Improving sustainability in pharma cold chain
Leveraging workflows with AI



Improving sustainability in pharma cold chain
Leveraging workflows with AI


tools elevating precision and efficiency in drug manufacturing

Ross supplies the largest Double Planetary Mixers in the Pharmaceutical Industry. Sanitary design, IQ/OQ documentation, SIP capability. Perfect for mixing and homogenous granulations.
Custom PLC recipe controls are available to optimize your batch data and meet CFR-21 requirements.
With units sized from 200mL to 4000L, Ross has the Double Planetary Mixer to fit your process.
Learn more at mixers.com. Call 1-800-243-ROSS or try our free online Knowledge Base & Product Selector web app at mixers.com/web-app.

























































































Where supply chain pros ome together to find solutions
Come get face-to-face with the people, technologies and ideas that are shaping this industry’s future at ProMat 2025, your unrivaled supply chain experience. From hands-on demonstrations from over 1,000 leading suppliers to 200 thought-provoking seminars and four inspiring keynote speeches, ProMat has everything you need to make your operations more resilient, transparent and sustainable. McCormick Place, Chicago | March 17-20, 2025
Learn more & register for free admission at promatshow.com


























EDITORIAL TEAM
andrea corona Senior Editor acorona@endeavorb2b.com
EDITORIAL ADVISORY BOARD
biKaSh chatterJee, Pharmatech Associates
emiL ciurcZaK, Doramaxx Consulting robert Dream, HDR Company
barrY hoLtZ, Phylloceuticals
eric LaNger, BioPlan Associates
ivaN Lugo, INDUNIV, Puerto Rico
giriSh maLhotra, Epcot International rich mYer, Tergus Pharma garY ritchie, GER Compliance
michaeL touSeY, Solid Dose Technology
DESIGN & PRODUCTION TEAM
michael annino Art Director mannino@endeavorb2b.com
rita fitzgerald Production Manager rfitzgerald@endeavorb2b.com
jennifer george Ad Services Manager jgeorge@endeavorb2b.com
This year’s Pharma Innovation Awards are setting new standards and quietly reshaping the future of pharma manufacturing
andrea corona Senior Editor

The saying “big shoes to fill” takes on a different weight when it’s not just about the person, but the legacy they’ve built on the page. Karen Langhauser’s voice in this column marked a before and after for our publication.
When she took the helm over a decade ago, the publication was already solid, but Karen wasn’t interested in solid. She was interested in excellence, constantly elevating both the magazine and the industry conversations we sparked.
Karen’s influence on me — and on all of us — was less about what she said and more about how she worked. A self-described perfectionist, “good enough” was never part of the equation.
Every idea, every angle, every word was an opportunity to go further. Under her guidance, I learned that innovation and excellence aren’t just about big moments or breakthroughs. It’s often the smaller, sharper improvements that change the game.
That spirit is at the heart of this year’s Pharma Innovation Awards. The products and technologies we’re recognizing aren’t just impressive, they’re essential.
These innovations might not make headlines on their own, but they’re the gears quietly driving the industry forward. These tools represent the kind of smart, incremental progress that moves the needle in ways that matter.

ADMINISTRATIVE TEAM
keith larson VP/Group Publisher klarson@endeavorb2b.com

endeavor business media, LLc
chriS ferreLL, CEO
JuNe griffiN, President
PatricK raiNS, COO
PauL aNDreWS, CRO
JacQuie Niemiec, Chief Digital Officer
tracY KaNe, Chief Adm. and Legal Officer
miKe chriStiaN, EVP, Industrial Group
1501 E. Woodfield Road, Suite 400N, Schaumburg, IL 60173
Phone: (630) 467-1300 • Fax: (630) 467-1179
Subscriptions/customer Service Local: (847) 559-7598
Toll free: (877) 382-9187 or PharmaManu@omeda.com
These awards are an opportunity to celebrate the technologies that enhance product quality, reduce risks, and boost manufacturing efficiency in pharma. At Pharma Manufacturing, we honor the vendors whose innovation drives lifesaving medicines and sets them apart as industry leaders.
And when you take a look at our Global Dose profile on Pittsburgh, it’s clear — real innovation doesn’t happen by accident.
Pittsburgh has methodically expanded itself from a steel powerhouse to a growing life sciences hub, building on its manufacturing legacy to bet on a new economy.
Pittsburgh’s reinvention is proof that when you build on what’s strong, you create space for something even greater.
In a similar sentiment, filling Karen’s shoes isn’t the goal, but continuing to build on the foundation she laid, is. And like this year’s innovators, the intention will continue to be to go above and beyond what’s expected, finding the real value in pushing limits past what was thought possible.
Andrea Corona Senior Editor
A rundown of 2024’s defining pharma moments, from game-changing therapies to billion-dollar deals
2024 was a year of breakthroughs.
Scientists developed the first graphene-based semiconductor, generative AI took center stage and NASA launched its Europa Clipper spacecraft to explore Jupiter’s moon to determine its astrobiological potential.
Meanwhile, pharma had its own standout moments. FDA approvals like Iovance’s Amtagvi, the first T-cell therapy for solid tumors, and BMS’ Cobenfy, the first antipsychotic targeting cholinergic receptors for schizophrenia, marked a new era of therapeutic approaches.
Here’s a look at the biggest moments in pharma this year.
Amtagvi for solid tumors
In February, the FDA granted accelerated approval to Iovance’s Amtagvi (lifileucel), the first T-cell therapy for solid tumors and for advanced melanoma post-anti-PD-1 therapy. This one-time immunotherapy uses T-cells to attack cancer and is indicated for adults with unresectable or metastatic melanoma who have already undergone PD-1 and BRAF inhibitor treatments.
Pfizer’s gene therapy for hemophilia B
In April, Pfizer’s Beqvez was approved as a one-time gene therapy for moderate to severe hemophilia B, reducing the need for frequent factor IX infusions. Priced at $3.5 million per treatment, Pfizer also launched a warranty
program to support its rollout and ensure accessibility.
Eli Lilly’s donanemab
Approved in July, Eli Lilly’s donanemab targets beta-amyloid plaques to slow early Alzheimer’s progression. Initially rejected for accelerated approval in 2023, it gained traditional approval after phase 3 trials showed significant cognitive decline reduction.
BMS’ KarXT
In September, Bristol Myers Squibb’s Cobenfy (xanomeline and trospium chloride) became the first antipsychotic targeting cholinergic receptors for schizophrenia, moving away from dopamine-targeting drugs. Trials showed significant symptom reduction but warned of side effects like urinary retention and liver issues.
Novo’s $16.5 billion Catalent acquisition
In February, Novo Holdings, the parent company of Novo Nordisk, announced its acquisition of Catalent for $16.5 billion in cash. As part of this deal, Novo Nordisk also bought three Catalent fill-finish sites for $11 billion to boost production of its popular weight loss drugs like Ozempic and Wegovy.
BMS’ $14 billion acquisition of Karuna Therapeutics
Finalized in March, this deal gave Bristol Myers Squibb ownership of KarXT, a novel antipsychotic drug approved later in the year. This acquisition adds a key psychiatric treatment to BMS’s pipeline, with significant financial implications as the transaction is expected to reduce the company’s 2024 earnings by about $0.30 per share due to financing costs.
J&J’s $13.1 billion Shockwave deal
In April, Johnson & Johnson completed its $13.1 billion acquisition of Shockwave Medical, a company known for its intravascular lithotripsy (IVL) technology used to treat calcified arterial lesions. This acquisition
for advertising sales or production information, contact the following
For subscription information call Local: (847) 559-7598 Toll free: (877) 382-9187 or PharmaManu@omeda.com
amy loria Group Sales Director (352) 873-4288 aloria@endeavorb2b.com
regina dexter
Strategic Account Manager (234) 201-8709 rdexter@endeavorb2b.com
betsy norberg
Strategic Account Manager (913) 956-1670 bnorberg@endeavorb2b.com
greg zamin Account Manager (704) 256-5433 gzamin@endeavorb2b.com
lori goldberg Advertising Coordinator lgoldberg@endeavorb2b.com REPRINTS
To purchase custom reprints or e-prints of articles appearing in this publication contact reprints@endeavorb2b.com (574) 303-8511
strengthens J&J’s position in cardiovascular intervention, expanding into calcified coronary artery disease (CAD) and peripheral artery disease (PAD) segments.
AbbVie finalized its acquisition of Cerevel Therapeutics in August, adding promising clinical-stage assets to its neuroscience portfolio. This includes treatments for schizophrenia and Parkinson’s, such as emraclidine and tavapadon, which are in advanced clinical trials. This deal helps AbbVie strengthen its presence in the neuropsychiatric treatment space.
Keytruda: Merck’s cancer immunotherapy is set to dominate the market, with projected 2024 sales of $30 billion. The drug continues to grow thanks to expanded indications across various cancers, solidifying its place as one of the top-selling therapies globally.
Ozempic: Novo Nordisk’s GLP-1 receptor agonist for diabetes and weight loss is expected to hit $14 billion in sales this year. Its popularity has soared due to its effectiveness in weight management, creating a booming market demand beyond diabetes treatment.
Eliquis: Co-marketed by BMS and Pfizer, this anticoagulant is projected to bring in $11 billion in 2024. It remains a key treatment for stroke prevention and blood clot management, especially in patients with atrial fibrillation.
Dupixent: Regeneron and Sanofi’s blockbuster anti-inflammatory drug is expected to reach $13 billion in
2024 sales. Originally approved for eczema, Dupixent has expanded into treatments for asthma, eosinophilic esophagitis, and other inflammatory conditions, driving its market growth.
Rocket’s rare disease gene therapy
In June, Rocket’s gene therapy for leukocyte adhesion deficiency (LAD-I) was delayed after the FDA issued a complete response letter requesting additional manufacturing data. This setback came after a three-month review extension.
Daiichi Sankyo, Merck’s lung cancer ADC
In June, the FDA issued a CRL for their lung cancer therapy, patritumab deruxtecan, due to manufacturing issues at a third-party facility. Despite the safety and efficacy of the drug, approval was stalled.
Lykos PTSD treatment
Lykos Therapeutics received a CRL from the FDA in June for its midomafetamine capsules for PTSD. The agency recommended further phase 3 trials to assess safety and efficacy, following concerns raised during an FDA Advisory Committee meeting.
The FDA rejected Vanda Pharmaceuticals’ application for tradipitant in September, citing insufficient evidence for approval. Despite data from placebo-controlled studies and real-world evidence, the FDA requested further studies, which Vanda argued aren’t necessary. The company also intends to submit a new application for tradipitant to treat motion sickness later this year.


