





Suspension:permanentlypreventcaking/sedimentation




Toothpaste:preventsdryingup/waterseparationwhile givingfinetexture
Emulsion:preventsemulsionbreakingwhilegivingfinetexture
Tablets:Improvesdissolution,binding&flowproperties


























































Suspension:permanentlypreventcaking/sedimentation




Toothpaste:preventsdryingup/waterseparationwhile givingfinetexture
Emulsion:preventsemulsionbreakingwhilegivingfinetexture
Tablets:Improvesdissolution,binding&flowproperties























































CustomToolRoom&MachiningSolutionsforEvolvingIndustries
AtKairishInnotech,ourfullyintegratedTool Room spans12,000sq.ft andishousedina temperature-controlledenvironment—ensuring idealconditionsforprecisionmachining,toollife, anddimensionalaccuracy.
Weserveawidespectrumofindustriesincluding pharmaceuticals,medicaldevices,packaging, foodprocessing,engineering,andautomation, offeringcustommachiningsolutionsforcomplex componentssuchas injectionmoulds,dies, tools,filling&washingneedles,changeparts, andmore.Whetherworkingwith plastics, metals,orhybridmaterials, wespecializeinfully bespokebuilds,tailoredtoyourfunctionaland operationalneeds.
01. 02.
08VMCs(VerticalMachining Centers)
3-axisand4-axisbyHAASandDMG
Moriforhigh-speed,high-precision manufacturing.
ElectricalDischargeMachining (EDM)
Fordetailedcavities,threading,and cylindricalpartmachining—ideal forintricateorhard-to-machine features.
03. 04. 05. 06.
Drilling&MillingMachines
Forreliable,repeatableshaping, cutting,andhole-makingacrossa rangeofsubstrates.
ChmerWire-CutEDM
Operatingwitha0.25mmwireand precisionupto±5microns,it deliversultra-finecutsinsteel, aluminum,copper,andalloys.
LaserWeldingSystem
Designedforultra-clean,highstrengthweldswithOD/ID concentricity<0.002mmand toleranceof0.005mm—essential forbothstructuralintegrityand aesthetics.
PrecisionLatheMachines
Enablingversatileturning,shaping, andsurfacefinishingwithtight tolerancesandhighrepeatability.

who helps you get to the finish line faster?
aquarius™ genesis
coating system

Who solves your customization needs? We do. Ashland Aquarius film coatings are carefully formulated and customized to achieve specific properties, depending on formulation requirements. And precise formulation is crucial for tablet appearance, performance, and processing. It’s also critical for achieving desired functionality like adhesion, strength, opacity, and more.
If you need technical support, customization, and a broad range of pharmaceutical film coating solutions, review our portfolio here Ashland.com/aquariusfilmcoatings and contact Ashland solvers –ashland.com/contact.














Chairman of the Board
ViveckGoenka
Sr.Vice President-BPD
Neil Viegas
Vice President-BPD
Harit Mohanty
Editor
Viveka Roychowdhury*
Editorial Team
Lakshmipriya Nair
Kalyani Sharma
Kavita Jani
Neha Aathavale
DESIGN
Art Director
Pravin Temble
Senior Designer
Rekha Bisht
Senior Artist
Rakesh Sharma
Marketing Team
Rajesh Bhatkal
Ashish Rampure
Production Co-ordinator
DhananjayNidre
Scheduling & Coordination
Pushkar Waralikar
CIRCULATION
Mohan Varadkar

26 STARTUPS: INDIA’S NEXTBIG BET ON PHARMA INNOVATION?
30 PRETTYRISKY: INDIA'S WELLNESS BOOM AND THE MISSING RULEBOOK


32
DIGITALTWINS: REVOLUTIONISING VACCINE TRIAL SITE PROCUREMENTIN DEVELOPING COUNTRIES

53 HOWPATIENT SUITES ARE RESHAPING THE FUTURE OF CLINICALTRIALS

P22: INTERVIEW DR SATISH WAGH CHAIRMAN,CHEMEXCIL, AND FOUNDER,SUPRIYA LIFESCIENCE
58 CAN STEM CELL THERAPYCURE DIABETES?
61 IIHMR UNIVERSITY'S ANNUALCONVOCATION 2025 FELICITATES 423 GRADUATES, HIGHLIGHTS GLOBALRECOGNITION AND ACADEMIC EXCELLENCE
Regd.With RNI No.MAHENG/2005/21398.Postal
Regd.No.MCS/164/2025 - 27.Printed and Published byVaidehi Thakar on behalf of The Indian Express (P) Limited and Printed at The Indian Express Press,Plot No.EL-208,TTC Industrial Area,Mahape,Navi Mumbai-400710 and Published at Mafatlal Centre,7th floor,Ramnath Goenka Marg,Nariman Point,Mumbai 400021.
Editor: Viveka Roychowdhury.* (Editorial & Administrative Offices: Mafatlal Centre,7th floor,Ramnath Goenka Marg,Nariman Point,Mumbai 400021) * Responsible for selection of news under the PRPAct.Copyright © 2017.The Indian Express (P) Ltd.All rights reserved throughout the world.Reproduction in anymanner,electronic or otherwise,in whole or in part,without prior written permission is prohibited.

“IDMA CARES should become what Indian pharma represents not just in molecules but in morals.”
This was Daara Patel, Secretary General, Indian Drug Manufacturers’ Association (IDMA), setting the tone on July 18, at the launch of IDMA CARES, an initiative which aims to strengthen workplace safety, Prevention of Sexual Harassment (POSH), and Environmental, Social, and Governance (ESG) principles. Express Pharma was a Media Partner for the launch and we’ll be featuring a detailed report soon but here are a few hard hitting comments that stayed with me.
Patel reiterated that “POSH, ESG are not just good ethics but smart business sense”, while Bharat Shah, National President, IDMA stressed that the industry cannot claim to save lives while polluting rivers, and must therefore deliver safe medicines while ensuring safe workplaces.
Neha Thakore, Chairperson, ESG Committee - IDMA, stressed that “doing good is no different from doing well” and urged pharma companies to consider laws like POSH as “reputational insurance”, and reframe their identity “as not just manufacturers but stewards of trust.”
For me, the highlight of the IDMA CARES launch was a panel discussion where Aditi Kare Panandikar, Managing Director, Indoco Remedies and P S R K Prasad, Director, Natco Pharma, shared how their companies have implemented sustainable practices. Meher Pudumjee, Chairperson,Thermax Group, highlighted that ESG is “not compliance but a competitive advantage” and is a “ticket to the world” with more regulators moving to cleaner, greener regulations around chemicals.
The panel was moderated by Sandeep Mohanty, PartnerSustainability Transformation, PwC India, who stressed that global clients are making it mandatory for suppliers and vendors to follow Scope 3 emission norms. Thus “sustainability does pay”, as it ensures ESG compliant companies have a competitive edge vis a vis those that do not follow global ESG norms.
While many pharma companies have started on the ESG path, we have a long long way to go. More importantly, the message has to filter down the line to suppliers and vendors. The recent explosion at Sigachi Industries where as many as 46 people died at its Pashamylaram plant in Sangareddy district, Telengana is the latest warning that workplace safety, a key part of ESG, needs to be top priority too.
The Sigachi Industries' Q1 FY26 results statement hints at the soul searching and introspection within senior ranks of the company, resulting in a ‘strategic realignment post Pashamylaram’. Amit Raj Sinha, MD & CEO, Sigachi Industries called the Q1 FY26 “an emotionally and operationally challenging quarter”, acknowledging that “the tragic incident led to the loss of lives and injuries among our workforce, and we extend our deepest condolences to all affected.”
The company has started the disbursal of interim compensation under the ex-gratia financial support package for the 46 families of the deceased and eight staff members who remain unaccounted for. Interim payments have also been made towards injury compensation and continues to support the families of three members still under medical care.
“This event has prompted a comprehensive review of our operational controls and risk governance," says Sinha. "Looking ahead, we are committed to a decisive reset, prioritising safety, accelerating cost improvements, focusing on margin-led portfolios, and rebuilding with global standards, resilience, and transparency. With the lessons behind us and our resolve strengthened, we are confident in our ability to deliver sustainable growth and improve

Proactively investing in employee and workplace safety is not just good for employees, but makes smart business sense
EBITDA margins.” This transparency is to be applauded and encouraged, and one hopes to see the company rebuild not just bigger but better than before.
Sigachi Industries is by no means the only company to have such incidents. On a recent post Q1 FY26 earnings call, Ashok Nair, Managing Director, RPG Life Sciences disclosed that the company’s API business had a temporary sales impact due to a fire incident at their Navi Mumbai-based API manufacturing plant in January. Thankfully there were no casualties, and as per Nair, “a dedicated team of 155 professionals is working on restoring the plant with a completion target in Q2.”
“RPG Life Sciences is built on a people-first, safety-first culture” and Nair credits the emergency response team, the RPG Group’s long standing manufacturing expertise across sectors from tyres to infrastructure and good insurance coverage that will allow the company to recover and get back on track. 85 per cent of APIs manufactured at the impacted plant were exported, 15 per cent were for the domestic market.
These statements underline the multiple ramifications of such incidents. At an individual level, such incidents leave a scar on the lives of the affected individuals and their families. At a company level, rebuilding physical infrastructure will have to go hand in hand with rebuilding trust with employees and clients. Thus proactively investing in employee and workplace safety is not just good for employees, but makes smart business sense.
However, one wonders how many such tragedies remain unreported in smaller unlisted companies, especially those in far flung industrial manufacturing zones? Do employees and their families receive adequate help with medical expenses and compensation for injuries and deaths? There are many MSMEs that serve the pharma industry, and not all of them are as visible as listed companies who are mandated to inform stock exchanges and answer to investors. Therefore, one has to assume that the incidence of such accidents and death tolls would be much higher.
While larger companies have the organisational bandwidth to disburse compensation (offset by partly insurance etc) and recover from the business setback, many other companies are not in the same position. They would prioritise a back-to-business policy, without rectifying faulty practices and employees would remain at risk.
Such incidents could tarnish the image of India’s pharma industry as a whole, just as promising bilateral agreements like the recent India-UK Free Trade Agreement (FTA) fructify after years of negotiation. UK PM Keir Starmer in fact reportedly termed it “the most significant deal since the UK left the EU.”
Namit Joshi, Chairman, Pharmexcil underlines that with India's pharma exports to the UK reaching $914 million in FY24, the India- UK FTA agreement strengthens supply chains, enhances access to affordable medicines, and drives Foreign Direct Investment (FDI).
According to his statement, this partnership paves the way for collaborations in bulk drug imports, CDMO, and joint research, empowering India’s competitive edge and promoting global partnerships.
India's pharma sector is already globally connected and has a reputation to uphold. Thus besides the moral case of putting employee safety first, workplace safety makes good business sense. If only to avoid disruptions to production schedules, revenue erosion and reputational damage.
VIVEKA ROYCHOWDHURY, Editor viveka.r@expressindia.com viveka.roy3@gmail.com





India’s chemical companies are undergoing a strategic transformation to be an integral part of global
Dr Satish Wagh, Chairman,CHEMEXCIL,and founder,Supriya Lifescience,discusses how the COVID-19 pandemic prompted Indian chemical exporters to rethink sourcing strategies, build domestic capacities,and embrace quality standards.He also shares insights on export trends,FTAutilisation,digital transformation,and policy recommendations for long-term sector resilience,in an interview with Viveka Roychowdury .Edited excerpts below…
Dr Wagh, as Chairman, CHEMEXCIL, and as the interface between government and industry, what are the learnings from the COVID era that have been incorporated by memberexporters connected to the pharma and life sciences sector?
The COVID-19 pandemic exposed vulnerabilities and offered important lessons for Indian chemical companies, shaping strategies to build resilience and competitiveness.
As a CHEMEXCIL chairman I have interacted with various sector companies. Heavy reliance on China for raw materials of key inputs led to shortages during lockdowns. Indian companies learned to diversify sourcing to countries like South Korea, Japan, and Europe, and prioritise local suppliers to overcome geopolitical and logistical risks.
Most Indian chemical and pharma companies rapidly expanded their inventory, stock, etc. Large scale manufacturing companies accelerated efforts to produce key inputs locally, reducing import dependency and ensuring supply stability.
IPA (isopropyl alcohol) shortages during the pandemic highlighted India’s overreliance on Chinese imports. It is sad to mention that some of the Indian companies manufacturing IPA are creating a monopolistic

India currently has 14 FTAs in effect,including those with EFTA, UAE,and Australia.In most of these FTAs chemicals are covered under preferential tariffs and are a good opportunity for Indian chemical exporters
situation by insisting that the department put anti-dumping duty on imports of IPA from China.
During COVID pandemic domestic companies inflated the cost of IPA and controlled the supply that ultimately burdened consumers. In fact, IPA prices in India had surged from ?65/kg to ?190/kg, (during March 2024).
As a CHEMEXCIL chairman I took the lead and strongly opposed the proposed imposition of anti-dumping duties on imports of IPA originating from China due to its potentially adverse consequences.
I appreciate the call for BIS standards which are important for domestic/pharma-grade material.
CHEMEXCIL as a part of capacity building initiative did the exporter engagement, organised various workshops, virtual sessions on BIS, QCO standards, ZED certification, etc. Liaised with competent authorities of BIS and Department of Chemicals and Petrochemicals, Govt of India on these issues.
What have been the cumulative export trends of your member companies in this category? Which are the segments showing an upward trajectory for the next few years?
Overall, the cumulative export trends for our CHEMEXCIL
member companies in the chemical sector have been quite robust over the past 20 years, demonstrating significant growth.
During 2004-2005, the exports of chemicals were USD 3.3 billion and now after 20 years it has reached USD 21 billion. (It includes the exports of Dyes, Dye intermediates, Basic Organic Inorganic Chemicals Including Agrochemicals, Cosmetics Soaps, toiletries, essential oils, Castor oil and Specialty Chemicals. It does not include petrochemicals).
While the chemical sector has faced some headwinds globally, our member companies have shown resilience in maintaining stable export levels.
The segments which will show an upward trend in the coming few years because of enough and added domestic capacities are Organic Chemicals, Organic Intermediates, Inorganic Chemicals especially Alkali Chemicals, Agrochemicals especially Herbicides, Insecticides and Fungicides, Cosmetics and Essential oil, Green Chemicals and Specialty Chemicals
What has been the impact of recent geopolitical moves like FTAs, tariffs etc on exports of your member companies?
Frankly the impact of recent
TranschemoffersBASF’sKollicoat® SR30Dand Kollidon® SRforpH-independentsustainedrelease formulations.
Kollicoat® SR30D isapolyvinylacetateaqueous dispersionstabilizedwithpovidoneandsodium laurylsulfate.
Thelowviscosityproducthasaweakcharacteristicodour andamilkywhiteorslightlyyellowishappearance.
•AmenableasaWetbinderforgranulationprocessesand/orcoatingapplicatio forsustainedrelease
•Providingconstantandreliabledrugreleaseinallintestinalfluids
•Drugreleaseprofilecanbeeasilyoptimizedwithcompatibleporeformers
•Providesmoderatetaste-maskinginmultipledosageforms
Kollidon® SR isaco-processedexcipientwitha compositionofpolyvinylacetateandpovidone.
Theproductisobtainedbyspray-drying.Itissuppliedas slightlyyellowish,free-flowingpowder.
•Thematrixformerprovidingsuperiorsustainedrelease
•Providingconstantandreliabledrugreleaseinallintestinalfluids
•Reliableprocessingduetoexcellentflowandcompressibilityfeatures
TranschemCorporationPharmaPvt.Ltd.
D421/422,NeelkanthBusinessPark,StationRoad,Vidyavihar(W), Mumbai-400086
Email:sales@transchemcorp.com
Phone:+91022-49711633
Web:www.transchemcorp.com
ons




geopolitical moves like FTA and tariffs is very positive on the Indian chemical sector.
From time-to-time the department is consulting CHEMEXCIL for negotiating the FTAs with partner countries. India currently has 14 FTAs in effect, including those with EFTA, UAE, and Australia. In most of these FTAs chemicals are covered under preferential tariffs and are a good opportunity for Indian chemical exporters.
But somehow most of the members (especially MSMEs) are not aware about these FTAs and rules of origin for preferential access. Hence FTAs are underutilised by Indian chemical companies. Hence, we organised a knowledge base session on FTA and its utilisation.
Hopefully in coming years this situation will be rectified and Indian chemical exporters can take the full advantage of these FTAs. As regards US reciprocal tariffs on Indian chemicals, Indian chemical industry has minimal impact on exports. There is no impact on imports of chemicals from the US to India. However, we have suggested that the department accelerate BTA with US covering preferential access to the US. This will boost Indian chemical exports.
How are your member companies ensuring that they are more integrated into global supply chains? How are digital tools helping to capitalise on identifying export opportunities?
Indian chemical companies are
undergoing a strategic transformation to be an integral part of global supply chains and explore new export opportunities across nontraditional markets by various initiatives.
Companies are investing in domestic feedstock production to reduce reliance on imports. They are also focusing and increasing investment in research and development.
Secondly, the Government is developing chemical hubs and upgrading port infrastructure to streamline logistics and reduce costs. And thirdly, the government is engaging with various countries and blocs for FTAs.
Digital transformation is playing an important role in helping Indian chemical companies. Analytics and
machine learning are being used to predict demand, optimise formulations, and identify high-potential export markets. Companies are using targeted digital marketing to reach global buyers and drive cross-border sales. Real-time tracking of shipments and inventory through advanced devices is enhancing the supply chain.
What are the policy suggestions from CHEMEXCIL to prevent future shortages of key input ingredients, etc?
To prevent future shortages of key chemical ingredients in India, the government and industry should come closer and address challenges like import dependency, infrastructure gaps, and
regulatory delays.
◆ Expand Domestic Feedstock Production by way of technology upgradation and R&D.
◆ Upgrade existing chemical clusters e.g., PCPIRs
◆ Streamline Environmental Clearance process
◆ Implement Production Linked Incentive Scheme for feedstock production
◆ Promote R&D in Chemical Sector by providing tax benefits
◆ Enhance skills of Industry people
◆ Improve port and logistic infrastructure.
◆ Negotiate Product Specific Rules in FTAs
Viveka Roychowdury, Editor viveka.r@expressindia.com viveka.roy3@gmail.com



