

































for use with Ruhof’s Prepzyme® Forever Wet Enzymatic Pre-Cleaner
The cleaning process begins at the point of use. When soils dry, they become harder to remove and increase the risk of corrosion, biofilm formation, and HAI’s. The Forever Wet Auto Sprayer delivers a consistent application of Prepzyme® Forever Wet in less than half the time—keeping instruments moist for up to 72 hours.

















JANUARY 2026
Sourcing & Logistics
8 > Stronger Supply Chains, Better Outcomes
DANIEL BEAIRD
Infection Prevention
12 > A Coalition for the Next Crisis
MATT MACKENZIE
Special Report
16 > Beyond the Back Room
MARK DURO
Sterile Processing
20 > Beyond Tray Counts: Building a Smarter, Measurable Future for Sterile Processing
KARA NADEAU
28 > Understanding Bowie-Dick Testing: Purpose and Usage in Daily Sterilization Practice
KAYLA OSTRANDER
32 > Wet Packs
ADAM OKADA

Departments

BY DANIEL BEAIRD
As we welcome 2026, we enter a moment of reflection and renewed purpose for healthcare systems across the country. This issue begins that conversation by examining the challenges ahead and highlighting areas where meaningful progress is already taking shape across public health, supply chain operations, and sterile processing.
Associate Editor Matt MacKenzie highlighted a change in public health initiatives in his feature article as these institutions continue to face turbulence. The Governors Public Health Alliance is a group of 15 state governors that has been formed to share data, communicate about emerging health threats, strengthen emergency preparedness, and align public health policies across their states. State coalitions are not new, but this alliance aims to advance coordinated, evidence-based public health strategies.
Just as states are recognizing the value of shared information and coordinated action, health systems are facing a similar imperative within their own operations as HPN discovered through a recent webinar series on strengthening supply chains.
Greater visibility into the healthcare supply chain post pandemic has helped systems mature, but it also brings new risks. Not every

fluctuation signals trouble, and quick reactions can sometimes create instability instead of preventing it. The next stage of resilience requires tools and strategies that help leaders interpret complexity, anticipate problems, and act with purpose.
This broader challenge of managing complexity is also evident much closer to the point of care. For years, sterile processing professionals have worked under increasing pressure. They are asked to speed up instrument turnaround, support growing surgical volumes, manage complex devices, and help protect patients from infection. Yet they often operate without standardized national metrics that would allow them to understand performance, measure quality, or justify the resources they need.
As Senior Contributing Editor Kara Nadeau found out, many SPD leaders must advocate for staffing, equipment, and technology without the data that other clinical departments rely on. Although healthcare is highly data driven, sterile processing continues to function with limited visibility into workloads, bottlenecks, or the true cost of reprocessing.
Across public health, supply chain operations, and sterile processing, a common need stands out. Healthcare requires transparency, stability, and clear information that supports confident decision making.
January 2026, Vol. 50, No. 1
Group Content Director Healthcare
Jennifer Breedlove jbreedlove@endeavorb2b.com
Editor-in-Chief
Daniel Beaird dbeaird@endeavorb2b.com
Associate Editor
Matt MacKenzie mmackenzie@endeavorb2b.com
Senior Contributing Editor Kara Nadeau knadeau@hpnonline.com
ADVERTISING SALES
Director of Sales Healthcare
Jennifer Hazen jhazen@endeavorb2b.com | 330-598-0308
East & West Coast
Kristen Hoffman khoffman@endeavorb2b.com | 603-891-9122
Midwest & Central
Deborah Baron dbaron@endeavorb2b.com | 917-763-7275
Advertising & Art Production
Production Manager | Ed Bartlett
Art Director | Kelli Mylchreest
Advertising Services
Karen Runion | krunion@endeavorb2b.com
Audience Development
Laura Moulton | lmoulton@endeavorb2b.com
Endeavor Business Media, LLC
CEO Chris Ferrell
COO Patrick Rains
CDO Jacquie Niemiec
CALO Tracy Kane
CMO Amanda Landsaw
EVP Infrastructure & Public Sector Group
Kylie Hirko
VP of Content Strategy, Infrastructure & Public Sector Group
Michelle Kopier
Healthcare Purchasing News USPS Permit 362710, ISSN 1098-3716 print, ISSN 2771-6716 online is published 7 times annually Feb/Mar, Apr/May, Jun/ Jul, Aug/Sep, Oct, Nov/Dec, Nov/Dec IBG, by Endeavor Business Media, LLC. 201 N Main St 5th Floor, Fort Atkinson, WI 53538. Periodicals postage paid at Fort Atkinson, WI, and additional mailing offices. POSTMASTER: Send address changes to Healthcare Purchasing News, PO Box 3257, Northbrook, IL 600653257. SUBSCRIPTIONS: Publisher reserves the right to reject non-qualified subscriptions. Subscription prices: U.S. $164.80 per year; Canada/Mexico $199.56 per year; All other countries $284.54 per year. All subscriptions are payable in U.S. funds. Send subscription inquiries to Healthcare Purchasing News, PO Box 3257, Northbrook, IL 60065-3257. Customer service can be reached toll-free at 877-382-9187 or at HPN@omeda.com for magazine subscription assistance or questions.
Printed in the USA. Copyright 2026 Endeavor Business Media, LLC. All rights reserved. No part of this publication should be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopies, recordings, or any information storage or retrieval system without permission from the publisher. Endeavor Business Media, LLC does not assume and hereby disclaims any liability to any person or company for any loss or damage caused by errors or omissions in the material herein, regardless of whether such errors result from negligence, accident, or any other cause whatsoever. The views and opinions in the articles herein are not to be taken as official expressions of the publishers, unless so stated. The publishers do not warrant either expressly or by implication, the factual accuracy of the articles herein, nor do they so warrant any views or opinions by the authors of said articles.
Jimmy Chung, MD, MBA, FACS, FABQAURP, CMRP, Chief Medical Officer, Advantus Health Partners and Bon Secours Mercy Health, Cincinnati, OH
Joe Colonna, Chief Supply Chain and Project Management Officer, Piedmont Healthcare, Atlanta, GA; Karen Conway, Vice President, Healthcare Value, GHX, Louisville, CO
Dee Donatelli, RN, BSN, MBA, Senior Director Spend symplr and Principal Dee Donatelli Consulting LLC, Austin, TX
J. Hudson Garrett Jr., PhD, FNAP, FSHEA, FIDSA, Adjunct Assistant Professor of Medicine, Infectious Diseases, University of Louisville School of Medicine
Melanie Miller, RN, CVAHP, CNOR, CSPDM, Value Analysis Consultant, Healthcare Value Management Experts Inc. (HVME) Los Angeles, CA
Dennis Orthman, Consulting, Braintree, MA
Janet Pate, Nurse Consultant and Educator, Ruhof Corp.
Richard Perrin, CEO, Active Innovations LLC, Annapolis, MD
Jean Sargent, CMRP, FAHRMM, FCS, Principal, Sargent Healthcare Strategies, Port Charlotte, FL
Richard W. Schule, MBA, BS, FAST, CST, FCS, CRCST, CHMMC, CIS, CHL, AGTS, Senior Director Enterprise Reprocessing, Cleveland Clinic, Cleveland, OH
Barbara Strain, MA, CVAHP, Principal, Barbara Strain Consulting LLC, Charlottesville, VA
Deborah Petretich Templeton, RPh, MHA,Chief Administrative Officer (Ret.), System Support Services, Geisinger Health, Danville, PA
Ray Taurasi, Principal, Healthcare CS Solutions, Washington, DC

Benchmark Report Identifies Fundamental Risks in Sterile Processing Operations
The report, published by Aesculap, Inc. and Ascendco Health, found that 76% of those leaders cite human error as the biggest challenge in tracking surgical instruments. Another 58% reported surgery delays caused by instrument sets that were missing, incomplete, or not ready on time.
Read on: hpnonline.com/55330330
SteriPro and Getinge Partner to Expand Off-Site Sterile Processing Capacity
SteriPro has expertise in “operating large-scale off-site reprocessing centers” and Getinge has established “proven leadership in sterile processing equipment, automation, and digital workflow solutions.” The companies will work to provide hospitals with an “end-to-end pathway for optimizing sterile processing—from designing and operating high-performance offsite centers to integrating technology that supports full visibility, traceability, and efficiency across the instrument lifecycle.”
Read on: hpnonline.com/55333037
UPS Expands Healthcare Logistics Footprint with $1.6B Acquisition of AHG
UPS has finalized its $1.6 billion acquisition of Canada-based Andlauer Healthcare Group (AHG), a leader in cold chain transportation and healthcare logistics. The move expands UPS’s global reach in managing complex, temperature-sensitive supply chains for pharmaceuticals, medical devices, and clinical trial materials.
Read on: hpnonline.com/55329745
CDC Website Changes Statement to Not Rule Out Link Between Infant Vaccines and Autism
Until yesterday, the CDC webpage stated that “studies have shown that there is no link between receiving vaccines and developing autism spectrum disorder (ASD). No links have been found between any vaccine ingredients and ASD.” Experts are characterizing the change to the new message as a weaponization of the agency to “promote [HHS Secretary] RFK Jr.’s anti-vaccine point of view.”
Read on: hpnonline.com/55332072
































































BY DANIEL BEAIRD











Editor’s Note: In a recent Healthcare Purchasing News webinar series, John Wright, chief operating officer of Advantus Health Partners, offered a look at what has changed, what remains vulnerable, and what must still evolve for healthcare supply chains.
Tom Redding, executive vice president of The St. Onge Company, focused on a changing workforce around talent pipelines, leadership evolution, and technological skills.




