IMPACT REPORT August 2024


Working Towards Animal-Free Safety Assessments, Together













Working Towards Animal-Free Safety Assessments, Together












Thank you to our members and our collaborators for your unwavering dedication to advancing animal-free safety assessments. Since the launch of the International Collaboration on Cosmetic Safety (ICCS) in early 2023, much has been accomplished.
ICCS is focused on advancing the adoption of animal-free science for human and environmental safety assessments of cosmetics and their ingredients through science, education & training, and regulatory engagement.
While animal tests on cosmetics are increasingly banned around the globe, there remain regulatory requirements in different regions of the world that still mandate animal tests, including non-cosmetic related regulations, instead of permitting animal-free new approach methodologies (NAMs). NAMs have been demonstrated as equivalent or better for fulfilling such requirements.
This drives a need for animal testing even where viable NAMs exist. ICCS aims to address this paradox by understanding regulatory needs, supplying sound scientific solutions based on innovative, animal-free approaches for human and environmental health, and addressing education and training needs to collectively build confidence and promote regulatory acceptance.
ICCS will work with all stakeholders, including industry, safety assessors, regulators, and policymakers around the world to share the collective experience of applying NAMs and next-generation risk assessments (NGRA) frameworks to ensure cosmetic safety. In doing so, ICCS will accelerate the transition to global acceptance and use of animal-free science for human and environmental safety assessments for cosmetics.
ICCS will develop universal confidence in robust animal-free NAMs and NGRA frameworks such that animal testing is no longer deemed necessary for human and environmental safety assessments or registration of cosmetics and their ingredients. ICCS will be a Center of Excellence and preferred partner in all aspects of NAMs and NGRA frameworks and serve as a model of a collaborative approach to eliminate the need for animal testing.
Accelerate the global acceptance of animal-free science for human and environmental safety assessments of cosmetics through our science, education & training, and regulatory engagement programs.
All ICCS activities should adhere to four core principles:
1. Prioritize activities focused on addressing regulatory needs of cosmetics
2. Collaborate with relevant stakeholders to leverage existing regulatory, scientific, and educational resources, avoiding duplication of effort a nd maximizing harmonization
3. Develop broadly accessible tools and resources to enable robust animal-free safety assessments that protect human and environmental health
4. Strive for integration of human and environmental NAMs into NGRA frameworks where possible
Warm regards,
Erin Hill President and CEO
ICCS will attain its mission by focusing on three pillars, each with its own individual goal and strategic elements: Science – Core Science Team (CST), Education & Training – Core Education Team (CET), and Regulatory Engagement – Core Acceptance Team (CAT). These three pillars will be closely interlinked to maximize cross-fertilization.

The members of our organization come from three sectors: NGOs, Cosmetic & Chemical Trade and Research Associations, and Cosmetic Product and Ingredient Manufacturers.




Cosmetic & Chemical Trade and Research Associations










Cosmetic Product and Ingredient Manufacturers

























CHAIRMAN
Charles-François Gaudefroy
Unilever
VICE CHAIRMAN
Stéphane Dhalluin
L’Oréal
Seoyoung Kim
Amorepacific
Robert Landsiedel BASF
Horst Wenck
Beiersdorf
Carlos Berzunza Sánchez CASIC
Véronique Scailteur
Chanel
Brian Slezak
Colgate Palmolive
Kathleen Edgar Edgewell
Andrea Maltagliati EFfCI
Wilfried Petersen Evonik
SECRETARY
Jon Lalko Estée Lauder
TREASURER
Michael Southall Kenvue
Darren Praznik Cosmetics Alliance Canada
John Chave Cosmetics Europe
Mylene Lanvin Coty
Tim Storey-Hall Croda
Monica Engebretson Cruelty Free International
Emma Meredith CTPA
Dan Selechnik Fragrance Creators Association
Tracey Spriggs Haleon
Simone Hoffmann-Doerr Henkel
Troy Seidle Humane Society International
Gregory Ladics IFF
Martina Bianchini IFRA
Kristie Sullivan IIVS
Ian Callan Innospec
Katie Sminkey Inolex
Junji Yamamoto JCIA
Javier Avalos Kao
Anne Laissus-Leclerc LVMH
Garrett Moran Oriflame
Esperanza Troyano P&G
Francine Lamoriello PCPC
Elizabeth Baker PCRM
Julia Baines PSCI
Joanna Rowland Reckitt
Anne Marie Api RIFM
Morihiko Hirota Shiseido
Ian Bartlett Syensqo
Martin Cowen Takasago

