CHEMISTRY

Chemical Bonding

CHEMICAL BONDING
BUILDING UPON PRIOR KNOWLEDGE
Chemical bonding plays a crucial role in designing and creating advanced materials with specific properties tailored to various applications. For instance, let's consider the development of high-performance composite materials used in aerospace engineering.

In aerospace, engineers seek to create lightweight yet strong materials to improve fuel efficiency and overall performance of aircraft. By understanding chemical bonding, researchers can design and manipulate materials at the molecular level to achieve desired properties.
One example is the development of carbon fiber-reinforced composites. These materials consist of carbon fibers embedded in a matrix of epoxy resin. The strong covalent bonds within the carbon fibers provide excellent tensile strength, while the resin matrix, with its weaker intermolecular forces, enhances toughness and flexibility.
The carbon-carbon bonds in the fibers, often arranged in a hexagonal lattice, create a highly stable structure. The arrangement of these bonds, along with the orientation of the fibers, determines the material's strength and stiffness. By controlling these factors, engineers can tailor the composite's properties to meet specific requirements for different aerospace components.
The following concepts are important for understanding the fundamentals of chemical bonding and should be reviewed first
1. Electronic configuration of elements : specially Non-metals
2. Orbital shapes : with wave Function diagram
3. Knowledge of EN
4. Lone pairs
5. Bond length
6. Valency Expansion.
7. Periodicity of atomic radius.
• Chemical Bond
• Ionic Bond
• Polar and Non Polar Covalent Bond
• VSEPR
• Valence Bond Theory
• Hybridisation
• Molecular Orbital Theory (MOT)
• Hydrogen Bond
KEY-TERMINOLOGY
1. Chemical Bonding: The process by which atoms combine to form molecules or compounds through the sharing, gaining, or losing of electrons.
2. Covalent Bond: A type of chemical bond formed by the sharing of electrons between atoms, resulting in the formation of molecules.
3. Ionic Bond: A type of chemical bond formed between ions with opposite charges, where electrons are transferred from one atom to another.
4. Polar Covalent Bond: A covalent bond in which the electrons are not shared equally between atoms, leading to partial positive and negative charges on the atoms.
5. NonpolarCovalentBond: Acovalentbondin whichtheelectronsareshared equallybetween atoms, resulting in no significant charge separation.
6. Valence Electrons: Electrons in the outermost energy level of an atom that are involved in chemical bonding.
7. Lewis Structure: A diagram that shows the arrangement of atoms and valence electrons in a molecule or ion.
8. Octet Rule: The concept that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight electrons in their outermost energy level (except for hydrogen and helium).
9. Hybridization: The mixing of atomic orbitals to form new hybrid orbitals that participate in bonding, often seen in molecules with double or triple bonds.
10. Metallic Bond: A type of bond formed between metal atoms, where their outer electrons are delocalized and can move freely throughout the material.
11. Dipole-Dipole Interaction: A type of intermolecular force between polar molecules, where the positive end of one molecule attracts the negative end of another.
12. Hydrogen Bond: A strong type of dipole-dipole interaction involving a hydrogen atom bonded to a highly electronegative atom (usually nitrogen, oxygen, or fluorine) and another electronegative atom.
13. Van der Waals Forces: Weak intermolecular forces that include London dispersion forces (temporary fluctuations in electron distribution) and dipole-induced dipole interactions.
14. Electronegativity: A measure of an atom's ability to attract electrons in a chemical bond.
15. MolecularGeometry: Thethree-dimensional arrangement ofatoms in amolecule,influenced by the type of bonding and lone pairs of electrons.
1. CHEMICAL BONDING
➢ The force of attraction that holds oppositely charged ions or atoms together in chemical substances is called a chemical bond
➢ Valence Electrons: Electrons present in outermost shell of an atom or ion.
➢ Octet Rule: Atoms combine to complete their octet.
➢ Ionic Bond: Octet is achieved by complete transfer of electrons. It is formed between metals and non metals.
Examples:
Na Na+ + e–[Ne]3s1 [Ne] + e–Cl + e– Cl–[Ne]3s23p5 + e– [Ne] 3s2 3p6 or [Ar].
Na+ + Cl– NaCl or Na+ Cl– joined by electrostatic force of attraction
Na+:Cl–:: : LewisdotstructureofNaCl
Know Yourself
Draw the Lewis structure of the following ionic compounds
➢ Covalent Bond: Formed by sharing of electron pair between atoms. It is formed between non metals
Example:
Key Concept
• Hydrogen tends to achieve duplet?
• Atoms that have less than four electrons in valance shall fall short of octet. Example:
Boronhassextetofelectrons
Steps to write Lewis dot structure of covalent compound
(i) Add valence electron of all atoms to find total no. of electrons.
(ii) For every negative charge add one electron and for every positive charge remove one electron.

Example:
CH3 –has4 + 3+1=8electrons
(1C) (3H) (1negativecharge)
Similarly CH3+ has 4 + 3 –1 = 7 electron
(iii) In general (not always) the least electronegative atom is central atom (atom with maximum covalency) All covalent bonds, are formed by surrounding atoms with the central atom.
Know Yourself
Identify the central atom in each of the following
(iv) Form one covalent bond of central atom with all surrounding atoms
(v) Litilize remaining electron to form multiple bonds or leave in the form of lone pair of electrons
Note: Lone pair is electron pair not involved in bond formation
Example: F
Know Yourself
Draw the Lewis Dot structure of the following covalent compounds [U]
➢ Formal Charge: Difference between number of valence electrons in free state and electrons assigned to atom in Lewis structure.
FC = V – L –1 2 S.
V: Valence electrons in free state
L: Lone pair of electrons.
S: Shared (or Bonding) electrons
Example:
CO. C O CO
V=4 S
F.C. on C = 4 – 2 –1 2(6) = –1
Similarly F.C. on O = 6 – 2–1 2(6) = +1.
Know Yourself
Draw Lewis dot structure along with formula charge on central atom in each of the following: [A]
Key Concept
In oxoacids (oxygen containing acids) H atom is generally (not always) attached to oxygen atom eg HNO3.
Limitations of Octet Rule: If does not explain:
(a) Incomplete octet of central atom:
Examples: BF3, BeH2 etc.
This occurs when valence electrons is less then four.
(b) Odd electron molecules
Examples: No, No2
(c) Expanded octet in 3rd period elements and beyond.
Examples: PF6, SF6, etc.
Summary: (To be reinforced mentally)
Know Yourself
1. Explain the formation of a chemical bond [I]

2. Write Lewis dot symbols for atoms of the following elements : Mg, Na, B, O, N, Br. [I]
3. Write Lewis symbols for the following atoms and ions:
S and S2–; Al and Al3+; H and H–
4. Draw the Lewis structures for the following molecules and ions : [U] H2S, SiCl4, BeF2, 2 3CO , HCOOH
5. Define octet rule. Write its significance and limitations [I]
6. Draw Lewis dot structure of [U]
.................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. ..................................................................................................................................



2. IONIC BOND
➢ From Lewis Theory it follows that formation of ionic band depends upon:
(a) Ease of formation of cation i.e. low value of ionization enthalpy metals of group 1 & 2
(b) Ease of formation of anion : i.e. highly negative value of electron gain enthalpy non metals of group 16 & 17
(c) The arrangement of cations and anions in crystalline lattice (three dimensional array of ions)
Examples: Na(g) Na+(g) + e–: ∆H = 495.8 KJ/mol
Cl(g) +e– :Cl– (g) : ∆H = –348.7 KJ / mol
• The sum of above two energy terms = 147.1 KJ / mol, yet formation of sodium chloride is exothermic.
The energy released when 1 mole of ionic compound is formed in CRYSTALLINE LATTICE in solid state from its constituent ions in gaseous state
Key Concept
• Quantitative measure of stability of ionic compound is provided by its enthalpy of lattice formation and not simply by achievement of octet of electrons around ions involved
• Combination of opposite charges is always exothermic


➢ Lattice Enthalpy: Energy required to separate one mole of crystalline solid into constituent gaseous ions.
NaCl (s) Na + (g) + Cl–(g) ∆H = 788 KJ /mol
➢ Energy released when one mole of ionic solid is formed from constituent ions in gaseous state.
Na+(g) +Cl– (g) NaCl (s) : ∆H = 788 KJ / mol
Key Concept
• Sign convention : Energy released – sign. Energy absorbed + sign
• Experimental observation: smaller the difference in size of cation and anion, greater the value of Lattice enthalpy.
Examples: LiF : ∆ H L.E. = – 616 KJ / mol
LiCl : ∆ H L.E. = – 408.6 KJ / mol
Illustration-2.1
In general, during the formation of an ionic compound the sum of electron gain enthalpy and ionization energy should be negative. Still in many cases the net value is positive. Which of the following comes to rescue this problematic situation?
(a) Attraction between ions
(b) Repulsion between ions
(c) Energy released during the formation of lattice (d) All of the above
Solution: (c)
I.E. of Na = +495.8 KJ mol–1
∆egH of Cl = –348.7
The sum = 495.8 – 348.7 = +147.1 KJ mol–1
Lattice energy = –788 KJ mol–1
Net total energy = –788 + 147.1 = –640.9 KJ mol–1
Illustration-2.2
Which of the following statements is incorrect?
(A) ∆egH is always –ve
(a) (A)
Solution: (a)
∆egH may be +ve or –ve.
(B) I.E. is always +ve
(b) (B)
(c) Both (A) and (B) (d) None of these
Illustration-2.3
Though lattice energy compensates in the formation of electrovalent bond yet this bond is easily formed when one atom has ______ and other has ______.
(a) low I.E., high E.A.
(c) low I.E., low E.A.
Solution: (a)
(b) high I.E., low E.A.
(d) high I.E., high E.A.
Ease of formation of cation is related with low I.E. and the formation of anion is related with high E.A.
Illustration-2.4
Enthalpy of lattice formation of NaCl is –788 KJ mol–1. This shows that:
(a) I.E. of Na is 788 KJ mol–1
(b) E.A. of Cl is 788 KJ mol–1
(c) 788 KJ energy is required to separate Na+ and Cl– ions of one mole of NaCl crystalline form
(d) 788 KJ energy is required for melting 1 mol of NaCl
Solution: (c)
Definition of Lattice Energy
2.1 Bond Parameters
(i) Bond Length : It is defined as the equilibrium distance between the centres of the nuclei of two bonded atoms in a molecule . R
rA rB
R = Bond length
R = rA + rB
Key Concept
Stronger the bond, closer the constituent particles and hence shorter the bond length.


