CELIACCANADA



A round-up of the latest clinical and scientific research updates from the International Celiac Disease Symposium
Celiac Canada wants to see a world without celiac disease. As part of our mission, we are keeping abreast of and leading research to help us continue moving towards this goal.
Executive Director
Melissa Secord and Dietitian Caleigh McAulay had the opportunity to learn first hand about the latest advancements internationally at the bi-annual International Celiac Disease Symposium. We are pleased to share our highlights for you in this report.
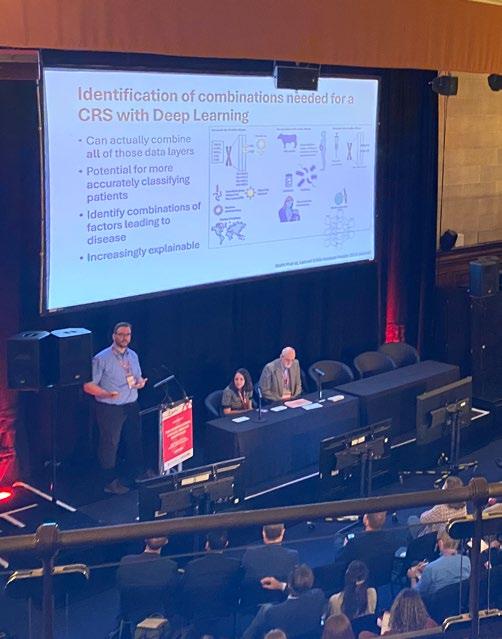

EPIDEMIOLOGY
Leonard’s
research
found that levels of the protein zonulin increased 18 months before a child’s diagnosis of CD

Children who received high levels of antibiotics early on in their life exhibited elevated zonulin levels.
Maureen Leonard’s study attempted to predict the onset of CD in young children over a period of several years by examining various factors—including the gut microbiome, environmental factors, maternal delivery, feeding practices and antibiotic use. Her research found that levels of the protein zonulin increased 18 months before a child’s diagnosis of CD. Additionally, children who received high levels of antibiotics early on in their life exhibited elevated zonulin levels. Her study suggests that incorporating gut microbiome analysis and gene and environmental testing could be used as part of risk assessment for CD in children. u
Screening of CD should be done for children getting routine bloodwork.
Gemma Viillasante suggested opportunistic screening for CD in children undergoing routine bloodwork, as the disease has so many different symptoms, including 12% of cases being asymptomatic or showing only minor symptoms. Carlo Catassi further proposed that mass pediatric screening is the best approach to catch cases early, particularly by screening children between the ages of 5 and 10 years, which captures most active cases of CD. In Italy, the government is already screening schoolage children for both CD and type 1 diabetes. It is hoped that the data from these screenings will help influence legislative changes in other countries, including Canada. u
Ravi Sargur emphasized that the only reliable method for determining food reactivity is through a food elimination process
EPIDEMIOLOGY
Antonio Carroccio discussed the overlap between disorders of gut-brain interaction (DGBI) and gluten-related disorders, emphasizing the importance of screening for CD in patients with irritable bowel syndrome (IBS). Currently, there are no genetic markers to distinguish between non-celiac gluten sensitivity (NCGS) and DGBI, but certain clinical features may help in diagnosis. Carroccio suggested that evaluating gut-brain disorders, particularly those presenting with extra-intestinal symptoms (such as neurological issues), could help in identifying more people with NCGS. u
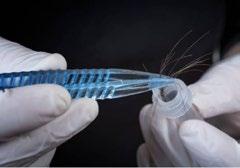
People can waste money on disproven tests for food intolerances, such as hair analysis.
Evaluating gut-brain disorders, particularly those presenting with extra-intestinal symptoms (such as neurological issues), could help in identifying more people with NCGS

Ravi Sargur shared his concern for people wasting their money on disproven tests for food intolerances, such as hair analysis, electrodermal testing— which he cautioned could be dangerous—and Diamine Oxidase (DAO) testing. He emphasized that the only reliable method for determining food reactivity is through a food elimination process. Sargur also advised against using the term “food allergy,” as there are no effective tests for accurately identifying food intolerances. u
Edwin Liu discussed the creation of a potential Combined Risk Score using fixed and variable data to help predict CD onset. Fixed data would be country born, sex and family history; and variable data would include lifestyle, infections, diet and occupation. The introduction of artificial intelligence can help in these predictions and risk factors. But the data needs to be right. Sharing health data across international borders for large studies is another major hurdle. u
... the overlap between T1D and CD was found to be lower than previously believed, suggesting other influencing factors may be involved
EPIDEMIOLOGY
MARIAN REWERS ON THE ASK AND TEDDY STUDIES, WHICH ARE LONGITUDINAL STUDIES EXAMINING CHILDREN’S HEALTH
Marian Rewers provided updates on the ASK (Colorado) and TEDDY (six international centres) studies, which are longitudinal studies examining children’s health. By age 15, 5% of participants had persistent tissue transglutaminase antibodies (TGA), and 3.3% were diagnosed with CD, a rate twice that of type-1 diabetes (T1D). The studies also found that introducing wheat, rye or barley too early (1-3 months) or too late (after 7 months) does not increase the risk of developing CD. Children who develop both T1D and CD are more likely to carry the HLA- DR3/4 genotype associated with “atypical CD.” Most children diagnosed with CD by age four were asymptomatic and showed normal growth.
The TEDDY study indicated a spike in CD cases around age 2, with a mean diagnosis age of 5.8 years, particularly notable in Sweden, where gluten is in-
Researchers are considering whether the quantity of gluten introduced, rather than just the timing, plays a role in risk
troduced earlier in baby food compared to the U.S. and other countries that favour rice-based formulas. Researchers are considering whether the quantity of gluten introduced, rather than just the timing, plays a role in risk. Additionally, the overlap between T1D and CD was found to be lower than previously believed, suggesting other influencing factors may be involved.
The TEDDY study indicated a spike in CD cases around age 2, with a mean diagnosis age of 5.8 years, particularly notable in Sweden, where gluten is introduced earlier in baby food compared to the U.S. and other countries that favour rice-based formulas
The biggest caution is that children can remain relatively asymptomatic even when presenting with severe Marsh scores, which assess damage to the small intestine (0-4 being the most severe damage). Additionally, researchers found that increased gluten intake is associated with a dramatic rise in the role of enterovirus, highlighting the complexities of diagnosing and managing CD.
Environmental predictors being monitored as triggers for CD in children
z Amount of gluten and age of introduction
z Rotavirus
z Enteroviruses
z Childhood viral GI infections
z 25-hydroxy vitamin D levels
z Caesarean-sections
z Maternal gluten intake late in pregnancy u
... fetuses are essentially exposed to a “soupy broth” of what mothers have previously consumed – Alfonso Herrera
EPIDEMIOLOGY

