

SUMMER FUN & SCIENCE the return of the CBR Amazing race
DRIVING EXCELLENCE
CBR and Canadian Blood Services renew collaboration




SUMMER FUN & SCIENCE the return of the CBR Amazing race
DRIVING EXCELLENCE
CBR and Canadian Blood Services renew collaboration

The CBR aims to improve the health and well-being of patients through innovative research in blood and blood-related processes.

Over the past year, donor support has helped us develop novel approaches to battle severe bleeding in rural areas, delineate the mechanisms of inflammatory diseases, and increase the quality of blood products used in transfusions — only a few examples among many pioneering discoveries. With your continued support, the CBR will further transform innovative ideas into life-enhancing solutions.
The CBR needs you to help fund our programs, which range from $50 to $100,000. We invite you to explore opportunities at the CBR where your partnership with us will result in positive impacts on education, training and meaningful research. Examples of initiatives that need your support include:
Reward leadership in students and staff with the Neil Mackenzie Mentorship Award
Expose trainees to diverse career opportunities with the CBR Career Night
Jumpstart a postdoctoral fellow’s career with the Postdoctoral Transition Award
Support a clinical fellow in Translational Research Studies
Make a CBR Symposium possible
Explore further: www.cbr.ubc.ca/support-us
Edward M. Conway, MD, PhD
Director, Centre for Blood Research
Tel: 604.822.4252 | Email: ed.conway@ubc.ca

CBR Research & Clinical Goals
• Improve the quality and safety of blood product collection, storage and delivery
• Create new knowledge to better treat bleeding and clotting disorders
• Develop novel approaches to modulate the immune system to treat inflammation and infections and promote wound repair

CBR Education Commitment
• Support student research through competitive undergraduate, graduate, and postgraduate awards
• Offer a range of stimulating educational symposia, workshops, and seminars
• Provide cutting-edge career development opportunities for our trainees

PUBLISHED BY
Knowledge Translation Committee
DESIGNER
Kaitlyn Chuong
CONTRIBUTING EDITORS
Alexandra Witt*
Dr. Emily Park*
Erik Lamoureux
Debajeet Ghosh
Kaitlyn Chuong
Kiran Toor
Jackie Hagstrom
John Perrier
Joyce Teodoro
Loulou Cai*
Marie-Soleil Smith*
Mohammeed Al-Seragi
Nastaran Davilu
Rhonda Thygesen
Sarah Bowers*
* indicates Editorial Board member
COVER ART “Is that Boba or Champange?” - Dr. Marine Theret BLOG cbr.ubc.ca
FACEBOOK @cbrubc
TWITTER @CBR_UBC
INSTAGRAM @cbr_ubc
LINKEDIN UBC Centre for Blood Research
The CBR magazine is published by the Centre for Blood Research, with many articles written and edited by the CBR Knowledge Translation (KT) Committee, a group of undergraduates, graduate students, postdoctoral fellows, research associates, and technicians who are interested in science writing, blogging, and mixed media communications. It is distributed free of charge to CBR and UBC alumni, friends, and the scientific community. Opinions expressed in the magazine do not necessarily reflect the views of the centre or the university.
Address correspondence to:
The Centre for Blood Research 4th Floor, Life Sciences Centre 2350 Health Sciences Mall Vancouver, BC, Canada V6T 1Z3
The KT Committee publishes at CBR News (cbr.ubc.ca) and covers a wide range of topics, from recent research highlights and opinion pieces on science and academia, to event coverage and CBR initiatives. If you are interested in participating in the KT Committee, email Kaitlyn at: kaitlyn. chuong@ubc.ca or talk to one of the members! All undergraduates, graduate students, PDFs, RAs, and technicians are welcome to join.
kaitlyn.chuong@ubc.ca
Driving excellence: CBR and Canadian Blood Services renew collaboration to advance blood science education and training
CBR finishes 3rd at the 2024 Vancouver Sun Run

Summer Fun and Science: The Exciting Return of the CBR Amazing Race Celebrating Young Researchers: A Look Back at CBR-LSI Research Day 2024
Three CBR scientists awarded CIHR project grants
CBR External Travel Awards: Norman Bethune Symposium 2024 – Fateme Babaha
Two CBR scientists are part of the interdisciplinary UBC team to train a new generation of immunoengineers
Federal funding boosts 25 UBC Faculty of Medicine research projects including one CBR scientist
Marie Johns awarded 2023-2024 William and Dorothy Gilbert Scholarship
Dr. Aly Karsan awarded funding from the CFI JELF and the Terry Fox New Frontiers Program Project Grant



UBC research pinpoints how early-life antibiotics turn immunity into allergy
Familial Alzheimer’s disease transferred via bone marrow transplant in mice
Transforming Transfusion: engineered platelets using lipid nanoparticles
Targeting a hitherto enigmatic protein could help to treat people with insulin resistance
Jefferies lab demonstrates potential of a novel cell-based immunotherapy for cancer
Antibiotic Failure – Beyond Antimicrobial Resistance
A novel sequencing panel for somatic hypermutation (SHM) status determination
Amenorrhea in Women with HIV: Unveiling Key Risk Factors for Menstrual Health
CBR-LSI 2024 Summer Students
CBR alumni: Marie-Soleil SmithGraduate Profile
Meet the CBR graduate award program (gap) cohort 2024-2025
National Postdoc Appreciation Week 2024: Celebrating CBR Postdocs and Research Associates
Meet the Researcher: Dr. Hayley Merkeley

THIS BLOG WAS ORIGINALLY
& PROGRAMS COORDINATOR, KAITLYN CHUONG, AND FORMER CANADIAN BLOOD SERVICES KNOWLEDGE BROKER, DR. TRAVIS SZTAINERT, WITH EDITS PROVIDED BY ABBY WOLFE.
After more than 20 years, the Centre for Blood Research (CBR) and Canadian Blood Services are proud to share that they have renewed their collaboration agreement which supports education, training and knowledge mobilization for the next generation of trainees and researchers in blood science and transfusion at CBR.
The collaboration agreement which is administered by Innovation and Portfolio Management at Canadian Blood Services has recently been extended for three more years to 2027. This agreement formalizes the next iteration of this long-standing partnership and is the result of continued commitment from both organizations to improve blood research.
This partnership emerged in response to recommendations made following Canada’s public inquiry into the contaminated blood crisis of the 1980s and 1990s. Thus, to address a recognized gap in transfusion science knowledge, in 2002, a group of visionary blood researchers at the University of British Columbia (UBC) established Canada’s first interdisciplinary blood research centre — an entirely unique and valuable resource for established and emerging blood scientists. With its creation, the
We are delighted to extend our partnership with the Centre for Blood Research through 2027. This renewal highlights our shared commitment to advancing education, training, and research in transfusion science. Together, we aim to drive innovation and improve transfusion practices, ensuring a safer and more effective future for the Canadian blood system.
DR. CHANTALE PAMBRUN, SENIOR MEDICAL DIRECTOR, INNOVATION & PORTFOLIO MANAGEMENT
The long-term relationship of Canadian Blood Services and the CBR has been nothing less than fantastic! By combining resources and sharing knowledge, education and research outputs, this unique partnership has resulted in more rapid and meaningful advances that are recognized locally, nationally and internationally. The entire transfusion medicine community has benefitted and will continue to do so.
DR. ED CONWAY, DIRECTOR OF THE CENTRE FOR BLOOD RESEARCH
CBR brought together a wide range of multi-talented investigators to develop highly specialized labs to study blood and transfusion science. Since its inception, the CBR has grown to include more than 35 basic science, applied science, social science and clinical investigators in 12 departments, representing 6 faculties at UBC, with a focus on advancing knowledge of transfusion science and medicine in the lab and in the clinic.
The partnership of Canadian Blood Services was integral to creating the Centre for Blood Research, providing essential start-up funding and continuing to support infrastructure for the centre and its training and education programs. Several Canadian Blood Services scientists are active members within the Centre for Blood Research including senior scientist Dr. Ed Pryzdial and adjunct scientists Dr. Ed Conway, Dr. Hongshen Ma and Dr. Jay Kizhakkedathu.
The CBR is grateful for Canadian Blood Services’ continuous support, without which its many educational, training and research programs would not be possible. This includes, for example, CBR’s Graduate Award Program and Summer Studentship Program, as well as the CBR’s weekly seminar series and the annual Earl W. Davie and Norman Bethune symposia.
THIS STORY IS REPUBLISHED FROM THE UBC FACULTY OF MEDICINE WEBSITE.
In a study published today in the Journal of Allergy and Clinical Immunology, a research team from the School of Biomedical Engineering (SBME) has identified a specific cascade of events that lead to allergies and asthma. In doing so, they have opened many new avenues for exploring potential preventions and treatments.
“Our research finally shows how the gut bacteria and antibiotics shape a newborn’s immune system to make them more prone to allergies,” said senior author Dr. Kelly McNagny, professor in the SBME and the Faculty of Medicine’s department of medical genetics. “When you see something like this, it really changes the way you think about chronic disease. This is a well-sculpted pathway that can have lasting consequences on susceptibility to chronic disease as an adult.”
Allergies are a result of the immune system reacting too strongly to harmless substances like pollen or pet dander, and a leading cause for emergency room visits in kids. Normally, the immune system protects us from harmful invaders like bacteria, viruses and parasites. In the case of allergies, it mistakes something harmless for a threat—in this case, parasites—and triggers a response that causes symptoms like sneezing, itching or swelling.
The stage for our immune system’s development is set very early in life. Research over the past two decades has pointed toward microbes in the infant gut playing a key role. Babies often receive antibiotics shortly after birth to combat infections, and these can diminish certain bacteria. Some of those bacteria produce a compound called butyrate, which is key to halting the processes uncovered in this research.
Dr. McNagny’s lab had previously shown that infants with fewer butyrate-producing bacteria become particularly susceptible to allergies. They had also shown that this could be mitigated or even reversed by providing butyrate as a supplement in early life. Now, by studying the process in mice, they have discovered how this works.
Mice with depleted gut bacteria who received no butyrate supplement developed twice as many of a certain type of immune cell called ILC2s. These cells, discovered less than 15 years ago, have quickly become prime suspects in allergy development. The researchers showed that ILC2s produce molecules that “flip a switch” on white blood cells to make them produce an abundance of certain kinds of antibodies. These
antibodies then coat cells as a defence against foreign invaders, giving the allergic person an immune system that is ready to attack at the slightest provocation.
Every cell, molecule and antibody described along this cascade increases dramatically in number without butyrate to dampen them.
Butyrate must be given during a narrow window after birth—a few months for humans, a few weeks for mice—in order to prevent the proliferation of ILC2s and all that follows. If that opportunity is missed and ILC2s multiply, then the remaining steps are assured and remain with somebody for life.
Now that researchers know what those other steps are, they have many more potential targets for halting the cascade, even after the supplementation window has closed.
“We can now detect when a patient is on the verge of developing lifelong allergies, simply by the increase in ILC2s,” said Ahmed Kabil, the study’s first author and a PhD candidate in the SBME. “And we can potentially target those cell types instead of relying on supplementation with butyrate, which only works early in life.”
As Dr. McNagny and study co-lead Dr. Michael Hughes point out, treating people’s allergies with antihistamines and inhalers relieves the symptoms but does not cure the disease. To achieve more lasting progress, researchers must target the cells and mechanisms that build this hypersensitive immune system. Until now, there hadn’t been a selective way to do that.
With this new understanding, patients can look forward to more effective, long-term solutions that address the root of the problem, paving the way for a future where allergies are managed more effectively, or perhaps avoided altogether.


