®
The Peer-Authored Management Source for Lab Professionals since 1969

®
The Peer-Authored Management Source for Lab Professionals since 1969
diabetes diagnosis and classification Page 8
PLUS Detection of Group B streptococcus in the lab
Page 22
Improving laboratory inventory management
Page 30

LAB INNOVATOR
Lisa-Jean Clifford President, Gestalt Diagnostics

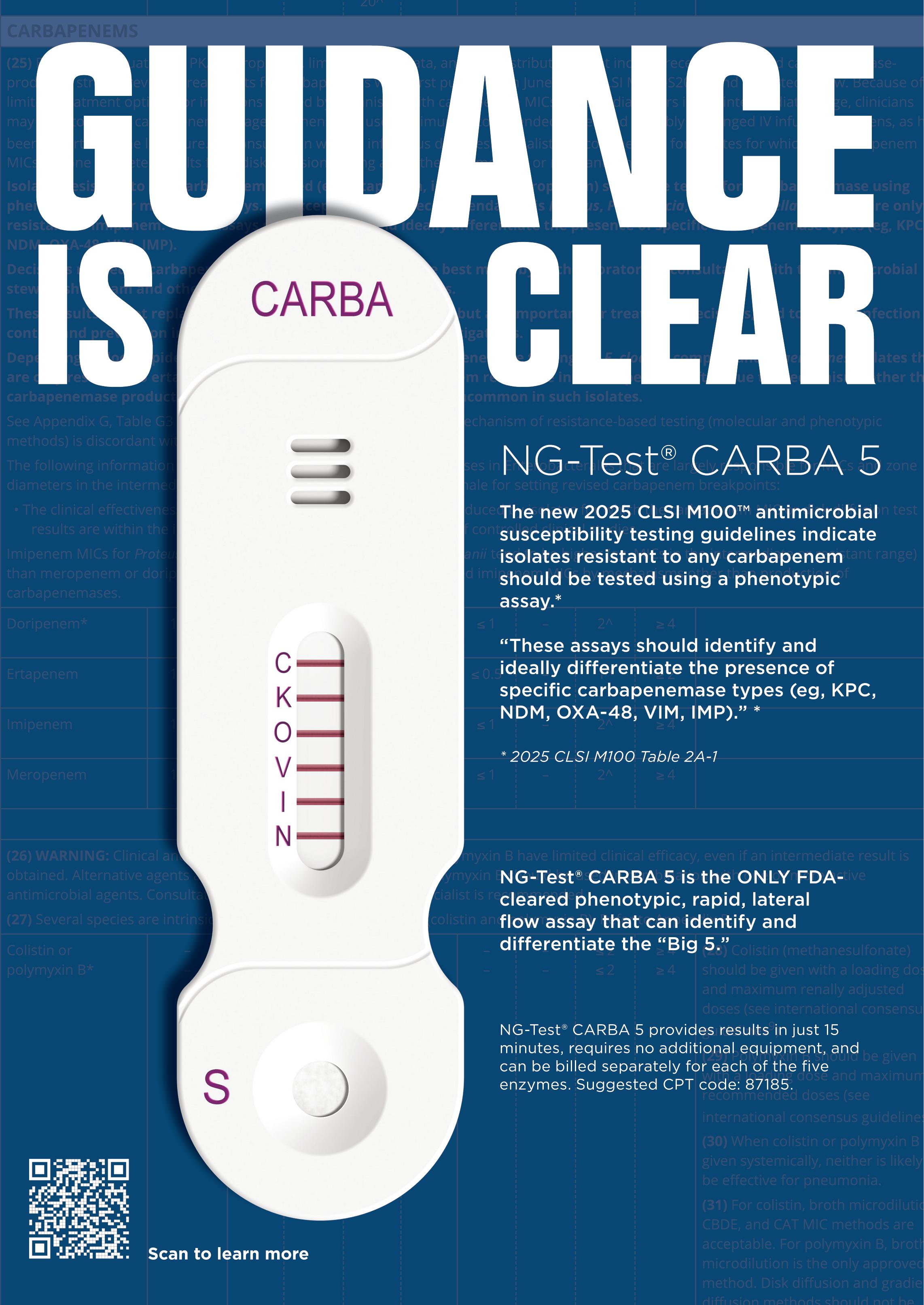




























Choosing the right partner can help you not only succeed, but surpass expectations. The partnership of Sysmex and CellaVision® — two global leaders in hematology diagnostics — can help your lab do just that.

At Sysmex, we are dedicated to our partners, because we’re all in this together.













See our expanded portfolio of solutions this year at ADLM Booth #2212 or visit Sysmex.com/ADLM

























































Informatics For 25 consecutive years Sysmex has rated highest for System Reliability & Service.*




Rajasri Chandra, MS, MBA
Tobin Efferen, MD,
Mark O. Colgin II MBA,

Empower your laboratory with the only FDA-authorized PCR assay to help prevent Candida auris outbreaks in healthcare settings.
The CDC is tracking increasing C. auris cases in the US.1 Identifying colonized individuals is crucial for infection control and preventing deadly outbreaks which can be life-threatening to vulnerable patients.
Your HAI testing solution for C. auris detection to enhance your clinical, operational, and economic outcomes.
Prompt identification of patients colonized with C. auris without compromising performance: Clinical sensitivity (PPA) of 94.8% and specificity (NPA) of 98.75% in clinical trials with results in under 2 hours.2
Simple PCR workflow on the versatile LIAISON® MDX system: No prior sample extraction. Run 1-8 samples on a reusable direct amplification disc.
Faster results may lead to better fiscal outcomes: Prevent the spread of this highly drug-resistant fungus, and reduce the need for extended hospital stays and treatment costs associated with hospital-onset infections.3





































































































































By Christina Wichmann
in Chief
July is my favorite month. When I think of summer, there are two primary things I love: sun and water. You really need water to enjoy summer…swimming, boating on a lake, watering the garden, bike riding along the river. It’s always been at the center of my family’s summer.
Drinking water on hot days is also very important. It helps maintain a normal body temperature and blood pressure, flushes out toxins, and cushions our joints. Our July Continuing Education article is on diabetes mellitus. Water is the perfect drink for people with diabetes. It has no added sugar like many beverages and can lower blood glucose levels. Adequate hydration also helps our organs perform at their best. For example, water helps the kidneys to filter and absorb excess glucose from the blood. There are also some studies that suggest a healthy lifestyle that includes drinking an adequate amount of water regularly can prevent prediabetes.
In the clinical laboratory, water is a key factor every day. On average, laboratories use hundreds of liters of water a day depending on the size of the lab, the types of tests performed, and the specific equipment used.
Water is used in the lab for numerous tests such as blood tests, tissue sample tests, toxicology tests, and microbiology tests. Water is the most frequently used reagent in the lab for lab analyzers, as it is used to prepare solutions or dilute manufacturers’ reagents.
Certain laboratory equipment needs water for operation and cooling, and water is used for the cleaning of lab surfaces, glassware, and equipment, including sterilization machines that produce steam. And obviously, water is used by laboratory staff for handwashing.
In the laboratory, the availability of pure water is essential, and while most outside the lab might consider tap water to be “pure,” laboratory scientists regard it as highly contaminated with impurities such as dissolved inorganic compounds and dissolved organic compounds. The quality of the water is extremely important in clinical diagnostics because it affects the chemistry of the tests and the general operation of lab analyzers, which would reduce the reliability of patient test results. There are standards regulating water in the laboratory and water purification systems are in all laboratories. Reagent water is the most pure and is categorized in types 1 through 3. Most labs use a water purification system that combines filtration, reverse osmosis, and deionization.
A day without water to enjoy in July would be a bummer, but a day without water in the lab would be a day without any work or tests being completed.
I welcome your comments and questions — please send them to me at cwichmann@mlo-online.com.
Vol. 57, No. 5
PUBLISHER Chris Driscoll cdriscoll@endeavorb2b.com
EDITOR IN CHIEF Christina Wichmann cwichmann@mlo-online.com
MANAGING EDITOR Erin Brady ebrady@endeavorb2b.com
PRODUCTION MANAGER Edward Bartlett
ART DIRECTOR Kelli Mylchreest
AUDIENCE DEVELOPMENT/LIST RENTALS Laura Moulton | lmoulton@endeavorb2b.com
ADVERTISING SERVICES MANAGER Karen Runion | krunion@endeavorb2b.com
ADVERTISING
DIRECTOR OF SALES
EAST COAST/MIDWEST SALES, CLASSIFIEDS Carol Vovcsko (941) 321-2873 | cvovcsko@mlo-online.com
SOUTH/WEST COAST/ILLINOIS SALES Lora Harrell (941) 328-3707 | lharrell@mlo-online.com
MLO EDITORIAL ADVISORY BOARD
John Brunstein, PhD, Biochemistry (Molecular Virology) President & CSO PathoID, Inc., British Columbia, Canada
Lisa-Jean Clifford, COO & Chief Strategy Officer Gestalt, Spokane, WA
Barbara Strain, MA, SM(ASCP), CVAHP Principal, Barbara Strain Consulting LLC, Formerly Director, Value Management, University of Virginia Health System, Charlottesville, VA
Jeffrey D. Klausner, MD, MPH Professor of Preventive Medicine in the Division of Disease Prevention, Policy and Global Health, Department of Preventive Medicine at University of Southern California Keck School of Medicine.
Donna Beasley, DLM(ASCP), Director Huron Healthcare, Chicago, IL
Anthony Kurec, MS, H(ASCP)DLM, Clinical Associate Professor, Emeritus , SUNY Upstate Medical University, Syracuse, NY
Suzanne Butch, MLS(ASCP)CM, SBBCM, DLMCM Freelance Consultant, Avon, OH
Paul R. Eden, Jr., MT(ASCP), PhD, Lt. Col., USAF (ret.) (formerly) Chief, Laboratory Services, 88th Diagnostics/Therapeutics Squadron, Wright-Patterson AFB, OH
Daniel J. Scungio, MT (ASCP), SLS, CQA (ASQ), Consultant at Dan the Lab Safety Man and Safety Officer at Sentara Healthcare, Norfolk, VA CORPORATE TEAM
CEO Chris Ferrell
COO Patrick Rains
CRO Paul Andrews
CDO Jacquie Niemiec CALO Tracy Kane CMO Amanda Landsaw EVP HEALTHCARE, CITY SERVICES & DIGITAL INFRASTRUCTURE Kylie Hirko 30 Burton Hills Blvd., Suite 185 Nashville, TN 37215 800-547-7377 | www.mlo-online.com
Medical Laboratory Observer USPS Permit 60930, ISSN 0580-7247 print, ISSN 2771-6759 online is published 10 times annually (Jan, Mar, Apr, May, Jul, Aug, Aug-CLR, Sep, Oct, Nov) by Endeavor Business Media, LLC. 201 N Main St 5th Floor, Fort Atkinson, WI 53538. Periodicals postage paid at Fort Atkinson, WI, and additional mailing offices. POSTMASTER: Send address changes to Medical Laboratory Observer, PO Box 3257, Northbrook, IL 60065-3257. SUBSCRIPTIONS: Publisher reserves the right to reject non-qualified subscriptions. Subscription prices: U.S. $160.00 per year; Canada/Mexico $193.75 per year; All other countries $276.25 per year. All subscriptions are payable in U.S. funds. Send subscription inquiries to Medical Laboratory Observer, PO Box 3257, Northbrook, IL 60065-3257. Customer service can be reached toll-free at 877-382-9187 or at MLO@ omeda.com for magazine subscription assistance or questions. Printed in the USA. Copyright 2025 Endeavor Business Media, LLC. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means,
• Liquid, ready-to-use

