Cardiff and Vale University Health Board Research & Development Performance Report 2020 - 2023
and Vale University Health Board Research & Development Performance Report 2020—2023
Cardiff
Contents Foreword from CVUHB Research & Development Director 3 Summary of Excellence 6 Clinical Board Recruitment Summary 8 Clinical Board Performance Reports All Wales Medical Genomics Service 11 Children and Women’s 14 Clinical Diagnostics & Therapeutics 25 Medicine 29 Mental Health 35 Primary, Community & Intermediate Care 39 Specialist Services 44 Surgical Services 72 Other 78 Appendix 80 Clinical Board Publications, Grants, Initiatives, Case Studies and Study Data Tables
Foreword
Foreword
Research & Development Director Foreword
Research & Development Director Foreword


Professor Matt Wise
Professor Matt Wise
This is the sixth edition of Cardiff & Vale University Health Board’s (CV UHB) Research and Development Performance Report and writing this foreword, particularly striking the correct balance between optimism and where we aspire to be, is a challenge. In what feels like an increasingly inhospitable environment the report nevertheless showcases the strength of research activity across our organisation. For the second consecutive year in excess of 7,000 patients have been recruited to a clinical trial or study. Cardiff & Vale UHB continues to be the leading organisation in Wales delivering both commercial and non-commercial clinical trials to patients.
This is the sixth edition of Cardiff & Vale University Health Board's (CV UHB) Research and Development Performance Report and writing this foreword, particularly striking the correct balance between optimism and where we aspire to be, is a challenge. In what feels like an increasingly inhospitable environment the report nevertheless showcases the strength of research activity across our organisation. For the second consecutive year in excess of 7,000 patients have been recruited to a clinical trial or study. Cardiff & Vale UHB continues to be the leading organisation in Wales delivering both commercial and non-commercial clinical trials to patients.
Recruitment is continuously updated for all studies open in Wales and can be accessed here
Recruitment is continuously updated for all studies open in Wales and can be accessed here
The Power BI report can also be used to look at recruitment nationally for any given study in which we are participating.
The Power BI report can also be used to look at recruitment nationally for any given study in which we are participating.
The research delivery team in conjunction with the Health and Care Research Wales (HCRW) performance team have also developed a local version for CAV UHB, access here
The research delivery team in conjunction with the Health and Care Research Wales (HCRW) performance team have also developed a local version for CAV UHB, access here
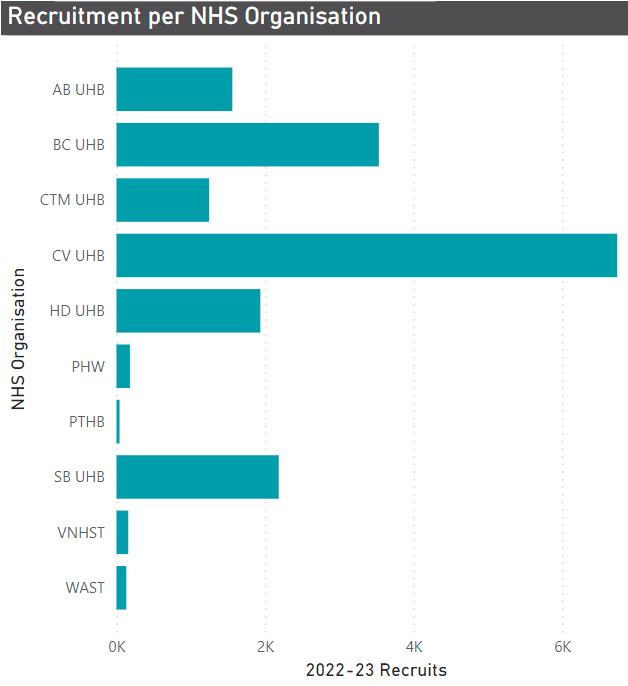
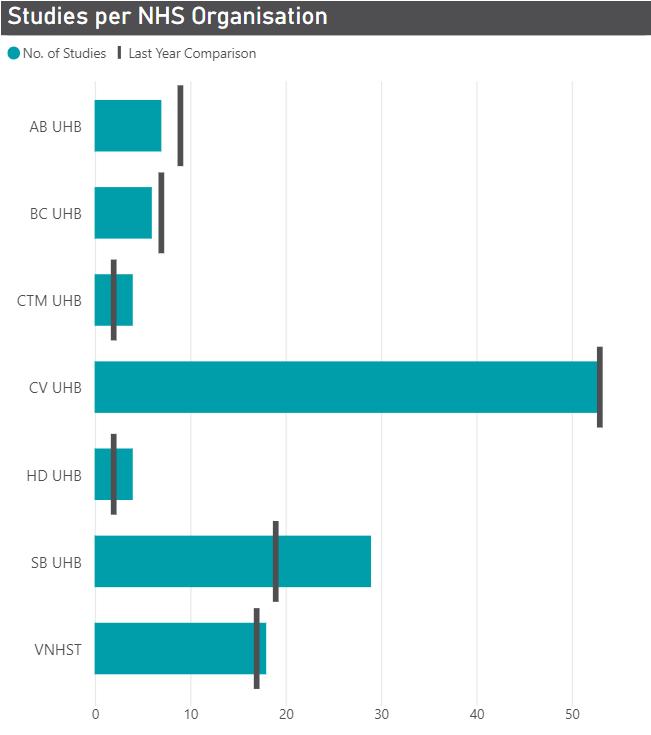
Figures 1a and 1b - Number of Open & Recruiting Non-Commercial studies (left) and Number of Participants Recruited (right) per NHS organisation (2022-23)
Figures 1a and 1b - Number of Open & Recruiting Non-Commercial studies (left) and Number of Participants Recruited (right) per NHS organisation (2022-23)
3
Professor Matt Wise Research & Development Director
2
This year Professor Keith Harding has been appointed as an independent member of the board in the role of research champion and whilst this may increase visibility of research, we are still a long way off from embedding research within the culture of the organisation. Our funding for delivering research is still largely dependent on an allocation from HCRW, which at around £6 million (in real terms accounting for inflation) represents around 25% of the amount the UHB received a decade ago. We seek to have greater autonomy of this budget and I hope that we can move towards this in the near future. Many of you will have heard me say over the years that we cannot rely on HCRW to grow our activity and meet the needs of our investigators. We need to be more adept at developing commercial links and writing grants. I am delighted to say, with respect to the latter, NHS investigators have had an outstanding year with five NIHR grants awarded totalling more than £11 million.
This year Professor Keith Harding has been appointed as an independent member of the board in the role of research champion and whilst this may increase visibility of research, we are still a long way off from embedding research within the culture of the organisation. Our funding for delivering research is still largely dependent on an allocation from HCRW, which at around £6 million (in real terms accounting for inflation) represents around 25% of the amount the UHB received a decade ago. We seek to have greater autonomy of this budget and I hope that we can move towards this in the near future. Many of you will have heard me say over the years that we cannot rely on HCRW to grow our activity and meet the needs of our investigators. We need to be more adept at developing commercial links and writing grants. I am delighted to say, with respect to the latter, NHS investigators have had an outstanding year with five NIHR grants awarded totalling more than £11 million.
• Julie Cornish - POLARiS (Pathway Of Low Anterior Resection Syndrome Relief after Surgery) an international multicentre (30) randomised clinical trial with £1.8 million from UK as well as an additional AUD1.3 million for Australia.
• Julie Cornish - POLARiS (Pathway Of Low Anterior Resection Syndrome Relief after Surgery) an international multicentre (30) randomised clinical trial with £1.8 million from UK as well as an additional AUD1.3 million for Australia
• Jared Torkington and Sadie Jones - The PICCOS Trial (PIPAC in Cancers of the Colon, Ovaries and Stomach) with £2 million funding from NIHR Efficacy and Mechanism Evaluation (EME).
• Peter Collins and Sarah Bell - Clinical and cost-effectiveness of a maternity quality improvement programme to reduce excess bleeding and need for transfusion after childbirth: the Obstetric Bleeding Study UK (OBS UK) Stepped Wedge Cluster Randomised Trial. A NIHR HS&DR funded study of £3,453,702.95 plus industry collaboration.
• Jared Torkington and Sadie Jones - The PICCOS Trial (PIPAC in Cancers of the Colon, Ovaries and Stomach) with £2 million funding from NIHR Efficacy and Mechanism Evaluation (EME).
• Peter Collins and Sarah Bell - Clinical and cost-effectiveness of a maternity quality improvement programme to reduce excess bleeding and need for transfusion after childbirth:
• Krishna Narahari - The ELIPSE Study - a randomised controlled trial comparing the clinical and cost-effectiveness of lymph node removal in patients undergoing curative surgery for localised high-risk prostate cancer. A NIHR HTA Researcher-led call Primary Research for funding of £2 million.
• Dave Bosanquet – PLACEMENT trial (Perineural local anaesthetic catheter after major lower limb amputation trial). A NIHR HTA £1,635,918.
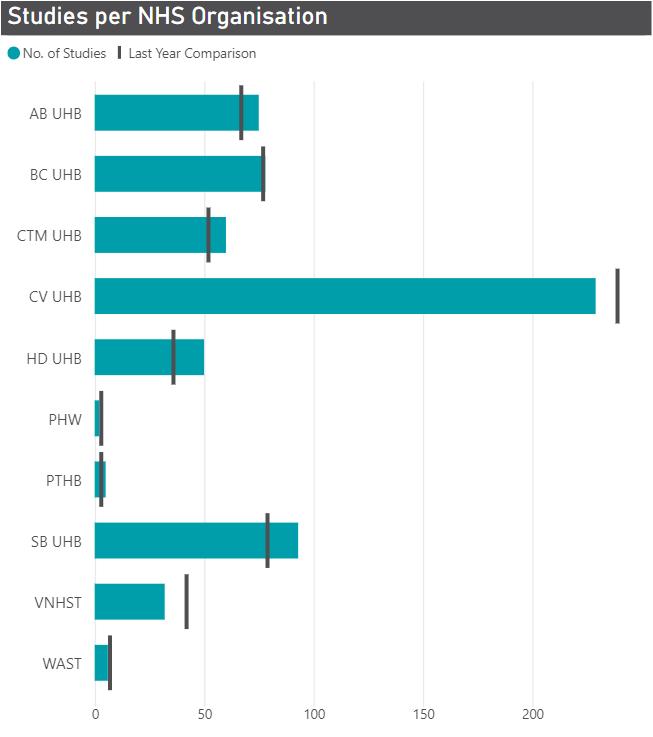
 Figures 2a and 2b - Number of Open & Recruiting Commercial (left) and Number of Participants Recruited (right) per NHS organisation (2022-23)
Figures 2a and 2b - Number of Open & Recruiting Commercial (left) and Number of Participants Recruited (right) per NHS organisation (2022-23)
3
Figures 2a and 2b - Number of Open & Recruiting Commercial (left) and Number of Participants Recruited (right) per NHS organisation (2022-23)
4
It has been clear that a key enabler of this success in several of these grants has been the placement of a clinical trials manager within the surgical clinical board and it is a model that we will be looking to develop further within the Health Board. Obtaining these multicentre grants also poses some new problems around handling large grants as the sponsoring organisation. We shall soon be appointing a finance officer within R&D which will not only facilitate this but give us better day-to-day oversight of R&D income and expenditure.
The recent publication of the Lord O’Shaughnessy review ‘Commercial clinical trials in the UK’ has led to a shift in emphasis in both NIHR and HCRW and is an attempt to address the fall in commercial trial activity in the UK. None of the report should come as a surprise but there is certainly, with the right investigators and R&D infrastructure, the opportunity to provide novel cutting-edge therapies to patients. Both Moderna and BioNTech are looking to invest in the UK, focussing particularly in vaccine trials. Moderna are investing around £2 billion in the manufacture and delivery of vaccines in the UK over the next decade, focussing initially on infectious diseases and later cancer and cardiovascular disease in their trial pipeline. This is a great opportunity not only for patients but also has obvious downstream benefits for preventative healthcare and the burden on the NHS in Wales. Although we wait to hear the final outcome we are hopeful that CV UHB will be able to lead the Moderna program in Wales with a vaccine centre based in the Clinical Research Facility led by Andrew Freedman. It also offers a fantastic opportunity to collaborate with the Systems Immunity Research Institute in Cardiff University.
Another important collaboration which continues to mature is the Cardiff Cancer Research Hub which is a collaborative between CAV, Velindre Cancer Centre (led by Mererid Evans) and Cardiff University (led by Awen Gallimore). This will not only provide a wider access to medium and high-risk early phase trials and advanced cell therapies but also bench to bedside therapies, reverse translation and novel diagnostics.
I hope that if you pick up and read the report that you won’t just focus on your own speciality but look at the breadth of activity within the organisation. Ideally this will stimulate people to seek out and collaborate with other like-minded investigators. As R&D director a key aspect of my job is to provide individual researchers with the appropriate infrastructure so that they can achieve their potential as investigators. This applies equally to the Chief Investigator of an international multicentre randomised control trial to those undertaking observational studies or being an Associate PI (Principle Investigator) as part of the NIHR scheme. Elevating the profile of research across the organisation not only allows patients better access to study participation but also the potential for novel therapeutics that can alter disease trajectory. The downstream benefits of trials include change in practice, education, clinical experience, financial gain, staff recruitment, retention and reputational growth. Unfortunately, many of these trial facets are frequently overlooked but they are key for an organisation to develop and improve patient outcomes. Changing and maintaining a proactive culture that maintains the importance of both research and education cannot be understated as goals for CV UHB. Given the size of the budget compared to the UHB as a whole, R&D punches well above its weight.
I would like to thank all our investigators and research staff for their continued hard work and contribution to improving the care of patients. Thanks also go to Sarah Ballard and Sara Shankland for producing this report. As ever, any other suggestions of how we can improve the report are welcome!
5
282
Studies Open & Recruiting
7072
Patients recruited this Financial Year
MOVE WALES 1 (NEUROLOGY)
UK’s Highest Recruiter
Top Recruiter in the UK
4514 (Frontier 2): Mim8 in adults and adolescents with haemophilia A
Study’s 1st Top Recruiter
Site to open and recruit in the UK REINFORCE (Surgery)
£3.2m
Awarded to Prof Dayan/Cardiff University by MRC for the LIONNS-D2 study in hypothyroidism (2023-26)
REPEAT phases 1 and 2 (Respiratory disorders)
Top Recruiter in the UK
LISTEN (Long COVID)
6
Abstract presented at the International Confederation of Midwives (ICM) congress in Bali (June 2023)
Study’s top recruiter
OPTIMAS trial (Stroke)
Shortlisted for the RCM Award for Excellence in Midwifery for Research
Cardiff & Vale Midwifery Research Team
UK’s Top Recruiter
Ver-A-T1D (Type 1 Diabetes)
Study’s
Top Recruiter
First site to recruit nationally/worldwide
LPRI-CF113 in the treatment of endometriosis
Trial Administrators from Research Delivery Team
Abstract presented at HCRW Support & Delivery event (March 2023)
Recruiter in the UK
Urine Biomarkers for Detecting Prostate Cancer
2nd Highest Add-Aspirin (Urology)
3rd Best Recruiter
out of 112 international sites for Prostate Cohort
7
mSep
Clinical Board Recruitment Summary
Clinical Board Recruitment Summary

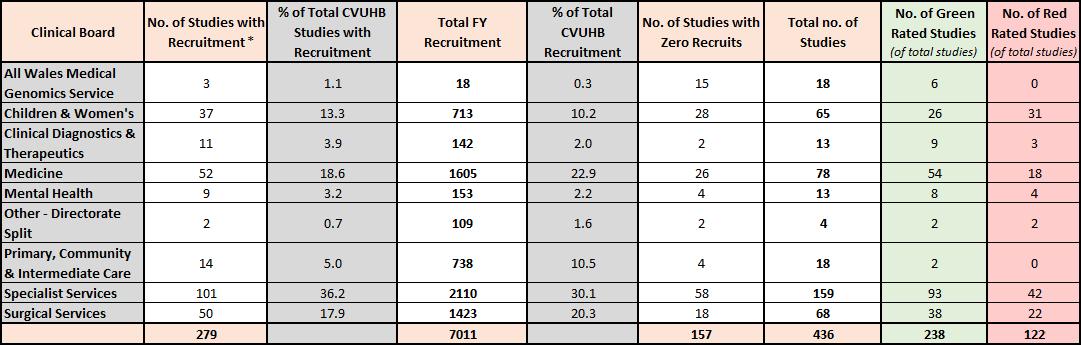
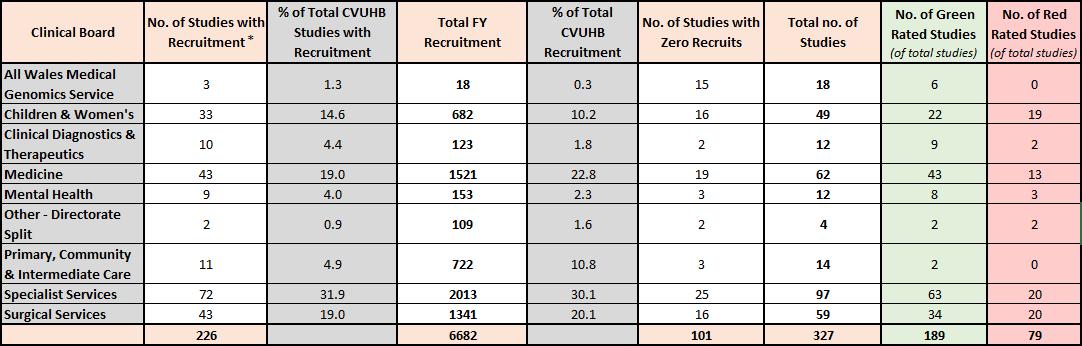
8 2020-21 Figure 1 - Non-Commercial and Commercial Financial Year Recruitment by Clinical Board (2020-21) 2021-22 Figure 2 – Non-Commercial and Commercial Financial Year Recruitment by Clinical Board (2021-22) All Wales Medical Genomics Service 3 2 10 0 19 22 Children & Women's 28 16 1662 28 24 52 Clinical Diagnostics & Therapeutics 4 2 39 1 7 11 Medicine 31 17 2139 36 37 68 Mental Health 5 3 48 1 3 8 Other - Directorate Split 2 1 7 0 2 4 Primary, Community & Intermediate Care 8 4 114 2 7 15 Specialist Services 75 42 1349 23 64 139 Surgical Services 24 13 602 10 38 62 180 5970 201 381 Clinical Board No. of Studies with Recruitment* % of Total CVUHB Studies with Recruitment Total FY Recruitment % of Total CVUHB Recruitment No. of Studies with Zero Recruits Total no. of Studies All Wales Medical Genomics Service 5 1.8 26 0.4 15 20 7 0 Children & Women's 38 13.4 1501 21.1 15 53 31 16 Clinical Diagnostics & Therapeutics 7 2.5 79 1.1 3 10 7 2 Medicine 54 19.1 1498 21.0 20 74 49 13 Mental Health 10 3.5 107 1.5 3 13 9 2 Other - Directorate Split 3 1.1 23 0.3 1 4 1 2 Primary, Community & Intermediate Care 8 2.8 346 4.9 6 14 4 1 Specialist Services 102 36.0 1929 27.1 55 157 97 34 Surgical Services 56 19.8 1613 22.6 20 76 44 23 283 7122 138 421 249 93 No. of Red Rated Studies (of total studies) Clinical Board No. of Studies with Recruitment % of Total CVUHB Studies with Recruitment Total FY Recruitment % of Total CVUHB Recruitment No. of Studies with Zero Recruits Total no. of Studies No. of Green Rated Studies (of total studies) * 8
Clinical Board Recruitment Summary

9
9
Clinical Board Recruitment Summary
2022-23
Figure 3 - Non-Commercial Financial Year Recruitment by Clinical Board (2022-23)
Figure 4 - Commercial Financial Year Recruitment by Clinical Board (2022-23)
Figure 5 – Non-Commercial and Commercial Financial Year Recruitment by Clinical Board (2022-23)
Clinical Board Recruitment Summary Clinical Board Recruitment Summary


Clinical Board Recruitment Summary



7
Figure 6 - Total
Non-Commercial
and Commercial Financial Year Recruitment by Clinical Board (2019-20)
Figure
7 - Total Non-Commercial and Commercial Financial Year Recruitment by Clinical Board (2020-21)
71 1637 170 617 176 5 303 1408 1454 10 1662 39 2139 48 7 114 1349 602 26 1501 79 1498 107 23 346 1929 1613
Figure 13 - Total Non-Commercial and Commercial Financial Year Recruitment by Clinical Board (2021-22)
10
Figure
6 - Total Non-Commercial and Commercial Financial Year Recruitment by Clinical Board (2020-21)
Figure
7 - Total Non-Commercial and Commercial Financial Year Recruitment by Clinical Board (2021-22)
10 1662 39 2139 48 7 114 1349 602 26 1501 79 1498 107 23 346 1929 1613 18 713 142 1605 153 109 738 2110 1423 10
Figure 8 - Total Non-Commercial and Commercial Financial Year Recruitment by Clinical Board (2022-23)
All Wales Medical Genomics Service Clinical Board
Summary from the AWMGS Clinical Board R&D Lead
The All Wales Medical Genomics Service (AWMGS) continues to support research in the fields of rare disease and precision medicine through its own initiatives and through collaborations with teams around Wales and the UK. Over the last 12 months, we have received grants for the advancement of precision oncology projects and contributed to the Interdisciplinary Precision Oncology Cardiff Hub (IPOCH) collaborative initiative with Cardiff University. Through the SWAN clinic, we have now developed an ethical framework for researchers to access stored genomic data within Wales via the Swansea University biobank, and in the next 12 months this will create a foundation for a significant expansion of our rare disease diagnosis research initiatives. There are also plans to move ahead with novel sequencing research for the undiagnosed patients from the SWAN cohort. We also support rare disease treatment research by facilitating the identification of candidate patients from across Wales in collaboration with other specialities. We have also opened the RECONNECT study for recruitment in collaboration with the Childrens and Young Adults Research Unit (CYARU) in Cardiff. We also continue to contribute towards multiple UK-wide and international research efforts towards understanding and diagnosing rare diseases in patients without a diagnosis from standard NHS testing.
11
All Wales Medical Genomics Service Clinical Board
All Wales Medical Genomics Service Clinical Board
12 Figure 1 – All Wales Medical Genomics Service Clinical Board Non-Commercial Recruitment Total Against Target (2020-21)
2 – All Wales Medical Genomics Service Clinical Board Non-Commercial Recruitment Total Against Target (2021-22) 191 183 154 44 28 2 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 50 100 150 200 250 Genetic mechanisms in polyposis of the bowel EMBRACE Genes and the Kidney in Tuberous Sclerosis Framing of decision-making in predictive and prenatal genetic tests Molecular genetic analysis of duodenal polyposis in FAP and MAP IHCAP Splicing and Disease A study of movement disorders in adults with 22q11 deletion syndrome EDEN Genetic Causes of Eye Malformations Genetics of Perrault Syndrome ISO SOLVE-RD BOLT EXE-T1D Genetic basis of cranial malformations Genetic disorders of growth, development and the brain HumGenDis IDFOW Lymres The SCOTTY Study Understanding the conditions of the RASMAPK pathway Recruitment total Recruitment Total Target 195 195 155 44 32 2 2 0 0 0 0 0 0 0 0 0 0 0 0 0 0 50 100 150 200 250 EMBRACE Genetic mechanisms in polyposis of the bowel Genes and the Kidney in Tuberous Sclerosis
of decision-making in predictive and prenatal genetic tests Molecular genetic analysis of duodenal polyposis in FAP and MAP IHCAP Splicing and Disease BOLT EDEN EXE-T1D Genetic basis of cranial malformations Genetic disorders of growth, development and the brain Genetics of OGID syndromes Genetics of Perrault Syndrome HumGenDis IDFOW ISO Lymres The SCOTTY Study Understanding the conditions of the RAS-MAPK pathway Recruitment total Recruitment Total Target 12
Figure
Framing
All Wales Medical Genomics Service Clinical Board
All Wales Medical Genomics Service Clinical Board
13
13 Figure
No
Recruitment
213 196 155 32 4 2 2 1 0 0 0 0 0 0 0 0 0 0 0 50 100 150 200 250 EMBRACE Genetic mechanisms in polyposis of the bowel Genes and the Kidney in Tuberous Sclerosis Molecular genetic analysis of duodenal polyposis in FAP and MAP Splicing and Disease IHCAP Investigating the molecular mechanisms of rare genetic disorders Genetic Disorders of Human Neurological and Immune Function EDEN Genetic basis of cranial malformations Genetic disorders of growth, development and the brain Genetics of OGID syndromes Genetics of Perrault Syndrome HumGenDis IDFOW Lymres The SCOTTY Study Understanding the conditions of the RAS-MAPK pathway Recruitment total Recruitment Total Target
3 – All Wales Medical Genomics Service Clinical Board Non-Commercial Recruitment Total Against Target (2022-23)
All Wales Medical Genomics Service Clinical Board
Commercial
in
2020-21, 2021-
22 or 2022-23
Children & Women’s Clinical Board
Summary from the Children & Women’s Clinical Board R&D Lead
This has been another year of new developments in the Children and Women’s Clinical Board.
The obstetric unit had a podium finish at the Royal College of Midwives R&D Team of the Year Award. There have been some significant challenges with staffing levels, but trial recruitment has been maintained which is a credit to the team.
In paediatrics there continues to be a wide portfolio of trials into a number of diseases, most notable being respiratory, endocrine, haematology and infectious diseases. The oncology department became one of the UK’s Experimental Cancer Medicine Centres in partnership with Welsh Government and CRUK. CYARU is opening a number of vaccine trials as part of the Moderna programme and has participated in the HARMONIE RSV trial as well as COMCOV3.
2023-2024 looks to be an exciting year!
14
Children & Women’s Clinical Board
Children & Women’s Clinical Board
Total Target
PNET 5 PHITT GEM3 Fetal Atrial Flutter &… FaR-RMS
The POOL study nSEP mSEP BLOOD MARKERS FOR INVESTIGATING… ROCkeTS Induced sputum in children with cystic… The 'Big Baby Trial' Cerclage after full dilatation caesarean… CASAP Clinical Characterisation Protocol for… CLOCS FIRST-ABC UK Childhood ITP Registry Azithromycin Therapy for Chronic Lung… RECOVERY trial UMBRELLA. CF START OASI2: PROTECTOR Comparing COVID-19 Vaccine Schedule… The Tommy's National Rainbow Clinic… MyeChild
Quality of life in paediatric sarcoma SMPaeds
Blueprint:Service Design for Children &…
Characterisation of Paediatric… AZTEC2 HOLDS Giant PANDA PRESSURE WILL DECRYPT FaR-RMS ICONIC NCCPG TDM 2018 PHITT REACH study C-STICH2 rEECur SIOP Ependymoma II
Fetal Atrial Flutter & Supraventricular… GEM3 Needle-free, pain medicine for young… PNET 5 Recruitment total
15
15
Against Target
Figure 1 - Children & Women's Clinical Board Non-Commercial Recruitment Total
(2020-21)
Against
2618 644 369 147 126 116 102 78 53 44 35 33 33 30 25 25 23 20 20 17 17 16 15 13 14 10 8 7 6 5 4 4 3 2 2 1 1 1 1 1 1 0 0 0 0 0 0 500 1000 1500 2000 2500 3000 The POOL study BuRN-Tool Grown in Wales ROCkeTS Induced sputum in children with… mSEP Pregnancy and Neonatal Outcomes… The 'Big Baby Trial' nSEP Evaluating Risk in Twin Pregnancies Clinical Characterisation Protocol for… UK Childhood ITP Registry FIRST-ABC BLOOD MARKERS FOR… CHIPS (HIV) Cerclage after full dilatation… PUMA UMBRELLA Calibration and Cross-Validation of… CLARITY CF START CASAP C-STICH CLOCS Azithromycin Therapy for Chronic… PROTECTOR MyeChild 01 RECOVERY TRIAL HOLDS SMPaeds Pharmacokinetic variation and… WILL Characterisation of Paediatric… ECUSTEC NCCPG TDM 2018 SIOP Ependymoma II rEECur Investigating the inflammatory… ICONIC Looking at the best needle-free,… DECRYPT
Recruitment
3398 196 193 161 155 126 99 96 57 52 43 33 33 32 28 22 18 18 18 16 15 14 13 12 9 7 6 6 5 5 5 3 3 2 2 2 2 1 1 1 0 0 0 0 0 500 1000 1500 2000 2500 3000 3500 4000
Figure 2 - Children & Women's Clinical Board Non-Commercial Recruitment Total
Target (2021-22)
Recruitment total
Recruitment Total Target
Children & Women’s Clinical Board
Children & Women’s Clinical Board
16 Figure
3568 399282220164133126104 68 52 49 45 43 33 28 25 25 25 23 20 19 16 16 15 15 14 13 13 12 10 9 7 7 7 5 5 4 4 4 4 2 2 1 1 1 1 0 0 0 0 0 0 0 500 1000 1500 2000 2500 3000 3500 4000 The POOL study nSEP mSEP BLOOD MARKERS FOR… ROCkeTS Cerclage after full dilatation… Induced sputum in children with… The 'Big Baby Trial' OASI2 Clinical Characterisation Protocol for… REINFORCE RECOVERY trial The iHOLDS Trial UK Childhood ITP Registry The Tommy's National Rainbow… PROTECTOR IMPORT Giant PANDA CF START Biomarkers for Ovarian Pathology ComCov3 SMPaeds CF-HomeSpIT Quality of life in paediatric sarcoma MyeChild 01 PRESSURE FIDO Experiences of end of life care AZTEC2 HOLDS Blueprint WILL Psychosocial access to kidney… Characterisation of Paediatric… PINPOINT Study Childhood cancer diagnosis SINEPOST study NCCPG TDM 2018 FaR-RMS CONTRACT 2 PHITT ICONIC VERITAS SIOP Ependymoma II rEECur C-STICH2 The SuPPORT Project REGAL trial PNET 5 International Congenital Lung… Fetal Atrial Flutter &… DOLFIN Recruitment total Recruitment total Target 16
3 - Children & Women's Clinical Board Non-Commercial Recruitment Total Against Target (2022-23)
Children & Women’s Clinical Board
Children & Women’s Clinical Board
17
17
Figure 4 - Children & Women's Clinical Board Commercial Recruitment Total Against Target (2020-21)
6 2 1 1 1 1 0 1 2 3 4 5 6 7 8 9 RMD Lophlex Powders Case Studies XLH Registry GSK's paediatric HZ vaccine in paediatric renal transplant patients Pediatric study of Gilteritinib in FLT3 positive R/R AML Phase 3b Study of ELX/TEZ/IVA in CF subjects 6 Through 11 Years of Age Pfizer Pediatric VTE Treatment study Cerliponase alfa Observational Study A Phase 3 Study of Erenumab in Children with Chronic Migraine A Phase 3 Study of Erenumab in Children with Episodic Migraine Recruitment total Recruitment Total Target 12 8 2 2 2 1 1 1 1 1 0 0 5 10 15 20 25 30 VX20-CFD-004 XLH Registry A Phase 3 Study of Erenumab in
Chronic Migraine GSK's
HZ
Pediatric
R/R AML Efficacy
Pediatric Immune
Cerliponase alfa Observational Study Galileo OLE Pfizer Pediatric VTE Treatment study Provention Bio's T1D Trial Evaluating CPeptide with Teplizumab A Phase 3 Study of Erenumab in Children with Episodic Migraine Recruitment total Recruitment Total Target
Figure 5 - Children & Women's Clinical Board Commercial Recruitment Total
Against
Target (2021-22)
Children with
paediatric
vaccine in paediatric renal transplant patients
study of Gilteritinib in FLT3 positive
and Safety of Avatrombopag in
Thrombocytopenia
Children & Women’s Clinical Board
Children & Women’s Clinical Board
18 Figure
Commercial
25 13 10 3 3 2 2 1 1 1 0 0 0 0 0 0 0 5 10 15 20 25 30 35 HARMONIE VX20-CFD-004 XLH Registry A Phase 3 Study of Erenumab in Children with Chronic Migraine LPRI-CF113 in the treatment of endometriosis GSK's paediatric HZ vaccine in paediatric renal transplant patients Pediatric study of Gilteritinib in FLT3 positive R/R AML Cerliponase alfa Observational Study Efficacy and Safety of Avatrombopag in Pediatric Immune Thrombocytopenia Pfizer Pediatric VTE Treatment study A Phase 3 Study of Erenumab in Children with Episodic Migraine A Placebo-controlled RCT Volixibat Intrahepatic Cholestasis Pregnancy CIP342 Lenny Study FORTIS Vedo 3024 UC Vedo 3025 CD Recruitment total Recruitment total Target 18
6 - Children & Women's Clinical Board
Recruitment Total Against Target (2022-23)
Children & Women’s Clinical Board
Children & Women’s Clinical Board
ACUTE CHILD HEALTH Children & Women’s Clinical Board
: Indicates study opened in Feb/March - towards the end of the financial year. : Indicates study Closed to Recruitment or Withdrawn during the relevant financial year. : Indicates study Suspended. Charts exclude studies in set-up.
Recruitment Total Target
The POOL study BuRN-Tool Grown in Wales ROCkeTS Induced sputum in children with… mSEP
Pregnancy and Neonatal Outcomes… The 'Big Baby Trial' nSEP
Evaluating Risk in Twin Pregnancies
Clinical Characterisation Protocol for… UK Childhood
ITP Registry FIRST-ABC
BLOOD MARKERS FOR… CHIPS (HIV) Cerclage after full dilatation… PUMA UMBRELLA Calibration and Cross-Validation of… CLARITY CF START CASAP C-STICH CLOCS
Azithromycin Therapy for Chronic… PROTECTOR MyeChild 01
RECOVERY TRIAL HOLDS SMPaeds
Pharmacokinetic variation and… WILL
Characterisation of Paediatric… ECUSTEC NCCPG TDM 2018 SIOP Ependymoma II
rEECur
Investigating the inflammatory… ICONIC Looking at the best needle-free,… DECRYPT PNET
5 PHITT GEM3
Fetal Atrial Flutter &… FaR-RMS Recruitment total
19
19
Against Target
Figure
1 – Children & Women’s – Acute Child Health Non-Commercial Recruitment Total
(2020-21)
Against Target (2021-22) 196 161 126 57 52 33 33 32 28 22 18 16 14 13 12 9 7 6 5 3 3 2 2 2 2 1 1 0 0 0 0 50 100 150 200 250 300 350 Nsep BLOOD MARKERS FOR… Induced sputum in children with… CASAP Clinical Characterisation Protocol for… FIRST-ABC UK Childhood ITP Registry Azithromycin Therapy for Chronic… RECOVERY trial UMBRELLA. CF START Comparing COVID-19 Vaccine… MyeChild Quality of life in paediatric sarcoma SMPaeds Blueprint:Service Design for Children… Characterisation of Paediatric… AZTEC2 PRESSURE DECRYPT FaR-RMS ICONIC NCCPG TDM 2018 PHITT REACH study rEECur SIOP Ependymoma II Fetal Atrial Flutter & Supraventricular… Needle-free, pain medicine for young… PNET 5 Recruitment total Recruitment Total Target 644 126 53 35 33 33 30 25 23 20 20 17 17 16 14 8 7 5 4 3 2 2 1 1 1 1 1 1 0 0 0 0 0 100 200 300 400 500 600 700 BuRN-Tool Induced sputum in children with… nSEP Clinical Characterisation Protocol… FIRST-ABC UK Childhood ITP Registry BLOOD MARKERS FOR… CHIPS (HIV) PUMA UMBRELLA Calibration and Cross-Validation of… CLARITY CF START CASAP Azithromycin Therapy for Chronic… MyeChild 01 RECOVERY TRIAL SMPaeds Pharmacokinetic variation and… Characterisation of Paediatric… NCCPG TDM 2018 ECUSTEC DECRYPT ICONIC Looking at the best needle-free,… Investigating the inflammatory… rEECur SIOP Ependymoma II FaR-RMS Fetal Atrial Flutter &… PHITT PNET 5 Recruitment total Recruitment Total Target
Figure 2 – Children & Women’s – Acute Child Health Non-Commercial Recruitment Total
12
Figure 1 - Children & Women's Clinical Board Non-Commercial Recruitment Total Against Target (2019-20)
1473 1061 644 369 260 144 126 86 55 40 37 33 33 28 25 23 20 16 14 12 12 9 8 6 6 6 5 4 4 4 3 3 2 2 2 2 1 1 1 1 0 0 0 0 0 0 0 0 0 500 1000 1500 2000 2500 The POOL study RHiNO BuRN-Tool Grown in Wales The SANDWICH Trial ROCkeTS Induced sputum in children with cystic… SenITA Phenotyping the Brecon Cohort The 'Big Baby Trial' CAP-IT UK Childhood ITP Registry BATCH Trial FIRST-ABC CHIPS (HIV) PUMA Calibration and Cross-Validation of… UMBRELLA. CF START ALDO Evaluating Risk in Twin Pregnancies DRN100 (TrialNet) C-STICH HOLDS MyeChild 01 Azithromycin Therapy for Chronic Lung… Euro Ewing 2012 Pharmacokinetic variation and toxicity… NEURO-PACK CASAP v1.0 PROTECTOR SHARES Study Characterisation of Paediatric… The OPTIMIST-A Trial ECUSTEC SMPaeds SIOP Ependymoma II rEECur Clinical Characterisation Protocol for… Investigating the inflammatory response… UMSCOM PNET 5 PHITT GEM3 Fetal Atrial Flutter & Supraventricular… Clinical & biological factors associated… NCCPG TDM 2018 Looking at the best needle-free, pain… Recruitment total Recruitment Total Target 2618 644 369 147 126 116 102 78 53 44 35 33 33 30 25 25 23 20 20 17 17 16 15 13 14 10 8 7 6 5 4 4 3 2 2 1 1 1 1 1 1 0 0 0 0 0 0 500 1000 1500 2000 2500 3000
Figure 2 - Children & Women's Clinical Board Non-Commercial Recruitment Total Against Target (2020-21)
Children & Women’s Clinical Board
Children & Women’s Clinical Board
ACUTE CHILD HEALTH
Children & Women’s Clinical Board
: Indicates study opened in Feb/March - towards the end of the financial year. : Indicates study Closed to Recruitment or Withdrawn during the relevant financial year. : Indicates study Suspended. Charts exclude studies in set-up.
The POOL study BuRN-Tool Grown in Wales ROCkeTS Induced sputum in children with… mSEP
Pregnancy and Neonatal Outcomes… The 'Big Baby Trial' nSEP
Evaluating Risk in Twin Pregnancies
Clinical Characterisation Protocol for… UK Childhood
ITP Registry FIRST-ABC BLOOD MARKERS FOR… CHIPS (HIV) Cerclage after full dilatation… PUMA UMBRELLA
Calibration and Cross-Validation of… CLARITY CF START CASAP C-STICH CLOCS
Azithromycin Therapy for Chronic… PROTECTOR
MyeChild 01
RECOVERY TRIAL HOLDS SMPaeds
Pharmacokinetic variation and… WILL
Characterisation of Paediatric… ECUSTEC NCCPG TDM 2018
SIOP Ependymoma II
rEECur
Investigating the inflammatory… ICONIC Looking at the best needle-free,… DECRYPT PNET 5 PHITT GEM3
Fetal Atrial Flutter &… FaR-RMS Recruitment total
399 220 126 52 45 33 25 23 19 16 16 15 15 14 13 13 12 9 7 7 5 5 4 4 4 4 2 2 1 1 1 0 0 0 0 0 50 100 150 200 250 300 350 400 450 nSEP BLOOD MARKERS FOR INVESTIGATING… Induced sputum in children with cystic… Clinical Characterisation Protocol for… RECOVERY trial UK Childhood ITP Registry IMPORT CF START ComCov3 CF-HomeSpIT SMPaeds MyeChild 01 Quality of life in paediatric sarcoma PRESSURE Experiences of end of life care FIDO AZTEC2 Blueprint Characterisation of Paediatric… Psychosocial access to kidney… Childhood cancer diagnosis PINPOINT Study CONTRACT 2 FaR-RMS NCCPG TDM 2018 SINEPOST study ICONIC PHITT rEECur SIOP Ependymoma II VERITAS DOLFIN Fetal Atrial Flutter & Supraventricular… International Congenital Lung… PNET 5 Recruitment total Recruitment total Target 20
Figure 3 – Children & Women’s – Acute Child Health Non-Commercial Recruitment Total Against Target (2022-23)
12
Figure 1 - Children & Women's Clinical Board Non-Commercial Recruitment Total Against Target (2019-20)
1473 1061 644 369 260 144 126 86 55 40 37 33 33 28 25 23 20 16 14 12 12 9 8 6 6 6 5 4 4 4 3 3 2 2 2 2 1 1 1 1 0 0 0 0 0 0 0 0 0 500 1000 1500 2000 2500 The POOL study RHiNO BuRN-Tool Grown in Wales The SANDWICH Trial ROCkeTS Induced sputum in children with cystic… SenITA Phenotyping the Brecon Cohort The 'Big Baby Trial' CAP-IT UK Childhood ITP Registry BATCH Trial FIRST-ABC CHIPS (HIV) PUMA Calibration and Cross-Validation of… UMBRELLA. CF START ALDO Evaluating Risk in Twin Pregnancies DRN100 (TrialNet) C-STICH HOLDS MyeChild 01 Azithromycin Therapy for Chronic Lung… Euro Ewing 2012 Pharmacokinetic variation and toxicity… NEURO-PACK CASAP v1.0 PROTECTOR SHARES Study Characterisation of Paediatric… The OPTIMIST-A Trial ECUSTEC SMPaeds SIOP Ependymoma II rEECur Clinical Characterisation Protocol for… Investigating the inflammatory response… UMSCOM PNET 5 PHITT GEM3 Fetal Atrial Flutter & Supraventricular… Clinical & biological factors associated… NCCPG TDM 2018 Looking at the best needle-free, pain… Recruitment total Recruitment Total Target 2618 644 369 147 126 116 102 78 53 44 35 33 33 30 25 25 23 20 20 17 17 16 15 13 14 10 8 7 6 5 4 4 3 2 2 1 1 1 1 1 1 0 0 0 0 0 0 500 1000 1500 2000 2500 3000
Figure 2 - Children & Women's Clinical Board Non-Commercial Recruitment Total Against Target (2020-21)
Recruitment
Total Target
Children & Women’s Clinical Board
Children & Women’s Clinical Board ACUTE CHILD HEALTH
For Acute Child Health Directorate Commercial Recruitment (2020-21 and 2020-22), please see Children & Women’s Commercial Studies’ charts on page 17 (all Commercial data during these years were under Acute Child Health)
For Acute Child Health Directorate Commercial Recruitment (2020-21 and 2020-22), please see Children & Women’s Commercial Studies’ charts on page 17-18 (all Commercial data during these years were under Acute Child Health)
21
21
25 13 10 3 2 2 1 1 1 0 0 0 0 0 0 5 10 15 20 25 30 35 HARMONIE VX20-CFD-004 XLH Registry A Phase 3 Study of Erenumab in Children with Chronic Migraine GSK's paediatric HZ vaccine in paediatric renal transplant patients Pediatric study of Gilteritinib in FLT3 positive R/R AML Cerliponase alfa Observational Study Efficacy and Safety of Avatrombopag in Pediatric Immune Thrombocytopenia Pfizer Pediatric VTE Treatment study A Phase 3 Study of Erenumab in Children with Episodic Migraine CIP342 Lenny Study FORTIS Vedo 3024 UC Vedo 3025 CD Recruitment total Recruitment total Target
Figure 4 – Children & Women’s – Acute Child Health Commercial Recruitment Total Against Target (2022-23)
Children & Women’s Clinical Board
Children & Women’s Clinical Board
Children & Women’s Clinical Board
OBSTETRICS & GYNAECOLOGY
: Indicates study opened in Feb/March - towards the end of the financial year. : Indicates study Closed to Recruitment or Withdrawn during the relevant financial year. : Indicates study Suspended. Charts exclude studies in set-up.
total
The POOL study BuRN-Tool Grown in Wales ROCkeTS Induced sputum in children with… mSEP
Pregnancy and Neonatal Outcomes… The 'Big Baby Trial' nSEP
Evaluating Risk in Twin Pregnancies Clinical Characterisation Protocol for… UK Childhood
ITP Registry FIRST-ABC BLOOD MARKERS FOR… CHIPS (HIV) Cerclage after full dilatation… PUMA UMBRELLA
Calibration and Cross-Validation of… CLARITY CF START CASAP C-STICH CLOCS
Azithromycin Therapy for Chronic… PROTECTOR
MyeChild 01
RECOVERY TRIAL HOLDS SMPaeds
Pharmacokinetic variation and… WILL
Characterisation of Paediatric… ECUSTEC NCCPG TDM 2018 SIOP Ependymoma II
Total Target
needle-free, pain…
rEECur
Investigating the inflammatory… ICONIC Looking at the best needle-free,… DECRYPT PNET
5 PHITT GEM3
Fetal Atrial Flutter &… FaR-RMS
22
22
Figure 1 – Children & Women’s – Obstetrics & Gynaecology Directorate Non-Commercial Recruitment Total Against Target (2020-21)
2618 369 147 116 102 78 44 25 15 13 10 6 4 0 0 500 1000 1500 2000 2500 3000 The POOL study Grown in Wales ROCkeTS Msep Pregnancy and Neonatal Outcomes in COVID-19 The 'Big Baby Trial' Evaluating Risk in Twin Pregnancies Cerclage after full dilatation caesarean section C-STICH CLOCS PROTECTOR HOLDS WILL GEM3 Recruitment total Recruitment Total Target 3398 193 155 99 96 43 18 18 15 6 5 5 1 0 0 500 1000 1500 2000 2500 3000 3500 4000 The POOL study mSEP ROCkeTS The 'Big Baby Trial' Cerclage after full dilatation caesarean section CLOCS OASI2 PROTECTOR
HOLDS Giant PANDA WILL C-STICH2 GEM3 Recruitment total Recruitment Total Target
Figure 2 – Children & Women’s – Obstetrics & Gynaecology Directorate Non-Commercial Recruitment Total Against Target (2021-22)
The Tommy's National Rainbow Clinic Study
12
Figure 1 - Children & Women's Clinical Board Non-Commercial Recruitment Total Against Target (2019-20)
1473 1061 644 369 260 144 126 86 55 40 37 33 33 28 25 23 20 16 14 12 12 9 8 6 6 6 5 4 4 4 3 3 2 2 2 2 1 1 1 1 0 0 0 0 0 0 0 0 0 500 1000 1500 2000 2500 The POOL study RHiNO BuRN-Tool Grown in Wales The SANDWICH Trial ROCkeTS Induced sputum in children with cystic… SenITA Phenotyping the Brecon Cohort The
Baby Trial' CAP-IT UK Childhood ITP Registry BATCH Trial FIRST-ABC CHIPS (HIV) PUMA Calibration and Cross-Validation of… UMBRELLA. CF START ALDO Evaluating Risk in Twin Pregnancies DRN100 (TrialNet) C-STICH HOLDS MyeChild 01 Azithromycin Therapy for Chronic Lung… Euro Ewing 2012 Pharmacokinetic variation and toxicity… NEURO-PACK CASAP v1.0 PROTECTOR SHARES Study Characterisation of Paediatric… The OPTIMIST-A Trial ECUSTEC SMPaeds SIOP Ependymoma II rEECur Clinical Characterisation Protocol for… Investigating the inflammatory response… UMSCOM PNET 5 PHITT GEM3 Fetal Atrial Flutter & Supraventricular… Clinical & biological factors associated… NCCPG TDM 2018 Looking at the best
Recruitment
2618 644 369 147 126 116 102 78 53 44 35 33 33 30 25 25 23 20 20 17 17 16 15 13 14 10 8 7 6 5 4 4 3 2 2 1 1 1 1 1 1 0 0 0 0 0 0 500 1000 1500 2000 2500 3000
Figure 2 - Children & Women's Clinical Board Non-Commercial Recruitment Total Against Target (2020-21)
'Big
Recruitment Total Target
Recruitment
Recruitment
total
Children & Women’s Clinical Board
Children & Women’s Clinical Board
OBSTETRICS & GYNAECOLOGY
Children & Women’s Clinical Board
: Indicates study opened in Feb/March - towards the end of the financial year. : Indicates study Closed to Recruitment or Withdrawn during the relevant financial year. : Indicates study Suspended. Charts exclude studies in set-up.
total
*REINFORCE counted under Other Clinical Board but recruitment split between Children & Women's and Surgical Services
associated… NCCPG TDM 2018
Looking at the best needle-free, pain…
The POOL study BuRN-Tool Grown in Wales ROCkeTS Induced sputum in children with… mSEP
Pregnancy and Neonatal Outcomes… The 'Big Baby Trial' nSEP
23
Figure 3 – Children & Women’s – Obstetrics & Gynaecology Directorate Non-Commercial Recruitment Total Against Target (2022-23)
3568 282 164 133 104 68 49 43 28 25 25 20 10 7 1 0 0 500 1000 1500 2000 2500 3000 3500 4000 The POOL study mSEP ROCkeTS Cerclage after full dilatation caesarean section The 'Big Baby Trial' OASI2 REINFORCE The iHOLDS Trial The Tommy's National Rainbow Clinic Study Giant PANDA PROTECTOR Biomarkers for Ovarian Pathology HOLDS WILL C-STICH2 REGAL trial Recruitment total Recruitment total Target
12
Figure 1 - Children & Women's Clinical Board Non-Commercial Recruitment Total Against Target (2019-20)
1473 1061 644 369 260 144 126 86 55 40 37 33 33 28 25 23 20 16 14 12 12 9 8 6 6 6 5 4 4 4 3 3 2 2 2 2 1 1 1 1 0 0 0 0 0 0 0 0 0 500 1000 1500 2000 2500 The POOL study RHiNO BuRN-Tool Grown in Wales The SANDWICH Trial ROCkeTS Induced sputum in children with cystic… SenITA Phenotyping the Brecon Cohort
Baby Trial' CAP-IT UK Childhood ITP Registry BATCH Trial FIRST-ABC CHIPS (HIV) PUMA Calibration and Cross-Validation of… UMBRELLA. CF START ALDO Evaluating Risk in Twin Pregnancies DRN100 (TrialNet) C-STICH HOLDS MyeChild 01 Azithromycin Therapy for Chronic Lung… Euro Ewing 2012 Pharmacokinetic variation and toxicity… NEURO-PACK CASAP v1.0 PROTECTOR SHARES Study Characterisation of Paediatric… The OPTIMIST-A Trial ECUSTEC SMPaeds SIOP Ependymoma II rEECur Clinical Characterisation Protocol for… Investigating the inflammatory response… UMSCOM PNET 5 PHITT GEM3 Fetal Atrial Flutter & Supraventricular… Clinical & biological factors
Figure 2 - Children & Women's Clinical Board Non-Commercial Recruitment Total Against Target (2020-21)
The 'Big
Recruitment
Recruitment
2618 644 369 147 126 116 102 78 53 44 35 33 33 30 25 25 23 20 20 17 17 16 15 13 14 10 8 7 6 5 4 4 3 2 2 1 1 1 1 1 1 0 0 0 0 0 0 500 1000 1500 2000 2500 3000
Total Target
Evaluating Risk in Twin Pregnancies Clinical Characterisation Protocol for… UK Childhood ITP Registry
BLOOD
Cerclage
dilatation…
UMBRELLA Calibration and
of…
Azithromycin
Chronic…
Pharmacokinetic
Characterisation
5
Fetal
&…
Recruitment total Recruitment Total Target
FIRST-ABC
MARKERS FOR… CHIPS (HIV)
after full
PUMA
Cross-Validation
CLARITY CF START CASAP C-STICH CLOCS
Therapy for
PROTECTOR MyeChild 01 RECOVERY TRIAL HOLDS SMPaeds
variation and… WILL
of Paediatric… ECUSTEC NCCPG TDM 2018 SIOP Ependymoma II rEECur Investigating the inflammatory… ICONIC Looking at the best needle-free,… DECRYPT PNET
PHITT GEM3
Atrial Flutter
FaR-RMS
Children & Women’s Clinical Board
Children & Women’s Clinical Board OBSTETRICS
& GYNAECOLOGY
24
24 No Obstetrics & Gynaecology Directorate Commercial Recruitment in 2020-21 or 2021-22
4 – Children & Women’s – Obstetrics & Gynaecology Directorate Commercial Recruitment Total Against Target (2022-23) 3 0 0 2 4 6 8 10 12 LPRI-CF113 in the treatment of endometriosis A Placebo-controlled RCT Volixibat Intrahepatic Cholestasis Pregnancy Recruitment total Recruitment total Target
Figure
Clinical Diagnostics & Therapeutics Clinical Board
Summary from the Clinical Diagnostics & Therapeutics Clinical Board R&D Lead
The strategic focus of Research & Development within the Clinical Diagnostics and Therapeutics
Clinical Board for 2022-23 has remained to encourage research led by Clinical Board staff with ensuring, where possible, capacity to support research from other parts of Cardiff & Vale UHB. Pandemic recovery and withdrawal of HCRW funding from R&D leads has created a challenging environment to foster greater R&D activity. However, the Podiatry/CEDAR collaboration to increase research capacity and has yielded an RfPPB application. The CD&T R&D forums have been promising and will hopefully improve research awareness in directorates with smaller numbers of researchactive staff. The strategy for 2022-23 will be to continue to develop such local initiatives and to ensure an appropriate emphasis on research delivery by those who are not medical consultants. The performance data for 2022-23 again highlight the small numbers of trials which are led by the Clinical Board and the recruitment difficulties of trials in recent times. It remains the case that CD&T support a high proportion of studies led from other Clinical Boards. We are currently considering the impact of such potential services changes as TrAMs and Community Diagnostics Centres on our future capacity to support clinical trials.
Publications in peer-reviewed journals from the clinical board include analysis of reports of carbon monoxide exposures and potential cyanide poisoning to the UK National Poisons Information Service. Also from the All Wales Therapeutics & Toxicology Centre was work on the costs of hypoglycaemic agents in primary care, and nirmatrelvir/ritonavir pre-hospital therapy. Therapies staff published work on the consensus statement on physical activity for cystic fibrosis, digital competency for allied health professionals, treatment of foot ulceration and the Nutrition Skills for Life programme.
