NO / NO. 3 / 2022 Share clinical knowledge and best practice to improve health and patient care. 35th NCDV 2022 Congress Highlights Page Dermatology12 inSexualitydermatologic nursing care Page 16 Atopic dermatitis and exposure to antibiotics in early life Page 40
Do you want to help... ...deliver medical care where the need is greatest? At Doctors Without Borders, we rely on medical staff, logisticians, administrators, and many other profiles to join forces and provide medical care to millions of people caught in crises worldwide. Currently, we are recruiting medical staff to work in our field projects. If interested, visit your country’s Doctors Without Borders website to see job openings and read about the essential requirements, compensation and benefits, life in the field and how to apply. Læger uden Grænser Danmark: Legerwww.msf.dkUtenGrenser www.lakareutangranser.seLäkarewww.legerutengrenser.noNorge:UtanGränserSverige: Photo credit: Alice martins Doctors Without Borders/Médecins Sans Frontières (MSF), 78 rue de Lausanne, Case Postale 116, 1211 Geneva 21, Switzerland
All content is made by clinicians for clinicians and edited by the ed itorial desk. We hope and encourage all of you to continue contrib uting to the development by asking questions and sharing your knowledge and research with your colleagues.
BestPractice Nordic is an independent journal published by: BestPractice Nordic ApS Teknikerbyen 5, 2. sal 2830 Virum, Denmark +45 4466 www.bpno.dkinfo@bpno.dk9210 Editor in chief: Jan Andreasen MD & Journalist +45 5151 Thejan@bpno.dk8831articlesin BestPractice Nordic are independent of interests. Most pieces are written from the author’s pro fessional perspective, and the message of the articles does not necessarily reflect an expression of the views of the editor. This magazine is distributed to Nordic dermatologists. Advertising: Ina ibo@bpno.dk+45Bøgkjær61305072 Copyright© 2022 BestPractice Nordic ApS Layout: Helle Rindom Our mission is to share clinical knowledge and best practice to improve health and patient care. Welcome to BestPractice Nordic 5041Trykksak0751 SVANEMERKET
Last year we shared more than 3.000 articles covering best practic es in dermatology, oncology, haematology, neurology, rheumatology, general practice, gastroenterology, psychiatry, pharmacy and now adding endocrinology to our portfolio.
Best Practice Nordic is a clinical platform that facilitates an arena for more than 35.000 active clinical specialists in the Nordic region. They all work to improve public health and patient care through dialogue and sharing best practices amongst doctors and healthcare profes Atsionals.BestPractice Nordic we hope to break down barriers between the fields to facilitate a more holistic patient view and care by commu nicating current and clinically relevant knowledge of new indications and treatments, studies, guidelines and best practice, all of which optimize patient care.
3 Welcome 4 Content 6 Editorial / Henrik Nielsen 8 New nordic clinical advisory board for dermatology 2022 10 Highlights from the 35th NCDV 2022 / Line Kibsgaard 13 Addressing sexuality in dermatologic nursing care / Astrid Blikstad 16 Interaction between hand eczema, HRQOL, and changed exposures for health care workers during the COVID-19 pandemic / Yasemin Topal Yüksel 20 The role of patients in the development of Nordic dermatology / Lars F. Werner 22 NCDV 2022 MEDtalk highlights 24 Proteomics in the dermatology of the future – inclusion of the skin proteome 26 Nail fold changes as a marker for psoriatic arthritis in psoriasis of the skin / Jørgen Guldberg-Møller and Mette Mogensen 30 Environmental factors and auto-immune diseases – a hypothesis / Henrik Nielsen and Merete Engelhart 34 The relationship between atopic dermatitis and exposure to antibiotics in early life / Mwenya Mubanga 36 Low prevalence of patients diagnosed with psoriasis in Nuuk: a call for increased awareness of chronic skin disease in Greenland / Sofia Hedvig Christensen Botvid and Carsten Sauer Mikkelsen ContentDermatology
eller
Målrettet
Referanser: 1. https://nyemetoder.no/metoder/dupilumab-dupixent-indikasjon-v (15.10.2021) 2. Dupixent SPC kap 5.1 24.06.2021 3. Dupixent SPC kap. 4.4 24.06.2021 Dupixent (dupilumab) 200 mg og 300 mg, oppløsning i en ferdigfylt sprøyte og ferdigfylt penn. Den ferdigfylte pennen med dupilumab er ikke beregnet til bruk hos barn under 12 år. Indikasjon: Atopisk dermatitt Voksne og ungdom: Behandling av moderat til alvorlig atopisk dermatitt hos voksne og ungdom (12 år og eldre) som er aktuelle for systemisk behandling. Atopisk dermatitt Barn 6 til 11 år: Behandling av alvorlig atopisk dermatitt hos barn 6 til 11 år som er aktuelle for systemisk behandling. Dosering: Voksne: 600 mg (2 injeksjoner à 300 mg) ved oppstart, etterfulgt av 300 mg hver 2. uke. Ungdom ≥12-17 år: over 60 kg: som voksen, under 60 kg: 400 mg (2 injeksjoner à 200 mg) ved oppstart, etterfulgt av 200 mg hver 2.uke. Barn ≥6-11 år: 15-60 kg: 300 mg på dag 1 og 300 mg på dag 15 ved oppstart, etterfulgt av 300 mg hver 4.uke (start 4 uker etter dag 15-dosen). Dosen kan økes til 200 mg annenhver uke. Over 60 kg: som voksen. Vanligste bivirk ninger: reaksjoner på injeksjonsstedet, konjunktivitt, blefaritt og hodepine. Interaksjoner: Interaksjoner med levende vaksiner er ikke undersøkt. Pasienter på Dupixent kan få inaktiverte eller ikke-levende vaksiner. Pakninger og priser: Injeksjonsvæske, oppløsning i ferdigfylt sprøyte: 200 mg: 2 × 1,14 ml 2 (ferdigfylt sprøyte m/automatisk nålebeskyttelse) kr 15 819,60. 300 mg: 2 × 2 ml2 (ferdigfylt sprøyte m/automatisk nålebeskyttelse) kr 15 819,60. Injeksjonsvæske, oppløsning i ferdig fylt penn: 200 mg: 2 × 1,14 ml (ferdigfylt penn) kr 15 819,60. 300 mg: 2 × 2 ml (ferdigfyltpenn) kr 15 819,60. Refusjon: 1. H-resept: D11A H05_1 Dupilumab. Refusjonsberettigetbruk: Vilkår: (216) Refusjon ytes kun etter resept fra sykehuslege eller avtalespesialist. Vennligst se SPC eller felleskatalogen.no for utfyllende informasjon. Sanofi og Regeneron samarbeider i et globalt forsknings- og utviklingsprogram og med markedsføring av Dupixent. blokkering av IL4 og IL13, viktige drivere av type 2-inflammasjon, som ved atopisk dermatitt effekt på alvorlighetsgrad, kløeintensitet
og livskvalitet2* Ingen krav om overvåkning av organtokisitet3* * p value <0,0001 vs placebo og/eller samtidig behandling med TCS 10/2021V.1.0MAT-NO-2100712 sanofi-aventis Norge AS, Postboks 133, 1366 Lysaker. www.sanofi.no
5BestPractice Nordic / Dermatology / NO. 3 / 2022
dermatitt
Målrettet biologisk behandling mot alvorlig
2* Dokumentert
ATOPISK DERMATITT
og hodepine. Pasienter som bruker Dupixent mot
Godkjent 21.06.21Beslutningsforumavtilbehandlingavalvorligatopiskdermatitthospasienterialderen611år.1▼
Utvalgt sikkerhetsinformasjon: De vanligste bivirkningene er reaksjoner på injeksjonsstedet, konjunktivitt, blefaritt alvorlig atopisk og som har komorbid astma skal ikke justere med lege. Forsiktighet bør utvises hos pasienter med astma, ved helmint og/eller vedvarende konjunjtivitt og keratitt. Pasienter bør rådes til å rapportere nyoppståtte forverrede øyesymptomer helsepersonell.
2
eller avbryte astmabehandling uten samråd
infeksjon, systemisk overfølsomhet
til
6 BestPractice Nordic / Dermatology / NO. 3 / 2022
New insights into gut-brain communication1 have emerged from studies that have sought to link the gut microbiome to diseases we do not yet understand, for example Myalgia Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS).
Henrik Nielsen / M.D., D.M.Sc., specialist in intern medicine and rheumatology.
As researchers, we are limited by the fact that it is not ethically justifiable to conduct experiments on humans. We simply must lean on statistics and considerations. Regardless, it is important that we do not let our actions be slowed down by lack of evidence.
Understanding the gut defense system
Greater focus should be put on the importance of gut/ skin biodiversity in relation to the health of the body. If we fail to do this, things will go as we see in nature – and the species could go extinct. The biodiversity that we have all acquired early in life affects the development of our immune system and is responsible for the health and development of diseases later in life.
The importance of protecting gut/skin biodiversity to preserve health
Today, thousands of people are living with diseases that are the direct consequence of negative environmental factors. This goes far beyond infections due to bacteria and viruses. People know of course about environmental fac tors like smoking, obesity, etc. that are harmful to health, but negative impacts also come from more hidden sources such as synthetic chemicals and pesticides in foods, and microparticles in urban air. These negative environmen tal factors can result in a breakdown of the body’s pro tection, impair the biodiversity in our mucous membranes, and make us more vulnerable to a wide range of diseases.
The role of skin microbiotas Skin-barrier structure and function are essential for health. Skin microbiotas play an integral role in the maturation and homeostatic regulation of keratinocytes and host immune networks, with systemic implications for the im portance of gut/skin biodiversity. Indeed, disturbances of the stratum corneum have been noted in allergic dis eases (eczema and food allergy), psoriasis, rosacea, acne vulgaris and with the skin-aging process. Impaired gut biodiversity Examples of diseases related to impaired gut biodiversity include chronic fatigue syndrome, post-traumatic stress disorder (PTSD), and Gulf War Illness. These have been in tensively studied over the years. Less well-studied are several chronic diseases that all have commonalities with late-COVID-19 symptoms: fatigue, memory discomfort, and diffuse pain. The factors surrounding these chronic diseases can only be evaluated with more time – cause and effect are not currently understood.
Editorial
The emergence of atypical chronic diseases such as ME/ CFS is a mystery, but when virus intrusion and degraded environmental conditions (leading to degraded gut/skin biodiversity) are taken into consideration, then we can focus on the question of whether biodiversity failure is a factor in these diseases. Studies of COVID-19 and the sur rounding environmental conditions, where the setting for disease outbreak was present before the pandemic, will bring us closer to understanding the gut defense system.
The gut/skin microbiome biodiversity is central to the body’s defense and disease presentations. During COVID-19, the decline in gut biodiversity has come into focus. The ques tion is how reduced gut/skin biodiversity may increase the risk of death or secondary manifestations from viral attacks. And what can we do to prevent a reduction in the body’s defense in the future?
Many studies have shown that dysbiosis (lack of biodiver sity of the mucous membranes) in infants leads to many diseases, such as lung diseases, asthma, food allergies, diabetes, obesity, and autoimmune diseases. Lack of ex posure to microorganisms in early life can cause aller gies. Studies have shown that children who grow up in natural environments have fewer allergy symptoms than children who grow up in urban areas. One of the reasons that children in urban areas have less diversity in their in testines is due to pollution from microparticles in the air.
The human gut microbiome Recently, many studies have shown the important role of the human gut microbiome. This includes, among other things, the ability to extract energy from food, to increase the harvest of nutrients, to change the appetite signal and to maintain the production of vitamins, to name just a few examples. The gut microbiome includes more than 100 trillion microorganisms and has 150 times more genes than the human genome itself. It is involved in basic bio logical processes, including the regulation of intestinal development, graduation of the individual metabolic na ture, and stimulation of innate immunity, as well as the production of antimicrobial substances that kill micro bial organisms (the organism’s own antibiotics). That’s why it’s important to have more research into the body’s gut-biodiversity.
