A NNUAL REPORT 2022









Now in its 6th year of operation, the Michigan-Pittsburgh-Wyss Regenerative Medicine Resource Center continues to advance promising therapies and serve as a resource hub for technology translation in the dental, oral, and craniofacial space. We are deeply grateful to all of our partners, who relentlessly dedicate their time and expertise in the pursuit of our mission to shepherd these technologies to the clinical marketplace.
This report is a showcase of achievements by the individual Interdisciplinary Translational Projects and by the Resource Center, which are made possible through the continued support and commitment from our community and NIH. We share this report with enthusiasm, and look forward to expanding our efforts to serve and make an impact on the broader regenerative medicine and the dental, oral, and craniofacial research ecosystems.
Thank you for your continued support of the Michigan-Pittsburgh-Wyss Regenerative Medicine Resource Center.
David H. Kohn, PhD | University of Michigan
William V. Giannobile, DDS, DMSc | Harvard University
David J. Mooney, PhD | Wyss Institute

Charles S. Sfeir, DDS, PhD | University of Pittsburgh
William R. Wagner, PhD | University of Pittsburgh
The Michigan-Pittsburgh-Wyss Regenerative Medicine (MPWRM) Resource Center is one of the two national Resource Centers established by the National Institute of Dental and Craniofacial Research (NIDCR)’s Dental Oral and Craniofacial Tissue Regeneration Consortium (DOCTRC) initiative, under award numbers U24DE026915 & U24DE029462. With the overarching goal of developing clinical trial-ready tissue engineering/ regenerative medicine products and protocols, the DOCTRC initiative provides funding and resources through the Interdisciplinary Translational Project (ITP) program administered by the two national Resource Centers.
The MPWRM Resource Center brings together a multi-disciplinary team of clinicians, engineers, scientists, and technology commercialization and regulatory experts from academia and industry. This team supports the regenerative medicine research community by providing resources and expertise to guide innovations that address unmet clinical needs for the regeneration or restoration of DOC tissues. Through its Core Services and Resources, the MPWRM Resource Center strives to accelerate the translation of promising technologies towards clinical trials and beyond.
The Interdisciplinary Translational Project (ITP) program seeks to identify promising technologies that address clear unmet clinical need with market potential in the dental, oral, and craniofacial (DOC) space, and to catalyze the clinical translation of these technologies towards FDA submissions. The long-term goal is to achieve high-impact outcomes in the clinical marketplace.
Cores are at the heart of the MPWRM Resource Center, and support ITP technology translation. In addition to the scientific/ technical/ analytical services, the network of experts that comprise the Resource Center provides an integrative approach to de-risk technologies. All ITP teams collaborate with the various cores to understand customer needs to position the technology for a successful commercialization and clinical adoption.
Identify and navigate strategic regulatory pathways, prepare and facilitate FDA submissions
Assist in the implementation of design controls
Protocol/ SOP/ study development and planning (exploratory, regulated)
Engagement with CROs (prequalification, due diligence, study monitoring, report review)
In-depth interviews with key opinion leaders to understand clinical/ industry needs
Development and facilitation of user handling studies
Pitch deck and investor/ partner outreach plan development and execution
Quantitative market modeling and market mapping
MicroCT, histology, biomechanics, materials fabrication/ characterization
Prototyping, manufacturing, method/ process development, drug release profiling
The MPWRM Resource Center brings together a multi-disciplinary team of clinicians, engineers, scientists, and technology commercialization and regulatory experts from academia and industry to support the regenerative medicine research community by providing resources and expertise to guide innovations that address unmet clinical needs for the regeneration or restoration of DOC tissues.
Expertise of the MPWRM Resource Center is available to the broader DOC research community through the Translational Research Consult Program.

The Translational Research Consult Program provides an in-kind one-hour teleconference consultation with one of the MPWRM Resource Center’s members with expertise in a relevant topic, in response to information provided by the applicant through an online application process.
How might the envisioned product be classified, what elements affect this classification, and what are the pathways associated with that classification?
What Good Laboratory Practice (GLP) protocol requirements might be relevant to the envisioned product?
For more information, please visit our website: https://mpwrm-doctrc.pitt.edu/core-servicesresources/translational-consult-program/
What factors should be considered as first target indication is defined?
• Ostiio receives SBIR award from NIDCR and publishes in Plast Reconstr Surg and J Craniofac Surg
• GreenMark Biomedical secures follow-on investment

• Dr. Taboas wins an award from the UPittsburgh grant program

• Oraxsys Therapeutics : ITP technology leads to new company formation with an investment partner
• DOCTRC presents a poster at the 2022 AADOCR Annual Meeting
• Patent issuance: Emergence Dental, US 11,317,955
• 2022 MPWRM Resource Center Retreat | virtual


