





























































WWW.HPNONLINE.COM CLINICAL INTELLIGENCE FOR THE HEALTHCARE ECOSYSTEM APRIL 2024 | VOL. 48, NO. 4 Diversity, Equity, and Inclusion in Supply Chain ... 6 Pharmaceutical & Nutritional Delivery/Injection Devices ... 10 Air & Surface Disinfection ... 16
2024 SPD OF THE YEAR ... 18

VISIT US AT HSPA BOOTH # 901


•
•


Enzymatic pretreatment humectant spray designed to keep instruments and rigid scopes moist for up to 72 hours. • EARN CE CREDITS • VIEW PRODUCT DEMONSTRATIONS

Multi-tiered • PICK UP GENEROUS FREE SAMPLES • RECEIVE A LIMITED-EDITION GIFT
enzymatic detergent with advanced proteolytic action & rust inhibitors for the effective decontamination of surgical instruments and endoscopes


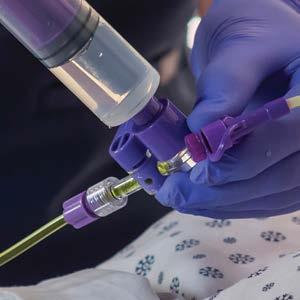
1 (800) 537-8463 • www.ruhof.com CLEAN CONFIDENTLY with The Next Generation of Contamination Monitoring Technology
SMART HANDHELD MOBILE PLATFORM
CLOUD-BASED
WI-FI CONNECTIVITY
INFINITE USERS & TEST POINTS
•
•
•
•
CUSTOMIZABLE DASHBOARD
RFID READER/BARCODE READER
USER FRIENDLY INTERFACE PREPZYME FOREVER WET ENDOZIME TRIPLE PLUS WITH APA
• AD-077 Rev0 013124




2 | April 2024 | Healthcare Purchasing News Sourcing & Logistics 6 > Diversity, Equity, and Inclusion Advance Supply Chain Operations JANETTE WIDER
> Making the Case for Standards and Scanning KAREN CONWAY Surgical/Critical Care 10 > Flexible Nutrition and Drug Delivery Supports PatientCentered Care KARA NADEAU Infection Prevention 16 > The Hidden Dangers on Hospital Surfaces MATT MACKENZIE Sterile Processing 18 > The Multiplier Effect of Success in Sterile Processing KARA NADEAU 24 > Water for cleaning medical devices JEANE APARECIDA GONZALEZ BRONZATTI AND RAFAEL QUEIROZ DE SOUZA 28 > Taking SP Leadership Journey Offers Big Gains DAVID TAYLOR 30 > Bone Cement and Black Light Detection - Part 1 STEPHEN M. KOVACH Departments 4 > New Look, Same Great Taste 5 > What’s on the Web 18 6 16 10 CONTENTS > APRIL 2024 153655482 © KOONSIRI BOONNAK DREAMSTIME.COM
32
DETECTO manufactures more unique models for each clinical product category than any other medical scale company. Giving you better choices means you never have to settle for a product that isn’t
DETECTO: A true vertically-integrated medical scale manufacturer – family owned and privately held – with factory and headquarters in Webb City, MO.


QUALITY WHERE CHOICE MEETS DETECTO | 102 East Daugherty St. | Webb City, MO 64870 | (800) 641-2008 | detecto@cardet.com | www.Detecto.com The Best Choice is the One That Gives You MORE Choices
the right fit for your application.
MORE
LEARN
New Look, Same Great Taste
BY JANETTE WIDER, EDITOR-IN-CHIEF
In 1985, Coca-Cola introduced “New Coke” in reaction to Pepsi’s “Pepsi Challenge,” an aggressive marketing campaign consisting of a blind taste test between Coca-Cola and Pepsi—and Pepsi was dominating. New Coke, which had a sweeter formulation than the original Coke, was found to outperform both classic Coke and Pepsi in top-secret taste tests.
As some of you may remember, the release of New Coke was not well received … at all.
An article from History.com says that “New Coke left a bitter taste in the mouths of the company’s loyal customers. Within weeks of the announcement, the company was fielding 5,000 angry phone calls a day. By June, that number grew to 8,000 calls a day, a volume that forced the company to hire extra operators. ‘I don’t think I’d be more upset if you were to burn the flag in our front yard,’ one disgruntled drinker wrote to company headquarters. At protests staged by grassroots groups such as ‘Old Cola Drinkers of America,’ consumers poured the contents of New Coke bottles into sewer drains. One Seattle consumer even filed suit against the company to force it to provide the old drink.”

Further, “The outrage caught Coca-Cola executives by surprise. They had hardly made a rash decision unsupported by data. After all, they had performed 190,000 blind taste tests on U.S. and Canadian consumers. The problem, though, is that the company had underestimated loyal drinkers’ emotional attachments to the brand. Never did its market research testers ask subjects how they would feel if the new formula replaced the old one.”
In July of that year, Coca-Cola announced it would be returning to the original formula, dubbed “CocaCola Classic.” Coca-Cola president Donald Keough said in a statement, “Our boss is the consumer. We want them to know we’re really sorry.”
As you’ve probably already noticed, this month Healthcare Purchasing News has a new logo, tagline, and magazine design. Unlike New Coke, HPN ’s “taste” will remain the same. HPN will continue to cover our many verticals including supply chain, sterile processing, infection prevention, surgical and critical care, environmental services, and more—everything you need to know about the healthcare ecosystem under one roof—just with a little more pizzazz.
Group Publisher
Chris Driscoll
cdriscoll@endeavorb2b.com | 978-880-8345
Editor-in-Chief
Janette Wider jwider@hpnonline.com
Associate Editor
Matt MacKenzie mmackenzie@endeavorb2b.com
Senior Contributing Editor Kara Nadeau knadeau@hpnonline.com
Advertising Sales
East & West Coast Kristen Hoffman khoffman@endeavorb2b.com | 603-891-9122
Midwest & Central April Bruffy abruffy@hpnonline.com | 713-992-0381
Strategic Accounts Sales
Chris Driscoll cdriscoll@endeavorb2b.com | 978-880-8345
Advertising & Art Production
Production Manager | Ed Bartlett
Art Director | Tracy Arendt
Advertising Services
Karen Runion | krunion@endeavorb2b.com
Audience Development
Laura Moulton | lmoulton@endeavorb2b.com
Endeavor Business Media, LLC
CEO Chris Ferrell | President June Griffin
COO Patrick Rains
CRO Paul Andrews
Chief Digital Officer Jacquie Niemiec
Chief Administrative & Legal Officer Tracy Kane
EVP Medical & Healthcare Technology Kylie Hirko
EVP Endeavor Business Intelligence Paul Mattioli
Healthcare Purchasing News USPS Permit 362710, ISSN 1098-3716 print, ISSN 2771-6716 online is published 11 times annually with an additional issue in November - Jan, Feb, Mar, Apr, June, Jun, Jul, Aug, Sep, Oct, Nov, Nov IBG, by Endeavor Business Media, LLC. 201 N Main St 5th Floor, Fort Atkinson, WI 53538. Periodicals postage paid at Fort Atkinson, WI, and additional mailing offices. POSTMASTER: Send address changes to Healthcare Purchasing News, PO Box 3257, Northbrook, IL 60065-3257. SUBSCRIPTIONS:
Publisher reserves the right to reject non-qualified subscriptions. Subscription prices: U.S. $160.00 per year; Canada/Mexico $193.75 per year; All other countries $276.25 per year. All subscriptions are payable in U.S. funds. Send subscription inquiries to Healthcare Purchasing News, PO Box 3257, Northbrook, IL 60065-3257. Customer service can be reached toll-free at 877-382-9187 or at HPN@ omeda.com for magazine subscription assistance or questions.
Connect with us:
facebook.com/hpnonline twitter.com/hpn_online linkedin.com/company/healthcare-purchasing-news/
Printed in the USA. Copyright 2024 Endeavor Business Media, LLC. All rights reserved. No part of this publication June be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopies, recordings, or any information storage or retrieval system without permission from the publisher. Endeavor Business Media, LLC does not assume and hereby disclaims any liability to any person or company for any loss or damage caused by errors or omissions in the material herein, regardless of whether such errors result from negligence, accident, or any other cause whatsoever. The views and opinions in the articles herein are not to be taken as official expressions of the publishers, unless so stated. The publishers do not warrant either expressly or by implication, the factual accuracy of the articles herein, nor do they so warrant any views or opinions by the authors of said articles.
EDITOR’S NOTE 4 | April 2024 | Healthcare Purchasing News
Editorial Advisory Board
Jimmy Chung, MD, MBA, FACS, FABQAURP, CMRP, Chief Medical Officer, Advantus Health Partners and Bon Secours Mercy Health, Cincinnati, OH
Joe Colonna, Chief Supply Chain and Project Management Officer, Piedmont Healthcare, Atlanta, GA; Karen Conway, Vice President, Healthcare Value, GHX, Louisville, CO
Dee Donatelli, RN, BSN, MBA, Senior Director Spend symplr and Principal Dee Donatelli Consulting LLC, Austin, TX
J. Hudson Garrett Jr., PhD, FNAP, FSHEA, FIDSA, Adjunct Assistant Professor of Medicine, Infectious Diseases, University of Louisville School of Medicine
Melanie Miller, RN, CVAHP, CNOR, CSPDM, Value Analysis Consultant, Healthcare Value Management Experts Inc. (HVME) Los Angeles, CA
Dennis Orthman, Consulting, Braintree, MA
Janet Pate, Nurse Consultant and Educator, Ruhof Corp.
Richard Perrin, CEO, Active Innovations LLC, Annapolis, MD
Jean Sargent, CMRP, FAHRMM, FCS, Principal, Sargent Healthcare Strategies, Port Charlotte, FL
Richard W. Schule, MBA, BS, FAST, CST, FCS, CRCST, CHMMC, CIS, CHL, AGTS, Senior Director Enterprise Reprocessing, Cleveland Clinic, Cleveland, OH
Barbara Strain, MA, CVAHP, Principal, Barbara Strain Consulting LLC, Charlottesville, VA
Deborah Petretich
Templeton, RPh, MHA,Chief Administrative Officer (Ret.), System Support Services, Geisinger Health, Danville, PA
Ray Taurasi, Principal, Healthcare CS Solutions, Washington, DC



