

















































The Next Generation Of Contamination Monitoring Technology
For the cleaning verification of surgical instruments, endoscopes, and surfaces, ATP Complete 2 by Ruhof a cloud-based cleaning monitoring system used to help hospitals and other healthcare organizations achieve optimal standardized cleaning levels.






















Sourcing & Logistics
8 > 2024 Supply Chain Department of the Year: University of Utah Health
JANETTE WIDER
16 > Optimizing Healthcare Procurement: Balancing Quality and Cost
SPONSORED
32 > Sustainable Healthcare: In Supply Chain’s Sweet Spot
KAREN CONWAY
Surgical/Critical Care
12 > Setting the Latest Standards in Room Cleaning and Disinfection
KARA NADEAU
Departments
4 > Why I Love Supply Chain
6 > What’s on the Web, Advertiser Index

Sterile Processing
18 > Key Considerations for Transitioning Scopes from HLD to Sterilization
KARA NADEAU
22 > Meeting the Challenges of Endoscope Reprocessing and Documentation
RHONDA THOMPSON
28 > Leverage Interdepartmental Relationships for SPD Quality
DAVID TAYLOR
29 > General Ultrasonic Cleaner Questions
STEPHEN M. KOVACH
Special Report
30 > Protecting Against Ransomware Attacks on Supply Chain


MATT MACKENZIE 8 12 30 18 CONTENTS > SEPTEMBER 2024
COVER PHOTO COURTESY OF CHARLIE EHLERT, UNIVERSITY OF UTAH
Front row, Left to Right: David Schiff, Brian Pollick, Mark Fawson, Cody Hansen, Nicholas Taylor
Second row: Jacob Vonk (black tie), Scott Brennan (green tie)
Third row: Destiny Burbank (blue blazer), Kraig Kirk, Laurel Bailey, Christian Chavez
Fourth row: Saundra Yates, John Kelly (maroon tie)
Fifth row: Donald Moosman (black shirt, grey facial hair), Spencer Brudnicki, Sheila Payne, D.J. Lambert, Matthew Glick (glasses, brown facial hair, red shirt)
Sixth row: Peter Marcelis (glasses, red shirt), Seth Moulton, Chandra Rai, Lydia Roll, Are Yount (pink shirt)
Seventh row: Kallie German (black shirt), Scott Merkley, Juan Sarena, Anton Ermeev, Edison Butters
Eighth row: David Canada (light grey shirt), Connor Brigante, Bhakti Bhurtel (green hat)
Ninth row: Mitchell Quintana (black shirt)
Back row, left to right: Bryan Kilpatrick, David Chinea, Abe Wallin (red hair), Austin Pollick

BY JANETTE WIDER, EDITOR-IN-CHIEF


Abefore I took over as Editor-in-Chief of Healthcare Purchasing News, I worked on both of our sister publications, Healthcare Innovation and Medical Laboratory Observer. These two publications only touch on supply chain and I was thrilled when the opportunity to come over to the supply chain side was presented to me.
Why, you ask? Because of my dad.
My father has worked in purchasing and supply chain for his entire career. I grew up hearing him talk about how he “managed procurement cycles from requisition to payment” and “established and developed viable, sustainable relationships with suppliers.” He’s worked in various industries, including spending several years at PerkinElmer (probably why I was so interested in joining the laboratory magazine!).
But what really drew me to this publication and brand are the hardworking people like my dad. Although he wasn’t an engineer or a sales representative, he always knew how (and still knows how) important
me my first job out of college as a technical writer at a company that designed and manufactured liquid level sensors. Seeing my dad in action when I was taking my first steps into the working world was something else—I’ve never seen his passion wane.
And this editorial is fitting because this month we’re featuring our 2024 Supply Chain Department of the Year. Every nomination, including the winning team (University of Utah Health), showed dedication and passion to supply chain in healthcare. It is truly the individuals working in these departments that make things happen and, therefore, improve patient safety and satisfaction.
So, congratulations to this year’s winner! If you organization missed the deadline or you have a story to share about why your supply chain department is crushing it, reach out to me directly at jwider@hpnonline.com. I’d love to feature your department in an upcoming editorial feature.
VP & Market Leader
Healthcare and Dental
Chris Driscoll
cdriscoll@endeavorb2b.com | 978-880-8345
Editor-in-Chief
Janette Wider jwider@hpnonline.com
Associate Editor
Matt MacKenzie mmackenzie@endeavorb2b.com
Senior Contributing Editor Kara Nadeau knadeau@hpnonline.com
Advertising Sales
East & West Coast
Kristen Hoffman khoffman@endeavorb2b.com | 603-891-9122
Midwest & Central
Brian Rosebrook brosebrook@endeavorb2b.com | 918-728-5321
Advertising & Art Production
Production Manager | Ed Bartlett
Art Director | Tracy Arendt
Advertising Services
Karen Runion | krunion@endeavorb2b.com
Audience Development
Laura Moulton | lmoulton@endeavorb2b.com
Endeavor Business Media, LLC
CEO Chris Ferrell | President June Griffin
COO Patrick Rains
CRO Paul Andrews
Chief Digital Officer Jacquie Niemiec
Chief Administrative & Legal Officer Tracy Kane
EVP Medical & Healthcare Technology Kylie Hirko
EVP Endeavor Business Intelligence Paul Mattioli
Healthcare Purchasing News USPS Permit 362710, ISSN 1098-3716 print, ISSN 2771-6716 online is published 11 times annually with an additional issue in November - Jan, Feb, Mar, Apr, June, Jun, Jul, Aug, Sep, Oct, Nov, Nov IBG, by Endeavor Business Media, LLC. 201 N Main St 5th Floor, Fort Atkinson, WI 53538. Periodicals postage paid at Fort Atkinson, WI, and additional mailing offices. POSTMASTER: Send address changes to Healthcare Purchasing News, PO Box 3257, Northbrook, IL 60065-3257. SUBSCRIPTIONS: Publisher reserves the right to reject non-qualified subscriptions. Subscription prices: U.S. $160.00 per year; Canada/Mexico $193.75 per year; All other countries $276.25 per year. All subscriptions are payable in U.S. funds. Send subscription inquiries to Healthcare Purchasing News, PO Box 3257, Northbrook, IL 600653257. Customer service can be reached toll-free at 877-382-9187 or at HPN@omeda.com for magazine subscription assistance or questions.
Printed in the USA. Copyright 2024 Endeavor Business Media, LLC. All rights reserved. No part of this publication June be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopies, recordings, or any information storage or retrieval system without permission from the publisher. Endeavor Business Media, LLC does not assume and hereby disclaims any liability to any person or company for any loss or damage caused by errors or omissions in the material herein, regardless of whether such errors result from negligence, accident, or any other cause whatsoever. The views and opinions in the articles herein are not to be taken as official expressions of the publishers, unless so stated. The publishers do not warrant either expressly or by implication, the factual accuracy of the articles herein, nor do they so warrant any views or opinions by the authors of said articles.

















































































The FDA recently introduced online resources to provide information about reprocessing single-use medical devices for healthcare facilities and FAQs. The page provides information to help healthcare facilities understand the use of reprocessed medical devices originally labeled for single-use, and to clarify the level of FDA’s regulatory oversight of these reprocessed single-use medical devices to remain as safe and effective as the original manufactured devices.
Read on: hpnonline.com/55130760
Guillermo serves as the Founder and CEO of NotiSphere, Inc., a healthcare technology startup transforming the traditional handling of medical supply disruption communications. On this episode, Guillermo and Editor-in-Chief Janette Wider discuss the current state of medical device recalls.
Listen at: hpnonline.com/55129883
The Joint Commission announced it has achieved renewed deeming approval from the U.S. Centers for Medicare & Medicaid Services (CMS) for its Ambulatory Surgical Center Accreditation Program, effective September 1, 2024, through September 1, 2030. Six years is the maximum term of approval granted by CMS. Accreditation is voluntary and seeking deemed status through accreditation is an option—not a requirement.
Read on: hpnonline.com/55130277

Jimmy Chung, MD, MBA, FACS, FABQAURP, CMRP, Chief Medical Officer, Advantus Health Partners and Bon Secours Mercy Health, Cincinnati, OH
Joe Colonna, Chief Supply Chain and Project Management Officer, Piedmont Healthcare, Atlanta, GA; Karen Conway, Vice President, Healthcare Value, GHX, Louisville, CO
Dee Donatelli, RN, BSN, MBA, Senior Director Spend symplr and Principal Dee Donatelli Consulting LLC, Austin, TX
J. Hudson Garrett Jr., PhD, FNAP, FSHEA, FIDSA, Adjunct Assistant Professor of Medicine, Infectious Diseases, University of Louisville School of Medicine
Melanie Miller, RN, CVAHP, CNOR, CSPDM, Value Analysis Consultant, Healthcare Value Management Experts Inc. (HVME) Los Angeles, CA
Dennis Orthman, Consulting, Braintree, MA
Janet Pate, Nurse Consultant and Educator, Ruhof Corp.
Richard Perrin, CEO, Active Innovations LLC, Annapolis, MD
Jean Sargent, CMRP, FAHRMM, FCS, Principal, Sargent Healthcare Strategies, Port Charlotte, FL
Richard W. Schule, MBA, BS, FAST, CST, FCS, CRCST, CHMMC, CIS, CHL, AGTS, Senior Director Enterprise Reprocessing, Cleveland Clinic, Cleveland, OH
Barbara Strain, MA, CVAHP, Principal, Barbara Strain Consulting LLC, Charlottesville, VA Deborah Petretich Templeton, RPh, MHA,Chief Administrative Officer (Ret.), System Support Services, Geisinger Health, Danville, PA
Ray Taurasi, Principal, Healthcare CS Solutions, Washington, DC
Tristel ULT delivers an industry-best, 2-minute HLD easily and effectively. No installed ventilation equipment, plumbing or electrical connections, service contracts, or dedicated facilities required! Just 4 pumps of Tristel’s FDA-approved, proprietary ClO2 foam applied to a transducer via a Tristel Wipe achieves HLD in just 2 minutes. Tristel ULT is a cost-effective solution, compatible with more than 950 endocavity, transvaginal, and generalpurpose transducer models from major ultrasound equipment manufacturers.
Reduced recycle time and increased throughput by processing closest to point-of-care.