– Keynote presentation: Fundraising for Advancing your DOC Technology Towards Commercialization and Successful Clinical Adoption
– Interactive discussions: Building a Compelling Pitch Deck
– Stakeholder listening sessions: ITPs



• Drs. Bertassoni & Yelick form a new company, RegendoDent
• Amend Surgical receives funding from the Institute for Commercialization of Florida Technology
• Patent issuance: Dr. Little/ Oraxsys Therapeutics, US 11,413,245
• DOCTRC hosts Innovator Networking Series: Medical and Dental Reimbursement
• Dr. Almarza, publishes Resource Centersupported GLP study in PlosOne



• MPWRM RC Operating Committee meetingwelcomes new OC members
• ITP teams present and attend Forsyth dentech
• Dr. Feinberg receives Best Abstract award at American Association of Oral and Maxillofacial Surgeons Annual Meeting, engages with The Tissue Foundry at BioFabUSA
• RevBio receives UK approval to initiate a clinical trial , NIH SBIR, selected as Accelerator Companies by MedTech Innovator

• MPWRM RC issues a request for proposals for the ITP program
• MPWRM RC launches Core of the Month newsletter section
• 3rd Annual DOCTRC Retreat | Ann Arbor, MI
– Keynote presentation: Building A HighPerforming Organizational Culture to Nurture Successful Innovations
– Panel discussion: Clinical Trial Planning & Funding

– ITP networking session
– Dental industry advisory meeting
– GLP certification session
• DOCTRC co-hosts 2022 AADOCR Fall Focused Symposium | Ann Arbor, MI



• RevBio’s bone adhesive flies on SpaceX CRS-26

Clinical Need – Over 1M dental bone grafting procedures are performed annually in the US, most frequently before dental implant placement. In the most challenging procedures, revision rates may reach ~25% due to the difficulty of reliably regenerating sufficient bone for implant placement. Currently used barrier membranes, meshes, and fixation systems are unable to offer the form-stability needed to protect healing grafting sites from mechanical insults, while also offering resorbability and gingival tissue friendliness. The inability of regenerative products to offer these three features result in dental bone grafting procedures that are highly technique-sensitive, prone to adverse events, and require invasive removal procedures.
Solution – The AmpliMag Bone Grafting System provides the form-stability and gingivaltissue friendliness needed to minimize adverse events and maximize bone regeneration. The system is fully resorbable which eliminates the need to retrieve hardware following healing. The AmpliMag system is based on a patented magnesium alloy developed by nanoMAG and device designs developed at the University of Pittsburgh.
Competitive Advantage – No other barrier membranes or meshes offer both form-stability and resorbability which, taken together, enable maximization of alveolar ridge augmentation while obviating the need for device removal.
ITP Support – The ITP program has provided financial support for design, manufacturing, and benchtop and pre-clinical testing activities for the AmpliMag barrier membrane. Additionally, the Resource Center has provided expert clinical, market, regulatory, and quality advice.
Brown et al. Porous magnesium/PLGA composite scaffolds for enhanced bone regeneration following tooth extraction. Acta Biomater 2015
US11,317,955 Magnesium enhanced/induced bone formation
FOUNDATIONAL PUBLICATION INTELLECTUAL PROPERTY ANTICIPATED
Device, initial de novo classification as one product system (mesh and fixation system) with a follow-on 510(k) to permit marketing of the fixation system separately
REGULATORY PATHWAY ANTICIPATED COMMERCIALIZATION STRATEGY

Michigan-Pittsburgh-Wyss Regenerative Medicine Resource Center is supported in part by the National Institute of Dental & Craniofacial Research of the National Institutes of Health under Award Number U24DE029462. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
 Andrew Brown, PhD Emergence Dental, Inc.
Stephen LeBeau, PhD nanoMAG, LLC
Andrew Brown, PhD Emergence Dental, Inc.
Stephen LeBeau, PhD nanoMAG, LLC
Clinical Need – Periodontitis is one of the most pressing oral health concerns today, affecting nearly half of adults over the age of 30 in the U.S. When left untreated, patients may require dental implants and bone grafting procedures. Antibiotics are currently used as an adjunct therapy to scaling and root planing (SRP), which remain the current gold standard of care for periodontitis. However, recent insights highlight the central role of chronic inflammation in the pathology of periodontal disease and it is this overactive immune response that is responsible for most of the damage and disease progression. Thus, new treatment modalities that target inflammation directly in the oral mucosa are greatly needed.
Solution – A team at the University of Pittsburgh led by Dr. Steven Little and Dr. Charles Sfeir, DDS has identified a non-antibiotic, controlled release microparticle systems that repairs the underlying immune-dysfunction responsible for tissue degeneration in periodontitis. This work has led to the founding of Oraxsys Therapeutics, a biotechnology company that is building on Dr. Little and Dr. Sfeir’s work to further develop a microparticle-based therapeutic that recruits regulatory T cells to the oral mucosa, controls chronic local inflammation, and induces immune-homeostasis, thereby reducing the destruction caused by periodontitis and promoting tissue regeneration.
Competitive Advantage – By targeting the underlying chronic inflammatory pillar of periodontitis (a novel treatment strategy that would synergize with SRP), an immune-modulatory, controlled release product, is thought to overcome the current limitations in the treatment of periodontal diseases, peri-implantitis, and other oral inflammatory conditions.

