
CLINICAL INTELLIGENCE FOR THE HEALTHCARE ECOSYSTEM MAY 2024 | VOL. 48, NO. 5 Laboratory Supply Strategies ...6 Reusable vs. Single-Use ... 14 Surgical Device Pre-Treatment ... 18 Recycling, Sustainability ... 28 WWW.HPNONLINE.COM CRITICAL CARE Operations Worth Watching ... 10

VISIT US AT APIC 24 #917

CLEAN CONFIDENTLY with The Next Generation of Contamination Monitoring Technology
• SMART HANDHELD MOBILE PLATFORM
• CLOUD-BASED
• WI-FI CONNECTIVITY
• INFINITE USERS & TEST POINTS
• CUSTOMIZABLE DASHBOARD
• RFID READER/BARCODE READER
• USER FRIENDLY INTERFACE

PREPZYME FOREVER WET
Enzymatic pretreatment humectant spray designed to keep instruments and rigid scopes moist for up to 72 hours.
• EARN CE CREDITS
• VIEW PRODUCT DEMONSTRATIONS

ENDOZIME TRIPLE PLUS WITH APA
Multi-tiered enzymatic detergent with advanced proteolytic action & rust inhibitors for the effective decontamination of surgical instruments and endoscopes

• PICK UP GENEROUS FREE SAMPLES
• RECEIVE A LIMITED-EDITION GIFT



• www.ruhof.com
(800)
1
537-8463
AD-077 Rev0 013124



2 | May 2024 | Healthcare Purchasing News Sourcing & Logistics 6 > Improving Lab Supply Strategies Requires Better Communication JANETTE WIDER 32 > AI and Healthcare: Matchmaking and Translation KAREN CONWAY Surgical/Critical Care 10 > This Critical Care Operation is Worth Watching JANETTE WIDER Infection Prevention 14 > Reprocessing Single-Use Devices Helps Orgs Achieve Cost Savings, Sustainability JANETTE WIDER Sterile Processing 18 > Tips for Point of Use (POU) Pre-Treatment Success KARA NADEAU 22 > From Novel to Established, a Journey of VHP Sterilization ARTHUR HENDERSON 26 > Qualities of Strong, Effective, QualityFocused SPD Leaders DAVID TAYLOR 27 > Bone Cement and Black Light Detection - Part 2 STEPHEN M. KOVACH Special Report 28 > The Way Forward for Sustainability in Healthcare MATT MACKENZIE Departments 4 > Good Things Come to Those Who Wait 5 > What’s on the Web, Advertiser Index 10 6 18 14 28 CONTENTS > MAY 2024

Good Things Come to Those Who Wait
BY JANETTE WIDER, EDITOR-IN-CHIEF
This month we covered a “critical care operation worth watching.”
When I’m not wearing my Editor-inChief hat for Healthcare Purchasing News, you will often find me watching a television series or a movie. And, of course, in true modern-day fashion, I’m obsessively looking up information on the next season or what other projects the actors are working on now.
Sometimes, I feel impatient waiting for certain media to drop (I’m looking at you, James Cameron and the Avatar films). Nevertheless, I’m always looking for updates online about the next big movie from certain actors or if a series I love has been renewed.
In my role at HPN, I’m doing something similar. I’m keeping tabs on different departments at hospitals across the country via press releases, articles, podcasts, and videos to see what new and innovative things they are implementing. Yet, in healthcare, I tend to be more patient.
On page 10, Columbus, Ga.-based Piedmont Columbus Regional is featured in a Q&A with HPN about its expansion plans for its ICU and the impact it will have on the community. Founded in 1836, Piedmont Columbus Regional is a healthcare provider in west Georgia serving the Columbus, Ga. area with a network of health and

medical services through its two hospital campuses. Piedmont Columbus Regional joined the Piedmont system on March 1, 2018.
During COVID, it became apparent to leaders at the organization that more space was needed, and they took action. The project will be completed in two years’ time and will now be able to provide the community with the most up-to-date ICU environment.
HPN plans to feature more stories like this one moving forward so our readership can learn from their peers and see what is happening in hospitals across the U.S.
We have one more exciting an nounce ment for the brand this month. HPN is launching its podcast, Healthcare Hodgepodge, later this month. The podcast will feature interviews with industry experts and article reads across our many verticals. Hosts of the podcast will include Janette Wider, Editor-in-Chief; Matt MacKenzie, Associate Editor; and Chris Driscoll, Publisher. Healthcare Hodgepodge will be available on your favorite podcasting app—subscribe today so you don’t miss the first episode!
Finally, if your department is doing something that is “worth watching,” reach out to me at jwider@hpnonline. com or connect with me on LinkedIn.
EDITOR’S NOTE 4 | May 2024 | Healthcare Purchasing News Connect with us: facebook.com/hpnonline twitter.com/hpn_online linkedin.com/company/healthcare-purchasing-news/ Group Publisher Chris Driscoll cdriscoll@endeavorb2b.com | 978-880-8345 Editor-in-Chief Janette Wider jwider@hpnonline.com Associate Editor Matt MacKenzie mmackenzie@endeavorb2b.com Senior Contributing Editor Kara Nadeau knadeau@hpnonline.com Advertising Sales East & West Coast Kristen Hoffman khoffman@endeavorb2b.com | 603-891-9122 Midwest & Central April Bruffy abruffy@hpnonline.com | 713-992-0381 Strategic Accounts Sales Chris Driscoll cdriscoll@endeavorb2b.com | 978-880-8345 Advertising & Art Production Production Manager | Ed Bartlett Art Director | Tracy Arendt Advertising Services Karen Runion | krunion@endeavorb2b.com Audience Development Laura Moulton | lmoulton@endeavorb2b.com Endeavor Business Media, LLC CEO Chris Ferrell | President June Griffin COO Patrick Rains CRO Paul Andrews Chief Digital Officer Jacquie Niemiec Chief Administrative & Legal Officer Tracy Kane EVP Medical & Healthcare Technology Kylie Hirko EVP Endeavor Business Intelligence Paul Mattioli Healthcare Purchasing News USPS Permit 362710, ISSN 1098-3716 print, ISSN 2771-6716 online is published 11 times annually with an additional issue in November - Jan, Feb, Mar, Apr, June, Jun, Jul, Aug, Sep, Oct, Nov, Nov IBG, by Endeavor Business Media, LLC. 201 N Main St 5th Floor, Fort Atkinson, WI 53538. Periodicals postage paid at Fort Atkinson, WI, and additional mailing offices. POSTMASTER: Send address changes to Healthcare Purchasing News, PO Box 3257, Northbrook, IL 60065-3257. SUBSCRIPTIONS: Publisher reserves the right to reject non-qualified subscriptions. Subscription prices: U.S. $160.00 per year; Canada/Mexico $193.75 per year; All other countries $276.25 per year. All subscriptions are payable in U.S. funds. Send subscription inquiries to Healthcare Purchasing News, PO Box 3257, Northbrook, IL 60065-3257. Customer service can be reached toll-free at 877-382-9187 or at HPN@omeda.com for magazine subscription assistance or questions. Printed in the USA. Copyright 2024 Endeavor Business Media, LLC. All rights reserved. No part of this publication June be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopies, recordings, or any information storage or retrieval system without permission from the publisher. Endeavor Business Media, LLC does not assume and hereby disclaims any liability to any person or company for any loss or damage caused by errors or omissions in the material herein, regardless of whether such errors result from negligence, accident, or any other cause whatsoever. The views and opinions in the articles herein are not to be taken as official expressions of the publishers, unless so stated. The publishers do not warrant either expressly or by implication, the factual accuracy of the articles herein, nor do they so warrant any views or opinions by the authors of said articles.
Jimmy Chung, MD, MBA, FACS, FABQAURP, CMRP, Chief Medical Officer, Advantus Health Partners and Bon Secours Mercy Health, Cincinnati, OH
Joe Colonna, Chief Supply Chain and Project Management Officer, Piedmont Healthcare, Atlanta, GA; Karen Conway, Vice President, Healthcare Value, GHX, Louisville, CO
Dee Donatelli, RN, BSN, MBA, Senior Director Spend symplr and Principal Dee Donatelli Consulting LLC, Austin, TX
J. Hudson Garrett Jr., PhD, FNAP, FSHEA, FIDSA, Adjunct Assistant Professor of Medicine, Infectious Diseases, University of Louisville School of Medicine
Melanie Miller, RN, CVAHP, CNOR, CSPDM, Value Analysis Consultant, Healthcare Value Management Experts Inc. (HVME) Los Angeles, CA
Dennis Orthman, Consulting, Braintree, MA
Janet Pate, Nurse Consultant and Educator, Ruhof Corp.
Richard Perrin, CEO, Active Innovations LLC, Annapolis, MD
Jean Sargent, CMRP, FAHRMM, FCS, Principal, Sargent Healthcare Strategies, Port Charlotte, FL
Richard W. Schule, MBA, BS, FAST, CST, FCS, CRCST, CHMMC, CIS, CHL, AGTS, Senior Director Enterprise Reprocessing, Cleveland Clinic, Cleveland, OH
Barbara Strain, MA, CVAHP, Principal, Barbara Strain Consulting LLC, Charlottesville, VA
Deborah Petretich
Templeton, RPh, MHA,Chief Administrative Officer (Ret.), System Support Services, Geisinger Health, Danville, PA
Ray Taurasi, Principal, Healthcare CS Solutions, Washington, DC


What’s on the Web
Human Case of H5N1 Bird Flu Reported in Texas
A person in Texas has tested positive for highly pathogenic avian influenza (HPAI) A(H5N1) virus (“H5N1 bird flu”) a er exposure to dairy ca le in Texas presumably infected with the viruses. CDC continues to consider the human health risk assessment for H5N1 bird flu in the U.S. general public to be low, but they urge that people with “close or prolonged, unprotected exposures to infected birds or other animals (including livestock), or to environments contaminated by infected birds or other animals, are at greater risk of infection.”
Read on: hpnonline.com/55001503

HHS Publishes White Paper on Supply Chain
The U.S. Department of Health and Human Services (HHS) announced via a press release that it has released a white paper highlighting steps HHS has taken to prevent and mitigate drug shortages and proposing additional solutions for policymakers to consider. Drug shortages have occurred in the nation’s healthcare system for several decades, largely due to market failures and misaligned incentives. The paper suggests solutions and says that HHS is prepared to work with Congress on this issue so patients do not go without needed meds.
Read on: hpnonline.com/53072187
Coming Soon: HPN’s New Podcast, Healthcare Hodgepodge
Healthcare Purchasing News is pleased to announce the launch of Healthcare Hodgepodge, a podcast featuring interviews with industry experts and article reads of our topical content across HPN’s many verticals including supply chain, infection prevention, surgical/critical care, sterile processing, healthcare IT and more. Healthcare Hodgepodge will be available on your favorite podcasting app in late May.