Three in Four Think AI Tech Will be Widespread Within Three Years
Berkeley Research Group (BRG) recently released a report entitled “AI and the Future of Healthcare Report.” The report found that three in four healthcare professionals surveyed believe that artificial intelligence (AI)-related technologies will be widespread within the next three years. A press release said that “The report finds that more than 4 in 10 healthcare provider respondents say AI has already been widely accepted and effectively implemented.”
Read on: hpnonline.com/53097927
Philips Expands Access to Nearly Helium-Free MRI
According to a press release, Royal Philips announced the expansion of access to virtually helium-free MRI to more patients in more places, with more than 1,000 systems now installed worldwide.
The press release says that Philips BlueSeal magnet is a1.5T fully sealed magnet and requires only 0.5% of the helium of a conventional Philips MR system.
Read on: hpnonline.com/53098133
Walmart Reduces Supply Chain Emissions
Recently, The Wall Street Journal reported that Walmart said its suppliers have removed 1 billion metric tons of greenhouse-gas emissions from their value chains, six years ahead of the scheduled target date.
In 2017, the organization began an initiative to encourage its suppliers to reduce their carbon footprint, aiming to avoid, reduce or sequester the 1 billion tons of emissions by 2030. Walmart has plans to improve and expand the project.
Read on: hpnonline.com/53097720
CDC Updates Guidance on Respiratory Viruses Like COVID, Flu, and RSV
CDC has released updated recommendations regarding protection from respiratory viruses, aiming to bring a “unified approach” to addressing COVID-19, flu, and RSV.
As part of this new guidance, CDC is emphasizing a number of core prevention strategies that are meant to apply to all respiratory viruses.
Read on: hpnonline.com/53098201
hpnonline.com | April 2024 | 5
679764821 © MARIA_LH | STOCK.ADOBE.COM What’s
This index is provided as a service. The publisher does not assume liability for errors or omissions. Index of Advertisers Aesculap ............................... C3 aesculapusa.com/aicon Cygnus Medical..................... 9 cygnusmedical.com Dale Medical Products 15 dalemed.com/samples DETECTO 3 detecto.com Healthmark Industries ......C4 hmark.com Marketlab ............................. 29 marketlab.com Owen Mumford 31 owenmumford.com Ruhof Corporation C2 ruhof.com
on the Web
Diversity, Equity, and Inclusion Advance Supply Chain Operations
Three supply chain professionals share their perspectives on the benefits of DEI programs and diversity when it comes to suppliers
BY JANETTE WIDER, EDITOR-IN-CHIEF
Diversity, Equity, and Inclusion (DEI) are important organizational frameworks for companies, especially in the healthcare industry. Over the past
several years, there have been many announcements and initiatives surrounding DEI in healthcare. Healthcare Purchasing News spoke to three supply chain industry professionals about
DEI to get the latest updates in this space. The experts discussed DEI programs within their organizations as well as the importance of supplier diversity in organizations.
Seema Bhansali, vice president of Team Schein Member Experience & Inclusion at Henry Schein, told HPN, “There is a lot of variety for DEI programs in supply chain. Some programs may specifically focus on suppliers or customer diversity, while others focus on internal team members and the concept of inclusion. From my

SOURCING + LOGISTICS 6 | April 2024 | Healthcare Purchasing News 162263426 © DESIGNER491 | DREAMSTIME.COM
perspective, DEI in supply chain is crucial. Supply chain is very interconnected, so inclusivity is vital because as individuals work closely together, there must be a mutual understanding of one another. By doing so, it can help promote effective collaboration and help individuals navigate the intricacies of the supply chain.”
Tina Vatanka Murphy, president and CEO, GHX, said, “I have seen health systems recognize the link between health equity, DEI programs, and the overall cost of healthcare in recent years, and how supply chain plays a significant role. Today, more healthcare organizations are actively seeking to engage a diverse range of suppliers, partners, and stakeholders from underrepresented groups. The link between a diverse supply chain and health equity is significant, extending beyond just businesses and their employees to positively impact the communities they serve.”
Vatanka Murphy added, “Diversifying the businesses engaged in the supply chain leads to increased job opportunities for individuals from different backgrounds and communities. This not only benefits the economic landscape but also helps enhance access to healthcare and essential resources, contributing to better health outcomes in the community. In the United States, health inequity costs $451B annually and is expected to balloon to $1 trillion in 2040 if the issue is not addressed. When it comes to health in the U.S., zip code often matters more than genetic code.”
DEI boosts innovation
When speaking about the importance of DEI programs, Henry Schein’s Bhansali said, “These programs are important for a variety of reasons. In Henry Schein’s case, our focus is on inclusivity. Inclusivity is an important part of our Team Schein culture because it allows for the diversity of thought and advanced creativity. As


a global company that operates in 33 countries, this enables us to create solutions for our customers, and the patients they serve, who represent every imaginable background and identity. It is essential that we create a place of knowledge and understanding, helping each other to feel comfortable having safe and open conversations. It’s important that people feel free to be who they are. The days of hiding your life, your culture, your accents, and even your hair because you feel you have to, should be over. Overall, at Henry Schein we’ve seen that by fostering a culture of inclusion, it brings together a variety of perspectives and experiences, ultimately leading to an engaged team.”
“As a distributor, our focus is enabling access to quality care,” Bhansali added. “We do this by supporting programs such as the American Dental Association’s Give Kids a Smile program and Special Olympics, among many others. Additionally, at Henry Schein we continue to advocate for diversity and inclusion in the healthcare profession by supporting national, state, local, and culturally diverse associations.”
GHX’s Vatanka Murphy noted, “Enhancing diversity in the supply chain boosts innovation and outcomes. Incorporating a variety of backgrounds and perspectives in decision-making results in better solutions, which is what we need to address healthcare’s most profound issues. A diverse outlook can help uncover untapped opportunities while fostering deeper connections with the communities we serve. By
emphasizing a diverse supply chain, organizations not only drive deeper innovation and fresher ideas, but also enhance employee retention and recruitment.”
“Healthcare organizations are being more deliberate in developing and maintaining a culture of diversity, equity, and inclusion — whether it’s the creation of employee resource groups and educational resources for staff, creating more diverse candidate pipelines, or driving supplier and economic diversity programs,” she said.
Current programs
Henry Schein’s Bhansali explained, “At Henry Schein, our emphasis on inclusion has brought our team together, fostering deep understanding and empowerment of one another. It has strengthened collaboration within Team Schein, leading to improved solutions for us and our customers.”
She added, “An example of our programs that have contributed to the strength of Team Schein are our six Employee Resource Groups (ERG), which are voluntary, employee-led networks that foster a diverse, inclusive workplace aligned with our Team Schein Values and business goals. Our ERGs are: The Women’s Leadership Network (WLN), which supports women, Pride and Allies, which supports the LGBTQ+ community, Black Legacy Professionals, which supports the Black community, COLEGAS, which supports the Hispanic and Latinx community, elevASIAN, which supports the Pan Asian community, and Veteran Engagement Team (VET), which supports veterans, members of the armed forces and their families. A seventh ERG will be created that is focused on individuals with disabilities & allies.”
GHX’s Vatanka Murphy told HPN about GHX’s program. She said, “At the start of 2021, we formed the Diversity & Inclusivity Advisory Council (now known as the DEI &
hpnonline.com | April 2024 | 7
Seema Bhansali Tina Vatanka Murphy
Culture Council). The group architected the company’s first diversity and inclusion roadmap, including the use of data to improve equity, as well as expanding mentorship opportunities, developing more robust D&I practices in recruitment and career development, and investing in dedicated D&I leadership, which resulted in the 2022 appointment of the company’s first vice president of D&I.”
“To date, nearly 70% of managers at GHX have gone through training on unconscious bias, and inclusive culture trainings have been rolled out across the company,” she added. “On average, GHX hosts 20 cultural awareness events and celebrations throughout the year, including Juneteenth, AAPI Heritage Month, Pride Month, and Hispanic Heritage Month. In many instances, GHX employees stepped up to lead these events, sharing their histories and cultural experiences with their peers.”


She added, “It increases the competition and the pull of suppliers when you can ensure that you’re getting that innovation as one of the key aspects that we see by having an inclusive supply chain from an economic impact. It supports the growth of small and minority owned businesses and flashing the economic development, which is the output of building an inclusive supply chain. So, you’re not only fostering economic development in those suppliers, but also in the communities where they are, in which most of the healthcare organizations operate. You’re building not only an ecosystem of suppliers that build more unique offerings and products, but
of the community engagement or stakeholder engagement map and provide a different alternative. There are also things about diverse suppliers creating diverse products and access to diverse products, so they tend to understand their own populations better. So, they understand the diversity stakeholders that are there more. One of these examples which we thought was really cool is we had a client who worked fora hospital that was procuring wheelchairs, and they were spending a fortune on these wheelchairs because the tires kept being damaged. And they were using a large kind of multinational, non-diverse owned company and as an unrelated part of their supply diversity outcomes, they switched their suppliers to a diverse wheelchair provider and that person was part of the community, understood the area outside of the hospital and professionally provided a different type of wheelchair because they understood either the patient needs there but also the physi-
“You’re not only fostering economic development in those suppliers, but also in the communities where they are, in which most of the healthcare organizations operate.”
THERESA HARRISON, GLOBAL ENVIRONMENTAL SOCIAL GOVERNANCE SERVICES LEADER, EYI
Diversity with suppliers
Theresa Harrison, EY’s Global Environmental Social Governance Services Leader, when asked about incorporating diversity into an organization’s suppliers, told HPN, “One of the things that it does if they incorporate supplier diversity into their supply chain is it offers them a wider range of products and services that they can offer. A lot of times we don’t have access to diverse suppliers who can provide new or innovative or cost-effective products or services that might not be available from our existing supply.”
also supporting the local communities where you work. And then we’ve seen also from an enhanced reputation, this demonstrates a commitment to diversity and inclusion. And so, it enhances the overall organization’s reputation among patients and employees in the community because they see it impacting where they live and operate or even the companies where they might buy specific products and services from as well.”
Cate Mork, EY, U.S. Supply Chain Sustainability Lead, regarding how diversity can improve the patient experience, said, “It can form part
cal grounds that’s out of the hospital. And so it resulted in actually less cost for the hospital because the tires on the wheelchair were not popping as often and not breaking.”
“It was a better patient outcome as well and all it came down to was that kind of knowledge of those populations, local populations and understanding of the geography outside of the hospital,” she added.
Where to get started
EY’s Mork commented on where organizations can get started. She said, “The place to start is to define that
SOURCING + LOGISTICS 8 | April 2024 | Healthcare Purchasing News
Theresa Harrison Cate Mork
ambition. So obviously that depends on who you are in that organization, and whether you have the authority to define that vision. But if you don’t, you can definitely influence it. I think defining the vision, is this something that you want to explore? Is this something you want to be a leader in within the industry? Do you want to come out straight at the gate and start linking this to health equity?”
“A lot of healthcare and life sciences organizations, particularly in the U.S., already have this program and some forms,” she mentioned. “So, it’s about then expanding and defining that vision, but the path would be the same. Step one would be to define that vision or influence that vision and take it to the person who does have the authority to pursue it. I think secondly, it’s really around taking stock about where you are and understanding the diverse suppliers that you currently have in your supply chain that you might not be aware of. Then, figure out what you need to do to close the gap between where you are today and the 2-3 year out vision.”
Mork added that this process takes time. At EY, she mentioned, Harrison’s program is 20 years in the making. Often, in the healthcare and life sciences sectors, you want to start small scale and be successful and then expand from there. She says starting small on things like looking at your organization’s category procurement categories and where there is opportunity to increase your diverse spend, and therefore the impact, can be helpful. Then over the next two to three years as part of your vision, double down on initiatives related to that before expanding into further initiatives.
GHX’s Vatanka Murphy gave some advice on how to speak to leadership about implementing DEI in one’s organization. She said, “One word: data. One practical approach is to gather and present use case data that
demonstrates the potential impact on clinical and financial outcomes, and underscore how the program will help the organization better understand the populations it serves. Drawing from real-life anecdotes is also helpful in illustrating value. Remember that it’s OK to start small – consider
proposing a pilot to test and refine, rather than rolling out a full-fledged program from the start. Another approach is to look for like-minded organizations in the community who may be interested in learning together, and partner to implement a DEI program.” HPN


