2






Over half a century of design.
7 Welcome 8 Our Expertise 10 Our Awards 12 Our People 14 Medical device development process 16 Medical and Scientific services 20 Our connected disciplines 22 Research and Strategy 25 Mechanical Engineering 27 Industrial Design 29 Human Factors and Usability 31 Interaction Design 33 Electronic Engineering 34 Software Engineering 37 Prototyping and Evaluation 44 The art of persuasion: Designing devices for patients who don’t want to adhere 60 Sustainability and drug delivery devices Drug delivery 9, 38-43, 50-59, 66-69, 74-77, 84-96, 104 Connected 74-77, 96, 103, 106-107, 160-161 Sustainability 58-59, 60-65 Other Medical 112, 116-119, 126-129, 134-135, 140-143 Scientific Instruments 18, 116-119, 120-125 Consumer Healthcare 130-133, 154-161, 168-189, 191-192 Commercial and Industrial 144-149, 194-195 Contents 70 Designing for uncertainty 78 Cybersecurity and connected drug delivery, an integrated risk-based approach 97 Don't develop a connected drug delivery device without reading this 108 How smart do smart medical devices need to be? 114 Why the IVDR is changing the way we develop diagnostic devices 136 The future of radiotherapy treatment 150 Beyond compliance. What is the role of human factors in medical device development? 162 Designing products that stand the test of time 196 Our location 201 Contact Medical and Scientific Overview 7

8
Welcome
Since the early sixties we have helped a wide variety of companies design and develop market leading products that users still value every day, ranging from the Stanley knife to the Eurotunnel Shuttle.
Today we focus on building long term relationships with large corporations in four market sectors: ‘Medical and Scientific’, ‘Consumer’, ‘Commercial and Industrial’, and ‘Transport’.
Founded in 1960, we are one of the world’s leading product design and development consultancies, operating globally from our campus in Warwick, UK.
9
Our Expertise
We do this through an intelligent approach to design, based on the transparent management of risk, informed decision making, true integration of disciplines and rigorous development processes.
We believe that the outstanding commercial success of the products we help create is dependent ultimately on delivering exceptional value to our clients customers.
We provide the right blend of strategic thinking and pragmatism to deliver our clients’ projects successfully.
We balance the creativity and the technical discipline needed to achieve commercially successful product innovation.
Every client is unique. To support our clients, we like to understand them, their place in the market and their ambitions thoroughly.
We add value by improving the success of product innovation.
10
Sanofi
SoloStar®

Disposable insulin pen injector
Design planning
Usability and HF
Mechanical engineering
Industrial design
Colour, material and finish
Instructional design
Graphic design
Prototyping
Testing and evaluation
Production support

11
Our Awards
A multi award winning design and development service .
12








gold winner 2015
Multi award winner
Multi award winner
Stanley Caplan
User-Centered Product Design Award
Multi award winner
13
Multi award winner


















































































































14
Our People
They combine to create a vibrant fusion of disciplines including mechanical engineers, electronics and software engineers, industrial designers, usability and interaction experts, researchers, strategists, prototyping technicians and specialist project managers. Each person is an expert in their own field, but has the curiosity, understanding and flexibility to reach
across traditional inter-disciplinary boundaries. Our organisational structures and team culture encourage this synergistic blending and integration of specialist skills. Our clients benefit not only from each individual’s depth of knowledge and experience but also from a team whose combined strength exceeds the sum of its individual members’ expertise.
DCA is a collection of over 130 extraordinary individuals. Intelligent, creative and thorough, our people make the difference to our clients’ projects.
15
How do you meet the challenge of delivering your new product to market quickly with the right performance and a strong intellectual property position, whilst industrialising robustly and cost effectively?
Medical
process 1 Foundation Pre-prototype Iteration Prototype Iteration Industrialisation Support 3 Design Output 2 Design Input 16
device development
It’s a complex problem, but one in which we have a great track record, with many commercially successful products delivered for clients in the pharmaceutical, medical device, consumer healthcare, hospital equiptment and scientific instrument markets.
An effective development process underpinned by informed decisionmaking is fundamental to our work. If a project is planned and structured correctly from the outset, then key risks can be identified early and managed towards a positive outcome.
Successful medical and scientific device development requires keen attention to detail, with commercial realities meaning that development effort must always be appropriately directed. You can have confidence that our creativity, technical rigour and sensitive design skills will be intelligently focussed to deliver exciting and effective solutions to the challenges you bring.
5
4
17
7 Production Support 6 Design Transfer
Design Verification Design Validation
Production Validation
Medical and Scientific services
We offer a comprehensive design service to the medical and scientific industries, with development processes aligned to standards and regulations in the EU and US.
18

19
Microbial Systems
CellFacts II
Modular real-time cell analysis instrument

Design planning
Mechanical engineering
Industrial design
Prototyping
Production support
20
With extensive experience of strategically important and technically demanding projects, our large multidisciplinary development team comprises mechanical engineers, electronics and software engineers, industrial designers, usability and interaction experts, researchers, prototyping technicians and specialist project managers. We provide fully integrated product development services from initial project direction through to detailed support for industrialisation. Our skills include product development strategy, design research, project planning and management,
intellectual property strategy, concept creation, prototyping, feasibility studies, design auditing, risk management, detailed design, IEC62304 compliant medical device software development, engineering analysis, evaluation testing, usability engineering, design verification, supplier selection and technical support for industrialisation. We can provide a complete turn-key development service, or staged input to a project.
Our design and development service is certified to ISO 9001 and ISO 13485.
Fully integrated product development services from initial project direction through to detailed support for industrialisation.
21
Our connected disciplines
DCA’s specialists offer robust tools and techniques in every field of product design and development, but it’s the connection between these different disciplines that we believe make us unique.
Our studios, laboratories and workshops have different disciplines working side by side. Our ability to connect and integrate the right disciplines, at the right time, in the right way is the cornerstone of our approach.
Since our foundation a multidisciplinary philosophy has been the cornerstone of our approach to product design and development.
22
Software Engineering
Interaction Design
Mechanical Engineering
Electronic Engineering
Industrial Design
Design Research & Planning
Prototyping
Human Factors & Usability
23
Research and Strategy
Research and Strategy at DCA exists to inspire and inform these decisions, providing the cultural and user insight on which to build great product strategy and designs.
Practised by a team with diverse experience we use a range of tools to build robust data and rich stories. No two projects are the same. We go wide and we go deep, gaining intimate knowledge of the relationship between people, brands, products and their environments.
Deciding which direction to take a design, or even what to design next, often proves one of the greatest hurdles in product development.
24

25

26
For us, this means employing the best engineers with a wealth of individual and collective experience. It means planning projects rigorously and applying individually tailored development processes during their implementation. It means using cutting-edge tools and techniques to develop and test our ideas. And it means integrating our engineering thinking, closely with
our other in-house product development skill bases to deliver unified project results.
World class engineering is at the heart of most projects we undertake and provides our clients with the highest probability of success, even with the most technically challenging developments.
To consistently deliver market leading products you need a world class approach to engineering.
27
Mechanical Engineering

28
Industrial Design
The space between medical devices and consumer products has become blurred and market tolerance for poorly executed visual design is low. Yet there are still some important differences between medical and consumer products. Safety must always be paramount, usability cannot be compromised and longer market lifecycles mean that visual design must transcend short-term fashions and trends.
In this context we believe industrial design should be informed and relevant. It should be highly creative and push what is technically possible.
We achieve this by integrating the industrial design team with research, usability and engineering disciplines and by employing designers who understand strategic context and are passionate about detail.
In an increasingly sophisticated world, external form and visual detailing have become an important expression of the quality and performance of a medical device, or scientific instrument.
29

30
Human Factors and Usability
We integrate human factors and usability throughout the design process, adopting domain-specific regulations and guidance from ISO 62366.
Emphasis is placed on moving beyond compliance to leverage the commercial benefits of more inclusive products and services that optimise system performance.
We inform ideas and their implementation through a deep understanding of the relationship between people, products, and their environment.
31

32
Interaction Design
In an increasingly connected world, new challenges have emerged in delivering compelling user experiences. Our multidisciplinary approach delivers product interactions across integrated physical and digital platforms that are simple, intuitive and a delight to use.
Our team combines interaction, graphic and industrial designers, researchers, electronics hardware and software engineers to develop
co-ordinated product experiences. Whether extending products with digital touchpoints or developing interactions for embedded hardware, we use an integrated approach to create future facing concepts and develop these through to production.
Our multidisciplinary approach delivers product interactions across integrated physical and digital platforms.
33

34
Electronic Engineering
Success in these areas depends on robust requirements definition and careful partitioning of functionality between electronic, mechanical and software sub-systems. The effective management of interfaces and interactions between sub-systems is key, and is greatly enhanced by an integrated team structure. For this reason our electronics engineers, designers, mechanical engineers and researchers work very closely together from the start of projects to capture, define and translate requirements into effective design solutions.
Whether electronic functions are an inherent part of your new product architecture, or an existing mechanical system needs to be enhanced with new electronic features, we have the skills and knowledge to meet your development challenge.
Our electronics team have developed medical devices and scientific instruments with functions ranging from simple automated monitoring to sophisticated electromechanical control, diagnostics and connectivity.
35
Software Engineering
Software must be carefully planned and diligently executed, but this does not mean that it has to be slow. DCA’s agile software development process is fully compliant with IEC 62304, but also draws on years of experience developing code efficiently for the consumer goods and automotive sectors.
With a powerful blend of experience, talent and rigour, our software engineers integrate seamlessly
with our electronics, mechanical engineering and interaction design teams to deliver products ranging from complex electro-mechanical systems through to more simple, but equally compelling devices.
A rigorous approach to software engineering is fundamental to safe, effective and successful medical device development.
36

37

38
Prototyping and Evaluation
Prototyping is at the heart of our business.
Since our foundation we have always had extensive workshop and prototyping facilities in the centre of our studios.
This enables us to explore, test and iterate concepts at increasing levels of resolution throughout a project and is a fundamental part of our product development and risk management processes.
39
DCA partnered Sanofi throughout the development of SoloStar®, applying our rigorous evidencebased approach to all aspects of the design. The result is a device that delivers leading performance in almost every respect. With superior levels of safety and comfort, the pen is sophisticated, yet simple to use.
Since its launch in 2007, SoloStar® has been adapted for use across a range of therapies and can now be found in almost every market around the world.

The multi-billion selling SoloStar ® pen injector is one of the world’s best known drug delivery devices.
40
Sanofi
SoloStar®
Disposable insulin pen injector

Design planning
Usability and HF
Mechanical engineering
Industrial design
Colour, material and finish
Instructional design
Graphic design
Prototyping
Testing and evaluation
Production support

41
Sanofi
Toujeo® SoloStar®
Disposable pen injector for concentrated insulin

Design planning
Mechanical engineering
Industrial design
Colour, material and finish
Instructional design
Packaging design
Graphic design
Prototyping
Testing and evaluation
Production support
42
Toujeo ® SoloStar ®
Building on the multi-award winning SoloStar® platform, DCA partnered Sanofi to develop a new range of pen-injectors for Toujeo®, a triple concentrated basal insulin.

Toujeo® SoloStar® contains 450 insulin units, deliverable in single unit increments. Due to the triple-concentrated formulation,
dose accuracy tolerance limits are three times tighter than for a standard pen, meaning that doses need to be delivered within a ±0.0033mL window.
Toujeo® SoloStar® was painstakingly engineered to achieve this exacting requirement and also to provide an ultra-low injection force.
43
Sanofi
Toujeo® Max SoloStar®
Disposable pen injector for concentrated insulin

Design planning
Mechanical engineering
Industrial design
Colour, material and finish
Instructional design
Packaging design
Graphic design
Prototyping
Testing and evaluation
Production support
44
Toujeo ® Max SoloStar ®
Toujeo® Max SoloStar® has a capacity of 900 insulin units, the largest of any long-acting insulin pen on market, and an increased maximum dose of 160 units, selectable in 2 unit increments.
This means fewer pen changes and fewer injections, making life easier for the increasing number of patients on higher insulin doses.

45
By some estimates, half of patients with chronic medical conditions fail to take their drugs as prescribed. For many, this is a conscious decision. Intelligent design of drug delivery devices can help to change this.
Within the context of drug delivery devices, the conventional approach to improving therapeutic compliance is often to think in terms of reminders or dose counters. While these devices have a clear role to play, they only tackle one part of the problem – unintentional nonadherence. They do little to explicitly challenge patients who elect not to take their drugs as prescribed. The scale of the problem is hard to quantify, but there is evidence that a significant proportion of patients with chronic medical conditions actively decide not to comply with their prescribed treatment; some estimates attribute intentional nonadherence as high as 70% of the issue ²
There is a consensus that the reasons for intentional nonadherence are complex and often idiosyncratic, meaning that it is unlikely that any single intervention will ensure that all patients take their medication as directed. Systemic solutions are needed that help patients to better understand and engage with their therapy, along with drug delivery solutions that cater for the patient’s emotional, as well as physical needs.
This article will explore what device developers can do to confront these issues within realistic commercial constraints that tend to favor ‘standardized’ device solutions.
The Non-Adherence Problem
Medication non-adherence is one of the biggest challenges facing health care providers. It is incredibly difficult to determine exactly how many people are affected, or why patients are not taking their drugs as prescribed, however, current estimates from the World Health Organisation 1 are that 50% of patients around the globe, with long-term illnesses, do not take their medications as prescribed. In the USA alone, it is estimated to cost $290 Billion a year 3, cause 125,000 deaths annually, and account for 10% to 25% of hospital and nursing home admissions 4
Intentional or Non-Intentional?
Unintentional non-adherence is usually related to some form of forgetfulness or confusion. The result is that patients may forget to take a dose, take the wrong amount, repeat a dose, or take the incorrect drug.
Intentional non-adherence, on the other hand, relates to situations where patients are aware of what drugs they should be taking and when. However, they decide that they do not wish to take their drugs as prescribed. This may mean that users fail to take any of the prescribed drugs, end a course
By some estimates, half of patients with chronic medical conditions fail to take their drugs as prescribed.
Published on 17th December 2015 46
The art of persuasion: Designing devices for patients who don’t want to adhere
prematurely, or take a different dose to that prescribed.
Behaviour Change
Ostensibly, the intentional nonadherence challenge is one of behavioral change. One commonly adopted approach is to address this challenge by viewing patients as the problem. Designs then focus on making patients more motivated, preventing them from doing something, or to persuade them to do something else. Fear tactics are one example of this; however, their efficacy is questionable, moreover, they can lead to patient anxiety, often in individuals who have no issues with adherence.
An alternative approach is to view the patient as a rational decision maker that has absorbed the information provided to them, considered it in the context of their
particular situation and decided to depart from the prescribed drug regimen. Like the reasons for non-adherence, the way in which individuals make decisions are largely idiosyncratic. However, there have been many attempts in the past to describe decision making activities. One model commonly used is the OODA loop. This describes a feedback loop where decision makers observe the information available to them, they then Orientate this information to their own lives and the specific context, Decide which of the available actions they should adopt, and then act.
Learning from decision making theory, it is evident that the presentation of information alone does not change user behavior, rather it is the process
of interpretation, or orientation, that is key. In order to both gain and maintain user engagement, users need to be able to relate the information provided to their own lives and the specific context of use.

