AbouttheAuthor
Dr.BahmanZohuricurrentlyworksfor GalaxyAdvancedEngineering,Inc.,a consulting fi rmthathestartedin1991when heleftboththesemiconductoranddefense industriesaftermanyyearsworkingasa chiefscientist.Aftergraduatingfromthe UniversityofIllinoisinthe fi eldofphysics, appliedmathematics,hewenttotheUniversityofNewMexico,wherehestudied nuclearengineeringandmechanicalengineering.HejoinedWestinghouseElectric Corporation,whereheperformedthermal hydraulicanalysisandstudiednaturalcirculationinaninherentshutdown,heat removalsystem(ISHRS)inthecoreofa liquidmetalfastbreederreactor(LMFBR)as asecondaryfullyinherentshutdownsystem forsecondaryloopheatexchange.Allthese designswereusedinnuclearsafetyand reliabilityengineeringforaself-actuated shutdownsystem.Hedesignedamercury heatpipeandelectro magneticpumpsfor largepoolconceptsofanLMFBRforheat rejectionpurposesforthisreactoraround 1978,whenhereceivedapatentforit.He wassubsequentlytransferredtothedefense divisionofWestinghouse,whereheoversawdynamicanalysisandmethodsof launchingandcontrollingMXmissilesfrom canisters.TheresultswereappliedtoMX launchsealperformanceandmuzzleblast phenomenaanalysis(i.e.,missilevibration andhydrodynamicshockformation).Dr. Zohuriwasalsoinvolvedinanalyticalcalculationsandcomputationsinthestudyof nonlinearionwavesinrarefyingplasma. Theresultswereappliedtothepropagation ofso-calledsolitonwavesandtheresulting
chargecollectortracesintherarefaction characterizationofthecoronaoflaserirradiatedtargetpellets.Aspartofhis graduateresearchworkatArgonneNational Laboratory,heperformedcomputationsand programmingofmultiexchangeintegralsin surfacephysicsandsolid-statephysics.He earnedvariouspatentsinareassuchas diffusionprocessesanddiffusionfurnace designwhileworkingasaseniorprocess engineeratvarioussemiconductorcompanies,suchasIntelCorp.,VarianMedical Systems,andNationalSemiconductorCorporation.HelaterjoinedLockheedMartin MissileandAerospaceCorporationasSenior ChiefScientistandoversawresearchand development(R&D)andthestudyofthe vulnerability,survivability,andbothradiationandlaserhardeningofdifferentcomponentsoftheStrategicDefenseInitiative, knownasStarWars.
Thisincludedpayloads(i.e.,IRsensor) fortheDefenseSupportProgram,theBoost SurveillanceandTrackingSystem,and SpaceSurveillanceandTrackingSatellite againstlaserandnuclearthreats.Whileat LockheedMartin,healsoperformedanalysesoflaserbeamcharacteristicsandnuclearradiationinteractionswithmaterials, transientradiationeffectsinelectronics, electromagneticpulses,system-generated electromagneticpulses,single-eventupset, blast,thermomechanical,hardnessassurance,maintenance,anddevicetechnology.
Hespentseveralyearsasaconsultantat GalaxyAdvancedEngineeringservingSandia NationalLaboratories,wherehesupported thedevelopmentofoperationalhazard
assessmentsfortheAirForceSafetyCenterin collaborationwithotherresearchersandthird parties.Ultimately,theresultswereincluded inAirForceInstructionsissuedspecificallyfor directedenergyweaponsoperationalsafety. Hecompletedthe firstversionofacomprehensivelibraryofdetailedlasertoolsfor airbornelasers,advancedtacticallasers, tacticalhigh-energylasers,andmobile/ tacticalhigh-energylasers,forexample.
HealsooversawSDIcomputerprograms,in connectionwithBattleManagementC3I andartificialintelligence,andautonomous systems.Heistheauthorofseveralpublicationsandholdsseveralpatents,suchasfora laser-activatedradioactivedecayandresultsof athrough-bulkheadinitiator.Hehaspublished
thefollowingworks: HeatPipeDesignand Technology:APracticalApproach (CRCPress); DimensionalAnalysisandSelf-SimilarityMethods forEngineeringandScientists (Springer); High EnergyLaser(HEL):Tomorrow’sWeaponin DirectedEnergyWeaponsVolumeI (Trafford PublishingCompany);andrecentlythebook onthesubject DirectedEnergyWeaponsand PhysicsofHighEnergyLaser withSpringer.He haspublishedotherbookswithSpringerPublishingCompanyandCRCinvarioussubjects relatedto: ThermodynamicsinNuclearPower PlantSystems (Springer);and Thermal-Hydraulic AnalysisofNuclearReactors (Springer),heatpipe technology,artificialintelligence,andsoon.He alsohasmorethan60softwarecopyrightsand patents.
Preface
Thisbookhaswrittenforscientistsand engineers,aswellasgraduatestudentsand seniorundergraduatestudentswhoneed tohaveabetterunderstandingofphysics ofcryogenicsandrelatedthermodynamics todotheirjoborgetajobinthis fi eld.This isabookwiththeintentionofbeinga desktopreference,containinghundredsof tablesandchartsofcryogenicdatagathered underoneroof,andtheyarehardtocome crossassumofmaterialsrelatedtosubject ofphysicsofcryogenic.
Thewriterassumesthatreadershave somefundamentalknowledgeofengineeringandscienceandalsosomeofcalculusof differentialequations.Itismoreconvenient forthemeasurementunitsoftradetobein SI,althoughsomesteamtablesarepresented inMKSunits.
Cryogenicprocessesdifferfromgeneral chemicalprocessesinseveralways.Theuse ofmultistreamheatexchangerswithinternal pinchpointsmakesitnecessarytouse somewhatdifferentapproachestosimulate mixedrefrigerantprocesses.Themethods forsimulatingandoptimizingcryogenic processesusingaprocesssimulatorarepresentedinChapter2.
Theneedforusingrefrigerantmixtures overpure fluidsispresentedinChapter3 withreferencetosimplerefrigerationand gas-coolingprocesses.Themorecomplex refrigerationprocessesarepresentedin Chapter4.Aunifieddesignapproachhas beenevolvedforoptimizingmixedrefrigerantprocessrefrigeratorsandliquefiersand ispresentedinChapter5.Thedifferent
naturalgasandnitrogenliquefactionprocessesarepresentedinChapters6and7, respectively.
Thisbookalso:
• Providesanoverviewofthehistoryofthe developmentofcryogenictechnology;
• Includesthelatestinformationonmicrocoolersformilitaryandspaceapplications;
• Offersdetailedinformationonhighcapacitycryogenicrefrigeratorsystems usedinapplicationssuchasfoodstorage,high-powermicrowaveandlaser sensors,medicaldiagnostics,and infrareddetectors.
Today’stechnologyofcryogenicsistakinganewturnandhasfounddifferent medicalapplicationsforthefuture,suchas preservingthebody,althoughthisseemsto beafantasyanddream,howeveritisafact thatsomeresearchersarelookingintoit.As canbeseeninthisbook,cryogenicfreezingis theprocessofpreservingadeadbodywith liquidnitrogen.
Advocatesbelievethatscientistswillone dayworkouthowtowarmthebodiesup andbringthembacktolife,bywhichtime doctorsmightbeabletocurecancersand otherdiseaseswhichareuntreatabletoday. Inthiscase,thefreezingneedstobeginas soonaspossibleafterthepatientdiesto preventdamagetothebrain;thebodyis first cooledinanicebathtograduallyreduceits temperature.
