

Engineered Organs

KalArm





New innovations are revolutionizing the field of medicine

Human organs are being synthetically designed using bio-degradable materials. This in itself is a creative innovation that is actually
proving to be beneficial. This bioengineered Organs' Initiative is a multi-disciplinary effort focused on constructing longer life.
Collaborative research at Carnegie Mellon University by a multidisciplinary research team is designing, creating, and testing a new generation of long-term replacement organs engineered from a combination of bioprinted cellular and synthetic materials. This lifesaving technology has the potential to one day eliminate the current organ transplant waiting list.
The collaborative research involves 3-D printing, tissue engineering, biomaterials, cellular mechanics, and artificial organs, that has the potential to support or replace diseased organs.
Kymriah developed by Novartis is the first-ever FDA-approved CAR-T cell therapy, that empowers patients’ immune systems to fight their cancer. It was developed in collaboration with the Perelman School of Medicine at the University of Pennsylvania, a strategic alliance between industry and academia, which was first-of-its-kind in CAR-T research and development.
Indian engineers create the First Bionic Hand designed and developed in Hyderabad, India. KalArm® is a 3Dprinted, EMG sensors embedded, lightweight and affordable bionic hand, to address the problems of 40 million people globally, of which India alone holds about 6 million in need of prosthetic assistance.
NAVEED HASAN
n.hasan@projectleadersmagazine.com






Engineered Organs


Scientists in the field of regenerative medicine and tissue engineering are now applying the principles of cell transplantation, material science, and bioengineering to construct biological substitutes that will restore and maintain normal function in diseased and injured tissues. Are engineered organs finally becoming reality in medicine? To answer this we have taken this as our prime focus this month.

THE PRIME FOCUS
LIFE SAVING APPROACH
The Engineered Organs approach is an interdisciplinary team attempt aimed at extending human life. The Carnegie Mellon University’ researchers are trying to take a
novel, all-encompassing way of addressing the unfulfilled necessity of organ transplants by developing the next generation of long-term new organs.
THE VISION IS TO FOCUS ON SAVING LIVES BY EXPANDING THE AVAILABILITY OF ORGANS TO THE PATIENTS.
The multidiscipline research group has been developing, manufacturing, and evaluating the generation of artificial organs made of bioengineered cellular and synthetic components. This life-saving technique offers great ability to remove the existing organ donor waitlist someday. Those certain genetically altered organs have the potential to transform overall survival for the thousands of patients worldwide experiencing terminal failure of the organs (Bioengineered Organs Initiative). Multiple organ replacement could now be conducted using tissue engineering. Using a bio-based framework, cells can be stimulated to assemble and set up fully functioning tissues. Autologous cells and an adequate scaffold can be used to develop artificial organs. This method has been used to grow urinary bladders. Stable organs including the kidneys, liver, heart, lungs, and lymphoid tissue are being grown. Bio-engineered inserts have the potential to multiply, redevelop, and react to damage.
Bio-Degradable Materials To Build Organs
The Carnegie Mellon University’ bio-engineers are using bio-degradable materials that contain both non-biological and biological elements. Because of the limitations of tissue substrates made entirely of biological materials, biomaterial resources have been explored for tissue engineering scaffolds.
Biopolymers often have interesting characteristics that enable them to interact with living cells, tissues, as well as organs without even being denied by the patient body (Sohn, 2020).
Bio-hybrid substances are now being investigated as a possible substitute for traditional therapeutic treatment for lifethreatening ailments. In comparison to the total number of people being cured, there are fewer extractable organs for transplant surgery.
A biopolymer can be made from metal alloys, ceramic materials, polymers, glass, or perhaps even living stem cells or tissue. They could be reconfigured into moldable or manufactured components, adhesives, natural fibers, coatings, foaming agents, and fabrics to be used in medical and biological products and applications. Biopolymers can take multiple shapes and be created from a wide range of materials. Biopolymers must also preferably be porous, with tiny holes that enable fluid, air, and sometimes cells to cross through configuration that also implies they have tiny pores that enable liquids, gases, and cells to continue moving through the pores, much like the tissue or organ they are trying to treat (Wang, 2018) .

