CHOICE. Choose the one alternative that best completes the statement or answers the question.
Which is an example of matter?
Which of the following is a chemical property of aspirin? 5) _______ A)
It melts at 135°C. B)
It does not decompose when protected from moisture. C)
It does not readily dissolve in water. D)
It is a white crystalline solid in pure form at room temperature. E)
It can be compressed into tablets when mixed with cornstarch.
Which of the following is a physical change?
A) the condensation of water vapor
the explosion of nitroglycerin C) the rusting of iron
the baking of a potato
Which statement describes a physical change? 7) _______ A) digesting your lunch B) turning on a flashlight C) lighting a match D) burning the morning toast E) winding an alarm clock 8
All of the following are examples of forms of energy except 8) _______ A) water.
heat.
electricity.
sound.
light.
Which of the following causes a chemical change?
_______
digging a hole
A)
B)
slicing a tomato
C)
pumping gasoline
D)
winding an alarm clock
E)
turning on a flashlight 1
A chemist is given an unknown gas sample. Which observation describes a chemical property of the sample?
10) ______
A)
It weighs 11.2 grams.
B)
It has a sharp, stinging odor. C)
It is colorless.
D)
It extinguishes a glowing splint. E)
Its density is greater than that of air. 1
Which example illustrates a form of potential energy?
11) ______ A)
charged battery
B)
running water C)
lighted match
moving truck
D)
E)
flying bird 1
Which example illustrates a form of kinetic energy?
12)
a moving stream B) a sleeping baby C) a flashlight battery D) a book on the top of a shelf E) a rock at the top of a hill
Which of the following is an example of matter? 13) ______ A) wisdom B) forgiveness C) jealousy D) light E) clothing
Which best describes the size and shape of a sample of gas? 14) ______ A) definite volume and definite shape B)
volume and shape are both determined by the container C)
definite volume, but shape is determined by the container D)
volume and shape cannot be described E)
volume determined by container, but definite shape
Which term does not describe a conversion between states of matter? 15) ______ A) melting B)
Chemistry is important to the study of which of the following subjects?
A chemist is given an unknown sample. Which of her observations is not a physical property? 17)
The sample is a colorless liquid.
B)
The density of the liquid is 0.789 g/mL.
C)
The sample has an odor similar to gasoline.
D)
The sample size is 55 mL.
E)
The sample is flammable. 1
1-butanethiol, one of the compounds giving skunks their distinctive odor, freezes at -115.7°C and boils at 98.5°C. What is its phase at 37°C, the normal body temperature of humans? 18)
a mixture of solid and liquid E)
a mixture of liquid and gas
1
Malic acid, a compound used to increase the acidity of fruit-flavored products, freezes at 128°C and boils at 150°C. What is its phase at 100°C, a temperature used in food processing applications? 19)
a mixture of solid and liquid
a mixture of liquid and gas
Which of the following is a physical property of aspirin?
20)
2
Aspirin does not decompose when tightly sealed in a bottle. B)
Aspirin can moderate some heart disorders when ingested. C)
Aspirin can be pressed into tablets when mixed with cornstarch. D)
Aspirin reacts with water to produce salicylic acid and acetic acid. E)
Aspirin yields carbon dioxide and water vapor when burned.
2 Which factor determines the state of matter in which a substance exists?
Barium sulfate is described as a white crystalline solid that melts at 1580°C and decomposes at 1600°C. At a temperature of 500°C, you would expect a sample of barium sulfate to be a
22) ______ A) yellow liquid. B) colorless liquid. C) white cloud of vapor. D) white crystalline solid. E) form that cannot be determined. 2
Which of the following is a pure substance? 23)
Which of the following observations demonstrates that a solid sample is a compound? 24) ______ A)
Heating the substance causes it to melt, then to boil.
B)
Heating the substance causes no visible color change.
C)
Crushing the sample does not affect its other properties.
D)
It cannot be broken down into simpler substances by chemical methods.
E) It cannot be broken down into simpler substances by physical methods.
Which of the following are states of matter?
2
25) ______ A) solution B) solid C) precipitate D) suspension E) all of the above 2 A pure substance
26) ______ A) is composed of more than one element. B) is chemically inert. C) always has the same elemental composition.
D) can be broken into its components by physical means.
E) changes color when placed in bright sunlight.
Which of the following is a mixture? 27) ______ A)
2
Which of the following can be classified as a pure compound? 28)
carbon, C
alcohol in water, OH in O C) sugar,
A chemical reaction occurs when 29)
A) a substance is heated. B) a substance is dissolved in water. C) a substance's structure is altered. D)
two or more substances are mixed. E)
all of the above
SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. 3
List and describe two differences between pure substances and mixtures.
30)
List three examples of pure substances and three examples of mixtures that have not been previously discussed in class. 31)
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
Consider the chemical reaction described as
3
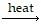
mercury(II) oxide mercury + oxygen.
Identify the reactant(s) in this example.
32) ______ A)
mercury + oxygen B) heat C) oxygen D)
mercury E)
mercury(II) oxide
Vegetable oil is a(an) ________, and is found in the ________ phase.