This year’s Pharma Innovation Awards are creating tools that elevate precision and efficiency in every step of the drug manufacturing process

From COVID-19 vaccines to curative cell and gene therapies to radiotherapeutics, every scientific leap is made possible by often-overlooked heroes — the manufacturing vendors who create the tools that bring precision and efficiency to every step of the drug development process.
Now in its twelfth celebration, this year’s Pharma Innovation Awards winners are the quiet forces at work that not only help secure each achievement but also lay a strong foundation that empowers drugmakers to keep pushing forward and reach new heights.
Transitioning products from the lab to commercial manufacturing is one of the most defining steps in a drug’s journey. Its success hinges on addressing challenges such as scalability, consistency and adherence to regulatory standards.
And advancements made in this stage can directly impact patients too. Companies that excel in process development can reduce time to market, improve product accessibility, and lower overall costs, making their therapies more affordable for patients.
Our first winner in this category, Watson-Marlow Fluid Technology Solutions’ WMArchitect range is a versatile single-use fluid management system designed to support biopharmaceutical processes from lab research through full-scale production. Its open-architecture design allows seamless integration into existing systems, giving drugmakers the flexibility to upgrade without costly infrastructure changes.
Yokogawa Electric Company CQ3000
The WMArchitect range also addresses one of the most significant challenges in scaling bioprocesses: reducing regulatory burden. By offering validation and regulatory support, this system helps companies meet regulatory requirements more efficiently.
Our second winner, Alphinity’s PIXER pump, is a single-use diaphragm pump designed to handle delicate biological materials such as high-value proteins and cells with minimal shear stress.

In bioprocessing biologically derived drugs, maintaining the integrity of their sensitive materials is a major challenge. High-pressure and high-viscosity processes can degrade their quality, potentially affecting the final product.
The PIXER pump solves this problem by preserving the integrity of these materials, ensuring consistent quality even as production scales up. Integrating sensors into the recirculation loops allows for PAT and closed-system integration of analytical instruments, supporting Quality by Design (QbD) manufacturing strategies to deliver life-saving therapeutics to patients faster. With flow rates ranging from a few mL/min to over 200 L/ min, PIXER mixing pumps offer scalability from lab bench experiments to full-scale GMP manufacturing.
Lastly, the CQ3000 from Yokogawa Electric Company provides a key development in cellular analysis by combining advanced microscopy with automated data analysis in a high-content imager.
By facilitating high-throughput analysis, the CQ3000 enables researchers to gather detailed observations into cellular and molecular responses to various compounds, reducing the time and costs associated with early-stage drug development.
Cell and gene therapy (CGT) continues to be one of the most promising treatment areas in pharma, with the global market expected to reach $97.33 billion by 2033.
And 2024, brought notable breakthroughs like Casgevy, the first gene therapy that uses CRISPR technology to treat sickle cell disease.
But even as the sector makes considerable progress, manufacturers still face persistent challenges related to high production costs, complex supply chains, and the scalability of these drugs. As more CGT therapies make it through FDA approval, innovation in manufacturing processes is crucial to keep pace with market needs.
Our first awardee in this category, Ori Biotech’s IRO, is designed with scalability in mind. IRO allows for a
M3DIMAKER 2

seamless transition from early process development and the ability to scale into GMP manufacturing with the same instrument, consumables and process.
One of the platform’s key features is its ability to automate and standardize key processes without sacrificing biological performance, offering closed and automated sterile fluid transfer, a novel bioreactor system, remote realtime data analytics, and flexibility in a single platform.
By streamlining tech transfer and reducing the cost of goods by up to 50%, the IRO platform not only accelerates the manufacturing process

but also helps to make CGT therapies more accessible. Additionally, with processing times reduced by up to 25%, the platform enables faster production, shortening the timeline for getting therapies from the lab to patients who need them most.
Also with the goal of shortening the timeline of CGT therapies, the M3DIMAKER 2 by FABRX is a revolutionary multi-printhead GMP pharmaceutical 3D printer designed to produce personalized medicines directly at the point of care.
Personalized medicine, especially in CGT, necessitates precise dosages tailored to individual patient needs. The traditional compounding process, which is labor-intensive and prone to variability, can introduce errors and inefficiencies, especially when manufacturing at scale.
The M3DIMAKER 2 addresses these challenges by automating the preparation of personalized dosages, minimizing the risks associated with manual compounding. By allowing for the production of custom formulations on-site, this printer simplifies the process of creating tailored treatments, reducing costs and improving patient safety.

From sterile environments to streamlined production processes, innovations in plant operations can have a significant impact on the overall efficiency and safety of drug manufacturing.
This year’s innovations focus on strengthening plant operations by improving cleanliness, production speed, and cost-effectiveness, making it easier for drugmakers to strike a balance between meeting regulatory requirements and growing demand.
AES Clean Technology’s CleanLock Module is poised to be a game-changer in cleanroom management. This modular airlock system is designed to enhance cleanliness and reduce contamination risks in pharmaceutical manufacturing environments. One of the key advantages of the CleanLock Module is its offsite-fabricated components, which allow for quick and cost-effective cleanroom expansions. The modular system also incorporates advanced HEPA filtration that captures 99.97% of airborne particles.
By making cleanroom expansion faster and more affordable, the CleanLock Module helps drugmakers adapt to changing production demands without compromising cleanliness or safety. Additionally, the CleanLock Module aligns with sustainability goals by reducing energy consumption, a growing priority for industry.
Sterility is another critical factor in plant operations, as the smallest amount of contamination can render a product unusable. In a seemingly tiny but mighty way, our second winner in this category, the Flush Fasteners’ F-Head Fastener, addresses this challenge by eliminating visible recesses in its design — common areas where bacteria can proliferate.
The simple yet effective design of the F-Head Fastener reduces the risk of bacterial growth, helping manufacturers avoid costly contamination issues that could compromise product quality.
Traditionally, the production of PPOP tablets involves multiple steps, including complex solvent granulation processes that can be time-consuming and costly. The Corelease OPL system simplifies this process by combining multiple ingredients into a

Another understated yet impactful innovation, Colorcon’s Corelease OPL aims to streamline the formulation process for push-pull osmotic pump (PPOP) tablets.
single, directly compressible powder, eliminating the need for solvent granulation altogether.

