









































































JULY 28 TH – AUGUST 1 ST CHICAGO, IL BOOTH #1413
Versatile biochemistry analyzers, offering the world’s largest test menu to suit all laboratory sizes.
Leading provider of complete quality control solutions including; daily quality control, calibration verification and proficiency testing for results you can trust.
Near patient care solutions delivering fast results and workflow efficiencies.
Comprehensive range of infectious disease testing solutions for inherited diseases, mutation analysis and SNP genotyping.
Extensive range of open channel biochemistry reagents facilitating routine and niche diagnostic testing.

 By Christina Wichmann
Editor in Chief
By Christina Wichmann
Editor in Chief
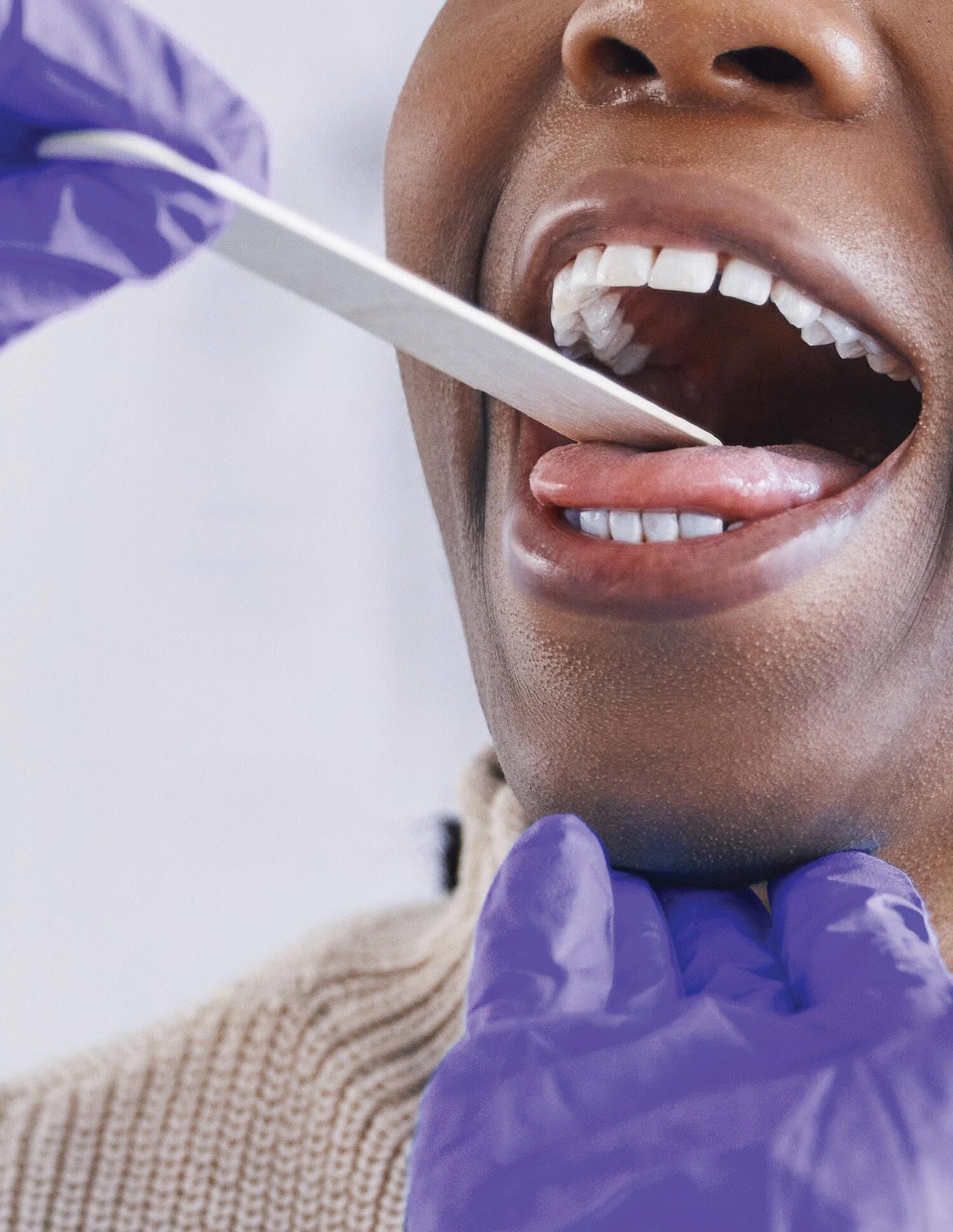
During the pandemic, I was tested for COVID-19 in a laboratory. This test really scared me. The lady testing me stuck the swab so far up my nose, I was afraid I was going to get hurt. I never wanted to get tested again. I wasn’t alone feeling this way — some people were even injured from too deep swab tests. When COVID-19 self-testing kits came out, I was relieved. It probably encouraged people to keep testing when they started showing symptoms of a respiratory illness. This spring, the U.S. Food and Drug Administration (FDA) approved the United States’ first human papillomavirus (HPV) self-collection tests for women. These tests will now allow women to self-collect vaginal specimens for HPV in healthcare settings, including physician offices, retail pharmacies, clinics, and mobile vans. The samples will then be sent to a laboratory for testing. Cervical cancer is preventable, and screening plays an important role in early detection. However, over half of the cervical cancer cases in the United States are among women who have never been screened or who do not participate in routine screening.1 Allison McMullen from Roche shared with me that there are many reasons for this: “Some may live in areas without many health resources. Some may have a history of a traumatic experience, cultural concerns, or are embarrassed to receive screenings.” Allison said, “By providing women the option to perform self-collection, we can reduce some of the barriers to participation and increase the level of screening.”
HPV is the cause of most cervical cancers, and there are many strains of HPV viruses, with some posing a higher risk for causing cancer than others. There is no cure or treatment for HPV, but there are treatments for the complications it can cause, such as genital warts, cancer-causing cells, and cervical cancer. According to the National Cancer Institute, the full public health impact of prophylactic HPV vaccination on reducing cervical cancer rates will not be realized for at least another generation.1 So improving screening access is very important in prevention efforts. Self collection for cervical cancer is already available in other countries such as Denmark, Sweden, the Netherlands, Kenya, Australia, and New Zealand.
The National Cancer Institute has been working in a public-private partnership called the Cervical Cancer “Last Mile” Initiative to improve cervical cancer screening to underserved and never‐screened/under‐screened women. Part of this initiative is the Self-collection for HPV testing to Improve Cervical Cancer Prevention (SHIP) Trial. Abbott, BD, and Roche self-collection devices are part of this trial. Dr. Jeff Andrews of BD shared that participant enrollment is scheduled to begin this summer, and the trial will evaluate the usability, acceptability, accuracy, and effectiveness of self-collection testing in healthcare and other settings, including at home.
I welcome your comments and questions — please send them to me at cwichmann@mlo-online.com.
REFERENCE
1. Sahasrabuddhe V. Addressing a “last mile” problem in cervical cancer screening. Nih.gov. Accessed June 11, 2024. https://deainfo.nci.nih.gov/advisory/joint/1219/Sahasrabuddhe.pdf.
PUBLISHER Chris Driscoll cdriscoll@endeavorb2b.com
EDITOR IN CHIEF Christina Wichmann cwichmann@mlo-online.com
MANAGING EDITOR Erin Brady ebrady@endeavorb2b.com
PRODUCTION MANAGER Edward Bartlett
ART DIRECTOR Kermit Mulkins
AUDIENCE DEVELOPMENT/LIST RENTALS
Laura Moulton | lmoulton@endeavorb2b.com
ADVERTISING SERVICES MANAGER Karen Runion | krunion@endeavorb2b.com
ADVERTISING
DIRECTOR OF SALES EAST COAST/MIDWEST SALES, CLASSIFIEDS Carol Vovcsko (941) 321-2873 | cvovcsko@mlo-online.com
SOUTH/WEST COAST/ILLINOIS SALES Lora Harrell (941) 328-3707 | lharrell@mlo-online.com
MLO EDITORIAL ADVISORY BOARD
John Brunstein, PhD, Biochemistry (Molecular Virology) President & CSO PathoID, Inc., British Columbia, Canada
Lisa-Jean Clifford, COO & Chief Strategy Officer Gestalt, Spokane, WA
Barbara Strain, MA, SM(ASCP), CVAHP Principal, Barbara Strain Consulting LLC, Formerly Director, Value Management, University of Virginia Health System, Charlottesville, VA
Jeffrey D. Klausner, MD, MPH Professor of Preventive Medicine in the Division of Disease Prevention, Policy and Global Health, Department of Preventive Medicine at University of Southern California Keck School of Medicine. Donna Beasley, DLM(ASCP), Director Huron Healthcare, Chicago, IL
Anthony Kurec, MS, H(ASCP)DLM, Clinical Associate Professor, Emeritus , SUNY Upstate Medical University, Syracuse, NY
Suzanne Butch, MLS(ASCP)CM, SBBCM, DLMCM Freelance Consultant, Avon, OH
Paul R. Eden, Jr., MT(ASCP), PhD, Lt. Col., USAF (ret.) (formerly) Chief, Laboratory Services, 88th Diagnostics/Therapeutics Squadron, Wright-Patterson AFB, OH
Daniel J. Scungio, MT (ASCP), SLS, CQA (ASQ), Consultant at Dan the Lab Safety Man and Safety Officer at Sentara Healthcare, Norfolk, VA
CORPORATE TEAM
CEO Chris Ferrell
PRESIDENT June Griffin
COO Patrick Rains
CRO Paul Andrews
CHIEF DIGITAL OFFICER Jacquie Niemiec
CHIEF ADMINISTRATIVE AND LEGAL OFFICER Tracy Kane
EVP CITY SERVICES & HEALTHCARE Kylie Hirko 30 Burton Hills Blvd., Suite 185 Nashville, TN 37215 800-547-7377 | www.mlo-online.com
Medical Laboratory Observer USPS Permit 60930, ISSN 0580-7247 print, ISSN 2771-6759 online is published 10 times annually (Jan, Feb, Mar, Apr, May, Jul, Aug, Aug CLR, Sep, Nov) by Endeavor Business Media, LLC. 201 N Main St 5th Floor, Fort Atkinson, WI 53538. Periodicals postage paid at Fort Atkinson, WI, and additional mailing offices. POSTMASTER: Send address changes to Medical Laboratory Observer, PO Box 3257, Northbrook, IL 60065-3257. SUBSCRIPTIONS: Publisher reserves the right to reject non-qualified subscriptions. Subscription prices: U.S. $160.00 per year; Canada/Mexico $193.75 per year; All other countries $276.25 per year. All subscriptions are payable in U.S. funds. Send subscription inquiries to Medical Laboratory Observer, PO Box 3257, Northbrook, IL 60065-3257. Customer service can be reached toll-free at 877-382-9187 or at MLO@ omeda.com for magazine subscription assistance or questions. Printed in the USA. Copyright 2024 Endeavor Business Media, LLC. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopies, recordings, or any information storage





COVID-19 eliminated a decade of progress in global level of life expectancy
The latest edition of the World Health Statistics released by the World Health Organization (WHO) reveals that the COVID-19 pandemic reversed the trend of steady gain in life expectancy at birth and healthy life expectancy at birth (HALE).

»10 years of progress in improving life expectancy was wiped out by the pandemic in two years.
–1.8 years from the global life expectancy (to 71.4 years) between 2019 and 2021 (back to the level of 2012).
–1.5 years from the global healthy life expectancy (to 61.9 years) in 2021 (back to the level of 2012).
Enforcement discretion granted for the use of conjunctival swabs with the CDC Human Influenza Virus Real-Time RT-PCR Diagnostic Panel, Influenza A/H5 Subtyping Kit
On May 24, 2024, the U.S. Food and Drug Administration (FDA) granted enforcement discretion for the use of conjunctival swabs as an acceptable specimen type with the CDC Human Influenza Virus Real-Time RT-PCR Diagnostic Panel, Influenza A/H5 Subtyping Kit.

>1 billion people aged five years and older were living with obesity in 2022.
Laboratories may now submit conjunctival swabs from patients that meet Epidemiologic criteria AND either Clinical OR Public Health Response criteria for novel influenza A virus testing to their state public health laboratories for use with the CDC assay alongside a paired nasopharyngeal swab (NPS).
The risk posed by novel influenza A viruses to the public still remains low.

>1/2 billion people were underweight in 2022.
https://www.who.int/news/item/24-05-2024-covid-19-eliminated-a-decade-ofprogress-in-global-level-of-life-expectancy
Contact flusupport@cdc.gov for any additional questions, comments, or concerns.
U.S. clinical trials begin for twiceyearly HIV prevention injection
Two clinical trials have launched to examine a novel longacting form of HIV pre-exposure prophylaxis (PrEP) in cisgender women and people who inject drugs.
The mid-stage studies will assess the safety, acceptability, and pharmacokinetics (how a drug moves through the body) of lenacapavir, an antiretroviral drug administered by injection every six months. The studies are sponsored and funded by Gilead Sciences, Inc. and implemented through the HIV Prevention Trails Network (HPTN).

The studies will take place at HPTN sites in the United States and enroll people who might benefit from taking PrEP. The first trial will enroll cisgender women, with a focus on making enrollment accessible to women who self-identify as Black and/or Latina. The second trial will enroll a diverse group of people who inject drugs. In both studies, participants will be randomly assigned to receive either injectable lenacapavir or an FDA-approved PrEP formulation consisting of oral tenofovir disoproxil fumarate and emtricitabine.

Participants will provide laboratory samples and give qualitative feedback on their experience taking each form of PrEP.
On May 21st, CDC Principal Deputy Director Nirav D. Shah laid out the agency’s recommendations that influenza surveillance systems continue operating at enhanced levels during the summer and to increase the number of positive influenza A virus samples submitted for subtyping to help detect even rare cases of human H5N1 virus infection in the community.
Shah emphasized the importance of remaining vigilant and outlined a nationwide influenza virus monitoring plan for the summer season, which is a time when influenza activity and testing typically decline. The goal of this plan is to maintain heightened awareness of circulating influenza viruses given the ongoing outbreak of H5N1 among poultry and U.S. dairy cattle.
Specifically, Shah asked jurisdictions to work with clinical laboratories to increase submissions of positive influenza virus samples to public health laboratories for subtyping. Subtyping is a process that determines whether the influenza A sample is a common, seasonal influenza virus or a novel virus like H5N1.
Prime Plus provides the most clinical value of any blood gas/critical care analyzer profile by adding essential tests for kidney function (BUN, Creatinine, eGFR), plasma volume (ePV), ionized magnesium (iMg) and MCHC.
Creatinine, eGFR, and BUN
Over 50% of patients admitted to the ICU develop some degree of acute kidney injury.1 Creatinine, eGFR, and BUN monitoring provides indication of changes in kidney function and helps guide therapy to prevent AKI.
Estimated Plasma Volume (ePV)
The plasma volume status of a patient is one of the top priorities in evaluating and treating critical illness including CHF, ARDS, AKI, and Sepsis.2-4
Ionized Magnesium (iMg)
Hypomagnesemia is a frequent finding in critically ill patients.5 Magnesium therapy guided by real time ionized magnesium monitoring has been shown to improve outcome in these patients.6
Mean Corpuscular Hemoglobin Concentration (MCHC)
Helps differentiate types of anemia.

Test Menu Includes
novabiomedical.com
1. Mandelbaum T et al. Outcome of critically ill patients with acute kidney injury using the AKIN criteria. Crit Care Med 2011;39(12):2659-2664.
2. Kobayashi M et al. Prognostic Value of Estimated Plasma Volume in Heart Failure in Three Cohort Studies; Clin Res Cardiol 2019;108(5): 549-561.