Future research aims to determine whether gluten-reactive cells exist in fetuses and whether this exposure provides protection against CD.
Dr. Alfonso Herrera studied the presence of gluten immunogenic peptides (GIP) in the womb of women with CD, finding that newborn urine samples contained measurable GIP. The researchers said that fetuses are essentially exposed to a “soupy broth” of what mothers have previously consumed. Future research aims to determine whether gluten-reactive cells exist in fetuses and whether this exposure provides protection against CD or increases susceptibility to inflammation. Additionally, the study raises questions about potential inflammatory events occurring after birth that have yet to be identified.
Definition Gluten immunogenic peptides (GIP) are resistant to gastrointestinal digestion and are then excreted via the stool and urine. –World Journal of Gastroenterology u
Daniel Agardh raised the question of whether CD is solely a pediatric condition or if some adults simply experience late-onset or late diagnosis. CD is prevalent in about 1% of the general population, and longitudinal studies like TEDDY and DAISY indicate that most cases typically develop in early childhood. These studies are tracking children over their lifetimes, which will help identify potential spikes in diagnosis in the coming years. Agardh suggested that we may observe secular or environmental trends, including varying triggers for different decades and ages. We’ll have to wait a few more years to see the results! u
Canadian researcher Jocelyn Silvester conducted a study in the U.S and Canada to evaluate a non-biopsy approach for diagnosing CD in children, following the European gastroenterology guidelines. The study found that 83% of children with symptoms and a tissue transglutaminase IgA (TTg-IgA) test >10 times the upper limit had celiac enteropathy (enteropathy is a disease of the intestinal tract), with a positive predictive value of 95.5% for enteropathy in all patients. However, she cautioned that 17% of seropositive children did not have active CD upon biopsy, highlighting discrepancies in the accuracy of various blood assays (collection brands) used in real-world settings. u
Dr. Sylvester is a former Celiac Canada JA Campbell Young Investigator Award recipient.
19% of patients with CD will have elevated liver enzymes. 83% of these cases resolve with a strict GFD – Alberto Rubio-Tapia
...
highlighted the increased risk of CD in individuals with Down syndrome,
who are six times more likely to develop CD, with some studies showing rates as high as
10%-18%
Marisa Stahl highlighted the increased risk of CD in individuals with Down syndrome, who are six times more likely to develop CD, with some studies showing rates as high as 10%-18%. The mechanism of increased risk is unknown. Not all villous atrophy in DS patients is CD; conditions like SIBO, peptic duodenitis, infections and medications may cause similar symptoms, and some cases remain unexplained. In some cases, patients with non-specific villous atrophy developed CD later, making repeat endoscopic evaluations necessary. An Italian study found that one-third of patients with DS and CD had no gastrointestinal symptoms, complicating diagnosis. Additionally, CD could be confused with DS regression disorder, which affects learning and speech. Managing CD in DS patients can also be more challenging due to co-existing conditions, multiple medications and the need for a coordinated case from several caretakers who need to learn and manage the GFD. Further research is needed to understand the unique characteristics of CD and duodenitis in DS patients. u
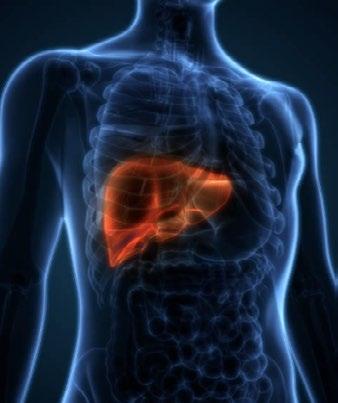
Alberto Rubio-Tapia discussed the association between CD and liver disease, noting that 19% of patients with CD will have elevated liver enzymes. He emphasized that 83% of these cases resolve with a strict GFD, while unresolved cases may require continuous monitoring to explore other underlying factors. u
The majority of liver abnormalities improve with a GFD