The specially engineered tissues are int polymeric materials instead of convent
Traditional manufacturing techniques categorize bioengineered organ manufacturing methods into three broad categories (Wang):
1.Completely automated ( 3D bio-printing)
2.Semi-automated (Mechanical Handling)
3.Hand-manipulated ( Bio-material Casting)
Bioengineered organs look organs that reinstate, kee cells or a whole organ. tissues or organs can offe body parts while also immunosuppressive drugs such as their manufactu have proved to be benefic to regenerate injured tissu cells or recovering previou tissues.

tended to use 3d bio-printing cells and ional plastics.
k to build fully functioning ep, or enhance damaged Biologically engineered er an insatiable supply of avoiding the need for s. Simple, vacuous organs, ring and transplantation, cial. It also allows the body ue by substituting injured sly irrecoverable organs or
As per CMU these bioengineered organs have the potential to improve life expectancies for the vast numbers of people in the United States struggling from endstage organ dysfunction. The demand for new organs is enormous and innovative methods to construct replacement organs proficient for restoration, complementing, or substituting long-term body functions are needed. Bioengineered organ development techniques are a collection of practices that could be used to create organs for transplant employing bioengineered precepts. Significant work has been done in the creation of numerous organ manufacturing techniques during the last decade.
Bioengineered organ manufacturing techniques can also be defined into many methods prefaced on the handling method:
1.Bottom-up manufacturing techniques
2.Inside out manufacturing techniques
3.Outside in manufacturing techniques
The main advantages of biomedical implants are that they allow for mass manufacturing and that sick people are much less prone to undergo transplant rejection. Based on modern technologies and healthcare potential, transplant waiting lists could be substantially eliminated or reduced. This is largely due to advancements in technology. Both renal and hepatic transplant recipients have a 95 percent each rate of survival for transplant patients performed between 2011 and 2014, especially in comparison to the 83 percent survival in 1994 to 1995 (Wynn, 2017). Relatively secure techniques to reconfigure a person's own tissues to effectively distinguish into operational substitute types of cells are one of the rapid advancements which could have a significant contribution in the field. It is possible that these tissues would be merged with organic, polymeric, and decellularized organ elements to make transplanted cell replacements. Hence, the engineered organs have revolutionized the biotechnology and medical field of research and proven itself as a future of the medicine.
The CMU University is trying to take a novel, all-encompassing approach to addressing the unfulfilled necessity of donated organs by accelerating the development of long-term artificial organs. The CMU researchers have designed bioengineered hearts, lungs, livers, and 3-D tissue constructs using 3dimensional Bioprinters. Even though scientific research should resolve obstructions before all these engineered organs can be used for daily medical facilities, incredible medical advances have been achieved. However, replicating what humans inherently have in a laboratory setting is a complex process.
BIOLOGICALLY ENGINEERED TISSUES OR ORGANS CAN OFFER AN INSATIABLE SUPPLY OF BODY PARTS WHILE ALSO AVOIDING THE NEED FOR IMMUNOSUPPRESSIVE DRUGS.
Bioengineered organs can maybe solve all problems; there would be a much reduction in the incidence of immune suppression, and the organs themselves might also possibly be produced in large quantities. Whilst also these organ transplants have indeed been conducted all around the world, they are reportedly used as a short-term fix before patients get an original organ. In the near future, as advances in technology and engineered organs, and transplants are becoming more prevalent, the additional supply of body parts is likely to decrease, if not remove, the waiting period.
As in long term the CMU researchers thinks that if these organ transplants can become a cost-effective way of treating serious diseases, anybody with coronary heart disease or centralized cancers may have the impacted organ changed, effectively reducing the occurrence of heart problems and forms of cancer. Verifying and evaluating these situations would necessarily require a vast range of potential assertions and might be time consuming.
KYMRIAH
by Novartis
Kymriah is a drug by Novartis, used to treat individuals with specific kinds of B-cell non-Hodgkin lymphoma and adults up to the age of twenty-five years with specific forms of B-cell acute lymphocytic leukemia. It is also being investigated as a treatment for other cancer types.