47
Many people in the drug development and distribution chain also have their role to play in increasing adherence.
Accordingly a key opportunity in helping users to adhere to their drug regimen lies in the orientate phase of this decision making cycle.
What is clear is that much of the patient information currently provided is often not being read or not being understood. A Danish study 5; found that 40% of elderly patients did not understand the purpose of the drugs they were taking, while only 21% understood the implications of the omission of a drug or dose. This is perhaps unsurprising when viewed in the context of how many drugs some people are taking – with 60% of over 65s in the US taking five or more medications 6 .

A recent study assessing the impact of text messaging on adherence 7; also provides some interesting insights. The study involved 303 participants; half of which received SMS alerts prompting them to take their drugs, while the other half, the control group, received no intervention. The first insight is that the reminders were helpful; in fact 60% reported that they were reminded at least once to take a dose that they may have forgotten. This is perhaps unsurprising, but validates the assertion that reminders have a role to play in the adherence challenge.
What gets a bit more interesting is what else the study found. When
In many cases, there are opportunities to simplify drug regimens by viewing them holistically and reducing the number of drugs required.
48
comparing drop out rates (i.e. those either stopping completely or taking less than 80% of their medication), it is apparent that the drop out rate was considerably lower for those in the test group (25% in the control group compared to 9% of those receiving text messages). Further examination reveals that those in the test group were asked to text back with any concerns that they might have. 15% of the test group reported concerns on at least one occasion, because of uncertainty over the need for treatment, concern over side-effects, or another medical illness. Each case was followed up with a telephone call to address these concerns. This intervention, simply by calling them up and explaining these concerns, resulted in 87% (13% of the test group) resuming treatment.
These findings of this study suggest that actively addressing patient concerns and uncertainty can have a marked impact on adherence rates.
For device manufactures the next logical question should therefore be how can medical devices, and the ecosystems that surround them, be designed to support this.
What Can Device Developers Do?
As a systems issue, health care providers are well placed to make changes to adherence levels. In many cases, there are opportunities to simplify drug regimens by viewing them holistically and reducing the number of drugs required. Likewise, the way patients receive their drugs can be simplified, a number of pharmacists are now offering clearly labelled sachets containing all of the drugs a patient should take at a given time.
Many people in the drug development and distribution chain also have their role to play in increasing adherence. Within the marketing team, the way drugs are presented and even named have the potential to impact adoption,

packaging design will undoubtedly have an impact on the way drugs are perceived, likewise the way drug devices look, feel, sound, taste, and function will all shape perceptions. Focusing on medical device development, and more specifically products that patients are using to self-manage their regimens, there remains much that can be done. As highlighted in the text message study, engagement has a key role to play in tackling the adherence challenges. This challenge can be split between gaining and maintaining engagement.
Maintaining Engagement
The concept of seeking to maintain engagement is often more familiar to device developers, involving topics that are well understood by those familiar with good usability engineering practice. This involves minimizing the impact on patients’ lifestyles.
49
Article by Dr D. Jenkins Research Lead Human Factors and Usability
Rob Veasey Senior Sector Manager Medical and Scientific
Matthew Jones Senior Sector Manager Medical and Scientific


Common tactics include focusing on:
• Convenience and flexibility of use (products should generally be unobtrusive on users’ lifestyles. Extending the time between doses and flexibility in the timing of doses. They should be transportable, allowing users to use them, and safely dispose of them in a wide range of scenarios. Patients should be supported in managing their drug regimen).
• Time taken (devices should minimize the time required to set up, use, and safely dispose).
• Complexity of use (devices need to be intuitive to use – matching the cognitive abilities and expectations of the target users).
• Physical effort and comfort (the forces and postures required to deliver a drug need to be carefully considered and controlled).
• Capturing and sharing information on adherence (can the device system communicate progress to the patient, providing feedback and reward? Where appropriate, can the system capture the level of adherence for health care providers or carers).
Gaining Engagement
Understanding the patient’s initial decision making process is central to gaining engagement. This is a topic that tends to receive less attention in standards (e.g. IEC 62366) and guidance documents (e.g. HE75).
Playing the decision cycle described in the OODA loop backwards can reveal some interesting insights. Explicitly considering the decisions that users are making, and how their specific view of their condition and context shapes this, is critical.
Ultimately, the way we design and present information to users should be driven by a clear understanding of the way they orientate themselves with this information.
In this context, the ‘information’ goes well beyond written instructions. It includes all sensorial aspects of the device that patients are interacting with. The form, the colour, the material, the way it feels, and the way it responds are all information prompts that shape the way users decide whether to engage.
A second opportunity lies in the decision making section of the model. Carefully controlling the options available to the patient can also assist in the process. Pre-metered doses or treatments in a single pill may help to reduce patients under dosing or overdosing. There are a number of activities that can help device developers to understand the orientate phase:
• Understanding the context (understanding the condition and the system of therapy, considering how and when patients are engaging with this product and other products used in conjunction).
• Developing an emotional connection (Getting products out of the medicine cabinet. Designers and developers should ask what makes users love and engage with a product and not want to hide it away. There are many lessons here that can be learnt from how traditionally ‘taboo’ consumer products are now packaged).

Ultimately, the way we design and present information to users should be driven by a clear understanding of the way they orientate themselves with this information.
This article was originally published on the MDT - Medical Design Technology website 50
References
• Information that the users can relate to (Devices should be designed to support simple instruction. Instructions should be limited to major points presented using clear, everyday language and photographs or pictograms, covering why they should follow each instruction, along with how).
What does it mean for Standardized devices?
Based on the guidance captured thus far, it is apparent that devices that resonate with individual needs, or certainly the needs of sub-groups of the population are important. This may be in the form of fun ‘funky’ products for children, or ruggedized products for those who are keen to take part in outdoor pursuits. However, this requirement for multiple variants of the same device presents significant challenges for medical device manufacturers. Even subtle differences in colour can mean separate regulatory submissions – resulting in additional cost and potential delays in getting drugs to market.
The result is that, in most cases, a single product must be found that balances the needs of its diverse user base. This requires detailed
1 World Health Organisation report (2003). Adherence to long-term therapies: evidence for action. ISBN 92 4 154599 2.
2 Reid. K (2012). The Heart Of The NonAdherence Epidemic. Available at http://www. atlantishealthcare.com/news-media/details/ the-heart-of-the-non-adherence-epidemic accessed 06/07/14.
3 CVS Caremark (2012). State of the States: Adherence report.
4 Smith DL. Compliance packaging: a patient education tool. Am Pharm. 1989;NS29(2): 42–45. 49–53.
consideration to ensure that product can be both standard while still meeting the needs of as wide a patient group as possible.
Arguably, the challenge of maintaining engagement is far better understood and well captured in guidance (e.g. HE75). The FDA focus on safety and efficacy seeks to ensure that the physical forces required and the complexity of use is appropriate for the user population. However, the process prescribed in IEC 62366 does far less to encourage explicit consideration of how devices can be optimized to gain engagement. For that, a different focus is required. There is a wide range of tools from the fields of human factors and design research that can help to structure this focus. Techniques such as ethnography and semistructured interviews allow device developers to gain a richer insight into the lives of device users. Furthermore, they can help inform how an emotional connection can be established. In addition, a detailed understanding of decision-making psychology can help structure what information is required, along with where, when and to whom it should be displayed.
5 Barat I, Andreasen F, Damsgaard EM (2001). “Drug therapy in the elderly: what doctors believe and patients actually do”. British Journal of Clinical Pharmacology 51 (6): 615–622.
6 Belcher VN, et al., (2006). View of older adults on patient participation in medication-related decision making. Journal of general Internal Medicine. 21 (4): 298-303.
7 Wald DS, Bestwick JP, Raiman L, Brendell R, Wald NJ (2014) Randomised Trial of Text Messaging on Adherence to Cardiovascular Preventive Treatment (INTERACT Trial). PLoS ONE 9(12).
Even subtle differences in colour can mean separate regulatory submissions – resulting in additional cost and potential delays in getting drugs to market.
51
Sanofi
Lyxumia®
Disposable pen injector for GLP-1

Design planning
Usability and HF
Mechanical engineering
Industrial design
Colour, material and finish
Instructional design

Packaging
Graphic design
Prototyping
Testing and evaluation
Production support
52
It is the product of an intensive, evidence-based development programme focused on improving the injection experience of patients, for whom Lyxumia® may be their first experience of self-injection.
Providing a device that is not only intuitive to use, but is also comfortable and reassuring was a primary consideration in the design.

The Lyxumia® pen is an easy to use disposable injector, intended to help people with type 2 diabetes.
53
Sanofi
Variable dose auto injector

Colour, material and finish
Industrial design
Insight and strategy
Mechanical engineering
Packaging design
Industrialisation support
Prototyping
Testing and evaluation
Pen
'3D'
54
‘3D Pen’ is a disposable pen injector with an unparalleled feature set for precise delivery of variable doses of medicine. It enables a class-leading maximum dose of 120U to be selected and delivered in single unit increments.
Unlike traditional pen injectors, the innovative spring-powered mechanism avoids the need for the button to wind out of the housing when selecting a dose. This means that very large doses can be comfortably delivered automatically, at the touch of a button.

The ‘3D’ variable dose pen injector with zero dial extension, automatic injection and innovative user feedback features, provides class-leading usability and flexibility.
55
AllStar ® is the first reusable insulin pen produced by a global pharmaceutical company in India.
The pen injector is the result of three years exemplary team work between DCA and Sanofi, with the sole purpose of offering a product that matches the needs of people living with diabetes in India and other developing markets.
AllStar® is a state-of-the-art device that is easy for patients to use and also supports physicians in early initiation of insulin therapy, for better glycaemic control and enhanced therapeutic outcomes.
56

57
Sanofi
AllStar®
Reusable insulin pen injector

Design planning
Usability and HF
Mechanical engineering
Industrial design
Colour, material and finish
Instructional design

Prototyping
Testing and evaluation
Production support
58

59
Sanofi
TouStar®


Reusable pen injector for concentrated insulin

Colour, material and finish
Industrial design
Insight and strategy
Mechanical engineering
Packaging design
Production support
Prototyping
Testing and evaluation
60
With a dedicated replaceable cartridge system, TouStar® can only be used with Toujeo® insulin cartridges. These contain a concentrated ‘U300’ formula with 50% more insulin than a regular cartridge, making them more convenient and longer lasting.
To prevent users from accidentally attaching incompatible insulin cartridges to TouStar®, the new dedicated cartridge system simplifies the cartridge exchange process and helps to prevent insulin mix-ups from occurring.

TouStar ® is the first reusable insulin pen for a concentrated insulin.
61
Sustainability and drug delivery devices

We are witnessing a global boom in the use of drug delivery systems. Valued at approximately $500 billion in 2016, this market is forecast to reach nearly $900 billion by 20251 . Increase in demand for self-administration and home healthcare devices has helped fuel this expansion, meaning that today drug delivery devices are not only much more widespread, they have become easier to use, safer and more effective. They form an essential part of our healthcare infrastructure, enabling delivery of countless therapies that save lives and improve patient outcomes on a vast scale.
As they have been targeted at wider audiences, drug delivery systems have become more disposable. This market trend has been driven primarily by the desire to improve safety and usability. Disposable devices typically require fewer operating steps than reusable ones and because they have a finite life, they are less susceptible to wear and contamination. A good example is the evolution of Dry-Powder Inhalers (DPIs). These began in the 1970s as relatively simple re-
usable devices, such as Spinhaler and Diskhaler in which users fitted replaceable capsules or blister packs containing the drug product. They are now predominantly disposable products, in which the primary pack is sealed for life.
Drug delivery devices have also evolved to become more mechanically sophisticated. For DPIs, the requirement to automatically manage drug primary packaging and the addition of safety features such as dose counters has driven this trend. A similar story is found with injection devices, where one of the latest generation spring-powered disposable insulin pen injectors has seventeen components. In contrast, when first developed in the late 80s and early 90s, disposable pen injectors typically had fewer than ten parts. As device complexity has increased, designers are also able to select from an ever-growing pallet of polymers. This has enabled improvements in performance and reliability, but the mix of materials now found in many devices adds to already significant challenges for recyclability.
In this article, Rob Veasey, Senior Sector Manager, Medical and Scientific, at DCA Design International, explores the environmental challenges facing the drug delivery device industry, and the opportunities they bring.
Published on 30th September 2020 62
The need for change
As scientific evidence of the environmental challenges we face becomes clearer, the necessity to improve the impact of products we use every day has become more pressing. We all share this responsibility, but governmental bodies have taken measures to drive adoption of more sustainable practices. Under the 2015 Paris Agreement, the UN is aiming to keep global temperature rise to below 2°C above pre-industrial levels. Nationally determined contributions will lay out how each country aims to reduce emissions and adapt to the impacts of climate change. The UK’s current target is a reduction in greenhouse gas emissions of at least 80% by 2050, relative to 1990 levels2. As countries develop and publish their individual strategies, the impact of commitments will become evident. It can be expected that changes will be needed to the way that most products are manufactured,
distributed, used and recycled.
The contribution of our industry to environmental damage is also beginning to receive greater attention. Within the last couple of years, reports have emerged describing the global warming effects of pressurised metered dose inhalers (pMDIs). For example, it has been estimated that the propellants used within these devices contribute a staggering 4% of the total carbon footprint of the National Health Service (NHS) in the UK3. ‘Greener’ alternatives are being developed, with at least two major players, AstraZeneca and Chiesi, recently announcing a commitment to develop pMDIs with near zero Global Warming Potential. This is one example of the steps our industry is taking, but further substantial actions will be needed and we must all start to plan for and develop more sustainable drug delivery systems.
A further concern that has recently received publicity is the dramatic increase in plastic waste entering and damaging marine environments. In response, the EU has taken action to ban an array of singleuse items, put in place new targets to encourage recycling of plastic products and mandated the use of more recycled polymers4. Due to the vital role that single-use plastics perform in healthcare and the difficulties in re-using many types of medical devices, this EU legislation does not apply to the medical industry. The performance and inherent safety of polymers, coupled with their cost-effectiveness, makes them ideal for medical applications. This is unlikely to change in the short term, but in the context of increased legislation and efforts within other sectors, it seems almost certain that our reliance on single-use plastics in healthcare will start to come under greater scrutiny.
 Figure 1 - Single-use plastics play a vital role in healthcare.
Figure 1 - Single-use plastics play a vital role in healthcare.
63
The contribution of our industry to environmental damage is also beginning to receive greater attention. Within the last couple of years, reports have emerged describing the global warming effects of pressurised metered dose inhalers (pMDIs).
The Circular Economy