Wprocess complexity, anticipate risk, and make smarter, not just quicker, decisions.

hen the world shut down in 2020, healthcare supply chains were thrust into a spotlight few anticipated. Nearly six years later, the ripple effects continue to reshape policy, technology, labor, and the architecture of how medical products move from manufacturing sites to patient bedsides.

Modern supply chains are drowning in data, including weather shifts, factory shutdowns, shipping bottlenecks, labor strikes, and countless other variables that move simultaneously. No human team can keep pace. That’s why AI has become a critical force multiplier.









The disruptions of Covid were not a one-off crisis. They were a wake-up call. The pandemic may have receded, but the instability it exposed is still very much alive. For years, supply chains were built around efficiency at all costs. Few stopped to ask where raw materials came from or how many international touchpoints stood between a product and a patient.



Wright emphasized that AI excels at surfacing weak signals, modeling risk, and issuing early warnings, but it is far from a crystal ball.



“We’ve become a more mature supply chain since Covid,” said John Wright, chief operating officer of Advantus Health Partners. “We’ve gained visibility into sourcing, materials, and upstream risks we didn’t know existed.”






“AI is only as useful as the structure you build around it,” he said. “Blind reliance just means making bad decisions faster.”





As labor shortages continue to reshape healthcare, systems are turning to automation to keep operations moving. Autonomous cleaning robots, automated delivery systems, intelligent inventory platforms, and robotic process automation are now essential tools, not optional enhancements. At the same time, supply chain teams are taking on stronger clinical partnerships, helping eliminate product variation and supporting clinicians who are stretched thinner than ever.




Today, health systems scrutinize manufacturing footprints, evaluate country-of-origin exposure, and dig deeper into the vulnerabilities buried within their suppliers’ suppliers. But increased visibility brings its own hazards.
“Not every blip is a crisis,” Wright cautioned. “If we overreact to signals, we drive the crisis ourselves. Excess demand can become its own crisis.”
The urge to react quickly to early warning signs can push organizations into overcorrection, creating the very instability they hope to avoid. This challenge of overreaction highlights a broader reality: in a world where disruptions can arise from countless sources, simply reacting faster isn’t enough. Organizations need tools that help them



While technology and automation are helping fill immediate gaps, they are only part of the solution. Sustaining a resilient healthcare supply chain also requires a workforce equipped with the skills, expertise, and strategic insight to navigate an increasingly complex and fast-changing environment.




Today’s supply chain complexity has far outpaced traditional staffing models. As veteran leaders retire and roles expand, attracting and retaining skilled professionals has become a growing struggle. Supply chain teams now drive enterprise strategy, analytics, logistics, and long-term planning, not just purchasing and distribution.


Do I have the right people? Do I have the right skills?


These are questions that now dominate executive agendas, according to Tom Redding, executive vice president

Editor’s Note: Dr. Jimmy Chung, chief medical officer of Advantus Health Partners, and Omar Devlin, executive director of supply chain planning for Stanford Medicine, joined the webinar series to discuss the patient-centered digital supply chain.
Framing patients as “customers” fundamentally reshapes healthcare, placing their safety, outcomes, and overall experience at the center of every decision.
“Patients have the right to be consumers,” emphasized Dr. Jimmy Chung, chief medical officer of Advantus Health Partners. “By prioritizing what ma ers most to them, like quality, reliability, and safety, we can make supply chain decisions that genuinely drive be er outcomes.”
Standardization has proven to be a powerful strategy. Limiting variability in procedures and products across clinicians not only cuts costs but also boosts reliability, reduces errors, and streamlines inventory management.
Equipped with real-time visibility into inventory, replenishment cycles, and alternative products, clinical teams can act decisively, ensuring optimal care.
Omar Devlin, executive director of supply chain planning for Stanford Medicine, highlighted the stakes with a personal example: a nurse struggling to locate a specific bandage.
“Frontline staff need clear visibility of what’s available, where it is, and when replenishment is coming,” he said. “Making that information transparent transforms the patient’s experience.”
• Contract Management: AI scans contracts for compliance with pricing, rebates, and obligations, maximizing value while dramatically cu ing manual workload.
Dr. Chung highlighted the clinical impact of AI through procedure analytics. By analyzing data across vendors and procedures, AI can identify standardized products or vendors that improve patient outcomes, reduce variability, and lower costs.
Predictive analytics is also reshaping supply chain strategy by enabling organizations to anticipate disruptions before they occur. Devlin shared Stanford Medicine’s experience using predictive tools to simulate scenarios, evaluate second-source suppliers, and optimize inventory levels.


This example underscores the vital role transparency and visibility play in the daily operations of healthcare supply chains. Building on that foundation, advanced technologies, especially AI and automation, are empowering teams to make faster, smarter decisions that have a direct, measurable impact on patient care.
AI, automation, and predictive analytics are transforming healthcare supply chain operations, driving efficiency, accuracy, and be er patient outcomes through:
• Standardization: AI harmonizes item descriptions and product data across multiple systems, reducing errors and ensuring consistency throughout the supply chain.
• Decision Support: AI-powered analytics provide actionable recommendations, ranging from vendor consolidation and pricing optimization to real-time inventory adjustments, based on clinical and operational data.
“Even supply shortages for critical items, like IV fluids, can now be modeled in advance,” Devlin explained. “This lets us proactively mitigate risks and maintain continuity of care.”
Dr. Chung added that predictive analytics extends beyond supplies. By forecasting patient admissions and procedure volumes, hospitals can allocate resources and staff more effectively, ensuring the right care is delivered at the right time.
Devlin envisions a future where AI and automation seamlessly integrate EHR, ERP, and other systems, eliminating tedious manual work and empowering smarter, faster decision-making. Dr. Chung echoed this vision, asserting that AI will enable clinicians and operational leaders to make decisions for patients as effortlessly as we use smartphones to snap a photo.
Digital transformation in healthcare supply chains is no longer optional, but it is essential. By combining transparency, AI, predictive analytics, and a patient-centered approach, hospitals and health systems can deliver care that is safer, more efficient, and higher in quality, all while controlling costs.
Healthcare supply chains are evolving from logistical backbones into strategic, patient-focused engines. As Devlin and Dr. Chung emphasized, leveraging digital tools and AI is critical to building supply chains that are smarter, faster, and more resilient, and ensure patient care is always safe, reliable, and efficient.
For healthcare leaders steering this transformation, the directive is clear: put patients at the center, embrace technology boldly, and standardize wherever possible. The future of healthcare supply chains is digital, and patients are driving the agenda.
of St. Onge, a world-recognized supply chain strategy and logistics consulting firm.
Over the past decade, the expectations for supply chain leaders have risen dramatically. Today’s leaders must demonstrate:
• Mastery of analytics platforms and automation tools.
• Deep logistics expertise as care extends into the home.
• Broad strategic and financial insight.
• Agility to navigate constantly shifting distribution models and volatile product availability.
Redding warned that many organizations remain unprepared for what lies ahead, particularly as healthcare decentralization accelerates and delivery models continue to evolve. Building a resilient workforce ecosystem demands close collaboration among GPOs, suppliers, consultants, and health systems.
“Every project, we learn something new,” Redding said. “And every project, we aim to leave the client in a stronger position than when we arrived.”
This continuous exchange of knowledge is essential for advancing the industry.
Strengthening the workforce ecosystem and fostering collaboration lays the foundation, but attracting and developing the next generation of talent is equally critical to ensure long-term resilience and innovation.
Younger professionals are naturally drawn to industries celebrated for supply chain innovation like retail, e-commerce, and manufacturing. Redding warned that healthcare cannot afford to lag in this competition for talent.
The link between supply chain roles and patient outcomes is a compelling story, particularly for Gen Z, but storytelling alone is not enough. Health systems must demonstrate real innovation, offer meaningful challenges, and create clear, achievable pathways for career growth.
Tomorrow’s supply chain professionals must be technically skilled, intellectually curious, and highly adaptable. While certifications provide foundational knowledge, Redding emphasized that intrinsic motivation and a drive to solve problems often separate the best performers. True modernization depends not just on technology, but on people, including leaders and teams that approach change with empathy, user-centered design, and training rooted in real operational needs.
As veteran leaders retire, many health systems, especially small and mid-sized ones, are discovering that their current organizational structures cannot support career advancement or succession. Redding envisions a future where supply chain leadership is more specialized and layered, creating clear progression paths and reducing
dependence on a single individual to manage sprawling operations.
Redding’s powerful advice for those starting their careers: be curious, stay proactive, ask plenty of questions, and don’t be afraid to take risks.
He said those qualities will shape the next generation of healthcare supply chain leaders, the ones who will spark innovation, build stronger systems, and make a real difference in the industry.