BY JOYCE TEODORO, PHD STUDENT, MA LAB (LEFT) & KIRAN TOOR, MASTERS STUDENT, BROWN LAB (RIGHT)


On April 18th 2024, the Centre for Blood Research (CBR) held their 12th annual Norman Bethune Symposium, a renowned research event that gathers scientists, clinicians, healthcare professionals, and trainees to discuss advancements in the field of blood research. The symposium was held at UBC Robson Square, on the traditional territory of the Musqueam, Squamish and Tsleil-Waututh people. The full day event consisted of expert talks on thalassemia, thrombocytopenia, sickle cell disease, and other bleeding disorders. Research trainees delivered impressive presentations on innovative topics including engineering cells for cancer immunotherapy and computational approaches to inhibit Klebsiella pneumoniae. The event showcased nearly 30 trainee research posters, demonstrating the breadth of ongoing research.
CBR Director, Dr. Ed Conway, began with the opening remarks, followed by the presentation by Dr. Christian Kastrup, Professor at the Medical College of Wisconsin and Senior Investigator at Versiti Blood Research Institute. Dr. Kastrup’s presentation focused on his work on genetically modifying transfusable donor platelets using
mRNA-lipid nanoparticles. Following this, there were a series of presentations on the topic of bleeding disorders. It was a research-filled day that honored the contributions of Drs. Don Brooks and Dana Devine.
Blood Research & Reflections:
The symposium was an amazing opportunity to learn about the exciting advances in blood research. It also served as a great platform for speakers to share their personal experiences with blood disorders, as well as for scientists to discuss their professional path in blood sciences.
Attendees were fortunate to hear Ritika Rakshit share her personal journey with thalassemia, a genetic blood disorder characterized by the body’s inability to produce enough hemoglobin. She detailed her experience from being diagnosed to her treatment plans including being on extramedullary hematopoiesis (EMH) and switching to luspatercept. She offered insights into her hesitancy to switch medications and highlighted the invaluable support of her clinical team. Ritika continues to be an advocate for thalassemia in her role on the patient advocacy board. It was an eye-opening opportunity to be able to hear from a patient reflect on their own journey and understand their perspective on living with a blood disorder.
Dr. Geraldine Walsh, a development scientist with the Canadian Blood Services (CBS), also gave an insightful talk describing her career path in blood
sciences. Drawing from her experiences, she shared several pieces of advice on navigating a career in science. She highlighted the importance of finding work that we are passionate about, building strong relationships, and learning from those around us. Dr. Walsh concluded her talk by encouraging attendees, especially research trainees, to explore various career opportunities, including those that may deviate from the conventional scientific career trajectory.



Celebrating Drs. Don Brooks and Dana Devine:
One of the highlights of the symposium was the talk by Dr. Cedric Carter, which celebrated Drs. Don Brooks and Dana Devine’s achievements and contributions. Drs. Don Brooks and Dana Devine are two of the founding members of the Centre for Blood Research (CBR). In addition to their significant and impactful contributions to the field of transfusion science and medicine, they have been instrumental in promoting research and developing initiatives through various leadership roles. Dr. Brooks has served as Associate Vice-President, Research at UBC and Founding Director of UBC’s Support Programs to Advance Research Capacity (SPARC). He has also served as a member of the Board of Directors for TRIUMF, Provincial Health Services Authority, and BC Emergency Health Services. Dr. Devine has held numerous leadership positions, which include her role as chief scientist at CBS, director of the CBR, president of the Association for the Advancement of Blood and Biotherapies (AABB), and editor-in-chief of Vox Sanguinis, a transfusion medicine journal. Their dedication and commitment to scientific discovery through research and development serve as inspiration for future generations of scientists.
Inaugural Don Brooks and Dana Devine Transfusion Science Innovation Address:
In honour of Drs. Don Brooks and Dana Devine’s scientific contributions to advancing transfusion science and medicine, the inaugural Don Brooks and Dana Devine Transfusion Science Innovation Address was presented by Dr. Stephen Withers. Dr. Withers, in collaboration with Dr. Jay Kizhakkedathu’s lab, worked on identifying efficient enzymes for cleaving terminal sugar structures on the surface of red blood cells (RBCs), which are responsible for determining blood types. By removing these terminal sugar structures, A and B type RBCs can be converted to the universal donor blood type O, which can increase blood supply for transfusion applications. By screening the human gut microbiome for enzymes that can cleave these
terminal sugar structures, they identified a pair of enzymes (FpGalNAc deacetylase and FpGalactosaminidase) that can convert type A to type O blood.
Posters & Awards:
This year, there were 29 posters presented by various graduate trainees, postdoctoral fellows, clinical fellows and research associates! These included members within the CBR, but we also had the pleasure of having presentees come from out of town. Multiple trainees from the University of Alberta, University of Toronto & Queen’s University were able to attend the symposium with the support of the CBR Travel Award. The Best Poster Presentation went to Dr. Georgina Butler from the Overall Lab for their poster on “SARS-CoV-2 main protease 3CLpro (nsp5), regulates the formation of tunnelling nanotubes by coordinating cytoskeleton reorganization.”
Our final speaker was Dr. Ed Pryzdial with an exciting talk on Dengue virus-induced thrombocytopenia. Afterwards, a reception was held and attendees got to network and socialize with each other to celebrate the end of another incredible symposium!
A huge thank you to everyone who attended, to our speakers and all presenters!
The Centre for Blood Research would like to thank their event sponsors, without whom the 12th Annual Norman Bethune Symposium would not have been possible: the Naiman-Vickars Endowment Fund, Canadian Blood Services, Novo Nordisk, GRIFOLS, CSL Behring, ALEXION, Sobi and Pfizer.

Attendees gathered after the symposium.


THIS
Familial Alzheimer’s disease can be transferred via bone marrow transplant, researchers show March 28 in the journal Stem Cell Reports. When the team transplanted bone marrow stem cells from mice carrying a hereditary version of Alzheimer’s disease into normal lab mice, the recipients developed Alzheimer’s disease—and at an accelerated rate.
The study highlights the role of amyloid that originates outside of the brain in the development of Alzheimer’s disease, which changes the paradigm of Alzheimer’s from being a disease that is exclusively produced in the brain to a more systemic disease. Based on their findings, the researchers say that donors of blood, tissue, organ, and stem cells should be screened for Alzheimer’s disease to prevent its inadvertent transfer during blood product transfusions and cellular therapies.
“This supports the idea that Alzheimer’s is a systemic disease where amyloids that are expressed outside of the brain contribute to central nervous system pathology,” says senior author and immunologist Wilfred Jefferies, of the University of British Columbia. “As we continue to explore this mechanism, Alzheimer’s disease may be the tip of the iceberg and we need to have far better controls and screening of the donors used in blood, organ and tissue transplants as well as in the transfers of human derived stem cells or blood products.”
To test whether a peripheral source of amyloid could contribute to the development of Alzheimer’s in the brain, the researchers transplanted bone marrow containing stem cells from mice carrying a familial version of the disease—a variant of the human amyloid precursor protein (APP) gene, which, when cleaved, misfolded and aggregated, forms the amyloid plaques that are a hallmark of Alzheimer’s disease. They performed transplants into two different strains of recipient mice: APP-knockout mice that lacked an APP gene altogether, and mice that carried a normal APP gene.

In this model of heritable Alzheimer’s disease, mice usually begin developing plaques at 9 to 10 months of age, and behavioral signs of cognitive decline begin to appear at 11 to 12 months of age. Surprisingly, the transplant recipients began showing symptoms of cognitive decline much earlier—at 6 months post-transplant for the APP-knockout mice and at 9 months for the “normal” mice.
“The fact that we could see significant behavioral differences and cognitive decline in the APP-knockouts at 6 months was surprising but also intriguing because it just showed the appearance of the disease that was being accelerated after being transferred,” says first author Chaahat Singh of the University of British Columbia.
In mice, signs of cognitive decline present as an absence of normal fear and a loss of short and long-term memory. Both groups of recipient mice also showed clear molecular and cellular hallmarks of Alzheimer’s disease, including leaky blood-brain barriers and buildup of amyloid in the brain.
Observing the transfer of disease in APPknockout mice that lacked an APP gene altogether, the team concluded that the mutated gene in the donor cells can cause the disease and observing that recipient animals that carried a normal APP gene are susceptible to the disease suggests that the disease can be transferred to health individuals.
Because the transplanted stem cells were hematopoietic cells, meaning that they could develop into blood and immune cells but not neurons, the researchers’ demonstration of amyloid in the brains of APP knockout mice
shows definitively that Alzheimer’s disease can result from amyloid that is produced outside of the central nervous system.
Finally the source of the disease in mice is a human APP gene demonstrating the mutated human gene can transfer the disease in a different species.
In future studies, the researchers plan to test whether transplanting tissues from normal mice to mice with familial Alzheimer’s could mitigate the disease and to test whether the disease is also transferable via other types of transplants or transfusions and to expand the investigation of the transfer of disease between species.
“In this study, we examined bone marrow and stem cells transplantation. However, next it will be important to examine if inadvertent transmission of disease takes place during the application of other forms of cellular therapies, as well as to directly examine the transfer of disease from contaminated sources, independent from cellular mechanisms,” says Jefferies.
This research was supported by the Canadian Institutes of Health Research, the W. Garfield Weston Foundation/ Weston Brain Institute, the Centre for Blood Research, the University of British Columbia, the Austrian Academy of Science, and the Sullivan Urology Foundation at Vancouver General Hospital.