3. Niedermeyer, et al. Calculated Plasma Volume Status Is Associated With Mortality in Acute Respiratory Distress Syndrome. Critical Care Explorations: September 2021, V3(9):1-9.
4. Kim HK et al. Prognostic Value of Estimated Plasma Volume Status in Patients with Sepsis. J Korea Med Sci 2020;9(37):1-10.
5. Soliman HM. Development of ionized hypomagnesemia is associated with higher mortality rates. Crit Care Med 2003;31(4):1082-7.
6. Wilkes NJ et al. Correction of ionized plasma magnesium during cardiopulmonary bypass reduces the risk of postoperative cardiac arrhythmia. Anesth and Analg 2002;95(4) 828-834.
Louis Pasteur likely predicted the concept of antimicrobial resistance (AMR) with his famous quote in the late 1800s, “Messieurs, c’est les microbes qui auront le dernier mot.” (Gentlemen, it is the microbes who will have the last word.)”1
Later, penicillin was discovered on September 28, 1928, by Alexander Fleming and approved for clinical use in 1941. During his 1945 Nobel Lecture, Flemming warned of resistance through the dangers of underdosing. “It is not difficult to make microbes resistant to penicillin in the laboratory by exposing them to concentrations not sufficient to kill them, and the same thing has occasionally happened in the body. The time may come when penicillin can be bought by anyone in the shops. Then there is the danger that the ignorant man may easily underdose himself and by exposing his microbes to non-lethal quantities of the drug make them resistant.”2
Throughout the next several decades, many classes of antibiotics were developed.3 (See Figure 1.) Interestingly, resistance to SalvarsanTM (the first clinically used antibiotic ~1910) took approximately 20 years to emerge.3,4 Resistance to sulfonamides and penicillin occurred much sooner [~12 years], as outlined in the timeline below. 3 Newer antibiotics approved since 2010 are listed in Table 1 and consist of combinations of current antibiotic classes or congeners of existing molecules.
Early in my training as a clinical pharmacist interested in infectious diseases, I can remember two specific examples of
See test online at https://ce.mlo-online.com/ courses/the-history-ofantimicrobial-resistance-andthe-important-role-diagnosticsplays-to-combat-it/ Passing scores of 70 percent or higher are eligible for 1 contact hour of P.A.C.E. credit.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
Scan code to go directly to the CE test.
Discuss the history of the discoveries and warnings of antibiotic use.
List the agencies and organizations involved in the development of antibiotic stewardship programs. Identify the top microorganisms and antibiotics that contribute to AMR.
Discuss the advances in antimicrobial laboratory testing and therapies and how they contribute to combatting AMR infections.
aggressive efforts by sales representatives to position an antibiotic as a preferred agent in the treatment of almost any type of infection. Such outlandish claims provide a perfect example of behaviors and perceptions within healthcare practice that contribute to the inappropriate use and overprescribing of antibiotics. These situations included a sales representative stating, “If you don’t know the bug, my new 3rd generation cephalosporin is the drug.”
At this time in the early 1990s, the sales representative knew that it could take at least 48–72 hours to get a blood or other culture result to begin to show sufficient pathogen growth to cross the threshold needed for identification and susceptibility testing (ID/AST). The sales representatives were likely trying to capitalize on the use of empiric broad-spectrum therapy to cover a wide variety of bacterial pathogens until
2011
2012
2014
2015
2016
2017
2018
• Fidoxamicin
• Bedaquiline
• Ceftolozane/tazobactam
• Delamanid
• Finafloxacin
• Pazulfloxacin mesylate
• Posiconazole
• Tedizolid
• Ceftazidime/avibactam
• Isavuconazole
• Zabofloxacin hydrochloride
• Nemonoxacin
• Delafloxacin meglumine
• Meropenem/ vaborbactam
• Ozenoxacin
• Eravacycline
• Omadacycline
• Plazimicin
• Alalevonadifloxacin mesylate
• Cefiderocol
• Imipenem/cilistatin/ relebactam
2019
2021
• Lascufloxacin hydrochloride
• Lefamulin
• Nadofloxacin
• Pretomanid
• Sarecycline hydrochloride
• Contezolid
• Ibrexafungerp
• Macrolide
• Diaryquinalone (Anti-mycobacterial)
• Cephalosporin/ betalactamase inhibitor
• Nitroimidazole
• Fluroquinolone
• Fluroquinolone
• Azole antifungal
• Oxazolidinone
• Cephalosporin/ class A beta-lactamase inhibitor
• Triazole antifungal
• Fluroquinolone
• Fluroquinolone
• Fluroquinolone
• Carbapenem/ betalactamase inhibitor
• Fluroquinolone
• Tetracycline
• Tetracycline
• Aminoglycoside
• Fluroquinolone pro-drug of levonadiafloxacin
• Cephalosporin
• Carbapenem/ betalactamase inhibitor
• Fluroquinolone
• Pleuromutilin
• Fluroquinolone
• Nitroimidazooazines class of antimycobacterials
• Tetracycline
• Oxazolidinone
• Antifungal: First-in-class triterpenoid Glucan Synthase inhibitor
ID/AST results became available, which could influence the use of their product.
In another memorable situation, I had a sales representative say to me “Don’t forget about my broad-spectrum fluoroquinolone for cold and flu season!”When I asked this salesperson if they realized they were potentially making an off-label claim that an antibiotic class prescription was not indicated for viral infections, he seemed offended.
These examples, while they initially may seem humorous, serve as strong representative examples of the inappropriate use of antimicrobial therapy.
As awareness and concern for antibiotic use grew, several governmental and professional organizations began raising awareness over the concern for the rising rates of resistant pathogens. These organizations leveraged the President’s Council of Advisors on Science and Technology (PCAST) to educate and enlighten government officials on antimicrobial resistance.5

Figure 1. Timeline showing the decade new classes of antibiotic reached the clinic. The antibiotics are coloured per their source: green = actinomycetes, blue = other bacteria, purple = fungi and orange = synthetic. At the bottom of the timeline are key dates relating to antibiotic discovery and antimicrobial resistance, including the first reports of drug resistant strains methicillin-resistant S. aureus (MRSA), vancomycin-resistant enterococci (VRE), vancomycinresistant S. aureus (VRSA) and plasmid-borne colistin resistance in Enterobacteriacee.
In 2007 the Infectious Diseases Society of America (IDSA) published guidelines on developing institutional programs to enhance the adoption of antimicrobial stewardship practices.6 Their goal was to establish realistic guidelines for antibiotic use, due to the rising rates of antimicrobial resistance and a shrinking pipeline of de novo antibiotic approvals.
In 2013, the Centers for Disease Control and Prevention (CDC) published a report to raise awareness and establish priorities concerning resistant pathogens entitled Antibiotic Resistance Threats in the United States. 7 This report set off a series of government actions in the fight against AMR (see Figure 2).8
In 2014, PCAST reported on combating antibiotic resistance to the President. An outcome of the PCAST report was the agreed goal to develop a working group of experts in antibiotic resistance in both human and veterinary sectors and to develop recommendations for the U.S. government to take actionable steps to curb the growing problem of AMR.
A Presidential executive order (13676: “Combating Antibiotic-Resistant Bacteria) was issued that accompanied the PCAST report in September 2014.9 The executive order set off a chain of initiatives, such as developing the Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria (PACCARB), to forge ahead in the fight against antimicrobial resistance (Figure 2).
In 2016, the IDSA and SHEA societies published guideline updates detailing how to effectively implement an antimicrobial stewardship program.10 By the end of 2017, the Centers for Medicare & Medicaid Services (CMS) was to have federal regulations requiring the development and implementation of “robust antibiotic stewardship programs” in hospitals, critical access hospitals, and long-term care, and nursing home facilities. Outpatient antibiotic use is stated to account for ~80% of antibiotic use,11 thus it was essential for outpatient antibiotic stewardship programs to soon follow suit as well.
In 2022, a systematic analysis was published entitled “The Global Burden of Bacterial Antimicrobial Resistance
in 2019.”12 This publication provided the first global estimates of the burden of bacterial AMR and the most comprehensive estimates to date. The systematic review included a broad range of pathogens and pathogen-drug combinations. The findings estimated that 4.95 million deaths were associated with bacterial AMR, which included 1.27 million deaths attributable to bacterial AMR.12 Estimations of deaths and disability-adjusted life-years were determined for 23 pathogens, 88 pathogen-drug combinations, and included 204 countries and territories.
Three infectious syndromes accounted for 79% of global AMR-associated deaths: lower respiratory tract infections (LRTI), bloodstream infections (BSI), and intra-abdominal infections (IAAI). Six pathogens accounted for 72% of Global AMR-associated deaths: E. coli, S. aureus, K. pneumoniae, S. pneumoniae, A. baumannii, and P. aeruginosa. Resistance to
Sept. 2013: CDC report: Antibiotic Resistance Threats in the United States, 2013
Sept. 2014: Executive Order Combating Antibiotic-Resistant Bacteria, National Strategy to Combat Antibiotic-Resistant Bacteria, PCAST Report on Combating Antimicrobial Resistantance
March 2015: National Action Plan for Combating AntibioticResistant Bacteria
June 2015: White House Forum on Antibiotic Stewardship
June 2016: CDC awards $26 million to Academic Medical Centers as Prevention Epicenters Program
July 2016: CDC provides $67 million to health departments through CDC’s Epidemiology and Laboratory Capacity for Infectious Diseases Cooperative Agreement (ELC)
Oct. 2016: CDC awards $14 million to fund new approaches to combat antibiotic resistance to support activities
Figure 2. Timeline of U.S. federal engagement in antimicrobial resistance.
beta-lactams and fluoroquinolones accounted for greater than 70% of Global AMR-associated death.12 Seven pathogen-drug related combinations account for >50,000 deaths, including Methicillin-resistant S. aureus (MRSA) [>100,000 deaths], multi-drug resistant (MDR) M. tuberculosis, 3rd generation cephalosporin-resistant E. coli, carbapenem-resistant A. baumannii, fluroquinolone-resistant E. coli, carbapenem-resistant K. pneumoniae, and 3rd generation cephalosporin-resistant K. pneumoniae.
The top three global infectious disease threats in 2019 were AMR, (1.27M), malaria (696K), and HIV/AIDS (690K).12
Antimicrobial resistance was noted as the highest-burden in low-resource settings. Several goals were identified to combat rising AMR rates moving forward:
• Infection prevention and control to avoid the spread of AMR
• Vaccinations to reduce the need for antibiotics
• Improve access to essential antibiotics where needed
• Reduce exposure to antibiotics unrelated to human disease: One Health
• Optimize the use of antibiotics (antibiotic stewardship) guided by diagnostics (“building infrastructure that allows clinicians to diagnose infection accurately and rapidly is crucial”)
• Maintain investment in the development of a pipeline for new antibiotics
• Integrate fighting AMR as a priority in national strategies
• Increase microbiological laboratory and data collection capacity to improve data collection and understanding the threat of AMR
Several key points identified in the Global Burden of Bacterial Antimicrobial Resistance in 2019 follow a common theme expressed by Alain Mérieux in 2017: “Without diagnostics, medicine is blind.”13
The evolution of microbial testing has evolved over centuries and is currently advancing rapidly with automation, computerization, and nanotechnology. The first major discovery in microbiology was made in 1674 by Anthony van Leeuwenhoek when he peered through a drop of lake water through a glass magnifying lens he carefully ground. 14 Although his lenses only magnified up to 300-fold, he was able to describe the three major shapes of bacteria: the cocci,
bacilli, and spirilla. Robert Koch is credited for developing the pure culture techniques we use today. In the late 1870s, Koch realized that the isolation of pure cultures would be simplified on a solid medium on which a single isolated cell could multiply in a defined area. Koch’s laboratory also developed the Petri dish and using agar.14
Conventional blood culture processes and systems remained in place until the late 1960s when automated systems were introduced.15 Critical factors and recommended guidelines for optimal recovery of pathogens in blood were defined and established during the latter part of the 20th century and helped to develop best practices for the collection, processing, and interpretation of blood cultures. Examples of such critical factors and guidelines include but are not limited to, adequate skin disinfection, volume of blood collected, number and timing of blood cultures, blood culture bottles/ media types, and duration of incubation and testing.16 With the establishment of critical factors came the need for better technology to process, monitor, and report on cultures and their results. Continuous blood culture monitoring systems were introduced and led to increased capacity of culture bottles, less manipulation, and earlier reporting of test results.15 The first automated blood culture systems were introduced in the early 1970s. With newer and faster technologies, the isolated rank order of recovery was also determined, which helped to provide input on automated testing development.15 DNA sequencing was developed in the late 1970s followed a decade later by the polymerase chain reaction (PCR) technology.21 These technologies are referred to as nucleic acid amplification tests (NAATs).17 PCR testing has evolved to include multiplex PCR (including and detecting multiple pathogens on a panel at once) and can provide results in about one hour.18
Applying automated diagnostic results to active antimicrobial stewardship has been shown to improve targeted antimicrobial therapy, improve patient care, and reduce antibiotic and length of stay costs (Figure 3). 19 Integration of antimicrobial and diagnostic stewardship programs has also been an important step in the fight against AMR.20 Recently, a more robust multiplex PCR system was launched. It is FDA-approved and CLIA-waived with a smaller footprint and faster results turn-around time of about 15 minutes.21
The major advancements and improvements in molecular biology that were transitionally incorporated into sequencing

Figure 3. Comparison of time to organism identification, availability of phenotypic antimicrobial susceptibility results, and first appropriate modification of antimicrobial therapy for the subset of study subjects with organisms represented on the rapid multiplex polymerase chain reaction (rmPCR) panel (n = 481). Time O is when the positive Gram stain result was reported. Median time in hours (interquartile range [IQ]) to organism identification: control 22.3 (17-28), both rmPCR and rmPCR + stewardship 1.3 (0.9-1.6); de-escalation: control 39 (19-56), rmPCR 36 (22-61), rmPCR + stewardship 20 (6-36); escalation: control 18 (2-63), rmPCR 4 (1.5-24), rmPCR + stewardship 4 (1.8 9). *P<.05 vs control; *P<.05 vs control and rmPCR groups.
technologies led to the second and third sequencing methodologies, commonly termed next-generation sequencing (NGS).17 The advantages of NGS compared with traditional sequencing methods include higher throughput (including multiplexing), higher sensitivity in detecting low-frequency variants, faster turnaround time for high sample volumes, and lower cost.22 Although NGS is not without limitations, it serves as a dramatic improvement in evaluating rare diseases, pathogen identification and antibiotic resistance profiles, and disease outbreak tracking in clinical settings (e.g., Ebola Virus, Malaria, and SARS-CoV-2).
Bacteriophage (phage) therapy has gained resurgent interest in recent years due to the lack of therapeutic options for patients unresponsive to conventional antimicrobials because of antimicrobial resistance.23 Phage therapy is considered compassionate, salvage therapy for patients with resistant pathogens from chronic/ recurrent infections such as urinary tract, respiratory, and skin/soft tissue infections. Other situations are implantable devices and joint replacements. Phage therapy is not FDA-approved and there is a need for more homogeneous randomized controlled trials to establish its place in patient care.23
Vaccines are a major achievement in medicine, but the development of more effective vaccines against infectious diseases is essential for the prevention and control of emerging pathogens worldide.24 Traditional vaccines, typically inactivated pathogens, have shown great success in the prevention or eradication of more than 30 infectious diseases.25
Additionally, mRNA vaccines have emerged as a revolution in vaccine fields due to their simplicity and adaptability in
antigen design, the potential to induce both humoral and cell-mediated immune responses, and the high efficiency, and rapid, low-cost production in using similar manufacturing platforms for different mRNA vaccines. Recent research in infectious disease includes emerging or reemerging infectious pathogens (e.g., HIV, respiratory syncytial virus, influenza, streptococcus, etc.). Cancer vaccine research is in phase I/II clinical trials (e.g., colorectal, glioblastoma, ovarian, prostate, etc.).25
In conclusion, with rising rates of AMR and a lack of de novo antibiotics, maximizing our diagnostic capabilities is critical to use rapid and targeted therapy, in addition to phage and vaccine therapies to optimize patient care.

Chris Groke, PharmD, BCPS, BCIDP serves as a Senior Medical Science Liaison for bioMérieux US Medical Affairs Division. His background is an Infectious Diseases Clinical Pharmacist with over 35 years of pharmacy practice and management in many health-systems. His Doctor of Pharmacy is from the Medical University of South Carolina, and his Bachelor of Science is from Auburn University Harrison College of Pharmacy.
References are available online at mlo-online.com/55054880.