Radiology have published several papers in 2022-23 on image enhancement and assessment with AI; analysing radiologists’ behaviour when assessing mammograms; management of patients with Hodgkin’s lymphoma, gallbladder polyps and Paget-Schroetter syndrome; electroconvulsive therapy; imaging of aortic dissection; and recruitment into trials of arterio-venous grafts for haemodialysis. Medical Physics & Clinical Engineering’s publications have all come from CEDAR, relating to PREMs in ophthalmology, the Hughes Abdominal Repair Randomised Trial, the All Wales Postpartum Haemorrhage Quality Improvement Initiative and two from NICE work (UroLift for Treating Lower Urinary Tract Symptoms of Benign Prostatic Hyperplasia; Parafricta bootees to prevent heel pressure ulcers). A chapter for the recently-published book ‘Patient Reported Outcomes and Quality of Life in Cardiovascular Interventions’ was also written by Kathleen Withers of CEDAR.
25
Clinical Diagnostics & Therapeutics Clinical Board Clinical Diagnostics & Therapeutics Clinical Board
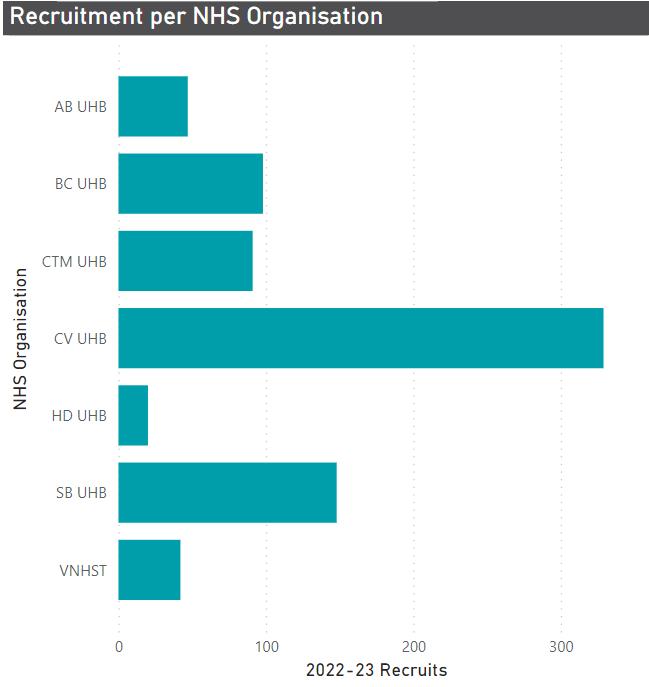
26 Figure 1 - CD&T Clinical Board Non-Commercial Recruitment Total Against Target (2020-21) Figure 2 - CD&T Clinical Board Non-Commercial Recruitment Total Against Target (2021-22) 1078 59 21 21 10 9 4 3 2 0 0 0 200 400 600 800 1000 1200 CR UK SMP ORION-4 LEAP-MS Single-Arm Feasibility BACKonLINE Genomic analysis of Helicobacter pylori and the upper gastrointestinal microbiome WORKWELL BCPPA IONA Patient perception on using salt treatment at their tube site BASIL-2 IFNopathy natural history study Recruitment total Recruitment Total Target 1090 68 24 21 19 7 5 3 3 0 0 200 400 600 800 1000 1200 CR UK SMP ORION-4 COVIDTrach LEAP-MS Single-Arm Feasibility Study COVID SALES EASE-P TrachVest IONA TIPTOP IFNopathy natural history study Recruitment total Recruitment Total Target 26
Clinical Diagnostics & Therapeutics Clinical Board
27
Diagnostics
27 Figure 3 - CD&T Clinical Board Non-Commercial Recruitment Total Against Target (2022-23) 104 34 20 13 12 9 8 3 2 2 2 0 0 20 40 60 80 100 120 ORION-4 TRAK-MSK feasibility The OSTRICH trial TIPTOP EASE-P TrachVest Over the rainbow IONA Adult SMA REACH Study DM PAD study DOLPHIN 2 IFNopathy natural history study Recruitment total Recruitment total Target
Clinical
& Therapeutics Clinical Board
Clinical
Clinical Diagnostics & Therapeutics Clinical Board 28 No CD&T Clinical Board Commercial Recruitment in 2020-21 & 2021-22 Figure 4 – CD&T Clinical Board Commercial Recruitment Total Against Target (2022-23) 19 0 5 10 15 20 25 30 35 Study of Lipoprotein(a) Levels in Patients With a History of ASCVD Recruitment total Recruitment total Target 28
Clinical Diagnostics & Therapeutics
Board
Medicine Clinical Board
Summary from the Medicine Clinical Board R&D Lead
In 2022-23, research continued to be delivered across the Medicine Clinical Board in areas of existing strength with growth into some new areas, providing more patients groups with access to research. Some highlights are below.
Colonoscopy: CONSCOP-2 is a bowel cancer screening study led by Dr Sunil Dolwani at Cardiff University trialling a new staining method to improve identification and subsequent removal of serrated polyps or cancers in the upper bowel. In 2022-23, 168 patients were recruited (248 in total to date) with gratitude to the Medicine Research Delivery team for sustaining support for the study over the course of a challenging year in terms of staffing capacity. The study is expected to lead to a change in international guidelines with global impact.
Cystic Fibrosis: The CF centre led by Dr Jamie Duckers at UHL has been selected as 1 of 6 early phase CF centres in the UK yielding a Phase I inhaled-mRNA trial currently in set-up. Later phase trials continue, with an open-label study recently being secured which will provide earlier access to very high cost CFTR modulator therapies for CF patients and costs fully covered by the company. Finally, Project Breathe combined remote monitoring via a smart phone app for CF patients with artificial intelligence (AI) to determine exacerbations prior to them becoming clinically apparent. 103 people with CF from across Wales used the remote monitoring service and the project has resulted in a change in clinical practice.
Emergency/Sepsis: The PRONTO study, conducted in the Emergency Unit at UHW by Dr Jonathan Underwood and Dr Nicholas Manville, investigates the use of a rapid bedside Procalcitonin test to aid diagnosis in patients with suspected sepsis. 209 participants were recruited in 2022-23 (372 to date), a great achievement especially considering the challenging environment in which the research is taking place. Special thanks to the ED research nurses Lauren Thomas and Non Smith for their exceptional work on this study. We are delighted that Research Delivery has recently confirmed support for the creation of a new full-time research nurse post in ED/Trauma and look forward to developing a new portfolio of research across this area.
Endocrinology: New therapies continue to be available to patients suffering from a wide range of endocrine conditions via trials at C&V. In the last year, commercial trials led by Professor Aled Rees in Congenital Adrenal Hypoplasia and Adrenal Insufficiency exceeded their recruitment targets, with support from the Early Phase Team. In Type 1 Diabetes, C&V are the UK leading recruiters (8) for the Ver-A-T1D (Verapamil) trial led by Professor Colin Dayan at Cardiff University.
Infectious Disease: On the back of the delivery of very successful and high impact COVID treatment and vaccine trials, the infectious disease portfolio has expanded into new areas. Dr Underwood is leading a new commercial trial in HIV which recently recruited its first patient, in addition to a Phase II Hepatitis B vaccine trial due to open very soon.
Long COVID: LOCOMOTION led by Dr Helen Davies with support from the Research Delivery team at UHL is a study to identify best practice in providing Long COVID services. In 2022-23, 322 patients were recruited making it the Clinical Board’s highest recruiting research study this year. A great achievement in a high priority area.
Stroke: Cardiff & Vale are the UK’s leading recruiters for the OPTIMAS trial, investigating whether an earlier initiation of anticoagulation medication after a stroke (within 4 days) is better than current guidelines (between 7-14 days). Well done to Dr Benjamin Jelley, the Stroke team and the Medicine Research Delivery team for the sustained and exceptional effort in achieving this, with 172 patients recruited to date.
29
Medicine Clinical Board
Medicine Clinical Board
Medicine Clinical Board
Medicine Clinical Board
: Indicates study opened in Feb/March - towards the end of the financial year. : Indicates study Closed to Recruitment or Withdrawn during the relevant financial year. : Indicates study Suspended. Charts exclude studies in set-up.
: Indicates study opened in Feb/March - towards the end of the financial year. : Indicates study Closed to Recruitment or Withdrawn during the relevant financial year. : Indicates study Suspended. Charts exclude studies in set-up.
: Indicates study opened in Feb/March - towards the end of the financial year. : Indicates study Closed to Recruitment or Withdrawn during the relevant financial year. : Indicates study Suspended. Charts exclude studies in set-up.
30
1032 588 291 303 264 214 159 159 135 120 95 88 65 64 60 60 59 58 57 47 41 37 34 34 31 28 26 25 25 23 21 18 17 14 11 10 10 9 8 8 8 8 6 6 6 6 5 4 4 3 2 2 1 1 1 1 0 0 0 0 0 200 400 600 800 1000 1200 Clinical Characterisation Protocol for Severe Emerging… CREATE SOCRATES RECOVERY TRIAL AD GENETICS ISTID BADBIR LungCAST BronchUK Study Extracellular vesicle transport in the circulation Determinants of Graves’ Disease Bio-markers of systemic treatment outcomes in Psoriasis Immune fingerprinting of infections in cirrhosis CLARITY OPTIMAS Trial FALCON C-19 DRN 552 ADDRESS-2 TACTIC-R (COVID-19) BSRBR IBD Bioresource PHOSP-COVID REACH HCV PBC Genetics Study Peptidia Lymph Node Monitor Virus Watch The Microbiome and Dementia IRONMAN CFHealthHub Data Observatory Treatment of Hidradenitis Suppurativa Evaluation Study AIP LAUGH EMPOWERED STOP-COVID19 Manuka honey sinus rinse study The I-DSD Registry InFORM StartRight Study Outcomes following Chest Trauma Score ATOMIC2 Biologics for Children with Rheumatic Diseases DLB Genetics CALIBRE Study HIIT v MISS in PCOS PROFILE PLUM TOPaZ study DAISy-PCOS PD COMM BSPAR ECS Should respiratory & sleep physiologists be based in… IASO ASPEN Study UK-Irish A*STAR A Prospective Study of Duodenal Disease in MAP INNODIA ATD Study PriDem Vismodegib resistance in basal cell carcinoma ToPPRA ENRICH-AF Spironolactone for Adult Female Acne Recruitment total Recruitment Total Target
Figure 1 - Medicine Clinical Board Non-Commercial Recruitment Total Against Target (2020-21)
30
1032 588 291 303 264 214 159 159 135 120 95 88 65 64 60 60 59 58 57 47 41 37 34 34 31 28 26 25 25 23 21 18 17 14 11 10 10 9 8 8 8 8 6 6 6 6 5 4 4 3 2 2 1 1 1 1 0 0 0 0 0 200 400 600 800 1000 1200 Clinical Characterisation Protocol for Severe Emerging… CREATE SOCRATES RECOVERY TRIAL AD GENETICS ISTID BADBIR LungCAST BronchUK Study Extracellular vesicle transport in the circulation Determinants of Graves’ Disease Bio-markers of systemic treatment outcomes in Psoriasis Immune fingerprinting of infections in cirrhosis CLARITY OPTIMAS Trial FALCON C-19 DRN 552 ADDRESS-2 TACTIC-R (COVID-19) BSRBR IBD Bioresource PHOSP-COVID REACH HCV PBC Genetics Study Peptidia Lymph Node Monitor Virus Watch The Microbiome and Dementia IRONMAN CFHealthHub Data Observatory Treatment of Hidradenitis Suppurativa Evaluation Study AIP LAUGH EMPOWERED STOP-COVID19 Manuka honey sinus rinse study The I-DSD Registry InFORM StartRight Study Outcomes following Chest Trauma Score ATOMIC2 Biologics for Children with Rheumatic Diseases DLB Genetics CALIBRE Study HIIT v MISS in PCOS PROFILE PLUM TOPaZ study DAISy-PCOS PD COMM BSPAR ECS Should respiratory & sleep physiologists be based in… IASO ASPEN Study UK-Irish A*STAR A Prospective Study of Duodenal Disease in MAP INNODIA ATD Study PriDem Vismodegib resistance in basal cell carcinoma ToPPRA ENRICH-AF Spironolactone for Adult Female Acne Recruitment total Recruitment Total Target
Figure 1 - Medicine Clinical Board Non-Commercial Recruitment Total Against Target (2020-21)
23
542 264 211 159 159 135 120 103 94 92 86 77 62 58 57 46 35 34 28 25 25 25 23 23 20 17 16 15 12 12 10 10 8 8 8 7 7 6 6 5 4 4 3 3 2 2 1 1 1 1 1 1 1 1 0 0 0 0 0 0 0 0 100 200 300 400 500 600 700 800 900 CREATE AD GENETICS ISTID BADBIR LungCAST BronchUK Study Extracellular vesicle transport in the circulation OASIS Determinants of Graves’ Disease Lymphocytes in liver disease and hepatocellular… Bio-markers of systemic treatment outcomes in Psoriasis Inflammation and Immune Regulation in Early… Immune fingerprinting of infections in cirrhosis DRN 552ADDRESS-2 BSRBR TIRED-UK Peptidia Lymph Node Monitor PBC Genetics Study IBD Bioresource HuMiD CFHealthHub Data Observatory IRONMAN Clinical Characterisation Protocol for Severe Emerging… MORe PREcISE REACH HCV Manuka honey sinus rinse study RECOVERY trial Function of novel lipids in skin disease StartRight Study FOMAxS TriMaster STRAP HIIT v MISS in PCOS DLB Genetics Biologics for Children with Rheumatic DiseasesThe… CALIBRE Study PROFILE PLUM TOPaZ study PD COMM APRICOT BSPAR ECS The Microbiome and Dementia IASO PREdiCCt ALPHA. Outcomes following Chest Trauma Score UK-Irish A*STAR ASPEN Study ATD Study INNODIA OPTIMAS Trial PriDem A Prospective Study of Duodenal Disease in MAP Should respiratory & sleep physiologists be based in… Vismodegib resistance in basal cell carcinoma Treatment of Hidradenitis Suppurativa Evaluation Study AIP DAISy-PCOS Hidradenitis Suppurativa long term outcomes following… InFORM Recruitment total Recruitment Total Target 30
Figure 1 - Medicine Clinical Board Non-Commercial Recruitment Total Against Target (2019-20)
Medicine Clinical Board
Medicine Clinical Board
Medicine Clinical Board
31
1181 691 620 312 237 201 163 160 139 134 115 107 97 88 80 77 70 70 67 58 57 37 36 34 34 32 27 25 25 24 21 21 17 17 17 17 15 15 14 11 9 9 8 8 8 7 6 6 6 6 5 5 4 4 3 3 3 3 3 2 2 1 1 1 0 0 0 0 200 400 600 800 1000 1200 1400 Clinical Characterisation Protocol for Severe Emerging Infection SOCRATES CREATE RECOVERY trial ISTID Extracellular vesicle transport in the circulation PRONTO BADBIR BronchUK Study OPTIMAS Trial Immune fingerprinting of infections in cirrhosis IBD Bioresource Determinants of Graves’ Disease Bio-markers of systemic treatment outcomes in Psoriasis CONSCOP 2 Inflammation and Immune Regulation in Early Inflammatory… AIP PHOSP-COVID DRN 552 –ADDRESS2 TACTIC-R (COVID-19) BSRBR HEAL-COVID trial The Microbiome and Dementia PBC Genetics Study Peptidia Lymph Node Monitor LOCOMOTION IRONMAN ASSIST Treatment of Hidradenitis Suppurativa Evaluation Study Acromegaly Register LAUGH EMPOWERED The I-DSD Registry HIIT v MISS in PCOS InFORM Manuka honey sinus rinse study Spitfire Is there a genotype-phenotype correlation in SDHB mutation… PiP DAISy-PCOS The CF STORM Trial Blood-brain barrier dysfunction following systemic infection ELECT2 Trial CALIBRE Study DLB Genetics PROFILE TOPaZ study ASPEN Study PLUM REPEAT phases 1 and 2 Spironolactone for Adult Female Acne ASEPTIC ToPPRA ENRICH-AF Health Professionals' Perspectives on Funding for Dementia Care BOPPP Trial Genetic risk factors for cerebral small vessel disease INNODIA TIPAL UK-Irish A*STAR ATD Study Vismodegib resistance in basal cell carcinoma A Prospective Study of Duodenal Disease in MAP PriDem Ver-A-T1D CIA COVID-19 antibody responses in Cystic Fibrosis patients The CLEAR Trial Recruitment total Recruitment Total Target
Figure 2 - Medicine Clinical Board Non-Commercial Recruitment Total Against Target (2021-22)
31 Figure
1181 691 620 312 237 201 163 160 139 134 115 107 97 88 80 77 70 70 67 58 57 37 36 34 34 32 27 25 25 24 21 21 17 17 17 17 15 15 14 11 9 9 8 8 8 7 6 6 6 6 5 5 4 4 3 3 3 3 3 2 2 1 1 1 0 0 0 0 200 400 600 800 1000 1200 1400 Clinical Characterisation Protocol for Severe Emerging Infection SOCRATES CREATE RECOVERY trial ISTID Extracellular vesicle transport in the circulation PRONTO BADBIR BronchUK Study OPTIMAS Trial Immune fingerprinting of infections in cirrhosis IBD Bioresource Determinants of Graves’ Disease Bio-markers of systemic treatment outcomes in Psoriasis CONSCOP 2 Inflammation and Immune Regulation in Early Inflammatory… AIP PHOSP-COVID DRN 552 –ADDRESS2 TACTIC-R (COVID-19) BSRBR HEAL-COVID trial The Microbiome and Dementia PBC Genetics Study Peptidia Lymph Node Monitor LOCOMOTION IRONMAN ASSIST Treatment of Hidradenitis Suppurativa Evaluation Study Acromegaly Register LAUGH EMPOWERED The I-DSD Registry HIIT v MISS in PCOS InFORM Manuka honey sinus rinse study Spitfire Is there a genotype-phenotype correlation in SDHB mutation… PiP DAISy-PCOS The CF STORM Trial Blood-brain barrier dysfunction following systemic infection ELECT2 Trial CALIBRE Study DLB Genetics PROFILE TOPaZ study ASPEN Study PLUM REPEAT phases 1 and 2 Spironolactone for Adult Female Acne ASEPTIC ToPPRA ENRICH-AF Health Professionals' Perspectives on Funding for Dementia Care BOPPP Trial Genetic risk factors for cerebral small vessel disease INNODIA TIPAL UK-Irish A*STAR ATD Study Vismodegib resistance in basal cell carcinoma A Prospective Study of Duodenal Disease in MAP PriDem Ver-A-T1D CIA COVID-19 antibody responses in Cystic Fibrosis patients The CLEAR Trial Recruitment total Recruitment Total Target
2 - Medicine Clinical Board Non-Commercial Recruitment Total Against Target (2021-22)
31
Medicine Clinical Board
Medicine Clinical Board
Medicine Clinical Board
32
1181 691 622 372372354 272252 248 201187172161160 139115 97 88 77 72 70 66 64 57 51 46 38 36 35 32 31 27 26 25 24 20 11 9 9 9 9 8 8 7 6 6 6 5 5 5 5 4 3 3 3 2 2 1 1 0 0 0 0 0 200 400 600 800 1000 1200 1400 Clinical Characterisation Protocol for Severe Emerging… SOCRATES CREATE RECOVERY trial PRONTO v1.0 LOCOMOTION CIA ISTID CONSCOP 2 Extracellular vesicle transport in the circulation IBD Bioresource OPTIMAS Trial The Physical and Psychological Impact of Stroke on Carers… BADBIR BronchUK Study Immune fingerprinting of infections in cirrhosis Determinants of Graves’ Disease Bio-markers of systemic treatment outcomes in Psoriasis Inflammation and Immune Regulation in Early… DRN 552 (ADDRESS-2) AIP REPEAT phases 1 and 2 ELECT2 Trial BSRBR Blood-brain barrier dysfunction following systemic infection HEAL-COVID trial Peptidia Lymph Node Monitor HIIT v MISS in PCOS PBC Genetics Study DAISy-PCOS COVID-19 antibody responses in Cystic Fibrosis patients The I-DSD Registry EXPLAIN Is there a genotype-phenotype correlation in SDHB… Acromegaly Register Spitfire The CF STORM Trial ASEPTIC CALIBRE Study ENRICH-AF Ver-A-T1D ASPEN Study UNDIES INNODIA Genetic risk factors for cerebral small vessel disease PLUM ToPPRA CASCADE Health Professionals' Perspectives on Funding for… TIPAL UK-Irish A*STAR BOPPP Trial Genetic Analysis to Predict the Development of Paget's… IBD-RESPONSE The CLEAR Trial ATD Study PREPARE A Prospective Study of Duodenal Disease in MAP UK-EDI CONGA PHEAST TB-DILI TOP HAT Recruitment total Recruitment total Target
Figure 3 - Medicine Clinical Board Non-Commercial Recruitment Total Against Target (2022-23)
32
1181 691 622 372372354 272252 248 201187172161160 139115 97 88 77 72 70 66 64 57 51 46 38 36 35 32 31 27 26 25 24 20 11 9 9 9 9 8 8 7 6 6 6 5 5 5 5 4 3 3 3 2 2 1 1 0 0 0 0 0 200 400 600 800 1000 1200 1400 Clinical Characterisation Protocol for Severe Emerging… SOCRATES CREATE RECOVERY trial PRONTO v1.0 LOCOMOTION CIA ISTID CONSCOP 2 Extracellular vesicle transport in the circulation IBD Bioresource OPTIMAS Trial The Physical and Psychological Impact of Stroke on Carers… BADBIR BronchUK Study Immune fingerprinting of infections in cirrhosis Determinants of Graves’ Disease Bio-markers of systemic treatment outcomes in Psoriasis Inflammation and Immune Regulation in Early… DRN 552 (ADDRESS-2) AIP REPEAT phases 1 and 2 ELECT2 Trial BSRBR Blood-brain barrier dysfunction following systemic infection HEAL-COVID trial Peptidia Lymph Node Monitor HIIT v MISS in PCOS PBC Genetics Study DAISy-PCOS COVID-19 antibody responses in Cystic Fibrosis patients The I-DSD Registry EXPLAIN Is there a genotype-phenotype correlation in SDHB… Acromegaly Register Spitfire The CF STORM Trial ASEPTIC CALIBRE Study ENRICH-AF Ver-A-T1D ASPEN Study UNDIES INNODIA Genetic risk factors for cerebral small vessel disease PLUM ToPPRA CASCADE Health Professionals' Perspectives on Funding for… TIPAL UK-Irish A*STAR BOPPP Trial Genetic Analysis to Predict the Development of Paget's… IBD-RESPONSE The CLEAR Trial ATD Study PREPARE A Prospective Study of Duodenal Disease in MAP UK-EDI CONGA PHEAST TB-DILI TOP HAT Recruitment total Recruitment total Target
Figure 3
-
Medicine Clinical Board Non-Commercial Recruitment Total Against Target (2022-23)
32
Medicine Clinical Board
Medicine Clinical Board
33
Target
Figure
Against Target
4 3 1 1 1 1 0 0 0 1 2 3 4 5 6 7 8 9 A Study Evaluating the Safety of Elexacaftor Combination Therapy A Study Evaluating the Long-term Safety of VX-445 Combination Therapy CC-90001-IPF-001safety and efficacy of CC90001 in patients with IPF M16-046 Heads-Up Ph3 adults with Moderate to Severe AD WN41874Gantenerumab Open Label Extension Study IMPACT Study STELLAR Teens CANCOVID Recruitment total Recruitment Total Target 15 7 4 2 1 1 0 0 2 4 6 8 10 12 14 16 PREF NET TriMaximize IMPACT Study ASPEN CC-90001-IPF-001 CHAMPAIN NBI-74788-CAH3003 Recruitment total Recruitment Total Target
Figure 4 - Medicine Clinical Board Commercial Recruitment Total
Against
(2020-21)
5
-
Medicine Clinical Board Commercial Recruitment Total
(2021-22)
33
Medicine Clinical Board
Medicine Clinical Board
(2022-23)
34 Figure
37 20 16 12 9 5 4 3 3 2 2 0 0 0 0 0 0 5 10 15 20 25 30 35 40 LUNAR Trajectory PREF NET TriMaximize MEDI7352 in PDN CHAMPAIN IMPACT Study A Phase 3 Study of VX-121 Combination Therapy in Subjects With Cystic Fibrosis Heterozygous A Phase 3 Study of VX-121 Combination Therapy in Subjects With Cystic Fibrosis Homozygous ASPEN NBI-74788-CAH3003 A Phase 2 Study to evaluate the safety and efficacy of Linsitunib M20-370 Phase 2 ABBV-154 study in PMR pts dependent on GC Tx MK8591A-052 SPARROW TCH-306 Recruitment total Recruitment total Target 34
6 - Medicine Clinical Board Commercial Recruitment Total Against Target
Mental Health Clinical Board
Summary from the Mental Health Clinical Board R&D Lead
The Mental Health Clinical Board had a 50% increase in recruitment numbers in the last financial year compared to 2021-2022. Cardiff and Vale remains by far the most research active health board for mental health in all of Wales. Particular areas of strength on the research portfolio are PTSD, early psychosis and the use of technologies to monitor and treat mental health conditions. We have a number of intervention studies that have allowed patients to access novel therapies including MDMA assisted psychotherapy for PTSD; the use of an antidepressant to prevent depression in people with a recent first episode of psychosis; guided self-help for depressed people with autism; and remotely conducted EMDR (eye movement desensitisation and reprocessing therapy) for military veterans. There has been increased involvement from clinicians in research this year with several new local PIs taking on studies. Work has been completed to allow CRIS (Clinical Record Interactive Search) to extract anonymised data from PARIS – the electronic medical records system used in the Mental Health Clinical Board. In 2023 all agreements should be in place to allow CRIS to assist in conducting research as well as quality improvement projects. We are pleased to include a list of some of the publications coming from the research done within the clinical board, which highlights the range of research taking place into conditions that often come with significant distress and disability, and illustrates the importance of the research activity.
35
Mental Health Clinical Board
Mental Health Clinical Board Clinical Diagnostics & Therapeutics Clinical Board
: Indicates study opened in Feb/March - towards the end of the financial year. : Indicates study Closed to Recruitment or Withdrawn during the relevant financial year. : Indicates study Suspended. Charts exclude studies in set-up.
: Indicates study opened in Feb/March - towards the end of the financial year. : Indicates study Closed to Recruitment or Withdrawn during the relevant financial year. : Indicates study Suspended. Charts exclude studies in set-up.
The influence of autism on restrictive eating disorders in
CD&T also work on PAVE (IRAS: 176799) – Please see 2019-20 recruitment in Specialist Services: Nephrology & Transplant Chart (p.99)
19
Figure 1 - CD&T Clinical Board Non-Commercial Recruitment Total Against Target (2019-20)
Target
1073 59 16 13 10 9 4 4 3 2 0 0 0 200 400 600 800 1000 1200 CR UK SMP ORION-4 LEAP-MS BACKonLINE Genomic analysis of Helicobacter pylori and the upper gastrointestinal microbiome BASIL-3 BCPPA WORKWELL IONA Patient perception on using salt treatment at their tube site BASIL-2 IFNopathy natural history study Recruitment total Recruitment Total Target 1078 59 21 21 10 9 4 3 2 0 0 0 200 400 600 800 1000 1200 CR UK SMP ORION-4 LEAP-MS Single-Arm Feasibility BACKonLINE Genomic analysis of Helicobacter pylori
the upper gastrointestinal microbiome WORKWELL BCPPA IONA Patient perception on using salt treatment at their tube site BASIL-2 IFNopathy natural history study Recruitment total Recruitment Total Target
Figure 2 - CD&T Clinical Board Non-Commercial Recruitment Total Against
(2020-21)
and
36
Against Target
Figure 1 - Mental Health Clinical Board Non-Commercial Recruitment
Total
(2020-21)
Against Target
226 188 33 13 10 7 6 1 0 50 100 150 200 250 NCMH NCISH Exploration of the relationship between social cognition and PTSD PREP Study
Figure 2 - Mental Health Clinical Board Non-Commercial Recruitment Total
(2021-22)
Gender dysphoria and Autism An Intergrated Analysis Of Mammillary Body Function WAND-P Recruitment total Recruitment Total Target 239 200 37 23 22 22 14 7 7 6 5 1 1 0 50 100 150 200 250 300 NCMH NCISH Exploration of the relationship between social cognition and PTSD Understanding Experiences of Feeling Exceptional: questionnaire study COVID-CNS TULIPS PREP Study Gender dysphoria and Autism TAPERS An Intergrated Analysis Of Mammillary Body Function ATP MIST WAND-P Recruitment total Recruitment Total Target 36
women
Mental Health Clinical Board
Clinical
37 Figure
Mental
Non-Commercial
295 223 55 31 30 26 5 4 4 4 1 0 0 50 100 150 200 250 300 350 NCMH NCISH Exploration of the relationship between social cognition and PTSD TULIPS COVID-CNS ADEPT-2 VC versus FTF EMDR for PTSD ADEPP ATP Service user & staff views on digital remote monitoring for psychosis WAND-P Social Environment and Early Psychosis: An Urban Mind Study Recruitment total Recruitment total Target 37
Mental Health
Board
3 -
Health Clinical Board
Recruitment Total Against Target (2022-23)
Mental Health Clinical Board
Clinical Board 38 No Mental Health Clinical Board Commercial Recruitment in 2020-21 or 2021-22 Figure 4
Mental Health Clinical Board Commercial Recruitment Total Against Target
0 0 1 1 2 2 3 MAPS Recruitment total Recruitment total Target 38
Mental Health
-
(2022-23)
Primary, Community & Intermediate Care (PCIC) Clinical Board
Summary from the PCIC Clinical Board R&D Lead
The Primary, Community, and Intermediate Care (PCIC) Clinical Board includes a wide range of services in the community settings. The Clinical Board delivers health and wellbeing services in patients’ homes, in the community and from a range of other facilities. Research has been identified by the PCIC board as a crucial component of evidence-based care provided within Primary Care, and research development and performance constitute core components of the UHB’s end of life strategy. The Sexual Health department has previously made a successful application for the NHS Research Time Award (NHS RTA) which aimed to build research capacity, and there are strategic plans to expand on research activity.
The number of studies with recruitment has significantly increased in 2022-23 and the recruitment has more than doubled compared to the previous years. With the restructuring of Primary Care research delivery and the initiatives taken together with Health & Care Research Wales (HCRW), there has been a sizable increase in research activity. PCIC being one of the largest directorates closely linked to all other departments, there is still a vast potential to enhance research activity within Primary Care and aid recruitment in other directorates within the health board. The restructuring of research delivery and development of academic and secondary care links is still underway, and we can expect further improvements in the future. We aim to continuously improve on the existing processes, making it more efficient and fit for purpose.
Within the Clinical Board, Primary Care, Palliative Care and Sexual Health have been the most research active departments. Development of research and academics in other departments such as nursing, optometry and pharmacy was previously identified as an area of focus that will need further work. The drive to achieve excellence through promotion of research culture within the directorate, underpinned by the principles of the health board research strategy, will be a continuous process.
39
Primary, Community & Intermediate Care (PCIC) Clinical Board
Primary, Community & Intermediate Care (PCIC) Clinical
Board
40
Figure 1 - PCIC Clinical Board Non-Commercial Recruitment Total Against Target (2020-21)
325 73 42 36 32 28 25 3 3 2 0 0 50 100 150 200 250 300 350 CAP Trial PRIMUS CovPall CLASP PRINCIPLE MERIT ARTIC PC CRaFT The MENAC Trial RAMBO Study The Nuclear Community Charity Fund Chromosomal study Recruitment total Recruitment Total Target 325 128 103 96 75 70 42 22 7 2 1 0 0 50 100 150 200 250 300 350 CAP Trial (CNRS) ComFluCov Positive Voices PRIMUS Active Brains Study PRINCIPLE BASIL+ PAM trial CRaFT RAMBO Study The MENAC Trial The Nuclear Community Charity Fund Chromosomal study Recruitment total Recruitment Total Target 40
Figure 2 - PCIC Clinical Board Non-Commercial Recruitment Total Against Target (2021-22)
Primary,
Community & Intermediate Care (PCIC) Clinical Board 41
465 325 192 126 98 76 73 17 10 10 8 4 0 0 0 50 100 150 200 250 300 350 400 450 500 Using Primary Care to Tackle Domestic Violence and Abuse (DVA) CAP TrialCNRS HEAR 2 Interpretation services for Refugees & Asylum Seekers Positive Voices PRIMUS Active Brains Study PRINCIPLE ATTACK DEVA T2T CRaFT Understanding the role and work of paramedics in primary care The Nuclear Community Charity Fund Chromosomal study Time Credits as a Social Prescription Recruitment total Recruitment total Target 41
Community & Intermediate Care (PCIC) Clinical Board Primary,
Figure 3 - PCIC Clinical Board Non-Commercial Recruitment Total Against Target (2022-23)
Primary, Community & Intermediate Care (PCIC) Clinical Board
Primary, Community & Intermediate Care (PCIC) Clinical
Board
42
Figure 4 - PCIC Clinical Board Commercial Recruitment Total Against Target (2020-21)
24 21 5 0 0 50 100 150 200 250 EX9536-4388 SELECT semaglutide cardiovascular outcome trial PRIM 4852 FLOW (NN9535-4321) Semaglutide renal outcomes trial PRIM 5039 Recruitment total Recruitment Total Target 14 5 0 2 4 6 8 10 12 14 16 PIONEER REAL FLOW (NN9535-4321) Recruitment total Recruitment Total Target 42
Figure 5 - PCIC Clinical Board Commercial Recruitment Total Against Target (2021-22)
Primary, Community & Intermediate Care (PCIC)
Primary, Community & Intermediate Care (PCIC)
Clinical Board
43
28 5 2 1 0 5 10 15 20 25 30 PIONEER REAL FLOW (NN9535-4321) Observational study in atrial fibrillation (AF) patients at high risk Maximising Adherence and Gaining New Information For Your COPD v1 Recruitment total Recruitment total Target 43
Clinical Board
Figure 6 - PCIC Clinical Board Commercial Recruitment Total Against Target (2022-23)
Specialist Services Clinical Board
Summary from the Specialist Services Clinical Board R&D Lead
Specialist Services as a Clinical Board has recovered well from the COVID pandemic in terms of research performance, with more studies being reactivated and restarted in this financial year. Most of the research nurses who were re-deployed have returned back to their research roles.
In terms of the number of studies that have been recruiting, Specialist Services has maintained over 100 open studies, with 2,173 patients recruited in total for Portfolio Non-Commercial studies - the highest in the health board. 96 of the studies are rated green in terms of ongoing recruitment.
There has been some spectacular recruitment in some studies, such as the Clinical Characteristics Protocol for Severe Emerging Infections in the Critical Care directorate which has recruited 1,233 patients and RADAR in Nephrology which has recruited 847 patients throughout their durations. There are also studies that involve very invasive protocols requiring considerable UHB support which are recruiting well in most of the directorates.
In 2022-23, there has been the highest number of Commercial trials enrolled in the Specialist Services Clinical Board. Highlights include the PNH Registry in Haematology with some of the highest numbers recruited. Important new practice-defining studies are ongoing in the Clinical Board also, including the ongoing pulmonary embolism (PE) treatments being actively researched and recruited into in the cardiothoracic directorate, which will help to inform and shape important future advances in PE treatments.
There have been vast numbers of important publications in high-impact peer-reviewed journals ranging from the New England Journal to the Lancet and the BMJ from the Clinical Board as a whole, with highlights outlined in the appendix.
Some directorates are still finding their overall research and development partially hindered by lack of full alignment with Cardiff University, in terms of staffing and joint supervision of potential research candidates. There is much progress to be made to ensure directorates can maximize their research potential through these avenues.
Despite all of these massive challenges, the directorates that make up Specialist Services had a hugely impressive year in not only delivering high quality clinical care but pushing forward with groundbreaking research embedded in a number of clinical services. Onwards and upwards!
44
Medicine Clinical Board
Specialist Services Clinical Board Specialist
Specialist Services Clinical Board
Services Clinical Board
: Indicates study opened in Feb/March - towards the end of the financial year. : Indicates study Closed to Recruitment or Withdrawn during the relevant financial year. : Indicates study Suspended. Charts exclude studies in set-up.
(2020-21)
45
0 100 200 300 400 500 600 700 800 900 Move Wales v1 RADAR Pregnancy choices with kidney disease Role of NFkB in CLL Enroll-HD DESEPTiW NIHR BioResourceRare Diseases SIMPLIFIED The role of the marrow microenvironment in the pathogenesis of AML V1: Development of a PRO Measure in Haematological Practice RaDaR (NephroS) Procoagulant phospholipids in arterial thrombosis CMVIR Platelet & microparticle PL in thrombotic and bleeding disorders UKAITPR Innate-like T cells in sepsis AML18 GenOMICC REMAP-CAP DELIVER MS AspiFlu Biology of Juvenile Myoclonic Epilepsy FiTPAD DOPPS TAME AML19 FLAIR GAPP HOT-ICU SC IL-1Ra in SAHphase III trial UKALL 14 UK TTP Registry LI-1 DECISIve Holistic experiences of living with a kidney transplant A2B Trial RECOVERY trial PACE The EMPA-KIDNEY Study TONIC Phase 3 TRIDENT DYNAMO MND Register for England, Wales and Northern Ireland UKEHL Outcome Registry BHF PROTECT-TAVI HEMOTION Trial Tonic 2 Phase 4 MS-STAT2 The A-STOP Study Tonic Phase 2 Prepare for Kidney Care COVID19 in recipients of allogeneic stem cell transplantation EuroNet-PHL-C2 COSI HAEFiT AMADEUS PETReA Cardiac CARE DOMINO-HD Evaluating protocols for identifying and managing patients with FH Prion Surveillance in Primary Immunodeficiency RapidNSTEMI Comparison of quantitative fMRI with FDG-PET in epilepsy ENRICH HighCALS The HOT 2 Trial QUACS IDRIS CATALYST Health Status and Quality of Life in Congenital Agammaglob WAND-NDE Almitrine bismesylate in COVID-19 ALL-RIC AID ICU LIPS MITHRIDATE Trial Version 2.0 ORBITA-2 Pacing in Heart Failure Study PITHIA MoTD CRYOSTAT-2 Eslicarbazepine and cognition in older patients FORVAD Huntington's Disease Fetal Tissue Collection PHIND Study STELLAR UKPID Registry Recruitment total Recruitment Total Target
Figure 1 - Specialist Services Clinical Board Non-Commercial Recruitment Total Against Target (2020-21)
Clinical Board Non-Commercial Recruitment Total Against Target
0 100 200 300 400 500 600 700 800 900 Move Wales v1 RADAR Pregnancy choices with kidney disease Role of NFkB in CLL Enroll-HD DESEPTiW NIHR BioResourceRare Diseases SIMPLIFIED The role of the marrow microenvironment in the pathogenesis of AML V1: Development of a PRO Measure in Haematological Practice RaDaR (NephroS) Procoagulant phospholipids in arterial thrombosis CMVIR Platelet & microparticle PL in thrombotic and bleeding disorders UKAITPR Innate-like T cells in sepsis AML18 GenOMICC REMAP-CAP DELIVER MS AspiFlu Biology of Juvenile Myoclonic Epilepsy FiTPAD DOPPS TAME AML19 FLAIR GAPP HOT-ICU SC IL-1Ra in SAHphase III trial UKALL 14 UK TTP Registry LI-1 DECISIve Holistic experiences of living with a kidney transplant A2B Trial RECOVERY trial PACE The EMPA-KIDNEY Study TONIC Phase 3 TRIDENT DYNAMO MND Register for England, Wales and Northern Ireland UKEHL Outcome Registry BHF PROTECT-TAVI HEMOTION Trial Tonic 2 Phase 4 MS-STAT2 The A-STOP Study Tonic Phase 2 Prepare for Kidney Care COVID19 in recipients of allogeneic stem cell transplantation EuroNet-PHL-C2 COSI HAEFiT AMADEUS PETReA Cardiac CARE DOMINO-HD Evaluating protocols for identifying and managing patients with FH Prion Surveillance in Primary Immunodeficiency RapidNSTEMI Comparison of quantitative fMRI with FDG-PET in epilepsy ENRICH HighCALS The HOT 2 Trial QUACS IDRIS CATALYST Health Status and Quality of Life in Congenital Agammaglob WAND-NDE Almitrine bismesylate in COVID-19 ALL-RIC AID ICU LIPS MITHRIDATE Trial Version 2.0 ORBITA-2 Pacing in Heart Failure Study PITHIA MoTD CRYOSTAT-2 Eslicarbazepine and cognition in older patients FORVAD Huntington's Disease Fetal Tissue Collection PHIND Study STELLAR UKPID Registry Recruitment total Recruitment Total Target 45
Figure 1 - Specialist Services
23
542 264 211 159 159 135 120 103 94 92 86 77 62 58 57 46 35 34 28 25 25 25 23 23 20 17 16 15 12 12 10 10 8 8 8 7 7 6 6 5 4 4 3 3 2 2 1 1 1 1 1 1 1 1 0 0 0 0 0 0 0 0 100 200 300 400 500 600 700 800 900 CREATE AD GENETICS ISTID BADBIR LungCAST BronchUK Study Extracellular vesicle transport in the circulation OASIS Determinants of Graves’ Disease Lymphocytes in liver disease and hepatocellular… Bio-markers of systemic treatment outcomes in Psoriasis Inflammation and Immune Regulation in Early… Immune fingerprinting of infections in cirrhosis DRN 552ADDRESS-2 BSRBR TIRED-UK Peptidia Lymph Node Monitor PBC Genetics Study IBD Bioresource HuMiD CFHealthHub Data Observatory IRONMAN Clinical Characterisation Protocol for Severe Emerging… MORe PREcISE REACH HCV Manuka honey sinus rinse study RECOVERY trial Function of novel lipids in skin disease StartRight Study FOMAxS TriMaster STRAP HIIT v MISS in PCOS DLB Genetics Biologics for Children with Rheumatic DiseasesThe… CALIBRE Study PROFILE PLUM TOPaZ study PD COMM APRICOT BSPAR ECS The Microbiome and Dementia IASO PREdiCCt ALPHA. Outcomes following Chest Trauma Score UK-Irish A*STAR ASPEN Study ATD Study INNODIA OPTIMAS Trial PriDem A Prospective Study of Duodenal Disease in MAP Should respiratory & sleep physiologists be based in… Vismodegib resistance in basal cell carcinoma Treatment of Hidradenitis Suppurativa Evaluation Study AIP DAISy-PCOS Hidradenitis Suppurativa long term outcomes following… InFORM Recruitment total Recruitment Total Target
Figure 1 - Medicine Clinical Board Non-Commercial Recruitment Total Against Target (2019-20)
Specialist
Services Clinical Board Specialist Services Clinical Board
Specialist Services Clinical Board
Specialist Services Clinical Board Non-Commercial Recruitment Total Against Target (2021-22)
46 0 100 200 300 400 500 600 700 800 900 Move Wales RADAR Pregnancy choices with kidney disease NEuRoMS Work Package 2i Observational Study ENLIST Vaccination sub-study Role of NFkB in CLL Enroll-HD SYMPLIFY SIMPLIFIED NIHR BioResourceRare Diseases The role of the marrow microenvironment in the pathogenesis of AML Platelet & microparticle PL in thrombotic and bleeding disorders CMVIR RaDaR: (NephroS) UKAITPR Innate-like T cells in sepsis PROTECT V GenOMICC BHF PROTECT-TAVI AML18 DELIVER MS COVID-19 ENLIST REMAP-CAP DOPPS FiTPAD BLING III AspiFlu AML19 Biology of Juvenile Myoclonic Epilepsy TAME SC IL-1Ra in SAHphase III trial DECISIve FLAIR GAPP A2B Trial HOT-ICU UKALL 14 UK TTP Registry DOMINO-HD Optimising Staff-Patient Communication in Advanced Renal Disease RECOVERY trial TRIDENT HEMOTION Trial MND Register PACE Almitrine bismesylate in COVID-19 Tonic Phase 3 DYNAMO QUACS MS-PROACTIVE COSI CANDID UKEHL Outcome Registry The A-STOP Study AMADEUS MS-STAT2 Tonic 2 Phase 4 PETReA MoTD Tonic Phase 2 COVID19 in recipients of allogeneic stem cell transplantation HAEFiT NEuRoMS Work Package 2ii Qualitative Study Multivessel TALENT Prepare for Kidney Care Psychological Impact of COVID-19 SOS trial FUTURE GB Cardiac CARE AID ICU Prion Surveillance in Primary Immunodeficiency Evaluating protocols for identifying and managing patients with FH ENRICH MND-SMART Comparison of quantitative fMRI with FDG-PET in epilepsy RapidNSTEMI ORBITA-2 BAT-X Study SIGNET RADAR (UK-MRA Myeloma XV) ALL-RIC WAND-NDE LIPS Biology and genetics of smouldering myeloma HDClarity CRYOSTAT-2 MITHRIDATE Trial Version 2 VICTOR PROMise FROM-16: ATMP Pacing in Heart Failure Study PITHIA STELLAR TURING ChariotMS Huntington's Disease Fetal Tissue Collection UKPID Registry Eslicarbazepine and cognition in older patients PHIND Study WAND-JME CARE pilot trial Recruitment total Recruitment Total Target
Figure 2 -
46 0 100 200 300 400 500 600 700 800 900 Move Wales RADAR Pregnancy choices with kidney disease NEuRoMS Work Package 2i Observational Study ENLIST Vaccination sub-study Role of NFkB in CLL Enroll-HD SYMPLIFY SIMPLIFIED NIHR BioResourceRare Diseases The role of the marrow microenvironment in the pathogenesis of AML Platelet & microparticle PL in thrombotic and bleeding disorders CMVIR RaDaR: (NephroS) UKAITPR Innate-like T cells in sepsis PROTECT V GenOMICC BHF PROTECT-TAVI AML18 DELIVER MS COVID-19 ENLIST REMAP-CAP DOPPS FiTPAD BLING III AspiFlu AML19 Biology of Juvenile Myoclonic Epilepsy TAME SC IL-1Ra in SAHphase III trial DECISIve FLAIR GAPP A2B Trial HOT-ICU UKALL 14 UK TTP Registry DOMINO-HD Optimising Staff-Patient Communication in Advanced Renal Disease RECOVERY trial TRIDENT HEMOTION Trial MND Register PACE Almitrine bismesylate in COVID-19 Tonic Phase 3 DYNAMO QUACS MS-PROACTIVE COSI CANDID UKEHL Outcome Registry The A-STOP Study AMADEUS MS-STAT2 Tonic 2 Phase 4 PETReA MoTD Tonic Phase 2 COVID19 in recipients of allogeneic stem cell transplantation HAEFiT NEuRoMS Work Package 2ii Qualitative Study Multivessel TALENT Prepare for Kidney Care Psychological Impact of COVID-19 SOS trial FUTURE GB Cardiac CARE AID ICU Prion Surveillance in Primary Immunodeficiency Evaluating protocols for identifying and managing patients with FH ENRICH MND-SMART Comparison of quantitative fMRI with FDG-PET in epilepsy RapidNSTEMI ORBITA-2 BAT-X Study SIGNET RADAR (UK-MRA Myeloma XV) ALL-RIC WAND-NDE LIPS Biology and genetics of smouldering myeloma HDClarity CRYOSTAT-2 MITHRIDATE Trial Version 2 VICTOR PROMise FROM-16: ATMP Pacing in Heart Failure Study PITHIA STELLAR TURING ChariotMS Huntington's Disease Fetal Tissue Collection UKPID Registry Eslicarbazepine and cognition in older patients PHIND Study WAND-JME CARE pilot trial Recruitment total Recruitment Total Target
46
Figure 2 - Specialist Services Clinical Board Non-Commercial Recruitment Total Against Target (2021-22)
Specialist Services Clinical Board
Specialist
Specialist Services Clinical Board
Services Clinical Board
47
0 100 200 300 400 500 600 700 800 900 Move Wales v1 RADAR NEuRoMS Work Package 3 ROAR Study NEuRoMS Work Package 2i ENLIST Vaccination sub-study Enroll-HD A study of Clonal Haematopoiesis in the Welsh population The role of the marrow microenvironment in the pathogenesis of AML SIMPLIFIED Platelet & microparticle PL in thrombotic and bleeding disorders NIHR BioResourceRare Diseases RaDaR: NephroS CMVIR UKAITPR BHF PROTECT-TAVI GenOMICC DELIVER MS Innate-like T cells in sepsis A2B Trial PROTECT V AML18 COVID-19 ENLIST REMAP-CAP DOPPS BLING III SC IL-1Ra in SAHphase III trial OSCAR QUACS AspiFlu Biology of Juvenile Myoclonic Epilepsy DECISIve FLAIR ADVENT GAPP MND-SMART HOT-COVID COSI HEMOTION Trial UK TTP Registry The A-STOP Study MND Register for England, Wales and Northern Ireland WAND-JME PROGROUP Feasibility Randomised Controlled Trial v1 TRIDENT TONIC Phase 3 AMADEUS CANDID PETReA MoTD UKEHL Outcome Registry FUTURE GB SIGNET TONIC Phase 4 Multivessel TALENT TONIC Phase 2 HAEFiT Prepare for Kidney Care SOS trial COSMOS iCorMicA Psychological Impact of COVID-19 RAPID-PROTECTION HIDDEN2 V1.0 MAST Trial RADAR (UK-MRA Myeloma XV) BRIGHTLIGHT_2021 ORBITA-2 FROM-16: ATMP MiNDToolkit Feasibility Study Prion Surveillance in Primary Immunodeficiency TURING The conNeCT study ALL-RIC PROMise CARE pilot trial ChariotMS LIPS MITHRIDATE Trial OptimalECRI 13 The PROTECT study VICTOR WAND-NDE ACHIEVE HDClarity Kidney transplant patient decision making Version 1 ProMMise STELLAR MOSAICC STUDY Pacing in Heart Failure Study PREDICT VF II SINIMA FEDORA HAVEN Huntington's Disease Fetal Tissue Collection VITDALIZE UK Recruitment total Recruitment total Target
Figure 3 - Specialist Services Clinical Board Non-Commercial Recruitment Total Against Target (2022-23)
47
0 100 200 300 400 500 600 700 800 900 Move Wales v1 RADAR NEuRoMS Work Package 3 ROAR Study NEuRoMS Work Package 2i ENLIST Vaccination sub-study Enroll-HD A study of Clonal Haematopoiesis in the Welsh population The role of the marrow microenvironment in the pathogenesis of AML SIMPLIFIED Platelet & microparticle PL in thrombotic and bleeding disorders NIHR BioResourceRare Diseases RaDaR: NephroS CMVIR UKAITPR BHF PROTECT-TAVI GenOMICC DELIVER MS Innate-like T cells in sepsis A2B Trial PROTECT V AML18 COVID-19 ENLIST REMAP-CAP DOPPS BLING III SC IL-1Ra in SAHphase III trial OSCAR QUACS AspiFlu Biology of Juvenile Myoclonic Epilepsy DECISIve FLAIR ADVENT GAPP MND-SMART HOT-COVID COSI HEMOTION Trial UK TTP Registry The A-STOP Study MND Register for England, Wales and Northern Ireland WAND-JME PROGROUP Feasibility Randomised Controlled Trial v1 TRIDENT TONIC Phase 3 AMADEUS CANDID PETReA MoTD UKEHL Outcome Registry FUTURE GB SIGNET TONIC Phase 4 Multivessel TALENT TONIC Phase 2 HAEFiT Prepare for Kidney Care SOS trial COSMOS iCorMicA Psychological Impact of COVID-19 RAPID-PROTECTION HIDDEN2 V1.0 MAST Trial RADAR (UK-MRA Myeloma XV) BRIGHTLIGHT_2021 ORBITA-2 FROM-16: ATMP MiNDToolkit Feasibility Study Prion Surveillance in Primary Immunodeficiency TURING The conNeCT study ALL-RIC PROMise CARE pilot trial ChariotMS LIPS MITHRIDATE Trial OptimalECRI 13 The PROTECT study VICTOR WAND-NDE ACHIEVE HDClarity Kidney transplant patient decision making Version 1 ProMMise STELLAR MOSAICC STUDY Pacing in Heart Failure Study PREDICT VF II SINIMA FEDORA HAVEN Huntington's Disease Fetal Tissue Collection VITDALIZE UK Recruitment total Recruitment total Target 47
Figure 3 - Specialist Services Clinical Board Non-Commercial Recruitment Total Against Target (2022-23)
Specialist Services Clinical Board Specialist Services Clinical Board
Specialist Services Clinical Board
Figure 4 - Specialist Services Clinical Board Commercial Recruitment Total Against Target (2020-21)
Recruitment Total Target
Recruitment Total Target
of AZD4573 in Relapsed or Refractory Haematological
observational study to evaluate usage of Hizentra
Emicizumab in Haemophilia A without FVIII Inhibitors GekoTM Pacemaker Interaction Study PDR 001 in Acute myeloid Leukaemia or high risk MDS RENA 4590 (ASCEND-ND)
Vedolizumab in Subjects Undergoing Stem Cell Transplantation aHUS REGISTRY Phase1b study for patients with Relapsed/Refractory AML 021FSG16010DUPLEX, Patients with FSGS
Patients with Acute Myeloid Leukemia. XATOA
Phase 1b/2 Study of KRT-232 in Patients with Acute Myeloid Leukemia. XATOA
BN40955 OLE study to evaluate the safety & tolerability of RO7234292 Phase I/IIa study to evaluate CCS1477 in haem. malignancies v1.0
BN40423 Generation HD 1 ECLIPSE Hookipa H-100-002
BN40955 OLE study to evaluate the safety & tolerability of RO7234292 Phase I/IIa study to evaluate CCS1477 in haem. malignancies v1.0 BN40423 Generation HD 1 ECLIPSE Hookipa H-100-002
Expanded Access Treatment Protocol: Remdesivir (RDV; GS-5734) for the…
Expanded Access Treatment Protocol: Remdesivir (RDV; GS-5734) for the…
PROTECT 021IGAN17001
PROTECT 021IGAN17001
A phase 1/2 study with Acalabrutinib and AZD6738 in high risk CLL CALLS IXchange Paradigm 8 HORIZON
A phase 1/2 study with Acalabrutinib and AZD6738 in high risk CLL
Characteristics and outcomes of FCS patients treated with volanesorsen
PCYC-1145-LTLong-term safety rollover for ibrutinib trials CPI-0610 with or without ruxolitinib
Characteristics and outcomes of FCS patients treated with volanesorsen
PCYC-1145-LTLong-term safety rollover for ibrutinib trials
Extended Access of Momelotinib in Myelofibrosis
CPI-0610 with or without ruxolitinib
Safety &Efficacy of Padsevonil in Subjects With DrugResistant Epilepsy
Extended Access of Momelotinib in Myelofibrosis
Uproleselan with chemotherapy vs chemo alone in adults with R/R AML
Safety &Efficacy of Padsevonil in Subjects With DrugResistant Epilepsy
A Phase 2a/b Study of KRT-232 in Polycythemia Vera ARTEMIS-IGAN
Uproleselan with chemotherapy vs chemo alone in adults with R/R AML
A Phase 2a/b Study of KRT-232 in Polycythemia Vera
CQGE031C2303: Ligelizumab in the treatment of CSU
DREAMM-7
ARTEMIS-IGAN
CQGE031C2303: Ligelizumab in the treatment of CSU
Efficacy and safety of LNP023 compared with rituximab in iMN
DREAMM-7
Four oral doses of BI 690517 in patients with Diabetic Nephropathy. Gazyvaro Short Duration Infusion (SDI) study
Efficacy and safety of LNP023 compared with rituximab in iMN
GRAVITAS-309
Four oral doses of BI 690517 in patients with Diabetic Nephropathy.