While India’s pharma startups can resolve scientific and systemic blind spots,funding remains the missing link blocking scalable impact,finds Neha Aathavale
While established pharma companies focus on strategy and scale, pharma-focused startups in India are quietly laying their groundwork. Not yet driven by scale, but certainly by possibility. These early-stage ventures are doing the work that bigger players sometimes overlook: filling out the missing parts of India’s pharma jigsaw.
Rather than positioning themselves as challengers to big pharma companies, pharma startups are emerging as its R&D scouts; de-risking early innovation, translating academic research into viable therapies, and plugging capability gaps in areas like drug delivery, repurposing, and AI-led discovery. With risk-tolerant capital cautiously entering the fold, public schemes nudging R&D beyond academia, and large pharma firms showing interest in early-stage partnerships, the foundations are being laid. Can India now build the kind of patient, high-stakes startup ecosystem pharma innovation needs; before this quiet ambition loses steam?
Numbers sayit all The Indian pharma startup ecosystem is grappling with a pronounced funding downturn. "The Indian pharma space witnessed its peak funding in 2018, with a total of $399M raised. Since then, the industry has experienced a declining trend,” says Neha Singh, Co-founder of Tracxn. As of May 31, 2025, total funding stands at $25.3 million—down 75 per cent and 52 per cent compared to $101 million and $53.1 million raised during the same period in 2024 and 2023, respectively.
Singh attributes this decline to a mix of global and local challenges, “The slowdown is likely driven by a mix of global pricing pressures, regulatory shifts, and changing export dynamics, including stricter



scrutiny of pharmaceutical exports from India and adjustments in domestic drug pricing policies.” She adds that policy developments in key export markets, aimed at reducing
The Indian pharma space witnessed its peak funding in 2018,with a total of $399M raised.Since then,the industry has experienced a declining trend
Neha Singh Co-founder of Tracxn
In India,while there is vibrant startup energy across technology and health tech,pharma-only startups have yet to secure sustained investor traction
Sudarshan Jain
Secretary General,Indian
Pharmaceutical Alliance (IPA)
cines. Another deal came from Exsure, which raised $347K in seed funding for its nano drug delivery platform targeting cancer.
While aggregate numbers remain modest, Singh emphasised that such deals signal India’s continued relevance in global pharma innovation, “Despite recent headwinds, the sector’s ability to attract substantial investments in cuttingedge areas highlights its underlying strength and the continued global relevance of Indian pharma innovation,” Singh adds.
The Indian pharmaceutical sector has not seen any significant "big-ticket" VC deals in recent times,contrasting sharply with the more robust funding environment in the US,UK,and China
Aurojyoti
Bose Lead Analyst at GlobalData
drug costs, have “further intensified the challenges” for Indian pharma players; making investors more cautious.
Yet, May 2025 offered some respite. Funding that month hit
the year’s highest, marking an 11 per cent increase over May 2024. This was largely due to a $25M Series C round by Pharmazz, a clinical-stage company developing critical care medi-
Sagar Pawar, Partner & Lead, Lifesciences & Medical Devices, Deal Advisory - M&A Consulting at KPMG India, echoed the cautious optimism, “the Indian life sciences startup ecosystem attracted approximately $307 Mn in funding in 2024, marking a 36 per cent increase over 2023.” This growth, he says, reflects “sustained investor confidence in the sector’s innovation trajectory.” Pawar points to strong momentum in three areas: diagnostics and POC testing, biopharma ventures, and AI-powered imaging and screening in medtech. “This is driven by the growing demand for personalised medicine and tech-enabled healthcare solutions.”
However, the size and scale of venture capital deals in India still lag behind global counterparts. “The VC funding landscape for Indian pharmaceutical startups has been notably smaller in scale compared to their counterparts in western markets such as the US and the UK, as well as regional peers like China,” says Aurojyoti Bose, Lead Analyst at GlobalData. “India did not see many VC deals in 2025 so far,” he notes, and the ones that did happen lacked the high-value transactions common in the West.
Bose points out that “the Indian pharmaceutical market is

Confidently power your scientific IP research with CAS Solutions. The STN IP Protection Suite™ connects you to quality IP insights that spark innovation, uncover opportunities, and mitigate risk.
Learn more at cas.org
characterised by a focus on cost-effective production rather than high-value innovation,” but believes this may shift as Indian firms boost their R&D capabilities.
Encouragingly, some investors are doubling down. “Mumbai Angels has invested in Mestastop Solutions, a firm focusing on cancer metastasis drug discovery, twice,” Bose highlights; once in 2020 and again in 2024, indicating “ongoing investor confidence.”
Srikanth Mahadevan, Director, Deloitte India, notes that the investment climate is gradually improving. “Private venture capital was slow to embrace this space - historically, only a small fraction of India’s 10,000+ biotech startups secured funding,” he says. But momentum is building; in May 2025 alone, Indian pharma startups raised around $254M, including one early-stage company securing over $150M in a single round. “This uptick underscores that institutional investors and VCs are actively exploring pharma opportunities now.”
Offering the pharma industry’s view, Sudarshan Jain, Secretary General, Indian Pharmaceutical Alliance (IPA) states, “Globally, 2024 has been a relatively encouraging year for pharma and biotech investments, particularly in sectors such as AI-driven drug discovery.” But in India, “pharmaonly startups have yet to secure sustained investor traction.” The reason? The sector’s long gestation periods and capital-intensive nature. Still, Jain acknowledges “green shoots are emerging in areas such as novel drug delivery platforms and deep-tech R&D”, early indicators of longterm potential if the right enablers are in place.
Whyriskcapital still hesitates
Despite growing interest in pharma innovation, early-stage startups in India face a steep climb, especially when it comes to securing funding.
“Bringing a new drug or therapy to market involves navigating complex clinical trial and approval processes under


agencies like the CDSCO,” says Mahadevan. “Market entry can be delayed by 2–3 years just in obtaining approvals for a novel drug.” This prolonged regulatory timeline not only slows down growth but adds significantly to costs. “Developing a new drug in India can cost upwards of ?1,500 crore (around $200 million),” he explains, with R&D and trials consuming both time and capital. That, combined with a high failure rate (many drug candidates never make it to market), makes many investors cautious. “In contrast to quick-tomarket tech startups, pharma ventures require patience and specialised expertise, so raising capital is a constant hurdle.”
Market dynamics present further complications.
“Pharma startups must compete with well-established domestic and multinational companies,” says Mahadevan. Carving out space in India’s competitive generics market demands major resources and
Pharma startups are increasingly becoming critical players in the broader pharmaceutical ecosystem,with strong potential to bridge key strategic gaps
Sagar Pawar Partner & Lead,Lifesciences & Medical Devices,Deal Advisory - M&AConsulting,KPMG India
“Investors in India’s pharma startup space are increasingly drawn to innovation across the value chain, with a strong focus on AI integration, real-time diagnostics, precision medicine, and digital therapeutics.”
Investors are gravitating towards pharma startups that either push the frontier of science (new therapies, biologics,precision medicine) or innovate on process and business model (platform technologies, outsourced R&D/manufacturing,or digital efficiency tools)
Srikanth Mahadevan Director,Deloitte India
brand credibility, assets most young firms lack. Even after launching a product, startups must contend with price controls on essential drugs, which cap margins and impact profitability. “This means even a startup with an approved product might face profitability challenges due to government price controls on drugs,” he notes.
Intellectual property (IP) is another pain point. “Securing patents in India is a lengthy process—often taking five years or more,” Mahadevan adds. During that time, startups are vulnerable to having their innovations reverse-engineered or copied. “Such IP delays and the threat of reverseengineering or counterfeiting discourage innovation.”
Beyond regulation and IP, structural challenges persist: access to efficient manufacturing, cold-chain logistics, and quality distribution is limited for smaller players. “Cuttingedge pharma R&D requires
highly skilled scientists and regulatory experts, and startups must compete with big pharma companies to hire and retain this limited talent pool,” Mahadevan says.
In short, the sector’s hurdles include “long regulatory cycles, capital intensity, funding constraints, IP delays, intense competition, pricing pressure, and talent gaps.” None are insurmountable, but they demand strategic agility. “Entrepreneurs need to form strategic partnerships, tapping government grants, or focusing on niche markets to gain traction despite these barriers,” he advises.
Even with the challenges, the appetite for pharma startups is growing. Particularly those innovating across the value chain with deep-tech capabilities, digital platforms, and sciencefirst approaches.
Pawar notes a marked shift,
He also highlights the rise of AI-as-a-service for pharma: startups are using proprietary engines and generative AI for drug discovery, lead optimisation, and molecular screening. Meanwhile, commercial pharma platforms are redefining how pharma reps engage with healthcare professionals (HCPs) through CRM-powered, data-driven tools, and a new generation of patient-facing startups is integrating digital therapeutics to manage chronic conditions.
Startups developing personalised therapies, especially those aligning with global precision medicine trends are also commanding attention. “Clinical innovation is not just a buzzword; it’s where the frontier lies,” adds Pawar.
Mahadevan underscores that biopharma innovation remains the most dominant theme, accounting for 42 per cent of all life sciences startup funding in recent years. “With an estimated $100+ billion worth of biologic drugs set to go off-patent globally by 2030, Indian startups in the biosimilars space are especially attractive,” he explains. Indian companies that can produce affordable, high-quality biosimilars are well-positioned to claim a global share. “Biosimilars in India are expected to grow at nearly 30 per cent CAGR through 2027,” notes Mahadevan, calling it one of the hottest verticals for investors.
“In summary,” Mahadevan notes, “investors are gravitating towards pharma startups that either push the frontiers of science (new therapies, biologics, precision medicine); or innovate on process and business model. These areas align with both India’s comparative advantages and global pharma trends, offering the most promise for high returns.”
Backing the breakthroughs While investor interest in




H6-10: High-speed fillingwithprecisionandcare
⚪ OptimizedforGLP-1:gentlehandlingandaccuratedosing
⚪ Provenreliabilityincommercialmanufacturing
⚪ Outputupto38,000/h

⚪ ProcessesRTUsyringes(glass&plastic),vials,andcartridges
⚪ Optionalvacuum filling&stoppering
⚪ StatisticalIPCforconsistentquality
⚪ Handleswaterytohighlyviscous,non-toxictotoxicproducts




OPTIMApharmaGmbH 74523SchwaebischHall|Germany|pharma@optima-packaging.com|www.optima-packaging.com/pharma
OPTIMAIndiaPackagingMachinesPvt.Ltd. unit110,1stfloor|BrigadeRubix,PlotNo.20|HMTmainroad|Bangalore560013|info-in@optima-packaging.com|Phone:+918046525900|www.optima-packaging.in
pharma innovation is clearly rising, the ecosystem still needs strong policy scaffolding to help startups translate their ideas into viable, scalable businesses.
According to Jain, one of the most immediate needs is more accessible capital; both domestic and international, including FDI, NRI funding, and cross-border venture capital.
Government efforts like the Promotion of Research & Innovation in Pharma MedTech (PRIP) scheme and the newly launched Research Development and Innovation (RDI) scheme are timely and welcome. However, he notes that the current funding cap of ?1 crore per startup under PRIP may not be sufficient. Suggests that grants in the ?5–8 crore range would be more effective in helping startups reach critical early milestones and build investor confidence. “These are
necessary to unlock subsequent rounds of funding and build investor confidence.”
Bridging the gap
With policy support firming up and investors gradually warming to science-driven ventures, the conversation naturally turns to the role of startups in India’s pharma ecosystem. Are they peripheral actors or could they become critical enablers of innovation?
According to Mahadevan, “pharma startups play a crucial, complementary role in India’s broader pharmaceutical ecosystem by filling innovation gaps and injecting agility into the industry.” While large pharma companies continue to dominate high-volume manufacturing and distribution, startups bring a sharper focus to untested ideas, emerging technologies, and high-risk research that big players may ini-
tially shy away from.
He adds that Indian startups often act as “innovation scouts for the industry,” working on new drug targets, AIpowered discovery tools, or advanced drug delivery systems. These ventures don’t just introduce new ideas, they often make them viable enough for scale, licensing, or acquisition. In doing so, they “significantly expand the pipeline of potential treatments that large pharma can eventually bring to market.”
However, despite this symbiotic potential, scalability remains a critical concern. Jain points out that India has “India has many essential pillars in place: world-class scientific talent, a growing base of academic–industry partnerships, and an evolving regulatory framework.” Yet startups often struggle to scale domestically. He highlights two barriers: lim-
ited domestic risk capital and a “cultural bias against failure.”
As a result, many startups register overseas to access what Jain calls “patient capital, mentorship, and global markets.”
To counter this trend, he recommends fostering a more supportive environment through policy and financial mechanisms. “Indian investors must adopt a higher-risk, highreward mindset, supported by policy guarantees like the RDI scheme, targeted grants, IP protections, and public–private translational hubs.”
Mahadevan reinforces this point, acknowledging that “pharma startup investment has lagged behind healthtech, primarily due to longer development timelines, higher costs, stricter regulation, and a historically lukewarm investor ecosystem for deep science ventures.”
However momentum is
building. Deloitte concludes on an optimistic note, “The good news is that this gap is starting to narrow as stakeholders recognise the immense longterm value pharma innovation can create. Investors are slowly gaining confidence as they see success stories and as government funds help de-risk early stages. Pharma entrepreneurship is inherently a long game, but with supportive trends now in motion, it may begin to catch up to the buzz of healthtech in the coming years.”
Together, these insights suggest that while Indian pharma startups are still finding their footing, they are well-positioned to plug innovation gaps, bring agility, and enable India’s shift up the value chain.
neha.aathavale@expressindia.com
nehaaathavale75@gmail.com
Swaroopa Bhatkar, Independent Researcher highlights that India’s aesthetic medicine and wellness industry,a fast-growing sector thrives in a legal grey zone,raising urgent questions about safety and accountability
Apandemic of oversight— but not for beauty
In 2020, as the world tackled emergency vaccine approvals and global headlines dissected clinical trials, in India, another kind of medical industry was flourishing in silence. Cosmetic clinics, offering facelifts, liposuctions, and hair transplants, continued to grow unchecked. Unlike pharmaceutical companies, which faced intense scrutiny during COVID, these beauty businesses operated in a legal grey zone, without dedicated regulations, licensing protocols, or standardized safety measures. Five years later, the story hasn’t changed.
The recent Express Pharma article, ‘Beauty Boosters and the Law: Who’s Watching India’s Wellness Market?’ (July 2025), reflects a regulatory landscape that seems to have remained largely unchanged over the past five years. The

concerns it raises, lack of oversight, the marketing of invasive treatments as wellness offerings, and blurred boundaries between medicine and consumer aspiration, strikingly mirror the patterns documented in a 2020 sociological study on India’s cosmetic surgery sector. That these parallels persist half a decade later suggests that the gaps noted then have not just endured, but perhaps deepened.
The question is no longer whether this industry is growing. It’s why we still aren’t watching.
During my master’s thesis (Engineered Beauty, 2020), I interviewed doctors and clients in Mumbai and Ahmedabad to study the cosmetic surgery boom. What I found was less about vanity and more about
social performance. Middleclass consumers were driven by workplace pressures, marriage markets, and digital self-presentation. Clinics operated like salons, offering EMI options and festival discounts. Regulation was almost invisible as patients signed consent forms, but risk disclosures were rarely explained in full.
Now, five years later, Express Pharma reports the same dynamics, only with new packaging. IV drips and injectable “wellness” boosters are marketed with the same aspirational language and unchecked claims. And just like in 2020, the rules are either too broad or too absent to matter. Additionally, influencers, not doctors, are shaping medical decisions.
Whythe regulation gap persists
A 2025 blog from SRMIST on aesthetic medicine confirms
that India has no specific regulatory framework for cosmetic or aesthetic procedures. They are loosely governed by the Indian Medical Council Act and general medical ethics, but no separate licensing body or regulatory mechanism exists to address this growing sector. This legal vacuum creates a two-tier reality; Firstly, Aesthetic procedures borrow the legitimacy of medicine, yet escape the strict oversight applied to pharmaceuticals or clinical healthcare. Secondly, Safety standards, advertising claims, and practitioner qualifications remain largely self-regulated.This raises an important question for the broader healthcare and pharmaceutical community: as a medically driven industry continues to expand rapidly, how can we ensure it is accompanied by frameworks that uphold quality, safety, and
Cosmetic medicine in India sits at the intersection of aspiration and clinical practice,but without a regulatory framework that reflects this duality.As the industry evolves,so must our approach to governing it,not to curb its growth,but to ensure that innovation is met with responsibility,and that choice is backed by care
ethical accountability?
The wellness boom: From surgeryto serums
The rise of injectable skin boosters and “glow” therapies represents the natural evolution of what I observed five years ago. The middle-class desire to “upgrade” the body has shifted from the scalpel to the syringe. While procedures are
marketed as “minimally invasive,” the risks - from infections to long-term side effects are real, especially in clinics where medical training is questionable.In 2020, the cosmetic surgery market was projected to grow exponentially. In 2025, the numbers are even larger, fueled by social media trends, influencer marketing, and post-pandemic wellness narratives. Yet,
the regulatory conversation lags far behind the business curve.
The unasked questions
Why does India hold pharma to rigorous compliance audits while allowing the aesthetic sector to operate in shadows?
Shouldn’t procedures that pierce skin, inject chemicals, or alter facial structure be moni-
tored as closely as any medical device?
These are not abstract questions. In both 2020 and 2025, the absence of oversight means the consumer bears the risks, often unknowingly. Cosmetic medicine in India sits at the intersection of aspiration and clinical practice, but without a regulatory framework that reflects this duality. As the industry evolves, so must our approach to governing it, not to curb its growth, but to ensure that innovation is met with responsibility, and that choice is backed by care. It’s not about slowing down the industry. It’s about giving it the structure it needs to grow safely, ethically, and with the confidence of those it serves.
The mirror may flatter, but it won’t protect. And a blind spot, left long enough becomes the norm.