Dr. Ines Pinto Sanchez, Celiac Canada Professional Advisory Council member, discussed irritable bowel syndrome (IBS), a gut-brain interaction disorder characterized by chronic abdominal pain and altered bowel habits, which can often be confused with non-celiac gluten sensitivity (NCGS). Around 80% of IBS patients report food-related issues, with gluten frequently identified as a trigger. While around 50% of IBS patients self-report symptom improvement on a gluten-free diet (GFD), research from McMaster found no significant symptom differences between IBS patients consuming gluten-containing or gluten-free bread. This suggests that a strict GFD may
not be necessary for GS-IBS patients.
Dr. Sanchez noted that collaboration with a dietitian is crucial when prescribing a GFD for IBS patients
It is also important to consider the potential downsides of the GF diet. In this study, 65% of IBS patients on a strict GFD were found to be deficient in micronutrients such as zinc, iron and vitamin D and experienced low fibre intake, leading to constipation. The GFD is also associated with excess fat and sugar intake, which can result in weight gain. The diet is also restrictive and costly. Dr. Sanchez noted that collaboration with a dietitian is crucial when prescribing a GFD for IBS patients. u
Early diagnosis is crucial, as gluten-related brain damage is often irreversible – Iian Croall
Deanna Kelly, an expert in schizophrenia, discussed the link between gliadin, inflammation and neurochemistry in schizophrenia. About 32% of individuals with schizophrenia have elevated anti-gliadin antibodies (AGA IgG), especially in early illness, with rates even higher in those with depression. A GFD has been shown to improve psychiatric symptoms, including psychosis, and reduce inflammation. In studies, patients on a GFD experience significant improvements in negative symptoms of schizophrenia, as well as cognitive function, which is a major factor in functional impairment. The GFD also led to improvements in inflammatory markers, further supporting its potential therapeutic role in schizophrenia. u
Iain Croall discussed the impact of gluten on brain health and the importance of diagnosing and monitoring gluten-related neurological issues. While gluten can trigger brain-related issues such as gluten ataxia and neuropathy, these issues occur on a spectrum, and not everyone with CD develops neurological symptoms, and vice versa. Antibodies such as gliadin, tissue transglutaminase (TTG) and transglutaminase 6 (TG6) play key roles in identifying gluten-related brain damage. TG6 is linked to brain atrophy and is especially important for diagnosing and monitoring neurological problems driven by gluten sensitivity. Early diagnosis is crucial, as gluten-related brain damage is often irreversible. u
Mara Floare discussed GA, a neurological condition caused by an autoimmune response to gluten, where the immune system mistakenly attacks parts of the brain, specifically the cerebellum, which controls coordination and balance. This can result in symptoms such as difficulty walking, poor coordination, tremors and, in some cases, issues with speech and vision.
GA is part of a broader spectrum of gluten-related neurological disorders, which includes cerebellar ataxia, encephalopathy, recurring headaches, peripheral neuropathy, epilepsy and depression. It can occur in individuals with or without CD, and in some cases, it
may be the only manifestation of gluten sensitivity.
Early diagnosis and adherence to a strict GFD can help stabilize symptoms and prevent further neurological damage. However, diagnosing GA is challenging, as it lacks distinct clinical features that differentiate it from other forms of ataxia, often leading to delayed diagnoses. Additionally, GA is underdiagnosed in gastrointestinal clinics, despite its high prevalence among patients with CD. Since the cerebellum cannot regenerate like the gut, understanding the disease’s progression is crucial for early management and treatment to prevent irreversible damage. u
Dr. Hadjivassiliou stated that there are three types of transglutaminases: TG6 - which is primarily expressed in the brain, TG3 - associated with dermatitis herpetiformis, and TG2 - used for CD diagnosis. TG6 antibodies are prevalent in neurological manifestations of gluten sensitivity and can be the only marker in some cases. Results from a study of patients with dermatitis herpetiformis show that TG6 was more common in those without enteropathy (60%) compared to those with it.
Among children with CD, 25% had TG6 antibodies, with the prevalence increasing to 61% in children with other autoimmune diseases. Most patients with classic CD show neurological symptoms, and a stricter GFD can reduce TG6 antibody levels over several years. However, even
with a GFD, 66% of patients with GA remain TG6 positive, albeit at lower levels, and 37% remain positive for antigliadin antibodies. TG6 antibodies can be reduced with immunosuppressive treatments like oral steroids.
Conclusions from Dr. Hadjivassiliou’s session
z TG6 antibodies are prevalent in patients presenting with neurological manifestations of gluten sensitivity (and can be the only marker of GS).
z TG6 autoimmunity is a late phenomenon in CD.
z The presence of TG6 antibodies in classic CD is often associated with brain damage.
z Given the slow elimination of TG6 antibodies on a GFD, the potential role of immunosuppression from the point of diagnosis is worth considering for managing these cases. u
Dr. Biagi expressed skepticism about non-celiac gluten sensitivity (NCGS), a condition where patients report symptoms triggered by gluten despite not having CD or a wheat allergy. Although NCGS is often thought to affect about 6% of people, it mainly relies on self-reported symptoms, making diagnosis difficult without clear biomarkers. Some studies suggest that fructans (a type of sugar), not gluten, may be the true culprit.
Dr. Biagi’s diagnostic approach involves ruling out CD, wheat allergy and other gastrointestinal conditions, only considering NCGS if the patient
spontaneously mentions gluten or wheat as a trigger and denies other food-related triggers.
Dr. Biagi’s diagnostic approach
z Excluding CD, wheat allergy and other gastrointestinal conditions, without directly asking patients if symptoms are related to specific foods.
z If the patient mentions gluten/wheat spontaneously, he asks whether other foods might induce the symptoms.
z If they deny other foods, he then takes NCGS into consideration. u

Penny Whiting emphasized the importance of targeted testing for CD in high–risk populations. Adults with conditions like dermatitis herpetiformis (DH), anemia, migraines, type 1 diabetes (T1D), and osteoporosis have a two-fold increased risk of developing CD. Similarly, individuals with thyroid disease, irritable bowel syndrome (IBS) and subfertility or recurrent pregnancy loss face a 1.5 to 2x increased risk of developing CD. The strongest predictors across all demographics, however, is a family history of CD.
Whiting also discussed cost-effective screening approaches, recommending serological and genetic testing to anyone with at least one predictor, such as a family history of CD or anemia.
2x higher risk :
z Dermatitis herpetiformis (DH)
z Migraines
z Anemia *
z Type 1 Diabetes (T1D)
z Osteoporosis
z Chronic liver disease
z Family history of CD *
* STRONGEST PREDICTORS
STRONGEST
z Adults
Whiting recommended serological and genetic testing to anyone with at least one predictor.
The biggest risk factor for CD in all groups is a family history of the disease
1. First Degree relative with CD
2. Anemia
z Children
1. First degree relative with CD
2. T1D
3. Turner syndrome
4. IgA deficiency u

The gluten challenge remains essential but must be applied with an understanding of its limitations
Kalle Kurppa highlighted complexity of using a gluten challenge for CD diagnosis. This approach is essential for individuals who have reduced their gluten intake or adopted a GFD without prior testing.
It is particularly relevant for those with:
z A family history of CD
z IBS-like symptoms or other clinical indicators
z Perceived health benefits from gluten avoidance
z Participation in clinical research
A GFD complicates CD diagnosis because symptom recurrence can take weeks to months, while serological (blood test) and histological (tissue biopsy) changes may take months to years. Importantly, symptoms and serology do not always align-individuals may have positive serological results without symptoms or vise versa.
z Typical intake: 5-15 grams of gluten per day
z Serological relapse within 1-12 months; histological changes within 3-12 months.
z The European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) advises starting with 10-15 grams/day. Monitor serology after one month and re-evaluate every three months if no relapse occurs.
z Endoscopy poses challenges, especially during critical growth phases, due to the need for anesthesia.
z A recommended intake of 6-10 grams of gluten per day for 1-3 months ensures accurate serological and histological assessment
z Serology alone is an unreliable predictor of intestinal damage.
FUTURE DIRECTIONS IN GLUTEN CHALLENGE
Kurppa also discussed emerging technologies that could replace traditional gluten challenges:
z ELISpot Assays Detect gluten-specific T cells in peripheral blood after a brief 3-day challenge.
z IL-2 Detection Measures cytokine responses from gluten-specific CD4+ T cells within hours of gluten ingestion.
z Flow Cytometry Identifies gluten-specific T cells using HLA-DQ: gluten tetramers
z Advances Diagnostics AI-driven histological analysis and omics approaches are on the horizon.
White these methods are still in early development, they represent a potential shift towards faster, less invasive diagnostic tools.
Testing for CD before starting a GFD is crucial to avoid diagnostic challenges. The gluten challenge remains essential but must be applied with an understanding of its limitations. u

