











congress@eahp.eu www.eahp.eu tel. +32 (0) 2/669.25.15 22-23-24 MARCH CONGRESS #EAHP2023
PROGRAMME BOOK



FROM DRUG DESIGN TO TREATMENT SUCCESS
WHAT REALLY MATTERS TO PATIENTS?
INDEX
27TH CONGRESS OF THE EAHP - LEADERSHIP & STAFF

GENERAL INFORMATION
FOREWORD BY THE PRESIDENT
ABOUT EAHP
IMPLEMENTATION OF THE EUROPEAN STATEMENTS OF HOSPITAL PHARMACY
EAHP’S CURRENT POLICY AND ADVOCACY PRIORITIES
CALL FOR ABSTRACTS – 2024 BORDEAUX
INFORMATION ABOUT ACPE ACCREDITATION
EAHP ACCREDITATION INITIATIVE
SCIENTIFIC PROGRAMME
PROGRAMME SCHEDULE: WEDNESDAY, 22 MARCH
PROGRAMME SCHEDULE: THURSDAY, 23 MARCH
PROGRAMME SCHEDULE: FRIDAY, 24 MARCH GOOD
3 General Information
INITIATIVES
PRESENTATIONS POSTER AWARD NOMINEES - ORAL PRESENTATIONS KEYNOTE PRESENTATIONS SEMINARS WORKSHOPS INTERACTIVE SESSIONS YOUNG PROFESSIONAL SESSION PHARMACOTHERAPY SESSION
SATELLITE : Non-Biologic Complex Drugs (and nano medicines) INDUSTRY SPONSORED SATELLITES SPEAKERS’ BIOGRAPHIES EXHIBITOR STAND LISTING EXHIBITION FLOOR PLAN FLOOR PLAN - GROUND FLOOR FLOOR PLAN - FIRST FLOOR | 5 | 7 | 11 | 13 | 17 | 20 | 21 | 22 | 26 | 29 | 33 | 38 | 40 | 43 | 45 | 47 | 53 | 77 | 81 | 85 | 87 | 89 | 90 | 107 | 163 | 164 | 166 | 168
PRACTICE
- ORAL
SYNERGY

4
INNOVATIVE
YOUR ASEPTIC
. STERILINE Srl Via Tentorio 30 - 22100 Como - Italy www.steriline.it | Booth N° 14
OUR STANDARD:
SOLUTIONS FOR
PROCESS
27 TH CONGRESS OF THE EAHP –LEADERSHIP & STAFF

ORGANISING COMMITTEE
Chairman
Dr. Nenad Miljković (Serbia) Director of Finance
Members
Dr. András Süle (Hungary) President
Prof. Dr. Thomas De Rijdt (Belgium)
Chairman of the Scientific Committee
Mrs. Jennie De Greef (United States)
Managing Director
SCIENTIFIC COMMITTEE
Chairman
Prof. Dr. Thomas De Rijdt (Belgium)
Members
Dr. Kornelia Chrapkova (Czechia)
Dr. Torsten Hoppe-Tichy (Germany)
Dr. Ulrika Gillespie (Sweden)
Dr. Lene Juel Kjeldsen (Denmark)
Prof. Dr. Raisa Laaksonen (Finland)
Mrs. Despoina Makridaki (Greece)
Prof. Branislava Miljković (Serbia)
Dr. Gunar Stemer (Austria)
Dr. Inese Sviestiņa (Latvia)
Dr. Juraj Sykora (Slovakia)
Dr. Ana Valladolid Walsh (Spain)
Dr. Francesca Venturini (Italy)
Dr. Virginia Silvari (Ireland)
Mr. Jonathan Underhill (United Kingdom)
Mr. Armando Alcobia (Portugal)
Dr. Fatma Karapinar (The Netherlands)
Dr. Stefanie Deuster (Switzerland)
Dr. Daniele Mengato (Italy)
Dr. Xandra Garcia Gonzalez (Spain)
Dr. Clément Delage (France)
EAHP HEADQUARTERS
Boulevard Brand Whitlock 87
Box 11 (4th floor) 1200 Brussels
email: congress@eahp.eu
website: www.eahp.eu
EAHP BOARD OF DIRECTORS
President
Dr. András Süle
Immediate Past President
Dr. Petr Horák
Vice President
Dr. Darija Kuruc Poje
Director of Finance
Dr. Nenad Miljković
Director of Professional Development
Mrs. Despoina Makridaki
Director of Professional Development
Mr. Tjalling van der Schors
Director of Professional Development
Dr. Piera Polidori
Director of Professional Development
Dr. Ana Lozano
Director of Professional Development
Ms. Claudia Plesan
Director of Professional Development
Dr. Louis Bertin
Management
Jennie De Greef, Managing Director
Policy & Advocacy
Stephanie Kohl, Policy & Advocacy Officer
Diogo Teixeira Pereira, Policy Assistant
Projects

Gonzalo Marzal López, Project Portfolio Manager
Events
Chris Irons, Events Assistant
Sharelynne Paras, Events Assistant
Nathalie Signe, Events Assistant
Accounting
Patricia Thonus, Senior Bookkeeper
5 General Information FROM DRUG DESIGN TO TREATMENT SUCCESS
WHAT REALLY MATTERS TO PATIENTS?
EAHP STAFF
QUALITY CONTROL IN SECONDS
Verify the identity and concentration of compounded injectable medications, quickly and efficiently, with DrugLog™ and PrepLog™.

6
pharmacolog.com VISIT US IN BOOTH 42
GENERAL INFORMATION
CONGRESS SECRETARIAT AND REGISTRATION DESK*
OPENING HOURS
Tuesday 21 March, from 12.00 to 17.00
Wednesday 22 March, from 07.00 to 17.00
Thursday 23 March, from 08.00 to 17.00
Friday 24 March, from 08.00 to 11.00
*(individuals, groups and exhibitors’ registrations)

COFFEE BREAKS AND LUNCHES
Coffee breaks and lunches will be available free of charge for all participants during the official congress days. Lunch will be served in the Exhibition Hall (Pavilion I & II) from 13.30 to 14.45 on Wednesday, and on Thursday from 13.30 to 15.00.
ADMISSION TO SESSIONS


There will be no ticketing for sessions. The doors will be closed to the sessions on time and will re-open 10 minutes after the session begins, for latecomers. Please note that the entrance to sessions will not be permitted within the last 30 minutes of each session. Name badges will be scanned as participants exit the room at the end of each session. Badges must be scanned in order to obtain continuing education points. No exception will be made
Please note that badge switching/sharing during the event is strictly prohibited and will subject badges to confiscation by security. We thank you for your cooperation.
POSTER SESSIONS
The deadline to deliver and hang your poster is Wednesday, 22 March from 10.15 to 17.00. Please note that there will be no access to the poster area to hang your posters before the indicated time. Please go to the poster area (Pavilion IV) and check-in with the hostesses on duty to find out where and how to hang your poster.
The posters will be displayed in Pavilion IV for the duration of the congress. Presenters are expected to be present at their poster during 2 coffee breaks (Thursday, 23 March from 10.30 to 11.00, and Friday, 24 March from 10.30 to 11.30). The presence of at least one of the poster authors is mandatory under disciplinary sanctions.
Abstract Award Nominees’ oral presentations will take place on Wednesday, 22 March from 10.30 to 12.00, in Auditorium VI, and Good Practices Initiatives’ oral presentations in Auditorium VII. The winners are announced at the closing ceremony on Friday, 24 March from 11.30 to 13.00. Authors must be present during the closing ceremony to win the prize.
Posters can be removed on Friday, 24 March between 13.00 and 14.00. Posters not removed after the dismantling deadline will be removed and discarded.
7 General Information
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
COMPACT LAMINAR FLOW ISOLATOR SYSTEM
SMALL SCALE, FULL COMPLIANCE
THE PERFECT ALLY FOR YOUR HOSPITAL PHARMACY
THE COMPACT LAMINAR FLOW ISOLATOR SYSTEM IS A MODULAR CGMP CLASS A/ISO 5 ISOLATOR SYSTEM SPECIFICALLY DESIGNED FOR SAFE HANDLING OF HAZARDOUS COMPOUNDS (CHEMOTHERAPY, ONCOLOGICAL APPLICATIONS) IN HOSPITAL PHARMACIES.

IT CAN NOW BE INTEGRATED WITH OUR BRAND NEW VPHP GENERATOR FOR A TOTALLY RELIABLE 6-LOG BIO-DECONTAMINATION PROCESS

Established in 1985, Tema Sinergie has become a world leader in the design and manufacture of shielded isolators for the Nuclear Medicine market.
The company enhances its position on the international market and promotes internal growth by efficiently responding to existing needs and anticipating industry demands with innovative ideas. Presently, the main focus is on the pharmaceutical and chemical sectors, for which Tema Sinergie manufactures customized high-quality stainless steel barrier isolator systems for containment and aseptic processes. VISIT US AT BOOTH #1 of EAHP!
GUARANTEED ASEPSIS
MODULAR BASED CONFIGURATION
AUTOMATIC LEAK TEST
EASE OF MAINTENANCE
ERGONOMIC DESIGN
GLOVE INTEGRITY TESTING
MAXIMUM SAFETY
8
CLF-IS
www.temasinergie.com
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
CONGRESS OPENING HOURS
Wednesday 22 March, from 07.00 to 20.00
Thursday 23 March, from 08.00 to 17.00
Friday 24 March, from 08.00 to 11.00
EXHIBITION
NOTE: Doors to the exhibition hall will have security as entry is not permitted until Wednesday, 23 March at 10.15.
Wednesday 22 March, from 10.15 to 20.00
Thursday 23 March, from 09.00 to 17.00
Friday 24 March, from 09.00 to 12.00
CLOAKROOM
The cloakroom is located at the entrance and will be available for use each day, free of charge. The cloakroom hours are as follows:
Wednesday 22 March, from 07.00 to 20.00
Thursday 23 March, from 08.00 to 17.00
Friday 24 March, from 08.00 to 11.00
WIFI ACCESS
Free WiFi service will be available throughout the congress centre. The WiFi network name is: EAHP2023
Password: eahpatlisbon2023
NEAREST PHARMACY
Farmácia Correia de Azevedo
Address: Rua Luís de Camões 42B, 1300-360 Lisboa, Portugal
Email: teresazevedosoares@gmail.com
Phone: +351 21 363 86 25
Website: https://www.farmaciasportuguesas.pt

Opening hours:
Monday and Wednesday: 09.00 to 13.00 and 15.00 to 19.00
Tuesday, Thursday and Friday: 09.00 to 19.00
Saturday: 09.00 to 13.00
CONGRESS FIRST AID NUMBER
+351 21 892 14 20
EMERGENCY NUMBERS
European Emergency: 112
Ambulance service: 112
Fire brigade: 112
Police:112
9 General Information




10 General Information
FOREWORD BY THE PRESIDENT
Dear Colleagues,
It is my great pleasure and privilege to welcome you all to the 27th EAHP Annual Congress in Lisbon. While these unprecedented times have kept on presenting new challenges and difficulties, our profession has prevailed and hospital pharmacists have never ceased to proactively adapt to a changing world in order to continuously serve our patients’ best interests.
Our team at EAHP has resiliently continued to work hard for our Congress this year as well, so that the international hospital pharmacy community could get together in person. We are delighted to offer you an unforgettable experience along with a valuable learning and networking opportunity.
These last few years have brought upon a turbulent era of multiple paradigm shifts for the European hospital pharmacy profession, during which our commitment to patient-care and medication safety, as well as to our dedication towards evidence-based medicine, was put to a major test. I believe that the hospital pharmacy profession steadily took to the challenge and prevailed. Moreover, the pandemic enabled novel roles for hospital pharmacists to be taken and, therefore, provided further recognition and expanded visibility for all the services our colleagues offer and fulfil.
Our moral imperative dictates our devotion toward helping those in need. It is our duty to provide the best possible care for our patients – spanning from the initial steps of drug design to our ultimate goal: treatment success. A success that is not solely defined by medical parameters, but also through the patient’s perceptions and engagement to play an active role in their own journey towards recovery.
The 2023 EAHP Congress will encompass this wide horizon and provide an insight into these different and distinct, but also overlapping, sub-specialities. I am sure that the event programme will effectively enable us all to grasp this rather complex landscape and to take home with us some valid answers.
I wish you all a memorable and joyful congress, and a wonderful stay in Lisbon!
 Dr. András Süle EAHP President
Dr. András Süle EAHP President

11 General Information FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY
MATTERS TO PATIENTS?
photo: Mariona Ribo




12 Revolutionize Your Pharmacy’s Workflow Capsa Healthcare is your partner for solutions that optimize your pharmacy’s workflow and ensure accuracy in medication dispensing, administration, and inventory management. • High speed, high volume central filling automation from RoboPharma • Automated medication dispensing solutions • Mobile medication delivery to the patient bedside Talk to Capsa Healthcare today about our range of pharmacy innovations. See us at EAHP Stand #9 CapsaHealthcare.com +1.614.864.9966 RoboPharma.com +31416347172 NexsysADC™ RoboPharma™ robotic dispensing MedLink™ Trio™
WHAT REALLY MATTERS TO PATIENTS?
ABOUT EAHP
The European Association of Hospital Pharmacists represents more than 25.000 hospital pharmacists in 35 European countries and is the only association of national organisations representing hospital pharmacists at European and international levels.
HOW HAS THE EAHP DEVELOPED?
At a scientific congress in Strasbourg a number of hospital pharmacists from different European countries decided that they should establish a new European association to represent their common interests. Thus in 1969, the seeds of EAHP were sown. On 6 March 1972, the representatives of six European countries signed the first Statutes of the European Association of Hospital Pharmacists, in The Hague, The Netherlands. The first President of EAHP was Marcel Lebas from France.
The first member countries were Belgium, Britain, Denmark, France, the Federal Republic of Germany and The Netherlands. In 1973, Ireland and Spain joined EAHP, soon followed by Norway, Greece, Austria, Sweden and Switzerland. In the 1990s, Italy, Portugal, Hungary, Luxembourg, Finland, Slovakia, Slovenia, Croatia and the Czech Republic became members. Estonia, Latvia, Lithuania, Poland, Serbia, Turkey, North Macedonia, Bulgaria and Bosnia Herzegovina followed. More recently, Iceland, Malta, Romania and Montenegro became members of EAHP.
Membership within EAHP is open to countries members of the Council of Europe.

On the 1st of August 2011, the EAHP officially became an International Not-for-Profit Organization, and adjusted its policy to better face modern challenges.
EAHP MISSION
EAHP represents and develops the hospital pharmacy profession within Europe in order to ensure the continuous improvement of care and outcomes for patients in the hospital setting. This is achieved through science, research, education, practice, as well as sharing best-practice and responsibility with other healthcare professionals.
WHAT ARE THE GOALS OF THE EAHP?
• In the interest of patients and public health, to promote and further develop hospital pharmacy and to obtain and maintain general joint pharmaceutical principles and a joint pharmaceutical policy;
• to foster research and education activities on behalf of hospital pharmacy, in order to allow hospital pharmacists in all countries which are members of the Council of Europe from time to time (hereinafter “Countries in the European Region”) to contribute optimally to public health and furthermore anything directly or indirectly related or beneficial thereto, all in the broadest sense of the word;

13 General Information FROM DRUG DESIGN TO
TREATMENT SUCCESS
• to promote cooperation with other organisations in the domain of public health;

• to promote the position and function of hospital pharmacists;
• to support and uphold the interests of hospital pharmacists at the European Union authorities;
• to support and uphold the interests of hospital pharmacists at the Council of Europe authorities;
• to support and undergo everything related to the above that may be conducive to carrying out the Association’s purpose.
EAHP SCIENTIFIC COMMITTEE – EDUCATIONAL MISSION
The European Association of Hospital Pharmacists is committed to providing educational innovation and training of hospital pharmacists to a level of specialisation and maintain continuing professional development (CPD). We will facilitate and enhance the professional growth of European hospital pharmacists and develop hospital pharmacy in order to promote the best and safest use of medicines and medical devices for the benefit of patients in Europe.
EAHP SCIENTIFIC COMMITTEE – EDUCATIONAL GOALS
• To identify the educational needs of EAHP members and prepare educational programmes to meet those needs;
• to provide knowledge and application based educational programmes to assist pharmacists who practice in hospitals meet their patient care responsibilities and expand their professional roles and goals;
• to share best practice, innovation and educational programmes that can be applied to daily practice;
• to promote hospital pharmacy practice research.
STRUCTURE OF THE EAHP
The prime governing body is the General Assembly, which meets annually and elects the Board of the Association. The General Assembly is a delegate conference at which each member state may have up to three delegates. Every delegation has one vote regardless of size.
The Board of Directors is the Executive Body of the Association and is elected for a three-year term of office, with the possibility to be re-elected. The responsibility for the core activities of the association are shared between the different directors. The Board normally meets four times a year in addition to meetings during the Congress and the General Assembly.
The Board carries out the policies agreed at the General Assembly, and designs and coordinates the implementation of the strategic goals of the association, with the support of the EAHP staff. In addition, the Board is closely involved in the control of the official journal of the Association, European Journal of Hospital Pharmacy (EJHP). The Board is also closely involved in the organisation of the annual EAHP Congress of Hospital Pharmacy, with one director chairing the congress organising committee and another chairing the scientific committee.
14 General Information
TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
Every year, at the General Assembly, the Director of Finances discloses all the expenses and revenues and explains them in detail. Members would have had the opportunity to study them before the General Assembly meeting as they receive the files at least 6 weeks prior the General Assembly meeting.
EAHP is funded by:

• membership dues
• revenues generated by the yearly congress
• advertising revenue related to the annual congress
• gifts
• educational grants
• subsidies and donations
• all other income legally obtained.
All accounts are audited both by EAHP auditors, appointed by the General Assembly and external auditors.
EAHP PARTNERS WITH CLIMATECARE
EAHP join forces with climate and sustainable development experts from ClimateCare to offset the carbon emissions associated with the air travel of the Association’s Board members to and from Board meetings. Through projects which tackle global climate change. By offsetting emissions through ClimateCare, EAHP is supporting projects that make a measurable difference to people’s lives. The LifeStraw Carbon For Water project relies on finance from offsetting to deliver safe water to 4 million people in Kenya. It was the first project to link water provision with carbon credits at scale and has been recognised by the United Nations. The Gyapa Stoves project supports local entrepreneurs to manufacture and distribute safe, efficient cookstoves to households in Ghana. Run on the ground by our partners Relief International, the Gyapa Stove cuts charcoal use by up to 50%, saving families money and reducing harmful smoke emissions.
15 General Information
DRUG
FROM
DESIGN
IMPLEMENTATION OF THE EUROPEAN STATEMENTS OF HOSPITAL PHARMACY

WHAT ARE THE EUROPEAN STATEMENTS OF HOSPITAL PHARMACY?
The European Statements of Hospital Pharmacy were formulated following an 18-month review process, which included two rounds of online Delphi consultations with EAHP’s member associations and an equal number of patient and other healthcare professional organisations. Final agreement on the wording of the statements was reached at a European Summit on Hospital Pharmacy, held in Brussels in May 2014. Only those Statements where there was a high level of agreement between patient groups, doctors, nurses, and pharmacists were accepted.
The primary purpose of the statements is to provide safer and more effective care where medicines are used in European hospitals. The Statements can also be used as a guide for safer and more effective use of medical devices as well, with the responsibility for medical devices being with Hospital Pharmacies in several of our member countries. The European Statements reflect the importance of the hospital pharmacist as a key stakeholder within the hospital teams for providing optimal and safe patient care. Therefore, the European Statements reflect what we believe every European health system should be aiming for when delivering hospital pharmacy services.
The Statements are divided into 6 different sections:
• Section 1 - Introductory Statements and Governance
• Section 2 - Selection, Procurement and Distribution
• Section 3 - Production and Compounding
• Section 4 - Clinical Pharmacy Services
• Section 5 - Patient Safety and Quality Assurance
• Section 6 - Education and Research
ADOPTION OF A COMMENTED VERSION OF THE STATEMENTS
In 2020, a review was conducted to assess the continued relevance of the Statements and that they effectively covered emerging issues. It was found that the document has remained remarkably resilient and a decision was made that the wording of the statements would not be changed, thereby staying true to the scientific principles used to develop and finalise them. What was agreed though, is that a small number of Statements would benefit from the addition of comments to further clarify their meaning.
The final version of the commented version of the Statements was approved by the EAHP General Assembly. The below are the Statements where comments were added:
S 1.6 “Hospital pharmacists should take the lead in coordinating the activities of multidisciplinary, organisation-wide Drug & Therapeutics Committees or equivalent. They should have appropriate representation as full members of these Committees which should oversee and improve all medicines management policies”
17 EAHP’s Policy and Advocacy FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
COMMENT – Hospital management, taking account of national guidelines, determines what structures are in place to assure the efficacy, safety and cost-effective use of medicines.
S 2.2 “Hospital pharmacists should take the lead in developing, monitoring, reviewing and improving medicine use processes and the use of medicine related technologies. Responsibility for using these processes may rest with other health care professionals and may vary according to the medicine, the medicine related technology, the health care setting and the multidisciplinary team delivering care”.
COMMENT – Hospital pharmacists have a key role, working with others, in ensuring continuous quality improvement for medicines use processes, including where information technology is utilised
S 3.5 “Hazardous medicines should be prepared under appropriate conditions to minimise the risk of contaminating the product and exposing hospital personnel, patients and the environment to harm”
COMMENT – To achieve this there will need to be a multidisciplinary risk assessment of the hazardous medicines to determine where and how it is best pre- pared.
S 3.6 “When the reconstitution or mixing of medicines takes place in a patient care area, a hospital pharmacist should approve written procedures that ensure staff involved in these procedures are appropriately trained”
COMMENT – Among healthcare professionals the hospital pharmacist is in the best position, because of their expertise in formulation, to advise on reconstitution or mixing of medicines. It is critical that any healthcare professional undertaking these tasks is competent.
S 5.1 “The “seven rights” (the right patient, right medicine, right dose, right route, right time, right information and right documentation) should be fulfilled in all medicines related activities in the hospital.”
COMMENT – This is not an exhaustive list of ‘rights’ and with the increase in use of personalised medicines the ‘right patient’ has an additional meaning beyond just identification of the individual, it is also now whether the medicine is genetically appropriate for that individual patient
EAHP SELF-ASSESSMENT TOOL

The EAHP Self-assessment tool allows hospital pharmacists to assess the level of implementation of the European Statements within their hospitals while providing tailored actions plans. EAHP is kindly asking all hospital pharmacies in Europe to assess their hospitals with the self-assessment tool to help us better understand gaps on implementation within Europe.
You can access the tool here: https://sat.eahp.eu/en/home
18 EAHP’s Policy and Advocacy
DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
OATH TO SOCIETY
The European Association of Hospital Pharmacists (EAHP) and the European Society of Clinical Pharmacy (ESCP) have collaboratively developed the “Oath to Society” that acts as a contract for excellence in providing compassionate patient care, working as part of the healthcare team and advancing the pharmacy profession, and showcasing how clinical and hospital pharmacists work every day. The Oath to Society is the promise that the members of EAHP and ESCP make to patients and the public they serve, the healthcare professionals they interact with and the health systems they work in. The Oath functions as a compass for pharmacists to adhere to the highest standards of ethics, integrity and professionalism, as they provide service to the community over the course of their careers. Touching on trust and respect, different aspects of the patient care pathway, the multidisciplinary care team, disease prevention and health promotion, education and the future development of pharmacy practice, the Oath to Society is all-encompassing.
The Oaths to Society can be found here: https://www.eahp.eu/hp-practice/oathtosociety
EAHP-ESCP Oath to Society
As a pharmacist, I vow to se by
• working to the highest of ethical standards, respecting and protecting all personal and health information entrusted to me
• treating all equally, fairly and with respect
• applying my knowledge, skills and experience, as part of the multidisciplinary care team, to ensure the best possible treatment, and achieve optimal person-centred care

• contributing to early detection of disease, promoting the adoption of healthy behaviours and engaging in public health initiatives
• educating patients, their caregivers and members of the multi-disciplinary care team about medication to ensure optimal use, managing adverse effects and responding to questions


• following evidence-based standards of care and engaging in continuous professional development and life-long learning
• ensuring continuity of care, preventing medication-related harm and overseeing and improving medication use processes to optimise their safety, effectiveness and cost-effectiveness
• providing compounded personalised medication tailored to individual needs


• supporting the advancement of standards of practice, research and education in pharmacy
• contributing to the education and training of the future pharmacy workforce ting the interests of the pharmacy practice amongst health care

19 EAHP’s Policy and Advocacy
FROM
Photo: Dutch Association of Hospital Pharmacists (NVZA)
implementation
of the


european statements of hospital pharmacy
EAHP POLICY PRIORITIES
PROFESSIONAL DEVELOPMENT
COMMON TRAINING FRAMEWORK
ACTIONS AGAINST MEDICINES SHORTAGES
COMBATTING INFECTIONS
EQUAL ACCESS FOR PATIENTS
Providing equal and timely access for all patients,







ENSURING SAFE THERAPIES
Ensuring safe and appropriate use of medicinal therapies

DIGITALISATION IN MEDICATION PROCESSES


Facilitating the proper adoption, implementation and usage of digital technologies, including automation and robotics
FUTURE-PROOFING THE WORKFORCE

Addressing the changing roles and resilience of the hospital pharmacy workforce in response to demographic challenges


20 EAHP’s Policy and Advocacy
Fostering
the continuous development and education of hospital pharmacists
Creating a Common Training Framework and enabling the implementation of a common competency framework for hospital pharmacy in Europe
Securing European level action on the global health threat of medicines shortages
Leveraging the role of the hospital pharmacist in combatting infectious diseases and actively participating in vaccination programmes
CALL FOR ABSTRACTS – 2024 BORDEAUX






28th EAHP CONGRESS

20-21-22
MARCH
2024
Bordeaux Bordeaux
Sustainable healthcareOpportunities & strategies



ABSTRACT SUBMISSION OPENS 1ST AUGUST 2023!
Original contributions from all fields of hospital pharmacy are encouraged and welcomed for poster presentation.
DEADLINE FOR SUBMISSION: 1ST OCTOBER 2023
During the review process, the award nominees will be selected, and the presenting author of the nominated abstracts will be invited to give an oral presentation after which the final judging will take place.
Please be sure to provide an email address which will not be blocked by spam servers so that EAHP may notify you for modifications and nominations.
(Abstracts may be submitted through the EAHP website’s online submission page.)
IMPORTANT NOTE: The online submission form does not recognise some symbols from certain keyboards. Therefore, please proof your abstract after it has been entered into the system and before your final submission.
Please visit the EAHP website at http://www.eahp.eu/congresses/abstract to view the guidelines and to submit abstracts for the Bordeaux Congress 2024.

Abstracts must be entered into the system by section according to the guidelines.
There will be 5 sections: Background – Purpose – Material and methods – Results – Conclusion
All abstracts must be linked to the European Statements on Hospital Pharmacy:
Section 1: Introductory Statements and Governance

Section 2: Selection, Procurement and Distribution
Section 3: Production and Compounding
Section 4: Clinical Pharmacy Services
Section 5: Patient Safety and Quality Assurance
Section 6: Education and Research

21 General Information FROM DRUG DESIGN TO TREATMENT SUCCESS
PATIENTS?
WHAT REALLY MATTERS TO
#EAHP2024
EAHP thanks the continued support of Corporate Partner Omnicell
CONGRESS
INFORMATION ABOUT ACPE ACCREDITATION

The European Association of Hospital Pharmacists (EAHP) is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
ACPE is the American agency for accreditation of professional degree programmes in pharmacy and providers of continuing pharmacy education, including certificate programmes in pharmacy. Accreditation is the public recognition granted to a professional degree programme in pharmacy or a provider of continuing pharmacy education, including certificate programmes in pharmacy that is judged to meet standards through periodic evaluations.
Note that only seminars and keynote presentations are accredited.

How can participants receive their ACPE certificates?
In order to receive your ACPE accreditation points, what you have learnt during this session will be assessed. At the beginning of their presentations, each presenter will ask 3 questions that will be answered in the content of their presentations. After the congress, you will receive an evaluation with the same questions, and this is when you will need to answer these questions in order to receive your accreditation certificates.
Please note that the ACPE certificate will not be sent automatically, but upon request only. EAHP will send you the certificate within 15 days after receiving your request.
More information on how to access the evaluation forms will be sent soon.
For licensed pharmacists and pharmacy technicians for the USA
ACPE and the National Association of Boards of Pharmacy (NABP) are developing a continuing pharmacy education (CPE) tracking service, CPE Monitor, that will authenticate and store data for completed CPE units received by US-licensed pharmacists and pharmacy technicians from ACPE-accredited providers as the EAHP. This service will be particularly helpful to the growing number of pharmacists who are licensed in multiple states, and thus may need to meet the varied CPE requirements of different state boards of pharmacy. The CPE tracking system will create a direct link for sending CPE data from the EAHP to ACPE and then to NABP, ensuring that all reported CPE units are officially verified by the EAHP. To view and track these credits, you must first set up an NABP e-Profile (www.nabp.net), obtain your NABP e-Profile ID, and register for CPE Monitor.
So, for each licensed pharmacist from the United States of America who attends the congress, their NABP e-Profile ID and date of birth are requested to be sent out to congress@eahp.eu, in order to notify NABP and ACPE of the number of CPE units collected by each US participant during the congress on 2023. After CPE units are processed by ACPE and NABP, you will be able to access information about your completed CPE through your NABP e-profile (www.nabp.net).
AFTER THE CONGRESS YOU WILL RECEIVE AN EVALUATION FORM. PLEASE FILL IN THE EVALUATION FORMS OF THE SESSIONS YOU ATTENDED IN ORDER TO RECEIVE YOUR CERTIFICATE OF ATTENDANCE.
Please note that the ACPE certificate will not be sent automatically, but upon request only. EAHP will send you the certificate within 15 days after receiving your request.
22 General Information
Activity Announcements Required Items
A. Objectives: verbs must elicit or describe observable or measurable behaviors on the part of participants. (Avoid “understand,” “learn,” etc.)*
B. Type of activity, i.e., knowledge, application, certificate program*
C. Target audience(s) that may best benefit from participation in the activity
D. Faculty member(s) name, degree, and title/position*

E. Fees for the activity
F. Schedule of the educational activities
G. The amount of CPE credit, specified in contact hours or CEUs
H. The official ACPE logo, used in conjunction with the statement identifying the accredited provider sponsoring the activity:

• “The [name of accredited provider] is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.”
• (Optional: listing the ACPE - accredited or non-accredited co-sponsor - if applicable)
I. The ACPE Universal Activity Number assigned to the activity with the appropriate target audience designation (‘P’ and/or ‘T’) in the activity UAN
J. A full description of all requirements established by the provider for successful completion of the CPE activity and subsequent awarding of credit
• (e.g., passing a post-test at specified proficiency level, completing an activity evaluation form, participating in all sessions or certain combinations of sessions that have been designed as a track, etc.).
K. Acknowledgment of any organization(s) providing financial support for any component of the educational activity of the CPE activity
L. For home study activities: the initial release date and the expiration date.
M. For Virtual events: Access to System requirements: The Internet browser(s) supported and minimum versions of each required by the learner to complete the online activity; The minimum memory, storage, processor, and internet speeds require by the learner to complete the online activity
CPE Activity A
*Note: for multi-day conferences, the learning objectives may be listed for the overall conference instead of individual activities on the activity announcement. The items with an asterisk must be listed in the final conference program if they are not listed on the activity announcement. If the items are not listed in the respective locations, then the item should be rated as ‘Needs Improvement.’
23 General Information FROM DRUG DESIGN TO TREATMENT
WHAT
MATTERS
PATIENTS? 2023 EAHP Congress ACPE Policy and Procedure 4.0
SUCCESS
REALLY
TO
Checklist
Monitoring Activity Announcements
x x x x x x x x x x x N/A N/A
ESCP


The European Association of Hospital Pharmacists (EAHP) and The European Society of Clinical Pharmacy (ESCP) have set up the European Council for Pharmacy Education Accreditation.