Even as organizations scramble to strengthen their internal capabilities, external pressures continue to test the system. Global dependencies and offshore production create vulnerabilities that no workforce alone can fully mitigate, making supply chain resilience a challenge that extends far beyond hospital walls.
Offshore production still overwhelmingly dominates the healthcare supply chain. The appeal of overseas sourcing persists, fueled by America’s limited manufacturing capacity, significantly lower foreign labor and production costs, and the long, expensive runway required to rebuild domestic facilities. At the same time, global volatility has become the rule, not the exception, and geopolitical conflicts, trade upheavals, weather pattern disruptions, logistical bottlenecks, and raw material shortages threaten product flow.
Federal support may play a role in boosting domestic manufacturing over time. Incentives or changes to CMS purchasing rules could help move the market, but Wright noted that it’s wise not to depend too heavily on that, especially after the Strategic National Stockpile struggled during COVID.
“Resilience can’t be outsourced,” he said. “It has to be built deliberately and internally.”
Even the strongest supplier partnerships can collapse when manufacturing is concentrated in a single region. Wright underscored that real diversification is not simply adding more suppliers, but it is a deliberate geographic strategy designed to withstand the next inevitable disruption.
From the vulnerabilities exposed by the pandemic to ongoing labor shortages, offshore dependencies, and accelerating digital transformation, the industry faces a landscape of constant disruption. Building supply chains that can withstand these challenges requires more than technology or strategy alone. It demands a workforce equipped with the skills, vision, and adaptability to navigate complexity and drive meaningful change. HPN
BY MATT MACKENZIE
Over the past year, news from federal health agencies has often been marked by confusion and rapid shifts.
HPN has reported on a range of these developments: leadership changes occurring within weeks, major advisory committees being restructured, recommendations to scale back certain vaccine development efforts, adjustments to long-standing vaccine formulations, and the unexpected removal—and quick reinstatement—of several prominent scientists. Together, these abrupt changes have raised broader questions about the overall direction and stability of key public health institutions.
Public confidence has declined as a result. According to an Axios/Ipsos American Health Index poll released in October, trust in the CDC fell from 66% in December of last year to 54% in the most recent survey. Respondents were also unsure about whether federal health guidance primarily reflects the work of career scientists or broader organizational leadership, with many reporting uncertainty. Support for CDC-recommended childhood vaccination schedules has also decreased. In the latest polling, 74% of Americans agreed that parents should follow these schedules, down from 81% in March. The share who “strongly agree” dropped from 51% to 39%. Survey responses also indicated mixed views about recent health-related policy claims, with many participants expressing uncertainty about the accuracy of specific health assertions.
The data additionally shows widening differences in how various groups perceive public health guidance. For example, levels of strong agreement with CDC vaccination schedules vary significantly across demographic segments. As overall trust
declines, it creates a gap in public confidence.
Enter the Governors Public Health Alliance. This group brings together the governors of 15 states, along with Guam, to coordinate their public health strategies. They have also established an advisory panel to help guide their work. The alliance is described as a forum for sharing data, communicating about emerging health threats, strengthening emergency preparedness, and aligning public health policies across member states.
While this type of collaboration is not new—state coalitions have formed in the past to address major public health challenges—the renewed emphasis on coordination reflects the current landscape, in which approaches to public health vary widely and trust in federal agencies is less assured.
Rodney Rohde, PhD, MS, SM(ASCP)CM, SVCM, MBCM, FACSc, Regents’ Professor at Texas State University System, provided some insight as to how exactly this new alliance could work and what it means for existing federal public health agencies and what it may imply about the state of the nation.
How can the Governors Public Health Alliance directly affect policy?
Rohde: The Governors Public Health Alliance can exert significant influence on state and national health policy by aligning executive leadership to advance coordinated, evidence-based public health strategies. Through bipartisan collaboration, the Alliance can directly affect:
• Public health infrastructure and funding. Advocate for sustained, long-term investments in laboratory capacity, surveillance systems, and the essential public health workforce.
• Data modernization and integration. Promote standardized, interoperable data systems that enhance real-time information sharing and outbreak response.
• Workforce development policies. Support legislation and initiatives that strengthen educational pipelines, credentialing, and retention of laboratory and public health professionals.
• Preparedness and response coordination. Influence comprehensive, statelevel emergency preparedness frameworks to ensure consistent, sciencedriven responses to emerging health threats. By leveraging the collective authority of governors, the Alliance can depoliticize public health, strengthen national resilience, and ensure equitable access to essential health protections across all communities.
How might public health agencies be affected when their work becomes a point of national debate?
Rohde: Public health, healthcare, research, and similar areas should never be politicized, in my professional opinion. I have long stated that infectious disease pathogens, and chronic disease issues as well, simply do not care who we vote for, what we look like, how much money we have, or what our cultural foundations are. Pathogens simply exist to infect, amplify, cause harm or death, and move on to its next host. Politicization diminishes an agency’s technical credibility and operational capacity—costing lives, degrading preparedness, and making future responses more difficult. Safeguarding scientific integrity, transparent communication, and
independent expert review is essential to prevent these harms. We should all collectively be working to support community public health because it is in our common good as humans. Without that support, we will continue to see eroding public trust which reduces the public’s willingness to follow health guidance and can lead to weaker compliance with NPIs and lower vaccine uptake. We will watch worse health outcomes and slower epidemic control. Lower vaccination and slower responses translate into more cases, hospitalizations, and deaths. There will be ongoing weakened scientific integrity and decision making. Political interference can sideline evidence, degrade guidance quality, and disrupt routine agency functions. Our current and future college majors will witness damage to the public health workforce and institutions as increased threats, staff departures, and loss of institutional expertise undermine preparedness. Finally, the global consequences will reveal widening health inequities and politicized access to resources.
Will these fractures influence infection prevention efforts across the country?
Rohde: The politicization of public health, research, healthcare, and subject matter expertise not only damages institutional credibility, but it directly impairs infection prevention, increases the likelihood of future outbreaks, and widens disparities in health outcomes. Restoring trust, scientific consistency, and interagency collaboration is critical to rebuilding a resilient national infection prevention infrastructure. When public health agencies and scientific institutions become politicized, the resulting erosion of trust and coordination directly undermines infection prevention at every level of the system.
The key effects will be a reduction in public adherence to prevention measures like we are currently witnessing in vaccine hesitancy. There will be inconsistent implementation of infection control standards and measures across jurisdictions. We are already seeing this impact with “public health alliances” in various regions of the U.S. With any natural disaster or health emergency, we need one trusted voice to reduce confusion and panic. Weakened surveillance and early detection can result in delayed data sharing and response coordination. Our public health and healthcare workforce, already at dangerously low staffing levels, will continue to face burnout, threats, and attrition due to being questioned by non-experts.
Finally, there will be an erosion of cross-sector partnerships. Early and effective infection prevention depends on trust among healthcare systems, laboratories, and public health authorities.
How can clinicians, hospitals, and health systems build trust with their patients in the wake of this confusion?
Rohde: Clinicians, hospitals, and health systems play a frontline role in restoring trust and reinforcing evidence-based infection prevention and health communication. Trust is built not through messaging alone, but through transparency, consistency, and authentic community engagement.
I would recommend a strategic initiative built on the following pillars:
• Communicate clearly, consistently, and locally.
• Show transparency in decision making.
• Empower clinicians as trusted messengers.
• Engage communities beyond the clinic.
• Model integrity and accountability.
• Invest in workforce education and communication training.
Restoring trust requires relationships, not just responses. When clinicians and health systems communicate with transparency, empathy, and scientific clarity, especially during uncertainty, they become stabilizing forces in an environment where public confidence has been shaken. Trust, once rebuilt, strengthens every facet of infection prevention and public health resilience.
What areas will be most affected by a fractured public health landscape?
Rohde: A fragmented public health system weakens the nation’s ability to prevent, detect, and respond to health threats. The impacts extend across multiple domains—operational, social, and scientific—with cascading effects on community health and national security. The most significant areas threatened in my professional opinion are: Infectious disease surveillance and response; healthcare, public health, and medical laboratory workforces; health communication and trust; rural and underserved communities; chronic disease prevention and community health programs; and research and data modernization and innovation.1
Where can people go for reliable public health information these days?
Rohde: Identifying trusted, evidence-based sources of public health information is essential. Reliable information must come from organizations grounded in science, transparency, and peer-reviewed data rather than opinion or ideology. The primary sources I utilize and trust are:
• Federal and global health agencies.
• State and local health departments.
• Academic and professional organizations.
• Academic health institutions and trusted experts.
• Science-based media outlets. I often implore my family, friends, and colleagues to “trust the data, not the drama” as well as remind them that “expertise still matters.”
Where do you see this situation going from here?
Rohde: It’s always tough to predict the future with so many changing variables. The path forward will depend on whether leaders, institutions, and communities choose to rebuild trust through science, transparency, and collaboration, or continue down a trajectory of division and misinformation. If current fractures persist, the U.S. risks a continued erosion of public trust, inconsistent public health responses, and widening inequities in health outcomes. Routine prevention measures—such as vaccination, surveillance, and antimicrobial stewardship—could weaken further, making the nation less resilient to emerging threats. The public health workforce and healthcare workforce such as medical laboratory, physicians, nursing, and infection prevention are already stretched, and may continue to lose talent and morale.
However, there is also a window of opportunity. The challenges of recent years have elevated public health in national consciousness, revealing both its fragility and its indispensability. With leadership, sustained investment, and renewed commitment to science communication, the U.S. can modernize its public health infrastructure and restore confidence in its institutions. We all must support policies and people who are committed to depoliticizing public health and healthcare, rebuilding our workforce capacity in these areas, modernizing our data
systems, reinforcing our community engagement, and promoting accurate health literacy.
Is there anything else you would like to share?
Rohde: One of my go to statements is “Public health, healthcare, research, and expertise matter all the time to everyone.” Public health without trust is just data on a piece of paper. Everyone must realize that science does not take sides. We should listen to our grandparents who understand the critical lifesaving value of our past scientific innovation and discovery that has saved millions of lives like clean drinking water, hand hygiene, vaccines, antimicrobials and antibiotics, and other measures. Our society has grown a bit spoiled by taking these things for granted and the microbes are just waiting for us to become lazy and uninformed. Confusion is
Healthcare Hodgepodge,
contagious too, but public health is the antidote.
Former CDC Director Dr. Tom Frieden

HPN also able spoke with former CDC Director Dr. Tom Frieden, who is now the president and CEO of Resolve to Save Lives, a non-profit organization whose mission is to prevent 100 million cardiovascular disease deaths by 2050.
HPN asked him what measures need to be taken in the wake of these developments.
Dr. Frieden: We need to block damage, patch gaps, and begin to build faster, more responsive health and public health systems. First, protect what protects us like disease tracking, labs, vaccination, outbreak response, and action to address the leading
HPN’s podcast, features interviews with special guests and article reads on our many verticals, including supply chain, sterile processing, surgical and critical care, infection prevention, and more.
Scan the QR code so you don’t miss an episode!

healthcarehodgepodge.podbean.com
causes of disease, disability, and death.
Second, fill gaps to the extent possible by pooling resources, coordinating, learning lessons, and collaborating for learning and efficiency. This is an important role for multi-state coalitions.
Third, start creating a modern public health that is quicker to find threats and act on them, better at listening and communicating, linked closely with primary care and grounded in strong partnerships, and focused on delivering results people can see, including cleaner water and air, healthier food, fewer asthma attacks, quicker control of outbreaks and more accessible and effective primary health care. HPN
References are available online at hpnonline. com/55332811.

