

Understanding Glucans: A Guide to Their Benefits for Cancer
Vaclav Vetvicka
Copyright © 2024 by Vaclav Vetvicka
All Rights Reserved
No part of this text may be reproduced in any form without the written consent of the author.
Author’s Disclaimer
This e-book is not intended as medical advice. It is written solely for informational and educational purposes. Please consult a health professional before taking any natural modulator. Because there is always some risk involved, the author and publisher are not responsible for any adverse effects or consequences resulting from the use of any of the suggestions or preparations described in this publication. Neither the publisher nor author advocate the use of any particular product, but believe the information presented in this book is important enough that it should made available to the public.
Table of Contents
Foreword
Despite my cooperation with numerous companies involved in glucan business, I would like to make one thing crystal clear - I am not selling glucan and I never have been. Similarly, I am not endorsing any particular brand of beta glucan. Therefore, I will not give you any advices about which glucan you should buy, all you can expect to find here is the honest information about what glucan is and what glucan can do. The decision to take (or not to take) glucan and which glucan to take is entirely up to you.
I wrote this e-book objectively and completely without any profit expectations. Therefore, all information is for educational purposes only. Any commercial name used in this book is mentioned only as a result of my own extensive testing. And when I did not personally perform the tests mentioned in this book, I provided the full reference for the relevant publication.
This e-book was written primarily to give the reader a complex overview of glucan, its potential, and mechanism of action. Most of attention was devoted to glucan activities in an area of cancer. I intentionally kept the text simple, but at the same time gave full details, including full scientific references, so the readers can judge the evidence for themselves. All scientific references (when possible) included either Digital Object Identifier (DOI) or PMID identification number, so readers interested in the particular study can find it on the internet.
Introduction
Glucans are type of soluble fiber found in the cell walls of certain plants, fungi, bacteria, and some cereals, such as oats and barley. For decades, these compounds have been studied for their various biological activities and potential health benefits. Glucans are a type of polysaccharide, which is a large molecule composed of multiple sugar units. These glucose molecules are linked together in specific ways, creating a complex carbohydrate structure.
Polysaccharides in general, and glucans in particular, have a long history as immunomodulators. As early as the beginning of the 18th century, it was known that certain infectious diseases showed a therapeutic effect on malignant processes. The dedicated use of such therapy dates from around the middle of the 19th century, at which time Bush performed experiments in search of curing sarcoma by infecting patients with an acute streptococcal infection of the dermis. Coley repeated these therapeutical procedures towards the end of the 19th century. Despite the limited success, the early researchers had no knowledge of the molecule responsible for the observed effects.
Much later, the attention was focused on molecule called zymosan. Although zymosan was able to strongly stimulate a nonspecific immune response, initially it was not clear what component of this rather crude composition was responsible for the activity. When zymosan was later examined in detail, glucan was identified as the component of primary effect. It was subsequently isolated and its immunological effects were investigated.
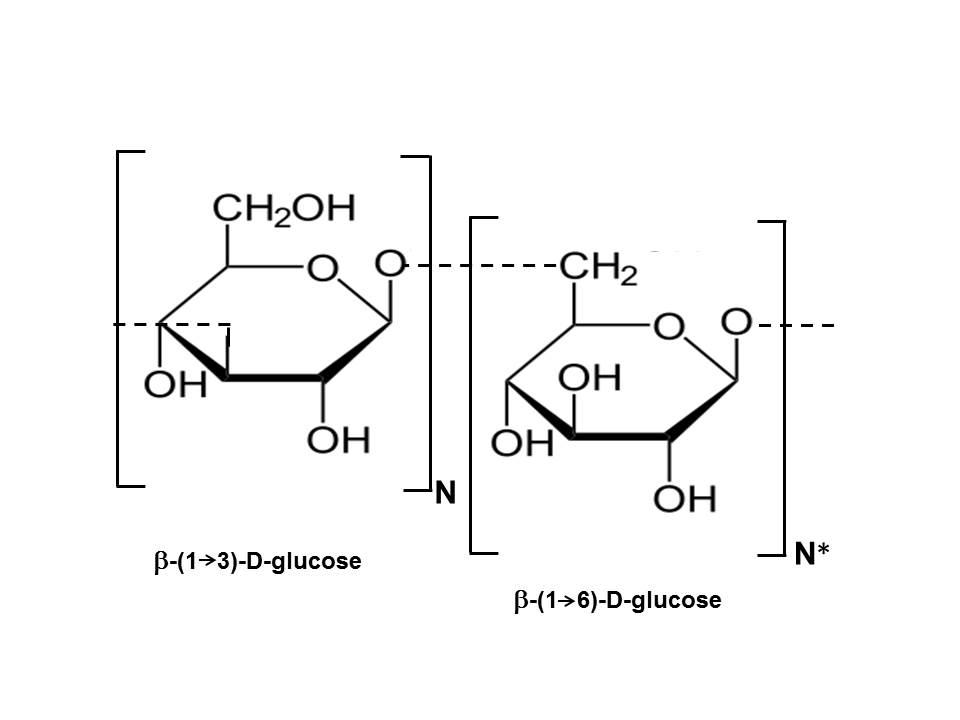
After several decades of rather low intensity studies, intensive research of biological activities of β-glucan (hereinafter referred to as glucan) was conducted in Japan and they arrived at glucan via a different route. In Asian medicine, consuming different medicinal mushrooms (shiitake, maitake, reishi, etc) has been a long tradition. In detailed studies of the biological effects of these mushrooms, in particular their anticancer action, glucans were found to be the main cause of nonspecific immunomodulation. This initial investigation was conducted by Goro Chihara at Teikyo University in Kawasaki, who isolated glucan from the shiitake mushroom, which he referred to as lentinan. This glucan, with some subsequent modification, was approved as a drug in 1983 and has been successfully used. It's important to note that the specific biological activities of glucans can vary depending on factors such as their source, structure, and molecular weight. Research in this field is ongoing, and while there is evidence supporting many of these potential benefits, more studies are needed to fully understand the mechanisms and to establish clear recommendations for their use. As with any dietary component or supplement, it's advisable to consult
Figure 1. Scientific diagram of glucan
with a healthcare professional before making significant changes to your diet or lifestyle.
During decades of research, numerous types of glucans have been isolated and described. In scientific literature or at the market, you can find hundreds of different components, all under the name glucan. Unfortunately, not all glucans were created equal and glucans widely differ not only in physicochemical properties such as molecular weight, branching or molecular weight, but also in biological properties. Some of the described glucans show little activity and some have no biological activities at all.
Yeasts are the major source of glucans in Western countries. The Far East (Japan, China and East Russia) traditionally focus on mushrooms (based on their folk remedy). A high amount of seaweed existing in western France resulted in interest in seaweed-derived glucans. Glucan derived from baker’s yeast (Saccharomyces cerevisiae) has been the most extensively studied and has produced the highest biological effects. We recommend that readers interested in the introduction of a significant number of biologically active glucans read an excellent review written by Harada and Ohno (2008).