ECPhA will establish a system for accrediting lifelong learning education in pharmacy in Europe and internationally. ECPhA’s goal is to help improve the quality of continuing education in pharmacy practiced in healthcare settings across Europe via accrediting live and online lifelong learning events throughout collaborating with national healthcare professional associations and accrediting bodies. The first events accredited by ECPhA will take place in 2023, stay tuned to learn more!
ECPHA applies high quality standards in the assessment of available educational programmes, which address the needs and current practice of pharmacists, pharmacy technicians and the pharmacy staff practicing in Europe and worldwide.
Do you want to learn more about ECPhA and the process to have your educational events accredited by the first European system for accrediting learning education in pharmacy? Contact the ECPhA team at info@ecpha.eu
General Information
European Society of Clinical Pharmacy
INFORMATION ABOUT ACPE ACCREDITATION

ADF Search Results
ADF Search Results

25 General Information FROM
DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
UAN Activity Title Cosponsor Release Date Expiration Date Hours (CEUs) Status Activity Type 0475-0000-23-001-L04-P Personalised medicine - opportunities for hospital pharmacists in clinical practice No Joint Providership 03/22/2023 03/22/2026 0.75 (0.075) Submitted Knowledge 0475-0000-23-002-L04-P Hospital support for pharmacy research activities No Joint Providership 03/22/2023 03/22/2026 1.5 (0.15) Submitted Knowledge 0475-0000-23-003-L04-P Medicines procurement � don't forget patients' needs! No Joint Providership 03/22/2023 03/22/2026 1.5 (0.15) Submitted Knowledge 0475-0000-23-004-L04-P Quality assurance of pharmacy preparations � a key for treatment success No Joint Providership 03/22/2023 03/22/2026 1.5 (0.15) Submitted Knowledge 0475-0000-23-005-L05-P PHARMACOTHERAPY SESSION - Safe medication use in patients with cirrhosis No Joint Providership 03/22/2023 03/22/2026 1.5 (0.15) Submitted Knowledge 0475-0000-23-006-L04-P The road to E-hospital pharmacy - are we there yet? No Joint Providership 03/22/2023 03/22/2026 1.5 (0.15) Submitted Knowledge 0475-0000-23-007-L04-P Green Hospital - The Role of Hospital Pharmacists No Joint Providership 03/22/2023 03/22/2026 1.5 (0.15) Submitted Knowledge 0475-0000-23-008-L04-P Advanced therapy medicinal products (ATMPs) - challenging opportunities for hospital pharmacy No Joint Providership 03/22/2023 03/22/2026 1.5 (0.15) Submitted Knowledge 0475-0000-23-009-L04-P Pharmacogenetic testing to optimise therapy No Joint Providership 03/22/2023 03/22/2026 1.5 (0.15) Submitted Knowledge 0475-0000-23-010-L04-P Patient reported outcome measures � what tools can be used? No Joint Providership 03/22/2023 03/22/2026 1.5 (0.15) Submitted Application 0475-0000-23-011-L04-P Non-Biologic Complex Drugs (and nano medicines) No Joint Providership 03/23/2023 03/23/2026 1.5 (0.15) Submitted Knowledge 0475-0000-23-012-L04-P The shortage pandemic � why we haven�t succeeded yet No Joint Providership 03/23/2023 03/23/2026 1.5 (0.15) Submitted Knowledge 0475-0000-23-013-L04-P Lay involvement in prescribing committees � hearing the patient�s voice No Joint Providership 03/23/2023 03/23/2026 1.5 (0.15) Submitted Knowledge 0475-0000-23-014-L07-P Is compounding (always) the answer to drug shortages? No Joint Providership 03/23/2023 03/23/2026 1.5 (0.15) Submitted Application 0475-0000-23-015-L05-P Improving the communication of risks and benefits to patients No Joint Providership 03/23/2023 03/23/2026 0.75 (0.075) Submitted Knowledge 0475-0000-23-016-L04-P From Benefit to Burden - safely discontinuing medicines at the end of life No Joint Providership 03/23/2023 03/23/2026 1.5 (0.15) Submitted Knowledge 0475-0000-23-017-L04-P How the pandemic changed hospital pharmacy management - moving forward No Joint Providership 03/23/2023 03/23/2026 1.5 (0.15) Submitted Knowledge 0475-0000-23-018-L05-P Become a medication safety pharmacist! No Joint Providership 03/23/2023 03/23/2026 1.5 (0.15) Submitted Knowledge Run Date: 02/14/2023 Page 1 of 2
Accreditation Council for Pharmacy Education 135 S. LaSalle Street, Suite 4100 Chicago, IL 60603-4810 Phone (312) 664-3575 Fax (312) 664-7008 http://www.acpe-accredit.org UAN Activity Title Cosponsor Release Date Expiration Date Hours (CEUs) Status Activity Type 0475-0000-23-019-L04-P Patients� individuality: challenges facing hospital pharmacists No Joint Providership 03/23/2023 03/23/2026 1.5 (0.15) Submitted Knowledge 0475-0000-23-020-L05-P The expanding role of the hospital pharmacists in the care of pre and post renal transplant patients No Joint Providership 03/23/2023 03/23/2026 1.5 (0.15) Submitted Knowledge 0475-0000-23-021-L04-P Clinical trials - getting actively involved No Joint Providership 03/24/2023 03/24/2026 1.5 (0.15) Submitted Knowledge 0475-0000-23-022-L04-P The art of estimating renal function in adult patient groups No Joint Providership 03/24/2023 03/24/2026 1.5 (0.15) Submitted Knowledge 0475-0000-23-023-L04-P Patient involvement in pharmacy practice research: no decision about me without me No Joint Providership 03/24/2023 03/24/2026 0.75 (0.075) Submitted Knowledge Run Date: 02/14/2023 Page 2 of 2
Accreditation Council for Pharmacy Education 135 S. LaSalle Street, Suite 4100 Chicago, IL 60603-4810 Phone (312) 664-3575 Fax (312) 664-7008 http://www.acpe-accredit.org
EAHP ACCREDITATION INITIATIVE
The annual EAHP congress is recognised as a valid continuing pharmacy education by the below national association of hospital pharmacists.
ACCREDITATION FOR AUSTRIAN PARTICIPANTS
The Weiterbildungskommission Krankenhausfachapotheker of the Austrian Chamber of Pharmacy, on request of the Austrian Association of Hospital Pharmacists (www.aahp.at), has accredited the 27th EAHP Congress. Austrian hospital pharmacists are therefore eligible to obtain continuing education points according to the KrankenhausfachapothekerWeiterbildungsordnung 2015.

Details on how to claim these points will be available in the member section of the Austrian Chamber of Pharmacists’ website (www.apotheker. or.at). ACPE certificate from participation of congress is needed. A total of maximum 18 credits can be earned.

ACCREDITATION FOR BELGIAN PARTICIPANTS
The Federal Public Service HEALTH, FOOD CHAIN SAFETY AND ENVIRONMENT has accredited the 27th EAHP Congress. Belgian hospital pharmacists are therefore eligible to obtain continuing education points. ACPE certificate from participation of congress is needed. A total of maximum 2 points per day (6 in total) in section can be earned.
CONTINUING EDUCATION CREDITS FOR CZECH PARTICIPANTS


The 27th EAHP Congress is accredited by the Czech Chamber of Pharmacists (CLnK). CLnK sets criteria for the life-long education of pharmacists in healthcare settings and accredits educational events and online content. To receive the credits for attendance at the EAHP Congress, Czech participants will submit a copy of their certificate of participation individually to the CLnK secretariat. A maximum of 2 credit points will be assigned for each 45 minutes, based on the attendance overview provided with the certificate.
ACCREDITATION FOR ESTONIAN PARTICIPANTS
The 27th EAHP Congress is accredited by the Estonian Society of Hospital Pharmacists. Participants have to be present at forthcoming EAHP congress and submit copy of their certificate of participation to ESHP which includes name and all seminars/workshops attended. One accreditation point will be given for every 45 minutes of educational event participated. (www.ehas.ee)

26 General Information
ACCREDITATION FOR GERMAN PARTICIPANTS
The German Society of Hospital Pharmacists (ADKA) acknowledges the high level and quality of scientific education provided by the 27th EAHP Annual Congress. Therefore, the 27th EAHP Annual Congress is accredited by the German accreditation system of Zertifizierte Fortbildung Klinische Pharmazie der ADKA.

ZeFoBi (ADKA) will accept the congress as a continuing education event and will give points on the basis of the ZeFoBi rules. (https://www.adka.de/)


ACCREDITATION FOR PORTUGUESE PARTICIPANTS
To obtain continuing education points, please submit to Ordem dos Farmacêuticos the EAHP programme, which is downloadable via the EAHP website along with your certificate of participation which is included with the name badges upon registration at the congress centre.

ACCREDITATION FOR SERBIAN PARTICIPANTS
The 27th Congress is recognized as a valid form of continuing education (International Congress) by the Pharmaceutical Chamber of Serbia. The following amount of credits will be awarded, in accordance with current regulations: invited lecturer - 15 points; oral presentation - 13 points; poster presentation - 11 points for the first author and 0.5 points for other co-authors; passive participation - 10 points.

ACCREDITATION FOR SLOVAK PARTICIPANTS
The 27th EAHP Annual Congress is being accredited by Slovak Chamber of Pharmacists (SLeK) as a continuing education for pharmacists. The Section of Hospital Pharmacists of the Slovak Chamber of Pharmacists acknowledges the high quality of scientific education provided by the EAHP Annual Congress. Participation will be evaluated according to the SLeK methodology depending on the number of hours spent on specific presentations and seminars within the congress. ACPE certificate of participation in congress is needed.

ACCREDITATION FOR SWISS PARTICIPANTS
The 27th EAHP Congress is accredited by the GSASA (Swiss Association of Public Health Administration and Hospital Pharmacists). Hospital Pharmacist Switzerland: 50 FPH credit points per day, and Clinical Pharmacist Switzerland 50 FPH credit points per day.
27 General Information FROM DRUG DESIGN TO
TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?




28 Increase accuracy and control with automation that helps advance patient safety and protect staff during production of hazardous and non-hazardous compounded sterile preparations. Compounding Automation Visit grifolsinclusiv.com to learn more. Join us in the Grifols booth at the 27th Congress of the EAHP 2023 to find out more about the inclusiv® IV compounding portfolio of integrated products and services. Solutions designed to increase your patients safety KIRO® Oncology Automated compounding of hazardous sterile preparations Gri-fill® 4 Semi-automated compounding for batch and patient-specific production of hazardous and non-hazardous sterile preparations KIRO® Fill Automated compounding of non-hazardous sterile preparations © 2023 Grifols All rights reserved January 2023 GNL-INCP-2200008
FROM DRUG DESIGN TO TREATMENT SUCCESS
WHAT REALLY MATTERS TO PATIENTS?
SCIENTIFIC PROGRAMME
THEME
FROM DRUG DESIGN TO TREATMENT SUCCESS - WHAT REALLY MATTERS TO PATIENTS?
KEYNOTES
KEYNOTE 1 - Personalised medicine - opportunities for hospital pharmacists in clinical practice
ACPE UAN: 0475-0000-23-001-L04-P - A knowledge-based activity
KEYNOTE 2 - Improving the communication of risks and benefits to patients
ACPE UAN: 0475-0000-23-015-L05-P - A knowledge-based activity
KEYNOTE 3 - Patient involvement in pharmacy practice research: no decision about me without me
ACPE UAN: 0475-0000-23-023-L04-P - A knowledge-based activity
SECTION 1: INTRODUCTORY STATEMENTS AND GOVERNANCE
SEMINAR IG1 - Hospital support for pharmacy research activities
ACPE UAN: 0475-0000-23-002-L04-P - A knowledge-based activity
SEMINAR IG2 - The road to e-hospital pharmacy - are we there yet?
ACPE UAN: 0475-0000-23-006-L04-P - A knowledge-based activity
SEMINAR IG3 - How the pandemic changed hospital pharmacy management - moving forward
ACPE UAN: 0475-0000-23-017-L04-P - A knowledge-based activity
SECTION 2: SELECTION, PROCUREMENT AND DISTRIBUTION
SEMINAR SPD1 - Medicines procurement - don’t forget patients’ needs!
ACPE UAN: 0475-0000-23-003-L04-P - A knowledge-based activity
SEMINAR SPD2 - The shortage pandemic - why we haven’t succeeded yet
ACPE UAN: 0475-0000-23-012-L04-P - A knowledge-based activity
SEMINAR SPD3 - Green Hospital - The Role of Hospital Pharmacists
ACPE UAN: 0475-0000-23-007-L04-P - A knowledge-based activity
SECTION 3: PRODUCTION AND COMPOUNDING
SEMINAR PC1 - Advanced therapy medicial products (ATMPs) - challenging opportunities for hospital pharmacy
ACPE UAN: 0475-0000-23-008-L04-P - A knowledge-based activity
SEMINAR PC2 - Quality assurance of pharmacy preparations - a key for treatment success
ACPE UAN: 0475-0000-23-004-L04-P - A knowledge-based activity
WORKSHOP 1 - Is compounding (always) the answer to drug shortages?
ACPE UAN: 0475-0000-23-014-L07-P - An application-based activity

29 Scientific Programme




COMECER S.p.A. Via Maestri del Lavoro, 90 48014 - Castel Bolognese (RA) - Italy t: +39 0546 656375 - f: +39 0546 656353 marketing@comecer.com - www.comecer.com Comecer booth #15 A BATCH APPROACH FOR PATIENT SPECIFIC ONCOLOGY DRUGS AUTOMATIC COMPOUNDING SYSTEM High performance • Up to 35 preparations/h • Liquid and powder drugs managed Total flexibility • More than 300 drugs managed • Ready doses in all possible final containers Significant savings • Zero drug residuals • Cost reduction
FROM DRUG DESIGN TO TREATMENT SUCCESS
WHAT REALLY MATTERS TO PATIENTS?
SECTION 4: CLINICAL PHARMACY SERVICES
SEMINAR CPS1 - Pharmacogenetic testing to optimise therapy
ACPE UAN: 0475-0000-23-009-L04-P - A knowledge-based activity
SEMINAR CPS2 - From Benefit to Burden - safely discontinuing medicines at the end of life
ACPE UAN: 0475-0000-23-016-L04-P - A knowledge-based activity
INTERACTIVE SESSION 1 - The expanding role of the hospital pharmacists in the care of pre and post renal transplant patients
ACPE UAN: 0475-0000-23-020-L05-P - A knowledge-based activity
INTERACTIVE SESSION 2 - The art of estimating renal function in adult patient groups
ACPE UAN: 0475-0000-23-022-L04-P - A knowledge-based activity
SECTION 5: PATIENT SAFETY AND QUALITY ASSURANCE
SEMINAR PSQ1 - Become a medication safety pharmacist!
ACPE UAN: 0475-0000-23-018-L05-P - A knowledge-based activity
SEMINAR PSQ2 - Lay involvement in prescribing committees - hearing the patient’s voice
ACPE UAN: 0475-0000-23-013-L04-P - A knowledge-based activity
SECTION 6: EDUCATION AND RESEARCH
SEMINAR ER1 - Patients’ individuality: challenges facing hospital pharmacists
ACPE UAN: 0475-0000-23-019-L04-P - A knowledge-based activity
SEMINAR ER2 - Clinical trials - getting actively involved
ACPE UAN: 0475-0000-23-021-L04-P - A knowledge-based activity
WORKSHOP 2 - Patient reported outcome measures - what tools can be used?
ACPE UAN: 0475-0000-23-010-L04-P - An application-based activity

OTHER SESSIONS
PHARMACOTHERAPY SESSION - Safe medication use in patients with cirrhosis
ACPE UAN: 0475-0000-23-005-L05-P - A knowledge-based activity
YOUNG PROFESSIONAL SESSION - Learning from the career journeys of others
ACPE Non-Accredited Activity
SYNERGY SATELLITES - Non-Biologic Complex Drugs (and nano medicines)
ACPE UAN: 0475-0000-23-011-L04-P - A knowledge-based activity
INDUSTRY SPONSORED SATELLITES

ACPE Non-Accredited Activity
GOOD PRACTICE INITIATIVES ORAL PRESENTATIONS
POSTER AWARD NOMINEES ORAL PRESENTATIONS
FILL IN THE EVALUATION FORMS OF THE SESSIONS YOU ATTENDED IN ORDER TO RECEIVE YOUR ACPE ACCREDITATION CERTIFICATE AND CERTIFICATE OF ATTENDANCE.
Please be reminded that you will only be able to download the certificates after the Congress. You will have 60 days from the date of the live activity to claim your ACPE accreditation points, the Certificate of attendance and your poster certificate (if applicable), through the EAHP congress portal! The personal logon code from your badge will be required to get the certificates.
31 Scientific Programme






32 Scientific Programme Automated dispensing systems Multipurpose compounding platforms Automated dispensing cabinets Product Portfolio Your Partner In Pharmacy Automation Viestikatu 1-3 70600 Kuopio, Finland +35810 3221 800 sales@newicon.fi Visit us at Booth 33 to discover the best automation solutions for your hospital! Unlock the full potential of your pharmacy operations with cutting-edge automation solutions from Finland, designed to optimize efficiency and accuracy.
FROM DRUG DESIGN TO TREATMENT SUCCESS
WHAT REALLY MATTERS TO PATIENTS?
SCIENTIFIC PROGRAMME SCHEDULE
EAHP confirms that the Speakers and the Scientific Committee members responsible for the development of the Congress programme have signed and submitted the Conflict of Interest Disclosure forms.
* Indicates speaker or SC member has stated a conflict of interest which has been reviewed and accepted. See speakers’ bios page for more information.
Tuesday, 21 March
Wednesday, 22 March
08.30 - 10.15 Opening Ceremony & Keynote 1 – Personalised medicine - opportunities for hospital pharmacists in clinical practice
R. Onatade
ACPE UAN: 0475-0000-23-001-L04-P - A knowledge-based activity
10.15 - 11.45 Coffee Break
10.30 - 12.00 Poster Award Nominees – Oral presentations

Section 3 – Production and Compounding
10.35 – Impact of agitation on pembrolizumab (keytruda®) safety and efficacy: aggregation and functionality - A. Torrente-López

Section 4 – Clinical Pharmacy Services
10.44 – Using machine learning to predict pharmaceutical interventions in a hospital setting - E. Johns
10.53 – Bedside check of medication appropriateness (bed-cma) as a risk-based tool for bedside clinical pharmacy services: a proof-of-concept study at the trauma surgery wardG. van de Sijpe
11.02 – Evaluating the potential clinical and economic impact of chemotherapy prescribing by pharmacists at a university teaching hospital - S. Nally
33 Scientific Programme
Time Meetings/Events Room 12.00 - 17.00 Registration
Main Entrance Foyer
opens (individuals, groups and exhibitors)
Time Meetings/Events Room 07.00 -
Main Entrance Foyer
17.00 Registration opens (individuals, groups and exhibitors)
Auditorium I
Pavilion I & II
Pavilion I & II
10.15 Exhibition opens
Auditorium
VI
11.11 – Consensus validation of a screening tool for cardiovascular pharmacotherapy in geriatric patients: the rasp_cardio list - J. Hias
11.20 – Sepsis code: improving outcomes for patients with sepsis - M.E. Martinez Nuñez
11.29 – Agamenon-seom model for the prediction of survival in patients with her2-positive advanced esophagogastric adenocarcinoma receiving trastuzumab-based first-line treatment - L. Macía-Rivas
Section 5 – Patient Safety and Quality Assurance
11.38 – Using a text-mining approach to identify the context variables language barrier, living alone, cognitive frailty and non-adherence from electronic health records (ehrs)F. Karapinar
10.30 - 12.00 Good Practice Initiatives – Oral presentations Auditorium VII

Section 2 – Selection, Procurement and Distribution
10.35 – Hospital Pharmacists pioneering in installation of an Automated Dispensing System in General Public Hospital, Chania, Greece - M. Petrongonas
10.42 – First Danish pharmaceutical tender with environmental criteria - L. M. Deleuran
Section 3 - Production and Compounding
10.49 – Preparation of Monoclonal Antibodies on the Pharmacy Benchtop - Risk Assessment and Practical Considerations - A. Morris
Section 4 – Clinical Pharmacy Services
10.56 – Pharmacist prescriber, embedded within medical team, improves patient care by timely and accurate discharge medication prescribing - F. Watson
11.03 – Implementation of a multidisciplinary personalized medicine unit for pharmacogenetic testing- J. Fernández-Fradejas
11.10 – Optimising anticoagulation counselling using video media - S. Al-Rawi
Section 5 – Patient Safety and Quality Assurance
11.17 – Software tool development for reconstitution and administration of parenteral antibiotics in hospitals - An international project - Z. Ćetković
11.24 – Assessment of the safety of advanced therapy medicinal products (ATMPs) process: A tutorial videos creation module - C. Jadoul
11.31 – Opioids room of horrors - An interactive learning to improve safety of drug administration - S. Hannou
Section 6 – Education and Research
11.38 – Development of a professional competency framework for clinical pharmacy in Sweden - M. Balgard
11.45 – A 3-Year Transformation of a Belgian Clinical Trial Pharmacy Team - M. Coenen
34 Scientific Programme
10.30 - 12.00
Special Interest Group (SIG) Dissemination
10.30 - 11.15
Investigation of Medication Errors in Intensive Care Units
B. Franklin & S. McCarthy
11.15 - 12.00
The EAHP roadmap toward eliminating avoidable harm
A. Mulac & S. Guntschnig
13.30 - 14.45
14.45 - 16.15
14.45 - 16.15
14.45 - 16.15
Auditorium III & IV
16.45 - 17.15
Lunch Pavilion I & II
Seminars
Seminar IG1 – Hospital support for pharmacy research activities
I. Spriet & D. Mengato
ACPE UAN: 0475-0000-23-002-L04-P - A knowledge-based activity
Seminar SPD1 – Medicines procurement - don’t forget patients’ needs!
D. Glintborg & E. Caccese
ACPE UAN: 0475-0000-23-003-L04-P - A knowledge-based activity
Seminar PC2 – Quality assurance of pharmacy preparations - a key for treatment success
H. Baião & F. Lagarce
ACPE UAN: 0475-0000-23-004-L04-P - A knowledge-based activity
Pharmacotherapy Session – Safe medication use in patients with cirrhosis
M. Aerts & S. Borgsteede*
ACPE UAN: 0475-0000-23-005-L05-P - A knowledge-based activity
Industry Sponsored Satellite
Micromedex (MERATIVE) – An intercollaborative practice model approach: supporting the patient experience in medicines management
ACPE Non-Accredited Activity
Equashield – Unprecedented Technology for Handling Hazardous Drug: The Future of Automated Compounding
- Cutting edge technology: Automated compounding at the next level (14.45 - 15.30) &

- Revolutionizing hazardous drug compounding with leading technology and uncompromised safety (15.30 - 16.15)
ACPE Non-Accredited Activity
Young Professional Session
Young Professional Session – Learning from the career journeys of others
T. Hoppe-Tichy*, S. Deuster*, V. Silvari, K. Chrapková & U. Gillespie
ACPE Non-Accredited Activity
Coffee Break
Room 5A+B
Auditorium VI
Auditorium VII
Auditorium VIII
Auditorium II
Auditorium III & IV
Room 5C
Pavilion I & II
35 Scientific Programme
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
17.15 - 18.45 Seminars
Seminar IG2 – The road to e-hospital pharmacy - are we there yet?

S. Amann & J.M. Martínez-Sesmero
ACPE UAN: 0475-0000-23-006-L04-P - A knowledge-based activity
Seminar SPD3 – Green Hospital - The Role of Hospital Pharmacists
A. Harjans & J. Baehr
ACPE UAN: 0475-0000-23-007-L04-P - A knowledge-based activity
Seminar PC1 – Advanced therapy medicinal products (ATMPs) - challenging opportunities for hospital pharmacy
A. Black* & C. Alonso-Martínez
ACPE UAN: 0475-0000-23-008-L04-P - A knowledge-based activity
Seminar CPS1 – Pharmacogenetic testing to optimise therapy
M. Lampert & J.J. Swen
ACPE UAN: 0475-0000-23-009-L04-P - A knowledge-based activity
Workshop 2 – Patient reported outcome measures - what tools can be used?
T. Graabaek
ACPE UAN: 0475-0000-23-010-L04-P - An application-based activity


17.15 - 18.45 Synergy Satellite
Non-Biologic Complex Drugs (and nano medicines)
(supported by an education grant from CSL Vifor)
J. De Vlieger, G. Stemer & M. Bañobre-López
ACPE UAN: 0475-0000-23-011-L04-P - A knowledge-based activity
Room 5A+B
Auditorium VI
Auditorium VII
Auditorium VIII
Room 5C
Auditorium II
-
19.30 - 21.00 Poster Walk – Join the Scientific Committee during their evaluation of the abstract posters selected for the Poster Walk
The European Association of Hospital Pharmacists (EAHP) is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
More information: https://www.eahp.eu/congresses/acpe
36 Scientific Programme
Pavilion I
19.00
21.00 Get Together Reception
& II
20.00
Pavilion
II
Gallery
Exhibition closes
I &
The Aural Apothecary Podcast


Live on stage at EAHP Congress 2023
Come and join the Three Apothecaries for a session filled with fun and laughter. We will welcome 2 guests from the conference audience to join us on stage and share their stories. Our guests will share their Memory Evoking Medicine for the Aural Apothecary ‘Formulary’; the anthem that soundtracks their career; and their book recommendation for the Aural Apothecary ‘library’.
The Aural Apothecary Podcast is essentially “pub/café” based CPD chat, taking an edgy yet light-hearted view on the world of medicines, pharmacy, and healthcare. Medicines are part of everybody’s life. It’s a podcast about people’s relationship with their medicines and healthcare. Everyone has a story to tell.
Developed and produced by the Three Apothecaries, the podcast, now in Series 5, has delivered over 45 episodes and 3 live show. It has been downloaded in over 50 countries worldwide. The podcast is a regular in the Apple Medical Podcast Charts around the world.

Three Apothecaries are:
Jamie Hayes, FRPharmS
Jamie is a certified executive coach, trained team facilitator and medical educationalist. Following a career as a clinical pharmacist, in both primary and secondary care, Jamie has focused his passion on behavioural change, culture, coaching and performance. Throughout his thirty-year career, he has had the privilege of listening to thousands of stories from clients, colleagues and patients.
Paul Gimson, FRPharmS
After a career in community and primary care pharmacy and a stint as a Director in the Royal Pharmaceutical Society, Paul graduated from the prestigious ‘Generation Q’ programme with the Health Foundation and now works to improve patient safety as the lead for Quality Improvement in CTM University Health Board, Wales, UK.
Steve Williams, FRPharmS
A clinician, Steve has worked as a clinical pharmacist for over 30 years, 25 in acute NHS hospitals and 7 in GP practices. He is a fierce advocate for medicines optimisation and is totally driven by the need to protect all patients from the biggest medication safety problem of all –problematic polypharmacy in an ageing population with multi-morbidity.
Follow us on Twitter https://twitter.com/AuralApothecary Listen to the podcast on all platforms: https://linktr.ee/auralapothecary Booth #57 Thursday, 23 March 2023 14:00 - 14:45
Thursday, 23 March
08.00 - 17.00 Registration opens (individuals, groups and exhibitors) Main Entrance Foyer
09.00 Exhibition opens Pavilion I & II
09.00 - 10.30 Seminars
Seminar IG1 – Hospital support for pharmacy research activities
I. Spriet & D. Mengato
ACPE UAN: 0475-0000-23-002-L04-P - A knowledge-based activity
Seminar SPD2 – The shortage pandemic - why we haven’t succeeded yet
M. Newton, B. Smith* & J.F. Ferreira
ACPE UAN: 0475-0000-23-012-L04-P - A knowledge-based activity
Seminar PC1 – Advanced therapy medicinal products (ATMPs) - challenging opportunities for hospital pharmacy
A. Black* & C. Alonso-Martínez
ACPE UAN: 0475-0000-23-008-L04-P - A knowledge-based activity
Seminar PSQ2 – Lay involvement in prescribing committees - hearing the patient’s voice
C. Schaefer & C. Pitkeathley
ACPE UAN: 0475-0000-23-013-L04-P - A knowledge-based activity
Workshop 1 – Is compounding (always) the answer to drug shortages?
K. Schimmel & R.P. Marques
ACPE UAN: 0475-0000-23-014-L07-P - An application-based activity

Room 5A+B
Auditorium VI
Auditorium VII
Auditorium VIII
Room 5C
09.00 - 10.30
Industry Sponsored Satellite
Omnicell – How a visionary Euro-Autonomous Pharmacy can be used to advance the medication use process
ACPE Non-Accredited Activity
ICU Medical – Transformational journey to a fully connected infusion system across multiple sites improving drug delivery and minimising errors
ACPE Non-Accredited Activity
10.30 - 11.00
11.00 - 11.45
Coffee Break and attended posters
Keynote 2 – Improving the communication of risks and benefits to patients
M.d.C. Climént Palmer
ACPE UAN: 0475-0000-23-015-L05-P - A knowledge-based activity
Auditorium II
Auditorium III & IV
Pavilion I & II
Auditorium I
12.00 - 13.30
Seminars
Seminar IG2 – The road to e-hospital pharmacy - are we there yet?
S. Amann & J.M. Martínez-Sesmero
ACPE UAN: 0475-0000-23-006-L04-P - A knowledge-based activity
Room 5A+B
38 Scientific Programme
Time
Meetings/Events Room
FROM DRUG DESIGN TO TREATMENT SUCCESS
WHAT REALLY MATTERS TO PATIENTS?
Seminar CPS2 – From Benefit to Burden - safely discontinuing medicines at the end of life
J. Hayes & L. Oboh
ACPE UAN: 0475-0000-23-016-L04-P - A knowledge-based activity
Seminar PC2 – Quality assurance of pharmacy preparations - a key for treatment success
H. Baião & F. Lagarce
ACPE UAN: 0475-0000-23-004-L04-P - A knowledge-based activity
Seminar CPS1 – Pharmacogenetic testing to optimise therapy
M. Lampert & J.J. Swen
ACPE UAN: 0475-0000-23-009-L04-P - A knowledge-based activity
Workshop 2 – Patient reported outcome measures - what tools can be used?
T. Graabaek
ACPE UAN: 0475-0000-23-010-L04-P - An application-based activity
12.00 - 13.30 Industry Sponsored Satellite
Amgen – Evaluating Biosimilars: Focus on the Rare Disease Paroxysmal Nocturnal Haemoglobinuria (PNH)
ACPE Non-Accredited Activity
Simplivia – Controlling Occupational Exposure to Hazardous Drugs - Detecting Contamination and the Need for CSTD in the Pharmacy Setting
ACPE Non-Accredited Activity
13.30 - 15.00 Lunch
Auditorium VI
Auditorium VII
Auditorium VIII
Room 5C
Auditorium II
Auditorium III & IV
Pavillon I & II
14.00 - 14.45 The Aural Apothecary Podcast - Live on stage EAHP Booth #57 (Exhibition area)

15.00 - 16.30
Seminars
Seminar IG3 – How the pandemic changed hospital pharmacy management - moving forward
D. Mozgis & A. Melo Gouveia
ACPE UAN: 0475-0000-23-017-L04-P - A knowledge-based activity
Seminar SPD3 – Green Hospital - The Role of Hospital Pharmacists
A. Harjans & J. Baehr
ACPE UAN: 0475-0000-23-007-L04-P - A knowledge-based activity
Seminar PSQ1 – Become a medication safety pharmacist!
T. Toivo & N. O’Hanlon
ACPE UAN: 0475-0000-23-018-L05-P - A knowledge-based activity
Seminar ER1 – Patients’ individuality: challenges facing hospital pharmacists
K. Vučićević & N. Jager
ACPE UAN: 0475-0000-23-019-L04-P - A knowledge-based activity
Interactive Session 1 – The expanding role of the hospital pharmacists in the care of pre and post renal transplant patients
T. van Gelder* & A. Devaney*
ACPE UAN: 0475-0000-23-020-L05-P - A knowledge-based activity
Room 5A+B
Auditorium VI
Auditorium VII
Auditorium VIII
Room 5C
39 Scientific Programme
15.00 - 16.30 Industry Sponsored Satellite
Sanofi – Beyfortus® (nirsevimab) - A novel immunization against Respiratory Syncytial Virus (RSV) Lower Respiratory Tract Disease (LRTD) in neonates and infants
ACPE Non-Accredited Activity
Baxter – Patient Care and Medication Safety: The Evolving Role of the Pharmacist in Parenteral Nutrition
ACPE Non-Accredited Activity
16.30 - 17.00 Coffee Break
Auditorium II
Auditorium III & IV
Pavilion I & II
17.00 Exhibition closes Pavilion I & II
Friday, 24 March
Seminar CPS2 – From Benefit to Burden - safely discontinuing medicines at the end of life
J. Hayes & L. Oboh
ACPE UAN: 0475-0000-23-016-L04-P - A knowledge-based activity
Seminar ER2 – Clinical trials - getting actively involved
M. Briel & K. Suter
ACPE UAN: 0475-0000-23-021-L04-P - A knowledge-based activity
Seminar PSQ1 – Become a medication safety pharmacist!
T. Toivo & N. O’Hanlon
ACPE UAN: 0475-0000-23-018-L05-P - A knowledge-based activity
Seminar ER1 – Patients’ individuality: challenges facing hospital pharmacists
K. Vučićević & N. Jager
ACPE UAN: 0475-0000-23-019-L04-P - A knowledge-based activity

Interactive Session 2 – The art of estimating renal function in adult patient groups
C. Franssen & M. Kerskes
ACPE UAN: 0475-0000-23-022-L04-P - A knowledge-based activity
10.30 - 11.30 Coffee Break and attended posters
11.30 - 13.00 Closing Ceremony & Keynote 3 – Patient involvement in pharmacy practice research: no decision about me without me
G. Hickey & K. Turner
ACPE UAN: 0475-0000-23-023-L04-P - A knowledge-based activity
Room 5A+B
Auditorium VI
Auditorium VII
Auditorium VIII
Room 5C
Pavilion I & II
Auditorium I
12.00 Exhibition closes Pavilion I & II
40 Scientific Programme
Time Meetings/Events Room 08.00 - 11.00 Registration opens (individuals, groups and exhibitors) Main Entrance Foyer 09.00 Exhibition opens Pavilion I & II 09.00
10.30 Seminars
-





Scientific Programme FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS
Read the latest issue online
European Jo peer review and innovat ejhp.bmj.co
We are a global pharmaceutical company that develops, manufactures and distributes generic, biosimilar and added-value medicines in over 85 countries worldwide, helping to improve access for patients to vital pharmaceutical care.
Our approach is agile and inventive, we’re driven to think differently by continuing to explore and deliver high quality medicines designed to benefit patients’ lives worldwide.