BY MARK DURO
In the high-stakes world of healthcare, success is often measured by what patients see— doctors, nurses, spotless rooms, and advanced surgical suites. But behind every successful procedure lies a group of highly skilled, often unseen professionals: sterile processing technicians (SPTs). These technicians are the first and last line of defense in patient safety, responsible for ensuring every surgical instrument and device is clean, sterile, functional, and safe for use.
Despite the complexity and critical nature of their work, sterile processing technicians are frequently grouped under the outdated category of “Central Services,” a
classification that undervalues their technical knowledge and expertise. As a result, these professionals are often underpaid, underrecognized, and excluded from the compensation and professional pathways afforded to similarly skilled healthcare roles.
This article explores why certification in sterile processing is essential, how misclassification under “Central Services” contributes to unfair pay, and why it’s time to reclassify, rebrand, and properly compensate this essential healthcare workforce. It also highlights how professional bodies like the Healthcare Sterile Processing Association (HSPA) are working to drive legislative change
that acknowledges sterile processing for what it truly is: a vital clinical discipline.
Sterile Processing: The silent cornerstone of patient safety
Every surgical procedure, from a simple biopsy to a complex open-heart operation, depends on clean, sterilized, and properly functioning instruments. Sterile processing departments (SPDs) are responsible for ensuring this. Their work involves more than just “cleaning tools”— they manage the full reprocessing cycle: decontamination, inspection, functional testing, assembly, sterilization, storage, and distribution. Key responsibilities of SPTs include:
• Decontamination: Using enzymatic cleaners, washers, and ultrasonics to remove organic and inorganic material.
• Inspection and assembly: Carefully inspecting each instrument for cleanliness, damage, or wear, and assembling sets to precise specifications. Often, device instructions for use (IFUs) are vague or misleading which requires critical thinking skills.
• Packaging and sterilization: Using steam sterilization, hydrogen peroxide, gas plasma, or ethylene oxide systems to eliminate microorganisms.
• Documentation and quality control: Recording sterilization data, monitoring biological/chemical indicators, and ensuring regulatory compliance.
Modern sterile processing requires proficiency in microbiology, anatomy, medical terminology, chemistry, infection control, engineering, and a deep understanding of hundreds of types of surgical instruments.
Yet many healthcare administrators still view SPD through a decades-old lens—grouping it with laundry, dietary, and environmental services under “Central Services.” This misclassification has devastating consequences for compensation, recruitment, retention, and, ultimately, patient safety.
Certification is not a formality. It is a standardized, evidence-based method to validate a sterile processing technician’s competency.
Leading organizations such as the HSPA and the Certification Board for Sterile Processing and Distribution (CBSPD) offer nationally recognized certification programs that assess knowledge in:
• Infection prevention and microbiology.
• Instrument identification and surgical procedure relevance.
• Manufacturer IFUs.
• Disinfection and sterilization principles.
• Quality assurance and risk management.
• Regulatory compliance (AAMI, CDC, OSHA, etc.). Certification should be mandatory because it:
• Reduces variability in practice across hospitals and facilities.
• Raises the bar for accountability and professionalism.
• Aligns sterile processing with other certified healthcare roles, such as surgical technologists or respiratory therapists.
• Demonstrates commitment to patient safety and regulatory compliance.
• Creates a benchmark for fair pay and job advancement.
Certification is not a formality. It is a standardized, evidence-based method to validate a sterile processing technician’s competency.
Yet, despite the technical knowledge required to pass certification exams, many hospitals still hire non-certified technicians, offer no financial incentive to become certified, and fail to require ongoing professional development.
How HSPA is leading the push for state-level certification laws
Recognizing the link between certification and patient safety, HSPA has been instrumental in advancing legislative efforts across the U.S. to mandate certification for sterile processing professionals. Their advocacy has already made a measurable impact. States where certification is now required or supported by law include:
• New Jersey: A trailblazer in this space, New Jersey mandates that all SPD staff be certified.
• New York: Requires SPD professionals to be certified and to maintain ongoing competency.
• Connecticut: Certification must be achieved within a defined time after hire.
• Tennessee: Recently passed a law requiring certification within two years of employment.
• Pennsylvania: Active HSPA-supported advocacy efforts are ongoing.
HSPA provides testimony, legislative consultation, and data-driven rationale to lawmakers, arguing that standardizing training and certification across states protects patients, improves outcomes, and reduces costs associated with surgical infections and OR delays. By promoting legislation that validates sterile processing as a clinical profession, HSPA is leading the charge to remove the outdated “Central Services” label and elevate sterile processing to its rightful place in the healthcare hierarchy.

One of the most damaging issues sterile processing professionals face is being grouped under the broad umbrella of “Central Services” or “Support Services,” a designation that encompasses food service, transport, linen, and housekeeping. While these departments are essential to facility operations, sterile processing technicians perform clinical, technical, and regulated work. Some consequences of misclassification include:
• Wage suppression: SPTs are often paid on par with custodial workers or dietary staff, despite the complexity of their roles.
• Limited career advancement: HR systems typically do not offer a defined promotion pathway for SPD professionals.
• Lack of respect and recognition: Clinical departments may view SPD as non-clinical or subordinate.
• Minimal onboarding or training: Some facilities onboard SPD techs with little to no formal instruction or certification support.
• Burnout and turnover: Low pay and lack of respect drive away talent, creating unsafe staffing levels. In contrast, certified sterile processing professionals in well-supported environments are often key players
in infection prevention teams, operating room logistics, and surgical readiness programs.
Canada has taken a progressive step by removing the term “service” from the profession’s name altogether. The standard term across Canadian hospitals is now Medical Device Reprocessing (MDR), which more accurately reflects the scientific and technical nature of the
Canada has taken a progressive step by removing the term “service” from the profession’s name altogether.
job. This new term matters because it shifts public and institutional perception from support work to clinical reprocessing; encourages integration with perioperative teams and infection prevention; facilitates standardization of training and career pathways; and lays the groundwork for appropriate compensation.
While the naming convention may seem trivial to some, it carries deep professional implications, much like the difference between calling someone a “clerk”
versus an “analyst.” Language shapes how institutions treat and compensate their staff.
The average wage for a certified sterile processing technician in the U.S. hovers between $18 to $26 per hour, depending on the region and healthcare system. However, these wages frequently fail to reflect:
•The level of certification and continuing education required.
•The legal liability and infection control responsibility.
•The surgical dependency on SPD output and turnaround times.
• The complexity of modern instrument design and reprocessing protocols.
Technicians in high-volume facilities may reprocess 10,000-plus instruments per week, with turnaround times measured in minutes, not hours. Instruments for robotics, endoscopy, neurosurgery, and orthopedics are intricate, delicate, and require exact specifications. Errors can, and do, lead to surgical delays, site infections, equipment damage, and patient harm. Yet, despite this pressure and precision, many SPD staff earn wages that barely compete with entry-level retail positions. This is a structural failure in healthcare policy that must be corrected.
The stakes are far higher than technician morale or payroll budget lines. Understaffed or undertrained sterile processing departments lead to:
•Retained surgical items.
•Improperly sterilized instruments.
•Case delays and cancellations.
•Surgical site infections.
•Hospital-acquired infections (HAIs).
•Regulatory citations or financial penalties.
Hospitals that fail to invest in certification, staffing, and professional development for SPD staff ultimately compromise patient outcomes and institutional reputation.
Five critical
•Reclassify the department. Hospitals must move sterile processing out of “Central Services” and create a dedicated classification—Medical Device Reprocessing or Surgical Instrument Reprocessing. This allows for customized job descriptions, pay structures, and career ladders.
• Mandate certification and reward it. Make certification a requirement within 12 to 24 months of hire. Provide financial incentives and continuing education opportunities. Certification should lead to higher pay and advancement potential.
•Benchmark compensation fairly. Conduct local and national pay studies comparing sterile processing to roles with similar training and risk. Adjust pay accordingly to attract and retain talent.
•Educate clinical and administrative leadership. Use internal education, case studies, and infection control data to educate OR directors, executives, and HR teams about the vital role of SPD.
• Support legislative action. Engage with HSPA and local lawmakers to support certification laws in your state.
Bring patient safety data to the table and help define standards that elevate the profession.
Picture a hospital where sterile processing technicians are fully certified, fairly compensated, and respected as vital members of the surgical team. Where job titles reflect skill, not history. Where no instrument leaves the SPD without a trained expert inspecting it, and where patient safety is enhanced because those experts are empowered and valued. This is not a fantasy—it’s a future within reach. But it requires action.
To hospital executives: Reclassify sterile processing. Invest in certification. Pay your technicians what they’re worth—not based on outdated labels, but on the complexity and impact of their work.