The environmental challenges we face are complex and multi-faceted, meaning there is unlikely to be a simple ‘one size fits all’ solution. So what factors should we be thinking about and what opportunities might these bring?
At the heart of sustainable thinking is the concept of the circular economy. This idea involves the gradual decoupling of economic activity from consumption of finite resources and seeks to remove waste from systems. The aim is to build longterm resilience, generate new economic opportunities and provide environmental and societal benefits.
One important aspect of this model is the distinction drawn between biological and technical cycles. The ultimate aim is that consumption happens only within biological cycles, where biologically derived materials can be returned to the system through processes like composting. In contrast, technical cycles should aim to recover and restore products, components, materials and chemicals through strategies like reuse, repair or recycling.
Whilst a fully circular economy will be hard to achieve, by searching out opportunities to minimise waste throughout the lifecycle of products, we can take steps towards this goal.
The environmental challenges we face are complex and multi-faceted, meaning there is unlikely to be a simple ‘one size fits all’ solution.
64
Figure 2 - At the heart of sustainable thinking is the concept of the circular economy.
Measuring sustainability
When you set out to improve a system, it is essential to define the metrics by which the improvement will be assessed. Without this, it is impossible to know if or when progress has been made. The first step in defining sustainability targets is therefore to decide what to measure and how to compare performance. In the context of the circular model, three key parameters are important:
• The energy consumed in manufacture, distribution and use of a product.
• The amount of material that is derived from renewable or recycled content.
• The amount of material that can be recovered for re-use at end of life.
In some instances, factors influencing the choices made in relation to these parameters may be conflicting, or they may conflict with other design requirements. It is therefore essential that we develop objective ways in which conflicts can be understood and resolved to achieve the best environmental profile for a product. This understanding is typically gained through life cycle analysis (LCA), following methods defined within the ISO14000 standards. Owing to the huge array of factors involved in manufacture and distribution of products, LCAs are complex and time consuming to compile. This makes them an impractical tool to inform design decisions in real-time and as a result, they are often used to analyse designs and production systems retrospectively.
To achieve most value from LCA, detailed analysis of existing product
solutions is best used to inform the development of new ones. This identifies where the biggest opportunities for improvement exist, enabling effort to be directed where it will be most effective. This can sometimes be in unexpected places; for example in the case of a device that requires cold-chain distribution, significantly more energy may be consumed in transporting and storing the product than manufacturing it. In this instance, improving secondary packaging to increase packing density may be the most effective way to improve the environmental profile.
Whilst LCA remains the ‘gold standard’ means for assessing the environmental impact of a product, the design industry needs other tools to help assess and inform design decisions rapidly during development. Such tools do not need to be fully comprehensive, but they should allow engineers to make informed decisions about options that might impact on the environment. Some promising early options, such as Eco-Indicator 995 have been developed, but to remain relevant and useful it is essential that these continue to be maintained and improved. Given the increasing focus on sustainable development, it seems inevitable that new tools will become available in coming years.
Developing more sustainable devices
If we restrict our analysis to mechanical drug delivery devices, two issues that are commonly encountered in relation to sustainable development are materials selection and product lifetime. Neither of these issues are straightforward, since environmental design decisions are never taken in a vacuum. Instead, these factors
must be weighed alongside many other requirements that safety critical products such as drug delivery devices must achieve.
Sustainable materials
Most drug delivery devices are made predominantly from plastics, so careful selection of these materials is important when targeting more sustainable solutions. Following the principles of the circular economy, we can break this down into three aspects for consideration.
• Renewable and recycled polymers
Devices are typically manufactured from ‘medical grade’ polymers. These can be traced back to their raw material batches and come with guarantees that the formulation will not change. Recycled polymers are not currently available with medical grade certification and so the most sustainable alternatives in the medium term are likely to be biopolymers. Derived from biological rather than petrochemical sources, a small range of biopolymers are starting to emerge. Unfortunately, in the short-term, device developers are likely to be faced with a lack of choice for biopolymer grade variants. They also carry a price premium compared with conventional polymers and some grades need to be separated from standard recycling streams. Because of the relatively small size of the medical sector in comparison with the wider polymer market, it is unlikely that our industry will drive the development of new sustainable materials. Instead, we should seek to be fast followers of other industries such as food packaging, that use greater quantities of polymer and for whom regulations and customers are demanding rapid adoption of greener solutions.
65
Most drug delivery devices are made predominantly from plastics, so careful selection of these materials is important when targeting more sustainable solutions.
Article by Rob Veasey Senior Sector Manager Medical and Scientific

• End-of-life solutions that enable better recovery and recycling
Recycling of polymer materials contained in drug delivery devices is challenging, leading to problems in establishing the infrastructure to do this safely and effectively. Firstly, they usually contain some residual drug product and may also be contaminated with biological materials. Secondly, because each component is optimised for its particular function, they typically contain a mix of polymer types as well as materials such as glass, aluminium and rubber. Thirdly, they are often designed to be inherently difficult to disassemble to deter tampering or counterfeiting. As a result, it is difficult and expensive to reprocess devices by any means other than incineration for energy recovery. Chemical recycling, in which polymers are broken down into more basic chemicals that can be reprocessed to create new highperformance polymers may be one option, but this technology is not yet widely established. To improve the recyclability of drug delivery devices, many of these issues will need to be addressed at a design level, so must become a requirement at the outset of new development programmes.
• Lower embodied-energy
Not all polymers are created equal and they have subtly different environmental profiles. Generally, more complex polymers require higher energy usage during their manufacture. For this reason, simple polyolefins such as HDPE and PP are usually considered more sustainable than alternatives such as ABS, PC or POM. Clever design and materials selection can optimise part count and the use of polymers, ensuring that more complex and highly refined materials are only used where they are absolutely necessary.
Extending device life
Given challenges in sourcing more sustainable materials, extending product life may be the most effective path to improving the environmental profile of drug delivery devices in the near-term. The longer a device can be used, the lower the environmental impact is likely to be in terms of material usage, waste and energy expenditure when assessed over a fixed period of therapy.
In this context, it is evident that re-usable drug delivery devices are likely to have better environmental profiles than disposable ones. At the beginning of this article, I outlined that recent trends have been in the other direction, so what can be done to reverse this?
• Reusable devices that are easier, safer and more convenient to use
A primary concern with reusable drug delivery devices has often been usability. Patient groups regularly contain large numbers of individuals with reduced manual dexterity or vision impairments. These patients can struggle to correctly replace a primary pack, or reset the operating mechanism. To address this, we should continue to make reusable devices easier to use, for example ensuring that the mechanism resets automatically when the old primary pack is removed, or when a new one is fitted.
• Reusable devices that are more appealing
In the past, there have often been no real advantages to selecting a reusable device over a disposable one. Indeed, as described above there have been some legitimate concerns in relation to usability; yet this should not be the case. With reusable devices, cost is typically offset against a usable life of years
In this context, it is evident that re-usable drug delivery devices are likely to have better environmental profiles than disposable ones.
This article was originally published in the September/October 2020 issue of ONdrugDELIVERY magazine. 66
References
rather than days or weeks, so there is an opportunity to specify better materials to achieve improved performance and to include more automated features. One interesting development that may help to tip the balance in favour of reusable devices is the advent of connected drug delivery systems. For many applications, the cost of electronic monitoring and control functionality is currently seen as a barrier to embedding this technology within disposable devices, making reusable solutions much more attractive. Connected systems may also help to balance some usability downsides of reusable devices, for example by providing warnings against potential use errors. For some applications it may remain impractical to offer fully reusable drug delivery devices. In these circumstances an alternative solution may be to develop disposable products that have greater dose capacity, so that their use-life is prolonged. This approach may of course bring challenges with drug stability and device affordability, but it is a trend that is already well established in some consumer markets and we are likely to see further developments of this sort within drug delivery in the future.
Closing thoughts
The environmental challenges we face are complex, but they also bring opportunities. By good design, there is no reason why more sustainable drug delivery devices cannot also be more cost effective and better for patients. But given the relatively long development cycles required for drug delivery devices, new environmental legislation may emerge that imposes targets that some businesses find hard to achieve. We are currently witnessing this in the automotive industry, where companies that have proactively developed sustainable product ranges are now in a much stronger position than those that left it late.
In many ways our industry is well equipped to deal with environmental challenges. We are systematic in our approach, data driven and highly analytical in our methods. Drug delivery devices are not subject to the whims of fashion; their performance and effectiveness must be comprehensively demonstrated before they enter the market, meaning that we tend not to embrace short-term thinking. To effect change, we will all need to adopt a more sustainable mind-set, in which we question the environmental impact of our decisions in the same way that we currently think about patient safety and therapeutic efficacy.
1 Size of the global drug delivery systems market in 2016 and a forecast for 2025, Statista Research, October 2018.
2 UK action following the Paris Agreement - UK Government, October 2016.
3 Progress on reducing F-gas Emissions - House of Commons Environmental Audit Committee, April 2018.
4 Press Release from the European Parliament, March 2019.
5 Eco-indicator Manual for Designers – a damage oriented method for life cycle impact assessment, October 2000.
By good design, there is no reason why more sustainable drug delivery devices cannot also be more cost effective and better for patients.
67
AllStar ® Pro premium reusable pen injector.
Sanofi have recently launched the AllStar® Pro pen injector in Europe and Canada. AllStar® Pro is a reusable pen injector, intended to help people living with diabetes. The pen uses replaceable cartridges, providing a convenient option for patients who inject regular doses of insulin. DCA partnered Sanofi throughout the development of
AllStar® Pro, targeting a product that delivers high quality with efficient use of materials. This important new device is the result of a rigorous development programme, which builds on the award winning AllStar® device platform.
68

69
Sanofi
AllStar® Pro
Reusable insulin pen injector

Design planning
Usability and HF
Mechanical engineering
Industrial design
Colour, material and finish
Instructional design
Prototyping
Testing and evaluation
Production support
70

71
Designing for uncertainty
Making decisions about the future direction of a product or service is not easy. Not only does it require commercial acumen and technical ingenuity, but it also requires an element of prediction – determining how the product or service will fit the future user and market needs.
Project teams can fall foul of one of two clear traps when deciding on the future direction for a product or service. Some teams limit the information collected, in favour of relying on intuition – progressing the design without a clear understanding of risk, while others collect too much, delaying decision making in a quest for clearer, more unequivocal, information. In the latter, there is a risk that analysis paralysis can set in – where decisions can be repeatedly deferred as additional questions are raised resulting in further research.
The pragmatic middle ground is to base decisions on a grounding of appropriate information, recognising when the information available is enough to progress for a given risk level. Perhaps more critically, the challenge is to ensure that the right information is sought.
While we cannot be certain of the future, we can make educated assumptions. Some assumptions will have high levels of certainty, others less so. Likewise, some assumptions will be critical to the design, others less so. Products stand the greatest chance of success if they are designed based on an explicit understanding of the assumptions that underpin key design decisions along with a description of their robustness and their criticality to the design. Furthermore, actively monitoring,
72
Article by Dr D. Jenkins Research Lead Human Factors and Usability
Rob Woolston Managing Director Malcolm Boyd Senior Sector Manager Medical and Scientific



and protecting, those assumptions plays an important role in increasing the likelihood of success.
Assumption-based design
The approach we have been refining over the past few years and describe as Assumption-based design creates an explicit, and auditable, link between the information available, the assumptions that are made based on this information, and design recommendations.
Understanding the links between information, assumptions, and design recommendations is critical. By linking assumptions to design recommendations, it is possible to understand which assumptions are more critical to the project, and which are less (or even irrelevant). Likewise, the information that is being used to direct future product recommendations can be explicitly highlighted.
When a rating of confidence is applied to the assumptions, the approach serves as a structured process for prioritising future research, focusing first on the
assumptions that have significant sway on design direction and those with lower levels of certainty.
The process can be summarised as follows:
1. Record information and insights collected
2. Record assumptions made
3. Link assumptions to information and capture a rating of assumption confidence
4. Record recommendations made
5. Link recommendations to assumptions and capture a rating of recommendation confidence
6. Identify critical assumptions
7. Determine the required processes to confirm and monitor information and assumptions
Information
The type of information collected will be dependent on the type of product being designed. However, it is likely to include a mixture of factors that can direct innovation:
Products stand the greatest chance of success if they are designed based on an explicit understanding of the assumptions that underpin key design decisions.
1 3 5 7 6 2 4 73
Needs
• Explicit stakeholder (end users, manufacturers, installers, maintainers, etc.) wants and needs
• Latent stakeholder needs
• Market demands (e.g. regulatory requirements, cost models)
Technology
• Latest component availability
• Current R&D pipeline
• Predicted technological innovations and costings (extrapolation of trends)
Category trends
• Descriptions of current competitor products
• Intelligence around competitor pipelines (what they are talking about coming next)
• Patent searches and landscaping
Macro trends
• Trends from parallel worlds (what is happening in other markets that tend to cascade down)
• Broader trends (e.g. attitudes towards disposable plastics, views on cashless transactions)
Assumptions
Assumptions are made based on the interpretation of one or more pieces of information.
As an example, for a given product, a number of information sources (such as ‘voice of the customer’ data and competitor portfolio mapping) may indicate the importance of a connected version of a product, leading to an assumption that a connected variant would be critical to the design.
We can be very confident about some of the assumptions that we make about a product or a service. Others can feel like little more than a guess. As such, it is important to have some way of capturing a description of their certainty, along with a link to the information source(s) used. This creates an auditable trail and allows assumptions to be revisited should the validity of an information source be subsequently questioned.
Recommendations
Recommendations can be treated in much the same way as assumptions.
74
When a rating of confidence is applied to the assumptions, the approach serves as a structured process for prioritising future research.
It is important to record what they are based upon, and the level of confidence in them. The adoption of a recommendation is likely to determine the importance of each of the linked assumptions and, in turn, the associated information elements. This may lead to further research to confirm the information.
Continuing with the example of a need for a connected device, this is likely to lead to a recommendation to develop a connected variant of a product. However, it may be critical to re-test this assumption throughout the development process to ensure that the product being developed is indeed meeting the needs of the consumer.
Improving the model
Once all of the assumptions are listed out, and linked to recommendations and information, it is then important to understand which are the most critical to the success of the product or service. This allows critical assumptions to be monitored and a focus to be placed on the assumptions that are critical to product success. Critical
assumptions can then be tracked, protected and hedged.
For example, if product success is linked to two core assumptions: that the product will have the lowest cost of goods (COGs) and that the cost will be a key driver in purchase decisions, then it may be critical to monitor competitor portfolios and innovation pipelines (e.g. patent searches) to understand if they are developing technologies or processes that may give them a cost advantage.
Cost advantages can be protected by further reducing COGs through cost reduction exercises (making it harder for the assumption to fail).
It can also be hedged by ensuring that the product has added value to consumers that would allow it to be a viable proposition even if the assumption were to fail (no longer the lowest cost on the market).
Conclusions
Our experience is that assumptionbased design provides a highly structured approach to product and portfolio planning. The explicit
nature of the approach provides a clear audit trail for decisionmaking providing a more efficient, transparent, evidence-based process.
This not only helps to guide product development, but it also helps to reduce instances of ill-informed decision-making and analysis paralysis.
This is particularly relevant when initiating a product in the face of uncertainty. Rather than delaying project kick-off in pursuit of further information, this approach can be employed to start the project based on a clear understanding of the assumptions made, resulting in a specification that is refined over time and allowing timelines to be met, while still managing risk and uncertainty.
75
We can be very confident about some of the assumptions that we make about a product or a service, others can feel like little more than a guess.
Sanofi
AllStar® Connect
Reusable connected pen injector