For manufacturers, this innovation translates into faster production times and lower costs. By reducing the number of steps involved in the formulation process, Corelease OPL allows manufacturers to increase efficiency and output without compromising the integrity of the final product.
In tablet manufacturing, KORSCH America’s X 5 Tablet Press is raising the bar for efficiency and versatility. Launched at this year’s INTERPHEX, this machine is designed for high-speed production, offering flexibility with its interchangeable turret, allowing manufacturers to produce single-layer, bi-layer, or tri-layer
tablets on the same machine. This flexibility is particularly useful for manufacturers that need to switch between different tablet types without experiencing lengthy production downtime.
The X 5 Tablet Press also enables quick changeovers, which helps to minimize disruptions and increase overall production output. Its highspeed capabilities make it an essential tool for drugmakers that need to produce large volumes of tablets while maintaining strict quality controls. One of our reviewers called it, “Science and art combined.”
With drugs becoming more complex, the demand for high-precision, automated packaging and inspection systems also grows. Manufacturers need solutions that can handle high volumes while ensuring that every product meets quality and safety requirements.

Advanced Quality Event Management (AQEM)
This year’s innovation in this category looks to create more reliable, efficient solutions for drugmakers, helping to improve both packaging and inspection processes.
looking to maintain strict quality controls while scaling up production.
As the demand for biologics and other temperature-sensitive therapies increases, manufacturers are seeking advanced solutions that can ensure reliable temperature control throughout the transfer of materials.
Single Use Support
RoSS.PADL

Antares Vision Group’s VRI Automatic Inspection Machine addresses this challenge by providing 100% automatic inspection of blow-fill-seal (BFS) containers at high speeds. BFS containers, often used for liquid pharmaceuticals, can pose unique challenges when it comes to inspection due to their complex shapes. The machine detects particles, cosmetic defects, and fill levels, ensuring that every container meets the necessary quality standards.
By automating the inspection process, the VRI Automatic Inspection Machine allows manufacturers to detect any imperfections or issues early, preventing defective products from reaching the market. Its highspeed capabilities and ability to handle large production volumes make it a competitive solution for companies
Innovations in cold chain logistics are driving improvements in temperature control, monitoring, and overall efficiency, ensuring that these sensitive drugs are safely transported and stored.
As the only awardee in this category, Single Use Support’s RoSS. PADL is a homogenization system designed to handle single-use bags for biopharmaceutical liquids. By combining cooling, heating, and kneading, RoSS.PADL ensures the uniform distribution of cell counts in pharmaceutical liquids in preparation of aliquoting into smaller single-use bioprocess containers, which is crucial for maintaining consistent dosages. This is particularly important in biopharmaceutical workflows where deviations in active ingredients can affect patient safety.
The RoSS.PADL system enhances the reliability, consistency and accuracy of dosing, reducing the risk of ingredient separation during storage and transport. Its ability to preserve the uniformity of active ingredients throughout the cold chain ensures that patients receive the correct dosages, improving the safety and efficacy of temperature-sensitive therapies.
Like most industries, pharma is becoming more digitized, and data management systems are helping manufacturers maintain quality standards, avoid errors, and streamline production processes. This year’s winners in the data management category are providing tools that make data handling more reliable, secure and automated.
Our first awardee in this category, MasterControl’s Advanced Quality Event Management (AQEM) platform is a user-friendly solution designed to streamline the management of quality events in pharmaceutical manufacturing. Quality events, such as deviations, non-conformances, and corrective actions, can be time-consuming and costly to manage manually. The AQEM platform automates these processes, allowing companies to create custom workflows that simplify the reporting and resolution of quality issues. By integrating AI and automation, the AQEM platform helps manufacturers maintain high standards of quality while speeding up their operations. Its no-code interface makes it easy for manufacturers to create and modify workflows without the need for extensive IT support, making it a flexible and efficient tool for managing compliance in a highly regulated industry.
Emerson’s AMS Asset Monitor for Life Sciences offers a predictive maintenance solution through a continuous,
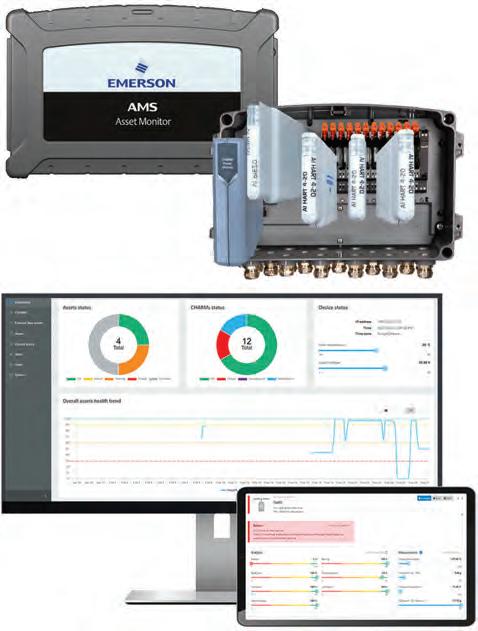
Emerson AMS Asset Monitor for Life Sciences
online condition monitoring system that helps drugmakers avoid equipment failures. The system monitors variables such as vibration, temperature, and pressure in real-time, providing insights into the health of manufacturing equipment. By identifying potential issues before they lead to equipment failures, the AMS Asset Monitor allows companies to schedule maintenance more effectively, reducing downtime and ensuring smooth production processes.
This approach to maintenance helps companies increase operational efficiency by minimizing unexpected equipment breakdowns and reducing repair costs. The AMS Asset Monitor also enhances safety by ensuring that
equipment operates within specified parameters, reducing the risk of accidents or product defects. For manufacturers looking to optimize their operations and reduce costs, the AMS Asset Monitor helps ensure the reliability and safety of production equipment.
With the conclusion of our 2024 Pharma Innovation Awards, we extend our sincere thanks to all the participating companies and the dedicated suppliers driving progress in pharma manufacturing. As we move into 2025, we are eager to learn about more transformative breakthroughs and look forward to seeing how these innovations will continue to elevate our industry.
When you think of Pittsburgh, you might still picture the towering steel mills, the iron giants of an industrial era long gone. But today, the foundation laid by those smokestacks is flourishing beyond metal.
Pittsburgh, the once steel capital of the world, is looking to expand its economic identity with life sciences at the heart of its growth. Because while the steel industry forged Pittsburgh’s past and present, its future is also being crafted in labs, biotechs, and research facilities.
“We’re building on our manufacturing legacy,” says Matt Smith, chief growth officer of the Allegheny Conference on Community Development. “It’s about creating opportunities for economic growth and developing our life sciences infrastructure.”
“Pittsburgh has a history of life-changing medical breakthroughs,” says
Andrea Corona Senior Editor
Building on its manufacturing legacy, Pittsburgh is now headed in a new direction — from steel to science
Smith. The city is home to the polio vaccine development, a moment that Smith adds, paved the way for the current vision, “That tradition of innovation continues today with cutting-edge work in cell and gene therapies,” he adds.
Pittsburgh isn’t just hoping for this transformation to unfold — it’s making it happen by tapping into its academic, industry, and government resources.
The city boasts two powerhouse institutions driving its life sciences sector: the University of Pittsburgh (Pitt) and Carnegie Mellon University.
Pitt ranks among the top 20 U.S. universities in terms of research expenditure, boasting over $1 billion in research funding. It stands out in health sciences, ranking in the top 10 nationwide for biological and biomedical sciences. Pitt also shines in its bioengineering and translational research, fueling start-ups in healthcare.
Meanwhile, CMU is renowned for its work at the intersection of AI, robotics, and life sciences, making it a leader in precision medicine and computational biology.
This blend of academic prowess and technological expertise has made Pittsburgh uniquely positioned to lead in life sciences.
Megan Shaw, president & CEO at the Pittsburgh Life Sciences Alliance (PLSA) sums it up, “We have real strengths in AI, data analytics, and chemical engineering.” And it’s this synergy that sets the city up for success, “When you put all of those together, we’re well-positioned to lead in both data-driven healthcare and the manufacturing of nextgen therapeutics.”
The PLSA is leveraging the region’s capabilities in clinical care, research, manufacturing and technology to establish it as a global leader in life sciences and a catalyst for economic
growth, pulling resources and people together.
Local companies have already tapped into the benefits of being in proximity to academic institutions. “We have always maintained a very strong relationship with the University of Pittsburgh, including licensing of technologies, taking advantage of some of the leading core facilities, and ongoing research collaborations with faculty,” says Steve Thorne, co-founder and Chief Scientific Officer at KaliVir Immunotherapeutics.
The biotech, established in 2019, focuses on developing oncolytic viral immunotherapies for cancer treatment, partnering with pharma companies like Astellas and Roche. “We are also looking to open clinical sites at University of Pittsburgh Medical Center and Allegheny Health Network to support clinical development of our products,” he adds.
With a focus on CGT manufacturing, Bioforge will be operational in 2027.
For Pittsburgh, expanding from an industrial hub to a life sciences leader isn’t just about infrastructure — it’s also about vision. And that vision includes nurturing collaboration at every level.
“Pittsburgh isn’t branded as a pharma hub, but there’s a lot of activity in biotech and biomanufacturing here,” says Smith. “While Philadelphia is considered Pennsylvania’s pharma hub, we have a lot going on in here as well,” he adds.
This collaborative spirit has been critical in attracting major investments from life sciences companies like Elevate Bio, Peptilogics and Krystal Biotech. The stakes are high, and the opportunities even higher.
According to Shaw, it’s this sense of working together that gives Pittsburgh its edge, “Pittsburgh is known for its down-to-earth, hardworking, and collaborative culture. Bringing that spirit together is our biggest opportunity.”
One of Pittsburgh’s flagship projects in its life sciences resurgence is
BioForge, a biomanufacturing facility funded by a $100 million grant from the Richard King Mellon Foundation and spearheaded by ElevateBio.
The Pitt BioForge building, currently under construction, will be owned by the University of Pittsburgh and will house both the BioForge organization and ElevateBio as tenants.
BioForge is set to open in 2027 and will focus on manufacturing cell and gene therapies — a fast-growing therapeutic market.
“BioForge is a great story of economic rejuvenation,” says Heidi Wagner, global head of government affairs at ElevateBio. “It’s a chance to bring cutting-edge science to an area that was pivotal to the country’s industrial development.”
A core mission of BioForge is to ensure that innovation stays right at home.
Wagner highlights a common issue faced by many life sciences hubs: the tendency for innovations to leave town once they take off. “A lot of ideas spin off from academia, but they often