The neurodegenerative processes that precede dementia begin to chip away at a person’s cognitive reserve many years before mental capacity is determined to be outside the normal population range.1 Mild cognitive impairment (MCI) represents a state of partial decline that may be evaluated by neuropsychological testing but falls short of the criteria for dementia. Alzheimer’s disease (AD) is the most common form of dementia and affects an estimated 6.9 million Americans above the age of 64. 2 In Europe, more than 10 million people are affected by MCI and roughly half are expected to progress to dementia within 5 years. 3 Due to the lack of routine cognitive screening, 50% to 70% of patients with symptoms of AD are not recognized or diagnosed in primary care.4 For early onset AD, in which patients and those around them are less likely to consider medical help, diagnosis may be delayed 2-3 years. 5 The diagnostic process is even less effective for those with subjective or mild cognitive impairment (See Figures 1, 2, 3). Therefore, more effective diagnostic solutions are needed. To facilitate the advancement of new solutions, numerous research projects are currently underway. This article provides a brief introduction to some recent initiatives.
The AI-Mid Connector project has enrolled more than half of its planned 1,000 patients in a study of AI tools for prediction of their risk to develop dementia based on high-density magneto- and electroencephalography combined with cognitive tests and genetic biomarkers.6 Wider use of wearable technologies for tracking physical activities, speech, and other biomarkers provides additional possibilities for detecting patterns that may relate to onset of dementia.
The CUBOId study was designed to acquire data from wearables and other fixed sensors from participants diagnosed with MCI or AD and their live-in partners in the United Kingdom. Deep learning methods will be utilized to analyze activity and speech patterns.7 Observed behaviors that may
be relevant to cognitive decline include patterns of room occupancy, wandering and partner shadowing, and sleep disturbances.
RADAR-AD is a European project funded by the Innovative Medicines Initiative8 focusing on use of technology to monitor the impact of AD on daily functioning. Its goal is to understand how AD affects daily activities and to explore how technologies like smartphones and sensors can track these changes. This project will involve research with 220 AD patients across Europe. It builds on previous work by the RADAR-AD consortium, which focused on developing a biomarker screening platform and identifying biosignatures for early AD diagnosis and interventions.11
A project incorporating a wholistic approach to determining an individual’s risk of dementia, entitled PREDICTOM (Prediction of Neurodegenerative Disease using a Biomarker Screening Platform), recently secured funding for a consortium of 30 partner organizations to develop an AI-based screening platform to identify individuals at risk of developing dementia, even before symptoms manifest. Identifying risks associated with AD is crucial for early diagnosis, as it enables timely interventions for early detection. Moreover, early identification of risk factors not only aids in prompt initiation of treatments but also maximizes the effectiveness of new disease-modifying therapies (DMTs) designed to target the early stages of the disease. A major goal of their approach is to enable individuals to perform screening themselves in the comfort of their homes. More than 4,000 participants will be enrolled in the initial trial. This will include individuals from previous projects including PROTECT UK, PROTECT Norway, and Radar-AD, as well as others. In addition to home collection of blood samples and use of digital biomarkers, additional laboratory tests, magnetic resonance imaging, and electrophysiological testing will be utilized to develop a generalized risk assessment method to prioritize access to preventative measures and to aid in earlier diagnosis and prognosis.9

One overall aim for such projects is to provide primary care physicians with the tools they need to assess disease risk for those in their care, and to also provide easily interpreted evidence for decisions leading to more efficient and effective follow up through specialists. Because there is no cure for AD today, care must be taken to understand how a diagnosis of dementia may affect various aspects of a person’s life. Strained relationships, continuing employment, access to healthcare, and psychological outcomes must all be considered. Such an undertaking requires a wide range of capabilities. As part of the Innovative Health Initiative, which is a European public-private partnership, a group of 30 academic, governmental,





HemoCell delivers workcell efficiency, quality and standardization for Hemostasis labs. Featuring ACL TOP® 750 LAS and HemoHub™ Intelligent Data Manager, it’s the only workcell to combine the leading Hemostasis testing system with specialized lab automation. And, because it can be customized to your lab’s specific needs, HemoCell lets you design the automation layout that’s right for you.

Make your lab a HemoCell lab.

For more information, contact your local Werfen representative.
werfen.com
and industry partners are working in a consortium spanning Europe, Asia, and the Americas. Advances at this scale of effort do not come cheaply and a total of €21M will be provided from November 2023 through to October 2027 to complete this work.
Several reasons for being optimistic that such groundbreaking work may lead to positive results exist:
• Advances in diagnostics methods: The ability to use self-collected capillary blood or interstitial fluid samples for health tracking increases the accessibility of fluid biomarkers for applications like screening for AD. Data on the collection of finger prick blood for biomarkers of neurodegeneration, in a manner similar to what is done for self-testing of glucose levels by diabetics, was presented recently.10

Figure 2. Misdiagnosed: 1 in 5 Alzheimer’s cases may be misdiagnosed. Alzheimer’s disease is often misdiagnosed, possibly causing undue stress for those who don’t have the disease but are told they do, and delays in treatment for others. https://www.cbsnews.com/news/alzheimerscases-misdiagnosed-men-women/
• Advancements in AI and machine learning: These technologies have matured significantly, enabling more accurate and nuanced analysis of complex multi-modal data.
• Widespread wearable device usage: With the ubiquity of fitness trackers, smartphones, and other technologies allowing personal monitoring, they are more easily adopted for clinical applications.
• Greater health and wellness awareness: Society is more aware of and proactive about cognitive health, increasing the demand for accessible monitoring tools.
• Clinical validity of new testing methods: Blood-based biomarkers have shown tremendous potential in early identification of neurodegenerative diseases. This combined with recent developments in digital biomarkers provides a strong foundation for success.
Transitioning from CSF (cerebral spinal fluid) to blood-based biomarker tests is game changing because they are less invasive, more accessible, cost-effective, and highly scalable. This transition is already underway and well documented in the context of specialized research institutions offering such testing today. Wider availability is expected to enable a precision diagnostic approach in neurology akin to what was established in the fields of molecular virology and oncology

Figure 3. ARIA Incidence: New disease modifying therapies for Alzheimer’s disease have emerged. However, the rate of incidence of their well-known side effect, Amyloid Related Imaging Abnormalities (ARIA), was 10-30% in clinical trials. Therapeutic monitoring will be key to patient safety. Yu, Fang F., “Invited Commentary: Radiologist’s Role in Anti-Amyloid Therapy for Alzheimer Disease.” RadioGraphics 43:9 (2023) https://doi.org/10.1148/rg.230205
in past decades. With clinical validation of scalable solutions, it is expected that use in memory clinics and primary care will be possible soon. While challenges exist, most notably in standardization of testing methods and development of kits for the highly automated, random-access instruments that are utilized in centralized laboratories, it is reasonable to assume that such tests will be used routinely in the near future.11
An interesting aspect of the PREDICTOM project is that, while it takes a practical and cost-effective approach to bringing diagnostics closer to the patient, it will also evaluate the most innovative technologies for risk identification — including advanced MRI methods, supplementary tools such as EEG and eye-tracking, and the utilization of blood-based biomarkers for comprehensive assessments. AI models will not only be used for data analysis, but also to direct those at high risk for dementia to personalized interventions that may slow or prevent further cognitive decline, and hopefully to prevent development of dementia.
Such a solution could enable those at risk for dementia to better plan in partnership with caregivers before a decline in mental capacity restricts their ability to make decisions. Furthermore, it could support preventative screening or more frequent disease monitoring, allowing for early interventions when DMTs are most potent. Pharmaceutical developers are investigating the utilization of DMTs in the pre-clinical or prodromal stage of disease, signaling a potential shift to a more proactive approach.
Beyond the direct personal impacts, the societal burden of dementia is significant. The care provided by family and other unpaid caregivers to dementia patients in the United States was recently estimated to be $339.5 billion. These costs combined with payments for professional services related to elders with dementia, raises the total to more than half a trillion dollars. Therefore, the returns in societal savings from earlier and more effective interventions could be significant.
Author Disclosures: Arejas J. Uzgiris is an employee of Siemens Healthcare Diagnostics Inc. and Gaby Marquardt is an employee of Siemens Healthineers AG. Both legal entities, Siemens Healthcare Diagnostic Inc and Siemens Healthineers AG are part of Siemens Healthineers, which is a partner in the PREDICTOM consortium. Arejas J. Uzgiris also owns stock in Siemens Healthineers, Siemens AG, and Bayer. Gaby Marquardt owns stocks in Siemens Healthineers and Siemens AG.
Acknowledgements: The authors would like to acknowledge several individuals for their review of the content for this article, including Lance Ladic and Thomas Benkert at Siemens Healthineers; Dag Aarsland at King’s College, London; and Nicholas Ashton at Banner Healthcare, Arizona.
1. Rossini PM, Miraglia F, Alù F, et al. Neurophysiological Hallmarks of Neurodegenerative Cognitive Decline: The Study of Brain Connectivity as A Biomarker of Early Dementia. J Pers Med. 2020;30;10(2):34. doi:10.3390/jpm10020034.
2. 2024 Alzheimer’s disease facts and figures. Alzheimers Dement 2024;20(5):3708-3821. doi:10.1002/alz.13809.
3. Haraldsen IH, Hatlestad-Hall C, Marra C, et al. Intelligent digital tools for screening of brain connectivity and dementia risk estimation in people affected by mild cognitive impairment: the AI-Mind clinical study protocol. Front Neurorobot. 2024;5;17:1289406. doi:10.3389/ fnbot.2023.1289406.
4. Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimers Dement. 2022;18(12):2669-2686. doi:10.1002/alz.12756.
5. Stojkovic´ T. Survival and pharmacotherapy delay in young onset Alzheimer’s disease. Alzheimer’s & Dementia. 2023;19.
6. Ai-mind.eu. Accessed May 28, 2024. https://www.ai-mind.eu/blog/ai-mindpasses-the-mark-of-500-research-participants-for-its-clinical-study/.
7. Kumpik DP, Santos-Rodriguez R, Selwood J, et al. A longitudinal observational study of home-based conversations for detecting early dementia: protocol for the CUBOId TV task. BMJ Open 2022;23;12(11):e065033. doi:10.1136/bmjopen-2022-065033.
8. Homepage. IMI Innovative Medicines Initiative. Accessed May 28, 2024. https://www.imi.europa.eu/.
9. Gonzalez-Ortiz F, Kac PR, Brum WS, et al. Plasma phospho-tau in Alzheimer’s disease: towards diagnostic and therapeutic trial applications. Mol Neurodegener. 2023;16;18(1):18. doi:10.1186/ s13024-023-00605-8.
10. Huber, H. et al. A finger prick collection method for detecting blood biomarkers of neurodegeneration — a pilot study (DROP-AD). AAIC Abstr. 80275 (2023).
11. Lista S, Mapstone M, Caraci F, et al. A critical appraisal of blood-based biomarkers for Alzheimer’s disease. Ageing Res Rev. 2024;96:102290. doi:10.1016/j.arr.2024.102290.
12. https://www.radar-ad.org/patient-engagement/radar-ad-nutshell
13. Figures 1, 2, 3 Empowering clinicians to transform Alzheimer’s Disease management - Siemens Healthineers USA (siemens-healthineers.com) The page is copyrighted as follows: Siemens Medical Solutions USA, Inc. ©2024.

Arejas J. Uzgiris leads the Center for Innovation in Diagnostics in North America for Siemens Healthineers His scientific focus is on combining in vitro diagnostic tests, imaging modalities, and machine learning techniques for earlier diagnosis to enable more effective treatment of neurodegeneration, cancer, cardiovascular and infectious diseases. His most recent work, in collaboration with Biogen, Novartis, and Johns Hopkins University, helped to translate neurofilament light chain (NfL) testing into use for groundbreaking clinical trials in multiple sclerosis, amyotrophic lateral sclerosis, and Alzheimer’s disease.

Gaby Marquardt is heading the Innovation Cell Erlangen, Germany at the Center for Innovation in Diagnostics at Siemens Healthineers . In her role, Gaby is responsible for managing an interdisciplinary team of scientists focusing on new and innovative technologies for the diagnostics business of the future. Her work involves technology and innovation scouting, with a particular emphasis on discovering novel technologies and biomarkers that will shape the diagnostics business of the future. Gaby and her team actively participate in cross-sectorial public funding projects, contributing to the company’s strategic objectives of improving healthcare access and medical expertise through innovative medical technology, digital transformation, and artificial intelligence.


We asked you, our laboratory partners, what you wanted in the best automated, triple-slide Gram stainer and then we built our new GramPRO 3™ based on your feedback. It is truly built for you. With the GramPRO 3™’s unique patented electric eye technology, there is no variation in each Gram stain, resulting in no under or over decolorization. The GramPRO 3™ will stain three slides at once; individually and in batch mode. And maybe the best part is that there is minimal cleaning or maintenance required! Even better, all processes occur automatically inside the unit, even methanol fixation.
Scan to learn more
Clinical laboratories are now confronted with the growing risk of cybersecurity attacks and are grappling with the task of sustaining operational functionality in the face of such incidents. Over the past decade, the sophistication of cyberattacks threatening the healthcare industry has increased dramatically with no signs these threats will subside.1 This article will review cybersecurity pitfalls and highlight events that could lead to a delay in laboratory operations, which impacts the financial stability of the health system and ultimately patient care. It is crucial to understand financial and operational stakes at risk during cybersecurity attacks, with the average cost of data breaches reaching on average $8 million per healthcare organization. 2 The strategic response to such an event is multifaceted and requires immediate, well-coordinated actions. Figure 1 presents the structured approach to managing a cybersecurity incident within the laboratory, starting with detection and moving through the subsequent stages of systems going offline, the adoption of manual documentation, the restoration of systems, the recovery phase, and concluding with the essential postevent learning outcomes.
Day-to-day challenges during a cybersecurity event: The immediate aftermath of a cybersecurity attack presents numerous operational hurdles. Clinical laboratories face the task of activating downtime procedures to replace automated/electronic systems. Making the switch from electronic documentation to downtime or manual documentation processes can be difficult. The laboratory staff must have a procedure for downtime operations as a guide, this will ensure proper recovery of operations when the system is back up and running. Operational workarounds become a daily reality, as staff members must adapt to manual data entry and the use of alternative equipment, often leading to increased workload and potential for errors. However, during a downtime event, this practice is paramount. If your downtime documentation is out of date or does not include planning for extended downtimes (i.e., more than two weeks), you risk the chance of staff burnout and/or revenue lost due to documentation issues such as recording of patient information, specimen data, and test results. In 2017, laboratories generated an estimated $87.3 billion in revenue, totaling 2.6% of annual healthcare spending in North America.3 This revenue figure highlights the vital role that laboratorians play in the healthcare system, emphasizing the necessity of robust documentation practices as manual processes inevitably slow operations, reducing the potential for revenue collection. Most importantly, manual processes increase the potential for human error, posing a threat to the quality of patient care.
Effective communication and coordination become vital during a cybersecurity event. Communication barriers become prominent due to system outages and email disruptions, necessitating an alternative means of communication. It is crucial to set up communication channels with key stakeholders, like department or section heads, who play a critical role in downtime management and can efficiently spread information. Quickly implementing alternative communication methods at the onset of unexpected downtime is essential for keeping your team informed with up-to-date information. A unified response across various teams is crucial for managing the crisis effectively. Teamwork stands as the cornerstone of effective downtime management, playing a critical role in navigating your laboratory through unexpected downtimes. Operational challenges and solutions: The transition to manual processes introduces specific operational issues such as the need for alphabetizing patient records manually, complexities in the patient readmission documentation in the laboratory, and the management of supplies, particularly the exhaustion of downtime labels. Laboratories should devise new systems for label printing and patient management, underscoring the importance of innovative solutions and efficient tactics in managing these challenges. Ensure your laboratory has a back-up system for generating downtime labels in the event you lose all middleware and laboratory information system functionality. Although it may seem unlikely, the possibility cannot be entirely dismissed. The crucial question remains: Are you prepared if it does happen?
Your lab should be prepared to run quality and control, result specimens, and troubleshoot downtime accessions that are not generated by electronic means.You should have a system in place to manually review QC before performing patient results, in the event your QC management software system goes down as well. Ensuring the notification of critical results during system downtimes is essential. It is important to have mechanisms established both for identifying critical results and for alerting the relevant department or floor when regular communication channels like phone lines, fax machines, and email are unavailable. It is also important to implement systems of communication with the floors/units regarding time blood draws, and blood culture collections. How can the laboratory inform the floors about the collection of blood specimens or cultures when there isn’t an electronic system available for confirmation?
Continuity and recovery planning: Preparing for such events through drills and training fairs is essential to ensure staff readiness. Some major healthcare organizations hold downtime drills hospital-wide monthly to ensure the hospital system is better prepared for downtime documentation methods. During a
downtime event, manual processes such as charting, or admissions can be notably time-consuming. It’s important to recognize that most admissions and nursing floors may not engage in downtime practices as frequently as the laboratory. Therefore, it’s vital to establish a system-wide downtime drill that includes the lab and other departments in the hospital, aiming to identify and address any miscommunications or ineffective practices that could lead to considerable delays during an actual downtime scenario. Post-event, the focus shifts to recovery strategies and learning from the incident to improve future preparedness. It’s essential to keep your downtime requisitions updated, making sure they include the most current test codes, CPT codes, and order identification numbers. Determining which departments can be consolidated onto a single requisition form is essential. This involves assessing if the Emergency Department (ED), Operating Room (OR), and Radiology (XRAY) need individual forms or if their testing requirements can be efficiently met with one comprehensive requisition.
When facing extended periods of downtime, it is worth considering if your downtime requisitions should include fewer tests to streamline processes, while leaving space for physicians to write in testing not listed. Additionally, implementing a color-coded system for each test on the requisition form can greatly assist non-lab professionals in identifying the correct tubes for specimen collection. Including the requesting floor/unit/department and a reliable contact number at the top of the requisition could enhance the efficiency of recovery efforts. This addition ensures clear communication pathways and facilitates prompt responses to any queries or issues that arise. Given the volume of specimens and requisitions laboratories receive, such measures are crucial in managing the extensive recovery process effectively.
Establishing a designated recovery area within your lab right at the onset of a cybersecurity event can significantly reduce your lab’s recovery time. This recovery room should be staffed by key personnel dedicated to leading the recovery efforts. It serves as a centralized location for organizing all results and original requisitions securely, facilitating a swift return to normal operations once systems are restored. This setup is also beneficial for rapid result readback to providers who request results from the period of downtime, which could span several weeks and are not yet available in the system for reference.
Collaboration with your IT and laboratory information system (LIS) teams is critical to determine the most effective method for recapturing lab testing orders. Charge recovery can follow various pathways, and the optimal approach will depend on your laboratory’s specific context, including the available personnel for recovery efforts and the capabilities of your IT and LIS infrastructure. For example, ABC Health System implemented a strategy for charge recovery using a bulk-charge sheet, enabling the laboratory to input multiple tests simultaneously into an Excel spreadsheet, provided the patient’s name, medical record number, and admission dates are correctly matched with the registration team’s inputs. This allows the laboratory information system (LIS) to upload the charge sheet and post lab charges, though it’s important to note that lab results will not be recorded in the system for the duration of the downtime.
It is important to note during a recovery, manual entry of results is always the preferred method in order to ensure patients’ lab results are accessible in the system. However, manually entering results following an extended downtime presents a significant challenge. Additionally, registration must create or modify patient stay accounts before the lab can post results, adding another layer of complexity to the recovery process.
Recovery from an extended cybersecurity event is a team effort, necessitating close coordination across the laboratory departments and the health system. Regular communication with hospital leadership is essential for addressing ongoing issues, such as unverified requisitions or illegible orders, specimen transport issues between inpatient and outpatient clinics. This collective approach underscores the importance of unity and collaboration in overcoming the challenges posed by downtime and ensuring the continuity of laboratory services. To enhance recovery processes in the clinical laboratory, the following recommendations are proposed:
1. Regularly update your laboratory requisitions at least once a year to ensure they reflect the latest tests, codes, and procedural changes.
2. Actively engage in or organize comprehensive downtime drills across the hospital. Follow up with meetings to address any issues encountered and discuss improvements.
3. Assess your laboratory’s current downtime protocols and confirm the availability of non-electronic backup solutions for label printing and other critical functions that typically rely on the laboratory information system (LIS) or electronic medical records (EMR).
4. Maintain an updated list of key contacts for quick communication with various hospital units during extended downtimes, so as to ensure minimal disruption to laboratory services.
5. Develop a contingency plan for delivering test results when conventional methods (tube system, phone lines, fax machines) are unavailable, incorporating manual delivery methods, as necessary.
6. Recognize the importance of sustaining staff morale, especially during challenging periods. Offering meals, wellness breaks, and incentive pay can significantly contribute to a positive work environment during extended downtimes.
7. Allocate a specific area or room within your laboratory for recovery efforts. This space should be equipped with computer workstations and have the resources to handle the sorting and processing of results and requisitions during and after downtime events efficiently.
In conclusion, this study has shed light on the significant impact that cybersecurity events can have on laboratory operations, emphasizing the critical need for effective recovery and continuity strategies. By highlighting common pitfalls and offering insights to mitigate disruptions, this research underscores the importance of a strategic, well-coordinated response to safeguard laboratory services, alleviate financial burdens on health systems, and minimize adverse effects on patient care. The findings demonstrate that preparedness, resilience, and adaptability are key to navigating the complexities of cybersecurity events in the healthcare sector. Through the implementation of the recommended strategies, laboratories can enhance their defenses against such disruptions, ensuring the continuity of care.