Thisbookprovidesapracticalprospective onthecryogenicsworld.Anhistoricaland currentpictureofcryogenicsinindustry,
government,anduniversitylaboratorieswill behelpfulinmanycareers.Thetechnical aspectsofboththeclassroomandlaboratory workwillprepareyoutoactuallybegin workinmanydifferenttypesofjobsin cryogenics,whichhasbecomeubiquitousin industry,governmentlabs,andmedical centers.Youwillbeabletoseetheequipmentandprocessesthatareprevalentinthe moderncryogenicworkplace.Youwillalso begiventhebackgroundthatwillenableyou tocontributetotheadvancementoftheartof cryogenicsinthefuture.
Thisbookisformattedinsuchawaythat Chapter1startswithanintroductionto cryogenicsasahigh-levelapproachand Chapters2 13describethefundamentalsof thermodynamicsandheattransferaswellas theconceptofheatexchangersinparticular compacttypes.Thisallowsgoingintothe granulardepthofthephysicsofcryogenics,
startingfromChapter14.Forthosereaders whohavesuchknowledge,theycaneasily skipforwardtoChapter14andscanthrough Chapters2 13foranynecessaryreminders. Takingthisapproachdoesnotgivethisbook thepresentationofaclassicalthermodynamicsbook,yetitgivesa flavorofwhatthe readerneedstoknowtobeabletoeasily followtheconceptofultra-low-temperature phenomena.Itisparticularlyhelpfulfor studentsatundergraduateleveltotakea courseinthissubject,withorwithout knowledgeofthermodynamics,heattransfer,etc.,solongastheyhavebasiccoursesin differentialandpartialdifferentialsubjects, aswelladvancedcalculus.
Albuquerque,NewMexico,2016 UnitedStatesofAmerica
B.Zohuri
CryogenicTechnologies
OUTLINE
1.1Introduction2
1.2LowTemperatureinScienceand Technology4
1.3Defi ningCryogenicFluidsor Liquids8
1.3.1TypeofCryogenicLiquids10
1.3.2ThermophysicalProperties13
1.3.3LiquidBoil-Off13
1.3.4CryogenUseforEquipment Cool-Down14
1.3.5PhaseDomains15
1.3.6PersonalProtectiveEquipment toBeWorn15
1.3.7HandlingCryogenicLiquids16
1.3.8StoringCryogenicLiquids16
1.3.9HazardsofCryogenicLiquids17
1.3.10GeneralHazardsofCryogenic Liquids17
1.4HeatTransferandThermalDesign18
1.4.1SolidConduction18
1.4.2Radiation19
1.4.3Convection21
1.4.4GasConduction22
1.4.5MultilayerInsulation22
1.4.6VaporCoolingofNecksand Supports23
1.5RefrigerationandLiquefaction25
1.5.1Thermodynamicsof Refrigeration25
1.5.2HeliumRefrigeratorsversus Lique fiers26
1.5.3RealCyclesandRefrigeration Equipment28
1.6IndustrialApplications33
1.6.1CryogenicProcessingforAlloy Hardening33
1.6.2CryogenicFuels34
1.6.3CryogenicApplicationin NuclearMagneticResonance Spectroscopy34
1.6.4CryogenicApplicationin MagneticResonanceImage35
1.6.5CryogenicApplicationin ElectricPowerTransmission WithinBigCities35
1.6.6CryogenicApplicationin FrozenFoodTransport36
1.6.7CryogenicApplicationin ForwardLookingInfrared37
1.6.8CryogenicApplicationin Space38
1.6.9CryogenicinBloodBanking, Medicine,andSurgery39
1.6.10CryogenicinManufacturing Process42
1.6.11CryogenicinRecyclingof Materials43
1.6.12CryogenicEnergyStorage43
1.6.12.1CESCharacteristics44
1.6.13CESinNuclearPowerPlants45
1.6.14CryogenicApplicationin Research47
1.7CryogenicFluidManagement47
1.7.1Benefits47
1.7.2ResearchOverview47
1.7.3Right:Lightweight, High-EfficientCryocooler48
1.7.4Background49
1.7.5Right:Lique fierDemoand CryogenicInsulationTest Facility49
1.8Conclusion50 References50 FurtherReading51
Cryogenicsisthesciencethataddressestheproductionandeffectsofverylowtemperatures.ThewordoriginatesfromtheGreekwords kryos meaning “frost” and genic meaning “toproduce.” Undersuchadefinition,itcouldbeusedtoincludealltemperaturesbelow thefreezingpointofwater(0 C).However,Prof.KamerlinghOnnesoftheUniversityofLeidenintheNetherlands firstusedthewordin1894todescribetheartandscienceofproducingmuchlowertemperatures.Heusedthewordinreferencetotheliquefactionofpermanent gasessuchasoxygen,nitrogen,hydrogen,andhelium.Oxygenhadbeenliquefiedat 183 C afewyearsearlierin1887,andaracewasinprogresstoliquefytheremainingpermanent gasesatevenlowertemperatures.Thetechniquesusedinproducingsuchlowtemperatures werequitedifferentfromthoseusedsomewhatearlierintheproductionofartificialice.In particular,ef ficientheatexchangersarerequiredtoreachverylowtemperatures.Overthe years,theterm “cryogenics” hasgenerallybeenusedtorefertotemperaturesbelow approximately 150 C(123.15K, 238.00 F).
1.1INTRODUCTION
Accordingtothelawsofthermodynamics,thereexistsalimittothelowesttemperature thatcanbeachieved,whichisknownasabsolutezero.Moleculesareintheirlowest,but finite, energystateatabsolutezero.Suchatemperatureisimpossibletoreachbecausetheinputpowerrequiredapproachesinfinity.However,temperatureswithinafewbillionthsofadegree aboveabsolutezerohavebeenachieved.Absolutezeroisthezerooftheabsoluteorthermodynamictemperaturescale.Itisequalto 273.15 Cor 459.67F.ThemetricorSI(InternationalSystem)absolutescaleisknownastheKelvinscale,whoseunitisthekelvin(not Kelvin),whichhasthesamemagnitudeasthedegreeCelsius.ThesymbolfortheKelvinscale isK,asadoptedbythe13thGeneralCouncilonWeightsandMeasures(CGPM)in1968.Thus, 0 Cequals273.15K.TheEnglishabsolutescale,knownastheRankinescale,usesthesymbolR andhasanincrementthesameasthatoftheFahrenheitscale.IntermsoftheKelvinscale,the cryogenicregionisoftenconsideredtobethatbelowapproximately120K( 153 C).Thecommonpermanentgasesreferredtoearlierchangefromgastoliquidatatmosphericpressureat thetemperaturesshownin Table1.1,calledthenormalboilingpoint(NBP).Inthistable,we
TABLE1.1 NormalBoiling,Triple,andCriticalPoints
Cryogen(K)( C)( R)( F)TriplePointCriticalPoint
haveincludedthetriplepointandcriticalpoint,whichwewillexplaintheminnextchapter. Suchliquidsareknownascryogenicliquidsorcryogens.Whenliquidheliumiscooledfurther to2.17Korbelow,itbecomesasuper-fluidwithveryunusualpropertiesassociatedwithbeing inthequantummechanicalgroundstate.Forexample,ithaszeroviscosityandproducesa filmthatcancreepupandoverthewallsofanopencontainer,suchasabeaker,anddrip offthebottomaslongasthetemperatureofthecontainerremainsbelow2.17K.
Themeasurementofcryogenictemperaturesrequiresmethodsthatmaynotbesofamiliar tothepublicingeneral.Normalmercuryoralcoholthermometersfreezeatsuchlowtemperaturesandbecomeuseless.Oneofthemetalelementsthathasawell-definedbehaviorof electricalresistanceversustemperatureisplatinumresistancethermometer.Itiscommonly usedtomeasureaccurately,includingcryogenictemperaturesdowntoabout20K.Certain semiconductingmaterials,suchasdopedgermanium,arealsousefulaselectricalresistance thermometersfortemperaturesdownto1Kandbelow,aslongastheyarecalibratedoverthe rangetheyaretobeused.Suchsecondarythermometersarecalibratedagainstprimary thermometersthatusefundamentallawsofphysicsinwhichaphysicalvariablechanges inawell-knowntheoreticalwaywithtemperature.