• Superior stability
• Fast, reliable results
Enhance efficiency with our robust liquid, ready-to-use reagents. Central to Werfen’s total Hemostasis testing solution, our comprehensive line of HemosIL reagents deliver superior onboard stability and fewer manual steps—from routine to specialty testing. Other key components include ACL TOP® Family 50 Series systems with automated pre-analytical sample integrity checks, and HemoCell Specialized Lab Automation, designed specifically for the automation of Hemostasis testing. Rely on HemosIL reagents for improved laboratory efficiency—for better patient care.
Simply efficient.
For more information, contact your local Werfen representative. werfen.com

By Rajasri Chandra, MS, MBA
Diabetes mellitus is a chronic, metabolic disease that occurs due to the body’s inability to produce enough insulin or to ineffectively utilize the insulin produced leading to hyperglycemia or elevated
levels of blood glucose (or blood sugar). If not controlled, diabetes can cause damage to the heart, blood vessels, eyes, kidneys, and nerves.1
Per the International Diabetes Federation’s Diabetes Atlas 11th edition
See test online at https://ce.mlo-online.com/courses/ evolving-paradigms-in-diabetes-diagnosis-andclassification-ADA-standards-of-care-2025-and-theuse-of-artificial-intelligence/.
Passing scores of 70 percent or higher are eligible for 1 contact hour of P.A.C.E. credit.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
Scan code to go directly to the CE test.
1. Describe the pathophysiologies in the classifications of diabetes.
2. Discuss the laboratory tests and their results in the diagnosis of the classifications of diabetes.
3. Differentiate confirmatory data of laboratory testing in the confirmation of diabetes.
4. Discuss AI strategies for the prediction and management of diabetes.
published in 2025, diabetes is one of the fastest growing global health emergencies in the 21st century with 1 in 9 adults having diabetes. In 2024, 588.7 million people had diabetes, and it is estimated that 852.5 million would develop diabetes by 2050 with a 45% growth. In the United States, 65.6 million people have diabetes, and it is estimated that number to reach 72.4 million by 2050 with a 10% rise.1
The field of diabetes care is evolving through new research, technology, and treatments to improve the health and well-being of people with diabetes. Since 1989, the American Diabetes Association (ADA) has been updating the Standards of Care recommendations annually to capture the most current state in the field of diabetes.
The America Diabetes Association (ADA) classified diabetes based on metabolic, genetic, and pathophysiology features, as below:2









• Type 1 diabetes (T1D) – due to autoimmune β -cell destruction, usually leading to total insulin deficiency, including latent autoimmune diabetes in adults
• Type 2 diabetes (T2D) – due to a non-autoimmune progressive loss of adequate β -cell insulin secretion, frequently causing progressive insulin resistance
• Specific types of diabetes due to other causes, e.g., monogenic diabetes caused from a mutation in a single gene; diseases of the exocrine pancreas; drug- or chemical-induced diabetes
• Gestational diabetes mellitus (GDM) – observed in the second or third trimester of pregnancy in some individuals
It is important to identify the type of diabetes — type 1 or type 2 — to render personalized therapy. Traditionally it is believed that type 1 occurs in children and type 2 in adults; however, that may not be the case always. For some individuals, it is difficult to clearly classify the diabetes type at the time of diagnosis, and misdiagnosis is common. About 40% of type 1 diabetes cases
are misdiagnosed as type 2 and many maturity-onset diabetes of the young (MODY) caused by monogenic syndrome may be misdiagnosed as type 1 diabetes.3
AABBCC is a clinical tool that may be used to determine if a newly diagnosed diabetic has type 1 diabetes based on the following criteria:
A) Age (e.g., for individuals <35 years old, consider type 1 diabetes)
A) Autoimmunity (e.g., personal or family history of autoimmune disease or polyglandular autoimmune syndromes)
B) Body habitus (e.g., BMI <25 kg/m2)
B) Background (e.g., family history of type 1 diabetes)
C) Control (preferred term is “goal,” i.e., the inability to achieve glycemic goals on noninsulin therapies)
C) Comorbidities (e.g., treatment with immune checkpoint inhibitors for cancer can cause acute autoimmune type 1 diabetes)
The American Diabetes Association encourages use of C-peptide and islet autoantibody testing in ambiguous adult-onset cases. The flowchart in Figure 1 helps to distinguish type 1 and type 2 diabetes
using age, BMI, autoantibodies, and insulin dependency.
Tests for screening and diagnosis (see Table 1)
• Fasting plasma glucose (FPG)
• 2-h plasma glucose (2-h PG) during a 75-g oral glucose tolerance test (OGTT)
• A1C
With diabetes impacting so many individuals across America, it is hard to see greater availability of screening tools as anything but a net positive.
The A1C test should be performed using a method that is certified by the National Glycohemoglobin Standardization Program (NGSP) (ngsp.org) and standardized or traceable to the Diabetes Control and Complications Trial (DCCT) reference assay. Point-ofcare A1C assays may be NGSP certified and cleared by the U.S. Food and Drug Administration (FDA) for use in both Clinical Laboratory Improvement Amendments (CLIA)–regulated and CLIA-waived settings.2
Unless there is an absolute match between the clinical diagnosis (e.g., individual with hyperglycemia or hyperglycemic crisis and random plasma glucose >200 mg/dL (>11.1 mmol/L), additional confirmatory tests are necessary. Diabetes can be confirmed with two abnormal screening test results measured either at the same time or at two different points of time and may be performed using two different types of tests, e.g., A1C and FPG.2
Criteria for diabetes in non-pregnant individuals includes one of the following:
• A1C ≥6.5% (>48 mmol/mol)
• Fasting plasma glucose (FPG) ≥126 mg/dL (≥7.0 mmol/L)
• 2-hour plasma glucose (2-h PG) ≥200 mg/dL (≥11.1 mmol/L) during OGTT
• Random plasma glucose ≥200 mg/ dL in symptomatic patients
Criteria for pre-diabetes in non-pregnant individuals includes one of the following:



Smart transfer system allows for a compact footprint: The AUTION EYE connects with the AUTION MAX AX-4060, and results in a very small footprint (41.9" x 25.6" x 23.6").
Automatic Dilution Function
Atlas Image Collection
Microscopic Automatic Image Collection
Compact Footprint
Automatic Cross-check Function
Gating Function
Cost Inexpensive and readily available in all labs More expensive and may not be available in all labs
Time frame of hyperglycemia measure Acute Chronic – spanning past 2–3 months
Pre analytic stability
Sample type
Assay standardization
Poor
Plasma, serum, whole blood (Measurements vary based on sample type)
Not standardized
Requirement of fasting Fasting required
Within variability
High
Factors affecting result Food intake, stress, recent illness, activity
Other factors affecting results Diurnal variation, medications, alcohol, smoking, bilirubin
Interferences
Depends on specific assay: sample handling/processing time, hemolysis, severe hypertriglyceridemia, severe hyperbilirubinemia
Table 1. Comparison between glucose test and A1C test.4
Characteristics
• Autoimmunity
• Normal blood glucose
• Pre-symptomatic
Diagnostic criteria
• Multiple islet autoantibodies
• No impaired glucose tolerance (IGT) or impaired fasting glucose (IFG), normal A1C
Table 2. Stages of type 1 diabetes.5
• A1C 5.7–6.4% (39–47 mmol/mol)
• Autoimmunity
Good
Whole blood
Well standardized
Fasting not required
Low
Not affected by food intake, stress, recent illness, activity
Altered erythrocyte turnover (e.g., anemia, iron status, splenectomy, blood loss transfusion, hemolysis, glucose-6-phosphate dehydrogenase deficiency, erythropoietin), HIV, cirrhosis, renal failure, dialysis, pregnancy
Depends on specific assay: hemoglobin variants, severe hypertriglyceridemia, severe hyperbilirubinemia
• Abnormal blood glucose
• Pre-symptomatic
• Multiple islet autoantibodies (usually)
• Abnormal blood glucose
• Fasting plasma glucose (FPG) 100 mg/dL (5.6 mmol/L) to 125 mg/dL (6.9 mmol/L)
• 2-hour plasma glucose (2-h PG) ≥200 mg/dL (≥11.1 mmol/L) during 2-h PG during 75-g OGTT 140 mg/dL (7.8 mmol/L) to 199 mg/dL (11.0 mmol/L) impaired glucose tolerance (IGT)
Type 1 diabetes
5–10% of diabetics have type 1 diabetes.2 For individuals with a family history of type 1 diabetes or other genetic risks, screening for presymptomatic type 1 diabetes (T1D) may be done by using standardized islet autoantibody test for detection of autoantibodies to insulin, glutamic acid decarboxylase (GAD), islet antigen 2 (IA-2), or zinc transporter 8 (ZnT8). Multiple confirmed islet autoantibodies are a risk factor for clinical diabetes. An individual may be in different stages of type 1 diabetes as depicted in Table 2
Prediabetes and type 2 diabetes
90–95% of diabetics have type 2 diabetes.2 Criteria to screen for pre-diabetes or type 2 diabetes is as follows:
• Autoimmunity
• Very high blood glucose
• Symptomatic
• Autoantibodies may become absent
• Diabetes by standard criteria
1. Adults who are overweight or obese (BMI 25 kg/m 2 or 23 kg/m 2 in individuals of Asian ancestry) and have one or more of the following risk factors:
• First-degree relative with diabetes
• High-risk race, ethnicity, and ancestry (i.e., African American, Latino, Native American, Asian American)
• History of cardiovascular disease
• Hypertension (130/80 mmHg or on therapy for hypertension)
• HDL cholesterol level <35 mg/dL (<0.9 mmol/L) and/or triglyceride level >250 mg/dL (>2.8 mmol/L)
• Individuals with polycystic ovary syndrome
• Physical inactivity
• Other clinical conditions associated with insulin resistance (e.g., severe obesity, acanthosis nigricans, metabolic dysfunction–associated steatotic liver disease)
2. Individuals with pre-diabetes should be tested annually.
3. Individuals who had GDM should be tested every 1–3 years.
4. For all others, testing should begin after age 35.
KCNJ11
Permanent or transient: intrauterine growth restriction (IUGR); possible developmental delay and seizures; responsive to sulfonylureas
INS Permanent: IUGR; insulin requiring
ABCCB Permanent or transient: IUGR; rarely developmental delay; responsive to sulfonylureas
6q24 (PLAGL1, HYMA1)
Transient: IUGR; macroglossia; umbilical hernia; mechanisms include uniparental disomy of chromosome (UPD6), paternal duplication, or maternal methylation defect; may be treatable with medications other than insulin
GATA6 Permanent: pancreatic hypoplasia; cardiac malformations; pancreatic exocrine insufficiency; insulin requiring
EIF2AK3 Permanent: Wolcott-Rallison syndrome: epiphyseal dysplasia; pancreatic exocrine insufficiency; insulin requiring
EIF2B1 Permanent: can be associated with fluctuating liver function7
FOXP3 Permanent: immunodysregulation, polyendocrinopathy, enteropathy X-linked (IPEX) syndrome: autoimmune diabetes, autoimmune thyroid disease, exfoliative dermatitis; insulin requiring
5. If results are normal, testing should be repeated at an interval of 3 years.
6. Individuals in other high-risk groups — people with HIV, exposure to high-risk medicines, history of pancreatitis — should be monitored closely
• Individuals having acute pancreatitis should be screened for 3 -6 months after an episode and annually thereafter
• Individuals with cystic fibrosis should be tested annually from the age of 10 - Post transplantation after the individual is stable
<5% of individuals harbor monogenic defects of β -cell dysfunction in neonates causing neonatal diabetes and maturity-onset diabetes of the young (MODY).
• Neonates diagnosed with diabetes in the first 6 months of life should have genetic testing for neonatal diabetes and
• Children and young adults with diabetes who do not have typical characteristics of T1D or T2D and family history of diabetes in successive generations should have genetic testing for MODY.
Genes causing monogenic diabetes syndrome are described in Tabl es 3 and 4 6
Gestational diabetes mellitus (GDM) is a metabolic disorder characterized by increased blood sugar levels during the second and third trimester of the pregnancy in some women.
Patty Eschliman, MHA, MLS(ASCP)DLM, CPC

