CE: Molecular testing in GI infections
Page 4
LIS buyer’s guide
Page 12
The evolution of respiratory diagnostics
Page 18


Yves Dubaquie, PhD Senior Vice President, Diagnostics, Revvity

Hemostasis innovation is here.

CE: Molecular testing in GI infections
Page 4
LIS buyer’s guide
Page 12
The evolution of respiratory diagnostics
Page 18


Yves Dubaquie, PhD Senior Vice President, Diagnostics, Revvity

Hemostasis innovation is here.



The ACL TOP Family 50 Series offers the most advanced automation and quality management for routine and specialty Hemostasis testing in mid- to high-volume labs. All models are standardized and offer automated pre-analytical sample integrity checks to identify under-filled sample tubes, abnormal sample aspiration potentially caused by clots, and assay-specific interference from hemolysis, lipemia and bilirubin. Plus, lab accreditation tools make compliance easier. Better efficiency and quality management for you—better quality care for your patients.
more information, contact your local Werfen representative. werfen.com
David Allen, PharmD, BCIDP
Rajasri Chandra, MS, MBA
Christina Wichmann




By Christina Wichmann
Editor in Chief
On December 28, 2024, a number of new and revised CLIA regulations go into effect, including new definitions and updates to numerous personnel requirements. These revised regulations (CMS-3326-F) were published in the Federal Register on December 28, 2023 and can be accessed at https://www.federalregister. gov/d/2023-28170.
A summary of some of these updates for December are below.
One revised (revised language is underlined) and four new definitions will go into effect. These are as follows:
• Continuing education (CE) credit hours means either continuing medical education (CME) or continuing education units (CEUs). The CE credit hours must cover the applicable laboratory director responsibilities and be obtained prior to qualifying as a laboratory director.
• Doctoral degree means an earned post-baccalaureate degree with at least three years of graduate level study that includes research related to clinical laboratory testing or advanced study in clinical laboratory science, medical laboratory science, or medical technology. For purposes of this part, doctoral degrees do not include doctors of medicine (MD), doctors of osteopathy (DO), doctors of podiatric medicine (DPM), doctors of veterinary medicine (DVM) degrees, or honorary degrees.
• Experience directing or supervising means that the director or supervisory experience must be obtained in a facility that meets the definition of a laboratory under this section and is not excepted under §493.3(b).
• Laboratory training or experience means that the training or experience must be obtained in a facility that meets the definition of a laboratory under § 493.2 and is not excepted from CLIA under § 493.3(b).
• Midlevel practitioner means a nurse midwife, nurse practitioner, nurse anesthetist, clinical nurse specialist, or physician assistant licensed by the State within which the individual practices, if such licensing is required in the State in which the laboratory is located.
Laboratory director qualifications and responsibilities have also been revised. Some of these changes are as follows:
• 20 earned CEs that cover laboratory director responsibilities for all laboratory directors except doctors of medicine and doctors of osteopathy who are certified in anatomic or clinical pathology, or both, by the American Board of Pathology or the American Osteopathic Board of Pathology.
• Where the requirements state a degree in “laboratory science,” acceptable degrees are biological, chemical, or laboratory science degrees. A degree in physical science is no longer considered acceptable in the regulations. Individuals without a biological, chemical, or laboratory science degree can meet the personnel requirements through an educational algorithm (explained on pages 90005–90006 of the final rule).
• Remove the residency provision; however, relevant experience in a residency or fellowship would continue to be acceptable experience and training for qualifying individuals.
• The laboratory director must be on site at the laboratory at least once every six months, with at least a four-month interval between the two on-site visits.
• There is a grandfather clause for all personnel affected by this final rule as long as they were qualified and serving in a CLIA-ceritfied lab as of December 28, 2024 and remained continuously employed in that position since December 28, 2024.
Again, the complete final rule can be accessed at https://www.federalregister. gov/d/2023-28170.
I welcome your comments and questions — please send them to me at cwichmann@mlo-online.com.
VP/MARKET LEADER
Vol.56, No. 9
HEALTHCARE & DENTAL GROUP Chris Driscoll cdriscoll@endeavorb2b.com
EDITOR IN CHIEF Christina Wichmann cwichmann@mlo-online.com
MANAGING EDITOR Erin Brady ebrady@endeavorb2b.com
PRODUCTION MANAGER Edward Bartlett
ART DIRECTOR Kermit Mulkins
AUDIENCE DEVELOPMENT/LIST RENTALS Laura Moulton | lmoulton@endeavorb2b.com
ADVERTISING SERVICES MANAGER Karen Runion | krunion@endeavorb2b.com
ADVERTISING
DIRECTOR OF SALES
EAST COAST/MIDWEST SALES, CLASSIFIEDS Carol Vovcsko (941) 321-2873 | cvovcsko@mlo-online.com
SOUTH/WEST COAST/ILLINOIS SALES Lora Harrell (941) 328-3707 | lharrell@mlo-online.com
MLO EDITORIAL ADVISORY BOARD
John Brunstein, PhD, Biochemistry (Molecular Virology) President & CSO PathoID, Inc., British Columbia, Canada
Lisa-Jean Clifford, COO & Chief Strategy Officer Gestalt, Spokane, WA
Barbara Strain, MA, SM(ASCP), CVAHP Principal, Barbara Strain Consulting LLC, Formerly Director, Value Management, University of Virginia Health System, Charlottesville, VA
Jeffrey D. Klausner, MD, MPH Professor of Preventive Medicine in the Division of Disease Prevention, Policy and Global Health, Department of Preventive Medicine at University of Southern California Keck School of Medicine. Donna Beasley, DLM(ASCP), Director Huron Healthcare, Chicago, IL
Anthony Kurec, MS, H(ASCP)DLM, Clinical Associate Professor, Emeritus SUNY Upstate Medical University, Syracuse, NY
Suzanne Butch, MLS(ASCP)CM, SBBCM, DLMCM Freelance Consultant, Avon, OH
Paul R. Eden, Jr., MT(ASCP), PhD, Lt. Col., USAF (ret.) (formerly) Chief, Laboratory Services, 88th Diagnostics/Therapeutics Squadron, Wright-Patterson AFB, OH
Daniel J. Scungio, MT (ASCP), SLS, CQA (ASQ), Consultant at Dan the Lab Safety Man and Safety Officer at Sentara Healthcare, Norfolk, VA CORPORATE TEAM
CEO Chris Ferrell
PRESIDENT June Griffin
COO Patrick Rains
CRO Paul Andrews
CHIEF DIGITAL OFFICER Jacquie Niemiec CHIEF ADMINISTRATIVE AND LEGAL OFFICER Tracy Kane
EVP CITY SERVICES & HEALTHCARE Kylie Hirko 30 Burton Hills Blvd., Suite 185 Nashville, TN 37215 800-547-7377 | www.mlo-online.com
Medical Laboratory Observer USPS Permit 60930, ISSN 0580-7247 print, ISSN 2771-6759 online is published 10 times annually (Jan, Feb, Mar, Apr, May, Jul, Aug, Aug CLR, Sep, Nov) by Endeavor Business Media, LLC. 201 N Main St 5th Floor, Fort Atkinson, WI 53538. Periodicals postage paid at Fort Atkinson, WI, and additional mailing offices. POSTMASTER: Send address changes to Medical Laboratory Observer, PO Box 3257, Northbrook, IL 60065-3257. SUBSCRIPTIONS: Publisher reserves the right to reject non-qualified subscriptions. Subscription prices: U.S. $160.00 per year; Canada/Mexico $193.75 per year; All other countries $276.25 per year. All subscriptions are payable in U.S. funds. Send subscription inquiries to Medical Laboratory Observer, PO Box 3257, Northbrook, IL 60065-3257. Customer service can be reached toll-free at 877-382-9187 or at MLO@ omeda.com for magazine subscription assistance or questions. Printed in the USA. Copyright 2024 Endeavor Business Media, LLC. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical,





























Side-Activated Safety Lancet Contact-Activated Safety Lancet Top-Activated Safety Lancet
Unistik® Safety Lancets are designed with you and your patient in mind. They are engineered with Comfort Zone Technology® to help reduce pain during the sampling process while consistently delivering the results you expect. Three activation methods are available in a variety of gauge sizes to provide solutions for all of your capillary sampling needs.
Choosing Unistik Safety Lancets ensures:
Supply Continuity
for your patients






By David Allen, PharmD, BCIDP
Diarrheal disease is a significant cause of morbidity and mortality, with the World Health Organization ranking it as the third leading cause of death in children under five years old globally.1 While acute gastroenteritis is infrequently fatal in the United States, it still poses a significant challenge to healthcare systems as a common and costly cause for healthcare visits.
One of the key difficulties in managing acute gastroenteritis is that the recommended treatment is usually supportive care despite significant symptoms affecting quality of life. This can often lead to pressure from patients who expect more from diagnostics since COVID-19 and who desperately want reassurances that everything is being done to identify the cause of their disease in hopes of a quick return to normalcy.



When testing is indicated, clinicians and laboratory professionals face mounting pressure to optimize diagnostic and therapeutic approaches to limit costs and antibiotic overuse. Molecular testing paired with diagnostic stewardship has revolutionized the management of gastrointestinal (GI) infections. This article explores how these cutting-edge strategies can enhance patient care, improve laboratory efficiency, and aid in the battle against antimicrobial resistance (AMR).
See test online at https://ce.mlo-online.com/ courses/transformingdiagnostics-in-gi-infectionsthe-role-of-molecular-testing/ Passing scores of 70 percent or higher are eligible for 1 contact hour of P.A.C.E. credit.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Discuss GI disease healthcare statistics and testing strategies.
2. Describe the benefits of molecular testing in the diagnosis and management of GI diseases.
3. Describe molecular panel sizes and the benefits and limitations of each.
4. Discuss diagnostic strategies and the reimbursement challenges that contribute to limitations.
Advancements in molecular diagnostics testing In recent years, extensive research has been conducted to evaluate diagnostic testing for infectious diarrhea, with molecular panels emerging as a powerful alternative to traditional stool cultures. These innovative panels offer superior diagnostic yield and dramatically reduce turnaround times, often delivering results within hours rather than the multiday turnaround time frequently required with conventional stool cultures.
Studies have consistently demonstrated the improved yield of molecular syndromic panels, with one study finding a 35.5% positivity rate over the 6% yield observed with traditional methods.2 Multiplex polymerase chain reaction (PCR) panels, in particular, have shown enhanced detection rates across various pathogen types: bacteria (7.8% versus 2.9%), viruses (11.4% versus 0.63%), and parasites (2.4% versus 0.15%) when compared to standard techniques.3
Faster and more precise identification of pathogens contributes to antimicrobial stewardship efforts by allowing for the use of better-targeted therapies and the reduction of unnecessary antibiotic use. Rapid detection can also inform the implementation of infection control measures, thus preventing further spread, improving patient satisfaction by decreasing unnecessary isolation, and allowing for earlier detection of potential outbreaks. The comprehensive nature of these panels also helps to facilitate improved epidemiologic tracking of local trends allowing for better resource deployment.
The 2017 clinical practice guidelines for infectious diarrhea, published by the Infectious Diseases Society of America (IDSA) and the American College of Gastroenterology (ACG), recommend targeted testing in patients presenting with moderate-to-severe diarrhea persisting beyond seven days to clarify the etiology of illness. This approach optimizes diagnostic yield and clinical utility when testing is performed
after a prolonged period of active symptoms while ensuring more judicious use of resources.4
While current guidelines acknowledge the value of molecular panels, they also highlight the scenarios where conventional culture methods are still necessary. They mention this in the context of recent trends in practice reflecting the growing demand for fast, accurate diagnostics that can facilitate timely treatment initiation and improve patient outcomes.
Molecular panels for gastrointestinal pathogens are typically stratified into three categories based on the number of targets they contain. The size of these panels (i.e., number of targets) may directly impact reimbursement by payors. Small panels, which focus on detecting the most clinically significant pathogens, comprise five targets or fewer, and are generally reimbursed. However, this approach can often lead to the need for additional visits and tests if the initial test is negative.
Medium-sized panels, targeting 6 to 11 pathogens, have variable reimbursement rates and clinical adoption. While they may not be comprehensive enough for many special populations, they are generally considered suitable for the average patient with fewer limitations than small panels.
Large panels, encompassing 12 or more targets, have been facing an erosion in outpatient reimbursement with an increase in restrictions pushing these panels to inpatients and specific high-risk outpatient populations. These most commonly include immunocompromised individuals, pediatric and elderly populations, and those with recent travel history.
For example, in the inflammatory bowel disease (IBD) population, the value of large panels has been demonstrated in comparative studies. One such study evaluated the use of a large GI PCR panel compared to traditional work-up and found a statistically significant reduction in IBD therapy
escalation (16% versus 29%; P < 0.01) and post-test endoscopic procedures (10% versus 18%; P = 0.04).6 (See Figure 1.) These findings suggest that the initial investment in advanced molecular diagnostics may lead to cost savings and improved patient outcomes in the long run for the IBD population.
Outside of special populations, the implementation of large PCR panels for inpatients has also resulted in significant benefits. For instance, in one study, a shorter duration of hospitalization was observed from the time of stool collection (3.4 days versus 3.9 days). This paired with a lower observed rate of additional stool testing orders and imaging studies underscores the potential to reduce unnecessary interventions and improve patient outcomes.
The incorporation of molecular panels in clinical practice represents a significant advancement in diagnosing gastrointestinal infections, offering superior detection rates across a spectrum of pathogens while also providing more information to support judicious antimicrobial use. Healthcare providers must navigate the balance between diagnostic comprehensiveness and economic considerations, particularly in light of reimbursement policies that may prioritize cost-effectiveness over clinical discretion. Ultimately, the integration of these advanced diagnostic tools requires an approach that ensures both improved patient care and practical constraints in healthcare delivery.
The diverse clinical presentations and pathogen types in GI infections necessitate a nuanced approach to diagnostic strategies. While PCR panels have gained prominence in many inpatient laboratory settings due to their high sensitivity and rapid turnaround time, they require supplementation with traditional diagnostic methods in some scenarios. Conventional techniques retain value when there is concern for novel pathogens or outbreak investigations. Moreover, while antibiotics are
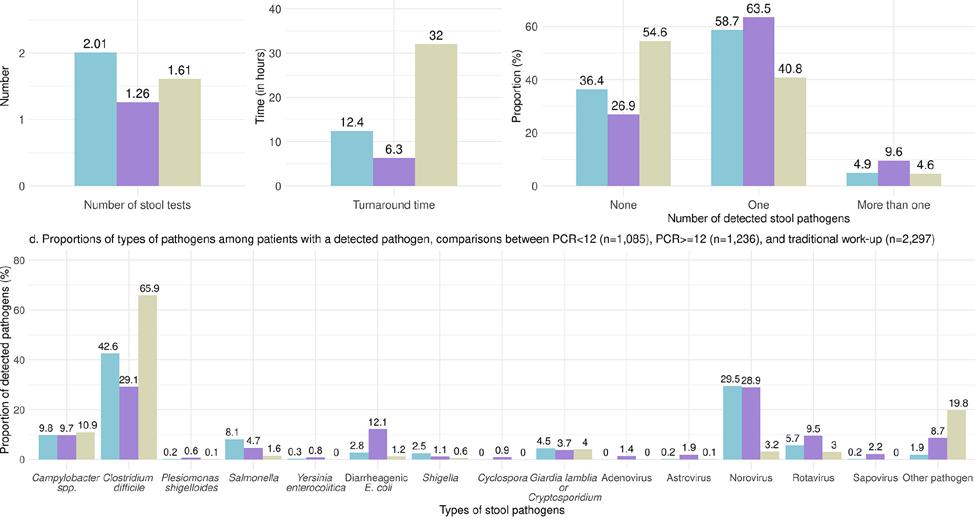
Figure 2. Results of a large retrospective cohort looking at the association between diagnostic method and healthcare resource use in 36,787 adult outpatients tested for acute infectious gastroenteritis. The figure highlights the reduced need for follow-up stool testing with PCR panels with ≥12 targets, the fast turnaround time, and the improved diagnostic yield.5

