




N F I D E N T LY

The Next Generation Of Contamination Monitoring Technology
For the cleaning verification of surgical instruments, endoscopes, and surfaces, Clean Read Handheld by Empire Bio Diagnostics is a cloudbased cleaning monitoring system used to help hospitals and other healthcare organizations achieve optimal standardized cleaning levels.



















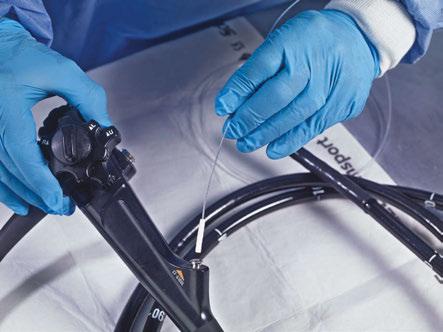



FEBRUARY 2025

Sourcing & Logistics
8 > The Art of Pediatric Supply Chain Management: Balancing Care and Costs
JANETTE WIDER
32 > Clinically-Driven Logistics Improve Costs, Care and Sustainability
KAREN CONWAY
Infection Prevention
12 > Preparing for the Next Pandemic
MATT MACKENZIE
Sterile Processing
16 > Why Water Quality in Sterile Processing Departments is Now a Top Priority
KARA NADEAU
24 > Education Nation: CurriculumBased In-Services
SARAH B. CRUZ
28 > SPD Ergonomics Planning Worth the Effort & Investment
DAVID TAYLOR
30 > Airflow
ADAM OKADA


Departments 4 > Pediatric Challenges
6 > What’s on the Web, Advertiser Index




Medication errors occur due to:
Confusing units (kilograms vs pounds)
Calculations due to inaccurate weight measurements
DETECTO protects you against both of these common issues:
OneWeigh units locking into LBs or KGs
NTEP certified weighing accuracy


DETECTO has received NTEP (National Type Evaluation Program) approval administered by the National Conference on Weights & Measures for most of the company’s major medical scales.
This verifies from a regulated governing body that DETECTO scales are manufactured to the highest accuracy and quality possible for medical scales used to treat patients.
BY JANETTE WIDER
According to a UNICEF report, published in late 2023, “Many countries face a myriad of challenges in their supply chain systems including limited data visibility, financing challenges, fragmented procurement processes, and limited warehousing, storage and distribution challenges. These are further compounded by an inadequately staffed and skilled supply chain workforce. These bottlenecks limit the availability of products, trigger service interruptions and undermine the safety of recipient communities, particularly hardto-reach populations. For example, in 2021, five million children globally missed out on basic vaccines, 13.6 million children under the age of 5 suffered from severe wasting, and 698 million lacked basic sanitation services at school.”
Although this data is not strictly hospital related, it is indeed troubling. And these statistics inspired me to write this month’s cover story in challenges in children’s hospital supply chain operations (see page 8).
Children’s hospital supply chain leaders face many challenges due to the unique nature of pediatric care. From specialized medical supplies and equipment and inventory management complexities to supply chain disruptions that are expected to continue for the foreseeable future, supply chain professionals have their work cut out for them—to say the least.

Yet, due to the unwavering dedication of these professionals, there is hope, particularly through strong relationships with facilities group purchasing organizations and vendors.
Cory Turner, CMRP, senior director, Healthcare Strategy, Tecsys, who was featured in the article, noted, “A key element in handling these complexities is strong communication with vendors. For any hospital, the key is adopting the right blend of self-distribution, safety stock and just-in-time inventory management. With pediatric hospitals, solving for that just-in-time component only works if the supply chain leaders and vendors are in sync, sharing information on consumption trends and understanding the unique demands of the pediatric population.”
He added, “From a supplier’s side, it’s not just about fulfilling orders; they need to understand the seasonal variations and consumption patterns unique to pediatric care. Strong data visibility is crucial here, allowing both hospitals and suppliers to forecast demand accurately and avoid disruptions.”
If you have a story about protecting vulnerable populations, like pediatrics, reach out to me at jwider@ hpnonline.com. In 2025 and beyond, I’d like to feature more real-world stories overcoming challenges with innovation and compassion for those who need it most.
February 2025, Vol. 49, No. 2
VP & Market Leader
Healthcare and Dental
Chris Driscoll cdriscoll@endeavorb2b.com | 978-880-8345
Editor-in-Chief
Janette Wider jwider@hpnonline.com
Associate Editor Matt MacKenzie mmackenzie@endeavorb2b.com
Senior Contributing Editor Kara Nadeau knadeau@hpnonline.com
Advertising Sales
East & West Coast
Kristen Hoffman khoffman@endeavorb2b.com | 603-891-9122
Midwest & Central
Brian Rosebrook brosebrook@endeavorb2b.com | 918-728-5321
Advertising & Art Production
Production Manager | Ed Bartlett
Art Director | Tracy Arendt
Advertising Services
Karen Runion | krunion@endeavorb2b.com
Audience Development
Laura Moulton | lmoulton@endeavorb2b.com
Endeavor Business Media, LLC
CEO Chris Ferrell
COO Patrick Rains
CRO Paul Andrews
CDO Jacquie Niemiec
CALO Tracy Kane
CMO Amanda Landsaw
EVP Medical & Healthcare Technology Kylie Hirko
EVP Endeavor Business Intelligence Paul Mattioli
Healthcare Purchasing News USPS Permit 362710, ISSN 1098-3716 print, ISSN 2771-6716 online is published 11 times annually - Jan, Feb, Mar, Apr, Jun, Jul, Aug, Sep, Oct, Nov/Dec, Nov/Dec IBG, by Endeavor Business Media, LLC. 201 N Main St 5th Floor, Fort Atkinson, WI 53538. Periodicals postage paid at Fort Atkinson, WI, and additional mailing offices. POSTMASTER: Send address changes to Healthcare Purchasing News, PO Box 3257, Northbrook, IL 60065-3257. SUBSCRIPTIONS: Publisher reserves the right to reject non-qualified subscriptions. Subscription prices: U.S. $160.00 per year; Canada/ Mexico $193.75 per year; All other countries $276.25 per year. All subscriptions are payable in U.S. funds. Send subscription inquiries to Healthcare Purchasing News, PO Box 3257, Northbrook, IL 60065-3257. Customer service can be reached toll-free at 877-382-9187 or at HPN@omeda.com for magazine subscription assistance or questions. Printed in the USA. Copyright 2025 Endeavor Business Media, LLC. All rights reserved. No part of this publication June be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopies, recordings, or any information storage or retrieval system without permission from the publisher. Endeavor Business Media, LLC does not assume and hereby disclaims any liability to any person or company for any loss or damage caused by errors or omissions in the material herein, regardless of whether such errors result from negligence, accident, or any other cause whatsoever. The views and opinions in the articles herein are not to be taken as official expressions of the publishers, unless so stated. The publishers do not warrant either expressly or by implication, the factual accuracy of the articles herein, nor do they so warrant any views or opinions by the authors of said articles.
“LIKE
Here’s what people are saying about their 50 Series Medical Device Dryers
“It saves us time.”
“Never thought a dryer could make such a difference.”
“Provides predictable drying times.”
“It makes our life easier.”
“It’s like we’ve got another FTE in the department.”

Minimize canceled low-temp sterilizer cycles with complete and predictable drying
Dry one caseload of robotic arms in 30 minutes

According to data from the CDC, the amount of acute respiratory illness causing people to seek healthcare is high. Nationally, emergency department visits with diagnosed RSV and influenza are very high and emergency department visits for COVID-19 are increasing from low levels. Influenza test positivity increased to 18.7%. COVID-19 test positivity has increased to 7.1%. RSV test positivity increased to 12.8%. On a national level, wastewater viral activity levels for COVID-19, influenza A, and RSV are at moderate levels.
Read on: hpnonline.com/55253373
Linen, Uniform, and Facility Services with Joe Ricci
TRSA | Association for Linen, Uniform, and Facility Services Industry President Joe Ricci shares insights with Editor-in-Chief, Janette Wider. The podcast highlights the importance of reusable healthcare PPE and Hygenically Clean when contracting the essential service offered by TRSA.
Listen at: hpnonline.com/55252373
Public health experts in the U.S. believe that available treatments and vaccines for H5N1 avian influenza A virus are “sufficient to prevent severe disease” for the time being. A new commentary published in the New England Journal of Medicine suggests that people find a balance between “enhanced vigilance and ‘business as usual’ with respect to HPAI H5N1.” In 2024, the virus caused “66 confirmed and 7 probable cases of influenza in people in the U.S. and one case in Canada.
Read on: hpnonline.com/55252456