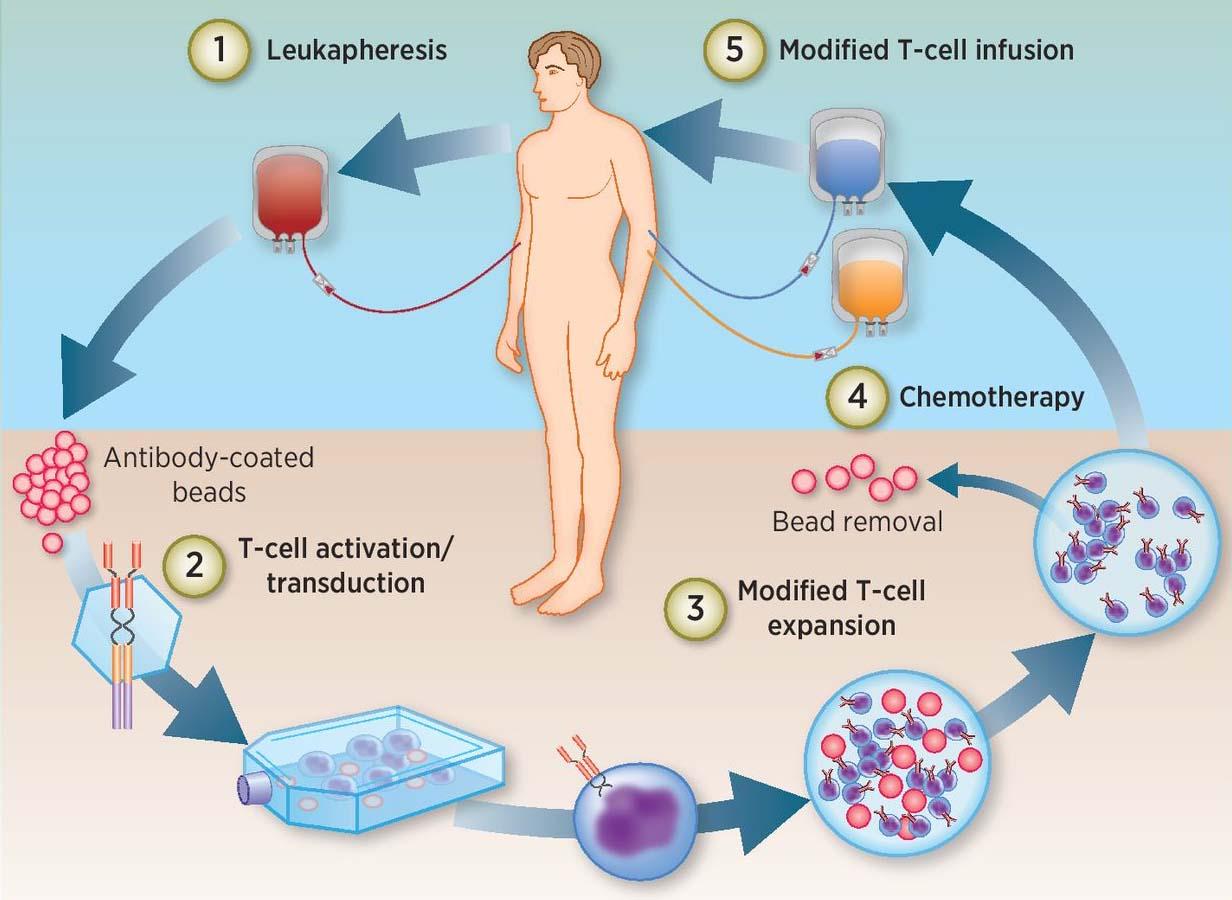
Kymriah is generated from T-cells (a type of immune system cell) of patients.
Kymriah, the registered trademark for tisagenlecleucel (comprising of genetically improved WBCs), has been authorized for relapsed or refractory follicular lymphoma patients who already have ended in failure in two or more treatments. Penn Medicine, which formed Kymriah over a decade earlier, made the announcement on its official site (FDA, 2019). It is a gene therapy product that is a form of advanced therapeutic drug. This is a kind of drug in which genes are delivered to the body of the patient.
It has been used to cure unique blood types of cancer, and has been identified as an orphan medicine (a drug used for uncommon diseases). Kymriah is made from the white blood cells of the patients, collected, and genetically altered in the research laboratory. It is administered as a single transfusion into the vein of the patient and can only be administered to a patient whose cells have been used to develop it. Prior to actually getting Kymriah, the patient must undergo a brief period of chemotherapeutic agents to clarify their white blood cells, and the person is given paracetamol as well as antihistamine drugs just before the infusion to mitigate the likelihood of infusion interactions (EMA, 2018).

