Real World Ideal World
Ref: UN Economic and Social Commission for Asia and the Pacific. Statistical yearbook 2012.
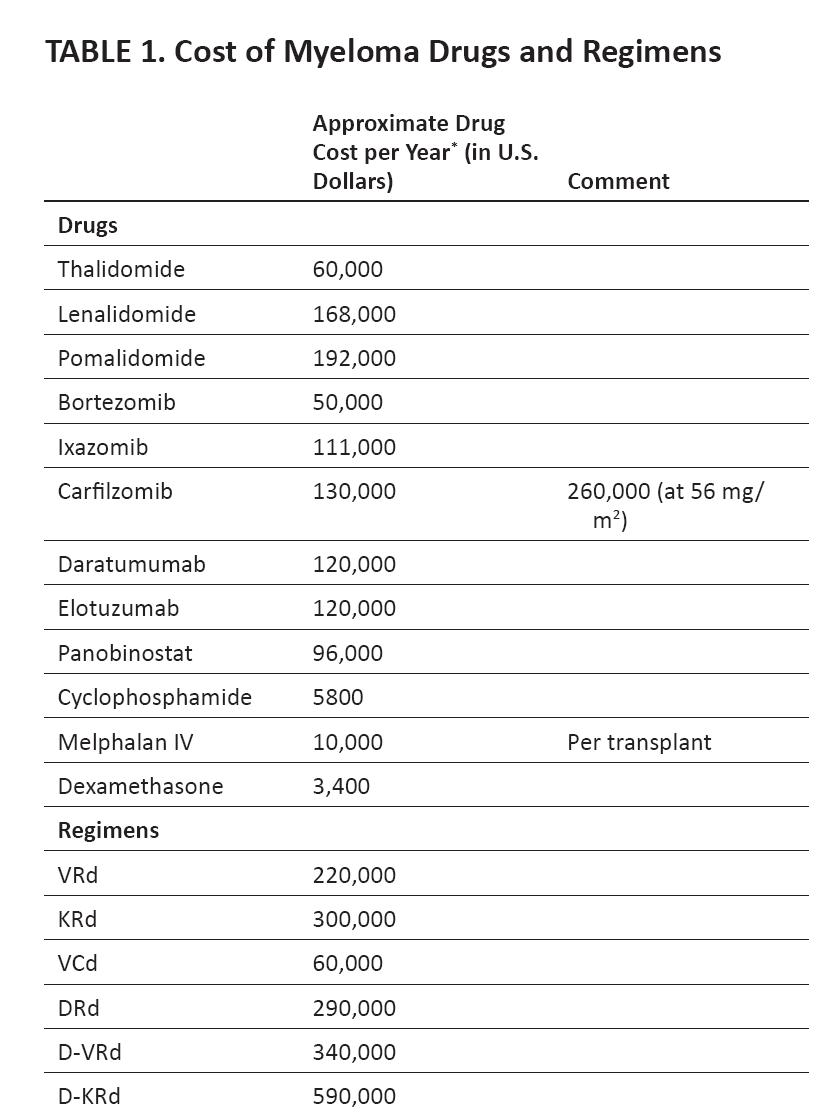

Worldwide Bortezomib and Lenalidomide Approval 2016

Stem-Cell Transplant Rate per 10 Million, 2010
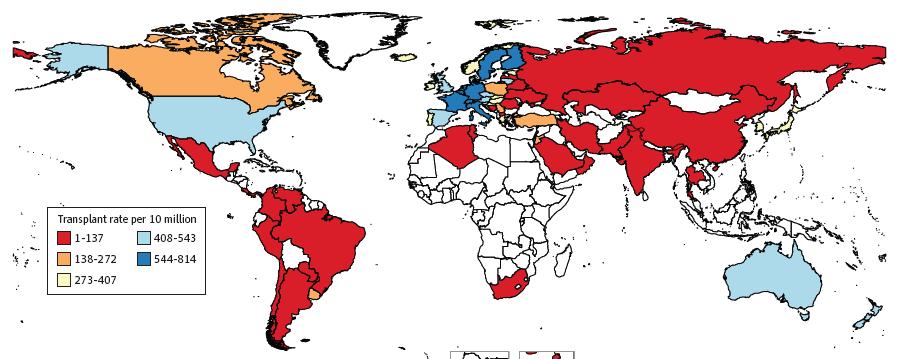 Cowan A, et al. JAMA Oncol. 2018;4(9):1221-1227.
Cowan A, et al. JAMA Oncol. 2018;4(9):1221-1227.
The Asian Myeloma Network- 2011




Agenda of Asian Myeloma Network
• Define the Asian myeloma
• Identify the unmet needs and challenges
• Bridge the gaps
• Improve quality of care for standardization with international myeloma community
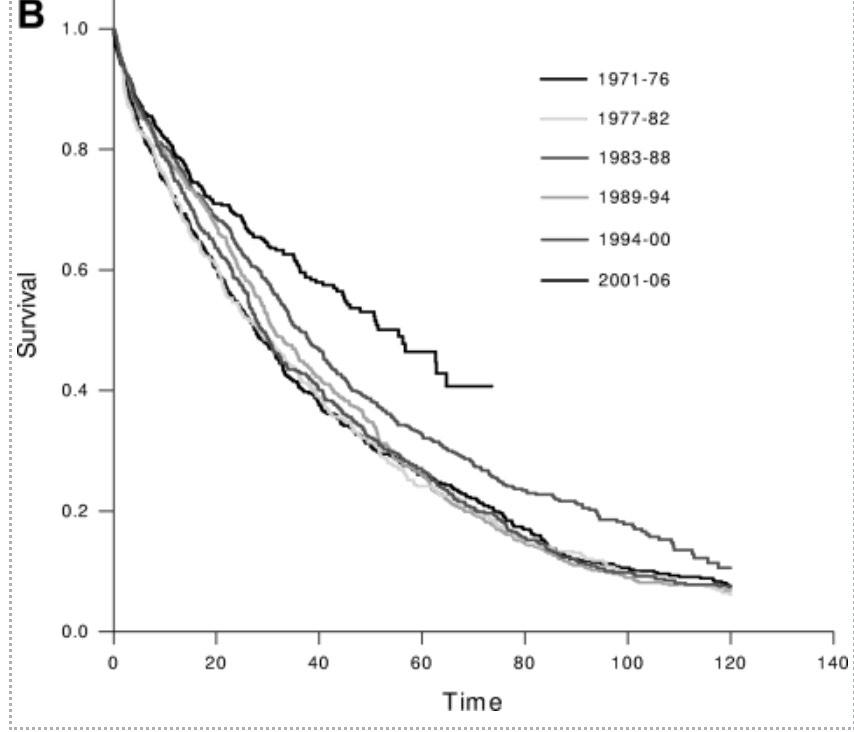

With adequate resources, Asian myeloma patients do just as well
Incidence and mortality of Multiple Myeloma in China, 2006–2016: an analysis of the Global Burden of Disease
Liu et al. Journal of Hematology & Oncology (2019) 12:136
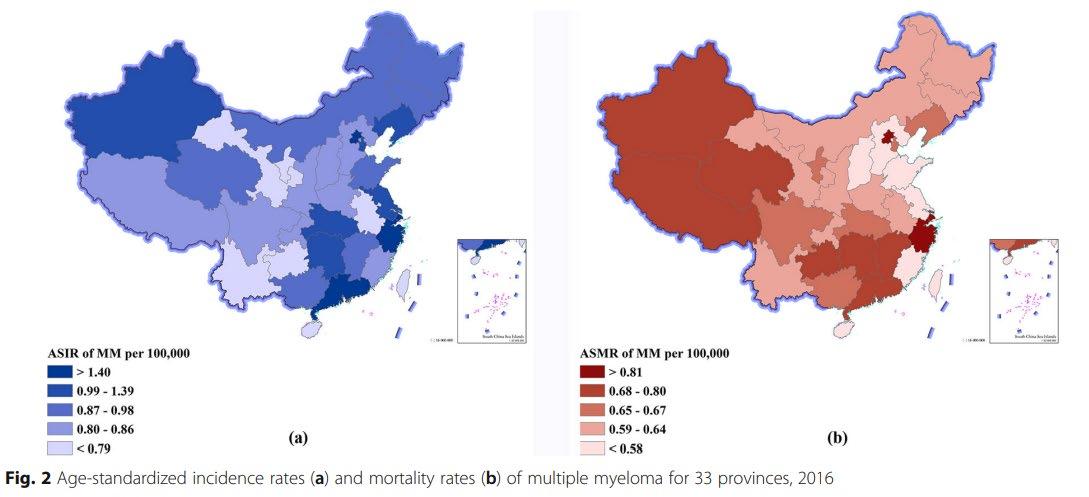
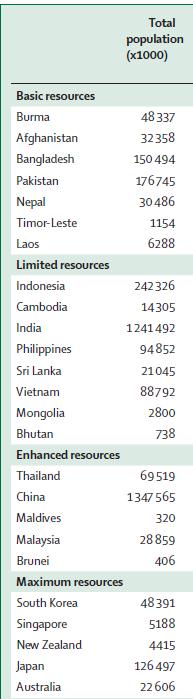
Ability to optimize available resources to achieve best outcomes for our patients

