Play with Your Food Before You Eat: Exploring the Effect of Dominance on Play
Initiation and Foraging
Varecia variegata rubra
Varecia variegata variegata
Priority in Red (Varecia variegata rubra) and Blackand-White (Varecia variegata variegata) Ruffed Lemurs (Primates, Lemuridae)
V Woolf
Senior Thesis | 2025
Play with Your Food Before You Eat:
Exploring the Effect of Dominance on Play
Initiation and Foraging Priority in Red (Varecia variegata rubra) and Black-and-White (Varecia variegata variegata) Ruffed Lemurs (Primates, Lemuridae)
V Woolf
Boston University Academy, Boston University. E-mail: vwoolf@bu.edu
Abstract— Female dominance behaviors define the social structures of many lemur species, including ruffed lemurs (Varecia spp.), influencing all aspects of their sociality and behavior. This study examines the interplay of dominance, play, and foraging behavior in a small population of captive black-and-white and red ruffed lemurs. The analysis focuses on how age and sex influence play initiation frequency and foraging priority behavior. Existing literature suggests that older females displayed higher aggression and often moderated group interactions, reinforcing matriarchal hierarchies. Play behaviors reflect dominance influences, with older females managing or vetoing play more often while younger males initiate play more frequently. Foraging priority refers to the order in which individuals access food, often reflecting dominance hierarchies, while foraging locomotion describes the movement and effort involved in finding and acquiring food. Dominance affects foraging priority by granting higher-ranking individuals first access to high-quality feed; in foraging locomotion, dominant individuals may expend less energy, as they can claim resources more directly, while subordinates often travel farther or search longer to find food, increasing their energy costs. In contrast, this study finds no significant differences in play initiation acceptance frequency between the females and males, while foraging priority varies between the groups and individuals. Several factors, including kinship, may contribute to these findings. The kinship of the red-ruffed lemur sisters may have mitigated aggression, fostering more egalitarian interactions. The increased variability in play behaviors between the red ruffed lemur pair further supports the importance of kinship ties in shaping social dynamics. Meanwhile, the black and white ruffed lemurs exhibited a less egalitarian social structure. These findings suggest that lemur dominance behaviors are shaped by a complex combination of innate social structures and environmental influences.
Keywords: Ruffed Lemurs Play Initiation Foraging Priority, Foraging Locomotion, Dominance, Captivity, Observational Study, Varecia variegata variegata, Varecia variegata rubra
INTRODUCTION
Female social dominance is a prominent feature of many lemur species, shaping their social structures and behaviors (Lewis 2019; Waeber 2003; Meyer 2000). While the evolutionary basis for female dominance in lemurs remains unresolved, hypotheses suggest it may be linked to environmental pressures, metabolic factors, or the high metabolic demands of reproduction (Lewis 2018; Dunham 2008; Meyer 2000). Some studies have emphasized female feeding priority in species with low basal metabolic rates and high maternal investment like ruffed lemurs (Meyer 2000; Overdorff 2005). However, other research, including findings from this study, suggest that female dominance may not be so straightforward. Lemur dominance appears to depend on a range of traits, including social behavior (Martin 1972; Seex 2024). The prevalence of female dominance in black-and-white and red ruffed lemurs in the existing literature posits that this behavior could be an instinctive aspect of their social organization.
Research into black-and-white ruffed lemur social dynamics can help in exploring the extent of dominance and foraging priority, especially in influencing key factors such as group dominance behavior and food access. However, consistency in results varies. In some cases, females lead the group to food sources and experience higher food intake, especially on first arrival (Overdorff 2005). On the other hand, complex group dynamics led to a shift in dominance and, therefore, foraging behavior; for Overdorff’s group, the remaining females did not lead the group or experience higher food intake than males. To further complicate the study, kinship relationships also play a large role in dominance and the stability of females within the hierarchy (Overdorff 2005). The variability in foraging priority dynamics in the literature suggests that rather than a clear-cut female dominant society, factors like group stability, the number of females, kinship, and reproductive status can shape dominance behavior.
Intragroup feeding competition can also influence foraging behavior, as dominant individuals influence food access and group foraging patterns. For example, when food resources are limited, dominant individuals are likely to control access to high-quality food, directing the group's movement toward these resources and ensuring the higher-ranked individuals reach food sources first (Garber 1987). This paper’s exploration into resource utilization and the following costs of locomotion also relate to dominant individual behavior, with their influence affecting food access in foraging. In some case studies, more dominant individuals receive first-priority access to higher quality feed with less energy spent on foraging locomotion (Garber 1987). Garber posits that foraging patterns can also reflect solutions to ecological challenges, and dominance hierarchies could provide an adaptive strategy in responding to resource scarcity or distribution, supporting this paper’s observations of the flexibility of dominance hierarchies based on environmental changes (Garber 1987). These findings suggest that lemur dominance behaviors are shaped by a complex combination of innate social structures and environmental influences and are not as clear-cut as previously posited in the existing literature.