According to the accelerated approval program of the Food and drug authority (FDA), the persistent marketing provision of tisagenlecleucel for enhanced follicular lymphoma is dependent on the outcomes of prospective confirmatory clinical studies. Among relapsed or refractory follicular lymphoma patients, the therapy produced an 86 percent excellent prognosis, with 68 percent of cases staying in full remission after 16 and half month’s follow-up. Kymriah is custom-made for every person by Novartis' Morris Plains, New Jersey facility, has created CAR-T cells for multiple patients.
Novartis has set up a repeatable, versatile, and confirmed manufacturing process predicated on years of continued clinical research expertise. They are created by taking T cells out from patients and genetically re-engineering the T-Cells in the research facility to generate proteins on the surface known as chimeric antigen receptors (CARs). They identify and attach to particular receptors or proteins on the surface of cancerous cells. (Novartis Oncology Business Use Only Kymriah®)

The miracle drug by Novartis tends to work by effectively attaching to a specific protein CD19 that is found on B cells. Kymriah initiates the immune function by linking to CD19.
Kymriah initiates the immune function by linking to CD19. This aids the immune system in attacking cancerous cells.
Kymriah is autologous T-cell immunotherapy that has been genetically altered.
Through a process called leukapheresis, the treatment is inferred from the patient's WBCs. After being changed, the cells are injected into the same patient to destroy cancer cells.
SIGNIFICANCE OF KYMRIAH
According to findings reported at the latest American Society of Clinical Oncology annual gathering, Novartis’s drug fully cleared obvious symptoms in 66 percent of the initially treated group, and 86 percent of participants encountered a reaction. Kymriah suspension for intravenous administration, previously CTL019, is now a CD19directed genetically engineered autologous Tcells treatment for cancer suggested for the therapy of patients of age about 25 years old with B-cell precursor acute lymphoblastic leukemia that is unresponsive or subsequent recurrence, young patients with refractory or relapsed massive B-cell lymphoma. Kymriah is not prescribed to treat sick people with main central nervous lymphoma.
This medication has a serious complication recognized as cytokine release syndrome that also induces fever, shivers, difficulty breathing, nausea, and other clinical signs. If this situation takes place, the therapists will also have medicine on the side to cure it as quickly as practical.
Tisagenlecleucel could also end up causing potentially deadly nerve impingement. Notify the practitioners or actively sought urgent medical treatment if the patients are having speech problems, reasoning, or recounting, are confused, or are having seizures.
The approval is based on information from the Phase II ELARA trial, a single-arm, openlabel clinical trial wherein ninety individuals were assessed for effectiveness over a 2-year period. Eighty six percent of people medicated with Kymriah had quite a response, with 68 percent providing a full response.

