Novavax Clinical Trial Experience

February 23, 2023
 Rajan K. Merchant MD
Rajan K. Merchant MD






February 23, 2023
 Rajan K. Merchant MD
Rajan K. Merchant MD




● COVID-19 - Total number of US cases >100 million. ● COVID-19 - Total US Deaths 1.1 million.
COVID-19 - Third leading cause of death: 2020, 2021 and 2022.




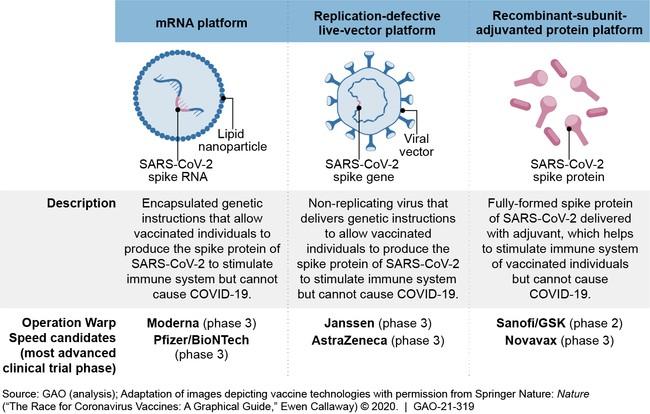


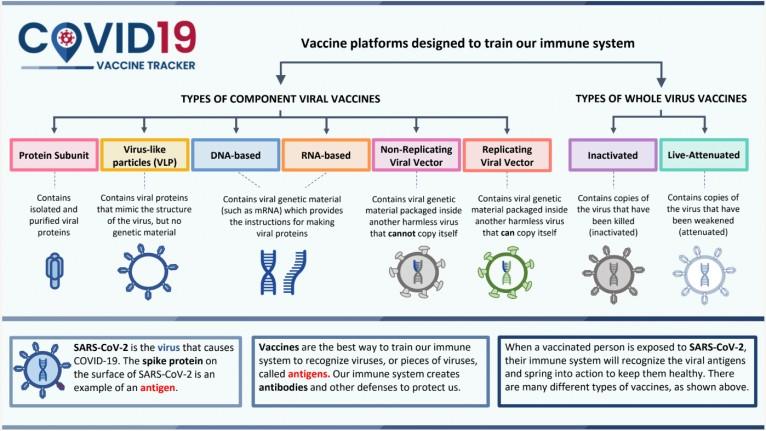

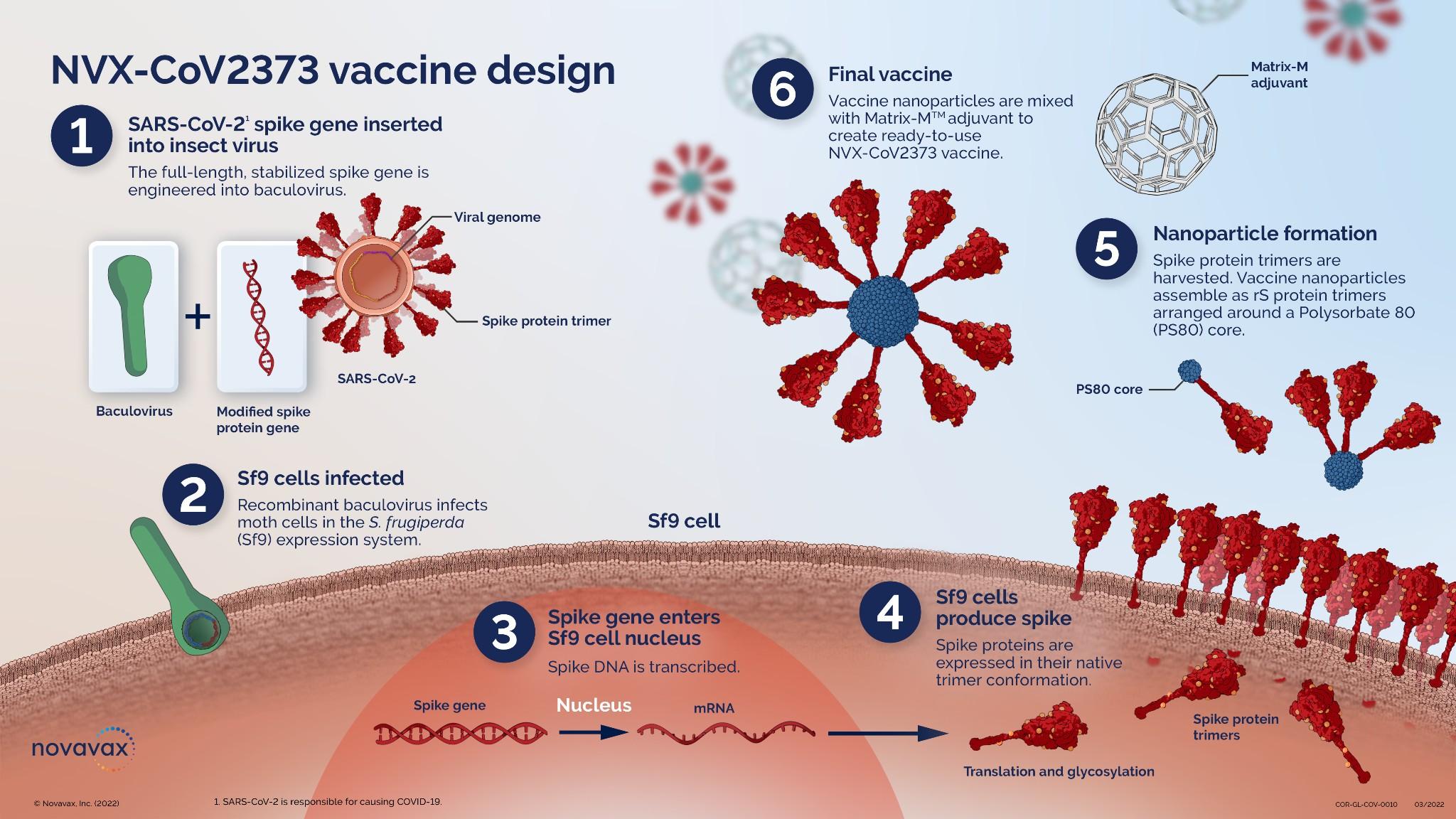

● Feasibility
○ Completed in July 2020 and selected 3 weeks later.
○ Goal was established to enroll 200 patients .
○ Anticipated Trial start in September 2020.
● Site Visit
○ Daily coordination and emails between clinic operations and research management.
Phones, refrigerators, storage, supplies, space, and personnel.

● Patient Selection
○ Worked on communication and messaging to ensure diversity of trial participants.
○ Informatics team help to identify high risk individuals with various socio economic standings.

● Budgets and Contracting
○ Spent several months to get sponsor, CRO, and legal teams at Dignity to agree on specific language . Lucrative budget was negotiated.
● Delay in Trial Start Date

● PREVENT 19 The PRE-fusion protein subunit Vaccine Efficacy Novavax Trial

● Phase 3 Randomized clinical trial of the NVX-2373 with Matrix M adjuvant
● Study was funded by Operation Warp Speed.
● The trial was initiated in December 2020. Two Year study
● Planned Completion April 2023.

● Participant demand
○
No marketing
○ No dedicated resources for patient experience or direct contact.
○ Using text messaging solution
● Team Background

● Enrollment Target
○ 30- 40 patients per week.

● Data Collection
○ EHR Tools
Protocol amendments
Communications

● Eligibility criteria
● Social Media






● NVX-CoV2373 achieved 90.4% efficacy overall and 100% efficacy against moderate to severe COVID -19
NVX-Cov2373 demonstrated a reassuring safety and tolerability profile.




NVX-CoV2373 WHO EUL and available in 170 countries.
Partnership with Serum Institute of India
US EUA Approved June 2022

November 2022
● World Health Organization Updates Emergency Use Listing for Novavax

○ Nuvaxovid COVID-19 Vaccine as a Primary Series in Adolescents
○ Booster in Adults

Retention
Vaccine Mandates
Vaccine Hesitancy
Vaccine Passports
Childhood and Adolescents Vaccination
Boosters ○ Reviewing eligibility.


● Incorporate more technology
○ Virtual recruitment