hpnonline.com | May 2024 | 5
Editorial Advisory Board 679764821 © MARIA_LH | STOCK.ADOBE.COM
This index is provided as a service. The publisher does not assume liability for errors or omissions. Index of Advertisers Aesculap ................................. 17 aesculapusa.com/aicon Cygnus Medical 13 cygnusmedical.com Dale Medical Products C4 dalemed.com/samples DETECTO 11 detecto.com Healthmark Industries ....... C3 hmark.com Medtrica 3 medtrica.com Ruhof Corporation C2 ruhof.com Uline 9 uline.com
Improving Lab Supply Strategies Requires Better Communication
BY JANETTE WIDER, EDITOR-IN-CHIEF
Laboratory services are imperative to the healthcare ecosystem. Laboratory procedures aid clinicians in diagnosing, managing, and treating patients—yet when an organization does not have successful laboratory supply chain strategies, patient care can become delayed, lost revenue can occur, and there can be extra burdens and stress on staff to keep up with demand.
Healthcare Purchasing News spoke with two industry experts in this space. Kevin Dunwoody, vice president laboratory national field sales, Medline, and Barbara Strain, MA, SM (ASCP), CVAHP, principal, Barbara Strain
Consulting LLC, formerly director of Value Management at University of Virginia Health System, and current member of HPN ’s Editorial Advisory Board.
When asked how lab supply strategies differ from other supply strategies, Dunwoody said, “For maximum lab efficiency and effectiveness, lab supply strategies should closely mirror other service line supply strategies in healthcare settings. Healthcare settings—including clinical labs— have processes and protocols that are very well defined. Lab team members know exactly what they need to run tests and which lab supplies need to be readily available. Most lab supply strategies could—and should—align with the strategies for everything purchased in volume

SOURCING + LOGISTICS 6 | May 2024 | Healthcare Purchasing News
throughout that hospital or healthcare system—whether it’s office supplies, maintenance repair and operations (MRO), or IT.”
He continued, “Minimally, healthcare and lab supply strategies should:
• Effectively standardize your product list to the degree possible
• Rationalize and consolidate a SKU list to decrease variability


• Deliver the right mix and quantity of products to match your demand profile and available storage
• Make shipments as full and infrequent as possible to minimize workflow disruption.”
Further, Dunwoody added, “At the end of the day, while accounting for the variety of required hazardous or temperature-controlled product storage solutions that address specific lab product attributes, there is not much justification for running a clinical lab supply chain that is not in sync with other supply chains in a hospital or healthcare setting.”
“Hospitals and health systems operate more efficiently and effectively when they align lab supply chain strategies
with other supply strategies within their hospitals or networks,” he said.
Dunwoody also commented on the biggest concern today when it comes to lab supplies. He said, “One of the biggest concerns for labs today is that many equipment and instrumentation manufacturers are requiring specific consumables, reagents, chemicals, etc. brands to be used with their products without demonstrating significant improvements to outcomes. This makes it more challenging for health systems and their labs to realize available efficiencies, particularly across service lines.”
“Labs are also in a unique and sometimes challenging position as they often cannot use a sub or move to another item if presented with a national backorder issue,” he continued. “Unlike medsurg, commodities and consumables where a robust auto sub program can help end users navigate through supply chain issues and backorders, labs often need to validate and update certain SOPs when looking at a new item. This only enforces the need for and importance of upstream data and proactive communication on inventory and supplies to the lab buyers and end users. Working
hpnonline.com | May 2024 | 7
Kevin Dunwoody
259896072 © GORODENKOFF | STOCK.ADOBE.COM, 55363932 © ARROW | DREAMSTIME.COM
Barbara Strain
together to plan and prepare for possible supply chain disruptions through data and communication will allow lab professionals more time to focus more on patient care and less on inventory management.”
Value analysis and the lab
When asked about the relationship between the laboratory and value analysis teams, Strain said, “It’s an interesting relationship.” She went on to explain that there are meetings where key members of the laboratory gather and speak, for example, about new methodologies. The team generally equates this to value analysis—looking at what the value of continuing a current methodology is or adopting a new one. Then, Strain said, it stops short of all the rest of the value.
Strain added, “What can help is when there is a topdown approach, where it becomes a strategic initiative in a hospital organization to look at how the best value can be provided to patients as well as to the organization. Then it becomes more evident and easier in a way that everyone is on board, whether they are in the laboratory, radiology, imaging, or nursing so that there is a process, and that is where value analysis, along with leaders, develops programs.”








“And then these teams are formed,” Strain commented. “You have to have folks that understand enough about the laboratory or any other area in a way that makes it meaningful to everyone. Right now, I think there’s a little bit more of a trend that there are value analysis teams for the laboratory.”
Current challenges
As for the biggest challenges for those responsible for lab supplies, Medline’s Dunwoody stated, “One of the biggest challenges is that healthcare space is at a premium. You walk into most labs and they are jammed with people, products, samples moving through. Often, you won’t see cohesive storage strategy. Hospitals and health systems are looking for ways to consolidate and store products being used for the variety of lab work—chemistry, biology, pathology, etc.—without negatively impacting the workflow or the samples coming through the space. Lab leaders are constantly looking to more effectively manage consumption of lab supplies in the given space.”
“Ideally, labs should be able to map out the areas where items are being used and disposed of along with best places to store those items to optimize workflow,” he added. “But that’s easier said than done.”
As for a possible solution, Dunwoody said, “Whether it’s creating more or re-engineering lab testing space, find costsavings or improving processes, bring in your lab teams and other stakeholders to identify what needs to be addressed and make a plan to act. Consult with distributors or business partners who have expertise in healthcare supply chain optimization, like Medline, who are committed to solving your bigger supply chain challenges with you, and can provide insight into how other clinical labs or healthcare systems have overcome similar challenges.”
Strain also added her two cents on current challenges. She said, “They [the lab] still won’t get what they need when they need it.”
Strain went on to say that there are often communication issues. For example, the lab often needs certain items—like test trips or reagents—by a certain time and things just don’t show up. So much depends on the laboratory; for example, if they don’t get a result out to someone in the emergency room or the operating room in a timely manner, it can mean the difference between the patient being helped effectively and the patient getting worse, or even dying.
Strain then commented on the importance of value analysis teams in regard to the laboratory when it comes to solving challenges. She said, “Value analysis listens to managers or leaders from, say, immunology or chemistry or hematology, and what they’re really planning, and then we help to look for their GPO contracts. What issues have
SOURCING + LOGISTICS 8 | May 2024 | Healthcare Purchasing News
eNews Print Digital Edition Industry-leading coverage of Supply Chain, Infection Prevention, Surgical/Critical Care and Sterile Processing products, services and practices SUBSCRIBE hpnonline.com/hpn-subscribe 2401HPN_HouseAd_Subscribe_14sq.indd 1 12/1/23 3:12 PM
you had with your current supplier? Can we help intervene there and figure out what we can negotiate to help make that better?”
Speaking from personal experience, Strain added, “And we actually then brought different divisions from the same manufacturer together that were siloed in different lab divisions so that they understood that we could work together for a common contract rather than all these individuals with all these different sort of rules and guidelines and things that they wanted to do. We were very helpful in bringing a lot of different people together, including end users of the lab services as well as the manufacturers that were providing them and other parts of the lab and some physicians on some cases and things like that to talk through. We’d understand what was happening with results and what was happening in the lab and how we could come together.”
“So, there’s some very good synergies for the laboratory with value analysis,” she asserted. “On the other hand, on the supply chain side, they would come in very early on with some inventory solutions and so labs can have full lean, exercises and assessments done and [learn] how to organize their supply rooms and how to do other types of inventory systems to make it easier. I remember going into one lab’s storeroom where they had things and we also had to understand what the Joint Commission and DNV and other inspection agencies’ guidelines were about putting boxes directly on the floor and things like that. So, we would get different types of shelving and different things for them so they could all be in compliance. It’s a very synergistic relationship when you put it all together.”
AI and the future
Medline’s Dunwoody then commented on what seems to be today’s million-dollar question in every sector of the healthcare industry right now: What is the deal with artificial intelligence (AI)?
“Our customers are definitely asking about AI solutions in lab supply chain to help identify and remove nonvalue added activities from lab processes,” he stated. “Currently, the more interesting lab AI solutions work with camera-based inputs and electronic IDs in storage areas. But, as with the rest of the healthcare industry, there are hurdles around patient privacy and security that are curbing the advancement of AI in lab supply chain at this time.”
And as for the future of this space, Dunwoody said, “Technology advancements and further investments into automation will have a big impact on the future landscape of clinical labs. Staffing shortages coupled with increased testing demands due to an aging population will continue to cause issues with time management for
lab professionals. Automation and new advanced testing platforms will not only allow labs to become more efficient and streamline a lot of their processes, but it also presents exciting opportunities in the form of early detection and better care of their patient population. 70% of all medical decisions are made and based on testing done within a clinical lab setting. This means investing not only in new technology but also people and future education and awareness on the importance of a lab professional is crucial and extremely impactful to the future of healthcare.”
“In addition, real estate in health systems and hospitals is becoming too valuable to do non-revenue generating, non-value added activities,” he added. “Every inch of a hospital or health system is being used to provide better service to patients, improve patient outcomes, and operate more effectively and efficiently.”
Dunwoody concluded by saying that, “More health systems are taking these non-value added services out of expensive health system real estate and are establishing multi-use central service centers designed to provide support operations and functions. More health systems will be using or operating consolidated service centers in 5-10 years.” HPN



hpnonline.com | May 2024 | 9 1-800-295-5510 uline.com ORDER BY 6 PM FOR SAME DAY SHIPPING π OUR SELECTION STACKS UP 2405HPN_Uline.indd 1 3/28/24 1:48 PM
This Critical Care Operation is Worth Watching
BY JANETTE WIDER, EDITOR-IN-CHIEF
Founded in 1836, Piedmont Columbus Regional is a healthcare provider in west Georgia serving the Columbus, Ga. area with a network of health and medical services through its two hospital campuses. Piedmont Columbus Regional joined the Piedmont system on March 1, 2018.
Located in Columbus, Georgia and serving patients from 21 surrounding counties, the Midtown Campus is a 583-bed tertiary care hospital providing an array of services to meet the
medical needs of the diverse community, including oncology services at the John B. Amos Cancer Center and the Family Medicine Residency Program at Midtown which was the first of its kind in Georgia in 1972 and is one of the first in the southeastern United States.
According to a March 14, 2023, press release, Piedmont Columbus Regional announced its Midtown Campus will undergo a renovation and expansion over the next two years to increase its ICU capacity.
The press release states that “The 99,038 square foot project, which was designed by Earl Swensson Associates and is being built by Brasfield & Gorrie, will include 43 new ICU beds along with a renovated 15-bed nursing unit. Construction is tentatively set to be completed in March 2026.”
Healthcare Purchasing News had the opportunity to speak with chief medical officer Christopher Edwards, M.D., critical care director Cindy Kinney, and Piedmont Fayette CEO Steve Porter about this massive project,
SURGICAL/CRITICAL CARE 30517931 © DANIL RUDENKO | DREAMSTIME.COM 91156500 © MARTIN BARRAUD | GETTYIMAGES.COM 10 May 2024 Healthcare Purchasing News
QUALITY WHERE CHOICE MEETS
DETECTO manufactures more unique models for each clinical product category than any other medical scale company. Giving you better choices means you never have to settle for a product that isn’t the right fit for your application.
DETECTO: A true vertically-integrated medical scale manufacturer – family owned and privately held – with factory and headquarters in Webb City, MO.


