
8 minute read
SeaSolv enables waste-free biorefinery of seaweed
(Photo: WUR)
Seaweeds ('macroalgae') are rich in valuable nutrients for food, nutraceuticals or pharmaceuticals. However, these are not easily extracted. In the SeaSolv project, Wageningen University & Research (WUR), together with international partners, has developed an innovative waste-free method for the multi-product biorefinery of these substances. This will use a new class of solvents: deep eutectic solvents (DES). Seaweed is a promising but underutilised green feedstock in Europe. It is mainly commercially exploited for food and for the production of phycocolloids (thickening agents). But industrial production is complex and inefficient and also uses acid and alkaline conditions, many chemicals and a lot of water and energy. This generates a considerable amount of waste, as other components are destroyed during the extraction of the phycocolloids. Even some of the phycocolloids themselves are degraded. Meanwhile, several new extraction techniques have been developed, for instance using ultrasound, microwaves, enzymes, homogenisation and supercritical extraction. These are still in their infancy and also have many drawbacks: they are expensive, have low yields, affect the final product and are difficult to scale up.
Advertisement
Green solvents
In SeaSolv, a more sustainable and cost-effective process will be developed that will allow the seaweed industry to significantly reduce its carbon footprint. The process uses deep eutectic solvents (DES). This new class of sustainable solvents offers many advantages, including low price, low toxicity and often biodegradability. They can be 'customised' and are even switchable during a desired process.
DES therefore represent a potential alternative to conventional organic solvents. They allow various components to be extracted from biomass one by one in a gentle manner, while maintaining the functionality of the end products. The result is a multi-product biorefinery
that generates little or no waste and consumes less energy. The technology will eventually also be usable for the extraction of metabolites from other types of land-based and aquatic biomass, such as plants and microalgae.
Text WUR> SeaSolv
The SeaSolv research will be developed in Wageningen University's Bioprocess Engineering (BPE) group, led by Dr Antoinette Kazbar. Partners in the project also involve industrial companies that are going to put the new techniques into practice: Hortimare from Heerhugowaard (Netherlands), Kelpblue from Zeist (Netherlands), Algaia from Saint-Lô (France) and the University of Aveira (Portugal). The research will been funded by the Open Technology Programme of the Netherlands Organisation for Scientific Research, NWO.
Making nanodiamonds out of bottle plastic
Extreme conditions inside ice giants such as Uranus and Neptune can result in peculiar chemistry and structural transitions, like the precipitation of diamonds or superionic water. To find out what happens inside such planets, an international team headed by the Helmholtz-Zentrum Dresden-Rossendorf (HZDR), the University of Rostock and France’s École Polytechnique conducted a novel experiment. They mimicked the extreme conditions by firing a laser at a thin film of simple PET plastic and investigated what happened using intensive laser flashes. The strong flashes that hit the foil-like material sample briefly heated it up to 6000 °C and thus generated a shock wave that compressed the matter to millions of times the atmospheric pressure for a few nanoseconds. The scientists were able to determine that tiny diamonds, so-called nanodiamonds, formed under the extreme pressure. One result was that the researchers were able to confirm their earlier thesis that it really does rain diamonds inside the ice giants at the periphery of our solar system. In addition to this rather fundamental knowledge, the new experiment also opens up perspectives for a technical application: the tailored production of nanometer-sized diamonds, which are already included in abrasives and polishing agents. In the future, they are supposed to be used as highly-sensitive quantum sensors, medical contrast agents and efficient reaction accelerators, for splitting CO2 for example. The group has presented its findings in the journal Science Advances (DOI: 10.1126/sciadv.abo0617).