Learn about 3T, a cloud-based intuitive compliance platform providing users with the tools to track and trace decontamination events in real time.
Schedule a demo: customerservice@parkerlabs.com
parkerlabs.com/products/tristel-ult
Process innovation, customer service, strategic planning and more make University of Utah Health deserving of this year’s award
BY JANETTE WIDER, EDITOR-IN-CHIEF
As they are every year, the nominations for Healthcare Purchasing News’ ( HPN ) Supply Chain Department of the Year were outstanding. Unfortunately, there can be only one department profiled. This year, we’ve chosen University of Utah Health (U of U Health).
The team is responsible for oversight for a network of five hospitals specializing in orthopedic, rehabilitation, cancer treatment, neuroscience, and general healthcare, alongside twelve community health centers. This team manages the task of receiving and distribution of medical equipment and commodities as well as intake of food, pharmaceuticals, building materials, and other goods essential for hospital and clinic operations. The team also supports receiving and delivery for multiple academic and research health partners across the University of Utah campus. The team handles fleet management and courier services for the above listed entities spanning the entire Salt Lake valley and a few locations beyond. Integral to their operations is the management of an automated inventory management system covering 192 distinct stocking locations and ensuring proper medical supplies are ordered
and supplied to clinicians who need them, when they need them. This team also oversees the maintenance, cleaning, and re-distribution of essential small patient care equipment between nursing units. This service covers small equipment such as IV and epidural pumps, sequential compression devices, crash cart and other specialty cart replenishments, and some custom kit creation for patient care.
Some key highlights from the team’s submission include:
• Non-Personnel Reduction : Through strategic sourcing, negotiating a contract in collaboration with their supply chain peers with a key strategic partner, they achieved a significant reduction in annual supply costs.
• Personnel Expense Optimization: By automating repetitive tasks and optimizing product intake with Medline, they decreased overtime expenses by 35% and increased productivity by 20% for the swing shift.
• Error Reduction and Improved Accountability: Implementing barcode scanning technology into their product intake processes reduced oversight of inventory shipment incongruencies by 50% with their former distribution
partner, leading to cost savings of an estimated $500K annually.
• Supplier Collaboration and Optimized Inventory Management:
The department has developed a strategic partnership with Medline, resulting in joint cost-saving initiatives and improved supply chain resilience. Datadriven strategies and collaboration have reduced their killed fulfillment rate by 40%
HPN had the pleasure of speaking with Mark A Fawson, MBA, BSN, RN, CMRP, director, Material Services about his submission and team. He broke down a few key components of his team’s initiatives and spoke with us about his leadership methodology.
The department has implemented several innovative approaches to streamline operations and improve efficiency. One project that particularly demonstrates process is their PAR Tracking.
The team created a program to supplement our inventory management system. PAR Tracking, as it has been dubbed, was formed using Google Sheets. The program intakes historic usage and ordering data, then computes it in a
defined algorithm with parameters we established, to provide an item par level modification report, a zero hits report, a frequent restock report, and other helpful graphs and information. This system has reduced inventory stock outs by 88% and reduced restocking nursing unit par rooms from our storeroom inventory by 80%.
Additionally, the team used Lean Six Sigma methodologies and conducted process improvement that now allows for 72–96-hour supply of goods in stocking bins, rather than 24-48 hours previously. This system also decreased the overall daily ordered lines by 33% which means that products are touched less frequently, even though they are receiving a similar volume of goods overall.
As an unexpected result from this project, other team members began making improvements of their own. The team was initially focused on low hanging fruit and PAR levels that they could modify without changing bin sizes in stocking locations. When team members saw the benefits of what we were doing, they began moving items in our storeroom to find better placement for efficiency of our operations overall. They also began better cleaning and maintaining our supply rooms in general, because they had more time to focus on value-added work.
The department has been managing the diverse needs of its internal customers, ensuring high levels of satisfaction and quality service through its Health Unit Coordinator (HUC) Tool.
The introduction of the HUC Tool significantly improved the accessibility and efficiency of ordering medical supplies for clinical staff. By allowing hospital staff to search for items using product Lawson numbers, common name, descriptions,
and manufacturer catalog/reference numbers, along with viewing a photo of the items, the tool has reduced the need for frequent phone calls and streamlined the ordering process.
The HUC Tool has facilitated easier training and onboarding for new staff. It has made it simpler for new
hires to familiarize themselves with the storeroom inventory, reducing the learning curve and improving overall staff productivity.
By addressing the difficulties faced by HUCs and other hospital staff in finding and ordering supplies, the HUC Tool has strengthened


customer relationships. The positive feedback and increased ease of use have demonstrated commitment to supporting hospital staff, enhancing their workflow, and improving overall satisfaction.
The flagship hospital distribution storeroom is open 24/7 and 365 days a year. The storeroom team regularly received phone calls from clinicians asking for product information so they could transmit an order to the storeroom for fulfillment. Distribution staff members don’t typically have a medical background, and so common terms for a clinician sometimes are not understood, leading to confusion on both sides. Having 1,457 unique items in our storeroom adds to the challenge. This year, the department wanted to tackle the problem by making a tool where a HUC, nurse, or other clinician could type a product number, item description, common name, or manufacturer catalog/reference number into a search tool to determine if that item was carried in our storeroom and have the information needed to transmit an order.
The basis for this tool came from the department’s Storeroom Dashboard project that was created in 2021. It was developed after observing similar difficulties within their own team when searching for products in the storeroom. This was especially true for new hires. The difficulty came from limitations in the two systems. One system was a search that could only be done using manufacturer catalog numbers or their own Lawson item numbers. The other system was a printed spreadsheet in a book that had the location in the storeroom. Without the item number first, the second tool was useless. The department concluded that it would build a system of their own, whereby material services staff could identify more readily products in question and know
their location quickly within the storeroom. From this, the Storeroom Dashboard was born.
This year, after noticing that hospital staff were having a hard time ordering items, the team began asking questions to identify just what those challenges were. The standout was that HUCs were having difficulty finding item information for the exact product(s) the clinician was requesting, thus, making it difficult for them to order it from the storeroom. The team realized they already had developed something similar to their own and hearing the suggestion of one of our material services staff members to add photos, they set out to create a tool for others to use. The “HUC Tool” was created as a simplified version of the formerly created Storeroom Dashboard. This HUC Tool had much of the same functionality as the Dashboard, since it had mostly the same information, but with certain things removed that others wouldn’t need to know.
Once created, the team began sending it out to some of the HUCs they interacted with regularly. They showed them how to use it and expected it just to spread naturally. At first, there were few searches but spreading the word about the HUC Tool paid off. Soon, a staff member from Professional Nursing Development reached out indicating she had seen the HUC Tool and asked if they would be willing to showcase it in a clinical staff education meeting. After the tool was presented at this meeting, there was a dramatic increase in the number of users for the HUC Tool and our team began hearing from HUCs and hospital staff that they could now find items much easier than they ever could before. It made training easier with new staff since they could search for the items to find what they needed.
This year, U of U Health’s department’s strategic planning efforts ensured seamless collaboration with partners across the supply chain continuum.
U of U Health staff effectively coordinate with a diverse range of partners across the supply chain, including raw material suppliers, manufacturers, distributors, group purchasing organizations, and service companies, to foster improved communication and joint efforts with provider groups/IDNs, and student groups to share best practices, career development, and learning opportunities
By engaging supply chain executives from various Integrated Delivery Networks (IDNs) and increasing board membership, U of U Health staff facilitate a more vibrant and strategic approach to meetings and events, ensuring a comprehensive and inclusive planning process. Through organizing impactful events like the Rocky Mountain Chapter of the Association for Healthcare Resources and Materials Management (RMAHRMM) learning sessions, networking events, service activities, and vendor fairs, UUH staff provide valuable educational content and foster strong professional connections, enhancing overall supply chain efficiency for the benefit of patients.
When asked about what the biggest challenges supply chain leaders and departments are facing today, Fawson said, “Backorders and other shortages of goods through manufacturing disruptions. This can be due to a variety of factors; political instability exacerbating challenges of moving goods, natural disasters causing raw material shortages, operating
too lean, mergers and acquisitions that lead to less options. You name it, backorders and product shortages are a major problem.”
Additionally, he added, “Cybersecurity and ensuring digital information shared for operations is not vulnerable. Tighter security measures slows implementation of new information technology to ensure sensitive information is protected.”
And finally, “Physician preference variability within institutional teams. Some practice groups are more collaborative and able to standardize their product selections,” Fawson noted. “This drives down cost due to that standardization and purchasing in economies of scale. Other groups may have higher provider turnover, departmental growth that adds additional providers and voices to the mix, or stronger and
more variable opinions within their group(s) leading to difficulty standardizing on fewer options.”
He also commented, “I’ve observed an informational gap with clinicians on understanding factors that should be considered when they want a new widget. One of the biggest is simply a lack of reimbursement knowledge when compared to the need to cover operational expenses. When we educate providers on these business details, they often marry that to the clinical decisions they have to make, and usually come to the best conclusions on their own. It is beautiful to behold when patient outcomes, quality of care, and financial viability are all in alignment. This is best achieved when multidisciplinary, interdepartmental collaboration takes place and broader understanding is achieved.” HPN Introducing the Brewer
Read how Teamowork makes the dream work at hpnonline.com/55131479






The first to offer a one-touch, whisper-quiet mobility system, Brewer makes it possible to reconfigure and clean exam rooms quickly and with zero disruption to patients and providers. Our advanced EasyGlide Mobility System is an available option on our Access® PLUS and FLEX™ PLUS Exam Tables. See our EasyGlide Mobility System in action and request a demo. A simple turn of a switch engages and disengages the EasyGlide Mobility System.