Gourab Ray, Category Buyer,Sanofi Healthcare India,draws from his experience and real-world challenges,to explain how digital twins bring predictive power and resilience to vaccine trial procurement
Having spent significant years navigating the complex landscape of pharma procurement across developing countries, I've witnessed firsthand the challenges that can make or break vaccine clinical trials. From equipment failures in the Philippines that compromised an entire cold chain to customs delays in Malaysia and Vietnam that held up critical trial supplies for weeks, these experiences have taught me that traditional procurement approaches simply aren't equipped for the unique demands of vaccine trials in resource-constrained environments.
The emergence of digital twin technology represents what I believe to be the most significant advancement in procurement risk management since the introduction of enterprise resource planning systems. Unlike conventional monitoring tools, digital twins create comprehensive virtual replicas of entire procurement ecosystems, enabling us to predict problems before they occur and optimise resource allocation in ways that were previously impossible.
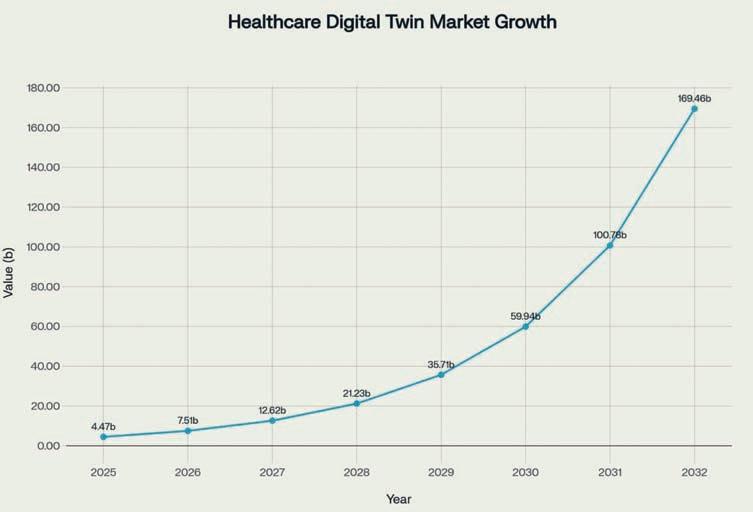
The global healthcare digital twin market's explosive growth—from $4.47 billion in 2025 to a projected $169.46 billion by 2032—reflects an industry-wide recognition that these technologies are no longer experimental but essential for competitive advantage¹. For vaccine trials in developing countries, where infrastructure limitations and regulatory complexities create cascading operational risks, digital twins offer a path toward truly resilient procurement operations.
My experience managing vaccine trial procurement across Bangladesh, Pakistan, Nepal, India, Vietnam, Philippines, Indonesia, and Malaysia has revealed consistent patterns of vulnerability that traditional approaches struggle to address.
The procurement of services, materials, goods, ancillary supplies, software, and medical devices for clinical trial sites involves coordinating hundreds of suppliers, managing complex regulatory requirements, and ensuring cold chain integrity across diverse geographical and infrastructure conditions.
The challenges extend far be-

but also the manufacturing schedules of local suppliers. In Nepal, the 18-month preparation time required to establish Phase
These aren't isolated incidents—they represent systemic challenges that require systemic solutions.
The COVID-19 pandemic amplified these vulnerabilities dramatically. Supply chain disruptions that might cause minor delays in developed markets can halt entire trials in developing countries, where alternative suppliers and logistics networks are limited. The interdependence of global supply chains means that a manufacturing disruption in China can affect API availability in India within days, while regulatory changes in one country can create bottlenecks that ripple across entire regional trial networks.
yond simple logistics. In Bangladesh, I've seen how unreliable electricity supply affects not just cold chain equipment
III vaccine trial capabilities demonstrates how human resource constraints compound procurement complexities².
Digital twin technology transforms procurement from a reac-
tive discipline into a predictive science. Rather than responding to disruptions after they occur, digital twins enable us to model potential scenarios, test mitigation strategies, and optimise resource allocation before problems manifest in the real world.
The technical foundation encompasses IoT sensors that monitor critical parameters such as temperature, humidity, location, and equipment status throughout vaccine supply chains. These sensors provide continuous data streams that feed into machine learning algorithms, enabling early detection of potential disruptions and proactive intervention strategies. Cloud infrastructure provides the computational power necessary for real-time simulation and scenario modeling, while integration with existing ERP systems ensures seamless data flow and comprehensive supply chain visibility.
What excites me most about this technology is its ability to address the specific challenges I've encountered in developing country procurement. When I consider the customs delays I've experienced in Vietnam, digital twins can model entire clearance processes, incorporating historical data on clearance times, current port congestion levels, and regulatory changes. This capability enables proactive identification of potential delays and implementation of alternative strategies before critical materials are affected.
Strategic framework: The enhanced Kraljic Matrix
The integration of digital twin technology with the Kraljic Matrix provides a robust framework for categorising and managing procurement items based
on supply risk and profit impact. This established tool, when enhanced with real-time digital twin capabilities, transforms static risk assessments into dynamic, predictive management systems.
Strategic items in vaccine trials include ultra-specialised cold chain equipment, rare vaccine components, and highly trained clinical personnel. These items are characterised by high supply risk and high profit impact, making their management critical for trial success. Digital twins enable predictive maintenance of refrigeration systems through continuous monitoring of compressor performance, temperature fluctuations, and power consumption patterns. Machine learning algorithms can identify subtle patterns indicating impending equipment failure, enabling proactive replacement before critical failures occur.

Bottleneck items such as specialized medical devices, regulatory documentation services, and logistics support services represent hidden vulnerabilities that can halt entire trials despite their relatively low cost. Digital twins excel at identifying and monitoring these vulnerabilities through comprehensive supply chain visibility and predictive analytics.
Leverage items including standard medical supplies, basic laboratory equipment, and commodity services represent opportunities where procurement teams can exercise significant buying power. Digital twins enhance leverage item management through real-time market intelligence and demand forecasting, enabling optimal timing of purchases and supplier negotiations.
Non-critical items often become critical in remote trial










•Monitoringoptimaltemperatureandhumiditylevelstoensure thehighestsafetyandqualityofsensitivegoods


•BluetoothconnectivityandUSB(optional),bothareavailable.


•16,000readingwitheasytraceabilityofallmeasurementvalues throughthefreetestoSmartApp
•Maximumflexibilitywithextremelysimpleoperation.
•Idealforpharmaceuticallab,storage,productionandhospital testoSmartApp forfreedownload




locations where replacement is difficult. Digital twins optimise inventory levels by balancing carrying costs with stockout risks in challenging logistics environments, incorporating consumption forecasting based on trial progress, seasonal variations, and local conditions.
Through my analysis of industry implementations and discussions with colleagues who have piloted digital twin technologies, the measurable improvements across critical procurement areas are remarkable. The data demonstrates significant enhancements in operational efficiency and risk mitigation capabilities.
Site capability assessment shows the highest improvement potential, with organisations reporting up to 75 per cent enhancement in their ability to evaluate and optimise site capabilities before trial initiation³. This improvement directly addresses one of the most significant challenges I've encountered—understanding true site capabilities beyond what appears on paper.
Equipment procurement planning benefits significantly from digital twin technology, with 65 per cent improvement in forecasting accuracy and supplier management. This enhancement is particularly critical for complex equipment like ultra-low temperature freezers and specialized laboratory instruments, where procurement lead times can extend 6-18 months in developing countries.
Cold chain management represents another area of substantial improvement, with digital twin technology enabling 60 per cent enhancement in temperature monitoring, predictive maintenance, and route optimization. This capability is essential for maintaining vaccine integrity throughout the supply chain, particularly in regions with unreliable electricity and challenging transportation infrastructure.
One of the most frequent questions I receive from colleagues
concerns the investment requirements for digital twin implementation. Based on my research and industry benchmarking, the framework requires clear understanding of scale, scope, and financial responsibility across different implementation levels.
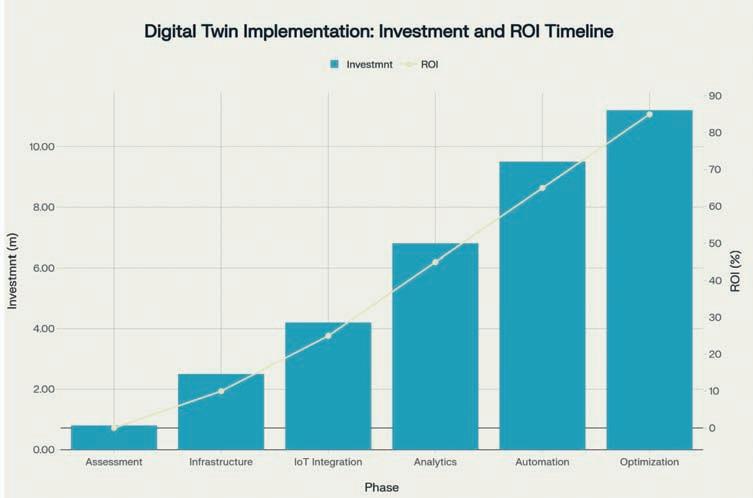
Per clinical trial site investment ranges from $25,000 to $50,000 per site in developing countries, covering IoT sensors, monitoring equipment, staff training, and basic connectivity infrastructure4. This represents only 0.1-0.2 per cent of typical Phase III trial costs while delivering 15-30 per cent efficiency improvements.
Per trial implementation for multi-site programmes requires $500,000 to $2,000,000 investment, covering single trials with 10-50 sites across multiple developing countries. This represents 1.5-6 per cent of Phase III trial budgets but delivers measurable procurement optimisation and risk reduction.
Company-wide digital twin platform requires $11.2 million total investment over 31 months, providing portfolio-wide capabilities across 5-10 concurrent trials. This represents 3.7-7.5 per cent of total portfolio value while delivering transformational capabilities and 85 per cent ROI upon full deployment.
The 2025 announcement requiring all new vaccines to undergo placebo-controlled clinical trials creates additional procurement challenges that digital twin technology can help address5. This "radical departure from past practices" increases trial complexity, duration, and resource requirements, particularly affecting developing country implementations where ethical considerations around placebo use are most complex.
Digital twin technology enables pharma companies to model the impact of extended trial durations on procurement requirements, optimise inventory management for longer study periods, and enhance supplier relationship management to ensure reliable supply throughout extended trials. The technology's simulation capabilities allow organisations to assess the procurement implications of placebo requirements before trial initiation.
Sanofi's substantial investment in digital twin technology provides compelling evidence of the technology's strategic value. The company's €900 million investment in EVolutive vaccine facili-
ties and advanced virtual patient population technologies demonstrates significant commitment to digital transformation6. The collaboration with Dassault Systèmes has enabled virtual testing of manufacturing processes before physical implementation, directly relevant to clinical trial supply chain challenges.
The company's digital twin implementations in manufacturing facilities—including predictive maintenance, process optimisation, and quality monitoring—provide direct parallels for site procurement applications. These capabilities enable remote adjustment of production parameters, virtual training for operators, and realtime supply chain optimisation, all of which translate effectively to managing procurement operations across distributed trial sites.
My experience across different developing country contexts has taught me that digital twin implementation must account for specific regional challenges and opportunities. South Asian countries including Bangladesh, India and Sri Lanka face particular challenges related to regulatory complexity, infrastructure reliability, and skilled personnel
availability.
India's substantial pharmal infrastructure provides opportunities for digital twin implementation at scale, but requires sophisticated approaches to manage complex supplier networks and regulatory requirements. The country's experience with clinical trials demonstrates both the potential for successful implementation and the need for comprehensive planning.
Southeast Asian countries including Vietnam, Philippines, Indonesia, and Malaysia present unique opportunities for digital twin implementation, particularly in cold chain management and equipment procurement. These countries' improving infrastructure provides a foundation for technology implementation while their growing pharma sectors create demand for advanced procurement capabilities.
African countries face the most significant infrastructure challenges but also represent the greatest opportunity for digital twin impact. The technology's ability to optimise procurement strategies in resource-constrained environments makes it particularly valuable for addressing the continent's vaccine trial procurement challenges.
Early digital twin adopters will gain valuable experience and develop capabilities that become increasingly difficult for competitors to replicate. The technology positions organisations for long-term competitive advantage in an increasingly digital healthcare landscape while contributing to improved global health outcomes.
Organisations should prioritise pilot implementations in high-risk, high-impact procurement categories where digital twin technology can demonstrate immediate value. This targeted approach enables rapid capability development while building organisational confidence for broader implementation.
Collaboration with established technology partners accelerates implementation timelines while reducing technical
risks. Strategic partnerships with proven digital twin providers offer access to validated platforms and implementation expertise, enabling faster time-to-value realisation for developing country vaccine trial operations.
Conclusion
The integration of digital twin technology with strategic procurement frameworks represents more than operational improvement—it's about fundamentally transforming how we approach the complex challenges of vaccine trial procurement in developing countries. My eight years in this field have convinced me that traditional approaches, while valuable, simply cannot address the dynamic, interconnected nature of modern global health challenges.
The convergence of market conditions, technology maturity, and proven business value creates an optimal adoption window for digital twin technology. Organisations that move decisively to embrace these capabilities will establish significant

Rather than responding to disruptions after they occur,digital twins enable us to model potential scenarios,test mitigation strategies,and optimise resource allocation before problems manifest in the real world
competitive advantages in vaccine trial procurement, ultimately contributing to improved global health outcomes and pharmaceutical innovation leadership. For those of us committed to advancing global health through innovative vaccine development, the question isn't whether to adopt digital twin technology, but how quickly we can implement it effectively. The technology offers a path toward truly resilient, predictive procurement operations that can navigate the complexities of developing country environments while ensuring the success of critical vaccine trials.
References
1. Markets and Markets. "Digi-

tal Twins in Healthcare Market by Application, Technology, End User - Global Forecast to 2030." Healthcare Market Research Reports, 2025.
2. HCP Live. "The Challenges of Vaccine Research in Developing Nations." Healthcare Communications, February 5, 2021.
3. Applied Clinical Trials. "How Digital Twins are Driving Sustainability in Clinical Supply Planning." Clinical Research Publications, June 18, 2025.
4. SSRN. "Real-Time Health Supply Chain Optimization Using Digital Twin Technology." Supply Chain Research Network, February 17, 2025.
5. The Vaccine Reaction. "'All New Vaccines' in the U.S. to Undergo Placebo-Controlled Safety Testing." Vaccine Policy Analy-
sis, May 28, 2025.
6. GSK. "Digital twin: using advanced technology to accelerate vaccine development." GSK Behind the Science Magazine, May 30, 2022.
7. PMC. "TWINVAX: conceptual model of a digital twin for immunisation services in primary health care centres."
PubMed Central, May 14, 2025.
8. Roots Analysis. "Digital Twins in Healthcare Market Size, Growth Analysis and Forecast 2024-2035." Healthcare Technology Reports, 2025.
9. IQVIA. "Clinical Data Analytics Solutions: Intelligent Document Review." Clinical Research Technology, 2025.
10. Deloitte. "End-to-End Digitalization of BioPharma Supply Chain." Healthcare Industry In-

Hall1,Booth:B01 18-20Sept,2025 HITEXExhibitionCentre Hyderabad
sights, 2025.
11. MIT Sloan Management Review. "Unlocking the Potential of Digital Twins in Supply Chains." Supply Chain Innovation, August 18, 2022.
12. Supply Chain Digital. "IoT: The Driving Force For Cold Chain Vaccine Distribution." Logistics Technology, July 11, 2021.
Disclaimer: The views, opinions, and insights expressed in this article are solely those of the author and are based on publicly available information and general industry practices. These views do not represent the official position, policies, or strategies of Sanofi Healthcare India Pvt. Ltd. or any of its affiliates. No proprietary, confidential, or trade secret information has been disclosed in this publication. The author has written this article in his personal capacity as an industry professional and any recommendations or conclusions are his own professional insights derived from experience in pharmaceutical procurement and supply chain management.




Website:www.srico-labworld.com|Telephone:+919900674407|Email:info@srico-labworld.com Bengaluru|Mumbai|Hyderabad|Bhubaneswar|Vadodara|Delhi
Chennai|Goa|Thiruvananthapuram|Pune|Visakhapatnam|Kolkata|Guwahati|Ahmedabad|Chandigarh|Lucknow



Industry leaders and innovators convene to decode the future of injectable therapies and showcase India’s growing strength in high-value injectables
The Injectable Innovations Conclave 2025 brought together leading minds to explore the next frontier in injectable drug delivery. The conclave featured breakthrough technologies, real-world case studies and thought-provoking discussions on manufacturing excellence, regulatory readiness, and the role of digital transformation in enhancing injectable therapies.
It began with a welcome address by Viveka Roychowdhury, Editor of Express Pharma, Express Healthcare, and Express Nutra, who outlined the vision behind the conclave. She highlighted the immense growth potential in the injectables market, driven by an aging global population, the increasing burden of chronic diseases, supportive policy shifts, and the wave of patent expirations opening up new opportunities for generics.
The welcome address was followed by the ceremonial lamp lighting, a moment that symbolised the official start of this knowledge-sharing initiative. Roychowdhury was joined by a distinguished group of industry leaders and partners.

L-R: Viveka Roychowdhury,Editor of Express Pharma,Express Healthcare,and Express Nutra; Klaus Braig,Global KeyAccount Manager, Uhlmann Pac-Systeme GmbH&Co.and MD,Uhlmann Switzerland;; Vidwans Rajendra Ramachandra,Senior Consultant – GMP,QA, and Sterile Dosage Forms,GAP& Consultant,Ami Polymer; ShaunakJ.Dave,MD & CEO,Antares Vision; Rajesh Bhatkal,GMExpress Pharma,Express Healthcare,and Express Nutra
Dr Pavan Bhat, CEO, Inventia Healthcare, set the tone for the day with a compelling presentation on the need to reimagine injectables through the lens of patient-centricity, technological precision, and environmental sustainability.
Dr Bhat emphasised that injectables are the lifeline of modern medicine, holding a dominant position in critical care, biologics, and vaccines. However, their growing importance is hindered by inherent challenges like working with fragile molecules, complex formulations, and the integration of increasingly sophisticated delivery devices.
As molecule diversity expands, so too does formulation complexity. This requires inn ovation that solves scien-

Dr Pavan Bhat,CEO,Inventia Healthcare
tific challenges while aligning with patient needs and considering environmental impact. Dr Bhat stressed the importance of aligning India’s pharma innovation agenda with a long-term vision that nurtures clusters to innovate, funding to de-risk, regulation to accelerate, and digital tools to scale.
By using the example of the evolution of semaglutide, from injectable to oral, from managing diabetes to tackling obesity, he explained how this stands as an example of what’s possible when scientific ingenuity, advanced delivery systems, and patient-focused design align. According to Dr Bhat, this kind of thinking is essential if India is to lead the next era of injectable innovation, not just for the patient, but for the planet.