Teamwork:
Leadership:
Personal growth:
How should I deal with a toxic co-worker?
How can I stay positive when the team seems so negative?
How can I ensure our ideas are heard by leadership?
How do I hold that difficult conversation?
What can I do to change the culture of my team or my organization?
What do I need to do to get ahead? How can I ask for a raise?
How can I increase my strengths and work with my weaknesses to be more successful?
What do I need to do to ensure I get that promotion or new job?
What skills are needed to build a cohesive team? Send your questions to editor@mlo-online.com
Patty’s answers to your confidential questions will be published in the April, September, and November 2025 issues.
HNF1A Progressive insulin secretory defect with presentation in adolescence or early adulthood; lowered renal threshold for glucosuria; large rise in 2-h PG level on OGTT (>90 mg/dL [>5 mmol/L]); low hs-CRP; sensitive to sulfonylureas
HNF4A Progressive insulin secretory defect with presentation in adolescence or early adulthood; may have large birth weight (macrosomia) and transient neonatal hypoglycemia; sensitive to sulfonylureas
HNF1B Developmental renal disease (typically cystic); genitourinary abnormalities; atrophy of the pancreas; hyperuricemia (high level of uric acid in blood); gout
GCK Higher glucose threshold (set point) for glucosestimulated insulin secretion, causing stable, nonprogressive elevated fasting blood glucose; typically does not require treatment; microvascular complications are rare; small rise in 2-h PG level on OGTT (<54 mg/Dl (<3 mmol/L])
Table 4. Genes that cause maturity-onset diabetes of the young.
It is associated with pancreatic β-cell dysfunction or delayed response to glucose levels and substantial insulin resistance due to release of placental hormones (human placental lactogen, estrogen, and progesterone).8 GDM poses risks for the mother, fetus, and neonate.2
Screening and diagnosis for GDM can be performed using either of the following two approaches:
Performing a 75-g OGTT, with plasma glucose measurement when an individual is fasting and at 1 hour and 2 hours, at 24–28 weeks of gestation in individuals not previously diagnosed with diabetes. The OGTT should be performed in the morning after an overnight fast of at least 8 hours.
The following results indicate GDM
• Fasting: >92 mg/dL (>5.1 mmol/L)
• 1 h: >180 mg/dL (>10.0 mmol/L)
• 2 h: >153 mg/dL (>8.5 mmol/L)
Step 1: Performing a 50-g glucose tolerance test (non-fasting), with plasma glucose measurement at 1 hour, at 24–28 weeks of gestation in individuals not previously diagnosed with diabetes. If the plasma glucose level measured 1 hour after the load is >130, 135, or 140 mg/dL (>7.2, 7.5, or 7.8 mmol/L, respectively), proceed to step 2 with 100-g OGTT.
Step 2: The 100-g OGTT should be performed when the individual is fasting. The individual is diagnosed with GDM if at least two of the following four plasma glucose levels (measured fasting and at 1, 2, and 3 hours during OGTT) are:9
• Fasting: >95 mg/dL (>5.3 mmol/L)
• 1 h: >180 mg/dL (>10.0 mmol/L)
• 2 h: >155 mg/dL (>8.6 mmol/L)
• 3 h: >140 mg/dL (>7.8 mmol/L)
Artificial intelligence (AI) has been transforming every field including the medical field. A new article published in the journal Healthcare and Rehabilitation mentions how AI is transforming diabetes care.10 By analyzing data from blood sugar levels, medical history, and even retinal scans, AI tools can predict diabetes subtypes, identify high-risk patients, and tailor solutions to individual needs — with improved accuracy, reducing healthcare costs and addressing critical gaps in diagnosis, treatment, and daily management.
AI-enhanced continuous glucose monitoring systems not only merely report glucose trends but also anticipate hypoglycemic events hours in advance, offering patients critical time to intervene.10 Intelligent insulin delivery systems are now available to predict glucose monitoring and personalized treatment plans.10
Though diabetes is on the rise, artificial intelligence and remote monitoring systems have the capability to proactively monitor patients, provide personalized care, and save lives. However, care needs to be taken to ensure the data are safe and secure. Hence, healthcare professionals, IT professionals, and regulatory authorities must work together to ensure that patients benefit from newer technologies and at the same time remain safe and secure.
Scan code to go directly to the CE test.
1. Diabetes Atlas 11th Edition 2025. IDF. Published 2025. Accessed May 28, 2025. https://diabetesatlas.org/media/uploads/sites/3/2025/04/ IDF_Atlas_11th_Edition_2025.pdf.
2. American Diabetes Association Professional Practice Committee. 2. Diagnosis and classification of diabetes: Standards of care in diabetes-2025. Diabetes Care. 2025;48(Supplement_1):S27-S49. doi:10.2337/dc25-S002.
3. Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2021;64(12):2609-2652. doi:10.1007/ s00125-021-05568-3.
4. Selvin E. Hemoglobin A1c-using epidemiology to guide medical practice: Kelly west award lecture 2020. Diabetes Care 2021;44(10):2197-2204. doi:10.2337/dci21-0035.
5. Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, Natural History, and Prognosis. Diabetes. 2017;66(2):241-255. doi:10.2337/db16-0806.
6. Carmody D, Støy J, Greeley SAW, Bell GI, Philipson LH. A clinical guide to monogenic diabetes. In: Genetic Diagnosis of Endocrine Disorders. Elsevier; 2016:21-30.
7. De Franco E, Flanagan SE, Houghton JAL, et al. The effect of early, comprehensive genomic testing on clinical care in neonatal diabetes: an international cohort study. Lancet. 2015;386(9997):957-963. doi:10.1016/S0140-6736(15)60098-8.
8. Mittal R, Prasad K, Lemos JRN, Arevalo G, Hirani K. Unveiling gestational diabetes: An overview of pathophysiology and management. Int J Mol Sci. 2025;26(5):2320. doi:10.3390/ijms26052320.
9. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7):768-773. doi:10.101 6/0002-9378(82)90349-0.
10. Ma S, Zhang M, Sun W, et al. Artificial intelligence and medical-engineering integration in diabetes management: Advances, opportunities, and challenges. Healthcare and Rehabilitation. 2025;1(1):100006. doi:10.1016/j.hcr.2024.100006.

Rajasri Chandra, MS, MBA is a global marketing leader with expertise in managing upstream, downstream, strategic, tactical, traditional, and digital marketing in biotech, in vitro diagnostics, life sciences, and pharmaceutical industries. Raj is an orchestrator of go-to-market strategies driving complete product life cycle from ideation to commercialization.