It’s a chance to bring cutting-edge science to an area that was pivotal to the country’s industrial development.
— Heidi Wagner, Global Head of Government Affairs, ElevateBio
end up in places like Boston or the Bay Area, where there’s more of an ecosystem,” she says. “We want to change that, with BioForge, we’re confident that businesses will see the benefit of staying local,” she adds.
And companies are already reaping the rewards of investing in the region. “The cost of lab space keeps us from needing to move to larger biotech hubs,” says Thorne. “We’ve built a strong team here and recently hired our first Chief Financial Officer,” he adds.
While the city’s universities provide a strong pipeline of highly educated graduates, Pittsburgh is focused on ensuring that there are opportunities for all workers — not just those with advanced degrees, and that the workforce ecosystem continues to grow.
“We’ve committed to at least 170 new jobs in Pittsburgh,” says Wagner. Some of those will require advanced scientific knowledge, but many won’t. “We’re working with the University of Pittsburgh to structure programs that can help people transition into these jobs,” she adds.
This effort goes beyond universities. Shaw and the PLSA are looking holistically at workforce development, understanding both current and future needs. “We’ve been focused on creating a demand-driven workforce,” says Shaw. “We’re collaborating with workforce developers in academia, apprenticeship programs, and training initiatives to ensure we have the right talent.”
And this endeavor is supported by biotech companies already rooted in Pittsburgh, like KaliVir. “We hire young, motivated employees, and the low turnover shows their commitment,” says Thorne.
With a pipeline of highly educated graduates from its universities and tailored workforce development programs, Pittsburgh provides companies with a steady stream of skilled labor, enabling them to scale operations efficiently without needing to relocate.
Pittsburgh’s affordability and quality of life are also major draws for both talent and businesses. “The cost of living is low, and the quality of life is consistently ranked among the top in the country,” says Shaw. “People move here and stay because it’s a great place to live. That helps us retain talent, which is crucial for longterm growth,” she adds.
No transformation happens without a little help from the top.
Pittsburgh’s life sciences rise has been bolstered by strong support from state and local governments. Pennsylvania has made life sciences a key pillar of its
economic development strategy, providing tax incentives, workforce training grants, and other benefits to attract and retain life sciences companies.
Wagner points to Governor Josh Shapiro’s clear commitment to the life sciences sector, “His economic plan is a healthcare plan,” she says. “He sees healthcare jobs as a key driver of economic growth, and that was a big factor in Elevate Bio’s decision to invest in Pittsburgh,” she says.
In addition to R&D and Job Creation tax credits, Pennsylvania offers programs like Pennsylvania First, which helps companies cover costs for infrastructure, equipment, and expansion. WEDnetPA plays a key role in workforce development, reimbursing businesses for training employees in critical industries like life sciences.
On top of that, early-stage companies in Keystone Innovation Zones (KIZ) can take advantage of tax credits aimed at boosting innovation in designated areas, making it easier for them to grow in the region.
The city’s leadership knows that the life sciences sector isn’t a one-off project — it’s a long-term commitment, followed by deliberate action to build a sustainable future whose benefits can be reaped by the local community.
“We see this as planting seeds,” says Wagner. “It’s about creating a pipeline of talent, innovation, and opportunity that will pay off for years to come.”
Even though BioForge will be fully operational by 2027, the effects of this transformation are already being felt across the city. “Pittsburgh is well on its way to becoming a major player in life sciences,” says Shaw. “And if we can deliver on this potential, the sky’s the limit.”
Alf Goebel CEO, advanco
Drugmakers are tackling sustainability challenges to ensure energy efficiency and reduced environmental impact

The cold chain is a vital component of the modern pharmaceutical ecosystem, with its roots traced back to the early 20th century.
It performs an essential function by ensuring the safe and efficient production, storage and shipping of key drugs that need to be kept at a very low temperature. These include some influenza and hepatitis vaccines, some specialty pharmaceuticals such as chemotherapy agents and ophthalmic solutions, or hormone and enzyme replacement therapies.
Attention to cold chain operations became heightened during the COVID-19 pandemic. Although this spotlight affirmed the cold chain’s status as a lifesaver, it also resulted in increased attention on its green credentials.
Indeed, many started to question just how sustainable the cold chain is, especially considering the significant energy and power needed to maintain such low temperatures.
Ensuring the pharmaceutical cold chain is sustainable involves various
strategies, all of which need to work in unison to secure its overall success.
Most of the overriding reasons for the historical unsustainability of the cold chain stem from the fact that vast amounts of energy are needed to power it.
Considerable resources are required to maintain stable temperatures throughout the entire supply chain. This includes refrigeration systems, freezers, and insulated containers, which will likely need to operate 24/7.
Furthermore, certain drugs have ultra-low temperature requirements, such as some vaccines requiring storage at -80°C that demand even more powerful cooling systems, which consume significantly more energy than regular refrigeration.
In addition to the units needed in manufacturing, packaging and storage units, when it comes to distribution, refrigerated transportation vehicles, including trucks, planes and ships