Captivity is another obstacle in the observational study of primates, especially lemurs. Research into captive ruffed lemurs highlights age, sex, and reproductive dynamics as key factors shaping social aggression, which affects social dominance behaviors (Cherevko 2020; Raps 1995). Some studies have found that older females exhibit the highest levels of aggression, particularly in breeding contexts, with aggression at a maximum between reproductive females (Cherevko 2020). However, this aggression is moderated in interactions using affiliative behaviors and kinship, suggesting that familial ties play a role in mitigating conflict (Cherevko 2020; Dunham 2008). Males, by contrast, show low rates of aggression, often adopting submissive behaviors that reinforce the matriarchal structure (Cherevko 2020; Kappeler
1993). These results support the effect of many factors to control dominance such as age, sex, and reproductive dynamics, and further posit that maintaining social structures varies within captive lemur populations.
Play also provides an important look into the dominance dynamics of ruffed lemurs, as play can be used to release stress, build bonds, and assert existing dominance roles. Studies of ringtailed lemurs, a species closely related to ruffed lemurs, further explore the link between female aggression and social play. Some case studies found that female aggression extended into play behavior, with females displaying more dominant and aggressive play styles, including increased biting and chasing (Grebe 2019; Pereira 1993). However, as individuals aged, overall rates of play decreased, and notable sex differences emerged: males initiated play more frequently, while females mainly vetoed play interactions (Grebe 2019). This highlights the influence of female aggression and the effect it has on dominance dynamics and social play, which may be influenced by hormonal and social mechanisms over time.
Together, these case studies provide a critical foundation for understanding the interplay between dominance, aggression, and social behavior in lemurs. This study continues the work in this field, focusing on play initiation frequencies in red ruffed lemurs (Varecia rubra) and black-and-white ruffed lemurs (Varecia variegata). We aim to examine how these interactions are influenced by age, sex, and social structure. We hope to further the understanding of dominance and aggression influences on behavioral expression in ruffed lemurs. This study examines the frequency of play initiation across four individual ruffed lemurs and the frequency that play was rejected or vetoed. Research on ruffed lemur social dynamics posits that female dominance and foraging priority influence food access; however, both in this study and in the existing literature, results have a large spectrum of variation. In some groups, females lead to food and consume more,
while in others, the status of the dominant female shifts foraging behavior. Kinship, reproductive status, and group stability also affect dominance and foraging patterns. Many case studies in the existing literature suggest that foraging behavior is shaped by a combination of social hierarchies and ecological factors, with dominance hierarchies providing adaptive strategies for responding to resource availability (Garber 1987; Overdorff 2005). This research’s outcome could provide further insight into how social roles and dominance hierarchies influence play behavior and foraging behavior within captive ruffed lemur group dynamics. We propose that the social structure, specifically in dominance hierarchies, significantly impacts all behavior of ruffed lemurs, and that the oldest and most dominant female(s) are expected to play a key role in moderating play interactions, by managing play requests and claiming top priority in foraging and foraging locomotion (Meyer 2000; Cheverko 2020; Grebe 2019; Overdorff 1987; Garber 2005). This demonstrates the influential role of dominant individuals in shaping group interactions while supporting the claims of existing literature for the complex shaping of social structure in captive ruffed lemurs.
MATERIALS
Population Details
The study was conducted with a population of four ruffed lemurs (Varecia spp.) housed at Capron Park Zoo in Attleboro, MA. The population consists of two red ruffed lemurs (Varecia rubra) and two black-and-white ruffed lemurs (Varecia variegata).
Housing Details
The black-and-white ruffed lemurs are housed on an outdoor island exhibit designed to encourage natural behaviors. This exhibit includes various forms of enrichment such as tire swings, ladders, and climbing boxes. The red ruffed lemurs were housed in their winter exhibit, a smaller enclosure with a chicken wire ceiling and front. While both environments allow for physical activity and climbing, the size and structural features differ, potentially influencing the frequency of interactions (González 2021).
Demographic Details
The age distribution of the individuals was divided into three groups: the two red ruffed lemurs (full sisters) are the youngest individuals in the group. The male black-and-white ruffed lemur is approximately one year older than the red ruffed lemurs. The eldest individual is the female black-and-white ruffed lemur, who is also the only previously parous individual in the group and is two years senior to the red-ruffed pair.
Social Context
The same sub-species pairs of lemurs are housed together in exhibits, providing opportunities for naturalistic social interactions, including play. Observations were conducted within this shared environment to account for the dynamics of group living.
Data Collection
Observational data was collected during two periods: September 22 & 23 and November 27 & 29. Focal studies were conducted to capture individual behaviors related to the focus, either play initiation or foraging behavior. Each focal observation lasted 20 minutes, with one red-ruffed lemur and one black-andwhite ruffed lemur observed per day for a total observational period of 20 hours.
In the behavioral ethogram, play initiation (Poᵢ) was defined as the successful initiation of a play event or bout involving two or more individuals. Play rejection (Poᵣ) was defined as the vetoing of a play initiation attempt by another individual, either through aggressive or non-aggressive rejection. A key limitation in this observation setup was the inability to differentiate between initiating play and continuing ongoing play. As a result, any instance of Poᵢ recorded without a corresponding instance of Poᵣ was classified as a bout of play in the dataset.
