
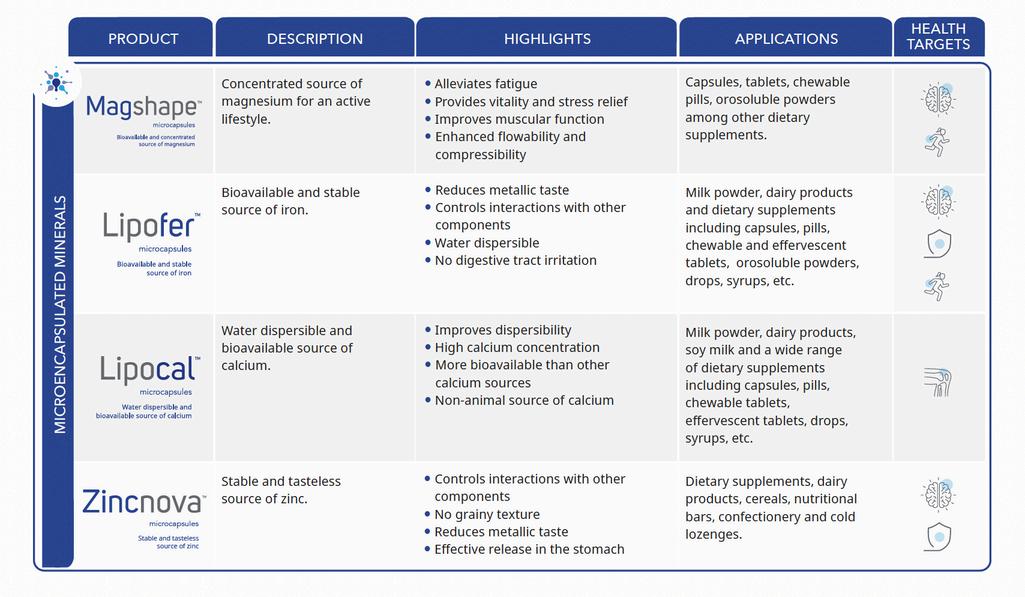
Enabling Differentiated Nutraceutical Solutions with Optimized Ingredient Delivery





Lubrizol's core microencapsulation technology offers several competitive advantages: Controlled interactions with other components


Enabling Differentiated Nutraceutical Solutions with Optimized Ingredient Delivery






Lubrizol's core microencapsulation technology offers several competitive advantages: Controlled interactions with other components





LipocalTM microcapsules is a water dispersible micronized source of calcium coated with lecithin which increases calcium dispersibility and absorption
There is a growing awareness of the importance of maintaining a high calcium intake throughout life, not just for bone health but also for the health of other body systems
•Improves dispersibility
•High calcium concentration
•More bioavailable than other calcium sources
•Non-animal source of calcium
COMPOSITION:
Identity preserved soy Lecithin*
PRODUCT FORMAT:
ACTIVE CONTENT:
DISPERSIBILITY:
White Powder or granules
33.7-39.6% elemental calcium
Highly dispersible
Calcium intake, particularly that during childhood, is a major determinant of bone mass in adults, and it also influences the rate of bone associated with aging Osteoporosis, a disease affecting many millions of people around the world, is characteri- zed by bone fragility that over time leads to bone fracture.
The major benefit of calcium is preventative, mitigating the risk of developing osteoporosis during the aging process Low dietary intake of calcium is also associated with higher risks of colon cancer and hypertension and may affect normal growth in children
Although dairy products account for about 70% of dietary calcium, total calcium intake remains inadequate This realization has led to the calcium fortification of an expanding number of foods Particularly pressing is the need to ensure adequate calcium intakes for vegetarians and for those with milk aversions.
LipocalTM microcapsules is a bioavailable non-animal source of calcium, suitable for vegetarians, that can be easily incorporated in a wide range of food applications

A study of dietary calcium absorption in guinea pigs was performed at the University of Barcelona, in order to compare the bioavailability of calcium from three different sources: Tricalcium Phosphate (TCP), LipocalTM microcapsules and dairy calcium
Four groups of five male Hartley guinea pigs weighing 400- 450g were orally administered a dose of 3 43 mg calcium/kg body weight, corresponding to two dairy yogurts of 100 grams for a person of 70kg. Blood samples were extracted before and after 10, 20, 30, 40 and 50 minutes of administration and the plasma calcium concentration was measured through Inductively Coupled Plasma Atomic Emission Spectrometry
The absorption profile of calcium during 50 minutes was obtained and the overall uptake per time was calculated

Lipocal
TM microcapsules
A faster calcium absorption profile is observed with LipocalTM microcapsules compared to other sources of calcium increases calcium blood concentration in animals faster
The corresponding area under the curve (AUC) for plasma calcium concentration for each formulation was estimated measuring the amount of calcium absorbed during the experimental period
Lipocal
TM microcapsules is a highlybioavailable calcium source
Lipocal
Results show microcapsules is 41% more bioavailable than TCP and 15% than milk calcium.
