Flexible Nutrition and Drug Delivery Supports Patient-Centered Care
BY KARA NADEAU, SENIOR CONTRIBUTING EDITOR
Chronic conditions, such as diabetes, cancer, cardiovascular, autoimmune, and inflammatory diseases, are on the rise. Biopharmaceutical companies continue to develop new drugs, therapeutics, and nutritional formulations to sustain and save lives.
In parallel, patients are demanding improved delivery modalities that offer ease of use and convenience, particularly for treatments requiring infusion or injection.
Healthcare Purchasing News explores some of the latest innovations in delivery systems for nutrition, drugs, and therapeutics designed to support safe, effective, and compliant patient care.
Nutritional delivery from hospital to home
Adequate nutrition is critical to patient health, whether it is in the prevention of chronic diseases or the management of existing medical conditions.
When a patient cannot consume enough calories and nutrients through the traditional route of eating, enteral nutrition (delivery of calories and nutrients into the gastrointestinal
tract) and parenteral nutrition (delivery of calories and nutrients into a vein) are vital interventions.
Advancements in enteral nutrition delivery
Numerous and often complex diseases can lead to the need for home enteral nutrition (HEN), such as swallowing disorders because of neurological diseases, chronic obstructive pulmonary disease (COPD), heart disease, chronic infections, and malabsorption/maldigestion because of liver, pancreas, or intestinal diseases. HEN is typically started during a patient’s hospital stay and continued post-discharge as a long-term home therapy.1
Like any transition of care from a high acuity to a low acuity setting, transitioning enteral nutritional delivery from hospital to the home has its challenges. Rachel Soriano, senior global portfolio manager of Nutritional Delivery/New Product Development at Cardinal Health, spoke to the continued technology innovation in this area, highlighting the Kangaroo OMNI Enteral Feeding Pump:
“As an industry leader in nutritional delivery, the Kangaroo brand
has been globally recognized for more than 30 years through its continuous innovation and improved offerings. The Kangaroo OMNI Enteral Feeding Pump was designed to help improve the lifestyle of enteral feeding patients and caregivers from hospital to home through ease of use, versatility, and portability. Kangaroo OMNI is the first and only attitude independent enteral feeding system in the U.S. designed to deliver thick*, homogenized and blended formulas.
“Additional new features include interruption monitoring to display nutrition missed when the pump has been turned off and a night mode that darkens the pump screen in low-light settings to help minimize feeding disruption,” Soriano continued. “By offering new enhancements, functionality, accessories, and options for nutritional delivery, Kangaroo OMNI is also helping enteral feeding patients and caregivers to meet their personalized needs throughout their enteral feeding journey.”
*Thick formula is defined as enteral fluid of smooth consistency that is categorized as level 2, 3, or 4 drinks within the International Dysphagia
“We are in the midst of a transformative shift in healthcare with an increasing number of patients receiving care at home.”
EMILY LEVY, CO-FOUNDER, PRESIDENT, CBO OF MIGHTYWELL
SURGICAL/CRITICAL CARE 10 | April 2024 | Healthcare Purchasing News
Diet Standardisation Initiative (IDDSI) framework.
Parenteral nutrition formulations
According to Cecilia Soriano, global president of Infusion Therapies and Technologies, Baxter, parenteral nutrition (PN) is a very dynamic category and a strong focus for the company. She said they are currently seeing shifts in PN formulations to meet the nutritional needs of patients throughout the care continuum.
“One shift is to higher protein formulations,” said Soriano. “With traditional formulations, patients would also be getting additional amounts of fluids, dextrose, and calories along with protein. That could lead to complications, such as hyperglycemia, related to the extra dextrose. To meet patients’ protein targets with less fluid and dextrose, we have brought to the U.S. market our CLINIMIX and CLINIMIX E formulations with higher protein. It contains the highest amount of protein, per liter, in any multi-chamber bag available in the U.S.”
“Another formulation shift we are seeing is with lipids,” Soriano continued. “Historically the formulations available were 100% soybean oil based. We are now seeing more mixed lipid emulsions. The Omega 6 fatty acids in 100% soybean oil formulations could have immunosuppressive effects. To manage that, some clinicians would dose only a few times a week, which could lead to underdosing, underfeeding, and other complications.”
To overcome this challenge, Baxter has developed its Clinolipid 20% lipid injectable emulsion for adult patients. It contains 80% olive oil, which is rich in immune-neutral Omega 9 fatty acids, and 20% soybean oil which provides patients with the needed essential fatty acids. It is the lowest soybean oil and highest olive oil lipid formulation available in the U.S.
Parenteral nutrition delivery
It is not only the formulations that are evolving to meet patient needs, but also the delivery methods, with the transition from individual bags to multi-chamber bags that hold multiple nutrients and components.
Gordon Sacks, PharmD, BCNSP, FASPEN, FCCP, senior director, Medical Affairs for U.S. Parenteral Nutrition at Fresenius Kabi, described the evolution of PN delivery since its development 50+ year ago:
“Delivery systems for PN (parenteral nutrition) have evolved extensively since being invented in the late 1960s. Historically, PN was administered to patients from multiple 1-liter glass bott les and these bott les were typically infused over 8 to12 hours. Dextrose and amino acids were combined in one bott le and lipid injectable emulsions were infused from a separate bott le. In the 1980s, there was a shift from bott les to IV bags for convenience and safety (i.e., avoiding breaking/cracking of glass containers).”
“At the same time, new research established that dextrose, amino acids, and lipid emulsions could be combined into 1 bag container to be administered over a 24-hour period (avoiding the need to infuse multiple containers within a 24-hr period),” Sacks continued. “During this time, PN was exclusively prepared and mixed by a pharmacist in a hospital or by a home infusion company on a daily or weekly basis according to strict aseptic compounding regulations. After preparation, compounded PN bags could be stored up to 9 days under refrigeration or 30 hours at room temperature. After these time periods, the bags would be discarded.”
Sacks highlighted how Fresenius Kabi’s 3-chamber bags (3CB) have simplified the PN ordering and administration process for both clinicians and patients. The bags are designed to deliver three macronutrients (dextrose,








hpnonline.com | April 2024 | 11
Kangaroo OMNI Enteral Feeding Pump
Baxter CLINIMIX E formulation with higher protein
Baxter Clinolipid 20% lipid injectable emulsion
Fresenius Kabi’s 3-chamber bag (3CB)
protein, and lipids) plus electrolytes in volumes and concentrations that meet the needs of most PN patients.
“Specifically, for clinicians, these products improve the ease and convenience of the prescribing process,”. Sacks explained. “They also minimize misinterpretation of prescription orders and calculations associated with compounded PN preparations. Ultimately, increases in cost savings from decreased pharmacist and prescriber time can result from the use of standardized, commercially available PN products such as Fresenius Kabi’s 3CB PN bags. For patients, the overall administration process is simplified since all nutrients and components are available in one container.”
Infused nutrition on the go Home parenteral nutrition (HPN) is the primary lifesaving therapy for patients with chronic intestinal failure (e.g., Crohn’s disease, short bowel syndrome) and may also be provided to patients as palliative nutrition in the late phases of end-stage diseases (e.g., cancer).2 HPN delivery systems have evolved to support the unique needs of patients receiving this therapy in outpatient settings, including the home.
“We are in the midst of a transformative shift in healthcare with an increasing number of patients receiving care at home,” said Emily Levy, co-founder, president, CBO of Mighty Well, which designs innovative medical products to make life simpler for patients. “Parenteral delivery systems are adapting to meet the unique needs of those relying on parenteral nutrition in a home setting. These advancements go beyond mere convenience; they signify a commitment to personalized and patient-centric healthcare. “
A leading innovation in this evolution is Mighty Well’s Fluid Motion Backpack, an infusion backpack that eliminates the need for patients to be
tethered to an IV pole for extended periods of time, providing them with greater mobility and dignity in their care.
“This innovation embodies a dedication to combining patient-centric design with compassionate care,” explained Levy. “The Fluid Motion Backpack serves as a powerful symbol of the ongoing revolution in homebased healthcare, reshaping how patients engage with and manage their parenteral nutrition and supplies. It represents the exciting shift towards the empowerment of patients in their continuum of care and is helping to redefine the quality of life for patients relying on home-based parenteral nutrition.”
Nutrition delivery monitoring
Accurate energy needs assessment is important along the patient’s care continuum so that underfeeding and overfeeding do not occur. Energy needs may vary from when a patient is mechanically ventilated, receiving PN in the intensive care unit (ICU) to the point they are discharged and receive PN at home. For this reason, Baxter offers Q-NRG+, a portable metabolic monitor, which provides quick and accurate resting energy expenditure (REE) measurements through indirect calorimetry (IC).
“Indirect calorimetry is considered the gold standard for assessing energy needs,” Angie Davanos, senior director of Medical Affairs, Baxter, explained. “Q-NRG+ can be used to measure the energy needs of the patient throughout their care journey as their conditions and needs change throughout the continuum of care.”
Drug and therapeutic delivery for convenience and compliance
The U.S. Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) approved 55 novel drugs in 2023, as well as approving
new dosage forms or drug formulations, and making some prescription drugs available over the counter.3 While some are administered in tablet or capsule form, others require injection or infusion.
New injectable drugs approved in 2023 include Talvey (talquetamabtgvs) to treat adults with refractory (treatment-resistant) or relapsed (recurring) multiple myeloma who have received other therapies, and Leqembi (lecanemab-irmb) to treat Alzheimer’s disease.
Newly approved infusion drugs include Loqtorzi (toripalimab-tpzi) to treat recurrent or metastatic (spreading) naso-pharyngeal carcinoma (NPC), a rare head and neck cancer, when used together with or following other therapies.
As more treatments expand outside of hospitals and physicians’ offices and into patients’ homes, manufacturers have responded with the development of convenient, easy to use delivery devices.
Home drug infusion management
As noted by the National Home Infusion Association (NHIA), “Infusing medications at home or elsewhere outside of an institutional setting reduces time spent away from normal activities, such as work, school, and hobbies while completing therapy.”4 But as Ben Noonan, general manager, Eitan Medical North America, pointed out, it also has its challenges. He stated:
“Drug delivery can be complicated and tedious. It requires patients to perform several steps to ensure successful administration, such as loading the drug, choosing the right settings and dosage for the specific device, ensuring sterility of both the device and the drug, and recording the dosage amounts and times given. To ensure patient-centric drug delivery in the home, minimizing patient interaction with the drug handling
SURGICAL/CRITICAL CARE 12 | April 2024 | Healthcare Purchasing News
process needs to be a top priority. This step helps prevent basic user errors that can lead to confusion, frustration, and non-compliance.”
“By enhancing ease-of-use factors, such as adding touch screens, intuitive protocol instructions and safety embedded software, home-based patients using either infusion or drug delivery devices will be better positioned for success,” Noonan added.
Last year, Eitan Medical launched Eitan Insights, a cloud-based infusion management system designed to meet the unique needs of home and specialty infusions. Eitan Medical’s Sapphire infusion platform, with its universal plug and play cellular accessory Sapphire Connect, and Avoset infusion pump with the AvosetGo app, connect and transmit infusion treatment data to Eitan Insights.
Eitan Insights provides near-real time prescription compliance data and pump geo location, enabling home infusion providers to optimize resources and reduce hospital readmission, helping improve both patient and caregiver experience, according to the company.
Self-injection devices
Application of self-injection devices, including vials and syringes, prefilled syringes, prefilled pens, and auto-injectors, has rapidly expanded to encompass a wide range of conditions and treatments.
In many cases, self-administration of subcutaneous drugs and therapies is more convenient for the patient compared with having to go to a healthcare facility for treatment. Patient administered self-injection is also a more cost-effective solution for healthcare delivery and helps alleviate today’s challenge of clinical staffing shortages.5
For example, biologics are increasingly used to treat specific types of cancer, autoimmune conditions, and inflammatory diseases. While in the
past patients had to receive intravenous infusion of a biologic in a doctor’s office or clinic, today technology has evolved so that patients can selfadminister the therapy via subcutaneous injection.
Pre-filled handheld autoinjectors are commonly used for selfadministration of biologics. These “spring-actuated mechanical devices, activated by pressing a button or pushing against the injection site,” are designed to “deliver a predetermined fi xed volume from a prefi lled syringe within 10 to 20 seconds.”5
Smart injection devices
Poor medication adherence is a significant problem among patients, with non-adherence estimated at 50% for chronic illnesses. In the U.S., poor adherence is responsible for an estimated 125,000 deaths annually and estimated to cause 10% of all hospitalizations.6
Take Type 2 diabetes, for instance, which affects an estimated 38.4 million people in the U.S. (11.6% of the population) and continues to rise in prevalence.7 Failure to adhere to insulin therapy regimens is one of the greatest barriers to Type 2 diabetes treatment.8
Manufacturers of injectable insulin delivery systems continue to evolve their devices to support better adherence. Delivery mechanisms have transitioned from vial and syringe to prefilled insulin pens, making it easier and less painful for diabetes patients to give themselves injections.
More recently, “smart” or “connected” insulin pens have been developed to help improve therapy adherence. They connect with smartphone applications to calculate and track doses, and provide helpful reminders, alerts, and reports.9 Smart connectivity and digital data collection also offer “insights into the impact of dosing behavior on glycemic outcomes” that can be helpful



