More at HZDR>
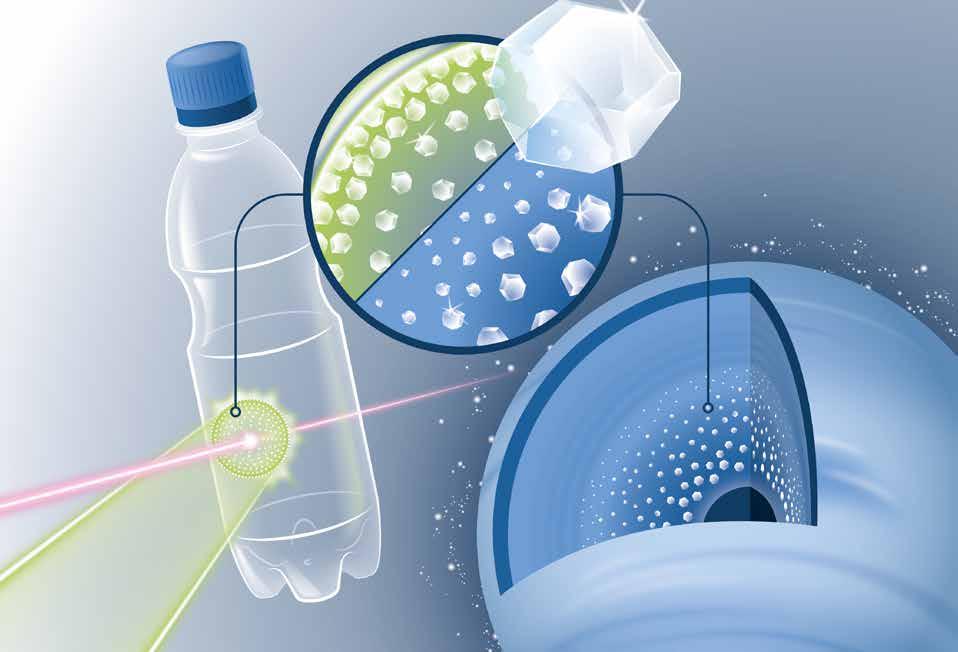
(Illustration: Blaurock / HZDR)

Micro nonwoven interlocks for enhanced wind power
During WindEnergy 2022 - trade fair for the international wind energy industry, last September in Hamburg, the German concern Freudenberg introduced its new Freudenberg Friction Inserts (FFIs). The FFI technology is based on a special very thin nonwoven carrier material that is coated on one side with hard particles. This increases the lateral stresses in a connection between components; which reduces the chance of failure. In this way, FFIs increase the power density of, for example, wind turbines. The idea is as follows. Bolted connections are in many applications exposed to lateral forces. If this lateral force reaches a critical value, it might cause the connection to fail because the parts slip, limiting the performance of the connection. According to Freudberg this can be prevented with FFI. The Friction Insert is applied between the two parts and the hard particles penetrate into the surface of both. The combination between the normal force and the increased friction coefficient between the two parts results in a significant higher friction force. This friction force withstands the occurring lateral force, allowing the bolted connection to be secured.
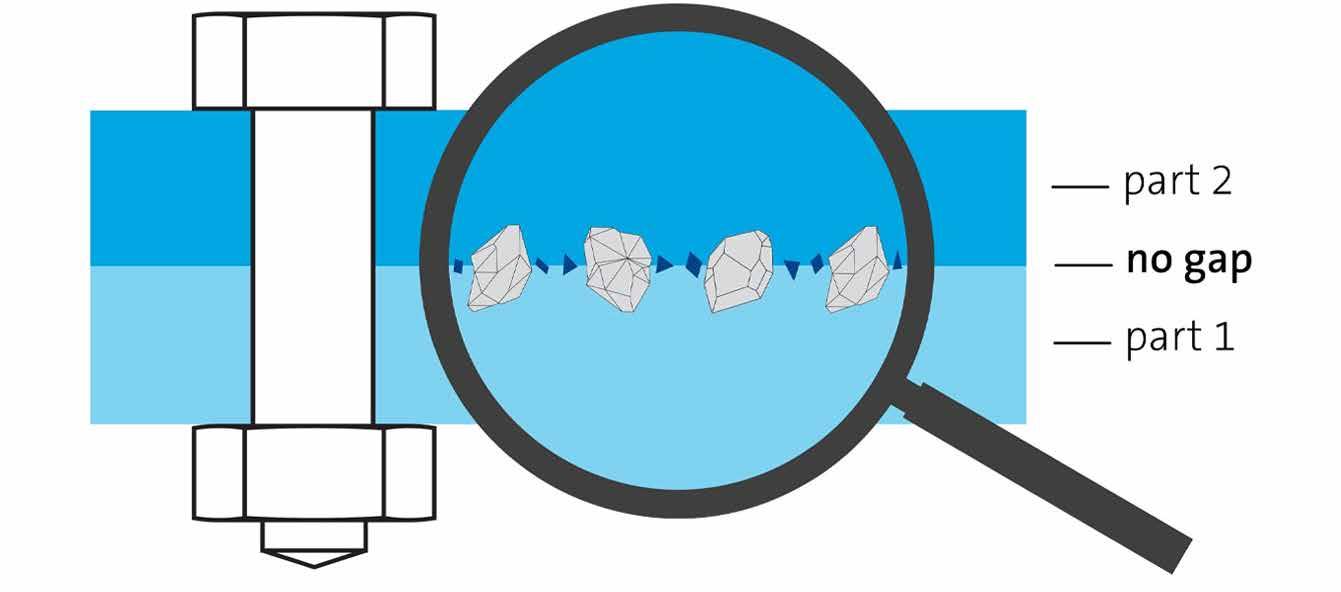
Not only can significantly higher torque and shear forces be transmitted, but noise and vibrations are also prevented.
This increase in performance and subsequent safety of the joints allows for use of thinner and lighter materials as well as new innovative material combinations, like connection of aluminium and plastic materials.
According to Freudenberg, FFIs help to improve the reliability of connections and thus of the entire wind turbine and also eliminate slipping and prevent the fretting of connections. According to Freudenberg FFI can thus significantly increase the performance of wind turbines.
More on Friction-Inserts>
Video
Durable coating kills COVID virus