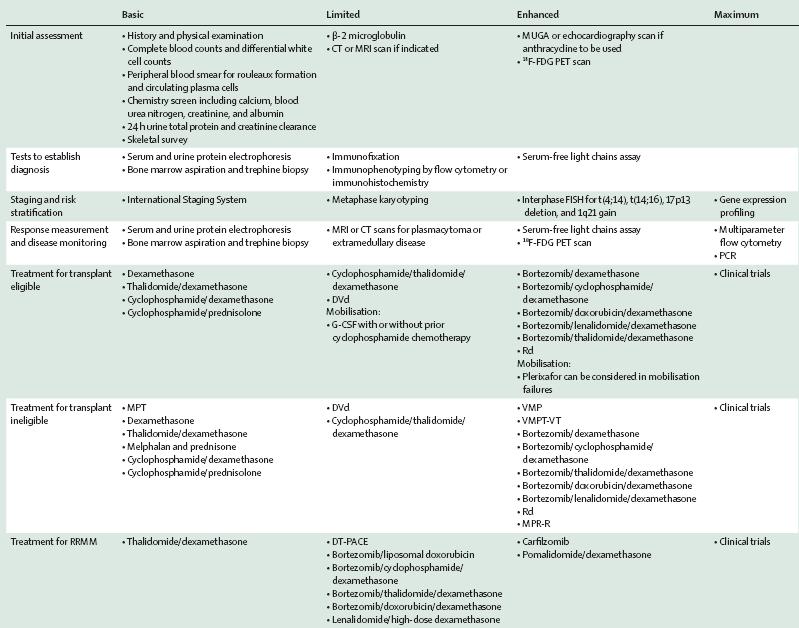
Level of Resources
Definition
Basic Core resources or fundamental services basic-level services typically are applied in a single clinical interaction.
Limited Second-tier resources or services that are intended to produce major improvements in outcome such as increased survival, and are attainable with limited financial means and modest infrastructure
Enhanced Third-tier resources or services that are optional but important; enhanced-level resources should produce further improvements in outcome and increase the number and quality of therapeutic options and patient choice.
Maximal High-level resources or services that may be used in some high-resource countries and/or may be recommended by myeloma guidelines that do not adapt to resource constraints but that nonetheless should be considered a lower priority than those resources or services listed in the basic, limited, or enhanced categories on the basis of extreme cost and/or impracticality for broad use in a resource-limited environment;
Developing a Resources-Stratified Treatment Guideline for Multiple Myeloma in Asia:
Improving drug access

Generics
Clinical trials

Drug Access in AsiaAMN Summit Oct
2021
R= Reimbursed SP= Self pay NPP=Named patient programme

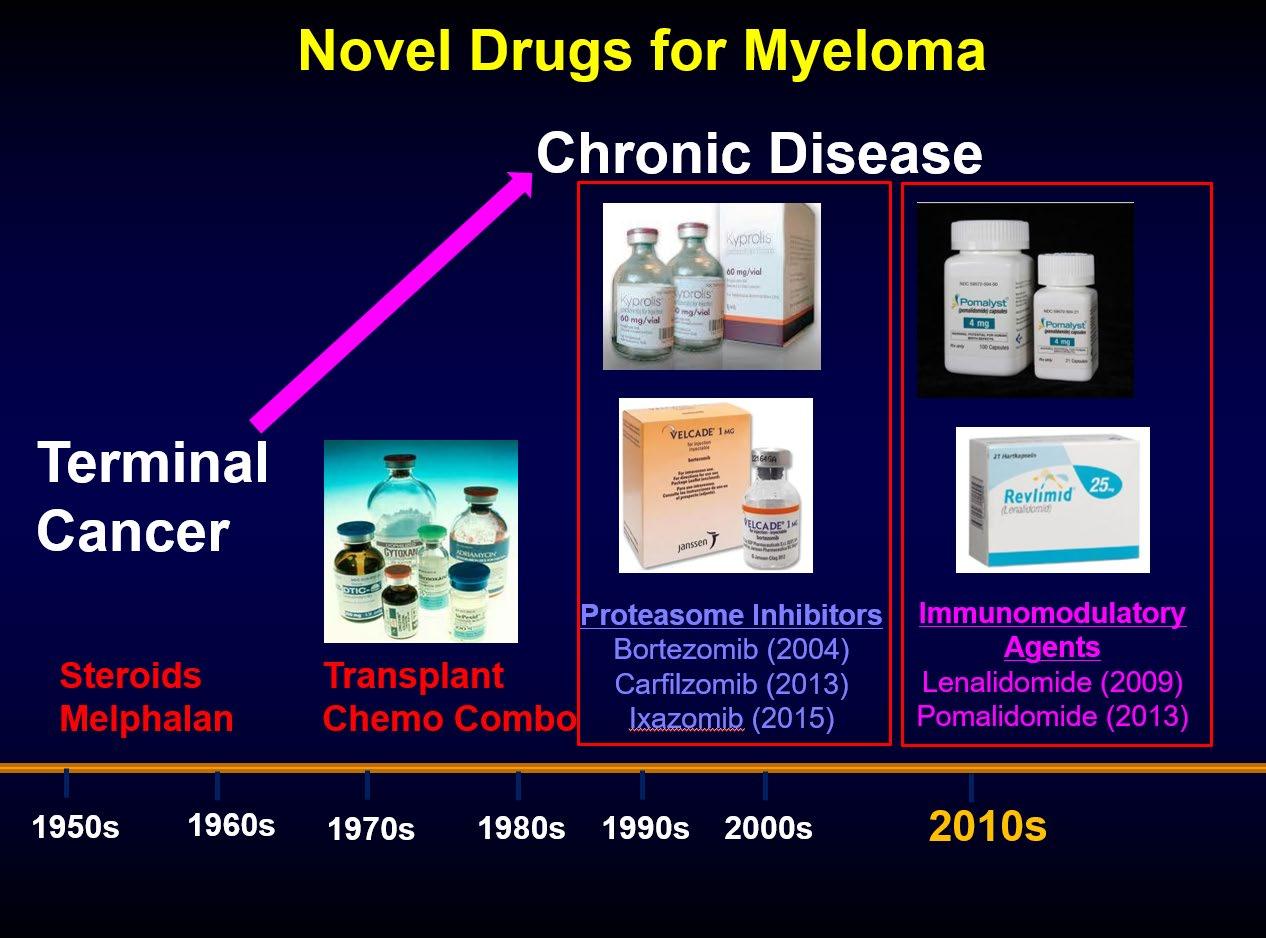





Prolonged survival and attainment of minimal residual disease negativity



Monoclonal Antibodies


Daratumumab (2015)
Isatuximab (2022)


Every Drug Class in Myeloma Treatment is Important for Optimal Survival




CAR-T


Bispecific T-cell Engagers
2020s

Improving
Drug Access for Myeloma Patients: Stakeholders
Health Authority
Pharmaceutical Industry
Key opinion leaders – (AMN)
Empowerment
Physician education
Improve treatment guidelines
Clinical trials
Patients – Advocacy





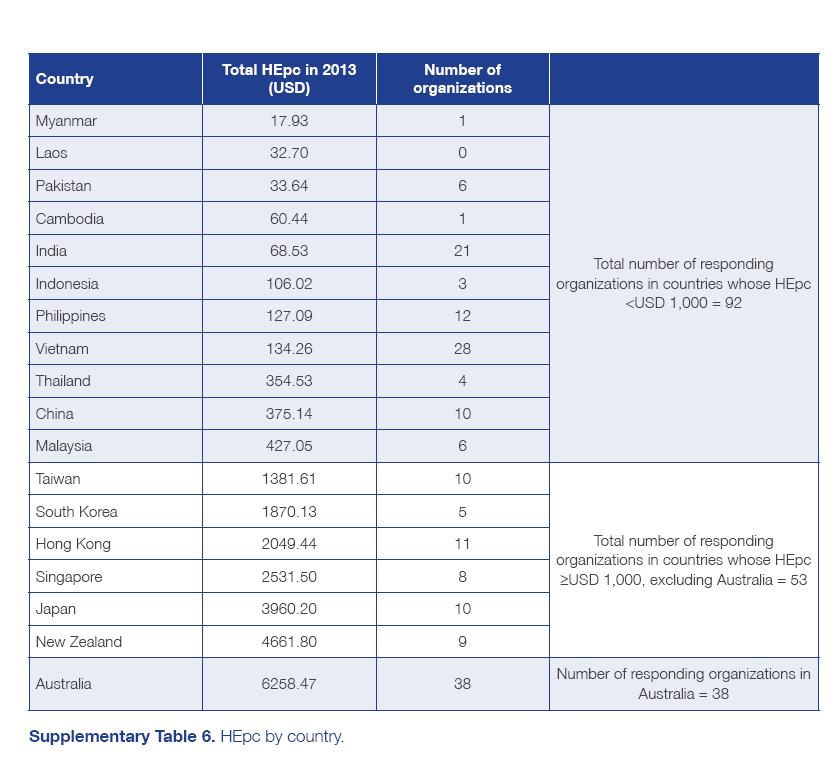
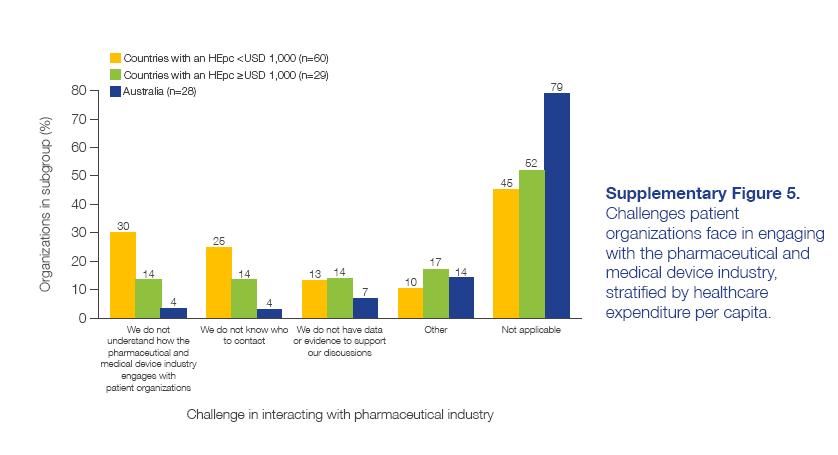
CANCER PATIENT ORGANIZATIONS IN ASIA Report of findings from a regional survey