Jimmy Chung, MD, MBA, FACS, FABQAURP, CMRP, Chief Medical Officer, Advantus Health Partners and Bon Secours Mercy Health, Cincinnati, OH
Joe Colonna, Chief Supply Chain and Project Management Officer, Piedmont Healthcare, Atlanta, GA; Karen Conway, Vice President, Healthcare Value, GHX, Louisville, CO
Dee Donatelli, RN, BSN, MBA, Senior Director Spend symplr and Principal Dee Donatelli Consulting LLC, Austin, TX
J. Hudson Garrett Jr., PhD, FNAP, FSHEA, FIDSA, Adjunct Assistant Professor of Medicine, Infectious Diseases, University of Louisville School of Medicine
Melanie Miller, RN, CVAHP, CNOR, CSPDM, Value Analysis Consultant, Healthcare Value Management Experts Inc. (HVME) Los Angeles, CA
Dennis Orthman, Consulting, Braintree, MA
Janet Pate, Nurse Consultant and Educator, Ruhof Corp.
Richard Perrin, CEO, Active Innovations LLC, Annapolis, MD
Jean Sargent, CMRP, FAHRMM, FCS, Principal, Sargent Healthcare Strategies, Port Charlotte, FL
Richard W. Schule, MBA, BS, FAST, CST, FCS, CRCST, CHMMC, CIS, CHL, AGTS, Senior Director Enterprise Reprocessing, Cleveland Clinic, Cleveland, OH
Barbara Strain, MA, CVAHP, Principal, Barbara Strain Consulting LLC, Charlottesville, VA Deborah Petretich Templeton, RPh, MHA,Chief Administrative Officer (Ret.), System Support Services, Geisinger Health, Danville, PA
Ray Taurasi, Principal, Healthcare CS Solutions, Washington, DC

Over 30 years creating proven solutions for patient care





Two supply chain leaders share insights on the children’s hospital supply chain landscape.
BY JANETTE WIDER, EDITOR-IN-CHIEF

Supply chain plays a crucial role in ensuring that hospitals are equipped to provide timely and lifesaving care. But for children’s hospitals, this task is compounded by unique challenges. From the specialized needs of pediatric patients to the complexities of securing products for a diverse range of age groups, the supply chain in these institutions is a high-stakes balancing act. Issues like product shortages, backorders, and limited suppliers often create a ripple effect, disrupting critical services and requiring quick, innovative solutions. With a focus on safety, efficiency, and care quality, the supply chain in children’s hospitals must navigate hurdles that are distinct from those faced by adult healthcare facilities, making it a constant challenge to meet the needs of the youngest and most vulnerable patients.
Healthcare Purchasing News had the opportunity to speak with two leaders in this space, Cory Turner, CMRP, senior director, Healthcare Strategy, Tecsys, and Gloria Graham, DNP, RN, CVAHP, manager, Value Analysis, Cincinnati Children’s Hospital Medical Center, shared their insights on challenges, trends, and what supply chain professionals need to know when it comes to children’s hospital supply chain challenges.
Turner said, “In healthcare, there’s always a delicate balance between cost and patient care, with hospitals continuously trying to manage expenses while ensuring quality outcomes. However, in pediatric settings, there’s a greater tendency to let cost considerations take a backseat more readily compared to the emphasis on delivering high-quality care. Ensuring that every procedure is equipped with the right supplies tailored to each child’s specific needs becomes the absolute priority.”
He added, “From my perspective, the biggest differences between supply chain operations in children’s hospitals and regular hospitals stem from the specialized needs of pediatric care. With a patient demographic that ranges from neonates to adolescents, children’s hospitals face added complexity in managing supplies because of the range of supplies needed for this population. The wide variety of SKUs needed for different ages, sizes, and medical needs means our supply chain has to be more versatile and precise compared to an adult hospital. This creates unique challenges, especially in departments like Cath labs, where limited space must accommodate a wider variety of SKUs than in adult facilities due to the range of equipment sizes required.”
Graham largely agreed and started by addressing the facility itself. She said, “There are clear differences in how we approach value analysis—how we evaluate and make decisions about supplies and equipment for the
hospital. This extends even to designing new facilities. For instance, we must consider room layouts that accommodate a family member staying overnight, as it’s common for someone—be it a parent, grandparent, or other relative—to stay with the patient 24/7. Even details like hallway design matter; the environment needs to be kid-friendly, with engaging pictures and interactive elements throughout.”
“Most children’s hospitals also have a family resource center,” she explained. “It’s a space where families can take a break, equipped with computers and other amenities to provide a moment of respite. We also ensure there are laundry facilities—washers and dryers—for families to use during their stay.”
Graham explained, “And that’s just the design aspect. When it comes to purchasing, we have to view products through a different lens to ensure they’re safe for both pediatric patients and adults. For example, consider a syringe with a cap. If the cap is accidentally left in the room, what’s the first thing a child might do? They see it in the bed, mistake it for candy, and put it in their mouth. Safety like this is always top of mind.”
When asked about vendor relations, Turner noted, “A key element in handling these complexities is strong communication with vendors. For any hospital, the key is adopting the right blend of self-distribution, safety stock and just-in-time inventory management. With pediatric hospitals, solving for that just-in-time component only works if the supply chain leaders and vendors are in sync, sharing information on consumption trends and understanding the unique demands of the pediatric population.”
He added, “From a supplier’s side, it’s not just about fulfilling orders; they need to understand the seasonal variations and consumption patterns unique to pediatric care. Strong data visibility is crucial here, allowing both hospitals and suppliers to forecast demand accurately and avoid disruptions.”
Graham highlighted the need to consider essential care items for infants and toddlers, saying, “We even have to think about potty training chairs for children in that developmental stage, as well as bedside commodes. In adult hospitals, there’s typically one size, but that doesn’t work for children—the adult size is far too big and impractical. Children are not just small adults, though many people and suppliers seem to think otherwise. We strive to educate everyone that pediatric patients have unique needs.”
“For example,” she continued, “due to our diverse population, we define certain age groups as follows: neonates are from birth to 28 days, infants are 29 days to 2 years, children are 2 to 12 years, adolescents are 12 to 21 years, and adults are over 21 years. We must accommodate sizes for every age group, which can get complex.”
Graham commented, “For example, we need specialized lancets in various sizes which is used to prick your finger for blood tests. For neonates, especially very small babies like preemies, we use the smallest lancets to collect capillary blood samples, typically from their heels. These babies are so tiny that only the smallest lancets will work. At the same time, we also need lancets suitable for adolescents and adults, which means maintaining a range of products to meet the needs of all age groups.”
Graham explained that the challenge is that in pediatrics, we often face limited product options, especially since our purchasing volume is much smaller compared to adult hospitals. This forces us to rely on multiple suppliers for the same product, as one supplier may only offer neonatal sizes, while another might carry the regular or adult sizes. This creates complications in standardizing products across our facilities. This also impacts clinical practice due to clinicians being competent on variations of the same type of product. She noted that a variation in product leads to a variation in practice which ultimately can lead to a variation in outcomes.
She continued, “Supply chain issues, such as backorders or product discontinuations, can make these challenges even worse. For instance, last year, a company that produces neonatal peritoneal dialysis sets went on backorder for over four months. With no ready-made solution available, we had to manually source 11 separate items from different suppliers to assemble a comparable set. This added significant work for both the supply chain and clinical staff, increasing the potential for errors and safety concerns.”
“Another example involves a specific type of catheter used by our cardiothoracic surgeons,” she said. “There was only one supplier, but due to low demand, they discontinued certain sizes of this catheter. This forced the surgeons to adjust their practices, impacting procedures and added complexity to patient care.”
Graham asserted, “We have to account for a wide range of variables when making decisions, which differs from the approach in adult hospitals, where the considerations are more straightforward.”
When asked about advancements in technology in this space, Turner said, “One of the ongoing challenges in pediatric hospitals, like many others,
is the lack of visibility in the supply chain due to legacy systems and outdated item masters. Managing the item master effectively can be a huge task, especially when dealing with outdated data, multiple SKUs, and fluctuating inventory needs. That’s why I believe the solution lies in leveraging technology — particularly AI and data analytics. At Tecsys, we’ve been actively working on AI-enabled tools that help healthcare systems clean up their item masters and improve inventory management. This means better data, more accurate forecasts, and fewer manual interventions.”
“Across the supply chain spectrum, inside and outside of healthcare, I see a growing reliance on data-driven solutions,” he said. “As more pediatric hospitals begin to work together with peers and technology partners, the entire healthcare supply chain will benefit. It’s not just about the technology — it’s about the community of supply chain professionals coming together to solve these challenges. I’m particularly impressed with the user network we’re building at Tecsys, because we’re combining the human element of collaboration with advanced technology, all with a clear-eyed focus on the CQO tenets. This is what I believe will drive better outcomes for patients and greater efficiency in supply chain operations across the board.”
As for teamwork, Graham said, “You quickly identify key personnel in situations like this. In value analysis, I collaborate closely with materials management. When an issue arises, such as a neonatal set being on back order, I immediately contact them. To gauge the urgency, they assess the remaining inventory to determine how many days of stock are left.
Further, “Once we know how much time we have left, we engage with the clinicians to determine their needs for continuing the procedure evaluating potential substitute products and assessing their impact on clinical
practice. Over my 35 years at Children’s, I’ve built strong relationships across different departments. For example, if it’s a dialysis product, I know exactly who to contact. We then collaborate with the clinicians to identify alternative components that would allow them to proceed with the procedure.
“In this case, we had all the necessary components available. I then coordinated with the materials management team to ensure they ordered enough to prevent shortages. The materials management staff assembled, bagged, and delivered them to the clinical team. The clinicians also put together educational materials to clarify the process.


