

Review of AAD Annual Meeting
Amy Paller and Yagiz
Matthew Akiska discuss their award-winning abstracts Interviews
JAK Inhibitors: A New Frontier in Dermatology Feature



Editorial Board
Editor-in-Chief
Dr Michael Gold Gold Skin Care Center, Nashville; Tennessee Clinical Research Center, USA
Editorial Board
Dr Todd Schlesinger Clinical Research Center of the Carolinas, South Carolina, USA
Dr Raj Chovatiya Rosalind Franklin University Chicago Medical School; Center for Medical Dermatology and Immunology Research, Illinois, USA
Dr Jacqueline Watchmaker U.S. Dermatology Partners, Arizona, USA
Dr Brian Hibler Dermatologist, Private Practice, New York, USA
Dr Neal Bhatia Therapeutics Clinical Research, California, USA
Dr Leon Kircik Indiana University Medical Center, USA; Icahn School of Medicine at Mount Sinai Medical Center, New York, USA
Aims and Scope
AMJ Dermatology is an open-access, peer-reviewed eJournal committed to helping elevate the quality of healthcare for skin, hair, and nail diseases. AMJ Dermatology endeavours to increase knowledge, stimulate discussion, and contribute to a better understanding of these conditions.
The journal is published annually, 6 weeks after the American Academy of Dermatology (AAD) Annual Meeting, and features highlights from this event, alongside interviews with experts in the field, reviews of abstracts presented at the event, and indepth features on congress sessions. The journal also covers advances within the clinical and pharmaceutical arenas by publishing sponsored content from congress symposia, which is of high educational value for healthcare professionals. This undergoes rigorous quality control checks by independent experts and the in-house Editorial team.
AMJ Dermatology also publishes peer-reviewed research papers, review articles, and case reports in the field. In addition, the journal welcomes the submission of features and opinion pieces intended to create a discussion around key topics in the field, and broaden readers’ professional interests. The journal is managed by a dedicated Editorial team that adheres to a rigorous double-blind peer-review process, maintains high standards of copyediting, and ensures timely publication.
AMJ endeavours to increase knowledge, stimulate discussion, and contribute to the delivery of world-class updates in the clinical realm. We do not publish veterinary science papers or laboratory studies that are not linked to patient outcomes. Further details on coverage can be found here: www.emjreviews.com
Editorial Expertise
AMJ is supported by various levels of expertise:
• Guidance from an Editorial Board consisting of leading authorities from a wide variety of disciplines.
• Invited contributors who are recognised authorities in their respective fields.
• Peer review, which is conducted by expert reviewers who are invited by the Editorial team and appointed based on their knowledge of a specific topic.
• An experienced team of editors and technical editors.
• A team of internal and independent medical writers.
Peer Review
Every review article, case report, feature, and research article published in AMJ undergoes peer review by at least two independent experts.
On submission, all manuscripts are assessed and undergo a technical check by the AMJ Editorial staff to determine their suitability for the journal and appropriateness for peer review. Editorial staff identify appropriate reviewers who are selected based on their specialist knowledge in the relevant area. All peer review is double-blind.
Following review, manuscripts are either accepted, returned to the author(s) for modifications, or rejected. Editorial staff ensure that necessary amendments have been made. The Editor of AMJ has final discretion over any proposed amendments. Manuscripts authored by members of the Editorial Board are subjected to the same double-blind process. Short opinion pieces are published following internal
review and publication is at the discretion of the Editor. Congress-associated content authored by the AMJ Editorial staff undergoes internal quality control checks. Congressrelated content sponsored or funded by our industry partners undergoes quality control checks independently. Industrysupported content that falls into any of the categories that are eligible for peer review, undergoes the same peer review process.
Submissions
We welcome contributions from professionals, consultants, academics, and industry leaders on relevant and topical subjects. Healthcare professionals wishing to discuss potential submissions, please email: editorial.assistant@emjreviews.com
To submit a paper, use our online submission site: www.editorialmanager.com/e-m-j
Submission details can be found through our website: www.emjreviews.com/contributors/authors
Reprints
All articles included in AMJ are available as reprints (minimum order 1,000). Please contact hello@emjreviews.com if you would like to order reprints.
Distribution and Readership
AMJ is distributed through controlled circulation to healthcare professionals in the relevant fields globally.
AMJ is available through the websites of our leading partners and collaborating societies.
AMJ journals are all available via our website: www.emjreviews.com
Open Access
This is an open-access journal in accordance with the Creative Commons Attribution-Non Commercial 4.0 (CC BY-NC 4.0) license.
Congress Notice
Staff members attend medical congresses as reporters when required.
This Publication
Launch Date: 2024
Frequency: Annually Online ISSN: 3033-3741
All information obtained by AMJ and each of the contributions from various sources is as current and accurate as possible. However, due to human or mechanical errors, AMJ and the contributors cannot guarantee the accuracy, adequacy, or completeness of any information, and cannot be held responsible for any errors or omissions. AMJ is completely independent of the review event (AAD 2024) and the use of the organisations does not constitute endorsement or media partnership in any form whatsoever. The cover photo is of San Diego.
Front cover photograph: San Diego, California © f11photo / stock.adobe.com
EMJ Podcasts
The EMJ Podcast aims to provoke conversations around the latest trends and innovations in healthcare, provide engaging and educational content for healthcare professionals, and hosts conversations with physician entrepreneur, Jonathan Sackier. Listen today www.emjreviews.com

Welcome letter
Editor
Evgenia Koutsouki
Editorial Managers
Anaya Malik, Darcy Richards
Copy Editors
Noémie Fouarge, Kirsty Hewitt, Katheeja Imani, Jenna Lorge
Editorial Co-ordinators
Natasha Meunier-McVey, Abigail Craig
Editorial Assistants
Victoria Antoniou, Ada Enesco, Helena Bradbury
Creative Director
Tim Uden
Design Manager
Stacey Rivers
Senior Designer
Roy Ikoroha
Designers
Steven Paul, Owen Silcox
Junior Designers
Dillon Benn Grove, Shanjok Gurung
Head of Sales
Robert Hancox
Senior Project Manager
Korey Coffey
Marketing Director
Kristina Mestsaninova
Chief Content Officer
Justin Levett
Chief Operating Officer
Dan Scott
Chief Commercial Officer
Dan Healy
Founder and Chief Executive Officer
Spencer Gore
Dear Readers,
 Evgenia Koutsouki Editor
Evgenia Koutsouki Editor
Welcome to the inaugural issue of Dermatology – The American Medical Journal. We are delighted to bring you the first issue of what promises to be the start of an exciting journey, where we delve into key topics in the field, especially for healthcare professionals based in the USA.
In this, the first of several American journals that we will publish throughout 2024, we bring valuable insights from the American Academy of Dermatology (AAD) Annual Meeting, which took place in vibrant San Diego, California, USA. It was an absolute privilege to be able to attend this meeting, and to talk to a plethora of prominent experts in person.
As well as giving you the key abstract highlights from the congress, we also have in-depth coverage of two exciting congress sessions on JAK inhibitors, and the treatment of psoriasis. JAK inhibitors have taken center stage in this issue, as we also feature an infographic exploring JAK inhibitors in the treatment of alopecia areata.
In addition to some great interviews with notable experts in the field of dermatology, we are delighted to introduce some members of our newlyfounded Editorial Board through a Q&A session.
Finally, do not miss our peer-reviewed articles, which include an observational study of cutaneous manifestations in patients with chronic kidney disease, and two case reports.
I would like to close by thanking our newly-founded Editorial Board, and our contributors and peer reviewers for ensuring a high standard in the first issue of the journal. Over the next year, we look forward to receiving your manuscript submissions, and we are already eagerly awaiting next year’s congress.
Sales


Foreword

Dear Colleagues,
Welcome to our first issue of Dermatology, The American Medical Journal (AMJ), featuring a range of peer-reviewed articles, interviews with field experts, and an infographic. Also included is our review of the American Academy of Dermatology (AAD) Annual Meeting 2024, held in San Diego, California, USA, from March 8–12. The review offers a detailed summary of the most significant highlights and content presented throughout the congress.
The AMJ team had the pleasure of speaking to various field experts, namely Amy Paller and Yagiz Matthew Akiska, who summarized their award-winning abstracts presented at AAD 2024, and shared valuable perspectives on promising advances within dermatology. Andrew Alexis shed light on his work surrounding dermatologic disorders that are prevalent in populations with skin of color. Interviews with several Dermatology AMJ Editorial Board members, namely Jacqueline
Watchmaker, Brian Hibler, and Neil Bhatia, provide fantastic insight into their career to date, the unique challenges presented by their respective fields, and their plans for shaping our new journal.
Our articles cover a diverse range of topics. Rajar et al. present their research investigating the effects of dermal fillers on vaginal laxity and sexual function, while Bhardwaj et al. report on the incidence of cutaneous manifestations in patients with chronic kidney disease. Tampouratzi et al. present the fascinating case of severe and unresponsive alopecia areata treated with baricitinib, a JAK inhibitor. This new class of drugs is further explored in our infographic and AAD congress review, which spotlights a session entitled ‘JAK Inhibitors: A New Frontier in Dermatology’.
I would like to express my gratitude to all those who have made valuable contributions to this inaugural issue of Dermatology AMJ. I hope you enjoy reading, and find it insightful.

 Dr Michael Gold Gold Skin Care Center, USA
Dr Michael Gold Gold Skin Care Center, USA
AAD 2024
Review of the 2024 American Academy of Dermatology (AAD) Annual Meeting
Location:
San Diego, California, USA
Date: March 8–12, 2024
Dermatol AMJ. 2024;1[1]:10-22.
Citation:
https://doi.org/10.33590/dermatolamj/FUXA6219.
In the sunny, coastal city of San Diego, California, USA, the American Academy of Dermatology (AAD) hosted the 2024 AAD Annual Meeting, bringing together the brightest minds in the dermatology space in the USA and Canada.
The number of attendees was close to 20,000, likely making the 2024 AAD Annual Meeting the largest dermatology meeting ever by attendance. The scientific program was alive with 339 sessions on dermatological topics ranging from hidradenitis suppurativa and atopic dermatitis, to clinical practice topics such as dermoscopy and bedside manner differences with patients.
The Plenary Session provided attendees with updates from Academy leadership and the chance to learn from award-winning named lecturers and select guest speakers on current topics that impact dermatologists, including changes in healthcare concerns and changes in educational delivery tools.
The Chair’s welcome was delivered by Hensin Tsao, Harvard Medical School, Boston, Massachusetts, USA, who tantalized the audience of 13,000 collaborators with the
promise of opportunities given at the Annual Meeting. The dermatology field is witnessing an unprecedented era of breakthroughs in therapies and technologies. These exciting and fundamental changes to its practice bring vast and complex challenges. Tsao encouraged the audience to rally together to support this revolution with collective wisdom: “Together we can accelerate the pace of innovation, share knowledge freely, and uplift our practice and to new heights.” He called on dermatologists to ignite and collaborate in a shared vision to heal, innovate, and to inspire using the Annual Meeting as a beacon of collaboration. Each year, the AAD Annual Meeting offers the invaluable experience of discussing and networking for new ideas to advance dermatological care and impact 300 million lives.
The first lecture in the Plenary Session, the ‘Clarence S. Livingood, MD Memorial Award and Lectureship: Photodermatology: Past, Present and Future’, was given by the award recipient, Henry W. Lim, Henry Ford Hospital, Detroit, Michigan, USA. Lim has served as the President of the AAD and several other notable bodies, and in 2020 was awarded the highest honor within the Academy, an Honorary Membership of the AAD. Lim presented
“Together we can accelerate the pace of innovation, share knowledge freely, and uplift our practice and to new heights.”

“While there may be a very difficult road ahead, there is no barrier, no challenge, no obstacle that we cannot surmount together when we work together as a united group of this nation's brightest and most talented physicians.”
his lecture on photodermatology, stating that this is an area of concern for him, and that if the discipline does not invest its time and resources into this research, there may be a risk of it slipping away.
The next person invited to the stage was Terrence A. Cronin Jr., Jackson Memorial Hospital, Melbourne, Florida, USA, for the President’s Address, ending his 1-year term as president of the organization. He reflected on his journey from a transformative year continuing to advance education and standards of care and providing healthcare for millions of Americans and beyond. He paid respect to the dedicated members and leaders of the Academy, commending the strong educational program and the cohesiveness of the many disciplines represented by the AAD, calling on members to work to preserve these assets, especially the focus on education to distinguish the AAD from other organizations.
Brian J. Druker, OHSU Knight Cancer Institute, Portland, Oregon, USA, was awarded the ‘Lila and Murray Gruber Memorial Cancer Research Award and Lectureship: Imatinib as a Paradigm of Targeted Cancer Therapies’. He shared highlights on the breakthrough research of the first products that targeted cancer specifically without harming normal cells. Having been a part of this breakthrough, he shared what made this possible and how this could be applied to many other diseases.
In what he deemed as the most important of his career, the President-Elect Address was delivered by Seemal R. Desai, University of Texas Southwestern Medical Center, Dallas, USA, in his inaugural speech as incoming president. As he picked up the baton for his dream role from his esteemed colleague Cronin Jr., he shared his message and his passion with the AAD members: “While there may be a very difficult road ahead, there is no barrier, no challenge, no obstacle that we cannot surmount together when we
work together as a united group of this nation's brightest and most talented physicians.”
The 'Marion B. Sulzberger, MD Memorial Award and Lectureship', established in 1983, was awarded to Brian S. Kim, Icahn School of Medicine at Mount Sinai, New York City, USA. The lecture, ‘Sensing Inflammation at the Barrier’, focused on mechanisms that underlie skin inflammation and the sensation of itch as a fundamental broad model paradigm for neuro-immunology.
The final lecture within the session was the 'John Kennedy Jr. MD Lifetime Achievement Award and Lectureship'. It was established in 2021 to recognize outstanding dermatologists, who throughout their career have been committed to improving the treatment of patients from underserved populations, including those scared of complications. The recipient of this award was Patricia Treadwell, Indiana University School of Medicine, Indianapolis, USA. She spoke about health equity in dermatology, and provided clarity and understanding on the role for individual dermatologists.
Finally, and remarkably, the Keynote Speaker for the meeting was William Shatner, the 93-year-old actor from Montréal, Québec, Canada, best known for his role in the Star Trek television series. He addressed the audience with an extraordinary account of his life, his acting career spanning seven decades, his diagnosis with Stage IV melanoma, and receiving surgical treatment followed by unspecified immunotherapy.
Read on for further coverage and late-breaking research from the 2024 AAD Annual Meeting. ●
Lutikizumab for the Treatment of Moderate-to-Severe Hidradenitis Suppurativa
LUTIKIZUMAB, a dual-variable-domain IL 1α/1β antagonist, can be used to treat patients with moderate-to-severe hidradenitis suppurativa (HS) who have failed anti-TNF therapy. However, the safety and efficacy has been seldom explored. Adults with a clinical diagnosis of HS who failed anti-TNF treatment, were recruited to a Phase II, mulitcenter, randomized, double-blind, placebocontrolled study to address this.
Patients were centrally randomized at baseline in a 1:1:1:1 ratio to one of four treatment groups, aiming for 40 patients in each treatment group. Group 1 received 300 mg lutikizumab every week, Group 2 received 300 mg every other week, Group 3 received 100 mg every other week, and Group 4 received a placebo every week. Across all treatment groups, the drug was administered at baseline and Weeks 1–15, with the final efficacy being assessed at Week 16.
Overall, 153 patients were randomized across 54 sites, and the majority (70.6%) had severe baseline Hurley Stage 3 disease. While the response rate for Group 3 was 27.0%, both Group 2 (59.5%) and Group 1 (48.7%) showed greater response rates over the placebo group (35.0%) in the primary endpoint at Week 16 of a Hidradenitis Suppurativa Clinical Response (HiSCR) of 50. Further results indicated a posterior probability of observing a positive treatment difference versus placebo of 98.5% and 89.3% in Groups 2 and 1, respectively. At the secondary endpoint, a higher proportion of participants achieved a skin pain response (NRS30) of NRS ≥3 in Group 2 and 1 compared to the placebo. Importantly, all doses were safe and well-tolerated.
"153 patients were randomized across 54 sites."
Overall, lutikizumab 300 mg administered every week and 300 mg administered every other week showed positive results when compared to a placebo in patients with moderate-to-severe, hardto-treat HS. ●