Starting in 2024, Italy will become the first country to systematically screen for CD and diabetes in pediatric populations, setting a precedent
Luisa Maerin discussed the no-biopsy approach to diagnosing CD in children, as outlined by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN), and how it has transformed the diagnostic landscape. With advancements in serological testing, biopsies may no longer be necessary for many pediatric patients, offering significant benefits and raising important considerations.
KEY POINTS FOR PEDIATRIC DIAGNOSIS
ESPGHAN guidelines
z 2012 guidelines
A non-biopsy diagnosis is appropriate if all the following criteria are met:
1. Symptoms consistent with CD.
2. TGA levels ≥10x ULN.
3 Positive EMA test in a second serum sample.
4. Presence of HLA-DQ2 and/or DQ8 genes.
z 2020 guidelines
Simplified criteria now exclude the need for symptoms or HLA testing. A biopsy can be avoided if:
1. TGA levels ≥10x ULN.
2. Positive EMA test in a second serum sample. This streamlined approach offers a faster, less invasive, and cost-effective diagnostic pathway, enhancing patient comfort and healthcare efficiency.
Benefits
z Patient comfort: avoids invasive procedures, improving the diagnostic experience for children.
z Cost Savings and Environmental Impact: Significant savings for healthcare systems and reduced carbon footprint.
Future potential: Point-of-Care (POC) Testing
z Rapid TGA tests show promise for future implementation, particularly in under-resourced areas.
– Similar to at-home COVID tests, these provide results within 10 minutes using a single drop of blood.
– Early studies indicate 92% diagnostic accuracy in children without biopsy confirmation.
– If scientifically validated, these tests could revolutionize early diagnosis, especially in resource-limited settings.
Global developments

z Italy leads the way: Starting in 2024, Italy will become the first country to systematically screen for CD and diabetes in pediatric populations, setting a precedent for global health policy.
The no-biopsy approach offers a promising future for pediatric CD diagnosis, providing significant cost, environmental, and patient care benefits. However, ongoing research and validation are crucial to fully realize its potential and address unforeseen challenges. u
The no-biopsy approach offers a promising future for pediatric CD diagnosis
DIAGNOSIS & HISTOPATHOLOGY
JEREMY WOODWARD ON SYMPTOMS & HISTOLOGY DISCORDANCE
Jeremy Woodward addressed the complex relationship between gluten ingestion, intestinal damage, and symptoms in CD. He highlighted a key reality: there is no consistent correlation between the severity of small bowel damage and symptom presence or severity.
z No clear correlation Evidence shows no direct link between the extent of tissue damage and symptoms. Patients may have tissue damage with mild, severe, or no symptoms at all.
z Response to a Gluten-Free Diet (GFD)
– Approximately 88% of patients report symptom improvement on a GFD.
– However, 7-30% continue to experience symptoms or lab abnormalities after 6-12 months on a strict GFD
– Persistent villous atrophy is observed in 25-43% of patients, despite adherence to a GFD.
z Residual symptoms without gluten exposure
50-65% of individuals with ongoing symptoms or tissue damage have no identifiable gluten ingestion, suggesting other factors may contribute.
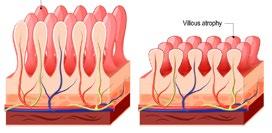
z Refractory Celiac Disease (RCD)
Affecting 0.6-4% of patients, RCD is characterized by persistent or recurrent symptoms and villous atrophy despite strict adherence to a GFD for over 12 months.
Understanding the disconnect between symptoms and histology is vital for effective CD management. Comprehensive follow-up, including biopsies, ensures accurate monitoring and support for patients navigating this complex condition. u
Marie Robert explored the challenges of interpreting mucosal biopsies for CD and potential innovative solutions. She noted that artificial intelligence (AI) is beginning to show promise in areas like prostate cancer biopsies and could revolutionize CD diagnosis.
However, barriers such as high costs, computational power needs and the requirement for large training datasets remain significant. Despite these challenges, early results are encouraging, suggesting AI tools could improve diagnostic accuracy in the future. u
The no-biopsy approach holds great promise for adult CD diagnosis, offering ... a patient-centered ... economical alternative to traditional methods
MOHAMED SHIHA ON THE NO BIOPSY APPROACH IN ADULTS – AN INTERNATIONAL
Mohamed started off by asking a question – why are we missing so many patients with CD at various steps in the diagnostic process?
These include patients with symptoms compatible with CD who do not receive testing, those with positive serology who aren’t referred for biopsy, and cases where biopsies are misinterpreted or not performed correctly. Notably, a third of patients with positive serology aren’t referred for biopsy, leaving their diagnosis unresolved.
Furthermore, only 4 out of 10 patients receive the recommended number of biopsies during endoscopy, often missing key areas such as the duodenal bulb. So, is a no-biopsy approach the solution?
WHILE PROMISING, THE NO-BIOPSY STRATEGY FOR ADULTS PRESENTS CHALLENGES
z Data limitations
– Compared to pediatric cases, there is less evidence supporting the predictive value of seroogy in adults.
– A positive predictive value (PPV) of 95% might still lead to misdiagnosis in some cases.
z Predictive value of serology
– Adults with TGA levels >10 times the upper limit of normal (ULN) are about 20 times more likely to have CD compared to those with lower levels.
– High TGA levels alone may be sufficient for diagnosis in many cases, especially in secondary care settings with moderate to high pre-test probability
PATIENT PREFERENCES MATTER
z Avoiding biopsies
– Patients prefer non-invasive testing methods due to factors like procedural discomfort and long wait times for endoscopy.
– An accurate and timely diagnosis is their primary concern.
z Cost Efficiency
– Endoscopies account for the majority of CD diagnostic costs.
– Implementing a no-biopsy approach could reduce costs by up to 75%.
z Reliable: High specificity and accuracy
z Non-invasive and cost-effective: Preferred by patients and reduces healthcare costs.
z Timely diagnosis: Allows quicker initiation of gluten-free treatment.
The no-biopsy approach holds great promise for adult CD diagnosis, offering a reliable, patient-centered, and economical alternative to traditional methods. However, ensuring robust serological protocols and addressing data gaps are essential for widespread implementation. u