Figure 3. Adjusted mean healthcare costs observed on index visit, 30-day follow-up, and in total among patients with acute infectious gastroenteritis who were tested outpatient. While traditional testing was associated with lower cost on the index visit this population had a higher cost on follow-up visits.5
often not indicated, antimicrobial susceptibility testing should be available for instances where the results would affect patient care by determining the most appropriate therapeutic options.
The reimbursement landscape for GI pathogen panels has grown increasingly complex, with many payors implementing tighter criteria based primarily on panel size. These size categorizations are not rooted in evidence but are largely determined by payor policies. This disconnect underscores the need for ongoing dialogue between healthcare providers, payors, and policymakers to ensure reimbursement policies are aligned with best clinical practices and patient outcomes.
As mentioned, large PCR panels often represent a higher upfront cost at the index visit compared to smaller panels or conventional diagnostic approaches but have been associated with significant downstream benefits. in one large retrospective cohort study, these included reduced mean 30-day follow-up costs and a lower risk of hospitalization and associated antibiotic use.5 (See Figures 2 and 3) These findings suggest that despite higher upfront costs, implementation of molecular panels could lead to improved clinical outcomes, reduced healthcare overutilization, and enhanced antimicrobial stewardship.
The arbitrary categorization of molecular diagnostics by payors necessitates careful consideration of regional patterns in pathogen prevalence, which can significantly impact the optimal design and composition of diagnostic panels. Consequently, there is a growing need for more adaptable testing platforms that can accommodate local epidemiologic trends.
In response to recent shifts in reimbursement trends, several diagnostic companies are actively developing mid-sized panels. This emerging market segment aims to bridge the gap between more limited small panels and comprehensive large panels, attempting to strike a balance between diagnostic breadth and cost-effectiveness for low-risk populations in the outpatient setting. As processes for GI pathogen detection continue to evolve, it is crucial to consider the diverse needs of both general and specialized patient populations in selecting diagnostic panels and designing testing algorithms with the flexibility to serve your patient population.
Implementing advanced molecular testing methodologies has emerged as a critical consideration in managing GI infections and the ongoing battle against AMR. Recent literature
provides compelling evidence supporting the superiority of molecular panels over conventional techniques for most patient populations. As the field of GI diagnostics continues to advance, the integration of these innovative tools promises to enhance patient care and infection control practices, improve laboratory efficiency, and contribute to global efforts in combating AMR.
Scan code to go directly to the CE test.
1. Diarrhoeal disease. Who.int. Accessed September 26, 2024. https:// www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease.
2. Cybulski RJ Jr, Bateman AC, Bourassa L, et al. Clinical impact of a multiplex gastrointestinal polymerase chain reaction panel in patients with acute gastroenteritis. Clin Infect Dis. 2018;13;67(11):1688-1696. doi:10.1093/cid/ciy357.
3. Axelrad JE, Freedberg DE, Whittier S, Greendyke W, Lebwohl B, Green DA. Impact of gastrointestinal panel implementation on health care utilization and outcomes. J Clin Microbiol. 2019;57(3). doi:10.1128/ JCM.01775-18.
4. Riddle MS, DuPont HL, Connor BA. ACG Clinical Guideline: Diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622. doi:10.1038/ajg.2016.126.
5. Moon RC, Bleak TC, Rosenthal NA, et al. Relationship between diagnostic method and pathogen detection, healthcare resource use, and cost in U.S. adult outpatients treated for acute infectious gastroenteritis. J Clin Microbiol. 2023;61(2):e0162822. doi:10.1128/ jcm.01628-22.
6. Hong S, Zaki TA, Main M, et al. Comparative evaluation of conventional stool testing and multiplex molecular panel in outpatients with relapse of inflammatory bowel disease. Inflamm Bowel Dis. 2021;27(10):1634-1640. doi:10.1093/ibd/izaa336.
7. Beal SG, Tremblay EE, Toffel S, Velez L, Rand KH. A gastrointestinal PCR panel improves clinical management and lowers health care costs. J Clin Microbiol. 2018;56(1). doi:10.1128/JCM.01457-1.

David Allen, PharmD, BCIDP is a Field Medical Director at bioMérieux . In this role, he works with key opinion leaders (KOLs) from large Integrated Delivery Networks and National Accounts on bridging the gap between the patient care needs of these KOLs and the pipeline development process. He is a graduate of Virginia Commonwealth University School of Pharmacy and previously practiced as an Infectious Diseases Clinical Pharmacy Specialist, with the majority of his time as the lead pharmacist for the antimicrobial stewardship program at the Inova Health System.

It’s
Introducing GEM Premier 7000 with Intelligent Quality Management (iQM3), offering hemolysis detection for the first time on a blood gas system. Providing quality assurance in real time, it can detect more sources of error at the point of care, improving the quality of critical results, including potassium (K+), for enhanced patient care.
Learn more about our latest innovation at werfen.com/GEMPremier7000





By Rajasri Chandra, MS, MBA


Sepsis is a systemic inflammatory reaction mainly caused by pathogen infection, which is often characterized by high fever, leukocytosis, and headache.1 Sepsis can lead to multiple organ dysfunction syndrome (MODS) and circulatory failure in critical condition. In 2016, The European Society of Intensive Care Medicine (ESICM) and the Society of Critical Care Medicine (SCCM) defined sepsis as a lifethreatening organ dysfunction caused by a dysregulated host response to infection. 2 The body’s reaction causes damage to its own tissues and organs leading to septic shock, multiple organ failure, and sometimes death, if not recognized early and treated promptly. The Centers for Disease Control and Prevention (CDC) reports that at least 1.7 million adults develop sepsis annually in U.S. hospitals and at least 350,000 adults who develop sepsis die during their hospitalization or are discharged to hospice. 3
Sepsis is usually caused by bacterial infections but infections from viruses such as COVID-19 and influenza, parasites or fungi, or noninfectious insults, such as traumatic injury, can also cause sepsis.3 Normally, to combat the infection or traumatic insult, the body releases chemical or protein immune mediators into the blood. If unchecked, those immune mediators trigger widespread inflammation, blood clots, and leaky blood vessels, causing impaired blood flow and organs becoming deprived of nutrients and oxygen, which leads to organ damage.4 The bacteria that have been predominantly found to be responsible for sepsis and septic shock are Gram-positive bacteria — Staphylococcus aureus and Streptococcus pneumoniae and Gram-negative bacteria — Escherichia coli, Klebsiella, and Pseudomonas spp.5 Previously, it was believed that Gram-negative bacteria was the major cause of sepsis and septic shock;6 however, presently, it has
Detecting and treating severe hypoglycemia promptly after birth is crucial due to its association with adverse long-term neurodevelopmental outcomes. However, limited data are available on the optimal timing of glucose screening in asymptomatic high-risk neonates prone to hypoglycemia. This webinar will address the incidence and the impact of individual risk factors on early hypoglycemia in asymptomatic high-risk neonates. Dr. Hanna will also discuss the importance of blood glucose screening in the first hour of life to help identify early severe neonatal hypoglycemia.

- Understand the optimal timing for initiating blood glucose monitoring and hypoglycemia long-term effects for asymptomatic at-risk neonates
- Learn the incidence of hypoglycemia in infants of diabetic mothers
- Explain the risk factors associated with developing early hypoglycemia
- Describe incidence rates of early hypoglycemia among asymptomatic neonates at risk of hypoglycemia
Primary Presenter
Dr. Nazeeh Hanna, M.D., Professor
Department of Pediatrics, NYU Grossman Long Island School of Medicine
Chief, Neonatology, NYU Langone Hospital
New York City, New York
Point-of-care (POC) testing is an essential diagnostic tool in neonatal and pediatric patient settings because infants and children experience more rapid changes in clinical status than adults. Dennis Begos, MD. Senior Director of Medical and Scientific Affairs at Nova, will describe the positive impact of accurate, interference-free POC glucose testing on outcomes and quality of care in neonatal settings.
Presenter
Dennis Begos, MD, FACS, FACRS
Senior Director
Medical and Scientific Affairs
Nova Biomedical
Webinar Dates: Thursday, December 5, 1:00 PM ET Thursday, December 19, 3:00 PM ET
Register Now at: novabiomedical.com/neonateglu-MLO
Educational Credits

This program offers 1 hour of P.A.C.E. continuing education credits. Nova Biomedical is approved as a provider of continuing education programs in the clinical laboratory sciences by the ASCLS P.A.C.E.® Program. This program has been approved by the American Association of Critical-Care Nurses (AACN), for 1.00 CERPs, Synergy CERP Category A, File Number 25253. Approval refers to recognition of continuing education only and does not imply AACN approval or endorsement of the content of this educational activity, or the products mentioned.
been shown that Gram-positive bacteria are the predominant cause of sepsis and septic shock.4,6 Among fungi, Candida species are predominantly responsible for causing sepsis and septic shock in immunosuppressed or neoplastic patients undergoing long-term treatment with chemotherapeutic and immunosuppressive drugs.8
The pathogenic Gram-positive and Gram-negative bacteria produce different types of toxins. Toxins from the bacteria helps the pathogenic bacteria to modulate host defenses, enabling them to escape the innate immune system to remote organs resulting in sepsis or septic shock. The outcome of the disease depends on the type of the toxin.9 Infection in sites that may lead to sepsis and their percent of occurrence are as follows: 8,10
• Respiratory tract/pulmonary parenchyma (43%)
• Urinary system (16%)
• Abdomen (14%)
• Head, which is associated with a fever of unknown origin (14%)
• Other sites/causes (13%)
People at high risk
The people who are at high risk of sepsis are the following: 11
• People older than 65 years old, newborns and infants, and pregnant women
• People having medical conditions such as diabetes, obesity, cancer, and kidney disease
• People with weakened immune systems
• People who are in the hospital for other medical reasons
• People with severe injuries, such as large burns or wounds
• People with catheters, IVs, or breathing tubes
• People with certain genetic variants are more prone to sepsis12
Stages of sepsis
There are three stages of sepsis: sepsis, severe sepsis, and septic shock.11
In the first stage, the immune response overreacts to an infection or injury resulting in a situation like systemic inflammatory response syndrome. However, at this stage it is very difficult to diagnose sepsis.
A patient having sepsis may have one or more of the following signs or symptoms: 2
• Clammy or sweaty skin
• Confusion or disorientation
• Extreme pain or discomfort
• Fever, shivering, or feeling very cold
• High heart rate or weak pulse
• Shortness of breath
Severe sepsis occurs when acute organ dysfunction begins and there is failure of one or more organs. Symptoms of severe sepsis are as follows:13
• Changes in skin color or patches of discolored skin
• Low or no urine output
• Disorientation, drowsiness, changes in mental ability, loss of consciousness
• Difficulty breathing
• Abnormal heartbeat
• Chills
• Extreme weakness
Septic shock is a serious condition that occurs when a body-wide infection leads to dangerously low blood pressure. Septic shock can affect any part of the body, including the heart, brain, kidneys, liver, and intestines. Symptoms of septic shock are as follows: 14
• Cool, pale arms and legs
• High or very low temperature, chills
• Lightheadedness
• Little or no urine
• Low blood pressure, especially when standing
• Palpitations
• Rapid heart rate
• Restlessness, agitation, lethargy, or confusion
• Shortness of breath
• Skin rash or discoloration
• Decreased mental status and confusion
For the mortality rate in sepsis stages, see Table 1.
• Increased breathing rate
• Urinary tract infections (UTI) or kidney problems
• If the immune system has gone into an over-reactive mode using certain blood tests
Because sepsis is highly complex and can progress to multiple organ dysfunction syndrome and death, various tools are used that incorporate clinical evaluation, vital signs, and laboratory results to screen, recognize severity, risk stratification prognosis and mortality. Some tools that have been used for this purpose are:
• Systemic Inflammatory Response Syndrome (SIRS) criteria,
• Quick Sequential Organ Failure Score (qSOFA),
• Sequential Organ Failure Assessment (SOFA) criteria,
• National Early Warning Score (NEWS), and
• Modified Early Warning Score (MEWS).17
The laboratory tests conducted for patients suspected of having sepsis are:18
• Complete blood count, including white blood count and platelet count
• Serum creatinine
• Bilirubin
• Serum lactate
• Procalcitonin (PCT)
• C-reactive protein (CRP)
• Urinalysis
• Blood culture followed by identification and antibiotic susceptibility testing if bacteria is detected
If viral or fungal infection is suspected, appropriate specimens need to be collected for their detection.
Table 1. Shapiro et al. conducted a study on in-hospital mortality and reported the mortality rate at different stages of sepsis.15
At present, there is not a single test to identify sepsis. Thus, doctors and healthcare professionals use a combination of tests and clinical signs to diagnose sepsis.16 Broadly, these tests determine the following:16
• The presence of an infection, if any. If detected, identify the organism and determine antibiotic susceptibility
• Very low blood pressure and high heart rate
Studies are being carried out to understand the occurrence and progress mechanism of sepsis at the genomic level and provide new targets for clinical diagnosis and treatment of sepsis. Studies have identified a few potential biomarkers markers of sepsis, such as ITK, CD247, MMP9, CD3D, MMP8, KLRK1, and GZMK.19 However, genetic test for sepsis are still not in practice. That said, rapid and sensitive molecular tests are often used to detect if there is any infection.
As sepsis and septic shock are medical emergencies, early identification and treatment initiation improve outcomes
in sepsis patients. These patients need constant monitoring and are often monitored and treated in ICUs. The treatment for septic and septic shock patients are as follows:5
First pillar of sepsis/septic shock treatment — antimicrobial therapy to control infection
To control the infection, antimicrobial therapy needs to be initiated immediately, ideally within one hour after admission. Microbiological specimens — blood, fluid or tissue from sites based on the clinical evaluation — need to be collected and analyzed as soon as possible for identification of infection. The choice of initial empiric antimicrobial therapy is based on clinical (i.e., site of infection, previous antibiotic use, immunosuppression, and risk factors for resistant organisms) and epidemiological criteria.
For patients proceeding toward septic shock, multidrug antimicrobial regimens with a wide spectrum of activity should be used (e.g., carbapenems and anti-Gram-negative antimicrobials with dual coverage). Dual coverage for Gram-negative organisms might be appropriate in cases of high suspicion for multidrug-resistant organisms (e.g., Pseudomonas aeruginosa or Acinetobacter baumanii). Dual coverage for Gram-positive organisms and methicillin-resistant Staphylococcus aureus (MRSA) should be considered for patients with a high risk of infection due to these pathogens.
The second pillar of treatment –intravenous fluid resuscitation
Intravenous fluid helps to increase depleted or functionally reduced intravascular volume that occurs in sepsis owing to vasodilated vascular network. Balanced crystalloids have been the fluid of choice with continuous hemodynamic monitoring to avoid fluid overload.
The third pillar of treatment – vasoactive agents
To maintain mean arterial pressure above 65 mmHg and reduce the risk of fluid overload vasoactive drugs are administered. Norepinephrine (NE) has been the first line of choice. Vasopressin (VP) and Epinephrine may be considered as second and third lines of treatment respectively for septic shock.
Oxygen and ventilation support
Patients may require oxygen or ventilation support, for example, when blood oxygen levels are low or the patient has sepsis-induced acute respiratory distress syndrome (ARDS).
Other treatment
Based on the clinical condition of the patient, it may be required to administer other medications to the patients including heparin, insulin, steroid, acetaminophen etc.
Conclusion
Sepsis is a very complex and challenging syndrome as it extends beyond the infection type and encompasses a spectrum of biological processes, including inflammation, coagulation, endothelial activation, and alterations in the microbiome.20, 21
Even though at present it is not possible to confirm sepsis and predict the prognosis using one single test, with the advancements in omics technologies (genomics, transcriptomics, proteomics, metabolomics, cytomics) coupled with artificial intelligence and machine learning, we can hope to detect sepsis, predict prognosis, and recommend personalized therapy to patients with sepsis in the future.
1. Evans L., Rhodes A., Alhazzani W., et al. Executive summary: Surviving sepsis campaign: International guidelines for the management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):1974-1982. doi:10.1097/CCM.0000000000005357.
2. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315(8):801. doi:10.1001/jama.2016.0287.
3. CDC. About. Sepsis. Published May 13, 2024. Accessed September 25, 2024. https://www.cdc.gov/sepsis/about/?CDC_AAref_Val=https://www. cdc.gov/sepsis/what-is-sepsis.html.
4. National institute of general medical sciences. National Institute of General Medical Sciences (NIGMS). Accessed September 25, 2024. https://www.nigms.nih.gov/education/fact-sheets/Pages/sepsis. aspx?ref=prendi-il-controllo-della-tua-salute.com.
5. Guarino M, Perna B, Cesaro AE, et al. 2023 update on sepsis and septic shock in adult patients: Management in the emergency department. J Clin Med. 2023;12(9):3188. doi:10.3390/jcm12093188.
6. Parrillo JE, Parker MM, Natanson C, et al. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113(3):227-42. doi:10.7326/0003-4819-113-3-227.
7. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003;348:1546–54. doi:10.1056/NEJMoa022139.
8. Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(21):2063. doi:10.1056/NEJMc1312359.
9. Ramachandran G. Gram-positive and gram-negative bacterial toxins in sepsis: a brief review. Virulence. 2014;5(1):213-8. doi:10.4161/ viru.27024.
10. Vakkalanka J.P., Harland K.K., Swanson M.B., Mohr N.M. Clinical and epidemiological variability in severe sepsis: An ecological study. J Epidemiol Community Health. 2018;72:741–745. doi:10.1136/ jech-2018-210501.
11. Cleveland Clinic. Sepsis. Accessed September 25, 2024. https:// my.clevelandclinic.org/health/diseases/12361-sepsis.
12. Engoren M, Jewell ES, Douville N, Moser S, Maile MD, Bauer ME. Genetic variants associated with sepsis. PLoS One 2022;17(3):e0265052. doi:10.1371/journal.pone.0265052.
13. Sepsis Alliance. Severe Sepsis. Published November 11, 2019. Accessed September 25, 2024. https://www.sepsis.org/sepsis-basics/ what-is-sepsis/severe-sepsis/.
14. MedlinePlus. Septic shock. Bethesda, MD: National Library of Medicine (US); Updated November 23, 2023. Accessed September 2024. Available from: https://medlineplus.gov/septicshock.
15. Shapiro N, Howell MD, Bates DW, Angus DC, Ngo L, Talmor D. The association of sepsis syndrome and organ dysfunction with mortality in emergency department patients with suspected infection. Ann Emerg Med. 2006;48(5):583-90, 590.e1. doi:10.1016/j. annemergmed.2006.07.007.
16. Sepsis. Yale Medicine. Published October 29, 2022. Accessed September 25, 2024. https://www.yalemedicine.org/conditions/sepsis.
17. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181-1247. doi:10.1007/ s00134-021-06506-y.
18. Fulton MR II, Zubair M, Taghavi S. Laboratory evaluation of sepsis. In: StatPearls. StatPearls Publishing; 2024.
19. Liang J, Wu W, Wang X, Wu W, Chen S, Jiang M. Analysis of sepsis markers and pathogenesis based on gene differential expression and protein interaction network. J Healthc Eng. 2022;12;2022:6878495. doi:10.1155/2022/6878495.
20. van der Poll T, Shankar-Hari M, Wiersinga WJ. The immunology of sepsis. Immunity. 2021;9;54(11):2450-2464. doi:10.1016/j. immuni.2021.10.012.
21. van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17(7):407-420. doi:10.1038/nri.2017.36.