use additional fuel and energy to power cooling units. These units run continuously, even when vehicles are not in motion, contributing to energy inefficiency. Furthermore, the international shipping of pharmaceutical products involves complex routes and long journeys, requiring sustained refrigeration, which increases fuel consumption.
Therefore, why can’t something be done? After all, innovations like energy-efficient refrigeration units and renewable energy sources are emerging.
The fact of the matter is that many cold chain systems still rely on older, less efficient technologies that consume more energy than necessary. There is a substantial legacy infrastructure across both individual companies, of all sizes, and across entire geographies. A lack of capital, competing business priorities and a lack of time will often mean that unless such systems are made mandatory, they may get relegated to the back of the queue for many manufacturers.
Other factors contribute to high energy use too, factors that are possible to control with better organization. For example, poor route planning, uncoordinated shipments, or storage mismanagement can lead to longer transit times, which directly correlates to increased energy demands. An unoptimized supply chain requires extra cooling over extended periods, driving up energy consumption.
As we have seen, maintaining a low temperature across all aspects of the pharmaceutical cold supply chain involves energy usage – this is a fact and there is no way around it.
Therefore, it is key to use the energy needed as smartly as possible, and of course, rely on renewable energy sources where they are available.
We have already touched upon the issue of older equipment and antiquated systems. However, all equipment should be regularly reviewed, with companies continuously looking
to the future to ensure they will be operating in the most efficient overall manner possible. This is especially true against the background of a much greater emphasis on the need for sustainability across all industries, not just pharma.
Drugmakers should at least be actively planning now on how to harness green energy in the future. Even if the changes won’t happen overnight, they ought to be factoring in future equipment upgrades to ensure they are using the most energy-efficient refrigeration units and storage facilities.
The modern equipment available today – and certainly in the future –will almost always have much better insulation and energy use metrics than were available even a few years ago. A smart manufacturer will be regularly reviewing the options open to them and planning how they can be seamlessly incorporated in the future. Where possible, renewable energy should be used to provide the power that cold stores rely upon, with solar or wind power becoming increasingly popular. The latest energy management systems can harness this renewable energy and optimize its usage even further by reducing wastage. Such systems comprise sophisticated tools and technologies designed to monitor, control, and optimize the performance of energy within buildings or facilities.
They play a critical role in improving energy efficiency, reducing costs, and minimizing environmental impact.
It is important to note that in some cases, the medicines themselves are changing, which is positively impacting the amount of energy needed to maintain their efficacy.
For example, innovation is taking place, meaning that some medicines, despite still needing to be kept very cold, do not require being kept as cold as they once did.
This shift is largely influenced by the development of new formulations and improved cold chain management systems. Insulin provides a good example. Traditionally requiring strict cold storage, newer formulations of insulin can now withstand higher temperatures for limited periods, reducing dependency on constant refrigeration.
For example, some types of insulin can be kept at room temperature (up to 86°F) for up to 28 days once opened. This reduction in the thermostat temperature correlates, in many cases, with less energy being used to maintain the new, less cold, required temperature.
Ensuring that packaging materials are sustainable supports the long-term viability of the pharmaceutical supply chain, making it more resilient and adaptable to future challenges.
The usage of sustainable packaging goes a long way to reducing the harmful environmental impact of the pharma cold chain.
Sustainable packaging often involves using recyclable or biodegradable materials, which helps reduce the amount of waste generated. This is particularly important in the pharmaceutical industry, where packaging waste can be substantial. Ecofriendly packaging materials also typically require less energy to produce and recycle, reducing the overall carbon footprint, with lighter packaging going some way to reducing transportation emissions. Furthermore, cold chain monitoring systems often rely on software that seamlessly, on a non-intrusive basis, is interoperable with existing enterprise IT solutions. This means that the carbon footprint continues to be controlled, as no extra servers or hardware is needed. The correct packaging solutions will also enhance levels of product integrity.
Sustainable packaging solutions are designed to provide effective thermal insulation, ensuring that temperature-sensitive pharmaceuticals remain within the required temperature range during transit. This is crucial for maintaining the efficacy and safety of the medications. Improved packaging designs can also offer better protection against physical damage and contamination, reducing the risk of spoilage and wasting the products contained within it. Sustainable packaging also aligns with global sustainability goals, such as the United Nations Sustainable Development Goals (SDGs). This contributes to broader efforts to combat climate change and promote sustainable practices. Ensuring that packaging materials are sustainable supports the long-term viability of the pharmaceutical supply chain, making it more resilient and adaptable to future challenges.
A key component of the pharma cold chain is transportation. This could be anything from relatively localized transport from one town to another within the same
country or sending a product halfway across the globe.
Ensuring a transport network is fully optimized involves several strategies which all need to work together in order to guarantee sustainable efforts are maximized.
One crucial aspect is route optimization. Advanced route planning, using sophisticated logistics software, will plan the most efficient routes, considering factors such as traffic, weather conditions, and delivery windows which will minimize travel time and fuel consumption.
Furthermore, dynamic routing will allow the implementation of real-time route adjustments based on current traffic conditions and other unforeseen variables to avoid delays and ensure timely deliveries.
Once the routes have been sorted, the temperature control technology needs to be correct. Vehicles will need to be equipped with advanced refrigeration units that maintain precise temperature control throughout the journey. They will also need to be fitted with temperature-controlled containers that use battery-powered systems or passive containers with phase change materials (PCMs) or dry ice to maintain the required temperature range.
Compliance with regulatory standards is also essential. Ensuring that transport solutions comply with international standards such as Good Distribution Practice (GDP) and the World Health Organization (WHO) guidelines for the cold chain should be an integral part of all planning. Manufacturers should also look at their Standard Operating Procedures (SOPs), developing and adhering to these without fail for all aspects of cold chain transport, including loading and unloading procedures, handling protocols, and emergency response plans.
The rise of modern systems has revolutionized the methods available for ensuring cold chain temperatures are kept at optimum levels. These systems leverage advanced technologies to provide real-time visibility, automated control, and proactive management, ultimately safeguarding product integrity and patient safety.
The use of Internet of Things (IoT) devices and sensors provide real-time monitoring of temperature conditions, ensuring that only the necessary cooling is applied. This is of course vital for conserving energy and ensuring that resources are not being used unnecessarily. Predictive analysis can furthermore anticipate and mitigate temperature excursions, reducing energy use by maintaining perfect conditions throughout the supply chain.
There are multiple benefits to be derived from systems such as this one. They include improved product safety by ensuring that pharmaceuticals are stored and transported within the correct temperature range, and enhanced traceability by providing end-to-end visibility, reducing the risk of temperature excursions and ensuring accountability.
Operational efficiency is also maximized through streamlining supply chain operations through automation, real-time monitoring, and proactive management, and cost savings by reducing waste and spoilage of highvalue products.
There are a host of other methods to ensure the cold chain benefits from higher sustainability levels.
These include much tighter coordination between the different suppliers and components of the supply chain, including suppliers, manufacturers,
and distributors. This will help to ensure timings are tighter all round, minimizing the need for prolonged storage. It will also help to improve inventory management which will reduce overstocking and the associated energy costs for storage.
It is also worth emphasizing that true end-to-end tracking, with just one point of contact, on a single item basis, enhances overall efficiency –saving time and energy – and a much lower overall total cost of ownership.
More emphasis on training and awareness is also important. Educating employees on the importance of sustainability and training them on best practices for maintaining an efficient and eco-friendly cold chain will ensure the entire sector benefits from shared standards. Engaging with stakeholders will ensure sustainability goals are aligned and supported across the supply chain.
Innovation and investment in research and development is also a factor. Research into new technologies that can improve the efficiency and sustainability of cold chain processes will ensure the future remains as sustainable as possible.
The pharma cold chain is key to ensuring that patients across the globe can rely on the medications being produced, stored and shipped to them in perfect conditions.
However, as with all aspects of the pharma sector, it cannot escape criticism from those who are concerned it needs to work harder to up its sustainability credentials.
Let’s not forget, the cold chain is essential. All parts of the pharma supply chain need to work together to ensure its sustainability credentials are as strong as its life-saving credentials.
Tatum O’Kennedy Senior Analytics Engineer, Seeq
How AI-inclusive advanced analytics platforms can enhance daily workflows by facilitating data collection, centralization and sharing