hpnonline.com | April 2024 | 13
Mighty Well Fluid Motion Backpack
Eitan Medical Sapphire Multi-Therapy Infusion System
Insulet Omnipod 5
Automated
Insulin
Delivery
System
© 2023 INSULET CORPORATION. ALL RIGHTS RESERVED.
West’s SmartDose 10 and 3.5 On-Body Delivery Systems
to diabetes care teams when developing patient counseling strategies.10
Taking it one step further, manufacturers have developed smart pen caps and attachments that can turn disposable insulin pens into smart devices when paired with smartphone applications.
Wearable injectors
Wearable injectors for subcutaneous drug delivery offer convenience for the patient by administering large volumes of drugs or other therapeutics over an extended time. The rising prevalence of chronic diseases such as diabetes, cancer, and autoimmune disorders that require long-term medication management is driving growth in this delivery modality, also known as “on-body delivery systems” (OBDS).11
One example is Insulet’s flagship innovation, the Omnipod 5 Automated Insulin Delivery System, which integrates with a continuous glucose monitor to manage blood sugar with no multiple daily injections and zero fingersticks. It can be controlled by a compatible personal smartphone or the Omnipod 5 Controller. The Omnipod Insulin Management System provides a unique alternative to traditional insulin delivery methods. With its simple, wearable design, the tubeless disposable Pod provides up to three days of non-stop insulin delivery, without the need to see or handle a needle.
Researchers report how wearable large-volume injectors open the door to new dosing options for patients. For example, evolocumab, a monoclonal antibody used to lower low-density lipoprotein cholesterol levels can be self-administered once a month within 5 minutes through a patientloaded wearable large-volume injector, as opposed to every two weeks within 15 seconds using a prefilled handheld autoinjector.12
“Our industry is experiencing a shift of treatment location from hospital to home for chronic diseases, driven by patient demand for more convenient treatment options, and the evolution of on-body delivery systems (OBDS),” said Atul Patel, vice president of Devices and Delivery Systems at West Pharmaceutical Services.
“West’s SmartDose On-Body Delivery System* was the first largevolume wearable to be approved as a combination product by the U.S. Food and Drug Administration and is now used with three approved therapies in the market. This technology has since evolved with multiple generations and different sizes with the goal of improving adherence and the patient experience.”
As wearable injector technology evolves, its applications broaden to improve the lives of chronic disease patients. In January 2024, researchers at the UNC School of Medicine announced their creation of a wearable patch featuring electrically triggered microneedles to deliver on-demand treatments for neurodegenerative disorders, including Alzheimer’s disease.13
“The beauty of this device is that it can house dozens, if not hundreds, of concentrated drugs and can program their sequential release automatically,” said lead researcher Juan Song, PhD, professor of pharmacology at the UNC School of Medicine.
*West’s SmartDose on-body injector platform is not independently cleared or approved by any regulatory body for general healthcare professional or patient use, nor is it available for general commercial purchase. Its distribution and use are subject to applicable regulatory requirements for clinical investigation, and for marketing authorization, as used in combination with a specific drug or biological product. Compatibility with any particular drug or biologic must be confirmed, and its ability
to achieve the desired patient benefits must also be confirmed, on a case-by-case basis. Important product and safety information and warnings available at: https:// www.westpharma.com/products/ self-injection-platforms/smartdose
Hydrogel injectables
While some chronic conditions can be treated through periodic large dose administration, others require controlled release of drugs over time. The ability to inject a drug and have its effects last weeks or even months is also more convenient for the patient.
Hydrogels have emerged as a modality to facilitate diffusion-based drug release. A drug encapsulated in a biodegradable hydrogel is injected into the patient where the gel slowly breaks down, releasing the drug over a desired period.
Beyond the benefit of controlled release, hydrogel injectables also facilitate more targeted drug delivery to a specific area of the body, for example, injection of immunotherapeutic agents directly into a tumor.
For Type 2 diabetes patients treated with GLP-1 drugs (e.g., Ozempic, Mounjaro, Trulicity, Victoza), a new hydrogel drug delivery system developed by Stanford University “transforms daily or weekly injections” of GLP-1 drugs “to just once every four months.”14
The new polymer-nanoparticle (PNP) hydrogel infused with a GLP-1 drug is injected under the patient’s skin. Over the course of four months, the hydrogel “melts away” releasing the drug molecules into the patient’s body.
Stanford researchers believe “such a system will greatly improve management of both diabetes and weight, improve patient drug compliance, and help those with Type 2 diabetes improve long-term health outcomes.” HPN References at hpnonline.com/53097916.
SURGICAL/CRITICAL CARE 14 | April 2024 | Healthcare Purchasing News










Represented Nationally by: Dale and ACE Connector are registered trademarks of Dale Medical Products, Inc. ENFit is a registered trademark of GEDSA used with their permission. ©2024 Dale Medical Products, Inc. All rights reserved. AD-102 Rev D QUALITY YOU CAN COUNT ON For a free sample call 800-343-3980 or visit dalemed.com/samples The Dale ACE Connector® 485 with ENFit® Connection Connect tubes for in-line feeding, suctioning, irrigation, gastric residual sampling and medication delivery with no need to disconnect Guards clinicians against potentially infectious gastric splashback Shields patient from external contamination ENFit styles to improve patient safety and reduce misconnections Simple on/off handle that is easy to operate
The Hidden Dangers on Hospital Surfaces
BY MATT MACKENZIE, ASSOCIATE EDITOR
Disinfection of both surfaces and air became even more complicated with the dawn of the COVID-19 pandemic in 2020. However, one silver lining came as a result of the pandemic –since hospitals and healthcare professionals across the globe were thrown immediately into a high-pressure situation with immediate implications for the care of their patients in the face of a highly contagious novel disease, the cracks in the existing systems became immediately apparent. Seeing where those cracks existed in such a transparent way has allowed professionals to narrow in on areas of need within the field.
A new study, published on Jan. 10, 2024, by the American Journal of Infection Control (AJIC), sounded the alarm on a number of pathogens that surfaces in hospitals tend to harbor
that were previously not known or expected to be particularly harmful to patients. New research suggests that those pathogens, including skin flora and environmental bacteria, can cause diseases as severe as meningitis and brain abscess in the right setting. Yet more alarmingly, surfaces that previously were not considered particularly high-risk, like manikins and bed rails, can harbor these bacteria despite adherence to routine disinfection procedures within hospital systems.
Healthcare Purchasing News spoke with two of the study’s authors –Piyali Chatterjee, PhD, Department of Research at Central Texas Veterans Health Care System, and Chetan Jinadatha, MD, MPH, Chief of Infectious Diseases Section at the Central Texas Veterans Health Care System – to address some of the concerns raised by the study, along with
possible innovations in the space that point a way forward.
Bugs and bacteria
When asked about the research group’s goals, Jinadatha said, “The group was looking to study the environment around patients and how that impacts infections in patients, and how we can keep the environment clean by using some of the latest technologies that are emerging, in terms of handheld UV devices, whole room disinfection, or new disinfectants.” As this new study makes abundantly clear, there is already a well-known cache of common organisms that are known to cause infection in patients in hospital settings, but there are also bugs and bacteria whose names are not nearly as well-known to either patients or even the workers in the hospitals themselves. The question the research team set out to answer was whether or not those bugs “actually mean something for our patients” and whether or not we need to pay attention to them for “future disinfection purposes.”
Chatterjee further characterized the research group’s goals as “quantitatively looking at or categorizing what types of bugs we see on high

INFECTION PREVENTION 16 | April 2024 | Healthcare Purchasing News 180850124 © MARTINMARK, 73207593 © EKATERINA CHERNYSHEVA DREAMSTIME.COM
touch surfaces” in order to further take the pulse of the “dynamic” environment in hospitals. Indeed, she and the other researchers found that “some of the uncommon organisms were found, in immunocompromised patients or in those who have comorbidities, some of these not-so-well-known pathogens can cause disease.”
Hand hygiene



Sounding the alarm regarding these lesser-known pathogens that nonetheless pose a significant threat to patients in hospitals necessitates a new emphasis on disinfection measures on those high-touch surfaces. Jinadatha highlights the importance of hand hygiene. He commented, “Best hand hygiene practices remain very effective, probably for all the bugs we know of and all the bugs we found in this study.” He also added that diligently washing hands whenever someone switches to a new surface or task is important because the hospital environment is “dynamic, so when you do disinfection, bacteria have the chance to reaccumulate because people are always moving around, touching different things.”
Even having the awareness to simply capitalize on moments of downtime to keep up on surface disinfection can make a huge difference. Reeducation can play an important role in this, according to Chatterjee. “Reinforcing [workers’] education on things like hand hygiene and highlighting that even if you are not touching the patient, but you are touching these high touch surfaces, you may end up bringing that with you to the next room or to the next patient,” she said.
The basics of hand hygiene are vital to continually reinforce, and healthcare professionals can do a lot with a litt le by simply remaining diligent
in washing their hands after interacting with patients and surfaces alike. That said, there are still some exciting technological innovations in this space that are continuing to be tracked. Jinadatha points out whole room disinfection as well as some progress in terms of understanding clean surfaces and how to track them.
“However, these are all supplemental technologies, not the replacement for manual disinfection,” he cautions. Diligence and adherence to disinfection policies across hospital workers themselves remains absolutely vital, no matter the technological advancements that supplement disinfection.
Jinadatha also makes note of the fact that COVID exposed the sheer human power necessary in keeping a hospital running. “I urge hospital CEOs to do their best to balance our routine requirements and care with what else we can do to keep on evolving as a sector that deals with patient lives,” he noted.
Where to look now
As far as next steps given the results of this study, Chatterjee believes that a look should be taken at some low touch surfaces that we don’t frequently use. If this research is any indication, indeed, there are lurking threats when it comes to infectious bacteria or pathogens that we may not yet fully understand. She mentions new technologies where hospital workers are able to physically see where they have already cleaned or disinfected an area by way of something color-based that lights up an area after it has been disinfected.
Keeping the surface itself and the hospital environment as a whole clean is so vital partially because it is so much easier to keep the spaces free of bacteria in the fi rst place rather than trying to predict which bacteria will cause disease or infection and when. An unintended side effect is that it can be difficult to decisively prove whether or not certain disinfection or sterilization practices empirically work because of how dynamic the healthcare environment can be. This emphasizes even further the need to take preventive steps.
HPN was also able to speak to current APIC president Tania Bubb, PhD, RN, CIC, FAPIC, and Senior Director of Infection Prevention & Control at the Memorial Sloan Kettering Cancer Center in New York, regarding some of the challenges healthcare workers face in surface disinfection. She said, “The type and complexity of the surfaces that are being cleaned post challenges, as some surfaces can be harder to clean than others.” Plus, some organisms simply “live on the surfaces for short periods of time while others live on surfaces for months.” Adherence to policies in place and a clear line of dialogue between healthcare workers and their infection prevention departments are vital strategies, as well.
Bubb also emphasizes the need for the space to innovate in order to tackle “multi-drug-resistant organisms of concern and even novel organisms” that we have not even discovered yet. She calls on the companies that produce these chemicals to “come up with solutions that are more feasible and accessible for the stuff to use to clean.” HPN
For a Q & A on air disinfection with Michelle Vignari, RN, BSN, CIC, Director of Highland Hospital Infection Prevention, continue reading online at hpnonline.com/53098141.
hpnonline.com | April 2024 | 17
Piyali Chatterjee Chetan Jinadatha Tania Bubb
The Multiplier Effect of Success in Sterile Processing
BY KARA NADEAU, SENIOR CONTRIBUTING EDITOR
While we refer to the Sterile Processing Department (SPD) of the year, and the accomplishments of the team, it is important to recognize that a successful department is the sum of its parts, namely the individual sterile processing (SP) professionals doing the work.
The story of Ann & Robert H. Lurie Children’s Hospital of Chicago, the 2024 HPN SPD of the Year Award winner, is one of investing in individual team members’ success to drive department-wide success and, ultimately, organization-wide success in support of the hospital’s mission of a “Healthy future for every child.”
“Our hospital CEO or COO will stop by our department and thank
our team for working so hard and a staff member will very earnestly say, ‘We have to take care of our children,’” said Lurie Children’s Hospital Senior Director, Surgical & Procedural Services John Olmstead, MSN, MBA, CNOR, FACHE. “They take personal pride and ownership of the safety of our patients.”
What’s equally impressive are the efforts of Lurie Children’s Hospital’s SPD team members to share their best practices with other SPD teams in the greater Chicagoland area and throughout the U.S. through presentations, events, and published works.
In the words of Thomas P. Shanley, MD, President and CEO of Ann & Robert H. Lurie Children’s Hospital of Chicago, “The outcomes of this
great effort have not only achieved the project goals but have also created a commitment to excellence which has extended quality enhancements far beyond the walls of the Sterile Processing Department.”
Those who judged this year’s 2024 HPN SPD of the Year nominations scored Lurie Children’s Hospital’s SPD team high across all five criteria categories: customer service, productivity, teamwork, education and training, and strategic outlook. They said of the nomination:
• “The standouts for me with Lurie Children’s Hospital are the level of detail and hard data provided throughout. I was especially impressed by the process and organizational changes that were