Theproductionofcryogenictemperaturesusuallyusesthecompressionandexpansionof gases.Intypicalairliquefactionprocess,theairiscompressed,causingittoheat,andallowed tocoolbacktoroomtemperaturewhilestillpressurized.Thecompressedairisfurthercooled inaheatexchangerbeforeitisallowedtoexpandbacktoatmosphericpressure.Theexpansion causestheairtocoolandaportionofittoliquefy.Theremainingcooledgaseousportionis returnedthroughtheothersideoftheheatexchanger,whereitprecoolstheincominghighpressureairbeforereturningtothecompressor.Theliquidportionisusually,distilledtoproduce liquidoxygen,liquidnitrogen,andliquidargon.Othergases,suchashelium,areusedinasimilar processtoproduceevenlowertemperatures,butseveralstagesofexpansionarenecessary.
Cryogenicshasmanyapplications.Cryogenicliquids,suchasoxygen,nitrogen,andargon areoftenusedinindustrialandmedicalapplications.Theelectricalresistanceofmostmetals decreasesastemperaturedecreases.Certainmetalsloseallelectricalresistancebelowsome transitiontemperatureandbecomesuperconductors.Anelectromagnetwoundwithawire ofsuchametalcanproduceextremelyhighmagnetic fieldswithnogenerationofheat andnoconsumptionofelectricpoweroncethe fieldisestablishedandthemetalremains cold.Thesemetals,typicallyniobiumalloyscooledto4.2K,areusedforthemagnetsofmagneticresonanceimaging(MRI)systemsinmosthospitals.Superconductivityinsomemetals
was firstdiscoveredin1911byKamerlinghOnnes,butsince1986,anotherclassofmaterials, knownashightemperaturesuperconductors,hasbeenfoundtobesuperconductingatmuch highertemperatures,currentlyuptoabout145K.Theyareatypeofceramic,andbecauseof theirbrittlenature,theyaremoredifficulttofabricateintowiresformagnets.
Otherapplicationsofcryogenicsincludefastfreezingofsomefoodsandthepreservation ofsomebiologicalmaterialssuchaslivestocksemenaswellashumanblood,tissue,andembryos.Thepracticeoffreezinganentirehumanbodyafterdeathinthehopeoflaterrestoring lifeisknownascryonics,butitisnotanacceptedscienti ficapplicationofcryogenics.The freezingofportionsofthebodytodestroyunwantedormalfunctioningtissueisknownas cryosurgery.Itisusedtotreatcancersandabnormalitiesoftheskin,cervix,uterus,prostate gland,andliver.
1.2LOWTEMPERATUREINSCIENCEANDTECHNOLOGY
Cryogenicsasitwasdescribedinprevioussectionisdefinedas thatbranchofphysics,which dealswiththeproductionofverylowtemperaturesandtheireffectonmatter, 1 aformulationthat addressesbothaspectsofattaininglowtemperaturesthatdonotnaturallyoccuronEarth andofusingthemforthestudyofnatureorthehumanindustry.Inamoreoperational way, 2 itisalsodefinedas thescienceandtechnologyoftemperaturesbelow 120K.Thereason forthislatterdefinitioncanbeunderstoodbyexaminingcharacteristictemperaturesofcryogenic fluidsasshownin Table1.1
Thelimittemperatureof120KcomprehensivelyincludestheNBPsofthemainatmosphericgases,aswellasofmethane,whichconstitutestheprincipalcomponentofnatural gas.Today,liquidnaturalgas(LNG)representsoneofthelargest andfast-growing industrialdomainsofapplicationofcryogenics(see Fig.1.1),togetherwiththeliquefaction andseparationofairgases(see Fig.1.2).Thedensificationbycondensationandseparation bydistillationofgaseswashistorically andremainstoday-themaindrivingforcefor thecryogenicindustry.Thisisexemplifiednotonlybyliquidoxygen,andbynitrogen
FIGURE1.1 A130,000m3 liquidnaturalgascarrierwithintegratedinvartank.
usedinchemicalaswellasmetallurgicalprocesses,butalsobythecryogenicliquidpropellantsofrocketengines(see Fig.1.3),withtheproposeduseofhydrogenasa “clean” energy vectorintransportation(see Fig.1.4).
Aswehavestated,cryogenictechnologyhastheneedforsmallercryocoolersbecauseof theadvancesintheminiaturizationofelectricalandopticaldevicesandtheneedforcooling andconductingef ficiency.Cryogenictechnologydealswithmaterialsatlowtemperatures andthephysicsoftheirbehavioratthesetemperatures.Inthisbook,wetrytodemonstrate theongoingnewapplicationsarebeingdiscoveredforcryocooledelectricalandoptical sensorsanddevices,withparticularemphasisonhigh-endcommercialapplicationsinmedicalandscientific fieldsaswellasintheaerospaceandmilitaryindustries.
(A)(B)
FIGURE1.3 Rocketsusingcryogenicliquidpropellants.(A)Ariane5(25tliquidhydrogen,130tliquidoxygen). (B)Spaceshuttle(100tliquidhydrogen,600tliquidoxygen).
FIGURE1.2 Cryogenicairseparationplantwithheatexchangeranddistillationcolumntowers.
Refrigerators,cryocoolers,andmicrocoolersareneededbyvariouscommercial,industrial, space,andmilitarysystems.Cryogeniccoolingplaysanimportantroleinunmannedaerial vehiclesystems,infraredsearchandtracksensors,missilewarningreceivers,satellite trackingsystems,andahostofothercommercialandmilitarysystems.
Nowwithnew-generationnuclearpowerplantsthatareknownasGEN-IV,alotofattentionisfocusedtowardmakingthemmoreefficientandcosteffective3 aswellasusingcryogenictechniquestoimplementenergystorageinnuclearplants. 4 Energystorageinnuclear powerplants(NPPs)residesonanovelmethodofintegrationofnuclearpowergeneration withcryogenicenergystorage(CES)toachieveaneffectivetimeshiftoftheelectricalpower output.CESstoresexcesselectricityintheformofcryogen(liquidair/nitrogen)throughan airliquefactionprocessatoff-peakhoursandrecoversthestoredpowerbyexpandingthe cryogenatpeakhours.5
Thequestforlowtemperatures,however, findsitsorigininearlythermodynamics,with Amontons’sgaspressurethermometer(1703)openingthewayfortheconceptofabsolute zeroinferredacenturylaterbyCharlesandGay-Lussacandeventuallyformulatedby Kelvin.Itis,however,withtheadventofBoltzmann’sstatisticalthermodynamicsinthe latenineteenthcenturythattemperature aphenomenologicalquantity-couldbeexplained intermsofmicroscopicstructureanddynamics.Considerathermodynamicsystemina macrostate,whichcanbeobtainedbyamultiplicityofmicrostates.Theentropy S ofthe systemwaspostulatedbyBoltzmannas
with kB x1 38 10 23 J/K.Thisformula,whichfoundedstatisticalthermodynamics,is displayedonBoltzmann’sgraveinVienna(see Fig.1.5).