Brittany Teeter, MS CLS MLS(ASCP)CM serves as an assistant professor in the Medical Laboratory Science Program at the University of Texas Health San Antonio, where she is also pursuing a Ph.D. in Health Sciences. Her commitment to the profession earned her a spot as a 2023 ASCP 40 Under 40 Honoree, highlighting her active involvement and volunteer efforts within the ASCP community.
References and acknowledgments are available online. mlo-online.com/55041556.
Group A streptococcal (GAS) infections have been on the rise since late 2022 and 2023 after an overall low incidence during the years of the COVID-19 pandemic.1 GAS infections are common among children and may be asymptomatic or produce mild infections such as pharyngitis, impetigo, and scarlet fever.1 Symptoms of GAS pharyngitis, also known as strep throat, include fever, pain when swallowing, sudden onset sore throat, red and swollen tonsils, white patches or pus on tonsils, tiny red spots on the roof of mouth, and swollen lymph nodes in the front of the neck. 2 GAS pharyngitis typically occurs in winter and early spring in temperate climates. Invasive GAS (iGAS) infections are potentially life threatening and clinical presentation of iGAS infections include sepsis, necrotizing fasciitis, streptococcal toxic shock syndrome, and other severe infections. Presently, iGAS infections affect 1.8 million persons worldwide, both young and old, with a mortality rate approaching 20%.1 iGAS infections may have non-specific symptoms such as fever, which makes clinical diagnosis problematic. Preliminary 2023 data from the U.S. Centers for Disease Control and Prevention (CDC) indicate that the number of severe infections caused by GAS reached a 20-year high.2 Similarly, non-invasive GAS, including GAS pharyngitis, has returned to similar or higher levels than those seen in the pre-COVID-19 pandemic years.2 The recent increase in GAS infections may be due to a number of factors, including increased circulation of more virulent strains, increased susceptibility in children due to reduced incidence during the COVID-19 pandemic, and/or increased susceptibility due to high rates of respiratory viral coinfection in the pediatric population.3
While acute pharyngitis is common, a minority of sore throats are caused by GAS infections. Because symptoms caused by the different agents of pharyngitis can overlap, clinical assessment alone may be insufficient for the differentiation of viral versus bacterial pharyngitis.4 Specific identification GAS as a causative agent of illness, especially in children, is integral to appropriate medical management of patients. Early and effective antibiotic treatment for GAS infections is essential to prevention of post-infectious sequelae, including iGAS infections and rheumatic fever, decrease of transmission to close contacts, and improvement of clinical symptoms.4 In the 2012 guidelines, the Infectious Diseases Society of America (IDSA) recommended rapid antigen detection tests (RADTs) for diagnosis of GAS in patients presenting with symptoms consistent with GAS pharyngitis; then backed-up by throat culture if the RADT is negative, due to the low sensitivity of the antigen test.4 In recent years, GAS identification using newly available molecular methods such as polymerase chain reaction (PCR) or other nucleic acid amplification tests (NAATs) conducted at point-of-care (POC) or ‘near patient’ locations is increasingly implemented due the relative simplicity, rapidity, and increased diagnostic sensitivity
Cobas LIAT Roche Waived GAS
ID Now Abbott Waived GAS
Xpert Xpress Cepheid Waived GAS
Alethia Meridian Bioscience Moderate GAS
Liaison MDX DiaSorin Moderate GAS
RevoGene Meridian Bioscience Moderate GAS
Solana GAS QuidelOrtho Moderate GAS
Solana Strep Complete QuidelOrtho Moderate Groups A & C/G Streptococcus
Xpert/Xpert Infinity Cepheid Moderate GAS
Lyra Direct Strep QuidelOrtho High Groups A & C/G Streptococcus
Table 1. Available GAS molecular tests.
of this approach (Table 1). Rapid result availability allows for diagnosis while the patient is still in the clinic, increasing the likelihood of appropriate antibiotic use. POC NAATs for GAS can take between 15–25 minutes for results, slightly longer than the typical result time of RADTs (5–15 minutes).5 Conversely, culture of throat swabs requires 18 – 48 hours for results and may be performed in a laboratory that is remotely located.5
The ability to appropriately prescribe antibiotics during the initial patient visit is an important benefit of POC RADT or NAAT testing compared to diagnosis by throat culture. In a meta-analysis of 15 primary care medical records in the United States. from 2018 to 2019, 99% of antibiotics were prescribed during the initial patient visit regardless of the diagnostic method used.6 A retrospective analysis using data from 2011–2015 found that during this time, 66% of the pharyngitis cases diagnosed by RADT plus culture began antibiotic therapy on the day of the patient’s initial visit.7 The implication of both analyses is that antibiotics were given before culture results were available and that treatment may have been unnecessary and ineffective. Inappropriate antibiotic use is associated with increased risk of adverse reactions in the patient as well as population-level increases in antibiotic resistance. Rapid molecular POC tests with field-verified high sensitivities and specificities have the potential to reduce inappropriate antibiotic prescriptions. Several U.S. studies compared PCR POC with RADT plus culture methods for appropriate antibiotic use and demonstrated that use of POC NAATs resulted in higher rates of appropriate antibiotic use when compared to the dual methodology due to the superior diagnostic accuracy of NAATs.5,6,8




We make diagnostics that matter

We recognize your passion for providing high quality care to patients displaying symptoms associated with respiratory infections, and we appreciate your efforts and resiliency working to reduce the rapid spread of these infections.
We are committed to providing high quality, molecular and antigen point-of-care tests for detecting the most common respiratory infections, so you can get the answers fast and your patients back to doing what they love.
Like you, we understand there is a patient behind every answer—and that’s what matters most.




Near-patient testing is defined as “an investigation taken at the time of the [clinical] consultation with instant availability of results to make immediate and informed decisions about patient care.”9 Near-patient testing has been used interchangeably with POC testing (POCT). Streamlined healthcare, relying on near-patient testing at community-based and one-stop clinics, has the potential to reduce healthcare disparities by providing earlier diagnosis, immediate patient counseling, and appropriate therapies.9 ‘One and done’ clinic visits may improve disease management, patient outcomes, patient satisfaction, and increase cost-effectiveness per visit.9 Studies in both clinical and pharmacy settings have shown that incorporating pharyngitis POCT improves both patient access and workflow. Results from near-patient pharyngitis NAATs improved the clinician’s confidence in the diagnosis compared to RADT and reduced the number of confirmatory cultures ordered.6 In the pharmacy setting, GAS near-patient testing improved patient access to care especially among the uninsured and patients without a primary care provider; 38% of the pharmacy visits were outside normal clinic hours and 54% did not have a primary care provider.10
of life days, patient satisfaction, treatment complications, staff time, access to treatment, and other healthcare resources. Many studies and meta-analyses recognize the potential for cost savings using POC NAATs but fail to quantify them in a meaningful way.
In a U.S. study which examined the cost-effectiveness and budget impact of POC NAAT for GAS, costs and outcomes were calculated using a decision tree model that compared POC NAAT with RADT plus culture methods.8 Outputs included quality-adjusted life-days lost, GAS morbidity, antibiotic complications, number of patients appropriately treated, and antibiotic utilization.8 The final analysis projected that a POC NAAT strategy would cost $44 per patient compared to $78 for RADT plus culture.8 Additionally, compared with RADT plus culture, POC NAAT would increase the number of appropriately treated patients, avert unnecessary use of antibiotics, and potentially yield cost savings to U.S. third-party payers.8

A POC testing approach may improve testing workflows in the clinical laboratory. Laboratories certified by the College of American Pathologists (CAP) are required to perform confirmatory testing for all negative GAS antigen tests performed on pediatric patients.11 In addition to ensuring clinicians collect and send specimens for confirmation, laboratories may need to demonstrate compliance to CAP inspectors by tracking rates of completed confirmatory testing for all associated clinics performing RADTs. Due to superior sensitivity, GAS NAATs do not require confirmatory testing, and use of CLIA-waived POC NAATs in near-patient locations confers workflow benefits to both the clinical and microbiology laboratory.
“Test-and-treat” workflow
Near-patient testing has the potential to eliminate unnecessary tests, waits, and return clinical visits. In a study where clinicians were discouraged from ordering throat cultures after a negative GAS NAAT result, follow-up throat cultures were ordered on 7% of patients with a negative GAS NAAT compared to 52% of patients with a negative RADT.6 An observational study of GAS near-patient testing found that implementation of this service in a community pharmacy could be achieved with minimal disruptions to the workflow and staff.12 Rates of additional patient follow-up visits for pharyngitis were not significantly different between nearpatient GAS NAAT and RADT plus culture test according to a 2019 report (data not shown).5 Further studies are needed to examine and optimize workflow with near-patient tests.
Implementation of POC NAATs is associated with increased test costs compared to RADTs, but these costs may be offset by increased test performance resulting in fewer throat cultures, increased clinician confidence, and decreased inappropriate antibiotic use. Calculating healthcare costs is complicated due to the many difficult-to-quantify factors involved, including quality
One cost-analysis study leaned more heavily on patient welfare and outcomes. Savings from POCT for respiratory infections (otitis media, sinusitis, pharyngitis and bronchitis) resulted from reductions in antibiotic use and reduction in complications from untreated infections and antibiotic side effects.13 Finally, a study evaluating near-patient GAS testing and treatment in community pharmacies suggested potential savings by reducing the patient burden on primary care.14 Further studies of near-patient GAS molecular tests in settings are needed to understand their full impact and potential benefits, especially concerning healthcare disparities in treatment.
Although more costly, POC or near-patient NAATs offer superior performance and a more streamlined workflow compared to traditionally used RADTs. Implementation of POC GAS NAAT use may lead to increased diagnostic accuracy and reduction of inappropriate antibiotic use in patients with acute pharyngitis.

Jane M. Caldwell, PhD is the executive director of the medical education company Medavera, Inc. and has over 25 years of diverse experience with research and development in multiple areas of epidemiology and molecular biology. She has published extensively in peer-reviewed journals, book chapters, and the popular press. Dr. Caldwell recently worked as a consultant to troubleshoot algorithms for qPCR detection of COVID-19 infections which improved the accuracy of the kits filed under the Emergency Use Authorization.

Rachael M. Liesman, PhD, D(ABMM) received her PhD in Microbiology and Immunology from the University of North Carolina at Chapel Hill, completed a fellowship in clinical microbiology at the Mayo Clinic, and is a Diplomate of the American Board of Medical Microbiology. She is currently an Associate Professor of Pathology and Director of the Clinical Microbiology and Molecular Diagnostics Laboratory at the Medical College of Wisconsin and Froedtert Hospital References
Don’t rule out group C/G Strep cases.
Group A strep may be the most obvious threat, but it isn’t the only one. The Solana Strep Complete Assay accurately detects group C/G strep in addition to group A without the need for culture confirmation. This ensures more patients get the right treatment the first time and helps put an end to “just in case” antibiotics use.
Great care doesn’t leave out G and C. Learn more at quidelortho.com/solanastrepcomplete or reach out to your Henry Schein representative.

Learn more about the Solana platform by scanning the QR code, visiting quidelortho.com, calling us at 800.874.1517, or reaching out to your QuidelOrtho representative.

Biomarkers serve as critical navigational tools, guiding physicians on their patients’ journey through the diagnosis, treatment, and management of their specific cancer. In oncology, a biomarker may be a molecule secreted by a tumor or a specific response of the of the body to the presence of cancer. It can help in identifying early-stage cancers, forecasting how aggressive a cancer might be, or predicting how well a patient will respond to treatment. Biomarkers are also used to predict or monitor cancer recurrence.
Prostate cancer often begins its journey with the detection of prostate-specific antigen (PSA) in the blood. This biomarker, a protein produced by both normal and malignant prostate cells, can be elevated in the presence of prostate cancer. PSA testing has become a keystone in the early detection of prostate cancer, offering insight into the biological state of the prostate. If a person has been diagnosed with cancer, biomarker testing may show whether the cancer is more likely to grow and spread, if certain cancer treatments are likely (or unlikely) to be helpful, and whether the cancer treatment is working.1
Once prostate cancer is diagnosed, the next step involves predictive biomarkers, which help in predicting the cancer’s behavior and potential response to treatments. Genes like ER, PR, and HER2/neu, although more commonly associated with breast cancer, have parallels in prostate cancer research, where molecular markers can predict the effectiveness of therapies such as hormone treatments or chemotherapy. One of the biggest areas of research is in immunotherapy, this is a treatment that manipulates the patient’s immune
system to fight cancer with drugs or modified immune T cells. In the past several years, immunotherapy has had great success in some patients with certain types of cancer, but not all patients respond to this type of treatment. I have written about this in previous articles I have done on cancer and treatments — I believe this type of treatment has great potential with further development.2
There is a significant amount of research focused on discovering biomarkers that could identify which patients are likely to respond to immunotherapy. In some types of cancer, the presence or absence of immune molecules in cancer cells has been associated with determining whether a patient will have a better or worse response to immunotherapy.
Biomarkers can also be used to help physicians determine if there are clinical trials available for their patients. Some trials, called basket studies, are based on the biomarkers in tumors and/or cells instead of the primary body site of the cancer. There are other trials that are using biomarkers to match treatments based on the gene characteristics or genetic changes in the patient’s cancer.
As treatment begins, prognostic biomarkers are an effective way of determining the efficacy of the chosen treatment. There are specific assays that can examine a panel of genes within the tumor biopsy to provide a recurrence risk score, thus offering a prognostic outlook on the likelihood of cancer returning.
Biomarkers have also shown benefits in monitoring how well a treatment is working over time. There is the potential that these biomarkers can be used as alternatives to the more common method of monitoring, that is, image-based tests, such as CT scans and MRIs. Such biomarkers are invaluable in tailoring patient-specific follow-up care and determining the frequency and type of follow up and continued care strategies.
The journey of a biomarker in prostate cancer is progressive. From the initial diagnosis through PSA levels to the nuanced understanding of the disease’s molecular landscape, biomarkers shape the course of treatment. They offer a personalized approach, ensuring that each patient receives care tailored to the unique characteristics of their cancer.
As research continues to evolve, biomarkers stand at the forefront of precision oncology, offering a ray of hope for patients who are receiving a devastating diagnosis. Their physicians are able to provide them with more information,
access to more options and a personalized approach to have better outcomes for their cancer journey.
How can patients advocate for biomarker testing?
Patients can advocate for biomarker testing by taking several proactive steps to ensure they receive the most personalized care possible. Here are some suggestions: Educate yourself: Learn about the types of biomarker tests available and how they can influence treatment decisions. Understanding the potential benefits can help you make informed requests for testing.
Discussion with healthcare provider: Bring up the topic of biomarker testing with your doctor. Ask about the tests that are relevant to your condition and how they might impact your treatment plan. Ask if you can speak with your pathologist. The pathologist who diagnosed you is the first line of care in your journey. If they use digital pathology, you may even be able to see images of your specific cancer tumor or cells as well as get a description and insights from the pathologist that are specific to your disease. This can be empowering for some patients.
Insurance coverage: Check with your insurance provider to see if biomarker testing is covered under your plan. If not, discuss with your healthcare provider about the necessity of the test, which might help in getting the coverage. Seek support: Contact patient advocacy groups or organizations that specialize in your condition. They can provide resources and guidance on accessing biomarker testing.
Legislative action: Support efforts to expand insurance coverage of comprehensive biomarker testing. Some states have enacted legislation to improve access to these tests.3
Financial assistance programs: Explore financial aid options through foundations and pharmaceutical programs that may cover the cost of biomarker testing.4
Any and all of the above actions can enable a patient to be an active participant in their care plan and to help them fight for the treatment and coverage that they and their physicians determine is best for them.
In summary, this article is meant to provide a view of the critical role biomarkers play in the journey of prostate cancer, from diagnosis to treatment and beyond. It highlights the transformative impact these biological indicators have on patient care, emphasizing the shift towards personalized medicine in oncology.
1. Biomarker tests and cancer treatment. Cancer.org. Accessed May 29, 2024. https://www.cancer.org/cancer/diagnosis-staging/tests/ biomarker-tests.html.
2. Clifford LJ. Key points of intersection in diagnosis and treatment of cancer. Medical Laboratory Observer. Published October 18, 2023. Accessed May 29, 2024. https:// www.mlo-online.com/disease/cancer/article/53073961/ key-points-of-intersection-in-diagnosis-and-treatment-of-cancer.
3. National. American Cancer Society Cancer Action Network. Accessed May 29, 2024. https://www.fightcancer.org/states/ national/actions.
4. Financial advocacy. Accessed May 29, 2024. https://www. accc-cancer.org/home/learn/financial-advocacy?.