33) ______ A)
element; liquid B)
mixture; solid C) compound; gas D)
mixture; liquid E) compound; solid
3
3
A reddish powder is heated gently in a loosely covered container. After the heating a silvery metal remains in the container, and a glowing wooden splint placed into the container bursts into flame. The original substance is a(an) ________, and the solid and gas produced are ________.
34) ______ A) compound; elements B) element; compounds
Each symbol denotes an element except
Which of the following is an element?
What is the chemical symbol for chlorine?
is the chemical symbol for calcium?
is the chemical symbol for copper?
is the chemical symbol for chromium?
chemical symbol represents a metalloid?
The most common element by mass percent in the human body is 42) ______ A) sulfur. B) phosphorus. C) carbon. D) oxygen E) hydrogen.
Xylene, a compound with the formula C8H10, is composed of 43) ______
A)
ten atoms of carbon and eight atoms of hydrogen.
B)
eight atoms of carbon and ten atoms of hydrogen. C) eight atoms of calcium and ten atoms of helium.
D)
any combination of atoms of carbon and hydrogen in a four to five ratio.
E)
any combination of atoms of carbon and hydrogen that add up to 18 total.
Which symbol does not denote a compound? 44) ______ A)
4
4
Which element is not essential for human life?
What is the symbol for tungsten?
What element is represented by the chemical symbol K?
element is represented by the chemical symbol Ag?
Of the elements listed, the most abundant by mass percent in the earth's crust is 49) ______ A) iron.
B) sodium.
C) aluminum. D) hydrogen.
E) silicon.
The formula for ammonia, , represents a compound composed of 50) ______ A)
one atom of nitrogen and three atoms of helium.
B)
three atoms of nitrogen and three atoms of hydrogen.
C)
one atom of nickel and three atoms of hydrogen.
D)
three atoms of nitrogen and one atom of hydrogen.
E)
one atom of nitrogen and three atoms of hydrogen.
The formula for sodium carbonate, , represents a compound composed of 51) ______
A)
two atoms of sodium and three atoms of carbonate.
B) six atoms of sodium, two atoms of carbon, and six atoms of oxygen.
C)
one atom of sodium and one atom of carbonate.
D)
two atoms of sodium, three atoms of carbon, and three atoms of oxygen.
E)
two atoms of sodium, one atom of carbon, and three atoms of oxygen.
The formula for calcium sulfate, Ca , represents a compound composed of
5
5
5
A)
one atom of calcium, four atoms of sulfur, and four atoms of oxygen.
B)
two atoms of calcium, four atoms of sulfur, and four atoms of oxygen.
C)
one atom of calcium and one atom of sulfate.
D)
one atom of calcium, one atom of sulfur, and four atoms of oxygen.
E)
one atom of calcium and four atoms of sulfate.
The formula for glucose, C6H12O6, represents a compound composed of 53)
A)
six atoms of carbon, ten atoms of hydrogen, and four atoms of oxygen.
B)
six atoms of carbon, twelve atoms of hydrogen, and six atoms of oxygen.
C)
one atoms of carbon, two atoms of hydrogen, and one atoms of oxygen.
D)
six atoms of carbon and six atoms of water.
E)
six atoms of carbon and two atoms of water.
The formula Ba represents a compound composed of 54)
A)
two atoms of barium, four atoms of sulfur, and four atoms of oxygen.
B)
one atom of boron, four atoms of sulfur, and four atoms of oxygen.
C)
one atom of barium, one atom of sulfur, and four atoms of oxygen.
D)
one atom of barium, four atoms of sulfur, and four atoms of oxygen.
E)
one atom of boron, one atom of sulfur, and four atoms of oxygen.
Element Z has the following properties:
a. noncombustible
b. unreactive
c. colorless, odorless gas at room temperature
5
5
5
d. nonconductor of electricity
Element Z can be classified as a ________ and will be found on the ________ of the Periodic Table.
55) ______
A)
non-metal; zigzag line
B)
metalloid; zigzag line
C)
non-metal; right
D)
metal; zigzag line
E)
metal; left
5
Amphetamine, a commonly abused drug, is composed of nine atoms of carbon, 13 atoms of hydrogen, and one atom of nitrogen. The chemical formula of amphetamine is written
56)
C9H13N1
C9H13
C13H93N0
C9H13N
C9H1N13
B)
C)
D)
E)
5
Caffeine, the active ingredient in coffee, is composed of seven atoms of carbon, ten atoms of hydrogen, and four atoms of nitrogen. The chemical formula of caffeine is written
57)
C7H10N4O2 B)
C7H10N4O
C) CHNO D)
C7H10N4
C7H12N4O4
E)
Which chemical symbol represents a non-metal?
Which chemical symbol represents a metallic element?
Element Z has the following properties: a. silvery-white solid at room temperature b. malleable c. used as a catalyst
d. conducts electricity Element Z can be classified as a ________ and will be found on the ________ of the Periodic Table.
______ A)
metalloid; zigzag line B) non-metal; zigzag line C) metal; left D) non-metal; right E) metal; zigzag line
MATCHING. Choose the item in column 2 that best matches each item in column 1. Match the following.
1. The composition of a pure substance is always the same, regardless of the source, but the composition of a mixture can vary.
2. Mixtures can be separated into their components by physical changes; some pure substances can be separated into components by chemical change. 31) Anything that makes sense in your class. 32)