“This back-and-forth is typical in value analysis. It’s about knowing who to connect with to resolve problems effectively. Even if you’re new to a situation or department, it’s important to reach out to your network to find the right person. For example, although I’ve never worked directly with dialysis patients, I’ve learned over time to rely on experts in those areas. This collaborative problem-solving is something we do every day.”
When Graham was asked about budgets and the bottom line, she said, “We often encounter situations where a product is off contract or has been discontinued, which is where we rely on our GPO for support. For example, if a product falls off contract, we work closely with our Vizient representative to explore the possibility of getting that supplier back on contract. In some cases, suppliers are willing to rejoin, but it really depends on the supplier.
“If they’re not willing to participate, our sourcing team steps in. Having the right people in value analysis is crucial for success. The sourcing team negotiates a local contract with the supplier, focusing on securing the best pricing to mitigate the impact of losing the previous contract pricing.
“Our sourcing team excels at handling these negotiations, ensuring we get the best possible price.” This effort, in collaboration with value analysis, ensures there is no loss or change in the quality, efficacy, and safety of the products. These principles are the foundational pillars of value analysis, guiding our commitment to maintaining high standards.”
It is clear that navigating the complexities of the supply chain in children’s hospitals requires a strategic and collaborative approach. From managing specialized product needs to negotiating contracts with suppliers, value analysis and sourcing teams play a crucial role in ensuring that hospitals can provide the best care possible, even when faced with shortages or disruptions. By leveraging partnerships with GPOs and working closely with suppliers, these teams help mitigate challenges, reduce costs, and maintain a steady supply of essential products. While the road can be challenging, the commitment to adaptability and problem-solving ensures that pediatric care continues uninterrupted, no matter the obstacles. HPN

The risk to the general public from bird flu is low, but the uptick in cases prompts a look at how prepared hospitals are for future outbreaks and pandemics.
BY MATT MACKENZIE, ASSOCIATE EDITOR

Nearly five years have passed since the start of the COVID19 pandemic, and at this point, there are plenty of data points surrounding which approaches worked and which may need tweaking should another pandemic come around.
The current outbreak of avian influenza in the U.S. has raised alarm bells and thrown into sharper focus the need for strong preparedness measures in the event of another pandemic-level event. At the point of this writing, the CDC continues to maintain that the risk to the general public is low, but there is still something alarming about the rising case numbers with memories of the beginning of the COVID pandemic so close at hand.
An article published in the New England Journal of Medicine on December 31, 2024, entitled “Highly Pathogenic Avian Influenza A(H5N1) Virus Infections in Humans” and written by Shikha Garg, et al, sought to describe characteristics of the human cases of avian influenza, or H5N1, that have spread throughout the country thus far. There have been 46 patients to date, and all but one of them were definitively exposed to infected poultry or infected or presumably infected dairy cows. The one who did not have an identified exposure “was hospitalized with nonrespiratory symptoms, and A(H5N1) virus infection was detected through routine surveillance.” However, among the 45 patients with animal exposures, “the median age was 34 years, and all had mild A(H5N1) illness; none were hospitalized, and none died.” The authors of the article concluded that “A(H5N1) viruses generally caused mild illness, mostly conjunctivitis, of short duration, predominantly in U.S. adults exposed to infected animals; most patients received prompt antiviral treatment. No evidence of human-to-human A(H5N1) transmission was identified. PPE use among occupationally exposed persons was suboptimal, which suggests that additional strategies are needed to reduce exposure risk.”
The article also makes the case that there are so many factors still unknown regarding H5N1 viruses currently circulating in dairy cows. Because of these uncertainties, “how long workers should be monitored is unclear.” High levels of the virus are also being found in “unpasteurized raw milk, which is probably an important source of transmission from cows to dairy workers.” Plus, looking at human cases across the globe reveals a “wide spectrum of clinical disease severity, ranging from asymptomatic illness, conjunctivitis, and mild upper respiratory tract symptoms to lower respiratory tract disease and critical illness, including death. Why recent U.S. cases have generally been clinically mild remains unclear; early detection and initiation of antiviral treatment may play a role.”
The amount that remains unknown about what a larger outbreak of bird flu would look like provides a useful opportunity to look at pandemic preparedness writ large to attempt to determine broadly what needs to be done to minimize adverse outcomes. The past several years of COVID provide plenty of data, both promising and not, about attempts to stop the spread of a respiratory pathogen. An article, called “Positive and negative aspects of the COVID-19 pandemic among a diverse sample of US adults: an exploratory mixed-methods analysis of online survey data”

and written by Stephanie A. Ponce, et al, published in BMC Public Health at the beginning of 2024, provided research and data surrounding both positive and negative experiences during the COVID-19 pandemic across “race-ethnicity, gender, and age.” The researchers used results from a survey of about 5,500 racially diverse adults living in the U.S. Most of the data surrounded social effects of the pandemic, but researchers did record “differences across age, gender, and race-ethnicity,” which lends further credence to the notion that recovery strategies need to be “tailored to community needs” if they wish to address inequities. Responses to any future pandemics or outbreaks, then, must attempt to account for “variation in access to economic and social resources” across different groups of people. The authors of the study conclude that having a “more nuanced understanding of such variation in the perceived positive and negative effects of pandemics can inform tailored public health efforts to mitigate potentially harmful factors.”
Healthcare Purchasing News was able to speak with Isis Lamphier, MPH, MHA, CIC, and the manager of Infection Prevention at Moffitt Cancer Center, about hospitals’ responses both to COVID-19 and to any future outbreaks. She also speaks to the possibility of a bird flu epidemic.
Are there any important lessons infection preventionists (IP) learned from the COVID pandemic?
Important lessons learned include the importance of standard precautions, early detection and response including screening protocols, preparedness, and risk management, and how misinformation can be an obstacle IPs face in their work.
What went well and what was lacking during the initial response to COVID?
The things that went well include rapid scientific advancements including testing, vaccine development, and innovations in treatment. Also, organizations quickly adapted to remote platforms to provide telemedicine and conduct meetings. In addition, there was an increase in public awareness of standard precautions such as hand hygiene and mask usage, which helped to drive down infections.
Some of the ways in which the response to COVID was lacking include unexpected supply chain failures and shortages of medical supplies, the spread of misinformation, and increased healthcare worker burnout.
As bird flu cases continue to grow in number, what do you think of the possibility that it could eventually reach epidemic levels?
I think bird flu is unlikely to reach epidemic levels in humans because historically there has been limited humanto-human transmission. After all, bird flu is a zoonotic disease that primarily spreads through humans coming into contact with infected animals or other animal-related contamination. We also have stringent monitoring and control measures in place to detect influenza outbreaks early, quarantine infected poultry, and vaccinate poultry.
What should hospitals and IP departments be doing now to gear up for the next pandemic?
As always, hospitals and IP departments should be informed of emerging pathogens by staying up-to-date with information from local health departments and organizations such as the CDC. Organizations should also have an established policy that discusses epidemics and pandemics. IP departments should run through possible scenarios, discuss the chain of command for emergency response, and have established plans to address issues such as PPE shortages, whether will they take or send patients to other hospitals, and quarantine measures.=
What measures are hospitals already taking to prepare?
The majority of hospitals already have established policies detailing emergency response and epidemic/pandemic preparedness.
What can everyday people keep in mind for how to behave when a new pandemic strikes?
Stay at home and avoid others if you feel sick, limit nonessential travel, take standard precaution measures, reinforce hand hygiene, and potentially use surgical masks depending on the organism causing the pandemic to limit transmission.
How do you feel generally about the U.S.’s preparedness for another pandemic?
We still have significant room for growth and much to learn from the COVID-19 pandemic. It is crucial to prioritize and reinforce the role of healthcare experts in providing accurate information and actively involving them in decision-making and policy development. To move forward effectively, we must shift attention away from the misinformation prevalent on social media and certain news outlets, focusing instead on the trusted expertise of public health officials. HPN

HPN’s podcast features interviews with special guests and article reads on our many verticals including supply chain, sterile processing, surgical and critical care, infection prevention, and more.

Scan the QR code so you don’t miss an episode!