Rajasri Chandra, MS, MBA is a global marketing leader with expertise in managing upstream, downstream, strategic, tactical, traditional, and digital marketing in biotech, in vitro diagnostics, life sciences, and pharmaceutical industries. Raj is an orchestrator of go-to-market strategies driving complete product life cycle from ideation to commercialization.
MLO’s annual LIS Buyer’s Guide showcases different LIS products from a variety of vendors. This year, MLO decided to expand its annual LIS guide and add lab data products from companies outside the LIS, which are featured in Tables 1-4.
By Erin Brady
Choosing the right laboratory information system (LIS) is a challenging process for laboratories everywhere. Recent technology advancements like artificial intelligence (AI) and machine learning, cyberattacks, and an increasing demand for cloud-based solutions have all affected the LIS market.
Medical Laboratory Observer (MLO) interviewed five LIS experts to better understand the current trends and challenges they’ve observed in the LIS market this year.
Gilbert Hakim, CEO of SCC Soft Computer said, “We saw a significant increase in genetic testing performed in client labs instead of sending it to reference labs. Of course, the labs need to understand that the EMR vendor cannot correctly bill for these specialized tests and should rely on the LIS vendor’s billing services to do so.”
Suren Avunjian, LigoLab Co-Founder and CEO highlighted the rise of cyberattacks in 2024. “This spike in attacks underscored the urgent need for enhanced cybersecurity measures. As we head into 2025, implementing strategies like two-factor authentication (2FA), immutable backups, and comprehensive disaster recovery plans are no longer optional but essential components of a robust cybersecurity posture.”
“One of the trends that we saw in 2024 was the fact that labs are having to deal with staffing shortages and are turning to technology to help them do that—using the LIS to gain workflow efficiencies, adopting more automation,” said Kim Futrell, MLS(ASCP), MSHI, CPMM, Senior Strategic Marketing Manager, Orchard Software.
Other trends she noted were, “the adoption of digital pathology is causing labs to begin moving to anatomic pathology laboratory information systems with the ability to optimize digital pathology workflows,” and “the growth in molecular testing continued to drive the adoption of more comprehensive, mature, and reliable molecular information systems.”
Technology advancements
MLO asked LIS experts how recent technology advances have impacted LIS and what future developments they are excited about.



AI is one of the most significant advancements in the history of pathology, according to Joseph Nollar, AVP of LIS Product Management, XiFin, Inc.“During the pandemic, we saw an increase in requests to integrate digital pathology solutions. This was driven by the FDA changing its guidelines to make it easier to install systems so pathologists could work remotely. Post-pandemic, the primary driver of the growth in digital pathology has been FDA-approved diagnostic algorithms driven by AI. We are particularly excited about the continuing growth and expansion of diagnostic algorithms that will provide additional diagnostic insights, assist pathologists in their diagnosis, mitigate risk, and improve lab productivity. The LIS has a key role in facilitating a fully integrated digital pathology workflow with the ability to integrate AI-generated diagnostic results.”
Ed Krasovec, Director – Clinical Solutions, LabWare, Inc. said, “Because
Product name URL
Unity Next Peer QC Qcnet.com/unity-next


Key Features Integration Capabilities
• Quality Control (QC) peer comparison
• On-demand peer reports
• Centralized QC software for data submission and monitoring
• Levey Jennings chart for continuous QC monitoring
• SPC Rules
Solutions the Service Provides
• QC data management software that integrates with all major instrument platforms
• Middleware
• LIS systems
• Unity Next Peer QC is a simple peer comparison tool that empowers labs to meet compliance with instant access to peer reporting in a centralized, user-friendly platform.
• Connect and compare QC results with over 38,000 peers.
• With comprehensive QC data processed from 68 million results monthly, QC performance can be evaluated across controls, instruments, and peers.
• This software helps optimize Quality Control processes by continuously monitoring and analyzing QC performance, and mitigating QC errors to increase confidence in patient test results.
Table 1. Lab Data Solution
of the need to transform the large (and increasing) volumes of data that are being tracked into actionable information, data analytics generally is a hot area of interest across many categories of software, including LIS.”
Product name
Atellica Data Manager
URL
https://www.siemens-healthineers.com/ diagnostics-it/atellica-diagnostics-it/ atellica-data-manager
Key Features
Increase visibility over multiple laboratories and centralize testing and quality management across a broad spectrum of core-lab instrumentation with:
• Rules-based standardized testing and automation workflows
• Realtime sample and test management dashboards
• Integrated QC management module
• Simplified and user-customizable review and edit areas
• Built-in and customizable result autovalidation
• Robust traceability, audit trails, and reporting
• Open connectivity to third-party instruments
Integration Capabilities
• The software can be easily configured to support unlimited instrument connections, users, and concurrent sessions. Connect and manage multiple labs, LISs, automation systems, and multidisciplinary instruments using ASTM or HL7 protocols.
• Atellica Data Manager is an open system with access to a growing library of device drivers supporting instrumentation from all major diagnostic manufacturers.
Relevant Certification/Compliance
• ISO 13485 - Medical devices Quality management systems, Requirements for regulatory purposes - is specifically for medical devices
• ISO 14971 standard (Medical Devices – Application of Risk Management to Medical Devices)
Solutions the Service Provides
• It can be time consuming, cumbersome, and expensive to work with individual analyzer software, to consolidate data in the laboratory information system, or to deal with functional gaps in immature middleware.
• Atellica Data Manager helps overcome these issues to deliver the results you care about!
Avunjian emphasized, “This is an exciting time as advancing technology supported by modern laboratory information system software has the power to push lab operations and finances to new levels of efficiency and improve patient care.”
He pointed to the following “positive impacts that AI and machine learning are already having on laboratory workflow management: Automated data entry, automated data entry and coding, workflow and process optimization, automated result validation, and predictive analytics for denial management.”
Navigating the change is one of the most common challenges laboratories face when integrating a new LIS, according to Nollar. “Lab staff are often resistant to change and may struggle with acceptance of a new system. Sometimes staff make requests to make the system behave like the old system that is being replaced, even when this undermines the new system’s operational improvements.”
He continued, “It is important to embrace your new system and all the new benefits and features that come with it. LIS providers, on the other hand, need to make sure that their system is adaptable to client needs regarding workflow and data configurations.”
https://www.ellkay.com/lkorbit Key Features
LKOrbit is an end-to-end solution to streamline ordering workflows, manage compendiums, map codes and data, and extend connectivity.
• Single point of connectivity between EHR Vendors, Labs, Imaging Centers, and other applications
• Ability to generate clean lab orders with up-to-date patient demographic and insurance data
• Ability to pull clinical data from the EHR to support prior-authorization workflows
• Interface Infrastructure for order and results
• Ability to provide ordering physicians with a robust ordering portal and results inbox
• Code Mapping and management for insurance codes, test compendiums, EHR data
• EHR compendium update service Integration Capabilities
• 700+ Labs/Radiology centers for orders and results
• 250+ EHRs for orders and results
• Deep integration capabilities with a network of EHRs
• HL7, FHIR, API, CCD, JSON, CSV, XML, other formats
• HIPAA Compliant
• HITRUST
• Penetration Tested
• ISO 27001
• Drop In Status with Labcorp Solutions the Service Provides
• Maximize revenue with accurate demographic, insurance, and clinical data from the EHR for clean, complete lab orders.
• Expedite accessioning via custom laboratory requisition and specimen labels.
• Streamline lab billing with access to clinical data.
Hakim and Krasovec both said resources are a barrier. “Common challenges include difficulty in devoting necessary resource bandwidth of key subject matter experts to the
Product name
Validation Manager
URL https://byg4lab.com/us/validation-manager/
Key Features
Say goodbye to exhausting verifications. Validation Manager by BYG4lab is the first fully digital, centralized software for method verifications/validations, enabling laboratories to:
• Complete verification/validation projects 6x faster than with traditional approaches
• Reduce data management time by 95%
• Cut routine quality work by 20%
• Standardize quality verification/validation reporting across all departments
• Validate your laboratory developed tests easily
• Validation and verification of new instruments and tests
• Validation of laboratory developed tests
• Measurement uncertainty, Total analytical error and Sigma
• Test performance overview and comparisons across laboratories and instruments
Integration Capabilities
• Integrate with countless analyzers, middleware and LIS systems across different departments and disciplines.
• Helps laboratories comply with the validation and verification requirements of ISO 15189, ISO 17025, CLIA, CAP, The Joint Commission and COFRAC.
implementation to provide input to the project, underestimating the effort related to inputting the lab’s master data dictionaries, and the tendency to replicate a legacy LIS solution rather than embracing the out of box behavior of the new LIS solution,” Krasovec said. Hakim added, “These systems are being replaced because they are antiquated, and the vendor is not advancing the system to accommodate today’s demands on an LIS. With laboratory staffing shortages, we focus on automation to reduce the amount of human intervention required to perform testing workflows. Often, we augment
customer staffing to implement the new laboratory and genetics software to meet timelines. To expedite implementation, we use a best-practice model database for dictionaries, instruments, and workflows to reduce the timeline and cost.”
Futrell pointed to the significance of data security to healthcare information systems and shared best practice strategies Orchard Software recommends for data management. “Orchard Software recommends that our solutions be deployed in a healthcare-centric data center that provides the secure design,
business continuity, and disaster recovery that is required by federal healthcare data regulations.”
Recent high profile ransomware attacks have highlighted the need for stronger cybersecurity measures because healthcare organizations, including clinical labs, are attractive targets for cybercriminals, said Avunjian. “To combat these threats, providers must first adopt their own robust cybersecurity strategies, and then, just as importantly, partner with laboratory information system companies that emphasize the importance of cybersecurity and offer practical and relatively inexpensive solutions.”
with common outpatient electronic medical-record systems part of your standard product offering?
Does your product have an automated process for
your product include barcode specimen tracking?
Does your product include features for inventory control and supply-chain management?
Does your product include a module for managing POCT?
Does your product include a module for genetic testing?
Does your product have a module for anatomical pathology?
your product have full bi-directional integration capabilities for data exchange with digital solutions?
Is your product interoperable with API’s or other proficiency testing providers?
SaaS model pricing available?
Does your platform provide AI-driven decision support or predictive analytics for diagnostics or lab performance?