M19-708 Venetoclax as Maintenance Therapy for AML ORATORIO HAND
Gazyvaro Short Duration Infusion (SDI) study
GRAVITAS-309
Phase 2 study of ACE-536 in MPN-associated myelofibrosis & anaemia
M19-708 Venetoclax as Maintenance Therapy for AML ORATORIO HAND
2 study of ACE-536 in MPN-associated myelofibrosis & anaemia
REGN1979 in patients with relapsed or refractory follicular lymphoma
The Radiance II Pivotal study in Stage II Hypertension.
Rare disease registry Program (Gaucher, Fabry, Pompe, MPS1)
48
0 50 100 150 200 250 300 350 ENSEMBLE 2 S-FLEX UK-II, Rev 1 SANTORINI SPYRAL HTN ON-MED MUSC 5224 PNH Registry CMV
patients, v4.0 AGHOS-UK Study
Figure 4 - Specialist Services Clinical Board Commercial Recruitment Total Against Target (2020-21)
in allogeneic hematopoietic stem cell transplant
Malignancies XPF-008-201 An
BO41423
A
CALLS IXchange Paradigm
HORIZON
8
Phase
The
Recruitment total
48
0 50 100 150 200 250 300 350 ENSEMBLE 2 S-FLEX UK-II, Rev 1 SANTORINI SPYRAL HTN ON-MED MUSC 5224 PNH Registry CMV in allogeneic hematopoietic stem cell transplant patients, v4.0 AGHOS-UK Study of AZD4573 in Relapsed or Refractory Haematological Malignancies XPF-008-201
Hizentra BO41423 Emicizumab in Haemophilia A without FVIII Inhibitors GekoTM Pacemaker Interaction Study PDR 001 in Acute myeloid Leukaemia or high risk MDS RENA 4590 (ASCEND-ND) Vedolizumab in Subjects Undergoing Stem Cell Transplantation aHUS REGISTRY Phase1b study for patients with Relapsed/Refractory AML 021FSG16010DUPLEX, Patients with FSGS A Phase 1b/2 Study of KRT-232 in
An observational study to evaluate usage of
REGN1979 in patients with relapsed or refractory follicular lymphoma The Radiance II Pivotal study in Stage II Hypertension. The Rare disease registry Program (Gaucher, Fabry, Pompe, MPS1) Recruitment total 48
Specialist Services Clinical Board
Specialist Services Clinical Board
Specialist Services Clinical Board
Total Against Target (2021-22)
Recruitment Total Target
Recruitment Total Target
total
patients BN40955 OLE study to evaluate the safety & tolerability of RO7234292
DREAMM-7 ECLIPSE Hi-PEITHO Hookipa H-100-002 ARTEMIS-IGAN
CC-92480 with Dexamethasone in Relapsed & Refractory Multiple Myeloma Frontier 2
CC-92480 with Dexamethasone in Relapsed & Refractory Multiple Myeloma Frontier 2
M15-954:Venetoclax and/or Azacitidine in Newly Diagnosed High-Risk MDS
PROTECT 021IGAN17001 The DAPA-MI Study 2-year ext. study to evaluate long-term effectiveness of Mavenclad AGAVE-201-
ELEVATE AHP Registry
GRAVITAS-309
M15-954:Venetoclax and/or Azacitidine in Newly Diagnosed High-Risk MDS PROTECT 021IGAN17001 The DAPA-MI Study 2-year ext. study to evaluate long-term effectiveness of Mavenclad AGAVE-201ELEVATE AHP Registry GRAVITAS-309 IFNg-study M19-708 Venetoclax as Maintenance Therapy for AML Paradigm 8
IFNg-study
M19-708 Venetoclax as Maintenance Therapy for AML Paradigm 8
Phase 3 Study of CPI-0610 in Myelofibrosis (Amendment 1, UK V2.1)
PEG-ADM in ARDS
Recombinant human alkaline phosphatase survival trial REFORM The Rare disease registry Program (Gaucher, Fabry, Pompe, MPS1)
Phase 3 Study of CPI-0610 in Myelofibrosis (Amendment 1, UK V2.1)
Recombinant human alkaline phosphatase survival trial REFORM
Uproleselan with chemotherapy vs chemo alone in adults with R/R AML BE HEARD
The Rare disease registry Program (Gaucher, Fabry, Pompe, MPS1)
Uproleselan with chemotherapy vs chemo alone in adults with R/R AML BE HEARD
Efficacy and safety of LNP023 compared with rituximab in iMN Haven 7 Phase 2 study of ACE-536 in MPN-associated myelofibrosis & anaemia Phase 2 Study of VIS649 for IgA Nephropathy
CARTITUDE-4
Efficacy and safety of LNP023 compared with rituximab in iMN Haven 7
Phase II study of sacubitril/valsartan in patients with nHCM
REGN1979 in patients with relapsed or refractory follicular lymphoma Remix 2
Phase 2 study of ACE-536 in MPN-associated myelofibrosis & anaemia Phase 2 Study of VIS649 for IgA Nephropathy Phase II study of sacubitril/valsartan in patients with nHCM REGN1979 in patients with relapsed or refractory follicular lymphoma Remix 2
SHP655-201: Pharmacokinetics, safety, and efficacy of SHP655 in aTTP SPARKLE Study of WVE-003 in Patients with HD (SNP3) URIROX-2 Recruitment total
49
0 20 40 60 80 100 120 140 S-FLEX UK-II, Rev 1 CARD 4750 SPYRAL HTN ON-MED MUSC 5224 Alexion PNH Registry M07-001 CMV in allogeneic hematopoietic stem cell transplant patients, v4.0 gekoTM Pacemaker Interaction Trial HORIZON SPYRAL-DYSTAL Pridopidine in Patients
Early Stage of Huntington Disease BO41423 Emicizumab in Haemophilia A
FVIII Inhibitors Phase I/IIa study to
CCS1477
haem. malignancies v1.0 The
Pivotal
Hypertension. Vedolizumab
aHUS REGISTRY An
CPI-0610
ruxolitinib ORATORIO HAND EFC16035 A
BN40955
DREAMM-7 ECLIPSE
ARTEMIS-IGAN
Figure 5 - Specialist Services Clinical Board Commercial Recruitment Total Against Target (2021-22)
with
without
evaluate
in
Radiance II
study in Stage II
in Subjects Undergoing Stem Cell Transplantation
observational study to evaluate usage of Hizentra
with or without
Phase 1b/2 Study of KRT-232 in Patients with Acute Myeloid Leukemia. A Phase 3 comparing BTKi to placebo in nrSPMS patients
OLE study to evaluate the safety & tolerability of RO7234292
Hi-PEITHO Hookipa H-100-002
49
0 20 40 60 80 100 120 140 S-FLEX UK-II, Rev 1 CARD 4750 SPYRAL HTN ON-MED MUSC 5224 Alexion PNH Registry M07-001 CMV in allogeneic hematopoietic stem cell transplant patients, v4.0 gekoTM Pacemaker Interaction Trial HORIZON SPYRAL-DYSTAL Pridopidine in Patients with Early Stage of Huntington Disease BO41423 Emicizumab in Haemophilia A without FVIII Inhibitors Phase I/IIa study to evaluate CCS1477 in haem. malignancies v1.0 The Radiance II Pivotal study in Stage II Hypertension. Vedolizumab in Subjects Undergoing Stem Cell Transplantation aHUS REGISTRY An observational study to evaluate usage of Hizentra CPI-0610 with or without ruxolitinib ORATORIO HAND EFC16035 A Phase 1b/2 Study of KRT-232 in Patients with Acute Myeloid Leukemia. A Phase 3 comparing BTKi to placebo in nrSPMS
Figure 5 - Specialist Services Clinical Board Commercial Recruitment
PEG-ADM in ARDS
CARTITUDE-4
Recruitment
SHP655-201: Pharmacokinetics, safety, and efficacy of SHP655 in aTTP SPARKLE Study of WVE-003 in Patients with HD (SNP3) URIROX-2 49
Specialist Services Clinical Board Specialist Services Clinical Board
Specialist Services Clinical Board
50
0 10 20 30 40 50 60 MUSC 5224 Alexion PNH Registry M07-001 The DAPA-MI Study BIOMICS study gekoTM Pacemaker Interaction Trial CELLCENTRIC ELEVATE AHP Registry Frontier 2 Efficacy and safety of finerenone in subjects with non-diabetic… Hi-PEITHO MAGNETISMM-5 TA-8995-304 aHUS REGISTRY ARTIOS CPI-0610 with or without ruxolitinib ORATORIO HAND The Radiance II Pivotal study in Stage II Hypertension. A Phase 1b/2 Study of KRT-232 in Patients with Acute Myeloid… AGAVE-201 ECLIPSE Estimating Risk of Selected AEs in Pts with VWD Treated with… Frontier 3 IFNg-study MANIFEST PERSEUS Remix 2 ARTEMIS-IGAN M15-954:Venetoclax and/or Azacitidine in Newly Diagnosed… Paradigm 8 PEG-ADM in ARDS Safety and Efficacy of AMT-130 in Adults with HD APeX-N NI-PASS evaluating Berotralstat in a real-world setting ASCENT CSL Behring AMR BoD study DEFINE GPS GRAVITAS-309 HERCULES Maternal PKU PTC-PIVOT HD Recombinant human alkaline phosphatase survival trial REFORM SNP3 SPARKLE The Rare disease registry Program (Gaucher, Fabry, Pompe,… VIALE-M WAY4001 ASTER BLU HARBOR CML with T315I mutationv00 CONVERT ENHANCE-2 Haven 7 MAGNOLIA Neurocrine NBI-921352-FOS202 OASIS F901318/0041 OBIC301OUS Phase 3 study to Compare T-Guard to Ruxolitinib for SR-… Phase II study of sacubitril/valsartan in patients with nHCM REGN1979 in patients with relapsed or refractory follicular… S.T.A.R. S.T.A.R.S. Extension Strike-PE Recruitment total Recruitment total Target
Figure 6 - Specialist Services Clinical Board Commercial Recruitment Total Against Target (2022-23)
50
0 10 20 30 40 50 60 MUSC 5224 Alexion PNH Registry M07-001 The DAPA-MI Study BIOMICS study gekoTM Pacemaker Interaction Trial CELLCENTRIC ELEVATE AHP Registry Frontier 2 Efficacy and safety of finerenone in subjects with non-diabetic… Hi-PEITHO MAGNETISMM-5 TA-8995-304 aHUS REGISTRY ARTIOS CPI-0610 with or without ruxolitinib ORATORIO HAND The Radiance II Pivotal study in Stage II Hypertension. A Phase 1b/2 Study of KRT-232 in Patients with Acute Myeloid… AGAVE-201 ECLIPSE Estimating Risk of Selected AEs in Pts with VWD Treated with… Frontier 3 IFNg-study MANIFEST PERSEUS Remix 2 ARTEMIS-IGAN M15-954:Venetoclax and/or Azacitidine in Newly Diagnosed… Paradigm 8 PEG-ADM in ARDS Safety and Efficacy of AMT-130 in Adults with HD APeX-N NI-PASS evaluating Berotralstat in a real-world setting ASCENT CSL Behring AMR BoD study DEFINE GPS GRAVITAS-309 HERCULES Maternal PKU PTC-PIVOT HD Recombinant human alkaline phosphatase survival trial REFORM SNP3 SPARKLE The Rare disease registry Program (Gaucher, Fabry, Pompe,… VIALE-M WAY4001 ASTER BLU HARBOR CML with T315I mutationv00 CONVERT ENHANCE-2 Haven 7 MAGNOLIA Neurocrine NBI-921352-FOS202 OASIS F901318/0041 OBIC301OUS Phase 3 study to Compare T-Guard to Ruxolitinib for SR-… Phase II study of sacubitril/valsartan in patients with nHCM REGN1979 in patients with relapsed or refractory follicular… S.T.A.R. S.T.A.R.S. Extension Strike-PE Recruitment total Recruitment total Target 50
Figure 6 - Specialist Services Clinical Board Commercial Recruitment Total Against Target (2022-23)
Specialist Services Directorate Recruitment:
Specialist Services Directorate Recruitment: ALAS (incl. Rehabilitation Engineering)
ALAS (inc. Rehabilitation Engineering)
51 No ALAS Directorate Non-Commercial Recruitment in 2020-21, 2021-22 or 2022-23 No ALAS Directorate Commercial Recruitment in 2019-20, 2020-21 or 2021-22 51
Specialist Services Directorate Recruitment:
CARDIAC SERVICES
Specialist Services Directorate Recruitment: CARDIAC SERVICES
: Indicates study opened in Feb/March - towards the end of the financial year. : Indicates study Closed to Recruitment or Withdrawn during the relevant financial year. : Indicates study Suspended. Charts exclude studies in set-up.
52
Figure 1 - Specialist Services - Cardiac Services Non-Commercial Recruitment Total Against Target (2020-21)
99 16 5 4 1 1 0 50 100 150 200 250 300 350 400 450 500 Procoagulant phospholipids in arterial thrombosis BHF PROTECT-TAVI RapidNSTEMI QUACS ORBITA-2 Pacing in Heart Failure Study Recruitment total Recruitment Total Target 70 19 11 5 5 1 0 50 100 150 200 250 BHF PROTECT-TAVI QUACS Multivessel TALENT RapidNSTEMI ORBITA-2 Pacing in Heart Failure Study Recruitment total Recruitment Total Target 52
Figure 2 - Specialist Services - Cardiac Services Non-Commercial Recruitment Total Against Target (2021-22)
CARDIAC SERVICES 53 Figure 3
Non-Commercial
Total Against Target (2022-23) 106 46 14 10 7 3 1 1 0 50 100 150 200 250 BHF PROTECT-TAVI QUACS Multivessel TALENT iCorMicA ORBITA-2 OptimalECRI 13 Pacing in Heart Failure Study PREDICT VF II Recruitment total Recruitment total Target 53
Specialist Services Directorate Recruitment: CARDIAC SERVICES Specialist Services Directorate Recruitment:
- Specialist Services - Cardiac Services
Recruitment
Specialist Services Directorate Recruitment: CARDIAC SERVICES Specialist Services Directorate Recruitment: CARDIAC
SERVICES 54
4
Specialist
Cardiac Services Commercial Recruitment Total Against Target (2020-21) Figure 5 - Specialist Services - Cardiac Services Commercial Recruitment Total Against Target (2021-22) 78 28 5 3 0 0 20 40 60 80 100 120 S-FLEX UK-II, Rev 1 SPYRAL HTN ON-MED GekoTM Pacemaker Interaction Study XATOA The Radiance II Pivotal study in Stage II Hypertension. Recruitment total Recruitment Total Target 128 45 15 13 5 3 2 1 0 0 20 40 60 80 100 120 140 S-FLEX UK-II, Rev 1 CARD 4750 SPYRAL HTN ON-MED gekoTM Pacemaker Interaction Trial SPYRAL-DYSTAL The Radiance II Pivotal study in Stage II Hypertension. Hi-PEITHO The DAPA-MI Study REFORM Phase II study of sacubitril/valsartan in patients with nHCM Recruitment total Recruitment Total Target 54
Figure
-
Services -
Specialist Services Directorate Recruitment: CARDIAC SERVICES 55 Figure 6
Commercial Recruitment
Target (2022-23) 18 15 15 5 5 4 1 1 0 0 0 0 10 20 30 40 50 60 The DAPA-MI Study BIOMICS study gekoTM Pacemaker Interaction Trial Hi-PEITHO TA-8995-304 The Radiance II Pivotal study in Stage II Hypertension. DEFINE GPS REFORM CONVERT Phase II study of sacubitril/valsartan in patients with nHCM Strike-PE Recruitment total Recruitment total Target 55
Specialist Services Directorate Recruitment: CARDIAC SERVICES
- Specialist Services - Cardiac Services
Total Against
Specialist Services Directorate Recruitment: CRITICAL CARE Specialist Services Directorate Recruitment: CRITICAL CARE
: Indicates study opened in Feb/March - towards the end of the financial year. : Indicates study Closed to Recruitment or Withdrawn during the relevant financial year. : Indicates study Suspended. Charts exclude studies in set-up.
56
Recruitment Total Against Target
2 - Specialist
- Critical Care Non-Commercial Recruitment Total Against Target (2021-22) 227 79 61 46 42 35 31 22 16 12 10 3 2 1 0 0 50 100 150 200 250 DESEPTiW Innate-like T cells in sepsis GenOMICC REMAP-CAP AspiFlu TAME HOT-ICU A2B Trial HEMOTION Trial The A-STOP Study RECOVERY trial CATALYST Almitrine bismesylate in COVID-19 AID ICU PHIND Study Recruitment total Recruitment Total Target 84 73 57 42 42 40 33 31 22 22 20 18 15 10 8 7 5 0 0 20 40 60 80 100 120 140 160 Innate-like T cells in sepsis GenOMICC REMAP-CAP BLING III AspiFlu TAME A2B Trial HOT-ICU HEMOTION Trial RECOVERY trial Almitrine bismesylate in COVID-19 CANDID The A-STOP Study Psychological Impact of COVID-19 SOS trial AID ICU SIGNET PHIND Study Recruitment total Recruitment Total Target 56
Figure
1 - Specialist Services - Critical Care Non-Commercial
(2020-21) Figure
Services
CARE 57 Figure 3 - Specialist Services - Critical Care Non-Commercial Recruitment Total Against Target (2022-23) 97 84 82 57 49 45 31 29 24 18 16 12 10 8 0 0 20 40 60 80 100 120 140 160 GenOMICC Innate-like T cells in sepsis A2B Trial REMAP-CAP BLING III AspiFlu HOT-COVID HEMOTION Trial The A-STOP Study CANDID SIGNET SOS trial Psychological Impact of COVID-19 MAST Trial VITDALIZE UK Recruitment total Recruitment total Target 57
Specialist Services Directorate Recruitment: CRITICAL CARE Specialist Services Directorate Recruitment: CRITICAL
Specialist Services Directorate Recruitment: CRITICAL CARE Specialist Services Directorate Recruitment: CRITICAL CARE
58
Figure 4 - Specialist Services - Critical Care Commercial Recruitment Total Against Target (2020-21)
2 0 1 2 3 Expanded Access Treatment Protocol: Remdesivir (RDV; GS-5734) for the Treatment of SARS-CoV2 (CoV) I Recruitment total Recruitment Total Target 1 1 0 5 10 15 20 25 PEG-ADM in ARDS Recombinant human alkaline phosphatase survival trial Recruitment total Recruitment Total Target 58
Figure 5 - Specialist Services - Critical Care Commercial Recruitment Total Against Target (2021-22)
Specialist Services Directorate Recruitment: CRITICAL CARE Specialist Services Directorate Recruitment: CRITICAL CARE
59
2 1 0 5 10 15 20 25 PEG-ADM in ARDS Recombinant human alkaline phosphatase survival trial Recruitment total Recruitment total Target 59
Figure 6 - Specialist Services - Critical Care Commercial Recruitment Total Against Target (2022-23)
Specialist Services Directorate Recruitment: HAEMATOLOGY & CLINICAL IMMUNOLOGY Specialist Services Directorate Recruitment:
60
CLINICAL
60 Figure 1 - Specialist Services - Haematology & Clinical Immunology Non-Commercial Recruitment Total Against Target (2020-21) Figure 2 - Specialist Services - Haematology & Clinical Immunology Non-Commercial Recruitment Total Against Target (2021-22) 336 138 123 109 83 79 66 38 34 33 31 26 25 25 21 18 17 9 9 8 8 8 8 7 6 6 5 4 3 2 1 1 0 0 0 0 0 50 100 150 200 250 300 350 400 Role of NFkB in CLL NIHR BioResourceRare Diseases The role of the marrow microenvironment… Development of a PRO Measure in… Platelet & microparticle PL in thrombotic… UKAITPR AML18 FiTPAD AML19 FLAIR GAPP UKALL 14 UK TTP Registry LI-1 PACE DYNAMO UKEHL Outcome Registry COVID19 in recipients of allogeneic stem… EuroNet-PHL-C2 COSI HAEFiT AMADEUS PETReA Cardiac CARE Evaluating protocols for identifying and… Prion Surveillance in Primary… ENRICH IDRIS Health Status and Quality of Life in… ALL-RIC LIPS MITHRIDATE Trial Version 2.0 MoTD CRYOSTAT-2 STELLAR UKPID Registry Recruitment total Recruitment Total Target 387 336 161 138 136 135 99 69 65 42 41 35 33 26 25 21 19 18 17 15 13 13 12 12 7 6 6 6 5 4 3 3 3 2 2 2 2 2 1 0 0 50 100 150 200 250 300 350 400 450 ENLIST Vaccination sub-study Role of NFkB in CLL SYMPLIFY NIHR BioResourceRare Diseases The role of the marrow… Platelet & microparticle PL in thrombotic… UKAITPR AML18 COVID-19 ENLIST FiTPAD AML19 FLAIR GAPP UKALL 14 UK TTP Registry PACE DYNAMO COSI UKEHL Outcome Registry AMADEUS PETReA MoTD COVID19 in recipients of allogeneic stem… HAEFiT Cardiac CARE Prion Surveillance in Primary… Evaluating protocols for identifying and… ENRICH BAT-X Study RADAR (UK-MRA Myeloma XV) ALL-RIC LIPS Biology and genetics of smouldering… CRYOSTAT-2 MITHRIDATE Trial Version 2 VICTOR PROMise FROM-16: ATMP STELLAR UKPID Registry Recruitment total Recruitment Total Target
HAEMATOLOGY &
IMMUNOLOGY
Specialist Services Directorate Recruitment: HAEMATOLOGY & CLINICAL IMMUNOLOGY
Specialist Services Directorate Recruitment: HAEMATOLOGY & CLINICAL IMMUNOLOGY
*ENLIST Vaccination sub-study and COVID-19 ENLIST recruitment (2021-22 and 2022-23) achieved by Nephrology & Transplant team
61
Figure 3 - Specialist Services - Haematology & Clinical Immunology Non-Commercial Recruitment Total Against Target (2022-23)
387 204 169 139 138 109 71 64 36 33 33 29 25 22 20 18 17 17 12 11 10 8 8 7 6 6 5 4 4 3 3 3 3 2 2 1 0 0 50 100 150 200 250 300 350 400 450 ENLIST Vaccination sub-study A study of Clonal Haematopoiesis in the… The role of the marrow… Platelet & microparticle PL in thrombotic… NIHR BioResourceRare Diseases UKAITPR AML18 COVID-19 ENLIST FLAIR ADVENT GAPP COSI UK TTP Registry PROGROUP Feasibility Randomised… AMADEUS PETReA MoTD UKEHL Outcome Registry HAEFiT COSMOS RAPID-PROTECTION HIDDEN2 V1.0 RADAR (UK-MRA Myeloma XV) BRIGHTLIGHT_2021 FROM-16: ATMP Prion Surveillance in Primary… The conNeCT study ALL-RIC PROMise LIPS MITHRIDATE Trial The PROTECT study VICTOR ProMMise STELLAR MOSAICC STUDY FEDORA Recruitment total Recruitment total Target 61
Specialist Services Directorate Recruitment: HAEMATOLOGY & CLINICAL IMMUNOLOGY
Specialist Services Directorate Recruitment: HAEMATOLOGY & CLINICAL IMMUNOLOGY
Recruitment total
MUSC 5224
Registry CMV in allogeneic hematopoietic stem cell… AGHOS-UK Study of AZD4573 in Relapsed or Refractory… BO41423 Emicizumab in Haemophilia A… PDR 001 in Acute myeloid Leukaemia or high… RENA 4590 (ASCEND-ND)
Vedolizumab in Subjects Undergoing Stem Cell… An observational study to evaluate usage of… Phase1b study for patients with… A Phase 1b/2 Study of KRT-232 in Patients with… Phase I/IIa study to evaluate CCS1477 in haem.… ECLIPSE A phase 1/2 study with Acalabrutinib and… CALLS Paradigm 8 HORIZON Characteristics and outcomes of FCS patients…
PCYC-1145-LTLong-term safety rollover for…
CPI-0610 with or without ruxolitinib
Extended Access of Momelotinib in…
Uproleselan with chemotherapy vs chemo…
A Phase 2a/b Study of KRT-232 in Polycythemia…
CQGE031C2303: Ligelizumab in the treatment…
DREAMM-7
Short Duration Infusion (SDI) study
Gazyvaro
GRAVITAS-309
M19-708 Venetoclax as Maintenance Therapy… Phase 2 study of ACE-536 in MPN-associated…
REGN1979 in patients with relapsed or…
The Rare disease registry Program (Gaucher,…
Recruitment Total Target
PNH Registry M07-001
Alexion
CMV in allogeneic hematopoietic stem cell…
HORIZON
BO41423 Emicizumab in Haemophilia A… Phase I/IIa study to evaluate CCS1477 in…
Vedolizumab in Subjects Undergoing Stem…
An observational study to evaluate usage of… CPI-0610 with or without ruxolitinib
Study of KRT-232 in Patients…
A Phase 1b/2
DREAMM-7 ECLIPSE
2
CC-92480 with Dexamethasone in Relapsed… Frontier
M15-954:Venetoclax and/or Azacitidine in…
AGAVE-201-
ELEVATE AHP Registry
GRAVITAS-309
IFNg-study
M19-708 Venetoclax as Maintenance…
Paradigm 8
Phase 3 Study of CPI-0610 in Myelofibrosis…
The Rare disease registry Program (Gaucher,…
Uproleselan with chemotherapy vs chemo… BE HEARD
CARTITUDE-4 Haven 7
Phase 2 study of ACE-536 in MPN-associated…
REGN1979 in patients with relapsed or… Remix
2
SHP655-201: Pharmacokinetics, safety, and…
SPARKLE URIROX-2
62
62
Figure 4 - Specialist Services - Haematology & Clinical Immunology Commercial Recruitment Total Against Target (2020-21)
224 37 23 21 14 10 9 5 5 5 5 4 4 3 3 3 2 2 1 1 1 1 1 1 1 0 0 0 0 0 0 0 0 0 0 50 100 150 200 250 300 350 ENSEMBLE 2 SANTORINI MUSC
Figure
5 - Specialist Services - Haematology & Clinical Immunology Commercial Recruitment Total Against Target (2021-22)
5224 PNH
Recruitment total Recruitment Total Target 23 22 20 13 6 6 5 4 4 3 3 3 2 2 2 1 1 1 1 1 1 1 1 1 0 0 0 0 0 0 0 0 0 0 5 10 15 20 25
Specialist Services Directorate Recruitment: HAEMATOLOGY & CLINICAL IMMUNOLOGY
Specialist Services Directorate Recruitment: HAEMATOLOGY & CLINICAL IMMUNOLOGY
63 Figure 6 - Specialist Services - Haematology & Clinical Immunology Commercial Recruitment Total Against Target (2022-23) 23 22 9 8 7 5 4 3 3 3 3 3 3 3 3 2 2 1 1 1 1 1 1 1 1 0 0 0 0 0 0 0 0 0 0 0 5 10 15 20 25 MUSC 5224 Alexion PNH Registry M07-001 CELLCENTRIC ELEVATE AHP Registry Frontier 2 MAGNETISMM-5 CPI-0610 with or without ruxolitinib A Phase 1b/2 Study of KRT-232 in Patients… AGAVE-201 ECLIPSE Estimating Risk of Selected AEs in Pts with… Frontier 3 IFNg-study MANIFEST Remix 2 M15-954:Venetoclax and/or Azacitidine in… Paradigm 8 APeX-N NI-PASS evaluating Berotralstat in a… ASCENT GRAVITAS-309 Maternal PKU SPARKLE The Rare disease registry Program (Gaucher,… VIALE-M WAY4001 ASTER BLU HARBOR CML with T315I mutationv00 ENHANCE-2 Haven 7 MAGNOLIA OASIS F901318/0041 OBIC301OUS Phase 3 study to Compare T-Guard to… REGN1979 in patients with relapsed or… Recruitment total Recruitment total Target 63
Specialist Services
Directorate Recruitment: NEPHROLOGY & TRANSPLANT Specialist Services Directorate Recruitment: NEPHROLOGY & TRANSPLANT
: Indicates study opened in Feb/March - towards the end of the financial year. : Indicates study Closed to Recruitment or Withdrawn during the relevant financial year. : Indicates study Suspended. Charts exclude studies in set-up.
64
64
2
Nephrology & Transplant Non-Commercial Recruitment Total
Target (2021-22) 763 432 141 102 101 75 48 23 10 1 1 0 100 200 300 400 500 600 700 800 900 RADAR Pregnancy choices with kidney disease SIMPLIFIED CMVIR RaDaR: (NephroS) PROTECT V DOPPS Optimising Staff-Patient Communication in Advanced Renal Disease Prepare for Kidney Care PITHIA TURING Recruitment total Recruitment Total Target 744 342 131 101 92 38 22 21 10 5 1 0 100 200 300 400 500 600 700 800 RADAR Pregnancy choices with kidney disease SIMPLIFIED RaDaR (NephroS) CMVIR DOPPS Holistic experiences of living with a kidney transplant The EMPA-KIDNEY Study Prepare for Kidney Care The HOT 2 Trial PITHIA Recruitment total Recruitment Total Target
Figure 1 - Specialist Services - Nephrology & Transplant Non-Commercial Recruitment Total Against Target (2020-21) Figure
- Specialist Services -
Against
NEPHROLOGY
TRANSPLANT 65
3
Transplant
Recruitment Total
Target (2022-23) *ENLIST Vaccination sub-study and COVID-19 ENLIST recruitment (2021-22 and 2022-23) achieved by Nephrology & Transplant team 805 141 114 110 76 52 47 12 6 2 2 0 0 100 200 300 400 500 600 700 800 900 RADAR SIMPLIFIED RaDaR: NephroS CMVIR PROTECT V DOPPS OSCAR Prepare for Kidney Care TURING ACHIEVE Kidney transplant patient decision making Version 1 HAVEN Recruitment total Recruitment total Target 65
Specialist Services Directorate Recruitment: NEPHROLOGY & TRANSPLANT Specialist Services Directorate Recruitment:
&
Figure
- Specialist Services - Nephrology &
Non-Commercial
Against
Specialist Services Directorate Recruitment: NEPHROLOGY & TRANSPLANT Specialist Services Directorate Recruitment: NEPHROLOGY & TRANSPLANT
66
66
4 - Specialist Services - Nephrology & Transplant Commercial Recruitment Total Against Target (2020-21) Figure 5 - Specialist Services - Nephrology & Transplant Commercial Recruitment Total Against Target (2021-22) 4 3 3 2 2 0 0 0 0 1 2 3 4 5 6 aHUS REGISTRY 021FSG16010DUPLEX, Patients with FSGS Hookipa H-100-002 PROTECT 021IGAN17001 IXchange ARTEMIS-IGAN Efficacy and safety of LNP023 compared with rituximab in iMN Four oral doses of BI 690517 in patients with Diabetic Nephropathy. Recruitment total Recruitment Total Target 4 3 2 2 0 0 0 1 2 3 4 5 6 7 8 9 10 aHUS REGISTRY Hookipa H-100-002 ARTEMIS-IGAN PROTECT 021IGAN17001 Efficacy and safety of LNP023 compared with rituximab in iMN Phase 2 Study of VIS649 for IgA Nephropathy Recruitment total Recruitment Total Target
Figure
67
6 4 2 1 0 2 4 6 8 10 12 Efficacy and safety of finerenone in subjects with non-diabetic CKD aHUS REGISTRY ARTEMIS-IGAN CSL Behring AMR BoD study Recruitment total Recruitment total Target 67
Specialist Services Directorate Recruitment: NEPHROLOGY & TRANSPLANT Specialist Services Directorate Recruitment: NEPHROLOGY & TRANSPLANT
Figure 6 - Specialist Services - Nephrology & Transplant Commercial Recruitment Total Against Target (2022-23)
Specialist Services Directorate Recruitment: NEUROSCIENCES Specialist Services Directorate Recruitment: NEUROSCIENCES
: Indicates study opened in Feb/March - towards the end of the financial year. : Indicates study Closed to Recruitment or Withdrawn during the relevant financial year. : Indicates study Suspended. Charts exclude studies in set-up.
68
68
Figure 1 - Specialist Services - Neurosciences Non-Commercial Recruitment Total Against Target (2020-21)
778 245 45 41 27 24 19 19 17 14 14 12 6 5 5 3 0 0 0 0 100 200 300 400 500 600 700 800 900 Move Wales v1 Enroll-HD DELIVER MS Biology of Juvenile Myoclonic Epilepsy SC IL-1Ra in SAHphase III trial DECISIve TONIC Phase 3 TRIDENT MND Register for England, Wales and Northern Ireland Tonic 2 Phase 4 MS-STAT2 Tonic Phase 2 DOMINO-HD Comparison of quantitative fMRI with FDG-PET in epilepsy HighCALS WAND-NDE Eslicarbazepine and cognition in older patients FORVAD Huntington's Disease Fetal Tissue Collection Recruitment total Recruitment Total Target 778 429 255 67 41 40 39 24 22 21 19 19 14 14 12 11 8 6 5 3 2 1 0 0 0 0 0 100 200 300 400 500 600 700 800 900 Move Wales NEuRoMS Work Package 2i… Enroll-HD DELIVER MS Biology of Juvenile Myoclonic Epilepsy SC IL-1Ra in SAHphase III trial DECISIve DOMINO-HD TRIDENT MND Register Tonic Phase 3 MS-PROACTIVE MS-STAT2 Tonic 2 Phase 4 Tonic Phase 2 NEuRoMS Work Package 2ii Qualitative… FUTURE GB MND-SMART Comparison of quantitative fMRI with… WAND-NDE HDClarity ChariotMS Huntington's Disease Fetal Tissue… Eslicarbazepine and cognition in older… WAND-JME CARE pilot trial Recruitment total Recruitment Total Target
Figure 2 - Specialist Services - Neurosciences Non-Commercial Recruitment Total Against Target (2021-22)
NEUROSCIENCES 69 Figure 3 - Specialist Services - Neurosciences Non-Commercial Recruitment Total Against Target (2022-23) 826 631 564 537 262 88 48 41 40 33 23 23 22 21 16 16 14 6 3 3 3 2 1 0 0 100 200 300 400 500 600 700 800 900 Move Wales v1 NEuRoMS Work Package 3 ROAR Study NEuRoMS Work Package 2i Enroll-HD DELIVER MS SC IL-1Ra in SAHphase III trial Biology of Juvenile Myoclonic Epilepsy DECISIve MND-SMART MND Register for England, Wales and Northern Ireland WAND-JME TRIDENT TONIC Phase 3 FUTURE GB TONIC Phase 4 TONIC Phase 2 MiNDToolkit Feasibility Study CARE pilot trial ChariotMS WAND-NDE HDClarity SINIMA Huntington's Disease Fetal Tissue Collection Recruitment total Recruitment total Target 69
Specialist Services Directorate Recruitment: NEUROSCIENCES Specialist Services Directorate Recruitment:
Specialist Services Directorate Recruitment: NEUROSCIENCES Specialist Services Directorate Recruitment: NEUROSCIENCES
70
70
(2020-21)
Figure 4 - Specialist Services - Neurosciences Commercial Recruitment Total
Against Target
Recruitment Total Against Target (2021-22) 7 3 3 1 0 0 1 2 3 4 5 6 7 8 XPF-008-201 BN40955 OLE study to evaluate the safety & tolerability of RO7234292 BN40423 Generation HD 1 Safety &Efficacy of Padsevonil in Subjects With DrugResistant Epilepsy ORATORIO HAND Recruitment total Recruitment Total Target 8 4 3 3 3 1 0 0 1 2 3 4 5 6 7 8 9 Pridopidine in Patients with Early Stage of Huntington Disease ORATORIO HAND EFC16035 A Phase 3 comparing BTKi to placebo in nrSPMS patients BN40955 OLE study to evaluate the safety & tolerability of RO7234292 2-year ext. study to evaluate long-term effectiveness of Mavenclad Study of WVE-003 in Patients with HD (SNP3) Recruitment total Recruitment Total Target
Figure
5 - Specialist
Services
- Neurosciences Commercial
Specialist Services Directorate Recruitment: NEUROSCIENCES
Specialist Services Directorate Recruitment: NEUROSCIENCES
71 Figure
4 4 3 2 1 1 1 0 0 0 0 1 2 3 4 5 6 ARTIOS ORATORIO HAND PERSEUS Safety and Efficacy of AMT-130 in Adults with HD HERCULES PTC-PIVOT HD SNP3 Neurocrine NBI-921352-FOS202 S.T.A.R. S.T.A.R.S. Extension Recruitment total Recruitment total Target 71
6 - Specialist Services - Neurosciences Commercial Recruitment Total Against Target (2022-23)
Surgical Services Clinical Board
Summary from the Surgical Services Clinical Board R&D Lead
We have had another great year in the Surgical Services Clinical Board for research, with more than £11 million of NIHR grants being awarded to 5 Chief Investigators across the board. This unprecedented level of success demonstrates the drive for home-grown research within the clinical board and our commitment to improving patient outcomes through research. The Chief Investigators come from a range of specialties, demonstrating the broadening of the drive for Cardiff-developed research with studies from urology, vascular, anaesthetics and colorectal surgery. The development of the in-house trials manager role has contributed to this and has led to other boards developing a similar role in their departments.
We have increased our commercial research portfolio, however this is something that will continue to be a focus in the coming 3 years as there is still more to do. We have continued to be a leader in delivering high quality research from other centres and have been in the top 5 recruiting sites for ROSSINI 2, POLARiS and PQUiP. We have been expanding the role of allied health professionals and trainees as Principal Investigators through the NIHR Associate PI scheme in several trials, and this is increasing the appetite for research participation in a multidisciplinary manner that adds strength to our holistic approach to research.
72
Surgical Services Clinical Board
Medicine Clinical Board
Surgical Services Clinical Board
Surgical Services Clinical Board
: Indicates study opened in Feb/March - towards the end of the financial year. : Indicates study Closed to Recruitment or Withdrawn during the relevant financial year. : Indicates study Suspended. Charts exclude studies in set-up.
: Indicates study opened in Feb/March - towards the end of the financial year. : Indicates study Closed to Recruitment or Withdrawn during the relevant financial year. : Indicates study Suspended. Charts exclude studies in set-up.
: Indicates study opened in Feb/March - towards the end of the financial year. : Indicates study Closed to Recruitment or Withdrawn during the relevant financial year. : Indicates study Suspended. Charts exclude studies in set-up.
1376 719 671 471 386 314 258 233 209 114 85 64 61 60 46 42 41 38 37 33 27 25 25 24 19 17 17 15 13 12 10 10 8 7 5 4 4 3 3 1 1 1 1 1 0 0 0 0 0 0 0 0 0 200 400 600 800 1000 1200 1400 1600 BBRC Versus ArthritisClinical Samples WHiTE Study OBS Plus BBRC Versus Arthritis(MOCAP) Perioperative Quality Improvement Programme WHiTE 8 COPAL UK Genetic Prostate Cancer Study International Impact of TALK Validation of patient centric measures in urolithiasis Add-Aspirin Patient experience of injections in age-related… PRIDA CIPHER The UK CAVIAR Study NSCCG BBRC Versus ArthritisMRI imaging for the joint HEAT PATCH MQUEST BBRC Versus ArthritisFluoroscopic imaging Reduction Of Surgical Site Infection using several… EMT2 START:REACTS ITACS Trial POSNOC WAX PITSTOP IntAct The HAPI Study ALLEGRO Economic burden and quality of life effect of OASIS PATHOS PIONEER WHiTE 9BeST (MOSES) AIR: RRP PRIMETIME SOFFT The FUTURE Study CPinBOSS MARS 2 STAR-TReC Surgery or Cast for Injuries of the EpicoNdyle in VIDEO Trial Best-Of COVER HUSHThe Humeral Shaft Fracture Trial NOSTRA OCCAMS Sarcopenia, frailty and nutritional status in… Surgery versus non-surgical splint treatment for… UNICORNS Recruitment total Recruitment Total Target
Figure 1 - Surgical Services Clinical Board Non-Commercial Recruitment Total Against Target (2020-21)
73
1376 719 671 471 386 314 258 233 209 114 85 64 61 60 46 42 41 38 37 33 27 25 25 24 19 17 17 15 13 12 10 10 8 7 5 4 4 3 3 1 1 1 1 1 0 0 0 0 0 0 0 0 0 200 400 600 800 1000 1200 1400 1600 BBRC Versus ArthritisClinical Samples WHiTE Study OBS Plus BBRC Versus Arthritis(MOCAP) Perioperative Quality Improvement Programme WHiTE 8 COPAL UK Genetic Prostate Cancer Study International Impact of TALK Validation of patient centric measures in urolithiasis Add-Aspirin Patient experience of injections in age-related… PRIDA CIPHER The UK CAVIAR Study NSCCG BBRC Versus ArthritisMRI imaging for the joint HEAT PATCH MQUEST BBRC Versus ArthritisFluoroscopic imaging Reduction Of Surgical Site Infection using several… EMT2 START:REACTS ITACS Trial POSNOC WAX PITSTOP IntAct The HAPI Study ALLEGRO Economic burden and quality of life effect of OASIS PATHOS PIONEER WHiTE 9BeST (MOSES) AIR: RRP PRIMETIME SOFFT The FUTURE Study CPinBOSS MARS 2 STAR-TReC Surgery or Cast for Injuries of the EpicoNdyle in VIDEO Trial Best-Of COVER HUSHThe Humeral Shaft Fracture Trial NOSTRA OCCAMS Sarcopenia, frailty and nutritional status in… Surgery versus non-surgical splint treatment for… UNICORNS Recruitment total Recruitment Total Target
Figure 1 - Surgical Services Clinical Board Non-Commercial Recruitment Total Against Target (2020-21)
23
542 264 211 159 159 135 120 103 94 92 86 77 62 58 57 46 35 34 28 25 25 25 23 23 20 17 16 15 12 12 10 10 8 8 8 7 7 6 6 5 4 4 3 3 2 2 1 1 1 1 1 1 1 1 0 0 0 0 0 0 0 0 100 200 300 400 500 600 700 800 900 CREATE AD GENETICS ISTID BADBIR LungCAST BronchUK Study Extracellular vesicle transport in the circulation OASIS Determinants of Graves’ Disease Lymphocytes in liver disease and hepatocellular… Bio-markers of systemic treatment outcomes in Psoriasis Inflammation and Immune Regulation in Early… Immune fingerprinting of infections in cirrhosis DRN 552ADDRESS-2 BSRBR TIRED-UK Peptidia Lymph Node Monitor PBC Genetics Study IBD Bioresource HuMiD CFHealthHub Data Observatory IRONMAN Clinical Characterisation Protocol for Severe Emerging… MORe PREcISE REACH HCV Manuka honey sinus rinse study RECOVERY trial Function of novel lipids in skin disease StartRight Study FOMAxS TriMaster STRAP HIIT v MISS in PCOS DLB Genetics Biologics for Children with Rheumatic DiseasesThe… CALIBRE Study PROFILE PLUM TOPaZ study PD COMM APRICOT BSPAR ECS The Microbiome and Dementia IASO PREdiCCt ALPHA. Outcomes following Chest Trauma Score UK-Irish A*STAR ASPEN Study ATD Study INNODIA OPTIMAS Trial PriDem A Prospective Study of Duodenal Disease in MAP Should respiratory & sleep physiologists be based in… Vismodegib resistance in basal cell carcinoma Treatment of Hidradenitis Suppurativa Evaluation Study AIP DAISy-PCOS Hidradenitis Suppurativa long term outcomes following… InFORM Recruitment total Recruitment Total Target 73
Figure 1 - Medicine Clinical Board Non-Commercial Recruitment Total Against Target (2019-20)
Surgical Services Clinical Board
Surgical Services Clinical Board
Surgical Services Clinical Board
burden and quality of life effect of IntAct TRANSLATE PATHOS The HAPI Study Sarcopenia, frailty and nutritional status in… (MOSES) PRIMETIME SOFFT Surgery versus non-surgical splint treatment for… AIR: RRP
or Cast for Injuries of the EpicoNdyle in CPinBOSS UNICORNS Exploring the epigenetic changes in diverticular… FEMuR III HEAR IT HUSH PERCEIVE study WHiTE 11FRUITI NOSTRA-Feasibility Study STAR-TReC BASIS
74
74 Figure 2 - Surgical Services Clinical Board Non-Commercial Recruitment Total Against Target (2021-22) 1378 751 686 627 474 379 339 270 209 206 126 118 112 100 68 65 50 46 42 39 38 37 34 33 31 30 28 28 26 25 25 24 23 20 19 18 18 17 14 13 10 8 6 5 5 4 4 3 3 2 2 2 2 2 2 1 1 0 0 0 0 0 0 0 200 400 600 800 1000 1200 1400 1600 BBRC Versus ArthritisClinical Samples WHiTE Study OBS Plus Perioperative Quality Improvement Programme BBRC Versus Arthritis3D motion analysis… Urinary Biomarker URO17 Study WHiTE 8 COPAL UK Genetic Prostate Cancer Study Validation of patient centric measures in… Urine Biomarkers for detecting prostate cancer Add-Aspirin Patient experience of injections in age-related… Reduction Of Surgical Site Infection using… SNAP3: Frailty & delirium Mapping the lipid envelope composition of… The CIPHER study MQUEST NSCCG BBRC Versus ArthritisMRI imaging for the joint EMT2 PATCH WHiTE 9 ALLEGRO BBRC Versus ArthritisFluoroscopic imaging The REST feasibility trial POLO Proximie Live Plus tele-mentoring for trainee… TIGER study ITACS Trial FLO-ELA PITSTOP WAX NOTACS POSNOC ObsQoR Economic
Surgery
DAVE L1FE SUPERSTAR
COACH
Recruitment total Recruitment Total Target
Study Best-Of
The
Trial
74 Figure 2 - Surgical Services
Recruitment
Target (2021-22) 1378 751 686 627 474 379 339 270 209 206 126 118 112 100 68 65 50 46 42 39 38 37 34 33 31 30 28 28 26 25 25 24 23 20 19 18 18 17 14 13 10 8 6 5 5 4 4 3 3 2 2 2 2 2 2 1 1 0 0 0 0 0 0 0 200 400 600 800 1000 1200 1400 1600 BBRC Versus ArthritisClinical Samples WHiTE Study OBS Plus Perioperative Quality Improvement Programme BBRC Versus Arthritis3D motion analysis… Urinary Biomarker URO17 Study WHiTE 8 COPAL UK Genetic Prostate Cancer Study Validation of patient centric measures in… Urine Biomarkers for detecting prostate cancer Add-Aspirin Patient experience of injections in age-related… Reduction Of Surgical Site Infection using… SNAP3: Frailty & delirium Mapping the lipid envelope composition of… The CIPHER study MQUEST NSCCG
joint EMT2 PATCH WHiTE
ALLEGRO
NOTACS
TRANSLATE
Clinical Board Non-Commercial
Total Against
BBRC Versus ArthritisMRI imaging for the
9
BBRC Versus ArthritisFluoroscopic imaging The REST feasibility trial POLO Proximie Live Plus tele-mentoring for trainee… TIGER study ITACS Trial FLO-ELA PITSTOP WAX
POSNOC ObsQoR Economic burden and quality of life effect of IntAct
PATHOS The HAPI Study
UNICORNS
FEMuR
STAR-TReC
SUPERSTAR
COACH
Recruitment total Recruitment Total Target
Sarcopenia, frailty and nutritional status in… (MOSES) PRIMETIME SOFFT Surgery versus non-surgical splint treatment for… AIR: RRP Surgery or Cast for Injuries of the EpicoNdyle in CPinBOSS
Exploring the epigenetic changes in diverticular…
III HEAR IT HUSH PERCEIVE study WHiTE 11FRUITI NOSTRA-Feasibility Study
BASIS Study Best-Of DAVE L1FE
The
Trial
Surgical Services Clinical Board
Surgical Services Clinical Board
Surgical Services Clinical Board
*REINFORCE counted under Other Clinical Board but recruitment split between Children & Women's and Surgical Services
*REINFORCE counted under Other Clinical Board but recruitment split between Children & Women's and Surgical Services
75
Services Clinical Board Non-Commercial Recruitment Total Against Target (2022-23)
Figure 3 - Surgical
1378 991 580 473 279 266 226 171 131 130 97 74 68 63 53 46 42 42 42 36 33 31 30 28 28 25 25 22 22 21 20 19 14 13 12 11 10 8 8 8 8 8 7 5 4 4 2 1 1 1 1 0 0 0 0 0 0 0 0 0 0 200 400 600 800 1000 1200 1400 1600 BBRC Versus ArthritisClinical Samples PQIP Urinary Biomarker URO17 Study BBRC Versus Arthritis3D motion analysis (MOCAP) UK Genetic Prostate Cancer Study Validation of patient centric measures in urolithiasis Urine Biomarkers for detecting prostate cancer ROSSINI 2 Add-Aspirin Mapping the lipid envelope composition of SARS-CoV-2 POLO TIGER study TRANSLATE POLARiS feasibility REINFORCE EMT2 BBRC Versus ArthritisMRI imaging for the joint FLO-ELA NOTACS Study ALLEGRO BBRC Versus ArthritisFluoroscopic imaging CUBRIC MRI Study of the prostate REVAMP ITACS Trial Proximie Live Plus Sarcopenia, frailty and nutritional status in colorectal… Using Artificial Intelligence to Diagnose ‘Glue Ear' in PATHOS VITAL IntAct EMCAT in hand surgery WHiTE 10LIT I-DECIDE BASIS Study MOSES PERCEIVE study Surgery versus non-surgical splint treatment for PPS… DEFINE SCIENCE SOFFT UNICORNS WHiTE 11FRUITI PASHiOn The ORION Trial AIR: RRP HUSH NOSTRA IONOE STAR-TReC SUPERSTAR WHiTE 12DUALITY Best-Of DAVE EndoNET ExPeCT OnCoRe ORiF Patient and staff experiences of perioperative cardiac… POETIC-A The COACH Trial Recruitment total Recruitment total Target
75 Figure 3 - Surgical Services Clinical Board Non-Commercial Recruitment Total Against Target
(2022-23)
1378 991 580 473 279 266 226 171 131 130 97 74 68 63 53 46 42 42 42 36 33 31 30 28 28 25 25 22 22 21 20 19 14 13 12 11 10 8 8 8 8 8 7 5 4 4 2 1 1 1 1 0 0 0 0 0 0 0 0 0 0 200 400 600 800 1000 1200 1400 1600 BBRC Versus ArthritisClinical Samples PQIP Urinary Biomarker URO17 Study BBRC Versus Arthritis3D motion analysis (MOCAP) UK Genetic Prostate Cancer Study Validation of patient centric measures in urolithiasis Urine Biomarkers for detecting prostate cancer ROSSINI 2 Add-Aspirin Mapping the lipid envelope composition of SARS-CoV-2 POLO TIGER study TRANSLATE POLARiS feasibility REINFORCE EMT2 BBRC Versus ArthritisMRI imaging for the joint FLO-ELA NOTACS Study ALLEGRO BBRC Versus ArthritisFluoroscopic imaging CUBRIC MRI Study of the prostate REVAMP ITACS Trial Proximie Live Plus Sarcopenia, frailty and nutritional status in colorectal… Using Artificial Intelligence to Diagnose ‘Glue Ear' in PATHOS VITAL IntAct EMCAT in hand surgery WHiTE 10LIT I-DECIDE BASIS Study MOSES PERCEIVE study Surgery versus non-surgical splint treatment for PPS… DEFINE SCIENCE SOFFT UNICORNS WHiTE 11FRUITI PASHiOn The ORION Trial AIR: RRP HUSH NOSTRA IONOE STAR-TReC SUPERSTAR WHiTE 12DUALITY Best-Of DAVE EndoNET ExPeCT OnCoRe ORiF Patient and staff experiences of perioperative cardiac… POETIC-A The COACH Trial Recruitment total Recruitment total Target 75
Surgical Services Clinical Board
Surgical Services Clinical Board
76
Figure 4 - Surgical Services Clinical Board Commercial Recruitment Total Against Target (2020-21)
69 4 3 2 2 1 0 0 0 0 0 10 20 30 40 50 60 70 80 90 100 MUSC 4535 (Degenerative Disease of the Hip) PluroGel and leg ulcers Tri-Solfen Version 3.0 Otonomy OTO4811 Debrisoft and healing Darolutamide roll-over study Open label study of BI 754132 in patients
Atrophy. PROTEUS REX-001 to
ischaemia
REX-001
(Category 5) Recruitment total Recruitment Total Target 32 28 6 5 4 3 3 2 2 0 0 0 0 0 5 10 15 20 25 30 35 METAPAIN PEP Study in nAMD and DME IPCOTT Tri-Solfen Version 3.0 PluroGel and leg ulcers Debrisoft and healing GR42691
ALLEVYN
Indicate
Open
OPN-FLU-CS-3205 Recruitment total Recruitment Total Target 76
Figure 5 - Surgical Services Clinical Board Commercial
Recruitment Total Against Target (2021-22)
with Geographic
treat critical limb
in adults with diabetes
to treat ischaemic ulcers in diabetic adults
AVONELLE-X BALATON COMINO
Gentle Border PMCF Study
Study
label study of BI 754132 in patients with Geographic Atrophy.
Surgical Services Clinical Board
Surgical Services Clinical Board
77
69 25 8 5 4 4 1 1 0 0 10 20 30 40 50 60 70 80 PROGRESS METAPAIN IPCOTT Tri-Solfen Version 3.0 A Prospective Natural History Study of Patients with ADOA Debrisoft and healing HZN-402 MARS study QB46C-H08 Recruitment total Recruitment total Target 77
Figure 6 - Surgical Services Clinical Board Commercial Recruitment Total Against Target (2022-23)
Other (Split) Clinical Board
Other (Split) Clinical Board
: Indicates study opened in Feb/March - towards the end of the financial year. : Indicates study Closed to Recruitment or Withdrawn during the relevant financial year. : Indicates study Suspended. Charts exclude studies in set-up.