ITP Support – The goal of the work under the ITP program is to develop clinicalgrade manufacturing and sterilization protocols to produce quality-controlled product for pharmacokinetic testing and toxicology studies in support of an IND submission and ultimately clinical testing.
Glowacki et al. Prevention of Inflammation-Mediated Bone Loss in Murine and Canine Periodontal Disease via Recruitment of Regulatory Lymphocytes. Proc Natl Acad Sci USA 2013
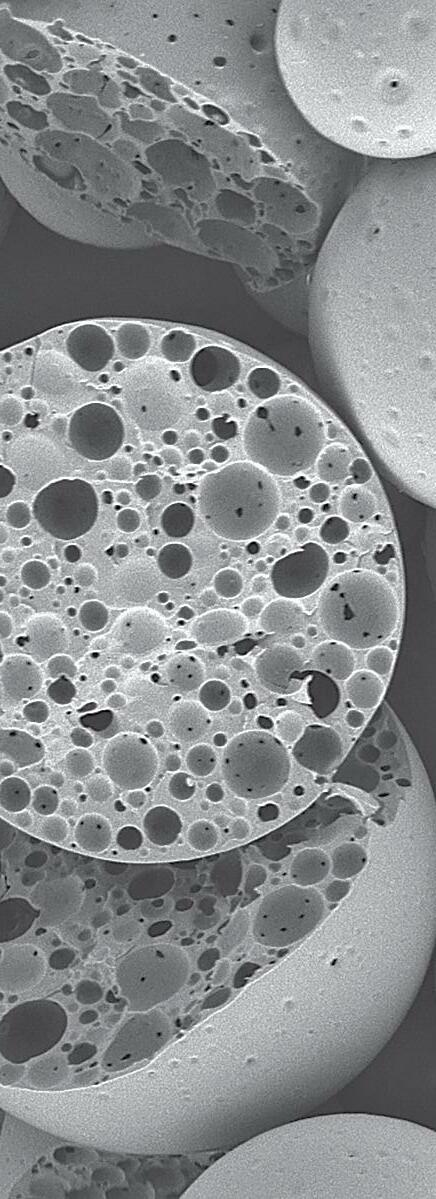
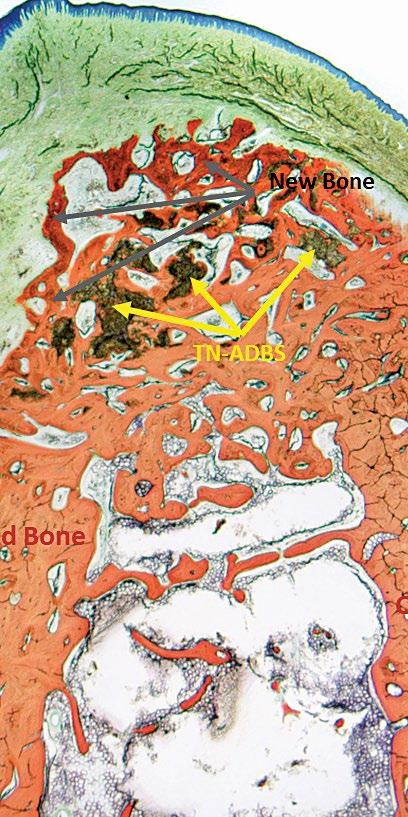
Clinical Need – Based on market research conducted by RevBio, almost half the patients that seek a dental implant supported crown suffer from chronic edentulism and require extensive bone grafting to rebuild their alveolar ridge. Over 30% of the time, these grafting procedures achieve suboptimal results and require some form of re-grafting adding to the overall cost, treatment time, and morbidity for these patients.