Introducing AlinIQ Insight and its family of applications— making your key diagnostic data more accessible and usable, because new levels of data visibility bring greater insight.
SIMPLE. INTUITIVE. INNOVATIVE.
• Easily sort, configure and set parameters to highlight what’s most important
• Instantly oversee application data integrated in real-time
• Quickly compare daily metrics to your own KPI targets


When asked what role cloud-based solutions will play in the future of LIS, Krasovec said that “many labs are finding advantages in outsourcing their IT infrastructure to cloud providers so that they can focus on their lab operations and not on IT.”
“The benefits of doing this are significant: improved security and compliance, reduced IT manpower, improved scalability & flexibility, and more,” he continued.
On the contrary, “The challenges we see include confusion related to differences between cloud hosting, SaaS (Software as a Service), IaaS (Infrastructure as a Service), and PaaS (Platform as a Service) and what the implications are for each of these options to the lab with regard to their IT responsibility and the flexibility or lack thereof to customize their LIS application.”
Hakim said, “Over the past four years, requests for cloud-based LIS solutions have significantly increased. Some of our largest commercial labs have now deployed our LIS and genetics software in the cloud. Many clients are shifting from “On-Prem” to cloud-hosted solutions. The most crucial factor is that the same software currently running in your LIS can be ported to a cloud-hosted solution. It is important not to introduce disrupters during this process. Most of our customers using courier, logistics, CRM, and supply chain management software are now cloud-hosted.”
Cloud-based solutions can increase the security of protected health information, Futrell added.“Offloading the bulk of security measures to a cloud vendor makes data more secure and reduces the burden on the organization’s IT staff.
What product updates do you anticipate releasing in 2025?
StratusDx — StratusDX — www.stratusdx.com
Integrated AI module for analytics.
XiFin, Inc. — XiFin LIS — www.XiFin.com
XiFin LIS in 2025 will see continued development to support the latest in molecular and anatomic pathology diagnostics. XiFin will be at the forefront of providing digital pathology workflows with integrated AI diagnostic results. XiFin’s cloud-based LIS allow labs to better scale operations, access data remotely, and reduce infrastructure costs. Additionally, XiFin LIS also enhances collaboration and interoperability, facilitating data sharing across healthcare systems and improving workflow efficiency.
Data Innovations — Instrument Manager — datainnovations.com
New versions of our Instrument Manager product.
LigoLab — LigoLab LIS & RCM Laboratory Informatics Platform — www.ligolab.com
Cloud Architecture, RCM 3.0, New User Interface, CRM Enhancements, Native Interface Engine, Internal Inventory Tracking.
Clinical Software Solutions — CLIN1 — clin1mobile.net
Updates are client driven.
Epic — Beaker — www.epic.com
Streamlining referrals and authorizations for reflex testing, OCR and AI processing for outreach, synoptics, and analytics.
LabWare, Inc. — Clinical Health Solution — www.labware.com
Launch of Hosted Software as a Service planned for 2025. Orchard Software — Orchard Enterprise Lab — www.orchardsoft.com
Improve the performance, stability, and security of our Enterprise solution. Improve and modernize the Outreach solution UI/UX. Implement digital pathology and incorporate workflow enhancements.
Table 6. LIS Buyer’s Guide (Part 2)
Healthcare organizations (HCO) that use a cloud-based information system can obtain a cost-effective IT solution without the capital layout or expenditure for internal IT staff to maintain and service the infrastructure. A subscription service levels the cost curve and eliminates costs peaks that occur across time. Cloud systems increase data redundancy and system availability (or uptime) by automating backups and disaster recovery options. This added data protection means that an HCO does not lose vital patient data and can minimize downtime.”
Article continued online at mlo-online.com/55233738.
“What is the most important trend you see on the horizon for LIS products in 2025?
StratusDx — StratusDX — www.stratusdx.com
Growing demand for automation and AI-driven analytics to enhance efficiency and streamline workflows. This will lead to increasing demand for seamless cloud-based integration and automation, driving both efficiency and scalability.
XiFin, Inc. — XiFin LIS — www.XiFin.com
In 2025, we will continue to see increasing adoption of cloud-based lab information systems, digital pathology solutions, and AI diagnostic solutions. Labs continue to shift toward cloud-based platforms for greater scalability, cost efficiency, and remote accessibility, making it easier to handle spikes in test demands, especially in high-volume and multi-location laboratories. AI and machine learning will continue to drive adoption of digital pathology. This functionality, combined with an integrated LIS digital pathology workflow, will continue to enhance automation, promote predictive analytics, and provide better clinical decision support tools.
Data Innovations — Instrument Manager — datainnovations.com
Moving to the cloud.
LigoLab — LigoLab LIS & RCM Laboratory Informatics Platform — www.ligolab.com
The industry is increasingly favored by all-in-one laboratory information systems (LIS) with integrated billing (RCM) modules. These comprehensive solutions ensure consistent and accurate data flow by maintaining a single source of truth for all technical and financial operations. By unifying laboratory activities, they maximize revenue and minimize compliance risks, eliminating the gaps that often occur in fragmented, siloed systems.
Clinical Software Solutions — CLIN1 — clin1mobile.net
Epic — Beaker — www.epic.com
Integrating laboratory professionals and pathologists with the rest of the healthcare ecosystem.
LabWare, Inc. — Clinical Health Solution — www.labware.com
Increasing importance of data analytics and prevalence of molecular and genomics testing.
Orchard Software — Orchard Enterprise Lab — www.orchardsoft.com
Customers moving to cloud-hosted environments.
SCC Soft Computer — SoftLab LIS/LIMS — www.softcomputer.com
Expansion of outreach, courier tracking systems, home care phlebotomy, telepathology, and business intelligence software to streamline billing and back-office activities.
NovoPath — NovoPath360 — www.novopath.com
The integration of AI as a decision support tool (DST).
Table 7. LIS Buyer’s Guide (Part 3)

Streamline your laboratory’s processes and workflow with intuitive tools
Fully integrate your LIS solution to promote organizational interoperability
Gain valuable analytic insights that boost lab productivity
Achieve system scalability that will allow your lab room to grow
Receive





Could a streamlined diagnostic algorithm for respiratory testing help you improve your workflow and do more for your patients? It did for Vanderbilt Health.
By Alesia McKeown, PhD
Respiratory diagnostics is complicated, especially lately. The COVID-19 pandemic sparked innovation, and for the past few years manufacturers have flooded the market with new options for respiratory diagnostics. As the diagnostic landscape continues to evolve, and with so many variables at play, the laboratorians and clinicians I meet across the country are asking some important questions about the optimal solutions for their institutions. They’re also looking at how diagnostic stewardship applies to each option, which makes decision-making even more challenging.
To optimize a respiratory testing strategy — and algorithm — that leads to the best patient outcomes, it’s important to consider several factors, including patient setting, risk stratification, and impact to patient management. Navigating all these factors is a major challenge for health networks; there really is no “one-size-fits-all” solution. Diverse care settings and patient populations require different types of tests. There are also issues
of workflow, timing, quality, managing ordering and inventory, and aligning stakeholders.
Fortunately, there are models that can provide a roadmap. The Association for Diagnostics and Laboratory Medicine (ADLM), for example, has published a guidance document on laboratory diagnosis of respiratory viruses that includes a suggested testing algorithm1 (see Figure 1). Additionally, when institutions share algorithms and best practices, it benefits everyone and enhances diagnostic stewardship.
In this article, which focuses on polymerase chain reaction (PCR) respiratory testing,2 we will follow the journey of one institution, Vanderbilt Health, to understand how its laboratorians and care providers approached the challenges of creating and revising both inpatient and outpatient testing algorithms for respiratory viruses in the post-pandemic era.3
Figure 1 (below). The Association for Diagnostics & Laboratory Medicine1 recently shared this sample algorithm for testing respiratory viruses. How does your organization’s model match up? Source: ADLM Guidance Document on Laboratory Diagnosis of Respiratory Viruses1, Figure 2
The ADLM recommends PCR testing because of its sensitivity and specificity, and because of PCR testing’s potential for automation, which is particularly useful
might be necessary for certain situations (e.g., surveillance screening or outbreak investigations). Additional considerations include symptoms onset, age of patients, sample types, and test availability.






We recognize your passion for providing high quality care to patients displaying symptoms associated with respiratory infections, and we appreciate your efforts and resiliency working to reduce the rapid spread of these infections.
We are committed to providing high quality, molecular and antigen point-of-care tests for detecting the most common respiratory infections, so you can get the answers fast and your patients back to doing what they love.
Like you, we understand there is a patient behind every answer—and that’s what matters most.




in high-volume settings, reducing contamination risk and hands-on time required by staff.1
Vanderbilt Health’s new respiratory algorithms were built with flexibility in mind because flexibility is a key factor in providing the right test for the right patient at the right time. In the future, as personalized medicine continues to transform healthcare, we expect to see new types of diagnostic tests, including tests that are faster, more customizable, and more aligned with principles of diagnostic stewardship.6 These increasingly more flexible tests have the potential to improve workflow, minimize costs, and overcome reimbursement obstacles.
PCR-based diagnostics are just one slice of the diagnostic pie, and PCR testing is a rich, complex slice. There are dozens of options to choose from in a variety of configurations, including singleplex (one pathogen), targeted multiplex (2–5 pathogens) and expanded multiplex (6+ pathogens). These options are further categorized by their complexity as it relates to Clinical Laboratory Improvement Amendments (CLIA), allowing them to either be performed at the point of care or in central labs. With so many options available, how can institutions determine how best to position these technologies to meet the needs of their diverse patient populations?
Vanderbilt Health operates seven hospitals and more than 200 clinics in the southeastern United States, and recently overhauled its algorithms for inpatient and outpatient testing (see Figure 2). It offers a variety of PCR testing options to suit the many providers in its system. However, in recent years that variety has swelled into “a bit of a hodgepodge,” according to David Gaston, MD, PhD, medical director of Vanderbilt Health’s Molecular Infectious Disease Laboratory (MIDL).3
“Everything was SARS-CoV-2 for a solid two years,” Gaston said.“The algorithm was built and then it expanded. Then the lab would bring in new technologies and new testing panels, and it would expand more.”3
It was time for a reset — and a more focused testing panel.
According to the ADLM, selection of the right test involves evaluation of test performance, testing volume, laboratory feasibility, cost versus value and the overall impact on clinical outcomes.1 Sometimes, selecting the right test requires rethinking existing algorithms.4
When Vanderbilt Health transitioned to a new and expanded off-site laboratory, the move created an opportunity to revamp the lab’s information systems and revise its testing algorithms.“The question was how to take some of the testing that had gotten away from a diagnostic stewardship focus and align it while we had the ear of the hospital and a lot of providers,”Gaston said.
Close examination of data revealed that “SARS-CoV-2 was still relevant, but less so, and influenza A was very prominent, as was RSV (respiratory syncytial virus),” Gaston said.“Showing providers that data was very compelling. It implied that perhaps broad respiratory testing for all patients wasn’t needed.”
The transformation at Vanderbilt also gave the clinical side a chance to step back and ask if they were meeting patient needs as efficiently as possible. “That was a big part of the success of [developing our new algorithm],” said Alisha Ezell, MHA, MLS (ASCP)CM, Vanderbilt Health’s enterprise point-ofcare manager for diagnostic laboratories. “It was a chance to integrate MIDL’s perspective and have point of care come together with our physicians to determine if we were making changes that made sense for the entirety of our patient care and all the patients that we serve.”
modified its algorithms for inpatient and outpatient respiratory diagnostics.
After years of experience dealing with the pandemic, the Vanderbilt Health team set out to create algorithms that were more sensitive, nuanced, and precise. Another change was to move away from the focus on point-of-care testing to “bringing as much as possible back to the lab” to allow providers and clinicians to focus on patients.
Key principles of diagnostic stewardship noted by the Society for Healthcare Epidemiology of America (SHEA)5 include multidisciplinary collaboration and including all workers affected by steps in the diagnostic pathway.5 Vanderbilt Health’s transformation is a strong example of following these practices, which improved the finished product while also making the people who were consulted feel more invested in, and receptive to, the new algorithm.
To reach that end, Gaston made sure that everyone even remotely impacted by the algorithm revision had a seat at the table. He sent “cold call” emails to myriad departments, including OB/GYN, emergency medical, and acute care oncology, asking for their thoughts on the algorithm revision. “We wanted to pull as many of those perspectives together as possible and find something that was flexible enough so that anyone could use it and not encounter roadblocks and restrictions,” he said.
Meanwhile, Ezell spent time determining who all of the stakeholders might be.“It starts with the clinicians and what they’re seeing and what impact the algorithm will have on them when we change it,” she said. “Boots on the ground” frontline medical workers need a voice, too. “Sometimes we forget that our nurses are right there with the patients, and that changes we make to the algorithm are going to impact their workflows,” she said. “We don’t want to make it harder for them to do their jobs. If we pull them away to do more work to get lab results, then we’re pulling them away from their patients.”
For algorithms to be of use, they need to “be in line with institutional standards but also flexible enough for providers to pursue different testing routes,” Gaston said.“I’ve had lots of conversations with providers where I’ve said, ‘I never would have thought of that use case. Let’s go back to the drawing board and figure out how we can fit this in and make it work.’”
To create new inpatient and outpatient respiratory testing algorithms for Vanderbilt Health (see Figure 2), Gaston and his team aligned their testing strategy with institutional goals set by multidisciplinary teams. They established that fourplex testing for SARS-CoV-2, RSV, influenza A, and influenza B should be the first step. “That’s really where I think most people should start,” he said.
Seasonality wasn’t a factor.“At this moment we’re not changing based on the respiratory season because we haven’t had a normal respiratory season for the past four years,” Gaston said.
As SHEA emphasizes, the way providers order tests is an important part of the equation because it allows for numerous interventions to improve test utilization.5
Gaston started with fourplex because “there’s been a lot of flu,” and that option is preselected in the test order set. For inpatients, all contact precautions are preselected and contacts with infection prevention are all automated, so the provider doesn’t have to click through a long list of selections.
“We’ve found that many providers would open the order set and just click ‘order,’” Gaston said. “So when a broad
multiplex panel is the first thing that comes up, that will be what’s ordered. That might be what the provider wants, but it might not be.”
Gaston wants to make it as easy as possible to order the test that the lab thinks will have the most clinical value for the greater population. The fourplex is at the top of the list so that the clinicians have a higher likelihood of selecting that without having to put a lot of thought into it.
“We’re not forcing them to make that selection,” Gaston said. “The provider just has to search a bit harder to find broader multiplex options.”
Changing the algorithms, including the way that tests are presented in the order panel, has made a positive impact throughout the Vanderbilt Health system, Gaston said: “We’ve gotten great feedback from providers, who are very much engaged and see this as something to which they want to contribute.”
The system is more efficient too. Changing from the previous respiratory algorithm to the current one has decreased clicks by anywhere from three to five, Gaston said. “For providers, that matters a lot.”

Alesia McKeown, PhD is a Scientific Partner for Infectious Disease in Medical and Scientific Affairs at Roche Diagnostics . She is the subject matter expert for Roche’s high-throughput and POC respiratory solutions. She also co-leads an interdisciplinary team focused on improving access and utilization of diagnostics in the respiratory disease area.
References are available online at mlo-online.com/55142842.































Hemostasis solutions from the #1 trusted brand in hematology*

Sysmex supports the Sustainable Development Goals.