Gavin Maxwell Unilever
Carsten Goebel Wella Company



Erin Hill* President & CEO

David Allen* Senior Director, Human Health Science & Operations

Donna Macmillan** Director, Education & Regulatory Engagement

Amelie Ott** Director, Environmental Sciences

Andreea Cuciureanu** Senior Project Manager, Human Health Sciences
*US Based
**Europe Based

Ryan Heisler* Senior Project Manager, Environmental Sciences

Develop NAMs and NGRA frameworks for cosmetics and ingredient safety assessments that will be protective of human and environmental health
Build a community to support continuous learning to establish confidence in, and application of, NAMs and NGRA for evaluating cosmetics

Accelerate the acceptance of NAMs and NGRA frameworks through standardization and validation activities, engagement with regulators, and supporting the global alignment of cosmetic regulatory requirements


Since the launch of ICCS in 2023, many member groups have been established to undertake the organization’s mission. The mission’s three pillars, each with its own individual goals and strategic elements, include: Science – Core Science Team (CST), Education & Training – Core Education Team (CET), and Regulatory Engagement – Core Acceptance Team (CAT). Work within the pillars is done by Delivery Teams (DTs) and Working Groups (WGs). Input and review of our efforts are sought from the Strategic Planning Team (SPT) and the Board of Directors (BoD).










Delivery Teams and Working Groups develop and oversee scientific programs in alignment with the ICCS strategy.



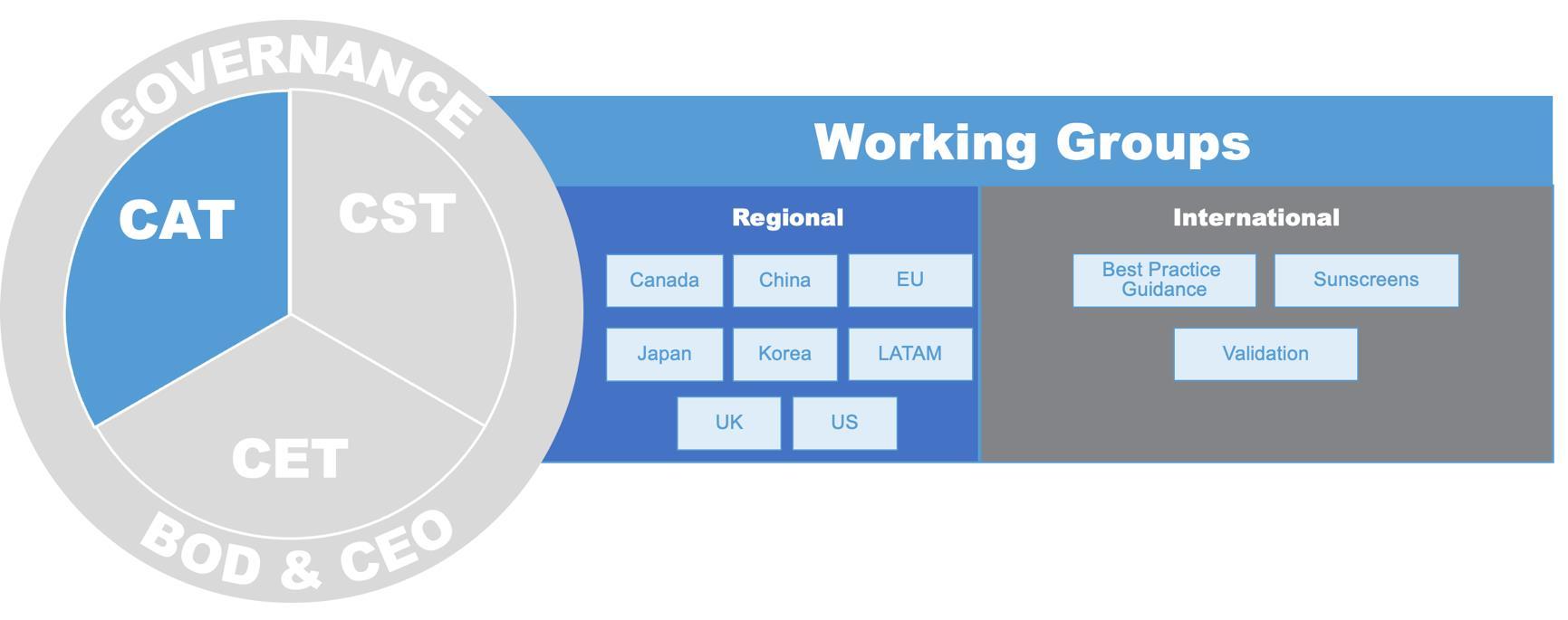
Working Groups focus on regional opportunities and challenges to implement NAMs and NGRA. International and Regional efforts focus on developing guidance and standardization of existing methods and approaches.