BY KARA NADEAU, SENIOR CONTRIBUTING EDITOR
The importance of cleaning and disinfection of contaminated surfaces in patient care areas is evidenced by the many stakeholders involved in these practices.
This includes the environmental services (EVS) professionals performing the tasks, the infection prevention (IP) practitioners collaborating with EVS leaders and teams, and both EVS and IP engaging with specialty department leaders and their teams to address unique sanitation aspects of their environments. All are guided by associations and agencies that publish
evidence-based standards for keeping patients safe.
HPN reached out to the Association for the Health Care Environment (AHE), the Association for Professionals in Infection Control and Epidemiology (APIC), the Association of periOperative Registered Nurses (AORN), the American Association of Critical-Care Nurses (AACN), and the American Society for Healthcare Engineering (ASHE) to learn the latest trends in room cleaning and disinfection.
We report on their news and insights for maintaining healthy
environments in care delivery settings, with specific considerations for perioperative and critical care spaces.
TJC updates infection prevention and control standards
The Joint Commission (TJC) approved new and revised requirements for infection prevention and control (IC) standards, effective July 1, 2024, that are “driven by a standardized approach that simplifies the requirements and eliminates those not providing value to healthcare facilities,” as reported by the AHE.1
In June 2024, Jonathan Flannery, MHSA, CHFM, FASHE, FACHE, senior associate director, Regulatory Affairs, ASHE, presented an AHE ondemand webinar on the TJC’s updated requirements, pointing out what is new, what is different, and what changes will likely have the greatest impact on EVS teams.2 ASHE and AHE are professional membership groups of the American Hospital Association (AHA). Flannery offered his insights on the requirements to HPN for this article.
“Even though there are changes, there is really not significant change within the new standards and requirements,” said Flannery. He explained some of the subtle differences, that while on the surface do not appear substantial, will have an impact on EVS and IP professionals moving forward.
For example, while healthcare facilities have been required by TJC to have in place written policies and procedures (P&P) for infection prevention and control, they now must specifically cite the relevant laws, regulations, guidelines, expert opinions, and other sources on which the P&Ps are based. It comes down to not just stating what measures a facility will take to prevent infections but also why they have chosen them.
“In my opinion, EVS professionals need to be the driving force behind improving those policies and procedures to make sure they’re meeting the proper regulations and guidelines, laws, and requirements,” said Flannery.
With regards to the TJC’s new protocols for highly contagious infectious disease (HCID) or special pathogens, Flannery also stressed the importance of EVS engagement. He noted how during the U.S. Ebola outbreak in 2014, the role of EVS was overlooked in some facilities, particularly those without strong EVS leadership.


“Because TJC’s new HCID protocols require IPs to conduct an organization-wide assessment, EVS professionals must be communicated with and trained properly on dealing with these issues,” noted Flannery. “EVS is part of the team of caregivers because they perform tasks critical to protecting patients and supporting their healing.”
When asked what EVS leaders should take away from the TJC’s new and revised requirements, Flannery stated:
“The key takeaway here is that your current programs are still valid but will require some expanding. It’s a perfect opportunity for organizations to go back and do a thorough review of their programs. Work this into your annual program evaluation, going step by step to identify improvements to your cleaning and disinfection practices, which ultimately impacts the bottom line - keeping patients safe and free from infection.”
Wendy Simpson, MSN, RN, CCRN, FCCS, Infection Prevention, Lt. Col. Luke Weathers, Jr. VA Medical Center, commented on the critical care implications of the new and revised TJC requirements. She highlighted IC.07.01.01, a new standard that addresses preparedness for high-consequence infectious diseases or pathogens, stating:
“This standard was inspired by the recent outbreaks of Ebola and COVID19. Though the standards leave decisions related to frequency and levels
of disinfection up to the individual facilities, each facility should have a robust cleaning program in place, particularly in our ICUs.”3
Karen deKay, MSN, RN, CNOR, CIC, FAPIC, senior perioperative practice specialist, AORN, offered her insights on how the new and revised TJC requirements impact cleaning and disinfection practices in the perioperative environment.
“The new and revised Joint Commission infection prevention and control standards are more streamlined and focus on the structures and processes that support a strong infection prevention and control program,” said deKay. “The reason for this restructuring is because infection prevention is not just about individual practices, but instead the overall framework in which infection prevention occurs.”
deKay highlighted these three elements of performance (EP) from TJC’s requirements that are particularly important to the perioperative space:3
IC.04.01.01 EP 2 recommends that hospitals foster a multi-disciplinary approach to infection prevention and control. “Communication and collaboration among various departments including infection prevention, nursing units, sterile processing, perioperative personnel, and environmental services are key,” deKay explained.
“Personnel from perioperative services may be asked to serve on a multi-disciplinary team to discuss challenges, share insights, and implement best practices.”
IC 04.01.01 EP 4 clearly identifies key elements for cleaning, disinfecting, and sterilizing medical equipment and surgical instruments that should be included in a hospital’s policies and procedures. “As surgical instrumentation, as well as a variety of noncritical devices and equipment, are utilized in
every surgical procedure, perioperative personnel should review these elements carefully,” said deKay.
“Some elements include following manufacturer’s instructions for use (IFU), using U.S. Environmental Protection Agency (EPA) registered disinfectants and U.S. Food and Drug Administration (FDA) approved liquid chemical sterilants, developing criteria for immediateuse steam sterilization, and documentation of device reprocessing cycles,” she added.
IC.07.01.01 EP 1 states facilities should have a protocol in place for recognizing, responding, and containing potential high-consequence infectious diseases or special pathogen outbreaks. “This is important for perioperative personnel because surgery for a patient with a confirmed or suspected infection with one of these pathogens may be necessary,” deKay commented.
“Protocols should include not only room preparation and PPE, but also how cleaning and disinfecting of the patient care area, surfaces, and equipment, as well as handling of waste and linen will occur,” she continued. “Education and training on the new protocols should be developed for all personnel, along with demonstration of those skills and tasks specific to their roles (e.g., cleaning operating rooms, sterilizing instruments).”
The importance of collaboration As TJC’s requirements demonstrate, collaboration among EVS, IP, perioperative, and critical care teams (and other clinical department teams as well) is critical in room cleaning and disinfection efforts.
“EVS is a profession and it’s a very important profession,” said Marci Butts, director of Environmental Services, West Hospital, Mercy Health, Cincinnati. “That’s why we

Marci Butts

Janet Flynn Mulroy
must have a seat at the table and a strong connection and relationship with our IPs and other teams responsible for patient care and safety. None of us can be on an island. We must work together and all be speaking the same language.”
Janet Flynn Mulroy, DNP, ACNP, CCNS, CCRN, Acute Care Nurse Practitioner, Threlkeld, Threlkeld, and Omer Infectious Disease Associates, spoke to the importance of this collaboration in critical care environments. She served on the national board for the AACN.
“Our critical care patients are at a much higher risk of infection than the average acute care patient, so maintaining a clean, anti-infective environment is essential to protect patients, family members, and staff,” said Mulroy. “The EVS team plays a crucial role in shaping the patient experience within a hospital setting. Even though EVS staff follow rigorous cleaning protocols with daily disinfection of high-touch surfaces in high acuity areas, the high traffic flow and the additional equipment found in critical care units create challenges unique to the ICU environment.”
“Utilization of AACN’s Healthy Work Environment (HWE) standards of skilled communication, true collaboration, and effective decision making with EVS, IP, and critical care clinicians is crucial to prevent spread of infection in the ICU,” Mulroy added.
Commenting on the impact of this collaboration in the perioperative environment, deKay stated:
“Explicit communication and collaboration between different
departments (e.g., EVS, IP, perioperative services) within a healthcare organization can significantly improve both patient and healthcare worker safety. The collaboration can play a crucial role in ensuring proper cleaning and disinfection of perioperative areas, as when everyone is on the same page, all work to make sure infection control protocols are followed consistently.”
“My own interpretation of TJC’s changes is that infection prevention must be integrated into all hospital departments and functions, including EVS, nursing and engineering,” said Marie Moss, MPH, RN, CIC, APIC Communications Committee Member. “Specific to EVS, consistent and timely communication around room contamination between IP and EVS leaders helps ensure EVS workflows remain in alignment with IP protocols.”
Simpson noted how TJC and several other advisory groups, such as APIC, recommend a system wide multidisciplinary approach to maintaining a safe and clean environment.
“It is beneficial to schedule regular multidisciplinary Environment of Care Rounds between EVS, IP, and critical care clinicians to evaluate current processes,” she stated. “The Environment of Care team members can discuss new and emerging diseases, assess knowledge gaps, provide needed education, and assure appropriate staffing and resources to provide a safe environment for everyone.”
Katelyn Harms, MPH, CIC, APIC Communications Committee member, Infection Prevention program manager, Performance Improvement Dept., UnityPoint Health, Meriter, Madison, Wis., spoke to the close relationship between EVS and IP leaders in her health system, stating:
“Our EVS workers are really our right hand in infection prevention. Acquisition of HAIs from environmental contamination is incredibly
common; therefore, we know routine disinfection practices are essential to providing safe patient environments. EVS workers partner with us to identify problem areas, often serving as our eyes and ears.”
UnityPoint Health’s EVS supervisors participate in the health system’s IP committees and annual risk assessments. They also routinely receive IP data around HAIs. Additionally, IP professionals participate in new EVS employee onboarding, orientation, and training.
“I love having face-to-face time with new EVS orientees so they know the IP team and understand we’ll have an ongoing collaboration throughout their employment,” she stated.
Harms and team review EVS competencies and training materials and infuse IP principles into EVS practices

