Introduction to command words
Commandwordsareverbsusedwithinthequestionsofexaminationpapersthatcangiveyouinsight inhowtogoaboutansweringthequestion.Thesewordscanhelpyoudeterminemuchaboutthe expectedanswerforthequestionthatisbeingpresented.Usedasclues,thesewordscanhelp determinethelengthanddepthofknowledgeananswerrequires,theappropriatestyleofresponse, anddifferentiatebetweensimilarquestionsthatneeddifferentresponses.Beingcomfortablewith thegeneraldefinitionsandstandardexpectationsforeachofthecommandwordslistedbelowcan reallyhelpyounavigateyourwaythroughyourpapers.
Analyse: examineindetailtoshowmeaning,identifyelementsandtherelationshipbetweenthem
Guidance: Thisisahigherlevel,andtypicallyhighermark,questionrequiringmultipledetails.Your responseto‘analyse’questionsislikelytobelongerthantoothertypesofquestions.
Assess: makeaninformedjudgement
Guidance: Thiscommandwordwilltypicallybeusedinascenariothatwasnotexpresslytaught throughthesyllabus.Youwillneedtopullrelatedinformationandapplyittothesituationtocreate yourjudgement.
Calculate: workoutfromgivenfacts,figuresorinformation
Guidance: Inthesequestions,youwillneedtodomathstoarriveattheanswer.Makesureto includeanyunitsandshowyourworking.
Comment: giveaninformedopinion
Guidance: Theseareshorterresponsequestionswhereyouwillapplygeneralknowledgetoanew scenario.Makesureyoujustifyyouropinionwithsupportingdetails.
Compare: identify/commentonsimilaritiesand/ordifferences
Guidance: Thesequestionsvaryinlengthsousetheprovidedmarkstodeterminehowmanypoints ofcomparisonyouneedtoprovide Makesureyoudonotrepeatthesamepointswithinyouranswer –eachmarkwillonlybegivenoncenomatterhowmanytimesyouhaverewordedit
Consider: reviewandrespondtogiveninformation
Guidance:Thesequestionsarehigherlevelandgenerallyrequiredataanalysisinthecontextofa casestudy
Contrast: identify/commentondifferences
Guidance: Thesequestionsvaryinlengthsousetheprovidedmarkstodeterminehowmanypoints ofcontrastyouneedtoprovide Focusentirelyonthedifferencesbetweentheprovidedsituations
Define: giveprecisemeaning
Guidance: Trytowordyourdefinitionsaspreciselyaspossiblewithallmajoridentifyingfeatures Thisisaconciseresponsethatshouldnotbelongerthanasentence
Demonstrate: showhoworgiveanexample
Guidance: Makesuretoprovideastep-by-stepmethodforhowtheprocessbeingaskedaboutworks oruseanexampletoillustratetheprocess
Describe: statethepointsofatopic/givecharacteristicsandmainfeatures
Guidance: Mostcommonlyconfusedwith‘explain’,‘describe’meanstoanswer what ishappening Thiscouldbeinreferencetodescribingthedata(forexample,itincreasesordecreases),the process,orthemeaningofaterm Atnopointshouldyoutrytowriteabouthoworwhysomethingis happening,asthatisthemeaningofthe‘explain’commandword
Develop: takeforwardtoamoreadvancedstageorbuildupongiveninformation
Guidance:Thistermisaskingyoutousebackgroundknowledgeinanovelsituationtopredictthe steporprocessinthesituation Thisquestionrequiresahigher-levelresponse
Discuss: writeaboutissue(s)ortopic(s)indepthinastructuredway
Guidance: Questionsusingthiscommandwordaregenerallylongerresponseandrequiredtheuse ofdetailstosupporttheyourknowledgeaboutatopic Ingeneral,responsesto‘discuss’questions requiretheuseofmultiplewell-writtenparagraphs
Evaluate: judgeorcalculatethequality,importance,amountorvalueofsomething
Guidance: For‘evaluate’questions,youwillneedtocreateanopinionorchooseasideaboutthe topicinquestion Youwillthenneedtosupportyourthesisusingdetailsfromeitheryourbackground knowledgeorinformationprovidedwithinthequestion
Examine: investigateclosely,indetail
Guidance: Thesequestionstypicallywillhaveyoulookoveradatasettopullinformationoutof Dependingonthestyleofthistypeofquestion,1-2sentencesisprobablysufficient,butusethe marksprovidedtodeterminehowmuchdetailisneeded
Explain: setoutpurposesorreasons/maketherelationshipsbetweenthingsevident/providewhy and/orhowandsupportwithrelevantevidence
Guidance: Oftenconfusedwith‘describe’,‘explain’questionsrequireyoutowriteaboutwhyorhow somethinghappens Inordertosucceedwiththesequestions,itisimportanttoprovidereasonsand clearconnectionswithinyouranswer Unlessexpresslystated,itisnotnecessarytooutline what is happening,just why or how
Give: produceananswerfromagivensourceorrecall/memory
Guidance: Questionsusing‘give’aregenerallyshortresponseandmaynotevenneedacomplete sentence Theymayrequireyoutorecallsomethingfrommemoryorlocateinonagraphordataset
Identify: name/select/recognise
Guidance: Thiscommandwordrequiresabrief,directresponse.
Justify: supportacasewithevidence/argument
Guidance: Usedtoencourageyoutoincludemanydetails,thiscommandtermwillbeseenwith high-markquestions.Youshouldprepareawell-organisedresponseofatleastoneparagraph.You mayalsoneedtocreateanopinionstatementbasedonthequestionasked,indicatingwhichpartof theargumentyouaresupporting.
Outline: setoutmainpoints
Guidance: Whileyoucanuseaparagraphtoansweran‘outline’question,adetailed,bulletedlistis alsoappropriate.Makesuretoincludeenoughdetailtoclarifyeachpoint.Thiscommandwordis oftenusedtogainanunderstandingofaprocessortheory.
Predict: suggestwhatmayhappenbasedonavailableinformation
Guidance: Questionsusingthiscommandtermdonotexpectyoutogettheinformationthrough recall.Rather,theexpectationisthatyoucanusebackgroundinformationinconjunctionwiththe informationordataprovidedinthequestiontomakeabestguessaboutwhatmayhappennext.For example,youmaysee‘predict’whendiscussingdataorbehavioroforganismsorthephysicalocean.
Sketch: makeasimplefreehanddrawingshowingthekeyfeatures,takingcareoverproportions
Guidance: Useapenciltodrawtheprocessorgraphbeingrequested.Makesuretolabelmajor featuresasnecessary.Ifdataisprovided,makesureyourproportionsareappropriatetorepresent thenumbersgiven(forexampletrophicpyramids).
State: expressinclearterms
Guidance: Thiscommandtermindicatesthattheresponseneededwillbeshort.Thiscanbeusedto haveyouidentifyimportantdatapointsonagraph,providethetermthatmatchesadefinition,orin othersituationswhereonlyaone-ortwo-wordresponsewouldbeappropriate.