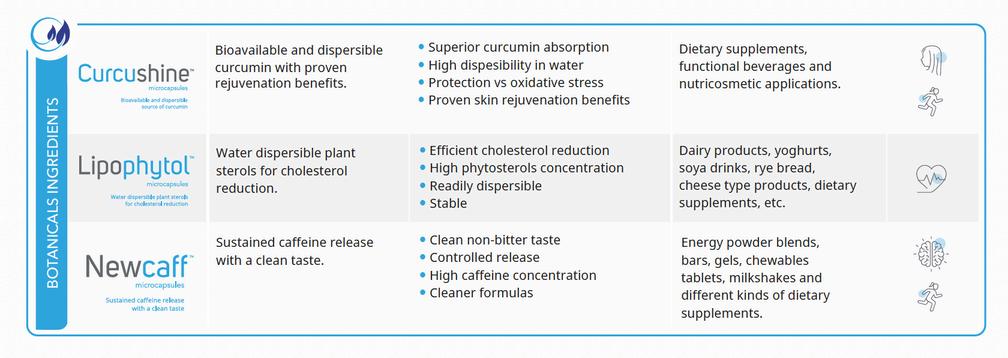
LipophytolTM microcapsules is a dispersible form of pine tree sterols which has been microencapsulated to make them water dispersible
•Efficient cholesterol reduction
•High phytosterols concentration
•Readily dispersible
• Stable
COMPOSITION:
Maltodextrin, Inulin, Sucrose Ester
PRODUCT FORMAT:
White to off-white powder
ACTIVE CONTENT:
DISPERSIBILITY:
68-75% or 87-94% phytosterols Highly dispersible
High blood cholesterol level is the first risk factor for coronary heart disease The cholesterol lowering effect of plant sterols is well documented in literature Consumption of 1 5 to 2 4 grams of plant sterols per day has been found to potentially lower LDLCholesterol by 7% to 10%.
The European Food Safety Authority (EFSA), has approved claims for plant sterols namely: i) ”plant sterols have been shown to lower/ reduce blood cholesterol with a daily intake of 1.5-2.4 g of plant sterols/stanols” and ii) ”plant sterols/stanols contribute to the maintenance of normal blood cholesterol levels with a daily intake of 0 8 g of plant sterols/stanols”
The U S Food and Drug Administration (FDA) approved the following claim for phytosterols: “foods containing at least 0 4 grams per serving of plant sterols, eaten twice a day with meals for a daily total intake of at least 0.8 grams, as part of a diet low in saturated fat and cholesterol, may reduce the risk of heart disease“
Due to their insolubility in water and lipids, phytosterols incorporation into foods and beverages formulations has been very challenging
In this respect, LipophytolTM microcapsules facilitates incorpora- tion of phytosterols in food matrices due to its high dispersibility
Cardiovascular Health
The cholesterol lowering effect of LipophytolTM microcapsules was studied in vivo using knock out Apo E mice in a study conducted by Dr Blanco at the Institut de Recerca de l’Hospital de la Santa Creu i San Pau in Barcelona.
Three groups of mice were fed either i) a high fat diet (control), ii) a high fat diet with LipophytolTM microcapsules or iii) a high fat diet with phytosterol esters
Serum analyses were determined for LipophytolTM microcapsules and raw
phytosterols diets, to assess their activity on triglyceride levels
Protective effect against aortic lesions
The effect of LipophytolTM microcapsules on aorta atherosclerotic lesions was examined on every group of mice after 8
weeks of treatment. Heart and proximal aortas were removed and atherosclerotic lesions quantified
Lipophytol
Lipophytol TM microcapsules TM microcapsules resulted in reduced damaged aorta, an indication of ability to protect arteries
Atherosclerosis damage test indicated that LipophytolTM microcapsules was the most effective in protecting mice arteries from the harmful effects of the high fat diet. significantly reduced the concentration of VLDL+LDL cholesterol in serum compared to the control
Results indicate that LipophytolTM microcapsules might enhance the activity of phytosterols against hypertriglyceridemia