Ethogram Behavioral Definitions
Dominance behaviors were also recorded using a standardized ethogram. These behaviors included tail pulling (Dtp), where an individual yanks another's tail to provoke a response; biting (Db), defined as an individual using their teeth to close on another's body to elicit a reaction; and hitting (Dh), characterized by forceful physical contact intended to provoke a response. Submissive locomotion (SL) was also observed, involving movement with a lowered head and tail, either approaching or avoiding another individual, often lagging behind when following. Additionally, instances where focus individuals were out of sight (OOS) were noted, with behaviors or states assumed based on contextual cues. Vocalizations were categorized based on their energy levels and their role in the interaction. Vocalization (V) refers to auditory sounds produced by an individual, either as an
initiation, communication, or reaction to another lemur’s actions or sounds. Vocalize Escalation (Vd) denotes the first instance of a new event or state within a sequence, characterized by a highenergy or loud volume approach. In contrast, Vocalize Placate (Vp) marks the first occurrence in a sequence, but with a low-energy or soft volume, typically in response to a non-vocal event or state.
Respond to Vocalization Escalation (RVe) involves an individual’s auditory response to another’s event, with an increase in energy or volume, while Respond to Vocalization Deescalation (RVd) represents a response with reduced energy or softer volume. These categories were used to analyze the dynamics of vocal communication in various states of escalation and de-escalation.
For the foraging priority hypothesis, the key behaviors were recorded as Foraging or Eating (F/E) which is defined as looking for, modifying, and masticating edible material either from the environment or via keeper placement, and Foraging Locomotion (FL) which is defined as motion around their area including: quadrupedal motion such as crawling and climbing, or non-quadrupedal motion like hanging or jumping. Arboreal or terrestrial, for the purpose or intent of foraging or ending in eating. Within Foraging, the ethogram defines the subcategory of social foraging, including Sharing Food (Ssf) and Mutual Foraging (Smf). Sharing food is defined as including or donating food to another lemur, either willingly or forced. Includes all edible materials, uneaten or eaten, while Mutual Foraging is defined as looking for, modifying, and masticating edible material either from the environment or via keeper placement with another individual within ~2 ft.
Data Cleaning Treatment
The recorded data was subsequently cleaned to remove inconsistencies and errors and then quantified for analysis, enabling a clearer understanding of the frequency and context of dominance behaviors within the group. The data was sorted from
non-necessary behaviors that were observed, and the bouts of behaviors observed were standardized into the 20-minute focal studies. The data was quantified into date, observer, study subject, bouts of desired behavior, priority if applicable, and sex. In the foraging priority and locomotion data, the quantification process included recognizing if priority was displayed during the desired behaviors. The three options for this column were 0, X, and Y. X represents the previously named dominant individual displaying priority, the Y is the previously named submissive individual and 0’s represented any time priority could not be determined. Although we also ran the 0s as a consideration of submissive behavior to explore that possibility and see if that would provide a different outcome than non-significant results.
Data Analysis Process
To analyze the play initiation dataset, the data was sent to two data processors, separated on hypothesis. The play initiation data was sent to Professor Schmitt, and the foraging priority and locomotion data was sent to Nathan Wu. The employed statistical methods were used to investigate patterns in play initiation. A ttest was conducted to compare the frequency of play initiation between sexes. To examine the influence of age on play initiation frequency, a one-way analysis of variance (ANOVA) was performed. Additionally, a factorial ANOVA was used to assess the interaction effects of age and sex on play initiation frequency, followed by post hoc Tukey pairwise comparisons to identify specific group differences. Before the analysis, data preprocessing was necessary to ensure compatibility with statistical tests. Missing values in the dataset were either replaced with zeros or imputed based on individual behavior data to maintain consistency. Column names were standardized to remove spaces and special symbols, facilitating smoother data manipulation and analysis.
The foraging priority and locomotion datasets were organized into two groups: dominant and submissive individuals, where the predefined dominance status of lemurs was applied to
the population. In this study, the older previously parous female black-and-white ruffed lemur was defined as more dominant than her male counterpart, and the older larger red ruffed lemur was defined as more dominant than her sister. Initially, based on the small instance of submission behavior in the data, only 3-2 instances of submissive behavior per group, 0s were included as submissive (Y) to increase the sample size. 0s in the quantified data were focus actions previously defined by the ethogram with no clear submissive or dominant position of the focal individual. For example, if the two red ruffed lemurs were foraging separately, it was considered a 0 dominance hierarchy. The proportion of dominant behavior (Y) was calculated for each group, with 0s representing egalitarian behavior (i.e., no submissive or dominant actions). For the entire group, the success rate (dominant behavior) was 64.4% (p = 0.644), with 35.6% of instances being submissive or egalitarian (q = 0.356). In the dominant group (B & D), the proportion of dominant behavior was 60% (p1 = 0.6), and submissive or egalitarian behavior was 40% (q = 0.4). In the submissive group (A & C), the proportion of dominant behavior was 69% (p2 = 0.69), and submissive or egalitarian behavior was 31% (q = 0.31). To test the difference between these groups, a zstatistic was calculated using the formula: ! = !! !" #!(% !)( ! #! ' ! #" ) .