Glucans can be isolated from almost every species of yeast and the reason for the popularity of Saccharomyces cerevisiae is based on availability, as this type of yeast is known as common barker’s or brewer’s yeast. Glucan forms part of the yeast cell wall, together with mannan, proteins, lipids and small amounts of chitin. Glucans represent a major structural component of the cell wall in fungi and some plants. Different physicochemical parameters, such as solubility, primary structure, molecular weight, branching and polymer charge, all play a role in determining whether the polysaccharide modulates immune reactions. Some conclusions can be made,
though. Branched or linear 1,4-β-glucans have very limited activity, if any. Glucans with 1,6 configurations usually have limited activity. The highest stimulation of defense reactions has been achieved with β-glucans that have a 1,3 configuration with additional branching at the position 0-6 of the 1-3 linked D-glucose residues. Among all glucans, those with a 1,3 configuration are best characterized in the literature. An excellent review of glucans as biological response modifiers and the relationship between structure and functional activity is given in (Bohn and BeMiller, 1995).
Since it was determined that the lethal dose of glucan is extremely high, glucan was found to be worthy of consideration in clinical practice. After detailed clinical trials, the Japanese government approved the use of glucan for the treatment of stomach and colorectal cancer, particularly when used together with common cytostatic chemotherapy drugs. Thus, glucan is clinically used since 1983.
The original studies on the effects glucan on the immune system focused on mice. Subsequent studies demonstrated that glucan has a strong immunostimulating activity in a wide variety of other species (see Table 1). Based on these results, it has been concluded that glucans represent a type of immunostimulant that is active over the broadest spectrum of biological species and that it is one of the first immunostimulants active across the evolutionary spectrum. Some experiments even show that glucan can help in the protection of plants such as tobacco or turmeric (Anasuya and Sathhiyabama, 2015). Glucan is therefore not only a biologically active polysaccharide with strong immunomodulating effects, but is also considered to be an evolutionary extremely old stimulant of a variety of defense reactions (Vetvicka and Sima, 2004).
Table 1
Glucan affects every biological species tested
Bees
Shrimps
Earthworms
Dogs
Rat
Rabbits
Fish Pigs
Chicken
Mice
Humans
Horses
Monkeys
Despite long and extensive investigations, no consensus on the source, size, or other properties of glucan has been reached. An important comparison of yeastderived and mushroom-derived glucans and their biological activities is given in (Kogan, 2000, Vetvicka and Vetvickova, 2007, 2010). In most cases, yeast-derived glucan consistently showed higher biological activity. One clear disadvantage of mushroom glucans is a common and usually strong smell, which can be unpleasant to many. Some manufacturers mask this smell by adding alcohol or more pleasantly smelling components.
One of the most interesting and important things about glucan is the fact that it effects wide variety of cell types and subsequently a wide number of biological processes (Figure 2).
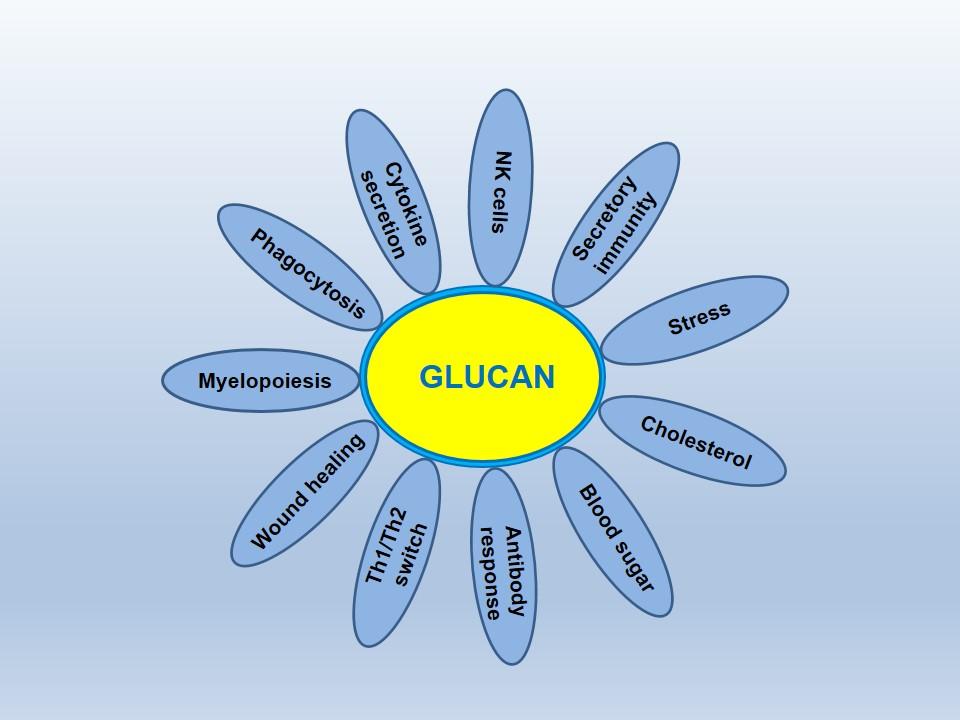
Figure 2
Here are some notable aspects of the biological activity of glucans:
1. Immunomodulation
Enhancement of immune function: Glucans are well-known for their immunomodulatory effects. They can stimulate the immune system, particularly by activating macrophages, which are important immune cells. This activation helps in the defense against infections and may contribute to improved immune responses. However, glucans stimulate various additional cells involved in immune reactions, namely B lymphocytes, monocytes, neutrophils, dendritic cells and natural killer (NK) cells.
2. Antioxidant Properties
The antioxidant activity of glucan was examined to assess potential new benefits associated with glucan, because oxidative stress is considered one of the primary causal factors for various diseases and aging. This antioxidant activity is thought to contribute to the overall protection of cells and tissues from oxidative stress Glucans exhibit antioxidant properties, helping to neutralize free radicals in the body. The amount of antioxidant activity was influenced by different physiologic properties (e.g., structure and molecular size) of glucan, which varied depending on the source and extraction method used. Yeast-derived glucans have the highest activity.
3. Cancer Treatment
After tumor cells are recognized by the immune system, specific antibodies are released and subsequently bind to the cancer cells. Upon this binding, the C3 fragment of complement coats the surface of cancer cells. Several types of glucan-primed cells (macrophages, neutrophils, NK cells) then recognize these antibody-C3 coated cells and kill them. In addition, glucan stimulates the production of tumor-specific antibodies and development of tumor-specific cytotoxic lymphocytes. Effects of glucan supplementation on tumor size are shown in Figure 3. More about glucan and cancer will be given in separate Chapter.
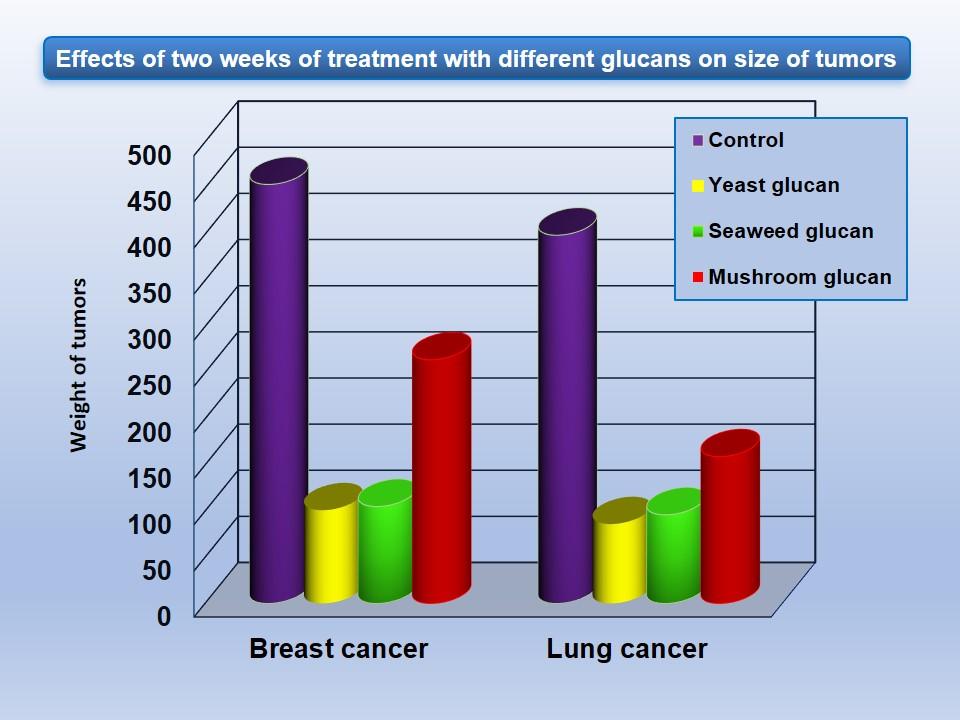
Figure 3
4
Anti-inflammatory Effects
Modulation of inflammatory responses: Glucans have been shown to have antiinflammatory effects. They can influence the production of pro-inflammatory cytokines and help regulate the body's inflammatory response. This property is of interest in the context of conditions associated with chronic inflammation. Numerous findings suggest that glucan can be used as an effective immune modulator to attenuate the progression of inflammatory disease (No et al., 2021).