Colour, material and finish
Industrial design
Insight and strategy
Mechanical engineering
Electronic engineering
Software engineering (IEC 62304)
Packaging design
Production support
Prototyping
Testing and evaluation
76
Developed to help the treatment of diabetes by automatically recording the details of every insulin dose delivered. Dose records can be automatically and seamlessly transferred wirelessly to a smartphone.
AllStar® Connect is operated just like the rest of the AllStar® device family on which it is based, with no added user steps for data sync. It maintains the same ergonomic features, such as large maximum dose, low dispense force, short thumb reach and simple cartridge exchange.
Integrated connectivity without compromise to usability.
77

78

79
Cybersecurity and connected drug delivery, an integrated risk-based approach
John Whitehouse, Rob Veasey and Shane Day, discuss the value of integrating cybersecurity into a holistic, multidisciplinary approach to risk management for connected medical devices.
We are all becoming increasingly aware of concerns about the security of digital information impacting our lives. Most people routinely communicate online and, in the wake of covid-19, many of us now also extensively work, shop, bank and socialise in the digital space. This inexorable trend is revolutionising the way we live and is impacting the medical industry as both healthcare providers and device companies embrace digital technology as a means to improve patient outcomes and streamline service efficiency.
Of course, electronically programmable medical devices have been around for decades; what is different now is the widespread integration of these devices with a patient’s own electronic products and systems, such as mobile phones and home networks. This integration significantly increases the vulnerability of personal medical data to cyber-snooping and raises the very serious prospect that malicious attacks could be made that disrupt safe and effective operation of devices that are critical to the health and well-being of patients.
In 2017, the WannaCry ransomware attack affected hundreds of thousands of computers around the world. Whilst this attack was not specifically targeted at medical systems, it exposed the vulnerability of large, interconnected healthcare providers, such as the UK’s NHS. The attack resulted in
the cancellation of thousands of appointments and operations within the NHS. It was also reported that some staff had to revert to pen and paper and the use of private mobile phones, as centralised IT systems had become completely disrupted. Perhaps even more alarmingly, reports by cybersecurity researchers have demonstrated the potential vulnerability of safety-critical devices, such as wireless-connected insulin pumps and pacemakers, to hacking¹, raising the genuinely sinister prospect of targeted, remote, life-endangering attacks on individuals.
Whether inadvertently or deliberately, it is clear that cyber-attacks have the potential to inflict serious harm on patients. In response, regulators expect that cybersecurity vulnerabilities are adequately identified and addressed by developers and manufacturers of all electronically programmable medical devices.
What Needs To Be Protected?
When determining how to protect the cybersecurity of a medical device, the first step is to understand the data assets that the device manages. Data records, especially sensitive patient data, need protection from snooping and manipulation for both privacy and safety reasons. Additionally, the software running on the device may be a key intellectual property asset that needs to be protected from theft or tampering.
Published on 29th September 2021 80
As a second step, one needs to consider the environment in which the device will be used. For example:
• Will the device be connected to the internet?
• Does sensitive data need to be transferred to, as well as from, the device?

• Does the device need to be operating at all times?
• Will the device be used in public or private spaces?
The answers to these questions will help to inform decisions on the most appropriate type of communications technology for the device, such as Bluetooth, near-field communication (NFC) or cellular, which in turn enables the developer to explore potential system risks and vulnerabilities.
Consider a hypothetical scenario, wherein a new drug delivery device is being designed with connectivity features to support a patient in
tracking their medication and to enable live monitoring by clinicians (Figure 1). In this scenario, a patient interacts with their device using an app on their smartphone via a short-range, personal area network (e.g. Bluetooth Low Energy), which allows the patient to read a log of their dose history. Additionally, the device has an internet connection that allows data to be uploaded to a cloud-hosted database server. The patient’s clinician can access the data from the database for remote patient monitoring. The device also includes a wired access port for device maintenance and diagnostics by the manufacturer.
An initial cybersecurity assessment identifies that there are a number of possible points of interest for a potential attacker. Data records, including the details of a patient’s medication history and any sensitive personal data, could be of interest to an attacker looking to profile or track an individual. Access to the software and configuration settings
that control the device’s behaviour, either via the wired access port or wirelessly, could provide an avenue for malicious attacks, as well as theft of intellectual property. The presence of an internet connection could also make the device vulnerable to a variety of attacks, such as “denialof-service”, where the device is flooded with superfluous requests in an attempt to make it unavailable to its intended users.
Whether inadvertently or deliberately, it is clear that cyberattacks have the potential to inflict serious harm on patients.
Figure 1: An example system containing a connected drug delivery device.
81
Identifying Vulnerabilities
Once the device and its system architecture are defined, threat modelling should be applied methodically to identify potential vulnerabilities that need to be addressed. By examining the potential for cyber-attacks, such as spoofing (disguising a communication from an unknown source as being from a known and trusted source), tampering, data repudiation (hidden manipulation or invalidation of data), information leaks, unauthorised use or denialof-service, the potential impacts on device behaviour can be explored. The device developer should aim to generate a comprehensive list of cybersecurity risks that require consideration and mitigation during the development of the detailed design for the device.
When evaluating the potential severity of cybersecurity risks and assessing possible risk controls, a common approach is to consider confidentiality, integrity and availability (CIA) for each scenario. The US National Institute of Standards and Technology (NIST)

defines these terms as follows²:
• Confidentiality: Preserving authorised restrictions on information access and disclosure, including means for protecting personal privacy and proprietary information.
• Integrity: Guarding against improper information modification or destruction, including ensuring information non-repudiation and authenticity.
• Availability: Ensuring timely and reliable access to and use of information.
The relative importance of each criterion will depend on the intended use of a medical device. For a connected drug delivery device, integrity of data, such as records of drug delivery activity, may often be considered more important than confidentiality or availability. However, availability of data might be more important in scenarios where the drug delivery device needs to provide real-time updates, such as alerting a clinician to an occurring problem.
82
Figure 2: The relationship between cybersecurity and safety risks.
83
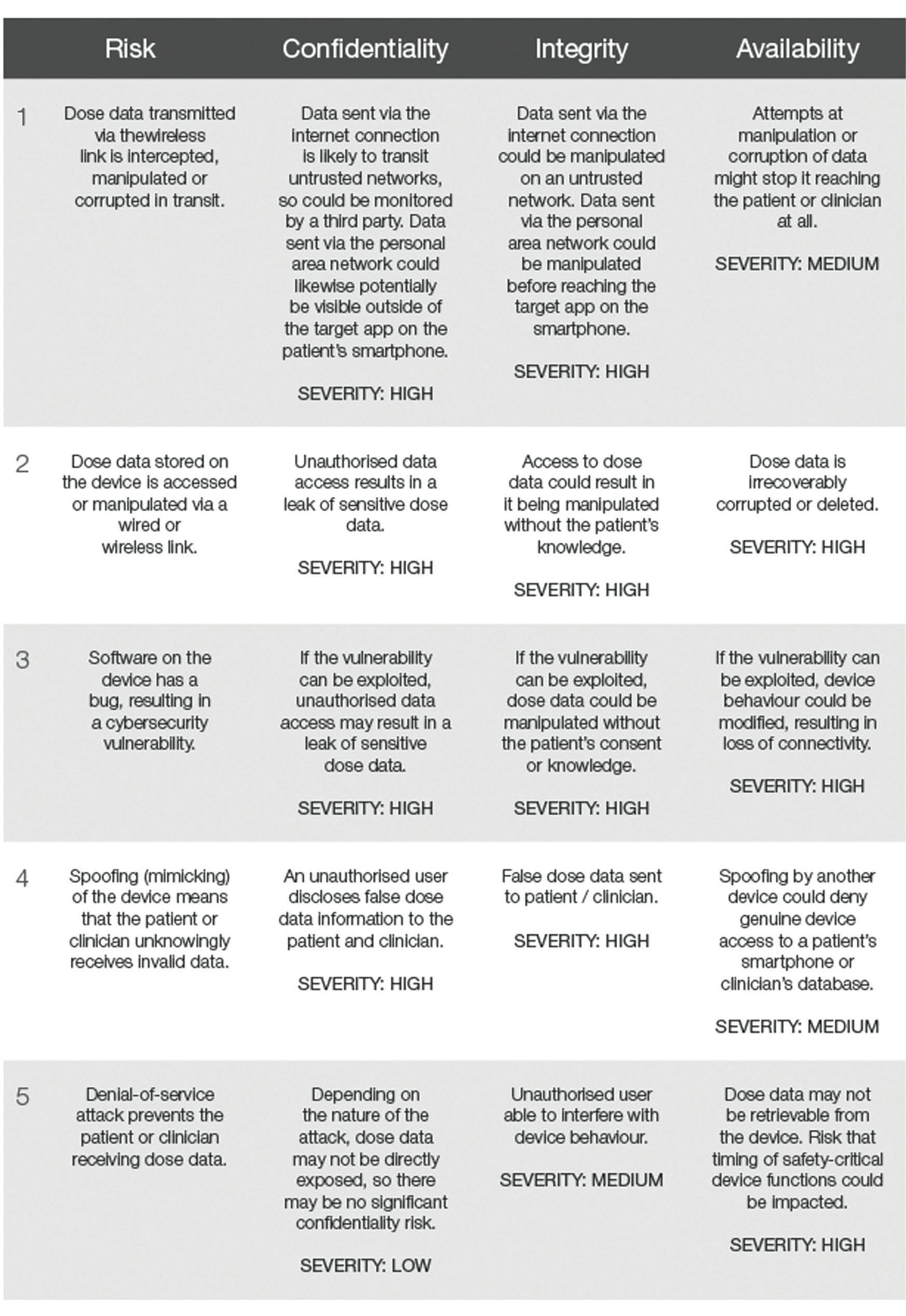
Table 1: Example cybersecurity risks identified using the CIA framework.
Article by John Whitehouse Senior Software Engineer


 Rob Veasey Senior Sector Manager Medical and Scientific
Shane Day Senior Skill Leader Electronics and Software
Rob Veasey Senior Sector Manager Medical and Scientific
Shane Day Senior Skill Leader Electronics and Software
Taking our hypothetical drug delivery device example, we have identified a few example cybersecurity risks and evaluated their potential impact using the CIA framework in Table1. Having identified cybersecurity risks in this way, they can then be resolved within the overarching connected device risk analysis.
When developing electronically programmable medical devices at DCA, the company also performs detailed research into known issues and published vulnerabilities for the hardware and software used in a medical device to support further risk identification. This includes examining supporting software documentation and assessing published information in open-source databases, such as the Common Vulnerabilities and Exposures (CVE) database. DCA also consults any appropriate guidance on the secure use of data communication protocols, such as Bluetooth Low Energy, that has been published by authorities like NIST.³
Cybersecurity As A Part Of Multidisciplinary Risk Assessment
After identifying potential cybersecurity risks, DCA’s approach is to manage and review the identified vulnerabilities as part of the overall risk management process for the device. This approach helps to ensure that all aspects of performance are considered and appropriately balanced. It is important to remember that a secure device is not necessarily a safe one, as shown in Figure 2, adapted from the Association for the Advancement of Medical Instrumentation’s (AAMI’s) technical report on the principles of medical device cybersecurity.4 The application of a cybersecurityfocused risk control measure in isolation from safety-related risk management could compromise essential performance of the device, for example by negatively impacting usability. One possible situation
where this might arise is if extra authentication steps are added to improve the security of the data shared from the device.
Returning to our hypothetical example device, let us consider some potential mitigations to the cybersecurity risks highlighted in Table 1 and the wider design impacts that their adoption could involve.
Risk 1 – Dose Data Transmitted Via The Wireless Link Is Intercepted, Manipulated Or Corrupted In Transit
In the case of dose data interception, manipulation or corruption in transit, one mitigation could be to specify and implement end-to-end encryption when dose data is transferred from the device to a smartphone or database. This could be supported by some form of pre-shared encryption key, though a better approach would probably be to use a secure key agreement protocol, such as Diffie-Hellman, for generating a shared encryption key across an insecure communications channel.
In reviewing this proposed mitigation, a relevant safety consideration would be whether the use of a computationally intensive encryption algorithm could impact on the timing of safety-critical functions, such as generating new dose activity records. This may require new design constraints to be specified to ensure that other device functions which impact patient safety are not compromised, such as the segregation of data transfer functionality from dose delivery or monitoring activities.
Risk 2 – Dose Data Stored On The Device Is Accessed Or Manipulated Via A Wired Or Wireless Link
When considering this risk, minimising the opportunities for data to be changed from outside of the device after manufacture would provide a useful mitigation. This could include restricting access
After identifying potential cybersecurity risks, DCA’s approach is to manage and review the identified vulnerabilities as part of the overall risk management process for the device.
article was originally published on the
This
MDT - Medical Design Technology website.
84
to dose data via the wireless and wired links, such as making it readonly. Adding integrity checks, such as error detection codes, could provide an additional detection mechanism in case of inadvertent data manipulation due to a device fault. In these cases, cybersecurity and safety mitigations are likely to be complementary, though the impact on essential performance should always be considered.
Implementing a user authentication scheme could provide a further mitigation for this risk, as well as for risks involving spoofing of a device. Authentication could, for example, involve the patient using their smartphone to scan a unique identifier printed on the device. Data from this identifier would subsequently be used to cryptographically confirm that the data is coming from the expected device. When reviewing this potential mitigation, however, there is a usability trade-off that needs careful consideration. The developer must assess whether the addition of this type of authentication means that the device remains usable and accessible for all target patients. Requiring additional authentication steps via a smartphone app may well be beyond the capabilities of some elderly or cognitively impaired users.
Risk 3 – Software On The Device Has A Bug, Resulting In A Cybersecurity Vulnerability
Where a software bug is published that may result in cybersecurity vulnerability, a couple of mitigation strategies can be employed. To improve monitoring and detection of such risks, a cybersecurity bill of materials(CBoM) can be prepared, which holds a list of software and hardware components that are, or could become, susceptible to cybersecurity vulnerabilities. The CBoM can be used to support risk
management through the device’s lifecycle. This includes assessment of purchasing controls and supply chains during manufacture and monitoring exposure to new vulnerabilities when the device is on the market.
Additionally, a device could be designed such that it supports remote software updates to patch software bugs associated with cybersecurity vulnerabilities. However, design of such a capability needs to be carefully considered to prevent the introduction of new cybersecurity risks. Such an update feature may provide a “back door” into the device for data manipulation, allowing pathways for unauthorised software changes or reloading of an old version of the software that has exploitable vulnerabilities. The remote software update protocol also needs to be sufficiently secure to avoid inadvertent loss of intellectual property. Microprocessor manufacturers are improving their capabilities for supporting secure remote software updates, but these should be carefully reviewed and evaluated as part of device risk management, as well as in design verification and validation planning.
Risk 4 – Spoofing Of The Device Means That The Patient Or Clinician Unknowingly Receive Invalid Data
Considering the risk of device spoofing, a potential cybersecurity mitigation could be to authenticate a patient’s device before accepting data from it. As with Risk 2, this could take the form of the patient using their smartphone to scan a unique identifier printed on the device, to confirm that the data is coming from the expected source.
Risk 5 – Denial-Of-Service Attack Prevents The Patient Or Clinician Receiving Data
Denial-of-service attacks can be mitigated by implementing a firewall to filter out opportunistic attacks
on the wired or wireless interfaces. Consideration of the intended use and careful design is then required to ensure that the risk is appropriately mitigated. Essential performance could still be impacted if most of the on-board computing resource on the device is required to service the firewall. A failsafe function could be considered in this situation too, which temporarily disables data communications to ensure essential performance is not compromised. However, this may not be appropriate where high availability is required; in this situation, a means of prioritising communications, such as alerts, might be required if the device needs to communicate whilst under a denial-of-service attack.
Conclusion
This overview only scratches the surface, as there are many technical solutions available to combat potential cybersecurity threats. When developing a connected drug delivery device, these solutions must be carefully considered in the context of the intended use, so that potential impacts on safety and usability are also appropriately balanced.
DCA believes that a detailed multidisciplinary approach to identifying and countering cybersecurity risks should be deployed throughout the development and lifecycle management of connected drug delivery devices, seeking to identify potential problems early, untangle conflicts and thereby achieve optimised design solutions. An effective development process is one that couples risk identification with informed design decision making to deliver safe, usable and cyber-secure connected devices.
References
1 Ngak C, “Black hat hacker can remotely attack insulin pumps and kill people”. CBS News, Aug 2011.
2 “Security and Privacy Controls for Information Systems and Organizations”. NIST Special Publication 800-53 Rev 5, Sept 2020.
3 Padgette J et al, “Guide to Bluetooth Security”. NIST Special Publication 800-121 Rev 2, May 2017.
4 “Technical Information Report: Principles for medical device security – Risk management”. AAMI, 16th Ed, 2019.
85
BD Libertas™
Wearable autoinjector for large-volume drug delivery