Standing: John Olmstead,
Seated: Ricky Williams, Courtney Hardy MD, Linda Darko, Charles Williams, Cherie Dominguez,


 Jonathon Ida MD, Brian Stahulak CNO, W. Zeh Wellington, Santino Bruno, Sayanet Yitbarek, Tyran Dumas
Gifty Agyekum, Linda Williams, Naa-Afi Amamkwah
Jonathon Ida MD, Brian Stahulak CNO, W. Zeh Wellington, Santino Bruno, Sayanet Yitbarek, Tyran Dumas
Gifty Agyekum, Linda Williams, Naa-Afi Amamkwah
STERILE PROCESSING - 2024 DEPARTMENT OF THE YEAR 18 | April 2024 | Healthcare Purchasing News
supported by photos and documentation. Their training was also detailed and innovative.”
• “Kudos to take the time to understand the time it takes to process each piece of instrumentation, multiplied by the number of pieces processed to develop the actual staffing plan. Back to the basics. Year over year continuous improvement.”
• “Their staffi ng model to increase staff was great. It is a good example for others in the same predicament. The workflow redesign is critical for any department to improve workflow and productivity. The organization of their extra wall instrument storage was awesome.”
“When we started out on our transformation journey three years ago, we had a lot of odds against us, but we said success is not linear and nothing of any significance is accomplished alone,” said Lurie Children’s SPD Director W. Zeh Wellington, DNP, RN, NE-BC. “Every single person in SPD has been our inspiration. It has been an amazing journey to where we are today, but there is so much

further we can go. As we tell everyone in SPD, ‘every tool, every tray, every scope that you touch, touches a child, this hospital, this community, this city, this state and it is powerful work we are doing.”
Rather than bulleting out accomplishments across the five award criteria categories, HPN offers a narrative on how Lurie Children’s Hospital’s SPD team has built upon its individual, departmental, and organizationwide successes.
Staffing for success
When Lurie Children’s SPD Leadership team kicked off its performance improvement initiative in 2020, one of the first considerations was staffing levels, which SPD Leadership described at the time as being “woefully inadequate” to support the department’s daily tasks.
The team conducted a comprehensive time study of each major job function in the SPD. Next, they created tools to calculate the total number of full-time employees (FTE) needed to support the average daily
workload, as well as allow time for staff members to engage in collaborative review with customer departments.
Lurie Children’s SPD leadership has since used this “Sterile Process Department Staffing Calculation Method” to justify staff increases each fiscal year as the volume of surgical cases and trays/instruments grows.
In the spirit of best practice sharing, they have documented their staffi ng calculation methodology in an article that is currently under review for publication in AORN Journal.
“As the Sterile Processing Department field has scant FTE benchmarking data, the hope is that this article may assist other SPD leaders in providing justification for adequate staffing,” Olmstead explained.
As noted by Olmstead, operational excellence demanded an increased layer of supervisory support. This led to the establishment of new positions in the SPD, shift supervisors and quality coordinators, and in turn a career ladder for highly motivated team members.




 JAN TERRY PHOTO — COURTESY OF LURIE CHILDREN’S HOSPITAL
JAN TERRY PHOTO — COURTESY OF LURIE CHILDREN’S HOSPITAL
hpnonline.com | April 2024 | 19
The three shift supervisors (termed resource coordinators) serve as point persons for OR/hospital stakeholder questions, vendor issues, and equipment troubleshooting. The quality coordinator is responsible for the SPD monitoring quality program and serves as a back-up resource coordinator (in case of absences).
Resource and quality coordinators routinely perform immediate education to staff involved in rarely encountered instrument issues and are readily available point persons for customers who are frustrated or confused about workflow processes.
Developing SP professionals
The Lurie Children’s SPD team is dedicated to individual staff member development with the goals of improving departmental and hospital performance and advancing the broader SP profession.
Recently, Cherie Dominguez, CSPDT, Lead Resource Coordinator, was invited by the Ann & Robert H. Lurie Children’s Hospital of Chicago Center for Quality & Safety to
participate in a yearlong Improvement Scholars Program.
This interdisciplinary “learn by doing” course empowers participants to learn and apply basic quality improvement science principles to improve functions, outcomes, and processes. Each participant leads an improvement project in their own local area.
Dominguez’s project was on tracing missing instrumentation, specifically missing items in the ENT Endoscopic Sinus Tray. With a price tag of $250,000, reducing missing instruments from this tray contributes to significant savings. Her project has already met its objective in the first quarter of 2024, decreasing missing instrumentation in this tray from 11 days down to 5 within three months.
By systematically continuing to collect data and utilizing a scale method involving SPD and Surgical Services, Dominguez and team will reduce the number of missing instruments by 65% by the end of 2024. The project is now being used as a roadmap for success for other complicated instrument
Table 1: Department Observation Study Outcomes
trays: neurosurgery, cardiology, urology, and pediatric surgery specialties.
Educating individuals
To support SP staff education and training, Lurie Children’s SPD is fortunate to have a dedicated educator in Krystal Westmoreland, BBM, AA, CSPM, CFER, CSPDT. Westmoreland facilitates full orientation for new team members on all department equipment and processes serving hospital and clinic needs. Additionally, she provides a personalized education plan for each team member that tailors the learning process to individual learning needs and speeds.
The outcome: 100% of Lurie Children’s SPD employees have passed the Healthcare Sterile Processing Association (HSPA) and Certification Board for Sterile Processing & Distribution (CBSPD) certification exams. They are the only department in the hospital that can boast 100% certification.
“Our director, manager, and leads are certified but for 100% of the staff to take ownership of their practice and say, ‘I believe in what I am doing, and I
ITEM TO BE PROCESSED DESCRIPTION MINUTES INVOLVED IN PROCESSING Trays Trays of surgical instruments; some are large (neurosurgical trays containing 50+ instruments) some are very small (dental trays containing five instruments) 10 Trays – Prep and Pack 15 Peel packs received from clinics/offices Individual instruments received from offices or clinics that centralize the processing of instruments in SPD versus handling them individually at the office / clinic 30/hour Peel packs received from OR/hospital departments Individual instruments received from the OR or other hospital departments 30/hour Autoclave runs Time involved in loaded, operating, and unloading the steam sterilizing autoclaves used to sterilize surgical instruments 10 Clinic flexible/rigid scopes/equipment Time involved in manually cleaning and sterilizing/high-level disinfecting equipment sent to SPD by offices or clinics that use centralized SPD services instead of performing the function in the individual office/clinic 30 OR/hospital scopes Time involved in manually cleaning and sterilizing/high-level disinfecting equipment sent to SPD by the OR or other hospital departments 45 Case carts Time involved in picking instrument trays from storage for use in the scheduled procedure 10 These times were then used to calculate the total FTE needs that would support the average daily workload. The minutes per activity were multiplied by the average number of task encounters, as illustrated in Table 2. Table 3 juxtaposes the new FTE needs calculation with the inadequate 2020 budgeted amount. STERILE PROCESSING - 2024 DEPARTMENT OF THE YEAR 20 | April 2024 | Healthcare Purchasing News
am going to earn certification to prove it,’ that is powerful,” said Olmstead.
Lurie Children’s SPD Leadership’s dedication to individual team member success is also demonstrated by its development of a training tool for department accreditation inspections. While all SP staff are taught department safety processes during their orientation, it’s no surprise newer SPD team members were nervous when they were told Joint Commission inspectors were planning to pull them aside for interviews during an upcoming inspection.
To help prepare them and alleviate their fears, Lurie Children’s SPD Leadership Team developed a formal Sterile Processing Inspection Preparation Guide. They use this tool to regularly review department processes for regulatory adherence and coach newer SPD employees in answering commonly asked questions by inspectors ahead of an inspection.
As part of the Leadership Team’s desire to share its work, they published the guide on the Association for the Advancement of Medical Instrumentation (AAMI) website on June 23, 2021. The advice about using this information to better prepare SPD staff for inspections was also published in a video blog/YouTube video by Whitman Partners on January 12, 2023.
Additionally, Lurie Children’s SPD Director W. Zeh Wellington, DNP, RN, NE-BC and Manager Charles Williams, CBSPD, gave a 3-hour presentation on their SPD team building practices during the October 2022 OR Manager National Conference.
Enhancing customer service starts from within All the performance improvement work initiated by Lurie Children’s SPD team – from education and training to productivity and efficiency – ultimately benefits end departmental
customers and their patients. This is certainly true for the team’s department redesign. Poorly positioned instruments and equipment led to many unnecessary steps in tray assembly. The average time spent in hunting replacements for missing instruments was 11 minutes.
To support SP team members in accurate and efficient tray assembly, the department replaced six traditional assembly desks with modern workstations, which have adjustable height to accommodate different employees more comfortably. They also placed printers and label makers at each station.
In addition, the team redesigned the instrument backwall, with labeled
hpnonline.com | April 2024 | 21
ITEM TO BE PROCESSED MINUTES USED MONTHLY AVERAGE ANNUALIZED HOURS NUMBERS MINUTES HOURS Trays – decontamination 10 7,433 74,330 1,239 14,866 Trays - Pre and Pack area 15 7,433 111,500 1,800 21,600 Peel packs received from clinics/offices 30/hr 2,500 — 83 1,000 Peel packs received from OR/hospital departments 30/hr 2,500 — 83 1,000 Autoclave / Low temp sterilizer runs 10 880 8,800 147 1,760 Clinic/office flexible/rigid scopes/ equipment 30 190 5,700 95 1,140 OR / hospital scopes 30 190 5,700 95 1,140 Case carts – per case 10 920 9,200 153 1,840 Totals 3,695 47,000 Adding 12% benefits 54,500 # FTEs using 6.5 hour days 32.0 Adding Shift Supervisors 2 Adding Liaisons 2 Total FTEs needed 36.0
Table 2: Fiscal year 2024 SPD FTE calculation figures
FISCAL YEAR 2022 2023 Planned for 2024 Supporting FTEs Manager FTE 1 1 1 Department Educator 1 1 1 Quality Coordinator 0 0 1 Resource Coordinators 0 3 3 Additional Runners, Mon-Fri • Two on AM shift • Two on PM shift 0 0 4 Productive FTEs Staff FTEs 17 23 24 Total budgeted FTEs 19 28 34
Table 3: Annual FTE comparison, before/after use of the revised calculation method
locations for all instruments. They next redesigned the instrument locations in the instrument tracking system software program. In doing so, team members know exactly where on the instrument wall to locate replacement instruments for trays and the corresponding instrument wall location assignment designations.The result was a 64% reduction in time spent locating replacement instruments, from an average of 11 minutes to just 4 minutes.
Furthermore, the new staffing model and department redesign have driven significant improvement in the department’s first pass yield completed trays percentage. Prior to this work, the percentage of trays that were filled per the tray contents instrument sheet hovered around 75%, on average.
Following the performance improvements, the average trays completed at first pass reached the department’s target of 95%, often exceeding this goal. This level has been sustained despite the surgical volume growth of 2022, when average daily surgical volumes grew from 55 to 60 cases per day to 75+ cases per day.