BY KARA NADEAU, SENIOR CONTRIBUTING EDITOR
The topic of water quality and management has been coming up more and more in my conversations with sterile processing (SP) professionals. While the ANSI/ AAMI ST108:2023 standard for water quality systems in medical device processing has certainly been a driver, concerns about water quality in the sterile processing department (SPD) aren’t new.
I reached out to those with expertise on this topic to understand why there has been growing interest and concerns about the water used in instrument and device reprocessing.
Based on these interviews, I aimed to answer high level questions those in the SP profession are likely to have about key differences between AAMI TIR34:2014/ (R)2021 and ANSI/AAMI ST108:2023 and the role SP professionals should play as part of their healthcare facility’s water management teams.
I also asked each expert contributor to provide one piece of advice to SP professionals when working toward ST108:2023 compliance, with this commentary wrapping up the article.
With SP teams struggling with so many aspects of their profession, from rising case volumes to lack of adequate instrument inventory, why should they take time to address water quality?
After speaking with experts, the answer is clear – water quality impacts virtually every aspect of SP operations. And it doesn’t stop there. The effects of
poor water management in instrument reprocessing snowballs to impact the operating room (OR), other procedural areas, and in severe cases, the reputation of the healthcare facility.
Most recently, media reports of U.S. hospitals with water quality issues in the SPD have heightened awareness of the problems that can occur.
“The headlines and social media frenzy from contaminated water incidents in healthcare facilities has spooked the public and internal healthcare leaders,” said Jhmeid Billingslea, CRCST, CIS, CER, CHL, CMRP, CST, HACP-IC, Managing Director Surgical Services, Advantage Support Services. “The overwhelming expense and service disruptions for instruments that do not come close to meeting their typical lifespans are killing SPD budgets. OR team members have also become more sensitive to wet packs, instrument staining and spotting, tray liner marks and other packaging variances caused by non-compliant water usage in SPD.”
“My opinion is that water quality in the SPD has moved front and center,” said Aimee Space, BSN, RN, Product Owner, U.S. Medical Content, Incision. “If you’re in the sterile processing world, it’s unlikely you haven’t seen the stories about facilities shutting down over water quality concerns. It’s not just a safety concern but also a financial concern. If you must shut down your sterile processing department because of poor water quality that’s a huge financial loss.”
The good, bad, and ugly of water quality
Jonathan A. Wilder, Ph.D., Managing Director, Quality Processing Resource Group, commented on the benefits of good water quality in the SPD:
“Water is a variable quantity when applied to processing of medical devices, depending upon where the facility is and the local water supply, and how the facility treats its water supply to generate water that


Angela Lewellyn
is suitable for processing. Good, corrosion-free results and good cleaning with good removal of residual detergent in the washer/ disinfectors and manual cleaning are a result of ensuring that the water used is up to the task and does not interfere with the action of detergent, or, if it does, that detergent is properly selected and dosed to compensate for this.”
Angela Lewellyn, Director of Development and Research, Advantage Support Services, pointed to issues such as stained instruments and chalk buildup in washers as indications of poor water quality, stating:
“These issues can mean the sterilization process isn’t working properly,
which could lead to patients getting infections. Some of the science and effects of water that SP is beginning to understand is that some bacteria, like Legionella or Pseudomonas, can grow in places where water sits still, like pipes or drains. They can also spread through things like contaminated water used for rinsing instruments or even during handwashing if faucets or drains are infected.”
Herb Kaiser, PhD, Senior Principal Scientist, Analytical Services and Development at STERIS Corporation, called attention to the fact that poor quality impacts not only devices and instrumentation, but also the equipment used to reprocess them. He stated:
“With everyone in healthcare worried about dollars, it is important to understand how poor water quality can shorten the lifespan of SPD equipment, including washer/disinfectors and sterilizers. When somebody tells me they are having trouble with their equipment, one of the first questions I ask is, ‘What does the inside of your washer or sterilizer look like?’ If it looks bad, with residue and deposits, that will negatively impact your instruments and devices.”

What are key differences between TIR34:2014/ (R)2021 and ST108:2023?
Wilder provided a summary of the evolution of water quality guidance and standards impacting the SPD.
“The effects of water on instrument processing were slow to be understood,” said Wilder. “The sequence of thought I have seen over my 34 years and one month in the industry from both the inside (in R&D and regulatory) was:
• Can we get it clean?
• Is our cleaning damaging the instruments?
• If our cleaning isn’t damaging the instruments, what is?”
“This is where TIR34 started,” Wilder explained. “It was first published in 2007. The second revision, which is the last revision, was published in 2014. These were both guidance documents, with a lot of progress made in understanding the effects of water between the 2007 and 2014 revisions.”
“Once the concept of the effects of water on processing was compiled into a TIR, it became clear to a group of AAMI committee chairs that enough was known to write a standard,” Wilder continued. “This led to the decision to create ST108. Detergent dosing, measures needed to provide good-quality critical water for final rinse, and how to make the best possible steam for sterilization depend greatly on the water supply.”
Kaiser offered his insights on the evolution of TIR34:2007 to TIR34:2014 to ST108: 2023, stating:
“The first version, TIR34:2007, featured many technical requirements
related to testing and analysis of water for impurities, which many felt were confusing to the end user. To address this issue, TIR34:2014 featured simplified charts of the requirements. Unfortunately, this simplification came at the expense of eliminating some of the values
As Kaiser noted, a major change in ST108:2023 compared with TIR34:2014 is that the standard defines three different categories of water important for device and instrument reprocessing:
• Utility Water: May be water as it comes from the tap but it may
“Water
is the number one tool we use in SP, and although we have had the technical report in TIR34, most SPDs did not take that information into consideration because SP relies on biomed engineers to monitor and take care of the water.”
—ANGELA LEWELLYN
needed to test and assess water samples. As a result, ST108: 2023 contains the values that were included in TIR34:2007 but with more detail for users to understand and apply them.”
When defining roles and responsibilities for SP professionals as part of a multidisciplinary team, Angela Lewellyn, Director of Development and Research, Advantage Support Services, suggests SP department (SPD) teams designate a “super user” to oversee water quality compliance. She defines this role as follows:
“In sterile processing, a super-user is a staff member with specialized expertise in water quality management. It is beneficial for SP to designate and initiate education to one or more super-users on the basics of water management solely in SP.”
“Their primary role is to monitor water quality by gathering and documenting test results, identifying issues, and ensuring the department meets compliance standards. They also serve as the go-to expert for troubleshooting water-related problems, such as black-stained instruments or wet loads, working to identify and resolve the root causes.”
“Super-users are vital because they ensure ongoing education and knowledgesharing within the department. By training other team members, they help build a sustainable culture of quality management, creating additional super-users to maintain consistent standards over time. This role not only helps address immediate issues but also supports long-term improvements.”
require further treatment at the facility to meet the specific requirements for Utility Water. This water is mainly used for flushing, washing, and intermediate rinsing (e.g., rinsing between cleaning and thermal disinfection in a washer/ disinfector).
• Critical Water: Water that generally requires extensive treatment by a multi-step process that can include a pretreatment, does include primary treatment (e.g., reverse osmosis (RO) and/or deionization (DI)), storage, distribution, and can include final treatment to provide a level of assurance that microorganisms and inorganic and organic material are removed from the water. This water is used for final rinsing and can be used for steam generation. ST108 has no requirements for what goes into steam generators – only what comes out.
• Steam: Vaporized water that is produced by a centralized boiler or a generator/heat exchanger near the sterilizer.
“Steam is separated out as having its own values in ST108, which are pretty much identical to critical water but it’s the mindset that steam is a different thing that needs to be tested,” Kaiser added.
Richard Parker, MBA, CHFM, CLSS-HC, FASHE, FACHE, Associate Director of Life Safety and Physical Environment, Accreditation Commission for Healthcare (ACHC), explained the difference between TIR34 and ST108, and what this means for healthcare organizations:
“The TIR is a Technical Information Report, a preliminary expert opinion statement,” said Parker. “It is not a requirement, but it contains information relevant and important to the industry and is vital for getting needed information out to the industry.”
“When it was advanced to a standard (ST108: 2023), it reached a consensus after public review and feedback,” he added. “This has been emphasized because the guidelines have advanced to an industry standard, and facilities are expected to progress to comply with the new standard.”
As Parker pointed out, the U.S. Food and Drug Administration (FDA) recognized ANSI/AAMI ST108:2023 as a standard in December 2023, which “suggests some momentum.” As of the writing of this article (December 2024), the U.S. Centers for Medicare & Medicaid Services (CMS) have not made a move to hold hospitals accountable for the standard.
He described nuances of the expanded requirements under ANSI/ AAMI ST108:2023 important to SP professionals:
Definitions and testing of water quality types (including steam)
“One of the water quality types defined in the ST108 requirements is that of Utility Water. This may be mistaken for the untreated water
from the local municipality but refers to softened water.”
Water system performance qualification
“This quality assurance step validates that the system designed and installed meets all predetermined requirements. It includes objective evidence, such as third-party confirmation that the water produced meets requirements.”
Communication/collaboration within a multidisciplinary water management team
“Sterile processing professionals need to know that their role is not passive. Participation and feedback are critical to ensuring that the objectives are met.”
Routine monitoring and continuous improvement
“Routine monitoring is a group effort. For example: SPD professionals will monitor the instruments during the packing process and monitor instruments that were not acceptable to the surgeon due to deficiencies. Facilities staff will likely be involved in the water quality monitoring and testing and provide this information to the team. Maintenance staff will monitor equipment performance and chemical effectiveness.”
“Like any preventive maintenance program for equipment, the intent is to maintain the system so that it provides the same quality as it did when it was installed,” Parker added.
Lewellyn commented on how the evolution of the TIR34 report to the ST108 standard has prompted some SPD teams to be proactive in water quality management, while in the past, they might have been more likely to be reactive to problems that arose.
“Water is the number one tool we use in SP, and although we have had the technical report in TIR34, most
SPDs did not take that information into consideration because SP relies on biomed engineers to monitor and take care of the water,” said Lewellyn. “Usually, most SPDs would get involved in water and steam quality if a problem arose, like wet loads or if instruments came back from the OR due to staining or blackened discolorations. But now SP has AAMI’s standard 108 that defines water, its uses and how best to test and monitor it.”
The establishment of a multidisciplinary water management team is a key element of the ANSI/ AAMI ST108:2023 standard. But who should be a part of this team? And what role should SP professionals play?
“Personnel who maintain sterilization equipment, such as clinical engineering or facilities staff,” Parker answered. “If equipment maintenance is outsourced, a maintenance representative from that company should be part of the water management team. This participation may involve documenting equipment performance, maintenance issues, and other observations.”
Wilder outlined the ST108 suggested team membership, describing their purpose and roles:
• Executive sponsors: This is the C-suite, who hold the purse strings
• Facilities engineering: This group is responsible for the infrastructure needed to get the water to SPD in its required state.
• IP/IC: This group is responsible for ensuring that anything that goes wrong and has potential or real effects on patient outcomes is recorded and the root cause determined.
• SPD/other processing personnel: They do the work using the water
for processing. They know when things are going wrong.
• Clinical engineering: They support the maintenance of the water system in a state where there are no pathogens or unexpected hindrances to water’s application.
• OR/procedure area personnel: This is the final QC test. Their observation of issues should be analyzed for trends and their needs for new instruments should be checked against the available processing capabilities.
Given the potential high cost of remediating water quality issues and the financial pressures on healthcare organizations today, Kaiser emphasized the importance of engaging hospital executive leadership in water quality management initiatives, stating:
“There definitely must be somebody involved from the executive team of the hospital. Somebody who can make decisions, take action, and allocate the resources.”
role should SP professionals play as part of this team?
For SP professionals interested in getting involved in their hospital’s water management practices, the experts interviewed for this article offered their insights.
Wilder noted the advantages of active SP professional participation in water management, and how the consequences of poor water quality can impact the department:
“Sterile processing is where the water is used and so the team should be involved in advocating for getting the water supplies to where they need to be,” said Wilder. “Sterile processing’s axes to grind here are:
• Good cleaning leading to good productivity with minimal rework
• Avoiding corrosion
• Avoiding staining
“Since SPD (and similar specialty departments that are similar in operation, like endoscopy) is where things happen for processing instruments, they will see issues first and, sadly, be blamed for them if they get to the point of use of the instruments,” said Wilder. “It is a matter of both quality assurance/control and self-preservation.”
Get educated and give feedback on water quality
Parker recommends SP professionals first learn how water is treated before it reaches their department.
“Specific criteria must be met for either RO or DI water, and as the end user, knowing the testing results provide insight,” he
Collaborate and communicate cross functionally
As part of a multidisciplinary water management team, SP professionals will have the opportunity to collaborate and communicate with the other disciplines. Lewellyn stressed the importance of relationship building between SP and facilities engineering teams in driving successful ST108 compliance.
“ST108 requires daily, weekly, monthly, and yearly testing of the three water types at different distribution loops,” she stated. “At Advantage Support Services, we encourage SP teams to partner with facility engineers by conducting regular cross-departmental rounds. These visits can be as brief as an