Reference: 1. König RS, Albrich WC, Kahlert CR, et al. The Gut Microbiome in Myal gic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS. Front Im munol. 2022. https://doi.org/10.3389/fimmu.2021.628741
Also, it is important that we make a significant effort to increase biodiversity in nature so it can “rub off” on us as human beings. Two positive steps would be to preserve more space for wild nature and to limit pesticides that exterminate insects and bees. This kind of action is necessary if we, as humans, have a chance of staying healthy. A call for action This is too serious an issue for us to slow our efforts be cause of a lack of evidence. We need to lean on hypoth eses and act now. There is a need for increased research into the health consequences of pollution from synthet ic chemicals and microparticles, and the accompany ing degradation of the gut/skin-biodiversity. From a political perspective, this means more research stimulation and funding. From a medical perspective, we need more focus on matters we do not understand; ME/ CFS has had long periods of neglect. General practition ers have not been trained in this disease, despite the fact it was the most ANA-positive disease back in 1986 in the Stanford lab where ANAs were defined (ANA = antinucle arTheantibodies).studydone by König RS et al as applied to ME/CFS serves as an example. We should use the same approach to study diseases such as lupus, Parkinson’s, and psori asis. Studies with similar assessments of biodiversity-loss due to environmental factors have the potential to bring us closer to understanding chronic diseases in general.
7BestPractice Nordic / Dermatology / NO. 3 / 2022
8 BestPractice Nordic / Dermatology / NO. 3 / 2022
Medical doctor at Zealand University Hospital since 2007. Specialty: AD. Country: Denmark.
Being part of the clinical advisory board is unpaid, but makes a great difference, not only by ensuring knowledge sharing between Nordic health professionals but also by supporting our collaboration with Doctors Without Borders. Hence, last year we were able to donate close to DKK 100,000 on behalf of all of our contribu Wetors.are looking forward to a great collabo ration with this new board of experts.
Usha Hartgill Specialist in dermatovenereology and leader at Olafiaklinikken in Oslo (sexual health clinic). Specialty: Venereology and genital dermatoses. Country: Norway. In this magazine, we are happy to intro duce our newly established dermatolo gy advisory board. The doctors who have taken on this job will act as sparring part ners to the editorial desk on topics, stud ies, and newest research. Our board members will ensure the high quality of our publications and keep our readers up to date within the field.
All content published through BestPrac tice Nordic’s channels is made by clini cians, for clinicians within the field. We hope and encourage all of you to con tinue to contribute to the development by asking questions and sharing your knowledge and research.
BestPractice Nordic proudlyKristinapresents:Ibler
Line Kibsgaard Specialist registrar at the Department of Dermatology, Aarhus University Hospital since 2020. Specialty: Venerology, genital dermatoses. Country: Denmark.
Kristian Kofoed Medical doctor, Ph.D. Specialist in dermatology, Hudklinikken, Rødovre, Denmark. Specialty: AD. Country: Denmark.
9BestPractice Nordic / Dermatology / NO. 3 / 2022
The new nordic clinical advisory board for Dermatology 2022.
Amra Osmancevic Medical doctor, PhD at Sahlgrenska University Hospital since 1997. Specialty: PsO. Country: Sweden.
Carsten Sauer Mikkelsen MD, specialist in dermato-venereology. GP and part of the research unit at Dept. of Der matology, Aalborg University Hospital. Speci alty: PsO, AD, NMMSC, HAE. Country: Denmark.
Dear colleagues, It is my absolute pleasure to share my obser vations from the 35th Nordic Congress of Der mato-venereology. So, lean back and enjoy my journey through this congress, which I hope will give you a picture of some of the highlights of special interest to you. The venue was the beautiful Tivoli Hotel in the centre of Copenhagen. The pre-programme started on April 19 with on-site board-meetings and an evening Network Event, hosted by the LEO Foundation as proud presenters of the new and visionary Skin Immunology Research Cen tre at the Maersk Tower in Copenhagen. The centre has collected biomaterials from 3,000 patients which will yield insights into how skin diseases develop over time. Day 1: Psoriasis, AD, and chronic urticaria
At NCDV 2022, Nordic and Baltic dermatologists came together to share the newest research and discuss hot topics. In this summary of events, Dr. Line Kibsgaard offers an of the 35th congress.
Highlights from the 35th NCDV 2022 LINE KIBSGAARD is MD and Ph.D. at the Department of Dermatovenereology, Aarhus Univer sity Hospital, Denmark. Her special interests include venereology, urticaria and hidrosadeni tis suppurativa.
One of my favourite learnings as a junior der matologist was the fact that itch sensations dif fer significantly between patients with AD com pared to patients with urticaria. This is easy to see in our clinics, as AD is often plastered by secondary excoriations, which is almost never seen in patients suffering from urticaria. In AD, itch is an inflammatory signal and part of the cascade of immunological reactions from the patient’s skin, which seeks to get rid of the sen sation by initiating a mechanical action in the form of scratch. The efferent sensation is led by the epidermal non-myelinated polymodal C-fi bres. The afferent mechanism is led by motor neurons that innovate and activate muscle con tractions to achieve relief from itch. Itch is a sensation that is rewarded with increased lev els of oxytocin in the postsynaptic nerve end ings of the CNS. In addition, functional MRI studies of itch have shown repeated activity in specific areas in the cerebral cortex. This can be imaged faster/ear lier than when the patient recognizes the visual signs of dermatitis on their skin. Hence, itch in AD should be referred to as an inflammatory, and even more important, non-histaminerge itch. In contrast, in urticaria the itch sensation is a con sequence of the rapid release of antihistamines and prostaglandins from epidermal mast cells. It is merely a consequence of the human innate immune response. In AD patients, JAK inhibitors
overview
10 BestPractice Nordic / Dermatology / NO. 3 / 2022
On Wednesday, April 20, the official programme started with the General assembly meeting of the Danish Dermatological Society (DDS). The chair position of DDS changed. Christian Vester gaard, Aarhus University Hospital is succeed ed by Simon Francis Thomsen, Bispebjerg Hos pital. Kristina Ibler, Bispebjerg Hospital be comes treasurer. The autumn meeting of DDS will be held in Aarhus on November 4-5 in a new and exciting format. All members are more than welcome to join. Understanding and treating itch Afternoon plenary sessions were split into par allel sessions on the two major chronic skin dis eases: Psoriasis and Atopic Dermatitis (AD). Each session had excellent speakers from across the Nordic countries. The “itch session” held by Jes per Elberling, Gentofte Hospital, was quite an audience attracter, as he manages to share his enthusiasm for getting to grips with the patho genesis of itch. Itch is one of the most frustrating parame ters in treating patients with AD. It’s frustrating for the patient and family members, and often also for the physician who tries to resolve the serious issues of their patient’s disease.
Day 2: HS, hot topics
11BestPractice Nordic / Dermatology / NO. 3 / 2022 and dupilumab (anti-IL13/anti-IL4) are first-line biologics; if using systemic anti-inflammatory treatments (MTX, azathioprine, or cyclosporine) does not afford acceptable disease control.
Thursday morning set off with a session on Hi dradenitis Suppurativa (HS), focusing on the worldwide epidemiology, treatment challenges, bio-eligibility, DLQI and when to consider sur gery for this group of patients, and future rec ommendations yet to come. According to the upcoming revision of the European guidelines, we will distinguish between active and inactive inflammatory disease, and measure disease activity according to the IHS4-scoring system, in which the number of nodules, abscesses, and draining fistulas are all considered.
Hot topics in Nordic dermatology
Other topics of interest were skin infections (COVID-19 skin manifestations), melanomas, work-related eczemas, and the increasing use of artificial intelligence in our daily work prac tice as Whendermatologists.itcomestofuture infections within our specialty, our concerns should not be limited to meat-eating bacteria and resistant staph ylococci; a general phenomenon in the Nordic countries is the rising prevalence of sexually transmitted diseases. In Denmark, the incidence of gonorrhea and syphilis are at their highest
Biologics targeting chronic urticaria Simon Francis Thomsen, Bispebjerg Hospital, el egantly followed up on this topic with his pres entation of future targets for treatment in chron ic urticaria patients (CSU) who do not respond or have a slow or insufficient response to omal izumab (type IIb CSU patients). In about 15% of all CSU patients, blockage of the FRI receptor is not sufficient to relieve symptoms. Small mol ecule treatments (cytokine and tyrosine kinase inhibitors) may be the answer in the future for this patient group, along with ligelizumab (an ti-IgE), benralizumab (anti-IL5), and mepoli zumab (anti-IL5). Hence, the future holds prom ise for the Xolair non- or insufficient respond ers, who may be suspected in cases with pos itive basophile histamine release tests and low levels of basophils, eosinophils, and total IgE.
In the following parallel sessions, some of the leading Nordic experts in AD and psoriasis gave presentations on comorbidities, pathogenesis, and treatment strategies. Patient-reported out comes were an emerging topic of interest, along with EASI scores that are about to match the re ported scores of PASI, for example EASI-50, EASI75 and EASI-90. Highlighting our international landmarks
All attending nationalities were – in a very unNordic manner – encouraged to be proud of their fellow Dermato-venereologists’ achieve ments throughout history. This was, in my opin ion, the most entertaining session of the whole congress.
The future of treatment in HS and biologics
Clinical trials have shown equal response to Doxycycline and the Clindamycin-plus-Rifam picin regimes. This gives us hope to offer bio logic treatments (anti-TNF α) much earlier than before, combined with the increasing know ledge and interests in biological treatments of patients with HCS, where secukinumab (anti IL17), bimekizumab (anti IL17A/F), spesolimab (an ti-IL36) and JAK pathway inhibitors are under investigation.Forpeople with a special interest in HS, the speakers were proud to announce the dates of the upcoming 2023 Conference of the Europe an HAS-society, which will be held February 8-10 in Florence.
Parallel to the morning session on Thursday was a brilliant pros-and-cons session on the themes of 1) Melanoma screening and 2) Emollients, both ongoing subjects of debate among pa tients, colleagues, and from a pharmaceutical and socioeconomic point of view, where it seems as if there are no uniform or final conclusions.
At a late-afternoon session on Wednesday, rep resentatives from Sweden, Norway, Iceland, Fin land, the Baltic countries and Denmark were each invited to give a talk on their respective na tions’ global contributions to Dermato-venere ology. This inspired a wide range of topics: fa mous Nordic dermatologists and venereologists from the 19th and 20th centuries, highlights of cohort-studies on syphilis from Norway and Sweden, the Norwegian contemplation of the Hanifin-Rajka criteria from 1979, the Danish No bel prize winner from 1903, Niels Finsen, who was the inventor of light therapy, highlights of the Baltic congresses of Dermato-venereology over the years, and finally the Icelandic “Keresis´ad venture”; A very fine presentation of the world’s 5th largest deliverers of skin transplants evolved from a fish skin model.
From a patient perspective: A way to receive expert assessments in one setting with close and expert follow up until complete disease con trol, where-after patients may again gain from the undebatable advantages offered by the pri mary health care sector. I would like to thank the organizing commit tee of this wonderful congress. For all of you who could not join us, I hereby give my warm est recommendations. I sincerely hope to meet you all at the next congress. With best wishes MD, Ph.D. Line Kibsgaard Department of Dermatovenereology Aarhus University Hospital, Denmark.
The Poster Walk on Thursday afternoon was con verted into short presentations of studies by some of our younger colleagues, who shared their enthusiastic and clever ideas. For me, this was the most inspiring moment of the congress, as it is in forums like these ideas come to life and the finest core of research becomes visi ble to Thursdayeveryone.night, the congress dinner was held at the Langelinie Pavilion next to the Little Mer maid. We arrived by boat, and Copenhagen showed itself from its most beautiful and sun ny side. Ideas and inspiring moments contin ued to be shared while glasses were raised in celebration of finally being able to meet faceto-face after two years of COVID-19-lockdown.
Lots of antibody and small molecule treat ments are on their way, and I have high expec tations for the future medical care of HS, urti caria, and AD patients, in particular. I think that multidisciplinary approaches are going to be the future “new black”, not only in treating cas es of psoriasis, dermatological cancer patients, and wound-care but also in the treatment of AD-, urticaria- and HS-patients. These are dis ease areas where initial multidisciplinary ex pert assessments may turn out to be the most cost-beneficial model from a long-term per spective and a way to plan treatment courses, which can be maintained by our excellent col laborators in the primary health care sector.