5 Cholesterol-Lowering Effects
Hypercholesterolemia is one of primary risk factors of cardiovascular disease, together with metabolic syndrome, hypertension and diabetes. Although progress has
been made, the search for novel methods of preventing and treating dyslipidemia is ongoing and current therapies for cardiovascular disease induce various side effects.
Reduction of LDL cholesterol: Some studies suggest that glucans, particularly those from oats and barley, can help lower levels of LDL (low-density lipoprotein) cholesterol, commonly known as "bad" cholesterol. This is believed to contribute to cardiovascular health.
Due to glucan structure, they are able to interact with innate immunity receptors, however they also act as dietary fibers in the digestive tract. As there are two forms of glucans, insoluble and soluble forms, they are able to interact with lipids and biliary salts in the bowel and consequently reduce cholesterol levels. Therefore, they may be developed as a suitable therapeutic option to treat patients with dyslipidemia, as they are natural molecules that do not induce any significant side effects.
Further research is required to more specifically define the role of glucan in modulating the immunological aspects of atherosclerosis. The current review strengthens the hypotheses that of all forms of dietary fiber, natural glucan molecules are the most promising to use as a method of treating patients with dyslipidemia. It remains unclear whether increasing β-glucan intake should be recommended to patients with severe hypercholesterolemia, to be used in addition to or even as an alternative to statins, as there have been very few direct comparisons between the effects of using glucans and statins on such patients. However, glucans may more effective as they have a different mechanism of action than statins; while statins block the action of the liver enzyme responsible for producing cholesterol, glucans promote a more physiologically-based rebalancing of cholesterol levels (for review see Sima et al., 2018).
6
Blood Sugar Regulation
Impact on glycemic control: Glucans may help regulate blood sugar levels. They can slow the absorption of glucose in the intestines, leading to improved glycemic control. This property is especially relevant for individuals with diabetes or those at risk of developing diabetes. This activity will be discussed in full details in following chapters.
7. Wound Healing
Promotion of wound healing: Some studies suggest that glucans play a role in wound healing, potentially by enhancing the immune response and promoting tissue repair. Topical applications of glucans are increasing, especially due to their pluripotent properties. Macrophages, keratinocytes and fibroblasts are considered the main target cells of glucans during wound healing. Glucans enhance wound repair by increasing the infiltration of macrophages, which stimulates tissue granulation, collagen deposition and reepithelialization. Glucan wound dressings represent a suitable wound healing agent, with great stability and resistance to wound proteases
In vivo studies and human clinical trials have provided compelling evidence that glucan preparations including hydrogels and nanofibers with incorporated glucan molecules promote moist wound healing and repair mainly due to the activation of the immune and cutaneous cells. Glucans can induce the proliferation and migration of keratinocytes and fibroblasts through several types of specific receptors such as Dectin-1, CR3 or TLRs. In addition, it was proposed that activation of wound macrophages and antigen-presenting cells by glucan represents a potential mechanism in eradicating bacterial load from chronic wounds. Besides therapeutic
benefits, treating chronic wounds with glucan hydrogels can help with reducing healing time and the total costs per treatment (for review see Majtan and Jesenak, 2018)
8. Leucopenia
The major side-effects of both traditional chemotherapy and/or irradiation are leucopenia (strong decrease of production of immunocytes) and resulting suppression of the immune system. These problems can be improved by glucan action (Turnbull et al., 1999). Glucan-induced recovery of bone marrow after irradiation is shown in Figure 4 (to be added), similar positive results were achieved also after chemotherapy and in the spleen and thymus.
9. Allergy
Allergic reactions represent several conditions caused by hyperactivity of the immune system. As one in three people suffers from allergy, the spreading epidemic of allergies and asthma led to increased interest in both research of the mechanisms and of the potential treatment. Allergic reactions include asthma, sinusitis, rhinoconjuctivitis, food allergy, atopic dermatitis, urticarial and anaphylaxis.
Several studies showed that administration of glucan during allergy season reduced the total number of allergy symptoms and rating of symptom severity, but had no effect on IgE concentration compared with placebo treatment in self-described ragweed allergy sufferers. Compared with pretreatment, glucan decreased study participants' ratings of tension, depression, anger, fatigue, and confusion and increased vigor. Glucan improved global mood states and had an improved effect on physical health, energy, and emotional well-being In addition, study participants reported a decrease in sleep problems, nasal and eye symptoms (Talbott et al., 2013).
Further studies suggest that glucan might be a promising candidate to be developed into a new anti-asthmatic agent. However, little attention has been focused on glucan in allergies. With only a limited number of observations, the exact mechanisms of glucan action in allergic diseases are difficult to interpret. Most published studies concur that based on the improved Th1/Th2 balance, glucan showed a potential for development as an adjunct to the standard treatment of in patients with allergies. Clearly, more studies, both animal and human, are necessary before we can reach a full conclusion (Sima and Vetvicka, 2017).
10. Other Effects
In addition to these most commonly recognized effects of glucan, several other biological effects exist. Recently, glucan has been shown to act a a strong adjuvant for numerous vaccines. In addition, glucan-collagen mixture is used for treatment of partial-thickness burns in children, where it increases tissue tolerance to ischemia and inhibits inflammation. A study of mice found that oral administration if glucan inhibited development of atopic dermatitis (Sugiyama et al., 2010), which supports the use of glucan in cosmetics, particularly when used as a part of a cream. Topical application of glucan resulted in 27% improvement of skin hydratation and 56% improvement in facial wrinkles. These effects of glucan on skin health are most probably the results of the interaction of glucan with Langerhans cells located within the epidermis. These cells are extremely sensitive to various environmental factors including polution and UV light. As we age, our skin is exposed to an excess of negative factors such as poor nutrition, smoking, UV light, or environmental toxins. All of which will compromise the protective ability of Langerhans cells. The glucan-induced protection is a combination
of direct activation of Langerhans cells and free radical scavenger capacity (Kougias et al., 2001).
When tested for treatment of periodontal disease, oral supplementation significantly reduced periodontal bone loss (Breivik et al., 2005). Glucan was also successfully used for treatment of various allergic problems including allergic asthma or seasonal allergic symptoms or atopic dermatitis lesions (for review see Jesenak et al., 2014).