Design planning

Mechanical engineering
Industrial design
Colour, material and finish
Prototyping
Testing and evaluation
86
In 2018 BD and DCA won a prestigious iF design award and a Good Design award for BD Libertas™. It was developed to administer biologic medicines for various chronic diseases over longer periods of time that cannot be administered through the use of auto injectors. The design prioritizes safety, convenience and usability while delivering high performance
with manufacturing efficiency. An exciting new device built on a foundation of pharmaceutical company needs, user understanding, and technical robustness, BD Libertas™ is a significant step forward for the safe and convenient delivery of biologics.
BD Libertas™ is a wearable injector capable of delivering large-volume and high-viscosity drugs.
87

88

89
Piccoject™
Autoinjector platform

Colour, material and finish
Industrial design
Mechanical engineering
Production support
Prototyping
Testing and evaluation
Usability and HF
90
PiccojectTM features press-on-skin activation and is compatible with 1mL and 2.25mL prefilled syringes. In addition to the features expected of this type of device, PiccojectTM targets usability improvements over other autoinjectors. The large window that surrounds the syringe makes inspection and monitoring of injection progress straightforward. A novel ‘status indicator’ changes colour when the injection is complete, providing clear, binary feedback.
Where traditional autoinjectors often have more than twelve components in addition to the syringe, PiccojectTM contains just eight. This simplifies manufacture and increases platform versatility.


An innovative, user focused, compact and fully featured autoinjector platform.
91

92

93

94
Achieving a design that performs consistently across the range of operating environments, is usable and intuitive, copes with foreseeable use and misuse conditions and functions correctly in all component tolerance combinations is a challenge. It’s a challenge that is magnified by the need to manufacture the device economically in very high volumes and within tight regulatory and delivered dose consistency constraints.
Success demands careful and considered management of the complex interactions that occur
between the device, the primary pack, the drug formulation and the user. This requires a rigorous, yet flexible approach, underpinned with a detailed understanding of the core operational principles of the design and sensitivity for user needs.
Our design and analysis work with global pharmaceutical and device companies includes pressurised metered dose inhalers, breath actuated inhalers, dry powder inhalers, dose counters, intra-nasal delivery systems and regimen assurance devices.
We understand just how deceptively complex it can be to successfully design and industrialise an inhaler or nasal drug delivery device.
95
Bespak
Pressurised metered dose inhaler with dose counter
Mechanical engineering

96
Major pharmaceutical company
Combination dry powder inhaler
Development of a mathematical model and early design verification to understand the lid foil compensation mechanism for an Inhaler.

97

98
Here, Matt Jones, Senior Sector Manager, Medical and Scientific, DCA –along with colleagues Richard Gledhill, Aidan O’Hare, Tony Smith, James May, Daniel Jenkins and Rob Veasey – summarises some of the key challenges and opportunities in the development of connected drug delivery devices, focusing in detail on deciding what a connected drug delivery device should do, designing for a coin cell, direct cellular connection and antenna design.
The market for connected drug delivery devices is in its infancy but many predict that it will grow at an astonishing rate1. At DCA, we think this is likely to happen – but only if these devices can demonstrably improve the lives and outcomes of the patients who use them.
The advent of new and exciting technology has brought with it a temptation to connect anything to everything, providing features that sometimes offer little real benefit. In this context, we believe the value proposition for new connected drug delivery devices must be clearly established at the outset.
emergent behaviours develop and which products win in the market. Some manufacturers opt to develop a 'minimum viable product' by simply connecting an existing product and exploring how users respond to the new proposition. This gets products to market fast – increasing the likelihood of taking early market share and even of establishing a new connected 'ecosystem'.
Matthew Jones Senior Sector Manager Medical and Scientific

One school of thought is that new connected objects should be created and launched to see what
This 'fail fast' approach has many success stories, particularly in the consumer products market, where development time, development costs, product lifespan and inherent product risk are often low.
For drug delivery devices, the picture is different. To make it to
Don’t develop a connected drug delivery device without reading this
99
market, new products need to be demonstrably safe and effective. Regulators demand extensive evidence of this and fears around data security and privacy are often far more critical than with consumer products. This creates challenges that are in turn compounded by the rapid evolution of underlying connectivity technologies.
So what’s the alternative to this 'suck it and see' approach? As obvious as it may sound, we think the best answer is found by first explicitly considering and defining the need before developing the product.
Why connect?
The ability to pass information to or from a drug delivery device enables features that can train and guide patients, monitor their usage, record side effects or assist in managing regimen changes. All of this can
allow interventions to improve patient compliance and reduce risks. It is also likely to change business models by providing data that facilitates outcome-driven payments.
What should a connected drug delivery device do?
It can be tempting to collect and present data simply because it is accessible. However, in the context of a medical device, it is imperative that the data is relevant, useful and accessible to everyone who will interact with it – whether they be a patient, carer, doctor or payer.
The functionality of connected devices should be informed by a detailed understanding of the conditions that they need to support. One place to start is by considering the use of the nonconnected legacy version of the product. Observing and interviewing stakeholders can reveal rich insights.

100
Figure 1 - Breaking down inclusiveness and usability.
The international standard for medical device usability (ISO 62366-1) advocates the use of task analysis to evaluate activities. This involves breaking down the activity into sub-tasks, which are then themselves broken down until baselevel operations are reached. Each base-level task can be examined to assess the demands it places on the user at a sensory, cognitive and physical level see Figure 1. This usability analysis will often throw up where new features or information can help support the user in carrying out a particular task. One approach is to then map the functionality of the system in a tabular form, comparing the legacy system with the proposed new connected system as shown in Figure 2.

The alternative or additional features or information sources can then be explored, assessed and filtered based on the benefits and potential risks to the stakeholders.
Generating a reasoned, useful and safe set of features is more likely to
yield a successful connected drug delivery device if the development team follows a structured process aligned to the relevant standards (e.g. ISO 13485, EN 62304, EN 62366 and ISO 14971). This should support an understanding of the needs and limitations of all stakeholders and help establish the device feature set at the start of the design process. This can then be reviewed and iterated through development.
The next step is to translate this design input into a successful device concept. For a connected drug delivery device, the development process often revolves around a few key challenges, first among which is usually the power source. There are many options available to the developer of a connected drug delivery device – off-the-shelf batteries, bespoke and flexible batteries, energy harvesting, printed batteries, etc. But for reasons of size, cost and availability, a simple, non-rechargeable coin cell is often selected.
Designing for a coin cell
For all its benefits, the very limited current capability and capacity of coin cells pose challenges that affect every aspect of the device design.
Sensors and storage
In one example, measuring physical characteristics such as pressure changes in a connected inhaler might be accomplished by using an optical transmitter and receiver. However, running an optical transmitter such as an LED continuously takes more current than the battery can provide. Pulsing the transmitter with short bursts of energy is an option. However, this can only go so far; if the pulses become too short, the transmitter no longer acts like a simple on/off switch and analogue side effects begin to make readings unreliable. At this point, a more sophisticated approach is required, such as precharging a capacitor to improve the turn-on speed of the LED and make the best use of the pulse length available.
We think the best approach is to first explicitly consider and define the need before developing the product.
101
Figure 2 - Comparing an existing system to its connected equivalent.
Once this problem has been solved, it is possible to take readings. Storing this information to non-volatile data storage would, in normal circumstances, be considered a relatively trivial exercise. However, when the memory is full, erasing data can use up considerable power, depending on the specification of the storage component. One potential solution is to dimension the storage memory to prevent the need to erase any records.
Data transmission
Once the measurements have been stored, this data often needs to be sent to a paired mobile device. The tiny current capability of a coin cell can generally be managed by the use of suitable capacitors between the battery and the RF transmitter chip. However, the leakage current through these capacitors can drain the battery. Therefore, the amount of data transmitted must be minimised to limit the number of capacitors and consequently the cost, printed circuit board (PCB) space required and background current drain. Similarly, care needs to be taken
when transmitting (relatively) large amounts of data. Sending a single reading may only be a small number of bytes but synchronising a device with a user’s new phone could easily result in a few thousand records being requested by the app. Sending that much data too fast could drag the battery voltage down, so intelligent throttling of the flow of data may be required.
The user interface
With the widespread adoption of mobile phones and their large displays, developers often decide to use a more basic interface on the device itself, such as a flashing LED or a sounder. This seems simple, yet the coin cell’s low voltage may mean it’s not capable of directly driving the LED.

It may be necessary to pulse the LED using a charge-pump system, for example, and vary the pulse duty cycle and speed depending on the battery voltage to achieve a consistent brightness.
Start-up
To achieve the battery life required for a disposable device, the
microprocessor must require an incredibly low current while the device is not in use. This means in most cases that the device is effectively coming out of reset each time, rather than a standby mode, and has to go from a standing start to taking the first measurements very quickly. This can be challenging and may drive the selection of an appropriate microprocessor.
Battery life – beginning and end
At the beginning of its life, microprocessor programming during manufacture can use up too much of the coin cell’s valuable energy unless carefully handled. As the battery comes towards the end of its life, its nominal (unloaded) voltage may appear reasonable but as soon as any current is drawn from it, it may drop significantly, potentially below the minimum operating voltage of the microprocessor. This could affect references and ranges for analogue inputs, the performance of sensors, and Bluetooth transmissions. Switched-mode power supplies will have to work harder to generate stable voltage supplies, further increasing the load on the battery and exacerbating the problem.
All aspects of the design of a connected drug delivery device that uses a coin cell are likely to revolve around the coin cell itself.
102
Figure 3 - Designing for a coin cell.
If these issues are not handled gracefully, various parts of the system may spontaneously reset or misbehave.
Finally, there is the issue of managing power 'on the shelf' between manufacture and first use. One option is to have a small strip of pull-tape between the battery and its contacts but this risks moisture ingress. It might also be necessary to have some changing data retained from manufacture, such as a real-time clock value. In either case, the current drawn must typically be virtually zero before first use to achieve an acceptable in-use life.
Designing for a coin cell: summary
All aspects of the design of a connected drug delivery device that uses a coin cell are likely to revolve around the coin cell itself. This is fundamentally different from designing devices with larger battery packs or a permanent power supply. New communication protocols bring exciting opportunities
A major part of the power budget of a connected drug delivery device is determined by the wireless communication protocol. Bluetooth Low Energy (BLE) and near field communication (NFC) are current favourites but new communication protocols are continually emerging. Currently, to connect our intelligent devices to the internet, we are dependent on the presence of a local network on our phones and tablets or around our buildings. However, exciting developments are being made in the shape of new low-power cellular Internet of Things (IoT) chipsets that use
one of two new radio transmission methodologies: 4G LTE-M and NB-IoT. They replace the current short-range wireless capability (e.g. BLE or Wi-Fi) with a direct cellular connection. This means devices no longer need to be connected through a phone or added to a local network (Figure 4) – opening up the potential for a wide range of new device features, vastly improved usability and greater adoption.
The worldwide network infrastructure to support 4G LTE-M and NB-IoT is currently incomplete but coverage is rapidly increasing. These new communication protocols provide opportunities to redefine the next generation of connected products and user experiences by:
1. Eliminating dependency on phones, Wi-Fi routers, etc. to form the intermediate communications link
2. Enabling lower power location of devices
3. Removing the need for multiple platform apps (e.g. for Apple or Android)

4. Simplifying the process of establishing a connection, which can be problematic for some users.
5. Providing greater opportunities for tighter data security control at the device
6. Improving the host server’s connection to the device, offering new feature opportunities (e.g. alerts if emergency medications are used).
The hardware platforms to support this functionality are now being made commercially available. This opens up opportunities to use their functionality to provide new features
103
Figure 4 - New low power IoT chipsets offer exciting opportunities.
and benefits to the end user.
Antenna design
The antenna is a key part of the product architecture for connected devices – while it is part of the electronic circuit, it also has a large influence on product packaging and mechanical design. As such, it is essential that the electronic, mechanical and visual design are all addressed simultaneously.
Large metal components near the antenna – such as batteries, motors or PCB ground planes – will reduce wireless communications range. If the product is handheld, poor antenna placement can lead to problems if the antenna can be masked by the user’s hands or even touched. Even if an off-the-shelf RF module with an integrated antenna is used, poor location of this within the device can severely reduce range.
Where device cost is particularly sensitive, it is attractive to use a printed antenna on the PCB. However, the board area required for the antenna itself and the necessary
separation from other parts of the circuit can be significant and cause the PCB to grow, affecting the overall product size or form factor. A surface mount component can be used with a smaller footprint than a printed antenna but this introduces additional parts and still limits the placement options to locations within the PCB footprint. By contrast, designing a bespoke antenna that is soldered to the PCB but extends beyond the board footprint can improve its location.