Congratulations to Dr. Jayachandran Kizhakkedathu, Dr. Kelly McNagny & Dr. Leonard Foster! UBC Faculty of Medicine researchers are leading 38 projects awarded more than $25 million in combined funding through the Canadian Institutes of Health Research Project Grant: Spring 2024 competition. The projects will address key health challenges including Alzheimer’s

Faculty of Medicine-led Project Grants
• Jayachandran Kizhakkedathu (Pathology and Laboratory Medicine) - Transfusion Without Blood Typing: Enzymatic Glycoengineering Towards ‘True’ Universal Blood
• Kelly McNagny (Medical Genetics/School of Biomedical Engineering) - Neonatal ILCs Sculpt Long-term Adult Immune Response
Faculty of Medicine-led Priority Announcement Projects
• Leonard Foster (Biochemistry and Molecular Biology) - Development and Commercialization of a Safe and Effective Mpox Subunit Vaccine with Global Impact. Priority Announcement Area: Pandemic Preparedness and Health Emergencies Research disease, cancer, multiple sclerosis, mental health and more.
Across the university, a total of 52 projects led by UBC researchers were awarded $34.2 million. On a national level, CIHR approved a total of 373 research grants, for a total investment of approximately $325 million. In addition, 68 priority announcement grants were funded for a total amount of $7,600,000 and 5 supplemental prizes were awarded for a total of $210,000.
We were so excited to welcome 20 summer students to this year’s CBR-LSI Summer Studentship Program. Between May - August 2024, these students took part in exciting workshops alongside their research projects. The CBR Summer Studentship Program provides undergraduate and medical students with an opportunity to get hands-on research experience during the summer months and to present their research at Research Day. This program also enhances the summer students’ learning with research skills workshops, career development events, and complementary social events.




















BY JOHN PERRIER, MASTERS STUDENT, PRYZDIAL LAB
Platelets are tiny cells with a big role in helping our blood clot. Serving as the first responders, they adhere to injured blood vessels when collagen or von Willebrand Factor become exposed. Once activated, platelets create aggregates that act as a plug. To strengthen this new blood clot, proteins called clotting factors join in and set up fibrin-generating factories to cast a strong net over the injury and hold everything together.
Platelet transfusion is the main form of treatment for actively bleeding patients or those with a low platelet count (thrombocytopenic). A new exciting avenue of transfusion research is being investigated by the Kastrup, Cullis, Jan, and Devine labs at the Centre for Blood Research. In this article by Leung et al., the authors delve into the possibility of genetically modifying platelets to express proteins that may offer greater therapeutic benefits. So how do you modify a platelet to upgrade their procoagulant activity? Since platelets have no nucleus, techniques used to express proteins that rely on DNA are not suitable. Instead, the group has identified lipid nanoparticles (LNPs) as a viable method of transfecting platelets with genetic information (mRNA) for protein synthesis! The composition of LNPs can be fine-tuned so that there is cellspecific delivery of the material inside.
Prior experiments showed that LNP-mediated mRNA transfection into platelets was a possibility, but protein expression was not detected. In this latest study, optimization of protein expression without platelet activation was the goal. To measure protein expression, the LNPs were loaded with mRNA encoding for the NanoLuc luciferase enzyme, a guide protein which produces light after reacting with its


substrate. This quantification was performed for variations of LNPs with different ionizable, helper and PEGylated lipids.
After screening several candidate lipids, they found that the ionizable lipid KC2 gave the highest amount of protein expression. When in combination with helper lipid POPC, only one ionizable lipid, CL4H6, met the criteria of producing detectable protein while minimally activating platelets. Transmission electron microscopy was used to ensure that the overall platelet shape remained the same following transfection by LNP-mRNA (Fig. 1), indicative of non-activation.
LNP-mRNA delivery was mildly associated with platelet activation; however, protein expression was not (Fig. 2, B and D). Rotational thromboelastometry (ROTEM) and an ex vivo model of dilutional coagulopathy (consumption of clotting factor proteins) were used to measure platelet activity, making sure that once transfected with mRNA, these platelets could still facilitate a blood clot. Platelets retained their ability to help form a clot following LNP-mRNA transfection, however one part of the coagulation pathway (the extrinsic pathway) took slightly longer.
In platelets that have been washed and incubated with the optimized LNPs we see protein expression, which is great! But can we target those platelets for increased protein expression in a more complex model, like in an animal? The experiments moved in vivo, using rats which have bleeding due to kidney injuries to model acute traumatic coagulopathy with impaired platelet function. With transfusion of platelets transfected with mRNA-LNPs following injury, it was shown that they were able to localize to the site of damaged vasculature and reduce blood loss.
This work is very encouraging for future projects which look to use LNPs as a way to express proteins of therapeutic benefit. The authors write, “platelets engineered with mRNA-LNP may be used to treat acute bleeding and can potentially be expanded for use in thrombolytics, bleeding disorders, and applications in oncology.” This leaves the door wide open for the treatment of numerous hemostatic disorders.
Link to paper: https://pubmed.ncbi.nlm.nih. gov/38039367/



After a five-year hiatus, the CBR Amazing Race returned! Under the slogan “Summer Students Run. CBR Members Host”, 9 CBR labs on campus united to host a one-of-akind science gaming event last Thursday afternoon.
This year, we were excited to welcome four Life Sciences Institute (LSI) summer students to join us in our race! 20 summer students arrived in the Life Sciences Centre (LSC) between 1:50 – 2:05pm. Here’s a tip, if you’re wanting students to arrive early, make sure you award them some bonus points! After partnering up and receiving their Amazing Race Passports, 8 teams were on their way to visit the labs. Many did not strategize and were out the door as soon as we said BEGIN! We knew that the students were eager when they all chose not to wait for the elevators and ran up the stairs all the way up to the 4th floor.
To earn their points, the summer students had to successfully navigate their way to the CBR labs and then complete an activity which ranged from: blood typing, crystallizing a protein, concentrating
a protein, assembling a microfluidic chip and various puzzles such as trivia, crosswords and anagrams!
Here were the final statistics:
• 4 teams made it to the lab outside of the LSC
• 4 teams made it to all but one lab
• 1 team made it to all labs
• 3 teams made it back on time at 3:15pm
Unfortunately for our late arrivals, there was a one-point deduction for every 5 minutes past 3:15pm. The last team to arrive showed up at 3:32pm.
To announce the win, we had CBR members join us for a post-race celebration with lots of yummy pizza to foster a festive atmosphere. Congratulations to our winners, Susan Zhang from the Pryzdial Lab and Alessia Palumbo from the Foster Lab! They were the ONLY team that made it to all labs. Although they showed up 4 minutes late, they still beat the runners-up by 3 points. As a prize, they received CBR tote bags, CBR umbrellas, CBR mugs and a goodie bag with snacks and drinks.
A BIG thank you to everyone who participated in and supported the CBR Amazing Race event. The event was only made possible because the CBR is lucky to have so many dedicated students, postdocs, research associates, PIs, staff, and the H&W Committee members working in all of its labs!



Award Recipient: Fateme Babaha, PhD Candidate, Pathology and Molecular Medicine, Queen’s University, Kingston, Ontario
Supervisor: Dr. David Lillicrap
Conference: Norman Bethune Symposium on April 18, 2024
Location: Vancouver, BC
Oral/Poster Presentation Title: Improving AAV-mediated FVIII transgene expression using enhancer sequences from the F8locus

I am delighted to share my experience at the 2024 Norman Bethune Symposium, which was made possible by the generous External Travel Award from the Centre for Blood Research (CBR). This was my first time attending and visiting the University of British Columbia and the beautiful city of Vancouver. During the symposium, I had the opportunity to learn about the latest research findings, innovative treatments, and improvement in patient care within the field of blood research. I really enjoyed networking with experts of the field and junior scientists during poster presentations and the speaker dinner. These events provided me with invaluable insights on my current project and future career path. I also received fantastic suggestions for exploring the city!
At the symposium, I presented a poster and gave a talk entitled “Improving Adeno-Associated Virus (AAV)mediated FVIII transgene expression using enhancer sequences from the F8 locus”. This is one of my ongoing PhD projects at the Department of Pathology and Molecular Medicine at Queen’s University, under the supervision of Dr. David Lillicrap. This project addresses the decline of FVIII expression following AAV gene therapy in hemophilia A patients. We aim to achieve increased and persistent transgene expression by employing a hyper-active element from F8 locus (FVIII-Padua) in the AAV expression cassette. We have confirmed that once inserted in a reporter plasmid, this

regulatory element is able to significantly increase downstream gene expression in different cell lines and mice. We are also interested in factors affecting vector uptake and its persistent in the nucleus and epigenetic remodeling of the transgene as a potential explanatory molecular mechanism responsible for the decline in FVIII expression. Through this six-minute oral talk, I practiced the skill of effective science communication, learning how to present the study, its objectives and results concisely yet informatively.
I am very happy that I came across CBR’s LinkedIn post about this symposium and am grateful to them for funding my attendance and giving me the opportunity to share my research findings. The event’s organization, the diversity of talks, and networking opportunities were all great. In the end, I felt like I found my science community and I am very much looking forward to reuniting with them at future CBR events.
*With funding from Canadian Blood Services & the Sheldon Naiman and Linda Vickars Endowment Fund, the CBR supports travel and accommodation expenses for trainees to attend and present at the EWD symposium.