DETECTO | 102 East Daugherty St. | Webb City, MO 64870 | (800) 641-2008 | detecto@cardet.com | www.Detecto.com
MORE
The Best Choice is the One That Gives You
Choices
LEARN MORE

other initiatives, and the future of Piedmont Columbus Regional.
Are there any current initiatives (other than the expansion) in critical care that Piedmont Columbus Regional is working on?
Edwards: In Columbus, we are working on our new free standing pediatric facility, the Bill and Olivia Amos Children’s Hospital. This will include pediatric critical care beds staffed by pediatric intensive care specialist physicians.
In general, what are the biggest challenges in the critical care space? How are organizations solving these challenges?
Edwards : As in all areas of healthcare, there are challenges with staffi ng. Critical care requires specialized training for physicians, nurses, and other levels of staff
as the patients are the sickest of the sick with complicated medical problems. Fortunately, we are rebounding from the critical staffing challenges that were the result of the COVID pandemic.
Regarding the expansion, can you please share with us how this project came to be? I see there was a need to increase ICU capacity. Can you expand on that?
Edwards: It became apparent during COVID that we needed more ICU space in order to manage surges during crises like the pandemic. But even during “normal” times our ICU at Piedmont Columbus Regional Midtown is typically full, and at times we are holding ICU patients in the Emergency Department. We needed to do what we could to eliminate the need to hold our admitted
critical care patients in the ED. We feel this expansion of ICU beds will accomplish this goal.
I see there will be a renovation as well as an expansion. What is being renovated?
Edwards: The entire ICU will be renovated; we are not just adding beds. At the end of the project, every ICU room will have been increased in size and completely redone. From a size standpoint, each room will be 2.5 times larger than the current rooms which will allow for a better space for patients, family, and staff. In addition, we are renovating and adding additional medical/surgical beds.
How will this new project benefit the community?


Edwards: The project will ensure that our community will have the most up-to-date ICU environment in the event of a critical care need. And by adding beds, we are helping to ensure we have the capacity to provide critical care locally at all times.
Are you using any new technologies in this space right now?
Kinney : The Real-Time Locating System (RTLS) is one of our newest technologies. Because our ICUs are currently located on three different floors, fi nding ICU equipment can be a challenge. The RTLS system allows us to tag our ICU devices and track
SURGICAL/CRITICAL CARE
12 | May 2024 | Healthcare Purchasing News
Headwall Footwall
PCR Midtown ICU
their locations using the RTLS webbased software. It’s a quick and efficient way to locate equipment.
What is in store for Piedmont Columbus Regional over the next 5-10 years?
Edwards: We are focused on patient safety and quality of care. If we provide the highest quality and safest care, our system will continue to thrive. Our goal is zero harm by 2026. We also want to provide the best access and patient experience for our communities. So for the next 5 to 10 years, we will focus on continuous improvement in these areas.
Anything else you’d like to share about critical care in your organization/the renovation and expansion?
Kinney : Because the physical design of the unit heavily impacts staff workflow, accessibility to equipment and supplies, and communication practices, feedback was sought from members of the ICU team early in the construction process. Their involvement helped to ensure that highly frequented areas like the storage, medication, and supply rooms were appropriately located and that telephones, computers, and the telemetry monitoring stations could be easily accessed by the staff. In the patient rooms, they provided input on the bed-ventilator-IV pump locations, configuration of the headwall, room lighting, and the type of patient lift system that would fit our needs. Our goal was to provide our staff and patients with the best ICU environment possible.
I recently saw that the American Association of Critical-Care Nurses recognized the Critical Care Unit at Piedmont Fayette with the silver-level Beacon Award for Excellence. Any comments on this achievement?


Porter : Earning the silver level Beacon Award for the second time demonstrates Piedmont’s commitment to consistently providing quality care. Our patients and our community can be confident in our critical care teams when they are needed the most. HPN

 Cindy Kinney Christopher Edwards
Cindy Kinney Christopher Edwards
Reprocessing Single-Use Devices Helps Orgs Achieve Cost Savings, Sustainability
BY JANETTE WIDER, EDITOR-IN-CHIEF
When costs are top of mind for every department in a hospital, the choice of single-use devices or reusable devices is an important one. Healthcare professionals today are not only understaffed but under constant pressure to keep costs low. Reprocessed single-use medical devices have been around for quite some time and many professionals
are taking a closer look at what their organization is doing in terms of reprocessing single-use devices. Not only can reprocessing single-use devices be cost effective, but it has environmental benefits as well.
Editor’s Note: Our special feature this month is about sustainability in the healthcare system, see page 28.
According to an article from APIC, “Increasing healthcare costs,
environmental concerns, and recognition of finite resources have prompted healthcare facilities to consider reprocessing devices labeled as single-use devices. At the same time, concerns over the safety of reprocessed single-use devices led the U.S. Food and Drug Administration to develop and publish regulations for this practice. Under these regulations, reprocessors, hospital-based or third party, must

INFECTION PREVENTION 14 | May 2024 | Healthcare Purchasing News 196942895 © PORAMATE CHEEWAPAT, 154742208 © POP NUKOONRAT | DREAMSTIME.COM
meet the same standards as the original manufacturer.”
The article adds that “For several decades, hospitals and private healthcare establishments have reprocessed various single-use devices (SUDs). The practice of using SUDs was promoted as labor-saving and cost-efficient. By 1982, at least two-thirds of all sterile devices used carried a label saying, ‘for single use only.’”
Further, “Concerns about healthcare costs, changes in the reimbursement system, and concerns about the environmental impact of disposing of these devices prompted healthcare facilities to evaluate the feasibility of reusing such devices. The U.S. Public Health Service approval of the practice of reusing hemodialysis filters led the way for current activity.”
“Manufacturers choose whether or not to label a device ‘single use,’ and they are not required to provide evidence to support their designation,” the article added. “Many manufacturers attach the single-use label to devices they previously marketed as reusable or currently market them as reusable outside the United States. At the same time, the U.S. Food and Drug Administration’s (FDA) Medical Device Reporting system has documented few adverse events associated with the reuse of SUDs.”
Healthcare Purchasing News had the opportunity to speak with Maureen Spencer, M.Ed, BSN, RN, CIC, FAPIC, infection preventionist consultant and Lars Thording, VP, marketing & public affairs, Innovative Health about their views on single use vs. reusable devices.
Regarding her background, Spencer says, “So my consulting experience is really more with companies. One of the things we focus on is bringing new vendors into infection prevention (IP) to try to help them navigate the world of IP and some things we’ve found useful to navigate that world.”


Instructions for use
As for best practices and guidelines, she said, “APIC has some guidelines and then of course we have the instructions for use (IFU) from the manufacturers when they’re going to label it single use, like harmonic scalpels or disposable blood pressure cuffs. Additionally, they’re now pushing endoscopy equipment that’s disposable but it’s very expensive.”
Spencer added, “We follow the IFUs, and some people are very strict about it. They don’t want any legal responsibility. Some hospitals say if it says disposable, single use then that is what we do. Then other hospitals have decided to look at various equipment from these companies that offer sustainability systems and have had success in reprocessing certain equipment like harmonic scalpels. The problem is they’re pushing disposable, like in the endoscopy area, because they can make a lot of money and they’re saying, ‘Well, then we won’t have these outbreaks.’”
She comments that, on endoscopes, there is data available on how to clean out, for example, crevices where rubber starts to crack after a period of time. “I would push for something like that rather than disposable endoscopes, because I think it is just too cost prohibitive for companies,” she added.
Going green
What about the environmental impact of reusing instruments or devices? Thording said, “There is a lot of interest in hospitals in becoming greener, but unfortunately road
maps and handbooks are few and far between. A couple of government agencies recently have tried to launch some toolboxes, but I would say in general, frankly, it’s been very hard for hospitals to adopt things like environmentally preferred purchasing.”
Thording added, “Meaning that in our consideration for what products to purchase and use in the hospital, we will favor products that are environmentally favorable. Because almost all purchasing decisions in healthcare are based on something else—costs. So, that is a major barrier.”
Best practices
“As for best practices in hospitals, I would say number one is creating tools so that everyone from physicians to administrators to technologists are aware of the environmental impact of what the hospital does,” Thording commented. “Transparency is a part of the solution itself. I think to some extent when physicians don’t take the environmentally preferred device, it’s probably because they’re not aware.”
Thording noted that best practice number two is to make environmental considerations a part of the purchasing decision making. And that’s something that easily fits within the purview of the Value Analysis Committee. The Value Analysis Committee gathers people from many different functional areas to make decisions about what products to adopt.
Thording stated, “Number three is the background. The vast majority of a hospital’s carbon emission footprint does not come from what the hospital does. It comes from what? The hospital buys so that means that most of the carbon emissions are coming from what is called scope 3 emissions. That is from the products and stuff that the hospital buys. So ask your suppliers to provide accounting of carbon emissions footprints of the
hpnonline.com | May 2024 | 15
Maureen Spencer Lars Thording
devices that they’re selling. It’s difficult to do, but it’s becoming more and more normal to be able to provide that kind of accounting and it really should be standard practice to demand that as a supply chain because it is a supply chain that is responsible for most of the waste and most of the carbon emissions.”
“And number four is that when there is a choice between reusable and single use devices to favor the reusable one, but in many cases, the fact is that that choice is not there,” he said. “That type of device is simply just single use or that type of device is not reusable. This not only relates to the infection preventionists, but it relates to OR directors, as well. If it involves surgical instruments like the harmonic scalpels of the trocars, they probably have a surgeon that
does that kind of surgery approve and give their input into it. This does involve teamwork: it involves a group of people together working with the vendor on what the vendor can do, and the process of collecting everything in those green bins to get those back to the loading dock and on the trucks that bring them to this facility. And then they reprocess them and get them back into stock. There are some surgeons when they see it’s a reprocessed harmonic scalpel, for instance, might reject it and say, ‘I don’t want use one that’s reprocessed. I want one that’s brand new.’ Sometimes that’s the influence of a vendor.”
Recycling
Thording also mentions a barrier to reprocessed single-use devices. “Now, when the purchasing department is
willing to look at environmental considerations, they get a conflicting message from leadership of the hospital: You need to reduce your costs and you need to pick the more environmentally friendly option,” he said. “The problem is that in most cases, environmentally friendly solutions are more expensive than the cheapest product in the market. So that’s definitely a barrier.”
Thording concluded by saying “Recycling is a big barrier to reprocessing. I just put the devices over here and somebody takes them, and it gets recycled. But the fact is now it’s out of circulation. So now it can’t be reused, which is expensive for the hospital and recycling usually does not yield really even close to the carbon emission results as reprocessing does.” HPN