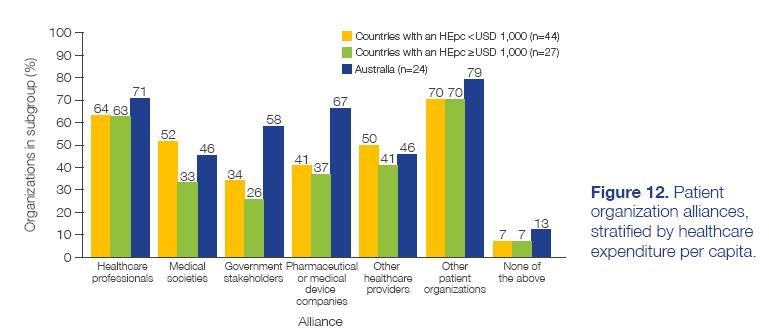
CANCER PATIENT ORGANIZATIONS IN ASIA Report of findings from a regional survey
CANCER PATIENT ORGANIZATIONS IN ASIA Report of findings from a regional survey
1st IMF Asian Myeloma Patient Virtual Forum






10 October 2021
AMN Asian Patient Support- Agenda
1) Identify current status of Support Group activities for each country/ region
2) Identify main obstacles faced by patients or support groups in each country/region
3) Introduce a common template for recommended support group activities / meetings
4) Empower patients with necessary knowledge (medical, administrative and advocacy)
China -Beijing




Taiwan


Singapore








South Korea



Japan
• Patient-championed > ‘IMF in Japan’autonomous-

• Industry support
• National group
• Engages industry, govt
• Partners with Japanese Society of MM > education (road show, MM guidebooks)
Hong Kong- HK Myeloma Care & Share Association



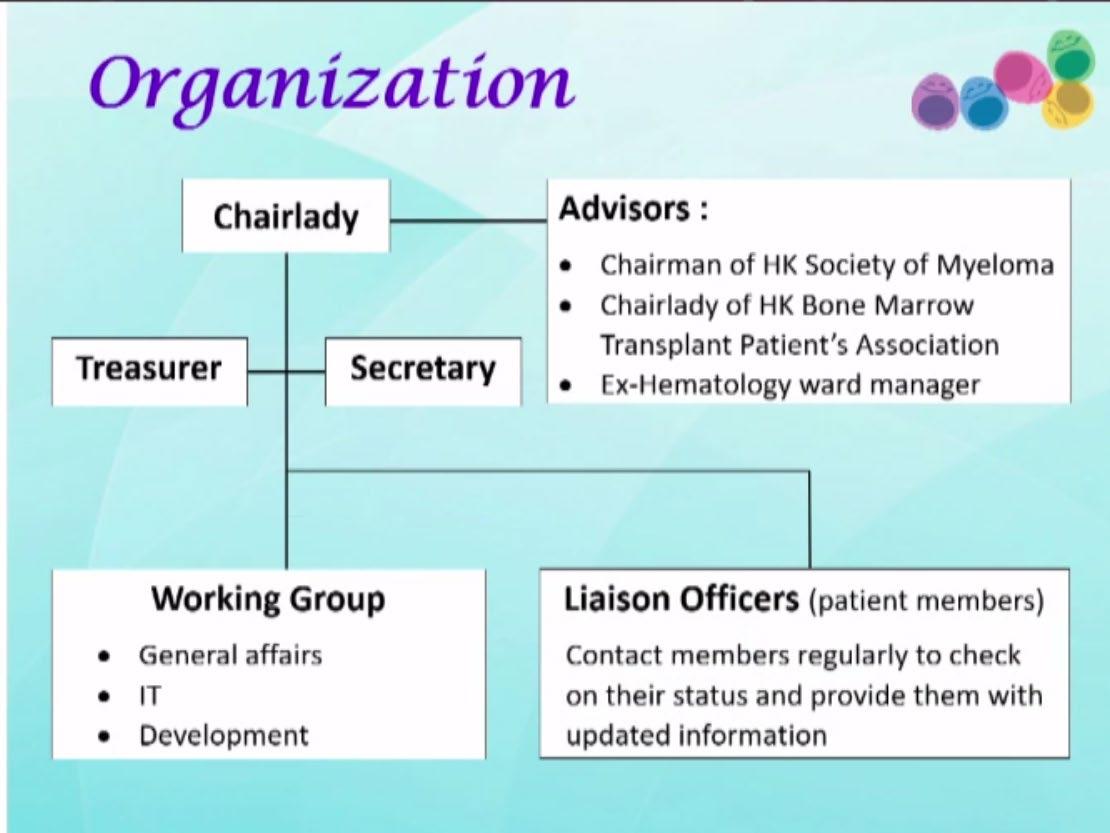
What has been the major strength of the myeloma support group in your country/region ?
What are the major shortfalls faced by the myeloma support group in your country ?
Lack of motivated patient /caregiver leaders
Only one dominant leader with no succession plan
Lack of members with organizational skills
Lack of members who knows how to engage stakeholders
Lack of physician oversight with no control over dissemination of inaccurate news
Heavily dependent on physician initiatives + +
Lack of funding
Membership fees + + +
Others
AMN Patient Support- Follow-up Activities
• Malaysia
•
• Thailand
• Identify pt/caregiver champion + core group
• Work on logistics for incorporation of MM pt support into legal entity
• Singapore
• Start-up kit and seed $ from IMF
• Taiwan : Taiwan Haematology Society’s myeloma working group to bring together myeloma physicians from various hospitals
• China : Multiple myeloma club > education, patient support
• South Korea : Work on myeloma specific support group
• Japan : Succession planning and to resolve leadership issues
• Hong Kong : Initiate regional collaboration , outreach to Chinese speaking countries/regions eg Taiwan, China
Evolution of Myeloma Patient Support Organizations
Physicians-driven
• Education
• Social gatherings
• No specific myeloma group

Patient Champions
• Lacks organization capability




• Lacks funding
Organization capability
• Lacks funding


Patient autonomy
• Lacks succession plan
• Self-sufficient
• Self-funded
Patient autonomy
• Legal entity

• Structured organization
• Engages government
• Engages industry
Patient autonomy
• Regional out-reach/ Collaborations
Conclusion
• Key to improved survival outcomes is the access to the latest novel therapeutics
• Escalation of costs involved in myeloma care may not be sustainable by many healthcare systems
• Engaging various stakeholders is key to finding solution for affordability and access
• Empowering our patients for better advocacy is paramount

Acknowledgements
AMN, IMF, Industry partners and our Patients

Thank You

Q&A

LUNCH BREAK

Thank you to our Sponsors!














Approaches to Relapse Therapy

Noopur Raje, MD
Massachusetts General Hospital
Approaches to Relapse Therapy
Noopur Raje, MD Director, Center for Multiple MyelomaMGH Cancer Center
Professor of Medicine
Harvard Medical School
Disclosures
• Consultant /Advisory Board: Celgene, Millenium
Takeda, Amgen-Onyx, Novartis, Janssen, BMS, Merck, Bluebird, Caribou Biosciences, Immuneel
• Research Funding: Bluebird bio
• Steering Committee: Amgen, Roche
Natural History of Multiple Myeloma
Myeloma Drugs Approved Since 2000
Zoledronic acid
Initial FDA approval date in multiple myeloma
2021: Ide-cel
2022: Cilta-cel
2023: teclistamab
Standard Practice in 2023
• Triplets in newly-diagnosed patients: RVD/KRD/VCD
– Phase II trial (2010)1
– Phase III data from SWOG S0777 (2016): RVd > Rd2
–
Phase II data (2013/2016)3
• Transplant and maintenance SOC4
• Daratumumab initially approved in 2015 in patients with ≥3 prior lines of treatment
– Now approved in first line with VMP5
Dara-Rd with Rd (MAIA)6
–
Ongoing trials comparing and Dara-RVd vs RVd (GRIFFIN)
• Triplets such as KRd or PVD > doublets7,8
• Quadruplets are under investigation and used more
• Continuous therapy is the accepted treatment paradigm for myeloma: Are we ready to question this?
Relapse MM
• With modern therapy, first relapse generally occurs ~ 3 to 4 years after initial diagnosis
• Many patients receive ≥ 5 lines of therapy over several years
• Duration of remission decreases with each regimen
• Treatment selection is affected by many factors
• Relapse is evolving as approved drugs are used in combinations and in the upfront setting
Defining Relapse
• Serum M component (absolute ↑ must be ≥ 0.5 g/dL)
• Serum M protein ↑ ≥ 1 g/dL, if the lowest M component was ≥ 5 g/dL
• Urine M protein (absolute ↑ must be ≥ 200 mg/24 hours)
• Involved and uninvolved FLC level difference (not ratio) in patients without measurable serum and urine M protein level†
• BM plasma cell percentage in patients without measurable serum and urine M protein levels and involved FLC levels†
• Appearance of a new lesion(s) by any imaging technique‡
• ≥ 50% increase in circulating plasma cells (minimum of 200 cells/μL)
*2 consecutive assessments are needed before new therapy.
†Absolute increase must be > 10 mg/dL
‡For KNOWN lesions: ≥ 50% increase from nadir in SPD > 1 lesion, or ≥ 50% increase in the longest diameter of a previous lesion > 1 cm in short axis.
Evaluation at Relapse
Bloodwork
•CBC and chemistry panel, including LDH, albumin, and corrected calcium
•Myeloma panel: quantitative immunoglobulins, SPEP, sFLC
•Consider 24-hour urine for total protein, CrCl, UPEP, and UIFE

Imaging
•At symptomatic relapse: skeletal survey, PET/CT (alone or if skeletal survey is negative), WB LDCT, WB MRI
Bone marrow
•Important to do a BM biopsy at the time of relapse
•Reassess karyotype and FISH
When to Initiate Therapy at Relapse
Asymptomatic biochemical relapse on 2 consecutive assessments
Asymptomatic high-risk disease or rapid doubling time
Symptomatic or extramedullary disease
Consider Treatment
Patient/Disease-Specific Monitor Carefully
Initiate Treatment
Consider Observation
Monitor Carefully
Not every relapse requires immediate therapy
Factors Impacting Therapy Selection
PatientRelated
TherapyRelated
Therapy Selection
DiseaseRelated
Principles of Treatment
• Improve symptoms
• Obtain a deep response: attain MRD negative disease
• Use your best drugs rather than reserve for later
• Consider risk even at relapse
• Balance efficacy and toxicity
• Consider clinical trials
Drugs for multiple myeloma




