KYMRIAH is only accessible through a constricted program under a Risk Management. and Mitigation Strategy called the KYMRIAH REMS despite the threat of neurotoxicity and CRS.

Kymriah's approval by FDA provides the patient with relapsed as well as refractory follicular lymphoma with the latest potential treatment and revived hope for good health outcomes. The accessibility of standard infusion procedure choice aids in altering the way medical professionals confront this form of blood carcinoma, and researchers applaud those that have helped contribute to the thrust of scientific investigation for the provision of quality care.


Novartis has an extensive, globally integrated CAR-T manufacturing presence, which improves the development and manufacturing process's adaptability, perseverance, and sustainable development.
Cancer treatment has seen significant advances as a result of the recent medical intervention revolution. It is common in clinical situations to discover that the same dosage of medication causes substantial differences in potency all over population groups.
Cancer genetic testing has gained prominence due to the possibility of individualizing cancer therapy while reducing toxicity and optimizing efficacy. Cancer personalized medicine facilitates the detection of patient populations at higher risk of serious toxic effects or those that are positioned to gain from therapeutic intervention, aiding the pursuit of the ultimate objective of personalized treatment of cancer.
Kymriah has transformed the cancer treatment procedures and gives a hope to the patients that innovative techniques and procedures are aiming to reduce or eliminate the cancer or tumors from the world. However, patients will need to be supervised by their medical professional for the rest of their lives after receiving Kymriah treatment, because they may develop supplementary cancers or relapse of cancer.
ASIA

KalArm originally developed and designed by Maker Hives, Hyderabad. It is a 3-dimensional printed, electromagnetic sensor integrated, portable, and cost-effective bionic hand aimed at both national and international markets of people with upper-limb below-elbow limb amputation, with the potential to deliver India a market edge in the prosthetic market (KalArmIndia’s First Bionic Hand). The bionic named after the founding members' inspiration, Dr. APJ Abdul Kalam, is the work of three inventors, Harsha, Pranav, and Suren. The bionic business intends to collaborate with hospitals and set up its assembly, with an ability of 1,500 hands annually.


KalArm has eighteen predefined grips and therefore is present in three palm sizes -
small, medium, and large with forearm connections differing from patient to patient, as well as the choice to personalize colour schemes of external casings to the patient's preference. It is a properly functioning, personalized, priced, and smartphone application controlled Bionic Hand that enables disabled people to transform from incapacitated to appealingly physically able and be a cause of admiration in the community.
The companyMakers Hive intends to develop advanced bionics including bionic skeletons, eyes as well as Parkinson's gloves.
The KalArm arrives with eighteen predefined grips such as a hanger grip to grab an object, a squeeze grip, and a tilting grip, among many others, and six specially designed grips that the consumer can set up using the app based on their specific requirements (Electronics et al., 2021).
It is among the most innovative bionic limbs on the market, as well as one of the most affordably priced. It is 1/10th the costs of foreign options available while offering outstanding properties.
The primary goal of KalArm is to support individuals with everyday activities. It is not designed to be used in severe conditions, but instead for daily use at a minimal cost. It is also incredibly fashion-conscious.


It is also incredibly fashion-conscious. Maker Hives claims that KalArm has also been checked with consumers of various gender groups, age groups, and amputation stages to evaluate its usability. A lot of consumers discovered KalArm to be simple to use, convenient, and hugely beneficial for day-to-day actions. All of the tests were conducted with the aid of a Medical Orthotist and Prosthetist.