CHRISTIAN COSTAS: A DIETITIAN’S PERSPECTIVE: WHY ADHERENCE IS NOT BLACK OR WHITE

Cristian Costas emphasizes that adherence to a GFD is not a binary decision but exists on a spectrum. Dietitians play a crucial role in supporting individuals with CD by assessing risks of gluten exposure and educating patients on managing and adhering to the GFD. Dietitians assess adherence across three key areas: label reading, at-home food preparation, and eating outside the home. However, barriers like lack of access to specialist dietitians, insufficient medical follow-up, and limited GFD education contribute to unintentional gluten exposure and difficulty maintaining the diet.
Patients are more likely to adhere to a GFD after receiving tailored advice from a knowledgeable dietitian, Continued on next page
Patients are more likely to adhere to a GFD after receiving tailored advice from a knowledgeable dietitian, addressing concerns such as affordability, mental health struggles, and misconceptions about the importance of adherence without symptoms. Costas emphasized the need for a shift in approach, meeting patients where they are, focusing on reducing perceived barriers ...

addressing concerns such as affordability, mental health struggles, and misconceptions about the importance of adherence without symptoms.
Costas emphasized the need for a shift in approach, meeting patients where they are, focusing on reducing perceived barriers, and offering continuous education and resources. To improve outcomes, greater access to specialist dietitians and standardized, cost-effective support systems are essential, ensuring GFD education receives as much priority as diagnosis.
z Risk of gluten exposure with labels
– Food and drink label reading habits
– Medication and supplements
z Risk of gluten exposure while eating at home
– Storage, cooking and preparation methods, use of cooking utensils and appliances, house hold education
z Risk of gluten exposure when eating food from outside the home
– Habits with restaurants takeouts and events with food (ie, weddings)
– Habits when eating at friends/family’s house
– Habits when traveling

Share tools to make GFD easier (resources, apps, label reading, cheaper foods ... )
1. I don’t have any symptoms
a. Clarify that symptoms are not a good predic tor of disease activity
b. Review all GI and non-GI previous symptoms. Patient may not associate them with CD.
c. Discuss benefits of following a GFD beyond symptom changes/discuss FU tests.
2. I can’t afford the GFD
a. Discuss food labels and ways to find cheaper naturally GF foods
b. Check if GF prescription foods are available or access to food banks
c. Tailor advice to patient and finances
3. I find the GFD too difficult
a. Share tools to make GFD easier (resources, apps, label reading, cheaper foods, etc.)
b. Focus on area they find most difficult (label reading, eating at home, eating out)
c. Are they prioritising their habits? Discuss pri orities and steps to overcome barriers
4. I’m struggling mentally
a. Acknowledge lifelong GFD adherence challenges and the psychological toll
b. Explore how mental health may be impacting adherence
c. Signpost to psychology services u
Rose Satherley’s presentation explored the psychological challenges and disordered eating patterns associated with CD and adherence to a GFD. The complexities of navigating a GFD— especially with inconsistent food labelling laws—can lead many individuals with CD to develop disordered relationships with food, ranging from hypervigilance and restrictive eating to diagnosable eating disorders like Anorexia Nervosa or Avoidant/ Restrictive Food Intake Disorder (ARFID).
A 2020 meta-analysis revealed that individuals with CD are 62% more likely to develop eating disorders compared to the general population.
For many, the fear of gluten contamination becomes profound, leading to severe anxiety, social isolation, and impaired quality of life (QOL). Common expressions include fear of eating outside the home, an inability to trust others’ food preparation, or extreme avoidance behaviors, such as

moving away from wheat fields or skipping meals entirely. While caution is natural during the initial adjustment to a GFD, longterm hypervigilance can become entrenched, increasing the risk of psychological distress.
Clinicians are encouraged to recognize the overlap between CD and disordered eating by asking questions about how the GFD affects daily routines. Signs to watch for include fear of social eating, refusal to consume anything not self-prepared, or an obsessive need to avoid gluten exposure. Effective interventions include psychoeducation about anxiety, tailored GFD education, and anxiety management strat-
egies. Self-help resources and psychological support have been shown to improve anxiety, depression, and QOL, highlighting the need for accessible mental health interventions for this population.
z Gluten will kill me
z I can go a few days without eating if I need to
z A speck of gluten will trigger cancer
z We don’t do Christmas anymore
z I’m too scared to go to the supermarket
z Food is my enemy
z My old house was by a wheat field, I had to move
z Gluten hides everywhere u
Negative effects of the GFD, such as hypervigilance, psychological distress, and social isolation, often contribute to non-compliance
Marie Robert highlights the value of follow-up biopsies in CD. These re-biopsies are typically performed 1-2 years after diagnosis to assess mucosal healing, especially in patients with persistent symptoms, new deficiencies, positive serology, or at patient request.
Pathologists use follow-up biopsies to assess mucosal recovery by comparing results to initial biopsy and determine whether inflammation has resolved, improved, or worsened. Recovery varies by age, with children more likely to experience full healing compared to adults.
z Normal biopsy results suggest successful healing, allowing clinicians to investigate other causes of persistent symptoms
z Partial or no improvement raises concerns about gluten exposure or diet adherence and the timing of the biopsy, as some patients are slow responders and heal more slowly.
z If no improvement is detected , clinicians may consider additional factors such as medications or, in rare cases, refractory celiac disease (RCD). While 10-30% of patients experience persistent symptoms, only a very small fraction develop RCD. Most cases involve other underlying factors affecting responsiveness. u
Dr. Luca Elli highlights the complex psychological and social challenges patients face when adhering to a strict GFD. While the diet aims to resolve symptoms, normalize antibodies and heal intestinal damage, these goals can take different timelines:
z Symptom recovery may take weeks
z Antibody levels can take months to normalize
z Mucosal healing can take years.
While the GFD is the cornerstone of CD treatment, adherence remains a challenge for 20-50% of patients. Negative effects of the diet, such as hypervigilance, psychological distress, and social isolation, often contribute to non-compliance, especially in individuals with mild or no symptoms.
Elli discussed the concept of gluten tolerance, questioning whether occasional or minimal gluten exposure might be acceptable for some patients without causing tissue damage. He referenced long-term observations of patients with sporadic gluten exposure who exhibited no tissue differences or permanent damage, challenging the rigid view of GFD adherence.
The presentation emphasized the need for a personalized approach to the GFD, taking into account the individual’s symptoms, lifestyle, and challenges. While the GFD remains the recommended therapy, future research may reveal that a subset of patients could safely tolerate small amounts of gluten, opening the door for more flexible management strategies in specific cases. u
Nick
presentation on gluten and FODMAPs in Irritable
(IBS) emphasized that symptom triggers vary widely
Nick Trott’s evidence-based presentation on gluten and FODMAPs in Irritable Bowel Syndrome (IBS) emphasized that symptom triggers vary widely among individuals. These triggers can include gluten, FODMAPs (particularly fructans), amylase-trypsin inhibitors (ATIs), and even psychological factors like the nocebo effect. Trott stressed the importance of tailoring dietary interventions to minimize symptoms without unnecessary dietary restrictions.