INFECTION PREVENTION 16 | May 2024 | Healthcare Purchasing News
WEBSITE hpnonline.com MAGAZINE hpnonline.com/subscribe NEWSLETTERS Sign up for monthly updates FACEBOOK hpnonline HPN_Online LINKEDIN healthcare-purchasing-news Scan Here to Subscribe to our FREE Magazine COMPLIMENTARY MAGAZINE SUBSCRIPTION 12 Issues — Including the Annual Reference and Buyers Guide Reporting the information, solutions and stories about supply chain management 2405HPN_HouseAd_Subscription_12h.indd 1 4/3/24 2:46 PM







AESCULAP Aicon™ Sterile Container System
Unwrap the future of sterile supply management. With features that help streamline processes and reduce the possibility of wet sets, Aesculap’s next generation rigid container is everything you’d expect from the market leader.
Visit aesculapusa.com/aicon to learn more about how this breakthrough technology can help your SPD Operate With Greater Precision.
OPERATE
PRECISION
WITH GREATER
Meet our newest STERILIZATION POWERHOUSE.
Aesculap, Inc. - a B. Braun company M e e t o u r n e w e s t
Tips for Point of Use (POU) Pre-Treatment Success
BY KARA NADEAU, SENIOR CONTRIBUTING EDITOR
It is well known that surgical/device pre-treatment is a point of contention between many sterile processing (SP) and operating room (OR) teams. When post-procedure pre-treatment isn’t performed correctly (or at all), SP teams are stuck with having to remove hardened biological/organic material, adding extra work, prolonging the reprocessing cycle, and jeopardizing patient safety (e.g., retained bioburden, instrument/ device damage).
Healthcare Purchasing News (HPN ) reached out to SP leaders, consultants and solutions vendors to solicit their opinions on why point of use (POU) pre-treatment is such a challenge and proven methods they have employed to improve compliance.
The battle with biofilms
Let’s start by getting back to the basics with biofilm. Preventing the formation of biofilms, defined as “surface-attached communities of
bacteria embedded in an extracellular matrix,” is a relentless battle in healthcare environments with the constant contamination of environment surfaces and objects.1
So-called “build-up biofilm” that forms on reusable medical devices “represents a great challenge for healthcare-associated infection control and prevention,” as noted by the editors of a Hygiene journal special edition on the topic.2 They go on to state:
STERILE PROCESSING 18 | May 2024 | Healthcare Purchasing News 516399284 © VZMAZE | GETTYIMAGES.COM
“Evidence-based strategies and practices to prevent biofilm formation and the chemical fi xation of existing biofilms, as well as to remove biofilms from environmental surfaces and reusable medical devices, are required to minimize pathogen transmission and, therefore, to deliver safer care to the patients.”
Performing cleaning and decontamination as soon as possible after an item has been used – at the point of use - is an evidence-based practice recognized throughout the healthcare industry (CDC, AORN, AAMI, APIC, etc.).
If POU pre-treatment of contaminated instruments and devices in the OR is a proven strategy to help prevent biofi lm formation, why isn’t compliance 100% across the board?
Challenges to compliance
Researchers from the Graduate School of Nursing, Uniformed Services University of the Health Sciences, in Bethesda, Md., conducted a literature review on POU treatment compliance and published their findings in the January 2024 issue of the journal Military Medicine. An interesting aspect of this research was the extrapolation of POU treatment noncompliance implications for Military Health System policies and future considerations.
If poor pre-treatment compliance makes decontamination more difficult when the OR and SPD are housed together in a stable, well-equipped hospital environment, just imagine the impact when compliance failures take place during “large-scale combat operations.”
With studies indicating “that drying times beyond 15 minutes significantly reduce the effect of subsequent cleaning to remove protein residues, including prions,” time is of the essence whether the surgical procedures are taking place in a hospital or on the battlefield.3 As the researchers





stated, “Completing POU treatment is critical to a successful surgical mission in both the hospital and austere environment.”
Based on their literature review, the researchers identified these barriers to POU treatment compliance: “complex POU processes, intricately designed surgical instruments and endoscopes, lack of healthcare worker (HCW) knowledge and competency, and inadequate or ambiguously written policies.” They pointed to “Training, competency assessments, and clearly written policies and procedures” as “cost-effective, evidence-based, and feasible solutions.”4
Turning back to the civilian healthcare delivery environment, contributors to this article commented:
“It can be difficult for any sterile processing department to achieve 100% compliance with all that we are responsible for,” said Randalyn Harreld, CRCST, CIS, CER, CHL, CSPDT, CASSPT, BLS, AAS, clinical education, manager, U.S., Belimed. “The amount of ‘best’ practices that have come into play, the variations in instructions and the entities providing guidelines and standards can be difficult to follow 100% of the time.”
“I believe POU pre-treatment is such an issue because people often will not prioritize a process change until it is mandated or a hospital is written up for noncompliance,” said Ascendco Health Co-Founder & CEO Brian Reed. “And they are getting written up.”
“When I perform audits, point of use treatment (POUT) failure is usually one of the top things that

is a compliance issue,” said David Jagrosse, CRCST, CHL, President, David Jagrosse Consulting. “To be clear, it is in the IFUs and AAMI standards but from a technical standpoint, it is not that we can’t process instruments without pre-treatment having been performed, but rather, it makes it almost impossible and much more challenging and very time-consuming.”
“OR staff may not understand the critical importance of immediate postprocedure pre-treatment of instruments,” said Joan Melendez, president & CEO, Xcelrate UDI. “Failure in this area risks patient safety, damages instruments, and delays processes.”
Explain the “why”
Explaining to the OR why their part in pre-treatment is important and benefits their workflows, and ultimately their patients, is key to gaining support.
“It’s like what Tom Cruise said in Jerry Maguire: ‘Help me help you,’” said Jagrosse. “The OR wants instruments turned over. They need them back for the second, third and fourth cases of the day. If they don’t do this pretreatment, it makes it almost impossible to turn these instruments over in a timely and efficient manner.”
Harreld refers to “obedience, accountability, and the inevitable ‘that’s not my job’ mentality.”
“Sometimes things don’t happen because people don’t want them to happen,” said Harreld. “More times than I’d like to admit I’ve seen many disregard point of use care, stating that it simply isn’t their job, they don’t
hpnonline.com | May 2024 | 19
David Jagrosse
Joan Melendez Brian Reed
Randalyn Harreld
have time, the spray is too much, too tedious, etc.”
“If the team understands ‘why’ something is being asked of them, and not just being asked to do it, usually the compliance rate will go up, and even better ideas can come into play all while increasing morale and outcomes,” she added.
Ongoing education and training are critical to long-term POU pre-treatment compliance success. Melendez recommends that hospitals “Prioritize joint sessions for the OR and SPD staff on correct sterilization techniques, regulatory updates, including FDA guidelines, and ISO 13485 standards. This approach ensures high-quality patient care through adherence to compliance, fostering a culture of excellence and continuous improvement,” she explained.
Understand the challenges
“As Sterile Processing professionals we should consider how we can help the OR reach pre-treatment goals,” said Jagrosse. “For example, one hospital purchased 4 oz spray bottles for pre-treatment application that the sterile processing team supplies on the case carts.”
“Have the right products and workflow that fit the need of your unique inventory,” said Harreld. “Make sure a point of use product process and workflow are set up. Many times, it might not work spraying liquid in the OR when the patient is still there, and no core is available or soiled utility space to complete the job. Maybe your workflow doesn’t allow for this to happen. Make sure we set our teams up for success to make POU pre-treatment mandatory and possible.”
Engage in collaboration
Harreld recommends SP teams partner with all areas responsible for POU pre-treatment and set a game plan together. She stated:
“Create a committee and let infection control be the driver of the safety component while a chief surgeon can also be the facilitator in the OR to help push the task. Implement a policy that addresses the process and include SPD during the education portion of this. Be consistent and hold the line when or if people start to push back.”
“Keep a team approach and mentality during this whole process and make sure to work together, as it’s the collective duty to take part in this important practice,” she added. “The procedure areas, clinics, operating room personnel, infection control reps, and doctors all need to collaborate with sterile processing to make this joint effort realistic and achievable.”
Melendez recommends strengthening communication between SP and OR teams through regular meetings and feedback sessions to facilitate a collaborative approach to address pretreatment issues.
Eddie Conklin, CRCST, CHL empha sized the vital roles of surgical technicians and decontamination technicians and the workflows that link them, stating:
“Being at the point of use, a surgical tech sees each individual instrument being used and knows the extent of bioburden, especially on complex or difficult instruments. Their AST standard addresses this; if the instrument is not precleaned at a minimum it should be separated for the decontam tech. Decontam techs’ workflows are large volume, and they work in batches to cover several ORs.”
“The instruments should be counted, the count sheet validated, and the instruments placed back in their original containers,” Conklin continued. “Extra items, such as peels, should be separated to avoid missing instruments at assembly and to improve first pass yields, quality, and efficiency. Indicators, pan locks, white arrows, tags, and labels should be removed.”
Back it with documentation
As Harreld pointed out, “Almost all recommendations, including guidelines from AST, AAMI and AORN, mention the importance of point of use care, so it is everyone’s responsibility. We need to be setting expectations and holding our teams to them to ensure they understand how it helps the longevity of the instruments and makes the cleaning process safer and more effective.”
Manufacturers’ instructions for use (IFU) are a vital resource for both the OR and SP teams to understand their roles in instrument cleaning and maintenance. Melendez recommends ensuring all OR team members have access to and understand manufacturers’ IFUs.
“Additionally, implement tray worksheets to accompany each instrument tray, serving as a checklist to ensure adherence to SOPs and IFUs,” she added.
Capture and measure compliance
According to Jagrosse, most hospitals will establish key performance indicators (KPI) for POUT. Then the question becomes, how do they set a baseline, measure compliance, and track improvements?
“Some organizations measure POUT compliance in decontamination on paper but departments with instrument tracking systems will have the ability to scan a case cart and scan a barcode to document deviations. Because if you don’t measure it or have a handle on it, it doesn’t exist.”
Address issues in a timely manner
Jagrosse pointed out that access to real-time data on POUT compliance enables timeliness of response, stating:
“Some places will collect the data and wait until the monthly meeting saying, ‘we had 72 instances of
STERILE PROCESSING 20 | May 2024 | Healthcare Purchasing News
non-compliance,’ but that means nothing when speaking in a crowd setting. In other instances, an SPD team will identify a deviation, attempt to address it with the OR team at the end of the day, which is again too late, and they may often answer ‘It wasn’t me, I was relieved for lunch,’ so now there is no ownership of the deviation.”
“There must be accountability and the best way to do that is in real time and not daily or monthly,” Jagrosse continued. “There are tracking systems that allow us to immediately identify deviations and even send text alerts to key leaders. If you get those real time notifications and directly address an issue with the corresponding OR team, then you will be able to engage with the team responsible for the deviation.”
According to Reed, Ascendco Health applies analytics to the
events captured and recommends to the hospital when and where they should perform audits (e.g., on certain trays, staff members, times of day).
“When hospitals are recording events and performing audits, they have seen an over 95% reduction in events,” said Reed. “It’s important to note that the tracking and auditing goes both ways. With our system, the OR team uses a dedicated smart phone or tablet to record events on trays coming from the SPD, such as missing/damaged instruments, holes in sterile wrap, etc. We didn’t invent event recording, but we made it easier to record events as it becomes part of the SPD and OR workflows.”
Keep patient safety as the focus
“The more hospitals push their care teams to attend to more patients and
increase surgical volumes, we must calculate more time for these additional responsibilities to take place and avoid the awful habits of taking shortcuts,” said Harreld. “This comes into play with priority sets and turnovers, inventory issues, etc. OR minutes equals money, but we also have safety to always consider.” HPN
References:
1. Biofilms on medical instruments and surfaces: Do they interfere with instrument reprocessing and surface disinfection, American Journal of Infection Control (AJIC), November 2023, https://www.ajicjournal.org/article/S01966553(23)00314-0/fulltext
2. The Impact of Biofilms on Cleaning, Disinfection of Surfaces and Reprocessing of Reusable Medical Devices, Hygiene, https://www.mdpi.com/journal/hygiene/special_issues/biofilm_cleaning
3. Rubak P, Lorenzen J, Ripadal K, Christensen AE, Aaen D, Nielsen HL, Bundgaard K. Can a humid storage environment of surgical instruments before reprocessing increase patient safety and durability of instruments? J Hosp Infect. 2022 Apr;122:64-71. doi: 10.1016/j.jhin.2022.01.012. Epub 2022 Jan 22. PMID: 35077808.
4. Eberhardt GL, Atwood BI, Smith JD. Point of Use Treatment for Medical Devices: From Bedside to Battlefield. Mil Med. 2024 Jan 9:usad499. doi: 10.1093/milmed/usad499. Epub ahead of print. PMID: 38198220.