12 BestPractice Nordic / Dermatology / NO. 3 / 2022 levels in 20 years, and it seems as if this is a trend that is not going to decrease without na tional interventions. Thus, it was very comfort ing to hear the talk of Usha Hartgill from Nor way, who has done important research on gon orrhea that gives us hope that a future vaccine against gonorrhea may become realistic.
Young colleagues sharing new ideas
Day 3: Microbiomes, rosacea, acne, and trends in psoriasis Microbiomes, rosacea, acne, and trends in pso riasis research were the topics on Friday morn ing in parallel with the second and well visited nurse section. It was a pleasure to observe how specialized dermatological care issues were shared and discussed on a highly competent level, where I am sure that we can all gain price less experiences, if we dare to share our per sonal stories of success and flaws with our neighboring countries who must face similar challenges, when it comes to socioeconomic, geographical and political issues within our field of expertise. Free communication and poster awards
CONFLICT OF INTEREST: Speaker at a LEO Pharma industrial symposium. Attendee at congress, sponsored by UCB. Attendee at symposium hosted by Eli Lilly. Attendee at symposium hosted by Abbvie.
Bright future for treatment of dermatological conditions In conclusion, the Congress left a great deal of joy and excitement among attendees about fi nally being able to meet again. We were able to share our common passion to treat, comfort, and relieve itch, sores, and the various agonies of our dermatological patients. There is no doubt that we are about to face an explosion of pos sible treatment strategies when treating AD pa tients with biologics, an explosion that is simi lar to the one psoriasis treatment has gone through during the last decade or two, and where EASI-90 is a realistic treatment goal in the fu ture to come.
The final sessions of the congress were two free communication sessions, where ten projects were presented by the conducting scientists with the opportunity to discuss methodology and future perspectives of their ideas. The ten most remarkable posters were selected in sev eral categories, from the most original idea (how to wash your socks to avoid fungal infections) to the most educational poster (Systematic re view on Prurigo Nodularis Hyde) assessed by the congress committee members.
Addressing sexuality in dermatologic nursing care
13BestPractice Nordic / Dermatology / NO. 3 / 2022
Clinical studies recognize that people living with different skin diseases are at risk of im paired sexuality.1 This issue should be addressed at an equal level with other kinds of treatment in a healthcare setting. However, studies have uncovered barriers, which include lack of knowledge, taboos, fear of negative feedback and the lack of clinical practice guidelines.2 Having uncovered these barriers also provides us with knowledge about how we can improve our clinical practice and assess sexuality more frequently with our patients. We can self-reflect, and we can reflect upon our practise together with our colleagues. Self reflection is, in my mind, a key to increase our communicative skills. This is important, and ideally, we should also create a safe and posi tive work environment where we can discuss sexual health topics and promote profession al development in our field of nursing.
ASTRID BLIKSTAD is Clinical Nurse Educator at Ward of Dermatology, Oslo University Hos pital and senior advisor for The Norwegian Psoriasis and Eczema Association (PEF).
Ask the question: “How do we assess sexual ity in my workplace?” If you don’t have an ans wer, find out why.
Addressing impaired sexuality
A holistic understanding of sexuality
A systematic assessment of the patient’s sexual health status gives us the opportunity to support sexuality by normalizing, providing information, and facilitating positive coping strategies.
Sexuality is a basic human need. It’s a part of our primal instincts. Its biological complexity makes us able to survive and reproduce as a species. We all know that sex is fun, too – alone or with others – regardless of our socio-cul tural norms that have tried to keep this fact a well-kept secret, especially for gay and female sexuality.Understanding what the term sexuality means is important. Sexuality is sex, health, gender identity, sexual orientation, fantasies, intimacy, reproduction, pleasure, identity and so much more. Just as important is to understand our own attitudes toward sexuality. As for many other aspects of our profession, it is important that we explore our own values, beliefs and disbeliefs, insecurities, and prejudices.
As nurses, we can analyse disease impact on sexuality in two assessment/intervention do mains – both the direct impact and indirect impact of sexuality. A direct impact would be Sexuality is a basic human need. However, sexuality is not always included in the nursing assessment. Studies have uncovered barriers that give us insights into how we can improve our clinical practice and assess sexuality more frequently with our patients. How can we assess sexuality by increasing our knowledge? And how do we promote a positive attitude toward sexuality, combined with standardized use of the “Dermatology Life Quality Index”?
Disease manifestations affecting sexuality
Indirectly, a skin disease regardless of wheth er occurring on the genitals or on other parts of the body can induce low self-esteem, altered body image, and self-perceived stigma, among other psychosocial factors.4 Itching is a com mon symptom that can affect the feeling of pleasure or the surplus energy to have sexual play. Psychological diseases such as anxiety and depression also play a role in an individ ual’s sexual health, and we now know that several chronic skin diseases are linked to an increased risk of mental health issues.3
In a variety of diagnoses, from cancer to bowel disease, nurses and health personnel describe in the literature barriers to why sexuality or sexual health is not always so well commu nicated between health personnel and their patients.2 Some of these barriers are lack of knowledge, embarrassment or taboo, fear of negative feedback, lack of angle or motive to initiate the talk, lack of time and suitable en vironment, or lack of procedures and routines.
We all know that the skin is a huge sensory organ as well as providing other vital functions of the skin. If we didn’t have skin, we would all be dead, and we would look horrible with our leaking bodies, with muscles and tendons hanging out on display – which would not mat ter because we would all be dead. But back to the sensory function. We communicate with our environment through touch, and socially with our skin itself. So how can a skin disease affect our sexuality?
DLQI in a multidisciplinary approach
A direct impact can be exemplified with in verse psoriasis where the genitals may be di rectly affected by the disease and hence make it painful to masturbate or have intercourse. For people with hidradenitis suppurativa, pain ful boils, wounds, and fistulas with secretion in the genital region make wanting or having sexual activities problematic. The same thing is the case for people living with other skin dis eases that affect the genitals directly like li chen sclerosis or genital ulcers.
To facilitate the assessment of sexual func tion in a dermatological setting, the idea is to standardize the use of the Dermatology Life Quality Index (DLQI) questionnaire. All adult pa tients can receive the questionnaire when ad mitted to the dermatological ward. Nurses can
physical changes causing a form of sexual impairment. And indirect would be the psy cho-social: : self-esteem, body image, shame, stigma, or identity changes.
Assessing and addressing sexuality in a ho listic and positive approach can give us the opportunity to facilitate our patient’s explo ration of sexuality – what it means for them.
14 BestPractice Nordic / Dermatology / NO. 3 / 2022
Sexuality is not only sexual activities like mas turbation or intercourse. It’s a very subjective experience and includes kissing, hugging, cud dling, and other forms of intimacy.
References: 1. Sampogna F, Abeni D, Gieler U, et al. Impairment of Sexual Life in 3,485 Dermatological Outpatients from a Multicentre Study in 13 European Countries. Acta Dermatovenereologica. 2017;97(4): 478-482. 2. Blikstad A, Falch- Koslung L,Tschudi-Madsen C. Samtaleverktøyet BETTER kan gjøre det lettere å snakke om seksualitet. (2020). Oslo: Sykepleien.no. 3. Dalgard F, Gieler U, Tomas- Aragones L. et al. The Psychological Burden of Skin Diseases: A Cross-Sectional Multicenter Study among Dermatological Out-Patients in 13 European Countries. J Invest Dermatol. 2015 Apr;135(4): 984-991. 4. Barisone M, Bagnasco A, Hayter M, et al. Dermatological diseases, sexuality and intimate relationships: A qualitative me ta-synthesis. J Clin Nurs. 2020;29(17-18): 3136-3153. 5. Finlay, A Y, Khan G K. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use.” Clin exp dermatolvol. 1994 May;19(3): 210-6.
CONCLUSION: We can inform, guide, and help the patient to adopt positive coping strategies, and normalize sexual chal lenges. Skin disease or not, many of us cherish our looks as a central aspect of our sense of sexuality. Not all patients want to talk about their sexuality and that’s fine! However, we can open the door. Using the DLQI questionnaire in a multidiscipli nary approach may help meet the patient’s needs and raise awareness of sexual health in dermatology. use the DLQI in the nursing assessment, and the form provides information about the pa tient’s Quality of Living (QoL) including sexu ality. Especially questions 2, 8, and 9 address thisQuestionissue.
15BestPractice Nordic / Dermatology / NO. 3 / 2022
2 investigates perceived embar rassment or self-consciousness, which may relate to the experience of stigma or shame from having the skin disease. Questions 8 and 9 address perceived problems with a partner and/or problems with friends and sexual dif ficulties.5 The qualitative and quantitative prop erties of the questionnaire are important for both the dermatological nurse and the der matologist.Asystematic assessment of the patient’s sexual health status gives us the opportunity to support sexuality by normalizing, providing information, and facilitating positive coping strategies. In our experience, it may accelerate referral to other types of health personnel who may be a support to our patients, such as a psychiatric nurse or a social worker.
A key aspect is to talk through the form to gether with the patient after documenting the score of the DLQI questionnaire. After handing out and collecting the form routinely at admission, collect the score and document it. Then give, let’s say a 15-minute timeframe. You can say to the patient: “I have 15 minutes. I want to hear more about your quality of life, including your sexual life”. In this way, you have a natural way of assess ing sexuality and QoL in a fair timeframe.
CONFLICT OF INTEREST: The Norwegian Psoriasis and Eczema Association (PEF) collaborates with LMI.
This article is based on my manuscript from the talk “Addressing sexuality in dermatologic nursing care” in nurse session 2 at the 35th NCDV 2022 in Co penhagen Friday April 22.
Figure 1A shows data from all healthcare workers. It shows that the use of ABHRs on wet skin and gloves on dry or wet skin, respective ly, increased more than it decreased during the pandemic, but the number of hand wash ings decreased more than it increased. The use of alcohol-based hand-rubs increased with
Participants were nurses, physicians, aux iliary nurses, biotechnicians, physiotherapists and midwives. More than half the target group were employed at departments with high ex posure to COVID-19 patients. Well-known risk factors for hand eczema
Interaction between hand eczema, HRQOL, and changed exposures for health care workers during the COVID-19 pandemic
Studies on the prevalence of hand eczema during the pandemic found that more than one-third of health care workers reported hand eczema.1-5 Higher prevalence was reported in studies on symptom-based hand eczema di agnosis.6,7 Suggested causes include the in creased use of hand washings and use of al cohol-based hand rubs (ABHRs) during the pandemic.Thisstudy from Denmark regarding hand ec zema and exposures is a prospective follow-up study where health care workers responded to a questionnaire at the beginning of the pan demic and again 11 months later. The aim was to evaluate changes in the prevalence of hand eczema, wet work exposures, and quality of life in healthcare workers with hand eczema.
The study sought, among other things, to find differences in exposure between healthcare workers with and without hand eczema.
The enormous workload and fear of being infected with COVID-19 have affected the wellbeing of many healthcare workers.13,14 Hand eczema is known to have a negative influ ence on the health-related quality of life.15 In addition, occupational stress is anticipated to worsen life quality further. However, data on health care workers with hand eczema dur ing the pandemic are limited.16,17
Studies on the prevalence of hand eczema during the pandemic found that more than one-third of health care workers reported hand eczema. But a 11-month follow-up study conducted during the COVID-19 pandemic with healthcare workers exposed to covid patients suggests that the links between changed exposures including hand hygiene procedures in patient care, and hand eczema are complex, and cannot be linked to a single factor.
16 BestPractice Nordic / Dermatology / NO. 3 / 2022
The use of alcohol-based hand rubs (ABHRs) has not previously been considered a risk fac tor for skin barrier damage10; however, irrita tion of the skin as an effect of ABHRs applied on wet skin has recently been suggested.11,12
The change of exposures with and without hand eczema
YASEMIN TOPAL YÜKSEL is MD and Ph.D. student at Department of Dermato-Venerology at Bispebjerg Hospital, Copenhagen, Denmark. She is also a board member of The Group of Young Researchers.
