27th EAHP Congress

Lisbon, Portugal
22-23-24 March 2023




From drug design to treatment success

What really matters to patients?



Lisbon, Portugal
22-23-24 March 2023




From drug design to treatment success

What really matters to patients?




THE EAHP INVITES YOU TO ATTEND THE 2023 SYNERGY SATELLITE SESSION:

Wednesday, 22 March 2023
17:15 to 18:45, Auditorium II
27th CONGRESS OF THE EAHP
Lisbon, Portugal
Facilitator
Speakers
Armando Alcobia
Jon de Vlieger
International Regulatory Advances for NBCDs and their follow-on products

Gunar Stemer
NBCDs – Considerations for hospital pharmacy practice


Non-Biological Complex Drugs (NBCDs) are drugs that comprise large high molecular weight molecules and, often, nanoparticles structures. They differ from typical small chemical molecules and also from biotechnologyderived medicinal products (large proteins).
For NBCDs, the entire complex is the active pharmaceutical ingredient and its properties cannot be fully characterized by physicochemical analysis. Most of these medicinal products will have to be managed in a hospital setting, which is why the interdisciplinary pharmacotherapy committees need to consider all levels of evidence generated, focusing specifically on data related to clinical safety and efficacy comparability, discussing interchangeability decisions more like a biosimilar than a generic approach.
Nanomedicine: revolutionizing medicine



Sponsored by an Educational Grant from CSL Vifor

Abstracts from the 2023 EAHP Congress
A1 National poster winner abstracts
A5 Section 1: Introductory statements and governance

A10 Section 2: Selection, procurement and distribution
A20 Section 3: Production and compounding
A37 Section 4: Clinical pharmacy services
A165 Section 5: Patient safety and quality assurance
A234 Section 6: Education and research
A251 Author index
Learning from the career journeys of others
Wednesday, 22 March 2023 14.45 - 16.15 - Room 5C



We’re pleased to announce that European Journal of Hospital Pharmacy now has a Journal Impact Factor of 1.652 and a Scopus CiteScore of 1.6.
Thanks to all the authors, reviewers and editorial team members who continue to help support our aim to publish the best evidence-based information with clinical impact.
Looking for somewhere to submit your next paper? Visit ejhp.bmj.com for our author guidelines.