To human resources: Create new titles and pay bands that reflect real-world skills. Support career ladders and require certification for advancement.
To policymakers and regulators: Partner with HSPA. Adopt laws requiring certification to ensure safe, standardized reprocessing across facilities.
To sterile processing technicians: Get certified. Share your stories. Advocate for your profession. Join the movement to raise the standard—for your patients, your peers, and yourself.
It’s time for the hidden heroes of the hospital to step into the light, not as support staff but as clinical professionals safeguarding every surgical moment from the shadows.
Mark Duro is a nationally recognized expert in sterile processing and surgical instrument reprocessing with over three decades of hands-on experience. He has held leadership roles at major healthcare institutions and global medical device companies, including New England Baptist Hospital, Lahey Clinic, Crosstex/Cantel Medical, and Terragene. Mark is a Fellow of the HSPA, a former President of the HSPA Foundation, and was honored as HSPA Educator of the Year in 2013.
BY KARA NADEAU
For years, sterile processing professionals have been asked to do more with less. They are expected to improve turnaround times, support surgical growth, manage complex instrumentation, and prevent infection — all while operating with limited visibility into what “good” performance really looks like.
Unlike surgical services or infection prevention, sterile processing departments (SPD) lack standardized metrics
and benchmarking. There are no widely accepted national indicators for quality, productivity, or cost.
As a result, SPD leaders are often flying blind, struggling to make a data-backed case for staffing, resources, and technology.
“Healthcare is highly data-driven in patient care, but decisions in SPD are often made without data,” said Ajay K. Jain, MBA, managing partner, SteriPro International. “Most hospitals have no idea what they spend on reprocessing, even though it may represent 2–4% of their total annual budget. Decisions are being made without knowing how many instruments exist, how long it takes to reprocess an instrument, or where the real bottlenecks are.”
The data gap: A persistent barrier to progress
When sterile processing leaders discuss productivity, the metrics vary widely. One common metric used is “number of trays processed,” which does not capture the complexity or variability of sterile processing work.
“We’re trying to measure productivity in a way that doesn’t reflect what our teams actually do,” said Carol Malone, AAB, CSPDT, CRCST, CIS, CHL, CER, sterile processing educator, The Cleveland Clinic. “One tray might have four instruments; another might have 600. You can’t compare those side by side and say that’s the same workload.”
In many hospitals, productivity metrics still trace back to operating room (OR) volume or case counts, which is data that ignores an SPD team’s full scope of work. Beyond reprocessing instrumentation for in-house surgical and other procedural services, it is common for sterile processing professionals to take on cart and tray transport, crash cart refills, instrument reprocessing for clinics, loaner tray reprocessing, and other myriad responsibilities.
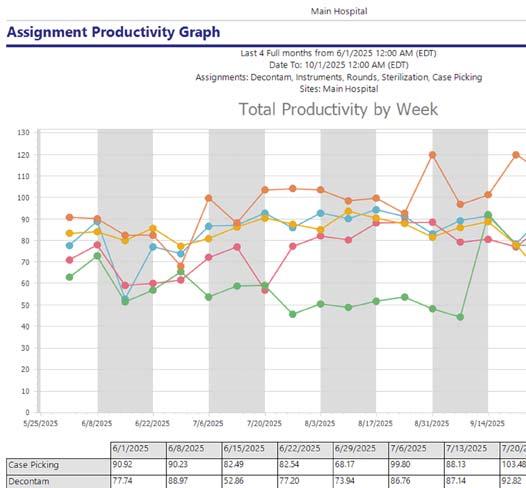
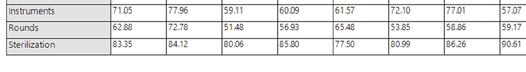
“Even within the same health system, facilities track productivity differently,” Malone explained. “Without standardized data, it’s nearly impossible to advocate for staff or resources.”
Data challenges also extend to quality measurement.
Edna Gilliam, DNP, MBA, RN, CNOR, NEA-BC, assistant vice president, Perioperative Services & SPD, DV, Nemours Children’s Hospital, contrasted sterile processing with infection prevention — where data such as surgical site infections (SSI) and central line-associated bloodstream infections (CLABSI) rates enable meaningful improvement and accountability — noting how SPD teams lack a comparable set of indicators for quality and productivity.
“In infection prevention, we have clear data on infection rates, but there’s nothing equivalent for sterile processing—no national benchmarks we can use to compare performance or advocate for resources,” Gilliam explained. “The sterile processing community needs consensus on meaningful data points—something that allows leaders to benchmark performance and advocate for staffing, training, and investment.”
Gilliam emphasized that quality reporting is often selfreported and inconsistently captured in tracking systems. “We’re relying on honesty and available time, which means
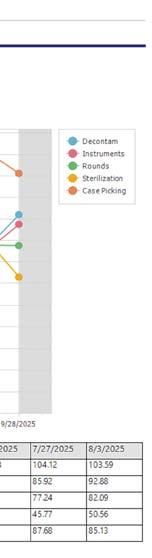
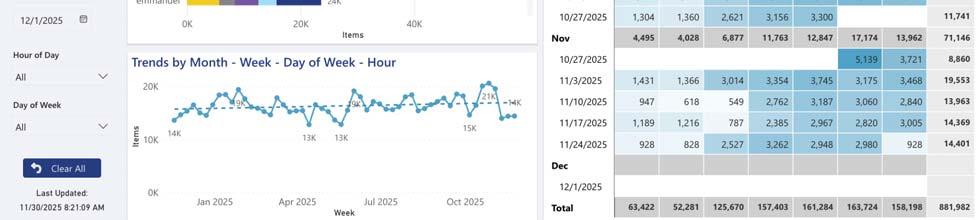
Total Productivity by Assignment
Workflow solution and asset tracking software view of SPD staff productivity (photos courtesy of STERIS)
some sites that report more issues may look worse on paper than those that don’t report at all,” she explained. “We need standardized definitions, automated reporting, and real transparency.”
She noted that another major data gap lies in defining acceptable levels of bioburden, stating: “Expecting zero percent bioburden isn’t realistic. Human factors will always play a role, but we need evidence-based guidance on what’s acceptable and achievable in the real world. Without consistent definitions or reporting, it’s hard to know how you’re performing compared to peers.”
The inability to calculate reprocessing costs remains one of SPD’s most persistent blind spots. Jain explained how
metrics that track economic and efficiency-based KPIs, including instruments processed per hour per technician, cost-per-instrument, and first-pass yield rates, link SPD operations to measurable business value.
“Without visibility into what reprocessing actually costs, you’ll never have reliable cost-per-case data, and that means you’re making financial decisions in the dark,” said Jain. “Once leaders calculate their costper-instrument — including labor, consumables, repairs, utilities, and equipment depreciation—they begin to see how SPD efficiency directly affects the bottom line. If you can tell your CFO what it costs to reprocess a single instrument, you’ll have their attention. That’s how SPD earns a voice in financial and operational planning.”
“Process mapping in SPD is simply documenting the work as it happens — from point-of-use in the OR through transport, decontamination,


inspection and assembly, packaging, sterilization, storage, and back to the OR,” Jain explained. “We timestamp each handoff, count the work units (trays, instruments, and cases), and record defects at the exact step they occur. That gives us a baseline for how the department truly performs day-to-day — how long each step takes, where labor load peaks, where backlogs form, and how work moves across people and equipment.”


Instrument tracking systems can provide rich operational data, but many are underutilized or inconsistently configured.
“Everybody’s all over the place,” said Malone. “Facilities collect scan points differently, which makes data inconsistent. So, how do we first get everybody on the same page as far as collecting the data of scan points. And then how do we turn that into productivity?”
John Kimsey, VP, Processing Optimization and Customer Success, STERIS, says every SPD leader who has an instrument tracking system should take the following six steps to extract data analytics that can be leveraged to optimize their department.
1. Map the instrument processing workflow and ensure you have scanning points for each process step – process steps are action steps such as cleaning, assembling, sterilizing, etc.
2. Create standard work for each process step and assign labor standards in the tracking system for staff to “earn” labor time when completing the process – this drives productivity reports.
3. Leverage technology-guided workflows to ensure staff compliance to standard work such as following the IFU or department policy.
4. Measure how long it takes products and instruments to travel through the process (known as cycle time) and then work to lean out the process to improve or reduce the time.
5. Map the inventory staging locations and ensure they are scan points – staging locations are non-action steps where inventory sits such as assembly receipt, out for repair, missing instrument holding, or sterile storage.
6. Track how long products sit at each inventory staging location and work to reduce staging or si ing time.”
“No ma er what data analytics are used, SPD leaders must make them actionable,” said Kimsey. “We measure to improve, not to report. The best SPD leaders understand the power of data and measurements to identify variance and opportunities. They want to know what’s not working right or performing to plan so they can target areas for improvement. Data should show you where your process is failing, where your team may not be working to plan, or where your next improvement project is needed. Data should be used to reduce and eliminate variance, eliminate root cause quality issues, and improve efficiencies.”
That lack of standardization makes it difficult to track tray turnaround times and technician throughput or process bottlenecks, which are key data points that could drive smarter staffing and flow optimization.
When asked how sterile processing departments can better leverage instrument tracking systems to track tray turnaround times, measure technician throughput, and identify process bottlenecks, Seamus Johnson, senior director of product development, Censis Technologies, stated:

“Modern instrument tracking systems give sterile processing departments real-time visibility into tray turnaround and staff productivity. By scanning trays at each step, the system logs timestamps, locations, and user activity, enabling managers to monitor processing times, identify delays, and balance workloads. Productivity reports highlight bottlenecks — such as trays waiting for a sterilizer or assembly bench — so leaders can target improvements.”