Hand washings, the use of alcohol-based hand rubs, and gloves are important preventive measures prohibiting the transmission of mi croorganisms.8,9 But hand washings and glove use are also well-known risk factors for the development of hand eczema.
Despite the increased focus on intensive hand hygiene measures and several studies sug gesting an increasing prevalence of hand ec zema during the pandemic, we found a slight ly decreasing prevalence during this period, how ever, an increasing severity of the hand eczema.
The prolonged use of gloves has previous ly been identified as a risk factor for hand ec zema,12,18 and the slightly increased exposure may have facilitated the skin barrier damage. The increased information campaigns on skin protective measures in relation to the pan demic, both in public media and in hospitals, might have resulted in a behavioral change in healthcare workers.19,20
The increased use of alcohol-based hand rubs on wet skin and increased use of gloves (on dry and wet skin) in health care workers were associated with hand eczema during the pandemic.
17BestPractice Nordic / Dermatology / NO. 3 / 2022 the same magnitude as it decreased. Figure 1B shows data from healthcare workers with hand eczema. The use of ABHRs on wet skin and gloves on dry or wet skin, respectively, in creased more than it decreased during the pandemic. Hand washings, the use of ABHRs, and nonoccupational wet work increased with the same magnitude as they decreased.
The increased use of alcohol-based hand rubs on wet skin and increased use of gloves in health care workers with hand eczema may have had an impact on the worsening of the hand eczema symptoms. Quality of life wors ened slightly, with hand eczema severity and frequent flares being risk factors for a reduced quality of life in healthcare workers with hand eczema.Thedecrease in the prevalence during the study period likely reflects the change of expo sures reported in our study. The reduced num ber of hand washings may have contributed to the lower hand eczema prevalence since it is a well-known risk factor for hand eczema.10,18 At the same time, the exposure to ABHRs on wet skin increased markedly and was significant ly associated with hand eczema at follow up. This is in alignment with an experimental study indicating that alcohol-based hand rubs may induce a skin barrier disruption when applied on wet or moist skin,11 as opposed to findings on ABHRs on dry skin.10 It can be anticipated that the risk of applying ABHRs on wet skin increas es with the increased use of ABHRs. Reflects the recommendations given by health authorities The change in the exposures reflects the ef ficacy of hand-hygiene recommendations of the Danish health authorities, who recommend fewer hand washings and increased use of al cohol-based hand rubs.
Slightly prevalencedecreasingofhand eczema
The onset of hand eczema during the pande mic was more often reported by healthcare workers with atopic dermatitis compared with those without atopic dermatitis. We found no association between changed exposures and hand eczema at follow-up in healthcare work ers with atopic dermatitis. During this study, the prevalence of hand ec zema declined from 16.0% to 13.0% at the fol low-up 11 months later. During this period, the number of hand washings decreased, where as the use of ABHRs on wet skin increased sig nificantly, together with an increase in the use of gloves. The increased exposure to ABHRs on wet skin was significantly associated with hand eczema.Compared to other data, the prevalence of hand eczema was 14.9% in German health care workers; however, the prevalence was between 29% and 33% in other European stud ies and up to 90.4% in Asian studies.3,5-7 Quality of life worsened slightly
*washingHand* ABHR*
B, Bar plot showing the change of exposures from baseline to follow-up in HCWs with HE (N = 93). The use of ABHRs on the wet skin and gloves on the dry and wet skin, respectively, increased more than it decreased during the pandemic. Hand washings, the use of ABHRs, and nonoccupational wet work increased with the same magnitude as they decreased. Statistically significant difference in change of exposures between baseline and follow-up is marked with asterisk (Wilcoxon Signed Rank Test). ABHR, Alcohol-based hand rub; HCW, health care worker; HE, hand eczema. ABHR use non-occupationalwetskin*onGloveswetskin*Glove use on wet skin
Figure 1 – The change of exposures in all health care workers with and without hand eczema.
skin Figure 1 - The change of exposures in all health care workers with and without hand eczema.
Figure 1 - The change of exposures in all health care workers with and without hand eczema.
onGloveswetskin*use on wet skin
ABHR use non-occupationalwetskin
18 BestPractice Nordic / Dermatology / NO. 3 / 2022
B, Bar plot showing the change of exposures from baseline to follow-up in HCWs with HE (N = 93). The use of ABHRs on the wet skin and gloves on the dry and wet skin, respectively, increased more than it decreased during the pandemic. Hand washings, the use of ABHRs, and nonoccupational wet work increased with the same magnitude as they decreased. Statistically significant difference in change of exposures between baseline and follow-up is marked with asterisk (Wilcoxon Signed Rank Test). ABHR, Alcohol-based hand rub; HCW, health care worker; HE, hand eczema.
B, Bar plot showing the change of exposures from baseline to follow-up in HCWs with HE (N = 93). The use of ABHRs on the wet skin and gloves on the dry and wet skin, respectively, increased more than it decreased during the pandemic. Hand washings, the use of ABHRs, and nonoccupational wet work increased with the same magnitude as they decreased. Statistically significant difference in change of exposures between baseline and follow-up is marked with asterisk (Wilcoxon Signed Rank Test). ABHR, Alcohol-based hand rub; HCW, health care worker; HE, hand eczema. ABHR use ABHR use non-occupationalwetskinwet
* washingHand ABHR*
A, Bar plot showing the change of exposures from baseline to follow-up in all HCWs (N = 795). The use of ABHRs on the wet skin and gloves on the dry and wet skin, respectively, increased more than it decreased during the pandemic. The number of hand washings decreased more than it increased. The use of ABHRs increased with the same magnitude as it decreased. Statistically significant difference in change of exposures between baseline and follow-up is marked with asterisk (Wilcoxon Signed Rank Test).
Glove
A, Bar plot showing the change of exposures from baseline to follow-up in all HCWs (N = 795). The use of ABHRs on the wet skin and gloves on the dry and wet skin, respectively, increased more than it decreased during the pandemic. The number of hand washings decreased more than it increased. The use of ABHRs increased with the same magnitude as it decreased. Statistically significant difference in change of exposures between baseline and follow-up is marked with asterisk (Wilcoxon Signed Rank Test).
A, Bar plot showing the change of exposures from baseline to follow-up in all HCWs (N = 795). The use of ABHRs on the wet skin and gloves on the dry and wet skin, respectively, increased more than it decreased during the pandemic. The number of hand washings decreased more than it increased. The use of ABHRs increased with the same magnitude as it decreased. Statistically significant difference in change of exposures between baseline and follow-up is marked with asterisk (Wilcoxon Signed Rank Test).
non-occupationalwetskin*onGloveswetskin*Glove use *washingHand* ABHR* on wet skin
onGloveswetskin*Glove use * washingHand ABHR* on
19BestPractice Nordic / Dermatology / NO. 3 / 2022
References:
1. Guertler A, Moellhoff N, Schenck TL, et al. Onset of occupational hand eczema among healthcare workers during the SARS-CoV-2 pandemic: com paring a single surgical site with a COVID-19 intensive care unit. Contact Dermatitis. 2020;83(2): 108-114. 2. Techasatian L, Thaowandee W, Chaiyarit J, et al. Hand hygiene habits and prevalence of hand eczema during the COVID-19 pandemic. J Prim Care Community Health. 2021;12: 21501327211018013.
3. Reinholz M, Kendziora B, Frey S, et al. Increased prevalence of irritant hand eczema in health care workers in a dermatolog-ical clinic due to increased hygiene measures during the SARS-CoV-2 pandemic. Eur J Dermatol. 2021;31(3): 392-395. 4. Erdem Y, Altunay IK, Aksu ¸Cerman A, et al. The risk of hand eczema in healthcare workers during the COVID-19 pandemic: do we need specific attention or prevention strategies?Contact Dermatitis. 2020;83(5): 422-423. 5. Hamnerius N, Pontén A, Bergendorff O, Bruze M, Bjök J, Svedman C. Skin exposures, hand eczema and facial skin disease in healthcare work ers during the COVID-19 pandemic: a cross-sectional study. Acta Derm Venereol. 2021;101(9): adv00543. 6. Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. J Am Acad Dermatol. 2020;82(5): 1215-1216. 7. Lin P, Zhu S, Huang Y, et al. Adverse skin reactions among healthcare workers during the coronavirus disease 2019 outbreak: a survey in Wuhan and its surrounding regions. Br J Dermatol. 2020;183(1): 190-192. 8. Pittet D, Allegranzi B, Sax H, et al. Evidence-based model for hand transmission during patient care and the role of improved prac tices. Lancet Infect Dis. 2006;6(10): 641-652. 9. Pittet D. WHO guidelines on hand hygiene in health care : a summary. World Heal Organ. 2009;30(1): 270. 10. Pedersen LK, Held E, Johansen JD, Agner T. Less skin irritation from alcohol-based disinfectant than from detergent used for hand disinfection. Br J Dermatol. 2005;153(6):1142-1146. 11. Plum F, Yüksel YT, Agner T, Nørreslet LB. Skin barrier function after repeated short-term application of alcohol-based hand rub following intervention with water immersion or occlusion 1. Contact Dermatitis. 2020;83(3): 215-219. 12. Yüksel YT, Ebbehøj NE, Agner T. An up date on the prevalence and risk exposures associated with hand eczema in Danish hospital employees: a cross-sectional questionnaire-based study. Contact Dermatitis. 2022;86(2): 89-97. 13. An Y, Yang Y, Wang A, et al. Prevalence of depression and its impact on quality of life among frontline nurses in emergency departments during the COVID-19 outbreak. J Affect Disord. 2020;276: 312-315. 14. Lai J, Ma S, Wang Y, et al. Factors associated with men tal health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3(3): e203976. 15. Nørreslet LB, Agner T, Sørensen JA, Ebbehøj NE, Bonde JP, Fisker MH. Impact of hand eczema on quality of life: metropolitan versus non-metropolitan areas. Contact Derma titis. 2018;78(5): 348-354. 16. Lee JY, Lee JY, Lee SH, et al. The experiences of health care workers during the COVID-19 pandemic in Korea: a qualitative study. J Korean Med Sci. 2021;36(23): e170. 17. Chernyshov PV, Kolodzinska L. Prospective study on hand dermatitis in nurses and doctors during COVID-19 pandemic and its improvement by use of adopted recommendations of the European Academy of Dermatology and Venereology Task Force on Con tact Dermatitis. Dermatol Ther. 2020;33(6): e14396. 18. Hamnerius N, Svedman C, Bergendorff O, Björk J, Bruze M, Pontén A. Wet work exposure and hand eczema among healthcare workers: a cross-sectional study. Br J Dermatol. 2018;178(2): 452-461. 19. Take care of the teacher’s hands: best advice from the re-searchers. BUPL. Accessed September 9, 2021. 20. Children should use moisturizers and wash hands carefully. Statens Serum Institut. Accessed September 9, 2021. https://www.sst.dk/da/nyheder/2020/boern-boer-bruge-haandcreme-og-vaske-haenderne-skaansomt.
CONCLUSION: Despite intense focus on hand hygiene during the pandemic, a slightly declining hand eczema prevalence was found in health care workers, but with a significant worse ning of the hand eczema severity. We found a reduction in hand washings, but an increase in the use of ABHRs on wet skin and glove use, which was significantly associated with hand ecze ma. Our findings suggest that the interaction between hand eczema and changed exposures is quite complex and cannot be linked to a single factor.
CONFLICT OF INTEREST: None. ORIGINAL PUBLICATION: Yüksel YT, Nørreslet LB, MD, Meulengracht EF, Ebbehøj NE, Agner T. Hand eczema, wet work exposure, and quality of life in health care workers in Denmark during the COVID-19 pandemic. JAAD International. 2022;7: 86-94.
LARS WERNER is Managing Director at the Danish Psoriasis Association and a long-term patient research partner and advisory board member in the field of psoriasis.
Certainly, within the area of psoriasis, the num ber of new treatments has increased signifi cantly over the past 20 years. The biological treatments have given hope to many patients.