78
Figure 1 - Split Clinical Board/Directorate Non-Commercial Recruitment Total Against Target (2020-21)
10 2 0 5 10 15 20 25 30 MANIFeST USTEKID FRONTIER IMPACT MULTI-AGENCY Recruitment total Recruitment Total Target 17 12 11 0 0 5 10 15 20 25 30 35 FRONTIER USTEKID ProJudge IMPACT MULTI- AGENCY Recruitment total Recruitment Total Target 78
Figure 2 - Split Clinical Board/Directorate Non-Commercial Recruitment Total (2021-22)
Other (Split) Clinical Board
Other (Split) Clinical Board
Figure 3 - Split Clinical Board/Directorate Non-Commercial Recruitment Total (2022-23)
*REINFORCE counted under Other Clinical Board but recruitment split between Children & Women's and Surgical Services
No Split Clinical Board/Directorate Commercial Recruitment in 2020-21, 2021-22 or 2022-23
79
11 4 0 0 5 10 15 20 25 30 35 ProJudge Determining Best Preventative Social Care Practice IMPACT MULTI-AGENCY Recruitment total Recruitment total Target 79
Appendix A
All Wales Medical Genomics Service Clinical Board
Publications
Edgerley, K., Bryson, L., Hanington, L., Irving, R., Joss, S., Lampe, A., Maystadt, I., Osio, D., Richardson, R., Split, M., Sansbury, F.H., Scurr, I., Stewart, H., McNeill, A. and Low, K.J. (2023). SOX5: Lamb–Shaffer syndrome—A case series further expanding the phenotypic spectrum. American Journal of Medical Genetics, 191(5), pp.1447–1458. doi:https://doi.org/10.1002/ajmg.a.63124.
Hamad, A., Sherlaw-Sturrock, C.A., Glover, K.J., Salmon, R., Low, K., Nair, R., Sansbury, F.H., Rawlins, L., Carmichael, J., Horton, R., Wedderburn, S., Edgerley, K., Irving, R.E.A., Callaghan, M., Mercer, C., McGowan, R., Roberts, L., Titheradge, H. and Naik, S. (2023). Expanding the phenotypic spectrum of Chromosome 16p13.11 microduplication: A multicentric analysis of 206 patients. European Journal of Medical Genetics, 66(4), pp.104714–104714. doi:https://doi.org/10.1016/j.ejmg.2023.104714.
Hopes, R., Croxall, J.A. and Morgan, C. (2022). Improved awareness of national genomic initiatives is needed to facilitate genomic education and training in Wales for pharmacists. Pharmacy Education, 22(1), pp.805–813. doi:https://doi.org/10.46542/pe.2022.221.805813.
Jezkova, J., Shaw, S., Taverner, N.V. and Williams, H.J. (2022). Rapid genome sequencing for pediatrics. Human Mutation, 43(11), pp.1507–1518. doi:https://doi.org/10.1002/humu.24466.
Lee, S., Menzies, L., Hay, E., Ochoa, E., Docquier, F., Rodger, F., Deshpande, C., Foulds, N.C., Jacquemont, S., Jizi, K., Kiep, H., Kraus, A., Löhner, K., Morrison, P.J., Popp, B., Richardson, R., Haeringen, A., Martin, E., Toribio, A. and Li, F. (2023). Epigenotype-genotype–phenotype correlations in SETD1A and SETD2 chromatin disorders. Human Molecular Genetics. doi:https://doi.org/10.1093/hmg/ddad079.
Mcvittie, L., Lumb, B., Davies, M., Conti, H., Hildebrandt, T., Hill, J., Davies, M. and Adams, M. (2023). INCIDENTAL FINDINGS IN SURVEILLENCE OF PAEDATRIC LI FRAUMENI PATIENTS - A ONE CENTRE EXPERIENCE. CCLG conference.
Rossi, A., Snijders Blok, L., Neuser, S., Klöckner, C., Platzer, K., Faivre, L.O., Weigand, H., Dentici, M.L., Tartaglia, M., Niceta, M., Alfieri, P., Srivastava, S., Coulter, D., Smith, L., Vinorum, K., Cappuccio, G., Brunetti-Pierri, N., Torun, D., Arslan, M. and Lauridsen, M.F. (2023). POU3F3‐related disorder: Defining the phenotype and expanding the molecular spectrum. Clinical Genetics. doi:https://doi. org/10.1111/cge.14353.
Thomas, R.H., Vinh, A.C., Davies, M., Conti, H., Hildebrandt, T., Morgan, C., Hill, J., Davies, M., Phillips, S. and Adams, M. (2023). 3 Year Experience of the All Wales Paediatric Cancer Predisposition Service. CCLG conference.
Vinh, A.C., Davies, M., Hildebrandt, T., Conti, H., Morgan, C., Hill, J. and Adams, M. (2022). All Wales Paediatric Predisposition Service – Adherence to Referral Guidelines. Welsh Paediatric Society Meeting.
80
Appendix A
All Wales Medical Genomics Service Clinical Board
Initiatives
Contributed by Dr Ian Tully
SAIL Biobank Collaboration
One of the pressing concerns in genomics is how best to utilise the increasing resource of stored genome data for patient benefit. Re-analysis of exome and genome data has the potential to increase diagnostic yield, even years after the dataset was acquired. As well as presenting a resource issue for the clinical laboratories, there are governance and ethical hurdles to clear.
We have initiated a collaboration with the Swansea SAIL biobank to allow transfer of genomic data for a cohort of well-curated patients via the SWAN clinic. Ethical approval has now been granted and a cohort of 30 patients selected for this investigation. This will provide pilot data for future re-analysis projects.
Musketeers Memorandum Studies
Much rare disease research is carried out on a national basis, with one centre obtaining ethical approval. The Musketeer Memorandum allows recruitment at other UK sites based on the central ethics approval.
We have undertaken a program of promoting these studies in the department to increase recruitment. This has included maintaining an up-to-date database of open studies, contact details and recruitment procedures; and inviting study leads from around the UK to talk about their work at our departmental meetings.
Commercial study promotion
Although Medical Genetics does not traditionally treat patients, commercial research opportunities are emerging in the rare disease sector. Opening to rare disease trials usually involves a small number of recruits (<5) from across Wales. The RECONNECT study is our first collaboration with CYARU to provide day-case care for an EMP in the treatment of Fragile X syndrome. We are also building links with commercial teams in the fields of Tuberous Sclerosis and Neurofibromatosis Type 1.
Syndrome Without a Name (SWAN) clinic – novel investigations
The SWAN pilot is in its second year and we are currently developing further analysis pipelines for patients who still do not receive a diagnosis after the SWAN assessment. These pipelines include data re-analysis (see above), whole transcriptome sequencing and methylation-sensitive sequencing. Research using this cohort will provide an opportunity to develop these novel approaches further for the benefit of patients across the health service.
Please note: all grants, publications, research impact case studies or initiatives have been provided by Clinical Board and Directorate R&D leads. Therefore, there may be further grants, publications, case studies or initiatives not mentioned in this report.
81
Appendix A –All Wales Medical Genomics Service Clinical Board
Appendix A All Wales Medical Genomics Service Clinical Board
Non-Commercial
Non-Commercial
5
Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitment total RAG EMBRACE 1358 20971 3027 Non-Commercial FALSE Observational Open To Recruitment All Wales Medical Genomics Service All Wales Medical Genomics Service 12/01/1998 31/01/2027 108 15 213 G Genetic mechanisms in polyposis of the bowel 14774 87399 8400 Non-Commercial TRUE Observational Closed to Recruitment, In Follow Up All Wales Medical Genomics Service All Wales Medical Genomics Service 09/07/2012 31/08/2022 31/08/2022 150 0 196 G Genes and the Kidney in Tuberous Sclerosis 19401 10073 9264 Non-Commercial TRUE Observational Open To Recruitment All Wales Medical Genomics Service All Wales Medical Genomics Service 08/03/2010 31/08/2023 1 0 155 G Molecular genetic analysis of duodenal polyposis in FAP and MAP 19065 158519 9223 Non-Commercial FALSE Observational Open To Recruitment All Wales Medical Genomics Service All Wales Medical Genomics Service 31/03/2017 31/08/2025 27 0 32 G Splicing and Disease 19058 49685 12886 Non-Commercial TRUE Observational Open To Recruitment All Wales Medical Genomics Service All Wales Medical Genomics Service 22/12/2015 31/12/2023 1 1 4 G IHCAP 14496 114546 9278 Non-Commercial TRUE Observational Open To Recruitment All Wales Medical Genomics Service All Wales Medical Genomics Service 11/07/2014 31/05/2025 1 0 2 G Investigating the molecular mechanisms of rare genetic disorders 37646 229483 16234 Non-Commercial TRUE Both Open To Recruitment All Wales Medical Genomics Service All Wales Medical Genomics Service 31/01/2022 31/03/2028 0 2 2 W Genetic Disorders of Human Neurological and Immune Function 10556 62971 9439 Non-Commercial TRUE Observational Open To Recruitment All Wales Medical Genomics Service All Wales Medical Genomics Service 11/10/2011 31/12/2028 0 0 1 W EDEN 31719 200297 13258 Non-Commercial TRUE Interventional Suspended All Wales Medical Genomics Service All Wales Medical Genomics Service 13/10/2016 31/08/2024 0 0 0 W Genetic basis of cranial malformations 7424 6086 12854 Non-Commercial TRUE Interventional Open To Recruitment All Wales Medical Genomics Service All Wales Medical Genomics Service 14/11/2014 30/04/2024 0 0 0 W Genetic disorders of growth, development and the brain 5539 15072 12862 Non-Commercial TRUE Interventional Open To Recruitment All Wales Medical Genomics Service All Wales Medical Genomics Service 27/04/2016 31/07/2023 0 0 0 W Genetics of OGID syndromes 42908 258623 13861 Non-Commercial TRUE Observational Open To Recruitment All Wales Medical Genomics Service All Wales Medical Genomics Service 05/02/2021 01/09/2029 0 0 0 W Genetics of Perrau lt Syndrome 20749 186802 13229 Non-Commercial TRUE Observational Open To Recruitment All Wales Medical Genomics Service All Wales Medical Genomics Service 09/05/2016 30/04/2025 0 0 0 W HumGenDis 7418 50895 9390 Non-Commercial TRUE Observational Open To Recruitment All Wales Medical Genomics Service All Wales Medical Genomics Service 25/01/2016 23/09/2023 0 0 0 W IDFOW 4917 58620 12845 Non-Commercial TRUE Observational Open To Recruitment All Wales Medical Genomics Service All Wales Medical Genomics Service 23/03/2015 01/10/2027 0 0 0 W Lymres 5756 96492 9391 Non-Commercial TRUE Observational Open To Recruitment All Wales Medical Genomics Service All Wales Medical Genomics Service 15/04/2016 01/03/2023 0 0 0 W The SCOTTY Study 31934 200475 9495 Non-Commercial TRUE Observational Suspended All Wales Medical Genomics Service All Wales Medical Genomics Service 08/11/2016 31/12/2025 0 0 0 W Understanding the conditions of the RAS-MAPK pathway 10486 7025 9444 Non-Commercial TRUE Observational Suspended All Wales Medical Genomics Service All Wales Medical Genomics Service 05/05/2016 31/12/2023 0 0 0 W 18 18 605
82
Appendix B
Children & Women’s Clinical Board: ACUTE CHILD HEALTH
Publications
Baioumi, A., Burrows, R., Hayward, R. and Pryce, R. (2022). Contrast media-induced hypothyroidism. Endocrine Abstracts, 85. doi: https://doi.org/10.1530/endoabs.85.oc8.5.
Birkebaek, N.H., Kamrath, C., Grimsmann, J.M., Aakesson, K., Cherubini, V., Dovc, K., de Beaufort, C., Alonso, G.T., Gregory, J.W., White, M., Skrivarhaug, T., Sumnik, Z., Jefferies, C., Hörtenhuber, T., Haynes, A., De Bock, M., Svensson, J., Warner, J.T., Gani, O. and Gesuita, R. (2022). Impact of the COVID-19 pandemic on long-term trends in the prevalence of diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes: an international multicentre study based on data from 13 national diabetes registries. The Lancet Diabetes & Endocrinology, 10(11). doi: https://doi.org/10.1016/s22138587(22)00246-7.
Bratina, N., Auzanneau, M., Birkebæk, N., de Beaufort, C., Cherubini, V., Craig, M.E., Dabelea, D., Dovc, K., Hofer, S.E., Holl, R.W., Jensen, E.T., Mul, D., Nagl, K., Robinson, H., Schierloh, U., Svensson, J., Tiberi, V., Veeze, H.J., Warner, J.T. and Donaghue, K.C. (2022). Differences in retinopathy prevalence and associated risk factors across 11 countries in three continents: A cross-sectional study of 156,090 children and adolescents with type 1 diabetes. Pediatric Diabetes, 23(8). doi: https://doi.org/10.1111/ pedi.13416.
French, R., Kneale, D., Warner, J.T., Robinson, H., Rafferty, J., Sayers, A., Taylor, P., Gregory, J.W. and Dayan, C.M. (2022). Educational Attainment and Childhood-Onset Type 1 Diabetes. Diabetes Care, 45(12), pp.2852–2861. doi: https://doi.org/10.2337/dc21-0693.
Jones, S. and Pryce, R. (2023). Monitoring the biochemical response in children with X linked hypophosphataemic rickets after starting Burosumab treatment and correlating with biochemical outcomes. RCPCH Spring Meeting.
Prigge, R., McKnight, J.A., Wild, S.H., Haynes, A., Jones, T.W., Davis, E.A., Rami-Merhar, B., Fritsch, M., Prchla, C., Lavens, A., Doggen, K., Chao, S., Aronson, R., Brown, R., Ibfelt, E.H., Svensson, J., Young, R., Warner, J.T., Robinson, H. and Laatikainen, T. (2022). International comparison of glycaemic control in people with type 1 diabetes: an update and extension. Diabetic Medicine, 39(5). doi: https://doi. org/10.1111/dme.14766.
Rees, S., Moss, S. and Pryce, R. (2022). An evaluation of the experiences with services in wales for children and young people and their families with prader willi syndrome (PWS). Endocrine Abstracts, 85. doi: https://doi.org/10.1530/endoabs.85.p69.
Williams, G.M., Leary, S., Leadbetter, S., Toms, S., Mortimer, G., Scorrer, T., Gillespie, K. and Shield, J.P.H. (2022). Establishing breast feeding in infants with Down syndrome: the FADES cohort experience.
BMJ Paediatrics Open, 6(1), p.e001547. doi: https://doi.org/10.1136/bmjpo-2022-001547.
83
Appendix B
Children & Women’s Clinical Board: ACUTE CHILD HEALTH
Paediatric Emergency Department
Ball, E., Purchase, T. and Morgan, J. (2022). Review of the 2021 Resuscitation Council United Kingdom guideline for the emergency treatment of anaphylaxis. Archives of disease in childhood - Education & Practice Edition, p.edpract-2022-324432. doi: https://doi.org/10.1136/archdischild-2022-324432.
Metezai, H., Wahid, A., Jones, C. and Evans, J. (2022). Fifteen-minute consultation: Rectal bleeding in children. Education and Practice Edition, 23, p.edpract-324626. doi: https://doi.org/10.1136/ archdischild-2022-324626.
Oruganti, S., Evans, J., Cromarty, T., Javaid, A. and Roland, D. (2022). Identification of sepsis in paediatric emergency departments: A scoping review. Acta Paediatrica, 111(12). doi: https://doi. org/10.1111/apa.16536.
Patel, S., Dadnam, C., Hewitson, R., Thakur, I. and Morgan, J. (2022). Fifteen-minute consultation: Recognition of sickle cell crises in the paediatric emergency department. Archives of disease in childhood - - Education & Practice Edition, 107(3), p.edpract-2020-321338. doi: https://doi.org/10.1136/ archdischild-2020-321338.
Smith, A.C., Jones, S.J. and Hanna, D. (2022). Epidemiology and mapping of child road casualties. Archives of Disease in Childhood, 107(6), pp.540–542. doi: https://doi.org/10.1136/ archdischild-2021-323331.
Tolhurst-Cleaver, M.F.J., Evans, J., Waterfield, T., Adamson, J., Marlow, R., Lyttle, M.D. and Roland, D. (2022). Periorbital and orbital cellulitis in children: a survey of emergency physicians and analysis of clinical practice guidelines across the PERUKI network. Emergency Medicine Journal: EMJ, 39(10), pp.766–770. doi: https://doi.org/10.1136/emermed-2021-211713.
Please note: all grants, publications, research impact case studies or initiatives have been provided by Clinical Board and Directorate R&D leads. Therefore, there may be further grants, publications, case studies or initiatives not mentioned in this report.
84
Appendix B –
Appendix B
Children & Women’s Clinical Board : ACUTE CHILD HEALTH
Children & Women’s Clinical Board: ACUTE CHILD HEALTH
Non-Commercial
Non-Commercial
85
9
Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Collaborating Directorates Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitment total RAG nSEP 42780 248404 13508 NonCommercial FALSE Observational Open To Recruitment Children & Women's Acute Child Health 02/03/2020 31/12/2023 100 180 399 G BLOOD MARKERS FOR INVESTIGATING COVID-19 AND SEPSIS IN CHILDREN 48037 250612 15299 NonCommercial FALSE Observational Closed to Recruitment, No Follow Up Children & Women's Acute Child Health 16/12/2020 30/06/2023 300 59 220 R Induced sputum in children with cystic fibrosis 14615 85645 8860 NonCommercial FALSE Interventional Open To Recruitment Children & Women's Acute Child Health 11/01/2012 31/12/2023 130 0 126 G *Clinical Characterisation Protocol for Severe Emerging Infection 14152 126600 12143 NonCommercial FALSE Observational Suspended Specialist Services Critical Care Acute Child Health; Internal Medicine; 30/03/2020 28/02/2023 2 0 52 S **RECOVERY trial 45388 281712 15098 NonCommercial FALSE Interventional Open To Recruitment Medicine Internal Medicine Acute Child Health; Haematology, Immunology and Metabolic Medicine; 18/03/2020 30/09/2030 1 0 45 G UK Childhood ITP Registry 14145 44138 8820 NonCommercial FALSE Observational Open To Recruitment Children & Women's Acute Child Health 19/07/2012 01/04/2031 100 0 33 R IMPORT 12457 62637 8902 NonCommercial FALSE Observational Open To Recruitment Children & Women's Acute Child Health 03/07/2013 31/01/2024 15 3 25 G CF START 31531 211995 10745 NonCommercial FALSE Interventional Open To Recruitment Children & Women's Acute Child Health 15/12/2017 30/04/2023 6 5 23 G ComCov3 50491 304450 16095 NonCommercial FALSE Interventional Open To Recruitment Children & Women's Acute Child Health 27/09/2021 05/12/2021 30 3 19 R CF-HomeSpIT 54158 281516 16535 NonCommercial FALSE Interventional Open To Recruitment Children & Women's Acute Child Health 08/09/2022 01/09/2025 80 16 16 G SMPaeds 40156 246557 12553 NonCommercial FALSE Interventional Open To Recruitment Children & Women's Acute Child Health 01/10/2019 31/10/2023 5 4 16 G MyeChild 01 19700 182694 10385 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Children & Women's Acute Child Health 04/04/2017 29/04/2022 28/06/2022 8 1 15 G Quality of life in paediatric sarcoma 50404 264472 13959 NonCommercial FALSE Observational Completed Children & Women's Acute Child Health 03/12/2020 31/12/2022 31/12/2022 100 2 15 R PRESSURE 48813 289545 15761 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Children & Women's Acute Child Health 06/12/2021 28/02/2024 16/12/2022 40 9 14 R Experiences of end of life care 51501 300913 16186 NonCommercial FALSE Observational Open To Recruitment Children & Women's Acute Child Health 22/02/2023 31/03/2023 10 13 13 G FIDO 53493 278080 16187 NonCommercial FALSE Observational Open To Recruitment Children & Women's Acute Child Health 31/10/2022 21/10/2023 100 13 13 R AZTEC2 44914 270464 13913 NonCommercial FALSE Observational Open To Recruitment Children & Women's Acute Child Health 18/06/2021 08/06/2023 25 0 12 R
Appendix B –
Appendix B
Children & Women’s Clinical Board : ACUTE CHILD HEALTH
Children & Women’s Clinical Board: ACUTE CHILD HEALTH
Appendix B –
Appendix B
–
Children & Women’s Clinical Board : ACUTE CHILD HEALTH
Children & Women’s Clinical Board : ACUTE CHILD HEALTH
*Clinical Characteristics Protocol for Severe Emerging Infection counted under Specialist Services Clinical Board .
*Clinical Characteristics Protocol for Severe Emerging Infection counted under Specialist Services Clinical Board
trial counted under
Clinical Board from 2023-24 onwards.
10 Blueprint 45240 272762 15209 NonCommercial FALSE Observational Completed Children & Women's Community Child Health 04/06/2021 30/04/2022 12/04/2022 8 0 9 G Characterisation of Paediatric Myelodysplasic Syndromes 20348 164398 9410 NonCommercial FALSE Observational Open To Recruitment Children & Women's Acute Child Health 06/06/2016 31/05/2025 5 0 7 G Psychosocial access to kidney transplantation in children 47330 270493 15490 NonCommercial FALSE Observational Open To Recruitment Children & Women's Acute Child Health 28/10/2022 01/09/2023 15 7 7 A Childhood cancer diagnosis 43772 258464 14027 NonCommercial FALSE Observational Closed to Recruitment, In Follow Up Children & Women's Acute Child Health 09/08/2021 31/03/2023 31/03/2023 100 4 5 R PINPOINT Study 48958 281336 15386 NonCommercial FALSE Observational Open To Recruitment Children & Women's Acute Child Health 01/04/2022 28/02/2023 60 5 5 R CONTRACT 2 50762 302249 16235 NonCommercial FALSE Interventional Open To Recruitment Children & Women's Acute Child Health 21/06/2022 30/04/2023 10 4 4 R FaR-RMS 42490 254931 13693 NonCommercial FALSE Interventional Open To Recruitment Children & Women's Acute Child Health 18/12/2020 28/02/2027 11 1 4 A NCCPG TDM 2018 40698 240311 13549 NonCommercial FALSE Observational Open To Recruitment Children & Women's Acute Child Health 07/08/2019 30/11/2023 5 2 4 A SINEPOST study 48937 294183 15787 NonCommercial FALSE Observational Closed to Recruitment, No Follow Up Children & Women's Acute Child Health 08/08/2022 31/10/2022 31/10/2022 21 4 4 R ICONIC 41346 254908 13652 NonCommercial FALSE Observational Open To Recruitment Children & Women's Acute Child Health 17/12/2020 31/01/2025 2 0 2 G PHITT 33836 212527 10464 NonCommercial FALSE Both Open To Recruitment Children & Women's Acute Child Health 08/11/2019 31/12/2023 1 0 2 G rEECur 17239 149572 10359 NonCommercial FALSE Interventional Open To Recruitment Children & Women's Acute Child Health 22/12/2016 30/09/2024 2 0 1 R SIOP Ependymoma II 18503 130101 9493 NonCommercial FALSE Interventional Open To Recruitment Children & Women's Acute Child Health 19/02/2018 12/02/2026 2 0 1 A VERITAS 41019 254424 13603 NonCommercial FALSE Interventional Suspended Children & Women's Acute Child Health 21/04/2022 31/03/2024 3 1 1 S DOLFIN 51833 303421 16246 NonCommercial FALSE Both Open To Recruitment Children & Women's Acute Child Health 30/03/2023 31/12/2026 30 0 0 R Fetal Atrial Flutter & Supraventricular Tachycardia Therapy Trial 32151 196628 13252 NonCommercial FALSE Both Completed Children & Women's Acute Child Health 02/03/2017 30/06/2022 30/06/2022 0 0 0 W International Congenital Lung Malformations Registry 43955 215924 14026 NonCommercial FALSE Observational Open To Recruitment Children & Women's Acute Child Health 31/03/2023 01/04/2028 5 0 0 R PNET 5 31891 111728 10453 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Children & Women's Acute Child Health 31/01/2019 10/01/2023 05/04/2022 1 0 0 R 35 336 1112
Medicine
**RECOVERY
Medicine
86
**RECOVERY trial counted under
Clinical Board from 2023-24 onwards.
Appendix B –
Appendix B
Children & Women’s Clinical Board : ACUTE CHILD HEALTH
Children & Women’s Clinical Board: ACUTE CHILD HEALTH
87
12 Commercial Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Collaborating Directorates Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitment total RAG HARMONIE 51978 1005180 16393 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Children & Women's Acute Child Health 16/12/2022 28/02/2023 28/02/2023 30 25 25 R VX20-CFD-004 47595 290795 15582 Commercial FALSE Observational Closed to Recruitment, No Follow Up Children & Women's Acute Child Health 30/03/2021 31/10/2022 06/07/2022 25 0 13 R XLH Registry 35793 229425 15272 Commercial FALSE Observational Open To Recruitment Children & Women's Acute Child Health 03/09/2020 31/08/2022 8 2 10 G A Phase 3 Study of Erenumab in Children with Chronic Migraine 38739 262647 13680 Commercial FALSE Interventional Open To Recruitment Children & Women's Acute Child Health 19/06/2019 03/10/2024 2 1 3 G GSK's paediatric HZ vaccine in paediatric renal transplant patients 36574 263468 13732 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Children & Women's Acute Child Health 12/12/2019 30/09/2024 31/08/2022 5 0 2 R Pediatric study of Gilteritinib in FLT3 positive R/R AML 43572 270337 14032 Commercial FALSE Interventional Open To Recruitment Children & Women's Acute Child Health Haematology, Immunology and Metabolic Medicine; 28/08/2020 30/09/2024 1 0 2 G Cerliponase alfa Observational Study 45091 268183 13951 Commercial FALSE Observational Closed to Recruitment, In Follow Up Children & Women's Acute Child Health 19/12/2019 24/09/2027 22/08/2022 1 0 1 G Efficacy and Safety of Avatrombopag in Pediatric Immune Thrombocytopenia 46968 1003418 15675 Commercial FALSE Interventional Open To Recruitment Children & Women's Acute Child Health 17/11/2021 16/01/2023 2 0 1 R Pfizer Pediatric VTE Treatment study 30279 225240 10659 Commercial FALSE Interventional Completed Children & Women's Acute Child Health 06/03/2019 01/12/2020 14/01/2023 2 0 1 R A Phase 3 Study of Erenumab in Children with Episodic Migraine 33055 262646 13705 Commercial FALSE Interventional Open To Recruitment Children & Women's Acute Child Health 19/07/2019 05/07/2023 2 0 0 R CIP342 Lenny Study 52934 316323 16629 Commercial FALSE Both Open To Recruitment Children & Women's Acute Child Health 21/02/2023 30/06/2023 5 0 0 R FORTIS 49318 1003968 16086 Commercial FALSE Interventional Open To Recruitment Children & Women's Acute Child Health 05/04/2022 30/04/2023 3 0 0 R Vedo 3024 UC 48044 1003562 15986 Commercial FALSE Interventional Open To Recruitment Children & Women's Acute Child Health 31/10/2022 31/05/2023 1 0 0 R Vedo 3025 CD 48172 1003594 15985 Commercial FALSE Interventional Open To Recruitment Children & Women's Acute Child Health 31/10/2022 31/05/2023 1 0 0 R 14 28 58 Commercial
Appendix C
Children & Women’s Clinical Board: OBSTETRICS & GYNAECOLOGY
Publications
Davies, L.C., Sharma, S., Rodrigues, P.R.S., Edkins, S., Strang, A., Shepherd, F., Oram, S., Siddall, K., Keeping, V., Simpson, K., Faggian, F., Bray, M., Bertorelli, C., Bell, S., Collis, R., McLaren, J.E., Zaher, S. and Ghazal, P. (2023). Pregnancy: An immunological stepping-stone to sepsis? Cardiff University
Infection and Immunity Meeting.
Sharma, S., Rodrigues, P.R.S., Zaher, S., Davies, L.C. and Ghazal, P. (2022). Immune-metabolic adaptations in pregnancy: A potential stepping-stone to sepsis. eBioMedicine, 86, p.104337. doi: https://doi.org/10.1016/j.ebiom.2022.104337.
Sharma, S., Rodrigues, P.R.S., Zaher, S., Davies, L.C. and Ghazal, P. (2023). Immune-metabolic adaptations in pregnancy: a potential stepping-stone to sepsis (review article). Cardiff University
Infection and Immunity meeting.
Sharma, S., Zaher, S., Rodrigues, P.R.S., Davies, L.C., Edkins, S., Strang, A., Chakraborty, M., Watkins, W.J., Andrews, R., Parkinson, E., Angelopoulos, N., Moet, L., Shepherd, F., Davies, K.M.M., White, D., Oram, S., Siddall, K., Keeping, V., Simpson, K. and Faggian, F. (2022). mSep: investigating physiological and immune-metabolic biomarkers in septic and healthy pregnant women to predict feto-maternal immune health – a prospective observational cohort study protocol. BMJ Open, [online] 12(9), p.e066382. doi: https://doi.org/10.1136/bmjopen-2022-066382.
Please note: all grants, publications, research impact case studies or initiatives have been provided by Clinical Board and Directorate R&D leads. Therefore, there may be further grants, publications, case studies or initiatives not mentioned in this report.
88
Appendix C
Appendix C - Children & Women’s Clinical Board: OBSTETRICS & GYNAECOLOGY
Children & Women’s Clinical Board: OBSTETRICS & GYNAECOLOGY
89
14
Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Collaborating Directorates Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitment total RAG The POOL study 39971 238743 12502 NonCommercial FALSE Observational Completed Children & Women's Obstetrics & Gynaecology 02/01/2019 30/06/2022 30/06/2022 1950 90 3568 G mSEP 45339 262062 13914 NonCommercial FALSE Observational Closed to Recruitment, No Follow Up Children & Women's Obstetrics & Gynaecology 05/10/2020 31/12/2023 31/12/2022 30 51 282 G ROCkeTS 17994 168143 9275 NonCommercial FALSE Interventional Open To Recruitment Children & Women's Obstetrics & Gynaecology 16/10/2015 31/03/2023 150 9 164 G Cerclage after full dilatation caesarean section 42833 261294 13787 NonCommercial FALSE Both Open To Recruitment Children & Women's Obstetrics & Gynaecology 23/11/2020 01/04/2024 121 37 133 G The 'Big Baby Trial' 36723 229163 11942 NonCommercial FALSE Both Completed Children & Women's Obstetrics & Gynaecology 06/08/2019 31/12/2022 14/10/2022 60 5 104 G OASI2 47083 282750 15429 NonCommercial FALSE Both Completed Children & Women's Obstetrics & Gynaecology 29/11/2021 29/11/2022 28/12/2022 1 48 68 G *REINFORCE 53026 311223 16555 NonCommercial FALSE Observational Open To Recruitment Other Executive / Central General & Vascular Surgery; Obstetrics & Gynaecology; Urology; 07/10/2022 01/07/2024 185 49 49 G The iHOLDS Trial 47006 278209 15667 NonCommercial FALSE Interventional Suspended Children & Women's Obstetrics & Gynaecology 20/05/2022 18/01/2023 48 43 43 S The Tommy's National Rainbow Clinic Study 45312 249991 15205 NonCommercial FALSE Observational Open To Recruitment Children & Women's Obstetrics & Gynaecology 18/05/2021 31/10/2024 30 10 28 G Giant PANDA 47078 284958 15785 NonCommercial FALSE Both Open To Recruitment Children & Women's Obstetrics & Gynaecology 10/11/2021 31/05/2023 50 20 25 R Non-Commercial
Non-Commercial
Appendix C - Children & Women’s Clinical Board: OBSTETRICS & GYNAECOLOGY
Appendix C
Children & Women’s Clinical Board: OBSTETRICS & GYNAECOLOGY
*REINFORCE counted under Other Clinical Board but recruitment split between Children & Women's and Surgical Services
90
15
PROTECTOR 39196 237992 12170 NonCommercial FALSE Interventional Open To Recruitment Children & Women's Obstetrics & Gynaecology 06/02/2019 31/12/2024 20 7 25 G Biomarkers for Ovarian Pathology 19111 127808 9221 NonCommercial FALSE Observational Open To Recruitment Children & Women's Obstetrics & Gynaecology 18/07/2022 01/01/2026 50 20 20 G HOLDS 31506 193293 9563 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Children & Women's Obstetrics & Gynaecology 24/06/2017 18/12/2022 08/11/2022 28 4 10 R WILL 39805 252294 13758 NonCommercial FALSE Interventional Suspended Children & Women's Obstetrics & Gynaecology 22/09/2020 31/12/2022 8 2 7 S C-STICH2 40203 239782 12538 NonCommercial FALSE Interventional Open To Recruitment Children & Women's Obstetrics & Gynaecology 19/10/2021 31/07/2024 4 0 1 R REGAL trial 50851 271703 15631 NonCommercial FALSE Interventional Open To Recruitment Children & Women's Obstetrics & Gynaecology 09/02/2023 31/01/2024 16 0 0 R The SuPPORT Project 55436 313908 16525 NonCommercial FALSE Observational Open To Recruitment Children & Women's Obstetrics & Gynaecology 08/07/2022 08/07/2024 500 0 0 W 17 395 4527
Appendix C
Appendix C - Children & Women’s Clinical Board: OBSTETRICS & GYNAECOLOGY
Children & Women’s Clinical Board: OBSTETRICS & GYNAECOLOGY
91
16 Commercial Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitment total RAG LPRI-CF113 in the treatment of endometriosis 52291 1005172 16531 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Children & Women's Obstetrics & Gynaecology 05/12/2022 31/12/2022 21/12/2022 10 3 3 R A Placebo-controlled RCT Volixibat Intrahepatic Cholestasis Pregnancy 46467 287442 15695 Commercial FALSE Interventional Closed to Recruitment, No Follow Up Children & Women's Obstetrics & Gynaecology 11/05/2021 25/10/2023 29/11/2022 9 0 0 R 2 3 3 Commercial
Appendix D
Clinical Diagnostics & Therapeutics Clinical Board
All Wales Therapeutics and Toxicology Centre
Publications
Coulson, J.M., Adams, A., Gray, L.A. and Evans, A. (2022). COVID-19 ‘Rebound’ associated with nirmatrelvir/ritonavir pre-hospital therapy. Journal of Infection, 85(4). doi: https://doi.org/10.1016/j. jinf.2022.06.011.
Gentile, D., Adams, R., Klatka, M., Bradberry, S., Gray, L., Thanacoody, R., Jackson, G. and Sandilands, E.A. (2022). Carbon monoxide exposures reported to the UK National Poisons Information Service: a 4-year study. Journal of Public Health, 44(3). doi: https://doi.org/10.1093/pubmed/fdab132.
Haden, M., Wheatley, N., Gray, L.A., Bradberry, S.M., Sandilands, E.A., Thanacoody, R.H. and Coulson, J. (2022). Potential cyanide poisoning reported to the UK national poisons information service: 2008–2019. Clinical Toxicology, 60(9), pp.1–8. doi: https://doi.org/10.1080/15563650.2022.2080074.
Taylor, P.J., Siah, Q.Z., Marei, O., McDade-Kumar, M., Rachedi, N., Bracchi, R., Boldero, R., Haines, K., Ali, M.K., French, R.J. and Dayan, C.M. (2022). Prescribing costs of hypoglycaemic agents and associations with metabolic control in Wales; a national analysis of primary care data. Diabetic Medicine, 39(9). doi: https://doi.org/10.1111/dme.14908.
Medical Physics & Clinical Engineering Publications
Bartlett, R., Absalom, R., Williams, O. and Withers, K.L. (2023). Development and pilot of a community low-vision, service-specific Patient-Reported Experience Measure. Ophthalmic and Physiological Optics. doi: https://doi.org/10.1111/opo.13130.
Dale, M., Bell, S.F., O’Connell, S., Scarr, C., James, K., John, M., Collis, R.E., Collins, P.W. and CarolanRees, G. (2022). What is the Economic Cost of Providing an All Wales Postpartum Haemorrhage Quality Improvement Initiative (OBS Cymru)? A Cost-Consequences Comparison with Standard Care. PharmacoEconomics - Open, 6(6), pp.847–857. doi: https://doi.org/10.1007/s41669-022-00362-2.
HART Collaborative (2022). Incisional hernia following colorectal cancer surgery according to suture technique: Hughes Abdominal Repair Randomized Trial (HART). British Journal of Surgery, 109(10), pp.943–950. doi: https://doi.org/10.1093/bjs/znac198.
Monteagudo-Vela, M., Panoulas, V. and Krasopoulos, G. (2022). ‘QOL and PROMS Following Transcatheter Aortic Valve Implantation’. In: T. Athanasiou, A. Darzi and A.Y. Oo, eds., Patient Reported Outcomes and Quality of Life in Cardiovascular Interventions. Switzerland: Springer Cham, pp.109–122.
O’Connell, S., Islam, S., Sewell, B., Farr, A., Knight, L., Bashir, N., Harries, R.L., Jones, S., Cleves, A., Fegan, G., Watkins, A. and Torkington, J. (2022). Hughes abdominal closure versus standard mass closure to reduce incisional hernias following surgery for colorectal cancer: the HART RCT. Health Technology Assessment, 26(34), pp.1–100. doi: https://doi.org/10.3310/cmwc8368.
Smith, L., Meggy, A., Watts, T., Knight, L., Torkington, J. and Cornish, J. (2022). Incisional hernia prevention: risk–benefit from a patient perspective (INVITE) – protocol for a single-centre, mixedmethods, cross-sectional study aiming to determine if using prophylactic mesh in incisional hernia prevention is acceptable to patients. BMJ Open, 12(12), p.e069568. doi: https://doi.org/10.1136/ bmjopen-2022-069568.
92
Appendix D
Clinical Diagnostics & Therapeutics Clinical Board
Pharmacy and Medicines Management Publications
All Wales Medicines Strategy Group (2023). Understanding unlicensed medicines. [online] All Wales Therapeutics and Toxicology Centre. Available at: https://awttc.nhs.wales/files/guidelines-and-pils/ understanding-unlicensed-medicines-pdf/.
Cauchi, M., Willis, M.E., Andrews, A., Backx, M., Brownlee, W.J., Ford, H., Gran, B., Jolles, S., Price, S., Rashid, W., Schmierer, K. and Tallantyre, E.C. (2022). Multiple sclerosis and the risk of infection: Association of British Neurologists consensus guideline. Practical Neurology, 22(5), pp.344–357. doi:https://doi.org/10.1136/practneurol-2022-003370.
Coulson, J.M., Adams, A., Gray, L.A. and Evans, A. (2022). COVID-19 ‘Rebound’ associated with nirmatrelvir/ritonavir pre-hospital therapy. Journal of Infection, 85(4). doi:https://doi.org/10.1016/j. jinf.2022.06.011.
Crawford , P., Jones , E., Smith , H., Miller, K., Barnett , N., Oboh , L., Darcy , C., McKee , H. and Agnew, J. (2022). An outbreak of blisters: exploring the increased promotion of monitored dosage systems for older people. Pharmaceutical Journal, [online] 308(7961). doi:https://doi.org/10.1211/ pj.2022.1.144839.
Cusens, C. and Jones, E. (2022). Charting new territory: medication administration records and transcribing in district nursing services. British Journal of Community Nursing, 27(12), pp.604–610. doi:https://doi.org/10.12968/bjcn.2022.27.12.604.
Dodington, S. and Speakman, A. (2023). Crisis management of HPN (home parenteral nutrition) patients affected by technical services production constraints. Clinical Nutrition ESPEN, 54, pp.721–722. doi:https://doi.org/10.1016/j.clnesp.2022.09.764.
Groves, T., Rees, M. and Alikhan, R. (2022). Apixaban for venous thromboembolism treatment in obese patients with weight >120kg or BMI >40kg m2. In: International Society on Thrombosis and Haemostasis Congress. ISTH 2022 Congress.
Jones, R. and Atkinson, A. (2023). An audit of the post-administration monitoring and documentation of subcutaneous daratumumab at the University Hospital of Wales. Journal of Oncology Pharmacy Practice, 29(1S), pp.S1–S94. doi:https://doi.org/10.1177/10781552231153542.
Jordan, S., Jones, E., Komninou, S., Loane, M. and Damase-Michel, C. (2022). Families need support to reduce the risk of adverse drug reactions from breastfeeding. Pharmaceutical Journal, [online] 309(7968). doi:https://doi.org/10.1211/pj.2022.1.166126.
Smith, S., Duncan, N., Scarfe, H., Davies, E., Shah, R., Maouche, N., Connolly, S. and Gabriel, S. (2022). High dose melphalan conditioning in autologous stem cell transplantation for multiple myeloma—a UK survey of practice. In: Bone Marrow Transplantation. The 48th Annual Meeting of the European Society for Blood and Marrow Transplantation: Pharmacist Committee – Poster Session. pp.451–457.
Wale, A., Ireland, M., Yemm, R., Hiom, S., Jones, A., Spark, J.P., Francis, M., May, K., Allen, L., Ridd, S. and Mantzourani, E. (2020). Unlicensed ‘Special’ Medicines: Understanding the Community Pharmacist Perspective. Integrated Pharmacy Research and Practice, 9, pp.93–104. doi:https://doi. org/10.2147/iprp.s263970.
93
Appendix D
Clinical Diagnostics & Therapeutics Clinical Board
Wale, A., Young, Z., Zhang, W., Hiom, S., Ahmed, H., Yemm, R. and Mantzourani, E. (2023). Factors affecting the patient journey and patient care when receiving an unlicensed medicine: A systematic review. Research in Social and Administrative Pharmacy, 19(7), pp.1025–1041. doi:https:// doi.org/10.1016/j.sapharm.2023.04.120.
Wyllie, T. and Girvin, J. (2022). P22 Unlicensed medicines use in neonates: do we know what we’re giving? Archives of Disease in Childhood, 107(5), pp.e25.24-e25. doi:https://doi.org/10.1136/ archdischild-2022-nppg.30.
Initiatives
Contributed by Elizabeth Hughes
Consultant Pharmacist Credentialing
The pharmacy department are encouraging experienced clinical pharmacists who have developed and demonstrated a high-level of expertise in their area of practice to complete the consultant pharmacist credentialing process. One of the five assessment domains for the credentialing process is research, including the practice of undertaking research to inform service innovation. This process is therefore a strong initiative to completing, publishing and celebrating pharmacy research. Three pharmacists have successfully credentialed at Consultant level during 2022-2023 and another has submitted their portfolio for review and is awaiting the outcome.
Core Advanced Pharmacist Credentialing
In November 2022 the Royal Pharmaceutical Society launched the credentialing process for clinical pharmacists at ‘Core Advanced’ level. Research is again one of the five assessment domains and so this will drive clinical pharmacists towards completing, publishing and celebrating pharmacy research as they seek to develop and advance their practice. The pharmacy department is actively encouraging pharmacists to undertake their credentialing journey through the establishment of awareness sessions and peer support groups.
Research Impact Case Study
Contributed by Elizabeth Hughes
SMPU is an NHS Pharmacy “Special’s” Manufacturing unit based in Cardiff and Vale UHB. Unlicensed medicines are used across the UK to treat an individual’s clinical needs when there are no appropriate licensed alternatives. Patients, carers and parents have reported facing challenges with unlicensed medicines at the points of transfer of care between settings, a key time when medication errors may occur. There is little known about the patient journey as a whole, or the factors affecting patient care when receiving an unlicensed medicine. To address this knowledge gap a PhD was initiated by SMPU in collaboration with Cardiff School of Pharmacy and Pharmaceutical Sciences, SMPU and Mayberry Pharmacy entitled “Unlicensed “special” medicines: improving the patients’ experience”. Semi-structured interviews were carried out with patients and a variety of Healthcare Professionals to identify themes relating to the use of special’s and the patient journey. Many issues were identified including aspects relating to: clinical decision making; knowledge and understanding; communication with patients; continuity of care during transfer between settings; and quality, ordering, supply and administration issues.
94
Appendix D
Clinical Diagnostics & Therapeutics Clinical Board
Aspects specifically relating to community pharmacists have been described [Wale, A., et al 2020] and a systematic overview [Wale, A. et al, 2023] as outputs of this research work together with successful completion and publication of the PhD in 2021. Further evidence of the impact and wide reaching effects of this research are demonstrated in the subsequent publication of an All Wales Medicine Strategy Group Guidance Document for improving the management of unlicensed medicines in all sectors of healthcare in NHS Wales, using output from this PhD.
Radiology Publications
Bashir, M., Jubouri, M., White, R.D., Tan, S.ZCP., Bailey, D.M. and Williams, I.M. (2022). Dynamic and Static Vessel Malperfusion as a Consequence of Acute Type B Aortic Dissection. Annals of Vascular Surgery. doi: https://doi.org/10.1016/j.avsg.2022.11.002.
Bashir, M., Tan, S.Z., Jubouri, M., Salahia, G., Whiston, R.J., White, R.D., Bailey, D.M. and Williams, I.M. (2022). A narrative review of the surgical management of Paget-Schroetter syndrome: case series and long-term follow-up. Cardiovascular Diagnosis and Therapy, [online] 12(5), pp.74455–74755. doi: https://doi.org/10.21037/cdt-22-158.
Howells, D., Rees, J., Townsend, R. and Kirov, G. (2022). Electroconvulsive Therapy Reverses Cerebral Hypoperfusion in a Patient With Psychotic Depression and Catatonia. The Journal of ECT, 38(2). doi:https://doi.org/10.1097/yct.0000000000000836.
Kingsmore, D.B., White, R.D., Mestres, G., Stephens, M., Calder, F., Papadakis, G., Aitken, E., Jackson, A., Inston, N., Jones, R.G., Geddes, C., Stevenson, K., Szabo, L., Thomson, P.C., Stove, C., Kasthuri, R., Edgar, B., Tozzi, M., Franchin, M. and Sivaprakasam, R. (2023). Recruitment into randomised trials of arteriovenous grafts: A systematic review. The Journal of Vascular Access, p.112972982311584112972982311584. doi: https://doi.org/10.1177/11297298231158413.
Lou, J., Lin, H., Marshall, D., White, R., Yang, Y., Shelmerdine, S. and Liu, H. (2022). Predicting Radiologist Attention During Mammogram Reading with Deep and Shallow High-Resolution Encoding. IEEE International Conference on Image Processing (ICIP). doi: https://doi.org/10.1109/ icip46576.2022.9897723.
Lou, J., Lin, H., Young, P., White, R., Yang, Z., Shelmerdine, S., Marshall, D., Spezi, E., Palombo, M. and Liu, H. (2023). Predicting Radiologists’ Gaze with Computational Saliency Models in Mammogram Reading. IEEE Transactions on Multimedia, pp.1–14. doi: https://doi.org/10.1109/tmm.2023.3263553.
Mehranian, A., Wollenweber, S.D., Walker, M.D., Bradley, K.M., Fielding, P.A., Huellner, M., Kotasidis, F., Su, K.-H., Johnsen, R., Jansen, F.P. and McGowan, D.R. (2022). Deep learning–based time-offlight (ToF) image enhancement of non-ToF PET scans. European Journal of Nuclear Medicine and Molecular Imaging, 49(11), pp.3740–3749. doi: https://doi.org/10.1007/s00259-022-05824-7.
Shankar, A., Hall, G.W., McKay, P., Gallop-Evans, E., Fielding, P. and Collins, G.P. (2022). Management of children and adults with all stages of nodular lymphocyte predominant Hodgkin lymphoma — All StAGEs: A consensus-based position paper from the Hodgkin lymphoma subgroup of the UK National Cancer Research Institute. British Journal of Haematology, 197(6), pp.679–690. doi: https:// doi.org/10.1111/bjh.18169.
Shelmerdine, S.C., White, R.D., Liu, H., Arthurs, O.J. and Sebire, N.J. (2022). Artificial intelligence for radiological paediatric fracture assessment: a systematic review. Insights into Imaging, 13(1). doi: https://doi.org/10.1186/s13244-022-01234-3.
95
Appendix D
Clinical Diagnostics & Therapeutics Clinical Board
Therapies
Publications
Beddard, M., Jones, N., Withers, K. and Morris, R. (2022). Towards Zero Deaths from Bowel Cancer in Wales. [online] Moondance Cancer Initiative. Available at: https://moondance-cancer.wales/researchinsights/towards-zero-deaths-from-bowel-cancer.
Frizzati, A., Jones, N., Palmer, R., Dale, M., Withers, K. and Morris, R. (2022). ‘Adferiad’ (Recovery) Long COVID Service National Evaluation (July 2022 update). Available at: https://cedar.nhs.wales/ files/adferiad-recovery-long-covid-service-national-evaluation-july-2022-update/
Jones, N.J. and Barnett, S. (2022). The role of Alprep Pad® in facilitating shared care of diabetic foot ulceration. The Diabetic Foot Journal, 25(2).
Tack, C., Holdsworth, L., Wilson, A., McComiskie, E., McCabe, P., Wilkinson, W. and King, M. (2022). Digital competency: a survey of UK allied health professionals. British Journal of Healthcare Management, 28(8), pp.1–15. doi: https://doi.org/10.12968/bjhc.2021.0123.
Twose, P., Terblanche, E., Jones, U., Firshman, P., Merriweather, J., Rock, C. and Wallace, S. (2023). Protected therapy services for critical care: A subanalysis of the UK-wide workforce survey. Australian Critical Care. doi: https://doi.org/10.1016/j.aucc.2022.11.011.
Williams, C.M., Barker, A.R., Denford, S., van Beurden, S.B., Bianchim, M.S., Caterini, J.E., Cox, N.S., Mackintosh, K.A., McNarry, M.A., Rand, S., Schneiderman, J.E., Wells, G.J., Anderson, P.J., Beever, D., Beverley, Z., Buckley, R., Button, B.M., Causer, A.J., Curran, M. and Dwyer, T. (2022). The Exeter Activity Unlimited statement on physical activity and exercise for cystic fibrosis: methodology and results of an international, multidisciplinary, evidence-driven expert consensus. Chronic Respiratory Disease, 19, p.147997312211216-147997312211216. doi: https://doi.org/10.1177/14799731221121670.
Williams, J.L. and Elliott, M. (2022). Promoting healthy environments, skills and communities in Wales: the Nutrition Skills for Life® programme. Perspectives in Public Health, 142(6), pp.316–318. doi: https://doi.org/10.1177/17579139221106948.
Please note: all grants, publications, research impact case studies or initiatives have been provided by Clinical Board and Directorate R&D leads. Therefore, there may be further grants, publications, case studies or initiatives not mentioned in this report.
96
Appendix D
Clinical Diagnostics & Therapeutics Clinical Board Appendix D - Clinical Diagnostics & Therapeutics Clinical Board
97
25 Non-Commercial Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Collaborating Directorates Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitm ent total RAG ORION-4 38382 240684 12353 NonCommercial FALSE Interventional Open To Recruitment Clinical Diagnostics & Therapeutics Laboratory Medicine - Med Biochemistry 15/04/2019 30/06/2023 59 36 104 G TRAK-MSK feasibility 52142 310033 16295 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Clinical Diagnostics & Therapeutics Therapies 03/10/2022 31/05/2023 20/02/2023 40 34 34 R The OSTRICH trial 46223 282832 15292 NonCommercial FALSE Interventional Open To Recruitment Clinical Diagnostics & Therapeutics Therapies 06/04/2022 30/09/2023 24 20 20 G TIPTOP 50306 289817 15830 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Clinical Diagnostics & Therapeutics Therapies 02/11/2021 17/05/2023 23/01/2023 60 10 13 R EASE-P 50594 303596 16023 NonCommercial FALSE Interventional Completed Clinical Diagnostics & Therapeutics Therapies 11/10/2021 04/07/2022 18/05/2022 5 5 12 G TrachVest 51009 291279 16031 NonCommercial FALSE Observational Completed Clinical Diagnostics & Therapeutics Therapies 08/11/2021 31/10/2022 30/09/2022 6 4 9 G Over the rainbow 55299 320283 16663 NonCommercial FALSE Observational Closed to Recruitment, In Follow Up Clinical Diagnostics & Therapeutics Therapies 16/01/2023 31/01/2023 03/02/2023 8 8 8 G Non-Commercial
Appendix D - Clinical Diagnostics & Therapeutics Clinical Board
26 Commercial IONA 18524 168706 12419 NonCommercial FALSE Observational Closed to Recruitment, No Follow Up Clinical Diagnostics & Therapeutics Therapeutics & Toxicology 19/02/2016 31/07/2024 31/03/2023 2 0 3 G Adult SMA REACH Study 49675 298064 15890 NonCommercial FALSE Observational Open To Recruitment Clinical Diagnostics & Therapeutics Therapies 18/01/2023 31/12/2023 8 2 2 G DM PAD study 50623 301408 16384 NonCommercial FALSE Interventional Open To Recruitment Clinical Diagnostics & Therapeutics Therapies 14/03/2023 28/02/2024 30 2 2 G DOLPHIN 2 47230 282478 15485 NonCommercial FALSE Interventional Open To Recruitment Clinical Diagnostics & Therapeutics Therapies Haematology, Immunology and Metabolic Medicine; 21/11/2022 31/01/2025 2 2 2 G IFNopathy natural history study 33429 207018 10720 NonCommercial TRUE Observational Open To Recruitment Clinical Diagnostics & Therapeutics Laboratory Medicine - Lab Genetics 06/12/2017 30/04/2023 0 0 0 W 12 123 209 Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitm ent total RAG Study of Lipoprotein(a) Levels in Patients With a History of ASCVD 50776 310751 16411 Commercial FALSE Interventional Closed to Recruitment, No Follow Up Clinical Diagnostics & Therapeutics Laboratory Medicine - Med Biochemistry 08/09/2022 31/05/2023 12/01/2023 30 19 19 R 1 19 19
Appendix D Clinical Diagnostics & Therapeutics Clinical Board Appendix D - Clinical Diagnostics & Therapeutics Clinical Board
26 Commercial IONA 18524 168706 12419 NonCommercial FALSE Observational Closed to Recruitment, No Follow Up Clinical Diagnostics & Therapeutics Therapeutics & Toxicology 19/02/2016 31/07/2024 31/03/2023 2 0 3 G Adult SMA REACH Study 49675 298064 15890 NonCommercial FALSE Observational Open To Recruitment Clinical Diagnostics & Therapeutics Therapies 18/01/2023 31/12/2023 8 2 2 G DM PAD study 50623 301408 16384 NonCommercial FALSE Interventional Open To Recruitment Clinical Diagnostics & Therapeutics Therapies 14/03/2023 28/02/2024 30 2 2 G DOLPHIN 2 47230 282478 15485 NonCommercial FALSE Interventional Open To Recruitment Clinical Diagnostics & Therapeutics Therapies Haematology, Immunology and Metabolic Medicine; 21/11/2022 31/01/2025 2 2 2 G IFNopathy natural history study 33429 207018 10720 NonCommercial TRUE Observational Open To Recruitment Clinical Diagnostics & Therapeutics Laboratory Medicine - Lab Genetics 06/12/2017 30/04/2023 0 0 0 W 12 123 209 Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitm ent total RAG Study of Lipoprotein(a) Levels in Patients With a History of ASCVD 50776 310751 16411 Commercial FALSE Interventional Closed to Recruitment, No Follow Up Clinical Diagnostics & Therapeutics Laboratory Medicine - Med Biochemistry 08/09/2022 31/05/2023 12/01/2023 30 19 19 R 1 19 19 98 Commercial
Appendix E - Medicine Clinical Board
Clinical Gerontology
Publications
Faheem, W., Nandra, T., Richardson, S., Saliu, D., Wilson, D., Jackson, T.A., Magill, L., McCluskey, L., Perry, R., Welch, C., Wilson, D., Copeland, C., Cunningham, E.L., Davis, D., Treml, J., Pinkney, T., Quinn, T., Nightingale, P., Jelley, B. and Gaunt, V. (2023). Increasing frailty is associated with higher prevalence and reduced recognition of delirium in older hospitalised inpatients: results of a multicentre study. European Geriatric Medicine, 14(2). doi: https://doi.org/10.1007/s41999-022-00737-y.