If a hospital performs one million tests per year and loses one in 1,400 tubes at a cost of $400–600 per tube, then the annual cost could be as much as $428K—not to mention the burden on patients and hospital.1
By implementing Indexor, you can trace samples starting at patient draw, monitor key quality indicators during transportation, and automate time-consuming lab operations.
By Tobin Efferen, MD, MS
Syphilis has reemerged as a growing public health concern, particularly within communities affected by substance use disorders. The opioid epidemic, driven by heroin and synthetic opioids such as fentanyl, has exacerbated this resurgence by contributing to high-risk behaviors including unprotected sex and needle sharing. Comprehensive testing protocols that incorporate both syphilis and drug abuse screening can facilitate the early detection of a condition once thought to be on the decline. With early detection, targeted intervention, and improved patient management can be enacted. Medical laboratory professionals are at the forefront of this initiative, leveraging advanced diagnostic technologies to support clinicians in identifying and treating affected individuals. How can thoughtful testing strategies better serve these vulnerable populations? And where in the patient journey can these patients be best identified, tested, and treated?
Syphilis—a resurgence
Syphilis was well controlled in the late 20th century, with a primary incidence of 2.1 per 100,000 individuals in the United States and 160 cases per 100,000 individuals worldwide after targeted control campaigns in the United States and elsewhere.1,2 Starting from 2011 onward, the incidence of syphilis began to rise with a reported incidence of 178 per 100,000 globally and 11.9 per 100,000 in the U.S. by 2021.1,2
New cases of syphilis have increased 79% since 2018, and new cases of congenital syphilis have increased 183% in that time frame according to the Centers for Disease Control and Prevention (CDC). Notably, CDC reports congenital syphilis has increased 755% since 2012.3
Syphilis is easily treated once identified, but if left untreated, it can progress to a complex and often debilitating disease. It is also highly transmissible, especially in vulnerable populations such as those exhibiting high-risk sexual behavior or chronic drug abuse.4,5 Syphilis progresses through four distinct stages. Primary and secondary syphilis occur within three months to one year from exposure. There is a prolonged latent period, often lasting years, during which the illness remains transmissible. In the later latent stage, while not transmitted sexually, syphilis can be transmitted from a pregnant female to her fetus, resulting in congenital syphilis. The final stage of syphilis, known as tertiary syphilis, is not transmissible but can have devastating consequences ranging from aortic aneurysms to central nervous system symptoms including seizures, dementia, psychosis, and depression.
Current recommendations from the CDC and the U.S. Preventative Services Task Force advise yearly testing for high-risk individuals such as those who engage in behavior that increases their risk for other sexually transmitted infections.6,7 Given that high-risk individuals often have minimal to no engagement with the healthcare system, reaching them can be a challenge.
Several studies have linked increased syphilis incidence to opioid misuse, particularly among individuals who engage in transactional sex to support their addiction. Additionally, opioid users face significant barriers to accessing both primary and emergency care, delaying diagnosis and treatment of acute and chronic conditions.5
Fentanyl is currently a major driver of the opioid crisis in the United States. As of the latest data, synthetic opioids like fentanyl are involved in approximately 68% of all opioid overdose deaths.8,9 This marks a significant increase over the past few years, reflecting the growing impact of fentanyl on opioid use and overdose rates. Undiagnosed and untreated syphilis contribute to higher transmission rates. The CDC has reported that opioid-related overdose deaths were six times as high in 2019 as they were in 1999, and the incidence of blood-borne infections have dramatically increased because of the opioid crisis.10 The introduction of fentanyl and ketamine into the illicit drug supply further complicates the landscape, as their dissociative and euphoric effects can impair judgment and increase risk-taking behaviors.
The ideal opportunity to intervene with the high-risk population would be during medically supervised detox admissions or admissions to inpatient rehabilitation facilities. At the time of such intake, drugs of abuse are often tested as part of the admission order. Given the intertwined nature of opioid addiction and syphilis, medical laboratories should prioritize integrated, comprehensive testing approaches that include syphilis screening via treponemal and non-treponemal assays to detect both active and past infections. In addition, laboratories should test for HIV and hepatitis, as these infections frequently co-exist with syphilis in opioid-using populations, and drug of abuse testing for substances such as fentanyl, heroin, and ketamine to monitor therapy and inform ongoing treatment plans. Implementing these assays as part of a comprehensive screening strategy during the admission process could lead to improved identification and treatment rates. Recent advancements in diagnostic technology have enabled comprehensive drugs of abuse and
infectious disease testing using high throughput clinical chemistry and immunoassay analyzers, which offer enhanced sensitivity and specificity for syphilis serology. In addition, rapid turnaround times support timely interventions to drive clinical decision-making. By leveraging these assays, laboratories can contribute to more effective public health surveillance and intervention efforts to combat syphilis.11,12
The effectiveness of comprehensive testing is enhanced when integrated into clinical workflows through electronic medical records (EMRs). The development of standardized intake order sets for solutions from companies like Epic System Corporation and Oracle Health (previously Cerner Corporation) can facilitate the ordering of bundled tests for at-risk populations and ensure consistency in testing protocols across healthcare facilities. An added benefit to this process would be to improve data collection and trend analysis to guide public health responses to emerging and reemerging infectious diseases. Clinical decision support tools within EMRs can also be used to alert providers to co-testing opportunities, thereby increasing testing rates and timely diagnoses.
The intersection of the opioid epidemic and syphilis resurgence necessitates a multidisciplinary approach to testing and intervention. Medical laboratories play a pivotal role in identifying affected individuals through comprehensive testing strategies. By integrating infectious disease and drug abuse testing into standardized EMR-order sets and implementing testing on advanced clinical chemistry and immunoassay analyzers, healthcare providers can enhance disease detection, improve patient outcomes, and support broader public health initiatives. As the crisis evolves, continued collaboration among laboratories, clinicians, and policymakers will be essential in addressing this dual epidemic.
1. Sexually transmitted infections surveillance, 2023. Centers for Disease Control and Prevention. Updated November 12, 2024. Accessed May 28, 2025. https://www.cdc.gov/ sti-statistics/data-vis/table-sticasesrates.html.
2. Global Health Observatory: Data on syphilis. World Health Organization. Accessed May 28, 2025. https://www.who.int/data/gho/data/ themes/topics/data-on-syphilis.
3. Rise in congenital syphilis cases concerns state legislators. National Conference of
State Legislatures. Updated February 7, 2025. Accessed May 28, 2025. https://www. ncsl.org/health/rise-in-congenital-syphiliscases-concerns-state-legislators.
4. Tucker JD, Cohen MS. The old foe syphilis strikes again: social responses and collective mobilization. American Journal of Public Health. 2022;112(9):1231–1232. doi:10.2105/ AJPH.2022.306997.
5. Acheampong AB, Striley CW, Cottler LB. Prescription opioid use, illicit drug use, and sexually transmitted infections among participants from a community engagement program in North Central Florida. J Subst Use. 2017;22(1):90-95. doi:10.3109/14659891.201 6.1144805.
6. Papp JR, Park IU, Fakile Y, Pereira L, Pillay A, Bolan GA. CDC laboratory recommendations for syphilis testing, United States, 2024. MMWR Recomm Rep. 2024;73(1):1-32. doi:10.15585/mmwr.rr7301a1.
7. US Preventive Services Task Force (USPSTF), Bibbins-Domingo K, Grossman DC, et al. Screening for syphilis infection in nonpregnant adults and adolescents: US preventive services task force recommendation statement. JAMA. 2016;315(21):2321. doi:10.1001/jama.2016.5824.
8. Provisional drug overdose death counts. Centers for Disease Control and Prevention. National Center for Health Statistics. Updated May 14, 2025. Accessed May 28, 2025. https://www.cdc.gov/nchs/nvss/vsrr/ drug-overdose-data.htm.
9. National Institute on Drug Abuse. Drug overdose deaths: Facts and figures. National Institute on Drug Abuse. Updated August 2024. Accessed May 28, 2025. https://nida. nih.gov/research-topics/trends-statistics/ overdose-death-rates.
10. Data summary: Vulnerable areas for infectious diseases in persons who inject drugs. Centers for Disease Control and Prevention. Published February 16, 2024. Accessed May 28, 2025. https://www.cdc.gov/persons-whoinject-drugs/vulnerable/index.html.
11. Loeffelholz MJ, Binnicker MJ. It is time to use treponema-specific antibody screening tests for diagnosis of syphilis. J Clin Microbiol. 2012;50(1):2-6. doi:10.1128/ JCM.06347-11.
12. Park IU, Fakile YF, Chow JM, et al. Performance of treponemal tests for the diagnosis of syphilis. Clin Infect Dis 2019;68(6):913-918. doi:10.1093/cid/ciy558.

Tobin Efferen, MD, MS is a Medical Director at Beckman Coulter and an emergency department physician with over 20 years of experience in emergency medicine. His work focuses on advancing diagnostics in acute care, scouting novel biomarkers, and developing machine learning–based algorithms for emergent and critical care settings. He holds a Doctor of Medicine degree and a master’s degree in Neurobehavioral Biology from New York University. He is deeply committed to medical education for the next generation(s) of clinicians. He completed his residency at the University of Chicago, where, as Assistant Medical Director, he managed the Quality Program and directed the Emergency Medicine clerkship for six Physician Assistant programs, mentoring over 400 students.

By Mark O. Colgin II MBA, CLS
Often regarded as the grandfather of quality control, Dr. Walter A. Shewhart made a groundbreaking contribution to the field of manufacturing quality with the publication, “Economic Control of Quality of Manufactured Product,” in 1931. This practically century-old work laid the foundation for modern statistical quality control (SQC) and introduced key concepts that continue to shape laboratory medicine.
In his book, Shewhart explored fundamental principles such as statistical control, design limits on variability, and the specification of standard quality. His idea that, “we can find and remove causes of variability until the remaining system of causes is constant, or until we reach that state where the probability that the deviations in quality remain within any two fixed limits is constant.”1 This concept would influence the development of Lean Six Sigma, Total Quality Management (TQM), modern process
improvement methodologies, and the birth of statistical process control. Statistical process control introduced the notion of control charts. This tool is now widely used to monitor process stability and detect disparities that require action by the operator. Shewhart’s work is the foundation for quality and played a crucial role in shaping modern quality management systems.
Building on Shewhart’s work, in the 1950s, two pathologists, Dr. Stanley Levey and Dr. E.R. Jennings, introduced the principles of statistical quality control (SQC) to the field of clinical laboratory science. They proposed applying Shewhart’s control chart to medical laboratories to improve the reliability and accuracy of diagnostic testing. Their revolutionary paper,“The use of Control Charts in the Clinical Laboratory,” laid the foundation for a more systematic
approach to monitoring laboratory performance, ensuring consistent and high-quality test results. As a result of their work, a type of control chart was developed and named in their honor.
The Levey-Jennings chart (LJ) is a graphical tool used to plot quality control data over time. Each point represents a QC measurement, which is compared against a predefined mean and control limit. By analyzing these values, laboratory personnel can quickly evaluate whether an assay or analytical instrument is functioning correctly. If the data points fall within the acceptable range, the test system is considered in control, however, if the points fall outside the control limits or display a recognizable pattern, corrective action is necessary to prevent inaccurate patient results. This innovation enhanced the accuracy, precision, and reliability of laboratory testing, allowing for early detection of systematic errors, reagent










inconsistencies, and potential equipment malfunctions.
In 1957 while working in the Bioscience Laboratories, clinical chemists Milton and Henry Segalove made another significant advancement in the field of laboratory quality control. They began applying the Levey-Jennings chart on a daily basis. Their biggest contribution involved their structured approach to monitor performance by using multiple levels of control values.
A few years later, the use of the Levey-Jennings charts gained a broader acceptance when researchers Freier and Rausch introduced an improvement to quality control methodology. Instead of relying on traditional patient samples, they recommended the use of serum pools. These pooled samples would provide a more consistent and standardized reference for evaluating assay performance. Initially, these pooled samples were referred to as “standards,” but as their application in quality control continued, they became known as “control samples.”
The adoption of consistent control samples transformed clinical laboratory quality assurance, enabling laboratories to improve accuracy, precision, and reproducibility of testing. The advancements made by Freier and Rausch set the foundation for traditional quality controls and formed the basic concepts for quality management systems (QMS) in the clinical laboratory.
In 1977, Dr. James Westgard and his colleagues introduced what would become the widely recognized and influential Westgard rules, a groundbreaking framework for internal quality control in laboratory testing. Their paper, “Performance Characteristics of Rules for Internal Quality Control: Probabilities for False Rejection and Error Detection,” gave rise to the development of modern quality assurance in clinical laboratories. In this study, Westgard examined two distinct groups of quality control rules. The first group, which would later be known as the Westgard rules, is applied when each individual control measurement is assessed independently to determine whether a test run should be accepted or rejected. As noted in the book by Westgard, “For this group, the probability of false rejection will increase as the number of control observations that are made during the run.”2 This statement highlights a key challenge in quality control, balancing the need
for error detection while minimizing unnecessary rejections.
It took four years, but in 1981 Westgard and his colleagues published “A Multi-Rule Shewhart Chart for Quality Control in Clinical Chemistry.” This paper defined “the control rule to indicate the criterion for judging whether the observed control measurements (or observations) represent typical or atypical (stable or unstable) performance of the analytical method.”3 By developing these statistical rules, Westgard transformed laboratory quality control. They provided a methodical approach to detecting errors and ensuring the reliability of test results. Today, the Westgard rules remain a cornerstone of clinical laboratory quality control practices worldwide.
The 1980s ended with the introduction of the Clinical Laboratory Improvement Amendments (CLIA) of 1988, a landmark regulation that set new standards for laboratory testing. Officially published in 1992, CLIA established stringent quality assurance and minimum quality control requirements to ensure accuracy and reliability in diagnostics. By standardizing these requirements CLIA enhances the consistency and dependability of laboratory results. The amendments also introduced comprehensive quality assurance programs to monitor test performance, equipment maintenance, and most importantly, provide ongoing training for laboratory personnel. These standards ensure the highest level of patient care.
Bill Smith introduced Six Sigma in 1986. Its introduction into healthcare marked a significant advancement in quality control. Six Sigma is a data driven approach designed to enhance process performance by minimizing variation. In essence, it is a management system aimed at achieving near perfect quality, allowing no more than 3.4 defects per million opportunities.
Implementing Six Sigma can greatly improve the quality of control materials in laboratories. Manufacturers adhering to Six Sigma principles continuously strive to eliminate errors, beginning with the selection of raw materials, thereby ensuring a higher quality final product. This, in turn, leads to more reliable and reproducible test results, reducing deviations that could compromise patient diagnoses. By applying traditional statistical methodologies, laboratories can
more accurately assess reagent performance, ensuring stability and effectiveness throughout the product’s shelf life. This approach minimizes inconsistencies, optimizes reagent selection, and ultimately enhances diagnostic accuracy.
The evolution of quality control in laboratory medicine has been shaped by decades of innovation, rigorous re-
Six Sigma is a data driven approach designed to enhance process performance by minimizing variation.
search, and the contributions of pioneering scientists. From Shewhart’s early work in statistical process control to the development of the Levey-Jennings chart, Westgard rules, and the introduction of Six Sigma, each advancement has played a crucial role in refining laboratory testing standards. The implementation of CLIA regulations further strengthened these efforts by standardizing quality requirements and ensuring greater accuracy and reliability in diagnostic medicine. Today, these foundational principles continue to guide laboratory professionals in their pursuit of excellence, ultimately improving patient care and advancing the field of clinical laboratory science. As technology and methodologies in laboratory medicine continue to advance, the commitment to quality control remains vital in driving progress and ensuring the highest standards.
1. Shewhart WA. Economic Control of Quality of Manufactured Product. 1931.
2. Westgard JO, Groth T, Aronsson T, Falk H, de Verdier CH. Performance characteristics of rules for internal quality control: probabilities for false rejection and error detection. Clinical Chemistry. 1977;23(10):1857-1867.
3. Westgard JO, Barry PL, Hunt MR, Groth T. A multi-rule Shewhart chart for quality control in clinical chemistry. Clinical Chemistry. 1981;27(3):493-501.