Addingreversiblyheat dQ tothesystemproducesachangeofitsentropy dS,witha proportionalityfactor T thatispreciselytemperature
Thus,alow-temperaturesystemcanbedefinedasonetowhichaminuteadditionofheat producesalargechangeinentropy,thatis,alargechangeinitsrangeofpossiblemicroscopic
FIGURE1.4 Automotiveliquidhydrogenfueltank.
configurations.Boltzmannalsofoundthattheaveragethermalenergyofaparticleina systeminequilibriumattemperature T is
Consequently,atemperatureof1Kisequivalenttoathermalenergyof10 4 eVor10 23 J perparticle.
Atemperatureisthereforelowforagivenphysicalprocesswhen kB T issmallcomparedto thecharacteristicenergyoftheprocessthatisconsidered.
Cryogenictemperaturesthusrevealphenomenawithlowcharacteristicenergy(Table1.2) andenabletheirapplicationwhensignificantlylowerthanthecharacteristicenergyofthe
TABLE1.2 CharacteristicTemperatureofLow-Energy Phenomena
PhenomenonTemperature(K)
DebyetemperatureofmetalsFew100
High-temperaturesuperconductors w100
Low-temperaturesuperconductors w10
Intrinsictransportpropertiesofmetals <10
CryopumpingFew
Cosmicmicrowavebackground2.7
Super fluidhelium42.2
Bolometersforcosmicradiation <1
LowdensityatomicBose-Einsteincondensates w10 6
FIGURE1.5 LvdwigBoltzmann’sgraveintheZentralfriedhofVienna,bearingtheentropyformula.
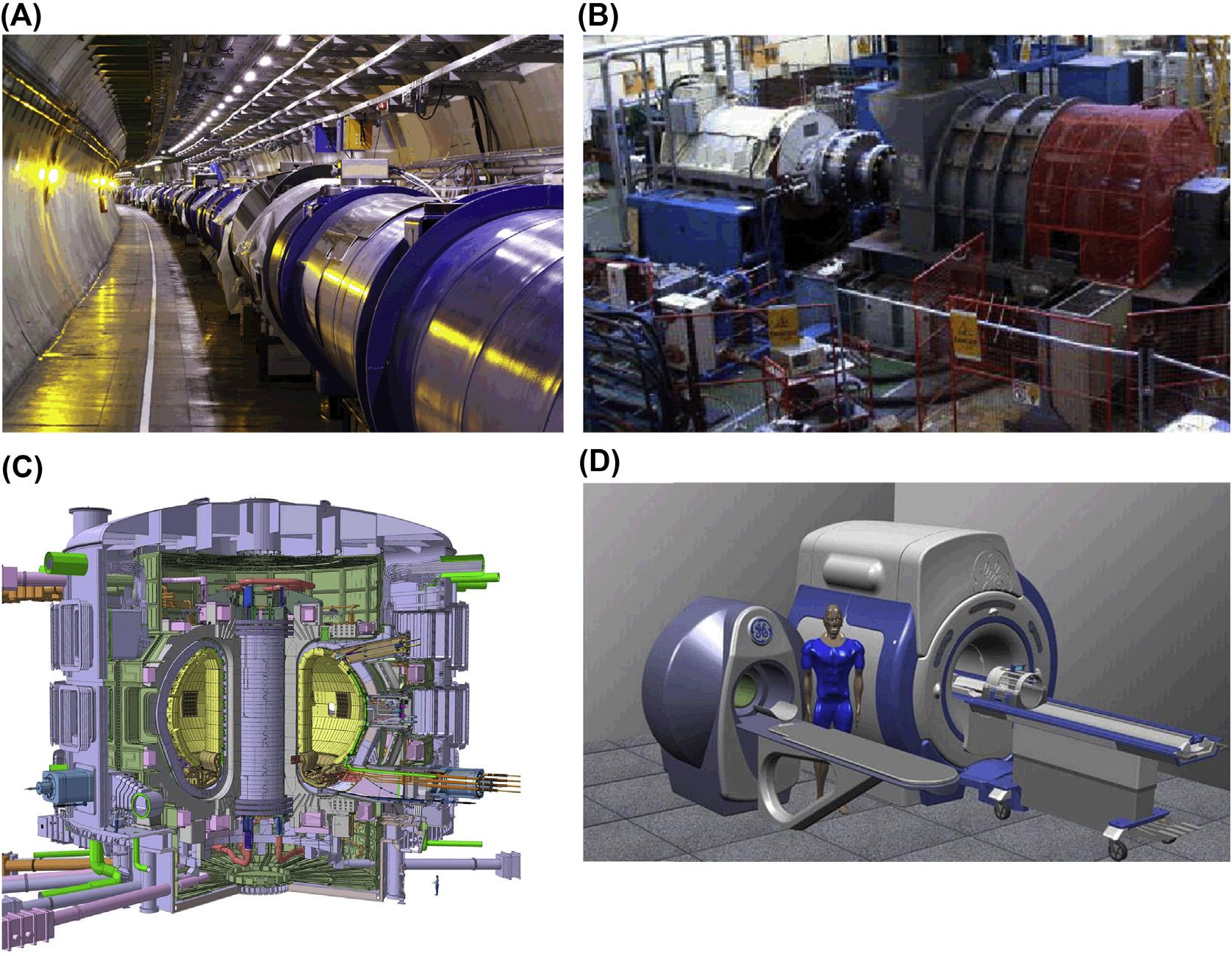
FIGURE1.6 Helium-cooledsuperconductingdevices.(A)largeHadroncollideratCERN,(B)5MVHTSship propulsionmotor(AMS),(C)Iterexperimentalfusionreactor,(D)whole-bodyMRIsystem(Bruker).
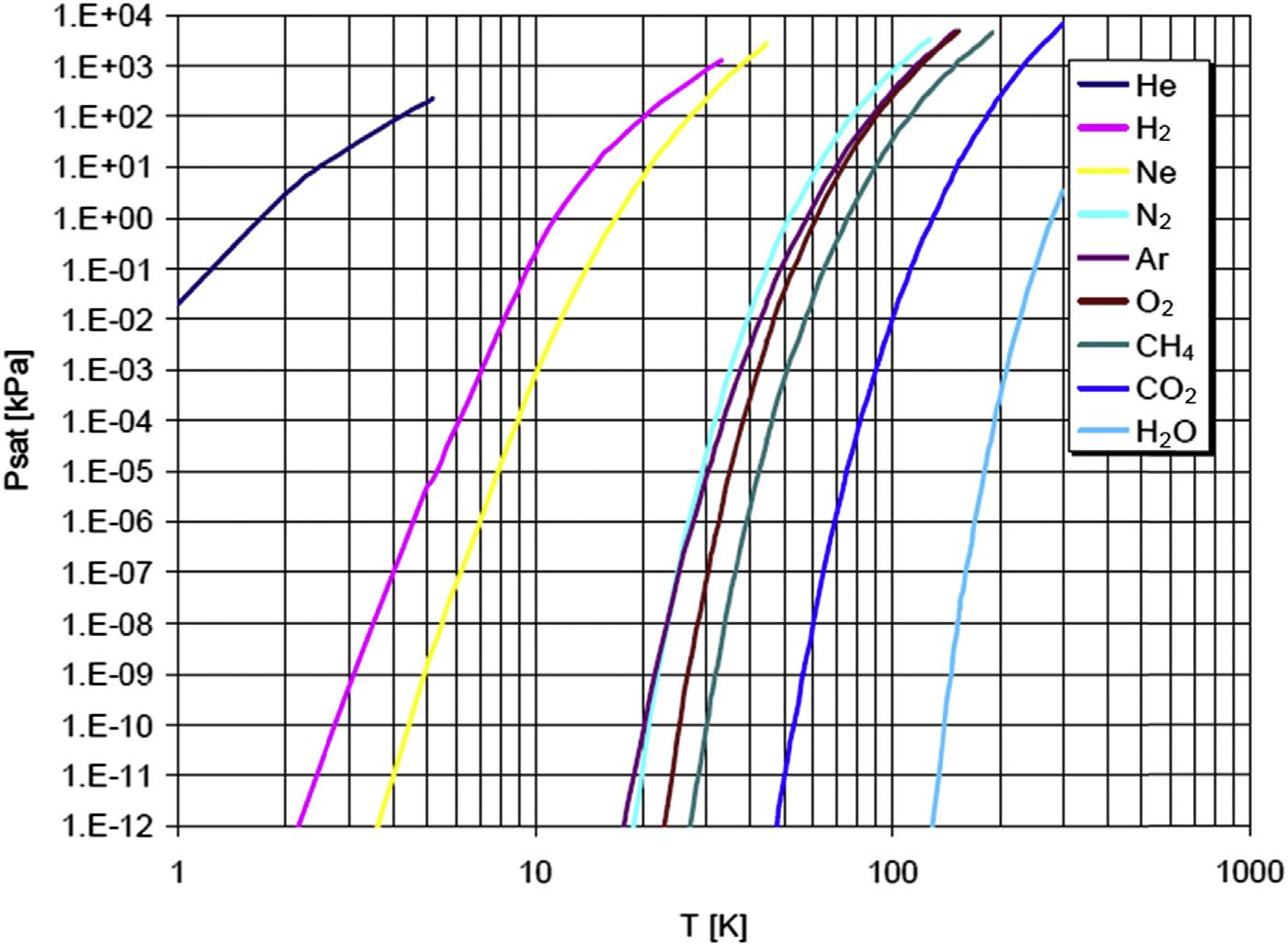
phenomenonofinterest.From Tables1.1and1.2,itisclearthat “low-temperature” superconductivityrequiresheliumcryogenics:severalexamplesofhelium-cooledsuperconducting devicesareshownin Fig.1.6.Consideringvaporpressuresofgasesatlowtemperature (see Fig.1.7),itisalsoclearthatheliummustbetheworkingcryogenforachieving “clean” vacuumwithcryopumps.
