
Ion exchange chromatography resins are chromatography media used to separate and purify proteins and other charged molecules from mixtures based on charge differences. These resins typically consist of a porous polymeric matrix with ionizable functional groups that can selectively bind and release charged molecules. The selection of resin depends on the specific characteristics of the target molecule and the composition of the sample matrix. In addition, depending on the nature of the sample matrix, different types of ion exchange resins can be utilized, including cationic, anionic or mixed-bed resins. Cationic resins bind negatively charged molecules whereas anionic resins bind positively charged molecules. Mixed-bed resins contain both cationic and anionic groups and can be used for more complex separations or removal of contaminants. Ion exchange chromatography is one of the most commonly used separation techniques in the biopharmaceutical industry due to its high selectivity and efficiency.
Different types of ion exchange chromatography





In strong cation exchange, the positively charged stationary phase (resin) attracts and binds to the negatively charged molecules in the sample solution, while the positively charged molecules pass freely. Meanwhile, the bound molecules can be eluted by adding a high concentration of salt or acid to the system. Strong cation exchange is a very powerful tool for the purification and separation of proteins, nucleic acids and other biomolecules based on their charge properties.

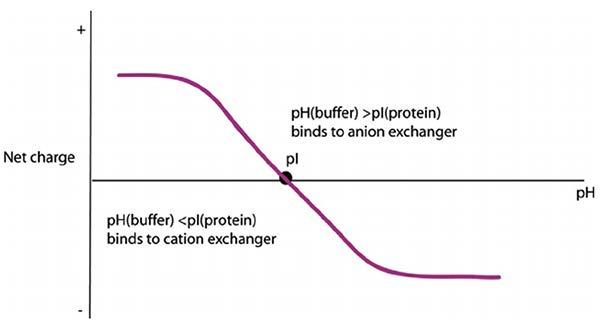
In strong anion exchange chromatography, the stationary phase is typically a resin with positively charged functional groups, such as quaternary ammonium, which attract and retain negatively charged analytes. The mobile phase is usually a buffer solution with a pH above the pKa of the buffer to maintain a suitable environment for separation. The strength of the interaction between the analytes and the stationary phase can be modulated by changing the pH or the ionic strength of the buffer solution, allowing the separation of analytes with different affinities for the stationary phase (Figure 1). Overall, strong anion exchange chromatography is a powerful technique for the separation and purification of complex biomolecules.
Weak cation exchange is a technique used in chromatography to separate and purify molecules based on their charges. In this method, the stationary phase is covered with charged molecules that have a weak affinity for positively charged target molecules in a sample. When a mixture is passed over this matrix, the negatively charged molecules will pass through the column while the positively charged molecules bind to the stationary phase. Once the negatively charged molecules have passed through, a buffer with a higher salt concentration is used to elute the bound molecules from the column. The main advantage of weak cation exchange is its ability to retain target molecules at specific pH ranges that cause weak binding under these conditions. Therefore, this method is an efficient way to separate charged molecules under mild conditions. In conclusion, the use of weak cation exchange has revolutionized the field of chromatography by enabling the separation of charged molecules with high purity and efficiency

In weak anion exchange chromatography, the positively charged stationary phase interacts with the negatively charged molecules in the sample, while the weakly charged or neutral molecules pass through the column. The bound molecules can be eluted by increasing the ionic strength of the mobile phase or by changing the pH. Weak anion exchange chromatography can be used to separate molecules with small differences in charge, such as protein variants, isomers, and mutants, and to remove contaminants with opposite charge properties. It is less demanding than strong anion exchange tomography and can be performed under mild conditions without denaturing the target molecule. Weak anion exchange chromatography is a common and effective tool in modern biotechnology and bioseparation science.
CD Bioparticles offers a range of ion exchange chromatography resins (Figure 2) suitable for industrial purification of biological molecules such as small proteins, polypeptides, nucleic acids, and antibiotics.


Features:
High flow rate and low back pressure
Shorten chromatographic purification cycle and improve production efficiency
High resolution
Stable high dynamic capacity at high flow rates and over a wide range of salt concentrations