Come and visit us at booth #30 accord-healthcare.com PT-01537 | January 2023
WE TOUCH MILLIONS OF LIVES EVERY DAY We make it better.
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?

GOOD PRACTICE INITIATIVES
ORAL PRESENTATIONS
WEDNESDAY, 22 MARCH 2023 – FROM 10.30 TO 12.00, AUDITORIUM VII
ACPE Non-Accredited Activity
Section 2 - Selection, Procurement and Distribution
10.35 - HOSPITAL PHARMACISTS PIONEERING IN INSTALLATION OF AN AUTOMATED DISPENSING SYSTEM IN GENERAL PUBLIC HOSPITAL, CHANIA, GREECE - M. Petrongonas
10.42 - FIRST DANISH PHARMACEUTICAL TENDER WITH ENVIRONMENTAL CRITERIA - L. M. Deleuran
Section 3 - Production and Compounding
10.49 - PREPARATION OF MONOCLONAL ANTIBODIES ON THE PHARMACY BENCHTOP – RISK ASSESSMENT AND PRACTICAL CONSIDERATIONS - A. Morris
Section 4 - Clinical Pharmacy Services
10.56 - PHARMACIST PRESCRIBER, EMBEDDED WITHIN MEDICAL TEAM, IMPROVES PATIENT CARE BY TIMELY AND ACCURATE DISCHARGE MEDICATION PRESCRIBING - F. Watson
11.03 - IMPLEMENTATION OF A MULTIDISCIPLINARY PERSONALIZED MEDICINE UNIT FOR PHARMACOGENETIC TESTING - J. Fernández-Fradejas
11.10 - OPTIMISING ANTICOAGULATION COUNSELLING USING VIDEO MEDIA - S. Al-Rawi
Section 5 - Patient Safety and Quality Assurance
11.17 - SOFTWARE TOOL DEVELOPMENT FOR RECONSTITUTION AND ADMINISTRATION OF PARENTERAL ANTIBIOTICS IN HOSPITALS – AN INTERNATIONAL PROJECT - Z. Ćetković
11.24 - ASSESSMENT OF THE SAFETY OF ADVANCED THERAPY MEDICINAL PRODUCTS (ATMPS) PROCESS: A TUTORIAL VIDEOS CREATION MODULE - C. Jadoul
11.31 - OPIOIDS ROOM OF HORRORS – AN INTERACTIVE LEARNING TO IMPROVE SAFETY OF DRUG ADMINISTRATION - S. Hannou

Section 6 - Education and Research
11.38 - DEVELOPMENT OF A PROFESSIONAL COMPETENCY FRAMEWORK FOR CLINICAL PHARMACY IN SWEDEN - M. Balgard
11.45 - A 3-YEAR TRANSFORMATION OF A BELGIAN CLINICAL TRIAL PHARMACY TEAM - M. Coenen
43 Scientific Programme

44 with Deenova, the leading European supplier of mechatronic solutions for closed-loop medication management and RFID-based medical devices traceability. The value of our technology is demonstrated by proven user benefits: Visit us at booth 41 deenova.com 1: Save related to reduce patient stay. Case study Ospedale Papa Giovanni XXIII, Bergamo 2: OPTIMED-ID Feasibility Project: Evaluation of Impact of the System – Updated Report from Extension Period for Data Collection, S Ahlberg Pilfold, P Hourd and N Medcalf (Loughborough University) 3: 2014 JCI Quality Award – Winner Project, ASL Alessandria Experience the potential of Infinite Control in pharmacy automation Cost savings Medication administration errors Reduction up to 0%estimated impact of 2.5 mln € save1 Supply of medications improved significantly2 Range between 15 and 25%3 Pharmacists time 0% -50% 15-25%
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?

POSTER AWARD NOMINEES
ORAL PRESENTATIONS
WEDNESDAY, 22 MARCH 2023 – FROM 10.30 TO 12.00, AUDITORIUM VI
ACPE Non-Accredited Activity
Section 3 - Production and Compounding
10.35 - IMPACT OF AGITATION ON PEMBROLIZUMAB (KEYTRUDA®) SAFETY AND EFFICACY: AGGREGATION AND FUNCTIONALITY - A. Torrente-López
Section 4 - Clinical Pharmacy Services
10.44 - USING MACHINE LEARNING TO PREDICT PHARMACEUTICAL INTERVENTIONS IN A HOSPITAL SETTING - E. Johns
10.53 - BEDSIDE CHECK OF MEDICATION APPROPRIATENESS (BED-CMA) AS A RISK-BASED TOOL FOR BEDSIDE CLINICAL PHARMACY SERVICES: A PROOF-OF-CONCEPT STUDY AT THE TRAUMA SURGERY WARD - G. van de Sijpe
11.02 - EVALUATING THE POTENTIAL CLINICAL AND ECONOMIC IMPACT OF CHEMOTHERAPY PRESCRIBING BY PHARMACISTS AT A UNIVERSITY TEACHING HOSPITAL - S. Nally
11.11 - CONSENSUS VALIDATION OF A SCREENING TOOL FOR CARDIOVASCULAR PHARMACOTHERAPY IN GERIATRIC PATIENTS: THE RASP_CARDIO LIST - J. Hias

11.20 - SEPSIS CODE: IMPROVING OUTCOMES FOR PATIENTS WITH SEPSIS - M.E. Martinez Nuñez
11.29 - AGAMENON-SEOM MODEL FOR THE PREDICTION OF SURVIVAL IN PATIENTS WITH HER2POSITIVE ADVANCED ESOPHAGOGASTRIC ADENOCARCINOMA RECEIVING TRASTUZUMAB-BASED FIRST-LINE TREATMENT - L. Macía-Rivas
Section 5 - Patient Safety and Quality Assurance
11.38 - USING A TEXT-MINING APPROACH TO IDENTIFY THE CONTEXT VARIABLES LANGUAGE BARRIER, LIVING ALONE, COGNITIVE FRAILTY AND NON-ADHERENCE FROM ELECTRONIC HEALTH RECORDS (EHRS) - F. Karapinar
45 Scientific Programme




KEYNOTE PRESENTATIONS
OPENING CEREMONY & KEYNOTE 1
Personalised medicine - opportunities for hospital pharmacists in clinical practice
ACPE UAN: 0475-0000-23-001-L04-P - A knowledge-based activity

Session: Wednesday, 22 March 2023 - from 8.30 to 10.15
Room: Auditorium I
Facilitator: Thomas De Rijdt
Presenter: Raliat Onatade
No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 1 - Introductory Statements and Governance: Statement - 1.1
Section 4 - Clinical Pharmacy: Statements - 4.1, 4.8
ABSTRACT
The EU Health Ministers define personalized medicine as “A medical model using characterization of individuals’ phenotypes and genotypes (e.g. molecular profiling, medical imaging, lifestyle data) for tailoring the right therapeutic strategy for the right person at the right time, and/or to determine the predisposition to disease and/or to deliver timely and targeted prevention” (1).
New technologies are developing to determine the individual patient’s genetic information, often referred to as “omics”, which assist in identifying the risk of developing certain diseases as well as selection effective medication treatments and avoiding medication side effects. This omics information combined with the patient’s age, organ function, comorbidities, existing medication treatment, diet and allergies provide the basis for selecting the best medication treatment for the individual patient. This information should be combined with the patient’s preference of medication treatment.
Indeed, a core part of the hospital pharmacist’s function is to individualise patient treatment based on objective data in collaboration with physicians and patients, hence the concept of personalized medicine provide ample opportunity for the hospital pharmacist in daily practice.

1: https://ec.europa.eu/health/medicinal-products/personalised-medicine_en
LEARNING OBJECTIVES
After the session, the participant should be able to:
• Define the concept of personalised medicine
• Describe the role of hospital pharmacists in personalised medicine
• Discuss current and future tasks for hospital pharmacists in personalised medicine
EDUCATIONAL NEED ADDRESSED
Hospital pharmacists are key placers in decision making regarding the optimal medication treatment of patients. Personalised medicine has in recent years become a hot topic with new opportunities to individualise medication therapy, and therefore it is important for hospital pharmacists to understand and operate within this field.
KEYWORDS
Personalised medicine, pharmacogenetics, individualised medication therapy
47 Scientific Programme FROM DRUG DESIGN TO TREATMENT SUCCESS
WHAT REALLY MATTERS TO PATIENTS?










48 Scientific Programme
KEYNOTE 2
Improving the communication of risks and benefits to patients
ACPE UAN: 0475-0000-23-015-L05-P - A knowledge-based activity

Session: Thursday, 23 March 2023 - from 11.00 to 11.45
Room: Auditorium I
Facilitator: Jonathan Underhill
Presenter: María del Carmen Climént Palmer
No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 1 - Introductory Statements and Governance: Statements - 1.1, 1.2
Section 4 - Clinical Pharmacy: Statements - 4.1, 4.6
Section 5 - Patient Safety and Quality Assurance: Statements - 5.1, 5.9
ABSTRACT
Choosing a treatment, deciding whether or not to have a surgical procedure or interrupting a treatment are all scenarios which can be influenced by how we present risks and benefits to patients.
A clear example is the recent case of the Astra Zeneca COVID vaccination, which was linked to an increased risk of developing thromboembolism. This risk was poorly communicated in many cases, leading some people to fear vaccination. Communicating risks and benefits is not straightforward; it is not simply about presenting the numbers you have to hand. It requires a series of strategies to ensure that these numbers have context, are only as precise as they deserve to be, are balanced, and are understandable.
The Winton Centre for Risk and Evidence Communication at the University of Cambridge, alongside other institutions, has researched closely with clinicians and patients to understand how risks and benefits are currently communicated in diverse health contexts, such as transplants, cancer therapies and vaccination. The Winton Centre’s team has developed strategies and tools to improve these practices to ensure that patients are supported to make shared treatment decisions based on information they can understand, as well as taking into account their own values and preferences and attitude towards risk.
This keynote will address the issues around the conventional ‘persuasive’ approach so often taken in risk communication to ‘maximise compliance’, and the importance of moving towards an informative style which clearly communicates risks and benefits. The keynote will cover crucial strategies that pharmacists can put in practice any time they communicate risks and benefits of treatments to patients. Some of these strategies are: never using just relative risks but adding absolute risks too; considering the format of how numerical information is presented; providing balanced information where consistent framing is essential; giving context to each situation; and, the use of visual aids and decision support tools. These points will be explained through examples on risks around medicines as well as other everyday examples of life. The audience will obtain practical advice for the next time they communicate risks and benefits, whether they talk about pain medicines, antibiotics or chemotherapy.
LEARNING OBJECTIVES
After the session the participant should be able to:
• Recognise the concept of risk in the health context
49 Scientific Programme FROM DRUG DESIGN TO TREATMENT
SUCCESS WHAT REALLY MATTERS TO PATIENTS?
• Distinguish between persuading and informing in communication to patients, and the ethical considerations of each of them
• Identify common mistakes in the media and medical practice when communicating health risks
• Apply strategies to communicate risks and benefits in the most understandable way possible
• Develop a more critical and holistic view of what good risk communication requires beyond the numbers
EDUCATIONAL NEED ADDRESSED
This keynote addresses the strategies needed by pharmacists to communicate risks and benefits of medical treatments in a precise, balanced and understandable way to promote free informed decisions from patients.

KEYWORDS
Clinical decision support, communication, counselling, cultural diversity, education, evidence-based medicine, health literacy, inter-professional collaborative practice, statistics




What to look out for at the congress
Personalised medicine - opportunities for hospital



50 Scientific Programme
4 27th Congress of the EAHP 27th EAHP CONGRESS
Welcome to the 27th Congress of the European Association of Hospital Pharmacists! This year’s 2023 Congress theme will once again endeavour to promote the roles and abilities of the hospital pharmacist, not only by providing advocacy and communication tools, but also engaging with hospital managers and the various departments by putting the spotlight on our adaptive reliability in the face of the unexpected and pulling through: Hospital pharmacists - changing roles in a changing world. This year’s programme offers a wide range of topics covering all aspects of the hospital pharmacists’ day-to-day activities: from disaster management to working smarter, from a more patient-centred approach and avoiding the prescription cascades to thinking global but acting local… and without forgetting research publications and the future of this crucial addition to any hospital by creating awareness to what our department entails. Over the course of the next three days, we will host 3 Keynote speeches, 5 Industry sponsored satellites sessions, Seminars, Interactive Sessions and Workshops, in addition to the Poster Walks, Abstract and Good Practice Initiatives’ Poster Awards (whose Oral presentations will take place today at 10.30 with the Awards handed out during the Closing Ceremony on Friday), the Young Professional Session and various networking opportunities the EAHP Congress offers, such as the Get Together Reception. There is much to look forward to with the EAHP, but if you still fancy a walk down memory-lane, we invite you to visit the History Walk right opposite our booth, in the Exhibition Hall. You are also invited to join us this evening, at 19.00 for our Get Together Reception. Welcome to our Lisbon Congress! Jennie De Greef - EAHP Managing Director
responsible for leading and advising on the strategic development of pharmacy services and medicines optimisation. She’s also Chief Pharmacist for the North Thames Genomic Medicines Service Alliance and therefore an expert speaker on this topic.
Onatade obtained her diploma as a pharmacist from the University of Ife in Nigeria, a masters degree in clinical pharmacy from the London School of Pharmacy, followed by a masters in clinical risk management from University College London. In 2019 Raliat obtained the degree of Doctor in Health from Middlesex University for her work in or to deliver timely and targeted prevention. New technologies are developing to determine the individual patient’s genetic information, often referred to as “omics”, which assist in identifying the risk of developing certain diseases as well as selection effective medication treatments and avoiding medication side effects. This omics information combined with the patient’s age, organ function, comorbidities, existing medication treatment, diet and allergies provide the basis for selecting the best medication treatment for the individual patient. This information should be combined with the patient’s preference of medication treatment. Hospital pharmacists, in collaboration with physicians and patients, are key players in decision making regarding the optimizing the medication treatment of patients, based on objective data. Personalised medicine has in recent years become a hot topic with new opportunities to individualise medication therapy, and therefore it is important for hospital pharmacists to understand and operate within this field. In this lecture we will define the concept of personalised medicine, we will describe the role of hospital pharmacists in this domain and discuss their current and future tasks. Prof. Dr. Thomas De Rijdt EAHP Scientific Committee Chairman EAHP NEWS The European Council for Pharmacy Education Accreditation: a new horizon for continuing pharmacy education The European Association of Hospital Pharmacists (EAHP) and The European Society of Clinical Pharmacy (ESCP) have collaborated to jointly set up The European Council for Pharmacy Education Accreditation (ECPhA). ECPhA will establish a system for accrediting lifelong learning education in pharmacy in Europe and internationally. ECPhA’s goal is to help improve the quality of continuing education in pharmacy practiced in healthcare settings across Europe via accrediting live and online lifelong learning events throughout collaborating with national healthcare professional associations and accrediting bodies. This will be achieved through applying high quality standards in the assessment of available educational programmes, which address the needs and current practice of pharmacists, pharmacy technicians and the pharmacy staff practicing in Europe. ECPhA will closely work with national and international accreditation bodies and international healthcare associations in order to exchange and improve existing practices in the assessment of available European and international educational programmes. It’s important to understand that ECPhA is an accreditation provider so the goal of ECPhA is to present an additional layer to the national accreditation systems and not be a substitute of it. Currently EAHP and most of the pharmacy organisations in Europe depend on the accreditation done by non-European bodies, whose standards might not always meet A European body, such as ECPhA, would ensure that accreditation of European continued education events is aligned with European practice. In addition, ECPhA could also facilitate the attendance of national education events by foreign pharmacists by ensuring the transferability of continued education points between countries. ECPhA’s accreditation is Contact the ECPhA team at info@ecpha.eu to learn more about ECPhA. The team is also looking for reviewers so contact us if you are interested to join. Gonzalo Marzal Lopez - EAHP Project Portfolio Manager AUDITORIUMI Wednesday08.3022March-10:15 photo: Mariona Ribo Wednesday 22 March 2023 The EAHP Welcome to the EAHP Daily Dose! Available hot off the press on Wednesday and Thursday of the Congress, this newspaper will come in handy to plan your day or brush up on some important issues. From a mixture of preview articles of sessions and seminars, to interviews with some of the key people behind the Congress and ‘at a glance’ overviews of the day’s schedule: the EAHP Daily Dose is a must–read for all attendees! Welcome to our 27th Congress! Dear Colleagues from Europe and beyond, It is with great pleasure and with humbling honour that welcome you all in Lisbon, for the 27th EAHP Congress. The whole EAHP team, the Scientific Committee and the Board of Directors are delighted to offer you this exciting event for which all our efforts have been utilised to make this an extraordinary and festive opportunity for all participants. These last few years have brought upon a significant era for the European hospital pharmacy profession, during which our commitment to patient care and medication safety, as well as our dedication towards evidence-based medicine, was put to a major test. I believe that our profession steadily stood up to the challenge and prevailed. Moreover, a multitude of novel roles for hospital pharmacists has been enabled and, therefore, provided further recognition and expanded visibility for all the services our colleagues offer and fulfil. Our moral imperative dictates our devotion toward helping those in need. It is our duty to provide the best possible care for our patients – spanning from the initial steps of drug design to our ultimate goal: treatment success. A success that is not solely defined by medical parameters, but also through the patient’s perceptions and engagement to play an active role in their own journey towards recovery. The scientific programme will encompass this wide horizon and provide an appreciation of these different and distinct, but also overlapping, sub-specialities. It will also be our pleasure to synthesize a comprehensive insight into the employment of evidence-based decisions and evidence-informed policies within reasonable timeframes, especially during times when available resources are limited and often uncertain. I am sure that the Congress programme will effectively enable us all to grasp this rather complex landscape and to take home with us some valid answers. Furthermore, your time with us in Lisbon is also going to be an ideal opportunity to meet your fellow colleagues, exchange experiences and knowledge, and find new professional connections and friends. Welcome to the 27th EAHP Congress! Welcome to Lisbon! Dr. András Süle – EAHP President Information that makes you feel good! Daily dose Engage in the social media debate using the hashtag #EAHP2023 SOCIAL MEDIA photo: Mariona Ribo MAT-GLB-2300117 1.0 01/2023 Beyfortus months of protection against RSV lower respiratory RSV, respiratory syncytial virus. Beyfortus Summary of Product Characteristics. AstraZeneca. Beyfortus is a long-acting antibody designed for all infants,* offering direct protection against RSV lower respiratory tract disease throughout their first RSV season.** With single dose, Beyfortus reduces the risk of medically attended RSV lower respiratory tract infections, including hospitalizations, in infants. The power to reduce the chaos of RSV season Discover Booth 29 or by attending Symposium Thursday March 23 4:30pm Beyfortus is indicated for the prevention of RSV lower respiratory tract disease in neonates and infants during their first RSV season. Beyfortus is contraindicated in infants with hypersensitivity to the active substances or to any of the excipients. medicinal product is subject to additional monitoring. new safety information. Healthcare professionals report adverse reactions. (nirsevimab) – Abbreviated Prescribing Information Beyfortus 50 mg and 100 mg solution pre-filled syringe containing 50 mg of nirsevimab in 0.5 mL (100 mg/mL) 100 mg nirsevimab in mL (100 mg/mL) respectively. Nirsevimab immunoglobulin G1 kappa (IgG1K) monoclonal antibody produced in Chinese (CHO) cells by recombinant DNA technology. Prevention Syncytial Virus (RSV) lower respiratory tract disease in neonates and infants RSV season. Beyfortus should be used in accordance Dosage and administration: The recommended dose is single dose infants with body weight <5 kg or 100 mg for infants ≥5 kg, administered Beyfortus should administered prior to commencement of from birth for born during the RSV season. For infants surgery cardiopulmonary bypass, an additional dose may is stable after surgery to ensure adequate after receiving the first dose of Beyfortus, dose 100 mg according to body weight. more 90 days have first dose, the additional dose could be single dose of 50 mg weight, to cover the remainder of the RSV season. There are no data available on repeat dosing. There are limited data available preterm infants (Gestational Age [GA] <29 weeks) less than weeks data available in infants with postmenstrual age (gestational age chronological age) of less than 32 weeks. Safety and efficacy in children established. Beyfortus for intramuscular injection only, preferably aspect of the Gluteal muscle should not be used routinely sciatic nerve Contraindication: Hypersensitivity to the active of the excipients. Warnings and precautions: traceability of biological medicinal products, record the name and Serious hypersensitivity reactions, including anaphylaxis, have been observed antibodies. signs and symptoms of clinically significant reaction anaphylaxis occur, immediately discontinue administration initiate appropriate medicinal products and/or supportive therapy. any other injections, nirsevimab should be given with caution with any coagulation disorder. can be with childhood vaccines. Nirsevimab should be mixed with same syringe or vial. When administered concomitantly with they should be given with separate syringes and at different Fertility, Pregnancy and Lactation: applicable. reported in clinical trials are uncommon: rash, injection site reaction, all therapeutic proteins, there is potential for immunogenicity. undesirable effects please refer to the Summary of Product Characteristics. care professionals asked to report any suspected adverse reactions reporting Marketing Authorisation Holder: AstraZeneca Södertälje, Legal Classification of the medicinal product medical Prescription Only Medicine. January Abbreviated Information based on the EU SmPC as of October Before product always refer to your full local prescribing information information may vary from country to country. Informação do Produto disponível em: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.ema. europa.eu/en/documents/product-information/beyfortus-epar-productinformation_pt.pdf Stay updated with our Daily Dose magazine. To be released during the congress on Wednesday and Thursday
Dr.
CLOSING CEREMONY & KEYNOTE 3
Patient involvement in pharmacy practice research: no decision about me without me
ACPE UAN: 0475-0000-23-023-L04-P - A knowledge-based activity

Session: Friday, 24 March 2023 - from 11.30 to 13.00
Room: Auditorium I
Facilitator: Raisa Laaksonen
Presenter: Gary Hickey, Kati Turner
No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 1 - Introductory Statements and Governance: Statement - 1.3
Section 4 - Clinical Pharmacy: Statement - 4.8
Section 6 - Education and Research: Statements – 6.4, 6.5
ABSTRACT
“No decision about me without me” - patients are experts in their own health or condition, and they make the decisions about using or not using medicines, or health or pharmacy services. Patients live with their condition 365 days in a year but rarely meet hospital pharmacists and use pharmacy services. Research intended to develop or improve pharmacy services offered to patients should be planned together with patients who are users of these services. Involving patients in planning research provides an opportunity to gain a better understanding of their lived experiences, needs, priorities, and beliefs. Patient involvement may significantly improve the research through their input to the study design, delivery and analysis, and ultimately the uptake of the service.
The National Institute for Health and Care Research has developed UK Standards for Public Involvement in Research. The standards recommend empowering patients and fostering their involvement through offering inclusive opportunities, working together, offering support and learning, sharing information that public involvement makes to research, using well-timed and relevant communications, and involving the public in research governance. How can pharmacists carry out pharmacy practice research with patients and the public? How can pharmacists involve patients and the public in research so that they become partners? How can involving patients in research improve the quality of research? In which parts of the research process could patients be involved to provide ‘best’ outcomes?
LEARNING OBJECTIVES
After the session, the participant should be able to:
• To describe how the lived experience of patients impacts on use of medicines and health services
• To demonstrate why patients should be and how they can be involved in pharmacy practice research to enhance research quality and, ultimately, hospital pharmacy services to patients
EDUCATIONAL NEED ADDRESSED
This session will introduce why and how to involve patients in pharmacy practice research to improve the quality of research and the pharmacy services offered in European hospital pharmacies.
KEYWORDS
Lived experience, patient involvement, clinical pharmacy, patient safety, research
51 Scientific Programme FROM DRUG DESIGN TO TREATMENT
SUCCESS WHAT REALLY MATTERS TO PATIENTS?






52 Scientific Programme Isolators performances for all your applications Design & Production Engineering Research & Development Modelling Assembly Qualifications & Trainings On-site installation IQ & OQ Cycle development User and technical trainings Maintenance & Assistance Preventative maintenances Troubleshooting Hotline Remote maintenance Our specialized ressources EuroBioConcept SAS ZA des Petits Carreaux · 4 Avenue des Coquelicots · 94380 Bonneuil-sur-Marne France Tél. + 33 (0)146 58 75 85 Fax : + 33 (0)146 58 76 46 contact@eurobioconcept.fr · www.eurobioconcept.fr Powders Clinical Trials Cytotoxics Monoclonal Antibodies Total Parenteral Nutrition Sterility Testing Sterile Compounding Visit us — Booth 47 Visit us – Booth 34
SEMINARS
SEMINAR IG1
Hospital support for pharmacy research activities
ACPE UAN: 0475-0000-23-002-L04-P - A knowledge-based activity
Session: Wednesday, 22 March 2023 - from 14.45 to 16.15
Thursday, 23 March 2023 - from 09.00 to 10.30

Room: Room 5A+B
Facilitator: Lene Juel Kjeldsen
Presenter: Isabel Spriet, Daniele Mengato
No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 1 - Introductory Statements and Governance: Statements - 1.3, 1.7
Section 6 - Education and Research: Statements - 6.4, 6.5
ABSTRACT
A symbiosis between hospital pharmacy practice and research should provide synergy in both disciplines with the aim of benefiting patient treatment, but how do we provide a research friendly setting in the hospital pharmacy?
Close collaboration with a university may provide a natural research environment for undergraduate and post graduate students as well as for PhD students and senior researchers. Also, a university hospital may accommodate research within many disciplines, which fertilises multidisciplinary research projects. However, some hospital pharmacies are not affiliated with a university, which may cause difficulties or maybe opportunities when starting and maintaining a research culture.
Core elements in a successful research culture include hospital management support, funding possibilities, networking opportunities and continued research support like access to a library including scientific journals. This session will give examples of how to achieve a successful research environment in a hospital pharmacy setting.
LEARNING OBJECTIVES
After the session, the participant should be able to:
• Recall examples of research projects conducted within the hospital pharmacy setting
• List facilitators of elements in a successful hospital pharmacy research environment
• Discuss the importance of collaboration and networking when conducting research
EDUCATIONAL NEED ADDRESSED
Research within the area of hospital pharmacy is essential for developing better ways of providing our services. However, research has traditionally not been a part of the hospital pharmacy profession, and consequently it is important to ensure the best possible strategies for including research within the hospital pharmacy practice.
KEYWORDS
Research support, hospital management, publication, research collaboration
53 Scientific Programme FROM DRUG DESIGN TO TREATMENT
SUCCESS WHAT REALLY MATTERS TO PATIENTS?
SEMINAR IG2
The road to e-hospital pharmacy - are we there yet?
ACPE UAN: 0475-0000-23-006-L04-P - A knowledge-based activity
Session: Wednesday, 22 March 2023 - from 17.15 to 18.45
Thursday, 23 March 2023 - from 12.00 to 13.30
Room: Room 5A+B
Facilitator: Ana Valladolid Walsh
Presenter: Steffen Amann, José Manuel Martínez-Sesmero
No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 1 - Introductory Statements and Governance: Statement - 1.7
Section 2 - Selection, Procurement and Distribution: Statement - 2.2
Section 5 - Patient Safety and Quality Assurance: Statement - 5.5
ABSTRACT
The provision of healthcare services supported by Information and Communication Technology (ICT) - such as computers, mobile phones, and satellite communications - is known as eHealth. This same definition can be applied to pharmaceutical services provided in hospitals in order to speak about e-hospital pharmacy.
In 2017, EAHP developed the “EAHP Position Paper on eHealth and mHealth”, referring to electronic and mobile health, urging national governments and health systems across Europe to take action towards:
• systematic and EU-wide achievement of electronic prescribing, administration and use of electronic medical records;
• ensuring barcoding of medicines to the single units in primary packages to enable more widespread take up of bedside scanning in European hospitals, thus improving patient safety;
• appropriate regulatory oversight mechanisms for mHealth applications to ensure that they have a positive impact and adequately protect patient data;
• provision of appropriate eHealth/mHealth training opportunities to healthcare professionals and promotion of digital health literacy;
• and involvement of hospital pharmacists in the design, specification of parameters and evaluation of ICT within the medicines processes.
Six years have gone by and much has been achieved, but we are constantly facing innovation and changes in healthcare.
Now the horizon is broadening, and we hear more and more about Digital Health, a term that includes not only eHealth, but also developing areas such as the use of advanced computer sciences in fields such as “big data”, genomics and artificial intelligence. Learning how these advances are being applied in hospital pharmacy and what else is in the horizon will let us know if we are at the end of the road...or just at the beginning.
LEARNING OBJECTIVES
After the session, the participant should be able to:
• Describe advances in e-hospital pharmacy
• Recognise the importance of the role that hospital pharmacists have in this area
• Understand how Digital Health can be applied to hospital pharmacy
EDUCATIONAL NEED ADDRESSED
Due to the rapid advance of eHealth technologies used within the medicines processes, hospital pharmacists are required to keep their competencies updated and receive necessary training.
KEYWORDS
eHealth, e-hospital pharmacy, digital health

54 Scientific Programme
WHAT REALLY MATTERS TO PATIENTS?
SEMINAR IG3
How the pandemic changed hospital pharmacy management - moving forward
ACPE UAN: 0475-0000-23-017-L04-P - A knowledge-based activity