Marie Moss

Katelyn Harms
so, in Harms’ words, “they know not only how to clean a room but why their work is so important to break the chain of infection.”
In conclusion, Harms stated: “Our message to EVS staff is clear - they have the power to impact patient safety.” HPN
References:
1. Analysis and Next Steps, The Joint Commission: New and Revised Requirements for the Infection Prevention and Control (IC) Chapter, AHE, https://www.ahe.org/joint-commission-newrequirements-infection-prevention-and-control-chapter-2024
2. Infection Control Standards Update, July 2024, AHE, https://www.ahe.org/joint-commission-new-requirementsinfection-prevention-and-control-chapter-2024
3. R3 Report: Requirement, Rationale, Reference, TJC, December 20, 2023, https://www.jointcommission.org/-/ media/tjc/documents/standards/r3-reports/r3_report_icrewrite-hap-cah.pdf
4. Association of periOperative Registered Nurses (AORN). Guideline for environmental cleaning. In: Guidelines for Perioperative Practice. Denver, CO: AORN, Inc;2024:197-226.
5. What is a Gemba Walk and Why is it Important? Six Sigma Daily, January 17, 2018, https://www.sixsigmadaily.com/whatis-a-gemba-walk/
There’s more online at hpnonline.com/55129636:
Story continues: VHA sets standard processes for UV-C radiation
Sidebars: Room Ceaning Considerations Considerations Specific to the Perioperative Environment




BY DELANEY REBERNIK
Editor’s note: This article is the first in a three-part series examining the challenges and opportunities facing healthcare procurement leaders as outlined in the 2024 Healthcare Procurement and AI playbook from Healthcare Purchasing News (HPN) and Staples Business Advantage (SBA). Respondents’ comments have been lightly edited for length and clarity.
Healthcare procurement has navigated a complex and volatile landscape in recent years. Confronted with tightening margins, rapid industry evolution, and geopolitical instability ranging from cybersecurity threats to climate change, procurement leaders have demonstrated exceptional resilience.
“We are facing challenges due to disruptions in the global supply chain, leading to delays in the delivery of essential medical supplies and equipment,” says one hospital supply chain executive in an April State of the Market survey from HPN and SBA. “These disruptions can impact patient care and operational efficiency.”
However, a path forward is emerging, driven by the potential of AI and advanced technologies. Nearly three-quarters of the 170+ senior executives and directors contributing to the 2024 Healthcare Procurement and AI report have adopted AI in procurement functions, with 92% reporting positive outcomes.
To build healthcare supply chains that stand the test of time (and tech disruption), leaders must balance innovation with proven best practices. According to survey respondents, three mandates will shape the industry’s future: quality, efficiency, and innovation (figure 1). This series will explore each in detail.
Securing high-quality partnerships and products is paramount. An overwhelming 83% of respondents say it’s a “critical” or “high” priority to find specific products that meet their organization’s needs, equaling the importance of reducing the overall cost of supplies.
Achieving a universal definition of “quality” within an organization is complex: “Some of the top challenges are just making sure that everyone is safe, everyone is happy, and everyone comes to a compromise with the products that are purchased,” one leader says.
Getting it right is crucial, given the broader implications for initiatives like talent retention and engagement. One respondent says they’re laser-focused on raising the spirits of employees and patients because their organization is expe-
dips.
Quality and cost are inextricably linked. One respondent summed up their top pain point as “trying to find affordable, high-quality options that please everyone” — a sentiment that survey respondents echoed again and again. For many, that means meeting rising standards for health, hygiene, and performance within constrained budgets.
While often viewed as competing priorities, quality and cost can yield substantial organizational benefits when addressed holistically. For instance, controlling rogue spending, a top concern for 76% of respondents, enhances process rigor and overall procurement efficiency.
“The whole value equation is improved,” says Jack Koczela, director of analytics and transformation, supply chain at Froedtert Health, a Wisconsin-based system with 10 hospitals and
more than 45 health centers and clinics. “We know that we’re increasing the overall experience of procurement, be that for the clinicians who are using it, or for our staff who experience a more automated purchasing process.”
Identifying the right supplier partner can be difficult. “The biggest challenge is to select reliable and stable suppliers with an excellent reputation in the industry,” one leader says. Another cites “vendor communication” as a big hurdle.
Aside from dependability, leaders are looking for diversity, swift handling of after-sales issues, and a broad or national footprint from their partners.
When it comes to the products themselves, quality assurance is a significant part of the equation. It’s also a big lift: Because the procurement team’s purview can cover everything from general breakroom supplies to specialized offerings like EVS cleaning solutions, “choosing safe, reliable, and hygienic products, as well as trustworthy suppliers, is essential,” one leader says.
As HPN reports, “Where in the past a hospital might purchase the cheapest product to cut upfront costs, today they are leveraging value analysis committees (VAC) and other key stakeholders to analyze the long-term impact of supply choices. They have learned that, in some cases, paying a little extra for a higher quality product at the time of purchase can lead to significant savings in the long run as a result of less waste, improved performance and enhanced safety.”
StaplesBusinessAdvantagethetrustedpartnerfor8of thetop10healthcaresystemsintheU.S.Partnerships withtopGPOsenableclientstoachievesignificant costsavingswhilestreamliningpurchasingacross multipleproductcategories,includingEVS,furniture, breakroom,officesupplies,andmore.Learnmore










BY KARA NADEAU, SENIOR CONTRIBUTING EDITOR
It has been over two years since publication of the American National Standards Institute (ANSI)/Advancement of Medical Instrumentation (AAMI) ST91:2021
Flexible and semi-rigid endoscope processing in health care facilities standard. What progress have healthcare organizations made in transitioning high-risk endoscopes – and other scopes - from high level disinfection (HLD) to sterilization as the standard of care?
Healthcare Purchasing News (HPN ) reached out to sterile processing (SP) professionals for their insights on the topic, where we are today, and key factors when considering a change from HLD to a sterilization modality.
Current state of scope sterilization
“There was a lot of talk in general around scopes and endoscopy during the Healthcare Sterile Processing Association (HSPA) 2024 Annual Conference, with sterilization being a big topic of discussion,” said Ash Crowe, director, Healthcare at St. Onge Company. “There is a push for manufacturers to design more scopes to be capable of sterilization.”
“From a patient perspective, sterile is better than disinfected,” Crowe added. “If I were going in for a procedure, I too would prefer it if my scope had been sterilized. I think everyone understands that, but then there is the challenge of how do you make that happen? How do you integrate more sterilized scopes into your workflows?”
While “evidence supports the sterilization of all flexible endoscopes, including those used in both semicritical and critical procedures,”1 even those healthcare organizations that want to standardize on flexible scope sterilization face the challenge of manufacturer instructions for use (IFU) that recommend only HLD for their products.
Some healthcare organizations that choose to continue HLD when given a choice in the scope manufacturers’ IFUs between HLD and sterilization take this route because they lack the resources to sterilize all scopes that can be sterilized. For example, they don’t have the recommended sterilization modality available, or the SP department simply can’t handle the added sterilization volume given staffing, equipment, and space constraints.
“As we know, flexible endoscopes are heat sensitive, so not compatible with steam and other sterilization modalities,” said John Whelan, BSN, RN, clinical education specialist, Healthmark, a Getinge company. “EtO sterilization, while compatible with many endoscopes, is not available to many facilities.”
The U.S. Environmental Protection Agency’s (EPA) March 14, 2024, issuance of its final rule to sharply cut toxic emissions of EtO marks one of the latest initiatives driving healthcare facilities to transition from EtO
to alternative low-temperature sterilization methodologies.2
“We have transitioned from HLD to terminal sterilization for some items, including bronchoscopes and obviously cystoscopes, which have always been terminally sterilized, and we are removing EtO as a sterilizer modality as we speak,” said Sean Weir, CRCST, CIS, CER, CHL, system sterile processing educator, UPMC.
But some manufacturers of flexible gastrointestinal (GI) endoscopes still recommend EtO sterilization in their IFUs.
“In my previous role, we transitioned all bronchoscopes, cystoscopes, and ureteroscopes from HLD to sterilization,” noted Hannah Schroeder, BSHA, CRCST, CHL, CIS, CER, clinical education specialist, Pure Processing. “We did not move GI scopes out of HLD because many of the manufacturers’ IFUs still indicate EtO as a recommended sterilant.”
“Even for a facility that has EtO, that cycle length is incredibly long, about 15 hours just in the sterilization chamber, so it is really not feasible for many facilities to go from HLD to sterilization for their GI scopes,” she added.
As Whelan pointed out, there are other sterilization options available beyond EtO for heat sensitive, flexible endoscopes if these modalities are recommended by manufacturers in their IFUs. He stated:
“Many scopes that are used in OR settings (e.g., ureteroscopes,