Approved August 2020
Approved March 2021
Myeloma Early Relapse –
Guideline Alternatives
Bortezomib Refractory
Daratumumab/lenalidomide/d
Daratumumab/carfilzomib/d
Carfilzomib/lenalidomide/d
Isatuximab/carfilzomib/d
Carfilzomib/pomalidomide/d
Daratumumab/pomalidomide/d
Isatuximab/pomalidomide/d
Ixazomib/pomalidomide/d
Lenalidomide
Lenalidomide
Refractory
Refractory
Daratumumab/carfilzomib/d
Daratumumab/bortezomib/d
Isatuximab/carfilzomib/d
Carfilzomib/pomalidomide/d
Daratumumab/pomalidomide/d
Isatuximab/pomalidomide/d
Pomalidomide/bortezomib/d
Ixazomib/pomalidomide/d
Other Options
Bortezomib/liposomal doxorubicin/d
Carfilzomib (twice weekly)/d
Elotuzumab/lenalidomide/d
Selinexor/bortezomib/d
Bortezomib/cyclophos/d
Carfilzomib/cyclophos/d
Cyclophos/lenalidomide/d
Daratumumab/cyclophos/ bortezomib/d
Pomalidomide/cyclophos/d
Elotuzumab/pomalidomide/d
Recent Phase 3 Clinical Trials in R/R MM vs PI/Dex
• *Experimental vs control, respectively; †Only 10% of patients had prior len exposure.
• a. Dimopoulos MA, et al. Lancet Oncol. 2016;17:27-38; b. Palumbo A, et al. N Engl J Med. 2016;375:754-766; c. San Miguel JF, et al. Lancet Oncol. 2014;15:1195-1206; d. Richardson PG, et al. Lancet Oncol. 2019;20:781-794; e. Dimpopoulos MA, et al. ASCO® 2020. Abstract 8501; f. Moreau P, et al. ASH 2020. Abstract 2316; g. Dimopoulos MA. ASH 2020. Abstract 2325.
Recent Phase 3 Clinical Trials in R/R MM vs Pom/Dex
Myeloma: First Relapse – IMWG Guidelines
First Relapse
Preferred options: DRd (or KRd)
Not Refractory to Lenalidomide
Refractory to Lenalidomide
Alternatives: DVd, Kd, DaraKd, Isa-Kd, IRd, Elo-Rd, PVd, SVd
When Dara, Isa, K not available: Rd, Vd, VTd, VCd, VMP
Preferred options: PVd DaraKd Isa-Kd
Second options DaraVd;Kd
Other options: KPd; DaraPd; Ipd
When Dara, isa, K or P not available: VCd, Vd, VMP
Consider salvage auto transplant in eligible patients
Early Relapse
• General principles
Use mechanisms of action not previously used
−
Do not continue to use lenalidomide if progressing on lenalidomide maintenance
− Triplets are preferred over doublets
• In real practice, most patients receiving VRD (Bortezomib-Lenalidomide-Dex) like regimens, first relapse is typically −
Daratumumab + Pomalidomide + Dex (APOLLO) −
Isatuximab + Pomalidomide + Dex (ICARIA)
Daratumumab + Carfilzomib + Dex (CANDOR)
−
Isatuximab + Carfilzomib + Dex (IKEMA)
−
Selinexor + Bortezomib + Dex (BOSTON)
Conclusions on Early Relapse
• There are more options than ever before! • We have phase 3 data with all the major classes of drugs • General principles
– Do not “save the best for last” – use the best options early on…
– Introduce new mechanism of action
– Use a triplet combination
– Select best combination based on patient, disease, and treatment characteristics • Soon, we will see CAR T-cell therapies and bispecific antibodies move up earlier in the disease course…
There is no “perfect” sequence!
Drugs for multiple myeloma: What is new?
T-cell engagers














Selinexor/SINE Mechanism of Action: XPO1
Inhibition
• XPO1 overexpression:
– Allows cancer cells to escape TSP-mediated cell cycle arrest and apoptosis induction
– Correlates with poor prognosis and drug resistance
• XPO1 inhibition impacts tumors by 3 mechanisms:
–
↑ nuclear levels and activation of TSPs
– Traps oncoprotein mRNA in nucleus leading to ↓oncoprotein levels
– Retains activated GR in nucleus

• Selinexor: oral, selective XPO1 inhibitor
• In preclinical data in MM models selinexor
– Reactivates multiple TSPs relevant to MM
– Inhibits NF-kB signaling
– Reduces cMYc levels
– Shows synergistic activity with BTZ, POM, and LEN in vitro and in vivo
STORM: Phase IIb Study of Selinexor + Dexamethasone for Triple-Class Refractory MM
Phase IIb study in patients with 7 median prior lines of therapy (range: 3-18)
— All patients exposed to lenalidomide, pomalidomide, bortezomib, carfilzomib, daratumumab, glucocorticoids, and an alkylating agent
— 96% refractory to carfilzomib, pomalidomide, and daratumumab, 100% refractory to their most recent therapy
BOSTON Trial: Selinexor-Vd vs Vd in Patients With MM Who Received 1-3 Prior Therapies (FDA Approved)
• Open-label, controlled, randomized phase III trial in patients with R/R MM after 1-3 prior therapies
HR: 0.70 (P = .0075)

Selinexor: Combined Options for R/R MM*
Safety All-grade: thrombocytopenia (73%), anemia (67%), neutropenia (40%), nausea (72%)
All-grade: thrombocytopenia (60%), anemia (36%), neutropenia (15%), nausea (50%)
*Several other combinations in phase IB/II: carfilzomib, lenalidomide and ixazomib.

Neutropenia, anemia, thrombocytopenia, leukopenia, fatigue
Thrombocytopenia, nausea, fatigue, anemia, neutropenia
1. Selinexor PI. 2. Chari. NEJM. 2019;381:727. 3. Grosicki. Lancet. 2020;396:1563.
4. White. ASCO 2021. Abstr 8018. 5. San-Miguel. Lancet Oncol. 2013;14:1055. 6. Gasparetto. eJHaem. 2021;5:56.
Selinexor: Toxicity Considerations
Selinexor
Select AEs to assess Thrombocytopenia, neutropenia (consider TPO/growth factor support)
Considered highly emetogenic
Consider longer-acting antiemetic (ie, olanzapine or rolapitant) as standing with 5-HT3 for breakthrough
Hyponatremia
Infections
Recommended monitoring Monitor patients at baseline and during treatment for cytopenia, neutropenia, hyponatremia, infections
Monitor patients’ body weight and potential for dehydration
Provide antiemetic (and antidiarrheal) prophylaxis; consider IV hydration for patients at risk of dehydration
Consider starting selinexor therapy at reduced dose of 80 mg once weekly
Recommended dose reductions
Starting dose (XVd): 100 mg once weekly
First: 80 mg once weekly
Second: 60 mg once weekly
Third: 40 mg once weekly
Discontinue after third reduction
Starting dose (Xd): 80 mg Days 1 and 3
First: 100 mg once weekly
Second: 80 mg once weekly
Third: 60 mg once weekly
Discontinue after third reduction

Venetoclax
• Mechanism of action: selective oral inhibitor of BCL2

– Binds directly to the BCL2 protein

– Displaces pro-apoptotic proteins and restores the apoptotic process
Key Administration Aspects in R/R MM Setting
Dosing 800 mg PO daily in 21-day cycles x 8 cycles, then 35-day cycles until progression
Renal/ liver dosing
Reduce by 50% for Child-Pugh class C
Adverse events Diarrhea, nausea, infections, thrombocytopenia, anemia, TLS neutropenia, pneumonia, edema, hyperglycemia, electrolyte abnormalities
Pearls Dose reductions for CYP3A4 inhibitors
Herpes zoster/PJP prophylaxis
Monitor TLS risk
Consider dose-escalation 400 mg daily (first wk), then increase to 800 mg daily
Not FDA approved Off label use in R/R MM w/ t(11;14) only
BELLINI trial
Phase III
Median follow-up of 18.7 mo
PFS: 22.4 vs 11.5 mo; P = .01,
Kaufman. Ven + d after 1+ line of tx (n = 31) in patients with t(11;14) demonstrated single-agent activity in patients who are refractory to CD38 mAb
Salvaris. Future Oncol. 2021;174:371. Kaufman. Lancet Oncol. 2020;21:1630.
Kaufman. Am J Hematol. 2021;96:418. Venetoclax PI.
Ven+ Vd vs Placebo+ Vd
ORR 85% vs 70%
PFS improvement at the cost of higher rate of fatal infections; OS favors placebo; higher CR rates in R/R MM with t(11;14)
Slide credit: clinicaloptions.com
BELLINI Final Survival Analysis: Study Design
• Double-blind, randomized 2:1, placebo-controlled phase III trial

Stratification by bortezomib sensitive vs naive and prior lines of therapy (1 vs 2-3)
Patients with R/R MM after 1-3 prior lines of therapy; not refractory to PI therapy
(N = 291)
Venetoclax 800 mg QD + Bortezomib 1.3 mg/m2 + Dexamethasone 20 mg (n = 194)
Placebo + Bortezomib 1.3 mg/m2 + Dexamethasone 20 mg (n = 97)
Cycles 1-8: 21-day cycles with bortezomib on Days 1, 4, 8, 11 and dexamethasone on Days 1, 2, 4, 5, 8, 9, 11, 12; cycles 9+: 35-day cycles, bortezomib on Days 1, 8, 15, 22 and dexamethasone on Days 1, 2, 8, 9, 15, 16, 22, 23.
• Primary endpoint: PFS (per IRC)
• Key secondary endpoints: ORR, ≥VGPR, OS, QoL/PRO parameters (PFS was investigator assessed in final OS analysis)
Slide credit: clinicaloptions.com
PFS and OS in Key Patient Subgroups