Injectable Polidocanol for Excess Submental Fat Reduction
CURRENT treatment modalities for excess submental adiposity, a common esthetic concern, may have poor efficacy, prolonged recovery, unpredictable results, and adverse events. Novel research, presented at the AAD 2024 Annual Meeting, shed light on the safety and efficacy of injectable polidocanol for submental fat reduction.
The team conducted a Phase IIB clinical trial to evaluate the safety and efficacy of injectable polidocanol, a non-ionic detergent and adipolytic. A total of 51 subjects were enrolled (intent-totreat population) and assigned to four dose cohorts, where they were administered either 2.0%, 3.0%, 4.5% polidocanol, or vehicle, up to six times, 4 weeks apart. Study endpoints included Clinician Submental Fat Score (CSFS) and Patient Submental Fat Score (PSFS) on a 0–4 point scale. The team also obtained local skin reactions, safety labs, and electrocardiograms from patients. Forty subjects completed all assessments and at least four treatments (completer population).
Out of the intent-to-treat patients in the 3.0% and 4.5% dose groups, 62% and 54% demonstrated a 2-Grade improvement in the CSFS/PSFS U.S Food and Drug Administration (FDA) composite endpoint (P<0.01), respectively. In the complete population, 80% of the 3.0% and 4.5% dose groups achieved a 2-Grade improvement in FDA composite endpoint (P<0.01). Polidocanol also demonstrated a rapid onset of efficacy, with an average of 1-Grade improvement after only two treatments. Furthermore, a total of 98% of local skin reactions were graded as 0 (absent) or 1 (mild), with the majority being classed as Grade 0.
Overall, the team noted that polidocanol showed a fourfold improvement in Grade 2 response relative to published data on deoxycholate, the only FDA-approved injectable drug for submental fat reduction. Polidocanol also demonstrated an improved safety profile compared to deoxycholate, with significantly lower frequency of adverse events, including pain, bruising, and edema. The study supports a superior efficacy and tolerability of injectable polidocanol compared to current products. ●
"A total of 98% of local skin reactions were graded as 0 (absent) or 1 (mild), with the majority being classed as Grade 0."
Comparing Office and Home Phototherapy for Psoriasis
HOME phototherapy may be the more effective method for treating psoriasis in the USA, according to new research presented at the AAD 2024 Annual Meeting. Previous studies have demonstrated that home phototherapy is less costly and often preferred by patients, compared to office phototherapy, which is cost-effective but difficult to access; however, there are limited clinical data to support this.
"Previous studies have demonstrated that home phototherapy is less costly and often preferred by patients."
In order to compare the efficacy of the two treatments, researchers carried out a randomized trial at 42 dermatology practices, involving a total of 783 patients (mean age: 48 years; 53% female; 11% on systemic treatment) with plaque or guttate psoriasis. Of this group, 393 patients were enrolled in home phototherapy, and 390 in office phototherapy. Co-primary endpoints of Physician Global Assessment (PGA) clear/almost
clear and dermatology life quality index (DLQI) ≤5 (mild) were assessed at Week 12. Enrollment was stratified by skin phototype (SPT; 350 patients in SPT I/II and III/IV yielding 80% power to establish subgroup non-inferiority).
When analyzing the results, the team found that, at Week 12, 33% of home and 26% of office patients achieved clear/almost clear (p=0.027) and 54% and 34% achieved DLQI ≤5 (p<0.001). Home phototherapy was further determined to be non-inferior for PGA and DLQI (p<0.001) for all skin types, the largest difference being clear/almost clear in SPT V/VI. Both home and office treatments were generally well tolerated, and no patients discontinued as the result of adverse events.
The team concluded that home phototherapy is more effective overall in real world practice than office phototherapy, due to difficulties accessing the latter treatment. As a result, they recommend that home phototherapy should be considered a first-line treatment option for plaque or guttate psoriasis. ●
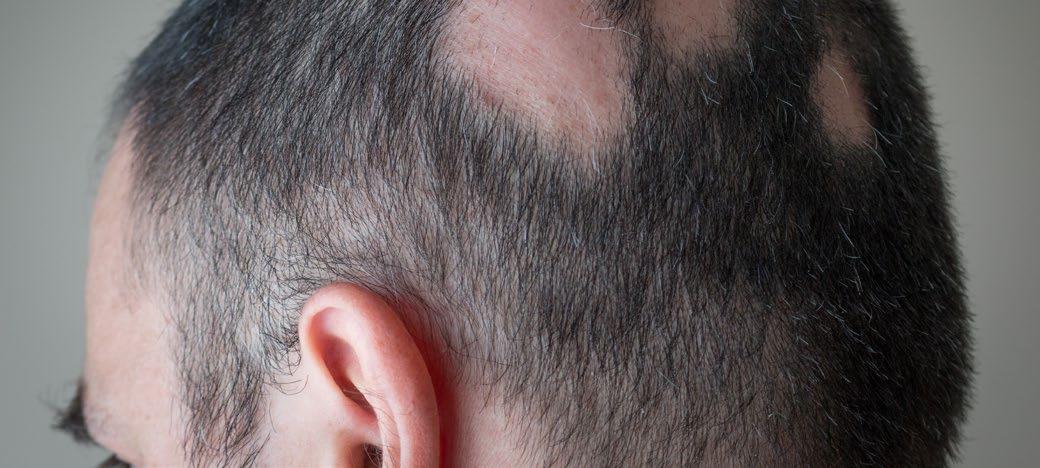
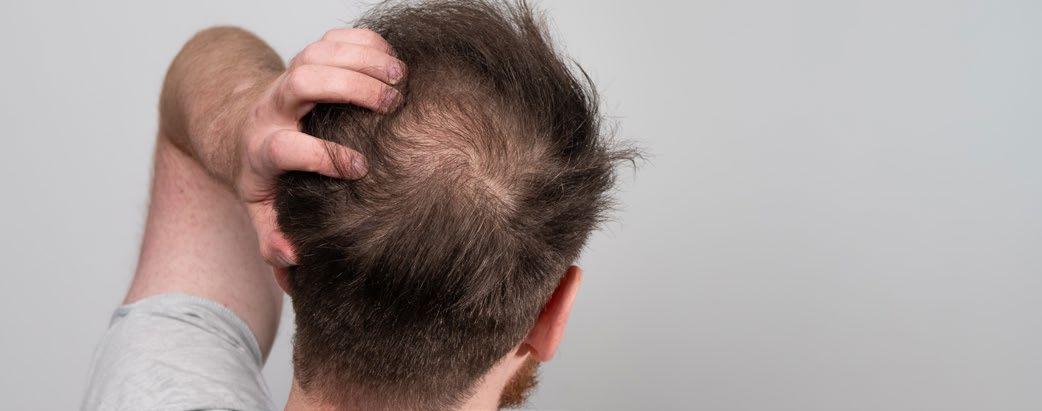
Treatment for Alopecia Areata Shows Promise in Children and Adolescents
IN a stride towards combating alopecia areata (AA), recent approvals of two JAK-inhibitors have marked an advancement in treatment options. However, these medications are designated for severe cases only, leaving a treatment gap for patients with moderate AA. Addressing this need, Coacillium® (Legacy Healthcare, Skokie, Illinois, USA) cutaneous solution has demonstrated a favorable benefit-risk profile for evaluation in both severe and moderate cases of AA, including in children.
The landmark RAAINBOW trial, a multinational, randomized, placebo-controlled, doubleblind, Phase II–III study was conducted. This trial targeted patients aged 2–18 years, with AA severity assessed through SALT (Severity of Alopecia Tool) scores ranging from 25–50 (moderate) and 50–95 (severe). The treatment regimen spanned 24 weeks, followed by a 24week treatment-free observation period to assess relapse post-discontinuation; concomitant treatments for AA were prohibited throughout the trial.
Of the 62 eligible patients, 42 received Coacillium, while 20 received a placebo. After 24 weeks, patients treated with Coacillium
exhibited a substantial mean change in SALT score of +22.87%, compared to -8.00% in the placebo group, and a treatment effect of 30.87% (p<0.0001). Improvements in quality of life metrics were observed to be positively associated with treatment efficacy.
"Coacillium demonstrated sustained remission without the need for ongoing treatment."
Following treatment discontinuation, 82% of Coacillium recipients continued to experience hair growth, with their mean SALT score decreasing from 43.6 to 29.0. By Week 48, 47% of Coacillium-treated patients achieved SALT scores of ≤20, compared with 9.1% observed in the placebo cohort (p=0.0031). Adverse events in the Coacillium group were predominantly mild or moderate, with one case of severe transient eczema being the only serious incident reported.
Coacillium demonstrated sustained remission without the need for ongoing treatment, making it a promising option for children and adolescents with moderate-to-severe AA. ●
Is Beremagene Geperpavec Safe?
RECENT data, presented at the AAD 2024 Annual Meeting, support the benefit-risk profile of beremagene geperpavec (B-VEC) for longterm treatment in patients with dystrophic epidermolysis bullosa, a rare genetic fragility disorder caused by mutations in COL7A1. This open-label extension study assessed the safety and tolerability of B-VEC, a herpex simplex virus 1-based gene therapy vector which topically delivers COL7A1 to patients. A Phase III study showed that this treatment significantly improved complete wound healing at 6 months compared to placebo, in 31 participants; however, there were no long-term data available yet.
"At the last visit, high levels of satisfaction were reported."
This open-label extension study included 47 subjects, 24 of whom participated in the previous
Phase III trial, and 23 of whom were treatmentnaïve. These patients were between 6 months–45 years old (mean: 16.5 years), and received a median of 565 days of treatment, up to 794 days.
Adverse events (AE) were reported by 35 subjects (75.5%). The majority of these were mild or moderate; however, 14 participants experienced serious AEs, which were not considered related to treatment. No AEs led to treatment discontinuation, and at the last visit, high levels of satisfaction were reported. Furthermore, there was an improvement in quality of life measures over time. High closure rates were maintained over time in rollover subjects and wounds that were treated with B-VEC during the Phase III trial (61.1–89.5%; Months 3–12). The open-extension study did not detect any new safety signals, supporting the benefit-risk profile of the treatment in the long term. ●

Efficacy of TYK2 Inhibitors for Moderate-to-Severe Plaque Psoriasis
LATE-BREAKING research on the efficacy and safety of ESK-001 was presented at the AAD Annual Meeting 2024. ESK-001 is an oral, highly selective tyrosine kinase 2 (TYK2) inhibitor, which blocks the TYK2 protein.
Lead study author Kim Papp, Division of Dermatology, Department of Medicine, University of Toronto, Ontario, Canada, and colleagues, led the Phase II randomized, double-blinded, placebocontrolled study, STRIDE. Adults with moderateto-severe plaque psoriasis were included, who scored ≥12 on the Psoriasis Area Severity Index (PASI), ≥3 on the Static Physicians’ Global Assessment (sPGA), or ≥10% on the Body Surface Area (BSA) assessment. The study created five arms, giving each a dose of ESK-001 between 10 mg daily to 40 mg twice daily, and comparing these to placebo. Researchers set the primary endpoint as PASI-75 at Week 12.
The key primary and secondary endpoints were met (p<0.00001) in the top three dose arms, where all patients experienced a dose-dependent response. In the arm who were given the highest
ESK-001 dose (40 mg twice daily), 64% achieved PASI-75, 39% PASI-90, and 15% PASI-100 (placebo: 0%). In this arm, 59% of patients also achieved sPGA 0/1, and 23% sPGA-0, both at Week 12 (placebo: 8%).
Treatment-emergent adverse events (AE) were mostly mild-to-moderate, and were similar across all study arms; the majority of these were upper respiratory tract infections, nasopharyngitis, and headache. The drug discontinuation rate for AE was <3%, with no serious or JAK-associated AEs, or deaths reported. No clinically significant laboratory trends were detected.
Currently, an open-label extension study is being conducted to evaluate those patients who completed the STRIDE study. Papp and team believe that their study “demonstrated clear, dosedependent effects,” and that these “supportive open-label extension findings indicate [that] best-in-class efficacy can be achieved safely with longer exposure.”●
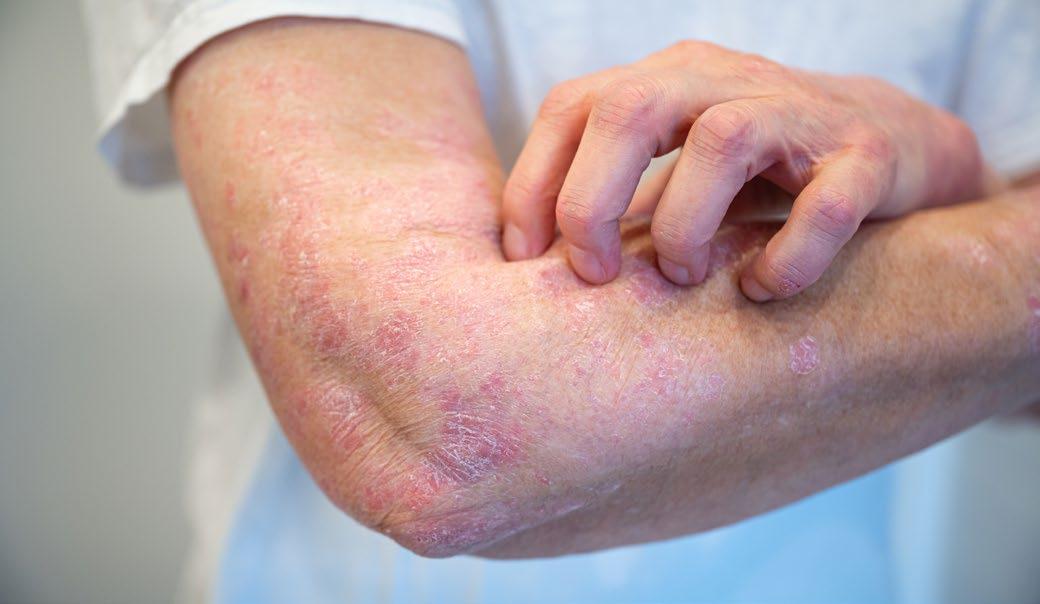
Brepocitinib in Patients with Cicatricial Alopecia
CICATRICIAL alopecias (CA) are progressive conditions causing scarring hair loss, with limited effective treatments available, despite significant impairment to quality of life. Activation of the Type 1 T helper (Th1)/JAK pathway in CA provides the rationale for a Phase IIA, double-blind, placebocontrolled trial investigating the inhibition of tyrosine kinase 2 (TYK2)/JAK1 by brepocitinib.
Patients with lichen planopilaris (LPP; n=16), frontal fibrosing alopecia (FFA; n=9), and central centrifugal cicatricial alopecia (CCCA; n=24) were randomly assigned in a 3:1 ratio to receive either 45 mg brepocitinib or placebo daily for 24 weeks, followed by an open-label 24-week period with brepocitinib. Safety assessments and changes in Th1 biomarkers at 24 weeks were co-primary endpoints. Scalp biopsies were collected from non-lesional and/or lesional skin at baseline, 24 weeks, and 48 weeks for analysis using RT-PCR.
Brepocitinib treatment resulted in significant improvements in the LPP activity index (LPPAI), with a 50.7% improvement at 24 weeks and a 76.2% improvement at 48 weeks from baseline
(p<0.01). Erythema reduction and improvements in patient-reported outcomes such as pruritus and pain were observed by 48 weeks (p<0.05). Frontal fibrosing alopecia severity index (FFASI) and CCCA change in hair loss grade (CHLG) improved by 32.1% and 37.1%, respectively, at 48 weeks compared to baseline (p<0.1). Placebotreated patients exhibited numerical worsening by 24 weeks. No new safety concerns were identified through 52 weeks. Biomarker analysis demonstrated significant downregulation of inflammatory markers, including Th1, Th2, and JAK3, in FFA, LPP, and/or CCCA (p<0.05).
"Brepocitinib treatment led to significant clinical improvements."
Overall, brepocitinib treatment led to significant clinical improvements in CA and demonstrated promising effects on biomarkers, suggesting the therapeutic potential of JAK inhibition in these conditions. ●

Efficacy and Safety of Remibrutinib in Moderate-toSevere Hidradenitis Suppurativa
HIDRADENITIS suppurativa (HS) is a chronic inflammatory skin disease causing abscesses and scarring of the skin. A non-receptor kinase, called Bruton’s tyrosine kinase (BTK), functions in intracellular signalling, proliferation, and migration of B lymphocytes and myeloid cells. Due to its involvement in immune response, inhibition of B cells has been seen as a treatment option for conditions such as rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, and most notably, HS.
Alexandra Boer Kimball, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA, and colleagues, conducted a randomized, doubleblind, placebo-controlled, Phase II study, to investigate the effect of remibrutinib, an oral BTK inhibitor, as a treatment option for moderateto-severe HS. The treatment was provided to subjects in varying doses: 25 mg (N=33), 100 mg (N=33), or placebo (N=11), twice daily for 16 weeks. Its effect was assessed against a simplified HiSCR rate (sHiSCR), consisting of a ≥50% reduction in abscess and inflammatory nodule count, with no increase in draining tunnels versus baseline.
Interestingly, at Week 16 remibrutinib exhibited greater sHiSCR (25 mg: 72.7%; probability: 0.999, and 100 mg: 48.5%; probability: 0.896), compared to placebo (34.7%). Responder rates at Week 16 were higher in the remibrutinib 25 mg and 100 mg treatment arms versus placebo (HiSCR: 69.7%, 48.5% versus 32.7%; HiSCR75: 42.4%, 27.3% versus 18.4%; HiSCR90: 36.4%, 15.2% versus 8.2%). Regarding adverse effects, remibrutinib was overall well tolerated with three serious AE events reported (N=1 per arm).
"These findings show great promise for the potential use of remibrutinib as an inhibitor BK tyrosine kinase, and thus treatment option for HS."
These findings show great promise for the potential use of remibrutinib as an inhibitor of BK tyrosine kinase, and thus treatment option for HS. ●
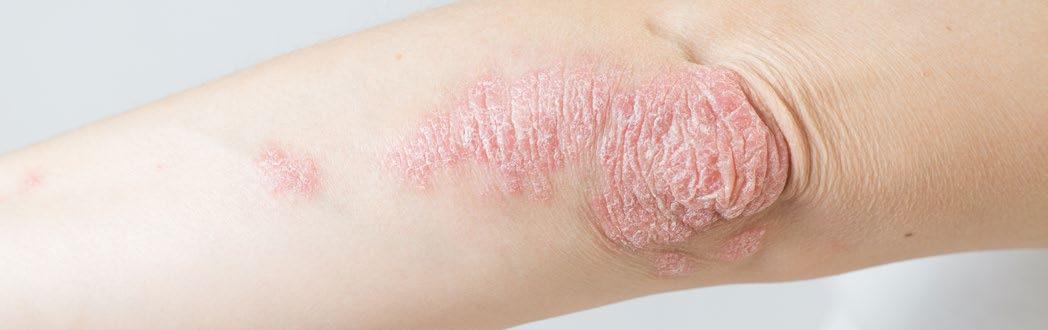
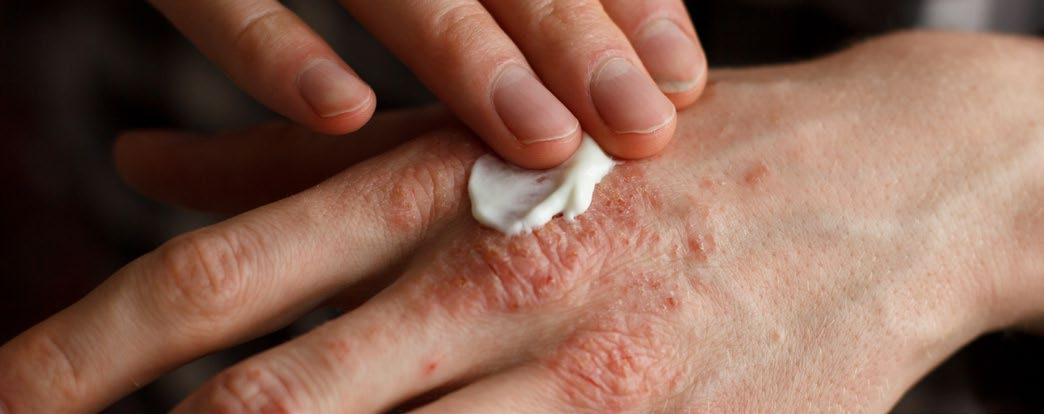
Efficacy of a Pan-JAK Inhibitor Cream in Patients with Chronic Hand Eczema
PHASE III, open-label extension, DELTA-3 trial results of pan-JAK inhibitor cream delgocitinib were presented at the AAD Annual Meeting 2024. Melinda Gooderham, Queen’s University, Kingston, Ontario, Canada, and team, checked the long-term safety and efficacy of delgocitinib cream in adults with chronic hand eczema (CHE).
The delgocitinib cream in patients with moderateto-severe CHE was well tolerated and showed significant improvement in all efficacy endpoints in DELTA-1 and DELTA-2. Volunteers who completed their 16-week treatment period in DELTA-1 and DELTA-2 were moved to DELTA-3. In DELTA-3, based on the individual need, volunteers (N=801) were given 20 mg/g delgocitinib cream twice a day, for a period of 36 weeks. Volunteers with an Investigator's Global Assessment for CHE (IGA-CHE) of 2 or above, received delgocitinib cream until they became clear or almost clear of symptoms (i.e., IGA-CHE 0/1 [clear/almost clear]). The number of treatment-emergent adverse events (TEAE) was the primary endpoint, and IGA-CHE 0/1 and improvement in Hand Eczema Severity Index of ≥75%/≥90% (HECSI-75/90) were the secondary endpoints. The Hand Eczema Symptom eDiary (HESD), captured worst severity of itch/pain reported by patients in the past 24 hours.
In DELTA-1 (N=325), DELTA-2 (N=313), and DELTA-3 (N=801), no safety concerns were
noticed after the use of delgocitinib cream. COVID-19 and nasopharyngitis were the most frequently noticed TEAEs in DELTA-3.
"No safety concerns were reported, and the efficacy improved in volunteers after using 20 mg/g delgocitinib cream."
In the DELTA-3 parent trial of delgocitinib cream, IGA-CHE 0/1, HECSI-75, HECSI-90, and ≥4-point itch/pain reduction showed improvement from 24.6%, 51.8%, 31.8%, and 50.6%/51.9%, respectively, at Week 0, to 30.0%, 58.6%, 36.6%, and 52.4%/55.4%, respectively, in 36 weeks.
Similarly in parent trials, the vehicle cream showed improvement from 9.1%, 23.7%, 12.0%, and 26.3%/32.3%, respectively, at Week 0, to 29.5%, 51.5%, 35.7%, and 41.3%/43.3%, respectively, at 36 weeks.
No safety concerns were reported, and the efficacy improved in volunteers after using 20 mg/g delgocitinib cream, making it possible to use delgocitinib cream in patients with CHE for a longer term based on their needs. ●
Promoting Hair Growth
by Activating Hair Follicle Stem Cells
PP405, a small molecule clinical candidate developed by Christina Weng, Baylor College of Medicine, Houston, Texas, USA, and team, promotes hair growth by activating the hair follicle stem cells (HFSC). The inhibition of pyruvate oxidation in past genetic and pharmacological studies in alopecic murine models showed lactate dehydrogenase (LDH) activity in hair follicles and that led to the stimulation of HFSC and hair growth.
Translational studies were conducted on human skin explants from facelift surgeries to determine the topical penetration, target engagement, and activation of HFSCs post-application of PP405. Punch biopsies, taken after 24 hours of single topical dose application, were embedded in optical coherence tomography (OCT), and frozen for conducting histology, immunostaining, and in situ LDH assays. Explant homogenates were also assayed for LDH activity.
In hair follicle stem cell niches, both telogen and anagen follicles, a dose-dependent LDH activity was noted. Topical doses of PP405 of 0.006% and 0.060% increased the activity of LDH in skin lysates by over 50% versus vehicle (p≤0.001). Twenty-four hours after the single topical dose of 0.006% and 0.060% PP405, a dose-related increase in cell proliferation was noted. This was indicated by Ki-67+ hair follicle stem cells and was also seen in both telogen and anagen follicles. With the help of mass spectroscopy, it was found that PP405, at a concentration of 0.6 µM, was able to activate LDH at the skin level.
Weng and colleagues believe that the ability of PP405 to activate HFSCs seen in this model guided the design of the first in-human Phase I clinical trial of PP405 in males with androgenetic alopecia. ●