cystoscopes, and bronchoscopes) already have compatibility with commonly available sterilization modalities (e.g., Sterrad).”
Recommendations for transitioning from HLD to sterilization
“Transitioning from HLD to sterilization is not a simple process,” said Schroeder. “We all love the idea of it because it is a safer alternative, but getting there is a puzzle and journey.”
“It can be a slow evolution because it’s not something you can do immediately,” said Stacy Johnson, APRN, CNS, CNOR, CCSVP, clinical education consultant, ASP. She noted how the move from HLD to sterilization requires stakeholders, including department managers, and infection prevention (IP) and SP professionals coming together to determine whether change is feasible. She added how vendor partners are another critical stakeholder in the mix as they can work alongside SP and clinical teams to provide support.
Schroeder, Johnson, and the other SP professionals interviewed for this article offered their recommendations and best practices for healthcare organizations considering a transition from HLD to sterilization for scopes that can be processed in this manner.
Form a multidisciplinary team
When it comes to who should be involved with decisions around scope HLD versus sterilization, Whelan suggests healthcare organizations “utilize a multidisciplinary team to review current state and provide input for proposed changes. This team should minimally
include processing professionals, infection prevention, customer clinicians, and administration.”
Weir emphasized the importance of collaboration among SP and IP teams regardless of the scope reprocessing modality, telling other SP leaders:
“If you don’t talk to your infection preventionists at least weekly, you need to reconnect. In my hospital, our infection preventionist and I have each other’s cell phone numbers so we can text questions back and forth. You’ve got to have a great relationship with your IP team. They are your biggest supporters on surveys and for compliance.”
As Whelan stated: “Start with a detailed inventory of the scopes within your institution. What models exist?”
In alignment with ANSI/AAMI ST91:2021, Whelan noted how prioritization must be given to “high-risk scopes” – those “…that have been associated with infectious outbreaks including those that are difficult to process.”
This includes bronchoscopes, cystoscopes, ureteroscopes, elevator channel endoscopes, and endobronchial ultrasound endoscopes.3
“Per Spauldings, for semi-critical reusable devices, it was not intended that HLD was the one and only option; but rather, if sterilization is not possible, then HLD,” said Whelan. “Where we landed over the years is having a predominance of flexible endoscopes undergoing HLD.”4
“Review IFUs to identify those scopes where compatibility with sterilization methods already exists,” Whelan continued. “Compare this information against the sterilization
modalities currently available within your facilities. A key point when it comes to sterilization for flexible endoscopes – sterilization is dependent on adequate cleaning, inspection, drying, and device preparation.”
“A multidisciplinary review may identify the need to adjust inventory levels for certain customer departments and/or consider options for fully disposable scopes,” Whelan added. “As much as this is a ‘big ask’ financially, it is far less expensive than the cost of device related exposures for patients.”
scope reprocessing locations
It is common for endoscope reprocessing to take place outside of the SP department and in clinical areas, such as endoscopy units and clinics (e.g., GI, ENT). Therefore, an important first step when considering a change from HLD to sterilization for scopes is to identify all areas where reprocessing currently takes place, the specific scopes being used and reprocessed, and the current reprocessing modalities.
“In the past, we’ve done surveys at hospitals just to figure out where all the HLD is happening,” Crowe noted. “Therefore, when considering a change from HLD to sterilization, an initial step is determining whether your sterile processing (SP) team knows where HLD is occurring outside their department. Where does HLD happen on your campus?”
“You know HLD takes place in the endoscopy suite,” Crowe added. “But other clinical areas, such as the emergency department (ED), uses scopes. There are also probes, such as ultrasound probes for OB/GYN, that also
require HLD. Unless SP has been managing HLD, they probably don’t know the scale of where it takes place.”
Evaluate staff resources, equipment, and workflows
Adding to the volume of sterilized reusable instruments and devices by shifting scopes from HLD to sterilization can have significant impacts on those individuals performing reprocessing, the facility’s reprocessing equipment, and the physical spaces where reprocessing takes place.
A major decision is whether to centralize all scope sterilization in the SP department or to equip scope users/ reprocessors in the various clinical departments where scopes are used.
According to Crowe, some health systems and hospitals are already making the move to centralize HLD processing of scopes within their SP departments, believing all scopes will shift to sterilization over time.
“There had been the idea that if endoscopy does their own HLD they are going to stay separate, but more and more, it feels like HLD is becoming part of the SP department,” Crowe explained. “There is a thought that if all flexible endoscope reprocessing is transitioning from HLD to sterilization eventually, let’s just make the move to centralizing scope processing now in the SP department.”
Crowe questioned: “But if you are going to bring HLD into the SP department, what happens to these departments when they eventually have to sterilize all the scopes? Most SP teams are busy. Their departments are full. How do they find the space? How do they build those workflows? And when building a new SP space today, HLD may be a completely separate function, so how do we plan for it to be part of sterilization in the future?”
“At this hospital, it all comes down to who is doing it and who can perform that task,” said Weir. “For example, do they have the right airflow, temperature,
and humidity for scope reprocessing? If we look at centralizing everything to the SP department, can they take on the sterilization of 20 more scopes per day? Would that require an additional FTE? How would the added volume impact the equipment?”
Weir added: “Rather than saying to the SP team, ‘you have to do this,’ it’s critical for SP, IP, and clinical leadership to take a step back and ask, ‘we need to make the move from HLD to sterilization the right way for patient safety, so how can we support the SP department in successfully making the change?”
“Before you jump the gun, the SP team and the end users of those scopes need to understand not only current procedural volumes but also projected volumes and how they will impact SP workflows,” said Schroeder. Asking questions like “What is your current sterilizer capacity? If you’re utilizing something like a hydrogen peroxide sterilization, chances are there’s other medical devices that are already being sterilized in that way. Where are the scopes going to fit into your day and into your priorities? What are average required turnaround times? What practices or plans do we need to consider so that we’re not delaying or cancelling procedures but still following compliance recommendations?”
Schroeder continued: “Do you have the space and the resources to sterilize and store some of your high-risk endoscopes early on? Or is this going to require a capital purchase? Even large facilities might have to bring in one or two new sterilizers depending on the required mode of scope sterilization.”
According to Johnson, no matter where sterilization takes place, whether it is in a clinical department or in the SP department, moving scopes previously reprocessed with HLD to sterilization will likely require changes in physical space and equipment. She stated:
“Everywhere, reprocessing areas are having to do some reconstruction and reconfiguring to make sure they have
the correct workflows for scope sterilization. Many SP departments and clinical areas (e.g., GI labs, clinics) still have older equipment or workspaces, so they need time to revamp before taking on scope sterilization tasks.”
As Johnson noted, endoscopy units and clinics that have historically processed their own scopes, using HLD or sterilization, could resist sending their scopes to the hospital or health system’s SP department. She stated:
“If you have an endoscope reprocessor in your department, it’s in your control and you can reprocess something quickly. I have seen rapid turnover clinics where a technician who played a role in the endoscopy procedure will perform the scope pretreatment at the point of use, then bring it to a reprocessing area and perform all the post-procedure steps there, including leak testing.”
“Now imagine an outpatient urology or ENT clinic sending their scopes off-site to their hospital’s SP department,” Johnson continued. “First, they face the challenge of getting them into the cleaning process within the recommended 60 minutes. Not only is it an issue of time, it’s also an issue of communication and coordination. It’s no longer someone in the procedure room transporting the scope and either that person or someone down the hall reprocessing it, but rather coordinating with an off-site SP team and having to document the hand-off.”
When it comes to tracking scope transport and documenting that the correct procedures have been followed (e.g., pretreatment, cleaning started within 60 minutes, etc.), Johnson notes how some healthcare facilities use manual processes (a report form that stays with the scope), while others use electronic tracking and documentation systems. HPN
There’s more expert recommendations online at hpnonline.com/55129311




















BY RHONDA THOMPSON, MANAGER OF EDUCATION AND TRAINING, SPM DIGITAL WORKFLOW SOLUTIONS WITH STERIS
Scope processing professionals are under constant pressure to produce endoscopes processed according to each device’s instructions for use and conforming to all industry standards and hospital guidelines requiring documentation on conforming outcomes, quality standards, and performance measurement. Contending with these factors while meeting the demands of a fast-paced endoscopy case schedule brings challenges to staff and facility processes. Medical device asset management software can help manage workflow documentation and safeguard quality outcomes.
1. List processing challenges and considerations for the seven essential steps of flexible endoscope processing
2. Identify how medical device asset management software can lead staff through complex tasks
3. Explain how data captured and documentation in software can improve quality, performance, and patient outcomes
Contributed by:

Properly processing an endoscope is extremely complex. Some endoscope manufacturers’ Instructions for Use (IFU) contain over 100 steps to leak test, manually clean, and render the endoscope ready to use on the next patient.
Processing endoscopes and capturing all necessary documentation is extremely complex and can be aided by medical device asset management software. Referencing manufacturer instructions for use and guidance documents is critical to a quality process and positive patient outcomes but creates difficulty in the decontamination environment. The wet environment ruins paper while laminated cards can be difficult to handle with bulky gloves. Electronic IFU and onscreen guidance protect instructions during use, allowing access while performing tasks.
A second challenge during endoscope processing is documentation. According to the Healthcare Infection Control Practices Advisory Committee (HICPAC), a federal advisory committee to the Center for Disease Control and Prevention (CDC), there are seven essential steps for flexible endoscope reprocessing: pre-cleaning, leak testing, manual cleaning, visual inspection, disinfection or sterilization, storage, and documentation. Endoscopy processing should “maintain documentation of adherence to these essential steps each time an endoscope is

reprocessed. Documentation is essential for quality assurance purposes and for patient tracing in the event a look back is necessary.” (HICPAC, 2016, p. 3). Medical device asset management software can help with documentation and quality processes.
Each step of reprocessing presents its own challenges. First, consider point-of-use treatment or precleaning. According to the Society of Gastroenterology Nurses and Associates (SGNA, 2018) guidelines, “Pre-cleaning occurs in the procedure room immediately after removal of the insertion tube from the patient and prior to disconnecting the endoscope from the power source. Pre-cleaning should be performed at point of use, before bioburden has an opportunity to dry and before complete decontamination.” (p. 16).
The Association for the Advancement of Medical Instrumentation (AAMI, 2021) concurs within the standard ANSI/AAMI ST91, which states, “To prevent buildup of bioburden, development of biofilms, and drying of secretions, point-of-use treatment is performed immediately after completion of use of the device. It is imperative that the manufacturers’ IFU for the endoscope, cleaning equipment, and cleaning solution are followed.” (Section 7.2.1).
Point-of-use treatment is the fi rst line of defense in preventing the formation of biofilms. Biofilm is a diverse community of bacteria and fungi that can congregate, attach to surfaces, and create their own extracellular matrix for protection, making them difficult to remove during cleaning. Point-ofuse treatment is critical in reducing bioburden and biofi lm buildup.
Though point-of-use treatment reduces bioburden, it does not eliminate it. Endoscopes must promptly begin the cleaning process so residual soils do not dry and remaining microorganisms do not have time to create biofi lm. Many endoscope IFU give a 60-minute window from the time of point-of-use treatment and the start of manual cleaning. If this time threshold is exceeded, the IFU may require extra processing steps such as extended soak times. Added processing steps and soak times extend the wait time for a patientready endoscope.
Manual documentation relies on staff to identify the endoscope’s remaining time and prioritize it upon receipt. This dependence can lead to delayed processing or the potential to use normal processing instructions for an endoscope requiring additional processing.
Electronic medical device asset management soft ware can allow for documenting the time of point-ofuse treatment completion and prioritization of endoscopes to prevent the need for delayed reprocessing. Some soft ware systems identify the need for delayed reprocessing at the time of manual cleaning, providing guides and steps for processing time delayed endoscopes.
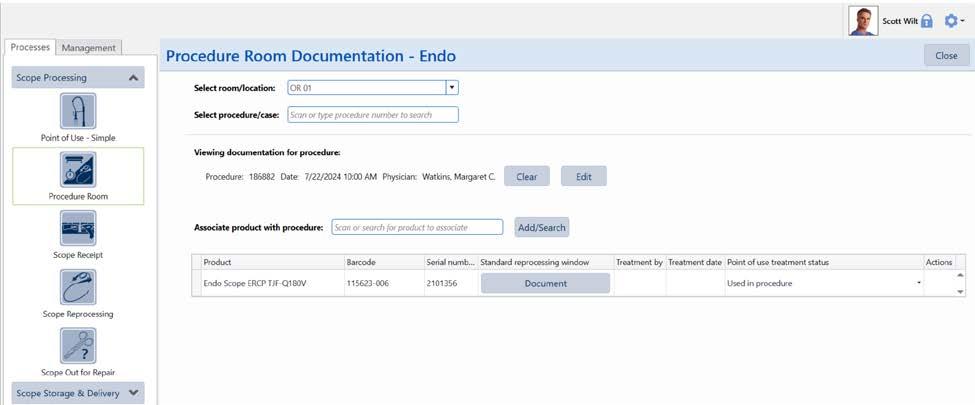
Some software allows procedure room staff to document the room they are serving and the procedure ID. They can scan the endoscope’s barcode to associate it with the procedure and document point-of-use treatment in the procedure room.
Following IFU, with some endoscopes having over 100 steps, is not simple. It is easy to see why steps might be missed. The complexity continues as cleaning instructions vary between models of the same type of endoscope. An Olympus bronchoscope may have different cleaning instructions from a Pentax bronchoscope. Even different models of bronchoscopes from the same manufacturer may have different cleaning instructions. As stated by SGNA (2018), “Any deviation from the reprocessing protocol can lead to the survival of microorganisms and increased risk of infection.” (p. 4) Documentation of each essential step is essential.
Manual checklists can help make sure each step is completed, but this is challenging in practice. Each endoscope make and model may need a different checklist. General checklists with branching instructions can lead to confusion and mistakes.
Some facilities may choose to complete manual documentation after cleaning. However, documenting after the fact can lead to errors. Staff must remember what they did and rework is necessary when missed steps are identified.
Capturing documentation requires a clear process and procedure. All endoscope processing staff must have sufficient training on the process for capturing documentation. They need to know what to document and how to document it.
Medical device asset management software can help staff follow the correct IFU for the endoscope being cleaned and document the manual cleaning process. Some software allows creation of standard work for each endoscope’s processing procedures. Processing guides provide step-by-step on-screen instructions during endoscope processing. Some software systems have guided workflows to help lead staff through each step. This step-by-step guidance

This lesson was developed by STERIS. Lessons are administered by Endeavor Business Media.
A er careful study of the lesson, complete the examination online at educationhub.hpnonline.com. You must have a passing score of 80% or higher to receive a certificate of completion.
The Certification Board for Sterile Processing and Distribution has pre-approved this in-service unit for one (1) contact hour for a period of five (5) years from the date of original publication. Successful completion of the lesson and post-test must be documented by facility management and those records maintained by the individual until recertification is required. DO NOT SEND LESSON OR TEST TO CBSPD. www.cbspd.net


Sterile Processing Association, myhspa.org, has pre-approved this in-service for 1.0 Continuing Education Credits for a period of three years, until July 23, 2027.
For more information, direct any questions to Healthcare Purchasing News editor@hpnonline.com.
can help technicians know what to do in what order, and include required interactions like checkboxes or confirming test results, such as leak test results.
Additional instructions can appear based on the result of a leak test or other interactions. With conditional documentation, instructions can appear on screen based on the test result. For example, if a technician documents that the leak test failed, instruction is provided indicating what to do next per facility policy.
Interactions built into a workflow confirm that critical steps, such as passing visual inspection, were completed. Each checkbox selected, comment made, test result recorded, and date entered is captured in the documentation.
Considerations for automated endoscope reprocessors (AER)
Automated processing of endoscopes can aid documentation of the essential steps. Automated endoscope processors can clean, high level disinfect, liquid chemically sterilize, or perform a combination of these activities. Many AERs have connectors specific to the make and model of the endoscope. As with any medical device, following the IFU is essential.
Cycle printouts and electronic files provided by AERs record and document
start times, cycle lengths, cycle parameters, chemistry lot numbers, and other critical factors. Equipment can alert staff to equipment or utility failures through cycle printouts.
Medical device asset management software can guide staff through the process of endoscope placement in the processor. Diagrams for hooking up the endoscope can be attached to the workflow for easy access.
Some AERs can electronically transfer cycle printouts and critical information such as cycle start and end times to medical device asset management software, linking cycle information to the reprocessing record of the endoscope.
Visual inspection occurs throughout the process. Staff may inspect external surfaces using lighted magnification and internal channels using a borescope.
Staff should document any damage discovered and subsequent repairs. Facilities must have policies and procedures describing actions required when damage is found. Considerations may include additional communications with the patient or the need to look back on previous cases.
Medical device asset management software allows staff to document

inspection results including attaching borescope images. Software can provide guidance and examples during inspection and assist in “look backs” when necessary.
Endoscopes must be dry prior to storage. Residual moisture left in lumens can provide an environment for microorganisms to grow and potentially create biofilms. Facilities should develop procedures to dry endoscopes prior to storage. Facilities may use borescopes or other moisture tests to confirm dryness. All such test results should be documented either manually or in medical device asset management software. Special storage cabinets can filter air entering the cabinet. Filtration helps reduce the exposure of the endoscope to environmental contamination. However, it is not sterile storage. Each facility should determine how long an endoscope can be stored before it is reprocessed again. The storage time should be documented prior to use of the endoscope. Endoscopes can be manually labeled with the storage time. Some cabinets track storage time providing alerts when the end of the storage time is approaching or has ended. Medical device asset management software can track storage time,


Processing guides provide staff step-by-step on-screen instructions and access to the IFU during endoscope processing
provide warnings when a scope is nearing its expiration, and receive information from storage cabinets to allow efficient scheduling of endoscope usage.
When the endoscope is processed and ready to store, a readily available electronic record of the essential processing steps is stored within the medical device asset management software, fulfilling the seventh essential step detailed by HICPAC (HICPAC, 2016). The record includes all process steps and outcomes, workflow test results, endoscope processor cycle documentation, and users associated with the process.
Staff competency is critical in cleaning and processing flexible endoscopes. Some medical device asset management software includes staff competency features. These features allow staff to demonstrate competency in endoscope cleaning and processing and limit endoscope processing work to qualified users.
SGNA (2018) states that facilities should have “Consistent oversight, compliance, documentation, and
process improvements in place to support a quality program.” (p.5)
A quality program helps the department consistently provide endoscopes ready for patient use. Facilities should ensure:
• Policies and procedures are followed
• Staff are competent
• Nonconformities, such as residual soils found after cleaning, are resolved
• Data is evaluated for negative trends With electronic data capture, quality reporting becomes easier and often more accurate than manual processes. These reporting tools allow review of