Immune Therapy
• CAR T-cell therapy
• Bispecific T-cell engagers
MoAb/drug/immunoconjugates: belantamab mafodotin, TAK
573
Emerging role of CelMods (iberdomide, CC92480)
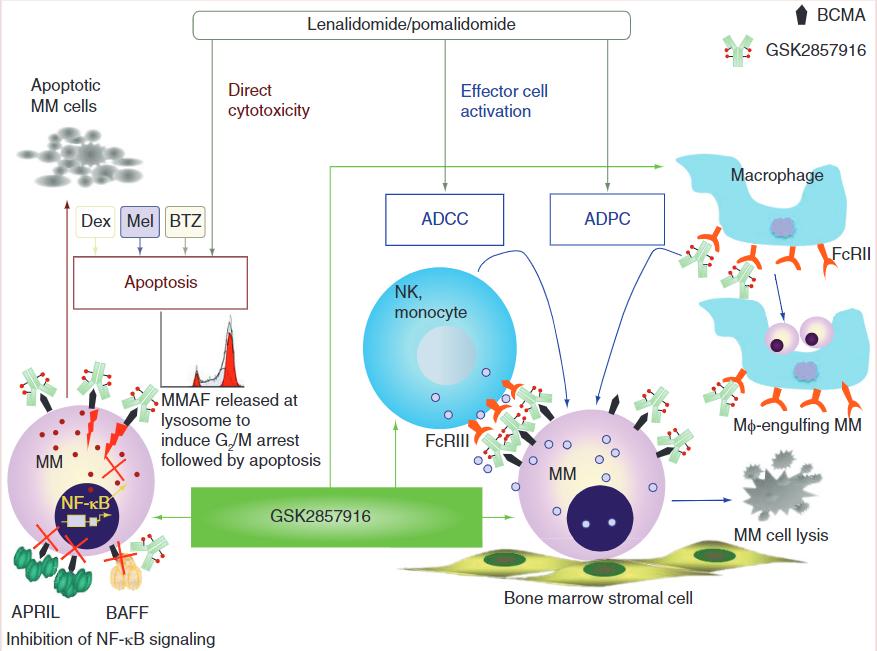
Immunotoxin Induces Strong Anti -MM Effects

Managing Belantamab Mafodotin Toxicity
Keratopathy
• Conduct ophthalmic exams at baseline, prior to each dose, and promptly if symptoms (dry eye, blurred vision) occur
• Counsel patients to use preservative-free lubricant eye drops and avoid contact lenses unless directed by an ophthalmologist
– However, prophylactic steroid eye drops DO NOT prevent or reduce risk of keratopathy
• Hold bela maf until improvement; resume or permanently discontinue based on severity
Grade Description Dose Modifications
1 Exam findings: mild superficial keratopathy
Change in BCVA: decline of 1 line on Snellen Visual Acuity

Exam findings: moderate superficial keratopathy
Continue at current dose
2
Change in BCVA: decline of 2-3 lines on Snellen Visual Acuity, not worse than 20/200
Exam findings: severe superficial keratopathy
Hold until improvement in exam and BCVA to grade 1, then resume at current dose
3
Dose Modifications
(see package insert for detailed instructions)
Starting Dose: 2.5 mg/kg IV every 3 wk
Dose reduction: 1.9 mg/kg IV every 3 wk
Discontinue if unable to tolerate 1.9 mg/kg dosing
Belantamab mafodotin PI.
4
Change in BCVA: decline of > 3 lines on Snellen Visual Acuity, not worse than 20/200
Exam findings: corneal epithelial defect
Change in BCVA: Snellen Visual Acuity worse than 20/200
Belantamab mafodotin is only available through REMS program
Hold until improvement in exam and BCVA to grade 1, then resume at reduced dose
Consider permanent discontinuation
If continuing, follow grade 3
recommendations
Slide credit: clinicaloptions.com
Modakafusp Alfa (TAK-573)
• A novel, first-in-class immunocytokine that delivers attenuated IFN to immune and tumor cells in highly targeted manner
• IFN is a potent cytokine with immunomodulatory, antiproliferative, and cytotoxic properties
• IFN is active in patients with MM, melanoma, and various lymphomas and leukemias
• Innovative attenuation and targeting is designed to improve efficacy and tolerability
• 1.5 mg/kg modakafusp alfa Q4W (N = 29)
– Median prior lines of therapy: 7 (range 3-16)
– 26 patients (89.7%) refractory to anti-CD38 mAb; 25 (86.2%) triple class refractory (to PI, IMID, and anti-CD38 mAb)
• Grade ≥3 TEAEs reported in 18 patients (75%)
– Grade 3-4 TEAEs: neutropenia in 20 patients (62%; grade 4 in 9 patients [31%]), leukopenia in 12 (42%), anemia in 9 (31%), and thrombocytopenia in 13 (45%; grade 4 in 5 [17%])
• ORR 38%; mPFS 5.7 mo
– CR n = 2; VGPR n = 6; PR, n = 3

– mTTR: 2 mo; mDoR: NR
– ORR 38% among 26 anti-CD38 mAb-refractory patients
–
ORR 75% (CR, n = 1; VGPR, n = 2) for 4 patients who received an anti-CD38 mAb as most recent line of therapy

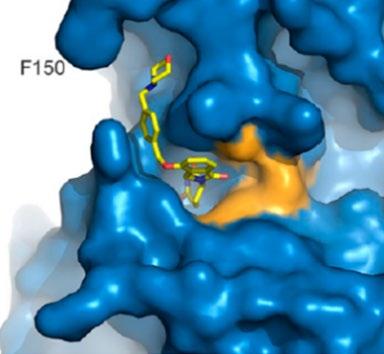

Iberdomide + Dexamethasone
Dose Expansion: Study Design
Open-label phase Ib/IIa trial
Adults with R/R MM; ≥3 prior therapies, including LEN, POM, PI, glucocorticoid, and anti-CD38 mAb; documented disease progression within 60 days of last therapy; refractory to an IMiD, PI, glucocorticoid, and anti-CD38 mAb
(N = 107)
Phase II (at RP2D)
Cohort D
Iberdomide* 1.6 mg + Dexamethasone†
Endpoints
• Primary: efficacy (ORR)
• Secondary: safety, additional efficacy (DoR, PFS, OS)
Adults with R/R MM; ≥3 prior therapies, including BCMA-targeted therapy, LEN, POM, PI, glucocorticoid, and anti-CD38 mAb; documented disease progression within 60 days of last therapy (or PD if CAR T-cell therapy was last therapy)
(N = 26)
Cohort I (post BCMA)

Iberdomide* 1.6 mg + Dexamethasone†
Primary: preliminary efficacy and safety
*Iberdomide (oral): Days 1-21 of each 28-day cycle.
†Dexamethasone (oral): 40 mg (20 mg if >75 yr) on Days 1, 8, 15, and 22 of each 28-day cycle.
Slide credit: clinicaloptions.com
Dose Expansion: Response
*PR or better. †2 patients in SD and MR discontinued tx due to death caused by COVID-19. ‡Includes all treated patients with post-BL efficacy assessment or patients who discontinued tx before any postbaseline efficacy assessment; 2 patients in cohort 1 with no post-BL efficacy assessments so were excluded from analysis.

CC-220-MM-001 Iberdomide + Dexamethasone

Dose Expansion: Overall Survival
• Among responders: – mOS: NR (95% CI: 11.3 mo -NR)
– Estimated event-free rate at 65 wk (14.9 mo): 74.5%
• Among nonresponders – mOS: 8.0 mo (95% CI: 6.6-10.7)
– Estimated event-free rate at 65 wk (14.9 mo): 28.6%
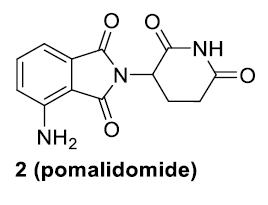
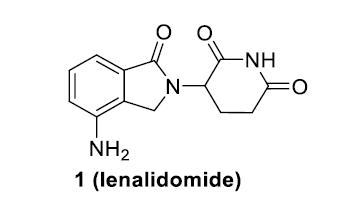
CC-92480
Is a Novel CELMoD Agent Specifically

Designed for Rapid Protein Degradation1,2

First-in-Human
Phase I Trial: CC-92480 + Dexamethasone in Heavily Pretreated R/R MM

characteristics
–
–
–
Median age 66 yr
Time since diagnosis: 7.2 yr
ISS stage ≥II (67.1%); EMP (36.8%)
• Prior therapies
–
–
–
–
–
Median prior therapies: 6
Lenalidomide refractory: 73.7%
Pomalidomide refractory: 78.9%
PI refractory: 73.7%
Anti-CD38 mAb refractory: 69.7%
• 50% of patients were triple-class refractory
• Primary endpoint: PK, safety, MTD/RP2D
Heavily Pretreated R/R MM: Best Response
• 7 of 11 patients at RP2D of 1 mg QD 21/28 days were triple-class refractory (to ≥1 IMiD, 1 PI, and 1 anti-CD38 mAb)
– Of these patients, 1 had CR, 1 VGPR, 2 PR, and 1 MR

Phase I/II CC-92480-MM-002: Response With CC-92480 + Bortezomib + Dexamethasone

• Median time to response: 1.17 mo (range: 0.7-4.9)
• Median DoR: 10.4 mo (95% CI: 9.5-NR)

Supportive Care
• Proteasome inhibitors/anti-CD38 antibodies: Shingles prophylaxis
• IMiDs: blood clot prophylaxis
• Corticosteroids: consider GI prophylaxis
• Bone disease: zoledronic acid or denosumab (preferred if renal dysfunction)
*pain control / interventions such as vertebroplasty / kyphoplasty
• Infection prophylaxis:
• Vaccines (pneumococcal, yearly influenza, shingles)
• Antibiotics are actively being studied
• IVIG if recurrent life-threatening infections or IgG<400
• Social Work
• Palliative Care
• Physical Therapy & Rehabilitative Medicine
Current Understanding and Future Directions
• Lots of options available
• No sequence is necessarily the right fit
• Make sure targeted options are considered
• All late stages options will move into earlier lines of therapy