“We listened to our team on what they needed for the department, and they were right,” said Wellington.
Collaborating with customer departments
SPD and OR team collaboration is critical to patient care quality and safety. The Lurie Children’s SPD Leadership team appointed service champions for the major operating room (OR) services to facilitate better interdepartmental communication. SPD staff who show interest in particular services are chosen as champions and given time to meet with the individual OR clinical coordinators of those services.
Coordinators review instrument tray issues with SPD service champions who in turn educate other SPD staff members on items pertinent to those services. To date, the department has established champions for cardiology, pediatric surgery, oncology, neurosurgery, and ophthalmology services.
“The days of SPD being in a silo downstairs is gone,” said Wellington. ”SPD is instrumental to the success of the OR. And having SPD work with our clinical coordinators, talk with the surgeons and learn what an instrument or device is for, that is powerful work.”
They have also applied their improved customer service mindset to the hospital’s emergency department (ED). Historically, the ED team would use a set of trauma instruments and send them to the SPD in hopes of receiving them back before additional traumas appeared. In numerous instances, the wait time averaged 12 to 24 hours.
The Lurie Children’s SPD Leadership team implemented an exchange program, which effectively eliminates turnaround time. They arrange duplicate instrument sets commonly used in trauma cases in the SPD. Following a case, an ED team member
transports the used instruments to the SPD and is given a replacement tray upon receipt.
Sharing best practices in Chicagoland and beyond
Throughout all their initiatives, Lurie Children’s SPD team has demonstrated its desire to share best practices with other healthcare organizations. Last year, the SPD Leadership team launched a program to facilitate collaboration among Chicagoarea SPDs. Olmstead summed up the spirit behind it when stating, “While businesses may compete, SPD professionals all face the same competitor: infection.”
On January 27, 2023, the Lurie Children’s SPD team held its Chicago SPD Forum Luncheon where they hosted the first ever Sharing, Promoting and Discussing Forum. In attendance were SP leaders from Advocate Christ Medical Center, Rush University Medical Center, Northshore University HealthSystem, Northwestern Memorial Hospital, and Prentice Women’s Hospital. Lunch was provided by a national vendor, and attendees engaged in conversations on opportunities and barriers to operations success.
The event was such a success that Lurie Children’s SPD Leadership team has decided to make it an annual event.
“We reached out to the community but didn’t know if anyone would show up. It turned out, everybody showed up,” recalled Olmstead. “If people want to benefit from what we are doing, we want to benefit from what they are doing as well.”
Looking ahead
Never content to rest on their laurels, the Lurie Children’s SPD team has in place a strategy for their future, which aims to leverage the superior education and training of the team to better support the growth strategy
STERILE PROCESSING - 2024 DEPARTMENT OF THE YEAR 22 | April 2024 | Healthcare Purchasing News
SPD Manager Charles Williams at OR Manager Conference, October 2022.
of the hospital. Key initiatives in 2024 include:
• Analytics: Development of an official SPD dashboard that will be reported monthly to surgical and hospital leadership, along with other operational dashboards such as the emergency department, infection prevention, and inpatient length of stay. The dashboard will be populated by a combination of electronic databases and will provide an instant summary of improvement activities.
• Clinic support: Lurie Children’s Clinical Services cover a host of physician offices across the Chicagoland area and serves an average of 2,400 patient visits per day. The larger clinics have built functional procedure rooms where minor examinations and surgical procedures are performed. The SPD team is centralizing the processing of used instruments at surgical department hubs. After sterilization, instruments are repackaged and returned to their respective clinic locations. Additionally, the SPD educator is serving as a regional consultant, providing education and direction for appropriate handling and storage for surgical instruments.
• ASC expansion support: The SPD Leadership team is also an integral part of the Ambulatory Surgery Center (ASC) Expansion Project. Due to maximum capacity constraints at the main hospital’s 21 ORs, hospital administration is coordinating with surgeons to double the number of patients treated in the northern and western ASCs, thereby freeing up valuable downtown OR time that will allow the reduction of back-logged surgery patients. This increase in ASC volume brings a host of instrument supply and processing issues, so the weekly consultation between ASC and SPD leadership will be critical to

the successful provision of surgery services in those centers.
“I want SPD to be a competitive advantage,” said Olmstead. “It is not just a service we provide but a bonus. I feel like it is now but with just a couple of things we have planned moving forward, the case will be stronger.”
A footnote to the story
The Lurie Children’s SPD team submitted their nomination to HPN at the time when “the damaging effects of the January 31, 2024 cyber-attack had disabled all computer networks at Lurie Children’s Hospital and associated clinics and ambulatory surgery centers.”
They had no access to the hospital’s electronic medical record (EMR) system; no communication between the EMR and associated partner programs, including their instrument tracking system; and no telephone access or Wi-Fi operated telephone access – communication was relegated to walkie-talkie and cell phone text messages.
Despite these challenges, the Lurie Children’s SPD team, led by Department Manager Charles Williams and
Resource Coordinator Cherie Dominguez instituted manual processes to meet all needs of the OR and hospital including:
• Implementing a manual doublecheck process to fill case cart needs minus the EPIC-SPM Preference Card interface information.
• Implementing a revised doublecheck method to reach manufacturer instruction for use (IFU) information should it be necessary to review.
• Innovating a method to print and make available all department policies for staff review.
• Implementing a series of paper logs to record all safety and biologic testing done to support sterility expectations.
“Charles and his team had to convert to 100% paper, no preference cards. I don’t know how they did it,” said Olmstead. “We do complex surgeries here and there has not been a single quality or safety issue or even delay from SPD during this cyberattack.” HPN
Visit hpnonline.com/53097994 for more photos and charts.
hpnonline.com | April 2024 | 23
Redesigned instrument backwall.
Water for cleaning medical devices
BY JEANE APARECIDA GONZALEZ BRONZATTI, RN, AND RAFAEL QUEIROZ DE SOUZA, RN
Cleaning is considered a fundamental step in the processing of healthcare products. It removes a variety of organic and inorganic dirt from instruments used in surgical procedures such as blood, proteins, lipids, bone, and ophthalmic viscosurgical devices, among others. In practice, medical devices are immersed, rinsed, or sprayed with water or solutions with surfactants to prevent the dirt from drying out. There are cases in which a pre-cleaning procedure in care areas needs to be carried out, for example, when there is a large amount of organic matter such as feces, blood, or other contaminants.1
To make the removal of dirt to safe levels possible, it is necessary to use either manual or automated cleaning methods - or a combination of both. In all cases it is necessary to use water,
Learning Objectives
1. Describe recommendations for adequate water quality for cleaning medical devices
2. Identify the impact of water quality on medical device reprocessing
3. Relate water quality to adverse events described in the scientific literature
Sponsored by:

which acts as a solvent, and is one of the essential elements of the Sinner’s circle2 along with four other factors: time, mechanical action, temperature, and chemical activity.3
Considering water as an essential element, its use occurs in several stages of processing: displacement of dirt through spraying in thermo-disinfecting washers and cavitation in ultrasonic washers, dilution of cleaning solutions, and in rinsing of medical devices. Thus, water quality control is essential for the cleaning process to be effective. (See Table 1.)
Additionally, there are documents that supplement the AAMI TIR34:2014 with product-specific recommendations as well as local guidelines and regulations. These additional guidelines should be consulted in the instructions for use of each product. For example, the TIR34 provides different indications for rinsing after high-level disinfection, while the Gastroenterological Society of Australia – GESA5 has specific and additional recommendations for the quality of water used in the processing of endoscopes (Tables 2 and 3).
In ophthalmology, there are specific recommendations due to the occurrence


of acute inflammations of the anterior chamber, or segment, of the eye following cataract surgery characterized as TASS: Toxic Anterior Segment Syndrome. That said, the guidelines of the American Society of Cataract and Refractive Surgery (ASCRS), American Academy of Ophthalmology (AAO), and Ophthalmic Outpatient Surgery Society (OOSS) recommends the use of tap water only when specified by the product’s instructions for use, the latest critical water rinse,6 as well as the Association periOperative Registered Nurses.7
Although there are differences in terms of recommendations, it is important to establish a control and monitoring program aimed at reducing risks for the patient and for maintaining instruments and equipment.
Water Quality Impacts on the Sterile Processing Department
Detergent interactions
Chemical activity is a component of the Sinner’s circle and is essential for efficient cleaning to be carried out.2 Detergent formulations contain surfactants, whose function is to allow
Specifications Units Utility water Critical water Hardness mg/L < 150 <1 Conductivity µS/cm < 500 <10 pH 6 - 9 5-7 Chlorides mg/L < 250 <1 Bacteria cfu/mL n/a (< 10)* <10 Endotoxin EU/mL n/a (< 20)* <10 *After high-level disinfection
2014.4 CONTINUING EDUCATION 24 | April 2024 | Healthcare Purchasing News
Table 1. Categories and recommended levels of water quality for medical device reprocessing
Source: AAMI TIR34,
water-insoluble dirt to become soluble through its molecule that contains a hydrophilic and a hydrophobic portion, acting as a bridge between water and dirt.8
Surfactants interact with water contaminants in the same way. During cleaning, the use of hard water with a high concentration of calcium and magnesium ions reduces the surfactant available for cleaning medical devices because of the formation of insoluble and chemically inactive salts, compromising the cleaning efficiency.8,9
Another important variable that can interfere with enzymatic activity is pH, especially in the cleaning steps when detergent is used, thus, the instructions for use of each solution must be considered so that the enzymatic activity is not compromised.4
Instrument conservation
Surgical instruments are made of stainless steel; however, they are not indestructible. Stainless steel is composed of iron, carbon, chromium, nickel, manganese, silica, and other metals. Surgical instruments made of stainless steel undergo a process called passivation, which makes them less “reactive” and therefore less susceptible to corrosion.10,11
The process of forming the passive layer can be carried out by treating the instrument’s surfaces with substances that remove iron from the surface, but maintain the chromium, which is the metal responsible for the “passive” characteristic of the instrument. When broken, the passive layer can “regenerate” when exposed to air, however
in the presence of dirt there will be no exposure to air and regeneration will not occur.11
Certain chemicals and contaminants in the water used in reprocessing can also damage the passive layer, in addition to various stains on instruments and equipment. Table 4 summarizes the most common problems related to poor water quality and their potential causes.
In general, the investigation to determine the causes of stains involves the following activities (Table 5).
In the case of new surgical instruments, they must be removed from the plastic packaging as this material allows condensation which can cause rust. Additionally, they must undergo reprocessing to remove oils and other residues derived from the manufacturing process.10
Adverse events
Adverse events resulting from contamination of the water used in the reprocessing of medical devices can be TASS, aseptic loss of implants, and pyrogenic reactions. Cases listed below will be described to illustrate the importance of using critical water for the last rinse, and also in the generation of steam as preventive measures.
Lesson:
Water for cleaning medical devices
April 2024
This lesson was developed by 3M Health Care. Lessons are administered by Endeavor Business Media.
Earn CEUs
After careful study of the lesson, complete the examination online at educationhub.hpnonline.com. You must have a passing score of 80% or higher to receive a certificate of completion.
Certification
The Certification Board for Sterile Processing and Distribution has pre-approved this in-service unit for one (1) contact hour for a period of five (5) years from the date of original publication. Successful completion of the lesson and post-test must be documented by facility management and those records maintained by the individual until recertification is required. DO NOT SEND LESSON OR TEST TO CBSPD. www.cbspd.net.