Figure 1 – Benefits of personalized medicine
Both “compliance” and “adherence” focus more on patient behavior regarding medica tion-taking. In contrast, “concordance” high lights the processes that underlie medica tion-taking, such as an equal and effective therapeutic relationship that supports the pa tient during the entire course of long-term treatment.Fromapatient perspective, concordance should be a standard operating procedure (SOP) in all corners of the health care system. Biomarker diagnostics personalizes treatment In years to come, patients may look forward to a more personalized treatment/medica tion that is driven by the implementation of biomarker diagnostics on a much larger scale than is the case today (figure 1).
At the recent Nordic Congress of Dermatology and Venereology held in Copenhagen, Denmark April 19-22, I was invited to give a small oral pres entation on the subject of “The role of patients in the development of Nordic dermatology”. I chose to focus on the recent development in treatment options, the life quality aspect, and patient empowerment.
20 BestPractice Nordic / Dermatology / NO. 3 / 2022
Concordance as guidance in treatment
How can we include the patient’s perspective in future dermatological treatment? Here is an update on hot topics in patient-centered treatment from the 35th NCDV Congress 2022 as seen from the patient’s perspective.
Adapted from Bayer Healthcare, “Personali zed Medicine”.
The role of patients in the development of Nordic dermatology
When it comes to communication between health care providers and patients, we have come a long way in achieving a mutually re spectful dialogue. The number of patients in the dermatological field seems to be endless, and it seems that it can be tempting some times to make consultations swift. However, we do see that the overall treatment result im proves by implementing the practice (or phi losophy, if you like) of concordance.
0.97
• Patient education – virtual.
1.68
I would also like to briefly touch upon the area of patient empowerment. WHO’s definition of patient empowerment is “A process through which people gain greater control over deci sions and actions affecting their health.”
1.74
Adapted from: Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The Psychological Burden of Skin Diseases: A Cross-Sectional Multicenter Study among Dermatological Out-Patients in 13 European Countries. Journal Invest Dermatol. 2015;135(4): 984–991. Table 3. 12
1.43 10.2174.00(0.55-3.74)(2.01-7.97)(4.07-25.41)2.40(1.67-3.47)1 Non-melanoma skin cancer InfectionsAcne skin AtopicNevi eczema Benign skin tumors Hand DermatologicalLegControlseczemaulcers out-patients
The psychological burden of skin diseases is well known. Here is the result of a 2015 study with the esteemed professor Jemec as one of the Overall,co-authors:10.1%ofdermatological patients were clinically depressed compared with 4.3% of con trols. And the numbers of depressed patients were higher for those with leg ulcers, hand ec zema, and psoriasis, as measured on the HADS score (Hospital Anxiety and Depression Scale).
Patient organizations in all the Nordic countries put a lot of resources to support this by developing:
• Patient education – in print
CONCLUSION: The next step toward more patient-centered treatments includes: A movement away from a narrow focus on compliance, and towards more concordance-based interaction. We also need development and implementation of biomarker diagnostics to supply precision medicine based treatments , and utilization of PROM to support patient empowerment.
2.65
2.14
Depression clinical case HADS ≥ 11% (n) clinical case HADS > 11 Crude OR1 depression clinical case HADS > 11 Adjusted OR2 depression Table 1. Depression in patients with common skin diseases and controls in percentages and ORs (95% confidence interval) N = 4,994 Psoriasis 13.8 (84) 4.8 (18) 8.9 (21) 8.0 (18) 5.7. (12) 6.0 (11) 10.1 (16) 4.8 (7) 15.1 (21) 24.3 (28) 10.1 (357) 4.3 (58) <0.001<0.001<0.001<0.001<0.0010.7290.0010.0070.3110.2150.587 3.23 (2.06-5.05) 1.21 11.232.051.792.59(0.62-2.36)(1.40-4.76)(0.89-3.59)1.53(0.74-3.13)(0.99-4.22)3.55(1.28-6.92)1.50(0.69-3.65)4.85(2.59-9.10)(5.71-22.09)2.69(1.88-3.84)1 3.02
3.27
CONFLICT OF INTEREST: None.
Patient Empowerment
As a patient organization, we know that many patients want to learn more about their skin disease, particularly how to control symptoms.
Life Quality – Patient-reported outcome measures
Eczema
One tool that is very useful to capture all aspects of skin diseases is Patient-Reported Outcome Measures (PROM), which is a ques tionnaire capturing relevant issues for both health care providers and patients. In 2022, we are implementing this tool for psoriasis in Denmark. This means that at least once a year, a psoriasis patient will also be asked ques tions related to how the skin disease affects the person’s daily life – his or her life quality.
• Patient education – face-to-face (e.g. patient schools)
Table 1 – Depression in patients with common skin diseases and controls in percentages and ORs (95% confidence interval) N = 4,994 Abbreviations: HADS, hospital anxiety and depression scale; MD, missing data; OR, odds ratio. Missing data hospital anxiety depression scale-depres sion = 109 (patients = 102; controls = 71). 1Without participants with missing data for one or more of the predictors. 2Regression model for each disease separately, adjusting for gender, age, socio-economic status, stress, and co-morbidity. Adapted from: Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The Psychological Burden of Skin Diseases: A Cross-Sectional Multicenter Study among Dermatological Out-Patients in 13 European Countries. Journal Invest Dermatol. 2015;135(4): 984–991. Table 3.
Abbreviations: HADS, hospital anxiety and depression scale; MD, missing data; OR, odds ratio. Missing data hospital anxiety depression scale-depression = 109 (patients = 102; controls = 71). Without participants with missing data for one or more of the predictors. Regression model for each disease separately, adjusting for gender, age, socio-economic status, stress, and co-morbidity.
21BestPractice Nordic / Dermatology / NO. 3 / 2022 Se annonce side 29.
Diagnosis P-value (1.86-4.90) (0.41-2.32) (1.39-5.06) (0.80-3.53) (0.73-4.17) (1.01-4.53) (1.61-6.62) overall
22 BestPractice Nordic / Dermatology / NO. 3 / 2022
Mattias Henning presented his latest study on whether the pandemic and the subsequent societal lockdown have affected different dermatoses sensitive to va rying degrees of stress. The results suggest that pa tients with hidradenitis suppurativa showed worse physical well-being during the pandemic; in contrast, patients with hyperhidrosis experienced a worsened mental well-being and a higher risk of stress inde pendent of the habitual HRQoL and stress.
Ida Vittrup Nielsen, MD, Ph.D. Student, Department of Der matology, Herlev and Gentofte Hospital, Denmark.
Adriana Caixinha took the audience through a case series and literature review on pyodermagangreno sum (PG) and peripheral arterial disease (PAD). Six out of seven of the patients had a poor outcome, ul timately requiring either bilateral or unilateral am putation, even though aggressive therapies were in itiated in the early stages. The data suggest that the presence of PAD is a risk factor for a poor prognosis of PG. The simultaneous presence of PG and PAD seems to aggravate the prognosis, resulting in am putation in a significant proportion of our patients. Thus, screening and diagnosis of PAD in the clinical setting of PG are essential for the prognosis. Howev er, the rapid development of severe PAD in patients with initial standard TBI could suggest that the pres ence of PG could trigger the action of PAD. More stud ies are required to optimize the management of this complex subgroup of PG patients and to investigate a possible causality effect between PAD and PG.
Adriana Caixinha, MD, Aarhus University Hospital.
This year BestPractice Nordic was present at the 35th NCDV congress, covering the latest news from der matology. On bpno.dk, bpno.no, and bpno.se some of the participants share highlights from their research with MEDtalks covering a wide range of topics. In the following, we will present some of the highlights all available through our platform.
PG could trigger the action of PAD
NCDV 2022 MEDtalk Highlights
It is well-known that AD is associated with school ab senteeism and social problems. But a new substan tial study presented by Ida Vittrup Nielsen, gives many insights into the link between AD and academic achie vement in both upper and lower secondary schools and which groups are most affected. One of her fin dings is that children in lower secondary school have a higher risk of receiving special educational assistance.
COVID-19 lockdown affected the well-being of dermatologic patients
Mattias Henning Dermatological Department, Zealand University Hospital, Roskilde. Academic achievements and atopic dermatitis
Rune Kjærsgaard Andersen presented a nationwide Danish study with data showing a 40% increased risk of cancer from multiple different organ systems with in patients suffering from hidradenitis suppurativa. This finding is important since the typical patients with hidradenitis suppurativa are young women. In general, this demographic group is less likely to de velop cancer however, this subset of patients with HS has an increased risk.
Itching is associated with most dermatological dis orders and is a significant challenge in dermatolog ical practice. Chief physician Jesper Elberling shared his vast knowledge of itching and the definition of how we understand – and neurologically perceive – itch ing and why the brain picks up a reward effect when we feel the need to scratch. He has high hopes for the development of treatments since some of the latest treatments, both the biological and small molecule, already target the itch neuron and result in treatments that can turn off the itching.
Rune Kjærsgaard Andersen MD, Ph.D., Dermatology depart ment, Zealand University Hospital.
Do patients with hidradenitis suppurativa have an increased cancer incidence?
PDE-4 inhibitor treatment may improve perception of illness in patients with HS Elisabeth H. Taudorf took a deep dive into a patient case with severe hidradenitis suppurativa where the patient was treated with a PDE-4 inhibitor. This phaseII study focused on reduced pain and increased qual ity of life following the treatment. After 57 days of treat ment, the study showed small indicators of improve ment, but more interesting was that the patient’s own experience of a reduced perception of illness, and pain index went from nine to six.
Jesper Vraamark Elberling, Chief Physician, Clinical Associate Professor, Ph.D., Department of Dermatology and Allergy, Herlev and Gentofte Hospital. SII could be lower in patients treated with biologics Psoriasis is considered a systemic inflammatory dis ease. The systemic immune-inflammation index (SII), based on peripheral blood, neutrophil, platelet, and lymphocyte counts, has shown to be elevated in pa tients with psoriasis compared to healthy controls. The results presented by Amanda Kvist-Hansen sug gest that SII is lower for patients treated with biolog ical treatments.
Amanda Kvist-Hansen, MD, Ph.D. student, Department of Al lergy, Skin and Venereal Diseases, Herlev and Gentofte Hospital.
Elisabeth H. Taudorf, MD, Zealand University Hospital.
23BestPractice Nordic / Dermatology / NO. 3 / 2022
Understanding itching in dermatological disorders
Medical diagnosis and treatment have tra ditionally been based on a combination of the patient’s medical history and objective find ings. In recent decades, biotechnological break throughs have opened the door to individu alized medical treatments tailored to the phys iology of each
Proteomics in the dermatology of the future – inclusion of the skin proteome in clinical research and practice
BJØRN KROMANN HANSEN is a doctor and Ph.D. student at the De partment of Allergy, Skin, and Venereal Diseases at Herlev and Gentofte Hospital. Bjørn’s research focuses on mapping characteristic protein compositions in inflammatory skin diseases, with a primary focus on psoriasis.
Developmentsindividual.ingenesequencing have made it possible to involve the genome both diag nostically and therapeutically; the genome is a stable foundation for an individual’s physiology and thus phenotypic expression. Because pro teins are end-products of the genome and perform all enzymatic processes, the proteome is an important part of the dynamic founda tion of a phenotype. Thus, a complete overview of the proteins will be necessary to obtain a full understanding of the physiology.
24 BestPractice Nordic / Dermatology / NO. 3 / 2022
BEATRICE DYRING-ANDERSEN is a doctor and Ph.D. in der matology. In addition, she is an associate professor at the Novo Nor disk Foundation Center for Protein Research. She is a group leader and researcher in molecular protein signatures for inflammatory skin dis eases, melanoma, and fungal infections.