Q&A

Immune Therapy Options
 Ajai Chari, MD
Ajai Chari, MD
University of California, San Francisco
Immune Therapy Options
Ajai Chari, MDProfessor of Clinical Medicine
Director of Multiple Myeloma Program
University of California, San Francisco
Progression Free Survival (PFS) and Duration of Response
(DOR) Delta of Recently Approved Therapies in RRMMa
In
Phase 1 studies of RRMM patients with a median of ~6 lines of therapy, T Cell Engagers are yielding Overall Response Rates
~60-100% with median PFS of 9+ months and DORs even longer?
aThis is not a head-to-head comparison and cross-trial comparisons should not be interfered from these data Data represent two populations, PFS includes all patients, DOR includes responding patients only

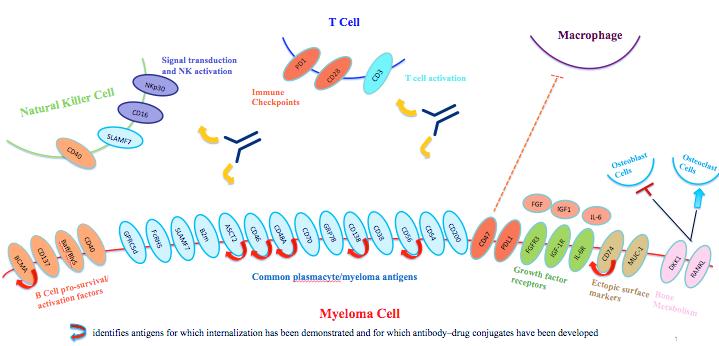
T/NK Cell Engager Targets in Multiple Myeloma



Complex Immunotherapies For Multiple Myeloma
• Bispecifcs – BCMA and non BCMA monotherapy + combinations

• CART – phase 1 dose escalation studies and randomized phase 3 studies
• BCMA sequencing
• Logistics of administering T cell redirection therapy
• CART vs bispecfiics
Monoclonal Antibodies Would Not be Possible Without Myeloma Cells – 1984 Nobel Prize!
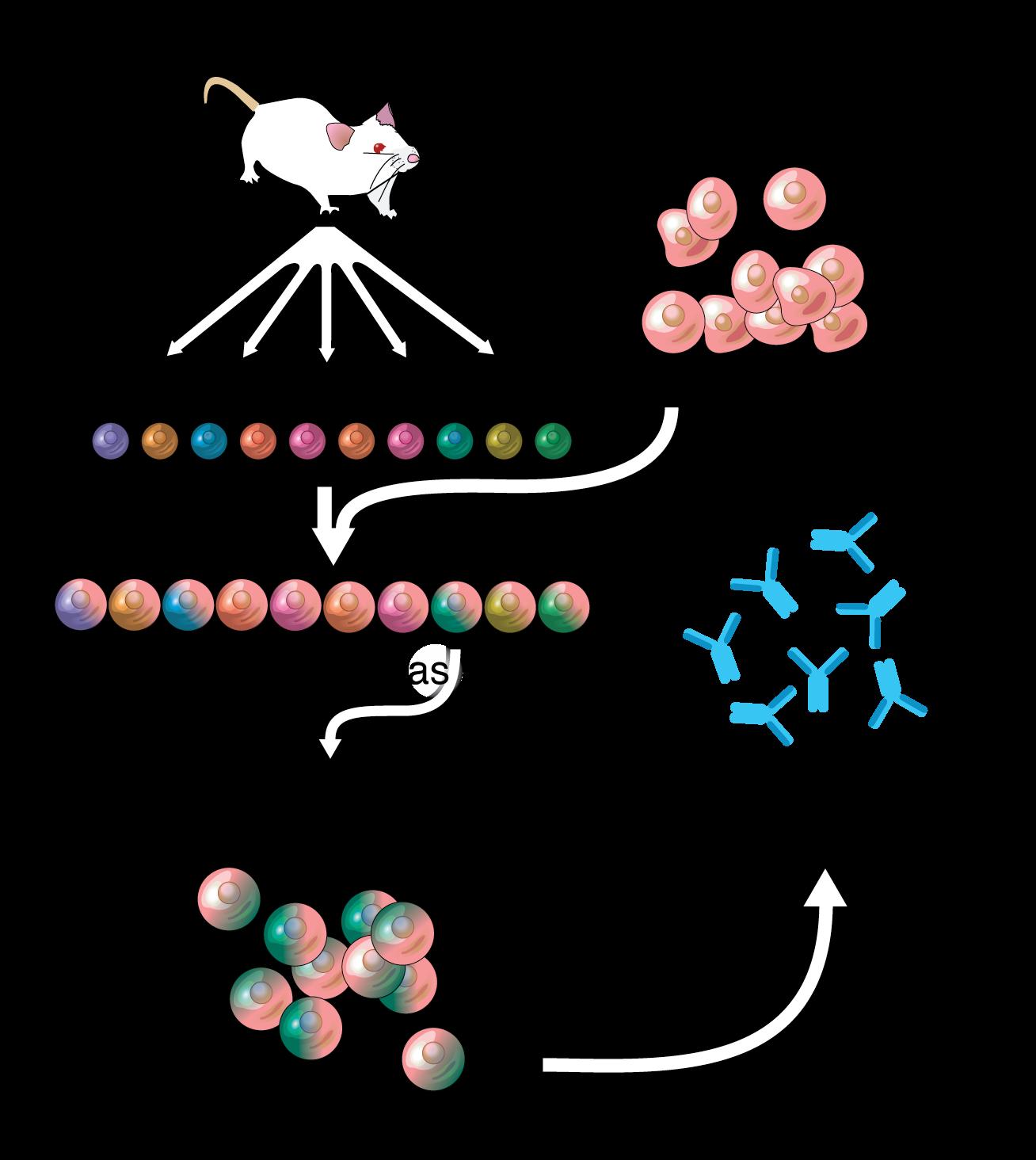
Gelboin, H et al. Trends in Pharmacological Sciences, 1999.
Wikipedia Adenosine
• ‘84 Georges Köhler, César Milstein, Niels Kaj Jerne shared Nobel Prize in Physiology or Medicine
• ’88 Greg Winter humanized mAbs eliminating the reactions that many monoclonal antibodies (mAbs) caused
• First mAb for MM not approved until 2015 - 31 years later!
• Currentlly approved antibodies for MM:
• Naked antibodies: daratumumab, isatuximab, elotuzumab
• Antibody drug conjugates: belantamb mafadotin

T/NK cell Engaging Antibody Clinical Trials in Multiple Myeloma
BCMA (B-cell maturation antigen)

• Receptor for BAFF and APRIL
• Expressed on mature B cell subsets, PC’s, and plasmacytoid DC’s
• Maintains plasma cell homeostasis
• BCMA-/- mice have normal B cell #s, impaired PC survival
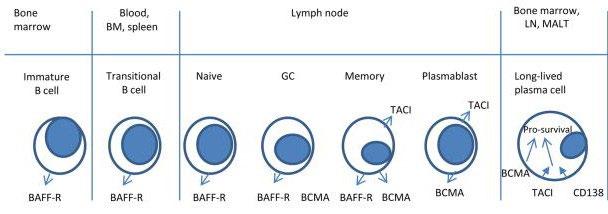
• Multiple agents in development, all demonstrating remarkable activity
• Unprecedented competition is good for the market and ultimately for patients!
Cytokine Release Syndrome

• CRS
• High rates of infectious complications, including severe infections- strategies
• IVIG, antibiotics
• fixed duration of therapy (can always retreat at time of relapse)
• Lower maintenance doses and less frequent schedule
G-protein–coupled Receptor Class 5 member D (GPRC5D) Expression

• G-protein–coupled receptor class 5member D(GPRC5D) is a type-C 7-pass transmembrane receptor protein
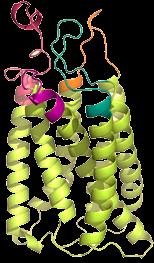
• Orphan receptor - ligand and signaling mechanism unknown
• No known shed peptides or extracellular domain shedding (reduced risk for sink effect)
Predominantly expressed in cells with a plasma-cell phenotype, including the majority of malignant plasma cells from patients with MM