Mark O. Colgin II MBA, CLS is the Core Laboratory Supervisor at Dignity Health Northridge, since 2020. He has been a licensed Clinical Laboratory Scientist (CLS) since 2012. Outside of work, he is a proud husband and father of two daughters, with a third on the way.










Respiratory season can pose a real threat to your patients, especially the elderly. Fast, accurate diagnosis and treatment is one way to protect them and get them back to doing what they love. Having the right diagnostics in your office can help you deliver a higher level of patient satisfaction and improve efficiency.
Our product portfolio includes high-quality, point-of-care molecular and antigen tests for diagnosing respiratory illnesses.
We make diagnostics that matter because we believe each test represents the health and well-being of a real person.




By Lt Col Paul R. Eden, MT(ASCP), PhD, USAF (retired); Christina Wichmann
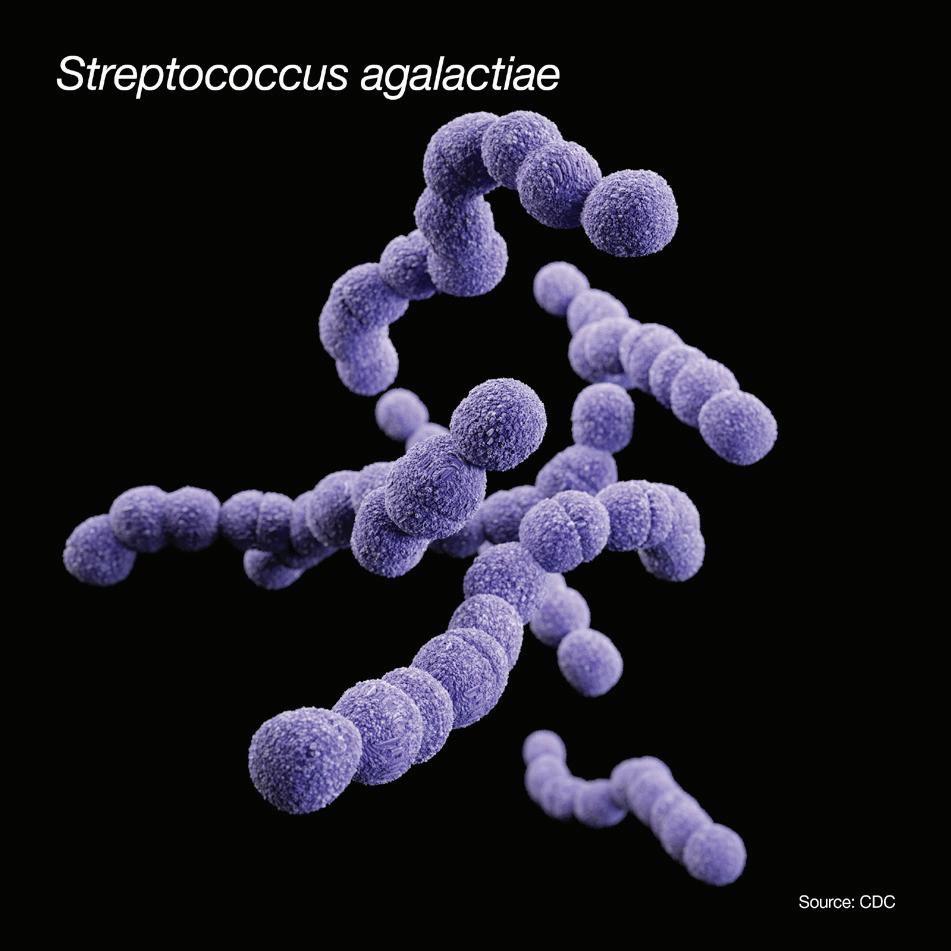
Streptococcus agalactiae , also known as Group B strep (GBS), is a Gram-positive colonizing bacterium that can cause life-threatening complications for newborns when it is passed to them from their mothers. This bacterium has been found in as many as 30 percent of healthy women,1 to whom it poses little danger. According to the American Pregnancy Association, Group B strep is found in as many as one in every 2,000 children born in the United States each year. 2
Women in the last few weeks of pregnancy are frequently tested for GBS due to the possibility of transmitting the bacteria to a newborn baby. Through screening, clinicians identify whether the baby may be at potential risk. Clinical laboratory professionals frequently test microbiology samples specifically for Streptococcus agalactiae. By utilizing established screening testing of late-term mothers, laboratory professionals assist obstetrical physicians in identifying potential threats to newborns, as well as their mothers.
The threat of Group B strep
Group B strep infection does not pose a considerable threat to the mother, but the organism may profoundly impact the health of a newborn. Presentation of GBS infection in the mother is typically found in the rectum/ lower intestinal tract and secondarily in the vaginal tract. See Figure 1 for the common sites of GBS colonization. The most common treatment is penicillin or ampicillin; in some cases, increased resistance to antibiotics have been noted. In addition, a recent study found “increased odds of MRSA carriage in GBS positive women.”1 The CDC recommends vaginal GBS screening of all women in the late stages of pregnancy.2 (Non-pregnant adults
who are immunocompromised due to cancer, diabetes, HIV, or increased age are also at-risk for GBS infection development.3)
Sites of GBS colonization in descending order
• Lower intestinal tract/rectum
• Lower vagina
• Cervix
• Urethra
Figure 1. Sites of GBS colonization. Vaginal colonization is intermittent; anorectal colonization is more constant. GBS in the urinary tract is indicative of heavy colonization.
GBS bacteria come and go naturally in people’s bodies. People may test positive for the bacteria at some times and not others. That’s why women get tested late in their pregnancy, close to the time of delivery. Hence, the purpose of screening pregnant women from 35 to 37 weeks gestation is to identify possible GBS infection for the mother and also to give an indication of the possibility that the newborn could be infected at the time of birth. Infection can occur either while the child is still in the placenta or during birth. Risk factors for neonatal GBS include prenatal colonization, premature delivery, prolonged rupture of membranes, intrapartum fever, and a prior baby with GBS.
Onset of neonatal disease is characterized by several possible health complications; however, the most common pathogenesis is meningitis. Meningitis symptoms can include fever, lack of appetite, excessive crying, vomiting, and a bulging soft spot (fontanelle). Other possible infections include sepsis (infection of the blood), pneumonia (infection of the lungs), skin and soft tissue infections, and bone and joint infections.4
Meningitis and sepsis in newborns can result in an increased mortality rate depending on complications and the child’s susceptibility to antibiotic resistance.
According to Chen et al, prior to the introduction of GBS screening in pregnant mothers, “GBS was responsible for substantial perinatal morbidity and mortality, with as many as one to three in 1,000 neonates affected.”3 Development of symptoms of GBS infection during the first six days of life is called early onset; development of symptoms between seven days and three months is called late onset.5 The addition of antibiotics to GBS-colonized women prior to the child’s birth has led to a decrease in mortality rates, from >50 percent in the 1970s to <10 percent by the 1990s.”3 However, GBS remains a significant cause of both neonatal bacterial meningitis and sepsis.”3 Testing by the clinical laboratory thus remains critical to bacterial identification and early prevention of transmission to the newborn child.
In 2021, the American Society for Microbiology (ASM) released new guidelines for detecting and identifying GBS to prevent disease in newborns.6 The GBS culture technique remains the gold standard. Nucleic acid amplification tests (NAAT) alone are not as sensitive and can produce false negatives.

Research indicates that GBS screening with NAATs offers a highly sensitive test with improved TAT and workflow compared to culture1 .
Studies have shown that the Panther Fusion® GBS assay detects 31% more positives than culture and has a shorter total turnaround time compared to other NAATs.2,3 These improvements can lead to a reduction in operating costs and more efficient use of laboratory resources, potentially saving up to $21,000 a year.3
Discover the benefits of NAAT GBS screening today.
* When compared to culture.
† When compared to other NAATs.
Detect 31% more positives*2 Turnaround in less time†2,3
Less hands-on time & sample prep3
References: 1. Filkins L, Hauser JR, Robinson-Dunn B, Tibbetts R, Boyanton BL, Revell P. American Society for Microbiology Provides 2020 Guidelines for Detection and Identification of Group B Streptococcus. J Clin Microbiol. 2020 Dec 17;59(1):e01230-20. doi: 10.1128/JCM.01230-20. PMID: 33115849; PMCID: PMC7771461. 2. Shin JH and Pride DT. Comparison of Three Nucleic Acid Amplification Tests (NAATs) and Culture for Detection of Group B Streptococcus (GBS) from Enrichment Broth. J Clin Microbiology. 2019;57(6):e01958-18. doi:10.1128/JCM.01958-18. 3. Berry GJ, et al. Comparison of the Panther Fusion and BD MAX GBS Assays for Detection of Group B Streptococcus in Prenatal Screening Specimens. J Clin Microbiol. 2019. ADS-04334-001 Rev. 001 © 2025 Hologic, Inc. All rights reserved. Hologic, Panther Fusion, and associated logos are trademarks and/or registered trademarks of Hologic, Inc. and/or its subsidiaries in the United States and/or other countries. All other trademarks are the property of their respective owners.
However, a modified testing technique that utilizes the broth enrichment step first increases sensitivity of NAAT. A brief description of the ASM guidelines is as follows:
• Use selective enriched broth that enhances growth of GBS. Incubate for 18–24 hours, 35–37 degrees.
• Culture media and GBS isolation methods should detect both hemolytic and non-hemolytic strains.
• Report GBS in any quantity from urine cultures from pregnant women during all trimesters.
• Acceptable phenotypic and proteomic methods of identification of candidate isolates include CAMP test, latex agglutination, and MALDI.
• Nucleic acid amplification–based identification of GBS from enrichment broth is acceptable, but not sufficient for all patients.
• Latex agglutination directly from enrichment broth and direct-from-specimen immunoassays are unacceptable methods for GBS detection.
• Perform antimicrobial susceptibility testing on all GBS isolates from pregnant women with penicillin allergy. For those without a known penicillin allergy, antimicrobial susceptibility testing is not required but should still be considered.
Laboratory testing for GBS has not changed significantly over the past decade and culture remains the gold standard method. However, disadvantages of culture are prolonged incubation and not allowing for point-of-care testing. Molecular methods from enrichment broth culture for GBS detection and identification are routinely used today. The shortened