hpnonline.com | May 2024 | 21
CONNECT WITH CLINICAL INTELLIGENCE FOR THE HEALTHCARE ECOSYSTEM Like us Follow us Link up eNews hpnonline.com Print 2405HPN_HouseAd_Connect_12h.indd 1 4/3/24 2:43 PM
From Novel to Established, a Journey of VHP Sterilization
BY ARTHUR HENDERSON, RN, SENIOR CLINICAL EDUCATION SPECIALIST, STERIS CORPORATION
For over 30 years, healthcare facilities have relied on vaporized hydrogen peroxide (VHP) to sterilize temperature sensitive medical devices. From its origins as a novel technology, VHP has gained the trust of healthcare, regulatory bodies, and standards organizations. With the release and Food and Drug Administration (FDA) recognition of a low temperature vaporized hydrogen peroxide standard (ISO 22441), the FDA classified vaporized hydrogen peroxide sterilization as an “Established Category A method of sterilization for products labeled sterile.”4 This is good news for manufacturers of sterile products looking to move away from Ethylene oxide (EO) as this puts VHP sterilization on equal terms with EO and steam sterilization processes.
FDA’s classification of VHP sterilization is part of a broader initiative to reduce the use of EO sterilization applications. This change is expected to increase adoption of VHP sterilization over the more environmentally hazardous EO sterilization applications used to sterilize products today. This leaves many healthcare facilities asking, “What does this mean to healthcare’s medical device processing?” The answer, increased adoption of VHP sterilization as a choice for reusable device sterilization.
Evolving to address sterile processing challenges
1993 to 2009 VHP Sterilization focus to replace EO
EO sterilization began as means to sterilize temperature sensitive materials incompatible with steam

sterilization. It has sterilized single use sterile items, reusable medical devices, and non-medical items like spices for many years. However, some of these materials easily absorb EO requiring extensive aeration times to remove the chemical from those materials. Sterilization cycle times ranged between 8 to 12 hours to complete mostly due to aeration needs. The long cycle times created bottle necks and required excess inventory to accommodate sterile processing needs. Additionally, EO’s carcinogenic and mutagenic nature created concerns and stricter regulation of its use. In 1993 the first VHP sterilizer was made available to healthcare facilities with the goal to provide a low temperature alternative to EO sterilization.
At that time, vaporized hydrogen peroxide sterilization was a novel

CONTINUING EDUCATION 22 | 2024 | Healthcare Purchasing News PHOTO COURTESY STERIS Contributed by:
Define what is meant by “established sterilization process”
List key breakthroughs in VHP sterilization development
Pair medical devices with the VHP sterilization cycle type
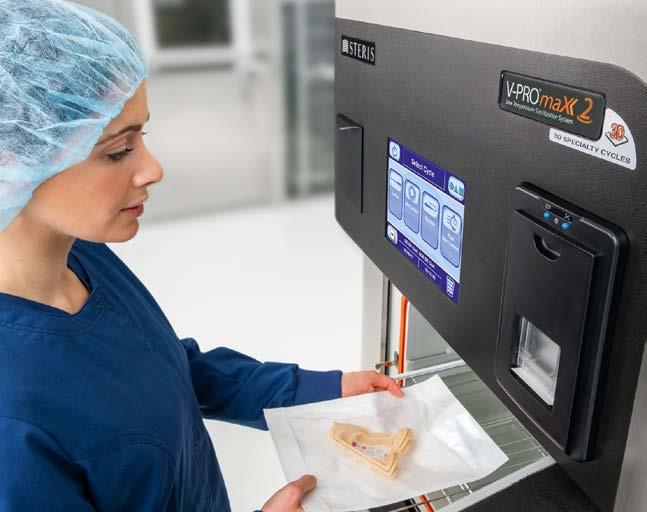
Figure 1: Sterile Processing Technician preparing to sterilize a 3D printed surgical guide
Learning Objectives 1.
2.
3.

sterilization method. It had proven safety and effectiveness but was a new form of sterilization with no established standard to define its use. Its popularity grew as manufacturers of rigid and small flexible endoscopes, batteries, and specialty surgical instrumentation began to include VHP sterilization in their instructions for use. By 2008, VHP sterilization was no longer ‘novel’ but ‘non-traditional’ as FDA had evaluated sterilizer data as part of a quality systems regulation evaluation and determined the methods to be adequate.
2011 to 2015 VHP Sterilization used to preserve device integrity
Medical devices continued to evolve as did their cost, increasing repair and replacement budgets. Facilities sought methods to preserve their medical device investment, one of which was VHP sterilization. Its low temperature process offered a broad range of material compatibility. Combined with a lesser vacuum, as compared with steam sterilization, it preserved the integrity of instrument adhesives and delicate optics. The demand to use VHP sterilization for a wide variety of rigid endoscopes brought a need to sterilize longer stainless-steel channels with smaller diameters.
Batteries previously sterilized through an immediate use steam sterilization cycle to limit exposure to high temperatures were sterilized through a low temperature, terminal VHP sterilization cycle preserving the battery’s life.
VHP sterilization is limited by the total number of channels within a load; the length and diameter of a channel; and the number of channels within a single device. Several new claims came during this time including multiple channel devices and narrow lumens typical of urology and pediatric devices.
2016 to 2018 Fast terminal cycle to help reduce immediateuse steam sterilization
The year 2013 defined the term Immediate-use Steam Sterilization (IUSS) and brought new policies intended to reduce its use. Quick turn processes and loaned equipment vendor policies met some success. However, late loaned trays and back-to-back cases continued. Added delays came from waiting for quick turn steam sterilized items to cool.
VHP sterilization responded to the need for speed by increasing load weights up to fifty pounds. With no need to cool sets after sterilization, sets return to use immediately after cycle completion. VHP sterilization also addressed the need for quick turns of terminally sterilized single items. For the time needed to run an IUSS cycle, VHP sterilization could complete and release a terminally sterilized instrument. By the end of 2018, VHP sterilization was a common sterilization method processing many of the same types of instrumentation as steam sterilization.
2023 First FDA cleared sterilization application for 3D printed medical models
Continued addition of instrumentation claims led to a major advancement outside the category of medical instrumentation. In 2023, a VHP sterilization platform became the first sterilizer with FDA cleared claims to sterilize 3D printed anatomical models and patient specific guides. Anatomical models allow visualization of surgical site anatomy. Patient specific surgical guides help surgeons precisely position cutting instruments for accurate osteotomies or pin and screw placement. Up until this point, facilities would have to develop and validate sterilization cycles for these items. Most healthcare facilities were not equipped to perform sterilization validation, toxic residual analysis,
Lesson:
From Novel to Established, a Journey of VHP Sterilization
April 2024
This lesson was developed by STERIS. Lessons are administered by Endeavor Business Media.
Earn CEUs
After careful study of the lesson, complete the examination online at educationhub.hpnonline.com. You must have a passing score of 80% or higher to receive a certificate of completion.
Certification
The Certification Board for Sterile Processing and Distribution has pre-approved this in-service unit for one (1) contact hour for a period of five (5) years from the date of original publication. Successful completion of the lesson and post-test must be documented by facility management and those records maintained by the individual until recertification is required. DO NOT SEND LESSON OR TEST TO CBSPD. www.cbspd.net