Biopharmaceutical manufacturers are plagued by high costs and lengthy timelines to bring new therapies to market. These factors can contribute to global drug shortages, causing delays in patients accessing necessary treatments.
The drug development process is intricate and can take decades, with a lengthy road from R&D to clinical trials to commercial manufacturing. Throughout this journey, organizations face numerous challenges in integrating and analyzing data, and a lack of real-time insights can slow critical decision-making as therapies progress.
In addition to market-specific pressures and regulatory obstacles, the pharmaceutical industry faces general manufacturing issues — such as equipment downtime and inefficient maintenance scheduling —that affect all process industries. To tackle these challenges, the sector must adapt and modernize their facility strategies by embracing emerging technologies like artificial intelligence (AI).
By adopting AI-inclusive advanced analytics platforms into daily workflows, organizations can implement predictive maintenance, enhance product development operations and scheduling, and accelerate delivery timelines. These proactive approaches not only improve operational efficiency, but also reduce maintenance and operational costs throughout facilities.
In pharma, as with any process industry, equipment failures are all too common, and unexpected asset failures can significantly delay production. For contract manufacturing organizations (CMOs) and large manufacturing facilities, where multiple production schedules are tightly balanced, the effects of these delays can ripple throughout the entire plant downstream to the supply chain. These delays prevent efficient delivery of therapies to patients who rely on them, often for life-critical needs.
These issues are often the result of insufficient equipment health visibility, which is required to obtain predictive insights. Additionally, comparing production behavior against historical patterns can help users understand long-term trends, but the process of aggregating the data necessary to do so can be time consuming and tedious.
As a result, manufacturing teams often schedule routine interval maintenance instead of condition-based maintenance. This prescheduled maintenance can be costly and excessive for some equipment, while equipment requiring more frequent maintenance may not receive adequate attention. Furthermore, interval-based maintenance ties up personnel and equipment resources that could be better used driving life-saving therapies to market.
Addressing these and other challenges, advanced analytics platforms empower pharma companies to effectively consolidate data throughout the entire drug product lifecycle. By centralizing data, these platforms facilitate the comparison of drug processing across critical development stages. This integration is crucial for maintaining key process parameters when transitioning operations from a development facility to a CMO for production.
Once data is in a centralized location for comparison, it can be challenging to isolate process inefficiencies or optimize process parameters. Advanced analytics platforms do not require users — consisting of
process engineers, managers, operators and more — to possess analytics expertise to garner key insights, enabling personnel of any discipline to leverage the software as efficiently as data scientists. Using tools, such as built-in AI assistants, users need only to key in or verbalize questions to gain insights about their processes. Similarly, no-code machine learning algorithms simplify analyzing historical data against real-time process parameters to predict and optimize operational outcomes.
In drug development, laboratory testing is critical for assessing drug efficacy and determining necessary process adjustments. However, lab equipment delays can hinder the timely availability of this information. Advanced analytics platforms help
A continuous process verification dashboard communicates behavior of critical process parameters (CPPs) with profile overlays built from historic data, streamlining batch review and regulatory reporting procedures.
address this issue as well by integrating historical lab data with real-time process data to create predictive analytics and forecast development outcomes. This empowers SMEs to make process adjustments in real-time without needing to wait for lab results, enabling early identification of potential operational issues to mitigate risks and avoid costly delays, such as a lost batch.
Furthermore, AI-enhanced platforms leverage continuous data monitoring in real-time to accelerate insights into key process metrics, providing an immediate and informed feedback loop to ensure consistent product quality. This guidance helps inform automated batch review reporting build-out (Exhibit 1).
Efficient process management with proactive adjustments help reduce R&D cycle times, while real-time monitoring and data-informed process improvements enhance product
quality. Collectively, these factors reduce the risk of batch rejections and compliance issues.
By minimizing the need for manual intervention, these platforms streamline batch review and help organizations maintain regulatory compliance. Additionally, data centralization through all stages of product development enhances collaboration among departments and companies, enhancing decision-making.
As process efficiency improves and re-manufacturing of failed batches becomes less of an issue, organizations can reduce operational costs. Savings previously spent on unnecessary trials and manufacturing adjustments can instead be redirected toward capital improvements and optimization efforts. This shift, driven by AI predictions, enhances output potential and accelerates the introduction of new drugs to the market.
By utilizing integrated, automated reporting and AI-enhanced
documentation, organizations ensure regulatory compliance throughout the entire development process, from R&D to large-scale commercialization. Improved documentation and validation procedures also streamline regulatory submissions in late-stage drug candidates.
These benefits empower technical teams with actionable insights about their processes, enabling informed decision-making at every step of development. This helps optimize processes and quickly address deviations, all while consolidating information into a single, accessible location.
With data spread across numerous equipment lines, or even among multiple facilities, maintaining real-time insights into production processes is critical. Advanced analytics platforms enable the required data integration across various sensors, control systems and plants to provide these insights,
A dashboard communicating key cycle time monitoring metrics, including phase, downtime events, related assets, and associated downtime costs.
generating excursion notifications before faults occur.
Equipment failures are often multivariate in nature, and therefore difficult to detect with standard tag-by-tag monitoring. The machine learning algorithms incorporated into routine advanced analytics workflows make equipment failures more predictable by leveraging pattern analysis and comparisons with historical equipment performance.
After combining trends across sensors and sites into a single platform feeding predictive algorithms, the next step is communicating key findings to plant personnel. This can be done by building out comprehensive analytics dashboards for display in centralized plant areas for operations and management staff alike. These dashboards can be customized to team- or site-specific needs, and they enable easy visualization of equipment health metrics and maintenance status.
When building dashboards in advanced analytics platforms, modern AI features can reduce the workload
burden even further. For example, users can simply ask the AI performance engine which key performance indicators (KPIs) are deviating, or have the AI populate trend and dashboard views with specific tables and visualizations (Exhibit 2).
While dashboards are useful for engineering and operations teams working in a facility, key stakeholders off the plant floor often cannot consistently monitor dashboards to stay informed of plant processes. Automated alerts and notifications fill this void, providing communication when a process trends out of specification, prompting action prior to impending failures.
With a shift towards proactive as opposed to reactive downtime and maintenance, scheduling teams can optimize production for ideal balance between producing therapies and ensuring reliable equipment operation. This facilitates equipment maintenance scheduling during low-impact times, with minimal disruptions to production.
AI can help engineers quickly and effectively implement predictive maintenance calculations and dashboards in production facilities. For example, one pharmaceutical manufacturer used an AI-inclusive advanced analytics platform to develop a pump health indicator for its CIP pumps. This prompted a complete shift to condition-based monitoring and maintenance, saving the company $200k per year.
Another manufacturer used the same platform to develop a similar health indication algorithm for critical heat exchangers throughout its facility. The team also configured alerts to notify plant personnel in the event that deviations from baseline production data occur. This saves the company an estimated $10k per year for each heat exchanger by eliminating unnecessary maintenance and significantly reducing downtime and associated production outages (Exhibit 3).
Deploying and streamlining predictive maintenance programs can quickly traverse facility fence lines, permeating entire organizations. Higher efficiency and optimized resource allocation help boost both productivity and overall operational efficiency, and data-based as opposed to time-based maintenance strategies enable critical maintenance prioritization. This enables scheduling around production needs to maximize uptime and production.
Additionally, performing equipment service based on actual needs can increase asset lifespans, reducing required expenditures on equipment replacement. Instead, these resources can be allocated toward process optimization, new therapy development, and facility expansion projects.
Pharmaceutical processors must adapt to market fluctuations and increasing demand for therapies by modernizing facilities with advanced analytics tools to accelerate and streamline outcomes. Integrating advanced analytics and AI platforms enables faster drug development, mitigates equipment failures, addresses inefficiencies, and shortens time-to-market. Furthermore, AI-driven insights and real-time monitoring ensure high production quality, reducing batch rejections and compliance issues.
By managing data across R&D, clinical trials and manufacturing phases within a unified system, organizations facilitate smooth transitions and break down communication barriers, enhancing decision-making and fostering innovation.
To stay competitive and deliver therapies quickly, pharma companies must keep their facilities up to date, leveraging insightful automation software tools to help maintain timely and high-quality patient care.