Gluten vs. FODMAPs in IBS
z Gluten is often blamed, but for IBS patients, fructans (a type of FODMAP) are more likely the true culprits.
z Research shows that anticipation of gluten consumption can heighten symptoms due to the nocebo effect (where negative expectations cause adverse reactions).
Dietary Challenges
z A GFD, while essential for celiac disease, can be nutritionally unbalanced if not properly managed. It often leads to higher fat intake and lower levels of essential micronutrients, highlighting the impor-
tance of long-term dietary assessment for individuals on a GFD.
z The low FODMAP diet is effective for IBS but complex to implement, requiring guidance from a specialist dietitian to ensure both safety and efficacy.
Tailored Approaches for IBS Management
z Many dietitians now favor a modified or gentle FODMAP approach over strict elimination diets. This strategy addresses adherence challenges and supports long-term success.
z For some IBS patients, a gluten-reduced diet may help alleviate symptoms without requiring complete gluten elimination.
2. Multidisciplinary Team (MDT)
z Effective IBS management involves a team of healthcare professionals, including a gastroenterologist, psychologist and dietitian. This multidisciplinary approach addresses both the physiological and psychological aspects of IBS.
No single dietary approach works universally for IBS. Both the GFD and low FODMAP diet can be effective, but personalization is key. Evidence-based guidance from a dietitian is crucial to navigating these diets, ensuring symptom relief and maintaining nutritional adequacy. u
Leda Roncoroni explored the potential benefits and limitations of a low FODMAP diet for persistent gastrointestinal symptoms in CD patients.
Leda Roncoroni explored the potential benefits and limitations of a low FODMAP diet for persistent gastrointestinal symptoms in CD patients. Despite strict adherence to a GFD) 30-40% of patients with CD experience ongoing symptoms, often due to other functional gastrointestinal disorders (FGIDs) like IBS.
Despite strict adherence to a GFD, 30-40% of patients with CD experience ongoing symptoms, often due to other functional
gastrointestinal disorders (FGIDs)
the low FODMAP diet may improve intestinal symptoms and quality of life but must be implemented carefully to avoid negative consequences.
Low FODMAP diet protocol
1. Elimination phase (2-4 weeks)
– Restriction of high-FODMAP foods to identify symptom improvements.
2. Reintroduction phase (6-8 weeks)
KEY POINTS
Persistent symptoms in celiac disease
z Common causes
– Poor adherence to GFD (most common).
– Refractory celiac disease (rare).
– Lymphoma or other serious conditions (rare)
– FGIDs (up to 50% of cases).
These persistent symptoms significantly impact quality of life and increase healthcare costs.
FODMAPs and celiac disease
z Research shows no direct link between FODMAP intake and persistent GI symptoms in CD.
z Low intake of FODMAP-rich fruits and vegetables in children has been linked to lower diet adequacy and nutritional quality.
z For celiac patients with IBS-like symptoms,
– Gradual reintroduction of FODMAP-contain ing foods, testing tolerance to each group (fructose, lactose, polyols, fructans, galacto-oligo saccharides).
3. Maintenance phase
– Personalization of the diet based on tolerated FODMAP, ensuring long-term balance and variety.
Benefits and limitations
z Positive effects
– Reduced GI symptoms and improved quality of life.
– No significant impact on nutrient intake when monitored by a dietitian.
z Negative effects
– Decreased bifidobacteria abundance, potentially impacting gut microbiota.
– Risk of reduced diet quality if poorly managed.
Roncoroni concluded that while the low FODMAP diet can be effective for celiac patients with IBS-like symptoms, it is not without risks
Considerations for long-term use
z The low FODMAP diet is a therapeutic tool, not a permanent solution. It requires regular dietetic monitoring to minimize risks, restore microbiota diversity, and ensure nutritional adequacy.
z Individualized approaches are crucial to optimize outcomes.
Roncoroni concluded that while the low FODMAP diet can be effective for celiac patients with IBS-like symptoms, it is not without risks. Dietitian supervision is vital to prevent negative impacts on gut health and nutrition. Clinicians must rule out other factors such as poor GFD adherence, refractory celiac disease, or other medical conditions before considering FODMAP restrictions. u



Adolescents often face more difficulties in adhering to a strict GFD compared to other age groups, as food plays a central role in social situations
Laura Kivela highlights the complexities faced by adolescents with CD as they transition to adulthood, particularly as they begin managing their condition independently. Between the ages of 8 and 15, children gradually learn to manage CD on their own, but this process often coincides with other significant life changes, complicating adherence. Adolescents often face more difficulties in adhering to a strict GFD compared to other age groups, as food plays a central role in many social situations, and the financial burden of the GFD can be a concern. Additionally, the long-term risks of untreated CD may feel distant.
The transition of care from pediatric to adult healthcare in CD management is not well-documented, with inconsistent practices in retrospective studies and no prospective data.
Experts in the US recommend
z Starting the transition process around age 12-13.
z Planning for the shift at age 14-15.
z Fully transferring care by age 18. A successful transition predicts better outcomes in adulthood and is defined by strict adher-

ence to the GFD, regular medical follow-ups, good quality of life (QOL), and a positive response to treatment without complications or persistent symptoms.
Persistent symptoms are common, with 15-34% of children and 18-38% of adolescents experiencing them during the transition to adulthood, while 30-52% of adults report ongoing symptoms. These symptoms
may significantly impact a patient’s quality of life.
Kivela emphasizes that early diagnosis and effective CD management during adolescence are critical to improving long-term health outcomes. Recognizing and addressing persistent symptoms, such as gluten exposure and other gastrointestinal conditions, can enhance patients’ QOL as they transition to adulthood. u
The financial burden of the GFD can be a concern. Additionally, the long-term risks of untreated CD may feel distant
People who adopt the GFD as a trend often view it extremely positively, fueled by celebrity endorsements.