The calculated z-value was -0.7217855732. The p-value was then determined using R, resulting in a p-value of 0.4704264. The pvalue was calculated using this function in R, resulting in a value of 0.4704264.
Figure 1: The p-value was calculated using this function in R, resulting in a value of 0.4704264. Since the test was two-tailed (to determine if there was a significant difference, not only to determine if p1 was greater than p2), the p-value was multiplied by 2. This is based on the ! = !! !"
) test formula.
RESULTS
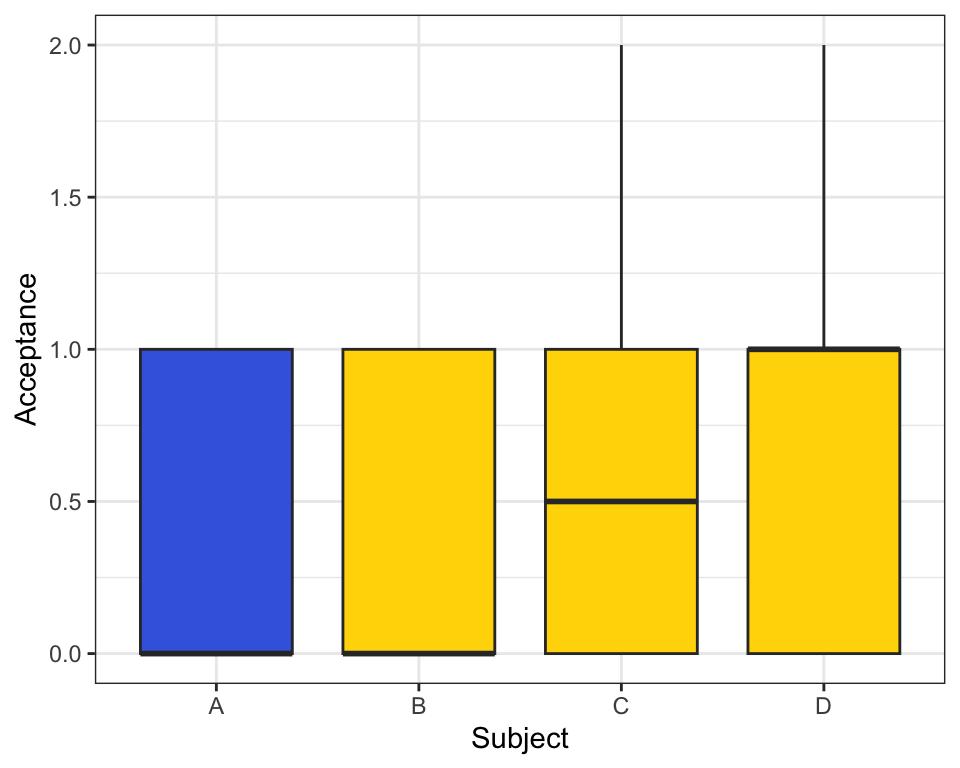
Figure 2: The bar plot illustrates the levels of Play Acceptance for four subjects (A, B, C, and D), with acceptance represented on the y-axis (ranging from 0 to 2) and subjects on the x-axis. Subject A, represented by a blue bar, corresponds to the male black-and-white ruffed lemur, while Subject B corresponds to the female black-andwhite ruffed lemur. Subjects C and D, also represented by yellow bars, correspond to the female red-ruffed lemurs. The height of each bar reflects the mean acceptance level for each subject.
Overall, the analysis revealed no significant differences in play acceptance between the subjects. The acceptance levels for the black-and-white ruffed lemurs (A and B) were relatively consistent, showing minimal variability. In contrast, while the red ruffed lemurs (C and D) exhibited greater variability in acceptance, this did not translate into a significant difference from the black-
and-white ruffed lemurs. In Figure Two, the error bars for subjects C and D reflect this variability, but the overall pattern suggests that both groups exhibit similar levels of acceptance. It is important to note that the lack of significant differences in play acceptance rates between subjects and sexes may be attributed to the small sample size in the dataset. With a larger sample, it is possible that a more substantial difference between the groups (C/D vs. A/B) could emerge. However, based on the current data, we conclude that the levels of play acceptance across subjects appear to be similar.