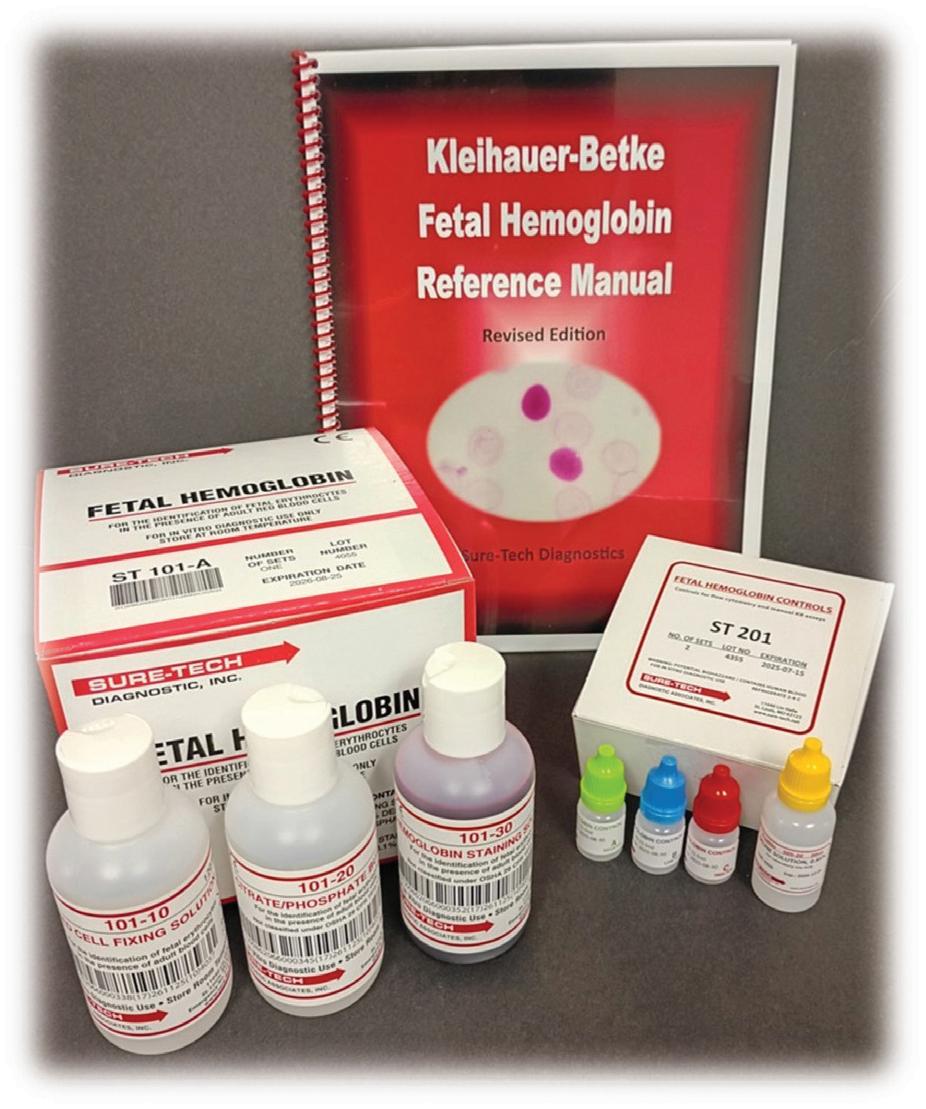
· TEST KITS
CONTROLS
REFERENCE MANUAL INFO@SURE -TECH.NET WWW.SURE - TECH.NET
turnaround time of about an hour after broth enrichment allows timely administration of appropriate antibiotic therapy for GBS positive patients.
1. Parriott AM, Brown JM, Arah OA. Predischarge postpartum methicillin resistant Staphylococcus aureus infection and group B streptococcus carriage at the individual and hospital levels. Infect Dis Obstet Gynecol. 2014;2014:515646. doi:10.1155/2014/515646.
2. Group B Strep Infection: GBS. American Pregnancy Association. Accessed May 22, 2025. http://americanpregnancy.org/ pregnancy-complications/group-b-strep-infection/.
3. Chen VL, Avci FY, Kasper DL. A maternal vaccine against Group B streptococcus: past, present and future. Vaccine. 2013;31 Suppl 4:D13-9. doi:10.1016/j.vaccine.2012.12.080.
4. Puopolo KM, Lynfield R, Cummings JJ. Committee on Fetus and Newborn, Committee on Infectious Diseases. Management of infants at risk for group B streptococcal disease. Pediatrics. 2019;144(2):e20191881. doi:10.1542/peds.2019-1881.
5. Group B streptococcal septicemia of the newborn. MedlinePlus. Updated November 5, 2022. Accessed May 22, 2025. https://medlineplus.gov/ency/article/001366.htm.
6. Guidelines for the detection and identification of Group B Streptococcus. ASM. Updated July 23, 2021. Accessed May 22, 2025. https://asm. org/guideline/guidelines-for-the-detection-and-identification-of.
This article was originally published in MLO’s July 2015 issue. It was updated in 2025 by Christina Wichmann.

Lt Col Paul R. Eden, MT(ASCP), PhD, USAF (retired) has over 25 years of laboratory experience managing both clinics and hospital laboratories including many years of applied research. He also served as Adjunct Assistant Professor, Pharmacology & Toxicology, Wright State University, Ohio.
*Also Distributed through Fisher Scientific & Cardinal Health


NEW PT PROGRAMS
• Blood Parasite (Competency) - #Q36
• Glycohemoglobin B (Verification) - #J95
• Traumatic Brain Injury (TBI) Assessment - #196
• Monkeypox (molecular) - #396
ENHANCED PT PROGRAMS
• HPV - #375 / 975
Additional analytes: HPV Genotypes (31, 33, 39, 67)
• Immunoproteins/C3 & C4 - #436
Additional analytes: Kappa light chain, Lambda light chain
NEW ADDITIONS YOU MAY HAVE MISSED
• Blood Culture - Limited - #310
• FebriDx Bacterial/Non-Bacterial Assay - #323
• Global Fever Panel - #395
• Infectious Disease Serology - #438
• M. tuberculosis Interferon-gamma - #450
• STI Panel- #392










By Alex Cameron
Laboratory professionals have long upheld a high standard of accuracy and reliability under increasingly difficult circumstances. Maintaining these outcomes will require not only perseverance, but also new approaches to sustain quality and efficiency.
Persistent staffing shortages in clinical laboratories, combined with rising test volumes, have created a critical inflection point for patient care; the effective combination of human expertise and advanced technology is setting a new benchmark for sustainable operations.
Recent data illustrates how staffing shortages are affecting laboratory professionals and highlights how automation is an essential strategy to offset the impact of staffing shortages on laboratory operations and enhance the lab’s valuable contributions to the overall healthcare system.
A recent survey conducted by the Harris Poll commissioned by Siemens Healthineers found that 39% of laboratory professionals cite limited staffing as one of their top operational challenges.1 Vacancy rates remain between 7% and 11% nationally and can reach as high as 25% in some regions. The workforce pipeline remains fragile: 28% of laboratory professionals over the age of 50 report plans to retire within the next three to five years.
Adding to the struggle, test volumes continue to rise, placing additional pressure on laboratory operations. Medical
laboratory technologists and scientists process more than 14 billion tests annually in the United States. With approximately 338,000 practicing professionals, the equivalent of one scientist now supports testing for roughly 1,000 Americans.2
It has become critically apparent that these challenges have a cumulative effect, creating operational inefficiencies that extend beyond the laboratory and affect the broader healthcare system. So, what is to be done about it?
Here are three ways laboratory automation can help solve for these pain points.
“Ensuring that employees can work at peak efficiency requires a workplace in which they feel supported. But it also requires the right tools, including automation and robotics. Automation and robotics can also create a safer workplace by reducing contamination risks.”
-Professor Christoph Keck, MD, Head of Medicover Laboratories Germany Diagnostics Services, Medicover
As Dr. Keck points out, laboratory automation is about more than volumes and turnaround time. It’s about how the lab needs to evolve to attract and retain talent. The latest research on Gen Z suggests the next generation of employees are indeed hard workers, but they don’t want to burn out doing uninspiring work.3



















The reduction in manual tasks such as sample sorting, decapping and recapping of tubes, centrifugation, verifying sample volume and integrity, and archiving and retrieving store specimens not only saves time, but can lead to increased employee satisfaction. In fact, the survey also highlighted these manual tasks as those that laboratory professionals would most like to see automated. These repetitive steps are time-consuming, physically taxing, risky due to exposure to biohazards, and prone to human error, especially under conditions of sustained staffing shortages:
• 14% of laboratory professionals reported making a high-risk error.
• 22% admitted to making a low-risk error related to documentation or repeat testing.
• 29% expressed concern about making mistakes due to feeling overworked.
Modern automation platforms are designed to address these challenges. New solutions consolidate or streamline up to 25 manual steps into largely hands-free workflows. These changes reduce time spent on otherwise tedious tasks, minimize risk associated with sample handling, and enable staff to focus on higher-value tasks that can contribute to higher colleague satisfaction—training and mentoring, for example.
One of the most common misconceptions is that automation is prohibitively expensive and only accessible to large, high-volume laboratories. Adoption of automation is widely supported by laboratory professionals themselves:
• 95% agree that automation can help improve patient care.
• 89% believe their laboratories need automation to keep up with demand.
• 91% feel that AI tools could help address unmet patient care challenges.
However, many lab managers assume that automation solutions are out of reach for small or mid-sized labs. Task-targeted automation can be a solution for labs looking for pre- and post-analytical capabilities but have space or staff limitations. Large-scale automation capabilities such as decapping, sealing, and sorting are built into standalone laboratory analyzers – even ones with a small footprint.
Whether you’re operating an independent lab, a network-affiliated lab, a

commercial reference lab, or a megalab processing thousands of samples per day, implementing laboratory automation is within reach.
Standardized, automated processes also enhance quality control and accelerate turnaround times, particularly for urgent or STAT testing. The impact of lab turnaround time on other hospital operations should not be overlooked. One multi-hospital study, for instance, found that slow lab results contributed to a 61% longer emergency department stay and a 43% treatment delay on average.4 Faster turnaround times that can improve operational efficiency enable physicians to act on results sooner, reducing idle wait times for beds or procedures—and ultimately contribute more positively to patient satisfaction scores.
3. Reinvest time where it matters most
“After more than 20 years working with total lab automation systems, I have learned from experience that although technology is important, we cannot leave people aside and that a well-automated laboratory must be a place where people can work feeling like people. For example, technicians at Hospital Clínic Barcelona now spend > 40 % of their shift on result validation and exception management instead of logistics.”
-Dr. José Luis Bedini, Core Laboratory Head at Hospital Clínic Barcelona, Spain
The real value automation offers laboratories is the ability to scale workflows more sustainably, in a way that enables more patient touchpoints, while at the same time relieving the burden
of repetitive manual work, improving safety, and enabling a greater focus on clinical oversight and expertise.
When asked how they would reallocate time saved through automation, laboratory professionals identified several high-impact activities:
• Training and mentoring employees (46%)
• Performing quality control troubleshooting (42%)
• Managing cross-departmental test sample workflows more efficiently (39%)
What’s noteworthy about lab professionals’ interest in pursuing these activities specifically—staff training, quality control, and communication with stakeholders—is that they align with industry best practices to help insulate labs from the unintended consequences of outsourcing their lab operations. As one white paper notes, “Healthcare organizations have no control over—let alone insight into—their outsourced laboratory’s hiring, training, or workforce upskilling efforts. This lack of oversight presents a real challenge to ensuring timely, quality results.”5
The antidote then is ensuring staff can focus on activities that amplify the lab’s value to stakeholders. This reallocation of time not only benefits laboratory operations, but also strengthens the clinical value laboratories deliver across the healthcare system, and can result in improved patient care.
Informatics solutions can bear the burden of automating workflows, minimizing errors, and enabling seamless data exchange that can provide labs
with unprecedented data to make timely, informed, and proactive decisions. Digitizing standard operating procedures into a smart IT system that drives automation can alleviate bottlenecks by directing testing and validating test results against rigorous quality checks and pre- and post-processing procedures.
Scaling operations to meet rising test demands and delivering at peak performance becomes the standard operating benchmark when the lab’s operations are improved with quantitative insights that can be acted upon.
A stronger future built on human and technological
Laboratory professionals have always been central to evidence-based clinical decision-making. Automation is a tool that can extend their impact, not replace it. The path forward is not about choosing between people and technology. It is about equipping laboratory professionals with the tools they need to revive their professional growth, sustain excellence, innovate with confidence, and continue serving as indispensable partners in patient care.
1. AACC whitepaper on overcoming lab staffing shortages. Myadlm.org. Accessed May 27, 2025. https://myadlm.org/advocacy-andoutreach/adlm-policy-reports/2023/ adlm-whitepaper-on-overcominglab-staffing-shortages.
2. Our lab testing capacity is getting dangerously low. Medpagetoday.com. Accessed May 27, 2025. https://www.medpagetoday. com/opinion/second-opinions/98415.
3. GWI. Gen Z: Exploring the behaviors, preferences, and priorities of a new generation. GWI. https://www.gwi.com/ reports/gen-z/explore?submissionGui d=e0baa08d-ce23-4706-9a0b-18615d700e09.
4. Dawande PP, Wankhade RS, Akhtar FI, Noman O. Turnaround Time: An Efficacy Measure for Medical Laboratories. Cureus. 2022;14(9):e28824. Accessed May 27, 2025. https://www.cureus.com/ articles/108313-turnaround-time-anefficacy-measure-for-medical-laboratories#!/.
5. ARUP Laboratories. The hidden costs of outsourcing digital pathology. Accessed May 27, 2025. https://go.aruplab.com/ l/127321/2025-01-22/57fcxw/127321/1737563 989v8O3lquh/Hidden_Costs_of_Outsourcing_Digital.pdf.