Session: Thursday, 23 March 2023 - from 15.00 to 16.30
Room: Room 5A+B
Facilitator: Inese Sviestina
Presenter: Dzintars Mozgis, Antonio Melo Gouveia
No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 1 - Introductory Statements and Governance: Statements - 1.3, 1.4, 1.5
Section 5 - Patient Safety and Quality Assurance: Statement - 5.1
ABSTRACT
During the last decade healthcare systems have survived different crises: economic, epidemiological (COVID-19 pandemic) and political (war in Ukraine and in other countries), which have influenced the capability of healthcare systems in general and hospital pharmacies in particular.
Traditional approaches, which have focused on identifying system failures, and understanding the causes of incidents, are still important. But such an approach may not be sufficient to assess the situation.
The concept of resilience has been advocated since the last decade as a new way for safety management in different systems including healthcare. It has been described as the ability of either organisational or larger systems and individuals to return to some “normal” state of functioning after a disaster (after the negative consequences of different disruptions?) and being able to function without compromising system performance. This means that both healthcare systems and hospital pharmacies must be flexible or ready to adapt to a new normal situation, where healthcare system or hospital pharmacy functioning is reorganised or enhanced in some way in response to the disruption they face especially because hospital pharmacies are often at the back of the queue of all hospital needs.
What hospital pharmacists have learned from previous crises?
What options are there to continue the development of hospital pharmacies and implement the European Statements of Hospital Pharmacy into daily life?
LEARNING OBJECTIVES
After the session, the participant should be able to:
• Describe the concept of resilience and financing of resilient health systems (is there a trade-off between efficiency and resilience?)
• Discuss how to adapt to new situations that affect hospital pharmacists’ work in the future
• Provide tools for hospital pharmacists to make their case with the hospital management (not be at the end of the line of priorities for resource allocation)
EDUCATIONAL NEED ADDRESSED
Ensuring a resilient health system is a key policy challenge worldwide for the years ahead, which require the sound data and analysis, evidence-based investment decisions, and careful redesign of health systems, one of the core components of which is pharmaceutical care and supply. Hospital pharmacists play a very essential role in patient care and they have to adapt to different situations including various crises. Therefore, it is important to have a broader view on processes and also to step out from the “just pharmacy” viewpoint.
KEYWORDS
Hospital pharmacist, patient care, pandemic, crises, resilience
55 Scientific Programme
FROM DRUG DESIGN TO TREATMENT SUCCESS
Comprehensive Solutions for Sterile Compounding & IV Infusion




Automated IV Compounding Systems (TPN / ONCO / CIVA)

Fluid Dispensing Pump
Pharmacy Operation & Order Entry Software (CPOE)
IV Workflow Management System (IV-WMS)
IV Compounding & Infusion Disposables

Modular Cleanrooms & Accessories


Biosafety & LAF Cabinets


www.kapsamsaglik.com.tr


56 Scientific Programme
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
Medicines procurement - don’t forget patients’ needs!
ACPE UAN: 0475-0000-23-003-L04-P - A knowledge-based activity
Wednesday, 22 March 2023 - from 14.45 to 16.15

Francesca Venturini
Dorte Glintborg, Erminia Caccese
No conflict of interest declared. Please reference speakers’ biographies.
Selection, Procurement and Distribution: Statements - 2.1, 2.4
The immediate concern of any hospital pharmacist is to ensure that every patient within the hospital receives the medications they need. For this reason, hospital pharmacists have a direct stake in the efficient functioning of the medicines supply chain. Pharmaceutical tendering is a complex process that involves different stakeholders and steps that are regulated at national level leading to diverse solutions in the different European countries.
Differently from medical devices, usually public tenders for medicines relies mainly on drug price and not on quality characteristics of the product, or on any supplemental service offered by the applicants. Some examples could be a more practical packaging, ease of mode of conservation, written patient information, patient time spend on hospital, etc. With the advent of telemedicine, the offer could take into account additional services, i.e. home delivery, home
In all this process the voice of the patient is fundamental, but barely taken into account. An approach to consider patients values and preferences could be through direct involvement of patients in development of guidelines and evaluation of new drugs, which lead to procurement.
LEARNING OBJECTIVES
After the session, the participant should be able to:
• Identify the methodology of medicine tendering, the different approaches and the price/quality option
• Identify the patient preferences in medicine characteristics and/or additional services that could influence the drug procurement process

EDUCATIONAL NEED ADDRESSED
Hospital pharmacists may have to deal with drug tendering process that are inefficient in taking into account patient preferences. This seminar will show attendees the different approaches to drug tendering and give examples of elements to consider in order to value patient preferences.
KEYWORDS
Medicine procurement, patient preferences, patient reported outcomes (PRO)

57 Scientific Programme
SEMINAR SPD2
The shortage pandemic - why we haven’t succeeded yet ACPE UAN: 0475-0000-23-012-L04-P - A knowledge-based activity
Session: Thursday, 23 March 2023 - from 09.00 to 10.30

Room: Auditorium VI
Facilitator: Gunar Stemer
Presenter: Max Newton*, Brian Smith*, Joao Ferreira
No conflict of interest declared. Please reference speakers’ biographies. *Conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 2 - Selection, Procurement and Distribution: Statements - 2.2, 2.5, 2.6
ABSTRACT
After many years the occurrence of medicines shortages still constitutes a steady challenge for hospital pharmacists in terms of various shortages’ management aspects. For example, when it comes to suggesting and sourcing therapeutic alternatives and accompanying the transition of medicines in various setting with expertise, information and education. If applied mitigation strategies fail, this challenge expands and escalates to other healthcare professionals and patients. The availability of therapeutically equivalent therapeutic options is considered a best-case scenario, whereas lack of adequate alternatives and resulting consequences (e.g. therapeutic delays) can be seen as a worst-case scenario.
In the past years, several different initiatives and projects, involving different stakeholders and other parties, were established, investigating causes and developing counteracting measures. Research on medicines shortages is conducted, as illustrated by many publications on various management aspects. On a political level, the problem of shortages is accepted as an essential and important one.
In 2020, the COVID-19 pandemic dramatically proved how fragile and susceptible supply chains of medicines still are and how quickly apparently stable situations can aggravate. After all achievements in the context of medicines shortages’ management prior to COVID-19, enormous efforts are still needed to counteract shortages and stabilise drug supply sustainably. Is it realistic to consider shortages an unsolvable problem? Did shortages come to stay?
LEARNING OBJECTIVES
After the session, the participant should be able to:
• Repeat key facts about current epidemiology and aetiology of medicines shortages
• Recall up-to-date and actual initiatives on an European and global level to address shortages and discuss various strategies against it
• State key learnings from the COVID-19 pandemic in the context of shortages management, both on a practice and political level
EDUCATIONAL NEED ADDRESSED
Hospital pharmacists are responsible for providing the right medicines to patients and therefore essentially rely on trusted availability of medicines. Extensive state-of-the-art knowledge on medicines shortages and all relating activities is key to fulfil this core responsibility of hospital pharmacy.
KEYWORDS
Medicines shortages, pandemic, counteracting strategies
58 Scientific Programme
SEMINAR SPD3
Green Hospital - The Role of Hospital Pharmacists
ACPE UAN: 0475-0000-23-007-L04-P - A knowledge-based activity
Session: Wednesday, 22 March 2023 - from 17.15 to 18.45

Thursday, 23 March 2023 - from 15.00 to 16.30
Room: Auditorium VI
Facilitator: Torsten Hoppe-Tichy
Presenter: Anna Harjans, Julian Baehr
No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 2 - Selection, Procurement and Distribution: Statements - 2.1, 2.2, 2.3, 2.4
Section 6 - Education and Research: Statement - 6.4
ABSTRACT
We all know about the benefits medicines can provide to our patients. This benefit is the highest priority in any decision we make around purchase and supply of medicines. Availability of the drug is the key for any drug treatment. Other priorities in the decision to put a drug in the formulary have always been safety issues and pharmacoeconomical reasons. But we are not living on an island and can just focus on the outcome of our patients alone. Climate change is a fact and we had to learn that health systems and so also hospitals have a great impact on climate change in producing a carbon footprint which is big. Discussions about carbon footprints are still controversial. It is always some kind of “what can we do if others do nothing or do worse“. We have to overcome those points and just take action in our own field. To be able to act we have to know the facts around the carbon footprint of hospitals, hospital pharmacies, medicines, to name a few. When we put the carbon footprint issue in our basis for decision-making the first step into a green medicines supply chain management is done. Another point where practice can influence the carbon footprint of hospitals is just pure logistic. This does not only focus on the inhospital supply chains but also the ordering process of hospital pharmacies at pharmaceutical industry and the outhospital supply chains and logistics. The speakers will give an overview about the impacts of different contributions to the carbon footprint of health systems and how we could include those in our decision-making process with the goal to positively influence the impact of hospitals to climate change. They will also show the role hospital pharmacist can take in this process and how to develop green practices around medicines.
LEARNING OBJECTIVES
After the session, the participant should be able to:
• List the different aspects of carbon footprints of health systems, including hospitals and hospital pharmacies
• Discuss the different measures to positively influence the impact of health systems to climate change
• Discuss possible measures regarding the supply chain of medicines
EDUCATIONAL NEED ADDRESSED
Climate change is real. Hospital pharmacists can have an impact on climate change. They should be able to discuss the impact of supply chain management, formulary decisions and the possibilities to influence the carbon footprint of hospitals.
KEYWORDS
Climate change, green house gas, carbon footprint, sustainability, medicines procurement, medicines supply chain, formulary decision-making
59 Scientific Programme FROM DRUG DESIGN TO
TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
THE MOST ADVANCED SOLUTION FOR LOGISTICS AND MEDICATION MANAGEMENT
CONNECTS EASILY TO OTHER HOSPITAL APPLICATIONS
AUTOMATES DISPENSING PROCESS WITH ROBOTIZED CARTS AND CABINETS

Allow healthcare operators to focus on the care of their patients
Maintain End to End traceability of the overall medication process
Deliver visibility and insights to your staff to make more informed, faster and accurate decisions
MAINTAINS END TO END TRACEABILITY OF DRUGS AND MEDICAL DEVICES
100% integration with your IT infrastructure

OPTIMIZES INVENTORY WITH AI FOR INTELLIGENT PLANNING
Eliminate dispensing errors, diversions, inventory stockout and waste


Create a fully automated and efficient environment
Ensure patient safety, providing the right dose, to the right patient, at the right time
Scientific Programme
SCAN HERE and discover how to support your healthcare organization with fully automated solutions www.antaresvisiongroup.com
Free your staff time and reduce operating costs
MEET US AT BOOTH 47
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
SEMINAR PC1
Advanced therapy medicinal products (ATMPs) - challenging opportunities for hospital pharmacy
ACPE UAN: 0475-0000-23-008-L04-P - A knowledge-based activity
Session:
Wednesday, 22 March 2023 - from 17.15 to 18.45
Thursday, 23 March 2023 - from 09.00 to 10.30

Room: Auditorium VII
Facilitator: Juraj Sykora
Presenter: Anne Black*, Carla Alonso-Martinez
*Conflict of interest declared. Please reference speakers’ biographies. No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 1 - Introductory Statements and Governance: Statement - 1.1
Section 3 - Production and Compounding: Statements - 3.3, 3.4
Section 5 - Patient Safety and Quality Assurance: Statements - 5.2, 5.4, 5.6
Section 6 - Education and Research: Statement - 6.4
ABSTRACT
With the release of Regulation (EC) No 1394/2007, a new framework for gene and cell therapy medicinal products and tissue-engineered products was established in the European Union. Advanced therapy medicinal products (ATMPs) are a dynamic and current topic for healthcare systems, with new products progressing to market at an increasing rate.
Advanced therapy medicinal products (ATMPs) are medicines for human use that are based on genes, tissues or cells. They offer groundbreaking new opportunities for the treatment of disease and injury. ATMPs can be classified into three main types:
• gene therapy medicines: these contain genes that lead to a therapeutic, prophylactic or diagnostic effect. They work by inserting ‘recombinant’ genes into the body, usually to treat a variety of diseases, including genetic disorders, cancer or long-term diseases. A recombinant gene is a stretch of DNA that is created in the laboratory, bringing together DNA from different sources;
• somatic-cell therapy medicines: these contain cells or tissues that have been manipulated to change their biological characteristics or cells or tissues not intended to be used for the same essential functions in the body. They can be used to cure, diagnose or prevent diseases;
• tissue-engineered medicines: these contain cells or tissues that have been modified so they can be used to repair, regenerate or replace human tissue.
In addition, some ATMPs may contain one or more medical devices as an integral part of the medicine, which are referred to as combined ATMPs. An example of this is cells embedded in a biodegradable matrix or scaffold.
ATMPs are medicines and so by definition, they fall under the responsibility of the hospital pharmacist. The hospital pharmacist should therefore be involved in procurement, production in the hospital, reconstitution, quality
61 Scientific Programme
control and logistics. The European Association of Hospital Pharmacist (EAHP) requires in its Position Paper on Pharmacy Preparations and Compounding that the management of ATMPs, as licensed medications, remains the responsibility of the hospital pharmacist. Pharmacists play an important role in the safe implementation of ATMPs. Wherever possible pharmacist advocate that medicines are issued to the clinical area in a ready-toadminister presentation. Some ATMPs arrive ready to administer but some require a preparation step to make them ready to administer to patients. The seminar will discuss the optimal location in which any preparation should be undertaken. It will discuss that this may vary depending on the type of ATMP and will ask whether pharmacy training is currently sufficient to safely handle cell or tissue-based products. Where preparation occurs in areas other than pharmacy aseptic suites it will discuss the oversight role of pharmacists who ensures, from a governance perspective, that the medicine is being handled in line with the Summary of Medicinal Product Characteristics or the Clinical Trial Protocol.
LEARNING OBJECTIVES
After the session, the participant should be able to:
• Explain the therapeutic, prophylactic and diagnostic effect of ATMPs
• Describe Regulatory Framework for ATMPs in Europe
• Apply the pharmacy-related aspects to implementation of ATMPs for use in clinical practice
EDUCATIONAL NEED ADDRESSED
To learn and understand the history, regulatory aspects, current clinical evidence, therapeutic, prophylactic and diagnostic indications, safety aspects of ATMPs and the role of the pharmacist in safe implementation of ATMPs.
KEYWORDS
Advanced therapy medicinal products (ATMPs), regulatory aspects, efficacy, safety, implementation, hospital pharmacy

62 Scientific Programme
WHAT REALLY MATTERS TO PATIENTS?
SEMINAR PC2
Quality assurance of pharmacy preparations - a key for treatment success
ACPE UAN: 0475-0000-23-004-L04-P - A knowledge-based activity
Session: Wednesday, 22 March 2023 - from 14.45 to 16.15
Thursday, 23 March 2023 - from 12.00 to 13.30
Room: Auditorium VII
Facilitator: Armando Alcobia
Presenter: Helena Baião, Frederic Lagarce
No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 1 - Introductory Statements and Governance: Statement - 1.2
Section 3 - Production and Compounding: Statements - 3.2, 3.3, 3.4, 3.5, 3.6
Section 5 - Patient Safety and Quality Assurance: Statements - 5.1, 5.3, 5.9, 5.11
ABSTRACT
The market for pharmaceutical products has been successively regulated as a result of several incidents that have caused hundreds of deaths and malformations. All the guidelines successively created about raw materials, drugs, medical devices, constitute one of the most complex and robust regulatory networks aimed at obtaining, safe and effective pharmaceutical products.
Good Manufacturing Practice (GMP) describes the minimum standard that a medicines manufacturer must meet in their production processes, and can be adapted to the hospital reality through the Guide to Good Practices for the Preparation of Medicinal Products in Healthcare Establishments - from Pharmaceutical Inspection Convention Pharmaceutical Inspection Co-Operation Scheme (PIC/S). Many parameters need to be mastered and monitored to ensure the quality of the products. In fact, the direct environment of the preparations should be controlled (quality of air, chemical and microbiological contaminations) and the skills of the technicians should be assessed. The European Medicines Agency (EMA) coordinates inspections to verify compliance with these standards and plays a key role in harmonising GMP activities at European Union (EU) level.
The available drugs for use in European hospitals have evolved in number and complexity and it is anticipated in the near future that new delivery systems (e.g. inactivated virus, microchips, lipid-based nanoparticles, etc.) may be used in our patients’ treatments. On the other hand, numerous medicines considered fundamental or very important for the treatment of multiple diseases are subject to increasingly frequent shortage or out-of-stock situations.
The hospital pharmacist is responsible for the quality of drug products and medical devices used in the wards, so quality assurance systems must keep up with these new realities, ensuring the ultimate goal to patients’ drug treatments: to exist, to be effective and safe.
LEARNING OBJECTIVES
After the session, the participant should be able to:
• Understand quality assurance evolution
• Recognise inspective pathways under hospital pharmacy scope
• Implement quality control systems adapted to daily operating practice in hospital pharmacies
EDUCATIONAL NEED ADDRESSED
New challenges are being posed to hospital pharmacists regarding the use of new classes of drugs or the need to replace the pharmaceutical industry in the preparation or adaptation of drugs. The need to ensure compliance with quality assurance criteria should be assumed as the key factor for the success of available treatments.
KEYWORDS
Quality Assurance (QA), Good Manufacturing Practices (GMP), Pharmaceutical Inspection Co-Operation Scheme (PIC/S)

63 Scientific Programme
FROM DRUG DESIGN TO TREATMENT SUCCESS
SO ARE YOUR NEEDS, SO IS




SEMINAR CPS1
Pharmacogenetic testing to optimise therapy
ACPE UAN: 0475-0000-23-009-L04-P - A knowledge-based activity
Session: Wednesday, 22 March 2023 - from 17.15 to 18.45

Thursday, 23 March 2023 - from 12.00 to 13.30
Room: Auditorium VIII
Facilitator: Kornélia Chrapkova
Presenter: Markus Lampert, Jesse J. Swen
No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 4 - Clinical Pharmacy: Statement - 4.1
Section 5 - Patient Safety and Quality Assurance: Statements - 5.1, 5.6
ABSTRACT
Over the past decade it has become increasingly apparent that genetically controlled variations in drug disposition and response are important factors for drug efficacy and safety. Testing patients for pharmacogenomic variants allows healthcare providers to provide their patients with a more personalised drug therapy and thus achieving the optimal therapeutic response, avoiding therapeutic failure and minimising side effects and toxicity.
Two broad approaches of pharmacogenetic testing can be taken: a point of care testing in which genotyping for specific variants is undertaken at the time of drug prescription, and a pre-emptive approach in which pharmacogenetic testing is performed for multiple variants that are thought to affect drug response. The information is then archived for later use when a target drug is prescribed. For drugs with known pharmacogenetic variations guidelines have been developed in order to help clinicians understand how available genetic test results could be used to optimise drug therapy.
Despite clinical and scientific advances of pharmacogenetic testing, its application into routine clinical practice remains limited. Potential barriers that need to be overcome may include cost-effectiveness of the testing, ethical concerns over the use of DNA and lack of education.
During this seminar we will address the current state of implementation of pharmacogenetic testing, the rationale for both approaches, impact on the cost-effectiveness and obstacles that must be overcome.
LEARNING OBJECTIVES
After the session, the participant should be able to:
• Define the benefits of pharmacogenetic testing
• Define the factors which influence the potential effectiveness of pharmacogenomic testing
• List the barriers for implementation of pharmacogenetic testing into clinical care
EDUCATIONAL NEED ADDRESSED
Hospital pharmacist knowledge and ability to understand and interpret genomic tests is important for optimisation of patient´s therapy.
KEYWORDS
Adverse drug reactions, pharmacogenomics, pharmacogenetic testing, personalisation of therapy
65 Scientific Programme
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
SEMINAR CPS2
From Benefit to Burden - safely discontinuing medicines at the end of life
ACPE UAN: 0475-0000-23-016-L04-P - A knowledge-based activity
Session: Thursday, 23 March 2023 - from 12.00 to 13.30
Room: Auditorium VI
Session: Friday, 24 March 2023 - from 09.00 to 10.30

Room: Room 5A+B
Facilitator: Jonathan Underhill
Presenter: Jo Hayes, Lelly Oboh
No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 1 - Introductory Statements and Governance: Statements - 1.1, 1.3, 1.4
Section 4 - Clinical Pharmacy: Statements - 4.1, 4.3, 4.5, 4.6, 4.7
Section 5 - Patient Safety and Quality Assurance: Statements - 5.1, 5.6
ABSTRACT
The burden of polypharmacy can represent an increased risk of adverse events, which can be particularly problematic at or near the end of life. Together with multimorbidity, the use of multiple medicines is common in palliative care. As conditions progress and the clinical context changes, the risk benefit ratio for medicines changes with some medicines moving from benefit to burden. When there is no or a limited evidence base for stopping medicines, a pragmatic and safe approach is needed. There is a need to explore the clinical reasoning that occurs, the barriers to deprescribing and conversations that take place.
OVERVIEW
A facilitated, interactive, case-based workshop which uses clinical case studies to explore medication review in palliative care. The cases will allow for brief discussions regarding the therapeutics at play. The session will also allow for and include brief discussion around decision making and communication in this complex area of healthcare, focusing on how to have meaningful conversations with patients, careers and healthcare professionals. The session will discuss how prescribers, faced with a changing clinical context, can become more confident in deprescribing medicines.
LEARNING OBJECTIVES
• Improve understanding of the problems of polypharmacy in palliative care
• Identify the principles of deprescribing for palliative care patients
• Learn to recognise and deal with specific circumstances for deprescribing in palliative care patients (e.g. cardiovascular medicines, anticoagulants, diabetic medicines and certain primary prevention medicines)
The seminar will also seek to explore the following:
• An increased confidence in dealing with medicines at the end of life
• Understand the clinical reasoning of a palliative medicine specialist and how shared decision making is employed at the bedside
66 Scientific Programme
FROM DRUG DESIGN TO TREATMENT SUCCESS
WHAT REALLY MATTERS TO PATIENTS?
• Improve your situational awareness and understanding what’s going on
• Identify tools to support deprescribing in an end-of-life population
• Better conversations for end of life care - identify scripts and phrases to improve conversations with patients and carers at the end of life
• How to learn and react, when it doesn’t go as expected
EDUCATIONAL NEED ADDRESSED
This session will address the value of having meaningful conversations with patients, careers and healthcare professionals and how this can enable pharmacists to become more confident in reviewing medicines and the end of life, and stopping them where appropriate and consensual.
KEYWORDS

Aging, communication, dementia, end of life, ethics, evidence-based eedicine, geriatrics, medication therapy management, pain management, pharmaceutical care, polypharmacy




67 Scientific Programme
and
eahpofficial eahpofficial Welcome to the 27th EAHP Congress in Lisbon, Portugal! #EAHP2023 #HPCARE4U Liked by You and 13 269 others See 923 comments Lisbon, Portugal
Visit EAHP at booth 57
impress your friends with the only “real” social picture!
44 COME AND VISIT US AT BOOTH
We serve all European countries and hospitals from our 4 hubs in
• Switzerland
• Denmark

• Lithuania
• Portugal
www.farmamondo.com YOUR TRUSTED PARTNER GLOBALLY SUPPLYING UNLICENSED MEDICINES
WHAT REALLY MATTERS TO PATIENTS?
SEMINAR PSQ1
Become a medication safety pharmacist!
ACPE UAN: 0475-0000-23-018-L05-P - A knowledge-based activity

Session: Thursday, 23 March 2023 - from 15.00 to 16.30
Friday, 24 March 2023 - from 09.00 to 10.30

Room: Auditorium VII
Facilitator: Raisa Laaksonen
Presenter: Terhi Toivo, Niamh O’Hanlon
No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 4 - Clinical Pharmacy: Statement - 4.8
Section 5 - Patient Safety and Quality Assurance: Statements - 5.2, 5.3, 5.5
ABSTRACT
Medication errors, such as administering a wrong medicine to a patient, are common worldwide. Tackling this problem requires seeking for systems based risk management approaches in healthcare. Coordinating and promoting medication safety is an important part of a medication safety pharmacist’s role. What do these medication safety pharmacists do and how have they developed their roles or how have their roles evolved? How do they work multi-professionally to develop patient and medication safety? What kind of medication safety initiatives have they developed and how have these initiatives been implemented? How do these medication safety pharmacists support each other and develop national or international networks? In this session, two pharmacists working within medication safety discuss their experiences.
In Finland, the second medication safety officer (MSO) working at a hospital, providing tertiary care services to nearly one million people, started in 2020. Before starting, the hospital’s Safe Medication Working Group and a survey to stakeholders had been used to identify the main and potential tasks for the MSO. Developing safe medication practices, and related guidelines, were prioritised. Networking and cooperation with healthcare professionals (e.g. doctors, nurses, pharmacists) plays a big role in getting started and to gain an insight into the current medication safety practices. To develop medication safety requires fostering and supporting change in practices: the support and commitment of the hospital management, the chief physician and the chief nurse is crucial.
The Irish Medication Safety Network (IMSN), a voluntary, independent group of hospital pharmacists from around 30 public and private hospitals, with an interest in medication safety, was established in 2007. The network’s principal aim is to improve patient safety with regard to the use of medicines. The network promotes the exchange of information on medication safety and facilitates national and global initiatives to help minimise risks to patients.
LEARNING OBJECTIVES
After the session, the participant should be able to:
• To demonstrate how to develop a job description for, and how to develop as, a medication safety pharmacist in hospital settings
• To identify medication safety initiatives, including national and international networks, that could be developed in the participants’ own working environments
• To describe how to design a medication safety programme in a hospital and how to address risks with the medication process (e.g. systems review and high risk medicines)
EDUCATIONAL NEED ADDRESSED
This session will introduce how to develop a job description for a medication safety pharmacist in hospitals, how to develop as a medication safety pharmacist and how to develop medication safety initiatives, including national and international networks, that could be implemented in European hospitals.
KEYWORDS
Patient safety, medication safety, risk management
69 Scientific Programme
FROM DRUG DESIGN TO TREATMENT SUCCESS




70
SEMINAR PSQ2
Lay involvement in prescribing committees - hearing the patient’s voice
ACPE UAN: 0475-0000-23-013-L04-P - A knowledge-based activity
Session: Thursday, 23 March 2023 - from 09.00 to 10.30

Room: Auditorium VIII
Facilitator: Jonathan Underhill
Presenter: Corinna Schaefer, Carole Pitkeathley
No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS

Section 1 - Introductory Statements and Governance: Statements - 1.1, 1.6
Section 2 - Selection, Procurement and Distribution: Statements - 2.3, 2.7
Section 4 - Clinical Pharmacy: Statements - 4.1, 4.6
Section 5 - Patient Safety and Quality Assurance: Statement - 5.1
ABSTRACT
There is much value and many benefits of having meaningful lay (patient) involvement in decision-making bodies about medicines. However, the intrinsic benefit of the lay perspective is its ability to ensure decisions are anchored in real life.
There are many ways to gather information about patient and public views to inform decision making on prescribing committees, health technology assessment or guideline development. These include using research findings and involving people through various methods of engagement to incorporate advice and feedback. In September 2021, the Guidelines International Network (GIN) Public Working Group launched an updated methodological toolkit with practical guidance and best practice examples to support organisations to encourage meaningful lay involvement. This session will showcase the value of lay people as members of these committees and, through case studies, will illustrate the significant advantages of meaningful lay participation beyond the usual guideline development arena.
LEARNING OBJECTIVES
• To demonstrate the intrinsic value and positive impacts of having patient and public involvement in decision making about medicines
• To present international case studies that highlight the key factors of successful patient and public involvement showing how different methods have been used successfully
• To signpost to a range of methods to involve the lay perspective
EDUCATIONAL NEED ADDRESSED
• Recognise the value of having meaningful lay involvement in prescribing committees
• Identify the principles of recruiting lay members and incorporating patients’ views
KEYWORDS
Cultural diversity, evidence-based medicine, health literacy, inter-professional collaborative practice, National Guidelines
71 Scientific Programme
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?





72 SOLUTIONS FOR HEALTHCARE Designing complete solutions for automated and safe drug management STAND N.22 Sinteco a Bucci Automations S.p.A. Division Zona Industriale Villanova, 32013 Longarone - Italy Phone: +39 0437 772146 | sinteco.it@bucci-industries.com healthcare.sintecorobotics.com The robotic system from logistics to patient SINTECO • BUCCI INDUSTRIES AUTOMATION FOR DRUG MANAGEMENT
SEMINAR ER1
Patients’ individuality: challenges facing hospital pharmacists


ACPE UAN: 0475-0000-23-019-L04-P - A knowledge-based activity

Session: Thursday, 23 March 2023 - from 15.00 to 16.30
Friday, 24 March 2023 - from 09.00 to 10.30
Room: Auditorium VIII
Facilitator: Branislava Miljković
Presenter: Katarina Vučićević, Nynke Jager
No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 4 - Clinical Pharmacy: Statements - 4.1, 4.3
Section 6 - Education and Research: Statements - 6.1, 6.2, 6.3
ABSTRACT
Patients respond differently to medications due to many reasons, which can either be intrinsic (age, race, weight, metabolic capacity, genetics) or extrinsic (co-medications, co-morbidities, etc.). These variations may be of pharmacokinetic or pharmacodynamic nature, implying that one dose does not suit everyone. To stratify dosing regimens for each individual patient, addressing the variability is essential. Interindividual differences in drug’s pharmacokinetics are easily observed, well documented and dosing regimens adjustments are required for clinically relevant variations. They imply that patients receiving the same dose of drug have different drug concentrations and/or exposures.
Since drug concentrations at target site are important for the response, relationship between drug’s pharmacokinetic and pharmacodynamic profile guide appropriate dosing regimen. Deviations in drug behaviour are observed between patients and healthy volunteers, but also among target patient population (such as age, disease, or other covariate-defined groups). However, in individual patients we may observe multiple factors influencing drug behaviour, and optimising dosing regimen requires the knowledge of the magnitude of the composite (combination of all factors) variability in individual patients.
Advanced computational methodologies have been developed to study and address the variations in drugs response, as well as to optimise dosing regimen to the patients’ needs. Since, clinical pharmacy services of the hospital pharmacists are oriented towards therapeutic decision-making processes, upgrading the knowledge of advanced tools of the covariates-based dosing is important aspect of continuing pharmacists’ training.
LEARNING OBJECTIVES
After the session, the participant should be able to:
• Recognise the reasons patient’s response differently to the same drug dosing regimen
• Understand the principles and tools used for drug dosing individualisation
EDUCATIONAL NEED ADDRESSED
Achieving optimal drug response in each individual patient requires education and training of hospital pharmacists regarding the sources of variability.
KEYWORDS
Variability, individualisation, computational tools
73 Scientific Programme
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?