"The ability of PP405 to activate HFSCs seen in this model guided the design of the first in-human Phase I clinical trial of PP405 in males with androgenetic alopecia."
Authors:
Citation:
JAK Inhibitors: A New Frontier in Dermatology
Abigail Craig, EMJ, London, UK
Dermatol AMJ. 2024;1[1]:23-26.
https://doi.org/10.33590/dermatolamj/PEOU2148.
JAK INHIBITORS (JAKi) “have changed dermatology, and will continue to change dermatology in ways that no other class of drugs has, or presently can do,” reported Brett King, Yale School of Medicine, New Haven, Connecticut, USA, during a fascinating session delivered at the American Academy of Dermatology (AAD) Annual Meeting 2024 held in San Diego, California, USA.
King further highlighted the rapid development of this class of drugs, explaining that in 2012, only two were approved by the U.S. Food and Drug Administration (FDA), but the number has risen to over 375 in 2023. Cytokines that signal through the JAK-STAT pathway co-ordinate a range of biological functions, from the growth and maturation of lymphoid cells, to mediating inflammation, to Th17 cell proliferation. They also have roles in driving skin diseases. JAKi can modulate the activity of these cytokines, improving patient outcomes.
ALOPECIA AREATA
Brittany Craiglow, Yale School of Medicine, started her session by describing alopecia areata (AA) as “traumatic,” and highlighted a patient who described how it “took a toll” on him mentally. To date, there have been five randomized control trials exploring both oral JAKi and topical JAKi, with promising results in the former, resulting in the FDA approval of baricitinib and ritlecitinib for patients with severe AA aged ≥18, and ≥12, respectively. Specifically, ritlecitinib helped 23% and 43% of patients achieve a Severity of Alopecia Tool (SALT) score of ≤20 at 24 and 48 weeks, respectively. Craiglow attributed some of this success to the high levels of patient adherence, despite the slow progress when treating AA. A 4 mg dose of barictinib, the first JAKi to be approved at two different doses, resulted in 35–39% of patients achieving a SALT score of ≤20 at 36 weeks, rising to 41% at 52 weeks. Craiglow also highlighted a new JAKi, deuruxolitinib, likely to be a twice-daily dose, that is demonstrating promising results in ongoing trials.
"JAKi can modulate the activity of these cytokines, improving patient outcomes."
In her concluding remarks, Craiglow explained that patients with less severe AA respond significantly better than those with a more severe disease. It is therefore important to consider dose, baseline severity, and the duration of the current episode, as these influence the response to treatment.
LUPUS, DERMATOMYOSITIS, AND STING-ASSOCIATED VASCULOPATHY
Tofacitinib was approved in 2012 for the treatment of rheumatoid arthritis. Ruth Vleugels, Brigham and Women’s Hospital, Boston, Massachusetts, USA, presented the case of a young patient with myositis treated with 10 mg tofacitinib twice daily, who responded “beautifully” 10 years ago. Since then, several cases of severe dermatomyositis have been successfully treated with this JAKi. This presented the question as to why JAKi improve

dermatomyositis. Vleugels explained that there is a strong interferon (IFN) signature driven by IFN-β in serious cases of myositis, but a low signature in patients with mild disease. Thus, JAKi can aid the alleviation of the interferon signal. Subsequently, dazukibart, an immunoglobulin G neutralizing monoclonal antibody directed against IFN-β, is currently enrolling for a Phase III global trial after achieving a Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) reduction of >5 points in 100%, and 96% of patients receiving 150 mg and 600 mg, respectively.
SARCOIDOSIS AND GRANULOMA ANNULARE
Sarcoidosis is considered a T cell-dependent disorder, with macrophages and myeloid cells playing significant roles in pathogenesis. Granuloma annulare (GA) shares similarities with sarcoidosis, but is clinically and histologically distinguishable. William Damsky, Yale School of Medicine, summarized the promise JAKi have in treating these conditions during his talk in this session. Specifically, data from open-label studies
and clinical trials using tofacitinib in patients with GA and sarcoidosis, respectively, demonstrated marked improvements in the conditions, and a reduction in inflammation.
PET scans, performed on patients with sarcoidosis prior to commencing treatment, permitted the assessment of the internal organs, and confirmed metabolic responses. Damsky stressed that not only did patients get better, but they were also able to taper off other medications, such as prednisone. Further molecular studies suggest IFN-γ is a key mediator in sarcoidosis pathogenesis, explaining the efficacy of JAKi. Specifically, the production of TNF-α, an inflammatory cytokine, occurs downstream of IFN-γ, a JAK STAT-dependent cytokine, thus controlling its production.
Regarding GA, molecular studies indicate the importance of IFN-γ, but also currently unknown secondary cytokines, possibly requiring broader therapeutic approaches. At present, there are no FDA approved treatments for GA, and Damsky stressed that with the exception of TNF inhibitors, current therapies are generally ineffective. Phase
I studies with tofacitinib gel in GA have been conducted, with the aim for larger controlled studies to establish efficacy conclusively.
VITILIGO
Khaled Ezzedine, Greater Paris University Hospitals, France, spotlighted the use of JAKi for the treatment of vitiligo, a skin condition characterized by loss of skin color. The rationale for using JAKi in vitiligo treatment stems from their role in the JAK1 and JAK2 pathway, which is implicated in the pathogenesis of the disease.
"Topical JAKi have shown promise in treating vitiligo."
Topical JAKi have shown promise in treating vitiligo, with studies demonstrating improvement in skin pigmentation. A Phase III study involving topical ruxolitinib showed significant improvement
in repigmentation of vitiligo lesions, with sustained effects even after treatment cessation. Specifically, at Week 52, the Facial Vitiligo Area Scoring Index (F-VASI75) was achieved by a greater percentage (52%) of patients applying ruxolitinib cream compared to a vehicle. However, safety concerns with topical JAKi include application site acne-like lesions, which can be bothersome to the patient, especially in visible areas. The recent addition of narrowband UVB phototherapy to topical JAKi treatment has shown to enhance repigmentation, though careful consideration of immunosuppression risks is warranted.
Four oral JAKi have also been explored in vitiligo treatment, with several drugs undergoing Phase II studies. Ritlecitinib specifically met key secondary endpoints when administered as a 50 mg daily dose, with or without an induction. Future challenges in vitiligo treatment include determining optimal dosing regimens, identifying maintenance strategies to prevent relapse, and addressing safety concerns associated with systemic JAKi therapy.

"Careful consideration of immunosuppression risks is warranted."
PSORIASIS
JAKi are a significant topic in psoriasis treatment, stressed Melinda Gooderham, SKiN Health, Peterborough, Ontario, Canada, who summarized the mechanism of action of JAKi, and their relevance to psoriasis treatment, during her talk.
Competitive inhibitors, which bind at the kinase domain, were initially explored, with tofacitinib being one of the first studied for plaque psoriasis, followed by trials of baricitinib and tofacitinib for psoriasis and psoriatic arthritis. While tofacitinib showed promising efficacy, it was not approved for plaque psoriasis, due to safety concerns and a narrow therapeutic index. Newer approaches shifted focus to allosteric inhibition, where drugs like deucravacitinib target the pseudokinase domain, allowing ATP-binding to proceed, maintaining normal JAK function. Deucravacitinib, a tyrosine kinase 2 inhibitor, has shown significant efficacy in plaque psoriasis, with 50% of patients achieving Psoriasis Area and Severity Index (PASI) 90, maintained for up to 3 years with a good safety profile.
Safety concerns with JAKi include risks such as venous thromboembolism and major adverse cardiovascular events, but the allosteric inhibition mechanism offers a better safety profile by allowing normal JAK function. In summary, JAKi, particularly deucravacitinib, offer a valuable treatment option for patients with psoriasis, providing efficacy comparable to biologics, with
a different safety profile. Gooderham suggests considering JAKi as an option, especially for patients who prefer oral medication, or have specific comorbidities, like psoriatic arthritis or palmoplantar psoriasis. Future research and ongoing trials aim to explore the efficacy of JAKi in other conditions, like hidradenitis suppurativa and palmoplantar psoriasis, expanding their potential therapeutic use.
CONCLUSION
Further topics discussed in this fascinating session include JAKi for the treatment of lichen planus, hidradenitis suppurativa, pyoderma, atopic dermatitis, and prurigo nodularis. King concluded the session by considering the safety profile of JAKi, emphasizing the need to balance the risks of adverse events with the suffering of patients with skin diseases. Delving into data comparing safety profiles across different diseases, King cautioned against overreliance on theoretical selectivity data, and advocated for decisions based on efficacy and safety data, despite the “unmatched ability” of this class of medicines in addressing inflammatory skin diseases. ●
"King advocated for decisions based on efficacy and safety data."
Author:
Citation:
Modern Approaches for Treating Psoriasis
Darcy Richards, EMJ, London, UKDermatol AMJ. 2024;1[1]:27-30.
https://doi.org/10.33590/dermatolamj/BSWH2237.
A COMPELLING session covered updates on the therapeutic pipeline and role for early treatment of psoriasis at the American Academy of Dermatology (AAD) 2024 Annual Meeting, held in San Diego, California, USA, between March 8–12.
NOVEL THERAPEUTICS AND TRIAL DATA
Bruce Strober, Yale University School of Medicine, New Haven, Connecticut, USA, opened the session by highlighting the recent U.S. approval of an IL17A and IL-17F monoclonal antibody, bimekizumab, capable of blocking all three types of IL-17A and IL17F homo- and heterodimers. Strober emphasized that treatment efficacy for psoriasis and psoriatic arthritis is enhanced by targeting both IL-17A and IL-17F. Noting this, Strober discussed the efficacy of bimekizumab in treating moderate-to-severe psoriasis. Presenting data from the BE READY trial,1 he showed that treatment with bimekizumab led to 90.8% of patients achieving a Psoriasis Area and Severity Index (PASI) score of 90 within 16 weeks of treatment, compared to just 1.2% for those given placebo. Further to this, he commented that evidence has shown IL-17A/IL-17F blockade to be fruitful in treating scalp psoriasis, with up to 85.0–88.0% of patients achieving scalp clearance.
Of note, the trial highlighted the increased risk of Candida infections in these patients as a challenge associated with IL-17F blockade.1 Strober commented that compared to traditional IL-17 inhibitors, where 1.0–3.0% will experience mucosal candidiasis, the rates are 10.0–15.0% when treated with IL-17A/IL-17F monoclonal antibodies. However, he noted that fungal infection incidence drops after the first year when looking at pooled data from five Phase III trials. This could be due to depletion of susceptible patients over time.
Spotlighting his real-world experience, Strober explained that in the approximately 30 patients he has initiated on bimekizumab, the rate of Candida infection has not been as high as that seen in the literature. He also emphasized the need to assess patients for a history of inflammatory bowel disease, in which case the drug is contraindicated.
The speaker also explored retention of therapeutic efficacy, touching on dosing strategies and their relation to this. Looking at data from a post hoc analysis of five Phase III/IIIb trials, he expressed that for those who achieve PASI 90 by Week 16 of treatment, there is an 82.0% likelihood of retaining this response over 2 years.
Strober further discussed that spesolimab, an anti-IL-36 receptor antibody, has been approved to treat flares of generalized pustular psoriasis as a single intravenous dose, and highlighted the Effisayil 1 study,2 which looked at the Generalized Pustular Psoriasis Physician Global Assessment (GPPGA) pustulation subscore 1 week after spesolimab injection. The study found that 54.0% of patients treated with spesolimab had a GPPGA pustulation subscore of 0 at Week 1, irrespective of disease severity at baseline. He further spoke on Effisayil 2,3 which looked at the use of subcutaneous spesolimab in prevention of flares, noting that this could potentially be approved in the USA in the near future. The study reviewed three different doses (high dose, medium dose, and low dose) and a placebo given regularly over 48 weeks in high-risk patients with generalized pustular psoriasis.3 In the high-dose group, no
flares occurred after Week 4, and there was an 84.0% risk reduction for developing a flare.3 Those in the medium-dose and low-dose groups achieved an intermediate response.
The presentation also discussed a Phase III, unapproved, investigational drug, TAK-279, which is a tyrosine kinase 2 (TYK2) inhibitor. Interestingly, Phase IIb data for use of TAK-279 in moderate-to-severe psoriasis showed that at Week 12, approximately 67.0% achieved PASI 75 when given at higher doses.4 Strober noted that deucravacitinib, another TYK2 inhibitor approved for use in psoriasis approximately 18 months ago, achieved a PASI 75 of approximately 45.0–50.0% at Week 12 and 58.7% at Week 16, which increased to 69.0% by 6 months.5 These early results indicate that TAK-279 could be more effective than deucravacitinib; however, there is a need to see if these results are replicated in Phase III studies. Acne and acneiform dermatitis, which occur with almost all TYK2 inhibitors, were noted as the adverse events associated with TAK-279 treatment.4
Strober also expressed excitement when discussing an unapproved oral peptide therapy (JNJ-77242113). This novel IL-23 receptor antagonist blocks the IL-23 receptor directly, which is a novel mechanism of action. Strober explained that JNJ-77242113 has gastrointestinal stability and can induce systemic IL-23 receptor blockade with only 1.0% bioavailability when taken orally. When looking at dosing and PASI 75, a dose response curve was seen. Nearly 80.0% of those who received the highest dose achieved PASI 75 by Week 16, compared to approximately 67.0% for TAK-279 at Week 12.6 Strober also highlighted that
in terms of PASI 90 and PASI 100, JNJ-77242113 is comparable to some biologic therapies, at 59.5% and 40.5%, respectively.6 Regarding safety, Strober explained that through Week 16, the safety is what would be expected from an IL-23 inhibitor, with a possible increase in upper respiratory tract infections at the higher doses.6 Phase III results are awaited.
VISIBLE,7 a novel study assessing an IL-23 inhibitor, guselkumab, in treating scalp psoriasis across all skin tones, revealed that a scalp-specific Investigator’s Global Assessment (ss-IGA) score of 0/1 (no or very mild disease) and Psoriasis Scalp Severity Index (PSSI) 90 scores were achieved in significantly more patients receiving guselkumab than placebo. There was also a greater mean change from baseline Psoriasis Symptoms and Signs Diary (PSSD) by Week 16 in patients treated with guselkumab, compared to placebo.7 Additionally, guselkumab improved patientreported impact of skin discoloration at Week 16 compared to placebo, as shown by a reduction in Skin Discoloration Impact Evaluation Questionnaire (SDIEQ) scores.7 This is a novel outcome measure for skin studies.
COULD EARLY TREATMENT IMPACT PSORIASIS OUTCOMES?
Following this, Richard Langley, Dalhousie University, Halifax, Nova Scotia, Canada, presented on the early treatment of psoriasis. Stressing its importance, Langley stated: “We should be looking at our patients who have significant psoriasis […] and trying to get them under effective control, however we can, as soon as possible.”
He noted that different guidelines and strategies for treating psoriasis exist in different countries, and emphasized the aspirational nature of his presentation, as this is important in advocating for patients.
Langley explored several key questions when discussing the value of early treatment in psoriasis, including whether early intervention can impact comorbidities, prevent disease progression, or induce remission. When considering comorbidities, he highlighted an example case of a 54-year-old male with psoriasis. The existing comorbidities included smoking, elevated BMI, elevated cholesterol, high blood pressure, and borderline diabetes. Langley discussed how this patient’s risk for a myocardial infarction would be 32.0%, but stressed that this calculation does not account for the psoriasis diagnosis. Alongside risk factor modification, treating the psoriasis could also help reduce this cardiovascular risk. Langley noted the importance of inflammation and that treating it could potentially decrease the mortality associated with psoriasis. He spotlighted striking data from the SOLAR registry, which looked at 12,000 patient years over a 7-year follow-up period. At baseline, many individuals had risk factors; however, a significant reduction in mortality was seen for
those treated with biologics and methotrexate. Anecdotally, he commented that this has changed the way he counsels patients about controlling their inflammation. Considering this, Langley explained that if patients have concerns about the side effects of treatments, clinicians need to discuss weighing the risk factors with the risk of not treating, and the potential impact this may have on both an individual’s quality and quantity of life.
Psoriatic arthritis is another major comorbidity of psoriasis. Langley noted that a number of studies have hinted at improvement of prevention following treatment with biologics. He highlighted the prospective PAMPA trial8 for prevention of psoriatic arthritis, utilizing ultrasound enrichment to determine if treatment prevents psoriatic arthritis. Langley commented that these types of study are needed, and that he is optimistic about the future.
The other area in which early treatment could impact psoriasis is disease progression and remission. Langley considered treating at an earlier stage, highlighting an individual case in which a 19-year-old patient with a 1-year history of psoriasis was given a single dose of rizankizumab, did not attend recall, yet remained PASI 100 for 7
years. He also highlighted drug withdrawal studies that found that half of patients did not have disease 8 months after drug withdrawal, which is longer than the drug half-life itself. He postulated on why some patients remain clear when off therapy and if there is a difference in treating early, exploring the concept of the molecular scar. Research has shown that tissue resident memory CD8+ T cells remain present in the skin and could cause disease recurrence. Treatment with secukinumab has shown normalization of molecular scar transcriptomes in moderate-tosevere psoriasis. This posed the question of whether early use of systemics could lead to prolonged remission. Exploring the ECLIPSE study,9 he highlighted that data have shown CD8+ T cells are reduced to a greater extent by guselkumab than secukinumab, and that this may help explain the durability of IL-23 inhibition.
Langley spoke on a study looking at molecular transcriptomes in patients with new-onset and chronic psoriasis. Whilst in both cohorts, reversion of skin transcriptomes to that of a non-lesional state occurred following 52 weeks of secukinumab treatment, normalization of these transcriptomes occurred more quickly in patients with new-onset disease, compared to those with chronic disease at 16 weeks versus 52 weeks, respectively.
Langley also discussed the GUIDE study, which looked at retreatment in super-responders
References
1. Gordon K et al. Efficacy and safety of bimekizumab in patients with moderate-to-severe plaque psoriasis: results from BE READY, a 56-week Phase 3, randomized, double-blinded, placebo-controlled study with randomized withdrawal. Poster 1116. AAD VMX, 12-14 June, 2020.
2. Bachelez H et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385(26):2431-40.
3. Strober B et al. Spesolimab for the prevention of generalized pustular psoriasis (GPP) flares: results from the randomized, placebo-controlled trial Effisayil 2 late-breaker session. LB01. WCD, 3-8 July, 2023.
4. Armstrong A et al. Efficacy and safety results from the randomized,
following guselkumab withdrawal and a long treatment-free period. Treatment free periods were shown to be longer for those with ultra-short disease duration (disease duration <15 months) than those with intermediate-short disease duration and long disease duration.
Langley concluded by stressing that early treatment can lead to higher response rates and increased likelihood of achieving remission; could contribute to disease prevention and comorbidity progression; and may result in enhanced life quality and quantity for patients.
"Early treatment can lead to higher response rates and increased likelihood of achieving remission."
CONCLUSION
Overall, Strober and Langley highlighted a buzz around the potential for treating psoriasis, with the development of multiple new therapeutics being tested at different trial/investigational phases, and promising research highlighting the possible impact that early therapeutic intervention could have on patient quality and quantity of life. ●
double-blind, placebo-controlled phase IIb trial of TYK2 inhibitor NDI-034858 in moderate-to-severe psoriasis. Oral abstract S025. AAD, 17-21 March, 2023.
5. Armstrong AW et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: Results from the phase 3 POETYK PSO-1 study. Session S033. AAD VM, 2325 April, 2021.
6. Bissonnette R et al. A Phase 2, randomized, placebo-controlled, dose-ranging study of oral JNJ77242113 for the treatment of moderate-to-severe plaque psoriasis: FRONTIER 1. Late-breaker session LB01. 2023. WCD, 3-8 July.
7. Alexis A et al. VISIBLE cohort
b: guselkumab demonstrates significant scalp clearance at week 16 in participants with moderateto-severe scalp psoriasis across all skin tones. Maui Derm Hawaii, 22-26 January, 2024.
8. Haberman RH et al. Efficacy of guselkumab, a selective IL-23 inhibitor, in preventing arthritis in a multicentre psoriasis at-risk cohort (PAMPA): protocol of a randomised, double-blind, placebo controlled multicentre trial. BMJ Open. 2022;12:e003650.
9. Muñoz-Elías E et al. Differential impact of IL-23 vs IL-17 blockade on serum cytokines, gene expression and immune cell subtypes in psoriatic skin: Results from the ECLIPSE study. Abstract D3T01.1D. EADV Congress, 9-13 October, 2019.
Abstract Highlights
The following abstracts have been selected to showcase award-winning abstracts presented at the American Academy of Dermatology (AAD) 2024 Annual Meeting. With a record number of abstracts submitted this year, the research summarized below represents the most exciting developments within the field of dermatology.
Citation:
Dermatol AMJ. 2024;1[1]:31-36.
https://doi.org/10.33590/dermatolamj/XFQN6712.