explained. “Provide feedback to the water management team on issues and trends with instrument processing. The SPD performs quality assurance activities on the process and instruments. This feedback affirms or identifies whether the methods of the water management plan are effective.”
hour but are highly impactful. We refer to this as going past the ‘wall,’ symbolizing the cinderblock barriers that house steam boilers and equipment in the facilities department—an area many SP staff have never seen.”
“During these visits, SP staff can gain valuable insight into how steam
and water systems are managed,” Lewellyn continued. “Similarly, facility engineers should visit the SP department to understand how water quality directly affects processes like decontamination.”
According to Lewellyn, regular communication and collaboration are critical for success. She recommends SP and facilities engineering stakeholders schedule regular meetings that focus on reviewing water and steam quality reports, discussing deviations, and planning corrective actions.
“Sharing resources, such as ST108 tables for performance standards and monitoring schedules, helps ensure both teams are aligned,” she added.
Keep on top of water quality testing
“Water quality results must be regularly monitored and shared between SP and facility engineering teams to maintain compliance with standards like ST108,” said Lewellyn. “Key tests include pH measurements, which should be taken at the point where the water distribution loop enters the processing area. These tests should be performed quarterly for utility water, monthly for critical water, and quarterly for steam. Additional tests, such as conductivity, total alkalinity, and total water hardness, should follow the same schedule. Bacterial testing is also essential, with samples collected quarterly for utility water and monthly for critical water.”
Lewellyn noted how the Advantage Support Services team, including a steam expert, provides a comprehensive evaluation of a facility’s water quality, including an in-depth analysis of its water system; tailored education on water quality fundamentals; and a comprehensive report that outlines any identified risks, detailed test results, and actionable recommendations.
Space said even if routine water quality testing is performed by the hospital’s facilities or engineering team, the SP team needs at the very least to have awareness that this testing is taking place. She also urges facilities and engineering staff members to keep the SP informed




of any issues that could impact the quality of their reprocessing water.
“Usually, facilities or engineering will be the ones who first learn of problems with city water, such as a water main break or repair,” Space explained. “They will also be aware of when boiler maintenance takes place












in the hospital. In these cases, they need to notify the SPD team so they can be on high alert for any impacts on instrument and device reprocessing.”
In January 2025, Incision launched an online course, The Importance of Water Quality in Sterile Processing, as part of a sterile processing e-learning program, which can be accessed through the company’s website.
Kaiser reminded SP professionals to consider seasonable variations that influence water quality when performing testing. He stated:
“Water changes in the spring, summer, fall, winter. Droughts or flooding can impact the quality of water in your facility. So, make sure you incorporate these factors when you are setting your test limits.”
For hospitals seeking knowledge and assistance with water testing, Kaiser highlighted STERIS water and steam testing services, Knowledge Center articles, and several webinars and study guides from STERIS University, all which can be found on the STERIS website.
When asked for one piece of advice they would give to SP professionals when it comes to water quality management and compliance with ST108, the experts interviewed for this article provided the following statements.

CRCST, CIS, CER, CHL, CMRP, CST, HACP-IC, Managing Director Surgical Services, Advantage Support Services
“Be tougher customers! The responsibility for producing and maintaining the systems that produce Utility and Critical Water lies in other departments, but ST108 gives us the tools to test that water, ask for reports, and join and contribute to the steering committee that oversees the water used in our facilities. It is up to us to take this opportunity to educate ourselves so that we can ask the right questions and advocate for water that ensures patient safety.”
HERB KAISER, PhD, Senior Principal Scientist, Analytical Services and Development at STERIS Corporation
“The first thing is to breathe. I hear and see a lot of panic out there around ST108, and believe it or not, there are others who have never even heard of TIR34. Compliance is a marathon, not a sprint. You can’t flip a switch, and all your requirements will be met. Purchase some copies of ST108 for stakeholders in your facility involved with water management and make sure they read it. Go slow with changes and make adjustments along the way. Turn to your SP equipment
vendors and water suppliers for assistance because they have the knowledge and the resources to help you.”

RICHARD PARKER, MBA, CHFM, CLSS-HC, FASHE, FACHE, Associate Director of Life Safety and Physical Environment, Accreditation Commission for Healthcare (ACHC) “Although accrediting organizations are not holding organizations accountable for this yet, many of the requirements for water quality have been an industry best practice for nearly a decade. Some requirements may also be found in the manufacturer’s instructions for use. The FDA recognized AAMI/ANSI ST108 on 12/18/2023.”
“My advice would be for sterile processing professionals to get involved with the water management team and begin a gap analysis to determine what steps are needed to achieve the requirements in ST108. If equipment is needed, this may need to be added to the capital equipment planning process. Often, installing the equipment has a space impact, and planning would be needed for that. SPD professionals need to get the conversation started.”
“The best water quality achievable will not fix a poor process. SPD professionals must continue efforts to validate each step of the process from the OR to the end of device sterilization.”