The newest role of glucan in activation of the immune system is the role in socalled trained immunity. The concept of trained immunity has become one of the most interesting and potentially commercially and clinically relevant ideas of current immunology. Trained immunity is realized by epi-genetic reprogramming of nonimmunocompetent cells, primarily monocytes/macrophages and NK cells, and is less specific than adaptive immunity; therefore, it may cross-protect against other infectious agents. Trained immunity differs from classical immunological memory of adaptive immunity in several important respects. It is performed by a row of cellular populations differing from each other by their origin and effector functions.
Originally, evidence for the existence of trained immunity in vertebrates was obtained from experiments with mice, which were protected against lethal bacterial infection with Staphylococcus aureus by nonspecific substances, such as glucan The basic idea behind these findings was that certain challenges promoted heightened response of myeloid cells upon subsequent infection with the same (and, in some cases, different) pathogens. Most studies have focused on changes in cells upon primary activation via numerous modulators such as glucan. Few were trying to determine if clinical infection induced trained immunity in humans. Using a Plasmodium falciparum infection model, monocyte response showed biphasic
pattern low levels of inflammatory cytokines followed by strong increase of IL-6 and TNF-a secretion after 36 days of the original stimulus (Walk et al., 2020). But it is certain that some structures of β-glucan act as a PAMP and enhance innate immunity (Figure 4), particularly the trained immunity by induction of epigenetic reprogramming bone marrow hematopoietic cells, and their myelopoietic differentiation into effector cell populations, mainly monocytes and macrophages In addition, in vitro experiments have shown that stimulation of monocytes by either BCG or glucan resulted in elevated levels of the same cytokines, mainly IL-6, IL-10, and TNF. This capacity to change the cytokine production was found to be identical in cells isolated from neonates or adults.
Detailed analysis of monocyte-macrophage differentiation upon glucan addition found a new population of long-lived monocyte-derived macrophages, but no clear differences in their function, only some elevated activities (Leonhardt et al., 2018).

Figure 4
One of the most interesting studies focused on a theory that glucan-induced trained immunity can start antitumor activity. Prophylactic treatment with glucan caused lower tumor growth (which has been observed repeatedly in other studies), but adaptive transfer of trained neutrophils into naïve animals suppressed cancer growth again. Detail evaluation found transcriptomic and epigenetic rewiring of neutrophils and entire granulopoiesis towards an anticancer phenotype (Kalafati et al., 2020). If confirmed, these findings might open a new window into cancer treatment, as glucan is already being used as a supplement or as an anticancer drug (Isoda et al., 2009). Kalafati’s research might result in recommending using glucan as a prophylactic. Numerous studies have confirmed the anticancer effects of glucans as an immune stimulant (for review see Wu et al. 2021), but as a confirmed inducer of trained immunity, glucan supplementation has gained another meaning (Geller et al., 2019)
7. Glucan and Cancer
Cancer is the general name for a group of more than 100 diseases in which cells in a part of the body begin to grow out of control. Generally, cancer is a name for a group of diseases characterized by uncontrolled growth and spread of abnormal cells. It involves malfunction of genes controlling cell growth and proliferation. Cancer is a leading cause of death worldwide. Approximately 20% of all deaths in the United States are caused by cancer. All cancers involve the malfunction of genes that control cell growth, division, and differentiation. Cancer can be caused by many factors, both external and internal. Among external factors are radiation, smoking, various chemicals, and infection factors. Among internal factors are mutations, hormones, and
conditions of the immune system. These factors can act individually or in conjunction. It is important to remember that ten or more years pass between exposure to these factors and clinical manifestation of cancer.
Despite decades of intensive research, cancer is still one of the most dangerous diseases. In the United States alone, hundreds of thousands of people die every year from various types of cancer. Despite great achievements and decades of intensive, labor-consuming and expensive research, the incidence of various tumors and cancers is still increasing at an alarming rate. Based on the National Cancer Institute estimates, slightly less than one-in-two men and little more than one-in-three women in the U.S. are likely to contract cancer in their lifetime. The reality is that anyone can develop cancer. The risk of being diagnosed with cancer significantly increases with age. Cancer is the second most common cause of death in the U.S. exceeded only by heart disease.
It has become clear that most cancers have external causes and, in principle, should be preventable. Studies of the association between diet and cancer have demonstrated that the differences in the rates at which various cancers occur in different human populations are often correlated with differences in diet. There is accumulating evidence to suggest that the composition of the diet has great impact on our immune system. Therefore, changing dietary compositions as a tool to improve the immune function is a current research focus. Glucans, as present in various foods, have been widely used as immunostimulating agents to promote immune responses.
Since the first direct scientific study forty years ago, the anti-tumor activity of glucan has been clearly demonstrated (Nakao et al., 1983). Since these pioneering studies, numerous animal and human trials have shown remarkable anti-tumor activity against a wide variety of different tumors including breast, lung, and gastrointestinal
cancer. Since the 1980s, two types of glucan have been successfully used as traditional medicine for cancer therapy in Japan and China. In Japan, glucan is already licensed as a drug effective in cancer treatment. In addition, at least 76 clinical trials are currently under way in the United States as well as in several European countries such as Turkey and France.
Based on the multiple biological effects of glucan, it is not surprising that this natural immunomodulator is also heavily involved in the fight against cancer. Despite the fact that most tumors are recognized by the immune system, the response is usually not strong enough to kill a cancer growth. Even a completely healthy immune system cannot adequately deal with fast-growing cancer cells, so the immune system needs some help. Glucan is extremely important, as it is able to cooperate with antibodies. After the tumor cells have been recognized as foreign, specific antibodies are released by B lymphocytes and subsequently bind to the cancer cells. Following this binding of antibodies, the C3 fragment of complement coats the surface of cancer cells. The glucan-primed cells, such as macrophages and specifically natural killer (NK) cells and neutrophils, then recognize these antibody-C3 coated cells and kill them. Without glucan, the destruction would not take place and the situation would be compounded very quickly.
In case our body will not form enough anti-tumor antibodies, current medicine found a way around this problem. Monoclonal antibodies are currently being evaluated in an increasing number of cancer types. Although many patients respond to the antibody treatment, remissions are often transient. For example, more than 50% of lymphomas recurrent after rituximab treatment, failed to respond the second time. The reasons for this resistance to the antibody treatment are currently unknown but might include loss of antigen, pharmacokinetic variations among individual patients, or
resistance to complement activity. It is clear, however, that for a truly reliable antibody treatment, there is a strong need for a synergetic support. Numerous recent studies have shown that glucan is extremely active in cooperation with antibodies that naturally occur in case of cancer (Hong et al., 2003, 2004). We have to keep in mind that antibodies alone cannot make tumor cells disappear. However, following the binding of antibodies on the surface of cancer cells, C3 fragments of complement coat the cancer cells. The glucan-primed cells, such as blood neutrophils, macrophages, and natural killer cells, then specifically recognize these complement-antibody complexes and kill the tumor cells. Despite the fact that most tumors are recognized by the immune system, the antibody response is usually light and often is not strong enough to kill a cancer growth. Here comes glucan to the rescue: glucan-activated immunocytes recognize and kill cancer cells coated with antibodies. Without the glucan-caused activation of immunocytes, the cancer cells remain coated with antibodies but no killing occurs. It is important to note that similar effects can be also observed with neutrophils.