(ii) Bond Enthalpy: It is defined as the energy required to break one mole of bonds of a particular type between two atoms in gaseous state.
Note: larger Bond enthalpy ⇒ stronger bond.
H2 (g) 2H(g) : ∆H = 435.8 KJ /mol.
O2 (g) 2O(g) : ∆H = 498 KJ /mol.
N2 (g) 2N (g) : ∆H = 946 KJ /mol.
Decreasing order of Bond strength : N2 > O2 > H2.
Decreasing order of Bond length (Connect with key concept of Bond length) H2 > O2 > H2
Key Concept
In case of polyatomic molecules, average bond enthalpy is used
Example:
H2O (g) H (g) + OH (g) : ∆H1 = 502 KJ /mol.
OH(g) O (g) + H (g) : ∆H2 = 427 KJ /mol.
Here bond energy changes as chemical environment changes
Average Bond Enthalpy = 502427 2 + = 464.5 KJ /mol.
(iii) Bond order : It represents the number of bonds between two atoms in a molecule.
Example:
H
HB. O. = 1; O = O; B. O. = 2
N N B. O. = 3
Greater the value of Bond order, stronger the bond.
(iv) Bond Angle:It isdefinedastheanglebetweentheorbitalscontainingbondpair ofelectronsaround the central atom
O Lonepair
1045° H Bondpair H
Bondangle
Bond angle helps in determining the shape of a molecule.
Examples: Water is bent or V shaped molecule.
Illustration-2.4
Atomic radii of C and H atoms are 77 pm (for single bond) and 37 pm respectively. The bond length of C–H bond is likely to be:
(a) 114 pm (b) 40 pm
(c) more than 114 pm (d) less than 114 pm
Solution: (d)
C–H bond length is 107 pm less than 114 pm (the sum of 77 pm and 37 pm).
Illustration-2.5
Solution: (d)
Average of energies of two ‘O – H’ bonds is taken.
Illustration-2.6
Which of the following is correct for bond energies of H
?
Bond energy is higher for higher bond order.

3. RESONANCE STRUCTURES
(i) Very often all properties of some molecules cannot be explained on the basis of a single Lewis structure
Key Concept
For all of the above there cases, we may generalize, whenever lone pair of electron is present adjacent to a multiple bond, the phenomenon of resonance operates.
➢
Rule to draw resonating structure : Convert lone pair into bond pair and simultaneously bond pair into lone pair of electron.
Structures Revisited : O
+ (2) (1)
O+ O
(1) : Lone pair into bond pair (2) : Bond pair into lone pair

(1) : Lone pair into bond pair (2) : Bond pair into lone pair
(2) (1)
O=C=O
(1)' (2)'
(1) : Lone pair into bond pair (2) : Bond pair into lone pair
(1)’ : Lone pair into bond pair (2)’ : Bond pair into lone pair
Illustration-3.1
In the structure of O3 the experimental bond length between two O-atoms is:

(a) 148 pm (b) 121 pm (c) 128 pm (d) 160 pm
Solution: (c)
O – O single bond length is 148 pm, O = O bond length is 121 pm but in O3 these bond length are 128 pm for each combination, showing resonance.
Illustration-3.2
For the following structures of ozone which statements (s) is/are correct?
(a) Structure (I) only is resonance hybrid.
(b) Structure (II) only is resonance hybrid.
(c) Structures (I) and (II) both are resonance hybrids.
(d) Structures (III) is resonance hybrid.
Solution: (d)
Structures (I) and (II) are called canonical structures.

4. POLAR AND NON POLAR COVALENT BOND
➢ Whenever a covalent bond is formed between similar atoms (e.g. H2, Cl2, F2, etc.) it is called a non polar covalent bond and the shared pair of electron is equality distributed between the participating nuclei.
➢ However, in case of different atoms, e.g. H-F partial charge arises on constituent atoms due to difference in electronegativities of constitutes atoms.
➢ H δ+ – F δ: The more electronegative fluorine attracts the shared pair of electron towards itself leading to creation of partial negative charge on fluorine and partial positive charge on H. Such a covalent bond is called a polar covalent bond.
➢ A polar covalent bond possesses dipole moment () which is a vector
(i) Whose direction is from positive to negative end.
(ii) Magnitude is equal to product of charge and distance of separation |q|d= i.e. q × d
➢ Diploe: Two equal and opposite charges separated by a small distance constitute a dipole.
➢ Unit of Dipole Moment : Debye
➢ 1 Debye = 3.33 × 10–30 Coulomb - metre.
In case of polyatomic molecules, the dipole moment of molecule is the vector sum of dipole moments of various bond.
➢ Thus in BeF2 : molecule = 0.
Explanation: F Be F (Linear) resultant = 0 BF
Due to bent structure of water, the resultant value of is non zero.
➢ Comparison of NH3 and NF3, : Both pyramidal shape.
LonePair:responsibleforcreatingadipolemomentwhichis directedfromnucleusofnitrogentowardslonepairofelectron.
Lonepairmoment
Resultantofbonddipolemoments
➢ The resultant of bond dipole moments and lone pair moment reinforce each other thereby increasing the overall resultant dipole moment.
Hencethelonepairmomentnullifiesresultantofbonddipolemoment
Thereforeoverallresultantdecreases.
Illustration-4.1
When two different atoms form a bond by sharing of electrons, the shared pair is attracted more by one, e.g., in HF it is fluorine that has higher attraction, the bond is termed as:
(a) ionic bond
(c) polar covalent bond
Solution: (c)
(b) non-polar covalent bond
(d) coordinate bond
Atom of higher attraction (higher electronegativity) acquires partial negative charge and other equal positive charge.
Illustration-4.2
Which of the following is incorrect w.r.t dipole moment?
(a) A small crossed arrow is used to show the dipole moment.
(b) The head of the arrow is on the + ve end.
(c) The direction of the crossed arrow is the same as the conventional direction of the dipole moment vector.
(d) The head of ⇸crossedarrowshowsthedirectionofshiftofelectrondensity.
Solution: (c)
The direction of head of crossed arrow is opposite to the conventional direction of dipole moment vector.
WF.d4J ==−
Illustration-4.3
Which of the following is/are expected to have zero dipole moment?
(I) BeCl2 (II) BCl3 (III) CCl4 (IV) CO2

(a) (I) and (II) (b) (II) and (III) (c) (I), (II) and (IV) (d) All of these Solution: (d)
All have μ = 0
Illustration-4.4
Which of the following is the correct decreasing order of dipole moments of HF, NF3, NH3, H2?
(a) HF > NH3 >NF3 > H2
(c) NH3 > HF > NF3 > H2
Solution: (a)
μ value of HF, NH3, NF3, and H2 are respectively
(b) NH3 > NF3 > HF > H2
(d) H2 > HF > NF3 > NH3
and 0D.
Know Yourself
1. Compare the dipole moment of H2O & F2O
2. Comment whether the following compounds are polar or polar. (assume B is more electronegative than A for each of these)
the plane oftriangle
3. Although both CO2 and H2O are triatomic molecules, the shape of H2O molecule is bent while that of CO2 is linear. Explain this on the basis of dipole moment. [A]
4. Apart from tetrahedral geometry, another possible geometry for CH4 is square planar with the four H atoms at the corners of the square and the C atom at its centre. Explain why CH4 is not square planar? [A]
5. Explain why BeH2 molecule has a zero dipole moment although the Be–H bonds are polar. [U]
6. Which out of NH3 and NF3 has higher dipole moment and why? [U]
4.1 Covalent Character in Ionic Bonds:
➢ All ionic bonds possess certain covalent character which is expressed in terms of FAJAN’S RULES.
➢ The rules help in qualitative (theoretical) comparison of covalent character present in ionic bonds.
➢ In general the positive charge density of cations is high due to the small size of cations. Consequently it has a tendency to attract the electron cloud of anion present nearby.
➢ The tendency of cation to distort the electron cloud of anion is called the polarization power of cation.
➢ Greater the charge and smaller the size of cation, greater is its polarization power.
➢ Similarly the tendency of electron cloud of anion to get distorted is called the polarizability of anion.
➢ Larger the size of anion and greater the magnitude of negative charge on anion, greater is its polarizability.
4.2 Fajans Rule:
With increase in polarization power of cation and polarizability of anion, the covalent character in ionic bond increases.
➢ This can be imagined as reversal of electron towards cation (or as sharing of electrons) which imparts covalent character to the bond.
Key Concept
In general the strength of ionic bond is greater than a covalent bond. Therefore, with increase in covalent character, the bond weakens.
➢ For the same cation covalent character increases on moving down the group due to increase in polarizability of anion.
LiF < LiCL < LiBr < LiI : Covalent character.
➢ For the same anion covalent character decreases on moving down the group due to decrease in polarization power of cation.
BeCO3 > MgCO3 > CaO3 > SrCO3 > BaCO3. : covalent character
Thermal stability follows the order
BeCO3 < MgCO3 < CaO3 < SrCO3 < BaCO3. :
➢ For cations of the same size and charge, the one with electronic configuration (n-1) dn n s°, typical of transition metals (called Pseudo noble gas configuration), is more polarising than the one with a noble gas configuration ns2np6, typical of alkali metal cations.
➢ This is due to poor screening effect of d orbital electrons, which results in higher polarization power of cation.

Example: melting point of cuprous chloride is lower than that of potassium chloride, even though both copper and potassium belong to the same horizontal period.
Illustration-4.5
The covalent nature in ionic bond is greater if:
(a) size of cation is small
(b) size of anion is large
(d) all of the above Solution: (d)
(c) charge on cation an anion, both, is large
Illustration-4.6
Which of the following is correct with respect to covalent character in ionic bond?
(a) For same size and charge of cation, the one with electronic configuration (n–1)dxns0 is more polarising than the cation having ns2 np6 electronic configuration.
(b) Cation polarises the anion.
(c) Electronic charge shifts towards cation to create covalent character.
(d) All of the above Solution: (d)
Know Yourself
1. Explain why lithium carbonate decomposes easily upon heating where as other group 1 carbonates are fairly stable towards heat. [U]
2. Amongst LiCr, RbCr, BeCl2, and MgCl2, the compounds with the greatest & lost ionic character respectively are [A]
(a) Licl and RbCl
(c) Rbcl and MgCl2
(b) RbCl and BeCl2
(d) MgCl2 and BeCl2
3. Arrange the following molecules in increasing order of covalent character. [A] AlCl3 MgCl2 NaCl?