Suggest: applyknowledgeandunderstandingtosituationswheretherearearangeofvalid responsesinordertomakeproposals
Guidance:Similarto‘predict’,‘suggest’asksyoutocombineinformationfromthequestionwith otherinformationlearnedduringthecourse.Generally,‘suggest’questionshaveawidevarietyof appropriateresponsesthatcouldbeusedtoproduceasuccessfulanswer.Makesuretoprovidea clearlineofreasoningforthesuggestionbeingmade.
Summarise: selectandpresentthemainpoints,withoutdetail
Guidance: Similarto‘outline’,abulletedlistcanbeappropriateforaquestionusingthe ‘summarise’commandterm.Makesuretolistoutalloftheimportantaspectsoftheprocessor theorybeingaskedabout,butrefrainfromaddingunnecessarydetailsorevidenceunlessspecifically askedforitinthequestion(thatis,iftheyaskyouto summarise and explain).


Chapter 1
Water
LEARNING INTENTIONS
Inthischapteryouwilllearnhowto:
usethekineticparticletheorytoexplainthechangesofstateinwater,betweensolid,liquidand gas
describehow thestructureofatomsleadstotheformationofdifferentbondssuchascovalent, ionic,andhydrogenbonds
explainhowhydrogenbondingprovidesspecialpropertiestowater
explain the terms solute, solvent, solution and solubility in order to apply these terms to the dissolutionofsubstancesintheocean
explain the impacts of physical factors, such as temperature and salinity and pressure, on the solubilityofsaltsandgasesinseawater
explainhowrun-off,precipitationandevaporationimpactthesalinityofseawater describethepHscaleandtechniquesusedtomeasurepHinwater
explain the effects on the density of seawater caused by water temperature, pressure and salinity
state why ice floats and explain why this is important as a thermal insulator and habitat to marineorganisms
describehowtemperatureandsalinitygradientsforminwatercolumnstoproduceoceanlayers andhowsubsequentmixingoftheselayersmayoccur
BEFORE YOU START
With a partner discuss your understanding of the term ‘atom’ Sketch a labelled diagram to show what you understand atoms to be made from Compare your drawing to others in class
• • • • • • • • • • •
Weusewatertocleanclothes,dishesandourownbodies Discusswithapartnerwhywateris sogoodforcleaning
Writeoneortwoquestionsaboutwhataffectsthedensityofseawateronastickynoteandpost them on the board With your class, compare and organise your questions into topics for discussion
THE STUDY OF
SEAWATER
Whenbeginningyourstudiesinmarinescienceithelpstogainascientificunderstandingofthe propertiesofseawater Afterall,71%ofourplanetiscoveredinwaterandthevastmajorityofthat water(about97%)isheldintheoceans Somepeoplemaythinkthatseawaterissimplysaltwater–theymaythinkthat,ifyouaddsometablesalttoaglassofwater,youcanrecreateseawaterthat issuitableforfishandotherorganismstolivein Butthisisnottrue
Seawaterisacomplexmixofchemicals.Infact,nearlyevery element inthePeriodicTablehas beenlocated,usuallycombinedwithotherelementsasmanydifferent compounds,withinthe ocean’svastwaters Wateritselfismadefromhydrogenandoxygen;itinteractswithalltheother substancesdissolvedorimmersedinit Thesesubstancesinteractwiththewater molecules to supportlifeinouroceans Theconcentrationsoftheseelementsandtheircompoundsdetermine whatorganismsarecapableoflivingwithindifferentmarineecosystems,andhowtheywill survive
Itisimperative,then,toknowhowthesesubstancesinteracttogetherandhowwatermolecules functiontosupportlife Tobeginthatstudy,wemustlooktothe atom andbuildourwayupfrom there
Questions
Do you agree that starting with the simplest form and working up is the best way to begin? Whyorwhynot?
Which of our senses can we use to try to determine what is in a sample of seawater? How reliable are these senses and can you suggest other methods or equipment to determine what isinthesampleofseawater?
• • 1 2
andreviewwithyourteacher
1.1 Particle theory and bonding
Anatomisthesmallestparticleanelementcanbeseparatedintoandstillbethesamesubstance Atomsaretheparticlesfoundineverythingaroundandwithinus
Atomic movement
The kinetic particle theory (particle theory) describesallmatterasacollectionofparticlesthat areinconstant,randommotion,evenifthosemovementsareonlysmallvibrations Theamountof movementaparticlehasisdeterminedbytheamountofenergyithas Thechairyouaresittingon, thewateryouaredrinking,andtheairyouarebreathingareallexamplesofmanyparticlesthat havejoinedtogethertocreatematerials Mattergenerallyexistsinthreestates:solid,liquid,orgas (Figure11) Asenergyistransferredawayfrom,ortransferredto,thesemolecules,thestateof mattermaychangeasthemovementofthe molecules withinthematterchanges

Figure 1.1: Thestatesofmatterandthearrangementoftheirparticles
Watermoleculescanbeusedtodemonstrateparticletheory Whenliquidwatercoolsdown,the movementofthewatermoleculesslowsuntiltheyarrangeinaregularstructurecalledalattice The moleculesbecomefixedinpositioninthislatticeresultinginasolid(ice)forming Asmoremolecules jointhelatticetheicecrystalsgrowlarger
Wheniceisheatedthewatermoleculesaregivenmoreenergy,resultinginthemvibratingfaster untiltheforcesholdingthemoleculestogetherstarttobreak Thewatermoleculesnearesttheouter surfacesoftheicecrystalsbreakfreeandareabletoflowawayfromthecrystals,takingtheshapeof thecontainertheyarein Thewatermoleculesarecloselypackedtoeachotherinaliquidbutableto movefreelypasteachother;thisexplainswhyliquidscantaketheshapeoftheircontainerbut cannotbecompressed–theyarealreadyveryclosetoeachother
Whenliquidwaterisheatedtheparticlesgainmoreenergy,makingthemmovefasterandslightly furtherfromeachother Someofthecollisionsbetweenwatermoleculestransferenoughenergyfor moleculesattheuppersurfacetoescapetheforcesattractingthemtoothermolecules,andtheycan evaporateintotheairabove.Thisprocessisessentialtothewatercycleandoccursfasterasthe surfacewaterbecomeswarmer.Whenwaterapproachesitsboilingtemperature(100°Cat1 atmospherepressure),allthewatermoleculeshaveenoughenergytobreaktheforcesholdingthem togetherandwaterrapidlyevaporates(boils). Water vapour isthetermgiventogaseouswater (bothfromevaporationandboiling).
A brief understanding of atoms
Atomsaremadeofthreesmallersubatomicparticlesthat,dependingontheirnumbers,givethe atomitscharacteristics(orproperties) Thesesubatomicparticlesare protons, neutrons and electrons Protonsarepositivelycharged,neutronsareneutral(theyhavenoelectricalcharge),and
electronsarenegativelycharged Anatomhasanequalnumberofelectronsandprotons,sotheyare neutral
Protons,neutrons,andelectronsarearrangedwithintheatomtoprovidestabilityandstructure(see Figure12) Atthecentreoftheatomisthe nucleus Thenucleusismadeoftheneutronsand protons Electronsmovearoundthenucleusinorbitscalled shells Theseshellsvaryinsizeand distancefromthenucleusdependingonhowmanyelectronsarepresent.Thefirstshellnearestthe nucleuscanholdtwoelectrons.Thisistheonlyshellpresentinbothhydrogenandhelium.Thenext twoshellsholduptoeightelectrons.Atomsareattheirmoststablewhentheiroutermostshell containingelectronsisfull.