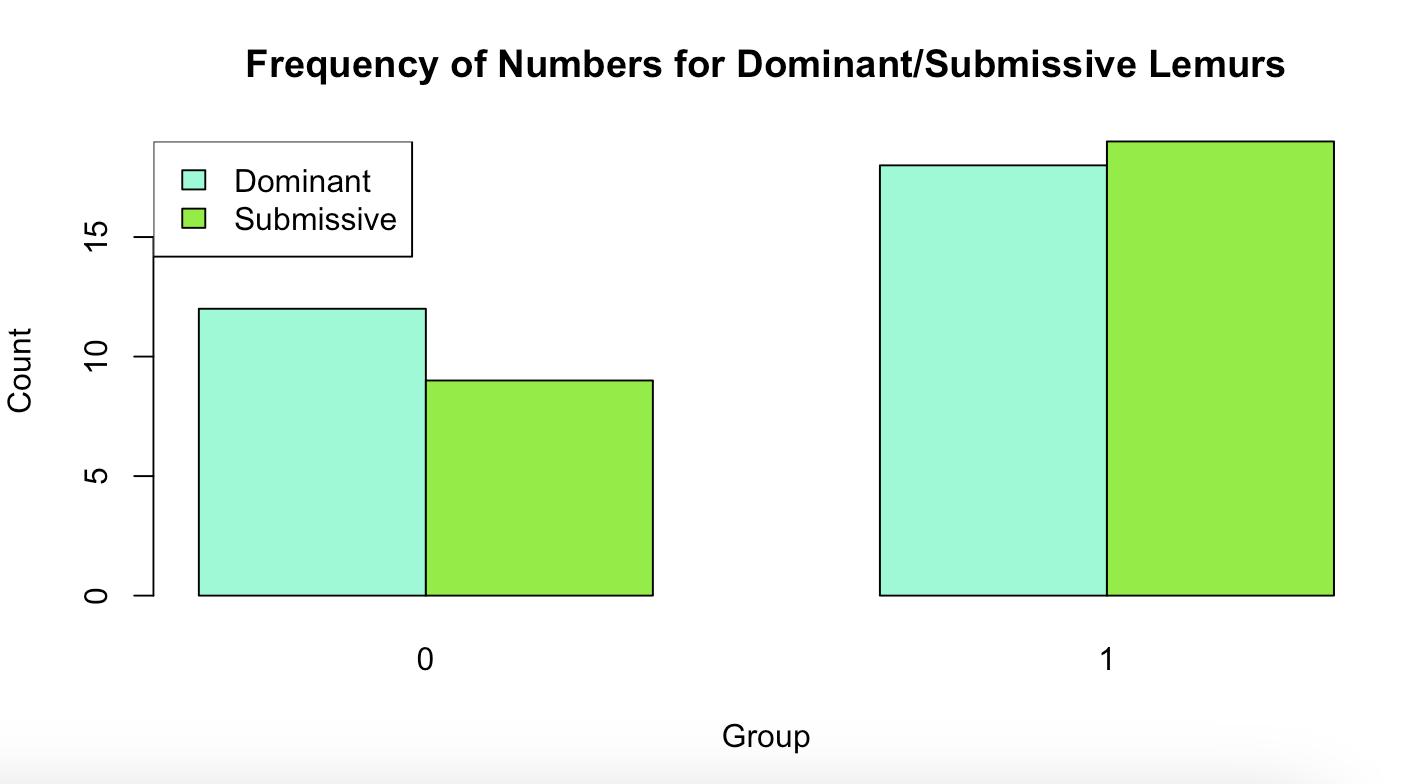
Figure 3: This graph displays the frequency of dominant and submissive actions in two previously status-determined lemur groups during foraging priority and foraging locomotion. The xaxis represents the two groups (Group 0 and Group 1), while the yaxis indicates the count of dominant and submissive actions. Dominant lemurs (B and D) are represented in light blue, while
submissive lemurs (A and C) are in green. In Group 0, dominant actions occur more frequently than submissive ones, whereas in Group 1, the counts of dominant and submissive actions are nearly equal. This suggests variability in foraging behavior across the groups.
For the foraging priority data, the analysis revealed patterns in the frequency of dominant and submissive behaviors across two different metrics: dominance/submissiveness on an individual basis (Figure Four) and dominance/submissiveness in foraging priority and locomotion (Figure Three). However, no significant outcomes emerged from the dataset in either case.
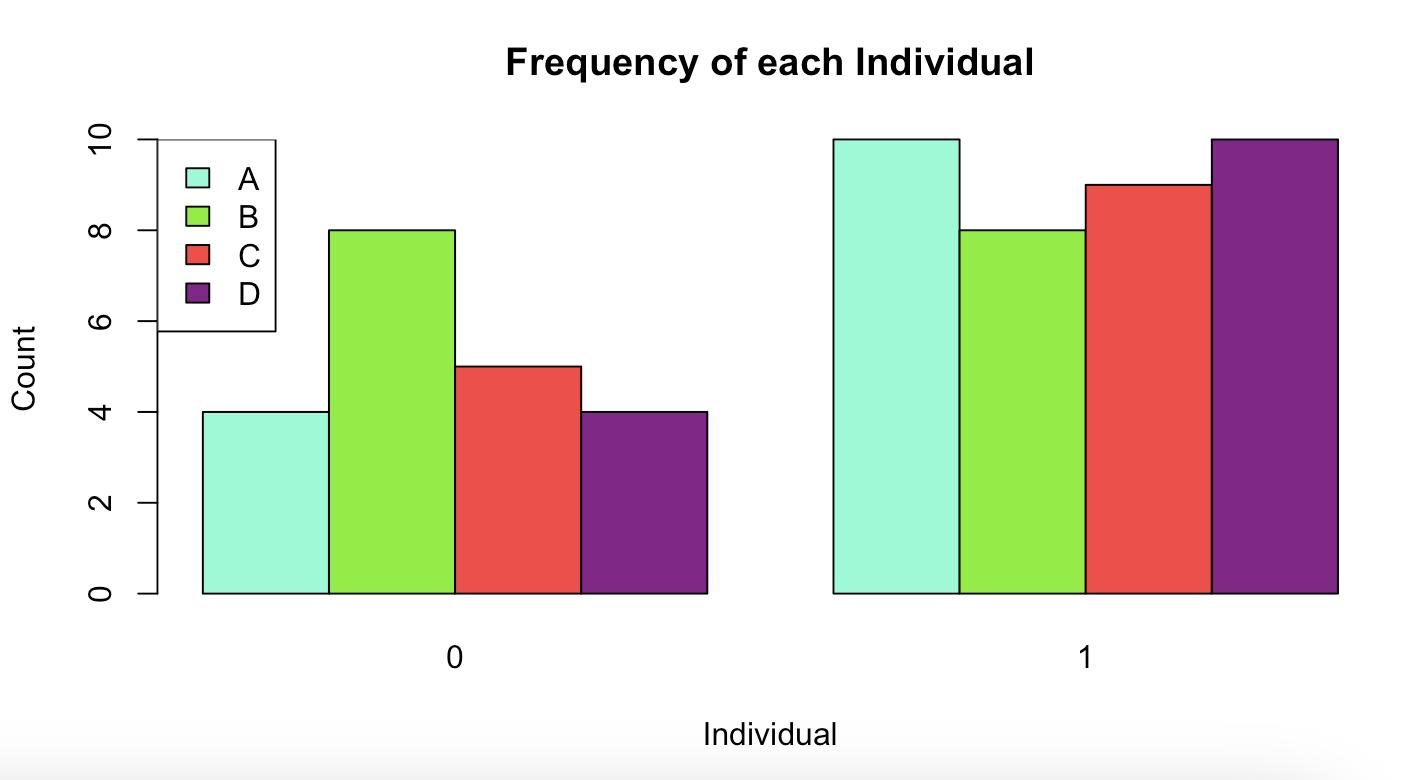
The data outcomes suggest that certain individuals may exhibit consistent behavioral roles. For instance, Individual B shows the highest frequency of submissive behavior, while Individual A displays the highest frequency of dominance. This could indicate role specialization within their social structure, where some individuals predominantly assume submissive or dominant roles even if it is not the hypothesized role for each individual as predicted by the theory. This contrast of the outcome to the hypothesis could suggest the similar patterns seen in the literature of variability.
The results may reflect an established social hierarchy. Individual A's dominance could indicate a higher-ranking position, whereas Individual B’s frequent submissiveness suggests a lower position in the hierarchy. On the other hand, the balanced dominance frequencies across B, C, and D on the dominant side (1) suggest that dominance behavior is not solely concentrated in one individual, except for A. This could mean that dominance is context-dependent and shared among individuals, with A possibly taking a lead role in specific scenarios. Intra-group dynamics may also help explain the lower submissive frequencies of Individuals
A and D. This variability could be indicative of individual personality traits or kinship relationships within the group.
Figure 4: The graph illustrates the frequency of individuals being submissive (0) or dominant (1) across four individuals while foraging: A, B, C, and D. On the submissive side (0), B displayed the highest count, followed by C, while A and D have lower frequencies. In contrast, on the dominant side (1), Category A has the highest frequency, with Categories B, C, and D displaying relatively balanced and similar counts. The categories are represented by distinct colors: light blue for A, green for B, red for C, and purple for D.