10.1136/ejhpharm-2023-eahp.1
Background Primaryhealthcarehasasignificantroleinpromotingtherationaluseofmedicines.Finland’shealthand socialservicesreformaimstostrengthenprimaryhealthcare servicesandpreventdiseaseswithmulti-professionalteamwork.Pharmacistsshouldbeinvolvedinthedevelopmentof themedicationmanagementprocessinprimarycare.
Purpose Theaimwastoidentifyriskfactorsinthemedication managementprocessinprimarycaretotargetaclinicalpharmacist’sworktaskswhichcouldimprovemedicationsafety.
MaterialandMethods Thisstudywasconductedinpublic healthcentersinEspoo,SouthernFinland.First,amedication safetyaudittoolforprimaryhealthcarewasdevelopedbased ontheaudittoolforhospitals.Second,medicationsafety auditswereconductedatfourpublichealthcenters.Inthe audits,thepharmacotherapyplanandthemedicationmanagementprocesswereobserved,andtherenewalprocessofelectronicprescriptionswasevaluated.Basedontheauditresults, aproposalwasmadeonthekeydevelopmentareasofthe medicationmanagementprocessandtheroledescriptionof theclinicalpharmacist.
Results Thekeydevelopmentareasidentifiedwiththemedicationsafetyauditswere1)updatingandimplementingthe unit-basedpharmacotherapyplans,2)standardizingthemedicationreconciliationprotocol,3)documentingandutilizing thepatient‘spharmacotherapyplan,4)standardizingtheprotocolforreviewinganddocumentingdrugallergies,and5)a needformedicationsafetyprotectionsinemergencycareservices.Furthermore,thelackofup-to-datemedicinesinformationwhenrenewingelectronicprescriptionswasidentified,as mostoftheprescriptionsarerenewedoutsidephysicians’ appointmentswithoutdirectcontactwithapatient.Thesuggestedcoretaskareasoftheprimarycareclinicalpharmacist atpublichealthcenterstoimprovemedicationsafetywere:1) pharmacotherapyplanandqualitycontrol;2)medicationrisk management;3)developmentofthemedicationmanagement process;and4)otherworktasksrelatedtoclinicalpharmacy.
Conclusions Withmedicationsafetyaudits,itwaspossibleto identifythemedicationsafetyrisksinthemedicationmanagementprocessandprioritizeaclinicalpharmacist’sworktasks thatimprovemedicationsafety.Theidentifieddevelopment areasandmedicationsafetyworkwouldsuittheprimarycare clinicalpharmacist’srole.
ConflictofInterest Noconflictofinterest
NP-002
OJouhet*,AGuibert,ACLagrave. Pharmacydepartment,PoissySaintGermainenLaye hospital,Poissy,France
10.1136/ejhpharm-2023-eahp.2
BackgroundandImportance StaffknowledgeofSterileMedical Deviceisweakenedbystaffturnover,acuteunderstaffing,constantchangesintheSMDfield,marketchanges,andnumerousmarketingshortagesanddiscontinuations.However,the dailyuseofmedicaldevicesisasourceofmisuseifimportant conceptsareunknown.Commentsandquestionsfromhospital staffrevealaneedfortrainingsessions.Wehadtoinnovateand buildnewcommunicationtoolsforstaffthatbebettertrained.
AimandObjectives Themainobjectiveofthisworkisto remindSMDstaffofimportantnotionstoimprovethequality andsafetyofSMDusebyavideotool.
MaterialsandMethods Wedefined50technicalorpractical notionsthankstoourinterventionshistoryaboutSMD.They havebeenclassifiedinto5themes:generalinformation,bandages,digestiveapproach,parenteralapproach,andmiscellaneous.Theirknowledgewasevaluatedamonghospitalstaffwith aweb-basedsurvey.Arateofknowledge(RK)wascalculated foreachnotionandtheme,consideredknownifthe RK>70%orunknowniftheRK<40%.Theunknownthemes willbethesubjectofaseriesoftrainingvideosproduced withAdobePremièrePro®.
Results Weobtained266answerstothesurvey.Theaverage rateofknowledgeofthe50conceptswas47%.Only11conceptswerewellknown,while19areunknown.Forexample, theinterviewedstaffdidnotknowthemeaningofthetwo stripeslogoorwhat-ENFitconnectivityis.Thetwotopics withthelowestlevelofknowledgeweregeneralinformation (RK=37%)andthedigestiveapproach(RK=36%).Thefirst videoofthe ‘CapsulePharma’ explainsgeneralitiesin4minutes. Itwassentonlineandtheoverallsatisfactionscorewas9,5/10. ConclusionandRelevance Thisstudyshowshowimportant continuoustrainingisthekeyforhospitalstafftobetter understandSMD.Theformatofshortvideoshasbeenchosen foritsattractivenessanditsunlimitedquickplaybackondifferentmedia.«CapsulePharma»willbecomeaninnovative andinstitutionalcommunicationtoolforSMDandother healthproducts.
NP-003 PHARMACEUTICALINFUSIONSCHEDULESASATOOL TOIMPROVEFLUIDRESTRICTIONINPICUPATIENTS
1MKleinlein*, 2MPNeininger, 1MHoeckel, 2TBertsche. 1Pharmacy,GesundheitNordhessen HoldingAG,Kassel,Germany; 2ClinicalPharmacy-InstituteofPharmacy-MedicalFaculty, LeipzigUniversityandDrugSafetyCenter-LeipzigUniversityandUniversityHospital,Leipzig, Germany
10.1136/ejhpharm-2023-eahp.3
Background Childreninintensivecareunit(PICU)areat increasedriskforfluidoverload,whichisassociatedwith increasedmorbidity.Therefore,unnecessaryfluidadministrationshouldbeavoided.ThevolumeneededforflushinginfusionlinesduringIVdrugadministrationisoftennot consideredinthedailycalculationoffluidintake.
AimandObjectives Theaimofourstudywastoreducethe dailyflushingvolumeandtherebyfluidoverloadinPICU patients.
MaterialsandMethods Aprospectiveinterventionstudywas conductedinourPICU(controlperiod:Jan-July2020;interventionperiod:Oct2020-Aug2021).Patientswith 2i.v. medications,>24hlengthofstay,andage0–18yearswere included.Primaryoutcomewastheoccurrenceoffluidoverload.Theinterventionwasthepreparationofpatient-specific infusionschedulesbyaclinicalpharmacist.Theschedules
1TKoskenkorva, 2CLinden-Lahti, 2LSchepel, 1ECelikkayalar, 2KKvarnström*. 1Universityof Helsinki,00790Helsinki,Finland; 2HUSPharmacy,UniversityofHelsinkiandHelsinki UniversityHospital,00290Helsinki,Finland ‘CAPSULEPHARMA’,ANINNOVATIVEDIGITAL TRAININGTOOLTOLEARNABOUTIMPORTANT STERILEMEDICALDEVICE(SMD)CONCEPTSindicatedtheIVaccessthroughwhichIVmedications,parenteralnutrition,andinfusionsolutionsshouldbeadministered toavoidincompatibilitiesandwhetherflushingoftheinfusion linewasrequired.
Results Inbothperiods,66patientseachwereincludedinthe evaluation.Flushingvolumewasreducedfromamedianof 0.68ml/kg/day(Q25/Q750.35/1.33)to0.31ml/kg/day(Q25/ Q750.05/0.74;p<0.001).Inthecontrolperiod,themedian fluidoverloadperpatientwas2.3%,while1.5%fluidoverloadoccurredintheinterventionperiod(p<0.001).Also, fewerpatientdayswithfluidoverloadof 10%occurredduringtheinterventionperiod.Fluidoverloadof 20%were onlyobservedinthecontrolperiod.
Conclusion Theuseofpharmaceuticalinfusionscheduleswith recommendationsforflushinginfusionlinesaccordingtocompatibilityhasreducedtheflushingvolume.Thiscanavoidthe administrationofunnecessaryIVfluids.Reducingfluidintake helpstoreducetheoccurrenceoffluidoverloadinPICU patients.
1SaraMerczel*, 1TiborBali, 2LajosBotz. 1SomogyCountyKaposiMórTeachingHospital, DepartmentofPharmacy,TalliánGyulaStreet20
32,7400Kaposvár,Hungary; 2University ofPécs,FacultyofPharmacy,DepartmentofPharmaceutics,HonvédStreet3,7624Pécs, Hungary
10.1136/ejhpharm-2023-eahp.4
ConclusionandRelevance Fromthisinvestigationweconcludedthattheactiveinvolvementofaclinicalpharmacist andtheinternationallyvalidatedclinicaldatabasesystemsare essential.Theyenhancetheclinicaleffectivenessofthemedicationbyreducingmultipledrugusesandbyeliminating adversedrugreactions.Ourreal-worldstudyishighlybeneficialfortheindividualisedmedicationofdementiapatients receivingchronichospitalcares.
NP-005
SELF-ASSESSMENTONTHEIMPLEMENTATIONOF RECOMMENDATIONSOFTHEPERIOPERATIVE PROCESS:INFECTIOUSRISKMANAGEMENTIN SURGERYSETTING
ElaMurrja,SaraPugliese,FrancescoCasoli,MilenaCasciari,SerenaNatalini, AlessandroCaraffa,AngelaGiuliani,CristinaPaolucci,MassimoFarina, AlessandroD’Arpino.
10.1136/ejhpharm-2023-eahp.5
BackgroundandImportance Surgicalsiteinfections(SSIs)are amongthemostcommoncomplicationinsurgery.Theyare associatedwithlongerpostoperativehospitalstays,maynecessitateadditionalsurgicalprocedures,requirelongantimicrobial treatmentleadingtoanincreasedantimicrobialresistancecontributingtoacostlyhealthcare.It’snecessarytoadopta healthcarepolicyaimedatamorerationaluseofantimicrobialstolimitantimicrobialresistance.
BackgroundandImportance
Thoseelderly,dementiapatients whoreceivetreatmentsfortheirvariouschronicdiseases belongtoahighriskcohort.Theirindividualisedmedication shouldavoidtreatmentwithmultipledrugsandwithactive substanceswhichposeahealthriskforthem.Thismayeliminatetheadverseeffectstowhichthesepatientsareparticularly susceptible.
Ouraimwastodevelopaself-assessmentontheimplementationoftherecommendations,inordertoidentifykeygaps andprovideguidanceandrecommendationsforimproving IPC(infectionpreventionandcontrol)practices.
MaterialsandMethods Amultidisciplinarycollaborationhas involvedinfectiousdiseasespecialists,hospitalpharmacists, microbiologists,intensivists,emergencysurgeons,nurses.Itwas conductedathoroughself-assessmentonthefourfollowing surgeryareas:generalsurgery,emergencysurgery,Orthopedic Surgery,CardiosurgeryUnitduringJuly2021 – March2022.
AimandObjectives
Thestudyevaluatesthemedicaltreatment ofdementiapatientsreceivingchronicandpalliativecares simultaneously.Wecollecteddataofindividualisedmedications fromhistoricpatientrecordsin2020–2021.Thestudywas approvedbytheresearchethicscommitteesoftheuniversity andthehospital(IG/02176-000/2022)
MaterialsandMethods Weexaminedthereal-worlddataof drugtreatmentindementiapatientsaged65orolderwho spentatleast5daysinthehospital.Weanalysedtheanonymised,aggregatedata.Weusedinternationaldatabasescompiledfrommeta-analysesandsystematicreviews(Beers Criteria,START/STOPP,WHO,EMAandUCSF).
Results Weanalysedthedrugtreatmenthistoryof108patients (74womenand34menwiththeaverageageof80.5±9 year),whometthepreliminaryselectioncriteria.Weclassified thepatientsintothefollowingcohorts:1.9%directiondiagnosis,20.4%basisofthemaindiagnosis,35.2%maindiagnosis, 38.9%comorbidityand3.7%diseaseunderlyingdeath.The distributionofdementiatypeswere:53.7%vascular,1.9% relatedtootherdiseasesand44.4%unspecified.Theaverage numberofmedicinestakenperdayperpatientwas10.8 pieces.Multipledrugtreatmentoccurredin86.1%ofpatients. 10%ofthepatientsreceivedmedicinetotreatdementia (donepezilin60%ofthecases,memantine40%ofthecases). Atleastonerequiredmedicationwasnotadministeredfor 38.9%ofdementiapatientsbecauseofitsadverseeffect.
Asummaryresultsoftherecommendationscorecomponentsself-assessmentwasprovidedbyascoredchecklistattributedtoaspecificlevelofrecommendationsimplementation (score0:notapplicable;1:noimplementation;2: £50%;3: >50%;4:100%implementation).
Thechecklistreport13macro-requisitestowhichascore isassigned;foreachrequirementwasreportedthenumberof improvementactions.
Results Followingtheassessment,31improvementactions wereidentified.Thecomparisonversustotalaverageofvalues shows4macrorequirementsunderthreshold:Screeningper S.Aureus;Preoperativebathing;mechanicalbowelpreparation andtheuseoforalantibioticsandthemaintenanceof adequatecirculatingvolumecontrol/normovolemia.
Thisself-assessmentreported8improvementactionsin EmergencySurgeon:10inOrthopedicSurgery,6actionsin GeneralSurgeryand7improvementactionsinCardioSurgery.
Furthermore,werehighlightedimportantshortcomingssuch asantimicrobialprophylaxisforthepreventionofSSIincolorectalsurgery:scored1,3(NA);screeningperS.Aureusin orthopedicsurgery:score1.
Conclusion Theassessmentallowedtheidentificationofthe priorityareasintervention,inordertosetinnovativestrategic actionstoimprovesafetyintheperioperativeprocess.
Inthefutureitwillbepossibletoimplementstrategies withproveneffectivenessandaglobalapproach.Theaimis
RETROSPECTIVESTUDYONINDIVIDUALISEDtoovercomeandrefiningguidelinesbyprovidingacomprehensiverangeofevidence-basedrecommendationsforthepreventionofSSIs.
administrationviaEFTmayleadtograftrejection.2 AppropriatedrugformsofISforadministrationviaEFTaremissingin ourcountry.
10.1136/ejhpharm-2023-eahp.6
BackgroundandImportance Theintroductionofimmunotherapyinthetreatmentofpatientswithnon-smallcelllungcancer(NSCLC),whosediseaseprogressedafterfirst-line treatment,wasconsideredanimportantadvance.Real-lifeuse dataforthesedrugsareessentialtomeasuretheirrealadded valueinthetreatmentofthesepatients.
AimandObjectives Ouraimwastostudytheeffectivenessof Atezolizumab(ATZ),Nivolumab(NVL)andPembrolizumab (PMB),inthesecond-linetreatmentofNSCLC,inrealclinical practiceandanalyzeitconsideringtheefficacydescribedin publishedclinicaltrials.
MaterialsandMethods Thisisanobservationalretrospective studyofpatientsdiagnosedwithlocallyadvancedormetastatic NSCLC,treatedinsecond-lineorlateruntiltheendof August2021,withoneofthefollowingdrugs:ATZ;NVLor PMB.EffectivenesswasevaluatedintermsofProgression-Free SurvivalandGlobalSurvival.
Results Thirty-twopatientstreatedwithATZ,46withNVL and17withPMBwereincluded.Ofthetreatedpatients, 59.4%forATZ,39.1%forNVLand100%forPMBhad positiveexpressionofPDL1(>1%).Themedianprogressionfreesurvivalcalculatedwas5.6monthsforATZ;8.4months forNVLand5.0monthsforPMB.Themedianoverallsurvivalcalculatedwas16.3monthsforATZ,15.7monthsfor NVLand32.6monthsforPMB.
ConclusionsandRelevance Theprogression-freesurvivaland overallsurvivalobtaineddemonstratethat,whenusedinclinicalpractice,thedrugsstudiedareeffective,withresultsnot lowerthanthosedemonstratedinclinicaltrials.Immunotherapyprovestobearelevanttherapyinthesecond-linetreatmentofNSCLC.
REFERENCE
1.1.Lancet2016387(10027):1540
1550;Lancet2017389(10066):255–265; NEJM2015;373(2):123
35;NEJM2015;373:1627–39
NP-007 RECOMMENDATIONSFORADMINISTRATIONOF IMMUNOSUPPRESSANTSVIAENTERALFEEDINGTUBE ACCORDINGTOTHEIR IN-VITRO ADMINISTRATION
1KLajtmanová*, 1KSzmicseková, 1,2SPorubcová. 1HospitalPharmacy,NationalInstituteof CardiovascularDiseases,Bratislava,Slovakia; 2DepartmentofOrganisationand ManagementinPharmacy,FacultyofPharmacy,ComeniusUniversity,Bratislava,Slovakia
10.1136/ejhpharm-2023-eahp.7
BackgroundandImportance Immunosuppressants(IS)areused inthetreatmentandpreventionofgraftrejectionaftersolid organortissuetransplantation.1 Theiradministrationviaan enteralfeedingtube(EFT)isproblematicregardingtheirnarrowtherapeuticindex,cytotoxic,teratogenicpotential,and occupationalhazard.Incompleteabsorptionduetoincorrect
AimandObjectives Despitemultiplepublishedguidelinesfor theadministrationofmedicinesviaEFT,availabledrugforms differbetweencountries.OuraimwastocreatelocalrecommendationsforthesafeadministrationofISviaEFTreflecting theavailablemedicinesinourcountry,whilepreventingEFT occlusionandpreservingoptimaleffect.
MaterialsandMethods Aliteraturesearchwasaimedtodeterminethesiteofabsorption,incompatibilities,andmeasuresto decreasetheoccupationalhazard.Thepracticalpartconsisted ofdissolvingtablets,capsules’ content,andtheiradministrationviaEFTsofdiameters10,8,and6Fr.TheadministrationofISwasrealizedbytheadaptedprotocolbyWhiteet al.,2015.3 Weevaluatedtherateofdisintegrationoftablets andtubeocclusion.
Results Onlyonebrandofmycophenolatemofetiltabletsand twobrandsofazathioprinetabletsdisintegratedinasyringe. Alltheothertabletsneedtobecrushed.Twoofthestudied IScausedtheocclusionofa6FrEFT,noEFTofwider diameterwasoccluded.Wesummariesourrecommendations inatable.
ConclusionandRelevance Crushingtabletsoropeningcapsules isoftentheonlypossibilityforISadministrationviaEFT.In thesecases,usingpersonalprotectiveequipmentisalways needed.Ciclosporin,mycophenolatemofetil,andazathioprine canbeadministeredrelativelysafely.Specialattentionis neededwhenanEFTof6Frisusedduetoitseasy occlusion.
REFERENCESAND/ORACKNOWLEDGEMENTS
OurprojectwassupportedbytheNationalInstituteofCardiovascularDiseases, EduPharmpriNÚSCH,BauschHealth,Novartis,Nutricia,Roche.
1.Hartono etal.,ColdSpringHarbPerspectMed.,2013.
2.Silva etal.,JClinPharmTher.,2020.
3.White etal.,Handbookofdrugadministrationviaenteralfeedingtubes,2015.
NP-008 EMERGENCYDEPARTMENTREVISITSOCOREBASED ONPHARMACOTHERPAY
1JesúsRuiz*, 2EmiliVela, 2DavidMonterde, 1LaiaLópez, 1MªAntoniaMangues, 1MireiaPuig, 2MontserratClérigues, 1AnaJuanes. 1HospitalSantaCreuiSantPau. Barcelona,Spain; 2Sistemasanitariintegrald’utilitzaciópúblicadeCatalunya.Barcelona, Spain
10.1136/ejhpharm-2023-eahp.8
BackgroundandImportance Drug-relatedproblems(DRPs)are acommonreasonforvisitingtheemergencydepartments (ED).However,theinformationavailableonriskfactorsassociatedwithnewEDvisitsbasedonthepatient‘spharmacotherapyislimited.
Objective TodevelopapredictivemodeloftheriskofrevisitingtheEDat30daysbasedonpatients’ treatmentat discharge.
Methods Retrospectivecohortstudyinvolvingadultpatients whoattendedtheEDinCatalonia(Period:2019)withatriagelevelof1–3.A30-dayreturnvisitpredictionmodelwas createdinareferralcohort(60%)usingalogisticregression model,beingvalidatedinavalidationsample(40%).Variables includedinthemultivariateanalysiswereassignedascore proportionaltotheregressioncoefficient.Thesociodemographicvariablesconsideredinthisstudywereage,sexand incomelevel,multimorbidityburdenbasedontheAdjusted
AnaSoaresArmandoAlcobia. Pharmacist,PharmacyDepartment,HospitalGarciadeOrta, PortugalMorbidityGroups(GMA).Forty-fourgroupsofdrugsassociatedwithDRPswereevaluated.
Results 851,649patientswereincluded[201,445(23.6%)with >9drugsprescribedatdischarge],ofwhom134,560(15.8%) visitedtheEDafter30days.Thefourvariablesevaluated (sex,age,GMA,andincomelevel)and34ATCgroupswere associatedwiththeriskofrepeatEDconsultationandwere combinedintoafinalscore(DRP-Score).Thedrugswiththe highestriskscorewereosmoticlaxatives(RR:1.421(95% CI:1.264–1.596)),b-lactamantibiotics(1.333(1.123–1.583)), digoxin(1.282(1.256–1.309)),heparins(1.150(1.112–1.190) andlithium(1,146(1.000–1.315))Themodelachievedan areaunderthereceiveroperatingcurve(AUC-ROC)valuesof 0.648(95%CI:0.646–0.650)inthereferencecohortand 0.647(0.644–0.649)inthevalidationgroup.Threeriskcategoriesweregenerated,withthefollowingestimatedrisksof revisitingtheEDat30days:lowrisk:10.2%,intermediate risk:18.3%,andhighrisk:28.4%.Thescorewasvalidatedin asampleof1437patientswhovisitedtheEDforDRPs, maintainingitspredictivecapacity.
ConclusionandRelevance TheDRP-scoreidentifiespatientsat highriskofreturningtotheEDwithin30daysbasedon pharmacotherapy,beingausefultoolforprioritizinginterventionsfromtheseunits.
Results 120patientswereincludedinthestudywithamedian ageof71years[IQR63.5 – 83.5].71.7%ofpatientswere taking 5medicationsbeforeadmission.Themedianpharmaceuticaltimerequiredtoperformthemedicationreconciliationactivitywas36minutes[IQR29 – 45].60.8%of admittedpatientshadatleastoneUMDonadmissionwitha medianof2perpatient[IQR1 – 3].Unintentionaldrug omission(67.3%)anddosemodification(21.2%)werethe mostfrequentlyencounteredUMD.88.5%ofidentifiedUMD werecorrected.Polymedication( 5medications)wasthe onlyvariableassociatedwith ‘presenceofanUMD’ atalevel veryclosetotheestablishedstatisticalsignificancelevelofp =0.05[OR2.24,p-value0.065].
ConclusionandRelevance Thisstudyconfirmsthemajorinterestofthemedicationreconciliationatadmissioninanorthopaedicandtraumadepartmentinanelderlyandpolymedicated population,exposedtohigh-riskmedicationsandtoarisky process.
NP-010 DEVELOPMENTOFA2%LIDOCAINEGELFORLOCAL ANAESTHESIAOFTHEEYEPRIORTOINTRAVITREAL INJECTION
10.1136/ejhpharm-2023-eahp.10
ADMISSION:APROSPECTIVESTUDYINTRAUMATOLOGY
1,2,3NRatsimalahelo*, 1,2NPerrottet, 4JDaSilvaRaposo, 4OBorens, 1,2,3FSadeghipour. 1ServiceofPharmacy,LausanneUniversityHospital,Lausanne,Switzerland; 2Centerfor ResearchandInnovationinClinicalPharmaceuticalSciences,LausanneUniversityHospital andUniversityofLausanne,Lausanne,Switzerland; 3InstituteofPharmaceuticalSciencesof WesternSwitzerland,UniversityofGenevaandUniversityofLausanne,Genevaand Lausanne,Switzerland; 4DepartmentofOrthopaedicsSurgeryandTraumatology,Lausanne UniversityHospital,Lausanne,Switzerland
10.1136/ejhpharm-2023-eahp.9
BackgroundandImportance Medicationerrorsleadingtopreventableadversedrugeventsoccurmainlyduringtransitions ofcare(admission/dischargefromahealthcarefacility,hospital interdepartmentaltransfers).Dataondrugreconciliationin surgicalwardsarescarce.
BackgroundandImportance Intravitrealinjectionisavery commoneyesurgery.Thepreparationoftheinjectionistimeconsumingandlabour-intensive,becausepatientsreceiveseveralophthalmicdrugsbeforehandlikelocallydisinfecting, pupildilatingandlocalanaestheticeyedrops.Additionally, eyedropscontainingoxybuprocainemustbeapplied3to5 timesatminuteintervalsforasufficientanaestheticeffect.
AimandObjectives Tosimplifytheprocess,alocalanaesthetic eyegelpreparationwasrequested.Theincreasedviscosity leadstoalongerlocalexposuretimeontheeye.Asingle doseisthereforesufficienttoachievetherequiredlocalanaestheticeffect.Asfarasweknow,acorrespondingproductis notavailableontheGermanmarket,soanin-houseproduct wasdeveloped.
AimandObjectives
Thepurposeofthisstudywastoassess theprevalenceofmedicationdiscrepanciesinpatientsadmitted toanorthopaedicandtraumadepartmentduringthemedicationreconciliationprocessperformedbyapharmacistat admission,andtoidentifypotentialriskfactors.
MaterialsandMethods
Thiswasaprospectivesingle-center observationalstudyconductedovera15-weekin2021.Eligiblepatientswereadultshospitalizedintwounitsofanorthopaedicandtraumadepartmentofatertiaryuniversityhospital inSwitzerland,admittedforadurationofhospitalization> 48hours,inthepresenceofachronicpathologyand/ora medicationatriskand/oronthephysicianinchargeofthe patient’srequest.TheBestPossibleMedicationHistorylist wasestablishedforeachpatientandcomparedtotheprescriptiononadmissiontoidentifymedicationdiscrepancies.These discrepancieswereclassifiedasintentional/unintentionalonthe basisofthemedicalrecordand,ifnecessary,adiscussionwith thephysician.Amultivariableanalysisbylogisticregression wasperformedtoidentifypredictorsofthe ‘presenceofan unintentionalmedicationdiscrepancy(UMD)’ .
MaterialandMethods Theactiveingredientlidocainehydrochloride2%(w/w)isdissolvedinhotWFIwith0.48%(w/w) sodiumchlorideasanisotonizingadditive.0.25%(w/w) sodiummonohydrogenphosphatex12H2O,leadstoapH valueof6-7inthefinishedgel.pH7mustnotbeexceeded, topreventprecipitationoflidocainebase.Hydroxyethylcellulose250(Natrosol250Gpharm®),asterilizablegellingagent, isincorporatedintothehotsolutionataconcentrationof 2.5%(w/w).Aftercooling,WFIisaddedtothefullbatch weight,thebatchisstirredvigorouslyandlefttostandcoveredovernight.Ahomogeneousgelofsuitableviscositydevelopsovernight.Thefollowingday,thegelisfilledinto Redipac® single-dosecontainerswithsubsequentautoclaving understandardconditions.
Theidentityandcontentofthepreparationischeckedby UV/VISspectroscopy.
Results Thepreparationdescribedachievesasufficientlocal anaestheticeffectaftersingleapplication,isfreeofpreservativesandcanbestoredatroomtemperature.
ConclusionandRelevance Thelidocainegelinsingle-dosecontainershassignificantlyacceleratedandsimplifiedthepreparationofintravitrealinjectionsintheUKSHEyeClinic.
1MRodriguezGoicoechea, 1VCaoViña, 2ETejedorTejada, 1NGarciaGomez, 1FHorno Ureña. 1ComplejoHospitalarioUniversitariode5Jaén,Pharmacy,Jaén,Spain; 2Hospital Clínic,Pharmacy,Barcelona,Spain
10.1136/ejhpharm-2023-eahp.11
BackgroundandImportance Inourhospitalthereare568 patientswithmultiplesclerosis(MS)inactivetreatment. Administrationofnatalizumabisevery4weeks,buttheneurologistsatourhospitalhaveoptimisedtheadministration every5or6weeks.
AimandObjectives Evaluateeconomicimpactfromnatalizumaboptimisation.
MaterialandMethods Retrospectiveobservationaleconomicstudyperformedinathird-levelhospitalbetween January2019andJune2022.Demographicdata(sex, age),clinicaldata(MStreatment,posologyandquantity ofcyclesadministered)andeconomicdata(Laboratory PurchasePrice(LPP)includingVAT)collectedfromprescribingprogrammeandecono micmanagementplatform. Patientsreceivingnatalizumabforatleast3cycleswere included.
Accordingtoposology,calculationofactivetreatmenttime andnumberofcyclessaved.Comparisonbetweentheorical economicimport(associatedtoadministrationevery4weeks) andreal.Analysisofchangesoftreatmentandcosts associated.
Results From568patientswithMS,43arereceivingnatalizumabinourstudyperiod.Only37receivedmorethan3 cyclesofnatalizumab.These37patientsinclude24women, withanaverageageof41.2years(23-65),4patientswere receivingnatalizumabevery6weeks,andtheothersevery5 weeks.111.5weeksofactivetreatmenttime(20-198)averaged,with21.7cycles(4-36)associated,meaning229vialsof natalizumabsaved.
Natalizumab ’ scostsaccordingtoLPP(C ¼ 1302)inour studyperiodcometoC ¼ 1.045.506,00.Ifnatalizumab wouldhavebeenadministeredevery4weeks,itscost wouldcometoC ¼ 1.343.664,00.Savingsamountto C¼ 298.158,00globally,ortoC ¼ 85.188,00annually.Of37 patients,4neededtochanget reatmentduetooutbreaks. Thenewtreatmentwasocrelizumab,withaPPLof C¼ 4.666,64/vial.Totalannualcostofpatients ’ ocrelizumab amounttoC ¼ 74.666,24.
ConclusionandRelevance Natalizumab’soptimisationwith administrationevery5weekshasmeantatotalsavingof C¼ 10.521,76,afterhavingreinvestedpartofthesavingsinthe newtreatmentswithocrelizumab,allowingourpatientsto accessinnovativetherapies.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
1ISG-002 MEDICATIONWASTEINANORTHOPAEDIC DEPARTMENT:EFFECTOFPATIENT’SOWN MEDICATIONUSEANDSELF-ADMINISTRATION DURINGHOSPITALISATIONANDTHEVIEWSOF PATIENTSANDHOSPITALSTAFF
1BMaat, 2ABenjaddi*, 1LVanHerpen-Meeuwissen, 3CDiekerhof. 1Elisabeth-Tweesteden Hospital,ClinicalPharmacy,Tilburg,TheNetherlands; 2UtrechtUniversity,Departmentof PharmacoepidemiologyandClinicalPharmacology,Utrecht,TheNetherlands; 3ElisabethTweestedenHospital,DepartmentofOrthopedics,Tilburg,TheNetherlands 10.1136/ejhpharm-2023-eahp.12
BackgroundandImportance Medicationwastehasdetrimental effectsontheenvironmentandhealthcarecosts.Pharmacotherapeuticchangescontributetomedicationwaste(e.g.substitutiontohospitals’ medicationformulary).Patient’ sown medication(POM)wherepatientsbringtheirownmedicines foruseduringadmissionisknowntopositivelyaffectmedicationwaste.Self-administrationofmedication(SAM)canbe combinedwithPOMuseandallowscapablepatientstomanagetheirmedicationregimenthroughouthospitalisation.Itis notclearhowthecombinationofPOMuseandSAMaffects medicationwaste.Furthermore,bothpatientsandhospital staffplayamajorroleinmedicationmanagementduringhospitalisation.Theirawarenesshereofmayaffectmedication waste,buttheirviewsonthisareunknown.
AimandObjectives TodetermineifPOMuseandSAM reducethevolumeandmonetaryvalueofmedicationwaste duringhospitalisation.Todeterminetheviewsofpatientsand hospitalstaffonmedicationwaste.
MaterialandMethods Aprospectivepre-postintervention studywasconducted,includingallpatientsadmittedtoan orthopaedicwardbetweenMarchandMay2022.InApril 2022,POMuseandSAMwereimplemented.Dataonvolume (inpieces)andmonetaryvalue(ineuros(C ¼ ))ofmedication wastewerecollected.A5-pointLikertscalesurveyonmedicationwastewasconductedamongpatientsandhospitalstaff. Datawereanalysedusingdescriptivestatistics.
Results Thevolumeofwastedmedicinesdecreased44.3% from477to331piecesper100inpatientdaysafterthe implementationofPOMuseandSAM.Themonetaryvalue inhospitalpurchasepriceofwastedmedicinesdecreased 151.8%fromC ¼ 283.80toC ¼ 112.70per100inpatientdays. 30patientsand78hospitalstaffmembersrespondedtothe survey.Themajoritywereawareofandinterestedinmedicationwaste.Interestingly,53%ofpatientsdidnotfeelthat theycontributetomedicationwasteasopposedto19%of hospitalstaff.Bothpatientsandhospitalstaffwerepositive towardsPOMuseandSAMasmeanstoreducemedication waste.
ConclusionandRelevance TheimplementationofPOMuse andSAMduringhospitalisationseemstohavethepotentialto reducemedicationwasteandconcomitantcostsatanorthopaedicward.Patientsandhospitalstaffseempositivetowards thistopic.Therefore,werecommendtofurtherimplement POMuseandSAM.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
1ISG-012 COST-MINIMISATIONANALYSIS:PROPHYLACTIC TREATMENTOFHAEMOPHILIATYPEA,WHATTO CHOOSEBETWEENFACTORVIII,RECOMBINANT FACTORSVIII(MOROCTOCOG-ALFAANDOCTOCOG) ANDEMICIZUMAB?
1,2HDaoudi*, 1,2OElQabissi, 1,2FZLasri, 1,2SElMarnissi, 1,2MAitElCadi. 1IBNSina HospitalUhcIbnSinaRabat,Pharmacy,Rabat,Morocco; 2FacultyofMedicineand PharmacyRabat,PharmacologyandToxicologyLaboratory,Rabat,Morocco
10.1136/ejhpharm-2023-eahp.13
BackgroundandImportance HaemophiliatypeAisahereditarybleedingdisorderlinkedtoadeficiencyinFVIII,treated byintravenousadministrationofFVIII.Emicizumabrepresents analternativetoFVIIIconcentrates,withoutanti-FVIIIinhibitor,administeredinasinglesubcutaneousdose.1
BackgroundandImportance Theongoingriseinhealthcare costsmakesitnecessarytoestablishcontainmentstrategies,in parallelwiththecommitmenttoimproveaccesstothemost effectiveandsafesttreatments.Theintroductionofbiosimilar medicinesisanopportunityforhealthsystems(HS)and patients.
AimandObjectives Theaimofthestudywastoevaluatethe useandcostevolutionofinfliximabandadalimumabinthe gastrointestinaldepartmentofatertiaryhospitaloverthelast eightyears.Inthisperiod,biosimilarmoleculesofbothdrugs havebeenincorporated.
MaterialandMethods Datawerecollectedbasedonconsumed unitsofadalimumabandinfliximabbetweenJanuary2014 andDecember2021.Wegroupedthedifferentpresentations oforiginalbrandandbiosimilarmoleculesavailableandthe costassociatedatthetimeitwasconsumed.
AimandObjectives
Theaimistoevaluatetheuseofemicizumabcomparedtothethreebiosimilarsusedforprophylaxisin haemophiliatypeA,inordertoensuregoodcarewitha goodqualityoflife.
MaterialandMethods Theanalysisofthecostofusingemicizumabcomparedtothethreebiosimilarswehaveinthehospital, namelyplasmaandrecombinantFVIII(Moroctocog-alfaand Octocog)bycalculatingthedirect,indirectandintangiblecosts, toassesstheadvantagesandconsequencesofemicizumabuse.
Results Accordingtothecostminimisationanalysis,wefound atotalannualcostforFVIIIC ¼ 125,293.7,Octocog
C¼ 252,183.7,Moroctocog-alfaC ¼ 2,753,70.6withasignificant intangiblecostbecausethefrequenttriptothehospitalmakes thepatienttiredandincreasesthenon-medicalcostandthe indirectcost,withthepossibilityin30%ofpatientsofdevelopinganti-FVIIIinhibitorsandthereforetheadministrationof highdoseplasmaFVIIIof6000-9000IUthreetimesaweek withanannualcostofC ¼ 366,565.8.
Ontheotherhand,emicizumabisindicatedevenfor patientswithananti-FVIIIinhibitorwhoseannualcostis C¼ 233,402.9,withagainofC ¼ 136,484.23,inadditiontoa goodqualityoflife.WededucethatplasmaFVIIIisuseful forpatientswithoutaninhibitorandemicizumabshouldbe reservedforhaemophiliacswithananti-FVIIIinhibitor.
ConclusionandRelevance Ourcostevaluationstudyisatool fordecisionsupportandreductionofuncertaintybetween fourdrugs,whichmakesitpossibletoadaptpurchasesaccordingtotheneedsexpressedforanoptimalallocationofresourcesfollowingtheevolutionofhealthexpenditure.
1.JoelL.Moake,MD,BaylorCollegeofMedicine,lemanuelMSD2022.
ConflictofInterest Noconflictofinterest
2SPD-001 USEANDCOSTEVOLUTIONOFINFLIXIMABAND ADALIMUMABOVER8YEARSINATERTIARY HOSPITAL
1AGómez, 1VCarrilloLópez*, 1MLopez, 2VRoyo, 1MMSantandreu, 1MFPérez, 2SKhorrami, 1MCIglesias, 1ODelgado, 2DGinard. 1UniversitaryHospitalSonEspases, Pharmacy,Palma,Spain; 2UniversitaryHospitalSonEspases,Gastroenterology,Palma,Spain
10.1136/ejhpharm-2023-eahp.14
Results Both,infliximabandadalimumab,consumptionhave graduallyincreasedoverthepasteightyears,from1,774to 2,765units(+55.9%)andfrom920to3,420unitsperyear (+271.7%),respectively.
Infliximabbiosimilarwasintroducedinthecentrein2015 andwasprogressivelyrolledoutinstartsandswitches, becomingthesolesince2021.Thishasledtoagradual reductionincosts,fromC ¼ 852,022in2014toC ¼ 497,235in 2021(-41.6%).
Adalimumabbiosimilarwasnotintroducedinthehospital until2019.Consumptionrosefrom920to2,153unitsper year(+134.0%)between2014and2018,intandemwith cost:fromC ¼ 442,745toC ¼ 936,175peryear(+111.5%).
Nevertheless,between2018and2021,consumptionincreases from2,153to3,420(+58.9%)withanabsolutecostreductionofC ¼ 563,683(-60.2%).Overall,adalimumabspending hasdecreasedby15.9%overtheeightyearsdespitethe increaseinconsumption.
ConclusionandRelevance Innovationinbiologicaltherapies,as wellastheincreaseincandidatestoreceivethem,hasgrown significantly.Itisassociatedwithanincreaseincoststhatmay becomeunaffordableforpublicHS.
Theintroductionoftwobiosimilarmoleculesinourcentre hasledtosignificantsavings,despitetheincreasein consumption.
Thecommercialisationofbiosimilarmolecules,alongside policiesthatallowtheirintroductioninhealthcarecentres, promotesthesystem’ssustainability,enablesaccesstoagreater numberofpatients,whileallowingforthecontinuedincorporationofinnovativemolecules.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
10.1136/ejhpharm-2023-eahp.15
BackgroundandImportance Boostinggenericsisanindispensableapproachinconductingcost-savingmanagementinhealthcaresystems.Infact,genericscanprovidesimilareffectiveness andsafetytooriginatorsbutwithlowercostsandcanincrease marketcompetition.
2SPD-002 COST-SAVINGIMPACTOFGENERICS:ALOCAL EXPERIENCEONLENALIDOMIDE JCDelRíoValencia*,RTamayoBermejo,COrtegadelaCruz,IMuñozCastillo. Regional UniversityHospitalofMalaga,PharmacyService,Malaga,SpainAimandObjectives
Thepurposeofthisworkwastodemonstratetheeconomicadvantageofagenericlenalidomidein realpractice,showingandcomparingcostsandconsumption duringtheperiod2021to2022.
MaterialandMethods Toconductthisanalysis,patients,type ofprescription(originatororgeneric),numberofpatients treated,numberofcycles,administeredmilligramsandpurchaseprices,duringtheperiodSeptember2021toAugust 2022,wereextrapolatedfrompharmacysoftwareand matched.
Results ComparedwithperiodfromSeptember2021toFebruary2022,duringMarchtoAugust2022,thenumberof treatedpatientsremainedsimilar(105vs104)andthenumberofcyclesadministered(388vs390). Abstract2SPD-002Table1
AimandObjectives Thepurposeofthisstudyistoanalyse themedicationerrors(ME)thathaveoccurredduringaspecificperiodoftime,throughouttheprocessofmedication delivery.Theaimisfindingcausesandpossibleimprovements. MaterialandMethods Wecarriedoutaretrospectivedescriptivestudy.TheerrorsthatoccurredbetweenJanuary2021 andAugust2022(20months)inthetelepharmacyprocess wereanalysed,takingintoaccounteverythingfromthepreparationinthehospitalpharmacytothecollectionofthemedicationbythepatientinthecommunitypharmacy.TheMEs werecollectedinalocaldatabase.Wedescribeddate,personal dataofthepatient,codesassignedtothesingleshippingroute anddestinationcommunitypharmacy,typeoferrorandstep inwhichtheMEwasdetected.
Results Intheperiodstudied,atotalof69MEswere recorded.Webreakthemdownintothefollowingtypes:20 caseswithaquantitativelackofmedication(28.99%),19 casesinwhichadifferentmedicationwassent(27.54%),15 withanotherpatient‘smedication(21.74%),10withmedicine withwrongdose(14.49%),2casesinwhichthemedicine wasnotsent(2.90%)andanother2inwhichthemedicine wassentbadlypackaged(2.90%),1caseinwhichtheonein whichthemisidentifiedmedicinewassent(1.45%)and1case inwhichalargerquantitywassent(1.45%).48MEswere detectedbythepatient(69.56%),15weredetectedinthe communitypharmacy(21.74%),4weredetectedinthehospitalpharmacy(5.80%)and2casesweredetectedduringthe transportationofthemedication(2.90%).Noneoftheerrors detectedhadconsequencesforthepatienttoourknowledge.
Thetotalexpenditureofgenericlenalidomidehasbeen C¼ 147,120andoriginallenalidomideC ¼ 1,204,839.15,therefore thetotalsavinghasbeen87.80%.
Likelihood,thegenericlenalidomidehasbeenaswelltoleratedasoriginallenalidomide.
ConclusionandRelevance Currently,costsavingsandrationalisationpolicyareplayinganessentialroleinhealthcaresystems,andgenericsrepresentagreatopportunitytoreallocate availableresources.Thisstudydemonstratedthatenhancinga genericlenalidomideisagoodstrategyforthesustainability ofcare.Lenalidomidecostsdecreasedwhilethenumberof patientsremainedsimilar.Insummary,genericsconstitutean efficientstrategyforthesustainabilityofnationalhealthservices,allowingresourcereallocationandaccesstocaretoa largernumberofpatients.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.Noconflictofinterest
ConflictofInterest Noconflictofinterest
ASánchezRuiz*,CMuñozCid,NGarciaGomez,JJerezRojas. HospitalUniversitariode Jaén,Farmacia,Jaén,Spain
10.1136/ejhpharm-2023-eahp.16
BackgroundandImportance Aftertheriseoftelemedicinewith theCOVID-19pandemic,atelepharmacyconsultationhas beenimplementedinourhospitalinthepharmacyoutpatient area,sendingmedicinestocommunitypharmacieswithina populationareaof600,000inhabitants.
ConclusionandRelevance AmongtheMEsdetected,themost commonwerethoserelatedtoaquantitydefectorlackofa medicationandthoseinwhichadifferentmedicationwas sent.Ingeneral,theyareerrorsthatcouldbeavoidedby automatingprocessesthatarecurrentlycarriedoutmanually.
REFERENCESAND/ORACKNOWLEDGEMENTS ConflictofInterest Noconflictofinterest
2SPD-004 ECONOMICEVALUATIONANDBUDGETIMPACTFOR AREGIONALHEALTHSERVICEASSOCIATEDWITHTHE INCLUSIONOFTHEFLUOCINOLONEACETONIDE INTRAVITREALIMPLANTINAREGIONAL PHARMACOTHERAPEUTICGUIDELINE
1MIZasGarcía*, 1MAGayosoRodríguez, 1AFernándezPérez, 2DLópezSuárez, 1JNúñez Rodríguez. 1HospitalValleDelNalón,HospitalPharmacyService,Langreo,Spain; 2Complejo AsistencialUniversitariodeLeón,HospitalPharmacyService,León,Spain
10.1136/ejhpharm-2023-eahp.17
BackgroundandImportance Duetothehighcostofthe implantoffluocinoloneacetonide(FAc)190 mg,itisespecially importanttorealiseaneconomicevaluationandbudget impactanalysisbeforeinclusioninthepharmacotherapeutic guideofanyhealthinstitution.
AimandObjectives Realiseaneconomicevaluationanda budgetimpactanalysistoassessitsinclusioninourregional pharmacotherapeuticguide,maintainingthefinancingconditionsofourNationalHealthSystem(NHS).
MaterialandMethods PubMedandreportsfromindependent evaluatorswereconsulted:NationalInstituteforHealthand CareExcellence(NICE)andtheScottishMedicinesConsortium(SMC)amongothers.
Results
Accordingtotheproductinformation,animplant releasesFAcforamaximumof36months,andanadditional implantcanbeplacedafter12monthsifvisiondecreasesor retinalthicknessincreases.PivotalstudiesandtheIRISSobservationalstudyconcludedintheneedtouse1.3implants/eye and1.13implants/eyeaffectedduringthefirst3yearsrespectively,thislastvaluebeingtheoneconsideredbytheERG (EvidenceReviewGroup).Takingthislastreference,thecost oftreatment/affectedeyeatC ¼ 1558.84/eye/yearorC ¼ 4676.53/ eye/3years.
Toestimatethetargetpopulation,weusedthecriteriaof theSMCevaluationreportinwhichtheyconsideredatotal of179patientswithpseudophakicchronicDMEeligiblefor treatmentinthefirstyear,increasingto186inthefifthyear. UnliketheSMC,ourNHSrestrictsitsfundingtothird-line, afteranti-angiogenicagentsandinpatientswithasuboptimal responsetovariousintravitrealdexamethasoneimplantsor pseudophakicpatients.
MakingaparallelismwiththeScottishpopulation,33.5 patients/1styear
34.8patients/5thyearwouldbecandidatesto receiveFAcinourregion.
NICEandtheERGfoundthatinclinicalpractice35%of patientswouldrequirebilateraltreatment.Thus12patients/ yearwouldneedtreatmentinbotheyesinourpopulation. Theeconomicimpactinourregionwouldrangebetween C¼ 5,3000.56/yearifitwereinsertedinonlyoneeyeand C¼ 71,706.64/yearinbotheyes.
ConclusionandRelevance Thefinancingconditionsofour NHSpositionthedruginthethird-line,whichinacertain waycontainsthebudgetimpact.
SinceSMCrestrictingtheconditionsofusemorethanour NHS,thebudgetimpactcouldbeunderestimated.
ConflictofInterest Noconflictofinterest
Eachvialwastransferredtoaclosedtestchamberconnectedtoavapourtrap.Toincreasedrugvapourisation,the chamberwasheatedto50°Candnitrogengaswasconstantly introducedintothevials.Anyvapourspotentiallyreleased fromtheChemfort™ VAweretrappedandthenextracted withsolvent.
QuantificationofCPwasperformedusingavalidatedLC/ MS/MSmethod.
Results NoCPwasdetectedforanyoftheVAswithintact Toxi-Guard® components,whethertestedimmediatelyor28 daysafterreconstitution,evenwhenheatandgasflowwere employedtoencouragetheproductionofvapoursandwhen theVAwasattheendofitsshelflife.Thelimitofdetection ofthemethodwasestimatedat0.02ng.Withoutanintact Toxi-Guard®,110.3ngofCPwerereleasedintothe environment.
ConclusionandRelevance ThemodelCSTDutilisingToxiGuard® air-cleaningtechnologycontaineddrugvapoursaftera 28-dayusageperiod,evenunderextremeconditions.Arecent studyproved28-daypreventionofmicrobialingressbythe sameCSTD.Takentogether,thetwostudiessupportpharmacists’ decisiontousedrugsfortheirfullshelflifeorto extendthebeyond-use-dateupto28dayswhenusingan appropriateCSTD,thusreducingcostandwaste.
ConflictofInterest Corporatesponsoredresearchorothersubstantiverelationships:
OferRazandElanaSlutskySmithareemployedbySimpliviaHealthcareLtd,themanufacturerofChemfortTM.Dekel NavarroandDanielEpsteindeclarenoconflictofinterest relatingtothematerialpresentedintheabstract.Fundingfor thisprojectwasprovidedbySimpliviaHealthcareLtd,the manufacturerofChemfortTM
1DNavarro, 1DEpstein*, 2ORaz, 2ESlutskySmith. 1NextarChempharmaSolutionsLtd., AnalyticalLaboratories,NessZiona,Israel; 2SimpliviaHealthcareLtd.,Designand Development,KiryatShmona,Israel
10.1136/ejhpharm-2023-eahp.18
BackgroundandImportance SeveralClosedSystemTransfer Devices(CSTDs)arecurrentlyapproveda7-dayusageperiod. Increasingpressuretoreducedrugcostsanddatasupporting stabilityofsomedrugsbeyond7dayscreateademandfor CSTDsthatcontainhazardousdrugvapoursfor28days.A previousstudyprovedthatamodelair-cleaningCSTDcontainsdrugvapoursfor7days.
AimandObjectives Theaimwastotestdrugvapourcontainmentofanair-cleaningCSTDunderextremeconditionsfor 28days.
MaterialandMethods Cyclophosphamide(CP)waschosenas therepresentativedrug.AmodelCSTD(Chemfort ™)Vial Adaptor(VA)wasconnectedtoeachvial,andCPwasreconstitutedusingtheCSTDSyringeAdaptor.VAsattheendof theirshelflife,representingextremeconditions,weretested bothimmediatelyfollowingand28daysafterreconstitution, withandwithoutintactToxi-Guard® air-cleaningsystems(an integralpartoftheChemfortTM VA).
2SPD-006 AREALLBIOLOGICAGENTSINTHETREATMENTOF ANKYLOSINGSPONDYLITISEQUIVALENT ALTERNATIVES?
1IGarcíaGiménez, 2OMonteroPerez, 1MRodriguezJorge*, 3SFenix-Caballero, 3EJAlegreDelRey. 1HospitalJuanRamónJiménez,PharmacyDepartment,Huelva,Spain; 2Instituto CatalandeOncologia,PharmacyDepartment,L’hospitaletdeLlobregat,Spain; 3Hospital UniversitarioPuertoReal,PharmacyDepartment,PuertoReal,Spain
10.1136/ejhpharm-2023-eahp.19
BackgroundandImportance Ninedrugsarecurrentlyapproved forthetreatmentofankylosingspondylitis(AS)inadults:adalimumab,certolizumabpegol,etanercept,golimumab,infliximab,ixekizumab,secukinumab,upadacitinibandtofacitinib. Tofacitinibwasthelastofthemtoreceiveitsapproval.However,therearenodirectcomparisonsbetweenthem.
AimandObjectives Toestablishwhetherthedrugsapproved forASinadultscanbeconsideredequivalenttherapeutic alternatives(ATE)inefficacyinAS.
MaterialandMethods Asearchofclinicaltrialsofthesedrugs inadultpatientswithASwasconducted,phaseIIorIII,double-blinded,controlledwithanotherdrugorplacebo.
Otherinclusioncriteriawere
. Endpoint:ASAS40(a 40%improvementandanabsolute improvementfrombaselineoftheAssessmentin SpondyloArthritisInternationalSociety).
. Follow-uptime:12-16weeks.
Forthosedrugswithmorethanonestudy,apreviousmetaanalysiswasperformedusingJoaquinPrimocalculator.An adjustedindirectcomparison(IC)ofthedrugsusedinASversustofacitinibwasperformedusingtheBuchermethod,using JoaquinPrimocalculator.Duetolackofdataintheliterature andconsideringthattherapyfailurecanberecoveredwith secondlines,halfoftheASAS40valueobtainedinmeta-analysiswastakenasdeltavalue.ATEguidewasfollowedinorder toestablishapositioning.
Results
Sixteenstudieswereincluded 4adalimumab,2golimumab,1 infliximab,1certolizumab,2etanercept,1upadacitinib,2 tofacitinib,1secukinumaband2ixekizumab.Thedifference inASAS40ofthedrugsbeforeversustofacitinibexpressedas RAR(IC95%)was:Adalimumab[4(-6,1;14,1)],certolizumab [-7,3(-25,1;10,5)],etanercept[2(-11,5;15,5)],golimumab[5(-16,3;6,3)],infliximab[8,43(-4,8;21,6)],ixekizumab[-9 (-20,6;2,6)],secukinumab[-2,7(-18,3;12,9)],upadacitinib[1,9(-17,8;13,9)].Adalimumab,etanerceptandtofacitinibare consideredATE.Infliximab,upadacitinib,secukinumab,golimumab,certolizumab,ixekizumabandtofacitinibcanalsobe consideredATE,beingtheprobabilityofclinicallyrelevantdifference<50%(mostofthe95%CIisintheequivalence range)andthefailuredoesnotinvolveserious/irreversible damage.
ConclusionandRelevance Tofacitinibandtherestofthese drugscouldbeconsideredATE.Foradefinitivestatement, thecriteriaofsafetyandadequacyshouldbeconsidered.
ConflictofInterest Noconflictofinterest
Theamountderivedfromthepurchaseofgenericlenalidomidecorrespondingtothestudiedperiod,comesupto C¼ 1,601.27.ThatresultsinanestimateofC ¼ 9,607.62for12 months.
Assumingthatthesamenumberofpatientsandtreatments withlenalidomidewerestablethroughouttheperiod,aswell astheexpenditureontherestoftheABCofdrugs,theeconomicimpactgeneratedwouldmeanasavingofapproximatelyC ¼ 1,005,278.84,whichwouldcauseasignificant decreaseinthechapterIIforourHospital(-14.25%).
ConclusionandRelevance Theeconomicimpactcausedbythe introductionofgenericlenalidomideinourHospitalwillproducesavingsofmorethanonemillioneuros.
Speedinguptheauthorisationprocessesforgenericmedicines,aswellasotherpricingpolicies,areessentialmanoeuvrestogetacohesivehealthsystemthatguaranteesequal accesstomedicines.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
2SPD-009 AVOIDEDCOSTSFROMTHEINCLUSIONOFBREAST CANCERPATIENTSINCLINICALTRIALS
1AMValleDíazdelaGuardia, 2SSadyrbaevaDolgova, 2CMontero-Vilchez*, 2MIArchilla Amat. 1HospitalUniversitarioVirgendeLasNieves,FarmaciaHospitalaria,Granada,Spain; 2HospitalUniversitarioVirgendeLasNieves,ServiciodeFarmacia,Granada,Spain
10.1136/ejhpharm-2023-eahp.21
FPAna*,MAGayosoRodríguez,MIZasGarcía,JNúñezRodríguez. HospitalValleDel Nalón,HospitalPharmacyService,Langreo,Spain
10.1136/ejhpharm-2023-eahp.20
BackgroundandImportance Breastcancerisoneofthe tumourswiththehighestincidenceinSpain,anditspharmacologicaltreatmentgeneratesahugeeconomicimpact.Clinical trialsareessentialforevaluatingtheefficacyandsafetyof newtherapies,andalsoprovideafinancialbenefittothepublichealthsystem.
AimandObjectives Theaimofthisresearchistocalculate thesavingcostsindrugs,derivedfromtheparticipationof breastcancerpatientsinclinicaltrials(basedonthedrugfree supportprovidedbythesponsorofeachstudy).
BackgroundandImportance
Theuseofgenericdrugsisone ofthemosteffectivetoolstoincreaseefficiencyintheeconomicmanagementofthehealthsystem.FromMarch2021 toFebruary2022,theacquisitionoftheoriginalmoleculeof lenalidomide(Revlimid®)representedthemainexpenseinthe ABCofdrugpurchases.Asofthisdate,agenericspecialty wascommercialisedandthePharmacyServiceproposeda replacementbetweenthem,giventhatbothsharethesame indicationsasinthetechnicaldatasheet.
AimandObjectives Quantifyingtheeconomicimpactinthe expensesofchapterIIofageneralhospital,causedbythe acquisitionofgenericlenalidomideinsteadofRevlimid® and itsrepercussiononthebudgetduring12months.
MaterialandMethods Althoughonlytwomonthsofevolution withthenewgenericmoleculeareavailable,wehaveextrapolatedthisdatatooneyearsothatwecancalculatetheeconomicaldifferenceswhenitcomestothebudget.
Results FromMarch2021toFebruary2022,thepurchaseof Revlimid® hasmeantanetamountofC ¼ 1,014,886.46,which represents9.8%ofthetotalexpenseinchapterII (C ¼ 10,246,115.23)andpositionsitasfirstspendintherankingofmedicinespurchasedinthisperiodof12months.
MaterialandMethods Aretrospectiveanalysiswasmadeofall breastcancerclinicaltrialsinitiatedinourhospitalsinceJanuary2020,andallpatientsincludedinthesetrialswere selected.Thedatacollectedwere:trialphase,investigational drug,numberofsubjectsenrolledandnumberoftreatment cyclesreceived.TheOncologyDepartmentwascontactedto discussthetherapeuticalternativeofchoiceanditstheoretical durationifthepatienthadnotparticipatedintheclinical trial.Thecostofeachoptionwascalculatedusingtheacquisitionpriceofthedrug(laboratorysaleprice – discount+4% VAT).Informationwasobtainedfromthedatabaseoftheclinicaltrialsunit.
Results Since2020,8breastcancerclinicaltrials(2phaseII and6phaseIII),wereinitiatedinourhospital.Were included10subjects,receivingatotalof106treatment cycles.Theinvestigationalmedicalproductsstudiedwere: trastuzumabandconjugates,pertuzumab,atezolizumab,olaparib,alpelisibandpalbociclib.Theoverallcostsavingwas C¼ 198.775,32.Thetrialwiththehighestcostimpactoffers asavingofC ¼ 8.269,48percycleofeachenrolledpatient. Thedrugwithhighestavoidedcostwaspemetrexed (C ¼ 32.890,54).
ConclusionandRelevance Clinicaltrialsinbreastcancer patients,inadditiontoofferingthepossibilityofaccessto
newtherapeuticalternatives,representaconsiderableeconomic savingandasignificantreductioninpharmaceuticalcosts.Itis importanttoimprovepatientrecruitmentinthesetypesof studies.
ConflictofInterest Noconflictofinterest
2SPD-013 INTERVENTIONALCARDIOLOGY:ANALYSISOF STERILEMEDICALDEVICE’SCONSUMPTIONAND ASSESSMENTOFDIFFERINGPRACTICES
SChabouni*,OChauvel,JLPons. VictorDupouyHospitalCenter,HospitalPharmacy, Argenteuil,France
1VGarciaLópez, 2AMoratallaRolanía, 2PHorsComadira, 1JMGuiuSegura*. 1Consortium ofHealthandSocialCareofCatalonia,PharmacyandMedicines,Barcelona,Spain; 2ConsortiumofHealthandSocialCareofCatalonia,CentralProcurementBody,Barcelona, Spain
10.1136/ejhpharm-2023-eahp.22
BackgroundandImportance Greenpublicprocurementisa processofcontractingproducts,services,andworkswith theleastpossibledamagetotheenvironmentduringtheir lifecycle.Inordertoimproveknowledgeontheapplicationofenvironmentalcriteriainhealthcareprocurement, itisnecessarytoassessthecurrentimplementation situation.
AimandObjectives Toreviewtheincorporationofenvironmentalcriteriainpublicprocurementproceduresfordrugs, medicaldevicesandnon-medicalequipment(paper,clothing, garbagebags,etc.)inagroupprocurementorganisation.
MaterialandMethods Aretrospectivestudywasperformedin whichallthetenderscarriedoutbythegroupprocurement organisationfrom2017tothefirstquarterof2022were reviewed.Alltendersthathadincludedenvironmentalcriteria intheevaluationcriteriawereidentified.Inordertoevaluate theimpactofthesecriteriainthesuppliers’ bids,itwasconsideredaspositiveifcompliancewiththeenvironmentalcriteriawasgiveninatleastoneoftheproductsoffered. Classificationofthesuppliers(drugs,medicaldevices,and non-medicalequipment)wasmadeonthebasisofsubject matteroftheprocurement.
Results Atotalof117tenderfileswerereviewed,where15 (12.8%)includedenvironmentalcriteriainthetechnical specifications:4(26.6%)fordrugs,6(40%)formedical devicesand5(33.3%)fornon-medicalequipment.Atotal of130supplierspresentedtender bidsinthe15tenders identified:80(61.5%)metoneo rmoreoftheenvironmentalcriteriaincludedinthespecifications.Regardingthesubjectmatterofthecontract,19companiessubmittedbidsto drugtenderfiles,55tomedicaldevicesand6tonon-medicalequipment.Duringtheperiod2018-2021,thehighest numberoftenderswithenvironmentalcriteriawerethose ofmedicaldevices.Overall,agrowingtrendwiththeincorporationofenvironmentalcriteriais observedoverthe years.
ConclusionandRelevance Theintroductionofenvironmental criteriainhealthcareprocurementisstilllowbutwithan increasingtrendtowardsahigherpercentageofthetendered contracts.Thecurrentsustainableprocurementpoliciesin Europeencourageforawiderintroductionofsocialandenvironmentalcriteriaintheprocurementofdrugs,medicaldevicesandnon-medicalequipment.
10.1136/ejhpharm-2023-eahp.23
BackgroundandImportance Therapeuticangioplasty(TA) allowsthedilatationofcoronarystenosis.Thisminimallyinvasiveprocedure,oftencombinedwithdiagnosticangiography, requiresvariouscostlymedicaldevices(MD).
AimandObjectives Thisstudywasconductedtohighlightif thereisadifferenceininterventionalcardiology(IC)practices betweenphysiciansandestimatetheassociatedcosts.
MaterialandMethods First,data(characteristicsofcardiac procedures(CP),numberandtypeofMD(nMD))were extractedfromthecardiovascularinformationsoftware(Cardioreport®)onaperiodofthreemonths.Then,Excel® was usedtocalculatetheaverageMDcost(AC)perprocedurefor eachoperator.Finally,datawereexploredinRStudio® using theMultiple-Regression,ClusteringwithK-meansandWard’ s method,inordertoclassifythesimilitudesandvisualisethe differencesinpractices.
Results Oursampleof74CPincludes11TAand63combinedprocedures.These,concerned63patients(averageage 68years),26%offemalesand76%ofmalesamongwhom 10hadatleast2CP.TheACestimatedperprocedureis C¼ 1125ofwhichC ¼ 602isnotcoveredbyadditionalpayments (NCA)whileC ¼ 523iscovered(CA).FivephysiciansA/B/C/D/ EoperateinICwitharespectivepercentageofactivityof 5%/9%/12%/22%/51%.
Abstract2SPD-013Table1
Multiple-RegressionshowsthatcostofCPisexplainedat 89%bynMDandNCAcostassignificantvariables(adjusted R²=0.891withP-value<5%(1.465e-12)sonull-hypothesis canberejected).ClusteringandWard’sdendrogramgrouped procedureswithcommoncharacteristicsandshowedthatthere weredifferencesinpracticesamongphysicians.Afterexcluding operators,AandE,clusteringshowsthatoperatorCissingularinhispractices(withahigherrateofcomplexprocedures definedaslongerthan90minutesforcombinedprocedures), whileBandDhavesimilaritiesintermsofchoiceofMD.
ConclusionandRelevance Thehospitalpharmacist,asMD expertplaysacentralroleinmanagingconsumptionanalysis. Expandingthesampletoconfirmtheresultswouldbemore relevant.Thus,itwouldbeinterestingtoexploretheimpact ofcommunicatingthisworktophysiciansinordertohomogenisetheirpractices.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
2SPD-014
1MDufossé*, 2FMartel, 3LBenard, 1APetit. 1CHUAmiens-Picardie,PharmacieÀUsage
Intérieur,Amiens,France; 2CHUAmiens-Picardie,CentraledeDésinfectionDesEndoscopes, Amiens,France; 3CHUAmiens-Picardie,ServiceBiomédical,Amiens,France
10.1136/ejhpharm-2023-eahp.24
BackgroundandImportance Inourhospital,weuseflexible ureteroscopesforlithiasistreatment,whichisathighriskof materialbreakage.Inthedevicespark,wehavesixadultdevices(inadequateinviewoftheactivity)andonepaediatric (obsolete),whichareoftenunavailable,fordisinfectionor repair.
AimandObjectives Tocompensateforunavailability,wecould usesingle-usedevices.Ourobjectiveistocomparereusable versussingle-usedevices’ costs,todetermineifreferencingsingle-usedevicesisrelevant.
MaterialandMethods Wesetaworkinggroup,includingurologists,pharmacists,biomedicalengineersandhealthexecutive fromdisinfectingcentre.Webasethecalculationofthecost on300usesperyear.Reusablecostgatherthepurchaseprice, theamortisationexpensefora3yearproductlifetime,disinfectionscost(products,equipment,staff),maintenancecontract andrepaircost.Single-usecostisassimilatedwiththepurchasepriceof300units.Themanufacturerprovidestheconsolefreeofcharge.Ourstudydoesnotconsiderwaste treatmentcost.
Results For300uses,reusableureteroscopescostC ¼ 133360 yearlypre-tax(C ¼ 445/unit):C ¼ 27813foramortisation expense,C ¼ 66000formaintenancecontract,C ¼ 20594for therepairs.DisinfectioncostsC ¼ 12900yearly,inadditionto C¼ 4353yearlyformaintenanceandC ¼ 1700foramortisation ofequipment.Ifweonlyusedsingle-useureteroscopes,it wouldcostC ¼ 184500yearly(C ¼ 615/unit).Theincremental costwouldbeC ¼ 51140yearly.
ConclusionandRelevance Ourresultsshowthat,inour case,single-useis moreexpensive,espec iallysincewehave anewdisinfectionfacilityforourreusableureteroscopes. Moreover,thesingle-useureteroscopes ’ picturequalityis lower,whichledthegrouptospeakinfavourofthepark increasetoeightunitsforusualuse.Inaddition,itrecommendspunctualpurchaseofsingle-use incasesofunavailabilityoractathighriskof materialbreakage.Indications forpaediatricusearerare,thegrouprecommendspurchasingafewsingle-useunitsandtowriteoffthereusable ureteroscope.
ConflictofInterest Noconflictofinterest
toprovideremotepharmaceuticalcareforvulnerablepopulations(elderly,socioeconomicormobilityproblems).
AimandObjectives Todescribetheimplementationofa THDPinahospitalthroughthecommunitypharmacy(CP).
MaterialandMethods Observationalretrospectivestudy between1October2021and30September2022.Patients thatvoluntarilyrequestedtobepartoftheTHDPwere evaluatedtomeettheestablishedcriteria:>3monthsof treatment,compliancewithconsultations,adherent,and properunderstandingoftheinformationontheTHDPand signinganinformedconsentform.Duetothehumanand economicresourcesavailable,p rioritywasgiventoolder patients(>65years),distancetothehospitalcentre,disabilityordependency.Neitherpathologynormedication weretakenintoconsideration.CPrequestedthemedication viaweb.Then,patientsreceivedfollow-upphonecallsby theHPafterreviewingtheelectronicmedicalrecords.The medicationwaspackagedindividuallywithbarcodelabels andsenttothenearestCPthroughapharmaceutical cooperative.
Results 8168patientsattendedtheoutpatientunit,444(5,4%) wereincludedintheTHDP.Rheumatoidarthritis(17.8%) treatmentswerethemostin-demandmedication,followedby multiplesclerosistreatments(10.1%)andantiretroviraldrugs (8.5%).
10.1136/ejhpharm-2023-eahp.25
BackgroundandImportance
TheSARS-CoV-2pandemicacceleratedtheimplementationofalternativewaysofremotepharmaceuticalcareanddispensation.Telepharmacyandhome deliveryprogrammes(THDP)allowhospitalpharmacists(HP)
Eightincidentsoccurred:dosingerror(25%),wrongdrug (12.5%),wrongformulation(62.5%),thatwereresolved. ConclusionandRelevance TheimplementationofTHDPhas beenanewchallengeforHP.Itenablesustoprovidedrugs topatientsintheirimmediateenvironmentwithoutextracost tothehealthcaresystem.However,theevidenceoftheimpact oftheseprogrammesissparse.
2SPD-017 TELEPHARMACYANDHOMEDELIVERYPROGRAMME FOROUTPATIENTSTHROUGHTHECOMMUNITY PHARMACY MRCantudoCuenca*,MArenasJimenez,AYSalmeronCobos,CMonteroVílchez, MIArchillaAmat. HospitalUniversitarioVirgendeLasNieves,Pharmacy,Granada,SpainCFernandezCuerva*,RAsensiDiez,IMuñozCastillo. HospitalRegionalUniversitariode Málaga,ServiciodeFarmacia,Málaga,Spain
10.1136/ejhpharm-2023-eahp.26
BackgroundandImportance Clostridiumdifficile isthemost commoncauseofinfectiousdiarrhoeainhospitalisedpatients andcausesgreatmorbidityduetothehighpercentageof recurrence.Bezlotoxumabwas thefirsthumanisedmonoclonalantibodyagainstC.difficiletoxinBapprovedforpreventionofrecurrent Clostridiumdifficile infection(CDI)in high-riskadultsinconjunctionwithstandardofcareantibiotics.Bezlotoxumabvialpoweris1,000mg,isgivenasaonetimeinfusioninrecommendeddoseof10mg/kgover60 minutes.NewguidelinesonthemanagementofCDIhave beenpublishedin2021:ACGandIDSA/SHEA,whichrecommendusingbezlotoxumabinpatientswithahighriskof recurrence.
Inordertoreduceeconomicimpactoftheadministration ofbezlotoxumab,ourcentrepromotedschedulingselected patientsonthesamedaytousetherestofthevials.
AimandObjectives Toassesstheeconomicimpactofthe appointmentstrategyinpatientstreatedwithbezlotoxumab.
MaterialandMethods Aretrospectiveanal ysisofpharmaceuticalexpenditureofbezlotoxumabprescribedforCDIwas conductedfromJune2019toAugust2022.Datacollected werenumberofpatientstreatedwithbezlotoxumab,weight, dateofinfusion,numberofvialsrequired.Datawerecollectedfromelectronicprescribingprogramandeconomic software.
Results 45adultpatientswereincludedinthestudy.Theyall receivedbezlotoxumabforCDIathighriskofrecurrence,in singleinfusionof10mg/kg.24patients(53.3%)werecited topreventvialwaste.21patients(46.7%)requiredacomplete vial:duetotheirweight,difficultiesinmakinganappointment,orboth.Of24patients,medianweightwas50kg (rank34-66kg).
Intheperiodofourstudy,35vialsofbezlotoxumabwere used.CostofbezlotoxumabvialisC ¼ 1,480.Estimatedexpenditurewas:C ¼ 51,800ifpatientswerecitedvsC ¼ 66,600if not.ThecostoftreatmentdecreasedbyC ¼ 14,800duetothe administrationappointmentstrategy.
ConclusionandRelevance BezlotoxumabisaneffectivetreatmentinpreventingCDIrelapseinhighriskrecurrence patients,followingguidelines.Administrationappointment strategyinselectedpatientshasproventobeefficientsince morepatientscanbetreatedwiththesamebudget.
1.ClinicalPracticeGuidelinebytheInfectiousDiseasesSocietyofAmerica(IDSA) andSocietyforHealthcareEpidemiologyofAmerica(SHEA):https://pubmed.ncbi. nlm.nih.gov/34164674/
2SPD-020 IMPACTOFCOVID-19TREATMENTOPTIONSON HOSPITALPHARMACY’SWORKLOAD:LESSONS
LEARNED
1SVonWinckelmann*, 2JMichaux, 1GVanDenBergh, 1VVerheyen. 1ImeldaHospital, HospitalPharmacy,Bonheiden,Belgium; 2CatholicUniversityLeuven,Faculty PharmaceuticalSciences,Leuven,Belgium
10.1136/ejhpharm-2023-eahp.27
BackgroundandImportance TreatmentguidelinesforCOVID19haverapidlybeenevolving.Differentdrugsagainst COVID-19haveurgentlyemergedtocontrolthepandemic, challenginghospitalpharmaciestomaketheseantiviraland immunomodulatorytherapiestimelyavailableforadmitted patients.
AimandObjectives Toanalysetheprescribingpatternsof COVID-19drugsinourhospitalanditsimpactonthepharmacy ’sworkload.
MaterialandMethods Weretrospectivelyanalyseddrugregistrationdatafrom1January2020to16March2022of COVID-19drugs(dexamethasone,remdesivir,baricitinib, casirivimab/imdevimabandsotrovimab)forhospitalised patients.Consumptiondatawereexpressedasnumberof patientsandnumberofpreparations.Todeterminepharmacy ’sworkload,wemeasuredtheaveragetimefordrug ordering,preparationanddispensing.Hydroxychloroquine andbaricitinibwereexcludedasthesearecommerciallyavailableoraldrugswhicharedistributedaccordingtostandard procedures.
Results ThevolumeofdispensedCOVID-19drugsfluctuated alongwiththehospitalisationwavesoftheCOVID-19epidemic.Oraldexamethasonewasthemostfrequentlyprescribeddrugthroughoutthewholeperiod,whichisconsistent withthestrongrecommendationinthenationalguideline. Remdesivir,introducedinourpracticesinceOctober2020, wasthesecondmostprescribeddrugdespitelowevidence. FromOctober2021untilDecember2021,41infusionsof remdesivirwereadministered,comparedto381infusions fromJanuary2022untilMarch2022.Comparedtodexamethasoneandremdesivir,monoclonalantibodies(casirivimab/ imdevimabandsotrovimab)werelesscommonlyused:48preparedinfusionsbetweenSeptember2021andMarch2022. Mostdrugsweregivenincombination.Remdesivirandmonoclonalantibodiesweremanuallyorderedtofulfilurgentneeds asthesupplyismanagednationwidebythegovernment.Infusionswerepreparedatonceduetolimitedstability.Ordering, preparinganddispensingrequiredanaverageof35minutes perpatienttocomplete.
ACGClinicalGuidelines
Prevention,Diagnosis,andTreatment ofClostridioidesdifficileInfections:https://pubmed.ncbi.nlm. nih.gov/34003176/
ConflictofInterest Noconflictofinterest
ConclusionandRelevance TheCOVID-19pandemicimpacted pharmacy ’sworkload.Wecouldhavemademoretimesaving decisionssuchastheuseofcommerciallyavailablemethylprednisoloneinsteadofdexamethasoneandbatchingremdesivirpreparations.Hospitalpharmacistsshouldbeinvolvedin developingnationalguidelinesandtakeintoaccountthe impactondailypractice.
1.Sciensano,InterimclinicalguidanceforadultswithconfirmedCOVID-19,July 2022,Version29
3PC-001 PREPACKEDBOXESFOROUTPATIENTPARENTERAL ANTIBIOTICTHERAPY(OPAT) – AQUESTIONNAIRE SURVEYONKNOWLEDGE,OPINIONANDWISHES
1TTruelshøj*, 2COlesen, 3LLundRøndbjerg, 4SPaarupKirkebyHerping. 1Hospital Pharmacy – CentralDenmarkRegion,Precurement,Dk-8000AarhusC,Denmark; 2Hospital Pharmacy
CentralDenmarkRegion,ClinicalPharmacy – Auh,Aarhus,Denmark; 3Hospital Pharmacy – CentralDenmarkRegion,Gødstrup,Herning,Denmark; 4HospitalPharmacy –CentralDenmarkRegion,ClinicalPharmacy – Hemidt,Silkeborg,Denmark
10.1136/ejhpharm-2023-eahp.28
BackgroundandImportance Tosupportasimpleandqualityassuredoutpatientparenteralantibiotictherapy(OPAT)workflow,thehospitalpharmacyoffersspeciallyprepackedboxes foruseinthepatient’shomeafterdischarge.Thereareboxes forthreedifferentantibiotics;benzylpencillin,cefuroximeand piperacillin/tazobactam,containingantibioticpowder,utensils andsolventforthreedaysoftreatment.Anotherboxcontains onlyutensilsandsolventforthreeadministrations.
AimandObjectives Theaimistoexploreknowledge,opinion andwishestotheprepackedantibioticandutensilsboxesin attempttofulfiltheneedsofthehospitalwards.
MaterialandMethods Inanelectronicquestionnairewith17 questions,onenurseateachhospitalwardwasaskedabout theirknowledge,opinionandwishestotheantibioticsand utensilsboxes.
AllhospitalswardsinCentralDenmarkRegionthatdischargepatientstoOPATreceivedaquestionnaire.Thequestionnairewasdesignedafterinterviewwithtwonursesand pilottestedbytwoothernursesondifferentwards. Results 39wardsof53(74%)respondedtothequestionnaire. TheresultsconfirmedthattheboxesarevaluedintheOPAT workflowonthehospitalwards.87%knewaboutsomeof theantibioticsboxes,59%knewabouttheutensilsbox.There wasagreement(97%)thattheuniformitythatcomeswiththe boxes,contributestopatients‘ safetyinprimary-care.
Therewasgeneralsatisfactionwiththenumberoftreatmentdaysintheboxes.Onethirdofrespondentswouldhave likedasupplementaryboxwithonedayofantibiotictreatment,enablingamoreflexiblesolutionandreduceddrug waste.25%wouldlikeboxescontainingotherantibiotics.
Severalcommentedontheavailabilityoftheboxesonthe wards,asafactorthatsometimespreventsuse.
ConclusionandRelevance Thesurveyshowsthattheboxes areknownandhighlyappreciated,butthereisaneedto increaseknowledgeaboutalltheboxesandimprovetheir availability.
Currentlytheexistingprepackedboxescovermostcasesof OPAT,butasupplementofaone-daytreatmentmayprovide amoreflexiblesolutionwithlessdrugwaste.
Toevaluatethewishesforotherantibioticsinprepacked boxes,moredataaboutuseofantibioticsforOPATpatientsis needed.
ConflictofInterest Noconflictofinterest
3PC-002
REDUCEEXPOSURE?
1PSessink, 2BTans, 2ISpriet. 1ExposureControlSweden,MonitoringandConsultancy, Bohus-Björkö,Sweden; 2UniversityHospital,Pharmacy,Leuven,Belgium
10.1136/ejhpharm-2023-eahp.29
BackgroundandImportance ExposuretoHazardousDrugs (HD)isapotentialhealthrisk.Multipleregulatoryagencies haveprovidedguidanceinvolvingenhancedcleaningproceduresandtheuseofClosedSystemTransferDevices(CSTDs) tominimisetheriskofexposure.However,despitethepotentialforsideeffectsinvolvingtheuseofantibiotics(ABs),guidancehasnotbeenprovidedforfacilitiestoreduceor minimisetheserisks.
AimandObjectives Theaimofthisstudywastoidentifythe levelofABandHDsurfacecontaminationinahospitalpharmacyandeightwardstoincreaseawarenessfortheneedfor enhancedcontrolsinvolvedinABuse.
MaterialandMethods SixHDswereanalysedinfoursurface wipesamplesfromapharmacyandtwowards.Samplingwas repeatedfourtimes(trials)overaperiodofeightmonths (288endpoints).ACSTDwasusedforHDpreparationduringtheentirestudy.EightABswereanalysedintwosurface wipesamplesfromsixwardscollectedduringthefourtrials (384endpoints).Samplingwasatthesametimepointsasfor HDsampling.ACSTDwasnotusedinABhandling. Enhancedcleaningwasimplementedfollowingthefirsttrial. Sampleswereanalysedwithliquidchromatographytandem massspectrometry.
Results HDsurfacecontaminationwasdetectedin6%ofthe samplescollectedduringthefourtrials.Sampleswithhigh levelsofcontaminationwerenotfound.ABsurfacecontaminationwasdetectedin68%ofthesamples.15%ofthesamplesshowhighlevelsofcontamination.Despiteenhanced cleaningprocedures,ABcontaminationwasincreasedinthe lasttrialscomparedtotheinitialtrials.
ConclusionandRelevance Thestudyillustratesthatinstitutionalguidance,involvingtheuseofaCSTDandeffective cleaning,hasproventobeeffectivetominimiseunintentional exposureofhealthcareworkerstoHDsurfacecontamination. Onthecontrary,guidance,controlsandcleaningwerenotsufficienttoreducesurfacecontaminationwithpotentialharmful ABs.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.AlertandactionlevelsforsurfacecontaminationwithHDsinTheNetherlands (https://www.dokterhoe.nl/fileadmin/user_upload/documents/cytostatica/meetstrategie-werkinstructie.pdf)
ConflictofInterest Corporatesponsoredresearchorothersubstantiverelationships:
ThestudywasfinanciallysupportedbyICUMedicalUSA. PaulSessinkisreceivinganhonorariumfromICUMedicalfor thispresentation.
10.1136/ejhpharm-2023-eahp.30
BackgroundandImportance Erenumabisanewmonoclonal antibodyforthetreatmentofmigrainethatbindstothecalcitoningene-relatedpeptide(CGRP)receptortoinhibititsfunction.Erenumabisadrugwithaconsiderableeconomicimpact onthehospital’sannualbudget.
practicalbeyondusedateofdrugvials,throughuseof CSTDs.
AimandObjectives ThisstudyaimedtotestiftheChemfortTM CSTDcanmaintainmicrobiologicalintegrityafter10 withdrawalsfromvialsoveraperiodof28days.
AimandObjectives
Toevaluatethebudgetaryimpactofthe redosificationofthecommercialdoseoferenumab140mg intodosesof70mg.
MaterialandMethods
Anobservational,retrospectivestudy wasconductedinatertiarycarehospitalwithaclean room.Allpatientstreatedwitherenumabbetween1January 2019and30August2022w ereincluded.Thevariablescollectedwere:sex,age,dosepreparedperpatient,numberof redosesperpatient,andnumberofsyringesoferenumab used.Tocalculatethebudgetaryimpactoferenumab,a pharmacoeconomicstudywascarriedoutinwhichthesavingsobtainedbytheredosificationof140mgindosesof 70mgwereevaluatedsincebothcommercialpresentations havethesameprice(PTRerenumab70mgand140mg= C¼ 200).Theactualcostofthetreatmentswithredosingand thetheoreticalcostwithoutredosingwerecalculated,consideringthenumberofdosesandthedurationoftreatment ineachpatient.Theinformationwasobtainedfromthecorporateprescriptionprogrammeandpatients ’ clinical records.
Results Atotalof281patientsweretreatedwitherenumab duringthestudyperiod.Themeanagewas46years(range 17-75),86.8%(n=244)womenand13.2%men(n=37).A totalof1,133syringesoferenumab70mg(mean:2;range 0-29)and1,875of140mg(mean4;range0-28)wereconsumed.Therealannualcostofthetreatmentswithredosing wasC ¼ 519,827;comparedtoatheoreticalannualcostof C¼ 629,282iftheredosinghadnotbeencarriedout.Therefore,theredosificationoferenumab140mginto70mghas saved547.28syringesoferenumab140mgperyear (C ¼ 109,455).AnestimatedsavingofC ¼ 389.52perpatientwas obtainedbytheredosificationoferenumab140mgdoseinto 70mg.
ConclusionandRelevance Theresultsshowthattherepackagingofthe140mgdoseinto70mgisagreateconomicsavingpracticeandeasytoimplementinhospitals.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
3PC-005 CLOSEDSYSTEMTRANSFERDEVICE(CSTD)EXTENDS
PRACTICALIN-USESHELFLIFETO28DAYSAFTER FIRSTPUNCTUREOFNON-PRESERVEDSINGLE-USEVIALSINBOTHCONTROLLEDANDUNCONTROLLED ENVIRONMENTS
1RTerkola*, 2CPietrzak, 3ASNebel. 1UniversityofGroningen,MedicalCenter,Groningen, TheNetherlands; 2UniversityofNaturalResourcesandLifeSciences,Food-AndBioTechnology,Vienna,Austria; 3FHCampusWien,AppliedLifeSciences,Vienna,Austria
10.1136/ejhpharm-2023-eahp.31
BackgroundandImportance Closedsystemtransferdevices (CSTD)wereoriginallydesignedtoprotectoperatorsfrom cytotoxic,mutagenic,andreprotoxicagents.Thereisincreasingpressuretoreducecostburdenbypreservingdrugs,especiallyinthefieldofoncology.Onesolutionisdrugvial optimisation,whichcanbeaccomplishedbyextendingthe
MaterialandMethods TestswereperformedinbothacontrolledGMPClassAenvironmentandanuncontrolledenvironment(350vialsineachenvironment).Environmental conditionsweremonitoredbycontinuousairsampling.The rubberstoppersofallvials,containingtrypticsoybroth(TSB) growthmedium,weredisinfectedpriortomountingChemfortTMVialAdaptors(VAs)onthevials.TheChemfortTMSyringeAdaptor(SA)wasattachedtoa10mLsyringeand subsequentlyconnectedtotheVA.TheseptaofboththeVA andSAweredisinfectedpriortoeveryconnection.Ten5mL aliquotswerewithdrawnfromeachvialat2-weekintervals (days0/3syringes,14/3syringes,and28/4syringes),incubated for7daysat20–25°Candthen7daysat30–35°C.After28 days,thevialcontainingtheremaininggrowthmedium(50 mL)wasalsoincubatedfor7daysat20-25°Candthen7 daysat30-35°C.Vialsandsyringeswereinspectedvisually forsignsofmicrobialgrowthduringeachincubation.Tenpositivecontrolcontainersweresubjectedtoagrowthpromotion test.
Results Nosignsofmicrobialgrowthwereobservedinanyof the7,000samples,norinthegrowthmediumremainingin thevialsaftertransferswereperformedineitheranuncontrolledorcontrolledenvironment.
ConclusionandRelevance Thedatapresenteddemonstratesthe abilityofthetestedCSTDtomaintainmicrobiologicalintegrityandsupportthedecisiontoextendthepracticalin-use shelflifeofdrugproductsforupto28dayswhenusedwith ChemfortTM ineitherasepticconditionsoruncontrolled conditions.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.ThisresearchwassupportedbySimpliviaHealthcareLtd.,Israel,andB.Braun AustriaGesmbH,Austria.
ConflictofInterest Noconflictofinterest
3PC-006 CONTAINERCLOSUREINTEGRITYTESTINGAND PROCESSVALIDATIONOFCLOSEDSYSTEMTRANSFER DEVICESFORASEPTICRECONSTITUTIONOFDRUG VIALSCONNECTEDTOFLUIDBAGS
1RVanDenBerg*, 2KAkgöl, 1ESwart, 2BNuijen, 1MCrul. 1AmsterdamUmc – Vrije UniversiteitAmsterdam,DepartmentofPharmacyandClinicalPharmacology,Amsterdam, TheNetherlands; 2NetherlandsCancerInstitute – AntoniVanLeeuwenhoek,Departmentof PharmacyandPharmacology,Amsterdam,TheNetherlands
10.1136/ejhpharm-2023-eahp.32
BackgroundandImportance Theclosureintegrityandprocess validationofclosedsystemtransferdevices(CSTD)shouldbe assuredbeforeimplementationinclinicalsettings.However, therearenogoldstandardmethodsforContainerClosure IntegrityTesting(CCIT)ofCSTDs.
AimandObjectives Weaimedtoinvestigatetheclosureintegrityandvalidatetheasepticprocedureoftwotypesof CSTDs(Vial-MatefromBaxter,hereaftercalledCSTDAand EcoflacConnectfromB.Braun,hereaftercalledCSTDB)by usingacombinationofthedyeingresstestandamediafill test.
MaterialandMethods
Thedyeingresstestwithmethylene bluewasusedasCCITforbothCSTDswithtensamplesof meropenemdrugvialsofthreebrands(n=60).Amediafill testwasperformedwithbothCSTDs(n=300perCSTD, 150carriedoutinasafetycabinetand150undernon-classifiedenvironmentalconditions).
Results InallsamplesofbothCSTDsmethylenebluewas absentaftervisualinspectionandspectrophotometricanalysis. ThenutrientmediaofonesamplewithCSTDA,reconstituted inasafetycabinet,wascontaminatedwhereasnoneofthe CSTDBsampleswithreconstitutioninaGMPgradeAenvironmentwerecontaminated.Undernon-classifiedenvironmentalconditions,onesampleofCSTDAandtwosamplesof CSTDBwerecontaminated.
ConclusionandRelevance Inconclusion,bothCSTDsconnectedtomeropenemvialsofthreebrandsareincompliance withtheclosureintegritybyusingthedyeingress.Theaseptic procedureofCSTBBwasvalidatedwiththemediafilltest whenreconstitutedinaGMPgradeAenvironment,butfailed forCSTDA.TheaddedvalueofCSTDsinahospital(pharmacy)remainsdebatablewithoutaclearlydemonstratedclosureintegritywhenbedsidereconstitutionisdone.Hospital pharmacistsarestronglyadvisedtoperformsufficientand adequateclosureintegritytestswithCSTDsbeforeimplementingtheminclinicaluse.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
3PC-008 SLOWANAKINRADESENSITISATIONPROTOCOL DESIGNFORDELAYEDHYPERSENSIBILITYREACTION
1HSuñer*, 1MMartínMarqués, 2OEstesoHontoria, 2CBadiaSantolaria, 1ASanjuánBelda, 1ISacanellaAnglès, 1DPascualCarbonell, 1SCondeGiner, 1CJCortésSánchez, 1AGarcia Molina, 3LCanadellVilarrasa. 1HospitalUniversitariJoanXXIII,Pharmacy,Tarragona,Spain; 2HospitalUniversitariJoanXXIII,Allergology,Tarragona,Spain; 3HospitalUniversitaryJoan XXIII,Pharmacy,Tarragona,Spain
10.1136/ejhpharm-2023-eahp.33
BackgroundandImportance Anakinra,arecombinanthuman IL-1receptorantagonist,isindicatedinrheumatoidarthritis (RA)withagoodsafetyprofile,nonethelessitsadministration hasbeenassociatedwithaseveredelayedinjection-sitereactionwithoutafullyunderstoodpathogenesis.Todealwith thatseveraldesensitisationschemeshavebeenpublishedinthe literature.
AimandObjectives Theaimistodescribethedesignofa slowdesensitisationprotocol(SDP)forsubcutaneous(SC)anakinraforpatientswhohavefailedtherapiddesensitisation scheme(RDP).
MaterialandMethods Weintroducea72year-oldpatient diagnosedforRAandtreatedwithSCanakinraafterfailing othertreatmentlineswhopresentssevereinjection-sitereactionsafter3weeksoftreatment.Anattemptwasmadeto desensitisequicklybutitwasnottoleratedeither.Asthere werenomorelinesoftreatmentavailable,itwasdecided,in collaborationwithallergists,todesignaSDP.
Itwasdesignedfor56dosesofincreasingconcentrations (until100mgdose).Lowerdosewas0,1mganddosechange wasperformedevery3-4days.Solutionswereelaboratedin thePharmacyService.Startingfromamothersolution(MS) of100mganakinrainphysiologicserum0,9%(SF)toafinal volumeof1mL(1:1solution)twoanakinradilutionswere
made:1:10,1:5.TheMSwaspreparedfromanakinra100 mg/0,67mlinjection.Thedilution1:10wasmadebytaking 0,5mlfromtheMSandSFuntil10ml(concentration5mg/ ml).Thedilution1:5waspreparedbydiluting1mlfrom 1:10dilutionuntil5mlfinalvolumewithSF(concentration1 mg/ml).
Topreventhypersensibilityreactionsitwasneededtoadd antihistaminesduringtheSDP.
Results AlthoughRDPwasnotwelltolerated,theproposed schemehadsatisfactoryresults.Atfirstthelowestdose(0,1 mg)wasnottoleratedbythepatient,soitwasdecidedto addantihistaminesduringtheprocess.Ifanydosecouldreact, thedosechangewasdoneinsteadof3after5-7days. Actually,thepatienthascompletedthedosesuntil50mg withoutadversereactions.
ConclusionandRelevance TheSDPproposedbyallergistin collaborationwithhospitalpharmacisthasallowedthesafe administrationofanakinra,avoidingalossofthelasttherapeuticlinepossibleinapatientwithRA.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
3PC-009 CYCLOPHOSPHAMIDESURFACECONTAMINATIONIN AROBOTICCHEMOTHERAPYCOMPOUNDING PROCESS
1,2ACRiestraAyora, 1MJTamés*, 1AIglesias, 1BGarcía, 1MJArgandoña, 1CRamajo, 1OOlariaga, 1MUrretavizcaya. 1OnkologikoaFoundation,PharmacyService,SanSebastián, Spain; 2UniversityofDeusto – FacultyofHealthSciens,DepartmentofMedicine,Bilbao, Spain
10.1136/ejhpharm-2023-eahp.34
BackgroundandImportance Thereisawildconsensusabout therisksassociatedtotheoccupationalexposuretohazardous drugs,butrecentstudieshaveshownthatthereisstillsurface contaminationinpharmaciespreparingantineoplasticdrugs. Themainreasonfortheimplementationofroboticcompoundingsystemsistoimprovesafety;forthepatientandfor healthcareworkers,avoidingrepetitivestraininjuriesandhazardousdrugsexposure.
AimandObjectives Theaimofthisstudywastoevaluate cyclophosphamideexposureofpharmacynursesduringthe roboticchemotherapycompoundingprocess.
MaterialandMethods Thesamplingareaswereselectedafter beingidentifiedasthehighestriskofpersonalcontamination inariskassessment.Wipesamplesweretakenfromvials, infusionbags,gloves,anddifferentlocationsoftherobotic system.Surfacemonitoringwasperformedusingasemi-quantitativedevicebasedonthinlayerimmunochromatography. Thesamplingwasperformedattheendoftheworkdayover severaldaysbeforecleaningprocesstoidentifythehighest potentialdegreeofcontaminationtowhichhealthcareworkers couldbeexposed.
Results Cyclophosphamidecompoundingwasperformedduring thestudydaysandseveralmonthsbefore.Therewasno cyclophosphamidespillinthethreemonthspriortothestudy. Externalcontaminationwasmeasuredon15vialsand10bags ofcyclophosphamideandon10glovesand5robotareas aftercyclophosphamidecompoundingduring5non-consecutivedays.TherewerenotCyclophosphamidecontamination overthedetectionlimitof0.5ng/cm2 innoneofthesamples
fromtherobot;vial,glovesandbagssampleswerealso negative.
ConclusionandRelevance Theroboticchemotherapycompoundingenablescyclophosphamidepreparationwithlowlevelsofpersonalexposure.Cyclophosphamideisagood standardformeasuringhazardousdrugscontaminationbecause itspreparationmethod,frequencyofuseandtheavailability ofoccupationalexposurestudies.
Toourbestknowledge,thisisthefirststudyinrobotic hazardousdrugcontaminationusingasemi-quantitative method.DespitethistechnologydoesnotallowprecisequantificationoftheamountofHDpresenttheuseofsemi-quantitativemethodscouldfacilitateitswidespreaddetermination duetoalowercostandimmediacyofresults,allowingthe implementationofcorrectivemeasures.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
EKasalova*,SKlovrzova,RKadlecova,MHojny. InstituteforClinicalandExperimental Medicine,InstitutionalPharmacy,Prague,CzechRepublic
10.1136/ejhpharm-2023-eahp.35
BackgroundandImportance Tacrolimusisanimmunosuppressiveagentusedinsolidorgantransplantation(SOT)forprophylaxisofrejection.InourhospitalSOTareperformed includingraremultivisceraltransplantations(MTVx).There areclinicalsituationswhenoraltacrolimuscannotbeadministeredtotheMTVxpatientbecauseofanon-functional bowel.Inhomecare,patientcouldbetreatedwithtacrolimus forsublingualuse.Thereisnocommercialproductforsublingualadministrationavailable,soformulationhastobe developed.
Tacrolimusispracticallyinsolubleinwater;however,suspensioncanbepreparedusingtacrolimuscapsulepowdercontent.Thisformulationisunstablewithriskofsedimentation, thereforeuniformdosecannotbeachieved.Weusedsolubilityoftacrolimusinethanolandpreparedthehomogenous sublingualsolutionfromsubstance.
AimandObjectives Toformulatesolutionoftacrolimus10 mg/mLbasedonethanolandglycerol.Todescribethepreparation,container,storageconditionsandshelf-lifeoftacrolimussolutionforsublingualuse.
MaterialandMethods Tacrolimusbelongstohazardousdrugs, H361.Biologicalsafecabinet(BSC)isrecommendedfor tacrolimuspreparation.
Solutionwaspreparedusingtheglassbeakerandstick. Tacrolimuswasdissolvedinethanol96%,thenglycerol85% wasslowlyadded.ThecitricacidwasusedtoadjustpH5-6, optimalforstabilityoftacrolimus.Orangeflavourwasadded forhigherpalatability.Theamberglasscontainerwithadapter fororalsyringewasused.
Results Turbidandhomogenoussolutionwithorangescent andpH6wasobtained.Concentrationoftacrolimuswas10 mg/mL.Shelf-lifeof30dayswasgiven,storedin15°C–25°C accordingtotheUSP<795>.Oraldosingsyringewasused toapplythesublingualsolution.
ConclusionandRelevance Theformulationoftacrolimuswas developed.Varietyofconcentrationsoftacrolimussolution maybeprepared.Thisallowsustopreparesolutionwith
higherconcentrationwiththesamevolume,ifneeded.Our MTVxpatienthadagoodtolerancetothissolution.Hehas takenthissolutionsinceNovember2021andhasremained stablewithoutrejectionoftransplantedstomach,liverand pancreas.
Itisnecessarytoworkfast,becauseofanairflowinBSC, theethanolevaporatesandthesolutionmayprecipitate.Clinicaleffectivenessmightbeinvestigatedtoconfirmtheutilityof thisformulation.
ConflictofInterest Noconflictofinterest
3PC-011 AVIRTUALSTERILISATIONAREA:ANINTERACTIVE TRAININGTOOL
ACrou*,DBoden,PPerot,GDesautez,VChevalet,RSchofield,AGhazouani. Hôpital MarieLannelongue,Haut-De-Seine,LePlessis-Robinson,France
10.1136/ejhpharm-2023-eahp.36
BackgroundandImportance Thetrainingofsterilisationtechniciansrepresentsamajorchallengetoensurethesafety, securityandlimitthecontaminationofreusablemedical devices.
AimandObjectives Theobjectivewastodevelopatoolto ensurebasicandcontinuoustrainingofsterilisationtechnicians,takingintoaccounttheirprofessionalbackgroundsand skills.
Thetoolaimstocontributetotheintegrationofnewcomersandthestandardisationoftraining,whichcurrentlyis mainlydonethroughmentoring.
MaterialandMethods Atrainingbookletwasdeveloped,coveringthedifferentstagesofthesterilisationprocess.Itwas usedasabasisfortherealisationofinstructionalvideos, showingtheentiresterilisationprocess.
Thevideoswerethenincludedinavirtualblueprintofthe sterilisationareamadewith3Dmappingsoftware.
Results TheguidewaswrittenfollowingtheFrenchSterilisationGuidelinesandtheinternalpracticesofthetechnicians.It wasdividedinto4mainparts,correspondingtothedifferent stepsofthesterilisationcircuit,whichare:individualoutfitting oftechnicians,washingofthemedicaldevices,assemblyof thesterileboxes,andunloadingsystemsforautoclaves.
Thesepartswereillustratedby4videos,whichwereintegratedintothedifferentroomsofthe3Dlayout.A3Dlayout wascreatedwithKozikaza®,3Dmappingsoftware,fromthe measuredblueprintofthesterilisationarea.Itreplicatedthe technicians’ workenvironmentasrealisticallyaspossible.To completetheirvirtualtraining,theagentdecideseithertofollowtheclassiccircuitortochooseonestepspecifically.Then, theyclickonthehyperlinkinthevirtualroomwhichrefers themtoavideocorrespondingtothestepofthecircuit.
ConclusionandRelevance Thisinteractivetoolallowscatering todifferentprofessionalbackgrounds,takingintoaccountthe technicians’ preferencesregardingtrainingmethods.Itenables animprovementofthequalityofthecircuit,ofsterilisation practices,andfacilitatesthetrainingofsterilisationtechnicians. Thistraining,systematicallyofferedtoemployeesupontheir arrivalandannuallytothewholeteam,willbeevaluatedto identifytheirneeds.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
1RTrittler*, 1AAbotaleb, 2CBöhlke, 3KOffner, 1MJHug, 2GBecker. 1UniversityMedical Centre,Pharmacy,Freiburg,Germany; 2UniversityMedicalCentre,DepartmentofPalliative Care,Freiburg,Germany; 3UniversityMedicalCentre,AnaesthesiologyandIntensiveCare, Freiburg,Germany
10.1136/ejhpharm-2023-eahp.37
BackgroundandImportance Opioidtherapyisstillnotoptimal.AsPCApumpswithcombinationsofopioidsand NSARareproducedinmanyhospitalpharmaciestominimisethedoseandthesideeffectsofopioids,thispolysubstanceuseisaccompaniedbyincompatibilityproblems. Severaladmixtureswithopioidsandmetamizolechange theircompositionduring administrationtime.Incaseof admixtureswithmorphineandmetamizole,wecoulddefine andisolatethemainreactionproduct ‘ metamorphine ’ . Dependentonmorphinecon centration,storagetemperature andstoragetime,PCA-pumpswithadmixturesofmetamizol andmorphinecancontain100%metamorphineinsteadof morphine.
AimandObjectives Asthestabilityproblemsdidnotresultin achangeoftheprescribingroutine,thepharmacologyofthis newsubstancewasinterested,especiallybecausethePCA pumpsstillhadtheiranalgesicpotencyandnewadverse effectswereneverreported.
MaterialandMethods AfterpermissionoftheEthicsCommitteeandinformedconsent,morphineandmetamorphinewere determinedinserumsamplesofpatientswithregularmorphine/metamizolePCAtherapy.
Results Uptonow,wehavedeterminedthemorphineand metamorphineconcentrationsinserumoffourpatients treatedwithadmixturesofmorphine/metamizole.Inthree ofthemwecouldidentifyor quantifymetamorphinebeside morphine.Inonepatient ’ sserumwefound0,75 mg/mL metamorphinebesideamorphineconcentrationof0,16 mg/ mL.Nolossoftheanalgesiceffectandnochangeof adverseeffectsduringPCAtherapyofthesepatientswas found.
ConclusionandRelevance Incompatibilitiesofpolysubstance useinPCApumpscanalsoleadtootheractivesubstances thanprescribed.Sincepatientsdonotnoticealossofthe analgesicpotencyorchangeofsideeffectsandtheserum levelofmorphinedecreasedsignificantly,itisverylikely thatmetamorphinehasanalgesicand/orspasmolytic potencyandcomparedtomorphinealoneitseffectsto m-, k -and d -opioidreceptorsmaybedifferent.Thestudyis relevanttounderstandasuccessful,well-establishedtherapyandleadspossiblytoanewoptimisedopioidtherapy infuture.
REFERENCESAND/ORACKNOWLEDGEMENTS
3PC-015 IN-USESTABILITYOFCOMIRNATYANDSPIKEVAX CLINICALSOLUTIONS:PFIZER-BIONTECHAND MODERNACOVID-19VACCINES:ACOMPARATIVE STUDYFROMAHOSPITALPHARMACYPERSPECTIVE 1JHermosillaFernández*, 1AAlonso-García, 2RPérezRobles, 1ATorrenteLópez, 1JRuiz Travé, 1NNavas, 3JCabeza, 3ASalmerónGarcía. 1BiomedicalResearchInstituteIbs. Granada,AnalyticalChemistrySciencesFaculty-UniversityofGranada,Granada,Spain; 2BiomedicalResearchInstituteIbs.Granada-FundacionParalaInvestigaciónBiosanitariade AndalucíaOrientalAlejandroOteroFibao,AnalyticalChemistrySciencesFaculty-University ofGranada,Granada,Spain; 3BiomedicalResearchInstituteIbs.Granada,ClinicalPharmacySanCecilioClinicalUniversityHospital,Granada,Spain
10.1136/ejhpharm-2023-eahp.38
BackgroundandImportance COVID-19emergedasanovel infectiousdiseasebylate2019,spreadingveryrapidlyand beingcategorisedasapandemicbyMarch2020bythe WHO.Severalvaccineshavebeenauthorisedandadministered worldwide,demonstratingefficacyandsafety,beingPfizerBioNTech(Comirnaty)andModerna(Spikevax)themostly administeredglobally.Althoughhavingdemonstratedefficacy andsafety,oneofthemajorissueshasbeentheirstability, fromwhichhardlyanystabilitydataisavailableinthepublic domain.Analysingthe in-use stabilityofthesenovelvaccines isparamountforensuringrationaleuseinhospitals.
AimandObjectives Thisstudyisaimedatassessingandcomparingthe in-use stabilityofComirnatyandSpikevaxclinical solutionsbycharacterisingtheparticulateprofileusing DynamicLightScattering(DLS).
MaterialandMethods Expiredandnon-expiredclinicalsolutionsofthevaccinesweresubjectedtodifferentstressconditions:visiblelightandmechanicalstresses.TheZaverageand thepolydispersityindex(PDI)ofthevaccinesclinicalsolutions wereevaluatedbyDLS,usingaZetasizerNanoZS-90(Malvern,UK).Forstatisticalanalysis,asimpleANOVAfollowed byDunnett’spost-hoctest,usingGraphPadPrism8Software wasused.Stressedsampleswerecomparedtocontrol(nonstressedsamples).Furthermore,differenceswereconsidered significantatap-value<0.05.Thestudywasconductedin triplicate.
Results ComirnatyDLSparametersweremainlyaffectedby mechanicalagitationandvortexstresses.Inthiscase,theZaverageandPDIincreasedsignificantly,evenintheexpired samples.Ontheotherhand,theDLSparametersweremaintainedinSpikevaxclinicalsamplesregardlessofthestressand theexpirationdate.
ConclusionandRelevance Thisstudyhighlightsthenecessity ofacarefulpreparationofthesevaccines,giventheirdemonstratedfragilityupongentlestress.However,Comirnatyhas proventobemorefragilethanSpikevaxintheirhandlingin real-useconditions.Previousliteraturecommentedonthe stabilityofComirnaty,havingpresentedsimilarresults.Nonetheless,nostabilitydatawereavailableonthein-usestability ofSpikevax.Therefore,thisdatawillbeofinteresttohospital pharmaciststowardsfollowingvaccinationcampaigns.
Acknowledgements TotheFarmacyUnitfromtheSanCecilio ClinicalUniversityHospital(Granada,Spain)tosupportand facilitatethisinvestigation.
ConflictofInterest Noconflictofinterest
10.1136/ejhpharm-2023-eahp.39
BackgroundandImportance HospitalsinNorwayproduce medicinalairforpatienttreatment.Themedicinalairisproducedbyacompressorcentralandsuppliedbyapipelinesystemforpatienttreatmentatthehospital.Thequalityofthe medicinalairiscontrolledannuallyaccordingtotheEuropean pharmacopoeia.ThemonographformedicinalairintheEuropeanpharmacopoeiaincludestestsforO2,C0,CO2,SO2, NOx,oilandH2O.Bothambientaircompositionandcomponentsinthecompressorcentral haveinfluenceonthemedicinal airquality.Thetimeforperiodiccontrolmaythereforeaffect theresult.Therearenopublicationspresentingresultsfromcontinuousmonitoringofhospitalproducedmedicinalairquality.
AimandObjectives Theaimofthestudywastoconfirmthat hospitalproducedmedicinalaircontinuouslyissafeandin compliancewiththeEuropeanpharmacopoeia.Basedona riskassessmentitwaschosentomonitorO2,C0andH2Oas indicatorsforairquality.OthertestintheEuropeanpharmacopoeiawasincludedintheperiodiccontrol.
MaterialandMethods Thecompressorcentralissituatedin Oslo,about500metresfromamainroad.Thecomponents ofthecompressorcentralarecompressor(AtlasCopcoZR 75VSD),pressuretank(Maskinspecialisten,typeB+F),adsorptiondryer(AtlasCopco,BD185+),carbonfilter/hopcalitecatalyst(AtlasCopco,QDTHOC185)andfilters(AtlasCopco, PDp).TheairqualitywasmonitoreddownstreamthecompressorcentralbydetectorsforO2,C0andH2O(Kimessa Monoline504/404andCS-instrumentsFA500).Theperiod formonitoringwastwoweekstoincludedailyvariations.
Results Theresultsfromthemonitoringcomplieswiththe Europeanpharmacopoeiaatalltimesduringthetestperiod.
BackgroundandImportance Pembrolizumab(Keytruda®)isa humanIgG4monoclonalantibody(mAb)fromthegroupof immunomodulators,whichbindstoprogrammeddeathreceptor1(PD-1).Givenitsstructuralcomplexity,physicalaggregationandchemicaldegradationcanoccurthroughoutitslife, andevenmodestenvironmentalstressescancauseextensive damagewhichmayaffectthesafetyandefficacyofthe medicine.1
AimandObjectives Toassesstheimpactofagitationonpembrolizumab(Keytruda®,25mg/mL)safetyandefficacythrough thestudyofaggregationandfunctionalitywhenmishandling inrealhospitalconditions.
MaterialandMethods Pembrolizumab(Keytruda®,25mg/mL) freshopenedvialswereused.Agitationstresswascarriedout inamechanicallaboratoryshaker(300rounds/min,24h, 25°C)andgentleagitationwasperformedmanually(1min, 25°C).AggregationwasassessedbyDynamicLightScattering (DLS)andSize–ExclusionUltra–High–PerformanceLiquid Chromatography(SE/UHPLC–UV).PembrolizumabfunctionalitywasevaluatedbyEnzyme-LinkedImmunosorbentAssay (ELISA).
Results Pembrolizumabcontrolsample(25mg/mL)showeda singleparticulatepopulationwithhydrodynamicdiameter (HD)of9.5±2.8nmcorrespondingtopembrolizumab monomers.SE/UHPLC–UVchromatogramsofthecontrolsamplerevealedamainchromatographicpeakassignedtopembrolizumabmonomersandasmalloneassignedtonative dimers.DLSandSE/UHPLC –UVshowedthatagitationstress didnotpromoteincreaseinaggregation.However,pembrolizumabfunctionalitywasaffectedafterapplyingagitationstress sinceELISArevealedasignificantlossoffunctionality.Asa consequence,agentleagitationofpembrolizumabwasperformedinordertoinvestigateifthislossoffunctionality couldalsohappeninlessstressfulconditions.Asaresult, ELISAalsorevealedasignificantlossoffunctionalityingently agitatedpembrolizumab.
Monitoringdata:O220,4
21,4%,CO<5 ppm,andH2O <67 ppm.
ConclusionandRelevance Themonitoringdatashowsthata hospitalcompressorcentralisabletocontinuouslydeliver medicinalairaccordingtotheEuropeanpharmacopoeia,even withdailyvariationsintheambientairqualityandcompressorsystem.Thisisrelevantinformationforpharmacistand technicalstaffwhenplanningqualitycontrolstrategiesfora compressorcentral.
1.TechnicalstaffatOsloUniversityHospital
ConflictofInterest Noconflictofinterest
1ATorrente-López*, 1RPérez-Robles, 1JHermosilla, 1JRuiz-Travé, 1MAHernández-García, 1ATorres, 2JCabeza, 2ASalmerón-García, 1NNavas. 1BiomedicalResearchInstituteIbs Granada,AnalyticalChemistry-ScienceFaculty-UniversityofGranada,Granada,Spain; 2BiomedicalResearchInstituteIbsGranada,ClinicalPharmacy-SanCecilioUniversity Hospital,Granada,Spain
10.1136/ejhpharm-2023-eahp.40
ConclusionandRelevance Theexposuretoagitationstressdid notinduceaggregateformationinpembrolizumab.Nevertheless,bothagitationstressandgentleagitationledtoalossof itsfunctionalitynotrelatedtoagitation.Thus,werecommend preventingpembrolizumabfromagitationwhenhandlingin hospitals.
REFERENCE
1.M.R.Nejadniketal.J.Pharm.Sc.107(2018)2013-2019.
Acknowledgements FundedbyprojectsP20-01029(Juntade Andalucía,Spain)andB-FQM-308-UGR20(Universidadde Granada,FEDER2020).A.T-LgrantsaFPUpredoctoralcontract(FPU18/03131,MinistryofUniversities,Spain).J.H benefitsaresearchcontract(P20_01029,JuntadeAndalucía andEuropeanRegionalDevelopmentFunds).R.P-Rholdsa postdoctoralposition(DOC-01694,JuntadeAndalucía, Spain).
ConflictofInterest Noconflictofinterest
3PC-018 TIMETOAVAILABILITYOFINJECTABLEANTICANCER DRUGSFOROUTPATIENTS:REASSESSMENTINA FRENCHCOMPREHENSIVECANCERCENTRE
1EGleditsch*, 2RNilsen. 1SykehusapotekeneHf – OsloHospitalPharmacy,Manufacturing, Oslo,Norway; 2OsloUniversityHospital,HospitalServices,Oslo,NorwayBackgroundandImportance Excessivewaitingtimeisoneof themaincausesofpatientdissatisfactioninoncologicdaily careunit(DCU).Leanmanagement,dosebanding,advanced prescriptionandautomatisationareusuallyusedinourhospitaltoimprovepatientcarepathway.InouradultDCU(>26 000patients/years),patientshavetowaitfortheirtreatment lessthananhour.
standardforpaediatricpatientsbecausetheyallowbody weightorienteddosingfordifferentagegroups.Sofar,there isnopublishedinformationregardingtheformulation,quality control,andstabilityofpharmacy-preparedbisoprololoral solutions.
AimandObjectives
Theaimofthisworkistoreassessthe timeofavailabilityinthisDCUandtoidentifythefactors influencingthistime.
AimandObjectives Theaimofthisprojectwastoformulate abisoprololfumarate0.5mg/mLoralsolutionforpaediatric use,establishsuitablequality-controlmeasures,andtoperform stabilitytests.
MaterialandMethods
Itisanambispectivemonocentricstudy inwhichhumanfactors(n=2),equipmentfactors(n=7), organisationalfactors(n=4),productivityfactors(n=16)and time-relatedfactors(n=6)wererecordedrandomlybetween September2021andApril2022(i.e.15daysstudied).Data werealsoextractedfromCHIMIO® softwareandfromour institutional ‘ LEANtool’ forreal-timemonitoringofpatients inoncologicDCU,inordertocalculatetimebetweenthe prescriptionofthedayandthedispensationofthe treatment.
Results Theaveragenumberofpatientsandpreparations manufacturedperdaywererespectively105(+/-7)and 146(+/-12);52%ofthesepreparationspreparedtheday before.Theaveragenumberofpreparationsnotprescribed inadvanceis49[18-62](34%)foranaveragenumberof 31patients[14-43](30%).Theaverag etimetoavailability was54min(+/-16)withamedianof60min.Onaverage,12[0-24]patientsperdaywaitedmorethananhour aftertheprescriptionwithamaximumwaitingtimeof 360min.
Fourdays(27%)wereidentifiedwithanaveragedispensingtimegreaterthan60min.Duringthesecritical days,apercentageofanticipatedpreparationslessthan 50%,withahighnumberofprescriptions(>30patients) andparticularlybefore9:45 a.m.orbetween12:00and 14:00p.m.wereobserved.Wenoticedalsoahigherproductivity([174-214]preparations),thelackofcoordination (2of4days),oradditionalproductions(analgesicsyringe preparations).
ConclusionandRelevance Mainimpactingfactorsseemtobe humanfactorsandproductivity.Timetoavailabilitybecame anessentialqualityindicatorofourcompoundinganti-cancer unit.Thisstudyshowedthatourworkingproceduresareefficientforamajorityofpatients,butnotforall.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
3PC-022 FORMULATIONANDQUALITYCONTROLOFA BISOPROLOL0.5MG/MLORALSOLUTIONFOR PAEDIATRICUSE
HLinxweiler*,JBoventer,AWalter,IKrämer. UniversityMedicalCenterJohannes GutenbergUniversityMainz,PharmacyDepartment,Mainz,Germany
10.1136/ejhpharm-2023-eahp.42
BackgroundandImportance Bisoprololisabetablockerindicatedforthetreatmentofheartfailureinpaediatricpatients. Therearenolicensedbisoprololcontainingpaediatricdosage formsavailableintheEU.Pharmacypreparationofpatient individuallydosedbisoprololcapsulesiscommonpracticeby usinglicensedbisoprololtabletsasstartingmaterial.However, thepreparationanduseofbisoprololoralliquidsarethegold
MaterialandMethods Bisoprololoralsolutionwasformulated inanalogytopropranololhydrochlorideoralsolution describedinNeuesRezeptur-Formularium2015/1,Germany. Efficacyofantimicrobialpreservationwastestedregardingto Ph.Eur.5.3.1byanexternallab.AstabilityindicatingRPHPLCmethodwasestablishedandvalidatedbasedonthe knownmethodofJoshietal.1
Results 100mLbisoprololfumarate0.5mg/mLoralsolution containbisoprololfumarate0.05gasactiveingredientas wellaspotassiumsorbate0.15g,anhydrouscitricacid0.07 g,sucrose25g,raspberryflavour0.1g,andpurifiedwater 84.33gasexcipients.Antimicrobi alpreservati onregarding Ph.Eur.5.3.1wasdemonstrated.Aftera6monthsperiod thebisoprololconcentrationamountedto103%±1%of theinitialconcentrationandthepHremainedunchanged (4.6).
ConclusionandRelevance Sweetenedandflavouredbisoprolol fumarateoralsolutionwassuccessfullydevelopedaspharmacy preparationsuitableforpreparationinstock.Adequatein-use preservationisgivenandstabilityisprovenforatleast6 months.Asecondversionofbisoprololoralsolutionwithout sucroseandraspberryflavourisunderdevelopment.
REFERENCE 1.JoshiSJetal.RP-HPLCmethodforsimultaneousestimationofbisoprololfumarate andhydrochlorothiazideintabletformulation. JPharmBiomedAnal.2010Jul 8;52(3):362–71.
ConflictofInterest Noconflictofinterest.
3PC-023 PATCHTESTSWITHETHAMBUTOL10%,ISONIAZID 15%ANDPYRAZINAMIDE25%:ACASEREPORT
1CLeitao, 1RAraújo, 1CFerreira, 2CFerreira, 2MVieira. 1CentroHospitalarTâmegaE Sousa-Epe,PharmacyDepartment,Penafiel,Portugal; 2CentroHospitalarTâmegaESousaEpe,ImunoallergologyDepartment,Penafiel,Portugal
10.1136/ejhpharm-2023-eahp.43
BackgroundandImportance A55yearoldmalepatient,developedaDRESS(drugrashwitheosinophiliaandsystemic symptoms)reactionafterstartingfirst-linetuberculosistreatmentwithrifampicine,ethambutol,isoniazidandpyrazinamide.Toassesstheresponsabilityofasuspecteddrugina DRESSreactionandposteriorsafereintroductionoftherapy, patchtests(PT)arethemostusefultool.Forthepurpose,the HospitalPharmacywasaskedtodevelopmagistralpreparationsofethambutol,isoniazidandpyrazinamide.ThePTwere performedwitheachtuberculostaticdrugdilutedin4IQ UltraChambers,appliedonthepatient’sskinatthebackand keptinocclusionfor48hours.Thereadingswereperformed atday2andday3.Onlyerythema,infiltration,papulesor vesicleswereconsideredpositivereactions.
AimandObjectives Developmentandvalidationofmagistral formulasfortopicalapplicationtoaccomplishpatchtestsof
ethambutol10%(w/w),isoniazid15%(w/w)andpyrazinamide 25%(w/w).
MaterialandMethods
Scarcebibliographicinformation Reviewarticlepublishedby theFrenchSocietyofDermatology,inwhichtheconcentrationsoftheactiveingredienttobeusedineachPTare established.
ApplicationofthegeneralrulesofGoodHandlingPractices,accordingtothePortugueseGalenicFormulary. Results ThepastesusedinthePTwereobtainedbygeometric dilutionofpulverisedethambutol400mg,isoniazid300mg andpyrazinamide500mgtabletsinwhitepetrolatum.
Afterquality-controlteststhatincludescolour,homogeneity andmassverificationassays,thepasteswereplacedina syringeforaneasierapplicationintheskin.Itwasgiven30 daysofstabilityatroomtemperature.
ConclusionandRelevance Thispreparationmadepossibleto developPTforthestudyofadelayedhypersensibilityreaction totuberculostaticdrugs,thatwasnotavailablebeforeinthe market,allowingasaferreintroductionofthetreatment.
AlthoughthePTswerenegativeinthispatient,itwaspossibletodevelopandvalidatethreecompoundingformulas withanadequatesafetyprofileandlowcost.Thisaccomplishmentwillbeusefulinfurthercases.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.Ingen-Housz-OroS,AssierH,etal.Hypersensibilitéretardéeauxtraitementsantituberculeux.Propositiond’uneconduiteàtenirpratiquedevantunexanthème: quandarrêter,quellesexplorationsallergologiquesetcommentréintroduirele traitement.AnnalesdeDermatologieetdeVénéréologie
2.PortugueseGalenicFormulary2001
ConflictofInterest Noconflictofinterest
Results 21patientsweretreatedwithinsulineyedrops1UI/ mL,sixofthemwithdiabetesmellitusandother15were non-diabetic.Administrationfrequencywas4timesinaday (QID).Theypresenteddifferentcornealdiseasesthatwere refractorytoconventionaltreatment.Themedianagewas74 (43-89)years.Atotalof52.4%werewomen.38.1%were diagnosedwithnon-herpetickeratitis,19%withherpetickeratitis,23.8%withcornealerosion,and19%withpersistentepithelialcornealdefect(PECD).Themediandurationof treatmentwas6months(2-9months).100%ofpatients respondedtotreatmentandcontinuedwithinsulineyedrops afterepithelialhealing.Allpatientspresentedepithelialhealing inabout30-60days,mostofthemreferredimprovedof symptomsduringfirsttwoweeks.
Nosignificantadverseeffectswerereported.Nonehypersensitivityreactionwerereportedbecauseofm-cresolpresence ininsulineyedrops.
ConclusionandRelevance Theinsulineyedropsformulation1 IU/mLadministeredQIDcanbeaquick,effective,andsafe optionfordifferentcornealdiseasesrefractorytotheusual treatmentsinbothdiabeticandnon-diabeticpatients.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
3PC-028 FORMULATIONOFANORALPLATELETLYSATEGEL TOTREATCHRONICGRAFTVERSUSHOSTDISEASE ASSOCIATEDORALMUCOSITIS:EFFECTIVENESSINA SERIESOFCASES
1ATorrent*, 1TLizondo, 1MMestre, 2MLozano, 1MCLópez, 1JRRoma, 1NFernández, 1MAlbanell, 1AEscolà, 1DSoy. 1HospitalClínicdeBarcelona,PharmacyService,Barcelona, Spain; 2HospitalClínicdeBarcelona,HemotherapyandHaemostasis,Barcelona,Spain
10.1136/ejhpharm-2023-eahp.45
CApezteguiaFernandez*,MPBautistaSanz,AMelgarejoOrtuño,EMatillaGarcia, BRodriguezVargas,CdeCaceresVelasco,MAAmorGarcia,RMorenoDiaz. Hospital UniversitarioInfantaCristina,ServiciodeFarmacia,ParlaMadrid,Spain
10.1136/ejhpharm-2023-eahp.44
BackgroundandImportance Epithelialcornealdefectsaredamagedareasofthecornealepitheliumasaconsequenceof injury.Theexistenceofinsulinandinsulin-likegrowthfactor receptorsincorneakeratocytesandepithelialcellscould explaintheincrementonthecornealepithelialhealingrates. Clinicalexperiencewithinsulineyedropsislimitedandmore evidenceinbothdiabeticandnon-diabeticpatientsisstill needed.
Recently,theinsulineyedropsformulation1IU/mLhas beenpreparedinPharmacyHospitalforpatientswithkeratitis,dryeyeandapersistentepithelialcornealdefect(PECD).
AimandObjectives Theaimistodescribeeffectivenessand toleranceofinsulin1IU/mLeyedropstreatmentfordifferent refractorycornealdiseases.
BackgroundandImportance Chronicgraftversushostdisease (cGVHD)associatedoralmucositisisacomplicationafter stem-celltransplantation.Corticosteroidsarethestandard treatment,butthereisnoconsensusincaseofrefractory lesions.Plateletconcentratesmaybeasafetreatmentoption. AimandObjectives Designasterileoralformulationableto releaseplateletlysate(PL)onoralcavity,andevaluateits effectivenessinaseriesofcases.
MaterialandMethods PLgel25%wascompoundedbymixinginasepticconditions1:1carboxymethylcellulosesodium base5%previouslyautoclavedwithPLalsodiluted1:1with sodiumchloride0.9%.PLgelwaspackagedin3mLaliquots usingoralsyringes,whichwerestoredinthefreezeruntil theiruse.Galenicvalidationwasperformed.
PatientswithcGVHDassociatedoralmucositisfrom November2021toAugust2022whoacceptedtoinitiateoral PLgelweremonitored.Effectivenesswasevaluatedbasedon severityoftheoralmucositis(NCI-CTCAEGrade1-4). Patientsatisfactionwasself-assessedinavisualscale0-10 accordingtothedegreeofpain/discomfort.Adherencewas assumedbasedonthenumberofsyringesdispensed.
MaterialandMethods
Retrospectiveobservationalstudyina tertiaryhospital.21patientswereincluded,treatedwithinsulineyedropsduringtheperiodbetweenFebruary2022–September2022.Thevariablescollectedwere:demographics, indication,durationoftreatment,clinicalresponseandadverse effects.Alldatawereobtainedfromtheelectronicmedical history.
Results PLgelobtainedwasslightlyyellow,translucent, pH=6,withmediumconsistencythatleadsadequatebioadhesivecharacteristics.NochangesofpH,colour,weight,or microbialgrowthwereobservedduringgalenicvalidations.A beyond-usedateof45daysat-20°Cwasgiven.
Sixpatientswithmoderateoralmucositis(grade3)who failedtofirst-linetopicalsteroidstherapystartedPLgel.Two
ofsixdiscontinuedafteronemonthbecausetheirlifestylepreventedthemfrompreservingthegelproperly.Fourpatients (threemen,onewoman)wentonwiththegelforanaverage of5months(range3-9).Clinicalevaluationshowedan improvementof1degreeinoralmucositisinthreepatients and2degreesinthepatientwiththelongesttreatment(9 months).Theself-assessmentscaleshowedanaveragedecrease ofpain/discomfortof2points.Estimatedadherencein patientswhoreceivedthetreatmentformorethanonemonth was80.8%(95%CI:56.8-104.9).
ConclusionandRelevance Theformulationofagelbasedon sodiumcarboxymethylcellulosewasadequatetoadministerPL ontheoralcavity.FourpatientswithcGVHDassociatedoral mucositisrefractorytostandardtreatmentweresuccessfully treated.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
3PC-029 MANAGEMENTOFACHEMOTHERAPYPRODUCTION
AFTERACYBER-ATTACKINAPUBLICHOSPITAL
SMuhammad*,EGaspéri,FBordet,MLMaëstroni. SudFrancilienHospital,Essonnes, Corbeil-Essonnes,France
10.1136/ejhpharm-2023-eahp.46
BackgroundandImportance InAugust2022,ourhospitalwas victimofamassivecyber-attack.Everysoftware,networkand connecteditemswereunusableincludingCHIMIO® which managesproductionofchemotherapyfromprescriptionto administration.OurChemotherapyProductionUnit(CPU) usuallyproducesabout19,000sterilepreparationsayear.
AimandObjectives Theobjectivewastopursuetheproductionofchemotherapyrespectinginmaximumtheusualproductionandqualityprocess.
maintainasafeandalmostnormalproduction.Excel® tool tracingpatientshistorypermitstodetectprescriptionserrors (doseadjustment,intervalsofadministration,protocols respect).Regularbackupsanddevelopmentofadegraded modeprotocolwillbeundertakensoon.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
3PC-031 ADELPHIMETHODTOSTANDARDISETHE PREPARATIONOFAUTOLOGOUSSERUMEYEDROPS?
1LChampmartin*, 2DLannoy, 3RPoulenard, 1PAgius, 1OMarqué, 1EBernikier, 4GMaillan, 3PYRobert, 5JJost, 1VRatsimbazafy. 1CHULimoges,Pui-UnitédesPréparations Galéniques,Limoges,France; 2CHRULille,Pharmacie,LilleCedex,France; 3CHULimoges, ServiceD’ophtalmologie,Limoges,France; 4CHULimoges,Pui-SecteurdePharmacotechnie, Limoges,France; 5CHULimoges,Pui-UnitéD’enseignementetdeRecherche,Limoges, France
10.1136/ejhpharm-2023-eahp.47
BackgroundandImportance Dryeyediseaseisafrequent causeofophthalmologyconsultation(5% – 34%ofpopulationworldwide).Severeforms,refractorytoconventional treatments(artificialtears,topicalcorticosteroids,cyclosporineA,contactlenses,punctualocclusion,systemicdiseases appropriatemanagement),areresponsibleforasignificant visualimpairmentanddisability.Autologousserumeye drops(ASEDs)arethenproventobeaninterestingtherapeuticalternative.First,inMarch2019wecarriedouta nationalinventoryofASEDp reparationspracticethathighlights:lowsupply(13producercentres)andproduction heterogeneity.
AimandObjectives
Generalobjective toimproveASEDsquality,safetyandsupply inourcountrycareinstitutions.
MaterialandMethods
Thefirst3daysprescriptionswere alreadyvalidatedandprintedatthepharmacy,servingas: patienthistory,prescriptionandprotocolmodel.Amolecule dataregisterwascreatedonExcel® listingcytotoxicdrugs data(stability,concentration ).First,ManufacturingSheets (MS)weretotallyhandwrittenthenanExcel® MSwasdeveloped,mimickingCHIMIO®.Atfirst,asinglemodelusing copy-pasteforlabelswasdeveloped.Then,severalmodelsfor bags,syringesorinfuserswerecreated,usingformulasto automaticallyfillthelabels,tosecureandspeeduptheprocess.Aschedulertracedallpreparationsbyauniquenumber. Finally,apatient’shistoryregisterwascreatedwithdata neededforpharmaceuticalvalidation.Asecondpharmacist double-checkedeveryMS.
Results 437preparationsweremadeindegradedmodeduring 6days(73/day).Only5%oftheproductionwasoutsourced inotherhospitals.Thefirst5MSwerehandwritten.Printing everyMSwiththefirstversionofExcel® MStookabout3h/ day.Then,improvingExcelMSreducededitionanddoublecheckingtimetoabout1h/day.Double-checkingMSdetected mostofeditingerrors.Duringfinalcheckingofpreparations, 3errors(<1%)weredetected.Twomajorswithwrong patient’snameanddose(-47%)andoneminorwithwrong schedulernumber.TherecoveryofCHIMIO® databasewas effectiveafter6days.TranscriptioninCHIMIO® foundonly oneundetectedprescriptionerror.
ConclusionandRelevance Developmentofasemi-automated Excel® toolanddoublecontrolofMShasallowedusto
Specificobjectives todefinetheconsensualitems,inorderto establishanationalstandardisedpreparationprotocol.
MaterialandMethods
Methodforconsensusreaching Delphimethod.Fourprotocol partsaborded:sampling,preparation,controls,conservation. Expertpanelrecruitedbyremobilisingcentresapproachedin 2019(ASEDsproducers,non-producers,ordidnotrespond). Localsteeringgroup:pharmacyresident,headofcompoundingunit,pharmacymethodologist.Circuit:questionnaireconstruction,mailingwithlinkaccesstoGoogleForms®,response analyses,consensusratecalculation(consensuswhen 80%), resultsynthesis,anonymousreferraltoexperts.Asmany roundsasnecessarytoachieveconsensus.
Results
Twelveansweringexperts After4rounds:outof39proposals initiallysubmitted,26validatedand10,abandoned.Insampling:15itemsvalidated,5dropped.Preparation:5validated, 1dropped.Control:3validated,4dropped.Conservation:3 validated.Fourroundstook86days.
ConclusionandRelevance AstandardisedprotocolASEDs preparationwillbeproposed.Thiscouldimprovethesupply ofcareacrossthecountry.Methodstrengths:Expertopinion solicitedontheinitialquestionnaire;qualifiedexpertsonthe topic;nogeographicallimitations;anonymityavoidingopinion leaderinfluence;applicabilitycriteria.Limitations:noophthalmologists,biologists,patientsinthepanel;noparticipationof thelargesteyedropproducer(despiterequests).
Acleardefinitionofthiseyedropstatus(pharmaceutical preparationornot)isalsonecessary.
Biochemicalqualitycontrols,abandoned,toberesubmitted (moleculessupposedtosupportASEDsefficacy).Supplementaryroundnecessarytodecidethefateofthelastitem(solutionvolumeineacheyedropbottle).
REFERENCESAND/ORACKNOWLEDGEMENTS
1.Acknowledgementstoallcolleaguesparticipatingto2019studyor/andDelphi method.
ConflictofInterest Noconflictofinterest
3PC-036 CENTRALISEDANDPERSONALISEDPREPARATIONOF INTRAVENOUSKETAMINEFORPATIENTSWITH RESISTANTDEPRESSION
FCostaChing*,ARSantos,SCastro,MBarata,EMarques. HospitalBeatrizÂngelo, Pharmacy,Loures,Portugal
10.1136/ejhpharm-2023-eahp.49
BackgroundandImportance Depressionisthethirdleading causeofdisabilityintheworldandabout1/3ofdepressive disordershaveresistancetosuccessivetreatments.
Intravenousinfusionofoff-labelketamineinsubanaesthetic doseshasfavourabletherapeuticresponsesinarelativelyshort evaluationtime.Accumulatedsafetyevidenceisconsideredan addedvalueinthetherapeuticarsenaltotreatthispathology.
3PC-035 GALENICDEVELOPMENTOFAGENERICSPECIALTY WITHCONVENTIONALRELEASEBASEDONACARBOSE
1MChedly*, 1KBenChaabane, 2FElkara, 3IBenJdidia, 4IBlouzaLimayem. 1HospitalHabib Thameur,Pharmacy,Tunis,Tunisia; 2LaboratoireNationalContrôleDesMedicaments, Chimie,Tunis,Tunisia; 3CentredeMaternitédeMonastir,Pharmacy,Monastir,Tunisia; 4InstitutSalahAzeiz,Pharmacy,Tunis,Tunisia
10.1136/ejhpharm-2023-eahp.48
BackgroundandImportance Inthecaseofthedevelopmentof agenericdrug,theapproachisbasedalmostexclusivelyon galenicandanalyticaldevelopments.However,tofacilitateformulation,itisstillnecessarytogothroughapre-formulation stage.Therefore,agenericdrugmustmeetthesamequality, safetyandefficacyrequirementsastheoriginatordrug.
AimandObjectives Theobjectiveofourworkconsistsona pre-formulationstagefollowedbyaformulationstageinorder toarriveatanoptimal,stableandeffectivegalenicformula andtodevelopagenericoralanti-diabeticdrugbasedon acarbose50mg.
MaterialandMethods Duringthedevelopmentofthisgeneric specialty,apreliminarystudyoftherawmaterialswasconducted(physico-chemicalcharacteristics,rheologicalproperties andcompatibilitystudy)inordertodeterminethequantitative formulaandthemanufacturingprocess.Then,6formulas werepreparedinordertoimprovetheflowtime.Thetablets obtainedweretestedforuniformityofmass,hardness,friability,disintegrationtimeanddissolutioninvitro.Subsequently,a comparativestudyofthedissolutionprofilesobtainedwith thatofthereferencedrugwasmadebycalculatingthedifferencefactorf2andsimilarityf1inordertodeterminethebest formula.
Results ThemethodforthedeterminationoftheactivesubstancebyHPLChasbeenvalidated.Therawmaterialhas beenwellstudiedandthechoiceofexcipientsandthe methodofmanufacturehavebeenjustified.FormulaF5havingafriabilitypercentageequalto0,16%,adisintegration time(5,9min)andadissolutionprofilesimilartothatofthe referencespecialty(f1<15%andf2>50%)wasselected.It wasconsideredtheclosesttotheprinceps.
ConclusionandRelevance Thegenericspecialtyformulated presentedanequivalenceintermsofinvitrodissolutionwith thereferencespecialty.Thus,comparativestudiesin3differentpHenvironmentsneedtobecompletedtojudgethisin vitroequivalence. REFERENCESAND/ORACKNOWLEDGEMENTS
Safetyissuesoftheuseofcentralanaestheticswithoutthe supportofanaesthesiologyareapivotaldriveforimplementingaclinicalprotocolthatincludesthepharmacy.Theuseof fixeddilutionsandrhythmsofadministrationaswellaspersonalisedcentralisedpreparationinthepharmacyovercomes mostconcernsabouttheregularandsafeuseofthisapproach onresistantdepression.
AimandObjectives Evaluatetheimplementedcircuit,characterisationofthepopulationandanalysisoftheimpactonthe effectivenessandsafetyofketamineinresistantdepression.
MaterialandMethods A19-monthretrospectiveanalysiswas madeontheuseofketamineinpatientswithresistantdepression.ThepharmaceuticalservicesdatabaseandtheSoarian Clinicals ® programmewereusedtocollectinformationandto consulttheelectronicclinicalprocessofpatientsthatusedthis therapeuticapproach.
Results Indicationforketaminetreatment,inadditiontothe absenceofcontraindications,meansthatthepatientisnot responsivetoatleastthreeantidepressantsSNRIsandatricyclic,apotentiationstrategiesandascore 9in thePatient HealthQuestionnaire-9(PHQ-9).
Thedatacollectedcorrespondtotheperiodbetween01/ 2021and07/2022andaresummarisedintable1.
Abstract3PC-036Table1
Totalofpatients9
SexF(%)77.8
Averageage45
Totalnumberofpreparations118
Numberofsessions(median)12
Averagedose(mg/kg)Total0.51
Initial0.27
Final0.66
Averagedurationoftreatment58days
Allcasesreportedpsychopathologicalimprovementrecognisedbythemselvesaswellasbyassistantpsychiatrists.
ConclusionandRelevance Ketaminehasshowntobeasafe alternativeprovidedthatlocalstrategiesarecreatedtoensurethe implementationof criteriainpatientselection,preparation,administration,andfollow-upprotocols.Theacceptanceandshort-term recognitionofthebenefitofthetreatmentbypatientsand professionalsallowforachievingthegoalofclinicaldischarge.
ConflictofInterest Noconflictofinterest
10.1136/ejhpharm-2023-eahp.50
BackgroundandImportance Erenumab,galcanezumabandfremanezumabwereapprovedin2019formigrainecrisisprevention.Efficacyandsafetyweredemonstratedinthree-months lastingclinicaltrials.Atpresent,long-termeffectivenessand safetycanbeanalysed.
AimandObjectives Toevaluatetheeffectivenessandsafetyof monoclonalantibodies(mAb)utilisedinmigraineaftertwoand-a-halfyearsofclinicaluse.