L-R: Sandeep Amritkar,Assistant Director,Lupin Pharmaceuticals (MODERATOR); Dr Amarender ReddyDonthidi,VP& Head - R&D Injectables and Ophthalmics,Amneal Pharmaceuticals; Dr Khalid Akhter Ansari,Senior Director,Rising Pharmaceutical; Mayurbhai Sankalia,VP- Research & Development,Invengene; Loganathan S,AVP,Onesource Biopharma; Dr Ramesh Nagarwal,VPComplexInjectable,BDR Pharma and Dr Rakesh Kumar Sinha,VP& Head - Drug Product Manufacturing,Biological E
The first panel at the Injectable Innovations Conclave centred on how the future of injectables is increasingly defined not only by the molecule but also by how effectively, painlessly, and precisely it reaches the patient. The experts across pharmaceutical formulation, device design, and drug delivery systems explored the shifting paradigm in injectable therapies.
The panel was moderated by Sandeep Amritkar, Assistant Director, Lupin Pharmaceuticals. Panellists included Dr Amarender Reddy Donthidi, VP & Head - R&D Injectables and Ophthalmics, Amneal Pharmaceuticals; Mayurbhai Sankalia, VP - Research & Development, Invengene; Dr Ramesh Nagarwal, VP Complex Injectable, BDR Pharma; Rakesh Kumar Sinha, VP & Head - Drug Product Manufacturing, Biological E; Loganathan S, AVP, Onesource Biopharma; Dr Khalid Akhter Ansari, Senior Director, Rising Pharmaceuticals
The panellists began by defining how injectable therapies are moving toward more refined, painless delivery systems that prioritise patient-centricity. This transition is especially vital as therapies become more targeted and biologically complex. The shift from traditional vials and syringes to prefilled syringes, autoinjectors, and needle-free systems reflects a broader industry commitment to ease of use and reduction of injection-related anxiety.
Long-acting and extended-release formulations also emerged as a key innovation frontier. By reducing the frequency of administration, these formulations are improving patient convenience, enhancing adherence and clinical outcomes. Panellists discussed how these solutions are particularly beneficial in therapeutic areas where consistent dosing is critical but often challenging to maintain with conventional regimens.
The discussion further highlighted that the design of the injectable deliv-
ery system has become a key differentiator in today’s pharmaceutical landscape. How the drug is being delivered is becoming increasingly important. Devices must now be intuitive, ergonomic, and designed with the patient in mind, ensuring precise, targeted delivery while upholding the therapeutic intent of the drug. Whether it’s a caregiver administering treatment at home or a patient self-injecting, the delivery device must offer ease, comfort, and confidence.
Accessibility was another crucial matter of discussion for the panellists. As therapies become more complex and personalised, panelists stressed the importance of innovating for affordability and scalability. The challenge lies in delivering high-tech solutions without inflating costs, especially in emerging markets like India. The panel advocated for designing with manufacturing in mind from the outset, ensuring that delivery systems are effective, while easy to produce, assemble, and
distribute at scale.
The panel discussed the rise of smart injectables and connected autoinjectors, and how these technologies enable real-time monitoring, personalised dosing, and data sharing with healthcare providers. The integration of digital technology with injectables is also fostering a proactive approach to treatment, where adherence, adverse events, and therapy responses can be monitored and managed remotely.
To round up the conversation, the panellists agreed that while innovation is inevitable, sustainability must become necessary. With increasing awareness of environmental impact, innovations such as green needles, autoinjectors, or interconnected injectable systems are gaining attention. Panellists concluded that the future of injectables lies in systems that are not only clinically effective but also sustainable, affordable, and centred on the end user. Together, these drivers are reshaping injectable therapies.
Vidwans Rajendra Ramachandra, Senior ConsultantGMP,QA, in Sterile, Non-sterile dosage forms, GAP & Consultant, Ami Polymer, offered a critical lens into recurring audit observations specific to injectable dosage forms, drawing from his extensive consulting experience. He highlighted that many Standard Operating Procedures (SOPs) in injectable manufacturing are often overly brief and vague, failing to offer the detailed, actionable guidance necessary for the consistent execution of complex and critical processes. This lack of specificity not only introduces variability but also exposes operations to compliance risks during audits.
He pointed out that while SOPs define alarm systems, there is frequently a gap in real-time monitoring and response, undermining real-time quality control and risk management. Another area of concern, stressed by Rajendra, was the weak implementation of data integrity programmes. In many facilities, the SOPs for data integrity lacked clarity on roles, review frequen-

cies, and procedures for audit trail checks, raising red flags about data reliability and regulatory compliance. Rajendra also underscored that Out-of-Specification (OOS) investiga-
tions were often incomplete or superficial, lacking a structured root cause analysis. This weakens the quality system and fails to generate preventive insights, making repeat deviations more
Many Standard Operating Procedures in injectable manufacturing are often overly brief and vague,failing to offer the detailed, actionable guidance necessary for the consistent execution of complex and critical processes
likely. His session served as a strong reminder to re-examine compliance fundamentals with greater rigour.
At the IIC 2025, Nitin Khaladkar, Head R&D (Rubber stopper), Bharat Rubber Works, delivered an insightful session on the critical role of Moisture Vapour Transmission Rate (MVTR) in pharmaceutical packaging, highlighting its direct impact on product quality, stability, and shelf life. He explained that MVTR is a key parameter in pharma, as it determines the ability of a container or closure system to prevent moisture ingress over time. This is especially important for moisture-sensitive formulations, where even minimal exposure can compromise product efficacy and safety.
Khaladkar emphasised that temperature and humidity are the primary factors influencing MVTR. Elevated temperatures increase molecular movement, thereby accelerating vapour

Nitin Khaladkar,Head R&D (Rubber stopper),Bharat Rubber Works
transmission through packaging materials. Similarly, a higher humidity gradient across the packaging barrier creates a stronger driving force for moisture to penetrate, making it essential to account for these variables during package design and material selection.
He further stressed that MVTR performance cannot be evaluated in isolation; it must be validated across the entire supply chain, from manufacturing through storage and distribution. Variations in environmental conditions at each stage can undermine the effectiveness of even the best-engineered packaging systems. Beyond being a technical consideration, MVTR compliance is a regulatory mandate, essential for ensuring product safety, maintaining efficacy, and adhering to global pharma and food safety standards.

Akey session at Injectable Innovations Conclave 2025 focused on solutions that enable sustainable, traceable, and flexible packaging for parenteral products in pharma manufacturing.
The session, titled 'Sustainable, flexible, and traceable solutions for parenteral packaging', was led by Klaus Braig, Global Key Account Manager, Uhlmann PacSysteme GmbH & Co. and Managing Director, Uhlmann Switzerland. The session addressed what innovations are shaping parenteral packaging, who is providing integrated solutions, where these systems are being deployed globally, when they are being adopted in manufacturing processes, why adaptability and traceability are critical for sterile products, and how modular designs are enabling operational agility.
Uhlmann Group, with over 75 years of experience and presence in 21 global loca-

tions, shared its capabilities in supporting pharma manufacturers with packag-
ing solutions tailored for parenteral dosage forms. The company provides
flexible packaging systems designed to accommodate varied product types and evolving regulatory requirements.
The session introduced Uhlmann’s Parenteral Tray Center (PTC 200), a modular packaging system that delivers up to 200 units per minute. The PTC 200 enables quick format changes and easy system expansion, allowing manufacturers to adapt production lines based on demand and product types.
Braig also covered how Uhlmann supports partners through the integration of automation, sustainability measures, and digital tools in the packaging process. These offerings enable traceability, operational efficiency, and regulatory compliance in sterile injectable production.
By combining modular system design with holistic support across the packaging lifecycle, Uhlmann contributes to building responsive, compliant, and efficient pharma packaging ecosystems.
The session titled, ‘New Frontiers of Inspection Technologies (Visual Inspection and CCIT) with Artificial Intelligence’, at the Injectable Innovation Conclave 2025 focused on how artificial intelligence is being used to advance inspection systems in sterile injectable manufacturing.
Presented by Shaunak J. Dave, Managing Director and CEO, Antares Vision. The presentation addressed what new technologies are being applied in inspection systems, who is leading these developments, where they are being deployed, when they are being used across the product lifecycle, why they are needed to ensure sterility and compliance, and how these systems operate across functions.
Antares Vision highlighted its wide portfolio of inspection systems that support sterile injectable manufacturing from early development to commercial production. These systems range from

ShaunakJ.Dave,MD & CEO,Antares Vision
lab-scale tools to fully automated highthroughput platforms. The company has strengthened its capabilities in this space through acquisitions such
as Convel. Dave also outlined key technological features in Antares Vision’s systems. These include Short-Wave Infrared
(SWIR) technology, which enables reliable fill level detection under challenging conditions, and Automatic Visual Inspection (AVI) systems that detect particulate matter, vial cracks, and seal issues that could affect sterility.
In addition to inspection, Antares Vision Group provides integrated platforms in traceability, product and supply chain data management, and generative AI. These platforms include tools such as serialisation, aggregation, real-time IoT, authentication, warehouse management, business intelligence, cloud computing, and big data analysis.
The company’s multi-technology hub spans camera-based systems, laser and absorption spectroscopy, high voltage, sensor-based detection, and microwave solutions. The session demonstrated how these technologies work together to ensure quality, safety, and traceability in injectable manufacturing.
At the recently concluded Injectable Innovations Conclave, Chiranjeevi Kondapaka, CEO, Steriline Asia, gave a compelling presentation titled “Inn ovative Solutions by Steriline.” The session showcased the company’s approach in addressing the evolving needs of the injectable pharmaceuticals market.
He presented Steriline’s “Zero Loss Philosophy” as a cornerstone of future-ready manufacturing. The approach fouses on minimising wastage across production stages through automation and integrated process control.
The session highlighted the increasing complexity in injectable product portfolios. Kondapaka pointed out how pharma manufacturers are expected to accommodate productspecific requirements, shorter turnaround times, and in-line sterilisation

Kondapaka,CEO,Steriline Asia
protocols without compromising on throughput or compliance.
He also informed that through advanced robotics and closed-system designs, the company aims to eliminate costly product losses and human error while enhancing batch integrity. These innovations are particularly relevant for high-value biologics, where even minor losses can result in significant cost implications.
Kondapaka also presented Steriline’s latest solutions that are designed to handle a variety of container formats, for pharma companies to swiftly adapt to changing production needs.
The session concluded with the speaker asserting Steriline’s commitment to continuous innovation in aseptic manufacturing. The company aims to be a key enabler in the transition toward next-gen injectable drug production.
❒ Express Pharma accepts editorial material for regular columns and from pre-approved contributors / columnists.
❒ Express Pharma has a strict nontolerance policy of plagiarism and will blacklist all authors found to have used/refered to previously published material in any form,without giving due credit in the industry-accepted format. All authors have to declare that the article/column is an original piece of work and if not,they will bear the onus of taking permission for re-publishing in Express Pharma.
❒ Express Pharma's prime audience is senior management and pharma professionals in the industry.Editorial material addressing this audience would be given preference.
❒ The articles should cover technology and policy trends and business related discussions.
❒ Articles for columns should talk about concepts or trends without being too company or product specific.

❒ Article length for regular columns: Between 1200 - 1500 words.These should be accompanied by diagrams, illustrations,tables and photographs, wherever relevant.
❒ We welcome information on new products and services introduced by your organisation for our various sections: Pharma Ally (News,Products, Value Add),Pharma Packaging and Pharma Technology Review sections. Related photographs and brochures must accompany the information.
❒ Besides the regular columns,each issue will have a special focus on a specific topic of relevance to the Indian market.
❒ In e-mail communications,avoid large document attachments (above 1MB) as far as possible.
❒ Articles may be edited for brevity,style, and relevance.
❒ Do specify name,designation,company name,department and e-mail address for feedback,in the article.
❒ We encourage authors to send their photograph.Preferably in colour, postcard size and with a good contrast.
Email your contribution to: The Editor, Express Pharma, Business Publications Division, The Indian Express (P) Ltd, Mafatlal Centre,7th floor, Ramnath Goenka Marg, Nariman Point,Mumbai 400021 viveka.r@expressindia.com viveka.roy3@gmail.com


L-R: Vidwans Rajendra Ramachandra,Senior Consultant -GMP,QA,in Sterile,Non-sterile dosage forms,GAP(MODERATOR); Leena Padala,Head-Packaging Development & OpEx- R&D, Eugia Pharma Specialities; Dr Bhaskar Chauhan,Leading R&D and QualityManagement,Puniska Injectables; Rajesh Kumar Chopra,President-Operations,ICHOR Biologics; Kiran Deshmukh,Site Head-Sterile Injectables,Lupin Pharmaceuticals; Tridip Mazumder,Head Packaging Development- Sterile,Dr Reddy's Laboratories; Prasadha Rao Lysetti,VP– Operational Excellence,Sterile Injectable Operations,Rising Pharmaceuticals
Apanel discussion on ‘Injectable Packaging 4.0: Securing Quality, Stability and Compliance’ was held as part of the inaugural Injectable Innovations Conclave 2025. The session focused on the role of injectable packaging in maintaining sterility, product stability, and ensuring safety across the lifecycle of injectable drugs.
The panel was moderated by Vidwans Rajendra Ramachandra, Senior Consultant - GMP and QA in sterile and non-sterile dosage forms, GAP. The panellists included Leena Padala, HeadPackaging Development & OpEx - R&D, Eugia Pharma Specialities; Dr Bhaskar Chauhan, Leading R&D and Quality Management, Puniska Injectable; Rajesh Kumar Chopra, President - Operations, ICHOR Biologics; Kiran Deshmukh, Site Head - Sterile Injectables, Lupin Pharmaceuticals; Tridip Mazumder, Head - Packaging Development - Sterile, Dr Reddy’s Laboratories; and Prasadha Rao Lysetti, VP – Operational Excellence, Sterile Injectable Operations, Rising Pharmaceuticals.
Injectable packaging plays a critical role in maintaining product stability,sterility, and safety throughout its lifecycle
The panel highlighted that injectable packaging is central to maintaining product sterility and stability. Ensuring the safety of patients requires consistent attention to packaging systems from development through delivery. The selection of packaging materials and systems must be guided by compatibility with the drug product, with particular attention to the risks of leachables and extractables, which can impact product stability and safety.
One of the key focus areas was the need for cost-effective design in self-injection devices. According to the panellists, such devices must be manufactured in a way that ensures affordability, ease of use, and accessibility. This would
enable wider adoption, particularly for therapies requiring frequent or longterm administration by patients themselves.
The session also underlined that developing safe and compliant packaging systems for injectables requires coordination across multiple functions. From partner selection and material sourcing to labelling, visual inspection, and cold chain management, collaboration is essential for maintaining product quality and compliance. The role of visual inspection, track and trace systems, and cold chain logistics was also discussed in relation to maintaining consistency across global markets.
Advancements in technology are
supporting the evolution of packaging systems. The use of robotic isolators, real-time particle monitoring, and automated machines is helping pharma companies improve sterility, precision, and operational efficiency. These technologies are becoming increasingly important in reducing human intervention and ensuring consistent manufacturing outcomes.
The panel also raised concerns related to the growing use of self-administered devices. Without adequate training and user support, these devices can pose risks in terms of incorrect administration. The experts emphasised the importance of educational interventions and support systems to ensure patient safety, adherence to prescribed therapy, and optimal therapeutic outcomes.
By focusing on safety, quality, collaboration, and innovation, the panel discussion provided a detailed view of how injectable packaging is evolving in response to new demands across the pharma industry.
At the Injectable Innovations Conclave 2025 in Hyderabad, one session was on, “A robust and next gen sterilisation packaging” by Kishor Kale, the Founder and Managing Partner of Sterile World Technologies.
Kale began the session by highlighting a critical challenge faced by pharma and medical device manufacturers, ensuring reliable, safe, and cost-effective packaging for products that require complete sterility. He pointed out how traditional packaging materials often fall short when it comes to performance, cost control, or consistency.
His company’s answer to this challenge is its newly developed White Line packaging material. Kale explained that White Line combines the softness and flexibility of fabric-like material with the strength and barrier protection of plastic films. This makes it ideal for packaging items that need to be kept sterile until the moment of use. It’s especially suited for steam sterilisation and has been designed to open smoothly and cleanly, without leaving any fibres or residue behind.
He informed that what makes White Line especially appealing is its ability to cut packaging costs by up to 20 per cent — a significant benefit for pharma companies managing high-volume produc-

Kishor B Kale,Founder & Managing Partner,Sterile World Technologies LLP
tion. It also lasts for up to five years under proper storage conditions and can be custom-printed with product details or usage indicators.
Another key highlight was its Sterility MZLINE range of header bags and pouches. Kale explained that many
pharma manufacturers face issues with current header bags, such as poor sealing and frequent failures during sterilisation. MZLINE addresses these problems with better design and material choices, offering significant cost savings while maintaining high reliability.
Rakesh Kumar Sinha, VP & HeadDrug Product Manufacturing, Biological E, gave a detailed session on, 'Contamination control strategy: What & How – A Practical Approach' at the Injectables Innovation Conclave.
The session focused on a practical and holistic approach to preve nt contamination in sterile facilities, especially in the context of aseptic filling and stopper hopper installations.
Sinha emphasised that contamination control isn’t just about equipment or protocols, it’s a mindset that must permeate every layer of sterile production. He also presented a case study that centered on the process of installing a sterilised stopper hopper into a Grade A barrier system.
The stopper hopper, which feeds

Rakesh Kumar Sinha,VP& Head-Drug Product Manufacturing,Biological E
Sterile World Technologies can help pharma organisations reduce packaging material by 15-20 per cent through its solutions like Whiteline and MzLine
Kale also spoke about Sterlie World Technologies’ recent partnership with VP Medical Packaging, a German packaging company. Throughout the session, Kale emphasised his company’s commitment to building smarter and safer packaging systems for the future of pharma.
Kale concluded that the company's goal is simple — to reduce cost, increase safety, and make India a global leader in pharma packaging.
rubber stoppers to vials on the automated line, is a vital component, but also a potential point of contamination.
Sinha gave a step-by-step breakdown of the sterilisation, transfer, installation and operational handling of the stopper hopper. He identified specific contamination risks across personnel, environment, method, materials, and machine, and presented control measures that can eliminate, prevent, reduce, or detect risks at each step.
He spoke on environmental monitoring trends, media fill simulations and historical deviations, highlighting how even minute lapses, such as improper gowning or glove integrity failures, can lead to batch rejections or product contamination. The talk concluded with a look at how to structure a robust Contamination Control Strategy (CCS) document.


L-R: Dr Mallinath S Harwalkar,VP- R&D,Hetero (MODERATOR); Raja Sekhar ReddyVanga,VP- Global RegulatoryAffairs,Biocon Biologics; Santosh Katti,VP- Drug Product Operations, Biocon Biologics; Dr Srikanth ReddySokkula,Head - RegulatoryAffairs,Jodas Expoim; Pavankumar R Gudi,Manager,Serum Institute of India
The last panel discussion at Injectable Innovations Conclave 2025 was on a highly pertinent topic, ‘Injectables in focus – Therapeutic areas driving market growth.’ With the injectable segment gaining momentum, the session brought together senior industry leaders to explore trends, challenges, and growth drivers shaping this fast-evolving sector.
The discussion featured a distinguished panel of experts: Dr Mallinath S Harwalkar, VP - R&D, Hetero (Moderator); Raja Sekhar Reddy Vanga, VPGlobal Regulatory Affairs, Biocon Biologics; Santosh Katti, VP - Drug Product Operations, Biocon Biologics; Dr Srikanth Reddy S., Head - Regulatory Affairs, Jodas Expoim; and Pavan Kumar R Gudi, Manager, Serum Institute of India.
The panel highlighted how injectables are becoming the preferred mode of treatment for chronic and high-burden conditions such as cancer, diabetes, autoimmune disorders, and pain. Injecta-
Injectables are now frontline options in several therapeutic areas.Their speed of action and targeted delivery are unmatched,pointed out the panelists.
bles are now frontline options in several therapeutic areas. Their speed of action and targeted delivery are unmatched, pointed out the panelists.
A major focus was on the shift towards patient-friendly formats. From prefilled syringes to auto-injectors and smart delivery devices, innovations are driving better patient adherence and convenience. The discussion highlighted how such formats are essential for market success, especially in self-administered therapies.
Biologics and biosimilars emerged as another key driver of injectable growth.
It was pointed out that the increased adoption of biosimilars is improving treatment access while demanding more sophisticated injectable formats and robust regulatory pathways. Innovation is not just about new molecules, but also about delivery formats that are scalable, affordable and patient-centric, opined the experts.
The discussion also addressed the importance of a resilient and agile supply chain. With many injectable drugs requiring cold chain storage and strict quality control, panelists called for endto-end visibility, real-time monitoring,
and seamless coordination across logistics, manufacturing, and regulatory teams. They emphasised that we need supply chains that are not only compliant but also connected, from factory floor to bedside.
Long-acting injectables were identified as another growth lever, especially in chronic and preventive therapies. These formats reduce dosing frequency, improve adherence, and support better clinical outcomes. They reduce the treatment burden for patients and operational burden for providers, informed the panel.
The session concluded with a consensus that injectables are at an inflection point. Advances in biologics, increasing chronic disease prevalence, and evolving patient expectations are reshaping the sector. Industry leaders stressed the need for continued R&D investment, regulatory agility, and cross-functional collaboration to fully capitalise on this opportunity.













