Risk-Based Quality Management Proactively Manage Risk Across the Clinical Trial Lifecycle

Part of the elluminate Clinical Data Cloud, elluminate RBQM provides clinical teams with a single platform for all study data sources to support a more focused risk monitoring effort that enables better trial oversight and ensures compliance with regulatory guidance (ICH E6 R2 and ICH E8 R1). Because the effectiveness of RBQM relies heavily on the integration of diverse data sources, taking a platform approach enables greater cost-efficiency, improves safety, and provides the flexibility to adapt to evolving challenges.
Benefits:
Data Unification & Integration
elluminate’s ingestion and standardization capabilities for all data types creates a single source of truth for all trial data – eliminating data silos and the need to access multiple applications for high quality data.
Enhanced Patient Safety & Data Quality
A holistic view of risks across data sources enhances study visibility and helps prioritize actions. Access to analytics and real-time insights enable early issue detection, improving data integrity and patient safety.
Scalability & Flexibility
elluminate RBQM offers a phased implementation approach that reduces adoption challenges, allowing trials of all sizes to benefit from RBQM while ensuring regulatory compliance.
Increased Efficiency & Cost Savings
A powerful role-based workflow engine centralizes issues and enables teams to address risks efficiently throughout the trial. By focusing on critical risks and automating processes, RBQM optimizes resources, reducing the cost and time spent on oversight.
Features:
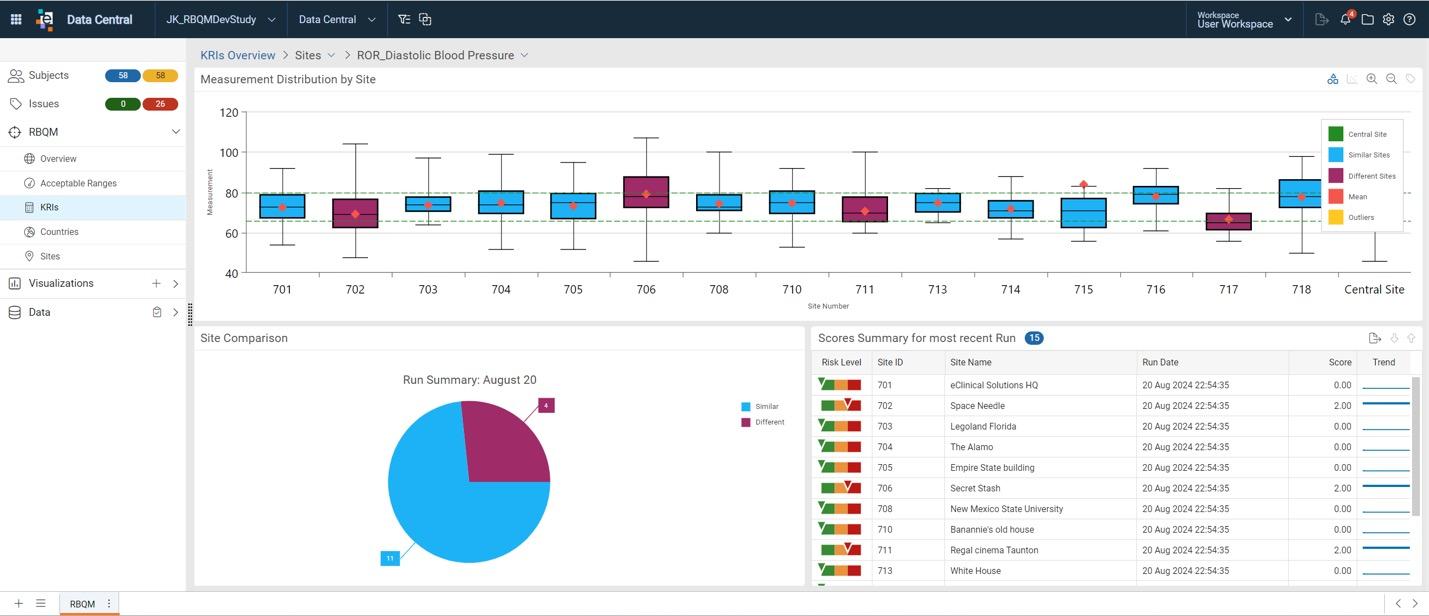
Key Risk Indicators (KRIs)
Configure KRIs to track performance and uncover issues across sites.