Healthcare Sterile Processing Association, myhspa.org, has pre-approved this in-service for
1.0 Continuing Education Credits for a period of three years, until January 29, 2027.
For more information, direct any questions to Healthcare Purchasing News editor@hpnonline.com.
hpnonline.com | April 2024 | 25
Quiz Answers: 1. B, 2. A, 3. B, 4. A, 5. A, 6. B, 7. B, 8. A, 9. A, 10. A
reprocessors
Substance or parameter Before disinfection Chemical purity As per manufacturer’s instructions Total viable count ≤ 10 cfu/100 mL Pseudomonas aeruginosa and atypical Mycobacterium species Nil detected/100 mL Endotoxin ≤30 EU/mL *Source: Gastroenterological Society of Australia, 2021.5
Table 3. Water quality for automated flexible endoscope
final rinse water*
Substance or parameter Before disinfection Water hardness < 150 mg/L Chloride < 120 mg/L *Source: Gastroenterological Society of Australia, 2021.5
Table 2. Water quality for pre-cleaning, cleaning and rinsing (before disinfection)*
Ophthalmology: The reported cases of TASS are associated with contamination of the instruments due to water. There are cases involving endotoxins12 and inorganic contaminants.13 One study evaluated the cytotoxicity of cannulas for hydrodissection subjected to challenge contamination, including
cleaning based on a validated standard operating procedure (SOP) and final rinsing in different water qualities, demonstrating the absence of cytotoxicity, regardless of the quality of water used in the last rinse. However, the authors highlighted that the data were obtained from new instruments, that is, in a good state
of conservation.14 Considering the importance of water for the conservation of instruments, the authors did not recommend the use of tap water, therefore, the TIR34 recommendations must be followed. Another relevant aspect observed by the authors is the importance of adherence to cleaning SOPs, due to
Problems
Residual dirt
Instrument surface damage:
Corrosion
Pitting
Rusting
Stress fracture
Loss of color
Discoloration
Gold-brown
Orange-brown
“Rainbow”
Black or purple staining (commonly observed after steam sterilization)
White staining or deposits (observed following drying or steam sterilization)
White, chalky buildup in the water lines, lumens, and valves of an automated processor
Biofilm
(Slime development over time, often appearing as different colors)
Source: Adapted from AAMI, 2014.4
Potential causes
Inefficient cleaning.
• Drying of dirt on the surface
• Exposure to some chemicals (e.g. saline solutions, chlorine, and low acidic or high alkaline chemistries)
• Chlorinated water (especially when heated) or high/low pH water
• Exposure to some chemicals (e.g. chlorine solutions, and low acidic or high alkaline chemistries)
• Chlorinated water (especially when heated) or high/low pH water
Excessive heating to stainless steel surfaces, combined with various water deposits
Phosphate layer developing on surface (from poor water quality and even some phosphatecontaining cleaning chemistries that are not rinsed correctly); often seen as orange-brown discoloration
Chromium oxide development observed as a “rainbow” stain that develops over time (and can include various blue-brown colors from the presence of copper and iron)
• High or low pH residuals remaining on the device following cleaning
• Can be from water quality or insufficient rinsing (or neutralization) with low acidic or highly alkaline cleaning chemistries
• Water hardness
• Combination with Other chemical contaminants (such as copper and iron) to give different colors
• Other chemical residuals (e.g., residuals from inadequate rinsing of cleaning chemistries, other water contaminants such as silicon oxide)
High volume of water with high mineral content, resulting in mineral buildup
• Ineffective maintenance of devices/equipment
• Inadequate contact (during cleaning/ disinfection) and poor water draining (e.g., pooling)
Inspect and remove instruments with rust Visible rust, corrosion, and pitting (rust may transfer and “seed” onto quality instruments.*)
Review storage conditions
(*instruments stored wet are subject to rust)
Test water quality used in the various stages of processing
*Seavey, 2015.10
Instrument and packaging humidity, ambient humidity within acceptable limits, furniture and surfaces clean and dry.
Control and monitoring of water according to each stage of reprocessing and the conditions required by the TIR34 and other applicable documents.
CONTINUING EDUCATION 26 | April 2024 | Healthcare Purchasing News
instruments: activities and verification items. Activity Items to be verified: Audit the instrument processing steps Point-of-use cleaning procedures, disassembly of surgical instruments for cleaning, bristles of cleaning brushes, dilution of chemical solutions, manual and automated cleaning procedures, drying, inspection, packing, instructions for use compliance. Review cleaning and sterilization equipment maintenance Maintenance and qualification of cleaning and sterilization equipment, steam generator,
pipes,
Table 5. Investigation of stains on surgical
steam supply network and
water treatment systems, instructions for use compliance.
Table 4. Examples of observed problems during device reprocessing that can be caused by poor water quality:
the high level of cytotoxicity of dirty instruments, since the last rinse will not compensate for poor cleaning.
Orthopedics: Instruments for orthopedic surgeries present a high level of complexity, favoring the retention of dirt15-18 and endotoxins after cleaning and rinsing with drinking water, especially intramedullary cutters and femur scrapes.19 In the case of flexible cutters, the retention of dirt can be cumulative between the steel blades that make up the flexible body and the toxicity of the accumulated residual is unacceptable for use.20 It’s likely this problem can be accentuated with endotoxins coming from rinse water when not properly treated. Endotoxins may also be related to the aseptic loss of implants, with adherence to cleaning and fi nal rinsing SOPs with critical water being essential.21,22
Cardiac catheterization: The main adverse event reported is the pyrogenic
reaction caused by endotoxins after cardiac catheterization, in which patients developed fever and chills, with or without hypotension.23, 24 A literature review on cardiac catheter reprocessing identified three articles reporting cases of pyrogenic reactions with a potential common cause: inappropriate water quality.25 In one of the studies, an increase in the amount of endotoxins and microorganisms was observed in distilled water stored by the hospital and used in the processing of catheters.23 The results demonstrated that water storage can be one of the critical factors in controlling water quality. In the studies found in the review, interventions to contain the cases were limited to the use of endotoxin-free water, sanitization of water distribution systems, and sterilization of catheters on the same day of reprocessing (possibly, the catheters would be less exposed to residual moisture
Water for cleaning medical devices - Practice Quiz
1. Cleaning is a procedure used to remove organic and inorganic matter, except hemoglobin.
A. True B. False
2. The Sinner cycle requires time, mechanical action, temperature, and chemical activity.
A. True B. False
3. Utility water is mainly used for flushing, washing, rinsing, and steam generation.
A. True B. False
4. Medical devices such as endoscopes and ophthalmic instruments may require additional guidance on precleaning and water quality. That said, additional manufacturer guidelines must be followed.
A. True B. False
5. Cleaning can be compromised by both the pH of the water and the hardness of the water.
A. True B. False
and consequent contamination). These results reinforce the need to use critical water to prevent pyrogenic reactions.
Conclusion
The quality of cleaning water must be controlled for the following reasons:
• Ensure the effectiveness of dirt removal, avoiding the reduction of detergent activity.
• Avoid recontamination of instruments with rinse water residues, including microorganisms and endotoxins.
• Preserve surgical instruments, preventing pitting, corrosion, and various stains.
• Maintain equipment efficiency, avoiding encrustations in pipes, chambers, and resistances.
• Ensure that medical devices are free from toxic waste capable of causing adverse events. HPN
Author bios and references at hpnonline.com/21289384
6. The passive layer of surgical instruments is formed by the enrichment of surface iron, which takes place through the removal of chromium, providing corrosion resistance.
A. True B. False
7. Excessive chlorine in the water is responsible for the formation of deposits on the instrument surface and the clogging of water pipes.
A. True B. False
8. Pooling in automated cleaning equipment, generally due to drainage failure, can induce the formation of biofilm on the equipment.
A. True B. False
9. Adverse events resulting from contamination of the water used in the reprocessing of medical devices can be TASS, aseptic loss of implants, and pyrogenic reactions.
A. True B. False
10. Instrument rinsing water can become contaminated during storage; therefore, this is one of the critical points in water quality control.
A. True B. False
All CEU quizzes must be taken online at: educationhub.hpnonline.com. The cost to take the quiz is $10.


hpnonline.com | April 2024 | 27 PURC HASI NG NE WS Healthcare ™ EDUCATION HUB
Taking SP Leadership Journey Offers Big Gains
BY DAVID L. TAYLOR, MSN, RN, CNOR, PRINCIPAL, RESOLUTE ADVISORY GROUP LLC
Sterile Processing (SP) professionals dedicated to advocating for and providing safe, efficient, high-quality customer service that promotes better patient outcomes have a great deal to offer—to their teammates, facilities, professional peers, and others within their communities and professional organizations. Opportunities to contribute to standards development, knowledge sharing, committee involvement, and greater participation within the Healthcare Sterile Processing Association (HSPA) or other professional associations are numerous.
Whether it comes in the form of networking to develop lasting personal and professional relationships, volunteering or being part of other efforts to enhance knowledge, competencies and work quality, active participation, and a commitment to service excellence not only can advance one’s career but also boost accountability and outcomes. Professional development requires a commitment to lifelong learning, inquiry, reflection, and response to new ideas and process changes. Rarely is the path to professional growth easy or clear. Still, if one’s goal is to become part of something bigger, leave a positive impression, and steer effective change, taking time to participate in professional organizations can make the journey less daunting and more fulfilling. It can also encourage new and upcoming generations of SP professionals to follow a similar path toward personal growth and professional excellence.
Serving a higher calling Professional healthcare organizations and associations are important not only for industry professionals but also the patients they serve. Their missions and work can also help generate ideas, steer improvements, and contribute to standard and health policy development. There is significant research and data demonstrating the benefits of not only joining a professional organization but serving in a deeper capacity. Local HSPA chapter membership and active participation is just one way SP professionals can make meaningful strides through networking and knowledge-sharing. Networking is vital for building professional connections, and peer-topeer interactions allow for direct collaboration, real-time feedback, and reflection. Additionally, networking is integral to professional development, helping participants explore new jobs or knowledge-sharing opportunities to take their career to the next level.
Chapter membership provides an opportunity to lead at the local level, and those opportunities can be further developed by volunteering in the local community to support the chapter (and profession) and spread the message about its purpose, mission, task forces, and committee work. Gaining membership, leadership, and other professional expertise within a chapter can help prepare SP employees for more roles and responsibilities within HSPA (and other associations), such as national committee involvement or perhaps even elected positions on the Board of Directors. Further,

that experience can lead to greater leadership opportunities within SPDs and perhaps even other organizations within the community.
Researching new ideas and creating content is another valuable facet of leadership. Submitting articles for HSPA’s publications or participating in podcasts or educational poster presentations, for example, are great ways to share knowledge, engage in scientific discovery, and share industry trends, successes, challenges, and more. Presenting at conferences can also have a profound effect on one’s career. There are local, national, and international speaking opportunities for skilled SP professionals; those with no or limited experience will benefit from starting public speaking on smaller stages, such as those at chapter meetings or events, or even at training events or other meetings within one’s own healthcare facility.
Committing to lifelong learning and knowledge sharing through association, committee, and membership involvement not only helps SP professionals gain a better understanding of the complexities and importance of their various roles and responsibilities, but also boosts confidence and career satisfaction. As importantly, building on one’s expertise has deep and positive effects on customer service, patient outcomes, and the organization’s success.
For more information about HSPA, its chapters, publications and other support offerings, and to learn how to get started, visit www. myhspa.org. HPN
ASSOCIATION: HSPA 28 | April 2024 | Healthcare Purchasing News


