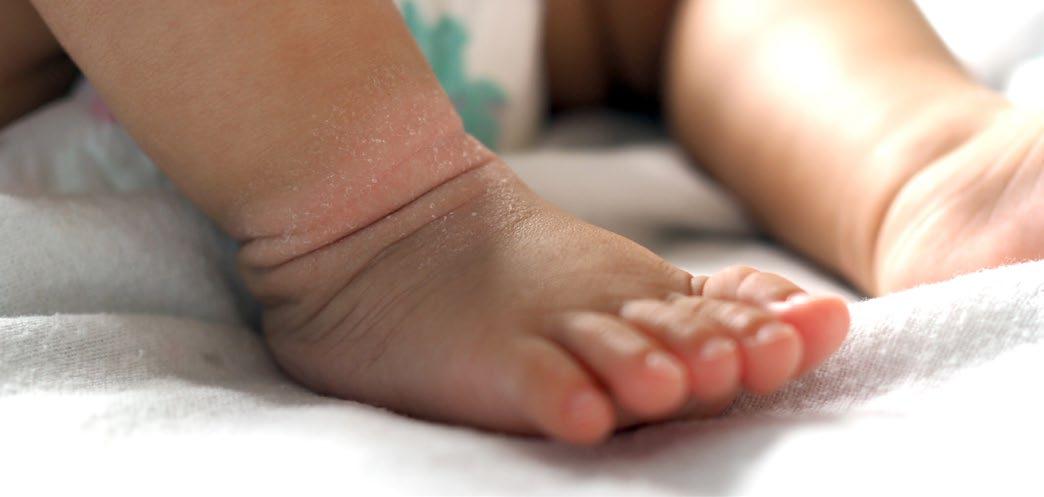
Growth Analysis in Children with Atopic Dermatitis
IMPACTS of atopic dermatitis (AD) on the growth of children have been revealed in a piece of research presented at the AAD 2024 Annual Meeting. This abstract took first place at the AAD poster awards, and described an analysis of height, weight, and BMI of children with AD in comparison to the general population, in order to clarify consequences for childhood growth.
A total of 1,329 children were included in the PEDISTAD clinical trial, which is an ongoing international study, incorporating patients aged <12 years with moderate-to-severe AD. This cohort was assessed against the Centers for Disease Control and Prevention (CDC)'s Learning Management System as a healthy control, comparing the percentage of patients above the 50th percentile, and the mean percentiles for height, weight, and BMI.
Looking at the results from this work, in the PEDISTAD study, 50% of males were above the 50th percentile for weight at baseline, but only 38% were above this for height. For their female counterparts, these figures were 51% and 52%, respectively. In participants aged between 5–12 years, only 28% of males and 47% of females were above the 50th percentile for height. Meanwhile, in the case of BMI, 69% of males and 71% of females were above the 50th percentile. The overall averages across all the age specific percentiles were 46th in height, 51st in weight, and 58th for BMI in the case of males; and 50th in height, 50th in weight, and 59th in BMI for females.
The researchers concluded, based on this evidence, that the data suggest moderate-to-severe AD has a negative impact on the growth of children aged <12 years. The explanations for this were hypothesized to be due to sleep deprivation, and/or long-term exposure to topical or systemic glucocorticoids and immunosuppressants. ●
"Moderate-to-severe AD has a negative impact on the growth of children aged <12 years."
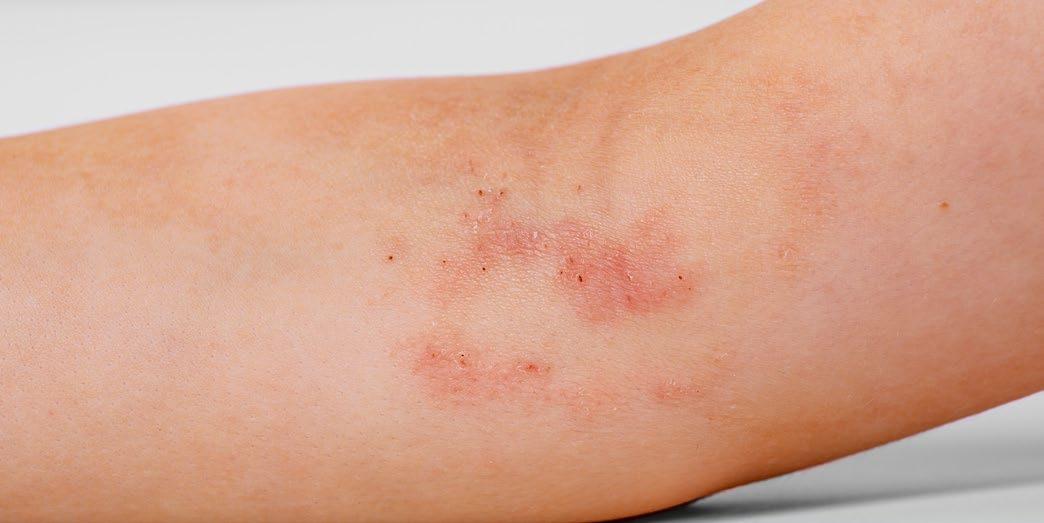

Link Between Maternal Hidradenitis Suppurativa and Childhood Hospitalization
A RECENT study revealed insights into the impact of maternal hidradenitis suppurativa (HS) on childhood health. Presented at the AAD 2024 Annual Meeting, this research revealed concerning associations with childhood morbidity, and took second place in the annual AAD poster awards.
HS has been associated with adverse pregnancy outcomes, yet its effect on offspring has remained unknown. The retrospective longitudinal cohort study in Québec, Canada, analyzed data from 1,275,593 children born between April 1, 2006–March 31, 2022, with the aim of uncovering the association of the condition with the risk of childhood hospitalization up to 16 years of age. Of the study group, 1,283 children were born to mothers diagnosed with HS, while the remaining 1,274,310 were unexposed.
Study results showed that children exposed to maternal HS had a higher rate of hospitalization compared to unexposed children, with the exposed group experiencing a rate of 53.2 per 1,000 person-years, compared with 36.6 per 1,000 person-years among unexposed children.
"Children exposed to maternal HS had a higher rate of hospitalization."
It was also revealed that maternal HS was associated with 1.31-times the risk of childhood hospitalization across various categories. The risk of hospitalization was elevated for respiratory issues by 1.21-times, and 2.64-times for metabolic conditions. Furthermore, the risk of hospitalization was higher for gastrointestinal disorders and mental and behavioral disorders among exposed children.
This study underscores the need for heightened awareness and monitoring of children at risk of exposure, as well as the impact of maternal HS on childhood health. ●
Systemic Janus Kinase Inhibitors: Are They Safe?
SYSTEMIC Janus kinase (JAK) inhibitors have a comparable safety profile to broader immunomodulators, according to new research presented at the 2024 AAD Annual Meeting, held in San Diego, California, USA.
JAK inhibitors are currently approved for the treatment of atopic dermatitis and alopecia areata, and have exhibited great efficacy for several other dermatologic conditions in ongoing clinical trials. However, safety concerns have arisen due to the U.S. Food and Drug Administration (FDA) issuing a black box warning on their risk for long-term adverse events (AE).
First author of this abstract, and awarded third place, Olivia Lamberg, University of Michigan Medical School, Ann Arbor, USA, and team, conducted a comprehensive literature review to assess the long-term AEs associated with oral JAK inhibitors, compared to traditional immunomodulators. The search focused on articles reporting medication exposure exceeding 1 year, and subsequent development of AEs, including malignancies, major adverse cardiovascular events, venous thromboembolism, and infections. The study encompassed several JAK inhibitors commonly used for dermatologic conditions, including tofacitinib, baricitinib, upadacitinib, and ruxolitinib, as well as traditional immunomodulators, such as cyclosporine, methotrexate, etanercept, adalimumab, and systemic steroids.
The team found no significant difference in the incidence rate of major adverse events (per 100 patients per year) between JAK and non-JAK inhibitors, for malignancies (excluding nonmelanoma skin cancer), venous thromboembolism, or serious infections. JAK inhibitors exhibited lower rates of non-melanoma skin cancer and major adverse cardiovascular events, but higher rates of Herpes zoster infection.
The study supports that JAK inhibitors have a comparable safety profile to non-JAK medications, and thus represent a therapeutic advancement, with more precise mechanisms of action compared to broader immunomodulators. A limitation of the study was that the methodology was not a systematic literature review, potentially leading to the omission of some relevant data. In future research, Lamberg and team will investigate specific patient populations, characteristics, and risk factors to better inform treatment decisions, as well as conduct a systematic review to summarize all data on long-term AEs. ●
"JAK inhibitors have a comparable safety profile to non-JAK medications."

Safety of Low-Dose Oral Minoxidil for Hair Loss
LOW-dose oral minoxidil (LDOM) has become a popular treatment alternative for patients with hair loss, for whom other treatments are logistically challenging, irritating, or ineffective. The safety and efficacy of LDOM for hair loss has not been extensively researched in larger studies, however, highlighting the need for expert, consensus-based guidelines. Thus, a research team, led by Yagiz Matthew Akiska, George Washington School of Medicine and Health Sciences, Washington, D.C., USA, sought to investigate this, with their research winning fourth place in the abstract awards at the AAD 2024 Annual Meeting.
Hair loss affects patients’ quality of life significantly, and can be hereditary, autoimmune, inflammatory, or secondary to medical, surgical, or emotional stressors, infection, or cancer therapies. Topical minoxidil (TX) is commonly used to treat hair loss due to its U.S. Food and Drug Administration (FDA)-approved indication; however, it can be ineffective or inconvenient for many patients. In order to test the efficacy and safety of LDOM, a popular alternative treatment, 43 experienced dermatologists, with an average of 18.26 years of post-residency dermatology experience and 6.29 years of experience with
LDOM for hair loss, took part in an initial survey. Three further rounds of questions were calibrated for Likert-scale responses, closing questions with ≥70% consensus and eliminating those with <60% consensus. Consensus was reached if ≥70% of participants indicated “agree” or “strongly agree” on a 5-point Likert scale.
In total, 94 items achieved Likert scale consensus, including diagnoses for which LDOM may provide direct or supportive benefit, and indications for LDOM over TX. Consensus was also achieved on adult and adolescent utilization, contraindications, precautions, dosing parameters, baseline evaluation and monitoring protocols, adjunctive therapy, and specialty consultation.
The research team concluded that LDOM has several direct benefits for patients with hair loss, and can be considered as a valid option when TX is more expensive, logistically challenging, no longer effective, causes product residue or skin irritation, or exacerbates inflammatory issues. More research is needed into the treatment, and clear guidelines should be laid out; however, preliminary research shows that LDOM is a good alternative to TX for patients with hair loss. ●
"LDOM has several direct benefits for patients with hair loss, and can be considered as a valid option."
Grading of Dermatologic Adverse Events from Anti-Cancer Therapies
INCLUDING dermatologists in the grading and classification of dermatologic adverse events (dAE) caused by cancer therapies may be more beneficial than consulting medical oncology physicians alone. New cancer therapies have brought a variety of dAEs beyond those seen with conventional chemotherapy, and Version 5.0 of the Common Terminology Criteria for Adverse Events (CTCAE v5.0) is the current, widely accepted classification and grading criteria used during medical care. There are little data available analyzing the application of this CTCAE, however. This study, which was highly commended in the AAD 2024 Annual Meeting abstract awards, aimed to look into its use across disciplines.
The study, carried out by Christopher Fay, Harvard Medical School, Boston, USA, and team, involved a survey of four cases of dAEs being sent to dermatologists and medical oncology physicians across the USA. Participants were asked to use photographs and text descriptions to grade and classify morbilliform, psoriasiform, and papulopustular rashes. The fourth case required the physicians to grade the condition using only a clinical text description.
In total, 182 medical oncology physicians and 81 dermatology physicians completed the survey. The researchers found that dermatologists were significantly more likely to provide correct responses when it came to classifying morbilliform and psoriasiform eruptions, with greater than 87% correct classification, while correct classification reached as low as 12% amongst the medical oncologists. Dermatologists were also far more likely to correctly grade psoriasiform, papulopustular, and written cases than medical oncologists. As a result of this survey, 87% of medical oncologists reported that they would be interested in additional educational tools on dAEs.
The research team concluded that medical oncologists may have more difficulty delivering appropriate treatment to patients with cancer dealing with dAEs. These results also raised the question of whether CTCAE v5.0 is the most suitable determinant of dAEs as cancer therapies continue to develop. Fay and team suggested that further research look into the potential role of involving dermatologists in grading and classifying these AEs, alongside medical oncologists. ●
"Participants were asked to use photographs and text descriptions to grade and classify rashes."
Congress Interviews
The AMJ team had the pleasure of speaking to Amy Paller and Yagiz Matthew Akiska, who summarized their abstracts that won first and fourth place, respectively, in the 2024 American Academy of Dermatology (AAD) Annual Meeting Poster Award. The pair also outline the future direction of their research, before summarizing the most exciting sessions at AAD 2024.

Citation:
Q1Amy Paller
Professor and Chair, Department of Dermatology, Northwestern University Feinberg School of Medicine; Professor of Pediatrics, Ann & Robert H. Lurie Children's Hospital, Chicago, Illinois, USA
Dermatol AMJ. 2024;1[1]:37-40.
https://doi.org/10.33590/dermatolamj/IPHQ4266.
Congratulations for receiving first place in the 2024 American Academy of Dermatology (AAD) Annual Meeting Poster Award for your submission, ‘Growth Analysis in Children Aged Less Than 12 Years with Moderate-to-Severe Atopic Dermatitis’.
Can you summarize the research presented in your poster?
The poster summarizes results to date of a 10-year observational study called PEDISTAD, which enrolls children under the age of 12 with atopic dermatitis (AD) that is severe enough to need systemic medication. There are many parameters that we're studying as we follow these children through the various visits. But one of the parameters that we're studying is growth. We know that individuals of this age group who have AD have chronic inflammation and that they don't sleep well, primarily because of their itch. In addition, many of them have used relatively
potent topical corticosteroids, and courses of oral corticosteroids. These are all factors that we know can affect linear growth.
Indeed, what we showed here is that when we followed 1,329 of these children aged under 12 years of age, we found that the weight and BMI were above the means for children of same age using the Centers for Disease Control and Prevention (CDC) percentiles. But the heights were below the average for age for both males and females. While database analyses have suggested that height in children with more severe AD may be low, this is the first real-world study through a registry.
I think that the results raise some very interesting questions, not just to further dive into what is driving this change, but also whether we can reverse this with effective management at this critical time of linear growth.
We now have an improved repertoire of medicine that's very safe and is being used increasingly in this 6–11 years of age patient population. It will be important to track not just the impact on inflammation, but ideally on sleep as well, and whether these improvements translate into improved linear growth for these children younger than 12 years of age.
Q2
Why did your research team choose to focus on AD in young children, and how do you hope your research will change the lives of these patients?
I happened to be the primary investigator of the PEDISTAD study, which is multi-center and sponsored by Regeneron. Children who are enrolled may stay on topical therapy based on shared decision making – and many have, while others may advance to a biologic or conventional systemic therapy. All are tracked, and there are no limitations on what the doctors prescribe – so it reflects real life practice. This gives us a change to compare responses and potential risks of use of our group of available agents, now and in the future. I hope that our data will help doctors and families compare among options for treatment, and will give us perspective about the answers to questions that may not have come up during trials of new medications, such as responses to live vaccines – as we are now enrolling another
500 children under the age of 5 years – and longterm safety of a variety of topical and systemic medications for AD.
Q3
What is next for your research team?
With respect to PEDISTAD study and its research team, having a longitudinal 10-year study presents an opportunity to study each participant in terms of the disease course. There are still many enrolled children who are on topical medications, providing the opportunity for a wonderful “control group” to compare with those who advance to systemic medications. I think it will be really key as this population grows to track their courses in response to various interventions, including of the development of comorbidities, which will be particularly interesting with respect to allergic disorders in those enrolled who are under 5 years of age.
We can look at durability of response, as well as long-term outcomes and risks. With respect to linear growth, recent data was presented from studies of dupilumab in 6–11 year olds that those on medication had signs of improved bone health, such as increased levels of alkaline phosphatase levels and bone biomarkers. Tracking linear growth in relationship to these biomarkers and AD improvement will be exciting.
Q4
You moderated a session at AAD 2024, focusing on AD. Could you tell us about this?
Well, this was a wonderful session, that really took the participants through all of the basic epidemiology, comorbidities, alterations of the barrier, immune system, and microbiome, and finally available and emerging topical and systemic therapies for AD. It’s a huge topic that is rapidly evolving. With respect to epidemiology, Aaron Drucker showed evidence that the prevalence of AD is now stabilizing after a period of increasing occurrence. Some more recently recognized comorbidities are autoimmune diseases and a link with cardiovascular disease in adults with AD.
"The prevalence of AD is now stabilizing after a period of increasing occurrence."
It is so clear that AD is a heterogeneous group of disorders with a similar phenotype, driven by Th2 immune skewing in the skin. Understanding biomarkers of subclassification that predict responses is going to be very important as we start to personalize therapies. Perhaps these will emphasize barrier issues with filaggrin or lipids –focus on different immunophenotypes (e.g. increased Th17 or reduced Th1 cells and products, in addition to the shared skewing towards Th2 pathway activation). The microbiome, with its reduction in commensal organisms and increases in Staphylococcus aureus, especially with flares, is another avenue of investigation with therapies in development to combat the shift in the microbiome without antibiotic use. To date, we do not know how variations in biomarkers affect responses.
But we do see certain changes in specific populations, such as the increase in Th17 pathway expression in children, individuals of Asian communities, and in lesions of nummular dermatitis. Would these groups of individuals benefit more from concurrent suppression Th17, or even an antimicrobial approach, since these surface organisms can be a driver of high Th17 responses?
I think what's most exciting to the participants is the new treatments, and how they differ from what is currently available. We talked about new topicals that have recently become available for psoriasis. One is a PDE4 inhibitor, and the other the first aryl hydrocarbon agonist; both really look like they're doing well for psoriasis and demonstrate very promising data for AD. Topical application of commensal organisms with potent anti-S. aureus effects are in development as well. New systemic medications are also emerging. Tralokinumab is most recently available for children as young as 12, lebrikizumab is coming out in the next year (already in Europe), and there are other biologics and JAK inhibitors in development. I think that the new kid on the block in terms of pathway is targeting the OX40 receptor and its ligand. Some long-term data in adults suggest potentially a more prolonged improvement off of these medicines after a treatment course, but more work is needed.
Q5
As the title of your abstract would suggest, you have dedicated a significant portion of your career to pediatric dermatology. What are the unique challenges associated with this specialism?
There are many challenges to treating children and bringing them new treatment options. Of paramount importance is safe management, especially for chronic diseases of the skin in which medications, whether topical or systemic, need to be used long-term, and potentially for a lifetime. I'm glad to see new medicines that have been very carefully studied in younger children. But we still have questions. The fact that we're going to have children on drugs for decades longer than adults raises questions about long-term safety that we cannot answer easily until decades have passed.
Of course, there are issues with drawing blood, and we're delighted to see some medications that do not require this that are very effective. There are also issues with injections. Many families, unfortunately, either cannot deal with doing the injections, or even coping with their children screaming while getting the injections in a doctor's office. Some of these children then are treated with agents we’ve had for decades, like low-dose methotrexate – and we have to remember that these are still effective, largely safe agents, even if they
require more careful monitoring, and should be used with caution long-term.
We also have to pay close attention to the psychosocial impact of chronic disease in children - the effect on stigma, on peer relationships, the potential development of anxiety and/or depression, and the impact of having a chronic skin disease on family dynamics, which also can profoundly affect a child long-term, beyond the disease itself. School-aged children and adolescents are very vulnerable to psychosocial impacts.
Q6 Reflecting on your extensive research career and substantial body of 650 publications, which areas of dermatology do you think require greater attention?
My personal areas of high interest are inflammatory and genetic skin diseases. I’ve been so thrilled to watch my patients who suffer from AD and psoriasis become clear, or virtually clear, and
have a dramatic change in their life and its quality. Areas among my patient population that require the greatest attention now are the rare diseases, for which there are often no treatments, and certainly not targeted treatments. Because they are uncommon, they receive very little attention from the scientific community, industry, and regulators. But that is changing. I am seeing a new interest in helping this needy population, whether it's topicals or systemics, and I am so eager to repurpose medications or work with developing new options, particularly as we discover new targets based on the underlying mechanism of disease. Anything that can help children who are suffering from chronic inflammatory diseases is very important to me, and I will continue to enjoy spreading exciting news to patients/families and the scientific community. It is especially important to develop safe medications that are easily embraced by the families we serve. ●
"Areas among my patient population that require the greatest attention now are the rare diseases."
Q1