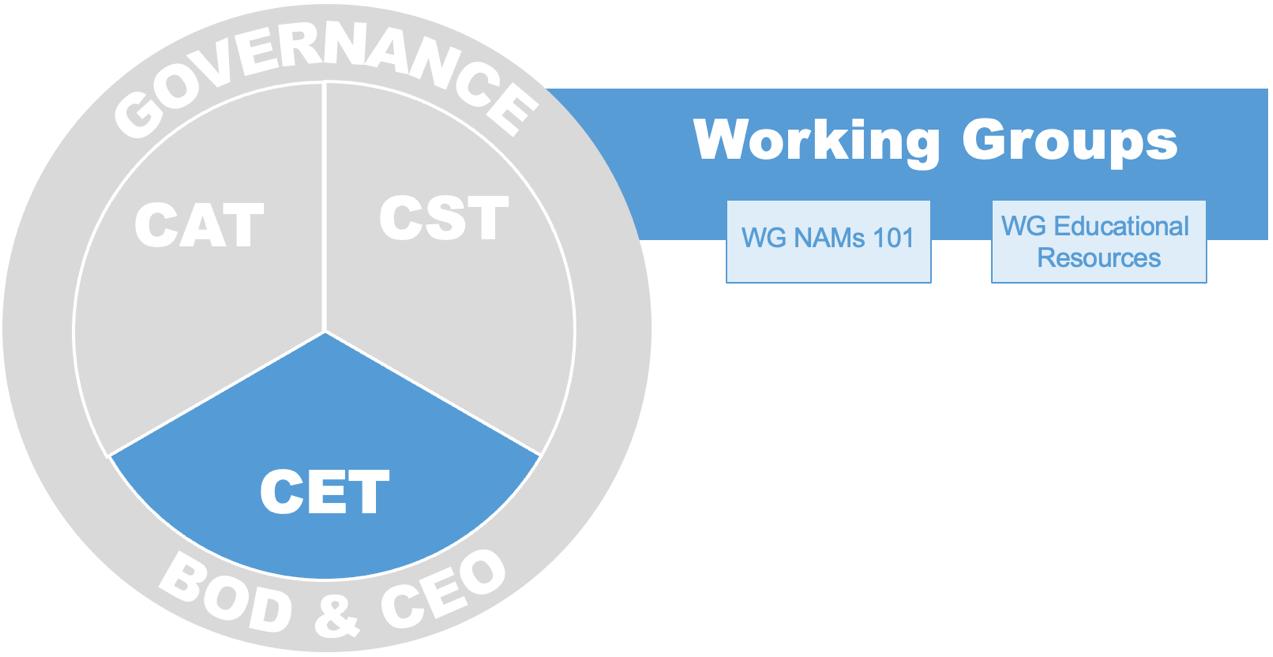
Educational Working Groups highlight existing educational materials and create resources to close gaps in education and training needs. The CET also responds to regional requests for support.
The CST, CAT, and CET are currently developing an integrated strategic roadmap to accelerate the global acceptance of animal-free science for human and environmental safety assessments.
Regulatory Implementation/ Acceptance of NGRA
ICCS
International/Regional Workgroups