An independent field of research called pro teomics covers several interrelated areas, in cluding studies of the total protein composi tion, the structure, function, and interactions of the proteins. There are several methods to study the composition of proteins in biological material. Liquid chromatography-tandem mass spectrometry (LC-MS / MS) is frequently used to day because it is superior to other technologies in identifying and at the same time quantifying as many proteins as possible in a single sam ple.2,3 This article focuses on this method and its application in the dermatological context. The future of mass spectrometry research Until now, immunohistochemical and anti body-based methods have been preferred for identifying and quantifying proteins in clin ical practice and research. Although these methods are reliable and validated, they have certain limitations. The most important of these is that the methods only allow examination of relatively few selected proteins at a time. With mass spectrometry, it is currently pos sible to identify thousands of proteins and their relative abundance in a single biological sam ple. The technology is constantly being improved An important part of the development of this technology is due to vastly expanded computer power, as large amounts of data are generated. The rapid development in the field has already resulted in several clinical applications in mi crobiology, screening of newborns for congen ital diseases, and urinary toxicology studies.4
Histology and immunohistochemical stain ing are also used for proteins and other com ponents as part of the diagnostic process in dermatology. Compared to modern prote omics, however, it must be noted that only a limited sample of the total protein composi tion can be examined with these methods. This article reviews current and future perspectives on the use of proteomics in a dermatological context. Proteomics refers to studies of the presence and physiological significance of proteins. This article focuses on mass spectrometry as an analysis method with great potential in both research and clinical practice.
In the future, mass spectrometric protein re search is expected to enable further findings of biomarkers of importance for diagnosis, prog nosis, or treatment response. Furthermore, there is potential to identify the presence of patho logical proteins that new drug candidates could target. LC-MS / MS is used in studies for protein analysis of human skin.
CONCLUSION: Mass spectrometric protein studies have al ready contributed to an increased understanding of physio logical and pathological changes in the protein composition of the skin. The technology is becoming further accessible and is expected in the future to increasingly support clinical practice and research.
Figure 1 – Techniques for collecting skin samples
References: 1. Fredman G, Skov L, Mann M, Dyring-Andersen B. Towards Precision Dermatology: Emerging Role of Proteomic Analysis of the Skin. Dermatology. 2022;238(2): 185-194. 2. Aebersold R, Mann M. Mass-spectrometric exploration of proteome structure and function. Nature. 2016;537: 347-355. 3. Sinha A, Mann M. A beginner’s guide to mass spectrometry-based proteomics. Biochem (London). 2020;42(5): 64-69. 4. Sabbagh B, Mindt S, Neu maier M, Findeisen P. Clinical applications of MS-based protein quantification. Proteomics Clin Appl. 2016;10: 323-345. 5. Dyring-Andersen B, Løven dorf MB, Coscia F, et al. Spatially and cell-type resolved quantitative proteomic atlas of healthy human skin. Nat Commun. 2020;11(1): 5587.
With different techniqu es, it is possible to select which skin layers are to be examined. Mass spectrome tric protein analysis can be performed on whole or se lected skin layers. Cell fractions can be isolated via fluorescence cytometry or laser microscopy.
Conversely, thousands of proteins can be iden tified and quantified in the skin via mass spec trometry.Inthisconnection, it is relevant to first know the proteome in healthy skin. In our research group, we have used surplus skin from plas tic surgery to characterize the proteome in four separate skin layers and four different cell types that occur in the skin. In total, we have identified 10,700 different proteins, which is the largest number of proteins found in healthy skin in a single study. Several unknown pro teins and proteins that have not previously been associated with the skin were found.5 Data from this study are publicly available at https://skin.science/.Usingproteomics,significant findings have also been made concerning the pathogene sis, diagnosis and/or prognosis for several skin conditions, including malignant melanoma, chronic hand eczema, psoriasis and atopic dermatitis.1 Challenges and perspectives Today mass spectromtric protein research is considered to be a fast and robust method for examining protein compositions in very small amounts of tissues or even few cells. However, there are still challenges and areas where the technology can be further developed. One of the challenges is that a very large amount of data is generated, and this plac es great demands on computer power. The fact that mass spectrometry is suitable for ex amining the protein composition in histolog ically-fixed tissue samples opens the possi bility of making analyzes on skin collected in a clinical context or from previous studies.
25BestPractice Nordic / Dermatology / NO. 3 / 2022
CONFLICT OF INTEREST: None. ORIGINAL PUBLICATION: Fredman G, Skov L, Mann M, Dyring-Andersen B. Towards Precision Dermatology: Emerging Role of Proteomic Analysis of the Skin. Dermatology. 2022; 238 (2): 185-194.
Up to 30% of patients with skin psoriasis (PsO) later develop symptoms compatible with pso riatic arthritis (PsA)1 with a time-lag of approx imately seven years.2 This delay provides a unique opportunity for early detection in this disease group already associated with high ly specialized treatment for their skin disease by a Alertnessdermatologist.forearly manifestations of psori atic arthritis is of utmost importance, as a de lay in diagnosis of as little as six months may be associated with a lower treatment response.3
The onset symptom of PsA is rarely a swollen joint or a sausage finger. Therefore, it is impor tant to be aware of other factors that may help identify at-risk patients. Psoriatic nail changes, especially the occurrence of onycholysis (nail plate solution) and nail pitting (thimble dots), are important prognostic markers for the de velopment of arthritis in the outer joints.4 Against this background, nail fold capillaros copy (NVK) by plain dermoscopy and optical coherence tomography (OCT) has the poten tial to diagnose PsA, and to differentiate the disease from PsO and even from osteoarthri tis of the outer joints (OA), where differential diagnostic plays a role. We aimed to assess the diagnostic pro perties of NVK and OCT in patients with PsA compared to patients with PsO and OA based on capillary patterns at the nail bed. Can patients with PsA be differentiated from patients with PsO and OA? We included 50 patients with PsA, 12 with PsO, and 13 with OA from rheumatology and der matological outpatient clinics in Zealand, a to tal of five centers.
26 BestPractice Nordic / Dermatology / NO. 3 / 2022
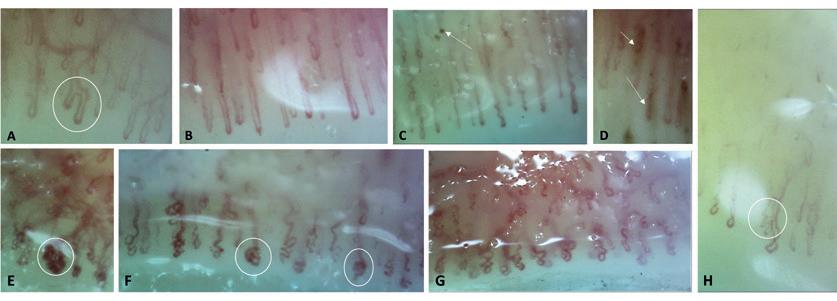
Nail fold changes as a marker for psoriatic arthritis in psoriasis of the skin
An early intervention with anti-inflammatory drugs can significantly improve the clinical and radiographic results. Dermatologists and general practitioners treating patients with skin psoriasis are primarily responsible for as sessing factors that could signal a transition from primary skin disease to also include joint and tendon attachments.
Prognostic factors for disease development
JØRGEN GULDBERG-MØLLER is a trained physiotherapist, doctor and PhD. He is employed as a ward doctor at Sjællands Universitetshospital in Køge as an ultrasound manager. He specializes in musculoskeletal diagnostic imaging and has a special interest in ultrasound. METTE MOGENSEN is a dermatologist. For more than 15 years, she has been working with the development of new advanced imaging diagnostics to detect skin diseases faster, easier, and more accurately by scan ning the skin – in close collaboration with the Technical University of Denmark and leading international research groups. She has a special focus on rapid diagnosis of breast cancer, but also imaging of autoimmune skin diseases and detec tion of vascular changes in the skin in patients with psoriasis and psoriatic arthritis, so-called nail fold capillaroscopy.
Patients with PsA could be differentiated from patients with PsO and OA by significantly lower capillary density and blood flow assessed on OCT, and by the presence of mi crobleeds in the nail fold at NVK.
Patients with psoriatic arthritis may have decreased capillary density and blood flow as well as microbleeds in the nail fold. However, no single diagnostic capillary pattern could identify psoriatic arthritis.
27BestPractice Nordic / Dermatology / NO. 3 / 2022 A: branched capillary arm in PsA. B: Extended capillaries in PsA. C: Implementing capillaries with microbleeds in PsA. D: Microbleeds in PsA E: Glomerular and dilated capillaries in OA. F: Glomerular and dilated capillaries in PSO. G: Crossed / meandering capillaries in PsA. H: Capillary branches in OA. Definition: Extended capillaries have diameters of 20-50 mm Figure 1: Qualitative NVK-morphology in patients with PsA, PsO and OAFigure 1 – Qualitative NVK-morphology in patients with PsA, PsO and OA A: branched capillary arm in PsA. B: Extended capillaries in PsA. C: Implementing capillaries with microbleeds in PsA. D: Microbleeds in PsA E: Glomerular and dilated capillaries in OA. F: Glomerular and dilated capillaries in PSO. G: Crossed / meandering capillaries in PsA. H: Capillary branches in OA. Definition: Extended capillaries have diameters of 20-50 mm. Table 1: Distribution of NVK and OCTA capillary morphology by PsA, PsO and OA NVK – rating (PsAn=188) PsO(n=42) (OAn=48) PsA vs PsO PsA vs OA lddKVNamoumCutoo Below mean capillary density, n (%) 95 (51 %) 14 (31 %) 24 (51 %) P=0 019 P=1 000 Irregularly enlarged capillaries, n (%) 42 (22 %) 14 (33 %) 12 (25 %) P=0 163 P=0 703 Giant capillary, n (%) 3 (2 %) 0 0 P=0 631 P=0 610 Microhaemorrhages, n (%) 24 (13 %) 3 (7 %) 1 (2 %) P=0 429 P=0.034 Capillary ramifications, n (%) 27 (14 %) 10 (24 %) 14 (29 %) P=0 162 P=0.020 Capillary disorganization, n (%) 84 (45 %) 24 (57 %) 23 (48 %) P=0 172 P=0 746 OCTA – rating PsAn=193 PsOn=48 nOA=52 PsA vs PsO PsA vs OA lddOCTAamoumCutoo Below mean capillary density, n (%) 103 (53 %) 10 (21 %) 17 (33 %) P<0.001 P=0.012 Irregularly enlarged capillaries, n (%) 66 (34 %) 27 (56 %) 10 (19 %) p=0 005 P=0 062 Giant capillary, n (%) 0 0 0 Microhaemorrhages, n (%) 0 0 0 Capillary ramifications, n (%) 0 0 0 Capillary disorganization, n (%) 108 (55 %) 22 (46 %) 25 (49 %) P=0 263 P=0 529 OCT Nail thickness*, mm , mean (SD) 0 62 (0 13) 0 73 (0 39) 0 59 (0 08) p=0 876 P=0 020 percentage, mean (SD) (2 94) (3 08) (3 30) OCTA blood flow 6 00 6 94 7 88 P=0 052 P<0 001 Capillary morphology PsAn=192 PsOn=48 nOA=52 PsA vs PsO PsA vs OA ddKVNamoumEULAR Tortuous/Crossed capillaries, n (%) 105 (55 %) 34 (71 %) 29 (56 %) P=0.050 P=1 000 Enlarged capillary diameter, n (%) 33 (17 %) 10 (21 %) 9 (17 %) P=0 674 P=1 000 Ramified capillaries, n (%) 24 (13 %) 7 (15 %) 10 (19 %) P=0 810 P=0 258 Microhaemorrhages, n (%) 32 (17 %) 3 (6 %) 4 (8 %) P=0 106 P=0 125 Elongated capillaries, n (%) 11 (6 %) 2 (4 %) 2 (4 %) P=0 747 P=0 741 Different capillary shapes, 5 (3 %) 6 (13 %) 1 (2 %) P=0 010 P=1 000glomerular, n (%) Data presented as a percentage of the capillary findings within the group, unless otherwise indicated. Differences between PsA and the other groups are presented as a two-sided p-value p <0.05 is considered significant. Table 1 – Distribution of NVK and OCTA capillary morphology by PsA, PsO and OA
A lower capillary density and low blood flow by OCT examination and the presence of microbleeds in patients with PsA raise excit ing questions for further research. Is there an expression of a higher inflammatory burden in PsA compared to PsO and OA?