processing steps, tests, and repair history down to an individual staff member or endoscope.
Some software includes quality features and the ability to document nonconformities. Quality management features may allow trending reports to identify areas for improvement, document corrective actions taken, and review quality events to drive continuous quality improvement.
Scope processing is a vital and complex process. Medical device asset management soft ware helps ensure all required endoscope processing tasks are completed in a structured, disciplined manner while capturing essential data for a quality program. Software can drive quality and ideal outcomes from point-of-use treatment through every reprocessing step
1. Which of these is true of some asset management software?
A. May give staff a guided, disciplined process to follow
B. Give technicians access to on-screen resources
C. Documentation on conforming outcomes can be easily captured as staff complete work
D. All of the above
2. What is the first line of defense in preventing the formation of biofilms?
A. Decontamination
B. Point-of-use treatment
C. Manual cleaning
D. Visual inspection
3. What is biofilm?
A. Procedural soils including blood and bacteria
B. The pre-sterilization population of viable microorganisms
C. Bacteria living on a surface captured in visual inspection
D. A diverse community of bacteria and fungi that can congregate, attach to surfaces, and create their own extracellular matrix
4. Which of these is NOT one of the essential steps in endoscope processing identified by HICPAC?
A. Transport
B. Visual inspection
C. Documentation
D. Storage
5. What step must happen within 60 minutes after an endoscopy procedure according to many IFUs?
A. Completing pointof-use treatment
B. Beginning manual cleaning
C. Placing the endoscope in the endoscope processor
D. Thoroughly drying the endoscope
6. What feature in some asset management software can help ensure only qualified users do the work of endoscope processing?
A. Quality events
B. Associate files such as IFUs
C. Staff competencies
D. Conditional workflows
All CEU quizzes must be taken online at: educationhub.hpnonline.com. The cost to take the quiz is $10.
to tracing the endoscope to a patient. Having all the information and tools in one place allows for efficiency in documentation, data retrieval, and process improvement, preparing facilities for the next credentialing audit while providing the best patient outcomes. HPN
Visit hpnonline.com/55131809 for references.
7. Which of these can occur using conditional documentation in some asset management software?
A. Instructions can appear on screen based on a test result
B. The result of a machine run leak test can be captured
C. A color-coded timer bar may appear
D. Resources such as IFU can be accessed in the workflow
8. Which of these is NOT something procedure room users document?
A. The room they are serving and procedure ID
B. The endoscope used in the procedure
C. Performing pointof-use treatment
D. Performing manual cleaning
9. Which of these does SGNA state need to be in place to support a quality program?
A. 30 days of training prior to new staff reprocessing flexible endoscopes independently
B. Manual documentation of each essential step in the process
C. Consistent oversight, compliance, documentation, and process improvements
D. On-screen access to resources such as IFUs, images, or instructional videos
10. Which of these can be documented in asset management software?
A. All process steps and outcomes
B. Workflow test results
C. Scope processor cycle documentation
D. All associated users
E. All of the above



nostril
Ideal for holding most nasogastric tubes, including nasoenteric, Salem Sump™, and Levin enteral tubes.
Skin-friendly adhesive stays in place for up to 3 days, yet is easy to remove.
Long single tab spirals around the nasogastric tube for superior tube security.
Non-adherent plastic tip on the end of the tab makes unwrapping and tube removal safer and easier, especially when wearing exam gloves.
Secure tube in either nare.
BY DAVID L. TAYLOR, MSN, RN, CNOR, PRINCIPAL OF RESOLUTE ADVISORY GROUP LLC
The key to a successful Sterile Processing department (SPD) is a qualified leader who is knowledgeable, supportive, and maintains service standards, all while overseeing the production and service within their department. They also build and foster effective relationships with their customers and other departmental leaders. Strengthening those relationships is crucial not only for improved collaboration and teamwork, but also for improved quality of instrument processing. SP professionals have many obstacles, which can include inadequate space, outdated or insufficient equipment and instrument inventories, limited staff, everevolving technology and challenging processes, and more.
One of the greatest obstacles, however, lies in establishing strong, effective interdepartmental relationships with leaders from the Operating Room (OR) and other procedural areas served by the SPD. Effective SP leaders are subject matter experts who know their areas of expertise and are willing to share that with others for the sake of quality, safety, process consistency, and the attainment of shared goals. Successful SP leaders take time to purposefully reach out to other department leaders to collaborate on mutually beneficial practices, processes, and workflows. Technical know-how will only take a leader so far. Strong leaders are selfaware, understand the importance of cultivating relationships, and can solve problems inside and outside the SPD to maximize positive outcomes. Effective leaders see the big picture.
They know their job, their customers’ needs, and how to work with their colleagues within an interconnected healthcare environment. All who rely on the SPD to support their operations is responsible for doing their part. Interdisciplinary collaboration and accountability are essential.
Managing human emotions is another skill successful SP leaders possess. Having high emotional intelligence (e.g., social skills, selfawareness, self-regulation, comprehension and reasoning) allows leaders to more fully engage in positive interaction and a spirit of collaboration. Cultivating interdisciplinary relationships improves the ability to influence compliance and help make necessary change.
Modern healthcare organizations continue to grow in complexity as new technologies, devices, and procedures are introduced. To boost efficiencies and prevent disruptions, real-time data analytics are needed to understand workflow and opportunities for improvement. When missteps happen, data can be used to demonstrate to the responsible parties why it is so important to follow correct processes and partner with the SPD. Collecting, measuring, analyzing, and quantifying data can paint a clearer picture for SP leaders about which areas work well and which need attention. This not only helps improve work within the SPD but also holds others accountable who are supported by SP technicians’ work. For example, data can identify the number of case

carts entering and exiting the SPD as well as the time case carts arrived in the decontamination area and when items were processed and returned for patient care. Further, it could also identify whether the instruments underwent point-of-use treatment prior to arrival in the SPD, while also steering quality improvement initiatives. Put more simply, harnessing data can help develop and streamline processes and lead to vital conversations with other departmental leaders about their impact on processes and quality outcomes. An SPD dashboard is an effective tool used to share data with other departmental leaders. For example, data could be disseminated about the percentage of trays sent to the SPD without undergoing point-ofuse treatment. Such details are important because they convey to leaders in user areas why devices may require extra processing time.
When instrumentation-related errors occur, SP professionals are often blamed even though each department bears some responsibility for ensuring proper processes and best practices are followed. SP leaders must commit to developing effective relationships with other departments to identify opportunities for improvement, promote education, and increase accountability. Effective leadership and effective outcomes do not occur in a vacuum. Collaboration and teamwork are imperative for quality, safety, growth, and progress. When leaders work together, better outcomes will follow. HPN
BY STEPHEN M KOVACH, BS, CFER, CLINICAL EDUCATOR EMERITUS, HEALTHMARK, A GETINGE COMPANY
Q“We have a tabletop ultrasonic cleaner that we must manually fill. My teammate told me if we fill it 3/4 full we get more cleaning power. Is that true?”
ALess is not more. Ultrasonic cleaners are fine-tuned for a particular fill level; underfilling the tank will not increase cleaning power. Rather, it can damage the unit and result in less-than-optimal cleaning.
To further your knowledge on this topic, I would recommend reading some great articles that deal with the importance of watts/gallon and how it correlates to ultrasonic cleaning.1
Q“I have heard that ultrasonic cleaners must be at a specific frequency for many medical devices. Is that true? How do I know what frequency my unit is (it is over 15 years old)?”
AFinding your frequency should be easy. Many units have it labeled. If you cannot find it, just call the manufacturer and ask them for that information on their letterhead for your records. Interestingly, ultrasonic units in SPD do have specific range, and it is based on research.
Published literature states, “. . . to cavitate a liquid medium, a frequency of 18 kHz is required. Ultrasonic cleaners are available in frequencies ranging from 18 to 100 kHz.”2 Other articles suggest that for metal cleaning a frequency range of 20–40 kHz is needed for proper cleaning. In an SPD, we clean many different types of metal medical devices. “Ultrasonic cleaning frequency has a significant effect with
respect to ultrasonic cleaning capability. Ultrasonic cleaning equipment manufacturers state that low frequency cleaners provide high power cleaning results and that a high-frequency cleaner provides more uniform cleaning results. Our study indicated that ultrasonic cleaners in the range of 40 kHz provide very uniform ultrasonic cleaning fields and a higher cleaning capability.”3 Thus, my observation over the years has shown most ultrasonics in the SPD marketplace to be in that 40 kHz range. Once you know the frequency of your department’s ultrasonic unit, you can then make sure it can be used for your various medical devices (based on their IFU). Thus, what I call the “matching game” begins.
Q“We ran out of our ultrasonic cleaning solution and my co-worker said to ‘Use anything we have; they all work the same.’ Can you explain why that is not a correct statement?”
AAs stated earlier, in an SPD, we clean many different metal medical devices. We purchase ultrasonic cleaners for cavitation, and you want to enhance cavitation any time you can. One way to do this is by using cleaning solutions formulated for ultrasonic cleaners. First, cleaning solutions can lower the surface tension inside the tank or bath solution, which can then increase cavitation within that tank/bath. Second, you want a lowfoaming cleaning solution—foaming or bubbles are air, and air can impede or reduce cavitation in that tank/bath.4,5