Figure 1.2: The atomic structure of helium showing the relative positions of the protons, neutrons andelectrons.
ThePeriodicTableyoumightseehangingonthewallinmanyscienceclasseslistsalltheknown typesofatoms Thesedifferenttypesofatomsarecalledelements Anelementismadeofatomsthat haveaspecificnumberofprotons This atomic number nevervariesandhelpsustoidentifythe characteristicsofelements Seawaterisamixtureofdifferentelementsandcompounds Examplesof elementswefindintheoceanincludecarbon,hydrogenandoxygen
Bonding properties of atoms
Whenindividualatomscometogethertoformdifferentsubstances,theyform bonds.Asubstance withaspecificratioofdifferentelementsbondedtogether,suchaswater,iscalledacompound.A watermoleculeistwoatomsofhydrogenbondedtooneatomofoxygen.Watermoleculesalways havea2[H]:1[O]ratio.Acompound’spropertiescanbeverydifferentfromthoseoftheelementsthat itismadeof.Forinstance,atroomtemperature,bothhydrogenandoxygenaregaseswhichrequire verylowtemperaturesandhighpressurestobecomeliquid.Whencombinedina2H:1Oratio, however,watercanbeformed,whichisliquidatroomtemperature.Thenewcharacteristicsa compounddevelopsthroughbondingarecalled emergent properties. Thetypeofbondformedbetweentheatomsofacompoundwillalsoplayapartintheemergent propertiesofthecompound Therearethreemajorcategoriesofbondsthatwewilldiscuss: covalent bonds, ionic bonds and hydrogen bonds Allthreebondtypesplayamajorroleinhow theoceanworksandhoworganismscanmaketheoceantheirhome
Covalent bonds
Acovalentbondformswhentwoatomsshareapairofelectrons Covalentbondingoccursinmost non-metalelements,andincompoundsformedbetweennon-metals
Becausetheatomsaresharingtheelectrons,bothatomshavecompleteoutershells.Thissharingof electronsalsomakesthistypeofbondbetweenatomsoneofthestrongest,requiringalargeamount ofenergytobreak.
Compoundswithcovalentbondsareabletoexistasasolid,liquidorgasatroomtemperatureand normalatmosphericpressure Therefore,itshouldcomeasnosurprisethatwaterisoneofthemost prevalentcovalentcompoundsonourplanet Eachwatermoleculecontainstwocovalentbonds connectingtheoxygenatomtoeachofthehydrogenatoms(Figure13) Thesebondsformwhenan oxygenatom,whichonlyhassixelectronsinitsoutermostshell,reactswithtwohydrogenatoms, withonlyoneelectroneach Thehydrogenatomssharetheirindividualelectronswiththeoxygen atom Thesharedelectronsorbitaroundtheatomsconnectedinthebond,fillingtheoutershellsof
theoxygenandthehydrogen Manycompoundsinseawaterhavecovalentbonds(Figure14) As seeninFigure14(b)carbondioxidehasfourcovalentbondsasthesinglecarbonmoleculeformsa doublebondwitheachoxygenmolecule Adoublebondoccurswhenmoleculessharetwopairsof electronsinsteadofjustone

Figure 1.3: The formation of the covalent bonds in water molecules The red circles represent electronsfromtheoxygenatomandthebluecirclesrepresentelectronsfromthehydrogenatoms.

Figure 1.4: Common covalently bonded molecules in seawater: (a) water (H2O); (b) carbon dioxide (CO2);(c)oxygen(O2);(d)glucose(C6H12O6);and(e)sulfurdioxide(SO2)
Carbondioxide,neededbyplantsforphotosynthesis,andoxygen,neededbyorganismsfor respiration,alsousecovalentbondstoholdtheiratomstogether Inphotosynthesis,theglucose producedthroughphotosynthesisisalsoacovalentlybondedcarbohydrate Thisisimportant becausealotofenergyisstoredinthecovalentbondsthatjoinmolecules,makingglucoseauseful moleculeforholdingchemicalenergy Inecosystemswherephotosynthesisisnotpossible,some bacteriausetheprocessofchemosynthesistobreakapartthecovalentbondswithinthemolecule sulfurdioxideinordertoobtaintheenergyneededforsurvival(seeChapter7) Ineachinstance, theseatomsaresharingoneormorepairsofelectronscreatingstrongchemicalbonds
Ionic bonds
An ion isanatomthathasgainedorlostanelectronfromitsoutershell.Thischangeinthenumber ofelectronsgivestheatomanelectricalcharge.Anelectronwillmovefromoneatom,whichresults ineitherfilledoremptyouterelectronshells.Ifanatomlosesanelectron,theioncreatedwillhavea positivechargebecausetheprotonsinthenucleus(positivecharge)nowoutnumbertheelectronsin theoutershell(negativecharge).Ifanatomgainsanelectron,theioncreatedwillbenegatively chargedduetoanexcessofelectronscomparedtoprotons.
So,howdoionicbondsform?Whenanionlosesanelectron,itspositivechargeisattractedtothe newlyformednegativeionthatgaineditselectron Thiselectrostaticattractioncausesanionicbond toform
TheprocessofforminganionicbondisseeninFigure1.5.Instep(a)sodiumandchloridebothhave incompleteoutershells,withsodiumhavingasingleelectronandchloridehavingseven.Instep(b)
sodium’ssingleelectronbreaksawayandmovestocompletechloride’soutershell,makingbothions morestableintheprocess Instep(c)sodiumhasapositivecharge,chloridehasanegativecharge Theelectrostaticattractionbetweenthepositivesodiumionsandnegativechlorideionscreatesan ionicbond

Figure 1.5: Theformationofanionicbondbetweensodiumandchlorideatoms.
Itisimportanttorememberthatmanyatomsofsodiumandchlorinereacttogetherinthisway,and theresultingpositiveandnegativeionscancreatesolidsthathaveathree-dimensionalioniclattice structure,asmallpartofwhichisshowninFigure16
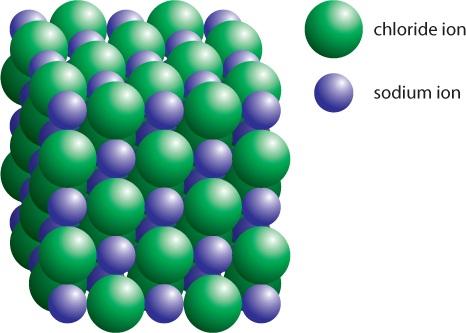
Figure 1.6: Ioniclatticestructureofsodiumandchlorideions
Saltsaremadefromions,whichareveryimportantcompoundsinouroceans,Therearemanytypes ofsaltsfoundintheocean,includingsodiumchloride(NaCl),calciumcarbonate(CaCO3),and magnesiumsulfate(MgSO4) Thesesaltsareallformedusingionicbonds Chemicaldiagramsofthe ionsinthesesaltscanbefoundinFigure17

1.7: Chemical diagrams of sodium chloride (a), calcium carbonate (b), and magnesium sulfate(c).