THIS STORY WAS ORIGINALLY POSTED ON THE SCIENCE NEWS DK WEBSITE, WRITTEN BY ELIZA BROWN. THIS FEATURES DR. ED CONWAY, PROFESSOR OF MEDICINE & DR. JAN ERIKSSON, PROFESSOR OF CLINICAL DIABETES RESEARCH & CLINICAL ENDOCRINOLOGY.
A new study highlights the CD248 protein as potentially being key in combatting insulin resistance, a core problem in type 2 diabetes. Experiments showed that mice lacking the CD248 gene were shielded from the obesity and insulin resistance normally caused by the high-fat diets fed to mice. The researchers noted similar effects in human fat cells (adipocytes), suggesting that CD248 is involved in insulin responsiveness. Although still far from clinical application, targeting CD248 represents a promising avenue for developing treatments for people with insulin resistance, offering new hope in fighting diabetes.
A little-known cellular protein called CD248 could hold the key to treating – and perhaps even reversing – the insulin resistance in type 2 diabetes, scientists say.
New research, published in eBioMedicine, found that mice with the CD248 gene removed were protected from the effects of a high-fat diet, including obesity and insulin resistance. And crucially, evidence indicates that CD248 may play a similar role in humans, since the study found that human fat cells with higher levels of CD248 are more resistant to insulin.
Although there is a long way to go before people could pick up CD248 silencers at their local pharmacy, the researchers say that CD248 is a promising target for drug development.
Insulin resistance and a bad-guy protein
As you digest a meal, cells in your pancreas release insulin – a hormone that instructs tissues all over the body to absorb sugar from the bloodstream and move it into storage in fat cells. But under certain conditions, some cells across the body become insulin resistant – meaning they do not follow insulin’s instructions or are less responsive to them. This can lead to high blood sugar, a marker of type 2 diabetes.
Despite the importance of insulin resistance in diabetes, no drug on the market targets it directly, says study author Ed Conway, a haematologist and Professor of Medicine at the Centre for Blood Research at the University of British Columbia,

Vancouver, Canada. And since CD248 sits on the surface of cells, it could be easy for drugs to reach.
The authors say that they happened on the relationship between CD248 and insulin resistance accidentally. About a decade ago, Conway was studying the role of CD248 in blood clotting when he encountered a Wikipedia report that mentioned off-the-charts levels of CD248 in fat cells. Simultaneously, researchers at Karolinska Institutet in Stockholm, Sweden had identified high levels of CD248 in a scan of fat cells from people who were obese and/or had diabetes.
“Nobody had noted that before,” Conway says, and not much was known about CD248’s function. More than 10 years later, it remains somewhat of a mystery.
Many diseases are caused by an important part of the body that becomes overzealous. For instance, some forms of arthritis are caused by an overactive immune system. But interestingly, that does not seem to be the case for CD248. Although this protein does not have a clear role in healthy cell functioning, high amounts of CD248 have been implicated in several clinical disorders. For example, it seems to be involved in promoting blood clotting and is present in high concentrations in the cells surrounding tumours.
“We do know that it promotes inflammation, scarring and cell growth, and these things are not necessarily always bad,” Conway explains.
“But the body must have redundant mechanisms because, at least in mice, a total lack of CD248 causes no apparent harm,” he says. Thus, for example, mice lacking the CD248 gene had normal liver and kidney function. In fact, the mice seemed to fare better overall without CD248 – they had a lower risk of the growth of some tumours, arthritis, blood clots and heart, liver and kidney conditions.
“It would be analogous to an appendix,” he says. “If taken away, it makes no difference, but we do not really know what the function is all by itself.” But just as researchers have discovered potential functions for the appendix over time – including supporting the immune system and as a reservoir of beneficial gut bacteria – Conway says we cannot rule out that CD248 has a role we have not pinned down yet.
After seeing the elevated levels of CD248 among people with insulin resistance, the researchers set out to determine whether knocking out CD248 could benefit insulin responsiveness and glucose regulation.
First, Conway and his team fed high-fat diets to both knockout mice and mice with an intact CD248 gene. That is how scientists typically provoke insulin resistance in mice and simulate type 2 diabetes.
The researchers found that the knockout mice were protected from the high-fat diet, Conway explains. They did not become obese and did not develop insulin resistance like their siblings with the intact gene did.
Even more striking was a series of experiments in which adult mice had the CD248 gene removed after diabetes onset. “If you make a mouse obese and with diabetes and then remove the CD248 gene, the diabetes reverses and glucose metabolism goes back to normal.”
The scientists even found a smoking gun: how CD248 disrupts a cell’s response to insulin. Using fluorescent

imaging, they determined that in immature fat cells of mice, CD248 sits very close to the insulin receptor, another membrane protein that is responsible for relaying insulin’s signal to the cell’s interior. Further experiments demonstrated that the close interaction of CD248 with the insulin receptor, makes it harder for insulin to bind to its receptor and to trigger the movement of glucose from the blood into the cell
But as researchers often joke, science has made mice immortal a dozen different ways – what matters for medicine is whether the same holds true for people. Co-author Jan Eriksson, Professor of Clinical Diabetes Research and Clinical Endocrinology at the Uppsala University Hospital in Sweden, and his research colleagues tested whether CD248 plays a similar role in human fat cells.
Eriksson took samples of belly fat from 10 people and exposed them to insulin. Just like in mice, the human fat cells with high levels of CD248 were less responsive.
Conway’s team has already identified one important potential limitation for CD248 as a drug target –CD248 interventions seem to affect male mice much more than females.
“Female mice still respond, but it is not as dramatic,” Conway says. “Another group in the United Kingdom did not find any effects of CD248 in female mice. We do not know the reason for these sex differences.” It is unclear how this sex-dependent difference could translate to people, but initial results from Eriksson’s lab suggest that women have higher rates of CD248 expression than men.
Scientists need to better understand the relationship between insulin resistance and CD248 before it becomes a human drug target, the authors say. “We do not really know why CD248 goes up,” Conway explains. Does CD248 promote fat accumulation, or does fat accumulation increase CD248 levels? “Some thin people have type 2 diabetes, for example. They have elevated levels of CD248 as well.”
In any case, the techniques used to remove or silence the CD248 gene in mice cannot yet be used on humans, Eriksson adds.
CD248 shows promise as a potential drug target – but “it will never be the complete solution” for insulin resistance, Eriksson emphasises. “These conditions are complex.”
“There are many, many genes and many, many molecular factors involved,” he says. “And the interaction with your behaviour and environmental situation is also extremely strong.”


What was your research about?
My PhD research utilized human embryonic stem cells to identify the effects of HIV antiretrovirals on embryonic development during pregnancy, particularly their impact on cellular and mitochondrial health as well as on early embryonic differentiation. My work aimed to improve health outcomes in infants exposed to HIV drugs in utero by addressing critical gaps in our understanding of HIV treatment safety in the context of pregnancy. I explored the molecular mechanisms underlying drug toxicity and identified potential safer therapeutic options for the treatment of HIV in women of reproductive age.
What inspired you to pursue graduate studies at UBC?
Having completed my undergraduate degree in Microbiology and Immunology, I have always been fascinated by the inner workings of the disease process. UBC is a hub of cutting-edge research, and I was particularly inspired by my PhD supervisor, Dr. Hélène Côté. Her groundbreaking work with HIV antiretroviral therapy and women’s health was directly in line with my research interests. I was especially excited to work on a project utilizing human embryonic stem cells, a model I was eager to explore further. You’ve been an active member in several committees at the CBR over the past years. Why did you join and what did you enjoy about them?
I conducted by PhD research at the UBC Hospital, and although it is geographically very close to the CBR, it often felt like worlds away. I first got involved with the CBR Health and Wellness committee after attending a very enjoyable and lively Aqua Zumba activity they organized in 2019. This experience opened the flood gates, leading me to meet many new people engaged in different but fascinating research, fostering a heightened sense of community. To further broaden my scientific network and contribute to the vibrant research community at UBC, I joined the Knowledge Translation committee to flex my writing and editing muscles. Being part of these committees allowed me to collaborate with a diverse group of researchers and professionals, enhancing my interdisciplinary knowledge and skills. I particularly enjoyed organizing activities such as the Stair Challenge with the Health and Wellness committee and editing a broad scope of fascinating articles produced by the Knowledge Translation committee.
Can you share one of the best memories you’ve had being a part of the CBR?
One of my best memories was with my CBR Graduate Award Program (GAP) cohort in the early days of the COVID-19 pandemic. One of the tasks assigned to GAP students is to organize an event. Our group originally planned to host an in-person video and board game night on March 19, 2020. However, due to the safety restrictions put in place, our event was canceled. Quick thinking and a dedication to giving back to the CBR community led us to shift from hosting an event to creating care packages for CBR members. Despite this pivot, the adversity we faced together highlighted our teamwork and resulted in a service that was well received by the community.
What is one piece of advice you would give a new grad student?
My advice to new grad students is to actively seek out and engage with the various opportunities that UBC and Vancouver have to offer. Whether it’s joining clubs, attending seminars, or exploring the city’s natural beauty, immersing yourself in these

experiences will enrich your graduate journey. Additionally, don’t be afraid to ask for help and collaborate with others. Building a support network and working with peers can significantly enhance both your personal and academic growth.
What’s next for you?
I’m excited to announce my move to Toronto and am actively seeking new opportunities! My primary goal is to further develop my expertise in stem cell biology while continuing to explore the fascinating intersection of infectious diseases and women’s health. I’m eager to apply my knowledge to innovative and challenging projects that can make a meaningful impact.
Is there anyone you’d like to thank or anything else you’d like to add?
I would like to express my heartfelt gratitude to my supervisor, Dr. Hélène Côté, for her unwavering support and guidance throughout my PhD journey. I also want to thank my family, friends, and colleagues for their encouragement and companionship. Lastly, I am grateful to the UBC and CBR communities for providing such a stimulating and supportive environment for my research and professional development.