Citation:
Yagiz Matthew AkiskaMD Candidate, The George Washington School of Medicine and Health Sciences, Washington, D.C., USA; 2022 Summer Research Fellow, University of California San Francisco, Department of Dermatology, USA
Dermatol AMJ. 2024;1[1]:41-42.
https://doi.org/10.33590/dermatolamj/BUPQ3738.
Congratulations on achieving fourth place in the 2024 American Academy of Dermatology (AAD) Annual Meeting Poster Award, for your submission entitled ‘Low-Dose Oral Minoxidil Initiation and Monitoring (LOMI) For Hair Loss: A Modified Delphi Consensus of Experts’. Can you summarize the research presented in your poster?
Low-dose oral minoxidil (LDOM) has been gaining traction internationally as an alternative to topical minoxidil. Our team saw an opportunity to provide expert-based consensus guidelines to dermatologists, as well as practitioners in other fields who may treat hair loss, so that LDOM can be safely and effectively prescribed to treat various hair loss conditions.
After a detailed literature review of oral minoxidil, its pharmacokinetic properties, its adverse effect profile, and its use for the treatment of hair loss, we recruited 43 dermatologists from 12 countries specializing in hair loss, to participate in four modified Delphi survey rounds. Ultimately, our research yielded 78 items of consensus on the safety, efficacy, dosing, and monitoring of LDOM for hair loss.
Our expert panel agreed that LDOM can be safely prescribed to adults and adolescents (>12 years of age), and provides direct benefit to hair loss conditions in which there is follicular miniaturization and hair cycle disruption. It can also offer supportive benefit for several forms of scarring alopecia. Our expert panel agreed that LDOM usually demonstrates efficacy within 3–6 months, and that, in the absence of precautions, routine baseline testing is not needed. Our expert
panel also reached consensus on when LDOM may be preferred over topical minoxidil, most commonly prescribed LDOM dosing ranges, screening (precautions and contraindications) and monitoring guidelines, as well as the use of adjunctive agents.
Q2
Why did you decide to focus your research on hair loss, and which challenges remain in this field?
I chose to focus on hair loss research because it is a topic close to my heart. Like many others, I've personally struggled with hair loss, and have tried numerous products and treatments. Despite my efforts, I experienced a plateau with my current regimens, which led me to question if there could be better options out there. This personal experience fueled my passion to explore alternative treatments, particularly LDOM, as it showed promise in clinical circles.
While small studies suggest LDOM's safety and efficacy for hair loss, at the moment, larger studies are lacking. As the patient demand increases, we hope these best practice guidelines will be helpful to our colleagues, until more robust evidencebased data emerges.
Q3
How do you hope your research findings will affect the treatment and quality of life of patients with hair loss?
We anticipate that our research findings will have a profound impact on the treatment of many different forms of hair loss.
By establishing consensus on key issues, such as LDOM safety, efficacy, and dosing, we hope clinicians will be empowered to make informed decisions, and tailor treatment plans to individual patient needs. Hair loss significantly affects mental health, as quality of life in patients suffering from hair loss can have a substantial impact on individuals' psychological wellbeing, personality, and social aspects. We hope these guidelines, by leading to improved treatment outcomes, will enhance patient satisfaction, and ultimately improve quality of life for individuals struggling with hair loss. Our research aims to fill a critical gap in the field, and contribute to advancing the standard of care for patients with hair loss.
Q4
What are you most looking forward to at AAD 2024?
As a physician in training, I am most excited about attending AAD 2024 as it offers a unique opportunity to immerse myself in the latest advances in dermatology. I'm excited to engage with experts, network with peers, attend lectures and workshops, and stay updated on cutting-edge research. Sharing my 2 years’ worth of research, and forging new professional relationships with future colleagues are all on the horizon, poised to contribute to my growth and development as a dermatologist.
Q5
Considering your wider career, you completed a Bachelor of Science in Biomedical Engineering. How did this educational background motivate you to pursue a medical degree, and what valuable transferable skills did you acquire from this course?
One of the fundamental concepts taught in biomedical engineering, as it differs from other engineering fields, is working within the clinical realm, fostering a mindset of identifying gaps, and devising innovative solutions. The core principles instilled by my engineering education, such as systematic thinking and problem-solving, helped me as I approached various design aspects of the LOMI project, including framing our initial research question, designing surveys, and co-ordinating with experts globally.
Q6
What is next for your research team?
We're excited to present our findings to another global audience at the 13th World Congress for Hair Research in Dallas, Texas, USA, this April. We are also preparing our project for publication. Looking ahead, we would be pleased to partner with our colleagues in pediatric dermatology to establish a similar set of consensus guidelines on the use of LDOM for pediatric hair loss. ●

Q1
Interviews

Introducing our new editorial board! The AMJ team caught up with Michael Gold, the Editor-in-Chief of Dermatology, The American Medical Journal, and board members Neal Bhatia, Brian Hibler, and Jacqueline Watchmaker, to discuss their careers to date, unique challenges presented by dermatology, and their plans for shaping the journal. We also had the pleasure of speaking to Andrew Alexis, who has dedicated a significant portion of his career to advancing patient care, research, and education pertaining to dermatologic disorders that are prevalent in populations with skin of color.

Citation:
Michael Gold
Medical Director, Gold Skin Care Center, Nashville; Medical Director Tennessee Clinical Research Center, Nashville, Tennessee, USA
Dermatol AMJ. 2024;1[1]:43-44.
https://doi.org/10.33590/dermatolamj/NJSO3857.
What initially sparked your interest in dermatology, and how has that passion evolved throughout your career?
My interest in dermatology comes from the fact that, as a teenager, I had bad acne. I was fortunate to have seen Albert Kligman, the innovator of tretinoin, who helped me more than anyone else had been able to help at that time. I became fascinated with what dermatology entails: medicine, surgery, and cosmetic therapies.
Q2
With the field of dermatology constantly evolving, how do you stay updated on the latest advancements and techniques?
Dermatology is evolving every day, both medically and aesthetically. I find myself reading more and more journal articles on important new
breakthroughs, perhaps more than I did years ago. I also am fortunate that I oversee several dermatology meetings and attend many; this is where we get our information on the latest trends and medicines.
Q3
As the Editor-in-Chief, how do you envision the future of dermatology research, and the role Dermatology AMJ will play in shaping the field?
I envision the medical dermatology realm to continue having explosive growth, and someday, we will have the ability to cure some of the skin diseases that are prevalent in our society. As a resident, a long time ago, that was not something that even came into our minds. ‘Cure’ is now closer than ever for a lot of our skin concerns.
Q4
In your opinion, what are the most pressing challenges and opportunities facing dermatology research and practice today?
The most pressing issue facing dermatologists today is the access to our wonderful medicines. We have to be able to get them to our patients, and at an affordable rate. If the prescription medicine costs 100,000 USD per year, and our patient’s co-pay is astronomical, then what is the point? If we have access to them, our patients, who are key, will benefit.
"The most pressing issue facing dermatologists today is the access to our wonderful medicines."
Q5
Dermatology often involves addressing both medical and cosmetic concerns. How do you balance the medical needs of your patients with their cosmetic desires?
I think those who practice dermatology can easily separate the medical and cosmetic worlds. I always tell the medical dermatology doctors that, if you are removing a skin cancer, you are looking for the best cosmetic result with that procedure; so, in essence, you are also a cosmetic dermatologist. We do have lasers and other energy-based devices that are playing more of a role in our patients with acne, rosacea, and with those affected by psoriasis and vitiligo. So, cosmetic devices are helping patients in medical dermatology. And, even with our neurotoxins, we have uses for them in the medical dermatology space, as well as in our normal cosmetic uses.
Q6
What do you find most rewarding about being a dermatologist, and conversely, what are some of the biggest challenges you face in your practice?
The most rewarding thing I do is to make someone better with their skin concern; taking someone suffering from a skin problem, and resolving that issue, and seeing them smile and get back to their normal daily routine. Patient care is what it is all about. The biggest challenge is the access to some of what I want to do.
Q7
In your opinion, what are some common misconceptions about dermatological conditions, and how do you work to educate your patients about them?
The misconceptions come from those who spend most of their time online trying to self-diagnose and treat themselves. They think at times they know more than we do. I always tell people not to be ‘Doctor Google’, as most dermatologists should be able to determine most/every rash or skin concern pretty easily, and come up with a treatment plan that should improve, or take care of, that concern.
Q8
With a portfolio boasting over 300 published articles, the establishment of a globally recognized conference on aesthetic medicine, and the founding of a skincare center, what accomplishment are you most proud of?
All of that is great, but none of it would have been accomplished without the support of my wife, Cindee. We have been married 42 years, and raised two wonderful kids, and they have been behind everything that I have done, and will do moving forward. ●
Q1

Neal Bhatia
Director of Clinical Dermatology, Therapeutics Clinical Research, San Diego, California, USA
Citation:

Brian Hibler Dermatologist, Schweiger Dermatology Group, New York, USA
Dermatol AMJ. 2024;1[1]:45-47.

Jacqueline Watchmaker Dermatologist, U.S. Dermatology Partners, Scottsdale, Arizona, USA
https://doi.org/10.33590/dermatolamj/UXAY5987.
How would you describe to our readers what you do?
NB:I am Director of Clinical Dermatology at Therapeutics Clinical Research in San Diego, California, USA, where my practice is 70% research and 30% clinic. I have been involved in the American Academy of Dermatology (AAD) as a board member and officer, and I am currently serving as chair of several committees. I also serve on multiple editorial boards. My background is in immunology, and I have interests primarily in medical dermatology and clinical research.
BH:I practice medical and cosmetic dermatology, meaning I treat various conditions which affect the skin, hair, and nails. My specialization is in lasers and cosmetic surgery. I combine various injectables, lasers, energybased devices, and other cosmetic treatments to address common aesthetic concerns, including lines and wrinkles, sun damage, broken blood vessels, uneven skin tone and texture, scars, skin laxity, excessive fat, laser tattoo removal, and hair removal, among many other conditions.
JW:I am a board-certified, fellowshiptrained dermatologist, who practices medical and aesthetic dermatology. My fellowship was in laser medicine, so I enjoy treating both cosmetic and medical conditions with lasers.
Q2 Why do you do what you do?
NB:I enjoy my work in advocacy, leadership, and as a teacher, but my job as a clinical trial investigator keeps me motivated and passionate to create new technologies and treatments. My passion is to translate the mechanism of action of diseases, and match them to the mechanism of action of therapies, to keep our specialty both relevant and vibrant.
BH:Medicine is the perfect mix of teaching, research, and patient care, all of which I enjoy. Dermatology is a very academic field, with a lot of advancements being made in both medical and cosmetic dermatology. The best part about dermatology is being able to take care of patients of all ages, helping them to feel more confident and comfortable in their skin.
JW:I find the field of dermatology very rewarding. The most rewarding part of my job is sharing knowledge with my patients, so that as a team, we can partner in how to best treat their medical or aesthetic concern. I also love being involved in clinical research, and staying up to date on the newest technologies and treatments, so I can provide the best possible care for my patients.
Q3
What are the unique challenges associated with providing care in your field?
NB:Between dwindling reimbursements, endless bureaucratic obstacles, the creeping scope of practice into our specialty, and the endless struggle to play defense against our patients’ internet searches, the challenges to stay sane and motivated are enough to make anyone burn out.
BH:Many dermatologic conditions are considered ‘cosmetic’, because they will not cause the patient physical harm; however, they can result in significant psychosocial distress, and affect their self-confidence. I enjoy being able to combine topical treatments, lasers, injectables, chemical peels, and energy-based devices to address my patients’ concerns.
JW:
Dermatology is a very visual field, so patients can see when their skin condition improves, but also when it worsens. Skin conditions often take weeks, if not months, to improve, which can leave some patients frustrated. Also, many conditions in the field of dermatology are chronic conditions, that can be managed, but not cured.
"Skin conditions often take weeks, if not months, to improve, which can leave some patients frustrated."
Q4 Considering your impressive career, which achievement are you most proud of?
NB:Despite being elected Vice President of the AAD, and Secretary-Treasurer of the Noah Worcester Dermatological Society, Indiana, Indianapolis, USA, my best achievement was finally becoming a father to my two wonderful sons, and enjoying the first 10 years of marriage, with hopefully many more.
JW:I am most proud of my ‘Intern of the Year Award’, as well as being selected as chief resident during my final year of dermatology residency. I’m also always honored to be invited to speak at conferences, both nationally and internationally.
Q5 What is one way your practice changed someone’s life that you didn’t expect?
NB:We have enrolled many unique patients into clinical trials, but there have been some amazing outcomes with some individuals who would not have received care without having found us.
BH:I have had patients come back and tell me that I have inspired them to pursue a career in a medical field, an impact I did not expect to have on others so early in my career. I always admired my pediatrician and other doctors growing up, so it feels extra special to be in this privileged position: to take care of patients, and be able to affect their future career path.
JW:I treated a patient who was severely scarred by a medical spa. After multiple laser treatments, she finally feels confident enough to return to work, and see her family. Which session(s) did you most enjoy at AAD 2024?
Q6 Which session(s) did you most enjoy at AAD 2024?
NB:Our session on ‘Managing Office Politics’ was a lot of fun.
BH:I enjoyed the hands-on laser session, where over 20 key opinion leaders and fellowship-trained cosmetic dermatologists taught the latest in lasers and energy-based devices.
JW:The hands-on laser course, and the ‘Complications’ session.
Q7
As an editorial board member, how are you hoping to shape the journal over the coming years?
NB:I hope to bring my experience as an editor to make the journal easy to read, compelling to follow, and attractive to sponsor.
BH:I hope to bring together high impact articles, which are meaningful and clinically relevant for the readership. Together, we will improve patient care by sharing sound scientific articles and late-breaking updates, that are important for dermatologists worldwide.
JW:The field of cosmetic dermatology continues to grow, and it is important that dermatologists stay abreast of the field, and of the latest technology. I hope to help incorporate well-done aesthetic studies and aesthetic commentaries. ●
Q1

Citation:
Andrew AlexisProfessor of Clinical Dermatology, Weill Cornell Medicine; Vice Chair for Diversity and Inclusion, Department of Dermatology, Weill Cornell Medicine, New York, USA
Dermatol AMJ. 2024;[1]1:48-49.
https://doi.org/10.33590/dermatolamj/OOAA9765.
After receiving your medical degree from Columbia University Vagelos College of Physicians & Surgeons, New York, USA, what inspired you to specialize in dermatology?
My inspiration to pursue dermatology begins with my mother, the late Mercy Alexis Akuffo, who was the first Black female dermatologist in Canada. She practiced dermatology in the greater Toronto area, and was on staff at the University of Toronto Women’s College Hospital, Ontario, Canada. Seeing firsthand the impact a dermatologist can have on patients of all ages and backgrounds, and the personal satisfaction my mother derived from being a dermatologist, certainly had an impact on my path to pursuing medical school, and a dermatology residency. A second major source of inspiration came from my medical school experience at Columbia. During my clinical rotations, I was amazed to see that, no matter what specialty I was rotating in, dermatological manifestations of numerous diseases and treatments would arise. It was remarkable that in these instances, the extraordinary dermatology consultation service at Columbia would be called for their input into often complex cases, and would uncover important findings on skin examination, that ultimately led to key diagnoses that changed the trajectory of the patient’s course of treatment and clinical outcomes.
Q2 You have dedicated a significant portion of your career to advancing patient care, research, and education pertaining to dermatologic disorders that are prevalent in populations with skin of color. Has this understanding and awareness changed throughout your career, and what challenges remain?
Over the past 20 years, there have been considerable advances in our understanding, awareness of nuances, and specific considerations for patient populations with skin of color. We have seen an increase in research studies, publications, educational content at continuing medical education meetings, and textbooks that highlight dermatologic conditions in patients with skin of color. We have particularly seen recent developments in pigmentary disorders, that have greatly expanded our therapeutic options for hyperpigmentation; new studies that investigate the treatment of psoriasis and atopic dermatitis (AD), specifically in patient populations with skin of color; and advances in hidradenitis suppurativa, to name a few. Some key challenges that remain understudied, and have limited treatment options, include keloids; scarring alopecias, such as central centrifugal cicatricial alopecia; erythema dyschromicum perstans; and lichen planus pigmentosus.
Q3
Your most recent publication, entitled ‘Integrating Skin Color Assessments Into Clinical Practice and Research: Review of Current Approaches’ concludes that there are significant deficiencies in skin classification instruments. What can the medical community do to combat this?
I think it is important for the medical community to recognize the limitations of our current classification instruments and approaches. For example, the Fitzpatrick skin phototype system is often used in a way that deviates from its original intent, which is to classify skin based on response to UV radiation, and can be imprecise with respect to classifying diverse populations with skin of color. It is very likely that we will see the increased
use of instrumentation, to objectively measure skin pigmentation in the clinical research setting, and this may also translate into the clinical setting.
Q4
Spotlighting atopic dermatitis, please can you outline variations in epidemiology, clinical presentation, and treatment in diverse racial and ethnic groups?
In the USA, there is a higher prevalence of AD in Black children versus White children (15.89% versus 9.70%), higher odds of persistence of AD from early- to mid-childhood in Black children versus White children, higher rates of absenteeism from school secondary to AD among Black and Hispanic children, and racial/ethnic disparities in healthcare utilization rates among patients with AD. In addition, patients with AD and more pigmented skin also tend to present with associated pigment alterations, such as hyper-and hypopigmentation, as long-term sequelae of their AD. This contributes to the overall burden and impact of AD on patients with skin of color.
Q5
You are due to present at many sessions at AAD 2024, including ‘Melasma in Skin of Color: 2024 Update’. What do you expect to be the key takeaways?
In this presentation, I highlight the differential diagnosis of melasma, including the importance of recognizing other disorders that present with hyperpigmentation on the face, such as lichen planus pigmentosus, medication-induced hyperpigmentation, and facial acanthosis nigricans. I also highlight advances in treating melasma and post-inflammatory hyperpigmentation, including trifarotene for post-inflammatory hyperpigmentation; tranexamic acid for melasma; and cosmeceutical/dermocosmetic ingredients, such as cysteamine and 2-mercaptonicotinyl glycine, to improve hyperpigmentation.
Q6
You serve on the Board of Directors for the Scarring Alopecia Foundation (SAF). What are the main focuses of this role?
Advancing awareness/education, research, and care for patients with scarring alopecias.
Q7
In editing a textbook focused on cosmetic procedures for individuals with skin of color, what essential factors should dermatologists take into account when providing care to these patients?
Recognize that there is no ‘one size fits all’; assess and treat each patient as an individual, and recognize their individual nuances in skin complexion, reactivity, clinical features, and cultural impact.
Q8
You make appearances on major television news programs such as ABC, CBS, NBC, and FOX, addressing issues like skin cancer awareness, and providing skincare guidance in the context of pandemic face mask usage. How crucial is this effort in augmenting public comprehension of dermatological concerns?
Participating in media interviews is an effective way to raise community awareness on several important health issues related to the skin, hair, and nails. I have had the privilege of sharing insights into skin cancer, sun protection, mask wearing during the COVID-19 pandemic, and scarring alopecia through numerous media engagements over the years.
Q9
Your work has been recognized by a wealth of awards, including being listed in the Castle Connolly Top Doctors list, which recognizes the top 7% of physicians in the USA. What has been your proudest achievement to date?
Being able to foster relationships with patients over a 20-year period, such that I can now treat patients’ entire families and loved ones through ‘word-of-mouth’ referrals.
It has also been very rewarding to see many of my former students and residents become successful dermatologists themselves. For many years, I would enjoy attending dermatology conferences with my mother, who would sit in the first row of my presentations, and even ask questions afterwards; this will remain one of my fondest memories. ●
JAK Inhibitors for Alopecia Areata
Citation: Dermatol AMJ. 2024;1[1]:50-51. DOI/10.33590/dermatolamj/10305279. https://doi.org/10.33590/dermatolamj/10305279.
Epidemiology of AA
AA is a common, chronic, tissue-specific, autoimmune disease affecting up to 2% of the global population1,6
Clinically manifests as non-scarring patches of alopecia1,3
Spotlighting JAK Inhibitors
• JAKi target chronic inflammatory skin conditions, like atopic dermatitis, AA, vitiligo, psoriasis, and lichen planus3
• Barictinib approved following clinical trials showing:4
CD4+ and CD8+ cytotoxic T lymphocytes are thought to mediate HF damage due to antigenic recognition3
Hair regrowth in ~1/3 of patients with severe AA
~80% scalp coverage in these patients
Eyebrow and eyelash regrowth
Targets of JAKi in the Treatment of AA
Follicular
Nucleus
Ritlecitinib
• JAKi interfere with inflammatory signals4 JAK3
Tofacitinib
Key:
AA: alopecia areata; FDA: U.S. Food and Drug Administration; HF: hair follicle; IFN: interferon; ISA: immunosuppressive agents; JAK: Janus kinase inhibitor; MHC: major histocompatibility complex RCT: randomized controlled trial; SALT: severity of alopecia tool.
References:
1. Pratt CH et al. Alopecia areata. Nat Rev Dis Primers. 2017;3:17011.
2. Liu M et al. Janus kinase inhibitors for alopecia areata: a systematic review and meta-analysis. JAMA Network Open. 2023;6(6):e2320351.
3. Sardana K et al. Which is the ideal JAK inhibitor for alopecia areata - baricitinib, tofacitinib, ritlecitinib or rfidancitinib - revisiting the immunomechanisms of the JAK pathway. Indian Dermatol Online J. 2023;14(4):465-74.
4. American Academy of Dermatology Association (AAD). JAK inhibitors: what your dermatologist wants you to know. 2023. Available at https://www.aad.org/public/diseases/a-z/jakinhibitors. Last accessed: 28 February 2024.
5. King BA, Craiglow BG. Janus kinase inhibitors for alopecia areata. J Am Acad Dermatol. 2023;89(2S):S29-32.
6. Zhou C et al. Alopecia areata: an update on etiopathogenesis, diagnosis, and management. Clin Rev Allergy Immunol. 2021;61(3):403-23.
Current Treatment Options
AA therapies are now more specific, due to improved pathophysiology understanding3
Ideal intervention: targeted small molecule therapy3
Various ISA for AA treatment act by modulating the immune response
Until recently, none of the approved drugs targeted the JAK pathway3
Baricitinib, an oral JAK1 and JAK2 inhibitor, gained FDA approval for the treatment of adults with severe AA3
Activated CD8+ T cells release IFN-γ, which signals via JAK1 and JAK2, to release IL-15, which mediates CD8+ T cells induction. This is an autocrine loop that activates CD8+ T cells via JAK1 and JAK3, to enhance the production of IFN-γ.
Future Directions
• There are several studies endorsing the efficacy of novel JAKi in AA (ritlecitinib, tofacitinib, and brepocitinib)
• A meta-analysis of 7 RCTs of JAKi identified 30–90% improvement in SALT scores from baseline compared with placebo2
• Although there are case reports of topical JAKi for AA, they have not demonstrated efficacy in clinical trials5
• This class of drugs can help replace the widespread use of steroids with its myriad regimens and doses, which lead to side effects3
• Ideal JAKi for AA remains unclear, despite acceptable safety and tolerability3
• Longer RCTs, larger samples, and head-to-head comparisons in AA needed3
• Further assessment of long-term recurrence required2
Correlation of Cutaneous Manifestations with the Severity of Disease in Patients with Chronic Kidney Disease and Effect of Hemodialysis: An Observational Study
Editor's Pick
Chronic kidney disease is an important public health issue and a growing contributor to global morbidity and mortality. Hemodialysis can improve survival in these patients. Jeswani et al. present a retrospective, observational study of 250 patients undergoing hemodialysis, and report on the prevalence of dermatological manifestations of chronic kidney disease, concluding that this is significantly associated with the duration of disease. The authors stress the importance of a multidisciplinary approach, specifically integrating dermatological assessments into routine evaluations to improve diagnostic accuracy and optimize treatment.
Michael Gold Gold Skin Care Centre, USAAuthors:
Disclosure:
Jitesh Jeswani,1 *Ankit Bhardwaj,2 Shuchi Bhatt3
1. Nephron Kidney Hospital, Nagpur, India
2. Department of Pharmacology, University College of Medical Sciences & Guru Teg Bahadur Hospital, Dilshad Garden, Delhi, India
3. Department of Radiology, University College of Medical Sciences & Guru Teg Bahadur Hospital, Dilshad Garden, Delhi, India
*Correspondence to drankitbhardwaj25@gmail.com
The authors have declared no conflicts of interest.
Received: 07.03.23
Accepted: 20.03.24
Keywords:
Citation:
Abstract
Aim:
Chronic kidney disease (CKD), cutaneous manifestations, hemodialysis.
Dermatol AMJ. 2024;1[1]:52-62.
https://doi.org/10.33590/dermatolamj/HZSA8667.