Hydrogen bonding
Ahydrogenbondisaweakerbondthatcanoccurbetweenmoleculescontainingahydrogenatom bondedtoanatomofoxygen,nitrogenorfluorine Waterisanexampleofsuchamolecule,asithas twohydrogenatomsbondedtoanatomofoxygen Whencreatingthiscovalentbond,theoxygenand hydrogenatomsshareelectronsunequally Theoxygenatomhasamuchstrongerattractiontothe bondingpairofelectronsbetweenoxygenandhydrogen,resultinginthesebeingpulledclosertothe oxygenatomthanthehydrogenatom Thisunequalsharingcausesapartialchargeontheatoms involvedinthebond:thehydrogenatomsarepartiallypositive(δ+),andtheoxygenatomispartially negative(δ ) Whenmoleculeshaveapartialchargeoneachendaswaterdoes,theyarereferredto as polar Duetothispolarity,themorepositivehydrogenatomsofonewatermoleculewillbe attractedtothemorenegativeoxygenatomsofanearbywatermolecule(Figure18)creatinga dipole–amoleculewithaseparationofpartialpositiveandnegativecharges

Whilehydrogenbondsareeasilybroken,theyhaveanincredibleimpactonthepropertiesofwater duetothesheernumberofwatermoleculesfoundwithinasingledropletofwater.Therefore,many hydrogenbondsarecontinuallyformingbetweenwatermolecules.
Oneofthereasonsseawaterisuniqueisbecauseofthemanydifferentelementsandcompoundsthat canbefoundwithinit Thisexceptionalvarietyisduetowater’spropertiesasa solvent Asolventis amaterialcapableofdissolvingothersubstances Thepartialchargesonthewatermoleculeallow watertointeractwithchargedionsfrommanyioniccompoundsaswellasmanycovalentsubstances, includingglucoseandgasesfromtheatmosphere Thepartialchargesonthewatermoleculeallowit toformbondswithanunusualnumberofsubstances,makingwateroneofthebestsolventsonthe planet Infact,waterisoftenreferredtoastheuniversalsolvent Moreinformationregarding water’ssolventcapabilitiescanbefoundinSection12
The density ofwaterisalsoimpactedbyhydrogenbonds.Aspreviouslymentioned,astheenergy withinmatterlowers,sotoodoesthemovementoftheindividualparticlesmakingupthatmatter.So, aswaternearsitsfreezingpoint(0°C),thewatermoleculesslowtheirmovementandgatherclosely together,allowingthehydrogenbondstobecomestronger.Thehydrogenbondsthenhelpkeepthe watermoleculesatperfectlysymmetricaldistancesfromeachother,formingacrystallattice-like shape.Theshapeformeduponfreezingactuallyspreadsthemoleculesoutfurtherthantheywere justpriortofreezing.Becausefewerwatermoleculescanfitintothesamespace,thedensityofsolid iceisactuallylessthanthatofliquidwater.Waterisoneofthefewsubstancesonearththatcan claimasolidstatethatislessdensethantheliquid.Moreinformationonwaterdensityandthe impactsoftemperaturecanbefoundinSection1.3.
Specific heat capacity,theamountofheatrequiredtochangeonekilogramofmassbyonedegree Celsius,isyetanotherpropertyofwaterthatrelatestoitshydrogenbondingproperties Waterhas oneofthehighestspecificheatcapacitiesduetothenumberofhydrogenbondsholdingthe moleculestogether Thispropertyallowswatertoactasagreattemperaturebufferandhelps moderateourplanet’sclimate Thesizeofouroceansallowsthemtoholdagreatdealofheatbefore
Figure
Figure 1.8: Hydrogenbondinginwater
theyactuallychangetemperaturecreatingmildclimatesalongcoastlinesworldwide
Test yourself
Sketchacarbonatom.Labelthenucleus,electrons,protonsandneutrons. Howdocompoundsdifferfromelements?
Whatdeterminesthenumberofcovalentbondsthatanatomcanform? Howwillyourememberthedifferencesbetweenthedifferenttypesofbonds?
1 2 3 4
1.2 Solubility in water
AswesawinSection11,asolventisamaterialcapableofdissolvingothersubstancesandwateris oneofthegreatestsolventsonearth Thesubstancethatisdissolvedbyasolventiscalleda solute Therearemanysolutesinseawater,suchassodiumchloride,carbondioxide,oxygenandcalcium carbonate Themixtureofsolutesandsolventisreferredtoasa solution
Understanding solubility
Solubility referstotheextenttowhichaparticularsolute,suchassodiumchloride,canbedissolved inasolvent,suchaswater Thiscombinationthencreatesasolution,suchasseawater Ingeneral, sodiumchlorideandothersolublesaltsdissolveeasilyintowaterthrough dissolution ofions Thisis aresultofthepolarityofthewatermoleculeanditsabilitytointeractwithionsfromionic substances AswesawinSection11,sodiumchlorideisanioniccompoundformedthroughthe attractionofsodiumionstochlorideionsafterthetransferofanelectronfromsodiumtochlorine Thechangeindistributionofelectronsgivesbothatomsanelectrostaticchargethatwatermolecules areattractedto Whenplacedinwater,thesodiumandchlorideionsareeasilydissolvedwhentheir ionicbondsarebrokenbythewatermolecules Atthispoint,thepartiallypositivehydrogenendsof thewatermoleculeswillsurroundthenegativelychargedchlorideionsandthepartiallynegative oxygenendsofthewatermoleculeswillsurroundthepositivelychargedsodiumions(Figure19)

Figure 1.9: Acomparisonofsodiumchloridesolidmolecularstructureanditsdissolvedstructurein water
Solubilitycanbeimpactedbyphysicalfactorswithintheseawater,particularlytemperature Repeatedstudieshavefoundthatasthetemperatureofseawaterrises,therateofdissolutionofsalts increasesaswell Thisisbecauseaswaterheatsup,theindividualwatermoleculesmovefaster This movementhelpsmixtheionsintothewatermakingiteasierforwatermoleculestobreaktheionic bondsandsurroundthepositiveandnegativeionsthatareleft So,thewarmerthewater,thesaltier itcanbe
Salinity
Whenstudyingthechemistryoftheocean,traditionallyyoustartbylookingatthe salinity of seawater Salinityisameasureoftheconcentrationofdissolvedsaltsinseawater Theunitusedfor salinityispartsperthousand(pptor‰) Thisunitwasoriginallyderivedusingthetraditional methodfordiscoveringthesalinityofasolutioncalledtotaldissolvedsolids(TDS) Usingthis method,scientistswouldboil1000gofseawateruntilallofthewaterpresenthadevaporated The solutesleftbehind,usuallyionscalledsalts,werethenweighed,makingthemthe‘parts’thatmake up1000gofwater Afterhundredsofyearsofwatersamples,scientistsnowknowthattheaverage salinityoftheopenoceanis35ppt
While35pptistheaveragesalinityofoceanwater,theactualsalinityatanygivenlocationdoesvary locallythankstothewatercycle. Precipitation (forexample,rainorsnow)lowersthesalinityofa

CORE PRACTICAL ACTIVITY
INVESTIGATING THE EFFECT OF SALINITY ON THE FREEZING POINT OF WATER
Introduction
Asthemoleculesofwaterarecooled,theirmovementslowsandtheyformalatticepatternthat createsthesolidice.Thetemperatureatwhichliquidwaterturnstosolidiceiscalledthefreezing point.Thispropertyofwaterhasmajorimplicationsformarineorganisms.Organismscannotswim throughice;however,muchofourArcticandSouthernOceanshavethrivingecosystemsdespite thelargevolumeofwaterthatfreezeseachyear.Thisisatleastpartiallyduetothepresenceof saltsinseawater.Throughoutthisinvestigation,youwillstudyhowincreasedsalinityimpactsthe pointatwhichwaterfreezes.