1.3DEFININGCRYOGENICFLUIDSORLIQUIDS
Cryogenicliquids,alsoknownascryogens,aregasesatnormaltemperaturesandpressures.However,atlowtemperatures,theyareintheirliquidstate.Theseliquidsare extremelycoldandhaveboilingpointslessthan 150 C( 238 F).Eventhevaporsandgases releasedfromcryogenicliquidsareverycold.Theyoftencondensethemoistureinair, creatingahighlyvisiblefog.Differentcryogensbecomeliquidsunderdifferentconditions oftemperatureandpressure,butallhavetwopropertiesincommon;extremelycoldand smallamountsofliquidcanexpandintoverylargevolumesofgas.Everyonewhoworks
Vaporpressureofcommongasesatcryogenictemperature.
withcryogenicliquidsmustbeawareoftheirhazardsandknowhowtoworksafelywith them. Fig.1.8 isapresentationofliquidnitrogen(LN).
Thediscoveryofsuperconductingmaterialswithcriticaltemperaturessignificantlyabove theboilingpointofliquidnitrogenhasprovidednewinterestinreliable,low-costmethodsof
FIGURE1.7
FIGURE1.8 Liquidnitrogen.
producinghigh-temperaturecryogenicrefrigeration.Theterm “hightemperaturecryogenic” describestemperaturesrangingfromabovetheboilingpointofliquidnitrogen, 195.79 C (77.36K; 320.42 F),upto 50 C(223.15K; 58.00 F),thegenerallydefinedupperlimitof studyreferredtoascryogenics.6 CryogenicistsusetheKelvinorRankinetemperaturescales presentinnature.
1.3.1TypeofCryogenicLiquids
Eachcryogenicliquidhasitsownspecificpropertiesbutmostcryogenicliquidscanbe placedintooneofthreegroups:
• InertGases:Inertgaseslargelytoanyextentdonotreactchemically.Theydonotburn orsupportcombustion.Examplesofthisgrouparenitrogen,helium,neon,argon,and krypton.
• FlammableGases:Somecryogenicliquidsproduceagasthatcanburninair.Themost commonexamplesarehydrogen,methane,carbonmonoxide,andliquefiednaturalgas.
• Oxygen:Manymaterialsconsideredasnoncombustiblecanburninthepresenceof liquidoxygen.Organicmaterialscanreactexplosivelywithliquidoxygen.Thehazards andhandlingprecautionsofliquidoxygenmustthereforebeconsideredseparately fromothercryogenicliquids
Itisgenerallyagreedthatcryogenic fluidsarethosewhoseboilingpoint(bp)atatmosphericpressureareabout120Korlower,althoughliquidethylenewithitsbpof170Kis oftenincluded.Alistofthecryogenic fluids,togetherwithsomeselectedproperties,isgiven in Table1.3.Detailedpropertiesareavailablecommerciallyoncomputerdisc.
Perhapsthemostimportantandwidelyused fluidsareLNG(bp ¼ w120K), liquidoxygen (bp ¼ 90.2K),andliquidnitrogen(bp ¼ 77.3K).
Theavailabilityofcryogenic fluidsformsanessentialpartoftheinfrastructureofamodern industrializedandcivilizedsociety.Oneofthemajorreasonsforusingliquidcryogensistoallow transportandstorageasliquidatatmosphericpressure,ratherthanashigh-pressuregasinthickwalledvessels,althoughthereisanenergypenaltyinvolvedin refrigeration.However,the distillationofliquidair(airseparation)enablestheproductionofveryhigh-purityoxygenand nitrogen.Plantsproducinguptoseveralhundredtonsperdayandmoreofoxygenarecommonplace,sometimesconnectedpermanentlytoachemicalplantorsteelworks.Liquidnitrogen formerlyabyproductoftheprocess isnowaproductinitsownright,beingusedprincipally asaconvenientsourceofrefrigeration,especiallyinthefrozenfoodindustry.
Theotherimportantbyproductofairseparationisliquidargon,which,again,canbeproducedataveryhighpurity.Forwelding,itisincreasinglybeingstoredasliquidatthefactory ratherthanbeingdeliveredinhigh-pressurecylinders.
Allcryogenic fluidsexcept helium and hydrogen behaveas “normal” fluids,theircommon distinguishingfeaturesingeneralbeingalowspeci ficheatandenthalpyofvaporization.All gaseouscryogensareodorless,andallliquidcryogensarecolorlessapartfromoxygen,which ispaleblue,and fluorine,whichispaleyellow.Theyarealldiamagneticexceptoxygen, whichisquitestronglyparamagnetic.
Withtheexceptionofoxygen,allthegasesareasphyxiates,andevenoxygenwillnotsupporthumanlifeinconcentrationsgreaterthanabout60%.Fluorineandoxygenarepowerful
TABLE1.3 SomePropertiesofCryogensatTheirNormalBoilingPoints
Normalboiling point(K)
4.2220.423.727.177.381.785.087.390.2111.6120.0165.0169.4
Liquiddensity(kg/m3)12571.0163120580979215021393114142324003040568
Liquiddensity/vapor density
Enthalpyofvaporization (kJ/kg)
Enthalpyofvaporization (kJ/kg-mol)
7.45371126175181267241255236270297272
20.424463018619921617516121351210896482
80.689912112333556560406659644167988206904212.60413.534
Volumeofliquid vaporizedbyenergyinput of1W-hr(cm3) 14101147433222114161517141313
Dynamicviscosityof liquid(mNsec/m2)
3.313.328.3124152 240260195119404506170
Surfacetension(mN/m)0.101.9 w34.88.99.614.812.513.213.25.518.316.5
Thermalconductivityof liquid(mW/mK) 18.7100 w100113135 1281521879474192
Volumeofgasat15 C releasedfrom1volumeof liquid
Pressureof1.01,323bar.