ICCS Activities
▪ 36 CST Projects
▪ Data Collection & Curation
▪ Landscape Analyses
▪ In-Kind Collaborations
▪ OECD & ICCR Efforts
▪ Dissemination & Training
Regulatory Engagement
International/Regional Stakeholder Engagement
ICCS Outputs
▪ Case Studies
▪ Updated Optimized Standardized NAMs Toolbox
▪ Best Practice Guidance
▪ OECD Test Guidelines
▪ Educational Materials
ICCS brings together internationally recognized scientists to advance animal-free safety assessments. Currently, the ICCS team is managing 36 projects focused on developing non-animal methods and frameworks for cosmetics and ingredient safety assessments that will be protective of human and environmental health.

The ICCS Science Advisory Committee is comprised of experts from human health and environmental safety holding key positions in regulatory agencies, research institutions, and validation centers worldwide. The SAC reviews and provides feedback on ICCS scientific strategies and project proposals.
Takao Ashikaga, Ph.D.
Division of Risk Assessment
JaCVAM
Tara Barton-MacLaren, Ph.D.
Research Manager
Health Canada
Scott E. Belanger, Ph.D.
Independent Senior Executive in Environmental and Sustainability Management of Chemicals
Natalie Burden, Ph.D.
Acting Head of Toxicology NC3Rs
Katie Paul Friedman, Ph.D.
Supervisory Toxicologist US EPA
Alison Harrill, Ph.D.
Associate Director for Toxicology US EPA
Nicole C. Kleinstreuer, Ph.D. NICEATM Director
National Institute of Environmental Health Sciences
Charlie Menzie, Ph.D. Environmental Scientist and Cultural Explorer Independent
Octavio Presgrave, Ph.D.
Coordinator of Brazilian Center for Validation of Alternative Methods (BraCVAM) Toxicology at ICTB/Fiocruz Brazilian CVAM
Kristin Schirmer, Ph.D.
Head of Department of Environmental Toxicology Cell Biologist/Toxicologist
Eawag - Swiss Federal Institute of Aquatic Science and Technology


Tomasz Sobanski, Ph.D.
Team Leader at Computational Assessments and Alternative Methods Unit European Chemicals Agency
Marize Campos Valadares, Ph.D. Full Professor of Toxicology Universidade Federal de Goiás/ ANVISA
Maurice Whelan, Ph.D. Head of the Systems Toxicology Unit and ECVAM European Commission, Joint Research Center (JRC)
Carole Yauk, Ph.D. Professor
University of Ottawa

The ICCS science projects represent a balanced portfolio with emphasis on moving projects from mapping to acceptance. Mapping/ Background Research
- Phase I metabolite testing
SS - Exploring existing NAMs
- Feasibility study
SS - Uncertainty in NGRA
GT - OECD TG RhE comet and MN assays
EI – Surfactant Testing
ED – in silico/in vitro methods
- Photogenotoxicity
SS - Difficult to test chemicals
- Metabolism
ED - PARC/VUB collab
SS - DA & NGRA framework reg acceptance
Effects &
and
CI:Cheminformatics
Endocrine Disruption
EI: Eye Irritation Exp: Exposure
GT: Genotoxicity OE: Overview Effects
P/Bio: Persistence/Biodegradation
PP: Pharmacology Profiling
RAX: Read across
SS: Skin Sensitization
TGx: Toxicogenomics
US: Underrepresented Species
Collaborations
ICCS member companies provide funding for selected science proposals aligned with the Roadmap each year. NGO members provide in-kind contributions of their expertise.