The global biologics drug market is booming, valued at $322.8 billion in 2021 and projected to be worth $689.59 billion by 2030, growing at a CAGR of 8.8%.2 The increasing prevalence of chronic diseases, such as cancer and autoimmune disorders3, 4 and advances in biomanufacturing technologies, such as single-use systems and continuous processing, have enabled more efficient and scalable production of biologics. These advancements have facilitated higher-volume sterile processing. Handling sterile actives in various volumes is still proving to be a challenge for manufacturers. They experience unique challenges in handling both small and large volumes of drug product (DP) for filling operations.

How to proactively manage contamination risks and ensure product safety and quality
Handling powders in bulk and not splitting batches into a larger quantity of smaller containers has advantages related to both productivity and risk management. This means that manufacturers rely on larger-volume SUT solutions — such as singleuse bags and closed connections — to handle higher volumes while maintaining sterile integrity. Expert aseptic processing technologies partners work closely with manufacturers to develop cuttingedge solutions that accommodate a wide range of volume transfers and packaging options, including standard and customized 2D and 3D formats.
As the biologics field continues to advance and the demand for these innovative therapies grows, it is expected that the need for efficient and scalable sterile processing solutions will continue to increase.
Custom single-use assemblies for sterile manufacturing, including aseptic handling of powders, offer numerous advantages in terms of efficiency, contamination control and cost reduction. They closely align with the needs and priorities of manufacturers of aseptic products, making them a popular choice in the industry as a way of keeping up with market demands and an answer to new trends. As these solutions are designed as closed systems, they minimize the potential for product exposure to the environment during transfer and processing, minimizing the risk of product loss. They are designed with optimized flow paths, reducing the amount of product that remains in the system after processing.
Experienced partners who can provide custom single-use assemblies work closely with manufacturers to design and configure their solutions to
meet specific process requirements. This includes selecting appropriate materials and connection configurations to reduce product loss, streamline operations and minimize contamination risks.
Many manufacturers are still working through specific strategies to adhere to the principles and requirements of the updated EU’s Good Manufacturing Practice (GMP) Annex 1, which provides guidelines for sterile medicinal products. Annex 1 outlines how a contamination control strategy (CSS) should be embedded in all aspects of sterile manufacturing, including personnel, premises, equipment, processes and materials.5
As these technologies continue to advance, there will be even greater adoption of automation in the aseptic processing industry, leading to safer, more efficient and cost-effective sterile products as well as benefits to workers.
Manufacturers have to ensure that they are meeting these standards and implementing all required control measures so they can establish a proactive and effective approach to managing contamination risks and ensuring the safety and quality of their aseptic products.
Single-use solutions have played a critical role in helping pharmaceutical organizations meet these stringent requirements in many ways:
• Reduced human intervention: These innovations can be integrated into automated processes, minimizing the need for manual interventions, which are potential sources of contamination. Closed automated systems help reduce human error and contamination risk.
• Flexibility and adaptability: Versatile containers with easy handling solutions and different choices of materials offer flexibility in design and operation, allowing manufacturers to quickly adapt to changing production needs. This aligns with Annex 1’s focus on quality risk management, where flexibility can be a key factor in mitigating risks.
By eliminating the need for cleaning and sterilization, which are major sources of contamination in traditional stainless-steel systems used in the aseptic manufacturing of pharmaceuticals, single-use solutions align with current CSS guidelines.
• Faster turnaround times: Innovative single-use equipment enables faster changeovers between batches, reducing downtime and increasing productivity. This can be particularly beneficial for smaller volumes of DP, which are becoming more
common in the industry with the rise of personalized therapies.
• Cost-effectiveness: Reduced cleaning, sterilization, and validation requirements mean that manufacturers can increase productivity and save costs.
Upgrading each production line and setup of existing sterile facilities has its unique logistical and technical challenges. Occasionally, there is not one single ideal solution, so using combinations of different technologies in new ways may be required. Experienced partners with expertise in aseptic processing can help manufacturers develop, enhance and qualify systems in new ways to ensure they remain compliant with evolving regulatory requirements.
Robotics and automation have been transforming pharmaceutical aseptic manufacturing. They have shown their potential in streamlining processes, increasing throughput, improving accuracy and reducing contamination risks to help ensure compliance. These technologies are used in various stages of aseptic manufacturing, including:
Robotics can aid in the precise filling of vials, syringes and cartridges with sterile drugs and sealing them to maintain product integrity. This minimizes human intervention, reducing the risk of contamination. Robots perform tasks with high precision and repeatability, ensuring consistent product quality.
Automated systems can transport materials within or between facilities, preventing cross-contamination and
ensuring smooth workflow. Recently, closed robotic powder handling solutions have increasingly been used in all powder applications in bioprocessing, fill-finish, solid dosage and drug substance manufacturing thanks to their ability to create semi-continuous production cells with readily available pre-engineered and off-the-shelf components that are ready to deploy. These solutions use existing systems for closed transfer between the process and the container, ensuring high performance and safety when sterile or even toxic materials are being handled.
Automated systems can inspect the product, ensuring consistent quality and compliance with regulatory standards. Automated systems can collect and analyze data throughout the manufacturing process, improving traceability and quality control.
Robotics can help package finished products, reducing the need for manual labor and improving efficiency.
The benefits of introducing robotics and automation into pharmaceutical aseptic manufacturing are substantial. These solutions speed up processes, improve efficiency, and reduce lead times, bringing products to market faster and safer. They eliminate staffing challenges and problems such as ergonomic issues that arise with extensive manual handling. As these technologies continue to advance, there will be even greater adoption of automation in the aseptic processing industry, leading to safer, more efficient and cost-effective sterile products as well as benefits to workers.
Companies specializing in aseptic processing technologies are collaborating with manufacturers to provide
tailored solutions and advance robotic manufacturing through innovation and partnership.
As aseptic pharma manufacturing grows rapidly, it’s set for major transformation driven by new developments. SUTs are expected to gain popularity for enhancing compliance, reducing contamination risks, and improving cost-effectiveness and flexibility. Innovations in materials and designs will further improve compliance with quality standards, broadening their use. SUTs not only support compliance by reducing contamination risks but also contribute to companies’ environmental sustainability strategies with their reduced water and energy consumption.
The integration of advanced robotics and automation into pharma production is set to expand, minimizing human intervention, reducing contamination risks and improving efficiency and consistency. Manufacturers will use robotic systems to perform complex tasks, like aseptic transfer and manipulation, streamlining processes and ensuring smooth workflows and consistent product quality. Integrating AI with robotics enables smarter automation, with AI algorithms analyzing data from sensors and cameras in real time, allowing robots to autonomously adjust and enhance reliability.
Collaborating with suppliers of equipment designed to meet stringent compliance and quality needs will help manufacturers stay at the forefront of innovation, ultimately ensuring patients receive safe, high-quality therapeutics faster.
For the complete list of references, visit pharmamanufacturing.com
Nate Bolton Engineering Design Manager, Genesis AEC