• Also expressed in
• bone marrow,
• heavily keratinized structures (skin, nails)
• salivary gland
Fc receptor-homolog 5 (FcRH5) Protein and mRNA expression
• Surface protein in immunoglobulin superfamily, closely related to Fc receptors
• Ligand(s) for FcRH5 are unknown, but implicated in proliferation and isotype expression in the development of antigen-primed B cells
• FcRH5 protein and mRNA over-expressed in malignant plasma cells
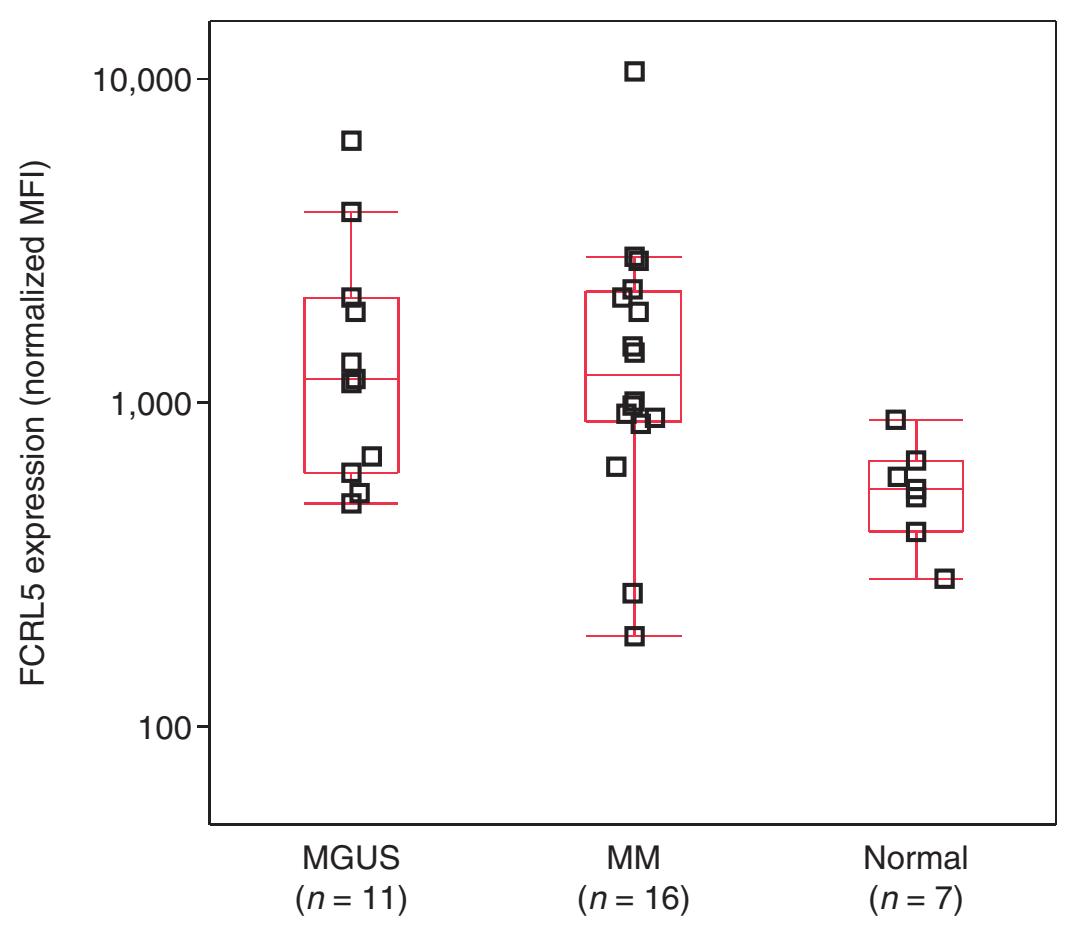

• Expressed on 100% of myeloma cells; expression increased in gain(1q)
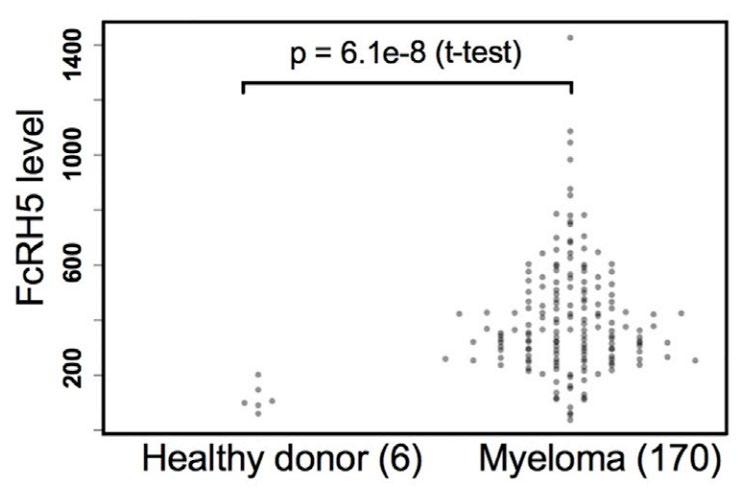
• FcRH5 protein expression by flow cytometry
FcRH5 protein expression by flow cytometry
FcRH5 mRNA expression in CD138+ plasma cells
MGUS, monoclonal gammopathy of undetermined significance
Non-BCMA-Targeted Bispecific Antibodies
• Not only competition within BCMA space but also non BCMA alternatives!
• Also highly active in phase 1 studies
Bispecific Antibody
Non-BCMA-Targeted Bispecific Antibodies
Talquetamab (JNJ-64407564)
Phase 1/2 MonumenTAL-1 Study
GPRC x CD3
Forimtamig (RG6234)
Phase 1
GPRC X CD3 (2:1)
Cevostamab (GO39775)
Phase 1
FcRH5 X CD3
AEs, (All/(Gr 3+)
CRS Infections
Neutropenia Anemia
Dysgeusia 48% (N/A) Skin 56% (0%) Nail 52% (0%)
72% (0.7%) 50% (12%) 28% (22%) 39% (25%) 27% (17%)
• CRS common, but not severe manageable
• ? Role for preventative medication tociluzuamb
• Perhaps less infections/lower of neutrophils
79% (2%) 46% (26%) 18% (16%) 49% (39%) 26% (19%) 12% (4%)
2 (1 due to AEs) NR
Mucosal 77% (5%) Skin 86% (23%)
Hair/nail 28% (0%)
+prophy toci/80% (2%) -> 36% (2.3%) /90%(3.6%)
43% (19%)
18% (16%) -> Gr3+ 64% vs 39%
32% (22%)
% not reported
6 (3.7%)
Diarrhea 26% (1%)
• This makes GPRC5d antibodies more combinable – with dara and also with tec, PFS 18-19 months!
• Other new target related toxicities with GPRC5d – taste, skin, nails
Enduring Responses After 1-Year of Fixed-Duration Cevostamab
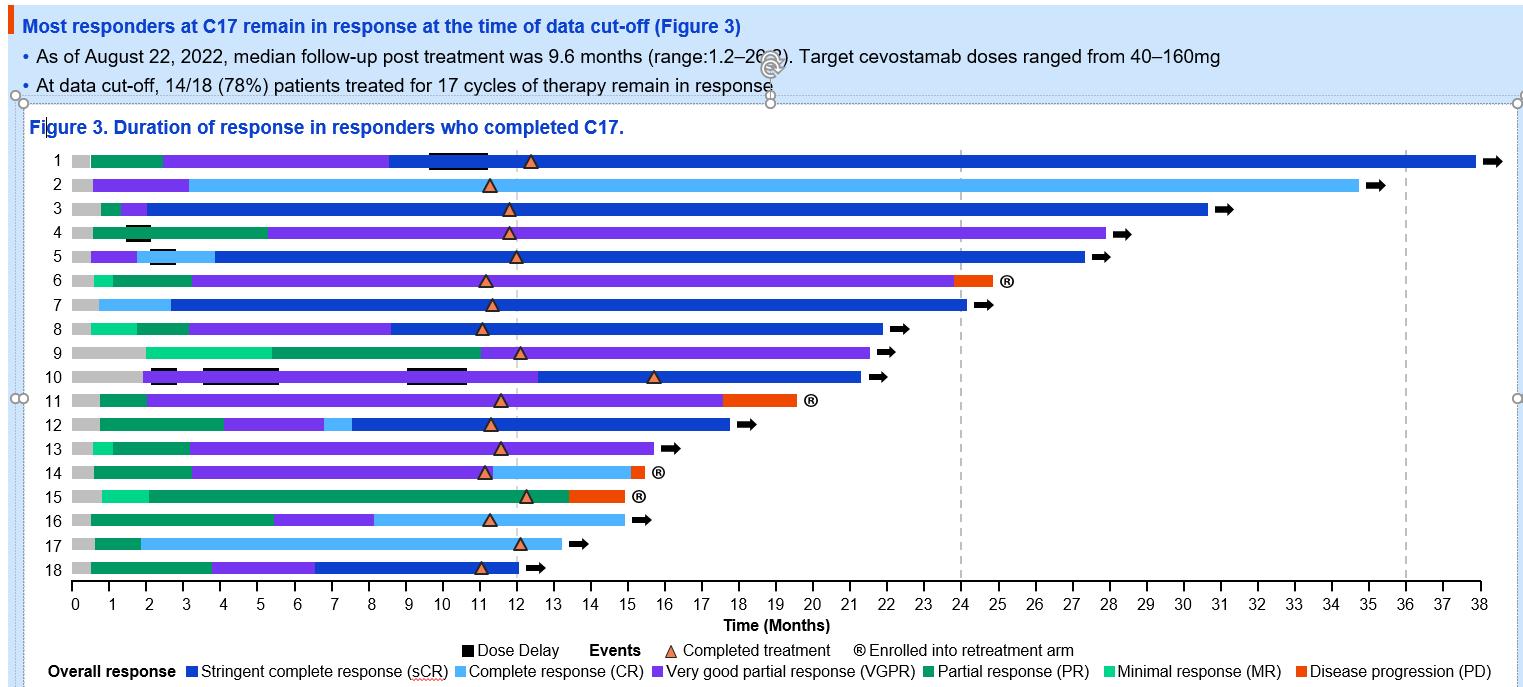
Therapy in Patients with Relapsed/Refractory Multiple Myeloma: Early Experience from a Phase I Study