Solution – RevBio developed Tetranite® (TN) Adhesive Dental Bone Scaffold (TN-ADBS), a synthetic, porous, cohesive organic-mineral bone scaffold with adhesive properties that resorbs and is replaced by bone on a timescale commensurate with existing graft materials but does not require ancillary fixation or containment devices. The TN-ADBS comprises a kit consisting of the formulation powders sealed in a mixing bowl, a vial filled with highly porous granules, an aqueous medium pre-filled syringe, and spatula for mixing and application to the site.
Competitive Advantage – Currently available particulate bone grafting products require significant surgical skill to apply. In contrast, TN-ADBS is both cohesive and adhesive which enables the placement of the product without the need for ancillary containment or fixation aids. The product will reduce the overall time necessary to perform ridge augmentation procedures, better maintain graft volume over time, and minimize the need for re-grafting, which adds time, cost, and risk to successful patient care.
ITP Support – ITP support has enabled RevBio to accomplish key product development and marketing milestones, including market surveys to validate the clinical need and the lack of any known competitive products with a comparable clinical value proposition, refinement of the preclinical animal model and product formulation, GLP preclinical studies and supplier audits, provision of advice from user handling trials, and regulatory guidance in developing and refining a cohesive strategy for the commercial approval of the product.
Clinical Need – Over $200 billion is spent globally on dental caries and its complications, the most prevalent chronic disease worldwide. The unmet clinical need is atraumatic treatment of caries through subsurface remineralization of enamel, as current high fluoride toothpastes and varnishes incompletely repair carious lesions. Our products treat both hypersensitivity and early-stage caries, to reduce discomfort, preserve precious enamel and improve oral health.
Solution – Our research has shown enamel lesions are negatively charged. We produce particles less than 400 nm in size from food-grade starch that have a positive charge, and load them with the enamel minerals, to target the subsurface and release minerals inside the lesion. In-situ degradation in saliva takes care of the rest. Our products, CrystLCare™ Biorestorative Fluoride-Free and Fluoride-Plus, are packaged in dry form and instantly disperse in less than a minute at time of treatment.

Competitive Advantage – While traditional fluoride treatments merely impact the extreme surface layer of enamel lesions, targeting the dominant subsurface lesion will enable a superior non-surgical dental treatment. Localized concentration of minerals and fluoride facilitate tooth structure regeneration through formation of apatite-like crystals, yielding superior remineralization of lesions compared to other available treatments.
ITP Support – Challenges led to innovation and easy-to-use dispersible film packaging. An AI-driven camera under development has shown correlation with mineral density and caries severity (Caries Research, 2022), supporting utility in future clinical studies. Dentinal occlusion was demonstrated by third-party testing. Two X-ray methods proved occluding material is mineral based, and remineralization is regenerating the apatite crystal structure. All third-party biocompatibility safety and biological risk assessments passed.

Clinical Need – Two million patients receive dental implants in the U.S. annually and up to 50% are post-menopausal women with osteoporosis. Dental implants are an effective treatment, but osteoporosis patients may be denied implant therapy due to compromised bone quantity and quality. Predictable treatments to regenerate lost tissues around teeth or implants are limited, and to date, there are no FDA-approved bone anabolic agents available for this indication.
Solution – A team of researchers led by Dean William Giannobile at the Harvard School of Dental Medicine is investigating the therapeutic potential of a systemic osteoanabolic drug, abaloparatide, to restore lost periodontium or enhance formation of implant-supporting alveolar bone. This approach offers easy dosing to regenerate lost periodontium or improve peri-implant bone density.
Competitive Advantage – By taking advantage of easy delivery of abaloparatide, which is already clinically approved for improvement of bone density in other indications such as osteoporosis, this approach may represent an improved access to drug therapies for periodontal and dental implant-related diseases that might otherwise not be as available due to limited reimbursement through typical dental insurance.
ITP Support – With the support of the ITP program, a preclinical experiment has been performed to assess the potential of abaloparatide in dental applications. Our team is now focused on the progression to a phase I/II human clinical trial to use on-label systemic abaloparatide to adjunctively treat alveolar bone loss and enable implant treatment for patients with compromised bone quality.