References: 1. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376(10): 957-970. 2. Tillett W, Charlton R, Nightingale A, et al. interval between the onset of psoriasis and psoriatic arthritis comparing the UK Clinical Practice Research Datalink with a hospital-based cohort. Rheu matol (Oxford). 2017;56(12): 2109-2113. 3. Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radio graphic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74(6): 1045-1050. 4. Love TJ, Gudjonsson JE, Valdimarsson H, Gudbjorns son B. Small joint involvement in psoriatic arthritis is associated with onycholysis: The Reykjavik psoriatic arthritis study. Scand J Rheumatol. 2010;39(4): 299-302. 5. Hoerth, Kundi, Katzenschlager, Hirschl. Qualitative and quantitative assessment of nailfold capillaries by capillaroscopy in healthy volunteers. Vasa. 2012;41(1): 19-26.
CONCLUSION: Even though we identified several characte ristic capillary features by NVK and OCT examination in the three patient groups, no single capillary pattern was associ ated with either increased or decreased likelihood of being diagnosed with PsA. OF INTEREST: None. ORIGINAL PUBLICATION: Guldberg-Møller J, Henriksen M, Ellegaard K et. al. Novel application of optical coherence tomography and capillaroscopy in psoriatic arthritis concerning psoriasis and hand osteoarthritis. Rheumatol Adv Pract. 2021;5(3). DOI: 10.1093 / rap / rkab065.
Although patients with PsO showed the most nail changes on clinical examination, patients with PsA could be differentiated from patients with PsO and OA by significantly lower capil lary density and blood flow assessed on OCT and by the presence of microbleeds in the nail fold at PatientsNVK.with PsO could be characterized by a high incidence of crossed and glomer ular capillaries assessed on NVK and irregu larly enlarged capillaries on OCT. The OA group showed a significantly high er incidence of capillary branches on NVK, which could perhaps be attributed to a high er age in this group and is also seen in 47% of healthyDespiteindividuals.5thesedifferences in capillary mor phology between the three patient groups, we could not identify a specific capillary pattern or a particular degree of blood flow that was significantly associated with an increased or decreased likelihood of being diagnosed with PsA.From a clinical perspective, the results may require greater collaboration between der matologists and rheumatologists to benefit the patient. With knowledge of common exami nation tools and a common understanding of the significance of nail changes and ar thritis in the outer joints in PsA, patients at risk can hopefully be identified earlier and avoid permanent joint damage with early treatment.
28 BestPractice Nordic / Dermatology / NO. 3 / 2022
CONFLICT
29BestPractice Nordic / Dermatology / NO. 3 / 2022 PsoriasisTreatmentTrajectory
For many years, the dialogue involving rheu matic diseases has focused on triggers involv ing environmental factors combined with viral components in the body; the triggers reactivat ing the sleeping virus, also called “the human intestinal virome.” But the interplay is poorly understood between environmental factors and possible viral components. The use of pes ticides, chemicals, and other factors may give us new insights into chronic diseases, espe cially if these diseases arise at an earlier living age resulting in new manifestations.
Organophosphate pesticides Organophosphate pesticides (OPs) are among the most widely used synthetic chemicals for the control of a variety of pests. Reactive oxygen spe cies (ROS) caused by OPs might be involved in one of several toxicities of various pesticides.
MERETE ENGELHART is a specialist in internal medi cine and rheumatology at Department of Rheumatology, Gentofte and Center for Rheumatology and Spine Diseases, Rigshospitalet, Denmark. Her research is focused on connective tissue diseases, Raynaud Phenomenon, and connective tissue lung diseases.
Environmental factors and autoimmune diseases – a hypothesis
30 BestPractice Nordic / Dermatology / NO. 3 / 2022
HENRIK NIELSEN is a rheumatologist and specialist in internal medicine and rheumatology. He holds several research and advisory positions in rheumatology. His professional interests cover environmental issues, and he works to promote aware ness of the negative impact of pesticide use on autoimmune diseases.
Previous studies have demonstrated that the reactivation of Epstein-Barr virus (EBV), la tent in the human body (>95%), could involve oxidative stress. In one study, OPs were able to reactivate EBV through ROS accumulation. Data showed that OP (chlorpyriphos, abbrevi ated as CPF) induces oxidative stress, accom The use of pesticides and chemicals may give us new insights and predictions about the human intestinal virome’s handling of chronic diseases, for example, COVID19, using cytochrome polymorphism as a measurement.
Studies have suggested OP compounds have an “immunotoxin” potential in human and non-target organisms, representing an unseen risk of escalating SARSCOV-2 pathology.
The prevalence of SLE is also significantly higher in African Americans compared to West Africans. Previous data has shown a two-fold increase of SLE in the urban population of Af rican American adult women. This suggests that environmental factors in western culture (e.g. OP or other factors) could play a key role in SLE pathogenesis in African Americans.11
As discussed, the decreased anti-viral de fense due to CYP polymorphism could further support the hypothesis that external triggers like OP affect different cultures disproportion ately. No data however has demonstrated a link with CYP polymorphism indicating a possible genetic factor or COVID-19/EBV prevalence. Environmental factors implicated in the development of SLE
Unfortunately, in many developing coun tries, OPs are being used indiscriminately in ways that could further amplify unintention al exposure to these compounds.
The gene/environment interaction between CYPs and OP exposure might induce oxida tive stress (OS) and increase the susceptibil ity to SARS-CoV-2 infection. Pesticides like or ganochlorines impair cellular and humoral immunity, increasing mortality by infectious diseases8. This represents a new potential trig ger with a badly understood mechanism. Contact with environmental chemicals can result in clinical warning signals that arise ei ther later or acutely. For example, exposure to carbamates (OP) is followed by hypersen sitivity reactions, inflammatory/chronic man ifestations, or cancers. Short-term exposure to atrazine, a widely used herbicide, can per sist for a longer duration even after termina tion of the exposure.9
The agricultural uses of OP pesticides are gaining in popularity, and they account for more than 30% of global insecticide sales. The global market for sales of OP pesticides is ex pected to grow by a compound annual growth rate of 5.5% during the period 2018–2023.10
31BestPractice Nordic / Dermatology / NO. 3 / 2022 panied by an increase in ROS production, DNA damage, superoxide dismutase and catalase activity. Moreover, CPF exposure significantly enhances the expression of BZLF-1.1 These results suggest that OPs could contribute to the reactivation of the EBV lytic cycle through ROS induction, a process that may play an im portant role in the development of EBV-associated diseases as well as other rheumatic diseases
EBV is a well-known factor in the mechanism in systemic lupus erythematosus (SLE)2, tak ing part in the crosstalk between the entero virus. But reactivation of EBV because of pes ticide exposure in SLE has not been reported. A postulate that rheumatoid arthritis could be an occupational disease has been open for discussion in past years3 – but bigger pop ulation studies are needed to confirm this hy pothesis.
Systemic lupus erythematosus
The etiology of SLE is not well understood, but it is known that certain demographic groups are more affected than others. SLE is signifi cantly more common among women; ap proximately 90% of patients diagnosed with SLE are women.
A major determinant of the biotransforma tion of OPs and other pesticides is our body’s detoxification system, the cytochrome (CYP) P450 gene families. Genetic polymorphism in CYPs is known to alter metabolic pathways and induce false cellular response. CYP polymor phism has been considered to be an impor tant risk factor for various pathological con ditions induced by chronic organochlorine ex posure.6 Racial disparity CYP polymorphism and mortality due to COVID-19 have been reported in the United States, where the mortality rate is 3.6 times higher in the African-American popu lation compared to other ethnic individuals.7
Over time, environmental factors that have been implicated in the development of e.g. SLE include crystalline silica, cigarette smok
OP compounds: “immunotoxin” potential in human and non-target organisms Due to the overuse of pesticides, OP-residues have contaminated drinking water, grains, veg etables, fruits, and other food items, and for many years this has provoked a global health concern about potential neurological disor ders.4 These chemicals block the activity of acetylcholine esterase at synapses, leading to a disproportionate accumulation of the neu rotransmitter acetylcholine. Moreover, recent investigations have linked OPs to several biological responses mediat ing severe toxic outcomes. Studies have sug gested OP compounds have an “immunotox in” potential in human and non-target organ isms5, representing an unseen risk of esca lating SARS-COV-2 pathology.
3. Murphy D, Hutchinson D. Is Male Rheumatoid Arthritis an Occupational Disease? A Review. Open Rheumatol J. 2017 Jul 27;11: 88-105. DOI:10.2174/1874312901711010088.
5. Rajak P, Ganguly A, Sarkar S, et al. Immunotoxic role of organophosphates: An unseen risk escalating SARSCoV-2 pathogenicity. Food Chem Toxicol. 2021 Mar;149: 112007. DOI:10.1016/j.fct.2021.112007. 6. Docea AO, Vassilopoulou L, Fragou D, et al. CYP poly morphisms and pathological conditions related to chronic exposure to organochlorine pesticides. Toxicol Rep. 2017 May 26;4: 335-341. DOI:10.1016/j. toxrep.2017.05.007.
4. Abou-Donia MB. Organophosphorus ester-induced chronic neurotoxicity. Arch Environ Health. 2003 Aug;58(8): 484-497.
2. Blank M, Shoenfeld Y, Perl A. Cross-talk of the environment with the host genome and the immune system through endogenous retroviruses in systemic lupus erythematosus. Lupus. 2009 Nov;18(13): 1136-1143. DOI:10.1177/0961203309345728.
32 BestPractice Nordic / Dermatology / NO. 3 / 2022 ing, environmental pollutants, infections, sex hormones and other factors.12 Studies of (NZB x NZW) F1 lupus-prone mice have pointed to a role of chemicals (silica) in the pathogen esis of SLE13 and human smoking14 although the evidence in human observational studies has been limited until now. Clear evidence has been missing for ethical reasons. Further studies are needed to examine any potential relationship between exposure to pesticides/chemicals via e.g. food intake and CYP polymorphism as exemplified by the SLE model. Whether or not changes in the human intestinal virome result in a defense or attack mechanism is just at the beginning of our un derstanding.15
1. Zhao L, Xie F, Wang T-T, et al. Chlorpyrifos Induces the Expression of the Epstein-Barr Virus Lytic Cycle Activator BZLF-1 via Reactive Oxygen Spe cies. Oxid Med Cell Longev. 2015;2015: 309125. DOI:10.1155/2015/309125.
CONFLICT OF INTEREST:
References:
CONCLUSION: To summarize, we have limited understan ding of whether diseases such as SLE or other manifestati ons (child adiposity,16 Gulf War illness (GWI),17 chronic fatigue syndrome18 …) present themselves at an earlier living age following exposure to environmental factors. Likewise, our understanding is limited as to whether environmental fac tors can alter the body’s natural defense mechanism – “the human intestinal virome” – so it will attack the body instead of defending it, thereby escalating SARS-COV-2 – pathoge nicity. None.
7. Parpia AS, Martinez I, El-Sayed AM, et al. Racial disparities in COVID-19 mortality across Michigan, United States. EClinicalMed icine. 2021 Feb 26;33: 100761. DOI:10.1016/j.eclinm.2021.100761.