ELEVATE YOUR TEAM’S EXPERTISE OVER LUNCH — ON US. Discover how our NEW Shift Kits and ergonomic brushes can redefine your SPD workflow while team members earn 1 CEU credit toward certification. Sign Up Your Facility by April 30 to Get Your Free Lunch. VISIT SHARN.COM/SPD
Bone Cement and Black Light Detection - Part 1
BY STEPHEN M KOVACH, BS, CFER, CLINICAL EDUCATOR EMERITUS, HEALTHMARK INDUSTRIES
Q“Recently, I read a posting on the Internet that one can use black lights to detect residual bone cement. Is this true,1 and could our department use a black light for this purpose?”
AWe all have watched forensics crime television shows where they use a “black light” to find blood and other substances. It must work for detecting bone cement, right? “Under the black light, substances not seen by the human eye may be visible.”2 Bone cement is hard for us to see, too, so we all want an easy tool to detect any residual residue. I read it on the Internet, so is it okay to use….?
Posts I see online do not provide clinically relevant evidence to back up this black light inspection method, nor are they sharing their policies about how to use the black light. Not saying it is not a useful tool for this purpose, but I do not see the same critical thinking used for other methods also being applied to this “black light method.”
As with anything, one must understand the tool to be used. Long ago, my mother, Irene, often reminded me to remember the 5 W’s (who, what, where, when, and why) and one H (how) when you do something.
If the black light shows something glowing on the medical device, what does it really mean? Better yet, what does a black light show when it is waved over the item?
What you see glowing under the black light is fluorescence. Whether it is your T-shirt, a poster, or maybe a medical device, the object must have some type of phosphor to show up and glow. Thus, what we are talking about is understanding fluorescence, and whether or not it can be applied to finding bone cement left on medical devices.3,4
UV-A, which is also called “black light” or “Long Wave” UV, spans wavelengths between 320 and 400 nm. Here are some questions I would like to raise.
• Is there a specific wavelength we need within this spectrum to pick up bone cement?
• Must the work area have certain il lu mination?
• Does background light have a direct or indirect impact on what we see?
• If no bone cement is detected, how do you confirm that?
• What is the detection limit?

Next, I know of at least four types of bone cement. Does it work on all of them? We know that if a substance contains phosphor, then a black light should pick it up, but how much phosphor do you need for it to be detected?
The next set of questions is based on bone cement. Does bone cement:
• Contain enough phosphor (or other radiopaque material) to be detected by a black light?
• Need a specific wavelength?
• Have a minimum detection limit and at what wavelength? Plus, we need to ask:
• Is 1.0 µ of phosphors detectable?
• What is the lowest level that is safe?
We also need to examine the word “cement,” as it “is a misnomer. The word cement is used to describe a substance that bonds two things together. However, …” in orthopedic surgery procedures, these substances “… act as a spacefiller that creates a tight space which holds the implant against the bone and thus acts as a ‘grout’.”5 For this article, we will use the term “bone cement,” which we all are accustomed to.
There are many different commercially available bone cements (polymethyl methacrylate (PMMA), calcium phosphate cements (CPCs), and glass polyalkenoate (ionomer) cements (GPCs)). Many of these bone cements also have barium sulphate (BaSO4) or zirconium dioxide (ZrO2) in the bone cement, which are a radiopaque material. These types of commercially available bone cement are successfully used in orthopedic and dental applications. The problem is that some cement gets left behind on the medical devices, and it is difficult to detect and clean off. The question is: Could a black light pick up that residual bone cement if left on the medical device? In a month, as the late American Radio Broadcaster Paul Harvey would say, “… the rest of the story,”6 in part 2! HPN
References
1. Jagrosse, D. (2023, February). Are you using a black light on SPD Instrument assembly to look for residual bone cement? Thoughts? Outcomes? [Image attached] [Post]. LinkedIn. https://www.linkedin.com/posts/jongoodwin3_apa2019-activity6569581103441682432-CN98
2. Brenner, L. (2018, April 26). What Kind of Invisible Stains Do Black Lights Detect? Sciencing. https://sciencing.com/kind-stains-black-lights-detect-5045775.html
References continue online at hpnonline.com/53097904.
STERILE PROCESSING INSIGHTS 30 | April 2024 | Healthcare Purchasing News
Venous Blood Collection
Designed for simple and safe blood collection.

Unistik® VacuFlip and Unistik® ShieldLock are engineered for intuitive blood collection, featuring easy to use safety mechanisms that can help reduce the risk of needlestick injuries. Unistik® Blood Collection devices are cost-effective and designed to not compromise quality or safety.









































BCAD23/OMI/0823/1/US For more information, call 1-800-421-6936 or visit owenmumford.com
AVAILABLE! Scan here to learn more!
NOW
Making the Case for Standards and Scanning
BY KAREN CONWAY, VICE PRESIDENT, HEALTHCARE VALUE FOR GLOBAL HEALTHCARE EXCHANGE (GHX)
In 2018, I wrote about a successful demonstration program in the U.K. that documented the value of using standardized GS1 barcodes to track inventory from the point of manufacture to the point of use. At the time, I questioned why more National Health Service (NHS) trusts had not adopted the program, given that the six demonstration sites achieved better operational efficiency, cost savings, and enhanced patient safety measures. The program is finally getting traction, even without government funding or mandates, and it provides lessons on the value of standards, barcode scanning, and going beyond what is required or funded.
What is Scan4Safety?
Under the Scan4Safety (S4S) program, healthcare delivery systems (called trusts in the U.K.) employ barcode scanners to capture data on patients and the products used in their care. In 2016, the U.K. government provided seed funding to six NHS trusts to measure how adopting and scanning GS1 barcodes could “enhance patient safety, improve operational efficiency, and reduce costs.” The results were overwhelmingly positive, with the trusts freeing up clinician time to spend on patient care, improving recall response time and costs, lowering costs through product standardization, and reducing inventory holding costs.
What is the current state of the program?
Despite the lack of funding or mandates, an increasing number of NHS trusts are implementing S4S, and NHS England is prioritizing the program to improve the use of supply chain data as a strategic asset.
Like its peers, East Lancashire Hospitals NHS Trust launched S4S in the operating theater, where it scans both implants and consumables at the point of consumption. Product replenishment is automated based on what was used; product picking takes minutes, not hours; and recalls are handled in seconds vs. days or weeks. As supply chain takes over tasks previously handled by clinicians, clinical teams have received 250 weeks of time

back to spend on patient care over two years. Finance enjoys higher stock values on the balance sheet, while inventory holding costs are down. The success of the initial rollout has enabled the integrated care system to have a common inventory management strategy, with opportunities to share stock and standardize processes across all locations where S4S is implemented.
At Manchester University NHS Foundation Trust, S4S has improved how inventory is managed. By educating clinical teams, the trust has reduced incidences of products being opened and not used or faulty products being thrown away vs. returned to supply chain and the manufacturer. The trust has seen measurable savings by increasing compliance with standardization initiatives and reducing the number of times the Trust is charged for products that have not been used.

Both trusts have linked their respective inventory management and electronic patient record systems, allowing for real-time data capture on the products used in patient care. At these and other trusts implementing S4S, the key to the accuracy of this data has been access to a product catalog that employs the GS1 Global Trade Item Number (GTIN) to identify products. GTINs are among the codes compliant with unique device identification (UDI) rules. While UDI is not yet a requirement in the UK, there is strong support within the NHS and among the S4S trusts for the adoption of GS1 standards, which, like in the U.S., are used to identify products and locations. In the UK, there is also a GS1 standard to identify patients, which combined with the other standards increases visibility into what products are used on which patients and where. This, in turn, supports an NHS objective to understand the role of products, particularly high-risk medical devices, in improving outcomes. The success of S4S offers an important lesson to health systems in the US and beyond that, even without government funding or regulatory requirements, there is a strong business case for investing in inventory management with barcode scanning and the use of standardized identifiers. HPN
VALUE. DELIVERED. 32 | April 2024 | Healthcare Purchasing News
259094020 © ANDREY CHERKASOV STOCK.ADOBE.COM







AESCULAP Aicon™ Sterile Container System
Unwrap the future of sterile supply management. With features that help streamline processes and reduce the possibility of wet sets, Aesculap’s next generation rigid container is everything you’d expect from the market leader.
Visit aesculapusa.com/aicon to learn more about how this breakthrough technology can help your SPD Operate With Greater Precision.
Meet our newest STERILIZATION POWERHOUSE.
OPERATE WITH GREATER PRECISION
Aesculap, Inc. - a B. Braun company M e e t o u r n e w e s t
Booth 801

Flexible Inspection Scope
Designed to visually inspect internal channels of potentially soiled or damaged items, this borescope provides enhanced light, vision & magnification. Visit our booth for a hands-on experience!




Detect and locate defects such as pinholes, cracks and bare spots in the jacket or coating of laparoscopic & bipolar electrosurgical instruments. Visit our booth for a hands-on experience!

McGan Insulation Tester Join Healthmark for In-Booth Education Sessions


Earn Continuing Education Units while learning about topics critical to infection prevention, sterile processing, endoscopy & more from our team of experienced industry professionals. Visit our team in booth 801 during HSPA 2024 to learn more!
Healthmark
Visit
at HSPA 2024 April 21 - 23 • Las Vegas, Nevada
hmark.com 800.521.6224







































































































































 Jonathon Ida MD, Brian Stahulak CNO, W. Zeh Wellington, Santino Bruno, Sayanet Yitbarek, Tyran Dumas
Gifty Agyekum, Linda Williams, Naa-Afi Amamkwah
Jonathon Ida MD, Brian Stahulak CNO, W. Zeh Wellington, Santino Bruno, Sayanet Yitbarek, Tyran Dumas
Gifty Agyekum, Linda Williams, Naa-Afi Amamkwah





 JAN TERRY PHOTO — COURTESY OF LURIE CHILDREN’S HOSPITAL
JAN TERRY PHOTO — COURTESY OF LURIE CHILDREN’S HOSPITAL











































