How the Department of Energy’s recent guidelines provide a clear roadmap for sustainable design and construction
The pursuit of net-zero emissions is gaining momentum across industries, and pharma manufacturing is no exception.
As facilities work to reduce their carbon footprint, the U.S. Department of Energy (DOE) has introduced a groundbreaking definition for zero emissions buildings (ZEB), offering a clear roadmap for sustainable design and construction.
To achieve net-zero, pharmaceutical companies need to address their greenhouse gas emissions across three scopes.
Firstly, Scope 1 emissions include direct emissions from owned or controlled sources, such as on-site boilers or company vehicles. Secondly, Scope 2 covers indirect emissions from purchased electricity, heat, or steam. Lastly, Scope 3 encompasses all other indirect emissions across the value chain, including those from suppliers, transportation, and product use.
Pharmaceutical manufacturing often has a significant Scope 3 footprint due to complex supply chains. While the DOE’s new ZEB definition primarily focuses on operational (Scope 1 and 2) emissions, it also promotes reductions in Scope 3 emissions by encouraging energy efficiency and the use of renewable energy.
The DOE defines a ZEB by three fundamental criteria:
• Energy efficient: The building must minimize energy consumption through high-performance design and materials, directly reducing Scope 2 emissions and potentially impacting Scope 3 emissions related to energy production.
• No on-site emissions: The building must eliminate all greenhouse gas emissions from its energy use by removing fossil fuel-powered systems and appliances, targeting Scope 1 emissions.
• Clean energy powered: The building must rely entirely on clean energy sources such as solar or wind, further reducing Scope 2 emissions.
While both ZEBs and zero energy buildings aim for sustainability, they are not the same.
ZEBs focus on eliminating operational carbon emissions, while zero energy buildings aim to produce as much energy as they consume over a year, often through on-site renewable sources. A ZEB can be a zero energy building, but not all zero energy buildings are ZEBs.
To determine if a building meets the criteria for zero energy status, building owners can use resources like the EPA’s ENERGY STAR Portfolio Manager tool. This tool helps document and analyze energy performance data. While the DOE’s ZEB program doesn’t offer a specific certification for zero energy buildings, it encourages
the use of Portfolio Manager or other methods that offer comparable or better data analysis. The goal is to show that a building can generate as much energy as it uses annually.
The DOE’s zero emissions building definition is crucial for pharmaceutical facilities for several reasons.
Being in alignment with ESG (environmental, social, and governance) goals is increasingly becoming a key performance indicator for investors and stakeholders. By adopting ZEB standards, companies can show their commitment to sustainability.
Another important benefit is cost savings. Energy-efficient buildings powered by renewable energy can lead to significant reductions in operating costs over the long term.
This combination of sustainability and economic efficiency makes ZEBs an attractive option for pharma companies seeking to reduce their environmental impact while boosting their bottom line. The DOE’s zero emissions building definition is a powerful tool in the fight against climate change. By embracing this standard, pharma facilities can create healthier, more sustainable buildings while contributing to a net-zero future.
Adopting these practices not only helps reduce operational emissions but also sets a benchmark for the entire industry, encouraging broader efforts toward environmental responsibility.
Harry Benson Director of Human Performance Services, CAI
It’s a transformational time for drug development, with radical new therapies and a wider variety of delivery methods coming to the market every day. However, ongoing drug shortages are affecting patients worldwide. Why is this happening, and what can companies do to stabilize drug production?
Pharma stakeholders invest heavily in developing breakthrough drugs and divert experienced staff to new manufacturing lines, often at the expense of meeting production goals for existing drugs. This lack of focus on ‘less exciting’ products can lead to GMP deviations and insufficient quantities of important therapies, including generics.
To address drug shortages, we need career paths in manufacturing that attract talent and promote retention and skill growth. Diverting top talent to drug development or new plant construction leaves current operations understaffed.
Talent shortages leave manufacturing sites without experienced leaders and operators, straining the workforce as non-compliance with FDA standards must be addressed before products can be released. The industry must grow the workforce and streamline operations to tackle this issue.
Fundamental to successful operations is a workforce capable of manufacturing drugs in a controlled, non-variable manner. They must be trained to have
exceptional attention to detail, be able to follow procedures meticulously and understand the processes they’re following well enough to recognize and act on problems.
To facilitate this, pharma must foster a culture of seeking continued education. Junior employees should feel confident, knowing they’ll receive the hands-on training necessary to advance in the workforce. It is essential to build a positive culture where employees feel comfortable speaking up when they don’t understand something. It fosters a hunger in workers to constantly improve, which enhances a manufacturing plant’s output in the long run.
Role clarity in a manufacturing plant means that every project step that requires specific skills and knowledge is assigned to a trained and qualified worker. All workers know who has been assigned each task, and therefore, every worker should understand their role and how it fits into the entire drug manufacturing process. However, a lack of role clarity is a common problem in plants.
Without role clarity, workers may take on too many responsibilities or may be unsure which tasks are most relevant for their roles. Either way, this leads to operational inefficiencies destabilizing manufacturing and supply. A culture of personal responsibility ensures minimal deviations in the manufacturing process that might prevent a drug product from reaching patients.

You don’t solve operational problems in a conference room. Minimizing silos between the C-suite and facility operations is critical to preventing manufacturing bottlenecks.
The C-suite naturally focuses on new development projects, but plant operations benefit when senior leaders engage with workers on the floor, addressing issues early to prevent delays. If leaders stay removed, teams may feel marginalized, workers may be shifted to development, and deviations can backlog, awaiting investigation.
Remember, feedback is a gift: it teaches us how to improve. In pharma, deviations are feedback about processes, but they’ve always been analyzed manually. Younger workers bring training in AI and advanced data systems, enabling the industry to gain deeper insight into why deviations occur and how to address them effectively.
Companies can mitigate drug shortages by dedicating enough resources to plants, people, and processes to consistently manufacture high-quality drugs. This requires prioritizing skill advancement, clarifying roles, and tracking inefficiencies to address them effectively.
Addressing shortages increases access to critical medications, helping patients receive the treatments they rely on to improve their quality of life.
Don Lazas MD, ObjectiveHealth

The age of precision medicine is upon us, with significant growth expected in the coming years as more physicians begin customizing treatments based on patient-specific genetic, lifestyle and environmental factors.
For pharma manufacturers, precision medicine represents a major opportunity to expand their market reach with specialized drugs and therapies that target populations of patients with specific genetic profiles. A flurry of new therapeutics, biologics and treatment options are already being introduced to treat cancer, neurological disorders, metabolic diseases and numerous other conditions.
Over the past several years, precision medicine has led to an explosion in clinical research as advanced biomarker diagnostics narrow the scope of each trial. But the clinical trial industry is increasingly struggling to keep up with this increase in demand, due in part to an aging population of principal investigators, who oversee all aspects of trials.
Yet, an emerging research model could present a solution that enables clinical research providers to continue keeping up with the output of pharma manufacturers. It expands clinical research from its traditional settings into more locations than ever before, while leveraging a previously untapped population of physicians who can fill the crucial role of principal investigators.
Traditionally, clinical trials have almost universally taken place at academic institutions or independent community research facilities.
In an academic setting, the principal investigator role is handled by physicians as a secondary responsibility, with their primary focus being research and education. In the community setting, clinical research takes place at standalone research centers that aren’t affiliated with medical practices.
Though doctors may have an interest in research, working directly on a trial often seems unrealistic. Managing a trial is too much alongside regular patient-care duties. Clinicians who establish their own research programs typically struggle with continuity, sustainability, and scalability.
But a new model of clinical research helps mitigate these concerns, broadening the scope of clinicians’ existing practices while effortlessly merging with their existing workflows.
The upside for physicians is providing cutting-edge treatments for patients who would otherwise lack access, while expanding services that differentiate their practices as centers of excellence. Doctors can nurture patient confidence by being at the forefront of new drug discovery and emerging technologies for hard-totreat conditions.
The trust in doctor-patient relationships is crucial to this model’s effectiveness. Historically, patient retention in clinical research has been around 70%, driven by factors like losing interest when no improvement is seen or finding trial participation inconvenient.
These challenges can be moderated by bringing clinical research to patients’ familiar medical care. Seeing research as an extension of their physician’s care, patients are more likely to complete trials. This model has achieved completion rates over 90%.
All of health care is becoming a research engine, with enrollment currently in progress for some 450,000 clinical trials. As the number of active investigators decreases, a new model of partnership between community practices and clinical research providers can benefit pharmaceutical manufacturers by providing a new source of qualified physicians to ensure that trials are run safely and meticulously. In addition, broadening into new community settings can deliver a level of patient diversity that is underrepresented in current research trials.
With clinical research becoming more critical than ever for continued medical progress, all of these factors combine to make innovative integrated research models a highly promising avenue for the future of medicine.

Compact pressure sensors and switches with 360° custom-color status display

www.vega.com/vegabar
Individually selectable: 256 colors
Measurement in progress
Sensor switching
Process malfunction