BackgroundandImportance
Thechemical-physicalstability, reportedamongthetechnicalcharacteristicsofthedrugs, indicatestheparameterstoberespectedforthesafetyuse ofthepreparationsbutoftentheconditionsofstorageof thedrugscanundergosignificantvariations.Thestability datareportedbythemanufacturersareoftenlimited whileinclinicalpracticeitisnecessarytoextendthe conditionsofuseandthevaliditytimesofthepreparations.Inreality,itmayhappenthatdrugsaretransported, storedandusedintemperatureconditionsotherthan thoseindicatedbythemanufacturerwithout,however, havingsufficientdataonsafetyandstabilityforuseoutsidethecertifiedconditions.
MaterialandMethods Prospective,observationalstudyconductedinatertiaryhospital(December2019toJune2022). Datawereobtainedfrompatients’ medicalrecords(approved byourEthicsCommittee).
AimandObjectives
Theobjectiveoftheanalysisperformedis toevaluatethechemicalandphysicalstabilityofdoxorubicin andepirubicinafterbeingstoredinthefreezer.
MaterialandMethods Theformulationsofdoxorubicinand epirubicinstoredinthefreezerforaperiodoftimeexceeding 48hwereanalysed.Thedrugsolutionswerethawedatroom temperatureandstoredintherefrigeratoruntilthetimeof thechemical-physicalanalysis.Foranalysis10microliterswere subsequentlydilutedfromeachvialandinjectedintoLC QTOFMS(n=4).
Results Dataobtainedfromtheanalysiscarriedoutwitha masschromatographictechniquehighlightedthechemicaland physicalstabilityofthedrugsanalysed.Themeasuredconcentrationofdoxorubicinfortheoverrangesamplewas1.995± 0.005mg/mlwhilefortheexternaldoxorubicinstandardwas 1.996±0.008mg/ml.Sometrendwasobservedforepirubicin,2.009±0.007mg/mlversus2.005±0.005mg/mlfor theoverrangesample.
ConclusionandRelevance Theanalysisshowedthechemicalphysicalstabilityofthecompoundsstudiedallowingtheiruse evenoutsidethestorageconditionsindicatedinthetechnical datasheet.Theresultsshowedthattherewerenostatistically significantdifferencesintheconcentrationofoverrangedoxorubicinandepirubicinsamplesevenafteraccidentalfreezing. Thisconsistsinareductionofdrugwasteinrealconditions. Aneasyaccesstomassspectrometryanalyticalplatformmay allowtheevaluationofdrugstability,redefiningthechemicalphysicalstabilitywithcertifieddata.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-002 EFFECTIVENESSANDSAFETYOFMONOCLONAL ANTIBODIESFORMIGRAINEPREVENTIONAFTERTWO ANDAHALFYEARSOFCLINICALEXPERIENCE
10.1136/ejhpharm-2023-eahp.51
Effectivenessandtoleranceareevaluated3monthsafter initiationandifitiseffectiveandwelltolerateditismaintainedupto12months.Responseisdefinedasadecreasein headacheand/ormigrainedayspermonth 50%comparedto baselineand/orasignificantimprovementinqualityoflife (measuredbyHIT-6andMIDASscales).Ifpartialresponse (PR)(decrease £50%)oradverseeffects(AE)anothermAb canbeemployedwithdifferentmechanismofaction.Iflack ofresponse(LR)(decrease £25%)treatmentissuspended.If responseisachievedduringthelastmonths,themAbcanbe maintainedforanotheryear.
Results 253patientsinitiatedtreatmentwithamAb.69% (n:175)completed12monthsoftreatmentwitheffectiveness (responders)and31%(n:78)stoppedatthird-monthevaluation(PR/LRpatientsandAE-sufferingpatients),42ofwhich changedtoasecondmAb.Endingreasonswere:PR/LR (n:52),PR/LRandAE(n:9),AE(n:8)andothersnotrelated tothetreatment(n:9).
Aftercompleting12months,140patientsstoppedthe treatment;25maintaineditforanothertreatmentcourse, someofwhichhavealreadystartedathirdcourse(median duration:23[17-37]),and10switchedtoasecondmAb.
Regardingsafety,33%(n:83)ofpatientsreportedatleast oneAEduringthetreatmentwiththefirstand/orsecond mAb,beingthereasonfordiscontinuationin7%(n:17)of patients(duetovertigoandconstipation,mainly).MostfrequentAEwerevertigo/dizziness(17%,n:45),constipation (13%,n:33)andskinrashesafterinjection(4%,n:11).
ConclusionandRelevance Anti-CGRPmAbareeffectiveand safetreatmentsthatimprovemigrainesufferingpatients’ qualityoflife.Asignificantpercentageofpatientscompletesthe treatmentcourseandonly7%ofpatientsdiscontinuesthe mAbduetointolerance.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-003 EXPERIENCESWITHABESTPOSSIBLEMEDICATION HISTORY(BPMH)CONDUCTEDBYPHARMACY STUDENTSINTHEHOSPITALSETTING:ASCOPING REVIEW
AWeidmann*,JSchintler. InnsbruckUniversity,ClinicalPharmacy,Innsbruck,Austria 10.1136/ejhpharm-2023-eahp.52
BackgroundandImportance Improvementofpatientsafetyat transitionofcarepointsisakeystrategicaimofthe3rd WHOGlobalPatientSafetyChallenge.1 Medicationreconciliationonadmissionintohospitalincreasespatientsafetyby reducingmedicationerrorsandadverseeventsandhasbeen showntoreducehospitalreadmissions.2 Collectionofan
3PC-037 LIQUIDCHROMATOGRAPHYMASSSPECTROMETRY 1VGarau*, 2MCrobeddu, 1GACarrucciu, 1PSerra, 2PCaboni. 1ABusinco’ Oncology Hospital,HospitalPharmacy,Cagliari,Italy; 2UniversityofCagliari,Pharmacy,Cagliari,Italy DFresan,AYerro,APastalle,CGarcia,MCalvo,IOrtega,ELacalle,RJuanbeltz,AIIdoate, MTSarobe,MMNoceda*. HospitalUniversitariodeNavarra,Pharmacy,Pamplona,Spainaccuratebestpossiblemedicationhistory(BPMH)isthefirst step.Thisisoftenresourceintensive.FinalyearpharmacystudentsarenowbeingassignedtoobtainBPMHs,asacosteffectivealternative.
AimandObjectives Theaimofthisscopingreviewwasto determinetheexperienceswithabestpossiblemedicationhistory(BPMH)conductedbypharmacystudentsinthehospital setting.
MaterialandMethods AscopingreviewwasconductedinvolvingPubMed,PubPharm,LIVIVOandWebofScience(20102021)includingoriginalstudiesandsystematicreviewsand theirreferencelists.OnlypapersinvestigatingpharmacystudentsBPMHcomparedtootherhealthcareprofessionalsin hospitalpracticewereincluded.Twoindependentreviewers screenedtitles,abstractsandfulltextarticlesandcompleted dataextractionwithdiscrepanciesbeingverifiedbyathird. Datachartingwasusedtoidentifyvariablescorrespondingto theresearchquestion.Reportingwascompletedinaccordance withPRISMA-ScR.
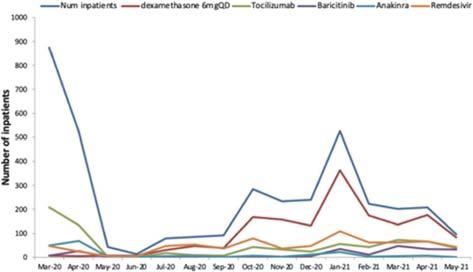
Results Outof235papers,18papersmettheinclusioncriteria.Australia(n=1);Canada(n=1)andtheUSA(n=16) includedatotalof7293patients.Pharmacystudentsusemore informationresources(77,6%;n=972)comparedtopharmacy technicians(58,4%;n=743);identifiedmoreprescription/nonprescriptionsdrugs(n=10,2)comparedtonurses(n=6,8)and medics(n=7,1);makefewermistakesidentifyingallergies/ intolerances(n=6)comparedtonurses(n=27)andreduced the30-dayre-admissionrate(0,6%).
ConclusionandRelevance Pharmacystudentsareabletoeffectivelycontributetopatientsafetybycarryingoutverydetailed bestpossiblemedicationhistories,offeringaneconomicalalternativetotechnicians,nurses,pharmacistsandmedicalhealthcareprofessionals.Inadditiontothebenefitstothehealthcare systemthisoffersadditionalopportunitiesforeducation/interdisciplinarytrainingbetweenpharmacyandmedical/nursing students.
1.WorldHealthOrganisation. GlobalPatientSafetyActionPlan2021-2030. Towardseliminatingavoidableharminhealthcare.2021.ISBN978-92-4003270-5.
2.MekonnenAB, etal.Effectivenessofpharmacistledmedicationreconciliationprogrammesonclinicaloutcomesathospitaltransitions. BMJOpen.2016; 6(2): e010003.
ConflictofInterest Noconflictofinterest
AimandObjectives Ouraimistoanalysethechangesinthe epidemiologyandprevalenceofuseofthedifferenttreatments usedagainstCOVID-19anditsclinicaloutcomesthroughout thepandemic(fromMarch2020toMay2021)inaretrospectiveunicentrestudy.Wepresentthedataofauniversity tertiaryhospital.
MaterialandMethods WeidentifiedallCOVID-19patients admittedtoourhospital>48hthroughtheelectronicmedical records(SAPMedication®).Weevaluateddemographicdata (ageandsex),clinicalfeatures(numberofadmissions/month inICUorregularwards,meanlengthofstayanddeaths includingthose<48h)aswellasmonthlydrugconsumption ofremdesivir,hydroxychloroquine,lopinavir/ritonavir,betainterferon,tocilizumab,baricitinib,anakinra,corticoids(dexamethasone6mg/dayand>20mg/day,methylprednisolone >40mg/day,prednisone>30mg/day,hydrocortisone>100 mg/day)andantibiotics.
Results Atotalof4406patientswithSARS-CoV-2infection wereadmittedofwhich3723mettheinclusioncriteria.The medianagewas66years,withhigherpercentageofmen (59%).ThenumberofpatientsadmittedtoICU,semicritical careoraregularwardwas,respectively20%,5,3%and 74,7%.Thepercentageofdeathsafterthelargepeakofmortality(15,2%)inMarchprogressivelydecreasedto7,7%in thefirsttrimester2021.ThemedianlengthofstayforICU/ semicriticalcareorregularcarewas26,2and8,7days. Trendsinmonthlyuseofthemostfrequentdrugsareshown inthefigurebelow.Morethan80%ofinpatientstooklopinavir/ritonavirandhydroxychloroquineatthebeginning,but consumptionwasdrasticallyreducedafter.Theuseofbeta interferonwasanecdoticalafterfirstmonths.Themostused antimicrobialswereceftriaxone(45,5%)andazithromycin (34,9%),followedbylevofloxacin(8,9%),amoxicillin/clavulanate(7,1%)andceftaroline(6,0%).
4CPS-004 TRENDSINTREATMENTSDURINGCOVID-19 PANDEMICINAUNIVERSITYTERTIARYHOSPITAL
1MAlbanell*, 1MTuset, 2FMeira, 1AEscolà, 1DSoy, 2ASoriano, 2CGarcía-Vidal, 3AMurgadella, 4IOriol. 1HospitalClínicdeBarcelona,Pharmacy,Barcelona,Spain; 2HospitalClínicdeBarcelona,InfectiousDiseases,Barcelona,Spain; 3HospitalMoisés Broggi,Pharmacy,Barcelona,Spain; 4HospitalMoisésBroggi,InfectiousDiseases, Barcelona,Spain
10.1136/ejhpharm-2023-eahp.53
BackgroundandImportance Pharmacotherapeuticmanagement ofSARS-CoV-2infectionfromthebeginningofCOVID-19 pandemictonowhasevolvedinaccordancewithresearch andclinicalexperience,improvingtreatmentsandthusclinical outcomes.
Abstract4CPS-004Figure1
ConclusionandRelevance TheuseofdrugsduringthepandemicofCOVID-19hasshownaclearevolutionovermonths towardsmorestandardisedtreatments,withremdesivirasantiviralanddexamethasone,tocilizumabandbaricitinibstanding outasanti-inflammatorydrugsinourcentre.Homogenisation andstandardisationofCOVID-19treatmentshavebeenmanagedasareflectionofthescientificevidenceaccumulated throughoutthepandemic.
ConflictofInterest Noconflictofinterest
4CPS-005
SUCCESSFULTREATMENTOFOSTEOMYELITISCAUSED BYDIFFICULT-TO-TREATRESISTANTPSEUDOMONAS
1ARodríguezEsquíroz*, 2EMorenoGarcia, 1MSarobeCarricas. 1UniversityHospitalof Navarre,Pharmacy,Pamplona,Spain; 2UniversityHospitalofNavarre,InfectiousDiseases, Pamplona,Spain
10.1136/ejhpharm-2023-eahp.54
BackgroundandImportance Cefiderocolisanewsiderophore cephalosporinwhicheffectivelypenetratestheoutercellmembraneofgram-negativebacteria.Althoughseveralstudieshave demonstratedtheefficacyofcefiderocolinthetreatmentof severeinfectionscausedbymultidrug-resistantgram-negative bacilli,currentinformationonefficacyinosteoarticularinfectionisscarce.
AimandObjectives Weaimedtoreportacaseofdifficult-totreatresistant Pseudomonasaeruginosa osteomyelitissuccessfullytreatedwithcefiderocolfor6weeks.
MaterialandMethods Thisisa64-year-olddiabeticmale patientwhodevelopeda P.aeruginosa osteomyelitissecondary toasurgicalwoundinfectionfollowingasupracondylaramputation.Itwastreatedwithmultiplesurgicaldebridementand severalantibioticseries(ciprofloxacin,piperacillin/tazobactam andmeropenem).Despitethis,culturesfromsurgicalsitecontinuedtogrow P.aeruginosa whichbecamemultidrug-resistant, (onlyitwassusceptibletocolistin,aminoglycosides,ceftolozane/tazobactamandcefiderocol).Ceftolozane/tazobactamdistributionwastemporarilystoppedatthistimeandamputation ofthelowerlimbwasbelievedtobetheonlyoption remaining.
Thepatientwastreatedwithcefiderocolasamonotherapy for6weeks(June-August2021)atatertiaryhospital,ata doseof2gevery8hoursadministeredina3-hourinfusion. Inaddition,foursurgicaldebridementswereperformedduring thistime.
Results After3weeksoftherapywithcefiderocol,thewound swabcultureswerenegative.Thepatientremainedafebrile duringandattheendoftheantibiotictherapy.Nodrugrelatedadverseeffectsorinfusionreactionswerereported. Therewasnoleukopenia,leucocytosis,orworseningrenal function.Theinflammatorymarkervaluesdecreaseduntilthey normalisedandthemagneticresonanceimprovedconsiderably after6weeksoftreatment.
Two-controlmagneticresonanceandbloodtestswereperformed,atweek15and45.Theyshowednoevidenceofpersistentorrecurrentinfectionandnoelevationsofacutephase reactants.Furthermore,thepatientwasfebrile,asymptomatic andpain-free.
ConclusionandRelevance Thiscaseaddsmoreexperienceto thescarceliteratureontheuseofcefiderocolin P.aeruginosa osteomyelitis.
Itssuccessinthetreatmentofosteomyelitissuggeststhat thisdrugpenetrateswellinbonetissueandcouldbeagood therapeuticoption,inconjunctionwithsurgicaldebridement.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-006 COMPLETECLINICALRESPONSEINMETASTATIC BREASTCANCERAFTERFRONT-LINETREATMENT WITHRIBOCICLIB/TAMOXIFEN
1JCDelRíoValencia, 1RTamayo-Bermejo, 2LRodelo-Haad, 1IMuñozCastillo. 1Regional UniversityHospitalofMalaga,PharmacyService,Malaga,Spain; 2HospitaloflaLineadela Concepción,OncologyService,laLinea,Spain
10.1136/ejhpharm-2023-eahp.55
BackgroundandImportance Endocrinetherapywithovarian suppressionorablationisthestandardfirst-linetreatmentfor perimenopausalorpremenopausalwomenwithhormone receptor(HR)positive,HER2-negative,advancedbreastcancer;however,endocrinetherapyresistanceanddiseaseprogressionoccurinmostcases.Ribociclibisaselective,small moleculeinhibitorofcyclindependentkinases(CDKs)4and 6hasshowedthatalongsideendocrinetherapycanimprove progression-freesurvivalandachievehigherproportionsof overallresponsesthanendocrinetherapyaloneinpremenopausalwomenwithHR-positive,HER2-negative,advanced breastcancer.
AimandObjectives Wepresentthecaseofawomanpatient diagnosedwithstage-IVHR+/HER2-breastcancer(Ki6725%)whoachievedcompleteresponsetofirst-lineribociclib treatment.
MaterialandMethods Thiswasanobservationalretrospective studyoftheuseofribociclibina60-year-womandiagnosed withHR+/HER2-metastaticbreastcancer.Datawere obtainedoftheelectronicmedicalrecords.
Results Thepremenopausal54-agedpatientwasdiagnosed withHR+/HER2-(Ki67-10%)localisedinfiltratingductalcarcinomaofleftbreast(1.5cm-sizetumour)inJuly/2015.She underwenttumorectomyandreceivedadjuvantradiotherapy andfive-yeartamoxifen20mgtreatment.InMarch2021,she sufferedfromlossofstrengthofleftupperlimb.CT-scan revealedamassintheleftaxillaryregionbetweenpectoral regionandfirstribandhypermetabolicbonelesionsinthe trochanteroftheleftfemur,compatiblewithbonemetastases. HR+/HER2-breastcancerwasconfirmedbytumourbiopsy. Ki67expressionwas25%.InJune/2021,thispremenopausal 60-yearwomanwastreatedwith3-monthly10.8mggoserelin,daily20mgtamoxifenandribociclib600mgoncedaily for21consecutivedaysfollowedby7daysofftreatment, resultinginacompletecycleof28days.InSeptember2021, sheachievedcompletemetabolicresponseofthelesions describedintheaxillaandbone,withoutcurrentfociofneoplasticdisease.InJune2022,thelastCT-scanrevealed absenceofneoplasticdisease,therefore,shecontinueswiththe sametreatmentwithoutdosemodificationsordelays.Side effects:treatmentwaswelltolerated;sheunderwentgradeI haematologicaltoxicity.
ConclusionandRelevance Thiscasereportdocumentsan exceptionaltumourresponseofafastgrowing,locally advanced,bonemetastaticHR+/HER2– denovobreastcancer treatedbyribociclib/tamoxifen/goserelincombinationtherapy. Treatmentsuccessislonglastingwithfewsideeffects.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-007
1EVillamañán*, 1CSobrino, 1CMateos, 1VCollada, 1AHoyo, 1SMallón, 2JPavón, 1IJiménez, 3YLarrubia, 1AHerrero. 1HospitalUniversitariolaPaz,Pharmacy,Madrid,Spain; 2HospitalUniversitariolaPaz,Pneumology,Madrid,Spain; 3HospitalInfantaSofía, Pharmacy,Madrid,Spain
10.1136/ejhpharm-2023-eahp.56
BackgroundandImportance Availabledatareporteddifferent resultsabouttheeffectofcolchicineinpatientswithCOVID19pneumonia(CN)provingtheneedformoreanalysis.Currently,manyofthesepatientsaretreatedwithhigh-costnew drugswithpoorresults.
AimandObjectives ToevaluatewhethertreatmentwithcolchicineaddedtothestandardtherapyforCNwasrelatedto deathsreduction.Secondaryobjectives:toanalysedifferences inlengthofstay(LOS)andcombinationofdrugsintreatment protocolswithbetterresults.
MaterialandMethods Multicentre,real-world,controlled,retrospectivecohortstudy(March-June2020).Inclusioncriteria: hospitalisedadultpatientswithCN.Admittedtocriticalcare unitswereexcluded.Experimentalgroup:Patientstreatedwith colchicinewhometinclusioncriteria(colchicinetherapygroup [CG]).Controlgroup:thosewhometinclusioncriteriaand didnotreceivedcolchicine(non-colchicinetherapygroup [NCG]).Patientswerematched1:1byage(±2years),sex, severityofthediseaseandcomorbidity.Toselectcontrols,we chosetheconsecutivelynextadmittedpatientafteronetreated withcolchicine.Thisallowedustoselectcontrolsubjectsata closetimeandplacetocases,thatis,undersimilarcircumstancesintermsofpatientcareprotocols.
Results 222(111treatedwithcolchicine)patientswereanalysed.Medianage79years[66–88](81years[66-87]inCG vs79years[66-88]inNCG,p=0.978).52.3%men(54.1% CGvs50.5%NCG;p=0.591).Primaryendpointofdeath occurredin19(17.1%)patientsintheCGascomparedwith 32(29.4%)intheNCG(OR:0.497;95%CI:0.261–0.946; p=0.031).HospitalLOSwasdichotomisedbythemedian value(10days),theuseofcolchicinewasassociatedwitha longerhospitalLOSwhencomparingwiththecontrolgroup (OR=1.856;95%CI:1.089–3.162;p=0.022).Proportionof deathswerehigherinNCGthaninCGinpatients 70years (p=0.012).Withrespecttosexandcomorbidity,distribution ofdeathsshowednosignificantdifferences.Almostallpatients receivedantimicrobials(91.9%)concomitantly,deathrate:19/ 50(38%)CGvs.31/50(62%)NCG;p=0.023),byantimicrobial:azithromycin(9/19)(47.4%)inCGvs.10/19(52.6%) NCG;p=0.517;ceftriaxone16/44(36.4%)CGvs28/44 (63.6%)NCG;p=0.022andlevofloxacin4/12(33.3%)CG vs8/12(66.7%)NCG;p=0.232.
ConclusionandRelevance Ourstudyshowedlowermortality inhospitalisedpatientswhoreceivedcolchicinetotreatCN. Thistreatmentwasparticularlybeneficialforelderlytreated withantibioticsconcomitantly.Findingsinourstudysupport theneedofmorerandomisedclinicaltrialsthatcouldfully elucidatethetypeofpatientswhomaypotentiallybenefit fromthislow-costdrug.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.KalilAC.TreatingCOVID-19-Off-LabelDrugUse,CompassionateUse,and RandomizedClinicalTrialsDuringPandemics. JAMA 2020;323(19):1897–1898.
ConflictofInterest Noconflictofinterest
4CPS-008 DOESCOMORBIDITYAFFECTADHERENCETO INHALERSINSEVEREASTHMAPATIENTSTREATED WITHBIOLOGICS?
1PGranda, 1EVillamañán*, 2CCarpio, 1CSobrino, 2DLaorden, 1EPérez, 1CLara, 1Sde Andrés, 1MEscario, 1AHerrero. 1HospitalUniversitariolaPaz,Pharmacy,Madrid,Spain; 2HospitalUniversitariolaPaz,Pneumology,Madrid,Spain
10.1136/ejhpharm-2023-eahp.57
BackgroundandImportance Comorbiditiesareoftenassociatedwithsevereasthmaincludingthosepatientstreatedwith biologics.Thatoftencontributestopoorlycontrolled asthma1 ,whichcouldberelatedtodeficientadherenceto inhalers.
AimandObjectives Toevaluateproportionofnon-adherence toinhalersinpatientswithsevereasthma(SA)treatedwith biologicsaccordingtotheircomorbidityandtocomparetwo methodstoassessnon-adherence.
MaterialandMethods Cross-sectionalretrospectiveobservationalstudyofpatientswithSArecruitedfromtheSAunitofa tertiaryhospitalinMadridfromJunetoDecember2020.We registereddemographicdata,comorbiditiesandconcomitant therapyforasthma.Non-adherencewasdefinedaspharmacy refilldata(PRD)<80%totheprimaryinhaler2 and/orTest ofAdherencetoInhalersquestionnaire(TAI)results<503 ConcordancewasassessedbydeterminingtheCohen’skappa statistic.Primaryvariable:Proportionofpatientsclassifiedas nothavingtherapeuticadherencemeasuredbybothofthefollowingmethods:PRD<80%intheprevious6months,and TAIquestionnaire:avalue<50.Comorbiditiesconsidered: rhinoconjunctivitis,nasalpolyposis,anxietyanddepression, gastroesophagealreflux,bronchiectasis,aspirin-exacerbatedrespiratorydisease(AERD)andallergicbronchopulmonary aspergillosis.
Results 53patientswereevaluated.Medianagewas61years (IQR51.8-67)and33(61%)werewomen.41(77%)had comorbidity:25(61%),rhinoconjunctivitis,16(38%)nasal polyposis,15(36%)anxietyanddepression,7(17%)gastroesophagealreflux,6(15%)bronchiectasis,5(12%)AERD and1(2%)allergicbronchopulmonaryaspergillosis.Thehighestnon-adherencewasdetectedinpatientswithrhinoconjunctivitisbythetwomethods:50%and55%accordingto TAIandPRD,respectively(k=0.02295%CI-0.256
0.3). Agreementofbothmethodswaslowinallcomorbidities; nasalpolyposis:42%vs23%(k=-0.04995%CI-0.421–0.519);anxietyanddepression:25%vs32%(k=095%CI -0.317–0.317);gastroesophagealreflux:8%vs10% (k=0.36495%CI-0.21-0.938)andAERD17%vs10% (k=-0.15495%CI-0.659–0.967).
ConclusionandRelevance Ourresultshighlightahighprevalenceofnon-adherencetoinhalersinpatientswithSAand othercomorbiditiestreatedwithbiologics.Therefore,hospital pharmacistsshouldfocusonthispatient´sadherenceto inhalers,especiallythosewithrhinoconjunctivitis,whenprovidingpharmaceuticalcaretoSAtreatedwithbiologicsin practice.
REFERENCESAND/ORACKNOWLEDGEMENTS
1. JPrecisRespirMed,2019;2:5–9.
2.Assessingadherencebycombiningthetestofadherencetoinhalerswithpharmacyrefillrecords. JInvestigAllergolClinImmunol 2021;31:58–64.
3.TestofAdherencetoInhalers. ArchBronconeumol 2017;53:360–1.
ConflictofInterest Noconflictofinterest
4CPS-009 D-9-TETRAHYDROCANNABINOLFORTHETREATMENT OFMULTIPLESCLEROSISSPASTICITY:EVALUATION OFEFFECTIVENESSANDSAFETY
10.1136/ejhpharm-2023-eahp.58
BackgroundandImportance Multiplesclerosis(MS)hasa rangeofsymptoms,suchasimpairedsleep,bladderdysfunctionandmobilityrestrictionslikespasticitythatworsenasthe diseaseprogresses.Spasticityiscommoninthepatients affectedofmultiplesclerosis.Itsimpactonpatientsfunctioningandqualityoflifeisprofound.Themanagementofspasticityisfocusedonfunctionimprovement,evaluatedwiththe ExpandedDisabilityStatusScale(EDSS).Themostofpatients becomeresistanttoantispasticdrugsornottolerate. D-9-tetrahydrocannabinol,oromucosalspraycontainingcannabinoid, improvesthespasticity 20-30%frombaseline,1 evaluated withanumericalratingscale(NRS).
AimandObjectives Theaimofthisstudyistoevaluateeffectivenessandsafetyof D-9-tetrahydrocannabinolinthepatients withMS.
MaterialandMethods Aretrospectivecohortstudywasconductedinallpatientswhobegan D-9-tetrahydrocannabinol betweenJanuary2021andAugust2022(18months)andthe datawasretrievedfromtheweb-basedregisteroftheItalian MedicinesAgencyandthroughtheanalysisofclinicalprescriptions.Thepatientswerebrokendownbygender,itwas calculatedtheaverageageandthevaluesofNRSandEDSS.
Results 213patientswereevaluated,58%ofthesewere female.Theaverageagewas58±11years,themeanNRS andthemeanEDDSscorebeforetreatmentwas6,82±1,35 and5,45±1,73respectively.Amediumcorrelationwas foundbetweenNRSandEDSSscore(R=0,458),whilealow correlationwasfoundbetweenageandNRSscore(R=0,119). TheNRSscoreaftertreatmentwas4,87±1,11(D=2,03± 0,86)witha30%mediumreductionofNRSscore.The adverseeffectdetectedwerenausea(7%),fatigue(6%),headache(5%)andvertigo(4%),and1%ofpatientshadtodiscontinuethetherapyforadverseeffects.
ConclusionandRelevance Thesymptomaticreliefofspasticity ledtoquantifiableandsustainablebenefitsintheabilityto performdailyactivitiesandimprovedtheirqualityoflife.The useof D-9-tetrahydrocannabinolwaseffectiveandwelltoleratedinthemanagementofthespasticityofpatientswithMS, andisaneffectivealternativefortheclassicalantispasticity medications.
1.ConteA,VilaSilvánC.ReviewofAvailableDatafortheEfficacyandEffectiveness ofNabiximolsOromucosalSprayinMultipleSclerosisPatientswithModerateto SevereSpasticity, NeurodegenerDis.2021;21(3-4):55
62.doi:10.1159/ 000520560
ConflictofInterest Noconflictofinterest
4CPS-010 DUPILUMABFORTHETREATMENTOFATOPIC DERMATITIS:EVALUATIONOFEFFECTIVENESSAND SAFETY
BackgroundandImportance Atopicdermatitis(AD)isa chronicinflammatoryskindisease.itaffects20%ofchildren and3%ofadults.Theclinicalstrategyusedinthetreatment ofmoderateandsevereformsofADistheuseofdupilumab inadultsandadolescentseligibleforsystemictherapy.Dupixentisusedforthetreatmentofsevereatopicdermatitiswith aEASI(EczemaAreaandSeverityIndex)score>=24,who haveanEASIscore>=24;evaluationofprurituswithNRS scale>=7;qualityoflifeassessmentwithDLQIindex>=10. AimandObjectives Theaimofthestudywastodetermine effectivenessandsafetyoftreatmentwithdupilumabin patientswithmoderatetosevereAD.
MaterialandMethods AretrospectivecohortstudywasconductedinallpatientswhobegandupilumabbetweenJanuary 2020andJune2022andthedatawasretrievedfromthe web-basedregisteroftheItalianMedicinesAgency.Theprimaryendpointwasthechangeinthedegreeofseverityof ADassessedbytheNRSscale,EASIscore,DLQIscoreand theevaluateofadverseeffectstoassesssafety.Theefficacyof dupilumabwasestablishedbyreductionaccordingtotheNRS, EASIandDLQIscale,fromthevalueatthebaselinetothe valueofthelastre-evaluationofthedisease,carriedoutafter 6months,andtheadverseeffectswereevaluatedduringthe wholeperiodconsidered.
Results 134patientswereevaluated,56%ofthesewere female.Themediumagewas40±19years,themean NRS,EASIandDLQIscorebeforetreatmentwas8,0± 2,0,26,0±4,0and15,0±7,0,respectively.TheNRS scoreaftertreatmentwas3,2±2,0( D=-5,0±2,0; <0,000).TheEASIscoreaftertreatmentwas5,0±4,0 ( D=-21,0±4,0; <0,000),andtheDLQIscoreafter treatmentwas3,0±3,0( D=-12,0±7,0; <0,000).90% ofpatientsobtainedareduction>20%oftheNRSscore; >50%oftheEASIscoreand33%reductionoftheDLQI score.Theadverseeffectdetec tedwerenon-infectiousophthalmological(45%),injection-sitereaction(12%),nausea (5%)andheadache(4%).
ConclusionandRelevance ThesymptomaticreliefofADled toimprovedpatient’squalityoflifeandledtoquantifiable benefitsintheabilitytoperformdailyactivities.Inagreement withotherstudies,theuseofdupilumabwaseffectiveand welltoleratedinthemanagementofatopicdermatitisandis aneffectivealternativefortheclassicalmedications.
ConflictofInterest Noconflictofinterest
MAToledoDavia*,NLabradorAndújar,ARRubioSalvador,CBlázquezRomero,LTorralba Fernández,CJiménezMéndez,RPrietoGalindo,PAguadoBarroso,EGómezFernández, PMoyaGomez. HospitalUniversitariodeToledo,HospitalaryPharmacy,Toledo,Spain
10.1136/ejhpharm-2023-eahp.60
BackgroundandImportance Inflammationplaysamajorrole intheprogressionofneoplasmssuchasnon-small-celllung cancer(NSCLC),soitisvitallyimportanttofindbiomarkers thatareeasilyapplicableandreproducibleinroutineclinical practicethatallowustoclassifypatientsaccordingtotheir forecast.
10.1136/ejhpharm-2023-eahp.59
GCasini,GPolito*,GBattistini,MFLioni,CCollice,EOrlandi,EMProli. AouPoliclinico UmbertoI,UocPharmacy,Rome,ItalyAimandObjectives
Toanalysetheinflammatorymarkerplatelet/lymphocyteratio(PLR)asapredictorofefficacyinimmunotherapytreatments;toassesswhetherthereisarelationship betweenPLRvalueandresponsetotreatment.
MaterialandMethods Retrospectiveandobservationalstudyof patientsdiagnosedwithNSCLCandtreatedwithpembrolizumabinatertiaryhospital,fromJanuary2018toDecember 2021.Wecollecteddemographicvariables(sexandage), ECOG,histology,presenceofmetastases,PD-L1expressionand previoustreatments.Progression-freesurvival(PFS)andoverall survival(OS)werecalculatedbytheKaplan-Meiermethodand log-rankashypothesistesting.;PLR(absoluteplateletcount/ absolutelymphocytecount)wascalculatedandPLR=200 wasconsideredthecut-offpoint.Coxregressiontestwas usedtoassesstheinfluenceofPLRontreatmentefficacy.
Results Seventy-threepatientstreatedwithpembrolizumab (80.8%male,n=59)andmedianage65[83-37]years. Adenocarcinomahistologywas90%(n=66);40patients ECOG=0,31patientsECOG=1and2patientsECOG=2;26 patientsPD-L1<50%,19patientsPD-L1>50%andfor28 patientsitwasunknown;12patientsCNSmetastasesand22 patientshadliver/bonemetastases.Significantdifferenceswere obtainedinthegroupofpatientswithliver/bonemetastasesin PFSwithmedianof6.3(2.9-9.6)CI95%vs17.3(11.4-23.2) CI95%months(p=0.03),andinthegroupofpatientswith CNSmetastasesinOSwithamedianof9.6(1.2-17.9)CI 95%vsat24.9(18.6-31.2)95%CImonths(p=0.003). MedianPFSwas15.6[10.15-21.1]95%CIforPLR<200vs 9.97[2.86-17.1]95%CImonthsforPLR>200(p=0.04); medianOSwas26.25[19.87-32.64]95%CIforPLR<200 vs11.31[3.86-18.79]95%CImonthsforPLR>200 (p=0.001).Coxregressiontest:HR=1.001(p=0.017)for PFSandHR=1.002(p=0.003)forOS.
ConclusionandRelevance PLRandthepresenceofmetastases correlateswithPFSandOS.PLR,withacut-offpoint=200, appearsusefulasaprognosticbiomarkerforpatientswith NSCLCtreatedwithpembrolizumab;higherPLRvalues,result inlowerPFSandOS(HR>1inPFSandOS).
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-014 COMPARISONOFTWOPROTOCOLSFORTHE ADMINISTRATIONOFLEUCOVORINRESCUESAFTER HIGHDOSEMETHOTREXATEINFUSIONOF24HOURS
AEscolàRodríguez*,NArranzPasqual,CBastida,MAlbanell,IMongeEscartín,PMaté Arbaiza,SRuizBoy,ECarceleroSanMartín,DSoyMuner. PharmacyService,Divisionof Medicines,ClinicBarcelona
UniversityofBarcelona,Barcelona,Spain
10.1136/ejhpharm-2023-eahp.61
BackgroundandImportance Therapeuticdrugmonitoring (TDM)ofmethotrexate(MTX)inplasmaisastandardproceduretoearlyidentifypatientswithdelayeddrugelimination andadjustleucovorindose.Adequateleucovorinrescues(LR) shouldstartwithin42-48hofthebeginningofhighdose (HD)-24h-MTXinfusiontoavoidMTXtoxicitybutextending LRmorethanneededcanreduceMTXantitumoureffect.
BeforeimplementationofnewPETHEMA-2019protocolat ourhospital,standardLRwereprescribedandMTXplasma concentrationwasdetermined48hafterinfusioncompletion. Followingnewprotocolrecommendations,pharmacistsstarted TDM.
AimandObjectives
Toassesswhethertheimplementationof thenewprotocolallowedreducingthetotalleucovorindose administeredafterHD-24h-MTXinfusion.Secondaryoutcomes:comparetheincidenceoftoxicityandthelevelof complianceofappropriateMTXsamplingtimesandLR betweenbothprotocols
MaterialandMethods Retrospectiveobservationalstudyconductedatauniversitytertiaryhospital.Adultstreatedwitha HD-24h-MTXinfusionastreatmentforacutelymphoblastic leukaemia(ALL)andBurkittlymphomafromMay2019to June2022wereincluded.Patientswerestratified(1:1)accordingtotheprotocolfollowed.Datacollectedwere:age,sex, haematologymalignancy,MTXdose,LRandserumcreatinine. Results Fifty-eightHD-24h-MTXinfusionswereanalysedcorrespondingto20patientsforthenewprotocol(75%males; mean±SDage49±15years;7withlymphoma,11ALLB,2ALL-T)andto20fortheoriginal(65%male;mean± SDage49±16years;10lymphoma;7ALL-B,3ALL-T). Themedian[interquartilerange]leucovorindoseadministered percyclefollowingtheoriginalprotocolwasan87%higher thanthedoseadministeredwiththenewprotocol(597mg/ m2[475,700]vs75mg/m2[45,180],p<0,001).Nephrotoxicityincidence(increaseof0,3mg/dlfrombasalcreatinine)was 21%intheoriginalprotocolvs19%inthenewone (p=0,84).SampleextractionsforTDMwerecorrectlydrawn in93%ofthecasesandLRwerecorrectlyadministeredin 76%ofthecaseswhenusingthenewprotocol,incomparison with97%and55%whenusingtheoriginalprotocol).
ConclusionandRelevance Implementationofthenewprotocol allowsasignificantreductionoftheleucovorindoseby87% withoutanincreaseinnephrotoxicity.Measurestoincrease adherencetothenewprotocolmaybeimplementedhereafter. REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-015 ROLEOFCLINICALPHARMACISTINTHE OPTIMISATIONOFNIRMATRELVIR/RITONAVIR PRESCRIPTIONINTHEEMERGENCYDEPARTMENT MDMSánchezSuárez,AMartínRoldan,MRCantudoCuenca,MIArchillaAmat, CMontero-Vilchez*. HospitalUniversitarioVirgendeLasNieves,Pharmacy,Granada,Spain 10.1136/ejhpharm-2023-eahp.62
BackgroundandImportance Nirmatrelvir/ritonavir(Paxlovid®) hasbeenrecentlyauthorisedfortreatingcoronavirusdisease 2019(COVID-19)inadultswhodonotrequiresupplemental oxygenandwhoareatincreasedriskforprogressingto severedisease.DuetomultipledrugsmetabolisedbyCYP3A mayhavesignificantinteractionswithritonavir,physiciansand pharmacistsshouldworktogetherforthesafeandeffective useofpaxlovid.
AimandObjectives Todescribethepharmacistinterventions (PIs)intheemergencydepartment(ED)regardingoptimisation ofpaxlovidprescriptionsinnon-hospitalisedCOVID-19 patients.
MaterialandMethods Anobservationalprospectivestudywas conductedfrom1April2022to31August2022ina1000beduniversityhospital.Clinicalvariableswereobtainedusing electronicmedicalrecords.Weregistereddemographicdata (sex,age),vaccinationstatusandcomorbidities,hospitalisation andprescriptionwithothertherapies(suchasremdesivirand baricitinib)afterpaxlovidtreatment,posology,potentialdrug
interactionsandcontraindications.PIswereclassifiedintothe followingtypes:(1)doseadjustment,(2)contraindication,(3) potentialinteraction,(4)non-compliancewiththeindication.
Wealsoidentifiedprimarynon-adherencetopaxlovid.
Results Weincluded77patients,56%female,medianageof 67years(IQR52-81).Mostpatients(87%)werefullyvaccinated(includingboosterdose),12%requiredsubsequenthospitalisationforCOVID-19,noneofthemdiedandonlyone patientrequiredremdesivirasothertherapies.Inrelationto comorbidities,86%ofpatientshadrespiratorydiseases,33% hypertension,30%cancertreatedwithchemotherapy,21% autoimmunediseases,17%renaldisease,16%diabetesmellitus,9%liverdisease.ThepercentageofpatientswithPIswas 70%.ThetotalofPIscarriedoutwas87:(1)31%,(2)13%, (3)33%,(4)23%.Forty-sixpotentialinteractionswere detectedbeingthemostfrequent:statins(33%),antihypertensives(11%),anticoagulants(6%),immunosuppressants(6%), amongotherdrugs,aswellas,14contraindications,inwhich statinsagainstoodout.Primarynon-adherencewasdetected in10%ofpatients.100%ofPIwereaccepted.
ConclusionandRelevance Hospitalpharmacistsarekeyinthe optimisationofpaxlovidprescriptionsintheED.Thisincludes assessingforpotentialdruginteractions,aswellascontraindications,amongotherPIs.Duetotherecentconditionalmarketingauthorisationofpaxlovid,itisimportanttoencourage multidisciplinaryworktoreducepotentialdosingerrorsand adversereactions,increasingpatientsafety.
ConflictofInterest Noconflictofinterest
4CPS-016 PREDICTIVEPERFORMANCEOFGLOMERULAR FILTRATIONRATEEQUATIONSBASEDONCYSTATIN C,CREATININEANDTHEIRCOMBINATIONIN CRITICALLYILLPATIENTS
1MAlbanell*, 1CBastida, 1ÁMarcos-Fendian, 1AEscolà, 2JMercandal, 3,4,5PCastro, 1,5DSoy. 1PharmacyService,DivisionofMedicines,HospitalClinicofBarcelona,Barcelona, Spain; 2SurgicalIntensiveCareUnit,AnaesthesiologyDepartment,HospitalClinicof Barcelona,Barcelona,Spain; 3MedicalIntensiveCareUnit,HospitalClinicofBarcelona, Barcelona,Spain; 4IDIBAPS,HospitalClinicofBarcelona,Barcelona,Spain; 5Universityof Barcelona,HospitalClinicofBarcelona,Barcelona,Spain
10.1136/ejhpharm-2023-eahp.63
BackgroundandImportance Twenty-fourhoururinecreatinine clearance(24h-ClCr)remainsthegold-standardforestimating glomerularfiltrationrate(GFR)incriticallyillpatients;however,ithasseveraldrawbacks.Serumcreatinine(SCr)isthe mostfrequentlyusedparametertoestimateGFR,however, Cystatin-C(CystC)mayreflectGFRchangesearlierthanSCr.
AimandObjectives Toassesstheperformanceofequations basedonSCr,CystC,andtheircombination(SCr-CystC)for estimatingGFRincriticallyillpatientsincomparisonto24hClCr.
MaterialandMethods Retrospective,observationalstudyina tertiary-carehospital(May2020toJuly2022).Patientswith CystC,SCrand24h-ClCrmeasurementswithin±2days wereincluded.Alteredthyroidstatusandcorticosteroidsuse for>5dayswererecorded,asbothcanalterCystCvalues.
24h-ClCrwasconsideredthereferencemethod.GFRwas estimatedusingSCr-basedequations:CKD-EPI-CrandCockcroft-Gault(CG);CystC-basedequations:CKD-EPI-CystCand CAPA;andCr-CystC-basedequations:CKD-EPI-Cr-CystC.
Bland-AltmanplotswereusedtocompareGFRestimations with24h-ClCr.Pearson´scorrelationcoefficientsandconcordancecorrelationcoefficients(CCC)werecalculated.Biaswas assessedas(estimatedGFR – 24h-ClCr);andprecisionasthe SDofbias.Furtheranalysiswasperformedwithstratifieddata into24h-CrCl<60mL/min/1.73m2,60-130mL/min/1.73m2 and 130mL/min/1.73m 2
Results Weincluded275measurements,correspondingto186 patients.Mean(SD)SCr,CystCand24h-ClCrwere1.3(1.1) mg/dL,1.8(1.2)mg/L,and77.0(57.7)mL/min,respectively. Theinfluenceofalteredthyroidstatus(N=22)andcorticosteroidstherapy(N=64)onCystCvalueswasstatisticallysignificant(p:0.0138andp<0.000,respectively);however,asboxplotwereoverlapped,wedidnotexcludethemfromthe analysis.
Bland-Altmanplotsareshowninfigure1.Intheoverall population,CKD-EPI-Crequationshowedthelowestbias (2.6)andbestprecision(33.1).Inpatientswith24h-CrCl <60mL/min/1.73m2 (N=124),CystC-basedequationsshowed thelowestbias(<3.0)andCKD-EPI-Cr-CystCwasthemost accurate(13.6).Inthesubgroupof60 £24h-CrCl<130mL/ min/1.73m2 (N=100),CKD-EPI-Cr-CystCwasthemostprecise(20.9).However,inpatientswith24h-CrCl 130mL/min/ 1.73m2 (N=51),CystC-basedequationsunderestimateGFR, whileCGoverestimatesit(22.8).CKD-EPI-Cr-CystCobtained thehighestPearson´scoefficient(0.742)andCKD-EPI-Crthe highestCCC(0.785).
Abstract4CPS-016Figure1 Bland-Altmanplotsshowingmean differencesbetweenestimatedGFRandmeasured24h-CICr
ConclusionandRelevance Ourstudyshowednoevidenceof superiorityofanyequationoverothersforallevaluated parameters.CystC-basedequationswerelessbiasedinindividualswithimpairedrenalfunction(GFR<60mL/min/1.73m2), CKD-EPI-Cr-CystCperformedproperlyinGFRfrom60130mL/min/1.73m2 andCGinpatients>130mL/min/1.73m2.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
1MAlfonsínLara, 1APérezLandeiro, 1NGarcíaBeloso, 1MCouñagoFernández, 1DRobles Torres, 1PPradoMontes, 1IAgraBlanco, 1ALópezLópez, 2SBaltazar, 1NMartínezLópez deCastro. 1HospitalÁlvaroCunqueiro,FarmaciaHospitalaria,Vigo,Spain; 2Institutode InvestigaciónSanitariaGaliciaSurIisgs,UnidaddeMetodologíaYEstadística,Vigo,Spain
10.1136/ejhpharm-2023-eahp.64
BackgroundandImportance AntimicrobialprescribingprevalenceinCOVID-19patientsisestimatedtobearound75%, whereasbacterialcoinfectionpr evalenceisestimatedtobe lessthan10%.Thisdatashowstheunnecessaryuseof antibiotics.
AimandObjectives TocomparetheevolutionofantimicrobialconsumptioninCOVID-19patientsbetweenthebeginningofthepandemicandthethirdCOVID-19waveinour hospital.
MaterialandMethods ObservationalretrospectivestudyconductedinatertiarycarehospitalduringMarchtoJune2020 andMaytoAugust2021inCOVID-19IntensiveCareUnit (CICU)andCOVID-19medicalward(CMW)patients.We extractedantimicrobialconsumptiondatafromthePharmacy database(Silicon)andbed-daysdatafromAdmissionService.
Westandardisedantimicrobialconsumptiontodefineddaily doses(DDD)/100bed-days.ThedescriptiveanalysiswasperformedwithSPSS.Weconductedanormality,anindependenceandacorrelationtest.
Results An8%decreaseinglobalantimicrobialusewas observed.However,wefounda30%decreaseinCMW,and a39%increaseinCICU.
Theantibioticuseinthetwoperiodsshowedasignificance correlation(p<0,001).
ConclusionandRelevance
. Thereisalightdecreaseofantimicrobialprescriptionsinall COVID-19patients.
. ThereisanimportantdecreaseinantimicrobialuseinCMW andaconsiderableincreaseinCICU.
. Theseresultssuggesttheneedformoreantimicrobial stewardshipprogrammesinCICU
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-019 QUALITYASSESSMENTOFTHEEVIDENCE UNDERPINNINGPHARMACIST-LEDANTIMICROBIAL STEWARDSHIPSINTERVENTIONS
1CCastaño-Amores*, 2IGarcía-Giménez, 1MNúñez-Núñez, 3JPérezdeRojas, 1JCabezaBarrera. 1HospitalUniversitarioSanCecilio,Pharmacy,Granada,Spain; 2HospitalJuan RamónJiménez,Pharmacy,Huelva,Spain; 3HospitalUniversitarioSanCecilio,Preventive Medicine,Granada,Spain
10.1136/ejhpharm-2023-eahp.65
BackgroundandImportance Antimicrobialresistance(AMR)is aglobalproblemthreateningpublichealth,securityandeconomicdevelopment.Antimicrobialstewardship(AMS)programmeshavebeenimplementedworldwideastheyhave demonstratedclinical,economicalandecologicalbenefitspromotingtheadequateuseofantibiotics.Pharmacist-ledantimicrobialstewardships(AMS)interventionsareproposedaskey strategiestooptimiseantibioticuseandreduceadverseevents, includingtheselectionofantimicrobialresistance.Systematic reviewsareatthehighestleveloftheevidencevalidityhierarchyandprovideinsightandsupportpolicy-makersinclinical practiceandresearch.
AimandObjectives Toevaluatethequalityofthesystematic reviewsmeasuringtheimpactofPHARMACIST-LEDAMS interventions.
MaterialandMethods Anumbrellareviewofthesystematic reviewsonAMSwasconductedfollowingthePRISMA-P(PreferredReportingItemsforSystematicreviewandMeta-AnalysisProtocols)guideline.Followingprospectiveregistration (ProsperoCRD42022333928),tworeviewersindependently searchedinPubMed,Scopus,CochraneLibraryandGoogle Scholar,withoutlanguageortimerestrictionsuntilJune2022. Weincludedsystematicreviewscoveringpharmacist-ledAMS interventions.TworeviewersindependentlyassessedmethodologicalqualityusingamodifiedAMSTAR-2toolandcollated themainfindings.
Results From1004citations,20reviewswereeligiblefor inclusionsummarisingatotalof648studies.Theoverall qualityof15(75%)reviewswascriticallylow.Fourstudies (20%)wereoflowqualityandonestudywasofhighquality (5%).Themostloss-makingdomainsinvolveprovidingalist ofexcludedstudies,measuringtheriskofbias(RoB)withan appropriatetool,toexplicitlystatethatthereviewmethods
werepreviouslyestablishedandtakingRoBintoaccountin theinterpretationofresults.Theonlyhigh-qualitystudy(5%) reportedonthesourcesoffundingforthestudiesincludedin thereviewandprovidedalistofexcludedarticles.
ConclusionandRelevance Systematicreviewsprovidethebest levelofevidence,buttheirqualitymustbeassured.Theoverallqualityofthesystematicreviewsmeasuringtheimpactof PHARMACIST-LEDAMSinterventionsislow.Thereisa needforhighlevelliteraturecoveringtheparticipationand implicationofpharmacistsinAMS.TherealimpactofAMS isunknowntosupportpolicymakersandefficientdesignsin bothclinicalpracticeandresearch.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-021 EFFECTIVENESS,PERSISTENCE,ANDADHERENCEOF BARICITINIBINRHEUMATOIDARTHRITIS:LONG-TERM REAL-WORLDEVIDENCESTUDY
1ACalvoGarcía*, 1ERamirezHerraiz, 2ILlorenteCubas, 3BVarasdeDios, 1AMorell Baladrón, 4JBenedíGonzález, 2RGarcíadeVicuña. 1HospitalUniversitariodelaPrincesa, Pharmacy,Madrid,Spain; 2HospitalUniversitariodelaPrincesa,Rheumatology,Madrid, Spain; 3HospitaldeSantaCristina,Rheumatology,Madrid,Spain; 4Universidad CompluetensedeMadrid,Farmacology,Madrid,Spain
10.1136/ejhpharm-2023-eahp.66
BackgroundandImportance Baricitinib(BAR)isaJanuskinase inhibitor(JAKi)selectiveforisoenzymes1and2.Itisusedin rheumatoidarthritis(RA)withaninadequateresponsetoconventionalsyntheticdisease-modifyingdrugs(csDMARD).
AimandObjectives Theobjectivewastoevaluatetheeffectiveness,persistence,andadherenceofBARinRAinareal-world setting.
MaterialandMethods Anambispectiveobservationalstudywas designedinathird-levelhospital.PatientswithRAwho startedBARbetweenSeptember2017andJune2021were includedandsignedaninformedconsent.Patientsparticipatinginaclinicaltrialwereexcluded.Patientswerefollowed upuntilDecember2021.EffectivenesswasevaluatedbyvariationoftheDiseaseActivityScore(28-jointcount)usingCreactiveprotein(DAS28PCR);andbythepercentageof patientsachievingtherapeutictarget:lowdiseaseactivity (LDA)(DAS28CRP£3.2)ordiseaseremission (DAS28CRP<2.6).Adherencewasanalysedusingthe5items Compliance-Questionnaire-Rheumatology(CQR5)appliedto patientsevery6months,andthemedicationpossessionratio (MPR).ThestudywasapprovedbytheInstitutionalReview Boardofthehospital.
Results 61patientswereincluded,51/61(83.6%)werefemale. Themeanagewas58.1(15.4)andthemeandiseaseduration was13.9(8.3)years.47/61(77.0%)and43/61(70.5%) patientspresentedanti-citrullinatedproteinantibodiesand rheumatoidfactor,respectively.44/61(72.1%)patientshad priorexposuretobiologicDMARDs.10/61(16.4%)patients wereonBARmonotherapy.Asignificantdecreasewas observedinDAS28PCRfrombaselinetotheendoftreatment/follow-up(3.9(0.9)vs2.7(1.3),adifferenceof1.2, p=0.000).Inaddition,6/61(9.8%)and37/61(60.7%) patientsachievedLDAorremission,respectively.31/61 (50.8%)patientsremainedontreatmentattheendoffollowup,withamedianpersistenceof31.3(14.1-47.7)months.
ThemeanMPRwas0.96(0.08),andallbutonepatientwere
adherent(MPR>0,8).AccordingtotheCQR5,allpatients were ‘goodadherers’
ConclusionandRelevance JAKiarethemostrecentalternative availableforRAtreatment.BARdemonstratedeffectivenessin ourstudycohort,withasignificantdecreaseinDAS28PCR,a highpercentageofpatientsreachingthetherapeutictarget, andapersistenceexceedingtwoyears.Adherencetotreatment wasveryhigh,almost100%.Morestudiesinreal-worldsettingareneededtoconfirmtheseresults.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-022 ADHERENCETOEVOLOCUMABANDITSIMPACTON LDLCHOLESTEROLREDUCTION
BBertrandelisBartolome*,MGonzálezSevilla,JMFerrariPiquero. HospitalUniversitario 12deOctubre,HospitalPharmacy,Madrid,Spain
10.1136/ejhpharm-2023-eahp.67
BackgroundandImportance Accordingtothelatestrecommendations,theneedtoachievelowercholesterollevelshas becomemoreimportant.Therefore,theuseofproteinconvertasesubtilisin/kexintype9(PCSK9)inhibitorshasbeen increasingrecently.
AimandObjectives Toestablishpatients’ adherencetoevolocumabtherapy,aPCSK9inhibitor,andtoanalysethereductionofpatients’ LDL-Clevels.
MaterialandMethods Descriptiveretrospectiveobservational studycarriedoutbetweenJanuaryandDecember2021ina third-levelhospital.Patientswiththreeormoredispensations ofevolocumabwereselected.Thenumberofprefilledpens andthedatewhenitwassuppliedwereconsideredtocalculatecompliance.Demographicsandclinicaldata(prescription andLDL-Cvalues:pre-treatment,after12weeks,andatthe endofthestudy)werecompiledthroughthemedicalrecord. Results 139patientswereincludedinthestudy,79males (57.25%)withamedianageof62.97years(IQR15.53).73 patients(52.90%)wereprescribedduetosecondarypreventionandtheremainderduetofamilialhypercholesterolemia. Allpatientsreceived140mgevery2weeks.
Patientsweredividedintothreegroups(1,2,and3)accordingtotheirmedicationadherence( 90%,75 – 89.99%,and <75%respectively).90patients(65.22%)wereingroup1, 30(21.74%)ingroup2and18(13.04%)ingroup3.
After12weeksoftreatment,areductionofLDL-Clevels byatleast50%wasobservedin71patients(78.79%)from group1,23(76.67%)fromgroup2,and11(61.11%)from group3.Thereductionpercentagemedianswere-69.18% (IQR26.69),-68.64%(IQR28.89),and-54.56%(IQR44.69) respectivelyforeachgroup.Resultsingroup1and2arebetterthanexistingliteraturedata(table1).Group3obtained worseefficacyresults.
Abstract4CPS-022Table1
PhaseIIIclinicaltrial(N)Reductionpercentage(CI95%)
20110114MENDEL-2(614)-58(-60,-55)
20110115LAPLACE-2(1896)-64(-66,-62)
20110117RUTHERFORD2(329)-63(-66,-59)
20110116GAUSS-2(307)-57(-61,-54)
ConclusionandRelevance Lowadherenceseemstodecrease LDL-Creductioncapacity,whilemoderatecomplianceseems tomaintainit.Furtherresearchisrequired,nevertheless,these resultswouldsupportthepossibilityofdecreasingthefrequencyofadministration,favouringtheadherencetotreatmentandreducingcosts.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-026 EFFECTIVENESSOFSODIUMZIRCONIUM CYCLOSILICATEINREDUCINGPOTASSIUM CONCENTRATIONSINHOSPITALISEDPATIENTS
DMarinDelgado*,RPuértolasVal,SGarcíaMuñoz,APupláBartoll,MMendozaAguilera, JMaiquesLlácer,VBosóRibelles,RFerrandoPiqueres. HospitalGeneralUniversitariode Castellón,HospitalPharmacy,Castellón,Spain
10.1136/ejhpharm-2023-eahp.69
4CPS-024 COMPARISONOFCHANGESINCLINICALVALUESOF INTENSIVECAREPATIENTSATVETERANSHOSPITAL ACCORDINGTOVARIOUSFATEMULSIONSFOR PARENTERALNUTRITION
JShin*,SSeob,SLima,SBaek,HJeonga. VHS,Pharmacy,Seoul,Korea-South 10.1136/ejhpharm-2023-eahp.68
BackgroundandImportance Thepurposeofthisstudywasto comparethechangesintheclinicalvaluesoffishoiland non-oil-basedTPNincriticallyillpatientsandtoprovidea clinicalrationaleforTPNadministration.
AimandObjectives Datawerecollectedfromcriticallyill patientswhoreceivedTNA(Fishoil-basedornon-FishoilbasedTNA,thelatterconsistingofolive-soybeanoil-based TNAorsoybeanoil-basedTNA)atVeteransHealthService medicalcentrefrom1June2019to31May2021.
MaterialandMethods Albumin,hsCRP,AST,ALT,TotalBilirubin(TB),PT.INR,WBC,Hb,Hct,PLT,Lymphocyte(LYT) levelswereanalysed.
Results Thisstudycollected224subjects(172fishoil-based TNA,45olivesoybeanoil-basedTNA,and7soybeanoilbasedTNA).Themeanchangesinhs-CRPbeforeandafter TNAinjectionwere-8.71,-47.48,and-33.33intheorderof fishoil,olive-soybeanoil,andsoybeanoil.Thealbuminlevel changeswere-0.26,+0.05,and-0.03,respectively.Other thanthat,therewerenostatisticallysignificantchanges.
Intheolive-soybeanoilgroup,thedecreaseinhs-CRP showedatendencytoincreaseasthenumberofprescription daysincreased.Onlyinthefishoilgroup,astheAPACHE2 scoreincreased,theTB(p<0.01)andAST(p<0.01)tended toincrease,andthethrombocytopeniatendedtoincrease (p<0.01).Intheolivesoybeanoilgroup(p<0.01)andsoybeanoilgroup(p=0.037),theincreaseinINRtendedto increaseastheBMIincreased.Inthefishoilgroup,ALT increasedwithage(p=0.014).
ConclusionandRelevance Asaresultofthestudy,therewas nosignificantdifferenceinclinicalvaluesbetweenthepreparationscontainingfishoilandthepreparationscontainingnonfishoilexceptforhs-CRPandalbumin.Therefore,itis judgedthatconsideringthenutritionalcomponentsandeconomicfeasibilityofTNApreparationswhenadministering TNAwillbehelpfulinimprovingthenutritionalstatusof patientsandreducingtheeconomicburden.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
BackgroundandImportance Hyperkalaemiaisacommonbut hazardouscomplicationinpatientswithchronickidneydisease (CKD).Recentstudiesshowedthatnewresinsareeffectivein reducingpotassiumserumlevelsbutitseffectivenessisusually testedaccordingtostandardrecommendationsofshockand maintenancedoses.Aretheserecommendationsfollowedin clinicalpractice?Ifnot,isitequallyeffective?
AimandObjectives Toevaluatetheuseandeffectivenessof sodiumzirconiumcyclosilicate(SZC)treatmentinroutineclinicalpractice.
MaterialandMethods Observationalandretrospectivestudy carriedoutinatertiarylevelhospitalthatincludedpatients admittedwithhyperkalaemiawhostartedtreatmentwith sodiumzirconiumcyclosilicatesinceDecember2021.
Drug’stechnicaldatarecommendsshockdoseof10g/8h foramaximumof72huntilnormokalaemiaandfromthere, maintenanceregimenwiththeminimumdosethatallowsconcentrationsbetween3.5-5mmol/L.TherapeuticPositioning Reportrecommendsreservingitsuseforpatientswithfailure orintolerancetoexchangeresinslikecalciumpolystyrenesulfonate(CPS).
Results 32patientswithamedianageof83years(IQR14) wererecruited,17men.MainunderlyingcauseofhyperkalaemiawasCKD,78%ofcases.
Only12.5%ofallpatientsreceivedashockregimenof 10g/8h,37.5%received10g/24h,6.3%received5g/8hand 43.8%didnotreceiveshockdose.
Regardingmaintenanceregimen,mostcommondosagewas 5g/24hin59.4%ofthepatients,followedby10g/24hin 9.4%and5g/48hin3.1%.Remaining28.1%didnotreceive amaintenance.44.4%werenevertreatedwithresinsand 3.7%showedintolerancetothem.
Meanpotassiumconcentrationbeforetreatmentwas5.9 ±0.7mmol/L.46.9%ofthepatientsreachedtargetpotassiumlevels(3.5-5mmol/L)at48hoftreatment,15.6%were below3.5mmol/L;and37.5%continuedwithconcentrations above5mmol/L,halfofwhomhadreceivedSZCasasingledose.
ConclusionandRelevance
Asignificantpercentageofpatients didnotreachthepotassiumconcentrationtargetaftertreatmentwithSZC,whichcouldberelatedtothelackofshock dose.Thisagreeswithavailableliterature,whichconcludes thatdoseshigherthan10g/dayleadtoagreaterpotassium depletion.Almostonethirdofpatientshadnotpreviously receivedresinssothemostefficientoptionwasprobablynot usedsince,ascost-comparisonstudiesclaim,CPShasa slightlybettercost-effectivenesscomparedtoSZC.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
1MDGil-Sierra, 2MDPBriceñoCasado*, 1CMoreno-Ramos, 1MABlanco-Castaño, 3CMCuadros-Martinez. 1HospitalUniversitariodePuertoReal,Pharmacy,PuertoReal, Spain; 2HospitalUniversitariodeJerezdelaFrontera,HospitalPharmacy,Jerezdela FronteraCádiz,Spain; 3HospitalUniversitarioJerezdelaFrontera,Pharmacy,Jerezdela Frontera,Spain
10.1136/ejhpharm-2023-eahp.70
BackgroundandImportance Appropriateoptimisationofbiologicagentsinimmune-mediatedinflammatorydiseasescan improvetreatmentefficiencybydecreasingnumberofdrug administrations.
AimandObjectives Toestimateeconomicimpactofoptimising theuseofetanerceptandadalimumabinpatientswith immune-mediateddermatologicalandrheumatologicaldiseases.
MaterialandMethods Adescriptiveretrospectivestudywas conductedbetweenNovember2021andSeptember2022. Patientswithpsoriasis,spondyloarthritis,rheumatoidarthritis andpsoriaticarthritistreatedwithetanerceptoradalimumab therapiesforatleast6monthsuninterruptedlywerescreened. Therapyoptimisationswerequantified.Optimisedtherapies weredefinedastreatmentswithextendeddosingregimensor treatmentdiscontinuationsduetoadequatepathologycontrol. Thesetherapyoptimisationswereappliedaccordingtoamultidisciplinaryprotocolofclinicaldecisionsbasedonbiochemicaltests(includingserumdruglevelsandpresenceofantidrugantibodies),pharmaceuticalinterviewsaboutpatients’ perceptionoftheirdiseasecourseandmedicalcriteria.Data recorded:medicaldepartments,drugs,biochemicaltests,pharmaceuticalinterviews,serumdruglevelsandpresenceofantidrugantibodies.Regardingeconomicimpact,savingsfrombiologicaltherapyoptimisationswereestimatedasthedifference betweencostsofrealdosesusedwithoptimisedregimensand thehypotheticalcostswithdosesusedpriortotreatmentoptimisations.Thenumberofdecreaseddrugadministrationswas estimated.
Results Thisstudyscreened256patients:182ofInternal MedicineDepartmentand74ofDermatology.Distributionof drugswas:171patientsreceivedetanerceptand85adalimumab.Therewere258biochemicaltestand258pharmaceutical interviews(2patientsrequired2biochemicaltestsand2pharmaceuticalinterviews).Serumdruglevelswereoutsidethe optimaltherapeuticrangesofdrugsaccordingtotheliterature in71.6%ofcases.Presenceofanti-drugantibodieswere foundin15patients.Treatmentoptimisationswereperformed in115patients:86(74.8%)ofInternalMedicineDepartment and29(25.2%)ofDermatology.Totaleconomicsavingsassociatedwithoptimisationofbiologicaltherapieswere C¼ 68804.96,C ¼ 53169,58savedinInternalMedicineDepartmentandC ¼ 15635.38savedinDermatology.Theaverage monthlysavingsforthesetreatmentoptimisationswasC ¼ 6255/ month.Numberofdrugadministrationsavoidedwas777.
ConclusionandRelevance Theoptimisationofetanerceptand adalimumabregimensinourpatientswithimmune-mediated dermatologicalandrheumatologicaldiseasesprovidedhigh efficiencybydecreasingthenumberofdrugadministrations.
REFERENCESAND/ORACKNOWLEDGEMENTS
None
ConflictofInterest Noconflictofinterest
4CPS-030 IMPACTOFPHARMACEUTICALPROPOSALSIN MULTIDISCIPLINARYPROGRAMMEFORCLINICAL DECISION-MAKINGINIMMUNE-MEDIATED INFLAMMATORYDISEASES
1MDGil-Sierra, 2MDPBriceñoCasado*, 1CMoreno-Ramos, 1MABlanco-Castaño, 3CMCuadros-Martinez. 1HospitalUniversitariodePuertoReal,Pharmacy,PuertoReal, Spain; 2HospitalUniversitariodeJerezdelaFrontera,HospitalPharmacy,Jerezdela FronteraCádiz,Spain; 3HospitalUniversitariodeJerezdelaFrontera,Pharmacy,Jerezdela Frontera,Spain
10.1136/ejhpharm-2023-eahp.71
BackgroundandImportance Pharmaceuticalproposals(PPs)in amultidisciplinaryprogramme(MP)forimmune-mediated inflammatorydiseasescouldimprovedrugeffectivenessand efficiencyofclinicaldecision-making.
AimandObjectives ToevaluatetheimpactofPPsinaMP forthemanagementofimmune-mediatedinflammatorydermatologicalandrheumatologicaldiseases.
MaterialandMethods Patientswithrheumatoidarthritis (RA),spondyloarthritis,pso riasisandpsoriaticarthritis(PA) receivingetanerceptoradalimumabforatleast6months continuouslywerescreenedinMPduringMay2021to September2022.Internists,dermatologists,pharmacologists andpharmacistscomposedtheMP.Bibliographicsearchon optimaltherapeuticranges(OTRs)ofdrugswasdeveloped andPPprotocolbasedonbiochemicalandclinicalcriteria wasdesigned.Biochemicaltestsonserumdruglevelsand anti-drugantibodieswereprovidedbypharmacists.Pharmaceuticalinterviews(PIs)aboutdiseaseevolutionwereconductedbeforePPs.PPsweretreatmentoptimisation(TO) basedonextendeddosingregim ensortreatmentdiscontinuations,drugswitching(DS) duetolossofeffectiveness,or unchangedtherapy(UT).PatientswithacceptedTOhadtelematicPIsafter1and3months(answers: ‘ goodcourse ’ / ’ milddisease ’ / ’ poorcourse ’ ).Recordeddata:drugs,multidisciplinarymeetings,biochemicaltestandPIs,drugslevels andanti-drugantibodies,typeofacceptedPPsandtelematic PIanswers.
Results MPincluded645patients.Drugsdistribution:51.8% etanerceptand48.2%adalimumab.Therewere25multidisciplinarymeetings.Uptostudycut-offtime,408biochemical testsandPIswereperformed.Resultsofbibliographicsearch presentedadalimumabOTRsof5-8 mg/mLforRAandPA, 3.2-7 mg/mLforpsoriasisand4.6-12 mg/mLforspondyloarthritis.EtanerceptOTRs:2-3 mg/mLforRAandspondyloarthritis,and2-7 mg/mLforPAandpsoriasis.Serumdruglevels wereoutsidetheOTRin72.9%ofpatients.Anti-drugantibodieswerefoundin18patients.PPsacceptedwere305: 183(60%)TO,52(17%)DSand70(23%)UT.Telematic PIsanswers1monthafterTOwere:81.8% ‘goodcourse’ , 3.6% ‘milddisease’ and14.6% ‘ poorcourse ’.At3months were:69.8% ‘goodcourse’,5.7% ‘milddisease’ and24.5% ‘ poorcourse ’ .
ConclusionandRelevance MostofacceptedPPsinourMP (DSandTO)canimproveeffectivenessandefficiencyoftreatmentsforimmune-mediatedinflammatorydiseasesinclinical decision-making.AlmostthreequartersofpatientswithTO presentedgooddiseaseevolution.
None
ConflictofInterest Noconflictofinterest
4CPS-031 THERAPEUTICDRUGMONITORINGOFCEFTAZIDIME/ AVIBACTAMADMINISTEREDBYCONTINUOUS INFUSION:PK/PDTARGETACHIEVEMENTAND
CLINICALOUTCOMES
1DFresan, 2SLuque, 2JMiedes*, 2CBosch, 3ABenítez-Cano, 4LSorlí, 2MDe-Antonio, 5NPrim, 6VVega, 4JPHorcajada, 2SGrau. 1HospitalUniversitariodeNavarra,Pharmacy Service,Pamplona,Spain; 2HospitalDelMar,Pharmacy,Barcelona,Spain; 3HospitalDel Mar,AnaesthesiologyandSurgicalIntensiveCare,Barcelona,Spain; 4HospitalDelMar, InfectiousDiseases,Barcelona,Spain; 5LaboratorideReferènciadeCatalunya,Microbiology Department,Barcelona,Spain; 6LaboratorideReferènciadeCatalunya,Analytical Department,Barcelona,Spain
10.1136/ejhpharm-2023-eahp.72
BackgroundandImportance Ceftazidime/avibactam(CAZ/AVI) isanovelbetalactamantibioticutilisedformulti-drugresistant(MDR)gram-negativebacteria.Therapeuticdrugmonitoring(TDM)ensuresthatCAZ/AVIlevelsachievethe pharmacokinetic/pharmacodynamic(PK/PD)target.Continuousinfusion(CI)hasbeenusedtooptimiseCAZ/AVI pharmacodynamics.
AimandObjectives ToanalysethecorrelationbetweenPK/PD targetattainmentofCAZ/AVIadministeredbyCI,clinicaloutcomesandtoxicity.
MaterialandMethods PatientstreatedwithCAZ/AVIadministeredbyCIandundergoingTDMoftheCAZplasmaconcentrationswereincluded.Definitions:
CAZ/AVIPK/PDtarget:
. timethatCAZfreeconcentrationsremain4timesabovethe minimuminhibitoryconcentration(MIC)ofthecausative pathogen(%fT>4xMIC).
. Overexposure:%fT>10xMIC.
. Clinicalcure:disappearanceofallsignsandsymptomsrelated totheinfectionandnorequirementforadditionalantibiotic treatmentinitiation(exceptaspartofde-escalationstrategy) forthediseasetobeinvestigatedwithin48haftercompletion ofthestudydrug.
. Thirty-dayall-causemortality:deathfromanycauseduring the30daysfollowingtheendoftreatment.
WhenrealMICwasnotavailable,aMICof8mg/Lwas assumed.
Results Thirty-onepatients(28males,median(range)ageof 64(37-78)years)infectedwithextensivelydrug-resistant Pseudomonasaeruginosa andextended-spectrumbetalactamase-producing Klebsiellapneumoniae wereincluded(26directed treatmentsand5empirical).
Twenty-six(83.9%)achievedthePK/PDtarget,15ofwhich presentedoverexposure.Only4(26.6%)overexposedpatients presentedadversereactions(3increasedliverenzymesand1 thrombocytopenia).
Twenty-one(67.7%)patientsachievedclinicalcure,18 (85.7%)ofwhichachievedthePK/PDtarget.Therewasa higherfrequencyofpatientswitha%fT>4xMICthatachieved clinicalcure(18/26(69.2%)inpatientswithclinicalcure vs 2/5(40%)withclinicalfailure,p=0.686).
The30-dayall-causemortalitywas19.4%(6patients).A lowermortalityratewasobservedinpatientsthatdidachieve a%fT>4xMIC(14.8%)inpatientswhosurvivedvs50%in thosewhodied,p=0.096.
ConclusionandRelevance CIseemsausefulstrategytoreach thePK/PDtargetofCAZ/AVI.Fewpatientswithoverexposure presentedadverseevents.Thereseemstobeacorrelation betweenPK/PDtargetattainment,clinicalcureand30-dayall-
causemortalitybutlargerstudieswithbiggersamplesare needed.
4CPS-034 ANALYSISOFTHEEFFECTIVENESSOFSOTROVIMAB INPATIENTSDIAGNOSEDWITHCOVID-19
BSánchezRodríguez,RGazquezPerez,MTGomezSánchez,DGamezTorres,MSánchez Valera,TMorenoDiaz*. HospitalUniversitarioTorrecárdenas,Pharmacy,Almeria,Spain 10.1136/ejhpharm-2023-eahp.73
BackgroundandImportance SotrovimabisindicatedintreatmentofCOVID19inadultsandadolescentswhodonot requiresupplementaloxygenandwhoareatincreasedriskof progressingtoCOVID-severe.Thedrugisadministered accordingtoprioritisationcriteriapublishedbytheSpanish AgencyofMedicinesandHealthProducts(AEMPS)1.
AimandObjectives Toanalysetheeffectivenessofsotrovimab andtoknowtheprofileofpatients.
MaterialandMethods Observational,retrospectiveanddescriptivestudyinatertiarylevelhospital.Patientswhohad receivedsotrovimabfromJanuary/2022-May/2022were included.Variables:sex,age,mild-moderate/severedisease,vaccination-COVID,riskfactors,hospitalisation/deathat29day. EffectivenesswasmeasuredasrateofpatientswithoutprogressiontoCOVID-severe(definedashospitalisation/deathat 29days).Variableswerecollectedfromdigitalmedicalrecords andin-hospitalelectronicprescribing.
Results Thirty-sevenpatientswereincluded,meanage=61 years(21-82),20women(54.05%).Twenty-ninepatients (78.38%)hadmild-moderateCOVID.29patients(78.38%) hadreceivedacompletevaccinationregimen(3doses),6 patients(16.22%)twodosesand2patients(5.41%)not vaccinated.Riskfactors:23hypertension(62.16%),13diabetes(35.14%),5obesity(13.51%)and4asthma (10.81%).Allpatientswereimmunosuppressed.17patients (45.94%)with2riskfactors, 9with3riskfactors (24.32%),7with1riskfactor(18.91%)and2patients (5.40%)with4riskfactors.AccordingtotheAEMPSprioritisationcriteria,allbelongedtothegroupof ‘ Immunocompromisedpersonsandhigh-riskconditions,regardless ofvaccinationstatus ’ .Thehigh-riskconditionswere:23 patients(62.16%)hadrecei vedsolidorgantransplantation withimmunosuppressivetreatment,13patients(35.14%) hadreceivedimmunosuppressivetreatmentwithantiCD20 intheprevious6months(100%rituximab)and1patient (2.7%)wasreceivingactivetreatmentwithmyelotoxicchemotherapy(inotuzumab)foracutelymphocyticleukaemia. 7patients(18.9%)werehospitalised/deadat29days(3 exitus).Allthesepatientshadreceivedrituximab.30 patients(81.1%)didnotprogresstosevereCOVID.Duringthestudyperiod,6patientsattendedtheemergency department,withoutadmission.
ConclusionandRelevance Mostpatientspresentedgood responseandtolerancetotreatment.Thisresultwasindependentofprevioustreatmentsorriskfactors.Previoustreatment withanti-CD20seemstoshowatendencytoprogressionto severeCOVID.Long-termstudiesareneededtoconfirm results
1.https://www.aemps.gob.es/medicamentos-de-uso-humano/acceso-a-medicamentosen-situaciones-especiales/criterios-para-valorar-la-administracion-de-las-nuevasalternativas-terapeuticas-antivirales-frente-a-la-infeccion-por-sars-cov-2/
ConflictofInterest Noconflictofinterest
1CMorenoRamos*, 1MDGil-Sierra, 2MDPBriceño-Casado, 1SFénix-Caballero, 1MABlanco-Castaño. 1HospitalUniversitariodePuertoReal,FarmaciaHospitalaria,Cádiz, Spain; 2HospitalUniversitarioJerezdelaFrontera,FarmaciaHospitalaria,Jerezdela Frontera,Spain
10.1136/ejhpharm-2023-eahp.74
BackgroundandImportance Psoriaticarthritis(PA)isacomplexinflammatorymusculoskeletalandskindisease.Nowadays,thereareseveraltherapeuticoptionstotreatthisdisease. AimandObjectives Toconductindirectcomparisons(ICs) betweenabatacept,brodalumab,guselkumab,ixekizumab, risankizumab,secukinumabandustekinumabusingacommon comparatorinpatientsdiagnosedwithPA.
MaterialandMethods AreviewinPubMedandEuropean MedicinesAgencydatabaseswasperformed.Inclusioncriteria: phaseIIIrandomisedclinicaltrials(RCTs)oftreatmentscited withadouble-blindandplacebo-controlleddesign,which includedpatientspreviouslytreatedwithanti-tumournecrosis factoragents.Exclusioncriteria:RCTwithoutacomparator commontoalternativesconsideredandrecruitingtreatmentnaivepatients.AmericanCollegeofRheumatology50% improvementcriteria(ACR50)at24weeksinRCTswere selectedasendpointtoestimateabsoluteriskreduction(ARR) foreachdrug.WeconductedadjustedICsusingBucher method.ThetherapeuticalternativewiththegreatestmagnitudeofeffectinRCTswasselectedasreferencetherapy.The maximumdifferencewithoutclinicalrelevance(D)wasdefined as±16%accordingtopreviouspublishedliterature.
Results
Sevenstudieswereincluded Alltreatments –exceptabatacept–showedbenefitoverplacebo.Regardingixekizumab80mg monthly(referencetherapy),ARRswere:-4.2%[95%confidenceinterval(CI),-15.43to7.03]vsbrodalumab210mg biweekly;-9.20%[95%CI,-22.53to4.13]vsguselkumab 100mgevery8weeks;-12.20%[95%CI,-32.37to7.97]vs secukinumab300mgmonthly;-13.60%[95%CI,-25.25to1.95]vsrisankizumab150mgevery12weeks;-19.5%[95% CI,-32.30to-6.70]vsustekinumab45mgevery12weeks; and-25.50%[95%CI-37.87to-13.13]vsabatacept125mg weekly.Ixekizumabshowedstatisticallysignificantbenefitcomparedtorisankizumab,ustekinumabandabatacept.Nevertheless,nostatisticaldifferencewasfoundcomparedto brodalumab,guselkumabandsecukinumab.Ixekizumabonly demonstratedaclinicallyrelevantbenefitversusustekinumab andabatacept.
ConclusionandRelevance OurICsprovidecomparativeefficacydatabetweencurrenttherapeuticalternativesforPAin termsofACR50.Nostatisticallysignificantbenefitwas observedbetweenixekizumab,brodalumab,guselkumaband secukinumab.Ixekizumabdidnotshowrelevantclinicalsuperiorityoverbrodalumab,guselkumab,secukinumaband
risankizumab.Theseresultscouldpromotepricecompetition betweenthesedrugsandimprovetheefficiencyofPA treatments.
4CPS-038 CHALLENGESRELATEDTOTRANSITIONINGFROM HOSPITALTOTEMPORARYCAREATASKILLED NURSINGFACILITY
LVRavn-Nielsen*. HospitalPharmacyofFunen,ClinicalPharmacyDepartmentandResearch Department,OdenseC,Denmark
10.1136/ejhpharm-2023-eahp.75
BackgroundandImportance Withdecreasingnumberofhospitalbeds,morepatientsaredischargedfromhospitalstotemporarycareatskillednursingfacilitiesrequiringhandlingof morecomplexandfrailcitizensinanon-hospitalsetting.
AimandObjectives Weaimedtosystematicallymapchallenges relatedtothetransitionofpatientsfromhospitaltotemporarycareataskillednursingfacilityinrelationto(i)medicationmanagement,(ii)responsibilityofthemedicaltreatment, and(iii)communication.
MaterialandMethods Thisdescriptivestudyincludedmedical orsurgicalpatientsadmittedtohospitalanddischargedto temporarycareataskillednursingfacilityfromMay-December2022.
Results Preliminaryresultsareavailablefor67patients(52% womenandmeanage77years).Anursefromtheskilled nursingfacilityusedinaverageatenminutephonecallto coordinatewithanursefromthehospitalbeforedischarge.In 100%(n=67)ofthepatientsthemedicationtothefirstday sentfromthehospitalwasused,eveniftherein30%(20of 67)wasproblemsduetomissingupdateoftheSharedMedicationRecord,changedstrength,missingorunidentifiedmedication,orotherdiscrepancies.Only58%(n=39)receivedall neededmedicationduringthefirstdayneededforfurther medicationdispensing.Thenursesmadeinaveragethree (range0-10)callsandsentthreeelectronicallycorrespondences perpatientaboutmedicationwithinthefirstfivedays.In36 of60(60%)patientsdidthediscrepancybetweenthedischargenoticefromthenursesandthedischargeletter,not resultinanyfurtheractionfromtheskillednursingfacility. Howeverin38%(n=9)ofthe24patientrecordsthat requiredextraactionfromtheskillednursingfacility,the actioncouldhavebeenavoidedifthenursesfromtheskilled nursingfacilityhadhadthedischargeletter.Fullresultsforan expected200patientswillbeavailableandpresentedatthe EAHPconference.
ConclusionandRelevance Weidentifiedchallengesrelatedto, inparticular,lackofneededmedicationandcommunication. Athirdoftheactionsrelatedtomedicationmanagementwere consideredavoidablewithimprovedpracticesaround communication.
ConflictofInterest Corporatesponsoredresearchorothersubstantiverelationships:Thestudywasfundedbythepublic healthresearchfundoftheRegionofSouthernDenmark.
4CPS-039 CLINICALANDECONOMICIMPACTOFMULTI-
1BMMuñozCejudo*, 2MRCantudoCuenca, 1BCancelaDíez, 1TChinchillaAlarcón, 1MLuqueJimenez, 1MAMoraMora, 1SGarcíaAgudo, 1GGilGonzález-Carrascosa. 1HospitalSanAgustin,Pharmacy,Linares,Spain; 2HospitalUniversitarioVirgendeLas Nieves,Pharmacy,Granada,Spain
10.1136/ejhpharm-2023-eahp.76
BackgroundandImportance Astheaccesstobiosimilarsat competitivepricesincreases,itisnecessarytoevaluatemultiple switchestoprovidedataontheirinterchangeability.Recently, theEuropeanMedicinesAgency(EMA)hasnotifiedthatmedicinesapprovedasbiosimilarsintheEUmaybeprescribed interchangeably.
AimandObjectives Theobjectiveistoassesstheefficiency andsafetyofswitchingfrominnovatoradalimumab(Humira®) tobiosimilaradalimumab(Imraldi®)andsuccessivetoanother biosimilar(Hyrimoz®)inareal-lifesetting.
MaterialandMethods Retrospectiveobservationalstudyconductedina200-bedhospital.Weincludedallpatientswho hadbeentreatedwiththeinnovatoradalimumabbetween1September-2019and31-March-2022andswitchedtotwo adalimumabbiosimilars.Variabl esanalysed:clinicalprescribingservice,disease,patientswhodiscontinuedtreatmentwith biosimilarandreason.Clinicalandeconomicdatawere obtainedfromelectronicmedicalrecordsandmanagement programs.
Results Thefirstswitchfrominnovatoradalimumabtothe firstbiosimilaradalimumabincluded114patients:47.4%prescribedbytherheumatologydepartment,28.0%bythedigestiveunitand24.6%bydermatologists.
Themostfrequentdiseasewasrheumatoidarthritis (26.3%),followingbyCrohn’sdisease(23.7%),psoriasis (17.5%)andpsoriaticarthritis(13.2%).
Thepercentageofpatientsthatdiscontinuedthefirstbiosimilarwas15.8%.While61.1%ofpatientsdiscontinueddue toinefficacy,38.9%hadadverseeffects.Atotalof96patients switchedtwice.Theretentionrateafterthesecondswitchwas 96.9%.Nomajorchangesindiseaseactivitywereobserved.
Inthefirstswitch(January2019),theacquisitioncostin ourhospitalofoneunitoftheoriginaldrugwasC ¼ 431.10, whilethatofthebiosimilar(Imraldi®)wasC ¼ 176.80.Inthe secondswitch(August2019)thepriceofImraldi® wasthe sameandforHyrimoz® wasC ¼ 158.0.Ifweconsiderthemost frequentposologyinourpatients(adosageeverytwoweeks), thefirstswitchresultedinannualsavingsofC ¼ 753,745.20 andthesecondswitchresultedinC ¼ 46,949.76.Themultiswitchingof96patientsresultedinatotalsavingof C¼ 681,682.56.
ConclusionandRelevance Theretentionrateaftermultiple switchesfrominnovatoradalimumabtoadalimumabbiosimilarsishigh.Consideringthismulti-switchingsuccessfulexperiencewithbiosimilarsregardingsafetyandeconomicimpact, interchangeabilitybetweenbiologicalmedicinalproductsshould becommoninclinicalpractice.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-040 ELECTRONICCLINICALDECISIONSUPPORTFOR PHARMACOTHERAPEUTICINTERVENTIONSTO REDUCEANTICHOLINERGICBURDENINOLDER HOSPITALISEDPATIENTS
1BMaat, 2MVanGeel*, 1TLeenders, 1AKeyany, 3DArnoldussen, 4TVanAsseldonk. 1Elisabeth-TweestedenHospital,ClinicalPharmacy,Tilburg,TheNetherlands; 2Utrecht University,DepartmentofPharmacoepidemiologyandClinicalPharmacology,Utrecht,The Netherlands; 3Elisabeth-TweestedenHospital,DepartmentofGeriatricMedicine,Tilburg,The Netherlands; 4Elisabeth-TweestedenHospital,DepartmentofNeurology,Tilburg,The Netherlands
10.1136/ejhpharm-2023-eahp.77
BackgroundandImportance Patients’ anticholinergicburdenis thecumulativeeffectoftakingoneormoreanticholinergic medications.Itisassociatedwithadverseoutcomessuchas falls,cognitiveimpairment,deliriumandincreasedmorbidity, especiallyinelderly.Previousstudiesdemonstratedthathospitalisationmayincreaseanticholinergicburden.Pharmacotherapeuticinterventionssupportedbyelectronicclinicaldecision support(eCDS)mayhavethepotentialtopreventthis.
AimandObjectives Theaimofthisstudywastoinvestigate whethertheanticholinergicburden,expressedasscoreonthe AnticholinergicBurdenScale(ACBscore),inolderhospitalised patientscouldbereducedthroughperformingeCDS-based interventionsduringhospitalisation.
MaterialandMethods
ProspectiveinterventionstudyinApril andMay2022.Studypopulation:patients 65yearswithan ACBscore 8andahospitalstayof 3days.AneCDStool wasusedtodetectpatientsthatmetinclusioncriteria. Patients’ anticholinergicmedicationwasreviewedandintervenedifpossible.Aninterventionconsistedofpharmacist-led advicetothepatient’sattendingphysicianbyphone.Primary outcome:numberandproportionofpatientswhoseanticholinergicburdenwasreducedbytheinterventions.Secondary outcomes:(i)acceptancerateofpharmacotherapeuticinterventionsbyattendingphysiciansand(ii)natureandfrequencyof anticholinergicsideeffects.Descriptivestatisticswereusedto analysetheresults.
Results 208patientswereincluded(44.7%female;meanage 75.7(±6.6)years).Anticholinergicmedicationof43 patientswasreviewedwhichledto43interventionsfor23 patients(53.5%,mean1.87(±0.81)interventionsper patient):7suggestionsfordosereduction(16.3%),4suggestionsforalternativemedication(9.3%)and32suggestions fordiscontinuationofmedication(74.4%).28ofthe43 interventionsweredirectlyacceptedbytheattendingphysician(acceptancerate65.1%)leadingtoatotalACBscore reductionof41pointsi.e.anaverageACBscorereduction of1.46points(±0.79)perintervention.33ofthe43 reviewedpatients(76.7%)experiencedoneormoreanticholinergicsideeffects.Constipationoccurredmostoften (45.2%).
ConclusionandRelevance Anticholinergicburdenwasreduced througheCDS-basedpharmacotherapeuticinterventionsin morethanhalfofreviewedpatientsandacceptancebyattendingphysicianswashigh,indicatingapromisingpotentialfor thisinitiativeinclinicalpractice.
MHernándezSánchez,MDNájeraPérez,JAGutiérrezSánchez,MACarvajalSánhez, PTorranoBelmonte,JIbanez-Caturla*,LFructuosoGonzalez,MGuillénDíaz,PPacheco López,AMartinezOrea. HospitalJoseMaríaMoralesMeseguer,Pharmacy,Murcia,Spain
10.1136/ejhpharm-2023-eahp.78
BackgroundandImportance Migraineaffects15%ofthe world’spopulationsoisnecessarytocarryoutinterventions tohelpimprovethequalityoflifeofpatients.
AimandObjectives Evaluationofqualityoflifeinmigraine patientstreatedwitherenumaborgalcanezumabbeforeadministration,threemonthsafterandoneyearlater.
MaterialandMethods RetrospectivestudyconductedinahospitalbyEQ-5D-5Lquestionnairesbeforedrugadministration, threemonthsandoneyearlater.Thefollowingdatawerecollected:sex,age,educationallevelandqualityoflifedimensions(mobility,self-care,activitiesofdailyliving,pain/ discomfortandanxiety/depression).Patientscompletedthevisualanaloguescale(VAS),graduatedfrom0(worstcondition) to10.
Results Ofthe67patients,45completedthequestionnaire afterthreemonths.16patientsdiscontinuedtreatmentbefore oneyear,sothepercentageofsurveysreceivedwas50%. Meanage48.9years(85.1%women).37.3%hadhighereducationand43.3%primaryorsecondaryeducation.Therest didnotprovidedata.
-Mobilitydimension 76.1%describedmildsymptomsand 2.98%severe.Afterthreemonths:55.22%and2.8%.One yearlater,mildsymptomsdecreased(28.87%).
-Mildself-caresymptomsweredescribedby91.1%and severeby2.98%.Threemonthslater:52.23%mildsymptoms. Oneyearlater:37.31%and0%.
-35.8%reportedmildproblemsinperformingdailyactivitiesand22.38%showedseverity.Threemonthslater,13.4% continuedwithsevereproblems.Atoneyear,mildsymptoms: 29.9%.
-Pain/discomfort severesymptomswere68.6%,threemonths later25.4%andoneyearlater4.5%.Mildsymptomsdidnot improve.
-Severeanxiety/depression initially26.9%.Threemonthslater 13.4%andoneyear6%.Mildsymptomsdecreasedfrom 52.2%to23.9%atoneyear.
OntheVASscale,amedianof5wasobtainedatthe beginningoftreatmentcomparedto6.25threemonthslater and7oneyearlater.
ConclusionandRelevance “Pain/discomfort’’ and ‘’depression/ anxiety ’’ arethemostaffecteddimensions.
Afterthreemonthsandafteroneyear, ‘’pain/discomfort’’ wasthemostimprovedand ‘’activitiesofdailyliving’’ was notaffected.
TheVASscaleshowedanincreaseinqualityoflifeafter threemonthsbyamedianof1.25pointsand2pointsafter oneyear.
Thisquestionnairehelpsustoassessthepatients‘ perspective,althoughwedonotyethavethetotalnumberof surveys.
RPlaPasán*,CRodríguezMoreta,AGanforninaAndrades,MVManzanoMartin,SSiles Morris. HospitalPuertaDelMar,Farmacia,Cádiz,Spain
10.1136/ejhpharm-2023-eahp.79
BackgroundandImportance Nonbullouscongenitalichthyosiformerythroderma(NBCIE)isararediseasethatpredominantlyaffectstheskin,courseswithpalmoplantarkeratoderma anhidrosis,andnaildystrophy.Inseverecases,thereisalopeciaincertainareas.
AimandObjectives Theobjectiveofthisreportistodescribe theoff-labeluseofustekinumabinapatientwithNBCIE,and toassessthesafetyofthetreatmentandtolerabilityprofile.
MaterialandMethods Weranadescriptivestudyof6year oldmanwithNBCIEdiagnosedatthetimeofbirth.The patientpresentedveryitchygeneralisedNBCIEaffecting patient’squalityoflife.Patient’sskinwasentirelyaffected withvisiblerednessofapprox90%ofbodysurface.Patient wentthroughseveraltreatmentswithnosuccess:emollients emulsions,topicalandoralcorticosteroidsandH1antihistamines.
InFebruary2021,anewoutbreakoccurredthatledthe Dermatologydepartmenttorequesttheoff-labeluseof Ustekinumab.
ThePharmacyandTherapeuticsCommitteeapprovedthe treatmentinitiationbasedonthepublishedefficacystudies.
NBCIEpatientshaveadominantprofileofTH17/IL-23and ithaspreviouslybeendemonstratedthesuccessfuluseofustekinumabinpatientsaffectedbyichthyosis.
EfficacywasevaluatedbasedonIASIscheme(Ichthyosis AreaSeverityIndex).
Results Thepatientstartedtreatmentwithustekinumabin May2021withadoseof1.2mg/kg.Dosingwasprovidedby theHospitalPharmacyDepartmentadjustedasperpatient’ s weight.Thefirst2months,thepatientreceivedmonthly doseswhilehereafterthedosingwasadjustedtoabi-monthly frequency.
Atthe3rd monthoftreatment,thepatientobservedamild improvementwhilepresentingdiffusederythemawithscaling androughskininthefolds,withanimpactof60%ofbody surface.Atmonth6,afourthdoseof27,6mgofustekinumabweight-adjustedwasprovided.Thereafter,thepatient observedasignificantimprovementthatdirectlyaffectedhis qualityoflife,achievinga60%reductionextensionaccording toIASIanda66%reductionerythemaaccordingtoIASIand nosideeffectswerereported.
NeverthelessDermatologydepartmentrequesttheoff-label useofdupilumab.
ConclusionandRelevance Resultsdemonstrateustekinumab providesonlypartialresponseatmonth6ofNBCIEtreatment,withmodestbutsignificantimprovementinthe patient’squalityoflifewithagoodsafetyprofile.
4CPS-043
JMiedes*,ARodriguez,JBarceló,DEcheverría,SGrau. HospitalDelMar,Pharmacist, Barcelona,Spain
10.1136/ejhpharm-2023-eahp.80
BackgroundandImportance Nirmatrelvir/ritonavirhasrecently beenapprovedfortreatingCOVID-19,butanelevatedriskof drug-druginteractions(DDI)hasbeenexposed.
AimandObjectives Theaimofthisstudywastoevaluate DDIwithnirmatrelvir/ritonavirandtheroleofhospital pharmacists.
MaterialandMethods Retrospectivestudyinatertiaryhospital betweenMay-September2022.Allpatientsthatreceivednirmatrelvir/ritonavirwereincluded.
Datacollected demographic,age-adjustedCharlsoncomorbidity index,medicaldepartment,concomitantdrugs.AllDDIand pharmacyinterventionswerescreenedandcategorised.
Continuousdataexpressedasmedian(IQR).U-MannWhitneyforcontinuousvariablesandChi-squareforqualitative data.
Results Atotalof48patientswith350concomitantdrugs wereselected.Female24(50%),age69(24-95)years,CHARLSON5(0-12).
DDIweredetectedin26(54.2%)patientsandin52 (14.9%)drugs.Seven(0-16)concomitantdrugsperpatient.
StatisticalsignificantdifferenceswerefoundwithATCand DDIcategory(p<0.001):cardiovascularsystemdrugshad moreX-categoryDDI(41.7%)andnervoussystemdrugshad moreC-categoryDDI(60.8%).
Haematologydepartmenthadmorepatientspresentingany DDI(23.1%,p=0.047).
NoDDIprovokedanyadverseeventduringtreatmentwith nirmatrelvir/ritonavir.
ConclusionandRelevance AhighriskforDDIwithnirmatrelvir/ritonavirwasfound,althoughmostofthemweremildand noneprovokedanyadverseevent.Cardiovascularsystemdrugs showedthemostsevereDDI.
Haematologypatientsandthosereceivingnervoussystem drugshadhigherprevalenceforDDI.
AlmosthalfofpharmacyrecommendationsweretodiscontinuethedrugpresentingtheDDI.Noneofthepharmaceuticalinterventionsinducedanyadverseeventderivedfromthe modificationofconcomitanttreatmentduringnirmatrelvir/ritonaviradministration.
ConflictofInterest Noconflictofinterest
4CPS-044 EFFECTIVENESSANDSAFETYOFDIFFERENTINITIAL DOSESOFARIPIPRAZOLEINTRAMUSCULARDEPOT MMoraCortes*,MDGil-Sierra,CMDominguez-Santana,MBlancoCastaño,GCano Martinez. HospitalUniversitarioPuertoReal,PharmacyDepartment,PuertoReal,Spain 10.1136/ejhpharm-2023-eahp.81
BackgroundandImportance Aripiprazoleintramusculardepot treatmentisusedformaintenanceofschizophreniainadult patientsstabilisedwithoralaripiprazole.Thisdrugcanbe startedwithsingleordoubleinjection(400or800mg).Real clinicaldataofthesetworegimenscouldberequiredtooptimisetreatments.
AimandObjectives Todescribeefficacyandsafetyoftwodifferentdosesforstartingaripiprazoledepottherapy.
Abstract4CPS-043Table1 21(40.4) 13(25) 5(9.6) 4(7.7) 3(5.8) 2(3.8) 2(3.8) 1(1.9) 1(1.9) MedicaldepartmentwithDDI,n(%) Haematology Oncology Nephrology Pneumology Emergencyroom
H-systemichormonalpreparations 6(23.1) 4(15.4) 3(11.5) 3(11.5) 3(11.5) Pharmacyinterventiononconcomitantdrugs,n(%) Discontinuation Adverseeventsmonitoring Dosereduction Substitution Efficacymonitoring
23(44.2) 15(28.8) 7(13.5) 5(9.6) 2(3.8)
MaterialandMethods Retrospectivedescriptivestudywith periodbetweenMarch2019andJuly2021.Hospitalised patientswhostartedtreatmentwitharipiprazoledepotwere included.Outcomeswerecollectedfrommedicalrecordsand electronicprescriptionprogramme:gender,age,diagnosis, startdoses,oralaripiprazoleconcomitanttreatment,concomitantantipsychotictherapy.Effectivenessendpointwaspercentageofpatientswithrequirementofhospitalisationfor singleanddoubleinjectiongroupswitharipiprazoleintramusculardepottreatmentat12and26weeks.Safetywasevaluatedbytherateofadverseeffects(AEs)andtypesineach scheme.
Abstracts A38 EurJHospPharm 2023;30(Suppl1):A1–A180
anxiety(18.8%),somnolence(18.8%)akathisia(12.5%)and others(24.9%).MostfrequentAEsindoubledosepopulation: akathisia(33%),anxiety(22%),sedation(11%),somnolence (11%)andothers(23%).
ConclusionandRelevance Startwithdoubledosearipiprazole depottreatmentshowedlowerpercentagesofre-hospitalisation thansingle-doseregimenformaintenancetreatmentofpatients withschizophrenia.SimilarAEswereobservedforboth regimens.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-046 AQUALITATIVESTUDYOFFEASIBILITYAND ACCEPTABILITYOFAPHARMACYPRIORITISATION
TOOLKITBYAFRAILTYFOCUSEDMULTIDISCIPLINARY TEAMINANACUTEHOSPITALEMERGENCY DEPARTMENT
EKennedy*,VSilvari. CorkUniversityHospital,Pharmacy,Cork,IrelandRep
10.1136/ejhpharm-2023-eahp.82
BackgroundandImportance Thenumberoffrail,older patientspresentingtotheemergencydepartment(ED)is increasing.Asfrailtyishighlylinkedtomedicationissues,a pharmacyprioritisationtoolkit(PPT),completedbyafrailty multidisciplinaryteam(MDT),iseffectivetoidentifypatients whowouldbenefitthemostfromthefrailtypharmacist’ s medicationreview.
AimandObjectives Toinvestigatefeasibilityandacceptability ofafive-questionPPTbytheMDTafterfourmonthsofuse.
MaterialandMethods Ananonymised,mixedmethodsquestionnaire(open/closedquestions)wasdistributedtotheMDT (consultant,registrarandclinicalnurseingeriatrics,dietician, occupational,physioandspeech&languagetherapists).The questionsaimedatestablishingbarriersandfacilitatorstothe PPT.Thestraightforwardnessofthetoolkitquestionswas rankedusingaLikertscale.Afocusgroupwasheldto expandontheresultsofthequestionnaireandinformfuture worktoenhancethetoolkituse.
Results Of8questionnairescirculated,7werereturned.Barriersidentified,inordermostmentionedthemetoleast:
·difficultyidentifyinghighriskmedications
·lackoffullmedical/medicationhistoryinED
·difficultyinterpretinghandwrittennotes
·timetakentocompletethetoolkit
AlthoughtimetakentocompletethePPTwasabarrier,5 respondentsreportedanaverageof4minutesforcompletion, whichwasdeemedacceptablewhendiscussingatthefocus group.Thegroupagreed,thatsomebarriersarenotmodifiablesuchlackoffullmedical/medicationhistoryinED.The mostcommonfacilitatorwasrecognitionthatthetoolclearly identifieswhenapharmacyreviewisneeded.Furthereducation,self-learningandpracticeofthetool,butalsoupskilling onhighriskmedicationsandfallsrelatedmedications,were consideredpotentialfuturefacilitators. ‘Regularuseofmore than10medications’ wasthemoststraightforwardquestionto answerinthetoolkitwhiletheleastwas ‘Reasonforadmissionpotentiallyrelatedtomedicationsoradmittedwithnonmechanicalfall’
ConclusionandRelevance Thetoolkitwasgenerallyaccepted bytheMDT,theconcisecompletiontimewasconsidered adequatetakingintoaccountthehighprevalenceof
medicationissuesinfrailpatients.Basedontheresponses,furthereducationtothefrailtyMDTisplanned,withmainfocus onrecognitionofhighriskmedicationsandfallsrelated medications.
ConflictofInterest Noconflictofinterest
4CPS-047 IMPACTOFCORTICOSTEROIDONTHEEFFECTIVENESS OFIMMUNOTHERAPY
AGándara*,CAparicio,SFuertes,AForneas,PNTerroba,AFernandez,RPampin, CDuran,CMartínez-Múgica. CabueñesUniversitaryHospital,PharmacyDepartment,Gijon, Spain
10.1136/ejhpharm-2023-eahp.83
BackgroundandImportance Symptomaticmanagementofcancerpatientsofteninvolvestheuseofcorticosteroids.Recently, anassociationhasbeenfoundbetweenbaselinecorticosteroid levelsandtheresponseratetoimmunotherapyinnon-smallcelllungcancer(NSCLC).
AimandObjectives Analysetheimpactofcorticosteroid administrationontheeffectivenessofimmunotherapy.
MaterialandMethods Retrospectiveanddescriptivestudy includingpatientswhoinitiatedimmunotherapybetween2014 and2021forthetreatmentoflocallyadvancedormetastatic stageNSCLC.Patientswithoutaresponseassessmentwere excluded.
Thefollowingvariableswerecollectedandanalysed throughtheoncologypatientmanagementprogrammeandthe electronicmedicalrecord:age,sex,EasternCooperative OncologyGroup(ECOG),histology,drug,durationof treatment.
Accordingtotheirpharmacydispensingrecord,patients wereclassifiedintotwogroups:thosewhohadreceivedcorticosteroidsatdoseshigherthan10mgofprednisoneorequivalentwithintwomonths(beforeorafter)ofimmunotherapy initiationthestartofimmunotherapyandpatientswhodid notreceivecorticosteroidsinthatperiodoftime.
EffectivenesswasassessedbycomparingProgressionFree Survival(PFS)betweenthetwogroupsofpatients.
Results Thestudyincluded144patients(103men)witha meanageof66years.97%ofpatientshadanECOG £ 1at baseline.Intermsofhistology,65%wereadenocarcinomas, 33%wereepidermoidandtheremaining2%wereundifferentiatedNSCLC.47%ofpatientsweretreatedwithpembrolizumab,51%withatezolizumabandtheremaining,26%with nivolumab.
Thecorticosteroidsprescribedwere prednisone(50%),dexamethasone(38%)andmethylprednisolone(3%).
Thegroupofpatientswhoreceivedcorticosteroidshada PFSof3,72months(95%CI;2,76-6),whilethegroupof patientswhodidnotreceivecorticosteroidshadaPFSof 5,52months(95%CI;4,56-9).Thedifferencesfoundwere statisticallysignificant(p=0,021).
ConclusionandRelevance Theuseofcorticosteroidsatdoses higherthan10mgprednisoneorequivalentwithintwo months(beforeorafter)ofimmunotherapyinitiationhasbeen showntoreducePFSofpatientswithNSCLC.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
MMartínez-Pérez,CCastaño-Amores*,MTNieto-Sánchez,MÁUrbano-Fernández, JCabeza-Barrera. HospitalUniversitarioSanCecilio,Pharmacy,Granada,Spain
10.1136/ejhpharm-2023-eahp.84
BackgroundandImportance TheWHOdefinestelemedicineas ‘theprovisionofhealthservices(wheredistanceisadeterminingfactor)byhealthprofessionalsthroughtheuseofinformationandcommunicationtechnologies(ICTs)fortheexchange ofinformationrelevanttodiagnosis,treatment,diseaseprevention,researchandevaluation,andforthecontinuingeducationofhealthprofessionals,withtheultimategoalof improvingthehealthofpopulationsandcommunities’.Telepharmacyispartofthetransformationprocessofourcurrent healthcaresystemthatallowsustoprovidepharmaceutical caretospecificgroupsofpatients,suchasfrailpatientsor thosewhohaveproblemstravelingtothehospital.
AimandObjectives Theaimofthisstudyistodescribeand analysetheimplementationofa telepharmacyconsultation in asecond-levelhospital.
MaterialandMethods ThestudywasconductedfromFebruary 2021toMay2022.Patientswereselectedascandidatestobe includedinthetelepharmacyconsultationforpharmacotherapeuticfollow-up,todetectandresolveanymedication-related problems,toanalyseandimprovepatientadherenceandto checkthatthefollow-upbythemedicalspecialistwas effective.
Results Atotalof262patientswereidentifiedascandidates toparticipateintheprojecttosendmedicationtotheir respectivehealthcentresduetodifficultiesinaccessingour hospital;247patients(94%)wereselectedforregularappointmentsandinterviewsinthetelepharmacyconsultationevery 3,6or12months.Atthetimeoftheconsultation,5.70% (n=15)ofthepatientscouldnotbecontacted.Theaverage telepharmacytimewas12h/monthwithanaverageof15 minutesperpatient.
In86(32.8%)patientsamedication-relatedproblem(MRP) wasdetected:23.2%occurrenceofadverseeffects,22.4%dispensingerrors,9.6%prescriptionerrors,8.0%insufficiently treatedhealthproblems,7.2%pooradherencetotreatment, 4%incorrectadministrationofmedication,0.8%inadequate storageofmedication,24.8%other.
ConclusionandRelevance Telepharmacyinvolvesimproving adherencetotreatmentanditsmonitoring,detectionofpharmacologicalinteractionsorsideeffects.Telepharmacyallows achievinginternaloptimisationofresourcemanagementand careburdenandimprovesaccessibilitytohealthservicesfor patients,byreducingtripstohospital,timeandresourceconsumption.Telepharmacyguaranteesacontinuous,patient-centredcaremodel.
ConflictofInterest Noconflictofinterest
4CPS-050 ANALYSISOFREAL-LIFEUSEOFIBRUTINIBAFTER RELAPSETOCONVENTIONALCHEMOTHERAPYIN PATIENTSWITHCHRONICLYMPHOCYTICLEUKAEMIA
AMartínRoldán,MDMSanchezSuarez,CAlarcón-Payer,CMontero-Vilchez*,AJimenez Morales. VirgendeLasNievesUniversityHospital,PharmacyDepartment,Granada,Spain
10.1136/ejhpharm-2023-eahp.85
BackgroundandImportance Ibrutinibhasrevolutionisedthe treatmentofchroniclymphocyticleukaemia(CLL).Clinical trialdatashowedsimilarsurvivalbetweenpatientsrandomised toibrutiniborchemoimmunotherapywithcrossovertoibrutinibatprogression.
AimandObjectives Outcomeanalysisofthereal-lifeuseof ibrutinibafterrelapsetoconventionalchemotherapyin patientswithchroniclymphocyticleukaemia.
MaterialandMethods Observationalretrospectivestudyof patientstreatedwithibrutinibassecondlinefrom2017to thepresentatatertiarylevelhospital.Clinicalvariables:sex, age,diagnosisdate,comorbidities,EasternCooperativeOncologyGroupscale(ECOG),BinetStagingSystem,cytogenetics (mutationTP53,immunoglobulinheavy-chainvariableregion gene(IGHV),chromosomedelection(11,13,12and17), treatment,duration,response(complete,partial)andrelapse, progression-freesurvival(PFS),adverseeffects,dosemodificationordiscontinuation.Datawasobtainedfromelectronic prescriptionwiththeapplicationPrisma® andelectronichealth recordswithDiraya®.
Results 31patientsweretreatedwithibrutinib(18patientsas secondlineand13asthird).Medianage71years(IQR6578),51.6%male.Medianageofdiagnosis2012(IQR20082014).29.5%ofpatientshadprevioushypertension,23.6% kidneydisease,17.3%diabetesmellitus,11.8%cardiacdiseasesand5.5%respiratorypathologies.41%ofpatientshad BinetStagingA,28.4%Band5.8%C.Allpatientshad ECOG0.TP53mutatedin16patients,15withunmutated IGHV,24with11qnegativeand18with13qand17qnegative.Treatmentsusedasfirstlinewerechlorambucil(9),fludarabine,cyclophosphamideandrituximab(7),bendamustine andrituximab(5).14patientsachievedcompleteresponse,4 partialand7discontinuedduetotoxicity.PFS19.17months. Assecondlineinpatientswithoutibrutinib,themostfrequent treatmentwasbendamustinewithrituximab(50%).Allexcept onestartedwith420mgdose.Mediandurationoftreatment was32months.11patientsreduceddoseduetotoxicity (66.6%diarrhoea,16.6%renalfailureandskintoxicity),7 suspendedindefinitelyduetocardiactoxicityand4temporarilyduetocardiacandgastrointestinaltoxicity.4patientsdied fromcausesotherthanthedisease.Nopatientlostresponse totreatment.
ConclusionandRelevance Treatmentwithibrutinibproved effectivenessassecondorthirdlineinCLL.However,adverse effectsrequiredoseadjustmentsandsometimes discontinuation.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-051 IMPACTOFTHENEWANTIEPILEPTICDRUG MONITORINGPROGRAMMEONTHEACTIVITYOFTHE PHARMACYANDNEUROLOGYDEPARTMENTS
1RJuvanyRoig, 1NMasBauza, 1PCleriesRovira*, 2MFalipCentellas, 1ÍGonzalez Caballero, 1CRiberaPuig, 1CPorredonAntelo, 3RRigoBonnin, 2JXSalaPadró, 1MBBadia Tahull. 1BellvitgeUniversityHospital,Pharmacy,L’hospitaletdeLlobregat,Spain; 2Bellvitge UniversityHospital,Neurology,L’hospitaletdeLlobregat,Spain; 3BellvitgeUniversity Hospital,ClinicalLaboratory,L’hospitaletdeLlobregat,Spain
10.1136/ejhpharm-2023-eahp.86
BackgroundandImportance In2016therapeuticdrugmonitoringprogramme(TDMP)beganfornewanticonvulsantdrugs
(NAD)forinpatientsandoutpatientsatourhospital.Weekly multidisciplinarymeetingswereheldtoreviseout-of-range troughdruglevels(TDL)onepilepticoutpatients,andto makeearlydrugadjustmentinterventions(EDAI)beforetheir scheduledclinicalfollow-up.
AimandObjectives ToevaluatetheimpactofNADTDMPon theactivityofthePharmacyandNeurologydepartments.
MaterialandMethods
Inpatients Quantificationofpharmacokineticinterventions(PI) andpatientsmonitoredbetween2016-2021.
Outpatients ActivityanalysisbetweenJuly2017andMay 2019:TDLrevision,patientsmonitoredandnumberofEDAI made.TDLandEDAIpercentagecalculationforeachdrug.
Results
Inpatients AnticonvulsantdrugPIwere56%ofallPI(6.067 of10.910)duringthestudyperiod.PIofclassicanticonvulsantdrugs(CAD)decreasedfrom934in2016to348in 2021(63%).In2021thepercentageofPIofNADandCAD were27%and16%(602and348outof2.209)respectively.
LevetiracetamandLacosamideaccountedfor63%(380)and 27%(163)ofallmonitoredNAD.RegardingCADmonitoring Valproatewasthemost86%(299)andFenitointheleast4% (15)monitored.
Outpatients 1.096TDLoutof2.324orderedwererevised (47%)whichbelongedto424patientsofatotalof877 monitored(48%).273TDL(25%)ledtoanEDAI,which affected196patients,thatis46%ofrevisedpatientsand 22%ofallmonitoredpatients.MostEDAIsupposedan increaseorreductionofdosage,51%and34%(139and 92outof273)respectively.Lev etiracetam,Perampaneland LacosamidewerethemostmonitoredNAD:26%(286), 13%(145)and10%(110)respectively,andthemost EDAI-prone:29%(79),27%(73)and11%(29) respectively.
ConclusionandRelevance InclusionofoutpatientstoTDMP allowedearlydrugadjustmentofalmosthalfoftherevised patients.
Thecreationofamultidisciplinaryteamthatincludespharmacistsandneurologistswithafocusonactivemonitoringof NADTDLmightbesignificanttobettercareforepileptic outpatients.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-052 LONG-TERMMONITORINGOFUREAASTREATMENT FORHYPONATREMIAASSOCIATEDTOINADEQUATE SECRETIONOFANTIDIURETICHORMONE(ISADH)
PTorrano-Belmonte,LFructuoso-González,JIbanez-Caturla*,MHernandez-Sánchez,
Sanchez,MVentura-Lopez. HospitalMoralesMeseguer,Pharmacy,Murcia,Spain
10.1136/ejhpharm-2023-eahp.87
BackgroundandImportance Normalbloodsodiumlevels(BSL) isbetween136-145mEq/L.ISADHcourseswithhyponatremia,plasmatichypoosmolality,highurineosmolality,andhigh natriuresis.Availabledrugsaredemeclocyclineandlithium, bothnephrotoxic,andvasopressinreceptorinhibitors,suchas tolvaptan,whichareeffectivebuthighlycostly.
AimandObjectives Todescribetheexperienceofuseofurea asanalternativefortreatmentofISADHandresultsmonitoredoneyearaftertreatment.
MaterialandMethods
Retrospectiveobservationalstudyin whichpatientstreatedwithurea(powderfororalsolution) wereanalysedalongtwoyears(January2020-December2021) andoneyearaftertreatment.Datacollectionof:age,sex, quantificationofBSL(atadmission,duringureatherapy,60 daysafterdrugadministrationandoneyearaftertreatment), initialtherapy,durationofureatreatmentandneedoftolvaptanuse.
Results Totalofpatientswas11.Meanagewas82years(7194years).45%werewomen.Averagedurationoftreatment was15days(3-60days).Initialtherapywashypertonicsaline solution,waterrestrictionand/orloopdiureticsorpotassium sparingagents.Onlyonepatientdidnottolerateurea.Dosage wasvariable:in54%was15gdaily,27%15gbidand18% 30gdaily.
Patientswereclassifiedaccordingtoinitialhyponatremia: 27%hadmildhyponatremia(130-135mEq/L),45%moderate (125-129mEq/L),and27%severe(<125mEq/L).
Resultswere
-Mildhyponatremia 66%recoveredBSL,while33%remained mildlyhyponatraemic.
-Moderatehyponatraemia,60%normalisedBSLand20% worsenedtoseverehyponatraemia.20%didnotanalised.
-Severehyponatremia,66%normalisedBSL.33%didnot haveanalyticalcontrol.
45%ofpatientsachievedBSLoncetreatmentended.27% requiredtreatmentwithtolvaptan15mgdaily.
50%ofpatientswithureaasmonotherapymaintainedBSL 60daysafterfinishingtreatmentand81.8%keptnormalBSL afteroneyearoftreatment.Just9%isstillintreatment.
ConclusionandRelevance Mostclinicalguidelinescontemplate ureaasanoptionforhyponatremiaforISADH,butitisnot clearitspreferencerespectotheralternatives.Ureaisshown tobeasafeandmoderatelyeffectiveoption,andalso,more effective.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-053 KOUNISSYNDROMESECONDARYTOMETAMIZOLE:A CASEREPORT
1COrtíJuan*, 1RAguilarSalmeron, 2CEscobarBolaños, 1XLarreaUrtaran, 1MBruguera Teixidor, 1CSubiranaBatlle, 1RSacrestGüell. 1HospitalUniversitariDr.JosepTrueta, PharmacyDepartment,Girona,Spain; 2HospitalUniversitariDr.JosepTrueta,Allergology Department,Girona,Spain
10.1136/ejhpharm-2023-eahp.88
BackgroundandImportance Kounissyndrome(KS)isanacute coronarysyndrome(ACS)triggeredbymastcellandplatelet activationinthecontextofanaphylacticreactions.ThediagnosisofKSrequiresahighindexofsuspicionandshouldbe consideredinpatientspresentingwithACS,plusotherassociatedsymptomssuchaspruritus,rash,urticariaorangioedema, shortlyafteradministrationofanewdrugorpossibleallergic stimulus.
AimandObjectives Todeterminethecontributionofpharmacistinallergicreactions.
MaterialandMethods A75-year-oldpatientwasadmittedtoa regionalhospitalforscheduledsurgeryforanteriorrectus dehiscence.Duringsurgery,coincidingwiththeadministration ofmetamizole,hepresentedhypotension,tachycardiaand decreasedoxygensaturation,sotheinfusionofthisdrugwas
JAGutierrez-Sánchez,MGuillén-Diaz,AMartínez-Orea,PPacheco-Lopez,MACarvajal-immediatelywithdrawn.DespiteadministrationofIVhydrocortisone,hypotensionanddesaturationpersist.Thepatient begantofibrillateandwentintocardiorespiratoryarrestand cardiopulmonaryresuscitationmanoeuvreswerestarted.The patientrequiredtheadministrationofadrenaline,amiodarone, noradrenaline,atropineanddobutamine.
Results Hewastransferredtoourcentreforintraoperative anaphylacticshockwithtroponinsincreasingfrom41ng/Lto 3144ng/Linthefollowingdeterminationandelevationof serumtryptaseconcentrationto15.4 mg/L,whichsupportsthe suspicionofanaphylaxissecondarytometamizole.Allergology Departmentperformsdiagnosticskintestsforlatexandmetamizoleallergy.Theskintestswereperformedaccordingto internationalguidelinesandincluded15-minutereadingsfor immediatereactions.PharmacyDepartmentperformedthe preparationforskintestssolutionsformetamizole,PRICK (400mg/ml)andintra-dermoreaction(IDR14mg/mland IDR210mg/ml)inthehorizontallaminarflowcabinet.The IDRisonlyperformedonthepatientifthePRICKskintest isnegative.
TheskintestwasnegativeforlatexandpositiveinIDR2 formetamizole.Pyrazoloneallergywasconfirmedandwas probablythecauseofKounissyndrome.
ConclusionandRelevance Drugallergiescansometimescause severereactionssuchasanaphylacticreactionsorKounissyndrome.Theprognosisofthesereactionsdependsonacorrect andimmediatediagnosisandrapidtreatment.
Electrocardiogramsanddifferentlaboratorymarkerssuchas tryptaseandtroponinsareavailablefordiagnosticorientation. SuspectedallergyshouldalwaysbeconfirmedbyallergytestingandthePharmacyDepartmentcanensurecorrect preparation.
ConflictofInterest Noconflictofinterest
AimandObjectives TodescribetheuseofintrathecalL-AmB inCandidameningitisinonepatient.
MaterialandMethods A59-year-oldwomanwithahistoryof obesitywithmetabolicsyndromewasadmittedtotheNeurosurgeryServiceforbilateralcerebellarischemicinfarction needingdecompressivecraniectomy.Duringherevolutionshe presentedasacomplicationCSFfistularequiringlumbar drainingofCSFandsubsequenturgentsurgicalintervention. CSFanalysisrevealedleukocytes1398/mm3,6.38mg/dLof glucoseand315mg/dLofprotein. C.albicans and Nakaseomycesglabrata (previouslynamed C.glabrata )wereisolatedin removedadiposeflapandCSF,respectively.Intravenousand intrathecalantifungaltherapywasrequiredandso,thePharmacyServicewasaskedtodevelopaL-AmBintrathecal injection.
Results TreatmentwithintravenousL-AmB(5mg/kg/day)and oralflucytosine(25mg/kg/6hours)wereinitiated.Afterten days,duetotheinabilityofremovingthelumbardrainand thepersistenceofCNSinfection,L-AmBintrathecalwas added(0.5mg/day,dissolvedin3mLof5%dextrose).Given thegoodevolution,itwasproposedtode-escalatetovoriconazole,flucytosineandintrathecalL-AmB.IntrathecalL-AmB wasdiscontinuedatthe20thdayoftreatmentwhentheCSF cellcount,glucoseandproteinlevelsreturnedtonormallevelsandthelastfourCSFcultureskeptsterile.L-AmBtreatmentwaswelltolerated,andnosideeffectswereobserved. ConclusionandRelevance Despitethelimitationsintheinterpretationofthiscasereport,theadministrationofintrathecal L-AmBmayconstitutealesstoxictherapeuticalternativeto conventionalAmB(deoxycholate)forCandidameningitis.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.DOI:10.1155/2015/340725.
1PGrandaLobato, 1MSánchezdeCastro, 1SGarcíaSánchez, 1SHeinzMorán, 1ÁmyusteGutiérrez, 1PSánchezLópez, 1PPratsOliván, 2GRamírezOlivencia, 1ACorrea Pérez, 1MHGonzaloSalado. 1HospitalCentraldelaDefensaGómezUlla,Pharmacy, Madrid,Spain; 2HospitalCentraldelaDefensaGómezUlla,InfectiousDiseases,Madrid, Spain
10.1136/ejhpharm-2023-eahp.89
BackgroundandImportance AmphotericinB(AmB)isastandardtreatmentforopportunisticfungalpathogenssuchascryptococcalmeningitis.Itstoxicityhasbeenreducedbyusing lipidformulationsofAmb(L-AmB),allowingtheadministrationofhigherdoses.However,AmBshowsslowandpoor penetrationtothecerebrospinalfluid(CSF)whenadministeredbyintravenousinjection.Toachievehigherconcentration inCSF,intrathecaladministrationofL-AmBhasbeensuccessfullyused.AppearanceofdifferentCandidaspeciesinCSF areinfrequentbutcritical.Therearestillsignificantknowledge gapsinpharmacodynamicsandpharmacokineticsastheexperienceofcentralnervoussystem(CNS)Candidainfections treatedwithL-AmBintrathecalliteratureislimitedtoone casereport.
4CPS-057 SEARCHINGFORATREATMENTFORPERIPHERAL TISSUEISCHEMIAINNEWBORNS:ACASEREPORT RDíazPerales*,LYunqueraRomero,RSaldañaSoria,CGallegoFernández. Hospital MaternoInfantildeMálaga,UnidaddeGestiónClínicadeFarmacia,Málaga,Spain 10.1136/ejhpharm-2023-eahp.90
BackgroundandImportance Peripheraltissueischemia(PTI)is acomplicationofvascularcatheterisationinnewborns.Conservativemeasuresareofteninsufficient.Topicalnitroglycerin hasbeenusedoff-labelassalvagetherapy.Wewererequested 2%topicalnitroglycerinointment(TNO)asacompounding formulaforPTIintwoprematures.Duetotheshortageof rawmaterial,aneffectiveandsafealternativehadtobe soughtout.
AimandObjectives Toidentifyanalternativefor2%TNOas therapyforPTI,andthus,todescribetheeffectivenessand securityofapplying0,4%rectalnitroglycerinointment(RNO) intheaffectedareas.
MaterialandMethods ProspectivestudybetweenJanuary-June 2022,of2newbornswithPTI.Patient1:female,31+5;2 days,1500g,ecchymosisin4fingers.Patient2:female,24 +6;5days,595g,markednecrosisinthepadsof5fingers.
Themanufacturinglaboratoryandotherhospitalswerecontactedtofindouttheavailabilityoftherawmaterial.Wealso
consultedtheSpanishPharmacyPreparationsandCompoundingGroup,andtheSpanishAgencyforMedicinesandMedicalProducts(AEMPS)databaseforalternatives.We systematicallysearchedMEDLINE,PubMed,Embase,Google Scholar.Aclosefollow-upwascarriedoutincoordination withpaediatricians,andelectronicprescriptionandcomputerisedmedicalrecordswereconsulteddaily,toevaluateeffectivenessandsecurity.
Results Duetothelackofrawmaterialandalternativesinits preparation,othermarketedpharmaceuticalformsofnitroglycerinwereevaluated(intravenoussolution,transdermal patches,sublingualtablets,rectalointmentandsublingual spray).Applicationof0.4%RNOintheaffectedareaswas consideredthemosteffective,safest,easytodose,andquickesttoacquirealternative.
Patient 1:ecchymosiscompletelydisappearedin48hoursof treatment.Noadverseevents,normalcontrolofmethaemoglobin.Goodperfusionwithoutvasoactives.Patient2:10daysto remitthemarkednecrosis.Welltolerated,initialslightdropin bloodpressure,needinganincreaseofdopamine.Lossofthe phalangeswasavoidedinbothpatients.
ConclusionandRelevance Commercialised0.4%RNOinPTI waseffectiveandsafeinlowbirthweightprematurenewborns.However,itisnecessarytobestudiedinmore patients.
Pharmacist’sroleinthepreparation,controlanddispensing ofmedicinesisessential,anditsintegrationinthemultidisciplinaryteamiscrucialtoensureaquickresponseinemergencysituations.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
parametricmethodswereperformedforhospitalstay, expressedbymedian(Q1-Q3).
Results Atotalof286patientswereincluded(105beforegroup,181after-group).Age69.9(12.7)years,women 40.6%.SAPSII28.8(8.1),FEV148.5(19.1).Nosignificant differenceswereobservedbetweengroups.
Pneumonia(18.7%vs18%,[CURB-652vs1,p=0.290]), COPDexacerbation(51.4%vs60%,p=0.351),bronchoaspiration(11.2%vs14%,p=0.120).Beta-lactams62.2%,quinolones31.1%,clindamycin2.1%.
Inafter-group,5.5%patientswereinsemi-criticalunit.No oxygentherapy27.1%,nasalcannula37.6%,ventimask 37.6%,noninvasivemechanicalventilation1.7%.
Hospitalstaywaslowerafterprotocolimplementation:8 (6-13)vs10(7-15)days(p=0.120),alsodaysofintravenous administration:8.3(4.9)vs12.3(19.1)days(p=0.007),days tooraladministration:3.6(2.3)vs5.3(2.9)days(p=0.000), andtreatmentduration:7.7(4.1)vs10.1(4.2)days (p=0.000).Nostatisticalsignificantdifferenceswerefoundin 7-daymortality,30-daymortality,30-dayreadmissionorclinicalcure.Clinicalfailurewasduetosuperinfection(11.1%vs 40%,p=0.165),inadequateempiricaltreatment(77.8%vs 60%,p=0.211)andfailureafterswitchtooraladministration (11.1%vs0,p=0.240).Nodifferencesobservedinthrombophlebitis,catheterbacteremiaorgastrointestinaltoxicity incidence.
ConclusionandRelevance Despitenotachievingstatisticalsignificance,probablybecauseofthelackofstatisticalpower,a 2-dayreductioninhospitalstaywasobserved.
Earlyswitchtooralantibiotherapywassafewithoutan increaseinadverseeventsorworseclinicaloutcome.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
ANTIBIOTHERAPYINPNEUMOLOGY:PROPENSITY SCORE-MATCHEDANALYSIS
1DEcheverría, 1ARodriguez, 2MDomínguez, 2PAusin, 2EBalcells, 1JMiedes*, 3SGómezZorrilla, 3JPHorcajada, 1SGrau. 1HospitalDelMar,Pharmacy,Barcelona,Spain; 2Hospital DelMar,Pneumology,Barcelona,Spain; 3HospitalDelMar,InfectiousDisease,Barcelona, Spain
10.1136/ejhpharm-2023-eahp.91
BackgroundandImportance Sequentialintravenous-to-oralantibiotictherapyhasbeenassociatedtonumerousbenefits.This strategyhasnotbeenstudiedinthecaseofpatientswithlung diseasewhoselateresolutionofrespiratoryfailurecoulddelay theswitchtooraltreatment.
AimandObjectives Theaimofthisstudywastoevaluatethe efficacyandsafetyofearlyswitchtooralantibiotherapyin pneumologydepartment.
MaterialandMethods Quasi-experimentalstudybefore-afterin atertiaryhospital.
TheprotocolwasestablishedbyAntimicrobialStewardship PrograminFebruary2018.
Inclusioncriteria intravenousantibiotictreatmentpotentially modifiabletooraladministrationatthetimeofadmissionto pneumology.Before-groupincludedpatientsfromDecember 2015toFebruary2018.After-groupfromMarch2018.
Dataexpressedbymean(standarddeviation)andabsolute frequencies.Propensityscorematchingadjustedbyage,pulmonaryhypertension,genderandpulmonaryfibrosis.Non-
4CPS-060 EFFECTIVENESSANDECONOMICANALYSISOF WEIGHT-BASEDVERSUSFIXEDDOSINGOF PEMBROLIZUMABINNON-MALLCELLLUNGCANCER 1JIbanez-Caturla*, 1PTorrano-Belmonte, 1LFructuoso-González, 1JAGutiérrezSánchez, 2PPachecoLópez, 1MÁCarvajalSánchez, 1MGuillénDíaz, 1AMartínezOrea, 1JLeónVillar, 1JCTitosArcos. 1HospitalMoralesMeseguer,PharmacyService,Murcia,Spain; 2Hospital RafaelMendez,PharmacyService,Lorca,Spain
10.1136/ejhpharm-2023-eahp.92
BackgroundandImportance Pembrolizumabisananti-PDL-1 monoclonalantibodywidelyusedinavarietyoftumouraldiseases.Initiallyusedinafixed-doseregime,trialsshowedthat aweight-baseddosingwasmorecost-effectiveandthusitwas authorisedbythePharmacyCommitteeinourcentre.
AimandObjectives Tocomparetheeffectivenessofpembrolizumab,eitherasafixed200mgdoseorasaweightbased2 mg/kgdoseeverythreeweeks,incombinationwithpemetrexedandplatinum,usedinthefirstlinesettingastreatment ofnon-squamousnon-small-celllungcancer(NSCLC),witha CPScount<50%.Inaddition,toevaluatetheeconomic impactofthisdosingchange.
MaterialandMethods Retrospective,observational,descriptive studyofallpatientstreatedwithpembrolizumab,pemetrexed andplatinuminnon-squamousNSCLCbetweenJanuary 2018-August2022.Collectedvariables:age,sex,weight,dosing,numberofcycles,bestresponse,progresion-freesurvival (PFS),overallsurvival(OS).Actualcostwascalculated
accountingforeachpatient,cycleandmilligramofpembrolizumabadministered.
Results Forty-sixpatients(38menand8women),witha medianof65yearsold(range39-77)wereincluded.26 patients(57%)receivedaweightbased,2mg/kgdose(WD group).Themedianbodyweightwas73kginbothgroups (range49-105).Overallresponserate(complete/partial response)was60%intheWDand50%intheFDgroup.
PFSwas13.24months(CI95%10-16.5)onaverageinthe WDgroupand13.7months(CI95%8.1-19.1)intheFD group.OSwas18.15months(CI95%14.3-22)intheWD groupand18.16(CI95%12.6-23.6)intheFDgroup.Nosignificantdifferenceswerefoundinthelog-ranktest.
PatientsintheWDgroupreceivedanaverageof148± 27mgofpembrolizumabforamedianof22cycles;the FDgroupreceived200mgandamedianof41cycles. Mostpatients(95%)receivedalowerdosethanthosein theFDgroup.Onaverage,thetotaltreatmentcostper patientwasreducedby34%intheWDgroup.TheestimatedsavedpublicexpenditurewasC ¼ 420852inthis pathology.
ConclusionandRelevance Pembrolizumabweight-baseddosing wasaseffectiveasthefixeddoseregimeandreducedcostsin patientswithNSCLC.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-061 APPLICATIONOFPROPORTIONOFDAYCOVERED (PDC)TOEVALUATEADHERENCEANDPERSISTENCE TOTREATMENTWITHFINGOLIMODINPATIENTS WITHMULTIPLESCLEROSIS
1CBotto*, 2APalazzo, 1MSantonocito, 1GCancellieri, 1EdeLuca, 3SRealmuto, 3DLo Coco, 2PPolidori. 1UniversitàDegliStudiDiPalermo,ScuolaDiSpecializzazioneInFarmacia Ospedaliera,Palermo,Italy; 2AoorVillaSofia – Cervello,UocFarmacia,Palermo,Italy; 3Aoor VillaSofia – Cervello,UocNeurologiaEStrokeUnit,Palermo,Italy
10.1136/ejhpharm-2023-eahp.93
BackgroundandImportance Multiplesclerosis(MS)isa chronic,neurodegenerativediseaseofthecentralnervoussystemwithanunpredictableandpotentiallydisablingcourse. AlthoughthereisnodefinitivecureforMS,thedisease-modifyingdrugs(DMDs)representavailablestrategiestoimprove thepatient’squalityoflifetreatingrelapses,modifyingthediseasecourseandmanagingsymptoms.Therapeuticadherenceis essentialtoobtaintheefficacyofthesetreatments:poor adherencereducesitsclinicaleffectivenesswhichcanadversely impactdiseaseprogression,MS-relatedhospitalisationand mortalityrates.
AimandObjectives Theaimofthisstudywastoevaluate adherenceandpersistencetotherapywithfingolimod,anoral DMD,inpatientsfollowedupbyaMSreferencecentre.
adherence(PDC<40%),partialadherence(PDC=40–79%) andadherence(PDC 80%)1
Results ThestudyfindingsshowedPDCvalues>80%in41 patients(89.1%),40%<PDC<80%in1patient(2.2%)and PDC<40%in4patients(8.7%).Amongthepatientswithlow adherence,twoofthesesuspendeddefinitivelythetreatment withfingolimod,twosuspendedittemporarilyduetobad compliance,whileonewaslostatfollowup.Anyway,41 patientsshowedpersistencetofingolimodtreatmentover365 days.
ConclusionandRelevance Fromthedataobtaineditispossible toassertthattheoraltherapywithfingolimodpresentsgood adherenceandcompliance,veryimportantfactorstogetclinicaleffectivenessofMSpharmacologicaltreatment.Thisstudy showedalsotheimportantroleofhospitalpharmacist, togetherwiththeclinician,inmonitoringmedicationadherence.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.DegliEsposti etal.ClinicoeconOutcomesRes.2022; 14:139–147.
ConflictofInterest Noconflictofinterest
4CPS-062 EVALUATIONANDFOLLOW-UPOFPAEDIATRIC PATIENTWITHSHORTBOWELSYNDROMEON TREATMENTWITHTEDUGLUTIDE:CASEREPORT MTGomezSanchez,FDFernandezGines,BSanchezRodriguez,DGamezTorres, MSanchezValera,TMorenoDiaz*. HospitalTorrecardenas,Pharmacy,Almeria,Spain
10.1136/ejhpharm-2023-eahp.94
BackgroundandImportance Shortbowelsyndrome(SBS)is anunusualdisordercausedbyresectionofapartofthe smallbowel.Frequentlythesepatientsaredependenton totalparenteralnutrition(TPN)becausetheysuffermalnutrition.Long-termTPNisassociatedwithcomplications suchasinfectionsandthr ombosis.Teduglutideisaglucagon-likepeptide(GLP-2)analoguethatincreasestheproliferationofintestinalmucosalcellscausinganincreaseinthe absorptionsurfaceareaanda reductioninthevolumeof TPN.
AimandObjectives Toevaluatetheeffectivenessofteduglutide inaPN-dependentpaediatricpatientwithSBS.
MaterialandMethods Aretrospective,observationaland descriptivestudyoftheonlyreferencedcasewasdesignedto evaluatetheeffectivenessoftreatmentwithTeduglutide.For thispurpose,thereductionofthevolumeofTPNuntilits withdrawalwasanalysed.Thiswithdrawalmustbecomplete toestablishthesuccessoftreatmentwithteduglutide.Data wereextractedfromtheclinicaldatabaseoftheAndalusian HealthSystem(Diraya).
MaterialandMethods
Withtheaimtoevaluatetherapeutic adherenceandpersistence,aretrospectivestudyhasbeenconductedincollaborationwithaneurologyward,byanalysing thefingolimodprescriptionsregisteredina12months’ period (June2021 – May2022).Thisstudyhasinvolved46patients. Datawereobtainedbyconsultinganinformaticprogramindicatingforeachpatient:age,therapystartandeventualend date,switchfromortootherdrugs.Adherencewascalculated asproportionofdayscovered(PDC)andclassifiedinlow
Results ThepatientneededaTPNperday.Thefollowing figureshowsthepercentageofvolumeofTPNvariedevery twomonthsfromthestartoftreatmentinourpatient.The volumeofTPNwas500mLwhenthefirstdoseofteduglutide(0.05mg/kg/day)wasadministered.However,thevolumedecreasedby45.6%(272mL)after14months. Subsequently,volumeincreasesofupto8%(month18) weredetectedduetoadmissionsfordiarrhealcrises.Finally, PNwassuspendedduetomultiplecomplicationsafter22 months.
Abstract4CPS-062Figure1
ConclusionandRelevance Inourcase,thepercentageofTPN volumereductionishighercomparedtootherstudiescollectedinarecentmeta-analysis.1 Moreover,theTPNwas totallywithdrawninlessti methandescribedinsome studies.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.Bioletto,F.,D’Eusebio,C.,Merlo,F.D.,Aimasso,U.,Ossola,M.,Pellegrini,M., Ponzo,V.,Chiarotto,A.,deFrancesco,A.,Ghigo,E.,Bo,S.EfficacyofTeduglutideforParenteralSupportReductioninPatientswithShortBowelSyndrome:A SystematicReviewandMeta-Analysis. Nutrients 2022, 14,796.https://doi.org/ 10.3390/nu14040796
ConflictofInterest Noconflictofinterest
1EJohns*, 2JGodet, 3AAlkanj, 1MBeck, 1LDalMas, 4BGourieux, 2EASauleau, 4BMichel. 1AgenceRégionaledeSantéGrandEst,DirectiondelaQualité-delaPerformanceEtde L’innovation,Strasbourg,France; 2HôpitauxUniversitairesdeStrasbourg,Départementde SantéPublique – GroupeMéthodesRechercheClinique,Strasbourg,France; 3Centrede RechercheEnBiomedecinedeStrasbourg,LaboratoiredePharmacologieEtdeToxicologie Neurocardiovasculaire – FacultédeMédecine,Strasbourg,France; 4HôpitauxUniversitaires deStrasbourg,ServicePharmacie-Stérilisation,Strasbourg,France
10.1136/ejhpharm-2023-eahp.95
BackgroundandImportance Thedigitisationofhospitaldrug prescriptionshasenabledthecollectionofahugeamountof data.Developingpharmaceuticaldecisionsupportsystemsis facilitatedthankstoartificialintelligenceandthecollected data.
AimandObjectives Buildapredictivealgorithmthatcan detectprescriptionsrequiringpharmaceuticalintervention (PI).
randomforeststatisticalmodelareencouraging,yetperfectible,especiallythesensibility.
Anewapproachofmodelconstructionisundergoing includingapharmaco-ontologygatheringthecharacteristicsof thedrugsbasedonthesummariesofproductcharacteristics. ThiswillallowthemodeltolearnthecontextoftheprescriptionleadingtoaPIanddetectPIswithnewdataina similarcontext.Suchpharmaco-ontologyexistsregrouping onlydrug-druginteractions.1
ConclusionandRelevance PharmaceuticaldecisionsupportsystemsusuallypredictPIsthankstorulesdesignedbypharmacists.Ourmodelaimstodetectthesehigh-riskprescriptions thankstomachinelearningandpreviousdatavalidatedby clinicalpharmacistsintheirdailypractice.Theontologywill helpassociateacontexttoeachPIpreviouslydetectedand predictPIsonnewdata.IntegratingthismodelintoprescribingassistancesoftwarewillmakeiteasierforclinicalpharmaciststodetectPIs.
Thepredictivealgorithmdevelopedinourresearchproject isnotasubstituteforpharmaceuticalanalysisofprescriptions. Itisanexpertsystemfortheidentificationofrisksituations thatwillbeintegratedintoateamapproachtoclinicalpharmacypractices.