Developed by a team of University of Michigan engineers and immunologists, there may soon be a new weapon in our centuries-old battle against germs: the first durable coating that can quickly kill bacteria and viruses and keep on killing them for months at a time. It proved deadly to SARS-CoV-2 (the virus that causes COVID-19), E. coli, MRSA and a variety of other pathogens. According to Michigan University the coating killed 99.9 % of microbes even after months of repeated cleaning, abrasion and other punishment on real-world surfaces like keyboards, cell phone screens and chicken-slathered cutting boards. The coating, which is clear and can be brushed or sprayed on, gets its durability and germ-killing power by combining classical ingredients in a new way. It uses antimicrobial molecules derived from tea tree oil and cinnamon oil, both used for centuries as safe and effective germ killers that work in under two minutes. The coating’s durability comes from polyurethane, a tough, varnish-like sealer that’s commonly used on surfaces like floors and furniture. The results of the study’s durability tests suggest that the coating could keep killing germs for six months or longer before its oil begins to evaporate and reduce its disinfectant power. But even
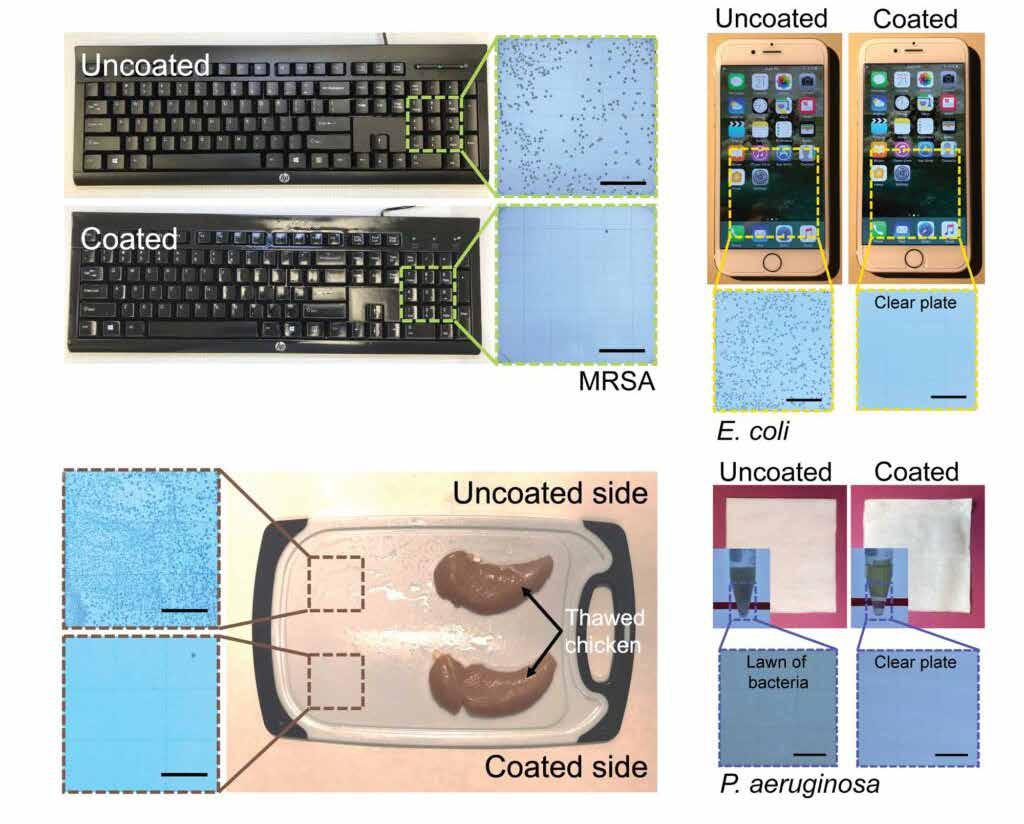
These images show the bacterial load on a coated and uncoated computer keyboard, cell phone and cutting board with raw chicken (Image: Anish Tuteja)
then, it can be recharged by wiping it with fresh oil; the new oil is reabsorbed by the surface, starting the cycle again.
The article ‘Surfaces with instant and persistent antimicrobial efficacy against bacteria and SARS-CoV-2’ was published in Matter last August. It is online>
Ultraconductive aluminum may beat copper
Copper is an excellent conductor and that is why the material is widely used in the world of electricity and electronics. That could be about to change. New research from Pacific Northwest National Laboratory (PNNL) has revealed a method that can significantly increase the conductivity of aluminum, making it economically competitive with copper. According to PNNL, the research may open the way to the development of an ultra conductive aluminium alternative to copper, which could revolutionize vehicles, electronics and the electrical grid.