However, the possible influence of external factors may have skewed the behavior or outcomes of the lemur groups. Categories such as resource availability, group composition, or
environmental stressors could have affected the captive lemur groups. For instance, A’s high dominance frequency could be linked to success in accessing or controlling resources. The observed patterns provide a foundation for exploring questions about the stability of these behaviors over time, the influence of age or sex on dominance roles, and how these interactions affect group cohesion or survival outcomes. Further data could also examine whether these trends persist across different contexts or environmental conditions. While these observations highlight potential behavioral differences between groups, the lack of significant outcomes suggests that these variations may not be robust or consistent. The small sample size in this dataset limits the ability to draw meaningful conclusions, and further studies with larger datasets may be required to clarify the role of group dynamics, dominance, and submissiveness in foraging and other social behaviors. Based on the current data, these patterns remain inconclusive, with no statistically significant outcomes to support strong claims about the behavioral dynamics of the groups.
DISCUSSION
These results are novel in their display of a more egalitarian social structure in ruffed lemurs, however, this is likely because of the experimental design and certain factors. One possible explanation for novel results against strong female dominance is the limited sample size in the current dataset. Given the observed trends, it is plausible that, with a larger sample size, a more pronounced difference between the groups could be detected, particularly between the opposite sexes. Additionally, the lack of significant findings could be related to the complex kinship dynamics within the red-ruffed lemur group, as both subjects C and D are full sisters, and kinship exceptions have been previously observed between female ruffed lemurs (Cheverko 2020). Sibling relationships may play a crucial role in shaping their interactions and acceptance behaviors, potentially leading to a more uniform level of acceptance and less distinct dominance behavior (Lewis 2019). This complicates the interpretation of their play acceptance data, as their kinship ties could increase variability and contribute to the observed lack of significant difference.