As a bonus answer, since air impedes or inhibits cavitation, this supports the concept of degassing. Degassing is a process which eliminates air trapped in solution when fresh solution is added to the bath.
Degassing conditions your bath for maximum efficiency. Be sure to expel dissolved air bubbles, as these bubbles will affect the cleaning effectiveness. It’s about ensuring enough cavitation to “keep those instruments clean.” Remember to test your ultrasonic cleaner each day it is used with a clinically relevant, evidence-based product. For example, as stated in a peer-reviewed article, the “SonoCheck is a vial with a solution that changes color within a few minutes due to ultrasound exposure, and can therefore be used as an ultrasonic activity indicator.”6 HPN
References:
1. John’s Corner (Fuchs, J.). (2013, January 22). Ultrasonics – Ultrasonic Power Density vs. Tank Size. PDF: Understanding Ultrasonic Power Requirements Based on Tank Size and Other Factors. https://techblog.ctgclean.com/wp-content/ uploads/Considerations-for-Ultrasonic-Power-Density-vs.Tank-Volume1.pdf. Cleaning Technologies Group. https:// techblog.ctgclean.com/2013/01/ultrasonics-ultrasonic-powerdensity-vs-tank-size/
2. U.S. Army. (Last accessed 2004, February 10). Background on Ultrasonic and Aqueous Cleaning. http://aec.army.rnil/ usaec/technology/p2compliance10.html. (No longer cached online.)
3. Gillespie, T.J. (1980, May 8–10). (GEPP-OP-501) Evaluation of Ultrasonic Cleaning Systems. p15. General Electric Co., Neutron Devices Department. St. Petersburg, FL. CONF-800538-3. From ACS 1980 Meeting-in-Miniature; Tampa, FL.
4. Upcorp. (n.d.). Basic Concerns with Ultrasonic Cleaning | Part 3: Cleaning Fluid. Ultrasonic Power Corporation. https:// www.upcorp.com/blog/basic-concerns-with-ultrasoniccleaning-part-3-cleaning-fluid/
5. Sandor, B. (2023, September 28). Ultrasonic Cleaner Degas: Why it’s Important. Tovatech. https://tovatech. com/blog/399/ultrasonic-cleaner/how-to-degas-ultrasoniccleaner-solution#:~:text=Bubbles%20and%20dissolved%20 air%20in,of%20an%20ultrasonic%20cleaning%20tank
6. Verhaagen, B., Fernández-Rivas, D. (2016). Measuring cavitation and its cleaning effect. Ultrasonics Sonochemistry , 29(2016), 619–628. https://www.sciencedirect.com/science/ article/pii/S1350417715000723/pdfft?md5=b21635f90f2a f9beb7767b5a262d1b47&pid=1-s2.0-S1350417715000723main.pdf

The impacts of a cyberattack on the supply chain could be significant, but many ways exist to protect against the worst case scenarios
BY MATT MACKENZIE, ASSOCIATE EDITOR
As the threat of ransomware and cyberattacks continues to grow, private companies like Microsoft and branches of the U.S. government like the Department of Health and Human Services (HHS) are rushing to ensure that hospital systems and healthcare organizations are duly protected against these increasing threats.
Healthcare Purchasing News had the opportunity to speak with Chad Wilson, CEO of LS Cyber. Wilson is a renowned leader in healthcare cybersecurity, known for his expertise in protecting patient data and securing information systems. He is a thought leader in the field, contributing valuable insights on evolving challenges and innovative solutions.
Could you tell our readers a bit about your background?
re-routing of financial transactions, or denial of logistics essential for operations. Plus, without computers available, communication internally and externally becomes challenging. Information may not be readily available, further compounding the ability of the organization to communicate effectively and in a timely fashion.

I’ve been practicing cybersecurity in healthcare for almost two decades now and cybersecurity in general for over three decades. I started a cybersecurity company quietly, called LS Cyber, and our goal is to bring a service and product to healthcare that helps integrate security within the environment as opposed to something that’s bolted on.
What would ransomware’s impact on a materials manager be?
Normal workflows for ordering, inventory control, shipping, and receiving are all reliant on computers. Ransomware disrupts the availability and confidentiality of the computer, and the information used by the organization. Normal workflows cease to be available. While ransomware removes resource availability, the bad actors controlling it also seek to access and steal all the information on the computer or neighboring computers.
Additionally, significant financial impacts can occur when the bad actor successfully disrupts operations. This can take several forms, such as denial of financial transactions,
If the supplier is attacked with ransomware, it will impact their operations with potential downstream impacts to their customers. All of the impacts I mentioned for the materials manager apply to a supplier organization – they may have difficulty getting paid, and they may have difficulty shipping materials because their systems are down.
Further, while they have to go through the same things a healthcare organization would go through in this situation, there is one notable exception – the supplier shouldn’t be thinking they’re in a vacuum all by themselves. They should be notifying their customers immediately, and they should know what their contractual obligations are. Say, I’m a supplier to healthcare, then I should be held accountable for all the same HIPAA regulations and responsibilities, even though I’m not a covered entity – I’m supplying to a covered entity. Those requirements should be contractually required by that covered entity for the supplier. The entity needs to know if their data is safe, or what data may have been compromised.
Also, a major hurdle is communication – who should be contacted, and how can they be contacted if typical email can’t be used? Contact information has to be in a format that is accessible when your computer is not available, and writing things on paper has its own old-school HIPAA requirements. When all the contact information is electronic, the time to back up that information is now, when it’s not an issue. HPN
Visit hpnonline.com/55131409 for the rest of the interview.

Bellwether League Foundation proudly inducts the Bellwether Class of 2024 into the Healthcare Supply Chain Leadership Hall of Fame at its 17th Annual Bellwether League Foundation Induction & Recognition Event (BLFIRE17) scheduled to be held at The University of Wisconsin-Milwaukee, Milwaukee, WI Monday, October 7, 2024.
Bellwether Class of 2024 to be inducted:
Ron Denton (1937-2023)
Dave Hunter
Gail L. Kovacs
Eugene S. Schneller, Ph.D.
Celeste West
Ammer Honoree Class of 2024 to be recognized:
Amanda Chawla, MBA, MHA, FACHE, CMRP
Anand S. Joshi, M.D., MBA
Future Famers Class of 2024 to be recognized:
Angie Bruns, MHA
Chico Manning, MHA
Corey Schmidt, CMRP, MBA
Learn more details about BLFIRE17 and the 17 Bellwether Classes, four Ammer Honoree Classes and 10 Future Famers Classes at http://www.bellwetherleague.org.
salutes all 148 healthcare supply chain innovators, pioneers and visionaries in Bellwether League Foundation’s Hall of Fame for Healthcare Supply Chain Leadership.
Honoring Supply Chain Leaders of Yesterday, Today and Tomorrow
BY KAREN CONWAY, CEO, VALUEWORKS
Over the years in this column, we have discussed how a supply chain mindset can support the move to valuebased healthcare, by better managing the finite resources available to any health system (or even society) to optimize the health and well being of patients and populations. Recently, I came across a publication¹ from the United Kingdom that supports this concept, highlighting the importance of creating a culture of resource stewardship. The publication, Sustainability in quality improvement: redefining value from the Royal College of Physicians, sets forth an expanded definition of “value” as one that supports the ability of the healthcare system as a whole to deliver quality care, not just for patients today, but for generations to come. Specifically, the authors suggest redefining value as the outcomes of care delivery relative to not only the economic but also the environmental and social impacts or what has been referred to as the “triple bottom line.”
and populations, including the social, economic and environmental determinants of health that play an even greater role on overall health than any clinical care received. It also considers the impact on the resources needed to keep patients healthy, now and in the future. In other words, it is a longerterm, systems-based approach.
The logic is sound, when you consider that even if a patient receives the best care possible in the hospital, the outcome of that care could easily be undermined if the patient goes home to an environment that is not conducive to healing. Further, when people do not have access to good food, live in impoverished and/or violent communities, or are exposed to air or water pollution (as examples), they often have higher rates of chronic disease, which make them higher risk patients, consuming greater amounts of resources during their care. All of this not only increases the costs of healthcare delivery but also the negative environmental impacts resulting from more inten-
I like this definition because it goes beyond more commonly used value equations that have focused narrowly on the quality and outcomes of a specific episode of care, divided by the cost of delivering that care. This definition takes into account all of the factors that impact health outcomes for people
sive healthcare operations, which are responsible for close to 10 percent of our nation’s total carbon footprint. To more effectively manage resources, the authors recommend starting with the carbon hotspots: pharmaceuticals (including those prescribed in primary care), medical devices, equipment and

instruments used in acute care, and the energy consumed in the operation of hospitals and other healthcare facilities.
Even more importantly, the authors speak to preventing the need for care in the first place by fostering community structures that support healthy populations and the ability of individuals to actively promote their own wellbeing. Then, when care is needed, the goal should be to increase access and efficiencies in the provision of highly effective interventions to those who need them, while limiting the use of treatments (and the associated resources) that are known to deliver little or no value.
Achieving the authors' recommendations is not the job of supply chain alone. It takes a concerted and coordinated effort across multiple functions, from clinicians and finance to operations and supply chain, as well as those community organizations with expertise in social, economic and environmental resource management. That said, supply chain is perfectly equipped to help align the various parties to achieve this broader definition of value. After all that’s what supply chain professionals do already, working with multiple stakeholders to understand the clinical, financial and increasingly social and environmental impacts of the resources procured and utilized in the delivery of health and healthcare. HPN
Reference 1. 1. Mortimer F, Isherwood J, Wilkinson A, Vaux E. Sustainability in quality improvement: redefining value. Future Healthc J. 2018 Jun;5(2):88-93. doi: 10.7861/futurehosp.5-2-88. PMID: 31098540; PMCID: PMC6502556.









The SafeStep ATP Monitor verifies the cleanliness of surfaces, endoscopes and cannulated instruments in just 15 seconds. By detecting ATP (adenosine triphosphate) – the energy molecule found in all human, animal, plant, bacterial, yeast, and mold cells –SafeStep gives you the testing power to minimize HAIs and improve patient and staff safety.







Unwrap the future of sterile supply management. With features that help streamline processes and reduce the possibility of wet sets, Aesculap’s next generation rigid container is everything you’d expect from the market leader.
Visit aesculapusa.com/aicon to learn more about how this breakthrough technology can help your SPD Operate With Greater Precision.