Since the preliminary experiments on animals were so promising, it is no wonder that these experiments are currently repeated in several clinical trials (among others, by researchers in The Memorial Sloan-Kettering Cancer Center and in the Brown Cancer Center in Louisville). Additional clinical trials focusing on soluble glucan Imprime PGGTM in combination with monoclonal antibody from ImClone System, ErbituxTM (CetuximabTM), and IrinotecanTM (chemotherapy drug from Pfizer) are currently under way. As the preliminary results are already exceeding all expectations, the manufacturer of Imprime PGGTM, has already started additional cancer clinical trials. Based on the latest information both current Phase II clinical lung cancer trials have achieved their stage one endpoints, demonstrating a significant level of efficacy
among the initial group of patients. Both trials are currently enrolling additional patients at 26 medical centers in the U.S. and Germany. These studies, which will enroll up to 90 patients each, further support the synergy of glucan and monoclonal antibody therapies. In addition, Biothera launched a Phase III clinical trial evaluating the combination therapy of Imprime PGGTM and ErbituxTM in colorectal cancer patients. This study will enroll up to 795 patients and will be conducted at 50 locations worldwide, including the U.S., France and Germany initially. However, cooperation with anti-tumor antibodies is not the only effects glucan have during cancer. There are multiple additional positive effects of glucan in tumor therapy. One is the direct stimulation of macrophages and NK cells. Macrophages serve as the first line of defense and protect our body against any type of invading cells including cancer cells. NK cells represent a special subtype of “bloodthirsty” lymphocytes and have an extremely important function to specifically recognize and kill tumor cells. Together, these cells form a defensive line that guards the integrity of our body.
Cancer patients observed additional benefits of glucan. A study by research teams from Germany and Turkey found that glucan induces proliferation and activation of monocytes in the peripheral blood of patients with advanced breast cancer (Demir et al., 2007). Similar findings were observed using yeast-derived glucan in prostate cancer patients (Magnani et al., 2010). These findings indicate that glucan not only helps bone marrow to overcome the negative effects of cancer, chemotherapy, and irradiation, but also increases the biological activities of these newly-formed immunocytes. An interesting approach used glucan to enhance the cytostatic activity of common cytostatic drugs cyclophosphamide, doxorubicin and actinomycin D. In all cases, glucan increased the effects of these chemotherapeutics (Badulescu et al.,
2009). Another study recently found that mushroom-derived glucan enhanced anticancer activity of bone marrow-derived dendritic cells, which might be used to enhance the immunotherapeutic effects of vaccine based on dendritic cells (Masuda et al., 2010). This glucan induced maturation of dendritic cells and antigen-specific response of T helper cells via activation of dendritic cells. When used in combination with lysate from dendritic cells, therapeutic and preventive effects have been observed. In additional study, glucan attenuated the ability of tumors to metastasize to lung tissue, probably by restraining macrophage polarization from M1 to M2 phenotype and by promotion of autophagic cell death by inhibiting Nur77 expression, AKT/mTOR signalling and inflammatory signals in breast tumor cells (Zhu et al., 2023).
Currently, most anti-tumor immunotherapies (except anti-tumor antibody therapy) are reserved for advanced patients that have failed conventional therapy. The challenges of antitumor immunotherapy lie in many aspects, including immune tolerance established by tumors. Although robust populations of immune effector cells can be generated ex vivo, clinical and pathologic complete responses remain rare in cancer patients treated with these modalities. Combined immunotherapy utilizing glucan and anti-tumor monoclonal antibodies is one means of breaking immune tolerance to tumors. The clinical usage of anti-tumor monoclonal antibodies continues to grow and will soon be incorporated into the standard of care for cancers of multiple organs. Increases in the number of these antibodies offer more opportunities to design versatile combinations with glucan therapy. This modality can also be used in synergy with most tumor vaccines as long as the antitumor humoral responses are elicited and the antibodies can bind to tumors resulting in complement activation. Combination of glucan and other chemotherapeutic agents have been assessed in several pre-clinical models such as chronic lymphocytic leukemia, lymphoma and lung and colorectal
cancer, where they show significant anti-tumor activities when compared to chemotherapy alone (Wilczak et al., 2024).
New insights into the mechanisms of how trained immunity could eradicate cancer was recently provided by Ding et al. (2023). The authors found that yeastderived glucan increased the responsiveness of lung interstitial macrophages to tumor-derived factors associated with the subsequent inhibition of tumor metastasis via enhanced cytotoxicity of cancer cells. The key pathway responsible for this phenomenon is sphingolipid-mitochondrial fission axis. Glucan-treated macrophages and monocytes exhibit a trained enhanced response upon direct contact with tumor cells. These data were confirmed using models of melanoma, lung and breast cancer. These findings represent a novel mechanism for trained immunity with direct implications for the treatment of primary and metastatic tumors. Newest data showed that glucan treatment restricted liver metastasis in pancreatic cancer (Thomas et al., 2022).
Food supplementation with glucan induced colonic antioxidant potential parameters, including an increase in total antioxidant status, a decrease in the superoxide dismutase activity and reduction in thiobarbituris acid release substance concentration. In addition, glucan supplementation decreased the levels of pro0inflammatory cytokines and C-reactive protein. Metabolomic studies confirmed the efficacy of glucan in early-stage colon cancer (Boulifa et al., 2024).
Another novel approach to treating cancer is the use of glucan-based vaccine, which showed increase of peripheral blood cell cytotoxicity against cancer, alleviation of chemotherapy side effects and restoration of balance of metabolic parameters (Ikewaki et al., 2023). A good example of manipulating the trafficking properties of glucan for the delivery of anti-cancer therapies was shown by Wang et al. (2018) in a
study where they conjugated a peptide antigen for a cancer biomarker to glucan in order to construct a vaccine. Only these conjugates elicited higher titers of antibodies. These studies suggested that by combining glucan with tumor antigens we may be able to stimulate components of both the innate and adaptive immune responses.
Readers keen on getting the newest data on glucan in cancer and the novel uses of this traditional therapeutic should read an excellent review written by Geller et al. (2019).
8. Practical applications
Here are examples of foods that are rich in glucans:
Oats and Oatmeal
Oats are one of the best sources of glucans. They contain a specific type known as beta-(1,3)-(1,4)-D-glucan. Eating oatmeal or adding oats to your diet can be a tasty way to incorporate glucans.
Barley
Barley is another cereal grain that contains glucans. Like oats, barley contains a type of glucan that has been associated with cholesterol-lowering effects.
Mushrooms
Certain mushrooms, such as shiitake and maitake, contain glucans. These can be included in various dishes, contributing not only to the flavor but also to the nutritional content.
Whole Grains
In addition to oats and barley, other whole grains like wheat, rye, and brown rice may contain varying amounts of glucans.
Seaweed
Some types of seaweed, such as laminarin from brown algae, contain glucans. Seaweed can be incorporated into salads, soups, or used as a wrap for sushi.
Baker's Yeast
Baker's yeast, often used in baking, is another source of glucans. It's used not only in bread but can also be found in certain dietary supplements.
Legumes
Certain legumes, including lentils and chickpeas, contain glucans. These can be included in soups, stews, salads, or as a side dish.
Nuts and Seeds
While not as high in bglucans as some of the previously mentioned sources, certain nuts and seeds, such as flaxseeds, chia seeds, and sunflower seeds, contain small amounts.
Vegetables
Some vegetables, like carrots and broccoli, also contain glucans. Including a variety of vegetables in your diet contributes to overall nutritional intake.