KalArm offers multiple modes like Workplace Mode, Cooking Mode, Athletics Mode, and Compact Mode, through a smartphone application to endorse and oversee the device's effectiveness, wellness, and functionality, allowing the user to achieve greater flexibility.
Consumers usually choose a mode needed for the job tasks, such as trying to work in the dining room, to swap between grasps. They achieve this by pushing a button.
Consumers can turn through the clutches in that configuration by using either single or the double open message once in that mode. Applying dimensional pressure shifts the thumb into such positions.
KalArm has a sensor for detecting the posture of the finger to ascertain what grip it can operate. The frequency and pressure of a prosthetic limbs hand's hand movement are dependent on the intensity of the patient's muscular sensor. This lets the user change the intensity of his arm due to the nature of the thing he is trying to grasp. KalArm is presently available in a single unisex shape. It measures about 450 grams.
In the journey from myoelectriccontrolled prosthetic limbs to socketbased advanced technologies, the discipline of bionics has developed exponentially.
As the era of bionics is evolving with the passage of time, artificial intelligence and related innovative technologies are progressing at an astonishing speed, as well as much research has been conducted to promote bionic implants.
Doctors, developers, and engineers all over the world are working to create more efficient prosthetic arms needed to aid prosthetics to feel touch employing textural feedback detectors and execute multiple tasks efficiently and effectively.
Bionic advances may require consumers to incorporate an outer skeleton onto their bodies. Incorporating devices with the body to enhance haptic feedback will depend on the skills of electromechanical engineers to achieve the next landmark.
When the bionic hand had first been revealed at Tirupati's BIRRD Hospital, a large number of pre-orders have been positioned.
As per founders, who plan to develop Parkinson's gloves as well as bionic eyes in the coming years, purchases are rising day by day.
It is constructed with the goal of providing direct connections to upper limb amputees from a number of units with over forty million individuals who need artificial limbs while preserving the financial challenges of the undeveloped nations in thought.
As an Innovation sta the project is consist and developing, according to the thr business owners.

The KalArm has fulfilled that dream, now people who have lost their hope and were in distress due to the loss of their body part are allowed to think about have fully functional bionic arm. By using that arm they can almost do the activities one can do with natural hands. KalArm is dream project not only for the founders but for the millions of people.

THE HYBRID WORK MODEL
WORKPLACE OF THE FUTURE
The term "hybrid work" refers to a flexible strategy which allows employees to divide
their hours between operating in the workplace and working remotely. This methodology entails tailoring tasks to how teams’ function most effectively and developing interactions which are as diverse and accessible as possible. In hybrid work model, the decision of how to work in the office versus from household is occasionally left up to the employee. There is not a hybrid model that works for everyone as it is based on the demands of the business as well as the requirements of an individual employee, through which each organization creates a hybrid model.
Although the hybrid model is frequently mentioned in the magazines but there is not a single well-defined example of hybrid model. In the end, it entails a mix of operating from an office and virtually (In, 2021). Although the hybrid model now appears distinct for each business, there are a few recurring features. Regardless of the specifics, businesses that decide to adopt a combined work approach, would encounter some difficulties.
Companies are discovering that their staff is reluctant to exit from remote work once offices begin to reopen. The majority of those who began to work remotely as a result of COVID-19 support it and wish to keep doing so. Firms can no longer claim that they cannot operate remotely, a typical justification for not allowing remote work prior to 2020, but they also cannot force people to return to work full-time due to continuous worldwide epidemic. The issue that every firm appears to be now battling is the most preferred approach known as hybrid work model. R E M O T E O F F I C E
If we consider the shortcomings of hybrid work model, this model is still evolving into the new working standard.
Walters, 2022
There are three basic structures of hybrid work model:
Remote-first
This work model cause firms to operate as a remotefirst business. Given that the workforce is not collocated across numerous nations and time zones, virtual interaction is the norm. Employers maintain offices for workers to use if they so desire, and offices are typically thought of as places where people can collaborate. If it is required for their job, some employees might be obliged to report to the office.
Office-first, remote allowed
Before COVID-19, a frequent paradigm was one in which some workers worked remotely while others did their work mostly in the office (Howspace, 2022). Because they miss the discussions, events, and relationships that are made for the vast percentage of workers operating on-site, remote workers in this system usually feel like second citizens.
Office-occasional
Employees who use the workplace hybrid work style occasionally visit the office. The workplace is a venue for both group and solitary work. Employees may be required to report to work on particular days of the week which can be each Monday and Wednesday or at minimum one day of their choosing each week, based on the industry.