AIMEE SPACE, BSN, RN, Product Owner, U.S. Medical Content, Incision
“You don’t need to be an expert in chemistry or engineering to play a role in water quality management within your hospital. There are many educational resources available, like the courses we are creating at Incision, to help you in your efforts. I encourage SP technicians to seek and learn practical aspects, so they can play their part in managing and maintaining quality.”
“And when you suspect a problem in your department, don’t be afraid to stop the line and ask a question or raise an issue to your manager. You will likely learn something and many times your manager will too.”
JONATHAN A. WILDER, Ph.D., Managing Director, Quality Processing Resource Group
“The standard was not created to increase the burden on SPD, but instead to help them get their work done more effectively. No one wants to do the same work twice. Water that meets the ST108 requirements will help reach that goal.” HPN
Reference:
1. ANSI/AAMI ST108:2023— Water for Processing Medical Devices, ANSI, November 13, 2024, https://blog.ansi.org/ansi-aami-st108-2023-water-quality-medical-devices/




• Mott Children’s Health Center
• HWL
• Clariti Solutions
• Acadiana Rehabilitation Hospital
• Prominence Advisors
• Capital Blue Cross
• TekTone Sound & Signal Mfg. Inc.
• Village Caregiving
• Pivot Point Consulting, a Vaco Company
• The Surgery Center LLC
Honorable Mention:
• Pacific Asian Counseling Svcs
• CloudWave
• Health Advocates Network, Inc.
• LifeFlight of Maine
• St. Clair County Community Mental Health Authority
• St. Tammany Health System
• Wabash General Hospital
• Metrolina Nephrology Associates
• Midland Memorial Hospital
• Hackensack Meridian Health
• CoxHealth
hcinnovationgroup.com/topcompaniestowork
BY SARAH B. CRUZ, CSPDT, CRCST, CHL, CIS IS PRESIDENT AND FOUNDER OF PRETREAT CSS
There are a number of free continuing education opportunities within the Sterile Processing (SP) industry. However, the specific standard works or standard operating procedures (SOPs) associated with department training are typically the responsibility of the facility’s Manager or Educator. Training comes with its own considerations like learning style, retention ability of the participant, and ease of performing the process. When SOPs are haphazardly created
due to poor, rushed, and/or reactive development, the frontline SP professional’s ability to retain key information can feel like a constant battle. Some of the most popular forms of training and retention in SP are kinesthetic and visual demonstration. These are more commonly referred to as hand-over-hand or see-one-do-one. A significant portion of new technician onboarding relies heavily upon this teaching application. Even more often, this is the only form of education provided. This results in the constant call
1. Demonstrate an effective curriculum based inservice.
2. Create a curriculum that quantifies comprehension.
3. Assemble necessary resources to research curriculum
4. Employ multiple approaches to incorporate all learning styles
Sponsored by:


of memory until department conditioning is achieved. If SP technicians don’t remember the process steps necessary to achieve the anticipated outcomes, then they typically defer to a more senior technician to retrain them. This form of practice reinforcement comes with its own limitations and opportunities for misinformation to become engrained, perpetuated, and normalized. This is a training version of the game ‘Telephone’, when one person relays information to the next repeatedly until the final


outcome is almost unrecognizable from its origins.
Retention and application go hand-inhand regardless of how the training is implemented. Facility leaders attempt to instill preferred practices with more robust demonstration via department in-services. While a compelling inservice is always the aim, they often rely heavily on textbook application and listening. While some professionals learn best auditorily and/or by written content, this is a small portion among the SP professional population. Therefore, the most opportune way to implement the desired department standards and facility expectations must incorporate all the primary learning modalities: kinesthetic, reading/writing, visual, and auditory. The composition of multilearning means also makes for a compelling and engaging in-service. By doing so, the training has a higher probability for on-the-job repeatability, consistent application, and staying power even after the time clock has been punched. By encompassing all the common forms of learning and ultimately retention, the in-service will become interactive. A speaking/reading component is important, true, but even more impactful when it becomes a dialogue. Dialogue is the first step to critical thinking development. The physical hand gestures that come with conversation also make the topic more engaging. If we are leading an in-service on reading instructions for use (IFUs), why not include an activity that requires the participants to pair up and try to refold the document? This physical activity will compel teamwork as well as introduce the contents of the document. Another approach may be to draw a sterilization table and ask each team to fill in the necessary information. The main goal of all this is to create an intrinsic way for the professionals to relate
to the content. To be more compelling is to ensure retention amongst the SP in-service attendees.
The ability to create and structure such inclusive learning activities resides in the creator’s ability to develop practical curricula that resonate with the group. This means the in-service developer must consider the professionals’ different personal backgrounds, experience levels, and previous understanding. These are the key components to whether or not the information will land, let alone stick.
The purpose of an ‘in-service’ is to encourage the participants to tap into a higher set of thinking skills in order to solidify their understanding of the subject. Thus, the three previously mentioned components are incorporated into curriculum-based content. Curriculum-based in-services require the content creator and/ or deliverer to indicate learning objectives and anticipated outcomes that create the roadmap to measurable outcomes.1 It is not enough to measure the effectiveness of an in-service with a one-dimensional outcome like whether or not the process is being performed. Effective curriculumbased in-services also create a way to determine what and why participants may not be applying the outcome of the newly taught discipline. This is referred to as curriculum-based measurement (CBM).2
A simple way to approach CBM is to start with the end goal or desired outcome. Then list the various concepts or skills that need to be taught to deliver that mastery consistently. This list will outline the areas that represent comprehension.3,4 Next, itemize ways to quantify this comprehension cohesively. The end result will be a checklist that determines not only how to achieve the desired skill or knowledge but how to successfully demonstrate it. Let’s take, for
Education Nation: Curriculum-Based In-Services
This lesson was developed by Solventum. Lessons are administered by Endeavor Business Media.
After careful study of the lesson, complete the examination online at educationhub.hpnonline.com. You must have a passing score of 80% or higher to receive a certificate of completion.
The Certification Board for Sterile Processing and Distribution has pre-approved this in-service unit for one (1) contact hour for a period of five (5) years from the date of original publication. Successful completion of the lesson and post-test must be documented by facility management and those records maintained by the individual until recertification is required. DO NOT SEND LESSON OR TEST TO CBSPD. www.cbspd.net.


Healthcare Sterile Processing Association, myhspa.org, has pre-approved this in-service for 1.0 Continuing Education Credits for a period of three years, until November 26, 2027.
For more information, direct any questions to Healthcare Purchasing News editor@hpnonline.com.

example, an in-service that aims to teach the team how to read an IFU. The desired outcome may be that SP professionals will learn to discern the correct sterilizer cycle. One way to tell this concept is not grasped is when an autoclave load is run on the incorrect cycle. This is not an effective CBM for many obvious reasons. However, without clear CBMs, it will remain the metric used to determine retention. More effective CBMs may include:
• Demonstrate the ability to locate the sterilization parameters in the IFU document
• Interpret the sterilization information table
• Determine which type of sterilizer is being used
• Apply previous training to select the appropriate sterilizer load These CBMs highlight specific areas that can be evaluated and can be addressed to ensure the sterilizer is run correctly and that the entire team is working in the same direction.
It goes without saying that the information given in the in-service must be accurate. While the confidence of the creator may be resounding, they must still ensure their practice and thought process emulates current standards, best practices, and regulatory expectations. Things change quickly in the world of Sterile Processing. The silver lining here is that there is always information available. Be sure the source of the information is noteworthy or supplemented by credible sources. This can also be done by referencing standard documents (AAMI, ANSI, SGNA, etc.) and even the facility policies.
Researching does not have to be a solo endeavor or as daunting as its reputation leads many to believe. An excellent place to begin research is through professional organization recommendations. The Association
of periOperative Registered Nurses (AORN), the Association of Surgical Techs (AST), and the Healthcare Sterile Processing Association (HSPA) are key industry organizations that provide best practice resources and guideline recommendations that influence SP department practices. Turning to these organizations and the documents they provide can also lead to a plethora of subject matter experts.
Industry professionals offer a tremendous amount of knowledge, and external input will help ensure the curriculum is robust and versatile. There is always the chance that there is a group of individuals searching for the same or similar information, as well. Other SP managers, educators, and industry consultants can provide tenured insight as well as offer a launching point for further investigation. Vendor partners and product educators are also a phenomenal resource for information. Many companies provide free training material, access to webinars, and even their own company-created in-services.
While this type of collaboration can provide up-to-date information, CBMs still need to be created. As the individual who brings in the vendor-led program, quality CBMs that encompass the educational points listed previously are very important. The primary goal of the vendor is to teach about the product and the creator’s duty is to ensure that the content is retainable, understandable, and can be put into action afterwards.
Quantifiable comprehension is a proactive and solidified way to determine if the content is absorbed effectively. This ensures that the team is not just going through the motions, but truly understanding what they are applying and why. The same list created to develop CBMs can be utilized in pre- and post-assessments. These assessments are used to gauge
comprehension before and after the inservice. The pre-assessment gauges the participants’ current understanding of the material, while the postassessment indicates what they have learned. Giving the same assessment again after a few days, then a week, and again after a month can also assist if follow-up or retraining needs to occur. These assessments can be in a number of formats, with the most common being a quiz. However, test anxiety or learning style can be a root cause of poor test performance. With that being said, the assessment can be given orally, in dialogue, and/or on a digital document.
Regardless of the modality, the purpose of these assessments is to demonstrate comprehension and retention. Post in-service observation is used to indicate the actionability of the training. The professionals need to be able to apply their learning effectively in the field. One technique is to casually ask questions of the individual in a conversational manner. For example, if the technician is selecting an autoclave load cycle, one could ask them to show them how to do it as if they were a new colleague. This will result in them explaining or demonstrating how to find the information. If it does not, then the area of improvement has been identified. Reiteration like this, along with repetition, help to cement concepts to actions.
Curriculum-based in-services supported by thorough research and measured by quantifiable comprehension are the foundation necessary to create quality in-services. With this strategic approach, learning styles, interest, and engagement are utilized to create retainable, interactive, and actionable educational content. A successful in-service must do more than require learning. It also needs to motivate and empower the SP professional in a way that resonates with them and connects them to the patient safety outcome. HPN