Equipment
Youwillneed:
•
• bodyofwaterbydilutingthesaltintheseawaterwithincomingfreshwater Afteritrains,thewater willflowoverorintotheEarth’ssurface Eventually,mostofthiswater,aspartofthehydrological cycle,findsitswaytotheoceansas run-off,eitherdirectlybyfirstflowingintoariver,orthrough flowsofgroundwater(watermovingthroughsoilandporousrocks) Inareasofoceanwithfreshwaterrun-off,thesalinitymaybemuchlowerthantheopenocean Thislowersalinityiscausedby theadditionoffreshwater,ratherthantheremovalofsalts Itisimportanttonotethatasrun-off flowsovercitystreetsorthroughfarmers’fields,itdissolvesmanysubstancesandcarriesthem along Thesesubstancescouldincludevitalnutrients,pesticides,fertilisers,oilsandotherpollutants capableofchangingthesalinityorqualityofthewateritisenteringaswell Evaporation willcausesalinitylevelstoriseduetotheremovalofwaterfromthesolutionrather thantheadditionofsalts.Itisimportanttonotethatonlywaterisremovedthroughevaporation;all solutesareleftbehind.Salinityhigherthan35pptisoftenfoundinregionswithabove-average evaporationratesandalimitedfresh-waterinflow.Whenthesaltsareevenmoreconcentrated,the waterisdescribedas hypersaline.ThemosthypersalineenvironmentintheworldisDonJuanPond inAntarctica.ScientistsrefertothisregionastheMcMurdoDryValleysduetothelackof precipitationinthearea.Thatlackofrainandsnowcausesthispondtohaveasalinityof440ppt, whichistwelvetimessaltierthantheocean.Thesalinityissohighthatevenin 50°Cweather,the ponddoesnotfreeze(Figure1.10).Formoreinformationaboutthefreezingpointofwaterand salinity,seeCorePractical1.1.
or digitalthermometers
Figure 1.10: AnaerialviewDonJuanPondlocatedintheMcMurdoDryValleysofAntarctica
1.1:
,1.5moldm
and2moldm
temperatureprobe
4×mediumtesttubesmarked0.5moldm 3,1.0moldm 3
3
3
4×100mlbeakersmarked05moldm 3,10moldm 3,15moldm 3 and2moldm 3
1litrebeaker or flask or graduatedcylinder
400cm3 beaker
freezer
icecubetrays
plasticbag
rubberhammer
digitalbalance
blackmarker
230gsodiumchloride accesstotapwater(tocreateice)
500cm3 ofdistilledwater(tocreatesolutions)
Safety considerations
Followallusuallaboratorysafetyrules.
Wearsafetygoggles,labcoatsandgloveswhileinthelaboratory Handleglasswarecarefully.
Before you start
Whyareyouusingdistilledwatertocreatethesolutionsinsteadoftapwater?
In this investigation, the independent variable is the amount of sodium chloride added to the water, and the dependent variable is the freezing point of the solution What are two other variablesthatwillneedtobecontrolledinordertoreceiveaccurateresults?
What do you predict will happen to the freezing point of water as sodium chloride is added to it?
Method
CreatearesultstableinyournotebooksimilartoTable1.1.
Createyourice
Using the beaker or graduated cylinder, mix 200g of Sodium chloride into 1dm3 of tap water.
Pourthesolutionintoicetrays
Placethetraysinthefreezerandleavethemovernight.
The next day, label the four beakers and test tubes as Sodium chloride 05moldm 3 , Sodium chloride10moldm 3,Sodiumchloride15moldm 3,Sodiumchloride20moldm 3
Prepareeachofthesolutionsbelowintheappropriatelylabelledbeaker:
Sodiumchloride05moldm 3 –mix100cm3 ofdistilledwaterwith29gofsodiumchloride
Sodiumchloride10moldm 3 –mix100cm3 ofdistilledwaterwith58gofsodiumchloride
Sodiumchloride1.5moldm 3 –mix100cm3 ofdistilledwaterwith8.7gofsodiumchloride
Sodium chloride 20moldm 3 – mix 100cm3 of distilled water with 116g of sodium chloride
Usingtheicecubespreparedthepreviousday,createanicebath.
Placethepreparedicecubesinalargeplasticbag
Usingtherubberhammer(orappropriatesubstitute)carefullycrushtheice
Fillthe400cm3 beakerwiththecrushedice
Pourthesolutionineachbeakerintoitsmarkedtesttubetothehalfwaymark.
Putadigitalthermometerintoeachtube
Addthetesttubestotheicebath
Results
Observe the test tubes and record the temperature when the first ice crystals begin to form alongthesurfaceintoacopyofTable11
• • • • • • • • • • • • • • • 1 2 3 1 2 a b c 3 4 a b c d 5 a b c 6 7 8 1
Table 1.1: Datatableformeasuringfreezingpoint
Create a line graph of Concentration of sodium chloride in solution (x-axis) against Freezing point of solution (y-axis)
Create a scale for each axis based on your data. Your scale should be large enough that yourdatatakesupmorethanhalfoftheavailablespacebothverticallyandhorizontally.
Asyourtemperatureswillbebelow0°C,your x-axisshould be atthe top ratherthan along thebottom,sothe y-axiscanbetterrepresentthenegativenumbers
Remember to use a ruler to create straight lines that will connect your carefully plotted datapoints.
Evaluation and conclusions
Identifythesolventandthesoluteinoursolutions.
Comparethesolubilityofthesodiumchloridewhenyouareadding29gtothesolubilityofthe sodiumchloridewhenyouareadding116g
Describethechangeintemperatureastheconcentrationofsodiumchlorideincreased.
Predictthefreezingpointofasolutionwith40gcm 3
How could you extend this experiment to test for other factors which may impact freezing point?