▪ Symposium on Environmental Degradation and Biodegradation of Water
Soluble or Dispersible Polymers
▪ Biodegradation Test Methods Based on Increased Cell Concentrations (ICC)
▪ Best Guidance Practices for the Mobility (M) Assessments of Unstable,
Reactive or Ionizable Substances
▪ Cosmetic Ingredient Applicability of the OECD TG 249: RTgill-W1 Fish Cell
Line Acute Toxicity Assay
▪ Case Studies to Develop & Test an Integrated Animal-Free Next
Generation Human and Environmental Safety Framework – Phase 1
▪ Informing Toxicologically Relevant Molecular and Cellular Targets Using Expert-Guided AI/ML Approach
▪ NAMs for Systemic Toxicity Landscape Analysis

ICCS, working with members and external collaborators, have offered PAT and MERCI as tools for environmental NAMs and NGRA.
PAT: Persistence Assessment Tool
Science: Freely available tool for better persistence/biodegradation assessments
Education & Training: In-person and virtual training sessions
Regulatory engagement:
▪ Close link with and feedback from ECHA and EU Member States
MERCI: Models to Evaluate the direct Release of Cosmetic Ingredients into natural water
Science: Freely available tools (in process) for environmental external exposure assessments (e.g. UV filters)
Education & Training: Ongoing for industry (members + external) & regulators
Regulatory engagement:
▪ In process for EU ECHA and US EPA
▪ Member application of MERCI in ingredient consortium

Collaborators



Collaborators


Over the past few decades, scientific advances in alternatives to animal testing, consumer demands, and animal test bans have driven the investment to ensure cosmetics and personal care product safety assessments can be performed ethically, without animal testing.
Regulatory bodies and policymakers set standards, develop guidance, and pass legislation on cosmetics and ingredients with the goal of protecting consumers and the environment. Although research and animal-free science methods have progressed, many policies and regulations have not yet incorporated them as complete replacements for animal tests. ICCS aims to close this gap by understanding regulatory needs and supplying sound scientific solutions based on innovative animal-free approaches.




ICCS communicates with international and regional authorities to understand regulatory data requirements. Through scientific dialogue, ICCS seeks to increase confidence and acceptance of animal-free approaches. Listed are examples of such organizations.







ICCS collaborated with colleagues in ICAPO, the United States, and Canada to submit a project proposal to the Organisation for Economic Co-operation and Development (OECD) which will advance non-animal approaches for systemic toxicity. The project, Progressing Integrated Approaches for Testing and Assessment (IATA) for Systemic Toxicity: Guidance to Advance the Evaluation of Next Generation Risk Assessments (NGRA)
Approaches for Systemic Toxicity was submitted to the Working Party on Hazard Assessment (WPHA), a group of regulatory and scientific representatives from OECD member countries tasked with creating tools and guidance for the assessments of chemicals, such as the OECD (Quantitative)
Structure– Activity Relationship [(Q)SAR] Toolbox and the IATA Case Studies Project.

While many guidances exist for application of standardized, validated methods, the guidance to integrate different approaches into a risk assessment framework is lacking.
ICCS is developing a series of BPG for safety assessors, aimed towards increasing the uptake and implementation of nonanimal methods in a range of contexts.
▪ Developed with input from global subject matter experts among the ICCS membership
▪ Clear and concise step-by-step guide on how to apply a process, beginning with problem formulation/decision context, suitable for global use
ICCS has been a contributor to the Stakeholder Session at the last two meetings of the ICCR. Through continued engagement, ICCS will seek input from ICCR on project deliverables, such as the Best Practice Guidance tool, to maximize their utility to regulatory authorities.