References:
1. Whitson D. Definitions of curriculum. University of Delaware. Available at: https://www1.udel. edu/educ/whitson/897s05/files/definitions_of_curriculum.htm. Accessed November 21, 2024.
2. Reading Rockets. What is curriculum-based measurement and what does it mean? Reading Rockets. Available at: https://www.readingrockets.org/topics/assessment-and-evaluation/ articles/what-curriculum-based-measurement-and-what-does-it-mean. Accessed November 21, 2024.
3. Cambridge Dictionary. Comprehension. Cambridge University Press. Available at: https:// dictionary.cambridge.org/dictionary/english/comprehension. Accessed November 21, 2024.
4. National Institute for Literacy. Adult education and literacy: Adult education and literacy at a glance. LINCS. Available at: https://lincs.ed.gov/publications/html/adult_ed/adult_ed_8.html. Accessed November 21, 2024.
1. What is a key responsibility of the inservice content creator?
A. To ensure that the in-service content is based solely on their personal experience and knowledge
B. To focus only on technical skills and ignore the development of critical thinking and retention
C. To avoid using any external resources or guidelines in favor of creating entirely original content
D. To develop a curriculum that considers the diverse learning styles, backgrounds, and experience levels of participants
2. Haphazard or poorly developed SOPs can make it difficult for Sterile Processing professionals to retain important information.
A. True
B. False
3. What is one major limitation of using only the “see-one-do-one” training method?
A. It provides clear written documentation for the participants
B. It assumes the information will be dedicated to memory quickly
C. It results in excessive time spent on training
D. It is universally effective for all learning styles
4. Which of the following best describes the purpose of incorporating all four primary learning modalities (kinesthetic, reading/writing, visual, and auditory) into an in-service?
A. To appeal to different learning preferences and improve retention
B. To make the in-service longer and more complex
C. To decrease the cost of training
D. To follow industry regulations
5. What is the primary purpose of quantifiable comprehension in Sterile Processing training?
A. To measure the speed at which tasks are completed
B. To determine if participants truly understand and can apply the training content
C. To assess whether participants can memorize all training materials
D. To evaluate how well participants can repeat information from memory
All CEU quizzes must be taken online at: educationhub.hpnonline.com The cost to take the quiz is $10.
6. What is the primary goal of a curriculumbased in-service in Sterile Processing?
A. To meet regulatory requirements
B. To ensure participants can memorize specific steps
C. To increase the speed of task completion
D. To encourage participants to use critical thinking and retain information
7. What is an example of a curriculum-based measurement (CBM) for evaluating an in-service on how to read an Instruction for Use (IFU)?
A. Checking if the sterilizer is running at the correct cycle
B. Asking the team to memorize the entire IFU document
C. Observing if the team can locate and interpret sterilization parameters in the IFU
D. Measuring if the technician can recite the IFU from memory
8. The effectiveness of an in-service can be determined by one-dimensional outcomes, such as simply completing the task correctly.
A. True
B. False
9. What is recommended as an effective strategy to ensure the in-service curriculum is both current and robust?
A. Relying only on internal facility policies to create training materials
B. Conducting research through professional organizations and collaborating with subject matter experts
C. Using only vendor-supplied content without modification
D. Relying on personal knowledge and interpretation
10. Pre-assessments and post-assessments assist with:
A. The current understanding of a topic
B. Indicate what was learned after the in-service
C. Identify areas that may need further training
D. All of the above

BY DAVID TAYLOR, PRINCIPAL, RESOLUTE ADVISORY GROUP LLC
Many healthcare facilities struggle to retain effective leaders, including those in the sterile processing setting. High turnover in this setting can disrupt daily operations, damage team morale and, more importantly, impact patient care quality and outcomes.
Leadership turnover can be incredibly costly, both in terms of financial outlay and organizational stability. Over the past five years, the average hospital turned over 106.6% of its workforce,1 and replacing a leader in an organization can cost between one-half to twice the leader’s annual salary, with some positions reaching
over 200% of their salary.2 This total takes into account direct recruitment and training expenses as well as costs tied to lost productivity and disruption to team dynamics during the transition period.
In sterile processing departments (SPDs), where quality outcomes depend largely on science-based knowledge and a focus on ensuring current standards, guidelines, instructions for use and policies and procedures are consistently followed, it becomes clear how the loss of effective, skilled, knowledgeable, and empathetic leaders has the potential for devastating results—including decreased employee morale, increased


staff turnover, and diminished productivity. Put simply, a single effective leader’s departure can trigger a domino effect that impacts the entire team and hospital’s performance.
To effectively retain healthcare leaders, organizations must prioritize and maintain positive cultures through open communication, transparency, and direct involvement in decisionmaking processes. Facility executives must cultivate an environment where leaders feel they are valued and appreciated for their contributions to the organization’s mission and vision. The following approaches are essential for retaining quality leaders:
• Facilitate open communication and feedback – Encourage leaders to openly share their thoughts, concerns, and ideas, and actively solicit their feedback through meetings and surveys to demonstrate that their input is valued.
• Promote transparency and foster active decision making – Allow and encourage leaders to share relevant information with their direct leadership regarding organizational challenges and opportunities. Doing so allows them to understand the rationale behind decisions, feel more connected to the process, and contribute their expertise and perspective on key issues.
• Foster alignment with the facility’s mission, vision, and values
– Strong, effective leaders desire to contribute to the organization’s overall purpose and goals. Executive leaders should promote a culture that engages its departmental leaders with a strong sense of purpose and commitment. Working toward a shared mission, vision, and values drives progress by clearly articulating the organization’s strategic goals while creating a sense of shared ownership amongst its leaders.
• Ensure effective cross-departmental collaboration – Successful, quality-focused organizations do not have cultures where individual departments work in siloes. Instead, they promote interdepartmental teamwork that taps each leader’s knowledge and strengths to build a stronger sense of unity and information sharing that
positively impacts operations and patient outcomes.
• Provide competitive compensation packages and promote a healthy work-life balance –Compensation goes beyond salaries. Organizations must also consider healthcare benefits, paid time off (PTO), retirement plans, wellness programs, flexible work arrangements (e.g., hybrid schedules), and performance-based bonuses. Supporting professional development through certification and participation in educational conferences can also enhance leaders’ satisfaction and contribute to their retention and value to the organization. Establishing a formal leadership recognition program to celebrate exemplary performance and achievements is another beneficial offering.
Leadership retention contributes to an organization’s growth, positive patient outcomes, reputation, and profitability. By prioritizing recruitment and retention of top-tier sterile processing leaders, organizations will create quality-focused departments and positive cultures that will make them better poised to attract and retain the best employees, all of which will promote safe, efficient, high-quality service and care. HPN
References:
1. https://www.nsinursingsolutions.com/Documents/Library/ NSI_National_Health_Care_Retention_Report.pdf
2. https://inflectionpoynt.com/leadership-turnovercost/#:~:text=Studies%20reveal%20a%20sobering%20 reality,projects%2C%20and%20potential%20revenue%20 losses.
David L. Taylor, MSN, RN, CNOR is an independent hospital and ambulatory surgery center consultant and the principal of Resolute Advisory Group LLC, in San Antonio, Texas.





