Alex Cameron is Head of Marketing for Atellica Solutions at Siemens Healthineers . He oversees global strategy and execution for the company’s automation, informatics, and systems portfolios, including Atellica Integrated Automation and the Atellica family of clinical analyzers.

Haptoglobin
Hemoglobin A1c
IgA, IgG, IgM
Insulin
Krebs von den Lungen-6
Lipoprotein(a)
Microalbumin
Prealbumin
Remnant Lipoprotein Chol.
Rheumatoid Factor
Transferrin UIBC

By Talia Hadad
Inventory management is seamless — until it isn’t. Suddenly, a mislabeled bottle causes a test to go wrong, or a missing reagent means that critical results are delayed.
Despite this, most labs continue to use manual inventory management methods that have been developed over the past hundred years. It is now time to explore the potential to not only streamline inventory management but also to focus on how inventory management can increase the speed, accuracy, and collaboration within a modern lab environment.
It’s time to take a new approach.
The microwave oven was invented through an aha moment when a researcher’s chocolate bar melted in his shirt pocket walking through a radar test room. There’s something in your shirt pocket, backpack, or on your desk that could transform inventory management —your mobile device.
Imagine being able to update stock levels mid-protocol without having to find pen and paper, enter data into a spreadsheet, or dash back to your desktop computer. Mobile apps used directly at the bench can streamline how stock is controlled, reported, tracked, and even reordered.
“Walking
Within any lab, it is the medical lab scientist’s job to scan, uncap, and measure, so they always know what’s happening
with inventory at any point in time. However, they are generally considered “reporters” only — entering the data into the system but not ensuring that actions are taken.
Instead of being passive members of the lab “supply chain,” laboratory staff can use mobile inventory management systems to not only report but also to act. When they add the data directly to their mobile devices concerning their usage of the dwindling supply of a reagent — directly from the bench — the system can use automation to add that reagent to the lab’s running shopping list within its inventory ordering system.
With real-time reporting and ordering, the medical laboratory scientists themselves can ensure that all supplies are on hand. More accurate stock levels mean fewer bottlenecks and faster and smoother operations. No one is waiting for that last solution to arrive before they can start their testing.
One lab, one MLS, and one test are definitely not how things work. In reality, labs have multiple teams working on a variety of tests — but only one can use the last bottle of XYZ reagent — because someone forgot to check one of the manual spreadsheets before placing the last order.
A mobile inventory management system works as a dynamic, self-correcting inventory system. Every time any
lab professional makes an update, those updates are correlated with actual inventory levels, lab requirements, and lab order systems — all in real time. Now multiply that across a few laboratory professionals in a single lab or an entire complex of lab professionals across many labs.
What is ideal about a mobile lab management system is that it doesn’t add to laboratory staff workloads (which may involve racing to other labs to see if they still have a batch of that critical reagent). Instead, it reduces stock discrepancies and outages.
Based on the lab type, compliance is either a minor hassle or a major challenge. When it is compliance audit time, what is easier — trying to collate and correlate inventory with spreadsheets for cold storage, freezer storage, hot storage and then cross referencing everything with the order…or downloading a real-time report from the inventory management system.
Laboratories need to be compliance ready at any point as well as improve their resource efficiency, streamline operations digitally , and grow testing operations — these can only benefit by “going mobile.” It is no longer a nice to have but a critical tool in the modern laboratory.
Managing remote, hybrid, and on-site
The “static” lab no longer exists – and the ways of “static” inventory systems are long gone.
Now, labs operate across multiple locations, with hybrid operations or flexible staffing. Agile mobile tools allow everyone to stay on the same page, coordinating activities, keeping everything up to date in real time, ensuring traceability, all while preserving the institutional memory.
Sharing responsibility while ensuring clarity
Inventory management should not be one person’s responsibility; it should be everyone’s. When using real-time inventory tracking with a mobile app, lab operations are smoother, smarter, and everyone is more accountable.
Thus, everything they need is in the right place at the right time, ensuring agility, efficiency, and accuracy – with easier regulatory compliance as a bonus.
REFERENCES
1. Digital transformation in life sciences: Rethinking clinical labs for the future. Deloitte. Accessed June 2, 2025. https://www2.deloitte.com.
2. Patel R, Shah A. Human error in laboratory inventory management: Causes and remedies. J Lab Autom. 2017;22(1):15–22. doi:10.1016/j. jala.2017.01.004.
3. Clark C, Watson H, Wyn S. GAMP 5 guide 2nd edition. ISPE | International Society for Pharmaceutical Engineering. Accessed June 2, 2025. https://ispe.org/publications/guidance-documents/gamp-5.
4. 21 CFR Part 11 – Electronic Records; Electronic Signatures. U.S. Food and Drug Administration. Published 2003. Accessed June 2, 2025. https://www.fda.gov/regulatory-information/ search-fda-guidance-documents.
5. Global Laboratory Informatics Market, Forecast to 2025. Frost & Sullivan. Accessed June 2, 2025. https://www.frost.com.
6. How digitization is transforming lab operations. McKinsey & Company. Accessed June 2, 2025. https://www.mckinsey.com.

Talia Hadad is product manager at Labguru, where she oversees Labguru’s complementing mobile application Labhandy and works closely with lab managers to simplify work on the go.



































By Angela Newman, MBA-HM, BSN, RN, CCRN, VA-BC
As the gold standard in healthcare for diagnosing and monitoring dangerous bloodstream infections, the number of blood cultures conducted annually in the United States has been reported to be over 30 million1 and as high as 58 million. 2 Blood draws for culture are primarily performed in hospitals, laboratories, and physician offices — with mobile and non-acute setting collection services currently on the rise.
Blood collection is crucial in patient care in every healthcare setting, with more and more providers recognizing blood culture kitting as a key strategy to improve patients’ experiences and outcomes, lower contamination rates,3 and promote clinical efficiency.
Lowering contamination rates, increasing efficiency, and reducing waste
Contamination and false positives in blood culture are persistent problems in healthcare. Inaccurate blood culture results can lead to delayed treatment or unnecessary antibiotic therapy, more procedures, longer lengths of hospital stays and even increased infection or harm to patients — all translating into additional healthcare costs.
Blood culture kitting simplifies decision-making, reduces touchpoint contamination, and decreases variation in practice by providing clinicians with a pre-loaded kit that contains all the components required for sample collection. Using kits also supports systems’ evidence-based collection protocols.
Standardization provided by kitting allows clinician and technicians — regardless of discipline, level of experience, or newness to a role or hospital system — to focus on patient care and clean collection practices versus supply gathering
and tracking. Clinicians that use kits know they have the right materials (and the correct amount) needed to do proper blood collection, minimizing trips back-and-forth to the supply area during collection.
Discussions about developing or procuring blood culture kits are typically triggered when a lab or system experiences an increase in blood culture contamination rates; when costs for collection materials are incongruent with numbers of tests performed; or, when labs or systems are looking for areas to improve quality and delivery of care overall. Kitting can also support staffing issues like staff shortages, burnout, and safety.
A good practice when launching a blood culture kitting project is for the laboratory to lead and invite representation from all the disciplines and departments that use or may be involved in procuring a blood culture kit, i.e., nursing, phlebotomy, perioperative, infection prevention, supply chain, materials management, and other stakeholders. Build off of your organization’s evidence-based practices around blood culture collection and examine current and potential industry compliance standards. Assess your organization’s quality initiatives, gathering benchmarks and establishing metrics against which success of the kitting project will be measured. Metrics can include blood culture contamination rate, kit utilization, and staff feedback.
For stakeholders who may evaluate kitting projects solely on increased costs, it is a good idea to break down how kitting costs compare to the time and resources spent pulling single-sterile items for every blood culture, addressing contamination issues impacting patient care along with re-doing work-ups, and accounting for supply waste generated by clinician error. Stakeholders may be interested in recent data indicating that using blood culture collection kits lowered both contamination rates and costs,3 resulting in an annual net savings of nearly $500,000.3 And, costs of one contaminated blood culture event have been estimated to be between $4,000 and $10,000.4 Keep in mind that building a blood culture kit goes a long way in supporting or reinforcing your organization’s sterility and aseptic techniques and protocols across all points of care.
One effective, hands-on exercise for kit building is to bring groups — by disciplines, divisions and care settings — into a space
displaying all the available options for the various components required for blood culture collection. Then, assign each team to build the “ideal” blood culture kit and compare the results. Identifying which items each group prefers or passes over, after handling the actual items, can be an indicator of the cost-effectiveness of including certain items in a kit and sets up a good-better-best scenario on proposed kits.
This exercise can identify any variation of technique in disciplines and can ultimately streamline best practices and eliminate the question of “what-do I-grab” for clinicians. In some systems, this exercise may reveal that more efficiency can be realized by developing more than one kit. For example, a blood culture kit for nurses may need to be different than a kit for phlebotomists.
Common blood culture kit items include needle, tourniquet, sterile blood culture bottles, antiseptic skin prep, disinfectant for the materials, and gloves. Including updated technology in kits for blood culture collection — such as blood diversion devices, diversion tubes and retractable safety needles — can also promote adoption and utilization of the safer collection components.
When effectively implemented and supported by continuous education and awareness among all staff, blood culture kitting can enhance patient outcomes and boost efficiency by optimizing workflows and minimizing waste.
Hospital labs rely on quality specimen collection to accurately diagnose patients. Sample contamination can lead to adverse events for the patient and facility. Collection kits are an efficient tool for standardizing collection processes and driving best practice that can lead to improved results.
1. Liaquat S, Baccaglini L, Haynatzki G, Medcalf SJ, Rupp ME. Patient-specific risk factors contributing to blood culture contamination. Antimicrob Steward Healthc Epidemiol. 2022;2(1):e46. doi:10.1017/ ash.2022.22.
2. Large-scale, peer-reviewed study quantifies multiple devastating patient harms associated with blood culture contamination and most significantly, a 74% increase in risk of in-hospital patient mortality. Magnolia Medical Technologies. January 24, 2023. Accessed May 16, 2025. https://magnolia-medical.com/news/large-scale-peer-reviewedstudy-quantifies-multiple-devastating-patient-harms-associated-withblood-culture-contamination-and-most-significantly-a-74-increase-inrisk-of-in-hospital-patient-mortalit/#.
3. Self WH, Talbot TR, Paul BR, Collins SP, Ward MJ. Cost analysis of strategies to reduce blood culture contamination in the emergency department: sterile collection kits and phlebotomy teams. Infect Control Hosp Epidemiol. 2014;35(8):1021-1028. doi:10.1086/677161.
4. Garcia RA, Spitzer ED, Beaudry J, et al. Multidisciplinary team review of best practices for collection and handling of blood cultures to determine effective interventions for increasing the yield of true-positive bacteremias, reducing contamination, and eliminating false-positive central line-associated bloodstream infections. Am J Infect Control 2015;43(11):1222-37. doi: 10.1016/j.ajic.2015.06.030.