REFERENCESAND/ORACKNOWLEDGEMENTS
1.CossinS,LebrunL,LobreG,LoustauR,JouhetV,GriffierR,MouginF,DialloG, ThiessardF.Romedi:AnOpenDataSourceAboutFrenchDrugsontheSemantic Web. StudHealthTechnolInform.2019Aug21; 264:79–82.doi:10.3233/ SHTI190187.PMID:31437889.
4CPS-066 BIOLOGICALTREATMENTSUSEDTOTREAT HIDRADENITISSUPPURATIVAINATERTIARYHOSPITAL MMMestreRibot*,RGonzálezGarcía,MTMartínConde,JRRomaMora,NArranz Pascual,DSoyMuner. HospitalClínicIProvincialdeBarcelona,Farmàcia,Barcelona,Spain 10.1136/ejhpharm-2023-eahp.96
BackgroundandImportance Hidradenitissuppurativa(HS)isa chronicinflammatoryskindiseasewhichcausespainful inflamedlesionsintheapocrinegland-bearingareasofthe body,withhighimpactonpatients’ qualityoflife.Treatment isbasedonacombinationofsurgicalandmedicaltherapies, withinbiologicalagentsplayakeyrole.Adalimumabiscurrentlytheonlybiologicapproved,whatleadstouseoff-label biologicaltreatmentswhenadalimumabfails.
AimandObjectives Ourobjectiveistoanalysetheprescription ofbiologicaltreatments,dosagesusedandadherenceinatertiaryhospitaltotreatHS.
MaterialandMethods
Thealgorithmwasdevelopedusing machinelearningtechniques.Datacollectedduringfour yearswereextractedfrompatients ’ recordsfromtheprescriptionassistancesoftwareofaselectedhospital.Various variableswereused,includingPIsgeneratedbyclinical pharmacists.
Results Weused1,961,176drugprescriptions,including 312,591PIs,todevelopthematrixofthepredictivealgorithm inR.Themodelclassifieseachdrugprescriptionaccordingto thepresenceortheabsenceofaPI.Theresultsaftera
MaterialandMethods Medicalchartsofpatientstreatedwith biologicaldrugsforHSwherereviewed.Demographicfeatures (sex,age,weight,height,smokingstatus),clinicalstage(hurley score)andbiologicaltreatmentused –includingdosages,numberofpreviouslinesandadherence– wererecorded.
Results Forty-onepatientswereincluded.Medianagewas43 (IQR30-52)andmedianbodymassindexwas27(IQR2433).Nineteenoutof41hadahurleyscoreof3(H3)and22 hadahurleyscoreof2(H2).Twenty-sevenpatientswereon adalimumab,includingallpatientsH2and5patientsH3.Sixteenoutof27wereon40mgq.wk,and11wereon80mg q.wk.TherestofH3patientswereon:infliximab10mg/kg (4),infliximab7.5mg/kgq.4.wk(1),subcutaneousinfliximab
ConflictofInterest Noconflictofinterest240mgq.wk(2),brodalumab210mgq.wk(3),tocilizumab 8mg/kgq.4.wk(1),guselkumab100mgq.4.wk(2),andustekinumab90mgq.8.wk(1).MedianpreviouslinesinH3 patientswere1(IQR1-3),andfiveofH3patientsdoesnot showasatisfactoryimprovementwithcurrenttreatment.Only 3patientsshowedanadherence<80%totreatmentbasedon recordeddispensations.
ConclusionandRelevance Mostpatientswithmoderateto severeHSdonotrespondatapproveddoseofadalimumab, forcingtousehigherdosesorswitchingtootherbiological treatments,whicharealsousedathigherdosesthanindicated intheSummatyProductCharacteristics.Unfortunately,these treatmentsarenotalwayseffective,andthereisnoconsensus abouthowtomanageit.Itisnecessarytokeepaclosefollow upofthesepatients,lookingforadverseeventsandlackof adherence.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
Hospitalmedicationchangesoccurredin79%ofpatients; 71%werecommunicatedtotheGPand42%tohomecare nurses.
Medicationreviewsrevealed55DRPsin67%ofpatients, mostlyrelatedtomedicationreconciliation,doseor interactions.
Follow-uptelephonecallson23patientsrevealedDRPsin 30%ofthese.
TestinfourGPs:
Seveninterviewswereperformed – oneperGP,threewith thepharmacistsinvolved(mean71minutes).
Clinicalstaffhadpositiveattitudestowardstheintervention andsawtheadvantagesofapharmacistwithashared employment.Economicswereidentifiedasabarrierforfuture implementation.
PharmacistsinsmallerGPclinicshadeasieraccesstocliniciansandfeltamoreintegratedmemberoftheteam.
Thelargerclinicsweremorestructuredandusedtointerdisciplinarycollaboration,allowingthepharmacistmorefreedomtoworkindependently.
4CPS-067 ACROSS-SECTORALPHARMACISTINTERVENTIONFOR PATIENTSINTRANSITIONBETWEENHOSPITALAND
GENERALPRACTICE:APILOTSTUDY
1CASørensen, 2LJeffery, 3JFalhof, 4PHarbig, 5KRoelsgaard, 6SGram, 1COlesen. 1Hospital PharmacyCentralDenmarkRegion,ResearchandDevelopment-ClinicalPharmacy,Aarhus N,Denmark; 2HospitalPharmacyCentralDenmarkRegion,ClinicalPharmacy,Silkeborg, Denmark; 3GeneralPractice,Laegefaellesskabet,Grenaa,Denmark; 4AarhusUniversity, PublicHealth,Aarhus,Denmark; 5RandersRegionalHospital,MedicalDepartment,Randers, Denmark; 6RandersRegionalHospital,Administration,Randers,Denmark
10.1136/ejhpharm-2023-eahp.97
BackgroundandImportance Drug-relatedproblems(DRPs) incross-sectoraltransitionsareoftenseen,primarilydue toinconsistentinformationaboutpatients ‘ medicinesat transfer.
AimandObjectives Totestacross-sectoralpharmacistinterventionforpatientsinhealthcaretransitions.
MaterialandMethods ThestudywasperformedinonehospitalandfourGeneralPractices(GPs).Thepharmacistshad sharedemploymentbetweentheHospitalPharmacyandthe GPs.
Intervention Transition GPtoHospital
Medicationhistory,medicationreconciliation,updatingthe SharedMedicationRecord(SMR).
Transition HospitaltoGP
Medicationreview,overviewofmedicationchanges,followuptelephonecalls,communicationwithGPonDRPs.
TheinterventionwastestedinoneGPandevaluated descriptively.
Afterwards,theinterventionwastestedinfourGPswith differingcharacteristicsandevaluatedqualitatively(semi-structuredinterviews).
Results TestinoneGP:
Transition GPtoHospital(n=14)
TheGPupdatedtheSMRin86%ofpatients.ThemedicationhistoryrevealeddiscrepanciesbetweenSMR-prescriptions andactualmedicationintakein64%ofthesepatients;91% ofdiscrepancieswereeasilysolvedbycorrectingtheSMR.
Transition HospitaltoGP(n=30)
ConclusionandRelevance GPshadlittlefocusonupdatingthe SMRpriortoadmission.Medicationchangesandfollow-up planswerenotalwayscommunicatedtothepatient,GPor homecareatdischarge.
Sharedemploymentwithuniqueaccesstohealthrecordsin bothsectorswasthemostimportanttoolinidentificationand resolutionofDRPs.
TheinterventionwastransferabletootherGPsandwas consideredacceptableandrelevantbyall.
REFERENCESAND/ORACKNOWLEDGEMENTS ConflictofInterest
4CPS-068 CONDUCTIONOFANAUDITTOREDUCETHE ECONOMICLOSSDUETOUNUTILISEDONCOLOGICAL DRUGPREPARATIONS
1MSantonocito*, 1GCancellieri, 1CBotto, 1EDELuca, 2SGambino, 2PPolidori. 1Università DegliStudiDiPalermo,Ssfo-ScuolaDiSpecializzazioneInFarmaciaOspedaliera,Palermo, Italy; 2OspedaliRiunitiVillaSofia – Cervello,UOCFarmacia,Palermo,Italy
10.1136/ejhpharm-2023-eahp.98
BackgroundandImportance Thecostsrelatedtounutilised oncologicaldrugspreparationshavethegreatestimpactonthe expenseofahospital.Inordertoreducewastes,itispossible toactonprocedurethatleadstofailuretoadministeran alreadycompoundedoncologicaldrug.Thisrepresentsaneconomiclossforhospital.
AimandObjectives Theaimofthestudyistoidentifythe reasonsthatledtothefailuretoadministerthecompounded oncologicaldrugs,inordertoreduceerrorsand,wastesand economicloss.
MaterialandMethods Theanalysiswasconductedbetween January-August2022.Datawerecollectedthroughanarray includingprotocol,dosage,wardandreasonfornon-administration.Italsoincludedwhetherthedrughadbeenreused (totallyorpartially)orthrownawayandtheeconomicloss. Toconducttheanalysisanauditwascarriedoutbetween doctors,pharmacistsandnursesaimedatidentifyingboththe reasonsthatcausestheeconomiclossandpossible improvements.
Results Of14.000preparations,92werenotadministered; 27/92weretotallyorpartiallyreused,65/92werethrown awaycausinganeconomiclossofC ¼ 31.461,09.Thereasonsthatledtothenon-ad ministrationweremainlyattributabletotheunsuitableclinicalconditionofthepatientat thetimeofadministration(64%-59/92).In19%(17/92)of casestheadministrationwasnotcarriedoutduetoerrors intheprescribingphase(therapeuticindicationinadequate totheprotocol,absenceofofflabelauthorisation,etc.).In 12%(11/92)ofcases,the causewasinadequatecommunicationbythedepartment(therapyconfirmedintheabsence ofthepatient).5%(5/92)ofcaseswerecausedbyinterruptionofadministrationduetoadversereactionsduringthe infusion.
ConclusionandRelevance Theresultsobtainedhavehighlightedtheinterventionsneeded.Itwouldbeadvisableforthe confirmationofthetherapytotakeplaceonthesamedayas thespecialistvisitandclinicaltests.Inthisway,wasterelated tothepatient‘snon-presentationand/orthepresenceofclinicalconditionsincompatiblewiththeadministrationwouldbe avoided.Itisalsoimportantthatthevalidationofaprotocol iscarriedoutbyatleasttwospecialists(includinganoncologist)inordertoavoidinappropriateprescriptions.
ConflictofInterest Noconflictofinterest
1CRhaymi*, 2AChaibi. 1MohammedVUniversity-FacultyofMedicineandPharmacy, Pharmacy,Rabat,Morocco; 2MohammedVUniversity-FacultyofMedicineandPharmacy, ClinicalPharmacy,Rabat,Morocco
10.1136/ejhpharm-2023-eahp.99
BackgroundandImportance Duringthehealthemergency periodrelatedtotheemergenceoftheCOVID-19pandemic,clinicalpharmacistshaveplayedavitalroleinmitigatingmedicationerrors,especiallyprescriptionerrorsin hospitals.
AimandObjectives Theaimofthisstudywastocarryouta descriptiveanalysisofthepharmaceuticalinterventions(PI)on theprescriptionsofthepatientsoftheCOVIDunitsofour establishment.
MaterialandMethods Aprospectivestudywasconductedon patientswithpositiveCOVID-19statusadmittedtoahospital COVIDunitoveraperiodoffourmonths.Thepharmaceuticalanalysispromptedinterventionstorectifymedicationrelatederrors.
Results Thestudyincluded108patients.Prescriptionanalysis ledto63PIs.Thesexratio(M/F)was0.5inafavourof femalepredominance.Hypertensionwasthemostcommon cardiovasculardisease,affecting34%ofpatients.Mostdrugrelatedproblemswereoverdoseaccountingfor38%(16/63). ThemostcommonPIin40%ofcaseswasdosageadjustment (18/63).Themaindrugclassesconcernedweregeneralantiinfectiveagentsforsystemicuse25%(16/63),followedby corticosteroids23%(15/63)andhydroxychloroquine19%(12/ 63)especiallyintheeventofinteractionwithdrugsthat lengthentheQTinterval.
ConclusionandRelevance Thecommitmentofclinicalpharmacyinsuchapandemicisthereforeimportant.Itspresence hasledtoareductionintheproblemsofdrugprescriptions.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.Noconflictofinterest.
ConflictofInterest Noconflictofinterest
4CPS-072 REAL-WORLDEXPERIENCEINHAEMOPHILIAB PATIENTSAFTERSWITCHINGTOFIXEXTENDEDHALFLIFEUSINGPHARMACOKINETICPOPULATION SOFTWAREANDMONOCOMPARTMENTALMODEL
1JCJuárez-Giménez*, 2OBenítez-Hidalgo, 3JARomero-Garrido, 4CMateos-Salillas, 5SGonzález-Piñeiro, 6JBMontoro-Ronsano. 1HospitalUniveristarioVallD’hebron, Pharmacy,Barcelona,Spain; 2HospitalUniversitarioVallD’hebron,Faramcia,Barcelona, Spain; 3HospitalUniversitariolaPaz,Farmacia,Madrid,Spain; 4HospitalUniversitariolaPaz, Faramcia,Madrid,Spain; 5ComplejoHospitalarioUniversitarioACoruña,Farmacia,A Coruña,Spain; 6HospitalUniveristarioVallD’hebron,Farmacia,Barcelona,Spain
10.1136/ejhpharm-2023-eahp.100
BackgroundandImportance NewstrategieshavebeendevelopedfortheprophylactictreatmentofpatientswithhaemophiliaB(HB)suchasextendedhalf-liferecombinantfactorIX concentrates(rFIXEHL).Theseproductshaveshownfavourablepharmacokineticproperties,attainingahalf-life3-to5foldlongerinrFIXEHLcomparedtostandardFIX concentrates
AimandObjectives Efficiencyofapharmacokinetic-basedtailoredprophylaxis-dosingscheduleversusstandarddosing(DS) iscompared,inHB,treatedwithtworFIX-EHL.
MaterialandMethods Observational,analytical,prospective, multicentrestudy,involvingHBpatients,beingtreatedwith rFIX-EHLlinkedtoalbumin(rFIX-FP)ortofragmentcrystallisable(rFIX-Fc).Demographicandclinicaldata,andDSand dosinginterval(DI)andactualFIXtroughlevelswere recorded.Pharmacokineticcharacterisationwasperformedfollowingbothapopulation(WAPPS-HEMO)andalinearonecompartment(OC)approach.ForeachapproachandrFIX preparation,anestimationofthetimetothetargettrough(5 IUFIX/dL)wasmade.Statisticalanalysiswasperformedby meansoftheStudent-Fishert-test.
Results Fifteenpatientswereincluded,ninebeingtreated withrFIX-FP(meanage,33years;weight60kg),andsix withrFIX-Fc(49years,86kg).MeanDSwas3222UI(SD, 1716)every11,9days(SD,4,4)forrFIX-FPpatients;and 4333UI(SD,606)every14,0days(SD,0,0)forrFIX-Fc patients.TheindividualtailoredDI,fora0,05UI/dLtrough targetwas:applyingOC;13,6days(SD,5,1),-1,8days(SD, 5,9)vsDS,representing240IU/day(SD,136,1)forrFIX-FP (p=0,40),and8,6days(SD,1,2),+5,4days(SD,1,2)vs DS,representing508IU/day(SD,65,6),forrFIX-Fc, (p<0,001).ApplyingWAPPS-HEMO;itwas15,0days(SD, 5,1),-3,1days(SD,5,3)vsDS,217IU/day(SD,114,7),for rFIX-FP(p=0,12),and10,2days(SD,2,5),+3,8days(SD, 2,5)vsDS,449,7UI/day(SD,129,1),forrFIX-Fc, (p=0,012).
ConclusionandRelevance EfficiencyofrFIX-EHLtreatment followingapharmacokinetic-basedtailoredprophylaxis-dosing scheduleversusDSinHBpatients,issignificantlybetter. Dependingonthecommercialpreparation,rFIX-FPorrFIXFc.Daily-adjusteddose,fora5IUFIX/dLtroughtarget, rangesbetween217-240IU/dayforrFIX-FP,or449-508IU/ dayforrFIXFc,accordingtothetwopharmacokinetic approaches(OCandpopulationbased).
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
4CPS-073 ANALYSISOFEFFECTIVENESSANDPOSITIVE PREDICTIVEVALUEOFANTIMICROBIALSTEWARDSHIP ALERTSUSINGACLINICAL-DECISIONSUPPORT
SYSTEM
1MÁAmor*, 1CdeCáceres-Velasco, 1AMelgarejo-Ortuño, 2XGarcía-González, 1EMatillaGarcía, 1BRodríguez-Vargas, 1PBautista-Sanz, 1CAApezteguia-Fernández, 3JAMeleroBermejo, 3MMateos-González, 1RMoreno-Díaz. 1HospitalUniversitarioInfantaCristina, ServiciodeFarmacia,Parla,Spain; 2HospitalGeneralUniversitarioGregorioMarañón, ServiciodeFarmacia,Parla,Spain; 3HospitalUniversitarioInfantaCristina,Serviciode MedicinaInterna,Parla,Spain
10.1136/ejhpharm-2023-eahp.101
BackgroundandImportance Clinical-decisionsupportsystems (CDSS)arecommonlyusedinclinicalpracticetogenerate antimicrobialstewardship(ASP)-alerts,whichcouldhelpimplementevidence-basedrecommendations.
AimandObjectives Toanalyseuse,effectiveness,andpositive predictivevalue(PPV)ofabundleofASPalertsgeneratedby CDSSinafirst-levelhospital.
MaterialandMethods Observational,retrospectivestudy.Inclusioncriteria:ASPalertsgeneratedbetween1November2021 and31August2022.Thebundleofalertsincluded(1)>7 daysofintravenousantimicrobialtherapy(IAT),(2)transition fromIATtooraltherapy,(3)antimicrobialdosageadjustment inrenalimpairment,(4)therapeuticantibioticmonitoring (TAM)and(5)durationofrestrictedantimicrobials(RA)(carbapenems,daptomycin,piperacillin/tazobactam,linezolid,tigecycline,ceftazidime/avibactam,echinocandinsandvoriconazole) >72hours.Totalnumberofgeneratedalerts,numberof patientswithatleastonealertduringtheirhospitalstay,type ofalertandantimicrobialrelatedthattriggeredthealertwere recordedandanalysed.
Effectivenesswascalculatedasaproportionbetweenalerts requiringinterventionandtotalnumberofalerts.PPVwas calculatedasaproportionbetweenacceptedinterventionsand totalnumberofalerts.Bothproportionswereexpressedas percentages(%).
Results Atotalof2,546alerts(on927patients)generated duringthetimeofstudy.Mostfrequentantimicrobialsthat triggeredthealertswere:28.6%piperacillin/tazobactam(727/ 2,546),13.6%meropenem(346/2,546),7.5%linezolid(190/ 2,546),6.7%levofloxacin(171/2,546)and6.2%ceftriaxone (158/2,546).ThetypeofASP-alertgeneratedwas:>7daysof AIT(32.0%),durationofRA>72hours(31.6%),antimicrobialdosageadjustmentinrenalimpairment(19.2%),transition fromIATtooraltherapy(13.2%)andTAM(4.0%).
Theeffectivenesswas14.5%,withaPPVof9.6%.By type,effectivenesswas9.5%(type1),21.1%(type2),11.0% (type3),19.6%(type4)and18.1%(type5).PPVforthese alertswas6.2%(type1),19.9%(type2),9.2%(type3), 11.8%(type4)and8.7%(type5).
ConclusionandRelevance ThemostfrequentlytriggeredASPalertsweredurationofIATandRA,andantimicrobialdosage adjustmentinrenalimpairment.However,thosealertswitha higherPPVweretransitionsfromIATtooraltherapyand TAM.FurtherstudiesareneededtodetermineASP-alertswith ahighereffectivenesstooptimisetheiruseandtoavoidalert fatigue.
4CPS-074 EFFICACYANDSAFETYOFSOTROVIMAB:RESULTSOF ARETROSPECTIVEOBSERVATIONALSTUDYINA FRENCHHOSPITAL
1TBrandin*, 1HCapelle, 2JAllemand, 3LFooCheung, 1CDumazer. 1CentreHospitalier EdmondGarcin,Pharmacy,Aubagne,France; 2CentreHospitalierEdmondGarcin, Infectiology,Aubagne,France; 3CentreHospitalierEdmondGarcin,NursingHome, Aubagne,France
10.1136/ejhpharm-2023-eahp.102
BackgroundandImportance COVID-19resultsinhospitalisationordeathinolderpatientsandthosewithunderlyingconditions.Sotrovimabisamonoclonalantibodythatwas designedtopreventprogressionofCOVID-19inhigh-risk patientsearlyinthecourseofdisease.
AimandObjectives Weaimedtoassesstheefficacyandsafety ofsotrovimabforadultsinfectedwithCOVID-19ina400bedFrenchhospital.
MaterialandMethods Thisisamonocentricretrospective observationalstudyconductedon36patients,whichreceived sotrovimabfromJanuarytoMarch2022inourhospital. Adultpatientswhohadapositiveresultonrt-PCRorantigen SARS-CoV-2testingandanonsetofCOVID-19symptoms withintheprevious5dayswereeligibletotreatmentby sotrovimab.Thepatientswereathighriskofprogression becauseofolderage( 80y)ordiabetes,obesity,chronickidneydisease,congestiveheartfailure,chronicobstructivepulmonarydisease,asthmaandcancer.Allthepatientsprovided writteninformedconsent.
Results Outofthe36patientstreated,meanagewas82.6± 9.5ywith80%patients 75y,BMI25.3±4.7andsexratio 0.3.Almostallofthemwerelivinginnursinghomes(30 patients).83%had 2conditionsconsideredtoberiskfactorsforprogressionofCOVID-19.Themostcommonrisk factorswere:age 80,congestiveheartfailureandcancer.30 patients(83%)hadreceivedacompleteschemaofCOVID19vaccineand18patients(50%)hadalreadybeeninfected withtheSARS-CoV-2virus.Amonghospitalisedpatients, nonewhoreceivedsotrovimabwasadmittedtoICU.Among thoselivinginnursinghome,nonewasadmittedtohospital. Mostofthepatientshadfewsymptoms.3patientshaddiseaseprogressionleadingtolowflowoxygenation.2patients diedthemonthfollowingtheinfection,including1related toCOVID-19.Amongunvaccinatedpatients,33%(2/6)had diseaseprogression.3patientsreceivedcorticosteroid,5anticoagulantand4antibiotictherapy.Adverseeventwas reportedfor1patient(itchyskinreaction)butnonehad seriousadverseevent.
ConclusionandRelevance Sotrovimabreducestheriskofdiseaseprogressiontohospitalisationordeathamongpatients withmild-to-moderateCOVID-19.Thisseemstobelegitin ourstudy,asdoesthesafety,especiallyforelderlypatients. Also,theeffectivenessofthisantibodyagainstdiseaseprogressionappearstobebetterforvaccinatedpatients.
4CPS-075
10.1136/ejhpharm-2023-eahp.103
BackgroundandImportance Weknowthatthelackofpersistenceofthetreatmentaffectsitsefficacyandcanleadtoan increaseinthedose,whichtriggersanincreaseinriskand cost.Knowingthepersistenceoftreatmentwithsecukinumab inpsoriaticarthritis(PsA),ankylosingspondylitis(AS)and psoriasis,couldleadtonewlinesofresearchthatcompare thedifferenttherapeuticalternativesforthesepathologies basedonthecostperpersistenttreatment.
AimandObjectives Todeterminethepersistenceoftreatment withsecukinumabandthecostofpersistenttreatmentinits approvedindications:PsA,ASandpsoriasis.
MaterialandMethods Descriptive,retrospectiveobservational study,whichincludedadultpatientswithPsA,ASandpsoriasistreatedwithsecukinumabbetweenNovember2017and August2021.Thedemographicvariablesofageandsexwere considered.Themainvariableswerethepersistenceofsecukinumabtreatmentandtheannualcostperpersistenttreatment. TreatmentpersistencewasanalysedusingtheKaplan-Meier testforeachindication.Thecostperpersistenttreatmentwas calculatedbasedontheprobabilityofpersistence,whichwas estimatedwiththeareaunderthecurveforeachofthethree curvesobtainedintheKaplan-Meieranalysis.Thesecondary variablescollectedwerediagnosis,durationandinterruption ofsecukinumabtreatmentandpreviouslinesoftreatment.
Results Weincluded138patientswithameanageof52.2± 13.9years,ofwhom67(48.6%)werewomen.ThemeanpersistenceofsecukinumabtreatmentinPsAwas36.5(95%CI 30.7-42.2)months,forASitwas39.7(95%CI34.3-45.2) monthsandinpsoriasisitwas40.4(95%CI35.1-45.7) months.Arelationshipbetweenage,gender,indication,and lineoftreatmentwithsecukinumabpersistencecouldnotbe established.Medianpersistencewasnotreachedforanyof thethreediagnoses.Theannualcostperpersistenttreatment, calculatedbasedontheprobabilityofpersistence,was C¼ 11,064forPsA,C ¼ 8,183forAS,andC ¼ 15,420for psoriasis.
ConclusionandRelevance Meansecukinumabpersistencewas higherforpsoriasiscomparedtoPsAandAS(p>0.05).The highestannualcostperpersistenttreatmentwasforpsoriasis. Morestudieswithreal-lifedataandlargersamplesizesare neededtoestablishthefactorsthatplayakeyroleinthepersistenceofsecukinumabtreatment.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
BackgroundandImportance Doravirineisanon-competitive, non-nucleosidereversetranscriptaseinhibitor(RTI),usedin combinationregimenswithotherantiretroviralsforthetreatmentofHIV-1withoutevidenceofresistancetonon-nucleosideinhibitors.
AimandObjectives Todescribetheclinical-epidemiological characteristicsandtheclinicalandanalyticalevolutionof DORAassociatedwith(ABC/3TC),(DTG)and(RPV).
MaterialandMethods ToassesstheefficacyofDORA,clinical responsewasanalysedthroughfollow-upconsultationsand serologicaltests,measuringviralload(VL),CD4-Tlymphocytes,liverprofile,andrenalfunction.Follow-upwasperformedat2,4and6monthsfromthestartoftreatment.
Results Wefollowedup36patients(31men),withamean ageof53.8years(26-64),20werebeingtreatedwith(ABC/ 3TC+DORA),9with(RPV+DORA)and7(DTG+DORA). 77%weresmokersand7ofthemdiagnosedwithalcohol habit.Atthebeginning,94.4%hadundetectableviralload (VL<50cop/ml),exceptfortwothatshowedVL>10x6cop/ ml,probablyduetonon-complianceorabandonmentoftreatment.VL<50cop/mlwereobservedduringthestudy,except forthosepreviouslymentionedthatachievedamaximum reductionof110and150cop/ml.Allwereclassifiedinstages A2andA3,excepttwoofthemclassifiedasB3.Themost commonsideeffectswerediarrhoea,nauseaand/orvomiting, andmildheadaches.Twoofthemreportedmyalgia,although wesuspectitwasunrelatedtoDORA,astheyweretreated withatorvastatin80mg/24hforhypercholesterolemia.The patientswith(RPV+DORA)camefrom(ABC/3TC+DORA), whowerereplacedbyRPVduetohypercholesterolemia,liver disordersorintakeofPPIsorNSAIDs.ThemeanCD4-Tlymphocytecountwas720/mL(262-1169/mL)andthemeancreatininewasnormalandbetween0.9and1.1mg/dl(laboratory range),exceptfortwopatientswith1.13mg/dland1.29mg/ dl.
ConclusionandRelevance Doravirinehasbeenshowntobea safeandeffectivetherapeuticalternativeforHIV-1infection, especiallyinpatientswithmetabolicdisordersorinteractions withotherdrugs.Theroleofhospitalpharmacistswasto guaranteeadherencetotreatmentandtodocumentthemost frequentsideeffectsbyreportingthemtotheLocalHIV Commission.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.Fichatécnica:https://cima.aemps.es/cima/pdfs/es/ft/1181332001/FT_1181332001. pdf
ThankstoInfectiousdiseaseUnit DORA:doravirine, RPV: rilpivirine, ABC/3TC:abacavir/lamivudine, DTG:dolutegravir, PPIs:proton-pumpinhibitors, NSAIDs:non-steroidalantiinflammatorydrugs ConflictofInterest Noconflictofinterest
4CPS-078 ASSESSMENTOFTHEQUALITYOFAHOSPITAL’S CLINICALTRIALINITIATIONVISIT
1FGomezdeRueda*, 2LRendóndeLope, 3BCancelaDíez. 1VirgenMacarenaUniversity Hospital,HospitalPharmacyExternalPatientsUnit,Seville,Spain; 2VirgenMacarena UniversityHospital,HospitalPharmacy,Sevilla,Spain; 3SanAgustinUniversityHospital, HospitalPharmacy,LinaresJaén,Spain
10.1136/ejhpharm-2023-eahp.104
1ETejedor*, 2JPeralta, 1MAlbanell, 1BGomez, 1DSoy. 1BarcelonaClinicHospital, Pharmacy,Barcelona,Spain; 2BarcelonaClinicHospital,Pharmacy,Barcelona,Spain
10.1136/ejhpharm-2023-eahp.105
BackgroundandImportance Theclinicaltrial(CT)initiation visitisthemeetingdesignedtopreparetheinvestigationalsite thatwillconductthestudy.Thisprocedureisperformedwith thesitepersonnelwhowillassumestudyresponsibilities.The
RSanchez*,MAlmiñana,CCortell,AGarcia,MAnton,AJimenez,BCorpa. Hospital ClinicodeValencia,Pharmacy,Valencia,Spainstaffisdividedintoaninvestigatorteamandapharmacy team.
AimandObjectives Qualityassessmentattheinitiationvisits ofaclinicaltrial.
MaterialandMethods Observational,prospective,single-centre, prospectivestudytoevaluatethequalityatthestartofaCE inatertiarylevelhospital.ThestudyperiodwasfromJune toAugust2022.A16-itemsurveywascarriedout,which includestheaspectstobetakenintoaccountintheperformanceofaCE.Thequestionscollectedwere:investigatorservice,phaseofthetrial,knowledgeofthepresentationand stabilityofthedrug,modeofpreparation,administrationand destructionoftheexperimentalproduct.Whenthetrialmonitor(CRA)knewthequestion,ascore=1wasassignedifhe/ shedidnotknow=0.Thetoolsusedwere:Excel® fordata collection,Fundanet® forEECCregistrationandGoogle Teams® formeetings.Themaximumscoreobtainedwas20 andapoorstartwasconsideredwithscoresbelow13.
Results ThirtyCTonsetswereanalysedduringthestudy period.Themainclinicalservicesunderinvestigationwere: oncology>dermatology>haematology.Thephasesofthe trialstobeinitiatedwere:III(14),II(10),I/IB(6)andIV (0).Themeanqualityscoreobtainedwas16.62.Therewere 4clinicaltrialswithascorebetween10-13and2trialswith ascoreoflessthan10.Thisledtoasecondreviewofthe CEbythesponsor,whichmeantadelayinthestartofthe investigation.
ConclusionandRelevance Althoughmostoftheclinicaltrials metthequalitycriteriaforinitiation,thereisanon-significant proportionwithpoorresults.Inthoseclinicaltrialsthatdo notmeettheminimums,adelayininitiationisnecessaryfor theresolutionofdoubtsonthepartofthesponsor.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.Liu,M.B.andDavis,K.;Chapter6:Monitoring. LessonsfromaHorseNamed Jim:aClinicalTrialsManualfromtheDukeClinicalResearchInstitute.Durham, NC:DukeClinicalResearchInstitute,2001.Print.
ConflictofInterest Noconflictofinterest.
inhalationtechniquemainlybytheirdoctor.Among50participants,30(60%)performedtheinhalationtechniqueincorrectly.Errorsweremostcommonatthestageofslow,full, deepbreathingasrecommendedbytheguidelines(70%),followedbynosprayagitation(15%).
ConclusionandRelevance Ourresultssupportpoorinhaler techniqueinchildrenthatmayhaveadverseconsequenceson therapeuticefficacy.Theeducationalroleoftheclinicalpharmacistisveryimportanttoimprovetheproperuseofthe inhalationtechniqueandthemanagementofpatients.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.Noconflictofinterest.
ConflictofInterest Noconflictofinterest.
4CPS-083 CREATININEANDCYSTATIN-BASEDESTIMATED RENALFUNCTIONINVANCOMYCINMONITORING 1ASCardoso*, 1CDuarteSilva, 1PASilva, 1,2APCarrondo, 1,2JPLopesDaCruz. 1Centro HospitalarUniversitárioLisboaNorte,PharmacyService,Lisbon,Portugal; 2PharmacyFaculty ofUniversityofLisbon,Imed.Ul-ResearchInstituteForMedicines,Lisbon,Portugal 10.1136/ejhpharm-2023-eahp.107
BackgroundandImportance Glomerularfiltrationrate(GFR) isusuallyestimatedbyusingrenalmarkerslikecreatinine(cr) orcystatinC(cysC),butresultsarenotalwaysoverlapping.
AimandObjectives EvaluatetheeffectofusingCockroft-Gault (CGcr)andChronicKidneyDisease-EpidemiologyCollaboration(EPIcr,EPIcysCandEPIcr/cysC)equationsinvancomycin monitoring.
MaterialandMethods Datafromthelast5yearswerecollectedretrospectively.Allpatients(n=34)whohadsimultaneouslycr,cysCandobservedvancomycinconcentrations(Cobs) obtainedwithinarangeof48h(n=47),wereincluded.PharmacokineticBayesianestimationwasperformedwithPKS
1CRhaymi*, 2AChaibi. 1MohammedVUniversity-FacultyofMedicineandPharmacy, Pharmacy,Rabat,Morocco; 2MohammedVUniversity-FacultyofMedicineandPharmacy, ClinicalPharmacy,Rabat,Morocco
10.1136/ejhpharm-2023-eahp.106
BackgroundandImportance Asthmaisarealpublichealth problem.Inhalationtherapyisthemainstayofthemanagementofthischronicdisease.oneofthemostimportantreasonsforfailedtherapyisimproperinhalertechnique.
AimandObjectives Demonstratethemostfrequenterrorsin thetechniqueofinhalationinasthmaticchildrentreatedin ourestablishment
MaterialandMethods Aprospectiveobservationalstudywas conductedwithasthmaticchildrenadmittedtoourestablishmentoveraperiodof2months.Wehavedevelopedanevaluationgridfortheinhalationtechnique.itwasconsidered correctwhenallstepswereperformedcorrectly.
Results Atotalof50patientswereincluded.Theaverageage was3.7years.Inhalersobservedweremetered-doseinhalers. Allpatientsdeclaredhavinghadademonstrationofthe
Abbott®.ForeachpairGFRs/Cobs,thepredictedconcentration (Cp)andthedailydoserequiredtoobtainamaximumand minimumconcentrationof25and15 mg/ml,respectively, weredetermined.Theabsoluteerror(E),E=Cobs-Cp=0,was usedasanindicatoroftheadequacyoftheequationsused. Results EstimatedGFRshowedstatisticallysignificantdifferences(mean±standarddeviation):CGcr=110.6±76.5, EPIcr=97.5±36.3,EPIcysC=42.8±18.6andEPIcr/ cysC=64.3±25.2ml/min/1.73m2 (p<<0.05).
CGcr,EPIcrandEPIcr/cysCequationsoverestimated(E>0) renalfunction:E=1.50±1.53(95%confidenceinterval[CI]: 1.05to1.95),E=1.62±1.35(95%CI:1.22to2.02)and E=0.47±1.14(95%CI:0.14to0.81) mg/ml,respectively. Renalfunctionwasunderestimated(E<0)withEPIcysC,E=1.06±1.54(95%CI:-1.51to-0.60) mg/ml.
Theestimateddifferencesindailydosesrangedfrom100 to1600mg/70Kg/day,consideringCGcrequationasreference. ConclusionandRelevance TheoverestimationofGFRwith equationsdependentoncr,CGcr,EPIcrand,toalesser extent,EPIcr/cysC,wasmarkedinpatientswithabnormally lowcr.Conversely,withEPIcysCequation,whichdependson cysC,abiomarkerindependentofmusclemass,GFRwas underestimated.Thismaybeduetofactorsthatincrease cysC,withoutrenalfunctionimpairment,suchashypertension,corticosteroidtherapyandmalignancy,allcommonin hospitalisedpatients,butpoordatadidnotallowtoexplore thisassociation.
ThedifferencesintheGFRestimatesareclinicallyrelevant ondosingadequacy,beingsuggestivethatinthepresenceof abnormallylowcr,equationswithcysCarepreferred.
Studiesareneededtoidentifythevariablesresponsiblefor theobservedvariability,inordertopreviouslyselectthemost appropriateequationforeachcase.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.TeafordHR,etal.CystatinC:APrimerforPharmacists.Pharmacy.2020Mar;8 (1):35
ConflictofInterest Noconflictofinterest.
4CPS-084 EFFECTIVENESSANALYSISOFPEMBROLIZUMABIN PATIENTSWITHADVANCEDNON-SMALL-CELLLUNG CANCERWITHVERYHIGHVSHIGHPD-L1 EXPRESSION
BSánchezRodríguez,MSánchezValera,RGazquezPerez,PNietoGuindo,TMoreno Diaz*,DGamezTorres. HospitalUniversitarioTorrecárdenas,Pharmacy,Almeria,Spain
10.1136/ejhpharm-2023-eahp.108
BackgroundandImportance Pembrolizumabshowedlonger overallsurvivalcomparedwithchemotherapyinthefirst-line treatmentofadvancednon-small-celllungcancer(aNSCLC)
AimandObjectives ToevaluatetheeffectivenessofpembrolizumabinhighlyexpressingpatientswithaNSCLC,comparing patientswithPD-L1expression 90%(veryhigh)vsthose withPD-L150-89%(high)inatertiaryhospital.
MaterialandMethods Observational,retrospectivestudy.Inclusioncriteria:patientswithNSCLCawithpembrolizumabfrom August2018toAugust2022.Theclinicaldatabaseofthe AndalusianHealthSystem(Diraya),itsanalyticalmodule (Modulab)andthepharmaceuticalvalidationprogram(FarmisOncofarm)wereconsulted.Variablescollected:sex,age, ECOG(initial),smoking(current/past/non-smoker),percentage ofPD-L1expressionanddateofadministration(first/last). StatisticalanalysisusingthenonparametricKaplan-Meier modelwithrandomcensoringstudyingwhetherthereisan increaseinoverallsurvivalprogressioninveryhighversus highPD-L1groups.ACoxregressionmodelwasincludedto analysewhethertherestofthevariablesstudiedaffectoverall survival.
Results 65patientsenrolled,40wereincluded,16withvery highPD-L1and24withhighPD-L1expression(excluded 14patientsPD-L1<50%and11with1singleadministrationofpembrolizumab).73.17%patientsweremalewith medianage43years[80-37]andECOG=1[0-2].39.02% werecurrentsmokers,53.65%wereformersmokersand 4.8%werenon-smokers.Me dianoverallsurvivalinPD-L1 (high)patientswas16.46month svs21.57monthsmedian overallsurvivalinPD-L1(veryhigh)patients.P=0.92is obtainedfromthePD-L1(High)versusPD-L1(veryhigh) survivalcurves.Currentsmokingistheonlyvariablewith p=0.49thatpositivelyaffectstheprobabilityofdeathwith respecttothosestudied(age,sex,ECOG,pastsmoking/ non-smoking,PDL).
ConclusionandRelevance SurvivalresultsinPD-L1patients ( 90%)comparedtolessexpressorswerepositivebutwithoutstatisticallysignificantdifferences.Itcouldbeduetothe smallstudysample.However,themediansurvivalobtainedis consistentwithdatafrompreviousstudies,itwouldbeadvisabletostudythishypothesisinlargercohorts.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
4CPS-088 CORRELATIONBETWEENIMMUNE-RELATEDADVERSE EVENTSANDEFFICACYINNON-SMALL-CELLLUNG CANCERTREATEDWITHNIVOLUMAB
1ACodonalDemetrio*, 1IMendozaAcosta, 1MBlancoCrespo, 1GICasarrubiosLázaro, 1EMartínezRuiz, 1PTardáguilaMolina, 1AMirandaDelCerro, 2LECharaVelarde, 1Pde Juán-GarcíaTorres. 1HospitalPharmacy,HospitalEmergencyDepartment,Guadalajara, Spain; 2Oncologist,HospitalEmergencyDepartment,Guadalajara,Spain
10.1136/ejhpharm-2023-eahp.109
BackgroundandImportance Nivolumab,animmunecheckpointinhibitor,hasshownarelationshipbetweenimmunerelatedadverseevents(irAEs)andefficacyindifferentstudies, althoughthesearenotveryconsistent.
AimandObjectives Theaimwastoassesstheassociation betweenirAEsandtheefficacyofnivolumabinadultswith locallyadvancedormetastaticnon-small-celllungcancer (mNSCLC)afterpriorchemotherapy.
MaterialandMethods RetrospectiveobservationalstudyincludingallpatientswithmNSCLCwhoreceivednivolumab3mg/ kgorflatdoseof240mgeverytwoweeksfromAugust 2015toJune2022inasecond-levelhospital.Datacollected weredemographic(age,sex)andclinical(histology,smoking habit,performancestatus(ECOG),lineoftreatment,response topreviouschemotherapyandirAEs).
Overallsurvival(OS)andprogression-freesurvival(PFS) analysiswasperformedusingKaplan-Meier.Theassociation betweenirAEsandOSwereanalysedbyCoxRegression. Results 67patients(88%men)withamedianageatthe beginningoftreatmentof67years(IQR:59-75)were included.Histologywassquamousin40%ofpatients.The smokinghabitwas:formersmokers(53%),smokers(39%) andnon-smokers(8%).52%presentedanECOG0-1.73% ofthepatientsreceiveditasasecondlinetreatment.Disease ControlRate(DCR)was78%.
MedianOSintheirAEpatientgroupwas12.1months (95%CI7.9-16.3;p<0.05)vs4.4months(95%CI1.9-7.0; p<0.05)inthenon-irAEpatientgroup;hazardratio:0.35, 95%CI0.2-0.6;p<0.05.ThemedianPFSwas8.7months (95%CI0-41.2;p<0.05)vs3.3months(95%CI1.9-4.7; p<0.05),respectively.
SubgroupanalysisoftheassociationbetweenirAEsandOS was:
Abstract4CPS-088Table1
IrAEstypeN=28irAEs(22patients)HR(95%CI)p Pneumonitis100.62(0.3-1.3)0.2 Endocrine60.23(0.1-0.7)0.014 Gastrointestinal60.59(0.2-1.6)0.3 Skin60.2(0.1-0.7)0.008
ConclusionandRelevance InthisstudyourdatasuggestarelationshipbetweenirAEsandincreasedOS,speciallyendocrine andskin.
Asourstudywasobservational,otherfeaturesassex, ECOGorsmokinghabitthatcouldbiasresultswerenotbalancedbetweenthestudygroups.
ConflictofInterest Noconflictofinterest
EFragaBueno*,ACasásMartínez,IRodríguezPenín.
10.1136/ejhpharm-2023-eahp.110
BackgroundandImportance Accordingtothe ‘Globalhealth sectorstrategyonviralhepatitis2016-2021’ publishedbythe WorldHealthOrganization(WHO),oneoftheobjectivesto beachievedbefore2030istodetect90%ofpeopleinfected byHepatitisCvirus(HCV)andprovidetreatmentto80%of them.
AimandObjectives TodescribeandanalysethecurrentsituationofHCV-infectedpatientstreatedwithdirect-actingantivirals(DAAs)inasecond-levelhospital.
MaterialandMethods Aretrospectiveobservationalstudyof allpatientstreatedwithDAAsin2021wasconducted.Data collectedfromtheelectronicalmedicalhistoryandelectronicprescriptionprogram mewere:demographicdata, dateandsettingofdetectionofHCVinfection,coinfection withhumanimmunodeficiencyvirus(HIV)and/orhepatitis Bvirus(HBV),viralload,degreeoffibrosis,previoustreatmentsforHCV,therapeuticoptionused,toleranceand effectiveness).
Results Thirty-sevenpatients(70%men)wereincluded,witha medianageof56years[interquartilerange(IQR):49-65]. Themediantimefromdiagnosistostartoftreatmentwas49 months(IQR:2-145).Only5patients(13%)hadbeenpreviouslytreated.
Diagnosiswasmadebythegeneralpractitioner(25 patients),acarecentrefordrugaddicts(4patients)and externalconsultationsofdifferentspecialties(8patients). ThreepatientswerecoinfectedwithHIV.Regardingthe degreeoffibrosis,F0-F1:19patients,F2:5patients,F3-F4: 12patients(6withcirrhosis).Themedianviralloadatthe startoftreatmentwas3,870,000IU/ml(IQR:1,160,0006,430,000).
Thetherapeuticoptionsusedincludedsofosbuvir/velpatasvir for12weeks(25patients),sofosbuvir/velpatasvirfor24weeks (1patientwithlivercirrhosiswithpreviousdecompensation, pretreatedwithpeginterferon/ribavirin),glecaprevir/pibrentasvir for8weeks(9patients),andledipasvir/sofosbuvir8weeks(2 patients).Therewasnotherapeuticfailurerequiringrescue withanotherDAA.Nopatientsufferedadverseeffectsrelated toantiviraltreatment.
ConclusionandRelevance Mostofthepatientsweredetected throughthescreeningprogramscurrentlyimplementedinthe differentcaresettingsofourhealtharea,whichmayallow achievingtheobjectivesoftheWHO.
Withtheseprogramsanearlydetectionoftheinfection wasachieved,whichleadstolessliverdamage.
Allourpatientsweretreatedaccordingtothepharmacotherapeuticoptionsofficiallyrecognisedasmorecost-effective.
4CPS-091 WHATYOUNEEDTOKNOWABOUTBRUGADA SYNDROMEIFYOUAREAHOSPITALPHARMACIST
RMorenoDiaz*,AMelgarejoOrtuño,MAAmorGarcía,CdeCáceresVelasco,EMatilla Garcia,MPBautistaSanz. HospitalUniversitarioInfantaCristina,HospitalPharmacy,Parla, Spain
10.1136/ejhpharm-2023-eahp.111
BackgroundandImportance Brugadasyndrome(BRS)isarare inheritedheartrhythmdisordercharacterisedbyST-segment elevationandapotentialriskoffatalarrhythmias.Itisadisorderoftransmembraneionchannelsthatpredisposesto arrhythmias.Channelopathiesarepureelectricaldiseasesthat arenotassociatedwithunderlyingstructuralheartdisease, makingearlydiagnosisdifficult.
AimandObjectives ReviewtheliteraturerelatedtocontraindicateddrugsinRBS;createanupdatedlisttofacilitatepharmaceuticalvalidationinthesepatientsandcomparethelist createdwiththeknownlistofQT-modifyingdrugs.
MaterialandMethods AcriticalanalysisofEMBASEand PUBMEDstudieswasperformed.Theterms ‘brugadasyndrome’ AND ‘drugs’ wereused.Includedstudiesmetthefollowingcriteria:reviews,withinthelast5yearsandinhumans only.Thelistofdrugsdescribedonthe brugadadrugs.org websitein2017wasusedasapreliminarybasis.Themedicines finallyidentifiedwereclassifiedintotwogroups,accordingto theirlevelofrisk.Thegroupofcontraindicateddrugs(should notbeusedunderanycircumstances)andgroupofpotentially dangerousdrugs(withinconclusivedata.Useshouldbeevaluatedonanindividualbasis).ThelistofQT-modifyingdrugs wasobtainedfromthe crediblemeds.org websiteandcompared withthelistofdrugsidentifiedforBRS.
Results Ninearticlesmettheinclusioncriteria.Themedicines classifiedinbothgroupsareshownintable.
Abstract4CPS-091Table1
ContraindicateddrugsinBRSPotentiallyhazardousdrugsinBRS
AjmalineAmiodarone
AlapinineAtropine
AcetylcholineBupropion
AmitriptylineCarbamazepine
BupivacaineCybenzoline
ClomipramineCyamemazine
DesipramineClotiapine
DopamineDesflurane
EtacyzineDexamethasone
ErgonovineDiphenhydramine
PhenylephrineDimenhydrinate
FlecainideDisopyramide
LevobupivacaineDobutamine
LithiumDosulepine
LoxapineDoxepin
MethoxamineEtomidate
NeostigminePhenytoin
NorepinephrineFluoxetine
OxcarbazepineFluvoxamine
PilsicainideGlycopyrrolate
PyridostigmineGranisetron
ProcaineImipramine
ProcainamideIndapamide
ComplexoHospitalarioUniversitario deFerrol,Pharmacy,Ferrol,SpainPropafenoneIsoflurane
PropofolIsoprotenerol
RopivacaineKetamine
TrifluoperazineLamotrigine
Lidocaine
Maprotiline
Metoclopramide
Ondansetron
Nitrousoxide
Paroxetine
Perphenazine
Propranolol
Sevoflurane
Sugammadex
Terfenadine/fexofenadine
Thiopental
Thioridazine
Tramadol
Verapamil
Vernakalant
ThedegreeofagreementobtainedwiththelistofQT-modifyingdrugswas29.21%.
ConclusionandRelevance Thelowconcordancewithrespect tothelistofQT-modifyingdrugsmakesitnecessarytodefine aspecificdruglistforpatientswithRBS.Thiscouldimprove thequalityoftreatmentvalidationbythehospitalpharmacist.
ConflictofInterest Noconflictofinterest
4CPS-092 STATISTICALRELATIONSHIPBETWEENBIOMARKERS WITHPROGNOSTICVALUEINANTI-PDL1 TREATMENTSINCANCERPATIENTS
MAToledoDavia*,NLabradorAndujar,ARRubioSalvador,CBlazquezRomero,LTorralba Fernandez,CJimenezMendez,RPrietoGalindo,ADominguezBarahona,PAguado Barroso,EGomezFernandez,PMoyaGomez. HospitalUniversitariodeToledo,Hospitalary Pharmacy,Toledo,Spain
10.1136/ejhpharm-2023-eahp.112
BackgroundandImportance Theprognosticvalueofbiomarkerssuchasneutrophil/lymphocyteratio(NLR),derived neutrophil/lymphocyteratio(dNLR)andplatelet/lymphocyte ratio(PLR)isincreasinglystudied,showingtheirusefulnessin patientswithdifferentanti-PDL1treatmentsinthecontextof oncologicalpathologies.
AimandObjectives Toanalysewhetherthereisastatistical relationshipbetweenthesethreeparametersandtoanalysethe biomarkersandtoanalysetheireffectonsurvival.
MaterialandMethods Observationalandretrospectivestudyin patientstreatedwithpembrolizumabanddiagnosedwithnonsmall-celllungcancer(NSCLC)inatertiarylevelhospital. Demographicvariables(sexandage)werecollected,NLRas neutrophil/lymphocytecount,dNLRasneutrophil/leukocyte/ neutrophilcountandPLRasplatelet/lymphocytecountwere calculated.Progression-freesurvival(PFS)andoverallsurvival (OS)werecalculatedusingtheKaplan-Meiermethodandlogranktestasahypothesistest.Thecut-offpointswere NLR=5,dNLR=3andPLR=200.Spearman’scorrelationtest wasusedtocheckthecorrelationbetweenthethree
biomarkers(previouslythenon-normalityofthesampleswas checkedbyKolmogorov-Smirnovtest).
Results Atotalof74patientstreatedwithpembrolizumab wereregistered,59men(80,8%)and14women,witha medianageof65[83-37]years.Medianneutrophilcountwas 5.45[6.1-1.5]x109neut/L,lymphocytecountwas1.45[3.90.2]x109linf/Landplateletcountwas174.7[56.92-1345] x109platelets/L.Table1showsthesurvivalresultsobtained.
Abstract4CPS-092Table1 ResultsofKaplan-Meiersurvival methodandlog-ranktest
Progression-freesurvival
Spearman’scorrelationtestshowedstatisticalsignificancein therelationshipbetweenthethreebiomarkersshowinga strongassociationbetweenthem,Spearman’scoefficients obtainedareshown:NLR-dNLR0.934(p=0),NLR-PLR 0.697(p=0)dNLR-PLR0.616(p=0).
ConclusionandRelevance Forthethreebiomarkersthereare significantdifferencesinsurvivaloutcomesfortheselected cut-offpoints,offeringprognosticvalueforourpatients. Spearman’stestindicatesthatthereisacorrelationbetween thebiomarkers.
MRodríguezMarín,EDelgado-Silveira,EMora-Rivas,LEMontes-Jovellar-Gonzalez, MMuñoz-Garcia,NVicente-Oliveros,CPalomar-Fernandez,AMAlvarezDiaz*. 1Hospital RamónYCajal,Pharmacy,Madrid,Spain; 2HospitalRamónYCajal,Otorhinolaryngology, Madrid,Spain
10.1136/ejhpharm-2023-eahp.113
BackgroundandImportance OropharyngealDysphagia(OD)is asymptomwherepatientswhopresentitusuallyhavemultiplenutritional,functional,morbidityandqualityoflifecomplications.Itisassociatedwithahigherincidenceof aspirationpneumonia.ODcanbecausedbyadverseeffectsof medications,suchasdopamineantagonists(DA),centralnervoussystemdepressants(CNSD),anticholinergicdrugs,which blocktheactionofacetylcholine,amongothers.
AimandObjectives Toanalysetheprevalenceofpolypharmacy( 5chronicdrugs)andinappropriatedrugs(anticholinergicsandCNSD)inpatientswithOD.Itwasalso
calculatedanticholinergicrisk(AR)usingdifferentanticholinergicscales(AS).
MaterialandMethods Aretrospectiveobservationalstudywas carriedoutinageneraltertiaryhospital.Datafrompatients diagnosedwithODwerecollectedfromtheotorhinolaryngologyconsultationofyears2019-2021.Demographical,clinical andpharmacotherapeuticdatawereobtainedfromtheelectronicmedicalrecord.ARwascalculatedusinganticholinergic scales(AS)withtheanticholinergicburdencalculator(available atwww.anticholinergicscales.es).
Results Sixtypatientswererecruited;4werelowduetonot havingtheirmedicationprescriptionrecord.Ofthe56 remainingpatients,28(50%)weremen.Theaverageagewas 73.2years[14.5-90.3].
Forty-three(76.79%)patientswerepolymedicated.461 drugswereanalysed,finding104(22.56%)potentialmedicationstocauseOD.Ofthese,91(19.74%)weredrugged withAR,13(2.82%)wereCNSDand7(1.52%)wereDA. WhenanalysingtheASscaleitwasfound:that12(21.42%) patientshadahigh-riskAR,15(26.78%)hadmediumrisk loadand3(5.36%)patientshadlowriskARbeingmostly men(56.66%).Themostrepeateddrugwastamsulosin (1.73%).
ConclusionandRelevance Itisobservedthatthereisahigh percentageofpatientswithODarepolymedicated.TheprevalenceofARishigh.AgoodpharmacologicalreviewwithAS mustbecarriedoutandtrytomakeadescription,toreduce theanticholinergicloadandthenumberofdrugs.
ConflictofInterest Noconflictofinterest
CLeGuen*,WAmmor,JClouet,KOSellal,DFeldman,CFronteau,FLindenberg. Nantes UniversityHospital,Pharmacy,Nantes,France
10.1136/ejhpharm-2023-eahp.114
BackgroundandImportance Intravenousadministrationisthe sourceofnumerousidentifiedrisksrequiringperiodicevaluationofprofessionalpractices.InFebruary2022,anobservationalauditinthehaematologyunitwascarriedoutinorder tooptimisetheinfusionsetups.
AimandObjectives Theobjectiveofthisauditistoevaluate theprofessionalpracticesofthenursingteamandthusto implementpermanentcorrectiveactions.
MaterialandMethods Anevaluationgridbasedonthegood infusionpracticesdefinedbythe ‘ObservatoireduMédicament,desDispositifsmédicauxetdel’InnovationThérapeutique’ Centrewasupdatedandvalidatedbyamultidisciplinary group.
InFebruary2022,twopharmacyinternsobserved62drugs administeredbyanalysingtheprescriptionsofallhospitalised patientsintheunit.
Results Regardingtheinfusionconfiguration,only90%ofthe peripheralinfusionlinewereclosedusinganadaptedplug. Nomisusewasobservedontheadministrationofparenteral nutrition.
Regardingflowrateproblems,onlyoneinfusionconfigurationexhibitsaninfusiondripchamberfilledbeyondthemaximumlimit.Interestingly,duringaflow-sensitivedruginfusion
andcontrarytoguidelines,absenceofnon-returnvalvewas observedin9%oftheinfusionconfiguration.
Apotentialriskofdrugincompatibilityhasalsobeenidentifiedwiththecurrentperfusionset-up.
ConclusionandRelevance Theresultsofthisauditappearto beverypositive.Thehaematologyunit,whosenursingteamis awareoftherisksassociatedwiththeadministrationofchemotherapy,isaunitaccustomedtotheavailabilityof pharmacists.
Thisauditallowedustoobservesomeerrorsduringinfusionpractice:inadequateprogrammedflowrate,absenceof plugsandabsenceofnon-returnvalveduringflow-sensitive drugsinfusion.
Inordertoimproveinfusionpractice,anewstandardised infusionset-upwillbeproposedtotheunitincludingnonreturnvalves.Thisset-upshouldmakeitpossibletoreduce therisks,particularlythoserelatedtoflowrateand incompatibilities.
However,thischangeinpracticewillrequiresupportfor theteamsandanewaudittoevaluatetheimpactofthis work.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-096 EVOLUTIONOFSELECTIVEIMMUNOMODULATE
1JBarceló-Vidal, 1XFernández-Sala, 1NCarballo, 2JFloresLe-Roux, 3AAldea-Perona, 3PDíaz-Pellicer, 1JMiedes*, 1OFerrández, 1SGrau. 1HospitalDelMar,Pharmacy, Barcelona,Spain; 2HospitalDelMar,Endocrinology,Barcelona,Spain; 3IMIM
InstitutMar D’investigacionsMèdiques,Pharmacology,Barcelona,Spain
10.1136/ejhpharm-2023-eahp.115
BackgroundandImportance Biologicaltherapyhassupposeda greattherapeuticalprogressonimmunomodulateddiseases. Nevertheless,somepathologieshavenolabelledindication. Therefore,medicationaccessonspecialsituationsareessential andmorefrequent.
AimandObjectives Theobjectiveofthisstudyistoanalyse therequestonimmunomodulatetherapyinspecialsituations amonglastyears.
MaterialandMethods RetrospectivestudyperformedinatertiaryhospitalbetweenJanuary2017-December2021.Off-label (OL)andcompassionateuse(CU)requestsonselectiveimmunomodulatorydrugsreceivedbythePharmacyandTherapeuticscommitteewereincluded(P&T).
Datacollected:number,typeanddrugsrequested,indication,clinicaldepartment,andapprovementbyP&T.Atemporalevolutiononthenumberofrequests,drugsandclinical departmentswasanalysed.Onthosewhichshowedan increase,anexhaustiveanalysiswasperformed.
Results Atotalof95requestswereidentified,78(82.1%)OL and17(17.9%)CU,representinga17.3%(95/549)ofall kindofrequeststotheP&T.Twenty-onedrugsand42differentindicationswereidentified.Eighty-seven(91.6%)were approved;sixweredeniedduetolackofevidenceandtwo duealackoffundingbythenationalhealthsystem.
Maindrugsrequested ustekinumab(18(18.9%)),dupilumab (15(15.8%)),rituximab(14(14.7%)),tofacitinib(9(9.5%)), tocilizumab(7(7.4%)),adalimumab(5(5.3%)).
Requestingclinicaldepartments dermatology(48(50.8%)), digestology(20(21.1%)),rheumatology(18(18.9%)),
nephrology(5(5.2%)),internalmedicine(2(2.1%)),pneumology(1(1.1%)),coronaryunit(1(1.1%)).
AnexponentialincreasewasobservedamongOLandCU duringthestudyperiod(requests/year:8/2017,12/2018,14/ 2019,21/2020,40/2021;y=5.2269e0.3778x,R2=0.9559).
Themaingrowthwasobservedindermatology (y=1.99e0.4277x,R2=0.768)anddigestology(y=1.5x-0,5, R2=0.9375).
Indicationsrequestedbydermatology:atopicdermatitis(15 (31.3%)),hidradenitissuppurativa(8(16.7%))folliculitisdecalvans(4(8.3%)),others(21(43.8%)).
Drugsrequestedbydermatology:dupilumab(15(31.3%)), ustekinumab(5(10.4%)),tofacitinib(4(8.3%)),mogamulizumab(4(8.3%)),adalimumab(4(8.3%)),secukinumab(3 (6.3%)),rituximab(3(6.3%)),infliximab(2(4.2%))and others(8(16.7%)).
Indicationsrequestedbydigestology:ulcerativecolitis(13 (65.0%)),Crohn’sdisease(4(20.0%)),collagenouscolitis(3 (15.0%)).
Drugsrequestedbydermatology:ustekinumab(13 (65.0%)),tofacitinib(4(20.0%)),vedolizumab(2(10.0%)), infliximab1(5.0%)).
ConclusionandRelevance
. Dermatologyperformedhalfofrequests,speciallyinatopic dermatitisandhidradenitissuppurativa,whichhaveobtained moreevidenceontheirtreatmentlastyears.
. Theexponentialincreaseonnumberofrequestsinspecial situations,speciallyoff-labelones,revealstheneedtoincrease theresourcesassignedtoevaluationcommittees.
REFERENCESAND/ORACKNOWLEDGEMENTS
Results 41patients,30ofthemmen,wereincluded.24 treatedwithnintedaniband17withpirfenidone,bothgroups hadamedianageof73yearsold(range54-89).
AveragedifferencefrombasalFVCwas+4,82%at6 months,+1,85%at12m,+1,85%at16mand-6,25%at 24mwithnintedaniband+2,4%at6m,-5,5%at12m,5,5%at16mand-18,5%at24mwithpirfenidone.
Mediandurationoftreatmentwas26monthswithnintedaniband45monthswithpirfenidone.Overallsurvivalwas65 months(CI95%57.5-73.9)onaveragefornintedaniband33 months(CI95%23.4-42.5)forpirfenidone(log-rank p=0.009).
Treatmentwaspoorlytolerated,withahighincidenceof AE(nintedanib:noAE:21%,G1:4%,G2:42%,G3:29%, G4:4%;pirfenidonenoAE:53%,G1:12%,G2:29%,G3: 6%).MostfrequentAEwasgastrointestinalreactionsin17 (71%)withnintedaniband6(35%)withpirfenidone,followedbyheadachein3(13%)withnintedaniband4(24%) withpirfenidone,hepaticenzymealterationin5(21%)with nintedanib,dermatological4(17%)nintedanib,renaltoxicity in2(8%)withnintedanib,haematological1(4%)with nintedanib.
AEcausedthediscontinuationoftreatmentin11(46%) patientswithnintedanibandin4(24%)withpirfenidone.
ConclusionandRelevance Nintedanibwassignificantlymore effectiveintermsofoverallsurvival,withaslowerdecrease inFVC,althoughpresentedworsetolerancethanpirfenidone, astreatmentofIPF.
4CPS-099 NINTEDANIBANDPIRFENIDONEINIDIOPATHIC PULMONARYFIBROSIS:COMPARATIVE EFFECTIVENESSANDSAFETYINATHIRD-LEVEL
HOSPITAL
1JIbanez-Caturla*, 1PTorranoBelmonte, 1LFructuosoGonzález, 1JAGutiérrezSánchez, 1MHernándezSánchez, 2PPachecoLópez, 1MÁCarvajalSánchez, 1MGuillénDíaz, 1AMartínezOrea, 1MDNajera-Perez. 1HospitalMoralesMeseguer,PharmacyService, Murcia,Spain; 2HospitalRafaelMendez,PharmacyService,Lorca,Spain
10.1136/ejhpharm-2023-eahp.116
BackgroundandImportance Idiopathicpulmonaryfibrosis (IPF)isachronicandprogressivediseasecharacterisedbya badprognosis.Theonlyavailablepharmacologicaltreatments aretwoantifibroticdrugs,pirfenidoneandnintedanib,which slowdownthedevelopmentofthediseasebuthavean unfavourablesafetyprofile,withahighincidenceofadverse effects.
AimandObjectives Tocomparetheeffectivenessandsafetyof thetwoavailableantifibroticdrugs,nintedanibandpirfenidone,usedastreatmentofidiopathicpulmonaryfibrosis.
MaterialandMethods Retrospective,observationaland descriptivestudyofallthepatientsdiagnosedwithidiopathic pulmonaryfibrosistreatedwithpirfenidoneornintedanib betweenJanuary2014andFebruary2022.Thecollectedvariableswere:age,sex,for cedvitalcapacity(FVC),durationof treatment,adverseeffects(AE)andgrade,andsurvival. Patientconfidentialitywaspreservedthroughoutthedata gathering.
4CPS-100 VALIDITY,RELIABILITYANDUSER-PRACTICABILITYOF ACLASSIFICATIONTOOLFORDRUG-RELATED PROBLEMSANDPHARMACISTINTERVENTIONS WITHINANUPPERAUSTRIANHOSPITALTRUST
TRedinger*,APointinger,AWeigl. KeplerUniversityHospital,PharmacyDepartment MedcampusIii,Linz,Austria
10.1136/ejhpharm-2023-eahp.117
BackgroundandImportance Inordertofullycapturethecontributionofclinicalpharmaciststopharmacotherapy,astandardisedandvalidatedclassificationtoolfordrug-related problems(DRP)andpharmacistinterventions(PI)isessential forbothresearchpurposesandmanagementtasks.Suchan instrumentisnotyetavailableinAustria.Therefore,thedocumentationsystem, ‘DokuTool’,hasbeendevelopedbyan UpperAustriahospitaltrustfollowingtheexpansionofits clinicalpharmacyservices.
AimandObjectives Thisstudyaimedtoassessthereliability, validityanduser-practicabilityoftheclassificationsystem, ‘DokuTool’,withinanUpperAustrianhospitaltrust.
MaterialandMethods Two-phasequantitativemethodology:1) Twenty-nineclinicalpharmacistsclassified24samplecases with ‘DokuTool’.Inter-raterandtest-retestreliabilitywas determinedusingthekappastatistic.Validitywasdetermined bycorrelatingtheindividualratingswithagoldstandard (majorityvoteofexperts)usingcontingencycoefficient.2) User-practicabilitywasassessedbyanonlinesurveyusinga5pointLikertscale.
Results ‘DokuTool’ achievedmoderateagreementintheinterraterreliabilitytestofthetwomaincategories ‘TypeofDRP ’
(k=0.528[95%confidenceinterval(CI):0.514 – 0.541]) and ‘CauseofDRP ’ (k=0.594[95%CI:0.587 – 0.601]).
Thecategory ‘PlannedPI’ showedsubstantialagreement(k= 0.638[95%CI:0.629
0.647]).Test-retestreliability achievedanalmostperfectagreementforallthreemaincategories: ‘TypeofDRP ’ (k=0.825[95%CI:0.734 – 0.915]),
‘CauseofDRP’ (k=0.896[95%CI:0.825 – 0.967])and
‘PlannedPI’ (k=0.891[95%CI:0.819 – 0.964]).The medianrater-specificcontingencycoefficientwas0.84[range: 0.75
0.89],0.95[0.94
0.96]and0.93[0.91 – 0.94].
‘DokuTool’ wasratedcomprehensive(median:2[interquartile range:1.75]),user-friendly(2[1])andpractical(2[1]).Time expenditurewasconsideredadequate(3[1]),butthecompletenessandclarityofthecategorieswereratednegatively(3 [2]).
ConclusionandRelevance Moderatetosubstantialinter-rater reliability,almostperfecttest-retestreliability,goodcriterion validityandacceptableuser-practicabilitydemonstratedthat
‘DokuTool’ isavalidandreliableclassificationinstrumentof DRPsandPIs,thatiswell-suitedforAustria.
However,theevaluationofusabilityledtosuggestionsfor improvementforfutureversions.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
treatment(25men(48%)versus27women(52%),median age56[IQR51 – 60]);whilethesecondcohortconsistedof 132patients(10.4%)whomaintainedsuchantiretroviraltreatment(107men(80.5%)versus25women(18.8%),median age45[IQR33 – 55]).Intotal266ADRsweredetected: 216probable,46possibleand4improbablerelationship.A 27.8%wereheadachesADRs,18.8%gastrointestinaleffects (diarrhoea,abdominalpain,nausea),16.2%sleepdisorders (insomnia)and37.2%others.
ConclusionandRelevance ThefrequencyofADRsofHIVpatientsintreatmentwithBIC/FTC/TAFislowandmostof peoplewhosufferitcancontinuewiththeirtreatment.Most ADRswereconsiderasprobablyandthemostcommonwas theheadaches,thegastrointestinaldisordersandtheinsomnia.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-103 PHARMACOKINETICINTERACTIONSTUDYOF OSIMERTINIBANDDIGOXIN:ACASEREPORT
1CMagroVázquez*, 2CGonzálezTrigueros, 3ALSalcedoMingoarranz, 1MMNoceda Urarte, 2MHerreroFernández, 1MTSarobeCarricas, 2GBaldominosUtrilla, 3BGarcíaDíaz. 1HospitalUniversitariodeNavarra,PharmacyDepartment,Pamplona,Spain; 2Hospital UniversitarioPríncipedeAsturias,PharmacyDepartment,AlcaládeHenares,Spain; 3HospitalUniversitarioSeveroOchoa,PharmacyDepartment,Leganes,Spain
10.1136/ejhpharm-2023-eahp.119
4CPS-101 CAUSALITYOFADVERSEDRUGREACTIONSINHIVPATIENTSTREATEDWITHBICTEGRAVIR/ EMTRICITABINE/TENOFOVIRALAFENAMIDE
1SJimenoAguado, 1SJimenoAguado*, 1MVélez-Díaz-Pallarés, 2AMMoreno-Zamora, 1MLavandeira-Pérez, 1HMartinez-Barros, 1PMartín-Sanz, 1EGemeno-López, 1MRodríguez-Marín, 1AMÁlvarez-Díaz. 1HospitalUniversitarioRamónYCajal,Pharmacy, Madrid,Spain; 2HospitalUniversitarioRamónYCajal,InfectiousDiseases,Madrid,Spain
10.1136/ejhpharm-2023-eahp.118
BackgroundandImportance Bictegravir/emtricitabine/tenofovir alafenamide(BIC/FTC/TAF)hasbeenpositionedasapreferencedruginthemainguidelinesinthetreatmentofHIV. BIC/FTC/TAFhasdemonstratedanexcellentsafetyandefficacyprofileinpivotalstudies.Inclinicalpracticefewstudies havebeenpublishedconcerningthesetwoaspects.
AimandObjectives Toassessthecausalityofadversedrug reactions(ADRs)ofHIV-patientstreatedwithBIC/FTC/ TAF.
BackgroundandImportance Tyrosinekinaseinhibitors(TKI) havemeantachangeofparadigminthetreatmentofnonsmall-celllungcancer(NSCLC)withdrivermutations.Many TKIdrugsinteractwiththedrug-effluxpumpP-glycoprotein (P-gp)involvedintheabsorptionand/ortransportofdrugs andxenobiotics.P-gpinhibitors,asosimertinib,mayincrease theserumconcentrationofP-gpsubstrate.Thisiskeyinnarrowtherapeuticrangedrugs,likedigoxin,aslevelshigher than1.2ng/mlareassociatedwithincreasedrisksofdeath. Althoughthisinteractionhasbeendescribedintheory,thisis thefirstcasereportinscientificjournals.
MaterialandMethods
Observationalandretrospectivestudy fromMay2019toMarch2021inageneralhospital.The patientswereclassifiedintotwocohortsbasedonwhether theycontinuedtreatmentornotafterreportingADRs.The variablesanalysedwere:age,sex,priorandsubsequentantiretroviraltreatment(ifapplicable).AllADRswereclassified accordingtotheorganclasssystem.ThecausalitywasevaluatedwithTheNaranjoAlgorithm.Thisisamethodto assesswhetherthereisacausalrelationshipbetweenanidentifieduntowardclinicaleventandadrug,usingasimple questionnairetoassignprobabilityscores.Thetotalscore are:definite(>9),probably(5-8),possible(1-4)anddoubtful (0).
Results Atotalof1,275patientswerecollected,ofwhich185 patients(14.5%)withatleastoneADRwereevaluated.The firstcohortconsistedof53patients(4.1%)whochangedtheir
AimandObjectives Todescribethepotentialdrug-druginteractionbetweenosimertinibanddigoxinmediatedbyP-gpina 77yearoldwomanwithahistoryofpermanentatrialfibrillation.ThepatientwasdiagnosedwitheGFRmutantNSCLC stageIIB.Leftlowerlobectomywasperformed.Subsequent tumourprogressionprovokedosimertinibtreatmentandafter thatanincreaseofpreviouslyinrangedigoxinlevelsis recorded.
Descriptiveandretrospectivecasereport Datawereobtained fromcomputerisedmedicalrecords.Mediwaresoftwarewas usedtomakepharmacokineticspredictionandadjustdosage recommendation.
Results Osimertinibtreatmentstartedatdosesof80mg/dayin March2022andaftertwomonthsitwasinterruptedbecause ofdiarrhoeaandmucositis.Twoweekslaterthepatientshows severehypomagnesemiarequiringhospitalisation.Laboratory resultsrevealedserumdigoxinlevelof1.38ng/ml,thus digoxindosewasreducedfrom125mcg/dayto100mcg/day. Athospitaldischargeosimertinibtreatmentwasrestartedwith half-dosereduction.Thenextdigoxinlevelswentupto1.9 ng/ml,sothePharmacyDepartmentrecommendedtoreduce thedigoxindoseto75mcg/day.Thereafter,digoxinlevels
increasedupto1.31ng/mland1.45ng/ml,requiringdose reductionto50mcg/day.
ConclusionandRelevance Inourcasereport,therapeuticdrug monitoringofdigoxinhasallowedforthedetectionof increasedlevelsofdigoxinandhigherrisksoftoxicity.Itcoincideswiththestartofosimertinibexposure,beingtheP-gp inhibitionthemostplausiblefactorforthisfinding.
ConflictofInterest Noconflictofinterest
4CPS-105 CEFIDEROCOLTREATMENTINCOVID-19POSITIVE PATIENTSCO-INFECTEDWITHPAN-RESISTANT PSEUDOMONASAERUGINOSA
CGastalver-Martín,OSerna-Romero*,SBuendia-Bravo,AIglesias-Bolaños,CCapillaMontes,IEscribano-Valenciano,TCruz-Cruz. HospitalUniversitarioDelSureste,Pharmacy Department,ArgandaDelRey,Spain
10.1136/ejhpharm-2023-eahp.121
MMuñozVillasur*,CRodríguez-TenreiroRodríguez,VJiménezGarcía,CLFernández Laguna,LMacíaRivas,IdelaFuenteVillaverde,SFernándezLastras,MEiroaOsoro, LOyagueLópez,ALozanoBlázquez. HospitalUniversitarioCentraldeAsturias,Hospital Pharmacy,Oviedo,Spain
10.1136/ejhpharm-2023-eahp.120
BackgroundandImportance Pharmaceuticalvalidationisnecessarytoachievemaximumclinicalbenefit.Thankstoclinical pharmaceuticalinterventions(CPI)manyprescriptionerrors, druginteractionsandadversereactionsareprevented.
BackgroundandImportance ImmunosuppressionduetoSARSCoV2infection(COVID19)hascausedanincreaseinidentificationofmulti-resistantorganismsinIntensiveCareUnits (ICU),amongwhichmulti-resistant Pseudomonasaeruginosa riseaboutothers.Cefiderocolisacostlynewcephalosporin againstextensivelyresistantGram-negativebacteria.
AimandObjectives Theobjectiveofthisstudyistodescribe thecharacteristicsandclinicalresultsofpatientstreatedwith cefiderocol,aswellasthedosageofthistreatment,inICU inpatientswithCOVID19pneumoniaandco-infectedwith pan-resistant Pseudomonasaeruginosa
AimandObjectives
ToanalyseCPIcarriedina1040-bedhospitalandtoassesstheacceptancerateoftheseinterventions.
MaterialandMethods Observationalandretrospectivestudyof CPIperformedbetweenJuneandAugust2022inhospitalised patients.TheywererecordedinthepharmaceuticalinterventionmoduleofPharmNetapplicationofMilleniumprogramme.Thevariablesevaluatedwere:episodenumber,date, typeofintervention,prescribingservice,drugandindication. Interventionsthatledtoachangeintheprescriptionwithin 48hoursoftheCPIwereconsideredaccepted.
Results Atotalof324interventionswereanalysedin293 patients,whichwere100%ofthoseperformed.Morethan halfoftheinterventionsweretherapeuticduplications (36.4%;n=118)anddosingerrors(25.9%;n=84)(overdose 62%andunderdose23%).Theywerefollowedinfrequency by:incompletemedicalorders(18.5%;n=60);drugsnot indicated(6.8%;n=22);druginteractions(4.6%;n=15); inappropriatedosageform(4.6%;n=15)andadverseevents (3%;n=10).Thedistributionofthenumberofinterventions accordingtoprescribingservicewas:cardiology(n=54);gastroenterology(n=44);pneumology(n=32);internalmedicine (n=30);vascularmedicine(n=29);neurology(n=23)and traumatology(n=28).TheacceptancerateoftheCPIwas 80,3%(n=260)withthefollowingservicedistribution:90% internalmedicine;87.5%pneumology;84%gastroenterology, 82.7%neurologyand79.3%cardiology.Drugswhichcaused mostinterventionswereantibiotics(17%),anti-inflammatory drugs(11.4%),cardiovascularagents(11.1%)andantidepressants(9%).
ConclusionandRelevance Theclinicalpharmaceuticalinterventionsproposedtotheprescribingserviceswerehighly accepted.Thisshowstheimportanceofpharmaceuticalvalidationbythehospitalpharmacisttobettermanagethequality andsafetyofpharmacologicaltreatmentprescribedtopatients duringtheirhospitalstay.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
MaterialandMethods Retrospectiveobservationalstudycarried outinageneralhospitalfromSeptember2020toDecember 2021.InpatientsatICUdiagnosedwithCOVID-19pneumonia thatweretreatedwithcefiderocoldueto P.aeruginosa infectionwereincluded.Collecteddatawere:daysadmittedin ICU,daysoftreatmentwithcefiderocol,concomitanttreatment,cefiderocoldosageandresultsofthetreatment.
Results Threepatientsfulfilledtheinclusioncriteriaamong70 patientsadmittedtoICUwithCOVID-19inthestudyperiod (4.3%).Allpatientsincludedweremenandthemedianage was66.6±6.5yearsold.Theypresentedascomorbidities obesity,hypertensionanddiabetesmellitus.Theywereadmittedduring87±28.6days,withdetectionofpan-resistant P. aeruginosa intherangeof32.5±2.1daysafteradmissionat ICU.Allofthesecultureswereonlysensitivetocefiderocol, beingresistanttoallothertestedantibiotics.Duetothat,all patientsreceivedcefiderocolduringtheirstayanddoseadjustmenttotheirrenalfunctionorrenalreplacementtherapy wereapplied.Everypatientreceivedabolusof2gramsin30 minutesandthemaintenancedoseinatleast3hours.The averageoftreatmentdayswas20.5±4.5days.Inallcases, theisolatedstrainsweresensitivetocolistin,socefiderocol wasusedincombinationwithit.Theresultsofthetreatment weredisparate:onecure,onedeath,andonedevelopmentof resistancetocefiderocol.
ConclusionandRelevance Cefiderocoluseformulti-resistant bacteriatreatmentrequirespriorknowledgeofitspharmacokinetics,takingintoaccountthephysiologicalfactorsofpatients initsdosage.Newtreatmentsarenotexemptfromthedevelopmentofresistance.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
10.1136/ejhpharm-2023-eahp.122
4CPS-107 EFFECTOFPATIENTBODYWEIGHTONTHE PHARMACOKINETICBEHAVIOUROFAMIKACIN 1OSerna-Romero*, 1CGastalver-Martin, 1SBuendia-Bravo, 1AIglesias-Bolaños, 1CCapillaMontes, 1IEscribano-Valenciano, 2ALSalcedo-Mingorranz, 1TCruz-Cruz. 1Hospital UniversitarioDelSureste,PharmacyDepartment,ArgandaDelRey,Spain; 2Hospital UniversitarioSeveroOchoa,PharmacyDepartment,Leganés,SpainBackgroundandImportance Obesityisadiseasethatinfluences numerousphysiologicalprocesses.Currentlythereislittle pharmacokineticdatainobesepatientsandextrapolateddata frompatientswithnormalweightareoftenused.Inorderto optimisethedosageofdrugsinobesepatients,itisnecessary todesignspecificpopulationmodelsinthisgroupofpatients.
AimandObjectives Toanalysethedifferencesinthepharmacokineticparametersofamikacininhospitalisedpatientsbased onbodymassindex(BMI).
MaterialandMethods Retrospectiveobservationalstudyin whichpatientstreatedwithamikacinbetweenJanuaryand August2022wereanalysed.Thevariablescollectedwere:age, weight,height,sex,serumcreatinine,dosageregimenandamikacinlevel.
PatientswereclassifiedaccordingtotheirBMI:lessthan 30Kg/m2(non-obese)andgreaterthan30Kg/m2(obese). Themeanandstandarddeviationofthevolumeofdistribution(Vd)andclearance(Cl)ofthetwogroupswerecalculatedusingapharmacokineticprogramme(MwPharm)based onasinglecompartmentmodel.
StatisticalanalysiswasperformedusingStudent’st-testfor independentsamples.
Basedonthedatacollected,BMIandcreatinineclearance (accordingtotheCockcroft-Gaultequation)werecalculated. Patientswithaglomerularfiltrationrateoflessthan30mL/ minwereexcluded.
Results 42patients(52%women)with156levelsofamikacin andameanageof69±28yearswereincluded.ThedistributionofpatientsaccordingtoBMIwas:59%normalweight and41%obese.
ThemeanandstandarddeviationofClofobesepatients andnormalweightwere2.67±1.41L/hand1.92±1.04 L/h,respectively.P-valuefromt-testwas0.04(p<0.05)for Cl.
Vddatawere0.314±0.068L/Kg(obese)and0.28± 0.034L/h(normalweight).P-valuewas0.648(p>0.05)for Vd.
ConclusionandRelevance Statisticallysignificantdifferences werefoundinClbetweenbothgroups:inobesepatientsamikacinClwashigherthaninpatientswithnormalweight.
NosignificantdifferencesinVdwerefoundbetweenthe twostudygroups.
Futurestudiesareneededtodesignpopulationpharmacokineticmodelsofamikacininobesepatients.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
Therefore,aclinicalpharmacyservicewasinitiatedonthe haematologywardatourhospital.
AimandObjectives Todeterminethesatisfactionrateofthe clinicalpharmacyserviceinpatientswithhaematological malignanciestreatedwithoralanticancertherapyandinhaematologists-in-training.
MaterialandMethods BetweenJanuaryandMay2022,a surveywasdevelopedtoassesspatientandhaematologists-in – trainingsatisfactionandperceivedvalueofhealthcareservicesprovidedbyclinicalpharmacistsatatertiarycarehospital.Thesurveywastakenbyapharmacistnotinvolvedin dailyclinicalpharmacypractice.Thesurveycontainedquestionsaddressingdemograph ic,typeoforalanticancertherapyandpharmacist-specificitems.Responseswereanalysed usingdescriptivestatistics.Satisfactionwasassessedby5 Likert-scalequestionsandeither8or4open-endedquestionsforcancerpatientsandforhaematologistsintraining, respectively.Weaimedtohaveasatisfactionrateofatleast 80%.
Results Atotalof65patientsand11haematologists-in-trainingparticipatedinthesurvey.Allpatients(100%)ranked thepharmacists ’ explanationaboutmedicationintakeand side-effectsaseitherverysatisfyingorsatisfying.Counsellingaboutdruginteractionswastheonlycriterionthatdid notresultintheachievementofthepredefined80%satisfactionrate,with27.6%ofpatientsbeingverysatisfiedand 51.7%ofpatientsbeingsatisfiedaboutthistopic,respectively.Overall,themajorityofpatients(89.7%)indicated thatpharmacistcounsellingandfollow-upvisitswereof addedvalue.All11includedhaematologistsintraining expressedhighlevelsofsatisfactionwiththeclinicalpharmacistservice.
ConclusionandRelevance Highlevelsofsatisfactionwiththe clinicalpharmacistservicewasreportedbybothpatientswith ahaematologicalmalignancyandhaematologists-in-training. Thissurveyidentifiedthatcounsellingondruginteractionsof oralcancertherapymightbeimproved.Furtherstudiesmay includeassessmentoftheassociationbetweenpatientsatisfactionandcomplianceandtreatmentoutcomes.Alsotheadded valueandcosteffectivenessoftheclinicalpharmacistservice needstobeinvestigatedinfutureresearch.
ConflictofInterest Noconflictofinterest
4CPS-109 THERAPEUTICDRUGMONITORINGOFVANCOMYCIN INONCOLOGICANDHAEMATOLOGICPATIENTS:
REAL-WORLDDATA
4CPS-108 PATIENTANDPHYSICIANS’SSATISFACTIONWITH CLINICALPHARMACYSERVICESONAHAEMATOLOGY WARDINALARGETERTIARYCAREHOSPITAL
1JNeefs, 1ESimons, 2AJanssens, 1,3ISpriet*, 1TVanNieuwenhuyse. 1UniversityHospitals Leuven,DepartmentofPharmacy,Leuven,Belgium; 2UniversityHospitalsLeuven, DepartmentofHaematology,Leuven,Belgium; 3KuLeuven,DepartmentofPharmaceutical andPharmacologicalSciences,Leuven,Belgium
10.1136/ejhpharm-2023-eahp.123
BackgroundandImportance Oralanticancertherapyisincreasinglyusedforthetreatmentofhematologicmalignancies. Despiteitsconvenience,severalchallengessuchasmedication adherencemayimpacttherapeuticeffectivenessandoutcome.
MBardollCucala,MGilabertSotoca,JRiusPerera,IManguesBafalluy,BMartinezCastro, MMirCros,AMoralesPortillo,JASchoenenbergerArnaiz. HospitalArnaudeVilanovade Lleida,ServiciodeFarmacia,Lleida,Spain
10.1136/ejhpharm-2023-eahp.124
BackgroundandImportance Vancomycinclearancetendstobe higherinpatientswithneutropenia1;consequently,therapeutic drugmonitoring(TDM)ishighlyrecommended.2
AimandObjectives Toassesstheachievementofatherapeutic pharmacokinetics/pharmacodynamics(PK/PD)targetofvancomycininoncologicandhaematologicpatientsusingtroughonlyTDM.
MaterialandMethods Weconductedaretrospectiveand descriptivestudythatincludedoncologicalandhaematological
patientsadmittedtoasecond-levelhospital,startingtreatment withvancomycinanddosingadjustmentguidedbyTDMat thePharmacyservice.
Demographicvariables,Cockcroft-Gaultcreatinineclearance (CrCl),initialdosage,doseadjustments,thefirsttroughlevel, durationoftreatment,andreasonforwithdrawalwerecollected.RenalimpairmentwasdefinedasCrCl<60ml/min. Dosagesof15-20mg/kg/doseandtroughlevelsbetween10 and20 mg/mlwereconsideredoptimalforintermittentinfusionschedules.TDMusedthePKS® software. Results Vancomycintroughlevelswereobtainedin49 patients;12wereoncological,and37werehaematological.
Dosageadjustmentwasnecessaryfor30patients(61%), 25/30duetosubtherapeuticlevel(troughlevel<10 mg/ml) and5/30duetosupratherapeuticlevel(throughlevel>20 mg/ mlwithorwithoutrenalimpairment).
Theinitialmeandosagewas13,7±2,5mg/kg/12h,except inthreepatientswhostartedevery24hduetorenalimpairment.Afterthedosageadjustment,therecommendedmean dosagewas14±3mg/kg/8hin18patientsand13,6±7,6 mg/kg/12hin12patients.
Themeandurationofantibiotictreatmentwas7±4,2 days.Thereasonsforstoppingthetreatmentwere:clinical improvement(n=29),switchtoatargettreatment(n=10), clinicaldeterioration(n=9)andnephrotoxicity(n=1).Nine patientsdied.
ConclusionandRelevance Morethanhalfofthepatientshad subtherapeuticvancomycinlevelsandrequiredantibioticdose adjustment.
Mostpatientsrequiredshorterdosingintervalsratherthan increaseddosestoreducetheincidenceofnephrotoxicity.
1.BuryD, etal.EurJClinPharmacol 2019;75:921–928
2.RybakMJ, etal.AmJHealth-SystPharm.2020;77:835–864
ConflictofInterest Noconflictofinterest
MaterialandMethods PatientswithHER2-negativeadvanced gastroesophagealadenocarcinomadiagnosedbetween2008and 2021fromamulticentreregistry(34centres)wereincluded. Patientsreceivedchemotherapybasedonplatinum(cisplatinor oxaliplatin)andfluoropyrimidine(5-fluorouracilorcapecitabine).Associationbetweenthefollowingbaselinevariables: specialtyoftheprescribingoncologist,ECOG-PS(Eastern CooperativeOncologicGroupPerformanceStatus),serum albumin,tumourlocation,Laurenclassificationandplatinum andfluoropyrimidineregimenswereevaluatedandChi2test wasperformed.
Results Atotalof1334patientswereregistered,66.49% (n=893)weremale.Seventypercentofourpopulationwas treatedalmostequallywithFOLFOX6(n=468)andXELOX (n=466),followedbyXP19%(n=252),FP3w7%(n=95) andinfewerpercentwithFUOXmodified3%(n=44),FP4w 1%(n=12)andFLO(n=6).Oxaliplatinwasthemostcommonlyusedplatinum(73%,p=971)whilebothfluoropyrimidineswhereadministeredinasimilarproportion(capecitabine 54%).Patientsweremainlytreatedbyanoncologistspecialisingingastriccancer(95%).Generaloncologistpreferredoxaliplatin-basedregimens(46%vs6%)andspecialistoptedmore forcisplatinandcapecitabineassociatedregimens(p=0.031). Patientswithworstperformancestatus(ECOG=2)were treatedtoagreaterextentthantheoverallpopulationwith schemesbasedonoxaliplatinand5-fluorouracil50%versus 38%ofthegeneralpopulation.ThosewithECOG=0received morethanexpectedschemeswithcisplatinandcapecitabine (21%,n=55).Patientswithbaselinehypoalbuminaemia(albumin<35g/dL)receivedintravenousfluoropyrimidinescheduleswithbothoxaliplatin(47%,n=156)andcisplatin(9%, n=3)inahigherproportionthanexpected(p<0.000).AccordingtoLauren`sclassification,therewasahigheruseofcapecitabineversus5-FUinintestinaltumours.Thistrendis reversedindiffusetumours(p<0.000).
ConclusionandRelevance Inthisstudywefoundanassociationbetweentheplatinumandfluoropyrimidineselectedin patientswithadvancedgastriccancerandcertainbaselinevariables.Futurestudiesareneededtoevaluatewhetherthis choicehasanimpactonpatientbenefit.
4CPS-110 ASSOCIATIONBETWEENBASELINECHARACTERISTICS ANDFIRST-LINECHEMOTHERAPYINADVANCED GASTRICCANCERPATIENTS
1AArias*, 2FJAlvarezManceñído, 3AMartinezTorron, 3LMaciaRivas, 4ACalvo, 5LVisa, 6MLLimón, 7GIglesiasÁlvarez, 7AMariñoMéndez, 8PPimentel, 3ALozano-Blázquez.
1UniversityofGranada/HospitalUniversitarioGermansTrias,DoctoralProgrammeIn Pharmacy,FacultyofPharmacy/PharmacyDepartment,Granada,Spain; 2Hospital UniversitarioCentraldeAsturias/UniversityofGranada,PharmacyDepartment/Doctoral ProgrammeInPharmacy,FacultyofPharmacy,Oviedo,Spain; 3HospitalUniversitarioCentral deAsturias,PharmacyDepartment,Oviedo,Spain; 4HospitalUniversitarioGregorio Marañon,DepartmentofMedicalOncology,Madrid,Spain; 5HospitalUniversitarioElMar, DepartmentofMedicalOncology,Barcelona,Spain; 6HospitalUniversitarioVirgenDel Rocío,DepartmentofMedicalOncology,Sevilla,Spain; 7HospitalUniversitarioCentralde Asturias-UniversidaddeOviedo-Ispa,DepartmentofMedicalOncology,Oviedo,Spain; 8HospitalGeneralUniversitarioSantaLucía,DepartmentofMedicalOncology,Cartagena, Spain
10.1136/ejhpharm-2023-eahp.125
BackgroundandImportance Thereisnostandardfirst-lineregimenforHER2-negativeadvancedgastroesophageal adenocarcinoma.
AimandObjectives Tostudythevariabilityinthechoiceof regimensaccordingtotumour,patientbaselinevariablesand prescribingphysician.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.ThisstudyispartofadoctoralthesisoftheDoctoralProgrammeinPharmacyof theUniversityofGranada.Thanksforyoursupportinthisresearch.
ConflictofInterest Noconflictofinterest
4CPS-111 IMPORTANCEOFIMPLEMENTINGACLINICAL PHARMACOKINETICUNITINHOSPITALPHARMACY SERVICE
1OSerna-Romero*, 1SBuendia-Bravo, 1CGastalver-Martin, 1CCapilla-Montes, 1AIglesiasBolaños, 1IEscribano-Valenciano, 2ALSalcedo-Mingorranz, 1TCruz-Cruz. 1Hospital UniversitarioDelSureste,PharmacyDepartment,ArgandaDelRey,Spain; 2Hospital UniversitarioSeveroOchoa,PharmacyDepartment,Leganés,Spain
10.1136/ejhpharm-2023-eahp.126
BackgroundandImportance Pharmacokineticmonitoringisa toolusedintherapeuticoptimisationtoachievethebestclinicalresultsandminimisetheincidenceofadverseeffects.
Itisparticularlyusefulindrugswithadose-dependentclinicalresponseandtoxicityrelationshipandwithanarrowtherapeuticmargin.Computingsoftwareareusedtointegrate
patientdataintopopulationmodelsthroughwhichpharmacistscanestablishtheoptimaldosageregimen.
AimandObjectives
Toanalysetheinfluenceofpharmacokineticreportsonclinicaldecision.
MaterialandMethods Aretrospectiveobservationalstudywas conductedfromJanuarytoAugust2022inageneralhospital.
Patientswithatleastoneplasmaconcentrationofamikacin, amitriptyline,carbamazepine,digoxin,phenytoin,phenobarbital,gentamicin,lithium,theophylline,tobramycin,valproic acid,andvancomycinwereincluded.
Collecteddataincluded gender,age,weight,height,serumcreatinine,drug,dosage,plasmaconcentrationsandconcomitant medication.
Apharmacokineticsoftwarewasused.ByBayesianestimation,optimaldosageregimenwascalculated.Basedonthese data,thepharmacistpreparedthepharmacokineticreportand dosagerecommendationsforthephysician.
Recommendationsmadebythepharmacistwererecorded andclassifiedaccordingtofollowingcriteria:Underdosing, intoxication,noadjustmentrequired,lackofadherenceand interactionadjustment.Thepercentageofacceptanceofthe interventionswasanalysed.
Results 182patientsand395interventionswereevaluated.
Clinicalservicesthatreceivedmorepharmacokineticreports wereinternalmedicine(48%)andpsychiatry(19%).Themost commonmonitoreddrugsweredigoxin(24%),valproicacid (22%)andvancomycin(18%).
In21%ofthepatientsadjustmentsweremadedueto underdosing,13%duetooverdosing,4%duetolackof adherence,and2%duetodrug-druginteractions.Therewas noneedtoadjustdosagein40%ofmonitoredpatients.The remaining20%wereinterventionsrelatedtoerrorsinthe extractionoftheanalytics.
71%oftherecommendationsaddressedtophysicianswere accepted.
ConclusionandRelevance Themostcommondosageadjustmentwasduetounderdosingsothattheefficacyofthetreatmentwascompromised.Itshouldalsobenotedthatthereis ahighpercentageoferrorsintheanalyticextractionprocedure.Healthprofessionalswhoperformthesamplecollection mustbeproperlytrained.
Clinicalpharmacokineticsisatoolthatallowsustooptimisethepatient‘sdosageregimen.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
AimandObjectives Developmentofawebtoolforthereview andanalysisofpolymedicatedpatients(>15drugs/month) whoattendoutpatientconsultationsinordertoimprovethe prescriptionofpolymedicatedpatientsandincreasethepresenceofthepharmacistinoutpatientconsultations.
MaterialandMethods AwebapplicationnamedVIGÍAwas developed(VIewerofPotentially InAdequatePharmacotherapeutic Groups).Itcancalculateadherencetotreatment accordingtopharmacydispensingrecordanddetectinadequaciesinpharmacotherapy:duplicities,prescribingcascades, drugswithlowtherapeuticvalue,drugsthatprolongtheQTintervalanddrugscontributingtoanticholinergicburden,givingascorenamedPotentialInadequacyIndex(PII):
PotentialInadequacyIndex(PII)
Duplicity1point
Lowtherapeuticvalue1point
Prescribingcascades0,5points QTintervalprolongation0,5points Anticholinergicburden0,5points
VIGIAcanfilterpatientsbyconsultationdate.Doctors havethereviewsavailableonlinewiththepharmacistrecommendations,beingableornottomodifytheprescriptionat theirchoice.
PIIbeforeandafterthestudywascalculated,comparing themeansthroughStudent’st-testfortwomeansofthesame population(twotails,significanceat5%).
Results Afterastudyperiodof120days,weelaborated486 reviewreportsfromrheumatologyanddigestiveconsultations, achievingtoreducethePIIscorefrom1.58to1.46,and averagenumberofmedicationswentfrom18to17.3.Student’sttestforthePIIvaluebeforeandafterthestudy periodwassignificant(p<0.05).
Abstract4CPS-115Table1
Prescribingcascades0.150.13
Drugswithlowtherapeuticvalue0.310.28
DrugsthatprolongtheQTinterval0.330.33
Drugscontributingtoanticholinergic burden 0.410.38
4CPS-115 DEVELOPMENTANDIMPLEMENTATIONOFAREVIEW PROGRAMMEASSISTEDBYTHEPHARMACISTTO IMPROVETHEADEQUACYOFTREATMENTIN POLYMEDICATEDPATIENTSINHOSPITALOUTPATIENT SETTING
AAlcalaSoto,MVázquezReal,DSRuizPérez,CMCuadrosMartínez*,JFSierraSánchez. HospitalUniversitarioJerezdelaFrontera,PharmacyService,JerezdelaFrontera-Cádiz, Spain
10.1136/ejhpharm-2023-eahp.127
BackgroundandImportance Ahighnumberofpolymedicated patientspassthroughoutpatientconsultationsandprescribers oftendon’thavethetimeorcapacitytodealwiththeir polypharmacy.
PII1.581.46**(p<0.05)
Averagenumberofmedications1817.3
ConclusionandRelevance Reviewofpolymedicatedpatientsby thepharmacistseemstoreduceinadequaciesoftheir pharmacotherapy.
ThisPIIscore,madeupofdifferentsituationsconsidered tobeatrisk,cangiveanideaofthebenefitofitsreduction, notonlyintermsofpatientsafetybutalsoeconomic,by reducingtheaveragenumberofdrugs.
4CPS-116 THEEFFICIENCYANDCOST-EFFECTIVENESSOF HEALTHCAREANDNUTRITIONALINTERVENTIONSIN THEMANAGEMENTOFPOST-STROKE
1SMarin*, 2OOrtega, 3MSerra-Prat, 1EValls, 4LPérez-Cordón, 1CCodina-Jiménez, 2PClavé. 1HospitalUniversitariGermansTriasIPujol,PharmacyDepartment,Badalona, Spain; 2HospitaldeMataró,GastrointestinalPhysiologyLaboratory,Mataró,Spain; 3Hospital deMataró,ResearchUnit,Mataró,Spain; 4HospitaldeMataró,PharmacyDepartment, Mataró,Spain
10.1136/ejhpharm-2023-eahp.128
BackgroundandImportance Post-strokeoropharyngealdysphagia(PS-OD)causessignificanthighcostsduringhospitalisation thatincreasewiththedevelopmentofmalnutritionandrespiratoryinfectionsatlong-term.Thisdatasuggeststhatthe appropriatemanagementofPS-ODincludingtheuseofearly detectionprogrammes,texture-modifieddiets,commercially thickenedfluids,domiciliaryenteralnutrition,andrehabilitationprogrammesincludingrestorativetreatmentscouldleadto cost-effectivereductionofclinicalcomplications.1
AimandObjectives Toassessliteratureontheefficiencyand cost-effectivenessofavailablehealthcareinterventionsonthe managementofPS-OD.
MaterialandMethods SystematicreviewfollowingPRISMA recommendations.MEDLINE,Embase,NHS-EEDandCEARegistryweresearchedupto30June2021toincludestudies onPS-OD.Outcomesofinterestweretheefficiencyandthe cost-effectivenessofhealthcareinterventionsonthemanagementofPS-OD.Economicevaluationstudieswereincluded. Oesophagealdysphagiaandnon-strokestudieswereexcluded.
Results 235studieswereidentifiedand10included.Svendsenet-al foundlowerhospitalisationcosts(HC)(USD12,556 CI95%9,751-15,361)whenPS-ODwasassessedduringthe first24hoursafteradmission.Liu-et-al didnotfinddifferencesinHCwhenPS-ODwasassessedwiththewaterswallowingvsvolume-viscosityswallowingtestifthewatertestfailed.
Schwartz-et-al foundanon-significantreductiononHC(Australiandollars18,053vs16,548,p=0.722)usingaprotocolto manageODafterthrombolysis.Wilsonet-alshowedvideofluoroscopyasthemostcost-effectivescreeningmethodcomparedtobedsideevaluationandacombinationofboth.
Khiaocharoenet-alandSuksathienet-alshowedcost-effective rehabilitationprogrammesthatincludedODmanagement.
Pelczarskaet-alshowedthattheuseoftexture-modifieddiets usingagum-basedthickener(NutilisClear®)wascost-effective (PLN21,387-20,977perQALY),andKoteckiet-althatcommerciallythickenedfluidsusewasmoreefficientthan insitu preparation.Eliaet-alshoweddomiciliaryenteralnutrition cost-effective(£12,817perQALY)andBeavan-et-al showed highernutrientintakeandlowHCincreaseusingloopednasogastrictube(5,20sterlingforevery1%increase).
ConclusionandRelevance Healthcareinterventionstomanage PS-ODwithapositiveclinicaleffecttendtobecost-effective. Futurestudiesassessingthecost-effectivenessofapplyingcompensatoryand/orrestorativestrategiesamongwithreporting cost-savingsbyappropriatePS-ODearlyevaluationandmanagementarene
1.MarinS, etal. Economicevaluationsofhealthcareinterventionsinoropharyngeal dysphagiaafterstroke:protocolforasystematicreview. SystRev.2022;11(1): 92.
MJLucasMayol,MRodriguezMorote,CMatosesChirivella,MIbañez,AMurciaLópez, ANavarroRuiz*. HospitalGeneralUniversitariodeElche,ServiciodeFarmacia,ElcheAlicante,Spain
10.1136/ejhpharm-2023-eahp.129
BackgroundandImportance Criticallypatientssometimessuffer fromgastrointestinaldisorderswhicharenecessarytotreatto improveclinicaloutcomes.Erythromycinisanantibioticwith prokineticactivityduetoitsagonistactivityonmotilinreceptors,acceleratinggastricemptying.
AimandObjectives Toevaluatetheefficacyofintravenous erythromycinasaprokineticincriticallyillhospitalised patients.
MaterialandMethods Retrospectiveobservationalstudyin criticallyillpatientsoveraperiodof12months(04/ 2021-03/2022).PatientdatawereobtainedusingFarmasyst ® andOrionClinic ® software:sex,age,weight,start andenddateoftreatment,dosage,clinicalservice,diagnosis,concomitantprokinetics(metoclopramide,dexpanthenol)andclinicalcourse.Theefficacyoferythromycin usewasassessedbytheabsenceofsymptomssuchas abdominaldistension,flatusandhydroaerialsoundsor nausea.
Results 39patientswerestudied,64%weremenand36% women,withameanageof64yearsandanaverage weightof71kilograms.85%wereinsurgicalintensive careunit,therestinintensivecareunit.Allpatientswere prescribederythromycinatdosesof250milligramsevery 8hours,maintainingtreatmentforanaverageof5days. Thediagnosesforwhicherythromycinwasprescribed were:weakperistalsisin13pat ients,absentperistalsisin 18,intolerancetoenteralnutritionin6patients,and uppergastrointestinalbleedingin2.Ontheotherhand, 39%ofallpatientshadalreadybeenprescribed10mgof intravenousmetoclopramideevery8hoursasaprokinetic priortostartingerythromycin,andthiswasmaintained whentreatmentwiththemacrolidewasinitiated.In41.5% ofpatients,metoclopramidewasprescribedtogetherwith erythromycin.Erythromycintreatmentwasendingin37 patientsduetoclinicalimprovementwithresolutionof abdominaldistension,auscultationofperistalsisandpresenceofstool,in1patientduetotolerancetoenteral nutritionand1patientdied.
ConclusionandRelevance Theuseoferythromycinasaprokineticinthepopulationevaluatedhasbeenshowntobe effectiveinimprovingintestinalmotility.Therewasnodifferencebetweengroupswhichwereadministeredmetoclopramideornotbeforeorduringthetreatmentwith erythromycin.Giventhevariabilityobserved,intermsof duration,concomitantprokineticsorindication,thereisa needtoestablishaprotocolfortheuseoferythromycinasa prokinetic.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-118
1XAntón*, 1JCasas, 1AdeBasagoiti, 2JGoikoetxea, 2EBereciartua, 1MLigros, 1JBarroso, 1BMoñino. 1HospitalUniversitarioCruces,HospitalPharmacyDepartment,Barakaldo –Biscay – BasqueCountry,Spain; 2HospitalUniversitarioCruces,InfectiousDiseases Department,Barakaldo – Biscay – BasqueCountry,Spain
10.1136/ejhpharm-2023-eahp.130
BackgroundandImportance Inrecentyears,hospitalpharmacistshavebeenapproachingpopulation-basedriskstratification modelsforselectedgroupsofpatients.Theimplementationof thesestrategiesasroutinewouldfacilitatetheadequationof thepharmaceuticalcaretopatientcomplexity.
AimandObjectives ToanalysethehealthoutcomesofHIV+ patientsonAntiretroviralTherapy(ART)inacomparative manneraccordingtotheirclassificationintheKaiserPermanentePyramid(KPP).
MaterialandMethods RetrospectiveobservationalstudyincludingallHIV+patientswithactiveARTon2022/01/03followedupintheoutpatientpharmacyofatertiaryhospital. Theresultsextractedon2022/01/03fromtheclinicalhistory wereanalysedaccordingtotheKPPriskstratificationmodel. Datacollected:sex,age,HIVViralLoad(VL),CD4+,polypharmacy( 6drugs,ARTincluded),ARTcost/patient/UndetectableVL(UVL;<50copies/mL),EmergencyDepartment Attendances(EDA)/previousyear,andstratumofKPP(General population:PromotionandPrevention(PP);Chronicpatients: Self-managementSupport(SS);High-riskpatients:IllnessManagement(IM);Patientswithseverecomplications:CaseManagement(CM)).
Results 947(68%men)withamedian(IQR)ageof54years [46-59]wereincluded.92%hadUVLand2%>200copies/ mL.5%had<200CD4+/mL,23%200-500CD4+/mLand 72%>500CD4+/mL.39%ofpatientshadpolypharmacy. EDA/previousyearwas:0,67%patients;1-3,29%patients; 4-8,3.5%;>8,0.5%.
ClassificationaccordingtoKPP:3.5%unclassified,3%PP, 45%SS,33%IMand15.5%CM.4%ofPP,16%SS,88%IM and85%CMhadpolypharmacy.
91%ofPP,93%SS,93%IMand87%CMhadUVL.NoPP patients,2%SS,1%IMand5%CMhadCV>200copies/mL.
NoPPpatients,3%SS,4%IMand10%CMhad<200 CD4+/L.82%ofPPpatients,79%SS,71%IMand57% CMhad>500CD4+/L.
EDA/previousyearwas0,77%PP-75%SS-65%IM-38% CM;1-3,23%PP-23%SS-33%IM-44%CM;4-8,NoPP-1% SS-2%IM-16%CM;>8,NoPPorSS-1%IM-2%CM.
TheART/patient/UVLcostwasthesameastheoverallcost inPPandIMpatients,9%lowerinSSand22%higherin CM.
ConclusionandRelevance Thestudyshowsaworseningin HIVhealthoutcomesandanincreaseinresourceconsumption aspatientcomplexityenhances.
TheKPPmodelallowsustoidentifypatientsatgreater riskofsickness-relatedcomplicationsandwithapotentially highconsumptionofresources,whomayrequireanindividualisedandmorespecificpharmaceuticalcareinoursetting.
ConflictofInterest Noconflictofinterest
4CPS-119 PNEUMONOLOGY-PHARMACYCOLLABORATIONIN THEPHARMACOTHERAPEUTICOPTIMISATIONOF MONOCLONALANTIBODIESINPATIENTSWITH SEVEREUNCONTROLLEDASTHMA
1AAlcalaSoto, 2FPérezGrimaldi, 2JGSotoCampos, 1CMCuadrosMartínez*, 1MTGómez deTravecedoYCalvo, 1JFSierraSánchez. 1HospitalUniversitarioJerezdelaFrontera, PharmacyService,JerezdelaFrontera-Cádiz,Spain; 2HospitalUniversitarioJerezdela Frontera,PneumologyService,JerezdelaFrontera-Cádiz,Spain
10.1136/ejhpharm-2023-eahp.131
BackgroundandImportance Inchronicdiseases,concernabout safetyandeconomicimplicationsoftreatmentwithbiological drugshaveraised,theneedtoadapt,byreducingdoses,the treatmentusedoncereachedtheindividualisedtherapeutic goalforeachpatient.
AimandObjectives Implementationofapharmaceuticalcare consultationforpatientswithSevereUncontrolledAsthma (SUA).
ToestablishacollaborationwiththePneumologyService forthereferralofcandidatepatientsforpharmacotherapeutic optimisation.
MaterialandMethods Pharmaceuticalcareconsultationswere scheduledforallSUApatients.
Candidatesforoptimisationwerethosetreatedwithany monoclonalantibodyformorethan1year,hadnoexacerbationsinthelast12months,ACTscore>20,FEV1>80%, withdrawaloforalcorticosteroids,hadgoodadherenceto treatmentmeasuredbytheTestofAdherencetoInhalersand thepharmacydispensingrecord.
Ifapatientmettheserequirementswasreferredtopneumologistwithatreatmentoptimisationproposal(lengthening theintervalbetweendosesorreducingthedose).Pneumologistswereabletoaccepttheoptimisationproposalornot.If therewasworseningafterdoseoptimisation,theinitialprescriptionwasreturned.
Results Duringa2-yearperiod,fromMay2020toMay 2022,38patientsreceivedMepolizumab,20Benralizumab,14 Reslizumaband59Omalizumab.125patientscametopharmacyconsultation.
35patientsthatmetthecriteriaforoptimisingtreatment andwereproposedtopulmonologist,withacceptanceofthe proposal:9withmepolizumabevery5weeks,1withbenralizumabevery9weeks,5withbenralizumabevery5weeks, and20withomalizumabathalfinitialdose.
InSeptember2022,25patientscontinuetobeoptimised, 10patientshavereturnedtotheusualdosebecausetheywere notfullycontrolledwiththeoptimisedregimen,noneof whomhadasthmaexacerbations.
ConclusionandRelevance Pharmacotherapyoptimisation exposespatientswithtotalcontrolofasthmatolessdrugand lessprobabilityofdevelopingadverseeffects,whileminimising costsinthehealthsystem.
Abstract4CPS-119Table1
MonoclonalantibodyNpatientsNoptimisation%optimisation Mepolizumab38923,7% Benralizumab2015% Reslizumab14535,7% Omalizumab592033,9% Totalpatients1313527%
Thecollaborationpneumology-pharmacyallowstheidentificationofpatientcandidatesforoptimisation,managingto optimisealmost1outofevery3patientsintreatmentwith monoclonalantibodies.
MGuerfali*,IFadhel,EFerchichi. UhclaRabta,PharmacyDepartment,Tunis,Tunisia 10.1136/ejhpharm-2023-eahp.132
BackgroundandImportance Antiretroviral(ARV)drugsare usedinthetreatmentandpreventionofHIVinfection,they haveimprovedtheprognosisofthedisease.1
However,ARVsexposetomanyadverseeffects,whichcan compromisethequalityoflifeandvitalprognosis.2
AimandObjectives Theaimofthisstudyistoevaluatethe frequencyandintensityoftheadverseeffectsofARVs observedwithPLHIV(peoplelivingwiththehumanimmunodeficiencyvirus)andtheactiontobetakeninordertoreduce theseeffects.
MaterialandMethods Itisaprospectivestudyconductedover aperiodof3monthson40patientsconsultingforHIVin theinfectiousdiseasedepartment.
Datacollectionwasdoneusingaquestionnaire:acollection sheetwith2sections:
. thefrequencyandintensityofadverseeffectsofARVs . whattodotoreducetheadverseeffectsoftheirantiretroviral treatment.
Thedatacollectedwasenteredintoadatabase(Excel 2007).
Results Thesampleiscomposedof45%(n=18)womenand 55%(n=22)men.
ThemainadverseeffectsofARVsobservedwithPLHIV aredizzinesswithafrequencyof(F=92%)andintensity (I=85%),diarrhoea(F=80%,I=75%),headache(F=78%, I=69%),sleepdisordersandskinproblems.
52.5%ofpatientsareuninformedonhowtoreduceside effects.
40%stoppedtheirtreatmentduetoadverseeffects,50% chosetheself-medicationandothersconsultedspecialistdoctorsbecause,forthem,certaineffectsarenotconsideredas warningsigns.
Only10%ofpatientsfeelthatsideeffectsarewellmanaged,whereasaremarkablepercentageof77.5%are ‘without opinion’
ConclusionandRelevance MostPLHIVdonottalkabouttheir sideeffectstotheirdoctororpharmacistdespitetheirhigh frequencyandintensity.Itisurgenttostrengthenandimprove informationtopatientonthemanagementofadverseeffects andespeciallytopassfrominformationtotherapeutic education.
1.DioufL.M.EffetssecondairesetcomplicationsliesauxtraitementsARVinAccess tocare13thICASA-NAIROBISeptembre24th-26th2003:[A54220];p132.
2.ASSALJP – Traitementdesmaladiesdelonguedurée:delaphaseaiguëau stadedelachronicité.Uneautregestiondelamaladie,unautreprocessusde priseencharge.EncyclMédChir,Thérapeutique,25-05-A-10,18pages
1ARodríguezEsquíroz*, 1NGLizamaGómez, 1AMGascónVillacampa, 1MPíoAsín, 2LLorzaGil, 2DPMillacoyAustenrritt, 3MSarobeCarricas. 1HospitalReínaSofía,Pharmacy, Tudela,Spain; 2HospitalReínaSofía,Haematology,Tudela,Spain; 3HospitalUniversitario Navarra,Pharmacy,Pamplona,Spain
10.1136/ejhpharm-2023-eahp.133
BackgroundandImportance Forthelastseveralyears,there hasbeenagrowingtendencyofadministeringferriccarboxymaltoseinhospitals.Thisstudyhasbeencarriedoutdueto thefactthatintravenousirontreatmentsrequireveryspecific occasions.
AimandObjectives Evaluatingtheamountofferriccarboxymaltoseadministeredtooutpatients.
MaterialandMethods Aretrospective,descriptivestudy.All patientsadministeredwithintravenousferriccarboxymaltose fromJanuary2022toJune2022wereincluded.
Thefollowingdatawascollected:demographicparameters (ageandsex),clinicsandbloodtest:administereddose,haemoglobin,ironprofile,comorbiditiesthataffectsaidprofile (kidneyfailure,heartfailure,immune-mediateddisorders, oncologicalprocedure,infection)andtheconcomitantuseof oraliron.
Theindicationwasassessedfollowingthedatasheet.Cases withdiscrepancieswererevisedbythehaematologyward.It wascheckedwhetheracontrolbloodtesthadbeencarried outwithinthreemonthsandwhetherironoverloadhad occurred.
Results 273patientswereincluded,60%werewomenwith anaverageageof63,7±19,03yearsold.26.4%of patientshadnormalvaluesofhaemoglobin.79.9%of patientshadtheirironprofilerequested.26.4%hadan oralirontreatmentand12.1%haditprescribeitafterwards.In29.7%ofpatients,thetreatment ’ seffectiveness wasnotprovensincetherewasnotasubsequentanalysis withinthenextthreemonths.Anironoverloadafterthe intravenousirontreatmentwasnoticedin2.2%of patients.
26%oftreatmentswerenotindicated:8.3%duetothe briefdurationoftheoraltreatment,56.3%duetotheinexistenceofapreviousironprofileand35.2%sinceanirondeficiencywasnotfound.
ConclusionandRelevance Thisstudyconcludedthatahigh percentageofpatientsreceivedintravenousirontreatment whenitwasnotindicated.Themainreasonswerethelackof anironprofileandtheabsenceofapreviousoralirontreatment.Anintravenousironusageprotocolshouldbesetin motioninthehospitaltoensureitscorrectuseandtocarry outasubsequentstudytoanalysetheresultsafterits implementation.
4CPS-120 ADVERSEEFFECTSOFANTIRETROVIRALS: EXPERIENCEOFPATIENTS.«TALKABOUTITTO BETTERMANAGEIT»4CPS-123 CASE-CONTROLSTUDYONTHEASSOCIATION BETWEENNOSOCOMIALBLOODSTREAMINFECTIONS ANDGLUCOCORTICOIDS,TOCILIZUMAB,SYSTEMIC ANTIBIOTICS,MECHANICALVENTILATIONAND LENGTHOFHOSPITALSTAYINCOVID-19
CCodinaJiménez*,SMarin,MÁlvarez,ETerricabras,LEstrada,EValls,CGarcíaCastiñeira,ABocos-Baelo,CQuiñones. HospitalUniversitariGermansTriasIPujol, PharmacyDepartment,Badalona,Spain
10.1136/ejhpharm-2023-eahp.134
BackgroundandImportance Hospitalisedpatientswith COVID-19areoftenexposedtoimmunosuppressiveandantiinflammatorydrugsinadditiontosystemicantibiotictreatments.Nosocomialbloodstreaminfections(nBSI)havebeen associatedwiththeneedformechanicalventilationorvenous catheterinsertion.However,thereiscurrentcontroversy regardingtheinfluenceofimmunosuppressive,anti-inflammatoryandantimicrobialdrugsonnBSIoccurrence.
AimandObjectives Assesstheassociationbetweenglucocorticoids,tocilizumab,systemicantibioticsandnonpharmacologic healthinterventionsandtheoccurrenceofnBSIinhospitalised patientswithCOVID-19.
MaterialandMethods Case–controlstudyincludingcasesof nBSIepisodesinadultinpatientswithSARS-CoV-2pneumonia overaone-yearperiodandcontrolswithoutnBSI.Sociodemographicandclinicaldatawerecollectedduringhospitalisation. Bivariableanalysiswasperformed.Numericalvariableswere comparedusingtheStudent’st-testortheMann-Whitneytest andcategoricalvariablesusingthe c2orFisher ’sexacttest. Variableswithap-value<0.1inbivariableanalysiswere includedinamultivariablelogisticregressionmodeltoassess thefactorsindependentlyassociatedwithnBSIoccurrence(pvalue<0.05).
Results 50caseswithCOVID-19and50controlswere included.Meanagewas63.0±12.4(66%men,2.3±2.1 meanCharlsonindexandcomparablebetweengroups).nBSI episodesshowedsignificantlyhigherlengthofhospitalstay (LOS)(OR1.173,95%CI:1.144-1.257, p<0.001),surgeries (OR10.80,95%CI:1.310-88.5, p=0.008),needformechanicalventilation(OR8.10,95%CI:3.31-19.8, p<0.001)antibioticandglucocorticoidstherapydays(OR1.166,95%CI: 1.112-1.122, p=0.017andOR3.20,95%CI:1.325-7.287, p=0.010,respectively),andtocilizumabuse(OR9.33,95% CI:1.115-77.125, p=0.017).Non-significanthighernumber ofchronicrenalfailurecaseswerepresentamongnBSIepisodes(p=0.1).Multivariateregressionanalysesshowed mechanicalventilation(aOR4.892,95%CI:1.206-19.845, p=0.026)andLOS(aOR1.231,95%CI:1.104-1.371, p<0.001)asindependentriskfactorsfornBSIwhencorrected forthepresenceofsurgeries,centralvenouscatheter,tocilizumab,chronicrenalfailureandthedaysofantibioticandglucocorticoidtreatment.
ConclusionandRelevance ThisstudyfoundnBSIindependentlyassociatedwithmechanicalventilationandLOSand didnotfindanassociationbetweennBSIandthepharmacologicalinterventionsassessed.However,giventhebivariateassociationbetweenthesepharmacologicalinterventions andnBSI,andpreviousinconclusiveliteratureonthe effectsofthesetreatmentsonbacterialandfungalinfectionsoccurrence,furtherinvestigationwithalargersample isrequired.
REFERENCE
1.Codina-JiménezC., etal.Riskfactorsfornosocomialbloodstreaminfectionsin COVID-19affectedpatients:protocolforacase-controlstudy. EurJHosp Pharm.2022
ConflictofInterest Noconflictofinterest
4CPS-125 PREEXPOSUREPROPHYLAXISINMENATHIGHRISK FORHIV-1INFECTION
1EMBarreiroFernandez*, 2CMDominguezSantana, 2ERiosSanchez, 3MABlancoCastaño, 4FJSalmeronNavas. 1HospitalUniversitarioPuertoReal,ServiciodeFarmacia,Cadiz,Spain; 2HospitalUniversitarioPuertoReal,FarmaciaHospitalaria,Cadiz,Spain; 3Hospital UniversitarioFarmacia,FarmaciaHospitalaria,Cadiz,Spain; 4HospitalPozoblanco,Farmacia Hospitalaria,Cordoba,Spain
10.1136/ejhpharm-2023-eahp.135
BackgroundandImportance ThePreexposureProphylaxis (PrEP)forHIVinfectionwiththedrugstenofovirandemtricitabine(FTC/TDF)isrecommendedbyWorldHealthOrganizationaspartofHIVpreventiontopeopleatsubstantialrisk HIVinfection.Manycountrieshaveincludeditintheir healthypolice.However,thereisalackofinformationonits implementationinrealpractice.
AimandObjectives Toevaluateadherence,theeffectiveness andsafetytotreatmentforPrEP.
MaterialandMethods Aretrospectiveanddescriptivestudyof alladultpatientswhousedFTC/TDFforPrEPfromSeptember2020toSeptember2022.Clinicaldatawereobtained fromdigitalclinicalhistoryandtheprescriptionsoftware Dominion®:sex,age,durationoftreatment,high-riskforHIV andadherencetotreatment.
Theadherencetotreatmentwasmeasuredusingthedispensingregistry.Effectivenesswasdeterminedbyrelative reductionofHIVincidence;HIVtestingwasperformedevery threemonthsduringthisstudy.Intermsofsafety,adverse events(AE)wererecorded.
Results Fortypatients,100%men,wereincluded,withan averageagedof35(20-57)years.Allpatientswerereceived (FTC/TDF),oncedaily.Theaveragedurationoftreatment was6months(1-30),8patientsreceivedonlytwomonths. AllpatientswereathighriskforHIV,definedas:sexualrisk behaviour(tenormoresexualpartnersandanybacterialsexuallytransmittedinfections(STIs)lastyear).25%patientshad discontinuedtherapyduetolackofadherence.
NoneofthesepatientswerediagnosedHIVduringstudy. 100%relativereductionofHIVincidence.
Notreatment-associatedadverseeffectswereobserved, although75%ofpatientshadPrPE-associatedbacterialSTIs. ConclusionandRelevance
. Aquarterofpatientswerenon-adherenttotreatment,a possiblealternativewouldbeon-demandregimeninthese cases.
. Intermsofefficacy,agreatertherapeuticresultwasobserved, becomingagoodtoolprevention.
. PrEPusedwasassociatedhighincreasedbacterialSTIs, probablyduetonotusingacondom.
4CPS-127
10.1136/ejhpharm-2023-eahp.136
BackgroundandImportance Theaimofpharmacokineticmonitoringistoimproveclinicaloutcomes.Aprotocolwasagreed betweenthepaediatricandthepharmacyservicestoestablish aninitialdosageinthispopulationtoreachatherapeutic benefit.
AimandObjectives Toevaluatetheinitialdosageoftheseantibioticsbycarryingoutpharmacokineticmonitoring.
MaterialandMethods Retrospectiveobservationalstudyfrom May2020toMay2022,includingpatientstreatedwithvancomycin,gentamicin,oramikacinfromthepaediatricsservice aged<1year.ThefollowingvariableswerecollectedatOrion Clinic®:dataonage(postnatal,gestational),weight,anddosage.Thepharmacokineticresults,creatinine,andpharmaceuticalrecommendationwerecollectedfromGestlab®.Theoptimal troughintervalsestablishedintheprotocolforvancomycin, gentamicin,andamikacinwere10-15mcg/mL,0.5-1.5mcg/ mL,2-5mcg/mL,andthedosageaccordingtopostnataland gestationalagewere10-12mg/kg/8h,2.5-4mg/kg/24h,15mg/kg/ 24-48h,respectively.
Results 231patientswereanalysed,50treatedwithvancomycin,169withgentamicinand12withamikacin.Themean weightwas2.58kg,2.52kg,and1.79kgforvancomycin,gentamicin,andamikacin,respectively.Regardinggestationalage (GA),inthevancomycingroup22patients<29weeks,23 between30-36,and5>37weeks.Forgentamicin,theGA was<29weeksin25patientsand>29weeksin144.The GAintheamikacingroupwas<30weeksin7patients, between30-34weeksin4,and>35weeksin1patient.For vancomycin,58%ofpatientsweretreatedforsuspectedsepsis,whilegentamicinandamikacinwerestartedempiricallyin 100%ofcases.Theinitialdosingregimenwasinlinewith theprotocolin86%,94%and67%patientsforvancomycin, gentamicinandamikacin,respectively.Afterthefirstmonitoring,30%patientstreatedwithvancomycinwerewithinthe targetrange,63%inthecaseofgentamicin,and33%for amikacin.Asecondmonitoringwasperformed,afterdosage individualisation,in35,19and6patients,ofvancomycin,gentamicinandamikacin,reachingtheobjectivein49%,68% and67%,respectively.
ConclusionandRelevance Inmostpatients,theinitialdosage ofthethreeantibioticswasadjustedtothehospitalprotocol.
Ahighnumberofpatientstreatedwithvancomycinrequired doseadjustment,incontrastwithgentamicinandamikacin. Theroleofthepharmacist,togetherwithpharmacokinetic monitoring,isappreciatedtoachieveoptimalconcentrations.
ConflictofInterest Noconflictofinterest
4CPS-128 CLINICALPRACTICE:ANTI-VEGFTHERAPYFOR RESISTANTMACULAROEDEMA
1EMBarreiroFernandez*, 2FJSalmeronNavas, 3CMDominguezSantana, 3MABlanco Castaño, 3ERiosSanchez. 1HospitalUniversitarioPuertoReal,ServiciodeFarmacia,Cadiz, Spain; 2HospitalPozoblanco,FarmaciaHospitalaria,Cordoba,Spain; 3HospitalUniversitario PuertoReal,FarmaciaHospitalaria,Cadiz,Spain
10.1136/ejhpharm-2023-eahp.137
BackgroundandImportance Therapyapprovedfordiabetic macularoedema(DME)areintravitrealranibizumab(IR), intravitrealaflibercept(IA)anddexamethasoneintravitreal (ID).Currentlythereisagapofinformationonitsusein unresponsivetoprevioustreatment.
AimandObjectives Toevaluateclinicaleffectivenessandsafety ofafliberceptorranibizumab(Anti-VEGF)therapyforresistant macularoedema.
MaterialandMethods Anobservationalretrospectivestudyof allpatientswithDMEunresponsivetopreviousanti-VEGF therapyfromSeptember2021toSeptember2022.Clinical datawereobtainedfromdigitalclinicalhistoryandtheprescriptionsoftware:sex,aged,pathology,previoustherapy,type treatment,numberinjectionsduringstudy,responseand adverseevents(AE).
Effectivenesswasdeterminedbycompleteorpartial response.Completeresponsewasdefinedasmaintenanceof visualacuity(VA)reductionofsubretinalfluidandinflammatoryactivity.Secondly,partialresponsewasconsideredifonly oneoftheseparameterswasobserved.Intermsofsafety, adverseevents(AE)wererecorded.
Results Thirty-fourpatients,53%women(n=18),were included,withanaverageagedof69(35-90)years.The populationwaspatientsdiagnosedwithresistantmacular oedema.Almostallpatientsreceivedtreatmentwithone-line anti-VEGFtherapy(80%aflibercept,20%ranibizumab),only onepatientreceivedtreatmentwithtwolinesanti-VEGF (bevacizumabandranibizumab).Duringthestudy,261injectionsofIR(median9,range3-12)wereadministeredinto 32eyescorrespondingto27patientsand35injectionsofIA (median5,2-7)wereadministeredinto9eyescorresponding to7patients.12%(n=4)forpatientswhoreceivedcombinedtherapywithID.Completeresponsewasobservedin 27%patients(n=9),partialresponsein26%(n=8)and non-response47%(n=17).
Notreatment-associatedadverseeffectswereobserved.
ConclusionandRelevance
. Theeffectivenesswasrelativelylowinunresponsiveto previoustreatment.Futurecontrolledtrialsareneededto confirmtheuseofthistypeoftreatmentsinunresponsive patients.
. Thesafetyprofileforuseofthetherapyshoweditwas tolerated.
ConflictofInterest Noconflictofinterest
MJLucasMayol,MRodriguezMorote,SGutierrezPalomo,LSorianoIrigaray,MMorante, ANavarroRuiz*. HospitalGeneralUniversitariodeElche,ServiciodeFarmacia,ElcheAlicante,Spain10.1136/ejhpharm-2023-eahp.138
BackgroundandImportanceremotemedicationdeliverysystems(telepharmacy)areincreasinglyusedbyhospitalsnowadays.Inourhospitalaninclusionandinterruptionprotocolis used,inordertoensurecorrectpharmaceuticalcare,safeand traceabledistributionanddispensingofmedications.Sinceits implementation,aprogressiveincreaseinthenumberoftelepharmacyrequestshasbeenobserved.Despitethis,itisstill unknownwhichkindofpatientswouldbenefitthemostwith thissystem.
AimandObjectivestoconductasocioeconomicanalysisof medicationdeliveryrequeststooutpatientsinatelepharmacy programme.
MaterialandMethodsretrospectiveobservationalstudy fromFebruary1toMay31,2022.Weanalysedwhetherthe averageincomeorthedistancetothehospitalineachlocality ofthepatientsinfluencedthenumberoftelepharmacy requestsbyperformingtwodispersionmapsofrequests:a mapoftheprovincewiththenumberoftelepharmacy requestsofeachlocalitypertotalinhabitantsandasecond mapoftheprovincewiththeaveragepercapitaincomeof eachlocality.
Results 2,842patientswereincludedwith14,833total requests.Accordingtothemapofrequestsfrequencydispersion,therewasnorelationshipbetweenthevolumeof requestsfortelepharmacyandthedistancetothehospital. Someofthemostdistantareasshowedfewerapplications, whileareasclosetothehospital,wereamongthelocations withmostapplicationsperinhabitant.Asshowninthemap ofaverageincomepercapita,wefoundarelationship betweenthenumberofrequestsfromeachlocalityandits averageincome.Theeasternzoneoftheprovince,which highestincomes,hadfewerapplicationsperinhabitant,while moreapplicationstendedtobeassociatedwiththewestern zone,whichhaslowerincomes.Thisrelationshipwasnot absoluteinalllocalities,althoughtherewasageneraltrend. ExceptionswereareassuchasBellavistaandSanlúcarde Guadiana,withhighincomesbutmanyapplications.
ConclusionandRelevancetelepharmacyperformsasocial functionbyfacilitatingaccesstomedicationforthepopulation withfewereconomicresources.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
amongdifferentlevelsofHealthcareSystemsandthatincrease patientmorbidityandmortality.
AimandObjectives ToanalysetheMRactivityonadmission bythePharmacyServiceofasecondlevelhospitaltodetermineitsusefulnessasamethodforpreventingmedication errors.
MaterialandMethods Retrospectivedescriptiveobservational study(January2022-July2022)ofthepharmaceuticalinterventions(PI)reviewedinrelationtoMR.Thevariables studiedwere:clinicalservice,pharmacotherapeuticgroup,type oferrorandacceptance.Weusedtheprogrammeofelectronic medicalrecordMambrinoXXI® forreviewingchronictreatmentsandthepharmaceuticalvalidationprogramme Farmatools®
Results Inthisperiodoftime,12.946admissionswerevalidatedand658PIaboutMRwereperformedonatotalof 516patients.TheclinicalserviceswithmorePIwere:Internal Medicine(N=287,43.62%),GeneralandDigestiveSurgery (N=78,11.85%),Digestive(N=57,8.66%)andNeurology (N=40,6.08%).Themostfrequenttypeofreconciliation errorwas:omission(N=523,79.48%),followedbychangeof dosageregimen(N=114,17.33%).Thepharmacotherapeutic groupswithmostPIwere:lipid-loweringagents(N=75, 11.40%),antihypertensives(N=69,10.49%),antidepressants (N=66,10.03%),urologicaldrugs(N=53,8.06%)and inhaledantiasthmatics(N=30,4.56%).Theacceptancerate was:43.92%(N=289),24.31%non-accepted(N=160)and 31.76%non-evaluable(N=209).Excludingnon-evaluable results,theacceptanceratewas64.37%.
ConclusionandRelevance AlthoughlessthanhalfofthePI wereaccepted,theroleofthepharmacistinMRisuseful. Thisactivitycouldbeoptimisedbythepresenceofthepharmacistbothintheemergencydepartmentandonthehospitalationunit,aswellasbyimplementingactionssuchaspatient interviews.Thedetectionofthemainclinicalservicesand pharmacologicalgroupsrequiringthistypeofintervention wouldmakeitpossibletoprioritiseMRcriteriaandcreate protocolsinordertoimprovethepatientsafetyandreduce theproportionofnon-evaluableresults.
4CPS-132 ANALYSISOFPHARMACEUTICALINTERVENTIONS REGARDINGADMISSIONRECONCILIATION
EGarcíaLópez*,SCanalesUgarte,JFernández-BravoRodrigo,LRubioAlonso,GPicazo Sanchiz,DBarredaHernández. VirgendelaLuzHospital,PharmacyDepartment,Cuenca, Spain
10.1136/ejhpharm-2023-eahp.139
BackgroundandImportance Medicationreconciliation(MR)is apharmaceuticalactivitythataimstoresolveerrorsthatoccur inthecontinuationofchronictreatmentatthetransition
4CPS-133 SAFETYANDEFFECTIVENESSOFGUSELKUMABON MODERATETOSEVEREPLAQUEPSORIASIS
1ETejedorTejada, 2MRodriguezGoicoechea*, 2AMorenoLopez, 2NGarciaGomez, 2MJBarberoHernandez, 2FHornoUreña. 1ClinicHospitalofBarcelona,HospitalPharmacy, Barcelona,Spain; 2HospitalaryComplexofJaén,HospitalPharmacy,Jaén,Spain
10.1136/ejhpharm-2023-eahp.140
BackgroundandImportance Psoriasisisachronicinflammatory diseaseassociatedwithvariouscomorbidities,whichrequires multidisciplinarytreatment.Inrecentyears,anti-IL-23drugs haveemergedasanewtherapeuticoptionforplaque psoriasis.
AimandObjectives
Toevaluatesafetyandeffectivenessof guselkumabinmoderatetosevereplaquepsoriasis.
MaterialandMethods
Multicentric,observationalandretrospectivestudyofpatientsdiagnosedwithmoderatetosevere plaquepsoriasis.StudyperiodofdatacollectionwasJune 2021-June2022,activepatientsintreatmentandpatients startingtreatment.Theanthropometricdatawereage,sex,
andpreviousbiologicaltreatments.Theeffectivenessvariables areaffectedbodysurfacearea(BSA)andpsoriasisarea severityindex(PASI)AND90%PASIclearance(PASI90)collectedatbaseline,andnextvisitswithdermatologist.The maintoolsused:Diraya©fortheclinicalhistory,Modulab© forlaboratoryvaluesandExcel©foranonymiseddatarecording.Theinformationwascollectedaccordingtodataminimisationpolicy,article5.1ofdataprotection.
Results 49patients(29men)includedwithameanageof 50.9years.Themainbiologicpre-treatmentswereetanercept (31),adalimumab(11),secukinumab(9)andustekinumab(9).
Averagedpre-treatmentBSA(13,6±10.27SD)andPASI(9.7 ±6.68SD).Nextdermatologist’scontrolat5months43 patientsaveragedBSA(3.9±9.27SD)andPASI(2.9±4.17 SD).PASI90wasreachedby48.8%ofpatients.Therewere fourtreatmentdiscontinuitiesduringthisperiod(1dueto lackofadherence,1duetoprimaryfailure,1duetosecondaryfailureand1duetotoxicity).At10months25patients averagedBSA(1.8±3.28SD),PASI(1.8±3.30SD),and PASI90wasreachedby72%.3treatmentdiscontinuitiesin thisperiod(1duetogestationaldesireand2duetosecondaryfailure).At18months15patientsaveragedBSA(0.9± 1.55SD)andPASI(0.5±0.91SD).PASI90wasreachedby 73%.Patientsnotcountedhadnotgonetodermatologycontrolyetwhenouranalysisweremade.
Safety: Onepatienthadtostoptreatmentduetostrongdiarrhoeasaftereachdose.
ConclusionandRelevance Accordingtotheresultsobtained,it ispossibletoevaluateguselkumabasaneffectiveandsafe alternativeinthetreatmentofmoderatetoseverepsoriasis resistanttoconventionaltreatments.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
Results 55CPNwerepreparedand32(58.2%)wereadministered.31newbornrequiredPNand100%receivedthe standardfirstdayoflifeCPN,18(58.1%)patientswere female,themeangestationalagewas28.5weeks,themean weightwas1138.2gand12(38.7%)weremultiplepregnancies.TheindicationofPNwas:23(74.2%)preterminfants born<32.0weekswithbirthweight<1500g,4(12.9%) pretermbabiesborn>32.0weekswith<1500gand4 (12.9%)patientsborn<32.0weekswithbirthweight >1500g.ThemeantimetostartCPNwas6:01h(range 1:13-22:54h),26(83.9%)babiesinitiatedwithin8hatthe latestand5(16.1%)patientsafter8hoflife(3duetoa lackofcentralline,1lackof2readytouseCPNfortwins and1delayedprescription).30patients(96.8%)startedtrophicfeedingwithbreastmilk(maternalorbank)withinthe first24hoflife.
ConclusionandRelevance StandardfirstdayoflifeCPNready tousehasconsiderablyreducedthetimetostartPNinnewbornpatients.However,CPNwasinitiatedafter8hoflifein 5patients(mostlyduetoalackofcentralline).Standardfirst dayoflifeCPNmetthenutritionalrequirementsofallnewbornrequiringPN,notneedingtoproduceindividuallytailoredCPNinanycase.
1.2018ESPGHAN/ESPEN/ESPR/CSPENguidelines
2.Neonatalparenteralnutrition.NICEguideline2020
ConflictofInterest Noconflictofinterest
4CPS-136 STANDARDFIRSTDAYOFLIFECENTRALPARENTERAL NUTRITION,EXPERIENCEINREALCLINICALPRACTICE
1MBoschPeligero, 2GGinovartGaliana, 1AAriasMartinez, 1ASilesBaena, 2MOcañaRico, 2RPortaRibera, 1AMartínVal, 1LLagunaMarmol, 1ETerricabrasMas, 1CCodina Jiménez*, 1CQuiñonesRibas. 1HospitalGermansTriasIPujol,PharmacyDepartment, Badalona,Spain; 2HospitalGermansTriasIPujol,NeonatalUnit – PaediatricDepartment, Badalona,Spain
10.1136/ejhpharm-2023-eahp.141
BackgroundandImportance Standardparenteralnutrition(PN) solutionsshouldgenerallybeusedoverindividualisedPNsolutionsinthemajorityofpaediatricandnewbornpatients, includingvery-low-birth-weightprematureinfants,1 startingas soonaspossibleandwithin8hatthelatest.2 In2021our PaediatricandPharmacyDepartmentsdesignedastandardcentralPN(CPN)tohavereadytouse,inordertomeetthe nutritionalneedsofmostnewbornpatientsintheirfirstday oflife.
AimandObjectives Evaluatetheuseofthestandardfirstday oflifeCPNanddescribeclinicaldataofpatientsandthetime frameforitsstart.
MaterialandMethods Observational,retrospectiveandlongitudinalstudyconductedbetweenMarch2022andSeptember 2022inatertiaryhospital.Adatabasewasdesignedtorecord allpreparedCPN,theiruseanddataofpatientswhoreceived them.
4CPS-137 EVALUATINGTHEPOTENTIALCLINICALAND ECONOMICIMPACTOFCHEMOTHERAPY
PRESCRIBINGBYPHARMACISTSATAUNIVERSITY TEACHINGHOSPITAL
1SNally*, 2KDalton. 1UniversityHospitalLimerick,Pharmacy,Limerick,IrelandRep; 2UniversityCollegeCork,SchoolofPharmacy,Cork,IrelandRep
10.1136/ejhpharm-2023-eahp.142
BackgroundandImportance Chemotherapyprescribingerrors representapotentiallyseriousriskofcausingpatientharm. Whilstpharmacistprescribinghasawell-establishedrolein manyclinicalsettingsworldwideandhasbeenshowntobe effective,thereisapaucityofresearchonpharmacistprescribingchemotherapy.
AimandObjectives Assessthepotentialclinicalandeconomic impactofpharmacistprescribingversusmedicalprescribingof chemotherapy(includingsupportivemedicines)atauniversity teachinghospital.
Quantifytheerrorrateinpharmacist-anddoctor-prescribedchemotherapyprescriptions.
ClassifyprescribingerrorsaccordingtothePharmaceutical CareNetworkEurope(PCNE)classificationframeworkfor drug-relatedproblems(DRPs).
Assessthepotentialseverityofprescribingerrorsmadeby thepharmacistsanddoctorsusingavalidatedtoolandpeer reviewpanel.
Evaluatethetimetakenforthechemotherapyprescribing processbydoctorsandpharmacistsandassigncoststothese times.Estimatethecostoftheprovisionofapharmacistprescribingserviceincomparisontothedoctorprescribing practice.
MaterialandMethods
Thiswasacomparative,prospective studythatexaminedthesamesetof155prescriptionspreparedbybothdoctorsandpharmacistsforthesamesetof patients.Thepotentialseverityandadversedrugevent(ADE) probabilityassociatedwiththeprescribingerrorswasassessed usingavalidatedtoolandpeerreviewpanel.Thecostavoidanceassociatedwiththeprovisionofpharmacistprescribing wasalsodetermined.
Results Inthecomparativesampleof155prescriptions,doctorsmadesignificantlymoreerrors(105in40.6%ofprescriptions)thanpharmacists(23in14.8%ofprescriptions); p<0.05.Noneofthepharmacists’ errorswereclassifiedas 'severe',whilst16.7%ofdoctors’ errorswere'severe'(n=17). Regardingcostavoidance,apotentialyearlynetcostbenefit ofC ¼ 1,254,347.72andacost-benefitratioofC ¼ 41.82wascalculatedfortheprovisionofapharmacistchemotherapyprescribingservice.
ConclusionandRelevance Thisstudyhasshownthathaving pharmacistsprescribing – andbetterusingtheirexpertskillset
resultsinfewerchemotherapyprescribingerrors.Whilethis minimiseshealthcareprofessionals’ workloadaswellasany potentialdelaysforpatientstoreceivechemotherapy,pharmacistprescribingmostimportantlyimprovespatientsafety,and thereforethisisultimatelywhythisinitiativeshouldbeconsideredforimplementationincancercareservicesonamuch widerscaleinfuture.
ConflictofInterest Noconflictofinterest
4CPS-138
RFusterTalens*,ABasCastillo,MIGilGómez,ROlivesCasasnovas,MDMBetoretVilar, PBlascoSegura. ConsorcioHospitalGeneralUniversitario,ServiciodeFarmacia,Valencia, Spain
10.1136/ejhpharm-2023-eahp.143
BackgroundandImportance Hyperkalaemia(K>5.5mEq/L)is anelectrolytealterationthatcandeterminefatalclinicalcomplications,themostseriousbeingcardiovascularandmuscular. Sodiumzirconiumcyclosilicatebindspotassiumthroughoutthe gastrointestinaltractreducingserumpotassiumlevelsand increasingfaecalexcretiontoresolvehyperkalaemia.
AimandObjectives Analysisoftheeffectivenessofsodiumzirconiumcyclosilicate(SZC,Lokelma®)forthetreatmentof hyperkalaemiainpatientstreatedinhospitalemergencyorin differenthospitalisationunitsinthefirst48hours.
patients(92.0%)and<30ml/min/1.73m2in41(62.0%). Thecausesofhyperkalaemiawere:chronickidneydisease (CKD)(47.0%,N=31),acutekidneydisease(AKD)(39.4%, N=26),iatrogenic(7.6%,N=5)andothercauses(6.0%, N=4).Thedrugscontributingtohyperkalaemiawereangiotensin-receptorblockers(41.0%,N=27),aldosteroneantagonists(28.8%,N=19),non-steroidalanti-inflammatorydrug (24.2%,N=16),andangiotensin-convertingenzymeinhibitors (16.7%,N=11).
Initialserumpotassiumconcentrationmeanwas6.4mEq/L (5.5-8.2),being>7.5mEq/Lin21patients(32.0%).Mean reductioninpotassiumconcentrationsat24hourswas14.1% (N=22)and22.5%(N=21)at48hours.24hoursafterstartingtreatmentwithSZC,potassiumconcentrationswerenormalisedin33.3%(N=22)ofpatientsandin31.8%(N=21) after48hours.
ConclusionandRelevance HyperkalaemicemergenciesarefundamentallyassociatedwithpatientswithAKD,CKDandin concomitanttreatmentwithdrugsinducinghyperkalaemia. SZCtreatmentisanalternativetobeconsideredinpatients withhyperkalaemicemergencies,contributingtothenormalisationofserumpotassiumlevelsinfirst24-48hoursafterstartingtreatment.
WAmmor*,CAuriault,MHorellou,SPetitgas. CentreHospitalierLoireVendéeOcéan, InternalUsePharmacy,Challans,France
10.1136/ejhpharm-2023-eahp.144
BackgroundandImportance SinceJuly2007,pharmaceutical teamwritesamonthlypharmaceuticalnewsletter(PN)tohospitalstaff(HS).Itcontainsinformationaboutdrugsormedicaldevices(pharmaceuticalnews,remindersofappropriate use,etc.).ItiscurrentlysenttohealthmanagersandHSby e-mailandisaccessibleonthehospitalwebportal.However, sinceitsimplementation,nostudyhasbeencarriedoutconcerningtheadherenceofHStothistool.
AimandObjectives TheaimistoassesstheadherenceofHS tothePNandtoproposeareasofimprovement.
MaterialandMethods
One-yearretrospectiveandobservationalstudywascarried,includingpatientstreatedinhospital emergencyoradmittedwithinitialpotassiumlevels 5.5 mEq/LwhoreceivedSZC.TheSZCregimenwas10gevery 8horally.Serumpotassiumconcentrationswereconsidered normalwithvaluesbetween3.3-5.1mEq/L.Thevariables collectedwereage,sex,diagnosisofheartfailure,serum potassiumconcentrations(at0,24,and48hoursafterstartingtreatmentwithSZC),thereasonforhyperkalaemia,glomerularfiltrationrate(GFR,estimatedwithCKD-EPI formula),concomitantdrugsthatcouldinfluencethe hyperkalaemia.
Results 66patients(63%men)withamedianageof79years (41-97)wereincluded.Heartfailurewasdiagnosedin27 patients(41.0%).TheGFRwas<60ml/min/1.73m2in61
MaterialandMethods Wedevelopedtwosurveysindigital andpaperformat:oneforthehealthmanagersandtheother forthereadersofthePN.Thesurveyforthemanagerswas firstsenttothemtofindouthowtheycirculatedthePNto thestaffintheirunits.Unitsforwhichmanagerialresponses hadnotbeencollectedwereexcluded.ThesurveywasconductinSeptember2022bytwopharmacyinterns.
Results 16healthmanagersrespondedtothesurvey:100% readthePN,81%distributedit(85%posteditinthedepartmentand15%bye-mail).
123readers(including40%ofnurses,20%ofnursing assistants,15%ofdoctors)from20departmentsrespondedto thesurvey.68%ofHSreadthePN:20%consulteditbyemail,30%readitonthehospitalwebportal,34%readit displayedintheunitand5%readitatthepharmacy.75% findituseful,83%aresatisfiedwithitscontent,83%withits presentationand63%withthedistributionchannel.Finally, 48%ofreaderswouldlikethePNtobedisplayedintheir
units,40%wouldlikeittobesentbyemailand15%would likeadedicatedwebsite.
ConclusionandRelevance ThemajorityofHSsupportthe PN,finditusefulandappreciateitscontentandpresentation. PartoftheHSdidnotknowthePN,whichshowsthatthe distributionmethodneededtobeimproved.Wehavethereforeupdatedthemailinglist.Thissurveyhasenabledusto highlightthesatisfactionwiththeHNandimproveits distribution.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-142 EVALUATIONOFPREMEDICATIONUSEINADVERSE DRUGREACTIONSOCCURRENCEINPATIENTSWHO RECEIVEDINFLIXIMABTOTREATINFLAMMATORY BOWELDISEASE
1XLarreaUrtaran*, 1QLópezNoguera, 2GEspinMartí, 2LDVTorrealbaMediana-, 1CDíez Vallejo, 1ÀCastellóNòria, 1MBrugeraTeixidor, 1ENoguéPujadas, 2DBusquetsCasal, 1RSacrestGüell. 1HospitalDr.JosepTrueta,PharmacyDepartment,Girona,Spain; 2Hospital Dr.JosepTrueta,DigestiveDepartment,Girona,Spain
10.1136/ejhpharm-2023-eahp.146
4CPS-141 PHARMACEUTICALINTERVENTIONSINAMEDICAL EMERGENCYDEPARTMENT:6-MONTHSEXPERIENCE
1,2OElQabissi*, 1,2FZLasri, 1,2HDaoudi, 1,2AChaibi, 2,3MAitelCadi. 1FacultyofMedicine andPharmacyofRabat-MohamedVSouissiUniversity-Rabat-Morocco,Pharmacy,Rabat, Morocco; 2IBNSinaHospital,Pharmacy,Rabat,Morocco; 3FacultyofMedicineand PharmacyofRabat-MohamedVSouissiUniversity-Rabat-Morocco,Toxicologyand Pharmacology,Rabat,Morocco
10.1136/ejhpharm-2023-eahp.145
BackgroundandImportance Medicationerrorsareamajor globalpublichealthproblemthatrequiresaveryimportant approachandcollaborativeworkbetweenphysicians,pharmacistsandnurses.Pharmaceuticalinterventions(PI)areaneffectivewaytofightdrugiatrogeny.
AimandObjectives Theobjectiveofthestudywastoanalyse pharmaceuticalinterventionsandtheirimpactonpatientshospitalisedinamedicalemergencydepartment
MaterialandMethods Thisisaretrospectivestudyconducted inamedicalemergencydepartmentoftheIbnSinaHospital inRabatoveraperiodof6months.Prescriptionswereanalysedandvalidatedaccordingtothemethodologyofthe FrenchSocietyofClinicalPharmacy(SFPC).Therelevanceof thePIswasassessedbytheiracceptanceratebytheprescribersandtheirclinicalimpactwasevaluatedaccordingtothe Hatoumscale.
Results Atotalof158Pharmaceuticalinterventionswere recordedoversixmonths.Ofthese,98%wereacceptedby theprescriber.Thesexratio(male/female)was1.35.The averageageofourpatientswas56.06±15.81years.86PIs (55%)concernedanantibiotic.Themainprescriptionproblemswereoverdose(29%).Ourinterventionsconcerneddosageadjustment(32.27%),optimisationofadministration modalities(22.15%).
ConclusionandRelevance Thisstudyhighlightstheimportance oftheclinicalpharmacistinthefightagainstdrugiatrogeny.
1.Hedira etal.Évaluationdel'activitédepharmaciecliniquedansuncentrehospitaliermère-enfantenTunisie – 53-LePharmacienHospitalieretClinicien.2018
2.A.Clementz etal.Miseenplaceetévaluationdesvalidationsd'ordonnanceset d'interventionspharmaceutiquesdansunserviced'urgencesadultes. LePharmacien HospitalieretClinicien,Juin2017; 52:e47–e53
3.AbdelazizH etal.Impactdesservicesdepharmaciecliniquedansuneunitéde courtséjourd'unserviced'urgencehospitalierauQatar. IntJClinPharm 2016;38:776
9
4.NielsenT etal.Servicedepharmaciencliniciendansleserviceaigu. IntJClin Pharm 2013;35:1137
51.
BackgroundandImportance Infliximabcancauseinfusionrelatedreactionslikedelayedhypersensitivityoranaphylactic shock.Usingcorticosteroidsorantihistaminesaspremedication canreduceadversedrugreactions(ADRs)frequency.
AimandObjectives Toevaluatepremedicationimpacton ADRsoccurrenceinpatientswithinflammatoryboweldisease (IBD)whoreceivedinfliximab.
MaterialandMethods Retrospectiveobservationalstudyin patientswithIBDwhoreceivedintravenousinfliximabfrom January2016toDecember2020.Thevariablescollected were:demographic(age,sex),clinical(typeofinflammatory boweldisease,HarveyindexBradshawinCrohn'sdisease, Mayoindexinulcerativecolitis),premedicationused(typeof drugandnumberofadministrations),numberofinfliximab administrationsandtheADRscharacteristics.Forthestatistical analysis,mean,standarddeviationandabsoluteriskwere used.
Results 119patientswereincludedwithanaverageageof46 ±17yearsand42%women.42patientshadulcerativecolitis,74patientshadCrohn'sdisease,and3patientshadindeterminatecolitis.Inthebaselinestudy,patientswithCrohn's diseasehadHarveyscoremeanof7.1±3.7andpatients withulcerativecolitishadpartialMayoscoremeanof3.7± 2.3.Atotalof1909infliximabinfusionswereadministrated andpremedicationwasusedin1185administrationin80 patients.Premedicationwasadministratedin21.2%(n=17) duringinductionphase,in32.5%(n=26)duringmaintenance phase,andin46.3%(n=37)duringbothphases.Glucocorticoidswereusedasapremedicationin97.5%ofcases.
25ADRswererecordedin21patients.Thepatients (n=17)whoreceivedpremedicationhad21ADRsandan absoluteriskof10.3%(CI95,0.7%-19.8%).Intheother group,thepatientswhodidnotreceivepremedicationhad4 ADRs(n=4)andanabsoluteriskof21.3%(CI95,12.3%30.2%).44%ofADRsoccurredininductionphaseand56% inmaintenancephase.ThemainsymptomsofADRSregisteredwereskinmanifestations(n=16),cardiovascular(n=6) andrespiratorysymptoms(n=3).
ConclusionandRelevance NolowerabsoluteADRriskwere observedinpatientswhoreceivedpremedicationcomparedto patientswhodidnotreceivepremedication.Morestudiesare neededinordertoevaluatetheimpactofpremedicationon ADRsoccurrence.
REFERENCESAND/ORACKNOWLEDGEMENTS
4CPS-144
10.1136/ejhpharm-2023-eahp.147
BackgroundandImportance Remdesivirwasthefirstantiviral authorisedbytheEuropeanMedicinesAgencyforthetreatmentofCoVID-19disease.
AimandObjectives Theaimistodescribetheeffectiveness andsafetyofremdesivirinpatientswithSARS-CoV-2infectioninrealclinicalpractice.
MaterialandMethods Observational,descriptive,retrospective studyinalevel-IIhospital.
HospitalisedpatientswithSARS-CoV-2infectionandprescriptionofremdesivirfromApril21-March22wereincluded. DatawereobtainedfromtheUnidosisFarmatools® module andMambrinoXXI®
Variables: sex,age,recommendationsofremdesivirdatasheet (timefromsymptomonsettoadministration £7-days,dosing regimen,durationoftreatmentandglomerularfiltrationrate (GFR)(contraindicatedif<30mL/min).
Effectivenessassessment: hospitalstay,IntensiveCareUnit(ICU) admission,clinicalrecoveryinpatientswith5-daytreatment.
Safetyassessment: elevatedtransaminases(pre-and-post-remdesivirlevels;contraindicatedif 5timesupperlimitofnormalLSN)
Results 59patientswereincluded,64%male,medianage67 (30-101)years.100%startedwithin7-daysofsymptomatology onset(median:3-days)andcompliedwiththerecommended dosingregimen.In93.2%thedurationwas5-days,one patientremainedontreatmentfor7-daysand3discontinued earlierduetoclinicalworsening.MeanGFR:79ml/min and96.6%compliedwiththerecommendation(GFR>30ml/ min).Themedianhospitalstaywas8-days(3-133).Twelve patientsrequiredadmissiontotheICU,twoofwhomdied. Clinicalrecoverywasachievedin91.1%ofpatientswhocompletedthe5-dayregimen.Duringthehospitalstay,7patients diedwithamedianageof85years(59-95).Priortoadministration,22.2%patientsshowedtransaminaselevelsabovethe LSN,includingonepatientwith5LSN.Afteradministration, transaminasesincreasedin31.1%,including5patientswith 5LSN,2ofwhomhadinitiallynormalvalues.
ConclusionandRelevance Allpatientsreceivedremdesiviras earlyasrecommendedandaccordingtotheconclusionsofthe pivotalclinicaltrial,wherethissubgroupwaspostulatedto havethegreatestclinicalbenefit.Althoughonethirdof patientshadelevatedtransaminasemia,nonerequiredtreatmentdiscontinuation.However,otherparameterswouldneed tobecollectedtoassesssafetymorecomprehensively.Despite thelimitationsofthestudy,inourexperience,remdesivir appearstohaveagoodeffectivenessandsafetyprofileand maybeatherapeuticalternativeinthetreatmentofCOVID19disease.
ConflictofInterest Noconflictofinterest
4CPS-145 CONSENSUSVALIDATIONOFASCREENINGTOOLFOR CARDIOVASCULARPHARMACOTHERAPYIN GERIATRICPATIENTS:THERASP_CARDIOLIST
1HdeSchutter, 1JHias*, 1LHellemans, 1KWalgraeve, 2JTournoy, 1LVanDerLinden. 1UniversityHospitalsLeuven-Belgium,HospitalPharmacyDepartment,Leuven,Belgium; 2UniversityHospitalsLeuven-Belgium,DepartmentofGeriatricMedicine,Leuven,Belgium 10.1136/ejhpharm-2023-eahp.148
BackgroundandImportance Cardiovasculartherapieshave beenidentifiedasmajorculpritsforadversedrugevents. Theirinappropriate(under)useputsgeriatricpatientsat increasedriskforavoidableharm.Thesetherapiesare usedfrequently,accountingforapproximatelyhalfofall prescribeddrugsingeriatricpatients.Paradoxically,most studiesonmedicationreviewsingeriatricpatientshave notspecificallytargetedcardiovasculartherapies.This mightbeowingtoalackofresources,trainingorexplicit supporttoperformtargetedmedicationreviews.Consequently,thereisaclearneedforanupdatedscreening tool,specificallytargeting cardiovasculartherapiesingeriatricpatients.
AimandObjectives Weaimedtoupdateandvalidatethecardiovascularsegmentofapreviouslydevelopedscreeningtool, theRASPlist.TheupdatedRASP_CARDIOlistwasintended foruseingeriatricpatientsbytrainedhealthcare professionals.
MaterialandMethods Athree-stepstudywasconductedbya collaborationofthepharmacy,geriatricmedicineandcardiologydepartmentsofalarge,academichospital.First,the cardiovascularsegmentoftheRASPlist(version2014)was updatedtakingintoaccountpublishedresearch,otheravailablescreeningtoolsandtheinputofend-users.Secondly, thisdraftwasreviewedduringthreepaneldiscussionswith fiveexpertcardiologistsandthreeclinicalpharmacists,all ofwhomhadrelevantexpertiseingeriatricpharmacotherapy.Thirdly,thecontentwasvalidatedusingamodified DelphiTechniquebyapanelofEuropeanhospitalpharmacists,cardiologists,geriatriciansandaninternalmedicine physician.Consensuswasachievedincaseof 80%agreementamongexperts.Thefinal constructwascomparedto thecardiovascularsegmentoftheSTOPP/STARTcriteria version2.
Results SeventeenexpertsfromfourEuropeancountries participatedintwovalidationrounds.Consensuswas achievedforallstatementsoftheRASP_CARDIOlist.One newstatementwasadded.Thefinalconstructcompriseda listof95statementsrelatedtopotentiallyinappropriate prescribingofcardiovascularagents.Approximately90% (29/32)ofthecardiovascu larstatementsofthesecondversionoftheSTOPP/STARTcriteriawereincludedinthe RASP_CARDIOlistandtheRASP_CARDIOlisthad66 additionalstatements.
ConclusionandRelevance TheRASP_CARDIOlistisan updatedandvalidatedexplicitscreeningtooltooptimisecardiovascularpharmacotherapyingeriatricpatients.
ConflictofInterest Noconflictofinterest
PHernandoMartínez*,GMarcosPérez,VLafargaLapieza,IMartínNiño,APortelaSotelo, BRealAragón,DBarredaHernández. HospitalVirgendelaLuz,PharmacyDepartment, Cuenca,Spain4CPS-146 ‘REALWORLD’ EXPERIENCEOFELEXACAFTOR/ TEZACAFTOR/IVACAFTORINTHETREATMENTOF CYSTICFIBROSIS:EFFECTIVENESSANDSAFETY EVALUATION
CFernandezCuerva*,JDParadasPalomo,TChinchillaAlarcon,IMuñozCastillo. Hospital RegionalUniversitariodeMálaga,ServiciodeFarmacia,Málaga,Spain
10.1136/ejhpharm-2023-eahp.149
BackgroundandImportance Cysticfibrosis(CF)isalife-limitingrecessivegeneticdisordercausedbypathogenicvariantsin theCFTR(cysticfibrosistransmembraneconductanceregulator)gene,resultinginincreasedviscosityanddifficultmucus clearance.Introductionofelexacaftor(ELX)/tezacaftor(TEZ)/ ivacaftor(IVA)toclinicalpracticehasbroughtachangeinthe clinicalapproachsincetheymodulateCFTR.
4CPS-147 REALWORLDDATA(RWD)ANALYSISONUSEOF IMMUNECHECKPOINTINHIBITORS(ICI)FORNONSMALL-CELLLUNGCANCER(NSCLC)
1SMasucci*, 2FSalerno, 3LRicci, 1MBellero, 1ABianco, 1GFFazzina, 1OSorrenti, 2GLacidogna, 2MDiMaio, 1AGasco. 1A.OOrdineMauriziano-UmbertoI,Hospital Pharmacy,Turin,Italy; 2A.OOrdineMauriziano-UmbertoI,OncologyDepartment,Turin, Italy; 3UniversityofTurin,Pharmacy,Turin,Italy
10.1136/ejhpharm-2023-eahp.150
AimandObjectives
Toassesseffectivenessandsecurityof ELX/TEZ/IVAinpatientsonatertiaryhospital.
MaterialandMethods
Observational,retrospectivestudycarriedoutbetweenMarch2020andSeptember2022,including alladultpatientstreatedwithELX/TEZ/IVA+IVAinour hospital.
Variablesincluded: age,sex,ageofdiagnose,pulmonaryfunction:measuredwith%pFEV1(medianpercentpredicted forcedexpiratoryvolumein1second)andpulmonaryexacerbations;treatmentadjustment,adverseeventsandtreatment suspension.
Datawerecollectedfromelectronicmedicalrecordsand pharmacydispensingprograms.
Results
Thirty-onepatientswereincluded: male45%(n=14),median of31yearsold(rank17-45),medianageofdiagnosisof4 months(0-38).BeforetakingELE/TEZ/IVA+IVA,45%(n=14) patientsreceivedTEZ/IVA+IVAasCFTRmodulator;55% (n=17)didnotreceiveanyCFTRmodulator.Medianlength ofELX/TEZ/IVA+IVAtreatmentatthemomentoftheanalysiswas9.43months(4.5-31.4).
%pFEV1duringtreatmentaugmentedin83%patients (n=26),slightlydecreasedin13%(n=3)anddidnotvaryin 1patient.Twopatients(6.5%)presentedpulmonaryexacerbationsthatrequiredantibiotictreatmentbutnothospital admission.
Twopatients(6.5%)requiredELX/TEZ/IVA+IVAadjustment:oneduetointeractionswithpotentCYP3A4inhibitors andotherbecauseofhepaticinsufficiency(Child–PughB). Nine(29%)patientspresentedanincreaseoftransaminase and/orbilirubininclinicalanalysis:onepatienttemporarily discontinuedtherapyandonesuspendedtreatmentdefinitely.
ConclusionandRelevance TheintroductionofELEX/TEZ/IVA toCFtreatmenthasbeenahopefuladvancethathasshown inourpopulationtohaveagoodsafetyprofile-whichcanbe managedwithregularcheck-ups-andwithagoodefficacy profile,achievinganincreaseof%pFVE1inashorttime.
REFERENCESAND/ORACKNOWLEDGEMENTS Noconflictofinterest.
ConflictofInterest Noconflictofinterest
BackgroundandImportance InItaly,monoclonalantibodies actingonprogrammedcelldeathprotein(PD-1),nivolumab(N)andpembrolizumab(P),oronthePD-L1ligand, atezolizumabordurvalumab, areauthorisedforthetreatmentofNSCLC.RegistrationRCTsmaynotgivedefinitiveanswersregardingtheoptimalICI'sdurationof treatment(DOT).Thereisevidencethattreatmentmay beinterruptedbeforeprogression,orbeforescheduled cyclesarecompletedfordifferentreasonsandthatpotentiallyaffectsefficacy.Arethecausesforpatientdiscontinuationtreatment(TDC)inRCTsandintherealworld comparable?
AimandObjectivesAim: evaluatetheappropriatenessoftreatmentchoicesbyanalysingDOTwithICIinacohortof patientswithNSCLC
MaterialandMethods For27monthsdatawererecordedon patientstreatedinI-linewithPorcombinationsofP+pemeetrexed+platinumchemotherapy(PPC),orinII-linewithN. ThepercentageofPD-L1expression(PD-L1el)wasobserved; medianDOTwasmeasured,andthedatawerestratified accordingtotreatmentdiscontinuationcauses.
Results Atotalof73patientsweretreated,62%men,38% women,29%smokers,3%non-smokers,40%ex-smokers, and28%n.a.Atthepresentdate5%ofthe73patientsare undergoingtreatmentand4%completedallcyclesoftherapy.Patientsweretreatedwith:33%N,49%P,and18% PPC.ThePD-L1elinthepopulationtreatedwasfor:N4% >50%,63%<1%,33%n.a.versusRTC77%>5%and 33%>50%(3);forP:<5%8%and>50%92%versus RTC100%>50%(4);forPPC:67%<5%and33%< 50%versusRCT-data<=50%63%and32%>50%. MedianDOTforP(8vs7.9months),N(5vs2.8months), andPPC(2vs9.8months)inRWDandRCTsrespectively. RWDTDC:96%7%(4%N,3%P17%C),progression 67%(79%N,53%Pand67%PPC)toxicity22%(13%N, 28%Pand17%PPC).FromRCTdata:death/progression (67%N,47%P,30.8%PC)andtoxicity(3%N,13.6%P, 13.8%PPC)
ConclusionandRelevance RCTandRWDdataareconflicting.MedianDOTforPandtheNdeath/progressionrate arecomparable.Thetreatmentchoicesmadewereappropriate,maximisingtreatmentefficacy,whilerespectingtherisk/ benefitprofileinapopulationdifferentfromthatofthe RCTs
REFERENCESAND/ORACKNOWLEDGEMENTS ConflictofInterest Noconflictofinterest
AChachulski*,CNiot,LSoubelet,LReal. CentreHospitalierD'arras,PasdeCalais,Arras, France
10.1136/ejhpharm-2023-eahp.151
BackgroundandImportance Inhealthinstitutions,painmanagementisanobligationfromdiagnosistotreatment.However,inmentalhealth,itisdifficulttotreatitbecause psychiatricdiseasesmayaltertheperceptionofthepainand therearedruginteractions(DI)betweenpsychotropicdrugs andanalgesics.
AimandObjectives Theaimofthestudyistofindguidelines onpainmanagementinpsychiatryandreviewthecurrent stateofanalgesicprescriptionsinourpsychiatricunits.
MaterialandMethods Abibliographicsearchonpainmanagementinpsychiatrywascarriedoutandanobservationalaudit ofanalgesicprescriptionswasdone,atagivenday,inthefive psychiatricunitsofourestablishment.
Dataareexpressedasaverage+/-standarddeviationand resultsaspercent.
Results Thebibliographicsearchofferspainassessmentscales inpsychiatryeveniftheyarenotspecifictothispopulation. Nevertheless,thereisnotanyconsensusonthetherapeutic painmanagementinmentalhealth,neitheratnationalnor internationallevel.
Thedayoftheaudit,on88patients,47(53%)were treatedwithanalgesics.Thesepatientswere50+/-17years oldandthesex-ratiowas1.04.
Fiftyprescriptionlinesforanalgesicswereidentified.The mainmoleculesfoundwere:paracetamol,prescribedalone on42prescriptions(90%),andtramadol,aloneon2prescriptions(4%)orco-prescribedwithparacetamolon2prescriptions(4%).Oneprescription(2%)includedparacetamol/opium +ibuprofen.
Ofallthepainkillers,90%wereprescribedconditionally, including79% ‘ifneeded/pain’;14% ‘ifAnalogVisualScale> 3,temperature>38°C ’;7% ‘ifAnalogVisualScale>3’
ADIanalysishasbeenperformedbetweenanalgesics/psychotropicsandasingleprescriptionwithanassociationnot recommended(tramadol/paroxetinewithriskofinefficiencyof tramadolduetometabolicinhibition)wasfound.Theabsence ofcontraindicationcanbeexplainedbythepharmaceutical analysisoftheprescriptions.
ConclusionandRelevance Followingthisaudit,across-referencedtableofexistingDIbetweenanalgesics/psychotropicswas madeandalternativetreatmentincaseofDIwasproposed. Theseworks,andalsoremindersofthescalesthatcanbeused inpsychiatrytoassesspainandthepossibilitiesoftreatment accordingtothementaldisorder,werepresentedtopsychiatristsduringasessiontofacilitatetheirpainmanagement.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-150 THEROLEOFCLINICALPHARMACISTINEMERGENCY DEPARTMENT
1LLópez-Vinardell*, 1RMerino-Mendez, 2JRuiz, 2AJuanes, 2CSocias-Cañellas, 1MBitlloch, 3MPuig-Campmany, 1LCampins. 1HospitaldeMataróConsorciSanitariDelMaresme, Pharmacy,Mataró,Spain; 2HospitaldelaSantaCreuISantPau,Pharmacy,Barcelona, Spain; 3HospitaldelaSantaCreuISantPau,EmergencyDepartment,Barcelona,Spain
10.1136/ejhpharm-2023-eahp.152
BackgroundandImportance Pharmacistroleintheemergency department(ED)hasexpandedoverthelastdecades.However,thereislimitedpublishedliteraturerelatedtotheinterventionscarriedoutintheseunits.
AimandObjectives Toperformadescriptiveanalysisofpharmaceuticalinterventions(PI)inED,theiracceptancerate,the mainprescribingerrors(PE)detectedandthemainAnatomicalTherapeuticChemical(ATC)groupsinvolved.
MaterialandMethods Aretrospectivemulticentricstudywas performedintheEDofasecondaryandatertiaryhospital thatserveabout685.000totalinhabitantswithanoverallof 228.550emergencyattendancesperyear.PIandPEwere documentedfromMondaytoFridayovera4-hourperiod betweenJune-September2022.Dosageandfrequencyadjustment,formularyanddrugmodification,medicationinitiation anddiscontinuation,andpharmacokineticmonitoringwerethe PIincluded.PEweredividedintothreegroups:lackofefficacy,potentialsafetyproblemornecessary/unnecessary treatment.
Results Outof857interventionsregistered,40.4%were relatedtodosageadjustment;32.0%medicationinitiation; 16.0%medicationdiscontinuation;5.6%drugmodification; 3.5%pharmacokineticmonitoring;1.5%frequencyadjustment and1.1%formularyinterchange.RegardingPI,71.9%were accepted,21.9%wererejectedand6.2%werenotevaluated becausepatientsweredischargedordead.AsforPE,37.8% wererelatedtonecessary/unnecessarytreatment,32.6%potentialsafetyproblemand29.6%toalackofefficacy.ThePE detectedwerereconciliationdiscrepancies(39.7%),underdose (21.4%),overdose(19.0%),duplicities(4.9%),contraindications(3.3%),adversedrugevents(1.5%)andinteractions (0.9%).ThemainATCGroupsinvolvedwerebloodand bloodformingorgans(B)(21.7%),anti-infectiveforsystemic use(J)(21.7%),cardiovascularsystem(C)(20.9%)andnervoussystem(N)(18.1%).
ConclusionandRelevance Dosageadjustmentsanddrugtherapyinitiationwerethemostcommondocumentedinterventions.MorethanhalfofPIwereaccepted.Themostfrequent PEwererelatedtonecessary/unnecessarytreatment.The majorityobservedPEwerereconciliationdiscrepancies.The mainATCgroupsinvolvedwereB,JandC.Thegreatnumberofinterventionsandthehighrateofacceptanceseemsto showthatEDpharmacist,asamemberofamultidisciplinary patientcareteam,isabletodecreasethenumberofmedicine errorsandtoimprovethequalityandsafetyofmedicalcare.
REFERENCESAND/ORACKNOWLEDGEMENTS
Thankyouall.
ConflictofInterest Noconflictofinterest
4CPS-151 ACETYLSALICYLICACIDDESENSITISATIONIN PATIENTSWITHCORONARYARTERYSYNDROME: LITERATUREREVIEW,RETROSPECTIVEANALYSISAND PATIENTFOLLOW-UPPROCEDUREINANIIALIAN CARDIOLOGICALCENTRE
10.1136/ejhpharm-2023-eahp.153
BackgroundandImportance Desensitisationprotocolsforthe treatmentofhypersensitivityto acetylsalicylicacid (ASA)
1AIezzi*, 1VTeso, 2DPinalli, 1SZitelli, 1GBallardini, 1BTebaldini, 1EOmodeoSalè. 1Centro CardiologicoMonzino,ServizioDiFarmaciaOspedaliera,Milano,Italy; 2UniversitàDegli StudiDiPavia,CorsoDiLaureaInFarmacia,Pavia,ItalyconsistintheadministrationofincreasingdosesofASAata settimeinordertosensitisethepatienttotheactivesubstanceandinitiateachronictreatment. Hypersensitivity to thedrugoccursinawiderangeofthepopulation,bothin healthysubjectsandpatientswithcoronaryheartdisease. Thisconditionmayaffectpatientcompliancetotherapyand increasetheriskofischemiceventsespeciallyinsecondary prevention.
AimandObjectives Theaimoftheworkisobtainingasystematicreviewoftheliteratureconcerningtheexisting desensitisationprotocols.Thepurposeistoconductadescriptive analysisofthepopulationandevaluatetheeffectivenessand safetyoftheprotocolovertheshortandlongterm.
MaterialandMethods Aretrospectiveanalysiswasconducted onagroupofpatientstreatedwith Rossini’sprotocol, 1 an increasingoraladministrationofASAto100mginfiveanda halfhours.
Results Theliterature'sreviewhasshowntheRossini’sprotocolhasthegreatestnumberofsampleandthebestefficacy andsafetydata.Theretrospectiveanalysisallowedtheevaluationofthegroupcomposedof30patientsaged>18 years,admittedtothecentrebetweenJanuary2020and April2022,diagnosedwithcoronaryarterysyndrome. 83.33%reportedahistoryofhypersensitivitytoASA,especiallywithskinmanife stations(n=8).Themostsensitive patientsrecei vedpre-medicationbeforeundergoingtheprocedure;despitetreatment,20%developedmildadversereactions.Atdischarge73.33%ofpatientsweretreatedwithan antiplatelettherapyofwhich77.27%withASA.50%ofthe patientsunderwentafollow-up,whichtookplaceonaverage after6months;uponre-evaluation60%wereontreatment withASA.
ConclusionandRelevance TheevidencesuggeststhattheRossini’sprotocoliseffectiveforawidespectrumofpatients. Thehospitalpharmacistinagreementwiththecardiologist willevaluatethepossibilitytoimplementasolution-basedformulationtotreatmorefragilepatients,whopresenthistoryof allergytoASA,dysphagiaorrequiringinterventional procedures.
1.R.Rossini, etal,Aspirindesensitizationinpatientswithcoronaryarterydisease: resultsofthemulticenterADAPTEDregistry, CircCardiovascInterv, 2017;10
ConflictofInterest Noconflictofinterest
appropriatenessandgivetreatmentsdataof2021andthefirst eightmonthsof2022inourhospital.
MaterialandMethods Theauthorspresenttheirroleinthe authorisationprocessforoff-labeluse,incompliancewith currentlegislation,andmonitoringdatawhicharecollectedfromspecialists ’ assessments/re-evaluations.Psychiatristscollectthepatient'sinformedconsent,filloutthe authorisationformanddeliverittopharmacists.Pharmacistsassesswhetherexistthec onditionsunderwhichthe ketamineinfusionissustainableintermsofbothappropriatenessandcosts.Oncethetreatmenthasbeenauthorised,thecollecteddataareenteredinadatabase periodicallyupdatedwitha uthorisationanddispensing information.
Results 37patientsweretreatedfrom01/01/21to31/08/22, 17in2021and20in2022.
In2021,4patientshadalreadyreceived1+treatmentsthe previousyear,whilst13patientsreceivedtheinductiondose. Ofthesepatients,10switchedtoastandardmaintenancedosageasrapidtherapeuticbenefitwasobserved;only3discontinuedtreatmentorhadadifferentdosageforclinicalreasons.
Between01/01/22and31/08/22,12patientsreceivedthe inductiondosewhile8hadalreadyreceived1+treatments thepreviousyear;ofthe12patients,10switchedtoastandarddoseasarapidtherapeuticbenefitwasobservedwhereas only2discontinuedtreatment.
ConclusionandRelevance Anintravenousslowinfusionofketamineissafeandeffectiveinthesymptoms ’ stabilisation.
Theroleofthepharmacywill betocontinuemonitoring andimproveadatabasetobeusedtoproposeketamine ’ s administrationindepressionfor inclusioninthelistof medicinessuppliedbytheNationalHealthServicetobe usedforatherapeuticindicationotherthantheauthorised ones.
4CPS-153 OFF-LABELUSEOFKETAMINEFORRESISTANT DEPRESSION:ROLEOFTHEHOSPITALPHARMACIST
1AMichielon*, 1ACorzani, 2MTBianco. 1UniversitàDiSiena,ScuolaDiSpecializzazioneIn FarmaciaOspedaliera,Siena,Italy; 2AziendaOspedaliero-UniversitariaSenese,UocFarmacia Ospedaliera,Siena,Italy
10.1136/ejhpharm-2023-eahp.154
BackgroundandImportance Anintravenousslowinfusionof ketamine,glutamatereceptorantagonist,hasemergedasan effective,safeandrapidlyactingantidepressantindifferent studies.Itsefficacyisreportedintreatmentofresistantrecurrentmajordepressionandbipolardepression.
Inourcountry,ketamineisnotcurrentlyauthorisedfor theseindicationsthereforeitisusedoff-label.
AimandObjectives Thepurposeistopresenttheroleof pharmacistsmonitoringketamine’soff-labelprescriptive
4CPS-155 CREATIONANDVALIDATIONOFAMEDICATION REVIEWSUPPORTTOOLFORPOTASSIUMCHLORIDE INJECTION(KCL-INJ)PRESCRIPTIONS
MBabin*,CDebanne,ACDesbuquois,MBoisgontier. CentreHospitalierCompiegne Noyon,60321,Compiègne,France
10.1136/ejhpharm-2023-eahp.155
BackgroundandImportance KCl-injisariskydrug,itsadministrationerrorisaNeverEvents.Limitingitsusetojustified situationscontributestoitssecurity.MedicationReview(MR) contributestothislimitation.Despiteawarenesscampaigns, non-compliantprescriptionspersist.DuringtheMR,thePharmaceuticalIntervention(PI)includesaPrescriptionProposal (PP):forKCl-injtheclinicalcontexthasastrongimpactand complicatestheMR.
AimandObjectives Creationandvalidationofasupporttool fortheMRofKCl-injprescriptionsallowingtakinginto accounttheentireclinicalcontextofthepatient.
MaterialandMethods Bibliographicresearchassociatedwith brainstormingonthevariousclinicalandbiologicalcriteriaof thepatientandtheirconsequencesallowedsettingupofa flowchart.
Forvalidation: experimentationofthetoolinaprescriptions prospectivestudy(foreachprescriptiontheproblemrelatedto
therapeutics,theproposedPPanditsacceptancearecollated inanExcelfile);thendiscussionandvalidationoftheresults inMedicinesandSterileMedicalDevicesCommission (MSMDC),inparticularforthenotacceptedPIs.
Results Theflowchartcriteriaarekalemia,oralintake,KCl-inj concentration,KCl-injinpreventionduringhigh-dosehypokalemictreatments,initiationoftreatment.Eachofthesituations identifiedislinkedtoaPIortheabsenceofPI.6axesofPP havebeenidentifiedincludingoralco-prescription,switchby electrolytesolution,andadaptationofthevolumeofsolvent.
Thestudyoveronemonthgives172lineswithaMR accordingtoourtool.85prescriptionswerecompliant.87PI formulatedincluding6withoutPP.ThePIacceptancerateis 43.2%,withamaximumof52%fortheoralrelayanda minimumof0%foradaptationofthevolumeofsolventor electrolytesolutionswitch.AttheendoftheMSMDC,our toolisvalidatedafteranagreementontheimportanceofpromotingtheuseofelectrolytesolution.
ConclusionandRelevance TheacceptancerateandtheconclusionsoftheMSMDCallowustovalidatetheflowchart.Its useimprovestherelevanceofPIs,theiracceptanceand reducestheuseofKCl-inj.Tofacilitatetheuseofthetool, anExcelfilethatidentifiesthePPsaccordingtothecriteriais beingdeveloped.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-157 COMPARISONOFTHEEFFECTIVENESSBETWEEN INTERLEUKIN-23INHIBITORSFORTREATMENTOF PSORIASISINATHIRDLEVELHOSPITAL
AMerchán,SCLucía,FMRaquel,GOMaríadeLosReyes,PMMaría,ABMaríaÁngeles*, PJMaríaDelPuerto,VMIsabel,AMMercedes,CPLucía,VHJoséManuel. HospitalClínico UniversitarioLozanoBlesa,Pharmacy,Zaragoza,Spain
10.1136/ejhpharm-2023-eahp.156
BackgroundandImportance Interleukin-23(IL-23)isacytokineinvolvedininflammatoryandimmuneresponsesinpsoriasis.Noveltherapiessuchastildrakizumab,guselkumab,and risankizumabinhibittheIL-23-receptorinteraction.
AimandObjectives TocomparetheeffectivenessbetweenIL23inhibitorsinpatientswithpsoriasisinathirdlevel hospital.
MaterialandMethods Anobservational,retrospective,descriptivestudywasconductedinpatientswithpsoriasistreated withtildrakizumab,guselkumaborrisankizumabbetween August-20andAugust-22.Demographic,clinical,andtreatmentspecificvariableswerecollected.Effectivenesswasdeterminedthroughthecomparisonofpsoriasisareaseverityindex (PASI)priorstartingIL-23inhibitorandafterthefirstvisit (betweenweeks4and16afterstart).
Results Thestudyincluded58patients[62.1%men,median age51(23-83)years]outofwhom8(13.8%)hadpsoriatic arthritiscomorbidity,11(18.9%)weretreatedwithtildrakizumab,20(34.4%)withguselkumaband27(46.5%)withrisankizumab.Medianoftreatmentlinewas3(2-5)with tildrakizumabandguselkumab,and2(1-12)withrisankizumab.Adalimumabwasthemostcommonprevioustherapy (54.5%,n=6fortildrakizumab;40.0%,n=8forguselkumab; 38.5%,n=10forrisankizumab)andthemediantimeoftreatmentwithpreviousdrugwas58.4(9.8-665.0),64.5(1.5921.0)and46.6(0.0-299.0)weeks,respectively.Reasonsfor
switchingtoIL-23inhibitorsweretreatmentfailure(100.0%, n=11fortildrakizumab;85.0%,n=17forguselkumab; 84.6%,n=22forrisankizumab),adverseevents(15.0%,n=3 forguselkumab;11.5%,n=3forrisankizumab)ordruginteraction(3.8%,n=1forrisankizumab).MediantimeoftreatmentwithIL-23inhibitorwas41.9(16.9-68.0),44.1(9.2168.0)and26.3(14.9-96.1)weeksfortildrakizumab,guselkumabandrisankizumab,respectively.MedianPASIbefore switchingtoIL-23inhibitortreatmentsvsafterfirstvisitwere 7.7(3.3-10.8)vs1.4(0.0-5.2)fortildrakizumab,8.9(1.029.1)vs0.9(0.0-6.8)forguselkumaband7.8(2.8-21.8)vs 1.2(0.0-10.4)forrisankizumab.7patients(35.0%)and10 patients(37.0%)intreatmentwithguselkumabandrisankizumabrespectivelyachievedPASI0,whileonly3patients (27.3%)intreatmentwithtildrakizumabdid.
ConclusionandRelevance Thedurationoftheprevioustreatmentwasprolonged.Treatmentfailurewasthemainreason toinitiateanIL-23inhibitortreatment.Datasuggestthat guselkumabandrisankizumabcouldbemoreeffectivetreatmentsbetween4and16weekscomparedtotildrakizumab.
ConflictofInterest Noconflictofinterest
4CPS-158 IMPROVEMENTINPATIENTCAREBYPHARMACIST PHONECALLAFTERSTARTINGTREATMENT
JUrdaRomacho,IBretonesPedrinaci,AJofrePeralta,MACastroVida*. Hospital UniversitarioPoniente,Pharmacy,Almeria,Spain
10.1136/ejhpharm-2023-eahp.157
BackgroundandImportance OutpatientPharmacyUnit(OPU) isthelastplacethatpatientgoeswithinthehospitalcircuit. Usually,patientarrivesoverloadedwithinformationandworriedabouthisnewdisease,notbeingabletoassimilateallthe informationthatisofferedtohimaboutthenewtreatment thathehastostart.
AimandObjectives Todevelopacommunicationproject betweenpatientsandOPUprofessionalstohelppatients understand,rememberandimproveadherencetotreatment prescribed,detectpossiblemedication-relatedproblems(MRP) andincreasethedegreeofsatisfactionwiththecarereceived attheOPU.
MaterialandMethods ProjectstartedinApril2019,inthe OPUofaregionalhospital.Threeprofilesofpatientswere included;Profile1:patientswho,afterarecentdiagnosis, mayhaveagreaterpsychologicalimpact;Profile2:those whostarttreatmentwithdevicesthatrequirespecific manipulationandProfile3:th osewho,duetotheirspecial conditions(language,age...)areconsideredtoneedreinforcementoftheinformationreceivedinthefirstvisitto thePharmacy(FVP).WhenthepatientcomestotheOPU forthefirsttime,heisofferedalltheinformationnecessary tostarthistreatmentandisincludedinafollow-upprogramme,doingaphonecall3to5daysafterbeginthe newmedication.OnthesecondvisittotheOPU,asatisfactionsurveyisgiven.
Results DatacollectedbetweenApril2019-December2021. Patientsincluded:142.Callsmadeto100%ofpatients,118 patients(83.1%)answeredthecall.52.1%ofthepatients wereclassifiedasProfile1;37.3%Profile2,and10.6%Profile3.49patients(34.5%)reportedadverseeffects,ofwhich 41(85.4%)evolvedfavorablyand8(14.6%)changed
treatmentduetopoortolerance.Regardingthesatisfaction survey,92.4%ofthepatientsreportedcallwasusefultothem 95.8%weresatisfiedorverysatisfiedwithcarereceivedat theFVPandatthephonecall.
ConclusionandRelevance Phonecallafterstartingtreatment reinforcestheinformationgiveninOPUduringtheFVP andallowsearlyinterventionindetectionandresolutionof MRP.Ahighpercentageofpatientsconsidertheproject useful,showingahighdegreeofsatisfactionwiththecare received.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-160 REAL-WORLDPERSISTENCEWITHDOLUTEGRAVIR/ LAMIVUDINEVERSUSBICTEGRAVIR/EMTRICITABINA/ TENOFOVIRALAFENAMIDEAMONGHUMAN IMMUNODEFICIENCYVIRUSPATIENTS
1LMartínZaragoza*, 1JSánchez-RubioFerrandez, 1AOntenienteGonzález, 1MGómez Bermejo, 1AAlcántaraPrado, 1LCarmonaJuárez, 2SJRodriguezÁlvarez, 2AMonereo Alonso, 1TMolinaGarcia. 1HospitalUniversitariodeGetafe,PharmacyService,Madrid, Spain; 2HospitalUniversitariodeGetafe,InternalMedicineService,Madrid,Spain
10.1136/ejhpharm-2023-eahp.158
BackgroundandImportance Persistencycanprovideinformationonthecomparativeeffectiveness,durabilityandtolerabilityinreal-worldpatientpopulations.
Littleisknownaboutcomparativepersistenceofdolutegravir/lamivudine(DTG/3TC)andbictegravir/emtricitabine/tenofovir-alafenamide(BIC/FTC/TAF),twopreferredantiretroviral treatmentsinourcountry.
AimandObjectives Tocomparepersistencebetweentwopreferredantiretroviraltherapiesandanalysereasonsfor discontinuation.
MaterialandMethods Weconductedaretrospective,non-interventional,longitudinalstudy.AllHIVpatientsover18years treatedwithDTG/3TCorBIC/FTC/TAFinourcentrewere included.
Persistencewasdefinedasthedurationoftimefrominitiationtodiscontinuationoftherapy(lastdispensingorendof thestudyinMarch2022).Persistencewasalsocalculatedasa dichotomousvariableattheconclusionofthefirstyearof therapy.Permissiblegap(daysbetweentwoprescriptionfills exceedingtheallowablerefillperiod)was90days.
Covariatescollectedfrommedicalrecordwere:age,gender, viralload(VL),CD4count,numberofpreviousantiretroviral medications,CharlsoncomorbidityindexandMedicationPossessionRatio(MPR).
Persistenceafterfirstyearwascomparedusingthe c²test. Kaplan-Meiersurvivalanalysiswasperformedanddifferences wereevaluatedusingthelog-ranktest.AdjustedriskofdiscontinuationwasassessedwithCoxProportionalHazardmodels.Significancelevelwas0.05.
Results Threehundredandsixty-twopatientswereincluded, 79.2%weremale.5.2%werenaive.Age(mean±SD)was 47±12years.91.2%hadVL<200copiesand10.1% CD4<200/ml.Numberofprevioustreatmentswas3.5±2.6. MPRwas95.4±11.1Charlsoncomorbidityindexwas1± 1.66.49.2%weretreatedwithBIC/FTC/TAF.
97.8%vs89.7%ofpatientswerepersistentafterthefirst forDTG/3TCandBIC/FTC/TAFrespectively[OR=5.1 (CI95%1.7-15.6);p=0.002].
Overall,meanpersistencedurationwas1.189days(CI 95%1.163-1.215).PersistencewithDGT/3TCwas1.231days (CI95%1.206-1.255)andpersistencewithBIC/FTC/TAFwas 980days(CI95%944-1.016);p=0.001.HoweverCox-model adjustedHRwas2.5(IC95%0.5-12;p=0.26).
ThemainreasonsfordiscontinuationforBIC/FTC/TAF weretolerability/toxicity(n=9)anddeath(n=3).Onlytwo patientswithdrawedDTG/3TC,duetotoxicity(n=1)and death(n=1).
ConclusionandRelevance Inourstudy,morepatientson DTG/3TCwerepersistentafterthefirstyearcomparedto BIC/FTC/TAF.However,therewerenodifferencesinoverall persistenceincovariate-adjustedanalysis.MainreasonforBIC/ FTC/TAFdiscontinuationwastolerability/toxicity.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-162 TRIPLEWHAMMYDRUG-DRUGINTERACTION: CLINICALRELEVANCEANDRESULTSOF PHARMACEUTICALINTERVENTION
ÁGonzálezGómez*,JAHernándezRamos,ACastroFrontiñán,JMCaroTeller, MDCanalesSiguero,JMFerrariPiquero. HospitalUniversitario12deOctubre,Pharmacy, Madrid,Spain
10.1136/ejhpharm-2023-eahp.159
BackgroundandImportance Acutekidneyinjury(AKI)isa highlyprevalentconditionamonginpatients,usuallyattributed topharmacologicalcauses.Oneofthemostclinicallyrelevant drug-druginteractions(DDI)inthiscontextisthetriple whammyinteraction(TWI),causedbytheadditionofthree potentialnephrotoxicgroupsofdrugs:Non-steroidalantiinflammatorydrugs(NSAIDs),diureticsandACEinhibitors/ angiotensinreceptorblockers(ARB).
AimandObjectives Toevaluateclinicalsignificanceofthe TWI,aswellastheroleofpharmaceuticalintervention(PI) inpreventingpossibleadverseeventsduetothisDDI.
MaterialandMethods Observationalretrospectivestudythat includedpatientswhowereprescribedtheTWIoveraperiod of4years(2018to2022).Datawerecollectedusingcomputerisedmedicalrecords,nurseadministrationregistryandPI database.ICUpatientswereexcludedfromthisstudy.Recommendationofmonitoringserumcreatinineandpotassium, aswellasdiscontinuingthetripletherapywascarriedoutin allpatients.IncidenceofAKIwascalculatedaccordingto AKINcriteria.ImpactofPIwasestimatedbasedonaverage numberofdayspatientsreceivedthecombinationandamount oftimeuntilcompleteresolutionofAKI.
Results 34patientswereincludedandstratifiedaccordingto theirriskfactorsfordevelopingAKI.87,5%patientswere consideredathighrisk.Thefirstcauseofadmissionwassurgeryin62%ofcases.MeanbasalSCrwas0,99(CI95% 0,82–1,15).AcceptanceofPIratewasestimatedin65,62%. IncidenceofAKIwas29,4%(10/34),8ofwhichwereclassifiedasAKIN1.Meandurationofthetripletherapywas 6,81days(CI95%=3,47-10,15)innon-acceptedPIgroup vs3,17days(CI95%=2,23-4,11)intheacceptedPIgroup. AKIwasdetectedmorefrequentlyinacceptedPIpatients(7/ 10).However,thesepatientsrecoverednormalrenalfunction fasterthanpatientswithnoapprovedPI:10days(CI95%= 5,41-14,58)vs14,33days(CI95%=8,52
20,14), respectively.
ConclusionandRelevance TheTWIcanparticipateinacute kidneyinjury,particularlyinhighriskpatients.Clinicalpharmacistsplayanimportantroledetectingpatientsatincreased riskofAKI,preventingadverseeventsduetoTWinteraction, monitoringAKIbiomarkersandrecommendingdeprescription ofpossiblenephrotoxicdrugs.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest
Noconflictofinterest
4CPS-163 CYTOCHROMEP4502C19GENOTYPINGFOR PERSONALISATIONOFPROTONPUMPINHIBITOR THERAPY
1,2JLDebattista*, 3JSchembri, 2CBarbara, 2GZahra, 1FWirth, 1LMAzzopardi. 1University ofMalta,DepartmentofPharmacy-FacultyofMedicineandSurgery,Msida,Malta; 2Mater DeiHospital,MolecularDiagnosticsUnit-DepartmentofPathology,Msida,Malta; 3Mater DeiHospital,Gastroenterology-DepartmentofMedicine,Msida,Malta
10.1136/ejhpharm-2023-eahp.160
BackgroundandImportance Protonpumpinhibitors(PPIs)are hepaticallymetabolisedprimarilybythecytochromeP450 2C19enzyme.PPIsaregenerallyconsideredeffective,however CYP2C19geneticpolymorphismsmayresultinpatientsnot respondingappropriatelytotreatment.CYP2C19genotyping andinterpretationofresultsmaybeacontributionbypharmaciststowardspersonalisationofPPItherapy.
AimandObjectives Theaimwastodeterminetheprevalence ofCYP2C19geneticpolymorphismsinacohortofpatients showingPPItherapyresistance.
doseincreaseandmonitoringforefficacyinthesepatients.In patientsatriskofside-effects(29%IMs,PMs),theguideline suggestsreductionindoseandcontinuedmonitoringforefficacy.Pharmacist-ledCYP2C19pharmacogenetictestingcanbe usedatooltoguidedosingandmonitoringinpatientstaking PPIs.
ConflictofInterest Noconflictofinterest
4CPS-164 ADEQUACYREVIEWINTHEUSEOFDAPAGLIFLOZIN FORTHETREATMENTOFHEARTFAILURE
MRodríguezMorote,MJLucasMayol,AGonzálezFernández,CMatosesChirivella,LPeral Ballester,ANavarroRuiz*. HospitalGeneralUniversiariodeElche,ServiciodeFarmacia, Elche,Spain
10.1136/ejhpharm-2023-eahp.161
BackgroundandImportance Protocolforuseofdapagliflozin wasapprovedfortheadulttreatmentofsymptomaticchronic heartfailurewithreducedleftventricularejectionfraction (LVEF)inpatientsuncontrolledwithfirst-linetherapies,angiotensin-convertingenzymeinhibitors(ACEI)orangiotensin receptorblockers(ARB)withbetablockers,andsecond-line therapies,aldosteroneantagonists.
AimandObjectives Toevaluatetheuseofdapagliflozininthe treatmentofheartfailureinhospitalisedpatients,assessingthe degreeofprescriptioncompliancewiththeprotocolagreed uponbythePharmacyandTherapeuticsCommittee.
MaterialandMethods
Patientsdiagnosedwithgastro-oesophagealrefluxdiseaseorpepticulcerdiseaseandwithdocumentedPPItherapyresistancewereidentifiedusing ambulatoryrefluxmonitoringandendoscopydatabases.An EDTAbloodsamplewascollectedfromeachpatient,followedbygenomicDNAextractionwiththeQIAcube(Qiagen).CYP2C19genotypingwasperformedwithreal-time polymerasechainreactionontheGeneAmpPCRSystem
9700thermalcyclerandreversehybridisationusingthe TwinCubatorwiththePGX-CYP2C19StripAssay® (ViennaLab).Genotypes(phenotypes)wereclassifiedas:*1*1(normalmetabolisers,NMs),*1*17(rapidmetabolisers,RMs), *1*2or*2*17(intermediatemetabolisers,IMs),or*2*2 (poormetabolisers,PMs).The2021ClinicalPharmacogeneticsImplementationConsortium(CPIC)guidelinewasused forgenotype-baseddosingrecommendations,whichsuggests thatNMsmaybeatincreasedriskoftherapeuticfailure comparedtoIMs/PMs,RMsareatincreasedriskoftherapeuticfailure,whileIMs/PMshaveincreasedchanceofefficacybutriskpotentialtoxicity.
Results Thirty-eightpatientswererecruited;allCaucasian;20 female,mode50-59years(n=11).Mostpatients(n=17) experiencedrefluxhypersensitivity,followedbypersistent oesophagitisdespitePPItreatment(n=10).PPItherapy includedesomeprazole(n=20),omeprazole(n=16)orlansoprazole(n=2).Themajorityofpatients(n=20)weregenotypedas*1*1(NM),followedby*1/*17(n=7,RM),*2*17 (n=6,IM),*1*2(n=4,IM)and*2/*2(n=1,PM).
ConclusionandRelevance Themajorityofpatientsinthis studymaybe(53%NMs)orare(18%RMs)atriskoftherapeuticfailure,andtheguidelinerecommendsconsideringa
MaterialandMethods Retrospectiveobservationalstudy betweenDecember2021andApril2022ofhospitalised patientswhostartedtreatmentwithdapagliflozin.Thestudy variableswere:sex,age,reasonforadmission,presenceof heartfailurewithLVEF<40%,concomitanttreatmentwith ACEI,ARB,betablockers,aldosteroneantagonists,positive inotropics,sacubitril/valsartanordiuretics,andpresenceofdiabeteswithorwithoutantidiabetictreatment.Clinicaldata wereobtainedfromtheOrion-Clinic® electronicmedical recordprogram.
Results Intheperiodevaluated,61patientsinitiateddapagliflozin10mgperday,42men(69%),withamedianageof 76years(IQR84-66).Atotalof46patients(75%)presented heartfailureonadmissionandtherestwereadmittedfor othercardiacpathology.Only38patients(62%)hadanLVEF registry,ofwhich22patients(36%)hadanLVEF<40% withamedianLVEFof32%(IQR35-25).Forty-fourpatients (72%)werediabeticand6patients(17%)weretreatedwith dapagliflozinincombinationwithmetformin.Forthestudyof concomitanttreatments:22patients(36%)wereprescribed ACEI/ARB,38patients(62%)betablockers,8patients(13%) positiveinotropics,21patients(34%)aldosteroneantagonist diuretics,41patients(67%)loop/thiazidediureticsand9 patients(14.8%)sacubitril/valsartan.Tohighlight,11patients (18%)werebeingtreatedwiththecombinationACEI/ARA-II +betablockers+aldosteroneantagonist.Finally,only35 patients(57%)continuedwithdapagliflozinasdischarge treatment.
ConclusionandRelevance Thedegreeofadequacyofdapagliflozinprescriptiontotheapprovedprotocolforusewashigh butanappreciablepercentageofpatientsdonotadhereto theinclusioncriteria,indicatingthattheprotocol
recommendationsshouldberevisedtoensureeffectiveuseof dapagliflozin.Onlyhalfofthepatientswhoinitiatedtreatment continuedafterdischarge.
1DRoblesTorres*, 1ECampeloSánchez, 1NLagoRivero, 2MSuárezSantamaria, 1MAlfonsínLara, 1PPradoMontes, 1MCouñagoFernández, 1IAgraBlanco, 1NMartínez LópezdeCastro. 1ALVAROCunqueiroHospital,HospitalPharmacy,Vigo,Spain; 2ALVARO CunqueiroHospital,ClinicalAnalysis,Vigo,Spain
10.1136/ejhpharm-2023-eahp.162
BackgroundandImportance Linezolidisanantibioticthat presentshighinter-andintra-individualvariabilityandthereforemaycompromiseitsclinicalefficacyorincreasetherisk ofassociatedtoxicity.
AimandObjectives Toestablishaprogrammeformonitoring linezolidplasmalevelsthatwillallowustoproactivelyidentifypatientswhocanbenefitmostfromitsuseandtoevaluateitsresultsinourcentre.
MaterialandMethods Aliteraturereviewwasperformedto definethecriteriathatallowedustoidentifypatientswho werecandidatesforpharmacokineticmonitoringoflinezolid.
Weestablishedthedeterminationofplasmaconcentrations beforetheadministrationofthe5thdoseandthenperiodicallyevery3-4daysuntiltheendoftreatment.Theefficacy andsafetycriterionwastomaintainthetroughplasmaconcentration(Cmin)inthetherapeuticrange(between2and8 mg/L).
Results Thecriteriaselectedfortheidentificationofpatients whowerecandidatestobepartofthemonitoringprogramme were:criticalpatients,transplantedpatients,severeburnsor cysticfibrosis,obesepatients(BMI>30),kidneyfailure(creatinineclearance<30ml/min)andliverfailure(ChildPugh C),renalreplacementtherapies,prolongedtreatments(>3 weeks)andtreatmentwithGlycoprotein-Pinducers.
FromJanuarytoApril2022,atotalof20patientsthat metatleastoneoftheaforementionedcriteriawereincluded intheprogramme.Allpatientsstartedtreatmentincritical careunitsandthechosenrouteofadministrationwasintravenous.Eighty-fivepercentofthepatientsweremen,the medianagewas69yearsandthemeandurationoftreatment was11.6days.
Atotalof50sampleswereanalysed(2.5samplesper patient).ThemeanCminwas5.3mg/L.Thirtysamples(60%) wereoutoftherapeuticrange. Fiftypharmacokineticreportswereperformed. In60%ofthe cases,modificationsofthedosingregimenweremade:17 weredoseincreasesand13weredosedecreases.
ConclusionandRelevance Incorporatingthisprogrammeinto clinicalpracticeallowsustoproactivelyidentifythepatients whocouldbenefitmostfromlinezolidmonitoring.
Theresultsdemonstratethehighvariabilityoflinezolid plasmalevelsandtheusefulnessofdosingrecommendations issuedbythePharmacyservicetoensurethattheCmin remainswithinthetherapeuticrange.
4CPS-167 CURRENTTRENDSINTHEUSEOFCHECK-POINT INHIBITORSFORNON-SMALL-CELLLUNGCANCER
AAlvarez-Yuste*,MPerez-Abanades,TGallego-Aranda,ELopez-Aspiroz,AIbañezZurriaga,AGarcia-Peralo,GEscudero-Sanchez,PDuque-Tebar,AMorell-Baladron. La PrincesaUniversityHospital,HospitalPharmacy,Madrid,Spain
10.1136/ejhpharm-2023-eahp.163
BackgroundandImportance Overthelastyears,immunotherapyhaschangedthetreatmentparadigmofnon-small-cell lungcancer(NSCLC).Thenumberofpatientstreatedwith immunecheck-pointinhibitors(ICI):atezolizumab,durvalumab,pembrolizumabandnivolumabhasdramatically increased.
AimandObjectives Toevaluatethecurrenttrendsintheuse ofICIforNSCLCinathirdlevelhospital.
MaterialandMethods Aretrospectiveobservationalstudy wasconducted,includingpatientswithNSCLCwhohad receivedtreatmentbetween2016and2021withchemotherapyorICI(atezolizumab,durvalumab,nivolumaborpembrolizumab).Thedatacollectedwasdrug,dateandnumberof administrations,daysbetweeneachadministrationandclinical response.
Results Duringthestudyperiod,therewere606patientsbeing treatedforNSCLC,and254ofthemreceivedICI(41.91%). ConclusionandRelevance Thetotalnumberofpatientstreated withICIforNSCLChasincreasedconstantlyduringthis periodoftime(49.15%increasebetween2016and2021). Moreover,immunotherapyentailthetreatmentofnearlyhalf oftheNSCLCpatients.
Duringthetimeperiodstudied,theuseofnivolumabhas decreased,favouringpembrolizumab,probablybecauseofthe riseofitsnewapprovedindications.Secondly,thenumberof patientswhohavereceivedatezolizumabanddurvalumabhas keptcomparable.
Abstract4CPS-167Figure1