There may be an existing metallic part in the device that can serve a dual purpose as the RF antenna – or, conversely, an external finish that degrades performance. The most appropriate approach needs to be evaluated on a case-by-case basis, as effective product design is about striking a balance between all functional characteristics of the device.
Conclusion
Connected drug delivery devices offer a huge range of opportunities
but come with an equal or greater number of challenges, which must be carefully negotiated to bring them to market successfully. Maximising the probability of success depends on deploying an experienced team and following a structured, evidence-based process. Due to highly constrained design challenges, multidisciplinary development teams must be tightly integrated and focused on common and well-understood goals. In this way, companies can deliver connected devices that really have the potential to improve people’s lives.
References 1 “Connected Drug Delivery Devices Market Worth $717.7 Million By 2025”. Grand View Research, 2018.
104
Figure 5 - Antenna design is critical for connected devices.

105
Major pharmaceutical company
Intra-nasal spray device

Complex tolerance analysis
Detailed mathematical modelling
Computational fluid dynamics
106





Packaging design Design strategy Inclusive design Usability and HF GSK Voltarol® Pain Relief Gel Pack design effectiveness bronze award winner 107
Connected pump for dermal treatments

Industrial design
Mechanical engineering
Electronic engineering
Software engineering
Prototyping
Aptar cDermal
108

109
How smart do smart medical devices need to be?
The devices we use on a daily basis are getting smarter. Devices that were once ‘dumb’ are now fitted with a range of sensors allowing them to work out what is happening around them, and how they are being used. For example, the latest generation of toothbrushes are able to determine when, and for how long, we are brushing our teeth. What’s more they can also evaluate our brushing technique, telling us if we are pressing too hard, or spending too much time in one region of our mouth.
Augmenting everyday devices with sensors, microprocessors, communication technologies, and algorithms provides the possibility to allocate tasks that were once the responsibility of the end-user to either a microprocessor and
software programme or another human elsewhere. Automation can offer a clear benefit by taking on the tasks that humans often perform poorly at, or would prefer not to engage in. Activities like continuous activity monitoring, or providing timely reminders may be better allocated to a microprocessor.
That said, it is important to remember that automation also comes at a cost. In the majority of cases, the same functions still need to be completed; however, they are simply passed from a human to a microprocessor or another human. Where data collection and decision making is distributed, it is imperative to ensure that the necessary communication is possible and assess the frequency and costs of this.
Published on 8th March 2017
110
For consumer devices, the latest technology is frequently used as a marketing driver. Smart products are often proposed as greater value than a non-connected version. This often comes with the promise of ability to control objects in our home remotely, or closely monitor what is happening and respond accordingly. From a commercial perspective, products are often made ‘smart’ in an attempt to encourage users into a wider ecosystem of products or services and collect rich and valuable data on user behaviour. In some cases, there is a strong push to upgrade products to smart devices without explicitly considering the additional benefits for the consumer.
While the value of these connected smart products is not always immediately clear to all of us, the demand is often unquestionable. Well-considered and well-designed connected devices have the potential to optimise the allocation of function and improve the
overall system’s performance in terms of efficacy, efficiency, safety, inclusiveness, satisfaction and flexibility.
For medical devices, adding intelligence to the device is often cited as a significant opportunity for enabling more patients to take control of their therapies. The idea of reminding users when to take their drugs, and recording what was taken and when, is clearly appealing. Likewise, the ability to collect rich, often continuous, data on biometrics (e.g. heart rate, blood glucose levels, blood pressure) can be invaluable. This has the potential to allow patients the ability to manage conditions in the home that once had to be handled by HCPs. Similarly, it provides HCPs with a more robust evidence-base with which to make diagnosis and track patient conditions.
Given these inherent advantages, there is a clear appetite to develop connected medical devices, albeit
111
For consumer devices, the latest technology is frequently used as a marketing driver. Smart products are often proposed as greater value that a non-connected version.
with some reservations about their implementation.
The role of smart phones
Smart phones have become the middleman in our relationship with the majority of the smart devices we interact with. Most smart consumer products take advantage of the advanced processing power, storage capacity, and relatively large, high-resolution, screens of our smart phones. By connecting these smart consumer devices to the ‘supercomputers’ in our pockets, the connected devices themselves can be kept relatively simple and cost effective.
For consumer products, shifting the ‘intelligence’ to the app (running on the smart phone) has a number of clear advantages.
1. It reduces the bill of materials
System Performance
cost of the smart device in terms of processing power, memory, and display.
2. It allows systems to be updated easily (via updates to the smart phone app) without having to make changes to the device.
3. User interfaces and even functionality can be highly customisable.
4. The smart device app can exchange information with other apps on the phone to gain greater contextual understanding (e.g. location, weather, calendar, health apps).
For medical devices, however, the picture is somewhat different, as different constraints are placed on the system. The two most obvious differences are:
1. The requirement for regulatory approval.
2. The development time of a medical device.
The impact for smart medical devices is that one has to question if the model used for consumer goods, where the intelligence resides in the app, remains fit for purpose. The advantages of a lower bill of material cost remain appealing; however, new challenges are introduced.
Firstly, the challenge of proving that the software is safe and effective is far more demanding if the software sits within a complex operating system. For any app, running on a mobile phone or similar, controlling a medical device it would need to be demonstrated that the function of the app and data integrity cannot
Effectiveness
Safety Flexibility Inclusiveness Satisfaction 112
Where Whom When How What
Article by Dr D. Jenkins Research Lead Human Factors and Usability
 Paul Draper Senior Sector Manager Medical and Scientific
Paul Draper Senior Sector Manager Medical and Scientific

be corrupted by the operating system. Phone and tablet operating systems tend to have major updates every 12 months with numerous smaller updates throughout the year. Each of these updates may require the app software to be updated. Furthermore, each time the app software is updated the system will need to be assessed for new risks and may need further regulatory approval.
With medical device development timelines covering multiple years, it’s unlikely that the operating system or even the smart phone, that the medical device is designed to work with at the start of the project will be the same as the one at launch. It is far more likely that there will be multiple changes of phone operating systems throughout the development process. What’s more, given the long development time and the high investment, medical devices are often expected to remain in the market for longer than consumer devices.
Thus, the intuitive answer is that medical devices need to be smart, much smarter than their consumer counterparts. Allowing them to be far more independent of the phone they may be connected to. Any interaction with smart phones and tablets needs to be carefully considered. Ideally any integration with a phone would be non-critical to the function of the medical device, reducing the regulatory approvals burden for the app and phone.
A smart approach to smart device development
Just like all connected devices, the first stage of developing a smart medical device should involve a detailed consideration of its purpose and the potential value of the connected system, above the legacy non-connected system. An explicit consideration should be made for the end-user and each of the stakeholders in the system.
An understanding of the information requirements should underpin the design of the system. This involves determining what information is required, when it should be displayed, where in the system, to whom and how (in what format).
In summary, the architecture of a connected medical device should be informed and driven by a combination of stakeholder needs, technological capability, appropriate risk, and the ability to gain and maintain regulatory approval. If a decision is made to allocate some tasks or functions away from the user to a ‘smart’ device, in the majority of cases, it makes sense that the intelligence lies in the physical device rather than the app. Apps can often offer a secondary view of this information; however, the regulatory overhead is likely to be reduced if the processing takes place on the device.
The intuitive answer is that medical devices need to be smart, much smarter than their consumer counterparts.
This article was originally published on the MDT – Medical Design Technology website
113
Provalis Diagnostics
in2it
Point of care diagnostic system for HbA1c measurement

Mechanical engineering
Electrical hardware
Software development
Industrial design
Interaction design
Prototyping
Testing and evaluation
Production support
114

115
Why the IVDR is changing the way we develop diagnostic devices

The In Vitro Diagnostic Regulation (IVDR) (EU) 2017/746 1 is the new EU legislation applicable to in vitro diagnostic (IVD) medical devices. Having come into force on the 25 May 2017 this started a five-year transition period for manufacturers as it replaces the In Vitro Diagnostics Directive (IVDD) 98/79/EC. From 26 May 2022 all new in IVD certifications must be under the IVDR, although existing certificates issued under the IVDD can have a grace period up to 27 May 2024.
There has been much discussion and even concern within the IVD community about just how significant the changes are in moving to the IVDR. BSI have identified four major changes 2:
1. Extension of the scope “to include ‘lifestyle tests’ by including the elements of ‘indirect medical purpose’ and ‘prediction’ in the definition to include ‘nutrigenetic tests and lifestyle tests’, which are not covered by the IVDD.
performed pre- and post- market”.
3. The conformity assessment routes for IVDs are amended to fit the new classification logic. “As a consequence 80% of all IVDs will need to be certified by a notified body under the IVDR, as compared to 20% currently under the IVDD”.
4. “Clinical performance studies will be required to support the CE mark under the IVDR. As a consequence IVD manufacturers will need to produce significantly more clinical evidence.”
One of the biggest impacts is on notified bodies, which face a huge increase in workload driven by the more stringent requirements of the IVDR.
Article by Chris Langley Senior Sector Manager Medical and Scientific
2. Introduction of a risk based classification system from A to D (low to high risk). “With notified bodies having to perform conformity assessment on all but class A devices, the landscape is dramatically changing in terms of files to be reviewed and audits to be
For IVD manufacturers these changes are also having significant impacts for those involved in preparing technical documentation for new IVD device designs, and for those updating technical documentation for existing IVDs that will need to be re-certified under the requirements of the IVDR to continue to be marketed when their existing IVD certification ends. Much of the administrative work falls on the shoulders of manufacturer’s regulatory and quality management departments, but designers and developers are impacted too.
on 24th May 2022
Published
116
Implications of the IVDR for designers and developers
From a historical point of view, the IVDR and MDR were a response to public scandals regarding poor devices that were not appropriately developed and controlled in manufacture. It was felt that there were also insufficient processes in place to effectively monitor clinical feedback from product in use. As a result, regulators have implemented a more comprehensive and elevated risk classification system that is now more significant for many IVDs. The IVDR requires developers to use more rigour and to gather better evidence for the effectiveness and safety of their devices, all of which must be well documented and suitable for notified body conformity assessments.
Of course, for product developers the technical skills required to develop a new IVD are not necessarily affected by the IVDR; but major aspects of the depth and manner in which it is done will almost certainly change, especially if the type of device being developed is effectively now in a higher risk class, requiring notified body involvement instead of selfcertification. Some key aspects of device development that need to be adjusted in light of new requirements from the IVDR are discussed below. Planning is essential to make sure that development projects are ‘IVDR ready’
It may seem obvious, but this is probably the most significant thing that can be done to help teams developing new IVD devices. Development procedures may need to be updated to reflect the need for increased design process controls, better design documentation and greater evidence of real world
device performance. Consideration should be given to the increased focus on clinical performance studies, meaning that additional prototypes may be required during development, and better evidence of the suitability and validity of these prototypes will also be needed. Notified bodies will want to review the development plan as part of the conformity assessment, so these plans need to be created properly at the project outset, as it is hard if not impossible to create the right evidence retrospectively.
Integration of risk analysis with design requirements
IWith increased emphasis on risk management, it is even more important to integrate risk analysis with the creation, development and documentation of design requirements. Increased emphasis on considering requirements for usability and in particular risks associated with use errors and handling difficulties should be expected. Design requirements must also include consideration of support for the device lifecycle, such as service, maintenance, updates and cybersecurity, end of life, etc.
An increased focus on development rigour and design documentation
Consideration needs to be given to the collation and documentation of evidence that design requirements and identified risk control measures are correctly implemented and verified. In order to support this, it is likely that more detailed engineering analysis, well documented tolerance analysis, mathematical modelling and computer aided simulations will be needed. Reasonable scenarios and permutations should be analysed as early as possible, which in our experience can often discover latent problems at significantly less
cost and time impact than finding a problem in late stage tests or worse, in the field. Additional technical skills and software tools may be needed to support the greater diligence that is now required compared with what was previously considered acceptable.
Evidence-based development and testing
From extensive experience of mitigating and refining design concepts that have been initiated by other parties, we have observed that it can be all too easy for development engineers to see and understand something about their design, but not to capture or record this sufficiently at the time. An evidence-based design philosophy is a cornerstone for our development work, underpinning and informing our important development decisions. But, of course, technical evidence is only of value if it is recorded and communicated clearly amongst key project stakeholders.
Summary
Ultimately the IVDR is about ensuring safe and effective devices reach the market. Proactive manufacturers should see the IVDR as an opportunity to encourage better planning, better understanding of requirements, more rigour in development and increased evidence to inform decision-making. Of course it is important that development programmes do not become mired in excessive process administration and documentation but the new requirements of the IVDR, when executed well, can only be a good thing for driving the development of safer and more effective devices that helps patients and healthcare professionals achieve better outcomes.
References
1 Official Journal of the European Union, REGULATION (EU) 2017/746 - https://eur-lex. europa.eu/legal-content/EN/TXT/HTML/
2 How to prepare for and implement the upcoming IVDR – Dos and don’ts - https:// www.bsigroup.com
117
Tissuemed
Tissuebond Applicator and 180 Light Source
Light activated surgical sealant system for cardio-vascular surgery


Mechanical engineering

Industrial design
Prototyping
118

119

120

121
Malvern Panalytical
Morphologi® 4 and Morphologi® 4-ID
Detailed automated imaging particle characterisation instrument range

Industrial design

Visual brand language
Colour, material and finish
Interaction design
Usability and HF
Graphic design
Production support
122