The 2024 Vancouver Sun Run attracted 45,517 participants this past Sunday, April 21st – the largest turnout since 2014. Among them, 13 CBR members of all levels of fitness and preparation took on the 10K team road race. We are very proud of this year’s team! The CBR team placed 3rd out of 51 teams in the 10K team results, education category. Congratulations, Team CBR!
The Vancouver Sun Run is Canada’s largest 10K road race and is now the 3rd largest timed 10K in North America. The Run is meant to promote running as a way to improve health and fitness, as well as support elite amateur athletics.
Allen Xu, a Postdoctoral Fellow in Jim Sun’s lab, had the fastest time of the CBR team at 39:15. Matthew Thibodeau, PhD Candidate, Strynadka Lab and John Perrier, Masters Student, Pryzdial Lab were both close in time as the second and third CBR finishers at 45:06 and 45:10. Lauren Puumala, Graduate Student, Cheung Lab was the fastest female runner on the CBR team with 46 minutes and 55 seconds.
Thank you to all runners for representing CBR at the Vancouver Sun Run 2024!
Participants (Alphabetically by first name)
• Allen Xu, Sun Lab
• Cheryl Pfeifer, Jefferies Lab
• Eliana Al Haddad, Jefferies Lab
• Erik Lamoureux, Ma Lab
• Hong Ma, Ma Lab
• Hugh Kim, Kim Lab
• John Perrier, Pryzdial Lab

• Kaity Ryan, Professor, UBC
• Lauren Puumala, Cheung Lab
• Matthew Thibodeau, Strynadka Lab
• Rachel Wang, Devine Lab
• Stephanie Besoiu, Jefferies Lab
• Wesley Mosimann, Strynadka Lab


THIS STORY IS REPUBLISHED FROM THE UBC FACULTY OF MEDICINE WEBSITE.
An interdisciplinary UBC team led by Dr. Peter Zandstra has received $1.65 million in federal funding to train a new generation of immunoengineers to innovate, develop and deliver new immunoengineering technologies for commercialization.
Dr. Hongshen Ma and Dr. Kelly McNagny, CBR Principal Investigators, are two of 11 members who are part of this interdisciplinary UBC team.
The training program, dubbed ImmunoE, will help prepare graduate students and postdoctoral fellows for the workforce to meet the needs of B.C. and Canada’s expanding bioeconomy. The funding was announced today by the Natural Sciences and Engineering Research Council of Canada (NSERC) through the Collaborative Research and Training Experience (CREATE) program.
“The COVID-19 pandemic highlighted increasing demand for advanced biotechnologies and immunotherapeutics,” said Dr. Peter Zandstra, Director, School of Biomedical Engineering (SBME), a partnership between the Faculties of Medicine and Applied Science. “ImmunoE will accelerate the biotechnology pipeline from research innovation to industry, and will train over 80 highly

qualified personnel with industry-ready technical expertise, problem-solving and professional skills, ready for productive careers in the biotechnology industry and addressing the shortage of highly qualified personnel in the life sciences sector.”
ImmunoE builds on an established network of interdisciplinary academic and industry life scientists and engineers with expertise in immunology, cell, molecular, and genetic engineering, and biotechnology that was developed under the UBC Immunotherapeutics Research Excellence Cluster and supports the work of Canada’s Immuno-Engineering and Biomanufacturing Hub.
THIS STORY IS RE-PUBLISHED FROM THE MICHAEL SMITH LABORATORIES (MSL) WEBSITE. PHOTOS CREDITED TO EMILY COOKE, COMMUNICATIONS COORDINATOR, MSL.

A new study from the laboratory of Professor Jefferies in the Michael Smith Laboratories, Department of Medical Genetics, Department of Microbiology and Immunology and the Centre for Blood Research highlights a promising, novel avenue for cancer immunotherapy using Type 2 innate lymphoid cells.
In recent years, significant strides have been made in the field of cell-based immunotherapy. However, while these advancements have offered a beacon of hope for cancer patients, the response rates to these therapies have unfortunately remained low.
Type 2 innate lymphoid cells (ILC2s) have previously been recognized for their ability to modulate immune responses, including response to parasitic infections. Now, a new study from the Jefferies lab, published in Frontiers in Immunology, is the first to demonstrate the potential of ILC2s to confer anti-cancer immune response and to modulate T-cell

response towards eradicating tumours in any preclinical model of cancer. In fact, in some settings, ILC2s transferred into mice were able to completely halt tumour growth during the study period.
“Solid tumours are often quite resistant to cancer immunotherapies. The fact that we observed a dramatic effect of the ILC2s in reducing the growth of solid tumours was exciting and somewhat unexpected,” highlights Professor Jefferies.
Working with Professor Martin Gleave, Director of the Vancouver Prostate Centre at UBC, the research team was also able to demonstrate that this mechanism may have direct relevance for patients with prostate cancer, also highlighting the potential of this immunotherapy for treatment across various cancer types.
While there are still many steps to take following these results, the team is encouraged by their new findings. In particular, not only were the ILC2s effective at reducing cancer growth, but when compared with CAR T-cell therapies, another cell-based therapy for cancer, they could be up to 150-fold more effective, and much less expensive to produce. If these findings can be translated to the treatment of human cancers, it could allow for more cancer patients to gain access to a more effective, widely applicable immunotherapy.
The entry of immune cells and mediators into the tumour appears to be part of the reason ILC2s are able to suppress tumour growth.
“The activation of the ILC2s further enhances the effects of anti-tumour treatments, and overcomes existing challenges of cell-based immunotherapy,” explains Dr. Iryna Saranchova, co-first author of the study.
“Solid tumours are resistant to cancer treatments because often times immune cells, including cell-based cancer immunotherapies like widely researched CAR T-cell therapies, are unable to penetrate into the tumour mass to exert anti-tumoural effects. With the transfer of ILC2s, we observe an influx of effector immune cells into the tumour mass as well as reduced tumour sizes. This is an exciting finding because it can potentially resolve the most difficult problem associated with most immunotherapies,” describes Clara Xia, also co-first author of the study.
However, while these findings are promising, the exact mechanism for how this infiltration occurs still requires further investigation. This underlies the current research endeavors of co-author and PhD candidate Stephanie Besoiu. She hopes her work will reveal the connections
between ILC2s and diminished tumour growth, with particular emphasis on how they modulate sentries in this process, like helper T-cells, and the consequential cascade of pro-inflammatory mediators and immune cell recruitment.
“It is known that ILC2s can activate helper T-cells, which can release various pro-inflammatory mediators, and our previous work demonstrated that ILC2s recruit other immune cells such as cytolytic T cells, the executioners of cancer cells. Our current research looks to expand our understanding of the other mechanisms underlying the connection between ILC2s and reduced tumour growth,” describes Besoiu.
Building upon these foundational proof-of-concept studies, the group hopes to progress to clinical trials, and ultimately, to create a new, clinically proven immunotherapy for cancer that addresses the challenges of current therapies.
“Existing CAR T-cell therapies for cancer can cost up to $1 million per patient. Type 2 innate lymphoid cells show promise as a cost-effective alternative, potentially filling this crucial gap in cancer treatment. Their affordability and potential for widespread use represent a significant advancement in making cancer immunotherapy more effective and accessible worldwide,” emphasizes Professor Jefferies.
This research was supported by the Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, MITACS Accelerate Fellowship, Zymeworks Fellowship, the Centre for Blood Research, the University of British Columbia, and by donations to the laboratory of WAJ through the Sullivan Urology Foundation at Vancouver General Hospital (https://www.urologyfoundation.ca).








BY JACKIE HAGSTROM, MOHAMMED AL-SERAGI AND NASTARAN DAVILU – SUMMER STUDENTS 2024
On August 15th, the CBR community gathered in-person and online to celebrate the hard work and research of this year’s summer students. The day kicked off with welcoming remarks, followed by oral presentations from students, where we heard from peers working on diverse projects. Each student had just 3 minutes to present their summer research projects, with the promise of a rubber chicken noise signaling if they exceeded their time limit.
It was inspiring to learn what these four months looked like for this year’s summer students. The collection of three-minute flash talks was a striking window into the immense breadth of research behind the twenty-one-membered cohort. Everything from cancer glycoimmunology to stem cell differentiation, blood disease pathology and the like were subjects of the twohour triage of presentations. More impressive than the research itself was how effectively everybody was able to collapse many weeks of work into a mere three minutes — something many students said they struggled with!
“The day provided both a platform to showcase our work and an opportunity to engage in meaningful conversations within the CBR community.” –Nastaran Davilu, CBR Summer Student 2024
This year’s Neil Mackenzie Mentorship Award was presented to Dr. Michael Sutherland who spoke on what excellent mentorship means to him, as he reflected on his 20+ years of working under Dr. Ed Pryzdial. He emphasized the value of giving students actionable advice when challenges arise, the importance of empathy, comradery and being understanding and even showcased his knowledge of “brat summer”, a term he learned from his students. A particularly memorable moment was Dr. Sutherland humorously mentioning being told he has no “rizz,” and shared his experience of checking if the term was offensive, only to have Reddit users call his question “cringe.” This lighthearted moment, featuring other classic Gen-Z phrases, added humor to the day and highlighted the inclusive and fun spirit of the CBR community.

To top it off, Dr. Brianne Kent, this year’s keynote speaker, delivered a highly insightful address on the intersection of learning, sleep, and circadian rhythms with dementia and what that means for our aging and vulnerable populations. Dr. Kent began by discussing novel biomarkers that could help predict the onset age of Alzheimer’s disease (AD), which may provide patients a better chance at benefiting from treatment. One such biomarker is disturbed sleep, with research suggesting that targeting sleep disturbances could prevent up to 15% of AD cases. She explored various topics, including the potential of the antidepressant Trazodone to slow cognitive decline. Overall, this research points to slow wave sleep as a key area of therapeutic intervention, and suggests that cognitive tasks assessing pattern separation are particularly sensitive to sleep disruptions and sleep aids. Maybe a bit more upending was Dr. Kent’s realization that students don’t sleep nearly as much as she thought, something that many students can attest to.
Following the keynote address, the event transitioned to the poster session in the LSC West Atrium. The CBR community circulated and engaged in in-depth discussions with students eager to delve deeper into their research. Poster judges provided valuable feedback, helping to explore future research directions, critiquing methodologies, and making connections to other related work happening at the CBR. With refreshments in hand, students also took the opportunity to explore their peers’ posters, gaining insight
into where their research paths might lead after the CBR Summer Studentship Program. The evening ended with a BBQ dinner shared with friends, families, and lab members, a fitting end to a summer filled with connections and opportunities.
“Ending the day off with poster presentations was an amazing representation of the collaborative atmosphere that permeated the event. It was clear that this year’s Research Day was more than just a platform for sharing results; it was a space for building connections, exchanging ideas, and sparking new collaborations.” – Sajida Chowdhury, CBR Summer Student 2024
Congratulations to all of the award winners at this year’s Research Day:
• Best Oral Presentation (voted by the students) –Madelyn Tisdale, Mizumoto Lab
• People’s Choice Award (voted by the audience) –Houria Afshar Moghaddam, Av-Gay Lab
• Best Poster Presentation (voted by the poster judges) – Simrat Binning, Cote Lab
“CBR Research Day was a hit in many more ways than the findings we were able to share. It was a time of learning but one of celebration as well. I have high promises for the
Research Days to come and hope to be around for them too!” – Mohammed Al-Seragi, CBR Summer Student 2024
Many thanks to our sponsors, without whom the CBR Summer Studentship Program and CBR Research Day would not be possible: the Neil Mackenzie Memorial Fund, the Naiman-Vickars Endowment Fund, Canadian Blood Services, GSK, AstraZeneca, GRIFOLS, Beigene, Stago and Sobi. We’d also like to say thank you to the Life Sciences Institute for partnering with us on this year’s program. Lastly, we are grateful for all the support and kindness of Kaitlyn Chuong, the CBR Communications & Programs Coordinator.