The CIR evaluates individual chemical compounds used in cosmetic products and publishes their safety assessments in the International Journal of Toxicology. The Expert Panel works towards gaining confidence in NAMs for all endpoints relevant to cosmetic ingredient risk/safety. ICCS participated in the 169th Meeting of the Expert Panel for Cosmetic Ingredient Safety, presenting “Identifying Eye Irritation Hazards without using Animals.” This was the first in a planned series of interactions with the Expert Panel.

ICCS seeks to grow our collective expertise in animal-free safety science within member companies and the wider community by promoting existing learning materials and creating new learning content to address gaps.
ICCS partners with existing training providers to deliver hands-on and virtual learning sessions designed to support safety assessors as they build their knowledge and increase their confidence in using animal-free approaches ensuring a more rapid and efficient transition to animal-free safety assessments.

To expand access to NAMs and NGRA concepts, ICCS is supporting a seminar organized by the Home and Personal Care Ingredients (Central and Eastern Europe) and IKW. A case study will be used to present how these approaches can be used in risk assessments.


To foster dialogue among industry, NGOs, and the regulatory community in developing countries, ICCS supported a session at CTDC to explore unique opportunities and challenges in developing countries with regards to NAMs and NGRA concepts.

While many high-quality educational and training materials exist, there are still unmet needs. ICCS works with subject matter experts among its members to create accessible content to fill those gaps.
Designed for an audience new to NAMs and NGRA frameworks, this tool will be available on the ICCS website and promoted via webinars in various regions.
ICCS staff participated in a Continuing Education (CE) course at the Society of Toxicology (SOT) Meeting in 2024 with a presentation titled, “Establishing Confidence in New Approach Methodologies for Inhalation Toxicity Testing.” ICCS will continue to work with members to create CE courses at major conferences such as the SOT, EuroTox, and SETAC.
To make our educational resources more impactful, ICCS and its members work to create content in native languages. ICCS collaborated with its members to create a video presentation of an NGRA case study in Japanese for the Convention of the Japanese Cosmetic Science Society. ICCS has also partnered with a Science Advisory Committee member to create a webinar series for LATAM in Spanish or Portuguese languages.
To further the ICCS mission, the staff collaborates with external stakeholders through presentations at scientific conferences, paper and poster sessions, as well as regularly meeting with ICCS members.


Here are examples of meetings in which ICCS has engaged with scientific and regulatory communities to discuss work in Science, Education & Training, and Regulatory Engagement. Communication and collaboration with these organizations aid in maximizing the impact of ICCS programs.















The Persistence Assessment Tool (PAT): Implementing a Methodology for Data Quality Evaluation and Weight of Evidence in Persistence Assessments
Biodegradable or Not? Developing a Standardized International Approach to Assessing the Biodegradability of Cosmetic Formulations
Breaking the Silos: Towards a Holistic Approach of AnimalFree Human and Environmental Health Safety Assessments of Cosmetics
Advances Towards a Harmonized Environmental Safety Analytical Toolbox for Cosmetic Ingredients (ATEST)
Biodegradation of WaterSoluble Polymers – Reviewing In Silico Methods
MERCI: A Tiered Exposure Modeling Framework to Support the Environmental Risk Assessments of Cosmetic Ingredients Directly Discharged into Natural Waters
Improving Understanding of Toxicologically Relevant Molecular and Cellular Targets: The ICCS Higher Tier Evaluation Working Group
Advancing Animal-Free Environmental Safety Assessments of Cosmetics
A Review of In Silico and In Vitro Methods for use in a Risk Assessment of a Substance Acting via Oestrogen or Androgen Modalities
ICCS creates hybrid team meeting opportunities at conferences to progress projects and maximize collaboration. The ICCS Core and Delivery Team chairs meet twice per year to ensure the organization’s activities are coordinated among the three pillars.