74 Scientific Programme
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
SEMINAR ER2
Clinical trials - getting actively involved
ACPE UAN: 0475-0000-23-021-L04-P - A knowledge-based activity
Session: Friday, 24 March 2023 - from 09.00 to 10.30
Room: Auditorium VI
Facilitator: Stephanie Deuster
Presenter: Matthias Briel, Katja Suter
No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 1 - Introductory Statements and Governance: Statement - 1.5
Section 2 - Selection, Procurement and Distribution: Statement - 2.6
Section 3 - Production and Compounding: Statement - 3.2
Section 6 - Education and Research: Statement - 6.5
ABSTRACT
Many hospital pharmacists are involved in clinical trials with medicinal products in their hospital. If a pharmaceutical company is the sponsor of the trial, the role of the hospital pharmacist covers mostly the logistics, storage and reconstitution of the investigational medicinal product (IMP). These basic routines, if performed in clinical trials, come with additional tasks regarding the drug accountability, the traceability, or the blinding of the IMP.
If hospital pharmacists are involved early in the planning of academic clinical trials, their contribution goes far beyond the above-mentioned tasks. Some clinical questions require blinded administration of the drugs being evaluated to ensure an unbiased assessment of effect. Although this fact is recognized by experts in clinical trial methodology, the availability of blinded drugs can be difficult and may require some trade-offs, or may even result in the discontinuation of research projects.
Particularly in the academic setting, pharmacists can make important contributions in developing IMPs tailored to the research question and in determining the appropriate strategy for blinding and emergency unblinding. As specialists for the production of medicinal products, hospital pharmacists also compile the IMP-related documentation for the submission to the competent authorities.
This seminar will provide the basic knowledge to perform these additional tasks and give examples of successful interprofessional collaboration. Besides, it will give insight into the design and realisation of investigator initiated clinical trials (IICTs) in the hospital setting and the challenges investigators and pharmacists may face during planning and conducting clinical trials.
LEARNING OBJECTIVES
After the session, the participant should be able to:
• Evaluate a clinical research project in terms of pharmaceutical requirements for the development and production of investigational medicinal products (intervention, comparator) in open or blinded manner
• Outline the key factors to be considered in organising the drug-related processes within a clinical trial
• Assess the different tasks and interprofessional interfaces in planning and conducting clinical trials (from the perspective of hospital pharmacists)
EDUCATIONAL NEED ADDRESSED
Hospital pharmacists need to know details regarding the design of investigator initiated trials with medicinal product and potential difficulties during the conduct of a clinical study in order to support the investigator with organising the medication related tasks.
KEYWORDS
Clinical trial, randomisation, blinding, investigational medicinal product, manufacture

75 Scientific Programme
American College of Clinical Pharmacy (ACCP)

Founded in 1979, the American College of Clinical Pharmacy (ACCP) is the leading clinical pharmacy organization in the world, now serving about 17,000 members. ACCP membership is composed of practitioners, the majority of whom are residency-trained, board certified clinical pharmacists; faculty; researchers; academic department chairs; deans; pharmacy directors; residency program directors; clinical managers; as well as pharmacy students, residents, and fellows in training.
ACCP provides international services as part of its mission “to advance human health by extending the frontiers of clinical pharmacy” around the globe.
International services include:
• Live programs for preceptors and practitioners seeking continuing professional development or specialty certification
• Clinical services development in all practice settings
• Professional degree program development
• Access to Pharm.D. degree and training programs

• Recruitment of qualified U.S. clinical faculty and clinical pharmacists for full-time positions outside the United States
• Organizational strategic planning
• Recognition as an ACCP-Approved Clinical Pharmacy Center/Center of Excellence
For more information, visit us in the exhibition hall, stand 51. You can also contact us at accp@accp.com.
Web Site: https://www.accp.com
76 Scientific Programme
WHAT REALLY MATTERS TO PATIENTS?
WORKSHOPS WORKSHOP 1
Is compounding (always) the answer to drug shortages?
ACPE UAN: 0475-0000-23-014-L07-P - An application-based activity

Session: Thursday, 23 March 2023 - from 09.00 to 10.30
Room: Room 5C
Facilitator: Thomas De Rijdt
Presenter: Kirsten Schimmel, Rui Pedro Marques
No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 1 - Introductory Statements and Governance: Statement - 1.3
Section 2 - Selection, Procurement and Distribution: Statement - 2.5
Section 3 - Production and Compounding: Statements - 3.1, 3.2, 3.3, 3.4
ABSTRACT
Since years drug shortages are the new reality in the pharmaceutical sector and will not disappear from the scene again. They may have their origin in increased demand, falling prices, lack of raw materials, logistical problems, administrative formalities, closed borders or even a pandemic. And they always cost hospital pharmacies hands full of money and personnel to manage. But even with extra staffing, it is not always possible to buy or to import a valid alternative to guarantee the continuity of therapy and pharmaceutical care.
Sometimes a registered drug is available on the market but at such a high price the healthcare payer cannot afford it, resulting in a practical shortage.
Fortunately, hospital pharmacists were trained in the art of compounding and can offer a way out of long term shortages of critical products. But is everything possible or are there limits to this solution?
In this workshop we will present different cases to discuss all possible hurdles and learn from best practices. Availability of raw materials, technical feasibility, operator safety, quality control, economic aspects, scalability and different legal requirements per country have impact on the equation.
LEARNING OBJECTIVES
After the session, the participant should be able to:
• To identify the possibilities and limitations of compounding as a solution to shortages
• To assess individual cases for feasibility
• To express the differences in European countries regarding hurdles and possibilities
EDUCATIONAL NEED ADDRESSED
Shortages are a new reality which hospitals have to deal with. Hospital pharmacists must be aware of the possibilities and limitations of compounding in order to resolve these shortages.
KEYWORDS
Drug manufacturing, pharmaceutical care, USP chapter 795, USP chapter 797, shortages, compounding
77 Scientific Programme
DRUG DESIGN TO
FROM
TREATMENT SUCCESS

WORKSHOP 2
Patient reported outcome measures - what tools can be used?
ACPE UAN: 0475-0000-23-010-L04-P - An application-based activity

Session: Wednesday, 22 March 2023 - from 17.15 to 18.45
Thursday, 23 March 2023 - from 12.00 to 13.30
Room: Room 5C
Facilitator: Ulrika Gillespie
Presenter: Trine Graabæk
No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 4 - Clinical Pharmacy: Statement - 4.1
Section 6 - Education and Research: Statement - 6.4
ABSTRACT
To assess the effects of clinical pharmacy interventions, in clinical practice or research projects, various outcome measures are being used. Examples of outcome measures include everything from “soft measures” such as satisfaction with services and health-related quality of life (HRQoL) to “hard measures” such as health service utilisation and mortality. When choosing an outcome measure it is essential to consider whether the intervention can indeed be expected to influence the proposed outcome(s). It has been suggested that the limited proof of effect for clinical pharmacy services such as medication reviews and efforts towards patient-education, -motivation, -partnership is mostly due to a lack of adapted outcome measures. Incidence of readmission is a commonly used outcome measure within the field of pharmacy practice research. This measure is however very multifactorial, affected by many aspects besides medicines optimisation, thus requiring large studies with long follow-up periods - and it remains uncertain to what degree these types of interventions actually influence hospitalisation.
A Patient-Reported Outcome Measure (PROM) is a report by a patient without a clinician’s interpretation. PROMs are being used more and more in line with healthcare striving to promote and practice in a person-centred manner. They aim to capture effects that are relevant and important for the individual, rather than the traditional medical and clinical effects. HRQoL measures are often used to assess patient-reported outcomes. These measures, however, have been developed to evaluate the impact of disease burden on patients’ life, not specifically the impact of pharmacotherapy. Recently, several outcomes measurement tools have been developed which focus specifically on patient reported drug-related quality of life or drug-related satisfaction. In this workshop you will get familiar with some of them and learn about how they can be used to evaluate your clinical pharmacy services.
LEARNING OBJECTIVES
After the session, the participant should be able to:
• Be familiar with the content and intended scope of several PROMs focusing on pharmacotherapy

• Describe how PROMs may be used practically and what the challenges and opportunities may be
• Describe how effects of clinical pharmacy services can be analysed and reported using different PROMs
EDUCATIONAL NEED ADDRESSED
Clinical pharmacists provide cognitive services to patients and physicians with the aim to optimise patients’ pharmacotherapy, medication use and well-being. The effects of these services are difficult to measure in practice and in research and commonly used outcome measures are often not sensitive enough. There is therefore a need to find alternative measures (such as PROMs focusing on pharmacotherapy) that these services can actually have an influence on and that are of most relevance to the patients.
KEYWORDS
Patient reported, outcome measures, research, clinical pharmacy services
79 Scientific Programme
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
The choice of the top cancer centers

Safe, reliable and trouble-free product
Security
DOSI-FUSER® Hard Shell and clear labeling to minimize errors
Drug compatibility
An extensive drug stability table is available
Simplicity
Easy-to-fill. Compatible with Closed Systems Transfer Devices (CSTD) and robotic systems. Clear and simple filling guidance in the app dosi-fuser.com
Versatility
Comprehensive range DOSI-FUSER® covers a huge amount of therapies

80
INTERACTIVE SESSIONS
INTERACTIVE SESSION 1
The expanding role of the hospital pharmacists in the care of pre and post renal transplant patients
ACPE UAN: 0475-0000-23-020-L05-P - A knowledge-based activity

Session: Thursday, 23 March 2023 - from 15.00 to 16.30
Room: Room 5C
Facilitator: Virginia Silvari
Presenter: Teun van Gelder*, Andrea Devaney*
*Conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 4 - Clinical Pharmacy: Statements - 4.1, 4.8
ABSTRACT
Renal transplantation is the treatment of choice for end-stage renal failure as it has been shown to reduce the high mortality observed in these patients. Both the perioperative medication management and the post-transplant long term medication management are crucial for the wellbeing of the renal transplant patients. Soon after the transplant these patients are commenced on multiple immunosuppressant medications which are characterised by a narrow therapeutic index, are responsible for frequent adverse drug events as well as drug-drug interactions. Thanks to the renal transplant, patients now live longer and inevitably they are exposed to immunosuppressants for a longer period of time. This prolonged exposure can potentially cause additional threats to the transplant patients, such as infections, malignancies, metabolic disorders, and hypertension. It follows that, in addition to the immunosuppressant agents, renal transplant patients require also non-immunosuppressant medications, not only to counteract the sideeffects of the immunosuppressants but also to treat the complications caused by them. The combination of complex immunosuppressants and non-immunosuppressants medication regimes exposes the renal transplant recipients to an increased risk of experiencing adverse drug events as well drug-drug interactions.
This seminar will focus on medication management strategies the hospital pharmacists uses to optimise the care of renal transplant recipient patients and improve patients safety.
LEARNING OBJECTIVES
After the session, the participant should be able to:
• Outline the essential role of the hospital pharmacist in the care of renal transplant recipient patients
• Describe the medication management strategies used to optimise the care of renal transplant recipient patients in the perioperative setting
• Discuss the long term medication management of renal transplant patients and its effects on patient’s care
EDUCATIONAL NEED ADDRESSED
This seminar addresses the advances on medication management strategies made in recent years to optimise the care of renal transplant patients.
KEYWORDS
Immunosuppressive agents, transplantation, medication therapy management

81 Scientific Programme


INTERACTIVE SESSION 2
The art of estimating renal function in adult patient groups
ACPE UAN: 0475-0000-23-022-L04-P - A knowledge-based activity
Session: Friday, 24 March 2023 - from 09.00 to 10.30

Room: Room 5C
Facilitator: Fatma Karapinar
Presenter: Casper Franssen*, Maria Kerskes
*Conflict of interest declared. Please reference speakers’ biographies. No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 1 - Introductory Statements and Governance: Statement - 1.1
Section 4 - Clinical Pharmacy: Statements - 4.1, 4.2, 4.3, 4.8
Section 5 - Patient Safety and Quality Assurance: Statements - 5.1, 5.5
ABSTRACT
Hospital pharmacists review patients’ medication daily and assess risk factors to reduce patient harm. One of those risk factors is the renal function. Pharmacists advise physicians on dose-adjustments of renally excreted medication or on the discontinuation of medication that could further decrease renal function. Before the introduction of formulas to estimate GFR (glomerular filtration rate), the most commonly used measure was the serum creatinine concentration. However, the serum creatinine is not only determined by glomerular filtration, but also dependent on many other factors such as muscle mass, meat consumption, medication use and malnutrition.
The formulas to estimate GFR use serum creatinine concentration and take into account several variables such as age, gender, weight and race, and are only validated in a stable chronic setting. The most commonly used formulas in daily practice are the Cockcroft-Gault formula, the Modification of Diet in Renal Disease (MDRD) formula and the Chronic Kidney Disease Epidemiology collaboration equation (CKD-EPI). These formulas have been developed in populations with specific characteristics, which hamper their use in various populations with a wide range of renal function. It is essential to understand the pitfalls of these formulas to allow correct interpretation of their results in adult patients. To address whether medication adjustments are needed hospital pharmacists need to distinguish between acute versus chronic renal failure. Also, the expected recovery of the renal function needs to be taken into account.
In this interactive session the characteristics of the several formulas are discussed. Also, the interpretation of these formulas in different patient groups - such as patients with obesity or older patients - is addressed using cases from routine clinical practice.
LEARNING OBJECTIVES
After the session, the participant should be able to:
• Describe which formulas are used for adult patients to estimate renal function in patients and the important pitfalls of these formulas
• Apply the right formula to special patient groups (e.g. older patients, patients with chronic renal failure, and patients with obesity)
EDUCATIONAL NEED ADDRESSED
Clinical pharmacists review patients’ medication daily. Based on the renal function of a patient pharmacists advice physicians on the pharmacotherapy. This seminar will focus on the pitfalls of estimating renal function for adult patients with common formulas used in daily practice.
KEYWORDS
Renal function, drug use evaluation, interventions, medication safety, medication error
83 Scientific Programme FROM DRUG DESIGN TO
TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
TURNKEY SOLUTIONS FOR PATIENT SAFETY TURNKEY SOLUTIONS FOR PATIENT SAFETY








Sterile


Quick Easy Safe
INERTA®




DUAL-MIX®

READYMIX®
ISO 7 ISO 7 CLEAN ROOM PRODUCTION

Made in France since 1972 www technoflex net
Flexible & Innovative Packaging solutions for pharma & compounding
YOUNG PROFESSIONAL SESSION
Learning from the career journeys of others
ACPE Non-Accredited Activity
Session: Wednesday, 22 March 2023 - from 14.45 to 16.15

Room: Room 5C
Facilitator: Clément Delage
Presenter: Torsten Hoppe-Tichy*, Stefanie Deuster*, Virginia Silvari, Kornélia Chrapková, Ulrika Gillespie
*Conflict of interest declared. Please reference speakers’ biographies. No conflict of interest declared. Please reference speakers’ biographies.
The Young Professional Session will offer participants the opportunity to learn from the career journeys of five hospital pharmacists who will describe their respective experiences and take time to answer questions student and young professionals may have.
This is an opportunity to get worthwhile information, advice and guidance to a future and exciting career in hospital pharmacy.

Join the Young Professional Session and be inspired!!!
The Speakers are:
Kornélia Chrapková, Head of Clinical Pharmacy Department, Institute for Clinical and Experimental Medicine, will talk about how she achieved her dream of working in a hospital pharmacy and becoming a clinical pharmacist. During her presentation, she will be talking about her professional journey starting in a community pharmacy, followed by studying and gaining experience abroad and other important milestones which helped her to implement and develop clinical pharmacy service in the largest transplant centre in Czech Republic and Slovakia.
Stefanie Deuster*, Head of the department quality assurance in the pharmacy, University Hospital Basel (Switzerland), whose career focusses on the preparation of drugs for the special needs of patients as well as the manufacturing of investigational medicinal products for clinical trials - and all the requirements for assuring the quality of the drugs. She will show possibilities to create and shape the general requirements for pharmaceutical preparation in pharmacies through cooperation and networks, and also talk about the different pharmacies (production and quality assurance). Having joined several working groups as Expert, on the national level with the Swiss authorities and also once on EU level in Strasbourg, she will discuss the needs and possibilities of pharmacy preparation with the authorities and to influence the requirements to facilitate production in pharmacies.
Torsten Hoppe-Tichy*, Chief Hospital Pharmacist at Pharmacy Department, Heidelberg University Hospital (Germany), who will focus his presentation on the reasons and motivations for his career path. He will recount the gaps and challenges, how he overcame them and also on the fun and pains of being a chief hospital pharmacist.
Ulrika Gillespie, Department Chief Pharmacist at Uppsala University Hospital, and Associate Professor at the Department of Pharmacy, Uppsala University (Sweden). As one of the first clinical pharmacists in Sweden, her career has focused on the development, implementation and evaluation of clinical pharmacy services in a hospital setting. In her presentation she will describe her journey, where she has used pharmacy practice research to promote and refine the clinical services and talk about her experiences - good and bad - of running everything from small student projects to large randomised controlled trials.
Virginia Silvari, Pharmacy Department, Cork University Hospital, Cork (Ireland), who will describe her experiences and the journey she could never have imagined. On how, since graduation, her pharmacy degree has allowed her to take so many different career paths: from research, to community pharmacy and finally to hospital pharmacy. In hospital pharmacy, she found the working environment that suits her best with a mixture of her previous roles.
85 Scientific Programme



86 Scientific Programme
PHARMACOTHERAPY SESSION
Safe medication use in patients with cirrhosis
ACPE UAN: 0475-0000-23-005-L05-P - A knowledge-based activity
Session: Wednesday, 22 March 2023 - from 14.45 to 16.15
Room: Auditorium VIII
Facilitator: Fatma Karapinar
Presenter: Maridi An Aerts, Sander Borgsteede*
No conflict of interest declared. Please reference speakers’ biographies. *Conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 1 - Introductory Statements and Governance: Statement - 1.1
Section 4 - Clinical Pharmacy: Statements - 4.1, 4.2, 4.3, 4.4, 4.8
Section 5 - Patient Safety and Quality Assurance: Statements - 5.1, 5.5
ABSTRACT
The biotransformation and transport of medication are dependent on liver function. Hence, impaired liver condition can influence the pharmacokinetics and pharmacodynamics of medicines, especially of medication with a high hepatic extraction such as some analgesics, antidepressants and antihyperlipidemic medication. In 2013, it was estimated that 29 million persons were suffering from a chronic liver condition in the European Union. One common liver condition is cirrhosis.
Cirrhosis is a slowly progressive disease and results from ongoing inflammation of the liver. Liver architecture is changed into structurally abnormal nodules, fibrosis and subsequently loss of hepatocyte density and function. The severity of cirrhosis is classified with the Child-Pugh score. Precautions in medication use with cirrhosis are often included as warnings in the Summary of Product Characteristics (SmPC) without detailed advice on when to adjust dosage exactly or when to avoid certain medications. Studies show that in patients with cirrhosis, dosages of medication are incorrect and many patients experience adverse drug reactions. For hospital pharmacists it is essential to understand when to intervene to prevent adverse drug reactions and toxicity. In this pharmacotherapy session, the characteristics of cirrhosis are highlighted together with practical advices on safe medication use in patients with cirrhosis.
LEARNING OBJECTIVES
After the session, the participant should be able to:
• Describe how the Child-Pugh score can define the severity of cirrhosis
• Know the most frequently prescribed ‘unsafe’ medicines in cirrhosis, and know ‘safe’ alternatives
• Find and apply the right safety advice and dosing strategies for patients with cirrhosis
EDUCATIONAL NEED ADDRESSED
Based on severity of cirrhosis, pharmacist could advice physicians on the right adjustments in pharmacotherapy. This seminar will focus on safe medication use in patients with cirrhosis and give guidance to pharmacists who review patients’ medication daily.
KEYWORDS
Cirrhosis, hepatic impairment, medication safety, drug use evaluation, interventions, pharmacology, clinical decision support

87 Scientific Programme FROM DRUG DESIGN TO TREATMENT
SUCCESS WHAT REALLY MATTERS TO PATIENTS?
SYNERGY SATELLITE EVENT
NBCDS
NON-BIOLOGIC COMPLEX DRUGS
AND NANO MEDICINES
Wednesday, 22 March 2023
17:15 to 18:45, Auditorium II
Lisbon, Portugal
Facilitator
Speakers
Armando Alcobia
Jon de Vlieger
International Regulatory Advances for NBCDs and their follow-on products

Gunar Stemer
NBCDs – Considerations for hospital pharmacy practice

Non-Biological Complex Drugs (NBCDs) are drugs that comprise large high molecular weight molecules and, often, nanoparticles structures. They differ from typical small chemical molecules and also from biotechnologyderived medicinal products (large proteins).
For NBCDs, the entire complex is the active pharmaceutical ingredient and its properties cannot be fully characterized by physicochemical analysis. Most of these medicinal products will have to be managed in a hospital setting, which is why the interdisciplinary pharmacotherapy committees need to consider all levels of evidence generated, focusing specifically on data related to clinical safety and efficacy comparability, discussing interchangeability decisions more like a biosimilar than a generic approach.
Manuel Bañobre-López
Nanomedicine: revolutionizing medicine
Sponsored by an Educational Grant from CSL Vifor
88 Scientific Programme
27th CONGRESS OF THE EAHP
DESIGN > www.biographia.it The European Association of Hospital Pharmacists (EAHP) is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education
eAhP inviTes you To ATTend The 2023 s ynergy s AT e lli T e s ession : ConTACT us | synergy@eahp.eu ACPE
- A knowledge-based activity
The
UAN: 0475-0000-23-011-L04-P
SYNERGY SATELLITE
Non-Biologic Complex Drugs (and nano medicines)
ACPE UAN: 0475-0000-23-011-L04-P - A knowledge-based activity
Session: Wednesday, 22 March 2023 - from 17.15 to 18.45
Room: Auditorium II
Facilitator: Armando Alcobia
Presenter: Jon de Vlieger, Gunar Stemer, Manuel Bañobre-López
No conflict of interest declared. Please reference speakers’ biographies.
LINKED TO EAHP STATEMENTS
Section 2 - Selection, Procurement and Distribution: Statement - 2.2
Section 4 - Clinical Pharmacy: Statement - 4.6
Section 5 - Patient Safety and Quality Assurance: Statements - 5.9, 5.11
Section 6 - Education and Research: Statement - 6.4
ABSTRACT
Non-Biological Complex Drugs (NBCDs) are drugs that comprise large high molecular weight molecules and, often, nanoparticles structures. In this category we currently include glatiramoids, iron oxide carbohydrate nanoparticles, liposomes, and polymeric micelles.


They differ from typical small chemical molecules, generally used as pharmaceutical active product ingredients and also from biotechnology-derived medicinal products (large proteins). For NBCDs, the entire complex is the active pharmaceutical ingredient and its properties cannot be fully characterised by physicochemical analysis.
NBCDs can also be characterised as complex drug products not being biologicals!
A recent appraisal of approval procedures for NBCDs “follow-on products” approved in Europe shows a diversity of regulatory pathways being followed (generic, hybrid or biosimilar) that must be discussed in order to incorporate the enlarged community of follow-on NBCD products that will enter the market.
The hospital pharmacist must be aware of the importance of clinical trials comparing the innovators and similar products in order to anticipate side events or any effectiveness gap. Most of these medicinal products (recombinant hepatitis B or HPV vaccines; ferric carboxymaltose; high molecular weight iron dextran; nab-paclitaxel, glatiramer acetate, liposomal doxorubicin or vincristine, aprepitant, paliperidone - some of NBCDs) will have to be managed in a hospital setting, which is why the interdisciplinary pharmacotherapy committees need to consider all levels of evidence generated, focusing specifically on data related to clinical safety and efficacy comparability, discussing interchangeability decisions more like a biosimilar than a generic approach.
LEARNING OBJECTIVES
After this synergy, participants should be able to:
• Explain their newly acquired understanding of the Non-Biological Complex Drugs
• Describe how pharmacists must be involved in NBCD evaluation
• Understand the different approvals pathways of NBCD
• List examples of NBCD
EDUCATIONAL NEED ADDRESSED
Hospital pharmacist should recognise that therapeutic equivalence is a critical question to NBCDs and will be more challenging when so many nanomedicines, nanoimaging agents and new drugs, that must be considered NBCD, will enter the market.
KEYWORDS
Non Biologic Complex Drugs, characterisation, evaluation, interchangeability

89 Scientific Programme FROM DRUG DESIGN TO
TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
INDUSTRY SPONSORED SATELLITES
Wednesday, 22 March
14.45 - 16.15
Micromedex (MERATIVE) - An intercollaborative practice model approach: supporting the patient experience in medicines management
Equashield - Unprecedented Technology for Handling Hazardous Drug: The Future of Automated Compounding
- Cutting edge technology: Automated compounding at the next level (14.45 - 15.30) &
- Revolutionizing hazardous drug compounding with leading technology and uncompromised safety (15.30 - 16.15)
Thursday, 23 March
Omnicell - How a visionary Euro-Autonomous Pharmacy can be used to advance the medication use process
ICU Medical - Transformational journey to a fully connected infusion system across multiple sites improving drug delivery and minimising errors
Auditorium II
Auditorium III & IV
12.00 - 13.30
Amgen - Evaluating Biosimilars: Focus on the Rare Disease Paroxysmal Nocturnal Haemoglobinuria (PNH)
Simplivia - Controlling Occupational Exposure to Hazardous Drugs - Detecting Contamination and the Need for CSTD in the Pharmacy Setting

Auditorium II
Auditorium III & IV
15.00 - 16.30
Sanofi - Beyfortus® (nirsevimab) – A novel immunization against Respiratory Syncytial Virus (RSV) Lower Respiratory Tract Disease (LRTD) in neonates and infants
Baxter – Patient Care and Medication Safety: The Evolving Role of the Pharmacist in Parental Nutrition
Auditorium II
Auditorium III & IV
Auditorium II
Auditorium III & IV
90 Industry Satellites
Time Meetings/Events Room
Industry Sponsored Satellite
Time Meetings/Events Room
Industry Sponsored Satellite
09.00 - 10.30
Industry Sponsored Satellite
Industry Sponsored Satellite
EVALUATING BIOSIMILARS:

FOCUS ON THE RARE DISEASE PAROXYSMAL NOCTURNAL HAEMOGLOBINURIA (PNH)
THURSDAY 23 MARCH 2023
12.00–13.30 WET, AUDITORIUM II
This highly informative symposium aims to support the vital role of the pharmacist as an educator of healthcare professionals and patients by providing important updates in the field of biosimilars.
We will provide an introduction to the rare disease PNH and consider factors of importance when evaluating and using biosimilars, with a particular focus on those relevant to the PNH setting. Looking to the future, we will also discuss the potential for evolution of both the biosimilar development process and the approach to biosimilar use in clinical practice.
PROGRAMME
Chair: João Gonçalves, Portugal
Welcome and introduction
Focus on the rare disease, paroxysmal nocturnal haemoglobinuria (PNH)
Key considerations for pharmacists when evaluating and using biosimilars
The future of biosimilars
Interactive panel discussion
Concluding comments and close
João Gonçalves, Portugal
Austin Kulasekararaj, UK
Niels Boone, The Netherlands
Gunar Stemer, Austria
All, moderated by João Gonçalves, Portugal
João Gonçalves, Portugal
AMGEN-SPONSORED SATELLITE SYMPOSIUM AT THE 27TH CONGRESS OF THE EAHP
Amgen (Europe) GmbH. CH 6343 Rotkreuz, Switzerland. www.amgen.com © 2023 Amgen Inc. All rights reserved. Date of preparation: January 2023. Job code: SC-PT-ABP 959-00001.
Micromedex
Join your peers for an interactive workshop
We will explore a collabora ve prac ce model approach in monitoring medica on counselling and therapeu c adherence in the pa ent care con nuum from admission to discharge. Problem-based learning derived from clinical prac ce guidelines will be shared to illustrate the broad evidence-based scope of Micromedex.
Weds 22nd March 14:45, Auditorium II
Visit the Micromedex exhibi on stand for details
Session lead by:
Interna onal Client Success Manager, Micromedex


92 Industry Satellites
Fabien Wecker, MSN, RN, MA, MHFA, CMI(c)
WHAT REALLY MATTERS TO PATIENTS?
MICROMEDEX
Evidence-based clinical decision support
- content you can trust
- updated every day
- accessible online, via EHR integra on and on mobile
Let the clinical team guide you through a bespoke demo on the Micromedex exhibi on booth
Learn More: mera ve.com/clinical-decision-support
Find out about a free trial: mera ve.com/micromedex-trial

93 Industry Satellites
DRUG DESIGN TO TREATMENT SUCCESS
FROM
Unprecedented Technology for Handling Hazardous Drugs
/ High Throughput
The EQUASHIELD® Pro streamlines pharmacy workflow with up to 70 individual doses per hour.


/ Uses EQUASHIELD® CSTD
The only compounding robot using a CSTD to further protect healthcare workers from exposure to hazardous drugs and prevent microbial contamination.
/ Small Footprint
EQUASHIELD® Pro can fit into your existing pharmacy space.
/ Patient Specific
Produces
patient-specific doses with Syringe Units, IV bags, and other finished dose forms.
/ Dosage Error Protection
EQUASHIELD®’s proprietary dose verification system includes high-tech image processing and artificial intelligence technology and ensures the accuracy of each dose to prevent incorrect drugs, mixing and labeling errors during compounding.
94 Industry Satellites
EQUASHIELD PRO
www.equashield.com
Join us for a live demo at B ooth #31
a
wide range of
EQUASHIELD
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
Unprecedented Technology for Handling Hazardous Drugs
We are pleased to invite you to the symposium session: The Future of Automated Compounding
Wednesday / 22 March 2023 / Auditorium III + IV
14:45 – 15:30
Cutting edge technology: Taking automated compounding to the next level
Agnieszka Drzazgowska, Head of Pharmacy University of Gdansk Hospital, Poland
15:30 – 16:15
Revolutionizing hazardous drug compounding with leading technology and uncompromised safety



Luis Filipe Guerreiro, Hospital Pharmacist Santa Maria Hospital, Portugal
Visit us at Booth #31
www.equashield.com

95 Industry Satellites
Omnicell invites you to attendthe 2023 Satellite Symposia :
How a visionary European Autonomous Pharmacy can be used to advance the medication use process?
Thursday, 23rd March 2023 | 9:00 - 10:30 am | Auditorium II 27th Congress of the EAHP

96 Industry Satellites
Dr Etienne Cousein – Valenciennes' General Hospital, France
Dr Etienne Cousein is in charge of the pharmacy department at the Valenciennes' General Hospital and is also vice-Chief Medical Officer. Dr Etienne Cousein's research is focused on preventing errors in medication prescription, dispending and administration, thanks to a better understanding of human-human and human-machine cooperation.
Prof Dr Martin J. Hug – Medical Center University of Freiburg, Germany

Prof Hug is currently Chief Pharmacist at the Medical CenterUniversity of Freiburg. He also serves as adjunct Prof. Clinical Pharmacy at the Institute of Pharmaceutical Sciences at the University of Freiburg.

Prof Hug's research focuses on the investigation of electrolyte transport across biological membranes. He is also involved in a number of projects dealing with the measurement of serum levels of novel drugs.

Dr Jordi Nicolás Picó – Hospital Universitario Mutua Terrassa, Spain
Jordi Nicolás Picó is Head of Department of Pharmacy at the Hospital Universitario Mutua Terrassa, Barcelona. He is currently the Vice President of the Spanish Society of Hospital Pharmacy SEFH.
He has been publishing and cited several times about his papers of pharmaceutical care, pharmacokinetics, and antimicrobial stewardship.

97 Industry Satellites FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
Auditorium
Thursday, 23rd March 2023 | 9:00 - 10:30 am |
II 27th Congress of the EAHP



98 Connect Smarter. Care Safer.™ ICU Medical brings you innovative IV therapy products designed to help safely, accurately, and efficiently deliver IV medications. 800.824.7890 | www.icumed.com © 2023 ICU Medical Inc. | P23-5921 Visit us at Stand 20 to learn how we can help you enhance IV medication safety. The product complies with current legislation and has the corresponding CE marking. For additional information, warnings and /or safety precautions, refer to the manufacturer’s Instructions for Use.
EAHP’s
As EAHP continues to receive requests regarding assistance in various areas of hospital pharmacy practice, EAHP is setting up SIGs to address the needs of patients. SIGs are comprised of professionals that focus on specific topics related to pharmaceutical care, medical devices and many other related areas. Members of the SIGs should be hospital pharmacists and other specialty pharmacists, doctors, nurses, patients and pharmaceutical industry leaders for the various subject-matter areas.
BENEFITS
• To bring professionals together with a common interest to advance patient safety and outcomes by sharing ideas, opinions and experience with their peers
• to provide pharmacists across Europe with direct access to information related to specific topics
• to provide the transfer of knowledge between healthcare, academia and industry professionals and researchers and to provide networking opportunities
• to incorporate the interest of healthcare professionals with those of patients
Groups will be formed based on information gathered via EAHP’s various surveys, input from the Education Executive Committee, Scientific Committee and Board Members. Funding will be necessary in order to facilitate this effort as there will be costs related to meetings, virtual platforms, web site and discussion forums, all of which will enable the SIGs to publish papers related to the specific topics in the European Journal of Hospital Pharmacy and provide other outcomes as requested by the GA/Board.
• to provide reliable health information resources for healthcare professionals and patients
• to increase awareness among stakeholders to strengthen partnerships in order to optimize patient care
• to facilitate access to information through the EAHP web site, newsletters, publications, etc.
EAHP currently has SIGs working on:
• the Investigation of Medication Errors in Intensive Care Units (financially supported by BD)
• Automated Medication Management (financially supported by Omnicell)
• the Use of Prefilled Syringes in Intensive Care Units and Operating Theaters (financially supported by BD)
BENEFITS
• To bring professionals together with a common interest to advance patient safety and outcomes by sharing ideas, opinions and experience with their peers
• the hospital pharmacist’s preparedness for in-vivo gene therapy medicinal products (financially supported by Pfizer)
• eliminating Avoidable Harm (financially supported by Baxter).
• to provide pharmacists across Europe with direct access to information related to specific topics
• to provide the transfer of knowledge between healthcare, academia and industry professionals and researchers and to provide networking opportunities
• to incorporate the interest of healthcare professionals with those of patients

• to provide reliable health information resources for healthcare professionals and patients
The first SIG on Hazardous Medicinal products (financially supported by Amgen) closed and is presenting their results during the EAHP Congress , Wednesday 23 March 2022 , 5:15 pm to 6:45 pm, Room –2.31
• to increase awareness among stakeholders to strengthen partnerships in order to optimize patient care
• to facilitate access to information through the EAHP web site, newsletters, publications, etc.