MagshapeTM microcapsules is a highly concentrated and bioavai- lable source of magnesium source effective at restoring and maintaining a healthy level of magnesium in human cells and bones.
•Helps to relax and alleviates fatigue
•Provides vitality and stress relief
•Improves muscular function
•Enhanced flowability and compressibility
COMPOSITION: Magnesium Oxide, Modified Starch, Sunflower Lecithin
PRODUCT FORMAT: White-off-white to cream powder granules
ACTIVE CONTENT: 30-34% elemental Mg
DISPERSIBILITY: Highly dispersible
Magnesium is an essential mineral involved in energy metabolism, respiratory function and maintenance of normal muscle contraction and relaxation. Magnesium deficiency may lead to the distortion of neuromuscular function, suggesting a possible association between magnesium and muscle cramps
However, despite magnesium’s critical role, maintaining its intake at an adequate level has been frequently overlooked, therefore magnesium supplementation has been widely recommended
In this respect, MagshapeTM microcapsules can contribute to an appropriate muscular function and an adequate energy level for better performance Furthermore, given its high magnesium content, more magnesium gets into the bloodstream to deliver its health benefits. Additionally, MagshapeTM microcapsules as contains magnesium oxide, it has more elemental magnesium, available to tissues
An improved stability, flowability, compressibility and pleasant taste compared to other magnesium sources, is also achieved thanks to the microencapsulation technology applied in the product
Active Nutrition
Tablets and capsules Powders
Imagined for life. Enabled by Science.™
MagshapeTM
The following parameters of were compared to the unencapsulated magnesium oxide salt to demonstrate the significant improvement in the rheological properties of the microen- capsulated product
In vitro efficacy - Muscular function improvement
High performance conditions were simulated (continuous stimulation at different frequencies for a long time) followed by induction of ramps (cramps) to study the effect of MagshapeTM microcapsules on muscle relaxation
Both muscle tissue bioprinted samples, control and, MagshapeTM microcapsuleswere stimulated at 10 Hz for 10 s and the response of the muscles was determined.
A randomized, double-blind, repeated crossover trial with 40 healthy adults involved four stages of single-day sampling at 7-day intervals, where blood samples were taken at 0, 1, 4, and 6 hours after fasting and consuming each of the four magnesium products





Its properties including pleasant taste, good flowability and compressibility, make MagshapeTM microcapsules an ideal magnesium source for nutraceutical products
MagshapeTM
MagshapeTM microcapsules microcapsules microcapsules outperforms unencapsulated magnesium oxide improve muscular function thanks to its relaxation capacity
After treatment with MagshapeTM microcapsules the muscle relaxes more quickly, so it can maintain a frequency of 10 Hz (simulation of cramp) without entering tetanic contraction. significantly increases magnesium levels within 1 hour and maintains elevated levels for at least 6 hours
The study found that MagshapeTM microcapsules significantly enhance magnesium absorption while reducing common gastric side effects compared to other magnesium sources
Ed



NewcaffTM microcapsules is a novel caffeine delivery system which has been designed to mask the bitter taste of caffeine and provide sustained release
•Clean non-bitter taste
•Controlled release
•High caffeine concentration
•Cleaner formulas
COMPOSITION: Caffeine, Mono and diglycerides of fatty acids
PRODUCT FORMAT: White to off-white or Off-white to light brown powder or granules
ACTIVE CONTENT: 56-60% or 73.3-78.3% caffeine
DISPERSIBILITY: Limited dispersibility
Caffeine is a methylxanthine alcaloid which is well-known for its properties in the central nervous system, its action as a metabolic stimulant, and a fatigue reducer Caffeine, however, is quickly absorbed and therefore its stimulating effect can be felt instantly after consumption leading to energy fluctuations. In addition, this compound has a bitter taste which compromises its addition into food systems
One of the trends driving the sports nutrition market growth is the sustained energy claim Following this trend, there is currently a need for caffeinated products which can continuously provide the desired benefits associated without the unwanted effects for a longer time.
This need can be met by NewcaffTM microcapsules whichisdesigned by using lipid hot-melt fluid bed microencapsulationtechnique to provide a controlled release of caffeine withtheadditional benefit of masking its objectionable bitter taste
Morphology and characteristics of microcapsules
physicochemical
NewcaffTM
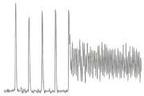
Scanning electron microscopy (SEM) observations show caffeine powdered particles with an angular shape and polyhedral appearance On the other hand, microcapsules containing caffeine have a round shape with little granules adhered to its surface forming the lipid insulating coating