Angela Newman, MBA-HM, BSN, RN, CCRN, VA-BC is the Senior Director of Clinical Services-Acute Care at Medline. She leads Medline’s perioperative, critical care, respiratory, and skin health clinical teams. Her clinical experience includes adult critical care. She is an active member of a variety of clinical professional organizations, including partnerships with the American Nurses Association, American Association of Critical Care Nurses, Sigma Theta Tau, Association for Vascular Access, and the Association for Professionals in Infection Control and Epidemiology. Angela holds a Bachelor of Science in Nursing from Rush University and an MBA in healthcare administration from Western Governors University.

wslhpt.org/mlo wslhpt.org/mlo

The D z-Lite c270 is a fully automated benchtop clinical chemistry analyzer capable of performing up to 270 tests per hour. It supports 1- to 4-reagent assays and offers a menu of over 80 specialty and general chemistry assays, all within a compact footprint suitable for labs of various sizes.
Diazyme

The DS2 2-plate open system offers sophisticated ELISA automation to deliver maximum performance and precise, reproducible results. It easily integrates into fastpaced laboratories with its incredibly small footprint and has the flexibility needed for a wide range of diagnostic applications, allowing you to customize your assay menu for increased testing demand.
DYNEX Technologies
Stat Profile Prime Plus whole blood critical care analyzers, now with micro-capillary sampling, provides blood gas, electrolytes, metabolites, renal, hematology, estimated plasma volume (ePV), and CO-Oximetry testing, with a full test menu of pH, PCO2, PO2, Na, K, Cl, iCa, iMg, Glucose, Lactate, BUN, Creatinine, Hct, Hb, SO2%, MCHC, CO-Ox, and ePV.
Nova Biomedical
The FDA-approved Auto-Pure 2400 liquid handling platform is designed to automate the T-SPOT.TB test, improving laboratories’ productivity while maintaining superior clinical performance in latent tuberculosis detection. The Auto-Pure 2400 combines liquid handling with magnetic cell isolation technology, reduces hands-on time, and delivers accurate, high-throughput TB testing— for in vitro diagnostic use.
Revvity

Sebia pairs the dIFine Automated Microscope with the dIFine P30 Processor. The P30 is our easy-to-use, efficient walk-away benchtop instrument for IFA slide processing. The dIFine Automated Microscope is the next-generation in IFA imaging, interpretation, and pattern recognition of Sebia IFA ANA HEp-2, dsDNA and ANCA slides.
Sebia

The QuantStudio 7 Pro Dx Real-Time PCR System is a standalone instrument with interchangeable 96- and 384-well blocks. It features RUO, IUO, and IVD modes, 1.5-fold sensitivity, and 10-order dynamic range. Its Applied Biosystems Diomni software supports multiple assays run with automated results interpretation, QC, LIS connectivity, sample tracking, and fleet management.
Thermo Fisher Scientific
Delivering consistent high-quality results, the RX imola is ideal for medium to high throughput laboratories. Capable of performing 400 photometric tests and 560 tests per hour including ISE, the RX imola comprises the RX series extensive test menu and excels in performance and functionality to provide rapid, comprehensive testing.
Randox


For the first time in point-of-care blood gas testing, hemolysis is flagged in just 45 seconds on the new GEM Premier 7000 with iQM3. Continuously monitoring the analytical process, before, during, and after each sample measurement, it detects, corrects, and documents sources of error, for quality-assured results. Werfen

















































Effortlessly upload results from your LIS and eliminate the #1 cause of PT failure--clerical errors.
• No changes to our firewall or security
• No interface required or software to install
• No additional fees—API DataDirect is free
Join the over 9 million results uploaded utilizing DataDirect!

American Proficiency Institute — Visit us at ADLM Booth #1234
Smart transfer systems allow for a compact footprint while allowing full automation through consolidation. The AUTION EYE can be operated stand alone or connected to the AUTION MAX series—resulting in a very small footprint (41.9” x 25.6” x 23.6”).

ARKRAY USA Inc.
Visit us at ADLM Booth #1463
NG-Test® CARBA 5 is the only rapid, multiplex, phenotypic, lateral flow, FDA cleared assay capable of detecting KPC, OXA-48-like, VIM, IMP and NDM carbapenemases produced by Enterobacterales and Pseudomonas aeruginosa. Detects the gene expression!
Hardy Diagnostics

Visit us at ADLM Booth #451

Repetitive manual Decapping and/ or Recapping of tubes exposes your staff to potential repetitive stress injuries. We offer a variety of models to fit any volume needs. Our Pluggo Decappers and KapSafe Recappers will eliminate potential injuries.
Universal and system specific kits available for your instrument such as Abbott, Beckman, Ortho, Roche, Siemens, and MORE!

AUDIT MicroControls
Visit us at ADLM Booth #3827

LIAISON PLEX® bloodstream infection assays provide the flexibility to only test for clinically relevant targets based on Gram stain of a positive blood culture. Learn more about the panels designed to support your diagnostic and antimicrobial stewardship programs.
Diasorin — Visit us at ADLM Booth #1431

The KRONUS 3-Screen Islet Cell Autoantibody ELISA Kit (KR7780) is for the simultaneous and non-differential detection of autoantibodies to GAD and/or
IA-2 and/or ZnT8 in a simple, highlyrobust, and user-friendly assay format.
KRONUS, Inc.

This test simultaneously detects and differentiates between COVID-19, Flu A, and Flu B in only 10 minutes, allowing healthcare providers to make more informed decisions when treating patients who have similar symptoms, but different treatment protocols.
SEKISUI Diagnostics
Visit us at ADLM Booth #1013

Sysmex’s innovative portfolio of hemostasis analyzers, reagents and controls are designed with quality, reliability and efficiency in mind. We provide advanced hemostasis technology across multiple platforms to match the needs of laboratories of all sizes.
Sysmex America Inc. — Visit us at ADLM Booth #2212
The Beckman Coulter DxH 900 hematology analyzer offers valuable advanced platelet analysis, providing high-quality platelet counts, without the need for additional modes, reagents and costs. Discover it at ADLM booth 2803.

Elevating performance for workflow optimization

ACL TOP® Family 70 Series Hemostasis Testing Systems optimize workflow with a powerful combination of quality and efficiency—for the entire laboratory network. Offering centralized data management, automation and connectivity, and automated pre-analytical sample checks.
Werfen — Hemostasis Visit us at ADLM Booth #2023


For more than two decades, Lisa-Jean Clifford has been a noteworthy leader in the high-tech healthcare solutions space. Lisa-Jean’s passion for making a positive impact on the lives of patients through technology can be traced back to her tenure at McKesson and IDX, now GE Healthcare, where she served in vital business development and marketing roles, and to Psyche Systems, an LIS solution provider, where she was the CEO for eleven years. She is currently the President at Gestal t Diagnostics
Now, recognized as an industry expert, she actively participates in numerous boards including the Association of Pathology Informatics where she serves as President and MLO’s Editorial Advisory Board. She is widely published in many top laboratory publications and noteworthy news sources, such as Forbes, CAPToday, Medical Laboratory Observer, and Health Data Management. Also, she is a highly sought-after speaker and focuses on delivering valuable content in critical areas such as lab automation including software and interoperability, digital pathology, AI in pathology, lab informatics, oncology, and women’s health.
The projected employment needs for pathologists are expected to grow by 5% through 2033 according to the Bureau of Labor Statistics. At the same time, there are shortages and burnout in the lab. How do you see technology helping with recruitment, retention, and employee satisfaction?
Technology provides a great way to leverage existing resources as well as to fill in gaps where tasks can be automated. There are several key areas:
By Christina Wichmann
• Digital pathology / IMS solutions are already proving their impact in the ability to leverage significant efficiency gains with pathologists and in reducing turnaround times and fatigue in mundane tasks. By automating many manual processes and being able to integrate with other applications in the lab to provide the pathologist with streamlined workflows and all of the information they need to diagnose their cases the first time they look at them, we are finding up to 40% efficiency gains and time savings.
• These solutions are also helping healthcare organizations and labs hire and retain pathologists by improving job satisfaction and by increasing their pool to draw from. By not requiring pathologists to relocate or enabling them to work part-time remotely, you are able to draw from pathologists regardless of geographic location. You are also able to leverage retired or outgoing pathologists who may still want to work a day a week or a week a month from their retirement location.
What are some of the artificial intelligence capabilities you see coming in the future of digital pathology?
AI algorithms are now being deployed in these areas:
• As we mainly know them in healthcare as a diagnostic aide. These are increasing speed and accuracy. They are also providing a second set of eyes for the pathologists enabling them to focus on the important areas of diagnosis while algorithms can quickly and accurately do computational work that used to take minutes and reduce that to fractions of a second.
• AI can also be used in things like data research and operational areas for healthcare organizations. For example, if a pathologist needs to research a particular finding or validate a complex diagnosis, they can use AI to find similar cases and information quickly.
• Another area that AI can be used in is for operational efficiencies within
an organization. For example, you can identify areas of consistent inefficiency to determine root cause and improve processes, you can generate reports for key findings automatically and compare those instantly to past outputs, again determining operational areas to focus on. Inventory management, reporting, staff retraining, billing, are all areas where AI can show vast improvements for healthcare organizations.
are ways that digital pathology can make a laboratory more competitive?
There are several — increase accuracy, reduce legal issues (missed diagnosis), increase pathologist job satisfaction (i.e., retention), improved ability to hire in a competitive environment, improve speed and efficiency, and increase business lines or lines of revenue. With digital you can increase your outreach work, provide access to cases for remote customers to diagnosis same day, and reduce shipping and courier costs and the staff associated with those. You can also build a data and image bank that you can leverage for increased revenue.
I read on your company bio that you have a horse farm! Could you tell us more about that?
I can talk all day about our home and horses! I have had horses since I could walk and was thrilled to find that both of my children shared that passion. We both rescue, and invest in, OTTBs (Off the Track Thoroughbreds) to retrain them and give them a second career as event horses or just as companions for easy riding. Whatever they are capable of after their lives on the track, either mentally or physically. My oldest daughter graduated from University of New Hampshire with an Equine Science degree, and she is my barn manager and trainer. Of course, with our land and buildings, people who know me also know I have a huge soft spot for any being in need or distress. So, I have somehow also ended up with a pair of Nigerian dwarf goat brothers — who just turned 21 and a father and son pair of American Guinea Hogs (similar to pot belly pigs) and two dogs. There is never a dull moment around here!












Calibration VerifiCation/linearity and daily QC
Providing value to our customers through:
• A broad line of superior quality universal & analyzer specific products.
• AUDITOR QC, a free and easy to use online data reduction service providing “instant reports”.