When incorporating glucan-rich foods into your diet, it's important to focus on a diverse and balanced eating plan. Consuming a variety of nutrient-rich foods ensures that you not only get glucans but also a wide range of essential vitamins, minerals, and other beneficial compounds. However, it is important to remember that despite the fact that numerous types of food contain glucan, our body has only very limited ability to absorb glucan directly from food. As glucan serves as improvements of
rigidity inside the cells, it is rather difficult to isolate it during the relatively short stay in our digestive tract. Therefore, supplement is much better choice.
Which Glucan Should We Choose?
Regardless of the country, there are dozens and most probably hundreds of different glucans available, differing in quality, source, purity, and price. All offer the best effects under the sun. Which one should we buy and which one can we safely ignore? Every reseller will stress the superiority of his product, but only few can offer proof of the biological effects of their product. Therefore, in order to make sure you will get the best product and not waste money on some inferior (or in the worst scenario completely untested) glucan, it is imperative to do your own research of available literature. Doing homework has its advantages, even in natural immunomodulators. The scientific literature (on glucan) running close to 50,000 papers, is the most extensive of all natural immunomodulators. In fact, there are more scientific papers on glucan than on all other natural immunomodulators combined.
In the past decades our research group focused on comparing many of the most successful commercially and easily available glucans on the market. We chose glucans widely sold and available around the globe, representing grain, mushroom and yeast-derived glucans in soluble and insoluble forms. The results of these comparisons were published in numerous peer-reviewed scientific journals (Vetvicka and Vetvickova, 2005, 2007, 2010, 2014 a,b,c, Vetvicka et al., 2008)
From these data and from careful comparison of other studies, it is evident that all glucans are not created equal. Yes, the Maserati and Ford Pinto are cars and both will get us to our destination eventually, but the level of comfort and pleasure will be substantially different. The same is true about glucans. The most important aspect is
to either manufacture or purchase glucan from a solid source and control the quantity, purity, and the biological activity. When you do not know where the glucan is manufactured, that might be the first red flag. In order to obtain the relevant information, it is imperative to get through the smoke and mirrors of unsupported or sometimes even pseudoscientific claims about the glucan offered by the companies There are some telltale signs that should immediately raise another red flag. One of them is a full database of glucan papers without any proof that the research was done using the beta glucan which the company is actually selling. It is common to find references about, let’s say, mushroom glucans, but if the company is selling yeastderived glucan, it is not really relevant, and it strongly suggests that there is no real research backing their claims. It is very easy to find dozens of completely legit papers describing the significant effect of glucan, but if the particular study is not done for the glucan of interest, it is not relevant.
9. References
Afiati, F., Firza, S.F., Lusmiati, L.S.A.: The effectiveness β-glucan of shiitake mushrooms and Saccharomyces cerevisiae as antidiabetic and antioxidant in mice Sprague Dawley induced alloxßan AIP Conf. Proc. 2120, 070006, 2019, Doi:10.1063/1.5115723
Anusuya, S., Sathiyabama, M.: Foliar application of b-D-glucan nanoparticles to control rhizome rot disease of turmeric. Int. J. Biol. Macromol., 72: 1205-1212, 2015. Doi:10.1016/j.ijbiomac.2014.10.043
Badulescu, M.-M., Apetrei, N. S., Lupu, A.-R., Cremer, L., Szegli, G., Moscovici, M., Mocanu, G., Mihai, D., Calugaru, A.: Curdlan derivatives able to enhance cytostatic
drugs activity on tumor cells. Rom. Arch. Microbiol. Immunol., 68: 201-206, 2009.
PMID:20583473
Bohn, J. A., BeMiller, J. N.: (1-3)-β-D-glucans as biological response modifiers: a review of structure-functional activity relatioships. Carbohydrate Polymers, 28: 3-14, 1995. Doi:10.1016/0144-8617(95)00076-3
Boulifa, A., Raftery, M.J., Franzen, A.S., Radecke, C., Stintzing, S., Blohmer, J.U., Pecher, G.: Role of beta-(1-3)(1-6)-D-glucan from yeast on natural killer (NK) cells and brest cancer cell lines in 2D and 3D cultures. BMC Cancer, 24:339, 2024, Doi: 10.1186/s12885-024-11979-3.
Breivik, T., Opstad, P. K., Engstad, R., Gundersen, G., Gjermo, P., Preus, H. L.: Soluble β-1,3/1,6-glucan from yeast inhibits experimental periodontal disease in Wistar rats. J. Clin. Periodont., 32: 347-362, 2005. PMID:15811050
Chakrabarty, S., Rajeswari, V.D.: Biomedical aspects of beta-glucan on glucose metabolism and its role on primary gene PIK3R1. J Functional Food, 99, 2022, Doi: 10.1016/j.jff.2022.105296.
Cloetens, L., Ulmius, M., Johansson-Persson, A., Akesson, B., Onning, G.: Role of dietary beta-glucans in the prevention of the metabolic syndrome. Nutrition Rev., 70: 444-458, 2012. Doi:10.1111/j.1753-4887.2012.00494.x.
Dedeepiya, V.D., Sivaraman, G., Venkatesh, A.P., Preethy, S., Abraham, S.J.K.: Potential effects of Nichi glucan as a food supplement for diabetes mellitus and hyperlipidemia: Preliminary findings from the study on three patients from India. Case rep., 2012, Doi:10.1155/2012/895370.
Demir, G., Klein, H. O., Mandel-Molinas, N., Tuzuner, N.: Beta glucan induces proliferation and activation of monocytes in peripheral blood of patients with advanced breast cancer. Internal. Immunopharm., 7: 112-116, 2007. PMID:17161824
Ding, C., Shrestha, R., Zhu, X., Geller, A.E., Wu, S., Woeste, M.R., Li, W., Wang, H., Yuan, F., Xu, R., Chariker, J.H., Hu, X., Li, H., Tieri, D., Zhang, H.G., Rouchka, E.C., Mitchell, R., Siskind, L.J., Zhang, X., Xu, X.G., McMasterm K.M., Yu, Y., Yan, J.: Inducing trained immunity in pro-metastatic acrohages to control tumor metastasis. Nat. Immunol., 24: 239-254, 2023, Doi:10.1038/s41690-022-01388-8.
Geller, A., Shrestha, R., Yan, J.: Yeast-derived beta-glucan in cancer: Novel uses of a traditional therapeutic. Int J Mol Sci., 20, 2019, Doi:10.3390/ijms20153618.
Geller, A., Shrestha, R., Yan, J.: Yeast-derived b-glucan in cancer: Novel uses of a traditional therapeutic. Int. J. Mol. Sci., 20:3618, 2019, Doi:10.3390/ijms20153618
Giuntini, E.B., Hoffmann Sarda, F.A., de Menezes, E.W.: The effects of soluble dietary fibers on glycemic response: An overview and futures perspectives. Foods, 11, 2022, Doi:10.3390/foods11233934.
