2 minute read
3D drawing
TECHNOLOGY
Advertisement
3D drawing
The world’s fi rst 3D printing pen allows you to draw freehand plastic sculptures
The 3Doodler pen, which is manufactured by WobbleWorks, is a handheld 3D printer that functions in a similar way to a hot glue gun. The pen uses melted, extruded plastic to build three-dimensional images.
The 3Doodler started life as a cannibalised 3D printer. The developers removed one of the plastic-extruding heads and added a handle to make a prototype that they called ‘the Teacup’. Further revisions of the new technology added a small fan at the tip, which blows cold air onto the plastic as it exits the device, cooling it down and enabling it to harden much more rapidly. Indeed, it solidifi es so quickly that the pen can be used to draw vertically in the air, creating instant 3D models.
The 3Doodler uses standard three-millimetre (0.12-inch)-thick strands of plastic specifi cally designed for 3D printing applications. They are fed into the nib, which can reach temperatures of up to 270 degrees Celsius (518 degrees Fahrenheit).
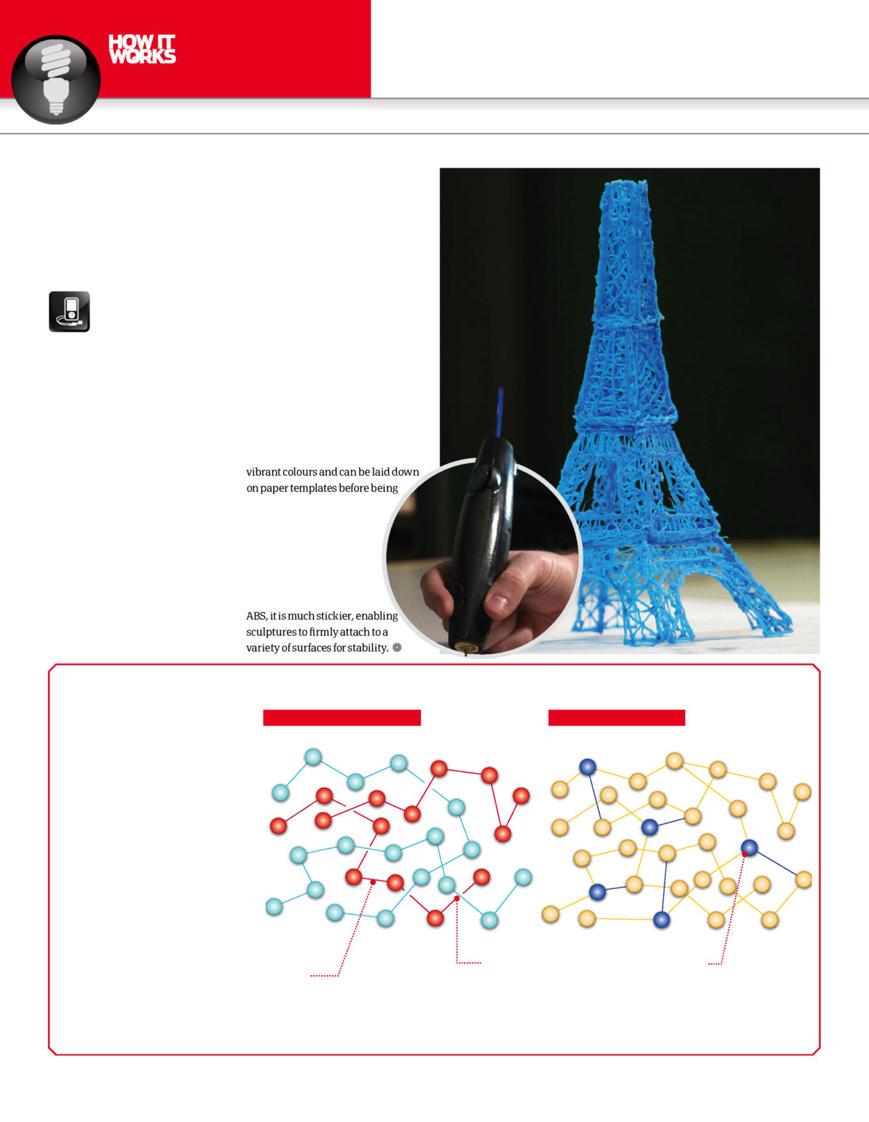
Artists have a choice of acrylonitrile butadiene styrene (ABS) or polylactic acid (PLA). ABS plastic is one of the most commonly used in this fi eld to make everything from toys to car parts; it sets rapidly, comes in many vibrant colours and can be laid down on paper templates before being peeled off to assemble more complicated structures.
PLA, on the other hand, is biodegradable, has a lower melting point and is made from cornstarch. Although it doesn’t solidify as quickly as ABS, it is much stickier, enabling sculptures to fi rmly attach to a variety of surfaces for stability.
All manner of things can be made with the 3Doodler from basic ornaments and decorations to more complex objects like this model of the Eiffel Tower
Plastic at a molecular level
3D printers use thermoplastics (aka thermosoftening plastics), which soften when heated and then solidify when cooled. In their solid state, the molecular chains that make up thermoplastics are held together by weak links. These are based primarily on differences in the charge of the atoms that make up the molecules, providing small-scale electrostatic attraction between adjacent chains. When heated, the increase in energy in the molecules means that they vibrate free of these weak interactions, melting the plastic. As it cools, the links can re-establish, producing a solid structure again. This is in contrast to thermosetting plastics, which form cross-links when they’re heated, permanently joining chains together to produce stronger 3D structures.
Thermoplastic elastomer
Chains
Plastics are made up of long chains, or polymers, with several thousand repeating units.
No bonds
There are no chemical bonds between chains in thermoplastics; instead they are held together by weak electrostatic interactions.
Thermoset elastomer
Cross-links
In thermosetting plastics, chemical bonds develop between chains when the plastic is heated, forming permanent links and preventing re-melting.