ADOPTING THE hybrid work model


Five companies which have acknowledged the future of workplace
Upwork
Spotify Microsoft Apple
Upwork runs a hybrid business model with a remote-first strategy and runs two offices in Chicago and San Francisco.
Although Spotify maintains multiple offices, staff members are free to work from anywhere. Employees who do not reside in a Spotify place are also reimbursed for shared space costs by Spotify.

Microsoft operates in a way that employees can spent 50% of the week remotely and they can also operate in a full remote way with permission from management.
Apple declared that its employees would have the option to work from home two days a week in addition to the three days they would spend in the office.
Twitter gives two options to its employees, whether they can work in the office or they can choose to work entirely remote.
People can work more effectively and creatively if the work environment is
changed to accommodate the flexible work style. For instance, implementing a flexible desk system where workers book their desk prior to arriving at the office might promote efficient use of space. It is possible that a company can operate at its best by assigning some teams to work on-site while others do so remotely. Alternately, they might elect to spread out people in house hours during the day.

Employee feedback can help in consistency with their individual requirements and preferences. Although hybrid employees might not engage with counterparts on a regular basis, digital network type cooperation enables employees to share information, commend colleagues for outstanding work practices, and poll the team about pressing issues as they come up. Particularly video conference, which enables users to profit from the complexities of being together face to face, is a crucial part of cooperation. Be available for staff to connect for coffee or to hang out via video chat. Building ties outside of the workplace can encourage and energize workers.


The hybrid work model can provide freedom and provide staff members the right to explore to their strengths, both of which increase output. Companies can achieve a solid combination of innovation and cooperation by fostering an environment that sees hybrid work as a beneficial alternative to finish profound projects in the office.
Workers may select when to work, which makes scheduling opportunity for continuous growth easier than it would be if they were remote or office employees. This is the attractiveness of the mixed work approach. Even though work and life are rarely perfectly balanced, a positive work-life balancing act is an essential component of any productive workplace. As a result, anxiety is lower, and exhaustion is less likely.
The environmental impact on the business can be significantly reduced by minimizing the need for individuals to commute to work.
Traffic congestion in cities can be reduced by allowing workers to stay in the home or travel less to operate from nearby coffee shops or coworking places.
To develop more natural conversations, build stronger professional relationships, and maintain the psychological aspect of conferences in mind by asking check in and keep a look at questions. The key is getting a shared virtual experience where staff members may interact, exchange ideas, help, and feel sense of belonging.
Lack of connection with coworkers is the main issue with hybrid or remote work.

In hybrid work employment, online meetings take up too much time. We have to adopt new policies and procedures where the actual work is completed on holidays and in the nights rather than during all working hours being spent in back-to-back meetings. By adopting an enabler's attitude, one can achieve this by knowing how interpersonal interactions function and crafting the task to best accommodate these routines and requirements.

The key component to making hybrid work model successful is office experience. It is critical to keep it from slipping. Even though your workforce might not be present on-site every day, it is crucial to make sure that each day at office is meaningful and meaningful.
For those who might be there to interact and communicate in person, the more individuals present, the better the experience.

Employee satisfaction at work is essential for a hybrid workplace to succeed. The hybrid approach is no more a substitute for other work practices. It will remain. However, there are positives and negatives to any employment arrangement. Firms must now accommodate their workforce's needs or potentially lose talent to organizations that do.
We can use this guidance as a professional in the workplace to implement hybrid working.
Keep in mind that allowing employees some freedom will boost their output and benefit the organization