5. VALENCE SHELL ELECTRON PAIR REPULSION THEORY(VSEPR)


➢ According to this theory theelectron pairsin valence shell of the central atom tend to orient themselves as far away from each other as possible
➢ As a consequence of this the electronic repulsion gets minimised and the energy of the system also gets minimised hence the stability gets maximised
➢ Electronic repulsions follow the order lone pair- lone pair repulsion > lone pair- bond pair repulsion > Bond pair- bond pair repulsion
Key Concept
As a result of bond pair bond pair repulsion the value of bond angle increases whereas due to loan pair bond pair repulsion the value of bond angle decreases
➢ The shapes of all standard molecules i.e. molecules in which lone pair of electrons is not present in valence shell of central atom, as per the valence shell electron pair repulsion theory, are as follows (The table on next page is to be thoroughly learnt in order to apply valence shell electron pair repulsion theory for determining the shape of molecules or ions)
Key Concept
In determining the shape of molecules in which loan pair of electrons are present in valence shell of central atom, only the relative arrangement of surrounding atoms with respect to the central atom is to be considered. All loan pairs are placed as per valence shell electron pair repulsion theory and the shape of the molecule is reported by hiding the lone pair of electrons.
➢ Example : For 3 electron pairs the geometry around the central atom will be trigonal planar as per VSEPR.
➢ If the molecule has form AB 2, then the arrangement of electron pairs around central atom will be trigonal planar and the shape will be bent
➢ Now we hide the lone pair of electrons and report, the shape of the molecule as bent.
➢ The chart on the following page represents the shape of the molecules in which lone pair of electron is present in the valance shell on central atom.
Geometry of Molecules in which the Central Atom has No Lone Pair of Electrons
Geometry of Molecules in which the Central Atom has No Lone Pair of Electrons
Shapes of Molecules containing Bond Pair and Lone Pair
4 1
Bent
should have been triangular planar but actually it is found to be bent or v-shaped. The reason being the lone pairbond pair repulsion is muchmoreascomparedto the bond pair-bond pair repulsion. So the angle is reduced to 119.5° from 120°.
Had there been a bp in place of lp the shape would have been tetrahedral but one lone pairispresentanddueto the repulsion between lp-bp (which is more than bp-bp repulsion) the angle between bond pairs is reduced to 107° from 109.5°.
The shape should have been tetrahedral if there were all bp but two lp are present so the shape is distorted tetrahedral or angular. The reason is lp-lp repulsion is more than lp-bp repulsion which is more than bpbp repulsion. Thus, the angle is reduced to 104.5° from 109.5°.
In (a) the lp is present at axial position so there are three lp bp repulsions at 90°. In(b) the lp is in an quatorial position, and there are two lp bp repulsions. Hence, arrangement (b) is more stable. The shape shown in (b) is described as a distorted tetrahedron, a folded square or a see-saw. AB
Tshape
In (a) the lp are at equatorial position so there are less lpbp repulsions as compared to others in which the lp are at axial positions. So structure (a) is most stable. (T-shaped).
In each of the above, how the bond angle changes due to lone pair- bond pair repulsion and also note how the shape of the molecule changes due to presence of lone pair of electrons instead of atom.
➢ Flow chart of VSEPR Theory
Statement order of repulsions shape without lone pair Distortion due to lone pair.
Illustration-5.1
Nyholm and Gillespie (1957) gave a new idea of VSEPR to decide the shape of a molecule, Postulates are summarised below, which one is incorrect?
(a) To decide the shape of a molecule, shared or unshared pairs of electrons are considered at the surface of a sphere around the central atom.
(b) These electron clouds repel one another to go maximum apart.
(c) Multiple bonds are taken equal to single bonds only.
(d) The decreasing order of repulsions is: bp – bp > lp – lp > lp – bp
Solution: (d)
The correct order of repulsion is as per VSEPR theory.
Illustration-5.2
According to VSEPR model what makes the shapes of CH4, NH3 and H2O different?
(a) The presence of different number of H-atoms in the central atom.
(b) Different central atoms.
(c) Different number of lone pairs of electrons on the central atoms which apply different total repulsion.
(d) All of the above
Solution: (c)
In CH4 there is no force of any lone pair. In NH3, one lone pair has the repulsive strong force. In H2O two lone pairs have maximum repulsive force among these cases. In CH4, NH3 and H2O angle at central atom are H – C – H = 109°28’, H – N – H = 107° and H – O – H = 104.5°.
Illustration-5.3
Select the incorrect statement out of the following:
(a) According to VSEPR model one type of molecules are in which the central atom has no lone pair.
(b) According to VSEPR model molecules have one or more lone pairs of electrons on the central atom.
(c) Lone pairs of electrons are localised on the central atom.
(d) VSEPR model cannot determine the shape of molecules where the energy difference between possible structures is very small
Solution: (d)
Correct statement is VSEPR model determine the shape of those molecules also where the energy difference between their structures is very small.
Illustration-5.4
Select the incorrect matching among the following where E stands for lone pairs of electrons:
(a) AB3E – Trigonal pyramidal shape
(c) AB2E2 – Tetrahedral shape
Solution: (c)
AB2E2 is bent shaped.
Illustration-5.5
AB2E – Bent shape
AB4E – See saw shape
Select the incorrect matching. Here E stands for lone pair of electrons.
(a) AB3E2 – T-shape
(c) AB4E2 – Square planar
Solution: (d)
AB5 (e.g., PCl5) is trigonal bipyramidal.
– Square pyramidal
– Square pyramidal
Know Yourself
1. Discuss the shape of the following molecules as per VSEPR theory. [U]
(a) BeCl (b) BCl3 (c) SiCl4 (d) AsF3
(e) H2S (f) PH3.
2. Whatdoyouunderstandbybondpairandloanpairofelectron. Explaineachwithasuitableexample [I]
3. Give one example which has the following geometry on the basis of VSEPR theory [I]
(a) linear (b) trigonal planar (c) tetrahedral
(d) Pyramidal (e) T- shape (f) Square planar
4. Arrange the following set of molecules in decreasing order of bond angle [A]
(a) SF6, H2O, NH3, CH4 (b) NH3, CH4, BF3, BeCl2
(c) BeH2, H2O, AlCl3, H2S
5. BeF2 is linear while SF2 is angular though both are triatomic. Explain [U]
6. Discuss the shape of the following molecules as per VSEPR theory. [A]
(a) BeCl2 (b) BCl3 (c) SiCl4 (d) AsF3
(e) H2S (f) PH3
7 Give one example which has the following geometry on the basis of VSEPR theory [I]
(a) linear (b) trigonal planar (c) tetrahedral
(d) Pyramidal (e) T- shape (f) Square planar

6. VALENCE BOND THEORY
➢ According to this theory, a covalent bond is formed by overlapping of atomic orbitals present in the valance shell of central atom and surrounding atoms.
➢ Whenever a covalent bond is formed, it is always followed by release of energy from the system. At the instance of bond formation the potential energy of the system gets minimised and stability gets maximised.
➢ The adjacent graph captures the essence of formation of covalent bond, with respect to H2 molecule

➢ When the atoms are very far from each other, the potential energy of the system is zero. As the two hydrogen atoms approach each other, the attractive force dominates over the repulsing force for a wide range of separation. At the point of bond formation the potential energy gets minimised Immediately after this point repulsive forces begin to dominate and the energy of the system steeply begins to rise. For the above system of H2 molecule, the essential bond parameters are
Bond length =74pm
Bond dissociation energy =435.8 KJ /mole
➢ Orbital Overlap Concept: According to this concept of covalent bond is formed by overlapping of atomic orbital is present in the valance shell of central atom and surrounding atoms
➢ This overlapping may occur by one of the following two methods
(i) Axial overlap or head on overlap, which is also called sigma bond
(i) Head on overlap or sideways overlap, which is also called pi bond
➢ The first covalent bonds form between two different atoms is always a sigma bond. Further, only one sigma bond is formed between two atoms. All other covalent bonds form between the two atoms already joined by a sigma bond are always pi bonds. Also the sigma bond is stronger of the two types of covalent bonds. Once a sigma bond is formed between two atoms, the orientation of orbitals does not allow head-on overlap. Hence all subsequent bonds are always pi bonds.
➢ Bonding interaction occurs only when overlapping occurs between orbitals that are in phase

➢ In the above diagram, the light and dark shade represents the different phases of wave function Whenever overlapping occurs between wave functions of same shades it results in formation of a covalent bond.
➢ Different aspects of sigma bond formation can be visualised through the following figures.
➢ All of the above are examples of sigma bond formation
Zero overlap occurs whenever same extent of orbital overlapping occurs between same phase and out of phase orbitals or orthogonal overlap that is overlapping in mutually perpendicular direction occurs

Zero overlap
Note: A sigma bond is always symmetric about the internuclear axis and hence rotation about sigma
bond is perfectly free.
➢ Rotation about pi bond on the other hand is restricted as it leads to rupture of pi bond
➢ Concept of ground state and excited state: As per valence bond theory the central atom can participate in bond formation either in ground state or in excited state .The necessary condition for formation of excited state is that vacant orbitals must be present within the same shell
Example:
CH4
C:
C:
:GroundState
:ExictedState
➢ Whether the central atom forms bond in ground state or an excited state is to be decided on the basis of requirement of surrounding atom. In the above example, four hydrogen atoms require four unpaired electrons which can only be possible in excited state of carbon. Also note that carbon can be converted into excited state as it has vacant orbital available in same shell.

➢ Coordinate Bond: A covalent bond formed in such a way so that both the electrons involved in bond formation are donated by the same atom is called a coordinate bond.
➢ Necessary condition: An atom should have a spare lone pair of electron. This is called donor atom or Lewis Base.

➢ Another atom should have a vacant orbital on it. This is called Acceptor atom or Lewis Acid.
Example:
Illustration-6.1
Which of the following is not explained by valence bond theory?
(a) Shape of molecule
(b) Formation and directional properties of bonds in polyatomic species
(c) Overlap and hybridisation of atomic orbitals
(d) Energy of molecule

Solution: (d)
Energy of a molecule cannot be calculated by valence bond theory.
Illustration-6.2
If A and B stand for two different H-atoms, in which of the following cases attraction exists? (N is nucleus, e is electron)
(a) (II) and (III)
(c) (I) and (IV)
Solution: (d)
(b) (V) and (VI)
(d) (II), (III), (V) and (VI)
There exist nucleus electron attractions
Illustration-6.3
Observe the following diagram and select the wrong matching:
(a) Bond energy – 74 units
(c) Both (a) and (b) are wrong
Solution: (c)
(b) Bond length – 435.8 units
(d) Both (a) and (b) are correct
Bond energy: Energy required to break the bond = 435.8 kJ mol–1
Bond length: The minimum distance between nuclei when the energy of the system is minimum = 74 pm.
Illustration-6.4
For the formation of bond, which of the following is correct?
(a) Atomic orbitals should have same charge.
(b) Atomic orbitals should have same phase and same orientation.
(c) Atomic orbitals should just touch each other.
(d) Atomic orbitals should have different orientations.
Solution: (b)
Atomic orbitals have nocharge. Thesign shownisthe direction of phase only. Overlap of orbitals ofsame orientation and same phase to the partial but maximum extent with electrons of opposite spins forms the bond.
Illustration-6.5
Which of the following is not showing the formation of sigma bond?
(a) (b)
(c) (d) Solution: (d)
It is showing π-bond.