EAHP currently has SIGs working on:
For more information
• the Investigation of Medication Errors in Intensive Care Units (financially supported by BD)
Jennie De Greef, Managing Director
European Association of Hospital Pharmacists (EAHP)
• Automated Medication Management (financially supported by Omnicell)
Boulevard Brand Whitlock 87, Box 11 (4th floor) – 1200 Brussels, Belgium
Tel: +32 (0) 2/669.25.10
• the Use of Prefilled Syringes in Intensive Care Units and Operating Theaters (financially supported by BD)
email: Jennie.DeGreef@eahp.eu
• the hospital pharmacist’s preparedness for in-vivo gene therapy medicinal products (financially supported by Pfizer)

• eliminating Avoidable Harm (financially supported by Baxter).

99 Speakers’ biographies FROM DRUG DESIGN TO TREATMENT SUCCESS
WHAT REALLY MATTERS TO PATIENTS?
Special Interest Groups (SIGs) gather and evaluate the evidence in specific, innovative and novel fields of hospital pharmacy practice with the aim to address patient needs and to advance the profession.
REV. 0 03/2022
When Healthcare & Simplicity Click

Simplivia’s CSTD provides a vial-to-vein closed solution offering broad protection for healthcare facilities and staff. Chemfort™ reduces the risk of exposure to hazardous drugs while maintaining drug sterility.


Simplivia's portfolio was tested with real cytotoxic drugs for vapor containment and found compatible with all known hazardous drugs. Simplivia products have been chosen by healthcare professionals as the most user-friendly CSTD*


*Comparative study of the different closed system transfer devices available in France for the preparation of injectable immunotherapies. Albaut V, Brobst M, Colliat F, Castel D. GERPAC POSTER 2021
learn
at
#3
To
about Simplivia's innovative drug delivery solutions Visit us
booth
View
Dr. Paul Sessink Founder and Managing Director of Exposure Control, Sweden
| March 23rd
Dr. Alan Wilkinson CEO at Biopharma Stability Testing Laboratory Ltd




simplivia.com Join Our Satellite Symposium: Thursday

101 Speakers’ biographies FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS
02 New Closed System Transfer Device Contains Real Drug Vapors for Up to 28 Days our posters
12:00-13:30 CET
01 Closed System Transfer Device (CSTD) Extends Practical In-Use Shelf Life to 28 Days After First Puncture of Non-Preserved Single-Use-Vials in Both Controlled and Uncontrolled Environments
Speakers
Dr. Robert Terkola MSc, PhD, aHPh Clinical Assistant Professor Oncology Pharmacist
Qualified Person CEO Health Concepts
Occupational Exposure to Hazardous Drugs - Detecting Contamination and the Need for CSTD in the Pharmacy Setting 2SPD-005 3PC-005
Dr. Elana Slutsky Smith Head of Product Science, Simplivia
Controlling
The power to reduce the chaos of RSV season

Beyfortus® is a long-acting antibody designed for all infants,* offering direct protection against RSV lower respiratory tract disease throughout their first RSV season.**,1

With a single dose, Beyfortus reduces the risk of medically attended RSV lower respiratory tract infections, including hospitalizations, in infants.1
Thursday March 23rd 3pm – 4:30pm
* Beyfortus® is indicated for the prevention of RSV lower respiratory tract disease in neonates and infants during their first RSV season. Beyfortus® is contraindicated in infants with hypersensitivity to the active substances or to any of the excipients.1
** Beyfortus® affords at least 5 months of protection against RSV lower respiratory tract disease.1 RSV, respiratory syncytial virus.
1. Beyfortus (nirsevimab), Summary of Product Characteristics. AstraZeneca. 2022.
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.

Beyfortus® (nirsevimab) – Abbreviated Prescribing Information
Presentation: Beyfortus 50 mg and 100 mg solution for injection in pre-filled syringe containing 50 mg of nirsevimab in 0.5 mL (100 mg/mL) and 100 mg of nirsevimab in 1 mL (100 mg/mL) respectively.
Nirsevimab is a human immunoglobulin G1 kappa (IgG1K) monoclonal antibody produced in Chinese hamster ovary (CHO) cells by recombinant DNA technology. Indication: Prevention of Respiratory Syncytial Virus (RSV) lower respiratory tract disease in neonates and infants during their first RSV season. Beyfortus should be used in accordance with official recommendations.
Dosage and administration: The recommended dose is a single dose of 50 mg for infants with body weight <5 kg or 100 mg for infants ≥5 kg, administered intramuscularly. Beyfortus should be administered prior to commencement of the RSV season, or from birth for infants born during the RSV season. For infants undergoing cardiac surgery with cardiopulmonary bypass, an additional dose may be administered as soon as the infant is stable after surgery to ensure adequate nirsevimab serum levels. If within 90 days after receiving the first dose of Beyfortus, the additional dose should be 50 mg or 100 mg according to body weight. If more than 90 days have elapsed since the first dose, the additional dose could be a single dose of 50 mg regardless of body weight, to cover the remainder of the RSV season. There are no safety and efficacy data available on repeat dosing. There are limited data available in extremely preterm infants (Gestational Age [GA] <29 weeks) less than 8 weeks of age. No clinical data available in infants with a postmenstrual age (gestational age at birth plus chronological age) of less than 32 weeks. Safety and efficacy in children 2-18 years not established. Beyfortus is for intramuscular injection only, preferably in the anterolateral aspect of the thigh. Gluteal muscle should not be used routinely due to risk of sciatic nerve damage.
Contraindication: Hypersensitivity to the active substance or to any of the excipients. Warnings and precautions: To improve traceability of biological medicinal products, record the name and batch number. Serious hypersensitivity reactions, including anaphylaxis, have been observed with monoclonal antibodies. If signs and symptoms of a clinically significant hypersensitivity reaction or anaphylaxis occur, immediately discontinue administration and initiate appropriate medicinal products and/or supportive therapy. As with any other intramuscular injections, nirsevimab should be given with caution to infants with thrombocytopenia or any coagulation disorder. Interactions: Nirsevimab can be given concomitantly with childhood vaccines. Nirsevimab should not be mixed with any vaccine in the same syringe or vial. When administered concomitantly with injectable vaccines, they should be given with separate syringes and at different injection sites. Fertility, Pregnancy and Lactation: Not applicable. Undesirable effects: Adverse reactions reported in clinical trials are uncommon: rash, injection site reaction, pyrexia. As with all therapeutic proteins, there is potential for immunogenicity. For a complete list of undesirable effects please refer to the Summary of Product Characteristics. Health care professionals are asked to report any suspected adverse reactions via their national reporting system. Marketing Authorisation Holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden. Legal Classification of the medicinal product regarding medical prescription: Prescription Only Medicine. Date of last review: January 2023.
Abbreviated Prescribing Information based on the EU SmPC as of January 2023. Before prescribing the product always refer to your full local prescribing information as this information may vary from country to country.
Informação do Produto em Português disponível em: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.ema.europa.eu/en/ documents/product-information/beyfortus-epar-product-information_pt.pdf
102
MAT-GLB-2300117 – 1.0 – 01/2023
Discover more on Booth 29 or
by attending our Symposium
Auditorium II
Satellite Symposium


A novel immunization against Respiratory Syncytial Virus (RSV) Lower Respiratory Tract Disease (LRTD) in neonates and infants



Thursday, March 23rd 2023 15:00-16:30
Moderator Speakers
Auditorium II
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
Beyfortus® (nirsevimab) – Abbreviated Prescribing Information Presentation: Beyfortus 50 mg and 100 mg solution for injection in pre-filled syringe containing 50 mg of nirsevimab in 0.5 mL (100 mg/mL) and 100 mg of nirsevimab in 1 mL (100 mg/mL) respectively. Nirsevimab is a human immunoglobulin G1 kappa (IgG1K) monoclonal antibody produced in Chinese hamster ovary (CHO) cells by recombinant DNA technology. Indication: Prevention of Respiratory Syncytial Virus (RSV) lower respiratory tract disease in neonates and infants during their first RSV season. Beyfortus should be used in accordance with official recommendations. Dosage and administration: The recommended dose is a single dose of 50 mg for infants with body weight <5 kg or 100 mg for infants ≥5 kg, administered intramuscularly. Beyfortus should be administered prior to commencement of the RSV season, or from birth for infants born during the RSV season. For infants undergoing cardiac surgery with cardiopulmonary bypass, an additional dose may be administered as soon as the infant is stable after surgery to ensure adequate nirsevimab serum levels. If within 90 days after receiving the first dose of Beyfortus, the additional dose should be 50 mg or 100 mg according to body weight. If more than 90 days have elapsed since the first dose, the additional dose could be a single dose of 50 mg regardless of body weight, to cover the remainder of the RSV season. There are no safety and efficacy data available on repeat dosing. There are limited data available in extremely preterm infants (Gestational Age [GA] <29 weeks) less than 8 weeks of age. No clinical data available in infants with a postmenstrual age (gestational age at birth plus chronological age) of less than 32 weeks. Safety and efficacy in children 2-18 years not established. Beyfortus is for intramuscular injection only, preferably in the anterolateral aspect of the thigh. Gluteal muscle should not be used routinely due to risk of sciatic nerve damage. Contraindication: Hypersensitivity to the active substance or to any of the excipients. Warnings and precautions: To improve traceability of biological medicinal products, record the name and batch number. Serious hypersensitivity reactions, including anaphylaxis, have been observed with monoclonal antibodies. If signs and symptoms of a clinically significant hypersensitivity reaction or anaphylaxis occur, immediately discontinue administration and initiate appropriate medicinal products and/or supportive therapy. As with any other intramuscular injections, nirsevimab should be given with caution to infants with thrombocytopenia or any coagulation disorder. Interactions: Nirsevimab can be given concomitantly with childhood vaccines. Nirsevimab should not be mixed with any vaccine in the same syringe or vial. When administered concomitantly with injectable vaccines, they should be given with separate syringes and at different injection sites. Fertility, Pregnancy and Lactation: Not applicable. Undesirable effects: Adverse reactions reported in clinical trials are uncommon: rash, injection site reaction, pyrexia. As with all therapeutic proteins, there is potential for immunogenicity. For a complete list of undesirable effects please refer to the Summary of Product Characteristics. Health care professionals are asked to report any suspected adverse reactions via their national reporting system. Marketing Authorisation Holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden. Legal Classification of the medicinal product regarding medical prescription: Prescription Only Medicine. Date of last review: January 2023.


Abbreviated Prescribing Information based on the EU SmPC as of October 2022. Before prescribing the product always refer to your full local prescribing information as this information may vary from country to country.
Informação do Produto em Português disponível em: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.ema.europa.eu/en/documents/productinformation/beyfortus-epar-product-information_pt.pdf

103
Paula Rebelo RTP Journalist, Portugal
Dr. Simon Nadel Consultant in Paediatric Intensive Care, United Kingdom
Dr. Alexandre Lourenço Hospital Administrator, Portugal
Professor João Gonçalves PhD, Professor and Principal Investigator at Faculty of Pharmacy, University of Lisbon, Portugal
MAT-GLB-2300119 – 1.0 – 01/2023
WET
Your Partner in Pharmacy Efficiency
ExactaMix is going PRO
Our new automated compounder combines the trusted features you have relied upon for 25 years with quad-core processing power, ease of use, a new intuitive interface and cyber security features for efficient and safe automated compounding.

Visit us for a demo!
Baxter Infusor –

helping you treat patients at home for over 30 years!
Baxter created the first elastomeric ambulatory infusion system more than 30 years ago. Since then, our Infusors supported by vast stability database, have given patients the option of receiving IV infusion therapy without being confined to a hospital bed.



Visit us to learn more!
C losed Loop Medication Mana gement
We are in an era where strict safety and accuracy are demanded in medical practice. What can an oral solid packaging system do for you?


Visit us to learn more!

* Product name may differ in different countries. Baxter and Infusor are registered trademarks of Baxter International Inc.
EMA-CN8-230002 v2.0 February 2023
Thursday, 23 March, 2023 / 15:00 - 16:30 WET
Auditorium III & IV
PATIENT CARE AND MEDICATION SAFETY: The Evolving Role of the Pharmacist in Parenteral Nutrition

PROGRAM OBJECTIVES:
• Describe the evolving role of the pharmacist in the parenteral nutrition (PN) pathway.
• Evaluate existing strategies to provide PN therapy according to patient needs, pharmacy resources and availability of PN solutions.
• Clarify the potential safety and cybersecurity risks to patients in the PN pathway in the digital era of interconnected devices.

FEATURING SPEAKERS:
Mattias Paulsson, PhD Hospital Pharmacist
Uppsala University Hospital
Uppsala, Sweden
Magdalena Piętka, MPharm
 Pharmacy Director of Compounding/Home
Pharmacy Director of Compounding/Home
Parenteral
Nutrition Center
Stanley Dudrick’s Memorial Hospital
Skawina, Poland
Baxter is a registered trademark of Baxter International Inc. GBU-CN00-230001 01/2023 Baxter International Inc. 1 Baxter Parkway, Deerfield, IL 60015 www.baxter.com
PLEASE VISIT US AT BOOTH 24 FOR ADDITIONAL INFORMATION. Join us for a scientific symposium

Increase the efficiency in your pharmacy Solutions for efficient preparation of drugs and parenteral nutrition B. Braun Melsungen AG | www.bbraun.com
SPEAKER’S BIOGRAPHIES
Aerts, Maridi An
PHARMACOTHERAPY SESSION - Safe medication use in patients with cirrhosis
Affiliation: UZ-Brussels
Country: Belgium
CURRENT STATUS, POSITION
Maridi An Aerts is MD, staff member at the gastroenterology department in UZ-Brussels, with a specialization in hepatology and interventional endoscopy.
EDUCATION
Maridi An Aerts is a gastroenterologist at UZ-Brussel. She has a specialization in hepatobiliairy diseases and subspecialization in advanced diagnostic and interventional endoscopy. She is also part of the palliative support team in her hospital. She is board member (treasurer) of the Belgian Society of GastroIntestinal Endoscopy (BSGIE) and Acta Gastro-Enterologica Belgica.
Education: VUB (2006) & UZ-Brussel (2012), Hepatology in UZ Ghent; Practice and experience: UZ-Brussel, UZ Gent.
Conflict of interest: No conflict of interest declared.
Alonso-Martinez, Carla
SEMINAR PC1 - Advanced therapy medicinal products (ATMPs)challenging opportunities for hospital pharmacy

Affiliation: Vall d’Hebron Hospital

Country: Spain
CURRENT STATUS, POSITION
Carla Alonso-Martinez is a hospital pharmacist at Vall d’Hebron Hospital in Barcelona, Spain. She works as a Clinical Pharmacist in the Haematology Department and is also responsible for the Advanced Therapies Unit of the Pharmacy Department. She is the Secretary of the Spanish Society of Hospital Pharmacy Advanced Therapies Working Group.
EDUCATION
Carla Alonso-Martinez studied Pharmacy at the University of Salamanca and graduated in 2014. She completed her residency in Hospital Pharmacy in the Vall d’Hebron Hospital in 2020 when she became a Specialist Hospital Pharmacist. She has presented scientific communications related to ATMPs in national and international congresses.

RESEARCH AREA
Carla Alonso-Martinez’s research focusses on clinical research with real-world data, specially in Oncohaematology, Advanced Therapies (ATMPs) and in Pharmacokinetics and Pharmacogenetics.
Conflict of interest: No conflict of interest declared.

107 Speakers’ biographies FROM DRUG DESIGN TO TREATMENT SUCCESS
PATIENTS?
WHAT REALLY MATTERS TO
Amann, Steffen
SEMINAR IG2 - The road to e-hospital pharmacy - are we there yet?
Affiliation: Munich Municipal Hospital Group

Country: Germany
CURRENT STATUS, POSITION
Steffen Amann is Chief Pharmacist, leading the team of the hospital pharmacy in the Munich Municipal Hospital Group. He was Board member of the German Society of Hospital Pharmacists (ADKA) for a decade and also a Board Member of EAHP from 2017 to 2021.

EDUCATION
Steffen Amann studied pharmacy at the University of Wuerzburg and became licensed in 1992. In 1997, he obtained a doctoral degree in Medical Microbiology at the University of Munich. He graduated as a specialist in clinical pharmacy in 1998.
RESEARCH AREA
Steffen Amann’s research focus is management systems and antimicrobial resistance
Conflict of interest: No conflict of interest declared.
Baehr, Julian
SEMINAR SPD3 - Green Hospital - The Role of Hospital Pharmacists
Affiliation: Technical University Darmstadt
Country: Germany
CURRENT STATUS, POSITION
Julian Baehr is a licensed pharmacist who additionally holds a Master’s degree in resource economics and Circular Economy. In his current role as PhD candidate at the Technical University Darmstadt, his research mainly focusses on methodological aspects of greenhouse gas accounting, with a special focus in the pharmaceutical sector.
EDUCATION
Julian Baehr studied pharmacy at the University of Münster, Germany, and became a licensed pharmacist in 2019. He then specialised in the field of Resource Economics and Circular Economy by graduating from the international EIT Raw Materials-labelled Master programme “Advanced Materials: Innovative Recycling (AMIR)“. He currently holds an M.Sc. double-degree from NOVA University Lisbon and Technical University Darmstadt. Julian has been a PhD candidate at TU Darmstadt focussing on carbon accounting methodologies since 2022.
RESEARCH AREA
Julian Baehr’s research mainly focusses on methodological aspects of greenhouse gas accounting with a special focus in the pharmaceutical sector.
Conflict of interest: No conflict of interest declared.

108
biographies
Speakers’
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
Baião, Helena
SEMINAR PC2 - Quality assurance of pharmacy preparations - a key for treatment success
Affiliation: INFARMED IP - National Authority for Medicament and Health Products

Country: Portugal
CURRENT STATUS, POSITION
• Regulatory and Scientific Advice Coordinator.
• She is a Pharmacist at Lisbon Pharmacy University.

• She worked in Macau Ministry of Health first as Hospital Pharmacist Assessor (coordinator of production, information/education and products management) and later as expert in drug assessment.
• GMP inspector (traditional and modern medicines) before joining INFARMED I.P. in 1997.
• Member of PIC/S Committee assessor and participated at Expert Circles, JVP, JRP among others and consequently organized PIC/S meetings.
• PIC/S co-rapporteur for EOF - Greece and MHRA - UK and JRP of Swissmedic - Switzerland and KALiechtenstein and JRP for Brazil’s application.
• Coordinator of Regulatory and Scientific Advice Office, at INFARMED I.P.
• National Coordinator of European Research Infrastructure Consortium EATRIS ERIC .Regulatory and Scientific Advice Manager and Quality Manager, at INFARMED I.P.
EDUCATION
• Master Degree on Pharmaceutical Science at the Lisbon University.
• GMP and GCP Inspector.
• WHO Adviser.
Conflict of interest: No conflict of interest declared.
109 Speakers’ biographies














Speakers’ biographies Hikma Injectablesmade in Europe We supply hospitals and pharmacies across our markets with generic injectables, supported by our manufacturing facilities. www.hikma.com Germany Italy Portugal
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?

Bañobre-López, Manuel
SYNERGY SATELLITE EVENT: Non-Biologic Complex Drugs (and nano medicines)

Affiliation: International Iberian Nanotechnology Laboratory


Country: Portugal
CURRENT STATUS, POSITION
Manuel Bañobre-López is currently a Research Group Leader and the former Coordinator of the Health Cluster at the INL. He leads research activities in the area of nanomedicine, both from the imaging and therapy sides.

EDUCATION
Manuel Bañobre-López studied Chemistry at the University of Santiago de Compostela in Spain, where he got his PhD in Solid State Chemistry in 2011. That same year, he joined INL to work on the development of functional nanostructures with application in the medical area.
RESEARCH AREA
Manuel Bañobre-López’ research focusses on the engineering of multifunctional (magnetic) nanostructures as medical platforms for diagnostic and/or therapeutic applications, mainly involving MRI contrast agents, magnetic hyperthermia effectors and smart drug delivery systems. Special interest is currently paid on stimuli-responsive programmed specific targeting for controlled drug delivery and activatable theranostics in nanomedicine.
Conflict of interest: No conflict of interest declared.
111 Speakers’ biographies
Black, Anne*
SEMINAR PC1 - Advanced therapy medicinal products (ATMPs) –challenging opportunities for hospital pharmacy
Affiliation: Specialist Pharmacy Service, NHS England Country: United Kingdom

CURRENT STATUS, POSITION
Anne Black is a Regional QA Specialist Pharmacist based in the Royal Victoria Infirmary in Newcastle upon Tyne. She is Chair of the Pan UK Pharmacy Working Group for ATMPs and a member of the National Pharmacy Clinical Trials Advisory Group.

EDUCATION
Anne Black studied Pharmacy in Sunderland University from 1988 - 1991, graduating with a first lass honours degree. She undertook further formal studies and obtained the Postgraduate Diploma in Pharmaceutical Technology and Quality Assurance from the University of Leeds. Anne currently teaches sessions on ATMPs at both the University of Manchester and University of Leeds.
RESEARCH AREA
Alongside her interests in clinical trials and safety of injectable medicines, she also developed an interest and expertise in Advanced Therapy Medicinal Products (ATMPs) as she became involved with the quality management of the Newcastle Cellular Therapies Facility. She has since used this expertise to emphasise the important role of Pharmacy to optimise patient safety in the delivery of these innovative medicines. Anne is Chair of the Pan UK Pharmacy Working Group for ATMPs and a Steering Group Member of the Northern Alliance Advanced Therapy Treatment Centre, as well as sitting on NHS Pharmaceutical QA Committee and the National Pharmacy Clinical Trials Advisory Group.
Conflict of interest: Anne Black has served on an advisory board which are funded by industry in the last 24 months
112
biographies
Speakers’
Borgsteede, Sander*
PHARMACOTHERAPY SESSION - Safe medication use in patients with cirrhosis
Affiliation: Health Base Foundation

Country: The Netherlands
CURRENT STATUS, POSITION
Sander Borgsteede is Pharmacist, clinical pharmacologist and epidemiologist and works as Science and Research Manager at Health Base Foundation in Houten. He is responsible for the development of new content and methodology for alerts in clinical decision support for medication and comprehensible patient information. Since 2016, he has developed recommendations for medication in patients with cirrhosis.
EDUCATION
Pharmacy (PharmD, 2000), PdD (2006) Epidemiology (MSc, 2009), Clinical Pharmacology (specialisation, 2019).
RESEARCH AREA
Sander Borgsteede’s research focusses on the development and performance of recommendations for safe use of medication, such as drug-drug interactions and drug-disease interactions.
Conflict of interest: Sander Borgsteede is employed at Health Base Foundation (HBF), an independent, noncommercial foundation, that maintains a drug information database (Pharmabase) and supports healthcare professional with a clinical decision support system. The information that is part of this education is subject to medical information provided by HBF. HBF has a scientific, non-commercial interest in the products being studied.
Briel, Matthias
SEMINAR ER2 - Clinical trials - getting actively involved
Affiliation: University Hospital Basel
Country: Switzerland
CURRENT STATUS, POSITION
Matthias Briel is currently leading the CLEAR Methods Center in the Division of Clinical Epidemiology at the University of Basel, Switzerland. He teaches health research methodology to under- and postgraduate audiences. He is also Adjunct Professor at the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Canada.

EDUCATION
Matthias Briel studied Medicine at the University of Freiburg (Germany) and completed a Doctoral Thesis in Medicine in 2000. He received a MSc in Health Research Methodology at McMaster University, Hamilton, Canada (2008) and a PhD in Epidemiology at the University of Basel (2018). In 2014, he completed his Swiss Board Certification in Prevention & Public Health.
RESEARCH AREA
Matthias Briel’s research focusses on various aspects of clinical trial methodology and evidence synthesis.
Conflict of interest: No conflict of interest declared.

113 Speakers’ biographies FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT
PATIENTS?
REALLY MATTERS TO
Speakers’
YOUR SWISS PARTNER

Based in Switzerland and privately-owned, Labatec is a pharmaceutical company with a 50-year track record of delivering high quality products to European and Emerging markets.
INJECTABLES PRODUCTS

Meeting the needs of the hospital sector, we deliver medicines that help to improve quality of life for patients. Manufactured in Europe, our growing product portfolio includes trusted European brand of injectables and orals for hospital use.
Since its acquisition, Labatec has rapidly expanded from its Swiss base to the emerging markets region, entering a dozen countries in the last twelve years, with presence in both retail and hospital segments.
114
biographies
OUR STORY si���������������������� OUR STORY si����������������������
Labatec Pharma was founded in Switzerland in 1957 and acquired in 2008 by Dr. Samih Darwazah, founder of Hikma Pharmaceuticals.
Caccese, Erminia
SEMINAR SPD1 - Medicines procurement - don’t forget patients’ needs!
Affiliation: ESTAR Tuscany region Italy

Country: Italy
CURRENT STATUS, POSITION
Erminia Caccese is currently a Hospital Pharmacist at ESTAR Florence. She is responsible for the innovative tenders of medical devices and drugs for the health service of the Tuscan region.

EDUCATION
Erminia Caccese studied Pharmacy at the University of Perugia and became licensed pharmacist in 1996. In 2005, she obtained a PhD in hospital pharmacy specialisation from Florence University for her work on Preparation of customized bags for cancer patients and application of pharmacoeconomic models for the definition of the “value-based price” of drugs and medical devices.
RESEARCH AREA
Erminia Caccese’s research focusses on value-based procurement of medical devices and definition of the price of drugs and innovative medical devices on the basis of cost-effectiveness.
Conflict of interest: No conflict of interest declared.
Chrapkova, Kornelia
YOUNG PROFESSIONAL SESSION - Learning from the career journeys of others
Affiliation: Institute for Clinical and Experimental Medicine
Country: Czech Republic
Kornelia Chrapkova, Head of Clinical Pharmacy Department, Institute for Clinical and Experimental Medicine, will talk about how she achieved her dream of working in a hospital pharmacy and becoming a clinical pharmacist.

During her presentation, she will be talking about her professional journey starting in a community pharmacy, followed by studying and gaining experience abroad and other important milestones which helped her to implement and develop clinical pharmacy service in the largest transplant centre in Czech Republic and Slovakia.
Conflict of interest: No conflict of interest declared.
115 Speakers’ biographies
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
de Vlieger, Jon
SYNERGY SATELLITE EVENT: Non-Biologic Complex Drugs (and nano medicines)
Affiliation: Foundation Lygature

Country: The Netherlands
CURRENT STATUS, POSITION
Jon de Vlieger is currently serving as Strategy Director at Foundation Lygature, a non-profit organization driving the development of new medical solutions for patients by managing public-private partnerships involving academia, industry and society. He is the Coordinator of the European Lead Factory, Europe’s largest drug discovery consortium, as well as coordinator of the Working Group on Non-biological complex drugs. He also serves as a Board Member at the Federation for Innovative Drug Research Netherlands.
EDUCATION
Jon de Vlieger studied Pharmaceutical Sciences at the VU University in Amsterdam and obtained a PhD in Bioanalytical Chemistry in 2011, as part of a public-private partnership in the Dutch Top Institute Pharma Programme.

RESEARCH AREA
Jon de Vlieger coordinates several international public-private partnerships, such as the European Lead Factory on early drug discovery and the Non-Biological Complex Drugs Working Group on regulatory innovation. He is a co-Editor of the book on NBCDs in the AAPS Advances in the Pharmaceutical Sciences Series, co-Author on a series of key-papers related to regulatory challenges for NBCDs and publishes on the value of public-private partnerships in drug discovery and development.
Conflict of interest: No conflict of interest declared.
116
biographies
Speakers’
del Carmen Climént Palmer, María
KEYNOTE 2 - Improving the communication of risks and benefits to patients
Affiliation: /
Country: Mexico
CURRENT STATUS, POSITION
María del Carmen Climént Palmer has just started a new role as Risk Communication Lead at the MHRA. Prior to that, Maria worked at the Winton Centre for Risk and Evidence Communication during four years, where she worked to improve risk communication in health and journalism.
EDUCATION
María del Carmen Climént Palmer studied Veterinary Medicine and got an MSc in Animal Health focused on breast cancer at the National Autonomous University of Mexico (UNAM). She graduated in 2011 and 2013, respectively. In 2015 she studied an MSc in Science Communication at the University of Sheffield. Before joining the Winton Centre in 2019, she worked for a decade as a science journalist on TV and radio.
RESEARCH AREA
María del Carmen Climént Palmer’s research focusses on improving risk communication within healthcare settings, particularly in transplants, COVID-19 and treatment options.


Conflict of interest: No conflict of interest declared.
Deuster, Stefanie*
YOUNG PROFESSIONAL SESSION - Learning from the career journeys of others
Affiliation: University Hospital Basel

Country: Switzerland
Stefanie Deuster, Head of the department quality assurance in the pharmacy, University Hospital Basel (Switzerland), whose career focusses on the preparation of drugs for the special needs of patients as well as the manufacturing of investigational medicinal products for clinical trials - and all the requirements for assuring the quality of the drugs. She will show possibilities to create and shape the general requirements for pharmaceutical preparation in pharmacies through cooperation and networks, and also talk about the different pharmacies (production and quality assurance). Having joined several working groups as Expert, on the national level with the Swiss authorities and also once on EU level in Strasbourg, she will discuss the needs and possibilities of pharmacy preparation with the authorities and to influence the requirements to facilitate production in pharmacies.
Conflict of interest: Family member working for industry
117 Speakers’ biographies FROM DRUG
TO
WHAT
DESIGN
TREATMENT SUCCESS
REALLY MATTERS TO PATIENTS?
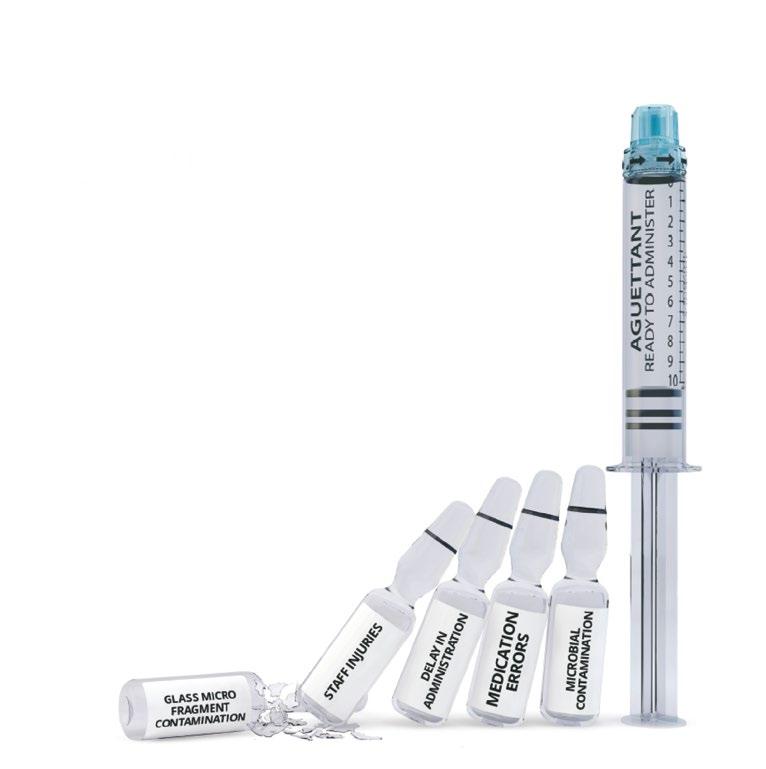
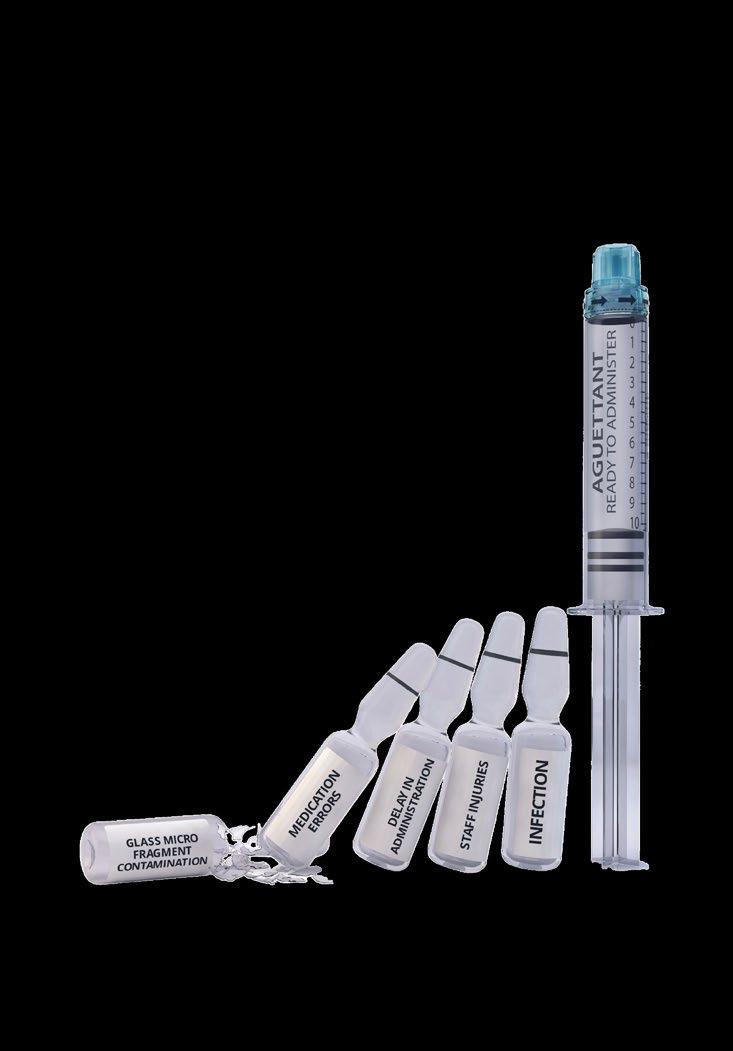
Devaney, Andrea*
INTERACTIVE SESSION 1 - The expanding role of the hospital pharmacists in the care of pre and post renal transplant patients
Affiliation: Oxford University Hospitals NHS Foundation Trust

Country: United Kingdom
CURRENT STATUS, POSITION
Andrea Devaney is a Consultant Pharmacist in Transplantation and Renal Services at the Oxford Transplant Centre, a supra-regional transplant centre within NHS South East. She is responsible for the renal pharmacy team delivery of a clinically led, patient-centred medicine management service to transplant and renal patients at Oxford University Hospitals NHS Foundation Trust. Her area of expert clinical practice is solid organ transplantation - kidney, pancreas and intestinal transplant.
EDUCATION
Andrea Devaney studied Pharmacy at the University of Wales, Cardiff (UWIST), and qualified as a pharmacist in 1989. She completed a diploma in clinical pharmacy in 2001 at University of Wales, Cardiff (UWCC), and an MSc in Pharmacy Practice at University of London (UCL) in 2009. She is an independent non-medical prescriber having gained a Practice Certificate in Independent Prescribing, University of Reading, in 2013. She was designated a Fellow of the Royal Pharmaceutical Society in 2014.