The bitter taste of caffeine particles in NewcaffTM microcapsules is masked
Caffeine particles received an uniform and stable wrapping via NewCaffTM microcapsules technology successfully masking the bitter taste of caffeine
In vitro
Caffeine release from the NewcaffTM microcapsules was tested using a standard method following the Health Canada official method of determination of the disintegration time DO-25 by being submitted to digestion process For this purpose, the analysis was carried out simulating in vitro digestive conditions at physiological temperature (37oC) and at physiological stomach and intestine pH
A sustained release of the caffeine from NewcaffTM microcapsules is observed
microcapsules
showed a good retention and an improved in vitro sustained release profile when compared to unencapsulated caffeine NewCaff

ZincnovaTM microcapsules is a non-reactive form of zinc oxide that has been microencapsulated in a food grade fat carrier to prevent moisture uptake and to delay undesirable interactions with other components of food formulations, thus preserving its bioavailability
•Controlled interactions with other components
•No grainy texture
•Reduced metallic taste
•Effective release in the stomach
COMPOSITION:
Glyceryl esters of fatty acids
PRODUCT FORMAT:
ACTIVE CONTENT:
White to Off-white powder
18-22% elemental zinc
DISPERSIBILITY:
Not dispersible
Immune Health
Zinc is one of the most important trace minerals in human nutrition. It is a structural component of more than 100 enzymes and is involved in numerous aspects of cellular metabolism, wound healing as well as in growth maintenance during childhood and adolescence.
It also plays a unique function in boosting immunity and proper functioning of the smell and taste systems It has also been shown to be effective in reducing the duration and severity of cold symptoms
Zinc deficiency can be due to inadequate intake of the mineral or to problems with absorption especially in people with gastrointestinal disorders
Various foods including oysters, red meats and poultry as well as some cereals and vegetables contain zinc naturally; however the presence of high levels of phytate in cereals can compromise the bioavailability of zinc from these sources Vegetarians are often at a high risk of zinc deficiency, thus the need to include zinc fortified cerals in their diets.
ZincnovaTM microcapsules comprises a novel encapsulated zinc oxide for an easy fortification of a wide range of food applications with this nutrient
Tablets and capsules Liquids Powders
Imagined for life. Enabled by Science.™
The morphology of fortifying agents may affect organoleptic properties of foods. The microcapsule structure of ZincnovaTM microcapsules was determined by Scanning Electron Microscopy.

A panel of volunteers tasted a commercial milk fortified with 0 1 mg/ml ZincnovaTM microcapsules The non-enriched milk was used as a control.
An orange juice was fortified with a concentration of 0 1 mg/ml as ZincnovaTM microcapsules or non-encapsulated zinc oxide The same commercial juice was used as a control The samples were homogenized and pasteurized and the changes in color were controlled by a colorimeter.
In order to study the availability of zinc after the digestion of a food fortified
with ZincnovaTM microcapsules, a released assay was performed following the “Determination of the disintegration time of tablets official method of Health Canada”
A simulated GIT release test was conducted using 0.1 mg/ml with or without pepsin (digestive enzyme) The pH was adjusted to 1 2-1 5 to simulate the stomach conditions.
ZincnovaTM
doesnotprovide a grainy texture
ZincnovaTM
does not alter the taste
Organoleptic stability (taste & color)
The small size and spherical geometry of ZincnovaTM microcapsules diminish the contact surface, minimizing the grainy texture in the mouth. Effective releaseinthe stomach with
The taste of the milk with ZincnovaTM microcapsules did not show any remarkable difference respect to the non-fortified one.
The color of the juice containing ZincnovaTM microcapsules was similar to that of the commercial juice
ZincnovaTM microcapsules microcapsules microcapsules
Results showed that 75% of zinc ZincnovaTM microcapsules was released from ZincnovaTM microcapsules in the absence of pepsin while almost 100% of the mineral was released after 2 hours in the presence of pepsin.