Know Yourself
1. Discuss the shape of the following molecules as per VSEPR theory. [U]
1. Draw orbital box diagram for formation of following compounds. Also identify whether bond formation occurs in ground state or in excited state of central atom.
2. Draw diagrams showing the formation of a double bond and a triple bond between carbon atoms in C2H4 and C2H2 molecules.
3. What is the total number of sigma and pi bonds in the following molecules? [U]
C2H2
C2H4
4. Considering x-axis as the internuclear axis which out of the following will not form a sigma bond and why?

7. PREREQUISITES: RULES OF ELECTRONIC CONFIGURATION, VBT, VSEPR
7.1 Hybridisation:
➢
As per valance bond theory,the four bondsin methanemust be non-identical asitinvolves overlapping of s and p orbital of carbon with hydrogen. However, experimentally, it is established that all four bonds in methane, are identical. This, and many other related phenomena, are explained on the basis of concept of hybridisation.
➢ The phenomena of intermixing of atomic orbitals present in the valance shell of central atom, having same or nearly same energy leading to the formation of new set of orbital that are exactly equivalent in terms of energy and shape is called hybridisation
➢ The hybrid orbitals are always same in number as the number of atomic orbital undergoing intermixing
➢ Further, the hybrid orbitals accommodate all sigma bonds and all lone pair of electrons present in the valance shell of central atom, but not pi bonds.
➢ The hybrid orbitals are oriented in space as per the valance shell electron pair repulsion theory.
➢ The hybrid orbitals possess unsymmetrical lobes, a bigger lobe for better overlap and a small lobe which does not participate in overlapping.
The Complete Table of hybridization and Geometry

➢ Number of Electron Domains (or "Number of electron pairs") =( Number of Other Atoms central atom is bonded To) +( Number of Lone Pairs in valence shell of central atom)
➢ Hybrid orbitals are used to form σ bonds and to hold lone pairs of electrons.
➢ Unhybridized p orbitals are used to form π bonds.
Key Concept
"Polar" refers to a molecule in which all terminal atoms are the same. In general, if terminal atoms are different, the molecule will be polar.
Shortcut for Hybridisation
• Count the number of atoms connected to it (atoms - not bonds!)
• Count the number of lone pairs attached to it.
• Add these two numbers together.
• If it's 4, your atom is sp3
• If it's 3, your atom is sp2
• If it's 2, your atom is sp. (If it's 1, it's probably hydrogen!)

Types of Hybridisation
➢ Sp Hybridisation: sp Hybridization is where when one s orbital and one p orbital intermix to form an sp hybridized orbital. Some key features are:

• The molecules formed are linear and have a bond angle of 180°
• sp hybridized orbital has an equal distribution of s and p orbital characteristics (50% – 50%).
• It is also called diagonal hybridization.
Examples of sp hybridization
• Beryllium compounds such as BeCl2 BeF2
• Carbon compounds containing triple bonds.
➢ sp2 Hybridization: sp2 Hybridization is where when one s orbital and two p orbitals intermix to form an sp2 hybridized orbital. Some key features are:

• The molecules formed are triangular planar in shape and thus have a bond angle of 120°
• sp2 hybridized orbital has 33% of s orbital characteristics and 66% of p orbital characteristics.
• It is also called trigonal hybridization.
Examples of sp2 Hybridization
• Boron compounds like BF3 and BH3
• All the carbon-containing compounds that have a carbon-carbon double bond such as Ethylene (C2H4)
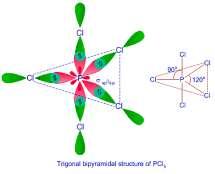
➢ sp3 d Hybridization: sp3 d Hybridization is where when one s orbital, three p orbitals and one d orbital intermix to form 5sp3 d hybridized orbitals. Some key features are:
• The molecules formed are trigonal bipyramidal in fashion and thus have a bond angle of 120° in the horizontal plane and 90° in the vertical plane.

• It is also called trigonal bipyramidal hybridization.
Examples of sp3d hybridization
• Phosphorus Pentachloride (PCl5).
➢ sp3 d2 Hybridization: Hybridization is where when one orbital, three orbitals and two orbitals intermix to form 6 sp3d2 hybridized orbitals. Some key features are:
• The molecules formed are octahedrons in fashion and thus have a bond angle of .
• It is also called Octahedral hybridization. Examples of sp3 d2 hybridization

• Sulphur Hexafluoride
➢ Other common examples:
Methane: By the interactions of C-sp3 with an H-1s, 4 equivalent C-H σ bonds can be formed.
Ethane: 6 C-H sigma σ bonds can be formed by the interaction of C-sp3 with an H-1s orbital and 1 CC sigma bond can be made by the interaction of C-sp3 with another C-sp3 orbital.
Formation of Ammonia (NH3) and Water (H2O): In the NH2 molecule, the nitrogen atom is sp3hybridized and one hybrid orbital possesses two electrons. Now to form an NH3 molecule, three 1sorbitals of three hydrogen atoms overlap with three sp3 orbitals. The angle between H-N-H has to be 109.50 but as there is one occupied sp3 -hybrid orbital, the angle decreases to 107.80. Therefore, the bond angle in the NH3 molecules is 107.80.



Formation of C2H4 and C2H2 molecules: In the C2H4 molecule, carbon atoms are sp2 - hybridized and one 2p- orbital remains out to hybridization which forms a p-bond. Whereas, sp2 - hybrid orbitals form sigma bonds.

Illustration-7.1
Which of the following is not essential for hybridisation?
(a) Orbitals of valence shell of an atom only are hybridised.
(b) These orbitals may or may not have almost equal energies.
(c) Promotion of electron prior to hybridisation is not essential.
(d) In hybridisation orbitals may be empty, half filled or even completely filled.
Solution: (b)
It is compulsory that combining orbitals should have very close energies.
Illustration-7.2
Which of the following is incorrect w.r.t. sp-hybridisation?
(a) It is also known as diagonal hybridisation.
(b) The hybrid orbitals are 180° angle to each other.

(c) The unit possesses linear geometry.
(d) In BeCl2, 1s and one 2p orbitals combine to give sp-hybridisations.
Solution: (d)
In BeCl2, 2s and one 2p get hybridised.
Illustration-7.3
Which of the following is not correct for sp3 hybridisation?
(a) One s and three p orbitals reshuffle their energies.
(b) Four new sp3 hybridised orbitals are formed.
(c) sp3 hybridised orbitals have 75% p-character.
(d) Examples are C2H4, PCl5, etc.
Solution: (d)
C2H4 is an example of sp2 hybridisation while PCl5 has sp3 d hybridisation.
Illustration-7.4
Which of the following is not correct for ethene?
(a) x is σ bond (b) y is π bond
(c) α is 116.6° (d) β is 112°
Solution: (d)
β is actually 121.7°.
Illustration-7.5
Which of the following is not correct for C2H4?
(
a) Carbon atoms are sp hybridised.
(b) Pure p-orbitals perpendicular to the plane of molecule form π-bond.
(c) Carbon-carbon bond length is 134 pm.
(d) C–H bond length is 108pm.
Solution: (a)
C-atoms in C2H2 are sp2 hybridised.
Illustration-7.6
Select the incorrect statement for C2H2:
(a) In sp hybridisation of C-atoms, the pure p-orbitals left ae 2py and 2pz
(b) Pure p-orbitals form two π-bonds between two C-atoms
(c) The triple bond between two C-atoms is made up of one sigma and two π-bonds
(d) C – C – H bond angle is 180°
Solution: (a)
The pure p-orbitals left are 2px and 2py
It is 2px that takes part in sp-hybridisation in C-atom.
Know Yourself
1. What is meant by hybridisation of atomic orbitals? Describe the shapes of sp, sp2, sp3 hybrid orbitals. [I]
2. Which hybrid orbitals are used by carbon atoms in the following molecules? [U]
(a) CH3 – CH3 (b) CH3 – CH = CH2 (c) CH3 – CH2 – OH
(d) CH3 – CHO (e) CH3COOH
3. Describe the change in hybridisation (if any) of the Al atom in the following reaction. [A] AlCl3 + Cl– AlCl4 –
4. Is there any change in the hybridisation of B and N atoms as a result of the following reaction? [A]
BF3 + NH3 F3 B. NH3

8. MOLECULAR ORBITAL THEORY (MOT)
➢ The magnetic behaviour of O2 is paramagnetic, associated with presence of unpaired electrons.VBT, howeversuggests,thatO2 shouldbediamagnetic.Manysuchresultsput aquestionmarkonapplication of VBT in explaining properties of molecules. Especially, in homonuclear diatomic systems (F2, O2, N2, etc. we tend to apply MOT
➢ Salient Features of MOT: According to molecular orbital theory, whenever two atoms approach each othertoformabond,thenre-distributioninenergyofall constituent orbitaloccursleadingtoformation of new set of orbitals that are called molecular orbitals.
➢ These molecular orbitals are formed by linear combination of atomic orbital. The constructive interferenceofatomicorbitalsleadstoformationofbondingmolecular orbitalsthat possesslessenergy as compared to atomic orbitals. Hence filling of electrons in bonding molecular orbitals is equivalent to formation of bond due to lowering of energy.
➢ The destructive interference of atomic orbitals leads to formation of anti-bonding molecular orbitals that possess higher energy as compared to atomic orbitals. Hence filling of electrons in anti-bonding, molecular orbital is equivalent to rupture of bond, already formed.
Diagrammatic Representation
(i) The electrons in a molecule are present in the various molecular orbitals as the electrons of atoms
(ii) The atomic orbitals of comparable energies and proper symmetry combine to form molecular orbitals.
(iii) While an electron in an atomic orbital is influenced by one nucleus, in a molecular orbital it is influenced by two or more nuclei depending upon the number of atoms in the molecule. Thus, an atomic orbital is monocentric while a molecular orbital is polycentric.
(iv) The number of molecular orbital formed is equal to the number of combining atomic orbitals. When twoatomicorbitalscombine,twomolecularorbitalsareformed.Oneisknownasbondingmolecular orbital while the other is called antibonding molecular orbital.
(v) The bonding molecular orbital has lower energy and hence greater stability than the corresponding antibonding molecular orbital.