Sysmex has been innovating hemostasis analyzers for more than 30 years, offering complete, easy-to-use hemostasis solutions for laboratories of all sizes and complexities.
The Sysmex CS-2500™ Automated Blood Coagulation Analyzer incorporates coagulation, chromogenic, immunoturbidimetric and aggregation** methodologies in full random access. Pre-analytical sample checks provide autovalidation without the need for manual sample handling.
A trusted reagent portfolio, coupled with the service you expect from Sysmex, ensures that the integration of hemostasis into your laboratory will be a pain-free experience.
* 2023 IMV ServiceTrak™ Annual Survey for Hematology ** For research use only, not for use in diagnostic procedures. RUO assays must be validated before use in clinical practice.
www.sysmex.com/us with A tru you of he expe
By Melanie Pollan PhD, MT (ASCP)
Medical laboratory scientists perform method verification to confirm manufacturers’ specifications for new instruments and assays are met prior to actively reporting patient test results for samples. For the most efficient process and to materialize results of greatest value to clinical stakeholders, a number of real-world factors must be carefully considered and assessed.
Method verification evaluations range from simple evaluations, such as when adding a new assay, to complex evaluations involving adoption of new analyzers and methodologies across multiple sites with the intent to standardize a health system. Performance evaluations may also need to be conducted after major maintenance on an analyzer or as an outcome of a root cause investigation to re-establish acceptability of an existing method. Regardless of when these activities are performed, considerable time and resources are invested to understand the analytical performance before initiating or returning an assay or an analyzer to clinical use for patient testing.
During initial evaluations of non-waived methods in the United States, as directed by CLIA, laboratories must conduct studies that assess accuracy, precision, analytic measuring range (AMR), and reference range. These studies confirm the expectations of the manufacturer and the laboratory are met prior to releasing patient results. These activities are essential quality control practices, as evidenced by the many CLSI standards available to detail the specifics of how to accomplish these studies. Even the most efficient studies require multiple days, so planning is essential to capture the data necessary to adequately meet regulatory needs, as well as any additional specific quality policies established by the laboratory director. While installation of new instrumentation is an infrequent occurrence, it typically requires the most extensive preparation and time.
Method comparison studies are the most common testing performed to assess accuracy. They also can be used to verify the AMR. Additionally, method comparison studies can provide valuable information about potential changes in diagnostic decision points or the need for re-baselining patients with new methods.
CLSI EP09c, third edition guidance recommends that method comparison studies include sufficient specimens (defined as at least 40) “…that span the common measuring interval of the measurement procedures.”1 The general rule of thumb for the ideal patient population (n=40) is to have 10 specimens at the low end, 10 specimens at the high end, and 20 specimens in the middle.
Locating such a wide range of specimens can be challenging for laboratories with low test volumes and those that serve primarily healthy populations. The rarity of samples having concentrations with extreme values can mean long lead times for specimen procurement. When specimens are not readily available to balance the distribution across the measuring range, the temptation is to complete the study quickly with specimens from a more narrow and unbalanced range.
Approaches to address these challenges can vary significantly among laboratories. Options include using remnant specimens frozen by the laboratory, sharing patient samples from a neighboring lab network, purchasing Institutional Review Board (IRB)–approved specimens from a vendor, and creating modified (e.g., dilution or spiking) or contrived samples. When moving away from optimal, freshly collected patient specimens and using either storage or sample






modification, care is necessary to ensure sample variables are not introduced into the comparison. These variables can delay test availability and redirect staff from their normal duties to troubleshoot unexpected results.
Inaccuracies can stem from degradation, matrix effects, or handling conditions. These differences may mimic bias and/or imprecision, leading to rejection of the performance evaluation data. The ideal method comparison study includes specimens with concentrations distributed across the measuring interval of the method(s). Additionally, the ideal outcome would find that all pairs of measured values lie along the line of identity (method a = method b) of a scatter plot resulting in an intercept of zero and a slope of 1. Rarely do such ideal conditions for method comparison studies exist within the real world—a result of the aforementioned sample procurement challenges combined with methodological differences and imprecision.
Tables 1–3 illustrate the importance of the proper patient sample balance. These data were taken from recent electrolyte
performance verifications with an initial narrow sample range that was supplemented with a broad sample range. Electrolyte assays were chosen because they are tightly controlled biologic analytes with standard reference ranges recognized by most manufacturers. All specimens were unmodified patient samples.
Broad range sampling covered >90% of the AMR, while narrow range sampling covered >30% (See Table 1). Analysis using broad sample ranges demonstrated slope nearer to 1.0, intercept closer to zero, and correlation coefficients closer to 1.0 (See Tables 2–3). The improvement is easily visualized in regression plot comparisons (See Figure 1).
These illustrations demonstrate that while individual sample comparisons may pass the criteria for accuracy, the narrow range method comparison may give an unclear picture of the clinical expectation for overall result interpretation. When considering the data in Figure 1E, one might expect a low bias for potassium levels, but the broad range study (Figure 1B) shows a nearly perfect correlation. These differences in correlation interpretation are even more stark for the sodium evaluation represented in Figures 1F and 1C.
In comparison of regression analysis methods, Stöckl et al similarly looked to electrolytes while examining using regression analysis to access adequacy or agreement in method comparison studies.2 They described the narrow physiological ranges tested resulted in poor estimates of regression statistics, impacting both slope and correlation coefficient, regardless of statistical analysis method.
Similar impacts on regression outputs are seen with narrower sampling ranges and smaller sample numbers, in both cases the impact is a broadening of the confidence intervals for the slope and intercept estimates. Additional visualizations such as bias plots and histograms can help assess whether assumptions about the data are valid and interpret any observed differences in the collected data. For non-standardized assays, concordance tables may provide another layer of understanding with regards to medical decision points. Together, these factors illustrate the critical attention required to have adequately designed studies to assess new instrumentation and methods.
Continued on page 39

By Kara Nadeau
The 2024 Medical Laboratory Observer (MLO) State of the Industry (SOI) survey on molecular diagnostics (MDx) queried medical laboratory professionals on MDx testing trends, including types of testing modalities used in their labs, methods for handling quality issues, and volume of respiratory testing compared with last year.
More than four years out from the height of the COVID-19 pandemic, we eliminated COVID-19 specific testing questions, although we continued to ask lab professionals about supply shortages given continued disruptions in the healthcare supply chain.
New for this year, the survey questioned lab professionals about their development of tools/processes to manage decreased access to laboratory developed tests (LDT). This topic is top of mind for many medical laboratorians given the U.S. Food and Drug Administration’s (FDA) final rule issued on April 29, 2024, which made explicit that:
“in vitro diagnostic products (IVDs) are devices under the Federal Food, Drug, and Cosmetic Act (FD&C Act) including when the manufacturer of the IVD is a laboratory.”1
Key 2024 survey findings:
• Testing modalities: A reported 5% increase in DNA/genetic testing compared with last year (20% in 2024, up from 15% 2023), while reverse transcriptase quantitative polymerase chain reaction (rRT-
qPCR) once again topped the list of MDx modalities employed (64% of respondents).
• Supply chain issues: The percentage of lab professionals reporting supply-related testing disruptions dropped again this year at 38% (down from 53% in 2023 and 85% in 2022).
• Quality assurance measures: There was a jump in several reported actions labs take to reduce the number of potential false positive test results, including:
• A 46% increase in those reporting to verify all preanalysis steps are performed correctly (68% in 2024, up from 22% in 2023).
• A 33% increase in those reporting to repeat the test with the same sample and new extractions (47% in 2024, up from 14% in 2023).
• A 26% increase in both those reporting to refer to quality assurance program guidance (34% in 2024, up from 7% in 2023) and those reporting to decontaminate the work/test area per laboratory procedures (59% in 2024, up from 33% 2023).
Nearly 100 lab professionals participated in the survey. Demographics changed little since last year, with most respondents working in Lab Manager, Administrator, Supervisor, or Lab
Director positions (60%) employed by hospital/health system labs (66%).
More than half of those surveyed work in labs that perform 100 or fewer molecular-based tests (non-COVID-19) annually (58%), while nearly one-quarter (22%) are in labs that perform 401+ MDX tests per year. Of the remaining respondents, annual lab volumes are as followed:
• 101-200 tests: 13%
• 201-300 tests: 5%
• 301-400 tests: 1%
Alongside the quantitative survey results data, we include commentary from medical laboratory professionals and suppliers in the MDx space.
When asked what types of MDx tests they use in their laboratories, reverse transcriptase quantitative polymerase chain reaction (rRT-qPCR) ranked highest, with 63% of respondents reporting its usage (62% in 2023).
Nearly half of respondents reported their labs’ usage of rapid molecular tests, down 10% since last year (49% in 2024, down from 59% in 2023). Close to half of those surveyed reported using rapid antigen tests, which was up 6% compared with last year (49% in 2024, up from 43% 2023).
There was a 5% increase in those reporting use of DNA/genetic testing (20% in 2024, up from 15% 2023), and a 3% increase in those with labs using flow cytometry (21% in 2024 up from 18% 2023).
Fewer medical lab professionals reported using:
• Reverse transcription loop-mediated isothermal amplification (RT-LAMP): 9% in 2024 (down from 12% 2023)
• Recombinase polymerase amplification (RPA): 4% in 2024 (down from 9% in 2023)
• CRISPR-based diagnostics: 0% in 2024 (down from 2% in 2023)
Reported usage of liquid biopsy and next generation sequencing was on par with last year’s survey results, with 6% reporting use of the former (5% in 2023) and 18% the latter (18% in 2023).
New for this year, we asked medical laboratory professionals about their use of mass spectrometry testing, with 8% reporting its usage in their labs.
Among the 11% of survey respondents selecting “other,” cited MDx modalities included:
• Reverse transcription-polymerase chain reaction (RT-PCR)
• Nucleic acid amplification test (NAAT)
• Thrombotic microangiopathy (TMA)
• Transferrin receptor testing (TFR)
• Indirect haemagglutination assay (IHA)
• Food intolerance
“We are moving away from traditional biochemical testing which, as a reference lab, have used for confirmation testing for rapid tests,” said Donna Ferguson, PHM, PhD, Public Health Laboratory Director, Monterey County Public Health Laboratory in Salinas, CA. “We are better equipped to test for outbreaks, and God forbid, future pandemics. We’ve purchased instruments for high throughput PCR testing including thermocyclers and instruments for extraction.”
“We have also implemented whole genome sequencing but are using it for non-diagnostic testing (i.e. surveillance),” Ferguson added. “We recently implemented diagnostic testing using the Bruker MALDI and purchased a ddPCR for both diagnostic and environmental testing.”
Lee Panton, former Lab Director from California who now serves as a consultant, commented on the pros and cons of various MDx instrument sizing, stating:
“The new, smaller MDx instruments are easier to use and feature a growing number of tests; therefore, more labs can find the space and personnel to run them. These often offer tests that are CLIA waived so RNs can run them. Larger instruments are coming up with a bigger variety of tests, but low volumes in small and medium sized labs may not make it cost effective.”
Mary Jo Blooflat, MT(ASCP), Director of Clinical Laboratory Services, Northfield Hospital, Northfield, MN, commented on the growing number of MDx tests for women’s health, noting how her lab has recently began using a vaginitis panel.
“We’ve had the chlamydia and gonorrhoeae panel for quite a while, but I would like to see something



for syphilis given we have to test all pregnant women twice for it during their pregnancies, during an early visit, and after they have delivered their babies,” she added.
“In critical access hospitals, I am seeing an increased use of Cepheid PCR technology,” said Steve Raymond, MLS, MS, MPA, Interim Lab Director, Northeast Montana Health Services Laboratory.
Nathan Patton, Vice President of Marketing for Near Patient Care at Roche Diagnostics commented on several key trends that are shaping the rapidly evolving MDx testing landscape, most of which include bringing testing closer to the patient.
“With the emergence of new technologies, decentralized healthcare sites such as urgent care centers, physician offices, and emergency rooms now have the opportunity to bring testing in-house that they may have previously sent out or managed via centralized laboratories. This shift to molecular point-of-care testing can provide advantages to clinicians and patients. It includes increased access to gold-standard PCR testing and reduced turnaround times, enabling clinicians to make accurate diagnoses and promptly determine the appropriate treatment.”



“Advancements in PCR-based diagnostics have enabled providers to test for multiple pathogens in a single multiplex assay and deliver results in 15 to 20 minutes, increasing these diagnostics’ applicability in POC settings,” Patton added.“Patients can also benefit from receiving their results during their office visit and leave with a prescription or a clear direction on their treatment plan.”
According to Jackie Weiss, PhD, Scientific Affairs Liaison at EUROIMMUN US (part of Revvity), molecular testing will continue to be a valuable tool in the fight against antimicrobial resistance (AMR). She stated:
“While efforts to curb AMR often focus on antibiotic resistance, the burden of antifungal resistances continues to increase. Historically, culture-based methods have been used to assess antifungal susceptibility. However, molecular techniques, such as PCR, produce results up to four weeks earlier than culture. In the case of invasive fungal infections, time is of the essence,
Has your lab experienced issues with maintaining a supply of testing products due to supply chain issues?
as delays in appropriate treatment are linked to increased mortality.”
“There is a growing trend towards the development and validation of multiplex PCR assays that can rapidly identify the most resistance-associated mutations in clinically relevant fungal species,” Dr. Weiss continued. “This is particularly important given the limited number of antifungal drug classes available and the rising incidence of antifungal-resistant infections.”
When asked how much their respiratory testing has increased in 2024 compared with 2023, nearly half reported a 0-25% increase (49%) and one-fifth (20%) a 26-50% increase. Additionally, 17% noted their respiratory testing had decreased since last year.
The remaining responses are as follows:
• 6% reported a 51-75% increase
• 7% reported a 76-100% increase
• 0% reported a 101+% increase
Raymond reported his lab is seeing an increase in respiratory testing, as well as C. diff testing.
Commenting on MDx testing trends in respiratory and other areas, Panton stated: “I am excited about the new testing for MTB and STDs. Speeding these up will make a big improvement in care. The GI tests and wide panels for respiratory disease are very good. Subacute units for respirator dependent patients benefit with these complete test menus. When they get the sniffles, we need to test them for serious illness versus a cold quickly. Patients coming to the ER want to know what they have specifically. Another area with a major need is testing for sepsis quickly. MDx will make major improvements in survival, LOS, and antibiotic stewardship.”
According to Patton, there is an increased demand for prompt detection of pathogens like SARS-CoV-2, influenza A/B, and RSV nucleic acid test, as well as tests for sexually transmitted infections (STI).
“Introducing multiplex PCR diagnostic tests with quick turnaround times into near-patient care environments such as emergency departments, urgent care facilities, and physician offices has the potential to provide swift results without sacrificing precision, expediting clinical decision-making processes,” he stated. “This approach can help reduce unnecessary antibiotic usage, facilitate targeted treatment strategies, and ultimately enhance patient outcomes and healthcare system efficiency.”2-7
What steps does your lab take to reduce the number of potential false positive test results?
Repeat the test with the same sample and new extractions
Repeat the test with another method and compare results
Verify all pre-analytic, analytic, post-analytic steps are performed correctly
Refer to quality assurance program guidance
Decontaminate work/test area per laboratory procedures
The FDA’s final rule to claiming regulatory authority over laboratory developed tests (LDT) has been met with concerns in the medical lab community and beyond. As reported by the American Society for Clinical Pathology (ASCP),“laboratories with new LDTs should expect a far more laborious, time consuming, and expensive FDA-approval process”8
When asked if their labs were developing tools/processes to manage decreased access to LDTs, 30% of survey respondents selected “yes,” 67% selected “no,” and 9% selected “other” with write in comments including:
• [We are working to] determine if any of our lab developed tests fall under any category of continuing enforcement discretion.
“As a public health laboratory, we still rely heavily on LDTs,” Ferguson commented.“We purchased two liquid handlers this past year to automate procedures that we were performing manually. We stay abreast of recent developments via webinars and Association of Public Health Laboratories (APHL). We plan on keeping our current LDTs and to go through the FDA approval process if necessary.”
George Manolopoulos, Clinical Lab Manager, Pathology Reference Lab, San Antonio, noted how his lab had “discontinued all LDTs way before the new LDT ruling and replaced them with FDA approved tests.” He added, “we will definitely think twice before deciding to bring an LDT on board in the future.”