Lisa-Jean Clifford is COO and Chief Strategy Officer of Gestalt Diagnostics . Clifford has more than 20 years of experience in high-tech industries, with over 15 of them specifically in high-tech healthcare.

Establishing overall quality goals for analytical performance is the first step toward assuring the quality of the analytical process and building an effective internal quality control (QC) system for your laboratory. This strategy sets the stage for creating a meaningful QC plan designed to meet basic accreditation requirements for quantitative tests. It is worth noting that many of the same principles will also apply to qualitative testing.
The laboratory should establish the level of risk they are willing to accept for reporting an erroneous patient test result. This goal should be the cornerstone of the quality plan for QC, and all aspects of the plan should be based on this.1
The laboratory should also define, in general terms, what it strives to achieve for analytical quality. These quality goals could be based on analyte-specific performance goals, such as total error, imprecision, and/or bias. To monitor these performance goals, the laboratory should establish policies on quality control testing, including the control materials and the process control system to be used.2
Analyte-specific performance goals
In developing analyte-specific performance goals, the laboratory may consider the following:
1. Which tests in the laboratory pose a higher risk of harm to the patient if an erroneous result is reported?
2. Should the laboratory plan make special provisions for higher-risk tests?
3. Is the laboratory aware of any tests that might be considered inconsistent performers requiring tighter control?
4. What is the expected frequency/probability of failure or malfunction (i.e., reliability) of the instrument, kit, or method?
5. How important is it to be alerted when a medically relevant analytical error occurs?
Develop a plan by defining laboratory policies
Once quality goals are established, a tactical plan designed to meet these goals should be prepared. The plan should be specific and identify QC measures for each test based on risk of reporting an erroneous patient test result and the severity of the outcome if one is reported. The plan should also consider assay limitations, the probability of device failure, and the level of technical expertise required to perform the test. In accordance with good laboratory practice, the plan should ensure that control materials are treated like patient samples during testing.
Elements of the plan may include the following:
1. Analyte-specific performance goals with the following characteristics:3
• Unique and defined by the individual laboratory, based on clinical outcomes
• Acceptable limits for bias, imprecision, and total error based on biological variation
• Based on best practices and
• Represented by long-term, between-run imprecision, as reported by the manufacturer or as measured by the laboratory

• Derived from regulatory agencies, professional organizations, or a proficiency testing organization
• Total error (TE) for the test, as determined by the manufacturer or laboratory
2. Frequency of including quality control materials for each analyte tested, based on a risk assessment. If electronic controls are used, both the use of electronic controls and the frequency of testing should be based on the risk assessment.
3. Concentrations (or levels) of quality control materials based on a risk assessment. Some countries support testing at least two different concentrations (usually a normal and abnormal concentration) depending on assay limits and the range of patient test results commonly reported. Other countries require controls covering the analytical range of the test.
4. An effective process control system for each analyte using appropriate statistical QC rules. The laboratory should avoid:
• Indiscriminate use of the 1:2s rule for run rejection
• Setting the same process control rule, or multi-rule, for all tests regardless of test capability or clinical utility
5. Statistical parameters for control materials — mean, median, standard deviation, CV%, total error — as established by the laboratory through repetitive testing. The plan should discourage long-term use of product insert values for establishing acceptable performance. Procedures should describe how to calculate a valid and reliable mean and standard deviation, i.e., setting target values and ranges of acceptable performance.2
6. Requirements for parallel testing of all new lots of controls alongside current validated lots to establish new target values and ranges of acceptable performance.
7. Specific intervals at which the laboratory will reassess the relevance and appropriateness of all statistical parameters used by the laboratory, with attention given to each test’s mean and standard deviation.
8. A comprehensive training program that covers the following:
• Basic QC statistics and interpretation
• How to handle control materials and prepare them for use: storage, reconstitution, or thawing
• How to interpret QC patterns: trends, shifts, random error, systematic error, error that requires action, and error that does not require immediate action
• How to resolve out-of-control situations
• How to log and maintain QC results and document that QC was performed
• Where to go to for additional troubleshooting assistance, if necessary
9. Participation in an external interlaboratory comparison program for all parameters tested in the laboratory. Such programs include those provided by commercial companies, government-run initiatives, and private individuals or organizations. If no comparison program is available for certain tests, the laboratory should have some other means of demonstrating the competency of laboratory staff and the reliability of test results.2
10. The nature of the control materials to be used. Many options are available, including electronic controls, commercial products, and patient pools. It may be appropriate to use a combination of different types of QC materials throughout the laboratory.
Control material selection
Electronic controls: The plan should identify which tests may be monitored using electronic controls and any additional measures needed to assure quality of patient test results.
If electronic controls are used, the laboratory should understand what portion of the analytical process is being monitored and if there is a need for additional controls to sufficiently mitigate the risk of reporting patient test results with medically important error.
Commercial control products: These include in-kit controls, instrument manufacturer controls, and independent third-party controls. The plan should describe when commercial control products are suitable materials for controlling the analytical process.
The laboratory should compare the effectiveness of in-kit, instrument manufacturer, and third-party controls at detecting trends, shifts, and medically important errors. Consideration should be given to control matrix (human versus non-human). Some accreditors/regulators may require the laboratory to know whether matrix effects are present that could potentially mask analytical errors.
In-kit or instrument manufacturer controls designed for specific test methods may not be suitable for other test methods or instruments. When in-kit or manufacturer controls are used to calculate assay cut-off ranges, some regulatory bodies may recommend the use of independent control materials to monitor the analytical process.
The plan should discourage use of control materials as calibrators and vice versa. This is not considered good laboratory practice. Sensitivity of the control product for detecting changes in the test system can be an issue for in-kit or instrument manufacturer controls when they are manufactured at the same time and from the same raw materials as the calibrator(s).
Note: Almost all commercially available control products are neither intended nor labeled for “trueness of measurement” and therefore the laboratory does not have to document traceability. However, if the control material chosen by the laboratory is labeled by the manufacturer as intended for “trueness of measurement,” the laboratory should document the metrological traceability of the product.4
The plan should describe when patient pools are suitable materials for controlling the analytical process. Some questions that might need to be answered include the following:
• Should all patient samples be tested for infectious diseases before mixing with the pool?
• Does the plan address national ethics’ regulations regarding patient consent before using a patient’s sample as part of a patient pool?
• Is it important to have pools with analyte concentrations at the medical decision points? Is it possible to achieve these concentrations?
• How will the laboratory achieve and maintain homogeneity of the material?
• How will the pool be stabilized and stored?
• What is the stability of the pool?
Process control systemsshould reflect current laboratory conditions and requirements. Conditions may change, which might require a reassessment of the QC plan for ongoing relevance, appropriateness, and effectiveness. Laboratory staff must understand their individual roles and responsibilities in implementing, maintaining, and modifying the plan as external factors in the laboratory change. Conducting reviews is the best way to evaluate relevance and effectiveness. Before a review is conducted, determine the frequency at which they will occur, and at what level(s): bench review, supervisor review, or departmental review.
Particular attention should be paid to the sensitivity of the system. If the system is too sensitive, it will likely generate an unacceptable number of false positive alerts leading to costly and unnecessary troubleshooting and repeats. Conversely, an insensitive system may miss important analytical errors. Consequently, the laboratory should have a feedback mechanism that provides data relative to the relevance and effectiveness of the process control system in use. It is critical that both laboratory staff and clinicians understand the performance characteristics of each test method.
1. CLSI. Laboratory Quality Control Based on Risk Management. 2nd ed. CLSI guideline EP23. Clinical and Laboratory Standards Institute; 2023.
2. CLSI. Statistical Quality Control for Quantitative Measurement Procedures: Principles and Definitions. 4th ed. CLSI guideline C24. Clinical and Laboratory Standards Institute; 2016.
3. Sandberg S, Fraser CG, Horvath AR, et al. Defining analytical performance specifications: Consensus Statement from the 1st Strategic Conference of the European Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem Lab Med. 2015;53(6):833-5. doi:10.1515/cclm-2015-0067.
4. In Vitro Diagnostic Medical Devices -Measurement of Quantities in Biological Samples-Metrological Traceability of Values Assigned to Calibrators and Control Materials

John Yundt-Pacheco MSCS is the Senior Principal Scientist at the Quality Systems Division of Bio-Rad Laboratories . He leads the Informatics Discovery Group, doing research in quality control and patient risk issues. He has had the opportunity to work with laboratories around the world — developing real time, inter-laboratory quality control systems, proficiency testing systems, risk management and other quality management systems.

Kody Andrew serves as Outbound Marketing Lead of the Clinical Diagnostics Group at Bio-Rad Laboratories . He is a passionate marketing leader specializing in clinical diagnostics, molecular diagnostics, and immune-mediated diseases and is dedicated to driving impactful marketing strategies that resonate with healthcare professionals and positively impact patient outcomes.
Laboratory errors can lead to errors in diagnosis and may cause increased costs, unnecessary testing and/or treatment, and adverse patient outcomes. Approximately 60–70% of laboratory errors are due to preanalytical factors.1 In vitro hemolysis is by far the most common preanalytical problem, accounting for 40–70% of unsuitable blood samples. 2-4 Hemolysis is defined as erythrocyte rupture, where red blood cell contents are released into the bloodstream.5 It can occur in vitro or in vivo. In vitro hemolysis is substantially more common and can cause spurious changes in the plasma or serum concentration of potassium and other substances released from the damaged red blood cell.5
The majority of in vitro hemolysis results from inappropriate techniques for collecting, handling, or transporting blood specimens.4,6 Table 1 details preanalytical errors that can cause hemolysis, with incorrect sample collection being the most common.Figure 1 details causes and frequencies of hemolysis in statistical/urgent testing when the healthcare provider requires test results as quickly as possible.
Prevalence of hemolysis
Hemolysis occurs frequently in samples obtained in the emergency department (ED), with reported rates ranging up to 18.1% in arterial blood gas samples, and in 8.8% of venous blood samples (Figure 2).8-12
Sample collection
• Excessive tourniquet
• Repeated fist clenching
• Excessively small needle gauge
• Traumatic venipuncture (squeezing the puncture site)
• Non-standard venipuncture site
• Inadequate site preparation
• Collection with syringe and needle rather than vacuum tube
• Excess blood flow rate (e.g., due to excess vacuum)
• Contamination with ethylenediaminetetraacetic acid (EDTA)
Sample handling and transport
• Overly vigorous sample mixing
• Delayed analysis or prolonged storage of uncentrifuged blood
• Transport of syringe in direct contact with ice
• Improper pneumatic tube settings
Central laboratory chemistry analyzers typically include automated spectrophotometric hemolysis detection and report a semiquantitative hemolysis index (HI).13,14 Hemolysis detection capabilities are much less common in point-of-care testing (POCT) analyzers commonly used in the ED. As a result, hemolysis in the ED can go undetected.14,15
Release of potassium and other substances from hemolyzed red blood cells can affect laboratory tests and cause reporting of erroneous results by the following:16
• Elevating plasma levels of potassium and other substances contained within the red blood cells
• Diluting the plasma
• Causing spectral interference
• Releasing enzymes that degrade analytes of interest
When hemolysis is detected:
• Potential sample redraw and retest in central lab
• Delayed turnaround time
• Patient discomfort
• Increased cost and consumption of resources
• Potential increased length of stay (LOS)
When hemolysis is present but not detected:
• Inappropriate treatment
• Unnecessary investigations
• Potential for increased LOS Hemolysis can require sample recollection, delay care, and increase costs. If hemolysis is not detected, inappropriate treatment and unnecessary investigations may result (Figure 3).16
Potassium is a key analyte for POCT in the ED because potassium abnormalities are common in patients in this setting











▪ Web-based program with NO software download required
▪ Instant Reports available with real-time peer data
▪ 24 hour access to historical reports
▪ Now including: Intralab Instrument Comparison!



and can cause life-threatening cardiac arrhythmias and other complications. Hemolysis can cause spurious elevations in plasma potassium levels. Because potassium levels are typically at least 20-fold higher in red blood cells than in plasma, even minor hemolysis can result in pseudohyperkalemia or mask hypokalemia.17 Several studies illustrate the potential clinical impact.
A study evaluated potassium levels and hemolysis index (HI) in 550 arterial blood gas samples. Of these, whole blood potassium levels were considered hypokalemic (<3.5 mmol/L) in 150 samples, normokalemic (3.5-4.5 mmol/L) in 328 samples, and hyperkalemic (>4.5 mmol/L) in 72 samples. 18% of samples had an HI >1, representing at least mild hemolysis. Correcting for the effects of hemolysis led to downgrading 22% of normokalemic samples and 14% of hyperkalemic samples. Hemolysis was associated with low sample volume (<0.5 mL).18
In an ED-based study of POCT in 100 unique ED admissions with elevated potassium, comparison of POC and central lab results found that 40 of the elevated potassium measurements represented pseudohyperkalemia due to hemolysis (39 patients) or contamination with IV fluid (1 patient). Review of medical records indicated that pseudohyperkalemia affected care in
When hemolysis is detected:
• Potential sample redraw and retest in central lab
• Delayed turnaround time
• Patient discomfort
• Increased cost and consumption of resources
• Potential increased length of stay (LOS)
When hemolysis is present but not detected:
• Inappropriate treatment
• Unnecessary investigations
• Potential for increased LOS
11 patients, including 6 with unnecessary follow-up testing, 3 with delayed treatment, and 2 with inappropriate interventions.19
Another ED-based study analyzed 472 arterial blood gas samples at POC for hemolysis and potassium. Of these, 12% were hemolyzed. Potassium levels were significantly higher in hemolyzed than in non-hemolyzed samples, with an increase of 15.29% (0.61 mEq/L), P<0.001.20 The authors recommended making POCT hemolysis detection an integral part of the diagnostic process.20
A study of 3,185 ED samples evaluated for hemolysis by visual inspection found that 495 samples (15.5%) were hemolyzed. Of the 2,518 tests ordered for these hemolyzed samples, 780 results (31%) were handled incorrectly (either incorrectly released or incorrectly suppressed).12 The tests with the highest risk for patient safety (i.e., those with high rates of hemolysis and high risk of patient harm) were potassium, troponin T, and total bilirubin.12
These errors can have considerable clinical and economic impact. A series of studies by Phelan et. al. estimated that hemolysis in the ED was associated with a 62-minute increase in a patient’s LOS in the ED.21 For a hypothetical typical ED, the direct cost of this increased LOS is estimated at $4 million per year. The ED was assumed to have 100,000 annual visits, with 40% of patients undergoing a blood draw for routine chemistry testing, and a 10% incidence of hemolysis for each blood draw.22
True hyperkalemia is rare among low-risk patients. A study of ED encounters, with 66 low-risk patients who presented with elevated potassium levels and hemolysis on initial testing, found that no patients had true hyperkalemia in a repeat test.23 Low-risk patients were defined as 18–65 years of age, not taking potassium-altering medications, had no hematologic malignancies, and had a repeat test within 12 hours.23 The median time between the initial and repeat blood draws was 145 minutes, representing an opportunity to decrease the ED LOS.23
Historically, EDs have been unable to detect hemolysis routinely at the POC. For whole blood potassium measurement, they may rely instead on clinical judgement, sample recollection, and/or HI measurement in the central lab. The lack of hemolysis detection presents several clinical challenges. These can be mitigated by POCT hemolysis testing (Figure 3). In recent years, stand-alone POCT hemolysis analyzers and a POCT blood gas analyzer with an integrated hemolysis detection capability have become available.
The prevalence and impact of hemolysis and the limitations of earlier approaches to hemolysis detection have led to calls for routine inclusion of hemolysis detection functionality in blood gas analyzers.24 In addition, the Clinical & Laboratory Standards Institute (CLSI) EP23 guideline notes that risk evaluation should include the ability to detect erroneous results and prevent medical action before they cause patient harm; and, that some hazards can be detected internally by the measurement system.25 The measurement system’s ability to detect hemolysis is an important consideration.25
Check
NEW PT PROGRAMS
• Blood Culture - Limited - #310
• FebriDx Bacterial/Non-Bacterial Assay - #323
• Global Fever Panel - #395
• M. Tuberculosis Interferon-y - #450
ENHANCED PT PROGRAMS
• ANA - #421
Anti-Centromere and Anti-Ribosomal P added
• Basic Bacteriology - #321
2 urine culture and susceptibility challenges per shipment
• Comprehensive Bacteriology - #328
2 urine culture and susceptibility challenges per shipment
• Educational Blood Bank - #518
Compatible with manual and automated methods
• Nail Fungus Panel - #391
5 samples
• Respiratory Panel - #370
Streptococcus dysgalactiae and Streptococcus pyogenes added
• STI Panel - #392
5 samples
• Urine Culture - #314
2 susceptibility challenges per shipment
• Vaginal Panel - #376
5 samples