7398308301412681806905824842613689520475
HandsBA,editor. CryogenicEngineering:AcademicPress;1996.
oxidizerseveninliquidform.Somecryogensare flammable;hydrogenisespeciallydelicate tohandle.
Hydrogenisanunusual fluidinthatthemoleculeexistsintwoformsknownas ortho and para,withsomewhatdifferentproperties.Theratioof ortho to para isdeterminedbyconventionalthermodynamicsandisdependentontemperature.Therearealsodifferentformsof isotopes(deuteriumandtritium)andthesetwoisotopesareusedindrivingfusionenergy productionviaeithermagneticconfinementfusion7 orinertialconfinementfusion.7
Anexplanationofthebehaviorofthehydrogenmoleculerequiresknowledgeofquantum mechanicsandwillnotbediscussedhere.Atlowtemperatures,equilibriumhydrogen(e-H2) isentirely para. Atroomtemperature,the ortho:para ratiois3.Theequilibriumstateatroom temperatureisoftenknownasnormalhydrogenorn-hydrogen.Thetransitionfromtheortho totheparastateinvolvesaheatofconversion whichcanbegreaterthantheenthalpyof vaporization sothatthevaporizationratesofhydrogenareoftenmuchlargerthanexpected.Itisforthisreasonthatacatalystisoftenincludedinahydrogenliquefiertoensure thatonly para hydrogenispresentintheliquid.8
Heliumisthecryogenic fluidthatcanbeclaimedtobeunique.Becauseofitslowmolecularweightandchemicalinertness,quantummechanicaleffectsareimportant.Therearetwo isotopicforms:thenaturalformHe4,whichhasanucleusconsistingoftwoprotonsandtwo
neutrons;,andthecomparativelyraremanufacturedformHe3,withonlyoneneutron.The twoisotopeshavemarkedlydifferentpropertiesduetotheirdifferentnuclearspins.He3 is notconsideredhere.
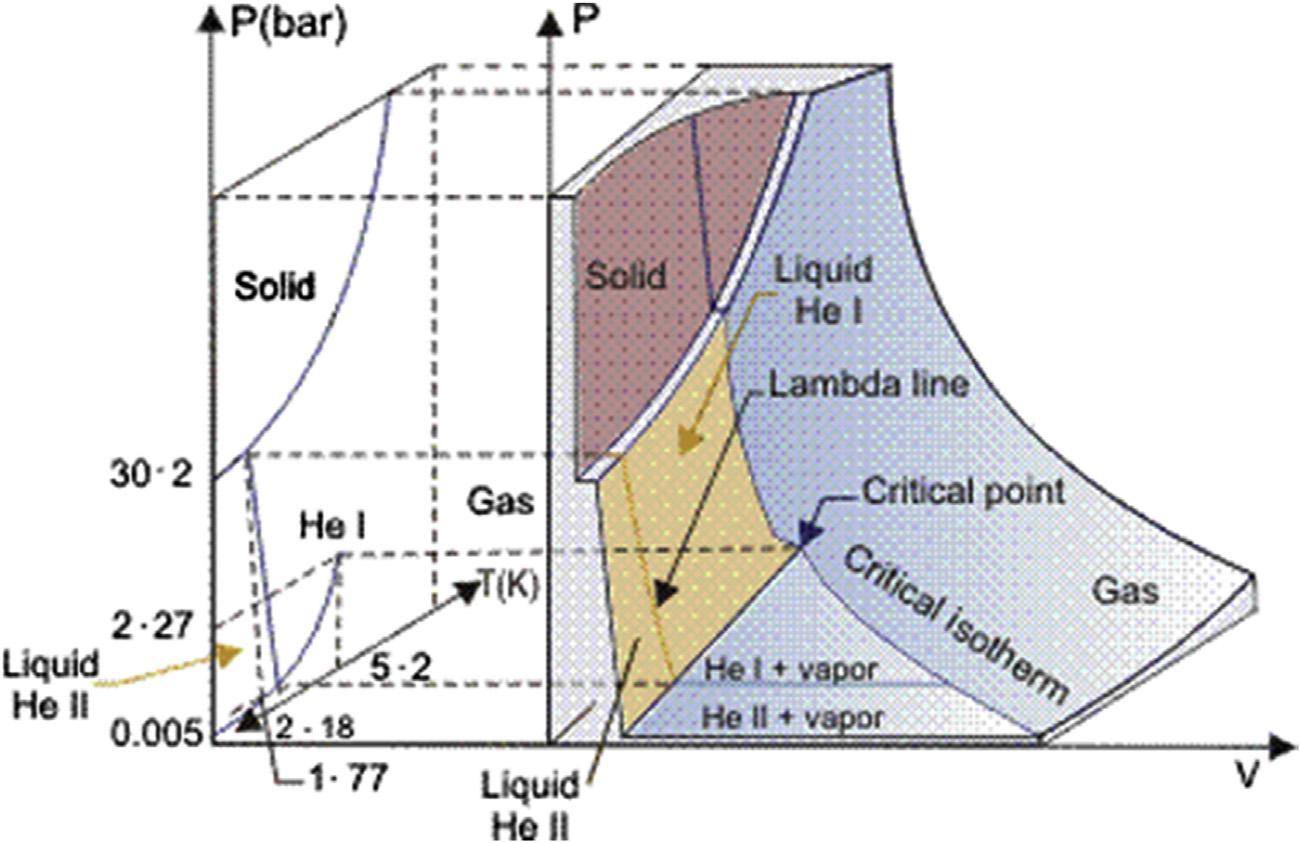
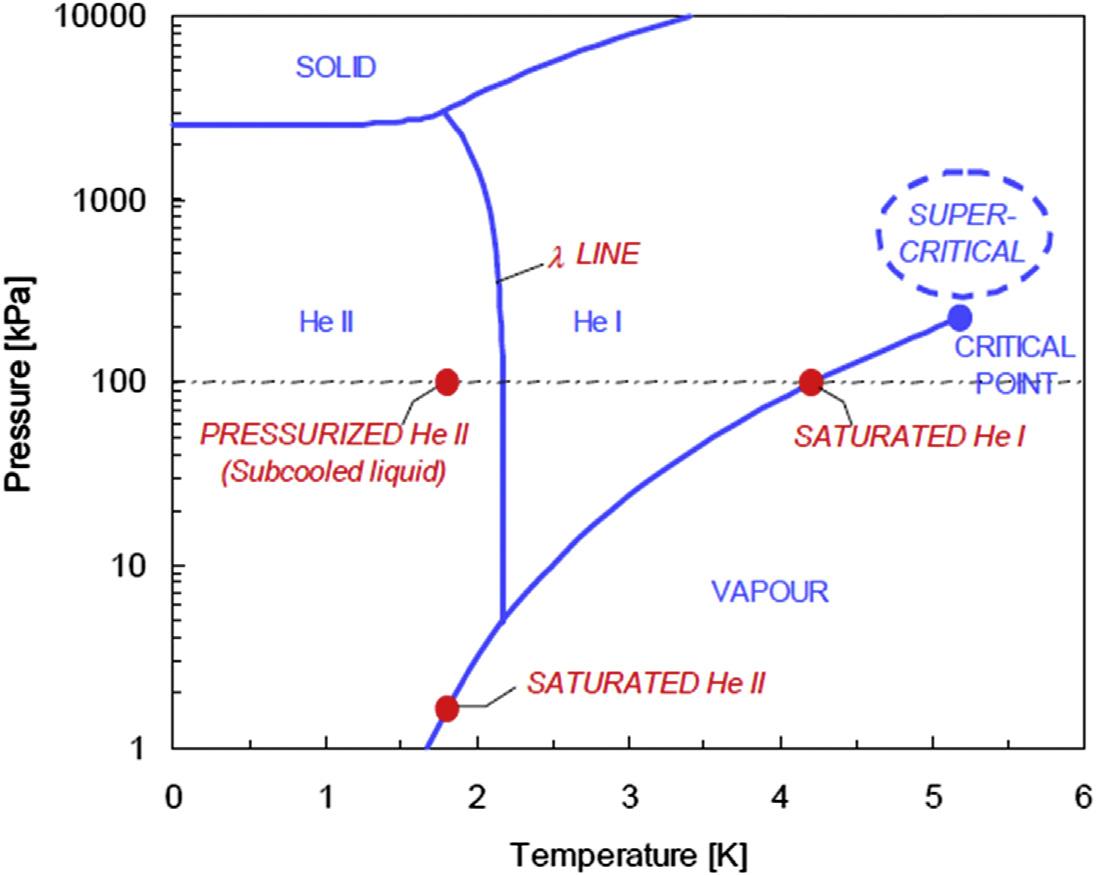
Below2.2K,He4becomes “ super fluid” andisoftenknownasHeII,the “normal” liquid beingknownas “’HeI.”’.ThelocusoftheHeI/HeIItransitionis,isknownasthe “’Llambda lineor l line”’ fromtheshapeofthecurveofspeci ficheatasafunctionoftemperature.The phasediagramofHe4 isshownin Fig.1.9,inbib8whichfeaturesofparticularinterestarethe absenceofatriplepointandthefactthattheliquidcanbesolidi fiedonlyunderpressure (greaterthanabout26bars).