Congratulations to Dr. Jayachandran Kizhakkedathu! His project is one of 25 Faculty of Medicine research projects who are receiving federal funding from the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Research Program.
UBC Faculty of Medicine researchers have been awarded more than $6 million in federal funding from the NSERC Discovery Grants program. A total of 157 new programs and projects led by UBC researchers were awarded more than $36.2 million through the 2024 NSERC Discovery Research Program competition.

The awards were announced by Yasir Naqvi, Parliamentary Secretary to the Minister of Health, and Ryan Turnbull, Parliamentary Secretary to the Deputy Prime Minister and Minister of Finance and Parliamentary Secretary to the Minister of Innovation, Science and Industry, on behalf of the Honourable François-Philippe Champagne, Minister of Innovation, Science, and Industry, as part of the Government of Canada’s investment of $693.8 million in funding for discovery and applied research.
The NSERC Discovery Grants program supports ongoing programs of research with long-term goals rather than a single short-term project or collection of projects. These grants recognize the creativity and innovation that are at the heart of all research advances.
Jayachandran Kizhakkedathu, Pathology and Laboratory Medicine
• Novel Biomaterials Design Concepts in the Development of Immunomodulating Materials and Bioactive Surfaces
BY LOULOU CAI, PHD CANIDATE, COTE LAB
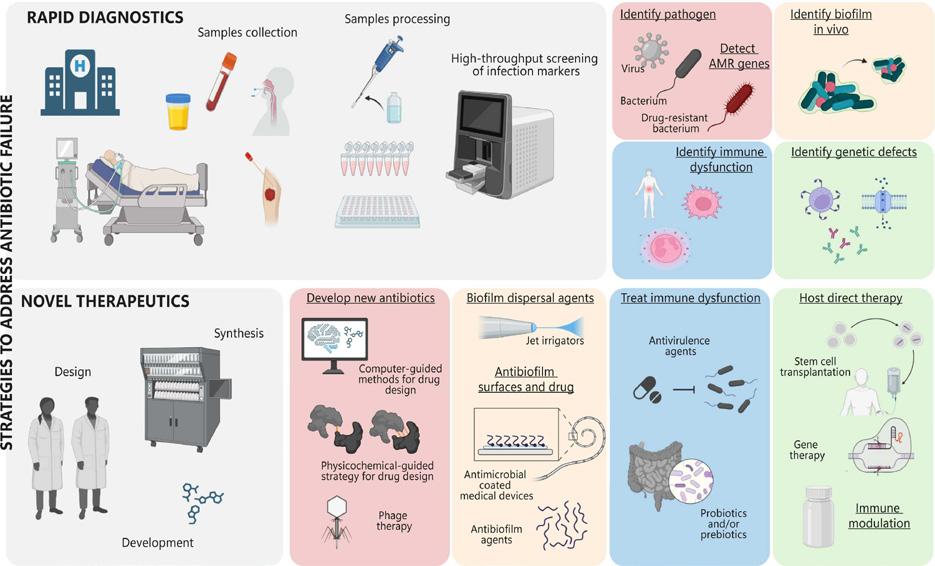
Each year, millions of lives are lost annually to bacterial infections. While antibiotics have revolutionized medicine, improved quality of life, extended lifespans, and made complex medical procedures possible, antimicrobial resistance is on the rise. A recent review by the Hancock Lab delves into antimicrobial resistance, with topics that include contributors to antibiotic failure, how these may be alleviated by rapid diagnostics, and alternatives to antibiotics.
Compromised immune and host defenses due to deficiencies in the innate or adaptive immune systems increase host susceptibility to frequent and/ or recurrent infections. Antibiotics are often necessary for immunocompromised patients to prevent long-term complications; however, immunodeficiency increases the chances of antibiotic failure as the effectiveness of antibiotics often depend on cooperation with the host immune response. In addition, antibiotics often change the composition of the host microbiome, thereby increasing susceptibility to other infections.
Biofilms are colonies of bacteria that grow in large clumps that aggregate typically on a surface. In the context of antimicrobial resistance, they often grow on surfaces of medical devices and/or implants. These cluster formations are potentially a stress adaptation, as they exhibit distinct transcriptional and proteomic profiles that enable them to survive in harsh environments, contributing to their resilience. Biofilm bacteria are 10-1000-fold more resistant to most antibiotics than their non-adherent, nonaggregating, so-called planktonic counterparts. Although antibiotic resistant biofilms are one of the most prominent infections in human health, no specific treatments have been approved.
It is widely appreciated that there is a general lack of understanding of underlying causes of antibiotic failure. Furthermore, and the scarcity of simple model systems to enable screening and testing of microbials makes it challenging to gain new knowledge. One potential counter is rapid diagnostics of factors that contribute to antibiotic resistance. Initial therapy is often empiric, whereby health care professionals consider the region of infection, the most likely bacteria, and known resistance patterns. However, this has the downside of over prescription. Authors believe the need for rapid molecular diagnostics, such as PCR (polymerase chain reaction) to distinguish

between viral and bacterial infections by differing host response or host markers and clinical symptomatology is required to counter resistance. New age methods such as multiplex PCR and other high-throughput molecular methods can detect most common classes of transmissible antibiotic resistance. While such methods are not yet in widespread practice, they can provide critical clinical information and therapeutic options that are faster than the traditional culturing of bacteria.
On the flipside, combination antibiotic therapies could help lower antibiotic resistance, as these may work in synergy and broaden the spectrum of action. Attacking multiple locations for bacterial replication and function in a simultaneous manner can offer a swift clearance. Other therapies include attenuating infection pathways or compensating for host immune deficiencies by boosting the innate or adaptive immune system. Despite the promise of these methods, they are more complex and expensive, requiring procedures such as exogenously supplied immunoglobulins, allogenic hematopoietic stem cell transplants, or injections of interfering RNA in conjunction with antibiotics.
Some non-medication-based alternatives to antibiotics can also aid in reducing antimicrobial resistance. In extreme low birth weight infants, dual-probiotic supplementation increased the eradication of Helicobacter pylori, decreased adverse events, and significantly reduced sepsis mortality. Prebiotic substances can also modify the gut microbiome and promote secretion of specific metabolites that facilitate proliferation of beneficial bacteria. Additionally, it has been noted that reversing anaerobicity of infectious sites by offering hyperbaric oxygen treatment can reduce bacterial load.

Non-antibiotic medicines for bacterial resistance are promising. Phage therapy, where the employment of viruses that specifically target certain bacteria is currently undergoing a phase III clinical trial to treat acute and non-complicated urinary tract infections. Treatment with lysins, enzymes that are synthesized by bacteriophages that disrupt the bacterial cell wall, is also promising, as this would provide high specificity, low risk of resistance development and minimal toxicity. Overall, this review addresses several critical issues of antimicrobial resistance, emphasizing the importance of understanding contributors to antibiotic failure and exploring advanced diagnostics and alternative treatments. By adopting rapid molecular diagnostics and innovative therapies, mitigation of resistance and improvement of patient outcomes are possible.

The CBR Graduate Award Program (GAP) is an educational development program available to MSc and PhD students. The program provides successful applicants with funding, as well as a chance to develop professional experience that is useful in and beyond academia. Meet the incoming CBR Graduate Award Program (GAP) Cohort for 2024-25! Over the next year, these students will take part in exciting professional development activities alongside their research. Learn more about them and their research below:








In honour of National Postdoc Appreciation Week (NPAW), we spoke with a couple CBR Postdoctoral Research Fellows and Research Associates to highlight their work, share their stories and celebrate their journeys.

Tell me about your research.
Organ transplants can save lives, but keeping the new organ from being rejected by the body’s immune system is challenging. In Canada alone, over 2,700 organ transplants were done in 2021. To make sure the body doesn’t reject the new organ, doctors use strong medications to suppress the immune system. While these immunosuppressive drugs help, they can also cause serious side effects.
One major issue is that the process of getting and preserving the organ can damage its blood vessels, making the body more likely to reject it. My research aims to solve this problem by using a new technique to protect the organ from this damage. I am working on special polymers that can help rebuild the organ’s natural defences. We’re testing this new approach with artery and kidney transplants to see if it works. If successful, it could make organ transplants less costly, improve patients’ lives, and potentially eliminate the need for those powerful immunosuppressive drugs.
What led you to the lab?
My journey into the lab began in my third year as an undergraduate when isolating
DNA from plant cells in a Molecular Biology class sparked my passion for lab-based research. This led me to Japan in 2015, where I completed my master’s thesis on developing micromotors for biosensing applications in a multidisciplinary lab. My excitement for combining diverse scientific fields continued through my PhD in Calgary, where I developed smart hydrogel scaffolds for cartilage tissue regeneration. Now, as a Postdoctoral researcher, I am focused on developing polymer-based glycoconjugates for organ engineering to prevent transplant rejection. I am driven not only by the technical challenges but also by the potential to solve complex problems and translate innovative solutions from the bench to the bedside. Multidisciplinary research excites me as it bridges fields like Physics, Chemistry, and Biology, providing diverse approaches to tackle critical human challenges.
What do you like to do outside the lab?
Outside the lab, I enjoy staying active and engaging in a range of extracurricular activities. I love playing cricket and occasionally ping-pong, going biking, and participating in various voluntary activities, including science-related outreach programs. These activities help me stay balanced and contribute to the community in meaningful ways.
What advice would you give to someone still searching for a program/postdoc position?
When searching for a program or postdoc position, seek diversity by stepping outside your comfort zone and exploring a range of opportunities. Aim for a position that aligns with your research interests and provides a dynamic, progressive lab environment. Additionally, consider roles that offer the chance to learn new tools and techniques, as this will enrich your technical skills and expertise. Balancing these factors will help you find a position that is both professionally fulfilling and supportive of your growth.