Why it matters: Data as a bridge between SPD and the C-suite
Few departments sit at the intersection of patient safety, operational efficiency, and cost the way sterile processing does. Yet without data, the SPD’s role in those outcomes remains largely invisible.
John Kimsey, VP, Processing Optimization and Customer Success, STERIS, outlined four core areas where data can transform sterile processing performance:
• Resource justification: Quantifying labor, equipment, and instrument inventory needs
• Outcome measurement: Measuring how well SPD is meeting external customer expectations
• Process measurement: Measuring how well SPD processes are predictable and follow standard work
• Efficiency measurement: Measuring how well SPD is performing to operational plan and where performance can be improved
“Understanding how to justify resource needs through data analytics is critical for every SPD leader to understand,” Kimsey explained. “Leveraging a tracking system’s data can provide workload volumes and how long it takes to process products through each step of the workflow. Variations in workloads by time of day, day of week, and season can be evaluated. Productivity by staff, shift, and department can be analyzed and allow the leader to show resource requirements based on facts and data.”
According to Kimsey, the ability to measure performance to expectations is core to managing any operation.
He stated, “Whether that’s measuring customer quality, internal process performance, or efficiency, strong leaders want to know how they are doing and where they can improve. Leaders who measure and hold their teams accountable to predictable standard work understand that product quality comes by managing internal process quality.”
guides technicians through the steps with electronic confirmation, you can report on that and show the data as proof of compliance.”
Even departments with tracking systems and dashboards can struggle to interpret what the data means or how to act on it.
Metrics reveal volume pa erns and technician output, helping managers align shi s with demand, justify FTE changes, and reduce overtime.
He pointed to the power of guided digital workflows to reinforce compliance and generate actionable metrics, stating, “Tracking systems providing guided workflows help measure staff compliance to standard work, IFUs, and department policies. If you develop an electronic workflow that
“Many tracking systems provide reporting and dashboard capabilities that allow for improved readability and trendsetting,” said Hannah Schroeder, BSHA, CRCST, CIS, CHL, CER, clinical education specialist, Pure Processing. “However, the availability of these data tracking
resources necessitates the need for education and training to understand how to use it to its greatest and fullest capabilities.”
As Schroeder points out, consolidating and integrating data points and watching trends and patterns is just the start. “One of the learning curves is understanding how to read, interpret, and then turn data into action items,” she said.
When looking at data points, Schroeder considers how she can get the metrics to tell a comprehensive story when integrated together.
“Sometimes it’s not about adding more but tying together what you have effectively,” she explained. “The concept of pulling the information together isn’t new; software and integrations allow for filtering and consolidating data to answer complex questions and to understand how one may influence the other. It’s becoming increasingly achievable with updates
in software capabilities and the integration of AI.”
When asked how SPD teams can turn instrument tracking system data into actionable improvements, Johnson responded:
“The real value lies in using this data to optimize staffing and workflows. Metrics reveal volume patterns and technician output, helping managers align shifts with demand, justify FTE changes, and reduce overtime. Time stamps pinpoint slowdowns, allowing SPDs to redesign layouts, add equipment, or adjust tasks to eliminate delays.
“Dashboards also support broader initiatives, from adding duplicate trays to reduce urgent reprocessing to improving training where errors occur. By turning insights into action— whether reallocating staff, fine-tuning workflows, or investing in resources— SPDs achieve faster turnaround, fewer bottlenecks, and more reliable instrument availability. Ultimately, datadriven decisions enhance efficiency, elevate patient care, and strengthen operational performance.”
Defining productivity and quality in a complex environment
Malone and Gilliam both emphasized the need for standardized definitions that reflect the true scope and complexity of sterile processing work.
“There’s nothing in the industry that defines what SPD productivity should look like,” said Malone. “The Association of periOperative Registered Nurses (AORN) has an OR staffing guideline, 2.5 FTEs per OR. That’s a clear benchmark. SPD doesn’t have that. Should it be based on tray counts? Case complexity? Turnaround times? Until we agree on what to measure, we can’t compare performance fairly or advocate for what we need.”
Gilliam shared that even among OR and SPD leaders, there’s confusion
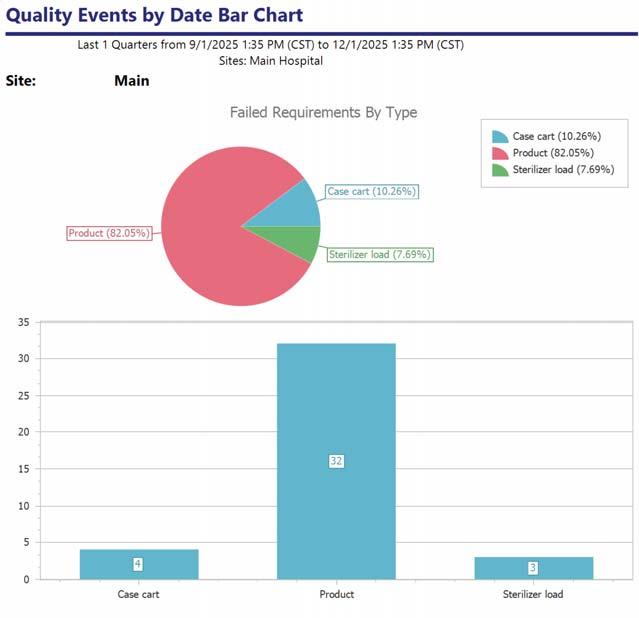
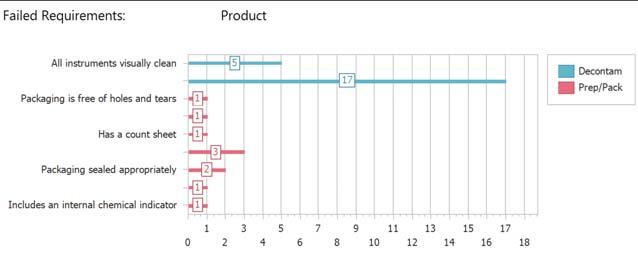
Failed Requirements:
All instruments visually clean
Packaging is free of holes and tears
Has a count sheet
Packaging sealed appropriately
Includes an internal chemical indicator Product
over what productivity means — some use “hours per patient day” or “number of cases,” neither of which accurately reflect the intricacies of sterile processing.
“Departments across the board rely on different metrics, yet none truly capture the complexity of our work,” she stated. “Sterilizing a spine tray involves far more intricacy than processing a tonsil tray, but our productivity measures fail to reflect that distinction. Without a standardized approach, we’re left creating our own definitions of productivity.”
When SPDs begin collecting standardized data and using it to justify resources, they start to earn recognition as essential partners in operational and financial planning.
“We have to stop thinking of SPD as a support function,” said Jain. “SPD enables surgery. Without sterile processing, there are no instruments — and without instruments, there are no surgeries. It’s like a restaurant with a Michelin-star chef. The surgeon is the chef, but without the right pots,
pans, and knives — the instruments — you’re not serving any food. Those tools have become highly complex and expensive, and they deserve respect in their own right.”
He noted that as surgical instruments and procedures become more complex, and hospitals move from global funding to per-case costing models, SPD performance directly affects case-level profitability.
“Without visibility into reprocessing costs, hospitals can’t make accurate financial or operational decisions. Once the current state is visible, leaders can remove bottlenecks, standardize steps, and track consistent indicators on a simple dashboard.”
“It’s not theory; it’s a practical way to make SPD performance measurable, predictable, and repeatable,” Jain added. “This level of clarity also supports smarter purchasing
and hiring decisions, enables targeted education and training, and allows a true corrective and preventive action (CAPA) program to be developed and implemented that is specific to the needs of that SPD.”
Here are three practical steps in the journey to meaningful SPD data based on insights from the experts:
1. Standardize scan points and definitions Every facility should define consistent scan points throughout the reprocessing workflow - decontamination, assembly, sterilization, storage, and delivery. These become the backbone for productivity and quality metrics.
2. Integrate data systems Tracking, scheduling, maintenance, and human resource systems should communicate. Integration allows SPD leaders to correlate tray turnaround times with staffing levels, surgical schedules, and quality outcomes.
3. Invest in data literacy Whether through education or hiring, SPDs need people who can interpret data and translate it into operational improvements.
“This is where a shift in department requirements could change and may require the addition of a data analyst into your team; specialized individuals who are trained to not only understand the data but see the story through experience, observation, and human connection,” said Schroeder. “Data has the potential to do really great things, but the greater benefit comes when we can connect the data to human impact.”
Kimsey believes that technology can make these goals achievable. “Most SPD departments already have the tools,” he said.
“It’s about using them to their fullest. When you map
Hannah Schroeder, BSHA, CRCST, CIS, CHL, CER, clinical education specialist, Pure Processing, offered these sources of data that SPD leaders can leverage for benchmarking, measurement, and reporting.
Quality management data
• Adverse event reporting
• Customer feedback
• Equipment maintenance & repairs
• Audit results
Staffing
• Rosters
• Schedules
• Competency
• Assignments
Productivity
• Throughput
• Decontamination
• Assembly
• Load records
workflows, assign labor standards, and measure cycle time, you create visibility — and visibility leads to improvement.”
Sterile processing has long been a behind-the-scenes discipline that is essential, but rarely quantified. That’s changing as healthcare becomes increasingly data driven.
With the right data, SPD leaders can demonstrate how process quality drives surgical outcomes, how staffing affects throughput, and how reprocessing efficiency impacts cost and resource utilization.
The data may still be imperfect, but the direction is clear. By defining what to measure and learning how to act on it sterile processing professionals can transform invisible work into visible value. HPN