(vi) Justastheelectronprobabilitydistributionaroundanucleusinanatomisgivenbyanatomicorbital, the electron probability distribution around a group of nuclei in a molecule is given by a molecular orbital
(vii) ThemolecularorbitalslikeatomicorbitalsarefilledinaccordancewiththeAufbauprincipleobeying the Pauli’s exclusion principle and the Hund’s rule.
➢ Qualitatively, the formation of molecular orbitals can be understood in terms of the constructive or destructive interference of the electron waves of the combining atoms. In the formation of bonding molecular orbital,thetwo electron waves of the bonding atomsreinforceeachother due toconstructive interference while in the formation of antibonding molecular orbital, the electron waves cancel each other due to destructiveinterference. As aresult, the electron density in a bonding molecular orbital is located between the nuclei of the bonded atoms because of which the repulsion between the nuclei is very less while in case of an antibonding molecular orbital, most of the electron density is located away from the space between the nuclei. Infact, there is a nodal plane (on which the electron density is zero) between the nuclei and hence the repulsion between the nuclei is high. Electrons placed in a bonding molecular orbital tend to hold the nuclei together and stabilise a molecule. Therefore, a bonding molecular orbital always possesses lower energy than either of the atomic orbitals that have combined to form it. In contrast, the electrons placed in the antibonding molecular orbital destabilise the molecule. This is because the mutual repulsion of the electrons in this orbital is more than the attraction between the electrons and the nuclei, which causes a net increase in energy.
➢ It may be noted that the energy of the antibonding orbital is raised above the energy of the parent atomic orbitals that have combined and the energy of the bonding orbital has been lowered than the parent orbitals. The total energy of two molecular orbitals, however, remains the same as that of two original atomic orbitals
➢ Conditions for combination of atomic orbitals
• The combining atomic orbitals must have the same or nearly the same energy.
• The combining atomic orbitals must have the same symmetry about the molecular axis.
• (By convention Z axis is taken as internuclear axis)
• The combining atomic orbitals must overlap to the maximum extent.
8.1 Types of Molecular Orbitals
➢ Molecular orbitals of diatomic molecules are designated as σ (sigma), π (pi), δ (delta), etc.
➢ In this nomenclature, the sigma (σ) molecular orbitals are symmetrical around the bond – axis while pi (π) molecular orbitals are not symmetrical. For example, the linear combination of 1s orbitals centered on two nuclei produces two molecular orbitals which are symmetrical around the bond-axis. Such molecular orbitals are of the σ type and are designated as σ1s and σ*1s
➢ If internuclear axis is taken to be in the z-direction, it can be seen that a linear combination of 2pzorbitals of two atoms also produces two sigma molecular orbitals designated as 2pz and *2pz.
➢ Molecular orbitals obtained from 2px and 2py orbitals are not symmetrical around the bond axis because of the presence of positive lobes above and negative lobes below the molecular plane. Such molecular orbitals, are labelled as π and π*
➢ The increasing order of energies of various molecular orbitals for O2 and F2 is given below:
2zx 1s*1s2s*2s2p2p2py*2p*2py*2pz
➢ However, this sequence of energy levels of molecular orbitals is not correct for the remaining molecules Li2, Be2, B2, C2, N2. For instance, it has been observed experimentally that for molecules with total electrons < 14, such as B2, C2, N2, etc, the increasing order of energies of various molecular orbitals is
z2x 1s*1s2s*2s2p2py2p*2p*2py*2pz
➢ The distribution of electrons among various molecular orbitals is called the electronic configuration of the molecule. From the electronic configuration of the molecule, it is possible to get important information about the molecule as discussed below.
➢ Stability of Molecules: If Nb is the number of electrons occupying bonding orbitals and Na the number occupying the antibonding orbitals, then
➢ Bond order : Bond order is defined as one half the difference between the number of electrons present in the bonding and the antibonding orbitals i.e., Bond order (b.o.) = 1⁄2 (Nb – Na)
A positive bond order (i.e., Nb > Na) means a stable molecule while a negative (i.e., Nb < Na) or zero (i.e., Nb = Na) bond order means an unstable molecule.
➢ Nature of the bond : Integral bond order values of 1, 2 or 3 correspond to single, double or triple bonds respectively as studied in the classical concept.
➢ Bond-length : The bond order between two atoms in a molecule may be taken as an approximate measure of the bond length. The bond length decreases as bond order increases.
➢ Magnetic Nature : If all the molecular orbitals in a molecule are doubly occupied, the substance is diamagnetic (repelled by magnetic field). However if one or more molecular orbitals are singly occupied it is paramagnetic (attracted by magnetic field), e.g., O2 molecule
8.2
Some Common Diatomic Molecules
➢ Hydrogen molecule (H2): Dihydrogen molecule belongs to the family of diatomic molecules, which consists of two hydrogen atoms bonded to each other by a covalent bond. According to the atomic number of hydrogen, it has only one electron in its 1s orbital. The electronic configuration of H2 molecule is given as:
Bond order = 1
Due to the absence of unpaired electrons in the hydrogen molecule, it is diamagnetic in nature.
➢ Lithium molecule (Li2) : Lithium molecule belongs to the family of diatomic molecules, which consists of two lithium atoms, bonded to each other by a covalent bond. The electronic configuration of Li2 molecule is given as:
Bond order = 1
Thus the Li2 molecule is stable and is diamagnetic in nature due to the absence of unpaired electrons.
➢ Carbon molecule (C2): Carbon molecule belongs to the family of diatomic molecules, which consists of two carbon atoms, bonded to each other by a covalent bond. The electronic configuration of the carbon molecule is given as, ( ) ( ) (
➢ Bond order = 2
➢ Due to the absence of unpaired electrons, it is diamagnetic in nature. Furthermore, due to the presence of four electrons in pi bonding orbitals, the double bond in C2 consists of both pi bonds.
➢ Oxygen molecule (O2): Oxygen molecule belongs to the family of diatomic molecules, which consists of two oxygen atoms, bonded to each other by a covalent bond. The electronic configuration of the Oxygen molecule is given as,
➢ Bond order = 2.
➢ Due to the presence of one unpaired electron, 2O molecule should be paramagnetic.
➢ Helium molecule (He2): According to the atomic number of helium, it has two electrons in 1s. The electronic configuration of the helium molecule according to molecular orbital theory is given as: ( )
; Bond order = 0.
➢ Thus, He2 molecule is unstable and does not exist.
Illustration-8.1
Which of the following is correct statement?
(a) Bohr’s explanation of spectrum is for species having one or more electron only.
(b) Calculations of ψ and values of quantum numbers from Schrodinger wave equation are based on one electron species only.
(c) Molecular orbital theory is for multielectron and multiatomic species only.
(d) Molecular orbital theory more accurately explains homonuclear diatomic systems. Solution: (d)
Illustration-8.2
Which of the following is wrong w.r.t. the salient features of MOT?
(a) In a molecule, electrons are present in molecular orbitals.
(b) In a molecule, an electron is influenced by two or more nuclei.
(c) Atomic orbitals of comparable energies and proper symmetry combine to form molecular orbitals.
(d) Two atomic orbitals combine to form two or more molecular orbitals.
Solution: (d)
Two atomic orbitals combine to form only two molecular orbitals.
Illustration-8.3
Which of the following is not correct w.r.t. the salient features of MOT?
(a) Two atomic orbitals combine to form two molecular orbitals. One is known as bonding molecular orbital and the other is called as antibonding molecular orbital.
(b) Bonding molecular orbitals have lower energy and stabilize the species than the corresponding antibonding molecular orbitals which destabilise the species.
(c) Electrons probability distribution around a group of nuclei in a molecule is given by a molecular orbital.
(d) Pauli’s principle and Hund’s rule do not apply on molecular orbitals while special Aufbau principle is applicable.
Solution: (d)
Pauli’s principle and Hund’s rule are obeyed as in atomic orbitals.
Illustration-8.4
Which of the following statements is not correct w.r.t. LCAO?
(a) ψMO = ψA ± ψB
(b) Electrons in bonding molecular orbitals remain between the two nuclei most of the time thereby decreasing the repulsion between nuclei.
(c) Electrons placed in bonding molecular orbitals tend to hold the nuclei together and stabilize the molecule.
(d) The energy of bonding molecular orbital is always slightly higher than either of the atomic orbitals that have combined to form it.
Solution: (d)
Bonding molecular orbitals always possess lower energy than them either of the atomic orbitals that have combined to form it.
Illustration-8.5
Which of the following conditions is not correct for the formation of molecular orbitals by LCAO?
(a) Combining orbitals must have the same or nearly same energy.
(b) The combining atomic orbitals must have the same symmetry about the molecular axis.
(c) The combining atomic orbitals must overlap to the maximum extent.
(d) px atomic orbital can form π-molecular orbital by combining with py atomicorbital.
Solution: (d)
It is a wrong concept and is not a part of conditions for the formation of molecular orbital by LCAO.
Illustration-8.6
Which of the following statements is not correct w.r.t. the types of molecular orbitals?
(
a) Molecular orbitals are designated as σ (sigma), π (pi), δ (delta), etc.
(b) σ-molecular orbitals are symmetrical around the bodn-axis.
(c) π-molecular orbitals are not symmetrical around the bond-axis.
(d) If Z-axis is taken as internuclear axis, 2pz orbitals of two atoms produce
Solution: (d)
If Z-axisistaken as internuclear axis, 2pz orbitalsoftwo atomsproduce
and not the pi molecular orbitals.
Illustration-8.7
z-axis is taken as internuclear axis. Which of the following will form σ bond?
Solution: (c)
will have linear overlap.
Illustration-8.8
Select the incorrect statement(s) out of the following:
(a) Bond order ( ) 1 2 baNN=−
(b) Molecule is stable if only Nb > Na
(c) B.O. values 1, 2 or 3 correspond to single, double or triple bonds, respectively as in classical concept
(d) Bond length ∝B.O.
Solution: (d)
Higher the bond length smaller is the bond order, i.e., 1
bondlength b.o.
Li2 (Z = 3) has MO electronic configuration (σ1s)2, (σ*1s)2, (σ2s)2. Which of the following is/are correct?
A → Li2 is paramagnetic B→Li2 has a single bond (in vapour state)
C→ Bond order of Li2 is 2
(a) A (b) B (c) C (d) All of these Solution: (b)
Li2 is diamagnetic as it does not have any unpaired electron. 1 421 2 =−=Bondorder[] B.O. = 1 ⇒Single bond.
Know Yourself
1. Write the important conditions required for the linear combination of atomic orbitals to form molecular orbitals. [I]
2. Use molecular orbital theory to explain why the Be2 molecule does not exist. [I]
3. Compare the relative stability of the following species and indicate their magnetic properties; 222 O,O,O+− (superoxide), 2 2O (peroxide) [U]