Jelley, B.J., Hughes, T.A.T. and Jones, P. (2023). The Evolution and Environmental Benefits of the All Wales Stroke Meeting (AWSM), a Video Conference Multidisciplinary Meeting for Stroke Physicians. Journal of Medical Sciences and Health, 9(1), pp.75–78. doi: https://doi.org/10.46347/jmsh.v9i1.22.512.
Lewis-Morton, R., Thomas, C., Mohamed, B., Mahon, S. and Williams, T. (2022). Developing a clinical psychology service for people living with Parkinson’s and their care partners: Reflections from people with Parkinson’s, carers and the multidisciplinary team. Clinical Psychology Forum, 1(359), pp.20–28. doi: https://doi.org/10.53841/bpscpf.2022.1.359.20.
Schroeder, B.E., Robertson, N.P. and Hughes, T.A.T. (2023). Cerebral amyloid angiopathy: an update. Journal of Neurology. doi: https://doi.org/10.1007/s00415-023-11631-3.
Grants Project Official Short/Full Title AI-enableD bone health Optimisation osteoPorosis for Treatment (ADOPT) IRAS ID 311094 CPMS ID 53288 Lead Investigator Dr Jane Turton Duration of Grant (Date from/to) 2022-2025 Funder Name/ Source of Funding NHS England & NHS Improvement: NIHR collaboration with NHS Grant Award (Total) £300,000 99
Appendix E - Medicine Clinical Board
Dermatology
Publications
Bates, J., Stanton, H., Cannings-John, R., Thomas, K.S., Leighton, P., Howells, L., Rodrigues, F., Howes, R., Collier, F., Harris, C., Gibbons, A., Thomas-Jones, E., Hood, K. and Ingram, J. (2022). Treatment of Hidradenitis Suppurativa Evaluation Study (THESEUS): protocol for a prospective cohort study. British Medical Journal, 12(4), pp.e060815–e060815. doi: https://doi.org/10.1136/bmjopen-2022-060815.
Bosma, A.L., Ouwerkerk, W., Heidema, M.J., Prieto-Merino, D., Ardern-Jones, M.R., Beattie, P., Brown, S.J., Ingram, J.R., Irvine, A.D., Ogg, G., Patel, P., Reynolds, N.J., Hearn, R.M.R., Wan, M., Warren, R.B., Woolf, R.T., Hyseni, A.M., Gerbens, L.A.A., Spuls, P.I. and Flohr, C. (2023). Comparison of real-world treatment outcomes of systemic immunomodulating therapy in atopic dermatitis patients with dark and light skin types. JAAD International, [online] 10, pp.14–24. doi: https://doi.org/10.1016/j. jdin.2022.09.006.
Garg, A., Dirr, M.A., Jemec, G.B.E., Ingram, J.R., Schmitt, J., Spuls, P. and Alam, M. (2022). Shifting focus from ‘what we do’ to the ‘impact of what we do’: Application of outcome measures to routine clinical care in dermatology. Journal of the American Academy of Dermatology, 87(2), pp.e83–e84. doi: https://doi.org/10.1016/j.jaad.2022.04.042.
Garg, A., Rawal, S., Akilov, O., Alavi, A., Ardon, C., Bechara, F.G., Cohen, A.D., Cohen, S.R., Daveluy, S., Del Marmol, V., Delage, M., Esmann, S., Fisher, S., Giamarellos-Bourboulis, E.J., Glowaczewska, A., Goldfarb, N., Gonzalez Brant, E., Grimstad, Ø., Guilbault, S. and Hamzavi, I. (2023). Factors associated with disease-specific life impact in patients with hidradenitis suppurativa: results from the Global VOICE project. British Journal of Dermatology, 188(6), pp.808–810. doi: https://doi.org/10.1093/bjd/ ljad069.
Garg, A., Zema, C., Ciaravino, V., Rolleri, R., Peterson, L., Garcia, L., Massaro, T., Jemec, G.B.E., Kirby, J.S., Thorlacius, L. and Ingram, J.R. (2023). Validation of the Hidradenitis Suppurativa Investigator Global Assessment. JAMA Dermatology. doi: https://doi.org/10.1001/jamadermatol.2023.0797.
Garg, A.X., Zema, C.L., Kim, K.K., Gao, W., Chen, N., Jemec, G.B.E., Kirby, J.S., Thorlacius, L., Villumsen, B. and Ingram, J. (2022). Development and initial validation of the HS-IGA: a novel hidradenitis suppurativa-specific investigator global assessment for use in interventional trials. British Journal of Dermatology, 187(2), pp.203–210. doi: https://doi.org/10.1111/bjd.21236.
Hasan, S.B., Gendra, R., James, J., Morris, D., Orenstein, L.A.V. and Ingram, J.R. (2022). Pain measurement in painful skin conditions and rheumatoid arthritis randomized controlled trials: a scoping review to inform pain measurement in hidradenitis suppurativa. British Journal of Dermatology. doi: https:// doi.org/10.1111/bjd.21821.
Ingram, J. (2022). A watershed moment for the BJD: Authors retain their article copyright. British Journal of Dermatology, 188(1), pp.1–2. doi: https://doi.org/10.1093/bjd/ljac006.
Ingram, J.R., Bettoli, V., Espy, J.I., Kokolakis, G., Martorell, A., Villani, A.P., Wallinger, H., Coak, E., Kasparek, T., Muscianisi, E., Richardson, C. and Kimball, A.B. (2022). Unmet clinical needs and burden of disease in hidradenitis suppurativa: real-world experience from EU5 and US. Journal of the European Academy of Dermatology and Venereology, 36(9), pp.1597–1605. doi: https://doi. org/10.1111/jdv.18163.
James, J.F., Madray, V.M., Salame, N., Hasan, S.B., White, M.S., Barron, J.R., Kirby, J., Ingram, J.R. and Orenstein, L.A.V. (2022). Demographic Gaps and Requirements for Participation: A Systematic Review of Clinical Trial Designs in Hidradenitis Suppurativa. Dermatology, 239(1), pp.45–51. doi: https://doi.org/10.1159/000526069.
100
Appendix E - Medicine Clinical Board
McCluskey, D., Benzian-Olsson, N., Mahil, S.K., Hassi, N.K., Wohnhaas, C.T., Burden, A.D., Griffiths, C.E.M., Ingram, J., Levell, N.J., Parslew, R., Pink, A., Reynolds, N.J., Warren, R.B., Visvanathan, S., Baum, P., Barker, J., Smith, C.H. and Capon, F. (2022). Single-cell analysis implicates TH17-to-TH2 cell plasticity in the pathogenesis of palmoplantar pustulosis. Journal of Allergy and Clinical Immunology, 150(4), pp.882–893. doi: https://doi.org/10.1016/j.jaci.2022.04.027.
Midgette, B., Strunk, A., Akilov, O.E., Alavi, A., Ardon, C.B., Bechara, F.G., Cohen, A.D., Cohen, S.P., Daveluy, S., Del Marmol, V., Delage, M., Esmann, S., Fisher, S., Giamarellos-Bourboulis, E.J., Głowaczewska, A., Goldfarb, N., Gonzalez Brant, E., Grimstad, Ø., Guilbault, S. and Hamzavi, I.H. (2022). Factors associated with treatment satisfaction in patients with hidradenitis suppurativa: results from the Global VOICE project. British Journal of Dermatology, 187(6), pp.927–935. doi: https://doi.org/10.1111/bjd.21798.
Ring, H.C., Yao, Y., Maul, J., Ingram, J.R., Frew, J.W., Thorsen, J., Nielsen, M., Wu, J.J., Thyssen, J.P., Thomsen, S.F. and Egeberg, A. (2022). The road to biologics in patients with hidradenitis suppurativa: a nationwide drug utilization study. British Journal of Dermatology. doi: https://doi.org/10.1111/ bjd.21673.
Shah, R., Finlay, A.Y., Salek, S., Nixon, S.J., Otwombe, K., Ali, F.M. and Ingram, J.R. (2023). Meaning of Family Reported Outcome Measure (FROM-16) severity score bands: a cross-sectional online study in the UK. British Medical Journal, 13(3), pp.e066168–e066168. doi: https://doi.org/10.1136/ bmjopen-2022-066168.
Tatovic, D., McAteer, M.A., Barry, J., Barrientos, A., Rodríguez Terradillos, K., Perera, I., Kochba, E., Levin, Y., Dul, M., Coulman, S.A., Birchall, J.C., von Ruhland, C., Howell, A., Stenson, R., Alhadj Ali, M., Luzio, S.D., Dunseath, G., Cheung, W.Y., Holland, G. and May, K. (2022). Safety of the use of gold nanoparticles conjugated with proinsulin peptide and administered by hollow microneedles as an immunotherapy in type 1 diabetes. Immunotherapy Advances, 2(1). doi: https://doi.org/10.1093/ immadv/ltac002.
van Straalen, K.R., Ingram, J.R., Augustin, M. and Zouboulis, C.C. (2022). New treatments and new assessment instruments for Hidradenitis suppurativa. Experimental Dermatology, 31(S1), pp.33–39. doi: https://doi.org/10.1111/exd.14609.
Vyas, J., Johns, J.R., Ali, F.M., Singh, R.K., Ingram, J.R., Salek, S. and Finlay, A.Y. (2023). A systematic review of 457 randomised controlled trials using the Dermatology Life Quality Index: experience in 68 diseases and 42 countries. British Journal of Dermatology. doi: https://doi.org/10.1093/bjd/ljad079.
Wainman, H.E., Lane, P.C. and Ingram, J.R. (2022). Hidradenitis suppurativa diagnosis and management in primary care: not just recurrent boils. British Journal of General Practice, 73(726), pp.43–45. doi: https://doi.org/10.3399/bjgp23x731733.
Willems, D., Hinzpeter, E.-L., Van der Zee, H.H., Sayed, C.J., Ingram, J., Beaudart, C., Evers, S.M.A.A. and Hiligsmann, M. (2023). Patient Preferences in the Management of Hidradenitis Suppurativa: Results of a Multinational Discrete Choice Experiment in Europe. Patient, 16(2), pp.153–164. doi: https://doi.org/10.1007/s40271-022-00614-7.
Willems, D., Sayed, C.J., Van der Zee, H.H., Ingram, J.R., Hinzpeter, E.L., Beaudart, C., Evers, S.M.A.A. and Hiligsmann, M. (2023). A discrete-choice experiment to elicit the treatment preferences of patients with hidradenitis suppurativa in the United States. Journal of Medical Economics, 26(1), pp.503–508. doi: https://doi.org/10.1080/13696998.2023.2194804.
101
Appendix E - Medicine Clinical Board
Williams, J.C., Alhusayen, R., Guilbault, S., Hills, N.K., Ingram, J.R., Kudlinski, M.V., Lowes, M.A., Marzano, A.V., Paul, M., Villumsen, B., Yannuzzi, C.A. and Naik, H.B. (2022). Biologic Therapy Is Not Associated with Increased COVID-19 Severity in Patients with Hidradenitis Suppurativa: Updated Findings from the Global Hidradenitis Suppurativa COVID-19 Registry. Dermatology, 239(2), pp.283–286. doi: https://doi.org/10.1159/000527401.
Yiu, Z.Z.N., Chi, C. -C., Ingram, J.R. and Flohr, C. (2022). Checking for update…living systematic reviews and clinical practice guidelines in the BJD. British Journal of Dermatology, 186(5), pp.761–762. doi: https://doi.org/10.1111/bjd.20896.
Integrated Medicine
Publications
Allen, L.A., Shrikrishnapalasuriyar, N. and Rees, D.A. (2022). Long-term health outcomes in young women with polycystic ovary syndrome: A narrative review. Clinical Endocrinology, 97(2), pp.187–198. doi: https://doi.org/10.1111/cen.14609.
Cauldwell, M., Steer, P., Adamson, D., Alexander, C., Allen, L., Bhagra, C., Bolger, A., Bonner, S., Calanchini, M., Carroll, A., Casey, R., Curtis, S., Head, C., English, K., Hudsmith, L., James, R., Joy, E., Keating, N., MacKiliop, L. and McAuliffe, F. (2022). Pregnancies in women with Turner syndrome: a retrospective multicentre UK study. BJOG: An International Journal of Obstetrics & Gynaecology, [online] 129(5), pp.796–803. doi: https://doi.org/10.1111/1471-0528.17025.
Daughters, K., Rees, D.A., Hunnikin, L., Wells, A., Hall, J. and van Goozen, S. (2022). Oxytocin administration versus emotion training in healthy males: considerations for future research. Philosophical Transactions of the Royal Society B: Biological Sciences, 377(1858). doi: https://doi. org/10.1098/rstb.2021.0056.
Elhassan, Y.S., Iqbal, F., Arlt, W., Baldeweg, S.E., Levy, M., Stewart, P.M., John A.H. Wass, Pavord, S., D. Aled Rees and Ronchi, C.L. (2023). COVID-19-related adrenal haemorrhage: Multicentre UK experience and systematic review of the literature. Clinical Endocrinology. doi: https://doi.org/10.1111/ cen.14881.
McCarthy, O., Pitt, J.P., Keay, N., Vestergaard, E.T., Tan, A.S.Y., Churm, R., Rees, D.A. and Bracken, R.M. (2022). Passing on the exercise baton: What can endocrine patients learn from elite athletes? Clinical Endocrinology, 96(6), pp.781–792. doi: https://doi.org/10.1111/cen.14683.
Panagiotou, G., Taylor, P.N., Rees, D.A. and Okosieme, O.E. (2022). Late offspring effects of antenatal thyroid screening. British Medical Bulletin, 143(1), pp.16–29. doi: https://doi.org/10.1093/bmb/ ldac018.
Perros, P., Basu, A., Boelaert, K., Dayan, C., Vaidya, B., Williams, G.R., Lazarus, J.H., Hickey, J., Drake, W.M., Crown, A., Orme, S.M., Johnson, A., Ray, D.W., Leese, G.P., Jones, T.H., Abraham, P., Grossman, A., Rees, A., Razvi, S. and Gibb, F.W. (2022). Postradioiodine Graves’ management: The PRAGMA study. Clinical Endocrinology, 97(5). doi: https://doi.org/10.1111/cen.14719.
Tschaidse, L., Reisch, N., Arlt, W., de la Perriere, A.B., Linden Hirschberg, A., Juul, A., Mallappa, A., Merke, D.P., Newell-Price, J.D.C., Perry, C.G., Prete, A., Rees, D.A., Stikkelbroeck, N., Touraine, P., Coope, H., Porter, J.B., Ross, R.J. and Quinkler, M. (2023). Modified-release hydrocortisone is associated with lower plasma renin activity in patients with salt-wasting congenital adrenal hyperplasia. European Journal of Endocrinology, 188(1). doi: https://doi.org/10.1093/ejendo/lvac006.
Whelan, C., Burnley-Hall, N., Morris, K., Rees, D.A. and James, P.E. (2022). The procoagulant effects of extracellular vesicles derived from hypoxic endothelial cells can be selectively inhibited by inorganic nitrite. Nitric Oxide, 122-123, pp.6–18. doi: https://doi.org/10.1016/j.niox.2022.02.002.
102
Appendix E - Medicine Clinical Board
Grants
Project Official Short/ Full Title
ICF:LIothyrONiNe Sulphate (T3S) use in hypothyroid subjects on T4 homozygous for the Thr92Ala Deiodinase 2 (D2) polymorphism - the LIONNS-D2 study
A clinical trial platform for combined beta cell preservation therapy
Preparing for the Future – extension of the UK T1D Immunotherapy Consortium. 2022-2023. $150k/£100k
Improving outcomes for patients with pituitary disease: advancing therapeutics and diagnostics using electronic health records and molecular imaging
Making up your mind: Obesity and Female Adolescent Brain development (O-FAB Trial)
Lead Investigator/s
Professor Colin Dayan
Professor Colin Dayan
Professor Colin Dayan
Professor Aled Rees
Dr Rachel Lord (Cardiff University)
Professor Aled Rees (co-applicant)
Duration of Grant 2023-2026 Sep 2022- Sep 2025 2022-2023 2021-2023 2021-2023 Funder Name/ Source of Funding MRC JDRF JDRF/Diabetes UK Anonymous Benefactor The Waterloo Foundation Grant Awarded (Total) £3.2m $2.6m £100,000 £252,953 £59,997.98 103
Appendix E - Medicine Clinical Board
Rheumatology Publications
Aletaha, D., Kerschbaumer, A., Kastrati, K., Dejaco, C., Dougados, M., McInnes, I.B., Sattar, N., Stamm, T., Takeuchi, T., Trauner, M., van der Heijde, D., Scholte-Voshaar, M., Winthrop, K.L., Ravelli, A., Betteridge, N., Burmester, G.R., Bijlsma, J.W.J., Bykerk, V.P., Caporali, R. and Choy, E. (2022). Consensus statement on blocking interleukin-6 receptor and interleukin-6 in inflammatory conditions: an update. Annals of the Rheumatic Diseases, 82(6), p.annrheumdis-222784. doi: https://doi.org/10.1136/ ard-2022-222784.
Galloway, J., Raine, T., Rivett, L., Roberts, J., Dews, S.-A. and Choy, E.H. (2022). Herpes zoster and Janus kinase inhibition in rheumatology and gastroenterology patients: managing risk and vaccination. Clinical and Experimental Rheumatology, 40(7). doi: https://doi.org/10.55563/ clinexprheumatol/0jdyse.
Guillo, L., Rabaud, C., Choy, E.H., D’Amico, F., Danese, S., Ng, S.C. and Peyrin-Biroulet, L. (2022). Herpes Zoster and Vaccination Strategies in Inflammatory Bowel Diseases: A Practical Guide. Clinical Gastroenterology and Hepatology, 20(3), pp.481–490. doi: https://doi.org/10.1016/j.cgh.2020.10.027.
Nagy, G., Roodenrijs, N.M.T., Welsing, P.M.J., Kedves, M., Hamar, A., Goes, M.C. van der, Kent, A., Bakkers, M., Pchelnikova, P., Blaas, E., Senolt, L., Szekanecz, Z., Choy, E.H., Dougados, M., Jacobs, J.W., Geenen, R., Bijlsma, J.W., Zink, A., Aletaha, D. and Schoneveld, L. (2022). EULAR points to consider for the management of difficult-to-treat rheumatoid arthritis. Annals of the Rheumatic Diseases. doi: https://doi.org/10.1136/annrheumdis-2021-220973.
Cystic Fibrosis Publications
Acaster, S., Mukuria, C., Rowen, D., Brazier, J.E., Wainwright, C.E., Quon, B.S., Duckers, J., Quittner, A.L., Lou, Y., Sosnay, P.R. and McGarry, L.J. (2022). Development of the Cystic Fibrosis QuestionnaireRevised-8 Dimensions: Estimating Utilities From the Cystic Fibrosis Questionnaire-Revised. Value in Health, 26(4), pp.567–578. doi: https://doi.org/10.1016/j.jval.2022.12.002.
Ayling-Smith, J., Speight, L., Dhillon, R., Backx, M., White, P.L., Hood, K. and Duckers, J. (2022). The Presence of Exophiala dermatitidis in the Respiratory Tract of Cystic Fibrosis Patients Accelerates Lung Function Decline: A Retrospective Review of Lung Function. Journal of Fungi, 8(4), p.376. doi: https://doi.org/10.3390/jof8040376.
Duckers, J., Fitzgerald, R., Proud, D., Addy, C. and Datta, D. (2022). Forewarned is forearmed: The cardiovascular time bomb in Cystic Fibrosis. Journal of Cystic Fibrosis, 21(3). doi: https://doi. org/10.1016/j.jcf.2021.11.008.
McNarry, M.A., Berg, R.M.G., Shelley, J., Hudson, J., Saynor, Z.L., Duckers, J., Lewis, K., Davies, G.A. and Mackintosh, K.A. (2022). Inspiratory Muscle Training Enhances Recovery Post COVID-19: A Randomised Controlled Trial. European Respiratory Journal, 60(4). doi: https://doi.org/10.1183/13993003.031012021.
Metcalfe, R.S., Swinton, P.A., Mackintosh, K.A., Berg, R., Shelley, J., Saynor, Z.L., Hudson, J., Duckers, J., Lewis, K., Davies, G.A. and McNarry, M.A. (2023). Heterogenous Treatment Effects Following Inspiratory Muscle Training during Recovery from Post-Acute COVID-19 Syndrome. Medicine & Science in Sports & Exercise, [online] Publish Ahead of Print. doi: https://doi.org/10.1249/mss.0000000000003207.
104
Appendix E - Medicine Clinical Board
Nye, C., Duckers, J. and Dhillon, R. (2022). Cefiderocol to manage chronic, multi-drug-resistant Burkholderia cepacia complex infection in a patient with cystic fibrosis: a case report. Access Microbiology, 4(10). doi: https://doi.org/10.1099/acmi.0.000413.
O’Leary, C., Edwards, V., Hardcastle, K.A., McCulloch, A. and Duckers, J.M. (2022). Adverse Childhood Experiences (ACEs) in Adults with Cystic Fibrosis. Psychology Research and Behavior Management, 15, pp.1601–1605. doi: https://doi.org/10.2147/prbm.s322425.
Riezk, A., Vasikasin, V., Wilson, R.K., Rawson, T.M., Mcleod, J.G., Dhillon, R., Duckers, J., Cass, A. and Holmes, A. (2023). Triple quadrupole LC/MS method for the simultaneous quantitative measurement of cefiderocol and meropenem in serum. Royal Society of Chemistry Journal (Analytical Methods), 15(6), pp.746–751. doi: https://doi.org/10.1039/d2ay01459a.
Roberts, A.E.L., Xanthe, C., Hopkins, A.L., Bodger, O., Lewis, P., Mahenthiralingam, E., Duckers, J. and Jenkins, R.E. (2022). A pilot study investigating the effects of a manuka honey sinus rinse compared to a standard sinus rinse on sino-nasal outcome test scores in cystic fibrosis patients. Pilot and Feasibility Studies, 8(1). doi: https://doi.org/10.1186/s40814-022-01175-0.
Shelley, J., Hudson, J., Mackintosh, K.A., Saynor, Z.L., Duckers, J., Lewis, K., Davies, G.A., Berg, R.M.G. and McNarry, M.A. (2022). Perceptions of inspiratory muscle training in adults recovering from COVID-19. PLOS ONE, 17(11), p.e0270620. doi: https://doi.org/10.1371/journal.pone.0270620.
Sutharsan, S., McKone, E.F., Downey, D.G., Duckers, J., MacGregor, G., Tullis, E., Van Braeckel, E., Wainwright, C.E., Watson, D., Ahluwalia, N., Bruinsma, B.G., Harris, C., Lam, A.P., Lou, Y., Moskowitz, S.M., Tian, S., Yuan, J., Waltz, D., Mall, M.A. and Aurora, P. (2022). Efficacy and safety of elexacaftor plus tezacaftor plus ivacaftor versus tezacaftor plus ivacaftor in people with cystic fibrosis homozygous for F508del-CFTR: a 24-week, multicentre, randomised, double-blind, active-controlled, phase 3b trial. The Lancet Respiratory Medicine, 10(3), pp.267–277. doi: https://doi.org/10.1016/ s2213-2600(21)00454-9.
Williams, D., Esan, O.B., Schlüter, D.K., Taylor-Robinson, D., Shantini Paranjothy, Duckers, J., Goodchild, N. and Phillips, R. (2023). Sharing decisions on reproductive goals: A mixed-methods study of the views of women who have cystic fibrosis. Journal of Cystic Fibrosis, 22(2), pp.207–216. doi: https:// doi.org/10.1016/j.jcf.2023.02.007.
105
Appendix E - Medicine Clinical Board
Grants
Project Official Short/Full Title
Eureka HRaas (Healthcare Robotics as a Service)
Lead Investigator/s
Dr Esyin Chew
Use of machine learning to predict acute pulmonary exacerbations in Cystic Fibrosis
CF Trust Fellow
Translation of Concept Scheme (Institutional Translational Partnership Award (ITPA) Ref: A rapid assay towards detection of Mycobacterium abscessus in clinical sputum samples from patients with cystic fibrosis
Dr Jamie Duckers (co-applicant)
Dr Jamie Duckers (co-applicant)
Dr Jamie Duckers (co-applicant)
Research Impact Case Study
Contributed by Dr Jamie Duckers
Project Breathe
Remote monitoring for people with cystic fibrosis (CF) and newly funded AI to determine exacerbations prior to them becoming clinically apparent. 103 people with CF from all across Wales on remote monitoring and has changed clinical practice.
Vertex VX21-522-001 first in human study of inhaled mrna therapy for cystic fibrosis
We have been selected as an Early Phase Centre and will be opening this early phase study for people with CF who cannot access the novel CF therapies available to date. We have been working to set up an early phase trial on the CF ward in Llandough and this is the first time this has happened. We now have a pipeline of early pahse studies for CF and bronchiectasis - all to be delivered in the ward setting.
Vertex VX20-12-104-OLS
We have secured an open label study for people with CF thus allowing earlier access to this CFTR modulator and provided by the company. These are very high cost therapies.
Duration of Grant Undisclosed Undisclosed One year Undisclosed Funder Name/ Source of Funding European Regional Development Fund NIHR AI CF Trust Wellcome Trust Grant Awarded (Total) £165,949.10 £1,575,265 £75,000 £19,196
106
Appendix E - Medicine Clinical Board
Endocrinology
Publications
Agrawal, M., Lewis, S., Premawardhana, L., Dayan, C.M., Taylor, P.N. and Okosieme, O.E. (2022). Antithyroid drug therapy in pregnancy and risk of congenital anomalies: Systematic review and meta-analysis. Clinical Endocrinology, 96(6), pp.857–868. doi: https://doi.org/10.1111/cen.14646.
Dayan, C.M., Lecumberri, B., Muller, I., Ganesananthan, S., Hunter, S.F., Selmaj, K.W., Hartung, H.P., Havrdova, E., LaGanke, C., Ziemssen, T., Van Wijmeersch, B., Meuth, S.G., Margolin, D., Poole, E.M., Baker, D.P. and Senior, P.A. (2023). Endocrine and multiple sclerosis outcomes in patients with autoimmune thyroid events in the alemtuzumab CARE-MS studies. Multiple Sclerosis Journal –Experimental, Translational and Clinical, 9(1), p.205521732211427-205521732211427. doi: https:// doi.org/10.1177/20552173221142741.
Eason, R.J., Thomas, N.J., Hill, A.V., Knight, B.A., Carr, A., Hattersley, A.T., McDonald, T.J., Shields, B.M., Jones, A.G., Simon, G., Ramos, A., Norris, A., Tan, K., Narendran, P., Ramtoola, S., Ali, A., Banerjee, M., Brooks, A., Chakera, A. and Johnson, A. (2022). Routine Islet Autoantibody Testing in Clinically Diagnosed Adult-Onset Type 1 Diabetes Can Help Identify Misclassification and the Possibility of Successful Insulin Cessation. Diabetes Care, 45(12), pp.2844–2851. doi: https://doi.org/10.2337/dc220623.
French, R., Kneale, D., Warner, J.T., Robinson, H., Rafferty, J., Sayers, A., Taylor, P., Gregory, J.W. and Dayan, C.M. (2022). Educational Attainment and Childhood-Onset Type 1 Diabetes. Diabetes Care, 45(12), pp.2852–2861. doi: https://doi.org/10.2337/dc21-0693.
Thomas, N.J., Hill, A.V., Dayan, C.M., Oram, R.A., Shields, B.M. and Jones, A.G. (2023). Age of Diagnosis Does Not Alter the Presentation or Progression of Robustly Defined Adult-Onset Type 1 Diabetes. Diabetes Care, 46(6), pp.1156–1163. doi: https://doi.org/10.2337/dc22-2159.
Research Impact Case Study
Contributed by Professor Aled Rees
A Phase III study of efficacy, safety and tolerability of Chronocort® compared with standard glucocorticoid replacement therapy in the treatment of congenital adrenal hyperplasia.
This phase III clinical trial compared the effectiveness of a new modified release formulation of hydrocortisone (Efmody/ Chronocort®) with conventional glucocorticoid replacement in controlling disease activity in patients with Congenital Adrenal Hyperplasia (CAH) due to 21-hydroxylase deficiency. Patients with CAH have a significant unmet health need. Whilst good disease control (with respect to biochemical normalisation of androgen levels and clinical symptoms) can be achieved in some patients with existing glucocorticoids, many others have suboptimal control whether assessed clinically (e.g. menstrual irregularity, sub-fertility, hirsutism, weight gain, adrenal rest tumours) or biochemically. This suboptimal control reflects the difficulty in regulating androgens on the one hand, whilst avoiding glucocorticoid over-replacement on the other. In some patients, good control of androgens (17-hydroxyprogesterone, androstenedione) can only be achieved at the expense of higher than ideal doses of glucocorticoid (resulting in weight gain and the longer-term sequelae [e.g. cardiometabolic risk, osteopenia] associated with excess glucocorticoid exposure).
107
Appendix E - Medicine Clinical Board
We recruited 4 patients with this rare disorder to the initial randomised trial and subsequent extension study. Efmody was shown to improve biochemical disease control in adults, with reduction in steroid dose over time and patient-reported benefit, leading to marketing authorisation being granted by the MHRA in 2021.
The trial also led to a high impact primary publication (Merke, D. P. et al, (2021). Modified-release hydrocortisone in congenital adrenal hyperplasia. J Clin Endocrinol Metab, 106(5), e2063-e2077) which was among the top 10% of articles (based on citation rate) published in this internationallyleading journal that year. A secondary publication has shown an additional benefit on lowered fludrocortisone replacement dose requirements consequent upon improved mineralocorticoid activity with lowering of 17-hydroxyprogesterone levels (Tschaidse, L., et al. (2023). Modified-release hydrocortisone is associated with lower plasma renin activity in patients with salt-wasting congenital adrenal hyperplasia. Eur J Endocrinol, 188(1), Ivac006).
The clinical and cost-effectiveness of Efmody for adults and children with CAH was reviewed by the All-Wales Medicines Strategy Group in 2022. They approved Efmody for use as a second-line treatment option in adults not adequately controlled on maximum guideline doses of immediaterelease hydrocortisone and/or prednisolone. This represents the first new drug therapy for patients with CAH for over 50 years. Our success as a centre has led to the capture of new studies with Efmody in primary adrenal insufficiency and of a novel corticotrophin releasing hormone receptor antagonist in patients with CAH.
Please note: all grants, publications, research impact case studies or initiatives have been provided by Clinical Board and Directorate R&D leads. Therefore, there may be further grants, publications, case studies or initiatives not mentioned in this report.
108
Appendix E - Medicine Clinical Board
Appendix E - Medicine Clinical Board
Non-Commercial
Non-Commercial
40
Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Collaborating Directorates Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitment total RAG *Clinical Characterisation Protocol for Severe Emerging Infection 14152 126600 12143 NonCommercial FALSE Observational Suspended Specialist Services Critical Care Acute Child Health; Internal Medicine; 30/03/2020 28/02/2023 2 0 1181 S SOCRATES 44661 273967 14946 NonCommercial FALSE Observational Closed to Recruitment, In Follow Up Medicine Internal Medicine (Rheumatology) 29/07/2020 30/09/2022 17/06/2022 280 0 691 G CREATE 13826 94479 10307 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine (Rheumatology) 01/08/2012 30/09/2023 500 2 622 G **RECOVERY trial 45388 281712 15098 NonCommercial FALSE Interventional Open To Recruitment Medicine Internal Medicine Acute Child Health; Haematology, Immunology and Metabolic Medicine; 18/03/2020 30/09/2030 1 10 372 G PRONTO v1.0 44264 268723 14994 NonCommercial FALSE Both Open To Recruitment Medicine Emergency Unit 02/07/2021 31/05/2023 380 209 372 G LOCOMOTION 51044 303623 16139 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 18/01/2022 31/07/2023 40 322 354 G CIA 46588 282164 15561 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 22/02/2022 30/04/2025 200 184 272 G ISTID 12849 80158 8310 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 25/03/2013 30/04/2025 15 15 252 G CONSCOP 2 44738 271876 14938 NonCommercial FALSE Interventional Open To Recruitment Medicine Endoscopy & Gastroenterology 28/06/2021 31/12/2023 240 168 248 G Extracellular vesicle transport in the circulation 17735 167697 9082 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 06/03/2015 31/12/2024 25 0 201 G IBD Bioresource 20664 173561 13505 NonCommercial FALSE Observational Open To Recruitment Medicine Endoscopy & Gastroenterology 25/10/2019 30/06/2025 85 78 187 G OPTIMAS Trial 40836 249552 13466 NonCommercial FALSE Interventional Open To Recruitment Medicine Clinical Gerontology 20/02/2020 31/01/2024 45 38 172 G The Physical and Psychological Impact of Stroke on Carers in Wales 50617 294363 15911 NonCommercial FALSE Observational Open To Recruitment Medicine Clinical Gerontology 15/12/2022 22/03/2024 20 161 161 G BADBIR 8090 32990 8073 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine (Dermatology) 15/01/2010 31/07/2025 195 0 160 A
109
Appendix E - Medicine Clinical Board
Appendix E - Medicine Clinical Board
41 BronchUK Study 18944 173303 9530 NonCommercial FALSE Observational Closed to Recruitment, In Follow Up Medicine Internal Medicine 20/02/2017 30/04/2022 30/04/2022 135 0 139 G Immune fingerprinting of infections in cirrhosis 40462 235693 12465 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Medicine Endoscopy & Gastroenterology 01/12/2018 01/09/2028 30/09/2022 120 0 115 HG Determinants of Graves’ Disease 14912 95037 8892 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 03/03/2014 01/08/2023 81 0 97 G Bio-markers of systemic treatment outcomes in Psoriasis 10646 71048 8382 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine (Dermatology) 15/01/2013 31/03/2024 50 0 88 G Inflammation and Immune Regulation in Early Inflammatory Arthritis 13767 74282 10306 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine (Rheumatology) 23/01/2013 30/09/2027 60 0 77 G DRN 552 (ADDRESS2) 9689 55225 8804 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 05/09/2012 31/07/2023 30 5 72 G AIP 4663 33446 9827 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 01/08/2013 31/12/2037 70 0 70 G REPEAT phases 1 and 2 49373 295614 16141 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 10/02/2022 31/10/2023 38 54 66 G ELECT2 Trial 51071 304751 16113 NonCommercial FALSE Interventional Completed Medicine Emergency Unit 21/02/2022 02/09/2023 24/02/2023 72 55 64 R BSRBR 7302 64202 8801 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine (Rheumatology) 01/04/2008 01/01/2028 57 0 57 G Blood-brain barrier dysfunction following systemic infection 50420 277598 15913 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 15/11/2021 09/11/2023 36 42 51 G HEAL-COVID trial 48890 294861 15776 NonCommercial FALSE Interventional Open To Recruitment Medicine Internal Medicine 21/06/2021 31/01/2024 5 9 46 G Peptidia Lymph Node Monitor 14851 103354 8887 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 03/04/2014 31/03/2024 16 4 38 G HIIT v MISS in PCOS 43743 262172 13599 NonCommercial FALSE Interventional Open To Recruitment Medicine Internal Medicine 31/12/2019 31/01/2024 40 20 36 G PBC Genetics Study 5630 32929 8058 NonCommercial FALSE Observational Open To Recruitment Medicine Endoscopy & Gastroenterology 25/03/2009 31/12/2024 50 1 35 A DAISy-PCOS 41566 256572 13586 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 03/03/2020 01/10/2024 15 19 32 G COVID-19 antibody responses in Cystic Fibrosis patients 52247 294037 15767 NonCommercial FALSE Observational Closed to Recruitment, In Follow Up Medicine Internal Medicine Acute Child Health; 21/03/2022 28/03/2023 28/03/2023 30 20 31 G
110
Appendix E - Medicine Clinical Board
Appendix E - Medicine Clinical Board
42 The I-DSD Registry 12729 269776 14982 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 01/09/2020 09/09/2024 40 7 27 G EXPLAIN 51331 305846 16172 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 09/09/2022 31/08/2023 50 26 26 A Is there a genotypephenotype correlation in SDHB mutation carriers? 45242 269678 15062 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 07/09/2021 01/04/2024 20 10 25 G Acromegaly Register 6687 33824 9921 NonCommercial FALSE Observational Suspended Medicine Internal Medicine 01/04/2008 31/12/2018 170 0 24 S Spitfire 51026 301001 15939 NonCommercial FALSE Interventional Completed Medicine Internal Medicine Haematology, Immunology and Metabolic Medicine; 16/08/2021 30/07/2022 10/06/2022 20 3 20 G The CF STORM Trial 48177 293186 15650 NonCommercial FALSE Both Open To Recruitment Medicine Internal Medicine Acute Child Health; 27/07/2021 31/03/2024 10 0 11 G ASEPTIC 42393 262176 13731 NonCommercial FALSE Interventional Open To Recruitment Medicine Endoscopy & Gastroenterology 08/06/2021 30/04/2023 8 4 9 G CALIBRE Study 39255 248487 13419 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Medicine Internal Medicine 05/03/2019 01/02/2023 31/08/2022 15 1 9 R ENRICH-AF 48029 277102 15207 NonCommercial FALSE Interventional Open To Recruitment Medicine Clinical Gerontology 25/03/2021 30/04/2023 3 5 9 G Ver-A-T1D 48712 291503 15602 NonCommercial FALSE Interventional Open To Recruitment Medicine Internal Medicine 06/08/2021 30/09/2023 2 8 9 G ASPEN Study 36392 231944 10713 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 04/09/2019 30/06/2023 6 2 8 G UNDIES 43148 265870 13884 NonCommercial FALSE Observational Open To Recruitment Medicine Endoscopy & Gastroenterology 01/09/2022 30/04/2023 5 8 8 G INNODIA 31472 210497 10463 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 05/06/2019 30/06/2023 1 4 7 G Genetic risk factors for cerebral small vessel disease 31627 200942 15829 NonCommercial FALSE Observational Open To Recruitment Medicine Clinical Gerontology 22/07/2021 31/12/2025 10 3 6 G PLUM 33029 201357 9549 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine (Dermatology) 13/06/2018 31/12/2023 6 0 6 G ToPPRA 43261 256930 13685 NonCommercial FALSE Interventional Open To Recruitment Medicine Internal Medicine (Rheumatology) 04/01/2021 31/07/2023 30 2 6 R CASCADE 52970 306443 16550 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 22/11/2022 31/12/2024 5 5 5 G Health Professionals' Perspectives on Funding for Dementia Care 51020 292705 15942 NonCommercial FALSE Observational Closed to Recruitment, No Follow Up Medicine Clinical Gerontology 10/01/2022 30/09/2022 30/09/2022 10 1 5 R 111
Appendix E - Medicine Clinical Board
Appendix E - Medicine Clinical Board
*Clinical Characteristics Protocol for Severe Emerging Infection counted under Specialist Services Clinical Board.
**RECOVERY trial counted under Medicine Clinical Board from 2023-24 onwards.
43
TIPAL 44455 269050 14987 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Medicine Internal Medicine 12/10/2021 30/04/2023 31/03/2023 5 2 5 G UK-Irish A*STAR 38716 237309 13308 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine (Dermatology) 06/01/2020 31/12/2023 12 2 5 R BOPPP Trial 40439 255446 13507 NonCommercial FALSE Interventional Open To Recruitment Medicine Endoscopy & Gastroenterology 19/10/2021 31/12/2023 20 1 4 R Genetic Analysis to Predict the Development of Paget's Disease 47992 259285 14838 NonCommercial FALSE Observational Open To Recruitment Medicine Clinical Gerontology 10/10/2022 31/12/2023 6 3 3 G IBD-RESPONSE 49964 295742 15931 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 01/02/2023 28/02/2024 34 3 3 R The CLEAR Trial 37574 214254 10731 NonCommercial FALSE Interventional Open To Recruitment Medicine Internal Medicine 07/02/2022 31/03/2024 1 2 3 G ATD Study 40951 250064 13483 NonCommercial FALSE Observational Closed to Recruitment, In Follow Up Medicine Internal Medicine 24/07/2019 31/12/2022 31/12/2022 1 0 2 G PREPARE 49615 289430 15892 NonCommercial FALSE Observational Completed Medicine Internal Medicine 08/08/2022 30/09/2022 03/10/2022 4 2 2 R A Prospective Study of Duodenal Disease in MAP 13304 84639 10260 NonCommercial FALSE Observational Suspended Medicine Endoscopy & Gastroenterology 12/07/2012 31/12/2031 12 0 1 S UK-EDI 42837 258093 14924 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 17/02/2023 01/06/2023 5 1 1 R CONGA 51886 298524 16242 NonCommercial FALSE Observational Open To Recruitment Medicine Internal Medicine 31/03/2023 01/05/2023 50 0 0 R PHEAST 50913 304658 16125 NonCommercial FALSE Interventional Open To Recruitment Medicine Internal Medicine 13/03/2023 30/11/2024 20 0 0 R TB-DILI 54081 1005097 16517 NonCommercial FALSE Both Open To Recruitment Medicine Internal Medicine 17/03/2023 14/11/2026 2 0 0 R TOP HAT 44305 266504 14963 NonCommercial FALSE Interventional Open To Recruitment Medicine Internal Medicine 14/03/2023 31/01/2024 5 0 0 R 63 1521 6563 112
Appendix E - Medicine Clinical Board
Appendix E - Medicine Clinical Board
44 Commercial Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitment total RAG LUNAR 52127 1005346 16366 Commercial FALSE Observational Open To Recruitment Medicine Internal Medicine 05/07/2022 30/09/2023 5 37 37 G Trajectory 50911 306934 16215 Commercial FALSE Observational Closed to Recruitment, In Follow Up Medicine Internal Medicine 25/04/2022 11/10/2022 14/09/2022 20 20 20 G PREF NET 47905 286528 15671 Commercial FALSE Observational Completed Medicine Endoscopy & Gastroenterology 24/06/2021 31/03/2022 14/06/2022 12 1 16 G TriMaximize 47923 294788 15806 Commercial FALSE Observational Open To Recruitment Medicine Internal Medicine 25/06/2021 30/06/2023 7 5 12 G MEDI7352 in PDN 46595 252169 15844 Commercial FALSE Interventional Closed to Recruitment, No Follow Up Medicine Internal Medicine 31/03/2022 31/03/2024 10/01/2023 1 9 9 G CHAMPAIN 49824 1003971 16033 Commercial FALSE Interventional Open To Recruitment Medicine Internal Medicine 15/12/2021 31/05/2023 1 4 5 G IMPACT Study 46090 285844 15352 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Medicine Internal Medicine 11/01/2021 01/06/2022 03/02/2023 2 0 4 G A Phase 3 Study of VX-121 Combination Therapy in Subjects With Cystic Fibrosis Heterozygous 49778 1003950 16227 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Medicine Internal Medicine 06/05/2022 26/08/2022 26/08/2022 3 3 3 G A Phase 3 Study of VX-121 Combination Therapy in Subjects With Cystic Fibrosis Homozygous 49779 1003951 16192 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Medicine Internal Medicine 10/05/2022 25/09/2022 25/09/2022 2 3 3 G Commercial 113
Appendix E - Medicine Clinical Board
Appendix E - Medicine Clinical Board
45 ASPEN 46948 1003433 15674 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Medicine Internal Medicine 26/11/2021 01/02/2023 01/02/2023 2 0 2 G NBI-74788CAH3003 46446 282848 15373 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Medicine Internal Medicine 19/07/2021 31/12/2022 31/12/2022 1 2 2 G A Phase 2 Study to evaluate the safety and efficacy of Linsitunib 51615 1004828 16369 Commercial FALSE Interventional Open To Recruitment Medicine Internal Medicine 26/01/2023 10/10/2023 2 0 0 R M20-370 Phase 2 ABBV-154 study in PMR pts dependent on GC Tx 49515 301154 16588 Commercial FALSE Interventional Closed to Recruitment, No Follow Up Medicine Internal Medicine (Rheumatology) 18/11/2022 30/12/2022 12/12/2022 2 0 0 R MK8591A-052 54585 1006916 16824 Commercial FALSE Interventional Open To Recruitment Medicine Internal Medicine 05/04/2023 31/08/2023 5 0 0 R SPARROW 1005795 16545 Commercial FALSE Both Closed to Recruitment, No Follow Up Medicine Internal Medicine 14/03/2023 01/07/2023 21/03/2023 1 0 0 R TCH-306 47367 289266 15686 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Medicine Internal Medicine 22/11/2022 31/12/2022 23/12/2022 1 0 0 R 16 84 113 114
Appendix F - Mental Health Clinical Board
Publications
Haddad, M. and Young, N. (2022). Self-harm and suicide: occurrence, risk assessment and management for general nurses. Nursing Standard, 37(6). doi: https://doi.org/10.7748/ns.2022.e11911.
Hayes, D., Hunter-Brown, H., Camacho, E., McPhilbin, M., Elliot, R., Ronaldson, A., Bakolis, I., Repper, J., Meddings, S., Stergiopoulos, V., Brophy, L., De Ruysscher, C., Okoliyski, M., Kubinová, P., Falgaard Eplov, L., Toernes, C., Narusson, D., Tinland, A., Puschner, B. and Hiltensperger, R. (2023). Recovery College characteristics, fidelity, commissioning models and unit costs: a cross-sectional global survey of 28 countries. doi: https://doi.org/10.31234/osf.io/kxtmb.
Howells, D., Rees, J., Townsend, R. and Kirov, G. (2022). Electroconvulsive Therapy Reverses Cerebral Hypoperfusion in a Patient With Psychotic Depression and Catatonia. The Journal of ECT, 38(2), pp.141–143. doi: https://doi.org/10.1097/yct.0000000000000836.
Psychology & Counselling
Adkins, K., Overton, P.G., Moses, J. and Thompson, A. (2022). Investigating the Role of Upward Comparisons and Self-compassion on Stigma in People With Acne: Cross-sectional Study. JMIR Dermatology, 6. doi: https://doi.org/10.2196/45368.
Adkins, K.V., Overton, P.G. and Thompson, A.R. (2022). A brief online writing intervention improves positive body image in adults living with dermatological conditions. Frontiers in Medicine, 9. doi: https://doi.org/10.3389/fmed.2022.1064012.
Anning, K.L., Langley, K., Hobson, C., De Sonneville, L. and Van Goozen, S.H.M. (2023). Inattention symptom severity and cognitive processes in children at risk of ADHD: the moderating role of separation anxiety. Child Neuropsychology, pp.1–25. doi: https://doi.org/10.1080/09297049.2023.21 90964.
Astill Wright, L., McElroy, E., Barawi, K., Roberts, N.P., Simon, N., Zammit, S. and Bisson, J.I. (2023). Associations among psychosis, mood, anxiety, and posttraumatic stress symptoms: A network analysis. Journal of Traumatic Stress, 36(2), pp.385–396. doi: https://doi.org/10.1002/jts.22916.
Bisson, J.I., Ariti, C., Cullen, K., Kitchiner, N., Lewis, C., Roberts, N.P., Simon, N., Smallman, K., Addison, K., Bell, V., Brookes-Howell, L., Cosgrove, S., Ehlers, A., Fitzsimmons, D., Foscarini-Craggs, P., Harris, S.R.S., Kelson, M., Lovell, K., McKenna, M. and McNamara, R. (2022). Guided, internet based, cognitive behavioural therapy for post-traumatic stress disorder: pragmatic, multicentre, randomised controlled non-inferiority trial (RAPID). BMJ (Clinical research ed.), 377, p.e069405. doi: https://doi. org/10.1136/bmj-2021-069405.
Clarke, E.N., Norman, P. and Thompson, A.R. (2022). How does self-compassion help people adjust to chronic skin conditions? A template analysis study. Frontiers in Medicine, 9. doi: https://doi. org/10.3389/fmed.2022.974816.
Dighton, G., Wood, K., Armour, C., Fossey, M., Hogan, L., Kitchiner, N., Larcombe, J., Rogers, R.D. and Dymond, S. (2022). Gambling problems among United Kingdom armed forces veterans: Associations with gambling motivation and posttraumatic stress disorder. International Gambling Studies, 23(1), pp.1–22. doi: https://doi.org/10.1080/14459795.2022.2063923.
115
Appendix F - Mental Health Clinical Board
Ferguson, C., Hobson, C., Hedge, C., Waters, C., Anning, K. and van Goozen, S. (2023). Disentangling the relationships between motor control and cognitive control in young children with symptoms of ADHD. Child Neuropsychology, pp.1–26. doi: https://doi.org/10.1080/09297049.2023.2190965.
Field, S.L., Fox, J.R.E., Jones, C.R.G. and Williams, M.O. (2023). ‘Work WITH us’: a Delphi study about improving eating disorder treatment for autistic women with anorexia nervosa. Journal of Eating Disorders, 11(1). doi: https://doi.org/10.1186/s40337-023-00740-z.
Green, C. and Cappleman, R. (2023). Staff views on reflective practice groups in an inpatient assessment and treatment unit for people with intellectual disabilities. Advances in Mental Health and Intellectual Disabilities, 17(2). doi: https://doi.org/10.1108/amhid-11-2022-0045.
Heapy, C., Norman, P., Emerson, L.-M., Murphy, R., Bögels, S. and Thompson, A.R. (2022). Mindful parenting intervention for parents of children with skin conditions: A single group experimental cases series. Behavioural and Cognitive Psychotherapy, 50(5), pp.462–480. doi: https://doi.org/10.1017/ S1352465822000170.
Hewitt, R.M., Ploszajski, M., Purcell, C., Pattinson, R., Jones, B., Wren, G.H., Hughes, O., Ridd, M.J., Thompson, A.R. and Bundy, C. (2022). A mixed methods systematic review of digital interventions to support the psychological health and well-being of people living with dermatological conditions. Frontiers in Medicine, 9. doi: https://doi.org/10.3389/fmed.2022.1024879.
Hill, C., Sims, S., ap Robert, M. and Collier, S. (2022). A thematic analysis of staff perspectives on the impact of a mental health nurse (RMN) in a critical care unit. Journal of the Intensive Care Society, p.175114372211164. doi: https://doi.org/10.1177/17511437221116474.
Howard, T.L.M., Williams, M.O., Woodward, D. and Fox, J.R.E. (2022). The relationship between shame, perfectionism and Anorexia Nervosa: A grounded theory study. Psychology and Psychotherapy: Theory, Research and Practice, 96(1). doi: https://doi.org/10.1111/papt.12425.
Howe-Davies, H., Hobson, C., Waters, C. and van Goozen, S.H.M. (2022). Emotional and socio-cognitive processing in young children with symptoms of anxiety. European Child & Adolescent Psychiatry. doi: https://doi.org/10.1007/s00787-022-02050-2.
Johns, G., Samuel, V. and Waddington, L. (2022). Prevalence and predictors of mental health outcomes in UK doctors and final year medical students during the COVID-19 pandemic. Journal of Affective Disorders, 311, pp.267–275. doi: https://doi.org/10.1016/j.jad.2022.05.024.
Knight, L. and Samuel, V. (2022). Acceptance and commitment therapy interventions in secondary schools and their impact on students’ mental health and well-being: A systematic review. Journal of Contextual Behavioral Science, 25, pp.90–105. doi: https://doi.org/10.1016/j.jcbs.2022.06.006.
Lavender, S.R., Waters, C.S. and Hobson, C.W. (2022). The efficacy of group delivered mentalizationbased parenting interventions: A systematic review of the literature. Clinical Child Psychology and Psychiatry, 28(2), pp.761–784. doi: https://doi.org/10.1177/13591045221113392.
Lynham, A., Knott, S., Underwood, J., Hubbard, L., Agha, S.S., Bisson, J.I., van der Bree, M.B.M., Chawner, S.J.R.A., Craddock, N., O’Donovan, M., Jones, I., Kirov, G., Langley, K., Martin, J., Rice, F., Roberts, N.P., Thapar, A., Anney, R., Owen, M.J. and Hall, J. (2023). DRAGON-Data: a platform and protocol for integrating genomic and phenotypic data across large psychiatric cohorts. British Journal of Psychology Open, 9(2). doi: https://doi.org/10.1192/bjo.2022.636.
116
Appendix F - Mental Health Clinical Board
Macbeth, A.E., Holmes, S., Harries, M., Chiu, W.S., Tziotzios, C., de Lusignan, S., Messenger, A.G. and Thompson, A.R. (2022). The associated burden of mental health conditions in alopecia areata: a population-based study in UK primary care. British Journal of Dermatology, 187(1), pp.73–81. doi: https://doi.org/10.1111/bjd.21055.
Moledina, Z., McPhee, M., Ravenscroft, J., Healy, E., Radley, K., Thomas, K.S. and Burden-Teh, E. (2023). How is oral isotretinoin prescribed for the treatment of acne vulgaris? Results from a UK Dermatology Clinical Trials Network (UKDCTN) and British Dermatological Nursing Group (BDNG) survey of health professionals. Clinical and Experimental Dermatology, 48(1), pp.20–23. doi: https:// doi.org/10.1093/ced/llac015.
Morrison, J., Williams, M.O. and Fox, J.R.E. (2022). Negative childhood events and the development of the anorexic voice: A grounded theory. Psychology and Psychotherapy: Theory, Research and Practice, 95(4). doi: https://doi.org/10.1111/papt.12416.
Muftin, Z., Gilbert, P. and Thompson, A.R. (2022). A Randomised Controlled Feasibility Trial of Online Compassion-focused Self-Help for Psoriasis. British Journal of Dermatology, 186(6), pp.955–962. doi: https://doi.org/10.1111/bjd.21020.