○ eConsents and eSignatures

○ Remote enrollment
○ Remote monitoring
● In person visits needed for IP
● Lab Samples and Collections
○ In person
○ Home collection
● Preparation plays a big role to be successful. The more we can prepare and use tools that are available to our advantage the easier it is to adapt to the rapidly changing dynamics. A key line from the movie Apollo 12. I don't care about what anything was DESIGNED to do, I care about what it CAN do.
● Being Flexible and adaptable is necessary to lead and succeed in any challenges big or small.
● Research possibilities are abundant and we need to create an environment that can help connect, initiate, and conduct research with the talent that this organization possesses.

● Learning strengths and weaknesses of individuals will create a more dynamic work environment and create a team that will allow you to achieve targets that may have seemed not possible .

● Communication, the most fundamental tool. When the challenges seem most insurmountable, effective communication is essential.
● Social media plays a big role in research communities and we will need to use these platforms in the future to help and also to do no harm to the studies being conducted.
● Recognizing that I am up here because of the team around me created the success
● The feeling of contribution to a greater cause and gratitude for being given the opportunity to be a part of this. Each and every day I showed up I knew that no matter what my role that day, I was contributing in some way and it gave immediate gratification knowing there was trust in the team we slowly built.
● To be able to jump in and help on such an important study for what was happening to all of us and the World.
● Vaccine finally getting approved.
● Appreciation from participants who are still actively participating and believing in the product.
● The complex protocol gave the research department an opportunity to work together and trouble shoot as a team.

● Our coordinating teams demonstrated their true passion for patient care (e.g., sacrificing their work-life balance, risking their own health).
● The experience restored their purpose to why they became research coordinators, actually being involved with a trial that had the potential to be cutting edge for health care deliverables.
● Coordinating team collaboration was developed across therapeutic areas and continues on today as a result of the trial.