Potassium results
Hyperkalemia
Normokalemia
True Hyperkalemia
Pseudohyperkalemia
Challenges without POC hemolysis detection
Unnecessary sample recollection
Altered treatment
Sample recollection with long TAT
Impact of POC hemolysis detection
Reduce unnecessary sample recollection
Reduce inappropriate treatment
Reduce testing TAT
Masked Hypokalemia
Hypokalemia
Conclusion
Hemolysis is a common problem in ED POCT analyses and can lead to spurious elevations in plasma levels of potassium and other analytes, potentially requiring sample recollection, delays, increased costs, and unnecessary procedures and treatment. Incorporation of automated hemolysis detection into routine POCT analyses can help mitigate these risks and improve patient safety (Figure 4).
References
1. Lippi G, Chance JJ, Church S, et al. Preanalytical quality improvement: from dream to reality. Clin Chem Lab Med. 2011;49(7):1113-26. doi:10.1515/CCLM.2011.600.
2. Plebani M. The detection and prevention of errors in laboratory medicine. Ann Clin Biochem. 2010;47(Pt 2):101-10. doi:10.1258/ acb.2009.009222.
3. Lippi G, Salvagno GL, Favaloro EJ, Guidi GC. Survey on the prevalence of hemolytic specimens in an academic hospital according to collection facility: opportunities for quality improvement. Clin Chem Lab Med. 2009;47(5):616-8. doi:10.1515/CCLM.2009.132.
4. Lippi G, Avanzini P, Pavesi F, et al. Studies on in vitro hemolysis and utility of corrective formulas for reporting results on hemolyzed specimens. Biochem Med (Zagreb). 2011;21(3):297-305. doi:10.11613/ bm.2011.040.
5. Buño A, Oliver P. POCT errors can lead to false potassium results. Adv Lab Med. 2021;30;3(2):142-152. doi:10.1515/almed-2021-0079.
6. Lippi G, Blanckaert N, Bonini P, et al. Haemolysis: an overview of the leading cause of unsuitable specimens in clinical laboratories. Clin Chem Lab Med. 2008;46(6):764-72. doi:10.1515/CCLM.2008.170.
7. Carraro P, Servidio G, Plebani M. Hemolyzed specimens: a reason for rejection or a clinical challenge? Clin Chem. 2000;46(2):306-7.
8. Nichols JH, Apple FS. Prevalence of Hemolyzed Whole Blood Potassium Results in Acute Care Settings. 28th AACC International CPOCT Symposium.; 2022.
9. Duhalde H, Skogö J, Karlsson M. Point-of-care hemolysis detection in blood gas specimens directly at the emergency department. Scand J Clin Lab Invest. 2019;79(5):283-287. doi:10.1080/00365513.2019.1612089.
10. Salvagno GL, Lippi G, Gelati M, Guidi GC. Hemolysis, lipaemia and icterus in specimens for arterial blood gas analysis. Clin Biochem 2012;45(4-5):372-3. doi:10.1016/j.clinbiochem.2011.12.005.
11. Lippi G, Plebani M, Di Somma S, Cervellin G. Hemolyzed specimens: a major challenge for emergency departments and clinical laboratories. Crit Rev Clin Lab Sci. 2011;48(3):143-53. doi:10.3109/10408363.2011.600228.
12. Luksic AH, Nikolac Gabaj N, Miler M, et al. Visual assessment of hemolysis affects patient safety. Clin Chem Lab Med. 2018;28;56(4):574-581. doi:10.1515/cclm-2017-0532.
13. Farrell CJ, Carter AC. Serum indices: managing assay interference. Ann Clin Biochem. 2016;53(Pt 5):527-38. doi:10.1177/0004563216643557.
Misdiagnosis
Reduce misdiagnosis
14. Dolci A, Panteghini M. Harmonization of automated hemolysis index assessment and use: Is it possible? Clin Chim Acta. 2014;15;432:38-43. doi:10.1016/j.cca.2013.10.012.
15. Simundic AM, Topic E, Nikolac N, Lippi G. Hemolysis detection and management of hemolyzed specimens. Biochem Med 2010;20(2):154-159.
16. McCaughey EJ, Vecellio E, Lake R, et al. Current Methods of Haemolysis Detection and Reporting as a Source of Risk to Patient Safety: a Narrative Review. Clin Biochem Rev. 2016;37(4):143-151.
17. Beilin LJ, Knight GJ, Munro-Faure AD, Anderson J. The sodium, potassium, and water contents of red blood cells of healthy human adults. J Clin Invest. 1966;45(11):1817-25. doi:10.1172/JCI105485.
18. Hawkins R. Measurement of whole-blood potassium--is it clinically safe? Clin Chem. 2003;49(12):2105-6. doi:10.1373/ clinchem.2003.027227.
19. O’Hara M, Wheatley EG, Kazmierczak SC. The Impact of Undetected In Vitro Hemolysis or Sample Contamination on Patient Care and Outcomes in Point-of-Care Testing: A Retrospective Study. J Appl Lab Med. 2020;1;5(2):332-341. doi:10.1093/jalm/jfz020.
20. Nigro M, Valli G, Marchionne ML, et al. Is There a Risk of Misinterpretation of Potassium Concentration from Undetectable Hemolysis Using a POCT Blood Gas Analyzer in the Emergency Department? Medicina (Kaunas). 2022;28;59(1):66. doi:10.3390/medicina59010066.
21. Phelan MP, Hustey FM, Good DM, Reineks EZ. Seeing Red: Blood Sample Hemolysis Is Associated with Prolonged Emergency Department Throughput. J Appl Lab Med. 2020;1;5(4):732-737. doi:10.1093/ jalm/jfaa073.
22. Phelan MP, Ramos C, Walker LE, Richland G, Reineks EZ. The Hidden Cost of Hemolyzed Blood Samples in the Emergency Department. J Appl Lab Med. 2021;1;6(6):1607-1610. doi:10.1093/jalm/jfab035.
23. Wilson M, Adelman S, Maitre JB, et al. Accuracy of Hemolyzed Potassium Levels in the Emergency Department. West J Emerg Med 2020;20;21(6):272-275. doi:10.5811/westjem.2020.8.46812.
24. Möckel M, Luppa PB. Why hemolysis detection should be an integral part of any near-patient blood gas analysis. Journal of Laboratory Medicine. 2021;45(4-5):193-195. doi:10.1515/labmed-2021-0076.
25. CLSI. Laboratory Quality Control Based on Risk Management. 2nd ed. CLIS guideline EP23™. Clinical and Laboratory Standards Institute; 2023.

Kim Skala, MLS(ASCP) is an Associate Project Manager, Customer Education Programs at Werfen . For more than 30 years, Kim served as a medical technologist and then point-of-care coordinator (POCC) at Advocate Christ Medical Center in Chicago. Kim co-chaired the ACL Laboratories POCT Technical Advisory Team, focusing on system standardization throughout Wisconsin and Illinois. In 2011, she was awarded POCC of the Year by the AACC (now ADLM). In 2014, Kim joined Werfen, where she provides education and training to laboratories, POCT programs, and other clinical areas throughout North America. Figure 5. Effects of













HbA1c Analyzer













Tosoh G8 delivers HbA1c results with a detailed chromatographic representation of the patient sample, allowing you to see all that is behind the A1c number. The G8 detects most commonly occurring hemoglobin variants, ensuring the most accurate diagnosis every time.
Quickly quantify A1c and provide an accurate diabetes diagnosis using the G8 HbA1c Analyzer
• Less than 2% CVs
• Gold Standard HPLC technology for A1c testing
• Clear separation between labile A1c and stable A1c
• Proven instrument reliability and low maintenance
Come see us at ADLM Booth 3801 for live demonstrations of our latest a dvancements in clinical diagnostics


Hepatitis B virus (HBV), despite widespread vaccination efforts, poses a global health challenge affecting around 2 billion people worldwide.1 Chronic HBV contributes to 30% of cirrhosis-related deaths and 40% of hepatocellular carcinoma-related deaths.4 The WHO recommends eradicating chronic viral hepatitis as a public health issue by 2030 through a 90% reduction of chronic HBV infection and a 65% decrease in associated mortality. 3
HBV transmission occurs through percutaneous (skin-penetrating) or mucosal contact with infectious blood or body fluids, such as semen and saliva. Transmission can occur during pregnancy or delivery from an HBV-positive mother to her baby, through sexual contact with an infected partner, and via injection drug use that involves sharing needles, syringes, or drug-preparation equipment.6
In the United States, there are approximately 60,000 new cases of HBV diagnosed annually, contributing to an already substantial chronic HBV population of over 2 million. The severity of the disease is further underscored by the fact that it causes 5,000 deaths annually, emphasizing the critical need for comprehensive prevention and healthcare strategies to address this significant health challenge.5
Chronic HBV disproportionately impacts U.S. residents born outside the country. Despite comprising only 14% of the overall U.S. population, non-U.S.-born individuals represent 69% of those living with chronic HBV infection in the country.6
In 2019, following a decade of consistent rates, there was a sudden 32% decline in the incidence of acute hepatitis B in the U.S. This reduction could be linked to a decrease in healthcare-seeking behavior during the COVID-19 pandemic.6
While not everyone newly infected with HBV will exhibit symptoms, some will experience fatigue, poor appetite, stomach pain, nausea, and jaundice. HBV can present as a brief illness or transform into a chronic infection, posing the risk of severe, potentially life-threatening health complications including liver disease or liver cancer.6
Age is also a factor. Approximately 9 out of 10 infants infected with hepatitis B will experience lifelong, chronic infections, with the risk decreasing as the child grows older. In contrast, most children aged 6 and older recover fully without progressing to chronic infection.6
The progression of chronic HBV does not always follow a linear course. Individuals may transition from having high viral levels without liver disease activity to experiencing active liver disease and, subsequently, returning to a state of no liver activity with low viral levels. This poses a significant challenge in the management of HBV.7
Screening involves testing serum for the presence of HBsAg to identify chronic infection. A diagnosis of chronic HBV infection is established when HBsAg persists for six months. Additionally, screening should incorporate the antibody to hepatitis B surface antigen (Anti-HBs) to identify individuals who have not been exposed, enabling them to be offered HBV vaccination.3
The CDC’s 2023 recommendations for HBV suggest screening adults aged ≥18 years at least once in their lifetime. It also recommends a triple panel test, which includes screening for HBsAg, anti-HBs, and total antibody to hepatitis B core











Table 1. Interpretation of screening test results for hepatitis B virus infection and recommended actions8
Acute infection Positive Negative Positive Positive Link to HBV infection care.
Chronic infection Positive Negative Positive Negative§ Link to HBV infection care.
Resolved infection Negative Positive Positive Negative Counsel about HBV infection reactivation risk.
Immune (immunity inferred from receipt of previous vaccination)
Negative Positive¶ Negative Negative Reassure if there is a history of HepB vaccine series completion; if partially vaccinated, complete the vaccine series per ACIP recommendations.
Susceptible, never infected Negative Negative** Negative Negative Offer HepB vaccine per ACIP recommendations.
Isolated core antibody-positive†† Negative Negative Positive Negative Depends on the cause of the positive result.
Abbreviations: ACIP = Advisory Committee on Immunization Practices; anti-HBs = antibody to hepatitis B surface antigen; HBcAg = hepatitis B core antigen; HBsAg = hepatitis B surface antigen; HBV = hepatitis B virus; HepB = hepatitis B; IgG = immunoglobulin G; IgM anti-HBc = immunoglobulin M antibodies to hepatitis B core antigen; total anti-HBc = total antibody to hepatitis B core antigen. Total anti-HBc measures both IgM and IgG antibodies to HBcAg. †Source: Abara WE, Qaseem A, Schillie S, et al. Hepatitis B vaccination, screening, and linkage to care: Best practice advice from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med 2017; 167:794–804. §IgM anti-HBc may be positive in persons with chronic infection during severe HBV infection flares or reactivation. ¶Immune if anti-HBs concentration is >10 mIU/mL after vaccine series completion. **Anti-HB concentrations might wane over time among vaccine responders (Source: Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: The Advisory Committee on Immunization Practices recommendations. MMWR Recomm Rep 2018;67[No. RR-1]:1–31). ††Can be the result of a past infection when anti-HBs levels have waned, occult infection, passive transfer of anti-HBc to an infant born to an HBsAg-positive gestational parent, a false positive, or mutant HBsAg strain that is not detectable by laboratory assay.
antigen (total anti-HBc or HBcT). The triple panel test can also be employed for periodic risk-based testing. Another approach is to begin with the anti-HBc test and, if positive, follow up with HBsAg and anti-HBs testing.6 For infants, healthcare professionals should conduct testing for both HBsAg and anti-HBs seromarkers if they are born to individuals who are HBsAg-positive. Lastly, for pregnant individuals, medical practitioners should conduct HBsAg testing during each pregnancy, ideally in the first trimester. This is recommended irrespective of vaccination status or past testing history. Pregnant individuals who have undergone appropriately timed triple panel screening and have not been exposed to new risks for HBV since screening should be tested for HBsAg.6
The American Association for the Study of Liver Diseases (AASLD) guidelines recommend screening for:
• Individuals born in regions with high or intermediate HBV endemicity (where the prevalence of HBsAg is ≥2%)
• U.S.-born individuals not vaccinated during infancy with parents born in regions where HBV is highly endemic (≥8% prevalence)
• Pregnant women
• Individuals requiring immunosuppressive medications (including those undergoing chemotherapy), blood donors, and patients with end-stage renal disease. 3 The World Health Organization (WHO) suggests:
• Widespread screening in countries where the seroprevalence of HBsAg is ≥ 2%
• Routine testing for all pregnant women attending antenatal clinics
• Regular screening of high-risk populations (such as sexual and household contacts of individuals with chronic HBV infection, those with HIV, injection drug users, men who have sex with men, sex workers, indigenous peoples, incarcerated individuals, and transgender persons)
• HBsAg screening of blood and organ donors
• HBsAg screening of adults showing signs and symptoms indicative of viral infection 3
Laboratory findings and interpretation of screening tests
When assessing a patient with suspected hepatitis, besides conducting a comprehensive history and physical examination, laboratory tests should encompass a complete blood cell
count and a complete metabolic panel. The metabolic panel should include ALT, AST, total bilirubin, alkaline phosphatase, albumin, creatinine levels, and INR assessments. Additionally, it is crucial to include markers indicating HBV replication, such as IgM antibodies against hepatitis B core (anti-HBc IgM), HBeAg, antibodies against hepatitis B e antigen (anti-HBe), and quantitation of HBV DNA.1
Furthermore, it is essential to obtain markers for potential coinfection with hepatitis C virus (HCV), hepatitis D virus (HDV), and human immunodeficiency virus (HIV) and assess immunity against HAV to determine the necessity for vaccination. The first identifiable viral marker is HBsAg, appearing in the serum 2 to 8 weeks before any elevation of aminotransferases. During the acute phase of infection, ALT and AST levels can surge to levels ranging between 500 and 5000 U/L, subsequently decreasing. Bilirubin levels in the serum seldom increase by more than 10 mg/dL; prothrombin times usually remain normal or exhibit mild elevation, and serum albumin levels are either normal or minimally depressed. Peripheral blood counts may indicate mild leukopenia, with or without relative lymphocytosis.1
Table 1 outlines the three primary serologic markers employed for determining HBV infection status: hepatitis B surface antigen (HBsAg), antibody to hepatitis B surface antigen (anti-HBs), and antibody to hepatitis B core antigen (anti-HBc).8
After identifying HBV infection, testing for other markers (HBeAg, anti-HBe, and HBV DNA) can offer insight into the degree of viral replication and infectivity, aiding clinical management:8
• HBV DNA measures viral load
• HBeAg denotes viral replication and heightened infectivity
• Anti-HBe can monitor response to treatment and progression of chronic HBV infection
Antiviral therapy is intended to reduce HBV DNA to undetectable levels. This is linked to improvements in liver inflammation and fibrosis, the reversal of cirrhosis, a decreased risk of HCC (hepatocellular carcinoma), and lower liver-related mortality. However, it’s important to note that none of the existing therapies offer a cure, and once treatment commences, it typically needs to be maintained for the foreseeable future. 6
Designed for simple and safe blood collection.