Dr. Yu-Hsuan Huang
Tell me about your research.
Chronic lung fibrosis is a debilitating, progressive disease for which there is no cure. Patients often have only 3-5 years after diagnosis before succumbing to an irreversible loss of lung function and death. Chronic lung fibrosis is a major health problem worldwide and there is an urgent need for effective new treatments that can stop or reverse this disease. Our study seeks to better understand the mechanisms of lung fibrosis and why it is a chronic disease. We use two mouse models of lung fibrosis: One where the fibrosis disappears over time (acute fibrosis) and the lungs recover, and one where lung damage is persistent and progressive (chronic fibrosis).
By comparing these two models, we plan to identify key differences between them, and then determine if these differences are responsible for driving either recovery or progression. We focus are on two cell types in the lung: macrophages and matrixproducing cells called mesenchymal cells. Macrophages are part of our immune system and protect the lungs from infection and damage, and matrix producing cells help repair the lungs when damage occurs. We have found differences in the macrophage populations and in the type of matrix produced between the acute and chronic models. By investigating these differences in more detail and testing whether specific cells, or the factors they produce, can slow the progression of fibrosis, we will discover new insights into the mechanisms of chronic fibrosis and how it progresses. This new information will change our
current understanding of chronic lung fibrosis and offer new opportunities for the development of more effective therapeutics for chronic lung fibrosis patients.
What do you enjoy most about science?
Science is like preparing a unique dish to share with other scientists: from putting together different ingredients to sitting down and enjoying the final meal, it requires a lot of trial and error, brainstorming and teamwork. It is a fun and rewarding process!
What do you like to do outside the lab?
Outside of the lab, I really like baking and hiking.
What is an award or achievement that you are proudest of?
Being a mentor to provide guidance, training, advice and support to the mentee is the greatest accomplishment and most rewarding part of doing what I do!

Dr. Yasin Tabatabaei Postdoctoral Research Fellow
lab
Tell me about your research. How would you describe it to a family member/ friend?
Approximately 2 million Canadians suffer from an autoimmune disorder. These diseases arise when the immune system mistakenly targets the body’s own cells, failing to recognize them as its own. My research focuses on the molecular mechanisms behind autoimmune diseases. The role of proteases like cathepsins in this process is one promising
area for further study.
Cathepsins play a crucial role in the immune response. Several proteases, including cathepsins, break down foreign substances into smaller fragments known as antigens when they enter the body. To combat the perceived threat, the immune system recognizes these antigens and produces antibodies.
We hypothesize that cathepsins could also be involved in generating new antigens that trigger autoimmune reactions. Studying cathepsin function and interaction with other proteins will shed light on the molecular origins of autoimmune disease by revealing whether they contribute to misidentification of self from non-self.
Our goal is to develop safer and more effective therapies and diagnostic tools against autoimmune disorders, but first we must understand the mechanisms that cause them.
What do you like to do outside the lab?
I enjoy spending time with my family, cycling, music and hiking.
What led you to the lab?
I have long been interested in the intersection of immunology and chemistry, especially in synthetic immunology. I am eager to tackle the challenge of developing new diagnostic tools and therapeutics for immunotherapy, particularly in underexplored areas of this field.
Joining Dr. Brömme’s lab offers an ideal opportunity to pursue this goal and combine my passion for understanding disease mechanisms with my background in organic chemistry.
What advice would you give to someone still searching for a program/postdoc position?
Two suggestions:
1. If a question doesn’t keep you awake at night, it might not be worth pursuing. If it doesn’t stir a deep passion, consider letting it go.
2. Marcel Proust said, “The real voyage of discovery consists not in seeking new landscapes but in having new eyes.”

Dr. Monika Kowatsch
Postdoctoral Research Fellow
Tell me about your research. Globally, over half (53%) of people with HIV are women and girls. Despite effective treatments, in Canada, women with HIV have a life expectancy 7 years shorter than their male counterparts and 5-10 years shorter than women without HIV. These women often face more agerelated conditions; while the reason is unclear, women living with HIV also tend to experience earlier decreases in sex hormones known to be protective of health. Addressing these disparities and understanding the aging experiences of women with HIV are key research priorities identified by the women living with HIV that we consulted and continue to work with as part of our study.
The British Columbia CARMA-CHIWOS collaboration (BCC3, CTN 335) merges two Canadian research cohorts—CARMA and CHIWOS—in a comprehensive study based at the Oak Tree Clinic and UBC. This CIHR-funded, community-based project
uses biological specimens, clinical data, and psychosocial information to explore women’s health across their lifespan. My Postdoctoral Fellowship project entitled, “The effect of hormones on cellular aging and comorbidities in women living with HIV.” aims to investigate how HIV affects sex hormones, inflammation, and cellular aging, and their links to concurrent conditions. This research will examine how key hormones — estradiol, estrone, progesterone, cortisol, and testosterone — are associated with markers of cellular aging, immune cells’ ability to respond to hormones, markers of inflammation, and age-related illnesses in women. Using a big data approach, we will integrate the biological, clinical, and social data collected in BCC3 to enhance our understanding of hormonal impacts and inform better care strategies.
Outside the lab, I enjoy handwork hobbies such as knitting/crochet and embroidery. I also enjoy bird photography and exploring all the habitats Vancouver has to offer for its wide array of permanent resident and migratory birds. I also enjoy the challenge of timing a photograph of a bird in motion.
During my PhD at the University of Manitoba, I was privileged to collaborate with two diverse communities: Sunshine House in Winnipeg, Canada and the Baba Dogo and Pumwani community clinics in Nairobi, Kenya. Working with these communities shaped how I see the role of basic science and how I see data and interpret the results. They’ve helped me see the real people behind each data point and understand data within the context of their lived experiences. These interactions have pushed me to think creatively about interpreting results and challenge any assumptions about a person’s health, well-being, or risk factors. As such this community-based research perspective is something I was looking for in my Postdoctoral Fellowship and in 2022, I met Dr. Melanie Murray at the International AIDS conference in Montreal and was immediately drawn to the community aspect of the BCC3 study and the strong community voices and commitment to is addressing key questions addressing key
health questions of women living with HIV that could not be accomplished elsewhere. What advice would you give to someone still searching for a program/postdoc position?
The best advice I could give to someone currently searching for a program/postdoc position is to talk to as many researchers and other postdocs as possible. Those connections are important for finding a lab in which you will excel. Take every opportunity to attend seminars/talks from researchers outside your department and apply for every conference opportunity to network with researchers from other institutions and countries. Finally, it’s okay not to have all the answers when entering your new position. Having a supportive lab means room to grow, taking your expertise and adding new opportunities, skills, and techniques. Embrace this opportunity as a valuable part of your professional journey and development.




BY DEBAJEET GHOSH, PHD STUDENT, KARSAN LAB
A Next Generation Sequencing (NGS) panel developed by Dr. Aly Karsan’s group in the BC Cancer Research Centre and UBC Centre for Blood Research allows for simultaneous detection of somatic hypermutation status (SHM) and other key clinical variants to inform treatment of Chronic Lymphocytic Leukemia1.
B cells, a special class of white blood cells, rely on a phenomenon called somatic hypermutation (SHM) to produce functionally competent antibodies (i.e. immunoglobulins). SHM introduces variation to antigen binding sites of antibodies, particularly the immunoglobulin heavy variable chains (IGHV), to improve their affinity for antigens on the target cell. When SHM processes are disrupted, as in cases of B-cell malignancies like Chronic Lymphocytic Leukemia (CLL), patients’ B cells have minimal or no variability in the IGHV, contributing to more aggressive disease than their functionally competent counterparts2. Hence, IGHV somatic hypermutation status determination is a routine prognostic test for CLL patients. Patients with a negative SHM or unmutated (U-CLL) status tend to have increased disease progression and shorter overall survival, compared to mutated patients (M-CLL)2.
While Sanger Sequencing has been a ‘gold standard’ for SHM status determination, the low throughput nature and limited sensitivity in clonality determination leaves more to be desired. Dr. Aly Karsan’s group at the BC Cancer Research Centre has devised a targeted capture


Next Generation Sequencing (NGS) panel that allows for simultaneous detection of SHM status along with other key clinical variants that dictate CLL treatment regimen3. The NGS panel designed by Grants et al. offers a powerful and versatile approach for comprehensive genomic profiling of CLL patients. The target capture technique with three different probe pools (described in detail below) allows for flexible customization of the panel, enabling the inclusion of virtually limitless additional variants – a marked improvement from its predecessor.
The published NGS pipeline featured 3 different probe pools:
• An IGHV SHM pool: Sequences and identifies SHM status and IGH V(D)J rearrangement in the IGHV 1-7 and IGHJ 1-6 gene families.
• An SNV/indel pool: Sequences and detects disruptive mutations in 11 different genes (such as TP53, MYD88 and NOTCH2) relevant to CLL in terms of outcome correlation, drug target determination, and diagnostic accuracy.
• A del(17p) pool: Sequences genes in the 17p chromosomal arm to detect if the patient bears the chromosomal band deletion which is associated with worse outcomes.
The panel’s NGS capabilities also allows SHM determination of multiple clonal populations, or clonotype, in patients exhibiting tumor heterogeneity – something not possible with Sanger Sequencing.
The NGS panel was validated using libraries constructed from 35 unique CLL patient samples. Compared to Sanger sequencing, the NGS panel results showed a very similar sequencing alignment for IGHV. For clonotype determination, the results were identical for all except two patients. For these samples, the NGS panel determined two different alleles of the IGHV gene that were not resolved by Sanger sequencing – highlighting its applicability in clonotype determination and accurate quantification. The panel showed high reproducibility in SHM determination, with a maximum variability of only 0.11% across technical replicates.
Increasing in SHM status detection sensitivity while simultaneously generating clues vital to CLL treatment regimens makes this targeted capture Next Generation Sequencing (NGS) panel from the Karsan lab a standout in diagnostic profiling tools.