RESEARCH AREA
Key interest areas include the role of the pharmacist in transplant assessment and out-patient clinics, medication adherence and medicine management strategies and generic immunosuppression. Andrea has authored several articles on the role of generic immunosuppression.
Conflict of interest: Member of the GSK Advisory Board, received a Speaker’s fee from Astellas and is an AHP Educational Faculty Member (Advisory) for Sandoz (Novartis)

119 Speakers’ biographies
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?

Ferreira, Joao
SEMINAR SPD2 - The shortage pandemic - why we haven’t succeeded yet
Affiliation: European Medicines Agency (EMA)


Country: The Netherlands
CURRENT STATUS, POSITION
Joao Ferreira is a Shortages Specialist at the European Medicines Agency (EMA). He has been part of EMA’s Supply and Availability Office, since 2021, dealing with shortages and availability of medicines in Europe. He is responsible for implementing EMA’s new extended mandate in medicine shortages. He joined EMA in 2014 and has 10+ years of experience in EU Medicines Regulation, Safety Monitoring and Transparency, as well as in Policy and Crisis Management & Preparedness. Prior to joining the EMA, he worked in (the field of) Regulatory Affairs and Pharmacovigilance consultancy.
EDUCATION
Joao Ferreira studied Pharmacy at the University of Algarve, registering as a pharmacist in 2013. He has been a Member of the Portuguese Pharmaceutical Society’s Youth Council since 2022.

RESEARCH AREA
Joao Ferreira specialises in Shortages and Availability issues working with the EU Medicines Regulatory Network and Stakeholders towards strengthening the availability of medicines/medical devices to protect the health of European citizens and animals by making medicines, medical devices and crisis-relevant products available. Currently, he is a Member of the EMA/HMA Task Force on Availability of Authorised Medicines (TF-AAM), and a Member of the EMA Executive Steering Group on Shortages and Safety of Medicinal Products (MSSG).
Conflict of interest: No conflict of interest declared.
Franklin, Bryony Dean
SPECIAL INTEREST GROUP (SIG) DISSEMINATION - Investigation of Medication Errors in Intensive Care Units
Affiliation: UCL School of Pharmacy / Imperial College NHS Trust
Country: United Kingdom
CURRENT STATUS, POSITION
Bryony Dean Franklin is Professor of Medication Safety at UCL School of Pharmacy and Executive Lead Pharmacist (Research) at Imperial College Healthcare NHS Trust. She is also a theme lead for the NIHR Imperial Patient Safety Translational Research Centre and Co-Editor-in-Chief of BMJ Quality and Safety.
EDUCATION
Bryony Dean Franklin studied pharmacy at the University of Bath and registered as a Pharmacist in 1991. She has subsequently obtained an MSc in Clinical Pharmacy, a BA in Theology and a PhD in Pharmacy Practice. Her clinical background is as a critical care pharmacist.

RESEARCH AREA
Bryony Dean Franklin’s research focusses on patient safety in general and medication safety in particular, with 30 years’ experience studying medication errors in both hospital and community practice.
Conflict of interest: No conflict of interest declared.
121 Speakers’ biographies
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
M ediM ix®
IMF GmbH is pleased to introduce the FIRST intelligent peristaltic pump able to facilitate ever yday pharmac y produc tion's tasks.

A Flexible, Efficient, Safe and Compac t solution needed in ever y pharmac y, the MediMix vigo was designed with the aim to reduce pharmac y technician's work load without compromising precision and traceability.
The automatic pharmac y solution for liquid transfer:
Repeats Repeats Repeats and and …
Ÿ High �lling speed up to 850 ml per minute
Ÿ Simple and intuitive operation through integrated touch screen
Ÿ Can be networked with common order entr y management systems
Flexible: Ideal for drugs reconstitution, IV transfer & mixing, plus filling of bags, syringes, elastomeric devices, and oral syringes
Efficient: Aimed to reduce pharmac y technician's work load

Safe: Allows full traceabilit y to the pharmacist, uses barcode technology to read formulations and verify the right ingredient is used and can be integrated with a scale for an automatic weight control reading
Compac t: With its ver y small footprint, offers solution for any size laminar flow cabinets or isolators
122 Speakers’ biographies
27th Congress of the EAHP 2023 Visit us at Stand 27
Franssen, Casper*
INTERACTIVE SESSION 2 - The art of estimating renal function in adult patient groups
Affiliation: University Medical Center Groningen

Country: The Netherlands
CURRENT STATUS, POSITION
Casper Franssen is a Nephrologist at the University Medical Center Groningen in The Netherlands. He is responsible for the CKD outpatient clinic and the dialysis unit.

EDUCATION
Casper Franssen studied at the University of Nijmegen and became licenced as a Nephrologist in 1994. In 1998, he obtained a PhD on the topic of vasculitis in the University Medical Center Groningen in The Netherlands.
RESEARCH AREA
Casper Franssen’s research focusses on the effects of chronic kidney disease and hemodialysis on the cardiovascular system.
Conflict of interest: Casper Franssen has served on the Pharvaris and Baxter advisory boards, which were funded by industry in the last 24 months.
Gillespie, Ulrika
YOUNG PROFESSIONAL SESSION - Learning from the career journeys of others
Affiliation: Uppsala University Hospital
Country: Sweden
Ulrika Gillespie is Department Chief Pharmacist at Uppsala University Hospital and Associate Professor at the Department of Pharmacy, Uppsala University (Sweden). As one of the first clinical pharmacists in Sweden, her career has focused on the development, implementation and evaluation of clinical pharmacy services in a hospital setting. In her presentation, she will describe her journey where she has used pharmacy practice research to promote and refine the clinical services and talk about her experiences - good and bad - of running everything from small student projects to large randomized controlled trials.
Conflict of interest: No conflict of interest declared.
123 Speakers’ biographies
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?



biographies Our MiniLab is an automated compounding concept to produce personalized medicines in pharmacies. Visit us at booth 48 www.curifylabs.com
Speakers’
Glintborg, Dorte
SEMINAR SPD1 - Medicines procurement - don’t forget patients’ needs!
Affiliation: Danish Medicines Council

Country: Denmark
CURRENT STATUS, POSITION
Dorte Glintborg is currently a Pharmacist at the Danish Medicines Council. She is working with development of guidelines and evaluation of new expensive drugs in order to get ‘most value of health for money’ spend on drugs in Danish hospitals.

EDUCATION
Dorte Glintborg graduated as MSc. Pharm. in 1996 and has been working with Rational Pharmacotherapy for over 20 years. She has been an employee at the Danish Medicines Agency and Danish Health Authority since 2001. In 2013, she joined the Danish Council for Expensive Hospital Medicine and transferred, in 2017, to the Danish Medicines Council.
RESEARCH AREA
Dorte Glintborg’s research are focused on pharmacoepidemiology investigating prescribing habits and rational use of drugs.

Conflict of interest: No conflict of interest declared.
Graabæk, Trine
WORKSHOP 2 - Patient reported outcome measures - what tools can be used?
Affiliation: Odense University Hospital
Country: Denmark
CURRENT STATUS, POSITION
Trine Graabæk is currently a Researcher at Hospital Pharmacy Funen in Denmark. She is also teaching pharmacy students at both the University of Copenhagen and the University of Southern Denmark.
EDUCATION
Trine Graabæk studied Pharmacy at the University of Copenhagen and became licensed pharmacist in 2008. In 2016, she obtained a PhD in clinical pharmacy and patient safety at University of Southern Denmark.
RESEARCH AREA
Trine Graabæk research focusses on clinical pharmacy activities, medication use among older persons, and communication within pharmacy practice.
Conflict of interest: No conflict of interest declared.
125 Speakers’ biographies
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
Global Healthcare Industry Statistics

66 % of healthcare organizations were hit by ransomware in 20213
61% of attacks were successful in encrypting data 3
~10,1 MILLION€ Average cost to hospital due to a data breach 4

94 % of healthcare organizations experienced major operational impacts from the attack 3


UP TO 1 MONTH
The time for 25% of organizations to recover following a cyberattack 3
65 % of data restored even after ransom paid 3
2. Conti Cyber Attack on the HSE. Health Services Executive. December 3, 2021. Accessed September 8, 2022. https://www.hse.ie/eng/services/publications/conti-cyber-attack-on-the-hse-full-report.pdf.
3. The State of Ransomware in Healthcare 2021. Sophos. 2021. Accessed February 2, 2022. https://www.sophos.com/en-us/medialibrary/pdfs/whitepaper/sophos-state-of-ransomware-in-healthcare-2021-wp.pdf.
4. Cost of a Data Breach Report 2022. IBM. Accessed August 19, 2022. https://www.ibm.com/downloads/cas/3R8N1DZJ

HELP PROTECT YOUR PATIENTS AND PHARMACY FROM CYBERATTACKS An added dose of security in every bag you compound. Advanced Cybersecurity from E xactaMix Pro Automated Compounder Baxter's ExactaMix Pro automated compounder brings advanced cybersecurity to the modern pharmacy. With the latest encryption technology, host-based firewall, role-based access, and certification to the FDA recognized UL 2900-2-1 cybersecurity standard, you can be confident that your compounder is well-protected against the next cyberattack. Healthcare system breaches present multiple risks As a key member of the clinical team, patients rely on you for the safety of their treatment and personal information. Healthcare equipment and systems, including those used in the pharmacy, are considered a major target for cyberattack. PATIENT SAFETY HEALTHCARE DATA AND PATIENT RECORDS ACCESS CLINICIAN ABILITY TO PROVIDE CARE PRESCRIPTION ACCURACY Baxter and ExactaMix Pro are trademarks of Baxter International Inc. Any other trademarks, product brands or images appearing herein are the property of their respective owners. GBU-CN8-220035 v1.0 09/2022 For the safe and proper use of the devices mentioned herein, refer to the appropriate Instructions for Use or Operator Manual. REFERENCES 1. Health Sector Cybersecurity: 2021 retrospective and 2022 look-ahead. U.S. Department of Health and Human Services (HHS). March 3, 2022. Accessed July 27, 2022. https://www.hhs.gov/sites/default/files/2021-retrospective-and-2022-look-ahead-tlpwhite.pdf.
Guntschnig, Sonja
SPECIAL INTEREST GROUP (SIG) DISSEMINATION - The EAHP roadmap toward eliminating avoidable harm
Tauernklinikum Zell am See
CURRENT STATUS, POSITION
Sonja Guntschnig, Mag. pharm., MSc currently works as a clinical pharmacist at Tauernklinikum Zell am See. She is part of the multidisciplinary ward round team, undertaking clinical pharmacy interventions and responsible for medicines information.

Sonja Guntschnig studied Pharmacy at the Karl-Franzens University in Graz. She obtained her MSc degree in “Advanced Clinical Pharmacy Practice” from Queen’s University in Belfast in 2019. She is currently a part-time PhD student at Ulster University and the Medicines Optimisation Innovation Centre in Northern Ireland.
Sonja Guntschnig’s research focusses on clinical pharmacy interventions, antimicrobial stewardship and improvement of patient safety.
Conflict of interest: No conflict of interest declared.
Harjans, Anna
SEMINAR SPD3 - Green Hospital - The Role of Hospital Pharmacists
Affiliation: Heidelberg University Hospital Germany



CURRENT STATUS, POSITION
Anna Harjans is currently working at Heidelberg University Hospital in production and compounding of nonsterile medicines as well as compounding of parenteral nutrition. Besides that, she deals with sustainability in the hospital pharmacy.

EDUCATION

Anna Harjans studied Pharmacy at Muenster University and became a licensed pharmacist in 2021. Since January 2023, she has been a PhD candidate in pharmaceutical technology at HHU Düsseldorf.
RESEARCH AREA
Anna Harjans research focusses on 3D printed drug formulations in the hospital pharmacy.
Conflict of interest: No conflict of interest declared.

127 Speakers’ biographies TO TREATMENT SUCCESS WHAT REALLY MATTERS
Speakers’ biographies



We personalize healthcare! individual composition flexible dose sustainable
Stand: 54
Solution
FLEXDOSE Printing!
The digital system of FLEXDOSE printer, software and pharmaceuticals for 2D and 3D printing of personalized drugs


The innovative technology for personalized precision medicine that brings measurable benefits to everyone
Become a part of this innovation

Let's talk about it!
FLEXDOSE Printing
Personalized Medicine with 2D and 3D printing
Our Vision:
„In the future, every patient should have access to personalized medicine. This means the desired active pharmaceutical ingredients are delivered in individual dosages and compositions, customizable at any time.
Individually prescribed by the doctor, printed in the pharmacy, often made possible by the health insurance companies in many countries.”
DiHeSys GmbH
Marie-Curie-Strasse 19
73529 Schwäbisch Gmünd
Phone +49 (0) 71 71 - 871 30 87
business-development@dihesys.com

Support hours:
Monday - Thursday: 08:30 a.m. to 5:00 p.m.
Friday: 08:30 a.m. to 3:00 p.m.
128
Hayes, Jo
SEMINAR CPS2 - From Benefit to Burden - safely discontinuing medicines at the end of life
Affiliation: Marie Curie Hospice Cardiff and the Vale Country: United Kingdom

CURRENT STATUS, POSITION
Jo Hayes is a Consultant in palliative medicine, Medical Director of the Marie Curie Hospice, Penarth and Course Tutor for the Cardiff University MSc in Palliative Medicine for Healthcare Professionals.

EDUCATION
Jo Hayes qualified in 1991 from the University of Wales College of Medicine and completed her MSc in Palliative Medicine in 2003.
RESEARCH AREA
Jo Hayes leads a community specialist palliative care team and regularly assists her patients to rationalise their medicines as they approach the end of their lives. She is currently leading on the writing of an e-learning module on deprescribing for the End of Life Care for All e-learning (e-ELCA) programme for Health Education England.
Conflict of interest: No conflict of interest declared.
Hickey, Gary
KEYNOTE 3 - Patient involvement in pharmacy practice research: no decision about me without me

Affiliation: University of Southampton Country: United Kingdom
CURRENT STATUS, POSITION
Gary Hickey is leading on the development of the ‘Agora Digital Centre: The online centre for connecting people with research’ at the University of Southampton. He is also a Senior Research Manager at the National Institute for Health and Care Research, providing advice and guidance on patient and public involvement and engagement in research.
EDUCATION
Gary Hickey has a PhD in Health Studies.
RESEARCH AREA
Gary Hickey provides advice and guidance on how to involve and engage with patients and public in research, including the co-production of research.
Conflict of interest: No conflict of interest declared.
129 Speakers’ biographies
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?



130 Speakers’ biographies Stand 52 Trusted, evidence-based medicines information to support decision-making about.medicinescomplete.com MedicinesComplete from Pharmaceutical Press, the Royal Pharmaceutical Society’s knowledge business, makes it easy for health professionals to access essential medicines information at the point of care. Scan the QR code to request a complimentary trial
Hoppe-Tichy, Torsten*
YOUNG PROFESSIONAL SESSION - Learning from the career journeys of others
Affiliation: Heidelberg University Hospital

Country: Germany
Torsten Hoppe-Tichy, Chief Hospital Pharmacist at Pharmacy Department, Heidelberg University Hospital (Germany), who will focus his presentation on the reasons and motivations for his career path. He will recount the gaps and challenges, how he overcame them and also on the fun and pains of being a chief hospital pharmacist.
Conflict of interest: Speaker advisory board: BD, Dailichi, PCM-Biosimilaris, Roche
Jager, Nynke
SEMINAR ER1 - Patients’ individuality: challenges facing hospital pharmacists
Affiliation: Radboudumc
Country: The Netherlands
CURRENT STATUS, POSITION
Nynke Jager is a Hospital Pharmacist and Clinical Pharmacologist at the Radboudumc in Nijmegen (The Netherlands), where she is responsible for the therapeutic drug monitoring process. She is also a Senior Researcher in the field of PKPD of antibiotic agents.
EDUCATION
Nynke Jager studied pharmacy at the University of Groningen (The Netherlands). She became a licensed pharmacist in 2010. In 2014, she obtained a PhD in bioanalysis and clinical pharmacology. Subsequently, she started her training in hospital pharmacy at the Amsterdam University Medical Center. After finishing this training, she was a visiting academic at the University of Queensland Centre for Clinical Research.

RESEARCH AREA
Nynke Jager’s research focusses on PKPD of antibiotic agents, with the aim to optimize therapy for the individual patient.
Conflict of interest: No conflict of interest declared.

131 Speakers’ biographies
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?


The Compounder Making parenteral medication safer Virtual Reality Demonstrations! EAHP 2023 BOOTH 13 Book your demo by e-mailing info@thecompoundingcompany.com The Compounding © Company thecompoundingcompany.com
Kerskes, Marieke
SEMINAR INT2 - The art of estimating renal function in adult patient groups
Affiliation: Catharina Hospital Eindhoven

Country: The Netherlands
CURRENT STATUS, POSITION
Marieke Kerskes is currently a Hospital Pharmacist at the Catharina Hospital in Eindhoven, the Netherlands. She works together with nephrologists, nurse practitioners and dieticians in multidisciplinary care for patients with renal failure and haemodialysis patients. She is also a member of the special interest group Nephrology of the Dutch Association of Hospital Pharmacists (NVZA).
EDUCATION
Marieke Kerskes studied Pharmacy at the University of Groningen and became licensed pharmacist in 2002 and licensed hospital pharmacist in 2006.

RESEARCH AREA
Marieke Kerskes’ research focusses on drug related problems in nephrology and on pharmacogenetics.
Conflict of interest: No conflict of interest declared.
Lagarce, Frederic
SEMINAR PC2 - Quality assurance of pharmacy preparations - a key for treatment success
Affiliation: Angers University Hospital
Country: France

CURRENT STATUS, POSITION
Frederic Lagarce is currently Professor of Pharmaceutical technology and biopharmacy at the faculty for health sciences in Angers. He is also the Chief Pharmacist of the pharmacy department of the Angers University hospital.

EDUCATION
Frederic Lagarce studied pharmacy in the faculty of Paris Saclay in France and followed a 4-year internship at Angers University Hospital. He became a Licensed Pharmacist in 2000 and then he entered a PhD programme. After receiving his PhD in pharmaceutical technology/pharmacology and pharmacokinetics in 2004, he was Researcher, then Assistant Professor in 2008 and Full Professor in 2012. In parallel, since 2005, he has worked as a pharmacist on drug preparations and was appointed as Chief Pharmacist in 2021.
RESEARCH AREA

Frederic Lagarce research area has focused on the biopharmaceutical aspects of nanomedicine for more than 20 years after his PhD on microparticulate systems for spinal delivery. His field of interest stands between rational design of drug delivery systems for the oral route and their evaluation in vivo or ex vivo. He also has an applicated field of research in pharmaceutical technology in hospital pharmacy and stability studies for new formulations.
Conflict of interest: No conflict of interest declared.
133 Speakers’ biographies
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?



































134 Speakers’ biographies Your Swiss global partner for any Unmet Medical Need all over the world Elixi International SA | elixi-int. | +41 91 682 20 40 • Unlicensed Medicines • Orphan Drugs • Oncologicals • siRNA / mRNA Medicines • Hemoderivates • Pharmaceuticals
Lampert, Markus
SEMINAR CPS1 - Pharmacogenetic testing to optimise therapy
Affiliation: Solothurner Spitaeler AG

Country: Switzerland
CURRENT STATUS, POSITION
Markus Lampert is a hospital and clinical Pharmacist. In his institution, he is responsible for clinical pharmacy services. He is also affiliated with the Pharmaceutical Care Research Group, University of Basel as a Senior Research Associate and Senior Reader in clinical pharmacy in undergraduate and postgraduate education.
EDUCATION
In 1992, following his studies in pharmacy at the University of Basel, Markus Lampert passed the federal exam and, in 1997, obtained his PhD. He then specialised in hospital and clinical pharmacy alongside with his academic activities which culminated in the habitation in clinical pharmacy, in 2021.

RESEARCH AREA
Markus Lampert’s research areas are mainly dedicated to the development and evaluation of clinical pharmacy services and interventions including the integration of pharmacogenetics.

Conflict of interest: No conflict of interest declared.
Marques, Rui Pedro
WORKSHOP 1 - Is compounding (always) the answer to drug shortages?
Affiliation: Hospital Santa Maria - Centro Hospitalar Universitario Lisboa Norte
Country: Portugal
CURRENT STATUS, POSITION
Rui Pedro Marques works as a Hospital Pharmacist at Hospital Santa Maria (Centro Hospitalar Universitario Lisboa Norte), where he is currently the supervisor of the Pharmaceutical Compounding Unit. He is also a member of the Commission for Health Technology Assessment (CATS) of INFARMED, Portuguese national regulatory agency, and Invited Professor at the Faculty of Pharmacy of the University of Lisbon.
EDUCATION
Rui Pedro Marques studied Pharmacy at the University of Lisbon and became licensed pharmacist in 2008. In 2020, he obtained a PhD in Pharmacoepidemiology at the University of Lisbon for his work on measuring and comparing clinical and patient-reported outcomes, at real-world conditions, from competing treatments in Oncology, complemented with systematic reviews and meta-analysis of clinical trials.
RESEARCH AREA
Rui Pedro Marques professional interests are mainly concentrated on drug management and compounding, particularly of anticancer therapies. His current research focusses on comparative effectiveness of competing treatment options in different settings.
Conflict of interest: No conflict of interest declared.
135 Speakers’ biographies
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?

136 Speakers’ biographies swisslog-healthcare.com The hidden dimension of pharmacy automation. Discover how automated medication management help you increasing efficiency and patient safety in your hospital. With Swisslog Healthcare the entire process from ordering, storage, and medication administration to documentation of medication is recorded and tracked. Visit us at booth 32 .
Martínez-Sesmero, José Manuel
SEMINAR IG2 - The road to e-hospital pharmacy - are we there yet?
Affiliation: Hospital Clinico San Carlos (HCSC) Country: Spain

CURRENT STATUS, POSITION
José Manuel Martínez-Sesmero is Director of Pharmacy HCSC; EAHP Spanish Ambassador; Innovation Manager at SEFH; Associate Professor at UCM.

EDUCATION
José Manuel Martínez-Sesmero studied Pharmacy at Universidad Complutense, Madrid, and became a licensed pharmacist in 1999. In 2012, he obtained a PhD in epidemiology, nutrition and cardiovascular health for his work at the Hospital Virgen de la Salud,Toledo. He has also been involved in different post-graduate courses like: Master Programme in direction and management of healthcare services, Master Programme in pharmacoeconomics, Master Programme in quantitative and analyticalmethods for evidence-based medicine, etc. He is also part of the Editorial Board of international pharmaceutical journal at International Pharmaceutical Federation (FIP).
RESEARCH AREA
Health Economics, Drug Evaluation, eHealth, Big Data, Artificial Intelligence.
Conflict of interest: No conflict of interest declared.
McCarthy, Suzanne
SPECIAL INTEREST GROUP (SIG) DISSEMINATION - Investigation of Medication Errors in Intensive Care Units
Affiliation: University College Cork Country: Ireland
CURRENT STATUS, POSITION
Suzanne McCarthy is currently a Senior Lecturer in Clinical Pharmacy at the School of Pharmacy, University College Cork.
EDUCATION
Suzanne McCarthy studied Pharmacy at the Robert Gordon University in Aberdeen, Scotland and became a licensed pharmacist in 2004. In 2009, she obtained a PhD in pharmacoepidemiology at the University of London. She has postgraduate qualifications in clinical pharmacy, statistics and medical education.

RESEARCH AREA
Suzanne McCarthy ‘s research focusses on medication safety and pharmacovigilance, across different clinical areas including paediatrics and mental health.
Conflict of interest: No conflict of interest declared.
137 Speakers’ biographies
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?

Melo Gouveia, Antonio
SEMINAR IG3 - How the pandemic changed hospital pharmacy management - moving forward
Affiliation: Hospital das Forcas Armadas, Polo de Lisboa
Country: Portugal
CURRENT STATUS, POSITION
Antonio Melo Gouveia is currently a Hospital Pharmacist at the Hospital das Forcas Armadas in Lisboa, where he is responsible for the area of oncology. He is also Vice President of the committee in charge of health technology assessment at the Portuguese medicine’s agency, INFARMED IP.

EDUCATION
Antonio Melo Gouveia got his degree in pharmacy in 1987 and has followed several post-graduations in management courses. He is regulatory affairs certified as Specialist in hospital pharmacy and regulatory affairs by the Order of Pharmacists, the regulator of the profession in Portugal.
RESEARCH AREA
• Hospital pharmacy practice
• Oncology
• Regulatory affairs

Conflict of interest: No conflict of interest declared.
Mengato, Daniele
SEMINAR IG1 - Hospital support for pharmacy research activities
Affiliation: Padova University Hospital
Country: Italy
CURRENT STATUS, POSITION
Daniele Mengato is currently working as a Hospital Pharmacist at the University Hospital of Padova, Italy. His principle activities fall within the field of infectious diseases, in which he coordinates clinical pharmacy and therapeutic appropriateness activities. He is also involved in clinical pharmacy activities in other areas, mainly in pediatrics, and in various researches connected with the activities of the Department of Pharmacy.
EDUCATION
Daniele Mengato graduated from the University of Padua with a degree in Pharmacy in 2013 and received his specialization in Hospital Pharmacy from the same university in 2017. He also obtained a Master of Science in Biostatistics in 2021.

RESEARCH AREA
Daniele Mengato’s research activities fall into several areas. Primarily, it is related to clinical pharmacy activities in the field of infectious diseases, with a focus on the real-world use of COVID-19 and/or HIV drugs and/or antibiotics. Secondly, he is involved in research on already published non-original data through the application of systematic reviews and meta-analysis.
Conflict of interest: No conflict of interest declared.
139 Speakers’ biographies
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
AntiVirus®

pneumatic tube meets robotics
Automated box and unit dose transport by Sumetzberger
Sumetzberger ultra-modern, computercontrolled pneumatic tube systems meets high tech box picking and unit dose machines.

Innovative interface solutions for automated, controlled, documented and safe transport of medicines. Also approved for narcotics.
JUST IN TIME Medication delivery
24/7
Unit dose transport and tracking Made-to-order medication for each patient.

Box picking, transport and tracking Centralized, space -saving drug distribution.
Would you like to learn more about innovative AntiVirus® pneumatic tube solutions? Contact us!




140
Ing. Sumetzberger GmbH. | Leberstrasse 108-110 | 1110 Vienna | +43 1 74035 0 | pt.sales@sumetzberger.at
Speakers’ biographies
Box Picking Unit Dose
PREMIUM QUALITY YEARS STAND 21
Mozgis, Dzintars
SEMINAR IG3 - How the pandemic changed hospital pharmacy management - moving forward

Affiliation: Riga Stradins University

Country: Latvia
CURRENT STATUS, POSITION
Dzintars Mozgis works as an Associate Professor in the Department of Public Health and Epidemiology at Riga Stradins University, in matters of healthcare management. He is also currently a Deputy Director of the Center for Diseases Prevention and Control of Latvia, where he is responsible for prevention and control of noncommunicable diseases and health promotion mostly. Also, he is still practicing as a pediatric surgeon in the State Emergency Medical Service.
EDUCATION
Dzintars Mozgis studied at the Medical Institute in Riga and became a pediatrician in 1982. In 1987, he obtained specialty pediatric surgery and in 2004, a PhD at Riga Stradins University for his work in research of anorectal malformations. In 2005, he was elected as an Assistant Professor, and in 2009 as an Associate Professor at Riga Stradins University. From 1995 to 2010, he served as CEO at the University Children Hospital in Riga, then worked as a medical expert in the State Emergency Medical Service until 2012. He has since then been working as a Deputy Director at the Centre for Diseases Prevention and Control of Latvia. He is also a member of the Management Board of the European Centre for Drugs and Drug Addiction form Latvia.

RESEARCH AREA
Dzintars Mozgis’research focusses on healthcare management, history of medicine, pediatric surgery, public health and comparative trends in the consumption of drugs.
Conflict of interest: No conflict of interest declared.
141 Speakers’ biographies
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
Speakers’ biographies
Ultra Phasys is a revolutionary automatic loading system capable of handling all packages that can be stored into the pharmacy automated warehouse RIEDL Phasys (including cylindrical items).
Developed in collaboration with leading research institutions, Ultra Phasys achieves the highest performances with up to 360 packs per hour. It is fully automatic, indeed it works even when the pharmacy is closed.

142
a brand of Visit us at booth No12 www.gpi.it A smart shelf system based on RFID technology for a safe and reliable traceability and control of your medical equipment and devices. Introducing
Mulac, Alma
SPECIAL INTEREST GROUP (SIG) DISSEMINATION - The EAHP roadmap towards eliminating avoidable harm
Affiliation: University of Oslo
Country: Norway
CURRENT STATUS, POSITION
Alma Mulac is a Postdoctoral Researcher at the University of Oslo. Her main responsibilities include research in the field of digital medication management with specific focus on safety aspects. She is also involved in teaching pharmacy and nursing students.