Unistik® VacuFlip and Unistik® ShieldLock are engineered for intuitive blood collection, featuring easy to use safety mechanisms that can help reduce the risk of needlestick injuries. Unistik® Blood Collection devices are cost-effective and designed to not compromise quality or safety.









































Savanna Real-Time PCR Testing Platform performs a simple, sample-to-result operation. A compact design delivers fast and customizable multiplex results. The unique design of the Savanna cartridge features syndromic panels with a range of targets, one of the first FDAcleared being Savanna HSV 1+2/VZV assay.
QuidelOrtho

The random-access IDS-i10 is a chemiluminescence (ChLIA) diagnostic solution. The compact fully automated immunoanalyzer is fast and efficient, offering a unique panel of immunoassays. 170 tests/ hour (assay dependent) may be loaded, samples can be added using a lab track –each sample can be processed efficiently with minimal hands-on time.

EUROIMMUN (Part of Revvity)
Atellica CI Analyzer, Model 1900 is an integrated clinical chemistry and immunoassay analyzer developed to bring world-class diagnostic capabilities into smaller laboratory environments. Measuring at 1.9 m 2 , this analyzer uses the same intelligent software, user-interface, reagents and consumables as our Atellica Solution, standardizing inventory, workflows, and clinical equivalence across all locations.
Siemens Healthineers
The Diesse MINI-CUBE is an automated erythrocyte sedimentation rate (ESR) analyzer that evaluates whole blood directly from EDTA tubes without consuming the patient sample. The MINI-CUBE produces results in 20 minutes, uses bar code scanning for positive patient identification and easily interfaces to LIS.
Streck


With new Applied Biosystems TaqMan QSY2 probes, users can now multiplex up to six targets in a single real-time PCR (qPCR) reaction with higher sensitivity, greater dynamic range, and better assay performance retention when scaling from 1-plex to 6-plex. The TaqMan QSY2 is equipped with the cyanine 5 and cyanine 5.5 long-wavelength dyes.
Thermo FisherWerfen’s GEM Premier 5000 system with integrated CO-Oximetry panel for POC and centralized laboratory testing, offers Arterial Blood Gas (ABG), Electrolytes, Glu, Lac, Hct, tHb, O2Hb, COHb, HHb, MetHb, sO2 , tBili, from a single whole blood sample. Self-contained GEM PAK cartridges incorporate all components for patient testing and are maintenance-free.
Werfen

The dIFine Family, a powerful partnership which pairs the Sebia Autoimmune & Infectious Diseases dIFine automated microscope with the dIFine P30 processor! The P30 is our easy-to-use and efficient walk-away benchtop instrument for IFA slide processing, designed to improve laboratory workflow and ensure high quality results… including cover slipping!
Zeus Scientific

When care can’t wait, hospitals rely on Acute Care Diagnostics from Werfen, a synergistic testing portfolio in point-of-care settings. Our integrative Blood Gas, Whole Blood Hemostasis and Patient Blood Management cartridge-based systems with quality management and connectivity solutions deliver fast, actionable results—simply and efficiently—so you can remain focused on patient care.
Emergency Department









Intensive Care Unit

Cardiac Catheterization Laboratory
Cardiovascular Operating Room









































For more information, contact your local Werfen representative or distributor. werfen.com








*Not Health Canada-licensed. Not available in all countries. GEM, Premier, GEM Premier ChemSTAT, ChemSTAT, GEMweb, iQM, Hemochron, VerifyNow, Avoximeter, and ROTEM are trademarks of Instrumentation Laboratory Company (d.b.a. Werfen) and/or one of its subsidiaries or parent companies and may be registered in the United States Patent and Trademark Office and in other jurisdictions. The Werfen logo is a trademark of Werfen and may be registered in the Patent and Trademark Offices of jurisdictions throughout the world. All other product names, company names, marks, logos, and symbols are trademarks of their respective owners. ©2023 Instrumentation Laboratory. All rights reserved.
From only one sample, the OSOM Flu SARS-CoV-2 Combo

Test simultaneously detects and differentiates between COVID-19, Flu A, and Flu B in only 10 minutes, allowing healthcare providers to make more informed decisions when treating patients who are symptomatic with viral infections that have similar symptoms, but different treatment protocols.
Sekisui Diagnostics

STAT sed rates are now possible. The miniiSED Erythrocyte Sedimentation Rate (ESR) analyzer significantly reduces the hands-on time and turnaround time required for ESR testing. The compact, automated analyzer generates results within 20 seconds from a capped EDTA sample tube while reducing subjectivity and biohazard exposure.
ALCOR Scientific
The combination of Revvity’s T-SPOT.TB test with the Auto-Pure 2400 liquid handler from Allsheng enables labs to efficiently perform accurate and reliable latent TB detection in a mid-high-volume setting. The platform tests up to 24 samples per run and completes day-1 T-SPOT.TB workflows in under 3.5 hours.
Revvity

Labs looking for blood culture identification panels and/or respiratory panels can utilize the cobas eplex system. Its upgraded design and features, leverage seven years of continuous input and 24 months of direct customer engagement through interviews and concept modeling.
Roche Diagnostics

Stat Profile Prime Plus is a blood gas/critical care analyzer. Blood oxygenation, tissue perfusion status, acid/base balance, electrolyte balance, fluid balance, glycemic control, and kidney function tests are available from two drops of blood in about 90 seconds at the point of care.
Nova Biomedical



A five-part differential hematology analyzer with built-in autoloader and a single closed tube sample mode. The hemoglobin analysis is performed using cyanide-free reagent. The analyzer processes up to 60 samples per hour and stores up to 100,000 results with histograms and scattergram. The barcode reader and optional LIS connectivity enable seamless sample data transmission. Mindray

KapSafe Recapper model 31011102-LS is a bench top 24” x 24” footprint for the automatic recapping of sample tubes, of various heights/diameters, at a throughput of 700 Tubes/Hour. KapSafe serves to eliminate exposure to repetitive stress injury during manual recapping. Uses muti-tier caps to fit various tube diameters. Other models available.
LGP Consulting
NG-Test CARBA 5 is one of the only rapid, multiplex, phenotypic, lateral flow, FDA cleared assays capable of detecting KPC, OXA-48-like, VIM, IMP and NDM carbapenemases produced by Enterobacterales and Pseudomonas aeruginosa . Detects the gene expression!
Hardy Diagnostics


The DZ-Lite c270 is a fullyautomated, open system, benchtop clinical chemistry analyzer with over 60 CLIA moderate complexity assays, comprising 15 urine drug screens, 28 special chemistries and 20 general chemistries. It offers up to 270 tests/hour with 36 reagent positions, and 30 sample positions. Carolina Liquid Chemistries



Registration Deadline: July 26th, 2024
healthcareinnovationsbestplacestowork.com

Over the past 20 years, clinical oncology has evolved in several important ways. Most excitingly, treatment strategies have become more personalized to the individual patient. This level of personalization is often only possible by implementing appropriate diagnostic tests to measure particular characteristics of an individual patient’s cancer.

and other factors, but involves periodic imaging tests, including computed tomography (CT) scans, magnetic resonance imaging (MRI) and colonoscopies, as well carcinoembryonic antigen (CEA) blood tests.
One of the fastest evolving and most exciting areas of personalized oncology care is minimal residual disease (MRD) testing for solid tumor cancers. In simple terms, MRD refers to cancer that may persist in a patient following curative-intent treatment, such as surgery. This residual cancer, or MRD, may be attributed to a microscopic deposit of cancer cells that is not yet large enough to be visualized by advanced radiographic tools. Left unchecked, these cells can lead to cancer recurrence or relapse that is eventually revealed by radiography (e.g., CT or PET scans).
MRD testing is often initiated after surgical resection to detect any residual disease that may persist and is otherwise unable to be visualized. Results in this setting may aid the oncologist to evaluate the suitability of additional treatments, such as chemotherapy.
Following treatment, MRD testing can be performed for several years afterward to monitor for cancer relapse and recurrence. MRD testing has been consistently shown to reveal recurrent cancer in advance of conventional approaches such as protein biomarker assessment and imaging techniques, which lack the resolution for identifying minute quantities of tumor and/or require a visit to the hospital for a radiology appointment. In certain settings, some patients neglect to come back to the hospital for routine surveillance testing with imaging. MRD testing, however, is much more convenient as it is performed with a simple blood draw that can be managed by mobile phlebotomy. This enables seamless access for the patient to important diagnostic testing.
In colon cancer, for example, as many as 30–40% of patients can recur after surgery with or without chemotherapy.2 Recurrence monitoring for colon cancer depends on the cancer stage
These conventional approaches have significant limitations. CEA testing identifies CRC recurrence in only about 59% of cases.3 Due to the poor sensitivity and specificity of CEA and limited resolution of imaging, these methods can miss cancer in early stages, when treatment has the greatest potential to eradicate disease.
In patients with a solid tumor cancer, such as colon or breast cancer, DNA molecules from the tumor are shed into the blood stream. These molecules can be detected using a socalled “liquid biopsy” test — in other words, a noninvasive test intended to identify tumor-derived material in bodily fluids, most commonly plasma derived from whole blood.
While normal cells also shed their DNA into the blood, circulating tumor DNA (ctDNA) is distinguished from normal cell-free DNA by the presence of cancer-specific mutations. The detection of ctDNA after treatment indicates that disease is still present and signifies a high risk of cancer recurrence in the absence of additional treatment. Because of the unique specificity of ctDNA for an individual cancer, a ctDNA-based MRD test with adequate sensitivity can enable identification of residual or recurrent cancer earlier than ever before (See Figure 1).
MRD testing is used to help answer the questions, “Did surgery remove all of my cancer,” and “Did my cancer return”? MRD testing can also be used to help monitor how a patient is responding to treatment, as indicated by changes in ctDNA levels during treatment. When used for treatment monitoring, MRD tests can help indicate when therapy is working and provide confidence that disease has been successfully treated. In contrast, increasing ctDNA levels may indicate a patient’s disease is not responding to the prescribed treatment and other interventions, including clinical trials, and should be explored.


The CE articles and accompanying CE tests remain online until the indicated deadlines have passed.
Take the monthly test online for immediate certification or download the printable test from www.mlo-online.com/ce (Mail-in tests may take up to 6 weeks to score.)
Completed tests and fees go directly to Northern Illinois University (NIU), and scores of 70% and higher qualify for one contact hour of P.A.C.E. credit.
Easy registration and payment options are available through NIU by following the link found at www.mlo-online.com/ce
MLO and NIU DeKalb, IL, are co-sponsors in offering continuing education units (CEUs) for each issue’s CE article. CEUs or contact hours are granted by the College of Health and Human Sciences at Northern Illinois University, which has been approved as a provider of continuing education programs in the clinical laboratory sciences by the ASCLS P.A.C.E. ® program. Approval as a provider of continuing education programs has been granted by the state of Florida (Provider No. JP0000496). Continuing education credits awarded for successful completion of this test are acceptable for the ASCP Board of Registry Continuing Competence Recognition Program.




Several clinical studies have shown that ctDNA can be an early marker of solid tumor cancer disease recurrence. For instance, one study found that individuals with negative ctDNA levels remained recurrence-free while 77% of patients with positive ctDNA levels relapsed during a median follow-up of 49 months. Notably, positive ctDNA preceded radiologic and clinical evidence of recurrence by a median of 3 months.4 While this and other data point to the significant potential of ctDNA-based MRD tests to aid clinical care, challenges remain. One crucial challenge with ctDNA analysis is the detection of extremely low numbers of tumor DNA molecules that may be present following treatment, which requires a highly sensitive testing technology for reliable detection.5
How a highly sensitive test can change the game
Haystack Oncology has developed a highly sensitive ctDNAbased MRD test to detect residual or recurrent cancer to better inform clinical decisions. Haystack MRD is a personalized ctDNA detection test that has been developed based on over two decades of landmark technology and clinical advances in the fields of cancer genomics and liquid biopsy led by Dr. Bert Vogelstein and his team at Johns Hopkins University. This team discovered the genetic basis of cancer, sequenced the first cancer exomes, and developed the first liquid biopsy techniques, laying the groundwork for the current era of personalized oncology and targeted therapies. The Haystack technology was developed specifically to detect ultralow levels of ctDNA typically seen in earlystage patients. Haystack MRD is tumor-informed, which means the test is custom-designed for each patient based on sequencing the patient’s tumor to identify up to 50 different variants in the patient’s ctDNA. This contrasts with tumor-agnostic methods, which use a single test to look for a fixed number of mutations in the blood without the benefit of personalization to the patient’s tumor. This lack of personalization of tumor-agnostic tests often identifies only a couple of variants in ctDNA, which is directly related to limitations in sensitivity yielding high false-negative rates and inconsistent results in clinical evaluations.
Another challenge of MRD testing is distinguishing tumor-derived DNA molecules from the normal (non-tumor) DNA molecules that are released into circulation from normal cells. For every molecule of ctDNA, there may be a million or more normal DNA molecules that obscure the cancer-specific signal. Therefore, a sensitive MRD test needs to be able to remove the background noise of normal DNA to detect those precious few ctDNA molecules in a tube of blood. As stated earlier, Haystack’s technology was designed specifically to detect these low-abundant ctDNA molecules, and so every aspect of the test, from the chemistry and workflow to the bioinformatics, has been purpose-built to reduce background noise and detect ctDNA.
With more Americans than ever before now being touched by cancer, detecting residual or recurrent disease and identifying better treatment options is essential to increasing survival rates and improving quality of life. Additionally, cancer patients experience both a clinical and economic burden, with treatment imposing side effects, requiring significant amounts of time and money and causing strain on personal relationships. This underscores the importance of identifying cancer as soon as possible and ensuring that the right treatment goes to the right patient at the right time.
One of the most exciting opportunities for MRD testing is to support clinical research, including the development of novel therapies. MRD testing can be leveraged for patients who have the highest risk of recurrence and therefore are more likely to benefit from therapy. Further, MRD testing can provide a real-time indication of patients’ disease response to treatment.
MRD testing has significant potential to guide treatment decisions following curative-intent surgery. The presence of post-surgical ctDNA indicates that surgery was not successful in the total eradication of disease. Across several studies, ctDNA has been shown to be predictive of cancer recurrence,
suggesting a role to better identify patients appropriate for adjuvant treatment.
Adjuvant treatment typically refers to chemotherapy, alone or with other treatments, such as radiation, following surgical resection — removal of a solid cancer tumor. This treatment essentially seeks to mop up any residual cancer cells that can remain in the body following surgery. However, many patients are successfully treated with surgery alone and do not need to undergo costly systemic therapy that is often fraught with side effects, from fatigue and nausea to neuropathy. The challenge in clinical practice is identifying those patients whose tumors have been completely surgically treated and can therefore avoid adjuvant therapy. MRD testing with an appropriate test has demonstrated an important role in this decision process.
The seminal DYNAMIC study suggests post-surgical MRD testing can dramatically improve the identification of patients for adjuvant therapy. Published in the New England Journal of Medicine in June 2022, DYNAMIC is the first completed randomized, interventional study to investigate the clinical utility of MRD testing to help guide the use of chemotherapy after surgical resection. The MRD test used in the study was based on a precursor to the current Haystack MRD technology. Among 455 stage II colon cancer patients, individuals with positive ctDNA results post-surgery were given adjuvant chemotherapy, whereas patients with negative ctDNA results did not receive chemotherapy. In contrast, patients in a group representing the standard of care received adjuvant chemotherapy based on traditional risk assessment using clinicopathological features such as tumor size and lymph node involvement. Results showed chemotherapy usage was halved in the ctDNA-guided group versus the standard of care group (with 15% of patients receiving therapy versus 28%, respectively) with no impact to two-year recurrence-free survival rates.6 This is the first study to demonstrate the clinical utility of using MRD testing to help guide the use of chemotherapy in cancer patient after surgery, providing strong evidence that ctDNA testing can help identify patients who are less likely to benefit from chemotherapy.
1. Colorectal cancer statistics. Cancer.org. Accessed May 23, 2024. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/ key-statistics.html.
2. Cafasso J. Colon cancer recurrence: Rates, treatment, and prevention. Healthline. Published September 14, 2022. Accessed May 23, 2024. https://www.healthline.com/health/colorectal-cancer/ colon-cancer-recurrence.
3. Liemburg GB, Brandenbarg D, Berger MY, et al. Diagnostic accuracy of follow-up tests for detecting colorectal cancer recurrences in primary care: A systematic review and meta-analysis. Eur J Cancer Care (Engl). 2021;30(5):e13432. doi:10.1111/ecc.13432.
4. Wang Y, Li L, Cohen JD, Kinde I, et al. Prognostic Potential of Circulating Tumor DNA Measurement in Postoperative Surveillance of Nonmetastatic Colorectal Cancer. JAMA Oncol. 2019;1;5(8):1118-1123. doi:10.1001/jamaoncol.2019.0512.
5. Galot R, Machiels JH. Current applications and challenges of circulating tumor DNA (ctDNA) in squamous cell carcinoma of the head and neck (SCCHN). Cancer Treat Rev. 2020;85:101992. doi:10.1016/j. ctrv.2020.101992.
6. Haystack MRDTM: Proven clinical utility. Haystackmrd. com. Accessed May 23, 2024. https://haystackmrd.com/ haystack-mrd-proven-clinical-utility.


ctDNA-based tumor informed MRD testing has emerged as a mainstream cancer diagnostic MRD testing is a fast-growing diagnostic approach that is expected to become an integral part of personalized cancer care. The ability to detect MRD can help physicians make better-informed decisions about their patients’ treatment. Per the current standard of care, many patients who are treated with surgery alone receive unnecessary adjuvant therapy and suffer associated toxicities and costs with minimal therapeutic benefit. The DYNAMIC trial showed that MRD testing can help identify patients that are more likely to benefit from adjuvant therapy by identifying the presence of ctDNA in their blood – an amount that may be indiscernible by traditional clinical or imaging assessments, yet still represents the presence of residual cancer. Additional research is underway across various cancer types and early evidence suggests MRD testing’s potential to reduce adjuvant therapy in other cancers beyond stage II colon cancer.
Hillary Sloane, PhD is the Director of Medical Affairs at Haystack Oncology, a Quest Diagnostics company. In her role, Dr. Sloane provides key clinical strategy support and primary oversight for the clinical development of Haystack’s cutting edge circulating tumor ctDNA minimal residual disease technology. She engages closely with the medical community to establish and execute clinical development initiatives that specifically address current and future needs to improve patient care. Hillary has an extensive background in molecular diagnostics and liquid biopsy, with experience in clinical development and medical scientific affairs roles in the oncology diagnostics industry. She holds a PhD in bioanalytical chemistry from The University of Virginia.