While the current results do not reveal any significant differences in play acceptance between the subjects, future studies with larger sample sizes and careful consideration of kinship dynamics may provide more robust insights. Further exploration of other factors, such as individual temperament or environmental influences, could offer a deeper understanding of play acceptance behavior in lemurs (Collins 2017; González 2017). In addition, the limitations of the experimental design, such as the acceptance constrained by the number of play initiation recorded per focal observation and the lack of differentiation between initiating play and play continuing, could be improved upon during a future study with modified experimental design. Similarly, for the foraging priority and locomotion, the observed behavioral patterns in this study align with previous findings in the literature, highlighting the potential for variability in dominance and submissive roles within
ruffed lemur social dynamics and against the established female dominance social dynamics. The results suggest that certain individuals exhibit consistent tendencies, with Individual B displaying the highest frequency of submissive behavior and Individual A showing the highest frequency of dominance. This role specialization may reflect an established social hierarchy, where Individual A occupies a higher-ranking position and Individual B assumes a lower-ranking role. However, the relatively balanced dominance frequencies across Individuals B, C, and D indicate that dominance behaviors, apart from A's prominence, may be context-dependent and shared among group members. This pattern is consistent with previous research suggesting that dominance hierarchies in lemur groups are not rigid but may vary according to environmental conditions and intra-group dynamics (Garber 1987; Overdorff 2005).
The lower submissive frequencies observed for Individuals C and D could be indicative of kinship relationships because they are full sisters. While the submissive frequencies of the black-andwhite ruffed lemurs (A and B) could be influenced by individual personality traits or their roles within the group. These findings may suggest that dominance behaviors are not strictly dictated by sex or age but rather influenced by a combination of individual and environmental factors. For example, A’s high dominance frequency could be linked to his success in resource acquisition or control, as dominance hierarchies often emerge as adaptive strategies in response to resource scarcity or competition (Garber 1987). In this context, the observed patterns may reflect the group’s adaptive responses to social and ecological pressures. However, it is important to note that the dataset did not yield statistically significant results, limiting the ability to draw robust conclusions about the dynamics of dominance and submissive behavior in this group. The small sample size likely contributed to this lack of significance. Furthermore, external factors may have complicated or skewed the interpretation of the
results. The findings remain consistent with the variability in dominance and foraging priority dynamics described by Overdorff (2005), emphasizing the need for larger datasets to better understand these complex social structures. Future research should aim to explore whether the observed behavioral patterns persist over time and across different environmental contexts.
Longitudinal studies and experiments with a larger sample size could help clarify the roles of individual traits, kinship, and ecological factors in shaping dominance hierarchies. Additionally, studies investigating the effects of reproductive condition, group stability, and feeding competition on dominance behaviors would provide further insight into the adaptive significance of these social dynamics. While the current findings are varied, they provide a foundation for understanding the variability in social and foraging behaviors within ruffed lemur groups and underscore the importance of considering complex factors within group social dynamics.
CONCLUSION
This study contributes to the broader understanding of social dynamics and dominance behaviors in lemur species, specifically within the context of captive ruffed lemurs (Dzierga 2014; Meyer 2000). Female social dominance is a welldocumented characteristic in many lemur species, and previous research has often attributed this dominance to ecological or physiological factors (Lewis 2019; Waeber 2003; Meyer 2000). However, the current findings suggest that the role of female dominance in ruffed lemurs may be more complex, potentially being an innate feature of their social structure rather than solely driven by external pressures (Martin 1972; Seex 2024). While no significant differences were found in play acceptance between the sexes, the variability in red ruffed lemurs' play acceptance, particularly between full sisters, points to the possible influence of kinship dynamics in shaping social interactions and dominance behaviors.
This research highlights the potential importance of considering kinship and familial relationships when investigating social hierarchies in primates, especially in captive and small population sizes (Dzierga 2014; Meyer 2000; Cheverko 2020). The observed kinship ties between the red ruffed lemur sisters may have moderated their social interactions, potentially reducing aggression and dominance behaviors typically observed in breeding females (Cheverko 2020). This suggests that, in species with complex social structures, understanding the nuances of kinship dynamics is crucial for interpreting broader patterns of dominance and aggression. In addition to kinship, other factors such as sample size, environmental conditions, and the design of observational studies must be considered when evaluating social behaviors in captive populations. The limitations of the current study, including the inability to distinguish between initiating and continuing play and the restricted number of play initiation observations, demonstrate the need for refined methodologies in
future studies within this field of research. Expanding sample sizes and addressing methodological limitations may help clarify whether the absence of significant findings is due to sample constraints or reflects true social dynamics in these species.
The outcomes of the foraging priority dataset highlight the variability in dominance and submissive roles in ruffed lemur society and challenge the notion of strict female dominance often seen in the literature. Individual A exhibited the highest dominance frequency, while Individual B displayed the highest submissiveness, suggesting potential role specialization and a flexible social hierarchy (Pereira 1993). The balanced dominance frequencies among Individuals B, C, and D imply contextdependent behaviors shared within the group. These findings align with some prior research positing that lemur dominance hierarchies are shaped by environmental conditions, kinship, and individual traits, compared to being based solely on sexual or age discrimination. (Greber 2005; Chevereko 2020; Overdorff 1987). While our results suggest adaptive strategies linked to resource control and social pressures, it is difficult to assert one interpretation of the data because of the lack of statistical significance, likely due to the small sample size. Future research with larger datasets and longitudinal studies is suggested to clarify how kinship, reproductive condition, and ecological factors influence dominance dynamics (Meyer 2000). These findings provide further support for understanding the novel complexities of ruffed lemur social behavior, which was previously undiscovered.