Determinants of the use of bispecifics
Safety- monotherapy
CRS
Off tumor (? On target) – incidence, time dependency (cumulative?), dose relationship
Infection especially COVID complications and deaths
Dysgeusia
Skin/Nail changes
Neuropathy
Safety in combination with mAbs, IMIDs, PIs, novel agents
Convenience
Inpatient vs outpatient
Cost
Reimbursement for drug (J code), inpatient hospitalization, toclluzumab (off label for bispecific), IVIg
Ability to give in community
Need for & number of priming doses
Route of administration – SQ > IV
Frequency of administration – q mo
Fixed duration vs Treatment to progression
Targets and Sequencing within bispecifics and relative to other agents – CARTs etc.
Time to market BCMA > GPRC5d
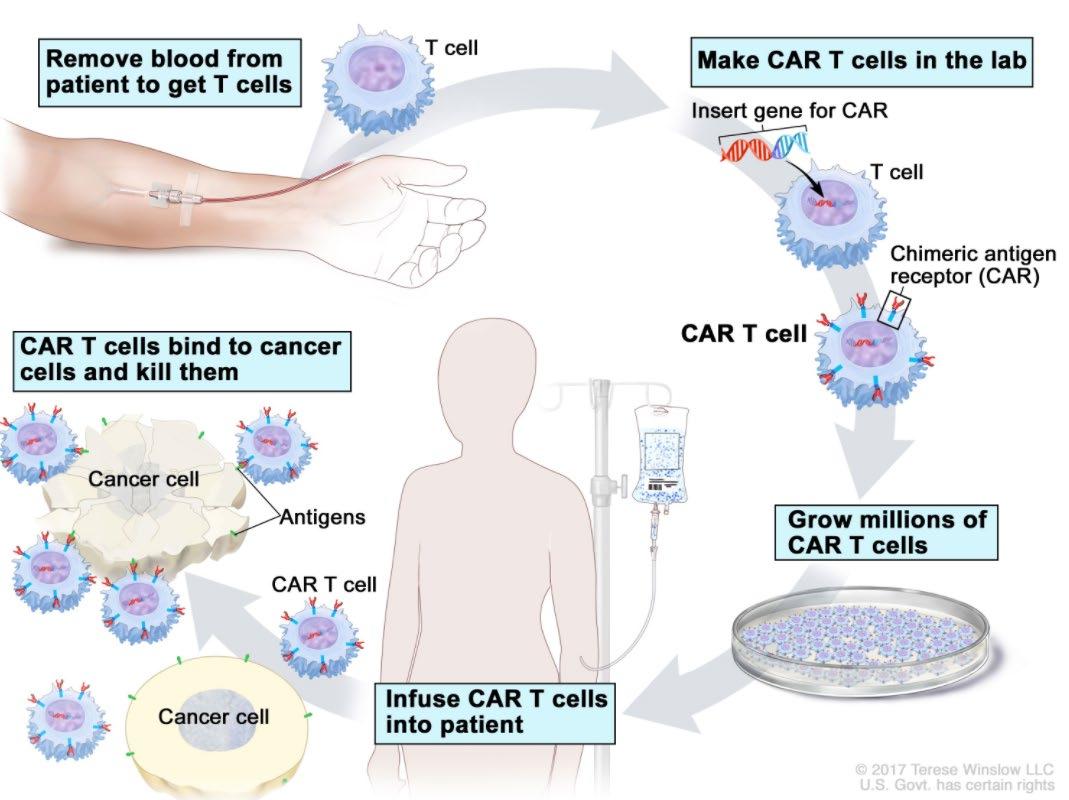
CAR-T cell therapy: What is it and how does it work? (2/3)
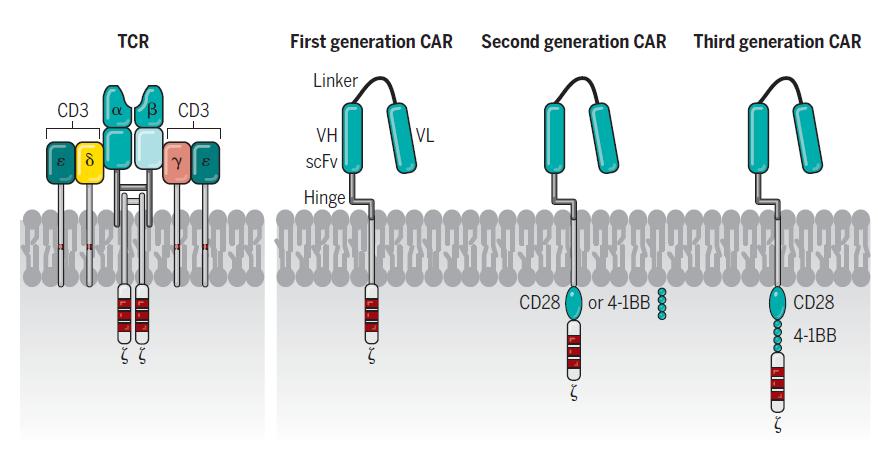
Structure of CAR domains
• Tumor binding domain
• costimulatory endodomains
• CD3 zeta
CAR-T cell therapy: What is it and how does it work? (2/3)
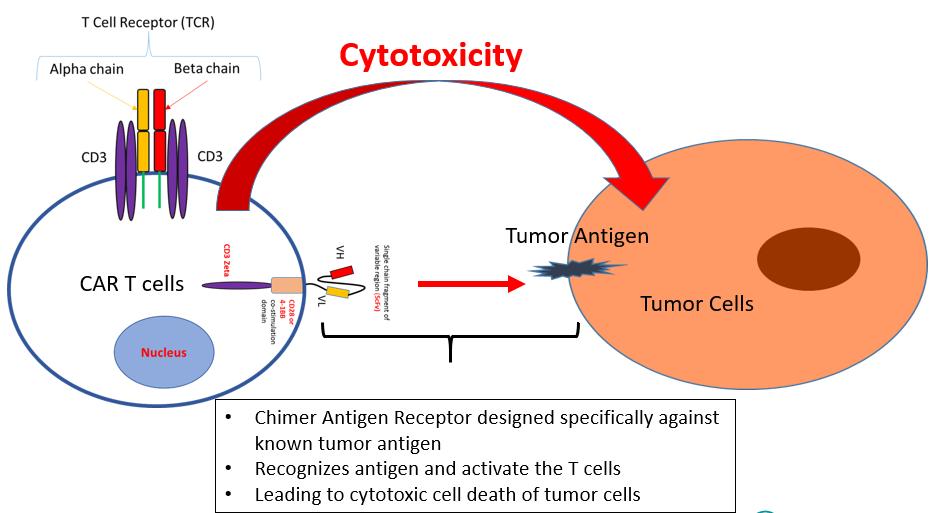
Approved CAR-T cells
Ide-cel KarMMa1 (n = 128)
Cilta-cel CARTITUDE-1 (n = 97) 2,3
Phase II Ib/II

Target BCMA BCMA
scFv Chimeric mouse Chimeric Llama
Age, (range) 61 (33-78) 61 (56-68)
# of lines 6 6
CAR-T cells in myeloma
*There, are no head-to-head comparisons of these data and naïve comparison should be conducted with caution BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor; EMD, extramedullary disease; HR cytog, high-risk cytogenetics; NA, not available; NR, not reached/not reported; ScFv, single-chain variable fragment; TCR, T-cell receptor; triple-R, triple-class refractory
CAR-T cells in myeloma
*There, are no head-to-head comparisons of these data and naïve comparison should be conducted with caution BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor; EMD, extramedullary disease; HR cytog, high-risk cytogenetics; NA, not available; NR, not reached/not reported; ScFv, single-chain variable fragment; TCR, T-cell receptor; triple-R, triple-class refractory

Factors to Consider in BCMA Sequencing
• BCMA therapy prior to even ciltacel – PFS is markedly reduced… so sequencing considerations include: mechanism of action, duration of prior BCMA therapy, time since prior BCMA therapy to apheresis/infusion
• Loss of BCMA genes

• BCMA expression (less likely) - ? Soluble > tissue
• Immune T cell fitness (PD-1, TIM3, CD38) and disease burden
• Availability of non BCMA directed therapeutics eg. GPRC5d, FCRh5
Pomalidomide/CART/Carfilzomib Randomized Phase 3 Studies
CART Side Effects
Bonifant et al. Molecular therapy oncolytics. 2016.

CARTITUDE-4
Cilta-cel vs Standard of Care
Grade 3+ AEs: 97% vs 94%
infections 27% vs 25%
cytopenias 94% vs 86%
CRS: 76% (1% grade 3; no grade 4/5)
ICANS: 5% (all grade 1/2)
movement and neurocognitive TEAE n=1
Considerations in Administration of T cell Redirection Therapies
• CRS/ICANS recognition – REMS Program for all involved parties
• Floor team - Vital sign frequency
• Front line provider- recognition and prompt order entry – consider order sets in EMR (see example on next slide)for
• Vitals – to avoid delays in recognition of hypotension/hypoxia requiring interventions
• Labs – inflammatory markers, coags, cytokines
• Therapeutics - toci, dex, anakinra, siltuximab
• Timely drug mixing/administration – pharmacy and floor nurse to coordinate
• Notification/training of consultants: neurology, neuroradiology, ICU, infectious disease, ER, outpatient oncology
CAR-T vs Bispecifics In Multiple Myeloma
• A hypothetical randomized study without bridging chemo
• a CART with a median PFS of < 11-12 mos unlikely to beat an off the shelf bispecific especially with explosive bulky disease
• Access and Choice Matter – many more patients have been dosed with bispecifics in clinical trials
• including age > 80, including more than several hundreds non-BCMA

•
• What if a CART has median PFS > 2 years?
• PFS, OS, QOL unsurpassed
• very limited data for TCE in CKD, EF< 40%, in elderly & frail patients, hx of CNS disorders
• Auto SCT widely available and cost-effective yet only 12% of MM in US receive it at accredited SCT centers- so until CART availability markedly increases and cost decreases, what % MM patients globally will benefit from CART?
CAR-T vs Bispecifics In Multiple Myeloma




 BCMA
GPRC5d
FCRh5
BCMA
Rolls Royce Phantom –rare , hard to get
Toyota Corolla – nearly 50 million sold globally
BCMA
GPRC5d
FCRh5
BCMA
Rolls Royce Phantom –rare , hard to get
Toyota Corolla – nearly 50 million sold globally
Conclusions: Treatment Selection for Penta-Refractory Multiple Myeloma
GOC/Pt preferences
Previous Rx w/therapies approved for TCR MM
Adequate organ function (CBC, Cr Cl, EF)
Teclistamab
Low ANC/plts due to MM & fit
Yes
Salvage Auto SCT (stem cells remaining)
96h infusional chemo
Rapid Progression
Near academic center
Bispecific study
Cel mod
Modakafusp alpha
Avoid anti-BCMA prior to BCMA CART if possible
Indolent Progression
Able to tolerate CRS/Neurotox
Far from academic center
CART study
Seli based triplet : (if cytopenia CD38/PI; all po - IMID)
Ide-Cel
Cilta-Cel











Q&A

Seminar Summary

Thank you to our Sponsors!














Breakout Sessions
Managing Myeloma Symptoms and Side Effects w/ Donna Catamero - Sunset

Care Partners w/ Robin Tuohy - Hollywood
Diagnosis and Treatment w/ Dr. Durie, Dr. Chari, Dr. Raje & Dr. Abonour – Beverly Ballroom