REFLECTION
AftercompletingCorePracticalActivity1.1,thinkaboutthesequestions:
Howcouldyouimproveyourresultsforthispractical?
Doyouthinkthispracticalhelpedyourunderstandingofthisconcept?Whyorwhynot?
Mixing of the layers
Thesurfacelayeroftheocean,fromzerotoaround200mdeep,isthebest-mixedareaoftheocean. Asthewindblowsacrossthesurfaceoftheocean,currentsandturbulencearecreated.Thiswater movementmixestheupper200morsooftheocean,makingitfairlyuniforminbothtemperature andsalinity.
Mixingofthelayerswithintheoceanistypicallydensitydriven Forexample,ifthesurfaceofthe oceancools,thedensityofthewaterwillincreaseduetothetemperaturechange Asthedensity increases,thewatersinks,carryingwithitallthenutrientsanddissolvedgasesthatitcontainedat thesurface,mixingwiththehigherdensitywaterthatisrising Thiscanhappenincoldoceanaswell whenthedensitychangesduetochangesinsalinitylevels Boththe halocline and thermocline are consideredareasofmixingwheresignificantchangesintheabioticfactorsoftheoceanhappen
Thedeepocean,orbottomlayeroftheocean,isuniform,likethesurface.Thedeepoceantendsto beverycold,saltyanddense.Thereisoxygenandothergasespresentinthislayer,buttheyare limited.
The pH scale
Potentialofhydrogen,morecommonlyreferredtoas pH,isanimportantabioticfactorforthe survivalofaquaticorganismsinamarineenvironment Thisisusedtomeasuretheconcentrationof hydrogenionsinwater Hydrogenionsarespontaneouslycreatedinpurewaterwhenthewater moleculeswillsplitintohydrogenions(H+)andhydroxideions(OH ) Solutionswithhigh concentrationsofhydrogenionscomparedtopurewaterare acidic (calledacids),whilesolutions withlowconcentrationsofhydrogenionsarecalled alkaline (youmayhavealsoseenthisreferred toas‘basic’) Becausepurewateristhepointofcomparison,itisconsidered neutral Forscientists
2 a b c 1 2 3 4 5 1 2 Concentration of sodium chloride in solution / mol dm 3 Freezing point of solution / °C 05 10 15 2.0
toeasilydeterminethepHofasolution,theycreatedthelogarithmic pH scale (Figure1.11).Using thisscale,solutionswithapH: below70areacidic at70areneutral above70arealkaline
ThepHoftheworld’soceanshasbeenslightlyalkalinesincebeforeweeverbegancollectingdata Historically,thepHoftheopenoceanhasbeenanaverageof82;however,therehasbeenarecent dropintheworldwideaveragetoan81pHduetoincreasinglevelsofcarbondioxide(CO2)inour atmosphere Thisseemslikeasmalldrop;butitiseasytomisjudgethereadingsonthepHscale becauseitisnotlinear Inalinearscale,a01decreaseinpHwouldindicatea1%increaseinacidity Instead,becausethisscaleislogarithmic,a01decreaseinpHisactuallya25%riseinacidity Small changesinpH,therefore,canbringdrasticissuestotheenvironment

1.11: The colour pH scale used with universal indicator paper to determine hydrogen ion concentrationswithinasolution.
ScientiststypicallyuseoneofthreemethodstomeasurethepHofwatersamples:litmusindicator, universalindicatorandpHprobes.Litmusindicatoranduniversalindicatorarebothsolutions,but theyarealsocommonlyusedaspaperswhereanabsorbentpaperhasbeensoakedinthesolution andallowedtodry,providingasimpleandeasiermethodtotestwiththeseindicatorsthatcanbe dippedintoasolution.Onceincontactwiththesolutionbeingtested,theindicatorbeginstochange colour.Litmusindicatoronlydeterminesifasubstanceinanacidoranalkali,itcannotshowhow stronganacidoralkaliis.Universalindicatorshowsarangeofstrengthsforacidsandalkalis: scientiststhencomparethecolourofthepapertothecolourpHscaleprovidedtogiveawhole numberpH.Thesemethodsarequick,butalsosubjective,leadingthescientisttotrytodetermine thepHascloselyaspossible ElectronicpHprobescanalsobeusedtodeterminepH Theseprobes measurethehydrogen-ionconcentrationwithinasolutiontoprovideanumericalread-outthatis morepreciseandlesssubjectivethantheothermethods
DETERMINING THE PH OF WATER
Introduction
Whenstudyingaquaticenvironments,liketheocean,itisimportanttounderstandhowchangesin pHcanaltertheabilityofsomeorganismstosurviveorreproducewithinanenvironment. Therefore,whencheckingwaterquality,scientistsoftentestthepHofthewaterusingoneofthree methods:universalindicator,litmusindicatororpHprobes.Eachmethodmeasuresthe concentrationofhydrogenions,H+,withinthesolutionbeingstudied.Aftertesting,eachmethod providesanumbervaluebetween0and14alongthepHscale.Thosenumbersbetween0and6.9 areconsideredacidic,a7.0isconsideredneutral,and7.1to14.0areconsideredalkaline.Asthe pHscaleislogarithmic,adecreaseof1indicatesthat10timesmorehydrogenionsarepresent withinthesolution.ThischangecangreatlyaltertheenvironmentfororganismssensitivetopH.
Equipment
Youwillneed:
• •
•
Figure
1.2:
CORE PRACTICAL ACTIVITY
distilledwater(upto60cm3 pergroupplusextraforrinsingtesttubesandpHprobe)
whitevinegar(10cm3 pergroup)
seawaterorasolutionof35gNaClperlitreoftapwater(20cm3 pergroup)
pondwater(20cm3 pergroup)
universalindicatorsolutionorpaperwithcorrespondingcolourchart
litmusindicatorsolution(orredandbluelitmuspapers)
bakingsoda(05mgpergroup)
pHprobe
5×testtubes(wideenoughtofitthepHprobe)
testtuberack
2×smallbeakers
droppingpipettes
Safety considerations
Wearsafetygoggles,labcoatsandgloveswhileinthelaboratory.
Handleglasswarecarefully
Check the Material Safety Data Sheets (MSDS) for the universal indicator solution used, and follownecessarysafetyprecautions.
Before you start
Which properties of distilled water make it appropriate to use for cleaning and calibrating equipment?
WhatpurposemightthevinegarandbakingsodaserveinapHexperiment?
Ocean acidification is a major concern for scientists due to potential impacts on marine organisms.Whatorganismsdoyouthinkareatthegreatestriskandwhy?
Method
For each solution listed in the results table, predict whether it will be acidic, neutral, or alkaline,andrecordyourpredictioninacopyofTable1.2.
Create a solution of vinegar and distilled water in one of the small beakers by combining 10 cm3 aceticacidwith10cm3 distilledwater Setaside
Createasolutionofbakingsodaanddistilledwaterinthesecondsmallbeakerbycombining5 mgofbakingsodaand20cm3 distilledwater Setaside
Pourapproximately1cmdepthofdistilledwaterintoatesttube
UsinguniversalindicatortomeasurepH:
Withsolution:
Addafewdropsofuniversalindicatortoeachsolution.