Gulcelik, M. A., Dincer, H., Sahin, D., Demir, O. F., Yenidogan, E., Alagol, H.: Glucan improves impaired wound healing in diabetic rats. Wounds – A Compendium Clin. Res. Pract., 22: 12-16, 2010, PMID: 25901457
Harada, T., Ohno, N.: Contribution of dectin-1 and granulocyte macrophagecolony stimulating factor (GM-CSF) to immunomodulating action of β-glucan. Int. Immunopathol., 8: 556-566, 2008. PMID:18328447
Hong, F., Hansen, R. D., Yan, J., Allendorf, D. J., Baran, J. T., Ostroff, G. R., Ross, G. D.: β-Glucan functions as an adjuvant for monoclonal antibody immunotherapy by recruiting tumoricidal granulocytes as killer cells. Cancer Res., 63: 9023-9031, 2003. PMID: 14695221
Hong, F., Yan, J., Baran, J. T., Allendorf, D. J., Hansen, R. D., Ostroff, G. R., Xing, P. X., Cheung, N. K., Ross, G. D.: Mechanism by which orally administered beta-
glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J. Immunol., 173: 797-806, 2004. Doi:10.4049/ jimmunol.173.2.797
Ikewaki, N., Devaprasad, V., Kadalraja, D., Kosagi-Sharaf, R., Vaddi, S., Osawa, H., Kisaka, T., Kurosawa, G., Srinivasan, S., Ramasamy, S., Kumar, B.,
Senthilkumar, R., Iwasaki, M., Preethy, S., Abraham, S.J.K.: b-Glucan vaccine adjuvant approach for cancer treatment through immune enhancement (B-VACCIEN) in specific immunocompromised populations (Review). Oncology Rep., 47, 2022, Doi:10.3892/or.2021.8225.
Innate immune training of granulopoiesis promotes anti-tumor activity. Cell, 183: 771785 e712, 2020, Doi:10.1016/j.cell.2020.09.058.
Isoda, N., Eguchi, Y., Nukaya, H., Hosho, K., Suga, Y., Suga, T., Nakazawa, S., Sugano, K.: Clinical efficacy of superfine dispersed lentinan (beta-1,3-glucan) in patients with hepatocellular carcinoma. Hepatogastroenterology, 56: 437-441, 2009, PMID: 19579616
Jesenak, M., Bonovcin, P., Rennerova, Z., Majtan, J.: b-Glucan in the treatment and prevention of allergic diseases. Allergol. Immunopathol., 42:149-156, 2014, Doi:10.1016/j.aller.2012.08.008.
Kalafati, L., Kourtzelis, I., Schulte-Schrepping, J., Li, X., Hatzioannou, A., Grinenko, T., Hagag, E., Sinha, A., Has, C., Dietz, S., de Jesus Domingues, A.M., Nati, M , Sormendi, S , Neuwirth, A , Chatzigeorgiou, A , Ziogas, A , Lesche, M , Dahl, A , Henry, I , Subramanian, P , Wielockx, B , Murray, P , Mirtschink, P , Chung, K J , Schultze, J L , Netea, M G , Hajishengallis, G , Verginis, P , Mitroulis, I , Chavakis, T.; Karamuthil-Melethil, S., Gudi, R., Johnson, B. M., Perez, N., Vasu, C.: Innate immune training of granulopoiesis promotes anti-tumor activity. Cell, 183: 771-785 e712, 2020, Doi:10.1016/j.cell.2020.09.058.
Karamuthil-Melethil, S., Gudi, R., Johnson, B. M., Perez, N., Vasu, C.: Fungal b-glucan, a Dectin-a ligand, promotes protection from Type 1 diabetes by inducting regulatory innate immune response. J. Immunol., 193: 3308-3321, 2014. Doi:10.4049/jimmunol.1400186.
Kida K., Inoue T., Kaino, Y., Ito, T., Matsuda, H., Elliott, R.B.: An immunopotentiator of b-1,6;1,3 D-glucan prevents diabetes and insulitis in BB rats.
Diabetes Res. Clin. Practice, 17: 75-79, 1992, Doi: 10.1016/0168-8227(92)90152-H
Kida, K., Inoue, T., Kaino, Y., Goto, Y., Ikeuchi, M., Ito, T., Matsuda, H., Elliott, R.B.: An immunopotentiator of β-1,6;1,3 D-glucan prevents diabetes and insulitis in BB rats. Diabetes Res. Clin. Pract., 17:75-79, 1992, Doi:10.1016/01688227(92)90152-H
Kogan, G.: (1-3,1-6)-β-D-glucans of yeast and fungi and their biological activity.
In: Studies in Natural Products Chemistry, Vol. 23, Ed. Atta-ur-Rahman, Elsevier, Amsterdam, p. 107, 2000.
Kouigias, P., Wei, D., Rice, P. J., Ensley, H. E., Kalbfleisch, J., Williams, D. L, Browder, I. W.: Normal human fibroblasts express pattern recognition receptors for fungal (1-3)-β-D-glucans. Inf. Immun., 69: 3933-3938, 2001, Doi: 10.1128/IAI.69.6.3933-3938.2001.
Leonhardt, J., Grosse, S., Marx, C., Siwczak, F., Stengel, S., Bruns, T., Bauer, R., Kiehntopf, M., Williams, D.L., Wang, Z.Q., Mosig, A S , Weis, S , Bauer, M , Heller, R.: Candida albicans beta-glucan differentiates human monocytes into a specific subset of macrophages. Front Immunol., 9, 2818, 2018, Doi:10.3389/fimmu.2018.02818.
Lobato, R.V., De Oliveira Silva, V., Andrade, E.F., Orlando, D.R., Zangeronimo, M.G., de Sousa, R.V., Pereira, L.J.: Metabolic effects of b-glucans (Saccharonyces
cerevisiae) per os adminstration in rats with streptozotocin-induced diabetes. Nutr. Hosp., 32:256-264, 2015, Doi: 10.3305/nh.2015.32.1.9013.
Lumaga, R. B., Azzali, D., Fogliano, V., Scalfi, L., Vitaglione, P.: Sugar and dietary fibre composition influence, by different hormonal response, the satiating capacity of a fruit-based and a beta-glucan-enriched beverage. Food Funct., 3: 67-75, 2012. PMID: 22057424
Magnani, M., Castro-Gomez, R. H., Aoki, M. N., Gregorio, E. P., Libos, F., Watanabe, M. A. E.: Effects of carboxymethyl-glucan from Saccharomyces cerevisiae on the peripheral blood cells of patients with advanced prostate cancer. Exp. Therap. Med., 1: 859-862, 2010.
Majtan, J., Jesenak, M.: b-Glucans: Multi-functional modulator of wound healing. Molecules, 2018, Doi:10.3390/molecules23040806.
Malunga, L.N., Ames, N., Zhouyao, H., Blewett, H., Thndapilly, S.J.: Betaglucan from barley attenuates post-prandial glycemic response by inhibiting the activities of glucose transporters but not intestinal brush border enzymes and amylolysis of starch. Front. Nutr., 8, 2021, Doi:10.3389/fnut.2021.62857.
Masuda, Y., Ito, K., Konishi, M., Nanba, H.: A polysaccharide extracted from Grifola frondosa enhances the anti-tumor activity of bone marrow-derived dendritic cell-based immunotherapy against murine colon cells. Cancer Immun. Immunother., 59: 1531-1541, 2010. Doi:10.1007/s00262-010-0880-7
Nakao, I., Uchino, H., Kaido, I., Kimuira, T., Goto, T., Kondo, T., Takino, T., Taguchi, T., Nakajima, T., Fujimoto, S., Miyazaki, T., Miyoshi, A., Yachi, A., Yoshida, K., Ogawa, N., Furue, H.: Clinical evaluation of schizophyllan (SGP) in advanced gastric cancer – a randomized comparative study by an envelop method. Jpn. J. Cancer Chemotherap., 10: 1146-1159, 1983. PMID:6223584
No, H., Kim, J., Seo, C.R., Lee, D.E., Kim, J.H., Kuge, T., Mori, T., Kumoto, H., Kim, J.K.: Anti-inflammatory effects of b -1,3-1,6-glucan derived from black yeast Aureobasidium pullulans in RAW264.7 cells. Int. J. Biol. Macromol., 193:592-600, 2021, Doi:10.1016/j.ijbiomac.2021.10.065.