Thetemperatureofthenormal/superfluidtransitiondependssomewhatonpressure.One endofthisboundaryformswithsolidHeIandHeIIthe “upperlambdapoint” (at1.77Kand 30.2bars).Theotherendoftheline(at2.18K,0.005bar),wherevapor,HeI,andHeIIcoexist, isknownasthe “lowerlambdapoint.”
HeIbehavesasaconventionalliquid(exceptwhennearthe l line)butrequiresmuchmore careinhandlingthandoothercryogenic fluids,principallybecauseofitsextremelylow latentheatofvaporization.HeIIisquitedifferent,havingavarietyofpropertiesthatare verydifferentfromthoseofanyotherliquid.Itwill,forinstance,climbupovertheedge ofacontaineranddripoffthebottom;ithasasmallorzeroviscosityandaverylargethermal conductivity.Flowvelocitythrough finecapillariesisindependentofthepressureheadand isgreaterintubesofsmallerdiameter.Flowmaybeinducedbyatemperaturegradientinthe absenceofanypressuregradient.Aconsequenceoftheveryhighthermalconductivityisthat belowthe l point,boilingceasesandtheliquidbecomes “quiescent,” althoughtherateof heattransferremainsveryhigh.Vinenhaspublishedabriefbutusefulreviewofthe propertiesofsuper fluidhelium.
1.3.2ThermophysicalProperties
Thesimplestwayofcoolingequipmentwithacryogenic fluidistomakeuseofitslatent heatofvaporization(e.g.,byimmersioninabathofboilingliquid).Consequently,theuseful temperaturerangeofcryogenic fluidsisthatinwhichthereexistslatentheatofvaporization, thatis,betweenthetriplepointandthecriticalpoint,withaparticularinterestintheNBP thesaturationtemperatureatatmosphericpressure.Thesedataaregivenin Table1.1.Inthis introductiontocryogenics,wewillconcentrateontwocryogens:helium,whichistheonly liquidatverylowtemperature,andnitrogenforitswideavailabilityandeaseofusefor precoolingequipmentandforthermalshielding.
Todevelopafeelingaboutpropertiesofthesecryogenic fluids,itisinstructivetocompare themwiththoseofwater(see Table1.4).Inbothcases,butparticularlywithhelium,applicationsoperatemuchclosertothecriticalpoint,thatis,inadomainwherethedifferencebetweentheliquidandvaporphasesismuchlessmarked:theratioofliquidtovapordensities andthelatentheatassociatedwiththechangeofphasearemuchsmaller.Duetothelow valuesofitscriticalpressureandtemperature,heliumcanbeusedasacryogeniccoolant beyondthecriticalpoint,inthesupercriticalstate.Itisalsointerestingtonotethatwhile liquidnitrogenresembleswaterasconcernsdensityandviscosity,liquidheliumismuch lighterandlessviscous.
Thislatterpropertymakesitamediumofchoiceforpermeatingsmallchannelsinside superconductingmagnetwindingsandthusstabilizingthesuperconductor.
1.3.3LiquidBoil-Off
Thefactorof10inlatentheatofvaporizationbetweenheliumandnitrogen,combined withthelowerdensityoftheformer,inducesalargedifferenceinvaporizationratesunder thesameappliedheatload(Table1.5).Thisillustratestheneedforimplementingmuchbetter insulationtechniquesinliquidheliumvesselstoachievecomparableholdingtimes.
Boil-offmeasurementsconstituteapracticalmethodformeasuringtheheatloadofacryostatholdingasaturatedcryogenbath.Insteadyconditions,thatis,providedtheliquidlevel inthebathismaintainedconstant,theboil-off mvap preciselyequalsthevapor flow mout
TABLE1.4 PropertiesofHeliumandNitrogenComparedtoWater
PropertyHeliumNitrogenWater
Normalboilingpoint(NBP)(K)4.277373
Criticaltemperature(K)5.2126647
Criticalpressure(bar)2.334221
Liquiddensity(kg/m3)125808960
Liquiddensityratioa 7.41751600
Heatofvaporizationa (kJ/kg)20.41992260
Liquidviscositya (mP1)3.3152278
aAtNBP.
TABLE1.5 VaporizationofLiquidHeliumandLiquidNitrogenat NormalBoilingPointUnder1WAppliedHeatLoad
Cryogen(mg/s)(1/hLiquid)(1/minGasNTP)
Helium481.3816.4 Nitrogen50.020.24
escapingthecryostat,whichcanbewarmeduptoroomtemperatureandmeasuredina conventionalgas flowmeter.Ondecreasingtheliquidlevel,though,partofthevaporwill takethevolumeinthecryostatpreviouslyoccupiedbytheliquid,whichhasvaporized, andtheescaping flowwillbelowerthantheboil-off.Moreprecisely,iftheboil-offvapor istakenatsaturationinequilibriumwiththeliquid
Theescapinggas flowmeasuredmust,therefore,becorrectedupwardtoobtainthetrue boil-off.Fromvaluesofsaturatedliquidtovapordensityratiosin Table1.4,thiscorrection factorisonly1.006fornitrogenandcanthereforebeneglected.Forhelium,though,it amountsto1.16andmustclearlybetakenintoaccount.
1.3.4CryogenUseforEquipmentCool-Down
Forboth fluids,thesensibleheatofthevaporoverthetemperaturerangefromliquidsaturationtoambientiscomparabletoorlargerthanthelatentheatofvaporization.Thisprovidesavaluablecoolingpotentialatintermediatetemperature,whichcanbeusedfor thermalshieldingorforprecoolingofequipmentfromroomtemperature.Theheatbalance equationforcoolingamassof,say,iron mFe ofspeci ficheat CFe ðT Þ attemperature T byvaporizingamass dm ofcryogenicliquidatsaturationtemperature Tv ,latentheatofvaporization Lv andvaporspeci ficheat C takenasconstant,isassumingperfectheatexchangewiththe liquidandthevapor.
Hence,thespeci ficliquidcryogenrequirementforcool-downfromtemperature T0 :
Theterm CðT Tv Þ addingto Lv inthedenominatorbringsastrongattenuationtothe speci ficliquidrequirement,providedthereisgoodheatexchangebetweenthesolidand theescapingvapor.Calculatedvaluesofspecificliquidcryogenrequirementsforironare givenin Table1.6,clearlydemonstratingtheinterestofrecoveringthesensibleheatofhelium vapor,aswellasthatofprecoolingequipmentwithliquidnitrogen.