9. HYDROGEN BOND
Definition :
➢ It is an electrostatic attractive force between covalently bonded hydrogen atom of one molecule and an electronegative atom
➢ It is not formed in ionic compounds
➢ Hydrogen bond forms in polar covalent compounds, (not in non-polar)
➢ It is also known as dipole-dipole attraction or ion-dipole interaction.
9.1 Main condition for Hydrogen bonding :
➢ Hydrogen should be covalently bonded with high electronegative element like F,O, N
➢ Atomic size of electronegative element should be small.
➢ Decreasing order of atomic size is-
N > O > F
Decreasing order of electronegativity is-
N > O > F (4.0) (3.5) (3.0)
➢ Intermolecular Hydrogen Bond: Hydrogen bond formation between two or more molecules of either the same or different compounds known as Inter molecular Hydrogen bonding These are two types.
(i) Homointermolecular :- Hydrogen bond between molecules of same compounds.
(i) Hetro intermolecular :- Hydrogen bond between molecules of different compounds. eg. alcohol, water
Intra molecular Hydrogen Bond:- It takes place within the molecule.
(i) Hydrogen bonded with electronegative elements of a functional group, form Hydrogen bond with another electronegative element present on nearest position on the same molecule.
(ii) This type of Hydrogen bond is mostly occured in organic compounds.
(iii) It result in ring formation (Chelation).
9.2 Effect of Hydrogen bond on Physical Properties :
(1) Solubility:
• Intermolecular Hydrogen Bonding
(i) Few organic compounds (Non-polar) are soluble in water (Polar solvent) due to Hydrogen bonding. Examples: alcohol, acetic acid etc. are soluble in water.
Other examples-Glucose, Fructose etc, dissolve in water.
(ii) Ketone, ether, alkane etc. are insoluble (no Hydrogen bond)
(iii) Solubility order- 333 CHOCHCHOH Primary amine secondary amine tertiary amine
• Intramolecular Hydrogen Bonding:
(i) It decreases solubility as it form chelate by Hydrogen bonding, so Hydrogen is not free for other molecule.
(iii) It can not form H-bond with water molecule so can not dissolves
Inter Molecular Hydrogen Bonding:
It can form Hydrogen bond with water molecule so it can dissolve
Viscosity: Hydrogen bond associates molecules together, so viscosity increases
water alcohol ether
➢ Melting point and boiling point
Due to intermolecular Hydrogen bond Melting Point & Boiling Point of compounds increases.
Illustration-9.1
Which of the following atom is not involved in hydrogen bonding?
(a) N (b) O (c) F (d) S
Solution: (d)
Only N, O and F can linear Hydrogen Bond formation
Illustration-9.2
Select the incorrect statement about hydrogen bond:
(a) It affects the structure and properties of the compound.
(b) It is strongest in solids and weakest in gases.
(c) It is of three types.
(d) Boiling point of H2O is highest among all hydrides of its family.
Solution: (c)
Hydrogen bond is of two types: intermolecular and intramolecular.
Illustration-9.3
In which of the following is the hydrogen bonding strongest?
(a) Solids (b) Liquids (c) Gases (d) All are equal
Solution: (a)
It depends upon distance
Illustration-9.4
Which of the following does not undergo hydrogen bonding?
(a) H2O (b) H2S (c) NH3 (d) HF
Solution: (b)
S does not make H highly +ve.
Know Yourself
1. Arrange the following in order of decreasing boiling point: [A]
n-Butane (II) n-Butanol (II) n-Butyl chloride (IV) Isobutane
2 Define hydrogen bond. Is it weaker or stronger than the van der Waals forces? [I]
3. Why KHF2 exists but KHCL2 does not? [A]
4 Explain why HF is less viscous than H2O [A]

1. Atomscombineinordertoachieve a more stable (Noble gas) configuration. A covalently bonded molecule can be represented by Lewis structure, which shows bonding and nonbondingelectronpairs
6. Molecular orbital theory describes bonding in terms of the combination and rearrangement of atomic orbitalstoformorbitalsthatare associated with the molecule as awhole.Moleculesarestableif the number of electrons in bonding molecular orbitals is greater than that in antibonding
MIND MAP CHEMICAL BONDING
2. According to VSEPR model, molecular geometry can be predicted from the number of bonding electron pairs and lonepairs.Itisbasedontheassumption that valence shell electron pairs repel oneanotherandtendtostay asfarapart aspossible.
3. Hybridization is the process of mixing up of two or more orbitals to form an equivalent number oforbitals having the same shape and energy. Common hybridizations are: sp,sp2,sp3,sp3d,sp3d2 etc.
4. Dipolemomentisameasureof the charge separation in molecules containing atoms of different electronegativities. The dipole moment of a molecule is the resultant of whatever bond moments are present.
5. Hydrogenbondisabondformedbetween hydrogen and a highly electronegative elementlikeN,OorF,eitherofthesame moleculeorofadifferentmoleculebutto whichthehydrogenisnotdirectlybonded byacovalentbondandthehydrogenitself should be bonded to a highly electronegativeelementlikeN2 OorF






MCQ with One Correct Answer Type Questions
1. When two atoms combine to form a molecule
(a) energy is released
(c) energy is neither released nor absorbed
(b) energy is absorbed
(d) energy may either released or absorbed
2. Most favourable conditions for electrovalent bonding are
(a) low ionisation potential of one atom and high electron affinity of the other atom.
(b) high electron affinity and high ionisation potential of both the atoms.
(c) low electron affinity and low ionisation potential of both the atoms.
(d) high ionisation potential of one atom and low electron affinity of the other atom.
3. Lattice energy of an ionic compound depends on
(a) charge on the ion only
(c) packing of the ion only
(b) size of the ion only
(d) charge and size of the ion
4. Which of the following statement is not true about covalent compounds?
(a) They may exhibit space isomerism.
(c) They show ionic reactions.
(b) They have low melting and boiling points.
(d) They show molecular reactions.
5. Polarisation is the distortion of the shape of an anion by the cation. Which of the following statement is correct?
(a) Maximum polarisation is done by a cation of high charge.
(b) A large cation is likely to bring large degree of polarisation.
(c) A smaller anion is likely to undergo a high degree of polarisation.
(d) Minimum polarisation is done by a cation of small size.
6. Which of the following has a giant covalent structure?
7. 104.5° is the bond angle present in
8. In which of the following molecules, the bond angle is maximum? (a)
9. Both ionic and covalent bonds are present in
10. The hydrogen bond is strongest in
11. The octet rule is not valid for the molecule
(a)
12. The type of bonds present in CuSO4·5H2O are
(a) electrovalent, covalent and coordinate
(c) electrovalent and coordinate
electrovalent and covalent
and coordinate
13. If a molecule MX3 has zero dipole moment, the sigma bonding orbitals used by M are
(a) pure p (b) sp hybrids (c) sp2 hybrids (d) sp3 hybrids
14. The molecule that has linear structure is
(a) CO2 (b) NO2
SO2 (d) SiO2
15. Which of the following would have a permanent dipole moment?
(a) SiF
16. The high boiling point of water is due to
(a) its high specific heat (b) hydrogen bonding between the molecules
(c) weak dissociation of water molecules (d) its high dielectric constant
17. Which of the following species is diamagnetic?
(a)
18. The number of σ-and π-bonds in l-butene-3-yne are
(a) 5 sigma and 5 pi
(c) 8 sigma and 2 pi
19. Number of bonds in benzene are
(a) 6 σ and 3 π
20. Resonance structure of a molecule should not have
(a) identical arrangements of atoms
(c) the same number of paired electrons
21. Which has the bond order 1/2?
(b) nearly same energy content
(d) identical bonding
(a) O2 (b) N2 (c) F2 (d) H2 +
22. Which carbon atom is most electronegative?
(a) Unhybridised carbon
(c) sp2 hybridised carbon
(b) sp hybridised carbon
(d) sp3 hybridised carbon
23. The bond angles of NH3, NH4 + and NH2 – are in the order
(a) NH2 – > NH3 > NH4 +
(c) NH3 > NH2 – > NH4 +
24. Coordinate covalent bond is formed by
(a) transfer of electrons
(c) donation of electrons
(b) NH4 + > NH3 > NH2 –
(d) NH3 > NH4 + > NH2 –
(b) sharing of electrons
(d) none of these
25. Pick out the isoelectronic structures from the following?
(i) CH3 +
(iii) NH3
(a) (i) and (ii)
(c) (i) and (iii)
(ii) H3O+
(iv) CH3 –
(b) (i) and (iv)
(d) (ii), (iii) and (iv)
26. The electronic structure of four elements a, b, c and d are:
(i) 1s2 (ii) 1s2, 2s2 2p2
(iii) 1s2, 2s2 2p5 (iv) 1s2, 2s2 2p6
The tendency to form electrovalent bond is greatest in
(a) (i) (b) (ii) (c) (iii) (d) (iv)
27. NH3 has a much higher boiling point than PH3 because
(a) ammonia has larger molecular weight
(b) ammonia undergoes umbrella inversion
(c) ammonia contains ionic bonds whereas PH3 contains covalent bond
(d) ammonia form hydrogen bonds
28. The molecule which has pyramidal shape is
(a) SO3 (b) PCl
29. T-type shape is exhibited by the molecule
(a) ClF3 (c) CCl4 (b) CHCl
30. The geometry of the molecule with 25 % s-character in hybrid orbital is
(a) plane triangular (b) linear (c) tetrahedral (d) octahedral
31. Number of sigma bonds in P4O10 is
(a) 6 (b) 7 (c) 17 (d) 16
32. Maximum bond angle is present in
(a) BF3 (b) BCl
33. In which of the following pairs, two species are isostructural?
(a)
34. Molecular shapes of SF4, CF4 and XeF4 are
same for all
(a) the same with 1, 1 and 1 lone pair of electrons respectively on the central atom.
(b) the same with 1, 0 and 2 lone pairs of electrons respectively on the central atom.
(c) different with 0, 1 and 2 lone pairs of electrons respectively on the central atom.
(d) different with 1, 0 and 2 lone pairs of electrons respectively on the central atom.
35. According to MO theory, which of the lists ranks the nitrogen species in terms of increasing bond order? (a)
36. A covalent molecule AB3 has pyramidal structure. The number of lone pair and bond pair electrons in the molecule are respectively
(a) 0 and 4 (b) 3 and 1
37. In which of the following molecules, the central atom does not have sp3-hybridization?
(a) CH4 (b) SF4 (c) BF4 – (d) NH4 +
38. Some of the properties of two species NO3 – and H3O+ are described below. Which one of them is correct?
(a) Dissimilar in hybridization for the central atom with different structures.
(b) Isostructural with same hybridization for the central atom.
(c) Isostructural with different hybridization for the central atom.
(d) Similar in hybridization for the central atom with different structures.
39. Which one of the following species does not exist under normal conditions?
(a) Be2 + (b) Be2 (c) B2 (d) Li2
40. Which of the following has a regular geometry?
(a) CHCl3 (b) SF4 (c) XeF6 (d) PCl5
41. According to Lewis and Kossel approach, which of the following molecule has complete octet of the central atom?
(a) LiCl (b) BeH2 (c) BCl3 (d) CO2
42. The group valency of the element is generally equal to the