Congratulations to Marie Johns, one of the recipients of the 2023-2024 William and Dorothy Gilbert Scholarship in Biomedical Sciences. This award was established in 1995 by Professor Terrance Snutch in honour of William and Dorothy Gilbert of Santa Fe, New Mexico. The award is presented annually to recognize outstanding doctoral students at the Michael Smith Laboratories.
Marie Johns joined the MSL in 2021 as a graduate student after completing her Bachelor of Science at UBC. Her interest in biology and love of the lab environment encouraged her to join the Jefferies lab (MSL, Department of Medical Genetics, Department of Microbiology and Immunology) as a Master’s student in Medical Genetics, before she transferred into the Medical Genetics PhD program.
“I’ve always been fascinated with biology, especially neuroscience and genetics. After joining the lab, I was very content with my supervisor, my lab mates, our research, and especially my thesis project, so when my supervisor suggested I fast-track to the PhD program, I was very excited to extend my studies that way,” shares Johns.
Johns’ research involves the use of both mouse and human stem cell-derived models to study Alzheimer’s disease. Her work aims to identify some of the molecular processes and mechanisms of Alzheimer’s, with the hope that this will lead to new targets for drug therapy. As part of this, Johns is looking for targets that might even be acted upon by existing drugs as a means of making new treatments available more quickly in the future.
Johns has particularly enjoyed how, through her work, she’s been able to identify and try to fill research gaps in real time. Her project grew from an extensive literature review to see what had been done before, and what still needed to be investigated in the field.
“It was really exciting to be able to design my PhD project

specifically around filling current Alzheimer’s disease research gaps, then put the project right into practice in the lab.”
Johns hopes that, once finished her PhD project, she may have the opportunity to follow-up on her findings, and to follow the progress that is made in this area in the future.
Reflecting on what this award means at this time in her studies, Johns emphasizes, “it’s really important that awards like this are available to students to receive recognition and support for their research, especially as early-career scientists. It’s a great encouragement to keep going even when the research can be challenging.”
Congratulations again to Marie! We’re excited to take part in celebrating her achievements thus far, and look forward to following the outcomes of her research projects.
Dr. Hayley Merkeley, MD, FRCPC, is a Principal Investigator at the CBR, co-director of the UBC Hematology Residency Training Program, clinical assistant professor in the Department of Medicine at UBC and a hematologist at St. Paul’s Hospital and Vancouver General Hospital. Dr. Merkeley completed medical school, internal medicine and hematology at UBC prior to completing a fellowship in red cell disorders at the University of Toronto. Her main research and academic interests are in sickle cell anemia, thalassemia, ITP and prophyria.
Dr. Merkeley joined the CBR just this past year and since then, she’s participated as a speaker at the Norman Bethune Symposium, presented at a CBR seminar and had a student partake in the 2024 CBR Summer Studentship Program.
Can you tell us a bit more about your research background?
I dabbled in research projects in medical school and internal medicine residency (mainly quality improvement). It was not until my subspecialty training in Red Cell Disorders and Master’s in Public Health that I started to do more clinical research as a means of improving the care of my patients. Currently, I am involved in clinical trials of new therapeutics as well as quality improvement projects for the patient populations that I care for.
What inspired you to become a hematologist and a researcher?
I love how visual and objective hematology is compared to many other specialties. In my subspecialty of red cell disorders, I can go down the lab and look under the microscope and generally know what kind of disorder my patient has based on the shape of their red cells. Being involved in research ensures that my patients are able to access novel treatments to improve their quality of life.
Could you tell us what a day in your life looks like?
Depends on the day! Most days I am in my outpatient office seeing patients and reviewing test results. I often have learners in my clinics and work quite closely with our allied health team of nursing, pharmacy and physiotherapy. Other days I am on call at the hospital caring for hematology inpatients receiving chemotherapy or other treatment, overseeing apheresis treatments and seeing new consults for hematologic issues.
I read that you completed your medicine degree, internal medicine and hematology training at UBC. What was your favourite thing about the UBC campus? Can you share your favourite place to eat at?
The endowment lands are a highlight for sure. In medical school I used to run through the woods and now I take my kids there to ride their bikes on rainy day weekends. So much has changed in terms of food services from when I was regularly on campus. I would have to say a cold pint and fries from Koerner’s pub is always a classic (which makes me feel a bit classic, since most of the other institutions are new from when I was on campus).

What advice would you have for emerging researchers?
Be open to a breadth of research experiences. I think it’s easy to think that all research is basic science and benchbased and sometimes be intimidated by that. Clinical research can be quantitative or qualitative and even small projects can meaningfully impact patients’ lives.
What excites you most about your work?
I love being able to collaborate with patients on their care. We can take treatments at the forefront of medical science like cellular therapy and radically transform people’s lives. Most topically to my practice is that there are now commercially available CRISPR treatments for sickle cell disease and thalassemia that provide functional cures.
What are the challenges of being a clinicianresearcher?
Time and energy. I don’t have any protected time or funds for research, so it’s often something that is added on to my clinical workload and using the free labor of the residents and medical students that I supervise. I can’t imagine not being involved in research as that would limit treatments to my patients and system improvements.
When you’re not working, what other activities do you enjoy?
I love hanging out with my kids, travelling, cooking and yoga/pilates. My undergraduate degree is in Spanish and I wish I had more time to keep it up. I guess I will just have to take a sabbatical in Spain one day!

BY RHONDA THYGESEN, CBR ALUMNI
Menstrual health serves as a key indicator of women’s overall well-being, and conditions like amenorrhea—characterized by the absence of menstruation—can point to serious underlying health concerns. Amenorrhea is defined as the absence of menstrual flow for 12 months or more post-menarche, not due to pregnancy, lactation, medication, or surgery2. New research from the UBC Centre for Blood Research and collaborators throughout UBC, examines the prevalence of early prolonged secondary amenorrhea in women with HIV and its associated biopsychosocial factors.
The study, “Associations of Early Prolonged Secondary Amenorrhea in Women With and Without HIV,” compares menstrual health outcomes between women living with HIV and HIV-negative women. Findings reveal a higher prevalence of amenorrhea among women with HIV, alongside other modifiable risk factors such as substance use and food insecurity.
In this study, the definition of amenorrhea was broadened to include cases of early menopause or premature ovarian insufficiency. While amenorrhea can be part of natural life changes, prolonged absence of menstruation before menopause can increase the risk of morbidity and mortality, making it a serious concern for women’s health.
The study analyzed data from two established HIV cohorts, focusing on women aged 16 and older who were not pregnant, lactating, or had a history of eating disorders like anorexia or bulimia nervosa. Out of 317 women with HIV and 420 women without HIV, the researchers found that 24% of women living with HIV experienced lifetime amenorrhea, compared to 13.3% of women who are HIV-negative.
This significant difference raises questions about how HIV influences menstrual health. According to the study’s multivariable analysis, HIV itself was found to be an independent predictor of amenorrhea, with an adjusted odds ratio of 1.70. This suggests that women with HIV may face a 70% greater likelihood of experiencing amenorrhea compared to women without HIV, even after accounting for other factors.
In addition to HIV status, several biopsychosocial variables were identified as important risk factors for amenorrhea. These include:
Older age: As expected, older women were more likely to experience amenorrhea, which may be related to natural declines in reproductive function.
White ethnicity: Women of white ethnicity were more likely to report amenorrhea, though the reasons for this are not fully

understood and warrant further investigation.
Substance use history: A striking finding was the strong association between substance use and amenorrhea, with women who had a history of substance use being over six times more likely to experience amenorrhea.
Food insecurity: Women facing food insecurity were twice as likely to experience amenorrhea, highlighting the role of socioeconomic factors in menstrual health.
The study’s findings underscore the importance of regular menstrual health assessments in women with HIV. With nearly one-quarter of women with HIV reporting amenorrhea, healthcare providers should be aware of the higher risks in this population and consider the broader social and behavioral factors that may be contributing to menstrual disturbances.
The modifiable nature of many of the risk factors—such as substance use and food insecurity—suggests that targeted interventions could reduce the prevalence of amenorrhea and improve overall health outcomes for women with HIV. Addressing these socio-structural determinants is critical in ensuring better health and quality of life for women living with HIV.
Ultimately, this research calls for more than just clinical intervention. It highlights the need for broader societal efforts to address food insecurity and substance use in vulnerable populations. By advocating for change at the intersection of health, social policy, and economic conditions, healthcare providers can help mitigate the risks of amenorrhea and promote menstrual health equity for all women, especially those living with HIV.

Congratulations to Dr. Aly Karsan! Dr. Karsan is one of 34 projects led by UBC researchers awarded a collective $7 million in funding provided through the CFI’s John R. Evans Leaders Fund (JELF). He is also one of two research teams led by UBC faculty of medicine professors that have been awarded more than $9 million in combined funding through the 2024 Terry Fox New Frontiers Program Project Grants (PPG) to expand cancer research and improve treatments.
Canada Foundation for Innovation (CFI) John R. Evans Leaders Fund (JELF)
Faculty of Medicine-led projects
Aly Karsan (Pathology and Laboratory Medicine) –Overcoming Therapy Resistance in Leukemia
Terry Fox New Frontiers Program Project Grant
One B.C.-based project, led by Dr. Aly Karsan, a professor in UBC’s department of pathology and laboratory

medicine, is receiving $7.5 million in renewal funding to take their work on leukemia to the next level, exploring epigenomic changes that influence the cancer’s behaviour and treatment response.
Project lead: Dr. Aly Karsan (Pathology and Laboratory Medicine)
Project: Strategies to divert malignant potentials in acute leukemia
Cancer Topic: Cancer Biology
Partners: Université de Montreal, UBC, BC Cancer Research Institute
We would like to thank the Naiman-Vickars Endowment Fund, Canadian Blood Services & The Faculty of Medicine for their continuous support, without whom the Norman Bethune Symposium and many CBR Programs would not have been possible:










