Figures like Victoria Beckham, Lady Gaga, and Novak Djokovic promote the diet as a secret to slimness, health, and athletic performance. This trend diminishes the seriousness of the diet for those with CD, who are often perceived as high maintenance or picky.
Anne Lee, RD, argues that while the GFD is sometimes portrayed negatively, it is not always the case. Historically, the GFD has only been used for the treatment for CD, which affects about 1% of the population. However, the market for gluten-free products has exploded, with global sales and products rising dramatically. This growth goes beyond the increase in diagnosed CD cases, with many people following the diet without a diagnosis. For instance, data from NHANES shows a rise in individuals adopting a GFD without CD, increasing from 0.52% in 2009-2010 to 1.68% in 2013-2014. This trend extends internationally, raising questions about what is driving the surge in GFD adoption.
Lee discusses how perceptions of the GFD vary depending on who you ask. Dietitians often express mixed feelings, focusing on potential nutritional concerns like higher fat, lower fiber, and
more processed foods, but also recognizing that a naturally GFD can align with healthy eating patterns, such as the Mediterranean diet. Patients with CD initially have a positive reaction to the GFD, experiencing relief from finally having a diagnosis, also referred to as the honeymoon phase. However, over time, typically after the first year, patients report a decline in quality of life (QOL), increased anxiety, and depression.
In contrast, people who adopt the GFD as a trend often view it extremely positively, fueled by celebrity endorsements. Figures like Victoria Beckham, Lady Gaga, and Novak Djokovic promote the diet as a secret to slimness, health, and athletic performance. This trend diminishes the seriousness of the diet for those with CD, who are often perceived as high maintenance or picky. Continued on next page
Trenders portrayal of the GFD as a lifestyle choice overshadows the medical necessity for those with CD, leading to social issues for patients
While the GFD is not always portrayed negatively, the positive portrayal is often tied to trends rather than the ...medical needs of patients with CD
Studies show that patients on a GFD for medical reasons experience high levels of social anxiety, with many reporting a significant impact on dating and overall quality of life.
Lee concludes that while the GFD is not always portrayed negatively, the positive portrayal is often tied to trends rather than the real medical needs of patients with CD. She stresses the importance of educating both patients and the public to differentiate medical necessity from dietary trends.
Celebrities’ perception of the GFD is extremely positive:
z Victoria Beckham: “helps you achieve a slim body shape”
z Lady Gaga and Emmy Rossen : “Secret to weight loss”
z Novak Jovovich: “Increased athletic performance”
z Thanks to Katy Perry we have a song “the one that got away” which she dedicated to all the pizzas she would never eat again because she feels better being GF
Trenders portrayal of the GFD as a lifestyle choice overshadows the medical necessity for those with CD, leading to misconceptions and social difficulties for patients. u
Yvonne Jeanes discussed the nutritional composition of the GFD, noting that poor dietary habits in individuals with CD often mirror trends in the general population.
z A study found that in both men and women with CD, fat and saturated fat intake remain consistent before and after diagnosis.
z Women often consume inadequate fiber, but this is also a challenge shared by the general population.
A major concern with adopting a GFD is the automatic removal of fortified cereals and grains, which are key sources of essential micronutrients such as iron and calcium. In the UK, 38% of adults’ iron and 31% of calcium intake come from fortified cereals and cereal products, a benefit lost with the GFD.
A significant issue is the lack of adequate micronutrient analysis in many gluten-free products, leaving their nutritional profiles largely unknown. This lack of data reduces the accuracy of individual and population-level nutrient assessments. Jeanes points out that the GFD is not a singular diet but rather highly varied. Many individuals with CD fail to meet healthy eating guidelines, but this mirrors trends in the general population. Improved micronutrient analysis of GF products is essential to ensure nutritional adequacy for those following a GFD. u




Jason Tye-Din discusses whether oats can be safely included in the GFD for individuals with CD. Globally, there are differing regulations: in the UK, Europe, and North America, oats can be part of a GF diet as long as they are labelled GF, while in Australia and New Zealand, they are excluded. These concerns stem from reports of adverse reactions and potential immune responses to oat proteins, known as avenin, among some individuals with CD.
1. Benefits of Oats
z Oats offer significant nutritional benefits – Rich in fiber, amino acids, and nutrients like calcium and iron.
z They help manage blood sugar and cholesterol levels and positively impact body mass index (BMI) and gastrointestinal health, making them a beneficial addition to a GFD.
2. Risks of Oats
z Contamination Many commercial oats are contaminated with wheat, barley, or rye during processing. However, when uncontaminated oats are used, research generally suggests they are safe for the majority of people with CD. In Finland, for example, 82% of individuals with CD consume oats with no adverse long-term effects and report an improved quality of life.
z Avenin Sensitivity While rare, some individuals with CD react to avenin (a protein in oats). In one study on avenin dose-response, it revealed that interleukin 2 (IL2) levels increased after oat consumption, indicating an immune response in 38% of individuals with higher doses of avenin correlating with more severe symptoms. However, extended consumption of oats for six weeks did not result in
small bowel damage or elevated celiac antibodies in most participants, indicating that while some may react to avenin, severe and long-term reactions are rare.
1. Include oats Oats are a healthy cereal that have positive health benefits for people with CD.
2. Avoid contaminated oats Individuals with CD should steer clear of standard commercial oat brands due to the risk of gluten cross contamination.
3. Monitor oat sensitivity Oat sensitivity is uncommon but those who experience symptoms should consult healthcare professionals for monitoring.
4. Informed choices Clear food labelling is essential to help individuals with CD make informed decisions regarding oat inclusion in their diet. u

In a study of
newly
diagnosed
CD
patients,
42% reported weekly headaches,
and adopting a GFD resolved symptoms in up to 75% of cases
Ludvig Sollid discusses the significant progress made in understanding CD, particularly when compared to other autoimmune conditions like Type 1 Diabetes (T1D). While advancements in T1D research have been slow, there have been major breakthroughs in CD, including the molecular understanding of its key processes. Insights into how transglutaminase 2 (TG2) is involved, the role of disease-driving gluten T-cell epitopes, and the understanding of T-cell and B-cell interactions have paved the way for potential treatments. Notably, the development of a transglutaminase 2 inhibitor for CD could be a significant therapeutic option.
Sollid emphasizes that the primary goal of all treatments and the GFD is mucosal healing, but histology alone is not an adequate measure. There is a need for better tools to assess mucosal healing more accurately. He also highlights the challenge of identifying the factors driving CD, stressing the importance of primary prevention strategies, though recognizing how complex this task is.
Solid concludes that curiosity-driven research should remain a top priority to advance CD treatment and prevention. u