BY KAYLA OSTRANDER
Today, the Bowie-Dick (BD) test is widely used and recognized as a valuable means of monitoring the performance of vacuum-assisted steam sterilizers. However, several aspects have changed since the original work was done in Britain in the early 1960s.1 Bowie et al. introduced a simple test to detect inadequate air removal in prevacuum steam sterilizers, because this air could lead to sterilization failure. They created a stack of porous towels with an indicator sheet placed at the center. When processed in an empty chamber, the indicator experienced a color change; an uneven
1.Explain the purpose and importance of the BowieDick test in sterilization processes.
2.Demonstrate the proper procedures for using the Bowie-Dick test effectively.
3.Describe the necessary steps to take when a Bowie-Dick test result indicates a failure.
Sponsored by:

color change indicated residual air or poor steam penetration. Their work demonstrated that sterilizers could pass routine checks yet fail to remove air effectively, highlighting the need for daily testing. This became the foundation for the BD test, which is now used globally as the standard for monitoring pre-vacuum sterilizer performance. Current industry standards for sterile processing recommend a BD test be performed daily, before the first processed load, and for all prevacuum steam sterilizers. BD test results are maintained as part of the batch record and quality control

system. The BD test has evolved in appearance since it was first introduced, and technology has enabled a new electronic BD test that eliminates the need for human interpretation of the printed indicator sheet; instead, it provides an automatic digital record keeping capability. Oversight of BD testing in the United States is provided by the American National Standards Institute(ANSI) and Association for the Advancement of Medical Instrumentation (AAMI) through ANSI/AAMI ST79, a comprehensive standard that serves as a guide to steam sterilization and sterility

assurance in health care facilities.2,3 This document establishes requirements for daily BD testing in pre-vacuum steam sterilizers, including test pack specifications, cycle parameters, and interpretation of results.
Objective 1: Explain the purpose and importance of BD testing in sterilization processes. Steam sterilizers use moist heat under pressure to kill microorganisms, including spores. A saturated
into daily sterilization practice, technicians can reliably monitor sterilizer performance, ultimately enhancing patient safety and compliance with industry standards.
Most of today’s sterilizers operate differently from those used by Bowie and Dick, which drew a single deep vacuum before beginning the sterilization cycle. Pre-vacuum sterilizers today typically have a series of steam injections and vacuum excursions before beginning the sterilization phase. In addition, the vacuum
Current industry standards...recommend a BD test be performed daily, before the first processed load, and for all pre-vacuum steam sterilizers.
steam sterilization cycle has at least three phases: condition, exposure, and exhaust. During the conditioning phase, air is removed from the sterilization chamber and saturated steam enters the chamber and begins heating the load. In the exposure phase, the chamber reaches the set temperature and pressure, and the load is held at these conditions for the required time to achieve sterilization. The steam is removed during the exhaust phase, so the pressure returns to ambient. The conditioning phase removes air from the sterilizer chamber. This allows the steam to make contact with all the surfaces in the load. Inadequate vacuum, air leak, or poor steam quality can create air pockets that compromise sterility by preventing steam penetration into the load.
The BD test is an essential Type 2 indicator used to verify the effectiveness of air removal in steam sterilization processes, particularly in pre-vacuum sterilizers.3 Type 2 indicators, which serve to assess specific performance characteristics of sterilization cycles, are vital for ensuring that sterilizers operate correctly and efficiently. By integrating the BD test
depth is not as great as in the older high vacuum sterilizers.
Monitoring with a BD test pack should be done daily, prior to running the first full load of the day. If a sterilizer has an inadequate vacuum, an air leak, or poor steam quality, air pockets may form inside the sterilizer and compromise sterility by preventing steam penetration into some of the packs in the load. The indicator sheet inside the BD test pack will not develop properly if air remains trapped inside the sterilizer chamber, providing a sensitive and rapid means of detecting air leaks, inadequate air removal, inadequate steam penetration, and non-condensable gases, which can be air or gases from boiler additives.2 If a BD test indicates a problem, the sterilizer should be taken out of service until the malfunction is identified and corrected. If the sterilizer is used continuously, the test may be performed at any time but should be performed at the same time every day.4 The BD test pack should be placed horizontally, label side up, on the bottom shelf of the sterilizer rack, over the drain in an otherwise empty chamber. It is not acceptable to

Understanding BowieDick Testing: Purpose and Usage in Daily Sterilization
January 2026
This lesson was developed by Solventum. Lessons are administered by Endeavor Business Media.
A er careful study of the lesson, complete the examination online at educationhub.hpnonline.com. You must have a passing score of 80% or higher to receive a certificate of completion.
Certification
The Certification Board for Sterile Processing and Distribution has pre-approved this in-service unit for one (1) contact hour for a period of five (5) years from the date of original publication. Successful completion of the lesson and post-test must be documented by facility management and those records maintained by the individual until recertification is required. DO NOT SEND LESSON OR TEST TO CBSPD. www.cbspd.net


Healthcare Sterile Processing Association, myhspa.org, has pre-approved this in-service for 1.0 Continuing Education Credits for a period of three years, until December 2, 2027
For more information, direct any questions to Healthcare Purchasing News editor@hpnonline.com.
Quiz Answers:

run the BD test in the same cycle as the biological indicator test pack.
Objective 2: Demonstrate the proper procedures for using the BD test effectively.
Before conducting the BD test, it is crucial for the technician to verify the sterilizer’s readiness to ensure accurate results. This begins with confirming that the sterilizer is empty and clean, as any residual materials could interfere with the test. Additionally, the technician should ensure that the sterilizer has completed any necessary warmup cycles. These initial checks establish proper baseline conditions, which are essential for the reliability of the test results. A well-prepared sterilizer sets the stage for effective testing, allowing for an accurate assessment of the sterilization process’s capability to eliminate air pockets and achieve proper steam penetration.
Next, technicians must inspect the integrity and expiration of the test pack or electronic device used in the BD test. It is important to check that the chemical indicator pack is sealed, undamaged, and still within its expiration date. This step helps prevent false results that could arise from compromised materials. In the case of electronic BD devices, technicians should confirm that the device is charged, calibrated, and functioning correctly. By thoroughly examining these components before the test, technicians can ensure that the test accurately reflects the sterilizer’s performance and that any potential issues are identified early on.
Once the test pack has been prepared, it is essential to follow standard operating procedures for placement and documentation. The test pack should be positioned horizontally over the sterilizer drain, which is typically the coldest and least penetrated area, to effectively challenge the sterilizer’s ability to remove air and allow steam penetration. When running the BD test, the sterilizer should be programmed to run a pre-vacuum cycle at 270-273ºF for 3 ½ - 4 minutes with ≤ 1 minute dry time.4
After the test, the technician evaluates the indicator result, checking for a uniform color change across the test sheet. Any inconsistencies, such as uneven colors or white spots, indicate a FAIL. Following this evaluation, the technician must document the result according to facility policy, recording necessary details such as test pack type, lot number, and placement location in the sterilizer log or tracking system. For electronic BD systems, while results may be automatically recorded, the technician should still ensure that the results are stored and traceable. If a test result indicates a failure, the technician must respond appropriately, initiating corrective actions to address any identified issues. If a major repair
of the sterilizer is required, qualification testing should be conducted before the sterilizer is put back into routine use. During sterilizer qualification testing, the BD test should be run in three consecutive cycles after running three consecutive biological indicator process challenge device cycles.
Objective 3. Describe the necessary steps to take when a BD test result indicates a failure.
Before conducting the BD test, it is crucial for the technician to verify the sterilizer’s readiness to ensure accurate results.
If a sterilization test results in a FAIL, the technician needs to take quick and careful steps to make sure everything is safe. First, the technician must quarantine the sterilizer, meaning it cannot be used for processing any instruments until the problem is fixed. This step is very important to avoid any risk of contamination. Next, the technician should immediately inform a supervisor or maintenance team about the failure. After checking for issues like air leaks, making sure the door seal is tight, or reloading the chamber properly, the technician must repeat the sterilization test. This process helps ensure that the sterilization is done correctly and highlights the importance of careful checks. It is recommended for the sterilizer to be properly heated before running the BD test to ensure accurate results and avoid false failures.
Electronic BD test systems are a big improvement in sterilization monitoring technology. These systems help technicians quickly see the results on a digital display or printout that clearly shows if the test is a PASS or FAIL. This technology removes the need for interpreting color changes, which could be confusing. With electronic BD systems, sensors and auto-readers provide fast and accurate results, reducing the chance of human error. This makes the process quicker and easier, allowing for better documentation. Overall, these electronic systems help keep instruments safe and ensure they are properly sterilized before being used.
BD tests help keep medical instruments safe by ensuring your sterilizers are working efficiently and are properly sterilized before being used. Ensuring the readiness of the sterilizer and the integrity of the test pack or electronic device is fundamental for conducting a reliable BD test, as these initial steps lay the groundwork