Waitforcolourchange
ComparecolourtoassociatedpHcolourchartandnoteresultsintable.
Withuniversalindicatorpaper:
Diptheindicatorpaperintothesolutionforabout1second. Allowthepapertodrycompletely
ComparecolourtoassociatedpHcolourchartandnoteresultsintable.
Foreachoftheremainingtesttubes,pourapproximately1cmdepthoftheremainingsolutions (vinegar solution, baking soda solution, seawater, and pond water) so that each test tube only containsonesolution
Repeatstep5foreachtesttube.
Rinsealltesttubeswithdistilledwater
Createnewsamplesofeachsolutionasbefore.
Uselitmusindicatorsolutionorpaperstoseeifeachsolutionisacidic,alkalineorneutral
Recordtheresultsofthelitmusindicatorinthetable(acidic,alkalineorneutral).
Rinsealltesttubeswithdistilledwater
Createnewsamplesofeachsolutionasbefore.
• • • • • • • • • • • • • • • 1 2 3 1 2 3 4 5 a i ii iii b i ii iii 6 7 8 9 10 11 12 13
UsingpHprobe,determinethepHofeachsolution
NoteallpHreadingsintheresultstable
Results
Prediction: acidic, neutral, alkaline
Colour and pH of universal indicator
Results of litmus indicator pH probe reading
distilledwater
vinegarsolution
bakingsoda solution
seawater
pondwater
Table 1.2: DatatableformeasuringpH
Evaluation and conclusions
Did each method provide uniform results for each solution? Why do you think different methodsmayhaveproducedslightlydifferentresults?
Pond water may be slightly acidic to slightly alkaline Where did your solution fall on the pH scale?Suggestareasonforthatreading
Predict what the pH would be if the vinegar solution were added to the baking soda solution Suggestanexplanationforyourprediction
REFLECTION
AftercompletingCorePracticalActivity12,thinkaboutthesequestions: Yourresultsmaybedifferentfromthoseofothergroups.Whymightthatbe? Whatproblemsdidyouencounterwhenworkingthroughthispractical?Howdoyouthinkthey couldhaveimpactedyourresults?
The solubility of gases in seawater
Gasesintheatmosphere(nitrogen,carbondioxideandoxygen)areinastateofequilibriumwiththe gasesdissolvedinoceanwater Astheconcentrationofaparticulargasintheatmosphereincreases (carbondioxide,forinstance),theconcentrationofthatgasinseawateralsorises Mixing,asaresult of turbulence andwaveaction,workstomaintainthisequilibrium Themoreturbulencethereis, theeasieritisforgasesintheatmospheretodissolveintotheocean Thiscanleadtohigher concentrationsofcarbondioxideandoxygenwithintheupper200mdepthoftheoceanthanare foundinthewaterbelowthis Factorscontributingtotheconcentrationofgasesinseawaterinclude thefollowing
Gas solubility
Carbondioxideisverysolubleinseawaterbecauseofitsabilitytoformcarbonicacid,aweakacid, whenintroducedtowater.Oxygen,however,hasalowsolubilitybecauseitdoesnotchemically combinewiththewatermolecules.Thismeansthatthelevelofcarbondioxideheldbyseawateris higherthanthatofoxygen.
Water temperature
Coldwatercandissolvemoregasthanwateratwarmertemperatures.Whenwaterincreasesin temperatureitsmoleculesmovefaster.Thisresultsindissolvedgasmoleculesevaporatingfromthe surfaceofwatermorequickly.Allgasesarelesssolubleinwarmerwaterforthesamereason.This meansthatwaterfoundnearthepoleswilldissolvemoreoxygenthanwaterfoundinthetropics (Table1.3).Theconcentrationofdissolvedoxygenisparticularlyimportanttoaquaticorganisms,so increasesintemperaturecanhaveasignificantimpactontherangeoforganismsthatthewatercan sustain.
14 15 1 2 3 1 2
Table 1.3: Relationshipbetweentemperatureandthemaximumconcentrationofdissolvedoxygenin freshwater.
Atmospheric pressure
Thesolubilityofgasesincreaseswithincreasingpressure.Atmosphericpressureplaysaroleinhow solublegasesareattheocean’ssurface.Whenatmosphericpressureincreases,theequilibriumof gasesintheatmosphereandthosedissolvedintheoceanchanges–thereisagreaterconcentration ofthegasintheatmosphere,thispushesmoreofthosegasmoleculestodissolveintheseawaterand increasetheconcentrationofdissolvedgasesinthesurfacewatersoftheocean.Whenatmospheric pressuredecreases,duringatropicalcycloneforinstance,thisequilibriumshiftstheotherwayand moreofthedissolvedgasmoleculesescapefromthesurfaceandentertheatmosphere.
Water pressure due to depth
Asyoutraveldeeperintotheocean,thepressureofthewateraboveyoucontinuestoincreasedueto thesheermassofthewater.Withthatincreasingpressure,gasesarebetterabletodissolveintothe waterandstaydissolvedinthewater.Notethat,whendescribingdepthofwaterbeverycarefulwith yourwording–trytousewordssuchas‘deeper’or‘shallower’insteadof‘higher’or‘lower’.A statementsuchas‘higherdepths’isambiguousbecauseahighvalueisdeeper,butalowvaluecould meansomethingishigherupintheocean.
The salinity of the seawater
Gasesarebetterabletodissolveinwaterwithlowerlevelsofsalinitybecausetherearelesssolutes takingupspacebetweenthewatermoleculesandsomorewatermoleculesareabletointeractwith gasmoleculesanddissolvethem Thiscanbeseenveryclearlyifyouaddateaspoonofsalttoa carbonateddrinksuchassparklingwaterorcola–whenthesaltisaddedthegasmoleculesare releasedveryquicklyastheliquidfizzesup Gases,likeoxygenandcarbondioxide,aremostsoluble infreshwaterenteringtheoceanfromrivers,suchasinestuaries;asthefreshwatermixeswithsalt waterinthesea,thesolubilityofthesegasesdecreases Therefore,youwouldexpecttofindhigher levelsofoxygeninanestuarythanintheopenocean
Impact of solubility on marine life
Organismsanddissolvedgasesareintricatelylinked Carbondioxideandoxygenarebothnecessary forthesurvivalofmarineorganisms Atthesurface,producerstakeindissolvedcarbondioxidefor useinphotosynthesisandthenreleaseoxygenasaresult Alllivingorganismsusedissolvedoxygen forrespiration Nitrogengasistransformedintoammoniabynitrogen-fixingbacteria,makingthe nitrogeneasierforotherorganismstouseforproteincreation
Asakeyrequirementforrespiration,theconcentrationof dissolved oxygen (DO) isincredibly importantinthemarineenvironment.Ingeneral,oxygenhasalowsolubilityinwater,andthis characteristicisimpactedfurtherbytemperature,salinityandpressurecausingtheconcentrationof DOtovarygreatlythroughouttheocean.Astemperatureandsalinityincrease,theconcentrationof DOdecreases.Thesolubilityofoxygengenerallyincreaseswithdepthduetoboththedecreasein temperatureandtheincreasingpressureofthewaterabove.