BY ADAM OKADA, CLINICAL EDUCATION SPECIALIST, HEALTHMARK, A GETINGE COMPANY
Q“The window that separates our decontamination area and our assembly and sterile storage areas was left open over the weekend (roughly 60 hours). We discovered it upon arrival on Monday morning. Do we need to re-sterilize everything in sterile storage?”
AFirst things first: great job catching the problem on arrival and recognizing that it was a problem! I have heard of (and seen firsthand) situations where things like this were simply corrected and “swept under the rug.” It’s much easier to close the window and ignore the possible patient (and staff) safety concerns than it is to open an investigation into the extent of potential risk.
You’ll need to perform a risk assessment (RA), and, in this case, RA will focus on (among other things) the potential patient safety concern of compromised sterile packages.
The main issue has to do with airflow in the two areas.
Decontamination is a “negative” airflow, meaning that the “dirty” air in decontam should flow up into the facility’s filtered vents.
Assembly/Inspection and Sterile Storage is meant to be “positive” so that air flows out when a door is opened to that area.
The idea here is that when a door (or window) between the two areas is briefly opened, the “clean” air should flow into the “dirty” side, and not vice-versa.
This airflow is maintained due to the delicate pressure balance in each room and relies on the doors and windows to these areas being closed. Positive airflow is created because air pressure inside the room is greater than the air pressure outside the room. This is achieved by adding more air into the room. Conversely, exhausting more air from a room creates negative pressure because

that room’s pressure is less than the pressure outside. The amounts of air pushed into a room (or exhausted from a room) are specific to the exact area of the room itself.
The longer that a door or window remains open between these two areas, the greater the likelihood the two rooms will “regulate” air pressure–essentially changing the size of the room so the air pressure will change with it. Free-flowing air from the contaminated side could flow into the “clean” area as a result.
Do you have electronic tracking and/or documentation of the airflow between the decontamination area and assembly/sterile storage areas? Some facilities are electronically tracking the positive and negative airflow between the two rooms, so they may be able to tell you when or if that airflow changed, to what extent, and for how long.
If there is no airflow tracking documentation, then you should assume

The ADI’S BALL-IN-THE-WALL® 1 detects threshold room directional differential pressure (DDP) measured in pascals (Pa). DDP is visually represented: red is negative, and green is positive.

the worst. Correct the airflow immediately by shutting that window as soon as you see it open. This should allow the HVAC system to return the rooms to the proper airflow. This applies to any situation (e.g., when techs in the decontamination area pass devices through the window) where that door/ window between the areas is left open.
To perform the RA, I would recommend involving your infection prevention (IP) team, risk management, and facilities management in the conversation. In addition to your sterile storage concerns, there are a few other considerations:
• All surfaces should be disinfected.
• You will likely need to have Environmental Services (EVS) come in to perform a terminal cleaning of the areas.
• Supplies in the assembly/inspection areas may have been contaminated and may need to be discarded.
• Accessories (e.g., computers, insulation testers, borescopes, etc.)
may also need to be cleaned and disinfected.
Some people may claim the risk for infectious transmission via airflow between these areas is very low. While I agree that the infection rate is proportionally low, I would argue we don’t fully understand the nature or extent of airborne pathogen transmission. Best to err on the side of caution. If the COVID-19 (SARS-CoV-2) pandemic taught us anything, it was the unforeseen viability of pathogens to travel long distances and survive in adverse environments.2,3,4
To sum up (based on the results of your team’s risk assessment):
• Correct the immediate problem.
• Gather your data and information.
• Make an informed decision. HPN
References:
1. ADI. (n.d.). BALL-IN-THE-WALL®. Airflow Direction Incorporated. https://airflowdirection.com/
2. Mikszewski, A., Stabile, L., Buonanno, G., & Morawska, L. (2022). The airborne contagiousness of respiratory viruses: A comparative analysis and implications for mitigation. Geoscience frontiers , 13(6), 101285. https://doi.org/10.1016/j. gsf.2021.101285
3. Short, K. R., & Cowling, B. J. (2023). Assessing the potential for fomite transmission of SARS-CoV-2. The Lancet Microbe, 4(6), e380–e381. https://doi.org/10.1016/S26665247(23)00099-X
4. Wang, Chia C., et al. (2021). Airborne transmission of respiratory viruses. Science 373, eabd9149. DOI:10.1126/ science.abd9149
Adam Okada has 18+ years of experience in Sterile Processing and is passionate about helping improve the quality of patient care by giving SPD professionals and their partners greater access to education and information. He has worked in just about every position in the Sterile Processing Department, including Case Cart Builder, SPD Tech I, II, and III, Lead Tech, Tracking System Analyst, Supervisor of both SPD and HLD, Manager, and now as an Educator. Adam is the owner of Sterile Education, the world’s first mobile application dedicated to sterile processing education, and a former Clinical Manager at Beyond Clean. He has published articles for HSPA’s Process magazine, is a co-chair on AAMI WG45 as well as co-project manager for the KiiP “Last 100 Yards” group, and is the former President for the Central California Chapter of HSPA. Adam is currently a Clinical Education Specialist at Healthmark, A Getinge company, where he works on Healthmark webinars, hybrid events, and educational videos, as well as the “Ask the Educator” Podcast with Kevin Anderson.
BY KAREN CONWAY, CEO, VALUEWORKS
Two significant healthcare trends – more care being delivered in the home and the rise in the number of specialty drugs – can deliver significant benefits, but they also come with risks for patients, the supply chain, and the environment. On the plus side, care in the home, including advanced or hospital level care, has been shown to lower the total cost of care, while reducing readmissions and increasing patient satisfaction. Specialty drugs are effective weapons in the fight against an epidemic of chronic disease, which is also a primary driver of an anticipated 20% increase in care at home over the next decade.
On the downside, the cold chain requirements of specialty drugs increase supply chain complexity, which, if not effectively managed, can increase risks for patients and the environment. For example, many of these drugs need to be kept at cold or even ultra-cold temperatures, which has typically been handled through the use of plastic-based packaging materials like Styrofoam and gel packs. There is also a risk to efficacy and patient outcomes if those cold chain requirements are not adhered to during the delivery process.

Phox Health, a start-up in the drug delivery space, is seeking to address several of these issues. Co-founder Amit Gir, MD, saw the potential for technology, such as mobile apps, to help patients adhere to their prescription regimens even before he began studying medicine. As a bioengineering student, he focused on the use of technology to improve both the patient experience and health, which eventually led him to medical school. Later, working as a physician in one of the Permanente Medical

Groups in southern California, the technology bug bit again, as hospital pharmacies began offering same day delivery to patients, a trend that skyrocketed during the pandemic. Not only did he see a business opportunity, but he also recognized the risks to patients as a result of delayed deliveries and deviations from required storage conditions.
When Phox Health first started, Gir envisioned using the major logistics carriers, like FedEx and UPS, but with his clinical background, he soon recognized the importance of medical grade logistics for these highly expensive drugs. In his words, “It’s like delivering a diamond ring that can melt.”
Phox Health holds its couriers, who can make up to six figures, to high standards, and uses technology to both support and track their deliveries to make sure all goes as planned. Taking a cue from Domino’s Pizza, Phox Health also deploys reusable totes in which the specialty drugs are transported, eliminating the need for excessive plastic-based packaging. The totes are also equipped with high grade, reusable ice bricks and temperature monitors to assure both the patient and the pharmacy that the drugs have not been compromised along the way. The move to reusable packaging is also saving hospitals money, to the tune of about 30% less than the disposable and often nonbiodegradable, non-recyclable materials.
Hospitals also benefit from no longer having to answer phone calls from patients about the status of their orders. Last year, the company fielded more than 10,000 such calls that would otherwise have gone to the pharmacies.
Clinically driven supply chain solutions like Phox Health will only become increasingly important with the rise in chronic disease and the need to reduce both the cost of healthcare and the level of waste it generates. Today, more than 60% of Americans suffer from one or more chronic conditions and that number is expected to grow to 80% in 10 to 15 years. Meanwhile, hospitals alone produce more than 13 tons of waste per day in the United States. The challenges are significant, and supply chain logistics will only become more important in addressing them. HPN


















Designed to protect both patients and healthcare professionals, while providing confidence in dose-delivery, Unifine® SafeControl® affords you the right balance of safety and control, from injection to retraction.
Unifine® SafeControl® provides:
• enhanced user control preventing premature activation1
• visual confirmation of dose-delivery1
• dual needle protection
• cost-effective solution

Scan here to learn more or request free samples!

It’s more than a box.
As the leading provider of rigid sterile containers, Aesculap has developed the AESCULAP Aicon® Sterile Container System to help ensure sterile security.
With a unique design that increases the aseptic area by 200%1, this innovative system significantly reduces the risk of contamination. Trust the AESCULAP Aicon Sterile Container System to uphold the highest standards of sterility in your operating room, providing a crucial line of defense for patient safety.
Scan to learn how the AESCULAP Aicon Sterile Container System can help your SPD Operate with Greater Precision.