(a) number of dots in Lewis symbol (b) eight minus the number of dots
(c) valence electrons (d) any of the above may possible
43. Ionic bonds will be formed more easily between elements with comparatively
(a) low ionisation enthalpy and high electron affinity
(b) high ionisation enthalpy and high electron affinity
(c) low ionisation enthalpy and low electron affinity
(d) high ionisation enthalpy and low electron affinity
44. Lewis dot structure of CO, 2NO and 2 3CO are I, II and III respectively
Which of the above structure(s) is/are wrong?
(a) Only I
Only II
None of these 45. Which of the following molecules has a triple bond? (a)

Which one of the following species has trigona planar shape? (a) BeCl
Number of π bond and σ bonds in the following structure is (a)
Which of the following is correct?
49. The molecule of hydrogen atom is formed due to the overlapping of orbitals of two hydrogen atoms. Which of the following types of overlapping takes place in the formation of H2 molecule?
(a) (b) (c) (d) All of these
50. Which of the following does not represent positive overlap?
(a) (b) (c) (d)
51. Diagonal hybridisation is the another name of (a) sp3 - hybridisation (b) sp2 - hybridization (c) sp - hybridization (d) All of these
52. Which of the following angle corresponds to sp2hybridisation?
(a) 90° (b) 120° (c) 180° (d) 109°
53. The decreasing order of the repulsive interaction of electron pairs is (Here, lp = lone pair, bp = bond pair)


(a) lp – lp > lp – bp > bp – bp
(d) lp – lp > bp – bp > lp – bp
(b) lp – bp > lp – lp > bp
bp
(d) bp – bp > lp – lp > lp – bp
54. Which of the following is responsible for the deviations occur from idealised shape of H2O and NH3 molecules?
(a) Same hybridisation
(c) Repulsive effect
(b) Different hybridisation
(d) None of the above
55. Polarity in a molecule and hence the dipole moment depends primarily on electronegativity of the constituent atoms and shape of a molecule. Which of the following has the highest dipole moment?
(a) CO2 (b) HI (c) H2O (d) BF3
56. The correct decreasing order of the boiling points of given compounds is
(a) HF > H2O > NH3
(c) NH3 > HF > H2O
(b) H2O > HF > NH3
(d) NH3 > H2O > HF
57. If Nb is the number of electrons occupying bonding orbitals and Na, the number of electrons occupying the antibonding orbitals, then the molecule will be stable if
(a) Nb > Na (b) Nb < Na (c) Nb = Na (d) Nb < Na
EXERCISE-2
Very Short Answer Type Question
1. Write Lewis symbols of Al and S2– ion.
2. Is ionic bond a directional?
3. What type of forces hold the atoms together in an ionic compound?
4. What type of orbitals can overlap to form a covalent bond?
5. Mention the favourable conditions for the formation of an ionic bond.
6. What is the valency of carbon in C2H4 and C2H2 ?
7. What types of bonds and how many of each are present in 4 + NH ?
8. Explain with one example, what is meant by polar covalent bond?
9. Why are the melting points of electrovalent compounds higher than those of covalent compounds?
10. Why are double bond compounds more reactive than single bond compounds?
11. What is hydrogen bond? Give one example?
12. What do you understand by a chemical bond?
13. Why do atoms combine to form compounds?
14. What is meant by hydrogen bonding
15. Write short note on sigma bond.
Short Answer Type Questions
1. Give reason for the following.
(i) Ionic compoundsaresoluble inwater whereas covalentcompoundsaremostlyinsolubleinwater
(ii) Ionic compounds have higher melting points than the covalent compounds.
2. Aluminium forms the ion Al3+ but not Al4+, why?
3. Which is more polar : CO2 or N2 O? Give reason
4. In each of the following pairs of compounds, which one is more covalent and why?
(i) AgCl, AgI
(iii) SnCl2, SnCl4
(ii) BeCl2, MgCl2
(iv) CuO, CuS
5. Why does formic acid exist as dimer? What is its one consequence?
6. With the help of molecular orbital theory, predict which of the following species is diamagnetic 2 222 H,O,O++ ?
7. Draw the shape of the following hybrid orbitals sp, sp2 and sp3
8. Discuss the hybridisation of Be in gaseous state and solid state
9. Using valence bond theory, draw the molecular structures of OSF4 and XeF4 indicating the location of lone pair(s) of electrons and hybridisation of central atoms
10. Explain why PCl5 is trigonal bipyramidal, whereas IF5 is square pyramidal?
11. Explain why carbon has a valency of four and not two. Also, why the four C – H bonds in methane are identical.
12. What is the total number of sigma and pi bond in the following molecules?
13. Which of the species have similar shape and why? 2223 NO,NO,CO,O −+
14. Explain why 2 3CO ion cannot be represented by a single Lewis structure. How can it be represented?
15. Arrange the following sets of molecules in the decreasing order of bond angle.
(i) SF6, CCl4, H2O, NH3 (ii) CH4, NH3, H2O, BF3
16. Define octet rule. Write its significance and limitations
17. Draw the Lewis structures for the following molecule and ions
H2 S, SiCl4, BeF2, 2 3CO HCOOH
18. (i) What factors favour the formation of ionic bond. Explain with examples
(ii) Arrangethefollowinginincreasingorder ofioniccharacterandalsogivethereason,NaCl, CaCl2, MgCl2, MgO.
19. (i) Why are some polar covalent bonds?
(ii) What is a dipole?
(iii) What are non-polar bonds?
(iv) How do dipole moments of molecules of CO2, CH4, H2O and NH3 help in ascertaining their structure?
20. What is an ionic bond? With two suitable examples explain, the difference between an ionic and a covalent bond?
21. (i) Discuss the significance/applications of dipole moment.
(ii) Represent diagrammatically the bond moments and the resultant dipole moment in CO2, NF3 and CHCl3.
22. What are the main postulates of valence shell electron pair repulsion (VSEPR) theory? What improvement was made by Nyholm and Gillespie?
23. Discuss the shape of the following molecules using VSEPR model.
234523BeCl,BCl,SiCl,AsF,H S,PH
24. (i) Draw the structure of following molecular shapes and give the various bond angles in the following:
linear, trigonal planar, tetrahedral and octahedral.
(ii) What are the shapes of 5336244342 PCl,NH,BCl,SFHO,CCl,SF,ClF,XeF , ,XeF
Directions In the following questions. an Assertion (A) is followed by a corresponding Reason (R) Use the following keys to choose the appropriate answer.
(A) Both (A) and (R) are correct and (R) is the correct explanation of (A).
(B) Both (A) and (R) are correct but (R) is not the correct explanation of (A).
(C) (A) is correct and (R) is incorrect.
(D) (A) is incorrect and (R) is correct.
1. Assertion: SF4 molecule has two types of structure,