'PARTNERS IN PROGRESS'












'PARTNERS IN PROGRESS'



'PARTNERS IN PROGRESS'

















'PARTNERS IN PROGRESS'












Anthony Mikulaschek, VP- DP&S Patient Commercial Strategy,IQVIA,suggests that to overcome challenges and reduce the potential for costly errors,clinical trials must implement an integrated data strategy utilising a patient suite
Developing a successful clinical trial is like laying a foundation — every brick must be placed with precision. Each tool, workflow and touchpoint must align seamlessly to support a reliable structure. Any misalignment introduces risks that can compromise the trial’s integrity and ability to deliver the endpoints required. In today’s increasingly complex research landscape, this foundation is digital.
From patient enrollment to final data capture, each phase of the patient’s clinical trial journey generates vast amounts of data. Capturing this data consistently, accurately and efficiently is crucial. According to the National Institutes of Health, an average 12-month trial involving 2,000 patients can generate up to three million data points. When handled manually, a trial of the same size has the potential to produce nearly 10 million opportunities for error.
Yet, many trials still rely on fragmented systems, outdated workflows and manual processes. These inefficiencies hinder progress and amplify the risk of data inaccuracies. To overcome these challenges and reduce the potential for costly errors, clinical trials must implement an integrated data strategy utilising a patient suite.
When thoughtfully designed and implemented correctly, a patient suite acts as the digital foundation of a clinical trial, streamlining efficient and accurate data capture, supporting regulatory compliance and enhancing the overall patient experience. In return, this reduces the po-

tential for costly delays and boosts the chances of trial success.
The patient journeyas the blueprint
A patient’s journey through a clinical trial is more than just a series of check-ins and assessments. It is a continuous stream of critical touchpoints, each generating data that impacts the trial outcome. This data must be captured, analysed, contextualised and readily accessible across the trial’s life cycle.
To ensure that trials can operate smoothly, sites and sponsors need systems that not only can capture this data but also reflect a deep understanding of the patient experience behind it. This understanding becomes the blueprint for improving trial efficiency, maintaining regulatory standards and safeguarding patient well-being.
The foundation: What is a patient suite?
A patient suite is not a single platform but a cohesive collection of advanced digital tools that work together to create a seamless experience for sponsors, sites and patients. When integrated, these tools can eliminate data silos, reduce operational friction and improve trial performance.
Think of the patient suite as the digital infrastructure of a clinical trial. When it operates behind the scenes, it remains centred on the patient, streamlining interactions, enhancing clarity and reducing burdens that can contribute to patient dropouts or disengagements.
For sponsors, a well-integrated suite supports the generation of cleaner data and faster timelines. It also enables smarter trial design and ensures compliance at every
step. Ultimately, a patient suite isn't just a convenience; it's a strategic imperative for modern clinical trials.
The core components of a patient suite
Each part of a patient suite contributes a different “brick” to the foundation. In total, there are four key bricks, or pillars, that enable the suite to function as an integrated system:
◆ Electronic clinical outcome assessments: Patientreported insights are essential for evaluating a treatment’s effectiveness, especially in trials focused on quality of life or symptom relief. Digitally capturing these insights via eCOAs brings significant advantages:
❑ High-quality data: Electronic entry reduces transcription errors and produces timestamped, traceable entries.
❑ Improved compliance: Mobile-friendly tools and automated reminders help patients complete assessments on time.
❑ Real-time monitoring: Sites can quickly gain visibility into patient data, helping identify issues, trends or missed assessments that could impact a trial’s outcome.
◆ Interactive response technology: As clinical trials scale across sites and regions, traditional processes can become a liability. To eliminate these challenges and risks, IRT systems can automate trial logistics, such as patient randomisation, drug supply management and visit tracking. A reliable IRT system forms the operational backbone of a trial, minimising
any manual burdens and prioritising smooth workflows.
◆ eConsent: Consent is a pivotal moment within the life cycle of a clinical trial. During this phase, both compliance and trust are established between the trial (sponsor and site) and the patient. Modern eConsent tools offer a simpler path for:
❑ Sites: Streamlined workflows, single sign-on functionality and easy version control reduce administrative burden and minimise the likelihood of introducing errors.
❑ Sponsors: A comprehensive eConsent program ensures global regulatory compliance, boosts recruitment and retention rates and provides data for optimising study design.
❑ Participants: The implementation of thoughtful, interactive and accessible content improves understanding and supports informed decision-making. Patients can mark sections for discussion, review on any device and access content tailored to diverse needs, including pediatric or cognitively impaired populations.
◆ Connected devices: According to a late 2024 report by Deloitte, 43 per cent of consumers (1) now use connected monitoring devices and digital tools for their health. These devices can empower the wearers with actionable insights while enhancing convenience by reducing the need for in-person visits. Additional benefits include:
❑ Continuous monitoring: Wearables capture data continuously, offering richer insight into treatment effects.
❑ Remote engagement: Pa-
tients stay connected without frequent site visits, which is especially valuable for rare diseases or global trials.
❑ Early detection: Devices can identify hidden trends in real-time, alerting sites to potential safety issues before they escalate.
Connected devices extend the reach and precision of a clinical trial, transforming how data is acquired, monitored and utilised.
Real-world benefits: From workflow to outcomesWhen eCOA, IRT, connected devices and eConsent are part of an integrated suite, the result is a robust digital foundation for clinical trials. Sponsors benefit from a unified stream of high-quality, realtime data that supports agile decision-making. For research sites, less time is spent
troubleshooting technology issues, and more time can be dedicated to delivering highquality patient care.
From a participant’s point of view, they experience a smoother journey, from enrollment to study completion, with fewer redundancies, clearer instructions and reduced reporting burdens. This increases retention rates, engagement and reliable trial outcomes.
This integrated approach also imp roves data quality by reducing errors and ensuring protocol adherence. Optimised digital workflows speed up critical phases like enrollment, randomisation and monitoring, leading to faster trial timelines. Finally, from a compliance perspective, builtin audit trails and automated reporting functions ensure
that trials are inspectionready from the outset.
When technology works in harmony across the entire clinical trial ecosystem, everyone benefits, from sponsors to patients.
Despite the benefits of an integrated and comprehensive patient suite, there are common missteps that can derail and hinder the success of a clinical trial. These include:
◆ Disjointed systems: When systems are not interoperable, data entry is often duplicated and manual, increasing error rates and nullifying digital efficiencies.
◆ Poor user experience: If a patient suite is poorly implemented, it will feel clunky,
create frustration and increase the risk of dropout.
◆ Site exclusion: Sites, like the patients, are just as deeply ingrained with the patient suite. Failing to include their requirements when selecting technology and deployment strategies undermines adoption and effectiveness.
The future Is built brick by brickConstructing a successful clinical trial, much like building a structure, starts with a solid foundation. Every tool, workflow and interaction with the patient must fit together with precision. When sponsors and sites invest in a fully integrated, patient-centred suite of technologies, they are not just adopting one-time use tools. They are laying the groundwork for expedited timelines, refined and actionable data,
and better overall outcomes.
Clinical success no longer hinges on isolated systems or ad hoc solutions. True success is the result of thoughtful design, collaboration and commitment to seamless execution. An integrated patient suite isn’t a future luxury — it’s a present-day necessity. For trials to thrive in today’s complex environment, the digital foundation for clinical trials must be rooted in interconnectivity and built with the patient and site in mind from day one.
References:
1. https://www2.deloitte.com/ us/en/insights/industry/health -care/life-sciences-and-healthcare-industry-outlooks/2025us-health-care-executiveoutlook.html?utm_source= chatgpt.com


Excerpts from the KPMG report reveal a blueprint for high-impact transformation in the life sciences sector,driven by the strategic adoption of AI.While life sciences organisations lead in AI adoption with a clear vision and robust data foundations,many struggle to achieve a high return on investment (ROI).The report explores how leading firms are bridging this 'ROI gap' by adapting operating models,breaking down silos,and fostering AI-driven agility
Foreward:
AI in life sciences: Closing the ROI gap
Life sciences organisations have established themselves as leaders in artificial intelligence (AI) adoption, with a clear vision, business-led implementation, and robust data and technology foundations. Despite this progress, generating a high return on their AI investments is proving challenging.
While AI is delivering operational improvements and strategic advantages, new research by KPMG International shows many organisations are reporting only moderate, low, break-even or no return on their AI investments. Looking ahead, many expect ROI to remain flat in the coming years, raising a critical question: How can life sciences organisations better bridge the gap from no to low or moderate returns to consistently high ROI?
Our research finds organisations that utilise hybrid operating models, an optimised mix of functional and agile, significantly increase their chances of high ROI. This finding highlights the importance of adaptability in driving AI-enabled value creation. If the operating model is the primary determinant of ROI success, then life sciences organisations should prioritise structural transformation to become more adaptable, flexible, and AI-ready.
The challenge is no longer whether AI can deliver value,but how companies can reshape their organisations to fully realise its potential.

In this report, we explore how leading life sciences firms are making this transition — adapting their operating models, breaking down silos, and fostering AI-driven agility. We also share actionable insights into how organisations can
take a value-based approach to AI that helps to accelerate innovation, unlock new growth opportunities, and maximise the impact of their AI investments.
Introduction: AI in life sciences is about more than just technology adoption — it’s about transforming the way life sciences organisations innovate, operate, and create
value. From accelerating R&D, innovating across product portfolios, optimising clinical trials to streamlining supply chains, AI can invigorate across multiple facets of the industry.
AI in life sciences is rapidly evolving — from traditional data analytics to generative AI and now toward agentic AI, capable of autonomous action within defined parameters. Generative AI (Gen AI) is already transforming R&D by accelerating drug development, generating novel compounds, and streamlining regulatory documentation.
The next leap — agentic AI — promises even greater impact, enabling systems that not only analyse data but proactively design experiments, manage trial workflows, and adapt in real time to new findings. This evolution is reshaping how life sciences organisations operate, collaborate, and deliver value — moving from static workflows to dynamic, AI-augmented ecosystems that accelerate innovation, reduce timeto-market, and personalise medicine at scale.
AI is already reshaping both scientific innovation and business operations. On the R&D front, AI is shortening drug discovery timelines and innovating the product portfolio with ‘around the pill’ and ‘around the device’ AI-enabled offerings. It’s changing precision medicine and digital therapeutics with personalised dosing and digital biomarkers and improving clinical trial design through realtime data analysis and predictive modeling. AI-driven
automation is even expediting regulatory submissions, ensuring compliance, and optimising drug manufacturing processes. Commercial and operational functions are also reaping significant benefits. AI is revolutionising supply chain management, improving demand forecasting, and personalising patient and healthcare provider engagement. AI agents are transforming customers, sales and field service teams by augmenting and automating engagement processes. AI-powered analytics are also enhancing market access strategies and salesforce intelligence, optimising pricing models, and identifying new opportunities for revenue growth.
This report is supported by exclusive KPMG research, providing findings from a survey of 183 senior life sciences AI leaders. It is designed to provide Csuite executives, technology leaders, and decision-makers across the life sciences industry with actionable insights and strategic guidance to help them navigate the complexities of AI adoption.
About the research:
To study the current state of AI adoption and technology related issues, KPMG International commissioned a quantitative survey of 1,390 decisionmakers in eight industries (life sciences, healthcare, insurance, technology, banking, retail, industrial manufacturing and energy), across eight countries (Australia, Canada, China, France, Germany, Japan, the United Kingdom, and the United States). Included in this multi-sector study are 183 senior life sciences leaders (51 per cent of whom held C-suite titles) representing large diagnostics firms and mid-sized pharmaceutical, biotech, medical device and pharma industry services companies.
A sector out in front
Among all industries exploring the potential of AI, our research shows that life sciences is one of the most advanced. Unlike sectors still experimenting or struggling to scale, life sciences organisations have em-
bedded AI deeply into their operations — from R&D and clinical trials to supply chains and commercial functions. For many, AI is not just a tool, but a core part of how they work.
This leadership stems from early investment. Most of the surveyed life sciences organisations have been using AI for over three years, and nearly all have a clear understanding of the AI projects they should invest in and why. Strategic thinking is strong, with twothirds of companies having a well-defined AI roadmap for the next five years. And perhaps most significantly, AI initiatives are usually businessled, not IT-led — ensuring alignment with core goals like speed to market, scientific innovation, and regulatory compliance.
Making AI workacross the enterprise AI is now embedded in day-today functions for many life sciences firms, not just in pockets. More than half of organisations are using AI across entire departments or value streams. Whether it’s speeding up clinical trial design, identifying promising compounds, or improving patient support, AI is being applied with clear purpose. This enterprise-wide adoption is made possible by thoughtful organisational design. High-performing firms use hybrid structures — blending functional expertise with agile teams — to drive both innovation and operational excellence. This balance enables teams to move fast when needed but stay grounded in the regulatory and scientific rigor the sector demands.
AI also requires the right technologybackbone
Most life sciences firms have invested in enterprise data platforms, analytics engines, and cloud-based infrastructure. These capabilities allow data to flow across teams, enabling better insights, automation, and decision-making. And critically, many organisations are training their people to understand data and AI — building a culture that’s ready for what’s next.
AI as a strategic and sustainable investment
Beyond immediate operational gains, life sciences firms are embedding AI into the core of their offerings. Sixty-five pe rcent of companies are systematically integrating AI into their products and services, and 69 per cent are investing in AI experimentation without immediate output expectations — a strong indicator of long-term commitment.
Despite AI’s strategic importance, sustainability remains a top priority. Seventy-eight per cent of life sciences organisations view meeting sustainability goals as an even greater strategic imperative than AI implementation, and 83 per cent have concrete plans to mitigate the increased energy demands of AI.
Looking ahead: Toward autonomous intelligence AI in life sciences is rapidly moving beyond traditional analytics. Generative AI is already being used for tasks like drafting clinical documents or modeling molecules, and many organisations are beginning to explore agentic AI — systems that can act autonomously within defined parameters.
These developments raise exciting possibilities. Eightynine per cent of organisations are comfortable with AI making end-to-end autonomous decisions for specific processes, and 85 per cent report significant or growing use of autonomous agentic systems. In the near future, intelligent agents could design lab experiments, monitor clinical trials in real time, or manage supply chain disruptions automatically without human oversight. Many leaders are ready for this shift, with high levels of comfort around AI making decisions in specific use cases.
The sector’s forward momentum is also evident in its mindset. Many organisations are investing in AI not just for immediate gains, but to help future-proof their operations. A majority are experimenting without expecting short-term payback — showing a deep commitment to innovation.
89 per cent - of organisations
are comfortable with AI making end-to-end autonomous decisions for specific processes
The data dilemma - Despite having strong digital foundations, data challenges remain a key barrier. Sixty-eight per cent cite issues like siloed data, inconsistent quality, and privacy concerns. Whether it’s patient information, clinical data, or regulatory content, accessing and integrating data across teams is still difficult.
These challenges create friction across the AI lifecycle — from training algorithms to generating actionable insights. And although confidence in AIgenerated outputs is relatively high, these barriers can slow adoption, limit scale, and increase the risk of bias or error.
Life sciences respondents rank measuring ROI as their third biggest AI adoption challenge. While most life sciences organisations believe in AI’s potential, quantifying its impact remains difficult. Many leaders report pressure from shareholders to demonstrate fast returns on AI investments — but less than a quarter of firms are currently seeing strong ROI. Around 38 per cent are seeing moderate gains, and one-third do not expect major improvements in the near term.
This disconnect is understandable. Many AI use cases are long-term plays — especially in R&D, where payback may take years. Nonetheless, the inability to consistently measure value remains a credibility issue, particularly in capital-intensive environments.
Many life sciences organisations are constrained by a growing AI skills gap. While 74 per cent of companies have begun training their workforce in AI, the demand for specialist skills — such as machine learning, data engineering, and AI governance — continues to outpace supply. This shortage is particularly acute in areas where scientific expertise and AI capabilities
must intersect, such as drug development, clinical informatics, and regulatory tech. Without sufficient internal talent, organisations often face delays in scaling initiatives, increased reliance on external vendors, and challenges in ensuring AI solutions are both scientifically sound and compliant.
Our research shows that operating model design is a major success factor. Organisations using hybrid structures — mixing functional expertise with agile squads — are twice as likely to achieve strong ROI from AI. These models enable faster innovation without sacrificing compliance or coordination. In contrast, firms that remain locked in traditional, functionally siloed models tend to struggle with collaboration, responsiveness, and speed. The takeaway is clear: to get the most from AI, life sciences organisations should rethink how teams are structured and how work flows across the enterprise.
Life sciences firms have moved beyond AI experimentation into real-world impact. They are building the culture, technology, and operating models needed to scale AI with confidence. The sector’s leadership position is not just about being early — it’s about being strategic, aligned, and forward-thinking.
Still, to unlock AI’s full potential and increase ROI, firms must continue breaking down data silos, improve how they measure success, and build agile organisations capable of navigating a rapidly evolving technology environment
Unlike traditional AI that requires human oversight, agentic AI can independently make complex decisions, adapt to changing environments and manage multi-step processes autonomously. This innovation is set to transform R&D, clinical trials and operational efficiency. Based on KPMG professionals’ technology and industry
experience working with life sciences organisations around the world, we anticipate that agentic AI will impact every area of life sciences.
In pharmaceuticals and biotechnologyAI will:
◆ Autonomously design experiments, optimise trial protocols, and adapt in real time to emerging data — Accelerating product development
◆ Independently manage regulatory documentation, align submissions with evolving guidelines across jurisdictions, and adapt strategies in real time based on feedback from agencies — Accelerating approval timelines and reducing compliance risks
◆ Continuously analyse realworld data and patient outcomes, tailor treatment protocols, suggest dose adjustments and flag adverse reactions in specific subpopulations — Enhancing therapeutic efficacy and safety post-launch
In MedTech AI will:
◆ Enable intelligent devices to self-calibrate, coordinate care workflows — Proactively guiding clinicians and patients based on continuous learning
◆ Connect devices to autonomously detect anomalies, escalate urgent findings and initiate next steps in care — Transforming chronic disease management and post-operative monitoring
◆ Forecast component wear, schedule preventive servicing in smart equipment, and adjust device settings — Maximising uptime, reducing costs, and ensuring uninterrupted clinical performance
Keyrecommendations:
The research reveals that those organisations that are realising the most value from their AI investments have focused on four strategic actions:
1. Design an AI strategy that aligns with core competencies:
The challenge is no longer whether AI can deliver value,but how companies can reshape their organisations to fully realise its potential
Life sciences organisations should develop an AI strategy that aligns with their commercial strengths. AI initiatives should be value-driven and deliver scalability, interoperability and measurable impact across the organisation.
Keyactions:
◆ Prioritise high-impact AI use cases:
Focus on AI applications that deliver immediate value in areas such as molecular modeling, biomarker discovery, realworld evidence analytics, regulatory automation and supply chain optimisation.
◆ Develop an AI governance and leadership framework: Establish a cross-functional AI steering committee that includes scientists, regulatory experts, IT leaders, data scientists and commercial stakeholders to oversee strategy, ethics and implementation.
◆ Ensure AI scalability and interoperability: Design AI solutions that seamlessly integrate with existing research platforms, clinical trial management systems, databases and biomanufacturing workflows, helping to ensure enterprise-wide scalability.
◆ Define clear AI metrics and ROI measurement: Establish quantifiable goals such as faster drug development timelines, reduced R&D costs, improved regulatory approval rates and optimised supply chain efficiency, helping to ensure AI delivers tangible business value.
2. Build trust into the AI transformation roadmap Trust is essential for AI adoption in life sciences. Engaging stakeholders early in AI development will help to ensure widespread adoption and longterm trust.
Keyactions:
◆ Implement explainable AI and bias audits: Help ensure AI models provide interpretable decisionmaking processes for scientists, regulators and clinicians by conducting bias detection and fair-
ness audits, particularly in areas such as genomics, clinical trials and drug efficacy predictions.
◆ Engage researchers, regulators and industry stakeholders in AI development: Involve scientists, pharmaceutical leaders, regulatory agencies and patient advocacy groups in AI model training and validation. Use continuous feedback loops to improve transparency and adoption.
◆ Establish AI ethics and compliance oversight:
Develop AI governance frameworks aligned with regulations, helping ensure privacy, security and accountability in drug development and patientcentric applications.
◆ Showcase proven AI success stories:
Share real-world case studies demonstrating AI’s impact on reducing R&D costs, accelerating clinical trial approvals and improving biomanufacturing efficiency.
3. Create a sustainable technology and data infrastructure for AI adoption:
High-ROI AI depends on scalable, interoperable and secure technology infrastructure. Organisations should modernise legacy systems, integrate fragmented research data sources and enable real-time AI applications across functions.
Keyactions:
◆ Modernise legacy IT and research infrastructure for AI readiness:
Transition to cloud-based, AI-native architectures that support real-time analytics and automation in drug development, compliance and supply chains.
◆ Unify and standardise data across platforms: Implement interoperable data standards (FHIR, HL7, OMOP, and CDISC) to integrate structured and unstructured life sciences data. Give AI
models access to comprehensive datasets across research, clinical trials and post-market surveillance.
◆ Invest in secure AI-driven data governance: Deploy AI-powered data management tools to automate de-identification of patient data, help ensure regulatory compliance and strengthen cybersecurity against IP theft and data breaches.
◆ Adopt federated learning for collaborative AI training: Enable privacy-preserving AI model training across life science companies, hospitals and research institutions so AI models can learn from diverse patient populations without compromising data privacy.
4. Build a culture that uses AI to elevate scientific and operational potential: AI should enhance human expertise, not replace it. Life sciences organisations should foster a culture where AI augments researchers, regulatory teams and commercial functions. Reskill the workforce, integrating AI into scientific education and reinforcing AI’s role in improving efficiency and innovation.
Keyactions:
◆ Integrate AI training into life sciences education and professional development: Embed AI literacy into biomedical research programs, clinical trial training, regulatory affairs courses and supply chain management certifications.
◆ Upskill life sciences professionals: Provide AI competency training for scientists, regulatory teams and commercial leaders.
◆ Position AI as an enabler, not a replacement: Clearly communicate AI’s role in accelerating research, improving decision-making
and automating routine tasks. Create tangible goals for the usage of AI — for example, “free up more time on X process, so we can spend more time on research” means it is less likely people will feel that they will be replaced.
◆ Encourage cross-disciplinary collaboration:
Foster partnerships between biologists, chemists, data scientists and regulatory experts to co-develop AI solutions that align with real-world research and business needs.
Conclusion:
The adoption of AI across the life sciences sector marks a transformative shift in how organisations innovate, operate, and deliver value. From accelerating drug development and optimising clinical trials to enabling precision medicine and enhancing supply chain agility, AI has become a present-day imperative. This report has highlighted how leading life sciences organisations are moving through three waves of AI maturity: enabling individual productivity, embedding AI across core operations for efficiency, reengineering processes for scaled value, and reimagining the entire ecosystem for sustainable growth and impact.
Yet, as the sector advances, new challenges emerge. Governance, interoperability and trust remain critical hurdles. Organisations must not only invest in AI technologies but also in the operating models, data strategies, regulatory alignment, and workforce skills needed to embed AI responsibly and at scale.
The future of AI in life sciences is agentic, multimodal, and deeply human-centric. Digital agents can automate complex workflows, generative AI can assist scientists in creative problem-solving, and predictive models can support physicians in delivering proactive, personalised care. To realise this potential, life sciences companies must act — establishing AI transformation roadmaps, modernising legacy systems, and fostering cross-functional collaboration.
AI is a strategic enabler — not just a technological upgrade and will be key to innovation and market leadership.
the latest advances in stem cell
of stem cells used,and the growing body of research supporting their use for the treatment of both type 1 and type 2 diabetes
Diabetes is quickly becoming one of the most urgent global public health crises. Currently, 540 million people worldwide have diabetes, and that number is projected to increase to 783 million by 2045. More than 90 per cent of cases are type 2 diabetes, which includes insulin resistance and beta-cell dysfunction.
Type 1 diabetes, also known as juvenile diabetes, is an autoimmune disorder where insulin-producing beta cells in the pancreas are destroyed, is also rising at alarming rates. In 2021, over 8 million people globally had type 1 diabetes, and that is expected to increase to as much as 17 million people by 2040.
When unmanaged, diabetes can lead to chronic complications affecting multiple systems, especially microvascular diseases such as retinopathy, nephropathy, and neuropathy, as well as macrovascular diseases including cardiovascular, cerebrovascular, and peripheral vascular diseases, all of which are associated with high morbidity and mortality. In 2021, diabetes and kidney disease due to diabetes caused over 2 million deaths globally, and around 11 per cent of cardiovascular deaths were linked to high blood glucose.
The conventional approaches to managing both type 1 and type 2 diabetes include continuous monitoring of blood sugar levels and maintaining a healthy lifestyle. Type 1 diabetes patients must rely on insulin supplements in the form of daily injections, while type 2 patients use oral medications like metformin, sulfonylureas, and DPP-4 inhibitors.
With the increasing prevalence of these diseases and the