123

Malvern Panalytical Morphologi® 4-ID 124

125
Malvern Panalytical
Zetasizer Ultra and Zetasizer Pro

High-resolution particle sizing
instrument range
Industrial design
Mechanical engineering
Visual brand language
Colour, material and finish
Interaction design
Usability and HF
Graphic design
Prototyping
Production support
126

127
Huntleigh Healthcare
Hydroven Flowtron
Compression therapy pump

Usability and HF
Industrial design
Colour, material and finish
Prototyping
Production support
128
Depuy
Surgical Jig

Tibial Jig
Design research
Usability and HF
Mechanical engineering
Industrial design
Instructional design
Packaging
Graphic design
Prototyping
129
Medlogic
Liquiband
Surgical adhesive applicator

Usability and HF
Mechanical engineering
Industrial design
Prototyping
Testing and evaluation
Production support
130

131
P&G
Hair colour measurement
Point of sale ‘hair colour analysis’ device
Design research
Usability and HF
Mechanical engineering
Electronic hardware

Software support
Industrial design
Colour, material and finish
Prototyping
Testing and evaluation
Production support
132

133
P&G
Hair friction comb
Point of sale ‘hair damage analysis’ device

Design research
Usability and HF
Mechanical engineering
Electronic hardware
Software support
Industrial design
Colour, material and finish
Prototyping
Testing and evaluation
Production support
134

135
MR-linac system

Design strategy and planning

Human factors and usability

Industrial design
Interaction design
Mechanical engineering
Prototyping
Elekta Unity
136

Elekta Unity has CE Mark and 510(K) clearance, but is not available in all markets. 137
The future of radiotherapy treatment
Millions of people worldwide benefit from radiotherapy every year, and the treatment cures more people than cancer drugs. Dan Jenkins and colleagues describe a project in which human factors played a critical role in the design of new equipment that delivers the therapy to patients.
Every so often, an opportunity arises to design systems that are truly transformative. Often as the result of the introduction of a fundamentally new technology, these revolutionary systems allow new tasks to be conducted or they allow existing tasks to be completed in a new way. The design of new systems opens exciting possibilities for human factors practitioners. It also brings up concerns and challenges as it’s difficult to predict how a new
technology system will shape future work. Observing current behaviour on legacy systems provides just part of the picture.
Elekta Unity, the first high-field MR-linac, is an example of groundbreaking technology because it overcomes the technical barriers that have hindered the integration of precision radiation therapy by combining magnetic resonance (MR) imaging with a linear particle accelerator for highly targeted, real-time radiotherapy. Fast moving, electrically charged particles are strongly influenced by a powerful magnetic field, so keeping them on track while near an MRI seemed like an impossibility before research found breakthroughs. It’s now a system that is being used by clinicians in healthcare institutions around the world.

138
The new MR-linac allows the exact location of tumours to be identified during treatment delivery. MR imaging provides radiotherapists with a much clearer description of the location of a tumour than is possible with more conventional computed tomography-based systems which use x-rays. What’s more, MR imaging is particularly adept at differentiating soft tissues making it especially relevant to tumours in the abdomen; the location of 65% of tumours. This increased confidence around the location of a tumour allows cancer cases to be treated with radiotherapy that was previously not viable because of the location of nearby critical tissue. The greater confidence in the location of dose delivery also opens the possibility of treating with fewer instances of higher doses.
The right tools for the job
Human factors practitioners have the skill and toolsets to help frame the design and its base architecture at the earliest stages of development where the objective is also to inspire and inform the design. Most explorations of human work draw on the same core data collection approaches:
1. Observations in a naturalistic setting (the ‘real world’).
2. Observations in a lab setting (simulations or user trials).
3. Interviews.
4. Self-reporting.
5. Literature reviews.
For revolutionary systems, observing and documenting current work (using descriptive models), or work as expected (using prescriptive models as described in standard operating procedures; SOPs),
only provides part of the picture. More formative tools, such as cognitive work analysis, are required to describe how work could be conducted.
As such, there is much that can be learnt from using a range of different tools. When new tools are introduced to a discipline, there’s often the tendency to compare them to more traditional approaches, highlighting the limitations and weaknesses of these established approaches. While this is an important part of discussing the value of the new, it can result in a complete rejection of the old – akin to ‘throwing the baby out with the bathwater’. In practice, it’s often advantageous to draw on the relative strengths of each of these method types.
In the case of Elekta Unity, a mixed methods approach was established that sought to learn from current work as prescribed using SOPs, work as disclosed via interviews, current work as done through observations, and future work as imagined using formative modelling, all at the earliest stages of the design process, seeking to maximise the value of the full toolkit.
This involved drawing from the same core sets of data collection approaches and analysing them with a diverse range of tools. The core data set was informed by studying several different areas: the current use of legacy equipment, Linacs using CT imaging across seven treatment centre visits spread across North America, South America and Western Europe; observations of over 360 patient treatment sessions; after-hours walk-throughs; over 50 stakeholder interviews; and extensive literature reviews.
139
The new MR-linac allows the exact location of tumours to be identified during treatment delivery.
The core methods used to process this data can be broadly segregated into descriptive and formative approaches.
The descriptive approach
Radiotherapy is typically a highly structured process that follows a well-rehearsed workflow. As such, Hierarchical Task Analysis (HTA) was a fitting backbone for the descriptive analysis. In the first instance, we used HTA to explore the variability in workflows, or work as done, by exploring the observed differences between treatment locations such as lung, prostate or breast, and geographic location, as well as treatment centre types, such as a large teaching hospital with many Linacs and a large radiotherapy department to regional cancer treatment centres with a single Linac and a small team. It soon became apparent that the variability was relatively limited. Where it did exist, it tended to be at the detailed ‘leaflevel’ of the task model or in the detailed ‘plans’ of the HTA.
Given the limited variability and the relatively close match between
work as prescribed and work as done, HTA proved to be a valuable approach. The main advantage of HTA was its large range of extensions, such as Critical Path Analysis and Link Analysis. The core model provided a common task description that could be explored in greater detail.
The temporal nature of the task was explored by assigning average baselevel task times recorded from over 350 observations to each sub-task in the HTA. Critical Path Analysis was then used to identify areas in the task flow that offered the greatest potential for efficiency savings.
Link analysis was used to time map the tasks in a spatial setting of a plan view of a typical treatment and control room. This revealed opportunities to optimise the layout of physical controls and objects that healthcare professionals and patients interact with, as well as the location of physical and digital information displays.
The HTA model also proved valuable in evaluating the safety of

the system, both from a physical, manual handling, perspective, using a tool called REBA or Rapid Entire Body Assessment, and from a cognitive level predicting opportunities for ‘error’ using TRACEr or Technique for the Retrospective and predictive Analysis of Cognitive Error.
The formative approach
At a more formative level, tools from cognitive work analysis were used to explore how work could be conducted. Hierarchies were constructed to explore the relationships between the physical objects in the system such as new and existing technology, and the higher order systems values of efficacy, efficiency, safety, inclusiveness, satisfaction and flexibility. Decision ladders were used to describe how information across digital displays, documentation, staff interactions, the physical environment and the verbal and nonverbal patient cues was currently being used to guide treatment sessions and to explore how it could be used in the future. The flexibility,
140
Image credit: The Royal Marsden
Article by Dr D. Jenkins Research Lead Human Factors and Usability
Malcolm Boyd Senior Sector Manager Medical and Scientific
 David Gilmore Director of User Experience at Elekta
David Gilmore Director of User Experience at Elekta

variability and resilience of the system were also explicitly explored.
Inspiring and informing design
The purpose of this detailed analysis was to inspire and inform the design of a vision for the future at the infancy of the project. This vision was created six years before the first patient was treated with the system; the intention was to form a basis for the detailed design that was technologically grounded and evidence-driven. Some of the notable features of the design, such as low table top or ‘couch’ that the patient lies on, were informed by anthropometric datasets and manual handling assessments of those assisting and positioning patients. Engineered safeguards were inspired and informed by ‘error’ predictions and carefully considered against their impact on system resilience.
The approach also provided a detailed description of the information requirements of the system. This ensured that the right information was displayed, in the right place, at the right time, to the right people, in a suitable format that
complements information drawn from human interactions and the physical environment.
The output was a three-minute video describing a vision for the patient experience for the future system, backed up by detailed reports. This formed the target for a fullscale development programme that resulted in the design of Elekta Unity, the world’s first high-field imaging MR-linac, that was used to treat its first patient in September 2018, ushering in a new era in the battle against cancer.

Author affiliations
Dan Jenkins leads the research team and Malcolm Boyd is a Senior Sector Manager at DCA Design international. See www.dca-design. com. David Gilmore is Director of User Experience at Elekta. The Elekta Unity project was awarded the 2018 HFES User Centred Design Award, a 2018 iF Design Award, and a 2018 Good Design Award. See www.elekta.com
Human factors helps frame, inspire and inform a design at the earliest stages of development.
141

142
Upright radiation therapy and radiology system
Our bodies move less in an upright position. When targeting a cancerous tumour, this reduction in motion allows greater accuracy in delivery of radiation. Being upright reduces setup times. Faster, simpler setups reduce treatment schedules and make treatment equipment available to more people. The system has also been designed to fit into existing facilities as well as new purpose built facilities, reducing the need for large hospital redevelopments and ultimately helping improve access to next generation therapy treatments.
In 2021 DCA and Leo Cancer Care’s work was recognised with a prestigious iF design award and a Good Design award. Leo Cancer Care’s upright patient positioning and imaging system is a major innovation anticipated to change the future of radiation therapy.

Colour, material and Finish Industrial Design Usability and HF
Enabling upright treatment of cancer with a ground breaking patient imaging and positioning system.
Leo Cancer Care MarieTM
143

144

145
Sluicemaster SOLO®
Bedpan macerator

Mechanical engineering
Industrial design
Prototyping
Testing and evaluation
Production support
Haigh
146

147
Watson Marlow
Qdos 30
A range of peristaltic pumps

Electronic hardware
Software development
Prototyping
Testing and evaluation
148
RTS Lifesciences
Smartstore
Laboratory based compound storage system

Mechanical engineering
Electronic hardware
Software development
Industrial design
Prototyping
Testing and evaluation
Production support
149
Sunrise Medical
Sunrise mobility scooter
Mobility Scooter

Mechanical engineering
Industrial design
Exterior styling
Prototyping
Testing and evaluation
150

151
Beyond
compliance. What is the role of human factors in medical device development?
The profile of human factors in medical device development has increased significantly, largely due to it playing a critical role in gaining regulatory approval for a medical device. However, for many, the focus on demonstrating safe and effective use can dominate the project involvement for human factors professionals. This article discusses how human factors tools and techniques can also help to define how to develop products that outperform their competition.
To be successful a medical device needs to overcome two challenges. Firstly, it needs to make it to market, and secondly, it needs to offer a recognisable advantage over its competitors.
Challenge 1: Making it to market
IEC 62366 is an international standard that outlines how human factors should be integrated into the process of medical device development. As compliance with the standard is critical for regulatory approval, the introduction of the standard has served to increase the salience of human factors within medical device development. So much so, that failure to adequately document the involvement of human factors is seen as a clear project risk.
Regulators such as the FDA focus on safe and effective use. The preferred method for demonstrating this is the simulated use test. This test involves putting the product in the hands of
Published on 27th January 2016
152
representative users and asking them to perform a set of predefined tasks. The test represents a clear barrier to project success. At best, failure means project delays and additional costs for design modifications, at worst; it results in the cancellation of the project and substantial financial losses. Accordingly, it is clearly understandable why such an importance is placed upon it. This focus on simulated use tests, and on safe and effective use, helps to ensure poorly designed products are kept off the market. What it doesn’t do; however, is explicitly seek to understand how the users feel about the device, nor does it seek to understand how the device performs in relation to its competitors.
Challenge 2: Establishing a competitive advantage
Whereas the first challenge, making it to market, posed the question is this acceptable for end users, the second challenge posed is more ambitious as it also strives to be better than its competition. But what does better mean? Most people involved in the medical device development process would like to think that they were in the business of making better devices. However, the interpretation of ‘better’ is likely to change between the diverse range of stakeholders. For those intimately involved in the manufacturing process, such as production engineers, there is likely to be a keen focus on the cost effectiveness of the devices. For others with a market focus, the emphasis may be on commercial viability.
Systems thinking
We can learn a lot about how good a medical device is by thinking of it as part of a system. At the most basic level, this system includes
the medical device and the patient. However, it could also include other people, such as healthcare professionals or carers, or other artefacts such as other devices, drugs, training materials, instructions for use, apps, etc. Additional values such as efficiency (how long it takes to setup the device), usability (how easy it is to use), and flexibility (how well the product fits the range of different lifestyles of its target population).
Measuring performance
The system’s values can serve as an excellent vehicle for comparing a proposed medical device against the product it is planned to replace, or its direct competition. Likewise, by thinking in more abstract terms, it is also possible to make a comparison with other types of devices or therapies used to treat the same condition. To aid these comparisons, it is advantageous if the differences in performance can be quantified. This is where the use of human factors tools and techniques comes in.
Efficiency
One of the most common techniques used within human factors is task analysis. This involves describing each of the core tasks that a user must conduct with a device. For example, this may include, unpacking, reading instructions, preparing the device, administering a dose, and disposal. Each of these high level tasks is further decomposed until a series of base level task steps is defined (e.g. rotate dial, slide button forward). The number of task steps alone is often a useful indication of the efficiency of a device and its complexity of use; however, more detailed assessments can be made by coding each task step. Time data can be used to provide a description
Human Factors tools and techniques can also help to define how to develop products that outperform their competition.
153
Planning, preparation and rigorous study design is key to gaining valid insights as is using a representative sample of the intended end users.
154
Article by Dr D. Jenkins Research Lead Human Factors and Usability
 Paul Draper Senior Sector Manager Medical and Scientific
Paul Draper Senior Sector Manager Medical and Scientific

of efficiency. Likewise, task steps can be represented on spatial arrangements using a tool called link analysis. For example, for medical installations this can be used to predict the number of operator footsteps required in a typical day.
Usability
The usability, or inclusivity, of a design can be assessed in a number of ways. A useful starting point is to consider each of the task steps against three aspects of human performance. (1) Sensory – the ability to see, hear, feel, smell or taste the device. (2) Cognitive – the ability to understand the device and remember how it works. And (3) Physical – the strength and dexterity required to use the device. There are a multitude of tools that can be used to quantify usability. Anthropometric datasets can be used to describe the percentage of a given population that would be excluded from use by the size of a product or the force required to actuate it. Likewise, data on those with sensory capabilities can also be used to determine how many users would be excluded by certain colour choices or text sizes.
Flexibility
Standardisation is a clear challenge for medical device developers. Even subtle changes to colour may require a separate regulatory submission. Accordingly, a single device system (e.g. device, labelling, packaging, IFU, training aids, support mechanisms) is often required to meet the many different ways of using the device. Imaginative solutions are required to build flexibility of use into the device system without introducing the burden of additional regulatory overhead.
Safety
Observations of representative users play an important role in assessing the safety of a device; however, the unsafe acts that can be considered are limited to those that can be observed. Given that medical devices can be manufactured in billions, and misuse can have adverse effects, low frequency errors are of obvious
concern. Accordingly, a structured and systematic approach to error prediction is needed. From a human factor standpoint, one starting point for this is at a task based level. For example where tasks such as dialling up a dose step can be subject to errors of omission, performing too much, performing too little, or performed in the wrong direction, etc.
Effectiveness
Simulated use trials provide a very useful indication of the influence of human factors on the effectiveness of a device – that is the ability of users to operate the device without impacting its efficacy. Planning, preparation and rigorous study design is key to gaining valid insights as is using a representative sample of the intended end users.
What should the role be?
So returning to the question posed in the title, what should the role of human factors be? The introduction of IEC 62366 makes it clear that the first challenge of demonstrating safe use is a minimum requirement.
Human factors is not simply a tool for regulatory compliance. The vast majority of medical devices operate in a competitive market, and while the product selection may not always lie with the end user, usability and system performance are increasingly shaping purchasing decisions.
Accordingly, the definition of system values and their quantification plays a critical role in informing the project direction and setting commercial, as well as regulatory, expectations for the device. Beyond compliance, the end-to-end integration of human factors tools and techniques in the design process is critical for designing a commercially successful device.
This article was originally published on the MDT - Medical Design Technology website 155