TABLE1.6 VolumeofLiquidCryogensRequiredtoCoolDown1kgofIron1
UsingLatentHeatOnly LatentHeatandEnthalpy ofVapor
Liquidheliumfrom290Kto4.2K29.50.75
Liquidheliumfrom77Kto4.2K1.460.12
Liquidnitrogenfrom290Kto77K0.450.29
1.3.5PhaseDomains
Typicaloperatingdomainswithcryogenicheliumareshownin Fig.1.10,superimposedon thepeculiarphasediagramofthesubstance:thesolidphaseexistsonlyunderpressureand thenormalliquidHeIundergoesatransitiontoanotherliquidphasebelow2.2K,HeII, insteadofsolidifying.Thereisnolatentheatassociatedwiththisphasetransitionbuta peakinthespeci ficheat,theshapeofwhichgavethename “l-line” tothephaseboundary. HeIIexhibitssuperfluidity,amacroscopicquantumbehaviorentailingveryhighthermal conductivityandverylowviscosity.WhileoperatinginsaturatedHeIprovides fixed(saturation)temperatureandhighboilingheattransferatmoderateheat flux,itmaydevelopinstabilitiesintwo-phase flowandispronetoboilingcrisisabovethepeaknucleateboiling flux (about1W/cm2).Theuseofmonophasesupercriticalheliuminforced-flowsystemsavoids theproblemsoftwo-phase flow.However,thestronglyvaryingpropertiesofthe fluidnear thecriticalpointmaycreateotherissues,suchasdensitywaveoscillations.Morefundamentally,supercriticalheliumexhibitsnolatentheat,sothatappliedheatloadsresultintemperatureincreases,whichmustbecontainedbyhigh flowrateorperiodicrecoolinginextended systems.Atlowertemperature,HeIIdemonstratesexcellenttransportproperties,which makeitacoolantofchoiceforadvancedsuperconductingdevices.9 Besidesthethermodynamicpenaltyoflowertemperature,theuseofHeIIimposesthatatleastpartofthecryogenic circuitsoperateatsubatmosphericpressure,thusrequiringefficientcompressionoflowpressurevaporandcreatingrisksofdielectricbreakdownandcontaminationbyairin-leaks. Thermophysicalpropertiesofcryogenic fluidsareavailablefromtables,graphs,andsoftware runningonpersonalcomputers,aselectionofwhichislistedinthebibliography(Fig.1.10).
1.3.6PersonalProtectiveEquipmenttoBeWorn
Aspartofsafetyrequirementtohandleanycryogenicliquids,weneedtoconsiderthe followingpersonalprotectiveequipment(PPE)andwearthemduringhandlingofsuch liquidstoprotecttheskinandeyes.
• Besuretoworkinawell-ventilatedareatopreventoxygen-deficientatmospheresunder 19.5%oxygen.
• Wearsafetyshoeswhenhandlingcontainersalongwithlong-sleevedshirtsandtrousers withoutcuffs.
• ALWAYSwearafull-faceshieldandsplash-resistantsafetygoggles.Contactlenses shouldnotbeworn.
Phasediagramofheliumshowingtypicaloperatingdomains.
• Wearalabcoatandanapronwhendispensingliquidnitrogen.
• Wearinsulatedorleathergloveswhenhandlingliquidnitrogenorlarge,coldobjects.
1.3.7HandlingCryogenicLiquids
Handlingthecryogenicliquidsrequiresthefollowingprecautionsaspartofstandard operatingprocedures:
• Neverallowanyunprotectedpartofthebodytotouchnoninsulatedpipesorvessels thatcontaincryogenic fluids.Tissuedamagethatresultsissimilartofrostbiteorthermal burns.
• Theextremelycoldmetalwillcause fleshtostickfastandtearwhenoneattemptsto withdrawfromit.
• Useasuitablehandtruckforcontainermovement.
• Donotdrop,tip,orrollcontainersontheirsides.Donotremoveorinterchangeconnections.Ifuserexperiencesanydifficultyoperatingcontainervalveorwithcontainer connections,discontinueuseandcontactsupplier.Usetheproperconnection. DONOT USEADAPTERS.
• Manysubstancesbecomebrittleandmayshatterwhencold,sendingpiecesofthe material flying.Avoidcommonglassandlarge,solidplastics.
1.3.8StoringCryogenicLiquids
Tostorethecryogenicliquids,considerthefollowingsteps:
• Storeandusewithadequateventilation.
FIGURE1.10
• Donotstoreinaconfinedspace.
• Cryogeniccontainersareequippedwithpressurereliefdevicestocontrolinternalpressure.Undernormalconditions,thesecontainerswillperiodicallyventproduct.Donot plug,remove,ortamperwithpressurereliefdeviceasthiscouldcauseanexplosion.
• Containersshallbehandledandstoredinanuprightposition.
• SmallquantitiesofliquidnitrogencanbestoredinDewarbottles.Dewarbottlesare hollow-walledglass-linedcontainers,whichprovideexcellentinsulation.
1.3.9HazardsofCryogenicLiquids
Hazardsofcryogenicliquidsarelisted:
• ExtremeColdHazard:Cryogenicliquidsandtheirassociatedcoldvaporsandgases canproduceeffectsontheskinsimilartoathermalburn.Briefexposuresthatwould notaffectskinonthefaceorhandscandamagedelicatetissuessuchastheeyes. Prolongedexposureoftheskinorcontactwithcoldsurfacescancausefrostbite.The skinappearswaxyyellow.Thereisnoinitialpain,butthereisintensepainwhenfrozen tissuethaws.Unprotectedskincansticktometalthatiscooledbycryogenicliquids. Theskincanthentearwhenpulledaway.Evennonmetallicmaterialsaredangerousto touchatlowtemperatures.Prolongedbreathingofextremelycoldairmaydamagethe lungs.
• AsphyxiationHazard:Whencryogenicliquidsformagas,thegasisverycoldand usuallyheavierthanair.Thiscold,heavygasdoesnotdisperseverywellandcan accumulatenearthe floor.Evenifthegasisnontoxic,itdisplacesair.Whenthereisnot enoughairoroxygen,asphyxiationanddeathcanoccur.Oxygendeficiencyisaserious hazardinenclosedorconfinedspaces.Smallamountsofliquidcanevaporateintovery largevolumesofgas.
• ToxicHazards:Eachgascancausespecifichealtheffects.Refertothe Myelodysplastic syndromesforinformationaboutthetoxichazardsofaparticularcryogen.
1.3.10GeneralHazardsofCryogenicLiquids
Thefollowingpointsareimportanttobearinmindwhenitcomestogeneralhazardsof cryogenicliquids:
• FireHazard:Flammablegasessuchashydrogen,methane,carbonmonoxide,andLNG canburnorexplode.Hydrogenisparticularlyhazardous.Itforms flammablemixtures withairoverawiderangeofconcentration.Itisalsoveryeasilyignited.
• Oxygen-EnrichedAir:Whentransferringliquidnitrogenthroughnoninsulatedmetal pipes,theairsurroundingacryogencontainmentsystemcancondense.Nitrogen,which hasalowerboilingpointthanoxygen,willevaporate first.Thisevaporationcanleave anoxygen-enrichedcondensateonthesurfacethatcanincreasethe flammabilityor combustibilityofmaterialsnearthesystem,creatingpotentiallyexplosiveconditions. Equipmentcontainingcryogenic fluidsmustbekeptclearofcombustiblematerialsto minimizethe firehazardpotential.