EDUCATION
Alma Mulac studied Pharmacy at the University of Sarajevo, Bosnia and Herzegovina, where she became an MPharm in 2011. She got her second master’s degree at the University of Oslo in 2016 and in 2022, obtained a PhD in Hospital Pharmacy at the University of Oslo for her work on medication errors in hospitals.
RESEARCH AREA
Alma Mulac’s research focusses on medication errors in hospitals, safety in the medication management process and the role of technology in medication safety.
Conflict of interest: No conflict of interest declared.
Newton, Max*
SEMINAR SPD2 - The shortage pandemic - why we haven’t succeeded yet
Affiliation: IQVIA

Country: Switzerland
CURRENT STATUS, POSITION
Max Newton is an experienced Consultant with 7+ years specializing in Pharmaceuticals and Life Sciences. He manages a team working with strategic partners (governments, national medical agencies, industry associations, pharma companies and suppliers) advising on commercial strategy and the impact of healthcare policies. Before joining IQVIA, he worked at GSK, and is also a guest lecturer at UCL School of Pharmacy.
EDUCATION
Max Newton studied BSc Medical Microbiology & Virology at Warwick University and obtained a MSc Drug Discovery & Pharma Management from the University College London School of Pharmacy focusing on the forecasting the impact of biosimilars within the Latin American market.
RESEARCH AREA
Max Newton’s research focusses on prominent policy projects notably supporting governments and policymakers with their understanding of the shortages issue using IQVIA datasets, having been involved in the UK’s HRT shortage issue, and the Commission’s publication on shortages in Europe.
Conflict of interest: Max Newton, via his employer IQVIA, works with pharmaceutical companies, governments, national competent authorities to provide services and data.
143 Speakers’ biographies FROM
WHAT
DRUG DESIGN TO TREATMENT SUCCESS
REALLY MATTERS TO PATIENTS?
Secures the care pathway
Ensures traceability




Simplifies dispensing




144
574, rue du Chat Botté - ZAC des Malettes - 01700 Beynost - FRANCE Tél. : +33 (0)4 72 25 95 09 - contact@nooddis.fr - www.nooddis.fr
AUTOMATIC NOMINATIVE DISPENSING MACHINE FOR LIQUID ORAL DOSES
O’Hanlon, Niamh
SEMINAR PSQ1 - Become a medication safety pharmacist!
Affiliation: Irish Medication Safety Network (IMSN) Country: Ireland


CURRENT STATUS, POSITION
Niamh O’Hanlon is the current Chair of the Irish Medication Safety Network, a voluntary, independent group of hospital pharmacists from public and private hospitals in Ireland, with the aim to improve patient safety with regard to the use of medicines. She is the Chief II Pharmacist, Medicines Information at St Vincent’s University Hospital, Dublin and a clinical lecturer for MSc in Hospital Pharmacy, Trinity College Dublin.
EDUCATION
• BPharm (Hons) University College London.

• PG Cert Pharm Prac University College London.
• MSc Health Services Management, Trinity College Dublin.
• Grad Dip Risk Management in Healthcare, University College Dublin.
RESEARCH AREA
Medicines Information & Medication Safety.
Niamh O’Hanlon is interested in quality improvement and optimising the safe use of medicines, and has special interest in VTE prevention. A member of international Pharmacists for Anticoagulation Care Taskforce (iPACT), Niamh was awarded Exemplar award from Thrombosis Ireland for her work on Venous thrombosis embolism awareness prevention. She has presented at IHI/BMJ International Forum on Quality & Safety in Healthcare.
Conflict of interest: No conflict of interest declared.
145 Speakers’ biographies
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
Innovative Products for The Compounding Pharmacy
















For over 50 years, IMI has designed medical devices that enhance medication security from the pharmacy to the patient. From Tamper Evident Caps to essential sterile compounding supplies such as connectors and needles, IMI manufactures American-made products to enhance pharmacy compounding operations and strengthen our shared mission of improved patient outcomes.
Visit our Booth to learn more about IMI partnerships and live product demos:
















Booth #49
























biographies IMI-501-AA-130 R1
Speakers’
IMIWEB.COM | 1.800.344.2554 To See All Our Products and Request Samples Visit
Oboh, Lelly


SEMINAR CPS2 - From Benefit to Burden - safely discontinuing medicines at the end of life

Affiliation: South East London Integrated Care Systems






Country: United Kingdom
CURRENT STATUS, POSITION
Lelly Oboh is currently the Overprescribing lead pharmacist, on an 18 month secondment in a clinical leadership role to drive the delivery and implementation of a national initiative to tackle overprescribing in South East London integrated care system. Her substantive role is working as Consultant Pharmacist, Care of older people at Guys and St Thomas NHS Trust. She also has a national role with the NHS Specialist Pharmacy Services to provide profession leadership, advice and support on medicines issues in older people across the UK.
EDUCATION
Lelly Oboh registered as UK Pharmacist in 1993 and completed a PGCert in Pharmacy Practice, School of Pharmacy, University of London in 1999. In 2001, she got a Postgraduate diploma in Community Clinical Pharmacy, Aston University, Birmingham, and in 2006, she did an independent non-medical prescribing qualification at Kings College London. In 2007, she was the first community-based Consultant Pharmacist in the UK and a Member of the Royal Pharmaceutical Society, Great Britain B Pharm (Hons) 22 Class, University of Benin, Nigeria.

RESEARCH AREA
Lelly Oboh’s specialty is medicines optimisation in older people, particularly those living with frailty, multimorbidity and polypharmacy in community-based settings Her work focusses on patient centred care, shared decision making, deprescribing, transitions of care, championing innovative clinical pharmacyled models of care to improve medicines use and facilitating system wide and collaborative integrated approaches to medicines optimisation.
Conflict of interest: No conflict of interest declared.
147 Speakers’ biographies
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
How can we help?
We help physicians, pharmacists and patients find medicines that can save lives.
WHO WE ARE
We are a pharmaceutical company that provides adaptable solutions for the safe and timely supply of pharmaceutical products, with a focus on hard-to-access medicines for patients with unmet medical needs.


WHAT WE DO
We can effectively respond to any needs by leveraging our established relationships within the pharmaceutical industry, which grants us acess to an extensive portfolio of medicines.
Our extended international network, combined with smart technologies and a streamlined supply chain, ensures that healthcare professionals get the right medicine for the right patient in the shortest timeframe, while also helping pharmaceutical companies reach new markets worldwide.
VISION

To be the global sourcing platform for medicinal products, connecting companies, healthcare professionals, and patients.

MISSION
Giving patients access to the medicines they need, through information technology and innovative services.
148 Speakers’ biographies
CONTACTS KEY FACTS WE SUPPLY
Medicinal products that are authorized but are not marketed or no longer commercialized Supply chain disruptions that limit the availability of authorized and marketed products Comparator sourcing, medicines and ancillaries Controlled and Narcotic drugs, temperature-sensitive medicines +351 216 092 994 Rua Leopoldo de Almeida, 11B 1750-137 Lisboa, Portugal info@rbpharma.pt www.rbpharma.pt
UNLICENSED MEDICINES SHORTAGES CLINICAL TRIALS SPECIAL DISTRIBUTION
Onatade, Raliat
KEYNOTE 1 - Personalised medicine - opportunities for hospital pharmacists in clinical practice
Affiliation: North East London Integrated Care Board; Barts Health NHS Trust; North Thames Genomic Medicine Service Alliance
Country: United Kingdom
CURRENT STATUS, POSITION
Raliat Onatade is Chief Pharmacist in North East London, responsible for leading and advising on the strategic development of pharmacy services and medicines optimisation. She is also Chief Pharmacist for North Thames Genomic Medicines Service Alliance.

EDUCATION
Raliat Onatade studied pharmacy at the University of Ife in Nigeria, qualifying in 1986. She obtained a Masters in Clinical Pharmacy from the London School of Pharmacy in 2002, followed by a Masters in Clinical Risk Management from University College London in 2010. In 2019, she obtained a Professional Doctorate in Health from Middlesex University for her work in advancing clinical pharmacy practice.
RESEARCH AREA
Raliat Onatade’s research interests cover clinical pharmacy practice and innovation, medication safety, and quality improvement and implementation science.
Conflict of interest: No conflict of interest declared.
Pitkeathley, Carole
SEMINAR PSQ2 - Lay involvement in prescribing committees – hearing the patient’s voice
Affiliation: National Institute for Health and Care Excellence

Country: United Kingdom
CURRENT STATUS, POSITION
Carole Pitkeathley is a Standing Lay Member of several NICE guideline development and technology appraisal committees, where she brings the patient voice to the heart of discussions and decisions. She is also a member of several NHS England strategic boards, representing the patient’s and service-user perspective, acting a critical friend to the organisation and challenging to ensure improved patient outcomes and the best patient experience.
Conflict of interest: No conflict of interest declared.

149 Speakers’ biographies
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
Our mission: to systematically detect and reduce the risks related to drug prescribing for your patients in real life

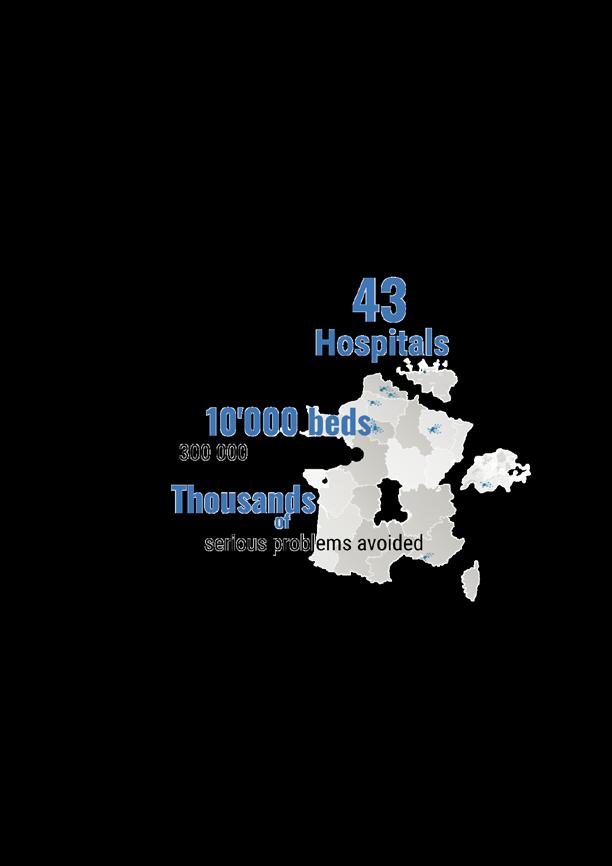


the first decision support system to provide detection and personalised and contextualised alerts truly relevant as well as risk prioritisation among your patients.
Eight years of research and development and three core areas of expertise in synergy for effective drug risk prevention Data intelligence, Bespoke usages and Health science
Real-time, relevant, personalised and contextualised alerts
Up to hospital stay secured
150
Schaefer, Corinna
SEMINAR PSQ2 - Lay involvement in prescribing committees - hearing the patient’s voice
Affiliation: German Agency for Quality in Medicine


Country: Germany
CURRENT STATUS, POSITION
Corinna Schaefer is the Deputy Director of the German Agency of Quality in Medicine and Head of the Departments for Evidence-based Medicine/Guidelines and Patients Information/Patient Involvement. She is responsible for the German National Disease Management Guidelines Programme, where patients and public involvement is mandatory.
EDUCATION
Trained as a Human Scientist, Corinna Schaefer has been working in healthcare research since 2006. She was chairing the Guidelines International Network’s Patient and Public involvement working group from 2010 to 2019, she is member of the German Guideline commission and is Chair of the German Health Literacy Network.

RESEARCH AREA
Corinna Schaefer’s work focusses on advancing methods for guideline development, especially methods for patient and public involvement, and on integrating shared decision-making aspects into guidelines development and clinical practice.

Conflict of interest: No conflict of interest declared.
Schimmel, Kirsten
WORKSHOP 1 - Is compounding (always) the answer to drug shortages?
Affiliation: Leiden University Medical Centre
Country: The Netherlands
CURRENT STATUS, POSITION
Kirsten Schimmel is currently Head of the GMP production facility at the Academic Hospital of Leiden. The facility has GMP licenses for conventional medicines, ATMPs, small molecules and radiopharmaceuticals. She is also Associate Professor, responsible for the teaching of ‘Production and Analysis’ within the Master of Pharmacy at the University of Leiden.
EDUCATION
Kirsten Schimmel studied Pharmacy at the University of Utrecht and became licensed pharmacist in 1999. She followed her internship in the Academic Hospital of Amsterdam and in 2004, she became a licensed hospital pharmacist. Since 2004, she has been Head of the production facility in the Academic Hospital of Leiden. In 2007, she obtained a PhD in medicine at the University of Leiden for her work on the cytostatic drug cyclopentenyl cytosine.
RESEARCH AREA
Kirsten Schimmel’s research focusses on the development and implementation of 3D printing in personalized medicine.
Conflict of interest: No conflict of interest declared.

151 Speakers’ biographies FROM
DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?

100% Exposure Control For a safer working environment Paxxo AB • Bjurögatan 35, SE-211 24 Malmö, Sweden www.paxxo.com • Tel: +46 (0)40-18 60 25
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
Silvari, Virginia
YOUNG PROFESSIONAL SESSION Learning from the career journeys of others
Affiliation: Cork University Hospital
Country: Ireland
Virginia Silvari, Pharmacy Department, Cork University Hospital, Cork (Ireland), who will describe her experiences and the journey she could never have imagined. On how, since graduation, her pharmacy degree has allowed her to take so many different career paths: from research, to community pharmacy and finally to hospital pharmacy. In hospital pharmacy, she found the working environment that suits her best with a mixture of her previous roles.
Conflict of interest: No conflict of interest declared.
Smith, Brian*
SEMINAR SPD2 - The shortage pandemic - why we haven’t succeeded yet
Affiliation: IQVIA

Country: United Kingdom
CURRENT STATUS, POSITION
Brian Smith is currently Chief Pharmacist in UK+I Healthcare Consulting at IQVIA. He is responsible for advising both pharma and NHS clients on market access across the lifespan of pharmaceutical and Med Tech products. He was previously an NHS Trust Chief Pharmacist with 25 years’ experience in the NHS.
EDUCATION
Brian Smith studied Pharmacy at Sunderland University, registering as a pharmacist in 1991. After a Postgraduate diploma in Clinical Pharmacy at the University of London, he studied for a Postgraduate Diploma in PTQA at Leeds University. He is a Member of the Faculty of Clinical Informatics and a Member of the British Oncology Pharmacy Association.
RESEARCH AREA
Brian Smith specialises in Market Access at pre-launch, post launch (regulation and reimbursement) and future product lifetime (uptake and LoE). He was previously an NHS Trust Chief Pharmacist in the North-West of England and a member of the GMMMG Joint Formulary Sub Committee.
Conflict of interest: Carrying out project work with multiple Pharmaceutical companies as part of confidential Market Access Projects, but has no individual or personal relationship with any of these clients and works exclusively for IQVIA.

153 Speakers’ biographies



Spriet, Isabel
SEMINAR IG1 - Hospital support for pharmacy research activities
Affiliation: University Hospitals Leuven and KU Leuven
Country: Belgium
CURRENT STATUS, POSITION
Isabel Spriet is Head of the Clinical Pharmacy group in the Pharmacy Department of the University Hospitals Leuven, where she is responsible for clinical pharmacy activities. She is also appointed as a Professor at the Pharmaceutical and Pharmacological Sciences department at KU Leuven.

EDUCATION
Isabel Spriet graduated as a pharmacist at the University of Ghent in 2002, and as a Hospital Pharmacist in 2003. She completed a Fellowship in Clinical Pharmacy in 2004. In 2011, she obtained a PhD in clinical pharmacy at the University of Leuven for her work on clinical pharmacokinetics of antimicrobials in critically ill patients.
RESEARCH AREA
Isabel Spriet’s research activities focus on antimicrobial dose optimization in special patient populations and on the development of new interventions for appropriate use of drugs in hospitalized patients.
Conflict of interest: No conflict of interest declared.
Stemer, Gunar
SYNERGY SATELLITE EVENT - Non-Biologic Complex Drugs (and nano medicines)
Affiliation: Vienna General Hospital - Medical University Campus
Country: Austria
CURRENT STATUS, POSITION
Gunar Stemer (PharmD, PhD) is currently:
• Deputy Chief Pharmacist in the University Hospital Vienna, Pharmacy Department

• Head of the medicines information and clinical pharmacy department
• Member of the Scientific Committee of the EAHP
• Associate editor, EJHP
EDUCATION
Gunar Stemer has completed his studies of Pharmacy at the University of Vienna and has an Mpharm Degree (Mag. pharm). He wrote his doctoral thesis on Renal clinical pharmacy services, degree (Dr. rer. nat) and has a specialisation in Hospital Pharmacy (approved Hospital Pharmacist). Gunar teaches pharmacology at the Vienna University of Applied Sciences. He holds an MBA degree in healthcare management (Vienna Business University) and is currently finishing his Master of Public Health studies.
RESEARCH AREA
Gunar Stemer’s research interests cover the impact of clinical pharmacy services, with a focus on nephrology patients, and the evaluation of medicines information services. Furthermore, he is interested in medicines pricing, reimbursement schemes and Market Entry Agreements.
Conflict of interest: No conflict of interest declared.

155 Speakers’ biographies
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?


156 Speakers’ biographies automationsalesuk@omnicell.com www.omnicell.com Do you want your pharmacy team to be equipped with the right, advanced technology so they can focus on patient care? Visit Omnicell on stand 18 The road to pharmacy excellence starts here. And most importantly, transform the delivery of patient care. Join Omnicell at EAHP Congress and discover a range of intelligent and connected technology and how these solutions can fulfil a vision of: zero errors 100% Data & Inventory visibility zero waste
Suter, Katja
SEMINAR ER2 - Clinical trials - getting actively involved
Affiliation: University Hospital Basel and University of Basel

Country: Switzerland
CURRENT STATUS, POSITION
Katja Suter works as Scientific Officer in the Department of Clinical Research at the University of Basel. As member of the scientific services, she supports clinical trials and research projects. Furthermore, she is Lecturer at the Department of Pharmaceutical Sciences and teaches evidence-based pharmacy.
EDUCATION
Katja Suter studied Pharmacy at the University of Basel and became a licensed pharmacist in 2003. She obtained a PhD in 2007, for her work on nasal application of midazolam.

RESEARCH AREA
At the Department of Clinical Research, Katja Suter is involved in knowledge transfer and methodological advice for research groups. Recruitment and the prevention of premature project discontinuation due to lack of recruitment is one of the thematic focal points.
Conflict of interest: No conflict of interest declared.
Swen, Jesse J.
SEMINAR CPS1 - Pharmacogenetic testing to optimise therapy
Affiliation: Leiden University Medical Center

Country: The Netherlands
CURRENT STATUS, POSITION
Jesse J. Swen is a Professor of clinical pharmacy and clinical pharmacist-clinical Pharmacologist at the Department of Clinical Pharmacy & Toxicology, Leiden University Medical Center. He is the Chair of the laboratory of the hospital pharmacy.
EDUCATION
Jesse J. Swen studied Pharmacy at Utrecht University. He trained as hospital pharmacist and clinical pharmacologist at the Leiden University Medical Center where he also obtained a PhD in pharmacogenetics.

RESEARCH AREA
The long-term central goal of his career is to improve the outcomes of drug treatment by gaining a better understanding of the genetic mechanisms that result in inter-individual variability in drug response.
Conflict of interest: No conflict of interest declared.
157 Speakers’ biographies
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
Speakers’ biographies
Turnkey Projects

Cleanrooms

Type Solution S.A. is composed by a multidisciplinary team committed to the development and implementation of solutions for a wide range of areas. We combine our experience in laboratory and industry to the most innovative technologies, allowing the development of solutions from Hospital Pharmacy and Radiopharmacy , Pharmaceutical Industry and Public Health and R&D Laboratories. In Hospital Pharmacy and Radiopharmacy, we design, build and revamp your laboratory in compliance with the latest standards and guidelines for Medicinal Product Preparations in Cleanrooms
With compliance to the latest ISO 14644 and GMP guidelines, your project is designed to respond to your current and future needs!
Together with our partner FASTER , we provide solutions and equipment for handling Medicinal Products under sterile and/or containment conditions
Faster S.r.l., based in Italy, is the European leader in the design and manufacture of laboratory equipment since 1984, specialized in Isolators , Laminar Airflow Cabinets and Chemical Fumehoods for Hospital Pharmacies, Pharmaceutical Industry and Research Laboratories




| Pharmaceutical Industry | Pharmacy and Radiopharmacy | Laboratory
Toivo, Terhi
SEMINAR PSQ1 - Become a medication safety pharmacist!
Affiliation: Tampere University Hospital, Hospital Pharmacy
Country: Finland
CURRENT STATUS, POSITION
Terhi Toivo is currently working as a Medication Safety Officer at Tampere University Hospital in Finland. She coordinates medication safety and its development; including e.g. safe medication practises, safety of patient information systems and employee competences. She also conducts research on medication safety, geriatric pharmacotherapy, high-risk medicines and coordination of care.
EDUCATION
Terhi Toivo studied Pharmacy at the University of Helsinki and became licensed pharmacist (M.Sc. Pharm) in 2005. In 2020, she obtained a PhD. Pharm in clinical pharmacy at the University of Helsinki. Her PhD study was about medication risk management of older home care patients. Terhi has an accreditation in comprehensive medication review.
RESEARCH AREA
Medication safety of older people (both home-dwelling and hospitalized patients): PIM, FRID-use, medication reconciliation and review, interprofessional coordination of care, risk assessment tools for patients.
Medication safety in hospital, high-risk medicines.
Risk-screening tools in management of drug-induced qt-prolongation and drug-drug interactions.
Conflict of interest: No conflict of interest declared.
Turner, Kati
KEYNOTE 3 - Patient involvement in pharmacy practice research: no decision about me without me
Affiliation: St George’s, University of London & South West London and St George’s Mental Health Trust


Country: United Kingdom
CURRENT STATUS, POSITION
Kati Turner is a researcher working from lived experience in mental health research. As part of her role, she provides advice and guidance to clinicians, researchers and academics on good practice in Public and Patient Involvement (PPI) in research. She also provides support and reflective spaces to people who use their lived experience in their research work.
EDUCATION
BA (Hons)
RESEARCH AREA
Kati Turner’s research is based in the mental health and social care field. Her area of specialist knowledge is in the field of Public and Patient Involvement (PPI) in research.

Conflict of interest: No conflict of interest declared.

159 Speakers’ biographies
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
Lexicomp: Drug information databases and clinical tools to help reduce adverse drug events

Lexicomp® from Wolters Kluwer is the only drug reference solution offering multiple sources of drug information and clinical tools in a single, easy-to-use interface.

• Adult, pediatric, neonatal, and geriatric drug information
• Renal and hepatic dosing content
• IV Compatibility*
• Drug and drug allergy interactions
• Poisoning and toxicology content
• Patient education (in up to 19 languages)
160 Visit us at Stand No. 7 to learn more!
*iv compatibility information: incorporates the Trissel’s™ 2 clinical pharmaceutics database by Lawrence A. Trissel.
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
van Gelder, Teun*
SEMINAR INT1 - The expanding role of the hospital pharmacists in the care of pre and post renal transplant patients
Affiliation: Leiden University Medical Center
Country: The Netherlands
CURRENT STATUS, POSITION
Teun van Gelder is Professor in Clinical Pharmacology at Leiden University Medical Center, in Leiden (The Netherlands). He is involved in the research theme Academic Pharma.
EDUCATION
Teun van Gelder was trained in internal medicine and nephrology at the Erasmus Medical Center, and in clinical pharmacology in Nijmegen. He completed his thesis in 1996 on the use of anti-interleukin-2 receptor monoclonal antibodies in solid organ transplantation. As a post-doctoral scientist, he worked in the Transplantation Immunology Laboratory of Dr. Randall E. Morris at Stanford University (1998 - 2000).
RESEARCH AREA
In December 2019, Teun van Gelder accepted a new position in Leiden University Medical Center (LUMC). He was appointed Professor in Drug Discovery & Development. LUMC, Leiden Institute of Chemistry, Leiden Academic Centre for Drug Research (LACDR), Centre for Human Drug Research (CHDR) and Leiden Bio Science Park (LBSP) together have the expertise and facilities for the design, development and testing of new innovative drugs. This environment is unique in the Netherlands, and provides an excellent basis for drug discovery and drug development

Conflict of interest: Speaker Buro for Astellas; Steering committee phase 3 trial for CSL Behring; Consultancy for Aurinia Pharma; Consultancy for Thermo Fisher Scientific
Vučićević, Katarina
SEMINAR ER1 - Patients’ individuality: challenges facing hospital pharmacists
Affiliation: University of Belgrade - Faculty of Pharmacy

Country: Republic of Serbia
CURRENT STATUS, POSITION
Katarina Vučićević is a full-time Professor at the University of Belgrade’s Faculty of Pharmacy, and a U.S. Fulbright Scholar alumni. She teaches pharmacokinetics and clinical pharmacy at master, doctoral and specialization studies, and supervises PhD research in pharmacometrics.

EDUCATION
Katarina Vučićević studied Pharmacy at the University of Belgrade and became licensed pharmacist in 2003. In 2010, she obtained a PhD in pharmacokinetics and her research was focused on studying the sources of pharmacokinetic variability of antiepileptic drugs using population modelling approach and TDM data.
RESEARCH AREA
Katarina Vučićević’s research focusses on the variability in clinical outcomes using population pharmacokinetic / pharmacodynamic modelling and simulations. Her goal is to contribute to the individualization of dosing regimen and to improve patient care by bringing complex PK-PD models to simple use in everyday clinical practice.
Conflict of interest: No conflict of interest declared.
161 Speakers’ biographies
Speakers’ biographies
The European Pharmaceutical Students' Association represents over 100.000 students from 35 different European Countries


EPSA Events

The Autumn Assembly is an event which takes place yearly in Autumn. It consists of a rich educational program, made up of symposia, workshops, public health campaigns and trainings focused on a relevant scientific topic, which happen in parallel with our General Assembly.
Other Projects


The Annual Congress is EPSA's main event. At an educational level, it is similar to the Autumn Assembly, albeit longer, which allows for aditional excursions and competitions. The General assembly, however, is different, as the elections of our board happen in this event.
The Summer University is more focused on developing the soft skills of our students. It is a shorter and ligther congress for students to enjoy mid summer, where the main objective is for participants to grow as future healthcare professionals and get to know eachother
The Annual Reception, which is held in Brussels in early spring, is our event of excellence. After a round of meetings with our members to discuss about our governance and advocacy, we call students and stakeholders to discuss a topic chosen each year with our priorities in mind.
The Lifelong Learning Platform (LLeaP) was specially designed for students to be able to have a more structured approach to their personal development.

The project has at its core a framework which aims for students to develop hard and soft skills that will help them excel in their future role as healthcare professionals. Any student can freely sign up for this platformand choose what to focus on according to their needs and interests, directly shaping their own development path.
The Chat with Professionals is held multiple times a year, and it provides pharmaceutical students with the opportunity to directly talk with professionals from various fields of pharmacy, from community and hospital pharmacy to military and astropharmacy.

The event starts with a short presentation of the speakers' career and is followed by a period of interactive talk where students are able to ask questions in the form of a conversation, which eases the contact in between them and the speakers.
Are you interested in becoming an EPSA Partner? Contact EPSA's Vice President of External Relations at vp.er@epsa.online.org!
162
epsa-online.org @epsa online @Epsa Online @epsaonline @epsaonline
EXHIBITOR STAND LIST Exhibitor List


163 Floor Plan
STAND NUMBER COMPANY NAME STAND NUMBER 43 International Pharmaceutical Federation (FIP) 55 51 IPSA 8 47 JCE Biotechnology 10 30 Kapsam Health Products 37 46 KEENTURTLE 53 58 Labatec Pharma SA 2 17 Leventon - A Werfen Company 40 45 Micromedex 50 28 NewIcon Oy 33 24 Nooddis 38 21 Omnicell 18 Hospital Pharmacy (EJHP) 57 Paxxo 39 Capsa Healthcare 9 Pharmacolog 42 Comecer 15 Picklog 19 Computer Engineering 10 Pharmaceutical Press 52 CurifyLabs 48 RB Pharma 5 Deenova 41 Sandoz 25 DiHeSys 54 Sanofi 29 EAHP / EPSA 57 SIDAM 26 Elixi International 6 Simplivia Healthcare Ltd. 3 Equashield 31 Sinteco - Bucci ind. 22 Eurobioconcept 34 Stockart 35 Farmamondo SA 44 Steriline 14 Fresenius Kabi 23 Sumetzberger 21 GPI SPA 12 Swisslog Healthcare 32 GRIFOLS 11 TECHNOFLEX 36 Hemedis 16 Tema Sinergie 1 Hikma Pharma GmbH 2 The Compounding Company 13 ICU Medical 20 Type Solution SA 4 IMI 49 7 Impromediform GmbH 27 Wolters Kluwer
EXHIBITION FLOOR PLAN

164 Floor Plan WALKWAY CONNECTION STAIRS TO LEVEL 1 SESSIONS ROOMS & POSTER AREA 48 57 58 55 54 53 52 51 49 50 47 42 40 39 35 34 33 38 37 36 17 16 41 43 44 6 7 9 10 22 21 20 19 12 13 14 11 15 32 Exhibitors Catering Point Restrooms 18
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
EXHIBITION ENTRANCE 2 AND

165 Floor Plan
STAIRS TO LEVEL 1 SESSIONS ROOMS & POSTER AREA
45 46 31 30 24 3 8 4 5 23 2 1 27 26 25 29 28 CONGRESS
ENTRANCES STAIRS TO LEVEL 1 SESSIONS ROOMS & POSTER AREA EXHIBITION ENTRANCE 1 REGISTRATION
REGISTRATION
CENTRE
FLOOR PLAN - GROUND FLOOR

166 Floor Plan
Auditiorium VI Auditiorium VII W.C Auditiorium VIII Auditiorium IV Auditiorium III Room 1.15 Restaurant Terrass Bar WALKWAY WALKWAY POSTER POSTER
and Auditiorium
STAIRS TO LEVEL 0 EXHIBITION AREA
POSTER AREA Rooms
Restrooms
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
STAIRS TO LEVEL 0 EXHIBITION AREA

167 Floor Plan
Auditiorium I Room 5.C. Room 5.A. + 5.B. Auditiorium II POSTER AREA POSTER
POSTER AREA
Room 1.10 Room 1.14 Room 1.13 Room 1.12 Room 1.11 Room 1.08 Room 1.07 Room 1.06 Room 1.05 Room 1.04 Room 1.03 Room 1.02
AREA
POSTER AREA
FLOOR PLAN - FIRST FLOOR
Rooms and Auditiorium Restrooms

168 Floor Plan
Auditiorium VI Auditiorium VII W.C Auditiorium VIII Auditiorium IV Auditiorium III
1.15 Restaurant Terrass Bar WALKWAY WALKWAY POSTER POSTER
AREA
Room
POSTER
STAIRS TO LEVEL 0 EXHIBITION AREA
FROM DRUG DESIGN TO TREATMENT SUCCESS WHAT REALLY MATTERS TO PATIENTS?
STAIRS TO LEVEL 0 EXHIBITION AREA

169 Floor Plan
Auditiorium I Room 5.C. Room 5.A. + 5.B. Auditiorium II POSTER AREA POSTER AREA POSTER AREA
Room 1.10 Room 1.14 Room 1.13 Room 1.12 Room 1.11 Room 1.08 Room 1.07 Room 1.06 Room 1.05 Room 1.04 Room 1.03 Room 1.02
POSTER AREA


EAHP 2023 Congress 22 — 24 March 2023 Lisbon Congress Centre Lisbon, Portugal Visit our booth to know more! www.fresenius-kabi.com
NOTES


















The European Association of Hospital Pharmacists (EAHP) is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. The European Association of Hospital Pharmacists represents more than 25.000 hospital pharmacists in 35 European countries and is the only association of national organisations representing hospital pharmacists at European and international levels. congress@eahp.eu www.eahp.eu tel: +32 (0) 2/669.25.15 EAHP thanks the continued support of Corporate Partner Omnicell 28th EAHP CONGRESS Bordeaux Bordeaux 20-21-22 MARCH Sustainable healthcareOpportunities & strategies 2024 #EAHP2024













































































































































































































 Pharmacy Director of Compounding/Home
Pharmacy Director of Compounding/Home



































































































































































