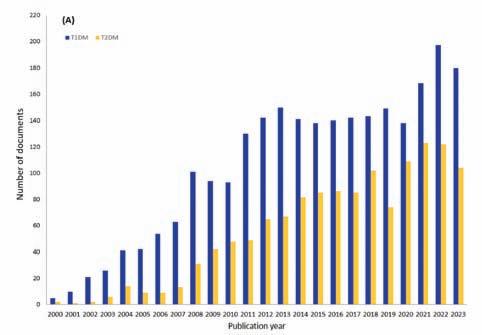
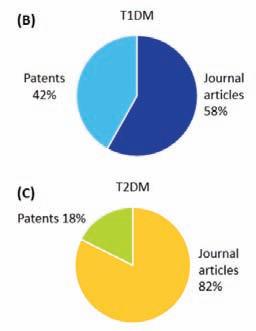
Figure 1:Publications related to stem cell therapyin diabetes treatment.(A) shows year-wise distribution of documents (journal articles + patents) related to type 1 diabetes (blue bars) and type 2 diabetes (yellow bars).(B) and (C) showrelative distribution of journal articles and patents related to type 1 and type 2, respectively.Source: CAS Content Collection

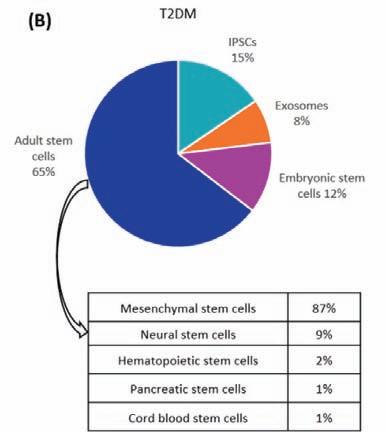
Figure 2:Relative distribution of various types of stem cells occurring in the diabetes dataset.(A) for type 1 diabetes and (B) for type 2 diabetes.Source: CAS Content Collection
aging of the overall global population,more cases of type 2 diabetes are expected, showing a need for better treatment options. In 2022, the FDA approved teplizumab to delay the onset of type 1 diabetes, and newer drugs like GLP-1 receptor agonists and SGLT-2 inhibitors are being explored for type 2.
However, more recent breakthroughs involve pancreatic islet cellular therapy to restore insulin secretion for patients with type 1 diabetes. The first successful attempts at differentiating stem cells into insulin-producing cells happened in the early 2000s, and clinical trials began in the 2010s. It was in 2023, however, that the FDA approved Lantidra, the first cellular therapy for type 1 diabetes patients. Recent studies also demonstrated the potential of stem cell-derived islet cells to restore insulin production in diabetes patients, reducing or even eliminating the need for external insulin injections.
New strategies are being explored to enhance the success and longevity of stem cell therapy, and these approaches could result in more effective and long-lasting treatments for type 1 and type 2 diabetes. We examined the CAS Content CollectionTM, the largest human-curated repository of scientific information, and found a marked increase in publications relating to stem cell therapy for diabetes from 2000 to 2023.
As seen in Figure 1 , there are more publications relating to stem cell therapy and type 1 diabetes than type 2. Furthermore, for type 1, the journal articles contribute 58 per cent and patents contribute 42 per cent of the total documents. For type 2, the number of journal articles is higher at 82 per cent while the number of patents is only 18 per cent.
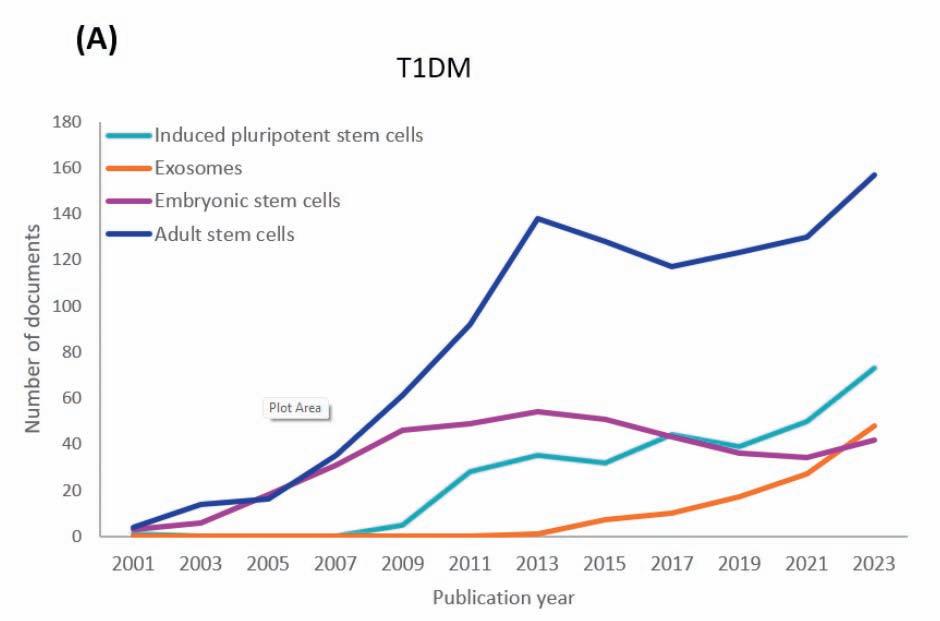

Types of stem cells showing potential for diabetes treatment
Stem cells are unspecialised cells capable of differentiating into various specialised cell types. There are three main types:
1. Adult stem cells: Multipotent cells found in tissues that can develop into a limited range of related cell types, such as hematopoietic stem cells (which can differentiate into various types of blood cells) and mesenchymal stem cells (which can differentiate into bone, cartilage, and fat cells).
2. Induced pluripotent stem cells (iPSCs): A type of pluripotent stem cell generated directly from adult (mature) somatic cells. These cells are reprogrammed back into an embryonic-like state, allowing them to differentiate into any cell type in the body.
3. Embryonic stem cells (ESCs): Pluripotent cells from early-stage embryos that can become almost any cell type.
We analysed the types of stem cells being explored for diabetes treatments and found that, for type 1 and type 2 diabetes, adult stem cells are the most consistently researched (see Figure 2) . Among adult stem cells, the most prominent type is mesenchymal stem cells (MSCs) followed by neural, hematopoietic, pancreatic, and cord blood stem cells.
Each type of stem cell has been the subject of important research,which we summarise in Table 1:
STEM CELLTYPE
MSCs
● MSC transplantation significantlylowers HbA1c levels and increases fasting C-peptide levels in type 1 patients.
● Autologous MSC treatment could preserve the function of ß-cell in new-onset type 1 patients.
● Fasting glucose,HbA1c,and insulin requirements decreased; fasting C-peptide and Cpeptide/glucose ratio increased in type 2 patients.
● The insulin requirement decreased; HbA1c increased modestly; glucagon-stimulated Cpeptide increased significantlyin type 2 patients.
iPSCs
ESCs
Exosomes derived from stem cells
● iPSCs reprogrammed into insulin-producing cells and injected into a patient,leading to stable insulinproductionwithout injections for over a year in type 1 patient.
● iPSCs can be differentiated into insulin-producing cells and have shown promise in preclinical studies.
● Promising results from an ongoing clinical trial showthat ESC-derived pancreatic islet cells successfullytransplanted into patients,achieving safetyand efficacybenchmarks.
● ESC-derived beta cells can restore insulin production and improve glycemic control in diabetic mice.
● MSC-derived exosomes shown to inhibit islet inflammation and alleviate the disease progression in type 1 diabetes studies in mice.
● MSC-derived exosomes restored islet structure and enhanced insulin sensitivityin type 2 diabetes studies in rats.
Table 1: Types of stem cells and findings from keystudies
The treatment candidate VX-880, derived from ESCs, is entering a phase III clinical trial (NCT04786262). VX-880 is an investigational allogeneic stem cell-derived, fully differentiated, insulin-producing islet cell therapy developed for the treatment of type 1 diabetes. There have been encouraging results with this candidate, and in November 2024, Vertex Pharmaceuticals announced the start of a pivotal phase III trial enrolling 50 patients. This demonstrates
the potential of many stem cell types to not just treat, but even to cure, diabetes.
In our analysis of the CAS Content Collection, we noted the year-wise trend of published documents related to each stem cell type for type 1 and type 2 diabetes research (see Figure 3) . For both types, initial studies investigated adult stem cells (mainly MSCs) and ESCs. However, research on adult stem cells has grown compared to ESCs. Documents related to iPSCs
also show steady growth for both types. Exosome-related documents or studies, which only started about a decade ago, are also on the rise.
Advantages and limitations of various stem cell types
◆ MSCs: These adult stem cells can be isolated from various sources (bone marrow, adipose tissue), and they have low immunogenicity since they lack MHC-II complex and exhibit lower expression of
MHC-I complex. They can modulate immune responses and inflammation and are relatively safe for clinical use with fewer tumorigenic risks. However, they have limited differentiation potential when compared to pluripotent stem cells, and there can be inconsistent cell quality and potency across donors. It’s also difficult to maintain these in their stem cell state in vitro, which makes scalability difficult. Lastly, there can be ethical concerns if they’re derived
from human donors.
◆ iPSCs: There are fewer ethical concerns with iPSCs since they are derived from somatic cells, and immune rejection can be avoided if they’re derived from the same patient. In terms of limitations, reprogramming these cells can be time-consuming, expensive, and challenging. There is also a risk of genetic mutations during the reprogramming process and high tumorigenic potential.
◆ ESCs: These exhibit high regenerative potential and are well-characterised and studied. They have high proliferation rates, which makes them suitable for large-scale production. They do, however, have a risk of teratoma formation, and there have been ethical concerns surrounding their use.
It’s possible that stem cell therapies could be used to treat many of the complications that come with diabetes.Today,conventional diabetes treatment options focus on regulating blood glucose levels, but they’re not efficient in reducing or treating the complications that can accompany this disease
It’s possible that stem cell therapies could be used to treat many of the complications that come with diabetes. Today, conventional diabetes treatment options focus on regulating blood glucose levels, but they’re not efficient in
reducing or treating the complications that can accompany this disease.
For example, a recent meta-analysis showed that diabetic foot patients can benefit from stem cell therapy, as indicated by various parameters like healing rate, amputation rate, pain scores, and new angiogenesis rate. Other studies have attempted to under-
stand the mechanisms and applications of stem cells in diabetic nephropathy and cardiovascular complications in diabetic patients.
Even though stem cells hold enormous potential in diabetes treatment, several challenges remain which may hinder their widespread application. One particular concern is immune rejection, es-
pecially if the cells are from another donor (an allogeneic source). Maintaining the viability and efficiency of transplanted stem cells is also a challenge, and there are risks of developing tumors. To address immunogenicity and teratogenesis after transplantation, scientists are using immune-isolation devices, encapsulation methods, or immunosuppressive therapies.
As researchers make progress on these challenges, it’s possible that we can develop not just treatments but cures for type 1 and type 2 diabetes. With these conditions becoming more prevalent, it is critical for the scientific community to keep making breakthroughs with innovative treatment options.


Showcasing a powerful reflection of women's empowerment in the healthcare landscape,the annual convocation at IIHMR University marked a milestone,with 58 per cent of the graduates being women,a strong sign of progress in healthcare education
IIHMR University, Jaipur, organised its annual convocation ceremony on July 19, 2025, at the university campus. Chief Guest Haribhau Kishanrao Bagde, Governor of Rajasthan shared, "I’m happy to observe that all four students who received gold medals at IIHMR University were females. He requested all the graduating students to apply the knowledge and skills acquired over the past two years towards the noble cause of nation-building. I also suggest that universities collaborate with national as well as international universities and institutions. By integrating traditional and modern knowledge, National Education Policy 2022 aims to foster the holistic development of students.”
Showcasing a powerful reflection of women's empowerment in the healthcare landscape, the annual convocation at IIHMR University marked a milestone, with 58 per cent of the graduates being women, a strong sign of progress in healthcare education.
Welcoming the dignitaries and students, Dr PR Sodani, President, IIHMR University, shared, “I extend my heartiest congratulations to our graduating students. Of 423 graduating students, 247 were women, outnumbering 176 male students. He said to the graduating students Always remember that you are the ambassadors of IIHMR University. Take our legacy of excellence in academics, innovations, and impactful research wherever you go. My key message to the graduating students is to lead with integrity and serve with compassion. Further sharing the university’s annual report, he


said that IIHMR University conducted various studies for the Government of India, the Government of Rajasthan, and other state governments to improve the health care delivery system.
Dr Sodani said, This year’s placement season marked another milestone for the university. The highest package stood at Rs 28.56 LPA, with 11 students securing interna-
tional placements in the UAE, Kenya, Indonesia, and Congo, highlighting the growing global recognition of IIHMR University’s talent pool.
Dr Rajeev Singh Raghuvanshi, Drugs Controller General of India, as the Guest of Honour, said, “India is a very important part of the global healthcare value chain. We provide medicines and trained manpower to more