8. Krzystyniak K, Tryphonas H, Fournier M. Approaches to the evaluation of chemi cal-induced immunotoxicity. Environ Health Perspect. 1995 Dec;103 Suppl 9(Suppl 9): 17-22. DOI:10.1289/ehp.95103s917. 9. Filipov NM, Pinchuk LM, Boyd BL, Crittenden PL. Immunotoxic effects of short-term atrazine exposure in young male C57BL/6 mice. Toxicol Sci. 2005 Aug;86(2): 324-332. DOI:10.1093/toxsci/kfi188. 10. Mordor Intelligence. Global organophosphate pesticide market – segmented by active ingredients, product type, application, and geography – growth, trends, COVID-19 impact, and forecasts (2021-2026). https://www.mordorintelligence.com/industry-re ports/organophosphate-pesticides-market [Accessed 15.3.2022]. 11. Williams JN, Chang SC, Sinnette C, et al. Pesticide exposure and risk of sys temic lupus erythematosus in an urban population of predominantly African-American women. Lupus. 2018 Nov;27(13): 2129-2134. DOI:10.1177/0961203318805844. 12. Barbhaiya M, Costenbader KH. Environmental exposures and the development of systemic lupus erythemato sus. Curr Opin Rheumatol. 2016 Sep;28(5): 497-505. DOI:10.1097/BOR.0000000000000318. 13. Bates MA, Brandenberger C, Langohr I, et al. Silica Trig gers Inflammation and Ectopic Lymphoid Neogenesis in the Lungs in Parallel with Accelerated Onset of Systemic Autoimmunity and Glomeru lonephritis in the Lupus-Prone NZBWF1 Mouse. PLoS One. 2015 May 15;10(5): e0125481. DOI:10.1371/journal.pone.0125481. 14. Leffers HCB, Troldborg A, Voss A, et al. Smoking associates with distinct clinical phenotypes in patients with systemic lupus erythematosus: a nationwide Danish cross-sec tional study. Lupus Sci Med. 2021 Apr;8(1): e000474. DOI:10.1136/lupus-2021-000474. 15. Carding SR, Hoyles ND. The human intestinal virome in health and disease. Aliment Pharmacol Ther. 2017 Nov;46(9): 800-815. DOI:10.1111/apt.14280. 16. Warner M, Ye M, Harley K, Kogut K, Bradman A, Eskenazi B. Prenatal DDT exposure and child adiposity at age 12: The CHAMACOS study. Environ Res. 2017 Nov;159: 606-612. DOI:10.1016/j.envres.2017.08.050. 17. Seth RK, Maqsood R, Mondal A, et al. Gut DNA Virome Diversity and Its Association with Host Bacteria Regulate Inflammatory Phenotype and Neu ronal Immunotoxicity in Experimental Gulf War Illness. Viruses. 2019 Oct 21;11(10): 968. DOI:10.3390/v11100968. 18. Naviaux RK, Naviaux JC, Li K, et al. Metabolic features of chronic fatigue syndrome. Proc Natl Acad Sci USA. 2016 Sep 13;113(37): E5472-5480. DOI:10.1073/pnas.1607571113.
The challenges in conducting research that inves tigates the use of antibiotics, specifically in early childhood, include studying small sample sizes, the use of homogenous populations, failure to con sider different types of antibiotics and insufficient information about both maternal and childhood factors.
The increased use of antibiotics has raised the question of their relationship to atopic dermatitis. This study investigated the antibiotic exposure among Swedish children during pregnancy and in the first year of life.
Theory of antibiotic exposure and risk of atopic dermatitis
MWENYA MUBANGA is PhD and Postdoc toral researcher at Department of Med ical Epidemiology and Biostatistics at Karolinska Institutet in Sweden.
The relationship between atopic dermatitis and exposure to antibiotics in early life
Atopic dermatitis is a persistent skin disease that commonly begins in childhood. It is characterized by intense itching, eczematous and irritating dry ness of the skin. Although a predisposition to at opic dermatitis may be inherited, there have been inconsistent findings in studies investigating the relationship between atopic dermatitis and com mon environmental factors, including exposure to antibiotics.
34 BestPractice Nordic / Dermatology / NO. 3 / 2022
We included all children born in Sweden be tween March 2006 and December 2012 and fol lowed them up to the study end of December 2015. This study is possible in Sweden because individ ual-level information on every resident is collect ed in centralized administrative registers that can be used for research. The registers contain infor mation such as mother-child pairs, age, area of residence, income, migration, prescribed medi cations and health outcomes.
In the total population which comprised more than 700,000 singleton children aged between 3 and 9 years, we found that exposure to antibiotics dur ing pregnancy or the first year of life was associ ated with a higher risk of atopic dermatitis during early childhood. However, when we accounted for familial and other environmental factors by sib ling analysis, we found that exposure to antibiot ics in pregnancy was not associated with a great er risk of atopic dermatitis. However, exposure dur ing the first year of life was still associated with a moderate risk of atopic dermatitis. Furthermore, when we consider the association of the number of doses prescribed to each moth er during the study period, there was a greater risk of atopic dermatitis in those who were prescribed more doses than those with few ones. However, this was not observed when we accounted for fa milial and environmental factors.
2. Grada A, Bunick CG. Spectrum of Antibiotic Activity and Its Relevance to the Microbiome. JAMA Netw Open. 2021;4(4): e215357. DOI:10.1001/jamanetworkopen.2021.5357.
CONCLUSION: Our findings are interesting in view of the increased use of antibiotics around the world. Further under standing is needed as to how exactly antibiotics affect the pathways that activate the onset of atopic dermatitis. It has been suggested that the effect of antibiotics may vary depending on how antibiotics act on the skin, the gut, duration of use, their mode of action and route of administration. It may also be important to see if the use of multiple drugs at the same time has a different effect than single dosed agents.2
35BestPractice Nordic / Dermatology / NO. 3 / 2022
CONFLICT OF INTEREST: None.
References:
1. Mubanga M, Lundholm C, D’Onofrio BM, et al. Association of Early Life Exposure to Antibiotics With Risk of Atopic Dermatitis in Sweden. JAMA Netw Open. 2021;4(4): e215245-e45. DOI:10.1001/jamanetworkopen.2021.5245.
Against this background, we recently published a scientific article in JAMA network open1 using a nationwide study to test the theory that the use of antibiotics during pregnancy or during the first year of life is associated with an increased risk of atopic dermatitis.
Additionally, we extended this investigation to a smaller population of sibling-pairs that we iden tified via the birth registers. This was done in order to help us determine whether results obtained in the general population were related to environ mental and familial factors, including genetic pre disposition, or could be mainly attributed to an tibiotic exposure. Exposure to antibiotics or environmental factors?
CARSTEN SAUER MIKKELSEN is MD and spe cialist in dermato-venereology. He is a private practitioner in Brønder slev and is part of the research unit at the Department of Dermatology, Aalborg University Hospital, Denmark.
Psoriasis around the world
Psoriasis is a multifactorial polygenic chronic, im mune-mediated inflammatory skin disease char acterized by the formation of sharply demarcat ed, scaly erythematous plaques.2 Psoriasis is es timated to affect 125 million people worldwide. In Europe, it is estimated that approximately 5 mil lion people suffer from psoriasis, while 7 million people in the US have psoriasis. In western countries, the prevalence of psoria sis is believed to be around 2–4%,3-5 while the high est prevalence of psoriasis is found in the Scan dinavian population. In Denmark, approximately 2.2–2.8% of the population suffer from psoriasis.6,7 The self-reported lifetime prevalence of psoriasis in northern Norway increased from 4.8% in 1979–1980 to 11.4% in 2007–2008, indicating an increased awareness of the disease over the last 40 years.8 Due to difficulties in accurately identifying and documenting the disease, psoriasis is believed to be underdiagnosed and undertreated in many countries around the world.5 Psoriasis in Inuit populations Very few studies describe the prevalence and in cidence of psoriasis among Inuit populations.9-11 Higher prevalence rates have been reported at This study set out to estimate the age- and gender-specific prevalence of psoriasis in Nuuk, Greenland. No overall gender-specific difference in prevalence was observed.
Greenland is the largest island in the world, with 85% of its 2.2 million square kilometers covered by ice. Approximately 56,500 people live along the ice-free coastline in Greenland, of which 19,261 live in the capital, Nuuk. The majority of the popula tion is of Greenlandic origin (90%). 10% are immi grants, mostly from Denmark, but also including 2.4% from the Philippines, Thailand, and Iceland.1
Further, the study showed a low prevalence of patients diagnosed with psoriasis in Nuuk. However, we speculate that the prevalence found in this study is underestimated, and further research should be conducted.
36 BestPractice Nordic / Dermatology / NO. 3 / 2022
SOFIA HEDVIG CHRISTENSEN BOTVID is MD, currently a Ph.D. student at National Research Center for Allergy as part of the Department of Al lergy, Dermatology, and Venereology at Gentofte Hospital, Denmark. She has recently finished working as a doctor in Nuuk and Paamiut, Greenland, for 15 months.
Low prevalence of patients diagnosed with psoriasis in Nuuk: a call for increa sed awareness of chronic skin disease in Greenland
ORIGINAL PUBLICATION: Botvid SHC, Hove LS, Backe MB, Skovgaard N, Pedersen ML, Mikkelsen CS. Low prevalence of patients diagnosed with psoriasis in Nuuk: a call for increased awareness of chronic skin disease in Greenland, Int. Journal of Circumpolar Health. 2022.81:1. DOI: 10.1080/22423982.2022.2068111
INTERESTS:
References: 1. Grønlands Statistik. [Online]. [cited 6 Mar 2022]. Available from: https://stat.gl/default.asp?lang=da. 2. Parisi R, Symmons DPM, Griffiths CEM, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2): 377–5. 3. Stern RS, Nijsten T, Feld man SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatis faction. J Investig Dermatol Symp Proc. 2004;9(2): 136–139. 4. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the UK: a population-based study. Arch Dermatol. 2005;141(12): 1537–1541. 5. Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiag nosed psoriasis in US adults: results from NHANES 2003-2004. J Am Acad Dermatol. 2009;60(2): 218-224. 6. Lomholt G. Prevalence of skin diseases in a population: A census study from The Faroe Islands. Dan Med Bull. 1964; 11:1–7. 7. Egeberg A, Skov L, Gislason GH, et al. Incidence and prevalence of psoriasis in Denmark. Acta Derm Venereol. 2017;97(7): 808–812. 8. Danielsen K, Olsen AO, Wilsgaard T, et al. Is the prevalence of psoriasis increas ing? A 30-year follow-up of a population-based cohort. Br J Dermatol. 2013 Jun;168(6): 1303-1310. 9. Harvald B. Genetic epidemiology of Greenland. Clin Genet. 1989;36(5): 364-367. 10. Connor WE. Effects of omega-3 fatty acids in hypertriglyceridemic states. Semin Thromb Hemost. 1988;14(3): 271-284. 11. Kromann N, Green A. Epidemiological studies in the Upernavik district, Greenland: incidence of some chronic diseases 1950–1974. Acta Med Scand. 1980;208(1–6): 401-406. 12. Farber EM, Nall ML. The natural history of psoriasis in 5,600 patients. Dermatology. 1974;148(1): 1-18.
CONFLICT
Results During the study period of 12 months, 175 patients (0.9%) were diagnosed with psoriasis in Nuuk, of which 79 (45%) were females and 96 (55%) were males. The prevalence of patients diagnosed with psoriasis in the adult population aged 20 years old or more in Nuuk was 1.1%. No overall gender-spe cific difference in prevalence was observed. Chronic diseases including diabetes, hyperten sion, and obstructive lung disease were observed more frequently among patients with diagnosed psoriasis (28.6%) in Nuuk compared to controls (20.9%) (p < 0.05). We found a low prevalence of patients with pso riasis in Nuuk. We speculate that the prevalence found in this study is underestimated. Thus, we call for an increased awareness of chronic skin dis ease in Nuuk, Greenland, particularly since chron ic co-morbidity to psoriasis was common.
37BestPractice Nordic / Dermatology / NO. 3 / 2022 higher latitudes, and also in Caucasians compared with other ethnic groups.12 In Greenland, the earli est mention of psoriasis stems from a book from 1940 based on observations done over 30 years by a doctor.10 Here, a girl in 1912 was described as having psoriasis. Furthermore, an epidemio logical study from 1980 found a low incidence of chronic diseases, including psoriasis, in the Uper navik district in northern Greenland in the years of 1950-1974.11 The current prevalence of psoriasis in Greenland is unknown. The objective of this study was to estimate the age- and gender-specific prevalence of psoria sis in Nuuk. Furthermore, we aimed to explore the common risk factors and co-morbidities for pa tients with psoriasis compared to an age- and gender-matched control group. The study was designed as a cross-sectional case-control study based on national high-quality data from medi cal records and population registers in Nuuk, from January 1, 2021, to January 1, 2022.
CONCLUSION: High prevalence rates of psoriasis have pre viously been reported among Inuit populations. However, this study found a low number of patients diagnosed with psoriasis in Nuuk, Greenland. Furthermore, no overall gender-specific difference was observed. Hence, we suggest that the prevalence found in this study is underestimated, and further research should be conducted. OF None.