Musa, N., Pang, N.T.P., Kamu, A., Ho, C.M., Waters, C., Berrett, J., Moghaddam, N. and Wider, W. (2022). The Development and Validation of the Comprehensive Assessment of Acceptance and Commitment Therapy Processes (CompACT)—Malay Version. International Journal of Environmental Research and Public Health, 19(15), p.9624. doi: https://doi.org/10.3390/ijerph19159624.
Parker, H., Carlton, E., Harris, S. and Daniels, J. (2022). Psychological predictors of health anxiety and pain in ambulatory presentations in a hospital emergency department. Behavioural and Cognitive Psychotherapy, 51(1), pp.11–20. doi: https://doi.org/10.1017/s1352465822000352.
Rawlings, G.H., Novakova, B., Armstrong, I. and Thompson, A.R. (2023). Can self-compassion help us better understand the impact of pulmonary hypertension on those with the condition and their carers? A cross-sectional analysis. Pulmonary Circulation, 13(1). doi: https://doi.org/10.1002/ pul2.12208.
Rawlings, G.H., Thompson, A.R., Armstrong, I. and Beail, N. (2022). What is the psychosocial burden of COVID-19 on people with pulmonary hypertension? Clinical Respiratory Journal, 17(2), pp.124–126. doi: https://doi.org/10.1111/crj.13570.
Rawlings, G.H., Thompson, A.R., Armstrong, I., Novakova, B. and Beail, N. (2022). Cognitive and behavioral processes predict anxiety and depression in patients with pulmonary hypertension. Pulmonary Circulation, 12(4). doi: https://doi.org/10.1002/pul2.12174.
Rawlings, G.H., Thompson, A.R., Armstrong, I., Novakova, B. and Beail, N. (2022). Coping styles associated with depression, health anxiety and health-related quality of life in pulmonary hypertension: cross-sectional analysis. BMJ Open, 12(8), pp.e062564–e062564. doi: https://doi. org/10.1136/bmjopen-2022-062564.
Roberts, G., Halstead, S., Pepper, R. and McDonnell, L. (2023). Social care professionals’ perceived barriers to implementing attachment and trauma-informed care training in their practice. Developmental Child Welfare, 5(1), pp.3–20. doi: https://doi.org/10.1177/25161032231153643.
Roberts, N.P., Lotzin, A. and Schäfer, I. (2022). A systematic review and meta-analysis of psychological interventions for comorbid post-traumatic stress disorder and substance use disorder. European Journal of Psychotraumatology, 13(1). doi: https://doi.org/10.1080/20008198.2022.2041831.
117
Appendix F - Mental Health Clinical Board
Rowe, R., Stride, C.B., Day, M.R., Thompson, A.R., McKenna, F.P. and Poulter, D.R. (2022). Why are newly qualified motorists at high crash risk? Modelling driving behaviours across the first six months of driving. Accident Analysis & Prevention, 177, p.106832. doi: https://doi.org/10.1016/j. aap.2022.106832.
Schut, C., Dalgard, F.J., Bewley, A., Evers, A.W.M., Gieler, U., Lien, L., Sampogna, F., Ständer, S., TomásAragonés, L., Vulink, N., Finlay, A.Y., Legat, F.J., Titeca, G., Jemec, G.B., Misery, L., Szabó, C., GrivchevaPanovska, V., Spillekom-van Koulil, S., Balieva, F. and Szepietowski, J.C. (2022). Body dysmorphia in common skin diseases: results of an observational, cross-sectional multicentre study among dermatological outpatients in 17 European countries. British Journal of Dermatology, 187(1). doi: https://doi.org/10.1111/bjd.21021.
Silver, J.H., Lewton, M. and Lewis, H.W. (2022). Mediators of negative content and voice-related distress in a diverse sample of clinical and non-clinical voice-hearers. British Journal of Clinical Psychology, 62(1). doi: https://doi.org/10.1111/bjc.12396.
Simon, N., Cunningham, E., Samuel, V. and Waters, C. (2023). Videoconference-delivered group acceptance commitment therapy for perinatal mood and anxiety disorders: facilitators views and recommendations. Journal of Reproductive and Infant Psychology, pp.1–15. doi: https://doi.org/10.1 080/02646838.2023.2180143.
Souch, A.J., Jones, I.R., Shelton, K.H.M. and Waters, C.S. (2022). Maternal childhood maltreatment and perinatal outcomes: A systematic review. Journal of Affective Disorders, 302, pp.139–159. doi: https://doi.org/10.1016/j.jad.2022.01.062.
Thompson, A.R., Eleftheriadou, V. and Nesnas, J. (2022). The mental health associations of vitiligo: UK population-based cohort study. BJPsych Open, 8(6). doi: https://doi.org/10.1192/bjo.2022.591.
Tinlin, R.M., Beckwith, H., Gregory, J.D. and Lomax, C.L. (2022). What is underneath all that stuff? A Q-methodological exploration of profiles of beliefs and vulnerabilities in hoarding disorder. Behavioural and Cognitive Psychotherapy, 50(5), pp.538–555. doi: https://doi.org/10.1017/ s1352465822000261.
Tour, S.K., Thompson, A., Howard, R.A. and Larkin, M. (2022). The Experience of Blogging About Visible and Long-Term Skin Conditions: An Interpretative Phenomenologicak Analysis. JMIR Dermatology, 5(2). doi: https://doi.org/10.2196/29980.
Waters, C.S., Cannings-John, R., Channon, S., Lugg-Widger, F., Robling, M. and Paine, A.L. (2022). The impact of a specialist home-visiting intervention on the language outcomes of young mothers and their children: a pragmatic randomised controlled trial. BMC Psychology, 10(1). doi: https://doi. org/10.1186/s40359-022-00926-1.
Whitmarsh, L., Player, L., Jiongco, A., James, M., Williams, M., Marks, E. and Kennedy-Williams, P. (2022). Climate anxiety: What predicts it and how is it related to climate action? Journal of Environmental Psychology, 83, p.101866. doi: https://doi.org/10.1016/j.jenvp.2022.101866.
Wilson, R.B., Thompson, A.R., Rowse, G. and Freeth, M. (2023). The experience of seeking, receiving, and reflecting upon a diagnosis of autism in the UK: A meta-synthesis of qualitative studies conducted with autistic individuals. Research in Autism Spectrum Disorders, 103, p.102135. doi: https://doi. org/10.1016/j.rasd.2023.102135.
Wilson, R.B., Thompson, A.R., Rowse, G., Smith, R., Dugdale, A.-S. and Freeth, M. (2022). Autistic women’s experiences of self-compassion after receiving their diagnosis in adulthood. Autism, p.136236132211367. doi: https://doi.org/10.1177/13623613221136752.
118
Appendix F - Mental Health Clinical Board
Wren, G., Baker, E.R., Underwood, J., Humby, T., Thompson, A.R., Kirov, G., Escott-Price, V. and Davies, W. (2022). Characterising heart rhythm abnormalities associated with Xp22.31 deletion. Journal of Medical Genetics, p.jmg-108862. doi: https://doi.org/10.1136/jmg-2022-108862.
Wren, G., Humby, T., Thompson, A.R. and Davies, W. (2022). Mood symptoms, neurodevelopmental traits, and their contributory factors in X-linked ichthyosis, ichthyosis vulgaris and psoriasis. Clinical Experimental Dermatology, 47(6), pp.1097–1108. doi: https://doi.org/10.1111/ced.15116.
Grants
Project/Grant Title
Investigating the benefits and harms of reduced daily dose oral isotretinoin in the treatment of acne: A parallel group, assessor blind, non-inferiority, multicentre randomised controlled trial, with an internal pilot (Acne-ID)
Developing and testing an online intervention to support self-management, improve outcomes and reduce antibiotic use in acne
The Autism Depression Trial-2 (ADEPT-2)
Lead Local Investigator Professor Andrew Thompson (Cardiff University; co-applicant) Professor Andrew Thompson (Cardiff University; co-applicant)
Dr Rona Aldridge
Please note: all grants, publications, research impact case studies or initiatives have been provided by Clinical Board and Directorate R&D leads. Therefore, there may be further grants, publications, case studies or initiatives not mentioned in this report.
Duration of Grant (Date from/to) September 2023September 2028 March 2022 –February 2027 September 2021December 2024 Funder Name/ Source of Funding NIHR HTA Programme PGfAR NIHR HTA programme grant National Institute for Health Research Health Technology Assessment Programme Grant Awarded (Total) £2,142,454.06 £1.901,577.00 £74,348.00 119
Appendix F - Mental Health Clinical Board
Appendix F - Mental Health Clinical Board
Non-Commercial
Non-Commercial
52
Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Collaborating Directorates Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitment total RAG NCMH 34524 155838 8754 NonCommercial FALSE Observational Open To Recruitment Mental Health Adult Mental Health (Incl. Addictions) Clinical Gerontology; 02/02/2017 31/03/2025 200 59 295 G NCISH 5655 185221 8005 NonCommercial FALSE Observational Open To Recruitment Mental Health Adult Mental Health (Incl. Addictions) 24/07/2012 31/03/2024 1 23 223 G Exploration of the relationship between social cognition and PTSD 43417 263222 13704 NonCommercial FALSE Observational Open To Recruitment Mental Health Psychology & Counselling 07/11/2019 31/10/2023 35 15 55 G TULIPS 42145 264686 14033 NonCommercial FALSE Both Closed to Recruitment, No Follow Up Mental Health Adult Mental Health (Incl. Addictions) 23/11/2021 31/12/2022 31/12/2022 31 9 31 G COVID-CNS 48229 313104 15864 NonCommercial FALSE Observational Closed to Recruitment, In Follow Up Mental Health Adult Mental Health (Incl. Addictions) 25/10/2021 01/09/2022 21/10/2022 30 8 30 G ADEPT-2 52734 310263 16402 NonCommercial FALSE Both Open To Recruitment Mental Health Psychology & Counselling 27/07/2022 31/12/2023 35 26 26 G VC versus FTF EMDR for PTSD 54173 301730 16326 NonCommercial FALSE Interventional Suspended Mental Health Adult Mental Health (Incl. Addictions) 01/11/2022 01/12/2023 12 5 5 S ADEPP 47000 279574 15415 NonCommercial FALSE Both Open To Recruitment Mental Health Adult Mental Health (Incl. Addictions) 30/06/2022 31/12/2023 5 4 4 G ATP 39882 248987 13784 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Mental Health Psychology & Counselling 11/05/2021 31/12/2023 07/06/2022 25 0 4 R Service user & staff views on digital remote monitoring for psychosis 53054 305509 16475 NonCommercial FALSE Observational Open To Recruitment Mental Health Adult Mental Health (Incl. Addictions) 01/03/2023 01/11/2023 4 4 4 G WAND-P 41814 250777 12579 NonCommercial FALSE Observational Open To Recruitment Mental Health Adult Mental Health (Incl. Addictions) 13/02/2019 02/10/2023 17 0 1 R Social Environment and Early Psychosis: An Urban Mind Study 44991 278399 16724 NonCommercial FALSE Observational Open To Recruitment Mental Health Adult Mental Health (Incl. Addictions) 28/02/2023 31/07/2023 12 0 0 R 12 153 678
120
Appendix F - Mental Health Clinical Board
Appendix F - Mental Health Clinical Board
53 Commercial Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitment total RAG MAPS 46660 260801 15096 Commercial FALSE Interventional Open To Recruitm ent Mental Health Adult Mental Health (Incl. Addictions) 13/12/2022 31/05/2023 2 0 0 R 1 0 0 Commercial 121
Appendix G - Primary, Community & Intermediate Care (PCIC) Clinical Board
Palliative Care Publications
Byrne, A., Torrens-Burton, A., Sivell, S., Moraes, F.Y., Bulbeck, H., Bernstein, M., Nelson, A. and Fielding, H. (2022). Early palliative interventions for improving outcomes in people with a primary malignant brain tumour and their carers. Cochrane Database of Systematic Reviews, 2022(1). doi: https://doi.org/10.1002/14651858.cd013440.pub2.
Gale, N., Hopkinson, J., Wasley, D. and Byrne, A. (2023). The promotion of homebased physical activity for people with lung cancer and cachexia, a qualitative study of healthcare professionals, patients and carers. Journal of Cancer Survivorship, 17, pp.677–685. doi: https://doi.org/10.1007/s11764-02301376-3.
Graham-Wisener, L., Nelson, A., Byrne, A., Islam, I., Harrison, C., Geddis, J. and Berry, E. (2022). Understanding public attitudes to death talk and advance care planning in Northern Ireland using health behaviour change theory: a qualitative study. BMC Public Health, 22(1). doi: https://doi. org/10.1186/s12889-022-13319-1.
Hussain, J.A., White, I.R., Johnson, M.J., Byrne, A., Preston, N.J., Haines, A., Seddon, K. and Peters, T.J. (2022). Development of guidelines to reduce, handle and report missing data in palliative care trials: A multi-stakeholder modified nominal group technique. Palliative Medicine, 36(1), pp.59–70. doi: https://doi.org/10.1177/02692163211065597.
Millward, C.P., Armstrong, T.S., Barrington, H., Brodbelt, A., Bulbeck, H., Byrne, A., Dirven, L., Gamble, C., Grundy, P.E., Islim, A.I., Javadpour, M., Keshwara, S.M., Krishna, S., Mallucci, C., Marson, A.G., McDermott, M.W., Meling, T.R., Oliver, K., Pizer, B. and Plaha, P. (2022). Opportunities and challenges for the development of ‘core outcome sets’ in neuro-oncology. Neuro-Oncology, 24(7), pp.1048–1055. doi: https://doi.org/10.1093/neuonc/noac062.
Retzer, A., Sivell, S., Scott, H., Nelson, A., Bulbeck, H., Seddon, K., Grant, R., Adams, R., Watts, C., Aiyegbusi, O.L., Kearns, P., Cruz Rivera, S., Dirven, L., Baddeley, E., Calvert, M. and Byrne, A. (2022). Development of a core outcome set and identification of patient-reportable outcomes for primary brain tumour trials: protocol for the COBra study. British Medical Journal Open, 12(9), pp.e057712–e057712. doi: https://doi.org/10.1136/bmjopen-2021-057712.
Selman, L.E., Sutton, E., Medeiros Mirra, R., Stone, T., Gilbert, E., Rolston, Y., Murray, K., Longo, M., Seddon, K., Penny, A., Mayland, C.R., Wakefield, D., Byrne, A. and Harrop, E. (2022). ‘Sadly I think we are sort of still quite white, middle-class really’ – Inequities in access to bereavement support: Findings from a mixed methods study. Palliative Medicine, 37(4), pp.586–601. doi: https://doi. org/10.1177/02692163221133665.
122
Appendix G - Primary, Community & Intermediate Care (PCIC) Clinical Board
Grants
Project Official Short/Full Title
Marie Curie Research Centre Cardiff Core Grant
Marie Curie Palliative Care Research Centre Transitional Core grant Patient Reported Core Outcomes in Brain Tumour Trials: the COBra study
Supporting people bereaved during COVID-19: a mixed methods study of bereaved people’s experiences and the bereavement services supporting them
Lead Investigator
Dr Anthony Byrne (co-applicant)
Dr Anthony Byrne
of Grant (Date from/to) 2023-2028 Grant Awarded (Total) £3,169,980 £468,815 £152,949 £355,328
Duration Funder Name/ Source of Funding Marie Curie Marie Curie The Brain Tumour Charity UKRI/ Economic and Social Research Council
Please note: all grants, publications, research impact case studies or initiatives have been provided by Clinical Board and Directorate R&D leads. Therefore, there may be further grants, publications, case studies or initiatives not mentioned in this report.
123
Dr Anthony Byrne Dr Anthony Byrne (coapplicant) 2022-2023 2020-2022 2020-2022
Appendix G - Primary, Community & Intermediate Care (PCIC) Clinical Board Appendix G - Primary, Community & Intermediate Care (PCIC) Clinical Board
Non-Commercial
Non-Commercial
56
Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Collaborating Directorates Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitment total RAG Using Primary Care to Tackle Domestic Violence and Abuse (DVA) 51138 302611 16748 NonCommercial FALSE Both Open To Recruitment Primary, Community & Intermediate Care Primary Care 21/11/2022 0 465 465 P CAP Trial - CNRS 8830 196036 10049 NonCommercial FALSE Both Open To Recruitment Primary, Community & Intermediate Care Primary Care 31/01/2006 31/12/2022 325 0 325 P HEAR 2 Interpretation services for Refugees & Asylum Seekers 50948 290877 15888 NonCommercial FALSE Observational Open To Recruitment Primary, Community & Intermediate Care Primary Care Internal Medicine; 01/05/2022 30/09/2022 200 192 192 A Positive Voices 14460 108483 9051 NonCommercial FALSE Both Closed to Recruitment, No Follow Up Primary, Community & Intermediate Care Primary Care 27/05/2014 31/03/2023 31/03/2023 50 23 126 G PRIMUS 36076 223941 10396 NonCommercial FALSE Both Open To Recruitment Primary, Community & Intermediate Care Primary Care 11/04/2018 31/03/2023 121 1 98 P Active Brains Study 44571 273507 14939 NonCommercial FALSE Interventional Open To Recruitment Primary, Community & Intermediate Care Primary Care 04/11/2021 28/02/2027 30 1 76 P PRINCIPLE 45457 281958 15131 NonCommercial FALSE Interventional Completed Primary, Community & Intermediate Care Primary Care 07/05/2020 31/03/2022 11/01/2023 1 4 73 P ATTACK 37100 228831 12480 NonCommercial FALSE Interventional Open To Recruitment Primary, Community & Intermediate Care Primary Care 21/06/2022 0 17 17 P DEVA 42794 269405 15073 NonCommercial FALSE Interventional Open To Recruitment Primary, Community & Intermediate Care Primary Care 06/04/2022 30/06/2023 10 10 10 G T2T 42053 263669 15662 NonCommercial FALSE Interventional Open To Recruitment Primary, Community & Intermediate Care Primary Care 28/07/2021 5 3 10 P CRaFT 41117 254366 13495 NonCommercial FALSE Observational Closed to Recruitment, No Follow Up Primary, Community & Intermediate Care Primary Care 04/11/2019 31/12/2022 31/05/2022 225 2 8 P
124
Appendix G - Primary, Community & Intermediate Care (PCIC) Clinical Board
Appendix G - Primary, Community & Intermediate Care (PCIC) Clinical Board
57 Commercial Understanding the role and work of paramedics in primary care 52138 308242 16317 NonCommercial FALSE Observational Open To Recruitment Primary, Community & Intermediate Care Primary Care 01/05/2022 0 4 4 P The Nuclear Community Charity Fund Chromosomal study 33847 215695 12269 NonCommercial FALSE Observational Suspended Primary, Community & Intermediate Care Primary Care 26/04/2018 30/09/2019 2 0 0 P Time Credits as a Social Prescription 44837 268253 14940 NonCommercial FALSE Interventional Closed to Recruitment, No Follow Up Primary, Community & Intermediate Care Primary Care 08/10/2020 30/07/2021 20/05/2022 187 0 0 P 14 722 1404 Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitment total RAG PIONEER REAL 47435 291357 15759 Commercial FALSE Observational Open To Recruitment Primary, Community & Intermediate Care Primary Care 02/06/2021 01/03/2022 10 13 28 P FLOW (NN9535-4321) 39317 257987 13533 Commercial FALSE Interventional Open To Recruitment Primary, Community & Intermediate Care Primary Care 01/07/2019 31/10/2020 6 0 5 P Observational study in atrial fibrillation (AF) patients at high risk 53313 316317 16572 Commercial FALSE Observational Open To Recruitment Primary, Community & Intermediate Care Primary Care 08/02/2023 0 2 2 P Maximising Adherence and Gaining New Information For Your COPD v1 42625 260690 16553 Commercial FALSE Observational Open To Recruitment Primary, Community & Intermediate Care Primary Care 0 1 1 P 4 16 36
57 Commercial Understanding the role and work of paramedics in primary care 52138 308242 16317 NonCommercial FALSE Observational Open To Recruitment Primary, Community & Intermediate Care Primary Care 01/05/2022 0 4 4 P The Nuclear Community Charity Fund Chromosomal study 33847 215695 12269 NonCommercial FALSE Observational Suspended Primary, Community & Intermediate Care Primary Care 26/04/2018 30/09/2019 2 0 0 P Time Credits as a Social Prescription 44837 268253 14940 NonCommercial FALSE Interventional Closed to Recruitment, No Follow Up Primary, Community & Intermediate Care Primary Care 08/10/2020 30/07/2021 20/05/2022 187 0 0 P 14 722 1404 Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitment total RAG PIONEER REAL 47435 291357 15759 Commercial FALSE Observational Open To Recruitment Primary, Community & Intermediate Care Primary Care 02/06/2021 01/03/2022 10 13 28 P FLOW (NN9535-4321) 39317 257987 13533 Commercial FALSE Interventional Open To Recruitment Primary, Community & Intermediate Care Primary Care 01/07/2019 31/10/2020 6 0 5 P Observational study in atrial fibrillation (AF) patients at high risk 53313 316317 16572 Commercial FALSE Observational Open To Recruitment Primary, Community & Intermediate Care Primary Care 08/02/2023 0 2 2 P Maximising Adherence and Gaining New Information For Your COPD v1 42625 260690 16553 Commercial FALSE Observational Open To Recruitment Primary, Community & Intermediate Care Primary Care 0 1 1 P 4 16 36
Primary, Community
Intermediate Care
Clinical Board Commercial 125
Appendix G -
&
(PCIC)
Appendix H - Specialist Services Directorate: ALAS (inc. Rehabilitation Engineering)
Publications
Green, A.J. (2022). Establishing and Improving compliance with the Pressure Management Pathway within a South Wales Posture and Mobility Service; a clinical audit. MSc Dissertation.
James, A.J. (2022). The application of tilt-in-space and backrest recline for tissue viability in wheelchair users: An evidence-based review. MSc Dissertation.
McGinty, G. (2022). The prevalence of musculoskeletal disorders amongst carers who push wheelchair users in South Wales. MSc Dissertation.
Wentworth, S.D. (2023). An investigation into an objective measure of muscle health: can electrical impedance myography measurements inform clinical decision making. DClinSci Thesis.
Please note: all grants, publications, research impact case studies or initiatives have been provided by Clinical Board and Directorate R&D leads. Therefore, there may be further grants, publications, case studies or initiatives not mentioned in this report.
126
Appendix H - Specialist Services Directorate:
CARDIAC SERVICES
Publications
Adlam, D., Zarebinski, M., Uren, N.G., Ptaszynski, P., Oldroyd, K.G., Munir, S., Zaman, A., Contractor, H., Kiss, R.G., Édes, I., Szachniewicz, J., Nagy, G.G., Garcia, M.J., Tomcsanyi, J., Irving, J., Sharp, A.S.P., Musialek, P., Lupkovics, G., Shirodaria, C. and Selvanayagam, J.B. (2022). A Randomized, double-blind, dose ranging clinical trial of intravenous FDY-5301 in acute STEMI patients undergoing primary PCI. International Journal of Cardiology, 347, pp.1–7. doi: https://doi.org/10.1016/j.ijcard.2021.11.016.
Ali, Z.A. and Sharp, A.S.P. (2022). The Bigger the Highway, the Less Likely the Traffic Jam. Circulation: Cardiovascular Interventions, 15(9). doi: https://doi.org/10.1161/circinterventions.122.012368.
Artico, J., Shiwani, H., Moon, J.C., Gorecka, M., McCann, G.P., Roditi, G., Morrow, A., Mangion, K., Lukaschuk, E., Shanmuganathan, M., Miller, C.A., Chiribiri, A., Prasad, S.K., Adam, R.D., Singh, T., Bucciarelli-Ducci, C., Dawson, D., Knight, D., Fontana, M. and Manisty, C. (2023). Myocardial Involvement After Hospitalization for COVID-19 Complicated by Troponin Elevation: A Prospective, Multicenter, Observational Study. Circulation, 147(5), pp.364–374. doi: https://doi.org/10.1161/ CIRCULATIONAHA.122.060632.
Atkinson, C., Hughes, S., Richards, L., Sim, V.M., Phillips, J., John, I.J. and Yousef, Z. (2022). Palliation of heart failure: value-based supportive care. BMJ Supportive & Palliative Care. doi: https://doi. org/10.1136/bmjspcare-2021-003378.
Azizi, M., Mahfoud, F., Weber, M.A., Sharp, A., Schmieder, R.E., Lurz, P., Lobo, M.D., Fisher, N.D.L., Daemen, J., Bloch, M.J., Basile, J., Sanghvi, K., Saxena, M., Gosse, P., Jenkins, J.S., Levy, T., Persu, A., Kably, B., Claude, L. and Reeve-Stoffer, H. (2022). Effects of Renal Denervation vs Sham in Resistant Hypertension After Medication Escalation. JAMA Cardiology, 7(12), pp.1244–1244. doi: https://doi. org/10.1001/jamacardio.2022.3904.
Azizi, M., Saxena, M., Wang, Y., Jenkins, J.S., Devireddy, C., Rader, F., Fisher, N.D.L., Schmieder, R.E., Mahfoud, F., Lindsey, J., Sanghvi, K., Todoran, T.M., Pacella, J., Flack, J., Daemen, J., Sharp, A.S.P., Lurz, P., Bloch, M.J., Weber, M.A. and Lobo, M.D. (2023). Endovascular Ultrasound Renal Denervation to Treat Hypertension: The RADIANCE II Randomized Clinical Trial. JAMA, 329(8), pp.651–661. doi: https://doi.org/10.1001/jama.2023.0713.
Azizi, M., Saxena, M., Wang, Y., Jenkins, J.S., Devireddy, C., Rader, F., Fisher, N.D.L., Schmieder, R.E., Mahfoud, F., Lindsey, J., Sanghvi, K., Todoran, T.M., Pacella, J., Flack, J., Daemen, J., Sharp, A.S.P., Lurz, P., Bloch, M.J., Weber, M.A. and Lobo, M.D. (2023). Endovascular Ultrasound Renal Denervation to Treat Hypertension: The RADIANCE II Randomized Clinical Trial. JAMA, 329(8), pp.651–661. doi: https://doi.org/10.1001/jama.2023.0713.
Banning, A.P., Serruys, P., De Maria, G.L., Ryan, N., Walsh, S., Gonzalo, N., Jan van Geuns, R., Onuma, Y., Sabate, M., Davies, J., Lesiak, M., Moreno, R., Cruz-Gonzalez, I., Hoole, S.P., Piek, J.J., Appleby, C., Fath-Ordoubadi, F., Zaman, A., Van Mieghem, N.M. and Uren, N. (2022). Five-year outcomes after state-of-the-art percutaneous coronary revascularization in patients with de novo three-vessel disease: final results of the SYNTAX II study. European Heart Journal, 43(13), pp.1307–1316. doi: https://doi.org/10.1093/eurheartj/ehab703.
Barbato, E., Azizi, M., Schmieder, R.E., Lauder, L., Böhm, M., Brouwers, S., Maria Bruno, R., Dudek, D., Kahan, T., Kandzari, D.E., Lüscher, T.F., Parati, G., Pathak, A., Ribichini, F.L., Schlaich, M.P., Sharp, A.S.P., Sudano, I., Volpe, M., Tsioufis, C. and Wijns, W. (2023). Renal denervation in the management of hypertension in adults. A clinical consensus statement of the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). European Heart Journal, 44(15), pp.1313–1330. doi: https://doi.org/10.1093/eurheartj/ehad054.
127
Appendix H - Specialist Services Directorate: CARDIAC SERVICES
Bourke, J.P., Turner, C., Bradlow, W., Chikermane, A., Coats, C., Fenton, M., Ilina, M., Johnson, A., Kapetanakis, S., Kuhwald, L., Morley-Davies, A., Quinlivan, R., Savvatis, K., Schiava, M., Yousef, Z. and Guglieri, M. (2022). Cardiac care of children with dystrophinopathy and females carrying DMD-gene variations. Open Heart, 9(2), pp.e001977–e001977. doi: https://doi.org/10.1136/openhrt-2022-001977. Das, A., Kelly, C., Teh, I., Nguyen, C., Brown, L.A.E., Chowdhary, A., Jex, N., Thirunavukarasu, S., Sharrack, N., Gorecka, M., Swoboda, P.P., Greenwood, J.P., Kellman, P., Moon, J.C., Davies, R.H., Lopes, L.R., Joy, G., Plein, S., Schneider, J.E. and Dall’Armellina, E. (2022). Phenotyping hypertrophic cardiomyopathy using cardiac diffusion magnetic resonance imaging: the relationship between microvascular dysfunction and microstructural changes. European Heart Journal - Cardiovascular Imaging, 23(3), pp.352–362. doi: https://doi.org/10.1093/ehjci/jeab210.
Davies, R.H., Augusto, J.B., Bhuva, A., Xue, H., Treibel, T.A., Ye, Y., Hughes, R.K., Bai, W., Lau, C., Shiwani, H., Fontana, M., Kozor, R., Herrey, A., Lopes, L.R., Maestrini, V., Rosmini, S., Petersen, S.E., Kellman, P., Rueckert, D. and Greenwood, J.P. (2022). Precision measurement of cardiac structure and function in cardiovascular magnetic resonance using machine learning. Journal of Cardiovascular Magnetic Resonance, 24(1). doi: https://doi.org/10.1186/s12968-022-00846-4.
De Filippo, O., Cortese, M., ascenzoF.D., Raposeiras-Roubín, S., Abu-Assi, E., Kinnaird, T., ArizaSolé, A., Manzano-Fernández, S., Templin, C., Velicki, L., Xanthopoulou, I., Cerrato, E., Rognoni, A., Boccuzzi, G., Montefusco, A., Montabone, A., Taha, S., Durante, A., Gili, S. and Magnani, G. (2019). Real-World Data of Prasugrel vs. Ticagrelor in Acute Myocardial Infarction: Results from the RENAMI Registry. American Journal of Cardiovascular Drugs, 19(4), pp.381–391. doi: https://doi.org/10.1007/ s40256-019-00339-3.
Domínguez-Rodríguez, L.M., Raposeiras-Roubín, S., Abu-Assi, E., Cespón-Fernández, M., MelendoViu, M., D’Ascenzo, F., Kinnaird, T., Ariza-Solé, A., Manzano-Fernández, S., Templin, C., Velicki, L., Xanthopoulou, I., Cerrato, E., Quadri, G., Rognoni, A., Boccuzzi, G., Montabone, A., Taha, S., Durante, A. and Gili, S. (2022). Ischemic-hemorrhagic balance in diabetic and non-diabetic patients after acute coronary syndrome. REC: CardioClinics, 57(3), pp.203–211. doi: https://doi.org/10.1016/j. rccl.2022.02.006.
Evans, M., Lewis, R.D., Morgan, A.R., Whyte, M.B., Hanif, W., Bain, S.C., Davies, S., Dashora, U., Yousef, Z., Patel, D.C. and Strain, W.D. (2022). A Narrative Review of Chronic Kidney Disease in Clinical Practice: Current Challenges and Future Perspectives. Advances in Therapy, 39(1), pp.33–43. doi: https://doi.org/10.1007/s12325-021-01927-z.
Evans, M., Morgan, A.R., Bain, S.C., Davies, S., Hicks, D., Brown, P., Yousef, Z., Dashora, U., Viljoen, A., Beba, H. and Strain, W.D. (2022). Meeting the Challenge of Virtual Diabetes Care: A Consensus Viewpoint on the Positioning and Value of Oral Semaglutide in Routine Clinical Practice. Diabetes Therapy, 13(2), pp.225–240. doi: https://doi.org/10.1007/s13300-021-01201-z.
Evans, M., Morgan, A.R., Patel, D., Dhatariya, K., Greenwood, S., Newland-Jones, P., Hicks, D., Yousef, Z., Moore, J., Kelly, B., Davies, S. and Dashora, U. (2020). Risk Prediction of the Diabetes Missing Million: Identifying Individuals at High Risk of Diabetes and Related Complications. Diabetes Therapy, 12(1), pp.87–105. doi: https://doi.org/10.1007/s13300-020-00963-2.
128
Appendix H - Specialist Services Directorate: CARDIAC SERVICES
Evans, M., Morgan, A.R., Whyte, M.B., Hanif, W., Bain, S.C., Kalra, P.A., Davies, S., Dashora, U., Yousef, Z., Patel, D.C. and Strain, W.D. (2022). New Therapeutic Horizons in Chronic Kidney Disease: The Role of SGLT2 Inhibitors in Clinical Practice. Drugs, 82(2), pp.97–108. doi: https://doi.org/10.1007/s40265021-01655-2.
Fisher, N.D.L., Kirtane, A.J., Daemen, J., Rader, F., Lobo, M.D., Saxena, M., Abraham, J., Schmieder, R.E., Sharp, A.S.P., Gosse, P., Claude, L., Song, Y. and Azizi, M. (2022). Plasma renin and aldosterone concentrations related to endovascular ultrasound renal denervation in the RADIANCE-HTN SOLO trial. Journal of Hypertension, 40(2), pp.221–228. doi: https://doi.org/10.1097/hjh.0000000000002994.
Fraser, A.G., Biasin, E., Bijnens, B., Bruining, N., Caiani, E.G., Cobbaert, K., Davies, R.H., Gilbert, S.H., Hovestadt, L., Kamenjasevic, E., Kwade, Z., McGauran, G., O’Connor, G., Vasey, B. and Rademakers, F.E. (2023). Artificial intelligence in medical device software and high-risk medical devices – a review of definitions, expert recommendations and regulatory initiatives. Expert Review of Medical Devices, 20(6), pp.467–491. doi: https://doi.org/10.1080/17434440.2023.2184685.
Ganesananthan, S., Zahid, A., Choudhry, A., Vadiveloo, T.V., Khan, N., Yang, T., Urrehman, H., Mahesh, S. and Yousef, Z. (2022). The Utility and Educational Impact of a Virtual Webinar to Deliver an International Undergraduate Cardiovascular Conference. Advances in Medical Education and Practice, 13, pp.993–1002. doi: https://doi.org/10.2147/amep.s376114.
Goss, M., Morgan, H. and Kinnaird, T. (2022). Personalisation of DAPT: from ‘bench’ to bedside. Journal of Prescribing Practice, 4(1), pp.20–22. doi: https://doi.org/10.12968/jprp.2022.4.1.20.
Götzinger, F., Lauder, L., Sharp, A.S.P., Lang, I.M., Rosenkranz, S., Konstantinides, S., Edelman, E.R., Böhm, M., Jaber, W. and Mahfoud, F. (2023). Interventional therapies for pulmonary embolism. Nature Reviews Cardiology, pp.1–15. doi: https://doi.org/10.1038/s41569-023-00876-0.
Hooper, S.M., Wu, S., Davies, R.H., Bhuva, A., Schelbert, E.B., Moon, J., Kellman, P., Xue, H., Langlotz, C.P. and Ré, C. (2023). Evaluating semi-supervision methods for medical image segmentation: applications in cardiac magnetic resonance imaging. Journal of Medical Imaging, 10(02). doi: https:// doi.org/10.1117/1.jmi.10.2.024007.
Hughes, R., Augusto, J., Knott, K., Davies, R.H., Shiwani, H., Seraphim, A., Malcolmson, J., Khoury, S., Joy, G., Mohiddin, S.A., Rocha Lopes, L., McKenna, W.J., Kellman, P., Xue, H., Tome, M., Sharma, S., Captur, G. and Moon, J. (2023). Apical Ischemia Is a Universal Feature of Apical Hypertrophic Cardiomyopathy. Circulation: Cardiovascular Imaging, 16(3), pp.e014907. doi: https://doi.org/10.1161/ circimaging.122.014907.
Jordan, A.N., Anning, C., Wilkes, L., Ball, C., Pamphilon, N., Clark, C.E., Bellenger, N.G., Shore, A.C., Sharp, A.S.P. and Valderas, J.M. (2022). Cross-cultural adaptation of the Spanish MINICHAL instrument into English for use in the United Kingdom. Health and Quality of Life Outcomes, 20(1), pp.1–12. doi: https://doi.org/10.1186/s12955-022-01943-9.
Jubouri, M., Al-Tawil, M.M., Yip, H.Y., Bashir, A.K., Tan, P., Bashir, M., Anderson, R.A., Bailey, D.M., Nienaber, C.A., Coselli, J.S. and Williams, I.D. (2022). Mid- and long-term outcomes of thoracic endovascular aortic repair in acute and subacute uncomplicated type B aortic dissection. Journal of Cardiac Surgery, 37(5), pp.1328–1339. doi: https://doi.org/10.1111/jocs.16349.
129
Appendix H - Specialist Services Directorate: CARDIAC SERVICES
Kandzari, D.E., Mahfoud, F., Weber, M.A., Townsend, R.R., Parati, G., Fisher, N.D.L., Lobo, M.D., Bloch, M.H., Böhm, M., Sharp, A.J., Schmieder, R.E., Azizi, M., Schlaich, M.P., Papademetriou, V., Kirtane, A.J., Daemen, J., Pathak, A., Ukena, C., Lurz, P. and Grassi, G. (2022). Clinical Trial Design Principles and Outcomes Definitions for Device-Based Therapies for Hypertension: A Consensus Document From the Hypertension Academic Research Consortium. Circulation, 145(11), pp.847–863. doi: https://doi. org/10.1161/circulationaha.121.057687.
Kinnaird, T., Gallagher, S., Farooq, V., Protty, M., Back, L., Devlin, P., Anderson, R., Sharp, A., Ludman, P., Copt, S., Mamas, M.A. and Curzen, N. (2023). Temporal Trends in In-Hospital Outcomes Following Unprotected Left-Main Percutaneous Coronary Intervention: An Analysis of 14 522 Cases From British Cardiovascular Intervention Society Database 2009 to 2017. Circulation: Cardiovascular Interventions, 16(1). doi: https://doi.org/10.1161/circinterventions.122.012350.
Kirtane, A.J., Sharp, A., Mahfoud, F., Fisher, N.D.L., Schmieder, R.E., Daemen, J., Lobo, M.D., Lurz, P., Basile, J., Bloch, M.J., Weber, M.A., Saxena, M., Wang, Y., Sanghvi, K., Jenkins, J.S., Devireddy, C., Rader, F., Gosse, P., Sapoval, M. and Barman, N.C. (2023). Patient-Level Pooled Analysis of Ultrasound Renal Denervation in the Sham-Controlled RADIANCE II, RADIANCE-HTN SOLO, and RADIANCE-HTN TRIO Trials. JAMA Cardiology, 8(5), pp.464–473. doi: https://doi.org/10.1001/jamacardio.2023.0338.
Klok, F.A., Piazza, G., Sharp, A.J., Áinle, F.N., Jaff, M.R., Chauhan, N., Patel, B., Barco, S., Goldhaber, S.Z., Kucher, N., Lang, I.M., Schmidtmann, I., Sterling, K.M., Becker, D., Martin, N., Rosenfield, K. and Konstantinides, S. (2022). Ultrasound-facilitated, catheter-directed thrombolysis vs anticoagulation alone for acute intermediate-high-risk pulmonary embolism: Rationale and design of the HI-PEITHO study. American Heart Journal, 251, pp.43–53. doi: https://doi.org/10.1016/j.ahj.2022.05.011.
Mahfoud, F., Kandzari, D.E., Kario, K., Townsend, R.R., Weber, M.A., Schmieder, R.E., Tsioufis, K., Pocock, S., Dimitriadis, K., Choi, J.W., East, C., D’Souza, R., Sharp, A.S.P., Ewen, S., Walton, A., Hopper, I., Brar, S., McKenna, P., Fahy, M. and Böhm, M. (2022). Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. The Lancet, 399(10333), pp.1401–1410. doi: https://doi.org/10.1016/S01406736(22)00455-X.
Millesimo, M., Elia, E., Marengo, G., De Filippo, O., Raposeiras-Roubín, S., Wańha, W., Abu-Assi, E., Kinnaird, T., Ariza-Solé, A., Liebetrau, C., Manzano-Fernández, S., Iannaccone, M., Henriques, J.P.S., Templin, C., Wilton, S.B., Velicki, L., Xanthopoulou, I., Correia, L., Cerrato, E. and Rognoni, A. (2022). Antithrombotic Strategy in Secondary Prevention for High-Risk Patients with Previous Acute Coronary Syndrome: Overlap between the PEGASUS Eligibility and the COMPASS Eligibility in a Large Multicenter Registry. American Journal of Cardiovascular Drugs, 23(1). doi: https://doi. org/10.1007/s40256-022-00554-5.
Mohamed, M., Kinnaird, T., Wijeysundera, H.C., Johnson, T., Zaman, S., Rashid, M., Moledina, S. and Ludman, P. (2022). Impact of Intracoronary Imaging-Guided Percutaneous Coronary Intervention on Procedural Outcomes Among Complex Patient Groups. Journal of the American Heart Association, 11(19). doi: https://doi.org/10.1161/jaha.122.026500.
Mohamed, M., Sirker, A., Chieffo, A., Avanzas, P., Nolan, J., Rashid, M., Dafaalla, M., Moledina, S., Ludman, P., Kinnaird, T. and Mamas, M.A. (2022). Temporal patterns, characteristics, and predictors of clinical outcomes in patients undergoing percutaneous coronary intervention for stent thrombosis. EuroIntervention, 18(9), pp.729–739. doi: https://doi.org/10.4244/eij-d-22-00049.
130
Appendix H - Specialist Services Directorate: CARDIAC SERVICES
Mullens, W., Auricchio, A., Martens, P., Witte, K., Cowie, M.R., Delgado, V., Dickstein, K., Linde, C., Vernooy, K., Leyva, F., Bauersachs, J., Israel, C.W., Lund, L.H., Donal, E., Boriani, G., Jaarsma, T., Berruezo, A., Traykov, V., Yousef, Z. and Kalarus, Z. (2020). Optimized implementation of cardiac resynchronization therapy: a call for action for referral and optimization of care: A joint position statement from the Heart Failure Association (HFA), European Heart Rhythm Association (EHRA), and European Association of Cardiovascular Imaging (EACVI) of the European Society of Cardiology. European Journal of Heart Failure, 22(12), pp.2349–2369. doi: https://doi.org/10.1002/ejhf.2046.
Papetti, D.M., Van Abeelen, K., Davies, R.H., Menè, R., Heilbron, F., Perelli, F., Artico, J., Seraphim, A., Moon, J., Parati, G., Xue, H., Kellman, P., Badano, L.P., Besozzi, D., Nobile, M.S. and Torlasco, C. (2023). An accurate and time-efficient deep learning-based system for automated segmentation and reporting of cardiac magnetic resonance-detected ischemic scar. Computer Methods and Programs in Biomedicine, 229, pp.107321–107321. doi: https://doi.org/10.1016/j.cmpb.2022.107321.
Patel, M.R., Jeremias, A., Maehara, A., Matsumura, M., Zhang, Z., Schneider, J.E., Tang, K., Talwar, S., Marques, K., Shammas, N.W., Gruberg, L., Seto, A.H., Samady, H., Sharp, A., Ali, Z., Mintz, G.S., Davies, J.E. and Stone, G.W. (2022). 1-Year Outcomes of Blinded Physiological Assessment of Residual Ischemia After Successful PCI: DEFINE PCI Trial. JACC Cardiovascular Interventions, 15(1), pp.52–61. doi: https://doi.org/10.1016/j.jcin.2021.09.042.
Patti, G., D’Ascenzo, F., De Filippo, O., Bruno, F., Leonardi, S., Chieffo, A., Iannaccone, M., Liebetrau, C., Manzano-Fernández, S., Gallone, G., Omedè, P., Cerrato, E., Kinnaird, T., Conrotto, F., Piroli, F., Henriques, J.P.S., Wańha, W., Elia, E., Dominguez-Rodriguez, A. and Raposeiras-Roubin, S. (2022). Safety and efficacy of different P2Y12 inhibitors in patients with acute coronary syndromes stratified by the PRAISE risk score: a multicentre study. European Heart Journal - Quality of Care and Clinical Outcomes, 8(1), pp.881–891. doi: https://doi.org/10.1093/ehjqcco/qcac002.
Protty, M., Gallagher, S., Sharp, A., Farooq, V., Egred, M., O’Kane, P., Ludman, P., Mamas, M.A. and Kinnaird, T. (2022). The Impact of Intracoronary Imaging on PCI Outcomes in Cases Utilising Rotational Atherectomy: An Analysis of 8,417 Rotational Atherectomy Cases from the British Cardiovascular Intervention Society Database. Journal of Interventional Cardiology, 2022, pp.1–9. doi: https://doi. org/10.1155/2022/5879187.
Protty, M., Gallagher, S., Sharp, A.S., Anderson, R., Ludman, P., Curzen, N., Nolan, J., Rashid, M., Mamas, M. and Kinnaird, T. (2022). Brachial arterial access for PCI: an analysis of the British Cardiovascular Intervention Society database. EuroIntervention, 17(13), pp.1100–1103. doi: https://doi.org/10.4244/ eij-d-21-00294.
Protty, M., Hussain, H.I., Gallagher, S., Al-Raisi, S., Aldalati, O., Farooq, V., Sharp, A., Egred, M., O’Kane, P., Ludman, P., Anderson, R., Mamas, M.A. and Kinnaird, T. (2020). Excimer laser coronary atherectomy during complex PCI: An analysis of 1,471 laser cases from the British Cardiovascular Intervention Society database. Catheterization and Cardiovascular Interventions, 97(5), pp.E653–E660. doi: https://doi.org/10.1002/ccd.29251.
Protty, M., Sharp, A.S.P., Gallagher, S., Farooq, V., Spratt, J.C., Ludman, P., Anderson, R., McEntegart, M.M., Hanratty, C., Walsh, S., Curzen, N., Smith, E., Mamas, M. and Kinnaird, T. (2022). Defining Percutaneous Coronary Intervention Complexity and Risk. JACC: Cardiovascular Interventions, 15(1), pp.39–49. doi: https://doi.org/10.1016/j.jcin.2021.09.039.
131
Appendix H - Specialist Services Directorate: CARDIAC
SERVICES
Protty, M., Sharp, A.S.P., Gallagher, S., Farooq, V., Spratt, J.C., Ludman, P., Anderson, R., McEntegart, M.M., Hanratty, C., Walsh, S., Curzen, N., Smith, E., Mamas, M. and Kinnaird, T. (2022). Defining Percutaneous Coronary Intervention Complexity and Risk: An Analysis of the United Kingdom BCIS Database 2006-2016. JACC: Cardiovascular Interventions, 15(1), pp.39–49. doi: https://doi. org/10.1016/j.jcin.2021.09.039.
Pruszczyk, P., Klok, F.A., Kucher, N., Roik, M., Meneveau, N., Sharp, A.J., Nielsen-Kudsk, J.E., Obradović, S., Barco, S., Giannini, F., Stefanini, G.G., Tarantini, G., Konstantinides, S. and Dudek, D. (2022). Percutaneous treatment options for acute pulmonary embolism: a clinical consensus statement by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function and the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention, 18(8), pp.e623–e638. doi: https://doi.org/10.4244/eij-d-22-00246.
Rashid, M., Kinnaird, T., Ludman, P., Keeble, T.R., Mamas, M.A. and Curzen, N. (2022). Variation in practice for out-of-hospital cardiac arrest treated with percutaneous coronary intervention in England and Wales. Catheterization and Cardiovascular Interventions, 100(3), pp.306–316. doi: https://doi.org/10.1002/ccd.30316.
Rashid, M., Stevens, C.V., Zaman, S., Pinilla-Echeverri, N., Velagapudi, P., Chieffo, A., Shoaib, A., Ludman, P., Mills, N.L., Nolan, J., Kinnaird, T. and Mamas, M.A. (2022). Sex Differences in Use of Intracoronary Imaging in Patients Undergoing Percutaneous Coronary Intervention. Cardiovascular Interventions, 15(12), pp.1290–1292. doi: https://doi.org/10.1016/j.jcin.2022.03.034.
Rashid, M., Zaman, M., Ludman, P., Wijeysundera, H.C., Curzen, N., Kinnaird, T., Moledina, S., Abbott, J.D., Grines, C.L. and Mamas, M.A. (2022). Left Main Stem Percutaneous Coronary Intervention: Does On-Site Surgical Cover Make a Difference? Circulation: Cardiovascular Interventions, 15(10). doi: https://doi.org/10.1161/circinterventions.122.012037.
Rehman, A., Kellman, P., Xue, H., Pierce, I., Davies, R.H., Fontana, M. and Moon, J. (2022). Convolutional neural network transformer (CNNT) for free-breathing real-time cine imaging. European Heart Journal-Cardiovascular Imaging, 23(Supplement_2). doi: https://doi.org/10.1093/ehjci/jeac141.001.
Reynolds, C.J., Pade, C., Gibbons, J.M., Otter, A.D., Lin, K.-M., Muñoz Sandoval, D., Pieper, F.P., Butler, D.K., Liu, S., Joy, G., Forooghi, N., Treibel, T.A., Manisty, C., Moon, J.C., Semper, A., Brooks, T., McKnight, Á., Altmann, D.M., Boyton, R.J. and Abbass, H. (2022). Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Science, 377(6603). doi: https://doi.org/10.1126/science. abq1841.
Routledge, H., Sharp, A.S., Kovac, J., Westwood, M., Keeble, T.R., Bathula, R., Eteiba, H., Grunwald, I.Q. and Curzen, N. (2022). Can Interventional Cardiologists Help Deliver the UK Mechanical Thrombectomy Interventional Programme for Patients with Acute Ischaemic Stroke? A Discussion Paper from the British Cardiovascular Interventional Society Stroke Thrombectomy Focus Group. Interventional Cardiology: Reviews, Research, Resources, 17. doi: https://doi.org/10.15420/icr.2021.35.
Sato, Y., Sharp, A., Mahfoud, F., Tunev, S., Forster, A., Ellis, M., Gómez, A.M., Dhingra, R., Ullman, J., Schlaich, M., Lee, D., Trudel, J., Hettrick, D.A., Kandzari, D.E., Virmani, R. and Finn, A.V. (2023). Translational value of preclinical models for renal denervation: a histological comparison of human versus porcine renal nerve anatomy. EuroIntervention, 18(13), pp.e1120–e1128. doi: https://doi. org/10.4244/eij-d-22-00369.
132
Appendix H - Specialist Services Directorate: CARDIAC SERVICES
Saxena, M., Schmieder, R.E., Kirtane, A.J., Mahfoud, F., Daemen, J., Basile, J., Lurz, P., Gosse, P., Sanghvi, K., Fisher, N.D.L., Rump, L.C., Pathak, A., Blankestijn, P.J., Mathur, A., Wang, Y., Weber, M.A., Sharp, A.S.P., Bloch, M.J., Barman, N.C. and Claude, L. (2022). Predictors of blood pressure response to ultrasound renal denervation in the RADIANCE-HTN SOLO study. Journal of Human Hypertension, 36(7), pp.629–639. doi: https://doi.org/10.1038/s41371-021-00547-y.
Seraphim, A., Dowsing, B., Rathod, K.S., Shiwani, H., Patel, K., Knott, K., Zaman, S., Johns, I., Razvi, Y., Patel, R., Xue, H., Jones, D.A., Fontana, M., Cole, G., Uppal, R., Davies, R.H., Moon, J., Kellman, P. and Manisty, C. (2022). Quantitative Myocardial Perfusion Predicts Outcomes in Patients With Prior Surgical Revascularization. Journal of the American College of Cardiology, 79(12), pp.1141–1151.
doi: https://doi.org/10.1016/j.jacc.2021.12.037.
Shamkhani, W., Kinnaird, T., Ludman, P., Rashid, M. and Mamas, M.A. (2022). Sex differences in high-risk but indicated coronary interventions (CHiP): National report from British Cardiovascular Intervention Society Registry. Catheterization and Cardiovascular Interventions, 99(2), pp.447–456.
doi: https://doi.org/10.1002/ccd.30081.
Shamkhani, W., Kinnaird, T., Wijeysundera, H.C., Ludman, P., Rashid, M. and Mamas, M.A. (2022). Ethnicity in Complex High-Risk but Indicated Percutaneous Coronary Intervention Types and Outcomes. The American Journal of Cardiology, 175, pp.26–37. doi: https://doi.org/10.1016/j. amjcard.2022.03.056.
Sharp, A.S.P., Tunev, S., Schlaich, M., Lee, D.P., Finn, A.V., Trudel, J., Hettrick, D.A., Mahfoud, F. and Kandzari, D.E. (2022). Histological evidence supporting the durability of successful radiofrequency renal denervation in a normotensive porcine model. Journal of Hypertension, 40(10), pp.2068–2075.
doi: https://doi.org/10.1097/HJH.0000000000003236.
Shoaib, A., Sharma, V., Spratt, J.C., Wilson, S., Hussain, S., Velagapudi, P., Siller-Matula, J., Rashid, M., Ludman, P., Cockburn, J., Kinnaird, T. and Mamas, M.A. (2023). Sex Differences in Clinical Profile and Outcome After Percutaneous Coronary Intervention for Chronic Total Occlusion. Cardiovascular Revascularization Medicine, 49, pp.34–41. doi: https://doi.org/10.1016/j.carrev.2022.12.005.
Shoaib, A., Spratt, J.C., Curzen, N., Wilson, S., Rashid, M., Ahmad, F., Ludman, P., Kinnaird, T. and Mamas, M.A. (2022). Clinical outcomes of percutaneous coronary intervention for chronic total occlusion by treated segment length. Catheterization and Cardiovascular Interventions, 99(2), pp.234–244. doi: https://doi.org/10.1002/ccd.30015.
Thornton, G., Musa, T.A., Rigolli, M., Loudon, M., Chin, C., Pica, S., Malley, T., Foley, J.R., Vassiliou, V.S., Davies, R.H., Captur, G., Dobson, L.E., Moon, J., Dweck, M.R., Myerson, S.G., Prasad, S., Greenwood, J.P., McCann, G.P. and Treibel, T.A. (2022). Association of Myocardial Fibrosis and Stroke Volume by Cardiovascular Magnetic Resonance in Patients With Severe Aortic Stenosis With Outcome After Valve Replacement: The British Society of Cardiovascular Magnetic Resonance AS700 Study. JAMA Cardiology, 7(5), pp.513–520. doi: https://doi.org/10.1001/jamacardio.2022.0340.
van Geuns, R.-J., Chang, C.-C., McEntegart, M., Меркулов, Е.В., Kretov, E., Lesiak, M., O’Kane, P., Hanratty, C.G., Bressollette, E., Silvestri, M., Wlodarczak, A., Barragan, P., Anderson, R., Protopopov, A., Peace, A., Menown, I., Rocchiccioli, P., Onuma, Y. and Oldroyd, K.G. (2022). Bioabsorbable polymer drug-eluting stents with 4-month dual antiplatelet therapy versus durable polymer drug-eluting stents with 12-month dual antiplatelet therapy in patients with left main coronary artery disease: the IDEAL-LM randomised trial. EuroIntervention, 17(18), pp.1467–1476. doi: https://doi.org/10.4244/ eij-d-21-00514.