Rozbulut, R., Sanlier, N., Doger, E., Bideci, A., Camurdan, O., Cinaz, P.: The effect of beta-glucan supplementation on glycemic control and variability in adolescents with type 1 diebetes mellitus. Diabetes Res. Clin. Practice 169:2020, Doi: 10.1016/j.diabres.2020.108464.
Sanchez-Golzales, D. J., Sosa-Luna, C. A., Vasquez-Montezuma, O.: Transfer factors in medical therapy. Med. Clin., 137: 273-277, 2011. Doi: 10.1016/j.medcli.2010.05.002.
Shen, X.L., Zhao, T., Zhou, T., Shi, X., Zou, Y., Zhao, G.: Effect of oat β-glucan intake on glycaemic control and insulin sensitivity of diabetic patients: A meta-analysis of randomized controlled trials. Nutrients, 8, 2016, Doi:10.3390/nu8010039
Sima, P., Vannucci, L., Vetvicka, V.: b -Glucans and cholesterol (Review). Int. J. Mol. Biol., 41:1799-1808,2018, Doi:10.3892/ijmm.2018.3411.
Sima, P., Vetvicka, V.: b-Glucan in allergies. Am. J. Immunol., 13: 73-80, 2017, Doi:10.3844/ajisp.2017.73.80.
Sugiyama, A., Hata, S., Suzuki, K., Yoshida, E., Nakano, R., Mitra, S., Arashida, R., Asayama, Y., Yabuta, Y., Takeuchi, T.: Oral administration of paramylon, a beta-1,3,-D-glucan isolated from Euglena gracilis Z inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. J. Vet. Med. Sci., 72: 755-763, 2010. PMID:20160419.
Talbott, S.M., Talbott, J.A., Talbott, T.L., Dingler, E.: b-Glucan supplementation, allergy symptoms, and quality of life in self-described ragweed allergy sufferers. Food Sci. Nutr., 1:90-101, 2013, PMID:24804018.
Thomas, S.K., Choi-Bose, S., Coho, H., Casella, C., Stone, M.L., Wattenberg, R.M., Beatty, G.L.: Beta-Glucan treatment restricts liver metastasis in pancreatic cancer. Cancer Res., 82:5633, 2022, Doi:10.1158/1538-7445.AM2022-5633.
Turnbull, J. L., Patchen, M.L., Scadden, D.T.: The polysaccharide, PCGglucan, enhances human myelopoiesis by direct action independent of and additive to early-acting cytokines. Acta Haematol., 103:66-71, 1999, PMID:10529508.
Vetvicka V., Vetvickova, J.: Immunostimulating properties of two different βglucans isolated from Maitake mushroom (Grifola frondosa). J. American Nutr. Assoc., 8: 33-39, 2005.
Vetvicka V., Vetvickova, J.: Physiological effects of different types of β-glucan.
Biomed. Pap. Med. Fac. Univ. Palacky, 151: 225-231, 2007 b, Doi: 10.5507/bp.2007.038
Vetvicka V., Vetvickova, J.: Physiological effects of different types of β-glucan.
Biomed. Pap. Med. Fac. Univ. Palacky, 151: 225-231, 2007 b. PMID:18345255.
Vetvicka, V., Sima, P.: β-Glucan in invertebrates. Invertebrate Survival J., 1: 60-65, 2004.
Vetvicka, V., Vashishta, A., Saraswat-Ohri, S., Vetvickova, J.: Immunological effects of yeast- and mushroom-derived β-glucans. J. Med. Food, 11:615-622, 2008, DoI:10.1089/jmf.2007.0588.
Vetvicka, V., Vetvickova, J.: A comparison of injected and orally administered beta glucans. J. American Nutr. Assoc., 11:42-48, 2008.
Vetvicka, V., Vetvickova, J.: An evaluation of the immunological activities of commercially available β1,3-glucans. J. American Nutr. Assoc., 10: 25-31, 2007
Vetvicka, V., Vetvickova, J.: Anti-stress action of an orally-given combination of resveratrol, b-glucan, and vitamin C. Molecules, 19: 13724-13734, 2014 b, Doi:10.3390/molecules190913724.
Vetvicka, V., Vetvickova, J.: Anti-stress action of an orally-given combination of resveratrol, b-glucan, and vitamin C. Molecules, 19: 13724-13734, 2014 c. Doi:10.3390/molecules190913724.
Vetvicka, V., Vetvickova, J.: Comparison of immunological effects of commercially available b-glucans. Applied Sci. Rep., 1: 2014 a. Doi:10.7243/20549903-1-2
Vetvicka, V., Vetvickova, J.: b1,3-glucan: Silver buller or hot air? Open Glycoscience, 3: 1-6, 2010, Doi: http://dx.doi.org/10.2174/1875398101003010001
Walk, J., Keramati, F., de Bree, L.C.J., Arts, R.J.W., Blok, B., Netea, M.G., Stunnenberg, H.G., Sauerwein, R.W.: Controlled human malaria infection induces long-term functional changes in monocytes. Front. Mol. Biosci., 7, 604553, 2020, Doi:10.3389/fmolb.2020.604553.
Wang, H., Yang, B., Wang, Y., Liu, F., Fernandez-Tejada, A., Dong, S.: betaGlucan as an immune activator and a carrier in the construction of a synthetic MUC1 vaccine. Chem. Commun., 55: 253–256, 2018, Doi: 10.1039/C8CC07691J.
Wilczak, J., Prostek, A., Dziendzikowska, K., Gajewska. A., Kopiasz, L., Harasym, J., Oczkowski, M., Gromadzka-Ostrowska, J.: Ota beta-glucan as metabolic regulator in early stage of colorectal cancer – A model study on azoxymethane-treated rats. Int. J. Mol. Sci., 25:4635, 2024, Doi:10.3390/ijms25094635.
Wu, L., Zhao, J., Zhang, X., Liu, S., Zhao, C.: Antitumor effect of soluble betaglucan as an immune stimulant. Int J Biol Macromol., 179: 116-124, 2021, Doi:10.1016/j.ijbiomac.2021.02.207.
Zavorkova, M., Vetvicka, V., Richter, J., Kral, V., Liehnova, I., Dobiasova
Rajnohova, L.: Effects of glucan and vitamin D supplementation on obesity and ipid metabolism in diabetic retinopathy. Open Biochem. J., 12:30-45, 2018, Doi:10.2174/1874091X01812010036.
Zhu, F., Zhang, Q., Feng, J., Zhang, X., Li, T., Liu, S., Chen, Y., Li, X., Wu, Q., Xue, Y., Alitongbieke, G., Pan, Y.: b-Glucan produced by Lentinus edodes suppresses breast cancer progression via the inhibition of macrophage M2 polarization by integrating autophagy and inflammatory signals. Immune Inflamm. Dis., 11: e876, 2024, PMID: 37249285.
Zurbau, A., Noronha, J.C., Khan, T.A., Sievenpiper, J.L., Wolever, T.M.A.: The effect of oat b-glucan on postprandial blood glucose and insulin responses: a systematic review and meta-analysis. Eur. J. Clin. Nutr., 75:1540-1554, 2021, Doi: 10.1038/s41430-021-00875-9