George Manolopoulos
[We are reviewing our] budget, looking for alternate funding sources, developing and implementing systems and processes to comply [with at] least Stage 1.
• We have created a team to work on LDT compliance but no plan regarding decreased access yet.
• We are sending [LDTs] to [our] sister laboratory.
Panton noted several questions medical lab professionals have on their minds in response to the FDA action on LDTs: “One question we all have is, for example, if the product insert says to use serum, can we also use plasma? Can we still validate it with a number of patient parallel samples? Such as collect 20 patient samples and test both? If the results are the same, can we modify? Is ANY modification prohibited?”

With long-read sequencing technology, labs can achieve greater insight than traditional methods and reduce the need for reflex testing. Experience the transformative power of a streamlined workflow.
Robert Cardwell, Chief Strategy Officer at the Genetics Institute of America (GIA), has been closely monitoring the impact of the FDA’s ruling on LDTs. He noted, “While many virology and microbiology assays are already available as FDA-cleared kits, the vast majority of human DNA and RNA studies remain laboratory-developed tests.”

smaller labs that might not have the resources to comply with the regulations. What I fear is consolidation where smaller university labs might start selling their LDTs to a large commercial lab, which leads to less competition. This could, in turn, result in higher costs for these tests, and CMS and private insurers will always try to avoid overpaying for anything.”
• Repeat the test, [verify] all steps, and/or send-out if results still questionable.
• Verify all pre-analytical, analytical, and post-analytical steps are performed correctly and repeat the test.
• Verify pre-analytical, analytical, and post-analytical steps, QC etc., then retest sample.
“This has raised significant concerns within the community,” Cardwell explained. “The assays that genetics and genomics laboratories rely on for personalized medicine — such as those used to characterize a patient’s tumor and identify response to therapeutics — are now subject to FDA review and approval. Pursuing regulatory clearance through either a PMA or a de novo application involves substantial costs.”
Cardwell added,“In the molecular diagnostic space, it’s an unusual position for me to say this, but innovation and care in other parts of the world may soon surpass what we have in the United States.”
Discussing GIA’s strategy in light of the FDA ruling, Cardwell highlighted how they have a robust pipeline for assay development and are continually expanding their test menu.
“We’ve reprioritized our molecular diagnostic development,” he stated. “Assays that are already FDA-cleared have been moved to the forefront of our pipeline. However, we will continue to develop appropriate LDTs.”
“With the new ruling, I believe the FDA is setting a baseline for quality with LDTs,” said Steven Kelly, Technical Product Manager, Streck. “There has been a lot of pushback, especially from
“There is a need for third party proficiency or third-party quality control that can help these labs prove to the FDA that their staff are proficient and their LDTs are accurate and effective,” he added.
The survey questioned lab professionals about their quality control measures, asking how they handle questionable results with MDx tests. Those reporting they repeat the test has continued trending downward (45% in 2024 down from 55% in 2023 and 65% in 2022).
Conversely, lab professionals who report verifying all pre-analytical, analytical, and/or post-analytical steps are performed correctly has continued trending upward (24% in 2024, up from 15% in 2023 and 7% in 2022).
Additionally, 15% reported repeating the test with a different employee/ equipment/test, which was not an answer option in the 2023 survey.

Those reporting their lab send results to another lab for verification and second test continued a downward trend (6% in 2024, 8% in 2023, 20% in 2022).
Among those who reported using “other” when handling questionable results with MDx tests, write-in responses included:
Is your lab developing tools/processes to manage decreased access to LDTs?
• Repeat the test and then send to our internal and external reference lab as they have other methods to verify testing.
• Notify administrators.
• Analyze QC with company, retest, correlate with NGS.
This year, more medical lab professionals reported taking the following actions to reduce the number of potential false positive test results:
• 68% verify all pre-analysis steps are performed correctly, a 46% increase over 2023.
• 59% decontaminate the work/test area per laboratory procedures, up 26% from 2023.
• 47% repeat the test with the same sample and new extractions, a 33% increase over 2023.
• 34% refer to quality assurance program guidance, up 27% from 2023.
Those survey respondents reporting to repeat the test with another method and compare the results held steady, at 14% in 2024, compared with 15% in 2023.
When asked if they use another step to reduce the number of potential false positive test results not listed above, survey respondents reported:
• Decontaminating after each test with bleach
• Performing contamination checks
• Performing wipe test of work area to confirm no contamination
Kelly spoke on the topic of MDx testing quality control and assurance as it relates to automation and lab tech shortages stating:
“With today’s MDx test platforms, the technician can simply place the sample and the platform will automatically do the extraction, purification, hybridization, or PCR, and then spit out the results. It’s the right time for this automation given the deficit in medical technologists and clinical lab scientists because it helps fill these talent gaps.”
On the other hand, Kelly points out how with automation, technicians don’t manually test each step for accuracy.





“Before these automated platforms, we had to do all those steps by hand, and we had to manually control for each step. For example, I could look at the amplification curve or the electropherogram of the sequencing and could tell if there’s garbage down at the bottom that showed it wasn’t a really clean sample. With automation, these platforms are more of a black box where the technician has to have faith that it is functioning as intended.”
Kelly added: “While the technicians running these machines can be generalists, which is positive given the shortages of techs with more advanced skills, it is still important that they how know a lab functions, the purpose of the lab test, etc. That way, the machine can do the heavy lifting, but the technician can tick off all the boxes to ensure they are doing everything according to their CAP checklist.”
Commenting on this year’s survey results, where more respondents report taking steps to reduce the number of potential false positive results (e.g., repeating the test, decontaminating the work area), Kelly believes this reflects a growing focus on quality control with MDx testing.
“Quality control to check work at each step is where we need to focus,” said Kelly. “So, let’s have our quality control systems take care of some of that heavy lifting of assuring the test is accurate and provides good data for treatment options. That’s what Streck provides. Our products evaluate the accuracy and precision of instruments and techniques to give lab teams confidence in the validity of their results.”
While there have been recent reports of supply shortages in various categories, most recently blood culture vials, the medical lab professionals who took part in this survey reported fewer issues with maintaining a supply of testing products due to supply chain issues compared with previous years’ responses.
When asked if a lack of sample-related products has impeded their testing capacity at times, 38% selected “yes,” down from 53% in 2023, and 85% in 2022. Conversely, 62% chose “no” indicating they have adequate testing supplies to meet testing demands, up from 47% in 2023, and 15% in 2022.
“Supplies and reagents have been arriving on time and in full quantities for the most part,” said Manolopoulos.“Only the occasional back orders on plastics but on items that we only order once
every few years, not on our regular ancillary supplies.”
While the number of medical lab professionals reporting supply chain issues has trended downward, it is worth noting that nearly two-fifths still report some level of supply disruptions impacting their work.

When asked which testing supplies they are having trouble sourcing due to supply chain issues:
• 27% selected controls/reagents
• 2 6% blood collection tubes
• 21% culture media
• 16% lab plastics not otherwise noted
• 13% winged blood collection sets
• 11% pipettes
• 11% testing kits for SARS-CoV-2
• 10% swabs/consumables
• 10% transport media
• 8% blood culture transfer devices
• 7% PPE
• 3% urine testing supplies
More than one-third of survey respondents (36%) reported no issue sourcing these supplies . Among the 4% of respondents who chose “other,” several note their trouble sourcing blood culture bottles, which was echoed by the lab professionals interviewed for this article.
“As of right now, the only thing we are on allocation for is blood culture bottles,” Panton commented. “We have some shortages in blood collection tubes and occasionally on some plastics. Overall, shortages in general are decreased.”
Blooflat too said her lab has been experiencing issues sourcing blood culture bottles, causing her team to “question doctors when they order blood cultures.” She explained:
“While the normal practice has always been to order two sets of blood cultures, we’ve been having to restrict our providers to just one set. They’ve been fighting it, of course, because they know this isn’t the best course of action. But what else can we do?”
Ferguson reports her lab has experienced shortages in blood culture tubes and PH testing reagents that are not commonly used by clinical labs. “We’ve also seen an increase in back orders and delays for a variety of supplies,” she added.
Odette Muriel-Ramos, Lab Manager, Keystone Urology Specialists, Lancaster, PA, said her lab’s supplier is currently switching them from the BD vacutainer gray topped urine culture
tube “that has been around for probably 50 years,” to a “sponge that gets dipped in the urine.” Commenting on this change, she stated:
“I’m still waiting to hear from the supplier on whether they are changing the methodology given they have changed the medium. We need to know these types of things so we can evaluate collection options appropriately. The supplier is moving faster than what we are being educated.”
Ferguson also spoke to how the high cost of supplier shipping fees and poor equipment quality impacts her lab’s operations, stating:
“We will need to increase our lab testing fees due to higher cost of shipping charged by vendors and rising costs of equipment, including maintenance and repair. We estimate shipping costs have increased by 30% and more. In some cases, shipping costs are higher than that of reagents/supplies. In our experience, some refrigerators, freezers, incubators, and dishwashers recently manufactured by reputable companies have been inferior in quality. Equipment that should last for 10 years or more are replaced more frequently.”
Cardwell also emphasized the growing importance of supply chain considerations with the FDA’s increased oversight of LDTs. Laboratories will need to comply with new design controls, purchasing controls, and acceptance activities, which differ significantly from the current CLIA regulations.
“These new requirements are quite different from what exists under CLIA, and most laboratories lack experience in these FDA-required areas,” Cardwell said.“Lab professionals should familiarize themselves with these requirements and integrate them into practice.”
Additional opportunities and challenges with MDx testing
Those individuals interviewed for this article spoke to other trends and issues they are experiencing with MDx testing not covered in the survey questions. Here are some of their comments:
Cardwell pointed to a shortage of molecular technologists with the skills needed for human genomic DNA testing, noting, “There is a real shortage of individuals skilled in next-generation sequencing, qPCR, and digital droplet PCR, where meticulous bench skills are crucial.”
The first FDA authorized targeted molecular assay for the detection of C. auris in colonized patients

The identification of patients colonized with Candida auris is essential to aiding infection prevention and control
Over the past decade C. auris has caused outbreaks in more than 35 countries. Both the WHO and US Centers for Disease Control (CDC) have identified C. auris as a significant healthcare concern that poses an urgent threat to antimicrobial resistance.1,2 Your HAI testing solution for C. auris












Simplified fungal workflow: Lower your probability of errors with CLIA moderate complexity; no DNA extraction
and



























































Flexible testing: Run as few as 1 and up to 8 samples at a time on a re-usable direct amplification disc (DAD). Learn more about the Simplexa C.auris direct kit. bit.ly/simplexa-cauris


Connectivity to maintain control
“As more solutions move into decentralized settings, connectivity solutions that can help customers maintain control and better manage the instruments and operators within these settings are critical,” said Patton. “In addition, connectivity solutions can enhance the efficient management of decentralized platforms by ensuring software remains up-to-date and automating the transmission of results.”
The role of physicians and other clinicians
According to Panton, while most labs are trying to add more molecular tests to their menus, because tests are costly, it is hard to justify these purchases with financial departments.
“Strong MD support (demand and orders) for the tests can help,” said Panton. “Administrators want to know how many FTEs will go away if we introduce these ‘rapid’ tests. With our current short staffing, it is not likely that these new tests will reduce any staff. In fact, they may need to add staff for performance and supervision. If we propose the MDX be installed as POC, then we need systems to train and manage a large nursing staff. Our MD support needs to show that we will have shorted LOS or lower drug usage with the advantages of MDx.”
Turning to running tests at the point of care (POC), she said this is “not always an advantage,” stating:
“It is hard for lab staff to manage the staff of other departments. RNs, aids and medical staff are not good at tracking tests, checking expiration dates, proper storage and even getting the results into the EMR and billing it.”
Muriel-Ramos has struggled to gain consensus among physicians that MDx testing is “the way to go,” given their reluctance to give up “old school cultures” and reimbursement concerns with new modalities.
“I’m fortunate that where I work now, the medical director loves genetics. He is one of the highest genetic ordering doctors in the United States. My goal is to bring PCR testing into the urology office but that requires securing approval from nine other doctors who had a poor experience when our office had attempted urine PCR in house testing in the past.”
“I found a company that does molecular for urine, I created an algorithm as to who gets sent out for culture versus who gets sent out for PCR, and a physician office policy where the lab can now decide which modality to use,” Muriel-Ramos added.“So, I feel like we’re getting there, but we’re not there yet because it is perceived among physicians as a big risk.”
Blooflat voiced her concerns around the volume of waste generated by MDx testing, noting its impact on the environment. She stated:
“Single use kits and cartridges are a good thing because they provide a control for contamination, but we are throwing away a lot of plastic. I think someday it’s going to have to be addressed. We’re going to have to find a way to decontaminate these items and get them into the recycling system somehow.”
MLO asked suppliers in the MDx space for their thoughts on testing trends in the year ahead and beyond.
“I see MDx testing becoming more personalized in the next year and into the future – if not to the individual then for groups of individuals,” said Kelly. “With better data from MDx, we can more rapidly gain knowledge about a specific cancer or a virus variant and more quickly determine the right treatment.”
“Looking ahead to 2025, laboratorians, clinicians, and leaders in healthcare settings can find value in molecular point-of-care testing,” said Patton.“With access to faster tests with high-quality PCR within decentralized settings, hospitals and health systems can truly rethink the patient care experience and support more efficient patient management and treatment.”
Patton said providers will also have more options for greater flexibility and customization of panel menus in the molecular lab, stating:
“This increased flexibility will also come with the potential for automatic or manual reflex options that will allow for multi-stage analysis and release of results for the necessary targets as defined for the individual patient. Improved flexibility has great promise to contribute to improved patient outcomes and diagnostic stewardship for the unique patient populations served by each institution.”
“At Revvity’s EUROIMMUN, we are continuously advancing in step with the evolving field to deliver innovative and effective solutions that address emerging public health needs,” said Dr. Weiss.“As part of this commitment, we are adapting our molecular assays to meet new clinical challenges. For example, we have updated our assays to detect Trichophyton indotineae and other resistant dermatophyte species, as well as Candida auris resistant strains. These advancements ensure we stay at the forefront of diagnostic technology, providing crucial support in managing emerging infectious threats.”
1. FDA takes action aimed at helping to ensure the safety and effectiveness of laboratory developed tests. U.S. Food and Drug Administration. Published August 9, 2024. Accessed September 26, 2024. https://www.fda.gov/news-events/press-announcements/fda-takesaction-aimed-helping-ensure-safety-and-effectiveness-laboratorydeveloped-tests.
2. May L, Robbins EM, Canchola JA, Chugh K, Tran NK. A study to assess the impact of the cobas point-of-care RT-PCR assay (SARS-CoV-2 and Influenza A/B) on patient clinical management in the emergency department of the University of California at Davis Medical Center. J Clin Virol. 2023;68:105597. doi:10.1016/j.jcv.2023.105597.
3. Hansen GT, Moore J, Herding E, et al. Clinical decision making in the emergency department setting using rapid PCR: Results of the CLADE study group. J Clin Virol. 2018;102:42-49. doi:10.1016/j.jcv.2018.02.013.
4. Berry L, Lansbury L, Gale L, Carroll AM, Lim WS. Point of care testing of Influenza A/B and RSV in an adult respiratory assessment unit is associated with improvement in isolation practices and reduction in hospital length of stay. J Med Microbiol. 2020;69(5):697-704. doi:10.1099/jmm.0.001187.
5. Garvey MI, Wilkinson MAC, Bradley CW, et al. Impact of a PCR point of care test for influenza A/B on an acute medical unit in a large UK teaching hospital: results of an observational, pre and post intervention study. Antimicrob Resist Infect Control. 2019;8(1):120. doi:10.1186/ s13756-019-0575-6.
6. Patel P, Laurich VM, Smith S, Sturm J. Point-of-care influenza testing in the pediatric emergency department. Pediatr Emerg Care 2020;36(11):515-518. doi:10.1097/PEC.0000000000002250.
7. Youngs J, Marshall B, Farragher M, et al. Implementation of influenza point-of-care testing and patient cohorting during a high-incidence season: a retrospective analysis of impact on infection prevention and control and clinical outcomes. J Hosp Infect. 2019;101(3):276-284. doi:10.1016/j.jhin.2018.11.010.
8. Overview of FDA Laboratory Developed Test Final Rule. ASCP. Published May 14, 2024. Accessed September 26, 2024. https://www.ascp.org/news/news-details/2024/05/14/ overview-of-fda-laboratory-developed-test-final-rule.