133
Appendix H - Specialist Services Directorate: CARDIAC SERVICES
Von Oppell, U., Szafranek, A. and Anderson, R. (2022). Cardiac surgery and percutaneous interventions in the elderly. Pathy’s Principles and Practice of Geriatric Medicine, 1, pp.455–481. doi: https://doi. org/10.1002/9781119484288.ch35.
Webber, M., Falconer, D., Alfarih, M., Joy, G., Chan, F., Davie, C., Howes, L., Wong, A., Rapala, A., Bhuva, A., Davies, R.H., Morton, C.L., Aguado-Sierra, J., Vázquez, M., Tao, X., Krausz, G., Tanackovic, S., Guger, C., Xue, H. and Kellman, P. (2022). Study protocol: MyoFit46—the cardiac sub-study of the MRC National Survey of Health and Development. BMC Cardiovascular Disorders, 22(1). doi: https:// doi.org/10.1186/s12872-022-02582-0.
Xue, H., Artico, J., Davies, R.H., Adam, R., Shetye, A., Augusto, J., Bhuva, A., Fröjdh, F., Wong, T.C., Fukui, M., Cavalcante, J.L., Treibel, T.A., Manisty, C., Fontana, M., Ugander, M., Moon, J., Schelbert, E.B. and Kellman, P. (2022). Automated In-Line Artificial Intelligence Measured Global Longitudinal Shortening and Mitral Annular Plane Systolic Excursion: Reproducibility and Prognostic Significance. Journal of the American Heart Association, 11(4). doi: https://doi.org/10.1161/jaha.121.023849.
Xue, H., Rehman, A., Davies, R.H., Moon, J., Fontana, M. and Kellman, P. (2022). CNNT DB-LGE: freebreathing dark blood late enhancement imaging using the convolutional neural network transformer speeds acquisition by 50%. European Heart Journal-Cardiovascular Imaging, 23(Supplement_2). doi: https://doi.org/10.1093/ehjci/jeac141.006.
Zaman, A., Prendergast, B., Hildick-Smith, D., Blackman, D.J., Anderson, R., Spence, M.S., Mylotte, D., Smith, D., Wilding, B., Chapman, C., Atkins, K., Pollock, K.G., Qureshi, A.C. and Banning, A.P. (2023a). An Update on Anti-thrombotic Therapy Following Transcatheter Aortic Valve Implantation: Expert Cardiologist Opinion from a UK and Ireland Delphi Group. Interventional Cardiology, 18(e13). doi: https://doi.org/10.15420/icr.2022.11.
Zaman, M., Stevens, C., Ludman, P., Wijeysundera, H.C., Siudak, Z., Sharp, A.S.P., Kinnaird, T., Mohamed, M.O., Ahmed, J.M., Rashid, M. and Mamas, M.A. (2023b). Intracoronary imaging in PCI for acute coronary syndrome: Insights from British Cardiovascular Intervention Society registry. Cardiovascular Revascularization Medicine. doi: https://doi.org/10.1016/j.carrev.2023.05.020.
Please note: all grants, publications, research impact case studies or initiatives have been provided by Clinical Board and Directorate R&D leads. Therefore, there may be further grants, publications, case studies or initiatives not mentioned in this report.
134
Appendix H - Specialist Services Directorate: CRITICAL
CARE
Publications
Andersen-Ranberg, N., Poulsen, L.M., Perner, A., Hästbacka, J., Morgan, M.Z., Citerio, G., Collet, M.O., Weber, S.-O., Andreasen, A.S., Bestle, M.H., Uslu, B., Pedersen, H.B.S., Nielsen, L.G., Damgaard, K., Jensen, T.B., Sommer, T., Dey, N., Mathiesen, O. and Granholm, A. (2022a). Agents intervening against delirium in the intensive care unit trial—Protocol for a secondary Bayesian analysis. Acta Anaesthesiologica Scandinavica, 66(7), pp.898–903. doi: https://doi.org/10.1111/aas.14091.
Andersen-Ranberg, N., Poulsen, L.M., Perner, A., Hästbacka, J., Morgan, M.Z., Citerio, G., Collet, M.O., Weber, S.-O., Andreasen, A.S., Bestle, M.H., Uslu, B., Pedersen, H.S., Nielsen, L.G., Damgaard, K., Jensen, T.B., Sommer, T., Dey, N., Mathiesen, O. and Granholm, A. (2023). Haloperidol vs. placebo for the treatment of delirium in ICU patients: a pre-planned, secondary Bayesian analysis of the AID–ICU trial. Intensive Care Medicine, 49(4), pp.411–420. doi: https://doi.org/10.1007/s00134-023-07024-9.
Andersen-Ranberg, N.C., Poulsen, L.M., Perner, A., Wetterslev, J., Estrup, S., Hästbacka, J., Morgan, M., Citerio, G., Caballero, J., Lange, T., Kjær, M.-B.N., Ebdrup, B.H., Engstrøm, J., Olsen, M.H., Oxenbøll Collet, M., Mortensen, C.B., Weber, S.-O., Andreasen, A.S., Bestle, M.H. and Uslu, B. (2022b). Haloperidol for the Treatment of Delirium in ICU Patients. New England Journal of Medicine, (26), pp.2425–2435. doi: https://doi.org/10.1056/nejmoa2211868.
Ashton, N.J., Moseby-Knappe, M., Benedet, A.L., Grötschel, L., Lantero-Rodriguez, J., Karikari, T.K., Hassager, C., Wise, M.P., Stammet, P., Kjaergaard, J., Friberg, H., Nielsen, N., Cronberg, T., Zetterberg, H. and Blennow, K. (2023). Alzheimer Disease Blood Biomarkers in Patients With Out-of-Hospital Cardiac Arrest. JAMA Neurology, 80(4), pp.388–396. doi: https://doi.org/10.1001/jamaneurol.2023.0050.
Borthwick, M., Granholm, A., Marker, S., Krag, M., Lange, T., Wise, M.P., Bendel, S., Keus, F., Guttormsen, A.B., Schefold, J.C., Wetterslev, J., Perner, A. and Møller, M.H. (2023). Associations between enteral nutrition and outcomes in the SUP-ICU trial: Protocol for exploratory post hoc analyses. Acta Anaesthesiologica Scandinavica, 67(4), pp.481–486. doi: https://doi.org/10.1111/aas.14194.
Burton, R.J., Cuff, S.M., Morgan, M.P., Artemiou, A. and Eberl, M. (2022). GeoWaVe: geometric median clustering with weighted voting for ensemble clustering of cytometry data. Bioinformatics, 39(1). doi: https://doi.org/10.1093/bioinformatics/btac751.
Crescioli, E., Klitgaard, T., Lone Musaeus Poulsen, Brand, B., Siegemund, M., Grøfte, T., Keus, F., Pedersen, U.G., Bäcklund, M., Karttunen, J., Morgan, M., Ciubotariu, A., Bunzel, A.-M.G., Vestergaard, S.R., Jensen, N.M., Jensen, T.S., Kjær, M.N., Georg, K., Lange, T. and Wetterslev, J. (2022). Long-term mortality and health-related quality of life of lower versus higher oxygenation targets in ICU patients with severe hypoxaemia. Intensive Care Medicine, 48(6), pp.714–722. doi: https://doi.org/10.1007/ s00134-022-06695-0.
Düring, J., Annborn, M., Cariou, A., Chew, M.S., Dankiewicz, J., Friberg, H., Haenggi, M., Haxhija, Z., Jakobsen, J.C., Langeland, H., Taccone, F.S., Thomas, M., Ullén, S., Wise, M.P. and Nielsen, N. (2022). Influence of temperature management at 33 °C versus normothermia on survival in patients with vasopressor support after out-of-hospital cardiac arrest: a post hoc analysis of the TTM-2 trial. Critical Care, [online] 26(1), p.231. doi: https://doi.org/10.1186/s13054-022-04107-9.
Eastwood, G., Nichol, A.D., Hodgson, C.L., Parke, R.L., McGuinness, S., Nielsen, N., Bernard, S., Skrifvars, M.B., Stub, D., Taccone, F.S., Archer, J., Kutsogiannis, D., Dankiewicz, J., Lilja, G., Cronberg, T., Kirkegaard, H., Capellier, G., Landoni, G., Horn, J. and Olasveengen, T. (2023). Mild Hypercapnia or Normocapnia after Out-of-Hospital Cardiac Arrest. New England Journal of Medicine, 389(1), pp.45–57. doi: https://doi.org/10.1056/nejmoa2214552.
135
Appendix H - Specialist Services Directorate: CRITICAL CARE
Fisher, B.A., Veenith, T., Slade, D.J., Gaskell, C., Rowland, M.J., Whitehouse, T., Scriven, J., Parekh, D., Balasubramaniam, M., Cooke, G.S., Morley, N., Gabriel, Z., Wise, M.E., Porter, J.C., McShane, H., Ho, L.-P., Newsome, P.N., Rowe, A., Sharpe, R. and Thickett, D.R. (2021). Namilumab or infliximab compared with standard of care in hospitalised patients with COVID-19 (CATALYST): a randomised, multicentre, multiarm, multistage, open-label, adaptive, phase 2, proof-of-concept trial. The Lancet Respiratory Medicine, 10(3), pp.255–266. doi: https://doi.org/10.1016/s2213-2600(21)00460-4.
Heimburg, K., Cronberg, T., Tornberg, Å., Ullén, S., Friberg, H., Nielsen, N., Hassager, C., Horn, J., Kjaergaard, J., Kuiper, M., Rylander, C., Wise, M.P. and Lilja, G. (2022). Self-reported limitations in physical function are common 6 months after out-of-hospital cardiac arrest. Resuscitation Plus, 11. doi: https://doi. org/10.1016/j.resplu.2022.100275.
Hookham, L., Fisher, C., Manson, J.J., Morgan, M., O’Hara, G., Riley, P., Tattersall, R.S. and Goodman, A.L. (2022). Understanding the diagnosis and management of multisystem inflammatory syndrome in adults (MIS-A) in the UK: results of a national Delphi process. Clinical Medicine, [online] 22(3), pp.266–270. doi: https://doi.org/10.7861/clinmed.2021-0700.
Lagebrant, A., Lang, M., Nielsen, N., Blennow, K., Dankiewicz, J., Friberg, H., Hassager, C., Horn, J., Kjaergaard, J., Kuiper, M.A., Mattsson-Carlgren, N., Pellis, T., Rylander, C., Sigmund, R., Stammet, P., Zetterberg, H., Wise, M.P., Cronberg, T. and Knappe, M.M. (2023). Brain injury markers in blood predict signs of hypoxic ischaemic encephalopathy on head computed tomography after cardiac arrest. Resuscitation, 184, pp.109668–109668. doi: https://doi.org/10.1016/j.resuscitation.2022.12.006.
Lang, M., Leithner, C., Scheel, M., Kenda, M., Cronberg, T., Düring, J., Rylander, C., Annborn, M., Dankiewicz, J., Deye, N., Halliday, T., Lascarrou, J.-B., Thomas, M., McGuigan, P., Morgan, M., Thomas, M., Ullén, S., Undén, J., Nielsen, N. and Moseby-Knappe, M. (2022). Prognostic accuracy of head computed tomography for prediction of functional outcome after out-of-hospital cardiac arrest: Rationale and design of the prospective TTM2-CT-substudy. Resuscitation Plus, 12. doi: https://doi.org/10.1016/j.resplu.2022.100316.
Law, D. and Morgan, M. (2021). Listening to patients in intensive care. Practical Neurology, 22(2), pp.96–97. doi: https://doi.org/10.1136/practneurol-2021-003255.
Moseby-Knappe, M., Levin, H., Blennow, K., Ullén, S., Zetterberg, H., Lilja, G., Dankiewicz, J., Jakobsen, J.C., Lagebrant, A., Friberg, H., Nichol, A., Ainschough, K., Eastwood, G.M., Wise, M.P., Thomas, M., Keeble, T., Cariou, A., Leithner, C., Rylander, C. and Düring, J. (2022). Biomarkers of brain injury after cardiac arrest; a statistical analysis plan from the TTM2 trial biobank investigators. Resuscitation Plus, 10. doi: https://doi. org/10.1016/j.resplu.2022.100258.
Ooi, S.Z.Y., Spencer, R.J., Hodgson, M., Mehta, S., Phillips, N.L., Preest, G., Manivannan, S., Wise, M.P., Galea, J. and Zaben, M. (2022). Interleukin-6 as a prognostic biomarker of clinical outcomes after traumatic brain injury: a systematic review. Neurosurgical Review, 45(5), pp.3035–3054. doi: https://doi.org/10.1007/ s10143-022-01827-y.
Pandey, M., May, A., Tan, L., Hughes, H., Jones, J.P., Harrison, W., Bradburn, S., Tyrrel, S., Muthuswamy, B., Berry, N., Pugh, R., Sutton, D., Campbell, A. and Morgan, M. (2022). Comparative incidence of early and late bloodstream and respiratory tract co-infection in patients admitted to ICU with COVID-19 pneumonia versus Influenza A or B pneumonia versus no viral pneumonia: wales multicentre ICU cohort study. Critical Care, 26(1). doi: https://doi.org/10.1186/s13054-022-04026-9.
Papathanasiou, A., Hibbert, A., Tallantyre, E., Harding, K., Selvam, A.P., Morgan, M., Quainton, C., Talaei, M., Arun, T., Ingram, G., Law, G.R. and Evangelou, N. (2023). Real-world annualized relapse rates from contemporary multiple sclerosis clinics in the UK: a retrospective multicentre cohort study. Neurological Sciences, pp.1–7. doi: https://doi.org/10.1007/s10072-023-06838-1.
136
Appendix H - Specialist Services Directorate: CRITICAL CARE
Ponsford, M.J., Burton, R.J., Smith, L., Khan, P.Y., Andrews, R., Cuff, S., Tan, L., Eberl, M., Humphreys, I.R., Babolhavaeji, F., Artemiou, A., Pandey, M., Jolles, S.R.A. and Underwood, J. (2021). Examining the utility of extended laboratory panel testing in the emergency department for risk stratification of patients with COVID-19: a single-centre retrospective service evaluation. Journal of Clinical Pathology, 75(4), pp.255–262. doi: https://doi.org/10.1136/jclinpath-2020-207157.
Rasmussen, B.S., Klitgaard, T., Perner, A., Brand, B., Hildebrandt, T., Siegemund, M., Hollinger, A., Aagaard, S., Bestle, M.H., Marcussen, K.V., Brøchner, A.C., Sølling, C., Poulsen, L.M., Laake, J.H., Aslam, T.N., Bäcklund, M., Okkonen, M., Morgan, M., Sharman, M. and Lange, T. (2021). Oxygenation targets in ICU patients with COVID-19: A post hoc subgroup analysis of the HOT-ICU trial. Acta Anaesthesiologica Scandinavica, 66(1), pp.76–84. doi: https://doi.org/10.1111/aas.13977.
Riddell, J.R., Jones, B.J., Fernandes, B.M., Law, D.J., Cooper, J.A. and Wise, M.P. (2022). Mechanical ventilation variables associated with high pulmonary artery pressures in ARDS patients: a post hoc analysis. Critical Care, [online] 26(1). doi: https://doi.org/10.1186/s13054-022-04282-9.
Saud, Z., Ponsford, M., Bentley, K., Cole, J., Pandey, M., Jolles, S., Fegan, C., Humphreys, I.R., Wise, M.P. and Stanton, R.J. (2022). Mechanically Ventilated Patients Shed High-Titer Live Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) for Extended Periods From Both the Upper and Lower Respiratory Tract. Clinical Infectious Diseases, 75(1), pp.e82–e88. doi: https://doi.org/10.1093/cid/ ciac170.
Shehabi, Y., Neto, A.S., Bellomo, R., Howe, B., Arabi, Y.M., Bailey, M., Bass, F., Kadiman, S., McArthur, C., Reade, M.C., Seppelt, I., Takala, J., Wise, M.P., Webb, S., Mashonganyika, C., McKee, H., Tonks, A., Donnelly, A.M., Hemmings, N. and O’Kane, S. (2023). Dexmedetomidine and Propofol Sedation in Critically Ill Patients and Dose-associated 90-Day Mortality: A Secondary Cohort Analysis of a Randomized Controlled Trial (SPICE III). American Journal of Respiratory and Critical Care Medicine, 207(7), pp.876–886. doi: https://doi.org/10.1164/rccm.202206-1208oc.
Simpson, R., Dankiewicz, J., Karamasis, G.V., Pelosi, P., Haenggi, M., Young, P., Jakobsen, J.C., BannardSmith, J., Wendel-Garcia, P.D., Silvio Taccone, F., Nordberg, P., Wise, M.P., Grejs, A.M., Lilja, G., Olsen, R.B., Cariou, A., Lascarrou, J.B., Saxena, M., Hovdenes, J. and Thomas, M. (2022). Speed of cooling after cardiac arrest in relation to the intervention effect: a sub-study from the TTM2-trial. Critical Care, 26(1). doi: https://doi.org/10.1186/s13054-022-04231-6.
Stefanizzi, F., Zhang, L., Salgado-Somoza, A., Dankiewicz, J., Stammet, P., Hassager, C., Wise, M.P., Friberg, H., Cronberg, T., Hundt, A., Kjaergaard, J., Nielsen, N. and Devaux, Y. (2022). Circular RNAs to predict clinical outcome after cardiac arrest. Intensive Care Medicine Experimental, 10(1). doi: https:// doi.org/10.1186/s40635-022-00470-7.
White, P.L., Springer, J., Wise, M.P., Einsele, H., Löffler, C., Seif, M., Prommersberger, S., Backx, M. and Löffler, J. (2022). A Clinical Case of COVID-19-Associated Pulmonary Aspergillosis (CAPA), Illustrating the Challenges in Diagnosis (Despite Overwhelming Mycological Evidence). Journal of Fungi, 8(1), p.81. doi: https://doi.org/10.3390/jof8010081.
Please note: all grants, publications, research impact case studies or initiatives have been provided by Clinical Board and Directorate R&D leads. Therefore, there may be further grants, publications, case studies or initiatives not mentioned in this report.
137
Appendix H - Specialist Services Directorate: HAEMATOLOGY & CLINICAL IMMUNOLOGY
Publications
Dillon, R., Maycock, S., Jackson, A.L., Fox, S., Freeman, S., Craddock, C., Thomas, C., Homer, E., Leahy, J., Mamwell, A., Potter, N., Russell, N., Wei, A., Beier Ommen, H., Hemmaway, C., Knapper, S. and Billingham, L. (2022). Venetoclax combined with low dose cytarabine compared to standard of care intensive chemotherapy for the treatment of favourable risk adult acute myeloid leukaemia (VICTOR): Study protocol for an international, open-label, multicentre, molecularly-guided randomised, phase II trial. BMC Cancer, 22(1). doi: https://doi.org/10.1186/s12885-022-10221-2.
Freeman, S.D., Thomas, A., Thomas, I., Vyas, P., Gilkes, A.F., Metzner, M., Jakobsen, B., Kennedy, A., Moore, A., Marquez Almuina, N., Burns, S., King, S., Andrew, G.M., Gallagher, K.M.E., Sellar, R., Cahalin, P., Weber, D., Dennis, M.M., Mehta, P. and Knapper, S. (2022). A Randomized Comparison of the Fractionated Versus Single Dose Schedule of Gemtuzumab Ozogamicin at Induction with Determinants of Benefit for Older AML Patients: UK NCRI AML18 Trial Results. Blood, 140(Supplement 1), pp.532–533. doi: https://doi.org/10.1182/blood-2022-162245.
Iland, H.J., Russell, N.H., Dillon, R., Schuh, A.C., Tedjaseputra, A., Wei, A.H., Khwaja, A.I., Knapper, S., Lane, S.W., Reynolds, J.V., McMullin, M.F., Martin, A.M., Tan, P., Taussig, D., Wong, A., Taper, J., Fraga, C.G.S., Kelly, R., Tawana, K. and Mehta, P. (2022). Characteristics and outcomes of patients with acute promyelocytic leukemia and extreme hyperleukocytosis at presentation. Blood Advances, 7(11), pp.2580–2585. doi: https://doi.org/10.1182/bloodadvances.2022007126.
Munir, T., Cherrill, L.-R., Webster, N., Dalal, S., Boucher, R.H., Sankhalpara, C., Yates, F., Fox, S., Macdonald, D., Fegan, C., McCaig, A., Schuh, A., Pettitt, A., Gribben, J.G., Patten, P.E.M., Devereux, S., Bloor, A., Fox, C.P., Forconi, F. and Rawstron, A.C. (2022). MRD4 Eradication at 6 Months and Early Clearance of MRD with Combination of Ibrutinib Plus Venetoclax Results in Sustained Clinical and MRD Responses: Exploratory Analysis of the Blood Cancer UK TAP Clarity Study. Blood, 140(Supplement 1), pp.222–223. doi: https://doi.org/10.1182/blood-2022-166700.
Mussai, F., De Santo, C., Cheng, P., Thomas, I.F., Ariti, C., Upton, L., Scarpa, U., Stavrou, V., Sydenham, M., Burnett, A.K., Knapper, S.K., Mehta, P., McMullin, M.F., Copland, M., Russell, N.H. and Dennis, M. (2022). A randomised evaluation of low-dose Ara-C plus pegylated recombinant arginase BCT-100 versus low dose Ara-C in older unfit patients with acute myeloid leukaemia: Results from the LI-1 trial. British Journal of Haematology, 200(5), pp.573–578. doi: https://doi.org/10.1111/bjh.18560.
Othman, J., Dillon, R., Wilhelm-Benartzi, C., Knapper, S., Batten, L.M., Canham, J., Hinson, E.L., Villiers, W., Kleeman, M., Gilkes, A.F., Potter, N., Overgaard, U.M., Mehta, P., Kottaridis, P., Cavenagh, J., Hemmaway, C., Arnold, C., Dennis, M.M. and Russell, N.H. (2022). Genomic Correlates of Outcome in a Randomised Comparison of CPX-351 and FLAG-Ida in High-Risk Acute Myeloid Leukaemia and Myelodysplastic Syndrome: Results from the UK NCRI AML19 Trial. Blood, 140(Supplement 1), pp.1036–1038. doi: https://doi.org/10.1182/blood-2022-159433.
Russell, N.H., Wilhelm-Benartzi, C., Knapper, S., Batten, L.M., Canham, J., Hinson, E.L., Overgaard, U.M., Gilkes, A.F., Othman, J., Potter, N., Dillon, R., Mehta, P., Kottaridis, P., Cavenagh, J., Hemmaway, C., Arnold, C., Freeman, S.D. and Dennis, M.M. (2022). FLAG-Ida Combined with Gemtuzumab Ozogamicin (GO) Improves Event Free Survival in Younger Patients with Newly Diagnosed Acute Myeloid Leukaemia (AML) and Shows an Overall Survival Benefit in NPM1 and FLT3 mutated Subgroups. Results from the UK NCRI AML19 Trial. Blood, 140(Supplement 1), pp.526–528. doi: https://doi.org/10.1182/blood-2022-162377.
138
Appendix H - Specialist Services Directorate: HAEMATOLOGY & CLINICAL IMMUNOLOGY
Wolf, J., Thomas, I., Stanworth, S., Knapper, S. and Griffin, J. (2022). Leukocytapheresis in the management of adults with acute myeloid leukaemia: A survey of AML treating centres highlighting variability in practice. Transfusion Medicine, 33(2). doi: https://doi.org/10.1111/tme.12929.
Wood, H., Bourlon, C., Kulasekararaj, A.G., Borg, A., Pavlu, J., Elder, P., Taussig, D., Veitch, S., Knapper, S., Othman, J., Dillon, R., Aries, J., Mitchell, E., Basheer, F., Leak, S., Murthy, V., Cross, J.W., Mehta, P., Jain, M. and Khan, A. (2022). Venetoclax-Based Non-Intensive Combinations Successfully Salvage Molecular Relapse of Acute Myeloid Leukemia and Are an Important Bridge to Cellular Therapy in Relapsed/Refractory Disease - Real-World Data from a UK-Wide Programme. Blood, 140(Supplement 1), pp.9016–9018. doi: https://doi.org/10.1182/blood-2022-167097.
Myeloma
Popat, R., Wilson, W.J., Camilleri, M., Ramasamy, K., Streetly, M., Sive, J., Bygrave, C., Benjamin, R., Chapman, M.A., Chavda, S.J., Phillips, B.M., del Mar Cuadrado, M., Pang, G., Jenner, R., Dadaga, T., Kamora, S., de Tute, R., Cavenagh, J.D., Clifton-Hadley, L. and Owen, R.G. (2022). Impact of Autologous Stem Cell Transplant (ASCT) Versus Consolidation on Health-Related Quality of Life (HRQoL) for Patients with Multiple Myeloma Receiving Carfilzomib Maintenance: Results from the Cardamon Trial. Blood, 140(Supplement 1), pp.10191–10192. doi: https://doi.org/10.1182/blood-2022-166344.
Rampotas, A., Vallance, G., Panitsas, F., Basker, N., Sangha, G., Salhan, B., Karim, F., Al-kaisi, F., Gudger, A., Ngu, L., Poynton, M., Lam, H.T., Morgan, L., Yang, L., Young, J., Walker, M.C., Tsagkaraki, I., Anderson, L., Chauhan, S.R. and Maddams, R. (2022). Isatuximab with Pomalidomide and Dexamethasone Provides Comparable Efficacy Outcomes in Frail Routine Care Myeloma Patients in a UK-Wide Cohort. Blood, 140(Supplement 1), pp.5206–5207. doi: https://doi.org/10.1182/ blood-2022-163025.
Royle, K.-L., Coulson, A.L., Ramasamy, K., Cairns, D.A., Hockaday, A., Quezada, S., Drayson, M., Kaiser, M., Owen, R.G., Auner, H.W., Cook, G., Meads, D., Olivier, C., Barnard, L., Lambkin, R., Paterson, A., Dawkins, B., Chapman, M., Pratt, G. and Popat, R. (2022). Risk and response adapted therapy following autologous stem cell transplant in patients with newly diagnosed multiple myeloma (RADAR (UKMRA Myeloma XV Trial): study protocol for a phase II/III randomised controlled trial. British Medical Journal Open, 12(11), pp.e063037. doi: https://doi.org/10.1136/bmjopen-2022-063037.
Walker, I., D’arcy, V., Rodriguez Romera, A., Anderson, G., Aubareda, A., Roy, J.P., Khandelwal, G., Wilson, W.J., Fitzsimons, E., Galas-Filipowicz, D., Foster, K., Popat, R., Ramasamy, K., Streetly, M., Bygrave, C., Benjamin, R., de Tute, R., Camilleri, M., Chavda, S.J. and Pang, G. (2022). Developing a Flow Cytometric Signature of Carfilzomib Responsiveness in Myeloma. Blood, 140(Supplement 1), pp.10106–10107. doi: https://doi.org/10.1182/blood-2022-169882.
Yong, K., Wilson, W., de Tute, R., Camilleri, M., Ramasamy, K., Streetly, M., Sive, J., Bygrave, C., Benjamin, R., Chapman, M., Chavda, S., Philips, E., del Mar Cuadrado, M., Pang, G., Jenner, R., Dadaga, T., Kamora, S., Cavenagh, J., Clifton-Hadley, L. and Owen, R. (2022). ASCT does not benefit patients who are MRD negative following induction therapy: updated results from the Phase 2 Cardamon study. In: International Myeloma Society. International Myeloma Society ASM.
139
Appendix H - Specialist Services Directorate: HAEMATOLOGY
& CLINICAL IMMUNOLOGY
Yong, K., Wilson, W., Tute, R.M. de, Camilleri, M., Ramasamy, K., Streetly, M., Sive, J., Bygrave, C.A., Benjamin, R., Chapman, M., Chavda, S.J., Phillips, E.H., Cuadrado, M. del M., Pang, G., Jenner, R., Dadaga, T., Kamora, S., Cavenagh, J., Clifton-Hadley, L. and Owen, R.G. (2023). Upfront autologous haematopoietic stem-cell transplantation versus carfilzomib–cyclophosphamide–dexamethasone consolidation with carfilzomib maintenance in patients with newly diagnosed multiple myeloma in England and Wales (CARDAMON): a randomised, phase 2, non-inferiority trial. The Lancet Haematology, 10(2), pp.e93–e106. doi: https://doi.org/10.1016/S2352-3026(22)00350-7.
Lymphoma
Kirkwood, A.A., Goulden, N., Moppett, J., Samarasinghe, S., Mee, J., Hough, R., Kearns, P.R., Lawson, S., Rowntree, C.J. and Vora, A. (2022). High Dose Methotrexate Does Not Reduce the Risk of CNS Relapse in Children and Young Adults with Acute Lymphoblastic Leukaemia and Lymphoblastic Lymphoma. Results of the Randomised Phase III Study UKALL 2011. Blood, 140(Supplement 1), pp.516–518. doi: https://doi.org/10.1182/blood-2022-160129.
Patel Wrench, B., Kirkwood, A.A., Zuborne Alapi, K., Clifton-Hadley, L., Lee, S., Marks, D.I., Moorman, A.V., Morley, N.J., Patrick, P., Rana, Z., Rowntree, C.J., Snowden, J.A. and Fielding, A.K. (2022). Final Results from UKALL60+ (NCT01616238), a UK National Cancer Research Institute Trial for Older People with De Novo Acute Lymphoblastic Leukaemia. In: American Society of Hematology. American Society of Hematology Annual Meeting.
Nurse-Led Research
Williams, E. (2022). How my CAR-T therapy research seeks to improve cancer care and empower nurses. Nurse Researcher, 30(2), pp.6–7. doi: https://doi.org/10.7748/nr.30.2.6.s2.
Williams, E. and Jones, C.H. (2022). The need for researchers and how their research can embed a positive research culture into practice. In: National PROMS Annual Research Conference.
Please note: all grants, publications, research impact case studies or initiatives have been provided by Clinical Board and Directorate R&D leads. Therefore, there may be further grants, publications, case studies or initiatives not mentioned in this report.
140
Appendix H - Specialist Services Directorate: NEPHROLOGY & TRANSPLANT
Publications
Clinical Nephrology
Sridhar, V.S. and Fraser, D.J. (2023). SGLT2 inhibition, glucose transport and peritoneal dialysis: Finding the sweet spot. Peritoneal Dialysis International: Journal of the International Society for Peritoneal Dialysis, 43(2), pp.115–118. doi: https://doi.org/10.1177/08968608231161178.
Heerspink, H.J.L., Radhakrishnan, J., Alpers, C.E., Barratt, J., Bieler, S., Diva, U., Inrig, J., Komers, R., Mercer, A., Noronha, I.L., Rheault, M.N., Rote, W., Rovin, B., Trachtman, H., Trimarchi, H., Wong, M.G., Perkovic, V., Alarmartine, E., Barratt, J. and Chae, D.W. (2023). Sparsentan in patients with IgA nephropathy: a prespecified interim analysis from a randomised, double-blind, active-controlled clinical trial. The Lancet, 401(10388). doi: https://doi.org/10.1016/S0140-6736(23)00569-X.
Herrington, W.G., Staplin, N., Wanner , C., Green , J.B., Hauske , S.J., Emberson , J.R., Preiss , D., Judge , P., Mayne , K.J., Ng , S.Y.A., Sammons , E., Zhu, D., Hill , M., Stevens , W., Wallendszus , K., Brenner , S., Cheung , A.K., Liu, Z.H., Li, J. and Hooi , L.S. (2022). Empagliflozin in Patients with Chronic Kidney Disease. New England Journal of Medicine, 388(2). doi: https://doi.org/10.1056/nejmoa2204233.
COVID-19
Asderakis, A., Khalid, U., Koimtzis, G., Ponsford, M.J., Szabo, L., Chalklin, C., Bramhall, K., Grant, L., Moat, S.J., Humphreys, I.R. and Jolles, S.R. (2022). An Analysis of Serological Response and Infection Outcomes Following Oxford-AstraZeneca (AZD1222) and Pfizer-BioNTech (mRNA BNT162b2) SARSCoV-2 Vaccines in Kidney and Kidney Pancreas Transplants. Transplantation, 106(7). doi: https://doi. org/10.1097/tp.0000000000004105.
Ponsford, M.J., Burton, R.J., Smith, L., Khan, P.Y., Andrews, R., Cuff, S., Tan, L., Eberl, M., Humphreys, I.R., Babolhavaeji, F., Artemiou, A., Pandey, M., Jolles, S.R.A. and Underwood, J. (2022). Examining the utility of extended laboratory panel testing in the emergency department for risk stratification of patients with COVID-19: a single-centre retrospective service evaluation. Journal of Clinical Pathology, 75(4), pp.255–262. doi: https://doi.org/10.1136/jclinpath-2020-207157.
Qualitative Research
Boadu, P., Mclaughlin, L., Al-Haboubi, M., Bostock, J., Noyes, J., O’Neill, S. and Mays, N. (2023). A machine-learning approach to estimating public intentions to become a living kidney donor in England: Evidence from repeated cross-sectional survey data. Frontiers in Public Health, 10. doi: https://doi.org/10.3389/fpubh.2022.1052338.
Faherty, G., Williams, L., Noyes, J., McLaughlin, L., Bostock, J. and Mays, N. (2022). Analysis of content and online public responses to media articles that raise awareness of the opt-out system of consent to organ donation in England. Frontiers in Public Health, 10. doi: https://doi.org/10.3389/ fpubh.2022.1067635.
McKeaveney, C., Noble, H., Courtney, A., Griffin, S., Gill, P., Johnston, W., Maxwell, A., Teasdale, F. and Reid, J. (2022). Dialysis, Distress, and Difficult Conversations: Living with a Kidney Transplant. Healthcare, 10(7), p.1177. doi: https://doi.org/10.3390/healthcare10071177.
141
Appendix H - Specialist Services Directorate:
NEPHROLOGY & TRANSPLANT
Transplantation
Bailey, P., Selman, L., Exley, C., Griffin, S., Hancock, A., Maxted, P. and Noyes, J. (2023). The IN-FAKT Study Protocol: Investigating the Experiences and Management of Individuals With Failing Kidney Transplants. International Journal of Qualitative Methods, 22, p.160940692311684. doi: https://doi. org/10.1177/16094069231168485.
Gittus, M., Bailey, P.K. and Griffin, S. (2023). Management of Patients with a Failing Kidney Transplant: a Survey of Uk-Based Renal Units. Nephrology Dialysis Transplantation. doi: https://doi.org/10.1093/ ndt/gfad084.
Mamode, N., Bestard, O., Claas, F., Furian, L., Griffin, S., Legendre, C., Pengel, L. and Naesens, M. (2022). European Guideline for the Management of Kidney Transplant Patients With HLA Antibodies: By the European Society for Organ Transplantation Working Group. Transplant International, 35. doi: https://doi.org/10.3389/ti.2022.10511.
Nimmo, A., Graham-Brown, M., Griffin, S., Sharif, A., Ravanan, R. and Taylor, D. (2022). Pre-Kidney Transplant Screening for Coronary Artery Disease: Current Practice in the United Kingdom. Transplant International, 35. doi: https://doi.org/10.3389/ti.2021.10039.
Dialysis
Damgov, I., Bartosova, M., Marinovic, I., Istanbuly, O., Kieser, M., Lambie, M., Davies, S.J., Schmitt, C.P., Vychytil, A., Raby, A.-C., Colmont, C., Aufricht, C., Johnson, D.W., Fraser, D., Eringa, E., Morelle, J., Valdivielso, J.M., Kratochwill, K., Jakulj, L. and Vervloet, M. (2023). IMPROVE-PD Finder: A WebBased Platform to Search and Share Peritoneal Dialysis Biobank, Registry, and Clinical Trial Metadata. Kidney International Reports, 8(4), pp.912–915. doi: https://doi.org/10.1016/j.ekir.2023.01.003.
Reproductive Health
Mc Laughlin, L., Jones, C., Neukirchinger, B., Noyes, J., Stone, J., Williams, H., Williams, D., Rapado, R., Phillips, R. and Griffin, S. (2023). Feminizing care pathways: Mixed-methods study of reproductive options, decision making, pregnancy, post-natal care and parenting amongst women with kidney disease. Journal of Advanced Nursing. doi: https://doi.org/10.1111/jan.15659.
Mclaughlin, L., Neukirchinger, B. and Noyes, J. (2022). Interventions for and experiences of shared decision-making underpinning reproductive health, family planning options and pregnancy for women with or at high risk of kidney disease: a systematic review and qualitative framework synthesis. BMJ, 12(8), pp.e062392. doi: https://doi.org/10.1136/bmjopen-2022-062392.
142
Appendix H - Specialist Services Directorate:
NEPHROLOGY & TRANSPLANT
Basic Science
Hamilton, K., Czajkowski, D., Kong, N.J., Tran, T.D., Gustafson, K.R., Pauly, G.T., Boyle, G.M., Simmons, J.L., Steadman, R., Moseley, R., Brooks, P., Ogbourne, S.M. and Russell, F.D. (2022). Anti-Fibrotic Potential of Tomentosenol A, a Constituent of Cerumen from the Australian Native Stingless Bee, Tetragonula carbonaria. Antioxidants, 11(8), pp.1604. doi: https://doi.org/10.3390/antiox11081604.
Sharma, S., Zaher, S., Rodrigues, P.R.S., Davies, L.C., Edkins, S., Strang, A., Chakraborty, M., Watkins, W.J., Andrews, R., Parkinson, E., Angelopoulos, N., Moet, L., Shepherd, F., Davies, K.M.M., White, D., Oram, S., Siddall, K., Keeping, V., Simpson, K. and Faggian, F. (2022). mSep: investigating physiological and immune-metabolic biomarkers in septic and healthy pregnant women to predict feto-maternal immune health – a prospective observational cohort study protocol. BMJ Open, 12(9), p.e066382. doi: https://doi.org/10.1136/bmjopen-2022-066382.
Grants
Project/Grant Title
Interaction between early CKD-associated endogenous ligands of TLRs and adaptive immune cells and its impact on atherosclerosis
Investigating novel mechanisms that drive cardiovascular disease in kidney patients, the relationship between systemic inflammation, alteration in hyaluronan matrix and arterial vascular calcification
What’s so special about the female kidney? Using single cell nuclear RNA sequencing to understand proximal tubular cell heterogeneity
Lead Investigator
Dr Siân Griffin
Dr Soma Meran, Professor Donald Fraser, Dr AnneCatherine Raby, Professor Dipak Ramji (Cardiff University; coapplicants)
Dr Tanya Smith
Duration of Grant (Date from/to) 2022 - 2025 2022 - 2025 2022 - 2025 Funder Name/ Source of Funding British Heart Foundation Kidney Research UK Kidney Research UK Grant Awarded (Total) £239,333 £74,983 £175,652 143
Appendix H - Specialist Services Directorate: NEPHROLOGY & TRANSPLANT
Project/Grant Title
Improving organ and tissue donation awareness within ethnic minority communities in Wales
Investigating nutritional amino acid regulation of antiviral immunity in kidney transplant recipients
National Unified Renal Translational Research Enterprise (NURTuRE) –
Acute Kidney Injury (two projects)
Investigating the experiences and management of individuals with failing kidney transplants: preparation for a randomised controlled trial (IN-FAKT)
Lead Investigator
Dr Farah Latif (Cardiff University
Professor Ian Humphreys (Cardiff University)
Professor Donald Fraser (Cardiff University)
Dr Siân Griffin (co-applicant)
Please note: all grants, publications, research impact case studies or initiatives have been provided by Clinical Board and Directorate R&D leads. Therefore, there may be further grants, publications, case studies or initiatives not mentioned in this report.
Duration of Grant (Date from/to) 2022 - 2023 2022 - 2025 2022 - 2025 2022 - 2024 Funder Name/ Source of Funding NHS Blood and Transplant Kidney Research UK Kidney Research UK NIHR RfPB programe Grant Awarded (Total) £10,000 £74,983 £22,000 £150,000
144
Appendix H - Specialist Services Directorate: NEUROSCIENCES
Publications
Altmann, A., Ryten, M., Di Nunzio, M., Ravizza, T., Tolomeo, D., Reynolds, R.H., Somani, A., Bacigaluppi, M., Iori, V., Micotti, E., Di Sapia, R., Cerovic, M., Palma, E., Ruffolo, G., Botía, J.A., Absil, J., Alhusaini, S., Alvim, M.K.M., Auvinen, P. and Bargallo, N. (2022). A systems-level analysis highlights microglial activation as a modifying factor in common epilepsies. Neuropathology and Applied Neurobiology, 48(1). doi: https://doi.org/10.1111/nan.12758.
Ambati, S., Wilson, C. and Hamandi, K. (2023). A case of Frontal Lobe Brain sagging syndrome. In: JNNP.
Attard Navarro, G. and Hamandi, K. (2022). Lessons from the video-EEG telemetry unit. Practical Neurology, 22(4), p.practneurol-2021-003313. doi: https://doi.org/10.1136/practneurol-2021-003313.
Beesley, R., Cauchi, M., Davies, L., Upcott, M., Norton, E., Loveless, S., Anderson, V., WynfordThomas, R., Pickersgill, T.P., Uzochukwu, E., Wardle, M., Robertson, N.P., Tallantyre, E. and Willis, M.D. (2022). Multiple sclerosis and COVID-19: Assessing risk perception, patient behaviors and access to disease-modifying therapies. Multiple Sclerosis and Related Disorders, 68, p.104121. doi: https:// doi.org/10.1016/j.msard.2022.104121.
Berkovic, S.F., Cavalleri, G.L., Koeleman, B.P.C. and International League Against Epilepsy Consortium on Complex Epilepsies (2022). Genome-wide meta-analysis of over 29,000 people with epilepsy reveals 26 loci and subtype-specific genetic architecture. medRxiv. doi: https://doi.org/10.1101/2022. 06.08.22276120.
Cauchi, M., Willis, M.E., Andrews, A., Backx, M., Brownlee, W.J., Ford, H., Gran, B., Jolles, S., Price, S., Rashid, W., Schmierer, K. and Tallantyre, E.C. (2022). Multiple sclerosis and the risk of infection: Association of British Neurologists consensus guideline. Practical Neurology, 22(5), pp.344–357. doi: https://doi.org/10.1136/practneurol-2022-003370.
Cohen, N.T., You, X., Krishnamurthy, M., Sepeta, L.N., Zhang, A., Oluigbo, C.O., Whitehead, M.T., Gholipour, T., Baldeweg, T., Wagstyl, K., Adler, S. and Gaillard, W.D. (2022). Networks Underlie Temporal Onset of Dysplasia-Related Epilepsy: A MELD Study. Annals of Neurology, 92(3), pp.503–511. doi: https://doi.org/10.1002/ana.26442.
Ferreira-Atuesta, C., de Tisi, J., McEvoy, A.W., Miserocchi, A., Khoury, J., Yardi, R., Vegh, D., Butler, J., Lee, H.J., Deli-Peri, V., Yao, Y., Wang, F.-P., Zhang, X.-B., Shakhatreh, L., Siriratnam, P., Neal, A., Sen, A., Tristram, M., Varghese, E. and Biney, W. (2022). Predictive models for starting antiseizure medication withdrawal following epilepsy surgery in adults. Brain, 146(6). doi: https://doi.org/10.1093/brain/ awac437.
Garjani, A., Liu, B.J.-Y., Allen, C.M., Gunzler, D.D., Gerry, S.W., Planchon, S.M., das Nair, R., Chataway, J., Tallantyre, E.C., Ontaneda, D. and Evangelou, N. (2022). Decentralised clinical trials in multiple sclerosis research. Multiple Sclerosis Journal, 29(3), p.135245852211004. doi: https://doi. org/10.1177/13524585221100401.
Harding, K.E., Ingram, G., Tallantyre, E.C., Joseph, F., Wardle, M., Pickersgill, T.P., Willis, M.D., Tomassini, V., Pearson, O.R. and Robertson, N.P. (2022). Contemporary study of multiple sclerosis disability in South East Wales. Journal of Neurology, Neurosurgery & Psychiatry, 94(4). doi: https:// doi.org/10.1136/jnnp-2022-330013.
145
Appendix H - Specialist Services Directorate: NEUROSCIENCES
Jacobs, B.M., Schalk, L., Dunne, A., Scalfari, A., Nandoskar, A., Gran, B., Mein, C.A., Sellers, C., Spilker, C., Rog, D., Visentin, E., Bezzina, E.L., Uzochukwu, E., Tallantyre, E., Wozniak, E., Sacre, E., HassanSmith, G., Ford, H.L., Harris, J. and Bradley, J. (2023). ADAMS project: a genetic Association study in individuals from Diverse Ancestral backgrounds with Multiple Sclerosis based in the UK. BMJ Open, 13(5), pp.e071656–e071656. doi: https://doi.org/10.1136/bmjopen-2023-071656.
Kinney, M.O., Smith, P.E.M. and Craig, J.J. (2022). Preventing Teratogenicity in Women with Epilepsy. Seminars in Neurology, 42(05), pp.679–692. doi: https://doi.org/10.1055/s-0042-1759579.
Kreft, K.L. and Robertson, N. (2022). Innovative biomarkers to predict unfavourable outcomes after initiating multiple sclerosis treatment. Journal of Neurology, 269(9), pp.5192–5193. doi: https://doi. org/10.1007/s00415-022-11300-x.
Larivière, S., Royer, J., Rodríguez-Cruces, R., Paquola, C., Caligiuri, M.E., Gambardella, A., Concha, L., Keller, S.S., Cendes, F., Yasuda, C.L., Bonilha, L., Gleichgerrcht, E., Focke, N.K., Domin, M., von Podewills, F., Langner, S., Rummel, C., Wiest, R., Martin, P. and Kotikalapudi, R. (2022). Structural network alterations in focal and generalized epilepsy assessed in a worldwide ENIGMA study follow axes of epilepsy risk gene expression. Nature Communications, 13(1). doi: https://doi.org/10.1038/ s41467-022-31730-5.
Lopes, M.A., Bhatia, S., Brimble, G., Zhang, J. and Hamandi, K. (2022). A Computational Biomarker of Photosensitive Epilepsy from Interictal EEG. eNeuro, 9(3), pp.ENEURO.0486-21.2022. doi: https://doi. org/10.1523/eneuro.0486-21.2022.
Lopes, M.A., Hamandi, K., Zhang, J. and Creaser, J.L. (2023). The role of additive and diffusive coupling on the dynamics of neural populations. Scientific Reports, 13(1). doi: https://doi.org/10.1038/s41598023-30172-3.
Lopez, S.M., Aksman, L.M., Oxtoby, N.P., Vos, S.B., Rao, J., Kaestner, E., Alhusaini, S., Alvim, M., Bender, B., Bernasconi, A., Bernasconi, N., Bernhardt, B.C., Caciagli, L., Caldairou, B., Caligiuri, M.E., Calvet, A., Cendes, F., Concha, L., Conde-Blanco, E. and Davoodi-Bojd, E. (2022). Event-based modeling in temporal lobe epilepsy demonstrates progressive atrophy from cross-sectional data. Epilepsia, 63(8), pp.2081–2095. doi: https://doi.org/10.1111/epi.17316.
MacIver, C.L., Tax, C.M.W., Jones, D.K. and Peall, K.J. (2022). Structural magnetic resonance imaging in dystonia: A systematic review of methodological approaches and findings. European Journal of Neurology, 29(11), pp.3418–3448. doi: https://doi.org/10.1111/ene.15483.
Park, B., Larivière, S., Rodríguez-Cruces, R., Royer, J., Tavakol, S., Wang, Y., Caciagli, L., Caligiuri, M.E., Gambardella, A., Concha, L., Keller, S.S., Cendes, F., Alvim, M.K.M., Yasuda, C., Bonilha, L., Gleichgerrcht, E., Focke, N.K., Kreilkamp, B.A.K., Domin, M. and von Podewils, F. (2022). Topographic divergence of atypical cortical asymmetry and atrophy patterns in temporal lobe epilepsy. Brain, 145(4). doi: https://doi.org/10.1093/brain/awab417.
Roebber, J.K., Lewis, P.A., Crunelli, V., Navarrete, M. and Hamandi, K. (2022). Effects of Anti-Seizure Medication on Sleep Spindles and Slow Waves in Drug-Resistant Epilepsy. Brain Sciences, 12(10), p.1288. doi: https://doi.org/10.3390/brainsci12101288.
Shakeshaft, A., Laiou, P., Abela, E., Stavropoulos, I., Richardson, M.P., Pal, D.K., Orsini, A., Howell, A., Hyde, A., McQueen, A., Duran, A., Gaurav, A., Collingwood, A., Kitching, A., Shakeshaft, A., Papathanasiou, A., Clough, A., Gribbin, A., Swain, A. and Needle, A. (2022). Heterogeneity of resting-state EEG features in juvenile myoclonic epilepsy and controls. Brain Communications, 4(4). doi:https://doi.org/10.1093/braincomms/fcac180.
146
Appendix H - Specialist Services Directorate: NEUROSCIENCES
Shakeshaft, A., Panjwani, N., Collingwood, A., Crudgington, H., Hall, A., Andrade, D.M., Beier, C.P., Fong, C.Y., Gardella, E., Gesche, J., Greenberg, D.A., Hamandi, K., Koht, J., Lim, K.S., Møller, R.S., Ng, C.C., Orsini, A., Rees, M.I., Rubboli, G. and Selmer, K.K. (2022). Sex-specific disease modifiers in juvenile myoclonic epilepsy. Scientific Reports, 12(1). doi: https://doi.org/10.1038/s41598-022-06324-2.
Shastin, D., Genc, S., Parker, G.D., Koller, K., Tax, C.M.W., Evans, J., Hamandi, K., Gray, W.A., Jones, D.K. and Chamberland, M. (2022). Surface-based tracking for short association fibre tractography. Neuroimage, 260, pp.119423–119423. doi: https://doi.org/10.1016/j.neuroimage.2022.119423.
Smith, P.E.M. and Pierri, M. (2022). Neurology teambuilding event. Practical Neurology, 22(6), pp.472–477. doi: https://doi.org/10.1136/practneurol-2022-003358.
Spitzer, H., Ripart, M., Whitaker, K., D’Arco, F., Mankad, K., Chen, A.A., Napolitano, A., De Palma, L., De Benedictis, A., Foldes, S.T., Humphreys, Z., Zhang, K., Hu, W., Zhang, K., Likeman, M., Davies, S., Güttler, C., Lenge, M., Cohen, N.T. and Tang, Y. (2022). Interpretable surface-based detection of focal cortical dysplasias: a Multi-centre Epilepsy Lesion Detection study. Brain, 145(11), pp.3859–3871. doi: https:// doi.org/10.1093/brain/awac224.
Tallantyre, E.C. (2022). Long-term treatment with anti-CD20 monoclonal antibodies is untenable because of risk: No. Multiple Sclerosis Journal, 28(8), pp.1175–1177. doi: https://doi. org/10.1177/13524585221091404.
Tallantyre, E.C., Scurr, M.J., Vickaryous, N., Richards, A., Anderson, V., Baker, D., Chance, R., Evangelou, N., George, K., Giovannoni, G., Harding, K.E., Hibbert, A., Ingram, G., Jolles, S., Jones, M., Kang, A.S., Loveless, S., Moat, S.J., Robertson, N.P. and Rios, F. (2022). Response to COVID-19 booster vaccinations in seronegative people with multiple sclerosis. Multiple Sclerosis and Related Disorders, [online] 64. doi: https://doi.org/10.1016/j.msard.2022.103937.
Tax, C.M.W., Genc, S., MacIver, C.L., Nilsson, M., Wardle, M., Szczepankiewicz, F., Jones, D.K. and Peall, K.J. (2023). Ultra-strong diffusion-weighted MRI reveals cerebellar grey matter abnormalities in movement disorders. Neuroimage Clinical, 38, p.103419. doi: https://doi.org/10.1016/j.nicl.2023.103419.
Topcu, G., Smith, L., Mhizha-Murira, J.R., Goulden, N., Hoare, Z., Drummond, A., Fitzsimmons, D., Evangelou, N., Schmierer, K., Tallantyre, E.C., Leighton, P., Allen-Philbey, K., Stennett, A., Bradley, P., Bale, C., Turton, J., das Nair, R. and NEuRoMS Collective (2022). Neuropsychological evaluation and rehabilitation in multiple sclerosis (NEuRoMS): protocol for a mixed-methods, multicentre feasibility randomised controlled trial. Pilot and Feasibility Studies, 8(1), p.123. doi: https://doi.org/10.1186/ s40814-022-01073-5.
Wagstyl, K., Whitaker, K., Raznahan, A., Seidlitz, J., Vértes, P.E., Foldes, S., Humphreys, Z., Hu, W., Mo, J., Likeman, M., Davies, S., Lenge, M., Cohen, N.T., Tang, Y., Wang, S., Ripart, M., Chari, A., Tisdall, M., Bargallo, N. and Conde-Blanco, E. (2022). Atlas of lesion locations and postsurgical seizure freedom in focal cortical dysplasia: A MELD study. Epilepsia, 63(1), pp.61–74. doi: https://doi.org/10.1111/epi.17130.
Zaben, M., Ved, R. and Manivannan, S. (2022). The Roles of HMGB1 Post-Brain Injury: Implications for Injury and Repair. In: HMGB1: Functions, Inhibitors and Clinical Significance. New York: Nova Science Publishers, pp.188–218.
Zaben, M., Ved, R., Manivannan, S. and Tasker, I. (2022). High mobility group box protein 1 and white matter injury following traumatic brain injury: perspectives on mechanisms and therapeutic strategies. Neural Regeneration Research, 17(8), p.1739. doi: https://doi.org/10.4103/1673-5374.332135.
147
Appendix H - Specialist Services Directorate: NEUROSCIENCES
Grants
Project/Grant Title
Exploring cell-type specific microstructural alterations in dystonia using diffusion-weighted MR spectroscopy 68200, AC68200UII
Making the Invisible Visible: a Multi-Scale Imaging Approach to Detect and Characterise Cortical Pathology
CRTF Fellowship (PhD Studentship)
Lead Investigator
Dr Kathryn Peall
Clinical Heterogeneity and Gene Environment
Stratification in Multiple Sclerosis (CHANGES in MS)
Professor Derek Jones
Dr Khalid Hamandi (coapplicant)
Professor William Gray, Ronak Ved
Dr Karim Kreft
Please note: all grants, publications, research impact case studies or initiatives have been provided by Clinical Board and Directorate R&D leads. Therefore, there may be further grants, publications, case studies or initiatives not mentioned in this report.