Theareaoftheoceanwiththegreatestconcentrationofdissolvedoxygenisthetop100mofthe ocean,knownasthesurfacelayer Withinthislayer,thedissolvedoxygenconcentrationcanreach ‘supersaturation’ Thismeansthereismoreoxygendissolvedintheseawaterthanitwouldnormally beabletocarry Twomajorfactorsworktogethertoincreasetheamountofdissolvedoxygento supersaturationlevel:themotionofthewaterandphotosynthesisbyproducers Themoreturbulent thewater,themoreoxygenismixedintoitbythemovementofthewaves Meanwhile,producers, like phytoplankton andalgae,carryoutphotosynthesisusinglightfromtheSuntocreateglucose
Temperature of water / °C Concentration of dissolved oxygen / mg dm–3 0 146 5 12.8 10 11.3 15 10.2 20 9.2 25 8.4
(fortheproducer)andgenerateoxygenasaby-productthatisreleasedintotheocean’swaters. Photosynthesiscanonlytakeplaceintheupperlayeroftheocean,calledthephoticzone,where lightisabletopenetrateandbeusedbyproducers Thisreleaseofoxygenincreasesthe concentrationofdissolvedoxygeninthesurfacelayer Dissolvedoxygenisremovedfromthesurface layerbytherespirationbyallorganisms TheconcentrationofDOcanalsovarytremendouslywith latitude,astropicalwatershavemuchhighertemperatures,reducingtheconcentrationofDOthat thewatercandissolve Polarwatersaremuchcolderandthereforeabletodissolveamuchhigher concentrationofDO
Belowthesurfacelayeroftheocean,theconcentrationofdissolvedoxygenchangesdramatically.As thedepthoftheoceanincreases,thelevelofdissolvedoxygendecreasesuntilitreachesthe oxygen minimum layer.Theoxygenminimumlayertypicallyoccursatadepthofaround500mbuthas beenfoundanywherebetween100mand1000mdeepdependingonlocation.Atthispoint,thelevel ofDOcansometimesnearlyreachzeroduetoalackofoxygenbeingintroducedintothewaterand consumersstillperformingrespirationtosurvive.
Someorganisms,suchasthevampiresquid,arecapableoflivingwithintheoxygenminimumzone, despitethelackofdissolvedoxygen,buttheydoneedspecialadaptationsforsurvival Mostofthe organismsfoundherearefairlyinactive,whichreducestheirneedforoxygen Thegillsofthefishin thisareaareincrediblyefficientatextractingoxygenfromwater,evenatthelowlevelspresentin thislayer Additionally,manyoftheorganismsherehaveaspeciallyadaptedformofhaemoglobin,a bloodproteinresponsibleforcarryingoxygenthroughoutthebody
Afterpassingthroughtheoxygenminimumlayer,theDOconcentrationbeginstoincreasewithdepth asexpected.Severalreasonsexistforthisincreaseinoxygenasyoumovedeeper(Figure1.12).
Falling detritus is colonised by bacteria that decompose the organic matter carrying out aerobic respiration which uses up oxygen As this detritus falls below the photic zone the oxygen cannot be replaced by photosynthesis Below the oxygen minimum layer this decomposition has been completedandthereislessrespirationcarriedoutbythesebacteria
The organisms found below the oxygen minimum layer are in an area with very few food resources. This lack of food reduces the need for the organisms to respire, so they survive with lessoxygen.
Thesolubilityofoxygenincreasesasthetemperaturedecreases Asyougodeeperintotheocean, thetemperaturedecreasestonear-freezing Thelowertemperaturemeansmoreoxygencanstay dissolvedinthewater
Aspressureincreases,thesolubilityofoxygenincreases Forevery10myousinkintotheocean, thepressureincreasesbyoneatmosphere

• • • •
Figure 1.12: Oxygen minimum layer in the eastern tropical Pacific Ocean and the biological processesresponsible.
7 Test yourself
Whatmakesseawaterasolution?Howcouldyoutestthistoverifyyouranswer?
Howdoesthesalinityofseawaterdifferinareaswhereprecipitationisgreaterthanevaporation, comparedtoareaswhereevaporationisgreaterthanprecipitation?
Howdoesthesolubilityofagasimpactitsavailabilitytomarinelife?
5 6
1.3 Density and pressure
Densityisthemassofadefinedvolumeofwaterdividedbyitsvolume Theformulafordensityis: density(kgm 3)=mass(kg)volume(m3)
Whendiscussingdensityinseawater,thedenserthewateris,theloweritwillsitinthe water column Theleastdensewaterwillrisetothesurfaceofthewatercolumnandthedensestwater willsinktothebottom Temperature,salinityandwaterpressureallplayaroleindeterminingthe densityofseawater
Temperature
Temperatureisthefactormostresponsibleforchangesindensity Asthetemperatureofseawater increases,densitydecreases Warmerwatertendstofloatnearthesurfaceofabodyofwater,and thisisexposedtoheatingbytheSun,causingthislayertogetevenwarmerandlessdense This warmlayerisoftenfairlyshallowandsitsontopofcolder,denserwater Betweenthetwolayersis anareawherethetemperatureabruptlychanges,knownasthethermocline(Figure113) Waterat thesurfacemayreach25°Corhigherintropicalseasbutismorelikelytobe1°Catdepthsof2000 mormore Inpolarseas,thetemperature gradient inathermoclineislessdrastic Intheseareas, thesurfacewaterislikelytobeclosetofreezingandremainatafairlyconstanttemperaturewith increasingdepth

Figure 1.13: Thermoclineinatypicaltropicalsea.
Whyisitthatthewateratthebottomoftheoceandoesnotfreeze?Theanswerhastwoparts: salinityanddensity Assalinityincreases,thefreezingpointofwaterdecreases,makingitmore difficulttocreateice Formoreinformation,seeCorePractical11 Whenwaterbeginstofreeze,the individualwatermoleculesbegintoarrangeintoalatticepattern(asyousawinSection11)dueto thehydrogenbondsholdingthemtogether Thesebondsholdthewatermoleculesataslightly greaterdistancefromoneanother,whichisdifferentfromthearrangementinliquidwaterwherethe moleculesofwaterareconstantlymakingandbreakinghydrogenbondswitheachotherasthey movepasteachother Thisreducesthedensityoficecomparedtoliquidwater,whichcausesiceto floatatthesurfaceofliquidwater Therefore,evenifthewaterfreezesonthebottomoftheocean, unlikelyduetothesalinity,itwouldfloattothesurfaceratherthanremainatthebottomofthe ocean
Thepropertyofwaterthatallowsicetofloatisofvitalimportanceformarineorganisms.Ifice stayedatthebottomoftheocean,theoceanwouldfreezeentirelyfromthebottomtothesurface, whichwouldleavemarineorganismsnowheretogointhedeadofwinter.Wheniceformsonabody





