Clinical Need – An intraoral wound overlay for soft tissue that is easy to apply, remains in place for the duration of wound healing, and can be removed without causing damage to underlying tissue. Current options for oral wound care are limited to difficult to use, uncomfortable, and largely ineffective glues and resins.
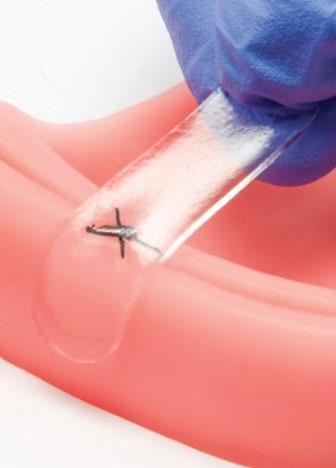
Solution – Amend Tissue Tape™ is a hydrogel-based adhesive comprised of two primary elements. The hydrogel consists of an interpenetrating network of alginate and polyacrylamide. The adhesive is composed of chitosan, which forms covalent bonds across the interface. When used together, the hydrogel and adhesive adhere to wet tissue and provide over five times the adhesion energy of cyanoacrylate while also providing a long duration mechanical barrier for the wound and flexibility to stretch with the wound without damaging the underlying tissue.
Competitive Advantage – There are limited products available that will safely adhere to sutured or non-sutured oral wounds. Periacryl, a cyanoacrylate-based product indicated as a dental cement, is often used off-label for wound closure. It requires a dry environment to fully set and is rigid, not stretching to accommodate movement or swelling. Amend Tissue Tape™ adheres to tissue in a wet environment, stretches with the wound, and stays in place for weeks, allowing wound healing to occur.
ITP Support – Market Assessment Core has facilitated handling demos and introductions with KOLs further validating the clinical need for Amend Tissue Tape™. The ITP program will support design and development activities and execution of a GLP animal study to evaluate wound healing and removal of test articles. This program will provide our project the clinical expertise and market and regulatory guidance leading up to FDA pre-submission.
Clinical Need – Dental caries is a common health concern worldwide. When caries damage the enamel deeply enough to reach the dentin, a resulting inflammatory response can damage the adjoining dental pulp. While current clinical treatments may slow the progression of resultant tooth decay, no treatments exist to regrow dental pulp and dentin lost to infection, decay, or trauma. What is needed is a regenerative therapy that activates the tooth’s inherent healing processes to regrow pulp and dentin.
Solution – RegendoGEL is a biodegradable, hydrogel-based product that contains natural tooth-derived bioactive molecules that stimulate “guided dental pulp and dentin regeneration”. Unlike currently used calcium and silicate cements, RegendoGEL renews tooth vitality by regenerating natural dental tissues that extend the life of patients’ teeth.
Competitive Advantage – As compared to conventional permanent, nondegradable cement-based products, RegendoGEL is biodegradable, and stimulates its replacement by regenerated natural dental pulp and dentin tissues. In vivo animal studies showed that RegendoGEL can regenerate dentin ~5x faster than existing products on the market. RegendoGEL’s biocompatible, non-inflammatory and biodegradable properties reverse the degenerative spiral of tooth decay, which normally progresses from a small cavity and filling to larger fillings, eventually leading to endodontic treatment and tooth replacement therapies.
ITP Support – The ITP program has supported various aspects of this project, including: Regulatory; Pre-Clinical Animal studies; and Micro-CT, Histology and Statistical Cores. Ongoing guidance on how best to bring our product to the market has been provided by the Market Assessment and IP/ Commercialization Cores.