Duration of Grant (Date from/to) Feb-July 2023 3 years November 2021November 2024 5 years Funder Name/ Source of Funding FLINR grant (Neurosciences and Mental Health Research Institute Medical Research Council MRC FEC Grant Awarded (Total) £4,980 £990,888 £200,000 £1,255,711
148
Appendix H - Specialist Services Clinical Board
Appendix H - Specialist Services Clinical Board
Non-Commercial
Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Collaborating Directorates Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitment total RAG Move Wales v1 17506 146495 9065 NonCommercial FALSE Observational Open To Recruitment Specialist Services Neurosciences 05/08/2015 01/04/2024 300 7 826 G RADAR 6726 162439 9924 NonCommercial FALSE Observational Open To Recruitment Specialist Services Nephrology & Transplant 19/03/2014 09/10/2024 100 42 805 G NEuRoMS Work Package 3 47750 287115 15593 NonCommercial FALSE Both Open To Recruitment Specialist Services Neurosciences 01/04/2022 30/09/2023 334 631 631 G ROAR Study 50943 276144 15731 NonCommercial FALSE Observational Open To Recruitment Specialist Services Neurosciences 22/02/2022 31/07/2024 500 454 564 G NEuRoMS Work Package 2i 44711 276570 14990 NonCommercial FALSE Observational Completed Specialist Services Neurosciences 23/06/2021 31/07/2022 31/05/2022 400 106 537 G *ENLIST Vaccination substudy 50305 16019 NonCommercial FALSE Observational Completed Specialist Services Haematology, Immunology and Metabolic Medicine Nephrology & Transplant; 25/03/2021 01/09/2022 01/09/2022 380 0 387 G Enroll-HD 16010 129743 8809 NonCommercial FALSE Observational Open To Recruitment Specialist Services Neurosciences 03/10/2014 01/08/2063 200 7 262 G A study of Clonal Haematopoiesis in the Welsh population 52793 280126 16183 NonCommercial FALSE Observational Completed Specialist Services Haematology, Immunology and Metabolic Medicine 07/06/2022 09/09/2022 21/09/2022 258 204 204 R The role of the marrow microenvironment in the pathogenesis of AML 36848 231974 10558 NonCommercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 06/11/2017 30/11/2024 120 33 169 G SIMPLIFIED 31929 192416 9556 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Nephrology & Transplant 15/11/2017 13/12/2024 100 0 141 G Platelet & microparticle PL in thrombotic and bleeding disorders 31196 208311 9420 NonCommercial FALSE Observational Completed Specialist Services Haematology, Immunology and Metabolic Medicine 01/06/2017 31/05/2022 31/05/2022 80 4 139 G NIHR BioResource - Rare Diseases 15941 131443 9145 NonCommercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 25/09/2014 30/11/2024 40 0 138 G RaDaR: NephroS 7945 29959 10015 NonCommercial FALSE Observational Closed to Recruitment, In Follow Up Specialist Services Nephrology & Transplant 28/05/2010 31/12/2022 31/12/2022 6 13 114 G CMVIR 38924 235946 12168 NonCommercial FALSE Observational Open To Recruitment Specialist Services Nephrology & Transplant 14/05/2018 01/08/2023 150 7 110 A Non-Commercial 149
Appendix H - Specialist Services Clinical Board
Appendix H - Specialist Services Clinical Board
92 UKAITPR 4961 92703 3160 NonCommercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 07/01/2013 30/06/2023 12 11 109 G BHF PROTECT-TAVI 45443 276396 15157 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Cardiac Services 08/12/2020 30/04/2025 200 36 106 G GenOMICC 30540 269326 13888 NonCommercial FALSE Observational Open To Recruitment Specialist Services Critical Care 14/04/2020 31/08/2029 80 25 97 G DELIVER MS 37650 243333 13321 NonCommercial FALSE Both Open To Recruitment Specialist Services Neurosciences 24/04/2019 30/06/2023 20 21 88 G Innate-like T cells in sepsis 36752 231993 10427 NonCommercial FALSE Observational Suspended Specialist Services Critical Care 15/11/2017 30/09/2023 150 0 84 S A2B Trial 40628 243640 12472 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Critical Care 27/05/2019 31/10/2023 20 49 82 G PROTECT V 47409 288652 15556 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Nephrology & Transplant 10/09/2021 31/12/2022 85 1 76 A AML18 15938 127379 8577 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 15/11/2014 31/12/2022 31/12/2022 40 2 71 G *COVID-19 ENLIST 50187 283297 15554 NonCommercial FALSE Observational Closed to Recruitment, No Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine Nephrology & Transplant; 29/12/2020 01/09/2022 01/09/2022 50 0 64 G REMAP-CAP 38197 237150 12274 NonCommercial FALSE Interventional Suspended Specialist Services Critical Care 15/04/2020 31/12/2023 1 0 57 S DOPPS 7855 182235 10011 NonCommercial FALSE Observational Open To Recruitment Specialist Services Nephrology & Transplant 19/04/2011 30/06/2023 25 4 52 G BLING III 37390 238151 13588 NonCommercial FALSE Interventional Completed Specialist Services Critical Care 27/04/2021 31/12/2022 11/07/2022 2 7 49 G SC IL-1Ra in SAH - phase III trial 35757 214739 10533 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Neurosciences Critical Care; 06/11/2018 30/09/2023 34 8 48 G OSCAR 49008 279084 15778 NonCommercial FALSE Observational Closed to Recruitment, In Follow Up Specialist Services Nephrology & Transplant 21/10/2021 31/12/2022 31/12/2022 47 23 47 G QUACS 41872 260891 13639 NonCommercial FALSE Observational Closed to Recruitment, In Follow Up Specialist Services Cardiac Services 15/01/2021 28/02/2023 28/02/2023 70 26 46 R AspiFlu 43440 271269 15217 NonCommercial FALSE Observational Open To Recruitment Specialist Services Critical Care 04/08/2020 01/05/2023 30 3 45 G Biology of Juvenile Myoclonic Epilepsy 34096 199351 11917 NonCommercial FALSE Observational Open To Recruitment Specialist Services Neurosciences 25/01/2018 30/06/2026 30 0 41 G DECISIve 43421 257949 13891 NonCommercial FALSE Observational Closed to Recruitment, In Follow Up Specialist Services Neurosciences 12/02/2020 06/05/2022 06/05/2022 28 1 40 G FLAIR 16675 126738 8544 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 22/04/2015 01/12/2022 31/01/2023 15 1 36 G 150
Appendix H - Specialist Services Clinical Board
Appendix H - Specialist Services Clinical Board
93 ADVENT 49867 285274 16396 NonCommercial FALSE Observational Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 11/11/2022 31/03/2023 01/02/2023 20 33 33 G GAPP 9858 76061 10152 NonCommercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 01/11/2011 30/04/2024 10 0 33 G MND-SMART 44601 227793 15078 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Neurosciences 07/12/2021 24/02/2023 25 27 33 G HOT-COVID 42009 254478 13582 NonCommercial FALSE Interventional Closed to Recruitment, No Follow Up Specialist Services Critical Care 23/06/2019 30/06/2024 08/03/2023 31 0 31 G COSI 41409 252254 13562 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 07/08/2020 01/04/2025 15/03/2023 50 11 29 R HEMOTION Trial 40308 241691 12394 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Critical Care 28/04/2019 31/03/2024 30 7 29 G UK TTP Registry 8435 5330 10035 NonCommercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 11/11/1997 31/12/2023 15 0 25 G The A-STOP Study 38139 234779 12422 NonCommercial FALSE Observational Closed to Recruitment, In Follow Up Specialist Services Critical Care 25/11/2019 01/06/2023 17/02/2023 12 9 24 G MND Register for England, Wales and Northern Ireland 20690 282429 14954 NonCommercial FALSE Observational Open To Recruitment Specialist Services Neurosciences 19/11/2019 25/11/2025 20 2 23 G WAND-JME 52190 271935 14829 NonCommercial FALSE Observational Open To Recruitment Specialist Services Neurosciences 02/03/2020 06/09/2024 30 23 23 G PROGROUP Feasibility Randomised Controlled Trial v1 50790 302670 16116 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 08/04/2022 31/05/2023 31/05/2022 16 22 22 G TRIDENT 39379 231800 12341 NonCommercial FALSE Interventional Completed Specialist Services Neurosciences 05/02/2019 31/12/2022 31/12/2022 18 0 22 G TONIC Phase 3 12497 12586 NonCommercial FALSE Observational Open To Recruitment Specialist Services Neurosciences 28/11/2018 30/06/2027 10 2 21 G AMADEUS 41275 253607 13541 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 14/10/2019 30/04/2023 29/03/2023 5 5 20 G CANDID 48425 289201 16137 NonCommercial FALSE Observational Completed Specialist Services Critical Care Adult Mental Health (Incl. Addictions); 13/01/2022 31/07/2022 16/06/2022 18 0 18 G PETReA 34767 221775 10599 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 20/09/2019 31/10/2022 2 6 18 G MoTD 46327 268363 15318 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 03/03/2021 30/11/2024 5 4 17 G 151
Appendix H - Specialist Services Clinical Board
Appendix H - Specialist Services Clinical Board
94 UK - EHL Outcome Registry 32890 206381 9557 NonCommercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 03/04/2017 31/12/2023 10 0 17 G FUTURE GB 45956 264482 15238 NonCommercial FALSE Both Open To Recruitment Specialist Services Neurosciences 24/05/2021 30/11/2023 30 8 16 A SIGNET 49404 288722 15866 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Critical Care 14/09/2021 31/03/2025 5 11 16 G TONIC Phase 4 35238 88372 11923 NonCommercial FALSE Observational Open To Recruitment Specialist Services Neurosciences 08/10/2018 01/08/2029 10 2 16 G Multivessel TALENT 47496 290834 15566 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Cardiac Services 11/05/2021 29/03/2024 22 3 14 A TONIC Phase 2 12495 12587 NonCommercial FALSE Observational Open To Recruitment Specialist Services Neurosciences 28/11/2018 30/06/2027 10 2 14 G HAEFiT 48503 251660 15328 NonCommercial FALSE Observational Closed to Recruitment, Follow Up Complete Specialist Services Haematology, Immunology and Metabolic Medicine 02/10/2020 02/10/2022 02/10/2022 10 0 12 G Prepare for Kidney Care 32254 215616 11952 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Nephrology & Transplant 19/02/2018 30/06/2023 18 2 12 R SOS trial 42169 260350 13770 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Critical Care 17/05/2021 01/12/2023 20 4 12 A COSMOS 46567 270077 15351 NonCommercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 20/07/2021 20/07/2026 5 8 11 G iCorMicA 50157 280206 15319 NonCommercial FALSE Both Open To Recruitment Specialist Services Cardiac Services 02/08/2022 30/06/2024 15 10 10 G Psychological Impact of COVID-19 47545 282400 15461 NonCommercial FALSE Observational Completed Specialist Services Critical Care 19/04/2021 01/06/2022 09/07/2022 10 0 10 G RAPID-PROTECTION 51571 1004764 16514 NonCommercial FALSE Interventional Suspended Specialist Services Haematology, Immunology and Metabolic Medicine Nephrology & Transplant; 13/01/2023 09/09/2023 10 10 10 S HIDDEN2 V1.0 51887 306532 16254 NonCommercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 02/11/2022 30/06/2023 100 8 8 R MAST Trial 46732 276415 15553 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Critical Care Neurosciences ; 07/11/2022 01/03/2024 20 8 8 G RADAR (UK-MRA Myeloma XV) 44923 265114 15016 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 21/07/2021 29/02/2024 9 4 8 G BRIGHTLIGHT_2021 49644 296932 16163 NonCommercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 15/06/2022 31/05/2023 50 7 7 R 152
Appendix H - Specialist Services Clinical Board
Appendix H - Specialist Services Clinical Board
95 ORBITA-2 43791 242451 13965 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Cardiac Services 04/02/2020 31/08/2026 20 2 7 A FROM-16: ATMP 51376 299383 16054 NonCommercial FALSE Observational Completed Specialist Services Haematology, Immunology and Metabolic Medicine 07/03/2022 31/03/2022 17/06/2022 8 4 6 R MiNDToolkit Feasibility Study 41888 260290 16177 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Neurosciences 28/04/2022 30/09/2023 30/11/2022 1 6 6 G Prion Surveillance in Primary Immunodeficiency 4617 198343 9825 NonCommercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 29/09/2007 31/03/2024 5 0 6 G TURING 41605 258589 13662 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Nephrology & Transplant 12/04/2021 30/06/2024 4 5 6 G The conNeCT study 43247 265706 14899 NonCommercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 28/11/2022 10/08/2023 5 5 5 G ALL-RIC 38207 236871 12256 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 03/09/2019 31/05/2025 30/09/2022 2 1 4 G PROMise 46423 261523 15454 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 28/04/2021 30/06/2023 5 2 4 A CARE pilot trial 49352 289197 15717 NonCommercial FALSE Both Open To Recruitment Specialist Services Neurosciences Acute Child Health; 10/11/2021 30/04/2023 3 3 3 G ChariotMS 47180 258909 15507 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Neurosciences 15/02/2022 01/12/2023 10 3 3 R LIPS 43073 238879 14840 NonCommercial FALSE Observational Completed Specialist Services Haematology, Immunology and Metabolic Medicine 23/07/2020 30/06/2022 30/06/2022 1 0 3 G MITHRIDATE Trial 39201 248335 15335 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 23/02/2021 25/10/2023 5 1 3 A Optimal - ECRI 13 45255 275334 14957 NonCommercial FALSE Both Open To Recruitment Specialist Services Cardiac Services 27/07/2022 30/06/2023 15 3 3 R The PROTECT study 49696 285290 16189 NonCommercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 31/05/2022 01/09/2026 40 3 3 R VICTOR 46867 277635 15425 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 01/06/2021 01/06/2023 4 1 3 A WAND-NDE 41649 250468 12506 NonCommercial FALSE Observational Suspended Specialist Services Neurosciences 01/04/2019 01/04/2024 30 0 3 S ACHIEVE 49300 261542 15624 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Nephrology & Transplant 14/09/2022 31/08/2023 10 2 2 R 153
Appendix H - Specialist Services Clinical Board
Appendix H - Specialist Services Clinical Board
*COVID-19 ENLIST & ENLIST Vaccination sub-study recruitment achieved by Nephrology & Transplant team.
96
HDClarity 32381 185506 10456 NonCommercial FALSE Observational Open To Recruitment Specialist Services Neurosciences 01/06/2021 01/08/2063 7 0 2 G Kidney transplant patient decision making Version 1 53811 313751 16413 NonCommercial FALSE Observational Open To Recruitment Specialist Services Nephrology & Transplant 21/09/2022 30/11/2023 7 2 2 R ProMMise 48133 1003553 15876 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 11/05/2022 31/03/2024 3 2 2 G STELLAR 38923 233709 13352 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 25/11/2019 31/05/2025 1 1 2 G MOSAICC STUDY 45162 272092 14826 NonCommercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 01/12/2022 31/03/2024 8 1 1 R Pacing in Heart Failure Study 39844 203878 10721 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Cardiac Services 01/10/2019 31/03/2024 10 0 1 R PREDICT VF II 37274 225117 16670 NonCommercial FALSE Observational Open To Recruitment Specialist Services Cardiac Services 05/01/2023 31/07/2023 10 1 1 R SINIMA 51077 305852 16118 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Neurosciences 01/05/2022 03/05/2025 10 1 1 R FEDORA 53036 1003972 16181 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 08/02/2023 30/11/2023 2 0 0 R HAVEN 44298 251987 14908 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Nephrology & Transplant 22/06/2022 30/09/2023 6 0 0 R Huntington's Disease Fetal Tissue Collection 16305 127941 9039 NonCommercial FALSE Observational Open To Recruitment Specialist Services Neurosciences 05/03/2014 31/12/2025 2 0 0 R VITDALIZE UK 46276 274476 15355 NonCommercial FALSE Interventional Open To Recruitment Specialist Services Critical Care 06/03/2023 30/09/2023 3 0 0 R 96 2013 6969 154
Appendix H - Specialist Services Clinical Board
Appendix H - Specialist Services Clinical Board
97 Commercial Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Collaborating Directorates Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitment total RAG MUSC 5224 20579 18724 12414 Commercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 18/07/2017 30/04/2023 20 0 23 G Alexion PNH Registry M07-001 48573 277801 12788 Commercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 29/08/2014 29/08/2029 10 0 22 G The DAPA-MI Study 45853 284188 15309 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Cardiac Services 24/01/2022 31/03/2023 08/03/2023 5 16 18 G BIOMICS study 50298 302843 16078 Commercial FALSE Both Closed to Recruitment, In Follow Up Specialist Services Cardiac Services 11/05/2022 31/03/2023 03/01/2023 10 15 15 G gekoTM Pacemaker Interaction Trial 50588 283281 15283 Commercial FALSE Both Completed Specialist Services Cardiac Services 28/09/2020 30/09/2022 22/08/2022 20 0 15 R CELLCENTRIC 41027 259880 13709 Commercial FALSE Interventional Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 21/01/2020 30/06/2023 3 3 9 G ELEVATE AHP Registry 49389 295247 15963 Commercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 03/03/2022 30/06/2023 5 7 8 G Frontier 2 45040 290498 15680 Commercial FALSE Both Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 09/02/2022 30/04/2023 31/01/2023 2 5 7 G Efficacy and safety of finerenone in subjects with non-diabetic CKD 47964 1005571 16440 Commercial FALSE Interventional Open To Recruitment Specialist Services Nephrology & Transplant 14/09/2022 05/05/2023 7 6 6 G Hi-PEITHO 49534 300758 15991 Commercial FALSE Both Open To Recruitment Specialist Services Cardiac Services 04/11/2021 01/12/2023 6 2 5 G MAGNETISMM-5 48432 1004688 16388 Commercial FALSE Interventional Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 24/06/2022 31/07/2023 3 5 5 G TA-8995-304 51086 1004686 16408 Commercial FALSE Interventional Open To Recruitment Specialist Services Cardiac Services Primary care; 01/09/2022 30/09/2023 10 5 5 A aHUS REGISTRY 49176 259317 15597 Commercial FALSE Observational Open To Recruitment Specialist Services Nephrology & Transplant 24/10/2017 01/07/2024 5 0 4 A Commercial 155
Appendix H - Specialist Services Clinical Board
Appendix H - Specialist Services Clinical Board
98 ARTIOS 41166 275978 15083 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Neurosciences 15/06/2022 31/10/2022 05/10/2022 3 4 4 G CPI-0610 with or without ruxolitinib 38192 245374 13324 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 22/05/2019 31/12/2022 30/11/2022 1 0 4 G ORATORIO HAND 41823 263484 13928 Commercial FALSE Interventional Open To Recruitment Specialist Services Neurosciences 23/03/2021 31/10/2023 5 0 4 G The Radiance II Pivotal study in Stage II Hypertension. 40587 252053 15584 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Cardiac Services 21/01/2021 30/04/2022 14/04/2022 5 1 4 R A Phase 1b/2 Study of KRT-232 in Patients with Acute Myeloid Leukemia. 43302 270645 13950 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 11/02/2020 01/10/2023 15/02/2023 2 0 3 G AGAVE-201 47474 292892 15863 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 14/12/2021 30/06/2022 09/08/2022 1 2 3 G ECLIPSE 39275 249896 13931 Commercial FALSE Observational Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 08/10/2019 31/05/2023 29/11/2022 3 0 3 G Estimating Risk of Selected AEs in Pts with VWD Treated with VEYVONDI® 51844 312058 16624 Commercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 26/12/2022 30/09/2023 2 3 3 G Frontier 3 48162 297112 16278 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 13/06/2022 31/03/2023 31/03/2023 1 3 3 G IFNg-study 47240 290322 15811 Commercial FALSE Observational Completed Specialist Services Haematology, Immunology and Metabolic Medicine 14/01/2022 15/10/2022 20/07/2022 15 2 3 R MANIFEST 47239 290367 15722 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 08/11/2021 28/02/2023 02/02/2023 3 2 3 G PERSEUS 42410 285295 15378 Commercial FALSE Interventional Open To Recruitment Specialist Services Neurosciences 13/04/2021 29/12/2023 2 0 3 G Remix 2 49987 1003916 16265 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 04/02/2022 03/02/2023 18/11/2022 2 3 3 G ARTEMIS-IGAN 38242 242364 13807 Commercial FALSE Interventional Open To Recruitment Specialist Services Nephrology & Transplant 19/10/2020 31/03/2023 1 0 2 G M15-954:Venetoclax and/or Azacitidine in Newly Diagnosed HighRisk MDS 46679 285849 15419 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 24/05/2021 30/09/2022 19/06/2022 5 0 2 R 156
Appendix H - Specialist Services Clinical Board
Appendix H - Specialist Services Clinical Board
99 Paradigm 8 264158 15104 Commercial FALSE Observational Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 06/11/2020 21/11/2022 10/11/2022 2 1 2 G PEG-ADM in ARDS 42208 1003856 15954 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Critical Care 14/03/2022 29/02/2024 01/03/2023 3 1 2 R Safety and Efficacy of AMT-130 in Adults with HD 47512 288821 15572 Commercial FALSE Interventional Suspended Specialist Services Neurosciences 05/05/2022 30/04/2023 2 2 2 S APeX-N NI-PASS evaluating Berotralstat in a real-world setting 51832 310778 16288 Commercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 26/08/2022 30/06/2025 1 1 1 G ASCENT 45867 293212 16431 Commercial FALSE Interventional Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 13/10/2022 26/05/2023 1 1 1 G CSL Behring AMR BoD study 51778 314629 16455 Commercial FALSE Observational Completed Specialist Services Nephrology & Transplant 20/09/2022 21/10/2023 03/11/2022 10 1 1 R DEFINE GPS 48372 294972 15987 Commercial FALSE Interventional Open To Recruitment Specialist Services Cardiac Services 28/11/2022 29/03/2024 50 1 1 R GRAVITAS-309 40948 255759 13553 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 02/07/2019 31/12/2022 01/09/2022 1 0 1 G HERCULES 44388 285728 15288 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Neurosciences 13/04/2021 01/12/2022 02/12/2022 4 0 1 R Maternal PKU 256857 16669 Commercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 06/02/2023 31/07/2024 1 1 1 G PTC-PIVOT HD 51614 1004960 16273 Commercial FALSE Interventional Open To Recruitment Specialist Services Neurosciences 01/08/2022 31/08/2023 5 1 1 R Recombinant human alkaline phosphatase survival trial 44949 284004 15354 Commercial FALSE Interventional Completed Specialist Services Critical Care 07/10/2021 01/07/2023 21/07/2022 20 0 1 R REFORM 44696 270648 15501 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Cardiac Services 08/04/2021 31/05/2023 14/10/2022 5 0 1 R SNP3 47765 292203 15858 Commercial FALSE Interventional Open To Recruitment Specialist Services Neurosciences 26/10/2021 30/05/2023 1 1 1 G SPARKLE 47136 270514 15100 Commercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 21/01/2022 31/12/2023 1 1 1 G The Rare disease registry Program (Gaucher, Fabry, Pompe, MPS1) 232441 13917 Commercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 13/09/2019 31/12/2035 10 0 1 R VIALE-M 43993 275346 15094 Commercial FALSE Interventional Suspended Specialist Services Haematology, Immunology and Metabolic Medicine 23/10/2020 31/10/2023 3 0 1 S 157
Appendix H - Specialist Services Clinical Board
Appendix H - Specialist Services Clinical Board
100 WAY4001 48447 289319 15798 Commercial FALSE Observational Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 13/04/2022 30/11/2035 1 1 1 G ASTER 51320 1004588 16484 Commercial FALSE Interventional Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 05/01/2023 1 0 0 B BLU HARBOR 49546 1003872 16332 Commercial FALSE Interventional Suspended Specialist Services Haematology, Immunology and Metabolic Medicine 26/09/2022 31/03/2024 3 0 0 S CML with T315I mutationv00 51239 307898 16216 Commercial FALSE Observational Completed Specialist Services Haematology, Immunology and Metabolic Medicine 15/03/2022 20/09/2022 22/09/2022 2 0 0 W CONVERT 47058 285414 15791 Commercial FALSE [NOT SET] Open To Recruitment Specialist Services Cardiac Services 17/11/2022 31/03/2023 5 0 0 R ENHANCE-2 47943 297376 15984 Commercial FALSE Interventional Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 14/02/2023 0 0 0 B Haven 7 47449 283985 15745 Commercial FALSE Interventional Completed Specialist Services Haematology, Immunology and Metabolic Medicine 04/05/2021 31/05/2022 31/05/2022 1 0 0 R MAGNOLIA 51538 1004746 16483 Commercial FALSE Interventional Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 05/01/2023 0 0 0 B Neurocrine NBI-921352FOS202 1005029 16713 Commercial FALSE Interventional Closed to Recruitment, No Follow Up Specialist Services Neurosciences 01/12/2022 31/03/2023 06/03/2023 3 0 0 R OASIS F901318/0041 50214 302060 16442 Commercial FALSE Interventional Open To Recruitment Specialist Services Haematology, Immunology and Metabolic Medicine 07/02/2023 30/11/2024 1 0 0 R OBIC301OUS 53755 1006592 16704 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 23/03/2023 30/06/2023 14/04/2023 5 0 0 R Phase 3 study to Compare T-Guard to Ruxolitinib for SR-aGVHD patients. 49662 301767 16081 Commercial FALSE Interventional Closed to Recruitment, No Follow Up Specialist Services Haematology, Immunology and Metabolic Medicine 23/09/2022 30/11/2024 09/01/2023 2 0 0 R Phase II study of sacubitril/valsartan in patients with nHCM 42896 275969 15203 Commercial FALSE Interventional Completed Specialist Services Cardiac Services 24/09/2021 14/03/2022 29/07/2022 3 0 0 R REGN1979 in patients with relapsed or refractory follicular lymphoma 36509 262118 13801 Commercial FALSE Interventional Suspended Specialist Services Haematology, Immunology and Metabolic Medicine 29/10/2020 30/11/2022 2 0 0 S S.T.A.R. 50410 304241 16195 Commercial FALSE Interventional Open To Recruitment Specialist Services Neurosciences 09/02/2023 3 0 0 R S.T.A.R.S. Extension 50508 304242 16202 Commercial FALSE Interventional Open To Recruitment Specialist Services Neurosciences 09/02/2023 3 0 0 R Strike-PE 52817 314721 16752 Commercial FALSE [NOT SET] Open To Recruitment Specialist Services Cardiac Services 31/03/2023 28/02/2025 25 0 0 R 62 97 210 158
Appendix I - Surgical Services Clinical Board
Perioperative Care Publications
Bailey, D.M., Halligan, C.L., Davies, R.G., Funnell, A., Appadurai, I.R., Rose, G.A., Rimmer, L., Jubouri, M., Coselli, J.S., Williams, I.M. and Bashir, M. (2022). Subjective assessment underestimates surgical risk: On the potential benefits of cardiopulmonary exercise testing for open thoracoabdominal repair. Journal of Cardiac Surgery, 37(8), pp.2258–2265. doi: https://doi.org/10.1111/jocs.16574.
Bailey, D.M., Rose, G.A., O’Donovan, D., Locker, D., Appadurai, I.R., Davies, R.G., Whiston, R.J., Bashir, M., Lewis, M.H. and Williams, I.M. (2022). Retroperitoneal Compared to Transperitoneal Approach for Open Abdominal Aortic Aneurysm Repair Is Associated with Reduced Systemic Inflammation and Postoperative Morbidity. Aorta, 10(5), pp.225–234. doi: https://doi.org/10.1055/s-0042-1749173.
Bell, S.F., Collis, R.E. and Collins, P.W. (2022). Comparison of haematological indices and transfusion management in severe and massive postpartum haemorrhage: analysis of a two-year national prospective observational study. International Journal of Obstetric Anesthesia, 50, p.103547. doi: https://doi.org/10.1016/j.ijoa.2022.103547.
Bell, S.F., de Lloyd, L., Preston, N. and Collins, P.W. (2023). Managing the coagulopathy of postpartum haemorrhage: an evolving role for viscoelastic haemostatic assays. Journal of Thrombosis and Haemostasis. doi: https://doi.org/10.1016/j.jtha.2023.03.029.
Brogly, N., Varvinskiy, A., Varosyan, A., Ateleanu, B., Engelhardt, W., Geldner, G., Madách, K., Ringvold, E.-M. . and Zerafa, M. (2022). On-Line Assessment (OLA) as a preparation for the European Diploma in Anaesthesiology and Intensive Care (EDAIC), a retrospective observational study on the results and the potential impact on the Part-I examination. Revista Española de Anestesiología y Reanimación (English Edition), 69(8), pp.454–462. doi: https://doi.org/10.1016/j.redare.2022.08.006.
Chandler, H.L., Stickland, R.C., Patitucci, E., Germuska, M., Chiarelli, A.M., Foster, C.E., BhomeDhaliwal, S., Lancaster, T.M., Saxena, N.K., Khot, S., Tomassini, V. and Wise, R.G. (2022). Reduced brain oxygen metabolism in patients with multiple sclerosis: Evidence from dual-calibrated functional MRI. Journal of Cerebral Blood Flow and Metabolism, 43(1), pp.115–128. doi: https://doi. org/10.1177/0271678x221121849.
Chiarelli, A.M., Germuska, M., Chandler, H.L., Stickland, R.C., Patitucci, E., Biondetti, E., Mascali, D., Saxena, N.K., Khot, S., Steventon, J.J., Foster, C.E., Rodríguez-Soto, A.E., Englund, E.K., Murphy, K., Tomassini, V., Wehrli, F.W. and Wise, R.G. (2022). A flow-diffusion model of oxygen transport for quantitative mapping of cerebral metabolic rate of oxygen (CMRO2) with single gas calibrated fMRI. Journal of Cerebral Blood Flow & Metabolism, 42(7), pp.0271678X2210773. doi: https://doi. org/10.1177/0271678x221077332.
Clayton-Smith, M., Narayanan, H., Shelton, C., Bates, L., Brennan, F., Deido, B., Donnellon, M., Dorey, J., Evans, B., Gower, J., Hamdaoui, Y., Hitchman, J., Kinsella, S.M., Knagg, R., Lawson, C., Morris, D., Pegna, V., Radcliffe, T., Schaff, O. and Sheppard, T. (2023). Greener Operations: a James Lind Alliance Priority Setting Partnership to define research priorities in environmentally sustainable perioperative practice through a structured consensus approach. BMJ Open, 13(3), pp.e066622. doi: https://doi. org/10.1136/bmjopen-2022-066622.
Collis, R. and Bell, S. (2022). The Role of Thromboelastography during the Management of Postpartum Hemorrhage: Background, Evidence, and Practical Application. Seminars in Thrombosis and Hemostasis, 49(2), pp.145–161. doi: https://doi.org/10.1055/s-0042-1757895.
Corner, H., Barley, M. and Metodiev, Y. (2023). The use of processed electroencephalography (pEEG) in obstetric anaesthesia: a narrative review. International Journal of Obstetric Anesthesia, 54, pp.103650. doi: https://doi.org/10.1016/j.ijoa.2023.103650.
159
Appendix I - Surgical Services Clinical Board
Dale, M., Bell, S.F., O’Connell, S., Scarr, C., James, K., John, M., Collis, R.E., Collins, P.W. and CarolanRees, G. (2022). What is the Economic Cost of Providing an All Wales Postpartum Haemorrhage Quality Improvement Initiative (OBS Cymru)? A Cost-Consequences Comparison with Standard Care. PharmacoEconomics - Open, 6(6), pp.847–857. doi: https://doi.org/10.1007/s41669-022-00362-2.
de Lloyd, L., Jenkins, P.P., Bell, S.F., Mutch, N.J., Freyer, J., Badenes, P.M., James, D., Ridgeway, A., Cohen, L., Roberts, T., Field, V., Collis, R.E. and Collins, P.L. (2022). Acute obstetric coagulopathy during postpartum hemorrhage is caused by hyperfibrinolysis and dysfibrinogenemia: an observational cohort study. Journal of Thrombosis and Haemostasis, 21(4), pp.862–879. doi: https://doi.org/10.1016/j. jtha.2022.11.036.
Hawkins, T., Agarwal, S. and Evans, C.R. (2023). Centre for Perioperative Care anaemia guideline: implications for anaesthesia. British Journal of Anaesthesia, 130(2), pp.115–119. doi: https://doi. org/10.1016/j.bja.2022.11.009.
Kelly, F.E., Frerk, C., Bailey, C.R., Cook, T.M., Ferguson, K., Flin, R., Fong, K., Groom, P., John, C., Lang, A.R., Meek, T., Miller, K.L., Richmond, L., Sevdalis, N. and Stacey, M.R. (2023). Human factors in anaesthesia: a narrative review. Anaesthesia, 78(4), pp.479–490. doi: https://doi.org/10.1111/ anae.15920.
Kelly, F.E., Frerk, C., Bailey, C.R., Cook, T.M., Ferguson, K., Flin, R., Fong, K., Groom, P., John, C., Lang, A.R., Meek, T., Miller, K.L., Richmond, L., Sevdalis, N. and Stacey, M.R. (2023). Implementing human factors in anaesthesia: guidance for clinicians, departments and hospitals. Anaesthesia, 78(4). doi: https://doi.org/10.1111/anae.15941.
Metodiev, Y. and Lucas, D.N. (2022). The role of total intravenous anaesthesia for caesarean delivery. International Journal of Obstetric Anesthesia, 51, p.103548. doi: https://doi.org/10.1016/j. ijoa.2022.103548.
Metodiev, Y. and Mushambi, M. (2023). The role of supraglottic airway devices in obstetric anaesthesia. Current Opinion in Anaesthesiology, 36(3), pp.276–280. doi: https://doi.org/10.1097/ aco.0000000000001241.
Minto, T., Abdelrahman, T., Jones, L.E., Wheat, J., Key, T., Shivakumar, N., Ansell, J., Seddon, O., Cronin, A., Tomkinson, A., Theron, A., Trickett, R., Sagua, N., Sultana, S., Clark, A., McKay, E., Johnson, A., Behera, K., Towler, J. and Kynaston, H. (2022). Safety of maintaining elective and emergency surgery during the COVID-19 pandemic with the introduction of a Protected Elective Surgical Unit (PESU): A cross-specialty evaluation of 30-day outcomes in 9,925 patients undergoing surgery in a University Health Board. Surgery Open Science, 10, pp.168–173. doi: https://doi.org/10.1016/j. sopen.2022.09.005.
Perry, H., Reeves, N., Ansell, J., Cornish, J., Torkington, J., Morris, D.S., Brennan, F. and Horwood, J. (2022). Innovations towards achieving environmentally sustainable operating theatres: A systematic review. The Surgeon, 21(3). doi: https://doi.org/10.1016/j.surge.2022.04.012.
Potter, T.L., Cronin, J.W., Kua, J., Nurmi, E., Wong, D.J.N., Ahmad, I., Cook, T., El-Boghdadly, K. and AeroComp Trainee Research Networks (2022). Aerosol precautions and airway complications: a national prospective multicentre cohort study. Anaesthesia, 78(1), pp.23–35. doi: https://doi. org/10.1111/anae.15851.
Rose, G.A., Davies, R.G., Appadurai, I.R., Williams, I.M., Bashir, M., Berg, R.M.G., Poole, D.C. and Bailey, D.M. (2022). ‘Fit for surgery’: the relationship between cardiorespiratory fitness and postoperative outcomes. Experimental Physiology, 107(8), pp.787–799. doi: https://doi.org/10.1113/EP090156.
160
Appendix I - Surgical Services Clinical Board
Rose, G.A., Davies, R.G., Torkington, J., Berg, R.M.G., Appadurai, I.R., Poole, D.C. and Bailey, D.M. (2023). Assessing cardiorespiratory fitness relative to sex improves surgical risk stratification. European Journal of Clinical Investigation. doi: https://doi.org/10.1111/eci.13981.
Sharma, S., Zaher, S., Rodrigues, P.R.S., Davies, L.C., Edkins, S., Strang, A., Chakraborty, M., Watkins, W.J., Andrews, R., Parkinson, E., Angelopoulos, N., Moet, L., Shepherd, F., Davies, K.M.M., White, D., Oram, S., Siddall, K., Keeping, V., Simpson, K. and Faggian, F. (2022). mSep: investigating physiological and immune-metabolic biomarkers in septic and healthy pregnant women to predict feto-maternal immune health – a prospective observational cohort study protocol. BMJ Open, 12(9), p.e066382. doi: https://doi.org/10.1136/bmjopen-2022-066382.
Vora, J., Leslie, D. and Stacey, M. (2022). Awake tracheal intubation. BJA Education, 22(8), pp.298–305. doi: https://doi.org/10.1016/j.bjae.2022.03.006.
Warde, D., Huckle, D. and Hatch, D. (2023). The first fifty years of The Association of Paediatric Anaesthetists of Great Britain and Ireland. Paediatric Anaesthesia, 33(7). doi: https://doi.org/10.1111/ pan.14676.
Grants Project Official Short/ Full Title OBS UK Lead Investigator Dr Sarah Bell Professor Peter Collins (Cardiff University) Duration of Grant (Date from/to) May 2023-Jan 2027 Funder Name/ Source of Funding NIHR Grant Awarded (Total) £3.65m 161
Appendix I - Surgical Services Clinical Board
Initiatives
Contributed by Dr Danielle Huckle
Clinical and cost-effectiveness of a maternity quality improvement programme to reduce excess bleeding and need for transfusion after childbirth: the Obstetric Bleeding Study UK (OBS UK) Stepped Wedge Cluster Randomised Trial
Excess bleeding is the most common complication of childbirth. Every year about 50,000 women in the UK lose 1 litre (almost 2 pints) of blood or more. Many women need a blood transfusion, are admitted to intensive care and find the experience of bleeding traumatic, developing mental health issues after having their baby. Women from ethnic backgrounds are disproportionately affected. We developed a care bundle for treating bleeding after childbirth called the ‘Obstetric Bleeding Strategy’ (OBS) and in a pilot study we rolled this out to maternity units, using quality improvement methods eg education and training. We saw a fall in the number of women having excess bleeding and fewer needed blood transfusion. Staff described improvements in teamwork. These results are encouraging, but since this was only a small study with limited data, we do not know whether these improvements were due to the change in care or whether the care bundle is value for money. To find this out, the OBS UK study will compare clinical outcomes, psychological wellbeing and cost of care for women in maternity units that use the care bundle with women receiving standard care.
We will use routine NHS data on about 189,000 women from 36 NHS maternity units over 30 months, including data about ethnicity. Women’s data will be included, whether they have excess bleeding or not. All maternity units will start with a period of standard care, they will then adopt the OBS care bundle using quality improvement methods over 9 months, followed by a period of OBS care. To measure how effective the OBS care bundle is, we will compare rates of blood transfusion before and after it is introduced. We will also study intensive care admission, hysterectomy and breastfeeding rates. Women with experience of excess bleeding have advised us on the study’s importance, its design and outcomes. Add-on studies will look at the effect of the OBS care bundle on the psychological wellbeing of women and birthing partners and how units adopt the care bundle, with special attention to ethnicity and organisational factors. This will include interviewing women, their birth partners and staff to understand their experiences of excess bleeding and whether the OBS care bundle changes how teams deliver care. The cost will also be studied.
Our research will establish whether (and how) the OBS care bundle improves outcomes and experiences of women giving birth. We will share our findings widely with the support of UK maternity providers and patient representatives.
Please note: all grants, publications, research impact case studies or initiatives have been provided by Clinical Board and Directorate R&D leads. Therefore, there may be further grants, publications, case studies or initiatives not mentioned in this report.
162
Appendix I - Surgical Services Clinical Board
Appendix I - Surgical Services Clinical Board
Non-Commercial
107
Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Collaborating Directorates Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitment total RAG BBRC Versus Arthritis - Clinical Samples 11302 51853 10107 NonCommercial FALSE Observational Closed to Recruitment, In Follow Up Surgical Services Trauma & Orthopaedics 14/10/2010 31/12/2022 31/12/2022 1000 0 1378 G PQIP 32256 215928 10749 NonCommercial FALSE Observational Open To Recruitment Surgical Services Perioperative Care 04/02/2019 31/10/2023 600 364 991 G Urinary Biomarker URO17 Study 49958 298341 15872 NonCommercial FALSE Observational Closed to Recruitment, In Follow Up Surgical Services Urology 07/07/2021 30/11/2022 30/11/2022 500 209 580 G BBRC Versus Arthritis - 3D motion analysis (MOCAP) 10478 51853 10099 NonCommercial FALSE Observational Closed to Recruitment, In Follow Up Surgical Services Trauma & Orthopaedics 14/10/2010 31/12/2022 31/12/2022 471 1 473 G UK Genetic Prostate Cancer Study 869 36812 2458 NonCommercial FALSE Observational Open To Recruitment Surgical Services Urology 18/02/2014 31/12/2027 268 9 279 G Validation of patient centric measures in urolithiasis 39841 217163 10493 NonCommercial FALSE Observational Open To Recruitment Surgical Services Urology 23/04/2018 31/12/2023 250 57 266 G Urine Biomarkers for detecting prostate cancer 47442 96199 15430 NonCommercial FALSE Observational Open To Recruitment Surgical Services Urology 06/05/2021 31/10/2023 225 20 226 G ROSSINI 2 39722 247285 13657 NonCommercial FALSE Interventional Open To Recruitment Surgical Services General & Vascular Surgery Urology; 13/11/2019 30/04/2023 150 61 171 G Add-Aspirin 18067 120104 8623 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Surgical Services Urology 10/11/2015 31/03/2023 31/01/2023 81 5 131 G Mapping the lipid envelope composition of SARS-CoV-2 51207 299479 16053 NonCommercial FALSE Observational Completed Surgical Services Hospital Dental 25/03/2022 31/12/2022 24/06/2022 35 96 130 G POLO 51370 301310 16032 NonCommercial FALSE Observational Open To Recruitment Surgical Services General & Vascular Surgery 15/10/2021 23/09/2023 90 54 97 G TIGER study 47861 279600 15721 NonCommercial FALSE Observational Open To Recruitment Surgical Services General & Vascular Surgery 01/06/2021 01/04/2023 15 46 74 G TRANSLATE 50362 293939 16017 NonCommercial FALSE Interventional Open To Recruitment Surgical Services Urology 21/01/2022 31/12/2023 1 51 68 G POLARiS feasibility 51890 307764 16252 NonCommercial FALSE Interventional Open To Recruitment Surgical Services General & Vascular Surgery 25/05/2022 30/06/2023 60 63 63 G Non-Commercial 163
Appendix I - Surgical Services Clinical Board
Appendix I - Surgical Services Clinical Board

108 REINFORCE 53026 311223 16555 NonCommercial FALSE Observational Open To Recruitment Other Executive / Central General & Vascular Surgery; Obstetrics & Gynaecology; Urology; 07/10/2022 01/07/2024 185 53 53 G EMT2 34700 199321 14013 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Surgical Services General & Vascular Surgery 22/06/2020 31/08/2023 01/09/2022 35 7 46 G BBRC Versus Arthritis - MRI imaging for the joint 10480 51853 10100 NonCommercial FALSE Observational Closed to Recruitment, In Follow Up Surgical Services Trauma & Orthopaedics 14/10/2010 31/12/2022 31/12/2022 40 0 42 G FLO-ELA 33869 214459 9593 NonCommercial FALSE Interventional Open To Recruitment Surgical Services Perioperative Care 17/06/2021 31/12/2023 30 17 42 G NOTACS Study 45298 278290 15109 NonCommercial FALSE Interventional Open To Recruitment Surgical Services Perioperative Care 20/08/2021 01/01/2023 20 19 42 G ALLEGRO 39231 231280 12292 NonCommercial FALSE Interventional Open To Recruitment Surgical Services General & Vascular Surgery 06/06/2019 30/04/2023 6 2 36 G BBRC Versus ArthritisFluoroscopic imaging 10481 51853 10101 NonCommercial FALSE Observational Closed to Recruitment, In Follow Up Surgical Services Trauma & Orthopaedics 14/10/2010 31/12/2022 31/12/2022 33 0 33 G CUBRIC MRI Study of the prostate 51683 300727 16117 NonCommercial FALSE Observational Suspended Surgical Services Urology 01/04/2022 01/03/2023 50 31 31 S REVAMP 52475 298826 16344 NonCommercial FALSE Observational Open To Recruitment Surgical Services Ophthalmolog y 01/08/2022 31/07/2025 63 30 30 G ITACS Trial 34443 217991 10494 NonCommercial FALSE Interventional Completed Surgical Services Perioperative Care 15/01/2018 31/12/2023 31/12/2022 36 0 28 R Proximie Live Plus 49086 281153 15487 NonCommercial FALSE Observational Closed to Recruitment, Follow Up Complete Surgical Services General & Vascular Surgery 01/05/2021 31/03/2023 31/03/2023 30 0 28 HG Sarcopenia, frailty and nutritional status in colorectal cancer 44421 231694 13711 NonCommercial FALSE Observational Open To Recruitment Surgical Services General & Vascular Surgery 02/03/2021 30/06/2023 10 15 25 G Using Artificial Intelligence to Diagnose ‘Glue Ear' in Children 52253 311902 16294 NonCommercial FALSE Interventional Open To Recruitment Surgical Services ENT 07/10/2022 31/05/2023 125 25 25 R PATHOS 18645 159836 9177 NonCommercial FALSE Interventional Open To Recruitment Surgical Services ENT 27/05/2016 31/10/2023 5 8 22 G VITAL 50027 297034 15944 NonCommercial FALSE Interventional Open To Recruitment Surgical Services Perioperative Care 01/12/2022 30/04/2024 70 22 22 G IntAct 34216 216411 10470 NonCommercial FALSE Interventional Open To Recruitment Surgical Services General & Vascular Surgery 12/06/2018 30/09/2023 16 3 21 G * 164
Appendix I - Surgical Services Clinical Board
Appendix I - Surgical Services Clinical Board
109 EMCAT in hand surgery 51091 302403 16168 NonCommercial FALSE Observational Completed Surgical Services Trauma & Orthopaedics 12/07/2022 14/01/2023 05/08/2022 20 20 20 G WHiTE 10- LIT 49156 16367 NonCommercial FALSE Interventional Open To Recruitment Surgical Services Perioperative Care Trauma & Orthopaedics; 03/05/2022 30/11/2025 56 19 19 G I-DECIDE 52153 311955 16324 NonCommercial FALSE Observational Closed to Recruitment, No Follow Up Surgical Services Trauma & Orthopaedics 12/07/2022 31/03/2023 28/02/2023 16 14 14 R BASIS Study 48820 291133 15769 NonCommercial FALSE Both Open To Recruitment Surgical Services Trauma & Orthopaedics 06/01/2022 28/02/2025 36 13 13 A MOSES 44086 256047 14923 NonCommercial FALSE Observational Open To Recruitment Surgical Services ENT 03/06/2020 01/12/2027 5 3 12 G PERCEIVE study 48050 290579 15620 NonCommercial FALSE Observational Completed Surgical Services General & Vascular Surgery 26/11/2021 31/03/2023 31/12/2022 10 9 11 G Surgery versus non-surgical splint treatment for PPS finger fractures 45052 277440 15039 NonCommercial FALSE Interventional Closed to Recruitment, In Follow Up Surgical Services Trauma & Orthopaedics 25/11/2020 01/03/2024 15/02/2023 20 5 10 R DEFINE 51814 309808 16240 NonCommercial FALSE Observational Completed Surgical Services General & Vascular Surgery 06/07/2022 01/08/2022 01/08/2022 20 8 8 R SCIENCE 41515 259931 13592 NonCommercial FALSE Interventional Open To Recruitment Surgical Services Trauma & Orthopaedics 04/07/2019 31/12/2029 2 4 8 G SOFFT 45217 276873 15189 NonCommercial FALSE Both Closed to Recruitment, In Follow Up Surgical Services Trauma & Orthopaedics 23/11/2020 31/12/2022 31/12/2022 4 3 8 G UNICORNS 44484 258638 15260 NonCommercial FALSE Observational Open To Recruitment Surgical Services Ophthalmolog y 02/02/2021 01/05/2024 5 4 8 G WHiTE 11- FRUITI 49158 287755 15712 NonCommercial FALSE Interventional Open To Recruitment Surgical Services Trauma & Orthopaedics 16/07/2021 01/04/2024 13 6 8 A PASHiOn 46724 280261 15418 NonCommercial FALSE Interventional Open To Recruitment Surgical Services Trauma & Orthopaedics 02/09/2022 30/04/2024 20 7 7 G The ORION Trial 50709 300449 16363 NonCommercial FALSE Interventional Open To Recruitment Surgical Services General & Vascular Surgery 11/10/2022 31/05/2023 10 5 5 R AIR: RRP 38013 164160 13506 NonCommercial FALSE Observational Closed to Recruitment, No Follow Up Surgical Services ENT 01/04/2019 19/12/2024 31/08/2022 4 0 4 G HUSH 45705 277059 15244 NonCommercial FALSE Interventional Open To Recruitment Surgical Services Trauma & Orthopaedics 24/11/2020 30/09/2023 10 2 4 R NOSTRA 38071 211232 13279 NonCommercial FALSE Observational Open To Recruitment Surgical Services General & Vascular Surgery 02/12/2019 31/12/2023 3 1 2 A IONOE 48244 280342 15737 NonCommercial FALSE Observational Open To Recruitment Surgical Services ENT 16/05/2022 31/12/2023 10 1 1 R STAR-TReC 31203 173279 9537 NonCommercial FALSE Interventional Open To Recruitment Surgical Services General & Vascular Surgery 19/07/2018 31/12/2023 4 0 1 R 165
Appendix I - Surgical Services Clinical Board
Appendix I - Surgical Services Clinical Board
*REINFORCE counted under Other Clinical Board but recruitment split between Children & Women's and Surgical Services
110
SUPERSTAR 50531 296972 16004 NonCommercial FALSE Observational Closed to Recruitment, No Follow Up Surgical Services ENT 04/02/2022 31/03/2023 18/10/2022 6 1 1 R WHiTE 12DUALITY 53416 16604 NonCommercial FALSE Interventional Open To Recruitment Surgical Services Trauma & Orthopaedics 02/12/2022 30/09/2024 15 1 1 R Best-Of 37926 223997 10723 NonCommercial FALSE Interventional Closed to Recruitment, No Follow Up Surgical Services ENT 16/10/2020 09/01/2023 09/01/2023 1 0 0 R DAVE 42168 265680 15003 NonCommercial FALSE Interventional Closed to Recruitment, No Follow Up Surgical Services General & Vascular Surgery 26/04/2021 30/09/2023 17/01/2023 4 0 0 R EndoNET 52372 1005155 16382 NonCommercial FALSE Interventional Open To Recruitment Surgical Services General & Vascular Surgery 13/04/2023 31/07/2027 10 0 0 R ExPeCT 50307 291897 15927 NonCommercial FALSE Interventional Open To Recruitment Surgical Services General & Vascular Surgery 11/11/2022 31/07/2023 3 0 0 R OnCoRe 37613 318679 14825 NonCommercial FALSE Observational Open To Recruitment Surgical Services General & Vascular Surgery 04/07/2022 31/10/2022 3 0 0 R ORiF 40063 248460 13446 NonCommercial FALSE Interventional Open To Recruitment Surgical Services Trauma & Orthopaedics 07/03/2023 31/12/2023 10 0 0 R Patient and staff experiences of perioperative cardiac arrest 49377 278219 15852 NonCommercial FALSE Observational Completed Surgical Services General & Vascular Surgery 16/12/2021 01/02/2023 29/06/2022 13 0 0 R POETIC-A 44805 271343 14989 NonCommercial FALSE Interventional Open To Recruitment Surgical Services General & Vascular Surgery 06/04/2023 30/09/2026 20 0 0 R The COACH Trial 50095 297574 15968 NonCommercial FALSE Interventional Open To Recruitment Surgical Services ENT 04/01/2022 28/02/2025 16 0 0 R 60 1394 5618 166
Appendix I - Surgical Services Clinical Board
Appendix I - Surgical Services Clinical Board
111 Commercial Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitment total RAG PROGRESS 49711 297700 16370 Commercial FALSE Observational Completed Surgical Services Hospital Dental 17/10/2022 31/12/2022 16/12/2022 68 69 69 G METAPAIN 47806 288863 15763 Commercial FALSE Interventional Closed to Recruitment, In Follow Up Surgical Services Hospital Dental 24/08/2021 31/05/2022 04/10/2022 20 4 25 G IPCOTT 46771 285979 15608 Commercial FALSE Both Open To Recruitment Surgical Services General & Vascular Surgery 24/03/2021 30/09/2023 4 2 8 G Tri-Solfen Version 3.0 45055 236852 12354 Commercial FALSE Interventional Suspended Surgical Services General & Vascular Surgery 03/01/2019 01/08/2022 5 0 5 S A Prospective Natural History Study of Patients with ADOA 51840 308673 16281 Commercial FALSE Observational Open To Recruitment Surgical Services Ophthalmology 06/09/2022 31/03/2025 4 4 4 G Debrisoft and healing 45058 263039 13804 Commercial FALSE Interventional Open To Recruitment Surgical Services General & Vascular Surgery 03/01/2020 01/08/2023 5 1 4 A HZN-402 50824 1004199 16270 Commercial FALSE Interventional Open To Recruitment Surgical Services Ophthalmology 13/10/2022 31/08/2023 2 1 1 A MARS study 52492 314752 16437 Commercial FALSE Interventional Open To Recruitment Surgical Services General & Vascular Surgery 13/12/2022 30/04/2024 10 1 1 R QB46C-H08 53709 1005472 16646 Commercial FALSE Interventional Open To Recruitment Surgical Services ENT 28/02/2023 31/05/2024 5 0 0 R 9 82 117 Commercial 167
Other (Split) Clinical Board
Other (Split) Clinical Board
Non-Commercial
*REINFORCE counted under Other Clinical Board but recruitment split between Children & Women's and Surgical Services
112
Short Title CPMS Study ID IRAS ID ReDA ID Commercial Status Musketeers’ Memorandum Study Design Local Status Clinical Board Lead Directorate Site Actual Recruitment Open Date Site Planned Recruitment Close Date Site Actual Recruitment Close Date Target 2022-23 Recruits Recruitment total RAG ProJudge 52310 279835 15458 NonCommercial FALSE Observational Open To Recruitment Other Nursing 05/02/2021 31/10/2023 30 0 11 R Determining Best Preventative Social Care Practice 51679 301786 16219 NonCommercial FALSE Observational Open To Recruitment Other Executive / Central 16/05/2022 31/05/2023 1 4 4 G IMPACT MULTIAGENCY 44249 266293 13898 NonCommercial FALSE Observational Completed Other Executive / Central 01/12/2020 31/08/2022 13/07/2022 2 0 0 R 3 4 15 Non-Commercial 168















 Figures 2a and 2b - Number of Open & Recruiting Commercial (left) and Number of Participants Recruited (right) per NHS organisation (2022-23)
Figures 2a and 2b - Number of Open & Recruiting Commercial (left) and Number of Participants Recruited (right) per NHS organisation (2022-23)