Centralized Statistical Monitoring
Use advanced statistical techniques and analytics to detect data errors, anomalies, and atypical patterns.
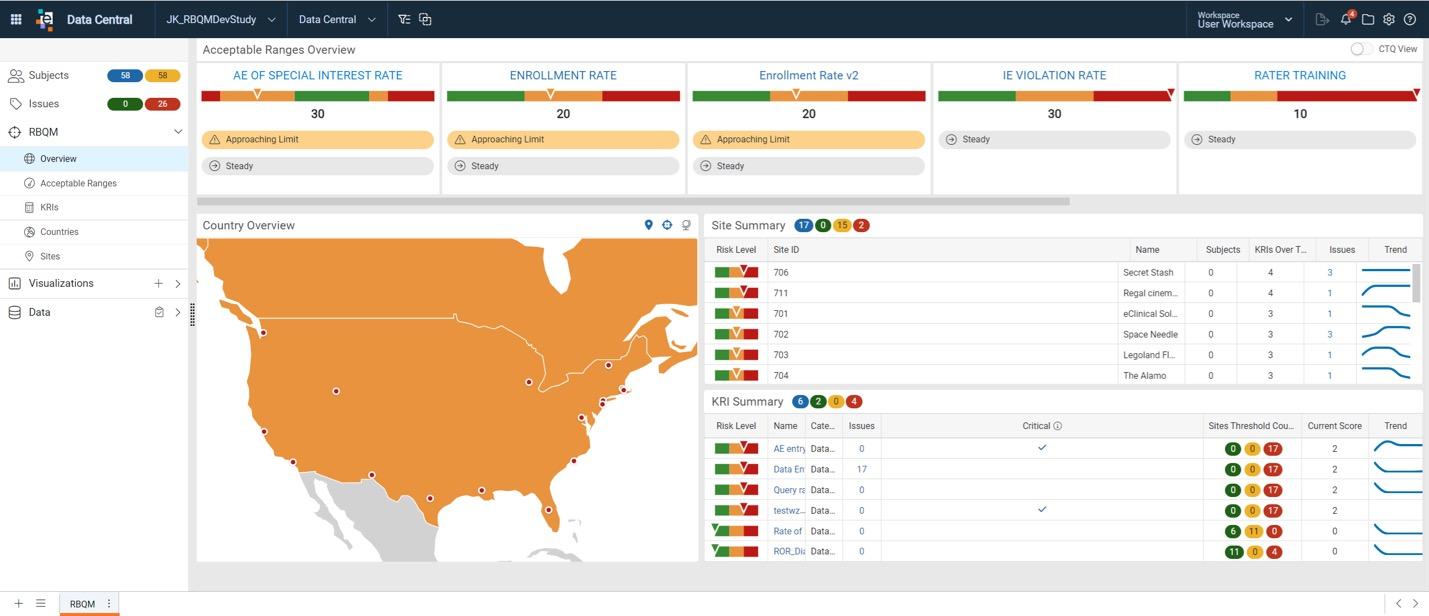
Critical to Quality (CtQ) Factors & Quality
Tolerance Limits (QTLs)
Define CtQ Factors and QTLs to track, evaluate, and take action when thresholds are nearing risk.
Risk Assessment and Categorization Tool (RACT)
Identify and assess study risk using a global library of risk assessment questions, study-specific risk tools, and past assessments.
Visualizations & Interactive Dashboards
View risk at the site and country level and further explore data dimensions with drill-down capabilities.
Issue Management
Take action to resolve issues faster with integrated issue management and role-based workflows.
About Us
eClinical Solutions’ industry-leading data & analytics platform, elluminate®, and biometrics services experts help biopharma researchers at large, mid-size, and emerging life sciences organizations manage trial complexity in less time and with fewer resources. Clients get accurate and timely data insights for better decision-making — enabling them to reduce cycle times, improve productivity, easily scale, and develop tomorrow’s breakthroughs with today’s resources.
Learn more at eclinicalsol.com and follow eClinical Solutions on LinkedIn.
T: 877-355-8668 (877-ELLUMN8) eclinicalsol.com