Nigel Hoggard examines the link between gluten, CD, and neurological issues, particularly migraines. His research involving brain scans shows that CD can affect white matter in the brain, increasing the risk of dementia, falls, and mortality. Notably, headaches—especially migraines—are common in people with CD. In a study of newly diagnosed CD patients, 42% reported weekly headaches, and adopting a GFD resolved symptoms in up to 75% of cases.
Migraines have a significant healthcare impact, with millions affected and many patients resistant to standard treatments. Hoggard suggests that screening migraine patients for CD could open new treatment possibilities, as undiagnosed CD may be an underlying cause. Given that CD is already part of routine checks for conditions like anemia and osteoporosis, expanding this to include chronic headaches could be beneficial.
There is a need for better tools to assess mucosal healing more accurately
The presentation also explores the link between migraines, depression, and gluten. People with migraines have a higher risk of depression, and those with gluten sensitivity often exhibit worse symptoms if not following a strict GFD. Reducing TG6 antibodies through dietary adherence can improve mood and overall functioning. Hoggard emphasizes the need for further research to better understand these connections, potentially leading to more effective, individualized treatments for neurological symptoms related to gluten. u
People who adopt the GFD as a trend often view it extremely positively, fueled by celebrity endorsements.
70% of Canadians with celiac disease received a biopsy-confirmed diagnosis ... 20% were diagnosed based on serology ... 10% relying on symptoms
Caleigh McAulay, RD presented two research posters highlighting insights from our State of Celiac Disease in Canada survey:
Canadians are diagnosed differently across the country.
While biopsy confirmation is the recommended diagnostic standard in North America, the survey results show that only 70% of Canadians with CD received a biopsy-confirmed diagnosis. Nearly 20% were diagnosed based on serology alone, with an additional 10% relying solely on symptoms. Notably, serology-only diagnoses have increased significantly over time, with just 4.4% diagnosed this way over 25 years ago, compared to 28.5% in the last two years. Additionally, patients with biopsy-confirmed diagnoses were more likely to have consulted and followed up with a gastroenterologist than those diagnosed by serology or symptoms alone. The findings showcase the need to improve alignment between clinical practices and diagnosis guidelines, encourage serologic testing before starting a GFD, and for biopsy confirmation of the disease.
Continued on next page
This poster focused on perceptions of GF labelling. Although products labelled “gluten free” are legally binding and must contain less than 20 ppm of gluten, results indicate that consumers with CD still experience confusion and label interpretation remains challenging: 23.9% report purchasing products labelled as “may contain wheat,” with that number rising to 45.6% when this statement was paired with a GF claim. Over half (52.9%) would buy a product with “manufactured in a facility with wheat,” but only 19.6% would buy products “manufactured on the same equipment as products containing wheat”. The results point to the need for clearer definitions of precautionary statements, ensuring that celiac consumers can make safe, informed choices. u For more information on our State of Celiac survey.
Thanks to generous research funding and Aeroplan mile donations, Executive Director Melissa Secord and Registered Dietitian Caleigh McAulay, joined this research event focused on the latest scientific and clinical advances in celiac disease.

Research confirms the day to day challenges living with celiac disease.
People who adopt the GFD as a trend often view it extremely positively, fueled by celebrity endorsements. Stay tuned as we continue to follow these developments, bringing hope for a brighter future
1. Modified Gluten
Scientists are working to create a form of gluten that triggers fewer immune responses in people with CD. Imagine gluten-free bread that tastes more like the real thing but is safe to eat!
2. Probiotics
Probiotics are live bacteria and yeast, similar to those found in yogurt. Research is exploring how these beneficial microbes might support gut health and reduce symptoms in CD.
3. Trigger Prevention
PrV101 is a molecule designed to prevent infections by the CVB virus, which is linked to the development of CD and type 1 diabetes. Although promising, this vaccine is currently not in active development.
4. Barrier Modulators
These drugs aim to strengthen the intestinal barrier, preventing gluten from triggering an immune response. Immunic is leading in this field, ready for Phase 2 trials.
5. Transglutaminase Inhibitors
Transglutaminase 2 (TG2) plays a key role in the harmful immune response to gluten. Inhibiting TG2 could prevent intestinal damage. ZED1227, developed by Takeda and partners, is the most advanced, currently in Phase 2B trials.
6. HLA Blockers
These therapies target HLA-DR molecules, which help the immune system recognize harmful substances. By blocking this process, these treatments may prevent the immune system from mistakenly attacking the gut in CD

7. Glutenases
Glutenases are enzymes designed to break down gluten in the digestive tract. Takeda’s Zamaglutenase (TAK-062) is entering Phase 2B trials, bringing hope for better gluten digestion in people with CD.
8. IL-15 and IL-25 Receptor Inhibitors
These inhibitors act like “off switches” for parts of the immune system that cause harmful inflammation. Several companies are preparing for Phase 2 trials to test their effectiveness.
9. T-Cell Modulators
These drugs regulate T-cells, which control the immune response. They can boost the immune system to fight diseases or calm it down to prevent attacks on the body. Research in multiple sclerosis could pave the way for CD treatments, with six companies currently in trials.
10. Antigen-Specific Immunotherapy
Often called the “Holy Grail” of treatments, this approach trains the immune system to tolerate gluten. Researchers hope it could stop CD at its root without affecting the rest of the immune system. This promising field involves 11 companies and draws inspiration from personalized cancer vaccines and childhood cancer research.
Stay tuned as we continue to follow these developments, bringing hope for a brighter future in CD management. u

Every year, Celiac Canada proudly awards two annual research grants, including the prestigious Young Investigator Award, to support cutting-edge research that improves the lives of those living with celiac disease.
Your donation helps fund critical studies that advance our understanding of this autoimmune disorder and bring us closer to better treatments, and improved diagnostics.

By supporting innovative Canadian scientists, you’re driving new discoveries and helping foster the next generation of leaders in celiac disease research.
DONATE