Chronic kidney disease is a non-communicable disease, and is the sixth fastest growing contributor of morbidity and mortality. Hemodialysis is one of the important therapeutic modalities that can improve survival in these patients, and can increase their life expectancy, but the cutaneous disorder can precede or follow the initiation of hemodialysis.
Methods:
This is a retrospective, observational study with a sample size of 250 patients, with a glomerular filtration rate <60 mL/min/1.73 m2 for a minimum duration of 3 months or more, undergoing hemodialysis. Patients post-renal transplant, HIV-positive cases, and pregnant patients were excluded from the study. Studied cases were recruited equally into two groups: the dialytic group (Group A) and non-dialytic group (Group B).
Results:
In the authors’ study, the prevalence of dermatological manifestations was 79% in the dialytic group and 75% in the non-dialytic group. The most common finding overall was xerosis (58%), which was more common in the dialytic (66%) group, both in number of patients and severity. The second most common finding was pallor (55%), which was seen more in the dialytic group (60%). Other major findings were pruritus (49%) and hyperpigmentation (37%). The intensity of pruritus was higher in nondialytic patients. Specific cutaneous manifestations, such as Kyrle’s disease, were seen only in eight patients. Skin infections were seen in 17% of patients overall, and there was no major difference seen in both groups. The prevalence of nail findings, mucosal changes, and hair changes was also high in the dialytic group. Other specific cutaneous manifestations, like calciphylaxis, uremic frost, and nephrogenic fibrosing dermopathy, were not seen in the authors’ study. Hemodialysis has increased the life expectancy of patients with end-stage renal disease and has also brought about a rise in the number of manifestations, by giving time for these changes to occur. The severity of symptoms was also higher in patients on dialysis. This could be because of the higher mean duration of disease in the dialytic group compared to the nondialytic group.
Conclusion:
Dermatological manifestations of chronic kidney disease were significantly associated with the mean duration of disease, which was higher in patients on dialysis. There was a higher prevalence of non-specific dermatological findings, such as xerosis, hyperpigmentation, nail findings, hair, and mucosal changes in the dialytic group, except pruritus. Any such cutaneous marker in the absence of a primary dermatological problem warrants a thorough search, including blood, urine, and radiological investigations, to rule out kidney disease.
Key Points
1. By analyzing dermatological signs and symptoms in patients with chronic kidney disease (CKD), the research aims to identify these manifestations as potential biomarkers for disease severity. This could lead to earlier detection and intervention, improving patient outcomes.
2. By comparing dermatological symptoms before and after hemodialysis, the research aims to inform clinicians about the holistic management of patients with CKD, to improve their overall wellbeing.
3. Healthcare professionals can adopt a multidisciplinary approach by recognizing the relationship between skin manifestations, disease severity, and hemodialysis. Integrating dermatological assessments into routine evaluations can improve diagnostic accuracy and optimize treatment, leading to an enhanced quality of life for individuals with CKD.
INTRODUCTION
Chronic kidney disease (CKD) is a noncommunicable disease that currently widely affects around 850 million people. Nowadays, it is an important public health issue, as it is the sixth fastest growing contributor of morbidity and mortality.1 Hemodialysis is one of the important therapeutic modalities which can improve survival in these patients, and can increase their life expectancy.2 Approximately 50–100% of patients with CKD have at least one associated non-specific cutaneous change.3,4 These cutaneous changes can precede or follow the initiation of hemodialysis therapy, and there are higher chances of developing newer cutaneous changes during the cycles of hemodialysis therapy. Pruritus is one of the most common characteristic symptoms of CKD, and is more severe in patients who are not on maintenance hemodialysis.5 Proposed etiologies for pruritus in CKD include changes related to xerosis, uremia, calcium and phosphate dysregulation, mast cell proliferation with high levels of histamine, hormonal derangement, and hypovitaminosis D.6,7 Other skin manifestations which are peculiar to dialysis are local complications, i.e., extravasation of blood, phlebitis, bacterial colonization of cannula, septicemia, allergic contact dermatitis to disinfectant, antiseptic solutions, and tape or bandages used to secure dialysis cannula or tubing.8,9 Complications associated with hemodialysis are bullous dermatosis of hemodialysis and gynecomastia.10
These cutaneous manifestations can precede or follow the initiation of hemodialysis therapy, and there are higher chances of developing newer cutaneous changes during the cycles of hemodialysis therapy, which may further affect the quality of life. The present study was conducted to evaluate the early cutaneous signs, and their prevalence and correlation, with the severity of disease in patients with CKD with or without hemodialysis, for early recognition of cutaneous signs, and to relieve further suffering, and decrease morbidity. The second objective of the study was to study the effect of hemodialysis on cutaneous manifestations.
PATIENTS AND METHODS
The retrospective, observational study was conducted in a multispecialty tertiary healthcare center from January 2017–June 2018 (total of 18 months). Taking the prevalence of cutaneous manifestations among patients with CKD as 80%,5 and absolute error of 5%, the sample size comes out to be 250.
Inclusion criteria for cases selected were as per the CKD definition, which is defined as kidney damage or glomerular filtration rate <60 mL/ min/1.73 m2 for a minimum duration of 3 months or more, undergoing hemodialysis irrespective of the cause, of either sex, in the age group of 10–90 years, and attending the Nephrology and Skin department. Patients post-renal transplant, HIV-positive cases, and pregnant patients were excluded from the study. Cases were recruited in the study and divided into two groups, the dialytic group (Group A) and the non-dialytic group (Group B), to study and compare dermatological manifestations in patients with CKD. Group A included a total of 125 patients with CKD on maintenance hemodialysis (pre-dialysis criteria for hemodialysis: serum creatinine: >4 mg/dL; blood urea >70 mg/dL; serum potassium >6.5). Symptomatic patients, irrespective of the abovementioned criteria, were considered for dialysis. The non-dialytic group included 125 patients with CKD. Detailed history and thorough physical examination, including a detailed assessment of skin, nails, hair, and mucous membrane for specific and non-specific cutaneous manifestations of CKD, were done in both groups and recorded on a predesigned proforma developed based on Skindex questionnaires. The Skindex questionnaire has 16 questions, but the authors opted for simpler modified question instruments based on Skindex-10, with their relevant subdomains for the patients on hemodialysis. Patients on maintenance hemodialysis were examined for any puncture marks, extravasation, and arteriovenous shunt dermatitis. Glomerular filtration rate was calculated for each patient using the Cockcroft–Gault formula, and staging of the severity of CKD was done accordingly. Various investigations were done, such as complete blood count, random blood sugar, renal function test, and serum calcium and phosphorous tests. Investigations such as Gram stain, Tzanck smear, potassium hydroxide mount,
bacterial culture and sensitivity, fungal culture, chest X-ray, and skin biopsies were done wherever indicated. Institute ethical committee approval, and written and informed consent from all participants were obtained before enrolling in the study.
STATISTICAL ANALYSIS
The data obtained were statistically analyzed using Microsoft Excel 2016 (Microsoft, Redmond, Washington, USA), and the statistical ꭓ2/Fisher exact test was used. The categorical variables were presented in number and percentage (%), and continuous variables were presented as mean±standard deviation and median. Quantitative variables were compared using the ꭓ2/Fisher exact test.
RESULTS
A total of 250 patients, primarily consisting of patients with CKD, and patients with CKD undergoing hemodialysis, were analyzed.
The groups were comparable, as there was no statistically significant difference between the sex, age, duration, CKD stage, and CKD etiologies. The mean hemoglobin level in both groups was 7.95±1.46 mg/dL in the dialytic group and 8.09±1.16 mg/dL in the non-dialytic group, and a maximum number of patients in the authors’ study had low hemoglobin levels. The mean blood urea level was 119.81±44.13 mg/dL in patients on dialysis and 115.50±42.45 mg/dL in non-dialytic patients. The most common underlying etiological cause of CKD in the studied participants was hypertension (36% in the dialytic group and 40% of the non-dialytic group), which was followed by diabetes (27% in the dialytic group and 22% in the non-dialytic group).
Both hypertension and diabetes were found in 17.6% in the dialytic group and 16.0% in the nondialytic group. In this study, most of the patients were in Stage 5 of CKD at the time of examination in both the dialytic (n=116; 92%) and pre-dialytic group (n=114; 91%). The mean duration of disease in dialytic and non-dialytic patients was 25.2 months and 12.4 months, respectively, with a wide variation in the duration of disease ranging from 5 months to 15 years. In the present study, nail
dystrophy was the most common nail finding in patients on dialysis (n=26; 20%) followed by SUB (n=22; 17%), nail discoloration (n=18; 14%), and half and half nail (n=15; 12%); while in nondialytic patients, subungual hyperkeratosis (n=25; 20%) was the most common nail finding, followed by nail dystrophy (n=19; 15%), nail discoloration, and onycholysis (n=16; 12%); Figure 1 and 2). In this study, the most common mucocutaneous finding was xerostomia (n=8; 6.40%) followed by macroglossia (n=6; 4.80%) in the analytic group, while in the non-dialytic group, the most common finding was macroglossia (n=6; 4.80%), followed by the fissured tongue (n=5; 4%; Figure 3). Xerosis was mild in most of the cases, and found more commonly in the dialytic group (n=86; 66.6%) in comparison to the non-dialytic group (n=60; 48.0%). Mild-to-moderate hyperpigmentation was seen in 56 (44%) dialytic cases and 38 (28%) nondialytic cases. Moderate-to-severe pruritus was seen mainly in all non-dialytic patients (n=74; 58%), while only 40% of patients of the dialytic group were having pruritus. Pallor was seen commonly in both groups. Among specific skin findings in CKD, associated perforating disorders were seen in five patients (4.0%) in the non-dialytic group and three patients (2.4%) in the dialytic group (Figure 4). In this study, dermatological manifestations of CKD were significantly associated with the mean duration of disease, which was higher in patients on dialysis. There was a higher prevalence of nonspecific dermatological findings such as xerosis, hyperpigmentation, nail findings, hair, and mucosal changes in the dialytic group, except pruritus. The other complication was dilation of the venous segment near the arteriovenous fistula, and eczema around the arteriovenous fistula, which was seen in 5.6% of cases.
CORRELATION
The authors observed that skin, nail, and hair manifestations showed a statistically significant correlation with the duration of dialysis (r2=0.9556 and 0.9910) during the first year of initiation of dialysis, while after 1 year this correlation becomes weaker (r2=0.2500). Cutaneous manifestation has an equally statistically significant correlation with all hemodialysis and peritoneal dialysis (r2=0.9473, 0.9578, and 0.9973). Similarly, there was a statistically significant correlation between the
CKD related skin manifestations in Group A & B Pruitus Grp B Pruitus Grp A
Grp B
Grp A
Grp B
Grp A
CKD related hair manifestations in Group A & B
CKD: chronic kidney disease; HYP: hyperpigmentation;
cutaneous manifestation with serum creatinine level (r2=0.9542, 0.9720, and 0.9230) and serum uric acid level (r2=0.9472, 0.9610, and 0.9910). This correlation is more significant in patients with serum creatinine level between 11–20 mmol/L and serum uric acid level above 200 mmol/L.
DISCUSSION
This retrospective observational study was done to study and compare dermatological manifestations in patients with CKD. In the present study, 79% of patients in the dialytic group, and 75% of patients in the non-dialytic group, had at least one dermatological manifestation. Similarly, in a study by Picó et al.,11 cutaneous manifestation was seen in all patients undergoing hemodialysis. Nunley et al.4 also found that 50–100% of patients with end-stage liver disease had at least one cutaneous lesion. In other Indian studies, the frequency of skin involvement ranged from 96–100%.5,12
disorder
infections
related dermal infections in Group A & B
Types of dialysis versus cutaneous manifestations y = -35.5x + 105.67
In the authors’ study, xerosis was the most common non-specific manifestation noted, with an overall prevalence of 58%, with the dialytic group (66%) having a statistically significantly higher prevalence (P=0.211) in comparison to non-dialytic patients (48%). Xerosis mainly affects the upper and lower limbs, back, and face. Diffuse xerosis was observed in 11% of overall cases. Similarly, in a study done by Gilchrest et al.13 and Yosipovitch et al.,14 high prevalence of xerosis was observed in 69% and 62% of non-diabetic patients, respectively, and 70% and 91% of patients on hemodialysis, respectively.13,14 The higher prevalence of xerosis in the authors’ study could be due to non-availability and inappropriate use of moisturizers, due to poor economic status. Other factors associated with xerosis in the Indian scenario could be poor diet, protein malnutrition, tropical climate with more sun exposure, and chronic dehydration. The other possible etiological factors for xerosis include a reduction in the size and functional abnormality of eccrine sweat glands, causing epithelial dehydration
Serum uric acid level versus cutaneous manifestations
y = -22x + 68
y = -21.5x +
and dehydration due to the use of chronic and high doses diuretics in patients with CKD.10,15 In the authors’ study, pallor was the second most common manifestation and observed in 55.20% of patients (dialytic group: 59.20%; non-dialytic group: 51.20%). No significant difference was found between the dialytic and non-dialytic groups. Similar findings were reported by Udayakumar et al.,5 Levillard et al.,16 Chanda et al.,17 and Pradhan et al.,18 who reported pallor in 60%, 70%, 57%, and 54% of patients with uremia, respectively.5,16-18
Chronic malnutrition superimposed on the anemia of chronic disease may have resulted in a higher prevalence of skin pallor. Pruritus was found in 49% of patients, with a higher prevalence in the non-dialytic group (59.2%) in comparison to the dialytic group (40.2%). The severity of pruritus varied in patients. Some patients gave a history of improvement of pruritus after dialysis. Similarly, a study by Chanda et al.17 reported a prevalence of pruritus higher in the non-dialytic group (60%) than the dialytic group (40%), similar to the authors’ study.17 The other studies reported prevalence of pruritus in the pre-dialytic period in patients ranging from 22% to as high as 85%.19,20 Proposed etiologies for pruritus in CKD include changes related to xerosis, uremia, calcium and phosphate dysregulation, mast cell proliferation with high levels of histamine, hormonal derangement, and hypovitaminosis D.6-7
In the authors’ study, hyperpigmentation was seen in 37% of patients, with a higher prevalence in the dialytic group (44.8%) than in the non-dialytic group (30.4%). Hyperpigmentation was more common in sun-exposed areas like the face and neck, while 30% of patients had diffuse hyperpigmentation. Skin manifestations were comparatively lesser in proportion, when compared to Picó et al.,11 Hajheydari et al.,3 and Udayakumar et al.,5 who reported pigmentary changes in 70.00%, 66.73%, and 53.00%.3,5,11 An increase in the amount of melanin in the basal layer of the epidermis is due to an increase in the concentration of the poorly dialyzable β-melanocyte-stimulating hormone.21,22
In the present study, skin infections were lesser in proportion than Udayakumar et al.5 and Sultan et al.,23 who reported a prevalence of 67% and 40% in their studies. The authors observed skin infections in 17.0% of the patients, 7.2% of which were fungal (tinea infection in 78% and pityriasis
versicolor in 22%), 4.4% were bacterial infections (in form of pyoderma), 3.0% viral infections (herpes zoster), and 3.6% parasitic (scabies). There was no statistically significant difference between the dialytic and non-dialytic groups. The high incidence of infections might be due to lymphopenia, decreased B cell activity, alteration of the T cell subsets, and other disorders associated with diabetes, low albumin, elevated intracellular calcium, acidosis, or repetitive vascular procedures.24
CKD-associated specific skin manifestations include acquired perforating dermatosis, metastatic calcification, uremic frost, nephrogenic fibrosing dermopathy, and bullous dermatosis of dialysis. Among the acquired perforating dermatosis, in the authors’ study, Kyrle’s disease was noted in eight patients (three dialytic and five non-dialytic). Other specific manifestations, like perforating folliculitis and perforating collagenases, were not found in the authors’ study.
Nail changes observed in the authors’ study were more significant in the dialytic group (54%) than in the non-dialytic group (38%). Nail dystrophy (21%), followed by subungual hyperkeratosis (17%), and half and half nail (13%), were most commonly found in the dialytic group, while in the non-dialytic group, subungual hyperkeratosis was most common (20%), followed by nail dystrophy (15%) and half and half nail (9%). Nail discoloration (13%), pitting (10%), Beau’s lines (9%), onycholysis (12%), absent lunula (9%), melanonychia (8%), koilonychia (4%), and leukonychia (4%) were the other nail findings seen in cases. Similarly, Shrestha et al.,25 Chanda et al.,17 Pradhan et al.,18 Asokan et al.,26 and Udayakumar et al.,5 reported 21–62% nail changes in their studies. Half and half nail was the most peculiar finding in patients with CKD, and was reported in between 9–40%.27
Hair changes were observed in 20.8% of patients (dialytic: 22%; non-dialytic: 20%), with no statistically significant difference between the two groups. Hair fall (11%), and sparse hair distribution (8%) with dryness and discoloration of hair, were the most common cases overall. This was significantly low in comparison to a study by Hajheydari et al.,3 which reported >50% of the patients with at least one hair disorder, out of which the most common is disseminated hair loss of the entire body, in particular of the lower limbs (9%).3 Udayakumar
Figure 2: Correlation between duration and type of dialysis, serum creatinine, and uric acid level with cutaneous manifestations.
CKD related hair manifestations in Group A & B
y = -41.5x + 121.33
R2 = 0.9556
y = -9.5x + 32.333
R2 = 0.9991
y
Skin manifestations
Nail manifestations Hair manifestations
Types of dialysis versus cutaneous manifestations
y = -35.5x + 105.67
R2 = 0.9473
R
Skin manifestations
Nail manifestations Hair manifestations
Manifestations
Figure 2: Continued Manifestations Manifestations
Values r2 >0.3 are considered statistically significant. HD: haemodialysis; PD: peritoneal dialysis.
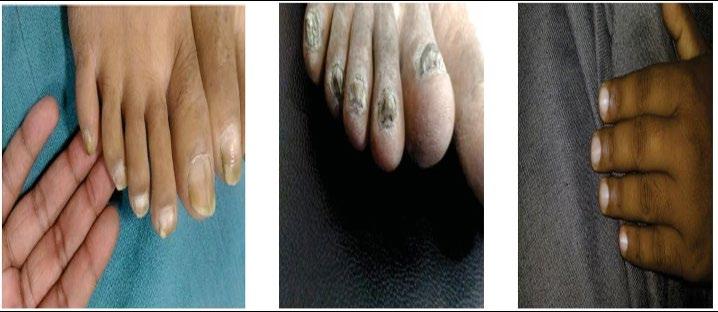
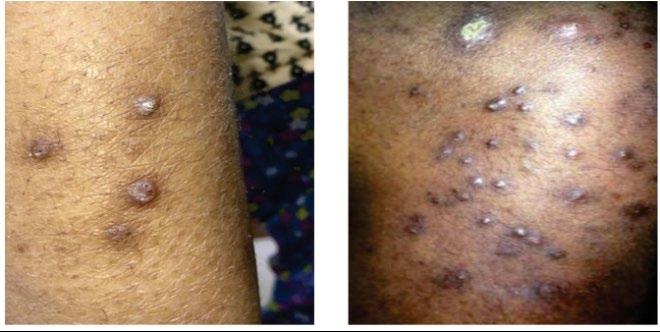
et al.5 reported sparse hairs in 11% of cases.5 The cause of sparse hair may be attributed to decreased secretion of sebum. The mucosal changes were observed in 14% of total patients in the present study, with no statistically significant difference between the dialytic and non-dialytic groups.
A similar mucosal change pattern was reported by Udayakumar et al.5 and Pradhan et al.18 (13–15%). Pradhan et al.18 reported a significantly higher number of cases (39%) with mucosal changes.
Xerostomia, macroglossia, fissured tongue, and angular cheilitis were the other changes reported.
In the authors’ study, local complications of dialysis were seen in 20.8% of the patients, while Masmoudi et al.28 and Chanda et al.17 reported local complications of dialysis in 33% and 74% of cases. In the authors’ study, the most common complication was dilation of the venous segment near the arteriovenous fistula, which was seen in 15% of cases, while Masmoudi et al.28 reported it in 22%.
Another complication was eczema around the arteriovenous fistula, which was seen in 5.6% of cases, and reported in 8.0% of patients with CKD by Udayakumar et al.5 Patients with diabetes as an etiological factor for CKD had a more significant prevalence of xerosis, pruritus, and hyperpigmentation. Attia et al.29 and Gilchrest et al.13 also reported the increased severity of skin manifestations in patients with CKD.13,29
However, their sample size was small (n=25), which was not statistically significant. In the authors’ study, cutaneous findings were higher in patients on dialysis than in the non-dialytic group, except for pruritus. With hemodialysis, life expectancy of patients with end-stage renal disease has increased, and has also brought about a rise in the number of manifestations, giving time for these changes to occur. The severity of symptoms was also higher in patients on dialysis. This could
References
1. Romão Junior JE. Doença renal crônica: definição, epidemiologia e classificação. J Bras Nefrol. 2004;26(3 Suppl 1):1-3.
2. Pastan S, Bailey J. Dialysis therapy. New Engl J Med. 1998;338(20):1428–37.
3. Hajheydari Z, Makhlough A. Cutaneous and mucosal manifestations in patients on maintenance hemodialysis: a study of 101 patients in Sari, Iran. Iran J Kidney Dis. 2008;2(2):86-90.
4. Medscape; Nunley JR. Dermatologic manifestations of renal disease. Available at: https://emedicine. medscape.com/article/1094846. Last accessed: 21 March 2024.
5. Udayakumar P et al. Cutaneous manifestations in patients with chronic renal failure on hemodialysis. Indian J Dermatol Venereol Leprol. 2006;72(2):119-25.
6. Balaskas EV, Uldall RP. Erythropoietin treatment does not improve uremic pruritus. Perit Dial Int. 1992;12(3):330-1.
7. Tapia L. Pruritus on hemodialysis. Int J Dermatol. 1979;18(3):217-8.
8. Nasser G, Ayus JC. Infectious complications of hemodialysis access. Kidney Int. 2001;60(1):1-13.
9. Hoen B et al. EPIBACDIAL: a
be because ofthe higher mean duration of disease in patients on dialysis compared to the non-dialytic group.
CONCLUSION
Based on the results of the present study, the authors propose that a routine skin examination of the patient with CKD receiving or not receiving hemodialysis is an efficient way of early detection of the CKD-related skin manifestations, and can improve care for the patients. A limitation of the present study is a small sample size, which limits generalizability. Another limitation of this study is related to the Skindex-10 questionnaire. In some cases, a non-applicable answer was given, which can lead to a false-negative result, potentially underestimating the impact of the disease. This was observed in 21% of patients on dialysis and 11% of patients with diabetes.
multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol. 1998;9(5):869-76.
10. Goel V et al. Cutaneous manifestations of chronic kidney disease, dialysis and post-renal transplant: a review. Indian J Dermatol. 2021;66(1):3-11.
11. Picó MR et al. Cutaneous alterations in patients with chronic renal failure. Int J Dermatol. 1992;31(12):860-3.
12. Khanna D et al. Comparison of cutaneous manifestations in chronic kidney disease with or without dialysis. Postgrad Med J. 2010;86(1021):641-7.
13. Gilchrest BA et al. Clinical and histological skin changes in chronic renal failure. Evidence for dialysis resistant, transplant responsive microangiopathy. Lancet. 1980;2(8207):1271-5.
14. Yosipovitch G et al. Sweat secretion, stratum corneum hydration, small nerve function and pruritus in patients with advanced chronic renal failure. Br J Dermatol. 1995;133(4):561-4.
15. Weisman K, Graham RM, “Systemic disease and the skin,” Champion RH et al. (eds.), Rook’s Textbook of Dermatology (1998) 6th edition, Oxford: Blackwell Science, pp. 2703-58.
16. Levillard DT, Kambil SM. Cutaneous
manifestations in chronic renal disease – an observational study of skin changes, new findings, their association with hemodialysis, and their correlation with severity of CKD. Int J Sci Res. 2015;5(3):2250-3153.
17. Chanda GM et al. Dermatological manifestations in chronic renal failure patients with and without hemodialysis: a study at a tertiary care centre. J Dr NTR Univ Health Sci. 2017;6(1):8-14.
18. Pradhan M et al. Cutaneous manifestations in patients with chronic kidney disease on hemodialysis and its correlation with renal function, dialysis cycle and haemoglobin. BJHS. 2018;3(2)6:468-74.
19. Young Jr AW, et al. Dermatologic evaluation of pruritus in patients on hemodialysis. N Y State J Med. 1973;73(22);2670-4.
20. Gilchrest BA et al. Clinical features of pruritus among patients undergoing maintenance haemodialysis. Arch Dermatol. 1982;118(3):154-6.
21. Smith AG et al. Role of the kidney in regulating plasma immunoreactive beta-melanocyte stimulating hormone. Br Med J. 1976;1(6014):874–6.
22. Smith AG et al. Plasma immunoreactive beta melanocytestimulating hormone and skin pigmentation in chronic renal failure.
Br Med J. 1975;1(5959):658-9.
23. Sultan M et al. Cutaneous manifestations in Egyptian patients with chronic renal failure on regular hemodialysis. J Egypt Women Dermatol Soc. 2010;7:49-55.
24. Avermaete A et al. Skin changes in dialysis patients: a review. Nephrol Dial Transplant. 2001;16(12):2293-5.
25. Shrestha P, Matheu M. Dermatological manifestations in
chronic kidney disease patients on hemodialysis. NJDVL. 2014;12(1): 34-40.
26. Asokan S et al. Cutaneous manifestations in chronic renal failure patients on hemodialysis and medical management. Int J Res Dermatol. 2017;3:24-32.
27. Amatya B et al. Pattern of skin and nail changes in chronic renal failure in Nepal: a hospital-based study. J Dermatol. 2008;35(3):140-5.
28. Masmoudi A et al. Cutaneous abnormalities in patients with end stage renal failure on chronic hemodialysis. A study of 458 patients. J Dermatol Case Rep. 2014;8(4):86-94.
29. Attia EAS et al. Cutaneous disorders in uremic patients on hemodialysis: an Egyptian case-control study. J Egypt Women Dermatol Soc. 2010;7:49-55.
Baricitinib Demonstrates Rapid Action Within Just 2 Months of Treatment in Severe and Unresponsive Alopecia Areata: A Case Report
Authors:
*E. Tampouratzi,1 K. Sfaelos,2 M. Pizimola,3 P. Rigatos,4 J. Katsantonis5
1. Tzaneio General Hospital, Piraeus, Greece
2. Department of Skin and Venereal Diseases, Faculty of Medicine, School of Health Sciences, University of Ioannina, Greece
3. Dermatologist-Venereologist, Rhodes, Greece
4. Dermatologist-Venereologist, Patra, Greece
5. Dermatology Department, Tzaneio General Hospital, Piraeus, Greece
*Correspondence to elefteria_tab@yahoo.gr
Disclosure: The authors have declared no conflicts of interest.
Received: 28.10.23
Accepted: 29.01.24
Keywords: Alopecia areata, baricitinib, severe.
Citation:
Erratum:
Abstract
Dermatol AMJ. 2024;1[1]:63-67.
https://doi.org/10.33590/dermatolamj/10307342.
This article was first published online March 25th 2024. Since then an erratum was made. The erratum can be seen here