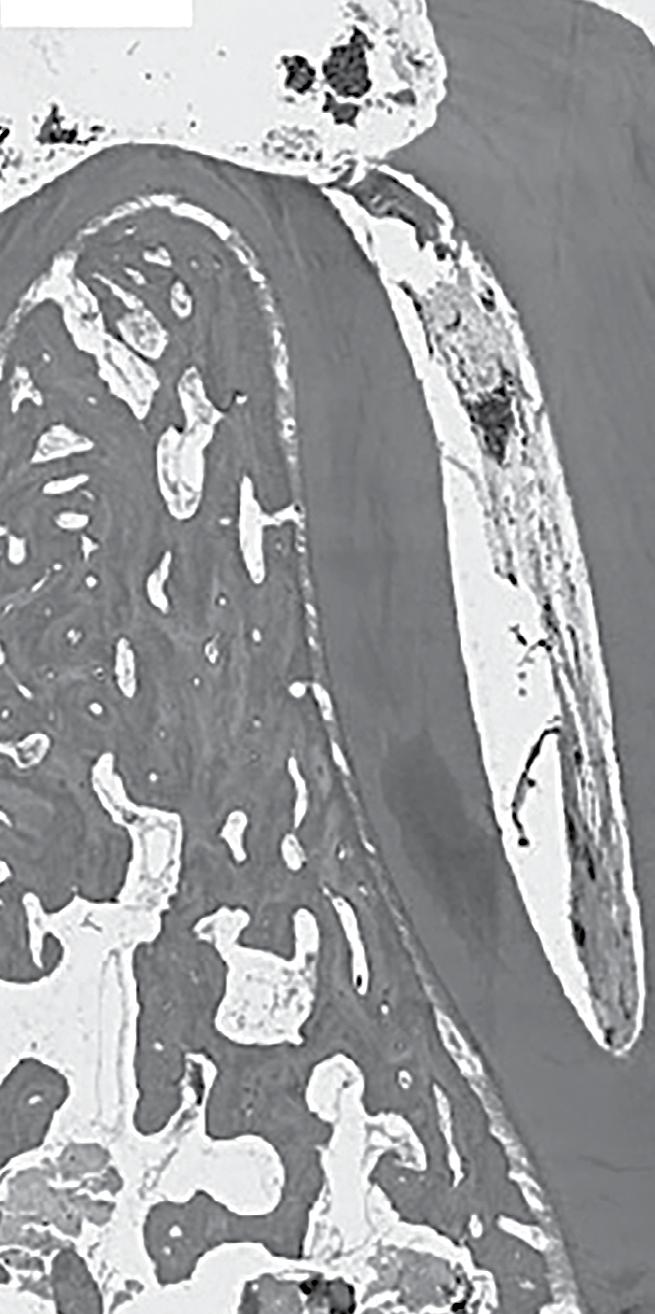


Clinical Need – Approximately 5 million procedures are performed in the USA each year to treat pulpitis of permanent teeth in the pediatric population. Children are often subject to multiple procedures, because the standard of care is to stabilize the tooth until more definitive treatment can be performed when the child’s growth stops. The outcomes are unideal, because the whole tooth is not revitalized after pulp removal, resulting in tooth discoloration, loss of tooth structure, and limited (if any) tooth growth.
Solution – Vital-Dent is an acellular, resorbable hydrogel scaffold intended for revitalization of pulp and maintenance of tooth vitality in immature permanent teeth treated with endodontic therapies. It is supplied as a powder in a single-use kit with sterile saline. The powder is rehydrated at the chairside using kit tools to make a clear liquid. The liquid is inserted into the instrumented canal space as would conventional sealers, and set in three minutes with a dental curing lamp to form the colorless Vital-Dent hydrogel. The tooth is then sealed with a bioceramic and restored with conventional techniques.
Competitive Advantage – Unlike current obturating materials, Vital-Dent is resorbable and promotes continued development of immature teeth, pulp revitalization and regenerative dentin, root strengthening, and long-term survival of the tooth. Vital-Dent eliminates difficulties of the only available revitalization procedure, revascularization therapy, with more consistent outcomes including root development, and a better fit with conventional clinic workflows.
University
ITP Support – With help from the Market Assessment Core, user needs and the indication for market entry were defined. With ITP funding, the device composition was frozen and efficacy in a dog model of pulpectomies demonstrated. In collaboration with the Regulatory Core, our regulatory strategy was defined and the design control processes implemented. Commercialization plans are in development with support from the Innovation Institute at the University of Pittsburgh and the Commercialization Core.
et al. Effect of the Periapical “Inflammatory Plug” on Dental Pulp
A Histologic In Vivo Study. J Endod 2020