Abstract4CPS-167Table1
Wecanseeasignificatedecreaseonthenumberof patientstreatedwithanICIbetweenMay2020andAugust 2020,possiblyinfluencedbythedecreaseinthenumberof patientsdiagnosedwithNSCLCduringtheCOVID-19 pandemic.
ThemeandaysbetweeneachICIadministrationwas slightlyabovetheapprovedposology,possiblyduetodelays becauseofadverseeffects.
REFERENCESAND/ORACKNOWLEDGEMENTS
Age50(43-58)years
Gender(woman)77%
Galcanezumabduration6(6-9)months
MDMmonth015(14-17)
MDMmonth35(3-6)
ORR>50%84%
HIT6month072(68-76)
HIT6month349(48-57)
-9%(5)ofthepatientscontinuewithactivetreatment, 100%maintaineffectiveness,medianMDM:3(2-6).
-91%(51)discontinuedtreatment:
Reasonfor suspension 67%
PTardáguilaMolina*,CDeanBarahona,MBlancoCrespo,EMartinezRuiz,AMirandaDel Cerro,GICasarrubiosLázaro,ACodonalDemetrio,FJProJimenez. GuadalajaraUniversity Hospital,Pharmacy,Guadalajara,Spain
10.1136/ejhpharm-2023-eahp.164
BackgroundandImportance Migraineisahighlydisabling neurovasculardisordercharacterisedbyasevereheadacheand trigeminovascularsystemactivation,involvingthereleaseof calcitonin-generelatedpeptide(CGRP).Galcanezumabisa humanisedmonoclonalantibodyblockingtheCGRP.
AimandObjectives
Analyze:
. Theeffectivenessofgalcanezumabintheprophylaxisof chronicmigraine
. Responsetootheranti-CGRPmonoclonalantibodiesafter galcanezumabfailure
MaterialandMethods Observational-retrospectivestudyfrom January2020toSeptember2022.Patientsinwhomatleast oneyearhadpassedsincethestartofgalcanezumabtreatment wereincluded.
Variablesanalysed:demographics,baselinemigrainedays/ month(MDM),threemonthslater,objectiveresponserate (ORR)>50%,duration,reasonforsuspension,andaction. Theheadacheimpacttest(HIT-6)wasperformedatbaseline vsafterthreemonthsoftreatment.Thisscorepresentsa rangebetween36and78(<49=littleornoimpact,50-55= certainimpact,56-59=importantimpact,>60=verysevere impact).
Quantitativevariableswereexpressedasmedian(interquartilerange).
Results 56patientswereincluded.
NªPatients191547
MDMmonth 0 15(12-16)15(15-17)15(15-20)
MDMmonth 3 4(3-5)15(12-17)15(7-20)
ORR>50%89%25%43%
HIT-6month 0 73(68-76)73(68-78)66(59-70)
HIT-6month 3 57(50-68)72(64-78)57(55-67)
*Medianmonthswithouttreatmentaftersuspension:7(5-11).
ConclusionandRelevance Ahighpercentageofpatientspresentedagoodresponsetogalcanezumab,withanimprovementintheHIT-6score.
Alargenumberofpatientswhoreceivedtemporaryprophylaxiswithgalcanezumabdidnotrequireanothervisitto theneurologist.MostofthepatientswhorequiredreintroductionofgalcanezumabreachedanORR>50%.
Lessthanhalfofthepatientswhorestartedtherapywitha differentanti-CGRPaftergalcanezumabfailure,achievedan ORR>50%.
Allpatientswhocontinuedwithgalcanezumabfromthe start,maintainedeffectivenessofthetreatment
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-171 METHADONEDRUG-DRUGINTERACTIONS
POTENTIALLYRELATEDTOCARDIOVASCULAREVENTS INCLINICALPRACTICE
10.1136/ejhpharm-2023-eahp.165
BackgroundandImportance Methadonecontinuestobethe drugofchoiceinmanagingopioidwithdrawal.However,itis knownthatitsuseisrelatedtoQTprolongation,torsadesde pointesandevensuddencardiacdeath.Theinteractionwith otherdrugscouldworsenthiseffect.
AimandObjectives Toquantifytheprevalenceofmethadone drug-druginteractionswithriskofQTintervalprolongation andtheincidenceofcardiovasculareventsduringadmission.
MaterialandMethods Weconductedaretrospective,descriptivestudythatincludedallpatientsreceivingmethadoneina tertiaryhospitalbetweenJanuary2021andSeptember2022.
Thevariablescollectedwere: age,sex,opioidabuse,treatment withmethadonepriortoadmission,methadonedose,cardiovascularhistory,numberofdrugsprescribed-inadditionto methadone-likelytoprolongQTduringadmission,anddevelopmentofcardiovascularcomplications.InteractionswereconsultedinLexicomp
Results Atotalof109patientswerecollected,themedianage of56(interquartilerange(IQR)50-60),and74.3%were male.82.6%ofpatientshadahistoryofsubstanceabuse recordedintheelectronicmedicalrecord,withprevious opioiduseexplicitin61.5%andwereonmethadonetreatment.Remainingpercentagewereonmethadonefor:respiratoryweaning(9.3%),analgesia(3.5%)andnewmanaging opioidwithdrawal(4.6%).Themedianmethadonedosewas 50mg(IQR35-80mg).Atotalof9.2%hadahistoryofcardiovasculardiseasepriortoadmission.
Patientsreceivedameanof1.8QT-prolongingdrugsin additiontomethadoneduringadmission.Inthiscohort, 93.6%ofpatientsreceivedanyQT-prolongingdrug,48.6% and21.1%twoorthreeQT-prolongingdrugs,respectively. ThemostfrequentlyprescribedQT-prolongingdrugswere quetiapine(24.8%),mirtazapine(19.3%)andondansetron (12.9%).Duringadmission,11.0%ofpatientssufferedacardiovasculareventwitharrhythmiasbeingthemostfrequent event(54.6%).Ahigherproportionofpatientswithprevious cardiovascularhistorysufferedanewcardiovascularevent (19.3%vs7.2%).
ConclusionandRelevance Ourresultsshowahighprevalence ofpatientsusingmethadoneconcomitantwithotherdrugs likelytoprolongQTduringadmission.
Amoresignificantproportionofpatientswithaprevious historyofcardiovasculareventssufferedaneweventduring hospitalisation.
ConflictofInterest Noconflictofinterest
4CPS-172 BRODALUMAB'SEFFECTIVENESSONMODERATETO SEVEREPLAQUEPSORIASISINREALPRACTISE
1MRodriguezGoicoechea*, 2ETejedorTejada, 3SCanoDominguez, 1AMorenoLopez, 1NGarciaGomez, 1MJBarberoHernández, 1FHornoUreña. 1HospitalaryComplexofJaén, HospitalPharmacy,Jaen,Spain; 2BarcelonaClinicHospital,HospitalPharmacy,Barcelona, Spain; 3UniversitaryHospitalVirgendeLasNieves,HospitalPharmacy,Granada,Spain
10.1136/ejhpharm-2023-eahp.166
BackgroundandImportance Plaquepsoriasisisachronicpathologywithanimportantimpactonpatients’ qualityoflife andemotionalhealth.Brodalumabwasthelastanti-interleukin17(IL-17)arrivingtopatients.
AimandObjectives
Toevaluatebrodalumabeffectivenessin realpractise
MaterialandMethods
Multicentric,retrospectiveandobservationalstudyperformedtoevaluatebrodalumabin patientswithmoderate – severeplaquepsoriasisbetween June2021andJune2022.Dataextractedfromclinical recordsapplicationandprescribingprogramme,demographicdata(age,sex),andclinical(previousbiologic treatmentlines,bodysurfacearea(BSA)andpsoriasisarea severityindex(PASI)before treatmentandineachdermatologiccontrol).Effectivenesswasmeasuredcomparing AMAGINEclinicaltrialsPASI75results(efficacycalculated withweightedaverage).
Results 41patientswithbrodalumabasactivetreatment,1 wasexcludedduetolackoffollowupandotherduetolate startoftreatment.39patientsincluded,52.4yearsaveraged, 66.7%weremen.Otherlinesoftreatmentsapprovedfor moderate-to-severeplaquepsoriasiswereusedasfirstlinein 19patients,as2ndlinein5,as3rdlinein3andas4thline in5patients.
AverageBSAandPASIatbaselinewere14.32and11.55 respectively.Afteramedianof23weeks,ourpatientsreached PASI75in56%,PASI90in51%andPASI100in49%of cases.Nopatientstoppedtreatment.
After40weeks,3patientshadchangedtheirtreatmentand 4hadnotreachedthenextvisittoDermatology.PASI90and PASI100keptin9of23patients(39%),asPASI75stillwas keptby11patients(48%).
Afteroneyearoftreatment,only15patientswereactive withtreatment,andPASI90andPASI100werekeptby5 patientsof10withrecordeddata.
Accordingtoclinicaltrials,brodalumabachievesaPASI75 in85%ofpatientsat12thweekandkeptaPASI75in68% ofpatientsat52ndweek.
Limitations: severalpatientsdidnothaverecordedintheir clinicalhistoryBSAandPASIafter12thweekvisit.
ConclusionandRelevance Ourfindingsshowthatbrodalumab islesseffectiveinrealpracticebutitcanbeconsideredasa potentantipsoriaticagentinclinicalpractise.Furtherandlongerstudiesshouldbemade.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-173 EVALUATIONOFINCLUSIONCRITERIAOF OUTPATIENTSINCLUDEDATHOSPITALMEDICATION DISPENSINGPROGRAMMETHROUGHCOMMUNITY PHARMACIES
CSubiranaBatlle,IGómezIbáñez,XLarreaUrtaran*,ADordàBenito,ÀCastellóNòria, COrtíJuan,MBrugueraTeixidor,QLópezNoguera,YOrtuñoRuiz,LViñasSagué, RSacrestGüell. HospitalDrJosepTruetadeGirona,PharmacyDepartment,Girona,Spain 10.1136/ejhpharm-2023-eahp.167
BackgroundandImportance Duringthecontextofthe COVID-19pandemic,inordertoavoidthepossibletransmissionofSARS-COV2,somehospitalsdevelopedanoutpatient hospitalmedicationdispensingprogrammethroughdeliveryto communitypharmacies.Toaccesstheprogramme,outpatients hadtomeetallthecriteriaestablishedbyHealthAuthorities: adherencetotreatment,livemorethan30kmfromthehospitalandpresentsomevulnerabilitycondition(age>65years, reducedmobilityorrespiratorypathology).Thisprogramme hasbeenmaintainedovertimeduetotheexcellentacceptance bypatients.
AimandObjectives
Toevaluatethecomplianceofourhospital withtheinclusioncriteriaandanalysepossibledeviations, assessingwhetheritisnecessarytomodifythembasedonthe currenthealthcontext.
MaterialandMethods Cross-sectionalobservationalstudyin whichallactiveoutpatientsintheprogrammebetweenJuly andSeptember2022wereincluded.
Thefollowingvariableswerecollected: demographic,distance betweenhomeandhospital,vulnerabilityconditionsand adherencetotreatment.
Results 95patientswereevaluated,94(98.9%)ofthemwere adherenttochronictreatment,81(85.3%)livedmorethan 30kmfromthehospital.Regardingthevulnerabilityconditions:68(71.6%)wereolderthan65yearsand14(14.7%) hadavulnerabilityconditionotherthanageover65years.
Ofalltheevaluatedpatients,75(78.9%)metalltheinclusioncriteria.20(21.1%)patientswereintheprogramme, butdidnotmeetsomecriteria:6(30.0%)patientslivedless than30kmaway,8(40.0%)didnothaveavulnerableconditionand6(30.0%)didnotmeetmorethanoneinclusion criteria.
ConclusionandRelevance Themedicationdispensingprogrammethroughcommunitypharmaciesoffersanoptionfor vulnerablepatientsand/orthosewithdifficultygoingtothe hospitaltocollecttheirchronicmedication,thusfacilitating therapeuticcomplianceoftreatment.
Althoughahighpercentageofpatientsmettheestablished criteria,deviationsweredetected.Thatmakeusconsiderthe needtomodifythesecriteriainordertoaccessintheprogrammeaccordingtocurrentneedsofoutpatients.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-175 SEPSISCODE:IMPROVINGOUTCOMESFORPATIENTS WITHSEPSIS
1MEMartinezNuñez*, 1NHerranzMuñoz, 2JBCachoCalvo, 3FJEstebanFernandez, 3FFerrereGonzalez, 2AGonzalezTorralba, 2DMolinaArana, 3GPerezCaballero, 3AMRodriguezBenavente, 1TMolinaGarcia. 1HospitalUniversitariodeGetafe,Pharmacy, Madrid,Spain; 2HospitalUniversitariodeGetafe,ClinicalMicrobiology,Madrid,Spain; 3HospitalUniversitariodeGetafe,InternalMedicine,Madrid,Spain
10.1136/ejhpharm-2023-eahp.168
BackgroundandImportance Sepsisisacommonandpotentiallylife-threateningconditiontriggeredbyaninfection.
CodeSepsis(CS)includesstandardisedSurviving-SepsisCampaignmanagementbundlesmeanttoguideearlyrecognitionandpromptgoal-directedtherapy,inordertoimprove clinicaloutcomes.
MultidisciplinaryCS-teamdailyevaluatesallpatientswith ‘CS-alert’ inordertoguaranteecompliancewithsepsisbundlesandpromotingappropriateantimicrobial-use.
AimandObjectives ToassesstheimpactofCSimplementation onclinicaloutcomesandantibiotictherapy.
MaterialandMethods ExperimentalstudyfromNovember2020toSeptember-2022.Allpatientswithconfirmedsepsis/ septicshockwereincluded.
Meanoutcome: overallandtrendofin-hospitalmortalityrate (MR).
Secondaryvariables:
. Medianlengthofhospital-stay(LOS)andIntensiveCareUnit stay(ICU-LOS).
. Severitycriteria:ICU-admission(%).
. Meanlengthofantibiotictherapy(LAT):overall, antipseudomonal-carbapenemsandantibioticsagainst resistant-gram-positivebacteria(daptomycin,vancomycinand linezolid).
Variableswereanalisedbytrimesters.Medianandinterquartile range(IQR)wereusedtodescribeallthequantitativevariables.Lineal-regressionwasperformedfortrendanalysis.
AllstatisticalanalyseswereassessedwithSPSS®V25.0.Significancelevelwas0.05.
Results Atotalof422CSalertwasactivatedin402patients. Medianage=79years(RIQ16),61.1%males.
Admissionward=12.8%surgical,81.5%medicaland5,7% ICU.
GlobalMRwas20.6%withasignificantlydownwardtrend (slope=-2.2;CI95%-3.4to-1.0).TheoverallMRwas reducedin53.8%(38.9%vs20.9%).
MedianLOSwas8days(RIQ12)andshowedanegative trend(slope=-0.4;CI95%-0.7to1.02).ThemedianICULOSstaywas6days(RIQ8.7)witha9.0%ofICU-admissions,whichalsodecreasedduringthestudy(slope=-0.2; CI95%-0.6to0.2).
TheoverallLATwas9.3days,withtrendtowardshorter courses(slope=-3.2;CI95%-0.9to0.2).Meandurationof antipseudomonal-carbapenemswas4.2days(slope=-2.2; CI95%-0.5to0.1),whereasanti-gram-positivewas5.4days (slope=-0.1;CI95%-0.8to0.6).
ConclusionandRelevance TheCSimplementationwasassociatedwithadecreasemortality,withanoverallreduce byupto50%.ThedownwardtrendinLOSandICUadmissionssuggeststhatanearlyrecognitionofsepsis andoptimised-treatmentarecrucialinpreventing complications.
Dailypatientsurveillanceandfollow-upbyamultidisciplinaryteampromotingantimicrobialde-escalation/discontinuationwasassociatedwithshortercoursesofantibioticswithout worseningclinicaloutcomes.
4CPS-176 EVALUATIONOFNIRMATRELVIR/RITONAVIRUSEAND EFFECTIVENESS
ABPousadaFonseca*,ISotoBaselga,NGarridoPeño,ISollanoSancho,IMorona Mínguez,JSolísOlivares,YMateosMateos,MRMengualBarroso,AGonzalezFuentes, BRubioCebrián,CMorielSánchez. HospitalUniversitariodeMóstoles,HospitalPharmacy, Móstoles,Spain
10.1136/ejhpharm-2023-eahp.169
BackgroundandImportance Nirmatrelvir/ritonavir(PAXLOVID)isarecentlyapproveddrugtopreventprogressionin high-riskCOVID-19-infectedpatients.
AimandObjectives Toevaluateprescribinganddispensingof PAXLOVIDandtheproportionofpatientswithhospitalisation ordeathfromanycauseat28day.
MaterialandMethods Descriptive,retrospective,observational studycarriedoutbetweenMayandAugust2022inasecondlevelhospital.AllpatientswithPAXLOVIDprescriptionwere selected.Sourcesofinformationwere:electronicmedical recordsandtheprescriptionprogramme.TheVariablesanalysedwere:sex,age,riskfactors,indications,interactions,
dispensation(yes/no)andfinaltreatmentreceived.Riskfactors wereevaluatedwithourcountry'sdrugregulatoryagency (DRA)recommendationstoassesedtheindication.Efficacy wasassessedbytheproportionofpatientsadmittedtohospitaland28-daymortality.
Results PAXLOVIDwasprescribedto34patients,14(41.2%) werewomen.Themedianagewas76.3yearsold[RIQ25.4].
MainindicationsforPAXLOVIDwere:tobeundergoing treatmentwithmyelotoxicchemotherapy(32.3%),corticosteroidsorotherimmunosuppressants(29.4%);beingover80 yearsofageandpresentingspecificRiskfactors(14.7%)and primaryimmunodeficiency(5.8%).21patients(61.8%)had somerelevantinteractionwiththeirusualmedication.The mostfrequentinteractionswerewithstatins(23.5%),analgesics(20.6%),oralanticoagulants(12%),antiarrhythmics (8.8%),antiplateletdrugs(5.8%),antidepressants(5.8%)and antidiarrhoeals(5.8%).
AfterValidatiónbythePharmacyService,11patients (32.4%)didnotreceivePAXLOVID,5becausetheydidnot meetDRAcriteria,2becausetheirglomerularfiltrationrate waslessthan30ml/minand4becausetheyhadincompatible interactions.4patientsfinallyreceived3days-remdesivir.
AmongpatientswhoreceivedPAXLOVID,82.26%received fulldoses,with4patients(11.76%)requiringadjustmentfor renalimpairment.3patients(13%)werehospitalisedinthe firstmonth,nonedied.
ConclusionandRelevance Themainindicationsforwhich PAXLOVIDwasprescribedwerepatientsundergoingchemotherapyand/orimmunosuppressivetreatments.Interactions withPAXLOVIDwerefrequentandinsomecaseslimited treatment.ValidationbyPharmacyServicepreventedaconsiderablenumberofpatientsfromreceivingPAXLOVIDwhenit wasno-indicatedorwhentheyhadinsurmountableinteractions,alsoallowedpatientstoreceivethedoseadjustedfor renalimpairment.PAXLOVIDwaseffectiveinavoidinghospitaladmissionandmortalityinthemajorityofpatients.
ConflictofInterest Noconflictofinterest
4CPS-177 OPTIMISATIONOFTHETHERAPEUTICMANAGEMENT OFPATIENTSONECMOINTHEPAEDIATRIC INTENSIVECAREUNIT
; 2CHAubagne,Pharmacie,Aubagne, France; 3AIXMarseilleUniversité,PharmacieClinique,Marseille,France
10.1136/ejhpharm-2023-eahp.170
BackgroundandImportance ExtracorporealMembraneOxygenation(ECMO)isalast-resortrescuetechniquethatallows thereplacementofcirculatoryand/orrespiratoryfunctions. Thepharmacokineticmodificationsgeneratedbythiscirculatoryassistancerequiretheadaptationofthedosageofcertain drugs
AimandObjectives Theobjectivewastocomparethedrug prescriptionofpatientsunderECMOwithdataavailablein theliteraturetoproposeappropriatedosages
thetypeandindicationofECMO,complicationsand adequacyofdosagescomparedtotheliteraturefor relevance
Results 14patientsunderECMOwereincluded:meanage 18months[0to168months],sexratio=1.Renalfunction wasimpairedin8patients(57%).Theaverageduration ofECMOwas15days[3-24days].6patientswere weaned,4ofwhomwerestillhospitalisedontheward (43%)and8patientsdied(57%).13patients(93%)were onveno-arterialECMO,followingacuterespiratorydistresssyndrome(8casesor61%),refractorycardiacarrest (3cases23%),cardiogenicshock(8%)orsepticshock (8%).1patient(7%)wasonveno-venousECMOfollowinganacuterespiratorydistresssyndrome(ARDS).11 patients(79%)developedcomplicationsrelatedtoECMO (9haemorrhages,8hemolysis,6oxygenationdifficulties,5 PAO,4stroke).Concerningthedrugmanagementofthese patients,wecounted16overdosesand2underdosesnot justifiedeitherbytheliteratureorbytherapeuticdrug monitioring(TDM)i.e.18nonconformitiesoutof73 linesanalysed(Vancomycin,Gentamicin,Fluconazole,Caspofungin,Voriconazole,Ganciclovir,Heparin,Morphine, Sufentanil,Midazolam,Cisatracurium,Hydrocortisone Hemisuccinate,Methadone)
ConclusionandRelevance Thepopulationsstudiedintheliteratureremaindifferentfromours,makingitdifficulttodiscuss ourclinicalresults.However,followingthenon-conformities ofdosagenoted,weproposeatableofdosageadaptation underECMOsynthesisingtheliteratureforthestudiedmoleculeswhichissystematicallyaccompaniedbyinstructionsto makeaTDM
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-180 VANCOMYCIN:CONCORDANCEOFDOSAGE ADJUSTMENTACCORDINGTOMINIMUMPLASMA CONCENTRATIONANDAREAUNDERTHECURVE/ MINIMUMINHIBITORYCONCENTRATION
APérezFácila*,TEdeSalinasMuñoz,JJSaizMolina,CNotarioDongil,RLópezAlvárez, MCCondeGarcía. HospitalGenerallaManchaCentro,FarmaciaHospitalaria,Alcázarde SanJuanCiudadReal,Spain
10.1136/ejhpharm-2023-eahp.171
BackgroundandImportance Thepharmacokinetic/pharmacodynamic(PK/PD)targetforvancomycinhasrecentlybeen definedasanareaunderthecurve(AUC)over24hours/minimuminhibitoryconcentration(MIC)of400-600.
AimandObjectives Toevaluatethedegreeofconcordanceof recommendationsafterdoseadjustmentofvancomycinaccordingtominimumplasmaconcentration(Cmin)andAUC/MIC ratio.
MaterialandMethods Retrospectivestudyinadultpatients whoweretreatedwithvancomycinadministeredbyintermittentperfusionandmonitoredbythePharmacyServiceata generalhospitalduringthemonthofAugust2022.
MaterialandMethods
Our6-monthprospectiveobservationalmonocentricstudyfocusesonpatientsinthepaediatricintensivecareunitreceivingECMO.Clinico-biological datawerecollectedfromthecomputerisedpatientrecord andbyourdailypresenceinthedepartment.Wenoted
Variablescollected: sex,age,weight,height,glomerularfiltrationrate(accordingtoCockcroft-Gault),totaldailydoseand recommendationissuedbasedonthedeterminationofCmin andAUC/MIC.
AppropriateCminwereconsidered15-20mg/mLincomplicatedinfection(endocarditis,nosocomialpneumonia,
1OHanafia*, 2HCapelle, 1JLeonelli, 3SHonore, 1PBertault-Peres. 1HôpitauxUniversitaires deMarseille,PharmacieTimone,Marseille,Francemeningitis,osteomyelitis/osteoarticularinfectionandwound infection/abscess)and10-15mg/mLinallotherinfections.For thecalculationofAUC/MIC,MIC=1mg/mLwasassumed. Interpretationofplasmalevelandindividualisedvancomycin adjustmentwasperformedusingMediWarePharm++® softwareusingabicompartmentalmodelandasinglevancomycin level(Cmin).
Results Vancomycintreatmentwasinitiatedandmonitoredin 42patients(52.4%female;72.3±12.3).Anthropometric parameters(weight:81.8±17.9kg;height:163.8±7.9cm; glomerularfiltrationrate:61.5±27.0ml/min/1.73m2);total dailydose:1,878.5mg±524.8mg.Therecommendation issuedwasconcordantviaCminandAUC/MICin35.7%.In thecaseofdiscordance,overexposurewasobservedin66.6% ofcases.
ConclusionandRelevance Approximately2outof3recommendationswerediscordantaccordingtothemethodused, withahighnumberofoverexposuresobservedinthecaseof recommendationsbasedonCmin.Therefore,despitethesmall samplesize,theimplementationofvancomycintherapeutic monitoringaccordingtoAUCisconsiderednecessaryforthe optimisationoftherapeuticmanagement.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
RAguilarSalmeron,ENoguéPujadas,MVilaCurrius,COrtíJuan*,XLarreaUrtaran, MBrugueraTeixidor,CSubiranaBatlle,YOrtuñoRuíz,IGómezIbañez,RSacrestGüell. HospitalUniversitariDrJosepTrueta,PharmacyDepartment,Girona,Spain
10.1136/ejhpharm-2023-eahp.172
BackgroundandImportance TherateofnewHIVdiagnoses duetoverticaltransmission(VT)inSpainisverylowandthe newcasesarerelatedtofailuresintheimplementationofpreventionmeasures.
discrepanciesregardingtheregimenreceived.Allchildren couldbeanalyticallyconfirmedtheabsenceofVT,insome casesafter18months.
ConclusionandRelevance Noneofthenewbornsbecame infectedwithHIV.Althoughthemajorityofmotherscarried outcontrolsduringpregnancy,theabsenceofARTbefore/duringpregnancystandsout,togetherwithdetectableCVsasthe mainRFdetected.Informationcampaignsarenecessaryfor thepreventionofVTviewingduringpregnancy,aswellas, trainingforprofessionalsandconstantupdatingofprotocols toguaranteethecorrectmanagementofchildrenexposedto HIV.
ConflictofInterest Noconflictofinterest
4CPS-182 EXPERIENCEOFDISCONTINUATIONTYROSINEKINASE INHIBITORSTHERAPYINPATIENTSWITHCHRONIC MYELOIDLEUKAEMIAINCLINICALPRACTICE
CMDominguezSantana*,MDominguez-Cantero,MMoraCortes,MBlancoCastaño, GCanoMartinez. HospitalUniversitarioPuertoReal,PharmacyDepartment,PuertoReal, Spain
10.1136/ejhpharm-2023-eahp.173
BackgroundandImportance Thetreatmentofchronicmyeloidleukaemia(CML)withtyrosinekinaseinhibitors(TKI) resultsinoptimalcytogeneticandmolecularreponses, improvinglifeexpectancy.Neverthelessasalifelongpharmacologicaltreatment,canleadtoadverseevents(AEs)that cansubstantiallyimpactthequalityoflife,adherenceand thereforethesuccesoftreatment.Nowadays,discontinuing treatmentinpatientswhoachievedasustaineddeepmolecularresponse(DMR)isthemaingoalinCMLtherapy,in ordertoachieveaTreatement-FreeRemision(TFR),leading toloweroccurrenceofdrug-relatedAEs,costreductionand feelingofcure.
AimandObjectives
Theaimsofstudyaretoidentifyand quantifyriskfactors(RF)forVTintheprenatal,intrapartum andpostnatalperiodsandtoevaluatetheadequacyofantiretroviral(ART)prophylaxis,theappearanceofadverseevents andfollow-upduringthefirstyearoflife.
MaterialandMethods Adescriptive,retrospectiveandobservationalstudywasdesignedwhichincludedallthechildrenwho werefollowedupinthehospitalduringthe2010-2020 period.ThemainRFsthatcouldcontributetoVTwere definedinthethreeperiodsanddemographicandclinicalvariablesofmothersandchildrenwerecollected.Thefollow-up wasrecordedduringthefirstyear.
Results Atotalof30children,of22HIV+mothers,were included.Theywereyoungwomen,mostlyfromimmigrant communitiesandwithouttoxichabits.17%ofthepregnant womenwerediagnosedduringthepregnancycontrolsandof theremaining,20%didnottakeARTtreatmentatthe beginningofpregnancy.Atthetimeofbirth,34.5%had detectableviralloads(CV).Regardingchildren,57%were bornbycesareansectionand13%werepremature.TheRF detectedcorrespondmainlytotheprenatalperiod(62.5%), followedbytheintrapartum(26.8%)andthepostnatal period.ThemostfrequentRFsweredetectableCVsfollowed byprematureruptureofmembranes.Allthechildren receivedprophylaxisthatwaswelltolerated,observing
AimandObjectives TodescribetheclinicalexperienceofdiscontinuingthetherapywithTKIinpatientsdiagnosedwith CML.
MaterialandMethods Retrospective,descriptive,singlecentre (350-beduniversityhospital)studyofpatientswithPhiladelphiachromosome(Ph)positiveCMLinchronicalphase, treatedwithTKItillaugust2022.Criteriafordiscontinuing thetreatment: 5yearswithTKItreatmentandDMR achieved(molecularresponse(MR) 4.0during 36 months).Outcomeswerecollectedfrommedicalrecords: gender,age,TKItreatment,follow-uptime,candidatesto discontinuation,timeelapsedtoreachMR,timebetween treatmentstartanddiscontinuation,TFRduration,percentage ofpatientswholostreponseandwerereintroductedtotherapy,timetolostofresponse,withdrawalsymptomsanddiseaseprogression.
Results Therewere48patients,70,83%male.Population presentedameanageof61(25-81)years.Allreceivedfirstlineimatinib,exceptonepatientwhoreceiveddasatinib.Follow-uptimemedianwas60months(3-243).25%werecandidatestodiscontinuation,mediantimetoreachMRwas15 months(3-50).Timebetweentreatmentstartanddiscontinuationshowedamedianof9years(3-16).TFRmedianwas 10months(3-108).Percentageofpatientswholostresponse andwerereintroductedtotherapywas25%.Mediantimeto lostofresponsewasthreemonthssincediscontinuation.Just
onepatientshowsawithdrawalsymptom(severeanemic) andnoneofthemshowsaprogressiontoadvanceddisease stages.
ConclusionandRelevance Highpercentageofcandidateswere safelydiscontinuedandcurrentlyremainuntreated.Reduction oftoxicitiesassociatedwithTKItherapycoulddrivetoaclinicalbenefitforCMLpatients,improvinglivingconditions.
4CPS-184 WHATISTHEADDITIONNALVALUEOF PHARMACEUTICALINTERVENTIONSON[123I]METAIODOBENZYLGUANIDINESCINTIGRAPHY?
1SChaïb*, 2AFlaus, 1MMeguennani, 1ELevigoureux, 1CBolot, 2MJanier, 3VBreant. 1Radiopharmacy,GroupeHospitalierEst-HospicesCivilsdeLyon,Lyon,France; 2Nuclear Medicine,HospicesCivilsdeLyon,Lyon,France; 3Pharmacy,GroupementHospitalierEstHospicesCivilsdeLyon,Lyon,France
10.1136/ejhpharm-2023-eahp.175
MAAllendeBandres*,MArenereMendoza,PGomezRivas,MAAlcaceraLopez,IVarela Martinez,RFresquetMolina,AFrutosPerez-Surio,LCazorlaPoderoso,TSalvadorGomez, JMVinuesaHernando,MDLRGarciaOsuna. HospitalClinicoUniversitarioLozanoBlesa, Pharmacy,Zaragoza,Spain
10.1136/ejhpharm-2023-eahp.174
BackgroundandImportance OnMarch28th 2022,nirmatrelvir/ritonavirwasmarketedinSpain.TheSpanishAgencyfor MedicinesandMedicalDevices(AEMPS)establishedcriteria toprioritiseitsadministrationinpatientsathighriskofprogressiontosevereCOVID.Dataregardingtheeffectiveness andsafetyofnirmatrelvirinpreventingseverecoronavirusdiseaseoutcomesarelimited.
AimandObjectives Toassesstheeffectivenessandsafetyof nirmatrelvir/ritonavirinpatientsathighriskforsevere COVID-19.
MaterialandMethods ProspectivedescriptivestudyfromApril toAugust2022ofpatientstreatedwithnirmatrelvir/ritonavir. Sociodemographicvariables,vaccinationstatus,hospitaladmission,highriskfactorsforprogressionandconcomitanttreatmentwererecorded.Readmissionswererecordedwithin30 daysoftheendofantiviraltreatment.
Results 53patientswereincludedwithameanageof64 years,51%womenand49%men.57%werevaccinatedwith 3doses,17%with2doses,9%with4doses,6%with1 doseand11%werenotvaccinated.34%(18/53)werehospitalisedatthetimeofinitiationoftreatment.
Themostprevalenthigh-riskcriteriawere:24%active treatmentwithmyelotoxicchemotherapy,21%treatmentin theprevious6monthswithanti-CD20drugs,14%over80 yearsvaccinatedwithsomeriskfactorforprogression,7% patientswithonco-haematologicaltreatmentand7%intreatmentintheprevious3monthswithinhibitorsoftheproteinkinase.3treatmentswereperformedoff-labelforpersistent covid.
Themeannumberofdaysfromtheonsetofsymptomsto thestartoftreatmentwas1.6days.23%ofpatientsrequired doseadjustmentduetorenalimpairment.
53%requiredadjustmentofchronictreatmentforinteractions,mainlywithmetamizole,statins,fentanylanddiazepam.
2patientsreceivedremdesivirandsotrovimab,2remdesivir andanothertwosotrovimab.
4(7%)patientswerereadmittedwithin30daysafterthe endoftreatmentwithnirmatrelvirritonavir,1ofthemwith persistentcovid.Onepatientstoppedtreatmentforhives.
ConclusionandRelevance Nirmatrelvirritonavirhasbeen showntobeasafeandeffectivedruginhigh-riskpatientsof progressiontoseverecovid.
BackgroundandImportance [123I]-metaiodobenzylguanidine (mIBG)scintigraphyisatooltoassesscardiacsympathetic innervation.Itisusedtodiscriminateparkinsoniansyndromes. However,manydrugsareknowntointerferewiththisradiopharmaceuticalthatcanleadtofalseresults1
AimandObjectives Theaimofthisstudywastotrytoassess theimpactofstoppinginterferingdrugswith[123I]-mIBGina retrospectivestudybeforetherecentintroductionofpharmaceuticalinterviewsinanuclearmedicinedepartment.
MaterialandMethods Aretrospectivestudyfrom01/01/2010 to31/03/2022wasconductedtofindoutifadruginteraction couldexplaindiagnosticmismatchesbetweena[123I]-Ioflupane and[123I]-mIBGscintigraphies,focusingontheneurological indicationi.e.thedifferentialdiagnosisofParkinson'sdisease. Onthenuclearmedicinesoftware,asearchofallthepatients whohadbotha[123I]-Ioflupaneanda[123I]mIBGscan2010 andJune2022wasperformed.Eachpatient’schartisanalysedandthediagnosisiscollected.
Results 81patientsunderwent[123I]-mIBGimagingforthedifferentialdiagnosisofneurodegenerativediseaseandamong them42hadnon-contributory[123I]-Ioflupaneimaging (51.9%).Adivergentdiagnosisbetween[123I]-mIBGand [123I]-Ioflupanewasfoundin31%ofcases,representing13 patients.Adruginteractioncouldexplainthismedicalinterpretationmismatchin2patients(15.4%).Concerningthelatter,drugsinvolvedwerecalciumchannelblockers.No abnormalityofthesympatheticinnervationwasfoundwhereas the[123I]-Ioflupanescintigraphyfoundanabnormalityofthe dopaminergictransmission.Theseresultsmaycomplement existingdatasuggestingthatcalciumchannelblockersinterferedincardiac[123I]-mIBGimagingthroughincreasedsympatheticactivity2
ConclusionandRelevance Thereisagreatmedicalinterestin continuingpharmaceuticalinterviewsbecausedruginteractions canleadtonon-contributoryorunconclusiveexaminations.In addition,settingupaclinicaltrialbyre-examiningthesetwo patientsbuttemporarilystoppingthedrugspotentially involvedcouldbeveryinteresting.Indeed,thisworkdemonstratesthecomplexityofassessingtheimpactofpharmaceuticalinterventions.Moreover,thisprocessshouldbeevaluated forothercategoriesofradiopharmaceuticals.
1.EmilioB,FrancescoG,CumaliA., etal.131I/123I-metaiodobenzylguanidine(mIBG) scintigraphy:procedureguidelinesfortumourimaging. EurJNuclMedMolImaging.2010Dec;37(12):2436–46.
2.AStefanelli,GTreglia,IBruno, etal. Pharmacologicalinterferencewith123I-metaiodobenzylguanidine:Alimitationtodevelopingcardiacinnervationimaginginclinicalpractice? EuropeanReviewforMedicalandPharmacologicalSciences.2013 May; 17(10):1326–33
ALuaces-Rodríguez*,PFeijoo-Vilanova,AMartínez-Pradeda,SRotea-Salvo,VGiménezArufe,LCaiero-Martínez,MMateos-Salvador,TVillalta-Andújar,BFeal-Cortizas,IMartínHerranz. ACoruñaUniversityHospitalComplex,Pharmacy,ACoruña,Spain
10.1136/ejhpharm-2023-eahp.176
BackgroundandImportance Persistentcornealepithelialdefects whichdonotimprovewithstandardsupportivetreatmentare challenging.Recently,insulineyedropshaveemergedasan alternativetreatmentastheyhaveshowntoimprovecorneal epithelialhealing.
AimandObjectives Todescribethecharacteristicsofthepopulationtreatedwithinsulineyedrops,itsuseinclinicalpracticeandsafety.
MaterialandMethods Retrospectiveobservationalstudyof patientstreatedwithcompoundingtopical1UI/mLinsulineye dropspreparedatthePharmacyDepartmentinatertiaryhospitalsinceDecember2020toJuly2022.
Thevariablescollectedwerepatientdemographics,ocular pathology,treatmentdurationandadversereactionsofinsulin eyedropsandconcomitantophthalmologicaltreatment.
Results 59patientstreatedwithinsulineyedropswere included(meanage68.7±17.1yearsold,49.2%women).
Pathologiestreatedwithinsulineyedropsinclude:keratitis(81.4%),ofwhich25.4%wereneurotrophickeratitis, 11.9%superficialpunctatekeratitis,10.2%herpetickeratitis,8.5%bacterialkeratitisandothers;cornealepithelial defectssecondarytokeratoplasty(8.5%),cornealopacity (5.1%)andothers.10.2%ofthepatientshaveaffected botheyes.
Insulineyedropswereprescribedfourtimesadayinall thepatientswhenfirstoptionswereineffective.40.7%ofthe patientscontinuewiththem,presentingamediandurationof treatmentof27.7weeks(min.5.1;max.79.1).Discontinuationwasobservedin59.3%ofthepatientsmostlybecauseof reepithelisationandsomebecausetheywereusedastransition treatmentuntilinitiationofautologousserumeyedrops. Noneofthepatientspresentedadversedrugreactions.
Regardingconcomitantoculartreatment,themeannumber ofeyedropsusedwere4.6±2.3perpatient.40.7%ofthe patientswerealsotreatedwithautologousserumeyedrops. Artificialtearsandlubricanteyedropswereusedin40.7% and88.1%ofthepatients.Otherprescribedeyedropsinclude drugssuchasanti-inflammatory(67.8%ofpatients),antibiotics(100%),antivirals(15.3%)andcycloplegicagents(35.6%).
ConclusionandRelevance Insulineyedropsaremainlyusedas secondarytreatmentforcornealdefects.Theyhaveprovento presentgoodtoleranceandbenefitsforthepatients.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
BackgroundandImportance Cilgavimab/tixagevimabaretwo recombinanthumanIgG1ĸ monoclonalantibodiesindicated forthepre-exposureprophylaxisofCOVID-19inadultsand adolescents 12yearsoldweighing 40kg.InSpain,potentialcandidatesarepeoplewithhighdegreeofimmunosuppression(duetopathologyortreatment),whodonotrespond adequatelytovaccination(anti-anati-Santibodies<260BAU/ ml).
AimandObjectives Toanalysetheeffectivenessandsafetyof cilgavimab/tixagevimabinatertiarycarehospital.
MaterialandMethods Descriptive,observational,retrospective study.Patientswhoreceivedcilgavimab/tixagevimabfromMay2022toAugust-2022wereincluded.Variablescollected:age, sex,riskconditionandCOVID-19infection.Theriskconditions,accordingtocriteriaoftheSpanishAgencyofMedicinesandHealthProductswere:1)haematopoieticprogenitor transplantrecipientorCART-T,inimmunosuppressivetreatmentorwithgraft-versus-hostdisease;2)solidorgantransplantrecipients;3)primarycombinedandB-cell immunodeficiencieswithabsenceofresponsetovaccinationCOVID-19;4)immunosuppressivetreatmentwithbiologic immunomodulators(anti-CD20,abatacept,belimumabor mycophenolate,mainly);5)solidorgancancerundertreatment withcytotoxicchemotherapyortreatmentsthatcarryahigh riskofsevereCOVID-19progression;6)peopleatvery-highriskofsevereCOVID-19whoarecontraindicatedfor COVID-19-vaccination.TheprimaryendpointwasCOVID-19infectionaftercilgavimab/tixagevimabadministration.Safety wasanalysedbyincidenceofadversereactions.
Results 43patientswereincluded.23men(53.5%),median age=64yearsold(27-77).36patients(83.7%)wereinrisk group4(26patientstreatedwithrituximab,6patientswith ocrelizumab,1patientwithadalimumaband1patientwith interferonbeta-1A)and7patientswereinriskgroup2(all kidneytransplant).4patients(9.3%)hadCOVID-19infection aftertreatmentwithcilgavimab/tixagevimab(3wereingroup 4and1wasingroup2).Themediannumberofdaysto COVID-19-infectionoccurrenceinthesepatientswas25days. 1patienthadadversereactionsaftertreatment(tachycardia, generalmalaise,hematoma,headache,nauseaanddiffuse abdominalpain).
ConclusionandRelevance Thetreatmentwaseffectiveinthe majorityofpatientsinourhospital.Thissupportstheuseof thedrugasprophylaxistopreventCOVID-19inpeoplewho donotrespondsufficientlytovaccination.Thetreatmentwas welltolerated,presentinglowincidenceofadversereactions. Longertermstudiesshouldbeperformed.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-187 PATIENTSWHOARECANDIDATESFORTREATMENT WITHMONOCLONALANTIBODIESFORPRE-EXPOSURE PROPHYLAXISOFCOVID-19
4CPS-186 EFFECTIVENESSANDSAFETYOFCILGAVIMAB/ TIXAGEVIMABINPRE-EXPOSUREPROPHYLAXISOF COVID-19
10.1136/ejhpharm-2023-eahp.177
RGazquezPerez,DGámezTorres,MSánchezVarela,BSánchezRodríguez,MTGómez Sánchez,TMorenoDiaz*. HospitalUniversitarioTorrecardenas,Pharmacy,Almeria,Spain
10.1136/ejhpharm-2023-eahp.178
BackgroundandImportance Cilgavimab/tixagevimabare recombinanthumanIgG1ĸ monoclonalantibodies,whichare indicatedforCOVID-19pre-exposureprophylaxisinadults andadolescents 12yearsofageweighing 40kg.
RGazquezPerez,BSánchezRodríguez,MTGómezSánchez,DGamezTorres,MSánchez Varela,TMorenoDiaz*. HospitalUniversitarioTorrecardenas,Pharmacy,Almeria,SpainAimandObjectives
Toassesspatientswhoarepotentialcandidatesfortreatmentwithcilgavimab/tixagevimabinatertiary carehospitalandtodescribethesearchstrategy.
2.1wasdevelopedtoaddressphysicalandemotional limitations.
MaterialandMethods
InSpain,potentialcandidatesfortreatmentwithcilgavimab/tixagevimabarepeoplewithahigh degreeofimmunosuppression(duetopathologyortreatment) whodonotrespondadequatelytovaccination.TheSpanish AgencyofMedicinesandHealthProductsestablishestheconditionsforpatientswhoarecandidatesfortreatmentwithcilgavimab/tixagevimab1 .Asearchforpatientswascarriedout, prioritisingthefollowingcriteria:haematologicalpatientson treatmentwithrituximabduringthelast9months,patients withsolidorgantransplant,patientswithmultiplesclerosison treatmentwithocrelizumab/rituximab,andpatientswithrecent infectionbyCOVID-19whobelongtoanyriskgroup.Allof themunderwentserology,includinginthestudythosewith negativeserology(anti-anati-Santibodies<260BAU/ml). Thosepatientswerescheduledforcilgavimab/tixagevimab administration.
Results 112patients(38=haematologicalpatientsonrituximabtreatment,50=multiplesclerosispatientsonrituximab/ ocrelizumabtreatmentand24=kidneytransplantation)were enrolled.72patientswereincluded,38women(52.8%), medianage59.5yearsold(27-77).Thecauseofexclusion waspositiveserologyinallcases.64patients(88.9%)were ontreatmentwithbiologicimmunomodulators(35haematologicpatientstreatedwithrituximab<9months,27patients withmultiplesclerosisontreatmentwithrituximab/ocrelizumab/interferonbeta-1Aand1patientontreatmentwithadalimumab)andtherestwerekidneytransplantpatients. Cilgavimab/tixagevimabwasadministeredto62patients (86.1%),7patientswithunknownreasons,2patientshad COVID-19infectionand1patienthadtobeexcludedfor deepveinthrombosisduetothedevelopmentofsymptomsat thetimeoftheappointment.
ConclusionandRelevance Morethanhalfofthepatients enrolleddidnothaveanadequateresponsetoCOVID-19 vaccination.Thesearchstrategywasagoodtoolforadministeringpre-exposureprophylaxisofCOVID-19tothesemore vulnerablepatients.Furtherstudiesareneededtoevaluatethe effectivenessofthetreatment.
1.https://www.aemps.gob.es/la-aemps/ultima-informacion-de-la-aemps-acerca-delcovid%E2%80%9119/prevencion-frente-a-la-covid-19/personas-candidatas-arecibir-evusheld-en-espana/#:~:text=En%20Espa%C3%B1a%2C%20son% 20potenciales%20candidatas,responden%20adecuadamente%20a%20la% 20vacunaci%C3%B3n.
AimandObjectives ToassesschangesinlongtermQoLin patientstreatedwithGalcanezumab.
MaterialandMethods Descriptivestudyofpatientswho receivedGalcanezumab(February2020toAugust2022).QoL datawerecollectedfrompatientsatweeks0,4,12and48 andfromtheelectronicclinicalhistory:sex,age,typeof migraine,numberofmonthlymigraineheadachedays(MHD) priortotreatmentanddurationoftreatment.ToassesseffectivenesswasusedMSQv2.1(14-itemquestionnairethatmeasuresQoLimpactsin3domains:RoleFunction-Restrictive (RFR),measureslimitationsinsocialandworkactivities;Role Function-Preventive(RFP),measuretheimpactthroughpreventionoftheseactivities;andEmotional-Function(EF),assess theemotionalimpact.HigherscoresindicatebetterQoL).The mainvariablewastherateofrespondersaccordingtoRFR definedaspatientswhoseaveragechangefrombaselinewas 25overweek48.Secondaryoutcomeswereresponders accordingRFRoverweek4and12,andmeanchangesfrom baselineinRFR,RFP,EFandMSQ-totalatweeks4,12and 48.
Results 34patientswereincluded,33woman,meanage45 years(29-69).Typeofmigraine:70,5%chronicmigraineand 29,5%highfrequencyepisodicmigraine.Meanmonthly MHDpriortotreatmentwere18days(8-30)andmeandurationoftreatmentof15(3-27)months.8patientsdidnot reach48weeks,treatmentwasdiscontinuedforineffectiveness.Mainoutcome:therateofresponderswas38,2%at week48.Secondaryoutcomes:34,2%and45,7%responders atweek4and12respectively.Thetableshowsaverage changefrombaselinescoreinMSQ-domainsandMSQ-total:
4CPS-188 QUALITYOFLIFEINPATIENTSONGALCANEZUMAB LONG-TERMTREATMENT
1CMDominguez-Santana, 2ERios-Sanchez*, 1EMBarreiro-Fernandez, 1MABlanco-Castaño, 1JMBorrero-Rubio. 1HospitalUniversitarioPuertoReal,Farmacia,PuertoReal,Spain; 2HospitalUniversitarioPuertoReal,Farmacia,PuertoRealCádiz,Spain
10.1136/ejhpharm-2023-eahp.179
BackgroundandImportance Galcanezumabisadrugindicated formigraineprophylaxis.Peoplewithmigraineexperiencesignificantfunctionalandqualityoflife(QoL)impairment.
Migraine-SpecificQuality-of-LifeQuestionnaire(MSQ)version
ConclusionandRelevance Inthisstudy,long-termgalcanezumabtreatmenthadamoderateeffectivenessinimprovingthe RFR-domainofQoL.Thenumberofrespondersdecreased overtime.Alldomainsimprovedfrombaselineoverthe weeksstudied.However,atweek48,qualityoflifeworsened comparedtoweeks4and12.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
4CPS-189 ANALYSISOFMEDICATIONPERSISTENCEIN MIGRAINEPATIENTSTREATEDWITHANTI-CGRP MONOCLONALANTIBODIES
1MMirCros*, 1FITorresBondia, 1SMCanoMarrón, 1PTabernerBonastre, 1CSantos Rodriguez, 1AMoralesPortillo, 2RCandeasAgusti, 3CGonzalezMingot, 3JSanahuja Montesinos, 1JASchoenenbergerArnaiz. 1HospitalUniversitariArnaudeVilanova,Hospital Pharmacy,Lleida,Spain; 2HospitalUniversitariArnaudeVilanova,PharmacyTechnician, Lleida,Spain; 3HospitalUniversitariArnaudeVilanova,Neurology,Lleida,Spain
10.1136/ejhpharm-2023-eahp.180
BackgroundandImportance Monoclonalantibodiestargeting thecalcitoningene-relatedpeptide(anti-CGRP)arerecently availableformigrainetreatment.Real-worlddataontheutilisationofthesedrugsinclinicalpracticeisscarce,butthis informationcouldhelphospitalpharmacistsaffordabetter selectionoftheavailabledrugs.
AimandObjectives Thestudyaimedtoexploredifferencesin medicationpersistenceinpatientswithmigrainetreatedwith erenenumab,ahumanmonoclonalantibodythatbindstothe receptorforCGRP,orfremanezumabandgalcanezumab, humanisedmonoclonalantibodiesthatbindCGRP.
MaterialandMethods RPTisadrugregistryofpatientswith migraineinitiatingbiologictreatmentinpublicuniversityhospitalsinCatalonia.Forthisstudy,weretrievedfromthe registrydataofpatientsinitiatingtreatmentafter01/02/2020 witherenumab,fremanezumaborgalcanezumab.Theprimary outcomesassessedwere:gender,age,discontinuationrate, timetodiscontinuation,andthecausesofit.Wealsocollecteddatatomeasurethetreatmentresponse,suchas migrainedayspermonthandthevalidatedqualityoflife scales(MigraineDisabilityAssessmentScaleandHeadache ImpactTest-6).
Retrieveddatawasdissociatedbeforeanyanalysis.Chisquarewasusedtocompareproportionsandt-Studentfor continuousvariables.
Results Datafrom131patientswasretrieved:55/131were treatedwitherenumaband76/131withgalcanezumab/fremanezumab.85%ofpatientswerewomen,withamedianageof 51.Medicationpersistencethreemonthsafterinitiatingtreatmentwas36/55witherenumaband57/76withfremanezumab/galcanezumab.Therewerenosignificantdifferences betweenthetwomechanismsofaction.
Themeantimetodiscontinuationinpatientstreatedwith erenumabwas8,9monthsandinpatientstreatedwithfremanezumaborgalcanezumab,6,8months,withoutsignificant differences.
2/19and3/19patientsdiscontinuedtreatmentduetotoxicitywitherenumabandfremanezumab/galcanezumab, respectively.
30/131patients’ treatmentwereswitchedtoadifferent mechanismofaction.Athree-monthfollow-upafterthetreatmentchangerevealedsignificantimprovementin15/30 patients.
ConclusionandRelevance Medicationpersistenceinmigraine treatmentwithanti-CGRPmonoclonalantibodiesseemssimilar forbothmechanismsofaction.
Moreextensivestudiesareneededtoclarifythedifference inresponsetodifferentanti-CGRPmonoclonalantibodies.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
4CPS-190 ANALYSISOFIBRUTINIBDOSEREDUCTIONIN PATIENTSDIAGNOSEDWITHCHRONICLYMPHOCYTIC LEUKAEMIA:AREWEDOINGITRIGHT?
JBooRodríguez*,ARosOlaso,IBeristainAramendi,AEceizaDíez,ALatasaBerasategui, CSaizMartínez,MJGarciadeAndoinBarandiaran,JLandaAlberdi,DGarcíaEcheverría, GLizeagaCundin. HospitalDonostia,Farmacia,Donostia,Spain
10.1136/ejhpharm-2023-eahp.181
BackgroundandImportance Theusualoraldosageofibrutinib inchroniclymphaticleukaemia(CLL)is420mgevery24h.
However,comorbidities,adverseeffectsanddruginteractions requireadosereduction(DR),andtheefficacyoftreatment maybecompromised.
AimandObjectives Toanalysethereasonsofibrutinibdose reductionanditsconsequencesondiseaseprogression/ death.
MaterialandMethods Retrospectiveobservationalstudythat includespatients(n=60)diagnosedwithCLLtreatedwith ibrutinibbetween09/16/2020-09/16/2022andnotinvolvedin aclinicaltrial.Thedemographiccharacteristicsofpatients werethefollowing:43males(72%),meanage72.9years (53-89).
DatacollectionincludeDRrequirements,DRreasons,treatmentsuspensions,diseaseprogressionanddeathwiththeir respectivedaterecording.
ThepercentageofpatientsrequiringDRandthereason thereofwerecalculated.Percentageofdiseaseprogressionand deathalso.Inaddition,mediantreatmentdurationswerecalculatedinmonthsandexpressedinpercentages;overall medianduration(OMD)andafterDRrequirement(DRMD).
Thedatawasobtainedfromtheelectronicmedicalrecord (OsabideGlobal)andtheelectronicprescriptionprogram (Onkobide).
Results 35%ofpatients(n=21)requiredDRduringthestudy period.ThemainreasonsforDRweretoxicity76,1% (n=16),pharmacologicalinteractions9,5%(n=2),efficacy 4,8%(n=1),aging4,8%(n=1)andpatientdecision4,8% (n=1).10%ofDRpatients(n=2)sufferedCCLprogression and29%(n=6)died.5%(n=2)ofpatientsnon-requiringDR sufferedCCLprogressionand13%(n=5)died.TheOMDof thetreatmentwas17months(0-73)andtheDRMDwas12 months(0-70).
ConclusionandRelevance TheibrutinibDRdoesnotinfluence thediseaseprogressionormortality,althoughthesamplesize isnotenoughforaformalstatisticalanalysis.Toxicitywas identifiedasthemostcommonreasonforDR.TheOMD andDAMDdatapresentedinthisworkarelowerthanthose commonlypublishedintheliterature(1)duetothetechnical limitationsonthesoftwaresystems.
1.Hardy-AbeloosC,PinottiandR,GabriloveJ,Ibrutinibdosemodificationsinthe managementofCLL. JournalofHematologyandOncology 2020;13:66Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7275592/pdf/ 13045_2020_Article_870.pdf
AVélezBlanco*,XCasasFernández,LOrtegaValín,IÁlvarezFernández,JCSáez Hortelano,SLlamasLorenzana,RVarelaFernández,EGutiérrezGutiérrez,JJOrtizde UrbinaGonzález. HospitaldeLeón,HospitalPharmacy,León,Spain
BackgroundandImportance In2020Spainwasinvolvedin theSARS-CoV-2pandemic.Thissituationentailedinthedispensingofdrugsfrompharmacyservicestopatients'homes. Thiswayofreachingthepatientfacilitatedtheaccesstoantiretroviraltreatment(ART)inthisdifficultsituation.However, duetothesocialstigmas,certainpatientsdidnotconsentto accessthisdispensingsystem.
10.1136/ejhpharm-2023-eahp.182AimandObjectives
Theobjectiveistostudyhowadherence toantiretroviraltreatmentwasaffectedinHIV-positive patientsduringthemonthsofthefirstalarmstateinSpain (March14toJune212020);becauseduringthoseperiod ARTwashomedispensation.
AimandObjectives Toanalysetheadherenceofpatientswith MSthatwereprescribedwithdisease-modifyingtreatment (DMT)andtoidentifyriskfactors.
MaterialandMethods
Observationalretrospectivestudy, includedpatientsHIV-positivewhoreceivedARTduringthe firstalarmstateinSpainduringCOVID-19pandemicandin thesameperiodof2019.
Collecteddatawere: sex,ageandvariablesrelatedtopharmacologicaltreatment(ARTintheselectedperiods,numberof dispensationsmade,galenicunitsdispensed).
Tomeasureadherence,anindirectmethodwasused,comparingthedispensationsmadeinthehospitalpharmacyofthe hospitalofLeónduringthestudiedperiodandthesamedates ofthepreviousyear.
%adherence=[dispensedgalenicunits/plannedgalenic units]x100
Results Weanalyse444patientswithamedianageof54 years(45-59)being77.93%(n=346)men.
Duringthestudyperiod83patients(18.69%)changed theirART.38.55%(n=32)carriedoutasimplificationof ARTin2020(fromatreatmentbasedonseveralpharmaceuticalformstoatreatmentbasedonasingleone).
Themeanadherenceintheperiodsstudiedin2019and 2020was91.89%(CI90.44-92.90)and90.25%(CI87.6192.90),respectively.In2019,67.12%(n=298)ofpatientshad adherencegreaterthan95%,comparedto86.71%(n=385)in 2020.
For38patientstherearenomedicationdispensations duringthe2020period.Of themajority(n=27)thereason fortheabsenceisunknown;6werenotdisposedoffrom thehospitalofLeónforspend ingtheconfinementoutside thecity;4havediedand1didnotaccepthome dispensation.
ConclusionandRelevance TheimplementationofhomedispensingcouldhavepositivelyinfluencedadherenceinHIVpositivepatients.Itisnecessarytoevaluateinthefuturethat theimplementationofnewtelepharmacyprogrammescan haveapositiveinfluenceonadherence.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1699-714X2020000300193
2.https://iris.paho.org/bitstream/handle/10665.2/51947/2020-cde-coronavirus-diseasehiv.pdf?sequence=1&isAllowed=y
ConflictofInterest Noconflictofinterest
MaterialandMethods Aretrospectiveobservationalstudywas conductedincludingMSoutpatientsunderactiveDMTin 2021.Variablescollected:gender,age,drugtype(subcutaneous interferonbeta1b,intramuscularinterferonbeta1a,subcutaneousinterferonbeta1a,teriflunomide,dimethylfumarate, fingolimod),routeofadministration(oralvsparenteral),polypharmacy(>5drugs/day),adverseeffects(AEs),typeofMS (relapsingremittingMS-RRMS-,secondaryprogressiveMSSPMS-,primaryprogressiveMS-PPMS-),timecourse, ExpandedDisabilityStatusScale(EDSS)scoreatonsetof DMT,numberofpreviousflaresandhospitalisations,and comorbidities.
AdherencewascalculatedthroughtheMedicationPossessionRatio(MPR)usingpharmacydispensationrecorddatabase.GoodadherencewasconsideredMPR 80%.A statisticalanalysiswasperformedwithIBMSPSSStatistics v21.0.
Results Atotalof214patientswereincluded[(62.1%female), meanage43.9(SD9.7)years].
Themostprescribeddrugwasteriflunomide(26.6%),followedbydimethylfumarate(20.6%),subcutaneousinterferon beta1b(14.5%),glatirameracetate(12.6%),fingolimod (12.1%),intramuscularinterferonbeta1a(7.0%),andsubcutaneousinterferonbeta1a(6.5%).Themostfrequentrouteof administrationwasoral(59.3%)vsparenteral(40.7%).38.3% ofpatientswerepolymedicatedand53.7%sufferedAEs.
95.3%ofpatientshadRRMSand4.7%hadSPMS. MediantimewithMSwas11(0.2 – 45)yearsandmedian EDSSwas1.5(0 – 8).Previousflare-upswere51.4%,hospitalisations39.3%andcomorbidities79.4%.
Goodadherence(MPR 80)wasdeterminedfor89.7%of thepatients.MedianMPRwas100(19 – 100).
Adherencewasinfluencedbyrouteofadministration (p=0.024)andcomorbidities(p=0.014)withstatisticallysignificantdifferences.Astatisticallysignificantdifferencewas notobservedfortheanyothervariable.
ConclusionandRelevance Adherencewassatisfactoryinmost patients.Determiningmodifyingfactorsofadherenceisimportanttoidentifypatientsatriskofnon-adherencewhoshall receivepersonalisedpharmaceuticalcareandoptimised treatment.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-192 ANALYSISOFADHERENCEANDASSOCIATEDRISK FACTORSINMULTIPLESCLEROSISPATIENTSUNDER DISEASE-MODIFYINGTHERAPY
1LSopena, 1AMagallon, 1AFrutos, 2MGarcés, 2CIñiguez, 1RFresquet, 1PGomez, 1JMVinuesa, 1AMerchan, 1TSalvador, 1ABMaríaÁngeles*. 1HospitalClínicoUniversitario LozanoBlesa,Pharmacy,Zaragoza,Spain; 2HospitalClínicoUniversitarioLozanoBlesa, Neurology,Zaragoza,Spain
10.1136/ejhpharm-2023-eahp.183
BackgroundandImportance Multiplesclerosis(MS)isoneof themostfrequentcausesofdisabilityamongyoungpeople. Understandingpatients’ adherencetotreatmentsisofgreat importancetoassesstheeffectivenessandsafetyoftheprescribedtreatments.
4CPS-193 SUITABILITYOFTHEDUALANTIPLATELETTHERAPY TOTHEGUIDELINESOFEUROPEANSOCIETYOF CARDIOLOGYINACUTECORONARYSYNDROME
HSuñer*,CJCortésSánchez,DPascualCarbonell,ASanjuánBelda,ISacanellaAnglès, AGarciaMolina,LCanadellVilarrasa. HospitalUniversitariJoanXxiii,Pharmacy,Tarragona, Spain
10.1136/ejhpharm-2023-eahp.184
BackgroundandImportance Thedualantiplatelettherapy (DAPT)consistingofacetylsalicylicacidplusoneP2Y12plateletreceptorinhibitorrepresentsthefirstlinetotreatpatients withdiagnosisofacutecoronarysyndrome(ACS).
AimandObjectives ToreviewtheDAPTprescribedtopatients withACSadmittedinathirdlevelhospitalandtoassesstheir
adequacygradetotheEuropeanguidelinesofcardiology (ESC).
MaterialandMethods
Observationalandretrospectivestudy donebetweenJanuary-June2022wheredataforpatientswith ACSdiagnosis:unstableangina(UA)ormyocardialacuteinfarctionwithandwithoutSTelevation(STEMI,NSTEMI)have beencollected.Studiedvariablesweredemographicandclinicalinformation(diagnostic,treatment,cardiovascularriskfactors(CVRF)).Foreachpatientischemicandhaemorrhagicrisk havebeencalculated(usingGRACEandCRUSADEscore). Patientswith3ormoreoftheCVRFdescribedhavebeen consideredfragilepatients.ESCguidelinesestablishedthe appropriateDAPTforeachpatientaccordingtotheACS's typeandpatient’sischemic-haemorrhagicrisk.Adequacywas assessedintermsofcomplianceornon-compliancewiththese recommendations.
DatawereexportedfrommedicalhistorythankstoSAP® informatics’ toolandSilicon® electronicprescriptionprogram. StatisticanalysiswasmadebyStata.v.15.0®.Qualitativevariableswereexpressedinpercentagesandabsolutefrequencies. Quantitativeoneslikeaverage±standarddeviation.
Results Atotalof95patientswerediagnosedwithACS
74,74%(71)ofwhichweremenwithanaverageageof 64,38±12,77years,the7,37%(7)withUA,44,21%(42) NSTEMIand48,42%(46)STEMI.Allwereundertreatment withDAPTandmoreoverthe21,05%(20)wereanticoagulatedafterpercutaneouscoronaryintervention.The51,58% (49)werelow,33,68%(32)mediumand14,74%(14)high ischemicriskypatients.Regardingthebleedingriskthe 53,86%(51)werelow,23,16%(22)mediumand23,16% (22)high.
The37,89%(36)oftheprescribedtreatmentsweren’tcomplyingwiththerecommendedDAPTinESCguidelines accordingtoACS'stypeandpatient’sriskfactors.Bydiagnosis,in42,85%(3/7)ofUApatients,42,85%(18/42)of NSTEMIand30,43%(14/46)ofSTEMItheprescriptionsdid notconformtoguidelines.
ConclusionandRelevance Percentageofnon-adequacyofprescribedDAPTtorecentpublishedESCguidelinesisconsiderable,leadingtodisparityofcriteriawithguidelinesand betweenprofessionalsandpossibletreatment'sinequity betweenpatients.Futurestudiescouldexploretheimportance ofpharmacistintegrationandvalidationtoavoidreported discrepancies.
ConflictofInterest Noconflictofinterest.
THERAPYTHROUGHUSEOFSGLT2IINACOHORTOF PATIENTADMITTEDWITHANACUTEMYOCARDIAL INFARCTION
NBenHajmessaoud*,PWright,SFhadil,SAntoniou. StBartholomew'sHospitalBarts HealthNHSTrust,Cardiology,London,UnitedKingdom
10.1136/ejhpharm-2023-eahp.185
BackgroundandImportance Therearearound100,000hospitaladmissionseachyearintheUKduetoacutemyocardial infarctions(AMI).Co-morbiditiesinthosewithischaemic heartdiseasearecommonandincludeheartfailure,diabetes andchronickidneydisease(CKD),theinterplaybetweenthese conditionsbeingrecentlytermedcardiometabolicsyndrome.
RecentupdatesinUKNICEguidancesupporttheuseof SGLT2iforthosewithtypeIIdiabetes(T2DM)andcardiovascular(CV)risk,fortreatmentinthosewithheartfailurewith reducedejectionfractionandmostrecentlyforCKD.
AimandObjectives AssesspatientsatalargeLondonbased cardiovascularcentre,beingpreviouslydischargedwithadiagnosisofAMItoidentifytheopportunitytooptimisetherapy throughprescribingSGLT2i.
MaterialandMethods RetrospectiveanalysisofpatientsadmittedwithanAMIbetweenJanuaryandOctober2021ata largeLondonbasedcardiovascularcentretocomparetheoptimisationofSGLT2iatdischarge(DC)andat12monthsin thosewithcardiometabolicriskfactors(i.eT2DM,HFand CKD).
Results 450patientswithAMIwerefollowedduring1year, averageagedof57.3yearsoldwith84%male,T2DM (25.7%),HF(23.5%),CKD(10%),43%smokersand3% withAF.
Atdischarge,SGLT2iwereprescribedin4.6%ofallAMI patients.InpatientswithT2DM,HFandCKD,therespective ratesofSGLT2iatdischargewere18%,3.7%and2.2%.
At12monthspost-discharge,T2DMincreasedto28%(11 newlydiagnosed),23.5%ofpatientswithHFand16%with CKD(26patientsnewlydiagnosed).SGLT2iwereprescribed in10.4%ofpatientswithrespectiveratesof30%,16%and 11.1%.
ConclusionandRelevance Thisdatasupportsanopportunity toimproveSGLT2iprescribinginourpost-MIcohortwith additionalcardiometabolicriskfactors.Therewasasmall increaseinprescribingnotedafter12monthsbutrecent updatesinUKpolicywouldsupportawideradoptionof SGLT2iuse,inparticularnotingthehighratesofT2DMand HFseeninthepost-AMIgroup.Strategiestofacilitateoptimisationincludeprotocolisationofinitiation,communicationfor 1rycarephysicianstostartshortlyafterdischargeandconsiderationofearlierinitiationpriortodischargeinthosewith cardiometabolicriskfactors.
ConflictofInterest Noconflictofinterest
4CPS-195 E-LUNGING:EVALUATIONOFANE-LEARNING PROGRAMINTENDEDFORHEALTHCARE PROFESSIONALSREGARDINGTHEMEDICATIONOF LUNGTRANSPLANTPATIENTS
1RSchofield*, 1OJouhet, 1DBoden, 1ACrou, 2GDauriat, 1VChevalet, 1LGutermann. 1HôpitalMarieLannelongue,Pharmacy,LePlessis-Robinson,France; 2HôpitalMarie Lannelongue,Pneumology,LePlessis-Robinson,France
10.1136/ejhpharm-2023-eahp.186
BackgroundandImportance Ourinstitutionisspecialisedin lungtransplantation(LT).Thedrugsassociatedwiththisprocedurearenumerousandofcomplexmanagement.However, thelong-termsuccessofLTisdirectlylinkedtopatientadherence.Inourinstitution,nursingstaffturnoverisfrequent,and thereplacementstaffisnotalwaysspecialisedinLT.This observationledtothedevelopment,incollaborationwiththe medicalandnursingteams,ofanonlinetrainingprogram(elearning)forhealthcarestaff,intendingtoreinforcethe appropriateuseoftransplantdrugsandbetterrespondto patients'queries.
AimandObjectives
Toevaluatetheimpactofourtrainingon theacquisitionofknowledgebynursingandpharmaceutical staff.
comprehensivemedicationreviewwasconductedbyahospital pharmacist.PIMsandPPOswereidentifiedusingtheSTOPPSTARTcriteria.
MaterialandMethods
Thecontentandformatofthee-learningweredecidedduringmultidisciplinarymeetings,andthe trainingwascreatedusingspecificsoftware(ArticulateStoryline®).Itisdividedinto5partsandcontainsanassessment questionnairetobecompletedbeforeandafterthetraining. Thee-learningwasdistributedtothenursingstaffofthe thoracicsurgeryandintensivecareunits,aswellastothe pharmaceuticalstaffofourhospitalgroup.Therateofcorrect answersobtainedbeforeandafterthetrainingwascollected andcompared.
Results 34peoplecompletedthetraining.Theaveragerateof correctanswersobtainedbeforeandaftercompletingthe trainingincreasedsignificantly,from75%to86%(p< 0.001).Themostrepresentedprofessionalcategorieswere pharmacystudents(15/34),pharmacyresidents(7/34),and nurses(7/34).
100%ofpeoplesaidtheywere ‘satisfied’ or ‘verysatisfied’ withthetrainingand97%wouldrecommendit.
ConclusionandRelevance Theincreaseintherateofcorrect answersbeforeandafterthetrainingshowsthatthenursing andpharmaceuticalstaffhasacquiredknowledge.Amainlimitationoftheprojectwasthedifficultyfornursestofinddedicatedtimetocompletethetraining.Theimpactondaily practicesinourinstitutionstillremainstobeevaluated.The distributionofthetrainingtocommunitypharmaciesthat treatlungtransplantpatientscouldprovevaluableto strengthenourcommunityrelations.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-196 POTENTIALLYINAPPROPIATEMEDICATIONSAND POTENTIALLYPRESCRIBINGOMISSIONSINOLDER PEOPLELIVINGWITHHIV
1JFernándezFradejas, 1MVélez-Díaz-Pallarés, 1EDelgado-Silveira, 1PGuijarro-Martínez, 1SRodríguez-Tierno, 2CRodríguez-González, 3FMartínez-DelaTorre, 4CMartínez-Nieto, 5EGonzález-Burgos, 1AMAlvarezDiaz*. 1HospitalUniversitarioRamónYCajal,Pharmacy, Madrid,Spain; 2HospitalGeneralUniversitarioGregorioMarañón,Pharmacy,Madrid,Spain; 3HospitalUniversitario12deOctubre,Pharmacy,Madrid,Spain; 4HospitalUniversitario InfantaSofía,Pharmacy,SanSebastiándeLosReyes,Spain; 5UniversidadComplutensede Madrid,PharmacologyDepartment,Madrid,Spain
10.1136/ejhpharm-2023-eahp.187
BackgroundandImportance DuetoahigherburdenofnonHIVcomorbiditiesandtheuseofmultiplemedicinesincomparisontonon-infectedpopulation,olderpeoplelivingwith HIV(PLWH)aremorelikelytobeatriskofdrug-related problems,includingpotentiallyinappropriatemedications (PIMs)andpotentiallyomittedprescriptions(PPOs).
AimandObjectives TodeterminetheprevalenceofPIMsand PPOsinolderPLWH.ToidentifythemaingroupsofmedicationsinvolvedinPIMsandPPOsaccordingtotheSTOPPSTARTcriteria.
MaterialandMethods Across-sectional,observational,multicentrestudywasconducted.OlderPLWH(aged65orolder) whowereonactiveantiretroviraltreatmentatfourdifferent hospitalsbetween1September2021and31December2021 wereincluded.Demographicandclinical-pharmacotherapeutic datawereobtainedfromelectronicmedicalrecords.A
Results Onehundredpatientswereincluded,83%male,mean age73.1years(SD6),meanVACSindex40.8(SD11),96% weremultipathological(meannumberofnon-HIVcomorbidities4.3,SD2).Meannumberofchronicdrugsperpatient (excludingantiretroviraltreatment),8.5(SD3.4),92%presentedpolypharmacy.Forthepatientsincluded,atotalof124 PIMsand119PPOswereidentified.Theglobalprevalenceof PIMsandPPOswas75%.TheprevalenceofPIMsandPPOs separatelywas53%and68%respectively.Seventeendifferent STOPPcriteriawereidentified.ThemostfrequentSTOPPcriteriawereA1(drugwithoutevidence-basedclinicalindication, n=51,41.1%),D5(benzodiazepinesfor4ormoreweeks, n=20,16.1%)andK1-K4(benzodiazepinesandZ-drugprescriptions,n=20,16.1%).TwentydifferentSTARTcriteria wereidentified.ThemostfrequentSTARTcriteriawereI1-I2 (influenzaandpneumococcalvaccine,n=56,47%),E4(bone antiresorptiveoranabolictherapyinosteoporosis,n=10, 8.4%)andG8(5-alphareductaseinhibitorwithsymptomatic prostatism,n=8,6.7%).
ConclusionandRelevance Threequartersofthepatients includedinourcohortofolderPLWHpresentPIMsor PPOs.ThemaingroupofdrugsinvolvedinPIMsandPPOs arebenzodiazepinesandvaccines.Medicationreviewisessentialtooptimisepharmacotherapyandpreventdrugrelated problemsinthispopulation.
REFERENCESAND/ORACKNOWLEDGEMENTS
STOPP-STARTcriteria(O’Mahonyetal.,2015).PMID25324330
ConflictofInterest Noconflictofinterest
4CPS-198 ANALYSISOFANTIPSYCHOTICDRUGSUSEATA SPANISHTERTIARYHOSPITAL
1RCastillejo*, 1LDLRuedaBermudez, 1GRamirezSoto, 1NRebolloDiaz, 2ÁLópezDíaz. 1ClinicalPharmacist,HospitalUniversitarioVirgenMacarena,Seville,Spain; 2Clinical Psychiatrist,HospitalUniversitarioVirgenMacarena,Seville,Spain
10.1136/ejhpharm-2023-eahp.188
BackgroundandImportance Theeffectivenessofsecondgenerationantipsychotics(SGA)hasbeenshowntobeequivalentto thefirst-generationantipsychotics(FGA),withdifferentside effectprofiles.
AimandObjectives Theaimofthisstudywastodetermine thepatternsofantipsychoticsprescriptionandrelatedcostsat auniversityhospital.
MaterialandMethods Oneyearretrospectiveobservational studyataSpanishuniversitygeneralhospital.Identificationof outpatientsundertreatmentwithantipsychoticsduring2021, whichhadbeenprescribedathospital.FGAandSGAdata consumptionwasobtainedfromMicrostrategy ® database. Comparativeanalysisinrelationtotheeuropeanpatternsof prescriptionandcostanalysiswereperformed.Mainoutcome measures:percentageofpatientswithSGA,FGAandlong-actingantipsychotics(LAI)andcostsperpatient.
Results Atotalof16.967patientsundertreatmentwithantipsychoticsduring2021wereidentified:9.01%FGAand 90.9%SGA.ThemostfrequentFGAwere:oralhaloperidol (44.4%),oralclotiapine(26.6%)andorallevomepromazine (17.9%).AmongtheSGA,themostusedwere:oral
quetiapine(37.3%),oralolanzapine(20.2%),oralaripripazole (15.7%)andoralrisperidone(11.4%).Regardingthetotal prescriptions,thepercentageofuseofLAIwas6.66%(3.43% and6.98%amongFGAandSGA,respectively).The95.31% totalLAIprescriptionswereSGA.TheuseofLAIathospital wasfoundtobelowerthaninotherEuropeancountries (15%accordingtoArangoC.etal2019).However,theproportionofsecondgenerationLAIwashigherthantheEuropeanaverage(34%).Themeancostperpatientwashigher forSGAthanforFGA(353.5C ¼ vs27.0C ¼ ).Likewise,incomparisontoconventionalinjectableantipsychotics,costsper patientwerehigherforLAI:155.1C ¼ vs15.8C ¼ forFGAand 2887.5C ¼ vs72C ¼ forSGA,respectively.
ConclusionandRelevance IncomparisonwithotherEuropean countries,apredominanceoftheuseofsecondgeneration LAIhasbeendetected.However,theuseofLAIislower. Cost-effectivenessstudiesregardingtheuseofSGAversus FGAandconventionalversusLAIantipsychoticsareneededin ordertooptimisethebenefitstothepatientandminimisethe economicburdenforthehealthsystem.
1.ArangoC etal.Long-actinginjectableantipsychoticsforthetreatmentofschizophreniainSpain. RevPsiquiatrSaludMent(EnglEd).2019; 12(2):92–105.
1SMousannif*, 2HMefetah, 3IZhim, 1MBouatia. 1MohammedVUniversity-Facultyof MedicineandPharmacy-PaediatricHospital,PharmacyDepartment,Rabat,Morocco; 2PaediatricHospitalRabat,PharmacyDepartment,Rabat,Morocco; 3MohamedVMilitary Hospital,PharmacyDepartment,Rabat,Morocco
10.1136/ejhpharm-2023-eahp.189
BackgroundandImportance DrugIntoxicationsinchildren,by itssocial-economicimplications,representamajorproblemof PublicHealth.Theyconstitutethemaincauseofemergency admissionsandalsooneoftheprincipalcausesofdeathin childrenandadolescents.
AimandObjectives Theaimofthisstudyistoevaluatethe pharmaco-economicimpactofdrugintoxicationsregistredin thepaediatricemergencydepartment.
MaterialandMethods Thisisastudyspreadoveraperiodof 12monthsfromJanuary1,toDecember31,2021,inthe paediatricemergencydepartment.Itisbasedontheanalysis ofcoststomanagealldrugintoxicationsrecordedinchildren foronedayofhospitalisationwhichincludethecostof:drugs andantidotesadministered,laboratoryandradiologicalanalysis,hospitalisationfees.
Thereferenceoftheidentifycostsisgivenbythebilling departmentofourhospital.
Results Duringthisperiod69casesofdrugintoxications wereadmitted.AccordingtoATCCSclassification,theclass N(NervousSystem)wasthemostcommonclassinvolvedin drugintoxications(50%)followedbyMusculo-SkeletalSystem(15%)thenGenito-UrinarySystemandSexHormones (11%),RespiratorySystem(8%)and16%forotherclasses.
Tomanagethesedrugintoxications,asymptomatictreatment andantidotesadministrationisregistredin32.5%ofcases (500C ¼ ),in35.5%ofcaseslaboratoryandradiologyanalysis weredone(1400C ¼ ).Thedistributionofthecostsforone
dayofhospitalisationrelatedtoeachinterventionandforall recordeddrugintoxicationsissummarisedinthetable below:
Onaverage,intoxicatedchildrenstayinthehospitalforat least48hoursundermedicalsupervision,thetotalcostof treatmentfordrugintoxicationbecomes6000C ¼ anditcan increasedependingontheseverityofintoxication.
ConclusionandRelevance Inourstudywehaveincludedonly thedrugintoxicationsandwehavefoundthattheirmanagementrepresentsaconsiderablepharmaco-economicimpact alsotheresearchhasallowedustoconcludethathalfofthe drugsusedbychildrenbelongtotheclassofthenervoussystemwhichconstitutesasignificantdanger.
4CPS-200 SUSTAININGAPHARMACEUTICALDECISIONSUPPORT SYSTEMBYDETERMININGTHECLINICALRISK’S LEVELOFDETECTEDDRUG-RELATEDPROBLEMS
1JBouet*, 1APotier, 2CMongaret, 3BMichel, 4MCillis, 5ADony, 1MAde, 5EDivoux, 1CViaud, 5EDufay. 1ChruNancy,Pharmacy,Vandœuvre-Lès-Nancy,France; 2ChuReims, Pharmacy,Reims,France; 3HuStrasbourg,Pharmacy,Strasbourg,France; 4ChuUclouvain, Pharmacy,Namur,Belgium; 5ChLunéville,Pharmacy,Lunéville,France
10.1136/ejhpharm-2023-eahp.190
BackgroundandImportance Pharmaceuticaldecisionsupport system(PDSS)isapositivetriangulationbetweenpatients’ data,modelledsituationsstandingfordrug-relatedproblems andareasoningsoftwaresendingalerts.Sothepharmaceutical interventionsbetterpreventadversedrugeventsandbetter reducehealthcarecosts.ButtobeoptimalthePDSShasalso tolinkthemodelledsituationstoaclinicalwell-definedrisk. Asconsequenceseachpharmaceuticalintervention’simpact willbedocumentedandthePDSS’sinterestinpatients’ safety sustained.
AimandObjectives Topresenttheresultsofane-Delphistudy duringwhichhealthprofessionalexpertsevaluatetheclinical risk’slevelof52modelledsituationsstandingfordrug-related problemsoradversedrugevents.
MaterialandMethods Twentyexpertsacross4francophone countrieswereinvolvedbecauseoftheirclinicalskills. Basedontheirexperience,physicians(5)orpharmacists (15)scoredthelikelihoodofoccurrenceofclinicalconsequencesanditsseverityforeachofthe52modelled patients ’ situationsusingafive-pointLikertscale.Thesesituationswerechosenamongapanelof199one,according totheirhighfrequencyinthehealthfacilities.Thedegree ofconsensusbetweenparticipantswasdefinedastheproportionthatgaveariskscoreinthesamecategoryasthe median.Consensuswasobtainedifthescorewas75%or more.Thenthe2mediansc ores-occurrenceandseverity-
werecombinedtoproducetherisklevelforeachsituation. Only2Delphiroundswerenecessary.
Results Afterthefirstroundaconsensuswasreachedfor8 situations.Expertsagreedonthelevelofriskassociatedwith 48outof52modelledsituations.Ahighorextremeconsensusrisklevelisdeterminedfor45modelledsituations.These situationsrepresentavarietyofdrug-relatedproblems.Overdosingwasthemostfrequentsituation[12(22%)].Cardiovascular,PsychiatricandEndocrinologicaldrugclassesarethe mostcommoninvolvedinrespectively[25(45%)],[7(13%)] and[5(9%)]situations.
ConclusionandRelevance Thesymbolicartificialintelligenceto detectdrug-relatedproblemsinpatients’ medicationswillbe muchmoresharedifpharmaceuticalalgorithmsincludingthe clinicalriskaredefinedthroughconsensus.
REFERENCESAND/ORACKNOWLEDGEMENTS
HealthRegionalAgency,InnovationDepartment,RégionGrandEst,France
ConflictofInterest Noconflictofinterest
QLopezNoguera,SGarciaRodicio,NRamonRigau,COrtíJuan*,ADordàBenito, RSacrestGüell. HospitalUniversitariDrJosepTruetadeGirona,PharmacyDepartment, Girona,Spain
10.1136/ejhpharm-2023-eahp.191
BackgroundandImportance Lungdiseaseprevalenceinthe prisonpopulationishigherthaninthegeneralpopulationof thesameage.Pharmaceuticalcaredetectsandreducesdrugrelatedproblemsbyhelpingintherapyoptimisationand improvingtreatmentadherence.
withoutexacerbationsoverthelastyear).28/36patients requiredpharmaceuticalcaretoimprovepatient’sinhalation technique(23non-adherentand5treatmentoveruse).
ConclusionandRelevance Pharmacistsplayakeyroletooptimisecomplextherapies.Thisstudyshowsusthatalmosthalf ofbronchodilatortreatmentsinprisoncanbeoptimised,and morethanthreequartersofthepopulationhavepooradherence.Aspecificpharmaceuticalcareprogrammeinprison shouldbecarriedouttoidentifydrug-relatedproblems.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-202 THEUSEFULNESSOFAPHARMACYRESIDENTSTAGE INTHECRITICALCAREUNIT
AMoralesPortillo*,MMirCros,MBardollCucala,MCuyBueno,BMartínezCatro, MMartínezSogues,MNevotBlanc,CSantosRodríguez,MGilabertSotoca, JASchoenenbergerArnaiz. HospitalUniversitarioArnaudeVilanova,Pharmacy,Lleida, Spain
10.1136/ejhpharm-2023-eahp.192
BackgroundandImportance Thepresenceofpharmacistsas membersofthemulti-professionalcriticalcareteamisincreasinglyacceptedandwelcome.However,theimpactofthis presenceisnotalwayseasytomeasuresincetheofferedserviceportfoliovarieswidelyfromhospitaltohospital.
AimandObjectives Thisstudymeasurestheintervention impactoftherotationofapharmacyresidentinthecritical careunitofahospitalafterassessingtheunit'scomplexity level.
MaterialandMethods Criticalcarecomplexitywasmeasured asthemeanMedicationRegimenComplexity-ICU(MRC-ICU) throughoutthestudyperiodandcomparedtoprevious studies.1
AimandObjectives
1/Toimprovethebronchodilatortreatmentofpatientswithasthmaorchronicobstructivepulmonarydisease(COPD)inprison.
2/Toidentifypatientswithlowadherenceinorderto checkthepatient’sinhalationtechniqueandensureproper administration.
MaterialandMethods Observational,descriptiveandretrospectivestudyofpatientswithasthmaand/orCOPDdiagnosisin August2022.Demographicdata(age,sex),clinicaldata(body massindex,smokinghabit,presenceofexacerbations)and typeoftreatmentwerecollected.Adherencewascalculated throughdispensingrecords(packagescollected/packagesprescribed)betweenAugust2021andAugust2022.Adherent patientwasdefinediftheyhad100-80%ofdispensations, non-adherentiftheyhad<80%,andpoorlycontrolleddueto bronchodilatortreatmentabuseiftheyhad>100%.
Results 46(6.7%)patientsunderbronchodilatortreatment wereidentifiedoutof686prisoners.10patientswere excludedbecausetheywerenotchronictreatment.The36 selectedpatientshadameanageof40±9yearsand8.3% werewomen.28patientshadasthmadiagnosis,6COPD diagnosisand2hadmixedpattern.33patientsweresmokers and24wereoverweightorobese.
Bronchodilatortreatmentcouldbeoptimisedin16/36 (44.4%)ofpatients:10patientswithasthma(5without inhaledshort-actingbronchodilator(SABA)and5usedinhaled corticosteroids),and6patientswithCOPD(3usedSABAas maintenancetreatmentand3usedinhaledcorticosteroids
Pharmacistinterventionsinthecriticalcareunitover7 weekswereprospectivelyrecorded.
Therewerethreetypesofinterventions:clinical(affecting thepharmacotherapyofanadmittedpatient),informative (regardinggeneralinformationofmedicines),andlogistical (regardingthecriticalunitorganisationandmedicines distribution).
Interventionswerealsoclassifiedbytheaddressee(medical, nurserystaff,orboth)andbyintensity(low,medium,or high),measuredaspreviouslydescribed.2
Acceptanceoftheinterventionswasalsorecorded.
Interventionsregardingparenteralnutritionandtherapeutic drugmonitoringwereexcludedfromthisstudysincethey werealreadystandardcareinourhospital.Results
ThemeanMRC-ICUscorewas10.46(standarddeviation 5.4).
Amongthe108interventionsrecorded,for83patients,75 (70%)wereclinical,22(20%)informativeand11(10%) logistical.In85cases(79%),theaddresseeoftheintervention wasthemedicalstaff,18(17%)thenurses,and5(4%)both. Regardingtheintensity,11/108(10%)wereclassifiedaslow, 37/108(35%)mediumand58(55%)ashigh.Theacceptance ofinterventionswashigh:106/108(98%).
ConclusionandRelevance Criticalcarecomplexityinthisstudy wasaboveaveragecomparedtopreviousstudies.1
Aclinicalpharmacist,evenatraineepharmacyresident,can improvecriticalhealthcareandclinicaldecision-makingbythe criticalcareteam.
Ahighinterventionacceptancerateshowshowvaluablethe restoftheprofessionalsintheintensivecareteamconsider theclinicalpharmacist.
REFERENCESAND/ORACKNOWLEDGEMENTS
1. CritCareMed 2022Sep1;50(9):1318–1328.doi:10.1097/ CCM.0000000000005585.
2. CritCareMed 2022Sep1;50(9):1318–1328.doi:10.1097/ CCM.0000000000005585.SupplementalDigitalContent – Table3.(http://links. lww.com/CCM/H141).
ConflictofInterest Noconflictofinterest
PCarlier, 1CNiot*,AVisticot,LRéal. CentreHospitalierD'arras,Pas-De-Calais,Arras, France
10.1136/ejhpharm-2023-eahp.193
BackgroundandImportance Themanagementofcomplex traumainchildrenandadolescentsisdifficultbecauseof hismultidimensionalnature.Researchintothisareaisparticularlychallengingandveryfewclinicalstudiesareavailable.Clonidineisusedoff-labelinourcountryforthis indication.
AimandObjectives Theaimofthisworkistostudytheprescribingpracticesofclonidineinchildrenandadolescentswith complextrauma:thepre-therapeuticassessment,thetargeted symptoms,thegalenicformulationandthetolerance.
MaterialandMethods Nationaldistributionofaquestionnaire viathemailingofthePsychiatryInformationCommunication networkandtheFederativeAssociationofPsychiatricStudents ofourcountrytofindoutaboutthepracticesofprescribing clonidineinotherhealthestablishments.Questionnairevalidatedupstreambythereferentchildpsychiatristthenextractionoftheanswersanddescriptiveanalysisofthedata collected.
Results 88responseswereobtained,58%frompsychiatrists and38%fromresidentsinpsychiatrywithatleastone responseperregion.TheanalysisshowstheuseofClonidine, especiallyintabletform,asalastresortandveryoftenin combinationmedication.Amongthe25peoplewhoanswered theentirequestionnaire,goodtolerancewasobservedin84% ofcases,theremaining12%reportedepisodesofhypotension orheadaches.Apre-therapeuticassessmentwithelectrocardiogram,bloodpressureandpulsearecarriedoutin72%of casesandclinicalefficacyisobservedin76%ofcases,inparticularonnightmares,insomniaandanxiety.
ConclusionandRelevance Preliminarydataseemtoindicate thatclonidinecouldhaveapositiveclinicalimpactoncertain symptomsofcomplextrauma.Amulticentre,double-blind clinicalstudy,Clonidineversusplacebo,onalargersample andtakingintoaccounttheenvironmentalcontextofthe child,couldmakeitpossibletoconfirmornotthis hypothesis.
1.BriereJ,ScottC.ComplexTraumainAdolescentsandAdults:EffectsandTreatment. PsychiatrClinNorthAm.sept2015;38(3):515–27.
2.LustigSL,BotelhoC,LynchL,NelsonSV,EichelbergerWJ,VaughanBL.Implementingarandomisedclinicaltrialonapaediatricpsychiatricinpatientunitata
children’shospital:thecaseofclonidineforpost-traumaticstress. GenHospPsychiatry.déc2002;24(6):422–9.
4CPS-206 EVALUATIONOFIRONCARBOXYMALTOSEVSIRON SUCROSEADMINISTRATIONFORTHECONTROLOF ANAEMIAINHOSPITALISEDPATIENTS
CCáceres-Velasco*,MÁAmor-García,AMelgarejo-Ortuño,RMoreno-Diaz,EMatillaGarcía,BRodriguez-Vargas,CAApezteguiaFernandez,MPBautistaSanz. Hospital UniversitarioInfantaCristina,PharmacyDepartment,Madrid,Spain
10.1136/ejhpharm-2023-eahp.194
BackgroundandImportance Ironcarboxymaltose(ICM)and ironsucrose(IS)aretwotypesofintravenousironusedfor thetreatmentofiron-deficiencyanaemia.Differencesbetween thedosingregimenandhospitallengthhaveledmanycentres toperformcost-effectivenessstudieswithvariableresults.
AimandObjectives Tocomparetheeffectivenessandcostof intravenousICMvsISforthecontrolofanaemiainhospitalisedpatients.
MaterialandMethods Retrospectivecohortstudyinanaemic patients(Hb£12g/dL)whoreceivedICM(500-1,000mg,single-dose)orIS( 3X100mg)betweenApril2021andApril 2022wasperformed.Demographicvariables(age,gender), totaldoseadministered,Hbpreandpost-treatment(>6days), patientswithincreasedHb 1g/dl,previoustreatmentwith oraliron,hospitaladmissionlengthanddirectcostwere collected.
Cohortswerematchedforbaselinecharacteristics(age,genderandhospitalservice)andinitialHbvalues.Selectedvariableswerecompiledfromtheelectronicmedicalhistoryand prescriptionandcomparedusingstudent'sttestwithSPSS v.22.0.
Results Atotalof98patients(63.3%women)wereincluded: 49receivedICMand40IS.Meanagewas75.5±13.8and 75.9±13.6yearsfortheICMandISgroupsrespectively.In theICMcohort,patientsreceivedameandoseof867.35± 223.0mgand36.7%hadpreviouslyreceivedoraliron. PatientsintheIScohortreceivedameandoseof438.8± 199.8mgand22.4%hadpreviouslyreceivedoraliron.
ThemeanpreviousHbwas:9.5±1.4g/dlintheICM groupand9.4±1.3g/dlintheISgroup.IntheICMgroup, 38.7%patientsshowedanincreaseHb 1g/dl,while24.5% didsointheISgroup.StatisticallysignificantvariationsinHb levelswereobservedinbothgroups(+0.71g/dlinICMvs +0.31g/dlinIS;p<0.05).However,theanalysisshowedno significantdifferencesbetweenbothcohorts(p=0.06).
HospitaldurationlengthmeanwasshorterintheISgroup (15.3±21.9vs19.9±22,9days)withoutsignificantdifferencesbetweencohorts(p=0.307).Ameancostof144.2± 37.1C ¼ /patientand5.0±2.3C ¼ /patientwasestimatedforthe ICMandISgroupsrespectively.
ConclusionandRelevance ICMandISadministrationproduced animprovementofHblevelsinbothcohortswithoutshowing asignificantdifferenceinthehospitaladmissionlength.ICM treatmententailedanincreaseofdirectcosts.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
MABlancoCastano*,MDominguezCantero,ERíosSánchez. UniversityHospitalofPuerto Real,Pharmacy,PuertoReal,Spain
10.1136/ejhpharm-2023-eahp.195
BackgroundandImportance Theemergenceofinformation andcommunicationtechnologieshasenabledthedevelopment oftelepharmacyprogrammes(TPP)asacomplementarytool topersonalcare,throughwhichpharmaceuticalcarecanbe providedwithouttheneedtovisitthehospital.TPPbeganin December2019withdeliveryofmedicationtoprimary healthcarecentrespreviouspharmaceuticalcarebytelephone fromthehospitalpharmacy.
AimandObjectives Describingthepharmaceuticalinterventions (PI)ofpatientsincludedinaTPP
MaterialandMethods Prospective,descriptivestudy,from december2019-september2022.Pharmacotherapyfollowupconsistedofstructuredtelephoneinterviewsscheduled every3months.Inclusioncriteria:durationoftreatment greaterthan3months,stablechronicdisease,adherence greaterthan90%,goodtolerancetomedicationand/or mobilityordependencyproblems.Exclusioncriteria:oncohaematologicaltreatment,andpatientswithcognitiveproblems,ortechnologicalbarr ierstotelephonepharmacotherapeuticfollowup.
PIwereclassifiedas:drug-druginteractions(DDI),clinical monitoring(CM),adversedrugreactions(ADR)and/orlack ofefficacy(LOF).Inaddition,theresultsofeachPIwere recordedas:temporary/permanentdiscontinuation(TPD), changeoftreatment(ChOT),changeofdosingregimen (ChDR)orcontinuationoftreatment(COT).Thedegreeof acceptanceofthePiwascalculated.
Results Atotalof4.497telephoneinterviewswereconducted with410patientsincludedintheTPP.Fifty-sevenpercentof treatmentswerebiologics,27%antiretrovirals,6%multiple sclerosis/amyotrophiclateralsclerosistreatment,3%lipid-loweringdrugs,3%somatropins,2%pulmonaryantihypertensives and2%otherdrugs.
88Piwereregistered,58%ofwhichwereacceptedbythe prescribingphysician.
Thepharmacist´sactivityinaTPPcancontributetoabetteruseofmedicines,aswellaspreventandsolvemedicationrelatedproblems.
4CPS-209 EXPERIENCEOFTHENOCEBOEFFECTINPATIENTS WITHSWITCHTOBIOSIMILARSINRHEUMATOID ARTHRITIS
1RFresquet, 2ABMaríaÁngeles*, 1OPascual, 1RGracia, 1LSopena, 1IVarela, 1PGomez, 1AMerchan, 1BBonaga. 1HospitalClínicoUniversitarioLozanoBlesa,ServiciodeFarmacia, Zaragoza,Spain; 2HospitalClinicoUniversitarioLozanoBlesa,ServiciodeFarmcia,Zaragoza, Spain
10.1136/ejhpharm-2023-eahp.196
BackgroundandImportance Thenoceboeffectisdescribedas theworseningofassociatedsymptomsoranincreasein adverseeffectsduetoanegativeattitudetowardsaparticular drugorpharmacologicaltherapy,inthiscasebiosimilartreatment.Lackofpatientknowledgeanddiscrepanciesinthe informationprovidedarethemaincausesofnegativeexpectationswithbiosimilarsandtheirexchangewiththeoriginaldrug. AimandObjectives Studyofthenoceboeffectinpatients withspondyloarthropathyandpsoriaticarthritisafterswitching fromtheoriginaldrugtothebiosimilarofadalimumabina tertiaryhospital.
MaterialandMethods Retrospectiveandobservationalstudy fromJanuary2020toOctober2021.Clinicalinformationwas obtainedfromtheelectronicmedicalrecord.Thefollowing clinicalanddemographicvariableswererecorded:age,sex, medication,typeofadversereaction,adherence,andfollow-up afterthechange.
Results Duringthestudyperiod,66switchesweremadefrom Humira® (originaldrug)toHyrimoz® (biosimilar),with72% biosimilaruseinthisclinicalcontext.In4%(3patients)of theswitches,aclinicalworseningwasobservedat6months, themeanagewas46years,male.Adherencetotreatment (Hyrimoz)wasover90%.Themostfrequentsymptomswere: skinsymptomswithpruritus,axialclinicalworsening,morning arthralgias.Inallcases,andafterdiscussionwiththeprescribingphysician,itwasdecidedtoswitchtotheoriginalbrand. Afterreturningtothereferencebrand,thepatientspresented animprovementofthesymptomatologyassociatedwiththe changetothebiosimilardrug.
ConclusionandRelevance Thenoceboeffectisanuncommon effect,butitcausesanincreaseinpharmaceuticalexpenditure, aswellasinmedicalvisitsandcomplementarytests.Dueto thesmallsamplesize,clinicalworseningcannotbeassociated withthenoceboeffectinthisstudy.Therefore,further researchonthistopicisrequired.Itmayalsoleadtothe administrationofnewdrugstocounteractthesymptoms causedbythenoceboeffect.Bettereducationofbothhealthcareprofessionalsandpatientsontheknowledgeofbiosimilarscanhelpreducethelikelihoodoftriggeringanocebo effect.
ConclusionandRelevance Pharmacotherapeuticmonitoringof patientsincludedintheTPPmainlyallowedforthedetection ofADRsandensuredadequateclinicalsupervisionofinpatientmedication.
TheoutcomeoftheinterventionswasmostlyCOTfollowedbymodificationoftheprescribedregimen.
REFERENCES
1.Horta-BaasG.Patient-reportedoutcomesinrheumatoidarthritis:akeyconsiderationforevaluatingbiosimilaruptake? PatientRelatOutcomeMeas.2022Mar 30;13:79–95.doi:10.2147/PROM.S256715.
ConflictofInterest
4CPS-210 EFFECTOFMONOCLONALANTIBODIESTOPREVENT PROGRESSIONTOSEVERECOVID-19DISEASE:REALLIFEDATAOFAUNIVERSITYHOSPITAL
1VRosafio*, 1ACorzani, 1VSDiVico, 1AMichielon, 2MTBianco. 1UniversitàDiSiena, ScuolaDiSpecializzazioneInFarmaciaOspedaliera,Siena,Italy; 2AziendaOspedalieroUniversitariaSenese,UocFarmaciaOspedaliera,Siena,Italy
10.1136/ejhpharm-2023-eahp.197
BackgroundandImportance SinceFebruary2021,our NationalMedicinesAgencyhastemporarilyauthorisedfor emergencyusethemonoclonalantibodiestotreatCOVID-19 disease.
Furthermore,firstauthorisedandmostusedonesinour Hospitalwerebamlanivimab-etesevimabmonoclonalantibody combination,casirivimab-imdevimabcombinationand sotrovimab.
MonoclonalantibodytherapyforCoronavirusdisease2019 (COVID-19)isrecommendedinmildtomoderatedisease patientswhoareatriskofprogressingtoseveredisease,with atleastoneriskfactor,includingageover65.
AimandObjectives Aimofthestudyistoevaluatetheeffect ofmonoclonalantibodytherapyforCOVID-19topreventdisease ’sprogression,hospitaladmissionsanddeaths.
MaterialandMethods Datarelatedtotreatedpatientsfrom 29/03/2021to02/05/2022werecollectedfromourNational MedicinesAgencydatabase.Thesedatawere:sex,age,outcomesofthetreatmentandantibodyadministered.
Results 336patientsweretreatedinourHospitalfrom29/03/ 2021to02/05/2022.
Patientstreatedwithbamlanivimab-etesevimab(700mg +1400mg)combinationwere117:48females(F);69males (M);64patientsagedover65.Thesepatientsweretreated withthiscombinationfrom29/03/2021to29/12/2021.The outcomeswere:112healings,3hospitalisationsoremergency departmentvisits,1death,1notavailable.
Patientstreatedwithcasirivimab-imdevimabcombination (1200mg+1200mg)were121:59Fand62M;72 patientsagedover65.Thesepatientsweretreatedwith thiscombinationfrom16/07/2021to31/12/2021.Theoutcomeswere:110healings,9hospitaldischarges(2 patients,treatedwithhighdosage(4000mg+4000mg), werehospitalisedforCOVID-19while7werehospitalised forotherreasons),2hospitalisationsoremergencydepartmentvisits.
Patientstreatedwithsotrovimab(500mg)were98:42F and56M;38agedover65.Thesepatientsweretreated withthisantibodyfrom29December2021to2May2022. Theoutcomeswere:96healings,1hospitaldischarge(hospitalisedforotherreasons)and1notavailable.
ConclusionandRelevance Theadministrationofmonoclonal antibodiesinpatientswithCOVID-19,withcomorbilities, whoareatriskofseveredisease’sprogressionreporteda reducedriskofhospitalisationordeath(only5hospitalisations oremergencydepartmentvisitsand1deathon336treated patients).
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
4CPS-212 SPECIALISTPHARMACIST-LEDMULTIDISCIPLINARY CAREPATHWAYFOROPTIMISINGLIPIDTHERAPY –SIXMONTHSINTERIMANALYSIS
1NHamedi*, 2SAli, 3JRobson, 4RPatel, 5HAlbert, 6IKhan, 5MKearney, 6BKrishek, 1SFhadil, 1PWright, 1SAntoniou. 1BartsHealthNHSTrust,Pharmacy,London,United Kingdom; 2SouthdeneSurgery,GpPractice,London,UnitedKingdom; 3ClinicalEffectiveness Group,QueenMaryUniversityofLondon,London,UnitedKingdom; 4BartsHealthNHS Trust,Cardiology,London,UnitedKingdom; 5Uclpartners,CardiovascularHealth,London, UnitedKingdom; 6RedbridgeClinicalCommissioningGroup,Pharmacy,London,United Kingdom
10.1136/ejhpharm-2023-eahp.198
BackgroundandImportance Cardiovasculardisease(CVD)isa leadingcauseofmortalityworldwideandaccountsforapproximately27%ofalldeathsinUnitedKingdom.TherelationshipofhypercholesterolemiatoCVDiswellestablishedand understoodintermsofatherogenesis.Reductionofatherogeniclipoproteins,inparticular,low-densitylipoproteinwith lipidmodificationtreatmentshasbeenshowntoreducethe riskofCVDeventsandmortality.
AimandObjectives Design,testanddevelopanintegrated carepathwaythatutilisesspecialistcardiovascularpharmacists workingwithprimarycareteams.Thisinvolvesoptimising secondarypreventionwithlipidmodificationtherapyinpeople withestablishedCVDacross42GeneralPractitionerpractices overone-yearpilotprogramme.
MaterialandMethods Specialistcardiovascularpharmacists werecommissionedtoworkwithprimarycarecliniciansto identify,reviewandoptimisesecondarypreventioninhigh-risk patientsnotreceivinglipidmodificationtherapy.
Eligiblepatients’ clinicalnoteswerereviewedtoconfirm CVDdiagnosis,historyoftreatment,bloodtestresultsand CVDriskfactors.Complexcaseswerereviewedbyavirtual lipidspecialistmultidisciplinaryteamtoagreeatreatment plan.Patientswerecontactedforavirtualconsultationtodiscussandinitiatetailoredlipidmodificationtherapy.
Results ApreliminaryreviewofpracticesCVDlistshowed 20%(2200/11233)ofpatientshadaCVDdiagnosisandwere notreceivinglipidmodificationtherapy.Asix-monthinterim analysisof1100outofthe2200clinicalreviewsconducted byspecialistpharmacists,identifiedthat60%(660/1100)were eligibleforstatintherapywithonly4%(44/1100)ofpatients havingatruestatinintolerance.
Theremaining36%(396/1100)werenotforlipidmodificationtherapy.Ofthesepatients:6%(66/1100)declinedtreatment,9%(96/1100)werepalliativeortheriskoftreatment outweighedthebenefits,8%(90/1100)hadnon-atherosclerotic CVD,9%(100/1100)hadincorrectCVDdiagnosisandthe remaining4%(44/1100)werenolongerpartofthepractice list.
ConclusionandRelevance Anintegratedcarepathwayusing specialistcardiovascularpharmacistssupportingamultidisciplinaryworkforcewithinprimarycarehasshownasignificant improvementinlipidmodificationtherapyprescribingto reducetheriskofmyocardialinfarction(MI)andstroke. Extrapolatingtheseresultsnationallywouldavert17,000MIs and5,000strokesovera5-yearperiod.
ConflictofInterest Noconflictofinterest
4CPS-213 CHARACTERISTICSOFMULTISYSTEMINFLAMMATORY
1OHanafia*,
10.1136/ejhpharm-2023-eahp.199
BackgroundandImportance SincetheCoronavirus(COVID19)pandemic,therehasbeenahighnumberofchildrenhospitalisedinthepaediatricintensivecareunit(PICU)forPaediatricMultisystemicInflammatorySyndrome(MIS-C) resemblingKawasakiDisease(KD)
AimandObjectives Theobjectivesofthisstudywereto describetheclinicandthetherapeuticsthatweusedinPIMS, comparedtothoseofKD.Todescribetheimpactofthe treatmentsusedanddiscusstheclinicalevolutionofour patients
MaterialandMethods Thisisaretrospectiveobservational studyinthepaediatricintensivecareunit,overa9-month periodfromApriltoDecember2020.Theclinical,biological andmedicationdatawascollectedviathecomputerised patientrecord,ourpresenceinthedepartmentandthanksto theprescriptionsoftwareforPIMSpatientsandcomparedto theKDdataofthescientificliterature
Results Weincluded12children,medianage8years[2-16 years]andsexratio=2,diagnosedwithMIS-C.Negative PCRtestsonadmissionandpresenceofanti-SRAS-CoV-2antibodiesinallpatients.Allpresentedfever,withameandurationof5days.5patientspresented2clinicalcriteria characteristicofKDinsufficienttodiagnosecompleteKD. Gastrointestinalsymptoms(10patients),rarelyseeninKD.All hadinflammatoryandcardiacmarkershigherthanthosein KD.Cardiacdamagewasobservedin10patients:50%had persistentsystemichypotensionand5hadECGabnormalities. Drugtherapywastoreduceinflammation.9patientsreceived intravenousimmunoglobulin(IVIG),5patientsreceiveda2nd doseofIVIGand2a3rddose.Corticosteroidtherapyfor4 dayswasadministeredto10patientsand9requiredantiinflammatorytreatmentwithacetylsalicylicacid.Thesetreatments,combinedwithvasopressorordiureticandanticoagulantsupport,werenecessary.Therewerenodeathsinour cohort,theaveragetimeofmanagementinthedepartment was6days[2-13days].
ConclusionandRelevance OurpatientsdescribedaclinicalpicturesuggestingKD,withabroadersymptomatologyand severity,muchmoremarkedinflammatoryandcardiac markers,ashorterfever,alowerplateletcount,morefrequent gastrointestinalinvolvement,themedianageofourcohort washigher.Thetherapeuticstrategy:IGIVandcorticosteroid therapyappearedtobeeffectiveinourstudy
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-215 DESCRIPTIONOFTHEPRE-EXPOSUREPROPHYLAXIS COVERAGEAGAINSTTHEHUMAN IMMUNODEFICIENCYVIRUS
XLarreaUrtaran*,ADordàBenito,ENoguéPujadas,ÀCastellóNòria,LViñasSagué, CSubiranaBatlle,COrtíJuan,MBrugueraTeixidor,IGómezIbáñez,YOrtuñoRuiz, RSacrestGüell. HospitalDrJosepTrueta,PharmacyDepartment,Girona,Spain
10.1136/ejhpharm-2023-eahp.200
BackgroundandImportance Pre-exposureprophylaxis(PrEP) againsthumanimmunodeficiencyvirus(HIV)infectionaimsto preventHIVtransmissioninpeopleatriskofacquiringthe infection,consistingofdailytenofovirdisoproxilfumarate withemtricitabine(TDF/FTC).
AimandObjectives TheaimofthestudyistodescribePrEP coverageandpatients’ baselinecharacteristicstakingPrEP.
MaterialandMethods Retrospective,descriptivestudy.Patients thatstartedwithPrEPfromOctober2020toApril2022 wereincluded.PatientswhotookPrEPlessthan6months wereexcluded.Demographicvariables(ageandsex),indicationcriteria,sexuallytransmittedinfections(STIs)(beforeand during),creatininevalues,seroconversiontoHIVandwithdrawalreasonswerecollected.Forthestatisticalanalysis,the mean,standarddeviation(SD)and t-student testwereused. Results 52patientsreceivedPrEPduringthestudyperiod.10 patientswereexcluded.Ofthepatientsincluded(n=42), 97.4%(n=41)weremenwithameanage±SDof35.8± 8.4years.
Theindicationsfortreatmentwere: 97.6%hadmorethan10 differentsexualpartnersinthelastyear;90.2%hadanalsex withoutacondominthelastyear;29.3%haddruguse relatedtohavingsexwithoutacondominthelastyear; 14.6%hadreceivedpost-exposureprophylaxisonseveral occasionsinthelastyearand36.6%hadatleastonebacterial STIinthelastyear.
66.7%(n=28)ofthepatientshadoneormoreprevious STIs.ThemostfrequentSTIwas Treponemapallidum (n=21) followedby Neisseriagonorrhoeae (n=12).Whilepatients weretakingPrEP,40.5%(n=17)ofthempresentedSTIs: 19.0%(n=8)had chlamydiatrachomatis;14.3%(n=6)had Neisseriagonorrhoeae and9.5%(n=4)had Mycoplasmagenitalium.Baselinemean±SDcreatininewas0.86±0.11mg/ dlandattheendofthestudywas0.90±0.11mg/dl (p=0.024).26.8%(n=11)ofthepatientsdiscontinuedPrEP (n=5duetostablecouple;n=2bytheirowndecision;n=2 duetolackoffollow-up;n=1duetochangeofcentreand n=1duetoproteinuria).Therewasnoseroconversionto HIVinanypatients.
ConclusionandRelevance ThemajorityofPrEPpatientsare youngmenwithriskysexualpractices.Duringtheuseof PrEP,STIswerefrequent.Therewasnoseroconversionto HIVduringthestudyperiod.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-217
REGULATORMODULATORSINPATIENTSWITHRARE MUTATION
1NMontiGuarnieri*, 2BFabrizzi, 1EAndresciani, 1AMFGarzone, 1SBagagiolo, 1IBartolucci, 1SGuglielmi, 1CCapone, 3CPolidori, 1APompilio. 1AouDelleMarche,Sod Farmacia,Ancona,Italy; 2AouDelleMarche,SosFibrosiCistica,Ancona,Italy; 3Università DegliStudiDiCamerino,ScuolaDiScienzeDelFarmacoEDeiProdottiDellaSalute, Camerino,Italy
10.1136/ejhpharm-2023-eahp.201
BackgroundandImportance CysticFibrosis(CF)isamonogenicandmulti-organdisease.Thisconditionisrelatedto mutationsinCysticFibrosisTransmembraneRegulator (CFTR),thegeneencodingtheepithelialionchannelthat normallytransportschlorideandbicarbonate.Therapeutic
1AGhandour, 2SHonoré, 1PBertault-Peres. 1HôpitauxUniversitairesde Marseille,PharmacieTimone,Marseille,France; 2AixMarseilleUniversité,Facultéde Pharmacie,Marseille,France THEUSEOFCYSTICFIBROSISCONDUCTANCEstrategiesdeeplychangedwhenIvacaftor(2015)andthe combinationtherapyIvacaftor/Tezacaftor/Elexacaftor(ETI) (2021)weremarketed.AtthismomentETItherapyis licensedtotreatCF’spatients>6yearswithatleastone F508delmutation,themostcommonone;however,patients withrarerCFTR’smutationsdon’ thaveaccesstothis therapy.
AimandObjectives Withthisworkwewouldliketoreport theuseofthecombinationtherapyIvacaftor-ETIintwo youngpatientswithrareCFTR’smutations:theN1303K/ 2183AA>GandtheW1282X/N1303K.
MaterialandMethods Startingfromtheoff-labelauthorisationsfromJanuary-2015toJune-2022byourHospitalCommittee(composedwithaClinician,aPharmacologistanda HospitalPharmacyst)inaccordtoLaw94/98,weidentified patientsthatrequiredoff-labelCFTRmodulators’ combinationtherapyduetotheirCFTR’ sraremutationsandinvitro responsetoETItherapy.Fortheseweanalysed:ageatthe beginningofthetherapy,gender,typeofmutation,clinical manifestations,periodoftherapy,AdverseDrugReactions (ADRs)notified.
Results Onlyin2022twopatientswereauthorisedtouseofflabelCFTRmodulators’ combinationtherapyduetotheirrare CFTR’smutations.Thefirstpatient(P1)wasafemale,20 years,W1282X/N1303Kmutations;herclinicalhistory showedmeconiumileus,seriouspneumopatyandsheoften requiredantibiotictherapyduetoherlungsinfections.The secondpatient(P2)wasafemale,19years,N1303K/ 2183AA>Gmutations;herclinicalhistoryshowedpancreatic andlungsinsufficiency,BMI<14,infectionsinducedbymultidrugresistantPseudomonasandMycobacteriumAbscessus,D hypovitaminosis.Atfirst,HospitalCommitteeauthorised3 cyclesoftherapyforP1and4cycles(28daysforeachcycle) forP2;bothofthemwereauthorisedtoprolongetheirtherapyduetoclinicalevidentefficacy.NosignificantADRs relatedtotreatmentwerenotified.
ConclusionandRelevance CFTRmodulatorsaresmallmoleculesthatdirectlyimpactandachievethefunctionofCFTR channel.Theygivelong-termimprovementsinclinicaloutcomesandwehopemoreresearchontheirefficacyin patientswithrarerCFTR’smutations.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
AimandObjectives TodeterminetheutilityofSingleNucleotidePolymorphismsofHLA-BandTNF-238,TNF857,TNF308,TNF-1031,TNFRSF1Basprognosticandpredictive markersinpatientsdiagnosedwithmoderate-severepsoriasis treatedwithadalimumab,etanerceptorinfliximab.Aswellas, toevaluatetheefficacyofanti-TNFtreatmentintheinduction phase.
MaterialandMethods Aprospectivecohortstudywasperformed.DataandDNAwereobtainedfromsalivasamplesof 103patientsresidingintheprovinceofGranadawithmoderateandseverepsoriasiswhohadbeentreatedwithanti-TNF. ThegenotypesweredeterminedbyTaqmanPCRRealTime. Results Patients'meanagewas54.19±13.65years;54male (54/103);100hadplaquepsoriasis(100/103),90locatedin trunkandextremities,and89onscalpandface,42with psoriaticarthritis(42/103),33smokers(33/103),36drinkers (36/103),62hadpsoriasisfamilyhistory(62/103).These103 patientshavebeentreatedwith135anti-TNF(adalimumab, ADA=80;etanercept,ETN=39;infliximab,INF=16).Also20 receivedoraladministrationoftheconcomitantmethotrexate (20/135).
Inreferencetoefficacy,74patientshadaresponsetoantiTNF(74/135),and61donotshowtheexpectedresponsein theinductionphase(61/135).ConcerningPASI75values,55 patientstreatedwithADAachievedPASI75at3-6months (55/80),12patientstreatedwithETN(12/39),and7patients treatedwithINF(7/16).
Furthermore,patientscarryingTNFRSF1B-rs1061622-G alleleanassociationwithADAresponseat3months (p=0.0026)andpatientscarryingTNFa-1031-rs1799964-Tan associationwithETNresponseat6months(p=0.0047),also patientscarryingTNFa-238-rs361525-GtreatedwithINF havearesponseat6months(p=0.045).
ConclusionandRelevance Inconclusion,responsetoanti-TNF drugswasassociatedwithdifferentsinglenucleotideallelic polymorphismsoftheTNFgene.Nonetheless,furtherstudies withlargecohortsofpatientshavetobeperformedtoconfirmthesedatainordertoapplyforthispersonalisedmedicineinroutineclinicalpractice.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-218 TNFGENEPOLYMORPHISMSPREDICTORSOF RESPONSETOANTI-TNFDRUGSINPATIENTS DIAGNOSEDWITHMODERATE-SEVEREPSORIASIS
1CMembrive-Jimenez, 2CZuñiga, 3CPérez-Ramirez, 4SArias-Santiago, 5AJimenez-Morales, 5CMontero-Vilchez*. 1PharmacogeneticsUnitPharmacyService,UniversityHospitalVirgen deLasNieves,Granada,Spain; 2UniversityofGranada,PharmaceuticalCare,Granada, Spain; 3UniversityofGranada,BiochemistryandMolecularBiology,Granada,Spain; 4UniversityHospitalVirgendeLasNieves,Dermatology,Granada,Spain; 5University HospitalVirgendeLasNieves,Pharmacy,Granada,Spain
10.1136/ejhpharm-2023-eahp.202
BackgroundandImportance Psoriasisisachronicinflammatory skindisease.Biologicaltreatmentsagainsttumournecrosisfactor(anti-TNF)areeffectiveintreatingthisdisease,however, notallpatientsrespondtothistreatment,anditcancause serioussideeffects.BiomarkersinvolvedintheTNFcytokine maybeimplicatedintheresponsetotheanti-TNFdrug.
4CPS-219 AGAMENON-SEOMMODELFORTHEPREDICTIONOF SURVIVALINPATIENTSWITHHER2-POSITIVE ADVANCEDOESOPHAGOGASTRICADENOCARCINOMA RECEIVINGTRASTUZUMAB-BASEDFIRST-LINE TREATMENT
1LMacía-Rivas*, 2CLFernández-Laguna, 2FJÁlvarez-Manceñido, 3AArias-Martínez, 4AMartínez-Torrón, 5IMacías-Declara, 6JMCano-Cano, 7MDiez, 8ACustodio, 9JLópezRobles, 2ALozano-Blázquez. 1UniversidaddeSantiagodeCompostela/Hospital UniversitarioCentraldeAsturias,DoctoralProgrammeInMedicineClinicalResearch/ HospitalPharmacy,SantiagodeCompostela,Spain; 2HospitalUniversitarioCentralde Asturias,HospitalPharmacy,Oviedo,Spain; 3UniversityofGranada/HospitalUniversitario GermansTriasIPujol,DoctoralProgrammeInPharmacy-FacultyofPharmacy/Pharmacy Department,Granada,Spain; 4UniversityofGranada/HospitalUniversitarioCentralde Asturias,DoctoralProgrammeInPharmacy-FacultyofPharmacy/HospitalPharmacy, Granada,Spain; 5HospitalUniversitarioParcTauli,MedicalOncology,Sabadell,Spain; 6HospitalGeneralUniversitariodeCiudadReal,MedicalOncology,CiudadReal,Spain; 7HospitalUniversitarioVallD’hebron,MedicalOncology,Barcelona,Spain; 8Hospital UniversitariolaPaz,MedicalOncologyDepartment/CiberoncCb16/12/00398,Madrid, Spain; 9HospitalUniversitarioMoralesMeseguer,MedicalOncology,Murcia,Spain
10.1136/ejhpharm-2023-eahp.203
BackgroundandImportance Trastuzumabassociatedwithchemotherapy(platinumandfluoropyrimidine)isthestandard first-linetreatmentinHER2-positiveadvancedoesophagogastricadenocarcinoma(AGA);however,itsbenefitsare heterogeneous.
AimandObjectives Todevelopandvalidateapredictivemodel foroverallsurvival(OS)andprogression-freesurvival(PFS)in patientswithAGAtreatedwithtrastuzumab.
MaterialandMethods PatientsfromtheSpanishSocietyof MedicalOncology(SEOM)-AGAMENONregistrywithHER2positiveAGAtreatedinfirst-linewithchemotherapyandtrastuzumabbetween2008and2021wereselectedforthisstudy. Anacceleratedtime-to-eventmodelwasdevelopedtopredict survivalandrepresentedasanomogramandanonlinecalculator.ThenomogramwasexternallyvalidatedinanindependentseriesfromTheChristieNHSFoundationTrusthospital inManchester,England.
Results 737patientswererecruited(AGAMENON-SEOM,n= 654;Manchester,n=83).Inthereferralcohortthemedian PFSandOSwere7.76(95%CI,7.13-8.25)and14.0months (95%CI,13.0-14.9),respectively.Patientsreceivedamedian ofsixcyclesofplatinum,eightcyclesoffluoropyrimidineand trastuzumabforamedianof7.6months(95%CI,7.10-8.30).
Inthevalidationcohort,themedianPFSandOSwere8.1 (95%CI,7.1-11.3)and12.8months(95%CI,10.3-20.4), respectively.Patientsreceivedchemotherapyforamedianof fivecyclesandtrastuzumabforamedianof6.3months.
SixcovariatesweresignificantlyassociatedwithOSand wereusedtoconstructthenomogram:neutrophil-lymphocyte ratio(timeratio(TR):0.73;95%CI:0.63-0.83),ECOGstatus (TR:0.59;95%CI0.48-0.73),Laurenhistologicsubtype (TR:0.73;95%CI0.57-0.94),HER2expression(TR:0.85; 95%CI0.73-1),histologicgrade(TR:0.87;95%CI0.721.07),andtumourburden(TR:1.69;95%CI1.34-2.13).The AGAMENON-HER2modeldemonstratedadequatecalibration andfairdiscriminatoryabilitywithac-indexforPFSandOS of0.606(95%CI0.58-0.64)and0.623(95%CI0.59-0.66), respectively.IntheManchestervalidationcohort,themodelis wellcalibrated,withac-indexof0.65and0.68forPFSand OS,respectively.
ConclusionandRelevance HER2-positiveAGApatientsreceivingtrastuzumabandchemotherapycanbestratifiedaccording totheirestimatedsurvivalendpointsusingtheAGAMENONHER2prognostictool.Thisnomogramcouldbeavaluable toolformakingtreatmentdecisionsindailyclinicalpractice.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-222 HAPLOIDENTICALHAEMATOPOIETICCELL TRANSPLANTATIONINPATIENTSAGED>55YEARS WITHACUTEMYELOIDLEUKAEMIA
COrtegadelaCruz*,RTamayoBermejo,JCDelRioValencia,IMMuñozCastillo. Hospital RegionalUniversitariodeMalaga,Pharmacy,Malaga,Spain
10.1136/ejhpharm-2023-eahp.204
BackgroundandImportance IntheelderlypatientswithAML, boththedevelopmentofreduced-intensityconditioning(RIC) regimensandtheuseofhaploidenticaldonorshaveimproved theiraccessibilitytoallo-HCT.
AimandObjectives Toanalysetheclinicalcharacteristicsand resultsofhaploidenticalfamilydonorallo-HCT,performedin
ourhospitalduringtheyears2014to2021,inpatientswith AML>55years.
MaterialandMethods
Retrospectiveobservationalstudy. Datacollected:age,sex, HCTstatus,timefromdiagnosistotransplant,ECOGPerformanceStatus,comorbidityindexes(HCT-CI,EBMTs,DRindex),haematopoieticprogenitorsource(HPS),CMV-mismatch, conditioningregimen,graft-versus-hostdisease(GVHD)prophylaxis,andpost-HCTcomplications.Overallsurvival(OS) andprogressionfreesurvival(PFS)wereanalysedusing Kaplan-Meier.
Results Thirtypatientswereincluded.Median(range):64 (56 – 71)years,57%women.70.3%infirstcompleteremission.Median(range)timefromdiagnosiswas6.5(3.4752.37)months.74%ECOG0.33%DRindexhighandvery high15%patients.TheEBMTs>4in26%andtheHCTCI 3in56%patients.HPSwasp eripheralbloodin52% andbonemarrowin48%.56%CMV-mismatch(donor-/ patient+).AllpatientsreceivedaRICregimenandpostHCTcyclophosphamideand 89%tacrolimusastheonly immunosuppressant.
Majornon-haematologicaltoxicitiesincludedmucositis, gastrointestinalandlivertoxicityin26%,19%and7%of patients,respectively.19%patientsdevelopedhaemorrhagic cystitis,onepatientunderwentthromboticmicroangiopathy,41%developedacuteGVHDand37%patientspresentedchronicGVHDcmvinfectionoccurredin78%of patients.
Median(range)follow-upwas21.55(1.67-89.80)months, OSat1yearwas65%(95%CI,46-83%),at2years56% (95%CI,36-75%).PFSat1yearwas61%(95%CI,4280%),at2years48%(95%CI,28-68%).48%arestillalive andallincompleteremission.
ConclusionandRelevance Thesmallsamplepreventsnumerous affirmationsfrombeingemphaticallyextracted,buttheresults obtained,whichareverycomparabletothepublishedexperiences,supporttheuseofthistypeofdonorinthispatient population.Currently,weshouldnotdelaytransplantationin elderlypatientswithAMLtryingtofindanHLA-identical donor.IftheexperienceoftheCentreisextensive,performingatransplantfromahaploidenticaldonorshouldbeconsideredinthealgorithmoftheAllo-HSCTprocedure.
ConflictofInterest Noconflictofinterest
4CPS-223 BOTULINUMTOXINTYPEA:THENON-INVASIVE SUCCESSFOROVERACTIVEBLADDERS
1ZImane*, 2SMousannif, 3LHamedoun, 4YBoukhlifi, 4MAlami, 5YTadlaoui, 6YBousliman. 1PharmacyUnit,DrugDepartment,Rabat,Morocco; 2Children'sHospital, PharmacyDepartment,Rabat,Morocco; 3MilitaryHospitalofInstructionMohamedV, UrologyDepartment,Rabat,Morocco; 4MilitaryHospitalofInstructionMohamedV,Urology Depatment,Rabat,Morocco; 5MilitaryHospitalofInstructionMohamedV,Drug Department,Rabat,Morocco; 6FacultyofMedicineandPharmacyRabat,Toxicology Laboratory,Rabat,Morocco
10.1136/ejhpharm-2023-eahp.205
BackgroundandImportance IntradetrusorinjectionsofbotulinumtoxintypeA(TBA)havesignificantlychangedthemanagementofoveractivebladder(OAB),allowingtheacquisition ofurinarycontinenceandcontrolofrenalrisks.Thistechniquemakesitpossibletoavoidbladderreplacementsurgeryby
enterocystoplasty.HAVincursdirectandindirectcoststo society.