156
RB
Scholl 2 in 1 corn express pen

Manual footcare tools
Design planning
Design research
Usability and HF
Industrial design
Prototyping
Testing and evaluation
Production support
157

158
RB
Scholl Pedi Perfect Wet & Dry
Waterproof and rechargeable electronic foot file

Design research
Industrial design
Visual brand language
Colour, material and finish
Packaging
Prototyping
Production support
159
RB
Scholl Gel Active
Range of insoles

Usability and HF
Industrial design
Colour, material and finish
Prototyping
Production support
160

161
Quit smoking wearable device

Colour, material and finish
Insight and strategy
Exterior styling
Industrial design
Interaction design
Mechanical engineering
Production support
Prototyping
Usability and HF
Visual brand language
GSK My Quit
162

163
Designing products that stand the test of time

The passage of time has a significant impact on the products we interact with – some products are like fine wines, they simply get better with age (or at least we perceive them to), others become outdated, or redundant as time passes, or the signs of use make them tatty and undesirable.
For many of us, some of the most cherished objects that we interact with are the oldest. They are perhaps the things that we have grown old with and formed memories around, such as a family table or a favourite mug. Others may represent specific events – such as a gift of a watch or an item of jewellery.
Other cherished objects may be viewed as just better, reminding us of simpler times. For me, this list
includes hand tools inherited from my grandfather and a watch from the 1950s. They may also include vintage furniture, motor cars or steam engines. These products often represent a simplicity, a focus on craftsmanship, and a commitment to use materials in a way that would be cost-reduced out of modern massproduction processes.
The modern drive for connectivity and smart products has undoubtedly influenced the lifespan of products. Moore’s law tells that processing power doubles approximately every two years, it is therefore easy to see how products are soon left behind, particularly if the ecosystem they are connected to is keeping pace with the latest technology. This results in smartphones that were once state of the art, becoming almost unusable
Published on 21st November 2018
164
Article by Dr D. Jenkins Research Lead Human Factors and Usability
five, or so, years later – often not as a result of the product itself degrading, but simply their failure to keep pace with the systems that they connect to.
A shorter product lifespan may be music to the ears of some retailers, as it allows more products to be sold. However, in many industries, such as public transport design or complex medical devices, products need to last for many decades in order to present a viable business case. Even where the business case does not demand it, an environmental conscience might. Furthermore, most product designers are also motivated to design things that will last – the cherished objects of the future.
This then begs the obvious question – how do we design products that stand the test of time? To develop products that last, and continue to be appreciated, we need to understand the impact that the passage of time will have on them. Both at a physical level (the physical behaviour of the product), as well as an emotional one – the relevance that the product has to those interacting with it.
Physically standing the test of time
The best classic cars are those that look like they could have rolled off the production line yesterday. They represent a snapshot of the past – they have almost no sign of wear and no modifications. Conversely, our expectations for a period property are quite different. We expect these to have been modernised – retaining period features and charms, but embracing modern living and comforts (e.g. central heating, open-plan fitted kitchens, and en-suite bathrooms).
From a physical perspective, there are two core approaches to managing the passage of time, (1) to design products so that they are resistant to changes due to time and the impact of wear, or (2) to design products that grow old gracefully, celebrating their signs of usage, and adapting to fit the changing context of use.

Patina is a word used commonly in design circles; it is used to describe the, often visual, signs or use and wears on a material’s surface.
Think of the much-loved leather sofa or, or perhaps a pair of jeans, that look better after being used and appreciated. Or perhaps more fittingly, the pair of shoes (or slippers) that, not only look better but actually mould to our feet – becoming more comfortable. A traditional wok is another good example. Not only does the product look better after many hours of use, the food actually starts to taste better when prepared in a well-used and well-cared-for wok (part of the reason non-stick versions are often avoided).
However, the idea of visual signs of use (patina) can be highly subjective. It may be celebrated for an intimate object such as items of clothing, however, they may be less well received for a communal object such as a train.
Interestingly, the idea of physical change as a result of usage does not necessarily translate directly to digital services.
At an emotional level
Engagement with a product typically comes from developing an emotional connection to it. This emotional connection may be formed in a number of ways. Its value may be linked to the way it was acquired (or first encountered) – creating a connection to someone involved in that process (a gift from a loved one) or a moment in time (a purchase on a special holiday or trip). Alternatively, its introduction may represent an investment in time and resources. The ‘IKEA effect’ is a cognitive bias in which consumers place a disproportionately high value on products they have, in part, created (See Norton et al 2012).
This phenomenon is well researched and understood, but it is limited to the start of the experiential journey. Just like our relationships with people, not all strong bonds are formed upon first meeting. Others are formed based on gradually building trust. Others still stand the test of time almost through attrition, because they adapt to fit changing requirements and needs – they remain relevant by changing their value proposition to fit the given environment and context.
The best classic cars are those that look like they could have rolled off the production line yesterday.
165
At a systems level
At a systems level, the whole idea of developing a relationship with a physical object may be called into question. In many markets, it could be argued that we are moving away from a connection to products towards a connection to experiences or brands – physical objects simply have too many constraints to adaptation – limiting their ability to remain relevant. Continuing with this argument, any given artefact (or product) is simply an embodiment of the brand that can be replaced or upgraded. For example, we may build a very strong and meaningful connection to a particular brand of smartphone, but be very happy to trade in our current model for the latest and greatest version every year or so – as the relationship is more with the service than the artefact itself.
That said, it’s fair to say the counter-argument against this disposable-culture is growing stronger. Environmental concerns are becoming far more mainstream. Furthermore, the role of the physical artefact in a meaningful relationship is becoming far clearer. In our haste
to embrace the clear advantages of the new, digital aspect of a brand ecosystem, we were, perhaps, too keen to disregard the merits of the lasting relations with physical highlycrafted objects – and the multisensory experiences that they bring.
It is argued that these physical relationships are key to lasting engagements. This can be evidenced by large brands, such as Google, Amazon, and now Facebook, who once lived exclusively in the digital world, investing in developing physical products. The physical assets representing not only a multi-sensory experience but also a commitment to an ecosystem.

Doing it…
So how do we design physical products that remain relevant and have the potential to become the cherished objects of tomorrow?
The simple answer is that “it depends…” the most appropriate solution will be dependent on the specifics of the project and the context of use. However, it’s fair to say that it will involve a consideration at a physical, emotional, and a systemic level.
Accurately predicting the future requirements of a product requires predicting the future. While we may not have a crystal ball, we do have structured processes to anticipate future use. There is much that can be learnt from looking back and exploring the variability of use in the past (over time) as well as exploring the variation in use today (between use cases).
Where products are relatively simple and have experienced little change in their use (such as hand tools), perhaps the simplest option is to look back in time and emulate the qualities of the cherished objects of the past. A return to more traditional materials and manufacturing techniques may create niche but viable business propositions – as long as the value, over much cheaper mass-produced alternatives, can be communicated. We are currently seeing a resurgence of more traditional tools such as brass razor handles that offer an alternative to single-use devices.
For physical products, that experienced greater variability in the ways that they are used, to stand the test of time, they need to remain
166
relevant as the world around them changes. In most cases, this means adapting. Products that change their value proposition based on their environment and context of use; have the potential to create a greater level of engagement. Just like the sympathetically modernised period home, they perhaps retain the charms of a particular era or aesthetic but remain relevant to any given movement in time.
Explicitly considering future use, and adaption, at the time of design is critical to this process. By understanding the past, current and future variability, along with far less transient human values, products can be developed that remain relevant.
Our recent work with Linn on the Selekt DSM Network Music Player is one such example of this.
The product has considered the passage of time in two main ways. At a more physical level, the product has been designed to be fully configurable, modular and upgradable. Allowing new functionality to be added at point of purchase, or upgraded and
even repaired with ease over time (by opening the casing and inserting new ‘cartridges’, each of which has been designed as a product in their own right).
This results in a product that is flexible to the user’s requirements and to new technologies that will become available as time progresses. On a digital level there are six customisable ‘smart buttons’ that adorn the front of the product and feel like piano keys beneath your fingers. These smart buttons can be programmed to perform a range of functions based on the user’s requirements allowing personalisation more common with a digital app while retaining a physical connection to the product. The result is a product that has been designed to last, at a physical, an emotional and systemic level.


167
Investing in early interaction prototyping can help reduce the UX changes later in the formal design development process.

168
Network Music Player

Interaction design
Industrial design
Colour, material and finish
Usability and HF
Prototyping
Linn Selekt DSM
169
DCA wins innovation award at CES
LifeFuels new smart nutrition bottle launched at CES 2018 and became a CES Innovation Award Honoree for the second time.
Designed by DCA for LifeFuels, this revolutionary smart nutrition bottle helps users understand how much water they should be drinking throughout the day and allows the user to prepare nutritional drinks on the go. Launched on 8th January at CES 2018, the world's largest technology show, LifeFuels has been
awarded the CES Innovation Award Honoree 2018 for Sports, Fitness and Biotech.
The system is made up of three parts: the bottle itself, the FuelPods and the LifeFuels app. The user selects three FuelPods, then inserts them into the bottom of the bottle. Using either the app or the button on the bottle, the user can dispense precise servings according to their taste and nutritional goals.
170

171
LifeFuels
Smart nutrition bottle

Industrial Design

Mechanical Engineering
Prototyping
Colour, material and finish
172

173
Huel

Powdered nutrition shaker
Industrial design
Design for manufacture

Usability
Visual brand language
174


175
GSK
Polident Dental Lab
Ultrasonic denture cleaning bath

Visual brand language
Usability and HF
Industrial Design
Packaging Design
Prototyping
Design for manufacture

Production support

176

177
GSK
Sensodyne mouthwash

Bottle and dosing cap
Design research
Usability and HF

Industrial design
Visual brand language
Packaging
Prototyping
Production support
gold winner 2015
178
GSK
Toothbrush
Sensodyne toothbrush


Design research
Industrial design
Visual brand language
Colour, material and finish
Prototyping
Production support
179
Kids toothbrush

Design planning
Design research
Usability and HF
Industrial design
Visual brand language
Colour, material and finish
Prototyping
Production support
GSK Aquafresh Kids
180
GSK
Aquafresh milk teether
Teether for soothing and cleaning

Design research
Usability and HF
Industrial design
Visual brand language
Colour, material and finish
Prototyping
Production support
181
Electrical toothbrush

Mechanical engineering
Industrial design
Prototyping
Packaging
Production support
GSK Dr. Best Vibration
182

183

184
RB
Veet Easywax

Electrical roll on wax applicator
Industrial design
Visual brand language
Colour, material & finish
Packaging
Prototyping
Production support
185

186
Clearasil perfectawash
No touch face wash dispenser

Design planning
Usability and HF
Industrial design
Visual brand language
Colour, material & finish
Packaging
Prototyping
Production support
RB
187






Unilever Axe/Lynx Deodorant body spray Usability and HF Mechanical engineering Packaging Prototyping Testing and evaluation Production support gold winner 2015 188

189
Unilever
Degree motionsense
Deodorant stick

Usability and HF
Mechanical engineering
Packaging
Prototyping
Testing and evaluation
Production support
190

191

192
Alternative Packaging Solutions (APS) MiniMist
Long duration spray pump
Mechanical engineering
Industrial design
Prototyping
Testing and evaluation
APS MiniMist
An innovative alternative to traditional aerosols.
DCA has helped APS to develop MiniMist, a new spray device which provides a great alternative to traditional aerosols and other spray dispensers.
MiniMist is able to produce a continuous spray without any chemical propellants, resulting in a significantly lower carbon footprint
than aerosols whilst remaining cost competitive. MiniMist’s spray characteristics and visual design are easily customizable to suit different brands and product categories.
193
Flonase nasal spray packaging

Packaging Prototyping



GSK Flonase
194
Mölnlyke
Biogel Gloves Packaging

Surgical glove packaging
Design research
Usability and HF
Industrial design
Packaging
Prototyping
Testing and evaluation
195
3M
Versaflo M-Series Headtops
Range of faceshields, hard hats and helmets with integrated respiratory protection

Design planning
Design research
Usability and HF
Mechanical engineering
Industrial design

Prototyping
Testing and evaluation
Production support
196

197
From

Our Location
our campus in the
town of Warwick, England,
develop products that
markets around the world. 198
historic
we serve clients internationally to
reach
From Birmingham International Airport

Travel time 25 minutes
From London Heathrow Airport
Travel time
1 hour 30 minutes
199



Helping clients achieve success through great product design.











































































































































































 Figure 1 - Single-use plastics play a vital role in healthcare.
Figure 1 - Single-use plastics play a vital role in healthcare.














 Rob Veasey Senior Sector Manager Medical and Scientific
Shane Day Senior Skill Leader Electronics and Software
Rob Veasey Senior Sector Manager Medical and Scientific
Shane Day Senior Skill Leader Electronics and Software




























 Paul Draper Senior Sector Manager Medical and Scientific
Paul Draper Senior Sector Manager Medical and Scientific





























 David Gilmore Director of User Experience at Elekta
David Gilmore Director of User Experience at Elekta






































