than 200 countries worldwide. Healthcare is not just a system but a promise of care, dignity, and equity for all. Uphold it with integrity, lead with humility, and serve with heart. Contributing to building society is our duty. Helping patients is the highest order of service.”
Dr Pramod Yeole, Vice Chancellor, Rajasthan University of Health Sciences, as the Guest of Honour, said, “Over 70 per cent of the Rajasthan population lives in rural areas; that statistic must not just inform policy, it must inspire action. Rural Healthcare is central to our development as a state and as a nation. You are graduating at a time when Healthcare is being redefined. Where public health is finally getting recognition with resources, and where the government is investing in grassroots infrastructure and workforce development. As you walk out of this gate, you will carry fire for knowledge, for service, for justice and change.”
Sudarshan Jain, Chairperson, IIHMR University, presided over the convocation ceremony and said that the convocation is a moment of joy and pride for everyone. He further advised students to continue their learning journey forever.
Dr Ashok Agarwal, Founder-Trustee of IIHMR, suggested that graduating students look at their jobs as learning tools. He also encouraged the graduates to enjoy life, remember their purpose, and see every challenge as a new lesson.
The graduating batch consisted of 225 students from MBA (Hospital & Health Management), 125 students from MBA (Pharmaceutical Management), 9 from MBA (Development Management), 2 from MPH (Implementation Science), 35 from MPH (Executive), and 18 from MHA (Executive). 9 doctoral students were also awarded with degrees.
India’s chronic delays in patent processing threaten its innovation potential and global trade ambitions.Introducing Patent Term Adjustment (PTA) could be a strategic solution to ensure fair patent protection,incentivise R&D,and align India’s IPsystem with global standards,highlights Milind Sathe, VP-IPand Tech,Themis Medicare
India experiencedchronic delays in patent examinations and grantwith average pendency periods among the highest in comparable economies despite recent improvements. Several sources detail the trends, causes, and precise figures about IP office statistics which more or less unanimously disclose declining number of patent applications progressing through examination stage in recent past, ultimately resulting in a reduced number of granted patents as compared to number of patent applications lodged and the pending backlog. The trend has ability to impact the image of India on global trade platform. Most of the sources converge on the main causes of delay as systemic resource shortages and surge in filings.
The annual report OF 202320242 gives encouraging figures of Granted patents while the number of examined applications is phenomenally low.
Underlying causes of delay
a) Manpower shortages i.e. far fewer patent examiners and controllers than required for the workload coupled with process bottlenecks such as proportion of Examiners to controllers, uneven workloads in different patent offices causing backlogs at final decision stage
b) Growing number of filings: Steady increase in applications, has outpaced staff increases
c) Procedural complexity: Multiple stages in prosecution, opposition, revocation, resource constraints, and legal processes add further delays
d) Quality and enforcement issues: Low-quality filings, unexpected decisions also im-

pact the timely processing of applications.
a) Loss of exclusivity period: Patentee often lose some years of their theoretical 20year exclusivity simply waiting for grants, with some patents receiving effective protection only towards the end of their term.
b) Uncertainty: Prolonged examination periods create commercial uncertainty, weaken deterrence against infringement, and delay product launches for Indian and foreign applicants.
c) Comparison to global norms: India’s grant times aretwo to three times longer than leading global patent offices.
d) Antagonises our dream of India becoming a superpower in global trade and a self-reliant economy because delays in patent grant discourage prospects of several business collaborations and joint ventures.
Recent developments: Ongo-
ing reforms and efforts to recruit more examiners/controllers, invest in digital systems, and streamline procedures possibly will improve situation but substantiated estimates are not available.
In summary,patent grant delays in India have averaged between four to six years over the past two decades, improving slightly with reforms, but regressing recently due to ongoing administrative bottlenecks.Delays remain a major obstacle to the effective protection and commercial use of inventions in India.
Though India is a leader in providing software solutions to global industry, developing and implementing sophisticated software to track the duration of patent application delays—whether due to patent office processing times or applicant response lapses—can be a time-intensive task.
As a progressive and reliable economy striving to lead global trade, continuously
dominating the generic pharma market and aspiring to forge ahead in novel pharmaceutical products, India can’t afford to fall short in ensuring transparency, accountability, and predictability in intellectual property rights (IPR) enforcement. To address the persistent delays in the examination or grant or refusal of patent applications, alternative solutions must be explored to enhance the efficiency and responsiveness of the patent system to retain India’s image, to augment its aspirations to take lead position in generic and novel pharmaceutical market, to attract more investments and to provide more effective medicines to prevailing and emerging disease profiles, globally.
One effective approach is the implementation of Patent Term Adjustment (PTA), a well-established legal and fair compensationmechanism in the United States compensating for delays caused by patent office processing primarily during examination or applicant response times. Accordingly, it extends the life of a patent beyond the standard 20-year termfrom the filing date, thereby ensuring that innovators receive a fair period of market exclusivity benefit promised by their patents. It is critical for protecting innovation, recovering research and development costs, and maintaining competitive advantage. Adopting similar measures in India could incentivise timely patent prosecution, enhance transparency, and reinforce India’s standing as a dependable leader in the global pharmaceutical landscape.
The patent examination process can be lengthy due to
backlogs at patent offices, longer reviews, or administrative inefficiencies. Without PTA, any delay by patent authorities shortens the effective period of exclusive patent rights, which unfairly penalises patentee.
PTA acknowledges procedural delays that are beyond the applicant’s control, preventing loss of effective patent term. It maintains the patent’s economic value, especially in sectors with long development cycles like pharmaceuticals and biotechnology where even a few months of additional protection can be financially significant.
◆ Extended market exclusivity: PTA prolongs patent term beyond 20 years from filing date during which a patentee can exploit the invention, p rev enting competitors from entering the market before expiry of adjusted and hence extended patent term.
◆ Increased revenue and investment incentives: For pharmaceutical industry, additional patent term due to PTA can translate into remarkable revenue and profits by delaying generic entry, improving ROI on R&D investments.
◆ Encourages innovation: By ensuring an adjusted and hence extended term of protection, PTA incentivises inventors to invent and investors to deploy or invest in new technologies and medicine development. It assures that market protection will not be eroded by bureaucratic delays.
◆ Alignment with international standards and expiries: PTA provisions can harmonise patent rights globally to a great extent, consistent with TRIPs or TRIPs
plus frameworks, improving the predictability of patent rights for international businesses.
◆ Supports strategic business planning: Companies can factor PTA into their IP strategies, successfully negotiate JVs, to maximise the commercial lifespan of key patents.
◆ Delay entry of generics: PTA postpones entry and the availability of cheaper generic versions, impacts public access to affordable medicines. It can be addressed by taking suitable steps to improve supply of alternate medicines.
◆ Complex calculation and disputes: The calculation of PTA involves multiple types of delays and applicant activities, which can be complex and lead to disputes between patent offices and applicants over the accurate adjustment period. Nonetheless, these disputes are capable of being resolved.
◆ Administrative burden: Monitoring and managing PTA claims require additional efforts from patent applicants, increasing costs and complexity. But to enjoy the benefits derived from PTA applicants have to take efforts else they would still be enforcing the right as per existing system. PTA is not compulsory but can be optional.
◆ Potential for strategic ma-
PTAacknowledges procedural delays that are beyond the applicant’s control,preventing loss of effective patent term.It maintains the patent’s economic value,especially in sectors with long development cycles like pharmaceuticals and biotechnology where even a few months of additional protection can be financially significant
nipulation: Some applicants may try to game the system to maximise PTA, possibly leading to inefficiencies or challenges for patent offices. The patent office should be able to p revent the misuse and abuse of PTA provisions, which is precisely their responsibility. If they are unable to effectively implement the PTA, the patent office should decide and disclose the processing timeframes for each stage of the patent application and periodically share progress updates with the public.
HowPTAhelps India in global trade and economy India, as a growing hub for global innovation and manufacturing, particularly in pharmaceuticals and biotechnology, stands to gain significantly from adopting and optimising PTA provisions to suit India. This is going to be territorial enactment and there can’t be



global interference.
◆ Enhances IP protection credibility: Implementing PTA assures both domestic and foreign investors that India protects IP more effectively than TRIPs provisions, fostering greater foreign direct investment and technology transfer.
◆ Boosts innovation ecosystem: PTA extends patent life and can provide Indian inventors and companies to better capitalise their innovations, motivating more R&D and boosting high-value industries.
◆ Improves India's position in global trade: PTA aligns India with TRIPs plus mechanisms, facilitating smoother trade negotiations and access to global markets. India will be able to negotiate Free Trade Agreements better.
◆ Drives economic growth and employment: Longer patent terms will encourage companies to commercialise innovations in India, leading
to higher economic output, job creation, and development of skilled workforce simultaneously satisfying public need.
◆ Balances public health and innovation: Though PTA extends exclusivity, careful policy design can mitigate impacts on access to affordable medicines through improved compulsory licensing, or more effective implementation of other duly amended sections of The Patents Act to balance public health concerns with innovation incentives. Inclusion of definition of Emergency, Medical emergency, national emergency, identifying reference Ministries and their reports therefor, can consolidate sovereign supreme to fulfill its duty towards subject population.
Summary
Advantages of PTA outweigh its disadvantages. By embracing appropri-
ately designed PTA regulations, India can strengthen its IP regime, address public health needs, encourage domestic and foreign innovation, attract more investments while fostering economic growth and competitiveness and enhance its role on the international stage.
References
1. https://www.indiaspend.com/ patent-delays-threaten-makein-india-67033
2. https://eacpm.gov.in/wp-content/uploads/2023/07/1-Patenting-Ecosystem.pdf
3. https://kankrishme.com/thesilver-lining-in-examination-delays-in-india
4. https://www.obhanandassociates.com/blog/indias-20-yearpatent-term-is-section-53-invincible-2
5. https://www.orfonline.org/research/current-trends-in-indias-patenting-landscape
6. https://spicyip.com/2025/05/ calcutta-high-court-refuses-tohold-20-year-patent-term-fromdate-of-application-as-unconstitutional.html
7. https://ipindia.gov.in/writereaddata/Portal/IPOAnnualReport/1_114_1_ANNUAL_REPORT_202223_English.pdf
8. https://ipindia.gov.in/writereaddata/Portal/Images/pdf/An nual_report_23-24_En.pdf

Pharma&LifeScienceExtrusion

Thesmallextruder
Formmastering big challen

-C3PROFILE:CLEAN,CLEVER,COMPACT
-EASYHANDLING,SIMPLE(DIS)ASSEMBLY,GOODCLEANABILITY

-SUITABLEFORTHROUGHPUTSOF50TO1,000G/H
-EASYSCALEUP
-INNOVATIVECOOLING/HEATINGSYSTEM
-HORIZONTALLYSPLITBARRELS


























*Foracompletelistofscientificresearchandfurtherinformationvisitourwebsiteatwww.pycnogenol.com.Pycnogenol®,Frenchmaritimepinebarkextract, isaregisteredtrademarkofHorphagResearchanditsapplicationsareprotectedbyU.S.patentsandotherinternationalpatents.©2024HorphagResearch.

































208,HillViewIndustrialPremises, AmrutNagar,Ghatkopar(W),Mumbai-400086,India. Mob.:9321965968/9869412342 E-mail:sales@mksilicone.com




Email: rajesh.bhatkal@expressindia.com rbhatkal@gmail.com






































PURIFIEDWATER/WFI
GENERATIONSYSTEMS
PW/WFIDISTRIBUTIONSYSTEMS
MCDP/PSGSYSTEMS
STORAGE/MIXINGTANKS
WFITANKS
HOSES,DRAINTRAPS
SANITARYPUMPS
SERVICES
-Electropolishing -PipingInstallation
-OrbitalWelding -Boroscopy -Instrumentation&Automation







purewaterengineers3@gmail.com
Nowavailable!
Best-in-classosmolalityperformance, designedwithyouinmind.

HIGHLIGHTEDFEATURES:
Offersthewidestrangeofosmolalitytesting(0–4000mOsm/kgH2O)
Supports21CFRpart11,GMPandEUAnnex11compliance
MeetsPharmacopeiaosmolalitytestingguidelines
3Leveluseraccessandpasswordprotection
Storage:unlimiteddatastorageforaccess
Audittrail:Preserveunlimitedresultsandevents
Databasebackup,protectsyourdatawithautomaticormanualbackup

No.127,BussaUdyogBhavan,TokershiJivrajRoad,SewriWest,Mumbai-400015, Maharashtra,Landline:+91022-24166630Mobile:+919833286615






































JRS Pharma’s PRUV® is the original sodium stearyl fumarate (SSF) introduced in the market over 20 years ago
Pharma lubricants are the inactive agents i.e. excipients added into tablet and capsule formulations in a very small quantity (usually 0.25-5.0 % w/w) to improve the processing properties of formulations. They decrease the friction at the interface between tablet’s surface and the die wall during the compression and ejection phase of the tableting so that the wear on punches and dies are reduced. They prevent sticking of tablets to punch faces as well as sticking of capsules to dosators and tamping pins. In terms of powder flow, lubricants can improve the flowability of blends and aid unit operations [1]
A good balance between hydrophobic and hydrophilic moieties in PRUV® (Sodium Stearyl Fumarate) makes it an ideal lubricant [2]
Most of the lubricants used in the pharma processes are boundary lubricants. Certainly, metallic salts of fatty acids such as magnesium stearate; stearic acid and sodium salt of fatty acid such as sodium stearyl fumarate are the most common ones.
A. Fatty acid esters: Fatty acid esters, including glyceride esters (glyceryl monostearate, glyceryl tribehenate, and glyceryl dibehenate), sugar esters (sorbitan monostearate and sucrose monopalmitate) and alcohol ester of stearic alcohol with fumaric acid, including sodium stearyl fumarate are often used as lubricants. In particular, sodium stearyl fumarate and glyceryl dibehenate are effective lubricants to replace magnesium stearate when the latter hampers dissolution and has chemical incompatibility issues. Relative to magnesium stearate, sodium stearyl fumarate has similar lubrication efficiency with a higher optimal concentration (around 2%,

w/w). In addition, the use of sodium stearyl fumarate does not affect compressibility [1]
B. Metallic salts of fatty acids: Use of the metallic salts of fatty acids as lubricants has a long history in the pharma industry and they are still the most dominant class of lubricants. Magnesium stearate, calcium stearate and zinc stearate are the three common metallic salts of fatty acids used [1]
C. Fatty acids: Fatty acids are also common lubricants used in the pharma industry with stearic acid as the most popular one. Chemically, stearic acid is a straight-chain saturated monobasic acid found in animal fats and in varying degrees in cotton seed, corn and coco. The commercial material of stearic acid has other minor fatty acid constituents such as myristic acid and palmitic acid [1] .
D. Inorganic materials and polymers: Inorganic materials and polymers are also used as lubricants when magnesium stearate cannot be used. In terms of inorganic materials, talc (a hydrated magnesium silicate (Mg3Si4O10(OH)2), is often used as a lubricant or as a glidant in formulations. Talc provides some essential lubricity for pharma operations because of its hydrophobicity and weakly bonded sheet structure [1][6]
Mechanism of lubrication:
There are four lubrication mechanisms: [1]
1. Hydrodynamic lubrication,
2. Elasto-hydrodynamic lubrication,
3. Mixed lubrication, and 4. Boundary lubrication.
As their names imply, the first three mechanisms are related to the usage of liquid lubricants to some extent. In the pharma industry, boundary lubrication is the most common mechanism functioning in unit operations.
occurs. Structurally, the lubricants commonly used for boundary lubrication are long chain molecules with active end-groups. The typical endgroups include:
1. –OH (long chain alcohol);
2. –NH2 (long chain amine);
3. –COOH (long chain fatty acids); and
4. Metal ions such as Mg2+.
Considerations for selecting a lubricant
There are many factors to be considered for selecting an appropriate lubricant for preparing solid dosage forms, including low shear strength, being able to form a durable layer covering the surface/particles, non-toxic, chemically compatible with APIs and other components in the formulation, low batch-to-batch variability, and
Solubility 0.5mg/100 ml at 250C/ 10 g/100 ml at 800C 20 g/100 ml at 900C
Melting point 224-2450C