Where structure (b) is more stable than (a)
Reason: Ip-bp repulsions decide the greater stability of structure (b)
(a) A (b) B (c) C (d) D
2. Assertion: Oxygen does not possess d-orbitals in their valence shell
Reason: The covalency of oxygen is two
(a) A (b) B (c) C (d) D
3. Assertion: PCl5 molecule has trigonal bipyramidal structure.
Reason: PCl5 contains 5 electron pairs and its hybridisation is sp3d.
(a) A (b) B (c) C (d) D
4. Assertion: BrF5 and SF6 both have same hybridisation
Reason: BrF5 is square pyramidal, while SP, molecule is octahedral
(a) A (b) B (c) C (d) D
5. Assertion: The values of dipole moment of CO2 molecule is zero.
Reason: CO2 molecule is liner
(a) A (b) B (c) C (d) D
6. Assertion: PbCl2 is more stable than PbCl4
Reason: PbCl4 is powerful oxidising agent.
(a) A (b) B (c) C (d) D
7. Assertion: NO+ and CN– both have same bond order and magnetism.
Reason: NO+ and CN– are isoelectronic species
(a) A (b) B (c) C (d) D
8. Assertion: LiCl is predominantly a covalent compound
Reason: Electronegativity difference between Li and Cl is too small.
(a) A (b) B (c) C (d) D
9. Assertion: Among the two O – H bonds in H2 O molecule the energy required to break the first O – H bond and the other O
H bond is not the same.
Reason: This is because the electronic environment around oxygen is the same even after breakage of one O – H bond
(a) A (b) B (c) C (d) D
10. Assertion: Ionic bond is non-directional
Reason: Each ion is surrounded by a uniformly distributed electric field.
(a) A (b) B (c) C (d) D
11. Assertion: In NH3, N is sp3 - hybridised, but angle is found to be 107°
Reason: The decrease in bond angle is due to repulsion between the lone pair on nitrogen and bond pair between N and H
(a) A (b) B (c) C (d) D
EXERCISE-3
Long Type Answer Questions
1. Explain the formation of H2 molecule on the basis of valence bond theory.
2. What ismeant by hybridisation of atomic orbitals.Describethe shapesof sp, sp2 and sp3 hybridorbitals
3. Which hybrid orbitals are used by C-atoms in the following molecules?
(i) CH3 – CH3 (iii) CH3 CH2OH (v) CH3 COOH
(ii) CH3 CH = CH2 (iv) CH3 CHO
4. DescribehybridisationinthecaseofPCl5 andSF6.Theaxialbondsarelongerascomparedtoequatorial bondsin PCl5 where as in SF6 bothaxial bondsandequatorial bond havethesamebond length Explain.
5. Use the molecular orbital energy level diagram to show that N2 would be expected to have a triple bond; F2 a single bond and Ne2, no bond.
6. How is molecular orbital different from atomic orbital? Give electronic configuration of
(i) 2H+ (ii) Li2, (iii) B2 and(iv) C2.Calculatetheir bond order and predict their paramagnetic behaviour.
7. (i) Discuss the concept of hybridisation. What are its different types in a C-atom?
(ii) What is the type of hybridisation of carbon atoms marked with star?
8. What is meant by an ionic bond? What are the favourable conditions for the formation of this type of bond? What are the characteristics of electrovalent compounds?
9. What are Fajan'srule? Explain youranswer with examples.
10. What is VSEPR theory? How does it explain the bond angles observed in CH4, NH3 and H2O molecules?
11. What is resonance? How is resonance energy calculated? Write the resonance structures of:
(i) Ozone
(ii) Sulphur trioxide
(iii) Nitrous oxide
12. Why do atoms combine to form molecules? What do you know about the octet theory of valency? Discuss its limitations.
Case Study Based Type Questions
1. Molecular orbital theory is a theory on chemical bonding, developed by F. Hund and R.S Mulliken to describe the structure and properties of different molecules. The valence bond theory failed to explain how certain molecules contains two or more equivalent bonds whose bond orders lie between that of a single bond and double bond, such as the bonds in resonance stabilised molecules. This is where the molecular orbital theory proved to be more powerful.
In simple terms, the molecular orbital theory states that each atom tends to combine together and form molecular orbitals. As a result of such arrangement electrons are found in various atomic orbitals and they are usually associated with different nuclei.
(i) Why 2Li+ molecule is more stable than 2Li ?
(ii) When O2 changes to 2O ion, then electrons are add in which orbital?
(iii) What is the bond order and magnetic characteristic of CN–?
(iv) Addition of electrons in antibonding molecular orbital destabilises the molecule. Why? OR
How many nodal planes are present in σ* s antibonding orbitals?
2. Chemical bonding, involves interactions that account for the association of atoms into molecules, ions, crystals, and other stable species that make up the familiar substances of the everyday world. When atoms approach one another, their nuclei and electrons interact and tend to distribute themselves in space in such a way that the total energy is lower than it would be in any alternative arrangement. If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, then bond together and the energy lowering is the bonding energy.
The ideas that helped to establish the nature of chemical bonding came during early 20th century, after the electron had been discovered and quantum mechanics had provided a language for the description of the behaviour of electrons in atoms. However, even though chemists need quantum mechanics to attain a detailed quantitative understanding of bond formation, much of their pragmatic understanding of bonds is expressed in simple intuitive models. These models treat bonds as primarily of two kindsnamely, ionic and covalent.
The type of bond that is most likely to occur between two atoms can be predicted on the basis of the location of the elements in the periodic table, and to some extent the properties of the substances so formed can be related to the type of bonding.
A key concept in a discussion of chemical bonding is that of the molecule. Molecules are the smallest unit of compounds that can exist. One feature of molecules that can be predicted with reasonable successistheir shape.Molecular shapes are of considerableimportancefor understandingthe reactions that compounds can undergo, and so the link between chemical bonding and chemical reactivity is discussed briefly here.
(i) According to molecular orbital theory, 2 2H molecule does not exist, why?
(ii) In the given compounds of chlorine. Which of them contains ionic as well as covalent bonds? NaCl, NaClO4, PCl3, POCl3
(iii) In 3 4P calculate formal charge on each oxygen atom and P – O bond order.
(iv) Acetophenone has higher dipole moment than ethanol. Explain? OR
On the basis of valence bond theory, explain the formation of H2 molecules from two H-atoms
3. Dipole moment is the measure of degree of polarity and is defined as the product of the magnitude of the charge and the distance between the centres of positive and negative charge. It is usually represented by a Greek letter μ (mu).
Mathematically, it is expressed as :
Dipole moment (μ) - charge (Q) × distance of separation (r)
It is usually expressed in Debye units (D).
1D = 3.33564 ×10–30 Cm where, C is coulomb and m is metre. or 1D=1×10–18 esu (or, stat Cm)
Here, esu = electrostatic unit stat C cm = static coulomb centimeter
It is a vector quantity and is represented by the crossed arrow (⇸) pointing towards the more electronegative atom.
F H– More electronegative atom
(i) The relation between resultant dipole moment of NH3 and NF3 is
(a) μ NH3 = μ NF3 (b) μ NH3 > μ NF3 (c) μ NH3 < μ NF3 (d) None of these
(ii) Inadiatomicmoleculethebonddistanceis1×10–8 cm.Itsdipolemomentis1.2D.Whatisthefractional electronic charge on each atom?
(a) 0.25 (b) 2.5 (c) 1 (d) 0.5
(iii) In water molecule, the two O – H bonds are oriented at an angle of 104.5° In BF3, the three B – F bonds are oriented at an angle of 120°, In BeF2, the two Be – F bonds are oriented at an angle of 180° Which will have highest dipole moment?
(a) BeF2 (b) BF3
(c) H2O (d) All have equal dipole moment
(iv) CO2 molecules is
(a) polar (b) non-polar (c) linear (d) Both (b) and (c) OR
Dipole moment is given by
(a) μ = q × d (b) μ = 2qd (c) qd 2 = (d) none of these
4. The molecular orbitals obtained by the subtraction of atomic orbitals are called antibonding molecular orbitals (AMOs) and are represented by σ* and π* In the formation of AMOs, the electron waves cancel each other due to destructive interference. In these orbitals, most of the electron density is located away from the space in between the nuclei.
Infact, they have a nodal plane (on which the electron density is zero) in between the nuclei and hence, the repulsion between the nuclei is high. In contrast to the bonding molecular orbitals, the electrons placed in these orbital destabilise the molecule because of more mutual repulsion of the electrons in thisorbitalascomparedtotheattractionbetweentheelectronsandthenucleiwhichcauseanetincrease in energy.
In these questions Nos. (i-iv) A statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
(A) Both (A) and (R) are correct and (R) is the correct explanation of (A).
(B) Both (A) and (R) are correct but (R) is not the correct explanation of (A).
(C) (A) is correct and (B) is incorrect.
(D) (A) is incorrect and (R) is correct.
(i) Assertion: The total energy of two molecular orbitals, however, remains the same as that of two original atomic orbitals.
Reason: The energy of the antibonding orbital is raised above the energy of the parent atomic orbitals that have combined and the energy of the bonding orbital has been lowered than the parent orbitals.
(a) A (b) B (c) C (d) D
(ii) Assertion: The combining atomic orbitals must have the same or nearly the same energy.
Reason: 1s-orbital can combine with another 2s-orbital but not with 1 s-orbital because the energy of 2s-orbital is appreciably higher than that of 1 s-orbital. This is not true if the atoms are very different.
(a) A (b) B (c) C (d) D
(iii) Assertion: F F bond has low bond dissociation energy
Reason: The fluorine has least reactivity
(a) A (b) B (c) C (d) D
(iv) Assertion: The order of bond energy is
Reason: C C < C = C < C C
(a) A (b) B (c) C (d) D
Assertion: Bond energy increases with increase in bond order.
Reason: The number of electrons in bonding molecular orbital and antibonding molecular orbital is equal.
(a) A (b) B (c) C (d) D
5. When anions and cations approach each other, the valence shell of anions are pulled towards cation nucleus and thus, shape of anion is deformed. The phenomenon of deformation of anion by a cation is known as polarisation and the ability of the cation to polarize the anion is called as polarizing power of cation. Due to polarization, sharing of electrons occurs between two ions to some extent and the bond shows some covalent character.
The magnitude of polarization depends upon a number of factors. The factors were suggested by Fajan and are known as Fajan's rules.
(a) Greater is the polarization in a molecule, more is covalent character.
(b) As the charge on cation increases, its tendency to polarize the anion increases.
(c) As the size of the cation decreases or size of the anion increases, the polarization increases.
(d) The cations with 18 electrons in the outermost shell bring grater polarization of the anion than those with inert gas configuration even both the cations have same size and same charge.
(i) Considering BeCl2, MgCl2, CaCl2, and BaCl2 predict which of the following statements is true?
(a) BeCl2 is least ionic out of the given chlorides.
(b) Covalent character increases as the atomic number of the metal atom increases.
(c) BeCl2 has the highest melting point among the given chlorides.
(d) All are highly ionic compounds.
(ii) In which of the halides, there is maximum polarization?
(a) AlF3 (b) AlCl3 (c) AlBr3 (d) All3
(iii) Which is most covalent in nature?
(a) NaCl (b) MgCl2 (c) AlCl3 (d) CaCl2
(iv) Non-aqueoussolventlikeetherisaddedtothemixtureofLiCI,NaCI,andKCI.Whichwillbeextracted into ether?
(a) NaCl (b) LICl (c) KCl (d) None
(v) Which has the minimum melting point?
(a) CaF2 (b) CaCl2 (c) CaBr2 (d) Cal2
6. Covalent molecule formed by heteroatoms bound to have some ionic character. The ionic character is due to shifting of the electron pair towards A or B in the molecule AB. Hence, atoms acquire small and equal charge but opposite in sign. Such a bond which has some ionic character is described as polar covalent bond. Polar covalent molecules can exhibit dipole moment. Dipole moment is equal to the product of charge separation, q and the bond length, d for the bond. The unit of dipole moment is Debye. One Debye is equal to 10–18 esu cm. Dipole moment is vector quantity. It has both magnitude and direction. Hence, dipole moment of molecules depends upon the relative orientation of the bond dipole, but not on the polarity of bonds alone. A symmetrical structure shows zero dipole moment. Thus, dipolemoments helpto predict the geometry of the molecules. Dipolemoment value can be used to distinguish between cis- and trans-isomers; ortho-, meta- and para- forms of a substance, etc. The percentage of ionic character of a bond can be calculated by the application of given ahead formula: % ionic character
100= Experimentalvalueofdipolemoment Theoretcialvalueofdipolemoment
(i) Which of the following compounds have zero dipole moments?



(ii) Which are non-polar molecules?
(a) XeF4 (b) BF3 (c) NHa (d) H2O
(iii) A diatomic molecule has a dipole moment of 1.2D. If the bond length is 1.0 × 10–8 cm, what fraction of charge does exist on each atoms?
(a) 0.1 (b) 0.2 (c) 0.25 (d) 0-3
(iv) Arrange the following compounds in increasing order of dipole moments, toluene (I), odichlorobenzene (II), m-dichlorobenzene (III) and p-dichlorobenzene (IV):
(a) IV<I<H<III (b) I <IV<II<III (c) IV<I<III<II (d) IV<II<I<III
(v) The dipole moment of NF3 is very much less than of NH3 because:
(a) number of lone pairs in NF3 is much grater than in NH3.
(b) Unshared electron pair is not present in NF3 as in NH3
(c) both have different shapes.
(d) of different directions of moments of N-H and N-F bonds.