AimandObjectives Assessdiseaseimpactinpatientsaffected withIBDsusingPROMs.
AimandObjectives
Ourstudyhastwomainobjectives:to evaluatetheimprovementofthehandicapofpatientswith urinaryincontinencebybladderhyperactivity,afterinjectionof botulinumtoxinAthentoevaluatethecosteffectiveness ratio.
MaterialandMethods
Aretrospectiveobservationalstudyof 74patients,whoreceivededucationonself-catheterisationand treatedwithTBAattheUrologyDepartmentofbetweenJanuary2018andAugust2022.AmodelwasdevelopedtoestimatecostsbycomparingthecostofTBAversusastandard protocol(involvingbehaviouraltherapy,incontinencepads, anti-cholinergictreatmentand,catheters)excludinglossof productivity.Aqualityoflifequestionnairewasalsoadministeredtopatientsatthefollow-upvisits.
Results ProfilesofTBAuse:Primo-injectionin83.78%.Forthe indication,AVHwithoutleakagein32.43%,urinaryincontinencebyAVHin35.14%,multiplesclerosisin13,51%and spinalcordinjuryin18.92%.Theinjectionswereperformed intheoperatingroom.Amedianparamedicaltimeof30min topreparethepatientandtheproduct.Injectionconducted endoscopicallylastedamedianof8minwithamedianhospital stayof2days.Clinicalimprovementin81%withamedian durationofefficacyof98days.Foradverseevents:hypoora contractilebladderrequiringself-catheterisation(n=81%),generalisedfatigue(n=40%)andmuscleweakness(n=35%).Calculatedcosts:Thecostofaninjectionis7000MAD(price producedwiththehospitalpackage).Thecostofstandard treatmentwithoutself-catheterisationis2340MAD(foranticholinergictreatmentassociatedwithbehaviouraltherapy).If useofcathetersthecostoftheinjectionis8340MAD.Ifurinaryretentionoccurs,thecostis13000MAD.Ourstudy showsthatthehospitalcostishigherthanthestandardtreatmentwithoutself-catheterisationandlessexpensiveifcatheterisationwaspreviouslyused,butwithasignificant improvementinthequalityoflifeaccordingtothequestionnaireresults.
ConclusionandRelevance Forourcentre,since2014,TBA representsanewtherapeuticoptioninsecond-linetreatment.
REFERENCESAND/ORACKNOWLEDGEMENTS
Theauthorsthankallthosewhocontributedtotherealisationofthiswork.
ConflictofInterest Noconflictofinterest
4CPS-225 HEALTHIMPACTOFTREATMENTFOR INFLAMMATORYBOWELDISEASEWITHBIOLOGICAL AGENTSFROMTHEPATIENT’SPERSPECTIVE:A CROSS-SECTIONALSTUDYUSINGPATIENTREPORTED OUTCOMEMEASURES(PROMS)
LEstrada*,SMarin,GCardona,LCarabias-Ané,AMorales,ETerricabras,ABocos-Baelo, CGarcía-Castiñeira,CCodina-Jiménez,EValls,CQuiñones. HospitalUniversitariGermans TriasIPujol,PharmacyDepartment,Badalona,Spain
10.1136/ejhpharm-2023-eahp.206
BackgroundandImportance Theclinicalmanifestationsof inflammatoryboweldisease(IBD)compromisepatient'sdaily life.Inthisregard,theuseofPatientReportedOutcome Measures(PROMs)todeterminehealthstatus,qualityoflife andtreatmenteffectivenessfromthepatient’sperspectivecan addsignificantvalueinclinicalpractice.
MaterialandMethods Cross-sectionalstudyincludingoutpatientstreatedwithbiologicalagentsforulcerativecolitis (UC)andCrohn'sdisease(CD) 18years.Socio-demographicandclinicalcharacteristicswerecollectedfromclinicalrecords:age,gender,typeofIBD,diagnosisyear, biologicaltreatment,startingdateofbiologicaltreatment, previousbiologicaltreatment,conco mitantimmunosuppressivetreatment,previoussurgeriesduetoIBDandsmoking habits.Weused2questionnairestoevaluatePROMs:IBDControl(IBD-Control-8sub -scoreplusvisualanalogscale (VAS),thatrangefrom0-16and0-100,respectively,higher scoresrepresentingbetterdiseasecontrol)andIBD-Disk (thatrangesfrom0-100,higher scorerepresentinghigher IBDdaily-lifeburden).
Results 42patientswithCDand21withUCwereincluded (meanage44.25±14.67,54%men).44patientswere treatedwithinfliximab(69.84%),9withustekinumab (14.29%),7withvedolizumab(11.11%),2withgolimumab (3.17%)and1withadalimumab(1.59%).22(34.92%)were previouslytreatedwithbiologicalagents.4werediagnosed duringthelast18monthswhileotherswerediagnosedbefore. 44patients(69.84%)tookoralimmunosuppressant.60were treated>6monthswiththeircurrentbiologicalagent,the other3casesfor3-5months.
MeanIBD-Control-8scorewas12.41±3.87.Mean VASscorewas87.19±18.17.MeanIBD-Diskscorewas 33.22±25.95(69.84%ofpatientsbeingbelow50 points).4outof63caseshadworseoverallmeasurements (IBD-Control-8score £7,VASscore £60andIBD-Disk score 63).3werewomenwithCDandsmokinghabits (2currentsmokersand1ex-smoker).3ofthemwere treatedwithinfliximaband1withvedolizumab(3requiringconcomitantimmunosuppressants).2requiredprevious surgery.
ConclusionandRelevance Thisstudyaddsnovelliteratureon healthstatusofthesepatientsusingPROMs.Measurements weregenerallyfavorablebut4patientsoutof63hadworse overallmeasurements.Literatureonthistopicisscarce. PROMsareusefultoolsthatcouldbeincorporatedinpharmaceuticalpractice.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-227 REALWORLDEVIDENCEOFTHEUSEOFDEFIBROTIDE FORPROPHYLAXISOFVENO-OCCLUSIVEDISEASE AFTERPOST-HAEMATOPOIETICSTEM-CELL TRANSPLANTATIONINCHILDREN
1MBettio, 1DMengato*, 2AFrancavilla, 1FVenturini. 1PadovaUniversityHospital, PharmacyUnit,Padova,Italy; 2UniversityofPadova,UnitofBiostatistics-Epidemiology-And PublicHealth-DepartmentofCardiac-Thoracic-AndVascularSciences,Padova,Italy
10.1136/ejhpharm-2023-eahp.207
BackgroundandImportance Hepaticveno-occlusivedisease (VOD)isalife-threateningconditioncausedbytheobstructionofliversinusoids.
Since2014,inItalythestandardofcareforthemanagementofVODisrepresentedbydefibrotide.Recentevidence suggestedthatdefibrotidecouldhelppreventingtheonsetof hepaticVODwhenallogeneichaematopoieticstemcell
transplantationisneeded.OnJune2022,however,a ‘direct healthprofessionalcommunication’ issuedbytheEuropean MedicinesAgency(EMA)invokednottousedefibrotideanymoreforVODprophylaxisduetolackofeffectiveness.
AimandObjectives Theaimofthisworkistoexplorethe differenceintheincidenceofVODsat30daysin2groups ofchildren,withandwithoutprophylaxistherapywithdefibrotidebeforeundergoinghaematopoieticstemcell transplantation.
MaterialandMethods Asingle-centre,retrospectivestudywas conductedataUniversityHospital.Alldatawerecollected fromelectronichealthrecords.Thesedatawerecross-checked withdatafromanintegratedanalyticsapplication(Qlikview®, QlikTechInternationalAB,KingofPrussia,USA).
Allpaediatricpatients(age<18years)undergoinghaematopoieticstemcelltransplantationforonco-haematologicaldiseasesandconsideredathigh-riskfordevelopingVODwere enrolled.Weobservedaninitialgroup,calledthe ‘intervention’ group,consistingofpatientswhohadreceivedthedrug, comparedwitha ‘historical’ controlgroupofpatientswith similarbaselinecharacteristicsbutwhodidnothaveaccessto defibrotide.
Results Between2020and2022,datawerecollectedfrom27 patients.Thebaselinecharacteristicsofthetwogroupwere similarregardingofage(9yearsoldforbothgroups),gender andonco-haematologicaldisease,allshowingnostatistically significantdifferences.Intermsofoutcome,wewitnessed onlyoneepisodeofVOD,inthetreatmentgroup(1of11 patients,9%),at30daysaftertransplantation.Noepisodes weredocumentedinthecontrols.
ConclusionandRelevance Accordingtotherecentstatement madebyEMA,ourdata – althoughnotdefinitive – show thatproportionofVODinchildrenundergoingbloodstem transplantationinpatientswhoreceivedaprophylaxistreatmentwithdefibrotidewascomparablewiththeoneinchildrenwherenoprophylaxisstrategyhasbeenadopted.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-228
ACasaldàliga,AFont, 1CJMoreno,EWilhelmi*,APieras,MVillaronga,FBossacoma, RFarré. HospitalSantJoandeDéu,Pharmacy,Barcelona,Spain
10.1136/ejhpharm-2023-eahp.208
BackgroundandImportance Statusepilepticus(SE)isassociated withhighmorbimortality.Earlytreatmenthasbeendemonstratedtodecreasetheriskofdeathandsequelae.WhenfirstlinedrugscannotsolveSE,therapeuticcomashouldbeinitiatedwithmidazolam(mostused),propofol,thiobarbitalor phenobarbital(bettertherapeuticprofilewithlowevidence, especiallyinpaediatrics)areusedforthispractice.
AimandObjectives Describinghigh-dosephenobarbital(HDPHB)usedintherapeuticcomainpaediatricrefractorySEand theirsideeffects.Exposingthepharmacokineticmonitoringto achievebarbituratecoma(BC).
MaterialandMethods Observationalretrospectivestudyofa third-levelpaediatrichospitalconductedbetween2012-2022. 51paediatricintensivecareunit(PICU)’spatientswho receivedintravenousphenobarbitaltreatmentwereincluded,6 ofthemunderwentBC.Variablescollectedwereage,weight,
numberofpreviousantiepileptictreatments,loadingandmaintenancedosesofphenobarbital,phenobarbitalplasmaticlevels duringcoma,BCdaysuntilresolutionofSE,exitusand adverseeffectsofHD-PHB.Alldatawereobtainedfromthe clinicalhistoryprogramme.
Results 51patientswereincluded,ofthem6(median9years [0.2-14.5]and20.2kg)weretreatedwithHD-PHBtoachieve BCduetothepresenceofseizuresrefractorytopropofolor midazolam:5hadaprevioushistoryofepilepsy,treatedwith amedianof3antiepilepticsathome.Theresolutionwasevaluatedbyencephalogram.Theinitialphenobarbitaldosesused toachieveBCwere60mg/kg/day[50-125].ReportedphenobarbitalplasmalevelsachievedintheBCphasewere 943mmol/L[743-1883].Patientswereincomaforamedian of4.5days[1-6]andinallofthemasuppressionburstwas observedintheencephalogram.GlasgowScalebeforecoma was9[7-13]andduringcomawas3[2-5].Afterresolutionof thestatus,taperingregimenwascarriedoutuntilphenobarbitalplasmalevelswerebelow350mmol/Landamaintenance dose(10mg/kg/12h[2-20])wascontinued.Theadverseeffects reportedwerehaematologicalin5patients(decreaseinhaemoglobinandhaematocritlevels)andhepaticin2patients(elevationoftransaminaseslevels).Onepatientdiedbefore6 monthspost-coma.
ConclusionandRelevance HD-PHBseemstobeaneffective therapeuticprocedureinpaediatricrefractorySE.Pharmacokineticsisimportanttoensurethemaintenanceofcomaand avoidtoxicity.Morepharmacokineticstudiesareneededto establishapopulationmodelandclearprotocolsforBC management.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-229 THEADDEDVALUEOFANATIONALELECTRONIC HEALTHRECORDFORTHEBESTPOSSIBLE MEDICATIONHISTORYOBTAINEDBYACLINICAL PHARMACIST
1ASzilvay*, 2ECzakó, 1PPázmány, 1KRichter. 1SzentBorbálaHospital,HospitalPharmacy Department,Tatabánya,Hungary; 2SzentBorbálaHospital,HospitalPharmacyDepartment, Tatabánya,Hungary
10.1136/ejhpharm-2023-eahp.209
BackgroundandImportance ObtainingtheBestPossibleMedicationHistory(BPMH)isanessentialstepinthemedication reconciliationprocess,thatshouldideallybebasedonthe mostappropriatesourcesofinformation,suchaspatient healthrecords,towhichaccessisoftenlimited.ImplementationofaNationalElectronicHealthRecord(NEHR)system aimsatstreamliningthisprocessbyconvergingrelevantdata intoasingulardatabase.
AimandObjectives Thisresearchaimedtoassesstheadded valueofNEHRtoBPMH.Inaddition,thequalityofNEHRbasedBPMHwascomparedtotheformerphysician/nurse-led StandardofCare(SoC),inordertoexploretheaddedvalue ofclinicalpharmacyservicesinobtainingBPMHs.
MaterialandMethods Thestudytookplacebetween05.202208.2022inthegeneralsurgerydepartmentofacountyhospital,enrollingpatientsover18yearsofage,admittedfrom theirhomes,withatleastoneregularlytakenprescribedmedicationandwithoutmajorcommunicationdifficulties.Medicationreconciliationwasinitiatedbyclinicalpharmacists,based
onthedocumentationavailableatthepoint-of-care(‘Hospital list’),whichinturngotvalidatedviaNEHRdata(‘NEHR list’),withthefinalstepbeingapatientinterview,formulating thefinalmedicationlist(‘BPMHlist’).Primaryoutcomemetricswerethefrequencyandtypesofmedicationdiscrepancies derivedfromthecomparisonoftheaforementionedlists, includingtheformerSoC.
Results Thestudyincluded100patients(52%female,average age=62years).231discrepancieswerefoundbetweenthe NEHRlistandtheHospitallist(median=2;IQR=4),64%of thepatientsbeingaffected.Themostcommondiscrepancy wasdrugomission(65%)andincorrectdailydose(26%). TherewasaninconsistencybetweentheBPMHlistandthe SoCin90%ofthepatients(median=3;IQR=3),themost commonerrorsbeingdrugomission(41%)andincorrectdaily dose(31%).
ConclusionandRelevance Basedontheseresults,theNEHR cancontributetothecompilationofamoreprudentBPMH duetoitsmorecomprehensivedatacontent.Thismethodologymay,inturn,facilitatethepreventionofmultiplemedication-relatederrors.Theseoutcomesalsounderlinethe legitimacyofpharmacists'accesstosuchnationalsystems.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-230 ALLOGENEICHAEMATOPOIETICCELL TRANSPLANTATIONINPATIENTSAGED<60YEARS WITHACUTEMYELOIDLEUKEMIA
RTamayoBermejo*,COrtegadelaCruz,JCDelRíoValencia,IMMuñozCastillo. Regional UniversityHospitalofMalaga,PharmacyDepartment,Málaga,Spain
10.1136/ejhpharm-2023-eahp.210
BackgroundandImportance Allogeneichaematopoieticcell transplantation(allo-HCT)isapotentiallycurativetherapeutic modalityforacutemyeloidleukaemia(AML),butitstillcarrieshighmorbidityandmortality;therearelimiteddata regardingoutcomes,soitisimportanttoresearchitsresults, andthefactorsthatinfluencethem.
21.6%haploidentical),35.1%unrelateddonor(21.6%HLAidentical,10.8%HLA9/10,and2.7%HLA8/10).70.3% allogeneicperipheralbloodstemcelltransplantation.64.9% reduced-intensityconditioning.16.2%retransplantation.Most donorsweremen>30years.37.8%receivedpost-transplantationtreatmentwithcyclophosphamide,tacrolimus,andmycophenolatemofetil.18.9%CMV-mismatch(patientpos/donor neg),56.8%ABO-compatible,54.1%developmentchronic GVHDand40.5%acuteGVHD.43.2%didnotrequire relatedhospitalisation.
PFSat12monthswas72%(95%CI,55-84%),and51% (95%CI,34-66%)at24months.OSat12monthswas78% (95%CI,61-89%)and62%(95%CI,45-76%)at24 months.MedianPFSandOSwerenotreached.Themedian follow-upforPFSwas33months[1-69]and34months[169]forOS.
PFSwassignificantlyhigherinpatientsin1stCR,EBMTscore £4,andlower-risk.
ConclusionandRelevance Patientsundergoingallo-HCTshow encouragingsurvival,althoughmoreextendedfollow-upis requiredtodefinemoreaccuratelytheirprognosis.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-232 COMPARATIVEEFFICACYOFRISANKIZUMABAND GUSELKUMABINMODERATETOSEVEREPLAQUE PSORIASIS
1MRodriguezGoicoechea*, 1BMoralesRivero, 2ETejedorTejada, 1NGarciaGomez, 1MJBarberoHernández, 1FHornoUreña. 1HospitalaryComplexofJaén,Pharmacy,Jaén, Spain; 2BarcelonaClinicHospital,HospitalPharmacy,Barcelon,Spain
10.1136/ejhpharm-2023-eahp.211
BackgroundandImportance Anti-interleukin23drugswere approvedinthelast5years.Theabsenceofcomparison betweenalternativessuchasrisankizumab(RIS)orguselkumab (GUS)needstobefulfilled.
AimandObjectives
Toassessthesurvivalofallo-HCTin AMLpatientsage<60years,describeitscharacteristics,and identifyfactorsthatarerelatedtothebestoutcomes.
MaterialandMethods
Retrospectiveobservationalstudy. Weincludedallpatientswith AML,aged<60years,whounderwentallo-HCTperformed atourcentrebetween2016-2019.
Weanalysedtheirage,sex,cytogeneticriskgroup,disease statusatthetimeoftransplantation,Karnofskyperformance status(KPS)score,comorbidityindexes(HCT-CIandEBMTscore),donortype,source,conditioningregimens,graft-versushostdisease(GVHD)prophylaxis,retransplantation,donor age,donorsex,CMV-mismatch,ABO-mismatch,development ofGVHD,relatedhospitalisations,progression,anddeath.
Overallsurvival(OS)andprogression-freesurvival(PFS) wereanalysedusingKaplan-MeierandLog-Ranktest.
Results
Thirty-sevenpatientswereincluded. Meanagewas44.81± 12.26[18-59]years.64.9%werewomen.51.4%intermediateriskand43.2%high-risk.70.3%infirstcompleteremission (CR).91.9%patientshadaKPSscoreover90%atthetime oftransplantation.54.1%HCT-CIbetween0-2,81.1%EBMT score £4.64.8%relateddonor(43.2%HLA-identicaland
AimandObjectives Toevaluatetheeffectivenessthroughindirectcomparisonsofrisankizumabandguselkumabinplaque psoriasis.
MaterialandMethods Multicentric,retrospectiveandobservationalstudy.Comparisonmadewithplaquepsoriasispatients withactivetreatmentwithrisankizumaborguselkumabfrom June2021andJune2022.Demographic(sex,age)andclinical(bodysurfacearea(BSA),psoriasisareaseverityindex (PASI)atbaselineandinsubsequentdermatologycontrols, PASIclearance(PASI100))datacollected.Comparisonmade throughPASI100andBSAandPASIreduction.
Results 59patientstreatedwithRIS,64%men,52,4±15,3 SDyearsoldaveraged,andBSAandPASIof11,4±8,2SD and8,7±4,2SDrespectivelyatbaseline.49patientstreated withGUS,59,2%men,50,9±12,1SDyearsoldaveraged, andBSAandPASIof10,25±10,27SDand8±6,69SD respectivelyatbaseline.
RISachievedatmean21,6±15,7SDweeksaBSAand PASIof2,24±6SDand1,81±3,7SDrespectively,with PASI100reachedby46%ofpatients.GUSachievedatmean 22,9±13,1SDweeksaBSAandPASIof3,87±9,28SD and2,89±4,26SDrespectively,withPASI100reachedby 45%.
At39,5±10,8SDweeks,RISobtainedBSA0,66±1,27 SDandPASI0,64±1,01SD,withPASI100in64%of
patients,whileGUSobtainedBSA1,82±3,28SDandPASI 1,89±3,3SD,withPASI100in50%ofpatientsin44,6± 17,5SDweeks.
After63,6±14,5SDweeks,RISachievedBSA0,68± 0,94SDandPASI0,9±1,14SD,andPASI100maintained by57%patients.GUSachievedBSA0,95±1,55SDand PASI0,53±0,92SD,andPASI100maintainedby67% patients.
ConclusionandRelevance RISandGUSareeffectivealternativesforplaquepsoriasistreatment,althoughitseemsthat afterayear,theactivityofRISstartstodecrease.Further studiesshouldbeperformedtodeterminethishypothesis.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-233 PD-L1EXPRESSIONANDHISTOLOGICALTYPEAS PREDICTORSOFRESPONSEINMETASTASICNONSMALL-CELLLUNGCANCER(NSCLC)PATIENTS TREATEDWITHPEMBROLIZUMABINFIRST-LINE
MTouris-Lores,MBusto-Iglesias*,LGarcía-Quintanilla,ACastro-Balado,ELopez-Montero, AMosquera-Torre,BBernardez-Ferran,SSantana-Martinez,SVazquez-Troche,HJMozoPeñalver,IZarra-Ferro. HospitalClínicodeSantiagodeCompostela,Pharmacy,Santiagode Compostela,Spain
10.1136/ejhpharm-2023-eahp.212
BackgroundandImportance InpatientswithNSCLCandprogrammeddeathligand-1(PD-L1)expression 50%,pembrolizumabasfirst-linetreatmenthasshownanincreaseinsurvival overplatinum-basedchemotherapy.Todate,itisnotknown whetherhigherPD-L1expressionisassociatedwithlonger survival.
ConclusionandRelevance Statisticallysignificantdifferencesin PFSbutnotOSwerefoundinpatientswithNSCLCandPDL1 80%expression.AdenocarcinomawithPD-L1 80% seemtobenefitthemostfrompembrolizumabtreatmentthan otherNSCLChistologies.ThesefindingscouldhaveimplicationsfortreatmentselectionbasedinNSCLChistology.Future researchisneeded.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.AguilarEJetal.,Outcomestofirst-linepembrolizumabinpatientswithnon-smallcelllungcancerandveryhighPD-L1expression.AnnOncol.2019oct1;30(10):16531659.
ConflictofInterest Noconflictofinterest
4CPS-236 LOSSTOFOLLOW-UPFACTORSOFPEOPLELIVING WITHHIV
1MVélez-Díaz-Pallarés, 2BMonteroLlorente, 2MÁParroMartín, 2MDMolinaMendoza, 2EGemenoLopez*, 3DHernándezHuerta, 4MJVivancosGallego, 5LDelCampoAlbendea, 5AMurielGarcía, 2AMÁlvarezDíaz. 1HospitalRamonYCajal,Pharmacy,Madrid,Spain; 2HospitalRamónYCajal,Pharmacy,Madrid,Spain; 3HospitalRamónYCajal,Psiquiatry, Madrid,Spain; 4HospitalRamónYCajal,InfectiousDisease,Madrid,Spain; 5Hospital RamónYCajal,ClinicalBiostatistics,Madrid,Spain
10.1136/ejhpharm-2023-eahp.213
BackgroundandImportance Lossofadherencetoantiretroviral treatment(ART)isoneoftheleadingcausesofvirological failureinpeoplelivingwithHIV(PLWHIV).Lackofadherenceisassociatedwithalossoffollow-upbythehealthsystem,particularlyinthePharmacyDepartment.
AimandObjectives ToidentifyfactorsinPLWHIVwhich causetheirfollow-uptofailbythePharmacyDepartment.
AimandObjectives
Theaimofthisstudyistoevaluatethe impactofPD-L1expressionlevelsonprogressionfreesurvival (PFS)andoverallsurvival(OS),inpatientsreceivingfirst-line pembrolizumabtreatmentforNSCLCanditsassociationto histologictype.
MaterialandMethods
Aretrospectiveanalysisofpatientswith metastaticNSCLCandPD-L1expressionlevelof 50%,who weretreatedwithpembrolizumabmonotherapyasfirst-line therapyinourcentrefromJanuary2020toJanuary2022 wascarriedout.ThedifferenceinresponsebetweenthehistologictypeofNSCLC(adenocarcinomaandnon-adenocarcinoma),andefficacyofpembrolizumabbylevelofPD-L1 expressionwasstudied.ROCcurvewasusedtoevaluatethe optimalPD-L1cut-offpointtoidentifyagreaterpossibilityof response.Event-timedistributionswereestimatedusing Kaplan–Meiermethodology.Log-ranktestswereusedtotest fordifferencesinevent-timedistributions.Allp-valuesare2sidedandCIsareatthe95%level,withsignificancepredefinedtobeatthe0.05level.
Results 49patientswereincludedinthestudy.36patients (73.5%)hadadenocarcinomahistology,10(20.4%)epidermoid,and3(6.1%)other.Acut-offof80%forPD-L1 expressionwasestablished.40(81.6%)hadPD-L1expression <80%and9(18.4%) 80%.MedianPFSwas14.7months (95%CI:7.0-15.1)inpatientswithPD-L1<80%and25.8 months(95%CI:notreached)inpatientswithPD-L1 80% (p=0.017).NodifferenceswerefoundinOS.Patientswith adenocarcinomaandPD-L1expression 80%obtainedbetter resultsinintermsofPFS:19.3months(95%CI:not reached,p=0.031).
MaterialandMethods Case-controlstudyconductedinatertiaryhospitalwhichattends3,000PLWHIV.Patientswhohad runoutofmedicationformorethanonemonth,accordingto pharmacyregistrationsbetweenSeptember2020andSeptember2021,wereidentifiedandnamedaftercasesifthereason tonotcometothePharmacywerenotjustified(death,hospitaltransfer,inclusioninaclinicaltrial,etc.).Weconducteda case-controlstudy(1:4),andcaseswerematchedaccordingto age(5years)anddateofthelastdispensation.
StatisticalanalysiswasperformedusingtheSTATA17.0 program(StataCorpLLC).Allmodelswereperformedunivariately,anda p<0.05wasconsideredsignificant.
Variablesstudiedwere: gender,age,regionofbirth,studies, stablehousing,routeofHIVtransmission,CD4nadir,years afterdiagnostic,typeofART,yearsonART,stage,adverse effectstoART,numberoflinesoftreatment,pharmacyregistrationsofadherence,alcoholuse,druguse,andpsychiatric problems.Datawereobtainedfromtheclinicaldatabase Results Sixty-onecaseswereidentifiedandmatchedwith244 controls.Statisticaldifferenceswerefoundingender,where cis-manhaveanOR=4.5(CI95%1.0 19.6,p=0.047)and trans-manhaveanOR=23.9(CI95%2.9 195.8,p=0.003)in comparisonwithwomen,andregionwhereLatin-American haveanOR=2.7(CI95%1.3 5.6,p=0.008).Patientswho failtoadheretotreatmentaccordingtotherecordsinPharmacyhaveanOR=0.04(CI95%0.01 0.11,p=0.000)and patientswhoarealcoholicsordrugabusers,havean OR=3.24(CI95%1.30 8.04,p=0.011)andanOR=2.01 (CI95%1.03-3.93,p=0.039),respectively.
ConclusionandRelevance Cliniciansshouldpayspecialattentiontocisortrans-men,LatinAmericans,historicbad
adherenceregistrationsbypharmacistsandalcoholicordrug abuserswhoaremorepronetolosingfollow-upintheirtreatments.Thisenhancestheimportanceofmultidisciplinaryteam approachtothesepatients.Clinical,pharmacistandnurse interventionsandinformationregistrationarecrucialtoidentifythesepatients.
ConflictofInterest Noconflictofinterest
ConclusionandRelevance Hyperkalaemiaismorefrequentin menandpatientswithKI.Thereisanassociationbetween PIDco-prescriptionandhyperkalaemiaepisodes.
Thedevelopmentofpharmaceuticalvalidationsupporttools suchasEAlocatorsprovidesthescreeningandmonitoringof disordersthatmighttriggerhealthconsequences.
ConflictofInterest Noconflictofinterest
1SSolisCuñado*, 1LRubio-Ruiz, 1RVázquez-Sánchez, 1NIbáñez-Heras, 1MHijazi-Vega, 2VBarroso-Torrejón, 2JSánchez-Valero, 1TMolina-García. 1GetafeUniversityHospital, HospitalPharmacy,Getafe-Madrid,Spain; 2AlcaladeHenaresUniversity,PharmacyDegree, AlcaládeHenares-Madrid,Spain
10.1136/ejhpharm-2023-eahp.214
BackgroundandImportance Hyperkalaemiaisafrequentelectrolytealteration(EA)inhospitalpatients(HP).Thus,close monitoringofplasmapotassiumlevels(PKL)andappropriately managementisnecessary.Highlevelsofpotassiummaylead toheartandmuscledisorders.
4CPS-240 FINGOLIMOD:ANALYSISOFUSEANDSAFETYIN PATIENTSWITHRELAPSING-REMITTINGMULTIPLE SCLEROSIS
1POrtizFernandez, 2MGilCandel, 2AMartinezSoto, 2AHerreroFernandez, 2PFernandezVillacañasFernandez, 3ISalarValverde, 2MGarciaCoronel, 2CPastorMondéjar, 2CCaballeroRequejo, 2EUrbietaSanz*. 1HospitalGeneralUniversitarioReinaSofia, Pharmacy,Murcia,Spain; 2HospitalGeneralReinaSofia,Pharmacy,Murcia,Spain; 3Hospital DoctorMoliner,Pharmacy,Serravalencia,Spain
10.1136/ejhpharm-2023-eahp.215
AimandObjectives
MainobjectivesaretoevaluateandmonitorhyperkalaemiainHP,tostudyriskfactorsandpotentially implicateddrugs(PIDs)andtoanalysethedegreeofacceptance(DA)ofthepharmaceuticalinterventionsonPKL normalisation.
BackgroundandImportance Fingolimodisusedwhenthediseaseremainsactivedespitetreatmentwithatleastoneother disease-modifyingtherapy,orissevereandgettingworserapidly.Ithadthebenefitofbeingtakenbymouthwhilemost otherdrugsaregivenbyinjection.
AimandObjectives Toanalysetheuseoffingolimodtreatment andanalysethecausesoffingolimod´streatment discontinuation.
MaterialandMethods
Observational,descriptiveandprospectivestudyfromOctober2021toJanuary2022.
Patientswithhyperkalaemia(K+>5.3mEq/L)inthefirst 24hourswereevaluatedwiththeassistanceofanEAlocator includedinthehealthrecordsystem.
PKLwereclassifiedasminor(5.3-5.9mEq/L),moderate(66.5mEq/L)orsevere(>6.5mEq/L).
Age,sex,basalPKLandmeasuredPKLfourdaysafter, prescribedPIDs,comorbiditiessuchaskidneyimpairment (KI),previoustherapeuticapproachordietarypotassium restrictions(DKR)werecollected.
DependingonthePKLandthepatientcharacteristics,differentrecommendationsweremade:discontinuationofpotassium-containingserums;PKLmonitoringandDKR considerationinminorhyperkalaemiacases;ion-exchangeresin (IER)evaluationwhenpatientswithmoderate-severehyperkalaemiatoleratedoralintake.Iftherewereanyprescribed PIDs,pharmacistsrecommendedanalternative.
PKLwereevaluatedafterinterventionsandDAwas determined.
Results Weanalysed87patients.64,4%weremenandthe averageagewas77.Themostacceptedrecommendations were:discontinuationofpotassium-containingserums(DA 100%),PKLmonitoringandDKR(DA64.2%)andIERprescription(DA46.15%).TheproposedalternativestoPIDshad notahighDA.ThePIDsprescribedwereheparin58.6%, renin-angiotensinsysteminhibitors39%,anti-inflammatory drugs27.9%andK-sparingdiuretics3.4%.66.7%ofthe patientsweretreatedwithmorethanonePID,41%ofthem hadKI.
Wemadeaninterventionin40,2%ofthecases.TheDA was65,7%witha60.8%ofPKLnormalisationversusa25% ofrecoveryinthosepatientswithnon-acceptedintervention.
MaterialandMethods Retrospectivedescriptivestudywasperformedinanareareferencehospital.Allpatientstreatedwith fingolimodfromitsinclusioninthehospital'spharmacotherapeuticguideinAugust2012tothepresentwereincluded. Datacollected:age,sex,previoustreatmentreceived,reason forprescription,dateofstartandendoftreatment,thereasonforsuspensionandclinicaldata(basal,finalorcurrent EDSS).WeusedExceltoanalysethedata.
Results Atotalof61patientswereincluded,onepersonwas excludedforreceivingonlyonedose,39(65%)werewomen, withamediaageof42±11years.Allpatientsweretheir heartactivitycloselymonitoredafterthefirstdose.7(10%)of patientsusedfingolimodasfirstline,whoseprescriptionreasonwas:fourforrapidandaggressiveevolutionandthree duetopositiveJCantibody.53(90%)ofpatientshadused otherdisease-modifyingtherapiesbefore,23(43.4%)glatiramer acetate,14(26.4)interferonbeta-1a,4(6.5%)dimethylfumarate,4(6.5%)teriflunomide,1(1.8%)interferónbeta-1band7 (13.2%)startedfingolimodafterfailuretonatalizumab. MedianEDSSwas1innaïvepatientsand1.5inpretreated patients.
Mediantimetodiscontinuationwas42.3[49.8]months.32 patients(53.3%)discontinuedtreatmentfordifferentreasons. Sideeffectswasthemaincause17(53.1%),followedbyinefficacy10(31.2%),forbothreasons2(6.2%)and2(6.2%) unknow.Lymphopeniarepresentedthemostprevalentofthe adverseevents(47.3%),followedbycefalea(21%),liver enzymelevels(21%)andotherlikearterialhypertension,atrioventricularblockandinfections.MedianEDSSincreasedone pointbothinthosewhodiscontinuedtreatmentduetoinefficacyandadverseeffects.
ConclusionandRelevance Therapeuticsuccessisnotassured, asitisadrugwithahighprevalenceofadverseeffects, whichmakesitnecessarytowithdrawtreatment.Isessential
detectingthesymptomsandsignsoftoxicityforavoid unwantedeffects,itispossiblebyfrequentvisitstothehospitalpharmacy.
2.MehtaRandOnatadeR.Contentvalidityofatoolforratingthesignificanceof pharmacists’ cliniccontributionsinhospitalsettings. UKCPASymposiumProceedings; 2016.
ConflictofInterest Noconflictofinterest.
1PGWright, 2MReena, 1RSloss, 3ROnatade*. 1BartsHealthNHSTrust,Pharmacy,London, UnitedKingdom; 2KingsCollegeHosptal,Pharmacy,London,UnitedKingdom; 3Barts HealthNHSTrust,Pharmacy,Lonodn,UnitedKingdom
10.1136/ejhpharm-2023-eahp.216
BackgroundandImportance Todatethereisnogoldstandard forratingclinicalsignificanceofpharmacycontributionsto care.
IMPACCTS(InstruMentforPhArmacyClinicalContributionsTocareSignificance)isbasedontheHatoumscale1 and consistsoffiveorderedcategoriesorlevels,eachunderpinned bydescriptivestatements(totalof45statements).
Arobustprocesstoensuresimplicityandclarityofthe instrumenthasbeenpreviouslyreported.2
AimandObjectivesAim:Tocompletethevalidationof IMPACCTS.
Objectives wereto:
. demonstratecomprehensivenessofIMPACCTS
. quantifyinterraterreliabilityofIMPACCTS
MaterialandMethods ThisstudywascompletedFebruary 2022.Thestudydidnotrequireethicsapproval.
Toassesscomprehensiveness,20seniorpharmacistswith priorexperienceofusingIMPACCTSwerepairedtoreview 45scenarios(450differentscenariosintotal)andaskedto findacorrespondingstatement,orfailingthat,asuitablesignificancelevel.
Forinterraterreliability,all20pharmacistsweregiventhe same15detailedscenariostorateclinicalsignificance.Intraclasscorrelationstatistics(two-way,randomeffects,absolute agreement,individual)werecalculatedusingStatav14.
Alldatawerecollectedviaawebsurveyplatform.
Results Comprehensiveness – forallscenarios,atleastone personfoundastatement.For441/450(98%)scenarios,both respondentsinapairfoundacorrespondingstatement.Out oftheninescenarioswhereonepersonfromthepairdidnot findastatement,alevelcouldbeassignedforeightofthese. Therefore,astatementand/orlevelcouldbeassignedfor449/ 450(99.8%)ofthescenariosbyallrespondentpairs.
Intraclasscorrelationwas0.71(95%CI=0.55,0.86) whichdemonstratesmoderatetogoodpharmacistinterrater agreement.
ConclusionandRelevance Thisstudydemonstratesexcellent comprehensivenessandmoderatetogoodinterraterreliability ofIMPACCTS.Thesedatasupportreadinessofthetoolfor useinresearchandpracticetoassessclinicalseverityofpharmacycontributionsinhospital.
1.HatoumHT etal. Evaluationofthecontributionofclinicalpharmacists:inpatient careandcostreduction. DrugIntelligenceClinicalPharmacy 1988; 22(3):252
9.
4CPS-242 ISAVUCONAZOLETREATMENTINTWOPAEDIATRIC PATIENTSDURINGEXTRACORPOREALMEMBRANE OXYGENATIONSUPPORT:THEROLEOFTHERAPEUTIC DRUGMONITORING
1APauParra*, 2MPujolJover, 3SMelendoPérez, 1AFernández-Polo, 1MMiarons, 2JIzquierdoBlasco, 1SGarcía-García, 3BFernándezLedesma, 1MJCabañas-Poy, 2JBalcells, 1SClemente-Baustista. 1VallD'hebronUniversityHospital,PharmacyDepartment, Barcelona,Spain; 2VallD'hebronUniversityHospital,PaediatricCriticalCareDepartment, Barcelona,Spain; 3VallD'hebronUniversityHospital,PaediatricInfectiousDiseasesand ImmunodeficienciesUnit,Barcelona,Spain
10.1136/ejhpharm-2023-eahp.217
BackgroundandImportance Extracorporealmembraneoxygenation(ECMO)mayleadtopharmacokineticalterationsof antimicrobials.Isavuconazoleisnotapprovedinpaediatric patients(PedP)(off-labeluse)anddataonpaediatricECMO arenon-existent.
AimandObjectives Todescribetwocasereportsusingtherapeuticdrugmonitoring(TDM)tooptimiseisavuconazoledosageinPedPduringECMO.
MaterialandMethods ProspectivestudyincriticallyillPedP treatedwithintravenousisavuconazolereceivingECMO(January2021toAugust2022).Biodemographic,clinicalandpharmacokineticdatawerecollected.Initialproposeddoseof isavuconazolebasewas5.4mg/kg(first48hq8h,followedby q24h;maximum200mg/dose).Isavuconazoletroughserum concentration(IsaCmin)of2.5-5 mg/mLwasconsideredas therapeuticrange(internalprotocol).Continuousvariables wereexpressedasmedian(range).
Results 1)A2-year-oldboy(11.5kg,90cm)lungtransplant recipient(pulmonarycapillaryhemangiomatosis)diagnosed withtracheobronchitiscausedby Aspergillusflavus (9months aftertransplant).Isavuconazolewasstartedataproposeddose andIsaCminremainedintherapeuticrange:5.1(2.5-5.5) mg/ mL.Secondaryprophylaxiswithisavuconazolewasmaintained (samedose),requiringECMOduetosevereacuterespiratory failure(multifactorial).DuringECMO(165days),itwasnecessarytoincreasethedoseto16.5(8.7-19.1)mg/kg/24hto achievetargetconcentrationofmedianIsaCmin2.82(1.3-6.5) mg/mL(24bloodsamples).Nonewfungalinfectionswere observedbutsadlythepatientdiedduetointracranial haemorrhage.
2)A11-year-oldgirl(70kg,158cm)admittedforinfluenza Ainfectionandnecrotisingpneumonia(Staphylococcusaureus),requiringECMO.Invasivefungalinfectionwasprobable (EORTCcriteria;positivegalactomannanandtrachealaspirate for Aspergillusniger)andisavuconazolewasstarted:loading doseof300mg/6h(suspectedinteractionwithpentobarbital duringfirst48h)andTDM-guidedmaintenancetherapy.DuringECMO(30days)medianmaintenancedosewas900mg (12.9mg/kg)/24h(variedwidelyrangingfrom200mg/12hto 250mg/4h)andmedianIsaCminremainedinthetherapeutic range:4.0(1.1-8.4) mg/mL(9bloodsamples).AfterECMO decannulation,isavuconazoledosewasreducedto200mg/1224handmedianIsaCminremainedinrange:3.9(2.8-11.4)
mg/mL.Shecontinuesisavuconazolemaintenancetreatment withapartialresponse.
ConclusionandRelevance
. PedPonECMOmayrequirehigherdosesofisavuconazoleto achievetherapeuticconcentrations,suggestingthatTDMmay beclinicallyuseful.
. FurtherstudiesincriticallyillPedP,especiallythoseon ECMO,arenecessarytoconfirmtheoptimalisavuconazole dosage.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
2022,thedelaywasreducedby20days(95%CI13.66to 27.26;p<0,001).
ConclusionandRelevance ThedelaytoARTinitiationhas beensignificantlyreducedinrecentyears.Factorsrelatedto thedecreaseindelayarelowerCD4c,startingtreatmentwith INSTIorPI/bvsNNRTIandbeingwithin2019-2022vs 2012-2018.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-246 TREATMENTANDNURSINGCAREOF
MUCORMYCOSISINPAEDIATRICS:ACASEREPORT
4CPS-243 DELAYEDHIVTREATMENTANDFACTORS
ASSOCIATED
1GICasarrubios*, 1AMiranda, 1EMartínez, 1CDean, 1ACodonal, 1PTardáguila, 1ALázaro, 2ADelgado, 2MTorralba. 1HospitalUniversitariodeGuadalajara,HospitalPharmacy, Guadalajara,Spain; 2HospitalUniversitariodeGuadalajara,InternalMedicine,Guadalajara, Spain
10.1136/ejhpharm-2023-eahp.218
BackgroundandImportance Clinicalpracticeguidelines(EACS, DHHS,Gesida)recommendstartingantiretroviraltherapy (ART)assoonaspossibleafterHIVdiagnosis,irrespectiveof CD4cellcount(CD4c).PostponingARTstartuntilcomplementaryassessmentsdependsonthesetting,medicalindicationsandriskoflossfromcare.
AimandObjectives Toanalysedelayintreatmentinitiation overthepasttenyearsandtounderstandfactorsassociated withdelayedARTinitiation.
MaterialandMethods Retrospectiveobservationalstudyin patientsdiagnosedwithHIVinfectioninanintegratedhealth areafromJanuary-2012toJune-2022.Variablescollected:age, sex,routeofinfection,healthcaresettingofdiagnosis,time fromdiagnosistoARTinitiation(delaytime),ART,AIDS stage,baselineVLandCD4c.
Datawerecollectedfromelectronicmedicalrecordsand outpatientdispensationprogram.StatisticalanalysiswasperformedusingStudent´st-testandlinearregressionmethod (dependentvariable:delaytime)bySPSS®v.15.0.
Results 108patientswereincluded,medianagewas34years (IQR29.2-42.7)and76.9%weremen.41.7%werediagnosed inprimarycareand58.4%inthehospitalsetting.38.9% wereinAIDSstageatdiagnosis.Thepredominantrouteof infectionwasmenwhohavesexwithmen(MSM)50.9%.
ARTwasinitiatedwithnucleosidereverse-transcriptase inhibitors(NRTI)combinedwithintegrase-strand-transfer inhibitors(INSTI)66.7%,non-nucleosidereverse-transcriptase inhibitors(NNRTI)13%andboostedproteaseinhibitors(PI/b) 20.4%.
ThemedianbaselinelogVLwas4.63(4.13-5.14)andCD4c was325(95-500).
Themediandelaywas21days(IQR9-55).Factorsassociatedwithdelay:baselineCD4c(forevery100CD4increase thedelaytimewasextendedby2.29days(95%CI0.56to 4.02;p=0.01);baselinelogVL(-3.25days95%CI1.57-8.08; p=0.18);AIDSatdiagnosis(-5.40days;95%CI3.30-14.10; p=0.2);useofINSTIorPI/bcomparedtoNNRTI(-31.28 days;95%CI7.85-54.71;p=0.016).Foreachyearofevolution,thetimetoARTinitiationwasreducedby3.05days (95%CI1.59-4.50;p<0.001).Comparing2012-2018vs2019-
1GPerez, 2EWilhelmi*, 2AFont, 2ACasaldàliga, 2CJMoreno, 2MVillaronga, 3JETorra, 2RFarré. 1HospitalSantJoandeDeu,P-Icu,Barcelona,Spain; 2HospitalSantJoandeDeu, Pharmacy,Barcelona,Spain; 3UniversitatdeLleida,NursingCollege,Lleida,Spain
10.1136/ejhpharm-2023-eahp.219
BackgroundandImportance Mucormycosisisaseriousfungal infectionthatcausesfastinvasion,especiallyinimmunocompromisedpeople.
Rhino-orbitalcerebralinvolvementmanifestswithoedema, sinusitis,periorbitalcellulitisandothers.
Treatmentoftenrequirescombinedendovenousandtopical therapy,anddependingontheinvolvement,surgery.
AimandObjectives Explainingthetherapeuticapproachand evolutionofaveryseverelesionwithdeepnecrosisinthe rightnostril,withrapidprogressionandinfectedbyAcinetobacterBaumaniiextremelymultidrug-resistant(ABXDR), AspergillusnigerandRhizopusarizus.
MaterialandMethods A13-year-oldpatient(45kg)withAtypicalHaemolyticUremicSyndrome,admittedinanothercentre whereshestartedtreatmentwitheculizumabandreceivecorticotherapy,wastransferredtoourcentreduetoclinical worsening.
Presentedarapidlyprogressionlesionwithdeepnecrosisin therightnostril.
WoundcultureisolatedAcinetobacterBaumaniiextremely multidrug-resistant(ABXDR)andAspergillusNiger.Subsequently,Rhizopusarizuswasisolatedinthebiopsyandadiagnosisofrhino-orbitalmucormycosiswasmade.Furthermore, ABXDRisisolatedinconjunctivalswabandtrachealaspirate.
Systemictreatmentwasstartedwithisavuconazole(loading dose:200mg/8hfor2daysandmaintenancewith200mg/24h, plasmaticlevels3.85mg/mL),liposomalamphotericin-B(225 mg/24h),meropenem2g/8hgivenasa4-hourextendedinfusionandnebulisedcolistin2MUIevery8h.
Locally,thewoundwasfirstsurgicallydebridedintwo stepsandtargetedtherapywasinitiated.Duetothelackof commerciallyavailableformulations,sterilegelsofamphotericinBdeoxycholate0.15%andcolistin0.5%wereprepared bythepharmacyservice;bothwerepreparedonawater-solublebasis.Theywereappliedevery4hoursalternately.
Duringadmission,topicaldressingswithsodiumhypochloritefomentation(MicrodacynR)plusbacteriostaticgel-based mesh(CutimedSorbactR)wereperformedevery24h.
Apharmacy-preparedcolistin0.2%/6hophthalmicgelwas appliedtotheeyes.
Throughoutthehospitalisation,thewoundwasclosely monitoredperformingsmearstodetectthemicrobialgrowth. Results ClinicalOutcomeswerearapidwoundreductionwith 80%granulationandnegativemicrobialculturesafter28days
ofcontinuoustreatment.Afteramonth,thepatientwasdischargedfromtheunit.
telepharmacy;pharmacokineticmonitoringandtelemedicine; carecoordination;patienthealtheducation;research,educationandtraining.Subsequently,theinitiativeswereprioritised basedontheirimpactonimprovingpatientcareandonthe feasibilityoftheirimplementation(scaleof1-5).
Results Twenty-eightinitiativeswereidentifiedandgroupedin sevenworkareas.Aftertheprioritisationoftheinitiatives,the expertsidentifiedfivepriorityinitiativesforHospital Pharmacy:
-Evaluationandselectionofmedicines:
. Incorporatethepatient'sperspectiveandopinioninHAE treatmentdecision-makingprocessesusingPROs(Patient ReportedOutcomes)andPREMs(PatientReported ExperienceMeasures).
. Participateinmultidisciplinarymeetingsfortheevaluation andselectionofdrugsforHAE.
-Carecoordination:
. Developaguidelineofrecommendationsforthecoordination ofthehealthcareprofessionalsresponsibleforthe managementofpatientswithHAE.
-Patienthealtheducation:
. Promotetheuseoftelepharmacytoolsforpatienteducation andinformationasacomplementtoface-to-facecare.
ConclusionandRelevance Rhino-orbitalmucormycosisisa veryseriousconditionthatrequiresspecifictargetedtreatment andthenursingcare,surgeryandpharmacyinvolvementasa teamisessential.
REFERENCESAND/ORACKNOWLEDGEMENTS ConflictofInterest Noconflictofinterest
4CPS-247 INITIATIVESTOIMPROVETHEMANAGEMENTOF PATIENTSWITHHEREDITARYANGIOEDEMABY HOSPITALPHARMACY
1JBMontoroRonsano*, 2JMMartínezSesmero, 3RLleonartBellfill. 1VallD’hebron UniversityHospital,HospitalPharmacyService,Barcelona,Spain; 2ClínicoSanCarlos Hospital,HospitalPharmacyService,Madrid,Spain; 3BellvitgeUniversityHospital, AllergologyService,HospitaletdeLlobregat-Barcelona,Spain
10.1136/ejhpharm-2023-eahp.220
BackgroundandImportance Hereditaryangioedema(HAE)is arare,hereditarydiseasewithanegativeimpactonthequalityoflifeofpatients.TheincreaseintheknowledgeofHAE andtheappearanceofnewtreatmentsinrecentyearshave contributedtomodifyingthecourseofthisdisease.Inthis scenario,hospitalpharmacistshaveacquiredamoresignificant role.
AimandObjectives Identifyandpromoteinitiativestoimprove themanagementofpatientswithHAEbyHospitalPharmacy andevaluatetheimportanceofcarecoordinationforamultidisciplinaryapproachtopatientswithHAE.
MaterialandMethods Initiativestoimprovethecareof patientswithHAEwereidentified,evaluatedandprioritised byamultidisciplinarypanelofexperts(agroupofhospital pharmacists,oneallergistandonenurse/HAEpatient).The initiativesweregroupedintosevenkeyareasofactivity:evaluationandselectionofmedicines;dispensationand
ConclusionandRelevance Fivepriorityinitiativesareproposed forthemanagementofpatientswithHAE,highlightingthe importanceofcarecoordinationtoimprovethemultidisciplinaryapproachofthesepatients.Fromthisstudy,specific actionshavebeenidentifiedthatcouldimprovetheapproach topatientswithHEAbyhospitalpharmacists.Thus,these professionalswillbeabletopromotepotentiallyimplementableinitiativesthatcouldhavearealimpactonpatients’ lives.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-249 RELATIONSHIPBETWEENRENALFUNCTIONAND ERTAPENEMPLASMACONCENTRATIONINADULT PATIENTS
1APauParra*, 1BMontoroRonsano, 1PGonzálezMoreno, 1DAnguitaDomingo, 2JVima Bofarull, 1MQGorgasTorner, 1DCampanyHerrero. 1VallD'hebronUniversityHospital, DepartmentofPharmacy,Barcelona,Spain; 2VallD'hebronUniversityHospital,Clinics LaboratoriesService,Barcelona,Spain
10.1136/ejhpharm-2023-eahp.221
BackgroundandImportance Ertapenemisaparenteral b-lactamantibioticwithpredominantlyrenalexcretion.Itpresents atime-dependentbactericidalactivityandusualdoseis1g every24h,butinpatientswithestimatedglomerularfiltration rate(eGFR)<30ml/minisrecommended0.5gevery24h.
AimandObjectives Ouraimistoevaluatetherelationship betweenrenalfunction(eGFR)andertapenemplasmatrough concentration(Cert).
MaterialandMethods Retrospectivecohortstudyconductedat atertiaryuniversityhospitalfromOctober2019toFebruary 2021.Adultpatientstreatedwithertapenemforatleast72 hoursandwhohadaCertdeterminationwereincluded.Biodemographic,analyticalandtreatment-relateddatawerecollected.Continuousvariableswereexpressedasmean± standarddeviation(SD)andcategoricalvariablesas
Abstract4CPS-246Figure1percentages(cases).High-performanceliquidchromatographyultraviolet(HPLC-UV)wasusedtomeasureCert.
Certwasmeasuredafteratleasttwodosesofertapenem (>48h)andbeforethenextdoseadministration(trough). RenalfunctionwasmeasuredaseGFRaccordingtoCKD-EPI (ChronicKidneyDiseaseEpidemiologyCollaboration).
Pearsoncorrelationcoefficient(R)wascalculatedtostudy thecorrelationbetweeneGFR(independentvariable)andCert (dependentvariable).Todeterminethestatisticalsignificance ofR,theanalysisofvariance(ANOVA)wasperformedandp valuewasobtained(IBMSPSSStatisticsV21.0).
Results 102patientswithCertdeterminationwereincluded, 53%malesex,with73.0±12.2yearsold.MeaneGFRwas 57.5±27.86mL/min/1,73m2.Certwasmeasured6.4± 4.04daysafterstartingertapenemandthemeandurationof treatmentwas15.5±11.4days.
Rvalorwas-0.436(R2=0.190)whichexplainsaninverse linearcorrelationbetweeneGFRandCertwithstatisticalsignificance(p=0.001).Influenceofothercovariates(albumin, platelets,ertapenemdose,samplingtime)ontherelationship betweenCertandeGFRwasstudied,withnosignificant impactobserved.
MeanCertforthedifferenteGFRrangesweresummarised inthetable:
eGFRcategory(mL/min/1,73m2)Cert(mcg/mL)
eGFR>90(n=24)7.3±12.1
eGFR60-90(n=24)14.1±10.1
eGFR30-60(n=29)19.4±19.5
eGFR<30(n=25)29.7±28.0
Total(n=102)17.8±20.3
ConclusionandRelevance
. DecreaseineGFRiscorrelatedwithanincreasedinCert, withapossibleoverexposureinpatientswithrenal dysfunction.
. Adoseadjustmentcouldbeconsideredinpatientswith compromisedrenalfunction,eveniftheeGFR>30mL/min/ 1,73m2
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
AimandObjectives UnderstandthepatientwithHAEpathway byidentifyingandassessingtheelementsthatcomprisethe burdenofthediseaseofpatients.
MaterialandMethods Descriptivestudy basedonabibliographicreviewandtheexpertiseofamultidisciplinarypanel of18professionalswithkn owledgeandexperienceinHAE (Allergology,Immunology,MedicalEmergency,Hospital Pharmacy,NursingandPatientAssociations).Thepatient pathwaywaselaboratedbyidentifyingtheelementsthat comprisetheburdenofthedisease.Thoseelementswere evaluatedfromthepatient ’ sandthehealthcaresystem ’ s perspectives.
Results ApatientwithHAEsuffersanaverageof5.8attacks peryear,althoughthereisgreatvariabilityamongpatients.It hasbeenestimatedthat35%ofpatientstakelong-termprophylaxis(LTP).
TheestimatedaveragecostofapatientwithHAEis C¼ 47,825/year,includingpharmacologicalcosts,admissions, medicalappointmentsandproceduresandindirectcosts(transportandlossofproductivity).Pharmacologicaltreatmentof LTPrepresents79%ofthetotalcosts;however,itdecreases thenumberofattacksby76%,andthereforereducingthe burdenofdisease.
Intermsoflostproductivity,itisestimatedthatapatient withHAElosses2.5daysofworkperyear,althoughthis variesdependingonthetreatmentandsituation.Thelossof productivityassociatedwiththelossofeducationalandprofessionalopportunitiesandtheemotionalimpactofHAEare importantcomponentsoftheburdenofthedisease.
TheprescriptionofLTPinpatientswithahighnumberof attacksandtheimplementationoftelepharmacy/telemedicine programsimprovesthequalityoflife,reducesvisitstohealth carefacilitiesanddecreasessickleaves.Thepossibilityofhavingthemedicationavailableathomeforself-administrationis animportantbenefitforpatientsandthehealthcaresystem. ConclusionandRelevance HAEhasahighimpactonpatients andthehealthcaresystem.Identifyingthekeyelementsat eachstageofthepatientpathwayisessentialtoimprovetheir qualityoflifewhileensuringthesustainabilityofthehealthcaresystem.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-251 HEREDITARYANGIOEDEMA:IMPACTOFTHEBURDEN OFDISEASEINSPAIN
1EMonteBoquet*, 2CBJosé, 3MNavarroBrugueras, 4ADEscobarOblitas, 5MTCaballero Molina, 6SSmithFlotz. 1HospitalUniversitarioYPolitécnicodelaFe,HospitalPharmacy Service,Valencia,Spain; 2HospitalUniversitarioClínicoSanCecilio,HospitalPharmacy Service,Granada,Spain; 3HospitalUniversitarioSantaMaría,HospitalPharmacyService, Lleida,Spain; 4HospitalUniversitarioSonEspases,ImmunologyService,PalmadeMallorca, Spain; 5HospitalUniversitariolaPaz,AllergologyService,Madrid,Spain; 6Asociación EspañoladeAngioedemaFamiliarAedaf,President,Madrid,Spain
10.1136/ejhpharm-2023-eahp.222
BackgroundandImportance Hereditaryangioedema(HAE)is ararediseasewithanegativeimpactonpatients'qualityof life.Understandingthepatientpathwaywouldcontributeto reducingtheburdenofthedisease.
4CPS-254 DAPAGLIFOZINPRESCRIPTIONPRACTICEINPATIENTS WITHCHRONICHEARTFAILURE
MGarcíaHervalejo*,AmLópez-González,RAparicioPeñacoba,ICondeGonzález, JCGarcíaCasanueva,MJOtero. HospitalofSalamanca,HospitalPharmacy-SalamancaSpain,Salamanca,Spain
10.1136/ejhpharm-2023-eahp.223
BackgroundandImportance Dapagliflozinisasodium-glucose cotransporter2(SGLT2)inhibitorauthorisedbytheSpanish MedicationandHealthcareProductsAgencyforchronicsymptomaticheartfailure(HF)withreducedleftventricularejectionfraction(LVEF).InthepivotalstudyDAPA-HF,therisk ofcardiovasculardeathorworseningoftheHFwasreduced withdapaglifozincomparedwithplacebo.
AimandObjectives Theobjectiveofthestudywastoevaluate theuseofdapagliflozininalevelfouruniversityhospitalfor HFindicationaccordingtotheDAPA-HFstudyinclusion
criteria,emergencyroomvisits,andhospitalreadmissionsdue toHFdecompensation,ordeathfromanycause.
MaterialandMethods ThiswasaretrospectivestudyJanuaryJuly2021thatincludedHFpatientswithatleastonedose ofdapagliflozin.Thevariablesrecordedwere:gender,age, LVEF,N-terminalB-typenatriureticpeptide(NT-proBNP), standardtreatment,HFclassificationaccordingtotheNew YorkHeartAssociation(NYHA),readmissions/emergency roomvisitsforHF,anddeath.Thefollow-upperiodlasted 14months.
Weevaluatedwhethertheprescriptionofdapaglifozinmet theinclusioncriteriaoftheDAPA-HFstudywhichwere: LVEF £40%,NT-proBNP 600pg/mL,NYHAclassII-IVand standardtherapy(angiotensin-converting-enzymeinhibitors, angiotensinIIreceptorblockersorsacurbitril/valsartan,plus betablockersandmineralocorticoidantagonists).
Results Wehad51patients(20%female)withanaverageage of71(49-88).Prescriptoradherencetoallofthecriteriawas achievedin30/51patients(59%).Adherenceforeachcriterionwas:LVEF £ 40%in46patients(90%),NT-proBNP 600pg/mLin44(86%),NYHAII-IVin38(74.5%)and adequatetreatmentwithstandardtherapyin45(88%) patients.
Seventy-sixpercent(39/51)ofpatientscontinuedwith dapagliflozinat14months.Duringthefollow-upperiod10/ 51visitedanemergencyroomand10/51werereadmittedfor HFdecompensation.Thecauseofdeathofthreeofthefour patientswhodiedwascardiovascular.
ConclusionandRelevance Morethanhalfoftheprescriptions fordapagliflozinmetthecriteriaforinclusioninthestudy. ThepercentageofHFdecompensationordeathfromcardiovascularcauseswasgreaterinourcohortthanintheclinical trialsample.
ConflictofInterest Noconflictofinterest
4CPS-255 ANTIPARKINSONIANMEDICATIONRECONCILIATION: HOWPREVENTINGMEDICATIONERRORSPROMOTES THERAPEUTICQUALITYANDSAFETY
AViudez-Martinez,AMRamirezLopez*,JLopez-Nieto,ECliment-Grana,GRiera. Hospital GeneralUniversitarioDrBalmisdeAlicante,Pharmacy,Alicante,Spain
10.1136/ejhpharm-2023-eahp.224
BackgroundandImportance Pharmacotherapyistheprimary treatmentforParkinsonDisease(PD).Theadministrationof PDmedicationneedstobecarriedoutataparticulartimeto avoidmissingdosesorinaccuratedosageschemesthatmay resultinmotorandnon-motorconsequences.One-thirdofall patientswithPDvisitanemergencydepartmentorhospital eachyear,yetabout70%ofneurologistsreportthatPD patientsdonotgettheirmedicationproperlywhenhospitalised.Besides,1in3patientswithPDisprescribedcontraindicateddrugsduringhospitalisationandseriouscomplications, mostlyneuropsychiatric,occurinmorethanhalfofthese patients.
AimandObjectives
Todesignandimplementamedication reconciliationprotocolledbyclinicalpharmaciststhatallowed toidentify,characteriseand,eventually,preventantiparkinsonianmedicationerrorstopromotetherapeuticqualityand safetyindailypractice.
MaterialandMethods
Thiswasaninterventional,singlecentre,one-year,prospectivestudyanalysingtheimpactof developinganantiparkinsonianmedicationreconciliation programme.Allthepatientswhowerehospitalisedand had,atleast,oneactiveprescriptioncontaininganantiparkinsoniandrugathospitalad missionwereincluded.The medicationreconciliationwasperformedbyfollowinga three-phasedcheck:inpatientelectronicprescriptionvalidationafterassessingtheoutpatientmedicationschedule, reviewofthelatestclinicalreportemittedbytheNeurology Department,andpharmacist-driveninterviewofthepatient and/orcaregivertoconfirmtheinformationregardingmedicationgathered.
Results 171admissionepisodesfrom132patientswereregisteredbetweenFebruary1,2021,andJanuary31,2022.Of 224prescriptionlinesinvolvingantiparkinsoniandrugs,179 contained,atleast,onemedicationerror(59.8%).Commission errors(91.62%)weremorefrequentthanomitteddrugs (8.38%).Themostcommonmedicationerrorswererelatedto timing(41.90%),frequency(21.23%),anddosing(19.55%). Theimplementationofthemedicationreconciliationprogrammepreventedtheerroneousadministrationof2716antiparkinsoniandoses,60%ofthetotalnumberofdoses prescribedduringthisperiod.Interestingly,asignificantrelationshipbetweenthenumberofmedicationerrorsandhaving levodopaprescribedwasevidenced(p<0.05).Acontraindicateddrugwasprescribedinalmostone-thirdoftheepisodes (29.82%).
ConclusionandRelevance Clinicalpharmacists'implementation ofanantiparkinsonianmedicationreconciliationprogramme sharplyreducedmedicationerrors,andcontraindicateddrugs prescription,thusimprovingtherapeuticsanddrugsafety.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-257 PREOPERATIVEINTRAVENOUSIRONTOTREAT ANAEMIABEFOREMAJORORTHOPEDICSURGERY
SAsenjoSegovia*,MSarobeCarricas,MNocedaUrarte. HospitalUniversitariodeNavarra, Pharmacy,Pamplona,Spain
10.1136/ejhpharm-2023-eahp.225
BackgroundandImportance Preoperativeanaemia,isarisk factorforpooroutcomeinpatientsundergoingsurgery.Sufficientdataexisttosupportintravenousironasefficacious andsafeifsurgeryisplannedfor<2-3weeksafterthe diagnosisofirondeficiency.Treatmentofpreoperativeiron deficiencyanaemiashouldbeimplementedasearlyasposible beforethescheduledsurgicalprocedure,mostmajorsurgeryis elective.
AimandObjectives Thepurposeofthisstudyistoreviewthe clinicaleffectivenessofIVIadministeredpreoperativelyfor irondeficientinadultpatientsundergoingelectiveorthopedic surgery
MaterialandMethods RetrospectiveObservationalstudyconductedbetweenJanuary2021andDecember2021
Eligibleparticipants,identifiedinpreoperativehospitalvisit wereolderthan18yearsofageandhadhaemoglobinless than13g/dLformenand12g/dLporwomen.
Preoperativeassessmentvisitscheduled1-2weeksbefore surgery,abletoreceiveinfusionatleast7daysbeforethe plannedoperationdate.
Intervention: Intravenousironwasadministeredasasingle 500-1000mgdoseofferriccarboxymaltose(FCM)
Endpointsincluded: demograficcharacteristics,clinicaleffectiveness(hemoglobinlevelbeforesurgery>13g/dL),time spanbetweenfirstFCMadministrationandsurgery,safety (rateofadverseevents).
Limitations: noevidencewithrespecttooutcomessuchas qualityoflife,post-operativecomplications,morbidityand mortalitywereidentified.
Results Werecruited165adultspatients(86,6%femaleand 13,3%male).Themedianagewas71,1years.Thetypeof orthopedicsurgerywas:hip66(40%),knee77(46,7%)and spine18(10,9%).Treatmentwithintravenousironwere administrateden79patients(48%)between7-15daysbefore surgery.
Intravenousironwasadministratedasasingle500mg dosein44patients(26,6%)andasingle1000mgin121 pacientes(73,3%)
Thedayofthesurgery,7,27%ofthepatientsreachedhaemoglobinconcentrationlevels 13g/dL
Patientsweremonitoredforadverseeventsorsignsof hypersensitivityduringandforatleast30minaftertreatment andnosevereadverseeventsrelatedtoFMCocurred
ConclusionandRelevance Theprimaryresultsofourstudy shownoevidenceofclinicalbenefitingivingintravenousiron preoperativelytopatientsundergoingmajorsurgery
Thestudysuggeststhatcurrentprotocolonpreoperative irontherapyshouldberevisedtoimprovetheresults.
ConflictofInterest Noconflictofinterest
1AMartínez*, 2LMoñinoDomingez, 2JCorderoRamos, 2VMerinoBohorquez. 1Clinical Pharmacist,HospitalPharmacy,Sevilla,Spain; 2HospitalUniversitarioVirgenMacarena, HospitalPharmacy,Sevilla,Spain
10.1136/ejhpharm-2023-eahp.226
BackgroundandImportance Invasivefungalinfectionsarean importantcauseofmorbidityandmortalityinimmunocompromisedpatients.Voriconazolehasvariablepharmacokinetics andchildrenusuallyrequirehigherdosestohavevoriconazole concentrationswithinthetherapeuticrange(TR)anddueto variability,closemonitoringofplasmaconcentrations(Cpvor) isrecommended.
AimandObjectives Todescribepharmacokinetic/pharmacokinetic(PK/PD)management,efficacyandsafetyofvoriconazole-inducedlivertoxicityinapaediatricpatient.
MaterialandMethods PK/PDmanagementwasperformedby clinicalpharmacistsandthegoalwastohaveplasmaCpvor withintheTR(1.5-5.5mg/L).Voriconazolehasvariable pharmacokineticslinkedtoage, cytochromeCYP2C19,hepaticdysfunctionanddruginteractions.Efficacyisdefinedas analytical,clinicalandradiographicimprovementandsafety astheabsenceofadversereactions.Cpvorweremeasured byavalidatedhigh-performanceliquidchromatography method.
Results An8-year-oldpaediatricpatientundergoingactive chemotherapyforacutemyeloidleukemia.Duringthe2nd consolidation(probableinvasiveaspergillosis)andafterthe 3rd(proveninvasiveaspergillosis)thepatientwas
hospitalisedandtreatedwithvoriconazole,reachingthe therapeutictargetwithvoriconazol20mg/kg/12horal/IV.In bothadmissions,separatedby8months,thepatientsufferedhepatictoxicity(incr easedtransaminases).Onboth occasionsthefollowingplanwasdeveloped:1)closemonitoringofCpvorand2)closemonitoringofliverfunction. Duringthefirsthospitalisation(Cpvor=1.23mg/L; ALT=90U/L;AST=58U/L;GGT=430U/L)itwasrecommendedtomaintainthedoseof20mg/kg/12horaland monitorliverfunction.At10daysCpvor=3.52mg/Land transaminasesdecreased.Duringthe2ndhospitalisation (Cpvor=9.7mg/L;ALT=35U/L;AST=72U/L;GGT=569U/ L)itwasrecommendedtodecreasethedosefrom20mg/ kg/12hIVto15mg/kg/12hIVandmonitorliverfunction. At10daysCpvor=1.58mg/Landtransaminasesdecreased. Thepatientwastreatedwi thoralandIVvoriconazole, oralbioavailabilitywasestimatedtovarybetween70100%.Treatmentwithvoriconazolewaseffective,the patientpresentedclinical,analyticalandradiographic improvement.
ConclusionandRelevance Voriconazolewaseffectiveinthe treatmentofprobableandprovenaspergillosis.Although voriconazole-inducedlivertoxicity isnotdose-dependent, onthesecondadmissionthe patienthadCpvorabovethe TR.Thepatientpresentedvori conazole-inducedhepatotoxicity,whichwasresolvedwithPK/PDmanagementonboth occasions.
REFERENCESAND/ORACKNOWLEDGEMENTS
4CPS-261 ANALYSISOFDRUGINTERACTIONSBETWEENORAL ONCOLOGICALTREATMENTOFPROSTATECANCER ANDCHRONICMEDICATION
1POrtizFernandez, 2EUrbietaSanz*, 2MGarciaCoronel, 2PFernandez-Villacañas Fernandez, 2AMartinezSoto. 1HospitalGeneralUniversitarioReinaSofia,Pharmacy, Murcia,Spain; 2HospitalGeneralReinaSofia,Pharmacy,Murcia,Spain
10.1136/ejhpharm-2023-eahp.227
BackgroundandImportance Potentialinteractionsaredetected withneworaltreatmentsforprostatecancer.Interactionswith thesedrugsneedtobereviewedinelderlypeoplearefragile, polypathologicalandpolymedicated
AimandObjectives Todetectandanalysetheinteractions betweentheselectiveinhibitoroftheenzyme17a-hydroxylase (abiraterone)andtheandrogenreceptorinhibitors(apalutamideandenzalutamide)withthechronicmedicationof patientswhocometotheoutpatientclinic.
MaterialandMethods ObservationalandtransversalstudycarriedoutintheOutpatientUnit(UPE)fortwomonths(JulyAugust2022)inanareareferencehospital.
Thepharmacistconductedaclinicalinterviewwithall patientsreceivingtreatmentwithabiraterone,apalutamide,and enzalutamidewhocametopickuptheirmedicationatthe outpatientclinic.Innecessarycases,werelyonthecomputerisedelectronicprescription.Inaddition,age,diseaseclassificationandtreatmentstartdatewerecollected.
ThedatacollectedwasanalysedusingtheLexicompdatabase,whichclassifiesinteractionsinto5categoriesaccording totherecommendation:CategoryAandB(Nofollow-upnecessary),C(Monitorthepatient),D(Considermodificationof therapy)andX.(Avoidcombination).Theinteractionsof
categoriesC,DandXhavebeenconsidered.Thedegreeof rigorandthereliabilityratingwerealsocollected.
Results Atotalof69menwereinterviewed.Themeanage was77years,allolderthan60years.31patientswere receivingtreatmentwithapalutamide,26withabiraterone and12withenzalutamide.Thepatientshadameanof12.6 ±15.1monthsoftreatment.88.5%took5ormore medications.
Atotalof709linesoftreatmentwereanalysed,finding that66.6%ofthepatientspresentedaninteractionintheir treatments,1.9interactionsperpatient.
Accordingtotheseverityoftheinteractions,76.2%(91) wereC,10.1%(12)Dand12.7%(15)categoryX.63.5%of theinteractionswerewithapalutamide,26.2%withenzalutamideand10.1%withabiraterone.4pharmacologicalgroups areresponsibleforcategoryDinteractionsand1isresponsibleforcategoryXinteractions(protonpumpinhibitors).
ConclusionandRelevance
. Thestudyhasallowedustodetectahighnumberof interactions,althoughtheproportionofpatientswith clinicallyrelevantinteractionsislow.
. Thepharmacistplaysaveryimportantroleintheprevention, detectionandmonitoringofinteractionsinthisgroupof patients.
Results Atotalof110responseswerereceivedfromdifferent countriesandpractitioners’ groups.Themajorityoftheparticipants(86.11%)statedtheywoulduseatoolforAchBassessmentifavailableandwhentheywereaskedtoratetheIACT againstothertools,amongst34responders,20.59%ratedit betterand8.82%rateditsignificantlybetter,44.12%ratedit neitherbetter,norworse,14.71%rateditworseand11.76% somewhatworse.
ConclusionandRelevance Thereisaneedforananticholinergicburdencalculatortoassesstheanticholinergicityofmedications.ToolssuchastheIACTpotentiallycouldmeetthis demanddueitsabilitytoassignscorestocurrentandnew medicationsappearingonthemarketbasedbothontheir chemicalstructureandreportedadversepharmacological effects.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
4CPS-262 ANOVELARTIFICIALINTELLIGENCE-BASEDTOOLTO ASSESSANTICHOLINERGICBURDEN:ASURVEY
1ASecchi*, 2CFox, 3HMamayusupova, 4SSami, 5IMaidment, 6SCoulton, 7PKMyint, 8CFox. 1KentandMedwayNHSPartnershipTrust,Pharmacy,Maidstone,UnitedKingdom; 2UniversityofExeter-CollegeofMedicineandHealth,UniversityofExeter,Norwich,United Kingdom; 3UniversityofEssex-Co43sq-UK,EssexUniversity,Essex,UnitedKingdom; 4UniversityofEastAnglia-Norwich-NR47TJ-UK,UniversityofEastAnglia,Norwich, UnitedKingdom; 5AstonUniversity,Pharmacy,Birmingham,UnitedKingdom; 6Universityof Kent,UniversityofKent,Kent,UnitedKingdom; 7AgeingClinical&ExperimentalResearch Team-InstituteofAppliedHealthSciences-UniversityofAberdeen-Aberdeen-ScotlandUK,InstituteofAppliedHealthSciences,Aberdeen,UnitedKingdom; 8UniversityofExeterCollegeofMedicineandHealth,CollegeofMedicineandHealth,Norwich,UnitedKingdom
10.1136/ejhpharm-2023-eahp.228
1AFésüs*, 2RBenkő, 2MMatuz, 2ZEngi, 2RRuzsa, 2HHambalek, 3ÁMFésüs, 4TBazsó, 4ZCsernátony, 5IBácskay, 6GKardos. 1UniversityofDebrecen,FacultyofPharmacy DepartmentofPharmaceuticalTechnology,Debrecen,Hungary; 2UniversityofSzeged, InstituteofClinicalPharmacy-FacultyofPharmacy,Szeged,Hungary; 3Universityof Debrecen,DepartmentofTraumatologyandHandSurgery-FacultyofMedicine,Debrecen, Hungary; 4UniversityofDebrecen,DepartmentofOrthopedicSurgery-FacultyofMedicine, Debrecen,Hungary; 5UniversityofDebrecen,FacultyofPharmacy-Departmentof PharmaceuticalTechnology,Debrecen,Hungary; 6UniversityofDebrecen,Departmentof Metagenomics,Debrecen,Hungary
10.1136/ejhpharm-2023-eahp.229
BackgroundandImportance Surgicalantibioticprophylaxisin orthopaedicjointarthroplastiesiscommonreasonforunnecessary,excessiveandirresponsibleuseofantibiotics.
AimandObjectives Thepurposeofthisstudywastoanalyse whetherthecontinuouspresenceofclinicalpharmacistonthe wardmayimproveSAPguidelinesadherenceandclinical outcomes.
BackgroundandImportance
Manymedicationspossessanticholinergicactivity.Theiruseisassociatedwithanumberof seriousadverseeffectsincludingcognitiveeffects.Thecumulativeanticholinergiceffect ofmedicationsasassessedby toolssuchastheanticholinergicburdenscale(AchB)can identifypeopleparticularlyatriskofanticholinergicsideeffects.Currently,morethan20toolsareavailableforclinicianstouse,butthereisnoconsensusonthemostappropriatetool.
AimandObjectives Toassesstheoverallneedforanassessmenttoolaswellastheusabilityofanewlycreatedtool,the InternationalAnticholinergicCognitiveBurdenTool(IACT),to assessanticholinergicburdenofmedications.
MaterialandMethods Anewlycreatedonlinetool,InternationalAnticholinergicCognitiveBurdenTool(IACT),basedon naturallanguageprocessingandchemicalstructureanalysis, wasdevelopedandmadeavailableforclinicianstotestits functions.Wecarriedoutasurvey(between8Februaryto31 March,2021)toassesstheoverallneedforanassessment toolaswellastheusabilityoftheIACT.
MaterialandMethods ThestudywasconductedatanOrthopaedicsDepartmentofatertiarycaremedicalcentre.Overallguidelineadherence(agent,dose,frequency,duration), clinicaloutcomes(lengthofstay-LOS,numberofsurgical siteinfections-SSIs),antibioticex posureanddirectantibiotic costswerecomparedbetweenpre-intervention(retrospective observational)andintervention(prospective)periods.The clinicalpharmacist ’ sinterventionsconsistedofproactively controllingantibioticprophylaxiseverydayonanindividual leveltoensurecompliancewithSAP(agentselection,dosage,andduration)guidelines,attendingsurgicalwardvisits, participatinginantibioticrelateddecisions,andproviding continuouscounsellingservice.SAPguidelineadherence, antibioticexposure,andcostsinthetwoperiodswerecomparedusingChi-square,Fisherexact,andMann-Whitney tests.
Results SignificantimprovementinoverallSAPguideline adherence(by56.2%,from2%to58.2%,p<0.001)was observed.SignificantreductioninSAPduration(by42.9%, 4.1±2.1vs2.1±1.9days,p<0.001),inSAPantibiotic exposure(by41%,from6.1±0.05to3.6±4.3DDD/ patient,p<0.001),andaverageprophylacticantibioticcost(by 54.8%,9278.8±6094.3vs3598.2±3354.6HUF/patient)
wereobserved.Moreover,prolongedprophylaxishasnobenefitonclinicaloutcomes(LOS:decreasedby37.2%,11.2±7 to7.62±3days,p<0.001;confirmedSSIs:deceasedby 1.8%,from3%to1.2%,p=0.21).
ConclusionandRelevance Continuouspresenceoftheclinical pharmacistiscrucialinoptimisingantibioticuse.Pharmacist ’ sinterventionledtoasignificantimprovementinSAP guidelineadherence,thatentailedalsothesignificantreductionofantibioticexposure,lengthofstay,andcosts.Additionalresearch,focusingonempiricalandtargetedantibiotic therapyandimplementationofoptimisingantibioticuse,is needed.
1.Fesus,A., etal.TheEffectofPharmacist-LedInterventiononSurgicalAntibacterial Prophylaxis(SAP)atanOrthopedicUnit.Antibiotics(Basel),2021: 10(12)doi.org/ 10.3390/antibiotics10121509
ConflictofInterest Noconflictofinterest
assurance
5PSQ-002 PHARMACISTINSECURINGDRUGCIRCUIT:FROM PRESCRIPTIONTOADMINISTRATION(ANALYSISAND ACTIONS)
1CMuziotti*, 1JFodimbi, 1CUnia, 2FSantin, 1LDol. 1CentreHospitalierD'hyeres,Service Pharmacie,Hyeres,France; 2CentreHospitalierD'hyeres,QualityService,Hyeres,France
10.1136/ejhpharm-2023-eahp.230
BackgroundandImportance Inamultidisciplinaryhospital with426beds,rolesofhospitalpharmacistarevariedand drugcircuitpresentsmanyrisksofmedicationerror.AccordingtotheWHO,therolesofpharmacistsare ‘theSevenstar Pharmacist’:caregiver,decisionmaker,communicator,leader, manager,lifelonglearnerandteacher.
AimandObjectives Objectiveofthisstudyistomeasureeffectivenessofactionstakenbypharmaciststoreducemedication errors:fromprescriptiontoadministration.
MaterialandMethods Between2019and2022,acompilation ofauditshavebeenmade.Variousstagesofdrugcircuitwere auditedusingpreviouslyvalidatedauditgrids.Eachaudithave beenmadeduring15daysforallnewprescriptions.Astatisticalanalysisofproportioncomparingtheerrorratebeforeand aftertheimplementationofimprovementactionswascarried out.Prescriptionofallinjectabledrugshasbeenformalised, newdoctorsarrivingatthehospitalaremadeaware.Concerningmedicationreconciliation:intheeventofadiscrepancy observed,doctorissystematicallyinformed,apharmacystudenthasbeenassignedtothesurgeryunit.Errorsnot detectedduringpharmaceuticalvalidationwerepresentedto allpharmacists.Measurestoreduceriskoftaskinterruption wereimplementedduringdispensing(dedicatedemergencytelephoneline,redefiningtasks).Concerningadministrationof medication:trainingworkshopdaysfornurseshavebeencreatedbypharmacists.
Results Resultsshowedastatisticallysignificantimprovement incertaincriteria(statisticalanalysisofproportion:comparingerrorratebeforeandafteractions;alpha=5%):medicationreconciliationrateincreasedfrom64%in2019to73% in2021(64%VS73%);errorsnotdetectedduring
pharmaceuticalvalidation(2%VS1%);dispensingerror(3% VS2%);lackofknowledgeoftheestablishment'sdrugadministrationprocedure(58%in2019VS33%in2022).Onthe otherhand,certaincriteriahavedeteriorated:prescription compliantin70%in2019and65%in2022.
ConclusionandRelevance Thisstudyhasmadeitpossibleto objectifythatactionsofpharmacistshavebeenbeneficialin managementofpatients.However,wefindthatactionstaken toimproveprescriptionofdrugshavenotbeeneffective.It wouldbeinterestingtosetupcontinuoustrainingfordoctorsontheuseoftheprescriptionsoftwareinour establishment.
ConflictofInterest Noconflictofinterest
5PSQ-003 PERFORMANCEOFACOLDMAINTENANCEDEVICE DURINGTHEIMPLEMENTATIONOFAPNEUMATIC CIRCUIT
CFerrari*,HModeste,PBesnier,RBaveux,CECollet,GSaint-Lorant. CaenUniversity Hospital,Pharmacy,Caen,France
10.1136/ejhpharm-2023-eahp.231
BackgroundandImportance Fewinformationareavailable abouttheperformanceofcoldmaintenancedevice.
Withintheframeworkoftheimplementationofapneumaticsysteminanewuniversityhospital,thefeasibilityof sendingdifferenttypesofmedicines,includingcoldproducts withapneumaticsystemwasstudied.
AimandObjectives Theobjectiveofthisstudyistoevaluate thecomplianceofacoldmaintenancedevicewithina pneumatic.
MaterialandMethods ThestudywasledinaFrenchUniversityHospitalwith1495bedsandmorethan80careunits betweenMayandSeptember2022.Theanalysiswasmade withkitsprovidedforthecartridgesdedicatedtocold transportandwithqualifiedelectronictemperaturerecordersLog-tags ® (C.M.IFrance,Neung-sur-Beuvon).Different conditionsweretested,oneconditionpertest,reproduced atleast3times:kitsplacedatroomtemperature,inthe fridge(2/8 °C)orinthefreezer,presenceornotofasecondarypackaging,eutecticplateorputtingthekitinthecartridge.Thesupplierhadcertifiedonhiscommercialleaflet adurationof50minbetween2and8 °Cunderthefollowingconditions:500mlinfusionbagstoredat5 °C,with thermalrecorderinsidethebag,placedinthekitthenin thecartridge.
Results Alltheresultsofthe9differenttests(onecondition pertest,reproducedatleast3times)donotmeetthe50 mindataindicatedbythesupplier.Themethodappliedby thesuppliershowsameandurationbetween2and8 °Cof 4.20min[4;5]Usingthesame startingconditions:freezing thekit,gaveanaverageof8.20min[7;9],usingasecondarypackaging,theaveragewas6.40min[6;7],outsidethe cartridge,theaveragewas4.40min[4;6],andaddingan eutecticplate,theaveragewas29.24min[11;60]butwith atemperaturebelow0 °C.Theaverageforalltestsis8.46 min.
ConclusionandRelevance Thisstudyshowedthatthesupplier ’sdeviceanddatadidnotcomplythegoodpracticesconcerningmanagementofhealthproductssubjecttocoldchain andthepatientsafety.
Variousstudieshavebeenundertakenatthelevelofthe Hospitalpharmacyandthecoldsuppliertoimprovethesuppliedisothermalenclosure.
RPlaPasán*,ISánchezLobón,MCorralesPaz,JTudelaTomás,MJHuertasFernández,
10.1136/ejhpharm-2023-eahp.232
BackgroundandImportance Epclusaisatwo-drugcombination administeredasasingledailypillcontainingVelpatasvirand SofosbuvirusedtotreatdeHepatitisC.Thetreatmentdurationis12weeksandthecureratesarefrom97%to100% inthosepatientswithoutcirrhosisorwithcompensated cirrhosis.
Basedondataobtainedfromphase3clinicalstudies,the percentageofpatientsexperiencinganyseriousadverseevent was3.2%.Themostcommonadversereactionsobservedare headacheandfatigue.
Pharmacovigilancecollectsinformation,andanalysesand notifiescaseofsuspectedadversedrugreactions(ADRs)to preventthemoccurringinthefuture
AimandObjectives Todescribeacaseofsleepinessina patienttreatedwithEpclusaandestablishitspossible association.
MaterialandMethods Wedescribeacaseofan72-year-old womandiagnosedwithhepatitisCwithcompensatedcirrhosis andtreatedwithEpclusa.InMay2022,beforestartingthe treatmentwithEpclusa,herhomemedicationwascheckedat thePharmacyDepartment,whichincludeatorvastatin,enalaprilandomperazole;pointingouttoseparatetheintakeof omeprazoleandEpclusa4hoursandprovingtherenowere anydruginteractions.After16daysreceivingthetreatment withEplcusa,shewasreferredtotheemergencydepartment presentingsleepinessandgeneraldeterioration.Asaresult, shewasdiagnosedwithcommoncoldandtreatedwithamoxicilin.Italsocoincidedwithconstipation,whichspontaneously resolvedwithintwodays.FinallyEpclusatreatmentwas stopped.
Results 4daysafter,shereportedimprovementinsleepiness afterdiscontinuationoftreatment,althoughtheiatrogenicorigincannotbeguaranteedsinceithasalsocoincidedwith catarrhalsymptomsandconstipation,bothsituationsinresolution.Naranjo’salgorithmsestablishthecausalityrelationship aspossible(scoreof2).TheSpanishpharmacovigilancecentre wasnotified.
ConclusionandRelevance TheEuropeanMedicinesAgency ’ s technicalsheetforEpclusadoesnotdescribesleepinessasan ADR.Patientcouldconfusefatiguewithsleepinessindealing withsubjectivesymptoms.TheRPCreportedthiscaseasthe onlyEpclusaADRnotifiedinourcountry.Thereportingof ADRsinhospitalsisveryimportantbecauseinnovativenew drugsareusuallyused,severeADRsaremostlikelytobe seeninhospitalsanditcanbedetectedearlyhelpingothers howtoact.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-005 ANALYSISOFANTI-ANGIOGENESIS-RELATED ADVERSEEVENTSASSOCIATEDWITHVASCULAR ENDOTHELIALGROWTHFACTORRECEPTOR-TYROSINE KINASEINHIBITORS(VEGFR-TKIS)INPATIENTSWITH METASTATICRENALCELLCARCINOMA
1,2NLee*, 3JLLee, 2JYLee. 1AsanMedicalCenter,DepartmentofPharmacy,Seoul,KoreaSouth; 2SeoulNationalUniversity,CollegeofPharmacyandResearchInstituteof PharmaceuticalSciences,Seoul,Korea-South; 3AsanMedicalCenter-UniversityofUlsan CollegeofMedicine,DepartmentofOncology,Seoul,Korea-South
10.1136/ejhpharm-2023-eahp.233
BackgroundandImportance Oralvascularendothelialgrowth factorreceptor – tyrosinekinaseinhibitors(VEGFR-TKIs) arestandardtreatmentsformetastaticrenalcellcarcinoma. TheVEGFpathwayplaysanimportantroleinthephysiologicalfunctionandhomeostasisofthecardiovascularand kidneysystems,resultinginanti-angiogenesis-relatedadverse events(AEs).Limitedstudieshaveevaluatedanti-angiogenesis-relatedAEsinvolvingVEGFR-TKIsusingreal-worlddata, whichmayprovideimportant evidencefordrugchoiceand monitoringinthetreatmentofmetastaticrenalcell carcinoma.
AimandObjectives Thisstudyaimedtoinvestigatetheincidenceandpatternsofanti-angiogenesis-relatedAEsassociated withtheuseofVEGFR-TKIsinpatientswithametastatic renalcellcarcinomausingreal-worlddata.
MaterialandMethods Thiscross-sectionalstudyincluded patientswithadiagnosisofmetastaticrenalcellcarcinoma whoreceivedaxitinib,cabozantinib,pazopanib,sorafenib,and sunitinibatthethirdlevelhospitalinSouthKoreabetween January2007andDecember2019.Anti-angiogenesis-related AEswererated ‘possible’ orhigherontheWHO-Uppsala MonitoringCentre(WHO-UMC)causalityassessmentscale. TheseverityofAEswasgradedusingtheCTCAEv.5.0.To comparetheincidenceofAEsassociatedwithdifferent VEGFR-TKIs,wedividedtheenrolledpatientsintothosewho hadnotpreviouslyreceivedaVEGFR-TKI(VEGFR-TKI-naïve) andthosewhohadpreviouslyreceivedaVEGFR-TKI (VEGFR-TKI-experienced).
Results Atotalof988patientswereincluded(75%men, median61years).644patientswereVEGFR-TKI-naïveand 314patientswereVEGFR-TKI-experienced.Anti-angiogenesisrelatedAEsofanygradeoccurredin65.1%ofVEGFR-TKInaïvepatientsand54.8%ofVEGFR-TKI-experiencedpatients. Inaddition,severeAEsoccurredin34.6%ofVEGFR-TKInaïvepatientsand36.0%ofVEGFR-TKI-experiencedpatients. Regardlessoftreatmenthistory,themostcommonAEwas hypertension,witha48.6%ofVEGFR-TKI-naïveand35.0% ofVEGFR-TKI-experienced.ForVEGFR-TKI-experienced patients,theoverallrateofanti-angiogenesis-relatedAEsfor sorafenib(24.3%)waslowerthanthatforotherVEGFR-TKIs (p<0.05).Femalegender(adjustedhazardratio[aHR]1.23, 95%confidenceinterval[CI]1.02-1.48)andhighbloodpressure(aHR1.47,95%CI1.23-1.76)wereriskfactorsfor VEGFR-TKI-associatedAEs.
ConclusionandRelevance Morethanhalfofpatientswith renalcellcarcinomareceivingVEGFR-TKIexperiencedantiangiogenesis-relatedAEs.AnygradeofAEsoccurredmorefrequentlyinVEGFR-TKI-naïvepatients,whilesevereAEs occurredmorefrequentlyinVEGFR-TKI-experiencedpatients.
ConflictofInterest Noconflictofinterest
MVManzanoMartin. HospitalPuertaDelMar,Farmacia,Cadiz,Spain1EChen*, 1VLamand, 1LCatala, 2WCaré, 2HNielly, 1CBroncard, 1BReynaud, 1ALAntoine. 1InternalUsePharmacy-BéginMilitaryTeachingHospital,Val-De-Marne, Saint-Mandé,France; 2DepartmentofInternalMedicine-BéginMilitaryTeachingHospital, Val-De-Marne,Saint-Mandé,France
10.1136/ejhpharm-2023-eahp.234
BackgroundandImportance High-alertmedications(HAMs) canleadtoseriousadverseeventswhenerrorsoccurduringthedrugmanagement.Toraiseawarenessamong healthcareprofessionals(HCPs),ourhospitalpharmacy hasdevelopedafuneducationaltoolinpuzzleform: PUIzzle(PUIistheFrenchacronymforinternaluse pharmacy).
AimandObjectives ToevaluatePUIzzle’simpactonHCPs’ knowledgeonHAMs,aswellasparticipants’ satisfaction.
MaterialandMethods Ourmonocentricstudytookplaceina 300-bedhospitalinParisregion(France)betweenJanuary andAugust2022.Noethicalapprovalforthestudywas requestedasparticipationwasvoluntaryandanonymous. PUIzzleconsistsof12piecescontaininggeneralinformation onHAMsandadescriptionoftheirmanagement,monitoringandantidotes.Aquestion-cardisassociatedwitheach puzzlepiece.Toassesstheirknowledge,participantscompletedapre-andpost-trainingquestionnaireconsistingof fivemultiple-choicequestions,withatotalscoreranging from0to5.Theyalsocompletedasatisfactionquestionnaire consistingoffouritemsratedfrom1 ‘ dissatisfied ’ to4 ‘ very satisfied’
Results Atotalof147participantsweretrainedduring39 sessions:92nurses,17pharmacytechnicians,17paramedicalstudents,12caregivers,7healthcarestudents,1pharmacistand1physician.Sessionmediandurationwas60 minutes[min=55;max=110].Theaverageknowledge scoresbeforeandaftertraining(AFT)wererespectively1.1/ 5and3.1/5(+40%).Beforetraining,54(37%)HCPshad ascoreof0,versus4(3%)AFT,43(29%)ascoreof1, versus20(14%)AFT,34(23%)ascoreof2,versus21 (14%)AFT,13(9%)ascoreof3versus42(29%)AFT,3 (2%)ascoreof4,versus35(24%)AFT.Twenty-fiveHCPs (17%)achievedthehighestscoreof5,onlyAFT.Asignificantimprovement(p<0.001)withanaverageincreaseof +2points( s=0.104)wasobserved.Regardingsatisfaction ofthetraining,participantsattributedanaveragescoreof 3.8/4( s=0.096).
ConclusionandRelevance PUIzzleisafuneducationaltool thatsignificantlyimprovesHCP'sknowledgeonHAMs.SuitabletoallHCPs,thistrainingwillgraduallybeextendedto morephysicians.Therefore,PUIzzlewillbepartofacontinuingeducationprogrammeondrug-inducedadverseevents implementedatourinstitution.
5PSQ-007 IMPACTOF PUIZZLE,APLAYFULEDUCATIONALTOOL ONHIGH-ALERTMEDICATIONSONHEALTHCARE PROFESSIONALS'KNOWLEDGE:WHAT'SNEWAND WHAT'SLEFT?
1EChen*, 1VLamand, 1LCatala, 2WCaré, 2HNielly, 1CBroncard, 1BReynaud, 1ALAntoine. 1InternalUsePharmacy-BéginMilitaryTeachingHospital,Val-De-Marne, Saint-Mandé,France; 2DepartmentofInternalMedicine-BéginMilitaryTeachingHospital, Val-De-Marne,Saint-Mandé,France
10.1136/ejhpharm-2023-eahp.235
BackgroundandImportance High-alertmedications(HAMs) havehigherrisksofcausingharmtopatients.Topreventthis, ourhospitalpharmacytrained147healthcareprofessionals (HCPs)onthistopicusingafuneducationaltoolinpuzzle form:PUIzzle(PUIistheFrenchacronymforinternaluse pharmacy),whichhassignificantlyimprovedshort-termknowledgeonHAMs.However,itsimpactonlong-termknowledge retentionisnotknown.1
AimandObjectives ToevaluatePUIzzle’simpactonHCPs’ knowledgeretentionanditscontributiontoprofessional practices.
MaterialandMethods Ourmonocentricstudytookplaceina 300-bedhospitalinParisregion(France)betweenJanuaryand August2022.Noethicalapprovalforthestudywas requested.HCPs’ knowledgewasassessedwithaself-questionnaireadministered3weeksafterthetraining(3WAT).The firstpartfocusedonprofessionalpractices.Thesecondpart wasidenticaltothepre-andpost-trainingknowledgequestionnaire,tocomparethescoresofthepre-training(PrT), post-training(PoT)and3WATquestionnaires.Thethirdpart hadfiveotherquestionsonHAMs.
Results Ofthe141eligibleHCPs,60completedthe3WAT (31nurses,13pharmacytechnicians,5paramedicalstudents, 4caregivers,6healthcarestudentsand1pharmacist)ina meantimeof3.6months(s=1.37),48werelosttofollowupand33didnotrespond.Fifty-two(87%)HCPscouldsituateoneofthetwoHAMs’ locationlist,and44(75%)could identifyHAMsontheprescriptionsoftware.Mostchangesin practicewereobservedattheprescribinglevel(n=31;52%). HCPsdeclaredbeingmoreattentivetoHAMs’ labelling.The meanscoreofPrT,PoTand3WAT’ secondpartwererespectively1.1/5(s=0.04),3.1/5(s=0.14)and2.5/5(s=0.33).A significantimprovementbetweenPrTand3WAT(p<0.03)and anon-statisticaldecreasebetweenPoTand3WAT(p<0.17) wereobserved.The3WAT’sthirdpartmeanscorewasidenticaltothesecondone(s=0.27).
ConclusionandRelevance PUIzzle’simpactispositiveon HCPs’ practicesandoverallknowledgeretentiononHAMs. Therefore,ourhospitalwillorganiseregulartrainingsessions, andthistrainingwillbetransposedintocontinuingprofessionaleducation.
1.E.CHEN,2022, Designofafuneducationaltoolfortrainingonhigh-alertmedicationsandevaluationofitsimpactonhealthcareprofessionals’ knowledge,pharmacythesis,Paris-CitéUniversity,France.
ConflictofInterest Noconflictofinterest
10.1136/ejhpharm-2023-eahp.236
BackgroundandImportance Medicationreconciliationhas becomestandardcaretoobtainacompleteoverviewofthe currentmedicationofapatient.However,itistime-consumingandlabour-intensive.Studieshaveshownpromisingresults foronlinemedicationreconciliationpreparationdoneby patients.Nonetheless,thereisaneedforenhancedpatient supporttomakethisprocessassimpleandeffectiveas possible.
AimandObjectives Toascertaintheexperiencesofpatients usingadigitalassistantforpre-visitonlinemedication reconciliation.
MaterialandMethods Thisstudyfollowedaqualitative, descriptivedesign.InMay2022,rheumatologyandneurology outpatientswereapproachedface-to-facebyarheumatologist/ neurologistduringtheirvisit,ifconsideredcapabletoparticipate.Theyreceivedaninformationletterexplainingthestudy. Participationwasvoluntary.Afterwrittenconsent,patients wereinstructedtouseadigitalassistantforverifyingand complementingtheirhomemedicationonline,afterwhich semi-structuredindividualinterviewswereconducted,audio recordedwiththeparticipant’spermission.Interviewdata wereanonymisedandevaluatedusinginductivethematicanalysisaccordingtothemethodofBraunandClarke.Awaiver wasobtainedfromtheregionalMedicalEthicsReview Committee.
Results Elevenpatientswereincluded.Thestudypopulation comprised2menand9womenwithamedianageof64.0 years(interquartilerange[IQR]50.0-70.0).Themainthemes identifiedamongstpatientexperienceswererelatedtousability,methodofinput,layout,safety,communication,perception andnecessity.Advantagespatientsmentionedwereplaceand timeindependence,efficiencyandincreasedawarenessoftheir medicationuse.Limitedinformationtechnology(IT)skills amongelderlywasthemostfrequentlymentionedbarrierfor usingthedigitalassistant.Suggestionsforimprovementwere relatedtousabilityofthedigitalassistant(e.g.largerfontstyle andascertainthattextsfitthedevice),layout(e.g.provide overviewofgivenanswers)andsafety(e.g.integratedigital assistantinonlinehospitalenvironmentandexplicitlystate thatpatientdataaresavedinasecureenvironment),amongst others.Themajorityofthepatientspreferredthedigitalassistantoveramedicationreconciliationconversationwithapharmacytechnician.
ConclusionandRelevance Overallexperiencesofpatientsusing adigitalassistantformedicationreconciliationwerepositive, demonstratingthereispotentialfortheuseofadigitalassistantinclinicalpractice.
ConflictofInterest Noconflictofinterest
5PSQ-010 DIGOXINADJUSTMENT:COMPARATIVEANALYSISOF THREEPHARMACOKINETICSOFTWARE
1ARodríguezEsquíroz*, 2CLeraltaGonzález, 3MJiménezMeseguer, 3ÁLSalcedo Mingorranz, 3BGarcíaDíaz, 1MSarobeCarricas, 2MFHurtadoGómez. 1Hospital UniversitariodeNavarra,Pharmacy,Pamplona,Spain; 2HospitalSanPedro,Pharmacy, Logroño,Spain; 3HospitalUniversitarioSeveroOchoa,Pharmacy,Madrid,Spain
10.1136/ejhpharm-2023-eahp.237
BackgroundandImportance Digoxinisadrugwithanarrow therapeuticindex(0.8-1.2ng/mL).Therapeuticdrugmonitoringisanimportanttooltoimprovetherapeuticsafetyand efficacy,especiallyinelderlypatients.
AimandObjectives Toestimatetheaccuracyandprecisionof threepharmacokineticsoftwaretoanalyseserumdigoxinconcentrations(SDC).
MaterialandMethods Retrospectiveobservationalstudyin elderlypatientsadmittedtoatertiaryhospitalandtreated withdigoxinin2020.Weexcludedpatientsover80yearsold.Variablesrecorded:sex,age,bodymassindex(BMI), SDC,creatinineclearanceevaluatedbytheCockcroft-Gault equation(CrCl),andconcomitanttreatment:protonpump inhibitors(PPIs)andnon-steroidalanti-inflammatorydrugs (NSAIDs).
SDCwereestimatedwiththreepharmacokineticsoftware: Mediware,PKSandNONMEM.
AccuracyandprecisionwereassessedusingSheinerand Beal'spredictionerrortheory.Accuracywiththemeanpredictionerror(MPE)andprecisionwiththemeanabsolutepredictionerror(MPAE)andthesquarerootoftherootmean squarepredictionerror(RMSE).
Twosubgroupswereanalysed: renalimpairmentpatients (CrCl<60mL/min)andpatientswithtwoormoreSDC.
Results 53patientswith130SDC,31women(58.5%), medianage75.5years-old(66.5-80.7).64%onconcomitant treatmentwithPPIsand41.5%withNSAIDs.
Accuracy: MPE0.002,-0.011,-0.081,forMediware,PKS andNONMEMrespectively.
Precision: MPAE0.193,0.201,0.243;RMSE0.331,0.345, 0.328forMediware,PKSandNONMEM.
Renalimpairment: 32patientswith64levels.
Accuracy: MPE-0.052,-0.028,-0.106forMediware,PKS andNONMEM.
Precision: MPAE0.192,0.246,0.275;RMSE0.330,0.416, 0.363forMediware,PKSandNONMEM.
2levels:36patients
Accuracy: MPE0.003,-0.010,-0.080forMediware,PKSand NONMEM.
Precision: MPAE0.205,0.211,0.235;RMSE0.347,0.360, 0.312forMediware,PKSandNONMEM.
ConclusionandRelevance Thethreesoftwareshowedsimilar accuracyandprecisionforanalysingSDC.
Mediwareisthebesttoolfordailyclinicalpracticein termsofeaseofuse.
ConflictofInterest Noconflictofinterest
1BMaat, 2ADijkmans*, 3KTenfelde, 3CVanDerLee, 1MKicken, 3JdeWit. 1ElisabethTweestedenHospital,ClinicalPharmacy,Tilburg,TheNetherlands; 2UtrechtUniversity, DepartmentofPharmacoepidemiologyandClinicalPharmacology,Utrecht,TheNetherlands; 3TilburgUniversity,DepartmentCommunicationandCognition-TilburgSchoolof HumanitiesandDigitalSciences,Tilburg,TheNetherlands1ISollano-Sancho*, 2SGarcíaMartínez, 2ALSalcedoMingoarranz, 2APovedaEscolar, 2IOrozcoCifuentes, 1CMorielSánchez, 2BGarcíaDíaz. 1MostolesUniversitaryHospital, HospitalPharmacy,Madrid,Spain; 2SeveroOchoaHospital,HospitalPharmacy,Madrid, Spain
10.1136/ejhpharm-2023-eahp.238
BackgroundandImportance Thereisaneedtocarefullyprescribedrugswithanarrowtherapeuticrangeandpharmacokineticreportsontheirserumconcentrationsarethenecessary toolforthiscommitment.
AimandObjectives Evaluatetheimpactofpharmacokinetic reportsonclinicaldecision.
MaterialandMethods Aprospectiveanalysiswasconductedin asecondarycarehospitalbetweenMarchandAugust2020 includingadultpatientswithatleastoneserumconcentration ofvalproicacid,amikacin,carbamazepine,cyclosporine, digoxin,phenytoin,phenobarbital,lithium,gentamicin,theophylline,vancomycinandvoriconazole.
Dialyzedandnon-admittedpatientswereexcluded.Data wasobtainedfrommedicalrecordsandpharmacokineticsoftware.Thevariablescollectedwere:age,sex,prescribeddrug, clinicaldepartment,pharmacokineticreportandmedical decision.
Results 166patientswith613pharmaceuticalinterventions wererecorded.Ninety-five(57.2%)werewomenwithamean ageof73years(28-100),meanweight66kg(40.5-139.2) andmeanserumcreatinine1mg/dL(0.3-12.5).
Thenumberofpharmacokineticreportsweredigoxin:265 (43.2%);vancomycin:139(22.7%);valproicacid:79 (12.9%);lithium:69(11.3%);amikacin:17(2.8%);carbamazepine:10(1.6%);theophylline:9(1.5%);phenytoin:8 (1.3%);gentamicin:8(1.3%);cyclosporine:4(0.7%);phenobarbital:3(0.5%);voriconazole:2(0.3%).
Pharmacokineticreportsaccordingtotheprescribingclinical department:internalmedicine:223(36.4%),psychiatry:100 (16.3%);andcardiology:71(11.6%)werethemainones.
Thephysician'sacceptanceofthepharmacokineticreports accordingtothedrugweredigoxin:112(37.6%);vancomycin:74(24.8%);valproicacid:43(14.4%);lithium:38 (12.8%);amikacin:13(4.4%);phenytoin:4(1.3%);theophylline:4(1.3%);gentamicin:3(1.0%);carbamazepine:2 (0.7%);cyclosporine:2(0.7%);phenobarbital:2(0.7%);voriconazole:1(0.3%).
Acceptanceofpharmaceuticalrecommendationsbymajor clinicalserviceswereinternalmedicine:110(36.9%);psychiatry:54(18.1%);andgeriatrics:32(10.7%).
Acceptedrecommendationsweredosemaintenance: 202 (75.4%);dosesuspension:26(72.2%);dosereduction:41 (68.3%);doseincrease:26(66.7%).
Thepharmacokineticreportsaccepted295(73.2%).8% werenotacceptedduetopatientdischargeordeath.
ConclusionandRelevance Apharmacokineticareasupports cliniciansinordertoestablishthesafestandmosteffective dosingregimens.
Ahighpercentageofpharmacokineticreportswere accepted,however,itisnecessarytoincreasethispercentage bytalkingtophysiciansandremarkingtheimportanceofthis activity.
5PSQ-012 CASEREPORT:INHALEDGRANULOCYTEMACROPHAGECOLONY-STIMULATINGFACTORFOR MILD-TO-MODERATEAUTOIMMUNEPULMONARY ALVEOLARPROTEINOSIS – 24MONTHSOFFOLLOWUP
1IOterino*, 2MJLinares-Asensio, 3IMorona-Minguez, 1SSanz-Márquez, 1MPérez-Encinas. 1AlcorconFoundationUniversityHospital,PharmacyDepartment,Alcorcón,Spain; 2Alcorcon FoundationUniversityHospital,PulmonologyDepartment,Alcorcón,Spain; 3Mostoles UniversityHospital,PharmacyDepartment,Mostoles,Spain
10.1136/ejhpharm-2023-eahp.239
BackgroundandImportance Autoimmunepulmonaryalveolar proteinosis(aPAP)isadiseasecausedbyIgGantibodies againstgranulocyte-macrophagecolony-stimulatingfactor(GMCSF).Thetreatmentofchoiceisbronchoalveolarlavage (BAL).
AimandObjectives TopresentaPAPevolutionduring24 monthsoftreatmentwithinhaledGM-CSF.
MaterialandMethods A37-year-oldmalediagnosedwith aPAPinApril2014hasrequired3BAL(October2014,February2016andMarch2019).InthelastBAL,hedeveloped majorcomplicationsthatrequiredadmissiontotheintensive careunit.InOctober2019,hepresentedanewworsening,so off-labeltreatmentwithsargramostim(250mginhaledevery12 hoursfor7dayseveryotherweek)waschosen.
Thepharmacyservicepreparedafavorablereportonofflabeltreatmentandrequesteddrugfromregulatoryagency.In thepharmaceuticalcareconsultation,theadministrationtechnique,stability,dosageregimen,storage(2-8°C)wereexplained anddoubtswereresolved.
Results Theclinicalandfunctionalevolutionoftheventilatory parametersandthesix-minutewalktestareshowninthe table1.
Thefigure1showstheradiologicalevolution(chestX-ray) fromthesituationbeforethirdBAL(1),furtherworsening after7monthsafterthirdBAL(2),improvementafter3 monthsoftreatmentwithinhaledGM-CSF(3)andstability after18monthsoftreatment(4).
growthfactorreceptor2-negative(HR+/HER2 )locally advancedormetastaticbreastcancer(LA/MBC).Neutropenia isthemostcommonadverseevent.Incontrasttoneutropenia inducedbychemotherapyagents,neutropeniaresultingfrom CDK4/6inhibitorsisreversibleanddosereductionsandmodificationsarerecommended.
AimandObjectives Theaimofthisstudywastoevaluatethe neutropeniaduetopalbociclibandtoanalysehowmodificationsintreatmentsaremadeinclinicalpractice.
MaterialandMethods Weconductedadescriptive,observationalandretrospectivestudy(April2016-July2022)ofpatients treatedwithPalbociclibinathirdlevelhospital.Thedata wereobtainedfromtheelectronicmedicalrecordsofthe patientsandtheFarmatoolsManagementprogramme.The parametersanalysedwere:demographicinformation,menopausalstatus,priorlinesoftherapytopalbociclib,frequency andgradesofneutropenia,timefromfirstdosetofirstepisodeonset,dosesreductions,cyclesdelays,useofhuman granulocytecolonystimulatingfactor(G-CSF),changesto otherCDK4/6inhibitoranddiscontinuationtreatment.Data wereprocessedbyMicrosoftExcelsoftware
Results 50womenwithHR+/HER2 MBCweretreatedwith palbociclib.Medianagewas62years.92%(46/50)waspostmenopausal.80%(40/50)receivedpriortherapytopalbociclib and58%(23/40)wasinthecontextofMBC.54%(27/50) receivedPalbociclibasfirst-linetreatment.Startingdosewere: 82%(41/50)125mg;12%(6/50)100mg;6%(3/50)75mg.
Abstract5PSQ-012Figure1
After24monthsoftreatment,thepatienthasnotpresentedanyadverseeventsandmaintainsanexcellentresponse withsignificantimprovementingasexchange,whichhas allowedhomeoxygentherapytobewithdrawn.
ConclusionandRelevance Inconclusion,ourcasesupports thatinhaledGM-CSFhasbeensafeandeffectiveinthetreatmentofaPAPandrepresentsatherapeuticoptionafterresistanceorcontraindicationtoBAL.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-013 PALBOCICLIBINMETASTATICBREASTCANCER TREATMENT:NEUTROPENIAMANAGEMENTIN CLINICALPRACTICE
1ARojasAlbarrán*, 2MDRivasRodriguez, 2AGilGarcia, 2MGrageraGómez, 2MDZambranoCroche, 2HVelazquezVazquez. 1UniversityHospitalComplexofBadajoz, HospitalPharmacy,Badajoz,Spain; 2UniversityHospitalComplexofBadajoz,Pharmacy, Badajoz,Spain
10.1136/ejhpharm-2023-eahp.240
BackgroundandImportance Palbociclibisaselectivecyclindependentkinase4/6(CDK4/6)inhibitorapprovedforthe treatmentofhormonereceptor-positive,humanepidermal
Thefrequencyofneutropenia(all-grade)was74%(37/50); 27%(10/37)wasgrade1-2;73%(27/37)wasgrade3-4.Time fromfirstdosetofirstepisodeonset(cycles)wasreportedin: 8,1%(3/37)first-cycle;56,7%(21/37)second-cycle;13,5%(5/ 37)three-cylcle;21,6%(8/37) fourth-cycle.Neutropenialed todosereductionin54%(20/37)ofpatients;32%(12/37) requiredadosereduction;21,6%(8/37)requiredtwodoses reductions.Cyclesdelaysoccurredin78%(29/37)ofpatients. 19%(7/37)wastreatedwithG-CSFassupportivetherapy. 5,4%(2/37)neededtochangetoanotherCDK4/6inhibitor. 10,8%(4/37)discontinuedtreatment.
ConclusionandRelevance Thefrequencyofneutropeniain ourpopulationwassimilartoclinicaltrials.¹Inclinicalpracticethistoxicitycanbemanagedwithdosereductionand cyclesdelayswithoutleadtodiscontinuationtreatment(only fourpatients)asitisdescribedinguidelines.²
REFERENCESAND/ORACKNOWLEDGEMENTS
1.PivotalstudyPALOMA-3
2.https://cima.aemps.es/cima/dochtml/ft/1161147003/FT_1161147003.html
ConflictofInterest Noconflictofinterest
5PSQ-014 IMPACTOFHISTAMINE-2ANTAGONISTSHORTAGE ONTHEINCIDENCEOFHYPERSENSITIVITY REACTIONSTOPACLITAXEL – TOWARDSCRISIS MANAGEMENTANDAPREMEDICATION RECONSIDERATIONINFRANCE(PACLIREACTSTUDY)
1GStrobbe*, 2LGaboriau, 1MAbele, 1AVillain, 3CAelbrecht-Meurisse, 3ACarnot, 4MCLe Deley, 4CLéguillette, 1ISakji, 1FFeutry, 1GMarliot. 1CentreOscarLambret,Servicede Pharmacie,Lille,France; 2ChuLille – CentreRégionaldePharmacovigilance,Servicede Pharmacologie,Lille,France; 3CentreOscarLambret,PoleD'oncologieMédicale,Lille, France; 4CentreOscarLambret,UnitédeMéthodologieEtdeBiostatistique,Lille,France
10.1136/ejhpharm-2023-eahp.241
BackgroundandImportance AtthebeginningofOctober 2019,aninternationalshortageofranitidineforcedusto adjustpaclitaxel-basedchemotherapypremedicationregimens.
Afterseveralmodifications,weimplementedananti-allergic premedicationprotocolbasedonDexchlorpheniramineashistamine-1antagonist(H1A),Methylprednisoloneascorticosteroid(Doubledoseatfirstinjection)andwithdrawalof histamine-2antagonists(H2A).
AimandObjectives Thisstudyaimedtodeterminetheefficacy ofthismodifiedregimenandassessthehypersensitivityreactions(HSRs)associatedwithit.
MaterialandMethods Weconductedasingle-centreobservationalretrospectivestudyofpaclitaxeladministrations(n=831 patients).Allincidentscharacterisedasdrugallergiesinthe prescribingsoftwarewereexhaustivelyrecordedoveratwoyearperiodfromJanuary2019toDecember2020(before andafterranitidineshortage,includingtheperiodwithoral Famotidineasatransitionalalternative).Tomodeltheriskof allergyateachinjectionaccordingtothetypeofinjectionand possibleconfoundingfactors,amixedlogisticregressionmodel wasimplementedtoaccountforrepeatedadministrationper patient.
Results Amongthe7146paclitaxeladministrations,therewere atotalof27HSRsoccurringin24patients,amongwhom threepatientshadtwoconsecutiveevents.Noprotectiveeffect wasobservedforH2Apremedicationregimens,neitherwhen comparingthetwotypesofH2A(famotidineorranitidine) separately(p=0.94)norwhencomparinginjectionswith H2ApremedicationversusinjectionswithoutH2A(OR:1.12, 95%CI,0.36-3.50,p=0.84).However,theriskofHSRs wassignificantlylowerforpaclitaxelinjectionswithcorticosteroidsthanforthosewithoutcorticosteroids(OR:0.08,95% CI:0.008-0.78,p=0.03).Inaddition,theriskofHSRwas significantlyhigherforthefirst,second,orthirdpaclitaxel injectionsthanforthesubsequentinjections(OR:10.1,95% CI:3.23-31.4,p<0.001).
ConclusionandRelevance Wedidnotfindevidenceofan increasedriskofHSRduetotheabsenceofH2AinthepremedicationprotocolsofPaclitaxel.Ourfindingssupportthe choiceofapremedicationprotocolwithoutH2A,despite whatishistoricallystatedinPaclitaxelmonographs.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-016 HUMANFACTORSROLEINMEDICATIONERRORS:
DILUTINGINTRAVENOUSMEDICATIONSATHOSPITAL WARDS – ASTUDYBASEDONINCIDENTREPORTS
1AMulac*, 2EHagesæther, 1,3AGGranås. 1UniversityofOslo,DepartmentofPharmacyThe FacultyofMathematicsandNaturalSciences,Oslo,Norway; 2OsloMetropolitanUniversity, DepartmentofLifeSciencesandHealth-FacultyofHealthSciences,Oslo,Norway; 3UniversityHospitalofNorthNorway,NorwegianCentreForE-HealthResearch,Tromsø, Norway
10.1136/ejhpharm-2023-eahp.242
BackgroundandImportance Humansmakemistakes,inadvertentlywhenmakingpoordecisions,beingdistractedor whennotperceivingriskwhilstmanagingmedications. Healthprofessionalsdonotmakemistakesonpurpose, yetmedicationerrorsremainthemostcommontypeof medicalerrors.Ahumanfactorsapproachcanbeapplied toaddressthecausationofmedicationerrorsfroma
processpointofviewwhileadd ressingourerror-prone humannature.Intravenousmedicationsarecomplexto prepareandadminister.Specifictasks,suchasdiluting intravenousmedicationsareatahigherriskofmedication errors.
AimandObjectives Thisstudyaimstoaddresshumanfactors inmedicationcalculationerrorsinvolvingdilutionofintravenousmedications.
MaterialandMethods Fromthemedicationerrorsreported in2016and2017totheN orwegianIncidentReporting System,wespecificallyscrutinisedmedicationcalculation errorsthatrequireddilution duringmedicationpreparation, dispensingandadministration.Weincludedrealeventsthat hadreachedthepatients,andwhichcontainedsufficient incidentdescriptiontoallowforcausalanalysis.Fromthe incidentdescriptions,weco nductedacontentanalysisof humanfactors.
Results Intotal,14incidentsmettheinclusioncriteriaand involvedthedilutionofmorphine,oxycodone,adrenalin, andnoradrenalin.Severalhumanfactorsexposedtheintravenouspreparationprocesstorisks.Forexample,performingtaskswithcognitiveloads,suchasdilution,followed bybedsidedosecalculationwhilstprovidingpatientcare. Somedilutionerrorswerecausedbynotknowingthe exactconcentrationafterdilution,whichresultedinone infantreceiving7mgofmorphineinsteadof0.7mg. Administeringfromasyringethatcontainsmorethanthe prescribeddosewasfoundasahigh-riskpractice.Most dilutionerrorsledtooverdosagesandresultedinpatient harm.
ConclusionandRelevance Thisstudydiscusseshowcognitive processingisrelatedtomedicationerrors.Addressinghuman factorsthatcontributedtomedicationerrorsshouldinvolve systemicmeasureswhichtakeinaccounthowhumansthink andprocessinformationtoavoidpatientharmfromdilution errors.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-019 ANALYSISOFTHEUSEOFIDARUCIZUMABINA
CRodriguez-TenreiroRodriguez*,MMuñozVillasur,VGarcíaJiménez,CLFernández Laguna,LMacíaRivas,IdelaFuenteVillaverde,SFernándezLastras,MEiroaOsoro, LOyagueLópez,ALozanoBlázquez. CentralUniversityHospitalofAsturias,Hospital Pharmacy,Oviedo,Spain
10.1136/ejhpharm-2023-eahp.243
BackgroundandImportance Theevaluationofanticoagulation reversalpracticesofdirect-actingoralanticoagulantsallows theiroptimisationbyimprovingtheirsafetyandefficiency.
AimandObjectives Toreviewtheuseofidarucizumabinthe reversaloftheeffectofdabigatranandtoevaluateitseffectivenessinthenormalisationofcoagulationparametersand clinicalevolutionofthepatient.
MaterialandMethods Descriptive,observational,retrospective studyofallpatientswhoreceivedidarucizumabintheperiod fromDecember2015toJune2022,inclusive,inatertiary hospital.Datawerecollectedfromtheelectronicmedical record.Variablesassessedwere:demographics(age,sex);coagulationparameters[activatedpartialthromboplastintime (aPTT)];indicationanddoseofdabigatran;reasonfor
prescriptionanddoseofidarucizumab;responsetotreatment (normalisationofaPTTandclinicalevolution).
Results Fifty-fourpatientsprescribedidarucizumabwereidentified.Onepatientwasexcludedbecauseactivetreatmentwas declined(n=53).Medianagewas82years(RIQ:75-88.5), 58.5%maleand41.5%female.Theindicationfordabigatran wasstrokepreventionandsystemicembolismduetonon-valvularatrialfibrillationin52patientsandstrokein1patient.
Thedosesofdabigatranreportedinthemedicalrecordswere: 150mg/12hin16patients,110mg/12in34patientsand 75mg/12in1patient(nodatain2patients).Thirty-six patientsreceivedidarucizumabformajorbleeding,12for urgentsurgery,3forurgentinvasiveprocedureand2for supratherapeuticlevelsofdabigatran.Inallcasestheindicationwasestablishedbythehaematologydepartment.Median aPTTbeforeantidoteadministrationwas46.95seconds(RIQ: 35.2-52.5)(n=52);1patienthadsupratherapeuticlevelsof dabigatran,showingincoagulable.MedianaPTTafteridarucizumabadministrationwas27.4seconds(RIQ:25-29.8)(no post-administrationaPTTvaluesin6patients).Thedoseof idarucizumabwas5ginallcases.Fourpatientsdied.In49 patientstreatmentwaseffectivewithnoepisodesofrebleeding orthromboembolism.
ConclusionandRelevance Idarucizumabwasmostlyusedin majorbleeding.Treatmentwaseffectivein92%ofthestudy population.
ConflictofInterest Noconflictofinterest
5PSQ-020 ANALYSISOFAPHARMACEUTICALINTERVENTIONIN POLYMEDICATEDPATIENTSTOINCREASETHE SAFETYANDADEQUACYOFTHEIRTREATMENT
BNucete,LGonzalez,AMarchena,MAGarciaLirola*. DistritoSanitarioGranada Metropolitano,Pharmacy,Granada,Spain
10.1136/ejhpharm-2023-eahp.244
BackgroundandImportance Polymedicationhaspotential healthrisksforpatientssuchasinteractionsandincreasedrisk ofadverseeffectsthatcanbefatal.
AimandObjectives Theaimofthisstudywastoanalysea pharmaceuticalinterventioncarriedoutbyagroupofpharmacistsinpatientstaking15ormoredrugsconcomitantlyto improvethesafetyandadequacyoftheirprescriptions.
MaterialandMethods Pre-poststudythatincludedpatientsof anyage,with15ormoredrugprescriptions,prescribedbya generalpractitoners(GPs)intheelectronicprescriptionsystem,fromJanuarytoDecember2021.Theinterventionwas performedby9pharmacistsin35primaryhealth-care centres(PHCC)and673GPs.Theyprovidehealthcareto 677,782inhabitants.First,ageneralsessionwasheldineach PHCC,presentingtheobjectivesandinformativematerial. Subsequently,individualmeetingswerescheduledwitheach physician,inwhichthepharmacistsprovidedtheprescribers withlistsofpolymedicatedpatients(PP)andvariouslocal documents,STOPP/START,Beerscriteriaandclinicalpractice guidelinestohelpreviewtreatments.Eachprescribeddrug wasevaluatedbasedonitsnecessity,effectiveness,appropriatenessandsafety.Inaddition,thepharmacistsalsoissued reviewreportsonpatientswithparticularlycomplexpathologies.ThereviewsperformedwererecordedbytheGPsin thedigitalhealthrecord.TheserecordsandlistsofPPwere
extractedthankstoalocalsoftwareapplicationandanalysed inExcel.
Results Pharmacistsprovided39grouptrainingsessionsand 387individualmeetingstotheGps.Atotalof1468patients metthecriteriaforPP.Meanage73.58years+-11.14(58% women).Prescriptionsof91.7%ofPPwerereviewedatleast oncein2021.Atotalof4,848reviewswereperformed.
In14.41%ofthecases,anewtreatmentwasstarted.In 14.73%oftherevisions,itwasnecessarytochangethedosage ortheprescribedtreatmentregimen.In27.81%ofthecases, theGPscancelledadrugfromthepatient´sprescriptions.In 54.68%ofthereviews,nochangeintreatmentwasmade ConclusionandRelevance Theinterventionhadahighlevelof acceptance.
Despitethehighpercentageofpatientsreviewed,itisstrikingthehighnumberofpatientsinwhom,nochangeintheir treatmentwasmade,whichraisesthequestionofwhetherthe reviewswerecorrect.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-021 AUTOMATIONMEETSTRACEABILITYTOOPTIMISE DRUGSANDMEDICALDEVICESLOGISTICS
GUARANTEEINGPATIENTSAFETYANDHOSPITAL STAFFWELL-BEING
1APiovanelli*, 2DTrojaniello, 3AFusco, 3DBonetti. 1AntaresVisionGroup,Digital Healthcare,TravagliatoBrescia,Italy; 2IrccsOspedaleSanRaffaele,CenterForAdvanced TechnologyInHealthandWellbeing,Milan,Italy; 3AntaresVisionGroup,DigitalHealthcare Department,TravagliatoBrescia,Italy
10.1136/ejhpharm-2023-eahp.245
BackgroundandImportance HospitalSanRaffaelewasseeking asolutiontoimprovethemedicationmanagementprocessand logistics,spanningfromcentralpharmacytothepatient’sbedside,inordertoavoidshortage,improvestaffwell-beingand patientsafetybyensuringthefiverightsofmedicationadministration(patient,drug,dose,timeandroute).
AimandObjectives TheCovidemergency,theshortageofpersonnelandtheneedtocontrolhealthcarespending,arekey driversinseekinginnovativesolutionstoimprovetheefficiencyindrugsandmedicaldeviceslogistics.
Thenewsystemincludesanewgenerationofautomated cartsandcabinets:beforeeachround,thesoftwarepredicts theoverallneedfordrugs/medicaldevices.
Thedrugsareautomaticallyloadedintothecartswithout anyhumanintervention.
Duringtheround,oncethepatientisidentified,theautomatedcartretrievesthedrug(s)tobeadministeredandplaces themdirectlyonthecountertop.
Thesystemtracksalltheoperations:whichdrugwas administered,atwhattimeandbywhom.
Thenewsystemenablesend-to-endtraceabilityensuringthe completevisibilitytothehospitalpharmacists/staffondrug flowsfromthecentralpharmacyuptomedicines’ administrationandbringingmanybenefitssuchas:logisticsoptimisation andinventoryaccuracybyavoidingwasteandshortage,while guaranteeingpreciserecalls/withdrawalsandhavingareal-time visibilityontheentirehospitalstocks.MaterialandMethods
ThestudycomparedthenewAUTOMATEDsolutionversustheTRADITIONALonebymeasuringdifferentmetrics andKPI.
Apanelofnurseswasselectedtoconductthetestwithdifferentprofiles(age,experience,andconfidencewithITapplications).Eachnursewasaskedtocarryonsomecyclesofthe therapydispensingandtoexpressevaluations,inaranking from1to5,onseveralparametersrelatedto:
. Trackingofalloperations
. PatientSafety
. Ergonomics
. Efficiency
Results
Abstract5PSQ-021Figure1
ConclusionandRelevance Theresultsshowthattheautomatic systemisprevailingoveralltargetmetrics,withparticularlya highgaponsafetyandefficiency,thankstothereductionof non-value-addedactivitiessuchasmanualdrugsreplenishment ofthestockswithincabinetsandcarts,enablingwhatreally matters:thePatientCare.
Thisprovidestothehealthcaresystemsanewdisruptive platformthatmakestheworkofhospitalstaffeasier,more efficient,reliablethusensuringpatientsafety.

REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
priortothebeginningoftreatmentandinthe6monthsafter treatmentof5patients(3womenand2men).DatawerecollectedfromtheclinicaldatabaseoftheAndalusianHealthSystem(Diraya).Allpatientshadahighdegreeofcomorbidity andVUswithuncontrolledpain.
Results Thefollowingfigureshowsthenumberoftimesthat patientsgotohealthservicesforuncontrolledpainassociated withvascularulcers.Thereisadecreaseinemergencycare visitsinallthepatientsstudiedafterthestartoftreatment.It shouldbepointedoutthatpatients2,4and5didnothave togotothehealthemergencyservices.
5PSQ-022 ANALYSISOFHEALTHCAREVISITSTOEMERGENCY SERVICESFROMPATIENTSWITHPAINFULVASCULAR ULCERSTREATEDWITHTOPICALSEVOFLURANE
MTGomezSanchez,FDFernandezGines,BSanchezRodriguez,MSanchezValera, TMorenoDiaz*,DGamezTorres. HospitalTorrecardenas,Pharmacy,Almeria,Spain
10.1136/ejhpharm-2023-eahp.246
BackgroundandImportance Patientswithchronicvascular ulcers(VUs)sufferpainthatisfrequentlymanagedwithsystemicanalgesicssuchasopioids,exposingthepatienttothe secondaryeffectsofthesedrugs.Therefore,thereisadecrease inthepatient'squalityoflifeandanincreaseinemergency healthcare.Recently,sevofluranehasbeenshowntohavea rapidanalgesiceffectwhenappliedtopicallyonVUs,providinganewtherapeuticalternativeinpainmanagement.
AimandObjectives Toevaluatetheanalgesiceffectivenessof topicalsevofluraneinpoorlycontrolledVUsusingacomparativeanalysisofemergencyandscheduledhealthcarebefore andafterthebeginningoftreatment.
Abstract5PSQ-022Figure1
ConclusionandRelevance Thisstudyseemstoshowadecrease inemergencyhealthcareafterapplyingtopicalsevofluranedue toitsroleasananalgesicinpatientsrefractorytoconventionaltherapies.Obviously,relevantclinicaltrialsarerequiered toadequatelyestablishtheroleoftopicalsevofluraneinthe painmanagement.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-023 SEVEREPHOTOTOXICITYREACTIONASSOCIATED WITHVANDETANIB:ACASEREPORT
1MDPBriceñoCasado, 2MDGil-Sierra, 2CMorenoRamos*, 3BdelaCalle-Riaguas, 1CMCuadros-Martinez. 1HospitalUniversitariodeJerezdelaFrontera,HospitalPharmacy, JerezdelaFrontera,Spain; 2HospitalUniversitarioPuertoReal,HospitalPharmacy,Puerto Real,Spain; 3HospitalNuestraSeñoraDelPrado,HospitalPharmacy,TalaveradelaReina, Spain
10.1136/ejhpharm-2023-eahp.247
BackgroundandImportance Vandetanibisatyrosinekinase inhibitorusedforthetreatmentofmetastaticmedullarythyroidcancer(MMTC).Thisdrughasbeenassociatedwith phototoxicity,butrarelysevere.
AimandObjectives Toreportacaseofseverephototoxicity reactionassociatedwithvandetanib.
MaterialandMethods
Theclinicalmanagementofacasewith rarephototoxicityadversereactionwasdescribed.Electronic medicalrecordswereusedtocollectpatientdata:baseline clinicalcontext,adverseevents,treatment,andclinicalevolution.Naranjoalgorithmwasusedbyhospitalpharmacistto stablishthecausalityofphototoxicity.
MaterialandMethods
Aretrospectivestudywasdesignedto quantifyurgentandscheduledcarevisitsinthe12months
Results An82-year-oldmannewlydiagnosedwithMMTC startedtreatmentwithvandetanib.After12days,he
presentedslightrashindorsalregion.Thepatientreceived oneradiotherapysession.Seventeendaysafterstartingvandetanib,hevisitedEmerg encydepartmentforgeneralised erythema(onface,neck,upperandlowerlimbs),flushing andpruritus,relatedtobriefsunexposure.Thepatientwas treatedwithsingledoseintramuscularmethylprednisolone andoraldexchlorpheniramine. Vandetanibandradiotherapy werediscontinued.Fivedayslater,thepatientpresented severedeterioration,progressi onoferythemaandintense oedemainhands,feetandface.Deflazacortwasprescribed. AfterdiagnosisbyDermatologydepartmentofacutephototoxiceruption,treatmentwasstartedwithprednisone45 mg/dayfor7dayswithprogressivedecrease,emollientsand topicalmethylprednisolone .Betweendays26-40,gradual improvementofoedemaanderythemawasobservedwithoutappearanceofnewtoxicity.Prednisonedosewas reduced.Progressively,des quamationandscabswere observedonbothhands,withimprovementoflegandfoot ulcers.Poorpaincontrol requiredtapentadol25mg/12 hours.Onday62,therewasaworseningwithincreased erythemasinceoralprednisonewasreduced.Treatmentwith Polypodiumleucotomos ,vitaminD,CandEwasinitiated. Onday68,therewasasignificantimprovementwithno itchiness.Threemonthsaftersymptomonset,itchingand erythemahadalmostdisappeared.Remaininghyperpigmentationoftheskinwasobserved.Naranjo'salgorithmdeterminedaprobablerelationsh ip(score5)andreintroduction ofvandetanibwasdiscouraged.
ConclusionandRelevance Hospitalpharmacistdetermineda probablerelationshipbetweenvandetanibandseverephototoxicityreactioninapatientwithMMTC.Theroleofhospital pharmacistsisessentialinpharmacovigilanceandininforming patientsaboutpossibleadverseeventsofdrugs.
ConflictofInterest Noconflictofinterest
5PSQ-024 HAZARDVULNERABILITYANALYSIS(HVA): EVALUATIONOFRISKINEXPERIMENTAL ONCOLOGICALDRUGSCOMPOUNDING
1GCancellieri*, 1MSantonocito, 1EdeLuca, 1CBotto, 2RGiammona, 2PPolidori. 1UniversitàDegliStudiDiPalermo,Ssfo – ScuolaDiSpecializzazioneInFarmacia Ospedaliera,Palermo,Italy; 2OspedaliRiunitiVillaSofia – Cervello,UocFarmacia,Palermo, Italy
10.1136/ejhpharm-2023-eahp.248
BackgroundandImportance Variousclinicaltrials,especiallyin oncologyandhaematology,involvechemiotherapicdrugscompounding.Thesepreparationsrequirestandardworkingproceduresforwhichhospitalpharmacistisresponsible.Oncological drugsusedinclinicaltrialsarecharacterisedby:lowtherapeuticindex;unknowntoxicity;dosagetobepersonalisedon patient;assignmentofnumberkit/placebotospecificpatient; associationswithotherdrugsnotknowninconsolidatedclinicalpractice.Alltheseelementscancontributetotheoccurrenceofpotentialerrors.
AimandObjectives TheaimofthisstudyistousedHazard VulnerabilityAnalysisinordertoclassify,intohigh,medium, lowrisk,experimentalprotocolsthatprovideforchemiotherapicdrugscompoundingandwhicharecurrentlyactiveour hospital.Forprotocolsclassifiedashighrisk,standardprocedureswillbeoutlinedtominimiserisks.
MaterialandMethods Inordertodeterminethepercentage risk(R%),iscalculated:probability(P)thatanerrorwilloccur, bycalculatingthenumberofpreparation-phases;magnitude (MA)bycalculatingcarcinogenicity,storagetimeofpreparationandchemicalincompatibilitybetweendrugsandmedical devices;mitigation(MI)bycalculatingdrugdosage,chemicalphysicalpreparationstability,possibleuseofsafety-devices.By applyingtheformulaR%=(P/3)*[(MA+MI)/18]*100,protocols aredefinedlow-riskifR%<30%,moderate-riskif30%£R%£ 60%,High-riskifR%>60%.
Results Among35activeclinical-trialsanalysed,18require chemiotherapicdrugscompounding.For33%(6/18)ofprotocolstheprobabilityislow;50%(9/18)ismoderate;17%(3/18) ishigh.For44%(8/18)ofprotocolsthemagnitudeislow; 50%(9/18)ismoderate;6%(1/18)ishigh.Finally,for6%(1/ 18)ofprotocolsthemitigationislow;88%(16/18)ismoderate;6%(1/18)ishigh.Byapplyingtheformulatocalculate percentageriskitwasfoundthat5/18protocolsarelowrisk, 10/18moderaterisk,3/18highrisk.
ConclusionandRelevance HVAprovidesasystematic approachtoanalysinghazardsthatmayaffecthospital service.Clinicalprotocolsclassifiedas ‘ highrisk ’ have beenmonitored,andstandardprocedureshavebeenoutlinedtominimisetherisks(e.g.proceduresformanaging vialaccidentalbrea king,coldchaincontrolforprepared drugs,useofsoftwaretocalculatedrugdosagebasedon bodysurface).Theseproceduresareaimedatallpersonnel involvedinpreparationphase,includingthehospitalpharmacist.Hospitalpharmacistiscoordinateswholeprocess, dealswithriskmanagementandensurespersonnel/patients safety.
ConflictofInterest Noconflictofinterest.
5PSQ-025 IMPACTANDEVALUATIONOFPHARMACOKINETIC MONITORINGINPRIMARYCARE
1GSJuanAntonio, 2ICJavier*, 2FGLydia, 2TBPaula, 2HSMaria, 2CSMiguelAngel, 2PLPilar, 2GDMaria, 2MOAdrian, 2MRNoemi. 1HospitalUniversitarioMoralesMeseguer, PharmacyService,Murcia,Spain; 2HospitalMoralesMeseguer,PharmacyService,Murcia, Spain
10.1136/ejhpharm-2023-eahp.249
BackgroundandImportance Monitoringofnarrow-margin drugsinprimarycareisimportanttooptimisetheefficacy andsafetyoftreatment.
AimandObjectives Toanalysetheimpactoftheactivityand repercussionsofmonitoringplasmalevelsofantiepileptics, lithiumanddigoxininprimarycarepatientscarriedoutby thePharmacokineticsArea-HospitalPharmacyService(PAHPS).
MaterialandMethods Two-monthretrospectiveobservational studyofthepharmacokineticreportsofallpatientswho requiredmonitoringoftheirplasmalevels.Thecircuitstarts witharequestfromtheprimarycarephysicianaskingforthe determinationoftheplasmalevel,thebloodsampleisanalysedbythelaboratoryandthePA-HPSinterpretsallthedata fromtheclinicalhistory,finallyproducingapharmacokinetic reportintegratedintheclinicalhistorytogetherwiththe analytical.
Thevariablesrecordedfromtheanalysesandclinicalhistorywere:age,sex,renalclearance,liverenzymes(GOT,GPT
REFERENCESAND/ORACKNOWLEDGEMENTSandgamma),monitoreddrugandplasmalevel,pharmacokineticreportsandtheirdegreeofacceptance.
Results Atotalof202pharmacokineticreportswereperformedtargeting191ambulatorypatients.Themeanageof thetotalwas42.33±16.46years(range:6-106)and51% werefemale.Only5patientshadestablishedrenalinsufficiencywithrenalclearance<60ml/minand3patientswith hepaticinsufficiency(liverenzymesgreaterthan3timesthe upperlimitofnormal).
Thepharmacokineticreportsproducedwerevalproic (43.56%),lithium(37.62%),carbamazepine(8.91%),digoxin (5.94%),phenytoin(2.47%)andphenobarbital(1.48%).Of thepatients,82.68%hadplasmalevelsintherapeuticrange, 14.85%weresubtherapeuticand2.47%weresupratherapeutic.Wehighlightadegreeofinterventionin17.32%ofthe pharmacokineticreportsmade,and10.93%ofthesereports requiredachangeinthedosingregimenordosinginterval togetherwithanewmonitoring.Thedegreeofacceptanceby thephysicianwas67%.
ConclusionandRelevance Itisimportanttoperforman adequatefollow-upofpatientswithactivetreatmentofdrugs withanarrowtherapeuticmarginforaconstantoptimisation ofthetreatment
Thedatareflecttheimportanceofthehospitalpharmacist aspartofthemultidisciplinaryteamandtheneedfordirect communicationwiththeprimarycarephysician.
Thehighdegreeofacceptanceofpharmacokineticreports showsthatthecircuitiswellreceived.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
5PSQ-026 MEDICATION-RELATEDOSTEONECROSISOFTHEJAWS AND CDK4/6INHIBITORS
1LDomínguezSenín, 2DMoralesPancorbo, 2MYRodríguezGarcés, 1MRodriguezJorge*, 1MDSantos-Rubio, 2JBayoCalero. 1HospitalJuanRamónJiménez,HospitalPharmacy, Huelva,Spain; 2HospitalJuanRamónJiménez,MedicalOncology,Huelva,Spain
10.1136/ejhpharm-2023-eahp.250
BackgroundandImportance Medication-relatedosteonecrosis ofthejaw(MRONJ)isarelativelyuncommonbutserious complicationofosteoclastinhibitorstherapywithintravenousbisphosphonatesandden osumab.Dose, schedule,and durationofinhibitionareassociatedwithMRONJrisk. Marcianòetal. 1 launchedanalertaboutapossibleassociationbetweenMRONJandcyclin-dependentkinase(CDK)4/ 6inhibitorsinbreastcancerpatientswithosteoclastinhibitorstherapy.
AimandObjectives EvaluatetheuseofCDK4/6inhibitorsas ariskfactorforMRONJinourcohortofpatientswithmetastaticcanceranddenosumab.
Retrospectiveobservationalstudy. Allpatientswithdenosumab (January2011toFebruary2022)wereincluded.Casesof MRONJfoundweredescribed.RelationshipbetweenCDK4/6 inhibitorsandMRONJwasanalysedwithaChi-square analysis.
Results 363patientswithdenosumabwereincluded.21cases ofMRONJweredetected:62.5%women,57.1%(12/21) withbreastcancer,19%(4/21)prostatecancer,and9.5%(2/ 21)lungcancer.42.9%withextraosseousmetastases.Median treatmentdurationfordenosumabwas19months(1-52).7
withCDK4/6inhibitors(3palbociclib,2abemacicliband2 ribociclib).MediantreatmentdurationwithCDK4/6inhibitors was27months(10-35).Themeantimefromthestartof denosumabtotheappearanceoftheeventwas23months (16-29).
Incidenceofthiscomplicationinpatientstreatedwith denosumabbutwithoutCDK4/6inhibitorswas5.24%(14/ 267)and7.29%(7/96)inpatientswithdenosumabanda CDK4/6inhibitor.AlthoughthegroupwithCDK4/6inhibitors hadahigherincidenceofMRONJcases,thedifferencewas notsignificant(0.461).
ConclusionandRelevance TheincidenceofMRONJinour cohortofpatientswithmetastaticcanceranddenosumabwas higherinthegroupofpatientswithCDK4/6inhibitors.However,thisdifferencewasnotsignificant.Ourdataaresomewhathigherthanthosereportedintheliteratureaccordingto whichtheriskofMRONJwithdenosumabis1.1%during thefirstyear,3.7%thesecondyearand4.6%peryearthereafter.StudieswithmorepatientswouldbenecessarytoconfirmtherelationshipbetweentheuseofCDK4/6inhibitors andMRONJ.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.Marcianò,A.;Guzzo,G.M.;Peditto,M.;Picone,A.;Oteri,G.Medication-Related OsteonecrosisoftheJawsandCDK4/6Inhibitors:ARecentAssociation.Int.J.Environ.Res.PublicHealth2020,17,9509.
ConflictofInterest Noconflictofinterest.
5PSQ-027 APPROPRIATENESSOFPRESCRIPTIONOFTRICYCLIC ANTIDEPRESSANTSACCORDINGTO STOPP CRITERIA: SYSTEMATICREVIEWOFTHECRITERIAREFERREDTO THEUSEOFTRICYCLICANTIDEPRESSIVESIN DEMENTIA
DGuerraEstévez*,MRomeroAlonso,CPalomoPalomo,MReyesMalia,EContreras Macías,AGanforninaAndrades,JEstaireGutiérrez. HospitalInfantaElena,Hospital PharmacyDepartment,Huelva,Spain
10.1136/ejhpharm-2023-eahp.251
BackgroundandImportance TheSTOPP-STARTcriteriaarea usefultooltodetectpotentiallyinappropriateprescriptions (PPIs).Fortricyclicantidepressants(TCAs),thereare6STOPP criteria.
AimandObjectives Toanalysetheadequacyaccordingtothe STOPPcriteriaoftheprescriptionofTCAsinpatientsolder than64yearsandtosystematicallyreviewtheliterature relatedtotheuseofTCAsinpatientswithdementia,analysingthesuitabilityoftheSTOPPcriteria.
MaterialandMethods Descriptivecross-sectionalstudythat includedallpatientsover65yearsofagereceivingTCAs. ThesystematicreviewwasconductedfollowingthePRISMA Declaration.
Results 63patients(50women)withamedianageof70 years(65-88)werereviewed.In21patients(33.3%),the prescriptionofTCAsaccordingtotheSTOPPcriteriawas notappropriate(9patientsreceivedconcomitanttreatment withopiates,4patientsdementiamedication,3hadprescribedcalciumantagonists,another3medicationforbenign prostatichyperplasiaand,finally,2forconstipation).Nosignificantdifferenceswerefoundintherelationshipbetween thenumberofprescribeddrugsandtheadequacyofthe TCAsprescription(p=0.74).Inthesystematicreview,
7articleswereincluded.Onestudyshowedthatinclinical practice,TCAsdispensationsweremaintainedafterthediagnosisofdementia.TwostudiesconcludedthatTCAsarethe antidepressantsleastassociatedwiththeonsetofdementia. Inanotherstudy,thelong-termuseofTCAswasassociated withadecreaseintheincidenceofdementia.Areviewby theCochraneGroupstatedthattheevidenceonthesafety ofantidepressantsinpatientswithdementiaisofmoderate quality,withlittledatafromtheantidepressantsubgroups. Thelasttwoarticlesassociatedtheuseofantidepressants withdementiawithoutdifferentiatingtheantidepressant groups.
ConclusionandRelevance Basedonthedatafromourpopulation,thehighinappropriatenessofTCAsprescriptionaccordingtotheSTOPPcriteriasuggeststhatthisisafieldwith ampleroomforimprovement.PPIscouldbereducedif STOPPcriteriawerecomputerisedinelectronicprescription programs.Sincetheresultsofthereviewarenotconsistent, webelievethattheSTOPPcriteriaregardingtheuseofTCAs inpatientswithdementiashouldbemoreflexible,assessing thebenefit-riskoftreatmentonanindividualbasisandclosely monitoringadverseeffects.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
Results 35patientswereincluded(100%male),medianage 53years(31-72),97.6%tookonetabletdaily,mediandisease evolution17years(0.5-33).
4telematicconsultationswerecarriedoutwitheach patient.
GlobalIEXPAC: 27patientshadabetterexperience,8 remainedthesame.ConditionalIEXPAC:30patientshada betterexperienceand5remainedthesame.TheWilcoxon testcomparedtheresultsofIEXPACbeforeandafterimplementingatelepharmacyprogramme(p<0.01).
ConclusionandRelevance Theimplementationoftelepharmacy programmesimprovestheexperienceperceivedbyHIV patientsofpharmaceuticalcare.
Telepharmacycouldbeausefultoolforthecontroland pharmacotherapeuticfollow-upofHIVpatientsandother pathologies,avoidingunnecessarytripsbyvulnerablepatients whohavedifficultyingoingtothehospital.
ConflictofInterest Noconflictofinterest
5PSQ-030 SAFETYANDSECURITYOFCICLOSPORINEYEDROPS INPATIENTSWITHXEROPHTHALMIA
MHerreraExpósito*,JIBretonesPedrinaci,JUrdaRomacho,MACastroVida. Hospitalde Poniente,HospitalPharmacy,ElEjido-Almería,Spain
10.1136/ejhpharm-2023-eahp.253
5PSQ-029 PERCEIVEDEXPERIENCEOFPATIENTSWITHHUMAN IMMUNODEFICIENCYVIRUS(HIV)AFTER IMPLEMENTINGATELEPHARMACYPROGRAMME
XCasas*,AVélezBlanco,LOrtegaValín,EGutiérrezGutiérrez,MSáezVillafañe,SLlamas Lorenzana,ZRodríguezFernández,RVarelaFernández,JCSáezHortelano,JOrtizde Urbina. ComplejoAsistencialUniversitariodeLeón,Farmacia,Leon,Spain
10.1136/ejhpharm-2023-eahp.252
BackgroundandImportance Telepharmacypromotescontinuous andqualityhealthcarebasedontheuseofnewtechnologies.
Usefulinpatientswithchronicdiseasesthatrequireapharmacovigilanceprogramme,suchasHIVpatients.
AimandObjectives Todetermineifatelepharmacymodel, improvestheperceivedHIVpatientexperiencecomparedtoa traditional(face-to-face)modelofhealthcare.
MaterialandMethods Prospectiveobservationalinterventional study(JanuarytoAugust2022).Included35HIVpatients withantiretroviraltreatment(ART)oflegalageunderfollowupbythepharmacist,withaccesstotechnologiestoreceive telepharmacyassistanceandwhogavetheirconsent.
Thestudywasdividedinto2stages:T-4pre-implementationoftelepharmacy(JanuarytoApril2022),T+4post-telepharmacy(MaytoAugust2022).
PatientswererecruitedduringtheT-4periodinthepharmaceuticalcareoffice,wheretheyweregiventhequestionnaire:InstrumentfortheEvaluationofChronicPatient eXperience(IEXPAC),a15-itemquestionnairewith11global questionsand4conditionalquestions,whichmakesitpossible toassessthepatient’sperceivedexperienceofhealthcare.
TheSPSS®programandWilcoxontestassessedwhether therearedifferencesintheIEXPAC(globalandconditional) inthesamepopulationbeforeandafterimplementingatelepharmacyprogramme.
Otherstratificationdatawere: sex,age,timesincediagnosis andnumberoftabletsperday.
BackgroundandImportance Ciclosporin1mg/mleyedropsis indicatedfortheuseofxerophthalmiainpatientswithsevere keratitisunresponsivetoartificialtears.Oculardrynessisa refractorysymptomofmanysystemicpathologies.Itisdifficulttomanageclinicallyandtherapeuticoptionsarelimited.
AimandObjectives Toreviewthetoleranceofpatientsto cyclosporine1mg/mleyedrops,aswellastherateofassociatedeyeinfectionsandthefeelingofimprovementevaluated bythepatienthimself.
MaterialandMethods Retrospectivestudycarriedoutina 350-bedgeneralhospital.Patientswhohadstartedtreatment withcyclosporine1mg/mleyedropsfrom2018to2022 andwhohadbeendiagnosedwithkeratoconjunctivitissicca (KS),Sjögren ’ ssyndrome(SS),Graves-Basedowsyndrome (GBS)withxerophthalmiawerestudied.Datacollected:sex, medianage[range],pathology,positiveSchirmertest(<5 mm),associatedeyeinfectionsduringtreatment,treatment oftheseinfections,discon tinuationofcyclosporinedueto infections,tolerancetotreatment,discontinuationdueto poortoleranceandclinicalimprovementperceivedbythe patient.Dataobtainedfromthedigitalmedicalrecord,the assistedelectronicprescriptionprogram(Dominion ® )and theclinicalinterviewwiththepatientinthepharmacy consultation Results 37patients.25women(67.57%).Medianage46[475].PatientswithSS14(37.84%),KS19(51.5%),GBS4 (10.81%).All(100%)ofthemwithpositiveSchirmertest(< 5mm).Associatedeyeinfectionsduringtreatment11 (29.73%),needforantibiotictreatment9(24.32%).Patients wholeftthetreatmentforanycircumstance20(54.05%),due topoortolerance14(37.84%).Patientsthatperceivedclinical improvement21(56.77%).
ConclusionandRelevance Xerophthalmiaisahardtocontrol symptominsystemicpathologies.Treatmentwithcyclosporine eyedropsisanalternativeforthosepatients.Somedonot
toleratethedrugcorrectlyanditisnecessarytoresortto othertreatmentstrategies.Associatedinfectionscouldbea riskfactorfordiscontinuingcyclosporineeyedrops,buteach patientmustbeevaluatedindividuallyandcloselymonitored forpossiblecomplicationsthatmayarisefromtreatment.The responsetociclosporintreatmentimprovedpatient’slife quality.
ConflictofInterest Noconflictofinterest.
1EMartinez*, 2ICorredor, 1PTardaguila, 1CDean, 1GICasarrubios, 1AMiranda, 1ACodonal, 1AMHorta. 1HospitalUniversitariodeGuadalajara,HospitalPharmacy, Guadalajara,Spain; 2HospitalUniversitariodeGuadalajara,HospitalEmergency Department,Guadalajara,Spain
10.1136/ejhpharm-2023-eahp.254
BackgroundandImportance Hospitalpharmacists’ activityis turningtowardsthedirectcareonclinicalunits.InEmergency Department(ED),medicationerrors(ME)mayoccurdueto multiplefactors:lackofcoordinationbetweenservicesorpressureinmedicalcare.Numerousstudies,highlightthebenefit ofpharmacistinterventioninthemultidisciplinaryhealth team.
AimandObjectives Theaimofthisstudywastoanalysepharmaceuticalinterventions(PIs)carriedoutinED,studiedthe ATCgroupofdrugsinvolvedandevaluatemedical acceptance.
MaterialandMethods Thistwomonth(April-May2022)prospectivestudywascarriedoutintheHalf-StayUnit(HSU)of theEDinasecondlevelhospital.
Inclusioncriteria: age 65yearsandpolypharmacy( 5 drugsinchronictreatment).
Variablescollected: demographic,PIs,causeofPIs,medical acceptanceandATCgroupofdrugsinvolved.
Dailylistofpatientswasobtainedthroughtheelectronic prescriptionprogramandPIswerenotifiedon-siteorusing thisprogram.
PIswereclassifiedaccordingtothesystemoftheConsensusofGranadamodifiedindrugdiscontinuation (unnecessary/duplicity/contraindication/interaction),drug change(contraindication/interaction),changeofdose,frequencyorschedule,initiationoftreatment(usualtreatment notprescribed/needadditionaltreatment),monitoring(determinationofplasmadruglevels andfollow-up)andprescriptionerrors.
PIswereconsideredacceptedwhendoctormodifiedtreatmentinmedicalorderordischargereport.
Results Finalanalysesincluded52patients.Medianagewas 82years(IQR:68-88),58%men.Duringthestudyperiod, 120PIswereperformedandthe77%wereaccepted.
46%ofPIscorrespondedtoinitiationoftreatment(usual treatmentnotprescribed),15%todiscontinuation(unnecessary drug),15%tochangeindosage,frequencyorschedule,14% toprescriptionerrorsand10%others.
ATCgroupsmostfrequentlyinvolvedwereCgroup(cardiovascularsystem)(35%)Bgroup(bloodandbloodforming organs)(25%)andNgroup(nervoussystem)(20%).
ConclusionandRelevance MostofPIscorrespondedtoinitiationofusualnon-prescribedtreatmentfollowedbydiscontinuationofunnecessarydrugs.
Medicalacceptancewashigh. HighlightPIscarriedoutaround groupC(lipid-loweringandantihypertensivedrugs).
Multidisciplinaryteamhelpsimprovepharmacotherapeutic profileandpatientsafety.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
5PSQ-033 ANTIVIRALTREATMENTDISCONTINUATIONIN PATIENTSWITHHEPATITISB
EMartinez*,SCorrales,CDean,ACodonal,PTardaguila,GICasarrubios,AMiranda, ALazaro. HospitalUniversitariodeGuadalajara,HospitalPharmacy,Guadalajara,Spain 10.1136/ejhpharm-2023-eahp.255
BackgroundandImportance Studiessuggestthesafeststrategy oftreatmentdiscontinuationwithnucleos(t)ideanalogues (NAs)againsthepatitisBvirus(HBV),isproposedafterloss surfaceantigen(HBsAg).Evidencesupportsthepossibilityof discontinuingNAsinthefollowingsituations:
. Patientswithpositiveeantigen(HBeAg)withoutcirrhosis: afternegativisationofHBV-DNAandHBeAgseroconversion, confirmedin2determinationsseparatedby3-6monthsand afterNAsatleast12months.
. PatientswithnegativeHBeAg,withoutadvancedfibrosisearly intreatment:afternegativisationofHBV-DNAforatleast3 yearsandHBsAgclearance(qHBsAg) £1000IU/mL.
AimandObjectives TheobjectivewastocharacterisethepopulationintreatmentwithNAsandanalysepatientswhomet requirementsfortreatmentdiscontinuation
MaterialandMethods Cross-sectional,descriptive,retrospective studyofpatientsunderactivetreatmentwithNAsbetween August2020-August2021.
Variablescollected: demographic,NAsused,treatmentdurationandclinical(positiveornegativeHBeAg,HBeAgseroconversion,HBV-DNA,qHBsAg,degreeofhepaticfibrosis, HBsAgloss,virologicalrelapse(RV)(HBV-DNA>2000IU/ml aftertreatmentdiscontinuation).
Results Weincluded50patients(70%men).Medianagewas 56years(IQR:48-66)andmedianoftreatmentdurationwas 66months(IQR:27-108).62%weretreatedwithtenofovir disoproxilfumaratoand38%withentecavir.
8%ofpatientshadpositiveHBeAgwithoutseroconversion andwithoutnegativeHBV-DNA.92%hadnegativeHBeAg withseroconversionandnegativeDNA-HBV.
32%ofpatientshadqHBsAg £1000IU/ml,28% 1000 IU/mland40%notdetermined.30%ofpatientshad advancedfibrosis.
In12%ofpatientswithpositiveHBsAg,treatmentdiscontinuationcouldbeconsidered.AllofthemhadHBeAgnegative,fibrosisF0-F1atthebeginningoftreatment,negative HBV-DNAmaintainedatleast3yearsandqHBsAg£1000IU/ ml.
HBsAglossoccurredin6%ofpatientswhohadnotdiscontinuedtreatmentand16%ofpatientshadtorestarttreatmentforRV.
. Studypopulationincludespatientswhomeetcriteriafor treatmentdiscontinuation.
. Treatmentdiscontinuationrequiresclosefollow-uptodetect RV.
. InpatientswithHBsAgloss,treatmentwasnotdiscontinued duetoadvancedfibrosis.
wouldreceive100mgdose,whichmeanshalfpriceofthe fixeddose)(Moniruletal,2020).
5PSQ-036 MEDICATIONERRORSRELATEDTOHIGH-ALERT MEDICATIONSINATERTIARYCAREPAEDIATRIC HOSPITAL – ANANALYSISOFREGISTER-BASEDDATA
1SKuitunen*, 2MSaksa, 2JTuomisto, 2ARHolmström. 1HelsinkiUniversityHospitalHus, HusPharmacy,Helsinki,Finland; 2UniversityofHelsinki,FacultyofPharmacy,Helsinki, Finland
5PSQ-034 FIXEDVSWEIGHT-BASEDDOSINGOF PEMBROLIZUMABFORPATIENTSUNDER80KG, BASEDONOBSERVEDADRSINONECANCER SETTING
MFortuna*,MKovačevič InstituteofOncology-Ljubljana-Slovenia,Pharmacy,Ljubljana, Slovenia
10.1136/ejhpharm-2023-eahp.256
BackgroundandImportance Monoclonalantibodiesareusually dosedbythekilogrammeofthepatient’ sweight,duetoperceivedcontributionofbodysizeinpharmacokineticvariability.Lately,especiallyduringtheCOVIDpandemic,alotof monoclonalantibodies,includingpembrolizumab,were switchedtofixeddosing.Pembrolizumabwasinitiallydosed at2mg/kg,fixeddosingincludes200mgeverythreeweeks or400mgeverysixweeks(Freshwater,2017).Thefixed dosingismoreconvenient,eliminatesthewaste,might improvepatient ’scomplianceandreducesdosingerrors. However,forthepatientsthatweightlessthan80kg,fixed doseisassociatedwithalmostmaximumexposure,whichis alsoassociatedwithgreateroccurrenceoftoxicity(CADTH, 2020).
AimandObjectives Theaimofthisretrospectivestudywasto determinewhetheritisbettertousefixedorweight-based dosingofpembrolizumabforpatientsunder80kginorderto avoidseriousADRs.
MaterialandMethods WeobservedADRsthatoccurredwith 391patientsreceivingpembrolizumabin2021,regardlessthe diagnosis.Wecollectedthedata,byreviewingpatients’ documentation.Thepatientsweredistributedacrossoncologyindications,includingNSCLC,melanoma,breastcancer,urothelial carcinoma,cervicalcancer,Hodgkin’slymphoma,headand necksquamouscellcarcinoma,oesophagealcancerandrenal cellcarcinoma.
Results Thepatientsweresplitintotwosubgroups,underand over80kginweight(group1and2).For29patients,data aboutweightwasnotavailable.198patientswereingroup1, whereasingroup2therewere164patients.TheADRs occurredin69patients(34,8%)fromgroup1and46patients fromgroup2(26%).ThemostcommonADRsoccurredwere skintoxicities,hypothyreosis,muscleandjointpain,diarrhoea andfatigue.Therewerenosignificantdifferencesinthe occurredADRsbetweengroup1and2.
ConclusionandRelevance Theresultsindicatedthatfor patientsunder80kg,weight-baseddosewouldnotonlybe betterintermsoflesstoxicity,butitwouldalsobemorecost effective.Theadaptationoffixeddosingregimenswouldlead totheestimated26%ofadditionalcost(e.g.50kgpatient
10.1136/ejhpharm-2023-eahp.257
BackgroundandImportance Paediatricpatientsareproneto adversedrugevents,includingmedicationerrors(MEs). Althoughhigh-alertmedicationsareoftenassociatedwithseriousMEs(1),fewerstudieshavefocusedondescribingthese errorswithinpaediatricpopulations(2–3).
AimandObjectives Theaimofthisstudywastoinvestigate theprevalenceandcharacteristicsofself-reportedMEsrelated tohigh-alertmedicationsinapaediatricuniversityhospital setting.
MaterialandMethods Thiswasacross-sectionalstudyofselfreportedMEs(n=2,404)inatertiarycarepaediatrichospital during2018–2020;743(31%)oftheMEsinvolvedhigh-alert medications(3).Aquantitativedescriptiveanalysis(frequencies andpercentages)wasperformedusingMicrosoftExcel®.The prevalenceofdifferenthigh-alertmedications,Anatomical TherapeuticChemical(ATC)groups,drugformulationsand administrationroutesappearinginthestudysamplewere defined.Finally,themostsevereMEswereidentifiedand summarised.
Results AmongthestudiedsampleofMEreports(n=743),71 differenthigh-alertmedicationswereidentified.Themost commonATCsubgroupswerebloodsubstitutesandperfusion solutions(B05;n=345,40%)antineoplasticagents(L01; n=139,16%),andanalgesics(N02;n=98,11%).Themost commonmedicationscomprisedparenteralnutrition(n=130, 15%),hypertonicsodiumchloride(n=93,11%),potassium chlorideconcentrate(n=66,8%),morphine(n=47,5%),and heparin(n=43,5%).Mosthigh-alertmedicationswereadministeredintravenously(n=636,73%).Moreover,IVpreparationswereadministeredviaoff-labelroutes(n=52,6%),such asoral,inhalationandintranasalroutes.MostseriousMEs (n=16,2%)wereassociatedwithanalgesics(N02)(n=8),antineoplasticagents(L01)(n=3),andantithromboticagents (B01)(n=3).
ConclusionandRelevance Accordingtothepresentandpreviousstudies,MEsonconcentratedelectrolytesandparenteral nutritionrepresentacentralrisktopaediatricmedication safety(1–2).WhilesevereMEsinthesegroupsremainedlow inthisstudy,ahighproportionofsevereMEsassociatedwith analgesicsandantineoplasticagentsrepresentedakeyfinding. Preventiveriskmanagementactionsshouldbetargetedon thesehigh-alertmedicationsaswellastosecuresafetyin intravenousadministrationandoff-labeldruguseinpaediatric patients.
2.Stavroudis, etal.JPerinatol2010;30(7):459–68
3.InstituteforSafeMedicationPractices.ISMPlistofHigh-AlertMedicationsinAcute
ConflictofInterest Noconflictofinterest.
1MVaraUrruchua*, 2CVilaGallego, 2MInclanConde, 2BBelioAguera, 2NGomez Echevarria, 2ZPerezEspaña, 2NMPardoSantos, 2AVAguirrezabalaArredondo. 1Basurto Hospital,Pharmacy,Bilbao,Spain; 2HospitalBasurto,Farmacia,Bilbao,Spain
10.1136/ejhpharm-2023-eahp.258
BackgroundandImportance Medicationreconciliationisakey tooltoincreasepatientsafetyandcanbeveryusefulinthe polymedicatedelderly.
AimandObjectives Theaimofthestudyistoassessthe impactofreconciliationandtoidentifyandpreventreconciliationerrors(CE)inpolymedicatedelderly.
MaterialandMethods Aprospectivestudywasconductedconsistinginamedicationreconciliationprojectatdischarge,with apharmacistincharge,inpatientsadmittedtothecareof MDIduringtheperiodOctobertoDecember2021.Patients over65yearsofageandpolymedicated(>5prescribed drugs)wereincluded.
Thepharmacistinchargewasresponsibleforcomparing themedicationprescribedintheupdatede-prescriptionwith thehospitale-prescriptionandthedischargemedicalreport. Herecorded,evaluatedandclassifiedthe ‘reconciliation errors ’,thosethatweremodifiedbytheprescriberafterthe pharmacist'swarningaccordingtotheSEFHConsensusDocumentonterminologyandclassificationinmedication reconciliation.
WeclassifiedtheCEsintoseven:dosagediscrepancy,omissionintheprescription,commission,prescriptionofadrug notindicatedorcontraindicatedduetothepatient'sclinical situation,incompleteprescriptionandduplicity.
Results Dischargereconciliationwasperformedin113 patients,51%ofwhomwerewomen.Themeanagewas82.4 years(62-100).Themeannumberofdrugsprescribedper patientwas11(5-22).Sixty-nine(61%)unjustifieddiscrepanciesweredetectedofwhich57(50%)werereportedtothe responsiblephysicianand50wereaccepted,88%ofthe reporteddiscrepanciesand72%ofthetotal,i.e.CEoccurred in72%ofthereconciledpatients.
Regardingtheclassificationofdiscrepanciesdetected,the majoritywereposologicaldiscrepanciesconstituting39.1% (27);followedby27.5%(19)maintainingadrugnotindicatedorcontraindicatedforthecurrentclinicalsituation; omissionofdrugsin18.8%(13);commission8.7%(6), incompleteprescription4.3%(3)andduplicity1.4%(1).
ConclusionandRelevance Pharmacotherapeuticreconciliation resultedinasignificantreductionintheincidenceofCDand itsimpact,constitutingastrategytoimprovesafetyinpolymedicatedelderlypatients.Thepresenceofapharmacistonthe hospitalwardisveryusefultocarryoutthistask,improving communicationbetweenprofessionalsandcontributingtoa moreeffectivereconciliation.
1JAna*, 1MPedemonte, 1CSocias, 1MSantos, 2MPuig, 1MAMangues, 1JRuiz. 1Hospital delaSantaCreuISantPau,Pharmacy,Barcelona,Spain; 2HospitaldelaSantaCreuISant Pau,Emergency,Barcelona,Spain
10.1136/ejhpharm-2023-eahp.259
BackgroundandImportance Drugrelatedproblems(DRPs) areoneofthemaincausesrequiringassistancetotheEmergencyDepartment(ED)infrailtypeople.Manyofthese patientsliveinnursinghomes(NH).IdentifyingthedifferentialcharacteristicsofpatientsandDRPsthatcauseconsultationinthissubgroup,canhelptoimprovethe pharmaceuticalcareprogramsimplementedinourenvironmentinNH.
AimandObjectives Toidentifydrugsthatareassociatedwith DRPsthatcausesconsultationofEDinpatientscomingfrom NHandcomparethedrugsinvolved,andthecharacteristics andcomorbiditiesofthesepatientswithnon-NHpatients.
MaterialandMethods Retrospective,descriptiveobservational studywasconductedbetweenFebruary21-May2022inthe EDofauniversityhospital.Weincludedadultpatientswho attendedEDforDRPs.
Thefollowingvariableswerecollectedandcompared betweenNHpatientsandnoNHpatients:age,sex,chronic pathologiesatadmission,numberofdrugsprescribedinthe electronicprescription,druginvolvedintheDRPsanddiagnosisrelatedtotheDRPs.
QualitativevariableshavebeencomparedbetweentheNH patientsvsnoNHpatientsusingtheChi-Squaretestand quantitativevariablesusingtheindependentdatat-test. Results 1029patientswereincluded.98ofthem(9.53%) werereferredfromNHnhpatientswereolder(84,6(8.9) yearsoldvs77,1(15.7)P<0.001*),mostlywomen[64 (65.3%)vs511(54.8%)P=0 ,046*],withahigherpercentageofcognitiveimpairment[59(60.2%)vs189(20.0%) P<0.001*],severefunctionaldep endence[68(69.3)vs216 (23.2)P<0.001*]and severepolypharmacy(>=10home medications)[53(54.0%)v s276(29.6%)P<0.001]than therestofthepatientswhoconsultedtheEDforDRPs. DRPsrelatedtotheATCgroupC(cardiovascularsystem) weremoreprevalentinNHpatients[21(21.4%)vs310 (33.2%)P=0.017*]aswellasdiagnosticsgastrointestinal motilitydisorders[23(23.4%)vs129(13.8%)P=0.011*] andconfusionalsyndromes[5(5.1%)vs17(1.8) P=0.031*]
ConclusionandRelevance NHpatientsthatconsultEDfor DRPswereolder,mostlywomenwithahighdegreeofsociofunctional,cognitivedependenceandextremepolypharmacy thannoNHpatients.DRPrelatedwithCATCgroupand diagnosisofconfusionalsyndromeandgastrointestinalmotility disordersarealsomoreprevalent.
CareSettings,2018.1BNucete, 2AMarchena, 2LGonzalez, 2MAGarciaLirola*. 1DistritoSanitarioGranadaMetropolitano,Pharmacy,Granada,Spain; 2DistritoSanitarioGranadaMetropolitano, Pharmacy,Granada,Spain
10.1136/ejhpharm-2023-eahp.260
BackgroundandImportance TheCOVID-19disease,declared apandemicinMarch2020,radicallychangedpeople’swayof life.Thehealthrisk,themeasuresofthestateofalarmand itsimpactatsocialandeconomiclevelhaveexposedthepopulationtoathreattotheirpsychologicalwell-being.
AimandObjectives Toanalysetherelationshipbetween COVID-19andchangesinthetrendofpsychotropicdrug consumption.
MaterialandMethods Descriptivedrugutilisationstudywhich included665,222inhabitants.Thispopulationisdistributedin anurban(UA)(275,990inhabitants)andrural,peri-urban (RA)(389,232inhabitants)area.ThestudyperiodwasJanuary 2018toDecember2021.Datawereobtainedfromthedatabaseofdispensedandbilledprescriptions.Theunitusedwas theDefinedDailyDose(DDD)andthemainvariablewasthe DDDper1000inhabitantsandday(DHD).Thetherapeutic groupsstudiedwerebenzodiazepines(N05BA,N05CA, N05CF)andantidepressants(N06AB,N06AX),accordingto theAnatomicalTherapeuticChemicalClassificationSystem (ATC).Mann–Whitneytestwasusedforstatisticalanalysis.
Results Thegroupofdrugswiththegreatestincreaseinconsumptionwasbenzodiazepines,followedbyantidepressants, thelatterbeinghigherinthe2ndand4thquarterof2020, coincidingwiththefirstandsecondwaveandhigherinrural areas.Inantipsychoticdispensations,aslightincreasewasonly observedinthemetropolitanarea(p<0,05).Duringtheyear 2021,theratesofbenzodiazepinesweredecreasing,ending theyearatvaluessimilartopre-pandemicrates.Incontrast, theincreaseinantidepressantusewassustainedduring2021.
BENZODIAZEPINES
UA:2018:86.71;2019:83.58;2020:86.16;2021:81.71
RA:2018:88.97;2019:88.95;2020:97.63;2021:87.85
ANTIDEPRESSANTS
UA:2018:38,79;2019:39,73;2020:40,16;202141,38
RA:2018:44.76;2019:45.58;2020:48.49;2021:47.85
-DHD4thQuarter
BENZODIAZEPINES
UA:2018:84.67;2019:83.15;2020:87.60;2021:82.00
RA:2018:88.42;2019:89.97;2020:97.38;2021:87.84
ANTIDEPRESSANTS
UA:2018:38.73;2019:39.72;2020:40.99;2021:43.14
RA:2018:45.12;2019:46.24;2020:48.91;2021:49.19
Itwasonlystatisticallysignificanttheincreaseintheconsumptionofantidepressants(P=0.019)intheperiods20202021vs2018-2019.
ConclusionandRelevance Theuncertaintyinthefirstmonths ofthepandemic,bereavement,isolationandtheeffectsofthe economiccrisismayhavefavouredanincreaseintheconsumptionofantidepressantsandbenzodiazepines.Itwouldbe necessarytoreorientclinicalpracticestrategies,promotingthe appropriateandsafeuseofthesedrugsintheprimaryand hospitalcaresetting.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
5PSQ-041 STERILEINTRAOCULARINFLAMMATIONAFTER INTRAVITREALAFLIBERCEPT
1MHernandoVerdugo*, 1AFijóPrieto, 1BÁlvarezGrande, 1MTSánchezSánchez, 2MILópezGálvez. 1HospitalClinicoUniversitariodeValladolid,PharmacyDepartment, Valladolid,Spain; 2HospitalClinicoUniversitariodeValladolid,OphtalmologyDepartment, Valladolid,Spain
10.1136/ejhpharm-2023-eahp.261
BackgroundandImportance Sterileintraocularinflammation (SII)isaknowncomplicationoftherapeuticintravitrealinjections,particularlywithallanti-vascularendothelialgrowthfactordrugs.Theseeventsusuallyoccurresporadicallybutthere hasbeenanincreaseinthenumberofreportedcasesdueto aflibercept 1 .
AimandObjectives ToanalysetheappearanceofSIIin patientswithintravitrealaflibercept.
MaterialandMethods Descriptiveobservationalstudyof patientswithSIIafterintravitrealafliberceptreportedfrom AugusttoNovember2021.Datacollected:sex,age,eye, pathology,pre-treatment,dosesandbatchofaflibercept,time topresentationanddescriptionofsymptoms,best-corrected visualacuity(BCVA)priortolastdose,aftertheonsetof symptomsandatsixmonthsoffollow-up.Patientstreated bilaterallywerecountedastwotreatments.
Results SIIwasobservedin14ofthe110patientstreated (12.7%);57%women;meanage:78.9±8.3years.
17eyesweretreated:right28.6%,left50.0%,bilateral21.4%. Pathology: neovascularage-relatedmaculardegeneration 85.8%,diabeticmacularoedema7.1%,branchretinalvein occlusion7.1%.
Patientspreviouslyreceivedranibizumab,bevacizumaband anaverageof11dosesofaflibercept.Alldoseswerefrom batchKT09625.
Meantimetopresentationsymptomswas22days,which includedmyodeopsis,precipitationinthevitreouswithcrystals ofaspectof ‘starrysky ’,vitreousinflammationanddecrease oftheBCVA.Nopatientpresentedinfectiousendophthalmitis andonerequiredvitrectomy.MeanBCVApriortolastdose andaftertheonsetofsymptomswas0.57±0.42logMAR and0.56±0.27logMAR,respectively,andtothesixmonths was0.66±0.39logMAR.
AlladverseeffectswerereportedtotheSpanishPharmacovigilanceSystem,manufacturerlaboratoryofafliberceptand DrugInspectionandControlDepartmentoftheSpanish AgencyforMedicinesandMedicalDevices.
ConclusionandRelevance
. SIIisassociatedwithintravitrealantiangiogenicdrugs, especiallywithaflibercept.1 However,thesuddenonsetof symptomsalertedtheOphthalmologyDepartment.
. Itwasinitiallysuspectedtoberelatedtothebatchof aflibercept,buttheresultsareinconclusive.
. Theclinicwasimportant,withoutshowingasharpdecrease inBCVA.
. Long-termmonitoringofthesepatientsisnecessarytoassess theresolutionoftheinflammation.
. Multidisciplinarypharmacovigilancecoordinationiscrucial forthedetectionofknownorunexpectedadverseeffects.
ConflictofInterest Noconflictofinterest.
1.GreenbergJPetal.Aflibercept-RelatedSterileIntraocularInflammationOutcomes. OphthalmolRetina.2019Sep;3(9):753-759.5PSQ-042
PPradoMontes,MCouñagoFernández*,IAgraBlanco,MAlfonsínLara,DRoblesTorres, NLagoRivero,EYRomeroVentosa,ICastroNúñez,NMartínezLópezdeCastro. Hospital ÁlvaroCunqueiro,Pharmacy,Vigo,Spain
10.1136/ejhpharm-2023-eahp.262
BackgroundandImportance Dysphagiaisahighlyprevalent syndromeintheelderlypopulation,andespeciallyinthose withcognitiveimpairment.Inadditiontoage-relatedfactors andcomorbidities,drugswithapotentialnegativeeffecton swallowingfunctionhavebeenidentified,manyofwhichare commonlyusedinpathologiesthatarealsoprevalentinthe elderly.Knowingandraisingawarenessaboutthedimension ofthisproblemcanhelpincreasethesafetyofpharmacologicaltreatmentinthesepatients.
AimandObjectives Todeterminetheprevalenceofmedication prescriptionwithapotentialnegativeeffectonswallowingin elderlyoutpatientswithcognitiveimpairmentanddiagnosisof dysphagia.
MaterialandMethods Observational,descriptiveandcross-sectionalstudyinwhichweanalysedthepharmacologicaltreatmentofpatientswithcognitiveimpairmentandadiagnosisof dysphagiaattendingthenutritionhospitalpharmacyclinicofa tertiaryhospital.Werecordedsociodemographic,prescribed medications,potentialeffectonswallowingfunctionandits mechanismdata.Medicationswithapotentialeffectonswallowingwereselectedfromtheexistingliteratureandthe informationcontainedinsummariesofproducts characteristics.
Results Weanalysed594prescriptionscorrespondingto68 patientswhosemeanagewas85,5.Weidentified170drugs belongingto12therapeuticgroups.66patients(97%)had beenprescribedsomemedicationwithapotentialnegative effectonswallowingfunction,andthemeannumberofthese medicationsprescribedperpatientwas3.6.246prescriptions (41,6%)correspondedtomedicationswithnegativepotential ontheswallowingfunction,mainlyduetotheirsedativeeffect (n=118,48%),followedbyproductionofxerostomia(n=44, 18%),neuromuscularaction(n=33,13.4%),directirritants (n=18,7.3%),andunknownmechanisms(n=4%).23prescriptions(9,3%)shareddifferentmechanisms.
ConclusionandRelevance Weobservedahighprevalenceof drugprescriptionswithapotentialnegativeeffectonswallowinginthissubgroupofpatients.Theseresultshighlight theimportanceofre-evaluatingtheclinicalneedforthese medicalprescriptionsinpatientswithdysphagia.Hospital pharmacyhasanimportantroleindetectingthesemedical prescriptionsandpromotingthesearchforalternativesto ensurethebestbenefit-riskratio.Theneedtoextendthe studytoothersubpopulationsofpatientswithdysphagia shouldbeconsidered.
ConflictofInterest Noconflictofinterest.
5PSQ-044 REAL-LIFESAFETYANDSATISFACTIONOFCFTR PROTEINMODULATORSINCYSTICFIBROSIS
1AIbáñezZurriaga*, 1ERamírezHerraíz, 2EPradillaBascuas, 2MRoderoNevado, 3RMGirónMoreno, 3VSanzCobeña, 1AGarciaPeralo, 1AÁlvarezYuste, 1GEscudero Sanchez, 1AMorellBaladrón. 1HospitalUniversitariodelaPrincesa,Farmacia,Madrid, Spain; 2UniversidaddeAlcala,Farmacia,AlcaladeHenaresMadrid,Spain; 3Hospital UniversitariodelaPrincesa,Neumologia,Madrid,Spain
10.1136/ejhpharm-2023-eahp.263
BackgroundandImportance Thenewtransmembraneconductanceregulator(CFTR)modulatordrugs(ivacaftor/tezacaftor/ elexacaftor)arebringingaboutamajorchangeinthetreatmentandqualityoflifeofcysticfibrosis(CF)patients.There isaneedtocollectinformationonpatientperceptionofbenefitandsafety.
AimandObjectives Toassessthesatisfactionandadverseeffect (AE)profilereportedbypatientsonivacaftor/tezacaftor/elexacaftortreatment.
MaterialandMethods Observational,prospective,single-centre studyfromMarchtoJune2022.CFpatientswithatleast onep.Phe508delmutationintreatmentwithCFTRmodulators.Variables:sociodemographic(ageandsex)andbiochemical(GOT-ASAT,GPT-ALAT,bilirubinandCPK)collectedfrom patients'medicalrecords.TreatmentSatisfactionQuestionnaire forMedication(TSQM1.4)with14itemsinfourscales assessingefficacy,sideeffects,convenienceandoverallsatisfactionfromthepatient'sownperspective,thehigherthescore, thehigherthesatisfaction.AdverseEffectsQuestionnaire(ad hoc),PatientInformedConsent.
Results Outof58patientsontreatment,43answeredthe questionnaires,17(40%)female,medianage30(26-37).For TSQMthemedianscoreforeachitemwas21(20-21)over 21;19(16-20)over21;21(18-21)over21and17(16-17) over17respectively.RegardingAEs:39.47%reported increasedappetite,31.58%rash,23.68%headache,13.16% runnynose,increasedbloodpressure,diarrhoeaanditchy skin,10.53%abdominalpainordiscomfort,commoncold anditchyorstingingeyesand £ 2%flatulence,memoryloss, yellowingofskinandeyes,musclepain,fluidretention, insomnia,acne,nauseaandvomiting.Onepatientpresented withsevererashrequiringdiscontinuationoftreatment.Three monthsafterstartingtreatment,onlythreepatientshadGPTASAT>3LSNandonlyonepatient>5LSN,onepatient hadincreasedCPK>5LSNandnopatienthadbilirubin>2 LSN.
ConclusionandRelevance Althoughahighpercentageof patientshaveexperiencedAEs,CFTRmodulatorsarewidely accepteddrugswithafavourableAEprofile.ThemostfrequentAEsreportedbypatientswereincreasedappetite,rash andheadache.TheAEsdescribedbypatientsaredescribedin thedatasheet.Morereal-lifestudiesareneededtoconfirm ourstudyandtoprovidefurtherevidence.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
1CGastalver-Martín,OSerna-Romero*,SBuendia-Bravo,AIglesias-Bolaños,CCapillaMontes,IEscribano-Valenciano,TCruz-Cruz. HospitalUniversitarioDelSureste,Pharmacy Department,ArgandaDelRey,Spain
10.1136/ejhpharm-2023-eahp.264
analysis,itreachesidentificationofpossibleinconveniences (failuremode)andeffectsonanentiresystem.
AimandObjectives OurpurposewasdetectingthemostcriticalphasesofParenteralNutritionbags(PNB)compounding processthroughanauditconsistingofhospitalpharmacists, doctors,nutritionistsandnurses.
BackgroundandImportance
Thehighhealthcareburdeninthe IntensiveCareUnit(ICU)duetotheSARS-CoV2Coronavirus pandemichascreatedaworkenvironmentthatincreasedmedicationerrors.Itisknownthatpharmaceuticalinterventions reducedmedicantionerrors.
AimandObjectives
Theobjectiveofthisstudyistoknowthe impactofpharmaceuticalinterventionincriticallyillpatients.
MaterialandMethods Retrospectiveobservationalstudycarried outinageneralhospital.Allthepharmaceuticalinterventions performedintheIntensiveCareUnit(ICU)betweenthe monthsofOctober2020andApril2021wereanalysed.It wasregisteredinadatabase:PositivediagnosisofCOVID-19 (SARS-CoV2coronavirusdisease),numberofinterventions, typeofinterventionandacceptanceoftheintervention.
Results Atotalof51interventionswereobtainedin169 patientsadmittedduringthe7monthsofthestudy(0.3interventions/patient).42.6%ofthepatientshadadiagnosisof COVID-19.17%ofthepatientsadmittedtotheICUhadat leastoneintervention,ofwhich38%hadmorethan1(mean 1.76interventionsperintervenedpatient).Themostfrequent reasonsforinterventionweredosemodificationduetoinappropriatedose(35.3%)andinappropriatechoiceofpresentationduetotherouteofadministration(21.5%).84%ofthe interventionswerecarriedoutinCOVID-19patients,withthe meannumberofinterventionsperformedinthesepatients higherthaninnon-COVID-19patients(1.87vs1.33).92% oftheinterventionsconductedbythepharmacistwere accepted.
ConclusionandRelevance Pharmaceuticalvalidationinthe IntensiveCareUnit(ICU)isessentialtooptimisethetreatmentofcriticalpatients,increasingsafetyandefficacyofmedicationstheyreceiveandreducingmedicationerrors.Patients diagnosedwithCOVID-19areespeciallylikelytobenefit frompharmaceuticalinterventions,whicharehighlyaccepted byphysicians.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-046 FMEA(FAILUREMODEANDEFFECTANALYSIS)
APPLICATIONTOPARENTERALNUTRITIONBAGS
MANUFACTURINGPROCESS:ROLEOFHOSPITAL
PHARMACIST
1EdeLuca*, 1GCancellieri, 1MSantonocito, 1CBotto, 2RGiammona, 2DLeonardiVinci, 2PPolidori. 1UniversitàDegliStudiDiPalermo,Ssfo
ScuolaDiSpecializzazioneInFarmacia Ospedaliera,PalermoPa,Italy; 2OspedaliRiuniti ‘VillaSofia-Cervello’,UocFarmacia, Palermopa,Italy
10.1136/ejhpharm-2023-eahp.265
BackgroundandImportance Correctidentificationofunexpected/unwantedprocessesvariabilityallowsusstudyingsolutionsforincreasinganysystemreliability.FMEA(Failure ModeandEffectAnalysis)isaninductivemethodthatprovidesa ‘bottomup’ approach;startingfromactivitiesprocess
MaterialandMethods FMEAwasappliedtoPNBcompoundingprocessinahospitalwith639beds.Theprocesswasdividedintofourphases(prescription,formulation,compounding, quality-control).Bymakinganestimateofseverity,probability anddetectability,wehavedefinedappropriateactionstobe takenforeliminatingpotentialproblems’ occurrence.The productbetweenpreviouslyidentifiedvalues(onscalefrom1 to10)providesRiskPriorityIndex(IPR),anoverallcriticality measure.
Results ThehighestIPRvalue(384)wasfoundinformulation phasewherebag’sosmolaritywashigherthanvenousaccess typechosen.AnIPR=168wasfoundinprescriptivephase concerningpatientincorrectselectionfromsoftware(homonymycases),followedbydataincompleteness,relatingto microelementsaddition(withanIPR=150).AnIPR=140was foundduringSiframixmachinesettingupregardingmicroelements’ housingsexchange(dangerduetoPotassiumreplacementwithmicroelementrequiredingreaterquantities). Finally,IPR=144wasfound,duringcompoundingphase,due toconfusionabout ‘lookalikesoundalike’ constituents(inframin/siframinreplacement);thisallowedPNBcompounding withaqualitative-quantitativecompositiondifferentfromthat requested.
ConclusionandRelevance Jointauditproposedsolutionsfor eachphase.Forprescriptiveone,itwouldbedesirabletotake advantageofasoftwarethatgivesaccesstomedicalrecords inordertocheckthatbagsuitedpatient'sneeds.Duringformulationphase,itisnecessarythatahospitalpharmacist(HP) performsadoublecheckbetweenworksheetdraftingand label,verifyingcorrespondence,completenessandoverlapping withdataindicatedinprescription.HPshavetocontrolprescriptionfeasibilityandthatvolumeissuitableforaccessprovidedforpatient.Forset-upphase,adoublecheckshouldbe carriedouttomakesurethateachhousingcontainscorrespondingnutrient;inaddition,atleasttwotechniciansshould bepresenttocarryouttheoperationsinduplicate.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
1ERodriguezMolins, 1APelaezBejarano, 1CJimenezDe-Juan, 1LGomezSayago*, 1LJimenez-Pichardo, 2JRodriguezCastilla. 1RiotintoHospital,HospitalPharmacy,RiotintoHuelva,Spain; 2PrimaryCarePharmacy,PrimaryCarePharmacy,Riotinto-Huelva,Spain
10.1136/ejhpharm-2023-eahp.266
BackgroundandImportance Precautionarycancellationisa newtoolforprimarycareandhospitalpharmacistthatallows themtocancelprescriptionsandavoiddispensingmedicinesat pharmacies.
AimandObjectives Analysetheprecautionaryannulments madeinahospitalandquantifythedegreeofacceptanceof thedoctor.
MaterialandMethods Prospectivestudylastingfivemonthsin acountryhospital.Allpatientsoutpatientswithonco-
haematologicalprescriptionsandhometreatmentwere included.Theprecautionaryannulmentswerecodifiedas safety:interactionbetweendrug(INT):categoryX(avoid combination)andcategoryD(modifytherapy),unnecesassary medication(UM),overdose(OD)andtherapeuticduplicity (TDUP).Thevariablescollectedwere:age,sex,prescribing service,typeofprecautionaryannulmentsanddegreeof acceptanceofthedoctor.Sourcesused:digitalclinicalhistory Diraya,corporatedispensingmodule,Uptodateinteractions andelectronicprescriptionprogramFarmisOncofarm v4.0.11.164.
Results Weanalysed35precautionaryannulments.Population ofmeanage52years(range46-87).71%werewomen.The prescribingserviceswereOncology(97.14%)andHematology (2.86%).Theprecautionaryannulmentswereofsafety:INT 80%(categoryX85.72%andcategoryD14.28%),UM 14.28%,OD2.86%andDUPL2.86%.Thedegreeofacceptanceofthedoctorwas88.57%andmodifiedthetreatment 11.43%.
ConclusionandRelevance Theresultsoftheseriesstudied showahighdegreeofacceptancebythedoctoroftheprecautionarycancellationsmadebythehospitalpharmacist.Itis ausefulsafetytool,emphasisingseriousinteractions.
REFERENCESAND/ORACKNOWLEDGEMENTS
AcknowledgementstotheCongress
ConflictofInterest Noconflictofinterest.
5PSQ-048 WHATDOONCOLOGISTSANDPHARMACISTSTHINK ANDWANTFROMA CAHMS-DRUGINTERACTION CHECKER?ABROADSCALESURVEYTOASSESS EXPECTATIONS
1ALammens, 2JNeefs, 2ESimons, 2,3ISpriet*, 4MDelforge, 4AJanssens, 2TVan Nieuwenhuyse. 1DepartmentofPharmacy-UniversityHospitalsLeuven-Belgium, DepartmentofPharmacy,Leuven,Belgium; 2UniversityHospitalsLeuven,Departmentof Pharmacy,Leuven,Belgium; 3KuLeuven,DepartmentofPharmaceuticaland PharmacologicalSciences,Leuven,Belgium; 4UniversityHospitalsLeuven,Departmentof Hematology,Leuven,Belgium
10.1136/ejhpharm-2023-eahp.267
BackgroundandImportance Theuseofcomplementaryand alternativeherbalmedicines(CAHMs)iswidespreadandpopularamongcancerpatientsfordifferentreasons.Unfortunately,CAHMscaninterferewithanticancertreatments leadingtobothtoxicityordecreasedefficacywiththerapeutic failure.Theavailabilityofatoolforthemanagementof potentialCAHM-druginteractions(CAHMDI)couldprovide healthcareprofessionals(HCP)withscientificevidence-based information.Itmayfacilitateopencommunicationabout potentialadverseeffectswithoutneglectingpatient’sbeliefs andpreferences.Suchatooldoesnotyetexistinour hospital.
AimandObjectives Theaimofthissurveywastoassess futureuser ’sexpectationsofapracticaltooltomanage CAHMDI.
MaterialandMethods Twoe-surveys,carriedoutinGoogle Forms,weresentto1)healthcareproviders(HCPs)ofall oncologicaldisciplinesinourhospitalandresearchdepartmentsand2)allhospitalpharmacistsofUHL.
Results Thesurveywascompletedby37HCPand27hospital pharmacists(HP).Theresultsclearlydemonstratedaninterest
inaCAHMDI,asconfirmedby94.6%and100.0%ofthe HCPandHP,respectively.Allrespondentsindicatedapreferenceforawebsiteratherthanatoolintegratedintheclinical decisionsupportsystem(51.0%HCPand46.4%HP,respectively).Intheircurrentdailypractice,themostcommonly consultedresourcesforcheckingCAHMDIbyHCPwereconsultingaclinicalpharmacist(33.9%)andLexicompDrug Interactions ® (21.4%).HPmentionedStockley ’sHerbalDrug Interactions ® (21.3%)andLexicompDrugInteractions® (21.3%).Keyrequirementsforthedevelopmentofatool weremanagementoptions,potentialclinicalconsequences, severitylevel,mechanismandlevelofevidence.
ConclusionandRelevance Developingauser-friendlyCAHMDI checkerwouldbehelpfulforHCPandHP.Alertingabout HDIcouldenhanceprescribers’ knowledgeandawareness aboutthistopicandenablethemtoinformpatientsaboutthe potentialadverseeffectsoftheseeasilyaccessibleCAHMs.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
1SBlondelle*, 2LLejeune, 1,2APardo. 1ChrHauteSenne,Pharmacy,Soignies,Belgium; 2UniversityofMons,FacultyofMedicineandPharmacyFaculty,Mons,Belgium 10.1136/ejhpharm-2023-eahp.268
BackgroundandImportance HighRiskMedicines(HRMs)are medicineswithanincreasedriskofsignificantharmtothe patientiftheyaremisused.
RegardingthestorageofHRMs,ourhospitalguidelinesare basedonthereferencesystemofouraccreditation organisation.
Compliancewithguidelinesisessentialtoensurethequality ofcare.
. Todeterminetherateofadversedrugeventsrelatedto HRMs.
. Toevaluatetheimpactoftheintroductionoflowconcentrationelectrolytes(KCl)ontheconsumptionof concentratedelectrolytes(KCl).
. Totesttheimpactofpharmaceuticalinterventionsonfour qualityindicatorslinkedtoHRMsstorageincareunits.
. Amongalltheadversedrugeventsencodedduringtheyear 2021,weidentifiedthoserelatedtoHRMs.
. AconsumptionanalysisofinjectablePotassiumChloride (KCl)concentratedandlow-concentratedsolutionswas performedduringtheyears2018-2022.
. AuditstargetingHRMswasconductedin6careunitsovera periodof3weeksinDecember2021.Theseauditsfocused onthefollowingitems:storage,quantity,labellingand expirydateofeachHRMstoredincareunit.Duringeach audit,apharmaceuticalinterventiontookplaceasfollows: tidying,relabelling,withdrawalofexpiredHRMs,feedback ofaudit,educationandawareness.Theimpactofthe pharmaceuticalinterventionswasfurtherevaluated.For comparisonbetweenthegroups(pre-testandintervention groups),datawereanalysedusingChiSquaretestforal HRMs.
5PSQ-049 ASSESSMENTANDOPTIMISATIONOFTHE MANAGEMENTOFHIGH-RISKMEDICINESINA GENERALHOSPITAL. TheLEANMethodologywasusedtodraftouractionplanto improveHRMspractices.
Results
. Weidentifiedthat14%ofadversedrugeventswererelated toHRMs.
. Ourconsumptionanalysisindicatedthattheintroductionof low-concentratedKClsolutionsincareunitswasnot followedbytheexpecteddecreaseintheprescriptionsof injectableKClconcentratedsolutions.
. Atotalof171HRMswereauditedincareunits.Theimpact ofthepharmaceuticalinterventionsperformedduringthese qualityauditswasevaluated,whichallowedtodemonstratea statisticallysignificantimprovement(p<0,05)intermsof storageandexpiryofHRMs.
ConclusionandRelevance Thisworkhighlightstheimportance ofthehospitalpharmacistasakeycontributorinthecontinuousqualityimprovementapproachtooptimisethemanagementofHRMsinahospital.
REFERENCESAND/ORACKNOWLEDGEMENTS
https://www.eahp.eu/24-5PSQ-161
ConflictofInterest Noconflictofinterest.
1MCalvoArbeloa*, 1AEgüesLugea, 2MPAnguianoBaquero, 1MMNocedaUrarte, 1MSarobeCarricas. 1HospitalUniversitariodeNavarra,PharmacyService,Pamplona,Spain; 2HospitalUniversitariodeNavarra,CriticalCareUnit,Pamplona,Spain
10.1136/ejhpharm-2023-eahp.269
BackgroundandImportance Sleepdisturbanceisveryprevalent incriticallyillpatients.Treatmentapproachestoimprovesleep havefocusedonbothnon-pharmacologicandpharmacologic strategies.Trazodoneisanatypicalantidepressantusedwith highlyfrequencyashypnotic.
Themainsideeffectsdescribedfortrazodoneareself-injuriousthoughts,anaemia,seizures,paraesthesia,confusionor dyspnoea.Itcaninhibitdopaminergicneurotransmissioninthe midbrainandasresult,causeextrapyramidaleffects.
AimandObjectives Todescribeacaseofparkinsonism inducedbytakingtrazodoneashypnoticinapatientadmitted inaCriticalCareUnit(CCU).
MaterialandMethods A57-yearoldmanwithnorelevant medicalhistorywasadmittedtoCCUinMay2021with pneumoniacausedbyCOVID-19disease.Thepatientsuffered frominsomnia.Thephysicianprescribedtrazodonestarting withadoseof50mgandthen100mg.
Results Thatafternoon,aftertakingtrazodone,thenurse describedslighttremorintensifiedwithmovementinupper extremities.Thephysicianondutywasnotifiedbuthedid notfindanyexplanation.Nextday,theofficialphysician checkedthemedicationwiththecriticalcarepharmacist.
Thesyndromewasnotexplainedbyanalyticsorother tests.Thepharmacistcheckedallpatient`smedicationssearchinginformationindifferentdatabases:theofficiallabelsand theclinicaltrials,PubMed® andUpToDate®.Inaddition,she checkedpossibleinteractionsinLexicomp® databasebutshe didnotfindnothing.Trazodonewastheuniquedrugassociatedwiththesyndrome.
Thephysicianandthepharmacistagreedtodiscontinuethe medicationtocheckifthesyndromedisappeared.
Thefollowingdays,thepatientcontinuedwithtrembleon movement.Thepatternofthemovementwassimilareach day.Itstartedatafternoonsanddisappearduringnights.The intensityofthemovementwasreducedeachday.Thesyndromedisappearedcompletelyoneweeklater.
Basedoncausalityassessmentofadversedrugreactionsby Naranjoetal.,weclassifythiseventasprobable/likely.The pharmacistnotifiedthisadverseeffecttopharmacovigilance. ConclusionandRelevance Trazodoneisconsideredsafeand usedfrequentlyinourmedicalsystem,sotheknowledgeof effectslikethatisimportant.Nevertheless,theparkinsonism inducedwasreverseanddisappearoneweeklateroncethe treatmentwasstopped.
REFERENCESAND/ORACKNOWLEDGEMENTS
Naranjoetal,ClinPharmacolTher1981.30:239-4.
ConflictofInterest Noconflictofinterest.
5PSQ-053 DESIGNOFAPRIORISATIONSYSTEMBYCOMPLEXITY OFTHEREVIEWINPOLYMEDICATEDPATIENTS: POTENTIALINADECUACYINDEX
AAlcalaSoto,MVázquezReal,DSRuizPérez,CMCuadrosMartínez*,JFSierraSánchez. HospitalUniversitarioJerezdelaFrontera,PharmacyService,JerezdelaFrontera-Cádiz, Spain
10.1136/ejhpharm-2023-eahp.270
BackgroundandImportance Inourhealtharea,whichserves 450,000patients,wehave>2,000polymedicatedpatients (PP)with>15drugs/month.Foranefficientapproachto thesePPitisnecessarytoestablishsomeprioritisationcriteria fortheirreview.
AimandObjectives Todesignanindexofprioritisationto reviewPPbasedontheinadequacyoftheirpolypharmacy, namedPotentialInadequacyIndex(PII).
StratifyallPP(>15drugs/month)accordingtothescoreof thePIIthroughanautomatedanalysisoftheirprescriptions. MaterialandMethods PIIismadeupofdifferentsituations thatcanoccurinthepharmacologicaltreatmentofPP:duplicities,prescribingcascades,drugswithlowtherapeuticvalue, drugsthatprolongtheQT-intervalanddrugscontributingto anticholinergicburdenwerechosenascomponentsofthePII, givingthemascoreincaseofappearance:
PotentialInadequacyIndex(PII)
Duplicity1point
Lowtherapeuticvalue1point Prescribingcascades0,5points QTintervalprolongation0,5points Anticholinergicburden0,5points
AllPPwerestratifiedaccordingtothePIIscore,review ’ s complexitydegreeofthepolymedicatedpatientandestimated timeforreviewareshown:
Review’scomplexity
DegreeofcomplexityPIIpunctuationEstimatedrevisiontime
Verylowcomplexity<110min
Lowcomplexity1to<215min
Moderatecomplexity2to<430min
Highcomplexity4to<890min
Veryhighcomplexity 8160min
Results 2,258PPwereincluded,withameannumberofmedicationsperpatientof16.78(95%CI14.65-18.79),andthe meanPIIscorewas2.01(95%CI1.96-2.06).Patients’ distributionbyreview ’scomplexityisshowninthefollowingtable:
Verylowcomplexity<13881717
Lowcomplexity1to<27293250
Moderatecomplexity2to<48803989
Highcomplexity4to<82281099
Veryhigh complexity >=82218100
All0a17,52247100100
ConclusionandRelevance Theautomatedanalysisoftheprescriptionsofpolymedicatedpatients,insearchofpotentialcriteriaofinadequacy,canfacilitateprioritisationinthereview ofpatients.
ThePIIcanhelpguidetheidentificationofthosepatients withthegreatestcareneeds.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
averageageof80.1years.84.8%(n=28)ofthevalveswere placedtransfemorally,6.1%(n=2)transapicallyand9.1% (n=3)transcarotidly.28patientshadsymptomaticsevere aorticstenosis(ASN),1patienthadasymptomaticASN,and4 patientshadcardiacdecompensationonASN.Contraindicationstosurgeryweredocumentedinthepatientrecordin 84.8%(n=28)ofcases.TheSocietyofThoracicSurgeons (STS)databasescorewasspecifiedin42.4%(n=14)ofthe casesandtheEuroscorewasnotspecifiedforanypatient. Multidisciplinaryconsultationswerecarriedoutin100%of cases,aswellaspre-andpost-TAVIassessments.Atotalof24 non-compliances(NC)wereobserved,including16patients with1NCand4patientswith2NC.Thefundingcriteria werenotrespectedin27.3%(n=9)ofcases.
ConclusionandRelevance Althoughmostofthepatientfiles stipulatecomorbiditiesconsistentwiththeplacementofa TAVI,thereisstillalackofformalisationoftheindications: theSTSscoreismentionedinonly42.4%ofthecases,even thoughitispartoftheFC.Areportwaspresentedtothe recruitingphysiciansandtheimportanceoftranscribingthe STSscoreinthepatientfilewasexplained.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-055 SAFETYTESTINGASSESSMENTFORTHEADHERENCE OFDOSEREDUCTIONINONCOLOGYTREATMENT FOLLOWINGCLINICALGUIDELINES
RECOMMENDATIONSINPATIENTSPRIORRECEIVING FLUOROPYRIMIDINES(5-FLUOROURACIL, CAPECITABINEANDTEGAFUR)
VChorro-Mari,ROnatade*,DChauhan. NorthThamesGenomicMedicinesServiceAlliance NHS,Pharmacogenomic,London,UnitedKingdom
10.1136/ejhpharm-2023-eahp.272
5PSQ-054 ASSESSEMENTOFOCCUPATIONALPRACTICES: ANALYSISOFTHEPRESCRIPTIONSOF TRANSCATHETERAORTICVALVEIMPLANTATION (TAVI)
JFouillet*,NPrisque,CFaure,JPerrey. ChudeMontpellier,PharmacieEuromédecine, Montpellier,France
10.1136/ejhpharm-2023-eahp.271
BackgroundandImportance Transcatheterimplantationofan aorticbioprosthesis(TAVI)allowsthereplacementofthe aorticvalvebyaprosthesiswithoutopensurgery.Likeany medicaldevice,theimplantationoftheseprosthesesmust complywiththeCEmark.AsthefundingofTAVIsisunder specificscriteria,thosemustalsoberespected.
AimandObjectives TocarryoutanoverviewofthecomplianceoftheTAVIprescriptionswiththefundingcriteria(FC).
MaterialandMethods 33patientswererandomlyselected fromthe300TAVIsimplantedin2021.Valvemodels,implantationroutes,andpatientdatawereextractedfromtheinternaltraceabilitysoftware(GILDAS)attheuniversityhospital andfromcomputerisedmedicalrecords(DxCare).Thesedata wereanalysedwithagriddevelopedfromtheFC.
Results Amongthe33patientsselected,15weremenet18 werewomen,ranginginagefrom68to94years,withan
BackgroundandImportance Fluoropyrimidinesinsolid tumoursaremetabolisedbydihydropyrimidinedehydrogenase (DPD)enzyme,encodedbyDPYDgene.Upto3-6%ofthe populationhaveaDPYDvariant,which,withoutappropriate dosereduction,willleadtoseveretoxicity/death[i].
Since2020theregulatoryagenciesinEuropeandtheUK recommendallpatientstobetestedforDPDdeficiencybefore initiationtominimisetheriskofthesereactions[ii],[iii]
FiveLondoncancerprovidingtrusts(CPT)assuredtesting wasbeingperformed[iv].
TheNorthThamesGenomicMedicinesServiceAlliance (NTGMSA)isoneofthesevenintheUKworkingona nationalprojecttoensureequitableimplementationofDPYD pharmacogenomictesting.
AimandObjectives ToestablishthatallCPTwithinNTGMSA aresafelyimplementingDPYDtesting.
MaterialandMethods Fivequestionsanalysedfromnational survey:
WhoisinvolvedincheckingtheresultoftheDPYD genetictest?;
Whomakesdoseadjustments?; Protocol?;
Ischemotherapyprescribedpriorthetestreport?;
Ischemotherapydelayedwhenresultsarepending?
Results WithinNTGMSAall14CPTresponded.Everyone providesDPYDtestingforallcancerindicationswhichinclude fluoropyrimidinetreatment.Multiplehealthcareprofessionals
checkandactionthetestresults,followingdosereductions, followingguidance.Chemotherapyisprescribedpriorreceiving thegeneticreportin10CPT.6hospitalswoulddelayadministrationwhenresultismissing.
ConclusionandRelevance Thereisarichmultidisciplinary involvementincheckingtheresultsofthetest,includingmakingthecorrectdoseadjustments.TheuseofDPYDteststo preventchemotherapytoxicityfollowsasafeandrobustpathwaywithinourregion.
REFERENCESAND/ORACKNOWLEDGEMENTS
[i]UKChemotherapyBoard, ‘PersonalisedMedicineApproachForFluoropyrimidinebasedTherapies,’ 2020.[Online].Available:https://www.theacp.org.uk/userfiles/file/ resources/dpd-testing-ukcb-july-2020-updated.pdf
[ii]EMArecommendationsonDPDtestingpriortotreatmentwithfluorouracil,capecitabine,tegafurandflucytosine|EuropeanMedicinesAgency(europa.eu)
[iii]5-fluorouracil(intravenous),capecitabine,tegafur:DPDtestingrecommended beforeinitiationtoidentifypatientsatincreasedriskofsevereandfataltoxicity
GOV.UK(www.gov.uk)
[iv]NijjarR,ShaunakN,MahmoudS,ThwaitesB,DesaiM,AjeditiC,BrownA,Yeoh I,PatelT,MasentoS.'AcollaborativeauditonDPYDtestingofallpatientsinitiatedonfluoropyrimidines(5-fluorouracil,capecitabineandoralprodrugtegafur) across5Londonteachinghospitals'Abstract;JournalofOncologyPharmacyPractice,2021Suppl&OralPosterPresentation
ConflictofInterest Noconflictofinterest
MTGomezSanchez,RGazquezPerez,MSanchezValera,DGamezTorres,TMoreno Diaz*,BSanchezRodriguez. HospitalTorrecardenas,Pharmacy,Almeria,Spain
10.1136/ejhpharm-2023-eahp.273
BackgroundandImportance Dexmedetomidineisanalpha-2 agonistwithsedativeeffects.Itisusedforthesedationof patientsintheIntensiveCareUnit(ICU)andsedationofsurgicalprocedures.InJune-2022,theSpanishAgencyforMedicinesandMedicalDevices(AEMPS)publishedasafetyletter reportinganincreasedriskofmortalityinpatients£65years ofagecomparedtostandardsedativeagents1
AimandObjectives Toanalysetheuseofdexmedetomidinein ourhospitalandtocomparetheheterogeneityoftheeffect onmortalityaccordingtoageinreallife.
MaterialandMethods Observational,descriptiveandretrospectivestudy.Patientstreatedwithdexmedetomidineorpropofol duringtheyear2021wereincluded.Variablescollected:age, sex,numberofdaysontreatmentwithdexmedetomidine/propofol,admissiondiagnosistotheICU,surgicalintervention duringICUstayand90-daymortalityfromanycause.Variableswerecollectedthroughthedigitalmedicalrecordandthe hospital'selectronicprescriptionprogram.Datawereanalysed usingExcel.
Results 403patientswereincluded(169=dexmedetomidine vs234=propofol).75.7% weremen(125=dexmedetomimidinevs180=propofol).Baseline patientcharacteristicsare showninthefollowingtable.Therewere74deathsat90daysinthecontrolgroupvs31deathsat90-daysinthe dexmedetomidinegroup,oddsratio(OR)=0,49[95%CI: 0,30 – 0,78].Inthe>65yearsgrouptherewere35vs13 deathsat90days(propofol vsdexmedetomidine,respectively),OR=0,39[95%CI:0,18 – 0,86].Deathsat90days inthegroupaged £65yearswere39vs18(propofolvs dexmedetomidine,respectively),OR=0,55[95%CI:0,30 –1,02].
Abstract5PSQ-056Figure1
ConclusionandRelevance Thedataobtaineddonotreproduce thoseobtainedinthestudyonwhichthealertreceivedwas based.Thismaybeduetolimitationsofourstudy.Evenso, theuseofdexmedetomidineinyoungpatientsshouldbecarriedoutwithcaution.Thepharmacyservicehascommunicatedthealerttothehospitalservices.
REFERENCESAND/ORACKNOWLEDGEMENTS
1https://sinaem.aemps.es/CartasFarmacovigilanciaDoc/2022/DHPC-dexmedetomidine. pdf
ConflictofInterest Noconflictofinterest
5PSQ-057 EFFICACYANDSAFETYOFADALIMUMABINTHE TREATMENTOFINFLAMMATORYFACIAL GRANULOMASECONDARYTOSILICONE
MLCasanovasMoreno-Torres*,PYanesSánchez,LMajuelosAicart,MJRevertAlarcon, VQuesadaMarques,MVMoralesLeon. ChuDoctorNegrin,HospitalaryPharmacist,Las PalmasdeGranCanaria,Spain
10.1136/ejhpharm-2023-eahp.274
BackgroundandImportance Theadministrationofsiliconeas afillermaterialisassociatedwiththedevelopmentofinflammatorygranulomaduetoanincreaseintheproinflammatory cytokinetumournecrosisfactor(TNF-a).Basedonthepathophysiologyofgranulomas,anti-TNF- a drugsarepostulatedas possibletherapeuticalternativeforpatientsnotrespondingto initialtreatments.
AimandObjectives Todescribetheefficacyandsafetyofthe useofadalimumabinpatientsdiagnosedwithinflammatory facialgranulomaduetofillermaterial(silicone).
MaterialandMethods A3-monthretrospectivedescriptive observationalstudyofapatientundertreatmentwithadalimumabforinflammatoryfacialgranulomaduetosilicone.
Studyvariablesincludednumberandsizeofgranulomas andadverseevents(AE)occurrencesassociatedwith adalimumab.
Results 62-year-oldwomanfollow-upbydermatologydepartmentduetoinflammationcompatiblewithsiliconeshowed threelesions,oneontheglabellaandtwoonthecheeks.She receivedasfirstlinetreatmentsystemiccorticosteroids(partial controloftheprocess),methotrexate(noclinicalresponseand evenworseningafter3weeks),doxycycline(noclinical responseafter6weeks)andfinallyhydroxychloroquinein associationwithdoxycycline(noclinicalresponse).Shestarts adalimumab40mg/2weeks.
-Response:After6doses ofadalimumabwereadministered(12weeksoftreatment)combinedwithdoxycycline 100mg/24handhydroxychloroquine400mg/24h.Since treatmentstartedpatientexperiencedadecreaseinthe
numberoflesionsandareductioninthesizeofthemasses: fromthreeinitiallesionsonlylesionattheglabellalevel remainsvisibleandpalpable.Afterobjectiveclinical improvementitwasdecidedto withdrawdoxycyclineand infiltrationofdexamethasoneatthepersistentlesion.Treatmentwithadalimumabtogetherwithhydroxychloroquine wasmaintained.
ThepatientdidnotreportanyAEassociatedwiththeuse ofadalimumab.
ConclusionandRelevance Theuseofadalimumabinthis patientshowedobjectiveclinicalbenefitsoverpreviouslyused alternativetreatmentsbyachievingasignificantreductionin thenumberandsizeoflesionsinareducedtreatmenttime withoutexperiencingAE.Togetherwiththeevidencecollected previouslytheuseofTNF-a inhibitors
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-059 REAL-WORLDCLINICALDATAOFPALBOCICLIBAND RIBOCICLIBINBREASTCANCERPATIENT
MDelVecchio*,CLauriaPantano,FZelante,BRe,VLadisa. FondazioneIrccsIstituto NazionaleDeiTumoriDiMilano,FarmaciaOspedaliera,Milano,Italy
10.1136/ejhpharm-2023-eahp.275
BackgroundandImportance Cyclin-dependentkinase(CDK)4/ 6inhibitors,blockthetransitionfromtheG1toSphaseof thecellcyclebyinterferingwithRbphosphorylationandE2F release,showingpotentantitumouractivityandmanageable toxicityinHR+/HER2 breastcancerpatients.
AimandObjectives Themainobjectiveofthisworkisto compareRealworlddata(RWD)betweenpalbocicliband ribociclibinordertoinvestigatethecontinuityintreatment andthefrequecyofheamatologicadverseevents(AEs)before andafterCDKinihibitorsdosereduction(DR).
statisticalmodeltoconfirmresults..Forclinicianusingribociclibismuchmorecomfortablethanpalboclib,duetothepossibilityofDMwithoutinterruptingtreatment
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-060 GLUTENINMEDICINES.APRESCRIPTIONHELPING TOOL
1PDuqueTebar*, 1SRuizGarcía, 1ERamírezHerraiz, 1AGarcíaPeralo, 1AAlvarezYuste, 1GEscuderoSánchez, 2ÁMorellMuñoz, 1AIbáñezZurriaga, 1MPérezAbánades, 3JCElvira Gómez, 1AMorellBaladrón. 1HospitalUniversitariodelaPrincesa,PharmacyDepartment, Madrid,Spain; 2UniversidadSanPabloCeu,FacultaddeFarmacia,Madrid,Spain; 3Hospital UniversitariodelaPrincesa,TechnologyDepartment,Madrid,Spain
10.1136/ejhpharm-2023-eahp.276
BackgroundandImportance Theuseofexcipientscontaining gluteninmedicinescanbeaproblemforceliacpatients,especiallyforthosewithchronicpathologies.Basedonthis,currentspanishlegislationrequirespharmaceuticallaboratoriesto declareexcipientscontainingglutenandthosethatmaycontaingluten.
AimandObjectives Toevaluatethepresenceofunsafeexcipientsforceliacspatientsinmedicinesandthequalityofthe informationregardingglutencontentforpatientsandprescriptors;aswellastocreateanapplicationthatfacilitatesprescriptionbyprofessionals.
MaterialandMethods
Acourtof128ptshasbeenanalyse frommedicalandpharmacyrecords,ofthese101treated withpalbocicliband27withribociclib.Patients(PTS)has beenobservedfrom2019to2021andtheresultswerecomparedwiththoseofpivottrials.TheDRwasdefinedas reducingpalbociclibdosefrom125mgto100mgor75mg ( 20%DR),whileinribociclibfrom600mgto400mgor 200mg.Inbothcases,DRiseffectiveinthemanagementof AE
Results RWDshowsthattimetofirstDRissimilarinboth cases:11and10monthsrespectivelyforpalbocicliband ribociclib.IfasecondDRisnecessary,itoccurrsbyth16,5 monthsforpalbocicliband16.6forribociclib.Of101pts treatedwithpalbociclib,50(49.5%)discontinuedforprogressiondisease(PD)andoneofthemformetastaticmelanoma.6/27ofpts(22.22%)intheribociclibsettingstopped forPD.Inbothcases,neutropeniaisthepriorAEtodose reductionasshowninreallifeandclinicaltrials.Itsfrequencydecreasesduringthefirstcyclefollowingthedose reduction,withareductionintheseverity.OtherAEs observedwere:haematologicdisorder,hepaticcytolysis,drug intolerance,anaemia,leukocytosis,febrileneutropeniaand fever.
ConclusionandRelevance Asshownbythepivottrials,both thetreatmentsareequalintermoftoxicityandduration.The proportionofptswithPDappearstobesuperiorinPalbociclibsetting,eventhoughneedadeeperstudywithagood
MaterialandMethods Adatabaseinatableformatwascreatedtodeterminethepercentageofpharmaceuticalpresentationswithexcipientsthatmaycontaingluten.Datacollected was:activeingredients,therapeuticgroup,typeofexcipient, andmarketingstatus.ThisdatawasobtainedfromtheprescriptionNomenclatortables(source:AgenciaEspañoladel MedicamentoyProductosSanitarios).Withthisdatabase,an applicationwascreatedtofindoutwhichpresentationsmay containtheseexcipientsandwhatalternativesareavailableon themarket.
Results 41319presentationswererecorded,ofwhich 19957werecommercialised.Thedatabaserevealedthat 8%ofthepresentationscommercialisedincludedexcipientsthatmaycontaingluten.Ofthese,93.05%correspondedtocarboxymethylstarchandsodium carboxymethylstarch,ofwhichitisdifficulttoknowthe sourceofthestarchanditspossibleglutencontent. Moreover,1.836%containedwheatstarch,whichcan havevariableamountofgluten.Theinformationfoundin thedatasheetswasvariableand,insomecases,insufficienttoacknowledgetherealrisk.
Withthisdata,anapplicationhasbeencreatedinwhichit ispossibletosearchbyactiveingredientortherapeuticgroup, providingspecialtiesthatcontainexcipientswithglutenorits derivatives,aswellastherapeuticalternativessuitableforceliacpatients.Inaddition,thisapplicationwarnsofthepresence oflactose.
ConclusionandRelevance Carboxymethylstarchandsodium carboxymethylstarcharethemostusedexcipientsthatmay containglutenandthereisagreatdifficultyinfindingreliable informationabouttheirorigin.Thissituationmakesprescriptiondifficultandshowstheneedfortoolsthatallowquick andeasyaccesstodata,guidingtowardsasaferprescription forceliacpatients.
ConflictofInterest Noconflictofinterest
RDíazPerales*,YDominguezRivas,ALunaHiguera,IMMuñozCastillo. HospitalRegional UniversitariodeMálaga,UnidaddeGestiónClínicadeFarmacia,Málaga,Spain
10.1136/ejhpharm-2023-eahp.277
BackgroundandImportance Approximately38%ofEuropean populationhasamentalhealthdisorderthatrequireschronic andcomplextreatment,whichhaveahighriskoflong-term toxicity.Moreover,inthetherapeuticgroupsused,itisadvisabletocarryoutaprogressivedecreaseinthedoseuntilthe drugiswithdrawn.
AimandObjectives Toevaluateamedicationreviewand deprescriptionprogrammeinpatientswhohaveprescribed threeormoredrugsforpathologiesundermentalhealthfollow-up.
MaterialandMethods Descriptiveandprospectivestudy,carriedoutwiththreecohortsineachofwhichpatientshadto havethreeormoreconcomitantprescriptionsof:antidepressants(A),neuroleptics(B)andbenzodiazepines(C);followed bythementalhealthunitofatertiaryhospital.
PharmacyserviceobtainedthelistsinMay2022through InformationProcessingModuletoknowtheconsumethrough electronicprescription,andpostedonacorporateapplication, sothateachdoctorcouldaccesstheindividualisedreviewduringthecurrentyear.Fourmonthslater,asectionwasmade tostudythedegreeofstrategy'simplementation.
Demographicdata(age;sex)andreview'spercentageswere collected,analysingdeprescription(one/twodrugs),treatment maintenance(byreasonofseverity/prescriptionondemand/deescalationphase/otherreasons),dosechangesandnewdrug's prescription(substitution/addition).
Results Studypopulationobtainedof338patients(meanage: 51years;men:55.3%):34(10.1%)(A),81(53.5%)(B)and123 (36.4%)(C).Theresultsobtained:53.9%reviewed[(A): 44.1%;(B):58%;(C):50.4%],34%pendingreview[(A):26.5%; (B):31.5%;(C):39.9%]and12.1%excluded(reviewnotapplicable).Somedrugswasdeprescribedin17.6%[(A):20%; (B):17.1%;(C):17.7%]:14.8%(one)and2.8%(two).Sameprescriptions'numberwasmaintainedin82.4%[(A):80%;(B): 82.9%;(C):82.3%]:75.3%severity,15.3%scheduleddemand, 8.7%de-escalationand0.7%other.Dosechangeswere reportedin12.1%[(A):6.7%;(B):15.2%;(C):8.1%]:allof themdecreased.Finally,1.7%ofnewprescriptionswere obtained[(A):6.7%;(B):1%;(C):1.6%]:allofsubstitution.Inno casewastheprescriptionsnumberincreased.
ConclusionandRelevance Thistoolprovidedhasallowedprescriberstoaccessandreviewthepopulationsusceptibleto deprescription.Thedegreeofacceptancehasbeengood.In themajorityofpatientstheprescriptionsweremaintained,but in1/5thepatient’smedicationdeprescriptionwasperformed. Thestudyshouldbeextendeduntilthereviewoftheentire selectedpopulation.
Periodicreviewscanmakeahighimpactonthesepatients' healthaswellasapositiveeconomicimpact.Furthermore,it wouldbeusefultocreateourowndrugreview/deprescription algorithmsandtoimplementthisstrategyinotherunits.
5PSQ-063 ACROSS-SECTIONALSTUDYONTHEPOTENTIALLY INAPPROPRIATEPRESCRIBEDAND CONTRAINDICATEDHIGH-RISKMEDICATIONIN HOSPITALISEDCHRONICCOMPLEXPATIENTS
ABocosBaelo*,CGarcía-Castiñeira,AVilariño,AMartin,AArias,CCodina–Jiménez, LEstrada,ETerricabras,EValls,SMarin,CQuiñones. HospitalGermansTriasIPujol, Pharmacy,Badalona,Spain
10.1136/ejhpharm-2023-eahp.278
BackgroundandImportance Increasedlifeexpectancyhas supposedahigherpresenceofcomorbiditiesleadingto polypharmacyrisingtheprevalenceofpotentiallyinappropriateprescriptions(PIPs)andhigh-riskmedication(HRM) burden.PIPscanbeacauseofharmornolongerprovide healthbenefitswhichiswhypharmacists-ledinterventions aimedatdetectingandred ucingithaveemergedduring recentyears.
AimandObjectives AssesstheprevalenceofPIPs,HRMand contraindicatedmedicationsinchroniccomplexpatients(CCP) towhompharmacist-ledin-hospitalreconciliationhavebeen performedandtodeterminedeHRMburdenconsequenceof PIPs.
MaterialandMethods Cross-sectionalstudyonhospitalised CCPbetweenMarchandApril2022.Pharmacist-ledmedicationreconciliation,PIPsidentification(usingtheListofEvidence-baseddeprescribingforchronicpatients(LESS-CHRON) criteria)andcontraindicated(usingtheSpanishdatasheet)and HRM(usingtheHigh-AlertMedicationsinchronicpatients InstituteforSafeMedicationPractices(ISMP)list)identificationwasperformed.
DemographicdatatogetherwithPfeifferandBarthelindex werecollectedfrompatients’ medicalrecords.Chi-squaretest wasutilisedtodeterminedifferencesintheproportionofPIPs betweenHRMandnon-HRM.
Results 60patientswereincluded,(43.3%women,meanage was76.8±9.8years).Pfeifferindexwas0-2(normalcognitivelevel)in35(58.3%)andBarthelindexwas60-99(low dependencelevelin26(43.4%)ofpatients.Meannumberof prescribedmedicationswas12.8±4.7.AtleastonePIPwas detectedin100%ofpatients(meannumberof4.7±4.1 PIPs).In11patients(18.3%)thedetectedHMRalsowas PIPs.Mostlyinvolveddrugswerebenzodiazepines(72.7%of cases),spironolactone(9.1%),vildagliptine(9.1%)andquetiapine(9.1%).In13cases(21.7%)HRMwasalsocontraindicated(23%oralanticoagulants,23%digoxineand15% eplerenone).Therewerenon-significantdifferencesintheproportionofPIPsbetweenHRMandnon-HRM(3.9%vs3%, p 0.05).
ConclusionandRelevance Consideringthesefindings,ahigh prevalenceofPIPswasfoundthroughpharmacist-ledassessmentinhospitalisedCCPaccordingtoLESS-CHRON criteria.
MoreoverfromHRMassessedbyIRMP,ahighnumber ofPIPSandcontraindicatedmedi cationwereidentified,of whichbenzodiazepinesandanticoagulantswerethemost detectedaccordingtothelit eratureandtheresults obtained.
Thisfacthighlightstheneedforpharmacists-ledtreatmentassessmentandoptimisationprogramsinthispopulation.
5PSQ-064 CONCOMITANTTREATMENTWITHATEZOLIZUMAB ANDENZALUTAMIDEFORMETASTASTICNON-SMALLCELLLUNGCANCERANDMETASTASTICPROSTATE
1AMelgarejo-Ortuño*, 1MPBautista-Sanz, 2ASánchez-de-Torre, 2EBernal-Hertfelder, 1CAApezteguia-Fernández, 1Cde-Cáceres-Velasco, 1MÁAmor-García, 1EMatilla-Garcia, 1BRodríguez-Vargas, 1RMoreno-Diaz. 1HospitalUniversitarioInfantaCristina,Pharmacy, Madrid,Spain; 2HospitalUniversitarioInfantaCristina,Oncology,Madrid,Spain
10.1136/ejhpharm-2023-eahp.279
BackgroundandImportance OnlyonephaseIIItrialofenzalutamidewithorwithoutatezolizumabinmenwithmetastatic prostatecancerwhoprogressedonabirateronehasbeen reportedintheliterature.Nocaseshavebeenreportedin clinicalpracticewithexperienceinthemanagementof patientswithlungandprostatecancerunderconcomitant treatmentwithatezolizumabandenzalutamide.
AimandObjectives Todescribetheefficacy,safetyandadherenceofconcomitanttreatmentwithenzalutamideformetastaticcastration-resistantprostatecancerandatezolizumabfor metastaticlungadenocarcinomainapatientcase.
MaterialandMethods Thiswasadescriptive,retrospective clinicalcase.Thedata(diagnostictests,therapyandclinical course)wereobtainedbyreviewofelectronicmedicalrecords. Adherencewasevaluateusingmedicationpossessionratio (MPR).
Results A72-year-oldmalepatientwithstageIVnon-smallcelllungcancer,negativeeGFR,ALKandPD-L1,diagnosedinJanuary2019,receivedafirstlinestandardchemotherapy.InSeptember2019,therewasevidenceof tumourprogressionandtreatmentwithatezolizumabwas started.InDecember2019,patientwasdiagnosisofprostateadenocarcinomawithpossibleganglionicinvolvement, surgerywasperformedandanti-androgentreatmentwas started.Thepatientcontinuesmaintenancetreatmentwith atezolizumabandinDecember2021,bonemetastasesof prostateoriginweredetected.Enzalutamidetreatmentis proposedforprostatecancerandmaintenanceatezolizumab forlungcancer.Nocaseshavebeenreportedintheliterature,butthereisonephaseIIItrial,Imbassador250,which atleastreportsconcomitantadministrationofthetwo drugsforprostatecancer.Giventhefavourablesafetydata fromthestudy,andtheefficacydatareportedforboth treatmentsfortheircorrespondingindications,enzalutamideisinitiatedwhiletreatmentwithatezolizumabis maintained.Notoxicityfrom thetreatmentshasbeen reported.Thepatienthasmaintainedbothtreatmentsto thepresentday,maintainingclinicalresponseforboth tumours.Thepatienthasshown100%adherencetooral andintravenoustreatment.
ConclusionandRelevance Thisisthefirstcasereportwithevidenceofefficacyofconcomitanttreatmentwithatezolizumab forlungcancerandenzalutamideforprostatecancer,withno additionaltoxicity.Itisimportanttoreportthesecasesinreal clinicalpracticebecausetheseconditionswillnotbepresent inclinicaltrials.
5PSQ-065 BEAHUMAN,NOTACASEREPORT:HOSPITAL PHARMACISTSMAKETHEDIFFERENCE
1,2ABrescia*, 3MDNaturale, 1,2GMMarrazzo, 1DCasuscelli, 1BSpinoso, 1SEsposito, 1CMonopoli, 1MZito, 3AdeFrancesco. 1AouMaterDomini,Pharmacy,Catanzaro,Italy; 2MagnaGraeciaUniversity,HospitalPharmacySchool,Catanzaro,Italy; 3AouMaterDomini, HospitalPharmacy,Catanzaro,Italy
10.1136/ejhpharm-2023-eahp.280
BackgroundandImportance Thehospitalpharmacyofour HealthInstituteiseligibletocarryoutphase1studytill 2017.InJuly2021amultidisciplinaryteam,whichincludes pharmacists,approvethechoicetoenlista61yearoldman of70kgaffectedbycoloncancerfourthstage,inoperable, withfailureofalldrugtherapiesandwithouttherapeutic treatment.
AimandObjectives Theaimofourworkwastocreateapersonalisedpharmacologicaltherapyinordertoimprove patient’slifeexpectancy,minimisingsideeffects.
MaterialandMethods Evaluationandc reationofacustom pharmacologicalprotcol,withcontinuousmonitoring patient ’ svitalparameters,before,duringandafterdrug administration.Thecalculateddosewas5mg/kg.Pharmacistswereinvolvedalsoinmonitoringofadversedrug reactionsschedulingperiodicalpatientinterviewandparticipatinginthereviewoftherapy withclinicians .Specifically 24hfromthefirstinjecton;7and15daysafterdrug administration.
Results Reductioninthevolumeofmorphologicallesionsafter amonthfromfirstinfusion,observedbycomputedtomography,accordingtoresponseevaluationcriteriainsolidtumours (RECIST1.1):supraclavicularlesionontheleft(cm1.6vs cm2.7);paratrachealformation(cm1.6vs1.4);formationof theaorta-pulmonarywindow(cm1.6vs1.8);decreasedhepaticformation(cm4.6vscm5.1).Afterninemonthsfrom firstadministration,weobservedthatreductionofmorphologicalvolumelesionsremainsconstant.Noadversereactions werepresentedinthewholeobservationalperiod.inaddition, thepatientinterviewedreportslessfatigueandincreased mobility.
ConclusionandRelevance Phase1study(eudract2017002615-33)involvestheuseofLNA-i-miR-221,anewmoleculesynthesisedtoinhibitmir-221,whichmayberesponsibleforcellulardysfunctionattributabletoincreased proliferationandinhibitionofapoptosis,whichhasalways beenallmarkersofcancer.Singledrugvialcontains35mg. TheForcalculateddosewas350mg,reconstitutedwith20 mlNaCl,infusedintotalvolumeof100mlfor30minutes. Therapypersonalisationandinterdisciplinarycollaboration provedtobeasuccessinensuringhelpandlimitingadverse effects.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.DiMartinoMT,et.alDose-FindingStudyandPharmacokineticsProfileoftheNovel 13-MerAntisensemiR-221InhibitorinSprague-DawleyRats.MolTherNucleic Acids.2020Jun5;20:73-85.
ConflictofInterest Noconflictofinterest
TESalinasMuñoz*,CNotarioDongil,APerezFacila,MDCGonzalezEscribano,AMarcos delaTorre,MLMorenoPerulero. HospitallaManchaCentro,Pharmacy,AlcazardeSan Juan,Spain
10.1136/ejhpharm-2023-eahp.281
BackgroundandImportance Thesevereaffectationbythe COVID-19virusiscausedbyaninflammatoryresponse triggeredintheindividual.Theuseofimmunosuppressive agentssuchastocilizumabmaybeeffectiveduetocytokinestormblockade.Sinceitisaboutoff-labeluse,experts recommenddevelopingmanagementprotocolsfrom hospitals.
AimandObjectives Todeterminetheefficacyoftocilizumab insevereCOVID-19diseaseandtheadequacytoaprotocol ofuse.
MaterialandMethods Aretrospective,observationaland descriptivestudyovera12-monthperiodwasconducted. AllCOVID-19patientswhoreceivedtocilizumabwere included.
Fortheprotocoldevelopment,followingcriteriawere included:1)Interstitialpneumoniawithrespiratoryinsufficiency andhigh-flowoxygen;2)Absenceofresponseto3bolusesof corticosteroids;3)Interleukin-6>40UI/L.Intheabsenceof interleukin-6,patientshadtomeetatleast3criteria:C-reactiveprotein(CRP)>10mg/dl;D-Dimer>1mg/ml;Ferritine>1000ng/ml;cytopeniaofatleast1series.Toevaluate response,CRPwasmonitored.
Collecteddatawereage,gender,administrationdate,vaccinationagainstCOVID-19,administereddose,CRP,exitus.
DatawerecollectedfromanelectronicprescriptionprogrammeFarmatools® andthecomputerisedmedicalhistory, MambrinoXXI®
Results Atotalof32patientswereanalysed,ofwhich90.6% adheredtotheprotocolforuse.Ofthosewhowerenot adhered9,4%duetoseverityofsuddenillness.
ThemeanvalueoftheinitialCRPwas5.96mg/dlreducing to1.14mg/dlafterthetocilizumabadministration.In81.3% ofpatientstherewasareduction.
Referingtodosebasedonweigh,60%ofthepatients receivedthe600mgdose,theremaining40%receivethedose of400mg.
Ofthetotalofpatients,43.75%hadnotbeenvaccinated againstCOVID-19,37.5%ofpatientstreatedthefinalresult wasexitus,allofthemvaccinated.
ConclusionandRelevance Ahighpercentageofpatientsmeet theprotocolcriteria.ThereasonwhyPatientsaccomplished theprotocolwasarapidevolutionofthedisease.
Ahighpercentageoftreatedpatientswerenotvaccinated. Ingeneral,thevaccineprotectsfromseveredisease.
Theroleofthehospitalpharmacistisimportantinthe developmentofprotocols,especiallyinthesecasesofoff-label usesforacorrecttreatmentapproachavoidingindiscriminate use.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-067 TRACKINGTHEOVERALINUSEANDSTRESS STABILITYOFROMIPLISTIM(N-PLATE®)BYTHE EVALUATIONOFASELECTEDSETOFCRITICAL QUALITYATTRIBUTES
1RPérez-Robles*, 2JRuiz-Travé, 2MMartinez, 3JHermosilla, 3ATorrente-López, 4ASalmerón-García, 4JCabeza, 3NNavas. 1Universityofgranada-fibao-ibsgranada, analyticalchemistry,granada,spain; 2UniversityofGranada,AnalyticalChemistry,Granada, Spain; 3Universityofgranada-ibsgranada,analyticalchemistry,granada,spain; 4Sancecilio universityhospital-ibsgranada,departmentofclinicalpharmacy,granada,spain 10.1136/ejhpharm-2023-eahp.282
BackgroundandImportance Romiplostim(N-Plate ® )isaFcfusionproteininwhichaTPOagonistpeptideisassociated withtheFcdomainofahumanantibody.Itcontainsof twoidenticalsubunitseachconsistingofanFcdomainof humanimmunoglobulinIgG1linkedtoapeptidecontaining twohumanTPOreceptorbindingdomains.Thisdrugis indicatedinthetreatmentofimmunethrombocytopenic purpura(ITP).Asproteinaceo usbased-medicine,romiplostimisindicatedtohavelowstability,thusthestudieson theeffectsofpossiblein-usem ishandlingandinstressconditionsarewelcomedtogetknowledgeuponitsstability anddegradation.
AimandObjectives ToevaluatetheimpactofinusemishandlingandforceddegradationonromiplostimchemicalstructurebyevaluationofseveralCriticalQualityAttributes (CQAs).
MaterialandMethods Vialsofromiplostim(N-plate,0.5mg/ mL)werereconstitutedasitisindicatedbythemanufacturer andsubmittedtoseveralstressstimuli:expositionat80 °C (2h),smoothshaking(12h),roomlightandtemperature (excursionaprox.20-24 °C)exposition(24h),acceleratedlight exposition(24h)and1freeze/thawcycle.TheCQAsevaluated were:(A)primarystructure,bypeptidemapping-RP/UHPLC(Orbitrap)MS/MS;(B)tertiarystructurebyIntrinsictryptophan fluorescencespectroscopy;(C)aggregationbySE-HPLC/DAD; andfunctionalactivity(asthecapacitytobindtoitstherapeutictarget)byELISA.
Results Changesintheprimaryandtertiarystructureandthe formationofaggregatesweredetectedafterromiplostimsamplesweresubmittedtohightemperatureandtoroomconditions.Thesechangesdetectedwereaccompaniedbyalossof functionality.Similareffectswerecausedwhenstressedby acceleratedlightexposition.Thesmoothshakeandfreeze/ thawcyclestimulididnotaffecttheCQAsstudied.
ConclusionandRelevance Thisstudyprovesthatromiplostimmustbereconstitutedandadministratedavoidinglongtimelightexposureandelevatedtemperaturesastheycan inducetheactivationofse veraldegradat ionpathways whichcauselossoffunctionali tyandaggregation,and thus,losingtheoriginalsafety,qualityandefficacyofthe drug.
ThisstudywasentirelyfundedbyProjectFIS:PI-17/00547(InstitutoCarlosIII,MinisteriodeEconomíayCompetitividad,Spain),whichmeansthatitwasalsopartially supportedbyEuropeanRegionalDevelopmentFunds(ERDF).
1ADordàBenito, 1XLarreaUrtaran*, 1ENoguéPujadas, 1ÀCastellóNòria, 1MVilaCurrius, 2FArtimeRodríguez-Hermida, 1CSubiranaBatlle, 1LViñasSagué, 1RSacrestGuell. 1Hospitaldrjoseptruetadegirona,pharmacydepartment,girona,spain; 2HospitalSanta Caterina,PharmacyDepartment,Girona,Spain
10.1136/ejhpharm-2023-eahp.283
BackgroundandImportance Nintedanibandpirfenidoneare theonlydrugsindicatedforthetreatmentofc(IPF).Both drugshavediarrhoeain62.4%and18.8%,respectively, describedintheSummaryofProductCharacteristics(SmPC)as afrequentadverseeffect(AE).
Incaseofdiarrhea,itisrecommendedtoreducethedose orstoptreatment.
AimandObjectives Toanalysethefrequencyofappearanceof diarrhoeaofthetreatmentwithpirfenidoneandnintedanib describedinSmPCwiththatofthepatientsinthestudyand todescribetheactioncarriedout.
MaterialandMethods Retrospectiveobservationalstudy betweenJanuary2017andAugust2022inwhichIPFpatients treatedwithnintedaniborpirfenidonewereincluded.
Thefollowingvariableswerecollected:age,sex,drug, durationoftreatment,withdrawalreason,existenceofdiarrhoeaandseverityaccordingtomedicalevaluationand,if necessary,thecorrectiveactiontaken(dosereduction,treatmentdiscontinuationorcontroloftheAEwithanother drug).
Forstatisticalanalysis,mean,medianandstandarddeviation(SD)wereperformed.Theoddsratio(OR)wascalculated withthedataobtainedandthosedescribedintheSmPCof eachmedication.
Results Thirtypatientswereincludedwithamean±SDage of72±8years,ofwhich23.3%(7)werewomen.80.0%(24) receivedtreatmentwithnintedanib(durationrange:37and 1953days),and20.0%(6)weretreatedwithpirfenidone (durationrange:124and1073days).
Ofthepatientstreatedwithpirfenidone,83.0%(5)discontinuedtreatment(noneduetoEA).17%(1)hadmilddiarrhoeathatwascontrolledwithloperamide.
66.7%(16)ofnintedanibpatientspresenteddiarrhoea(7 severe,7moderate,and2mild).Ofthese,37.5%(6)were treatedwithloperamidemaintainingthedose,18.7%(3)discontinuedtreatment,and43.8%(7)underwentadosereduction.Thisadjustmentallowedtreatmenttocontinuein71.4% (5/7)ofthepatients.TheORofdiarrhoeainstudypatients, comparedtodescribedinSmPC,withnintedanibwas1.21 CI95(0.51-2.86)andwithpirfenidonewas0.86CI95(0.107.47).
ConclusionandRelevance Theappearanceofdiarrhoeain bothdrugsisveryfrequent.Nostatisticallysignificantdifferenceswereobservedinthefrequencyofonsetofdiarrhoeain patientsatourhospitalcomparedtothosedescribedinthe SmPC.
Inmostcasesdiarrhoeawascontrolledbydosereduction orloperamideadministration.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-070 PHARMACEUTICALCAREINANONCOHAEMATOLOGICALCLINICALTRIALSUNIT
1LGarcíaBasas*, 2CVaron-Galcera, 2PGarciaOrtega, 1ESerramontmanyMorante, 1SGimenezGiner, 1MCarreresPrieto, 1PRoviraTorres, 1ICidonchaMuñoz, 3MQGorgas Torner. 1HospitalUniversitarioVallD’hebron,Pharmacy – VallD’hebronInstitutode OncologíaVhio,Barcelona,Spain; 2HospitalUniversitarioVallD`Hebron,Pharmacy – Vall D’hebronInstitutodeOncologíaVhio,Barcelona,Spain; 3HospitalUniversitarioVall D’hebron,Pharmacy,Barcelona,Spain
10.1136/ejhpharm-2023-eahp.284
BackgroundandImportance Thepharmacist’sroleinthe healthcareforpatientsparticipatinginclinicaltrials(CTs) goesbeyondtellingthemhowtotakethemedicationatthe startoftreatment.CTprotocolsareverycomplexandcompliancewithalltheirrequirementsisessential.
Patientsalwayshaveavailableapharmacydirectphoneand emailinordertomakeiteasiertosolveanyqueriesthey mayhaveaboutdrug-druginteractions,sideeffectsordietary anddailylivingrecommendations.
AimandObjectives Toevaluateallpharmaceuticalcarequeries addressedtothepharmacistteaminaCTsunit.
MaterialandMethods Retrospectivestudycarriedoutina tertiaryuniversityhospitalovera7-monthsperiod(January – July2022).Allthecon sultationswereevaluatedandclassifiedaccordingtothephaseoftheCTinwhichthe patientwasincluded,sex,pathology,personwhoasked (patient,oncologist,etc.),communicationmediaandreason ofconsulting.
Results Atotalof596querieswerereceived,mostlyabout patientsincludedinphaseItrials(58,6%).Thenumberof consultationsincreasedsteadilyfromJanuary(37)toJuly (115).
Accordingtosexandpathology,559queriescouldbeevaluated.Morethanhalfwereaboutwomen(58,9%).Mostfrequentconsultationswereaboutpatientswithbreast(17,4%), lung(14,1%)andgenitourinary(13,8%)cancer.
Ofthefullyevaluablequeries(494),40,7%weredoneby patients,followedbystudycoordinators(32,8%)andphysicians(25,9%).Mostoftheconsultations(57,7%)were receivedbyemail.Relatedtothetopic,almostallofthe querieswererelatedtointeractionswithconcomitanttherapies (92,5%),followedbyinstructionsonhowtotakethedrug (3.8%).
ConclusionandRelevance Pharmacistsreceiveahugenumber ofconsultationsaboutdrug-druginteractions.Bothpatients andhealthcareprofessionalsneedtohaveaquickwayto havetheirconsultationssolved.
Continuouspharmaceuticalattentionisvitaltoensurethe efficacy,safetyandcontrolleduseoftheinvestigationaldrug andtofacilitatecompliancewithCTprotocols.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
1ISoto, 2IGonzález, 1MRMengualBarroso, 1BRubioCebrian, 1PSanMiguelTorvisco, 1ISollano-Sancho*, 1IMoronaMinguez, 1APousadaFonseca, 1JSolisOlivares, 1YMateos Mateos, 1CMorielSanchez. 1HospitalUniversitariodeMóstoles,FarmaciaHospitalaria, Móstoles,Spain; 2HospitalUniversitariodeMóstoles,FarmaciaHospitalaria,Móstoles,Spain
10.1136/ejhpharm-2023-eahp.285
BackgroundandImportance Manypatientsbringmedication withthemduringtheiradmissiontohospital,whichisa sourceoferror.
AimandObjectives Analysetheprescriptionofmedicinesprovidedbythepatientandevaluatetheircorrectuse.
BackgroundandImportance Tofacitinib,baricitinib,upadacitinibandfilgotinibareJanuskinaseinhibitors(IJAKs)indicated inrheumatoidarthritis(RA).TheEMAnotifiedthatin patientswithRAwhowere 50yearswithatleastonecardiovascularriskfactorhadanincreasedriskofmajoradverse cardiovascularevents(MACE),andmalignancieswithuseof tofacitinibrelativetoTNF-alphainhibitor:https://www.ema. europa.eu/en/documents/dhpc/direct-healthcare-professional-communication-dhpc-xeljanz-tofacitinib-increased-risk-major-adverse_en.pdf Althoughitisbeingevaluated,itisstillunknownifthis riskissharedbyotherIJAKs.
MaterialandMethods
Cross-sectionaldescriptivestudyof patientsadmittedtoasecondlevelhospitalon11-11-2021 whichhadtreatmentsprescribedas ‘medicationprovidedby thepatient’ (MPP).Thesourcesofinformationusedwere:the electronicmedicalrecordandtheprescriptionprogramme. Thevariablescollectedwere:age,sex,prescribingservice, whetherornotthemedicationwasprovided,patientknowledgeofthedosageofthemedicationprovided,numberof totalactiveingredientsprescribedperpatient,andmedications provided,numberoftherapeuticduplicationsinthecomplete treatment.
Results Atotalof96patientshadaprescriptionforaMPP, representing28.92%ofthepatientsadmittedtothehospital atthattime.Ofthistotal,afterexcludingthosewhocould notbeinterviewedduetotheirclinicalsituation(Intensive Care,Resuscitation,Psychiatry,Emergency,HomeHospitalisationUnitandisolatedpatients),42patientswereanalysed, withamedianageof74.5years[RIQ70-80.75],59.52% beingmale.Themainprescribingservicewasinternalmedicine(59.52%)followedbysurgery(16.67%)andtraumatology (16.67%).Ofthetotalnumberofpatientswithprescribed MPP,85.71%actuallyprovideditand97.22%wereawareof itsdosage.Themediannumberofactiveingredientsprescribedperpatientwas13[RIQ11-17],withthemediannumberofMAPsbeing2[RIQ1-2.75].Therapeuticduplication wasfoundbetweenthemedicationprovidedandthatofthe admissionin2patients.
ConclusionandRelevance Aconsiderablepercentageof patientsadmittedtothehospitalprovidemedication,withthe majorityofpatientsbelongingtotheInternalMedicine Department.Aftertheinterview,itwasobservedthatmostof themcontrolledtheirmedication;however,asignificantpercentage,despitehavingmedicationprescribedasprovided,did nothaveitduringtheiradmission.Forthisreason,weconsiderthatthepatientshouldnotprovidemedicationasfaras possible,inordertotrytopreventmedicationerrorsduring thehospitalstayandtoadjusthistreatmenttothehospital pharmacotherapeuticguide.
ConflictofInterest Noconflictofinterest
AimandObjectives Todescribeandcomparethesafetyof tofacitinib,baricitinib,upadacitinibandfilgotinibinpatients withRAinareal-world-setting.
Secondaryobjective toanalyseifthereisarelationship betweenMACEandmalignancieswithapatientprofilewitha higherriskofdevelopingthemasestablishedinthealert.
MaterialandMethods Retrospective/prospectiveobservational studyofRApatientsundertreatmentwithtofacitinib,baricitinib,upadacitinibandfilgotinibuntilSeptember2022.
Safetywasdeterminedbasedontheadverseevents(AEs) reported.
Variables sex,ageatstart,time-of-treatment,reasonfordiscontinuation,riskfactor´sMACE,riskfactorsformalignancies, andAEs.
Statisticalanalysis adescriptionofcharacteristicsandevents thatoccurredinthecohortwascarriedout.Associationswere laterexplored.
Results 124patients(80.6%women)wereenrolled.Mean age:55.8(SD11.8)years.
Treatmentsreceived tofacitinib(n=60),upadacitinib(n=49), baricitinib(n=21)andfilgotinib(n=14),because19patients (15.3%)weretreatedwithmorethanoneIJAKsequentially.
Medianoftreatment;tofacitinib:399(171-884)days,baricitinib:308(210-632),upadacitinib:287(130-477)and93 (60-171)forfilgotinib.
Weidentified110patienttreatmentswithanincreasedrisk ofMACEormalignances.
AEswerereportedin39(31,5%)treatments(21,9,7,and 2caseswithtofacitinib,upadacitinib,baricitinibandfilgotinib) beingthemostcommonherpeszoster.Only2patientssufferedaMACEinthetotalcohort(bothwithtofacitinib).
Therewere79end-of-treatmentbecauseofinefficacy (n=46),AE(n=22),both(n=7)orforbeingconsidereda riskpatient(n=4).
Noassociationcouldbeestablishedbetweenriskpatientand thedevelopmentofadverseevents,neitherminorormajor. ConclusionandRelevance Therefore,itisstillunknownifthe exchangestrategybetweenthemisadequatetoreducethe risk.Limitation:alargersamplesizeandlongerfollow-up timearerequiredtodetectmajorAEsandtheirassociation withpatientsatrisk.
ConflictofInterest Noconflictofinterest
1MTGómezLluch*, 1PGarcíaLloret, 2MGonzálezBoronat, 3CVAlmeidaGonzález. 1HospitalUniversitarioVirgendeValme,ServiciodeFarmacia,Sevilla,Spain; 2Universidad deSevilla,Farmacia,Sevilla,Spain; 3HospitalUniversitarioVirgendeValme,Bioestadística deInvestigación,Sevilla,Spain
10.1136/ejhpharm-2023-eahp.286
5PSQ-074 INTERACTIONSDETECTEDWITHTHEUSEOF NIRMATRELVIR/RITONAVIRINATERTIARYHOSPITAL RManzanoLorenzo*,JCTallonMartinez,NSanchez-OcañaMartin,PPastorVara, JCorazonVillanueva,AFernandezRuiz-Moron,LYbañezGarcia,JMMartinezSesmero. HospitalClinicoSanCarlos,Pharmacy,Madrid,SpainBackgroundandImportance
Theincorporationofnirmatrelvir/ ritonavirintothetherapeuticarsenalforthetreatmentof SARS-CoV2infectionhasmadeitnecessaryforPharmacy departmentstoactivatecircuitsandtoolsthatallowusto adequatelyreviewthepotentialmultipleinteractionsthatritonavircanproduce.
AimandObjectives Todescribetheinteractionsdetectedsince thebeginningoftheuseofnirmatrelvir/ritonavirinatertiary hospital.
MaterialandMethods Allpatientswhoreceivednirmatrelvir/ ritonavirfromApriltotheendofAugust2022were included.Thepatient‘susualtreatmentwasconsultedinthe electronicprescriptionsystemoftheregionofMadrid,aswell asaninterviewwiththepatient,andthemedicalhistorywas consultedwhendeemednecessary.Forthedetectionofinteractions,the ‘COVID-19DrugInteractions’ platformofthe UniversityofLiverpoolwasusedandFarmaweb,anapplicationoftheMadridHealthService,wasusedtovalidatethe dispensingofmedication.Ifthereareanyinteraccionesthe pharmacistnotifiestheprescribingphysician,aswellasthe necessaryadjustments,thesetreatmentmodificationsarealso explainedtothepatientwhenthemedicationisgivento them.AnExceltablewasusedtorecordwhetherthepatient hadanyinteractionand,iftherewereany,thedrugswere recorded.
Results Duringthestudyperiod,thesedrugsweredispensed toatotalof81patients,andinteractionswiththepatient‘ s usualmedicationweredetectedin61.73%(50patients).18 patientshadoneinteraction,21patientshad2interactions,6 patientshad3,4patientshad4andonepatienthad5potentialinteractions.Themostcommonlydetectedinteractionwas withatorvastatin(19)followedbymetamizole(11),simvastatin (7),amlodipine(6)andtramadol(4).
ConclusionandRelevance Thepercentageofpatientswith interactionsisveryhigh,anditisveryimportanttoreview theusualtreatmentaswellasaninterviewwiththepatientto identifywhetherthepatientistakingotherunregisteredmedicationthatcouldinteract.
Thishashighlightedtheimportanceofinterdisciplinarycollaborationbetweenthemedicalteam(mainlyintheemergency department,wheremostofthesedrugshavebeenprescribed) andthepharmacyteamtoensurethecorrectuseofthis drug.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-075 PROPOSALFORTHEADJUSTMENTAND OPTIMISATIONOFTHEMEDICATIONPROVIDEDBY THEPATIENT
1IGonzález, 2ISotoBaselga, 2MRMengualBarroso, 2PSanMiguelTorvisco, 2BRubio Cebrian, 2ISollano-Sancho*, 2IMoronaMinguez, 2APousadaFonseca, 2YMateosMateos, 2CMorielSanchez, 2JSolisOlivares. 1HospitalUniversitariodeMóstoles,Farmacia Hospitalaria,Móstoles,Spain; 2HospitalUniversitariodeMóstoles,FarmaciaHospitalaria, Móstoles,Spain
10.1136/ejhpharm-2023-eahp.288
BackgroundandImportance Manypatientsbringmedication withthemduringtheiradmissiontohospital,whichisa sourceoferror
AimandObjectives ToadaptthePharmacotherapeuticGuide (PTG)tohometreatmentandpatientconformitytotherapeuticexchangeduringadmission.
MaterialandMethods
Cross-sectionaldescriptivestudyof patientsadmittedtoasecondlevelhospitalon11-11-2021 withtreatmentsprescribedasMedicationProvidedbythe patient(MPP).Thesourcesofinformationusedwere:the electronicmedicalrecordandtheprescriptionprogramme. Thevariablescollectedwere:age,sex,therapeuticgroupof thePPMaccordingtotheATCclassification,inclusionstatus inthePTGand/orinthetherapeuticexchangeprotocol,and patientagreementwiththeexchangeforanotheravailable.
Results Atotalof96patientshadaprescriptionforMPP,representing28.92%ofthepatientsadmitted.42wereanalysed afterexcludingthosewhocouldnotbeintervieweddueto theirclinicalsituation,withamedianageof74.5years (IQR70-80.75),59.52%beingmale.OftheMPPs,themost frequenttherapeuticgroupwasC(38.82%)followedbyN (20%)andR(15.29%)amongothers.AnalysinggroupC,the mostfrequentsubgroupwas:agentsactingontheReninAngiotensinSystem(RAS)(33.33%),lipid-modifyingagents (21.21%).47.06%oftheMAPswereincludedinthePT. Amongthosenotincludedintheguidelines,84.78%were includedinthetherapeuticexchangeprotocolwhile15.22% werenot,whichwererecommendedtobemaintainedduring admission.80.56%ofthepatientsshowedcompliancewith thechangeforanothermedicationavailableinthehospital ConclusionandRelevance Astrikingpercentageofpatients admittedtothehospitalbringmedication,themostfrequent therapeuticgroupandsubgroupwerethoserelatedtothecardiovascularsystemandtheRAS,respectively.AhighpercentageoftheMPPwerefoundinthePTG,andcouldhavebeen dispensedbythePharmacyService.Thosemedicinesnotavailableinthehospitalwereincludedinthetherapeuticexchange protocol;Fornon-interchangeabledrugs,wasrecommendedto maintainduringadmission.Mostpatientswouldhaveno objectiontotheirmedicationbeingexchangedduringadmission.Weconsiderthatthebestapproachwouldbetoavoid thesupplyofmedicationbypatients,withallmedication beingdispensedbythePharmacyService.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-076 PATIENTS’ SATISFACTIONAFTERCHANGINGFROM 150MGTO300MGSECUKINUMABPEN PRESENTATION
PCastroSalinas*,VCharques,ARetamero,SMendiola,AFigueras,JSerrais,JMartínez, DFerrandez. HospitalUniversitariodeIgualada,Pharmacy,Igualada,Spain
10.1136/ejhpharm-2023-eahp.289
BackgroundandImportance Secukinumabisananti-interleukin-17drugusedforpsoriasis,psoriaticarthritisorspondyloarthritis.Recently,ourhospitalchangedfrom150mgto 300mgsecukinumabpenpresentationinordertosimplify treatmentandfacilitateadministration.However,aspatients oftenhaveotherexpectations,desiresandprioritiesevaluating thedegreeofsatisfactionallowsustoidentifydeficienciesand causesofdissatisfaction.
AimandObjectives Todeterminepatients’ satisfactionafter changingfrom150mgto300mgsecukinumabpen presentation.
MaterialandMethods Retrospectivestudycarriedoutina regionalhospital.Patientsontreatmentwithsecukinumab 2x150mg/monthwhochangedpresentationto300mg/month
duringNovember-December2021wereincluded.Patientswho hadn´ttakenbothpresentationsforatleast4monthsand patientsimpossibletolocatewereexcluded.Thosewhogave theirverbalconsentunderwentatelephonesurvey.Variables collected:sex,age,drugindication,treatmentduration,selfadministration,painmeasuredwithVAS(VisualAnalogueScale) withbothpresentations,presenceofadministrationsitereactionswithbothpresentations,satisfactionwithpenchange measuredfrom0to10(0minimum-10maximum),300mg pendiscontinuationandreason.Qualitativevariableswere expressedasfrequencyandpercentageandquantitativeones asmeanandstandarddeviation.StatisticalanalysiswasperformedwithExcel(v.12.0).
Results Totalnumberofpatientswith300mgpenpresentation:33.Included:24(72.2%).Women:9(42.9%).Age:49 (13.9).Patientswithpsoriasis:19(79.2%),psoriaticarthritis4 (16.7%)andspondyloarthritis1(4.2%).Treatmentduration (months)38.7(22.6).Patientswhoself-administeredmedication:23(95.8%).VASwith150mgpresentation1.8(1.2)and with300mgpresentation2.2(1.9).Regardingthe150mgpresentation,2(8.3%)patientsreportedhavingbruisesatthe injectionsiteandregardingthe300mgpresentation,3(12.5%) reportedhavingsufferedswellingthatrevertedspontaneously. Two(8.3%)hadtodiscontinuethe300mgpresentationdueto severepainduringadministration.Regardingchangesatisfaction,1(4.1%)referredtothechangeasindifferent,2(8.3%)as notsatisfactoryand21(87.5%)assatisfactory,withtheaverage satisfactionbeing8.0(2.2).
ConclusionandRelevance
. Changingfrom150mgto300mgsecukinumabpen presentationwasconsideredsatisfactoryfor87.5%of patients.
. Twopatientssufferedgreaterpainduringadministration, leadingtoareturntothepreviouspresentation.
. Itwouldbeadvisabletocarryoutadditionalfollow-upin ordertodetectpossiblereactionsattheadministrationsiteor greaterpainafterthechangeofpresentation.
ConflictofInterest Noconflictofinterest
1,2PRozsívalová*, 2JMinaříková, 1MMikešová, 1LBeková, 3Ľ Slimáková, 4A Štricová, 2EZimčíková, 1MHeislerová, 5P Šmahel, 2JMaly, 6VKoblížek. 1UniversityHospitalHradec Králové,HospitalPharmacy,HradecKralove,CzechRepublic; 2FacultyofPharmacyIn HradecKrálové-CharlesUniversity,DepartmentofSocialandClinicalPharmacy,Hradec Králové,CzechRepublic; 3UniversityHospitalBratislava,HospitalPharmacy,Bratislava, Slovakia; 4UniversityHospitalBanskáBystrica,HospitalPharmacy,BanskáBystrica,Slovakia; 5UniversityHospitalHradecKrálové,DepartmentofInfectiousDiseases,HradecKralove, CzechRepublic; 6UniversityHospitalHradecKrálové,DepartmentofPulmonaryMedicine, HradecKralove,CzechRepublic
10.1136/ejhpharm-2023-eahp.290
BackgroundandImportance AtheightofCOVID-19pandemic surgeofdeltavariant,monoclonalantibodiesbecameavital treatmentoptionforSARS-COV-2positiveoutpatientsathigh riskofseverediseaseprogression.Casirivimabandimdevimab (C/I)wereusedunderEMAemergencyuseauthorisation (EUA)andtherewaspaucityofreal-worlddataonsafetyand effectiveness.
AimandObjectives
Thestudyaimedtodescribedrugsafety, self-reportedsymptomburdenandvaccinationstatusinSARSCOV-2positiveoutpatientswithin90dayspost-C/Iinfusion.
MaterialandMethods
Prospectivemulticentricsurveyof SARS-CoV-2positiveoutpatientswithmildsymptomsathighriskofsevereCOVID-19progression(definedcriteriaunder EUAauthorisationforC/Iambulatoryadministration)wasconductedfromSeptember2021tillJanuary2022inthreeteachinghospitals.Thedatacollectedusingelectronicmedical recordscomprised:patientdetails,vaccinationstatus,dateof SARS-COV-2positivetest,indication,adversedrugreactionto infusion,hospitalisation.Structuredtelephonequestionnaire withsymptomscoringadaptedfromBLAZE-1trialwasused onD(day)0,D+7,D+29andD+90post-C/Iinfusion. DatawereanalysedusingMSExcel.Ethicscommitteeapprovalwasobtained.
Results Withinstudiedperiod404outof471patientswere included(medianage66years;57.4%females).Excluded patientsincludedprophylacticC/I,notconsentedordropped out.396patientshadthefirstCOVID-19episode.Themost frequentindicationsincludedageover65years(55.5%), hypertension(56.8%),diabetesmellitusII(19.4%).C/Iinfusionwasadministeredwithameanof2.3days(range0–11 days)sinceviruspositivity.62.4%patientshadcompletevaccination(2or3dosesComirnaty,1doseJanssenvaccine)prior C/Iinfusion.Adverseeventswerereportedby11.6%of patients,mostcommonlychills,fever,diarrhea.SubjectiveworseningofsymptomsafterC/Iinfusionwasreportedby3.4% subjectsbyD+7.11.6%patientsobservednodifferencein symptomscorebetweenD0andD+7.Altogether85%;92% and93.6%patientsreportedimprovementinsymptomburden scorebyD+7,D+29andD+90respectively.
ConclusionandRelevance Wedescribereal-lifeoutpatientutilisationofC/Iintermsofpatientcharacteristics,self-reported symptomburdenandadverseevents.TherapeuticvalueofC/I timelyadministrationisevidentinhigh-riskpatientswithcompletedvaccination.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.NEnglJMed.2021Jan21;384(3):229-237
ConflictofInterest Noconflictofinterest
5PSQ-079 FONDAPARINUXINANINFANTWITHSUSPECTED HEPARIN-INDUCEDTHROMBOCYTOPENIA.ACASE REPORT
1EWilhelmi*, 2SFerro, 1AFont, 1ACasaldáliga, 1CJMoreno, 1ÁPieras, 1MVillaronga, 1RFarré, 3RBerrueco. 1HospitalSantJoandeDeu,Pharmacy,Barcelona,Spain; 2Hospital UniversitarioLucusAugusti,Pharmacy,Lugo,Spain; 3HospitalSantJoandeDeu, Hematology,Barcelona,Spain
10.1136/ejhpharm-2023-eahp.291
BackgroundandImportance A3-month-oldinfant(3kg)was admittedinthepaediatricintensivecareunitforextracorporealmembraneoxygenation(ECMO)afterapulmonary lobectomy.
Anticoagulanttreatmentwasperformedwithunfractionated heparin(UFH).
DuringtreatmentwithUFH,thepatienthadasustained decreaseinplateletcount(>50%ofbasal)andinferiorcava deepvenousthrombosis(DVT).OnceECMOwasfinished, anticoagulanttreatmentwasmodifiedtoenoxaparin.
DuetopersistentthrombocytopeniaandDVT,heparininducedthrombocytopeniawassuspected.Anticoagulantwas replacedtofondaparinux,whoserecommendeddoseinpaediatricsis0.1mg/kg/day.
AimandObjectives Toshowtheneedtoredosefondaparinux inpaediatrics,asregisteredpresentationsdon’tallowfractionation:theyaresingle-dosepre-filledsyringesbasedontwoconcentrations:5mg/mland12.5mg/ml.
Toverifythestabilityofthepreparationthroughthestudy ofthepharmacotherapeuticeffect,indirectlymeasuredby plasmalevelsofanti-Xafactor(antiXa).
MaterialandMethods Subcutaneousfondaparinuxwasstarted atadoseof0.3mg/day(0.06mL).Tofacilitateadministration, thepreparationwasinitiallydiluted1mg/mLinnormalsaline understerileconditions.Thedosewaspackagedin1mldead spacefreesyringewithapurgedneedle.Accordingtothe datasheet,thepreparationisstablefor24hatroom temperature.
AntiXawasmonitored3hoursafteradministrations.The dosewasadjustedaccordingtoTable1untilthetargetlevel (0.5mg/l)wasreached.
Subsequently,asthedoseincreaseallowed,theundiluted dose(0.4mg/0.08ml)wasfractionatedfromcommercialpresentation.Stabilityof7daysintherefrigeratorwasdefined accordingtotheriskmatrix(lowrisk)oftheGoodPharmaceuticalPracticesforthepreparationofsteriledrugs.
Results ThedosewasadjustedaccordingtoantiXa(Table2). ThemonitoringofantiX,necessaryfortheclinicalfollow-up, allowedustoobtainindirectdataonthestabilityofthefractionateddrug,maintainingcorrectlevelsthroughouttreatment, asshowningraph.
Afterfondaparinuxinitiation,theplateletcountincreased tonormalvalues.Anticoagulationtherapywasdiscontinued afterthreemonths,uponconfirmationofDVTresolution.
Abstract5PSQ-079Table1
ConclusionandRelevance IndividualiseddosingoffondaparinuxbydilutionorfractionationhasallowedDVTtreatment, usingacommercialpresentationunsuitableforpaediatrics.
Weverifystabilityofthefractionateddosewiththetherapeuticeffect.
ICavadaCarranza*,XGarcía-González,SIbáñez-García,DGómez-Costas,AHerranzAlonso,MSanjurjo-Saez. HospitalGeneralUniversitarioGregorioMarañón,Pharmacy Department,Madrid,Spain
10.1136/ejhpharm-2023-eahp.292
BackgroundandImportance Thepreoperativesettingisan areawithhighriskformedicationerrorswithpotentially severeconsequences.Pharmaceuticalcareprogrammes(PCP) canhelptoachieveanadequatepreoperativepharmacological management,toensurepatientsreachsurgeryinoptimalpharmacologicalconditions.Adequatecoordinationwithotherspecialistssuchassurgeonsandanaesthetistsisparamountto guaranteepatientsafety.
AimandObjectives ToevaluatetheimpactofaPCPin patientsundergoingcardiacsurgeryinpreventingmedication errorsafter4yearsofimplementation.
MaterialandMethods Retrospective,observational,descriptive study.Timeofstudy:July2018-July2022.Allpatientsscheduledforcardiacsurgerywereinterviewedbyaclinicalpharmacist24-72hbeforethesurgery.Interviewswereconducted byphone.Duringtheinterview,patients’ completemedication list,includingoverthecountermedicinesandherbalproducts, wascollectedandinstructionsforadequatepreoperativemedicationmanagementaccordingtocurrentguidelinesandanaesthetistinstructionswerereinforced.
Avoidedmedicationerrorswerecategorisedaccordingto Overhage-classificationandtheirseveritywasanalysedaccordingtoNCC-MERP.
Savingswerecalculatedbymultiplyingtheprobabilityof adverseeventoccurrencewiththeerror(NCC-MERP F:high riskofadmissionorprolongedhospitalstay)byavoidedcost (6.745C ¼ accordingtoMinistryofHealth,Consumerand SocialWelfare).
Results Duringthetimeofstudy,1020pharmacistpreoperativeinterviewswereperformed.Meanagewas66.8(sd:12.6) yearsand65.8%oftheinterviewedpatientsweremales.
41.8%ofpatientsweretakingatleastonedrugthat neededtobediscontinuedbeforesurgery.Themostfrequent wereangiotensin-convertingenzymeinhibitors,angiotensin-II receptorsblockersanddiuretics(23.6%),anticoagulantsand antiplatelettreatment(22.2%)andhypoglycaemictreatment (11.4%).43.5%ofpatientsneededheparinbridgetherapy.
Atotalof807pharmacyinterventionswereconducted with94.2%ofacceptancerate:533requirementstodiscontinuedrugsbeforesurgery(70.1%),81doseerror(10.7%),49 drugomission(6.4%),32associatedwithduration,frequency orindication(4.2%).
673seriouserrorswereavoided,236(31.1%)ofthese errorscouldhaveresultedinpermanentharm(G/H),277 (36.4%)intemporaryharm(E/F)and160(21.1%)monitoring patientstoconfirmnoharm(D).
Potentialmedicationerrorsavoidedanestimatedcostof 992.130C ¼ .
ConclusionandRelevance APCPinpatientsundergoingcardiacsurgerywassuccessfullyimplemented,ensuringacorrect preoperativedrugmanagement,with0.8severemedication errorsavoidedperpatientthatwasinterviewedandpotential savingsof992.130C ¼ .
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-084 ASSESSINGQUALITYOFLIFEOFPATIENTSWITH SEVEREASTHMAMEASUREDBYPATIENTREPORTED OUTCOMES
1MMuñozGarcía:, 1HMartínez-Barros, 1PGuijarro-Martínez, 2ASantamaria-Gadea, 3AdeAndrés-Martín, 1DGonzález-de-Olano, 4AMorales-Tirado, 5DAntolín-Amérigo, 6SSánchezCuéllar, 1AMAlvarezDiaz*. 1HospitalUniversitarioRamonYCajal,Pharmacy,Madrid, Spain; 2HospitalUniversitarioRamonYCajal,Otorhinolaringology,Madrid,Spain; 3Hospital UniversitarioRamonYCajal,Inmunology,Madrid,Spain; 4HospitalUniversitarioRamonY Cajal,PediatricsService,Madrid,Spain; 5HospitalUniversitarioRamonYCajal,Allergy, Madrid,Spain; 6HospitalUniversitarioRamonYCajal,Neumology,Madrid,Spain
10.1136/ejhpharm-2023-eahp.293
BackgroundandImportance Severeasthma,whichaffects approximately5-10%ofpeoplewithasthma,isaheterogenous,chronicandcomplexdisease.Itusuallyhasanegative physical,mental,emotionalandsocialimpact.
AimandObjectives Toanalysequalityoflifeofpatientswith severeasthmacurrentlytreatedwithbiologicaldrugsusing PatientReportedOutcomesMeasures(PROM).
Toidentifythedomainsandspecificquestionsmostfrequentlyaltered,byusingmini-AQLQ.MaterialandMethods
Adescriptivecross-sectionalstudybetweenApril-May2022 inatertiaryhospitalwascarriedout.Adultpatientswith severeasthmaonactivetreatmentwithabiologicaldrugfor atleastoneyearwhoprovidedinformedconsentwere included.
Electronicmedicalrecordswerereviewedtoobtain:
. Age,sex
. Numberofasthma-associatedcomorbidities
. FEV1andFEV/FVC
Atelephoneinterviewwasalsoconductedtorecordthe PROMs:
. Mini-AQLQ(maximumscore:7)
. EQ5D-5L(maximumscore:1)(withVisualanaloguescale, score0-100(VAS))
Results Fifty-fivepatientswhomettheinclusioncriteriawere identified.38whoagreedtoparticipatewerelocated.Median agewas65years(56-71.5)and60.5%(23)werefemale. Mediannumberofasthma-relatedcomorbiditieswas2.5(1-4). FEV1andFEV/CVFwere76.3%(SD=3.2)and69% (SD=1.3)respectively.
ThemeanscorefortheEQ5D-5Lwas0.924(0.818-1), whilethemedianVASwas70(60-85).
Theaveragescoreforthemini-AQLQwas6.1.By domains,environmentalhadtheworstmean(5(4.3-6.3)),followedbylimitationofactivities(6.1(5.5-6.7)),symptoms(6.2 (5.8-7))andemotional(7(5.3-7)).
Three(0.8%)patientsdidnothaveanydisturbancesinthe responses,buta81.6%(31)hadalteredlimitationofactivities,79.3%(29)environmental,73.7%(28)symptomsand 55.3%(21)emotional.
Specifically,thequestionthatmostfrequentlyreceivea scorebelow7was ‘didyoufeelthattobaccosmokebothered youordidyouhavetoavoidaplacebecauseoftobacco smoke?’ in76.3%(29)patients.
ConclusionandRelevance Thequalityoflifeofpatientswith severeasthmatreatedwithbiologicaldrugsisgood,according tospecificasthmaquestionnairesusedasPROM,althoughfew patientsdonothaveanyalteredsphere.
Themostalteredspherewasenvironmental.Tobaccois consideredamajorthreat.
ConflictofInterest Noconflictofinterest
5PSQ-086 ANALYSISOFPOTENCIALLYINAPPROPRIATE PRESCRIPTIONINANURSINGHOME
MRodriguezJorge*,RSánchezdelMoral,IGarcíaGiménez. HospitalJuanRamónJiménez, Pharmacy,Huelva,Spain
10.1136/ejhpharm-2023-eahp.294
BackgroundandImportance Potenciallyinappropriatemedicationprescriptioncanincreasetheriskofadversedrugreactions(ADRs).Therefore,multipletoolshavebeendeveloped todetectinappropriateprescriptions.STOPP(ScreeningTool ofOlderPerson’sPrescriptions)/START(ScreeningToolto AlerttoRightTreatment)criteriaisoneofthem.
AimandObjectives Toanalyseinappropriateprescriptions(IP) ortheneedofpotencialprescriptionsinpolymedicatedinstitutionalisedpatientsinordertoimprovepatientssafety.
MaterialandMethods Adescriptive,transversalstudywasperformedinSeptember2022.Weincludedallpolymedicated residents(>6drugs)ofanursinghomeattachedtoaPharmacyDepartment.Datacollectwereage,sex,numberofmedications/residentanddrugsprescribed.STOPP/STARTcriteria v.2.wasappliedtodetectinappropriateprescriptionsorthe needofpotencialtreatment.DatawerecollectedfromelectronicprescriptionprogrammeATHOS-PrismaandcomputerisedmedicalrecordDiraya.
Results Atotalof50patientswereincluded,66%men.The medianagewas73years(range:69-83).Averagedrugsprescribedbyresidentswas10(6-21).
Seventy-twopercentoftheresidents(36)presentedatleast oneSTOPPcriteria.TotalIPswere142,withanaverageof5 IPsperresident(0-7).Mostprevalentweretreatmentduration longerthandefined(72%),prolongeduse(>4weeks)of benzodiazepines(72%),drugsthatadverselyaffectfallers (mostrelatedtobenzodiazepines)(72%)andprescriptionof twodrugswithinthesameclass(22%).
RegardingSTARTcriteria,23residents(42%)presented anyprescriptioninitiationcriteria.Thetotalpotencialprescribingomissionwere26,withanaverageof1perresident(02).Themostcommonwere:useoflaxativesinpatientswith opioidtreatments(47,8%)andvitaminDsupplementsin olderpatients(34,8%).
ConclusionandRelevance STOPPcriteriawasthemostfrequentlyfound.Themajorityrelationedwithinappropriate durationorduplicityofbenzodiazepintreatment.
ForSTARTcriteria,theindicationoflaxativesforpatients receivingopioidsonaregularbasiswasthemostfrequent potencialprescribingomission.
TheuseofSTOPP/STARTcriteriacouldimprovepatients safety,whichareabletodetecttheinappropriateprescription
ofsomedrugsinadditiontotheomissionofpotencialindicatedmedication.
ConflictofInterest Noconflictofinterest
ConflictofInterest Noconflictofinterest
BMoñinoBlazquez*,MLigrosTorres,JBarrosoCastro,MDuqueRodriguez,ACalleja Bueno,XAntonMendez,AFernandezPeña,MLopezGillete,BSanJoseRuiz. Cruces UniversityHospital,PharmacyService,Barakaldo,Spain
10.1136/ejhpharm-2023-eahp.295
5PSQ-091 TRIFECTA™ BIOPROSTHESES:EVALUATIONOFTHE SAFETYBASEDONTHESTUDYOFDEGENERATIONS ACCORDINGTOTHEVARC-3CLASSIFICATION
1ORichez*, 2YHun-Chabry, 3DMalaquin, 3JHudelo, 2TCaus, 1APetit. 1Amiens-Picardie TeachingHospital,Pharmacy,Amiens,France; 2Amiens-PicardieTeachingHospital,Cardiac Surgery,Amiens,France; 3Amiens-PicardieTeachingHospital,Cardiology,Amiens,France 10.1136/ejhpharm-2023-eahp.296
BackgroundandImportance
TheuseoflamivudineasprophylactictreatmentinpatientswithpasthepatitisBvirus(HBV) infectionandontreatmentwithdrugsconsideredathighrisk ofreactivationhasbeenassociatedwithahigherlikelihoodof reactivationcomparedtotheuseofotherantivirals.
BackgroundandImportance In2021,cardiologistsreportedto themedical-devices-vigilancesectorseriousincidentsinfour patientswithafirst-generationTrifecta ™ bioprosthesisthat resultedinthreeaorticvalvereplacement(AVR)andone death.Thequestionofdegenerationoftheirbioprosthesis arose.
AimandObjectives Theaimwastoevaluatetheintrinsic imputabilityofTrifecta™ fordysfunctioninpatientsimplanted andtoreassesstheirreferencinginourcentre.
AimandObjectives
Toevaluatetheeffectivenessoflamivudine intheprophylaxisofHBVreactivationinpatientswithhaematologicaldisease,undergoingimmunosuppressiveorchemotherapytreatmentandpresentingpositiveserologyforHBV.
MaterialandMethods
Observationalandretrospectivestudy includingallhaematologicalpatientsover18yearsofagewho startedHBVprophylaxisbetweenJanuary2018andDecember 2020inatertiaryhospital.Follow-upwasperformedfrom thestartoftreatmentuntilDecember2021toobserve whetherHBVreactivationoccurred.
Electronicmedicalrecordswerereviewedandthefollowingvariableswerecollected:demographicdata(ageandsex), haematologicaldiagnosis,immunosuppressiveorchemotherapytreatmentreceived,analyticaldata(HBsAg,HBcAc, HBeAc,HBVDNA,transaminases)andHBVprophylactic treatment.
Results Inthestudyperiod,65patientsstartedHBVprophylaxis,ofwhich3patientswereexcludedduetofalsepositive. Sixty-twopatients(33women)werereviewed,withamedian age(range)of70years(20-91).Diagnoseswerelymphomas (26patients),monoclonalgammopathies(13),chroniclymphoproliferativesyndromes(7),autoimmunediseases(6),acute leukemias(5),chronicmyeloproliferativesyndromes(4)and bonemarrowaplasia(1).
Outofthe62patients,60patientswereHBsAgnegative andanti-HBcpositiveattheinitialserologicalcontrol.Allof whichreceivedlamivudineprophylaxis.Theother2patients hadchronicHBVinfectionatthestartofprophylaxis,with positiveHBsAg,positiveanti-HBeandundetectableHBV DNA.Oneofthemstartedprophylaxiswithtenofovir,and theotherreceivedlamivudineasprophylaxis.
Ofthepatientswhostartedlamivudineprophylaxis,60.7% werebeingtreatedwithdrugsconsideredathighriskofreactivation(rituximab,doxorubicinoridarubicin).
Nopatienthadeitherclinicalreactivationordetectable HBVviralloadduringthestudyperiod.Fourteenpatients diedduringfollow-upduetonon-HBVcauses.
ConclusionandRelevance Inourpatients,60.7%ofwhom receivedhigh-riskdrugs,noreactivationeventoccurred.LamivudinehasproventobeeffectiveintheprophylaxisofHBV reactivationinourstudypopulation.
MaterialandMethods Aretrospective,single-centreandobservationalstudyofcomputerisedpatientrecords(CPR)wasconductedbetween02/04/2011,dateofourcentre’sfirst implantation,and12/31/2016tohave5yearsoffollow-up perpatient.
Trifecta ™ valvesanddatarelatedtotheimplantationwere extractedfromthetraceabilitysoftware.Thecollectionof echographicandclinicalfollow-updatawasbasedontheCPR withanextendedfollow-upperioduntil03/31/2022.
DysfunctionswereclassifiedaccordingtotheVARC-3classificationcriteria:structuralvalvedeterioration(SVD),nonstructuralvalvedysfonction(NSVD),thrombosisand endocarditis.
Thestudywasapprovedbyourlocalresearchdepartment. Results Atotalof382bioprostheseswasimplantedin378 patients,meanage73.0yearsand60.7%male.Datawere missingfor253bioprosthesesand15patientsdiedperioperatively.Amongthe114bioprostheseswithconclusivedata,50 functionnedproperly(meanfollow-uptimeof6.6years)and 64presenteddysfunctions:34SVD,10NSVD(8paravalvularregurgitation,2prosthesis-patientmismatches)and20 endocarditis.AVRoccurredfor20patientsfollowingSVDand for11patientsfollowingendocarditis(4ofwhomhadasecondTrifecta™)withinameantimeof6.7yearsand3.4years, respectively.
ConclusionandRelevance TheclassificationoffailuresaccordingtoVARC-3allowedustoconfirmtheintrinsicimputabilityoftheTrifecta™ bioprosthesesregardingtothenumberof SVD-typedysfunctions.Althoughthisstudyhaslimitations,it showstheunderstatementofmedical-devices-vigilancecasesby themedicalstaff.The64fileswithdysfunstionswillbetransmittedtothenatonalhealthautority.Thepatientswillbe reviewedtocompletethedataandperformanechographic follow-up.Accordingtothemanufacturer,degenerationscould berelatedtotheexpansionsystemthatwasimprovedinthe second-generationTrifecta™ marketedin2016.Sincethis study,theTrifecta ™ hasbeenremovedfromthehospital formulary.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
1ISánchezLobón*, 2MDCJiménezde-Juan, 1JTudelaTomás, 1VManzanoMartín, 1RPla Pasán. 1PuertaDelMarUniversityHospital,Pharmacy,Cádiz,Spain; 2RiotintoHospital, Pharmacy,Huelva,Spain
10.1136/ejhpharm-2023-eahp.297
BackgroundandImportance Zolpidemisabenzodiazepine-like hypnoticthatactsonGABA-omegareceptorsinthecentral nervoussystem.Itisindicatedfortheshort-termtreatmentof insomniainadults.
In2014,aninformativenotewaspublishedbyTheSpanish MedicinesandMedicalDevicesAgency(AEMPS)recommendingadoseof5mg/dayinelderlypatients(over65yearsold), insteadof10mg(usualdose),inordertoreducethenumber ofcasesofalterationsinattentionandconcentrationcapacity, includingparasomnias.
AimandObjectives Evaluatetheimpactofhospitalpharmaceuticalintervention(PI)ontheprescriptionofzolpidemafter thepublicationoftheAEMPSinformativenote.
MaterialandMethods Multicentreandprospectiveintervention studywhichincludesallpatientsadmittedtotreatmentwith zolpidem.ThestudyintervalwasfromSeptember2021to September2022.Thevariablescollectedwerethefollowing: age,sex,dosageandprescriptionofzolpidemashometreatment.Clinicalrecords(Diraya®)andtheelectronicprescriptionprogram(Prisma®)werereviewed.TheIFconsistedof sendinganinformativenotetothedoctorresponsibleforthe patientswhodidnotcomplywiththeAEMPSrecommendation.
Results Atotalof62patientswereincluded(meanage:72± 15years;sex:37men).PIwasperformedin59.7%(37/62) becausetheprescriptionwasnotadjustedtotheAEMPSalert. Regardingthe37patientswithinappropriateprescription,the dosewasreducedto5mg/dayin37.8%(14/37)ofthecases. Thedoseoftherestofpatients,62,2%(23/37),wasnot change,ofwhich87%(20/23)hadtheoriginoftheprescriptionattheprimarycarelevel.
ConclusionandRelevance TheacceptanceofthePIwasperformedinalownumberofcasesduetothefactthattheoriginofzolpidemprescriptionsisprimarycare.Thiscreatesthe needtoestablishchannelsofcommunicationbetweentheprimarycarephysicianandthehospitalpharmacisttoreportpossibleerrorsdetectedintheirprescriptions.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
AimandObjectives 1)Toevaluatethehaematologicaltoxicity ofvenetoclaxduringdoseescalationand;2)TodescribeAE associatedwithvenetoclaxduringtreatment.
MaterialandMethods Multicentre,observational,retrospective studyinpatientswhoinitiatedvenetoclaxuntil01/06/2022 withatreatmentperiod 3months.Variablescollected:sex, age,diagnosis,treatmentschedule,hemoglobin,neutrophiland plateletlevelsatbaselineandafterdoseescalationand;AE developedduringtreatmentappearanceaswellasitsgravity accordingtoCommonTerminologyCriteriaforAdverse Events(CTCAE)version5.0.
Hematologictoxicityduringescalationwasanalysedusing Student’st-test(SPSSStatistics25.0).
Results 41patientsinitiatedvenetoclax,ofwhom33maintainedtreatment 3months(63.6%male,mean68.7±9.7 years).Diagnosis(CLL:20,AML:10,myelodysplasticsyndrome:3),treatmentschedule[monotherapy:2;bitherapy (rituximab:16,azacitidine:13,decitabine:1,obinutuzumab: 1)].5patientsrequireddoseadjustmentduetoconcomitant useofazoles(posaconazole:2,voriconazole:2,fluconazole: 1).
Meanhemoglobinatbaselineandafterdoseescalation (10.6±1.9vs10.8±2.1g/dL;p=0.282),meanneutrophils atbaselineandafterdoseescalation(1,667.6±1,064.9vs 1,237.3±1,011.5/mL;p=0.001),meanplateletsatbaseline andafterdoseescalation(120,060.0±77,662.3vs116,121 ±77,012.0/mm3;p=0.697).AEdevelopedduringtreatment: anaemia(G2:3,G3:4),neutropenia(G1:1,G2:6;G3:6,G4:4), thrombocytopenia(G2:1,G3:4),asthenia(G1:2,G3:1),bradycardia(G2:1),diarrhoea(G1:1),fever(G1:1),hypertransaminemia(G2:1),mucositis(G1:1),pneumonia(G2:2,G3:3), tumourlysissyndrome(G3:2).Duringtreatment,15patients requireddiscontinuationoftreatment(restarts:7)and5 requireddosereduction.
ConclusionandRelevance Duringdoseescalation,themain haematologicaltoxicityofvenetoclaxwasneutropenia.This adverseeffectalsooccurredmorefrequentlyduringmaintenancetreatment.Weconsideritrelevanttocarryoutserial haematologicalcontrolsinpatientstreatedwithvenetoclax.
Limitationsofthestudy:retrospectivestudywithasmall samplesize;therefore,itisconsiderednecessarytoperform morestudiestoconfirmtheresultspresented.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.CommonTerminologyCriteriaforAdverseEvents(CTCAE)Version5.0.27 November2017.
ConflictofInterest Noconflictofinterest
5PSQ-094 TOXICITYINPATIENTSTREATEDWITHVENETOCLAX. ASAFETYSTUDYINREAL-WORLDPRACTICE
1APérezFácila*, 2PCiudadGutiérrez, 2MFalcónCubillo, 1CNotarioDongil, 1JJSaiz Molina, 1NAndrésNavarro. 1HospitalGenerallaManchaCentro,FarmaciaHospitalaria, AlcázardeSanJuanCiudadReal,Spain; 2HospitalUniversitarioVirgenDelRocío,Farmacia Hospitalaria,Sevilla,Spain
10.1136/ejhpharm-2023-eahp.298
BackgroundandImportance Venetoclaxactsasaninhibitorof theanti-apoptoticproteinBcl-2,whichisincreasedin ChronicLymphocyticLeukemia(CLL)andAcuteMyeloid Leukemia(AML).Itisdescribedonitslabelthefrequent occurrenceofhaematologicaltoxicity,amongotheradverse events(AE).
5PSQ-095 CARDIACCONDUCTIONDISORDERSASSOCIATED WITHTHEUSEOFTRICYCLICANTIDEPRESSANTSIN THEELDERLY
DGuerraEstévez*,MReyesMalia,CPalomoPalomo,MRomeroAlonso,EContreras Macías,AGanforninaAndrades,JEstaireGutiérrez. HospitalInfantaElena,Hospital PharmacyDepartment,Huelva,Spain
10.1136/ejhpharm-2023-eahp.299
BackgroundandImportance Tricyclicantidepressants(TCAs) blocksodiumchannelsintheheart,whichcanprolongthe QTintervalandcausearrhythmias.Patientsover64yearsof ageareatincreasedriskofthesesideeffects.
AimandObjectives ToconductasystematicreviewofthecardiaceffectsofTCAsinpatientsolderthan64years.Asasecondaryobjective,thefrequencyofTCAsprescriptionsin patientsolderthan64yearswithcardiacconductiondisorders (CCD)wasanalysed,reviewingconcomitanttreatments.
MaterialandMethods Asystematicreviewofthepublished scientificliteraturewasconductedfollowingPRISMADeclaration.Inaddition,adescriptivecross-sectionalstudywas carriedout,includingallpatientsover64yearsofage receivingTCAstreatment.Ananonymiseddatabasecontainingthevariablesage,sex,andprescribedmedicationswas used.
Results Afterthesearch,5articleswereincludedinthequalitativesynthesis.AstudyconcludesthatTCAscauseCCD, butwithoutclinicalcompromise.ThesecondshowsanassociationbetweentheuseofTCAsandsuddendeathin patientswithpreviousheartdisease(HD).Anotherstudy concludesthatnormaldosesofTCAsinpatientswithsevere HDareequivalenttotoxicdosesinpatientswithoutHD. Thefourthshowsnocorrelationbetweenserumsodiumlevels,electrocardiogramchanges,andseverityofTCAstoxicity.
ThelateststudyshowsthatprolongedexposuretoTCAsis alsorelatedtotheoccurrenceofcoronarydiseaseeventsin patientswithoutknownHD.Theprescriptionsof63patients receivingTCAswithamedianageof70(65-88)yearswere reviewed.Nopatienthad prescribedtreatmentsforCCD, however,49,2%ofpatientshadprescribed³1drugthatprolongstheQTinterval.
ConclusionandRelevance Theliteraturereviewedreveals CCDcausedbyTCAs.InthedatasheetofTCAs,theiruse iscontraindicatedinpatientswithpreviousHD.Inoursample,theprescriptionofTCAsisappropriate;however,we recommendthatinpatientsover64yearsofagewithout CCD,electrocardiogramsbeperformedbeforestartingtreatmentwithTCAsandperiodically.Inaddition,afterverifying thehighfrequencyofprescriptionofdrugsthatprolongthe QTinterval,webelievethatitisessentialtoreviewtheconcomitantmedication,lookingfortherapeuticalternativesfor thesedrugs.
ConflictofInterest Noconflictofinterest
1COrtíJuan*, 1CToroBlanch, 2MAGispertAmetller, 1APérezPlasencia, 2CLechaOchoa, 1QLópezNoguera, 1RSacrestGüell. 1Hospitaluniversitaridrjoseptrueta,pharmacy department,girona,spain; 2Hospitaluniversitaridrjoseptrueta,emergencydepartment, girona,spain
10.1136/ejhpharm-2023-eahp.300
BackgroundandImportance Prescribingerrors(PE)arean importantcauseofmedication-relatedadverseeventsinthe EmergencyDepartments(ED)butlimiteddataareavailablein EDwith electronic prescribingand administration(ePA)systems.KnowingthefrequencyandtypesofPEcanhelp healthcareprofessionalstopreventandreducetheriskof themoccurring.
AimandObjectives TodeterminetherateofPEintheED,to classifyincidenttypesandtoidentifycriticalpointswhere measuresshouldbeimplementedtoimprovepatientsafety.
MaterialandMethods Prospective,observationalandcross-sectionalstudyinanEDwithePAsystemduring6workingdays (May-June2021).Theinclusioncriteriawerepatientsstayed morethan8hoursintheEDandallpatientsawaitinghospitalisation.Prescriptionswereanalysedbyamultidisciplinary teammadeupoftwopharmacists,anemergencyphysician andthepersoninchargeofthehospital’smedicationerrors committee.PEwerereportedtothehospital’spatientsafetyrelatedincidentnotificationsystem.
Results Ofthe65prescriptionsrevisedduringthestudy period,PEwerereportedin84casesand15situationswith thecapacitytocauseerrorsweredetected.Theaverageageof patientswas67±(SD=17,9)yearsandeachprescriptionhad anaverageof8.4medications.TherateofPEwas1.52 errorsperpatient,beinghigherinlessseverepatientsthan monitoredpatients(1.09vs2.0PEperpatient,respectively). ThemostcommontypesofEPwereomissionoftheusual medication(60.7%),wrongdose(15.5%),wrongfrequency (7.1%)anddrugisnotindicated(7.1%).Noadversereactions relatedtoEPweredetected.AccordingtotheSpanishconsensusabout MedicationReconciliationinEmergencyUnits, 47.1%ofomissionsofusualmedicationweredrugsthat shouldbereconciledduringthefirst4hoursintheED.The resultsofthestudyandtheimportanceofmedicationreconciliationarehighlightedinasessionintheED.
ConclusionandRelevance ThePErateintheEDwas1.52 perpatientandthemaintypewasomissionoftheusualmedication.Acrosssectionalstudywillbemadeinthefutureand comparedtothecurrentonetoestablishtheimpactofthe implementedmeasuresonthePErate.
PHARMACEUTICALINTERVENTION
ISánchezLobón*,RPlaPasán,AGanforninaAndrades,VManzanoMartin,MSaldaña Valderas,MJHuertasFernández. PuertaDelMarUniversityHospital,Pharmacy,Cádiz, Spain
10.1136/ejhpharm-2023-eahp.301
BackgroundandImportance Tofacitinibisaselectiveinhibitor ofthejanuskinasefamilyindicatedforthetreatmentofvariousrheumatologicalpathologiessuchasrheumatoidarthritis (RA)andpsoriaticarthritis(PsA)andused,offlabel,inpathologiessuchasalopeciaareata(AA).
TheSpanishMedicinesandMedicalDevicesAgency (AEMPS)releasedinJuly2021asafetyalertstatingthat patientsover65yearsofage,smokersorex-smokersand withcardiovascularriskfactorsorwithapredispositiontothe developmentofneoplasms,shouldnotreceivetreatmentwith tofacitinibunlessnootheravailabletherapeuticalternativecan beused,basedintheresultsfromtheORALSurveillanceclinicaltrial.HealthpolicyinAndalucíaestablishestheneedto follow-upontheapplicationofthesafetynotesissuedbythe AEMPSregardingprescriptionsofdrugs.
AimandObjectives Evaluatethepharmaceuticalintervention onthereviewoftofacitinibprescriptionstoensuretheiradaptationtothecriteriaestablishedbytheAEMPS,accordingto theAndalusianregionalregulations.
MaterialandMethods Retrospectivereviewoftofacitinibprescriptioninatertiaryhospital.Allpatientsontreatmentwith
tofacitinibfromJuly2021toFebruary2022wereincluded. Variablescollectedwereage,sex,riskfactorsincludedinthe healthalertandcontinuationordiscontinuationoftreatment.
Results Atotalof71patientsreceivingtofacitinibtreatment wereincluded(meanage:41±16;sex:74.6%women).The treatmentwasdiscontinuedin25.4%(18/71)ofthepatients duetoinefficacy,adversereactionsorpresentingatleastone riskfactor.However,74.6%(53/71)ofthepatientscontinued treatment,with43.4%(23/53)havingatleastoneriskfactor. ResultswereshowntothePharmacyCommission,wherethe pharmacistdevelopedaprotocolregardingtofacitinibsafety issues.
ConclusionandRelevance Thisisthefirstexperienceinour hospitalregardingtheglobalmonitoringofsafetynotes releasedbytheAEMPS,endorsedbyautonomicregulation. Despitethepresenceofriskfactors,tofacitinibwasnotwithdrawnnorjustifiedinahighpercentageofpatients.Thisfindingunderlinestherelevanceofsystematicpatientsfollow-up andtheneedtodevelopprotocolsagreedbythepharmacists andinvolvedphysicians.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-099 ADEQUACYOFSOTROVIMABPRESCRIPTIONINSARSCOV-2INFECTIONINAUNIVERSITYHOSPITAL
TBlancoEspeso,MFloridoFrancisco,RSerranoGiménez,MRodriguezJorge*. Hospital JuanRamónJiménez,HospitalPharmacy,Huelva,Spain
10.1136/ejhpharm-2023-eahp.302
BackgroundandImportance TheSpanishMedicinesand HealthProductsAgency(AEMPS)hasdevelopedcriteriato adapttheprescriptionofsotrovimab1,duetothepandemic situationandthelimiteddrugstock.
AimandObjectives Todescribethepatients´populationon treatmentwithsotrovimabandtoassesstheadequacyofthis prescriptionaccordingtothecriteriaestablishedbythe AEMPS.
MaterialandMethods RetrospectiveobservationalstudyanalysingallsotrovimabprescriptionsinpatientswithSARS-CoV-2 infectionfrom01/25/2022to08/31/2022.
DemographicvariablesanddatarequiredbytheAEMPS forsotrovimabprescriptionwerecollected:Omicronvariant infection,SARS-CoV-2vaccinationstatus,serology[anti-Santibody<260BAU(bindingantibodyunits)/mL].Also,patients hadtobelongtooneofthefollowinggroups:
. Group1:Immunocompromised,regardlessofvaccination status.
. Group2:>80yearsunvaccinated.
. Group3:>65years(regardlessofvaccinationstatus)and 1 riskfactorforprogression.
Prescriptionsforsotrovimabwerecollectedandanalysedto determinewhethertheymetthecriteriaandwhetherthey wereaccepted.Datacollectedfromelectronicmedicalrecords andprocessedusingExcel2019®.
Results Fiftypatientswereincluded,62%male;medianage 69years(IQR=60-76).100%hadtheOmicronvariant.Vaccinationstatus:84%complete,6%incompleteand10%unvaccinated.Serology:96%(<260BAU/mL)and4%(>260BAU/ ml).92%belongedtogroup1(39%solidorgantransplantation,29%activemyelotoxicchemotherapy,13%non-cytotoxic
onco-haematologicaltreatmentswithneutropenia/lymphopenia, 13%treatmentwithbiologicalimmunomodulators,2% Down’ssyndrome,2%haematopoieticstemcelltransplantationorCAR-T,2%HIVinfection(with £200cells/mL).Two percentbelongedtogroup2.Theremainingpatients(6%) didnotbelongtoanygroup.Tenpercentoftheapplications didnotmeetthecriteria:fourofthemwerenotaccepted (patientsdidnotbelongtoanyriskgroup);onewasaccepted, althoughitwasawell-controlledHIV.
ConclusionandRelevance Themainprofileofpatientstreated withsotrovimabismenwithsolidorgantransplantation,vaccinatedandwithnegativeimmunitytoSARS-CoV-2.Although theappropriatenessoftheprescriptionishigh,itisnecessary tocontinueprotocolisingtheuseofthisdrugtoensureits rationaluse.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.1.https://www.aemps.gob.es/medicamentos-de-uso-humano/acceso-a-medicamentos-en-situaciones-especiales/criterios-para-valorar-la-administracion-de-las-nuevasalternativas-terapeuticas-antivirales-frente-a-la-infeccion-por-sars-cov-2/
ConflictofInterest Noconflictofinterest
5PSQ-100 UTILITYOFSOCIALMEDIAASASOURCEOF
PAEDIATRICDRUGSAFETY,ASYSTEMATICREVIEW 1IVilimelis*, 2,3APérez-Ricart, 1,2MBoschPeligero, 4ACalvo, 2EVallsSánchez, 2CCodinaJiménez, 2SMarin, 1JMSuñéNegre, 2CQuiñonesRibas, 5JCGiménez-Juárez. 1Universitat deBarcelona.,PharmacyFacultyCampusDiagonal.,Barcelona,Spain; 2HospitalUniversitari GermansTriasIPujol-.,PharmacyDepartment,Badalona,Spain; 3ServeiCatalàdelaSalutÀreaMetropolitanaNord-RegióSanitàriadeBarcelona,PharmacyUnit,SantCugatDel Vallès,Spain; 4UniversitatPolitècnicadeCatalunya,ComputerScience,Barcelona,Spain; 5Centred’informaciódelmedicamenthospitaluniversitarivalld’hebron,pharmacyservice, barcelona,spain
10.1136/ejhpharm-2023-eahp.303
BackgroundandImportance Paediatricpopulationarepredisposedtohavemoreadversedrugreactions(ADR)andother drugrelatedproblems(DRP).Socialmedia(SM)couldbean innocuoussourceofpharmacovigilance.
AimandObjectives AssessADRandDRPevidencereportedin SM.
MaterialandMethods AsystematicreviewaccordingPRISMA recommendationswasconductedinMEDLINE,Embaseand LILACS.ArticlesinEnglish,SpanishandCatalanlanguages frominceptionuptoSeptember2021werereviewedusing searchtermsrelatedtopaediatricage,SMandDRP.Inthe screeningphase,articlesnotmentioningpaediatricsandSM wereexcludedincludinggreyliterature.Intheeligibility phase,articlesrelatedtonon-pharmacologicaltreatments/substances,surveys,recruitmentprotocols,sociologicalstudies, professionaluseofSMandtechnologicalimplementationwere excluded.Articlesincludinginformationaboutcommonlyused drugsinpaediatricswereevaluated.Demographicvariables,SM platforms,medicinesandtypeofinformation(ADR,DRPor experiencesandopinions(EO))wereanalysed.
Results 6079articleswereassessedand28(0,4%)metthe inclusioncriteria.16(57%)studieswerequalitative,6(21%) quantitativeandqualitativeandquantitative6(21%).When mentioned,mostarticlesanalyseddatafromparents/caregivers (10;36%)andadolescents(2;7%).GenderofSMuserwasnot systematicallyreportedbutfemaleswerereportedin7(25%) articlesinarangeinof22-77%,inanarticle245females comparedto74malesandonereferredthatpostswere
mostlyfrommothersofyoungchildren.Mostarticlesincluded datafromforums(13;46%),Twitter(5;18%)andFacebook (6;21%).17(61%)reportedinformationaboutvaccines,3 (11%)asthmamedicationsand8(28%)othermedicines.8 articles(28%)reportedanADRincludingtremor,auto-injector woundsandvaccineADR.Onlyinonearticletheseverity wasreported.EOwerereportedin25(89%)studiesand10 (36%)articlesmentionedaDRP.Studiesreportedlackof adherence(4;14%),difficulties(3;11%)ordoubts(2;7%) aboutdrugadministrationofasthmainhalers(2;7%),epinephrineauto-injector(1;4%),antibiotics(1;4%),oraldrugs (1;4%),ophthalmicdrugs(1;4%)andtopicaldrugs(1;4%).
ConclusionandRelevance Articlesevaluatingpharmacological drugsinpaediatricsfocusedmostlyonEOandscarcedata aboutADRandDRPwerementionedinSM.Consequently, morestudiesarerequiredtotakeadvantagefromSMasa potentialtoolinpaediatricpharmacovigilance.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
WGuibane*,ZSaoudi,NHagui,MGargouri. HospitalPharmacyDepartment-CenterFor TraumatologyandMajorBurns-Tunisia,BenArous,2013,Tunisia
10.1136/ejhpharm-2023-eahp.304
BackgroundandImportance Oneofthemostcommonorthopedicoperationsistheprostheticreplacementofthehipjoint. Tojudgethechoiceofaprosthesis,weoftenlackfactual data.
AimandObjectives OurobjectiveistoassesswhethertherecommendationsoftheHighHealthSecurityarerespectedand toanalysetheiraveragecoststooptimisepharmaceuticalvalidationbycreatingaprescriptionsheetfororthopedic implants.
MaterialandMethods Censusofthevariousinterventionswas carriedoutduringtheyear2019bytypeofact:TotalHip Prostheses(THP);IntermediateProsthesis(IHP);Repeats (REP);Rebuilds(REB).
Analysisofthedifferentfrictioncouples(head/insert): Ceramic/polyethylene(C/PE);Metal/PE(M/PE);Ceramic/ Ceramic(C/C).
ThesoftwareusedfordatacollectionisACCESSandfor statisticalanalysisisSPSS.
Results 140hipinterventionsin2019including:34HIP;88 THP;16REP;2REB.Agesaccordingtothetypeofoperationscarriedout:25.71%(£50years);12.85%(50-70years); 61.44%( 70);withthedistributionaccordingtothetypeof prosthesis:THP(meanage75years);PIH(82years);REP (47years).Clinicalindicationswerecoxarthrosis39.23% (THP);Femurfracture14.28%(HIP,THP);Osteonecrosis 7.14%(THP);Rheumatoidarthritis10.71%(IHP);Dislocation 17.81%(REP).FrictioncouplesusedwereC/PE3.57%;M/M 13.71%;C/C82.72%distributedaccordingtoCLASrating: competition(100%C/C),activity(78.7%C/C),leisure(80.8% M/PE),sedentary(100%M/PE).Breakdownoftheaverage costaccordingtotheallocatedbudgetwas62.85%THP, 24.28%HIP,8.57%REP,1.42%REB.
ConclusionandRelevance Thestudyshowedthatthemost frequentinterventionsareIHPandTHP,IHPareplacedin veryelderlypatientsinwhomosteosynthesisisnotpossible,theC/Ccoupleisreservedforpeopleundertheageof
50,withalevelofactivityandalifeexpectancy.Finally, wenoticedthatthemostcostlyinterventionisTHP. Resultsareinaccordancewiththerecommendationsofthe HAS.Thisstudyallowedustocreateaprescriptionsheet fromtheanalysisofthesedata indicating:identificationof thepatientspecifyingageandactivity;clinicalindication andtypeofprosthesisandfriction.Thisprocedureoptimisesthepharmaceuticalvalidati onbydirectingthecliniciantowardstherightmedicalandpharmacoeconomic choiceoftheprosthesis.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-102 SAFETYEVALUATIONOFMIDLINECATHETERSUSED FORANTIBIOTICTHERAPY
1AEgüésLugea, 1ARodríguezEsquíroz*, 2BFernandinoZubieta, 3BGalarragaIñarra, 4MTimonedaCompany, 5REscobedoRomero, 1MSarobeCarricas. 1HospitalUniversitario deNavarra,Pharmacy,Pamplona,Spain; 2HospitalUniversitariodeNavarra,Home-Care Unit,Pamplona,Spain; 3HospitalUniversitariodeNavarra,Digestive,Pamplona,Spain; 4HospitalUniversitariodeNavarra,InternalMedicine,Pamplona,Spain; 5Hospital UniversitariodeNavarra,IntensiveCare,Pamplona,Spain
10.1136/ejhpharm-2023-eahp.305
BackgroundandImportance Inpatientswithdifficultintravenousaccessorthosewhorequireshort-termintravenousdrug administration,midlinecatheters(MLC)canbeasafealternativetoperipherallyinsertedcentralcatheters(PICCs).
AimandObjectives Theobjectiveofthisstudyistodescribe outcomesinpatientswhohadamidlineplacedfortheindicationofantibiotictherapy.
MaterialandMethods Thiscohortstudyanalyseddatafrom hospitalregistryincludingpatientswhohadamidlineplacementforintravenousantibiotictherapy.Patientdemographics andclinicaldata(diagnoses,comorbidities,medications,laboratoryvalues,antibioticuseanddurationofinfusiontherapy), anddevicevariable(placementarmandveinofinsertion, cathetergauge,andnumberofcatheterlumens)were abstracteddirectlyfrommedicalrecords.Datawereanalysed fromJune2021toSeptember2022.
Results Sixty-ninepatientswithMLCwereincludedforanalysis,68%weremaleandmeanagewas70years(range28–96).Themostcommondiagnoseswerebloodstreaminfection (46.4%),respiratorytractinfections(17.4%)andurinarytract infections(14.5%).Themostprescribedantimicrobialswere piperacillin-tazobactam(52.2%),ertapenem(19%)andmeropenem(11.6%).
Intotal69MLCwereplaced,totaling952catheter-days, withandaveragemidlinedwell-timeof14days(range=243days;median=12days).Totalcomplicationswere31.9%, includingfour(5.8%) ‘leak’,fourteen(20.3%)catheter obstructions,two(2.9%)phlebitisandone(1.45%)thrombosis.Inaddition,onepatientpresentedagradeIinfiltrations (INSInfiltrationScale).Therewerenoconfirmedorsuspected bloodstreaminfection.
ConclusionandRelevance Inthisstudy,theMLCcomplication ratewas31.9%.Thecomplicationsweremostlymechanical (81.8%)anddidnotrequirethesuspensionoftheantibiotic therapyorthewithdrawalofthecatheter.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
1IVilimelis*, 2,3APérez-Ricart, 1,3MBoschPeligero, 4ACalvo, 3CCodina-Jiménez, 3EValls Sánchez, 1JMSuñéNegre, 3CQuiñonesRibas, 5JCGiménez-Juárez. 1Universitatde barcelona.,pharmacyfacultycampusdiagonal.,barcelona,spain; 2ServeiCatalàdelaSalutÀreaMetropolitanaNord-RegióSanitàriadeBarcelona,PharmacyService,SantCugatDel Vallès,Spain; 3HospitalUniversitariGermansTriasIPujol,PharmacyDepartment,Badalona, Spain; 4UniversitatPolitècnicadeCatalunya,ComputerScience,Barcelona,Spain; 5Centre d’informaciódelmedicamenthospitaluniversitarivalld’hebron,pharmacyservice, barcelona,spain
10.1136/ejhpharm-2023-eahp.306
BackgroundandImportance SocialMedia(SM)couldbea sourceofunmetneedsofparentsaboutdruguseinpaediatrics.Knowingsubjectiveinformation(SI)canleadtoimprove pharmaceuticalcare.
AimandObjectives Analysecontentofpostsfromparent forums(PF).
MaterialandMethods Observational,ambispectivestudyonPF relatedtoprimarycaremedicinesinpaediatrics.PFwere selectedifincludedchild-healthsectionsinCatalan,Spanish orEnglishandpermissionwasobtained.Dataminingsoftware wasdevelopedusingontologiesfromSpanishAgencyofMedicinesandMedicalDevicesandMedicalDictionaryforRegulatoryActivities.Postswereexcludedifwrittenbyprofessionals, referredtonon-pharmacologicaltreatments,adults,pregnancy, hospitaldrugs,non-originalentriesorduplicates.SIwasclassifiedintopositive,negativeordoubtsaccordingtoneand adjectivesexpressed.
Results 3572postsfromtwoPFweredownloaded,821 (26%)analysed.Excludedentries(94;11%):non-pharmacologicaltreatments(42;5%),hospitaldrugs(12;1%),adults (12;1%),non-originalentries(9;1%),pregnancy(2;0,02%)or duplicates(2;0,02%).444(72%)usersmentionedSIin591 posts(1,3SI/post).Notifierweremainlyparents(177;40%) andcaregivers(233;52%).SIpostscontainedneutral (223;38%),doubts(259;44%),negative(63;10%),positive (47;8%)SI.Postsreferredto453children,infants(28days23months)(156;26%)andchildren(3-11years)(107;18%) andnotclassified(190;56%).Reporteddrugs:antibiotics (214;36%),respiratory(99;17%)andnervoussystem (78;13%)medications,other(200,34%).Routes:oral (330;56%),parenteral(123;21%),other(138,23%).Topics reportedwere:
Positive(47)Negative(63)Doubts(256)
Effectiveness37(79%)10(16%)12(5%)
Accessibility(24;4%)
Vaccines0(0%)0(0%)7(3%)
Other0(0%)5(8%)12(5%)
ConclusionandRelevance Doubts,negativeattitudestowardsa futuremedicineandpositiveopinionsaboutdrugeffectiveness werethemostSIexpressedbyPFusers.Pharmacistscanhave amainroleprovidingmoreinformationandknowledgeto parentsaboutdrugs.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-106 SAFETYPERCEIVEDBYPROFESSIONALSAFTERTHE INNOVATIONOFTHEMEDICATIONUSECIRCUITINA
NEONATALINTENSIVECAREUNIT
1DCanales*, 1CGarcía-Muñoz, 1JMCaro, 1FMartinez, 1AGonzález, 1ACastro, 2MTMoral, 2INúñez, 1JMFerrari, 2CRPallás. 1Hospital12deOctubre,ServiciodeFarmacia,Madrid, Spain; 2Hospital12deOctubre,ServiciodeNeonatología,Madrid,Spain
10.1136/ejhpharm-2023-eahp.307
BackgroundandImportance Recently,aredesignhasbeentakingplaceinçcircuitofprescription,dispensing,preparation andadministrationofmedicationsinNeonatalIntensiveCare Unit(NICU).Thesechangesareaimedatimprovingthesafety ofthemedicationuseprocess.
AimandObjectives Toevaluatethesafetyrelatedtotheuse ofmedicationsperceivedbyprofessionalsaftertheimplementationofimprovementmeasuresinthecircuit.
MaterialandMethods Aquestionnairewasdevelopedfornursingstafftoassesstheperceivedsafetyin:prescriptionby assistedelectronicprescription(AEP),dispensingthroughan automatedmedicationdispensingsystemintegratedwithAEP, anddrugadministrationthroughsmartpumps.Secondly,a questionnairewasdevelopedformedicalstafftoassessthe perceivedsafetyofEAPandpharmaceuticalvalidation.In both,questionswereincludedaboutmeasurestobeimplementedtoimprovecircuitsafety.
Thequestionnairesconsistof17(nursingstaff)and14(medicalstaff)questionswithanaveragecompletiontimeof2 minutes.BothweredesignedinGoogleForms® togivemaximumdiffusion.
Attitudes(32;5%)
Futuremedicine2(4%)20(32%)0(0%)
Currentmedicine5(11%)5(8%)0(0%)
Druginformation(270;46%)
Pharmacokinetics0(0%)1(2%)7(3%)
Posology0(0%)0(0%)16(6%)
Avoided/delayed/discontinuedmedicine0(0%)3(5%)23(9%)
PeerAdvice0(0%)0(0%)97(39%)
Vaccinationschedule0(0%)0(0%)34(13%)
Alternativetherapies0(0%)9(14%)20(8%)
Routeofadministration3(6%)1(2%)8(3%)
Reconstitution0(0%)0(0%)2(1%)
Conservation0(0%)0(0%)1(0%)
AdverseEvents0(0%)9(14%)15(6%)
Contraindications0(0%)0(0%)1(0%)
Interactions0(0%)0(0%)1(0%)
Results Responseratewasof60%(42peoplefilledoutthe questionnaire,26fromnursingstaffand16ofmedicalstaff). RegardingAEP,nursingstaffagreedthatitprovidesgreater safetythanmanualprescription,although7.7%(n=2)consideredthattheinformationisnotalwaysclearandcomplete. Regardingmedicalpersonnel,88%considerthattheAEPprovidesgreatersecurity.
Regardingpharmaceuticalvalidation,100%ofmedicalstaff believethatitisanimprovementinthequalityofcareand thatitprovidessecuritytotheprocess.
Regardingdispensing,96%considerthatmedicinesare moreeasilyfoundwithrespecttotheplantmedicinecabinet systemand85%considerthattheintegrationwithAEPallows unequivocallyobtainingtheprescribedmedication.
Intheadministration,85%ofnursingstaffconsiderthat smartmedicationinfusionpumpspreventexceedingtherapeuticdoses.
Finally,intermsofareasforimprovement,themajorityof nursingstaffconsidersthatthemeasuresshouldfocuson preparation(57.7%)andthemedicalstaffconsidersthatthey shouldfocusonadministration(75%).Administrationbybarcodeisthemeasuremostvotedforbothgroupstoworkon incomingyears.
ConclusionandRelevance TheperceptionofsafetybyNICU staffofmeasuresimplementedishigh.Therearestillareas forimprovementsuchaspreparationoradministration.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-107 SAFETYASSESSMENTOFERENUMABAND
GALCANEZUMABINCLINICALPRACTICE
MDCGonzalezEscribano*,APerezFacila,JJSaizMolina,TdeSalinasMuñoz, MDMAlañonPardo,PAraqueArroyo. HospitallaManchaCentro,Pharmacy,Alcazarde SanJuan,Spain
10.1136/ejhpharm-2023-eahp.308
BackgroundandImportance Erenumabandgalcanezumabare twomonoclonalantibodies(mAbs)administratedsubcutaneouslyindicatedformigraineprophylaxisinadults.Asthese arenewlyapproveddrugs,itisimportanttoknowtheirsafety profile.
AimandObjectives Toanalysetheadverseeffects(AE)of thesemAbsinreallifeinatertiaryhospital.
MaterialandMethods Observational,retrospective,30-month study(March2020
September2022).Thestudyincluded allpatientsdiagnosedwithchronicmigraine(CM)orepisodic migraine(EM)andwhoreceivedtreatmentwithgalcanezumab orerenumabrespectively.
Thefollowingvariableswerecollected sex,age,typeof migraine,durationoftreatmentandAE.
Datawerecollectedthroughtheoutpatientmoduleofthe Farmatools® softwareandtheelectronichealthrecord,MambrinoXXI®