Alopecia areata (AA) is a form of nonscarring alopecia, and is the most common immune-mediated cause of hair loss worldwide. Numerous therapeutic schedules available as off-label options have demonstrated only limited results. However, in 2022, baricitinib, a selective JAK1 and JAK2 inhibitor, was approved as an oral administered systemic therapy for severe AA. Based on this, the authors used it in a 21-year-old White female, who presented with a 15-year history of severe AA (Severity of Alopecia Tool score [SALT]: score 88) and immense psychological burden. After laboratory examinations within normal limits, baricitinib was administered as monotherapy with a 4 mg daily dosage. The severe AA improved rapidly after the first month, and resulted in total hair restoration just after the second month under baricitinib treatment. Besides clinical improvement, SALT score impressively reduced to 30 and 10, respectively, in 2 and 6 months. Six months later, the patient is keeping up the same treatment with no sign of relapse, and is on a 2-month follow-up schedule. In the authors’ patient, almost total hair restoration was achieved in less than 3 months of treatment, which strongly advocates for the addition of baricitinib in the dermatologic armament as a safe, adequate, and fast AA remedy.
Key Points
1. Alopecia areata (AA) has an extremely negative impact on a patient’s quality of life, especially young patients, making the need for new treatments such as baricitinib imperative.
2. Daily clinical practice improves response in severe AA, with total hair restoration achieved in less than 3 months of treatment.
3. JAK inhibitors may represent the drug of choice for AA, as they have revolutionized the therapeutic outcome of this devastating disease.
INTRODUCTION
Among the different types of alopecia, alopecia areata (AA) is the most common reason of hair loss worldwide that is attributed to an immunemediated process.1,2 The documented AA global incidence is within the spectrum of 0.57–3.80% from hospital-based studies, and 1.7–2.1% from general population studies that attempt to calculate the lifetime incidence of AA.3 A total of 10–20% of people who are affected by AA have a positive family history of the disease, indicating a strong genetic background.4 Atopic dermatitis, vitiligo, and other relevant autoimmune disorders, such as systemic lupus erythematosus, are linked with AA, indicating common, but to this day unidentified, pathogenic mechanisms.5,6 AA can affect any region that bears hair, and can present in a wide spectrum that can range from patchy diffuse alopecia to alopecia totalis or universalis.7 The disease impacts quality of life (QoL) with major psychological effects, such as self-esteem issues, depression, and social anxiety, both in males and females.8,9
There are several therapeutic options available, including off-label ones (corticosteroids, cyclosporine, contact immunotherapy, hydroxychloroquine), but these have demonstrated only limited results, and highlight the autoimmune nature of AA.10,11,12 However, in 2022, regulatory authorities approved baricitinib, a selective JAK1 and JAK2 inhibitor, as per os systemic treatment for severe AA.13 Two pivotal randomized, placebocontrolled, Phase III clinical trials showed good clinical efficacy in severe disease versus the placebo treatment arm, with regard to hair regrowth at the 36 weeks timepoint, with favorable safety profile.14 Based on these results, the authors administered baricitinib in a female patient of
young age suffering from severe AA, who presented inadequate response to previous applied treatment modalities, with excellent and quick response in less than 3 months of treatment.
PATIENT INFORMATION
A 21-year-old White female patient presented with a 15-year history of severe AA and immense psychological burden. The severity of disease was assessed with the Severity of Alopecia Tool (SALT) score, with an initial value of 88 in this patient.15,16 Regarding the impact on the patient’s QoL, the Dermatology Life Quality Index (DLQI) questionnaire was utilized, scoring 21 during baseline evaluation. An informed consent form was obtained from the patient. No ethical approval was necessary for the specific case report.
CLINICAL FINDINGS
A generalized hair loss in round and oval areas with no apparent inflammation was noticed across the entire scalp, on the vertex area of the head, and in the parietal and occipital regions, as shown in Figure 1.
TIMELINE
The patient had a strong genetic predisposition, with a positive family history of AA from her mother, and onset of the disorder in her brother as well. The rest of her medical history was free of other systemic diseases, except for the severe emotional burden of AA, and a baseline DLQI score of 36. Prior to her visit, she had consulted many dermatologists,
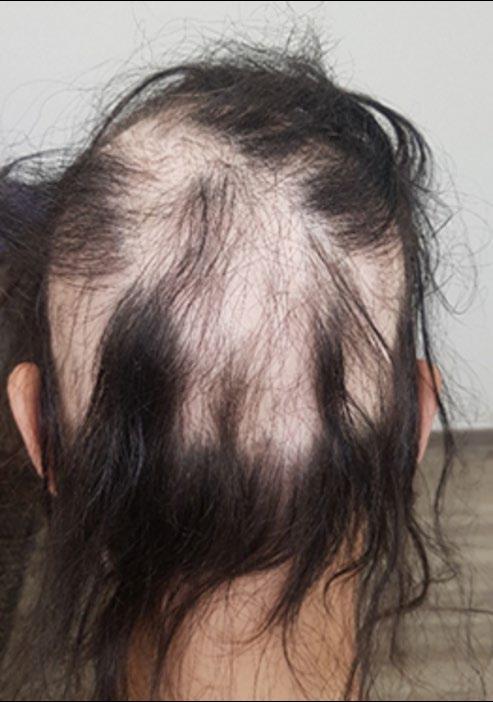
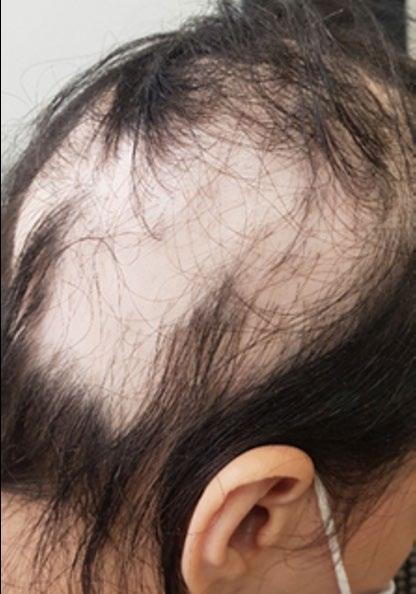
and had been exposed to various treatment modalities, including topical, intra-lesional, and oral corticosteroids; contact immunotherapy with diphenylcyclopropenone; topical minoxidil; cystine vitamin; and other immunosuppressive agents like cyclosporine that were proven insufficient, further worsening her overall QoL.
DIAGNOSTIC ASSESSMENT
The patient underwent a complete laboratory control (full blood count with differential comprehensive metabolic panel, lipid profile after fasting, along with HIV, tuberculosis, and hepatitis B and C screening), before initiating treatment on oral JAK inhibitor baricitinib. The drug was administered as monotherapy in a 4 mg daily dosing scheme.
THERAPEUTIC INTERVENTION AND FOLLOW-UP OUTCOMES
Signs and symptoms of severe AA were rapidly improved after the first month (SALT score: 30), leading to total hair restoration after 2 months on baricitinib treatment (SALT score: 10). Six months following initiation of treatment, the patient is maintaining the same treatment without any sign of
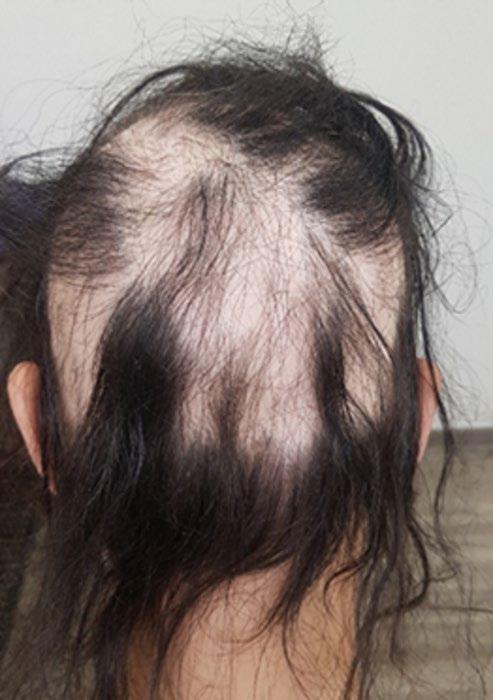
relapse. In addition, the laboratory results are within normal limits at the 6-month follow-up evaluation. The patient’s SALT score and clinical status at 6-month follow-up are indicated in Figures 2 and 3. Regarding the impact of treatment from a QoL perspective, DLQI assessment provided a score of 5 and then 0, at 1-month and 6-month evaluation, respectively. The patient is still on a 4 mg daily dosing scheme, and she is continually on a 2-month re-evaluation schedule in order to monitor progress of treatment.
DISCUSSION
Baricitinib, a first generation oral JAK inhibitor, with a mode of action that inhibits JAK1, JAK2, and to a lesser extent JAK3, interrupts the cytokine signaling pathways that have a key role in the pathogenesis of AA.17
Ιn the pivotal randomized, placebo-controlled, Phase III clinical trials BRAVE-AA 1 and BRAVE-AA 2, 38.9% of enrolled patients in the first study and 35.9% in the second achieved a SALT score of 20 or less at Week 36 under baricitinib treatment at a 4 mg dosage.14 In the authors’ case study, the patient achieved a SALT score of 30 in 4 weeks, and 10 in 8 weeks on the same dosage.
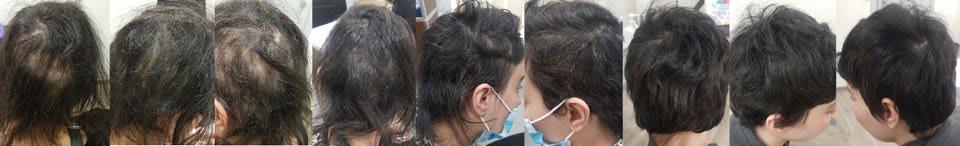
Another systematic review and meta-analysis, which included data from five randomized studies regarding patients with AA that were defined as >50% scalp hair loss, treated with baricitinib 4 mg once per day, presented the highest efficacy compared to other oral JAK inhibitors (ruxolitinib, tofacitinib, ritlecitinib, brepocitinib, and delgocitinib).12,18-21
Besides clinical trials, systematic reviews, and case study series, isolated case reports, such as Jabbari et al.’s22 from 2015, reported complete hair regrowth after 9 months of treatment with baricitinib on an 11 mg daily dosing scheme. Similar findings were observed by Olamiju et al.23 in 2019, with almost complete hair regrowth after 8 months of therapy with baricitinib 4 mg daily. Finally, Wang et al.,24 in 2022, observed response to baricitinib therapy at 5 months, which is the shortest report available to date.24
In addition to efficacy, safety data are already supported since 2011, by Shi et al.,25 who observed that per os administration of baricitinib in healthy controls either produced no or mild adverse effects (reduced reticulocyte count and neutropenia).25 There were no malignancy cases reported in relation to baricitinib use. Based on findings from a study that assessed potential carcinogenicity in mice models, baricitinib was found to have no carcinogenic effect.26 Furthermore, in BRAVE-AA 1 and BRAVEAA 2 trials, in 52 weeks of follow-up, there were no new safety signals compared to the short-term results.27 The rates of side effects with baricitinib generally reflect the inherent risk of the disease populations being treated, and are found more often in rheumatic patients who have an burden of disease.28-30 Additionally, imaging techniques such as line-field confocal optical coherence tomography can be used to observe and monitor early, subclinical hair regrowth during treatment with baricitinib, as has been recently demonstrated by Verzì et al.31
Figure 3: Hair regrowth after 1 month, 2 months, and 6 months, following treatment initiation with baricitinib.As elucidated by reviewing the above available references, the authors reasonably suggest that we could anticipate even faster response to baricitinib for severe AA cases. The authors’ patient achieved almost total hair restoration within 3 months of
References
1. Solimani F et al. Emerging topical and systemic JAK inhibitors in dermatology. Front Immunol. 2019;10:2847.
2. Strazzulla LC et al. Alopecia areata: disease characteristics, clinical evaluation and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78(1):1-12.
3. Uzuncakwak TK et al. Demographic and clinical features of 1,641 patients with alopecia areata, alopecia totalis, and alopecia universalis: a single-center retrospective study. Skin Appendage Disord. 2021;7(1):8-12.
4. Johnson R et al. Fitzpatrick’s color atlas and synopsis of clinical dermatology (2017) 8th edition, New York: McGrawHill Education.
5. Rodriguez TA et al. Concordance rate of alopecia areata in identical twins supports both genetic and environmental factors. J Am Acad Dermatol. 2010;62(3):525-27.
6. Alkhalifah A et al. Alopecia areata update: part ll. Treatment. J Am Acad Dermatol. 2010;62(2):191-202.
7. Dillon L. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata. Clin Cosmet Investig Dermatol. 2021;14: 691-714.
8. Villasante Fricke AC, Mitera M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Invest Dermatol. 2015;24:397-403.
9. Okhovat JP et al. Association between alopecia areata, anxiety and depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2023;88(5):1040-50.
10. Suchonwanit P et al. Alopecia areata: an autoimmune disease of multiple players. Immunotargets Ther. 2021;10:299-312.
11. Zheng C, Tosti A. Alopecia areata: new treatment options including janus kinase inhibitors. Dermatol Clin. 2021;39(3):407-15.
12. Sedeh BF et al. Comparative efficacy and safety of janus kinase
treatment, despite the fact that she had AA for more than 8 years without any hair regrowth during that time period. This strongly supports the addition of the specific treatment option in the dermatologic armament as a safe, adequate, and fast AA remedy.
inhibitors used in alopecia areata: a systemic review and metaanalysis. Acta Derm Venereol. 2023;103: adv00855.
13. Food and Drug Administration (FDA). FDA approves first systemic treatment for alopecia areata. 2022. Available at: http://www.fda.gov/ news-events/press-announcements/ fola-approved-first-systemictreatment-alopecia-areata. Last accessed: 1 November 2023.
14. King B et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386(18):1687-99.
15. Olsen EA et al. Alopecia areata investigational assessment guidelines part ll. National Alopecia Areata Foundation. J Am Acad Dermatol. 2004;51(3):440-4.
16. Olsen EA et al. Objective outcome measures: collecting meaningful data on alopecia areata. J Am Acad Dermatol. 2018;79(3):470-8.
17. Triyangkulsri K, Suchonwanit P. Role of Janus kinase inhibitors in the treatment of alopecia areata. Drugs Des Develop Ther. 2018;12:2323-35.
18. King B et al. Phase 2 randomized, dose-ranging trial of CTP-543 a selective janus kinase inhibitor in moderate to severe alopecia areata. J Am Acad Dermatol. 2022;87(2):306-13.
19. Guttman-Yassky E et al. Ritlecitinib and brepocitinib demonstrate significant improvement in scalp alopecia areata biomarkers. J Allergy Clin Immunol. 2022;149(4):1318-28.
20. King B et al. A phase 2a randomized, placebo controlled study to evaluate the efficacy and safety of the oral janus kinase inhibitors ritlecitinib and brepocitinib in alopecia areata: 24 week results. J Am Acad Dermatol. 2021;85:379-87.
21. King B et al. Efficacy and safety of the oral janus kinase inhibitor baricitinib in the treatment of adults with alopecia areata: phase 2 results from a randomized controlled study. J Am Acad Dermatol. 2021;85(4):847-53.
22. Jabbari A et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. E Bio Medicine. 2015;2(4):351-55.
23. Olamiju B et al. Treatment of severe alopecia areata with baricitinib. JAAD. 2019;5(0):892-94.
24. Wang Y et al. Efficacy and safety of baricitinib in patients with refractory alopecia areata. Dermatol Ther. 2022;35(12):e15845.
25. Shi J et al. The pharmacokinetics, pharmacodynamics and safety of orally dosed INCB018424 phosphate in healthy volunteers. J Clin Pharmacol. 2011;51(12):1644-54.
26. Carfagna M et al. Carcinogenicity assessment of baricitinib in Tg rasH2 mice and Sprague-Dawley rats. Regul Toxical Pharmacol. 2018;92:458-71.
27. Kwon O et al. Efficacy and safety of baricitinib in patients with severe alopecia areata over 52 weeks of continuous therapy in two phase lll trials (BRAVE-AA1 and BRAVE-AA2). Am J Clin Dermatol. 2023;24(3):443-51.
28. Bieber T et al. A review of safety outcomes from clinical trials of baricitinib in rheumatology, dermatology and COVID-19. Adv Ther. 2022;39(11):4910-60.
29. Taylor P et al. Baricitinib safety for events of special interest in populations at risk: analysis from randomized trial data across rheumatologic and dermatologic indications. Adv Ther. 2023;40(4):1867-83.
30. King B et al. Integrated safety analysis of baricitinib in adults with severe alopecia areata from two randomized clinical trials. Br J Dermatol. 2023.188(2):218-227.
31. Verzì AE et al. Subclinical, early hair regrowth in alopecia areata patients under treatment with baricitinib detected by line-field confocal optical coherence tomography evaluation. J Eur Acad Dermatol Venereol. 2023;DOI: 10.1111/ jdv.19623.
Effects of Dermal Fillers on Vaginal Laxity and Female Sexual Function: A Minimally Invasive Procedure for Vaginal Rejuvenation
Authors:
*Uzma Dost Muhammad Rajar,1 Asher Ahmed Mashhood,2 Sumayya Qazi,3 Uzma Tiwana Ayub4
1. Dermatology Department, Isra University Hyderabad, Pakistan
2. Dr Asher’s Aesthetic, Nelson’s Medical Complex, Rawalpindi, Pakistan'
3. Department of Biochemistry, Isra University, Hyderabad, Pakistan
4. UTA Clinic DHA, Karachi, Pakistan
*Correspondence to uzmarajar1@gmail.com