for accurate assessments of the sterilization process. Proper placement of the test pack within the sterilizer is critical to effectively challenge the system’s ability to eliminate air and achieve adequate steam penetration, which directly impacts the reliability of the test results. Thorough documentation and appropriate responses to test outcomes, especially in the case of failed results, are essential components of a robust sterilization protocol, promoting accountability and continuous improvement in infection control practices.
1.Bowie JH, Kelsey JC, and Thompson PR. The Bowie and Dick Autoclave Tape Test. Lancet 1963; 1:586.
2.ANSI/AAMI/ISO 11140-1:2004 Sterilization of health care products — Chemical indicators — Part 1: General Requirements. International Organization for Standardization. (2014).
3.ANSI/AAMI/ISO 11140-5:2007 (R2012) Sterilization of health care products — Chemical indicators — Part 5: Class 2 indicators for Bowie and Dick air removal test sheets and packs. International Organization for Standardization. (R2012).
4. Comprehensive guide to steam sterilization and sterility assurance in health care facilities. ST79. Association for the Advancement of Medical Instrumentation. (2017).
Kayla Ostrander BS, MS, CRCST, CHL, CER; Solventum, Maplewood, MN, USA
1.How often should the Bowie-Dick test be run?
A.Weekly
B.Monthly
C.Daily before the first processed load
D.During routine maintenance
2.The Bowie-Dick test is designed to evaluate the effectiveness of the air removal system of which kind of steam sterilizer?
A.Gravity-displacement
B.Prevacuum
C.Both a and b
3.The Bowie-Dick test is designed to detect residual air in the sterilizer chamber due to:
A.Inadequate air removal
B.Air leaks
C.Non-condensable gases in the steam
D.All of the above
4.The appropriate placement of a BowieDick test in the sterilizer is:
A.On the floor over the drain
B.On a basket over the drain
C.Horizontally, label side up, on the bottom shelf of the sterilizer rack, over the D.drain in the first full load of the day
E.Horizontally, label side up, on the bottom shelf of the sterilizer rack, over the drain in an otherwise empty chamber
5.It is recommended for the sterilizer to be properly heated before running the Bowie-Dick test to ensure accurate results and avoid false failures.
A.True
B.False
6.To save time, it is acceptable to run the Bowie-Dick test pack in the same cycle as the biological indicator test pack.
A.True
B.False
7.When running the Bowie-Dick test, the sterilizer should be programmed to run which of the following cycles?
A.270-273ºF prevacuum for 4 minutes or longer with 20 minute dry time
B.270-273ºF prevacuum for 3 ½ - 4 minutes with 1 minute dry time
C.270-273ºF gravity-displacement for 3 ½ - 4 minutes with 1 minute dry time
8.If the Bowie-Dick test result shows a FAIL (x), what should you do?
A.Use the sterilizer and schedule routine maintenance
B.Take the sterilizer out of use and notify your supervisor
C.Ignore the result
9.If a major repair of the sterilizer is required, qualification testing should be conducted before the sterilizer is put back into routine use.
A.True
B.False
10.During sterilizer qualification testing, the Bowie-Dick test is:
A.Run in three consecutive cycles after running three consecutive biological indicator process challenge device cycles
B.Run in three consecutive cycles before running three consecutive biological
C.indicator process challenge device cycles
D.Run once
E.Not needed


BY ADAM OKADA
Q: In January 2025, you wrote an article in HPN about wet loads and the potential causes for them. Do you have more information on how to address wet packs specifically?
A:You know I love a good callback!
In that article (HPN, January 2025)1, we were discussing “wet loads” and the routine cause for them. Much of the same information will apply here.
For example, are you documenting the configuration and taking pictures of the sterilization rack and contents? This can be helpful in identifying a root cause. Load configuration can be one of the causes of wet loads if rigid containers are placed over wrapped items/linen packs or if containers/trays are placed too closely together. Items should be evenly spaced (I make sure that I “slice” between the trays with the edge of my hand, like a karate chop), with rigid containers and heavier trays on the bottom rack, while lighter wraps and peel pouches should be on the top. I would recommend taking a picture of the sterilization rack and examining the configuration to see if anything jumps out visually.
Overpacked Tray: It could be an overpacked tray with dense metal mass or potentially some heavy sets weighing over 25 pounds. What were the contents of the load? All sterilizers have a weight limit for cycles, so it could be possible that you’re overloading the sterilizer itself. All questions that can be investigated with proper wet load documentation.

Loading Overly Wet Items: You may also want to see if items are being loaded into the sterilizer wet. Too much water in the chamber before sterilization can throw off the optimum wet/dry steam ratio (97–98% dry to 2–3% wet), so make sure you are drying items before packaging them for the autoclave. Yes, we know not to load the low-temp sterilizers with any moisture, but it’s important to think about that drying step with the steam sterilizer as well.
Specific Product Consideration: Say you’ve gone through all the troubleshooting steps and you’re still having issues with sporadic wet trays in your loads. You may need to con-

Wet/Dry Steam Ratio: Of course, the primary point of the January 2025 article1 was to consider the wet/dry steam ratio. Steam should be 97–98% dry saturated steam and 2–3% water. At one of my previous facilities where I was dealing with wet loads, testing revealed that we had 92–93% saturated steam and 7–8% water. This was the cause of our wet loads, and it was solved with some new steam traps installed on the boiler intake line.
Autoclave Chamber Drain: It could be a blocked or clogged autoclave drain, which would conceivably slow down the vacuum process in pre-vacuum sterilizers. Make sure to check and clean your chamber drain as recommended by the manufacturer.
sider specific products designed for drying. For example, you might have a loaner tray with dense metal mass and multiple layers. This hypothetical tray will cause more of an issue with drying than its standard counterparts simply because of its construction.
Moisture Wicking: This is a good point to talk about the concept of moisture wicking. Here’s the way it’s been explained to me. Moisture accumulation will occur during the steam sterilization process because about 2–3% of the steam content will be water. To assist with the drying process, it’s helpful to have moisture-wicking


























SafeGuard Dry Sterilization Wrap. Healthmark, A Getinge company.6
materials (e.g. towels or liners) under the trays. The moisture can accumulate on these materials instead of the tray, spread out over the full surface area of the material, and then be dried easier than if the moisture was sitting on plastic, polymer, aluminum, or another tray/ container surface.
Towels and Lint: In the olden days of my youth in sterile processing, we used towels to assist with moisture wicking. This was problematic for multiple reasons, but mostly for the complications that can arise from lint. In another article, callback from May 2025 (HPN, May 2025)2, I discussed the incredible research from Dr. Wava Truscott:
“In an article by Dr. Truscott in Biomedical Instrumentation and Technology called “Lint Fiber–Associated Medical Complications Following Invasive Procedures”3, she writes, “The most prevalent complications associated with lint contamination of surgical wounds include thrombogenesis (blood clot formation), infections (increased frequency, severity, biofilm formation), amplified inflammation, poor quality wound healing, granulomas, and adhesions.”
Suffice to say, lint inside surgical trays is very bad, and we should avoid laundered reusable towels inside our trays if it’s at all possible.
Disposable Liners: So, what is left if we get rid of our reusable towels? I recommend disposable liners (e.g. Underguard Dry™ Advanced 5) that can accomplish the same result as towel wicking, arguably even more effectively.
Dual-layer Liners: For those dense, multi-layer trays (like the hypothetical loaner tray mentioned earlier), I would recommend liners with dual-layer construction specifically designed for these types of difficult-to-dry sets.
Specialized Wrap: There are also special wraps on the market designed to deal with wet pack issues. Instead of being dual-bonded SMS layers, one layer of Healthmark’s SafeGuard Dry Sterilization Wrap is “wetlaid.”
Wetlaid material is a type of nonwoven fabric produced using a process similar to papermaking, where short fibers are suspended in water and then dried on a moving wire to form a uniform web, which is then bonded

Underguard Dry™ Advanced tray liner. Healthmark, A Getinge company.5
together to create a final product. This wetlaid process allows for the formation of highly precise, uniform, and porous materials from a variety of fibers, including natural, synthetic, and even glass fibers. Wetlaid materials are used in many applications, such as filters, teabags, and structural components, as well as medical materials like sterilization wrap.
Because of the unique properties of the wetlaid layer, SafeGuard Dry Sterilization Wrap6 can be a great option for facilities dealing with dense, heavy, or multi-level trays and complicated wet pack situations. HPN
1.Okada, A. (2024, Dec. 24). Wet Loads. Healthcare Purchasing News (HPN ). https://www. hpnonline.com/sterile-processing/article/55246796/wet-loads
2.Okada, A. (2025, April 25). Lint-free or Non-Linting? Healthcare Purchasing News (HPN). https://www.hpnonline.com/sterile-processing/article/55285901/lint-free-or-non-linting
3.Truscott, W. (2023). Lint Fiber-Associated Medical Complications Following Invasive Procedures.Biomedical instrumentation & technology,57(s1), 5–10. https://doi.org/10.2345/08998205-57.s1.5
4.Truscott, W. (2014). The Clinical Issue: Foreign Debris In Post-Surgical Complications [Photograph: Lint fiber with bacteria on p 2], Knowledge Network Kimberly-Clark Healthcare Education, Issue 8, 1–8.
5.Healthmark. (n.d.). Underguard Dry™ Advanced. Healthmark, A Getinge company. https:// www.hmark.com/product/underguard-dry-advanced/ 6.Healthmark. (n.d.). SafeGuard Dry Sterilization Wrap. Healthmark, A Getinge company. https://www.hmark.com/product/safeguard-dry-sterilization-wrap/
Adam Okada has 18+ years of experience in Sterile Processing and is passionate about helping improve the quality of patient care by giving SPD professionals and their partners greater access to education and information. He has worked in just about every position in the Sterile Processing Department, including Case Cart Builder, SPD Tech I, II, and III, Lead Tech, Tracking System Analyst, Supervisor of both SPD and HLD, Manager, and now as an Educator. Adam is the owner of Sterile Education, the world’s first mobile application dedicated to sterile processing education, and a former Clinical Manager at Beyond Clean. He has published articles for HSPA’s Process magazine, is a co-chair on AAMI WG45 as well as co-project manager for the KiiP “Last 100 Yards” group, and is the former President for the Central California Chapter of HSPA. Adam is currently a Clinical Education Specialist at Healthmark, A Getinge company, where he works on Healthmark webinars, hybrid events, and educational videos, as well as the “Ask the Educator” Podcast with Kevin Anderson.
Your SPD Team is Doing Stellar Work. Your Tools? Not So Much.
A new wrap has landed and it’s out of this world. SafeGuard Dry is ready to join the mission against wet packs.

Let’s beat the wet pack invasion together. Request a sample of SafeGuard Dry.























































































































































































As the largest manufacturing and supply chain event in 2026, MODEX showcases supply chains from every angle, featuring over 1,000 exhibits, 200+ educational sessions and four keynotes. MODEX’s unique combination of hands-on product and technology demonstrations, education and face-to-face connections makes it the can’t-miss experience for supply chain professionals in 2026.











Learn more and register for free admission at MODEXSHOW.com.