SETAC 34th Europe Annual Meeting Hybrid Environmental DT Meeting


To continue the impact of our mission, ICCS staff serve on many scientific advisory panels, expert working groups, and project steering committees.
▪ Advisory Group: Multi-Stakeholder Roundtable on the Commission Roadmap Towards Phasing Out Animal Testing for Chemical Safety Assessments
▪ ECETOC Biodegradation Simulation Workshop
▪ EPAA Environmental Safety Assessments Group for EU Commission Roadmap
▪ Expert Panel for Cosmetic Ingredient Safety Members and Liaisons
▪ HESI Initiated ecoNAM Network and Workshop
▪ Steering Committee for The World Congress on Alternatives and Animal Use in the Life Sciences (WC13)
▪ OECD Expert Group on Alternative Methods for Skin/Eye Irritation and Phototoxicity
▪ OECD Expert Group for Defined Approaches for Skin Sensitisation
▪ OECD Working Party on Hazard Assessment
▪ OECD Expert Group for New Marine Biodegradation Test (MaP) - via Newcastle University
▪ SETAC Persistence Science Interest Group
ICCS staff work to communicate the mission utilizing the web, social media, the press, and informational meetings. Following are examples of these efforts.



The Educational Resources section on the ICCS website is under construction and will soon be published, showcasing ICCS resources and highlighting existing resources.
In the past year we have tracked global engagement and inquiries per the analysis of the ICCS website, newsletter, and social media accounts.
Mission Reach Across Social, Newsletter, and Website
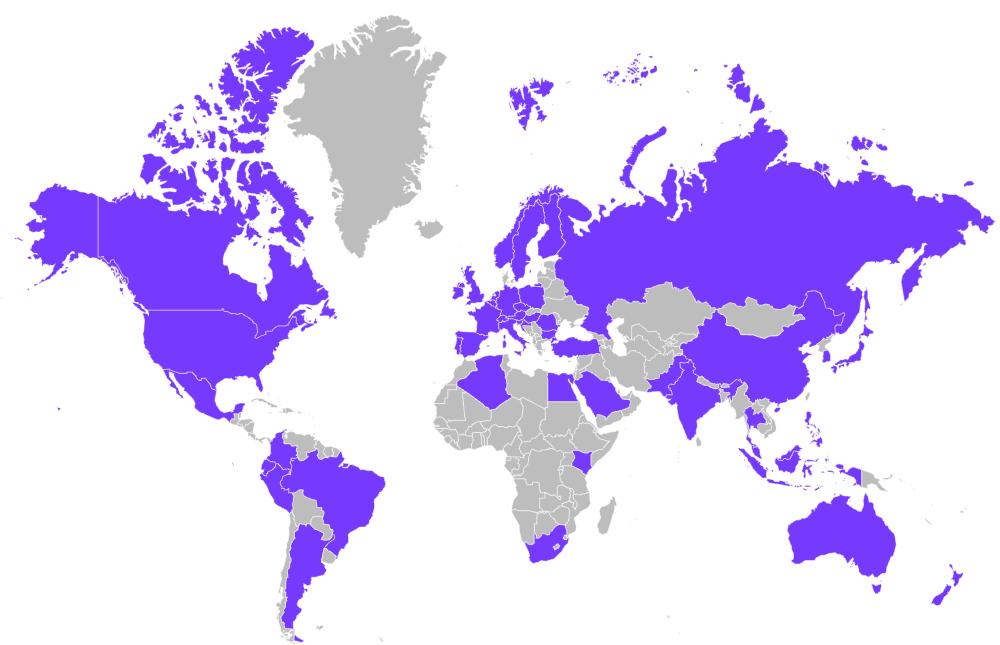






More than 200 participants from member organizations, interested companies, contract research organizations, and consulting companies attended our first Town Hall meeting. ICCS will hold these on a biannual basis to communicate our programs to members and the broader stakeholder community.


Media stories about the ICCS mission
have appeared globally. Here are some examples.






