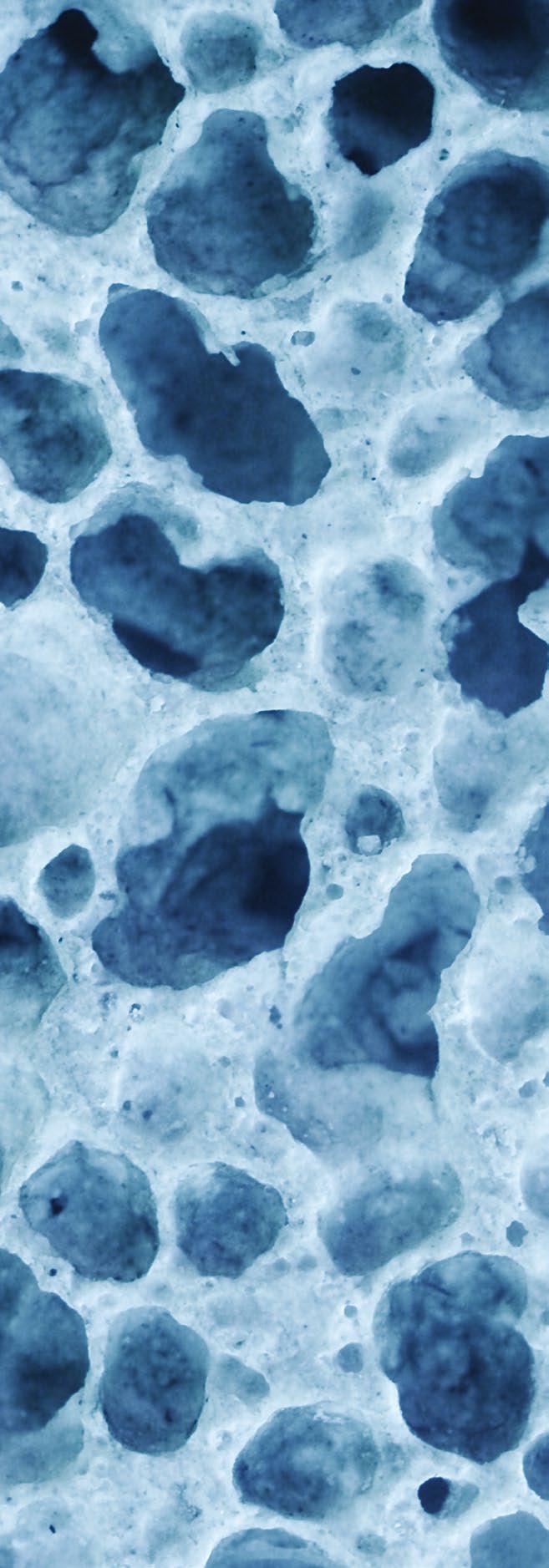
Clinical Need – Each year >200K newborns suffer from conditions that restrict growth of the skull or jawbone. Left untreated, these can have life threatening outcomes in an otherwise healthy child. However, treatments are complex and traumatic. Distraction is a gentler therapy that uses a device to slowly expand abnormal bone, but distractors are semi-buried, increasing complication risk; manual expansion is performed by parents, leading to noncompliance; and surgeons are blinded to treatment, forcing weekly X-rays and exams.
Solution – Ostiio’s integrated therapy uniquely solves pain points of distraction to improve patient outcomes and experience while reducing cost. The fully buried implant is magnetically expanded without contact, removing the complication risk of semi-buried devices. The automated driver simplifies expansion to a button push, reducing parental noncompliance. The remote monitoring platform allows surgeons to track treatment progress and address complications early on, reducing post-op follow-up.
Competitive Advantage – Although distraction is the segment within the craniomaxillofacial device market that offers differentiation opportunity, major competitors have been iterating upon technology that was first introduced to market >20 years ago with a focus on providing greater flexibility to the surgeon in the OR. However, treatment takes place at home. Ostiio instead will transform how distraction is provided by parents and monitored by surgeons to improve patient outcomes and experience while reducing cost.
ITP Support – The ITP program has enabled Ostiio to advance its integrated therapy from product conceptualization to late-stage prototypes. Working within an ISO 13485 certified quality management system, Ostiio has developed functional prototypes that exceed the most critical product specification by more than 2x. In parallel, Ostiio has progressed key regulatory activities and completed important market validation work.
Clinical Need – In the treatment of head and neck cancers, radiotherapy is commonly executed in conjunction with other modalities, such as surgery and/or chemotherapy. Because of the anatomical proximity, salivary glands receive secondary radiation damage, which results in xerostomia. While intensity-modulated radiotherapy significantly reduces the incidence of radiation-induced xerostomia, a need still exists for patients suffering from xerostomia, especially those in whom amifostine leads to significant side effects.
Solution – The method of ultrasound-assisted gene transfer (UAGT) was developed to deliver the AQP1 gene in irradiated salivary gland for the amelioration of radiation-induced xerostomia. This non-viral gene delivery is based on sonoporation generated by the ultrasound, enabling gene transfer into radiation-surviving salivary gland cells. The delivery of the water channel AQP1 to the parotid glands in a mini-swine model restored salivary flow post-radiation to pre-treatment levels, demonstrating the efficacy of our non-viral AQP1 gene transfer approach.
Competitive Advantage – While a recent clinical trial using a viral-based AQP1 gene delivery demonstrated an increase in saliva production, this approach has not advanced beyond Phase I/II trial due to side-effects generated by the adenovirus vector. With our non-viral based approach, it is anticipated that enhanced safety is provided and that serial dosing is feasible to provide patients with AQP1 gene therapy throughout their lifetime.


ITP Support – Through the ITP support, the team has demonstrated safety of UAGT-plasmid delivery and has collected data on the vector dosing range. With support from the Cores, suppliers were qualified, and documents are in preparation for the IND package. In addition, a detailed commercialization plan was established. Future work is focused on obtaining GLP toxicology data.
Wang et al. Ultrasound-assisted nonviral gene transfer of AQP1 to the irradiated minipig parotid gland restores fluid secretion. Gene Ther 2015
Clinical Need – Soft tissue deformities and volume/ contour deformities from craniofacial trauma, congenital anomalies, and cancer treatment are difficult to correct. Current standard of care includes injectable fillers, implants, and soft tissue flap procedures, which have limitations and often involve operations with significant risk. As such, autologous fat transfer is being explored as a lower risk alternative. However, as optimal results with fat transfer often require at least two treatments, there is a need for an on-site preservation of harvested tissue for subsequent procedures to minimize donor site morbidity and encourage fast recovery.
Solution – A team of researchers at the University of Pittsburgh led by Dr. Peter Rubin has previously validated the use of autologous fat transfer as a minimally invasive therapy for the restoration of craniofacial form. In order to facilitate fat transfer with minimal donor site morbidity, the team has developed a novel device to cryopreserve adipose for storage at the treatment facility, which can directly be used for the subsequent fat transfer(s).