Disclosure:
Received:
Accepted:
The authors have declared no conflicts of interest.
19.12.23
18.03.24
Keywords: Female health, Female Sexual Distress Scale-Revised (FSDS-R), muscle tone, vagina.
Citation:
Abstract
Introduction:
Dermatol AMJ. 2024:1[1]:68-73.
https://doi.org/10.33590/dermatolamj/FEGR7111.
For improving vaginal rejuvenation and minimizing vaginal laxity, the increased demand for soft tissue fillers may give females seeking such therapies the chance to have better general health and sexual performance. To address a wider range of female health issues, and support a more all-encompassing approach to personal wellbeing as cosmetic treatments improve, it is crucial to research the possible repercussions of these minimally invasive operations.
Methodology:
The strength of pelvic floor muscles was measured using the Laborie Peritron 9300 (Portsmouth, New Hampshire, USA) perineometer. A 110 mm long conical vaginal probe with a 26 mm diameter, that may extend to 33 mm under pressure, is a characteristic of this device. The vaginal probe is connected to the perineometer's main body by an 80 cm plastic tubing. When the probe is squeezed, the gadget has a pressure sensor that tracks vaginal pressure. A silicone rubber sensor loaded with air is included into the probe itself, to detect pressure in cmH2O. The Female Sexual Distress Scale-Revised (FSDS-R) was used to measure the participants' degrees of sexual distress, and this method made it easier to gauge the participants' pelvic floor muscle strength.
Results:
The participants' mean age was 39.43±2.12 years, and further demographic characteristics provided evidence that 44% of the participants were married for 10 years and had children; 36% were married for 5 years with no children; and 20% had been married for less than 5 years.
Conclusion: Soft tissue fillers, which have shown a rise in popularity, are a viable means of resolving issues with vaginal elasticity and rejuvenation.
Key Points
1. The study focuses on the importance of dermal filler therapy in improving both physical and emotional aspects of female health, addressing issues like vaginal laxity and sexual distress effectively, and less invasively.
2. The combination of perineometer and the Female Sexual Distress Scale-Revised (FSDS-R) demonstrates effective results, promoting a holistic approach to female wellbeing.
3. This study is not only filling a gap in the current research, but also providing a viable option for females experiencing vaginal laxity and sexual distress, which is also non-invasive.
INTRODUCTION
Female health has become one of the most discussed issues today. Many problems, like uterine prolapse, birth injuries, incontinence, and general vaginal health concerns cannot be neglected.1 Though vaginal laxity is associated with aging, and is rare in young females, it can occur due to many factors, including childbirth, hormonal imbalance, the intake of certain medications, or any pelvic surgery.1 Over 20 million females suffer from these conditions, due to reasons like increasing age, with factors like vaginal relaxation syndrome affecting wellbeing.2 Until recently, these topics were considered taboo, even among medical professionals.2,3 A survey conducted on female health reported that 95% of doctors assume vaginal laxity negatively impacts sexual function, yet only 14% of females opt for help regarding female sexual dysfunction, while experiencing psychological distress.4 However, public awareness campaigns have neutralized this taboo a lot. Doctors have taken education initiatives, including media coverage, leading to increased treatment demand.6,7 Due to increased knowledge, alternate interventions are being introduced, such as nonsurgical procedures, with a 30% rise in vaginal rejuvenation treatments between 2005–2006.7 There has been an increased approach towards cosmetic
vaginal rejuvenation from 3.90% in 2012, to 28.97% in 2015.8 Minimally invasive procedures are preferred, with significant growth in botulinum toxin Type A operations (759%) and soft tissue fillers (274%) since 2000.9 Although significantly lower from 2014, by 9%, microdermabrasion has nevertheless had an overall increase of 8% since 2000.10 An increasing tendency in favor of non-surgical solutions for improving one’s beauty and general wellbeing is highlighted by this move towards the least invasive procedures.10,11 It is important to explore the impacts of these minimally invasive procedures to address a wider variety of female health concerns, and promote comprehensive wellbeing as cosmetic procedures advance.
METHODOLOGY
Study Design
A quasi-experimental study.
Study Setting
The study was conducted at Skin and Laser Clinic, Hyderabad, Pakistan.
Sample Size and Target Population
The study included a total of 25 females, who attended a dermatology clinic for vaginal rejuvenation and opted for dermal filler intervention.
Inclusion and Exclusion Criteria
Married females aged between 20–50 years in good health, who had provided consent to undergo filler cosmetic procedure for vaginal rejuvenation, were included; whereas pregnant females, and those below and above the given age limits, were excluded.
Intervention Strategies
The injection site was initially numbed with 1–2 mL of mepivacaine 2%, injected with epinephrine 1/100,000 on both sides, to promote a comfortable and less intrusive experience. For the technique, mannitol, a strong antioxidant recognized for its capacity to prevent the degradation of hyaluronic acid (HA), was added to pre-filled syringes containing cross-linked, non-animal HA. A filler with a peri 19 mg/mL HA content (referred to as ‘19 mg/mL HA filler’) was used to treat moderate hypotrophy. A filler with a concentration of 21 mg/ mL HA mixed with mannitol (referred to as ‘21 mg/mL HA filler’) was favored in situations with moderate-to-severe hypotrophy. A 30-gauge needle was used to inject the first filler, and an 18- or 21-gauge cannula was used to insert the second filler. An initial entry point was made using a 19G needle in cases when the cannula was required. Two layers were engaged in the injection procedure. One-third of the filler was deliberately injected between the fibrous tunic and the lip dartos (a layer of smooth muscle cells found just beneath the skin), with the remaining two-thirds going into the subcutaneous layer. The longitudinal axis of the labia minora was aligned with the fan-shaped arrangement of these injections. In order to get the best results, the operator was able to spread the filler uniformly. Recommendation was made for the regular use of topical antibacterial and antimycotic therapy with phenethyl alcohol for 1 week. A 6-day course of antibiotic prophylaxis with amoxicillin clavulanate was advised for patients who had received 21 mg/ mL HA filler via an 18- or 21 gauge cannula. At each session, there is an involvement of a total
of four injections as a treatment protocol. Each injection consisted of 0.125 mL of the solution, making it certain that the delivery of HA filler is precisely controlled. This standardized approach allowed for consistent dosing and distribution of the filler across the treatment site, optimizing the therapeutic outcome for patients undergoing the procedure. This strategy was motivated by the realization that yeast and germs from the vagina and anus might potentially contaminate the vulvar region. However, Candida albicans generally produced vulval itching, Trichomonas vaginalis and Gram-negative bacteria were linked to vaginal discharge, and yeast and fungus were present in the vaginal flora of 21% of females, often without symptoms. Beyond these instances, germs and fungus often did not cause symptoms or abnormal discharge. Additionally, considering that greater cutaneous access often heals without sutures, antibacterial prophylaxis was provided to individuals who had received 21 mg/mL HA filler infiltration. HA was softly and progressively injected, with aspiration serving as a regular check to ensure that intravascular injection was avoided. Having a blunt tip was desirable when utilizing a cannula. Consideration of hyaluronidase usage was advised in cases of severe, excessively superficial, or non-homogeneous infiltration, as well as when patients displayed fast skin darkening, discomfort, or reticulated erythema.
Outcome Measures
During the study, the Laborie Peritron 9300 (Portsmouth, New Hampshire, USA), was used to measure the strength of the pelvic floor muscles. This perineometer has a conical vaginal probe that is 110 mm in length, with a measured length of 55 mm, and 26 mm in diameter (33 mm when pressurized). An 80 cm plastic tube connects the vaginal probe to the perineometer’s main body. A pressure sensor inside the gadget monitors the vaginal pressure when the probe is compressed by vaginal pressure. The probe itself has a silicone rubber sensor that is filled with air, and measures pressure in cmH2O. The perineometer’s values of occlusive pressure are substitute indicators of pelvic floor muscle strength. This methodology made it possible to measure the research participants’ pelvic floor muscle strength. The participants’ sexual distress was assessed using the Female Sexual Distress Scale-Revised (FSDS-R).
The perineometer was used twice to test muscle strength at baseline, and at the end of study.
Data Analyses Strategies
Data were analyzed using SPSS version 26 (IBM, Armonk, New York, USA). Demographic details were illustrated in the form of frequency and percentage tables, whereas for inferential statistics paired t test at 95% confidence interval was applied.
Ethical Considerations
The study was carried out according to the guidelines of the Belmont report, as laid down for human subjects. All participants were informed regarding the purpose of study prior to induction, written consent was taken, and autonomy was given to leave the study at any time without assigning any reason. Moreover, principles of beneficence and non-malfeasance were maintained throughout the study, and keen steps were taken to maintain the confidentiality of participant’s information obtained during the course of study.
RESULTS
The analyses of the findings revealed that the mean age of the participants was 39.43±2.12 years, and further demographic characteristics provided evidence that 44% of the participants had been married for 10 years and had children; 36% were married for 5 years with no children; and 20% had been married for less than 5 years. Detailed demographic features are provided in Table 1.
The effects of intervention were determined on vaginal muscle tone using a perineometer. Analyses of the findings revealed that at baseline, the vaginal muscular tone was 23.25±3.09 cmH2O, and improved to 27.56±2.11 after intervention. Inferential statistics revealed that the difference between the mean was significant (p<0.05), hence suggesting beneficial effects of dermal filler treatment in improving vaginal laxity (Table 2). Further, the effects of treatment were also determined on FSDS-R, and the findings revealed that at baseline, the values were 29.65±2.31, and were reduced significantly to 15.45±1.25 (p<0.05). Detailed descriptions of the findings are illustrated in Table 2.
Table 2: Within group analyses to determine the effect of intervention on outcome measures.
FSDS-R: Female Sexual Distress Scale-Revised; SD: standard deviation.
DISCUSSION
In this study, the authors examined the influence of dermal filler therapy on female sexual discomfort, as well as its effects on vaginal muscle tone using a perineometer. Results showed that the intervention significantly improved the muscle tone of the vagina. The initial assessment of vaginal muscle tone was 23.25±3.09 cmH2O; however, following the intervention, this value significantly increased to 27.56±2.11 cmH2O (p<0.05). The FSDS-R was used to assess the effect of the therapy on the participants’ levels of sexual distress. The subjects’ first baseline FSDS-R scores were recorded at 29.65±2.31, indicating a significant level of sexual discomfort. However, following the intervention, there was a marked decline in these scores, with a statistically significant drop to 15.45±1.25 (p<0.05). These results point to the potential of dermal filler therapy as a valuable therapeutic option for enhancing the physical and emotional aspects of patient health, in addition to beneficial effects on vaginal muscle tone and sexual distress in the participants.
A study was performed to treat females with vaginal laxity, genitourinary syndrome of menopause, and vulvar cosmetic problems, through a non-surgical radiofrequency method paired with an injection of HA into the labia majora skin.12 The therapy considerably improved clinical results, as seen by better scores on the Vaginal Laxity Questionnaire (VLQ), Vaginal Health Index (VHI), and Female Sexual Function Index (FSFI),
including 20 females with ages ranging from 36–72 years (mean age: 53.4).12 These 20 patients reported of sexual discomfort in the form of dyspareunia. Histological study showed greater levels of elastin and collagen in the vulvar skin and vaginal wall following the surgery (vulvar skin: elastin 61%, collagen 27%). Patient satisfaction was also significantly higher. These results demonstrate the effectiveness and safety of this radiofrequency and HA combination therapy, providing a viable approach to resolving the physiological, psychological, and cosmetic issues frequently connected to genitourinary syndrome of menopause, vaginal laxity, and vulvar health.12
The many uses of HA in the field of cosmetology have been explored in research, which highlighted its critical function in both non-invasive skincare through topical preparations and injectable procedures, such as dermal fillers. It also discusses the less important, but still significant functions, of HA in oral supplementation and vaginal preparations.13,14 The results show a growing understanding of HA’s esthetic potential among prospective beauty spree customs. While HAbased topical skincare treatments are in demand for their capacity to preserve skin moisture and counteract transepidermal water loss, HA injections, typically targeting regions like the lips, are favored for restoring skin firmness and smoothness. Rare side effects are frequently caused by immunological reactions.15 Temporary localized soreness at the injection site was the most frequent side effect; no significant adverse events were observed.
Multiple studies have suggested that HA functions as a versatile tool in cosmetology, enhancing skin vitality, and satisfying social demands for increased beauty and wellbeing.16,17 Multiple studies also suggest the benefits of HA for vaginal laxity.12,19-20 A well-defined approach, with in-depth explanations of the intervention and outcome measures, is one of the study’s many strengths. The findings are given more credibility by the use of objective tests, like the FSDS-R, and the Peritron 9300 perineometer. A dedication to patient safety is shown by the extensive safety precautions used, such as an antibiotic prophylaxis, and considerations for the use of hyaluronidase. But there are some restrictions that need to be understood. The study used a quasi-experimental methodology that could introduce bias and restrict causal inference by omitting randomization and control groups. Its Hyderabad single-center
References
1. Aulia I, Valeria M. Current perspectives in vaginal laxity measurement: a scoping review. Arch Plast Surg. 2023;50(5):452-62.
2. Barbara G et al. Vaginal rejuvenation: current perspectives. Int J Womens Health. 2017;21(9):513-9.
3. Erdogan G. Experience of vaginoplasty for enhancement of sexual functioning in a center in Turkey: a before and after study. Cureus. 2021;13(4):e14767.
4. Krapf J et al., "Genitourinary and sexual health," Geraghty P (ed.), Each Woman’s Menopause: An Evidence Based Resource (2022), Berlin: Springer, pp.257-82.
5. Fatton B et al. Pelvic organ prolapse and sexual function. Nat Rev Urol. 2020;17(7):373-90.
6. Hoss E et al. Noninvasive vaginal rejuvenation: radiofrequency devices. Skinmed. 2019;17(6):396-8.
7. Goodman MP. Female genital cosmetic and plastic surgery: a review. J Sex Med. 2011;8(6): 1813-25.
8. Jindal A et al. Cosmetic gynecologyan emerging field for the
location might make it harder to generalize to a larger population. Questions concerning the longevity of reported results are raised by the study’s brief or undefined follow-up time.
CONCLUSION
In conclusion, the outcomes of this study are positive. The intervention showed great promise in addressing physical elements of female health by considerably increasing vaginal muscle tone. Together, these results indicate that dermal filler therapy has potential as a useful and less invasive therapeutic alternative, not only for improving physical features but also for fostering mental and sexual wellbeing in females.
dermatologist. J Cosmet Dermatol. 2023;22(1):111-8.
9. Desai SA et al. Vaginal rejuvenation: from scalpel to wands. Int J Womens Dermatol. 2019;5(2):79-84.
10. Gonzales-Alabastro C et al. Female cosmetic genital reconstruction: a review of current trends, treatments, and techniques. Current sexual health reports. 2019;(11):44-51.
11. Choudhury I et al. Female genital rejuvenation surgery: a study of 34 cases. BSAPS Journal. 2021;2(1):18.
12. Kolczewski P et al. Hyaluronic acid and radiofrequency in patients with urogenital atrophy and vaginal laxity. Pharmaceuticals (Basel). 2022;15(12):1571.
13. Ratajczak P et al. Directions of hyaluronic acid application in cosmetology. J Cosmet Dermatol. 2023;22(3):862-71.
14. Matecka M et al. Subjective evaluation of the results of injectable hyaluronic acid fillers for the face. Clin Interv Aging. 2020;(15):39-45.
15. Mckee D et al. Effective rejuvenation with hyaluronic acid fillers: current advanced concepts. Plast Reconstr Surg. 2019;143(6):1277e-89.
16. Trévidic P et al. Injection guidelines for treating midface volume deficiency with hyaluronic acid fillers: the ATP approach (anatomy, techniques, products). Aesthet Surg J. 2022;42(8):920-34.
17. Rauso R et al. Cross‐linked hyaluronic acid filler hydrolysis with hyaluronidase: different settings to reproduce different clinical scenarios. Dermatol Ther. 2020;33(2):e13269.
18. Buzzaccarini G et al. Hyaluronic acid in vulvar and vaginal administration: evidence from a literature systematic review. Climacteric. 2021;24(6):560-71.
19. Amori P et al. Primavera: a new therapeutical approach to vulvovaginal atrophy. Dermatol Ther. 2018;31(6):e12678.
20. Tarabini F et al. A novel hyaluronic acid filling technique for restoring volume of the labia majora. Cureus. 2023;15(9):e45728.