Kara Nadeau has 20+ years of experience as a healthcare/ medical/technology writer, having served medical device and pharmaceutical manufacturers, healthcare facilities, software and service providers, non-profit organizations and industry associations.

Working together with laboratories, Thermo Fisher Scientific played a critical role in helping solve the world’s greatest challenges during the pandemic. As a leader in the industry, we will continue to deliver trusted molecular diagnostic solutions that help you guide effective patient outcomes. Leveraging gold-standard, PCR-based molecular detection that enables accurate and actionable results—as well as our robust supply chain, broad portfolio of tests to detect infectious disease pathogens, and reliable global support services—Thermo Fisher is here for you. Whether you have research or clinical needs, we can help your testing laboratory evolve, helping enhance the quality of patient care and shaping the future of healthcare best practices.


VITEK REVEAL enables AST results directly from blood culture, delivering results in approximately 3 hours, according to BMX.1.129899 Clinical Trial Summary Report (proprietary, bioMérieux). Load after ~3 minutes of sample preparation. It offers antimicrobial coverage for gramnegative bloodstream infections. The design allows for high throughput in a small tabletop footprint. bioM érieux

The GloCyte Automated Cell Counter for CSF delivers accurate cell counts for cerebrospinal fluid (CSF) samples in under 5 minutes. By eliminating cell count variability and reducing the turnaround time on every sample, GloCyte aims to ensure reliable diagnostics and enhance both lab productivity and patient care. Advanced Instruments
With a user-specific, customizable homescreen, CGM LABDAQ is designed to streamline laboratory workflows and assist in maximizing reimbursements, connectivity, patient safety, regulatory compliance, data integrity, security, and scalability. New in 2024, CGM LABDAQ v24.4 includes improvements to auditing, microbiology rules, and billing rules. CompuGroup Medical

Validation Manager by BYG4lab is the first fully digital, centralized software for method verifications/validations, enabling laboratories to:
• Complete verification/validation projects 6x faster than with traditional approaches
• Reduce data management time by 95%
• Cut routine quality work by 20%
• Standardize quality verification/validation reporting across all departments BYG4lab
Bio-Rad’s Specialty Immunoassay Plus (SPIA Plus) is a liquid immunoassay quality control designed to monitor the precision of laboratory specialty IA testing procedures. Available in barcoded, load-and-go InteliQ and standard vial Liquichek configurations; it features high-demand, clinically relevant analytes, including Procalcitonin, IL-6, Active Vitamin B 12 , TRAb, TSI, Fructosamine, and more. Bio-Rad

The UNIQO 160 (model no. YG 2900-0101) is a fully automated system designed for immunofluorescence testing, processing up to 160 samples within one device. It supports a range of testing protocols, executes up to 18 workflows per run, and includes EUROLabOffice 4.0 software for seamless integration with LIS networks.

EUROIMMUN (Part of Revvity)
The Tosoh G8 Analyzer utilizes the Ion-Exchange HPLC method of HbA1c measurement with less than 2% CVs. Suitable for high throughput HbA1c testing in the laboratory or clinic environment, the Tosoh G8 is designed to provide a reliable, accurate, and precise result. The compact analyzer detects and presumptively identifies commonly occurring hemoglobin variants. Tosoh


Detect the “Big 5” in 15 minutes
NG-Test CARBA 5, available exclusively in the U.S. from Hardy Diagnostics, is the only rapid, multiplex, phenotypic, lateral flow, FDA cleared assay capable of detecting KPC, OXA-48-like, VIM, IMP, and NDM carbapenemases produced by Enterobacterales and Pseudomonas aeruginosa. Detects the gene expression! Hardy Diagnostics

Control LQ HbA1c (K067M-8) is intended to simulate human patient whole blood samples for the purpose of monitoring the precision of laboratory testing procedures for the following assays: Glycohemoglobin A1c. Package Size: 8 x 1.0 mL. Vial Stability: 50 days at 2-8°C. AUDIT MicroControls

DPYD mutation detection
The Seraseq DPYD DNA Mutation Mix (Material Number: 0750-9502) is a highly multiplexed reference material designed for the validation and performance evaluation of molecular assays, detecting 39 clinically significant DPYD gene mutations. It features variants at clinically relevant frequencies in a well-characterized genomic background (GM24385), verified by digital PCR and NGS. LGC Group

a-1 Microglobulin
Anti-Streptolysin O
Apolipoprotein AI, B, E
Apolipoprotein AII, CII, CIII
b-2 Microglobulin
Complement C3, C4
CRP, hs-CRP
Cystatin C
D-Dimer
Factor XIII
Ferritin
Fibrinogen
H. pylori
Lower cost Expanded test menu
Outstanding customer support
Haptoglobin
Hemoglobin A1c
IgA, IgG, IgM
Insulin
Krebs von den Lungen-6
Lipoprotein(a)
Microalbumin
Prealbumin
Remnant Lipoprotein
Cholesterol
Rheumatoid Factor
Transferrin
UIBC
Nova’s Next Generation StatStrip Glucose Hospital Meter System, the only meter FDA-cleared for use with all critically ill patient samples, includes features that make hospital glucose testing easier and cybersecure. New features include a Linux OS, wireless charging, RFID data entry, sealed battery casing, hematocrit range 5%75%, and rugged external meter casing. Nova Biomedical


The PATHFAST Analyzer is designed to deliver quick, accurate, and dependable results to inform clinical decisions based on Chemiluminescence and Magtration technologies. PATHFAST runs the only POC high sensitivity Troponin I in the US, with 6% CV at the 99th percentile of URL, with optimal precision at very low concentrations in 17 minutes. Polymedco

Sapio LIMS is an end-to-end informatics platform designed to support high-throughput clinical and molecular diagnostics labs. It is 21 CFR Part 11 and EU Annex 11-compliant and features a physician portal, automated accessioning and order processing, configurable no-code workflow creation, automated reporting, built-in scientific tools, and much more. Sapio Sciences
Shimadzu’s Nexera QX Multiplex LC-MS/MS System eliminates waiting for column equilibration and system flushing by using multiple, alternating sample introduction streams for continuous operation of the mass spectrometer. Dedicated software provides an automated single point of control for multiple UHPLC streams and the mass spectrometer for a multiplexed workflow. Shimadzu Scientific Instruments


Puritan sterile, patented HydraFlock featuring multilength microfibers, each with a floret structure that effectively absorbs and elutes more specimens when compared to traditional spun fiber swabs. Available in various tip shapes and sizes, ideal for pediatrics, nasopharyngeal sampling, and several other collection sites when maximum absorbency and elution are required. Puritan Medical Products

Unistik 3 Side Activated Safety Lancets are designed to reduce pain for patients during sampling while giving healthcare professionals complete confidence and control during capillary blood sampling. Unistik 3 permanently retracts to help protect against accidental needlestick injuries and features laser-etched lot numbers for complete traceability. Owen Mumford

The SARSTEDT Tempus600 is a dedicated system for sending specimen tubes quickly and reliably to the laboratory. Sample tubes are placed directly into a sending station and arrive within seconds to the laboratory for processing without carrier batching. Samples arrive continuously as sent, reducing peaks for faster, more predictable workflow. SARSTEDT

Atellica CI Analyzer with Sample Handler is a mid-volume integrated chemistry and immunoassay clinical testing analyzer, featuring a broad menu; advanced sample management functions built-in such as sorting, archiving, onboard refrigerated calibration and QC, decapping, and sealing. The analyzer processes up to 1,120 test results per hour. Siemens Healthineers
In cases where the methods being compared lack standardization or have different reference ranges, regression analysis may be less desirable. For these assays, it is usually a good idea to put results into a concordance table to ultimately determine the positive, negative, and overall agreement of the two methods. Method comparison is useful for determining potential changes to clinical interpretation. While the methods may not give the same, or even similar, numeric results, the concordance of the interpretation should be the same. This also can be a good indication that re-baselining is necessary for patients who are monitored with a non-standardized assay. For example, oncology markers can be widely variable among manufacturers.
For novel lab tests that may not have a comparator method available, alternatives to method comparison studies include testing for accuracy with samples of known values including calibrators, assayed quality control materials, proficiency materials, or previously assayed patient specimens from another laboratory.4 The AMR verification can be performed with fewer samples of known concentration, or even diluted specimens of known concentration that span the measuring range.
Factors such as components of error and their sources, data collection qualities (e.g., sample number and range), and statistical analysis methods are all important components in interpreting method comparison study results.1-3 Additional information about the statistical underpinnings of regression analysis and goodness of fit, correlation, and sample effects can be found in statistical or analytical textbooks.
For the first time in point-of-care blood gas testing, hemolysis is flagged in just 45 seconds on the new GEM Premier 7000 with iQM3. Continuously monitoring the analytical process, before, during, and after each sample measurement, it detects, corrects, and documents sources of error, for quality-assured results. Werfen

With due diligence and careful consideration of these factors, in addition to accuracy and range verification, labs can efficiently and effectively provide clinical information that is effectively interpreted and of greatest value for clinical colleagues. Robust planning is a key component to successful method evaluation study preparation and post-evaluation test implementation success. Review of manufacturers’ Instructions for Use can help to understand differences in the test methods and standardizations that may impact the type of analysis most appropriate.
1. CLSI. Measurement procedure comparison and bias estimation using patient samples. 3rd ed. CLSI EP09c. Clinical and Laboratory Standards Institute; Wayne, PA: 2018.
2. Stöckl D, Dewitte K, Thienpont LM. Validity of linear regression in method comparison studies: is it limited by the statistical model or the quality of the analytical input data? Clin Chem. 1998;44(11):2340-2346. doi:10.1093/clinchem/44.11.2340.
3. Linnet K. Performance of Deming regression analysis in case of misspecified analytical error ratio in method comparison studies. Clin Chem. 1998;44(5):1024-1031. doi:10.1093/clinchem/44.5.1024.
4. Clinical Laboratory Improvement Amendments (CLIA). Verification of Performance Specifications. April 2006.

Melanie Pollan PhD, MT (ASCP) serves as Senior Director of Medical & Scientific Affairs, Diagnostics, Siemens Healthineers and was a co-author of the ADLM poster presentation A-101 “Is That Unexpected? Education on Handling Real World Complications with Tightly Controlled Analytes During Method Evaluation” with S.A. Love, J. Aguanno, J. Melchior, et al from which this article is derived.


Puritan® specimen collection and transport systems. Choose from a full line-up of sampling products, including foam and patented flock swabs, as well as dry transport tubes and liquid transport media. All crafted for convenience and patient comfort.







By William K. Dettwyler, MT, MLS
This is the story of my life as a lab tech, from a boy leaving the farm, to working in a clinical laboratory. I was born in 1933, so I am now 91 years old. I still remember going down the path to the barn that my father built to help milk and take care of the cows and feed the calves, wanting to work instead in a lab. When I was about 14 years old, I saw a doctor who did his own lab work. He showed me the counting chamber with the red and white cells in it that he was counting — I was very fascinated. Right after that, I wanted to do that type of testing, but there was little chance of that happening to me as the lone eighth grade graduate in my class of a small country school.
The family farm
In Oregon at that time, high school was required for all students under 16 years of age, but it was not enforced. So, at 14, I did not go to high school, but continued to assist my family on the farm, which consisted of father, mother, six boys, and four girls. I was number three in the sibling order.
I had already read about all the books in our school library and was starting to go to the Silverton Library for books such as Men Against Death, Microbe Hunters, Hunger Fighters, and almost all of Paul De Kruif’s series of medical books. Also, books on Madame Curie and Charles Lindbergh. I read no novels. I felt they were a waste of time, just made-up stories. My first love was airplanes, but being pragmatic, I could see that working in a lab was going to be more attainable. I was a great fan of Charles Lindbergh and Amelia Earhart. I also read all of Admiral Richard E. Byrd’s books about his polar explorations. But that, I knew, was way beyond my attainment. And anyway, by then exploring was almost over, so it was lab work that held the greatest chance of both exploring and a meaningful occupation. It would also get me off the farm. It was not that I disliked farmwork, but I was excited with the microscopic wonderland, just as Anton van Leeuwenhoek was when he first saw bacteria and called them “wee beasties.”
Our family doctor at that time was a great lover of cream and would come to our farm about weekly to buy cream from our family, and I eventually was able to go to his office in Silverton to assist the lab tech. I was about 17 at the time.

After working at home all day, I would go to Silverton in the evening and wash and dry all the glassware that the lab tech Eddie Condon had used.
In the fifties we did not have plastic labware; it was all glass. From the small pipettes for WBC and RBC counts, and the glass pipettes for transferring fluid for chemistries, it was all glass. At that time, it was all mouth pipetting, there were no automated pipettes as today, and even the 20% sodium cyanide that we pipetted, when performing the Uric Acid test was all mouth pipetted. Mr. Condon indicated that if I washed his glassware, he would help me learn to be a lab tech. Of course this was all free for him, as I was not being paid. I soon found that I was being used, as I was not learning that much about testing, but was good at washing glassware for him.
During this time, besides working full time during the day for my father, I was later at night taking a home study high school course from the American School to work toward getting a high school diploma. Besides that, I was also taking a home study course on Clinical Lab Technique by the Imperial Technical Institute in Texas. At about 19 years of age, I saw washing glassware was not getting me ready for a job, so I quit that and concentrated on my home study courses and of course working on the farm full time. So, I was quite busy with all these things and really burned the midnight oil. During this time, I was also helping my father with logging and hauling logs to the local sawmill. We also had 45 acres of hops, which took up a lot of our time in the spring working in the field. Then in the summer and fall, I worked in the hop house (A hop house is a large building where hops are dried, processed, and baled prior to being sold to brewers). The year that I was 20, I worked at the hop house almost around the clock, usually putting in about 20 hours a day and just sleeping on a plank, covered with burlap, for a few hours a night. That year, two of us baled over 700 two-hundred-pound bales of hops, as a two-man crew.
During the State Fair that summer, the Oregon Technical Institute in Klamath Falls had a display at the Fair showing some med tech students pipetting and performing lab tests that really got my interest. Here was a school right here in