Competitive Advantage – With the on-site cryopreservation and storage of the fat tissue, the device is envisioned to reduce patient and clinician burden for tissue harvest. The utilization of the device obviates the need for repeat tissue grafting procedures, and is anticipated to lead to reduction in treatment costs as the fat transfer injections may be performed outside of an operating room in a less acute setting.
ITP Support – The work supported by the ITP program is focused on the generation of market-ready prototype cryopreservation/ storage devices. Towards that end, project plans include prototype development and validation, as well as the development of a regulatory strategy and commercialization plan.

Clinical Need – The intended product assists in the repair/regeneration resulting from traumatic avulsion injuries or secondary to cancer ablative surgery involving a sphincter/ stoma/lumen/opening that result in loss of volumetric muscle mass and function in the body such as the face (lips, eyelids) and urogenital tract (anal sphincter, vagina).
 DDS, PhD University of Michigan
DDS, PhD University of Michigan
Solution – The product consists of a pre-fabricated innervated pre-vascularized pre-laminated (PIPP) composite soft tissue microvascular free flap that is composed of autogenous oral-skin-dermis-muscle that can be used in functional reconstruction of human lips. The in vitro produced muco-cutaneous “laminate” is composed of autogenous oral and skin keratinocytes cultured on an acellular dermal matrix and is placed on latissimus dorsi muscle using the body as an “in situ bioreactor” to finalize the tissue engineering medical product. The stoma/opening in the PIPP flap is maintained with a proprietary biocompatible obturator.
Competitive Advantage – The gold standard is the radial forearm free flap with the palmaris longus tendon, which creates an immobile, stiff lip, or an allogeneic cadaver graft that requires immunosuppression. The ultimate source of the cells to develop PIPP will come from the patient, thus making the construct autochthonous and obviates the need for immunosuppression seen with allogeneic grafts.
ITP Support – The program has supported the creation of an autogenous mucocutaneous construct to use as a laminate for the PIPP flap, development and fabrication of a biocompatible proprietary obturator to maintain the stoma opening, and the development of an athymic rat model for use in preclinical GLP studies. Regulatory guidance and consultation for preparation of an IND submission for a first-in-human clinical trial are supported by the program.
Eng Part C
Towards building and sustaining the translational research pipeline for the development of regenerative products that positively impact patient care in the dental, oral, and craniofacial space
With support from NIDCR, the MPWRM Resource Center has developed a unique ecosystem that coalesces expertise across translational research and commercialization domains, specifically in the regenerative medicine and dental, oral, and craniofacial space. Since its inception in 2017, the Resource Center has strived to accelerate the translation of regenerative technologies by providing the expertise and resources to shepherd them towards FDA submissions and commercialization. As we consider the horizon beyond the original award from the NIDCR, we are exploring ways to support the continuation of the Resource Center and its services.
ITP teams Investigators/ Researchers Clinicians Patients
Professional organizations Regulatory agencies Standard-setting organizations
Federal agencies Industry Investors Philanthropy
Incubators
one-on-one interviews facilitated by a Resource Center member understand stakeholders’ needs/ interests
identify areas of aligned interests — advisory meetings

Jeanne Ambruster
The Avenues Company
Connie Chang
ONL Therapeutics

Kay Fuller MDRS LLC

William Giannobile
Harvard University
Ken Hargreaves
University of Texas Health Science Center San Antonio
Darnell Kaigler
University of Michigan
David Kohn
University of Michigan
Paul Kostenuik
Phylon Pharma Services
David Mooney Wyss Institute
Katie Moynihan Indiana University Ventures
Vicki Rosen Harvard University
Charles Sfeir University of Pittsburgh
Tony Torres University of Pittsburgh
William Wagner University of Pittsburgh
Interdisciplinary Translational Project (ITP) Program
Mutsumi Yoshida, PhD
University of Michigan – School of Dentistry
1011 North University Avenue, Room 3303 Ann Arbor, Michigan 48109 (734) 764-4622 | yoshidam@umich.edu
Program Administration
Patrick Cantini
University of Pittsburgh/McGowan Institute
450 Technology Drive, Suite 300 Pittsburgh, Pennsylvania 15219 (412) 624-5209 | cantinip@upmc.edu
Image credits from our ITP teams: S. Feinberg, L. Bertassoni, P. Yelick, Oraxsys Therapeutics, W. Giannobile, J. Taboas