Healthcare Sterile Processing Association, myhspa.org, has pre-approved this in-service for 1.0 Continuing Education Credits for a period of three years, until March 22, 2027.
For more information, direct any questions to Healthcare Purchasing News editor@hpnonline.com.
hpnonline.com | 2024 | 23
Quiz Answers: 1. B, 2. E, 3. A, 4. B, 5. A, 6. D, 7. A, 8. D, 9. C, 10. B

cytotoxicity, and material analyses. Using an FDA cleared cycle saves facilities both time and money while supplying assurance of the sterilization process.
Given the long history of safety and effectiveness coupled with its growth and evolution, it is easy to see why this once novel sterilization process is now seen as established.
Established yet different when sterilizing medical devices
VHP sterilization joined steam and EO as established sterilization platforms, but it is different from them in many ways. The obvious differences include the sterilant, exposure time, and aeration requirements. The not so obvious difference is the determination of which devices to process within the sterilization platform.
Steam and EO sterilizers have defined sterilization cycle parameters that have been validated using a specified load configuration. The load configuration is defined within standards and guidelines and does not include specific instrumentation characteristics. Steam, for example, defines the worst-case load based on the weight of the metal mass included within the sterilizer using wrapped trays and a total load capacity. It does not include testing of lumen lengths, diameters, or instrumentation itself. This requires each device manufacturer to select the steam sterilization cycle best suited for their instruments and validate that the sterilization cycle parameters previously cleared by the FDA for the sterilizer are right for their instrument. The instrument vendor must provide this information to the user as part of the instrument’s instructions for use (IFU). If a steam or EO sterilization cycle is not listed in the IFU, it cannot be used for that device. VHP sterilizers are different. These sterilizers define the instrument characteristics and packaging for each
sterilization cycle as well as the largest load configurations for each cycle. Instruments that have the specified characteristics are tested and validated by the sterilizer manufacturer. Any instrument that falls within the defined instrument characteristics for the sterilization cycle validated by the VHP sterilizer manufacturer can be sterilized within it. The device manufacturer does not have to test and validate the instrument in the VHP sterilizer for a user to be able to use the VHP sterilizer cycle. The user must confirm that the instrument meets the cycle’s requirements and is compatible. The critical instrument characteristics to review include:
• Length and diameter of lumens and channels
• Number of lumens and channels in the instrument
• Compatibility of the instrument’s materials
• The type of sterile packaging
Obtaining this information from the device manufacturer can be challenging. To help, VHP sterilizer manufacturers have worked with device manufacturers to create searchable lists of instrumentation that can be sterilized within their respective sterilizers.
What can be sterilized?
VHP sterilizers provide sterile processing departments flexibility when managing workloads and workflow. Requiring only electricity and sterilant, VHP sterilizers can serve as a backup sterilization process when steam sterilizers go down such as during a boil alert, flood, or other emergency event. Understanding what can be sterilized within a VHP application is critical when planning for general workflow and emergency situations. VHP sterilizers have various sterilization cycles based upon the types of devices. The cycles and cycle claims will vary between sterilizer manufacturers. Refer to your sterilizer
operator’s manual for the types of devices that can be sterilized in any given cycle.
The general instrumentation cycle is the first type of cycle. This cycle sterilizes instrumentation with diffusion restricted spaces, such as hinges and box locks but not with lumens. “Non Lumen” and “Express” cycle are common names. Instrumentation can be composed of a variety of materials compatible with VHP. Forceps, light cords, cameras, and da Vinci endoscopes are some of the devices that are sterilized in general cycles.
The second cycle type sterilizes instruments with stainless steel lumens. This cycle type has a wide variety of names including “Lumen” and “Standard” cycle. The length and diameter of the lumen is important and not every cycle can sterilize the same lumens. These cycles typically allow mix loads of lumened and non-lumened instrumentation. Some sterilizer cycles are FDA cleared to sterilize stainless steel instruments that have two or three channels. Typical instrumentation includes rigid cystoscopes, orthopedic drills, trocar sheaths and some eye instrumentation.
Flexible endoscope cycles are the third type of sterilization cycle. As the name suggests, these cycles sterilize a variety flexible endoscopes. Typically cycle names include “Flexible” and “Flex” cycle. The lumen lengths within endoscopes play an important part in deciding if an endoscope can be sterilized within a cycle. Typical flexible endoscopes include bronchoscopes and surgical flexible endoscopes. Large flexible endoscopes, such as choledochoscopes and colonoscopes, contain a dry lubricant within the interstitial space of the endoscope that is incompatible with VHP sterilization.
The fifth and last cycle type is a specialty cycle designed to sterilize anatomical models and patient
CONTINUING EDUCATION 24 | 2024 | Healthcare Purchasing News
specific guides that were 3D printed within the hospital. It is important that the material used to construct the model is compatible with the system and that any lumens within the model meet the diameter and length requirements specified for the special cycle.
VHP sterilizer and device manufacturers often give sterilization information based on a single device but rarely is a device sterilized alone. Building sets for VHP sterilization will require knowledge of the
requirements of all the instrumentation within the set, the tray or container used to hold the instrumentation and all accessories including wraps, instrument organizers, and items used to protect wraps from punctures and tears. The set, in its entirety, should be compatible and packaging materials validated for VHP sterilization. Packs that hold items that can be sterilized in different sterilization cycles should be reconciled to the cycle that is common between all items within the set.

VHP sterilization into the future
Vaporized hydrogen peroxide has rapidly advanced over its short thirty plus years of use as a healthcare sterilization choice. Its continued application and new advancements in 3D printed models and guides is just the fi rst of many new sterilization applications to come in this established sterilization process’s future. HPN
References online at hpnonline.com/55001566.
From Novel to Established, a Journey of VHP Sterilization - Practice Quiz
1. What is an “established sterilization process”?
A. A 510(k) cleared sterilization cycle
B. A sterilization process listed in an Instructions for Use
C. A category of sterilization cycles listed by the FDA
D. A sterilizer sold in the United States
2. What were the challenges of EO sterilization addressed by VHP sterilization?
A. Compatibility issues with EO sterilization
B. Long cycle times
C. Chemical hazards of EO
D. All of the above
E. B and C
3. Why is the low temperature, lesser vacuum of VHP sterilization cycle preferable for rigid endoscopes?
A. Preserves the integrity of instrument adhesives and optics
B. Creates shorter sterilization cycles
C. Allows sterilization of more than one channel
D. Reduces dependence on aeration
4. How many stainless-steel lumens in single device can some cycles sterilize?
A. 1
B. 2-3
C. 4-5
D. 6
5. Which sterilization process does not require time to cool instruments prior to use?
A. VHP Sterilization
B. Steam Sterilization
C. Dry Heat Sterilization
D. Steam Formaldehyde Sterilization
6. How do healthcare facilities benefit from using VHP sterilization to sterilize 3D printed medical models?
A. There is no benefit
B. Cycle times are faster
C. The 3D printer runs faster
D. Save money on costly validations
7. Healthcare facilities can choose to sterilize a device in VHP sterilization cycle based on the device’s characteristics and sterilization packaging.
A. True
B. False
All CEU quizzes must be taken online at: educationhub.hpnonline.com.
The cost to take the quiz is $10.
8. What is considered when deciding a medical device’s ability to be sterilized in a VHP sterilization cycle?
A. Lumen length and diameter
B. Compatibility the instrument
C. Sterilizer manufacturer’s device matrix
D. All of the above
9. Which VHP sterilization cycle type can be used to sterilize a bronchoscope?
A. General cycle type
B. Stainless-steel Lumen cycle type
C. Flexible cycle type
D. Specility cycle type
10. When building a set, which sterilization cycle should be used?
A. The cycle most recommended
B. The cycle common to all instruments
C. The shortest cycle time
D. The longest cycle time


hpnonline.com | 2024 | 25
PURC HASI NG NE WS Healthcare ™ EDUCATION HUB
Qualities of Strong, Effective, QualityFocused SPD Leaders
BY DAVID L. TAYLOR, MSN, RN, CNOR, PRINCIPAL, RESOLUTE ADVISORY GROUP LLC
Sterile Processing (SP) leaders, like business leaders in other segments, must possess many skills—and know how to apply them consistently and effectively to be successful. Strong leaders not only strive to inspire and motivate their teams, but they also oversee considerable production cycles and critical safety requirements to ensure safe, high-quality, and well-functioning devices and exceptional service are delivered to their many healthcare customers within the organization.
Certainly, all leaders are not created equally. Although there are both great and poor leaders, virtually any dedicated, quality-focused SP professional can step up as a leader in their own unique way. They can become a Certified Instrument Specialist, for example, an unofficial mentor for fellow technicians, or serve as a liaison between the Sterile Processing department (SPD) and Operating Room (OR). Even the most experienced leaders should aim to continually broaden and improve their leadership skills to support their team and customers. Any departmental or business leader can make or break an organization, either promoting good outcomes or contributing to avoidable risks. This is why organizations must make hiring and developing excellent leaders (and mentoring and supporting future ones) a top priority.
Core priorities for effective SP leaders
SP leadership priorities are vast and will vary slightly from one facility to the next; however, some fundamentals should always be emphasized. These can include:
• Building dedicated teams of trained and certified staff that proactively solve problems in real time, while planning for incidents before they arise (and potential mitigation strategies).
• Improving or maintaining staff engagement by creating a positive and supportive culture where employees receive ongoing education and training and are proud of the work they do each day.
• Emphasizing safety and quality practices and empowering staff to raise issues or concerns that may affect the quality of their work and compromise patient care.

• Assessing instrument set inventories and streamlining same-day instrumentation turnaround, while also reducing error rates for surgical and procedural instruments.
• Being prepared to adapt to changes, tackle unexpected challenges, and seize on emerging opportunities.
Successful leaders also possess certain essential qualities, including integrity, accountability, and empathy. They hold high moral and ethical standards, are honest, and foster trust within their team. They take responsibility for their actions and their team’s performance, whether good or bad, and are supportive and encouraging, bringing out the best in their employees. They are also compassionate, respectful, and know how to connect with others, regardless of their backgrounds and differences.
Further, excellent leaders are experts at cultivating interdisciplinary relationships and collaboration, including with departmental and facility executives. They are competent at managing departmental and interdepartmental conflict and understand the need to partner for quality and safety. The best SP leaders are also confident in their knowledge and skillsets and know how to bring about the best in their teams and colleagues. They understand how their interdisciplinary colleagues work within the interconnected, broader healthcare ecosystem and lean on effective communication skills to clearly and concisely address problems, seek and adopt solutions, and foster compliance, motivation, and engagement.
Finally, effective SP leaders have good vision and strategic foresight. They plan effectively, stay current with industry, regulatory, or standard changes, set clear goals that will benefit their teams, customers, and organization, and keep a finger on the pulse of technological changes and innovations that will impact SP operations now and in the future.
Conclusion
Becoming a strong leader is always an ongoing journey, and any dedicated employee can become a leader, even informally. Effective leaders, regardless of their official title, are dedicated to sharing their knowledge with others, lending valuable support and collaborating for improved quality, safety, and efficiency. HPN
ASSOCIATION: HSPA 26 | May 2024 | Healthcare Purchasing News
Bone Cement and Black Light Detection - Part 2
BY STEPHEN M KOVACH, BS, CFER, CLINICAL EDUCATOR EMERITUS, HEALTHMARK, A GETINGE COMPANY
QThe original question from last month: “Recently, I read some posting on the Internet that one can use black lights to detect residual bone cement. Is that true,1 and can our department use a black light for this purpose?”
A“Now, the rest of the story…”2
My basic research has led me to consider the following:
• Some “bone cements” have phosphates {calcium, magnesium, beta tri-calcium, etc.} and other materials like barium sulfate in their design.3
• Users should know the type of bone cement used at their facility.
• Can staff perform their own testing on devices to find an acceptable level for detection?
• What type of “black light” ultraviolet (UV) light can be used?
• Does the IFU of the purchased black light state the device can be used for detecting medical bone cement?
• What does the literature say about using the black light method for the purpose they want?4,5,6 (Note: I have not found any peer-review literature supporting this practice.)
• Why are professionals using a black light without real data to support their practice?
Again, the question is: What does the black light pick up (show/glow) if a department is using it as an inspection tool? Because we do know that “Proteinaceous materials emit fluorescence when illuminated by UV light.”5
We all know the use of black light technology is helpful in hand hygiene. Some companies even say it can be used on a hard surface to help determine whether proper cleaning was performed.4 This technology requires not only a black light but a specific gel or powder to be used to show how effective your hand washing process is or how thoroughly a hard surface has been cleaned.4,7 I did not find anything related to using this process on medical devices. As I asked in April, “Could a black light pick [up] residual bone cement if left on the medical device?” That is, residual bone cements the natural eye cannot perceive. I would find answers to at least the questions I raised (you might have more) using this black light as an