Oregon, but still about 220 miles away from me, where I could go and learn to be a real med tech. I did not let that encounter go to waste. I checked it out and decided that I would go there and take the Medical Laboratory Technique course that fall. This was going to be a change for my family, one less worker at home, but I had four younger brothers and three younger sisters that could help my dad by then.
On a Thursday near the end of September, we picked sweet corn by hand for the cannery, all day. The next morning on a Friday, the last day that a student could still get into the fall classes at Oregon Tech, we got up real early and my folks took me to Klamath Falls and dropped me off at the Registrar’s office. I met with Mr. Smith, the Dean of Students, and presented my qualifications. Of course I did not have a high school diploma, as was required. I knew that that would be a problem, but I came prepared. I had a letter from both my doctor and dentist, saying that I could do the work. He accepted that information and allowed me to start the lab program. Of course, I was starting almost two weeks after classes had already begun, so I was behind the rest of the students, but my advantage was that I already had some experience working in a lab, so I caught up quickly.
That first evening, I already had a part-time job working for the concessions at the football game. I also was able to get a part-time job as a janitor. Our two lab instructors were well informed. One was a retired naval Chief Warrant Officer, who was an MT, RN, and a pharmacist. He was also a parasitologist who previously taught parasitology at the Armed Forces Institute of Pathology. The other instructor had worked with Dr. Rueben Kahn, who developed the first major non-complement fixation test for syphilis, the Kahn test, which was the standard test for syphilis in 1953. At that time, not every lab could perform the complement fixation test or the Kolmer, as they were quite complex, most used the Kahn or later the VDRL. We practiced drawing blood on each other. That was easy for me as I had already performed many venipunctures in the lab in Silverton. Our textbook was the Fifth Edition of Clinical Laboratory Methods and Diagnosis written by R.B.H. Gradwohl, M.D. D.Sc., an ex-Commander, Medical Corp, of the U.S. Navy. This was a two-volume set of 2,400 pages, and it covered lab testing from A-Z and then some. Interestingly, the whole campus of the Oregon Technical Institute (OTI) was a self-contained town
of Oretech, with its own library, theater, post office, fire department, coal-fired heating plant, gymnasium, cafeteria, barracks for the students and faculty housing. All located a mile high, in a little valley above the city of Klamath Falls. The entire site was purchased from the military by the state of Oregon for 1 dollar in 1946 and revamped into a technical school, starting classes in 1947. It was built by the military, just before the end of World War II, to accommodate the many military personnel returning from the South Pacific who had malaria and filariasis. The military felt these personnel needed to be isolated to prevent the spread of those diseases to the civilian population. The military had a concern that if the military personnel were back in the general population and were bitten by mosquitoes, they could pass on these diseases to the civilians. It is a fact, that the malaria spreading mosquito, Aedes Aegypti, cannot fly above 5,000 feet, so placing the military recuperation center at a mile high would protect the civilians. It was used only about a year, and then they no longer had any use for it. It even included the largest swimming pool west of the Mississippi. But after the state of Oregon purchased it, they never even filled the swimming pool as it would have been too expensive for the school to pump all that water up the 1,000-foot OTI hill to fill the pool, so it was instead used as a running track. I completed the two-year Med Lab Tech program in 1955. During my second year there, I was the editor of the school newspaper, The Miler, and I also worked at the local hospital every evening five days a week during the spring term. I performed the tissue technique, sectioning and staining the tissues for the pathologist, as I had already taken Tissue Technique and was able to work before I graduated. For Parasitology, we would collect stool specimens from the students and from local slaughterhouses. From our own students, we found Giardia lamblia, Entamoeba coli, and Endolimax nana. From the animal specimens, we found many Fasciola hepatica, Ascaris lumbricoides, and pork and beef tapeworms. We also microscopically identified many of the worm eggs from the animal stool specimens. The instructor, Mr. Martin, supplemented those specimens with some that Dr. Ernest Carroll Faust sent to him from his Tropical Medicine collection.
We also performed the sodium and potassium analytes using the chemistry method of Kramer and Tisdall, which was a two-day procedure, as most labs at that time did not yet use the flame photometer. So, there was no such thing as stat electrolytes in a lot of labs at that time. We also did thyroid tests with the BMR (basal metabolic rate) instrument, where we would determine the oxygen consumption and from a chart determine whether the person was normal, hypothyroid, or hyperthyroid. We learned to perform ECGs (electrocardiograms) using an instrument invented by Willem Einthoven through a gold-plated quartz string that would capture the shadow of the electrical impulse movement of the quartz string on a roll of photographic paper. This would then be taken to the radiology department, and it would be developed using the tanks that they developed the x-ray film in. Direct writers were probably available by then, but the school could not buy all the latest equipment.
We, at that time, performed all hematology using the small RBC and WBC pipettes. By using diluting fluid and placing the fluid on a hemocytometer, we would visually count the number of red and white cells on separate sides of the chamber and then by calculation come up with the actual blood count. Mr. Wallace Coulter perfected the Coulter Counter in 1948, but it was many years after that before the instrument was available for small labs to use. Our white cell differential counts were also performed visually for many more years after the Coulter Counter came out, as that technology to determine the WBC differential was much more complex.
We also learned how to perform intubations for gastric analysis. At that time, the instructor had us use a plastic tube with an olive-sized metal bulb on the end, which as a weight allowed it to go down easier, and we would pass it through the mouth, not the nose, as we did later. Once when one of the students put it down my mouth and tried to aspirate the gastric fluid, he could not get any. He asked the instructor what was wrong, our instructor put the tube to his ear and said, “you had better take it out of his lungs.” Which he did. Then he put it in again, and he did get gastric fluid, but when he tried to take it out of my stomach, it was stuck. He again asked Mr. Martin what to do. He said that we could cut it off and let it go through, but we would have to pay him for the tube. Or, he suggested,“You can go down to the hospital and have the pathologist take it out.”
40’s where they developed the atomic bomb, so he was not convinced easily. I was insistent, so he tested me with an x-ray machine, it did not make a sound if I was x-rayed or not. I passed that test, and he was beside himself.Years later, I think I discovered why I was feeling the ionizing rays. When I was in lab school, I always wore an orlon shirt, similar to nylon, and when I would go to radiology, they would have me take it off and put on a gown. What I was feeling was the effect of the ionization of the air, as the rays hit my arm, as I was ionically charged by taking off the orlon shirt. When the x-rays went through me, I was feeling the change of the ionic charge on my arms. This is only my theory; it has not been substantiated by any real research. No, I did not glow in the dark.

The method of performing pregnancy testing in the 50’s was to use a rabbit or frog. A woman, wishing to know if she was pregnant, was to bring in a urine specimen. We would inject, either under the skin or into a vein in the rabbit’s ear, about 5–10 milliliters of urine. After 48 hours, we’d kill the rabbit and observe the ovaries to see if they were hemorrhagic. If they were, we would indicate that the woman’s pregnancy test was positive. When we did this test in class, we had a classmate beg for some urine from a pregnant friend, and we did the test. After 48 hours, we killed the rabbit, opened it up and looked at the ovaries and they were hemorrhagic. That was quite exciting. Then our instructor gave us another project, he told us to take out the lungs and process the lung tissue, and from it, he had us extract thromboplastin. We then processed this further and made our own thromboplastin, standardized it, and were able to perform prothrombin times with it. But that was not the end of the rabbit. I skinned it out, threw away the hide, and saved the meat for my dinner. Some years later we started performing the pregnancy test using male frogs. We would concentrate the woman’s urine and then inject it into the dorsal lymph sac of the frog. After waiting 3–6 hours, we would bounce the frogs behind on a glass microscopic slide and look for spermatozoa under the microscope. If we found sperm, we would report it as a positive; no sperm, it was a negative result. Using a female frog for the pregnancy test was a similar technique, but the end result was different. If she laid eggs, the woman’s test was positive. I cannot confirm that any of the pregnancy tests were 100% accurate — we did not perform any proficiency testing on the various pregnancy tests.
I did not want to pay for a new tube. One of the students offered to take me to the pathologist. He had no trouble, and it was a great learning experience for us. He gave me a glass of water, and as I swallowed the water, he quickly pulled it out. As the gastroesophageal sphincter opened to allow the water through, the olive-sized bulb came right up through the sphincter. Then we went back to class, but being a glutton for punishment, the first thing I did was to have someone put the gastric tube in again, and the same thing happened, it was stuck. But now we had learned what to do, I drank a little water, swallowed, and it came right out.
As the radiology students were right next door, we were frequently asked to be their patients, as they needed people to practice on. I did notice that I was able to feel the x-rays. I mentioned that to the radiology instructor, and he did not buy it. He said that we cannot hear, feel, smell, taste, or see x-rays. And he had worked on the Manhattan Project in the
Near the end of the second year, all the students would spend the last six weeks in a lab residency, where we worked in a real lab and were able to perform testing on patients under supervision. I spent my six-week residency in a large clinic in Bend, Oregon. There I watched the Lab Manager make autogenous vaccines for patients that had allergies. (We did not learn this in lab school.) He had previous experience working with Dr. Reuben Kahn, the one who developed the Kahn test for syphilis. He would have patients bring in things that they may be allergic to, such as cat hair, floor dust, vacuum cleaner contents, what have you, and make an autogenous vaccine from that material. It would be processed into a vaccine, sterilized, diluted, and put in a small vial for the doctor to then use on that patient. No proficiency testing on this concoction either.
Visit https://mlo-online.com/55142199 to read the rest of William K. Dettwyler’s story about his 75-year labora tory career.

Yves Dubaquie is SVP of Diagnostics at Revvity, responsible for developing and executing the company’s long-term strategy for its broad specialty diagnostics portfolio, which includes solutions that span immunodiagnostics, infectious disease testing, reproductive health and genomics.
Yves is an accomplished executive with over 25 years of broad healthcare industry experience. He is fascinated by technology and the process of deploying science to positively impact human health. Over the course of his career, he has also held various senior leadership and business development roles at Bristol-Myers Squibb, Novartis Molecular Diagnostics, and GE Healthcare, among other companies.
Why do labs need to embrace automation, and what should they consider before implementing it?
Before the COVID-19 pandemic, staffing shortages already posed significant challenges. During the pandemic, lab automation became integral to handle the rapidly increasing surge of samples, establishing a“new normal”for scientists and lab technicians who had to depend on automated data management systems for accurate reporting and sample traceability.
In the post-pandemic era, rising healthcare costs and a heightened focus on developing advanced medicines will further increase reliance on automation in drug discovery and development. Automation offers numerous benefits, especially in the face of ongoing staffing shortages. It significantly reduces human error and enhances efficiency, allowing scientists to dedicate more time to more
By Christina Wichmann
valuable tasks such as research and innovation, where their expertise is crucial.
A mindset shift is essential for fully embracing and adapting to automated tools and instruments. Lab leaders must consider several factors, including training, for reassurance that automation will not replace human jobs but rather redirect efforts toward critical areas of research and innovation, and the establishment of efficient protocols for resolving queries.
Beyond continuous communication around automation’s benefits, scalability must also be a key consideration during implementation. Sample volumes often vary based on specific scenarios, necessitating that automation be flexible and adaptable enough to provide tailored solutions for different needs. This is particularly important in a period of clinical laboratory consolidation, which is requiring scalable automation solutions for labs as their volumes change. For example, Revvity’s EUROIMMUN offers a range of immunofluorescence test (IFT) platforms, including the IF Sprinter for small and medium sample volumes, Sprinter XL for medium to high volumes, and EUROLabWorkstation, the highest volume IFT processor available in the world, which is designed to serve the largest lab operations. Additionally, UNIQO 160, a new fully automated IFT solution with integrated sample preparation and imaging capabilities, is ideal for medium-sized laboratories. Finally, for newborn screening (NBS), Revvity provides solutions such as the GSP instrument and the VICTOR2 D instrument (combined with the DELFIA Trio), which cater to both high and low throughput requirements, respectively.
What are some examples of what we can do today because of automation that we couldn’t do just a few years ago?
Automation never operates in isolation. Effective automation in laboratory workflows requires seamless integration of associated software, reagents, consumables and relevant instruments, often connecting to a LIMS (laboratory information
management system). These components work together to connect and streamline multiple steps within the lab’s workflow, significantly reducing manual workloads.
For example, the UNIQO 160, the automated IFT system for autoimmune disease diagnostics, integrates seven workflows into a single system. It alleviates many common laboratory challenges by reducing hands-on time and creating efficiencies through end-to-end automation, including automated barcode scanning and sample assignment.
For research-use next generation sequencing (NGS) sample preparation, the BioQule system is designed to simplify the isolation of nucleic acids and the generation and quantitation of NGS libraries, even for operators without prior automation experience. It automatically measures library concentrations, streamlining the quality control process.
Another example is the Auto-Pure 2400 liquid handler for latent tuberculosis (TB) detection, which offers excellent sensitivity and specificity while enabling efficient lab workflows. With less than 10 minutes of hands-on time required, the automated workflow makes previously labor-intensive tasks, such as PBMC isolation and normalization, both quick and straightforward.
What role has artificial intelligence played in this evolution? What will it play down the road?
AI is playing a critical role in driving innovation, particularly by reducing time-consuming, repetitive tasks that were historically performed by humans. For instance, in prostate biopsy imaging, where 10-12 samples are typically analyzed, most results are negative. With AI, negative results with similar patterns are filtered out, allowing pathologists to focus more specifically on those results that indicate potential positives. Another example is that when UNIQO 160 generates fluorescence images and proposes test result interpretations, scientists are still needed to validate and sign off on these results.
These processes require precise differentiation between high-quality results and problematic ones. With proper training, AI will become increasingly adept at distinguishing results based on their quality. Rule-based decisions regarding cut-off ranges, quality control results, calibrations, and reference standards are now routine in medical labs, helping to filter out subpar samples. By eliminating the need for manual intervention, the potential for human-related errors is substantially reduced.
As a global leader in NBS, Revvity has screened approximately 800 million babies for life-threatening diseases across 110 countries cumulatively. The process begins in the hospital after delivery with a heel prick where blood is captured on specialized paper cards. These cards are then sent to laboratories performing NBS tests where automation plays a crucial role, followed by confirmatory testing. In order to identify inborn illnesses early enough to initiate potentially life-saving intervention, samples in the U.S. are typically processed within five days after birth.
We are developing an AI-integrated method for our dried blood spot (DBS) cards, particularly for optical reading. The DBS cards are manually marked with important information immediately after a baby’s birth, which needs to be entered into the LIMS and processed promptly. AI will swiftly capture this information, enabling much faster interpretation than previously possible, identifying inconsistencies or errors, and therefore saving valuable time.
Looking ahead, we anticipate that AI will suggest reflex testing based on initial test results, which could shorten the diagnostic journey and improve diagnostic quality. With AI-enabled faster operations, we expect enhanced accuracy and throughput, enabling clinical laboratories to support clinicians to make critical, time-sensitive decisions around the clock.
Revvity’s purpose is to expand the boundaries of human potential through science. Why is automation essential in achieving this?
Revvity has undergone a significant transformation, bringing together the ecosystem needed to realize the promise of precision medicine. The foundation of precision medicine lies in a deeper understanding of both the disease and the patient, including how the patient will respond to specific treatments.
Diagnostic testing is key to this improved patient characterization, critically relying on the interrogation of a broader range of biomarkers and conducting more specialized testing across various samples. In order to handle this increase in testing procedures, automation will play an important role in delivering timely results (e.g., through multiplexing) in an economically viable manner. Additionally, automation not only saves time but also minimizes human errors.
In essence, automation seamlessly integrates modular steps, creating a much more efficient workflow from screening to treatment.




MLO’s Lab of the Year Award celebrates medical laboratories that demonstrate their extraordinary commitment to quality patient care. Submissions will be judged on achievements in five areas. A panel of judges selected from MLO’s Editorial Advisory Board will select the winner and two runners-up. All will be featured in the April 2025 issue of MLO, in print and online, and awarded a display wall plaque, with the winner featured on the issue cover.
Medical laboratories, utilizers of a lab’s service and non-vendor affilities are welcome to submit. Submission requirements are at: https://www.mlo-online.com/55234457













“Beginning with the effective date of this final rule,* individuals with nursing degrees will only be able to qualify for personnel positions listed in subpart M when a nursing degree is specifically listed in the regulatory qualifications. Nursing degrees will qualify under moderate complexity testing personnel. However, individuals with nursing degrees will no longer be able to qualify as high complexity testing personnel.”