One thing is certain: Uncovering insights into residual or recurrent cancer is vital to helping patients overcome this deadly disease. It is essential for oncologists and laboratorians to stay abreast of innovations and developments like ctDNA-based MRD testing to ensure all cancer patients receive the most advanced care for optimal outcomes.








Continued from page 34
Eight approved drugs for treating chronic hepatitis B are currently available. These can be broadly classified into:
• Interferon preparations (standard interferon alfa-2b, peginterferon alfa 2a [peg-alfa 2a])
• Nucleos(t)ide analogs (lamivudine, adefovir, entecavir, telbivudine, tenofovir disoproxil fumarate, and tenofovir alafenamide)
Both WHO and AASLD guidelines recommend entecavir (for children ≥2) and tenofovir (for children ≥12) as first-line therapies for chronic HBV infection. AASLD also includes tenofovir alafenamide, an orally available prodrug of tenofovir with similar antiviral efficacy but lower renal and bone toxicity. Antiviral treatment is typically unnecessary for uncomplicated symptomatic acute HBV, as about 95% of immunocompetent adults experience spontaneous recovery. Therefore, the use of antivirals should be exercised with caution.1

Youssef Maakaroun is Senior Director MSL (Medical Affairs) in Siemens Healthineers where he supports the lab diagnostics division. Prior to Siemens, he worked at Sebia (Clinical Electrophoresis) setting up the international distribution network (Asia, Middle East, Africa, North America) and later starting the Medical Affairs department in Sebia inc. (Atlanta) where he participated in developing and launching a complementary diagnostic for a multiple myeloma immunotherapy.

Jim Aguanno, PhD received a Ph.D. in Biochemistry from Memphis State University. Following his Ph.D., he did two post-doctoral fellowships one at the University of Pittsburgh School of Medicine in Biochemistry and a second fellowship in Laboratory Medicine at Washington University School of Medicine and Barnes Hospital in St. Louis, Missouri. Dr. Aguanno was Director of the Core Laboratory at Baylor University Medical Center in Dallas, Texas for 24 years. Dr. Aguanno joined Siemens Healthineers in January of 2004 and is currently Senior Clinical Consultant in the Clinical and Scientific Affairs group.
1. Dekker SE, Green EW, Ahn J. Treatment and Prevention of Acute Hepatitis B Virus. Clin Liver Dis. 2021;25(4):711-724. doi:10.1016/j.cld.2021.06.002.
2. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;17;386(10003):1546-55. doi:10.1016/S0140-6736(15)61412-X.
3. Vittal A, Ghany MG. WHO Guidelines for Prevention, Care and Treatment of Individuals Infected with HBV: A US Perspective. Clin Liver Dis 2019;23(3):417-432. doi:10.1016/j.cld.2019.04.008.
4. Hepatitis B. Who.int. Accessed May 7, 2024. https://www.who.int/news-room/ fact-sheets/detail/hepatitis-b.
5. Tripathi N, Mousa OY. Hepatitis B. StatPearls Publishing; 2023.
6. CDC. Hepatitis B. Centers for Disease Control and Prevention. Published August 29, 2023. Accessed May 7, 2024. https://www.cdc.gov/hepatitis/hbv/ index.htm.
7. McMahon BJ. Natural history of chronic hepatitis B. Clin Liver Dis 2010;14(3):381-96. doi:10.1016/j.cld.2010.05.007.
8. Conners EE, Panagiotakopoulos L, Hofmeister MG, et al. Screening and Testing for Hepatitis B Virus Infection: CDC Recommendations - United States, 2023. MMWR Recomm Rep. 2023;10;72(1):1-25. doi:10.15585/mmwr.rr7201a1.

Mario Gutierrez Casale, MD obtained his MD degree from the Autonomous University of Guadalajara School of Medicine and a Master of Public Health from New Mexico State University. With over a decade of experience in the pharmaceutical industry and medical affairs, he has held pivotal roles at Arizona Liver Health and Summit Clinical Research. Currently, he serves as an MSL at Siemens Healthineers . Dr. Gutierrez Casale’s expertise spans a wide range of areas, focusing on Gastroenterology and Hepatology, and has cultivated an extensive network of Key Opinion Leaders (KOLs) at regional, national, and international levels.

Ashley Cheshire is the North American Marketing Manager for infectious diseases and sepsis assays (including HBV) at Siemens Healthineers . She earned her MBA in Healthcare Leadership from the Haslam School of Business, University of Tennessee, Knoxville. Her 15-year career in marketing medical technologies, spanning ophthalmology, orthopedic revisions, molecular imaging, and now core lab technologies, fostered a uniquely interdisciplinary perspective of the U.S. healthcare system and the patients it serves.

Avoid

If a hospital performs one million tests per year and loses one in 1,400 tubes at a cost of $400-600 per tube, then the annual cost could be as much as $428K – not to mention the burden on patients and hospital1.



SEE HOW INDEXOR STREAMLINES AND AUTOMATES SAMPLE TRACKING FOR BETTER RESULTS. VISIT US AT ADLM 2024 IN BOOTH #1201 TO LEARN MORE. • Trace samples from patient draw to arrival in the lab • Monitor key quality indicators • Automate to save time and improve service levels AT PHLEBOTOMY SITES DURING TRANSPORT IN THE LAB

1. Wiwanitkit, V. Types and frequency of preanalytical mistakes in the first Thai ISO 9002:1994 certified clinical laboratory, a 6 – month monitoring. BMC Clin Pathol 1, 5 (2001).
Alinity ci-series

Abbott’s Alinity systems are flexible and scalable with an increased throughput and capacity, allowing you to easily add modules as volume grows without having to replace your systems.
Abbott
Visit us at ADLM Booth #1201
Universal and system specific kits available for your instrument such as Abbott, Beckman, Ortho, Roche, Siemens, and MORE!


Effortlessly upload results from your LIS and eliminate the #1 cause of PT failures—clerical errors.
• No changes to your firewall or security
• No interface required or software to install
• No additional fees—API DataDirect ia free
Join the 7 million results uploaded utilizing API DataDirect!
American Proficiency Institute — Visit us at ADLM Booth #3146

The LIAISON PLEX® Respiratory Flex Assay is now FDA cleared on the next-generation, multiplex, sample-to-answer molecular diagnostic platform—the LIAISON PLEX® System. The flexible respiratory assay allows for customizable results based on patient needs.
AUDIT MicroControls
Visit us at ADLM Booth #3628

The KRONUS 3-Screen Islet Cell Autoantibody ELISA Kit (KR7780) is for the simultaneous and non-differential detection of autoantibodies to GAD and/or IA-2 and/or ZnT8 in a simple, highlyrobust, and user-friendly assay format.
KRONUS, Inc.

Acusera Smart QC optimizes your workflow by automating quality control processes. It reduces human error, minimizes operator handling time, and offers convenient onboard storage. Simplify your QC process and enhance efficiency today. Learn more: marketing@randox.com
Randox Laboratories — Visit us at ADLM Booth #1413
Diasorin — Visit us at ADLM Booth #1424

The NEW Unistik® venous blood collection portfolio is designed for safe and simple blood collection. Unistik ShieldLock, safety winged set, and Unistik® VacuFlip, safety needle, are intuitively designed utilizing tried-andtested blood collection techniques that healthcare professionals know. Unistik® ShieldLock and Vacuflip are a cost-effective alternative and available in a broad range of sizes.
Owen Mumford — Visit us at ADLM Booth #1826

The Tempus600® is a dedicated system for sending specimen tubes quickly and reliably to the laboratory. Sample tubes are placed directly into a sending station and arrive within seconds to the laboratory without carrier batching.
SARSTEDT Inc.
Visit us At ADLM Booth #3409

This test simultaneously detects and differentiates between COVID-19, Flu A, and Flu B in only 10 minutes, allowing healthcare providers to make more informed decisions when treating patients who have similar symptoms, but different treatment protocols.
SEKISUI Diagnostics
Visit us at ADLM Booth #1809

The Diesse MINI-CUBE is an automated ESR analyzer that evaluates whole blood directly from EDTA tubes. It produces results in 20 minutes, does not consume patient samples, requires no reagent and easily interfaces to LIS.
Streck — Visit us at ADLM Booth #821
G8 HbA1c Analyzer on the Sysmex

Increase your laboratory’s workflow efficiency by connecting the G8 A1c analyzer to the Sysmex automation track line.

Solana is a simplified molecular testing platform that helps make molecular diagnostics faster and easier than ever without sacrificing performance. Solana combines QuidelOrtho’s proprietary helicase-dependent amplification (HDA) with fluorescence detection to quickly deliver results you can trust.
Solana ® Molecular Testing Platform | QuidelOrtho
Visit us at ADLM Booth #1819

Sysmex’s innovative portfolio of hemostasis analyzers, reagents and controls are designed with quality, reliability and efficiency in mind. We provide advanced hemostasis technology across multiple platforms to match the needs of laboratories of all sizes.
Sysmex America Inc. — Visit us at ADLM Booth #413

Tosoh Bioscience
Visit us at ADLM Booth #3801

ACL TOP® Family 50 Series Hemostasis Testing systems offer the most advanced automation, quality management, and routine to specialty assays for mid- to high-volume clinical laboratories, including those with lab automation tracks.
Werfen - Hemostasis — Visit us at ADLM Booth #2813
GEM ® Premier TM 5000 blood gas system with iQM®2 assures quality before, during and after every sample in lab and POC testing—for improved patient care. All-in-one, multi-use cartridge offers advanced simplicity.
Werfen – Acute Care
Visit us at ADLM Booth #2813


Maggie Heider is an NGS Development Scientist at New England Biolabs . She received her BA in biology from the College of the Holy Cross and her PhD from the University of Massachusetts Medical School. Her graduate work focused on characterizing the protein complexes that regulate intracellular membrane trafficking and developing novel tools and assays to study their activity. Upon joining NEB in 2016, she turned her attention to enzymology and has spent the last several years developing NGS library preparation reagents and workflows including DNA library prep kits, enzymatic DNA repair, enzymatic fragmentation, PCR reagents, and multiplex oligos. Most recently, her work has centered on developing reagents and workflows for clinical sample types, particularly FFPE DNA samples.
What are some of the most difficult clinical sample types for sequencing-related projects and why?
Patient tissue is predominantly preserved as formalin-fixed, paraffin-embedded (FFPE) samples because the sample type is optimized for histology-based diagnostics and enables more cost-effective room temperature storage. However, the method is known to severely compromise the quality of nucleic acids. In fact, FFPE tissue samples are some of the most challenging to use for sequencing projects. The chemical fixation and harsh nucleic acid extraction processes impart extensive damage on already limited quantities of DNA. Depending on the preservation protocol, sample age, and extraction method used, FFPE DNA may incur varying degrees of damage, from crosslinking and fragmentation to nicks, gaps, abasic sites, cytosine deamination, and oxidative damage.
How do the challenges that clinical laboratories face with these sample types impact downstream processes and results?
Clinical laboratories often need to perform all required tests on limited quantities of tissue. This is particularly challenging when only small quantities of often highly damaged DNA are available. The damage present in FFPE DNA is problematic for two major reasons:
Low sample quality increases failure rates: DNA damage can inhibit the activity of enzymes required for the preparation of next-generation sequencing (NGS) libraries, limiting the rate of conversion of DNA molecules into the sequencing library. As a result, FFPE DNA libraries can suffer from insufficient molecular diversity and sensitivity to reliably detect patient mutations. Compounding this challenge, the impact of DNA damage on sample quality can be highly variable, so clinical labs face higher failure rates for library preparation when using a single protocol for all samples.
Damaged FFPE DNA can result in false positives: Several types of damaged DNA bases, notably deamination and oxidative damage, result in sequencing artifacts that present as false positives. The abundance of false positives makes the bioinformatic analysis of FFPE samples particularly complex.
What recent innovations to NGS should clinical labs be aware of pertaining to challenging clinical samples?
FFPE DNA samples still typically require higher DNA input than highquality samples. However, increasingly efficient methods of NGS library preparation have resulted in higher data quality from increasingly low FFPE sample input amounts. Additionally, the cost of sequencing continues to decrease. Cheaper sequencing is now enabling many labs to explore the utility of whole genome sequencing, when previously, they were constrained to targeted sequencing with hybrid capture panels or ampliconbased methods. The increasing adoption of spatial genomic approaches is another exciting application for which pre-fixed tissue samples, such as FFPE, are already well suited.
What role has New England Biolabs played in the development of solutions for NGS library preparation from clinical samples like FFPE (formalin-fixed, paraffin-embedded)?
At NEB, we’ve developed several new enzymatic solutions to address the challenges posed by FFPE samples, including a new enzymatic DNA repair mix, enzymatic DNA fragmentation mix, and PCR master mix. We have combined these solutions into an NGS library preparation kit — called the NEBNext UltraShear FFPE DNA Library Prep Kit — which was designed to be streamlined and sample quality agnostic, a key feature for clinical labs that often have no control over sample quality or the ability to tweak protocols based on individual samples. This workflow converts more damaged DNA into the NGS library, enabling higher coverage and, therefore, higher sensitivity for patient mutation detection. Furthermore, the kit improves library quality metrics — such as mapping rate, chimeric reads, and properly paired reads — increasing the amount of usable data for higher sensitivity and more cost-effective sequencing. Finally, the repair of DNA damage reduces sequencing artifacts, increasing the confidence in variant calls obtained from FFPE DNA. While the workflow provides many benefits for challenging samples such as FFPE, it is also compatible with high-quality DNA samples, allowing for the convenience of using a single kit regardless of the sample type obtained.
What elements of your approach lend themselves to successful innovation?
Our expertise at NEB lies in enzymology. We focus on ways we can leverage our deep knowledge of the unique properties of each enzyme in our portfolio to address molecular biology challenges, such as the many damage types in FFPE DNA. Not simply removing but fully repairing damaged DNA bases in a highly specific way enables accurate sequencing data, while also allowing researchers to maximize the diversity of DNA molecules present in their patient samples. Additionally, our new enzymatic fragmentation solution NEBNext UltraShear was designed specifically with FFPE samples and clinical laboratories in mind, improving the quality and quantity of usable data, as well as providing a convenient, automationfriendly workflow for clinical labs.








Empower your lab with primary vial loading from a trusted partner
The Roche cobas® 5800 System is built to offer a fully automated workflow that encompasses primary vial handling for cervical and sexual health samples, transfer and preparation, amplification and detection, result calculation and delivery to the laboratory information system (LIS).
Designed to bring efficiency, flexibility and simplicity to laboratories of all sizes, enabling greater patient access.
Learn more about the cobas® family of molecular systems today.