inspection/detection tool. Once they are all answered, I would then make a policy on how to use this tool within my department for inspection purposes—if the research called for such a policy
Personally, I do think there might be some truth to the notion that a black light could detect residual bone cement material…but what material(s) of the bone cement is it detecting, and how low is the sensitivity of the detection level? Currently, I have no idea. Some of these bone cements contain barium sulfate or phosphorus that are sensitive to UV light but maybe not specifically UV from a flashlight black light source. You might need a higher / lower UV than a little flashlight can provide, and perhaps that kind of light is not safe.
Put these ideas and products through the same rigorous process you would for anything else you use in your department. Critically think it through and assess the benefits. If you find you can use it at your facility, do so because you will have the documentation to back up your decision to use it properly. Thus, to market it as a standalone tool would be a bit presumptive at this time based on my limited research. Remember, you want to use clinically relevant evidence-based products (at least that is what I would want) to back up using a black light as an inspection tool.
I know that residual bone cement is an issue for the medical device reprocessing profession, and we all want to find simple, easy solutions for our team members to provide a clean and functional medical device. For me, I need to see more evidence. I recently read an interesting article on bone cement titled “Strategies for Eliminating Retained Bone Cement” in HSPA’s PROCESS publication.8 I would highly recommend reading it to find more information. Maybe someone can do a research paper on this topic and shed some real light on the subject.
“And now you know…the rest of the story.”2 HPN
References
1. Jagrosse, D. (2023, February). Are you using a black light on SPD Instrument assembly to look for residual bone cement? Thoughts? Outcomes? [Image attached] [Post]. LinkedIn. https://www. linkedin.com/posts/jongoodwin3_apa2019-activity-6569581103441682432-CN98
2. Jones, C. D., (2002). What Paul Harvey Used to Say, and Why We Say It Again . Callaway-Jones. https://callawayjones.com/restofthestory/ Full references online at https://www.hpnonline.com/55000287.
STERILE PROCESSING INSIGHTS hpnonline.com | May 2024 | 27
The Way Forward for Sustainability in Healthcare
BY MATT MACKENZIE, ASSOCIATE EDITOR
Healthcare Purchasing News was able to speak with Amy Piser, lead clinical educator at Daniels Health, in order to gain some insight into how to best implement sustainability measures in hospitals to maximize success and efficiency.
What are the biggest challenges hospitals tend to face in adopting sustainability measures?
In my experience, the biggest challenge that hospitals face in adopting sustainability measures is a lack of data to back and confi rm their efforts. It’s one thing to make pledges or commitments around sustainability, but tracking and reporting on specific Key Performance Indicators (KPIs) such as waste per adjusted patient days is significantly more difficult. I always tell people “what you can measure, you can manage,” and the number one question we’ve been getting recently is around how we can track initiatives around waste reduction, CO2 offsets, and other measurable outcomes, which is a great first step. Although organizations like Practice Greenhealth exist to provide
reporting tools and best practices, the hospitals themselves still have to build and report on their own sustainability data in partnership with their vendors. This lack of data visibility from vendors and partners is still a significant challenge for many hospitals.
What are some measures hospitals have taken that have been particularly successful?
More than anything, hospitals that take a proactive approach to supply chain management tend to have the most success driving sustainable outcomes. Likeminded vendors are typically more than happy to partner on sustainability initiatives, but it’s critical for hospitals to start with building achievable and repeatable KPIs into the brief. From there, vendors can ensure they are tracking the appropriate data points, supporting with business reviews on a regular basis to track outcomes. There are many other successful strategies we often see specifically within the hospital waste management space. For example, optimizing waste streams typically leads to a reduction in excess regulated medical waste, which is more energy intensive to dispose of. Similarly, developing a stringent pharmaceutical waste program keeps medications out of wastewater and trash. Additionally, adopting reusable products where

SPECIAL REPORT 28 | May 2024 | Healthcare Purchasing News 210307570, 210307570 © VECTORMINE, 139400069 © ELIZALIV | DREAMSTIME.COM
possible, such as waste containers, is increasingly successful for many hospitals. While this is sometimes a point of conflict with infection prevention and risk, there is rarely any infection risk associated with certain reusables. Hospitals are increasingly focusing on identifying those products that have a more sustainable lifecycle without threatening patient or staff safety.

How can hospitals educate their workers on acting and working more sustainably?
A successful educational program starts with a strong work plan backed by specific goals and vision. This sets clear topdown expectations and enables key stakeholders to regularly engage clinical and educational teams with consistent learning messages. Consistent and continual education is increasingly important, especially as many hospitals see a rise in employee turnover, traveling support, and contractor support. Focusing on sustainability in the onboarding process is also critical. Lastly, auditing current programs and metrics to determine success, and then adapting education based on findings, ensures that efforts are continuously focused on driving results and updated to meet the needs of the sustainability program.
What do you anticipate for the future in this space?
Whether through voluntary commitments like the Department of Health and Human Services Healthcare Resilience Pledge (to which Daniels Health is a signatory) or regulatory requirements like the new Securities and Exchange Commission ruling for public companies to
disclose climate-related information, there is a growing trend toward data-based carbon emissions reporting. While this hasn’t happened yet, it is not hard to imagine a world where Medicare and Medicaid reimbursements are impacted by sustainability measurements, similar to HAI [healthcare-associated infections] reporting. Additionally, many accreditation organizations like the Joint Commission are adding focus to sustainable outcomes. The bottom line is that this trend is not going anywhere, and with public perception at an all-time high, hospitals have nothing to lose by starting to plan now.
Are there any developments you’re tracking that seem particularly promising?
I am very excited by technology advances allowing us to develop better solutions for sustainable waste management. Hazardous and regulated medical waste require intensive disposal treatments that are not necessarily environmentally friendly, so developments in alternative treatment options like hydrogen destruction and waste to energy are very promising. While the technology is not yet universal, it is promising to see a roadmap for a world where medical waste management has a more positive impact on the environment. Additionally, within the space of waste management, we are seeing an ongoing shift away from paper to digital when it comes to manifesting, which is another step in the right direction. Combined with the adoption of optimization software, this digital first approach not only reduces paper waste, but enables tracking and reporting capabilities that support a deeper environmental impact. HPN
Healthcare Purchasing Isn’t Going as Green as You Think
By Lars Thording, VP of Marketing & Public Affairs at Innovative Health LLC
Is healthcare purchasing going green?
The industry headlines might suggest so. But the reality witnessed by bootson-the-ground healthcare professionals suggests otherwise.
According to the Commonwealth Fund, in the United States, the healthcare sector is responsible for 8.5% of total greenhouse gas emissions, and emissions increased 6% from 2010 to 2018. Today, U.S. hospitals generate more than 4.7 million pounds of waste annually (which equates to roughly
27 pounds of waste per staffed hospital bed in America per day), and they dispose of 2 million pounds of unused supplies each year, at a cost of $15 million annually. Further, according to a Modern Healthcare report, more than 70% of a health system’s greenhouse gas emissions are embedded in the products and services they buy (i.e., scope 3 emissions).
With this background, one would think that healthcare purchasing would be adopting rules for preferring environ-

mentally friendly products, given the level of attention being paid to battling climate change across industries. However, when looking closer, it seems that the healthcare supply chain has succeeded in insulating itself from any such considerations.
Supply chain professionals in healthcare today will tell you that even though hospital leadership is pushing for greater environmental sustainability, they will default
hpnonline.com | May 2024 | 29
Amy Piser
to an uncompromised focus on cost when push comes to shove. The cheapest product wins, whether it is green or not. In fact, multiple supply chain professionals directly spoke to this conundrum at a recent roundtable in Scottsdale: While climate impact is top of mind in the industry, only green initiatives that are cost neutral or reduce costs are actually being considered.
Cost above all other considerations
For decades, the healthcare supply chain has been focused solely on one thing: driving down costs. This came with efforts to negotiate single-source contracts and pushing for just-in-time inventory policies. With this sole task in mind, healthcare purchasing has played a less-than-glorious role in the hospital, which has increasingly struggled with severely strained bottom lines.
The pandemic changed that – for a minute. Supply chain shortages in vital product categories like gloves and face masks shone a light on the healthcare supply chain and suddenly made the purchasing and inventory function of the hospital arguably the most important function in healthcare. Healthcare purchasing, having lived a life out of the limelight, became critical in the acquisition and availability of products.
In this way, the pendulum shifted from just-in-time inventories to just-in-case inventories, and the cost focus shifted to a focus on resource availability. Solesource contracting (in some cases) was abandoned and changed to dual-source arrangements to enhance the resilience of the supply chain. During the pandemic, in other words, the sole focus on price faded. Other procurement criteria, such as environmental considerations, were invited to play a role.
However, since the pandemic, the pendulum has swung right back to where it started, with the healthcare supply chain solely focused on cost savings. We are back to single-source contracting and procurement decisions made to reduce costs.
Why green decisionmaking has stalled
Of course, the environmental and resilience discussion has not completely disappeared, and hospital leaders are eager to demonstrate that their hospitals are making an effort to combat climate change. After all, doing so helps to comply with political signals, build the hospital’s “brand,” and strengthen their hiring and retention efforts. To this end, most hospitals have hired sustainability directors.
Given the pervasiveness of this theme and the focus of hospital leadership, why is the healthcare supply chain still struggling with notions such as environmentally preferred purchasing? There are two main reasons why:
Supply chain executives do not drive all procurement decisions. Specifically, physicians and nurses often have the clout to insist on buying new or preferred technologies, for clinical reasons or otherwise. Some have suggested that physicians’ incomes and the fact that physicians direct most healthcare spending (80% is a frequently used number) are the real culprits in rising health care costs. We call this “physician-induced demand, a documented phenomenon that results in overtreatment and contributes to high health care costs.” So, for some of the most expensive products, supply chain staff is left to simply execute – not question.
When supply chain staff does drive procurement decisions, they are focused on their main task: to drive down costs. In the minds of supply chain decisionmakers, the environment is considered when the environmentally friendly alternative is cost neutral or reduces costs, but otherwise not. In many cases, the environmentally preferable product is also the more expensive one. (A noteworthy exception is single-use device reprocessing, which reduces the carbon emissions footprint by up to 50% and reduces costs by at least 40%.) When hospital leaders ask the supply chain to demonstrate effort to combat climate change, they are
essentially giving hospital supply chain executives conflicting instructions, since environmentally preferable purchasing often runs counter to the standing instruction to reduce costs. As a consequence, supply chain executives will ignore hospital leadership’s calls for greening the hospital.
The path to balancing costs with environmental sustainability
Given the importance of costs, what can supply chain executives in U.S. hospitals do to reduce their hospital’s environmental footprint? It must be recognized that they have to apply a common-sense approach; costs cannot be ignored. However, some basic initiatives would help:
Vendors typically don’t provide information about the environmental impact of their products. Why? Because the hospital does not ask. Simply asking for sustainability performance metrics for every supplier, contract, and product to appear alongside price would go a long way toward creating the conditions for balanced or “common sense” decision-making. Ideally, sustainability metrics would be based on life-cycle analyses (LCAs).
More fundamentally, while most hospitals have value analysis committees designed to balance clinical, operational, and financial considerations in contracting, they do not play the role they should in showing environmental stewardship. Simply bringing together the right people and providing sustainability metrics is well within the power of the supply chain professional. As usual, knowing the facts helps.
A joint platform for decision-making that considers – among other things –climate impact can be the foundation for supply chain leaders to “democratize” the procurement decision and create shared responsibility and accountability among different hospital functions. It may be time to re-write the charter of the value analysis committee.
SPECIAL REPORT 30 | May 2024 | Healthcare Purchasing News