Results Ninety-fivepatients(92%female,8%male)witha medianageof50years(18-73)wereincluded.Ofthese,72% hadCMand28%hadEM.45%and55%ofpatients receivederenumabandgalcanezumabrespectively.
48patients(44%erenumab,56%galcanezumab)experiencedsometypeofAEduringtreatment,consideredmildmoderateinseverity.Fourpatients(75%erenumab,25%galcanezumab)hadtodiscontinuetreatmentduetopoortolerabilitydespiteprophylactictreatment.17(41%erenumab, 59%galcanezumab)hadinjectionsitereactionorpain,27 (48%erenumab,52%galcanezumab)constipationand4(25% erenumab,75%galcanezumab)nauseaandvomiting.AEswere morefrequentamongpatientswithCM(65%)vsEM(35%).
Comparingthedataobtainedwiththosedescribedinother clinicaltrials,itwasobservedthattheproportionofAEswas verydifferentfromthatreportedinthetrials.Inaddition, therewerenocasesofnasopharyngitisorrespiratorytract infectiondescribedascommoninthetrials.Nocardiovascular AEswereobserved.
ConclusionandRelevance Basedontheresultsofourstudy,it wasobservedthatgalcanezumabanderenumabAEswerecategorisedasmild-moderate.TheincidenceofAEswashigher forthegroupofpatientsreceivinggalcanezumab.Inaddition, asmallnumberofpatientsdiscontinuedtreatmentdueto AEs.Itisessentialtoknowthesafetyprofileofnewly
approveddrugsinclinicalpracticesoastocomparethem withthosedescribedinclinicaltrialsandtoseepossibledifferencesbetweenthemthatcontributetogeneratenew evidence.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-108 COMPUTERISEDPHYSICIANORDERENTRYWITH CLINICALDECISIONSUPPORTINPREVENTING WRONGDOSEERRORSINPAEDIATRICMEDICATION ORDERS:ASYSTEMATICREVIEW
1HRuutiainen*, 2EKunnola, 2ARHolmström, 1SKuitunen. 1HelsinkiUniversityHospitalHus, HusPharmacy,Helsinki,Finland; 2UniversityofHelsinki,FacultyofPharmacy,Helsinki, Finland
10.1136/ejhpharm-2023-eahp.309
BackgroundandImportance Prescribingisaspecifichigh-risk taskwithinthepaediatricmedication-useprocess,whichis whydefensesareneededtopreventorstoperrors.Suchsystem-centricbarriersincludeelectronichealthrecord(EHR) systemswithcomputerisedphysicianorderentry(CPOE). Clinicaldecisionsupport(CDS)toolscanbeintegratedinto theCPOEsystemstoassistsafeprescribing.
AimandObjectives Theobjectiveofthissystematicreview wastoexaminetheeffectsofCPOEsystemswithCDSfunctionsonpreventingwrongdoseerrorsinpaediatricmedicationorders.
MaterialandMethods ThisstudyfollowedthePreferred ReportingItemsforSystematicReviewsandMeta-Analyses (PRISMA)2020criteriaandSynthesisWithoutMeta-analysis (SWiM)items.ThestudyprotocolwasregisteredinPROSPERO.TheliteraturesearchwasconductedinMEDLINE Ovid,Scopus,WebofScienceandEMBReviewsinJanuary 2022.Studyselectionanddataextractionwerecarriedoutby twoindependentreviewers.Afterthis,thequalityofevidence oftheincludedstudieswereassessed.Finally,votecounting methodwasusedtoevaluatetheeffectivenessofCPOE-CDS systemstoreducewrongdoseerrors.
Results Atotalof18studiespublishedin2007–2021metthe inclusioncriteria.ThemostcommonCDStoolsappearingin thestudiesweredoserangecheck(n=14/18),dosecalculator (n=8/18)anddosingfrequencycheck(n=8/18).Inninestudies,aspecificalertfunctionwasaddedtotheCDStool, whereasalertswererecordedin15studies.Astatisticallysignificantreductioninwrongdoseerrorswasfoundineight studies.Noneofthestudiesrepostedanoverallincreaseof wrongdoseerrors.
ConclusionandRelevance CPOE-CDSsystemshaveagreat potentialtopromotepaediatricmedicationsafety.Systemcustomisationforpaediatricpopulations,implementingCDS alerts,andtheuseofdoserangecheckseemtobemostusefulinterventionstoreducewrongdoseerrors.However, CPOE-CDSsystemscannotpreventallwrongdoseerrorsas humanerrorscontinuetooccur.Implementationofnewtechnologycanalsoposenewmedicationsafetyrisks,suchasalert fatigue.Therefore,furtherstudiesandsystematicdevelopment activitiesareneededtooptimisethesafeuseofCPOE-CDS systemsinpaediatriccaresettings.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
MLourenço,CCanário,GCosta,HDuarte,AAlcobia*. HospitalGarciadeOrta,Pharmacy, Almada,Portugal
10.1136/ejhpharm-2023-eahp.310
BackgroundandImportance Theinformatisationofprocesses hasincreasedthesusceptibilityofthehealthcaresectorto computerattacksthroughransomware.Thesecanmanifestby theimpossibilityofaccessingtheinternet,andcomputersystems,withconsequentinterruptionofelectronicprescription, registrationinpatientdiary,consultationofpreviousclinical data,requestformeansdiagnostictools,amongothers.PharmaceuticalServicesareparticularlyvulnerabletotheseattacks.
AimandObjectives Theaimofthisworkwastosystematise thestrategiesadoptedduringthecyberattack,minimisingthe errorandallowingtheworktobecarryoutattheCPU,with theelaborationofaguidelinetobeadoptedinafuture cyberattack.
MaterialandMethods RetrospectivestudybetweenApril26 andMay10,2022.
Results CPUwasrestructuredinordertoguaranteeitsfunctionality.ThroughtheinformationonpaperfromtheproductionmapsofApril2022,chemotherapyprotocols,chartswith reconstitution/dilutionofuseddrugs,literatureandcoordinationofinformationwiththenursingandmedicalteam,itwas possiblepreventthecollapseoftheunit.Itwasperformeda dailysurveyofpatientsmarkedinagenda,andrespective informationaboutthecycle.
Inthefirst15daysofattack,28.8%(n=132)ofscheduled patients(n=458)wereuncheckedduetolackofaccessto complementarymeansofdiagnosisandhistoryofchemotherapyprotocols.Thepreparationofcytotoxicswaspossible throughtheelaborationofmanuallabels(n=615),usingthe validationofpaperprescriptions.Inthefirstweek(April26 to29)41.9%(n=67)ofthepatients(n=160)wereunchecked andinthesecondweek(May2to6)25%(n=54)ofpatients (n=216).Intheinitialdaysofcyberattacknonewpatients werescheduled.
ConclusionandRelevance Facedwiththerealityofacomputer attack,theCPUprioritywastoensureasafetypreparationof chemotherapy.Ontheotherhand,thisattackshowedthatit iscrucialtohavemechanismsofreplacementofinformation suchasthechemotherapyprescriptionsfile.Anticipatingfuture cyberattacks,aguidelinehasbeendevelopedtoensurecircuit safetyincaseofcomputerfailure.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.Notapplicable.
ConflictofInterest Noconflictofinterest
5PSQ-111 MEDICATIONERRORS,THEIRPERCEPTION,ANALYSIS,
1HKomjathy*, 2VGogolova, 1KTóthová, 1DTárnoková, 1KHajtmanová, 1AHorváthova, 1CGálova, 3LMasaryková. 1HospitalAgelKomárno,HospitalPharmacy,Komárno,Slovakia; 2Expiro,Pharmacy,NovéZámky,Slovakia; 3FacultyofPharmacyComeniusUniversity Bratislava,DepartmentofOrganisationandManagementofPharmacy,Bratislava,Slovakia
10.1136/ejhpharm-2023-eahp.311
BackgroundandImportance Amedicationerrorisamistake intheuseofamedicinethatcanresultinharmtothe patient.Medicationerrorscanincreasehospitalisationcosts andprolonghospitalstays.Whereasthemedicationerrorsare preventable,itisimportanttoidentifythemostvulnerable steps.
AimandObjectives Theaimofourworkwastoanalysethe perceptionofmedicationerrorsbyhealthcareprofessionalsin ourhospitalconcerningparenteralmedicines.Subsequentlyto findtheroleofhospitalpharmacistsintheprocessofeliminatingmedicationerrors.Furthermore,toidentifythemedicinesinwhichamedicationerrorcanoccurmostoften.
MaterialandMethods Retrospective,observationalstudyina publichospitalfromAugust2021toDecember2021.The studywasconductedusingquestionnairesadministeredbyhospitalpharmacistsfilledbyhealthcareprofessionals(doctors andnurses).Thequestionsfocusedonmedicationerrors,their causes,andtheirfuturesolutions.Severalquestionswere focusedonspecificdrugsregardingthepreparationand administrationofspecificparenteraldrugs.
Results 47doctorsand72nursesfromthedifferentdepartmentsparticipatedinthesurvey.72%doctorsand46%nurses encounteredmedicationerrors.Increasedworkload(87%doctors,79%nurses)andnegligence(61%doctors,47%nurses) wereidentifiedasthemostcommoncauses.Themostfrequentlyobservedmedicationerrorsofparenteraldrugs occurredduringtheprocessofprescription(75%doctors, 54%nurses)andadministration(68%doctors,50%nurses). Whiledoctorsmostoftenconsultedtheircolleagues(78%)or lookfortheliterature(55%),nursesconsulteddoctors(81%) whensolvingissuesconcerningparenteraldrugspreparation andadministration.Ontheotherhand,upto49%ofdoctors alsoapproachedthehospitalpharmacist,inthecaseofnurses itwasonly22%.Morethan87%ofdoctorsand76%of nurseswouldwelcomelecturesandtrainingfrompharmacists focusedonthecorrectadministrationandprescriptionof parenteraldrugs.
ConclusionandRelevance Ourresultsconfirmtheliterature data,whichsaythatthemostcommonmedicationerrors occurduringprescribingandadministeringdrugs.Hospital pharmacistswiththeirknowledgecansignificantlycontribute totheeliminationofmedicationerrorsandincreasethesafety ofhospitalisedpatients.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-113 REAL-LIFEEFFECTIVENESS,SAFETYANDADHERENCE OFDUPILUMABINPATIENTSWITHATOPIC DERMATITIS
1CSociasCañellas*, 1PRiera, 1MMasip, 2ESerra-Baldrich, 2JSpertino, 2ERoé-Crespo, 1NPagès-Puigdemont. 1HospitaldelaSantaCreuISantPau,PharmacyDepartment, Barcelona,Spain; 2HospitaldelaSantaCreuISantPau,DermatologyDepartment, Barcelona,Spain
10.1136/ejhpharm-2023-eahp.312
BackgroundandImportance Dupilumab,ananti-IL-4/13,isa monoclonalantibodyapprovedforthetreatmentofmoderateto-severeatopicdermatitis(AD).Sofar,fewstudieshaveevaluateddupilumabeffectivenessandsafetyinrealclinical practice.
ANDPROPOSALOFSOLUTIONSINAPUBLIC HOSPITALToevaluatetheeffectiveness,safetyand treatmentadherenceofdupilumabinpatientswithADinclinicalpractice.
MaterialandMethods Weconductedaretrospectivestudycarriedoutinatertiaryhospital.WeincludedallADpatients treatedwithdupilumabwithaminimumfollow-upof52 weeks.
Wecollectedthefollowingdatafromelectronicmedical records:age,gender,previoustreatments,eczemaareaand severityindex(EASI)anddermatologylifequalityindex (DLQI)atbaselineandat52weeksoffollow-up,adverse effectsandtreatmentadherence(calculatedbymedicationpossessionratio[MPR]).
EffectivenesswasdeterminedbythechangeintheEASI andDLQIvaluesat52weekscomparedtobaseline.Safety endpointswerethenumberandtypeofadverseeffects(AE) duringthefollow-upperiod.
Results Intotal,61patientswereincludedinthestudy.The meanage(±SD)was40(±18)years.Thirty-fivepatients (57%)weremen.
Asprevioustopicaltreatments,100%ofpatientshad receivedcorticosteroids;whereas49%,tacrolimus.Besides, 70%hadunderwentphototherapy.Regardingsystemictreatment,79%hadreceivedcorticosteroids;70%,cyclosporine; 25%,mycophenolatemofetil;25%,azathioprine;and28%, methotrexate.
Mean(±SD)EASIandDLQIbaselinevalueswere33± 11and19±5,respectively.At52weeksfollow-up,these indexeswere2±3and4±5,respectively.Thereduction inEASIandDLQIwasstatisticallysignificant(p<0.001).Duringthisperiod,AEwerereportedin22patients(36%):conjunctivitis(20%),arthralgia(5%),herpesvirusinfection(5%) andparadoxicalpsoriasis(3%)werethemostcommonones. Threetreatmentswerediscontinuedduetoineffectiveness,4 duetoAEand2becauseofclinicalremission.
ThemeanMPR(±SD)was100±14%,whichdemonstratesgoodratesoftherapeuticadherence.NopatientpresentedaMPR<75%,sowecouldnotdeterminetheimpact ofthisvariableontreatmenteffectiveness.
ConclusionandRelevance Ourstudyshowsthatdupilumabis aneffectiveandsafedrugformoderate-to-severeDA.Our cohortexperiencedastatisticallysignificantimprovementin EASIandDLQIat52weeksoftreatment.Additionally,therapeuticadherencewasveryhigh.
ConflictofInterest Noconflictofinterest
10.1136/ejhpharm-2023-eahp.313
BackgroundandImportance Bisphosphonatetreatmentlasting morethan5years(BP5y)inpatientswithoutpreviousfractures and/orlowriskoffracturedoesnotconferadditionalbenefits. Theantiresorptveeffectismaintainedforatimeafterdrug discontinuationandtheside-effectsriskisminimised.The increasedriskofrareandlong-termside-effectassociatedwith theprolongeduseofbisphosphonateshasbeenreasonof
safetynotesissuedbytheSpanishAgencyforMedicinesand HealthProductsandotherregulatoryagencies.So,are-evaluationofthetreatmentisnecessaryconsideringthebenefits andrisksforthepatientindividually,especiallyafter5years ofuse.
AimandObjectives TheaimofthepresentstudywastoidentifypatientsintreatmentwithBP5yandtoevaluatethe acceptanceofpharmaceuticalintervention(PI)overdeprescribinginprimaryprevention.
MaterialandMethods Bymeansoftheprimarycaredata exploitationplatform(Digitalis®)andafterreviewingtheelectronicprescription,patientsontreatmentwithBP5ywere identifieduntilDecember-2021.Patients>85-years-oldwere selectedduetotheirincreasedfrailty,comorbiditiesandpolypharmacy.Thephysicianswereinformedviatheinformative sheetsoftheprimarycareinformationsystem(Turriano®) aboutthesusceptibleconditiontodeprescribingaftermore than5yearsofcontinuoustreatmentinprimaryprevention. Sevenmonthslater,acceptancedegreeofPIwasassessed.
Results Atthestartofthestudy,186patientswereidentified intreatmentwithBP5y,51ofwhomwere>85years-old. Finally,PIwasperformedon43patientsbelongingtothe selectedhealthcentres.Sevenmonthslater,bisphosphonate withdrawalwasobservedin10patients,withaprescribing doctorsacceptancerateof23%(10/43).Currently,33patients continuewithsuchtreatmenteitheroutofnecessityordueto lackofknowledgeofPI.Onlyoneofthe8patientswithout PI(12.5%)hadbisphosphonatediscontinuation.
ConclusionandRelevance Themonitoring,analysisoftreatmentswithBF5yandthecorrespondingPIhavepromoted thedeprescriptioninalmostaquarterofthecases,creating theneedtoextendthestudytotherestofthepatients.The importanceofthepharmacistinthereviewoftreatmentsis highlighted,aswellastheinterdisciplinarycollaborationwith physicianstoachieveasafeuseofthedrug.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-116 PERSISTENCEOFINHIBITORSOFINTERLEUKIN-23 (ANTI-IL-23)FORTHETREATMENTOFMODERATE-TOSEVEREPSORIASIS(MSPS)INTHEROUTINECLINICAL PRACTICECONDITIONS
1LDomínguezSenín, 2SCamachoParreño, 2ESánchezGómez, 2MRodriguezJorge*, 2MDSantos-Rubio. 1HospitalJuanRamónJimenez,HospitalPharmacy,Huelva,Spain; 2HospitalJuanRamónJiménez,HospitalPharmacy,Huelva,Spain
10.1136/ejhpharm-2023-eahp.314
BackgroundandImportance Anti-IL-23haveemergedassafe andeffectiveoptionsforthetreatmentofmsPs.
AimandObjectives Weaimedtoevaluatethepersistenceof anti-IL-23(guselkumabandrisankizumab)inpatientswith msPs.Secondarily,thesepatients’ clinicaloutcomesandhealthrelatedqualityoflife(HRQL)andthesafetyprofilewerealso assessed.
MaterialandMethods Retrospectiveobservationalstudyfrom January2019toSeptember2022.PatientswithmsPsreceiving anti-IL-23wereincluded.Demographic(sex,age)andclinical data(previousbiologicaltreatments,therapydurationand baselinePsoriasisAreaandSeverityIndex(PASI))werecollectedfromthedigitalmedicalrecord.Non-persistencewas definedastreatmentdiscontinuationoratreatmentgap>90
VLafargaLapieza*,PHernandoMartinez,CMartiGil,DCaniegoRodrigo,EGarciaLopez, DBarredaHernandez. HospitalVirgendelaLuz,Farmacia,Cuenca,Spaindays.Thecumulativeprobabilityoftreatmentpersistencewas analysedbyKaplan-Meiermethod.Secondaryendpoint: PASI90responseat1year,changeinHRQLthroughdermatologylifequalityindex(DLQI)at1year,andsafetyprofile. Results 44patientswereincluded(26women),30received guselkumaband14risankizumab.Meanagewas53.5years. 93.2%receivedbiologictherapiesbefore,and86.3%conventionalsystemictreatment.Atdatacut-offtime,73.3%and 92.8%patientsremainedonguselkumabandrisankizumab respectively.Themaincauseofdiscontinuationwasprimary failure.In13.3%ofguselkumabpatients,doseintervalwas extended>8weeksandin7.1%ofrisankizumabpatientswas extended>12weeks.Thecumulativeprobabilityofguselkumabtreatmentpersistencewas79.7%at1yearandforrisankizumab92.6%.ThemedianPASIscorewas8and9at guselkumabandrisankizumabtreatmentinitiationrespectively. 50%ofguselkumabpatientsand64.3%ofrisankizumab patientsachievedPASI90improvementat1year.44.8%of guselkumaband71.4%ofrisankizumabpatientsachieveda minimalclinicallysignificantdifference(>4-pointreduction)in DLQIscoreat1year.Onepatientexperiencedoneadverse reaction(ARs)relatedtoguselkumab:headacheandtworisankizumabpatientsexperiencedincreaseintransaminases.
ConclusionandRelevance OurcohortshowsamoderatepersistencerateandPASIimprovementat1yearwithguselkumabandamoderatebenefitinimprovingHRQL.High persistencerateandmoderatePASIimprovementwasreached withRisankizumabandasubstantialimprovementinHQRL. Noimportantadversereactionswerefound,withouttreatment withdrawals.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
5PSQ-118 PATIENTS’ EXPERIENCEWITHSUBCUTANEOUS INJECTIONSELF-ADMINISTRATIONANDTHEROLEOF VIRTUALREALITY
MGómezBermejo*,RVázquezSanchez,AOntenienteGonzalez,JCCiezarRodriguez, MGarciaParaje,ADomingoBuzon,GMDelgadoLopez,TMolinaGarcia. Hospital UniversitariodeGetafe,HospitalPharmacist,Getafe,Spain
10.1136/ejhpharm-2023-eahp.315
BackgroundandImportance Thenumberofpatientstreating themselvesviathesubcutaneous(SC)administrationroutehas widelyincreasedinrecentyears.Althoughself-medicationcan reducewaitingtimesandsavemoney,isapublichealthconcernthatitmaycarrysomepotentialrisksassociatedwith inappropriatemanagement.Gettingthecorrectmethodof administrationisessentialtoensurethedrug’seffectiveness andminimisetheriskofcomplications.
Weproposetotakeadvantageofthebenefitsthatnew technology,suchasvirtualreality(VR),couldprovidefor patients’ performance.
AimandObjectives Thisinvestigationaimedtoexplore patients’ perceptionsoftheirexperienceswithSCinjection self-administrationandtheirwillingnesstoimplementVRto improvetheirlearningprocessofthemethodof administration.
MaterialandMethods Anobservationalandtransversalstudy wasperformed.Theadultswhoattendedforsubcutaneous medicinedispensingwereincluded.Ayes/nosurveywasconductedregardingtomedicationfirstself-administration
knowledge,handlingskills,administrationerrors,riskperception,clarityofinformationreceivedandwhetheraVRenvironmentwouldhelptheirlearning.
Results
Forty-fivepatientswereincluded Mean±SDagewas51± 12years.Mostofthepatientsinterviewedwereintreatment withdrugsforimmune-mediatedinflammatorydisorders.The firstadministrationwasdonebyahealthprofessionalin 53.3%ofthecases,44.4%weredonebythemselvesand 2.2%weredonebyafamilymember.Although95.6%ofthe participantsconsideredthattheinformationgivenbythepharmacistwasclearenough,15.6%ofthemdiscardedtheinjectionsduetohandlingfailuresand66.7%reportedinjection sitereactions.Finally,75.6%ofparticipantsbelievedthatVR mayhelptolearntheadministrationprocess.
ConclusionandRelevance Althoughtheinformationandtrainingprovidedbythepharmacistwereclearenough,some patientsdonotfeelconfidentwiththeirfirstself-administrationhavingtodiscardthemedicationduetosomehandling failures.
TheVRrepresentsapotentialalternativeforpromotinga safeenvironmenttoimprovetheknowledge,skillsandattitudesinSCinjectionself-administrationthroughreproducing environmentsclosetotherealone.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
5PSQ-119 ANEWPHARMACEUTICALCAREPROGRAMMEFOR COVID-19PATIENTSTREATEDWITHPAXLOVID®: IMPLEMENTATIONANDSAFETYOUTCOMES REPORTED
1MFerrisVillanueva*, 2EChamorrodeVega, 2CGGonzalezRodriguez, 2BTorrobaSanz, 2JVicenteValor, 2AHerranzAlonso, 2MSanjurjoSáez. 1GregorioMarañónUniversity GeneralHospital,HospitalPharmacy,Madrid,Spain; 2GregorioMarañónGeneralUniversity Hospital,HospitalPharmacy,Madrid,Spain
10.1136/ejhpharm-2023-eahp.316
BackgroundandImportance TheCOVID-19pandemichas highlightedtheimportantrolethathospitalpharmacistsplay inimprovingpharmacotherapyoutcomes.Paxlovid® (Nirmatrelvir/ritonavir)wasrecentlygrantedanEmergencyUse AuthorisationforthetreatmentofmildtomoderateCOVID19.However,theuseofPaxlovid® withcertainotherdrugsin high-riskpatientsmayresultinpotentiallysignificantdrugdruginteractions(DDI)andadversedrugevents(ADE).
AimandObjectives Toassesstheimpactofacomprehensive pharmaceuticalcareprogram(CPCP)focusingonthepreventionofDDIandADE,initiatedinahospitalpharmacyfor patientswithmildtomoderateCOVID-19treatedwith Paxlovid®.
MaterialandMethods Design:Quasi-experimentalstudyperformedbetween1Mayand31July2022.Pharmacistswere responsibleforproposingCOVID-19localguidelinestophysicians,monitoringadherencetoguidelines,managingDDIand ADE,providingpatienteducation,andevaluatinghealthoutcomes.Atelephoneconsultationwascarriedout10daysafter theendofPaxlovid® treatment.
PotentialDDIweredetectedaccordingtoLexi-Comp ® and LiverpoolCOVID-19databases.Paxlovid-relatedADEreported weregradedaccordingtoCommonTerminologyCriteriafor AdverseEvents,version4.
Results 140patients(60.7%outpatients)initiatedPaxlovid® andwereenrolledintheCPCP.Adherencetolocalguidelines fortheuseofPaxlovid® was100%.
Overall,232DDIweredetectedin111(79.3%)patients, 142(61.2%)ofwhichrequiredspecificmanagement(34.5% discontinuationoftheconcomitantdrugand65.5%dose adjustment).
Pharmacistsmade267interventionsthatledtothepreventionof177ADE(1.3/patient),96(54.2%)ofwhichwere gradeG-H(NCCMERPclassification).
Atday10,96ADEswerereportedin42patients(26.1% ofwhichweregrade 3),beingdysgeusiaanddiarrhoeathe mostcommon.PrematurediscontinuationofPaxlovid® dueto ADEswasnecessaryin4(2.8%)patients.
ConclusionandRelevance TheimplementationofaCPCP developedbyhospitalpharmacistsforpatientstreatedwith Paxlovid® wasaneffectiveapproachformonitoringadherence toguidelines,managingDDI,providingpatienteducation,and evaluatingsafetyoutcomes.Paxlovid® showedanacceptable safetyprofile.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
5PSQ-120 ANALYSISOFTHEPHARMACEUTICALINTERVENTIONS PERFORMEDONONCO-HAEMATOLOGICALPATIENTS THROUGHANONCO-HAEMATOLOGYPHARMACY CONSULTATION
CAlarcon-Payer*,AMartínRoldan,MDMSánchezSuárez,CMonteroVílchez,AJiménez Morales. HospitalUniversitarioVirgendeLasNieves,ServiciodeFarmacia,Granada,Spain
10.1136/ejhpharm-2023-eahp.317
BackgroundandImportance Intheareaofonco-haematology, medicationerrorsareofgreatimportancebecauseoralantineoplasticdrugshaveanarrowtherapeuticmargin,complex dosingregimens,possibleinteractionswithotherdrugsand foods,andlowsupervisionoftheirself-administrationby healthcareprofessionals,increasingtheriskofmedication errors.
drugs,25%relevantdruginteractions,18%omissionofthe drug,10%incorrectfrequencyofadministrationand2% detectedadversereactions.Themostfrequentdoseerrors werepooradjustmentforrenalfunction(40%),failureto writethedoseinthepatient‘sclinicalcourse(30%),failureto adjustforliverfailure(20%),pooradjustmentforbodysurfacearea(10%).100%oftheerrorsweredetectedinthe pharmaceuticalvalidationprocessduringthedispensingoforal cytostatics.100%ofthepharmaceuticalinterventionswere enteredinthepatient‘sclinicalhistoryasaclinicalreport. 97%wereacceptedandprevented97%ofmedicationerrors inpatients.
ConclusionandRelevance Pharmaceuticalinterventionshave proventobeaneffectivetooltocontributetotheachievementofthepatient‘stherapeuticgoals.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.JOncolPract2011;7(1):7–12.
ConflictofInterest Noconflictofinterest.
5PSQ-122 COVID-19VACCINEVIGILANCE:COMPARATIVESTUDY BETWEENHOSPITAL,REGIONALANDNATIONAL DATA
Camerino,Italy
10.1136/ejhpharm-2023-eahp.318
BackgroundandImportance FollowingAIFA’ sauthorisationof firstmRNAvaccineon27/12/2020,C OVID-19vaccination campaignstartedinItalytogetherwithvaccinevigilancein ordertoindividuateexpectedandunexpectedAdverseEvents-Following-Immunisation(AEFIs)andthebenefit/risk balance.
AimandObjectives Theaimofthisworkiscomparingreports ofvaccinevigilanceinourHospitalfromDecember2020to June2022tonationalandregionaldata.
AimandObjectives
Toanalysethepharmaceuticalinterventions performedononco-haematologypatientsseeninanOnco-haematologyPharmacyconsultation.
MaterialandMethods Prospectiveobservationalstudyof onco-haematologypatientsinatertiaryhospitalforaperiod ofoneyear.Toidentifythetypeofinterventionperformed, adatabasewascreatedusinganExcel ® spreadsheettorecord andcategoriseit.Onceidentified,itwasenteredasanepisodeinthepatient ‘sclinicalhistoryintheDirayaClinica ® programmesothatthecliniciancouldconsultitinthe patient ‘sevolution.Finally,errors,interactionsandadverse reactionsavoidedbyperformingtheseinterventionswere recorded.
Results Atotalof35onco-haematologypatientsunderwent pharmaceuticalinterventions.55%menand45%women.The medianagewas64years.Thepatientsbelongedtotwoclinicalservices,40.8%toHaematologyand59.2%toOncology. Theonco-haematologicalpathologieswheremostinterventions wereperformedwere:ProstateCancer(30%),ColonCancer (25%),ChronicLymphaticLeukaemia(16%),MultipleMyeloma(10%),OvarianCancer(7%),BrainTumours(5%),Lung Cancer(4%),BreastCancer(3%).45%ofthepharmaceutical interventionsperformedwereincorrectdosesofantineoplastic
MaterialandMethods StartingfromdataoftheNationalSystemofPharmacovigilance(RNFV),weanalysedreportsby age,sex,severityofreaction,reporterandSystem-Organ-Class (SOC)involved(Meddraclassificationsystem).Finally,we comparedresultswithtwelfthvaccinesurveillancereportpublishedonJune2022bytheItalianAgencyofDrugs(AIFA) andto2021annualregionalreport.
Results IntheperiodourHospitaladministeredabout111000 doses(99%Comirnaty,0.3%Vaxzevria,0.6%Spikevax).176 reportswerecollected:69(39%)concernedCovidvaccination (reporting-rateRR0,06%).52(75,4%)ofCovid-reportswere notsevereand17(24,6%)weresevere;amongthosesevere,2 casesofineffectivevaccination(Comirnaty),1caseofheart attack(Spikevax),1caseofadrenalhematoma(Vaxzevria)and 1episodeofdeepveinthrombosis(Comirnaty).59(85,5%) involvedwomenand10(14,5%)men.65(94,4%)involved Comirnaty(23%severe,andfurther9%ofseverereaction aregivenbyassociationwithotherdrugs,RR0,06%),2 (2,9%)Spikevax(50%severe,RR0,6%),2(2,9%)Vaxzevria, (50%severe,RR0,3%).216AEFIswerecollected;83(38%) generaldiseasesandconditionsrelatedtositeofinjection(13 fever,9asthenia);37(17%)nervoussystemdiseases(26headache);30(14%)generalisedmuscularpains(8myalgia).Little
1IBartolucci, 1NMontiGuarnieri, 1AMFGarzone, 1EAndresciani, 1SBagagiolo, 1ECocci, 2CPolidori, 1APompilio*. 1AouDelleMarche,SodFarmacia,Ancona,Italy; 2Università DegliStudiDiCamerino,ScuolaDiScienzeDelFarmacoEDeiProdottiDellaSalute,percentageinvolvedalsovision,skinandrespiratorysystem. 49%ofreportswerefromdoctors,30%frompharmacists, 15%fromotherhealthworkerandlast6%patients.35(51%) AEFIswerefromfirstdose,32(46%)fromsecondand2 (3%)formthird.Almostallreportsinvolvedagerange18-64.
ConclusionandRelevance ResultsonComirnatyareinline withAIFA’sandregionalones;SpikevaxandVaxzevriashow alteredpercentagebecauseoflittlenumberofreports.Reportingratesarecomparable.Mostofreportsconcernednot severereactions,mainlyrelatedtositeofinjection.Itis importanttounderlinetheessentialroleofvaccinevigilance toidentifyredflagsforpublichealthinordertocontain mainseverereactions.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
prescribedinthesecasesafterTURwasbemiparinataprophylacticdoseof3500IUevery24hours.
59.5%ofpatientswereattendedatEmergencyDepartment (ED)afterTURwithhaematuriadiagnostic.
ConclusionandRelevance Althoughanticoagulationwasnot reintroducedastheprotocolestablished,morethan50%of patientswerereadmittedintheEDforhaematuria.Therefore, ourstudyconfirmsthatappropriateinterruptionofanticoagulationintheperioperativeperiodisadelicatebalancingact betweencomplicationsofbleedingandthrombosis.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-127 PHARMACOLOGICALRISKFACTORSFORDRUG-DRUG INTERACTIONSINPEOPLELIVINGWITHHIV:A SYSTEMATICREVIEW
5PSQ-125 BRIDGINGANTICOAGULATIONINPATIENTSWITH ATRIALFIBRILLATIONAFTERATRANSURETHRAL RESECTION:PATIENTMANAGEMENTISDONE APPROPRIATELY?
10.1136/ejhpharm-2023-eahp.319
EValls*,SMarin,CCodina-Jiménez,ETerricabras,LEstrada,ABocos-Baelo,CGarcíaCastiñeira,GCardona,ÀAndreu,CQuiñones. HospitalUniversitariGermansTriasIPujol, PharmacyDepartment,Badalona,Spain
10.1136/ejhpharm-2023-eahp.320
BackgroundandImportance
Themanagementofanticoagulationinpatientsundergoingsurgicalproceduresliketransurethralresection(TUR)ischallenging.Abalancebetween reducingthromboembolismriskandpreventingexcessive bleedingmustbereached.Thisriskisaggravatedinpatients treatedwithanticoagulants.
BackgroundandImportance Improvedsurvivalofpeopleliving withHIV(PLWH)increasescomorbiditiesburdenleadingto polypharmacyanddrug-druginteractions(DDIs).DDIssuppose ahigherconcerninPLWHduetoantiretroviraltherapy(ART). Presencesoriskfactors(RF)fordevelopingDDIsareofinteresttodetectcasesneedingforpharmaceuticalassessment.
AimandObjectives Assessliteratureonthepharmacological RFfordevelopingDDIsinPLWH.
AimandObjectives
Theaimofthestudywastoassessthe adequacybridginganticoagulationafterTURinpatients treatedwithdirect-actingoralanticoagulants(DOACs)orVitaminKantagonists(VKAs)topreventstrokeinatrialfibrillation(AF).
MaterialandMethods
Retrospectiveobservationalstudycarried outinanareareferencehospitalservingapopulationof 200,000inhabitants,fromJanuary2021toJune2022. PatientswhounderwentTURwithdiagnosticofAFwere included.DatawereobtainedfromMinimumBasicDataSet (CMBD).Wereviewedwhetherpatientswereanticoagulated, thetypeofanticoagulantdrugprescribed(VKA,DOAC)and theprescribeddrug(acenocoumarol,warfarin,dabigatran, rivaroxaban,apixaban,edoxaban).Weverifiedwhetherthe reintroductionofanticoagulanttreatmentafterTURwas appropriatetohospitalprotocolandtherateofsubsequent readmissionsduetobleeding.
BecauseofthemoderatebleedingriskofTUR,theprotocolforreintroducinganticoagulantmedicationafterTURin thecaseofpatientstreatedwithVKAsconsistsofadministeringbemiparinorenoxaparinatanticoagulantdoses24hours afterTURtogetherwiththeusualdoseofacenocoumarolor warfarin.InthecaseofpatientstreatedwithDOAC,theprotocolconsistsofreintroducingtheirmedicationattheusual dose24hoursafterTUR.
Results Themeanageofthe37includedpatientswas81±6 years.94.6%weremale.89.19%ofthepatientswereanticoagulated(60%AVK,40%DOAC).
Theprotocolforreintroducinganticoagulanttreatmentwas notfollowedin100%ofanticoagulatedpatients.Thedrug
MaterialandMethods FollowingthePRISMArecommendations,asearchcombiningtermsassociatedwith ‘ART’ , ‘DDIs’ and ‘RF’ wasconductedinMEDLINEdatabaseforrelevant English-andSpanish-languagearticlesfrom2006throughJanuary2022.Longitudinalandcross-sectionalstudieswere included.ArticlesnotmentioningdataonDDIsbetweenART andnon-ARTwereexcludedinafirstscreeningphase.Ina subsequentselectionphase,articleswereexcludediftheydid notcontaininformationonRFforDDIs.Theoutcomeof interestwasthepharmacologicalRFforDDIs(orgroupedby severity)betweenARTandnon-ARTinPLWH 18years. Datawassynthesisednarratively.
Results 349articleswereidentifiedand10included(4longitudinaland6cross-sectional).Kunimoto-et-al, foundanassociationbetweentheoccurrenceofpotentialDDIsandnumber ofcomedications(OR=1.52[1.16–1.99]),similarcorrelationwas reportedbyOkoli-et-al(OR=1.3[1.2–1.3]),Pontelo-et-al (OR=1.13[1.11–1.15])andBastida-et-al(OR=1.18[1.14-1.22]).
ElMoussaoui-et-al foundthatthenumberofcomedications independentlyassociatedwithorange-(OR=1.8[1.6–2.0])and red-flag(OR=1.4[1.3–1.6])DDIs.Relatedtocomedication, Kunimoto-et-al foundpolypharmacyasasevereRFforDDIs (OR=11.69[3.01–45.40]),thisalsoreportedbyLópez-Centeno-et-al forred-(OR=2.65[1.98–3.54])andorange-flag (OR=2.17[1.90–2.47])DDIs.Halloran-et-al reportedthat ART-regimenscontainingproteaseinhibitors(PIs)weremore likelytohaveDDIscomparedwiththosecontainingnonnucleosidereversetranscriptaseinhibitors(NNRTI)-andintegraseinhibitors(II).ThisincreasedriskofIP-regimenswasalso notifiedbyChen-et-al(OR=2.54[1.25-5.16])andBastida-et-al (OR=1.18[1.14-1.22]),insteadFernándezCañabate-et-al found itinPI-regimens(OR=8.82[4.07–19.14])asalsoNNRTI-
AHerrerosFernández,EUrbietaSanz*,PFernandez-VillacañasFernandez,POrtiz Fernandez,AMMartinezSoto. ReinaSofiaHospital,Pharmacy,Murcia,Spainregimens(OR=2.65[1.25–5.16]).Moreover,ElMoussaoui-et-al foundPIsasanindependentRFforred-(OR=7.9[3.2-19.5]) andorange-flag(OR=7.5[4.5-12.5])DDIswhileNNRTI (OR=2.4[1.5-4.0])andtheII(OR=1.6[1.0-2.6])onlyitwere fororange-flag.ThisriskofPIsofweremoreinvolvedin red-flag/contraindicatedwasalsoreportedbyLópez-Centenoet-alandHoltzman-et-al.
ConclusionandRelevance Thisisthefirstsystematicreview summarisingliteratureinthisfieldandishelpfultostratify patientsatneedforspecialisedmanagementtoreduceDDIs andpolypharmacyburden.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
Constipation(38.7%)anditchiness(3.2%)werethemost frequentadverseeventsduringthestudyperiod.
ConclusionandRelevance Ourfindingsin20treatment-resistantpatientsindicatedthatswitchingbetweenCGRPmAbs couldbebeneficialtosomenon-responderstoainitialmAb.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
MIGRAINE
1CRiberaPuig, 1PCleriesRovira*, 1NMasBauza, 1SGamarraCalvo, 2JCRojasAlvero, 2JCampdelacreuFumado, 1MComasSugranes, 1MAlonsoMoreno, 1MMuñozBolaño, 3NPadullésZamora. 1BellvitgeUniversityHospital,Pharmacy,Barcelona,Spain; 2Bellvitge UniversityHospital,Neurology,Barcelona,Spain; 3BellvitgeUniversityHospital,PharmacyBellvitgeBiomedicalResearchInstituteIdibell,Barcelona,Spain
10.1136/ejhpharm-2023-eahp.321
BackgroundandImportance Monoclonalantibodies(mAb) againstcalcitoningenerelatedpeptide(anti-CGRP)andits receptor(anti-CGRP-receptor)areeffectiveintheprophylaxis ofmigraine.However,studiestodetermineeffectivenessand safetyonswitchingbetweentheminnon-respondersare scarce.
5PSQ-132 PHARMACOGENETIC-GUIDEDTREATMENTIN PATIENTSWITHDYHYDROPIRYMIDINE
DEHYDROGENASEDEFICIENCY
1JFernándezFradejas, 2MMorín-Rodríguez, 1EGemeno-López, 1EDelgado-Silveira, 2MÁMoreno-Pelayo, 1AMAlvarezDiaz*. 1HospitalUniversitarioRamónYCajal,Pharmacy, Madrid,Spain; 2HospitalUniversitarioRamónYCajal,Genetics,Madrid,Spain
10.1136/ejhpharm-2023-eahp.322
BackgroundandImportance Certainpolymorphismsin DPYD geneareassociatedwithpartialorcompletedeficiencyof dyhydropyrimidinedehydrogenase(DPD)enzymeandare linkedtoagreaterriskofseveretoxicitiesafterfluoropyrimidines-basedtreatment.In2020,theEuropeanMedicines Agencyrecommendedthatpatientsshouldbetestedforthe deficiencyofDPDpriortotreatmentwithfluorouracil,capecitabineortegafur.
AimandObjectives Toassesstheprevalenceof DPYD variants linkedtoDPDdeficiencyincancerpatientswhoarecandidatestotreatmentwithfluoropyrimidinesandtoevaluatethe safetyofpharmacogeneticguidedtreatmentinpatientswith DPDdeficiency.
AimandObjectives
Toevaluatethereal-worldclinicaleffectivenessandsafetyofmAbswitchinmigrainepatients.
MaterialandMethods
Retrospectivecohortstudyofadult patientswhoswitchedbetweenmAbinatertiaryhospital fromDecember2019untilSeptember2022.Sociodemographic andclinicaldatawererecorded.Outcomemeasures:the reductionofHeadacheImpactTest(HIT-6)scalepunctuation andthereductionofmonthlymigrainedays.
Results Weanalysed147patientstreatedwithanti-CGRPor anti-CGRP-receptor.Amongthese,20patients(13.6%) switchedbetweenmAbandhadatleastonefollow-upvisit afterswitching.16patients(80%)sufferedfromchronic migraine(CM)withabaselinemediandaysofmigrainea monthof15[13-24],medianRegicorscaleof2%[1-3%]and medianHIT-6of67[62.5-72.3].19(95%)werefemale.
Outofthese20patients,15(75%)startedwithErenumab and5(25%)withGalcanezumab.FirstmAbswitchingwas performedafteramedianof7.4monthstreatment[5.9-11.8] (12fromErenumabtoGalcanezumab;3fromErenumabto Fremanezumab;2fromGalcanezumabtoErenumaband3 fromGalcanezumabtoFremanezumab).5patientsrequireda secondswitch,andonereceivedathirdmAb.Reasonsfor firstswitching:12(60%)non-response,7(35%)lossof responseand1(5%)adverseevent.1patient(5%)discontinuedmAbtreatingduringthestudyperiodduetolackof effectiveness.
MedianreductioninHIT-6afterfirstandsecondswitching was-2[-11.5-0],and-3.5[-11.8-0],respectively.Medianreductionofmonthlymigrainedaysafterfirst,andsecondswitchingwas-4.15[-7-0]and-4.8[-6.5to-0.6],respectively.
MaterialandMethods Prospective,observationalstudyata thirdlevelhospital.CancerpatientswhounderwentgenotypingtestforDPDdeficiencybetween1November2021and 15September2022wereincluded.Demographicandclinical datawerecollectedfromelectronicmedicalrecords.Thepolymorphismsstudiedwerers3918290,rs55886062,rs67376798 andrs75017182.DNAwasobtainedfromperipheralblood samplesandapharmacogeneticanalysiswasperformedusing areal-timepolymerasechainreactiontechnique.Patientswere classifiedasnormal,intermediate,andpoormetabolisers accordingtotheresultofthetest.Severetoxicities(grade3-4 CTCAE5.0)inintermediateandpoormetaboliserswere screenedduringthefirsttwocyclesoftreatment.
Results Atotalof345patientswereincluded,52.6%male, meanage68.3years(SD11.7).Themostfrequentdiagnoses werecoloncancer(43.8%),rectalcancer(18.9%),pancreatic cancer(9.8%),breastcancer(8.0%)andgastriccancer(7.1%).
Overall,14patientswereclassifiedasintermediatemetabolisers:8patientswereheterozygousforrs75017182,3 patientswereheterozygousforrs67376798,2patientswere heterozygousforrs3918290andonepatientwashomozygous forrs75017182.
Elevenoftheintermediatemetabolisersweretreatedwith fluoropyrimidine-basedchemotherapy(threepatientsdidnot starttreatment)withaninitial50%dosereductionandfurtheradjustmentbasedoninitialtolerancetotreatment.During followup,thesepatientsunderwenttreatmentwithoutsufferinganygrade3-4adverseevent.Nofurtherdosereductions ortreatmentdelayswererequiredinthisgroupofpatients.
ConclusionandRelevance Overall,4.1%ofthepatientsofour cohorthadpartialDPDdeficiency.Treatmentindividualisation
basedon DPYD genotypingcanhelptoavoidsevereadverse eventsinpatientstreatedwithfluoropyrimidines.
5PSQ-134 UNITDOSEINACYBERATTACKSCENARIO
AMSoares*,AMSimões,PSantos,PAlmeida,MRodrigues,AGusmão,AAlcobia. HospitalGarciadeOrta,PharmacyDepartment,Almada,Portugal
1FMortillaro*, 1AListro, 2SDominici, 2MPastorello. 1UniversityofPalermo,Graduate SchoolInHospitalPharmacy,Palermo,Italy; 2ProvincialHealthCompanyofPalermo, IntercompanyPharmaceuticalDepartment,Palermo,Italy
10.1136/ejhpharm-2023-eahp.323
BackgroundandImportance Medicationreconciliationand medicationreviewareindispensableinstrumentsinthepreventionofclinicalrisk.Inclinicalpractice,suchmethodsarenot alwaysused.Thisexposesthepatient,intreatmenttransitions, toDRPs,includingAdverseDrugReactions(ADRs),which couldcausehisrehospitalisation.Howmanyclinicalsymptoms arerelatedtodiseaseorhiddenADRs?TheClinicalpharmacist,throughremotemonitoringprovides,cansupporttothe GPbyaperiodicanalysisofthetherapytakenbytheindividualpatient.
AimandObjectives Theobjectiveofthestudywastooutline apharmaceuticalcareanddrugmonitoringmethodologybased onPharmacist-GPcollaborationtoidentifyDRPsthatcould generatepredisposingclinicalconditionsthatcanbeidentified assignsofhiddenADRs.
MaterialandMethods FromApriltoSeptember2022,we establishedateamworkbetweenPharmacistsandGPsina LocalHealthAuthority,selectingpatients>65yearsofage receiving>4drugs.Patient-relateddrugprescriptionsonthe healthcardwereanalysed,excludingherbalproducts,homeopathicproducts,andsupplements.Treatmentduplications, ATCtherapyswitchesanddruginteractionswereexamined, simultaneouslyverifyingdosingschedules.Appointmentshave beensetupwithGPstocomplementtheinformation.Final reportswerepreparedforindividualpatienttobedeliveredto theGPontheclinicalalertstobemonitored.
Results N.24/1304(%1,84)GPswereinvolved,n.149patients wereidentified(average72years)andn.1348drugsanddosingscheduleswereanalysed.Duplicationsidentified:13/1348 (%0,96).Unmotivateddrugswitches23/72(%31,94),drug alertsforinteractions:n.2357.Ex.fluoroquinolone-quetiapine, statin-clopidogrel,ASA-omega-3.Weidentifiedn.10hidden ADRs,subsequentlyregisteredonthePharmacovigilance NationalNetwork.
ConclusionandRelevance TheidentificationofhiddenADRs inpolytreatedpatientsavoidedtheinclusionofanewdrugto treattheclinicalsymptomnotrelatedtoanewdisease.The nextgoalistointegratethepatientintothepath,avaluable sourceofinformationcurrentlyunavailable,thusimplementing territorialhealthcarethroughnarrativepharmacovigilancethat willallowacompletepictureoftheindividualpatient.The aimistoanenhancedcaremodelwiththetopthepatient betweenGPandpharmacist.
10.1136/ejhpharm-2023-eahp.324
BackgroundandImportance Atdawnonthe26thofApril 2022,ourhospitalsufferedacyberattack.Allhospital´scomputersystemsandapplicationswereinaccessible,andthenetworkandmostworkstationsinoperable.Theonlyfew computersthatremainedoperationalwerestandalone,thatis, notconnectedtoanetwork.Theinstitutionalemailwasonly availableonmobilephones.Atthattime,wewereconsideredapaper-freehospital,totallycomputerised,withelectronicpatientrecordsandonlineprescriptiontotally implemented,andpharmaceuticalprocedureshighlydependentontechnologyandautomationso,itwasparticularly challengingtocontinuetoprovidepharmaceuticalcarein thisscenario.
AimandObjectives Descriptionofproceduresimplementedin ascenarioofcyberattackbythepharmacydepartmentand establishmentofpreventivemeasuresforthefuture.
MaterialandMethods Thisstudyisadescriptionofacase.
Results Duetolackofaccesstoclinicalandpharmacotherapeuticprofileofpatients,itwasnecessarytoreversethe prescriptionforpapersupport, ininpatientwards.TheKardexSystemremainedoperational,havingbeendisconnected fromthenetworkinatimelymanner,allowingthereconstitutionofthehistorytreatmentofpatientsthroughthepreviousdaytherapeuticmapfiles.MicrosoftExcelfileswere createdforallpatientsadmittedtoserviceswithunitdose distribution,usinglaptops stand-alone.Thecommunication withthenursingteamwasmadedaily,bytelephone,with conferenceofallthepatients.TheExcelfileswiththetranscriptionoftheprescriptions,perpatient,weremanually codedbyservice,patientanddrug,and,attheendofthe day,transformedintotheapp ropriateformattobecorrectly readbyKardexsystem,transferredtoitbypen-drive, allowingtheUnitDosepreparation.Contactwasstrengthenedwiththemedicalandnursingstafftoavoidduplication ofdrugsorinadequateposologyerrors.Paperfilefolders werecreatedbyserviceforallprescriptionsmade,and updateddaily.AllExcelfileswereposteriorlyaccountedfor regularisationofconsumption.
ConclusionandRelevance Inthiscyberattackcontext,itwas evidentthedifficultyinreversingtheprescriptionsforpaper support,especiallybyyoungdoctors.Itwillbenecessaryto implementvalidatedprocedureswithperiodicmeasures, includingtrainingincontingencyprotocolsandcloudbackup informationmaintenance.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.CanadianMedicalAssociationJournal2020;192(4):E101-2.
ConflictofInterest Noconflictofinterest
5PSQ-135
1,2OUrbina*, 2,3JCGainzarain, 2,4ACanut, 2,3JPortu, 2,3ZOrtizdeZárate, 2,3ESáezde Adana, 5MCampos, 1JJGarcía, 1EGómez, 1MDMartinez, 1CMartinez. 1ArabaUniversity Hospital,PharmacyDepartment,Vitoria-Gasteiz,Spain; 2Bioaraba,InfectiousDiseases ResearchGroup,Vitoria-Gasteiz,Spain; 3ArabaUniversityHospital,InfectiousDiseases Department,Vitoria-Gasteiz,Spain; 4ArabaUniversityHospital,MicrobiologyDepartment, Vitoria-Gasteiz,Spain; 5UniversityofMurcia,FacultyofComputerScience,Murcia,Spain
10.1136/ejhpharm-2023-eahp.325
BackgroundandImportance Theincreasinguseofantimicrobialsandtheglobalsurgeofantimicrobialresistanceisa majorpublichealthconcern.
AntimicrobialStewardshipProgrammes(AMSP)arean importantsecuritystrategyinhospitalsbecausetheirimplementationpromotesanoptimaluseofantimicrobials,improvingpatientoutcomeswhiledecreasingtheriskofadverse eventsaswellasantimicrobialresistance.
AimandObjectives ToevaluateifanAMSPhadanimpactin theoverallconsumptionofantibiotics,measuredasnumberof defineddailydosesper100stays(DDD/100s),inanacute carehospitalduringthefirstyearofimplementation.
MaterialandMethods AMSPstartedinArabaUniversityHospitalinOctober2020.
TheAMSPwasconducted3daysperweekbyahospital pharmacistandaninfectiousdiseasespecialistwiththepossibilityofconsultingamicrobiologistbytelephone.
AnAntimicrobialStewardshipProgrammeSupportSystem (AMSPSS)wasusedtoalertofantibioticprescriptionsthat needarevision.Thesealertswerepreviouslydesignedbythe AMSPteam.
Antibioticrecommendationsweremadeinthehealthelectronicrecordorbytelephonetothepatientresponsibledoctor.TheywereregisteredintheAMSPSSaswell.
Weretrospectivelyanalisedinterventionsandmeasuredthe globalantibioticconsumptionasDDD/100susingthepharmacydispensationregistersfromJanuarytoDecember2021. Results 1206alertsoftheASPSSwerereviewedbytheAMSP teamand434ofthem(36%)generatedprescriptionrecommendations(oneormore).
Atotalof820antibioticrecommendationswereperformed withaglobalacceptanceof78,3%.
Areductionof10,6DDD/100swasfoundin2021comparedto2020(58,42DDD/100svs69,02DDD/100s).
ConclusionandRelevance AftertheimplantationoftheAMSP, therewasadecreaseintheantibioticusein2021.Although otherfactorsmayhavealsocontributedtothisreduction,we confirmthatadailyAMSPisausefultooltooptimiseantimicrobialconsumption.
Itisnecessarytocontinuewiththeimplementationofthe AMSPtoguaranteetheproperuseofantimicrobials.
REFERENCESAND/ORACKNOWLEDGEMENTS
None
ConflictofInterest Noconflictofinterest
1AMiranda*, 1CLGemaIsabel, 1EMartínezRuiz, 1ACodonalDemetrio, 1PTardáguila Molina, 1CDeanBarahona, 1ALázaroLopez, 2MTorralbaGonzalezdeSuso. 1Farmacist, HospitalPharmacist,Guadalajara,Spain; 2MedicalDoctor,InternalMedicin,Guadalajara, Spain
10.1136/ejhpharm-2023-eahp.326
BackgroundandImportance Clinicalpracticeguidelinesrecommendinitiationofantiretroviraltherapy(ART)assoonaspossibleafterdiagnosisofHIVinfectionwithacombinationof nucleosidereversetranscriptaseinhibitors(NRTI)withintegraseinhibitors(INSTI),non-nucleosideNNRTIorprotease inhibitorspharmacologicallyboosted(PI/b).
AimandObjectives ComparethepotencyofdifferentcombinationsofNRTIwithNNRTIs,INSTIsorPI/bs.
MaterialandMethods Retrospectiveobservationalstudyof naivepatientsdiagnosedbetweenJanuary-2012andJune2022.Variablesanalysedwereage,sex,routeofinfection, ART,AIDS,viralload(VL)andtimetoreachundetectable VL(<50copies/ml).
Datawerecollectedfromtheelectronicmedicalrecords (MambrinoXXI®)andoutpatientdispensingsoftwareDPE Farmatools®.
Statisticalanalysiswasperformedusingalinearregression method(dependentvariable:potencyofthecombinationcharacterisedasthereductioninVLcorrectedforthetime (months)inwhichundetectableVLisachievedandananalysis ofvariance(ANOVA)usingSPSS® v.15.
Results Ninety-sixpeoplewerediagnosedwithHIVinfection. Medianage:34years(RIC30-43),78%male.AIDSstage waspresentin34%.Themostcommonrouteoftransmission wasmensexmen(MSM)53%.
InitiationofARTNRTIcombinedwithINSTIwas73%, NNRTI7%andIP/b20%.ThemeanlogVLbaselinewas 4.63(SD:0.93).
ThemeanVLreductionpermonthoftreatmentinpatients treatedwithNRTI+INSTIwas2.45copies/ml/month,NRTI +PI/bwas1.72copies/ml/monthandNRTI+NNRTIwas 1.63copies/ml/month.Thesignificanceoftheanalysisofvariancesofthemeansobtainedwas0.112.
ConclusionandRelevance INSTIspotencywashigherthanthe otherTARcombinations,althoughthedifferenceswerenot significant.
Studyheterogeneityinthefollow-uptimesbetweendiagnosisandthedateofVLanalysisaswellasthenumberof patientstreatedwithNNRTIs,andPI/bswaslowerthanthe INSTIsgroupmayexplainthenon-significantresults.
Itwouldbeinterestingtoextendthesamplewithamulticentrestudytovalidatetheresultsobtained.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
5PSQ-137
RTamayoBermejo*,BMoraRodríguez,MEspinosaBosch,IMMuñozCastillo. Regional UniversityHospitalofMalaga,PharmacyDepartment,Málaga,Spain
10.1136/ejhpharm-2023-eahp.327
BackgroundandImportance Dihydropyrimidinedehydrogenase (DPD)encodedbytheDPYDgene,istherate-limitingenzyme offluoropyrimidinescatabolism.
Amongaround450missenseDPYDsingle-nucleotidepolymorphisms,onlyapproximatelytwentyofthemacquirea functionalsignificance.Fourofthesevariantsareconsidered tobeofclinicalrelevanceforrecognisedeffectsontheprotein,theiridentifiedhigherriskofseveretoxicity,andfor theirpopulationfrequency.
AimandObjectives ToanalyseDPYDgenemutationsinall patientswhoarecandidatesforreceivingafluoropyrimidinebasedregimensandtheirinfluenceontheindividualisationof cancertreatment.
MaterialandMethods Retrospectiveobservationalstudyfrom July/2020-July/2022.Allpatientswhounderwentagenotyping testforDYPDwereincluded.Demographicvariableswere recorded.Theloss-of-functionvariantsintheDPYDgene wereanalysed:c.1905+1G>AthatidentifiestheDPYD*2A haplotype,andc.1679T>GthatidentifiestheDPYD*13haplotype.Italsostudiesthevariantsofreducedfunction: c.1129-5923C>GthatidentifiestheHapB3haplotypeand c.2846A>T.Thefrequencyofeachofthesevariantswere determined,andtherecommendationsoftreatmentindividualisationwerecollected.
Results Weanalysed638requestsforDPYDgenedetermination,meanagewas62.65±12.58years,and52.98%were men.Thirty-two(5,0%)hadsomemutationintheDPYD gene.Four(0,6%)patientswereheterozygousfortheloss-offunctionvariantc.1905+1G>Aandone(0,16%)patient washeterozygousforthevariantc.1679T>G.Twenty-three (3,6%)patientswereheterozygousforthedecreasedfunction variantc.1129-5923C>G,andfour(0,6%)patientswereheterozygousforthereducedfunctionvariantc.2846A>T.
Allofthemwereintermediatemetabolisers,whoifthey startedtreatmentwithfluoropyrimidines,theyshouldstart treatmentwithadosereducedtoapproximately50%and thenescalatethedoseinlatercyclesifnotoxicitywas observed.
Therecommendationofindividualisationoftreatmentwas: sixteenpatientsstartedtreatmentat50%ofthedose,inseven patientsthechemotherapyregimenwerechanged,inseven patientsadjuvanttherapyweredismissed,onepatientwasnot treated,andonepatientreceivedradiatiotherapyalone.
ConclusionandRelevance ThedeterminationofDPYDpolymorphismspriortothestartoftreatmentwithfluoropyrimidines,allowstoidentifyDPD-deficientpatients,andavoid mayexperienceserioussideeffectswhentreatedwithfluoropyrimidines;andthusclinicians ’ decisionsareinfluencedby theresultsofDYPDgenotyping.
ConflictofInterest Noconflictofinterest
MCSánchezArgaiz*,MJGándaraLadróndeGuevara,MISierraTorres,CMonteroVilchez, AJimenezMorales. HospitalUniversitarioVirgendeLasNieves,HospitalPharmacy, Granada,Spain
10.1136/ejhpharm-2023-eahp.328
BackgroundandImportance Chemotherapyerrorsrepresenta potentiallyseriousriskofpatientharmbecauseofthenarrow therapeuticwindowofantineoplasticandtheirhightoxicity. Pharmaceuticalvalidationaimstooptimisechemotherapytreatmentinordertoobtainthebestresultsforpatients‘ health andtominimisepotentialprescriptionerrors.
AimandObjectives Todescribethechemotherapyprescription errorsofintravenouscytostaticdetectedduringthepharmaceuticalvalidationandtheinterventionsmadetoavoidpotentialharmtothepatient,helpingpreventmistakes.
MaterialandMethods Observational,descriptive,retrospective studyofchemotherapyprescribingerrorsdetectedbypharmaceuticalvalidationthatresultedinaprescriptionchangein intravenouschemotherapy.
TheywererecordedbetweenNovember2020toSeptember 2021andtheprogrammeusedforprescribingandrecording wasOncoFarm.®
Theerrorswereclassifiedinto10groups:1)Upper/lower dose<10%,2)Upperdose>10%,3)lowerdose>10%,4) Inappropriatecyclefrequency,5)Relevantinteractionor adverseeffect,6)Doseadjustmentordelayadministration (renalandhepaticimpairment,haematologictoxicity),7) Incorrectprotocol,8)Missingdrug,9)Excessdrug,10) Others.
Results Duringthestudyperiod,180chemotherapyerrors weredetectedamong53.243dosesofintravenouschemotherapypreparedinahospitalpharmacyfor2.944patients(63% fromoncologyservice,32%haematologyservice,3%urology service;2%others).Theseerrorsdetectedthroughpharmaceuticalprescriptionreviewinducedchangesinchemotherapy prescriptions:88inhaematologyprescriptions,83inoncology prescriptions,6inurologyprescriptionsand3inonco-haematologyprescriptions.
Classification 6Upper/lowerdose<10%,60upperdose >10%,7lowerdose>10%,17inappropriatecyclefrequency, 4relevantinteractionoradverseeffect,14doseadjustmentor delayadministration(renalandhepaticimpairment,haematologictoxicity),20incorrectprotocol,12missingdrug,18 excessdrug,22others
Themostcommonerrorswereattributedtoerrorprescriptionofupperdose>10%,in36%werecarboplatindosemistakes;andalsoerrorsinprescribingandprotocolfrequency.
ConclusionandRelevance Despitethenumberofdetectedchemotherapyerrorsdoesnotrepresentalargevolumeinthe totalnumberofpatientstreatedinalmosttwoyears,theyled toaprobablereductioninadversedrugevents,toxicitiesand patientsoverdose.Thisgivesusanideaofthebenefitandthe importanceofpharmaceuticalvalidationinchemotherapy treatmentoptimisationandpatientsafety.
ConflictofInterest Noconflictofinterest
5PSQ-139
USINGATEXT-MININGAPPROACHTOIDENTIFYTHE CONTEXTVARIABLESLANGUAGEBARRIER,LIVING ALONE,COGNITIVEFRAILTYANDNON-ADHERENCE
1STenHoope, 2KWelvaars, 1MEveraars-Klok, 3SVanSchaik, 1FKarapinar*. 1Olvg Hospital,ClinicalPharmacy,Amsterdam,TheNetherlands; 2OlvgHospital,DataScience, Amsterdam,TheNetherlands; 3OlvgHospital,Neurology,Amsterdam,TheNetherlands
10.1136/ejhpharm-2023-eahp.329
BackgroundandImportance ElectronicHealthRecords (EHRs)containfreetextfieldssuchasclinicalnotes.These textfieldsfrequentlycontainvaluableinformationaboutthe contextofpatients.Nevertheless,thisinformationisoften unusedastextfieldsaretime-consumingtoread.Thecontextvariableslanguagebarrier,livingalone,cognitivefrailty andnon-adherenceareassociatedwithunplannedhospital readmissions.Previousstudieshavenotexploredwhether text-miningcouldhelptoidentifythesevariablesfromfree text.
AimandObjectives Theprimaryaimofthisstudywasto identifythefourcontextvariableslanguagebarrier,living alone,cognitivefrailty,andnon-adherencefromtheEHRs usingtext-mining.
MaterialandMethods Thestudypopulationwasfroma databaseofn=1,120unplannedhospitalreadmissions (30-days)atOLVGhospital.Amanualstandardwascreatedbyextractinginformation fromclinicalnotesandcategorisingeachpatientforeachvariable(induplo).Forthe simpletermslanguagebarrierandlivingalone,arulebasedalgorithmwasused,seefigure1.Forthemorecomplextermscognitivefrailtyandnon-adherence,aNamed EntityRecognition( NER)algorithmwasused,seefigure2. Eachalgorithmwasvalidated againstthemanualstandard untilahighpercentageagree mentwasachievedforamaximumoffiveiterations.Theprimaryoutcomewasthepercentageagreementandkappavaluebetweenthemanual standardandthealgorithm .Descriptivedataanalysiswere used.
Results Therule-basedalgorithmforlanguagebarrierhada percentageagreementof96.8%andaKappaof0.90.Forlivingalonethepercentageagreementwas76.8%andtheKappa 0.53.TheNERmodelforcognitivefrailtyhadapercentage agreementof95.1%andKappaof0.83,andfornon-adherencetheagreementwas91.9%andKappa0.37.Generally, themodelsoverestimatedthenumberofpatientswithacontextvariable(e.g.afamilymemberwithalanguagebarrier ratherthanthepatienthimself).
ConclusionandRelevance Inthisstudy,text-miningwasable toidentifycontextvariablesfromEHRs,withagoodkappa forthevariablelanguagebarrierandcognitivefrailty.Future studiesshouldexplorehowoverestimationintext-mining couldbereduced.Text-miningcouldhelphealthcareprofessionalstoanticipateonpatientcontextinthefuturetooptimise care.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
5PSQ-140 FREQUENCYOFBLEEDINGEVENTSREQUIRING HOSPITALISATIONINPATIENTSUNDERGOINGHOME TREATMENTWITHDIRECT-ACTINGORAL ANTICOAGULANTS(DOACS)
SGutiérrezPalomo,AAndújarMateos,MJLucasMayol,CMatosesChirivella,GMiralles Andreu,ANavarroRuiz*. HospitalGeneralUniversitariodeElche,HospitalPharmacist, Elche,Spain
10.1136/ejhpharm-2023-eahp.330
BackgroundandImportance DOACsweredevelopedtominimisethedrawbacksoforalvitaminKantagonists(VKAs).The goalofthenewdrugswastokeeporimprovetheefficacyas wellasthesafetyinthebleedingmonitoring.Pivotalclinical trialsshowedafavourablebenefit/riskratio.However,these studieshadmethodologicallimitations.
AimandObjectives Todeterminethefrequencyofbleeding eventsthatrequirehospitaladmissioninpatientsreceiving DOACtreatment,analysingthecharacteristicsofthepatients andclassifyingthebleedingeventsaccordingtothetypeof DOACandthesiteofbleeding.
MaterialandMethods Retrospectiveobservationalstudythat includesallpatientstreatedwithDOACswhorequiredhospitaladmissionduetoableedingevent(ICD-10-CM:T45.51). ThestudywasperformedbetweenJanuary2016andMarch 2022.Thevariablescollectedwere:sex,age,DOACandtype ofhaemorrhageresponsibleforhospitaladmission.Datawere collectedfromcomputerisedmedicalrecordsandtheywere analysedwiththestatisticalprogramSPSS®.
Results 53hospitaladmissionswereincluded.Themeanage was79.3±7.1years.37(69.8%)episodescorrespondedto men.Ifbleedingeventwasassociatedwiththetypeof DOAC,itwasfound:20(37.7%)episodesfordabigatran,17 (32.1%)forapixaban,8(15.1%)forrivaroxabanand8 (15.1%)foredoxaban.Regardingtypeofhaemorrhage,23 (43.4%)episodeswererelatedtolowergastrointestinalbleeding(LGIB),11(20.8%)tohaematuria,9(17%)touppergastrointestinalbleeding(UGIB)and7(13.2%)tointracranial haemorrhage(ICH).
ThemostprevalentDOACineachhaemorrhagewas studied.Dabigatranwasthemostfrequentwith10(43.5%) eventsinLGIBandwith5(71.4%)casesinICH.However, apixabanwasthemostfrequentwith4(44.4%)episodesin UGIBandwith4(36.4%)casesofhaematuria.
Regardingtypeofbleedingbysex,LGIB(OR=1.68;CI =0.74-3.83)andICH(OR=2.19;CI=0.98-4.90)weremore frequentinwomen,whilehaematuria(OR=1.41;CI=1.061.90)andUGIB(OR=1.35;CI=0.99-1.85)weremorecommoninmen.
Byagerange,percentagesofUGIB,haematuriaandICH caseswerehigherinpatientsaged80yearsorolder,being 55.6%,54.5%and57.1%respectively.LGIBoccurredin patientsyoungerthan80years(56,5%).
ConclusionandRelevance Patientswhorequiredhospitalisation wereelderly,havingahigherriskofsufferingdifferenthaemorrhageswhenwereover80yearsold.Statisticallysignificantdifferencesbetweenhaematuriaandmenwereobserved. Gastrointestinalhaemorrhagesandhaematuriaswerethemost
frequentdiagnosis.Dabigatranwasthecauseformostofthe hospitaladmissions,beingmainlyinvolvedinLGIBandICH, followedbyapixaban,relatedwithUGIBandhaematuria.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
5PSQ-143 THEROLEOFTHEPHARMACISTININCREASING HEALTHVIGILANCEAMONGHEALTHPROFESSIONALS: ANGIOEDEMAFOLLOWINGTHEADMINISTRATIONOF RITUXIMAB
1,2HDaoudi*, 1,2FZLasri, 1,2OElQabissi, 1,2SElMarnissi, 1,2MAitElCadi. 1IbnSina HospitalUhcIbnSinaRabat,Pharmacy,Rabat,Morocco; 2FacultyofMedicineand PharmacyRabat,PharmacologyandToxicologyLaboratory,Rabat,Morocco 10.1136/ejhpharm-2023-eahp.332
1IMoronaMinguez*, 2CMMeseguerBarros, 2IOterinoMoreira, 2LJamartSanchez, 1ISollano-Sancho, 1CMorielSanchez. 1HospitalUniversitariodeMóstoles,Pharmacy, Móstoles,Spain; 2DirecciónAsistencialOestedeAtenciónPrimaria,Pharmacy,Móstoles, Spain
10.1136/ejhpharm-2023-eahp.331
BackgroundandImportance Fallsintheelderlyhaveamultifactorialcomponent,amongthesefactors,oneofthemain causesischronicconsumptionofbenzodiazepines(BZD).
AimandObjectives TodescribetheprevalenceofchronicconsumptionofanxiolyticBZDinelderlypeoplewhohavesufferedfallswithinhospitaladmissionschemes.
MaterialandMethods Cross-sectionaldescriptiveandobservationalstudyinahealtharea.WeidentifiedthroughtheMinimumBasicDataSet(CMBD)patientsolderthan64years withhospitaladmissionwithcodeW19.XXXA(Unspecified acutefall,initialcontact)accordingtotheInternationalClassificationofDiseasesversion10,between2017and2021.Variablescollected:dateofbirth,sex,comorbiditiesandVan WalravenComorbidityIndex.
Chronicconsumption(morethan4weeks)ofanxiolytic BZD(ATC-WHOcodeN05BA)recordedintheprescription billingsystemwasanalysedinthesepatients.Patientswhohad pickedupBZDatthecommunitypharmacyduringthefall episodeweretheonesselected.
DatawereanalysedusingStata/BEv17statisticalsoftware. Results 1585patients(63.8%female)withacutefallcodehospitaladmissionbetween2017and2021wereidentified. Medianageatadmissionwas82.6[IQR11.5].Andmedian ofVanWalravenComorbidityIndexwas5.0[IQR11.0], mainly:hypertension(49.0%),arrhythmias(29.5%)anddiabetes(22.4%).Patientsthathadmorethanonefallepisoderepresented6.5%oftotal,withamedianof7.0[IQR7.4]days ofhospitalisation.ChronicanxiolyticBZDuseduringthefall episodewasobservedin23.3%(77.3%female)ofpatients. ThemostfrequentlyusedanxiolyticBZDwerelorazepam (48.6%),bromazepam(29.4%)anddiazepam(14.3%),the firsttwobeingofshort/intermediatehalf-lifeanddiazepamof longhalf-life.
ConclusionandRelevance Almostaquarterofthestudypopulationwithunspecifiedacutefallswerechronicanxiolytic BZDusers,mainlywithashort/intermediatehalf-life.Because BZDuseintheelderlyisacausativefactorinfalls,itisnecessarytoadjusttreatment,recommendingde-prescriptionor gradualdosereductionwherepossible.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
BackgroundandImportance Angioedemaisarapidswellingof theskinandmucousmembranesintheheadandneckarea andshouldbetreatedasanemergency.1 Rituximabisachimeric monoclonalantibodyusedinchemotherapyagainsttheCD20 surfacemolecule.2
AimandObjectives Thisworkisaimedtoevaluatetheefficacy andthesafetyofrituximabadministrationbydeterminingthe causalityofsuspectedangioedemainpatientsreceiving chemotherapy.
MaterialandMethods WearereportingtwocasesofangioedemaonRituximab:
. A66yearsoldmanwithDLBCLwhoreceivedfourcourses ofRCHOP(Rituximab,Cyclophosphamide,Doxorubicinand Vincristine).Onthefifthcourseand15minutesafterstarting administrationofrituximab,hedevelopedangioedema,after that,hereceivedhydrocortisoneandadrenalineandwas quicklytransferredtotheintensivecareunit,12hourslater, hewaspronounceddead
. A52yearsoldwomanwithahistoryofpulmonary tuberculosistreated18yearsago,treatedformarginalzone lymphomawithRCHOPprotocol,shepresentedan angioedematwohoursafterthestartoftherituximab infusionduringthe2ndcourseoftheprotocol.Thepatiente receivedhydrocortisoneandadrenalineandsherecovered well.
Thecause/effectassessmentwascarriedoutaccordingto theFrenchmethodafterathoroughinvestigation.3
Results Forbothcases,theresultsshowedthatrituximabwas incriminatedwithanintrinsicimputabilityscoreofI5andan extrinsicimputabilityscoreofB4,causedbyadministrationof ahighrateofrituximab(200mg/h)atthestartofthe infusion.
Toavoidthistypeofadverseevent,thehospitalpharmacist adjustedtherituximabinfusion,startingwithinfusionrateof 50mg/hfor30minutesandthenincreasingby50mg/hevery 30minutestoreachamaximumof400mg/h.
ConclusionandRelevance Thisobservationillustratestherole ofthehospitalpharmacistinmakingnursesanddoctorsaware oftherisksofadministeringdrugsthatcancauseangioedema, inparticularrituximab,topreventtheriskofincidenceand improvevitalprognosis.
1.PeterJ.Delves,Quincke’sedemaMSDmanuals
2.PloskerGL,FiggittDP,«Rituximab:areviewofitsuseinnon-Hodgkin’slymphomaandchroniclymphocyticleukaemia»Drugs2003;63:803-843 3.MooreN,etal.Adversedrugreactionmonitoring:DoingittheFrenchway1NBarreras*, 2SRamos, 1ECastillo, 2RCórdoba, 1MHernandez, 1JBécares. 1Hospital UniversitarioFundaciónJiménezDíaz,PharmacyService,Madrid,Spain; 2Hospital UniversitarioFundaciónJiménezDíaz,Haematology,Madrid,Spain
10.1136/ejhpharm-2023-eahp.333
BackgroundandImportance PROMSbegintomakeaplacein theworldofclinicalcare,forthisreason,itwasimplemented inourhospital.
AimandObjectives Theprimaryobjectivewastocomparethe adverseevents(AEs)profilereportedintheelectronicmedical record(EMR)andthosereportedbypatientsthroughavalidatedquestionnaire(PRO-CTCAE ®).Secondary,weretoanalysetheimpactinthereductionofvisitstoEmergencyRoom (ER).
TSteindl-Schönhuber,GGittler*. KrankenhausBarmherzigeBrüderLinz,HospitalPharmacy, Linz,Austria
10.1136/ejhpharm-2023-eahp.334
BackgroundandImportance Sincethebeginningofthe COVID-19pandemicdrugdistributionintheHospitalSt. JohnofGod,Linz,hasbeenswitchedtoautomatedunitdose packaging.Weintendedtocreateevidenceforpatients’ satisfactionwithpharmacydeliveredblistersachets,asliterature onthistopicislimitedandourserviceissofaruniquein Austrianhospitals.
MaterialandMethods
Patientswithdiagnosisofnon-Hodgkin’slymphomaintheneedofIVtherapybetweenJanuary 2019andDecember2021wereincluded. ‘E-ResSalud’ was launchedinJanuary2020.Patientsincludedin2019werethe controlarm.PRO-CTCAE® waselectronicallysentthroughthe appafter1st,3rd,and6th monthoftherapy.Thosesymptoms oflowintensityweretoreceiverecommendationsautomaticallythroughtheapp.Thosesymptomsofhighintensitywere toreceiveateleconsultationcallbythenurse.ASankeydiagramwasbuilttodepictflowsofseverityofsymptoms.Twosidedtestandp-values<0.05wereconsideredstatistically significant.
Results Amongthe201patientsincludedinthestudy,76 patients(37.8%)reportedoutcomesintheePROMprogram. MostfrequentlyAEsreportedintheEMRwerehaematological(73%),gastrointestinal(62%)andpsychological(38%).In contrast,themostfrequentlypatient-reportedadverseevents werecutaneous(47%),gastrointestinal(44%)andoral(26%), accordingtoPRO-CTCAE® categories(p<0.01).
Afterthefirstcourseofchemotherapy,46%ofpatients reportedsymptomsofhighfrequency,intensityorimpact intheirQoL.Atthirdmonththeproportionwassignificantlyhigher(67%vs46%;p<0.05).Differenceswere alsostatisticallysignificantbetweenfirstandsixthmonth (p<0.01).
ThosewhowereadherenttotheprogramhadfewernumberofvisitstoER(19.2%vs55.2%;p<0.01)andrequired fewerunscheduledhospitaladmissions(15.8%vs37.6%; p<0.01).Whenanalysingoutcomesofpatientswhowere calledbyanursereducedtheproportionofpatientswhovisitedtheERvsthosewhodidnotreportanyorlowintensity symptoms(18.8%vs53.8%;p<0.01).Survivalamongpatients visitingERwassignificantlyshorterthanamongthosewho didnot(hazardratio,2.26;95%[CI],1.11to4.63;p= 0.025).
ConclusionandRelevance BetterunderstandingofpatientreportedsymptomscouldaidpharmacisttodevelopanindividualisedtreatmentdoseadjustmentandreductionofERvisitsshouldbeakeytargetforhaematologistsasitmayimpact insurvival.
AimandObjectives Weperformedapatientsatisfactionsurvey toinvestigatethestatusquoaswellaspotentialneedsfor improvementandtoprovidethebasicdataforfurther analyses.
MaterialandMethods Patientswereinterviewedinhousewith aninternallydevelopedquestionnaire.Itsmixeddesign – 12 multiple-choicequestionsandfieldsforcomments – enabled quantitativeandqualitativefindings.
Patientsnotfamiliarwiththeblistermedication(e.g.no oraldrugs)ornot(mentally)fitenoughwereexcluded. Withinaperiodoftwoweekshospitalpharmacistscarriedout 38face-to-faceinterviews.
Results Patientsatisfactionwiththeblisterswashigh;Transparencyinadministereddrugtherapywasconsideredimportant.Patientsnotorrathernotsatisfiedstateddifficultiesin handlingtheblisters(20%).Poorphysicalconditions,vision deficiencyandhigheragecorrelatedwithutilisationproblems andlowersatisfaction.Onein10patientshadnotbeen capableofopeningtheblistersachetsandtakingthemedicationwithoutassistance.Two-thirdsfoundunitdosedrugdistributionpreferableorequaltotraditionalpilldispensers. Somepatientscommentedontheenvironmentaleffectsof theplasticsachets.
Respondingtothereporteddifficultiesweplacedinfographicsinthepatientroomsillustratingthelabellingand handlingoftheunitdosesachets.Thestaffonthewards weretrainedtogivefurtherinformationtopatientsandassistanceinopeningandemptyingtheblisters.
ConclusionandRelevance Studiesontheeffectsofunitdose supplyusuallyfocusoncost-effectiveness,medication safetyandnursingstafftimeandsatisfaction.Ourresults addinformationonthepatientperspectiveandwere importantforqualityimprovement:Thispilotstudynot onlyallowedforimmediatelyimplementedactions(graphic depictionsforpatientsandstafftraining)butisalsoa guidanceforthedesignofalargerstudy(patientselection, interviewtechnique, reliableandvalidq uestions)toobtain sufficientstatisticalpowerandquantifiableandactionable data.
YNLin*. TainanMunicipalHospitalmanagedbyshowChwanMedicalCareCorporation, Pharmacy,Tainan,Taiwan,RepublicofChina
10.1136/ejhpharm-2023-eahp.335
BackgroundandImportance Thepublicwasfarlessawareof adversedrugreactionsthantheefficacyofdrugs.Everyone neededtotakecareoftheirownmedicationsafety.
AimandObjectives Todevelopaninteractivegametoevaluate publicunderstandingofadversedrugreactions.
MaterialandMethods Wedesignedaninteractivegame throughtheuseof ‘Wordwall’ onlinetemplate: ‘Quiz’ for ‘Adversedrugreactions’.Thecorrectanswerofeachquestion couldbeshownautomaticallyattheendofthegame.The outcomeswerecollectedduringJuly2022andevaluatedwith t-testbySPSS(StatisticalProductandServiceSolutions)23.0. Results 46peoplewereincludedinthegameandthetotal correctratewas81.74±18.29%.Thelackofknowledge aboutadversedrugreactionswasfound,forexample,26.08% peoplethoughtthatadversedrugreactionsmustoccurwhen takingmedicine.Besides,41.30%peoplethoughtthatthe medicationmustbediscontinuedifanyadversedrugreaction occur.17.39%peopleagreedthataddingonotherdrugsmay increasetheincidenceofadversedrugreactions.Finally, 6.52%peopledidnotknowtheycouldfeedbacktoprescribingphysiciansandpharmaciststomarktheadversedrugreactioninmedicalrecords.
ConclusionandRelevance ‘Wordwall’ wasaneasy-to-playand user-friendlygame.Ourresultsindicatedthatgamificationwas wellacceptedamongpeopleandhelpedpharmacistsunderstandwhatpeoplereallythinkaboutadversedrugreactions.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
6ER-004 EFFECTIVENESSANDSAFETYOFCOVID-19 VACCINATIONINPATIENTSWITHIMMUNE-MEDIATED DISEASESONBIOLOGICALTHERAPY
1CBarcaDiez*, 1ICProupinCantelar, 2LGonzalezFreire, 1AMDeusCasas, 1ABVeiga Villaverde, 2CCrespoDiz. 1ComplejoHospitalarioUniversitariodePontevedra,Pharmacy, Pontevedra,Spain; 2Complejohospitalariouniversitariodepontevedrafundaciónbiomedica galiciasur.,pharmacy,pontevedra,spain
10.1136/ejhpharm-2023-eahp.336
BackgroundandImportance Theeffectivenessandsafetyof COVID-19vaccineshasbeendemonstratedinthepivotaltrialsthathaveledtotheirapproval.However,thereisnospecificinformationavailableregardingCOVID-19vaccinationin patientswithimmune-mediateddiseases(IMD).
AimandObjectives Evaluatetheeffectivenessandsafetyof COVID-19vaccinesinpatientswithIMDwhoarebeing treatedwithbiologicaldrugs(BD).
MaterialandMethods Prospectivedescriptiveobservational studyofpatientswithIMDtreatedwithBDwhohave receivedatlistonedoseofanyoftheCOVID-19vaccines commercialised.
Variablescollected:age,sex,IMD,BD,post-vaccination COVID-19infection,adversereactionsobservedaftervaccination.
Demographicandclinicaldatawereobtainedfromthe medicalrecords.
Toassesseffectiveness,wecheckedthenumberofpatients whobecameinfectedwithSARS-CoV-2aftervaccinationand whethertheinfectionwasasymptomatic,withmildsymptoms orrequiredhospitaladmission.
Toassesssafety,astandardisedinterviewofadversereactionsobservedinthefirstsevendaysafterCOVID-19vaccinationwasconductedduringroutinepharmacypractice.
ThisstudywasapprovedbytheEthicsCommitteeof ResearchwithMedicinesundercode:2021/435.
Results 106patients(52.8%female)wereincluded,witha medianageof53years(21-76).ThemostfrequentIMD were:rheumatoidarthritis(33%),psoriaticarthritis(15%), psoriasis(15%)andCrohn’sdisease(11.3%).ThemostcommonlyusedBDswere:adalimumab(33.9%),etanercept (25.5%),abatacept(7.5%),ixekizumab(6.6%),secukinumab (6.6%),golimumab(5.7%)andustekinumab(4.7%).
Twenty-twopatients(20.75%)wereinfectedafterreceiving dosesofCOVID-19vaccines:2afterthefirstdose,6after theseconddoseand14afterthethirddose.Infectedpatients hadmildsymptoms(77.3%)orwereasymptomatic(22.7%). Nopatientrequiredhospitaladmission.
Themostcommonadversereactionswere:painatthe injectionsite(79.2%),fatigue(48%),malaise(42.4%),myalgia (35.8%),headache(33%),arthralgia(25.5%),fever(21.7%), pruritus(11.3%),nauseaorvomiting(9.4%),andlymphadenopathy(9.4%).
ConclusionandRelevance 79.25%ofthepatientsstudiedwere notinfectedwithSARS-CoV-2aftervaccination.Mostofthe infectedpatientshadmildsymptomsandnoneofthem requiredhospitaladmission.
Adversereactionsweresimilartothosedescribedinthe generalpopulation,themostfrequentbeingpainattheinjectionsite,fatigueandmalaise.
COVID-19vaccineswereeffectiveandsafeinpatientswith IMDtreatedwithBDincludedinthestudy.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
6ER-005 EFFECTSOFADHERENCETOTHEMEDITERRANEAN DIETINPATIENTSWITHAUTOIMMUNEDISEASES
AMartínRoldán*,MDMSanchezSuarez,CMonteroVilchez,MIArchillaAmat. Virgende LasNievesUniversityHospital,PharmacyDepartment,Granada,Spain
10.1136/ejhpharm-2023-eahp.337
BackgroundandImportance Adherencetoahealthydietary patternhasbeenshowntobeinverselyassociatedwithmetabolicsyndrome.LowadherencetotheMediterraneandietis directlyassociatedwithaworseprofileofplasmaticinflammationmarkers.Somestudieshaveshownthatthisdietmay reducetheriskofautoimmunediseases.
AimandObjectives ToevaluateadherencetotheMediterraneandietinpatientswithautoimmunediseasesaswellas theirqualityoflife.
MaterialandMethods Retrospective,descriptivestudyofthe adherencetotheMediterraneandietinpatientswithautoimmunediseasesduringJanuarytoMarch2021.Variablescollected:demographic(sex,age),diagnosis,bodymassindex (BMI),biologicaltherapy,lifestyle,cholesterol,triglycerides, glucose,ferritin,calprotectinandC-reactiveproteinlevels.
AdherencewasmeasuredbythePREDIMEDquestionnaire. Qualityoflifewasdeterminedby:VisualAnalogScalefor Pain(VAS),ChecklistIndividualStrength(CIS)andTheFunctionalAssessmentofChronicIllnessTherapy–Fatigue(FACITF).Informationsources:electronicprescriptionandcomputerisedmedicalrecord.StatisticalanalysiswithR® software. Results 66patientswereincluded(50%women),medianage 48(IQR38-56).MedianBMI26.3(IQR26-39.1).Mostfrequentdiseases:rheumatoidarthritis(18),Crohn’sdisease(10), ankylosingspondylitis(8)andmultiplesclerosis(7).42%of patientshadnopreviouscomorbidity,28%hadarterialhypertension,13.6%hypercholesterolemiaand6%depression.The mediandiagnosisyearofthediseasewas2012(IQR20022016).37.8%ofpatientshavehadtwolinesoftreatment, 24.2%threelines,4.5%fourlines.Themostfrequentdrugs wereanti-TNFtherapy(19adalimumab,4certolizumab,4etanercept),tocilizumab(5)secukinumab(4)andtofacitinib(4). MedianscaleVASwas4(IQR1-6),CIS83(IQR76-91)and FACIT-F16(11-24).MedianofthePREDIMEDquestionnaire was7(lowdietaryadherence).NostatisticallysignificantdifferenceswerefoundbetweenadherencetotheMediterranean dietandscoresonqualityoflifequestionnaires.Statistically significantdifferenceswerefoundwithcalprotectinlevelsand glomerularsedimentationvolume.78.7%ofpatientsarenot awareoffoodswithpotentialanti-inflammatoryproperties and87.8%wouldliketoreceivedietaryrecommendations fromhealthcareprofessionals.
ConclusionandRelevance Althoughmorestudiesareneeded tolinkdiettoautoimmunediseases,itistruethatanappropriatedietreducestheriskofmultiplepathologies.Patients demandinformationandashealthprofessionalswemustgive ittothemandreinforceadherencetogooddietarypatterns suchastheMediterraneandiet.
ConflictofInterest Noconflictofinterest.
6ER-008 CORRELATESOFONE-YEARMORTALITYAMONG PATIENTSLIVINGWITHHIVACCORDINGTOTHE STRATIFICATIONLEVELOFTHEPHARMACEUTICAL CAREMODEL
1EContrerasMacías*, 2FALaoDomínguez, 2PGarcíaLloret, 2RMorilloVerdugo. 1Hospital InfantaElena,HospitalPharmacy,Huelva,Spain; 2HospitalUniversitarioVirgendeValme, HospitalPharmacy,Sevilla,Spain
10.1136/ejhpharm-2023-eahp.338
BackgroundandImportance Thesuccessofhighlyactiveantiretroviral(ARV)therapyhasallowedpeoplelivingwithHIV (PLWH)tohaveanear-normallifeexpectancy.However,the increaseinlifeexpectancyhasgeneratedanewsetofchallengesinthesepatients,whooftenexperienceage-related comorbiditiesand,withit,polypharmacywiththenegative consequencesthatthisentails.
AimandObjectives Toanalysetheeffectthatthelevelof stratificationhasonmortalityresultsatoneyearanddevelop apredictivemodelinPLWHonactiveARV
HIVpatientmodelpublishedbySEFH).Asurvivalanalysis wasperformedtoassesshowthelevelofstratificationpredictedmortalityatoneyear.Thesurvivalratewasestimated usingKaplan-Meieranddifferencesbetweenlevelswereevaluatedusingalog-ranktest.Afterverifyingtheproportional hazardassumption,aCoxregressionwasruntoestimatehazardratios(HR).Toevaluatethediscriminatorypowerofthe model,thecalculationoftheareaundertheROCcurve (AUC-ROC)wascarriedout.Theanalysiswascarriedout usingtheSPSSv.28.0software.
Results Atotalof428PLWHwereincluded.Morethan90% ofthepatientshadadequateimmunovirologicalcontrol.The distributionofpatientsaccordingstratificationmodelwas: level3(83%),followedby12%and5%forlevel2and1, respectively.Attheendoffollow-up,5patientsdied.The resultsoflog-rankanalysisshowedsignificantdifferences regardinglevelofstratificationformortalityatoneyear (p=0.02).Coxregressionidentifiedlevelofstratificationasa riskfactorformortality,wherepatientsstratifiedaslevel1 hada99.7%higherrisk(HR:0.003;95%CI:0.001-0.027). TheAUC-ROCwas0.98(95%CI:0.96-1.00).
ConclusionandRelevance Patientsclassifiedaslevel1inpharmaceuticalcarestratificationmodelhaveahigherriskofmortalityatoneyear.Thepredictivemodeldevelopedhighlights theimportanceofthisconceptandtheneedforbothindividualisedpharmaceuticalcareandcomprehensivemonitoring.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
6ER-009 COMPARATIVEEFFICACYOFABEMACICLIBAND PALBOCICLIBASADJUVANTTREATMENTINPATIENTS WITHEARLYBREASTCANCER
1AGanforninaAndrades*, 2ASalgueroOlid, 3EJAlegreDel-Rey, 3SFenixCaballero. 1Puerta DelMarUniversityHospital,Pharmacy,Cádiz,Spain; 2OsunaHospital,Pharmacy,Osuna, Spain; 3PuertoRealUniversityHospital,Pharmacy,PuertoReal,Spain
10.1136/ejhpharm-2023-eahp.339
BackgroundandImportance Abemaciclibincombinationwith endocrinetherapy(ET)hasrecentlybeenauthorisedforadjuvanttreatmentofpatientswithhumanepidermalgrowthfactorreceptor2(HER2)negativeandluminalearlybreast cancer(EBC)athighriskofrecurrence.
AimandObjectives Toassessthecomparativeefficacybetween abemaciclibandpalbociclibinHER2-negative,highriskof recurrenceandluminalEBCpatientsandtoestablishwhether thesedrugscanbeconsideredequivalenttherapeuticalternatives(ETA),throughanadjustedindirecttreatmentcomparison (ITC).
MaterialandMethods
Asingle-centre,cross-sectionalstudy thatincludedPLWHonactiveARVwhoattendedPharmaceuticalCareoutpatientbetween1Januaryand15March 2021andwerefollowedupforaperiodof1year.Demographic,clinical,pharmacotherapeuticvariableswerecollected andpharmaceuticalcare,levelofstratification(accordingto
MaterialandMethods Abibliographicsearchwasconductedto identifyphaseIIIclinicaltrialswithabemacicliborpalbociclib asadjuvanttreatmentinasimilarEBCpopulation(luminal type,HER2-negativeandhighriskofrecurrence),duration andendpoints.Theprimaryendpointwasinvasivedisease-free survival(IDFS)andETwasusedasacommoncomparator. Similarclinicaltrials,consistentresultsandefficacydemonstrationagainstthecommoncomparator(ET)wererequiredfor theadjustedITC.
Results Twotrialswereincluded,oneofeachdrug.Bothof themwerephaseIIItrials,randomised,inpatientswith HER2-negative,highriskandluminalEBC.Differenceswere foundinthetrialdesign(abemaciclibopen-labelvspalbociblib
double-blind),numberofpatientsincluded(abemaciclib N=5637vspalbociclibN=1250),treatmentduration(abemaciclibtwoyearsvspalbocicliboneyear)andpercentageof patientspretreatedwithtaxane,anthracyclineorboth(abemaciclib37%vspalbociclib99%).Clinicaltrialswerenotsimilar duetothesedifferences.
AbemaciclibwaseffectiveinHER2-negative,highriskand luminalEBC.However,palbociclibwasnot.IDFSabemaciclib groupwasstatisticallysignificant(HR=0.70;95%CI:0.590.82;p<0.0001)withamedianfollow-upof27months (90%patientscompletedtreatment).Incontrast,IDFSpalbociclibgroupwasnotstatisticallysignificant(HR=0.93;95%CI: 0.74-1.17;p=0.525)withamedianfollow-upof43months (92%patientscompletedtreatment).
Regardingconsistresults,2-yearIDFSratewasdifferent too:abemaciclib93%vspalpociclib88%.Inshort,relevant methodologicallimitationsweredetectedsoadjustedITCwas notpossible.
ConclusionandRelevance Abemaciclibandpalbociclibcannot beconsideredETAinHER2-negative,highriskandluminal EBC,althoughabemaciclibdemonstratedefficacyasadjuvant treatmentinthesepatients.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
6ER-010 EVOLUTIONOFONCO-HAEMATOLOGICALCLINICAL TRIALSFROM2016TO2021:EXPERIENCEFROMA
TERTIARYHOSPITAL
HMartinezBarros,ÁDíazGago,MRodriguezMarin,EGemenoLopez*,CPueyoLópez, MLavandeiraPerez,APovedaEscolar,AMAlvarezDian. HospitalUniversitarioRamonY Cajal,Pharmacy,Madrid,Spain
10.1136/ejhpharm-2023-eahp.340
BackgroundandImportance Previousworkhasdescribed changesinthetrendsinonco-haematologicalclinicaltrialsin recentyears,describinganincreaseintheuseofsurrogate endpoints,changesintheirfundingoragreaternumberof non-randomisedtrials(1,2).
AimandObjectives Todescribeandcomparethecharacteristics ofonco-haematologicalclinicaltrialsopenedinatertiaryhospitalin2016and2021.
MaterialandMethods Allinterventionalclinicaltrialsinitiated inourhospitalin2016and2021wereincluded.Thefollowingvariableswerecollected:title,funding,tumoursite,blinding,control,randomisationandprimaryendpoint.Datawere comparedusingthePearson c2.Resultsweredeemedstatisticallysignificantatp<0.05.Statisticalanalysiswasperformed usingSTATA(StataCorp,Texas,USA).
Results Wefound89interventionalclinicaltrialsstartedin 2016and71studiesin2021.ThemajoritywereintheMedicalOncologyservice(93.6%and83.1%).Breastcancer accountedforthelargestnumberoftrialsinitiated(22.5% and19.7%).Inbothstudyperiods,mostclinicaltrialswere industry-sponsored,withanincreaseovertime(82.0%vs 94.4%;p=0.019).Morethanhalfofthestudiesinitiated werecontrolled(58.4%vs54.9%;p>0.05),randomised (59.6%vs66.2%;p>0.05)andopen-label(78.7%vs67.6%; p>0.05),withnostatisticallysignificantdifferencesbetween 2016and2021.Anincreaseinthenumberofphase3clinical trialswasobserved(37.0vs54.9%;p=0.017),withapredominanceofopen-labeldesign(54.6%vs51.3%;p>0.05)
andtheuseofsurrogateendpointsasprimaryoutcomes(54.5 vs69.2%;p>0.05).Notrialhadqualityoflifeasaprimary endpoint
ConclusionandRelevance Mostphase3clinicaltrialsusedan open-labeldesignandsurrogateendpointsasprimary outcomes.
Althoughthisisasingle-centreanalysis,sometrends observedbyotherauthors,suchasahighernumberofindustry-sponsoredstudies,wereobserved.
Noneofthe160clinicaltrialsinitiatedhadqualityoflife asaprimaryendpoint.
1.DelPaggioJCetal.EvolutionoftheRandomizedClinicalTrialintheEraofPrecisionOncology.JAMAOncol.2021May1;7(5):728-734.
2.WessonWetal.Characteristicsofclinicaltrialsforhaematologicalmalignancies from2015to2020:Asystematicreview.EurJCancer.2022May;167:152-160. ConflictofInterest Noconflictofinterest.
6ER-011 EFFICACYOFTHERAPIESINNON-SMALL-CELLLUNG CANCERWITHEGFREXON20INSERTION MUTATIONS:ASYSTEMATICREVIEW
1MDGil-Sierra, 2MDPBriceñoCasado*, 1CMoreno-Ramos, 1MABlanco-Castaño, 3CMCuadros-Martinez. 1HospitalUniversitariodePuertoReal,Pharmacy,PuertoReal, Spain; 2HospitalUniversitariodeJerezdelaFrontera,HospitalPharmacy,Jerezdela FronteraCádiz,Spain; 3HospitalUniversitariodeJerezdelaFrontera,Pharmacy,Jerezdela Frontera,Spain
10.1136/ejhpharm-2023-eahp.341
BackgroundandImportance Patientswithnon-small-celllung cancer(NSCLC)andepidermalgrowthfactorreceptor (EGFR)exon20insertionmutationshavepoorprognosisand fewtherapeuticalternatives.
AimandObjectives Todevelopasystematicreviewofplatinumpre-treatedNSCLCharbouringeGFRexon20insertions toassessefficacyoftreatmentsandscientificqualityof studies.
MaterialandMethods PreferredReportingItemsforSystematic ReviewandMeta-analysis(PRISMA)guidelineswasappliedin bibliographicreview.SearchwasconductedinPubMed® databaseupto15September2022.Filter ‘clinicaltrial’ ontypes ofarticleswasappliedtothefollowingreviewstrategy:(exon 20insertion)AND(Therapy/broad[filter]).Inclusioncriteria: Randomisedclinicaltrials(RCTs)evaluatingtreatmentsin patientsdiagnosedwithadvancedormetastaticNSCLCharbouringEGFRexon20insertionswhohadpreviously receivedplatinum-basedchemotherapy.Efficacyendpointsconsideredwereobjectiveresponserate(ORR),progression-free survival(PFS)andoverallsurvival(OS).Datarecorded:publicationdate,studydesign,comparatorarm,therapies,sample size,treatmentline,efficacydata.
Results Fortysearchresultswerefoundinreview.Twelve RCTswereincluded.Publicationdatesofstudieswere betweenApril2015andJuly2022.Designofstudies:9 (75%)phaseIIRCT(onewasbaskettrial)and3(25%)phase I/II.Noneofthempresentedacomparatorarm.Therapies assessed:poziotinib,osimertinib(highandlowdoses),pertuzumab-trastuzumabcombination,mobocertinib,amivantamab, erlotinib-onalespibcombination,luminespib,ado-trastuzumab emtansineanddacomitinib.SamplesizeofRCTsrangedfrom 10to114patients.Bothuntreatedandplatinum-pretreated patientswererecruitedin4(25%)RCTsandtherest
comprisedexclusivelyplatinum-pretreatedpopulation.Ado-trastuzumabemtansineshowedthebestnumericalresultsaccordingtoORR(54.5%),buttheworstPFS(2.8months;95% CI1.4-4.4)andOS(8.1months;95%CI3.5-13.2)ofall therapeuticalternatives.Thehighestnumericalefficacyresults wereachievedbyamivantamab[PFS=8.3months(95%CI 6.5-10.9);OS=22.8months(95%CI14.6tonotreached)] andmobocertinib[PFS=7.3months(95%CI5.5-9.2);OS =24.0months(95%CI,14.6-28.8)].
ConclusionandRelevance Resultsofamivantamabandmobocertinibsuggestedahighernumericalefficacyforclinicallyrelevantendpointsinplatinumpre-treatedNSCLCharbouring EGFRexon20insertions.However,comparativeRCTswith largersamplesizesarenecessarytoobtainreliabledata.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
ConclusionandRelevance Theclinicalevaluationsystemfor internshipstudentsinthepharmacyserviceisexpectedtobe veryusefulforpharmacistsinthePharmacyService.Thanks tothisexamtheywillhaveobjectiveinformationabouttheir teachingrolewiththesestudents,thusdetectingpointsfor improvement.Inaddition,thestudentlearnsaboutclinical reasoning,decision-making,problemsolving,andinterpersonalrelationshipskills.
ConflictofInterest Noconflictofinterest.
6ER-019 THESTUDENTPHARMACISTEXPERIENCEOF ENHANCEDCLINICALPLACEMENTS
1,2CThompson*, 1,2SWilliams. 1UniversityofBrighton,MedicinesUseResearchGroup, Brighton,UK; 2UniversityofBrighton,SchoolofAppliedSciences,Brighton,UK
10.1136/ejhpharm-2023-eahp.343
AMValleDíazdelaGuardia,SSadyrbaevaDolgova,MIArchilla*,CMontero-Vilchez. HospitalUniversitarioVirgendeLasNieves,ServiciodeFarmacia,Granada,Spain
10.1136/ejhpharm-2023-eahp.342
BackgroundandImportance
Structuredassessmentforfinal yearstudentsisateachingtoolbasedontheMiller´sPyramid thathasbeenimplementedinSpainformanyyears.Tocarry outthisevaluationsystemforstudentsdoinginternshipsin thehospital’sPharmacyServiceisveryinnovative.
AimandObjectives Todescribetheprocessofdesigninga structuredevaluationforstudentswhoaredoingtheirinternshipinthehospitalPharmacyService.Thepurposeofthe objectiveassessmentistoverifythatthestudentscandemonstratewhattheyhavelearnedduringtheirhospitalpractice. Todothis,differentclinicalskillsandtechnicalskillswillbe evaluated,simulatingrealsituationsrelatedtotheworkofthe PharmacyService.
MaterialandMethods Sixtestshavebeenestablished:pharmaceuticalcareforoutpatients,validationofmedicalprescriptions,stockmanagement,reconciliationofmedicationon admission,preparationofamasterformulaandoncology pharmacy.Alltestsarerelatedtodailyassistanceactivitiesof thehospitalpharmacy.Ineachtest,thestudenthasalimited timetoperformthetaskthatisindicated.Thequalification methodistotallyobjective,throughapreviouslydefined checklist.Aschedulehasbeenscheduledforeverythingtobe readyinJanuary2023.
Results Theobjectiveclinicalevaluationhasbeenstructuredin 6tests,inwhichatotalof24studentsmaybeexamined. Thepresenceof6evaluatorsand3actorswillbenecessary. Thecostofeachtestwillbeminimalbecausemostofthe materialsaredonationsfrompharmaceuticallaboratoriesand othercompanies.Thequalificationisimmediate,andthedurationofthetestwillbeabout3hours.Thechecklistofeach testwillbereviewedbytwoevaluators,andmustinclude itemsonclinical,technicalandinterprofessionalcommunicationskills.
BackgroundandImportance Theroleofapharmacistiseverexpandingwithanincreasingneedfortheprovisionof enhancedhealthservices.InresponsetotheGeneralPharmaceuticalCouncil’srecentannouncementfor ‘prescribing ready ’ pharmacygraduatesby2025,HealthEducationEnglandhas,thisyear,broadenedthecl inicaltariffforeducation providerstoincludepharmacygivinganopportunityto enhancetheclinicalplacementsofferedtopharmacystudents intheUK.
AimandObjectives Theaimofthisprojectwastoassessthe overallexperiencestudentshadonanEnhancedClinical Placementsoveravarietyofclinicalsettings.
MaterialandMethods Theplacementwaspatient-facing(5days)undertheprimarysupervisionofaprescriber,witha pre-placementinduction(15hoursofblendedlearning)consistingofsimulatedclinicalactivities,andapost-placement conference(1day).Duringtheplacement,studentshadthe opportunitytodevelopanextendedrangeofclinicalskills andobserveanddiscusstheprescribingdecisionsmadeby theirprescribingsupervisorwhicharecurrentlyoutsidethe scopeofthepharmacydegree.
Twofocusgroups(n=10andn=9)wereheldwithstudentsatthepost-placementconference.Studentswereasked abouttheirinductionandplacementexperience.Focusgroups weretranscribedandanalysedusingThematicAnalysis. Results Fourmainthemesemergedfromthedata,whichwere namedVariety,Consolidationofpriorlearning,Professional identity,andLogistics.Studentsexpressedanappreciationfor theECPinprovidingthemwithadditionalclinicalexperience overawidervarietyofsettingsthantheyhadseenbefore. TherewasarecognitionthattheECPhelpedtoconsolidate learningtheyhadgainedonthetaughtcoursesandthatit heightenedtheirprofessionalidentitybutstudentsalsoraised someareasforimprovementintermsofthegenerallogistics oftheplacement.
ConclusionandRelevance Thiswasausefulexercisetoprovidestudentswitharangeofexperiences,helpingthemto promoteanunderstandingoftheirprofessionalvalueandrole withinamulti-disciplinaryteam.Futureimplementationneeds toconsiderthelevelofstandardisationbetweenplacements
andtheimportanceofhavingclearexpectationsforstudents andproviders.
ConflictofInterest Noconflictofinterest.
10.1136/ejhpharm-2023-eahp.344
BackgroundandImportance TheimpactofCOVID-19andits influenceinthemanagementofhospitalisedpatientshasbeen indisputable.Manypublicationspresentcombinationsofdifferentantimicrobialstotreatthepatientsinfections,andtheliposomalamphotericinb(Amb-L)isanexampleofoneofthe mostprescribed.
AimandObjectives Tocomparetheprescriptionandindication ofAmB-Linatertiaryhospitalbeforeandduringthe COVID-19pandemic.
MaterialandMethods Observational,retrospective,descriptive studyofpatientsprescribedAmB-LfromMarch-2020to March-2021,andthecomparisiontotheyearbeforethe pandemic.
Results 58patientsanalysed:40(69%)men,medianage71 years(IQR54.5-75.2),and18(31%)women,medianage 63.5years(IQR49.5-71.25).Themonthsinwhichmore patientsreceivedAmB-Lwere:July2020(6/56),December 2020(7/56)andFebruary2021(12/56).
-39(69.6%)CRITICALpatients.Outofthese:22witha coviddiagnosis,14non-covidand3onco-haematological.26/ 39patientsreceivedAmB-LasatargetedtreatmentforCandidaGlabrataandAlbicans(16/26),AspergillusFumigatus(6/ 26)andMucor(4/26).Asaconcomitanttherapy,anidulafunginandisavuconazolewerethepreferentones.ThemostprescribeddoseofAmB-Lwas400mg(5mg/kg)withamedian of7daysoftreatment(IQR4-17.5).86.4%outofthetotal experienceddeath.
-17(30.4%)NON-CRITICALpatients:0covidpatients,6 (35.3%)non-covidand11(64.7%)onco-haematological patients.10(58.8%)patientsreceivedAmB-Lasempirical treatmentforfebrileneutropenia,withposaconazoleanditraconazoleasthemostcommonlyusedantifungals.Themost prescribeddosewas200mg(3.3mg/kg)foramedianof9 days(IQR6-16).
Inthepreviousyear(March2019toFebruary2020)we observed:17patientsreceivedtreatmentwithAmB-L,53% (9/17)onco-haematological,12menwithamedianof53years (IQR:38.2-59.1).Mostprescribeddose:180mg(3mg/kg).
ConclusionandRelevance Thedataobservedinthisperiod reflectshowtheprescriptionofAmB-Ltripledcomparedto thepreviousyear.Ittargetsacompletelydifferentprofile: unstablepatients,withinvasivelungdisease,riskfactorsin criticalcareunits,treatedwithhighdosesofAmB-L.Thefact ofbeinganantifungalwithahighcost/dayperpatient,the wayofmonitoringthesituationofthistypeofpatientisa crucialstrategytoguaranteeefficiencyandoptimisepharmaceuticalspending.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
6ER-021 RETROSPECTIVEOFDRUGINNOVATIONDURINGTHE SARS-COV2PANDEMIC:DEVELOPMENTOFAGAMEBASEDTRAINING
STollec*,VTeixeira,ALefrançois. OrléansRegionalHospital,Pharmacy,Orléans,France
10.1136/ejhpharm-2023-eahp.345
BackgroundandImportance Hospitalpharmacieshavecontributedtotheresearchanddevelopmentofremediesagainst coronavirusdisease2019(COVID-19),bymanagingmany drugs,off-label,inclinicaltrial,orinearlyaccessprogram. Withintheframeworkofcontinuingeducationofpharmacy technicians,aretrospectiveofthisdruginnovationprocess, withashortandplayfulformat,wasproposed.
AimandObjectives Todevelopandevaluateagame-based training,forthepharmacytechnicians,inordertounderstand thedruginnovationprocess,duringtheSARS-Cov2pandemic. MaterialandMethods Regardlessoftheirstatus,32medications,usedagainstCOVID-19,inourhospital,fromMarch 2020toMay2022,wereidentified.Foreachmedicine,a playingcardwascreatedwithonthefront:International Non-ProprietaryName(INN)andprinceps,andontheback: INN,princeps,drugstatus,pharmacologicalclassandfamily, dateoffirstdispensing.2teamsof3playerscompetedto aligntheplayingcardsinchronologicalorder,thenthetrainer debriefedthegame.Apresentationsupportofthetraining wasdone,detailingthepedagogicalobjectives,therulesofthe gameandthetheoreticalknowledge.Aself-assessmentanda feedbackformwerecreated.
Results 2one-hour(30minutesofplay,30minutesofdebriefing)sessionswereconducted.34healthcareprofessionals, from14hospitals,participatedintraining.94%ofparticipants completedquestionnaires.Attheendofthesession,100% improvedtheirknowledge,84%couldchronologicallylocate thedrugsusedagainstCOVID-19(against16%atthebeginningofthesession)and97%couldexplainthestagesofdrug innovationduringthepandemic(against3%atthebeginning ofthesession).Regardingthefeedbackform,100%appreciatedthecontentand97%therhythmofthegame.Theoverallsatisfactionratewas97%(goodorverygood).
ConclusionandRelevance Thisgamificationoftrainingwas verymuchappreciated.Theformatcombinesconvivialityand cooperation,whileprovidingseriouscontent.Theexperience couldbereplicated,duringcontinuingeducation,withother themes.
ConflictofInterest Noconflictofinterest.
6ER-022 CLINICALIMPACTOFTHEUSEOFGLUCOCORTICOIDS FORTHETREATMENTOFCOVID-19ININTERMEDIATE RESPIRATORYCAREUNITS
MAyllón*,VLCollada,EVillamañán,MEscario,LGarcía,AHerrero. HospitallaPaz, HospitalPharmacy,Madrid,Spain
10.1136/ejhpharm-2023-eahp.346
BackgroundandImportance Duringthepandemic,patients admittedtointermediaterespiratorycareunits(IRCU) receivednon-invasiverespiratorysupportandpharmacological treatment,mainlyglucocorticoids(GC).Dexamethasoneisthe onlyonethathasshownreducingmortality;however,there arenocomparativeefficacystudiesbetweenthedifferentGC.
PBlancoGarcia*,MAntonMartinez,AFijóPrieto,MTSánchezSánchez. HospitalClínico UniversitariodeValladolid,HospitalPharmacy,Valladolid,Spain REFERENCESAND/ORACKNOWLEDGEMENTSAimandObjectives
Todeterminethepossibleinfluenceofthe typeanddoseofGConthepatients’ evolutionwithSARSCoV-2pneumoniaadmittedtotheIRCUduringthefirstand secondwaveofthepandemic.
MaterialandMethods
Descriptive,observationalandretrospectivestudyofpatientswithSARS-CoV-2infectionadmittedto theIRCUinatertiarycarehospitalsinceMarchuntilDecember2020.Demographicvariables,comorbidities,GCtherapy receivedandfinalresolution(improvement,transfertoICU, ordeath)wereanalysed.Thedatawereobtainedfromthe clinicalhistoryandtheelectronicprescription.
Results 135patients(62.5%men)wereincludedwithamean ageof67.00(SD:13.16)years.69.31%ofthemhadoverweightand29.41%respiratorypathologies.
89.63%ofthepatientsadmittedtotheIRCUreceived treatmentwithGC,withinthem,89receivedtreatmentwith asingleGC,27receivedthecombinationoftwoandonly3 patientsreceivedthreeGC.64GC-treatedpatientsimproved, receivingameanprednisoneequivalentdoseof65.43 (SD:88.77)mgdailyforameanof13.40(SD:7.02)days.
The19patientstransferredtotheICUreceivedamean doseof89.18(SD:71.81)mgdailyfor6,00(SD:5.19)days. The38patientswhodiedinIRCUtreatedwithGCreceived ameandoseof114.18(SD:90.39)mgdailyforameanof 8.92(SD:6.17)days.
ThemostusedGCorcombinationswere:dexamethasone (76patients),dexamethasoneandprednisone(13patients), methylprednisolone(11patients),dexamethasoneandmethylprednisolone(8patients),andmethylprednisoloneandprednisone(5patients).100%ofpatientstreatedwith dexamethasoneandprednisoneimproved,followedbydexamethasoneandmethylprednisolone(62.5%)andmethylprednisoloneandprednisone(60%).27.27%ofthepatientstreated withmethylprednisolonealoneimproved,with63.64%dying.
ConclusionandRelevance Mostofthepatientsadmittedto theIRCUwithcoronavirusreceivedGCandtheresultssuggestsomeimprovementinthosewhoreceivedlowerdosesof GCforlongerperiods.
TheGCcombinationwasassociatedwithahigherrateof improvement,especiallywithdexamethasoneandprednisone. Treatmentwithmethylprednisolonealonehadthehighest deathrate.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
6ER-024 COMPARISONOFREDUCTIONSINMONTHLY MIGRAINEDAYSBETWEENNEWSMALLMOLECULE CGRPRECEPTORANTAGONISTS(GEPANTS)AND MONOCLONALANTIBODIESTARGETINGCGRP/CGRP RECEPTOR
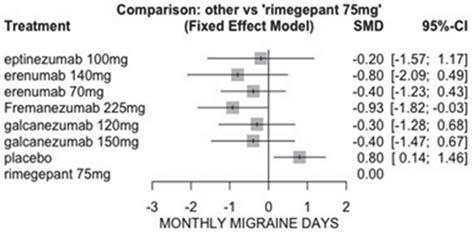
MRomero-González*,JCorderoRamos,MValeraRubio,VMerinoBohorquez,LMartin Casado. HospitalUniversitarioVirgenMacarena,HospitalPharmacy,Seville,Spain
10.1136/ejhpharm-2023-eahp.347
BackgroundandImportance Migraineischaracterisedby repeatedheadacheattackslastinghoursordaysandusually accompaniedbyotherassociatedsymptoms.Accordingtothe InternationalHeadacheSociety,itcanbeclassifiedinto
migrainewithaura,withoutauraandchronicmigraine.Atargetpathwaytotreatorpreventmigraineisthecalcitonin gene-relatedpeptide.Availabletreatmentsinourcountrythat actinterferingthatpathwayareerenumab,fremanezumab,galcanezumab,iptenezumabandrimegepant.
AimandObjectives Toanalysewhetherthedifferenttherapeuticoptionsareequivalentalternativesthroughanadjusted indirectcomparison.
MaterialandMethods Thetherapiesincludedwerefound afterasystematicsearchperformedinPubMed.Theanalysis includedrandomised,double-blind,phase2and3,controlled trials,prophylaxistherapiesandnumberofmigrainedays reducedmeasurementafter12weeksoftreatment.TheanalysiswasperformedusingtheR® softwaretoestimateBayesianstatistics,withrimegepanttakenasareferenceforthe comparison.Adeltavalueof1day,asprovidedbytheregulatoryagenciesFDAandEMA,wasusedtodeterminethe margin(maximumacceptabledifferenceasanon-inferiority criteria)andtheaveragenumberofmigrainedaysreduced. Toestablishthetherapeuticpositioning,theNationalEquivalentTherapeuticAlternativesPositioningGuidecriteriawere applied.
Results AsshowninFigure1,thedifferenceinthemean numberofmigrainedaysreducedpermonthversusplacebo wasfavourableinallcases.Eachtreatmentreduced migrainebybetweenonetotwodayspermonth,showing statisticallysignificantdifferences.Themostoutstanding beingfremanezumab(-1,73[-2.33;-1.12]).Basedonthe resultsobtained,asubsequentanalysiswascarriedoutcomparingfremanezumabwiththeotheralternatives.Inthis case,erenumab140mgshowedthemostsimilarefficacy result(0.13[-1.14;1.39]).Nevertheless,itdidnotshowa statisticallysignificantdifferenceagainstanytreatment, exclusivelyagainstplacebo.Nodifferenceswerefoundin termsofsafety.
Abstract6ER-024Figure1 Forest-plotofthedecreaseinaverage numberofmigrainedayspermonth.Comparator:rimegepant75mg/ 48h.SMD:standardmeandifference.95%CI:95%confidenceinterval
ConclusionandRelevance Nostatisticallysignificantdifferences werefoundbetweenrimegepantandmonoclonalantibodies againsttheCGRP/CGRPreceptorexceptforfremanezumab. Fremanezumabpresentedastatisticallysignificantmorepronouncedresponseinthedecreaseofmigrainedayspermonth at12weeksoftreatment.
ConflictofInterest Noconflictofinterest
6ER-025
10.1136/ejhpharm-2023-eahp.348
BackgroundandImportance Autologousserumeyedrops (SAED),apharmaceuticalformulationpreparedfrompatient’ s blood,areusedincornealsurfacepathologies.Sincealternativetherapiesarelimited,itsprescriptionhasincreasedin recentyears.
AimandObjectives AnalyseeffectivenessandsafetyofSAED inpatientsdiagnosedwithcornealsurfacepathologies.
MaterialandMethods Observational,retrospectivestudyina secondaryhospitalbetweenJanuary2019andMarch2022 includingpatientstreatedwith20%SAED.
Variables:demographicdata,diagnose,concomitantdiseases, durationoftreatment,ocularaffectation(lefteye(LE),right eye(RE),botheyes(BE)),subjectiveclinicalimprovement (SCI),adverseeffects(AE),concomitanttreatments,visual acuity(VA)atmonths0,3and6oftreatment.
EffectivenesswasevaluatedbySCIandVA,measuredona decimalscale,atthreeandsixmonthsoftreatment.Safety wasevaluatedbyAEdocumentedinmedicalrecords.
Results Thirty-fivepatients(77%women)wereincludedwith meanage61years(20-96).Principalsdiagnoseswere:dryeye syndrome(n=15),superficialpunctuatekeratitis(n=10)and Sjögren’ssyndrome(n=9).Forty-eightpercentofpatientspresentedconcomitantdiseases,highlightingfibromyalgiainsixof them.
Meantreatmentlengthwas500±348days.Tenpatients (28%)discontinuedtreatmentduringthestudy.Thereasons were:reactionto20%SAED(n=4),remission(n=4),death notassociatedwiththetreatment(n=1)andchangeofhospital(n=1).
Twenty-ninepatients(82%)hadaffectationinBE.SCIwas observedin82%ofpatientsatmonthsthreeandsix.PrincipalsAEwere:conjunctivalhyperaemia(n=4),blepharitis (n=2),stinging(n=1)andtearswithexcessmucus(n=1). Artificialtears(51%)andcorticosteroidseyedrops(11%) werethemainconcomitanttreatments.
VAdatawasavailablein14patients(40%).MeanVAin REwas0.80±0.29,0.80±0.31and0.82±0.25at months0,3and6respectively.MeanVAinLEwas0.85± 0.25,0.83±0.23and0.87±0.15respectively.
ConclusionandRelevance AccordingtoSCIandVA’ sprogressiveimprovementoverthemonthsandalowincidenceof AE,20%SAEDareaneffectiveandsafetreatmentforcornealsurfacepathologies.
ConflictofInterest Noconflictofinterest.
6ER-026 SPECTRUMOFHEARTFAILUREIN16SUB-SAHARAN AFRICANCOUNTRIES:TREATMENTSANDINHOSPITALOUTCOME
1PCavagna*, 2CKouamKouam, 3IBDiop, 4ELimbole, 5LAllawaye, 4JLTakombe, 6AKTraore, 7RN’Guetta, 8MSIkama, 9XJouven, 1MAntignac. 1PitieSalpetriereAphp UniversityHospital,Pharmacy,Paris,France; 2RegionalHospitalofBafoussam,Cardiology, Bafoussam,Cameroon; 3FannUniversitaryHospital,Cardiology,Dakar,Senegal; 4Ngaliema Hospital,Cardiology,Kinshasa,CongoRepublicOf; 5HopitalGeneraldelaReference Nationale,Cardiology,Ndjamena,Chad; 6HospitalofSikasso,Cardiology,Sikasso,Mali; 7AbidjanInstituteofCardiology,Cardiology,Abidjan,CoteD’ivoire; 8NationalUniversity HospitalofBrazzaville,NationalUniversityHospitalofBrazzaville,Brazzaville,Congo Brazzaville; 9EuropeanGeorgesPompidouHospital-Ap-HpCentre-UniversityofParis, Cardiology,Paris,France
10.1136/ejhpharm-2023-eahp.349
BackgroundandImportance Heartfailure(HF)isthemost commonprimarydiagnosisforpatientsadmittedtohospital withheartdiseaseinsub-SaharanAfrica(SSA).However,little isknownaboutthemanagementofHFinhospitalisationin SSA.
AimandObjectives Todescribeinhospitaldrugsstrategiesto manageHFin36cardiovascular(CV)departments.
MaterialandMethods WeconductedatransversalandlongitudinalstudyinCVdepartmentsof36hospital(publicandprivate)in16SSAcountries.TheFebruarystudyisanongoing observatoryincludedallinpatientsinFebruaryfromeachyear since2016.Dataincludingsocio-demographicandclinical characteristics,CVriskfactors,causesofadmission,medicationandlengthofstaywerecollectedduringhospitalisation byphysicians.Patientwealthindexwasassessedbyphysicians aslow,middleandhighaccordingtopatientcapacityto affordhospitalisation.Allanalyseswereperformedwithrandomeffectoncountriesandthroughscriptsdevelopedinthe Rsoftware4.0.3.
Results Overall,2084patientswereadmittedforHFinthe Februarystudy.HFrepresenting47.9%ofallpatients included.Themeanagewas57±17.4yearsand53.8% weremen.ProportionsofpatientsadmittedforHFvaried acrosscountriesfrom21.4%inBurundito66%inCongo (p<0.01).Averagelengthofstayinhospitalswas11daysand mortalityratewas13%.AmongHFpatients,74%ofpatients hadCVriskfactorsandhypertensionwasreportedin55.8% ofpatients.Duringhospitalisation,88.8%ofpatientswere treatedwithdiureticsfollowedbyangiotensin-converting enzymeinhibitors(ACEI)(61.8%),anticoagulant(47.8%)and betablockers(BB)(34.6%)(figure).Monotherapywereused in14%,combinationoftwodrugs,threedrugsandfour drugsstrategieswereusedin35%,33%12%respectively. Diureticsweremostlyprescribedinpatientwithlowwealth indexwhereasACEI,BBandanticoagulantinhighwealth index(p<0.05).
MCanoAlonso*,APlanas-Giner,NAlmendros-Abad,ASosa-Pons,LCardonaRoca, LCoronelCordero,MRoigSoronellas,NRudiSola. HospitalGeneraldeGranollers, PharmacyDepartment,Granollers,SpainAbstract6ER-026Figure1
ConclusionandRelevance HFtreatmentaccessvariedsignificantlyacrosscountriesandaccordingtopatientwealthindex.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
1JEClark*, 1GUlangkaya, 1MWatler, 2JValdesLedsma. 1UniversityofSouthFlorida,Taneja CollegeofPharmacy,Tampa,USA; 2UniversityofSouthFlorida,TanejaCollegeof Pharmacy,Tamp,USA
10.1136/ejhpharm-2023-eahp.350
BackgroundandImportance Studiesindicatethatgapsin knowledgeaboutunderservedpatientcareissuesmaybeassociatedwiththelevelofcomfortandattitudesofpharmacy studentscaringforunderservedpatients.
AimandObjectives Theobjectiveofthisstudywastoassess theattitudes,perceivedknowledge,andskillsofpharmacystudentstodelivercaretounderservedpopulations.
MaterialandMethods 385pharmacystudentswereeligibleto participateinthestudy.StudentscompletedamodifiedversionoftheHealthProfessionals’ AttitudesTowardthe HomelessInventoryadministeredbetweenDecember2020 andJanuary2021.Eachparticipantwasaskedtoratetheir levelofagreementwith8statementsconcerningattitudes towardtheunderservedand8statementsregardingperceived skilfulnessincaringfortheunderservedinprovidingmedicationreconciliationservicesandpatientcounsellingonascale from1to5.
Results Theresponseratewas22%(n=85).Moststudentsfelt comfortableprovidingmedicationtherapymanagement(78%), medicationreconciliation(79%),andpatientcounselling(78%) services.88%felttheyknewhowtocommunicatewith patientsfromdifferentculturalbackgrounds.Theaverageperceivedskilfulnessincompletingmedicationreconciliationactivitiesvariedlongitudinallyacrossclassyears(P1,3.4±1.08; P2,4.27±1.01;P3,4.8±1.01;P4,5.0±0.89).Theaverageperceivedskilfulnessinaddressingpatientsfromdifferent culturalbackgroundswashighestforstudentsintheP1years (4.09±1.12)andlowestforstudentsintheP2classyear (3.91±1.21).
ConclusionandRelevance Theattitudesandcomfortlevelsof studentstowardunderservedpopulationsdidnotdiffersignificantlybetweenclassyears.Theperceivedskilfulnessincreased longitudinallybetweenfirstandfourth-yearstudentsinthe areasofconductingmedicationreconciliationactivities,counsellingandassessingmedicationunderstandinginunderserved patients.StudentsintheP1classyearperceivedskilfulnessin caringfortheunderservedwashigherthanstudentsintheP4 classyear.
REFERENCESAND/ORACKNOWLEDGEMENTS
1.BuckDS,MonteiroFM,KneuperS,etal.DesignandvalidationoftheHealthProfessionals’ AttitudesTowardtheHomelessInventory(HPATHI). BMCMedEduc 2005;5(1):2.(2)LupuAM,ConnorSE,JonkmanL.Pharmacystudents’ actual andperceivedknowledgerelatedtounderservedpopulationsacrosstheprofessionalcurriculum. CurrentsinPharmacyTeachingandLearning 2013;5(6):526540.
ConflictofInterest Noconflictofinterest.
1MSerrano*, 2ELópez-Aspiroz, 1EGarcía-Martín, 1JPBarro-Ordovas, 1AMartínezHernández. 1HospitalUniversitarioInfantaSofía,Pharmacy,SanSebastiandeLosReyes, Spain; 2HospitalUniversitariolaPrincesa,Pharmacy,Madrid,Spain
10.1136/ejhpharm-2023-eahp.351
BackgroundandImportance Interferonalpha-2bisanoption oftreatmentinmalignantocularpathologiessuchasocular squamoussurfaceneoplasia(OSSN)butitsuseisless extendedtobenigndiseaseslikepterygium.Inourcountrywe hadavailableIntrona® tomadeeyedropsuntil2021June, butitsproductionwasdiscontinuedandanotherdrug(Bioferon®)withfewdataofsafetyinocularadministrationwas imported.
AimandObjectives Toevaluatetheefficacyandsafetyoftwo differentdrugs(Introna® andBioferon®)inthetreatmentof ophthalmicpathologies.
MaterialandMethods Allpatientswhounderwenttreatment witheyedropsofinterferonalpha-2binourhospitalfrom Aprilof2009untilAugustof2022wereselected.Weregisteredage,typeofpathology,timeoftreatment,adverse events,recurrencesandresponse(partial,completeorsurgery immediatelyafterorbeforetreatment).
Aliteraturesearchwasdonetomaketheeyedropsfrom Bioferon® andfinallyweusedwaterforinjectiontoreconstitutethevialandbalancedsalinesolutiontocomplete10mL ofvolume(concentrationof10mg/mL).
Results Thirty-sixpatientsreceived38treatments(twopatients hadbotheyesaffected).Bytypeofpathology,24werepterygium,8OSSN,2papilloma,1epidermoidcarcinomaand1 clearcellcarcinoma.Fromthetotalofpatientswithpterygium,54%receivedsurgery,21%hadpartialresponseand 25%hadnoresponse;fourpatientswithmalignantpathology (OSSNandcarcinomas)hadcompleteresponse,2hadpartial responsesand4wereoperated.Allpatientswithpapilloma underwentsurgery.
Ongroupsofmalignantpathologyandpapilloma1patient hadrecurrenceateachone.Evaluationofrecurrencesin pterygiumgroupwashardduetolackoffollowupafter
surgery,butwiththedataavailable70%ofthemhadno recurrences.
Onlyoneofallpatientshadanadverseevent(ocular irritation).
TwopatientsreceivedBioferon®,bothwithOSSN.Onehad completeresponseandtheotherpartialresponsewithno adverseeffects.
ConclusionandRelevance Wecanconcludethateyedropsof interferónalfa-2baresafeandeffectivetotreatmalignant pathologiesandtheformulationwiththenewdrugBioferon seemstomaintainsafetyandefficacy,butweneedmore patientstoconfirmit.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
6ER-031 KNOWLEDGEABOUTHUMANIMMUNODEFICIENCY
VIRUS(HIV)TRANSMISSIONINPEOPLELIVINGWITH HIVINANTIRETROVIRALTHERAPY
1ÁLópezGarcía*, 1LMartín-Zaragoza, 1LRubio-Ruiz, 2CMartínez-Nieto, 3MVelez-DiazPallares, 4PSanmartin-Fenollera, 5JAPeña-Pedrosa, 1AOnteniente-González, 6LMBedoyadel-Olmo, 6IIglesias-Peinado, 1JSánchez-Rubio-Ferrández. 1HospitalUniversitariode Getafe,PharmacyService,Getafe,Spain; 2HospitalUniversitariodelaPrincesa,Pharmacy Service,Madrid,Spain; 3HospitalUniversitarioRamonYCajal,PharmacyService,Madrid, Spain; 4HospitalUniversitarioFundaciónAlcorcón,PharmacyService,Alcorcón,Spain; 5HospitalClínicoSanCarlos,PharmacyService,Madrid,Spain; 6UniversidadComplutense deMadrid,PharmacyFaculty,Madrid,Spain
10.1136/ejhpharm-2023-eahp.352
BackgroundandImportance HumanImmunodeficiencyVirus (HIV)infectionisnowadayschronicduetoantiretroviraltherapy(ART).
KnowledgeaboutHIVtransmission(KHIVT)empowers peoplelivingwithHIV(PLWHIV)toengageinART.
AimandObjectives TodescribeKHIVTamongPLWHIVon ARTandtoidentifyfactorsassociatedwithloweraccessto thisinformation.
MaterialandMethods Multicentre(5centres),observational, prospectiveandcross-sectionalstudy.Weincludedadult PLWHIVonARTwith>3monthssincediagnosis.
KHIVTwasevaluatedusingan adhoc questionnaireof20 statements,tobereplied ‘true’ or ‘false’.Resultsarethepercentagesofcorrectanswers,consideringasoptimalknowledge results 80%.
Factorscollectedweresexualorientation,genderidentity, racialisation,religion,socialsupport,educationallevel,relationshipandeconomicstatus,socialvisibility,druguse,and involvementinsexwork.
Associationsbetweenquantitativeandqualitativevariables wereanalysedwithStudent’sTtestorMann-WhittneyUtest basedonnormalitytests.Spearmancorrelationcoefficient(r) wasusedbetweenquantitativevariables.
P-values<5%wereconsideredstatisticallysignificant.
Results Weenrolled169participants,aged20-81yearsold (x=46.6±12.2);147men,19women,and3non-binary people.
KHIVTobtainedanaverageresultof87.2±10.4%. 77.52%ofparticipantshadoptimalknowledge.
Threeofthefourstatementswiththeworstresultswere thatrelatedtoHIVuntransmissibilityinPLHIVwithundetectableviralload(U=U).
Womenachievedworseresultsthanmen(Dx=8.16| CI95%:3.3-13.0|p=0.001).
Heterosexualmenachievedworseresultsthanhomosexual men(Dx=6.1|CI95%:2.7-9.5|p=0.001).Therewerenosignificantdifferencesbetweenbisexualmenandothermen.
PLWHIVwithno/onlyprimaryeducationobtainedworse results(Dx=7.5|CI95%:3.2-11.8|p=0.000).
PLWHIVwithanincome<1,000C ¼ /month(gross)obtained worseresults(Dx=3.7|CI95%:0.5-6.8|p=0.015).
AgewasinverselycorrelatedwithKHIVT(r=-0.367| p=0.000).
ConclusionandRelevance AboutaquarterofPLHIVhavesuboptimalKHIVT.Furthermore,thepremiseU=Uisnotyet sufficientlywidespread.
Women,heterosexualmen,olderpeople,peoplewithlow educationlevelandthosewithalimitedeconomicalincome havegreaterdifficultyaccessingthisinformation.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest
6ER-032 HOWCANDUTCHUNIVERSITYHOSPITALS CONTRIBUTETOAFFORDABLEMEDICINESANDCOST CONTAINMENTOFTOTALHOSPITALDRUG EXPENDITURE:ADELPHISTUDY
1ADane*, 2ARamlal, 3MOvergaag-vanHemert, 1PRoos, 4CPost, 5CUyl-deGroot, 1HVan DerKuy. 1ErasmusMc,HospitalPharmacy,Rotterdam,TheNetherlands; 2NoAffiliation,N/ A,DenHaag,TheNetherlands; 3NZA,N/A,Utrecht,TheNetherlands; 4AmsterdamUmcLocatieUniversityofAmsterdam,Oncology,Amsterdam,TheNetherlands; 5Erasmus UniversityRotterdam,ErasmusSchoolofHealthPolicyandManagement,Rotterdam,The Netherlands
10.1136/ejhpharm-2023-eahp.353
BackgroundandImportance IncreasingexpenditureonpharmaceuticalsisofgrowingconcerntotheaffordabilityofhealthcaresystemsacrossEurope.AsstatedintheEuropean Commission(EC)’sPharmaceuticalStrategyforEuropeall stakeholdersshouldbeinvolvedintacklingthisproblem.The EChasindicatedthatsolutionsalongtheentiredruglifecycle (DLC)shouldbeconsideredasitoffersamorecomprehensive andintegratedviewtoaddressthistopic.Beingastakeholder, universityhospitals(UH)areengagedinmultiplephasesof theDLC,consistingof(pre-)clinicalresearch,marketauthorisation,pricingandreimbursement,manufacturing,procurement,prescribing,dispensingandmonitoringreal-world effectiveness.
AimandObjectives
WeaimedtoexplorewhichactivitiesUHs performorshouldperformtocontributetocostcontainment ofmedicines.
MaterialandMethods WeusedaDelphitechniqueand assembledanexpertpanelof31pharmaceuticalexpertsof DutchUHs(i.e.,doctors,researchers,hospitalpharmacists, directors),healthinsurersandgovernmentalauthorities.Inthe firstround,weexploredactivitiesUHscurrentlyperformor shouldperformthroughouttheDLCandwhatbarriersor dilemmastheyencounter.Inthesecondround,weaskedour panelona5-pointLikertpointscaleto(dis)agreewithall mentionedactivitiesandbarriers.Thethirdroundwasused toreachconsensusonactivitiesandbarrierswhichwere(dis) agreeduponlessthan50%.
Results Thepanelagreedthat,considering(pre-)clinical research,UHsshouldincreaseinvolvementindrugrepurposingandmonitoringofreal-worldeffectivenessofmedicines. Furthermore,whileprescribingmedicinesisreservedfor medicalspecialistsUHsshouldraisemoreawarenessoncosteffectiveprescribingbydoctorsviamoreactiveinvolvement ofhospitalpharmacists,adjustmentofnationalprescribing guidelinesandextendingpharmacotherapyeducation.Finally, costcontainmentcouldbeenhancedbyreducingspillage,e. g.,efficientdosing.Controversyamongthepanelremained onthenotionofUHsbuildingmoreknowledgeonregulatoryaffairsformarketingauthorisationandincreasingtheir effortonself-manufacturingofmedicines.AgreeduponbarriersrestrictingUHstoexpand theiractivitieswereinsufficientfinancialresourcesan dlegalandentrepreneurial expertise.
ConclusionandRelevance UHsshouldincreasetheireffortsto reducecostsofmedicinesthroughoutthewholeDLC,but especiallyonactivitiesregardingdrugrepurposing,avoidance ofspillageandcost-effectiveprescribing.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
6ER-033 PEMBROLIZUMABANDATEZOLIZUMABASPOSSIBLE EQUIVALENTFIRST-LINETHERAPEUTICALTERNATIVES INPD-L1-EXPRESSINGTRIPLE-NEGATIVEBREAST CANCER
MDRivasRodríguez*,ÁGilGarcía,ARojasAlbarrán,MGrageraGomez,HVázquez Velázquez. BadajozUniversityHospital,Pharmacy,Badajoz,Spain
10.1136/ejhpharm-2023-eahp.354
BackgroundandImportance Recentstudieshaveestablished theinfluenceoftheimmunesystemondiseaseprogressionin triplenegativebreastcancer(TNBC)patients.
AimandObjectives Todetermineifpembrolizumabandatezolizumabcanbeconsideredequivalentfirst-linetherapeutic alternatives(ATE)byusingacommoncomparator,for patientswithlocallyrecurrentunresectableormetastaticunresectableTNBCinadultswhosetumoursexpressPD-L1and whohavenotreceivedpriorchemotherapy.
MaterialandMethods Abibliographicsearchwasconductedto selectphaseIIIrandomisedclinicaltrialsoffirst-linetreatmentsforTNBC.Theindirectcomparisonwasperformed withtheBuchermethod.Thevariableselectedtodetermine clinicalequivalencewasprogression-freesurvival(PFS),dueto thelackofmaturitywithrespecttotheoverallsurvivalvariable.Themaximumacceptabledifferenceasaclinicalnon-
inferioritystandardDelta(D),anditsinverseweresetat0.65 and1.54,respectively.TheywereestablishedbyESMO-MagnitudeofClinicalBenefitScale.
Toestablishthepositioning,weappliedthecriteriaofthe guideontherapeuticalternatives.
Results Accordingtotheclinicalstudiesreviewed,apotentialtherapeuticequivalenttopembrolizumab,atezolizumab combinedwithnab-paclitaxel(IMp assion130)wasidentified forthetreatmentofTNBCwhosetumoursoverexpressPDL1 1%andwhohavenotreceivedp riorchemotherapyfor theirmetastaticdisease.Althoughinourcase(KEYNOTE355),thePD-L1 10subgroupwasconsideredthereference subgroupforthestudy,wehavedatafromthePDL1 1 subgroupinpatientstreatedwithpembrolizumabincombinationwithchemotherapythatallowustomakethe comparison.
AfterapplyingtheBuchermethod,aHR=0.85(95%CI 0.63to1.16)wasobtainedforpembrolizumab+chemoterapyversusatezolizumab+nab-paclitaxel.Consideringthe standarddeltaestablished,thisisaprobableclinicalequivalence.WehavetoresortinthiscasetoShakespeare’scalculatorwhichstatesthatthereisa4.25%probabilitythatthe valueisbelow0.65.Sincethisisaprobabilityoflessthan 17%,wecanconcludethattheseareequivalenttherapeutic alternatives.
ConclusionandRelevance Pembrolizumabandatezolizumab couldbeconsideredATE,however,recentstudiessuchasthe Impassion131bringagreatdealofuncertaintytothis determination.
REFERENCESAND/ORACKNOWLEDGEMENTS
ConflictofInterest Noconflictofinterest.
6ER-034 USEOFCLOSEDSYSTEMTRANSFERDEVICESWITH INVESTIGATIONALDRUGPRODUCTS
1LValdeolmillos, 2CGarciaPastor*, 2MSerranoAlonso, 2CLacasaArregui, 2EMolíns Castiella. 1ClinicaUniversidaddeNavarra,Pharmacy,Pamplona,Spain; 2ClinicaUniversidad deNavarra,PharmacyDepartment,Pamplona,Spain
10.1136/ejhpharm-2023-eahp.355
BackgroundandImportance Investigationaldrugproducts (IDP)shouldbetreatedashazardousdrugs(HD)asitisnot frequenttohavehazardstudiesavailableortheinformation aboutsafetyisusuallyinsufficient.Thisisahandicapfor pharmacists,whomustguaranteethesafetyofprofessionals duringthehandling,preparationandadministrationofIDPas wellasdrugquality.
RecommendationsbyNIOSHandUSPincludetheuseof closedsystemtransferdevices(CSTDs)inthehealthcaresettingtoreduceoccupationalexposuretoHD.
FrequentlythereisalackofinformationaboutthepotentialimpactofusingCSTDsonproductquality.Thismaybea challenge,especiallywhentheyareusedwithIDP,monoclonal antibodies(mAb)anddrug-conjugatedmAb.
AimandObjectives Toreviewthescientificevidencerelatedto theuseofCSTDswhencompoundingandadministering IDPs,inordertodeterminethemainchallengesrelatedtoits useandtoestablishtheusecriteriaindailypractice.
MaterialandMethods AcomprehensivesearchinPubMed databasewasperformed.Thesearchstrategywasbasedona combinationofthefollowingterms:closedsystemtransfer devices,drugdevelopmentandbiologicproducts(meSH
term).WeincludedstudiesevaluatingCSTD,safehandling anddrugquality.
Results Weincluded7articles(onesystematicreview,four reviewsandtwoprospectivestudies)thatshowedthefollowingcriticalissues:
. ThereisawidevarietyofcomponentsinCSTDsthatcan potentiallycauseincompatibilityissues,physicalandchemical instabilitiesaswellasdruglossandpoorqualityproductdue toadsorptionontoCSTDmaterials.
. CSTDsareassociatedwithhigherincidenceofinsolublefine particlesrelatedtosiliconeoildroplets.MAbareknownto formaggregateswhenCSTDsareusedthatcouldbe potentiallydetrimentaltopatientsafety.
. CSTDsholdupvolumerangefrom0,04to1mLwhichhas animpactondeliverabledrugdosewhichisespecially worryinginlowvolume-doseIDP.
ConclusionandRelevance Frequently,thereisinsufficientinformationtoexcludesafetyconcernsforIDPleadingtobroad useofCSTDsaccordingtoguidelines.
Thereisanurgentneedtoincreaseknowledgeaboutthe hazardofnewtherapiesandtoassessCSTDsimpactonproductquality,clinicaltrialoutcomeandpatientsafety.
REFERENCESAND/ORACKNOWLEDGEMENTS ConflictofInterest Noconflictofinterest.
Thenumbernexttotheauthorindicatesthepagenumber,nottheabstractnumber.
AbeleM,A115
AbotalebA,A17
AcramelA,A18
AdeM,A90
AdrianMO,A119
Aelbrecht-MeurisseC,A115
AgiusP,A21
AgraBlancoI,A30,A77,A126
AguadoBarrosoP,A27,A53
AguilarSalmeronR,A41,A82
AguirrezabalaArredondoAV,A124
AitElCadiM,A6,A69,A159
AkgölK,A14
AlañonPardoMDM,A148
AlamiM,A97
Alarcón-PayerC,A40
Alarcon-PayerC,A152
AlbanellM,A20,A24,A28,A29,A49
AlbertH,A94
AlcántaraPradoA,A75
AlcaceraLopezMA,A83
AlcalaSotoA,A60,A62,A129
AlcobiaA,A149,A155
Aldea-PeronaA,A54
AlegreDel-ReyEJ,A162
Alegre-DelReyEJ,A8
AlfonsínLaraM,A30,A77,A126
AliS,A94
AlkanjA,A45
AllawayeL,A167
AllemandJ,A48
AllendeBandresMA,A83
AlmeidaGonzálezCV,A137
AlmeidaP,A155
Almendros-AbadN,A167
AlmiñanaM,A49
AlonsoMorenoM,A154
Alonso-GarcíaA,A17
AlvarezDianAM,A163
AlvarezDiazAM,A53,A78,A89,A141,A154
AlvarezManceñídoFJ,A59
AlvarezYusteA,A132
ÁlvarezDíazAM,A101
ÁlvarezFernándezI,A86
ÁlvarezGrandeB,A125
ÁlvarezM,A64
ÁlvarezYusteA,A126
Alvarez-YusteA,A77
Álvarez-DíazAM,A56
Álvarez-ManceñidoFJ,A96
AmmorW,A54,A68
AmorGarcíaMA,A52
AmorGarciaMA,A20
AmorMÁ,A48
Amor-GarcíaMÁ,A92,A134
AnaFP,A9
AnaJ,A124
AnaSoaresArmandoAlcobia,A3
AndújarMateosA,A158
AndrésNavarroN,A143
AndrescianiE,A95,A152
AndreuÀ,A153
AnguianoBaqueroMP,A129
AnguitaDomingoD,A105
AntónX,A62
AntignacM,A167
AntoineAL,A112
Antolín-AmérigoD,A141
AntonM,A49
AntonMartinezM,A165
AntonMendezX,A142
AntoniouS,A88,A94
AparicioC,A39
AparicioPeñacobaR,A106
ApezteguiaFernandezC,A20
ApezteguiaFernandezCA,A92
Apezteguia-FernándezCA,A48,A134
AraújoR,A19
AraqueArroyoP,A148
ArchillaAmatMI,A9,A11,A28,A161
ArchillaMI,A164
ArenasJimenezM,A11
ArenereMendozaM,A83
ArgandoñaMJ,A15
AriasA,A59,A133
AriasMartinezA,A67
Arias-MartínezA,A96
Arias-SantiagoS,A96
ArnoldussenD,A36
ArranzPascualN,A45
ArranzPasqualN,A28
ArtimeRodríguez-HermidaF,A136
AsenjoSegoviaS,A107
AsensiDiezR,A12
AuriaultC,A68
AusinP,A43
AyllónM,A165
AzzopardiLM,A76
BabinM,A73
BácskayI,A109
BadiaSantolariaC,A15
BadiaTahullMB,A40
BaekS,A32
BáezN,A13
BagagioloS,A95,A152
BalcellsE,A43
BalcellsJ,A103
BaldominosUtrillaG,A56
BaliTibor,A2
BallardiniG,A72
BaltazarS,A30
BarataM,A22
BarbaraC,A76
BarberoHernándezMJ,A79,A100
BarberoHernandezMJ,A66
BarcaDiezC,A161
BarcelóJ,A38
Barceló-VidalJ,A54
BardollCucalaM,A58,A91
BarredaHernándezD,A66,A70
BarredaHernandezD,A150
BarreiroFernandezEM,A64,A65
Barreiro-FernandezEM,A85
BarrerasN,A160
Barro-OrdovasJP,A168
BarrosoCastroJ,A142
BarrosoJ,A62
Barroso-TorrejónV,A102
BartolucciI,A95,A152
BasCastilloA,A68
BastidaC,A28,A29
BattistiniG,A27
BautistaSanzMP,A20,A52,A92
Bautista-SanzMP,A134
Bautista-SanzP,A48
BaveuxR,A110
BayoCaleroJ,A120
BazsóT,A109
BécaresJ,A160
BeckM,A45
BeckerG,A17
Bedoya-del-OlmoLM,A169
BekováL,A139
BelioAgueraB,A124
BelleroM,A71
BenChaabaneK,A22
BenHajmessaoudN,A88
BenJdidiaI,A22
Benítez-CanoA,A34
Benítez-HidalgoO,A47
BenardL,A11
BenedíGonzálezJ,A31
BenjaddiA,A5
Benkő R,A109
BereciartuaE,A62
BeristainAramendiI,A86
Bernal-HertfelderE,A134
Bernardez-FerranB,A101
BernikierE,A21
BerruecoR,A139
Bertault-PeresP,A81,A95
BertrandelisBartolomeB,A31
BertscheT,A1
BesnierP,A110
BetoretVilarMDM,A68
BettioM,A98
BiancoA,A71
BiancoMT,A73,A94
BitllochM,A72
BlázquezRomeroC,A27
BlancoCastañoM,A38,A82
BlancoCastañoMA,A64,A65
BlancoCastanoMA,A93
BlancoCrespoM,A51,A78
BlancoEspesoT,A145
BlancoGarciaP,A165
Blanco-CastañoMA,A33,A35,A85,A163
BlascoSeguraP,A68
BlazquezRomeroC,A53
BlondelleS,A128
BlouzaLimayemI,A22
BocosBaeloA,A133
Bocos-BaeloA,A64,A98,A153
BodenD,A16,A88
BöhlkeC,A17
BoisgontierM,A73
BolotC,A83
BonagaB,A93
BonettiD,A117
BooRodríguezJ,A86
BordetF,A21
BorensO,A4
Borrero-RubioJM,A85
BosóRibellesV,A32
BoschC,A34
BoschPeligeroM,A67,A145,A147
BossacomaF,A99
BottoC,A44,A46,A119,A127
BotzLajos,A2
BouatiaM,A90
BouetJ,A90
BoukhlifiY,A97
BouslimanY,A97
BoventerJ,A19
BrandinT,A48
BreantV,A83
BresciaA,A134
BretonesPedrinaciI,A74
BretonesPedrinaciJI,A121
BriceñoCasadoMDP,A33,A118,A163
Briceño-CasadoMDP,A35
BroncardC,A112
BrugeraTeixidorM,A69
BrugueraTeixidorM,A41,A79,A82,A95
BrunM,A18
Buendia-BravoS,A57,A59,A127
BusquetsCasalD,A69
Busto-IglesiasM,A101
CaballeroMolinaMT,A106
CaballeroRequejoC,A102
CabañasGimenoCC,A13
Cabañas-PoyMJ,A103
CabezaJ,A17,A18,A135
Cabeza-BarreraJ,A30,A40
CaboniP,A23
Cáceres-VelascoC,A92
CachoCalvoJB,A80
Caiero-MartínezL,A84
CallejaBuenoA,A142
CalvoA,A59,A145,A147
CalvoArbeloaM,A129
CalvoGarcíaA,A31
CalvoM,A23
CamachoParreñoS,A150
CampanyHerreroD,A105
CampdelacreuFumadoJ,A154
CampeloSánchezE,A77
CampinsL,A72
CamposM,A156
CanárioC,A149
CanadellVilarrasaL,A15,A87
CanalesD,A147
CanalesSigueroMD,A75
CanalesUgarteS,A66
CancelaDíezB,A36,A49
CancellieriG,A44,A46,A119,A127
CandeasAgustiR,A85
CaniegoRodrigoD,A150
CanoAlonsoM,A167
CanoDominguezS,A79
CanoMarrónSM,A85
CanoMartinezG,A38,A82
Cano-CanoJM,A96
CantudoCuencaMR,A11,A28,A36
CanutA,A156
CaoViñaV,A5
CapelleH,A48,A81
Capilla-MontesC,A57,A59,A127
CaponeC,A95
CaréW,A112
Carabias-AnéL,A98
CaraffaAlessandro,A2
CarballoN,A54
CarceleroSanMartínE,A28
CardonaG,A98,A153
CardonaRocaL,A167
CardosoAS,A50
CarlierP,A92
CarmonaJuárezL,A75
CarnotA,A115
CaroJM,A147
CaroTellerJM,A75
CarpioC,A26
CarreresPrietoM,A136
CarrilloLópezV,A6
CarrondoAP,A50
CarrucciuGA,A23
CarvajalSánchezMÁ,A43,A55
CarvajalSánhezMA,A37
Carvajal-SanchezMA,A41
CasásMartínezA,A52
CasaldáligaA,A139
CasaldàligaA,A99,A104
CasanovasMoreno-TorresML,A131
CasarrubiosGI,A104,A122
CasarrubiosLázaroGI,A51,A78
CasasFernándezX,A86
CasasJ,A62
CasasX,A121
CasciariMilena,A2
CasiniG,A27
CasoliFrancesco,A2
Castaño-AmoresC,A30,A40
CastellóNòriaÀ,A69,A79,A95,A136
CastillejoR,A89
CastilloE,A160
CastroA,A147
CastroFrontiñánA,A75
CastroNúñezI,A126
CastroP,A29
CastroS,A22
CastroSalinasP,A138
CastroVidaMA,A74,A121
Castro-BaladoA,A101
CasuscelliD,A134
CatalaL,A112
CausT,A142
CavadaCarranzaI,A140
CavagnaP,A167
CazorlaPoderosoL,A83
CelikkayalarE,A1
ChaïbS,A83
ChabouniS,A10
ChachulskiA,A72
ChaibiA,A47,A50,A69
ChamorrodeVegaE,A151
ChampmartinL,A21
CharaVelardeLE,A51
CharquesV,A138
ChauhanD,A130
ChauvelO,A10
ChedlyM,A22
ChenE,A112
ChevaletV,A16,A88
ChinchillaAlarcónT,A36
ChinchillaAlarconT,A71
Chorro-MariV,A130
CidonchaMuñozI,A136
CiezarRodriguezJC,A151
CillisM,A90
CioffiV,A27
CiudadGutiérrezP,A143
ClériguesMontserrat,A3
ClarkJE,A168
ClavéP,A61
Clemente-BaustistaS,A103
CleriesRoviraP,A40,A154
Climent-GranaE,A107
ClouetJ,A54
CocciE,A152
CodinaJiménezC,A64,A67
Codina–JiménezC,A133
Codina-JiménezC,A61,A98,A145,A147,A153
CodonalA,A104,A122
CodonalDemetrioA,A51,A78,A156
ColladaV,A26
ColladaVL,A165
ColletCE,A110
ColliceC,A27
ComasSugranesM,A154
CondeGarcíaMC,A81
CondeGinerS,A15
CondeGonzálezI,A106
ContrerasMacíasE,A120,A143,A162
CorazonVillanuevaJ,A137
CorderoRamosJ,A108,A166
CórdobaR,A160
CoronelCorderoL,A167
CorpaB,A49
CorralesPazM,A111
CorralesS,A122
CorreaPérezA,A42
CorredorI,A122
CortésSánchezCJ,A15,A87
CortellC,A49
CorzaniA,A73,A94
CostaChingF,A22
CostaG,A149
CouñagoFernándezM,A30,A77,A126
CoultonS,A109
CrespoDizC,A161
CrobedduM,A23
CrosC,A18
CrouA,A16,A88
CrulM,A14
Cruz-CruzT,A57,A59,A127
CsernátonyZ,A109
CuadrosMartínezCM,A60,A62,A129
Cuadros-MartinezCM,A33,A118,A163
CustodioA,A96
CuyBuenoM,A91
CzakóE,A99
D’ArpinoAlessandro,A2
DaSilvaRaposoJ,A4
DalMasL,A45
DaltonK,A67
DaneA,A169
DaoudiH,A6,A69,A159
DauriatG,A88
deAndrésS,A26
deBasagoitiA,A62
deCáceresVelascoC,A52
deCáceres-VelascoC,A48
deCaceresVelascoC,A20
deFrancescoA,A134
deJuán-GarcíaTorresP,A51
delaCalle-RiaguasB,A118
delaFuenteVillaverdeI,A57,A116
deLucaE,A44,A119,A127
deSalinasMuñozT,A148
deSalinasMuñozTE,A81
deSchutterH,A70
deWitJ,A113
de-Andrés-MartínA,A141
De-AntonioM,A34
de-Cáceres-VelascoC,A134
DeanBarahonaC,A78,A156
DeanC,A104,A122
DebanneC,A73
DebattistaJL,A76
DelCampoAlbendeaL,A101
DelRíoValenciaJC,A6,A25,A100
DelRioValenciaJC,A97
DelVecchioM,A132
DelforgeM,A128
DelgadoA,A104
DelgadoLopezGM,A151
DelgadoO,A6
Delgado-SilveiraE,A53,A89,A154
DesautezG,A16
DesbuquoisAC,A73
DesmarisR,A18
DeusCasasAM,A161
DiMaioM,A71
DiVicoVS,A94
DíazGagoÁ,A163
DíazPeralesR,A42,A133
Díaz-PellicerP,A54
DiekerhofC,A5
DiezM,A96
DíezVallejoC,A69
DijkmansA,A113
DiopIB,A167
DivouxE,A90
DolL,A110
DomínguezBerraqueroG,A66
DomínguezM,A43
DomínguezSenínL,A66,A120,A150
DomingoBuzonA,A151
DominguezBarahonaA,A53
DominguezCanteroM,A93
DominguezRivasY,A133
DominguezSantanaCM,A64,A65,A82
Dominguez-CanteroM,A82
Dominguez-SantanaCM,A38,A85
DominiciS,A155
DonyA,A90
DordàBenitoA,A79,A91,A95,A136
DuarteH,A149
DuarteSilvaC,A50
DufayE,A90
DufosséM,A11
DumazerC,A48
DuqueRodriguezM,A142
DuqueTebarP,A132
Duque-TebarP,A77
DuranC,A39
EceizaDíezA,A86
EcheverríaD,A38,A43
EgüésLugeaA,A146
EgüesLugeaA,A129
EiroaOsoroM,A57,A116
ElMarnissiS,A6,A159
ElQabissiO,A6,A69,A159
ElkaraF,A22
ElviraGómezJC,A132
EngiZ,A109
EpsteinD,A8
EscalupL,A18
EscarioM,A26,A165
EscobarBolañosC,A41
EscobarOblitasAD,A106
EscobedoRomeroR,A146
EscolàA,A20,A24,A29
EscolàRodríguezA,A28
Escribano-ValencianoI,A57,A59,A127
EscuderoSánchezG,A132
EscuderoSanchezG,A126
Escudero-SanchezG,A77
EspinMartíG,A69
EspinosaBoschM,A157
EspositoS,A134
EstaireGutiérrezJ,A120,A143
EstebanFernandezFJ,A80
Esteban-CartelleB,A78
EstesoHontoriaO,A15
EstradaL,A64,A98,A133,A153
Everaars-KlokM,A158
FabrizziB,A95
FadhelI,A63
FalcónCubilloM,A143
FalhofJ,A46
FalipCentellasM,A40
FarinaMassimo,A2
FarréR,A99,A104,A139
FaureC,A130
FazzinaGF,A71
Feal-CortizasB,A84
Feijoo-VilanovaP,A84
FeldmanD,A54
FenixCaballeroS,A162
Fenix-CaballeroS,A8
Fénix-CaballeroS,A35
FerchichiE,A63
FernándezFradejasJ,A89,A154
FernándezLagunaCL,A57,A116
FernándezLastrasS,A57,A116
FernándezN,A20
FernándezPérezA,A7
Fernández-BravoRodrigoJ,A66
Fernández-LagunaCL,A96
Fernández-PoloA,A103
Fernández-SalaX,A54
FernandezA,A39
FernandezCuervaC,A12,A71
FernandezGinesFD,A44,A118
FernandezPeñaA,A142
FernandezRuiz-MoronA,A137
Fernandez-VillacañasFernandezP,A102,A108, A153
FernandinoZubietaB,A146
FerrándezO,A54
FerrandezD,A138
FerrandoPiqueresR,A32
FerrariC,A110
FerrariJM,A147
FerrariPiqueroJM,A31,A75
FerreiraC,A19
FerrereGonzalezF,A80
FerrisVillanuevaM,A151
FerroS,A139
FésüsÁM,A109
FésüsA,A109
FeutryF,A115
FhadilS,A88,A94
FiguerasA,A138
FijóPrietoA,A125,A165
FlausA,A83
FloresLe-RouxJ,A54
FloridoFranciscoM,A145
FodimbiJ,A110
FontA,A99,A104,A139
FooCheungL,A48
ForneasA,A39
FortunaM,A123
FouilletJ,A130
FoxC,A109
FragaBuenoE,A52
FrancavillaA,A98
FresanD,A23,A34
FresquetMolinaR,A83
FresquetR,A87,A93
FronteauC,A54
FructuosoGonzálezL,A55
FructuosoGonzalezL,A37
Fructuoso-GonzálezL,A41
Fructuoso-GonzálezL,A43
FrutosA,A87
FrutosPerez-SurioA,A83
FuertesS,A39
FuscoA,A117
FusterTalensR,A68
GaboriauL,A115
GainzarainJC,A156
GalarragaIñarraB,A146
GallegoFernándezC,A42
Gallego-ArandaT,A77
GálovaC,A149
GamarraCalvoS,A154
GambinoS,A46
GamezTorresD,A34,A44,A51,A84,A118,A131
GámezTorresD,A84
GándaraA,A39
GándaraLadróndeGuevaraMJ,A157
GanforninaAndradesA,A37,A120,A143,A144, A162
GarauV,A23
GarcésM,A87
GarcíaAgudoS,A36
GarcíaB,A15
GarcíaBasasL,A136
GarcíaBelosoN,A30
GarcíaCasanuevaJC,A106
GarcíaDíazB,A56,A113,A114
GarcíadeVicuñaR,A31
GarcíaEcheverríaD,A86
GarcíaGiménezI,A8,A141
GarcíaHervalejoM,A106
GarcíaJiménezV,A116
GarcíaJJ,A156
GarcíaL,A165
GarcíaLópezE,A66
GarcíaLloretP,A137,A162
GarcíaMartínezS,A114
GarcíaMuñozS,A32
GarcíaPeraloA,A132
GarcíaSánchezS,A42
García-CastiñeiraC,A64,A98,A133,A153
García-GarcíaS,A103
García-GiménezI,A30
García-GonzálezX,A48,A140
García-MartínE,A168
García-MuñozC,A147
García-QuintanillaL,A101
García-VidalC,A24
GarciaA,A49
GarciaC,A23
GarciaCoronelM,A102,A108
GarciadeAndoinBarandiaranMJ,A86
GarciaGomezN,A5,A7,A66,A79,A100
GarciaLópezV,A10
GarciaLirolaMA,A117,A125
GarciaLopezE,A150
GarciaMolinaA,A15,A87
GarciaOrtegaP,A136
GarciaOsunaMDLR,A83
GarciaParajeM,A151
GarciaPastorC,A170
GarciaPeraloA,A126
GarciaRodicioS,A91
Garcia-PeraloA,A77
GargouriM,A146
GarridoPeñoN,A80
GarzoneAMF,A95,A152
GascónVillacampaAM,A63
GascoA,A71
GaspériE,A21
Gastalver-MartínC,A57,A127
Gastalver-MartinC,A57,A59
GayosoRodríguezMA,A7,A9
GazquezPerezR,A34,A51,A84,A131
GemaIsabelCL,A156
GemenoLopezE,A101,A163
Gemeno-LópezE,A56,A154
GhandourA,A95
GhazouaniA,A16
GiammonaR,A119,A127
GilCandelM,A102
GilGómezMI,A68
GilGarcíaÁ,A170
GilGarciaA,A115
GilGonzález-CarrascosaG,A36
Gil-SierraMD,A33,A35,A38,A118,A163
GilabertSotocaM,A58,A91
Giménez-ArufeV,A84
Giménez-JuárezJC,A145,A147
GimenezGinerS,A136
GinardD,A6
GinovartGalianaG,A67
GirónMorenoRM,A126
GispertAmetllerMA,A144
GittlerG,A160
GiulianiAngela,A2
GleditschE,A18
GodetJ,A45
GogolovaV,A149
GoikoetxeaJ,A62
GomezB,A49
GomezdeRuedaF,A49
GomezEchevarriaN,A124
GomezFernandezE,A53
GomezP,A87,A93
GomezRivasP,A83
GomezSánchezMT,A34
GomezSanchezMT,A44,A118,A131
GomezSayagoL,A127
GómezA,A6
GómezBermejoM,A75,A151
GómezdeTravecedoYCalvoMT,A62
GómezE,A156
GómezFernándezE,A27
GómezIbáñezI,A79,A95
GómezIbañezI,A82
GómezLluchMT,A137
GómezSánchezMT,A84
Gomez-BayonaE,A78
Gómez-CostasD,A140
Gómez-ZorrillaS,A43
GonzálezA,A147
GonzálezBoronatM,A137
GonzálezFernándezA,A76
GonzálezGómezÁ,A75
GonzálezGarcíaR,A45
GonzálezI,A137,A138
GonzálezMorenoP,A105
GonzálezSevillaM,A31
GonzálezTriguerosC,A56
González-BurgosE,A89
González-de-OlanoD,A141
González-PiñeiroS,A47
GonzalezCaballeroÍ,A40
GonzalezEscribanoMDC,A135,A148
GonzalezFreireL,A161
GonzalezFuentesA,A80
GonzalezL,A117,A125
GonzalezMingotC,A85
GonzalezRodriguezCG,A151
GonzalezTorralbaA,A80
GonzaloSaladoMH,A42
GorgasTornerMQ,A105,A136
GourieuxB,A45
GraciaR,A93
GrageraGómezM,A115
GrageraGomezM,A170
GramS,A46
GranåsAG,A116
GrandaLobatoP,A42
GrandaP,A26
GrauS,A34,A38,A43,A54
GrisantiS,A4
GuerfaliM,A63
GuerraEstévezD,A120,A143
GuglielmiS,A95
GuibaneW,A146
GuibertA,A1
Guijarro-MartínezP,A89,A141
GuillénDíazM,A37,A43,A55
Guillén-DiazM,A41
GuiuSeguraJM,A10
GusmãoA,A155
GutermannL,A88
GutiérrezÁmyuste,A42
GutiérrezGutiérrezE,A86,A121
GutiérrezPalomoS,A158
GutiérrezSánchezJA,A37,A43,A55
GutierrezPalomoS,A65
Gutierrez-SánchezJA,A41
HagesætherE,A116
HaguiN,A146
HajtmanováK,A149
HambalekH,A109
HamediN,A94
HamedounL,A97
HanafiaO,A81,A95
HarbigP,A46
HeinzMoránS,A42
HeislerováM,A139
HellemansL,A70
HermosillaFernándezJ,A17
HermosillaJ,A18,A135
HernándezHuertaD,A101
HernándezRamosJA,A75
HernándezSánchezM,A37,A55
Hernández-GarcíaMA,A18
HernandezM,A160
Hernandez-SánchezM,A41
HernandoMartínezP,A70
HernandoMartinezP,A150
HernandoVerdugoM,A125
HerranzAlonsoA,A151
HerranzMuñozN,A80
Herranz-AlonsoA,A140
HerreraExpósitoM,A121
HerreroA,A26,A165
HerreroFernándezM,A56
HerreroFernandezA,A102
HerrerosFernándezA,A153
HeymannC,A4
HiasJ,A70
Hijazi-VegaM,A102
HochbrüggeH,A4
HoeckelM,A1
HojnyM,A16
HolmströmAR,A123,A148
HonoréS,A95
HonoreS,A81
HorcajadaJP,A34,A43
HorellouM,A68
HornoUreñaF,A5,A66,A79,A100
HorsComadiraP,A10
HortaAM,A122
HorváthovaA,A149
HoyoA,A26
HudeloJ,A142
HuertasFernándezMJ,A111,A144
HugMJ,A17
Hun-ChabryY,A142
HurgonA,A18
HurtadoGómezMF,A113
IbañezM,A61
IbáñezZurriagaA,A126,A132
Ibanez-CaturlaJ,A37,A41,A43,A55
Ibáñez-GarcíaS,A140
Ibáñez-HerasN,A102
Ibañez-ZurriagaA,A77
IdoateAI,A23
IezziA,A72
IglesiasÁlvarezG,A59
IglesiasA,A15
IglesiasMC,A6
Iglesias-BolañosA,A57,A59,A127
Iglesias-PeinadoI,A169
IkamaMS,A167
ImaneZ,A97
InclanCondeM,A124
IñiguezC,A87
IsabelVM,A74
IzquierdoBlascoJ,A103
JamartSanchezL,A159
JanierM,A83
JanssensA,A58,A128
JavierIC,A119
JefferyL,A46
JeongaH,A32
JerezRojasJ,A7
Jiménezde-JuanMDC,A143
JiménezGarcíaV,A57
JiménezI,A26
JiménezMéndezC,A27
JiménezMeseguerM,A113
JiménezMoralesA,A152
JimenezA,A49
JimenezDe-JuanC,A127
JimenezMendezC,A53
JimenezMoralesA,A40,A157
Jimenez-MoralesA,A96
Jimenez-PichardoL,A127
JimenoAguadoS,A56
JofrePeraltaA,A74
JohnsE,A45
JoséCB,A106
JoséManuelVH,A74
JostJ,A21
JouhetO,A1,A88
JouvenX,A167
Juárez-GiménezJC,A47
JuanAntonioGS,A119
JuanbeltzR,A23
JuanesA,A72
JuanesAna,A3
JuvanyRoigR,A40
KadlecovaR,A16
KarapinarF,A158
KardosG,A109
KasalovaE,A16
KearneyM,A94
KennedyE,A39
KeyanyA,A36
KhanI,A94
KhorramiS,A6
KickenM,A113
KleinleinM,A1
KlovrzovaS,A16
KoblížekV,A139
KomjathyH,A149
KoskenkorvaT,A1
KouamKouamC,A167
Kovačevič M,A123
KrämerI,A19
KrishekB,A94
KuitunenS,A123,A148
KunnolaE,A148
KvarnströmK,A1
LabradorAndújarN,A27
LabradorAndujarN,A53
LacalleE,A23
LacasaArreguiC,A170
LacidognaG,A71
LadisaV,A132
LafargaLapiezaV,A70,A150
LagoRiveroN,A77,A126
LagraveAC,A1
LagunaMarmolL,A67
LajtmanováK,A3
LamandV,A112
LammensA,A128
LandaAlberdiJ,A86
LannoyD,A21
LaoDomínguezFA,A162
LaordenD,A26
LaraC,A26
LarreaUrtaranX,A41,A69,A79,A82,A95,A136
LarrubiaY,A26
LasriFZ,A6,A69,A159
LatasaBerasateguiA,A86
LauriaPantanoC,A132
LavandeiraPerezM,A163
Lavandeira-PérezM,A56
LazaroA,A122
LázaroA,A104
LázaroLopezA,A156
LeDeleyMC,A115
LeGuenC,A54
LéguilletteC,A115
LeónVillarJ,A43
LechaOchoaC,A144
LedesmaBFernández,A103
LeeJL,A111
LeeJY,A111
LeeN,A111
LeendersT,A36
LefrançoisA,A165
LeitaoC,A19
LejeuneL,A128
LeonardiVinciD,A127
LeonelliJ,A81
LeraltaGonzálezC,A113
LevigoureuxE,A83
LigrosM,A62
LigrosTorresM,A142
LimónML,A59
LimaS,A32
LimboleE,A167
LinYN,A161
Linares-AsensioMJ,A114
Linden-LahtiC,A1
LindenbergF,A54
LinxweilerH,A19
LioniMF,A27
ListroA,A155
LizamaGómezNG,A63
LizeagaCundinG,A86
LizondoT,A20
LlamasLorenzanaS,A86,A121
LleonartBellfillR,A105
LlorenteCubasI,A31
LoCocoD,A44
LopesDaCruzJP,A50
LópezAlvárezR,A81
LópezDíazÁ,A89
LopezGilleteM,A142
LópezGálvezMI,A125
LópezGarcíaÁ,A169
LópezLópezA,A30
LópezLaia,A3
LopezM,A6
LópezMC,A20
LopezNogueraQ,A91
LópezNogueraQ,A69,A79,A144
LópezRanchalR,A13
LópezSuárezD,A7
Lopez-AspirozE,A77
López-AspirozE,A168
López-GonzálezAm,A106
Lopez-MonteroE,A101
Lopez-NietoJ,A107
López-RoblesJ,A96
López-VinardellL,A72
LorzaGilL,A63
LourençoM,A149
LozanoBlázquezA,A57,A116
LozanoM,A20
Lozano-BlázquezA,A59,A96
Luaces-RodríguezA,A84
LucíaCP,A74
LucíaSC,A74
LucaEDE,A46
LucasMayolMJ,A61,A65,A76,A158
LunaHigueraA,A133
LundRøndbjergL,A13
LuqueJimenezM,A36
LuqueS,A34
LydiaFG,A119
MaatB,A5,A36,A113
MacíaRivasL,A57,A116
Macía-RivasL,A96
Macías-DeclaraI,A96
MaciaRivasL,A59
MaëstroniML,A21
MagallonA,A87
MagroVázquezC,A56
MaidmentI,A109
MaillanG,A21
MaiquesLlácerJ,A32
MajuelosAicartL,A131
Maly J,A139
MalaquinD,A142
MallónS,A26
MamayusupovaH,A109
MancillaMonteroE,A13
ManguesBafalluyI,A58
ManguesMªAntonia,A3
ManguesMA,A124
ManzanoLorenzoR,A137
ManzanoMartínV,A143
ManzanoMartinMV,A37,A111
ManzanoMartinV,A144
MaríaÁngelesAB,A74,A87,A93
MaríadeLosReyesGO,A74
MaríaDelPuertoPJ,A74
MaríaPM,A74
MarchenaA,A117,A125
MarcosdelaTorreA,A135
MarcosPérezG,A70
Marcos-FendianÁ,A29
MariñoMéndezA,A59
MariaGD,A119
MariaHS,A119
MarinDelgadoD,A32
MarinS,A61,A64,A98,A133,A145,A153
MarliotG,A115
MarquéO,A21
MarquesE,A22
MarrazzoGM,A134
MartínCondeMT,A45
MartínMarquésM,A15
MartínNiñoI,A70
MartínRoldánA,A40,A161
MartínRoldanA,A28,A152
MartínValA,A67
MartínZaragozaL,A75
Martín-HerranzI,A84
Martín-SanzP,A56
Martín-ZaragozaL,A169
MartínezA,A108
MartínezCatroB,A91
MartínezE,A104
MartínezJ,A138
MartínezLópezdeCastroN,A30,A77,A126
MartínezOreaA,A43,A55
MartínezRuizE,A51,A156
MartínezSesmeroJM,A105
MartínezSoguesM,A91
Martínez-BarrosH,A141
Martínez-DelaTorreF,A89
Martínez-HernándezA,A168
Martínez-MúgicaC,A39
Martínez-NietoC,A89,A169
Martínez-OreaA,A41
Martínez-PérezM,A40
Martínez-PradedaA,A84
Martínez-TorrónA,A96
MartelF,A11
MartiGilC,A150
MartinA,A133
MartinCasadoL,A166
Martin-SanzP,A78
MartinezBarrosH,A163
MartinezC,A156
MartinezCastroB,A58
MartinezE,A122
MartinezF,A147
MartinezM,A135
MartinezMD,A156
MartinezNuñezME,A80
MartinezOreaA,A37
MartinezRuizE,A78
MartinezSesmeroJM,A137
MartinezSotoA,A102,A108
MartinezSotoAM,A153
MartinezTorronA,A59
Martinez-BarrosH,A56,A78
MasBauzaN,A40,A154
MasarykováL,A149
MasipM,A149
MasucciS,A71
MatéArbaizaP,A28
MateosC,A26
MateosMateosY,A80,A137,A138
Mateos-GonzálezM,A48
Mateos-SalillasC,A47
Mateos-SalvadorM,A84
MatillaGarciaE,A20,A52
Matilla-GarcíaE,A48,A92
Matilla-GarciaE,A134
MatosesChirivellaC,A61,A76,A158
MatuzM,A109
MefetahH,A90
MeguennaniM,A83
MeiraF,A24
MelendoPérezS,A103
Melero-BermejoJA,A48
MelgarejoOrtuñoA,A20,A52
Melgarejo-OrtuñoA,A48,A92,A134
Membrive-JimenezC,A96
MendiolaS,A138
MendozaAcostaI,A51
MendozaAguileraM,A32
MengatoD,A98
MengualBarrosoMR,A80,A137,A138
MercandalJ,A29
MercedesAM,A74
MerchánA,A74
MerchanA,A87,A93
MerczelSara,A2
MerinoBohorquezV,A108,A166
Merino-MendezR,A72
MeseguerBarrosCM,A159
MestreM,A20
MestreRibotMM,A45
MiaronsM,A103
MichauxJ,A12
MichelB,A45,A90
MichielonA,A73,A94
MiedesJ,A34,A38,A43,A54
MiguelAngelCS,A119
MikešováM,A139
MillacoyAustenrrittDP,A63
MinaříkováJ,A139
MirCrosM,A58,A85,A91
MirallesAndreuG,A158
MirandaA,A104,A122,A156
MirandaDelCerroA,A51,A78
MoñinoB,A62
MoñinoBlazquezB,A142
MoñinoDomingezL,A108
ModesteH,A110
MolínsCastiellaE,A170
MolinaAranaD,A80
MolinaGarciaT,A75,A80,A151
MolinaMendozaMD,A101
Molina-GarcíaT,A102
MonereoAlonsoA,A75
MongaretC,A90
MongeEscartínI,A28
MonopoliC,A134
MonteBoquetE,A106
MonterdeDavid,A3
MonteroLlorenteB,A101
MonteroPerezO,A8
MonteroVílchezC,A11,A152
MonteroVilchezC,A157,A161
Montero-VilchezC,A9,A28,A40,A96,A164
Montes-Jovellar-GonzalezLE,A53
MontiGuarnieriN,A95,A152
MontoroRonsanoB,A105
MontoroRonsanoJB,A105
Montoro-RonsanoJB,A47
Morín-RodríguezM,A154
MoraCortesM,A38,A82
MoraMoraMA,A36
MoraRodríguezB,A157
Mora-RivasE,A53
MoralMT,A147
MoralesA,A98
MoralesLeonMV,A131
MoralesPancorboD,A120
MoralesPortilloA,A58,A85,A91
MoralesRiveroB,A100
Morales-TiradoA,A141
MoranteM,A65
MoratallaRolaníaA,A10
MorellBaladrónA,A31,A126,A132
MorellMuñozÁ,A132
Morell-BaladronA,A77
MorenoCJ,A99,A104,A139
MorenoDiazR,A20,A52
MorenoDiazT,A34,A44,A51,A84,A118,A131
MorenoGarciaE,A25
MorenoLopezA,A66,A79
MorenoPeruleroML,A135
MorenoRamosC,A35,A118
Moreno-DíazR,A48
Moreno-DiazR,A92,A134
Moreno-PelayoMÁ,A154
Moreno-RamosC,A33,A163
Moreno-ZamoraAM,A56
MorielSánchezC,A80,A114
MorielSanchezC,A137,A138,A159
MorilloVerdugoR,A162
MoronaMínguezI,A80
MoronaMinguezI,A137,A138,A159
Morona-MinguezI,A114
MortillaroF,A155
Mosquera-TorreA,A101
MousannifS,A90,A97
MoyaGomezP,A27,A53
Mozo-PeñalverHJ,A101
MuñozBolañoM,A154
MuñozCastilloI,A6,A12,A25,A71
MuñozCastilloIM,A97,A100,A133,A157
MuñozCejudoBM,A36
MuñozCidC,A7
MuñozGarcía:M,A141
MuñozVillasurM,A57,A116
Muñoz-GarciaM,A53
MuhammadS,A21
MulacA,A116
MurciaLópezA,A61
MurgadellaA,A24
MurielGarcíaA,A101
MurrjaEla,A2
MuziottiC,A110
MyintPK,A109
N’GuettaR,A167
NájeraPérezMD,A37
Najera-PerezMD,A55
NallyS,A67
NataliniSerena,A2
NaturaleMD,A134
NavarroBruguerasM,A106
NavarroD,A8
NavarroRuizA,A61,A65,A76,A158
NavasN,A17,A18,A135
NebelAS,A14
NeefsJ,A58,A128
NeiningerMP,A1
NevotBlancM,A91
NiellyH,A112
NietoGuindoP,A51
Nieto-SánchezMT,A40
NilsenR,A18
NiotC,A72,A92
NocedaMM,A23
NocedaUrarteM,A107
NocedaUrarteMM,A56,A129
NoemiMR,A119
NoguéPujadasE,A69,A82,A95,A136
NotarioDongilC,A81,A135,A143
NuceteB,A117,A125
NuijenB,A14
NúñezI,A147
NúñezRodríguezJ,A7,A9
Núñez-NúñezM,A30
OcañaRicoM,A67
OffnerK,A17
OlariagaO,A15
OlesenC,A13,A46
OlivesCasasnovasR,A68
OmodeoSalèE,A72
OnatadeR,A103,A130
OntenienteGonzálezA,A75
OntenienteGonzalezA,A151
Onteniente-GonzálezA,A169
OriolI,A24
OrlandiE,A27
OrozcoCifuentesI,A114
OrtíJuanC,A41,A79,A82,A91,A95,A144
OrtegadelaCruzC,A6,A97,A100
OrtegaI,A23
OrtegaO,A61
OrtegaValínL,A86,A121
OrtizdeUrbinaGonzálezJJ,A86
OrtizdeUrbinaJ,A121
OrtizdeZárateZ,A156
OrtizFernandezP,A102,A108,A153
OrtuñoRuízY,A82
OrtuñoRuizY,A79,A95
OterinoI,A114
OterinoMoreiraI,A159
OteroMJ,A106
Overgaag-vanHemertM,A169
OyagueLópezL,A57,A116
PaarupKirkebyHerpingS,A13
PachecoLópezP,A37,A43,A55
Pacheco-LopezP,A41
PadullésZamoraN,A154
Pagès-PuigdemontN,A149
PalazzoA,A44
PallásCR,A147
Palomar-FernandezC,A53
PalomoPalomoC,A120,A143
PampinR,A39
PaolucciCristina,A2
ParadasPalomoJD,A71
PardoA,A128
PardoSantosNM,A124
ParroMartínMÁ,A101
PascualCarbonellD,A15,A87
PascualO,A93
PastalleA,A23
PastorMondéjarC,A102
PastorVaraP,A137
PastorelloM,A155
PatelR,A94
PauParraA,A103,A105
PaulaTB,A119
PavónJ,A26
PázmányP,A99
Peña-PedrosaJA,A169
PedemonteM,A124
PelaezBejaranoA,A127
PeralBallesterL,A76
PeraltaJ,A49
PérezAbánadesM,A132
PerezCaballeroG,A80
PérezdeRojasJ,A30
PérezE,A26
PerezEspañaZ,A124
PérezFácilaA,A81,A143
PerezFacilaA,A135,A148
PerezG,A104
PérezGrimaldiF,A62
PérezLandeiroA,A30
PérezMF,A6
PérezPlasenciaA,A144
PérezRoblesR,A17
Perez-AbanadesM,A77
Pérez-CordónL,A61
Pérez-EncinasM,A114
Pérez-RamirezC,A96
Pérez-RicartA,A145,A147
Pérez-RoblesR,A18,A135
PerotP,A16
PerreyJ,A130
PerrottetN,A4
PetitA,A11,A142
PetitgasS,A68
PicazoSanchizG,A66
PierasÁ,A139
PierasA,A99
PietrzakC,A14
PilarPL,A119
PimentelP,A59
PinalliD,A72
PíoAsínM,A63
PiovanelliA,A117
PlaPasánR,A37,A111,A143,A144
Planas-GinerA,A167
PointingerA,A55
PolidoriC,A95,A152
PolidoriP,A44,A46,A119,A127
PolitoG,A27
PompilioA,A95,A152
PonsJL,A10
PorredonAnteloC,A40
PortaRiberaR,A67
PortelaSoteloA,A70
PortuJ,A156
PorubcováS,A3
PostC,A169
PotierA,A90
PoulenardR,A21
PousadaFonsecaA,A137,A138
PousadaFonsecaAB,A80
PovedaEscolarA,A114,A163
PradillaBascuasE,A126
PradoMontesP,A30,A77,A126
PratsOlivánP,A42
PrietoGalindoR,A27,A53
PrimN,A34
PrisqueN,A130
ProJimenezFJ,A78
ProliEM,A27
ProupinCantelarIC,A161
PuértolasValR,A32
PueyoLópezC,A163
PuglieseSara,A2
PuigM,A124
PuigMireia,A3
Puig-CampmanyM,A72
PujolJoverM,A103
PupláBartollA,A32
QuesadaMarquesV,A131
QuiñonesC,A64,A98,A133,A153
QuiñonesRibasC,A67,A145,A147
QuitteB,A18
RamajoC,A15
RamirezHerraizE,A31
RamírezHerraízE,A126
RamírezHerraizE,A132
RamirezLopezAM,A107
RamírezOlivenciaG,A42
RamirezSotoG,A89
RamlalA,A169
RamonRigauN,A91
RamosS,A160
RaquelFM,A74
RatsimalaheloN,A4
RatsimbazafyV,A21
Ravn-NielsenLV,A35
RazO,A8
ReB,A132
RealAragónB,A70
RealL,A72
RéalL,A92
RealmutoS,A44
RebolloDiazN,A89
RedingerT,A55
ReenaM,A103
RendóndeLopeL,A49
RetameroA,A138
RevertAlarconMJ,A131
ReyesMaliaM,A120,A143
ReynaudB,A112
RhaymiC,A47,A50
RiberaPuigC,A40,A154
RicciL,A71
RichezO,A142
RichterK,A99
RieraG,A107
RieraP,A149
RiestraAyoraAC,A15
RigoBonninR,A40
RiosSanchezE,A64,A65
RíosSánchezE,A93
Rios-SanchezE,A85
RiusPereraJ,A58
RivasRodríguezMD,A170
RivasRodriguezMD,A115
Roé-CrespoE,A149
RobertPY,A21
RoblesTorresD,A30,A77,A126
RobsonJ,A94
Rodelo-HaadL,A25
RoderoNevadoM,A126
RodríguezEsquírozA,A25,A63,A113,A146
RodríguezFernándezZ,A121
RodríguezGarcésMY,A120
RodríguezMarínM,A53,A78
RodríguezMoretaC,A37
RodríguezMoroteM,A76
RodríguezPenínI,A52
Rodríguez-GonzálezC,A89
Rodríguez-MarínM,A56
Rodríguez-TenreiroRodríguezC,A57
Rodríguez-TiernoS,A89
Rodríguez-VargasB,A48,A134
RodriguesM,A155
RodriguezÁlvarezSJ,A75
RodriguezA,A38,A43
RodriguezBenaventeAM,A80
RodriguezCastillaJ,A127
RodriguezGoicoecheaM,A5,A66,A79,A100
RodriguezJorgeM,A8,A66,A120,A141,A145, A150
RodriguezMarinM,A163
RodriguezMolinsE,A127
RodriguezMoroteM,A61,A65
RodriguezVargasB,A20
Rodriguez-TenreiroRodriguezC,A116
Rodriguez-VargasB,A92
RoelsgaardK,A46
RoigSoronellasM,A167
RojasAlbarránA,A115,A170
RojasAlveroJC,A154
RomaJR,A20
RomaMoraJR,A45
RomeroAlonsoM,A120,A143
RomeroVentosaEY,A126
Romero-GarridoJA,A47
Romero-GonzálezM,A166
RoosP,A169
RosOlasoA,A86
RosafioV,A94
Rotea-SalvoS,A84
RoviraTorresP,A136
RoyoV,A6
RozsívalováP,A139
RubioAlonsoL,A66
RubioCebriánB,A80
RubioCebrianB,A137,A138
RubioSalvadorAR,A27,A53
Rubio-RuizL,A102,A169
RudiSolaN,A167
RuedaBermudezLDL,A89
RuizBoyS,A28
RuizGarcíaS,A132
RuizJ,A72,A124
RuizJesús,A3
RuizLaraLM,A13
RuizPérezDS,A60,A129
RuizTravéJ,A17
Ruiz-TravéJ,A18,A135
RuutiainenH,A148
RuzsaR,A109
SacanellaAnglèsI,A15,A87
SacrestGüellR,A41,A69,A79,A82,A91,A95, A144
SacrestGuellR,A136
SadeghipourF,A4
SadyrbaevaDolgovaS,A9,A164
SáezdeAdanaE,A156
SáezHortelanoJC,A86,A121
SáezVillafañeM,A121
Saint-LorantG,A110
SaizMartínezC,A86
SaizMolinaJJ,A81,A143,A148
SakjiI,A115
SaksaM,A123
SalaPadróJX,A40
SalarValverdeI,A102
SalcedoMingoarranzAL,A56,A114
SalcedoMingorranzÁL,A113
Salcedo-MingorranzAL,A57,A59
SaldañaSoriaR,A42
SaldañaValderasM,A144
SalernoF,A71
SalgueroOlidA,A162
SalinasMuñozTE,A135
SalmerónGarcíaA,A17
Salmerón-GarcíaA,A18,A135
SalmeronCobosAY,A11
SalmeronNavasFJ,A64,A65
SalvadorGomezT,A83
SalvadorT,A87
SamiS,A109
SanJoseRuizB,A142
SanMiguelTorviscoP,A137,A138
SanahujaMontesinosJ,A85
SánchezArgaizMC,A157
SánchezdeCastroM,A42
SánchezdelMoralR,A141
SánchezGómezE,A150
SánchezLópezP,A42
SánchezLobónI,A111,A143,A144
SanchezR,A49
SanchezRodriguezB,A44,A118,A131
SánchezRodríguezB,A34,A51,A84
SánchezRuizA,A7
SanchezSuarezMDM,A40,A161
SánchezSánchezMT,A125,A165
SánchezSuárezMDM,A28,A152
SanchezValeraM,A44,A118,A131
SánchezValeraM,A34,A51
SánchezVarelaM,A84
Sánchez-CuéllarS,A141
Sánchez-de-TorreA,A134
Sanchez-OcañaMartinN,A137
Sánchez-RubioFerrandezJ,A75
Sánchez-Rubio-FerrándezJ,A169
Sánchez-ValeroJ,A102
SanjuánBeldaA,A15,A87
SanjurjoSáezM,A151
Sanjurjo-SaezM,A140
Sanmartin-FenolleraP,A169
Santamaria-GadeaA,A141
Santana-MartinezS,A101
SantandreuMM,A6
SantinF,A110
SantonocitoM,A44,A46,A119,A127
SantosAR,A22
SantosM,A124
SantosP,A155
SantosRodríguezC,A91
SantosRodriguezC,A85
Santos-RubioMD,A66,A120,A150
SanzCobeñaV,A126
Sanz-MárquezS,A114
SaoudiZ,A146
SarobeCarricasM,A25,A63,A107,A113,A129, A146
SarobeCarricasMT,A56
SarobeMT,A23
SauleauEA,A45
SchembriJ,A76
SchepelL,A1
SchintlerJ,A23
SchoenenbergerArnaizJA,A58,A85,A91
SchofieldR,A16,A88
SecchiA,A109
SellalKO,A54
SeobS,A32
Serna-RomeroO,A57,A59,A127
SerraP,A23
Serra-BaldrichE,A149
Serra-PratM,A61
SerraisJ,A138
SerramontmanyMoranteE,A136
SerranoAlonsoM,A170
SerranoGiménezR,A145
SerranoM,A168
SessinkP,A13
ShinJ,A32
SierraSánchezJF,A60,A62,A129
SierraTorresMI,A157
SilesBaenaA,A67
SilesMorrisS,A37
SilvaPA,A50
SilvariV,A39
SimõesAM,A155
SimonsE,A58,A128
Slimáková Ľ,A139
SlossR,A103
SlutskySmithE,A8
ŠmahelP,A139
SmithFlotzS,A106
SoaresAM,A155
SobrinoC,A26
SociasC,A124
SociasCañellasC,A149
Socias-CañellasC,A72
SolísOlivaresJ,A80
SolisCuñadoS,A102
SolisOlivaresJ,A137,A138
SollanoSanchoI,A80
Sollano-SanchoI,A114,A137,A138,A159
SopenaL,A87,A93
SørensenCA,A46
SorianoA,A24
SorianoIrigarayL,A65
SorlíL,A34
SorrentiO,A71
Sosa-PonsA,A167
SotoBaselgaI,A80,A138
SotoCamposJG,A62
SotoI,A137
SoubeletL,A72
SoyD,A20,A24,A29,A49
SoyMunerD,A28,A45
SpertinoJ,A149
SpinosoB,A134
SprietI,A13,A58,A128
Steindl-SchönhuberT,A160
ŠtricováA,A139
StrobbeG,A115
StrobelHG,A4
SuárezSantamariaM,A77
SuñéNegreJM,A145,A147
SuñerH,A15,A87
SubiranaBatlleC,A41,A79,A82,A95,A136
SwartE,A14
SzilvayA,A99
SzmicsekováK,A3
TabernerBonastreP,A85
TadlaouiY,A97
TakombeJL,A167
TallonMartinezJC,A137
TamayoBermejoR,A6,A97,A100,A157
Tamayo-BermejoR,A25
TamésMJ,A15
TansB,A13
TardáguilaMolinaP,A51,A78,A156
TardáguilaP,A104
TardaguilaP,A122
TárnokováD,A149
TebaldiniB,A72
TeixeiraV,A165
TejedorE,A49
TejedorTejadaE,A5,A66,A79,A100
TenHoopeS,A158
TenfeldeK,A113
TerkolaR,A14
TerricabrasE,A64,A98,A133,A153
TerricabrasMasE,A67
TerrobaPN,A39
TesoV,A72
ThompsonC,A164
TimonedaCompanyM,A146
TitosArcosJC,A43
ToledoDaviaMA,A27,A53
TollecS,A165
ToroBlanchC,A144
TorraJE,A104
TorralbaFernándezL,A27
TorralbaFernandezL,A53
TorralbaGonzalezdeSusoM,A156
TorralbaM,A104
TorranoBelmonteP,A37,A55
Torrano-BelmonteP,A41,A43
TorrealbaMediana-LDV,A69
TorrentA,A20
TorrenteLópezA,A17
Torrente-LópezA,A18,A135
TorresA,A18
TorresBondiaFI,A85
TorrobaSanzB,A151
TóthováK,A149
Touris-LoresM,A101
TournoyJ,A70
TraoreAK,A167
TrittlerR,A17
TrojanielloD,A117
TruelshøjT,A13
TudelaTomásJ,A111,A143
TuomistoJ,A123
TusetM,A24
UlangkayaG,A168
UniaC,A110
Urbano-FernándezMÁ,A40
UrbietaSanzE,A102,A108,A153
UrbinaO,A156
UrdaRomachoJ,A74,A121
UrretavizcayaM,A15
Uyl-deGrootC,A169
ValdeolmillosL,A170
ValdesLedsmaJ,A168
ValeraRubioM,A166
ValleDíazdelaGuardiaAM,A9,A164
VallsE,A61,A64,A98,A133,A153
VallsSánchezE,A145,A147
VanAsseldonkT,A36
VanDenBergR,A14
VanDenBerghG,A12
VanDerKuyH,A169
VanDerLeeC,A113
VanDerLindenL,A70
VanGeelM,A36
VanHerpen-MeeuwissenL,A5
VanNieuwenhuyseT,A58,A128
VanSchaikS,A158
VaraUrruchuaM,A124
VarasdeDiosB,A31
VarelaFernándezR,A86,A121
VarelaI,A93
VarelaMartinezI,A83
Varon-GalceraC,A136
VázquezRealM,A60,A129
VázquezSanchezR,A151
VázquezVelázquezH,A170
Vázquez-SánchezR,A102
Vazquez-TrocheS,A101
VegaV,A34
VeigaVillaverdeAB,A161
VelaEmili,A3
VelazquezVazquezH,A115
VélezBlancoA,A86,A121
Vélez-Díaz-PallarésM,A56,A89,A101
Velez-Diaz-PallaresM,A169
Ventura-LopezM,A41
VenturiniF,A98
VerheyenV,A12
ViñasSaguéL,A79,A95,A136
ViaudC,A90
VicenteValorJ,A151
Vicente-OliverosN,A53
VieiraM,A19
VilaCurriusM,A82,A136
VilaGallegoC,A124
VilariñoA,A133
VilimelisI,A145,A147
VillainA,A115
Villalta-AndújarT,A84
VillamañánE,A26,A165
VillarongaM,A99,A104,A139
VimaBofarullJ,A105
VinuesaHernandoJM,A83
VinuesaJM,A87
VisaL,A59
VisticotA,A92
Viudez-MartinezA,A107
VivancosGallegoMJ,A101
VonWinckelmannS,A12
WalgraeveK,A70
WalterA,A19
WatlerM,A168
WeidmannA,A23
WeiglA,A55
WelvaarsK,A158
WethmarU,A4
WilhelmiE,A99,A104,A139
WilliamsS,A164
WirthF,A76
WrightP,A88,A94
WrightPG,A103
YanesSánchezP,A131
YbañezGarciaL,A137
YerroA,A23
YunqueraRomeroL,A42
ZahraG,A76
ZambranoCrocheMD,A115
Zarra-FerroI,A101
ZasGarcíaMI,A7,A9
ZelanteF,A132
ZhimI,A90
ZimčíkováE,A139
ZitelliS,A72
ZitoM,A134
ZuñigaC,A96
EJHP
The only official journal of European Association of Hospital Pharmacists (EAHP)
Editor in Chief
Phil Wiffen (UK) editor.ejhp@bmj.com
Deputy Editor
Tommy Eriksson (Sweden) tommy.eriksson@mah.se
Disclaimer
While every effort is made by publishers and editorial board to see that no inaccurate or misleading data, opinions, or statements appear in this Journal, they wish to make it clear that the data and opinions appearing in the articles and advertisements herein are the responsibility of the contributor or advertiser concerned.
The Editor of European Journal of Hospital Pharmacy has been granted editorial freedom and European Journal of Hospital Pharmacy is published in accordance with editorial guidelines issued by the World Association of Medical Editors and the Committee on Publication Ethics. European Journal of Hospital Pharmacy is primarily intended for healthcare professionals and its content is for information only. The Journal is published without any guarantee as to its accuracy or completeness and any representations or warranties are expressly excluded to the fullest extent permitted by law. Readers are advised to independently verify any information on which they choose to rely. Acceptance of advertising by European Journal of Hospital Pharmacy does not imply endorsement. Neither the European Association of Hospital Pharmacists nor BMJ Publishing Group Limited shall have any liability for any loss, injury or damage howsoever arising from European Journal of Hospital Pharmacy (except for liability which cannot be legally excluded).
Copyright: © 2023 European Association of Hospital Pharmacists (EAHP). All rights reserved; no part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without prior permission.
European Journal of Hospital Pharmacy is published bimonthly by BMJ Publishing Group Ltd, and printed in the UK on acidfree paper from sustainable forests.
European Journal of Hospital Pharmacy (ISSN No: 2047-9956) is distributed in the USA by Air Business Ltd. Periodicals postage paid at Jamaica NY 11431. POSTMASTER: send address changes to European Journal of Hospital Pharmacy, Air Business Ltd, c/o Worldnet Shipping Inc., 156-15, 146th Avenue, 2nd Floor, Jamaica, NY, 11434, USA.
Associate Editors
Pascal Bonnabry (Switzerland)
Witold Brniak (Poland)
Cristina Garcia Yubero (Spain)
Ilko Getov (Bulgaria)
Irene Kraemer (Germany)
Raisa Laaksonen (Finland)
Paul Le Brun (The Netherlands)
Liv Mathiesen (Norway)
Hein van Onzenoort (The Netherlands)
Social Media Editor
Madalina Butuc (Romania)
International Advisory Board Members
Mike Allwood (UK)
Lona Christrup (Denmark)
Susan Goodin (USA)
Robert Janknegt (Netherlands)
Carlo Polidori (Italy)
Sonia Prot-Labarthe (France)
Gunar Stemer (Austria)
Judith Thiesen (Germany)
Hugo van der Kuy (The Netherlands)
Anita Wiedmann (Austria)
Steven Williams (UK)
Editorial Office
EJHP Editorial Office, BMA House, Tavistock Square, London, WC1H 9JR, UK
E: info.ejhp@bmj.com
Publishing Executive Caitlin Alder
E: calder@bmj.com
Customer Support
For general queries and support with existing and new subscriptions:
T: +44 (0)20 7111 1105 https://myaccount.bmj.com/myaccount/ customerservice/support-home.html
Jimmy Jose (Oman)
Lihong Liu (China)
Judy Mullen (Australia)
Guidelines for Authors and Reviewers
Full instructions are available online at https://ejhp. bmj.com/pages/authors/. Articles must be submitted electronically http://mc.manuscriptcentral.com/ejhpharm. Authors are required to transfer copyright in their work to the European Association of Hospital Pharmacists. Included in Science Citation Index.
EJHP is published bimonthly (subscribers receive all supplements)
Print (includes online access at no additional cost)
£277
Online only
£240
ISSN 2047-9956 (print), 2047-9964 (online)
Personal print or online only and institutional print subscriptions may be purchased online at https://jech.bmj.com/pages/subscribe/
Residents of some EC countries must pay VAT; for details, call us or visit support@bmj.com
Permissions http://www.bmj.com/company/productsservices/rights-and-licensing/permissions/
Senior Production Editor
Malcolm Smith
E: production.ejhp@bmj.com
Supplement Enquiries
E: calder@bmj.com
Subscriptions
T: +44 (0)20 7111 1105 http://ejhp.bmj.com/pages/subscribe/
Display Advertising Sales – Rest of World
Sophie Fitzsimmons (Sales Manager)
T: +44 (0)20 3655 5612
E: sfitzsimmons@bmj.com https://www.bmj.com/company/ for-advertisers-and-sponsor/
Online Advertising Sales
Marc Clifford (Sales Manager)
T: +44 (0)20 3655 5610
E: mclifford@bmj.com http://group.bmj.com/advertising/
Display & Online Advertising Sales –North America
American Medical Communications
T: +1 973 214 4374
E: rgordon@americanmedicalcomm.com
Author Reprints
Reprints team
E: admin.reprints@bmj.com
Commercial Reprints
Nadia Gurney-Randall
M: +44 (0)7866 262344
E: ngurneyrandall@bmj.com
Commercial Reprints (USA & Canada)
Ray Thibodeau
T: +1 267 895 1758
M: +1 215 933 8484
E: ray.thibodeau@contentednet.com
For all other EJHP journal contacts http://ejhp.bmj.com/pages/contact-us/
European Journal of Hospital Pharmacy offers a high-quality, peer-reviewed platform for the publication of practical and innovative research and aims to strengthen the profile and professional status of European hospital pharmacists