The thing is, copper's properties make its application inevitable, at least so far. In addition, the demand for copper currently exceeds availability, driving up its prize. This is expected to get worse with the rising number of electric vehicles (EVs), which need twice as much copper as traditional vehicles. Plus, copper is heavy, which drives down EV efficiency. Aluminium is just one-third the price and weight of copper, but it is only about 60 % as conductive. Aluminium’s relatively low conductivity can be a limitation in some real-world applications. So increasing aluminium’s conductivity would be a game-changer. PNNL-team's first step was to find out how far the conductivity of aluminium could be increased by the influence of temperature and structural defects. Next, the researchers wanted to develop an atom-by-atom recipe to increase conductivity. The researchers looked to semiconductors for inspiration because previous research had successfully simulated conductivity in these silicon-based materials and some metal oxides. The team adapted these concepts to work with aluminium and simulated what would happen to the metal’s conductivity if individual atoms in its structure were removed or rearranged. These tiny changes added up to big gains in total conductivity. Now that the recipe for changing the conductivity of metal is theoretically clear, the team want to apply the concept in practice on a laboratory scale. They are also exploring the possibility of increasing the conductivity of other metals using the same simulations. The research team expects that more conductive aluminium would have far-reaching implications - any application that uses electricity or copper could benefit from the development of affordable, lightweight, ultra-conductive aluminium.
The paper ‘Electrical conduction processes in aluminium: Defects and phonons’ (Kashi N. Subedi, Keerti Kappagantula, Frank Kraft, Aditya Nittala, and David A. Drabold) was published earlier this year in the journal Physical Review (doi. org/10.1103/ PhysRevB.105.104114)