https://educationhub.hpnonline.com/ Enroll. Read the articles. Take the test.

All online, in one easy-to-use portal. Continuing Education for Sterile Processing Professionals

PURCHASING NEWS Healthcare ª EDUCATION HUB
AI and Healthcare: Matchmaking and Translation
BY KAREN CONWAY, CEO, VALUEWORKS
Many articles have addressed how artificial intelligence (AI) can improve supply chain management, from automating repetitive tasks to demand planning. A recent webinar on AI and the future of healthcare got me thinking about a few additional applications that could further supply chain’s role in fostering a more value-based health system. The webinar was presented by IIA Healthcare, an AI-driven technology company in the hiring and recruiting space, and featured two experts:
• Former IBM executive and AI expert Beth Rudden, CEO of Bast AI
• Boston Children’s Hospital AI engineer, Dinesh Rai, MD
Rai explained how generative AI applies algorithms to large sets of data to identify patterns and predict what comes next. That could be the next word in an application like ChatGPT or, if we consider supply chain opportunities, determining the supplies required to meet a specific patient need or diagnosis.
Rudden added that while machines can consume and analyze far more data than any one person could, humans add the specific context and emotional intelligence, both critical to translating medical knowledge into actual care and healing. For example, a highly trained oncologist may understand the kind of cancer a patient has and how to treat the disease, but he may struggle to explain things to a Vietnamese grandmother
in a manner that builds trust and compliance. Beyond medical information, AI can help the doctor and team communicate effectively. The care team can then provide a healing measure of compassion.
That got me thinking about how AI might help supply chain professionals when sourcing culturally sensitive products and services. For example, the appropriateness of the food and personal hygiene products provided in a patient’s room can have a significant impact on whether a patient feels seen and valued.
Rai described how AI can help match a patient’s social needs with the specific resources available to them, such as eligibility for food assistance. This is essentially what supply chain does: matches resources to meet specific needs. That made me wonder if AI could query the myriad products and vendors in different categories to find those that meet specific needs beyond cost and efficacy, such as those provided by a certified diverse supplier or that lower the carbon footprint?
AI’s translational capabilities can also support workforce development, including efforts to hire more individuals from disadvantaged communities. For example, Rudden says it could be used to help a mother on government assistance identify that she has skill sets that can be applied in a working environment, such as negotiating with vendors to manage cash flow. AI could also be

used to retrain individuals by transferring knowledge in a manner that best matches individual trainees’ unique backgrounds and experience and how they learn.
AI could also address the long-held desire to identify products the same way, e.g., using the unique device identifier (UDI) across multiple databases and processes. Rai says AI can write queries to create more consistency across multiple databases. That, in turn, could help identify when products are the same, even if not identified as such, and assign the UDI, facilitating a more holistic view into the role specific products play in lowering the cost of care, improving quality and outcomes, and/or optimizing reimbursement.
Rudden sees the potential for each of us having our own personalized AI that could, for example, analyze the results of a medical test in the context of everything else happening in and around us (physiologically, emotionally, socially, etc.). The challenge, she says, is not the technology involved, but rather how we – as humans – view and manage data. Beyond inaccuracies and incompleteness, she says we need to solve for data sovereignty, including patient rights to their own data. If we want to address the whole person, and meet their clinical and social needs, patients must be comfortable providing the data. And there is no better way than respecting their rights. HPN
VALUE. DELIVERED. 32 | May 2024 | Healthcare Purchasing News
ProFormance™ Cleaning Verification
Clearly Visible, Easy to Interpret, Objective Tests of Cleaning Methods




SonoCheck™
When the ultrasonic cleaner is supplying sufficient energy and condi�ons are correct, SonoCheck™ will change color. Problems such as insufficient energy, overloading, water level, improper temperature and degassing will increase the �me needed for the color change. In the case of major problems the SonoCheck™ will not change color at all.
TOSI®
Reveal the hidden areas of instruments with TOSI® - the easy to use blood soil device that directly correlates to the cleaning challenge of surgical instruments. TOSI® is the first device to provide a consistent, repeatable, and reliable method for evalua�ng the cleaning effec�veness of the automated instrument washer.
LumCheck™
The LumCheck™ is designed as an independent check on the cleaning performance of pulse-flow lumen washers. Embedded on the stainless steel plate is a specially formulated blood soil which includes the toughest components of blood to clean.
HemoCheck™ & ProChek-II™
Go beyond what you can see with all-in-one detec�on kits for blood or protein residue. HemoCheck™ is simple to interpret and indicates blood residue down to 0.1μg. The ProChek-II™ measures for residual protein on surfaces down to 0.1μg.
hmark.com
| 800.521.6224
| healthmark@hmark.com
For more of Healthmark’s intelligent solutions for instrument care & infection control, visit
hmark.com
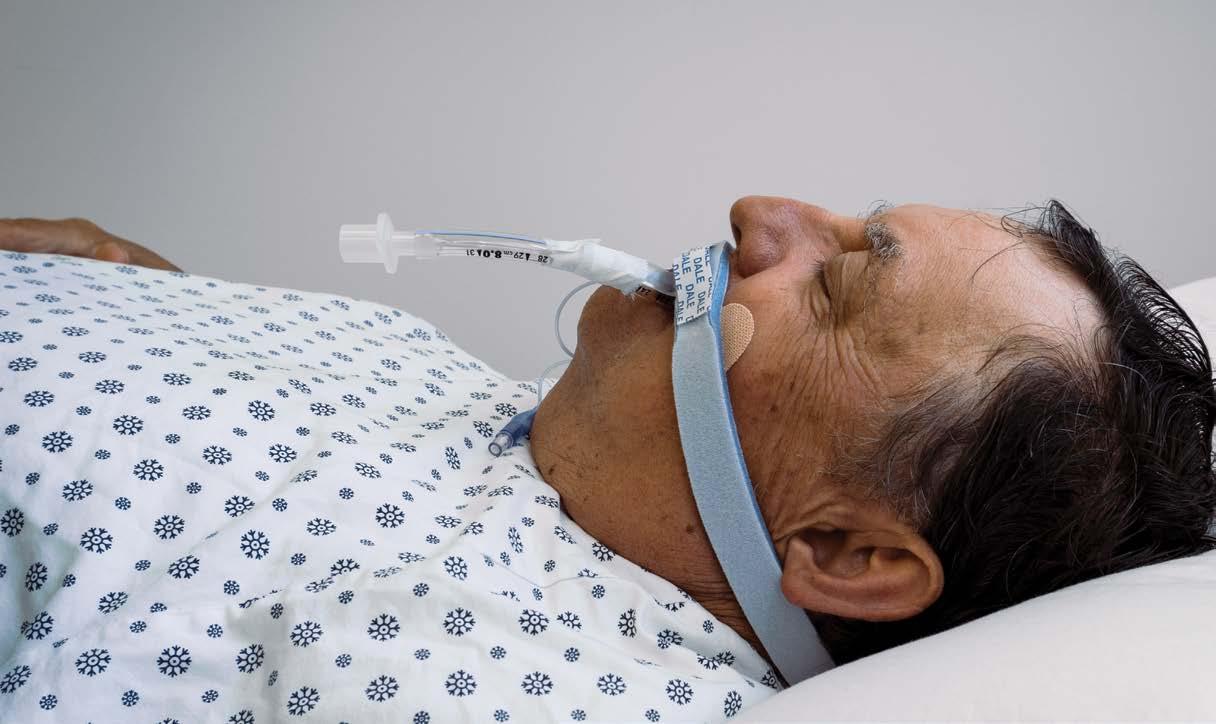


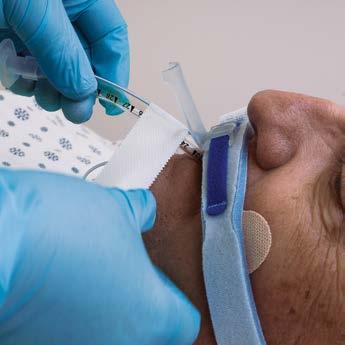
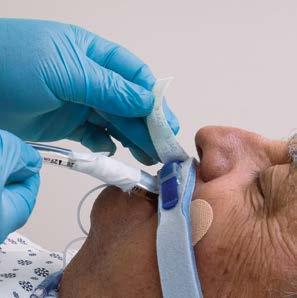

Provides reliable stabilization of endotracheal tubes (sizes 7.010.0mm) following intubation.
The low profile design and cushioned neckband are soft and comfortable on the face and neck.
It can be used in supine or prone position.
Slide allows easy repositioning of ET tube placement.
Allows easy access for oral care.
Prevents kinking or flattening of tube.
Minimizes unintentional ET tube movement and helps prevent accidental extubation.
AD-115 Rev C Represented Nationally by: Dale and BreezeLock are registered trademarks of Dale Medical Products, Inc. ©2024 Dale Medical Products, Inc. All rights reserved.
Dale BreezeLock® Endotracheal Tube Holder
The
QUALITY YOU CAN COUNT ON For a free sample call 800-343-3980 or visit dalemed.com/samples














































 Cindy Kinney Christopher Edwards
Cindy Kinney Christopher Edwards

































