For boundary lubrication, a lubricant typically forms layers/film between surfaces or at interfaces to reduce friction, where the penetration of the lubricant into surface asperities
having minimum adverse effects on the performance of the finished dosage forms. [1][3]
In addition, the optimal concentration and mixing time are also needed to be taken into
consideration when selecting a lubricant because both of these two parameters greatly affect the performance of pharma products and processes. [1][6]
After understanding what are lubricants, commonly used lubricants in pharma formulation, mechanism of lubricants and considerations for selecting the best lubricant, let’s focus on more detail regarding PRUV® (Sodium Stearyl Fumarate) lubricant from JRS Pharma.
PRUV® (Sodium Stearyl Fumarate) characteristics:
◆ Highly efficient lubricant and anti-adherant
◆ More water-soluble than Magnesium stearate
◆ Well-defined particle size and specific surface area
◆ High melting point (230°C)
◆ Lamellar structure
◆ High purity and batch-tobatch consistency
HowPRUV® works?
◆ PRUV® reduces inter-particulate friction during tablet manufacturing while acting as a boundary lubricant in the formulation.
◆ PRUV® facilitates lubrication during blending through shearing.
◆ PRUV® is more hydrophilic, dissolution is not compromised wherever facing dissolution issue with magnesium stearate.
◆ PRUV® goes beyond lubrication and it also accelerates dissolution.
Applications of PRUV®
PRUV® can be used in dry granulation as well as wet granulation technology to avoid sticking of intra-granular blend to roller or RMG wall. PRUV® can also be used in capsule dosage by reducing friction between the particles. PRUV® is a perfect lubricant for highspeed tableting/continuous manufacturing because it is less sensitive to heat. PRUV® can also be used in hot melt
extrusion due to its higher melting point (224-245oC).
Following are the case studies of PRUV® to evaluate the effect on physio-chemical properties on tablet dosage form [4]
In this study, the effect of different lubricants on Acetaminophen formulation was studied. Acetaminophen API blended with PROSOLV SMCC HD 90 for 15 min and afterwards blended with sieved lubricants for three minutes. Different lubricants like PRUV,
Magnesium stearate, stearic acid and sodium stearate are used. Tablets were compressed to 800 mg weight. tablet.
Magnesium stearate is the most widely used lubricant in the pharma industry. Hence, PRUV® is compared with Magnesium stearate in the following section:
PRUV® helps to avoid API incompatibilities and enhances API stability. With a few exceptions, PRUV® can be applied to any formulation for lubrication,
particularly those in which API stability or tablet taste is compromised due to magnesium stearate. Magnesium cation (Mg2+) is electrophilic, it interacts with the free electrons of an API and forms insoluble salts. This is one of the many causes of API incompatibility with magnesium stearate. [4]
Electrostatic Properties: Magnesium stearate shows higher voltage and retention time than PRUV®. Low electric charge and retention improve lubricant dispersion during blending. As a result, PRUV® due to its low voltage and retention can be considered a superior lubricant with improved lubricant uniformity.
PRUV® was carefully designed to consistently deliver the following functional characteristics:
LUBRICANTS: PRUV® (SODIUM STEARYLFUMARATE),MAGNESIUM STEARATE, STEARIC ACID,SODIUM STEARATE)
Tablet Hardness/Compactability
In this study,the effect of different lubricant on tablet hardness was evaluated
Lubrication efficiency
In this study,the effect of lubrication efficiencyof different lubricants was evaluated
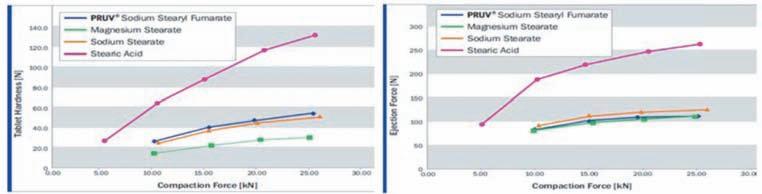
Observation: The kind of lubricant used had a significant influence on the hardness of the tablets. While tablet lubricated with stearic acid resulted in the highest tablet hardness,those made from magnesium stearate exhibited the lowest hardness. Tablets made from PRUVshowintermediate hardness.
Disintegration time
In this study,the effect of different lubricant on disintegration time was evaluated
Observation: As for compactibility,a similar trend observed in the ejection forces.The tablets lubricated with stearic acid showed the highest ejection forces,while magnesium stearate was found on the lower end of the ejection force spectrum.Tablets made with PRUVexhibited the same lowejection forces as compared to magnesium stearate.
Dissolution behaviour
In this study,the effect of different lubricants on dissolution behaviour was evaluated
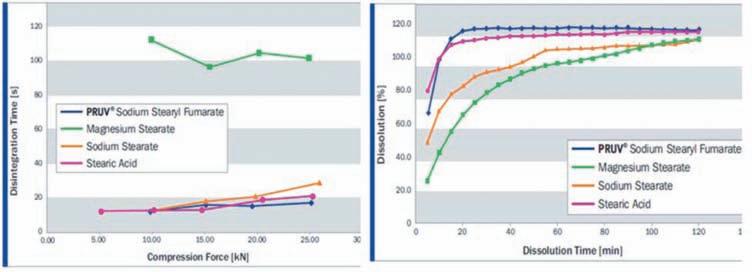
Observation: Tablets lubricated with magnesium stearate needed byfar the longest time for disintegration.All other tablets were found to have disintegration times in the same range
Observation: Tablets containing magnesium stearate showed byfar the slowest dissolution rates.Sodium stearate and stearic acid-lubricated tablets released the API much faster.The faster drug release was observed for tablets lubricated with PRUV®
PARTICLE SIZE AND SPECIFIC SURFACE AREAFOR DIFFERENTGRADES OFSSF[4]
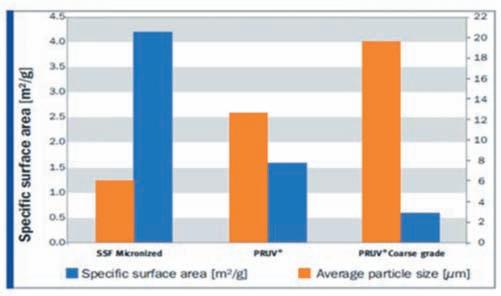
◆ Tight particle size distribution
◆ Well-defined specific surface area
◆ Reproducible particle morphology
The outstanding performance of PRUV® is based on its well-controlled particle size and shape. Following studies show the effect of deviating from the ideal values.
Conclusion: Beyond tablet lubrication properties of PRUV®: It shows:
◆ Improved API stability
◆ Superior blending properties
◆ Faster disintegration
◆ Faster dissolution times
The choice of lubricant can influence the quality of the tablets as well as the dissolution rates. Since APIs tend to be less water-soluble and difficult to compress, choosing the right lubricants continues to become an even more important task.
Most commonly available lubricants are very hydrophobic and thus increase dissolution times significantly. In such cases, a less hydrophobic lubricant can help to decrease the dissolution times as well as increase the API release.
PRUV® Sodium Stearyl Fumarate complies with Ph.Eur., NF and JPE. It has the ideal particle size and specific surface area to offer a perfect balance between all functionality
aspects. It is the preferred choice over magnesium stearate in terms of improving disintegration time and dissolution.
Furthermore, different particle sizes are available, which help to fine tune tablet formulation resulting in the desired dissolution profiles.
Regulatory status of PRUV®:
◆ Ph.Eur., NF, JPE, GRAS status
◆ C-DMF is available for PRUV®
◆ Non-animal origin
◆ BSE/TSE-free
◆ GMO-free
◆ OVI-free (USP<467>) and conforms to the residual solvents requirement of Ph.Eur.(5.4) and USP <467>
◆ QBD dossier available
◆ Elemental impurity statement available
References:
1. Jinjiang Li, Yongmei Wu. Lubricants in Pharmaceutical Solid Dosage Forms. Lubricants 2014, 2, 21-43.
2. Sonja Bauhuber, Maria Launer, Gernot warnke.Influence of Different Lubricants on Tableting Characteristics and Dissolution Behavior. Influence of Different Lubricants on Tableting Characteristics and Dissolution Behavior- JRS Pharma
https://www.jrspharma.com/ph arma_en/technicalinfo/brochures/technical-info/lu-
bricants.php
3. Hölzer, A.W, Sjögren, J. Evaluation of sodium stearyl fumarate as a tablet lubricant. Int. J. Pharm.1979, 2, 145–153.
4. JRS Pharma PRUV® | Sodium Stearyl Fumarate- JRS Pharma https://www.jrspharma.com/ph arma_en/products-services/excipients/lubricants/pruv.php
5. Rowe, R.C., Sheskey, P.J.; Quinn, M.E. (2009) Handbook of Pharmaceutical Excipients. 6th Edition, Pharmaceutical Press, 667-669.
6. Leon Lachman, Herbert A.
Lieberman, Joseph L. Kanig. The theory and Practice of Industrial Pharmacy, Varghese publication house, 3rd edition, 1990, 123-144. QR Code: Scan the QR code for more details regarding PRUV® from JRS Pharma.

Mr Prashant Bhangdiya Technical Manager,Pharma Business unit,Rettenmaier India Prashant Bhangdiya, Technical Manager, Pharma Business Unit, Rettenmaier India, is responsible for the JRS Pharma’s Excipients Business in South India, East India and Bangladesh, including but not limited to, supporting the business development team, customer service and logistics team.
He completed M Pharmacy from Poona College of Pharmacy, Pune (Bharati vidyapeeth). He has also completed Diploma in Intellectual Property (IP) rights from Symbiosis International, Pune. He has over 10 years of experience in the field of formulation development and pharma excipients.

Optima Pharma offers fully integrated turnkey solutions from a single source – combining filling systems, isolators, and freeze-dryers into harmonized production platforms. Designed for vials, syringes, and cartridges, these systems are backed by decades of industry expertise and engineered to meet the highest standards in process reliability, safety, and efficiency.
At the heart of the turnkey approach lies the seamless integration of technologies, paired with a deep understanding of pharmaceutical production requirements. Optima supports projects across the entire product life cycle with tailored or standardized solutions, comprehensive qualification services, and accelerated commissioning through its CSPE methodology, increasing production safety.
A key topic is the safe and efficient processing of GLP-1 products, which are transforming the treatment of Type 2 diabetes and obesity. These sensitive formulations require precise handling, especially in pre-filled syringes (PFS) and cartridges.
To meet this demand, Optima offers high-performance filling systems like the H6-10 and SV125. Both platforms are optimized for GLP-1 products,


featuring advanced sensorbased filling, process flexibility, and full GMP compliance.
The H6-10 achieves outputs
of up to 38,000 containers per hour, while the SV125 is ideally suited for smaller batch sizes up to 20,000/h. Both systems
support all common ready-touse container types as well as double-chamber cartridges, and handle products ranging
from watery to highly viscous –safely, gently, and with maximum precision.
Whether for clinical scaleup or commercial production, Optima’s integrated approach ensures consistent quality and reduced project risk.
More about Optima’s turnkeyapproach at Pharma Pro&PackIndia
Optima will be showcasing its turnkey expertise at Pharma Pro&Pack, taking place from September 18-20, 2025 in Hyderabad. Visit Optima in Hall 6, Booth H6-H41 to learn more about integrated turnkey systems and solutions for the processing of GLP-1 products.
Testo, a leader in measurement technology, introduces the new testo 174 BT (BT is Bluetooth enabled) series mini data loggers, designed to optimize indoor climate monitoring and ensure precise temperature and humidity control. The range offers smart, intuitive features that save time, reduce costs and provide hassle-free digital documentation for a wide range of industries, including food production, pharmaceuticals, and industrial sectors.
Innovative features for enhanced efficiency
The new testo 174 mini data loggers come in multiple models: testo 174 H BT, testo 174 T BT, testo 174 H, and testo 174 T. These loggers support Bluetooth and USB-C connectivity, providing seamless integration with mobile devices or PC software for easy data access and management. These loggers are designed to meet stringent quality assurance requirements and simplify compliance with regulatory standards.
Bluetooth-enabled data loggers: convenient and efficient
The testo 174 H BT (for temperature and humidity) and testo 174 T BT (temperature only) are equipped with Bluetooth, allowing for convenient wireless monitoring. Users can easily configure, record and evaluate measurement data using the free Testo Smart App. The app connects directly to the loggers from up to 30 meters away, enabling real-time data retrieval without the need to remove the device from its monitoring location. This wireless functionality ensures more efficient operations and significant time savings.
With automatic data backup, even if the battery is replaced, all previously recorded data remains intact. The Testo Smart App allows for seamless excel

exports, as well as the generation of professional PDF reports with signature and comment functionality. These features eliminate the need for manual record-keeping, reducing the risk of human error and ensuring paperless documentation. Both Bluetooth models are HACCP certified, ensuring they meet food safety standards, and the testo 174 T BT also holds NSF certification and complies with EN 12830, making them ideal for industries that require stringent monitoring, such as food production and pharmaceuticals.
For users who prefer traditional PC-based data management, the testo 174 H (temperature and humidity) and testo 174 T (temperature only) models with USB-C offer an intuitive solution. These loggers feature a large measurement range, from -30 °C to +70 °C for the Testo 174 T and -20 °C to +70 °C for the Testo 174 H, ensuring precise readings for a wide variety of applications. With a data storage capacity of up to 16,000 readings, these



devices can operate for extended periods, ensuring continuous monitoring. The data can be easily accessed and analysed using the ComSoft Basic software, included with the devices, for fast configuration and readout. ComSoft Professional offers advanced features for batch programming, data analysis, and report generation, making it easier to handle complex quality assurance tasks.
Like the Bluetooth models, these USB-C loggers also meet HACCP standards and EN 12830 requirements and the testo 174 T is NSF certified, ensuring reliable product safety in sensitive industries.
◆ Time & cost efficiency: Digital documentation and easy data re-
trieval reduce manual labour and the risk of human error, saving time and costs.
◆ Wireless connectivity: Bluetooth-enabled models offer convenient data readout via the Testo Smart App, streamlining processes.
◆ Large data storage: With up to 16,000 readings, these loggers support extended measurement periods without the need for constant monitoring.
◆ HACCP and NSF certification: Compliance with international standards ensures reliable product safety in food, pharmaceutical and industrial environments.
◆ User-friendly software: The free Testo Smart App and ComSoft software make data management simple and efficient.
Whether you're ensuring the quality of temperature-sensitive
goods or maintaining optimal indoor climates, the testo 174 series mini data loggers provide a reliable, efficient, and intuitive solution for all your measurement needs.
Service & Calibration: Located at our Head Office in Pune, Testo India has a high quality NABL accredited service & calibration lab. Instruments of any brand/make can be calibrated and serviced locally maintaining international standards. All calibrations are traceable to highly accurate international standards of the PTB Germany.
We undertake calibration jobs for temperature: contact as well as non-contact, humidity, pressure, air velocity, RPM, sound, flue gas & dew point instruments of any brand/make.
Testo India Testo India Pvt. Ltd. is a 100% subsidiary of Testo SE & Co. KGaA (headquartered in Titisee, Germany) which was established in 2006 and has shown phenomenal growth over the last 18 years with its head office in Pune, & a PAN India network.
Courtesy: Testo India Pvt. Ltd.For more info: Write to info@testo.in or visit www.testo.com
Top Syringe Mfg.Co.(P) Ltd is a leading manufacturer of glass syringe and glass tube engineering Products for decades
Ideal for laboratory liquid handling of various liquids including Plastic Sensitive Materials, hazardous chemicals, Adjuvant solutions, petroleum derivatives, glues, synthetic dyes, perfumes, essential oils and organic chemicals.
Features
● Glass Barrels & Pistons are made from borosilicate glass that provide protection against thermal shock.
● Graduation marks are permanently fused into a glass barrel.
● Luer locks are NickelChrome plated brass or stainless steel (SS316) as per ISO 594/1.
● Precision inner diameter ensures improved leak free smooth operation.
● Outstanding Chemical Resistance being of 3.3 Borosilicate Glass.
● Colored fiducial line at the tip of plunger facilitates dosage measurements.
● Inert liquid Path – Glass and Nickel-chrome plated brass.
● Autoclavable at 121oC at 15 PSI (1.05 kg/cm2) pressure.
● Reinforced truncated flange prevent syringe rolling.
● Interchangeable part of the syringe provide instant assembly without any sorting during bulk sterilization and hence improves operational efficiency.
● We Supply in private label terms as well (OEM), Special Graduation on request.
Top Syringe exports its products to over 40 countries worldwide and counting, keeping a single quality standard for local and export products.
The interchangeable Glass syringe is manufactured 100% in India by Top Syringe Mfg Co (P) Ltd. We are fully committed to supporting you with our full product range, spares, and technical assistance.

For more information, Contact:
Top Syringe Compound, W.E. Highway, Pandurangwadi, Mira road (East), Thane 401107 India. Mob: 81046 82560
Email : sales@topsyringe.com website: www.topsyringe.com
There is a wide range of processing technologies
Aseptic connections, they can be supplied as either discrete components or more often as pre-validated, pre-sterilized single-use systems –ready to open and use. They are used to connect single-use fluid paths – these fall into two basic categories based on how the connection is achieved: those that connect by welding or fusing together two fluid paths and those that mechanically couple two components installed in the fluid pathway.
The advantages of aseptic connectors are not only that they provide more robust sterility assurance, the connectors also permit the operation to be conducted within a lower classification for the surrounding cleanroom (for example, where a traditional connector would require ISO class 5 / EU GMP Grade A, and ISO class 7 / EU GMP Grade B area could be used).
Sterile connectors for aseptic processing (commonly referred to as ‘aseptic connectors’) enable two lines of tubing to be joined while maintaining a ster-

ile fluid pathway. Each line of tubing is pre- connected to one connector end. At the end of the connector is a removable membrane which facilitates the two pieces of tubing to connect (mechanical coupling). Once the two connectors ‘connect’ the membrane is removed. Through the act of connection, the process line is maintained as closed and sterility ideally assured. Furthermore, whereas the risk with traditional connectors includes the operator and hence finger plates are typically taken, the aseptic connector does not require finger plates to be taken as the impact of the operator and the level of bioburden carried on gloved hands will not affect the assurance of sterility as the connection is made.
The ImaSteriConnect™ is a single-use, genderless aseptic connector designed for fast, secure, and sterile fluid transfer between two fluid paths in controlled environments. Its intuitive design allows for reliable connections in upstream and downstream bioprocessing, including media and buffer preparation, cell culture, and fill-finish operations.
Traditional biologics like vaccines and mAbs as they have been early adopters of aseptic connectors and single-use technologies (SUT) overall. Some of the main benefits that led to SUT adoption are:
◆ Cost: Reduced manufacturing cost by elimination of cleaning and sterilisation steps
◆ Speed: Time and labour savings during setup and between operational cycles
◆ Sterility: Elimination of cross contamination between batches
Thermoplastic elastomer tubing and Sterile welder
To create an aseptic connection between the two lengths of complementary thermoplastic tubing, sterile tubing welders were built. Two ends of tubing from the same module are firmly inserted into the tube clamp mechanism as part of the welding process configuration. Sterile tubing welders make connections quickly and safely while maintaining a functionally closed system, whether dry tubing or wet tubing is being attached. Cross- contamination is limited because each wafer is thrown after a single-use. A functionally closed system is maintained by the ideal sterile welder.
A general question that can arise is how to determine if you should use a tube welder or an Aseptic connector. Tube welders are commonly used when a small number of connections are to be made per day and when only one dimension of tubing is used. As the number of connections increase use of aseptic connectors is preferred as they provide more flexibility of using multiple tube
sizes and take less time as tube welding takes more time.
Aseptic connectors are available in both gendered and genderless versions.
Gendered connectors are composed of two different connectors (typically male and female component) connected together to create a fluid pathway.
In Genderless connectors two components brought together to make a connection are identical.
Genderless connector offers various significant advantages over gendered connector leading to time saving, reduced inventory, increased operational and design flexibility.
Sterile connectors make operation easier and faster than other methods of connections in an aseptic environment, such as open manipulations, while completing the path of a sterile closed system.
Nonetheless, due diligence is required to ensure that an appropriate connector type is selected and tested to satisfy the user, product requirements, system integrity, and safety.
prabhat.b@amipolymer.com 9512235356
In pharmaceutical manufacturing, the threat of moisture isn’t always visible — but its impact can be significant. Whether it’s the clumping of powders, compromised tablet hardness, microbial activity, or rejected batches, excess humidity quietly undermines quality, shelf life, and compliance.
The challenge becomes even more acute during monsoons or in high-humidity zones where HVAC systems alone may fall short in maintaining the required RH levels — especially in areas like compression rooms, blister packing zones, or cold stor-
age for APIs. What’s needed is not just cooling, but precision air management — down
to the last decimal.
Over the years, industry leaders have turned to pur-
pose-built dehumidification systems to bridge this gap.
Companies like Bry-Air, with

decades of experience in delivering engineered air solutions, have quietly earned trust across pharma facilities. Their P95 dehumidifiers, known for energy efficiency and reliability, are often behind the scenes ensuring stability where it matters most.
As regulations tighten and the margin for error narrows, humidity control isn’t a line item — it’s a safeguard. An investment in consistency, not just compliance.
If you’d like to explore BryAir’s solutions for humidity control in pharmaceutical environments, write to: bryairmarketing@pahwa.com
Complete Washable, Greater Sealing and Pressure Resistant.
When it comes to pharmaceutical facilities and laboratories, clean rooms’ hygiene and protection from environmental contamination are the most important factors to consider. Gandhi Automation's PRIME NEO High Speed Doors are designed to provide superior sealing and resistance to pressure differences for clean rooms. And the added advantage is that it is absolutely washable.
PRIME NEO High Speed Doors assures:
Minimized Contamination: The reinforced polymer material is smooth-surfaced and jointfree. The door structure has been designed with full accessibility for water cleaning. Contamination risks are very minimal, making it fully compliant with



the requirements of clean environments.
Just-in-time Opening Cycle: Its smart design and German technology cause no delay in operation. Opening and closing in time reduces exposure time to a minimum, resulting in a reduction in energy costs and the risk of airborne contamination.
Sealing and Resistance to Pressure Differences: The door has a flexible curtain with horizontal FRP stiffeners guided by vertical guides. Pressure is evenly distributed over the whole curtain, pushing it against the guides, giving a perfect seal. This design allows the door to operate even under pressure differences up to 50 Pa in a controlled room environment.
Safety: PRIME NEO reduces the risk of accidents and damage. The free, flexible, and soft bottom edge minimizes hitting impact. The radar detector and inbuilt photocells reopen the door at the slightest impact.
Windows: The transparent material allows people to see coming traffic. Long-term transparency is assured, as the material is anti-fatigue PVC fabric.
Multi-Composite Structure:
The toughness of the side guides and their ability to absorb an impact avoid high repair costs. PRIME NEO is anti-corrosion and paint-free, which ensures durability in corrosive and aggressive environments.
Auto-reset: The door is equipped with auto-reset technology. In the event of an accidental crash, the curtain sets itself again. This unique system avoids time loss and reduces the risk of high repair costs.
To know more about PRIME NEO, write us at sales@geapl.com or call 022 6672 0200/0300

REGD.WITH RNI NO.MAHENG/2005/21398,POSTAL REGD.NO.MCS/164/2025 – 27,PUBLISHED ON 5TH EVERY MONTH, POSTED ON 9TH,10TH,AND 11TH EVERY MONTH POSTED AT MUMBAI PATRIKA CHANNEL SORTING OFFICE,MUMBAI – 400001



