Overall, this paper’s research outcome reinforces the idea that social structures in lemurs are influenced by a complex combination of traits, which includes instinctive behaviors, ecological factors, and complex social relationships (Lewis 2019; Waeber 2003; Meyer 2000). We suggest that further research into the role of kinship, age, sex, and environmental factors could potentially help researchers gain a deeper understanding of social behavior in ruffed lemurs and other lemur species. This paper
strives to contribute to the broader field of lemur and primate sociality as a whole, as the limits of the experimental design provide unique challenges for studying our focal species.
STATEMENTS:
Acknowledgements:
I would like to acknowledge and express my gratitude to Professor Schmitt for the support in writing this paper, as the first hypothesis of this thesis was written as part of his course, CASAN336: Primate Behavioral Ecology, and for his valuable assistance in analyzing my data. I would also like to express my gratitude to Nathan Wu for his work in my second hypothesis data analysis; the results and discussion sections would not have been possible without him! Lastly, I would like to acknowledge my sister, Ashleigh Woolf, for her assistance in aiding my data cleaning process as someone with experience in R and statistics as a whole.
Conflict of Interest Statement
The author has no conflicts of interest to declare.
REFERENCES
Cherevko, L. S. (2020). Aggressive behavior of black-and-white ruffed (Varecia variegata variegata) and red ruffed (Varecia variegata rubra) lemurs (Primates, Lemuridae). Biology Bulletin, 47(8), 1032–1042. https://doi.org/10.1134/S106235902008004X.
Collins, C., Corkery, I., Haigh, A., McKeown, S., Quirke, T., & O'Riordan, R. (2017). The effects of environmental and visitor variables on the behavior of free‐ranging ring‐tailed lemurs (Lemur catta) in captivity. Zoo Biology, 36(4), 250260.
Dunham, A. E. (2008). Battle of the sexes: cost asymmetry explains female dominance in lemurs. Animal Behaviour, 76(4), 1435-1439. (Duplicate)
Dzierga, B. M. (2014). Zutrition: Analyzing and evaluating diets fed to captive mammals at Capron Park Zoo.
Garber, P. A. (1987). Foraging strategies among living primates. Annual review of Anthropology, 16, 339 González, Gabriel Robinson. (2021). I like to move (it): Use of outdoor space in a mixed exhibit of ring-tailed lemurs (Lemur catta) and red ruffed lemurs (Varecia rubra) at Furuvik Zoo.
Grebe, Nicholas M., Courtney Fitzpatrick, Katherine Sharrock, Anne Starling, and Christine M. Drea. (2019). Organizational and activational androgens, lemur social play, and the ontogeny of female dominance. Hormones and Behavior, 115, 104554. https://doi.org/10.1016/j.yhbeh.2019.07.002.
Kappeler, P. M. (1993). Sexual selection and lemur social systems. In Lemur social systems and their ecological basis(pp. 223240). Boston, MA: Springer US.
Lewis, R. J. (2018). Female power in primates and the phenomenon of female dominance. Annual Review of Anthropology, 47(1), 533-551.
Lewis, R. J. (2019). Female power: A new framework for understanding “female dominance” in lemurs. Folia Primatologica, 91(1), 48-68.
Martin, R. D. (1972). Review lecture: Adaptive radiation and behaviour of the Malagasy lemurs. Philosophical Transactions of the Royal Society of London. B, Biological Sciences, 264(862), 295-352.
Meyer, Christoph, Taryn Gallo, and Stewart T. Schultz. (2000). Female dominance in captive red ruffed lemurs (Varecia variegata rubra). Folia Primatologica, 70(6), 358–361.
https://doi.org/10.1159/000021718.
Overdorff, D. J., Erhart, E. M., & Mutschler, T. (2005). Does female dominance facilitate feeding priority in black‐and‐white ruffed lemurs (Varecia variegata) in southeastern Madagascar? American Journal of Primatology, 66(1), 722.
Pereira, M. E. (1993). Agonistic interaction, dominance relation, and ontogenetic trajectories in ringtailed lemurs. Juvenile Primates: Life History, Development, and Behavior. Oxford University Press, New York, 285-305.
Raps, S., & White, F. J. (1995). Female social dominance in semifree-ranging ruffed lemurs (Varecia variegata). Folia Primatologica, 65(3), 163-168.
Seex, L. (2024). The self-organisation of lemur social systems. [Thesis fully internal (DIV), University of Groningen]. University of Groningen.
https://doi.org/10.33612/diss.973674249
Waeber, K., & Hemelrijk, C. (2003). Female dominance and social structure in Alaotran gentle lemurs. Behaviour, 140(10), 1235-1246.